CHAPTER 69 Encephalitis, Myelitis, and Meningitis
GENERAL CONSIDERATIONS
Bacterial, viral, protozoal, mycotic, rickettsial, and parasitic pathogens are all recognized as etiologic agents of inflammatory central nervous system (CNS) disease in dogs and cats. In addition, a variety of meningitis syndromes that have no identifiable etiology but are presumed to have an immunologic basis exist in dogs. These include a steroid-responsive meningitis-arteritis (SRMA) of young dogs, granulomatous meningoencephalomyelitis (GME), and necrotizing meningoencephalitis (NME).
The clinical signs of CNS inflammation vary and depend on both the anatomic location and the severity of inflammation. Individual syndromes may have characteristic constellations of clinical signs. Cervical pain and rigidity are common in dogs with meningitis of any etiology, causing a reluctance to walk, an arched spine, and resistance to passive manipulation of the head and neck (Fig. 69-1). Fever is common. Inflammation of the spinal cord (myelitis) will cause associated upper motor neuron (UMN) or lower motor neuron (LMN) deficits in the limbs, depending on the spinal cord region involved. Animals with inflammation in the brain (encephalitis) can experience vestibular dysfunction, seizures, hypermetria, or disorders of consciousness reflecting the distribution of intracranial lesions.
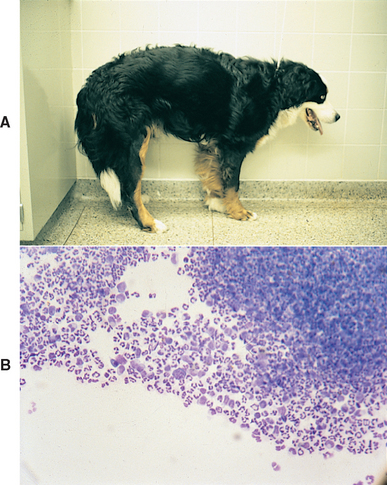
FIG 69-1 A, A young Bernese Mountain Dog with steroid-responsive meningitis arteritis stands with an arched spine and is reluctant to walk because of pain. B, Cerebrospinal fluid from this dog is inflammatory, with a dramatic neutrophilic pleocytosis.
(From Meric S et al: Necrotizing vasculitis of the spinal pachyleptomeningeal arteries in three Bernese Mountain Dog littermates, J Am Anim Hosp Assoc 22:463, 1986.)
A thorough physical and ophthalmologic examination and search for systemic abnormalities should be performed. Dogs and cats with bacterial meningitis/meningoencephalitis usually have an infected site from which the infection has spread to the CNS. Animals with viral, protozoal, fungal, or rickettsial meningitis/meningoencephalitis may have involvement of other organs, such as the lung, liver, muscle, or eye, which may aid in diagnosis. Cerebrospinal fluid (CSF) analysis is necessary to confirm a suspected diagnosis of CNS inflammatory disease. Analysis of the cells found in the CSF, together with the clinical and neurologic findings, may aid in determining the etiology of the inflammation in an individual case (see Box 64-3). Analysis of CSF protein, CSF culture, measurement of serum and CSF antibody titers for likely infectious agents, and CSF polymerase chain reaction (PCR) analysis may also be of diagnostic value. These results, with the use of other appropriate ancillary diagnostic tests, allow diagnosis of a specific disorder and the initiation of prompt appropriate treatment (Table 69-1).
 TABLE 69-1 Ancillary Tests in the Diagnosis of Infectious Inflammatory Central Nervous System Disease
TABLE 69-1 Ancillary Tests in the Diagnosis of Infectious Inflammatory Central Nervous System Disease
| DISORDER SUSPECTED | ANCILLARY DIAGNOSTICS |
|---|---|
| Acute distemper (D) | |
| Bacterial (D, C) | |
| Toxoplasmosis (D, C) | |
| Neosporosis (D) | |
| Feline infectious peritonitis (C) | |
| Cryptococcosis (D, C) | |
| Rocky Mountain spotted fever (D) | |
| Ehrlichiosis (D) |
D, Dog; C, cat; RT-PCR, reverse-transcriptase polymerase chain reaction; CSF, cerebrospinal fluid; CT, computed tomography; MRI, magnetic resonance imaging; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK, creatine kinase; PCR, polymerase chain reaction; CBC, complete blood count; IFA, immunofluorescent antibody analysis.
NECK PAIN
Neck pain is a sign commonly associated with compressive or inflammatory diseases of the cervical spinal cord. Animals with neck pain typically have a guarded horizontal neck carriage and are unwilling to turn their neck to look to the side; they will instead pivot the entire body. As part of every routine neurologic examination, the presence or absence of cervical hyperesthesia should be assessed by deep palpation of the vertebrae and cervical spinal epaxial muscles and by resistance to flexion, hyperextension, and lateral flexion of the neck (Fig. 63-21). Anatomic structures that can cause neck pain include the meninges, nerve roots, intervertebral disks, joints, bones, and muscles. Neck pain has also been recognized as a clinical symptom of increased intracranial pressure, particularly as a result of forebrain mass lesions (Box 69-1).
 BOX 69-1 Causes of Neck Pain in the Dog
BOX 69-1 Causes of Neck Pain in the Dog
MUSCLE: Myositis (immune, infectious), muscle injury
BONE: Fracture/luxation, diskospondylitis, vertebral osteomyelitis, neoplasia
JOINT (facetal joints): Polyarthritis (immune, infectious), degenerative joint disease (osteoarthritis)
INTERVERTEBRAL DISK: Disk degeneration/prolapse
NERVE ROOT: Neoplasia, compression (by disk, tumor, fibrous tissue)
NON-INFECTIOUS INFLAMMATORY DISORDERS
STEROID-RESPONSIVE MENINGITIS-ARTERITIS
SRMA is the most common form of meningitis diagnosed in most veterinary hospitals. An immunological cause is suspected, resulting in vasculitis/arteritis affecting the meningeal vessels throughout the entire length of the spinal cord and brainstem. This disorder has also been called steroid-responsive suppurative meningitis, necrotizing vasculitis, juvenile polyarteritis, pain syndrome, and aseptic meningitis. Affected dogs are usually juveniles or young adults (6 to 18 months of age), but middle-aged and older dogs are occasionally affected. Large-breed dogs are most commonly affected. SRMA may be seen as a breed-associated syndrome in Beagles (Beagle pain syndrome), Bernese Mountain dogs, Boxers, German Shorthaired Pointers, and Nova Scotia Duck Tolling Retrievers. Clinical signs of SRMA include fever, cervical rigidity, and vertebral pain that may wax and wane early in the course of disease. Affected dogs are alert and systemically normal, with a common owner complaint being that the dog will not eat or drink unless the bowl is raised to head level. Neurologic deficits (e.g., paresis, paralysis, ataxia) are rare but can develop, particularly in chronically affected or inadequately treated dogs, as a result of concurrent myelitis, spinal cord hemorrhage, or infarction.
Laboratory changes typically include a neutrophilic leukocytosis with or without a left shift. Spinal fluid analysis shows an increased protein concentration and a neutrophilic pleocytosis (often >100 cells/μl; >75% neutrophils). Early in the course of the disease, when neck pain is intermittent, CSF may be normal or minimally inflammatory. Within 24 hours of administration of a single dose of prednisone, CSF may be normal or show a predominance of mononuclear cells; therefore CSF should always be collected for diagnosis when a dog is symptomatic before initiating therapy. High IgA concentrations are found in the CSF and serum of many dogs with SRMA, aiding diagnosis. Some dogs with SRMA have concurrent immune-mediated polyarthritis (IMPA). Bacterial cultures of the CSF and blood are negative. To date, no etiologic agent has been identified.
Treatment with corticosteroids consistently and rapidly alleviates the signs of fever and cervical pain. Dogs that are not treated early in the course of the disease occasionally develop neurologic deficits associated with spinal cord infarction and meningeal fibrosis; treatment may not resolve the resultant neurologic signs in these dogs. Corticosteroids should be administered initially at immunosuppressive dosages and then tapered to alternate-day therapy and decreasing dosages over a period of 4 to 6 months (Box 69-2). Dogs that do not respond completely to prednisone and dogs that relapse during prednisone tapering may benefit from the addition of azathioprine (Imuran; Burroughs Wellcome; 2.2 mg/kg/PO q24h) to their treatment for 8 to 16 weeks. The prognosis for survival and complete resolution is excellent. Older dogs and Beagles, Bernese Mountain dogs, and German Shorthaired Pointers with breed-associated SRMA may have disease that is more difficult to control, so treatment with prednisone and azathioprine from the outset and more prolonged tapering of prednisone dose may be warranted in those dogs. Some affected Beagles develop systemic manifestations of vasculitis, thyroiditis, and amyloidosis of the spleen, liver, or kidneys.
 BOX 69-2 Treatment Recommendations for Steroid-Responsive Meningitis Arteritis
BOX 69-2 Treatment Recommendations for Steroid-Responsive Meningitis Arteritis
If clinical signs have resolved, the dose of prednisone is gradually tapered:
If clinical signs are present or if they recur during tapering, return to step 2 and add azathioprine (2 mg/kg/day) to treatment for 8 to 16 weeks. Continue prednisone, tapering after signs resolve.
GRANULOMATOUS MENINGOENCEPHALITIS
GME is an idiopathic inflammatory disorder of the CNS that is believed to have an immunologic basis. GME occurs primarily in young adult dogs of small breeds, with Poodles, toy breeds, and Terriers most commonly affected. Large-breed dogs are occasionally affected. Most dogs with GME are 2 to 6 years of age, although the disease may affect older or younger dogs. Cats are not affected.
There are three distinct forms of GME. The ocular form is the least common and results in optic neuritis with an acute onset of blindness and dilated nonresponsive pupils (see Chapter 66). The focal form induces clinical signs suggestive of a single enlarging space-occupying mass with slowly progressive neurologic signs similar to a tumor. This form is most likely to affect the pontomedullary region, the forebrain, or the cervical spinal cord. The diffuse form of GME causes rapidly progressive signs of multifocal or disseminated disease affecting the brainstem, cerebrum, cerebellum, cervical spinal cord, or meninges.
Clinical signs reflect the location and nature of the lesion. Prominent features may include cervical pain, suggesting meningeal involvement, or brainstem signs such as nystagmus, head tilt, blindness, or facial and trigeminal paralysis. Ataxia, seizures, circling, and behavior change are also common. Many dogs with the diffuse form of GME have a fever and peripheral neutrophilia but no other evidence of systemic disease. The disseminated form of the disease has an acute to subacute progression over weeks to months, with 25% of the cases dead within 1 week. The focal form is more insidious, with progression over 3 to 6 months.
CSF analysis reveals an increase in protein concentration and a mild to marked mononuclear pleocytosis. Lymphocytes, monocytes, and occasional plasma cells predominate (Fig. 69-2). Anaplastic mononuclear cells with abundant lacy cytoplasm are sometimes present. Neutrophils are seen in two thirds of the samples, usually making up less than 20% of the cells. A single sample of CSF is sometimes normal. CSF electrophoresis typically shows evidence of blood-brain barrier disruption, and chronically affected dogs have dramatically increased intrathecal production of gamma globulins. Evaluation for infectious causes of meningoencephalomyelitis through culture and appropriate serum and CSF titers and a systemic search for neoplasia should precede a presumptive diagnosis of GME. Computed tomography (CT) or magnetic resonance imaging (MRI) usually shows a solitary contrast-enhancing mass in the brain or spinal cord with focal disease and may be normal or demonstrate patchy ill-defined regions of contrast enhancement with diffuse disease. Definitive diagnosis requires biopsy or necropsy for histologic examination.
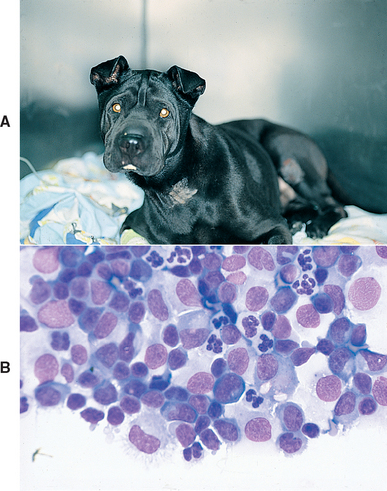
FIG 69-2 A, A young Chinese Shar-Pei with incoordination, depression, vertical nystagmus, and a slight head tilt resulting from disseminated granulomatous meningoencephalomyelitis. B, Cerebrospinal fluid from this dog has increased cellularity—primarily lymphocytes, monocytes, plasma cells, and neutrophils.
Corticosteroids can occasionally halt or reverse the progression of clinical signs, particularly in animals with slowly progressive clinical signs associated with focal disease. The administration of prednisone (1 to 2 mg/kg/PO q24h) may cause a dramatic response, but clinical signs often recur quickly, with the median survival time highly variable depending on type and location of disease, ranging from longer than 12 months in dogs with focal forebrain GME to 8 days in dogs with diffuse GME. Improvement in clinical signs and survival can sometimes be seen when more aggressive chemotherapy protocols are used. Recommended drugs and protocols are outlined in Box 69-3. Radiation therapy may also greatly benefit some dogs with focal intracranial masses resulting from GME. With any protocol the best results are seen in patients with focal disease and those that receive treatment before neurologic signs are severe. Comparative efficacy between protocols is difficult to assess because of disease and patient variability and the failure to obtain a definitive pretreatment diagnosis in most patients. Most affected animals improve with treatment, but the prognosis for permanent recovery is poor.
 BOX 69-3 Chemotherapy Options for Presumed Granulomatous Meningoencephalitis
BOX 69-3 Chemotherapy Options for Presumed Granulomatous Meningoencephalitis
PO, By mouth; SC, subcutaneous.
Prednisone
1 mg/kg PO q12h for 2 weeks, then 1 mg/kg PO q 24h for 4 weeks, then 1 mg/kg q 48h forever
Cytosine arabinoside (Cytosar; Upjohn Pharma)
50 mg/m2 body surface area SC q12h on 2 consecutive days every 21 days
NECROTIZING MENINGOENCEPHALITIS
NME is a breed-specific idiopathic inflammatory condition affecting the brain of Pugs (pug encephalitis), Malteses, and Yorkshire Terriers (necrotizing leukoencephalitis). No infectious agent has been detected, and a genetic predisposition is likely. Necrosis and nonsuppurative necrotizing meningoencephalitis (NMG) and leptomeningitis occur, affecting primarily the cerebral cortex in Pugs and Malteses and the cerebral cortex and brainstem in Yorkshire Terriers. Affected dogs first show clinical signs between 9 months and 7 years of age.
Dogs with rapidly progressive cerebral cortical disease caused by NME are presented with seizures and neurologic signs referable to the cerebrum and meninges. They may have difficulty walking or may be weak or lack coordination. Circling, head pressing, cortical blindness, and neck pain are common. Affected Yorkshire Terriers may have a head tilt and cranial nerve abnormalities. Neurologic deterioration is rapid, and within 5 to 7 days the dogs develop uncontrollable seizures or become recumbent, unable to walk, and comatose.
Dogs with a more slowly progressive form of NME are also commonly presented with a generalized or partial motor seizure, but these dogs are neurologically normal after the seizure. Seizures then recur at varying intervals from a few days to a few weeks, followed by the development of other neurologic signs referable to the cerebral cortex. Survival times are generally only a few weeks, with a maximum survival time of less than 6 months from the time of initial presentation.
A diagnosis of NME should be suspected on the basis of signalment and characteristic clinical and clinicopathologic features. Hematologic and serum biochemistry findings are unremarkable. Imaging studies are consistently abnormal, with focal hypodense areas within the brain parenchyma visible on CT and areas of high signal intensity seen on MRI. CSF analysis reveals a high protein concentration and an increased nucleated cell count, with the predominant cell type being the small lymphocyte. Definitive diagnosis requires autopsy or brain biopsy.
No specific treatment exists for this disease. Treatment with antiepileptic doses of phenobarbital may decrease the severity and frequency of the seizures for a short period of time. Corticosteroids are commonly administered (as for GME) but do not appear to alter the course of this disease. There are some anecdotal reports of improvement after the administration of mycophenolate mofetil (20 mg/kg, administered orally q12h for 30 days, then 10 mg/kg q12h for the remainder of the animal’s life), but the prognosis for improvement and survival must be considered poor.
FELINE POLIOENCEPHALOMYELITIS
A nonsuppurative encephalomyelitis with no etiologic agent identified occasionally causes progressive seizures or spinal cord signs in young adult cats. Affected cats range from 3 months to 6 years of age, with most cats being younger than 2 years old. Affected animals have a subacute to chronic progressive course of neurologic signs. Pelvic limb hyporeflexia may accompany ataxia and paresis of the pelvic limbs, and intention tremors of the head and seizures may occur. Seizures and behavior change may be the only signs observed in some cats.
Clinicopathologic findings are normal in most cats. CSF analysis reveals a mild increase in CSF mononuclear cells and a normal or slightly increased CSF protein concentration. Definitive diagnosis can be confirmed only at necropsy. Lesions are confined to the CNS and are found in the spinal cord, cerebral cortex, brainstem, and cerebellum. These lesions include perivascular cuffing with mononuclear cells, lymphocytic meningitis, neuronophagia, and the formation of glial nodules. White matter degeneration and demyelination are also present. The prognosis is poor, although reports exist of spontaneous recovery from a clinically similar disorder in a few cats.
INFECTIOUS INFLAMMATORY DISORDERS
FELINE IMMUNODEFICIENCY VIRUS ENCEPHALOPATHY
Neurologic abnormalities associated with feline immunodeficiency virus (FIV) encephalopathy in cats include behavioral and mood changes, depression, persistent staring, inappropriate elimination, seizures, twitching of the face and tongue, and occasionally paresis. A presumptive diagnosis of FIV encephalopathy is made on the basis of suggestive clinical signs and positive FIV serology, but because FIV-infected cats have increased susceptibility to numerous neoplastic and infectious causes of encephalitis, it is important to carefully exclude other neurologic diseases. CSF analysis reveals an increase in lymphocytes and normal or only slightly increased CSF protein concentration. FIV antibodies can be demonstrated in the CSF of most affected cats. Care must be taken to keep from contaminating the CSF with blood during collection because serum antibody titers are higher than those in the CSF. Culture of freshly collected CSF may yield the virus. Zidovudine (AZT: 5 mg/kg, administered orally q12h) administration may reduce the severity of neurologic impairment in some cats.
BACTERIAL MENINGOENCEPHALOMYELITIS
Bacterial infection of the CNS is uncommon in dogs and cats. It may result from direct extension of infection from an extraneural site such as the middle ear, eye, sinus, or nose or because of a penetrating injury to the skull. Hematogenous dissemination from extracranial foci occurs rarely, except in neonates with omphalophlebitis and dogs and cats with severe immunodeficiency. In contrast to people, bacterial meningitis and meningoencephalomyelitis in dogs and cats is not caused by microorganisms having a specific predilection for the nervous system. Bacterial infections of the CNS are instead associated with the wide variety of organisms infecting primary sites.
Clinical signs of bacterial infection of the CNS commonly include pyrexia, neck pain, vomiting, and bradycardia. Neurologic abnormalities reflect the location of damaged parenchyma and may include seizures, coma, blindness, nystagmus, head tilt, paresis, or paralysis. The clinical course is usually rapidly progressive and frequently fatal. Affected animals are almost always systemically ill. Shock, hypotension, and disseminated intravascular coagulation are common. Routine laboratory tests often reflect the underlying inflamatory process.
CSF analysis reveals increased protein concentration and a predominantly neutrophilic pleocytosis, with cell counts often >500 cells/μl. Neutrophils in the CSF may appear degenerate, and occasionally intracellular bacteria are seen (Fig. 69-3). Treatment with antibiotics before CSF is collected may lower the CSF cell count and result in a predominance of mononuclear cells. The rate of organism recovery is improved by inoculation of CSF into broth enrichment media, but fewer than 50% will have positive CSF cultures. Whenever bacterial meningitis is suspected, diagnostic evaluation should include CSF cytologic analysis; CSF anaerobic and aerobic bacterial culture; blood and urine bacterial cultures; ophthalmologic and otic examination; screening radiographs of the spine, skull, and thorax; and abdominal ultrasound examination. MRI can be used to identify defects in the skull or infections or tumors extending into the cranial vault from the ear, eye, sinus, or nose. The presence of systemic bacterial illness or the identification of an extraneural focus of infection in a dog or cat with inflammatory CSF should prompt immediate treatment for suspected bacterial infection of the CNS. If the focus of underlying infection can be determined, that site should be cultured. Therapy usually is initiated before culture results are available.
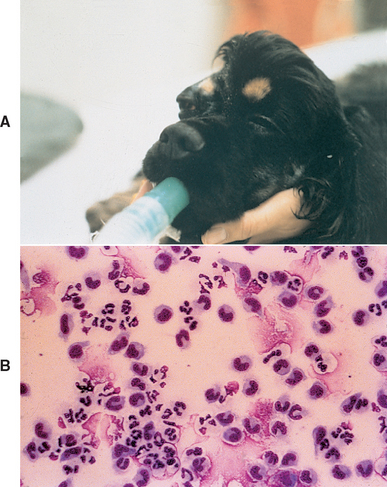
FIG 69-3 A, A 4-year-old Cocker Spaniel with a chronic retrobulbar abscess developed fever and severe depression. B, Cerebrospinal fluid from this dog reveals septic inflammation. Postmortem examination confirmed communication between the retrobulbar abscess and the central nervous system.
Bacterial meningitis is a life-threatening infection and requires rapid and aggressive treatment. Appropriate therapy of CNS infections is based on identification of the causative organism and selection of an appropriate antimicrobial agent that will reach high concentrations in the CSF and CNS tissues. Enrofloxacin and third-generation cephalosporins (e.g., ceftriaxone, cefotaxime) are good choices for gram-negative infections, and metronidazole can be used for anaerobic infections. While inflammation persists, ampicillin and amoxicillin with clavulonic acid are also effective and may be the best choice for gram-positive infections. Initial treatment with a combination of ampicillin (22 mg/kg, administered intravenously q6h), cefotoxime (20-40 mg/kg, administered intravenously q6h), and metronidazole (15 mg/kg administered once intravenously, then 7.5 mg/kg intravenously q8h or 10-15 mg/kg orally q8h) may be warranted if the infectious agent is unknown. Whenever possible, antibiotics should be administered intravenously for 3 to 5 days to achieve high CSF concentrations, and oral therapy should be continued for 4 weeks after recovery. Concurrent intravenous fluids and systemic support are important, and anticonvulsants should be administered to patients having seizures (see the discussion of status epilepticus in Chapter 67). Antiinflammatory drugs or corticosteroids (dexamethasone, 0.2 mg/kg IV q12h) are sometimes administered for the first 2 days of antibiotic treatment to minimize the inflammatory consequences of antibiotic-induced bacterial lysis.
The response to antibiotic therapy is variable, and relapses are common. The prognosis should be considered guarded because even with appropriate therapy many animals die. However, treatment should be attempted because some individual patients respond dramatically to therapy and have complete resolution of their neurologic defects.
CANINE DISTEMPER VIRUS
Canine distemper virus (CDV) is a paramyxovirus that commonly affects the CNS of dogs. Widespread vaccination has substantially decreased the incidence of clinically apparent CDV infections in many regions, but outbreaks still occur among unvaccinated dogs and sporadically in vaccinated dogs. Clinical signs vary, depending on virulence of the virus strain, environmental conditions, and host age and immune status. Most CDV infections are probably subclinical or are associated with mild signs of upper respiratory tract infection that resolve without therapy. Young, immunocompromised, and unvaccinated dogs are most likely to develop severe generalized distemper.
Progressive generalized infection with CDV most commonly affects unvaccinated puppies between 12 and 16 weeks of age. The first sign of infection is a mild serous to mucopurulent ocular and nasal discharge followed by a dry cough and sometimes tonsillitis. The cough becomes moist and productive as pneumonia develops. Affected dogs are depressed, inappetent, and often febrile. Diarrhea develops and may be mild or severe. Hyperkeratosis of the footpads and nose may occur. Neurologic signs begin 1 to 3 weeks after dogs start to recover from systemic illness and may include dementia, disorientation, seizures, cerebellar or vestibular signs, tetraparesis, and ataxia. Neck pain is uncommon. Seizures can be of any type, depending on the region of the brain affected, but “chewing gum” seizures caused by polioencephalomalacia of the temporal lobes are commonly described. Myoclonus, a repetitive rhythmic contraction of a group of muscles resulting in repetitive flexion of a limb or contractions of the muscles of mastication, is often referred to as distemper chorea and is very common in dogs with distemper encephalomyelitis. Anterior uveitis, optic neuritis, or chorioretinitis occurs in some infected dogs. Dogs surviving mild CDV infection before eruption of their permanent teeth will often have irregular dental surfaces and brown discoloration of their teeth subsequent to virus-induced enamel hypoplasia. Older animals occasionally develop chronic encephalomyelitis months to years after prior CDV infection and recovery (old dog encephalitis), with neurologic abnormalities that include progressive tetraparesis or vestibular dysfunction in the absence of systemic signs.
CDV is diagnosed on the basis of history, physical examination, and laboratory findings. In many animals a history of mild to severe gastrointestinal and respiratory illness precedes the onset of neurologic signs. Results of a CBC may be normal or may reveal a persistent lymphopenia; distemper inclusions can sometimes be found in the circulating lymphocytes and erythrocytes. Optic neuritis, chorioretinitis, and retinal detachment may be detected during an ophthalmologic examination. Irregular, ill-defined, gray-to-pink densities in the tapetal or nontapetal region suggest acute or active chorioretinitis, whereas well-defined hyperreflective regions are more indicative of chronic infection with scarring.
Early in an infection, immunofluorescent techniques, using anti-CDV antibodies, may reveal CDV in cytologic smears prepared from conjunctival, tonsilar, or nasal epithelium. Virus may be detected past these initial stages in epithelial cells and macrophages obtained from the lower respiratory tract by tracheal wash. The virus persists for up to 60 days in the skin, footpads, and CNS; thus immunohistochemical techniques can be applied to biopsy or necropsy specimens for diagnosis. Biopsy of the haired skin of the dorsal neck can be used for antemortem immunohistochemical testing to confirm acute and subacute infection with CDV. Reverse-transcriptase polymerase chain reaction (RT-PCR) can also be used to detect CDV RNA in whole blood, buffy coat preparations, CSF, and tissues of affected dogs.
Distemper meningoencephalitis characteristically causes an increase in protein concentration and a mild lymphocytic pleocytosis in the CSF; occasionally, the CSF is normal or more indicative of an inflammatory process (increased neutrophils). Increased protein concentration in the CSF has been identified primarily as anti-CDV antibody. Measured CDV antibody titer in the CSF may be increased relative to the serum titer (C-value, see Box 64-4).
Treatment of acute CDV meningoencephalomyelitis is supportive, nonspecific, and frequently unrewarding. Progressive neurologic dysfunction usually necessitates euthanasia. Anticonvulsant therapy has been recommended to control seizures. Antiinflammatory doses of glucocorticosteroids (0.5 mg/kg q12h PO for 10 days, then taper) may be used to control other neurologic signs in the absence of systemic disease; however, their beneficial effects are not well documented.
Prevention of CDV infection through routine vaccination is usually very effective. CDV can, however, develop with exposure following stress, illness, or immunosuppression, even in a currently vaccinated dog. Meningoencephalitis has been reported in a few dogs 7 to 14 days after vaccination with modified live virus-canine distemper vaccines (MLV-CDV). Particular batches of vaccines may be implicated, but vaccination of immunosuppressed neonates, particularly those with a known or suspected parvoviral infection, should be avoided.
RABIES
Rabies virus infection in dogs and cats is almost always the result of a bite from an infected animal that has rabies virus in its saliva. Most dogs and cats are infected through contact with wildlife vectors (e.g., skunks, raccoons, foxes, bats). Although the prevalence of wildlife rabies has been increasing, cases of rabies in pet dogs and cats have been decreasing as a result of routine vaccination protocols. The incubation period from the time of the bite to the onset of clinical signs is extremely variable (1 week to 8 months), with average incubation 3 to 8 weeks. Once neurologic signs develop, the disease is rapidly progressive, with death occurring within 7 days in most animals.
Rabies can have a wide range of clinical signs, which makes it difficult to differentiate from other acute, progressive encephalomyelitis syndromes. Because of its public health significance, rabies should be on the list of differential diagnoses considered in every animal with rapidly progressing neurologic dysfunction and precautions should be taken to minimize human exposure. Rabies infection has classically been divided into two major types: furious and paralytic. Dogs and cats typically undergo an early prodromal phase lasting 2 to 3 days during which they may be apprehensive or nervous and may lick or chew at the site of inoculation. This can be followed by a furious or psychotic phase (1 to 7 days) in which animals are increasingly irritable and excitable, often snapping at imaginary objects and biting at their cage or surroundings. They become incoordinated and may exhibit generalized seizures, progressing to death. Animals with the paralytic or dumb type of rabies develop generalized LMN paralysis progressing from the site of inoculation to involve the entire CNS within a few (range 1 to 10) days. Cranial nerve paralysis may be the first sign seen (especially if the bite was on the face). Difficulty swallowing, excessive drooling, hoarse vocalization, diminished facial sensation, and dropped jaw may be seen.
Any unvaccinated animal with an acute, rapidly progressive course of neurologic disease should be suspected of having rabies. Ancillary testing should be performed with caution, minimizing exposure of personnel. CSF analysis reveals increased mononuclear cells and protein concentration, as might be expected with any viral encephalomyelitis. Rabies antibody may be increased in CSF compared with serum. Biopsies obtained from the dorsal skin at the nape of the neck or the maxillary sensory vibrissae may be positive for rabies virus antigen; however, although positive results are reliable, negative results are not. Definitive diagnosis of rabies encephalitis is through the demonstration of rabies virus antigen by immunohistochemical techniques in the brain tissue (thalamus, pons, and medulla) of an infected animal postmortem. Because of the risk associated with inadvertent human exposure, it is recommended that all unvaccinated animals with progressive neurologic dysfunction of unknown origin undergo postmortem evaluation for rabies.
Fortunately, vaccinations have been extremely effective in reducing the prevalence of rabies in pet dogs and cats and in decreasing the incidence of rabies infection in humans. Inactivated products and recombinant vaccines are available and are relatively safe and effective when used as directed. Dogs and cats should receive their first rabies vaccine after 12 weeks of age and then again at 1 year of age. Subsequent boosters are administered every 1 to 3 years, depending on the vaccine used and local public health regulations. Rarely, soft tissue sarcomas have developed in cats at the site of rabies virus prophylactic inoculation. Postvaccinal polyradiculoneuritis causing an ascending LMN tetraparesis has also been reported occasionally in dogs and cats.
FELINE INFECTIOUS PERITONITIS
Progressive neurologic involvement is common in cats affected with the dry form of feline infectious peritonitis (FIP). Neurologic signs may include seizures, cerebellar signs, vestibular dysfunction, and paresis. Most affected cats have a fever and systemic signs such as anorexia and weight loss. Concurrent anterior uveitis, iritis, keratic precipitates, and chorioretinitis are common and should raise the suspicion of this disease. Careful abdominal palpation will occasionally reveal organ distortion caused by concurrent granulomas in the abdominal viscera.
Typically, the complete blood count is inflammatory and serum globulin concentrations may be very high. Serum tests for anticoronavirus antibodies are variable. MRI and CT may reveal multifocal granulomatous lesions and secondary hydrocephalus or may be normal. Typical findings on CSF analysis include a marked neutrophilic or pyogranulomatous pleocytosis (>100 cells/μl; >70% neutrophils) and an increase in CSF protein concentration (>200 mg/dl). In a few cases, however, CSF will be normal or only slightly inflammatory. Coronavirus antibody will usually be positive in the CSF, and coronavirus can sometimes be detected in the CSF and affected tissue using RT-PCR. The prognosis for cats with CNS FIP is very poor. Some palliation may be achieved with immunosuppressive and antiinflammatory medications (see Chapter 97 for more information on FIP).
TOXOPLASMOSIS
Toxoplasma gondii infections can be acquired transplacentally, through ingestion of tissues containing encysted organisms, or through ingestion of food or water contaminated by cat feces containing oocysts. Most infections are asymptomatic. Transplacentally infected kittens may develop acute fulminating signs of liver, lung, CNS, and ocular involvement. Disease in older animals results from reactivation of a chronic encysted infection. Infection is evident in the lung, CNS, muscle, liver, pancreas, heart, and eye in cats. In dogs lung, CNS, and muscle infections predominate.
CNS toxoplasmosis can cause a variety of signs, including behavioral change, seizures, circling, tremors, ataxia, paresis, and paralysis. Muscle pain and weakness caused by Toxoplasma myositis is discussed in Chapter 72.
Routine labwork may be normal in dogs and cats with CNS toxoplasmosis, or a neutrophilic leukocytosis and eosinophilia may be seen. Serum globulins may be increased. Liver enzymes are increased when there is hepatic infection, and creatine kinase (CK) is increased in animals with myositis. Cats commonly have concurrent uveitis or chorioretinitis. CSF analysis typically reveals increased protein concentration and a mild to moderately increased nucleated cell count. Lymphocytes and monocytes usually predominate, but occasionally the pleocytosis is neutrophilic or eosinophilic. The CSF concentration of antibody directed against T. gondii may be increased relative to serum concentration, suggesting local production of specific antibody and an active infection. Rarely, cytologic examination of the CSF reveals T. gondii organisms within host cells, allowing a definitive diagnosis of toxoplasmosis.
Antemortem diagnosis of CNS toxoplasmosis may be difficult. If other organ systems are involved, finding organisms in samples from affected extraneural tissues allows definitive diagnosis. A fourfold rise in IgG titer in two serum samples taken 3 weeks apart or a single elevated IgM titer in a patient with neurologic signs supports a diagnosis of toxoplasmosis, but antibody titers are negative in some animals with severe disease (see Chapter 99). CSF titers should be interpreted in conjunction with evidence for blood-brain barrier disruption, calculating the antibody coefficient or c-value (see Box 64-4). PCR can sometimes be used to identify Toxoplasma in blood, aqueous humor, CSF, muscle, or nervous system tissue from affected dogs and cats.
Recommended treatment for meningoencephalomyelitis caused by toxoplasmosis in dogs and cats consists of clindamycin hydrochloride (10 mg/kg PO q8h for at least 4 weeks). This drug has been shown to cross the blood-brain barrier and has been used with success in a limited number of animals. Trimethoprim-sulfadiazine (15 mg/kg, administered orally q12h) can be used as an alternate anti-Toxoplasma drug, especially in combination with pyrimethamine (1 mg/kg/day), but if this is used for long-term treatment, folic acid supplementation should be considered. The prognosis for recovery is grave in animals with profound neurologic dysfunction. Affected cats should be routinely tested for concurrent feline leukemia virus (FeLV) and FIV infections. Neurologic, ocular, and muscular manifestations of toxoplasmosis are not usually associated with patent infection and oocyte shedding in cats, so isolation of affected animals is not necessary.
NEOSPOROSIS
Neospora caninum is a protozoan parasite that causes neuromuscular disease in dogs. Domestic dogs and coyotes are definitive hosts, shedding oocysts in their stool after ingestion of N. caninum cysts in muscle from intermediate hosts (primarily deer and cattle). The predominant route of transmission is transplacental, causing acute symptomatic infection in some puppies and subclinical infection leading to encystment in neural and muscle tissues in others. Young puppies 6 weeks to 6 months of age typically develop weakness, loss of patellar reflexes, and finally LMN paralysis of the rear limbs as a result of inflammation of the muscles and nerve roots (Fig. 69-4). Multiple puppies from a litter may be affected. If treatment is not initiated promptly, severe atrophy and contracture of affected muscles fixes the rear limbs in rigid extension (Fig. 69-5). Most affected puppies are bright and alert and otherwise normal. Disease in older animals usually results from reactivation of a chronic encysted infection acquired congenitally or through ingestion of tissue cysts. These dogs commonly have signs of multifocal CNS involvement. Paraparesis, tetraparesis, cerebellar signs, seizures, and cranial nerve abnormalities are reported. Some dogs have concurrent myositis. Rarely, a rapidly progressive diffuse LMN paralysis similar to acute idiopathic polyradiculoneuritis has been reported. Most affected dogs are systemically normal, but occasionally systemic neosporosis will occur, causing fever, pneumonia, hepatitis, pancreatitis, esophagitis, or pyogranulomatous dermatitis.
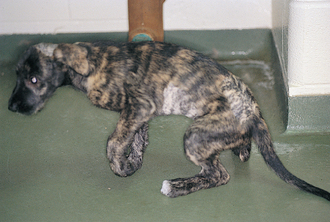
FIG 69-4 A 10-week-old Irish Wolfhound puppy with a crouched rear limb stance, quadriceps muscle weakness, and atrophy and patellar areflexia caused by Neospora caninum myositis and lumbar radiculoneuritis. This dog recovered after clindamycin treatment.
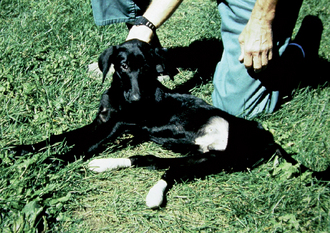
FIG 69-5 A young Labrador Retriever with rigid extension of the rear limbs caused by pediatric neosporosis.
Hematologic and biochemical findings vary and depend on the organ systems involved. With muscle disease, serum CK and aspartate aminotransferase (AST) activities are increased. Serology can be used to support the diagnosis, but there is no correlation between serum titer and severity of clinical signs. Puppies may have maternally derived antibodies without being infected; however, these should be gone by day 32 of life. CSF may be normal or may have mild increases in protein concentration (20 to 150 mg/dl) and leukocyte count (10 to 100 cells/μl), with monocytes and lymphocytes predominating; some neutrophils and eosinophils may be present. Specific antibodies may occasionally be detected in the CSF. Immunocytochemical staining can be used to identify Neospora and differentiate it from Toxoplasma in muscle biopsies antemortem and in muscle and CNS tissues postmortem. Treatment with clindamycin hydrochloride (10 mg/kg PO q8h for at least 4 weeks) is most effective in dogs without severe neurologic signs. Multifocal signs, rapid progression of signs, pelvic limb rigid hyperextension, and delayed treatment are all associated with a poor prognosis for recovery.
LYME DISEASE
Lyme neuroborreliosis, resulting from infection of the CNS by the spirochete Borrelia burgdorferi, has been well documented in people, but there are few reports of dogs with neurologic signs convincingly caused by Lyme disease. Most affected dogs have concurrent polyarthritis, lymphadenopathy, and fever. Reported signs of neurologic system involvement include aggression, other behavior changes, and seizures. CSF may be normal or only slightly inflammatory, and there may be an increase in anti-B. burgdorferi antibody in the CSF compared with serum. Although it is rare, Lyme neuroborreliosis should be considered in the differential diagnosis of disease involving the CNS in dogs from endemic regions. Early antibiotic treatment may be effective, but it is important to select an effective antibiotic that is capable of reaching high concentrations in the CSF. Ceftriaxone (25 mg/kg, administered intravenously or subcutaneously q24h for 14-30 days), doxycycline (10 mg/kg, administered orally q12h for 30 days), and amoxicillin (20 mg/kg, administered orally q8h for 30 days) have all been recommended.
MYCOTIC INFECTIONS
Disseminated systemic mycotic infections may occasionally involve the CNS and eyes. Clinical signs depend on the fungus involved and include gastrointestinal, respiratory, or skeletal problems in conjunction with neurologic and ocular signs. The most common neurologic signs are depressed mentation, behavior change, seizures, circling, and paresis. Ocular examination may reveal uveitis, chorioretinitis, retinal detachment, or optic neuritis. Typical abnormalities on CSF analysis include a neutrophilic pleocytosis and increased protein content. Diagnosis is usually by finding the organism in extraneural infected tissues. Therapy may be attempted; however, the prognosis is poor when the nervous system is involved.
It is uncommon for systemic mycoses to present with only neurologic signs. The exception is infection caused by the encapsulated yeasts Cryptococcus neoformans and Cryptococcus gatti. These organisms have a predilection for the CNS in the dog and cat. Infection occurs via extension from the nose through the cribiform plate and via hematogenous dissemination of severe disease in the dog or cat.
In cases of cryptococcal meningoencephalitis, CSF analysis reveals increased protein concentration and cell counts. A neutrophilic pleocytosis is most common, but eosinophils have been reported. Organisms can be visualized in the CSF in approximately 60% of cases. Fungal culture of the CSF should be considered in dogs with inflammatory CSF in which no organisms are visible. Detection of capsular antigen in the CSF or serum of affected animals using a latex agglutination test may also be a useful aid to diagnosis. Cytologic examination of nasal exudate, draining tracts, enlarged lymph nodes, and granulomas located extraneurally may yield the diagnosis. The organism is readily visible using Gram’s stain, India ink, or Wright’s stain. Treatment of CNS cryptococcus is usually attempted using amphotericin B or fluconazole, both of which penetrate the CNS. Itraconazole is sometimes effective (see Chapter 98 for more information).
RICKETTSIAL DISEASES
Rocky Mountain spotted fever (RMSF), caused by Rickettsia rickettsii, and ehrlichiosis, caused by Ehrlichia canis, commonly involve the CNS of dogs, causing meningoencephalomyelitis. Neurologic signs are seen in approximately 30% of dogs with both diseases, but the signs are most severe in dogs with RMSF. Neurologic abnormalities in dogs with RMSF tend to be more acute and progressive than those seen with ehrlichiosis. Neurologic signs with either disease include neck pain, mental changes, ataxia, vestibular signs, stupor, and seizures. Neurologic abnormalities have not been recognized in dogs without concurrent systemic disease. Signs of systemic disease depend on the degree of involvement of other organ systems but may include fever, anorexia, depression, vomiting, oculonasal discharge, cough, dyspnea, and lymphadenopathy. Hematologic abnormalities including anemia, thrombocytopenia, leukocytosis, and hyperglobulinemia are common and should prompt consideration of tick-borne illness in dogs from endemic regions with neurologic signs. The organisms of granulocytic ehrlichiosis (Ehrlichia ewingii and Anaplasma phagocytophilia) also cause thrombocytopenia, polyarthritis, and meningitis in dogs.
Although the number of cases reported is small, neutrophils seem to predominate in the CSF of dogs with RMSF, whereas lymphocytes or neutrophils predominate in ehrlichiosis; the CSF is normal in some dogs with each disease. In some dogs with granulocytic ehrlichiosis, neutrophils in the blood or in the CSF may contain morulae. Serologic testing or PCR (blood or CSF) is essential to confirm the diagnosis of rickettsial infection and to differentiate between these diseases. Treatment with doxycycline (5 to 10 mg/kg, administered orally or intravenously q12h) is effective in most cases. Short-term treatment with corticosteroids may also be warranted. Dramatic clinical improvement should be expected within 24 to 48 hours of initiating treatment. The presence of neurologic signs may slow recovery, and in some cases the neurologic damage is irreversible (see Chapter 96 for more information on rickettsial diseases).
PARASITIC MENINGITIS, MYELITIS, AND ENCEPHALITIS
Meningitis and meningoencephalitis caused by aberrant parasite migration have been reported in the dog and cat. In these diseases migration and growth of parasites can result in extensive damage to the neural parenchyma. An eosinophilic CSF pleocytosis should prompt consideration of parasitic migration through the CNS, although several more common neurologic disorders should also be considered, including intracranial neoplasia, toxoplasmosis, neosporosis, and GME. An apparently immune-mediated eosinophilic meningitis has also been described in young dogs, particularly Golden Retrievers. Diagnostic evaluation of animals with eosinophilic CSF should include a fundic examination, complete blood count, serum biochemistry profile, urinalysis, serum and CSF titers for Toxoplasma and Neospora, thoracic and abdominal radiographs, abdominal ultrasound, fecal flotation, and heartworm antigen testing. CT and MRI may document necrosis along the path of parasite migration within the CNS. Definitive diagnosis of parasitic CNS disease requires pathologic demonstration of the parasite in the CNS. Empirical treatment with ivermectin should be considered if parasite migration is likely (200 to 300 μg/kg, administered orally or subcutaneously every 2 weeks for three treatments). Antiinflammatory treatment with prednisone may also be indicated.
Adamo PF, Adams WM, Steinberg H. Granulomatous meningoencephalitis in dogs. Comp Cont Educ Vet. 2007;29:679-690.
Cizinauskas S, Jaggy A, Tipold A. Long-term treatment of dogs with steroid-responsive meningitis-arteritis: clinical, laboratory and therapeutic results. J Small Anim Pract. 2000;41:295.
Crookshanks JL, et al. Treatment of canine pediatric Neospora caninum myositis following immunohistochemical identification of tachyzoites in muscle biopsies. Can Vet J. 2007;48:506.
Dubey JP, Lappin MR. Toxoplasmosis and neosporosis. In Greene CE, editor: Infectious diseases of the dog and cat, ed 3, St Louis: Elsevier, 2006.
Greene CE, Appel MJ. Canine distemper. In Greene CE, editor: Infectious diseases of the dog and cat, ed 3, St Louis: Elsevier, 2006.
Greene CE, Rupprecht CE. Rabies and other Lyssavirus infections. In Greene CE, editor: Infectious diseases of the dog and cat, ed 3, St. Louis: Elsevier, 2006.
Higginbotham MJ, Kent M, Glass EN. Noninfectious inflammatory central nervous system diseases in dogs. Comp Cont Educ Vet. 2007;29:488.
Kent M. Bacterial infections of the central nervous system. In Greene CE, editor: Infectious diseases of the dog and ca, ed 3, St Louis: Elsevier, 2006.
Munana KR. Head tilt and nystagmus. In: Platt SR, Olby NJ, editors. BSAVA manual of canine and feline neurology. Gloucester: BSAVA, 2004.
Radaelli ST, Platt SR. Bacterial meningoencephalomyelitis in dogs: a retrospective study of 23 cases (1990–1999). J Vet Intern Med. 2002;16:159.
Thomas WB, et al. Retrospective evaluation of 38 cases of canine distemper encephalomyelitis. J Am Anim Hosp Assoc. 1993;29:129.