chapter 16 Managing visual and visuospatial impairments to optimize function*
After completing this chapter, the reader will be able to accomplish the following:
1. Understand how visual information is processed by the central nervous system.
2. Understand how everyday living is affected if visual and spatial impairments are present.
3. Be aware of procedures to perform a visual screening after a brain injury.
4. Implement at least five intervention strategies focused on decreasing activity limitations and participation restrictions for those living with visual and spatial impairments.
“Vision is our dominant sense: More than just sight is measured in terms of visual acuity; vision is the process of deriving meaning from what is seen. It is a complex, learned, and developed set of functions that involve a multitude of skills. Research estimates that eighty to eighty five percent of our perception, learning, cognition and activities are mediated through vision.”41
Visual processing during functional activities
The visual system is commonly impaired after brain damage. Typical visual impairments include visual field deficits, loss of ocular alignment or control, diplopia, and changes in visual acuity.2,47 Further complex impairments include spatial relations impairments as is discussed later, visual agnosia (see Chapter 19), neglect of visual information contralateral to the brain injury (see Chapter 19), and so on. In order for one to use vision to support participation in daily activities, visual information must be correctly received and recognized (Table 16-1).
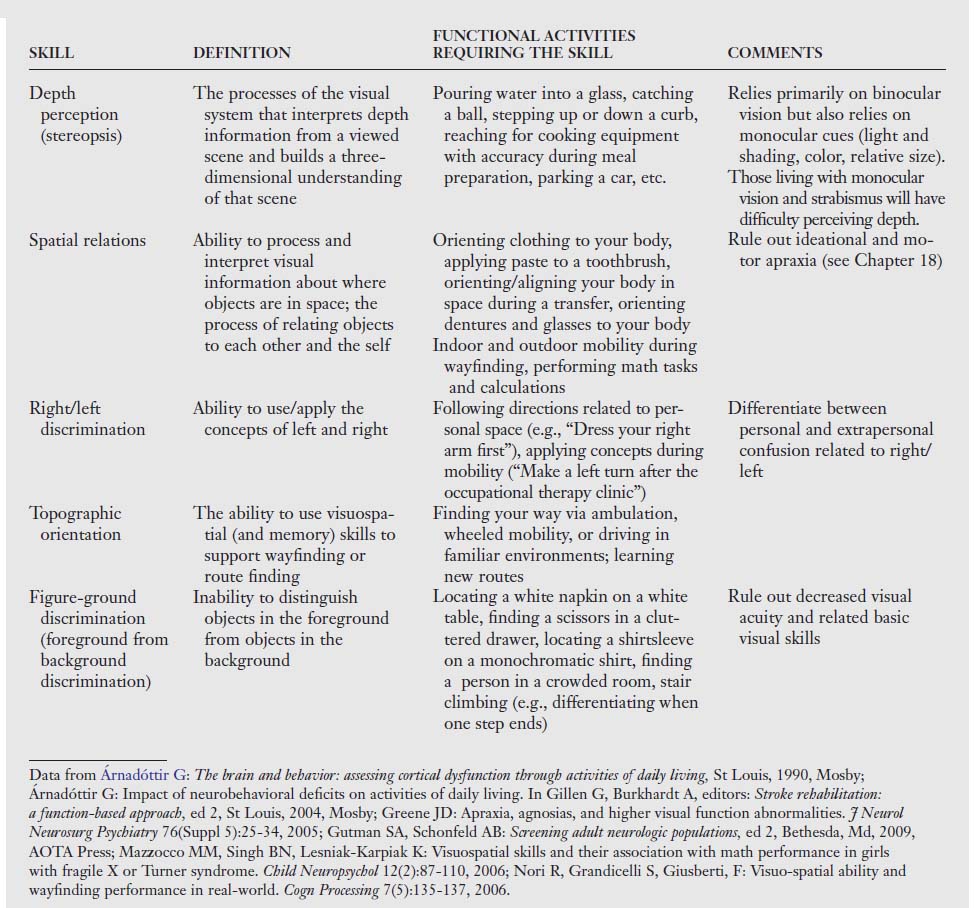
Table 16-1 Visual Skills and Their Associated Functions and Resulting Dysfunctions after Stroke2
| VISUAL SKILL | VISUAL FUNCTION | VISUAL AND PERCEPTUAL DYSFUNCTIONS |
|---|---|---|
| Visual acuity | Clarity of vision at near point and distance; 20/20 refraction | Vision blurred in one or both eyes consistently or inconsistently; visual fatigue; task incompletion |
| Accommodation | Process of focusing whereby the lens changes curvature so that various viewing distances remain clear | Blurred vision; inattention; poor concentration; eyestrain; visual fatigue |
| Visual fields | The peripheral area of vision up, down, in, and out when both eyes are positioned straight forward | Inability to read or starting to read in the middle of the page; ignoring of food on one half of the plate; difficulty orienting to stimuli in specific areas of space |
| Oculomotor range of motion; fixation; saccades and pursuits | Ability of both eyes to move within the six cardinal positions of gaze (right, left, inferior, superior, inferior oblique, superior oblique); maintenance of gaze for 10 seconds; small precise eye jumps; following a moving stimulus | Excessive head movement; frequent loss of place; skipping of lines; poor attention span; slow copying; difficulty when driving, reading, writing; difficulty tracking in all planes |
| Vergence | The ability to bring the eyes together smoothly and automatically along the midline to observe objects singly at near distance (convergence) or to move the eyes outward for single vision of distant objects (divergence) | Difficulty focusing; decreased depth perception; difficulty and confusion in interpreting space; decreased eye-hand coordination in self-care and hygiene; difficulty in driving, sports, communication, and ambulation |
| Strabismus | Deviation of one eye or one eye at a time from the object of regard, where the eye not in use is turned | Esotropia (inward turn); exotropia (outward turn); hyperopia (upward turn); hypopia (downward turn); double vision or suppression; decreased eye-hand coordination during mobility tasks; overreaching or underreaching; difficulty with reading and near tasks |
| Functional scanning | Ability to read or write from left to right precisely and smoothly without errors | Omitting letters, words, numbers; losing place when returning to next line; exaggerated head movement; using finger as pointer; abnormal working distance |
| Color perception | Ability to perceive colors | Muddy or impure color; color may fade out; difficulty finding items by color |
| Stereopsis | Depth perception and its relationship to spatial judgment | Problematic binocular system; deficits in three-dimensional perception; decreased spatial judgment, especially in fine motor areas |
The ultimate function of visual processing is to support participation in daily activities via appropriate motor and/or cognitive response. A relationship exists between visual impairments after acquired brain damage and difficulties with activities of daily living (ADL), increased risk of falls, and poor rehabilitation outcome.17 Visual processing involves a complex system of peripheral and central structures. Compromised integrity of any of the structures impedes functional performance. To illustrate this complexity, the following examination of processing visual information is based on the example of searching for a gallon of milk that is stored in the left side of the refrigerator. Fig. 16-1 outlines the visual pathways within the central nervous system.
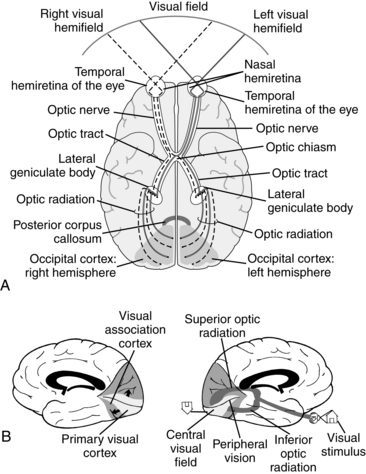
Figure 16-1 The visual pathways. A, Inferior view depicting flow of information from the visual fields to the visual cortex (visual fields = 180 degrees). B, Medial view of components of the visual cortex and visual processing.
(A, From Aloisio L: Visual dysfunction. In Gillen G, Burkhardt A, editors: Stroke rehabilitation: a function-based approach, ed 2, St Louis, 2004, Mosby. B, From Árnadóttir G: The brain and behavior: assessing cortical dysfunction through activities of daily living, St Louis, 1990, Mosby).
Once the refrigerator is opened, a variety of eye movements occur to locate the milk. This usually systematic visual search is supported by rapid intermittent eye movements (saccades) that occur when the eyes fix on one point after another in the visual field. Each eye is controlled by six muscles (Fig. 16-2). These muscles in turn are controlled by three cranial nerves (cranial nerve III or oculomotor, IV or trochlear, VI or abducens).
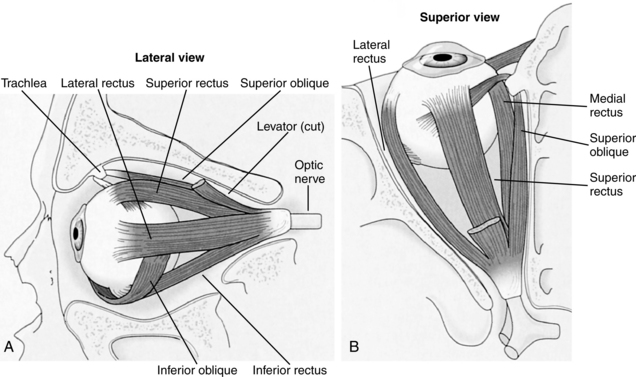
Figure 16-2 The origins and insertions of the extraocular muscles. A, Lateral view of the left eye with the orbital wall cut away. B, Superior view of the left eye with the roof of the orbit cut away.
(From Goldberg ME: The control of gaze. In Kandel ER, Schwartz JH, Jessell TM, editors: Principles of neural science, ed 4, New York, 2000, McGraw-Hill.)
The frontal eye fields within the premotor cortex support visual search and guide gaze shifts. The image “lands” on the nasal hemiretina of the left eye and the temporal hemiretina in the right eye once the milk is located in the left visual field. The information is mobilized posteriorly via the optic nerve. At the point of the optic chiasm, information from the right eye’s temporal hemiretina remains ipsilateral in the right hemisphere, and the information from the left eye’s nasal hemiretina crosses into the right hemisphere.2,58 Therefore, visual information from the left visual field is processed in the right hemisphere. The optic tract projects to the lateral geniculate nucleus of the thalamus because the lateral geniculate nucleus is the principal subcortical structure that carries visual information to the cortex.58 The optic radiation “fans out” and carries the visual information to the primary visual cortex around the calcarine fissure in the occipital lobe.
During the radiation, fibers carrying information from the inferior visual field run posteriorly through the parietal lobe, whereas fibers carrying information from the superior visual field loop around the temporal lobe on their way to the visual cortex in the occipital lobe.2,58 Any lesion in this retino-geniculate-cortical pathway will result in a loss of visual fields (Fig. 16-3). The distribution (e.g., nasal, temporal, inferior, superior, homonymous) of the visual field loss is usually determined by the point of injury. The function of the pathway discussed thus far is to move the visual information from the retina to the cortex, and the direction of flow is primarily anterior to posterior.
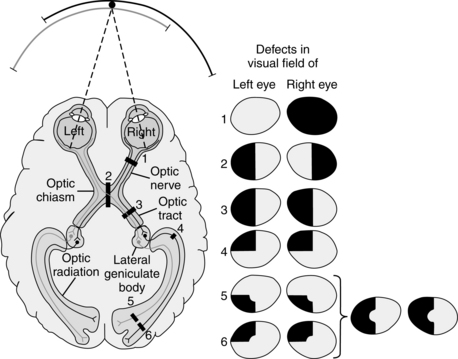
Figure 16-3 Deficits in the visual field produced by lesions at various points in the visual pathway. The level of a lesion can be determined by the specific deficit in the visual field. In the diagram of the cortex, the numbers and the visual pathway indicate the sites of lesions. The deficits that result from lesions at each site are shown in the visual field maps on the right as black areas. Deficits in the visual field of the left eye represent what an individual would not see with the right eye closed rather than deficits of the left visual hemifield. (1) A lesion of the right optic nerve causes a total loss of vision in the right eye. (2) A lesion of the optic chiasm causes a loss of vision in the temporal halves of both visual fields (bitemporal hemianopsia). Because the chiasm carries crossing fibers from both eyes, this is the only lesion in the visual system that causes a nonhomonymous deficit in vision (i.e., a deficit in two different parts of the visual field resulting from a single lesion). (3) A lesion of the optic tract causes a complete loss of vision in the opposite half of the visual field (contralateral hemianopsia). In this case, because the lesion is on the right side, vision loss occurs on the left side. (4) After leaving the lateral geniculate nucleus the fibers representing both retinas mix in the optic radiation. A lesion of the optic radiation fibers that curve into the temporal lobe (Meyer loop) causes a loss of vision in the upper quadrant of the opposite half of the visual field of both eyes (upper contralateral quadrantic anopsia). (5) and (6) Partial lesions of the visual cortex lead to partial field deficits on the opposite side. A lesion in the upper bank of the calcarine sulcus (5) causes a partial deficit in the inferior quadrant of the visual field on the opposite side. A lesion in the lower bank of the calcarine sulcus (6) causes a partial deficit in the superior quadrant of the visual field on the opposite side. A more extensive lesion of the visual cortex, including parts of both banks of the calcarine cortex, would cause a more extensive loss of vision in the contralateral hemifield. The central area of the visual field is unaffected by cortical lesions (5) and (6), probably because the representation of the foveal region of the retina is so extensive that a single lesion is unlikely to destroy the entire representation. The representation of the periphery of the visual field is smaller and hence more easily destroyed by a single lesion.
(From Wurtz RH, Kandel ER: Central visual pathways. In Kandel ER, Schwartz JH, Jessell TM, editors: Principles of neural science, ed 4, New York, 2000, McGraw-Hill.)
At this point the visual information has reached the primary visual cortex in the occipital lobe around the calcarine fissure involved in reception of the visual information. If damage occurs bilaterally around the calcarine fissure, the presentation is usually that of cortical blindness.3,5 Those living with cortical blindness can usually detect lights and movement but otherwise the visual impairment is severe. Following the processing that occurs in the primary visual cortex, the visual information is mobilized to the visual association cortex. Two pathways allow for sophisticated examination of incoming visual information:2,3,5,58
1. The ventral stream or inferior occipitotemporal pathway functions include object recognition via vision, perception of color (e.g., the milk is in a red container), recognition of shapes and forms (the milk is in a rectangular carton), and size discrimination (a quart of milk is smaller than a half gallon). Information from this pathway helps to answer the question, “What am I looking at?”
2. The dorsal stream or the superior occipitoparietal pathway functions include visuospatial perception (the milk is on the top shelf toward the left and behind the butter) and detection of movement. Information from this pathway helps to answer the question: “Where is the object located?”
Visual screening
Several authors have described the components of a vision screening.2,55,56 Prior to developing an intervention plan, a clinician must determine whether difficulties engaging in functional activities are due to a visual deficit, a cognitive or perceptual deficit, or a combination of both. Many dysfunctional behaviors observed or mistakes made during attempts at performing a functional activity can be attributed to one or several underlying impairments that must be differentiated. A person who is having difficulty searching for paperclips in a cluttered drawer may be presenting with poor visual acuity (a decrease in the clarity of vision) versus living with figure-ground impairment (the inability to differentiate foreground from background), necessitating visual acuity testing prior to developing an intervention plan. Similarly, a person who misses the glass when pouring juice from a container may be presenting with a spatial relations impairment related to judging depth or distance versus living with diplopia (double-vision) versus living with monocular vision (information is only obtained via one eye). Finally, not being able to identify an object on a bathroom sink by vision alone may be an issue related to decreased visual acuity versus living with a figure-ground impairment (e.g., not able to identify a white bar of soap on a white sink) versus living with poor contrast sensitivity versus not recognizing the visual information received by the cortex (visual agnosia).
A correlation study of adults who sustained a stroke and received occupational therapy examined the relationship between basic visual functions (defined as acuity, visual field deficits, oculomotor skills, and visual attention or scanning) and higher level visual-perceptual processing skills such as visual closure and figure-ground discrimination. The study suggested that a positive relation exists (r=0.75) between basic visual functions and visual-perceptual processing skills. The authors further concluded that the results suggest that evaluation of visual-perceptual processing skills must begin with assessment of basic visual functions so that the influence of these basic visual functions on performance in more complex tests can be taken into consideration.47 Therefore, it is recommended that a visual screening occur prior to or in conjunction with a full cognitive and perceptual evaluation (Box 16-1). Examples of components of a visual screening include near and far acuity, visual field testing, ocular range of motion or control, ocular alignment, contrast sensitivity, and the like. These skills are often considered the foundation skills for visual processing.2,53,54
Box 16-1 Components of a Vision Screening
The following is a description of vision screening processes, which should be administered in a well-illuminated room free of glare and reflection.
8. Visual Fields: The Confrontation Test
Data from Aloisio L: Visual dysfunction. In Gillen G, Burkhardt A, editors: Stroke rehabilitation: a function-based approach, ed 2, St Louis, 2004, Mosby; Gianutsos R, Suchoff IB: Visual fields after brain injury: management issues for the occupational therapist. In Scheiman M, editor: Understanding and managing vision deficits: a guide for occupational therapists, Thorofare, NJ, 1997, Slack; Gutman SA, Schonfeld AB: Screening adult neurologic populations, ed 2, Bethesda, Md, 2009, AOTA Press; and Warren M: Evaluation and treatment of visual deficits following brain injury. In Pendleton H, Schultz-Krohn W, editors: Pedretti’s occupational therapy: practice skills for physical dysfunction, ed 6, St Louis, 2006, Elsevier Science/Mosby.
Specific visuomotor abilities that should be assessed include the following:
 Fixation: The ability to steadily and accurately gaze at an object of regard (e.g., examining the detail of a painting in a museum).
Fixation: The ability to steadily and accurately gaze at an object of regard (e.g., examining the detail of a painting in a museum).
 Pursuits: The ability to smoothly and accurately track or follow a moving object (e.g., watching your dog run through the yard).
Pursuits: The ability to smoothly and accurately track or follow a moving object (e.g., watching your dog run through the yard).
 Saccades: The ability to quickly and accurately look or scan from one object to another (e.g., reading or watching a soccer game and trying to locate a certain player).
Saccades: The ability to quickly and accurately look or scan from one object to another (e.g., reading or watching a soccer game and trying to locate a certain player).
 Accommodation: The ability to accurately focus on an object of regard, sustain focusing of the eyes, and change focusing when looking at different distances (e.g., maintaining focus when you look from up from a textbook to a clock and back to the textbook).
Accommodation: The ability to accurately focus on an object of regard, sustain focusing of the eyes, and change focusing when looking at different distances (e.g., maintaining focus when you look from up from a textbook to a clock and back to the textbook).
 Vergence: The ability to accurately aim the eyes at an object of regard and to track an object as it moves toward and away from the person (e.g., watching people walking toward you [convergence] and away from you [divergence] in the mall).
Vergence: The ability to accurately aim the eyes at an object of regard and to track an object as it moves toward and away from the person (e.g., watching people walking toward you [convergence] and away from you [divergence] in the mall).
The Brain Injury Visual Assessment Battery for Adults (biVABA)55 is an example of a battery that includes standardized assessments for evaluation of the visual functions important in ensuring that visual perceptual processing is accurately completed:
Managing visual acuity impairments
Assessment of visual acuity has been described in Box 16-1. Visual acuity refers to clarity and sharpness of sight. It is commonly measured using the Snellen chart (or text cards for near acuity) and noted, for example, as 20/20, 20/60, 20/200, and so on. Modifications such as using picture charts or a “tumbling E” chart are available for those with aphasia. A visual acuity of 20/20 means that a person can see detail from 20 feet away the same as a person with normal eyesight would see from the same distance. If a person has a visual acuity of 20/60, that person is said to see detail from 20 feet away the same as a person with normal eyesight would see it from 60 feet away. Visual acuity becomes impaired in various refractive conditions (i.e., impaired focusing of the image on the retina), the most typical being myopia (nearsighted), hyperopia (farsighted), astigmatism (mixed), and presbyopia (age-related decrease in acuity).2 Chia and associates9 found that noncorrectable visual acuity impairment (defined as acuity less than 20/40) was associated with reduced functional status and well-being, as measured by the Medical Outcomes Study Short Form-36 (SF-36) (a measure of quality of life, see Chapter 3). Tsai and colleagues51 documented a relationship between poor visual acuity and depression using the Geriatric Depression Scale. Visual impairment was specifically associated with feelings of worthlessness and hopelessness.
A decrease in visual acuity can result in multiple difficulties in all functional domains. Examples include difficulty reading labels on pill bottles, doing crosswords, unsafe driving, increased fall risk, and so on. A focus on this impairment is warranted to improve participation in daily activities. In general, if visual acuity is worse than 20/40, a referral to an eye care specialist is warranted for evaluation of prescriptive lenses.2 Other interventions are in line with low-vision rehabilitation techniques. They are pragmatic yet effective and have been outlined by Warren:56
 Increase illumination: In general, increasing the amount of light can improve function. Particular attention should be placed on areas of high risk, where activities requiring precision are performed such as cooking, sorting pills into a pill box, and needlework. Task-specific lighting is recommended. Warren warns about maintaining the balance between increasing the amount and intensity of illumination while not increasing glare and recommends halogen, fluorescent, and full-spectrum lights to eliminate casting shadows.
Increase illumination: In general, increasing the amount of light can improve function. Particular attention should be placed on areas of high risk, where activities requiring precision are performed such as cooking, sorting pills into a pill box, and needlework. Task-specific lighting is recommended. Warren warns about maintaining the balance between increasing the amount and intensity of illumination while not increasing glare and recommends halogen, fluorescent, and full-spectrum lights to eliminate casting shadows.
 Increase contrast: Specifically background colors that contrast with objects used for function. Examples are purchasing colored soap to place on a white sink, using dark placemats and white dishes, and placing strips of colored tape on the edge of steps.
Increase contrast: Specifically background colors that contrast with objects used for function. Examples are purchasing colored soap to place on a white sink, using dark placemats and white dishes, and placing strips of colored tape on the edge of steps.
 Decrease background pattern: Increased patterns on household objects can further increase the difficulty of finding necessary objects. For example, finding a white sock on a patchwork quilt is much more difficult than finding the same sock on a solid colored bedspread.
Decrease background pattern: Increased patterns on household objects can further increase the difficulty of finding necessary objects. For example, finding a white sock on a patchwork quilt is much more difficult than finding the same sock on a solid colored bedspread.
 Decrease clutter and organize the environment: A focus should be placed on a having necessary objects placed out neatly and not overlapping.
Decrease clutter and organize the environment: A focus should be placed on a having necessary objects placed out neatly and not overlapping.
 Increase size: Commercially available magnification devices, labeling with bold markers, reprinting instructions or daily planners in larger fonts, changing personal computer settings to a larger font are just a few example of this intervention.
Increase size: Commercially available magnification devices, labeling with bold markers, reprinting instructions or daily planners in larger fonts, changing personal computer settings to a larger font are just a few example of this intervention.
Managing visual field deficits with an emphasis on hemianopsia
The visual fields extend approximately 65 degrees upward, 75 degrees downward, 60 degrees inward, and 95 degrees outward when the eye is in the forward position.15 Aloisio2 summarized that:
 The visual fields are essential areas of the visual system that allow the individual to orient effectively to stimuli in specific areas of space.
The visual fields are essential areas of the visual system that allow the individual to orient effectively to stimuli in specific areas of space.
 In terms of function, they are used when driving, walking, reading, eating, and in all daily living skills.
In terms of function, they are used when driving, walking, reading, eating, and in all daily living skills.
 In terms of impairment, inferior field loss causes difficulty with mobility, including poor balance, tendency to trail behind others when walking, walking next to walls and touching them for balance, trouble seeing steps or curbs, shortened and uncertain stride while walking, and trouble identifying visual landmarks. In addition, superior field deficit causes difficulty in seeing signs, reading, and writing; misreading of words, poor accuracy, slow reading rate, inability to follow lines of text, and inaccurate check writing are additional difficulties.
In terms of impairment, inferior field loss causes difficulty with mobility, including poor balance, tendency to trail behind others when walking, walking next to walls and touching them for balance, trouble seeing steps or curbs, shortened and uncertain stride while walking, and trouble identifying visual landmarks. In addition, superior field deficit causes difficulty in seeing signs, reading, and writing; misreading of words, poor accuracy, slow reading rate, inability to follow lines of text, and inaccurate check writing are additional difficulties.
Hemianopsia, or hemianopia or hemiopia, means “half-blindness” or a loss of half the fields of vision in both eyes.38 Homonymous visual field impairments are seen frequently in the clinic after an acquired brain injury. Thirty percent of all clients with stroke and 70% of those with a stroke involving the posterior cerebral artery present with hemianopsia. In addition, those with subarachnoid hemorrhages, intracerebral bleeds, and head trauma also commonly present with this impairment.34
Zhang and coworkers60 examined the medical records of more than 900 people presenting with visual field loss. The authors found that 37.6% were complete homonymous hemianopsias, whereas 62.4% were incomplete. Homonymous quadrantanopsia (29%) was the most common type of incomplete hemianopsia, followed by homonymous scotomatous defects (13.5%), partial homonymous hemianopsia (13%), and homonymous hemianopsia with macular sparing (7%). The causes of homonymous hemianopsias included stroke (69.6%), head trauma (13.6%), tumor (11.3%), after brain surgery (2.4%), demyelination (1.4%), other rare causes (1.4%), and unknown etiology (0.2%). The authors found that the lesions were most commonly located in the occipital lobes (45%) and the optic radiations (32.2%). Almost every type of hemianopsia was found in all lesion locations along the retrochiasmal visual pathways.
The amount and distribution of visual field loss (i.e., nasal, temporal, inferior, superior, homonymous) depends on the location of the lesion. If the optic nerve itself is damaged (i.e., the area between the retina and the optic chiasm), the presentation will be that of monocular visual loss. Damage to the optic tract will result in contralateral hemianopsia. If damage occurs posterior to the lateral geniculate body, the typical presentation is that of either quadrantanopsia or hemianopsia depending on the lesion site (see Fig. 16-3). Although the characteristics of visual field defects can be helpful in lesion location, specific visual field defects do not always indicate specific brain locations.60
Zihl62 summarized that those living with hemianopsia cannot process visual information as compared with those with intact visual fields. Specifically, they demonstrate numerous visual refixations, have inaccurate saccades and disorganized scanning, require longer visual search times, and omit relevant objects in the environment. In addition, they focus on their intact hemifield; their saccades are less regular, less accurate, and too small to allow rapid, organized scanning or reading.35 The majority of basic and instrumental ADL have the potential to be adversely affected without proper intervention. Reading may be particularly problematic. For example, in those living with a complete right homonymous hemianopsia, rightward saccades during text reading are disrupted (“hemianopsic alexia”), which interrupts the motor preparation of reading saccades during text reading.25
In terms of recovery, Zhang and coworkers59 longitudinally followed 254 clients with homonymous hemianopsia secondary to a variety of brain lesions. The authors documented spontaneous visual field deficit recovery in less than 40% of the cases. They also noted that the likelihood of spontaneous recovery decreased with increasing time from injury to initial visual field testing (p = 0.0003). The probability of improvement was related to the time since injury (p = 0.0003) with a 50% to 60% chance of improvement for cases tested within one month after injury. This chance for improvement decreased to about 20% for cases tested at six months after surgery. In most cases, the improvements occurred within the first three months after injury. The authors warned that spontaneous improvement after six months should be interpreted with caution because it may be secondary to improvement of the disease or to improvement in the client’s ability to perform visual field testing reliably. They recommended that visual field rehabilitation strategies should most likely be initiated early after injury.
The most objective test for mapping the available field is perimetry. This automated test is usually conducted while the person being tested is seated and looking straight ahead at a central target. The person is instructed to press a buzzer when he or she becomes aware of a small light within the visual field. The accuracy of the test depends on the person’s being alert and able to concentrate on the central target. The results from this test are printed out by the computer, objectively mapping blind spots in the visual field. A screening technique that grossly measures the visual fields is a confrontation test, which is described in Box 16-1. Although it is common for hemianopsia to occur in conjunction with neglect, there exists a double dissociation between the two impairments—each can occur separately or can coexist. As compared with those living with neglect, awareness of visual filed deficits tends to be better. Nonetheless, clients may benefit from awareness training to make connections between how this impairment will affect a variety of functional activities and to understand the importance of compensating for it (see Chapter 19).
Several interventions are available to those living with visual field loss. The methods are compensatory in nature. These methods include learning oculomotor compensation strategies, strengthening the person’s attention to the blind hemifield, improving the ability to direct gaze movements toward the involved side, exploring the involved side more efficiently, improving saccadic exploration toward the blind hemifield, using prisms, and so on.*
Some of the most useful approaches to the treatment of hemianopsia are based on compensating for visual field loss by oculomotor compensation. This training involves psychophysical techniques aimed at strengthening the client’s attention to the blind hemifield and improving his or her ability to explore the visual field with saccadic movement.6 Kerkhoff18 suggests three types of saccadic training: train people to make broader searches (“visual search field”) in the blind hemifield, train people to make large-scale eye movements toward the blind hemifield, and train people to make small-scale eye movements with the goal of improving reading.
In terms of specifically training reading, the minimum visual field required for reading is 2 degrees to the left and right of fixation. This is the area where the text is seen clearly and covers 10 to 12 letters of print at a distance of 25 cm. For fluent reading, the visual span must be extended in the reading direction up to 5 degrees or 15 letters. People with hemianopsia need a minimum of 5 degrees to both sides of fixation to read normally. Less than that amount affects people differently based on whether they are living with a right or a left hemianopsia. Less than 5 degrees preempts proper reading of a given line of text by those with right hemianopia and decreases the ability to locate the beginning of the next line of text by those with left hemianopsia.48-50 Those with right hemianopsia tend to perform worse on reading tasks and take longer to respond to treatment. Pambakian and Kennard35 suggest teaching to perceive each word as a whole before reading it. They specifically suggest that those with left hemianopsia should shift their gaze first to the beginning of the line and the first letter of every word in that line. In contrast, those with right-sided hemianopsia are discouraged to read a word before they have shifted their gaze to the end of it. Wang57 reported the case of a 65-year-old woman who presented with a right homonymous hemianopsia secondary to a left occipital lobe tumor. She was most concerned about her inability to read sheet music and developed an effective compensatory strategy to improve her reading ability. By turning her sheet at right angles (i.e., left-to-right became above-to-below), she could read a line almost as well as prior to the loss of vision. Another possible intervention to assist those with hemianopsia to participate fully in reading tasks is to teach the use of a ruler to assist in keeping track of each line of reading and using the ruler to increase the accuracy of the saccadic eye movements.
Specifically training visual search strategies is also recommended. Pambakian and associates36 examined 29 subjects with homonymous visual field deficits. Using a videotape, visual search images were projected on a television in subjects’ homes for 20 sessions over a one-month period. Prior to beginning the search, subjects fixated on a target in the middle of the screen. Random targets were projected among distracters, and subjects indicated when they appeared. During the training they were encouraged to not move their heads. The researchers found that the subjects had significantly shorter mean reaction times related to visual search after training (p < 0.001). The improvements were confined to the training period and maintained at follow-up. In addition, subjects performed ADL tasks significantly faster after training and reported significant subjective improvements. The researchers found no enlargement of the visual field, but there was a small but significant enlargement of the visual search fields. Findings led the authors to conclude that people with homonymous field deficits can improve visual search with practice and that the underlying mechanism may involve the adoption of compensatory eye movement strategies.
Compensatory visual field training has been tested by Nelles and colleagues.31 The authors examined 21 subjects with hemianopsia. Compensatory visual field training was accomplished using a 1.25 by 3.05 m training board with right- and left side-wings. Forty red lights were distributed across the board in four horizontal lines with 10 lights in each line. Clients sat 1.5 m away from the board so that visual fields of subjects were filled out by the board. The subject’s heads were kept midline. When the stimulus of the light was presented, the subjects reacted by pressing a button. Training was carried out under two conditions: (1) subjects were required to fixate on a central point on the board and to react to single visual stimuli, and (2) multiple stimuli were randomly presented on the board. Clients were asked to identify a target stimulus (e.g., square of four lights) in each hemifield with use of exploratory eye movements, but without head movements. Detection of and reaction time to visual stimuli were measured during the two conditions. The subjects showed an improvement of detection and reaction time during condition two, but minimum or no change during condition one. Improvements were maintained eight months after training. ADL skills also improved in all clients. Of note was that the size of scotoma (blind area) on computerized perimetry remained stable. Training improved detection of and reaction to visual stimuli without a change of the visual field impairment.
Pambakian and coworkers34 suggested three steps to improving visual exploration. People with hemianopsia should first practice making large, quick saccades (of amplitude 30 to 40 degrees) into their blind field, to enhance the overshoot of the target. They are then taught to scan for targets among distracters in a systematic way. Finally, these strategies are practiced during real-world activities. These strategies have been tested by Zihl,61 whose subjects increased their visual field searches from 10 to 30 degrees after four to eight sessions. More recently, Kerkhoff and colleagues19 had similar findings after examining 92 people with hemianopsia and 30 with additional neglect. Treatment focused on the practice of large saccades to targets in their blind hemifield. Additional focus was on adopting a systematic scanning strategy, either horizontal or vertical scanning. The subjects also practiced searching for targets on projected slides. Training was carried for 30 sessions, and the mean search field size increased from 15 to 35 degrees in those living with hemianopsia. Those with neglect required 25% more training over two to three months to achieve a similar result. At follow-up, almost two years later, there were no further significant changes. The effect of the treatment was independent of variables such as time since lesion, type of field defect, field sparing, and client age. Two noteworthy findings were that those with more severe impairments benefited most from training and that the mean number of required treatment sessions increased dramatically with the frequency and extent of head movements during training. Pambakian and Kennard35 noted that this finding contradicts the assumption that head movements are helpful to the compensatory mechanisms for those with hemianopsia as is sometimes claimed. The concept of using excessive head movements to compensate for a visual field deficit warrants further investigation.
Optical devices such as prisms also have been used for those with visual field loss. When a prism is applied to glasses, it shifts the peripheral image toward the central area of the retina. Rossi and associates43 examined the effects of using 15-diopter press on Fresnel prisms on subjects with homonymous hemianopsia and neglect. They found significant improvements on impairment tests of visual perception such as the Motor Free Visual Perception Test, Line Bisection, and Letter Cancellation tests. They found no difference in ADL and mobility scores as measured by the Barthel Index. These findings make sense because the improvements were found only in tabletop measures (i.e., measures that by definition do not encompass large visual fields). The visual image is only subtly shifted when wearing a prism, perhaps not enough to make a positive change in activities such as gait or wheelchair mobility, which require broader visual scans. Tabletop ADL have not been objectively tested, but based on these findings perhaps activities such as balancing a checkbook, doing a crossword puzzle, or leisure reading may be positively affected. On the other hand, several problems are related to wearing prisms, including double vision, a potential blocking of the central field, discomfort, disturbances in spatial orientation, and confusion from the distorted visual image. Prisms may consist of a straight-edged segment of press-on prism applied to the side of the field loss on both lenses or round prisms applied to the lens over one eye. Consultation with an optometrist, ophthalmologist, or neuro-opthamologist mandatory.
Managing diplopia
Diplopia, or double vision, is an all too common visual impairment after a neurological event. During intact processing of visual information, when people look at an object with both eyes, the visual image falls on the fovea (a spot located in the center of the macula, which is responsible for sharp central vision) in each eye, and a single image is perceived. When the eyes are not in alignment, the object we are looking at falls on the fovea in one eye and on an extrafoveal location in the other eye. When this occurs, two images are perceived (i.e., binocular diplopia).37,44 Diplopia typically resolves completely with monocular vision (i.e., covering one eye). If diplopia is present with monocular viewing, it is unlikely to be neurological in origin.44 Diplopia may present as the following:11,44
 Horizontal (secondary to impaired abduction or adduction of an eye involving the lateral or medial rectus or both)
Horizontal (secondary to impaired abduction or adduction of an eye involving the lateral or medial rectus or both)
 Vertical (secondary to impaired elevation or depression of the eye)
Vertical (secondary to impaired elevation or depression of the eye)
 Worse in a particular directional gaze (suggestive of ocular motility being impaired in that direction)
Worse in a particular directional gaze (suggestive of ocular motility being impaired in that direction)
 Worse while viewing objects far away (usually found in conjunction with impaired abduction or divergence of the eyes)
Worse while viewing objects far away (usually found in conjunction with impaired abduction or divergence of the eyes)
 Worse while viewing near objects (usually found in conjunction with impaired adduction or convergence)
Worse while viewing near objects (usually found in conjunction with impaired adduction or convergence)
Binocular diplopia is most likely caused by “ocular misalignment” that can be gross or subtle and warrants investigation as to the cause by an optometrist or neuroophthalmologist. The most common causes of misalignment of the visual axes are extraocular muscle dysfunction (see Fig. 16-2).11
Ocular alignment should be evaluated in those living with diplopia. Strabismus, or tropia, is a visible turn of one and may result in double vision. The person is unable to keep the eye straight with the power of fusion. In strabismus one eye may turn outward (exotropia), inward (esotropia), upward (hypertropia), or downward (hypotropia).2 Strabismus may be noncomitant strabismus (the amount of misalignment depends on which direction the eyes are pointed) or comitant (the amount of turn is always the same regardless of whether the person is looking up, down, right, left, or straight ahead). Newly acquired strabismus from a neurological insult is usually noncomitant (i.e., the eye turn changes depend on the direction in which the eyes are looking). Aloiso2 states that “strabismic disorder may result in an inability to judge distance, underreaching or overreaching for objects, covering or closure of one eye, double vision, head tilt or turn, ‘spaced-out’ appearance, difficulty reading, and avoidance of near tasks.” The term phoria is used when there is tendency for the eye to deviate but is controlled with muscular effort. It is not noticeable when a person is focusing on an object.56 The eyes remain straight as long as fusion is present.
In terms of assessing diplopia, scanning assessments such as convergence and ocular range of motion or ocular mobility should be examined to help determine the weak ocular muscle(s).2,15 Ocular mobility and convergence assessments as described in Box 16-1 should be evaluated to determine the available ocular range of motion and the observed range of motion lags. During the assessment, the clinician should be aware of the corresponding muscles responsible for the patterns of movements:
 The medial rectus adducts and rotates the eyes inward.
The medial rectus adducts and rotates the eyes inward.
 The lateral rectus abducts and rotates the eyes outward.
The lateral rectus abducts and rotates the eyes outward.
 The superior rectus uses elevation and intorsion to move the eyes upward.
The superior rectus uses elevation and intorsion to move the eyes upward.
 The inferior rectus uses depression and extorsion to move the eyes downward.
The inferior rectus uses depression and extorsion to move the eyes downward.
 The superior oblique uses depression and intorsion to rotate the eye downward and outward.
The superior oblique uses depression and intorsion to rotate the eye downward and outward.
 The inferior oblique uses elevation and extorsion to rotate the eye upward and outward (see Fig. 16-2).2,14
The inferior oblique uses elevation and extorsion to rotate the eye upward and outward (see Fig. 16-2).2,14
In addition, the cranial nerves that innervate the various muscles should be considered. The lateral rectus is innervated by the abducens nerve (cranial nerve VI). The medial, inferior, and superior recti and the inferior oblique muscles are innervated by the ocular motor nerve (cranial nerve III). The superior oblique muscle is innervated by the trochlear nerve (cranial nerve IV).2,14
Involvement of cranial nerve III results in exotropia, exophoria, convergence insufficiency, accommodative insufficiency, ptosis, and a fixed and dilated pupil. The affected eye is in a down and out position. Damage to the cranial nerve IV results in hypertropia, vertical diplopia, and limited downward gaze. Finally damage to cranial nerve VI manifests as esotropia, esophoria, divergence insufficiency, horizontal diplopia, and limited abduction of the affected eye.2,11
In terms of assessment, the Cover-Uncover Test is based on evoking a fixational eye movement and is appropriate for those living with diplopia. If a person is living with an ocular misalignment, only one of the eyes fixates on the particular object while the other eye deviates. If the fixating eye is covered, the deviating eye must refixate in order to align with the particular object. In the cover-uncover test, the person fixates on a distant object, then covers one eye. The examiner observes whether the uncovered eye makes a fixational movement and notes the direction of the movement. Then the occluder is removed and placed in front of the other eye. Again the examiner observes for fixational movements of the uncovered eye. If both eyes are aligned, no movement will be seen during the cover-uncover test (i.e., the test is negative). A positive test is documented if the uncovered eye moves to take up fixation. If refixation is observed, it can be assumed that under binocular viewing conditions the eye is not aligned with fixation, and a deviation is present. Based on the direction of the affected eyes, movement when the nonaffected eye is covered can indicate the type of misalignment. Inward movement of the uncovered eye indicates an exotropia, whereas an outward movement is an esotropia. A vertical deviation may be either a hypotropia or a hypertropia, depending on whether the eye moves up or down.2,11,56 The Alternate Cover Test is more dissociating than the Cover-Uncover Test, and it may demonstrate phoria more readily.11 In the Alternate Cover Test, the eyes are rapidly and alternately occluded—from one eye to the other and then back again. This procedure causes breakdown of the binocular fusion mechanism and will reveal refixation movements of each eye now of uncovering. If no tropia is present and the uncovered eye shows refixation during the alternate cover test, the client presents with phoria.
Holmes and coworkers16 developed a valid, reliable, and responsive questionnaire to quantify diplopia. This self-report measure asks, “Do you always, sometimes, or never see double?” for seven gaze positions (straight ahead, up, downstairs, right, left, reading, any position). The diplopia questionnaire score then ranges from 0 (no diplopia) to 25 (constant diplopia everywhere) and can easily be rescaled to 0 to 100 by multiplying the score by 4 (Fig. 16-4).
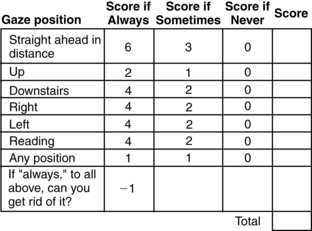
Figure 16-4 Diplopia questionnaire.
(From Holmes JM, Leske DA, Kupersmith MJ: New methods for quantifying diplopia. Ophthalmology 112[11]: 2035-2039, 2005.)
In terms of interventions, the overall goal of managing diplopia is to establish clear and comfortable binocular single vision to support engagement in meaningful activities. A typical way to manage diplopia is to apply a patch (i.e., full occlusion or “pirate patching”) over one eye. This technique does in fact result in single vision but causes several other problems: issues related to cosmesis and self-image, imposed loss of peripheral vision, eye fatigue, rendering the person monocular, mobility impairments, and safety concerns. Therefore, this technique is not recommended for long-term use.
More recently partial visual occlusion has been used. Proper use of partial occlusion can result in comfortable single vision without the negative side effects of full occlusion, particularly preserving peripheral vision. The “spot patch” is a type of partial visual occlusion. It is a round patch made of translucent tape that is placed on the inside of the client’s glasses (corrective or nonprescriptive lens) and directly in the line of sight. The size of the spot patch is approximately 1 cm in diameter, but this varies based on clinical presentation. In general, use the smallest size possible that decreases double vision. The spot patch is effective in eliminating double vision because it blurs central vision in the partially occluded eye.40
Another suggested method for partial visual occlusion is to apply a strip of opaque material such as surgical tape to the nasal field of one eye (i.e., the peripheral field is left unoccluded) over prescriptive or nonprescriptive glasses.56 Similar to the spot patch, this technique results in single vision while sparing the peripheral field. The clinician applies strips of tape systematically to a pair of glasses starting at the nasal field and progressively toward the center until a single image is obtained. In general, when using occlusion as an intervention strategy, the nondominant eye is occluded.56 To determine the nondominant eye, have the person focus on a far target through a 1-inch-diameter hole cut in the center of a piece of white paper. Ask the person to close one eye at a time. Depending on which eye is closed, the target will be visible through the hole. For example, if the person closes the right eye and the left can still see the target through the hole, the left eye is dominant. When the same person closes the left eye while looking through the paper, the target will not be seen with the right eye. Both versions of partial visual occlusion warrant further empirical investigation (Fig. 16-5).
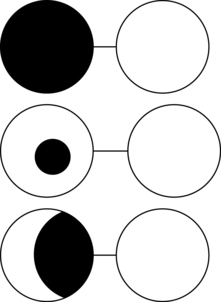
Figure 16-5 Visual occlusion techniques for diplopia. Top: Full visual occlusion (e.g., “pirate patch”) will result in the person seeing one image, but secondary complications include loss of peripheral vision, body image issues, and so on. Middle and lower figures represent partial visual occlusion such as spot patching with translucent tape (middle) and occluding the nasal field of the nondominant eye.
Optical aids such as prisms have been suggested for those with diplopia. Fresnel press-on plastic prisms may be helpful for clients with binocular diplopia up to 40 prism diopters in magnitude. The prisms are available in 1-diopter increments from 1 to 10 and then in 12, 15, 20, 25, 30, 35, and 40 diopters.44 Rucker and Tomsak recommended placing the Fresnel prism in front of the paretic eye and on only one lens of a person’s glasses to minimize blurring of vision. Prisms can be temporary (press-on plastic versions) or permanent (ground into the lens) depending on the trajectory of recovery. Further empirical testing of this intervention related to diplopia that occurs secondary to brain injury is necessary.
The support for eye exercises (orthoptics) in the literature is limited to improving convergence insufficiency.20,45 Scheiman and associates45 compared vision therapy/orthoptics, pencil pushups, and placebo vision therapy/orthoptics as treatments for symptomatic convergence insufficiency in adults ranging from ages 19- to 30-years-old by way of a randomized multicenter trial. The intervention lasted 12 weeks. There were three arms of the trial. The first arm was pencil pushups, in which the subject was instructed to hold a pencil at arm’s length directly between his or her eyes, and an index card was placed on the wall 6 to 8 feet away. Each subject was instructed to look at the tip of the sharpened pencil and to try to keep the pencil point single while moving it toward the nose. If one of the cards in the background disappeared, the person was instructed to stop moving the pencil and blink his or her eyes until both cards were present. The client was told to continue moving the pencil slowly toward the nose until it could no longer be kept single and then to try to regain single vision. If the person was able to regain single vision, he or she was asked to continue moving the pencil closer to the nose. If single vision could not be regained, the client was instructed to start the procedure again. The exercises were performed 20 times, three times per day (approximately 15 minutes per day) for 12 weeks.
In the second arm, the vision therapy/orthoptics group received therapy administered by a trained therapist during a weekly, 60-minute office visit, with additional procedures to be performed at home for 15 minutes a day, five times per week for 12 weeks. The exercise protocol46 included accommodative facility, Brock string exercises, vectograms, computer-assisted orthoptics, and so on.
In the third arm—the placebo office-based vision therapy/orthoptics—clients received therapy administered by a trained therapist during a 60-minute office visit and were prescribed procedures to be performed at home, 15 minutes, five times per week for 12 weeks. The procedures were designed to simulate real vision therapy/orthoptics procedures without the expectation of affecting vergence, accommodation, or saccadic function. Examples included using stereograms monocularly to simulate vergence therapy, computer vergence therapy with no vergence changes, and monocular prism (instead of plus and minus lenses) to simulate accommodative treatment.
The authors found that only clients in the vision therapy/orthoptics group demonstrated statistically and clinically significant changes in the near point of convergence (p = 0.002) and positive fusional vergence (p = 0.001). In addition, clients in all three treatment groups demonstrated statistically significant improvement in symptoms with 42% in office-based vision therapy/orthoptics, 31% in office-based placebo vision therapy/orthoptics, and 20% in home-based pencil push-ups. Although the vision therapy/orthoptics group was the only treatment that produced clinically, more than half of the clients in this group were still symptomatic at the end of treatment; however, their symptoms were significantly reduced.
Rawstron and colleagues42 systematically reviewed the current evidence regarding the efficacy of eye exercises. The authors reviewed 43 refereed studies (14 were clinical trials [10 controlled studies], 18 review articles, two historical articles, one case report, six editorials or letters, and two position statements from professional colleges). Based on their review, the authors summarized that “eye exercises have been purported to improve a wide range of conditions including vergence problems, ocular motility disorders, accommodative dysfunction, amblyopia, learning disabilities, dyslexia, asthenopia, myopia, motion sickness, sports performance, stereopsis, visual field defects, visual acuity, and general well-being. Small controlled trials and a large number of cases support the treatment of convergence insufficiency. Less robust, but believable, evidence indicates visual training may be useful in developing fine stereoscopic skills and improving visual field remnants after brain damage. As yet there is no clear scientific evidence published in the mainstream literature supporting the use of eye exercises in the remainder of the areas reviewed, and their use therefore remains controversial.”
Visuospatial and spatial relations impairments
Participating in daily living tasks in a meaningful and safe manner relies on higher-order visual processing such as perceiving depth, interpreting spatial relations, and differentiating foreground from background, for example. (Table 16-2). Visuospatial impairments are reportedly one of the most common impairments observed after stroke with a prevalence reported as high as 38%.32 These deficits have also been reported in those living with Huntington disease,26 Parkinson disease,28 traumatic brain injury,30 and multiple sclerosis.39
The presence of visuospatial impairments has been associated with a significant increase in falls,33 decreased performance of basic ADL and mobility after stroke as measured by the Barthel Index,32 impairments in both ADL and motor function in those living with Parkinson disease,27 and difficulties with dressing such as putting one’s arm in the correct sleeve52 (Fig. 16-6).
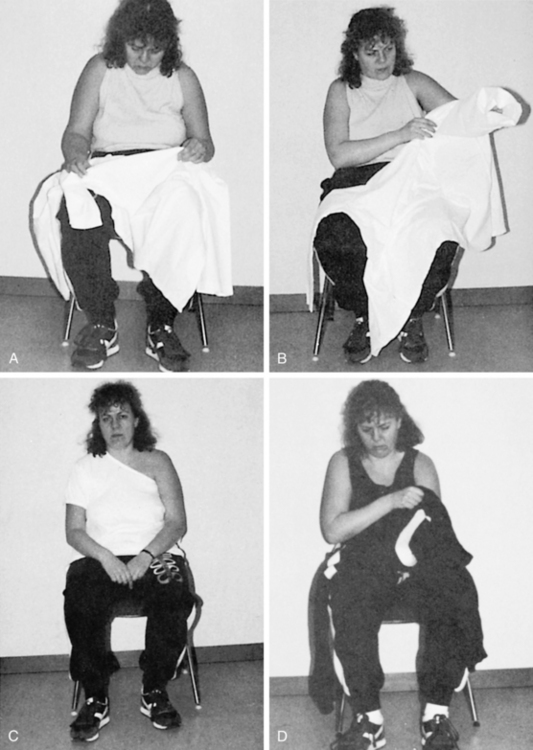
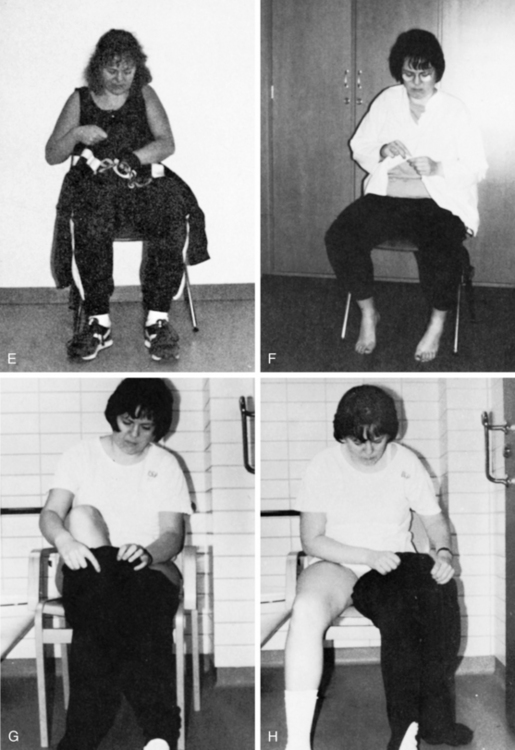
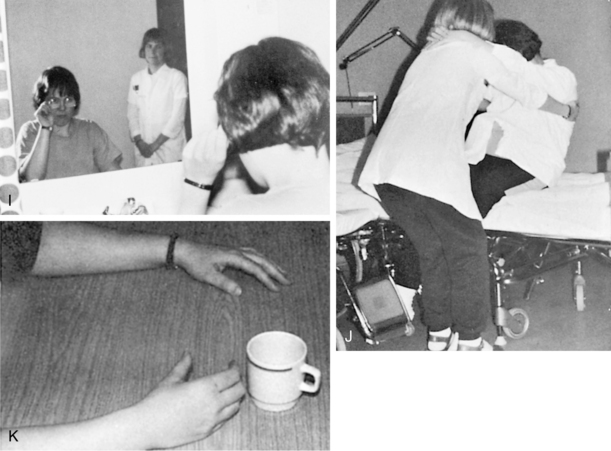
Figure 16-6 Spatial impairments: the effect on everyday living. A, Difficulties in differentiating foreground from background. The client has trouble finding the sleeve of a unicolor shirt. B, The client is unable to find the right armhole. C, The client may start at the wrong hole, placing her arm through the neckhole instead of the left sleeve. D, The client is unable to guide the paralyzed arm into the right hole. Pulling more on the shirt at the top of the arm than under it will result in the arm going past the right hole. This deficit can also be related to perseveration. E, The client’s arm goes through the neckhole instead of the armhole. F, The client matches buttons incorrectly with buttonholes. G, The client puts both legs through the same leghole. H, The client notices that the pants are turned wrong front to back, with the label at the front, and attempts to correct the mistake by turning the pants with the leg in the leg hole. Ideation also interferes with the client’s performance in attempting to correct for the error. See Chapter 18. I, The client puts the glasses on upside down. J, The client leans backward instead of forward while the therapist attempts to transfer her to a wheelchair. Such a client can be dangerous for the therapist if she is unaware of the problem because the client’s actions are unpredictable and often the opposite of what is expected. K, Spatial-relation difficulties manifested in underestimation of distances when reaching for the cup.
(From Árnadóttir G: The brain and behavior: assessing cortical dysfunction through activities of daily living, St Louis, 1990, Mosby.)
A qualitative study22 of those living with visuospatial impairments documented “three main themes comprising six characteristics of how the physical world was experienced in a new, unfamiliar, and confusing way that interfered with the participants’ occupational performance and with their experiences of being an individual ‘self-person.’” Specific everyday problems that the participants reported included confusion related to space and objects, difficulty reaching for objects, feelings that one’s arms were too short, not being able to figure out how to get one’s body into a car, feeling unsafe, familiar objects now being unfamiliar, difficulty finding everyday objects, and difficulties with wheelchair maneuvering, for example.
The majority of common instruments to measure the presence of spatial dysfunction use two-dimensional contrived tasks such as overlapping figures, design copying, and so on. The Motor Free Visual Perception Test (MVPT)10 is only one example of this level of impairment testing. The ability of these types of test to predict performance of everyday tasks performed in context is not clear, and results should be interpreted with caution.8,29 Specifically validity data have not been collected comparing MVPT scores with real-world tasks requiring visual perception.29 For example, a retrospective study examined21 individuals living with a stroke who completed the MVPT and an on-road driving evaluation. The MVPT scores ranged from 0 to 36, with a higher score indicating better visual perception. A structured on-road driving evaluation was performed to determine fitness to drive. A pass or fail outcome was determined by the examiner based on driving behaviors. The author’s results indicated that, using a score on the MVPT of less than or equal to 30 to indicate poor visual-perception and more than 30 to indicate good visual perception, the positive predictive value of the MVPT in identifying those who would fail the on-road test was 60.9%. The corresponding negative predictive value was 64.2%. The authors concluded that the predictive validity of the MVPT is not sufficiently high to warrant its use as the sole screening tool in identifying those who are unfit to undergo an on-road evaluation.21
An error analysis approach has been suggested to document the effects of impairments on daily living skills.3,5,52 The Árnadóttir OT-ADL Neurobehavioral Evaluation (A-ONE)3-5 is one of a select group of standardized assessments that document the effects of spatial impairments on daily living tasks such as mobility, feeding, grooming, and dressing. Specific impairment test items that are scored based on functional observations include spatial relations, visuospatial agnosia, impaired right and left discrimination, and topographic orientation (see Chapter 18). The Assessment of Motor and Process Skills (AMPS)12,13 may be used to document functional limitations of those living with a variety of impairments, including visual and spatial impairments (see Chapter 21). The Structured Observational Test of Function (SOTOF)23,24 is a valid and reliable tool that assesses the following:
 Occupational performance (deficits in simple ADL)
Occupational performance (deficits in simple ADL)
 Performance components (perceptual, cognitive, motor, and sensory impairment)
Performance components (perceptual, cognitive, motor, and sensory impairment)
 Behavioral skill components (reaching, scanning, grasp, sequence)
Behavioral skill components (reaching, scanning, grasp, sequence)
 Neuropsychological deficits (spatial relations apraxia, agnosia, aphasia, spasticity, memory loss)
Neuropsychological deficits (spatial relations apraxia, agnosia, aphasia, spasticity, memory loss)
 Specific visual and spatial impairments (in addition to the above impairments), including figure-ground discrimination, position in space, form constancy, spatial relations, depth and distance perception, visual acuity, visual attention, visual scanning, visual filed loss, and neglect. These impairments are detected by the structured observation of simple ADL (eating from a bowl, pouring a drink and drinking, upper body dressing, washing and drying hands).
Specific visual and spatial impairments (in addition to the above impairments), including figure-ground discrimination, position in space, form constancy, spatial relations, depth and distance perception, visual acuity, visual attention, visual scanning, visual filed loss, and neglect. These impairments are detected by the structured observation of simple ADL (eating from a bowl, pouring a drink and drinking, upper body dressing, washing and drying hands).
This relatively quick tool aims to answer the following questions:
 How does the subject perform ADL tasks?
How does the subject perform ADL tasks?
 What behavioral skill components are intact? Which have been affected by neurological damage?
What behavioral skill components are intact? Which have been affected by neurological damage?
 Which perceptual, cognitive, motor, and sensory impairments are present?
Which perceptual, cognitive, motor, and sensory impairments are present?
Although presented here, the SOTOF is appropriate for a variety of the problem areas.
Despite the prevalence of these impairments and the substantial effect on function, little empirical evidence is available to guide interventions focused on decreasing activity limitations and participation restrictions. It has been suggested that a functional approach is the most appropriate intervention for this population.4,52 This may consist of task-specific training, strategy training, and environmental modifications (Table 16-3). It also has been suggested that interventions that consist of engaging clients in everyday occupations that are presented to challenge the underlying impairment should be incorporated into treatment.1,4,7 Abreu and colleagues1 have proposed an integrated functional approach. In this approach, areas of occupation and context are used to challenge processing skills. With this integrated functional approach, treatment may be focused on a subcomponent skill such as spatial, but daily occupations are used as the modality. Box 16-2 lists potential activity choices.
Table 16-3 Potential Strategies to Improve Function in Those Living with Visuospatial Impairments
| DOMAIN OF FUNCTION | POTENTIAL INTERVENTIONS* |
|---|---|
| Dressing | Deemphasize visual demonstrations during dressing training. Focus on verbal descriptions to retrain the task. Decrease the use of spatial-based language (i.e., “under,” “over,” “right,” “left,” “behind”) when teaching dressing skills. For example, instead of saying “Your left arm is in the right sleeve,” say “Wrong sleeve” or “Other sleeve.” Use cues that facilitate insight into the spatial impairment and that assist in strategy development. For example, if a person puts on the shirt backward, start with a general cue such as, “Are you sure you are finished?” then progress to more specific cues. Use clothing that provides cues that can be used to orient the article of clothing to the body. A monochromatic blue T-shirt may be more difficult to orient correctly compared with a baseball jersey in which the sleeves are a different color than the body of the shirt. Teach spatial orientation strategies before the client starts to dress, for example, using the label to differentiate front from back or finding a decal on the front shirt. Use an audiotape (i.e., does not rely on visual skills) to cue the sequence of dressing. The therapist should sit next to and parallel to the person relearning how to dress so that they are working in the same spatial plane. |
| Meal preparation | Use tactile feedback to increase accuracy when reaching for needed objects (e.g., slide hand across the counter to reach for a pot). Decrease clutter. Keep drawers organized to improve foreground from background discrimination. Use contrasting colors such as dark dishes on a white counter and vice versa. Label or color code needed items or ingredients that are difficult to recognize. Organize the kitchen so that cooking equipment is always in the same place. This decreases the amount of time spent search and locating objects. Place a piece of colored tape at the edge of the countertop. Place colored tape on the handle of the refrigerator and stove controls to ease in spatial localization. Use tactile cues before pouring. For example, find the lip of the measuring cup by touch before pouring oil into it. Encourage the person to work slowly to ensure safety. Label cabinets based on contents. |
* May be applied to other functional domains as well; all require further empirical testing.
Box 16-2 Examples of Functional Activities Presumed to Challenge Visuospatial Skills* Based on Activity Analysis
Reaching for groceries on shelves of varying distances
Wayfinding/route finding in familiar and new environments
Sports activities such as playing catch, basketball, or golf
Organizing a workspace such as desk or kitchen counter
* Note: This relationship requires further empirical testing.
Review questions
1. Name three compensatory interventions that may be used for a person with decreased performance in grooming secondary to spatial impairment.
2. What are the components of a visual screening?
3. Describe the clinical reasoning process to determine why a person cannot locate a spoon in a utensil drawer.
4. Describe three different methods of visual occlusion that may be used with a person presenting with diplopia.
5. What are the potential impairments and the effect on function if a person develops a pathology that adversely affects the dorsal stream (occipitoparietal pathway)?
1. Abreu, Duval M, Gerber D, et al. Occupational performance and the functional approach. In: Royeen CB, editor. AOTA self-study series: cognitive rehabilitation. Rockville, MD: American Occupational Therapy Association, 1994.
2. Aloisio L. Visual dysfunction. In Gillen G, Burkhardt A, editors: Stroke rehabilitation: a function-based approach, ed 2, St Louis: Mosby, 2004.
3. Árnadóttir G. The brain and behavior. assessing cortical dysfunction through activities of daily living. St Louis: Mosby. 1990.
4. Árnadóttir G. Clinical reasoning with complex perceptual impairment. In: Unsworth C, editor. Cognitive and perceptual dysfunction: a clinical reasoning approach to evaluation and intervention. Philadelphia: FA Davis, 1999.
5. Árnadóttir G. Impact of neurobehavioral deficits on activities of daily living. In Gillen G, Burkhardt A, editors: Stroke rehabilitation: a function-based approach, ed 2, St Louis: Mosby, 2004.
6. Bolognini N, Rasi F, Coccia M, et al. Visual search improvement in hemianopic clients after audio-visual stimulation. Brain. 2005;128(Pt 12):2830-2842.
7. Brockmann-Rubio K, Gillen G. Treatment of cognitive-perceptual impairments. a function-based approach. Gillen G, Burkhardt A, editors. Stroke rehabilitation: a function-based approach, ed 2, St Louis: Elsevier Science/Mosby, 2004.
8. Brown GT, Rodger S, Davis A. Motor-Free Visual Perception Test-Revised. an overview and critique. Br J Occup Ther. 2003:66;4:159-167.
9. Chia EM, Wang JJ, Rochtchina E, et al. Impact of bilateral visual impairment on health-related quality of life. the Blue Mountains Eye Study. Invest Ophthalmol Vis Sci. 2004:45;1:71-76.
10. Colarusso RP, Hammill DD. Motor-free visual perception test, ed 3. Novato, CA: Academic Therapy Publications; 2003.
11. Danchaivijitr C, Kennard C. Diplopia and eye movement disorders. J Neurol Neurosurg Psychiatry. 2004;75(Suppl 4):24-31.
12. Fisher AG. Assessment of motor and process skills. vol. 1. development, standardization, and administration manual, ed 5. Fort Collins, CO: Three Star Press. 2003.
13. Fisher AG. Assessment of motor and process skills. vol. 2. user manual, ed 5. Fort Collins, CO: Three Star Press. 2003.
14. Goldberg ME. The control of gaze. In Kandel ER, Schwartz JH, Jessell TM, editors: Principles of neural science, ed 4, New York: McGraw-Hill, 2000.
15. Gutman SA, Schonfeld AB. Screening adult neurologic populations, 2nd ed. Bethesda, MD: AOTA Press; 2009.
16. Holmes JM, Leske DA, Kupersmith MJ. New methods for quantifying diplopia. Ophthalmology. 2005;112(11):2035-2039.
17. Jones SA, Shinton RA. Improving outcome in stroke patients with visual problems. Age Ageing. 2006;35(6):560-565.
18. Kerkhoff G. Neurovisual rehabilitation. recent developments and future directions. J Neurol Neurosurg Psychiatry. 2000:68;6:691-706.
19. Kerkhoff G, Münssinger U, Haaf E, et al. Rehabilitation of homonymous scotomas in clients with postgeniculate damage of the visual system. saccadic compensation training. Restor Neurol Neurosci. 1992;4:245-254.
20. Kerkhoff G, Stogerer E. Recovery of fusional convergence after systematic practice. Brain Inj. 1994;8(1):15.
21. Korner-Bitensky NA, Mazer BL, Sofer S, et al. Visual testing for readiness to drive after stroke. a multicenter study. Am J Phys Med Rehabil. 2000:79;3:253-259.
22. Lampinen J, Tham K. Interaction with the physical environment in everyday occupation after stroke. a phenomenological study of persons with visuospatial agnosia. Scand J Occup Ther. 2003:10;4:147-156.
23. Laver AJ. Clinical reasoning with simple perceptual impairment. In: Unsworth C, editor. Cognitive and Perceptual Dysfunction: A Clinical Reasoning Approach to Evaluation and Intervention. Philadelphia: FA Davis, 1999.
24. Laver AJ. The structured observational test of function. Gerontol Special Interest Sec Newslet. 17(1), 1994.
25. Leff AP, Scott SK, Crewes H, et al. Impaired reading in clients with right hemianopia. Ann Neurol. 2000;47(2):171-178.
26. Lemiere J, Decruyenaere M, Evers-Kiebooms G, et al. Cognitive changes in clients with Huntington’s disease (HD) and asymptomatic carriers of the HD mutation. a longitudinal follow-up study. J Neurol. 2004:251;8:935-942.
27. Maeshima S, Itakura T, Nakagawa M, et al. Visuospatial impairment and activities of daily living in clients with Parkinson’s disease. a quantitative assessment of the cube-copying task. Am J Phys Med Rehabil. 1997:76;5:383-388.
28. Marinus J, Visser M, Verwey NA, et al. Assessment of cognition in Parkinson’s disease. Neurology. 2003;61(9):1222-1228.
29. McCane SJ. Test review. motor-free visual perception test. J Psychoeduc Assess. 2006:24;3:265-272.
30. McKenna K, Cooke DM, Fleming J, et al. The incidence of visual perceptual impairment in clients with severe traumatic brain injury. Brain Inj. 2006;20(5):507-518.
31. Nelles G, Esser J, Eckstein A, et al. Compensatory visual field training for clients with hemianopia after stroke. Neurosci Lett. 2001;306(3):189-192.
32. Nys GM, van Zandvoort MJ, de Kort PL, et al. Cognitive disorders in acute stroke. prevalence and clinical determinants. Cerebrovascular Dis. 2007:23;5–6:408-416.
33. Olsson RHJr, Wambold S, Brock B, et al. Visual spatial abilities and fall risk. an assessment tool for individuals with dementia. J Gerontol Nurs. 2005:31;9:45-53.
34. Pambakian A, Currie J, Kennard C. Rehabilitation strategies for clients with homonymous visual field defects. J Neuroophthalmol. 2005;25(2):136-142.
35. Pambakian AL, Kennard C. Can visual function be restored in clients with homonymous hemianopia. Br J Ophthalmol. 1997;81(4):324-328.
36. Pambakian AL, Mannan SK, Hodgson TL, et al. Saccadic visual search training. a treatment for clients with homonymous hemianopia. J Neurol Neurosurg Psychiatry. 2004:75;10:1443-1448.
37. Pearce JM. Diplopia. Eur Neurol. 2005;53(1):54.
38. Pearce JM. Hemianopia. Eur Neurol. 2005;53(2):111.
39. Piras MR, Magnano I, Canu ED, et al. Longitudinal study of cognitive dysfunction in multiple sclerosis. neuropsychological, neuroradiological, and neurophysiological findings. J Neurol Neurosurg Psychiatry. 2003:74;7:878-885.
40. Politzer T. Visual function, examination, and rehabilitation in clients suffering from traumatic brain injury. In: Jay GW, editor. Minor traumatic brain injury handbook. Boca Raton, FL: CRC Press, 2000.
41. Politzer T. Introduction to vision and brain injury, (website). www.nora.cc/client_area/vision_and_brain_injury.html, May 1, 2007. Accessed
42. Rawstron JA, Burley CD, Elder MJ. A systematic review of the applicability and efficacy of eye exercises. J Pediatr Ophthalmol Strabismus. 2005;42(2):82-88.
43. Rossi PW, Kheyfets S, Reding MJ. Fresnel prisms improve visual perception in stroke clients with homonymous hemianopia or unilateral visual neglect. Neurology. 1990;40(10):1597-1599.
44. Rucker JC, Tomsak RL. Binocular diplopia. A practical approach. Neurologist. 2005;11(2):98-110.
45. Scheiman M, Mitchell GL, Cotter S, et al. A randomized clinical trial of vision therapy/orthoptics versus pencil pushups for the treatment of convergence insufficiency in young adults. Optom Vis Sci. 2005;82(7):583-595.
46. Scheiman M, Wick B. Clinical management of binocular vision. Heterophoric, accommodative and eye movement disorders, ed 2. Philadelphia: Lippincott Williams & Wilkins. 2002.
47. Suchoff IB, Kapoor N, Waxman R, et al. The occurrence of ocular and visual dysfunctions in an acquired brain-injured client sample. J Am Optom Assoc. 1999;70(5):301-308.
48. Trauzettel-Klosinski S. Reading disorders due to visual field defects-a neuro-ophthalmological view. Neuroophthalmology. 2002;27(1):79-90.
49. Trauzettel-Klosinski S, Brendler K. Eye movements in reading with hemianopic field defects. the significance of clinical parameters. Graefes Arch Clin Exp Ophthalmol. 1998:236;2:91-102.
50. Trauzettel-Klosinski S, Reinhard J. The vertical field border in hemianopia and its significance for fixation and reading. Invest Ophthalmol Vis Sci. 1998;39(11):2177-2186.
51. Tsai SY, Cheng CY, Hsu WM, et al. Association between visual impairment and depression in the elderly. J Formos Med Assoc. 2003;102(2):86-90.
52. Walker CM, Sunderland A, Sharma J, et al. The impact of cognitive impairment on upper body dressing difficulties after stroke. a video analysis of patterns of recovery. J Neurol Neurosurg Psychiatry. 2004:75;1:43-48.
53. Warren M. A hierarchical model for evaluation and treatment of visual perceptual dysfunction in adult acquired brain injury, part 1. Am J Occup Ther. 1993;47(1):42-54.
54. Warren M. A hierarchical model for evaluation and treatment of visual perceptual dysfunction in adult acquired brain injury, part 2. Am J Occup Ther. 1993;47(1):55-66.
55. Warren M. Brain injury visual assessment battery for adults. Birmingham, UK: visABILITIES Rehab Services; 1999.
56. Warren M. Evaluation and treatment of visual deficits following brain injury. In Pendleton H, Schultz-Krohn W, editors: Pedretti’s occupational therapy: practice skills for physical dysfunction, ed 6, St Louis: Elsevier/Mosby, 2006.
57. Wang MK. Reading with a right homonymous haemianopia. Lancet. 2003;361(9363):1138.
58. Wurtz RH, Kandel ER. Central visual pathways. In Kandel ER, Schwartz JH, Jessell TM, editors: Principles of neural science, ed 4, New York: McGraw-Hill, 2000.
59. Zhang X, Kedar S, Lynn MJ, et al. Homonymous hemianopias. clinical-anatomic correlations in 904 cases. Neurol. 2006:66;6:906-910.
60. Zhang X, Kedar S, Lynn MJ, et al. Natural history of homonymous hemianopia. Neurology. 2006;66(6):901-905.
61. Zihl J. Neuropsychologische rehabilitation. In: Von Cramon D, Zihl J, editors. Neuropsychologische rehabilitation: grudlagen, diagnostic, behandlungsverfahren. Berlin: Springer-Verlag, 1988.
62. Zihl J. Visual scanning behavior in clients with homonymous hemianopia. Neuropsychologia. 1995;33(3):287-303.