CHAPTER 83 Anemia
DEFINITION
Anemia is defined as a decrease in the red blood cell (RBC) mass. In practical terms it can be defined as a decrease in the packed cell volume (PCV) or hematocrit (HCT), the hemoglobin (Hb) concentration, or the RBC count below reference values for the species. In the context of this chapter, PCV and HCT are used interchangeably. In special circumstances, anemia is diagnosed in a given patient with a HCT that has decreased over time even though it may remain within reference values. For example, Greyhounds rarely have HCT values less than 50%, so an anemic Greyhound may have a HCT within the reference range for the dog. Because the reference values reflect the actual status in 95% of the feline and canine population, occasionally an “abnormal” value is indeed normal for a particular animal, prompting a needless evaluation in search of other abnormalities. Of note, anemia does not constitute a primary diagnosis; therefore every effort should be made to identify its cause.
CLINICAL AND CLINICOPATHOLOGIC EVALUATION
When interpreting the HCT, Hb concentration, or RBC count, the clinician should keep in mind that in some situations these values are above (e.g., sight hounds) or below (e.g., puppyhood, pregnancy) the reference value for the species. From a practical standpoint, when evaluating the erythroid series, the clinician does not need to assess all the values in the complete blood count (CBC) because several of them provide identical information. For example, the HCT, Hb concentration, and RBC count provide the same type of information (i.e., an increase in the number of RBCs usually results in an increased HCT and Hb concentration, and vice versa). Thus when evaluating the erythron in a CBC, the HCT is typically used as an indirect index of the RBC mass (or number).
The main clinical manifestations of anemia in cats and dogs include pale or icteric mucous membranes, lethargy, exercise intolerance, pica (mainly in cats), and decreased overall activity (Box 83-1). These clinical signs can be acute or chronic and can vary in severity; the duration of the clinical signs may not reflect the mechanism of anemia. For example, “acute” clinical signs are common in cats with chronic anemia; most cats with chronic anemia compensate by shifting the oxyhemoglobin dissociation curve to the right, thus releasing oxygen to the tissues more readily. Therefore cats are clinically stable until their HCT level gets below a specific percent and they develop “acute” signs. Owners may also detect some of the adaptive changes to anemia, such as tachycardia or an increased precordial beat. Following are several important questions to ask the owner of an anemic cat or dog:
 BOX 83-1 Clinical Manifestations of Anemia in Cats and Dogs
BOX 83-1 Clinical Manifestations of Anemia in Cats and Dogs
FeLV, Feline leukemia virus; FIV, feline immunodeficiency virus.
In addition to these questions, a detailed travel and pharmacologic history should be obtained. Certain infectious diseases associated with anemia may have geographic distribution (e.g., babesiosis in the southeastern part of the United States); however, because dogs frequently travel throughout the United States, the geographic disease distribution is becoming less common. Some drugs and toxins that have been associated with anemia in cats and dogs are listed in Box 83-2.
 BOX 82-2 Drugs and Toxins Associated with Anemia in Cats and Dogs
BOX 82-2 Drugs and Toxins Associated with Anemia in Cats and Dogs
Antiinflammatories (nonsteroidal)
When evaluating a patient with pallor, determine whether it is attributable to hypoperfusion or anemia (i.e., not every patient with pale mucous membranes is anemic). The simplest approach is to evaluate the HCT and the capillary refill time (CRT). Dogs and cats with cardiovascular disease and hypoperfusion usually have normal HCT values and additional clinical signs, whereas symptomatic anemic dogs have low HCT. Dogs and cats with congestive heart failure occasionally have dilutional anemia caused by intravascular fluid retention. The CRT may be difficult to evaluate in anemic cats and dogs because of the absence of contrast from the pallor.
The clinician should also look for petechiae, ecchymoses, and evidence of deep bleeding in animals with pallor. These findings are suggestive of a platelet or clotting factor deficiency (as seen in animals with Evans syndrome, disseminated intravascular coagulation [DIC], or acute leukemias; see Chapter 87), resulting in bleeding and secondary anemia. Particular attention should be paid to the lymphoreticular organs, such as the lymph nodes and spleen, because several disorders associated with anemia may also result in lymphadenopathy, hepatosplenomegaly, or both (Table 83-1). Abdominal radiographs in a dog with intravascular hemolysis may show metallic foreign bodies in the stomach, a potential source of zinc that frequently results in RBC lysis.
 TABLE 83-1 Disorders Commonly Associated with Anemia and Hepatomegaly, Splenomegaly, and/or Lymphadenopathy
TABLE 83-1 Disorders Commonly Associated with Anemia and Hepatomegaly, Splenomegaly, and/or Lymphadenopathy
| DISORDER | FREQUENCY | SPECIES |
|---|---|---|
| Lymphoma | F | D, C |
| Mycoplasmosis | F | C > D |
| Acute leukemias | F | C, D |
| Ehrlichiosis | F* | D > C |
| Systemic mast cell disease | R | C > D |
| Bone marrow hypoplasia | R | C, D |
| IHA | F | D > C |
| Hypersplenism | R | D, C |
F, Frequent; R, rare; D, dog; C, cat.
The degree of anemia may be helpful in establishing its cause. To this end, anemias are graded according to HCT level as follows:
| Dogs | Cats | |
|---|---|---|
| Mild | 30%-36% | 20%-24% |
| Moderate | 18%-29% | 15%-19% |
| Severe | <18% | <14% |
For example, if an anemic dog or cat has severe anemia, certain causes (e.g., bleeding, anemia of chronic disease, anemia of renal disease, IDA) can immediately be ruled out because none of those mechanisms is likely to result in such a severe decrease in the HCT; therefore the patient most likely has hemolysis or a bone marrow disorder (see below). The severity of the clinical signs usually also correlates with the pathogenesis of the anemia. For example, a dog or cat with severe anemia and mild to moderate clinical signs more likely has a chronic cause of anemia (e.g., bone marrow disease); acute causes of severe anemia (e.g., hemolysis) result in clinical signs of marked severity because the adaptive compensatory changes have not yet occurred.
As part of the evaluation of a patient’s HCT, the plasma should be examined for evidence of icterus or hemolysis and the protein content should be determined with a refractometer. The microhematocrit tube should be carefully inspected for evidence of autoagglutination (see p. 1215) and a slide agglutination test should be performed (see below). A blood smear should be evaluated to detect morphologic changes that may point the clinician toward the cause of the anemia.
A common issue that comes up often is whether a general practicing veterinarian should do CBCs in-house or send them to a referral laboratory. The recent introduction of accurate, user-friendly, benchtop hematology analyzers has revolutionized the practice of small animal hematology. Most of these instruments are trouble free and provide accurate results. However, when values are outside the reference range or are flagged, the clinician or technician should evaluate a blood smear from the patient in question.
Once the patient has been established as anemic, it should be determined whether the anemia is regenerative or nonregenerative. This is accomplished by obtaining a reticulocyte count during a routine CBC (some of the in-house analyzers, such as the LaserCyte from IDEXX Laboratories, Westbrook, Maine, provide reticulocyte counts) or by evaluating a blood smear for the presence of polychromasia. This reflects the pathogenesis of the anemia, thereby dictating the most logical diagnostic and therapeutic approach (Box 83-3).
 BOX 83-3 Pathogenetic Classification of Anemias
BOX 83-3 Pathogenetic Classification of Anemias
IDA, Iron deficiency anemia; ACD, anemia of chronic disease; ARD, anemia of renal disease.
In brief, regenerative anemias always stem from extra-marrow causes because the presence of reticulocytes or polychromatophilic RBCs (i.e., immature RBCs) in the circulation is a clear indication of a functional bone marrow. Regenerative anemias can result only from hemolysis or blood loss. Nonregenerative anemias can be caused by bone marrow or extra-marrow disorders, such as erythroid hypoproliferation, chronic inflammatory disease, chronic kidney disease, and acute hemorrhage or hemolysis (first 48 to 96 hours). Although IDA is traditionally classified as nonregenerative, most dogs with chronic blood loss leading to iron deficiency display a mild to moderate degree of regeneration, and the RBC indices are different than in other nonregenerative anemias (see below). Therefore I prefer to classify IDA in a separate category. Regenerative anemias are usually acute, whereas nonregenerative anemias are either peracute (i.e., blood loss or hemolysis of less than 48 hours’ duration) or, more often, chronic.
During the initial clinical evaluation of an anemic patient, examination of the blood smear usually suffices in determining whether the bone marrow is responding appropriately to the anemia (i.e., whether the anemia is regenerative or not). Several pieces of information can be acquired during the examination of a good-quality, properly stained blood smear, including the RBC size and morphology, the presence of autoagglutination, the approximate numbers and morphology of white blood cells and platelets, the presence of nucleated RBCs, the presence of polychromasia (indicative of regeneration), and the presence of RBC parasites. The clinician should perform this cursory evaluation of the blood smear; a blood sample should be submitted to a diagnostic laboratory for further analysis and evaluation by a clinical pathologist if the diagnosis is still uncertain after evaluating the blood smear. Some of the abnormalities detected during a careful examination of the blood smear and their clinical implications are summarized in Table 83-2. This evaluation should be conducted under oil immersion lens in a monolayer field, in which the erythrocytes are in a single layer and 50% of the cells are touching.
 TABLE 83-2 Interpretation of Morphologic RBC Abnormalities in Cats and Dogs
TABLE 83-2 Interpretation of Morphologic RBC Abnormalities in Cats and Dogs
| MORPHOLOGIC ABNORMALITY | COMMONLY ASSOCIATED DISORDERS |
|---|---|
| Macrocytosis | Regeneration, breed-related characteristic (Poodles); FeLV or FIV infection; dyserythropoiesis (bone marrow disease) |
| Microcytosis | Iron deficiency; breed-related characteristic (Akita, Sharpei, Shiba Inu); portosystemic shunt; polycythemia (erythrocytosis) |
| Hypochromasia | Iron deficiency |
| Polychromasia | Regeneration |
| Poikilocytosis | Regeneration; iron deficiency; hyposplenism |
| Schistocytosis (fragments) | Microangiopathy; hemangiosarcoma; DIC; hyposplenism |
| Spherocytosis | IHA; mononuclear phagocytic neoplasm; zinc toxicity |
| Acanthocytosis (spur cells) | Hemangiosarcoma; liver disease; hyposplenism |
| Echinocytosis (burr cells) | Artifact; renal disease; pyruvate kinase deficiency anemia |
| Elliptocytosis | Congenital elliptocytosis (dogs) |
| Heinz bodies | Oxidative insult to RBCs |
| Howell-Jolly bodies | Regeneration; hyposplenism |
| Autoagglutination | IHA |
| Metarubricytosis | Breed-related characteristic (Schnauzer, Dachshund); extramedullary hematopoiesis; regeneration; lead toxicity; hemangiosarcoma |
| Leukopenia | See text |
| Thrombocytopenia | See text |
| Pancytopenia | Bone marrow disorder; hypersplenism |
RBC, Red blood cell; FeLV, feline leukemia virus; FIV, feline immunodeficiency virus; DIC, disseminated intravascular coagulation; IHA, immune hemolytic anemia. Modified from Couto CG et al: Hematologic and oncologic emergencies. In Murtaugh R et al, editors: Veterinary emergency and critical care medicine, St Louis, 1992, Mosby.
A CBC and a reticulocyte count in an anemic patient provide more absolute data by which to assess the degree of regeneration. However, the information presented below must be used cautiously because the number of reticulocytes should increase proportionally to the decrease in the HCT. For example, a reticulocyte count of 120,000/μL or 4% represents an appropriate response for a dog with an HCT of 30% but not for one with an HCT of 10%. The following points generally hold true:
As part of the evaluation of a patient with regenerative anemia, it is beneficial to determine the serum or plasma protein concentration because blood loss usually results in hypoproteinemia and hemolysis does not. Other physical examination and clinicopathologic findings that help distinguish blood loss from hemolytic anemias are listed in Table 83-3.
 TABLE 83-3 Criteria for Differentiating Blood Loss from Hemolytic Anemias
TABLE 83-3 Criteria for Differentiating Blood Loss from Hemolytic Anemias
| VARIABLE | BLOOD LOSS | HEMOLYSIS |
|---|---|---|
| Serum (plasma) protein concentration | Normal-low | Normal-high |
| Evidence of bleeding | Common | Rare |
| Icterus | No | Common |
| Hemoglobinemia | No | Common |
| Spherocytosis | No | Common |
| Hemosiderinuria | No | Yes |
| Autoagglutination | No | Occasional |
| Direct Coombs test | Negative | Usually positive (in IHA) |
| Splenomegaly | No | Common |
| RBC changes | No | Common (see Table 83-2) |
Reprinted from Couto CG et al: Hematologic and oncologic emergencies. In Murtaugh R et al, editors: Veterinary emergency and critical care
IHA, Immune hemolytic anemia; RBC, red blood cell.
medicine, St Louis, 1992, Mosby.
PRINCIPLES OF MANAGEMENT OF THE ANEMIC PATIENT
The first basic principle of the management of anemic (or bleeding) patients is to collect all blood samples before instituting any therapy. Because the condition in most of these patients may constitute a true emergency at the time of presentation, samples often are not collected until the patient has been completely stabilized, resulting in treatment-induced changes in hematologic or serum biochemical values.
As a general rule, because of the acute onset of these disorders, patients with regenerative anemias (i.e., blood loss or hemolysis) require more aggressive therapy than those with nonregenerative forms. Specific therapy should be instituted once the clinician has determined that the patient’s condition is stable and whether or not the anemia is regenerative. The diagnosis and management of different forms of anemia in cats and dogs are discussed throughout the remainder of this chapter.
REGENERATIVE ANEMIAS
BLOOD LOSS ANEMIA
Acute blood loss in otherwise normal dogs and cats results in reticulocytosis (i.e., regeneration) within 48 to 96 hours. Therefore animals evaluated shortly after a traumatic injury and severe blood loss usually have nonregenerative anemias with low-to-normal serum (plasma) protein concentrations. The source of bleeding should be identified and the bleeding stopped; if the patient is bleeding as a result of a systemic hemostatic defect, it should be identified and specific treatment should be initiated (see Chapter 87). Aggressive intravenous (IV) fluid therapy with crystalloids or colloids or the transfusion of blood or blood products is often required in patients with anemia caused by acute blood loss.
HEMOLYTIC ANEMIA
In human beings the bone marrow is capable of undergoing hyperplasia until its production rate is increased approximately sixfold to eightfold; the same is probably true for dogs and cats. As a consequence, a considerable number of RBCs must be destroyed before anemia develops. As is the case in cats and dogs with blood loss anemia, patients with peracute hemolysis can be in a nonregenerative state at the time of presentation because the bone marrow has not yet been able to mount a regenerative response. In addition, in some dogs with immune-mediated hemolysis, the destruction of erythroid precursors in the bone marrow results in a lack of regeneration.
On the basis of their pathogenesis, hemolytic anemias can be classified as extravascular (i.e., the RBCs are destroyed by the mononuclear phagocytic cells) or intravascular (i.e., the RBCs are lysed by antibody-complement, drugs, toxins, or by hitting fibrin strands). On the basis of the age of the animal at onset, anemias can be classified as congenital or acquired (Table 83-4). Most dogs and cats with hemolytic anemia seen at my clinic have acquired extravascular hemolysis.
 TABLE 83-4 Causes of Hemolytic Anemia in Dogs and Cats
TABLE 83-4 Causes of Hemolytic Anemia in Dogs and Cats
| DISORDER | SPECIES | BREED |
|---|---|---|
| Congenital (Inherited?) | ||
| Pyruvate kinase deficiency | D, C | Basenji, Beagle, West Highland White Terrier, Cairn Terrier, Poodle, Dachshund, Chihuahua, Pug, American Eskimo, Abyssinian, Somali, domestic short-haired cat |
| PFK deficiency | D | English Springer Spaniel, Cocker Spaniel |
| Stomatocytosis | D | Alaskan Malamute, Miniature Schnauzer |
| Nonspherocytic hemolytic anemia | D | Poodle, Beagle |
| Acquired | ||
| IHA | D > C | All |
| Neonatal isoerythrolysis | C | British breeds, Abyssinian, Somali (other type B cats) |
| Microangiopathic hemolytic anemia | D > C | All |
| Infectious | ||
| Mycoplasmosis | C > D | All |
| Babesiosis | D > C | All |
| Cytauxzoonosis | C | All |
| Ehrlichiosis | D > C | All |
| Hypophosphatemia | D, C | All |
| Oxidants | ||
| Acetaminophen | C | All |
| Phenothiazines | D, C | All |
| Benzocaine | C | All |
| Vitamin K | D, C | All |
| Methylene blue | C > D | All |
| Methionine | C | All |
| Propylene glycol | C | All |
| Drugs that Can Cause Immune Hemolysis | ||
| Sulfas | D > C | Doberman, Labrador Retriever |
| Anticonvulsants | D | All |
| Penicillins and cephalosporins | D > C | All |
| Propylthiouracil | C | All |
| Methimazole | C | All |
| Antiarrhythmics? | D | All |
| Zinc | D | All |
PFK, Phosphofructokinase; IHA, immune hemolytic anemia.
Modified from Couto CG et al: Hematologic and oncologic emergencies. In Murtaugh R et al, editors: Veterinary emergency and critical care medicine, St Louis, 1992, Mosby.
In extravascular hemolysis, RBCs are phagocytosed by the mononuclear-phagocytic system (MPS) in the spleen, liver, and bone marrow. Stimuli that trigger RBC phagocytosis consist mainly of intracellular inclusions, such as RBC parasites or Heinz bodies (the latter are commonly seen in cats) and membrane coating with immunoglobulin (Ig) G or M (common in dogs). Congenital RBC enzymopathies can also precipitate extravascular hemolysis. Once abnormal RBCs are recognized, the MPS rapidly phagocytoses them, resulting in a decrease in the number of circulating RBCs and the generation of cells with specific morphologic changes (e.g., spherocytes). Anemia develops if the destruction of RBCs continues. Spherocytes are RBC “leftovers,” in that after a mononuclear-phagocytic cell takes a “bite” of cytoplasm and membrane, the membrane is resealed; the RBC then loses its redundant membrane and consequently its central pallor. Spherocytes are characteristic of immune hemolytic anemia (IHA), although they can occasionally be seen in other disorders, such as Babesia gibsoni infection or zinc toxicity. Immune hemolysis is the most common cause of extravascular hemolytic anemia in dogs at my hospital. Drugassociated hemolysis (e.g., βlactam antiiotics) and mycoplasmosis (formerly known as haemobartonellosis) are the two most common causes in cats, although IHA is now more common in this species. Other causes of extravascular hemolytic anemia in dogs and cats are listed in Table 83-4.
Intravascular hemolysis can occur as a consequence of direct RBC lysis caused by antibodies that activate complement (e.g., immune-mediated hemolysis), infectious agents (e.g., Babesia canis infection), drugs or toxins (e.g., zinc in pennies minted after 1983, in pet carrier bolts, other hardware, and zinc oxide–containing ointments), metabolic imbalances (e.g., hypophosphatemia in dogs and cats with diabetes mellitus treated with insulin), or increased shearing of RBCs (e.g., microangiopathy, DIC). Intravascular hemolysis is considerably less common in dogs and cats than extravascular hemolysis, with the notable exception of DIC in dogs with hemangiosarcoma, zinc toxicity, and hypophosphatemia. Certain congenital enzymopathies (e.g., phosphofructokinase [PFK] deficiency) in dogs also result in intravascular hemolysis.
Dogs with congenital (frequently familial) hemolytic anemias may have relatively prolonged clinical courses at the time of presentation, with the notable exception of English Springer Spaniels with PFK deficiency–induced hemolysis, in which acute hemolytic episodes occur after they hyper ventilate during excitement or field work (i.e., alkaline hemolysis). Dogs and cats with acquired hemolytic anemias are usually evaluated because of acute clinical signs consisting of pallor, with or without icterus (in my experience, only approximately half of dogs and a lower percentage of cats with hemolytic anemia are icteric); splenomegaly may be a prominent finding. If the patient has associated thrombocytopenia (e.g., Evans syndrome, DIC), petechiae and ecchymoses may be present. Clinical signs and physical examination findings associated with the primary disease can also be present in cases of secondary hemolytic anemias; however, as opposed to human beings, they are extremely rare in dogs and cats.
In the evaluation of dogs or cats with hemolytic anemia, a careful examination of the blood smear is mandatory. Morphologic abnormalities pathognomonic for or highly suggestive of a particular etiology are often detected with this method (see Table 83-2). The sample should also be tested for autoagglutination; this is done by placing a large drop of anticoagulated blood on a glass slide at room temperature and at 4° C. Agglutination can be distinguished from rouleaux formation by diluting the blood 5 : 1 or 10 : 1 in saline solution (this disaggregates rouleaux); rouleaux formation is common in cats but rare in dogs. A direct Coombs test to detect RBC-bound Ig should always be performed in dogs and cats with suspected hemolysis (see below). As a general rule, the presence of Ig coating on the RBCs indicates immune-mediated hemolysis. A positive Coombs test result should be interpreted with caution, however, because certain drugs and hemoparasites can induce formation of antibodies that bind to the RBCs, thus causing secondary immune hemolysis (e.g., cats with mycoplasmosis). The pretreatment of an animal with corticosteroids may also result in decreased binding of Ig molecules to the surface of the RBC, thus resulting in false-negative results. Direct Coombs tests are usually not necessary in animals with autoagglutination because this phenomenon connotes the presence of Ig on the surface of the RBCs (i.e., biologic Coombs test). Cryoagglutination (i.e., the agglutination of RBCs if the blood sample is refrigerated for 6 to 8 hours) occurs in a large proportion of cats with mycoplasmosis and is usually associated with IgM coating on the RBCs.
If an etiologic agent cannot be identified (e.g., RBC parasite, drug, pennies in the stomach), the patient should be treated for primary or idiopathic IHA while further test results (e.g., serologic tests or polymerase chain reaction [PCR] for hemoparasites) are pending. As previously mentioned, primary IHA is considerably more common in dogs than in cats; thus every effort should be made to identify a cause of hemolysis in cats, such as drugs or hemoparasites. A detailed discussion of IHA is presented below.
Hemolytic anemias not associated with immune destruction of the RBCs are treated by removal of the cause (e.g., drug, infectious agent, gastric foreign body) and supportive therapy. Corticosteroids (see below) can be administered to suppress MPS activity while the etiologic agent is being eliminated, although this is not always beneficial. Doxycycline (5 to 10 mg/kg PO q12-24h for 21 to 42 days) usually results in resolution of the signs in dogs and cats with mycoplasmosis and in dogs with ehrlichiosis.
Immune Hemolytic Anemia
IHA constitutes the most common form of hemolysis in dogs (see Chapter 104). Although two pathogenetic categories of hemolytic anemia are recognized (primary, or idiopathic, and secondary), most cases of IHA in dogs are primary (i.e., a cause cannot be found after exhaustive clinical and clinicopathologic evaluation). The immune-mediated destruction of RBCs can occur in association with drug administration (e.g., βlactam antibiotics, barbiturates) or vaccination. With the exception of the immune hemolysis secondary to hemoparasitism, IHA is rare in cats (although its prevalence is higher than 5 years ago). The clinical course in dogs is typically acute, but peracute presentations are also common.
In IHA, the RBCs become coated primarily with IgG, which leads to the early removal of the coated cells by the MPS, mainly in the spleen and liver. As a consequence spherocytes are generated; therefore the presence of spherocytes in the blood smear of a dog with anemia is highly suggestive of IHA. Spherocytes are difficult to identify in cats.
The typical patient with IHA is a middle-aged, female spayed Cocker Spaniel or small breed dog, although I have recently noticed a higher prevalence of IHA (and other immune-mediated cytopenias) in Golden Retrievers. Clinical signs in dogs with IHA include depression of acute (or peracute) onset, exercise intolerance, and pallor or jaundice, occasionally accompanied by vomiting or abdominal pain. Physical examination findings usually consist of pallor or jaundice, petechiae and ecchymoses (if immune thrombocytopenia is also present), splenomegaly, and a heart murmur. As previously noted, jaundice can be absent in dogs with IHA. A subset of dogs with acute (or peracute) IHA with icterus (and usually autoagglutination) shows clinical deterioration within hours or days of admission, resulting from multifocal thromboembolic disease or a lack of response to conventional therapy. I treat these dogs more aggressively than the typical dog with IHA (see next page).
Hematologic findings in dogs with IHA typically include strongly regenerative anemia, leukocytosis from neutrophilia with a left shift and monocytosis, increased numbers of nucleated RBCs, polychromasia, and spherocytosis. The serum (plasma) protein concentration is usually normal to increased, and hemoglobinemia or bilirubinemia may be present (i.e., pink or yellow plasma). As previously noted, autoagglutination is prominent in some dogs. Thrombocytopenia is also present in dogs with Evans syndrome or DIC.
The presence of polychromasia with autoagglutination and spherocytosis in a clinically ill dog with anemia of acute onset is virtually pathognomonic of IHA. In these cases a direct Coombs test is usually not necessary to confirm the diagnosis. In dogs that lack some of these physical examination and hematologic findings, a direct Coombs test should be performed to detect Ig adsorbed to the RBC membrane.
The direct Coombs test is negative in approximately 10% to 30% of dogs with IHA, yet they tend to respond to immunosuppressive therapy (see below). In these cases enough Ig or complement molecules may be bound to the RBC membrane to induce the MPS to stimulate phagocytosis but not enough to result in a positive Coombs test. Hemolysis can occur in human beings with approximately 20 to 30 molecules of Ig bound to the RBC, whereas the direct Coombs test can only detect more than 200 to 300 molecules of Ig per cell. Another explanation for the findings in this subset of patients is that the previous administration of exogenous corticosteroids has resulted in decreased antibody binding to the surface of the RBCs.
Immunosuppressive doses of corticosteroids (equivalent to 2 to 4 mg/kg of prednisone q12-24h in the dog and up to 8 mg/kg q12-24h in the cat) constitute the treatment of choice for primary IHA. Although dexamethasone can be used initially, it should not be used as maintenance therapy for prolonged periods because of its higher potential to cause gastrointestinal tract ulceration or pancreatitis; in addition, if given on an alternate-day basis, it causes interference with the hypothalamic-pituitary-adrenal axis. In equivalent doses dexamethasone does not appear to be more beneficial than prednisone in dogs. In cats with IHA, I have used dexamethasone (4 mg/cat, PO, q1-2wk) with a high degree of success.
A high percentage of dogs treated with corticosteroids shows a marked improvement within 24 to 96 hours (Fig. 83-1). Corticosteroids act mainly by three different mechanisms: they suppress MPS activity, decrease complement and antibody binding to the cells, and suppress Ig production. The first two effects are rapid in onset (hours), whereas the third effect is delayed (1 to 3 weeks).
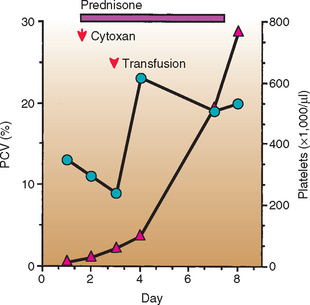
FIG 83-1 Response to treatment in a dog with immune hemolytic anemia (IHA) and immune-mediated thrombocytopenia (Evans syndrome). PCV, Packed cell volume;  , PCV;
, PCV;  , platelets; ↓, treatment administered.
, platelets; ↓, treatment administered.
I have observed a high number of dogs with acute or peracute IHA generally associated with icterus and autoagglutination that show a rapid deterioration and that usually die of thromboembolism of the liver, lungs, or kidneys despite aggressive corticosteroid therapy. Such animals are treated with cyclophosphamide (Cytoxan) at a dosage of 200 to 300 mg/m2 given orally or intravenously in a single dose over a 5- to 10-minute period in conjunction with a single IV dose of dexamethasone sodium phosphate (1 to 2 mg/kg). I also advocate the use of prophylactic heparin therapy because dogs with hemolysis are at high risk for DIC and thrombosis. In my practice heparin therapy of 50 to 75 IU/kg is routinely given subcutaneously every 8 hours. These dosages of heparin usually do not result in therapy-related prolongation of the activated clotting time (ACT) or the activated partial thromboplastin time (Aptt), tests used routinely to monitor heparinization. The use of low- or mini-dose aspirin (0.5 mg/kg q24-48h) has been associated with lower mortality rates in dogs with IHA. Because dogs with IHA are at high risk for thromboembolic events, I refrain from placing central venous lines; thrombosis of the anterior vena cava commonly leads to severe pleural effusion in these dogs. Aggressive fluid therapy should be administered in conjunction with these treatments in an attempt to flush the microaggregates of agglutinated RBCs from the microcirculation (Note: circulating blood does not clot). Importantly, however, is that depending on the degree of anemia, the resultant hemodilution may be detrimental to the patient. If deemed necessary, oxygen therapy should also be used, but it is rarely beneficial unless the HCT or Hb can be increased.
I have been using human intravenous IgG (HIVIGG-0.5 to 1.5 g/kg IV infusion, single dose) with a high degree of success in dogs with refractory IHA. This treatment is aimed at blocking the Fc receptors in the MPS with a foreign Ig, thus minimizing the phagocytosis of antibody-coated RBCs. This treatment appears to have other immunomodulatory effects as well. However, the product is moderately expensive (approximately $300 to $400 per dose for a 10-kg dog). This approach has had such an impact, however, that I frequently use it as the first line of therapy in dogs with severe IHA.
Drugs used for the maintenance treatment of dogs with IHA include prednisone (1 mg/kg PO q48h) and azathioprine (50 mg/m2 PO q24-48h), used either singly or in combination. Azathioprine is associated with few adverse effects, although close hematologic and serum biochemical monitoring is necessary because of its potential to suppress bone marrow function and cause mild hepatopathy. A dose reduction is necessary if myelosuppression or hepatotoxicity occurs; occasionally azathioprine must be discontinued in dogs with hepatotoxicity. In cats, chlorambucil is an effective immunosuppressor with very low toxicity; I have used it successfully in cats with IHA, immune-mediated thrombo cytopenia, or other cytopenias at a dosage of 20mg/m2 PO q2wk. In general, dogs and cats with IHA require prolonged (often lifelong) immunosuppressive treatment. Whether an animal requires continuous treatment is determined by trial and error; decremental doses of the immunosuppressive drug(s) are administered for a given period (usually 2 to 3 weeks), at which time the patient is reevaluated clinically and hematologically. If the PCV has not decreased or has increased and the patient is clinically stable or has shown improvement, the dose is reduced by 25% to 50%. This procedure is repeated until the drug is discontinued or the patient relapses. In the latter case, the previously used dosage that had beneficial effects is used again. In my experience, more than two thirds of dogs with IHA require lifelong treatment.
Alternative treatments for dogs with refractory IHA include danazol (5 to 10 mg/kg PO q12h), cyclosporine (10 mg/kg PO q12-24h), and possibly splenectomy. However, splenectomy has rarely been of benefit in dogs with IHA treated at my clinic.
Chlorambucil (20 mg/m2 PO q2wk) appears to be the best induction and maintenance agent in cats with IHA refractory to corticosteroids or in those who develop corticosteroid-induced diabetes mellitus. In my experience azathioprine causes pronounced myelosuppression in this species and should not be used.
One of the biggest dilemmas the clinician faces in the treatment of a dog with IHA is whether to administer a transfusion of blood or blood products. As a general rule, a transfusion should not be withheld if it represents a lifesaving procedure. However, because patients with IHA are already destroying their own antibody-coated RBCs, they may also be prone to destroying transfused RBCs (although this has not been scientifically proven). My recommendation is to administer a transfusion to any animal with IHA that is in dire need of RBCs (i.e., withholding a transfusion would result in the animal’s death). I usually pretreat these patients with dexamethasone sodium phosphate (0.5 to 1 mg/kg IV), administer fluids through an additional IV catheter, and continue the heparin therapy. Although cross-matching is indicated, time is usually of the essence; therefore non–cross-matched universal donor blood is frequently administered; moreover, if autoagglutination occurs, the results of a cross-match may be difficult to interpret.
Another issue pertaining to transfusion in dogs with IHA autoagglutination deals with blood typing; if blood typing cards are used, the results will be false-positive for DEA 1.1 (see Principles of Transfusion Therapy, p. 1221). Finally, no rule of thumb exists (e.g., PCV value, lack of response to oxygen therapy) regarding when to administer a transfusion. The clinician should use his or her best clinical judgment to determine when a transfusion of blood or blood products is necessary (e.g., does the patient exhibit tachypnea, dyspnea, or orthopnea?). If available, universal donor packed RBCs should be used instead of whole blood because they deliver a high oxygen-carrying capacity in a smaller volume and administration usually does not result in hypervolemia.
A polymer of bovine Hg has been available for use in dogs with acute anemia that are in dire need of oxygen-carrying capacity (Oxyglobin, Biopure Corp., Cambridge, Mass.). This compound has a long shelf life; it does not require refrigeration, blood typing, or cross-matching. Administration of Oxyglobin typically results in clinical improvement of the signs associated with anemia, but the duration of response is limited (2 or 3 days). Because of the nature of this compound, the PCV does not increase after infusion (the Hg concentration does increase). Some laboratory test results may be difficult to obtain after infusion of Oxyglobin because of interference with colorimetric analysis. Unfortunately this product is not readily available for veterinarians at this time.
NONREGENERATIVE ANEMIAS
With the exception of anemia of chronic disease (ACD), nonregenerative anemias do not appear to be clinically as common as regenerative forms in dogs, whereas the opposite is true in cats.
Five forms of nonregenerative anemia are typically recognized in cats and dogs (see Box 83-3). Because IDA can be mildly to moderately regenerative and the RBC indices are typically different from those in other forms of nonregenerative anemia (microcytic, hypochromic versus normocytic, normochromic; see Boxes 83-3 and 83-4 and Tables 83-2 to 83-4), I prefer to classify it in a separate category. Anemia of endocrine disease is typically mild and usually is an incidental finding in dogs with hypothyroidism or hypoadrenocorticism (see Chapters 51 and 53). In general, most nonregenerative anemias and IDA in cats and dogs are chronic, thus allowing for physiologic adaptation to the decrease in the RBC mass. As a consequence, these types of anemia may be detected incidentally during the routine evaluation of a cat or dog, which to the owner is asymptomatic. In many cases (e.g., ACD) the anemia is mild and clinical signs are absent. Although most nonregenerative anemias are chronic, two situations are commonly encountered in which this form of anemia is acute: acute blood loss (first 48 to 96 hours) and peracute hemolysis. In these two instances the bone marrow has not yet had time to mount a regenerative reticulocyte response.
 BOX 83-4 Classification and Causes of Nonregenerative Anemia in Cats and Dogs
BOX 83-4 Classification and Causes of Nonregenerative Anemia in Cats and Dogs
ACD, Anemia of chronic disease; ARD, anemia of renal disease.
When evaluating dogs and cats with symptomatic nonregenerative anemias of acute onset, the clinician should try to answer the following questions:
Most clinical and clinicopathologic abnormalities in cats and dogs with nonregenerative anemia have been discussed (see p. 1209). In general, the RBCs in dogs and cats with nonregenerative anemias are normocytic and normo-chromic; however, the RBCs are usually macrocytic and normochromic in cats with FeLV- or FIV-related hypoproliferative anemias. As previously discussed, the RBC indices are microcytic and hypochromic in dogs and cats with IDA.
The clinical evaluation of a cat or dog with nonregenerative anemia differs radically from that of a patient with regenerative forms because the absence of regeneration reflects primary or secondary bone marrow abnormalities (e.g., bone marrow disorder, ACD). Therefore after extra-marrow causes have been ruled out by performing a physical examination and a serum biochemical profile and urinalysis, a bone marrow aspiration or biopsy is indicated in these patients.
ANEMIA OF CHRONIC DISEASE
ACD is the most common form of nonregenerative anemia in cats and dogs; however, because it is mild, it almost never results in clinical signs of anemia and the patients are usually evaluated as a consequence of their primary disorder (e.g., cancer, infection). ACD develops secondary to a variety of chronic inflammatory, degenerative, or neoplastic conditions. Although the term anemia of chronic disease implies a chronic onset, it has recently been established that cats can develop ACD in as little as 2 weeks. However, some of those cats were receiving fluid therapy that may have resulted in hemodilution (Ottenjan et al., 2006). In most cats with ACD the PCV percentage values range from the high teens to the mid-20s, whereas in dogs they range from the mid-20s to the low 30s. Therefore ACD can usually be excluded in dogs with PCVs of less than 20% and in cats with PCVs of less than 17% to 18%. The RBC indices are normocytic and normochromic, and the CBC may also reflect the nature of the primary problem (e.g., leukocytosis, neutrophilia, monocytosis, hyperproteinemia resulting from a polyclonal gammopathy); some cats with ACD have microcytic hypochromic RBC indices, a condition that mimics IDA.
Sustained inflammatory or neoplastic processes cause iron to be sequestered within the bone marrow MPS, and it is therefore not available to the erythroid precursors for normal erythropoiesis. This unavailability of iron is mainly mediated by lactoferrin and other acute-phase reactants released from neutrophils during inflammation. In cats and dogs with ACD, the serum iron concentration and total iron-binding capacity (TIBC, or transferrin concentration) are usually decreased and the Hb saturation is low, but iron stores in the bone marrow are increased (Table 83-5). Although serum ferritin concentrations are the main feature that distinguishes ACD from IDA (i.e., high in ACD and low in IDA) in human beings, the results of ferritin assays in dogs and cats with IDA and ACD are not as clear cut. Therefore, to conclusively differentiate ACD from IDA, evaluation of bone marrow iron stores by Prussian blue staining is important. After a diagnosis of ACD has been confirmed, every effort should be made to identify the cause of the problem if it is not already evident.
 TABLE 83-5 Distinguishing Features of ACD and IDA in Dogs
TABLE 83-5 Distinguishing Features of ACD and IDA in Dogs
| PARAMETER | ACD | IDA |
|---|---|---|
| Serum iron concentration | ↓ | ↓↓ |
| Total iron-binding capacity | N | N↑ |
| Percentage saturation | ↓ | ↓↓ |
| Bone marrow iron stores | ↑ | ↓- |
| Platelet count | N, ↓-, ↑ | ↓, ↑↑ |
| Fecal occult blood | N | +(−) |
| Ferritin | N | ↓- |
ACD, Anemia of chronic disease; IDA, iron deficiency anemia; ↓, low; ↓↓, markedly low; ↑, high; ,↑↑ markedly high; N, normal; +(−), positive or negative.
Dogs and cats with ACD usually do not require specific or supportive therapy because treatment of the primary disorder causes the anemia to resolve. Although some have advocated the use of anabolic steroids in dogs and cats with ACD, these agents appear to be of little or no benefit.
BONE MARROW DISORDERS
Neoplastic, hypoplastic, or dysplastic bone marrow disorders can result in anemia and other cytopenias. In these conditions a “crowding out” of the normal erythroid precursors by neoplastic or inflammatory cells (myelophthisis), a paucity or absence of erythroid precursors (hypoplasia or aplasia, respectively), or a maturation arrest of the erythroid precursors (dysplasia) occur. All these disorders, with the exception of pure RBC aplasia (PRCA) (see following section), typically affect more than one cell line and the patients are bicytopenic or pancytopenic (see Chapter 86). In general, these disorders are chronic and the clinical signs are those of anemia (see p. 1209) with or without signs of the underlying disorder. Although some information regarding the pathogenesis of this type of anemia can be obtained by evaluating the clinical and hematologic data, a definitive diagnosis is usually made on the basis of the cytologic or histopathologic appearance of a bone marrow specimen and, possibly, the results of serologic tests or PCR for infectious agents (e.g., FeLV, FIV, Ehrlichia canis).
Bone Marrow (or Erythroid) Aplasia-Hypoplasia
Bone marrow aplasia-hypoplasia is characterized by aplasia or hypoplasia of all the bone marrow cell lines (bone marrow aplasia-hypoplasia or aplastic pancytopenia) or the erythroid precursor (RBC aplasia-hypoplasia or PRCA). This form of anemia (or combined cytopenias) can be caused by a variety of agents or disorders (see Chapter 86) (Box 83-5). The following discussion pertains to PRCA.
 BOX 83-5 Bone Marrow Disorders in Cats and Dogs
BOX 83-5 Bone Marrow Disorders in Cats and Dogs
FeLV, Feline leukemia virus; FIV, feline immunodeficiency virus; D, dog; C, cat.
Clinically, dogs and cats with PRCA are evaluated because of the clinical signs already discussed. In contrast to ACD, in which the degree of anemia, and thus the severity of the clinical signs, is mild, cats and dogs with PRCA usually have a PCV of less than 15% and are therefore symptomatic. Hematologically, severe (normocytic normochromic) nonregenerative anemia is usually the only abnormality; macrocytosis in the absence of reticulocytes is a consistent finding in cats with FeLV- or FIV-related PRCA, and mild microcytosis can occasionally be present in dogs with PRCA. The large RBC volume in cats with retroviral infections is attributed to the erythroid dysplasia or dyserythropoiesis induced by the virus. Dogs with PRCA occasionally have circulating spherocytes, pointing toward an immune basis for the anemia. The direct Coombs test is also positive in more than half of these dogs, and their anemia responds to immunosuppressive therapy. Cats and dogs with bone marrow aplasia-hypoplasia are pancytopenic (see Chapter 86).
In addition to the above, FeLV and FIV testing should be done in cats with PRCA. A bone marrow aspiration or biopsy specimen should also be obtained to rule out other bone marrow disorders.
The FeLV envelope protein p15E suppresses erythropoiesis in vitro and is postulated to cause PRCA in FeLV-infected cats. The anemia in these cats is usually chronic and severe (a PCV of 5% to 6% is relatively common), and despite supportive therapy the condition of the patient deteriorates, leading the owners to request euthanasia. The supportive treatment of these cats includes whole blood or packed RBC transfusions as needed; the interval between transfusions usually shortens with each transfusion until the cat needs transfusions weekly. Anabolic steroids may be beneficial in some cats, although no clinical evidence supports this. Interferon administered orally may improve clinical signs (without resolution of the anemia) in some of these cats (see Chapter 102).
FeLV-negative cats with PRCA often have a positive direct Coombs test and frequently benefit from immunosuppressive doses of corticosteroids; I typically use 4 mg of dexamethasone (per cat) once every 1 to 2 weeks instead of the conventional prednisone or prednisolone daily to every other day. This steroid formulation is safe and effective, and I have not yet seen secondary diabetes mellitus in the cats treated. The use of human recombinant erythropoietin (Epo) (see below) does not appear to be indicated in these cats because their endogenous Epo activity is higher than that of normal cats. In addition, the long-term use of human recombinant Epo may lead to the development of anti-Epo antibodies and resultant refractory anemia.
PRCA of presumptive immune origin is relatively common in dogs and cats. The postulated mechanism is similar to that of IHA, except that in PRCA the antibodies (or cell-mediated immunity) are directed against the erythroid precursors. Humoral factors (antibodies) that block erythropoiesis in vitro have been well characterized in dogs with PRCA. As previously discussed, the direct Coombs test result is positive in some of these dogs (60%) and cats (50%), and they respond well to immunosuppressive and supportive therapy. Bone marrow aspirates in dogs and cats with PRCA reveal either erythroid hypoplasia or hyperplasia of the early erythroid precursors and a maturation arrest at the rubricyte or metarubricyte stage. This poses an interesting situation because most clinical pathologists use the term “PRCA” only for the dogs and cats that have erythroid hypoplasia and “IHA with delayed erythroid regeneration” for those with erythroid hyperplasia and a maturation arrest. However, from a clinical standpoint both situations behave the exact way and respond to the same treatment. Therefore I prefer to use the term PRCA for dogs and cats with either of these bone marrow cytologic findings.
The same treatment as that used during the maintenance phase of IHA is recommended for these dogs (prednisone 2 to 4 mg/kg PO q24-48h and/or azathioprine 50 mg/m2 PO q24-48h). In cats, I have successfully used dexamethasone alone (as previously discussed) or in combination with chlorambucil (Leukeran) at a dosage of 20 mg/m2 given orally every 2 weeks. Responses occur in approximately 70% to 80% of the patients, but clinical and hematologic recovery may take 2 to 3 months; long-term (lifelong) treatment is usually required. Supportive treatment and transfusions of blood or packed RBCs are sometimes necessary. Because these patients are normovolemic, the latter is preferable. In addition, because transfusions may need to be administered on an ongoing basis, cross-matching is recommended before the administration of each transfusion. Of note, in dogs one of the mechanisms of adaptation to chronic hypoxia (e.g., anemia) is an increase in the intraerythrocytic 2,3-diphosphoglycerate (2,3-DPG) concentration, resulting in a lower oxygen affinity (i.e., the delivery of oxygen to the tissues is facilitated). Therefore, because stored RBCs have lower concentrations of 2,3-DPG, the transfused cells have a higher affinity for oxygen. As a result the transfusion of stored blood to a patient with chronic anemia may result in transient decompensation because approximately 24 hours is usually required for the transfused, stored RBCs to regain 50% of the normal 2,3-DPG concentrations and get “recharged.”
Myelophthisis, Myelodysplastic Syndromes, Myelofibrosis, Osteosclerosis-Osteopetrosis
These disorders are discussed in Chapter 86.
ANEMIA OF RENAL DISEASE
The kidney is the main site of production of Epo, the principal stimulus of erythropoiesis. In addition, in dogs and cats with chronic renal failure, the life span of RBCs is considerably shorter and subclinical to clinical gastrointestinal tract bleeding is present; high concentrations of parathyroid hormone also suppress erythropoiesis. Because of these factors, anemia is common in such patients. The anemia is usually normocytic and normochromic, with few or no reticulocytes. HCT levels in dogs and cats with anemia of renal disease (ARD) are usually in the 20% to low 30% range, although HCT levels in the teens are common. Of note, the HCT in these patients is usually that low only after they have undergone intensive fluid therapy (i.e., on presentation the anemia is not that severe because the patients are markedly dehydrated).
Improvement in renal function may result in marginal increases in the RBC mass. Anabolic steroids are rarely beneficial in improving the anemia in these patients. Human recombinant Epo (Epogen, Amgen, Thousand Oaks, Calif.) has been used successfully to treat anemia in cats and dogs with chronic renal failure. A dose of 100 to 150 IU/kg given subcutaneously twice weekly is administered until the HCT returns to a target value (usually 20% to 25%); the interval between injections is then lengthened for maintenance therapy. The HCT usually returns to normal within 3 to 4 weeks of the start of treatment. Given the fact that this Epo is foreign to dogs and cats, an appropriate antibody response usually nullifies the beneficial effects of long-term therapy (6 to 8 weeks) in more than 50% of the patients.
ACUTE AND PERACUTE BLOOD LOSS OR HEMOLYSIS (FIRST 48 TO 96 HOURS)
After an acute episode of blood loss or hemolysis, bone marrow takes approximately 48 to 96 hours to release enough reticulocytes to result in regeneration. Therefore blood loss and hemolytic anemias are nonregenerative during the initial phases of recovery.
In most dogs and cats with acute blood loss, profound bleeding is either historically or clinically evident. If no obvious cause of bleeding is found or if the patient is bleeding from multiple sites, the hemostatic system should be evaluated in search of a coagulopathy (see Chapter 89). Sites of internal bleeding should be evident after a complete physical examination is performed.
Once the bleeding has been stopped, the anemia typically resolves within days to weeks. The initial management of a bleeding episode should include supportive therapy and IV crystalloids or plasma expanders. If necessary, blood or packed RBCs or Hg solutions should be administered.
The management of dogs with peracute hemolysis was discussed earlier in the chapter.
SEMIREGENERATIVE ANEMIAS
IRON DEFICIENCY ANEMIA
IDA is traditionally classified as nonregenerative even though mild to moderate regeneration usually occurs. Moreover, as previously discussed, the RBC indices in dogs and cats with IDA are microcytic and hypochromic, distinguishing it from other forms of nonregenerative anemia, which are normocytic and normochromic. When evaluating the CBC of a dog with microcytic hypochromic anemia, the clinician must remember that microcytosis occurs in some breeds (e.g., Akita, Shiba Inu, Sharpei) and in dogs with other disorders, such as portosystemic shunts (see Table 83-2).
This form of anemia is well characterized in dogs with chronic blood loss. In cats, IDA has been well documented only in weanling kittens, in whom iron supplementation results in rapid resolution of the clinical and hematologic abnormalities. IDA is extremely rare in adult cats, and I have seen it primarily in association with chronic blood loss in cats with gastrointestinal (GI) lymphoma. Given its rarity in cats, the following discussion of IDA pertains primarily to dogs.
Chronic blood loss leading to iron depletion is common in dogs with GI tract bleeding caused by neoplasia, gastric ulcers, or endoparasites (e.g., hookworms) and in those with heavy flea infestation. Other causes of chronic blood loss, such as urogenital bleeding and iatrogenic bloodletting, are extremely rare. In my experience the most common cause of symptomatic IDA in dogs that present for evaluation of signs associated with anemia is GI neoplasia.
Dogs with IDA are typically evaluated because of the signs of the anemia or because of GI tract signs such as diarrhea, melena, or hematochezia. Mild IDA is occasionally recognized during the routine evaluation of heavily parasitized dogs (mostly pups). Hematologically, most dogs with IDA have microcytic, hypochromic indices, mild reticulocytosis (1% to 5%), a high RBC distribution width (RDW) with an occasional bimodal population of RBCs, thrombocytosis, low serum iron and TIBC (transferrin) concentrations, an extremely low percentage of saturation (usually less than 10%), a low serum ferritin concentration, and low iron stores in the bone marrow (see Box 83-5). The RDW generated by a particle counter represents a histogram of RBC sizes; a high RDW is indicative of anisocytosis. The typical tetrad of hematologic abnormalities in dogs with IDA is microcytosis, hypochromasia, mild regeneration, and thrombocytosis.
Because the most common cause of IDA in adult dogs is chronic GI tract bleeding, the stools should always be evaluated for occult blood with commercially available kits (see Chapter 29); if the results are negative, they should be evaluated again two or three times during a period when the animal is not eating canned dog food (myoglobin in canned dog food can occasionally result in false-positive reactions). If occult blood is present in the stool, a GI tract neoplasm should be ruled out. Tumors commonly associated with IDA in dogs include GI stromal tumors (GISTs), such as leiomyomas, leiomyosarcomas, and true GISTs; lymphomas; and carcinomas. In dogs with IDA, positive fecal blood test results, and lack of clinical signs associated with the GI tract, the most likely diagnosis is a jejunal tumor (usually a GIST); I refer to these tumors as the “silent” GI neoplasms.
Another condition that can lead to IDA is chronic upper GI tract bleeding secondary to gastroduodenal ulceration, although most of these dogs have overt clinical signs associated with the GI tract (e.g., vomiting, hematemesis, weight loss). In pups or kittens with IDA, fecal flotation or a direct smear for hookworms and a thorough physical examination (to search for fleas) are mandatory because these are the two most common causes of IDA in young dogs and cats.
IDA usually resolves within 6 to 8 weeks after the primary cause has been eliminated. Oral or intramuscular iron supplementation is usually not necessary to hasten the resolution of the hematologic abnormalities; a sound commercial diet usually achieves the same effect. As a general rule, if the cause can be eliminated, I do not use iron supplementation. The dietary iron requirement for adult dogs and cats is approximately 1.3 mg/kg/day.
PRINCIPLES OF TRANSFUSION THERAPY
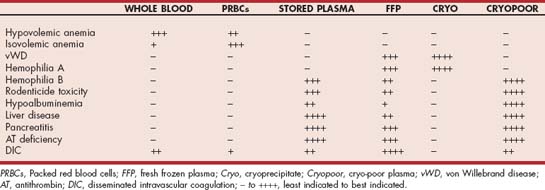
In the past 2 decades veterinary transfusion medicine has advanced radically. Several commercial blood banks are now available for pets, and most of them store blood components derived from processing units of whole blood or collected by apheresis. In a typical situation a unit of blood is spun immediately after collection, and packed RBCs (pRBCs) and fresh frozen plasma (FFP) stored at 20° C to30° C are prepared; the pRBCs are preserved by adding a nutrient solution, and can be stored for up to 5 weeks. After 1 year of storage at 20° C to 30° C, FFP loses the labile clotting factors (V and VIII) and is referred to as stored plasma (SP) or frozen plasma (FP). Some blood banks prepare platelet-rich plasma (PRP) or platelet concentrates by apheresis. If FFP is allowed to warm up in a refrigerator, when it reaches approximately 4° C to 6° C a sludge forms in the bottom of the bag. That sludge can be separated by a short centrifugation, yielding cryoprecipitate (CRYO), a small volume rich in factor VIII, fibrinogen, and von Willebrand factor (vWF); the supernatant is referred to as cryo-poor plasma.
The transfusion of whole blood or blood components (e.g., pRBCs, PRP, FFP, CRYO, or SP) is indicated in several clinical situations. Whole blood or pRBC transfusion is most commonly required to restore the oxygen-carrying capacity in patients with anemia. Whole blood should be used if the anemic patient is hypovolemic or if it needs clotting factors, whereas pRBCs are recommended for normovolemic dogs and cats with anemia (i.e., PRCA, ARD, hemolysis). Transfusion therapy should be used with caution in animals with IHA (see p. 1217) because a massive transfusion reaction may occur; in those patients, Hg derivatives may be a better alternative if available.
Clotting factor deficiencies (see Chapter 87) resulting in hemorrhage can be corrected through the administration of whole fresh blood (if a considerable blood loss has occurred) or, more ideally, FFP or SP. Cryoprecipitate contains a high concentration of factor VIII and vWF, so it is typically used in dogs with hemophilia A or von Willebrand disease. Cryo-poor plasma is a good source of clotting factors (except for factor III and vWF) and albumin. PRP or platelet transfusions, if available, can be used in dogs and cats with severe thrombocytopenia resulting in spontaneous bleeding (Table 83-6). However, the platelet count of the recipient is rarely raised enough to halt bleeding. PRP and platelet transfusions are of no benefit in patients with peripheral platelet destruction (e.g., immune-mediated thrombocytopenia) because the platelets are removed from the circulation immediately after the transfusion. Transfusion with whole fresh blood, PRP, or FFP is also indicated for the management of patients with DIC (see Chapter 87).
Less frequently, plasma is prescribed to correct hypoalbuminemia. However, only rarely can relevant increases in the recipient’s serum albumin concentration be achieved. Colloids or human albumin solutions are more effective in restoring plasma oncotic pressure.
BLOOD GROUPS
Several blood groups have been recognized in dogs; these include dog erythrocyte antigen (DEA) 1.1 and 1.2 (formerly known as blood group A) and DEA 3 through 8. Dogs do not have naturally occuring antibodies against blood group antigens; therefore they can only acquire them after receiving a transfusion or after pregnancy. Transfusion reactions can occur if blood positive for DEA 1.1, 1.2, or 7 is transfused, so donors should be negative for those antigens. However, clinically relevant acute hemolytic transfusion reactions are extremely rare in dogs. Transfusion of blood from a donor who has not been typed and has never been pregnant or transfused to a recipient, independently of their blood types, is generally safe.
Blood groups in cats include A, B, and AB. Cats tested in the United States have almost exclusively been type A; the prevalence of type B cats varies greatly from region to region and among breeds. Breeds in which 15% to 30% of the cats are type B include Abyssinian, Birman, Himalayan, Persian, Scottish Fold, and Somali; breeds in which more than 30% of cats are type B include the British Shorthair and the Devon Rex. Because fatal transfusion reactions commonly occur in type B cats receiving type A blood, cats should always be cross-matched or typed before receiving a transfusion. In those cases a type B cat should be used as a donor. All the type B cats seen in our clinic in the past 5 years have been domestic short-haired cats. Blood typing is also vital in cattery situations to prevent neonatal isoerythrolysis in type A or AB kittens born to type B queens.
CROSS-MATCHING AND BLOOD TYPING
Cross-matching is an alternative to blood typing in in-house donors or animals that have had prior transfusions, in cats, or in animals that will require multiple transfusions. Cross-matching detects many incompatibilities but does not guarantee complete compatibility. Rapid, cage-side blood typing cards for DEA 1.1 in dogs and for groups A and B in cats are commercially available (RapidVet-H, DMS Laboratories, Flemington, NJ). A kit for rapid cross-matching will soon be commercially available.
BLOOD ADMINISTRATION
Refrigerated blood may be warmed before or during administration, particularly in small dogs or cats; excessive heat should be avoided, however, because fibrinogen precipitation or autoagglutination may occur. I typically do not warm blood or pRBCs before administration. The administration set should have a filter in place (Baxter International, Deerfield, Ill.) to remove clots and other particulate matter, such as platelet aggregates. The blood is usually administered by way of the cephalic, saphenous, or jugular veins. However, intraosseous infusion may be performed in small animals, neonates, or animals with poor peripheral circulation. To administer fluids or blood intraosseously, the skin over the femur is surgically prepared and the skin and periosteum of the femoral trochanteric fossa are anesthetized with 1% lidocaine. A bone marrow needle (18 gauge) is placed into the marrow cavity parallel to the shaft of the femur. Suction with a 10-mL syringe should yield marrow elements (fat, spicules, and blood), confirming correct placement of the needle. The blood is administered through a standard blood administration set.
The recommended rate of administration is variable but should not exceed 22 mL/kg/day (up to 20 mL/kg/hr can be used in hypovolemic animals). Dogs and cats in heart failure may not tolerate a rate of more than 5 mL/kg/day. To prevent bacterial contamination, blood should not be exposed to room temperature during administration for longer than 4 to 6 hours (blood is considered to be contaminated if it is at room temperature for more than 6 hours). If necessary, two smaller volumes of blood can be administered in succession. Blood should never be administered with lactated Ringer’s solution because of the calcium chelation with citrate and consequent clot formation that may occur. Normal saline solution (0.9% NaCl) should be used instead. A simple rule of thumb to predict the increase in the recipient’s HCT is to remember that 2.2 mL/kg (or 1 mL/lb) of transfused whole blood will raise the HCT by 1% if the donor has an HCT of approximately 40%.
COMPLICATIONS OF TRANSFUSION THERAPY
Transfusion-related complications can be divided into those that are immunologically mediated and those that are of nonimmunologic origin. Immune-mediated reactions include urticaria, hemolysis, and fever. Non–immune-mediated complications include fever resulting from the transfusion of improperly stored blood, circulatory overload, citrate intoxication, disease transmission, and the metabolic burden associated with the transfusion of aged blood. Signs of immediate immune-mediated hemolysis appear within minutes of the start of transfusion and include tremors, emesis, and fever; these are extremely rare in dogs but common in cats receiving incompatible blood products. Delayed hemolytic reactions are more common and are manifested primarily by an unexpected decline in the HCT after transfusion over days, in association with hemoglobinemia, hemoglobinuria, and hyperbilirubinemia. Circulatory overload may be manifested by vomiting, dyspnea, or coughing. Citrate intoxication occurs when the infusion rate is too great or the liver is not able to metabolize the citrate. Signs of citrate intoxication are related to hypocalcemia and include tremors and cardiac arrhythmias. If signs of a transfusion reaction are recognized, the transfusion must be slowed or halted.
Andrews GA. Red blood cell antigens and blood groups in the dog and cat. In: Feldman BF, et al, editors. Schalm’s veterinary hematology. ed 5. Philadelphia: Lippincott Williams & Wilkins; 2000:767.
Authement JM, et al. Canine blood component therapy: product preparation, storage, and administration. J Am Anim Hosp Assoc. 1987;23:483.
Balch A, Mackin A. Canine immune-mediated hemolytic anemia: pathophysiology, clinical signs, and diagnosis. Compend Cont Educ. 2007;29:217.
Birkenheuer AJ, et al. Serosurvey of antiBabesia antibodies in stray dogs and American pit bull terriers and American Staffordshire terriers from North Carolina. J Am Anim Hosp Assoc. 2003;39:551.
Birkenheuer AJ, et al. Efficacy of combined atovaquone and azithromycin for therapy of chronic Babesia gibsoni (Asian genotype) infections in dogs. J Vet Intern Med. 2004;18:494.
Birkenheuer AJ, et al. Geographic distribution of babesiosis among dogs in the United States and association with dog bites: 150 cases (2000–2003). J Am Vet Med Assoc. 2005;227:942.
Boyce JT, et al. Feline leukemia virus–induced erythroid aplasia: in vitro hemopoietic culture studies. Exp Hematol. 1981;9:990.
Brazzell JL, Weiss DJ. A retrospective study of aplastic pancyto-penia in the dog: 9 cases (1996-2003). Vet Clin Pathol. 2006;35:413.
Callan MB, et al. Canine red blood cell transfusion practice. J Am Anim Hosp Assoc. 1996;32:303.
Duvall D, et al. Vaccine-associated immune-mediated hemolytic anemia in the dog. J Vet Intern Med. 1996;10:290.
Feldman BF, et al. Anemia of inflammatory disease in the dog: clinical characterization. J Am Vet Med Assoc. 1981;42:1109.
Giger U. Erythrocyte phosphofructokinase and pyruvate kinase deficiencies. In: Feldman BF, et al, editors. Schalm’s veterinary hematology. ed 5. Philadelphia: Lippincott Williams & Wilkins; 2000:1020.
Giger U, et al. Transfusion of type-A and type-B blood to cats. J Am Vet Med Assoc. 1991;198:411.
Gurnee CM, Drobatz KJ. Zinc intoxication in dogs: 19 cases (1991-2003). J Am Vet Med Assoc. 2007;230:1174.
Harvey JW. Pathogenesis, laboratory diagnosis, and clinical implications of erythrocyte enzyme deficiencies in dogs, cats, and horses. Vet Clin Pathol. 2006;35:144.
Harvey JW, et al. Chronic iron deficiency anemia in dogs. J Am Anim Hosp Assoc. 1982;18:946.
Jacobs RM, et al. Use of a microtiter Coombs’ test for study of age, gender, and breed distributions in immunohemolytic anemia in the dog. J Am Vet Med Assoc. 1984;185:66.
Jonas LD, et al. Nonregenerative form of immune-mediated hemolytic anemia in dogs. J Am Anim Hosp Assoc. 1987;23:201.
Klag AR, et al. Idiopathic immune-mediated hemolytic anemia in dogs: 42 cases (1986–1990). J Am Vet Med Assoc. 1993;202:783.
Klein MK, et al. Pulmonary thromboembolism associated with immune-mediated hemolytic anemia in dogs: ten cases (1982–1987). J Am Vet Med Assoc. 1989;195:246.
Mason N, et al. Cyclophosphamide exerts no beneficial effect over prednisone alone in the initial treatment of acute immune-mediated hemolytic anemia in dogs: a randomized controlled clinical trial. J Vet Intern Med. 2003;17:206.
Ottenjan M, et al. Characterization of anemia of inflammatory disease in cats with abscesses, pyothorax, or fat necrosis. J Vet Intern Med. 2006;20:1143.
Stokol T, et al. Pure red cell aplasia in cats: 9 cases (1989–1997). J Am Vet Med Assoc. 1999;214:75.
Stokol T, et al. Idiopathic pure red cell aplasia and nonregenerative immune-mediated anemia in dogs: 43 cases (1988–1999). J Am Vet Med Assoc. 2000;216:1429.
Weinkle TK, et al. Evaluation of prognostic factors, survival rates, and treatment protocols for immune-mediated hemolytic anemia in dogs: 151 cases (1993-2002). J Am Vet Med Assoc. 2005;226:1869.
Weiser MG. Correlative approach to anemia in dogs and cats. J Am Anim Hosp Assoc. 1981;17:286.
Weiss DJ. Antibody-mediated suppression of erythropoiesis in dogs with red blood cell aplasia. Am J Vet Res. 1986;12:2646.
Weiss DJ. Bone marrow necrosis in dogs: 34 cases (1996-2004). J Am Vet Med Assoc. 2005;227:263.
Weiss DJ. A retrospective study of the incidence and the classification of bone marrow disorders in the dog at a veterinary teaching hospital (1996-2004). J Vet Intern Med. 2006;20:955.
Weiss DJ. Hemophagocytic syndrome in dogs: 24 cases (1996-2005). J Am Vet Med Assoc. 2007;230:697.
Weiss DJ, Smith SA. A retrospective study of 19 cases of canine myelofibrosis. J Vet Intern Med. 2002;16:174.