Chapter 27 The search for naturally derived anticancer agents*
In the endeavour to discover effective drugs for the treatment of various cancerous diseases, the natural kingdoms, especially the plant kingdom, have been extensively researched. The research involved has been enormous and although the number of successful outcomes appears very modest, the effective drugs produced rank among the most common chemotherapeutic agents employed. Also, the wide diversity and complexity of the compounds isolated have afforded valuable material for the manufacture of semi-synthetic derivatives, often less toxic and clinically superior to the original isolate.
It has been estimated (2005) that over 60% of the anticancer drugs in current use are in some way derived from plants and microorganisms; marine products are in the process of evaluation. A successful anticancer drug should kill or incapacitate cancer cells without causing excessive damage to normal dividing cells. This ideal is difficult, or perhaps impossible, to attain and is why cancer patients frequently suffer unpleasant side-effects when undergoing treatment.
PLANTS IN CANCER TREATMENT
Plant materials have been used in the treatment of malignant diseases for centuries; a comprehensive survey of the literature describing plants used against cancer listed over 1400 genera (Hartwell, 1967–71, see Lloydia, 1971, 34, 427 for index). Recent phytochemical examination of plants which have a suitable history of use in folklore for the treatment of cancer has indeed often resulted in the isolation of principles with antitumour activity. Podophyllum was used over 2000 years ago by the ancient Chinese as an antitumour drug, and resins from the root of the plant Podophyllum hexandrum (syn. P. emodi) and the related American species, the May-apple (P. peltatum) have yielded a number of lignans and their glycosides having antitumour activity. Although the major constituents from these two species, podophyllotoxin and the peltatins, are unsuitable for systemic drug use, two semi-synthetic derivatives of podophyllotoxin, etoposide and teniposide, gave particularly good results in clinical trials. Etoposide is currently available for the treatment of small-cell lung cancer and testicular cancer, and teniposide is used in paediatric cancers, though both compounds have a similar anticancer spectrum. Other podophyllotoxin-related analogues have been developed and tested. Podophyllotoxin itself may be used topically, and is most effective in the treatment of venereal warts. From the time of Galen (about AD 180), the juice expressed from woody nightshade (Solanum dulcamara) has been used to treat cancers, tumours and warts, and references to its use have appeared in the literature of many countries. The active tumour-inhibitory principle has been identified as the steroidal alkaloid glycoside β-solamarine. Various lichens, e.g. species of Cladonia, Cetraria and Usnea, also have a history of use in folk medicine against cancer since about AD 970. These are all rich sources of usnic acid, a compound which has been recognized for many years as an antibacterial and antifungal agent, but only more recently as an antitumour compound. Similarly, many centuries ago, the druids claimed that mistletoe (Viscum album) could be used to cure cancer; protein fractions with marked antitumour activity have been isolated from mistletoe extract. Mezereon (Daphne mezereum), despite its toxic properties, has also been used in many countries for the treatment of cancer. The active antitumour constituent of this plant has been identified as a diterpene derivative mezerein, which is structurally very similar to the toxic principle daphnetoxin.
Very successful higher plant materials used in cancer chemotherapy are the alkaloids of Catharanthus roseus. Research on this plant, the Madagascan periwinkle, was stimulated by its mention in folklore,not as a cure for cancer, but in the treatment of diabetes. No hypoglycaemic activity was detected, but treated test animals became susceptible to bacterial infection, and this led the researchers to undertake extensive examination for possible immunosuppressive principles causing these effects. A number of bisindole alkaloids showing antileukaemic activity have subsequently been isolated and two of these, vincaleukoblastine (vinblastine) and leurocristine (vincristine), are now extracted commercially from Catharanthus roseus and used, either alone, or in combination with other forms of therapy for cancer treatment. Another important, more recent, addition to the list of anticancer drugs is paclitaxel (Taxol), a diterpene derivative isolated initially from the bark of the Pacific Yew, Taxus brevifolia. Although reportedly used by Native North Americans for various conditions, it does not appear to have had any traditional cancer usage and was obtained as part of the random collection programme of the US National Cancer Institute (NCI). Taxol and related taxanes are treated below.
METHODS OF INVESTIGATION
An intensive survey of plants, microorganisms and marine animals (starfish, corals, etc.) for antitumour activity began in the late 1950s, mainly because the United States National Cancer Institute (NCI) instigated and funded a major screening programme. A random- selection screening programme was adopted, since novel compounds may be found anywhere in the plant or animal kingdom, and it is known that some natural products are restricted to a single genus, or even species. Random mass-screening is naturally an expensive operation, and probably only justified in certain areas, where our present range of drugs is seriously inadequate or inefficient. Cancer was considered to be in this category.
Since the beginning of the programme, which continued until 1983, a vast number of extracts from various sources has been tested for antitumour activity. About 4% of the extracts tested have shown reproducible activity. Over about 25 years, some 114 000 plant samples representing 40 000 species were tested. Different parts of a plant—seeds, leaves, roots, etc.—were separately examined wherever possible. It is estimated that between a quarter and half a million plant species exist worldwide, and thus the plant kingdom still represented a vast untapped source of material.
The isolation of biologically active constituents, probably minor constituents, from a crude plant extract involves techniques differing from those of conventional phytochemical evaluation. With these, it was customary to study those chemicals which were most easily separated from a plant extract; these were usually those present in the largest quantities and which crystallized readily, or those which represented the researcher’s field of interest, e.g. alkaloids, terpenoids, phenols, etc. Only after characterization of their structures were such compounds subjected to biological testing, e.g. for hypotensive, antibacterial, anticancer activities, etc., and this would depend on sufficient material being available. Countless medicinally useful compounds have been missed in this type of approach.
In the more recent systematic studies for useful plant constituents, every portion of the plant and every fraction of the extract is tested biologically before any constituent is isolated and characterized. Usually only those fractions showing biological activity are studied further. Thus, one may isolate almost any class of compound as an active constituent, and it may not be one traditionally associated with a particular plant family. Even procedures involving continuous monitoring of fractions for biological activity are not free from anomalies. It is quite well known that isolated constituents of a plant drug may not give the same clinical response as a crude preparation of that plant drug. Very often, the total therapeutic activity is greater than, or different from the therapeutic activities of the individuals. Synergism or antagonism (see Chapter 7) due to the complex nature of the extract are probably the causes of such observations. It is thus possible that a fraction from a plant extract, although showing significant biological activity, possesses no single constituent with this activity. Conversely, a fraction showing no activity may still contain an active constituent. A further complication is that crude fractions may contain additional substances with delayed toxicity, causing test animals to die at about the same time as control animals.
An effective screening procedure in which a large number of crude plant extracts are to be assayed for biological activity should fulfil several criteria. It should be sufficiently selective to limit the number of leads for follow-up evaluation yet it should be highly sensitive in order to detect low concentrations of active compounds, and it should be specific so that the assay is not affected by a wide variety of inactive compounds. The preliminary screens employed in the NCI studies changed during the lifetime of the programme to avoid detecting weakly active common plant products such as polyphenols, tannins, saponins and sterols not capable of being developed into useful drugs; such dereplication (Chapters 8, 9) is now commonly employed. The routine testing of extract fractions for antitumour activity is frequently done via an in vitro cytotoxity assay, although in vitro cytotoxicity is not always an effective or reliable means of predicting in vivo antitumour activity. However, since the in vitro cytotoxicity bioassay is rapid and inexpensive, and only small amounts of extract are necessary, it is the popular method for initial tests.
Promising chemicals are subsequently tested against a range of standard experimental neoplasms, and then considered for preclinical toxicological studies if these results are sufficiently encouraging. At this stage, relatively large amounts of material will be required, and larger-scale extractions and fractionation may be necessary. Very few compounds will reach clinical trials. A low or very narrow therapeutic index (the ratio of maximum tolerated dose to minimum effective dose), undesirable side-effects or high toxicity can outweigh beneficial tumour-inhibitory activity. From some 25 000 screens conducted annually by the NCI (including both synthetic and natural materials), only eight to twelve compounds are likely to be selected for preclinical testing, and perhaps only six to eight go on to clinical trials. Slightly less than half of these may be plant-derived.
Should a plant-derived natural product or derivative be considered worthy of development as a drug, the availability of future supplies of the plant becomes critical. Collections from the wild may be exploited if the plant is common, but mass cultivation should be considered. With slow-growing crops, e.g. trees, this could mean a considerable delay before significant supplies are available. Alternative plants might be richer sources of the compound, or be more accessible; other species of the same genus or closely related genera from the same family should also be analysed. Thus, wild sources could not supply the huge amount of Taxus brevifolia bark needed to satisfy demand for the manufacture of taxol but the discovery of baccatin III and deacetylbaccatin III (readily convertible to taxol) in the leaves of the common yew (T. baccata) has ensured the future supply of the drug. For commercial exploitation, agreements giving some slice of any profit arising from the sale of a product derived from wild plant material must be arranged with the country of origin.
Plant tissue cultures might provide a reliable source. If total synthesis of the active chemical is feasible, this will always be the preferred option. However, the extremely complex structures of most bioactive natural products frequently preclude satisfactory commercial syntheses.
Although the random-selection screening programme for natural products was terminated by the NCI in 1983, the number of cytotoxic and antitumour agents identified was enormous, and these have increased our understanding of the cancer process, and the mechanisms of action of the agents. Synthetic work has enabled structure–activity studies to be undertaken, and there is every hope that synthetic or semi-synthetic analogues may, in time, be developed and become useful drugs.
Over the years, separation techniques (TLC, HPLC, chiral chromatography, etc.) and chemical structure determination (MS, NMR, UV, X-ray crystallography) have reached high levels of sophistication. For the examination of large numbers of samples, high-throughput screening is now employed (Chapter 9).
Following on from the above, partly as a result of new screening techniques, the NCI in 1986 revived its collection of plants, focusing on tropical and subtropical species which had local medicinal use.
Considerable recent research has been, and still is, carried out by National Cooperative Drug Discovery Groups (NCDDGs); these groups result from cooperative agreement awards funded essentially by the NCI, which supports all aspects of preclinical anticancer drug discovery and treatment strategies. In 2003, there were 13 funded groups of which five were natural-product based. Such groups may involve the participation world-wide, of universities, research centres and industry, together with the resource countries of plant or marine materials. The complex nature of such groups is evident from the number of authors involved in ensuing publications. For updated reports from a number of such groups, see ‘Further reading’. For an article giving explanatory details of the NCDDGs project, see Y. F. Hallock and G. M. Cragg, Pharm. Biol., 2003, 41(supplement), 78.
Another multinational group involving collaboration of seven S. American countries and supported by the Organization of American States has prioritized 314 Latin American plant species for screening for cytotoxic properties. Results for some 70 species from 40 families tested against breast, lung and central nervous system human cancer cell lines have been reported (A. I. Calderon et al. (11 authors), Pharm. Biol., 2006, 44, 130).
For cytotoxicity testing, human cancer cell lines obtained from the NCI are frequently employed. Plant selection for screening can be assisted by initially choosing those from an ethnomedical database and submitting them to a search for biological and chemical information in the libraries held by NAPRALERT (see Chapter 2), industry or other research centres.
Research groups in which there is a commercial interest should establish agreements with the countries of origin of the plant material giving the latter a fair share of any profits that might arise from the marketing of any successful products. This follows from the 1992 UN Convention on Biological Diversity, which calls for recognition of the sovereign rights of countries to control the utilization of their natural resources and genetic materials. Further, some countries, e.g. the Philippines, have instituted their own regulations concerning bio-prospecting.
ESTABLISHED NATURAL PRODUCTS AS TUMOUR INHIBITORS
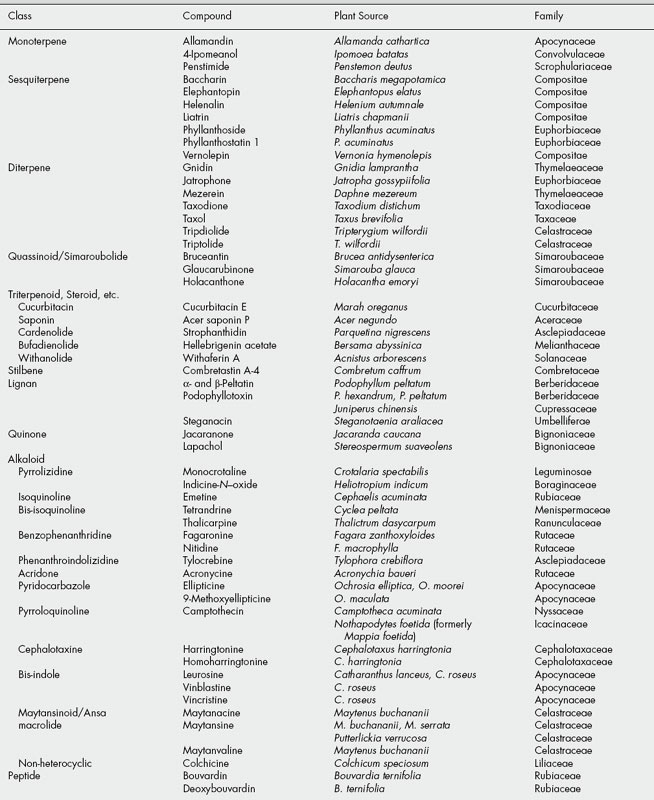
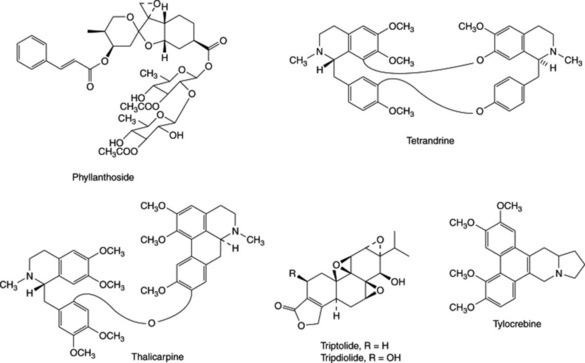
The tumour-inhibitory principles isolated in screening tests are usually new natural products, spanning a wide range of structural types. Examples of these, subdivided into phytochemical groups, are listed in Table 27.1, and structures of the more promising chemicals which were subsequently evaluated in clinical tests are shown in Fig. 27.1. However, a number of the compounds isolated were, in fact, previously known natural products. These were presumably compounds which had not been subjected to rigorous testing for biological, particularly antitumour, activity. Amongst these are usnic acid, ellagic acid, the anthraquinone aloe-emodin, the quinones juglone and lapachol, pyrrolizidine alkaloids retronecine and monocrotaline, the nitrophenanthrene aristolochic acid (see ‘Serpentary’), the bufadienolide hellebrigenin acetate, the Colchicum alkaloids colchicine, demecolcine and 3-demethylcolchicine (see ‘Colchicum’). Other known compounds that proved active but were rejected on terms of toxicity, low therapeutic index, etc. were the alkaloids of Senecio, indicine N-oxide from Heliotropium indicum and the cucurbitacins. Further examples established as tumour inhibitors at much later dates than the above were the alkaloids acronycine, ellipticine, emetine and nitidine. Acronycine, from Acronychia baueri, failed in phase 1 clinical trials. The pyridocarbazole alkaloids ellipticine and 9-methoxyellipticine from Ochrosia elliptica and other related plants, together with a number of synthetic analogues appeared useful but exhibited unacceptable side-effects. However, the quaternization of 9-hydroxyellipticine to give the water-soluble 9-hydroxy-2-N-methylellipticinium acetate (elliptinium acetate) (Fig. 27.2) has produced a highly active material, of value in some forms of breast cancer, and perhaps also in renal cell cancer. A variety of such quaternized derivatives is being tested, and some water-soluble N-glycosides also show high activity. Cephaelis ipecacuanha has been used for many years as an emetic and expectorant and the principal alkaloid, emetine, was shown to have antitumour properties. Clinical usefulness was marginal, however, and some toxic effects were noted. Nitidine is a benzophenanthridine alkaloid isolated from Zanthoxylum nitidum, and has more recently been obtained from screens of Fagara species (Rutaceae). Nitidine was selected for development based on its exceptional antileukaemic activity, but was dropped owing to erratic toxicity. The closely related alkaloid fagaronine is less toxic than nitidine. Similar benzophenanthridine derivatives are present in Chelidonium majus (Papaveraceae), a plant with substantial folklore history of use in the treatment of cancers. More recently, NK 109 (Fig. 27.2), a synthetic isomer of fagaridine found in Fagara xanthoxyloides, has been found to have greater antitumour activity than any of the natural benzophenanthridine structures, coupled with excellent stability, and was entered for clinical trials in Japan.
NEW NATURAL PRODUCTS WITH ANTITUMOUR ACTIVITY
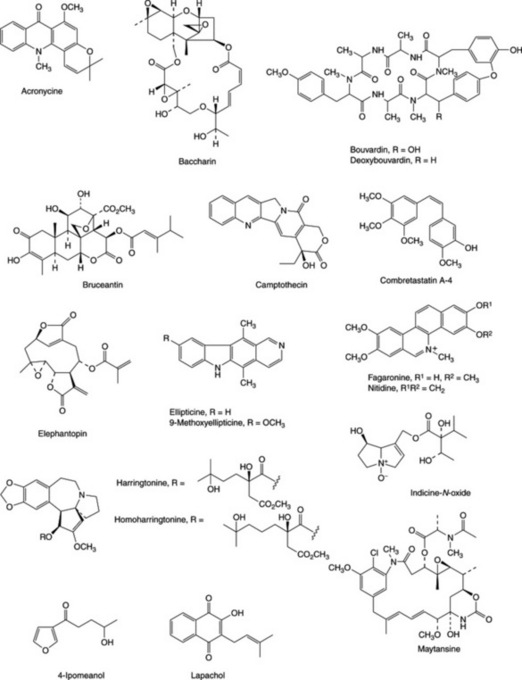
The systematic studies have also resulted in the isolation of many new natural products exhibiting antitumour activity, and a number of these were considered sufficiently active for clinical studies to be commenced (Fig. 27.1 and Table 27.1). Tylocrebine, a phenanthroindolizidine alkaloid from Tylophora crebiflora was sufficiently active for further development, but in a clinical trial unmanageable CNS effects precluded continuation of the studies. Two bis-benzylisoquinoline alkaloids, thalicarpine from Thalictrum dasycarpum and tetrandrine from Cyclea peltata appeared particularly promising and were selected for development. Clinical trials showed no antitumour activity, and these compounds were dropped from further study. Camptothecin and derivatives, alkaloids from the Chinese tree Camptotheca acuminata, showed broad-spectrum activity and produced a fair response inlimited clinical trials, but toxicity and poor solubility were problems. The natural 10-hydroxycamptothecin is more active than camptothecin, and is used in China against cancers of the neck and head. Synthetic analogues 9-aminocamptothecin and particularly the water-soluble derivatives topotecan and irinotecan (Fig. 27.2) showed good responses in a number of cancers; topotecan and irinotecan were made available for the treatment of ovarian cancer and colorectal cancer, respectively. Irinotecan is a carbamate pro-drug of 10-hydroxy-7- ethylcamptothecin, and is converted into the active drug by liver enzymes.
The quassinoids or simaroubolides are a group of terpenoid-related compounds isolated from a variety of plants in the Simaroubaceae (q.v.). Many of these plants have folk-medicine history, particularly for antiamoebic activity, and a number of the isolated quassinoids are currently of interest for their antitumour properties. Thus, Brucea antidysenterica is used in Ethiopia in the treatment of cancer, and systematic fractionation of this plant has led to the isolation of bruceantin, which shows high antileukaemic activity at low dosages, and over a wide dose range. Bruceantin acts through inhibition of protein synthesis, and has undergone clinical trials in man. No significant therapeutic activity has been noted in these early studies, but research on a whole range of quassinoids related to bruceantin continues. Maytenus serrata (Celastraceae) and other species of Maytenus contain maytansine, an ansa macrolide, which was regarded as an antitumour agent of exceptional promise. It is active against several of the experimental neoplasms at very low dosage and shows a favourable therapeutic index. It acts through inhibition of mitosis. In clinical trials, maytansine was a big disappointment, and showed few beneficial effects. Synthetic or semi-synthetic derivatives may offer more hope. Other maytansinoids isolated from Maytenus are also highly active; maytansine was originally chosen for study simply because of its relatively higher concentration in the plants. There is now the opportunity of producing maytansinoids via microorganisms. A species of Nocardia has been shown to produce ansamitocin, which is a mixture of esters of maytansinol, the parent alcohol of maytansine. This compound could then serve as starting material for semi-synthesis of a whole range of derivatives, and there is still potential for developing these compounds.
Several other natural products have also proved sufficiently interesting to justify clinical trials, or toxicological testing prior to further study. The diterpenes triptolide and tripdiolide isolated from Tripterygium wilfordii are potent antileukaemic agents that contain a reactive triepoxide system. The plant is not readily accessible and contains only small amounts of these compounds; large-scale isolation thus delayed their evaluation, and no further development occurred. Nevertheless, in China crude extracts of T. wilfordii are used in inflammatory and immune disorders, and triptolide underwent extensive evaluation in the treatment of these conditions. Of many sesquiterpene lactones tested, few show useful in vivo antitumour activity, but several of the best in vivo active compounds, e.g. the germanacrolide elephantopin from Elephantopus elatus, have been evaluated. Baccharin is a trichothecene sesquiterpene isolated from the Brazilian plant Baccharis megapotamica and is closely related to the fungal trichothecene toxins (q.v.). This and some of the fungal-derived compounds have undergone thorough evaluation. A series of novel alkaloidal esters from Cephalotaxus species are currently being isolated on a large scale for toxicological studies preliminary to clinical trials. The parent alkaloid cephalotaxine is inactive, but the esters harringtonine and homoharringtonine from C. harringtonia show good activity in a number of systems. Chinese researchers have reported favourable results in clinical studies using alkaloidal fractions of C. harringtonia, and homoharringtonine in particular is active in patients with leukaemia resistant to existing chemotherapies. Homoharringtonine is only a minor constituent in Cephalotaxus, but can be obtained by semi-synthesis from the more abundant cephalotaxine. Tissue cultures of Cephalotaxus also synthesize cephalotaxine and the active esters and may offer potential access to these alkaloids in useful quantities. The Central American tree Phyllanthus acuminatus contains in its roots a complex mixture of glycosides, two of which, phyllanthostatin 1 and phyllanthoside, have demonstrated marked antitumour properties. Phyllanthoside has undergone early clinical trials. The cis-stilbene combretastatin A-4 is one of the most potent antimitotic agents from about 20 active substances isolated from the African tree Combretum caffrum. A water-soluble phosphate pro-drug (CA4) has shown promise in early clinical trials and a number of other CA4 mimics are now under investigation.
The milk-thistle (Silybum marianum), established as a hepatoprotective remedy, contains a mixture of flavonolignans termed silymarin (Chapter 29). Recent research has shown this mixture and its individual components to possess anticancer activity; a phase I clinical study involving prostate cancer patients was recently reported (T. W. Flaig et al., Invest. New Drugs, 2007, 25, 139). The antitumour-promoting effects of seven silymarin flavonolignans on Epstein–Barr virus activation has been studied and in this research silychristin B proved to be the most active compound (A.-S. Lin et al., Pharm. Biol., 2007, 45, 735).
The fractionation schemes employed in the NCI programme are based on the rationale that a balance between hydrophilicity and lipophilicity is important for substances to reach biological receptors. Thus, extremely polar plant extracts (aqueous) or extremely non-polar extracts (petrol) are unlikely to show as much activity as extracts of intermediate polarity (ethanol, chloroform, etc.) which are normally screened. Recently, many peptides and proteins from microorganisms have shown high antitumour activity, as have some water-soluble polysaccharides, and consideration is thus being given to the testing of aqueous extracts from plants. Already, screening of the Mexican plant Bouvardia ternifolia has yielded the bicyclic hexapeptides bouvardin and deoxybouvardin as active principles. Bouvardin was selected for further development, but was subsequently dropped because of its narrow spectrum of activity. The mistletoe proteins mentioned earlier offer a further example. Future work on plant peptides could thus prove most valuable.
Numerous research papers regularly appear in the literature citing cytotoxicity tests for extracts and constituents of plants. As an example, N. R. Monks et al. (Pharm. Biol., 2002, 40, 603) scanned 145 Brazilian plants (538 extracts) from 34 families for antitumour activity against two human cell lines. Families containing a high proportion of active species were the Anacardiaceae, Annonaceae, Asteraceae, Celestraceae, Leguminosae (Fabaceae), Meliaceae and Myrtaceae.
An NCDD Group have reported on novel strategies for the discovery of plant-derived anticancer drugs; examples of plants containing alkaloids, diterpenoids, naphthoquinones, polyacetylenes, phenols, flavones, stilbenes and xanthones add to the list previously cited in Table 27.1 (A. D. Kinghorn et al. (16 authors), Pharm. Biol., 2003, 41(supplement), 53). For a review of a project devoted to the investigation of anticancer agents from unique natural product sources (includes fungi), see C. M. Ireland et al. (23 authors), Pharm. Biol., 2003, 41(supplement), 15.
The search for new antitumour compounds in nature is by no means confined to plants. Microorganisms, widely employed as sources of antibiotics (see Chapter 30), produce a considerable number of metabolites having antitumour activity. Many of these also have antibiotic properties. Amongst materials in general use against cancers are dactinomycin (actinomycin D), bleomycin, doxorubicin (adriamycin), daunorubicin, mithramycin and mitomycin C. Microorganisms have particular advantages over plants as far as ease of culture and the opportunity for genetic manipulation are concerned. The recent identification of epothilones from the bacterium Sorangium cellulosum has thus generated considerable interest, since these agents mimic the effects of taxol, and some analogues are much more potent than taxol, especially towards multidrug-resistant cell lines.
Marine animals, e.g. corals and starfish, may also be a fruitful source of potentially useful anticancer agents and this area of research is also producing a number of natural products with antitumour activity. Compounds of particular note are the depsipeptide didemnin B, isolated from the Caribbean sea-squirt Trididemnum solidum, the bryostatins, a group of macrocyclic lactones from the marine bryozoan Bugula neritina, and dolastatin 10, a linear peptide from the sea hare, Dolabella auricularia. Didemnin B shows activity against several human cancers (including prostate, lung and brain cancers and lymphomas) and bryostatin I has been found to bind to and activate protein kinase C which mediates growth of cancer cells. Dolastatin 10 is a very potent inhibitor of microtubule assembly, and synthetic material is now available for testing. All three compounds are in clinical trials. An NCDD Group has reported on new anticancer compounds derived from cultured and collected marine organisms; these include cancer cell growth inhibitors from marine invertebrates (sponges), selected marine microalgae (dinoflagellates), marine fungi and bacteria (W. Fenical et al. (21 authors), Pharm. Biol., 2003, 41(supplement), 6).
PLANTS CONTAINING ANTICANCER AGENTS IN CURRENT USE
CATHARANTHUS ROSEUS
The Madagascan periwinkle, Catharanthus roseus, has been variously designated Vinca rosea and Lochnera rosea (Apocynaceae). It is indigenous to Madagascar but is now widely distributed throughout warm regions and is much cultivated as an ornamental; it grows profusely in southern Florida. Commercial supplies of the drug are obtained from both wild and cultivated plants produced in various locations, including Africa, India, Thailand, Taiwan, eastern Europe, Spain, USA and Australia.
Characters
C. roseus is a herbaceous subshrub, 40–80 cm high, becoming woody at the base. The leaves are oppositely arranged,
oblong with a petiolate acute base, a rounded or mucronate apex and an entire margin. In form the flowers resemble those of the common periwinkle Vinca major and are coloured violet, rose, white (var. albus) or white with a red eye (var. ocellatus). The fruit is a divergent follicle. Tetraploid plants are reported to have a more vigorous growth habit and larger flowers than diploid ones.
History
Although the plant has a certain reputation in folk medicine for the treatment of diabetes, modern investigators have been unable to confirm this property. Instead Canadian workers, during 1955–1960, discovered that extracts of the leaves produced leukopenic actions in rats. These observations led researchers at Eli Lilly to undertake an intensive phytochemical investigation of the plant with a view to the isolation of constituents of value in cancer chemotherapy. Six alkaloids proved active in this respect and two are now available commercially (see R. L. Noble, Biochem. Cell Biol., 1990, 68, 1344–1351).
Catharanthus is an example of a drug plant which has been introduced into medicine during recent years, and it is used for the isolation of pure substances rather than for galenical preparation. Indeed, simple galenicals, prepared from the dried plant material and containing a wide spectrum of alkaloids, would be quite useless therapeutically. Hence, in normal circumstances, the raw material is handled by the manufacturer and does not reach the pharmacist as such.
Constituents
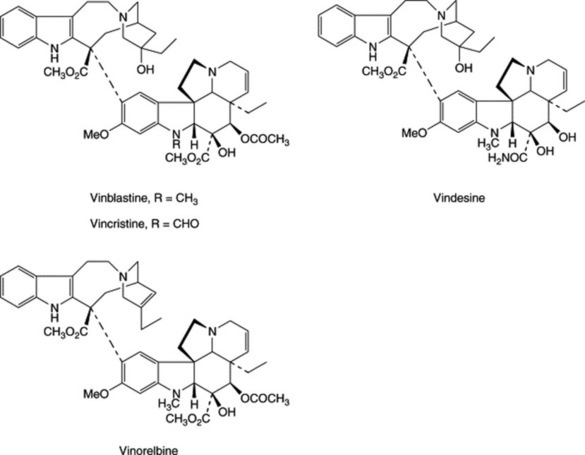
About 150 alkaloids have now been isolated from C. roseus; some, for example, ajmalicine, lochnerine, serpentine and tetrahydroalstonine, occur in other genera of the family. Of particular interest is a group of about 20 bisindole alkaloids which contains those having antineoplastic activity, including leurocristine (vincristine) and vincaleukoblastine (vinblastine). Vinblastine is produced by couplingof the indole alkaloids catharanthine and vindoline, both of which occur free in the plant. Formation of 3′,4′-anhydrovinblastine from these monomers has been effected with peroxidase isozymes isolated from C. roseus suspension cultures and with commercial horseradish peroxidase (see J. P. Kutney, Nat. Prod. Rep., 1990, 7, 85–103). Vincristine is structurally similar to vinblastine, but has a formyl group rather than a methyl on the indole nitrogen in the vindoline-derived portion. Because these alkaloids are only minor constituents of the plant (vincristine is obtained in about 0.0002% yield from the crude drug), large quantities of raw material are required and chromatographic fractionations are extensively employed in the isolation procedures. In addition, there is a growing demand for vincristine rather than vinblastine, but the plant produces a much higher proportion of vinblastine. Fortunately, it is now possible to convert vinblastine into vincristine either chemically, or via a microbiological N-demethylation using Streptomyces albogriseolus.
Cell cultures
In efforts to improve the production of alkaloids, cell cultures of C. roseus have received considerable attention (Chapter 13). Success has been achieved in obtaining total alkaloid yields corresponding to 0.1–1.5% dry weight cultured cells, but cultures produced catharanthine and tabersonine, and not vindoline, so lacked one of the essential precursors for formation of the bisindole alkaloids. A similar problem arises with transformed root cultures although the feeding of loganin alone at the early stationary phase has been shown to increase the ajmalicine production 2.3-fold and the serpentine 1.8-fold when compared with control cultures; catharanthine levels are unaffected by a single feed of the precursor. Research is still necessary to find means of inducing the production of the useful alkaloids.
Uses
Vinblastine is used mainly for the treatment of generalized Hodgkin’s disease, and non-Hodgkin’s lymphomas. Vincristine is used principally in the treatment of acute lymphocytic leukaemia in children. It has other applications for lymphomas, small-cell lung cancer, cervical and breast cancers. The semi-synthetic vindesine is also used in the treatment of acute lymphoid leukaemia in children. Vincristine has a superior antitumour activity compared to vinblastine, but is more neurotoxic. Vinorelbine is a newer, orally active, semi-synthetic anhydro derivative of 8′-norvinblastine with a broader anticancer activity and lower neurotoxic side-effects than the other Catharanthus alkaloids.
PODOPHYLLUM AND PODOPHYLLUM RESIN
Podophyllum (Podophyllum Rhizome, May-apple Root, Wild Mandrake) consists of the dried rhizome and roots of Podophyllum peltatum (Berberidaceae, sometimes Podophyllaceae), a perennial herb common in moist shady situations in the eastern parts of Canada and the USA. The drug is collected in Virginia, Kentucky, North Carolina, Tennessee and Indiana.
Collection
The rhizome, which is about a metre in length, is dug up, cut into pieces about 10 cm in length, and dried.
History
The drug has long been used by the North American Indians as a vermifuge and emetic, and was introduced into the 1864 Pharmacopoeia. Podophyllin, a crude resin obtained from the rhizomes and roots, was subsequently employed as a purgative, but usage declined, until in 1942 podophyllin was recommended for the treatment of venereal warts. Since then, extensive research has led to an appreciation of podophyllum’s antitumour properties, and the development of successful anticancer agents.
Macroscopical characters
Podophyllum occurs in subcylindrical reddish-brown pieces about 5–20 cm long and 5–6 mm thick. The outer surface is smooth (autumn rhizome) or wrinkled (summer rhizome). The nodes are enlarged to from two to three times the diameter of the internodes. On these swellings the remains of the aerial stems are visible on the upper surface as large cup-shaped scars surrounded by the remains of the cataphyllary leaves, some of which have buds in their axils. On the lower side of each node are about 5–12 root scars or portions of roots. The latter, if entire, are 2–7 cm in length and about 1.5 mm in diameter. The drug breaks with a short fracture and shows a starchy or horny interior. The transverse section of the internode shows a starchy bark and pith and a ring of 20–30 small fibrovascular bundles. The latter are not radially elongated (cf. Indian podophyllum). A section of the node is similar but shows branches from the ring of bundles running upwards to the cup-shaped scar of the aerial stem or downwards to the roots. Odour, slight; taste, disagreeably bitter and acid.
Microscopical characters
A transverse section of the rhizome shows a dark-coloured epidermis, one or two layers of cork cells, a large collenchymatous and parenchymatous cortex and pith, and a ring of small vascular bundles. The small vessels of the latter have simple pores or reticulate thickening. Many of the cells of the ground tissue contain reddish-brown masses of resin, cluster crystals of calcium oxalate, and starch. The cluster crystals are 30–60–100 μm in diameter, many exceeding 60 μm (cf. Indian podophyllum). The starch occurs in simple grains 3–15–25 μm in diameter and in compound grains with 2–15 components. In those rhizomes breaking with a horny fracture the starch shows gelatinization. The roots have a central wood occupying about one-sixth of the total diameter.
Constituents
The active principles of podophyllum are contained in the resin, podophyllum resin or ‘podophyllin’, which is prepared by pouring an alcoholic extract of the drug into water and collecting and drying the precipitate. American podophyllum yields about 2–8% and Indian podophyllum (see below) about 6–12% of resin. Podophyllum Resin of the USP was obtained solely from the American drug but that of the BP may be either American or Indian, although the resins from these two sources are not identical.
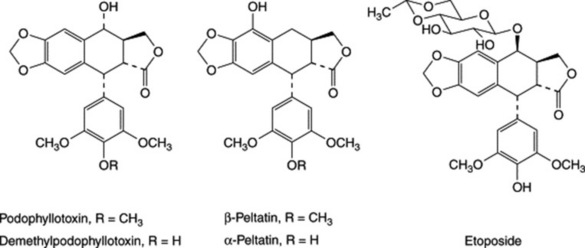
The chief constituents of the root belong to the group of lignans, which are C18 compounds derived biosynthetically by dimerization of two C6–C3 units (e.g. coniferyl alcohol) at the β-carbon of the side-chains. The most important ones present are podophyllotoxin (about 0.25%), β-peltatin (about 0.33%) and α-peltatin (about 0.25%) (see D. E. Jackson and P. M. Dewick, Phytochemistry, 1984, 23, 1147–1152). In the root, all of these occur both free and as glucosides. Preparation of the resin results in considerable losses of the glucosides. The root also contains smaller amounts of the closely related 4′-demethylpodophyllotoxin and its glucoside, desoxypodophyllotoxin and podophyllotoxone. These compounds all possess cytotoxic or antitumour activity, but activity is lost on mild base treatment. Epimerization, α to the carbonyl, results in the formation of the thermodynamically more stable cis-fused lactone ring, rather than the severely strained trans arrangement of the natural compounds.
C. Canel et al. (Planta Medica, 2001, 67, 97) have suggested that to avoid destruction of the natural population of P. pelatum by root collection the harvested leaves of cultivated plants be utilized. The authors found that rehydration of the powdered dried leaves and subsequent
organic solvent extraction, gave yields of 5.2% podophyllotoxin exceeding levels previously reported from any source. This increase in yield resulted from hydrolysis of lignan 4-O-β-O-glucosides in situ during the rehydration period.
Uses
Podophyllum resin has long been used as a purgative but has largely been replaced by less drastic drugs. It has a cytotoxic action and is used as a paint in the treatment of soft venereal and other warts. Podophyllotoxin is also used for this purpose. Etoposide (4′-demethylepipodophyllotoxin ethylideneglucoside) is a lignan derivative obtained semi-synthetically from podophyllotoxin and used in the treatment of small-cell lung cancer and testicular cancer as well as lymphomas and leukaemias. The water-soluble pro-drug etopophos (etoposide 4′-phosphate) is also available. The related thenylidene derivative teniposide has similar anticancer properties and though not as widely used as etoposide has value in paediatric neuroblastoma, lymphocytic leukaemia, and brain tumours in children (see H. Stähelin and A. von Wartburg, Cancer Res., 1991, 51, 5–15).
INDIAN PODOPHYLLUM
Indian podophyllum consists of the dried rhizome and roots of Podophyllum hexandrum, syn. P. emodi (Berberidaceae), a perennial herb found in Tibet, Afghanistan and the Himalayan areas of Pakistan and India. The drug is collected in India, Pakistan and China.
Macroscopical characters
The drug, at first glance, shows little resemblance to American podophyllum. The roots frequently break off and some samples consist almost entirely of rhizomes, while others consist largely of roots.
The rhizomes occur in much contorted pieces of an earthy brown colour, about 2–4 cm long and 1–2 cm in diameter. The internodes are much shorter than in the American drug, with the result that each piece bears the remains of about 3–6 branches ending in cup-shaped scars and about 20–40 roots or root scars. The rhizome is hard and somewhat difficult to break. Internally it is pale brown in colour and horny (usually) or starchy. The general arrangement of the tissues resembles that found in American podophyllum, but the vascular bundles are more elongated radially. The odour and taste resemble those of the American drug.
Microscopical characters
The calcium oxalate cluster crystals are fewer and smaller, 20–30–60 μm. The starch grains are simple or 2–20 compound; individual grains 2–7–34 μm (cf. American podophyllum).
Constituents
Indian podophyllum contains more resin (about 6–12%) than the American drug and the percentage of podophyllotoxin in the resin (up to 40%) is much higher. The root contains about 4% podophyllotoxin, 0.45% 4′-demethylpodophyllotoxin and smaller amounts of related lignans (see D. E. Jackson and P. M. Dewick, Phytochemistry, 1984, 23, 1147–1152). Only traces of the peltatins are present, but the range of constituents is much the same as in the American resin.
Root cultures of P. hexandrum have been shown to contain higher proportions of podophyllotoxin than normal roots (B. P. S. Sagar and R. Zafar, Pharm. Biol., 2005, 43, 404).
Uses
Indian podophyllum is used for the preparation of the resin and isolation of podophyllotoxin for drug use and semi-synthesis of etoposide. Other less common species of Podophyllum (e.g. P. pleianthum) and related genera (e.g. Diphylleia) also contain podophyllotoxin and structurally related lignans (see A. J. Broomhead and P. M. Dewick, Phytochemistry, 1990, 29, 3831–3837).
TAXUS BREVIFOLIA AND TAXOL
A note on nomenclature: the name taxol was given to a diterpene ester with anticancer properties when it was first isolated in 1971 from Taxus brevifolia. When this compound was subsequently exploited commercially as a drug, Taxol was registered as a trademark. Accordingly, the generic name paclitaxel has been assigned to the compound. The literature now contains an unhappy mixture of the two names, though the original name taxol is most often employed.
The Pacific yew, Taxus brevifolia (Taxaceae) is a slow-growing shrub/tree found in the forests of North-West Canada (British Columbia) and the USA (Washington, Oregon, Montana, Idaho and N. California). Although the plant is not rare, it does not form thick populations, and needs to be mature (about 100 years old) to be large enough for exploitation of its bark. At this age, the tree will be some 6–9 m high, and have a trunk of about 25 cm in diameter. The bark is removed from mature trees during the period May–August. The wood of T. brevifolia is not suitable for timber, and in some areas, plants have been systematically destroyed to allow cultivation of faster-growing commercially exploitable conifers. Harvesting is now strictly regulated. Although taxol is currently extracted from the dried bark, it is realized that this cannot be expected to provide a satisfactory long-term supply of the drug. It requires the bark from about three mature 100-year-old trees to provide one gram of taxol, and a course of treatment may need 2 grams of taxol. Current demand for taxol is in the region of 100–200 kg per annum.
Constituents
All parts of Taxus brevifolia contain a wide range of diterpenoid derivatives termed taxanes, which are structurally related to the toxic constituents found in other Taxus species, e.g. the common yew, Taxus baccata. Over a hundred taxanes have been characterized
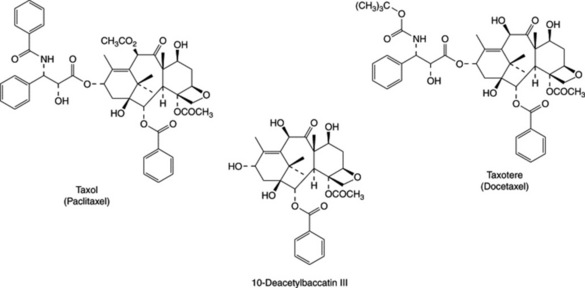
from various Taxus species, and taxol is a member of a small group of compounds possessing a four-membered oxetane ring and a complex ester side-chain in their structures, both of which are essential for antitumour activity. Taxol is found predominantly in the bark of T. brevifolia, but in relatively low amounts (0.01–0.02%). Up to 0.033% of taxol has been recorded in some samples of leaves and twigs (see N. C. Wheeler et al., J. Nat. Prod., 1992, 55, 432–440), but generally the taxol content is much lower than in the bark. Significant variation in taxol content depending on season, geographical location, and environmental factors as well as individual populations of trees have been noted. The content of some other taxane derivatives in the bark is considerably higher, e.g. up to 0.2% baccatin III. Other taxane derivatives characterized include 10-deacetyltaxol, 10-deacetylbaccatin III, cephalomannine and 10-deacetylcephalomannine.
TAXUS BACCATA AND OTHER TAXUS SPP
A more satisfactory solution now employed for the long-term supply of taxol and derivatives for drug use is to produce these compounds by semi-synthesis from more accessible structurally related materials. Both baccatin III and 10-deacetylbaccatin III may be efficiently transformed into taxol. 10-Deacetylbaccatin III is readily extracted from the leaves and twigs of Taxus baccata, and although the content is variable, it is generally present at much higher levels (up to 0.2%) than taxol can be found in T. brevifolia. Taxus baccata, the common yew, is widely planted as an ornamental tree in Europe and the USA and is much faster growing than the Pacific yew; it therefore provides a sustainable source of raw material. Five new taxanes and forty known ones have been reported from T. baccata grown in Israel (Q.-W. Shi et al., J. Nat. Prod., 2004, 67, 168). T. baccata pollen contains taxine alkaloids (yield 0.08%) and the taxoids taxol, baccatin III and 10-deacetylbaccatin III (overall yield 0.004%). It is suggested that exposure to yew pollen could be the origin of the atopic manifestations attributed to the tree (M. Vanhaelen et al., Planta Medica, 2002, 68, 36).
New taxane analogues have been reported from the needles of T. canadensis (J. Zhang et al., J. Nat. Prod., 2001, 64, 450; Q.-W. Shi et al., Nat. Prod., 2003, 66, 470). This species (Canada yew), occurring wild in the north-eastern United States and eastern Canada, is harvested commercially for its content of paclitaxel and 10-deacetylbaccatin III. S. L. Cameron and R. F. Smith (Pharm. Biol., 2008, 46, 35) have reported on taxane levels in both older and younger components of twigs throughout a season and find that the lowest levels occur during periods of active growth (April–July), with peak levels following; overall, the preferable harvesting time is August and September. For a review of the chemistry and biological activity of the taxoids (120) of T. cuspidata (Japanese yew), see H. Shigemori et al., J. Nat. Prod., 2004, 67, 245. Three new oxetane-ring-containing taxoids have been isolated from T. chinensis; the availability of such C-14 oxygenated taxoids with an oxetane functionality has great potential, allowing the synthesis of additional oxygenated derivatives of taxol (F.-S. Wang et al., J. Nat. Prod., 2004, 67, 905).
Advances in the identification of the genes involved in the biosynthesis of taxol have been reported. By 2001 five cDNA encoding pathway enzymes had been isolated from a Taxus cDNA library and functionally expressed from an appropriate vector or in bacteria or yeast as host (K. Walker and R. Croteau, Phytochemistry, 2001, 58, 1).
Cell cultures of Taxus species also offer excellent potential for production of taxol of 10-deacetylbaccatin III; taxol yields of up to 0.2% dry weight cultured cells have been reported. Taxus cuspidata (Japanese yew): large-scale cell cultures have produced approximately 3 mg/1 of taxol and 74 mg/1 total taxanes after 27 days of growth (S. H. Son et al., Plant Cell Rep., 2000, 19, 628).
A number of reports deal with the effect of various added precursors on taxol production; species so studied include T. baccata, T. brevifolia, T. chinensis, T. cuspidata and T. wallichiana, see C. Veersham et al., Pharm. Biol., 2003, 41, 426. Abietane diterpenoids have been isolated from callus cultures of T. baccata (B. Monacelli et al., Planta Medica 2002, 68, 764).
Uses
Taxol® (paclitaxel) is being used clinically in the treatment of ovarian cancers, breast cancers and non-small-cell lung cancer. It may also have potential value against other cancers. Taxotere®(docetaxel) is a side-chain analogue of taxol, which has also been produced by semi-synthesis from 10-deacetylbaccatin III. It has improved water-solubility and is used in treatment of breast cancers.
Banerjee S, Wang Z, Mohammad M, Sarkar FH. Efficacy of selected natural products as therapeutic agents against cancer. J. Nat. Prod.. 2008;71:492-496.
Canel C, Moraes RM, Dayan FE, Ferreira D. Molecules of interest—‘Podophyllotoxin’. Phytochemistry. 2000;54(2):115-130.
Cassady JM, et al. Recent developments in the maytansinoid antitumour agents. Chemical and Pharmaceutical Bulletin. A review listing 27 compounds. 2004;52(1):1-26.
Cragg GM, Newman DJ. Plants as a source of anti-cancer agents. J Ethnopharmacology. 2005;100:72-79.
Ge G-B, et al. Chemotaxonomic study of medicinal Taxus species with fingerprint and multivariate analysis. Planta Medica. 2008;74:773-779.
Itokawa H, Lee KH, Hardman R, editors. Medicinal and aromatic plants, Vol 32. Taxus–the genus Taxus. Boca Raton, FL: CRC Press, Taylor and Francis Group, 2003. A comprehensive coverage, 1584 refs
Kinghorn AD. Novel strategies for the discovery of plant-derived anticancer agents. Pharmaceutical Biology. 2003;41(supplement):53-67. and 15 other authors