CHAPTER 88 Lymphadenopathy and Splenomegaly
APPLIED ANATOMY AND HISTOLOGY
The lymph nodes and spleen constitute the main source of immunologic and mononuclear-phagocytic (MP) cells in the body. Because these lymphoid structures are in a constant dynamic state, they continuously reshape and change in size in response to antigenic stimuli. In general, the response of the cells within a lymph node to different stimuli is similar to that occurring in the spleen. However, the spleen responds primarily to blood-borne antigens (mainly nonopsonized organisms), whereas the lymph nodes respond to antigens arriving through the afferent lymphatics (i.e., local tissue response). The response of the lymph nodes and spleen to different stimuli is briefly reviewed in this chapter.
The canine and feline lymph nodes are reniform, encapsulated, well-developed structures responsible for filtering lymph and participating in immunologic reactions. Fig. 88-1 depicts the basic microscopic anatomy of a lymph node in a carnivore. It is composed of a capsule, subcapsular spaces, cortex, paracortex, and medulla. Each of these areas has specific functions. The capsule surrounds and supports all other structures within the node (stroma). The subcapsular spaces (or sinuses) contain mainly MP cells responsible for “filtering” particles arriving through the afferent lymphatics and presenting the antigens to the lymphoid cells. The cortex contains mainly B-cell areas in the germinal centers. The paracortex is composed primarily of T cells and is therefore involved in cell-mediated immunity. The medulla contains the medullary cords, where the committed B cells persist and may expand to solid areas of plasma cells in response to antigenic stimulation. Between the medullary cords, the medullary sinuses form an endothelial sieve containing varying numbers of MP cells, which “screen” the efferent lymph. The lymph flows from the medulla to the efferent lymphatics in the hilus.
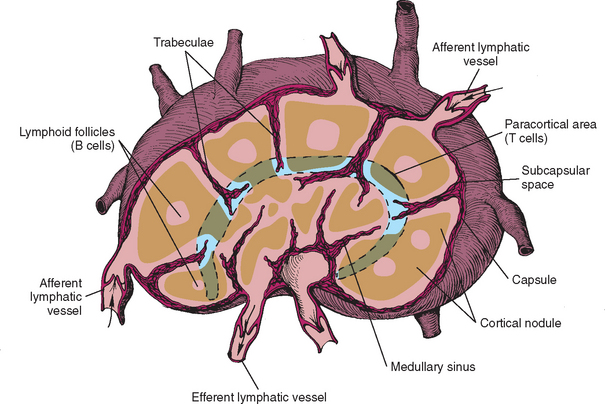
FIG 88-1 Microscopic anatomy of a typical lymph node in a carnivore.
(Reprinted from Couto CG: Diseases of the lymph nodes and spleen. In Ettinger SJ, editor: Textbook of veterinary internal medicine—diseases of the dog and cat, ed 3, Philadelphia, 1989, WB Saunders.)
An understanding of the different histologic and functional characteristics of these anatomic areas aids in understanding the pathogenesis of lymphadenopathy. For example, a lymph node reacting to a bacterial infection has primarily B-cell hyperplasia characterized by increased numbers of secondary follicles. This histologic/functional compartmentalization should be kept in mind when interpreting cytologic or histopathologic lymph node specimens.
FUNCTION
The two main functions of the lymph nodes are to filter particulate material and participate in immunologic processes. Particulate material is filtered as lymph flows through the areas rich in MP cells while it moves from the afferent to the efferent lymphatics. During this transit, particulate material is taken up and processed by the MP or antigen processing (AP) cells and presented to the lymphoid cells to generate a humoral or cellular immune response.
The spleen has multiple functions, including hematopoiesis, filtration and phagocytosis, remodeling of red blood cells (RBCs), removal of intraerythrocytic inclusions, storage of RBCs and platelets, metabolizing of iron, and immunologic functions. Because of its nonsinusal nature, the feline spleen is less efficient at removing intracellular inclusions than its canine counterpart.
LYMPHADENOPATHY
Etiology and Pathogenesis
In this chapter lymphadenopathy is defined as lymph node enlargement. According to the distribution, the following terms are used to characterize lymphadenopathy. Solitary lymphadenopathy refers to the enlargement of a single lymph node. Regional lymphadenopathy is an enlargement of a chain of lymph nodes draining a specific anatomic area. Generalized lymphadenopathy is a multicentric lymph node enlargement affecting more than one anatomic area. Lymphadenopathies can also be classified as superficial or deep (or visceral) according to their anatomic location.
Lymph nodes enlarge as a consequence of the proliferation of normal cells that normally reside in the node, or infiltration with normal or abnormal cells. Rarely, lymph nodes enlarge as a result of vascular changes (e.g., hyperemia, congestion, neovascularization, edema).
When normal cells proliferate within a lymph node in response to antigenic stimuli (e.g., vaccination, infection), the term reactive lymphadenopathy (or lymph node hyperplasia) is used. Lymphoid and MP/AP cells proliferate in response to immunologic and infectious stimuli, although occasionally a clinician evaluates a dog or cat in which a cause for the reactive lymphadenopathy cannot be identified. Because these lymphoid structures are usually presented with many antigens simultaneously, the cell proliferation that occurs in reactive lymphadenopathies is polyclonal (i.e., a wide variety of morphologic types of lymphoid and MP/AP cell types are present in a cytologic or histopathologic specimen).
When polymorphonuclear leukocytes or macrophages predominate in the cellular infiltrate, the term lymphadenitis is used. This is usually, but not always, a result of infectious processes. Depending on the predominant cell type in the infiltrate, lymphadenitides are classified as suppurative (neutrophils predominate), granulomatous (macrophages predominate), pyogranulomatous (macrophages and neutrophils predominate), or eosinophilic (eosinophils predominate). A focal area of suppurative inflammation with marked liquefaction (i.e., pus) is referred to as a lymph node abscess. The etiologic agents that commonly cause the different types of lymphadenitis are listed in Table 88-1.
 TABLE 88-1 Classification of Lymphadenopathies in Dogs and Cats
TABLE 88-1 Classification of Lymphadenopathies in Dogs and Cats
| TYPE | SPECIES |
|---|---|
| Proliferative and Inflammatory Lymphadenopathies Infectious | |
| Bacterial | |
| Actinomyces spp. | D, C |
| Borrelia burgdorferi | D |
| Bruceila canis | D |
| Corynebacterium spp. | C |
| Mycobacteria | D, C |
| Nocardia spp. | D, C |
| Streptococci | D, C |
| Contagious streptococcal lymphadenopathy | C |
| Yersinia pestis | C |
| Bartonella spp. | D, C |
| Localized bacterial infection | D, C |
| Septicemia | D, C |
| Rickettsial | |
| Ehrlichiosis | D, C |
| Anaplasmosis | D, C |
| RMSF | D |
| Salmon poisoning | D |
| Fungal | |
| Aspergillosis | D, C |
| Blastomycosis | D, C |
| Coccidioidomycosis | D |
| Cryptococcosis | D, C |
| Histoplasmosis | D, C |
| Phaeohyphomycosis | D, C |
| Phycomycosis | D, C |
| Sporotrichosis | D, C |
| Other mycoses | D, C |
| Algal | |
| Protothecosis | D, C |
| Parasitic | |
| Babesiosis | D |
| Cytauxzoonosis | C |
| Demodicosis | D, C |
| Hepatozoonosis | D |
| Leishmaniasis | D |
| Neospora caninum | D |
| Toxoplasmosis | D, C |
| Trypanosomiasis | D |
| Viral | |
| Canine viral enteritides | D |
| Feline immunodeficiency virus | C |
| Feline infectious peritonitis | C |
| Feline leukemia virus | C |
| Infectious canine hepatitis | D |
| Unclassified | |
| Pneumocystis carinii | D |
| Noninfectious | |
| Dermatopathic lymphadenopathy | D, C |
| Drug reactions | D, C |
| Idiopathic | D, C |
| Distinctive peripheral lymph node hyperplasia | C |
| Plexiform vascularization of lymph nodes | C |
| Immune-mediated disorders | |
| Systemic lupus erythematosus | D, C |
| Rheumatoid arthritis | D |
| Immune-mediated polyarthritides | D, C |
| Puppy strangles | D |
| Other immune-mediated disorders | D, C |
| Localized inflammation | D, C |
| Postvaccinal | D, C |
| Infiltrative Lymphadenopathies Neoplastic | |
| Primary hemolymphatic neoplasms | |
| Leukemias | D, C |
| Lymphomas | D, C |
| Malignant histiocytosis | D, C |
| Multiple myeloma | D, C |
| Systemic mast cell disease | D, C |
| Metastatic neoplasms | |
| Carcinomas | D, C |
| Malignant melanomas | D |
| Mast cell tumors | D, C |
| Sarcomas | D, C |
| Nonneoplastic | |
| Eosinophilic granuloma complex | C, D |
| Mast cell infiltration (nonneoplastic) | D, C |
D, Dogs; C, cats; RMSF, Rocky Mountain spotted fever.
Modified from Hammer AS et al: Lymphadenopathy. In Fenner NR, editor: Quick reference to veterinary medicine, ed 2, Philadelphia, 1991, JB Lippincott.
Infiltrative lymphadenopathies usually result from the displacement of normal lymph node structures by neoplastic cells and, more rarely, from extramedullary hematopoiesis. Neoplasms affecting the lymph nodes can be either primary hematopoietic tumors or secondary (metastatic) neoplasms. Lymph node infiltration by hematopoietic malignancies (i.e., lymphoma) constitutes one of the most common causes of generalized lymphadenopathy in dogs.
Clinical Features
From the clinical standpoint, familiarization with the location and palpation characteristics of normal lymph nodes, which should always be evaluated during a routine physical examination, is important. The following lymph nodes are palpable in normal dogs and cats: the mandibular, prescapular (or superficial cervical), axillary (in approximately half of animals), superficial inguinal, and popliteal (Fig. 88-2). Lymph nodes that are palpable only when markedly enlarged include the facial, retropharyngeal, mesenteric, and iliac (sublumbar) lymph nodes.
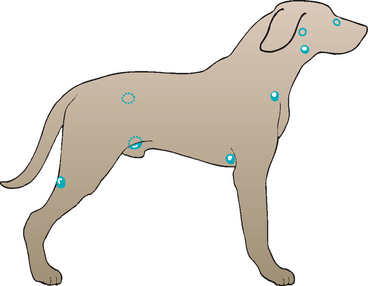
FIG 88-2 Anatomic distribution of clinically relevant lymph nodes in a dog. The nodes are in the same general location in cats. The lymph nodes depicted by the darkened circles include, from cranial to caudal, the mandibular, prescapular, axillary, superficial inguinal, and popliteal lymph nodes. The lymph nodes depicted by the open circles include, from cranial to caudal, the facial, retropharyngeal, and iliac or sublumbar lymph nodes.
(Reprinted from Couto CG: Diseases of the lymph nodes and spleen. In Ettinger SJ, editor: Textbook of veterinary internal medicine—diseases of the dog and cat, ed 3, Philadelphia, 1989, WB Saunders.)
When evaluating dogs and cats with lymphadenopathy or diffuse splenomegaly, the clinician can glean important information from the history. Certain diseases have a defined geographic or seasonal prevalence, including leishmaniasis in the Mediterranean region of Europe, salmon poisoning in the Pacific Northwest, and some systemic mycoses, such as histoplasmosis in the Ohio River Valley. Systemic (constitutional) clinical signs are usually present in dogs with systemic mycoses, salmon poisoning, Rocky Mountain spotted fever (RMSF), ehrlichiosis, bartonellosis, leishmaniasis, and acute leukemia. Clinical signs are rare or absent in dogs and cats with chronic leukemias, anaplasmosis, most lymphomas, and reactive lymphadenopathies occurring after vaccination; cats with idiopathic reactive lymphadenopathy (see the following section) are usually asymptomatic.
Clinical signs in dogs and cats with lymphadenopathy or splenomegaly are vague and nonspecific and are usually related to the primary disease rather than the organ enlargement; they include anorexia, weight loss, weakness, abdominal distention, vomiting, diarrhea, polyuria-polydipsia (PU/PD) (the latter in dogs with lymphoma-associated hypercalcemia), or a combination of these. Enlarged lymph nodes can occasionally result in obstructive or compressive signs (e.g., dysphagia resulting from enlarged retropharyngeal nodes, coughing resulting from enlarged tracheobronchial nodes, edema).
The distribution of the lymphadenopathy is also of diagnostic relevance. In patients with solitary or regional lymphadenopathy, the area drained by the lymph node(s) should be examined meticulously because the primary lesion is generally found there. Most cases of superficial solitary or regional lymphadenopathy in dogs and cats result from localized inflammatory or infectious processes or from metastatic neoplasia (less commonly), whereas most cases of deep (i.e., intraabdominal, intrathoracic) solitary or regional lymphadenopathy result from metastatic neoplasia or systemic infectious diseases (e.g., systemic mycoses). Most cases of generalized lymphadenopathy are caused by systemic fungal or bacterial infections (dogs), nonspecific hyperplasia (mainly cats), or lymphoma (dogs) (Table 88-2).
 TABLE 88-2 Correlation between Clinical Presentation and Etiology in Dogs and Cats with Lymphadenopathy in the Midwestern United States (in Relative Order of Importance)
TABLE 88-2 Correlation between Clinical Presentation and Etiology in Dogs and Cats with Lymphadenopathy in the Midwestern United States (in Relative Order of Importance)
| SOLITARY/REGIONAL | ||
|---|---|---|
| GENERALIZED | SUPERFICIAL | INTRACAVITARY |
A, Abdomen; T, thorax.
The characteristics of the lymph nodes on palpation are also important. In most dogs and cats with lymphadenopathy, regardless of the distribution, the lymph nodes are firm, irregular, and painless; their temperature is normal to the touch (cold lymphadenopathies); and they do not adhere to the surrounding structures. However, in patients with lymphadenitis the lymph nodes may be softer than usual and more tender and warmer than normal; they may also adhere to surrounding structures (fixed lymphadenopathy). Fixed lymphadenopathies may also be the presenting feature in dogs and cats with metastatic lesions, lymphomas with extracapsular invasion, or ceratin infectious diseases (e.g., mycobacteriosis).
The size of the affected lymph nodes is also important. Massive lymphadenopathy—lymph node size five to 10 times normal—occurs almost exclusively in dogs with lymphoma or lymphadenitis (lymph node abscess formation). In cats the syndrome of distinctive lymph node hyperplasia usually results in massive lymphadenopathy. Rarely, metastatic lymph nodes exhibit this degree of enlargement; the main example of massive metastatic lymphadenopathy is the apocrine gland adenocarcinoma metastases to the sublumbar lymph nodes. Recognizing that lymph nodes of normal size may contain metastatic neoplasia is important. Dogs with salmon poisoning may also have marked generalized lymphadenopathy as the presenting feature, preceded by or in conjunction with bloody diarrhea. Mild to moderate lymph node enlargement (two to four times the normal size) occurs mostly in a variety of reactive and inflammatory lymphadenopathies (e.g., ehrlichiosis, bartonellosis, anaplasmosis, RMSF, systemic mycoses, leishmaniasis, immune-mediated diseases, skin diseases) and in leukemias.
As previously discussed, the area draining the enlarged lymph node(s) should always be thoroughly examined, paying particular attention to the skin, subcutis, and bone. In dogs and cats with generalized lymphadenopathy, evaluation of other hemolymphatic organs is important, including the spleen, liver, and bone marrow.
SPLENOMEGALY
Etiology and Pathogenesis
Splenomegaly is defined as a localized or diffuse splenic enlargement. The term localized splenomegaly (or splenic mass) refers to a localized, palpable enlargement of the spleen. Diffuse splenic enlargement occurs as a consequence of either the proliferation of normal cells or infiltration with normal or abnormal cells. Rarely, diffuse splenic enlargement can occur as a result of vascular changes (e.g., hyperemia, congestion). Focal splenomegaly is more common in dogs, and diffuse splenomegaly is more common in cats.
Diffuse splenomegaly is classified into four major categories in terms of its pathogenesis: lymphoreticular hyperplasia, inflammatory changes (i.e., splenitis), infiltration with abnormal cells (e.g., lymphoma) or substances (e.g., amyloidosis), and congestion (Table 88-3).
 TABLE 88-3 Pathogenetic Classification of Splenomegaly in Dogs and Cats
TABLE 88-3 Pathogenetic Classification of Splenomegaly in Dogs and Cats
| TYPE | SPECIES |
|---|---|
| Inflammatory and Infectious Splenomegaly Suppurative splenitis | |
| Penetrating abdominal wounds | D, C |
| Migrating foreign bodies | D, C |
| Bacterial endocarditis | D, C |
| Septicemia | D |
| Splenic torsion | D |
| Toxoplasmosis | D, C |
| Infectious canine hepatitis (acute) | D |
| Mycobacteriosis (i.e., tuberculosis) | D, C |
| Necrotizing splenitis | |
| Splenic torsion | D |
| Splenic neoplasia | D |
| Infectious canine hepatitis (acute) | D |
| Salmonellosis | D, C |
| Eosinophilic splenitis | |
| Eosinophilic gastroenteritis | D, C |
| Hypereosinophilic syndrome | C, D |
| Lymphoplasmacytic splenitis | |
| Infectious canine hepatitis (chronic) | D |
| Ehrlichiosis (chronic) | D, C |
| Pyometra | D, C |
| Brucellosis | D |
| Hemobartonellosis | D, C |
| Bartonellosis | D, C |
| Leishmaniasis | D |
| Granulomatous splenitis | |
| Histoplasmosis | D, C |
| Mycobacteriosis (i.e., tuberculosis) | D, C |
| Leishmaniasis | D |
| Pyogranulomatous splenitis | |
| Blastomycosis | D, C |
| Sporotrichosis | D |
| Feline infectious peritonitis | C |
| Mycobacteriosis (i.e., tuberculosis) | D, C |
| Bartonellosis | D, C |
| Hyperplastic Splenomegaly | |
| Bacterial endocarditis | D |
| Brucellosis | D |
| Discospondylitis | D |
| Systemic lupus erythematosus | D, C |
| Hemolytic disorders (see text) | D, C |
| Congestive Splenomegaly | |
| Pharmacologic (see text) | D, C |
| Portal hypertension | D, C |
| Splenic torsion | D |
| Infiltrative Splenomegaly | |
| Neoplastic | |
| Acute and chronic leukemias | D, C |
| Systemic mastocytosis | D, C |
| Malignant histiocytosis | D, C |
| Lymphoma | D, C |
| Multiple myeloma | D, C |
| Metastatic neoplasia | D, C (rare) |
| Nonneoplastic | |
| EMH | D, C |
| Hypereosinophilic syndrome | C, D |
| Amyloidosis | D |
Modified from Couto CG: Diseases of the lymph nodes and the spleen. In Ettinger S, editor: Textbook of veterinary internal medicine, ed 3,
D, Dogs; C, cats; EMH, extramedullary hematopoiesis.
Philadelphia, 1989, WB Saunders.
The spleen commonly reacts to blood-borne antigens and RBC destruction with hyperplasia of the MP/AP and lymphoid components. This hyperplasia has been referred to as work hypertrophy because it usually results in varying degrees of splenic enlargement. Hyperplastic splenomegaly is relatively common in dogs with ehrlichiosis, bacterial endocarditis, systemic lupus erythematosus, or chronic bacteremic disorders such as discospondylitis and brucellosis, and in cats with mycoplasmosis or immune-mediated cytopenias.
RBC phagocytosis by the splenic MP system in human beings has been recognized to lead to hyperplasia of this cell population, resulting in splenomegaly. The same seems to occur in dogs and cats with certain hemolytic disorders, including immune-mediated hemolytic anemia, drug-induced hemolysis, pyruvate kinase deficiency anemia, phosphofructokinase deficiency anemia, familial nonspherocytic hemolysis in Poodles and Beagles, Heinz body hemolysis, and mycoplasmosis (see Chapter 83). Rarely, an area of focal splenomegaly is diagnosed histopathologically as hyperplasia after performing a splenectomy.
As in the lymph nodes, if polymorphonuclear leukocytes or macrophages predominate in the cellular infiltrate, the term splenitis is used. The infiltrates are also classified according to the cell type as suppurative, granulomatous, pyogranulomatous, or eosinophilic. Splenic abscesses can also form, often in association with a perforation by a foreign body. Necrotizing splenitis caused by gas-forming anaerobes can occur in dogs in association with splenic torsion or neoplasia. Lymphoplasmacytic splenitis cannot be distinguished cytologically from splenic hyperplasia. The etiologic agents for different types of splenitis are listed in Table 88-3.
Infiltrative splenomegalies are also common in small animals. Marked splenomegaly is a common finding in dogs and cats with acute and chronic leukemias (although it is more common in dogs), in dogs and cats with systemic mastocytosis, and in dogs with malignant histiocytosis. In addition, diffuse neoplastic infiltration of the spleen commonly occurs in dogs and cats with lymphoma and multiple myeloma. Diffuse splenomegaly may be the only physical examination and imaging finding in cats with monoclonal gammopathies; fine-needle aspiration (FNA) of the spleen reveals diffuse infiltration with plasma cells and is a common presentation for myeloma in this species. Metastatic splenic neoplasms usually result in focal splenomegaly but are rare.
Nonneoplastic causes of infiltrative splenomegaly are uncommon, with the exception of extramedullary hematopoiesis (EMH), which is more common in dogs than in cats. Because the spleen retains its fetal hematopoietic potential during adult life, a variety of stimuli—such as anemia, severe splenic or extrasplenic inflammation, neoplastic infiltration of the spleen, bone marrow hypoplasia, and splenic congestion—may cause the spleen to resume its fetal hematopoietic function and produce RBCs, white blood cells, and platelets. Finding EMH in percutaneous FNA of the spleen is extremely common in dogs and cats with diffuse or focal splenomegaly; the presence of hematopoietic blasts may lead to an erroneous diagnosis of lymphoma in some of these patients. I have also observed splenic EMH in dogs with pyometra, immune-mediated hemolysis, immune-mediated thrombocytopenia, several infectious diseases, and a variety of malignant neoplasms as well as in seemingly healthy dogs. Another disorder that commonly results in prominent infiltrative splenomegaly is the hypereosinophilic syndrome of cats (and some dogs, such as Rottweilers), a disease characterized by peripheral blood eosinophilia, bone marrow hyperplasia of the eosinophil precursors, and multiple-organ infiltration by mature eosinophils (see Chapter 85).
The canine and feline spleens have a great capacity to store blood, and under normal circumstances they store between 10% and 20% of the total blood volume. However, tranquilizers and barbiturates can cause splenic blood pooling to increase by relaxing the smooth muscle of the splenic capsule, leading to congestive splenomegaly. The blood that has pooled in an enlarged spleen can account for up to 30% of the total blood volume. Anesthetics such as halothane also may result in marked decreases of 10% to 20% in the packed cell volume and plasma protein concentrations in dogs as a result of the same mechanism.
Portal hypertension can lead to congestive splenomegaly; however, such splenic congestion does not appear to be as common in dogs and cats as it is in human beings. Causes of portal hypertension that may lead to splenomegaly in small animals include right-sided congestive heart failure; obstruction of the caudal vena cava as a result of congenital malformations, neoplasia, or heartworm disease; and intrahepatic obstruction of the venae cavae. Splenic vein thrombosis is a common incidental finding in dogs; it is usually associated with administration of corticosteroids and is typically of no clinical relevance. Ultrasonographic evaluation in these patients usually reveals markedly distended splenic, portal, or hepatic veins or thrombi.
A relatively common cause of congestive splenomegaly in dogs is splenic torsion. Torsion of the spleen, either by itself or in association with gastric dilation-volvulus syndrome, commonly results in marked splenomegaly caused by congestion. Splenic torsion can occur independently of gastric dilation-volvulus syndrome. Most affected dogs are of large, deep-chested breeds, primarily Great Danes and German Shepherd dogs. Clinical signs can be either acute or chronic. Dogs with acute splenic torsion are usually evaluated because of acute abdominal pain and distention, vomiting, depression, and anorexia. Dogs with chronic splenic torsion display a wide variety of clinical signs, including anorexia, weight loss, intermittent vomiting, abdominal distention, PU/PD, hemoglobinuria, and abdominal pain. Physical examination usually reveals marked splenomegaly, and radiographs typically reveal a C-shaped spleen. Ultrasonography of the abdomen in these patients may show greatly distended splenic veins. Hematologic abnormalities usually include regenerative anemia, leukocytosis with a regenerative left shift, and leukoerythroblastosis. Disseminated intravascular coagulation appears to be a common complication in dogs with torsion of the spleen. A high percentage of dogs with splenic torsion have hemoglobinuria, possibly as a consequence of intravascular or intrasplenic hemolysis. Dogs with splenic torsion and hemoglobinuria seen at our clinic occasionally have a positive direct Coombs tests. The treatment of choice for dogs with splenic torsion is splenectomy.
Splenic masses are more common than diffuse splenomegaly in dogs, whereas the opposite is true for cats. Most splenectomies in dogs are done to remove splenic masses. Because splenic masses in cats are extremely uncommon, the following discussion pertains primarily to localized splenomegaly in dogs.
Splenic masses can be classified according to their histopathologic features and biologic behavior as either neoplastic or nonneoplastic. Neoplastic splenic masses can be benign or malignant and mainly include hemangiomas (HAs) and hemangiosarcomas (HSAs), although the former are less common than the latter. Other neoplastic splenic masses that are occasionally found are leiomyosarcomas, fibrosarcomas, leiomyomas, myelolipomas, metastatic carcinomas or sarcomas, malignant histiocytic tumors, and occasionally lymphomas. Nonneoplastic splenic masses include primarily hematomas and abscesses, although splenic infarcts are occasionally described as splenic masses in dogs. As previously discussed, a splenic mass is occasionally diagnosed as a hyperplastic nodule on histopathology after splenectomy.
HSAs are malignant vascular tumors of the spleen; they are extremely common in dogs, constituting the most common primary neoplasm in surgically collected splenic tissues (i.e., splenectomy). These neoplasms are extremely rare in cats.
Clinical Features
The history-taking and physical examination in dogs with splenomegaly are similar to those in dogs with lymphadenopathy. The clinical signs in dogs with splenomegaly are vague and nonspecific and include anorexia, weight loss, weakness, abdominal distention, vomiting, diarrhea, PU/PD, or a combination of these. PU/PD is relatively common in dogs with marked splenomegaly, particularly in those with splenic torsion. Although the pathogenesis of the PU/PD is unclear, psychogenic polydipsia provoked by abdominal pain and distention of the splenic stretch receptors may be a contributory mechanism. Splenectomy in these dogs usually results in prompt resolution of the signs. Other signs associated with splenomegaly result from the hematologic consequences of the splenic enlargement and include spontaneous bleeding caused by thrombocytopenia, pallor caused by anemia, and fever caused by neutropenia or the primary disorder.
During a routine physical examination in pups and cats, the normal spleen is easily palpated as a flat structure oriented dorsoventrally in the left anterior abdominal quadrant. In some deep-chested dogs (e.g., Irish Setters, German Shepherd dogs), the normal spleen is also easily palpated during routine examination, either in the ventral midabdomen or in the left anterior quadrant. This is also the case in Miniature Schnauzers and in some Cocker Spaniels. The fullness of the stomach determines to what extent a normal spleen is palpable in other breeds of dogs. It is easily palpated postprandially because its contour conforms to the greater curvature of the stomach, such that it lies parallel to the last rib. However, not all enlarged spleens are palpable, and not every palpable spleen is abnormal. The characteristics of the spleen on palpation vary. In dogs an enlarged spleen can be either smooth or irregular (“lumpy-bumpy”). In most cats with marked splenomegaly, the surface of the organ is smooth; a diffusely enlarged, lumpy spleen in a cat suggests systemic mast cell disease. As previously discussed, animals with hematologic abnormalities secondary to splenomegaly may also have pallor, petechiae, or ecchymoses.
APPROACH TO PATIENTS WITH LYMPHADENOPATHY OR SPLENOMEGALY
Clinicopathologic Features
A complete blood count (CBC) and a serum biochemistry profile should be obtained, particularly in dogs and cats with generalized or regional lymphadenopathies and those with diffuse splenomegaly. Changes in the CBC may indicate a systemic inflammatory process (e.g., leukocytosis with neutrophilia, left shift, monocytosis) or hemolymphatic neoplasia (e.g., circulating blasts in acute leukemia or lymphoma, marked lymphocytosis suggestive of chronic lymphocytic leukemia or ehrlichiosis). Occasionally the etiologic agent may be identified during examination of a blood smear (e.g., histoplasmosis, mycoplasmosis, trypanosomiasis, babesiosis). Polymerase chain reaction for clonality and immunophenotyping with flow cytometry are commonly used in our clinic in patients with lymphadenopathy or splenomegaly and circulating abnormal cells or lymphocytosis.
The spleen exerts a marked influence on the CBC, resulting in two patterns of hematologic changes in dogs and cats with splenomegaly: hypersplenism and hyposplenism, or asplenia. Hypersplenism results from increased MP activity, is rare, and is characterized by cytopenias in the presence of a hypercellular bone marrow; these changes resolve after splenectomy. Hyposplenism is more common and results in hematologic changes similar to those seen in splenectomized animals, such as thrombocytosis, schistocytosis, acanthocytosis, Howell-Jolly bodies, and increased numbers of reticulocytes and nucleated RBCs.
Anemia in dogs and cats with lymphadenopathy or splenomegaly can occur as a result of the several mechanisms already mentioned. In brief, anemia of chronic disease can be seen in inflammatory, infectious, or neoplastic disorders; hemolytic anemia is usually present in patients with hemoparasitic lymphadenopathies or splenomegaly and in some dogs with malignant histiocytosis or hemophagocytic syndrome. Severe nonregenerative anemia may be seen in dogs with chronic ehrlichiosis, in cats with feline leukemia virus–related disorders or feline immunodeficiency virus–related disorders, and in dogs and cats with primary bone marrow neoplasms (e.g., leukemias, multiple myeloma).
Thrombocytopenia is a common finding in patients with ehrlichiosis, RMSF, anaplasmosis, sepsis, lymphomas, leukemias, multiple myeloma, systemic mastocytosis, or some immune-mediated disorders. Pancytopenia is common in dogs with chronic ehrlichiosis or systemic immunemediated disorders; in dogs and cats with lymphoma or leukemia; and in cats with disorders associated with retroviral infections.
Two major serum biochemical abnormalities are of diagnostic value in dogs and cats with lymphadenopathy or diffuse splenomegaly: hypercalcemia and hyperglobulinemia. Hypercalcemia is a paraneoplastic syndrome that occurs in approximately 10% to 20% of dogs with lymphoma and multiple myeloma, although it may also occur in dogs with blastomycosis. It is extremely rare in cats with these diseases. Monoclonal hyperglobulinemia commonly occurs in dogs and cats with multiple myeloma and occasionally in dogs with lymphoma, ehrlichiosis, or leishmaniasis (see Chapter 89). Polyclonal hyperglobulinemia commonly occurs in dogs and cats with systemic mycoses; in cats with feline infectious peritonitis; and in dogs with ehrlichiosis, anaplasmosis, or leishmaniasis (see Chapter 89).
Serologic and microbiologic studies should always be conducted in dogs and cats with suspected infectious lymphadenopathy-splenomegaly. Serologic tests or polymerase chain reaction for canine ehrlichiosis, RMSF, brucellosis, and systemic mycoses may help diagnose regional or systemic lymphadenopathies. Lymph node specimens for bacterial and fungal cultures should also be obtained if necessary.
Imaging
Radiographic abnormalities in dogs with lymphadenopathy can be related to the primary disorder, or they can reflect the location and degree of lymphadenopathy. In general, plain radiographs or computed tomography (CT) are beneficial in dogs and cats with solitary lymphadenopathy to search for primary bone inflammation or neoplasia, in those with generalized peripheral (superficial) lymphadenopathy to detect intrathoracic or intraabdominal lymph node enlargement, and in those with deep regional lymphadenopathy involving the thoracic cavity to determine the distribution and size of the affected nodes and the changes in the pulmonary parenchyma and pleural space.
The spleen is normally well visualized on plain abdominal radiographs, but its appearance can vary widely. On dorsoventral or ventrodorsal views, the spleen is seen between the gastric fundus and the left kidney. The size and location of the spleen are more variable on lateral radiographs than on ventrodorsal or dorsoventral projections. In some breeds, such as Greyhounds, the spleen appears to be large on plain radiographs and ultrasonography. On plain radiographs, large splenic masses usually appear in the caudal abdomen or the midabdomen. Tranquilization or anesthesia usually results in a diffuse congestive splenomegaly, making radiographic interpretation of splenic size extremely difficult. CT is a useful diagnostic tool in dogs with focal or diffuse splenomegaly.
Ultrasonography is the noninvasive procedure of choice to evaluate intraabdominal lymphadenopathy and splenomegaly because it can accurately image and show the size of both enlarged lymph nodes and the spleen so that the patient’s response to therapy can be monitored. In addition, ultrasound-guided FNA or biopsies can be performed with minimal complications. Abdominal ultrasonography can reveal diffuse splenomegaly, splenic masses, splenic congestion, hepatic nodules, or other changes; in addition, color-flow Doppler allows evaluation of splenic blood flow. A major issue a clinician frequently must deal with is the incidental splenic nodule in an older dog; these lesions are common and usually clinically irrelevant, but they tend to cloud the clinical picture in a patient with intraabdominal neoplasia. If possible, splenic nodules should be aspirated and evaluated cytologically. Of note, however, is that the presence of hepatic nodules in a dog with a splenic mass does not constitute a valid reason for an owner to decline treatment or request euthanasia because regenerative liver nodules are indistinguishable from metastatic lesions. Moreover, hypoechoic splenic nodules are frequently found in normal dogs.
Radionuclide imaging of the spleen (and less commonly of lymph nodes) using technetium-99m–labeled sulfur colloid has become an accepted method of splenic imaging in human beings and small animals. However, this technique only evaluates the spleen’s ability to clear particulate matter and rarely provides a morphologic diagnosis.
Additional Diagnostics
Evaluation of bone marrow aspirates or core biopsy specimens may be beneficial in dogs and cats with generalized lymphadenopathy or splenomegaly caused by hemolymphatic neoplasia or systemic infectious diseases. For example, acute or chronic leukemia in dogs may be difficult to diag nose on the basis of lymph node cytologic findings alone because the diagnosis is usually that of lymphoma (with the presence of well-differentiated or poorly differentiated lymphoid cells). In those cases, the combination of hematologic and bone marrow findings is usually diagnostic. Bone marrow evaluation should always be done before splenectomy in patients with cytopenias because the spleen may assume the primary hematopoietic function in dogs and cats with primary bone marrow disorders such as hypoplasia or aplasia. Splenectomy in these animals could remove the sole source of circulating blood cells, leading to death.
Cytologic evaluation of lymph node and splenic aspirates provides the clinician with a wealth of information and often constitutes the definitive diagnostic procedure in animals with lymphadenopathy or diffuse splenomegaly. In my experience, cytologic evaluation of appropriately collected specimens yields diagnostic findings in approximately 80% to 90% of dogs and 70% to 75% of cats with lymphadenopathy and in approximately 80% of dogs and cats with diffuse splenomegaly.
Although superficial lymph nodes can be aspirated with minimal difficulty, the successful aspiration of intrathoracic or intraabdominal lymph nodes or spleen requires some expertise and occasionally must be done under the guidance of imaging techniques (e.g., fluoroscopy, ultrasonography, CT) (see Chapter 75). To obtain an FNA of a superficial node, the area does not have to be surgically prepared. However, the aspiration of intrathoracic and intraabdominal structures (e.g., spleen) requires surgical preparation of the area and adequate restraint of the animal. Certain intraabdominal lymph nodes (e.g., markedly enlarged mesenteric or iliac nodes) are easily aspirated transabdominally by using manual isolation of the mass. Iliac lymph nodes can also be aspirated transrectally with a 2- to 3-inch (5 to 7.5cm) needle. Splenic aspirates are obtained with the animal in right lateral or dorsal recumbency with manual restraint or mild sedation. Transabdominal splenic FNA in dogs or cats chemically restrained with phenothiazine tranquilizers or barbiturates usually yields blood-diluted specimens as a result of splenic congestion.
In a patient with generalized lymphadenopathy, the clinician must decide which lymph node to aspirate. Obviously aspiration of a node in which the tissue changes are representative of the ongoing disease is important. Therefore do not obtain a specimen from the largest lymph node because the central necrosis in such a node usually pre-cludes a definitive diagnosis. Because clinical and subclinical gingivitis are common in older dogs and cats, mandibular lymph nodes should not be routinely aspirated because they are usually reactive and findings may obscure the primary diagnosis. The techniques of FNA are described in Chapter 75.
Several reviews of the cytologic evaluation of lymphoid tissues have appeared in the veterinary literature (see Suggested Readings). In brief, normal lymph nodes are composed primarily of small lymphocytes (80% to 90% of all cells); a low number of macrophages, medium or large lymphocytes, plasma cells, and mast cells can also be found. Normal spleens are similar except that RBCs are in high concentration given this organ’s vascularity. Reactive lymph nodes and hyperplastic spleens are characterized by variable numbers of lymphoid cells in different stages of development (small, medium, and large lymphocytes; plasma cells); hematopoietic precursors are common in dogs and cats with splenic hyperplasia. The cytologic features of lymphadenitis-splenitis vary with the etiologic agent and the type of reaction elicited. Etiologic agents can frequently be identified in cytologic specimens from nodes with lymphadenitis. Metastatic neoplasms have different cytologic features depending on the degree of involvement and the cell type. Carcinomas, adenocarcinomas, melanomas, and mast cell tumors are easily diagnosed on the basis of cytologic findings. However, the cytologic diagnosis of sarcomas may be difficult because the neoplastic cells that comprise this tumor do not exfoliate easily. Primary lymphoid neoplasms (lymphomas) are characterized by a monomorphic population of lymphoid cells, which are usually immature (showing a fine chromatin pattern, one or more nucleoli, basophilic cytoplasm, vacuolation) (Fig. 88-3). For a more detailed description of cytologic changes, see Chapter 75.
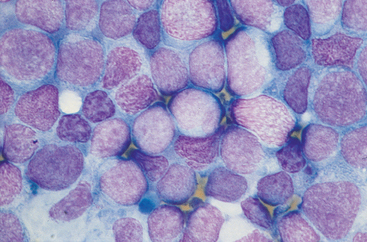
FIG 88-3 Cytologic characteristics of a lymph node aspirate from a dog with massive generalized lymphadenopathy (lymphoma). Note a monomorphic population of large, round cells with a lacy chromatin pattern (neoplastic cells) intermixed with small, darker, normal lymphocytes. (Wright-Giemsa stain, ×1000.)
When the cytologic examination of an enlarged lymph node or spleen does not yield a definitive diagnosis, excision of the affected node or incisional or even excisional splenic biopsy to obtain a specimen for histopathologic examination is indicated. Excision of the whole node is preferable because core biopsy specimens are difficult to interpret because the lymph node architecture is often poorly preserved. A wedge of tissue can be obtained during a splenic biopsy or, if the surgeon deems it necessary, a splenectomy can be performed. Care should be taken in handling the tissues during surgical manipulation because trauma may induce considerable artifactual changes, which would preclude interpretation of the specimen. The popliteal lymph nodes are easily accessible and are the ones usually excised in dogs and cats with generalized lymphadenopathy.
Once a node is excised, it should be sectioned in half lengthwise, impression smears made for cytologic analysis, and the node fixed in 10% buffered formalin in a proportion of one part of tissue to nine parts of fixative. The specimen is then ready to be sent to a laboratory for evaluation. Samples can also be saved for cytochemical or immunohistochemical evaluation, ultrastructural studies, or microbiologic evaluation, including polymerase chain reaction. The same guidelines apply to the preparation of splenic specimens.
MANAGEMENT OF PATIENTS WITH LYMPHADENOPATHY OR SPLENOMEGALY
As previously discussed, no specific treatment exists for dogs or cats with local, regional, or generalized lymphadenopathy or diffuse splenomegaly. Treatment should be directed at the cause(s) of the lymphadenopathy or splenomegaly rather than at the enlarged lymph nodes or spleen. Exploratory celiotomies provide considerable information regarding the gross morphologic characteristics of an enlarged spleen and adjacent organs and tissues. However, direct visualization of these structures may be misleading because differentiation of some benign splenic masses (e.g., hematoma, HA) from their malignant counterpart (e.g., HSA) on the basis of gross morphology alone may be impossible. As discussed in the section on imaging, the surgeon may recommend to the owners that the animal be euthanized on the operating table because it has a splenic mass and nodules in the liver, only to find out that the hepatic nodules represent nodular hyperplasia or EMH and the primary mass was benign (e.g., HA or hematoma).
Splenectomy is indicated in the event of splenic torsion, splenic rupture, symptomatic splenomegaly, or splenic masses. The value of splenectomy is questionable in dogs with immune-mediated blood disorders, dogs and cats with splenomegaly caused by lymphoma in which chemotherapy has not induced splenic remission, and dogs and cats with leukemias. Splenectomy is contraindicated in patients with bone marrow hypoplasia in which the spleen is the main site of hematopoiesis.
Although rare, a syndrome of postsplenectomy sepsis has been documented in approximately 3% of dogs that undergo this surgical procedure in our clinic. The syndrome is similar to its human counterpart. Most dogs with postsplenectomy sepsis evaluated at our clinic were undergoing immunosuppressive therapy at the time of surgery or had undergone splenectomy for a neoplasm. This sepsis is usually rapid in onset (hours to days), so prophylactic bactericidal antibiotic therapy is recommended postoperatively. We routinely use cephalothin (20mg/kg intravenously [IV] q8h) with or without enrofloxacin (5-10mg/kg IV q24h) for 2 to 3 days postoperatively. All dogs with clinically recognized postsplenectomy sepsis at our clinic have died within 12 hours of onset despite aggressive treatment.
The clinician occasionally encounters a patient in which the enlarged lymph node mechanically compresses or occludes a viscus, airway, or vessel. This may result in marked clinical abnormalities, such as intractable coughing, caused by tracheobronchial lymphadenopathy; colonic obstruction, caused by iliac lymphadenopathy; or anterior vena cava syndrome, caused by cranial vena cava and thoracic duct obstruction. Several treatment options are available for these situations. If the lymph node is surgically resectable, excision or drainage should be attempted. If the node is not surgically resectable or if surgery or anesthesia poses a high risk for the animal, one or more of the following can be used:
Ballegeer EA, et al. Correlation of ultrasonographic appearance of lesions and cytologic and histologic diagnoses in splenic aspirates from dogs and cats: 32 cases (2002–2005). J Am Vet Med Assoc. 2007;230:690.
Clifford CA, et al. Magnetic resonance imaging of focal splenic and hepatic lesions in the dog. J Vet Intern Med. 2004;18:330.
Couto CG. A diagnostic approach to splenomegaly in cats and dogs. Vet Med. 1990;85:220.
Couto CG, et al. Diseases of the lymph nodes and spleen. In Ettinger SJ, et al, editors: Textbook of veterinary internal medicine—diseases of the dog and cat, ed 4, St Louis: WB Saunders, 1995.
Fife WD, et al. Comparison between malignant and nonmalignant splenic masses in dogs using contrast-enhanced computed tomography. Vet Radiol Ultrasound. 2004;45:289.
Gamblin R, et al. Lymphadenopathy and organomegaly. In: Fenner WR, editor. Quick reference to veterinary medicine. ed 3. Philadelphia: Lippincott Williams & Wilkins; 2000:91.
Gamblin RM, et al. Nonneoplastic disorders of the spleen. In: Ettinger SJ, Feldman EC, editors. Textbook of veterinary internal medicine: diseases of the dog and cat. ed 5. St Louis: Saunders; 2000:1857.
Hammer AS, et al. Disorders of the lymph nodes and spleen. In Sherding RG, editor: The cat: diseases and clinical management, ed 2, New York: Churchill Livingstone, 1994.
Mills JN. Diagnosis from lymph node fine-aspiration cytology. Aust Vet Pract. 1984;14:14.
Mooney SC, et al. Generalized lymphadenopathy resembling lymphoma in cats: six cases (1972–1976). J Am Vet Med Assoc. 1987;190:897.
Moore FM, et al. Distinctive peripheral lymph node hyperplasia of young cats. Vet Pathol. 1986;23:386.
O’Brien RT, et al. Sonographic features of drug-induced splenic congestion. Vet Radiol Ultrasound. 2004;45:225.
O’Keefe DA, et al. Fine-needle aspiration of the spleen as an aid in the diagnosis of splenomegaly. J Vet Intern Med. 1987;1:102.
Spangler WL, et al. Prevalence and type of splenic diseases in cats: 455 cases (1985–1991). J Am Vet Med Assoc. 1992;201:773.
Spangler WL, et al. Prevalence, type, and importance of splenic diseases in dogs: 1,480 cases (1985–1989). J Am Vet Med Assoc. 1992;200:829.
Spangler WL, et al. Pathologic factors affecting patient survival after splenectomy in dogs. J Vet Intern Med. 1997;11:166.