chapter 12 Edema control
After completing this chapter, the reader will be able to accomplish the following:
1. Describe the proposed three theories of types of stroke hand edema and relate them to appropriate criteria for clinical treatment technique selection.
2. Provide neurological and anatomical rationales for treatment selection.
3. Be familiar with current research outcomes of treatment techniques for stroke hand edema reduction and be able to implement and expand data for clinical application problem-solving
4. Integrate realistic edema reduction expectations into treatment planning from material read in the case studies.
Research indicates that poststroke hand edema can range from 16%21 to 82.8%49 depending on the definition of edema, length of time since stroke occurred, and study design and methodology. Research also reports a broad range of time during which edema may develop, often from two weeks to two months poststroke.43 There are numerous theories regarding the development of edema. Boomkamp-Koppen and colleagues7 found that the loss of muscle activity, hyposensibility, and hypertonia link to edema, with hypertonia being the most significant predictor of hand edema.
There is no consensus on the etiology of poststroke hand edema38 or on the most effective management technique.7,23 Therefore, there are few guidelines for occupational therapists to employ with their clients. It is widely acknowledged that edema, particularly in the subacute and chronic stages, affects a client’s range of movement, sensation, dexterity, and function. There are also correlations between edema and joint fibrosis and, in stroke, there is increasing evidence on the relationship between edema and Chronic Regional Pain Syndrome (CRPS).30 Occupational therapists need to maximize their input into the multidisciplinary team to prevent CRPS and to minimize these barriers to rehabilitation.43 It is also imperative to consider the impact of stroke on movement, cognition, perception, communication, and psychological aspects of individuals. These areas form the basis for most rehabilitation poststroke, with edema and sensation often being lower priorities. However, given the limitations imposed by stroke on the above areas, one must consider how these impairments may interplay with edema management and functional outcomes, such as neglect, strength, and learned nonuse (Fig. 12-1).
This chapter will focus on exploring available research regarding the etiology and treatment of poststroke hand edema and will present it in a format to enable the therapist to apply research data to clinical problem solving.
Etiology of stroke hand edema
The edema that therapists treat is defined as an excess accumulation of fluid in the interstitium. It occurs on the capillary level (microcirculation level) when there is an imbalance of pressure between the arterioles, venules, and interstitium, or an obstruction of the lymphatic system.17,50 This is also known as an imbalance in Starlings Equilibrium. It is important to note that the vascular system refers not only to the venous and arterial capillaries but also to the lymphatic capillaries. All these structures influence poststroke arm edema.
A review of the literature has resulted in two major theories of poststroke hand edema: sympathetic vasomotor dysfunction due to the stroke, and venous congestion. The concept of vasomotor dysfunction as part of the stroke autonomic disturbance theory was proposed as early as 1930.55 This theory has been expanded on in light of more recent research,34 though, the role of the sympathetic vasomotor dysfunction deletion of post stroke hand edema formation remains unclear.38 The second theory proposes that poststroke hand edema results from venous congestion due to lack of, or decreased, limb motor function and dependency positioning.38 Aspects of both of these theories will be presented in the chapter when relative to a specific treatment technique.
Anatomical overview of the venous and lymphatic systems related to stroke hand edema and its etiology
The venous congestion and limb dependency theory is most relevant to the treatment techniques this chapter will present. Thus, it is important to take an anatomical look at the vascular system, which consists of both arterial and venous structures and the lymphatics. Both vascular structures, the venule and lymphatic capillaries, remove excess fluid from the interstitium and can be simultaneously activated in specific instances. Yet depending on the type of edema, in certain instances, each system must activate in its own unique way in order to reduce edema. Both the venous and lymphatic systems neurologically are controlled by the autonomic nervous system.17,35 However, both systems rely on the muscle motor pump to remove tissue fluid from the interstitium.17,35 Thus, with total or partial lack of motor function to an arm poststroke, swelling occurs.
Venous and lymphatic absorption of tissue fluid occurs on the microcirculation level. In the interstitium (interstitial spaces), the arterial and venule histologically join in an arc. The initial lymphatic (also called a lymphatic capillary or lymphatic net) is independent from the venule arterial arc, it is a “netlike” structure in the interstitium and is much larger than the venule.17,28 On this microcirculation level, plasma proteins, fluid, electrolytes, nutrients, and a few other elements are excreted from the arteriole, because it has a pressure of 35 mm Hg.6 These are the substances needed for surrounding cell metabolism. Ninety percent of what remain from the metabolism are small molecules that enter the venule via the process of osmosis and diffusion.27,28 The remaining 10% of the molecules, such as plasma proteins, are too large to be absorbed by the venule and must be absorbed by the lymphatic capillaries. The artery system via arteriole filtration and diffusion excretes tissue fluid into the interstitium.28 From the interstitium there are two structures, venules and lymphatic capillaries, that join with larger veins and lymphatic structures to bring fluid back to the heart (Fig. 12-2).17
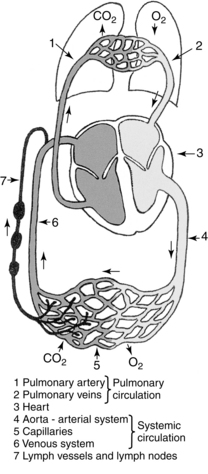
Figure 12-2 Blood and lymph circulatory systems space.
(From Foldi M, Foldi E, Kubil S: Textbook of lymphology for physicians and lymphedema therapists, Munich, 2003, Urban & Fischer Verlag.)
The absorption process by the venule and lymphatic capillaries differ from each other. The wall of a venule is thin and absorbs small molecules via osmosis and diffusion.17 Thus, elevation, light retrograde massage, muscle contraction, and compression will facilitate this absorption.
Lymphatic molecule absorption in the interstitium begins in the one cell initial lymphatic capillary that, most superficially, is part of a netlike structure located in the dermis layer of tissue. This initial lymphatic capillary is pencil shaped (tube closed on one end) and lined with one layer of overlapping endothelia cells (Fig. 12-3).28 Anatomically, fluid cannot be physically “pushed” into the lymphatic capillaries, nor does it move from the interstitium by osmosis into the lymphatic capillary. Tissue fluid and large molecules can only be absorbed by the lymphatic capillary when changes occur in interstitial fluid pressure or by movement of the elastic anchor filaments that extend from an endothelial cell to connective tissue.28,35 Then the junctions of these over lapping endothelial cells open like a trap door admitting large molecules from the interstitium into the pencil shaped lymphatic capillary.28,35 These pressure changes occur with movement of skin, light compression, muscle contraction, and respiration.14,28 The elastic anchor filaments extending from connective tissue to the endothelial junctions will open the junction flaps when pressure is put on their elastic filaments, such as from fluid congestion in the interstitium.17
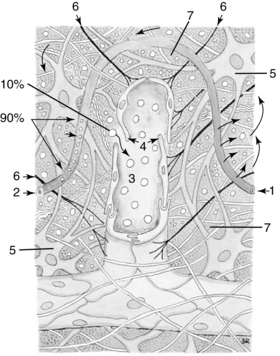
Figure 12-3 Incorporation of the lymph capillary into the interstitium: 1, Arterial section of the blood capillary. 2, Venous section of the blood capillary. 3, Lymph capillary. 4, Open intercellular groove-swinging tip. 5, Fibrocyte. 6, Anchor filaments. 7, Intercellular space.
(From Foldi M, Foldi E, Kubil S: Textbook of lymphology for physicians and lymphedema therapists, Munich, 2003, Urban & Fischer Verlag).
Lymphatic absorption is stimulated by respiration. The deepest and largest lymphatic structure is the thoracic duct. It lies anterior to, and parallels, the spinal column running from L2 to T4.56 Changes in thoracic pressure cause a proximal negative pressure vacuum and draws fluid proximally from the periphery. This is also called the pulmonary pump.17 The thoracic duct operates according to hydrodynamic laws.35 Therefore, inhalation and exhalation from diaphragmatic breathing cause changes in the thoracic duct pressure drawing the lymph within the duct toward the subclavian veins. These pressure changes in the thoracic duct then create a vacuum (suction), pulling lymph from peripheral structures centrally.14,35,36 The result is fluid from the periphery moves out of the area, and edema is reduced distally in a domino effect. Once the lymph enters the subclavian veins, it then becomes part of the venous system and continues on to lungs, heart, and other parts of the body.
The differences and similarities of the venous and lymphatic system of fluid absorption from the interstitium should be considered when choosing the appropriate edema reduction technique for poststroke arm and hand edema.
Three proposed theories of etiology and types of stroke hand edema
An in-depth exploration of the types of Complex Stroke Hand Edema theories is necessary in order to make treatment choices.
Dependency edema theory
Dependency edema is due to a combination of the involved flaccid or hemiparetic upper extremity hanging in a dependent position, plus potentially the impairment of sympathetic controlled muscle function.38,55 Thus tissue fluid pools distally. Often consistent elevation, daily light retrograde massage, and a light compression glove and/or elastic stockinette tube on the arm will reduce this edema. However, even after diligently following these methods, edema can persist. How much does this persistent edema relate to prolonged trunk immobility, lack of scapular movement, and lack of thoracic pressure changes?
This initial edema consists of small molecules that are readily absorbed by the venous system. However, the venous system has a maximum volume capacity. When this capacity is reached, the lymphatic system will carry off the excess. Often, the lymphatic system is referred to as the overflow system. Dependent venous edema has a soft “spongy feel” when pitted, rebounds quickly; and often reduces easily with elevation. This type of edema is often seen when poststroke edema first becomes evident.
Combined edema theory
When the lymphatic system acts as a safety valve or overflow system for the venous system, it carries out of the interstitium both the small molecule products that the venous system usually absorbs and the large molecules only removed by the lymphatic system. The lymphatic system also has a maximum load capacity. When the system reaches this capacity, there will be lymphatic congestion. Clinically, lymphatic congestion presents as viscous and has a very slow rebound time from being pitted of 20 to 30 seconds or more. At this point, the stroke edema is a combined venous and lymphatic edema that minimally reduces with elevation. Elevation alone will not reduce lymphatic congestion, because the large molecules do not go into the lymphatic capillary via osmosis and the overlapping endothelia cells surrounding the lymphatic capillary have to be stimulated to open and close. With combined edema, the reduction treatment has to be a combination of lymphatic proximal trunk stimulation (muscle contraction, diaphragmatic breathing), superficial tissue stimulation (creating absorption into the initial lymphatics), and elevation to aid in venous (low protein) return.
Minor trauma edema (inflammatory subacute edema) theory
Minor trauma to tissue is often caused by the arm or hand being bumped, getting caught on something, or from overzealous and improper exercise by the client or caregiver.8,18,30,33 This accidental trauma often occurs due to an impaired visual field or perception, neglect, lack of limb position in space awareness, decreased sensation, and from learned nonuse once motor function returns. Inflammation from trauma becomes a third component to persistent edema. On a microvascular level, trauma causes high capillary permeability leading to a wound healing sequence in the involved joints and tissue. If the limb is already congested due to a dependency and/or a combined venous and lymphatic overload, there is an increase in the colloidal osmotic pressure in the interstitium. This causes an imbalance in Starling equilibrium resulting in an excess of plasma proteins trapped in the interstitium for a prolonged period. Casley-Smith and Gaffney16 found that when excess plasma proteins stayed in the interstitium 64 days or longer, they caused chronic inflammation. Fibroblasts are activated by the proteins trapped in the tissue and produce collagenous tissue.32 This in turn can lead to the eventual shortening, scarring, and possible fibrosing of soft tissue and joints.12,15 Only the lymphatic system can remove the excess plasma proteins. Thus, the lymphatic system has to be specifically activated to reduce the trapped plasma proteins and break the cycle to pain, scarring, and possible fibrosis of tissue.
Chronic regional pain syndrome edema*
Reflex Sympathetic Dystrophy (RSD), the original term used in literature and is now known as Chronic Regional Pain Syndrome (CRPS) Type I, may be seen post stroke (central lesion damage).41,52 In the literature, Shoulder Hand-Syndrome (SHS) is used synonymously with CRPS Type I.41 CRPS Type II has the same clinical symptoms but occurs because of peripheral nerve involvement.41 It is defined as an exaggerated pain response to injury characterized by intense pain, trophic changes, and vasomotor changes in the involved limb.52 CRPS progresses through three phases, each causing increased hand dysfunction. Clinically, in the first phase the hand presents as edematous, hyperesthetic, warm, perspiring, having burning pain, tenderness at the wrist and finger joints, and an increased blood flow to the extremity.30,52 Poststroke edema occurs most frequently between two and four months after the stroke, as does the occurrence of RSD (CRPS Type I).30,31 However, clients who developed RSD during this period showed a greater degree of edema than the non-RSD edematous stroke hands.30 Another possible predictor of RSD researchers found was hand swelling occurring during the first month poststroke.30
The statistics for development of CRPS poststroke range anywhere from 1.56 %52 to 25%49 of stroke hand edema cases, using both clinical evidence and a three- phase triple bone scan. This great range of statistics occurs because of timing of inclusion factors, methods of evaluation, when treatment began, and the type of treatment received. The researchers documenting the 1.56% incidence concluded that their incidence of CRPS was low because rehabilitation poststroke began early at 16 days after the first stroke, and treatment included proper positioning, early mobilization, and sensory stimulation.52 Therapists can play a critical role in early identification and possible prevention or reversing of CRPS Type I. When evaluating and treating clients, occupational therapists should scan for neglect, sensory impairments, shoulder subluxation, and decreased visual field awareness. Pertoldi and colleagues41 found that the presence of these increased the risk of CRPS. Also, early initiation of a treatment program to prevent trauma (proper positioning, functional use, and mobilization) to the involved shoulder and extremity is critical to preventing CRPS I. See Chapter 10.
In a detailed review of research from 1973 to 1998 on the etiology and treatment of poststroke hand edema and SHS, it was found that the shoulder was only involved in half the cases with a swollen painful hand.23 Thus, a new term of wrist-hand syndrome has been coined.23 This same study found that in SHS, trauma causes aseptic joint inflammation.23
Edema evaluation methods
Volumetric measurement
Volumetric measurement is a water displacement method measuring hand and lower arm composite mass. The container called a volumeter is filled with enough room temperature water to flow out the container spout. When the water stops dripping out the spout, the client submerges his or her arm into the volumeter with the palm facing him or her, thumb facing the spout, and the web between the middle and ring fingers resting on the plastic stop bar. The therapist holds a beaker to catch the flowing water from the spout and then measures it in a graduated cylinder (Fig. 12-4). Care must be taken that the client does not lean his or her arm against the side of the volumeter or move the arm while water displacement is taking place, the container and cylinder sit on flat level surfaces, and measurements are consistently taken seated or standing and at same time of day and after same amount of activity. Tests have shown the volumeter measurements to be accurate within 10 mL, or 1% of the volume of the hand, when following the manufacturers’ directions.53 Measurements are then taken of the uninvolved hand for comparison sake. This method shows generalized, not site specific, changes in edema. A 12-mL change over time is considered clinically significant.48 Volumetric measuring has shown to be more accurate than visual inspection for determining presence of edema because it shows small increments of change.43 Clinically, it can be difficult to consistently position the client with a flaccid or spastic hand.
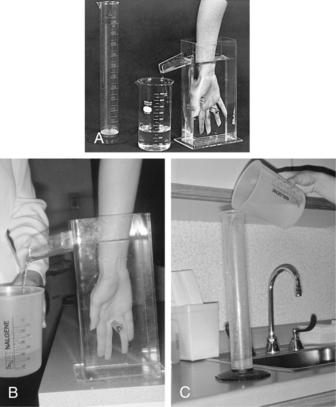
Figure 12-4 Volumeter, collection beaker, and graduated cylinder used to perform volumetric hand edema assessments.
(From Fess, E: Documentation: essential elements of an upper extremity assessment battery. In Mackin EJ, Callahan AD, Osterman AL, et al, editors: Rehabilitation of the hand and upper extremity, ed 5, St Louis, 2002, Mosby.)
Circumferential measurement
Results from using a nonweighted or spring loaded tape measure can vacillate greatly because of the inconsistency of tension put on the tape. The preference is to use a measuring tape with a weight on one end, or one having a spring loaded device. These devices will give a more consistent pull on the tape and therefore provide a more reliable, repeatable measurement. Circumferential measurements have the advantage of showing site specific changes in edema. Jeweler’s rings can also be used to circumferentially measure digits
Cognitive and perceptual assessments
Occupational therapists are skilled in prioritizing person-centered goals and addressing the many personal and environmental factors that alter function poststroke. Cognition and perception may affect a client’s ability to integrate the affected limb into normal tasks and to understand their role in preventing or managing edema (see Chapters 18 and 19). The caregiver may need education on how these areas influence function and a person’s ability to follow an edema management program, such as the importance of positioning, safe ranging, and gentle edema massage.
Upper limb assessments
Many clinics use an upper limb or neurophysical screen to comment on range, strength, shoulder integrity, coordination, pain, and edema, among other things. When conducting a standardized assessment of upper limb quality of movement or function, it is important to consider not only how motor recovery may affect the outcome, but also how edema may influence the results. Further discussion of upper limb assessments can be found in Chapter 10.
Sensibility testing
There is an old saying, “an insensitive hand is a blind hand.” Hand insensitivity or decreased sensation facilitates decreased use and possible injury to the extremity, which can be further complicated by a unilateral neglect. The monofilament method tests for the degree of sensibility present and can indicate a sensory deficit ranging from decreased light touch to loss of protective sensation and to loss of deep touch. Various size monofilaments on individual rods are slowly pressed against the tissue until the monofilament bends, and then the monofilament is slowly lifted from the skin. Once the monofilament bends, no further pressure can be exerted. If the client doesn’t detect “touch” from the monofilament, the therapist then uses a larger size monofilament for the next test. The client’s vision is occluded during the test.
Testing is important because edema puts pressure on nerve endings decreasing sensibility. As edema decreases sensitivity should improve. Results can be related to safety and activity of daily living (ADL) function. It is imperative to follow directions for accurate, reliable, and repeatable testing. Checking for sensibility can be an important predictor of edema. Boomkamp-Koppen and colleagues7 found that poststroke clients with hyposensibility, hypertonia, and motor impairment were 50% more likely to develop edema.
Rebound test
This is a subjective test, but gives an indication if congested edema is softening and decreasing from an area. The therapist places a one ounce weight or the weight of his or her thumb (enough weight to begin blanching the therapist’s finger nail) on the edematous area and counts to 10. This light pressure creates a pit in the edema. Then the therapist counts the time the tissue takes to rebound to the height of the adjacent tissue. Lymph congested tissue presents as slow to rebound and is clinically seen as significant with a 20- to 30- second rebound or slower. After doing edema reduction treatment, the test is repeated. If the rebound time is faster, then it is assumed that there has been some lymph decongestion in the area.
This is a quick easy test to do between more objective circumferential or volumetric measurements. It must be noted that tissue that does not pit is usually fibrotic.
Edematous tissue visual and tactile evaluation
Edema present one month poststroke should be watched closely for early signs of CRPS Type 1. Most hand edema begins two to four months poststroke, and CRPS has been observed to start at the same time but tends to be more extensive.30
Tissue in early stages of CRPS Type I presents as edematous, hyperesthetic, warm, perspiring, red and white blotching of skin, pain and tenderness of the wrist and finger joints, having a constant burning pain, and having an increased in blood flow to the extremity.30
This is rare in the upper extremity, but a therapist must be suspicious of any sudden onset of swelling that is accompanied by pain with a specific muscle movement, including tenderness and warmth. Do not treat the client; seek physician advice immediately.
Infected tissue acutely presents as red, warm, swollen, and painful to touch or movement, and often the client has a fever. If there is an infected open wound, the drainage may be opaque and have a pungent odor, in addition to the proceeding symptoms. Seek physician help immediately. Do not treat the limb until the physician has given the approval to resume treatment.
Routinely check for excessively dry skin from sensory impairment. Dry skin cracks and can be a source for bacterial infections.
Check client’s history regarding having had a mastectomy in the past. Approximately 15% to 20% of clients develop lymphedema postmastectomy node removal.42 However, because there is a decrease in the number of lymphatic structures, clients are always at risk to develop lymphedema. A stroke survivor with dependent edema involving the arm on the mastectomy side could potentially compromise the deficient lymphatic system and cause lymphedema. If this occurs, a therapist certified in manual lymphatic drainage techniques must be sought out to treat the lymphedema.
 Swelling from cardiac problems or from low protein edemas such as renal dysfunction, malnutrition, and liver disease
Swelling from cardiac problems or from low protein edemas such as renal dysfunction, malnutrition, and liver disease
Swelling from cardiac problems, such as chronic heart disease or congestive heart failure (CHF), can be characterized by bilateral ankle swelling, and the tissue is often slightly pinkish. Therapists should check the client’s chart regarding a possible cardiac condition. Edema reduction massage should not be performed because of the potential to send too much fluid back to the heart and further compromising the heart. This type of edema has to be controlled medically. Edema from renal disease, liver disease, and malnutrition is a low protein edema.27 This too has to be reduced by medication. Edema reduction massage can potentially overload and decrease the function of the already compromised organs in this case.
Current poststroke hand edema treatment methods
Manual lymphatic and venous absorption stimulation methods
Elevation and retrograde massage
In the early stages of poststroke edema, elevation and light retrograde massage followed by use of an elastic glove on the hand and cotton/elastic stockinette tube on the arm, if needed, can be effective for reducing hand/arm edema. The rationale is that elevation decreases arterial hydrostatic pressure and thus reduces the flowing of fluid into the interstitial spaces.14,50 The elastic glove and stockinette give light pressure to prevent or lessen refilling of tissue. An active muscle pump is needed to intermittently compress venous and lymphatic structures to return fluid back toward the heart. Without an active or fully functioning muscle pump, dependent limb edema results. Furthermore, over time the volume of fluid increases distally and less edema reduces with elevation and compression. At this point, it is theorized that both the venous and lymphatic systems have reached their maximum capacity, and a combined edema situation exists.
Clinical treatment considerations.
Avoid having the elastic glove or elastic/cotton stockinette tube being too tight and collapsing the initial lymphatics. This would prevent absorption of fluid from the interstitium. One clinical guideline is to be able to stretch the glove one eighth of an inch out from either side of a digit. The elastic/cotton stockinette tube should be firm but still allow a therapist’s hands to fit under the stockinette at the tightest point. The goals are to provide compression, not to collapse the initial lymphatic net along without causing tissue trauma with application and removal. Both the venous and lymphatic systems take fluid out of the interstitium, but lymphatic absorption is stimulated in ways previously discussed. If sensory or vascular insufficiencies exist in the involved arm and hand, the therapist must take appropriate precautions.
Anecdotally, therapists have seen distal hand edema reduction while doing extensive active, active-assistive trunk and scapular work. Anatomically, this is logical because trunk and scapular movement activates changes in thoracic pressures and thus activates the thoracic duct pump of the lymph system. Thus, applying the elastic glove and cotton/elastic stockinette tube on the extremity immediately following active trunk and scapular exercise prevents or lessens refill after an edema reduction has been achieved.
When elevating the arm, precautions should be taken if the client has medical conditions such as right-sided heart weakness or Raynaud disease. The latter diagnosis involves arterial vascular insufficiency, so elevation of the extremity further decreases the blood flow to the extremity and quickly increases the symptoms such as dysesthesia and further blanching of the finger tips.10 Also, extreme elevation of the right upper extremity, especially in supine, to reduce edema would not be advisable if the client had a comorbidity of right-sided heart weakness.10 This could potentially send fluid faster into the right side of the heart than it can be pumped into the left side to be reoxygenated, thus further compromising the heart.
Manual edema mobilization
Manual Edema Mobilization (MEM) was first introduced by Artzberger in 1995 with subsequent publications.1-5,29,44 It recognizes that swelling that lasts longer than one week and presents as slow to rebound, i.e., 20 to 30 seconds or more to reach surrounding tissue height when pitted, indicates a lymphatic congested system. MEM teaches specific concepts to stimulate and quickly decongest the lymphatic system for postorthopedic trauma and poststroke extremity edema. Treatment begins in the trunk, creating a vacuum drawing peripheral lymph proximally, toward the trunk. Treatment for the sedentary patient begins with pretreatment exercises of stretching for the trunk and shoulders to facilitate proximal decongestion. The MEM program is initiated with diaphragmatic breathing, trunk exercise, and light trunk massage, and then proceeds distally in sections toward the hand. Active or passive exercise of muscles in each section just massaged is essential to pump the lymph proximally. At the end, along with distal to proximal exercise, light flow massage from hand to arm to the trunk is completed. Keys to success include diaphragmatic breathing, starting treatment at the trunk, exercise at specific intervals, use of a technique called MEM Pump Points, light massage strokes, a home self-massage and exercise program, and low stretch bandaging and/or chip bags as needed. This is designed exclusively for the patient with an intact lymph system (not status post mastectomy where nodes have been removed). Treatment usually takes 20 minutes and is incorporated into a patient’s regular treatment program. The MEM technique includes specific guidelines and precautions, especially for the stroke survivor who often has many comorbidities, so taking a formal two-day MEM course is necessary.
When poststroke edema no longer reduces with elevation, light retrograde massage, and compression, it is theorized that both the venous and lymphatic systems are overloaded, and a combined edema exists. Thus, there has to be a specific activation of the lymphatic system to help reduce this type of edema. MEM is an appropriate treatment providing there are no medical contraindications relative to the technique. The following paragraphs will elaborate the key elements of MEM.
Manual edema mobilization begins at the trunk.
This follows the previously discussed hydraulic, vacuum principle of first moving lymph centrally into the venous system (subclavian veins), which then draws lymph proximally from the periphery. Diaphragmatic breathing facilitates this process through changes in thoracic duct pressure that then moves lymph proximally.14 Diaphragmatic breathing entails breathing air through the nose into the lower abdomen that pushes the naval area outward, and then slowly exhaling through pursed lips, bringing the lower abdomen inward.
Exercise muscles in area just massaged.
According to Guyton and Hall,27 the collector lymphatics move lymph ten to thirty times faster with exercise. MEM light massage techniques facilitate absorption of large molecules, permeable only to the lymph system, into the initial lymphatic net by exercising muscles under the tissue just massaged. This muscle pumping moves lymph faster through the system, and theoretically space is created for more absorption.
Light massage strokes.
Since 65 mm Hg pressure39 has been shown to begin collapsing the initial lymphatic net where absorption begins in the dermis layer of tissue, therapists are instructed to use a pressure no greater than half the weight of their hand. To quote a stroke survivor being taught a home program, “light is right.” Strokes are “U” shaped beginning proximally (top of the “U”), moving the skin distally, and then back upward to where begun. It is emphasized that the hand does not slide on the skin but remains in place moving the skin over underlying structures. Terms used in the massage performed by the therapist are clear and flow.
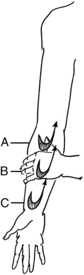
Clearing “U”s consist of five “U”s done in each of three segments of a section of an extremity, i.e., volar forearm. In this case, the “U”s start proximal at the elbow and end at the wrist (Fig. 12-5) These stimulate absorption into the initial and collector lymphatics.
Flow “U”s begin at the distal end of the segment just cleared and end proximal to where clearing started, hopefully at a set of nodes. Only one, not five, “U”s are done in each section of the segment. These are repeated five times from the distal end of the segment and ending proximal to where clearing started. This action is believed to stimulate absorption, help to prevent refluxing of lymph, and create a proximal flow.
Because the “flowing” concept can be difficult to teach a patient for his or her home program, the term sweep is used. The patient is instructed to very lightly slide his or her fingers and palm over the involved extremity, starting distally and moving proximally (Fig. 12-6).
Manual edema mobilization pump points.
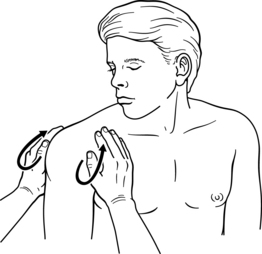
The upper extremity has five specific pump point locations, which are sites of lymph nodes or bundles of lymphatic structures (initial and collector lymphatics). A therapist uses both hands to simultaneously massage a set of nodes and lymphatic bundles in a “U” shaped pump pattern (see Figs. 12-7 and 12-8). Clinically, these seem to provide a faster flow of lymph versus the usual “clear” and “flow” technique, especially for patients with the combined type of edema. Because of the effect, an increased volume of lymph flow could have another existing medical conditions, such as cardiac and pulmonary. It is necessary to complete a MEM course because pump point application is thoroughly discussed for the stroke survivor. Box 12-1 lists the upper extremity pump points.
Box 12-1 The Five MEM Upper Extremity Pump Point Hand Placement Areas
1. First hand is placed on deltoid pectoral node area of trunk and anterior deltoid of upper arm. Second hand is placed on teres minor, posterior axilla, and posterior deltoid of upper arm. A praying hands position (Fig. 12–7).
2. First hand is placed on teres minor, posterior axilla, and posterior deltoid of upper arm, as in Pump Point One. Second hand is placed on medial side of antecubital crease of elbow—elbow node location. Middle finger lies across antecubital crease and thumb on back of arm above elbow.
3. First hand is placed on medial antecubital crease as in Pump Point Two. Second hand is placed on the posterior of the upper arm just above the back of the elbow (Fig. 12–8).
4. First hand is placed on antecubital crease as in Pump Points two and three. Second hand is placed on the volar forearm at wrist.
5. First hand is placed at the volar forearm at the wrist. Second hand is on the dorsum of the hand.
Manual edema mobilization home self-management program.
A home MEM program is essential to keep lymphatic structures open, and lymph flowing for long-term edema reduction. Patients are given a simplified version of the program the therapist used in the clinic. Often simple proximal to distal node massage from trunk to elbow, “sweeping” from hand to uninvolved axilla, and exercise of the arm and trunk is enough. These can easily be incorporated into the patient’s ADL tasks, such as daily hygiene and functional upper limb retraining, e.g., wiping the table.
Low stretch bandages.
Low stretch bandages look like the high stretch bandages often used postsports injury, frequently called ACE bandages; however, low stretch bandages have no elastic fibers and are 100% cotton. Because they have minimal stretch, they facilitate a “pumping” action on the initial lymphatic net with muscle contraction and relaxation.14 For more details, see the Bandaging Methods section.
Chip bags.
Chip bags are often placed under low stretch bandages or an elastic/cotton stockinette tube on areas of excessive swelling, especially areas of hard tissue. They consist of various densities of foam pieces one inch in size placed in a cotton stockinette with the ends sewn shut (Fig. 12-9). It is theorized that hard tissue is softened due to the neutral warmth that builds up under the foam pieces.4 See Bandaging Methods Section for ideal lymph flow temperatures. It appears that the various densities of the foam further help to soften and stimulate lymphatic uptake because of the tissue pressure differentiation they cause.
Clinical treatment considerations.
MEM techniques have been shown to reduce edema.44 However, for the flaccid extremity, this reduction will not last because the lymphatic system, like the venous system, needs an active muscle pump system to continually move the lymph.22 Light massage and passive exercise both put a stretch on the anchor filaments of the initial lymphatics (lymphatic net) and alter interstitial pressure, which will open the junctions of the endothelial cells, admitting molecules into the initial lymphatic. From there the collector lymphatics have a peristaltic pumping action that is controlled by the sympathetic and parasympathetic systems to conduct the lymph proximally.17 However, some authors believe that the autonomic system can be neurologically impaired by the stroke.55 This combined with lack of an active muscle pump causes lymph congestion. Proximal trunk exercise and diaphragmatic breathing stimulate the lymphatic system and will draw lymph proximally.14 Thus, even without knowing MEM techniques, a therapist can reduce the lymphatic congestion with diaphragmatic breathing, extensive trunk and scapular exercise and activation of the proximal noninvolved musculature. The decongestion then facilitates peripheral lymph absorption. By reducing edema, the occupational therapist may improve the patient’s perception and awareness of the affected upper limb and increase the functional dexterity of the hand. Providing a clear and meaningful home program may increase the patient’s ownership of his or her occupational therapy program.
Bandaging methods
There are two types of bandaging systems: elastic (high stretch) and low elastic (low stretch).14 Both look alike in thickness and color, but the low elastic (low stretch) bandages are usually 100% cotton and have no elastic fibers.14 The Casley-Smiths14 point out that the initial lymphatic net will only pump when compressed against something solid such between a contracting muscle and a solid counter-force (low elastic bandage). A tissue pressure differentiation, or pumping action, is created facilitating lymphatic absorption with muscle contraction against a counter-force (the lymphatic net is caught between the contracting muscle and the resistive bandage) and then relaxation of the muscle. The Casley-Smiths14 refer to low stretch bandages as having “high-working and low resting pressures.” An elastic bandage stretches and does not produce this counter-force.
Low stretch bandages are “rolled on,” not pulled tight, in order not to collapse the initial lymphatic net. Miller and Seale39 found that the initial lymphatic net begins to close as 60 mm Hg pressure and is completely closed at 75 mm Hg pressure. Graduated pressure with low stretch bandages is thus obtained not by pulling tightly, but by layers of bandages in an area.
Clinical treatment considerations.
Low stretch bandages create a pump action facilitating lymphatic absorption and prevent tissue refill.14 The neutral warmth (body temperature) that builds up under bandages softens indurated (hard) tissue facilitating fluid absorption. Kurz36 states that the ideal temperatures to facilitate lymph flow is between 22 degrees and 41 degrees C (71.6 F and 105.8 F). Please note that temperatures above 98.6 F or 37 degrees C will increase blood flow to the area and increase edema, so a therapist would not use these high temperatures when trying to reduce edema. Most importantly, when applied properly, the short stretch bandages do not collapse the lymphatic net, which prevents excess tissue fluid absorption, and they can be worn during periods of rest. Unfortunately, low stretch bandages are not often practical and have limitations for poststroke hand and arm edema, because they can potentially cause neurovascular problems or can limit function when applied too tightly by an untrained person; if an active muscle movement causes the desired excess tissue fluid absorption, the extremity loses girth, the bandages have to be reapplied, and most stroke survivors require assistance to reapply bandages due to their cognitive, perceptual, or motor limitations; and bandages may limit sensory retraining.
When there is minimal to no active muscle contraction, a cotton/elastic stockinette tube is more practical for stroke edema. Because the cotton stockinette tube is elastic, it only prevents or lessens tissue fluid refill, and, if loose enough, it will not collapse the initial lymphatic net. To ensure that an elastic/cotton stockinette tube is not too tight, the therapist should be able to get both hands in the tube on either side of the patient’s arm.
Rolling down of the elastic/stockinette tube can be a problem. Suggested ways to prevent this include: (1) Double the elastic stockinette tube, but make sure that the pressure is not too tight; (2) Loosely place a totally stretched out 3 inch-wide piece of Coban circumferentially 1 inch below the proximal end of the elastic stockinette tube and “cuff” the 1 inch proximal end over the Coban (Fig. 12-10). This can also be done with a loosely placed one inch foam splinting strap instead of the Coban. To achieve graded compression, place one piece of the cotton/elastic stockinette tube, for instance, from palm to elbow and a second smaller piece from palm to mid-forearm. Then stitch the two pieces together enabling the patient or caregiver to pull it on in one piece. When introducing bandaging, therapists must educate and closely monitor the patient and caregiver for appropriate application to prevent rolling down of the elastic/cotton stockinette tube that would then increase distal swelling. Chip bags can be placed under the elastic/cotton stockinette tube to soften hard edema or to prevent refill at a specific site.
Continuous passive motion
In 1990 Giudice25 published an article reporting hand edema reduction outcomes comparing 30 minutes of hand elevation and 30 minutes of hand elevation with continuous passive motion (CPM). Eleven of the 16 subjects had hemiplegia. Edema reduction was significantly greater with the combination of elevation and the use of the CPM machine. However, when the CPM was discontinued, the edema returned to its former rate.25
More extensive use and evaluation of use of the CPM machine was reported by Dirette and Hinojosa in 1994.20 In their ABA single subject design study, two clients one month poststroke received CPM treatment for two hours daily for one week. Results showed a continuous significant reduction of edema during the treatment week. During the withdrawal week, the edema increased, leveled off, but did not return to evaluation week edema volume.
Clinical treatment considerations and rationale.
The CPM provides gentle and nonexcessive motion to the hand, thus eliminating microscopic tearing of tissue that can lead to edema and potential fibrosis of tissue and joints. The passive movement stretches the elastic anchor filaments of the initial lymphatic net and causes changes of interstitial pressure, all facilitating opening of the endothelial cell junctions and absorption of fluid into the lymphatic net. It has been suggested that the CPM might have more pumping and drainage action on the dorsal hand lymphatics if it was set to flex metacarpophalangeal (MCP) joints to near normal flexion range.22 Because the CPM is on the hand, increased attention the involved limb may be noted during that period of usage.
Pneumatic pump and air splints
Pneumatic intermittent compression pumps were first introduced to reduce venous leg edema, such as from varicose veins, and were then expanded to usage with the lymphedematous extremity. Leduc37 reported that pneumatic pumps only force water back into blood and do not remove excessive protein from tissues. In 1999 Roper and colleagues47 reported on their study of 37 clients with stroke hand edema who received a two two-hour session two times a day of intermittent pneumatic compression for one month. Compression was 50 mm Hg. They found no change in hand volume in the treated group.47
Clinical treatment considerations, rationale, and potential future research ideas.
1. A Casley-Smith and Bjorlin13 research study concluded that 45 mm Hg pump pressure would not collapse the initial lymphatics. Would a graded sequential pump, meaning progressive chamber pressures from 40 mm Hg at the hand to 10 mm Hg at the axilla, be more effective versus 50 mm Hg pressure up the entire arm?
2. Did the Roper and colleagues47 study include early or combined edema?
3. If it were combined edema, would central trunk clearing performed before pumping positively affect the results? Recently new pumps have been developed that use lower pressures and begin massage at the trunk. Would these be more effective because they start drainage centrally, massage in a proximal to distal segment sequence, and then distal to proximal? Raines and colleagues45 found that the pneumatic pumps could only reduce edema, even temporarily, only if the venous drainage is normal. According to the vasomotor dysfunction theory of stroke edema development, venous drainage is impaired.55
If a pneumatic pump is used, precautions should be observed. It should not be used if there is a blood clot or any suspicion of a clot, infection, cellulitis, symptoms of CHF or chronic obstructive pulmonary disease, dizziness, lightheadedness, or headaches.14 Beta blockers in combination with pumping have been known to cause hypotension.11 The pneumatic pump or air splints should not be used on stroke clients who are on anticoagulant medications that can drop their platelet level below 120,000 mm.19
Clinically, the rationale of using air splints to reduce edema should be evaluated and appropriately applied. They provide single chamber circumferential compression that can push fluid both distal and proximal because there is no grading of compression. The air splint compression pushes tissue fluid back into the interstitium, which may work to reduce edema in the early stages when it is a low protein edema. Also, the neutral warmth that builds up under the plastic splint could soften indurated tissue. However, when the edema becomes a combined edema, this method will only push fluid out of the interstitium temporarily. The hydrophilic plasma proteins remain in the lymph, because anatomically they cannot be physically “pushed out.” They will reattract the water molecule, and swelling will return. In fact, if the pressure is above 40 mm Hg, it could collapse the lymphatic net.
Splinting
There is some evidence that splinting reduces edema. Garcies and colleagues26 found that a custom-made lycra garment with flexible plastic inserts when worn three hours a day provided a continuous stretch of spastic muscles and reduced edema. There are some clinical arguments for the use of a wrist cock up splint to reduce edema, to protect joints, and to minimize pain.24 Burge and colleagues9 conducted a randomized trial of a neutral functional realignment orthosis on 30 clients with subacute hemiplegia, with the orthosis group wearing it for six hours a day. They found the orthoses prevented pain but had little effect on edema or mobility. They also stated that they used circumferential measurements as directed by Leibovitz.9
Clinical treatment considerations and rationale.
The splint used by Gracies26 allowed for restricted movement, thus preventing overzealous passive movement of the hand and arm to cause trauma edema. Also, the ability to move the hand while it is in the splint helps to increase attention to the affected limb. Consideration should also be given to the role the combination of the elastic lycra and muscle contraction play to move low protein edema from the periphery centrally or in preventing further filling of tissue. See Chapter 13.
Exercise and positioning
Research regarding poststroke development of SHS, CRPS, and elbow-hand syndrome repeatedly show that the incidence can be reduced by half or more if inflammation of tissue can be avoided.7,8,30,33 Braus and colleagues8 reduced the frequency of SHS from 27% to 8% in their study by extensively educating everyone involved in client care on how to prevent trauma to the involved shoulder and extremity. Their regimen included immediately repositioning the hand/arm/shoulder if pain occurred; performing passive humeral motions of abduction and external rotation only after fully mobilizing the scapula; having not only the therapist perform the motion during treatment, but having other hospital services do so when handling the involved limb as part of their treatment, such as computerized tomography, electroencephalography, or when relatives assist with care; and avoiding needle sticks in the involved hand/arm.8
In their research article, Kondo and colleague33 included a passive exercise protocol for both the therapist and client to follow to prevent SHS. This article detailed a controlled passive movement regimen by a trained therapist and restriction of passive movement by the client for a minimum of four months poststroke. Restricted passive motion not only included shoulder-scapula protective ROM, but also included preventing the client from repeatedly hyperextending his fingers, which causes trauma to the finger joints. Clients with impaired sensation were more likely to excessively range or hyperextend their fingers.33 Another study also showed that the hand stayed edematous, even if the client had active motor return and did not use it.7 Furthermore, studies have shown that prolonged positioning of the wrist and fingers in flexion will exacerbate swelling, because it impedes venous and lymphatic flow at the wrist.7
Avoiding microtrauma to tissue is difficult if the client also has unilateral neglect and/or visual field deficits. Wee and colleagues54 found that 80% of those with shoulder-hand problems had unilateral neglect. They concluded that the neglect predisposed the client to shoulder-hand problems.54
Microscopic tearing of tissue that occurs from repeated mishandling and mispositioning of a nonfunctioning to a minimum functioning arm causes trauma to tissue. This will cause a wound healing sequence to occur to the involved joints and tissue. With the invasion of excess plasma proteins into tissue from trauma to a hand or arm with a preexisting diminished motor function and/or dependency edema, the cycle to possible fibrosis is established. Only the lymphatic system can remove these excess plasma proteins and thus has to be specifically stimulated.
Clinical treatment considerations and rationale.
Diligence is recommended to avoid causing tissue inflammation during all aspects of client care and rehabilitation. The client, family, nursing, nursing assistants, and even x-ray and lab technicians from the facility have to be trained in proper handling and positioning of the arm at all times during treatment and care, including bed mobility and walking. Pain to the involved limb has to be avoided or immediately corrected, such pain as from improper positioning. Education and repeated use of educational material is essential. Suggestions include: wheelchair lap tables should be at the appropriate height or include an arm wedge to support the affected limb in neutral; when moving the flaccid arm, even for bed positioning, support and glide the scapula at the same time; do not pull the affected arm during transfers and bed mobility; begin proper scapular and shoulder ROM glides as soon as possible after the stroke; position on pillows to support the shoulder complex; support the arm and shoulder during transfers and ambulation to prevent stretching on the shoulder capsule or dependent arm positioning; thoroughly and repeatedly educate the client and family not to exuberantly exercise the shoulder, wrist, and fingers, and to force extremes of range; all personnel involved with client care must glide the scapula concurrently while the humerus is moved when working with the client; and prevent wrist and fingers from assuming a flexed position for a prolonged period of time.
Diligently avoid head, arm, and trunk positioning or exercise that can cause tissue inflammation of the brachial plexus. CRPS Type II involves peripheral nerve lesions. Overzealous shoulder capsule stretching or prolonged subluxation of the glenohumeral joint can cause brachial plexus inflammation and potential nerve damage that could potentially lead to CRPS Type II.41 See Chapter 10.
Scalenus anticus syndrome is a cervical neurovascular (brachial plexus and subclavian artery) impingement syndrome involving the scalenus anticus muscle. It is facilitated by prolonged sitting with a forward head position, inwardly rolled shoulders, and flexion of the spine causing cervical and brachial plexus inflammation.10 Clients present with mild neck and shoulder pain including tingling sensation in the fingers.51 A corrective position is achieved by positioning the clients pelvis into a neutral tilt, placing a small rolled pillow or towel at the lower back to get a lumbar curve, which will then facilitate a normal shoulder external rotation position and head alignment above the trunk.
Clients who complain of bilateral arm pain, paraesthesia, and arm weakness with activities that require overhead reaching should be evaluated for thoracic outlet syndrome (TOS). For the client who has decreased proprioceptive or kinesthetic sensation in the involved arm, an activity such as weight-bearing on that arm with an unsupported shoulder girdle having poor scapula stability could cause or exacerbate a TOS.10 Furthermore, a client who repeatedly over stretches the arm above the shoulder without scapular gliding not only can cause microscopic tearing of the shoulder capsule soft-tissue structures, but could cause an impingement and inflammation at the thoracic outlet as well.
Begin treatment sessions with diaphragmatic breathing (or activities that cause changes in thoracic pressure such as laughing) and extensive trunk and scapular exercise to activate the lymphatic pump centrally drawing venous and lymphatic fluid forward. Remember that even passive exercise anatomically stimulates lymphatic absorption in the extremity, but absorption begins with central clearing as described previously.
For the client who has some motor return, emphasize the importance of frequent short hand exercise sessions and functional usage throughout the day to reduce hand edema. Relate exercise to functional tasks. Boomkamp-Koppen and colleagues7 found hand edema in 17.6% of their clients who had good hand function. They concluded that these clients were unwilling to perform active exercises with the hemiparetic hand as much as the noninvolved hand. Hemi neglect, visual field limitations, sensory limitations, and “learned” neglect all contribute to nonuse.
Clients with unilateral neglect have to be taught various compensation methods or use of safety devices to prevent microtrauma to the involved arm. Suggested methodologies have included modifications of the home and work environments to enable safe functional task performance; position in space awareness cuing and sensory cuing; auditory warning signals; and proprioceptive and visual correction techniques (see Chapter 19).
Electrical stimulation
Over the last 25 years, there has been considerable interest in and evolving research and clinical usage of Short Term Electrical Stimulation for neurological stimulation poststroke for pain reduction, muscular stimulation, muscle strengthening, and tone reduction. Recognizing the role the venous and lymphatic systems play for reducing edema and the effect the muscle pump has on these two systems to reduce edema, Faghri22 designed a research study using neuromuscular stimulation (NMES) to facilitate the muscle pump for edema reduction in the flaccid/paralyzed edematous poststroke hand. His study showed that edema reduction with 30 minutes of NMES of the flaccid/paralyzed wrist and finger flexors and extensors was significantly greater than 30 minutes of limb elevation alone. However, when the NMES was discontinued, the edema returned to its former volume in the limb. This study is very significant because it addresses the two theories of stoke edema: neurological impairment and dependency edema due to lack of an active muscle motor pump.
Neuroprosthetic functional electrical stimulation.
Faghri’s22 study involved only 30 minutes of treatment daily, and edema reduction occurred with electrically induced muscle contraction. A study done by Ring and Rosenthal also showed a result of edema reduction.46 This study involved clients six months poststroke, using one group with a flaccid hand and a second group with some motor return in the involved hand. In addition to their regular therapy, the subjects wore a neuroprosthesis on their involved forearm/palm for 50 minutes three times daily for six weeks. This neuromuscular stimulator stimulated five forearm muscles, activating the wrist and fingers, and the stimulation modes alternated finger flexion and extension. Those with some motor return were encouraged to actively carry out movement during stimulation, such as grasp and release. Results for the flaccid extremity group showed greater decrease in spasticity and greater improvement in proximal limb active ROM, compared to the control group. The group with some motor return demonstrated significant gains in hand function, a decrease in spasticity, and increased voluntary motion, as compared to the control group. Outcomes also showed that existing hand edema reduced in the neuroprosthesis study group but not in the control group. Long-term continuance of the gains made by usage of the neuroprosthesis unit was not assessed in this study. The authors cite a similar study40 of clients in the flaccid hand category that showed all gains made were lost within two weeks once the stimulation was removed.
Clinical treatment considerations and rationale.
Neurologically, usage of a multihour electrical stimulation device shows much promise for the clients with hand spasticity, a hemiparetic hand, or flaccid hand. More research is needed to determine the optimum lengths for daily and total usage to get the longest carry over when the treatment is discontinued. This will help the treating therapist decide if this treatment is applicable for their particular poststroke clients with hand edema.
Use of neuroprosthesis devices promotes gentle active and passive motion and does not cause microtrauma to tissue. Potentially multihour usage of the device helps to lessen hemineglect if the client uses it for functional tasks.
Functional activities
Edema and the associated deficits it causes, such as reduced sensation and range of movement, may limit a person’s integration of the affected limb into normal tasks and may reinforce learned nonuse. Occupational therapists can grade and provide cuing in daily functional tasks to address the cognitive, perceptual, sensory, and motor aspects of performance, and can facilitate use of the affected limb. Through task analysis, occupational therapists can highlight to a client what the client can do to maximize independence and reduce the impact of edema. Boomkamp-Koppen and colleagues7 found a significant relationship between edema and hand function when paresis was controlled in a statistical analysis. Clinically, edema may mask the motor and sensory potential of the upper limb may and limit progress of a client’s goals. Gilmore and colleagues24 advocated the use of purposeful activities that position the shoulder complex in normal alignment or facilitated scapulohumeral rhythm to minimize pain and trauma.
Clinical treatment considerations and rationale.
Occupational therapists are in the position to select meaningful tasks with their clients for therapy and to set specific functional goals. As the lymphatic system is activated by muscle pumping, the use of the affected upper limb in normal tasks within a safe range of movement will facilitate lymphatic flow. Reinforcing to the client and caregivers what activities, or parts of activities, a client can undertake with the affected upper limb can assist in this process.
Summary
It is essential to screen for and address poststroke upper limb edema as soon as possible. By effectively managing edema the incidence of CRPS Type I, pain, stiffness, and possible joint contractures can be reduced. However, most important, edema must decrease in order to facilitate functional arm and hand usage for occupation, especially as motor function return occurs. Unfortunately, there is no specific consensus on which of the discussed treatment technique is most effective for reducing poststroke hand edema.7,23,38 However, it is hoped that this chapter will give therapists a foundation for client treatment planning, critical problem-solving, and a basis to do further research of techniques.
CASE STUDIES
In this Australian setting, clients receive intensive neurological rehabilitation as directed by a multidisciplinary team. The occupational therapist’s role is to evaluate and maximize performance in activities of daily living, domestic roles, community safety, driving, work, and leisure. Numerous treatment frameworks are used. In these three case studies, the author provided intensive edema treatment to address functional goals.
Evaluation criteria
All edema measurements were conducted by the same occupational therapist to increase intrarater reliability. It was clinically reasoned that a volumeter was not a reliable way of measuring edema given the limitations of consistently positioning the stroke upper limb. Circumferential measurements were chosen to increase consistency of measurement and to identify change in anatomical regions. Measurements of the affected limb were taken at consistent landmarks with the client’s limb in the same position.
CASE STUDY 1 Subacute Stroke with Hemiplegia and Motivation
S.O. was 56-years-old when he suffered a left basal ganglia and corona radiata stroke. Two weeks after his stroke, he was transferred from an acute hospital to a specialized neurological rehabilitation setting. His main deficits were right hemiplegia, mild dysarthria, mild expressive aphasia, and mild memory impairment. He was right dominant. Prior to the stroke, S.O. lived independently in a country town and worked in the mining industry operating machinery. He enjoyed dancing and socializing and was motivated to return to independent living and driving.
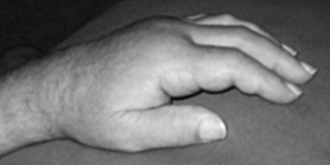
Approximately two months poststroke, S.O. developed edema in his right arm that did not respond to elevation and massage alone. The primary occupational therapist identified S.O. as a good candidate for MEM because his edema was limiting grasp and manipulation and release of objects, he had no medical contraindications limiting participation in MEM, and he had the cognitive ability to complete a self-MEM program. He was motivated to participate in all aspects of therapy and hospital life. S.O. was concerned that his edema was “holding him back” from using his arm; for example, he felt that he could not grasp a flannel shirt as his fingers felt “. . . like sausages.” At initial assessment, S.O. had nonpitting edema over his hand and upper limb, and his hand was hot to touch (an indication of tissue fluid congestion as no infection was present). Sensation was grossly intact, and he had some minor shoulder pain. Elbow extension was normal, but his wrist and finger extension were limited by his edema and increased flexor skeletal muscle activity. He was independent in self-care in the ward and used an electric wheelchair for mobility.
During the first session, S.O. was educated regarding the theoretical background of MEM and treatment progressed through Pump Point Two. The importance of light and “U” shaped strokes were emphasized, rather than the rough massaging of “up and down” the dorsum of the hand that he had been doing in an effort to reduce his swelling. S.O. was advised to complete a basic home program three times a day, which consisted of diaphragmatic breathing, exercises, axilla and terminus massage (supraclavicular area), and sweeping, in addition to his standard occupational therapy and physiotherapy sessions. An edema glove was tried, but S.O. did not tolerate it, saying it was uncomfortable. Instead, he used a foam wedge on his wheelchair arm trough to elevate his arm as much as possible.
In the second session, the therapist noted that there was increased flexor skeletal muscle activity in the forearm. MEM was used on all five Pump Points, and traditional retrograde massage was used on the hands and fingers to facilitate further clearance. S.O. could use the technique on his fingers himself, and he reported that his arm felt “lighter” by the end of the second session. The therapist noted post-MEM a decrease in flexor skeletal muscle activity, improved supination to wash his face, and an increase in wrist skin folds.
By the final two sessions, S.O.’s right hand was the same temperature as his left (fluid decongestion had occurred as the edema reduced), the circumference of his elbow and axilla had reduced by 1.3 and 5.4 cm respectively, and there was a reduction in flexor activity. S.O. had increased range of finger abduction and adduction, thumb extension, and composite flexion, which he could use in grooming tasks. S.O.’s primary occupational therapist was aware of MEM treatment principles and continued to use these when working with S.O. As he noted improvements, S.O. began to complete his self-MEM program three times a day without prompting by his occupational therapist.
MEM was a technique that S.O. could independently use and what he preferred when compared to the medical suggestion of bandaging, which was likely to restrict his progress in grooming. It appeared that the combination of diaphragmatic breathing, stretches, flowing (“sweeping”) up the arm, and elevation contributed greatly to the reduction of edema and subsequent functional goals. There were reductions of over 1 cm at the wrist and MCP joints and small changes over the digits. At the time of writing, S.O. had returned to independent living and was beginning to have the dexterity to write with his dominant hand.
CASE STUDY 2 Chronic Stroke with Minimal Hand Movement and Increased Skeletal Muscle Activity
K.P. was 55-years-old when he had a left middle cerebral watershed infarct at home. After acute and rehabilitation inpatient stays, K.P. could walk independently and was discharged home to live with his supportive long-term partner, who supervised him with ADL. Prior to his stroke, K.P. worked as a bus driver and enjoyed visiting his young granddaughter, woodworking, and sailing. K.P. received home-based occupational therapy and was then referred for outpatient occupational therapy. K.P. was then four months poststroke and was beginning to achieve active upper limb movement in his affected right arm. He was left dominant.
K.P. was driven by his partner for an hour each way, twice a week to attend occupational therapy and physiotherapy. K.P. had memory deficits and reduced attention. His partner used prompting with K.P. at home and had set up cue cards to assist in routine tasks around the house. His primary occupational therapy goal was to use his right hand in leisure activities. K.P. presented with poor trunk, shoulder, and head symmetry, both with standing and sitting. His rehabilitation had been limited by his lack of awareness, body positioning, and attention. As K.P. developed elbow extension and finger extension, a short thumb postsplint was fabricated for him to use for functional grasp.
It was noted that K.P. had significant pitting edema at his hand and wrist, which would fluctuate and appeared to restrict wrist extension and contributed to poor upper limb, head, and trunk dissociation and clonus in his upper arm. By this stage, K.P. was six months poststroke. Five MEM sessions were provided by the author to assist the primary occupational therapist. At the first session, subluxations were noted at the shoulder and wrist, and there was pitting edema over the dorsum of the hand. The first MEM session focused on treatment to Pump Point 3 and education for K.P.’s partner on the importance of light massage strokes. See Figs. 12-7 and 12-8 in Box 12-1. K.P. and his partner were motivated to continue therapy at home and were taught a basic MEM home program to do at least twice a day.
At the second session, MEM was expanded to include all pump points and the hand. Wrist and elbow extension range increased after the second session to enable reaching to furniture and large objects, and an increase in skin folds was noted. After the third session, there was no pitting edema. His partner reported that they were doing the home program at least once a day and that she was encouraging K.P. to use his hand in activities.
On the fourth session, slight pitting at the MCP joints was noted, and K.P.’s partner reported that they had done less of the home program, so treatment focused on Pump Points 4 and 5, rather than the fingers. Particular emphasis was placed on wrist and hand pump points and treatment of his fingers to facilitate grasp. See Figs. 12-11 and 12-12 for pre- and post-MEM views of K.P.’s hand and body position. These sessions were complemented by facilitated reaching to objects, sliding items, and separation of the forearm flexor and extensor muscle bodies.
By the final session, it was noted that most gains were maintained, and there was no pitting edema. Other benefits included a reduction of overactive skeletal muscle activity at the wrist, and K.P.’s elbow extension lag had improved by 10 degrees. K.P. also reported some improvement in sensation in his hand. Overall, the greatest edema changes were evident at the thumb and index finger. It appeared that the exercises and caregiver education on MEM were of great benefit to K.P. At time of writing, K.P. had achieved a lateral pinch grasp and was working on leisure goals. He and his partner still did MEM at home if his edema increased.
CASE STUDY 3 Chronic Stroke with Neuropathic Pain
T.W. was 56-years-old when he had a left middle cerebral artery stroke, nine months prior to receiving intensive edema treatment. After acute rehabilitation, T.W. was transferred to a specialist neurological rehabilitation ward for one month, primarily to address his expressive and receptive aphasia and apraxia. At discharge, he was independent in ADL, mobility and had developed some communicative skills. The ward multidisciplinary team then referred T.W. for home-based therapy for four weeks, followed by further outpatient occupational therapy.
T.W.’s goals included using cutlery bilaterally, increased ease of bed mobility, to shop and cook independently, and use a computer mouse. T.W. presented with severe expressive aphasia, moderate receptive aphasia, ideational apraxia, and gross upper limb movement, but also poor strength and dexterity, reduced sensation, right sided neuropathic pain, and chronic edema. Formal cognitive assessment was limited due to T.W.’s aphasia; however, reduced speed of processing and reduced scanning efficiency were evident in functional tasks. T.W. also attended outpatient physiotherapy, speech pathology, and an education center.
Prior to his stroke, T.W. lived independently in another state and had retired. He was involved in darts and enjoyed fishing and sports. Poststroke, T.W. decided to move back to the same state as his family and live with his mother. His mother completed the majority of the domestic ADL. T.W. was not motivated to resume any domestic or household maintenance roles himself and was socially isolated. He reported little community involvement and tended to stay home to watch TV or go on the computer. The outpatient team was concerned regarding his loss of roles.
T.W.’s pain and edema continued to limit his rehabilitation, and he was identified as a candidate for intensive MEM at five months poststroke. At initial assessment, T.W. presented with chronic neuropathic pain and dystrophic changes. His mobility and overall task efficiency were limited by his guarded pain postures. Brawny, pitting edema was evident throughout the hand, restricting his function to gross grasp. T.W. reported neuropathic pain down his entire right side and was initially tense during therapist’s contact. He reported less pain as each session progressed and always tolerated light touch.
Treatment on the first session was MEM up to Pump Point 2 and the posterior Big “V” (Fig. 12-13). T.W. was provided with an off-the-shelf Isotoner glove to wear at night and a basic home program of exercises, deep breathing, terminus and axilla massage, and sweeping. Between the first and second sessions, T.W.’s primary occupational therapist used MEM with him and reported reduction of nearly 1 cm at the wrist and MCP joints. On the second session, T.W. reported that he had done his home program once in the four days between sessions and had not used his affected arm in many bilateral tasks. T.W. had significant edema pooling behind his scapula and poor scapula stability during reaching tasks. MEM was conducted to Pump Point 4, and emphasis was placed on low functional reach and shoulder movements. He was also provided with a elastic/cotton stockinette tube to wear with the glove and ideas to increase his hand use.
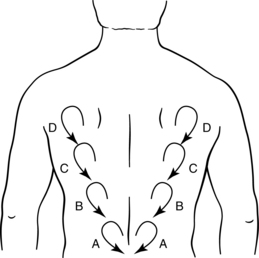
Figure 12-13 Manual Edema Mobilization Posterior “V” “clear” (A through D) and “flow” (D through A).
By the third session and reported daily following of his home program, T.W. no longer had brawny edema, and there was an increase in skin folds, particularly over the dorsal web spaces. MEM was done at all pump points and the posterior Big “V” (see Fig. 12-13). Functional activities were used at the end of treatment, including grasp and release of a cup and practice of a computer mouse. On the fourth session, there was an increase in pretreatment measurements, but this may have been due to the hot weather on that day and an apparent increase in T.W.’s neuropathic pain. The increase in pain only occurred once and reduced with MEM. Light bandaging was also tried overnight to reduce distal edema. On the final session for the case study, T.W. indicated that the bandaging was tolerable, but it did not result in significant measurable change.
After five intensive edema treatment sessions, T.W. had maintained reduction of his wrist and MCP joints edema. Edema at his axilla continued to respond well to treatment, but gains were not maintained between sessions. Treatment involved MEM to all pump points, the hand, the posterior Big “V,” and the neck. Sternal nodes were not treated due to an extensive scar. MEM was progressed gradually due to medical contraindications, although his consultant approved of the intervention. Progress was enhanced by trunk stretches and functional movement within pain, and was limited by aphasia, pain, and learned nonuse. T.W. reported doing his home exercise and MEM program, but only once every few days. Edema changed from brawny to only pitted on the ulnar side of the hand, and the skin at the upper arm had improved color and temperature. There was a slight functional increase in T.W.’s right grip strength, and his elbow extension lag had decreased from 10 degrees to neutral, without any passive ranging or facilitation at the elbow joint. T.W.’s primary occupational therapist continued with once to twice weekly sessions focusing on the above and functional hand use.
T.W. was discharged three months later from all outpatient services, given his limited involvement with home programs and limited progress. However, his pincer grip had improved, and he was able to pick up a glass and use a computer. T.W. was referred to a community-based acquired brain injury service for long-term follow-up, with goals of commencing volunteer work and resuming leisure interests. The occupational therapist recommended that T.W. be referred to a pain specialist as his neuropathic pain contributed to learned nonuse.
Review questions
1. Describe two key points unique to each of the three proposed theories of types of poststroke hand edema.
2. Treatment of poststroke hand edema is often impeded by what other neurological and sensory conditions?
3. Since trauma to the arm or hand poststroke could lead to edema and/or CRPS, list five ways that caregivers and treating staff can prevent trauma to the involved arm.
4. Describe how a functional treatment approach can decrease edema.
1. Artzberger S. Edema reduction techniques. a biological rationale for selection. Cooper C, editor. Fundamentals of hand therapy: clinical reasoning and treatment guidelines for common diagnoses of the upper extremity. St Louis: Mosby/Elsevier, 2006.
2. Artzberger S. A critical analysis of edema control techniques. Am Occup Ther Assoc Physical Disabilities Special Interest Section Quarterly. 2005;28(2):1-3.
3. Artzberger S. Hand manual edema mobilization. overview of a new concept. SAJHT. 2003;1:1. (edoc)
4. Artzberger S. Manual edema mobilization. treatment for edema in the subacute hand. Mackin E, Callahan A, Skirven T, et al, editors. Rehabilitation of the hand and upper extremity, ed 5, St Louis: Mosby, 2002.
5. Artzberger S. Edema control. new perspectives. Am Occup Ther Assoc Physical Disabilities Special Interest Section Quarterly. 1997;20:1.
6. Berne R, Levy M. Physiology, ed 4. St Louis: Mosby; 1998.
7. Boomkamp-Koppen H, Visser-Meily J, Post M, et al. Poststroke hand swelling and oedema. prevalence and relationship with impairment and disability. Clin Rehabil. 2005:19;5:552-559.
8. Braus D, Krauss J, Strobel J. The shoulder-hand syndrome after stroke. a prospective clinical trial. Ann Neurol. 1994:36;5:728-733.
9. Burge E, Kupper D, Finckh A, et al. Neutral functional realignment orthosis prevents hand pain in clients with subacute stroke. a randomized trial. Arch Phys Med Rehabil. 2008:89;10:1857-1862.
10. Burkhardt A. Edema control. In Gillen G, Burkhardt A, editors: Stroke rehabilitation: a function-based approach, ed 2, St Louis: Mosby, 2004.
11. Burkhardt A. High stakes from the clinical horizons in the treatment of lymphedema —to Pump or not pump. Occupational Therapy Forum. 1990;44:1-5.
12. Casley-Smith JR. Modern treatment of lymphoedema. Modern Medicine of Australia. 1992;35(5):70-83.
13. Calsey-Smith JR, Bjorlin M. Some parameters affecting the removal of oedema by massage—mechanical or manual. In: Calsey-Smith JR, Piller NB, editors. Progress in Lymphology X. Adelaide: Univ Adelaide Press, 1985.
14. Casley-Smith JR, Casley-Smith JR. Modern treatment for lymphoedema, ed 5. Adelaide: Lymphoedema Association of Australia; 1997.
15. Casley-Smith JR, Casley-Smith JR. The pathophysiology of lymphoedema and the action of benzo-pyrones in reducing it. Lymphology. 1988;21(3):190-194. 190
16. Casley-Smith JR, Gaffney RM. Excess plasma proteins as a cause of chronic inflammation and lymphodema. quantitative electron microscopy. J Pathol. 1981:133;3:243-272.
17. Chikly B. Silent waves. theory and practice of lymph drainage therapy. Scottsdale, AZ: I.H.H. 2001.
18. Davis PM. Steps to follow with hemiplegia, ed, 2. Berlin: Springer; 2000.
19. Davis PM. Steps to follow. a guide to the treatment of hemiplegia. Heidelberg: Springer-Verlag. 1985.
20. Dirette D, Hinojosa J. Effects of continuous passive motion on the edematous hands of two persons with flaccid hemiplegia. Am J Occup Ther. 1994;48(5):403-409.
21. Exton-Smith A, Crockett D. Nature of oedema in paralysed limbs of hemiplegic patients. BMJ. 1957;2(5056):83-1280.
22. Faghri P. The effect of neuromuscular stimulation-induced muscle contraction versus elevation on hand edema in CVA patients. J Hand Ther. 1997;10(1):29-34.
23. Geurts A, Visschers B, van Limbeek J, Ribbers G. Systematic review of aetiology and treatment of post-stroke hand oedema and shoulder-hand syndrome. Scand J Rehab Med. 2000;32(1):4-10.
24. Gilmore P, Spaulding S, Vandervoort A. Hemiplegic shoulder pain. implications for occupational therapy treatment. Can J Occup Ther. 2004:71;1:36-46.
25. Giudice ML. Effects of continuous passive motion and elevation on hand edema. Am J Occup Ther. 1990;44(10):914-921.
26. Gracies JM, Marosszeky J, Renton R, et al. Short-term effects of dynamic lycra splints on upper limb in hemiplegic patients. Arch Phys Med Rehabil. 2000;81(12):1547-1555.
27. Guyton A, Hall J. Textbook of medical physiology, ed 9. Philadelphia: Saunders; 1996.
28. Hole JW. Human anatomy and physiology, ed 4. Dubuque, Iowa: William C Brown; 1987.
29. Howard S, Krishnagiri S. The use of manual edema mobilization for the reduction of persistent edema in the upper limb. J Hand Ther. 2001;14(4):291-301.
30. Iwata M, Kondo I, Sato Y, et al. Prediction of reflex sympathetic dystrophy in hemiplegia by evaluation of hand edema. Arch Phys Med Rehabil. 2002;83(10):1428-1431.
31. Iwata M, Kondo I, Sato Y. Considerations on hand edema in hemiplegia. Arch Orthop Surg Trauma. 1990;34:4-401.
32. Kasseroller R. Compendium of Dr. Vodder’s manual lymph drainage. Heidelberg, Germany: Haug; 1997.
33. Kondo I, Hosokawa K, Soma M, et al. Protocol to prevent shoulder-hand syndrome after stroke. Arch Phys Med Rehabil. 2001;82(11):1619-1623.
34. Korpelainen J, Sotaniemi K, Myllyla U. Autonomic nervous system disorders in stroke. Clin Autonom Res. 1999;9(6):325-333.
35. Kubik S. Anatomy of the lymphatic system. In: Foldi M, Foldi E, Kubik S, editors. Textbook of lymphology for physicians and lymphedema therapists. Munich: Urban & Fischer, 2003.
36. Kurz I. Textbook of Dr. Vodder’s manual lymph drainage, vol 2, ed 4. Heidelberg, Germany: Haug; 1997.
37. Leduc A, Bastin R, Bourgeois P. Lymphatic reabsorption of proteins and pressotherapies. Partsch H, editor. Amsterdam: Progress in Lymphology-XI. Elsevier, Excerpta Med Int Cong Ser 779. 1988:591-592.
38. Leibovitz A, Baumoehl Y, Roginsky Y, et al. Edema of the paretic hand in elderly post-stroke nursing patients. Arch Gerontol Geriatr. 2007;44(1):37-42.
39. Miller GE, Seale J. Lymphatic clearance during compression loading. Lymphology. 1981;12(161):161-168.
40. Pandyan A, Granat M, Stott D. Effects of electrical stimulation on flexion contractures in the hemiplegic wrist. Clin Rehabil. 1997;11(2):123-130.
41. Pertoldi S, Benedetto P. Shoulder-hand syndrome after stroke. a complex regional pain syndrome. Eura Medicophys. 2005:41;4:283-292.
42. Petrek JA, Pressman PI, Smith RA. Lymphedema. current issues in research and management. CA Cancer J Clin. 2001:50;5:292.
43. Post M, Visser-Meily J, Boomkamp-Koppen H, et al. Assessment of oedema in stroke patients. comparison of visual inspection by therapists and volumetric assessment. Disabil Rehabil. 2003:25;22:1265-1270.
44. Priganc V, Ito M. Changes in edema, pain, or range of motion following manual edema mobilization. a single-case design study. J Hand Ther. 2008:21;4:326-334.
45. Raines J, O’Donnell T, Kalisher L, et al. Selection of patients with lymphedema for compression therapy. Am J Surg. 1977;133(4):430-437.
46. Ring H, Rosenthal N. Controlled study of neuroprosthetic functional electrical stimulation in sub-acute post-stroke rehabilitation. J Rehabil Med. 2005;37(1):32-36.
47. Roper TA, Redford S, Raymond CT. Intermittent compression for the treatment of the oedematous hand in hemiplegic stroke. a randomized controlled trial. Age and Aging. 1999:28;1:9-13.
48. Stanley BG, Tribuzi SM. Concepts in hand rehabilitation. Philadelphia: FA Davis; 1992.
49. Tepperman PS, Greyson ND, Hilbert L, et al. Reflex sympathetic dystrophy in hemiplegia. Arch Phys Med Rehabil. 1984;65(8):442-447.
50. Vasudevan S, Melvin J. Upper extremity edema control. rationale of the techniques. Am J Occup Ther. 1979:33;8:520-524.
51. Wang J, Yang C, Liaw M, et al. Suppressed cutaneous endothelial vascular control and hemodynamic changes in paretic extremities with edema in the extremities of patients with hemiplegia. Arch Phys Med Rehabil. 2002;83(7):1017-1023.
52. Wannapha P, Weiss D, Patel R. Reassessment of the incidence of complex regional pain syndrome type 1 following stroke. Neurorehabil Neural Repair. 2000;14(1):59-63.
53. Waylett-Rendal J, Seibly G. A study of the accuracy of a commercially available volumeter. J Hand Ther. 1991;4(10):10-13.
54. Wee J, Hopman W. Comparing consequences of right and left unilateral neglect in a stroke rehabilitation population. Am J Phys Med Rehabil. 2008;87(11):910-920.
55. Weiss S, Ellis LB. The circulation and unilateral edema in cerebral hemiplegia. J Clin Invest. 1930;9:17-18.
56. Weissleder H, Schuchhardt C. Lymphedema diagnosis and therapy, ed 2. Bonn: Kagerer Kommunikation; 1997.