LIGNANS AND LIGNIN
Lignans are dimeric compounds formed essentially by the union of two molecules of a phenylpropene derivative. At one time it was thought that these compounds were early intermediates in the formation of lignin but it is now recognized that they are offshoots of the principal lignin biosynthetic pathway. Unlike lignin they are optically active compounds and probably arise by stereospecific, reductive coupling between the middle carbons of the side-chain of the monomer. Some 300 lignans have been isolated and categorized into a number of groups according to structural features. Important pharmaceutical examples are the lignans of Podophyllum spp. (q.v.) which appear to be formed from two molecules of coniferyl alcohol or the corresponding acid with subsequent modification; apparently, a sinapic acid derivative, as might be expected by the inspection of the podophyllotoxin molecule, is not involved.
Some medicinal plants which contain lignans and which illustrate some of the structural types of this class of compound are given in Table 21.7. The lignans cited are not, however, necessarily the therapeutically active constituents of the plant.
Table 21.7 Occurrence of lignans in medicinal plants.
| Species | Lignans | Notes |
|---|---|---|
| Guaiacum officinale, G. sanctumSource of Guaiacum Resin |
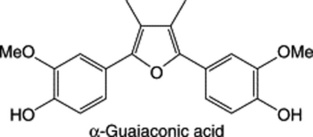 |
A furano-type lignan. See ‘Guaiacum resin’ for other details. (+)-Neo-olivil of similar structure is the principal lignan of Urtica dioica |
| Myristica fragrans (Nutmeg) |
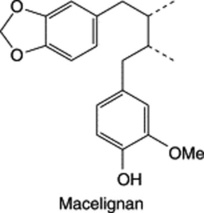 |
A dibenzylbutane-type lignan |
| Piper cubeba (Tailed pepper) |
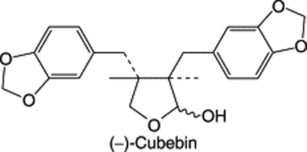 |
A tetrahydrofuran-type lignan. Fruits contain c. 2% of (−)-cubebin together with related compounds |
| Podophyllum spp. |
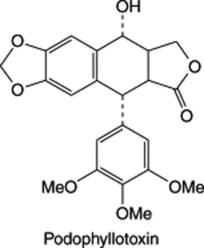 |
This aryltetralin-type lignan and other related compounds are the principal active constituents of Podophyllum root and rhizome (see Chapter 27). Also found in linseed, q.v. |
| Schisandra chinensis |
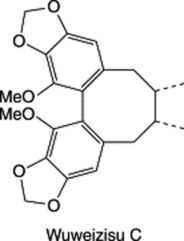 |
A dibenzocyclooctadiene-type lignan. This and other lignans of the same class have antihepatotoxic activity—see Chapter 29. Similar compounds are found in Korean Red Ginseng, q.v. |
| Silybum marianum | Silybin and others | Flavonolignans (see Chapter 29) |
| Urtica dioica (Stinging nettle) | Neo-olivil derivatives | Tetrahydrofuran-type lignans |
| Viscum album (Mistletoe) Eleutherococcus senticosus (Acanthopanax senticosus) |
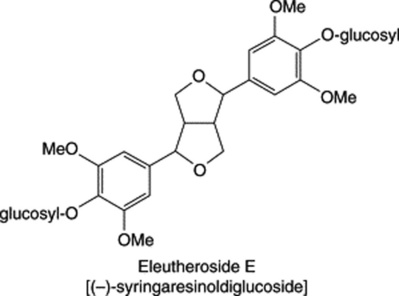 |
A tetrahydrofurofuran-type lignan. Occurs in a number of plants together with related compounds including eleutheroside D, the diastereoisomer |
| Zanthoxylum clava-herculis (Prickly ash bark) | (+)-Asarinin | A tetrahydrofurofuran-type lignan. Occurs in prickly ash bark together with alkaloids, coumarins, amides, resins etc. Plants of this genus used in Western, Indian and Chinese herbal medicine |
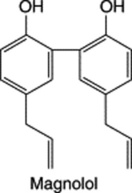
Neolignans are also derived from the same units as lignans but the C6–C3 moieties are linked head to tail or head to head and not through the β-β′ carbons. They occur in the heart-woods of trees of the Magnoliaceae, Lauraceae and Piperaceae. Magnolia officinalis and M. obovata are used in Chinese medicine. Magnolol, a neolignan isolated from the bark, has the following reported activities: CNS depressant and muscle relaxant, antiplatelet, antimicrobial, antitumour, anticancer, insecticidal, etc. (S. D. Sarker, Fitoterapia, 1997, 63, 3).
Lignin is an important polymeric substance, (C6–C3)n, laid down in a matrix of cellulose microfibrils to strengthen certain cell walls. It is an essential component of most woody tissues and involves vessels, tracheids, fibres and sclereids.
Lignins from different biological sources vary in composition, depending on the particular monomeric units of which they are composed (see Fig. 21.2). Variations in lignin constitution also arise as a result of random condensations of the appropriate alcohols with mesomeric free radicals formed from them by the action of a laccase-type (oxidase) enzyme. As there is no template for this non-enzymic condensation the lignin molecules formed vary in structure and so it is not possible to isolate lignin as a compound of defined composition.
The diagnostic value of lignin in crude drug analysis is covered in Chapter 42.