Chapter 29 An overview of drugs with antihepatotoxic and oral hypoglycaemic activities
The botanicals considered in this chapter are ones which, although not normally encountered in orthodox medicine, have important roles in traditional medicine for the treatment of two widespread and life-threatening conditions. In recent years new technologies have led to rapid advances in the biological testing and chemical elucidation of the active constituents of many of these plants. Much, but not all, of the research is attributable to workers in Asian institutions. In the space available here it is possible to provide a few examples only to illustrate the wide variety of chemical structures involved. For further information readers are referred to the various reviews and references quoted.
PLANTS IN THE TREATMENT OF LIVER AND BILIARY TRACT DISEASES
The liver, the principal organ of metabolism and excretion is subject to a number of diseases which may be classed as liver cirrhosis (cell destruction and increase in fibrous tissue), acute chronic hepatitis (inflammatory disease) and hepatitis (non-inflammatory condition). Jaundice, a yellow discoloration of the skin and eyes caused by bile in the blood is a symptom of blockage of the bile duct, or disease within the tissue of the liver itself.
The predominant type of liver disease varies according to country and may be influenced by local factors. In 1989 it was estimated that there were some 200 million chronic carriers of the hepatitis B virus of which 40% were expected eventually to die of hepatocellular carcinoma and 15% of cirrhosis. Causative factors of liver disorders include: virus infection; exposure to, or consumption of, certain chemicals, e.g. the excessive inhalation of chlorinated hydrocarbons or overindulgence in alcohol; medication with antibiotics, chemotherapeutic agents and possibly plant materials such as those containing pyrrolizidine alkaloids (q.v.); contaminated food containing toxins such as aflatoxins (q.v.) or peroxides in oxidized edible oils; ingestion of industrial pollutants, including radioactive material. Drug abuse in Western society and poor sanitary conditions in Third World countries are contributing factors to the above.
Except for the use of the appropriate vaccine for the treatment of hepatitis caused by viral infection, there are few effective cures for liver diseases. It is not surprising therefore that there has developed a considerable interest in the examination of those numerous world-wide traditional plant remedies which are used for such treatment and that in recent years in vivo and in vitro test models have been developed for the evaluation of plants for their antihepatotoxic activities. These systems measure the ability of the test plant extract to prevent or cure in rats or mice liver toxicity induced by various hepatotoxins. The latter include carbon tetrachloride (the most commonly used), D-galactosamine (this produces liver lesions comparable to those found in viral hepatitis) and peroxides. In such liver damage the serum level of the liver enzymes, particularly serum glutamic-pyruvic transaminase, is raised and the extent of its control by the antihepatotoxic drug under test is used as a basis for estimation. Other effects of induced liver damage which can also be used in the evaluation of plant extracts are: the prolonged lengthening of the time of lost reflex induced by short-acting barbiturates; reduction of prothrombin synthesis giving an extended prothrombin time; reduction in clearance of certain substances such as bromosulphalein.
In order to reduce the number of animal experiments involved in these surveys H. Hikino and colleagues developed (1983–85) assay methods for antihepatotoxic activity using hepatotoxin-induced cytotoxicity in primary cultured hepatocytes. The fractionated plant extract under test and the hepatotoxin (e.g. CCl4) are added to the hepatocyte medium and incubated; the activity of the transaminases released intothe medium are then determined. The results obtained by this method were comparable with the in vivo assays. (For further details and references readers should consult H. Hikino in Biologically Active Natural Products, eds K. Hostettman; P. J. Lea, Proc. Phytochemical Soc. Europe, 1987, No. 27, p. 143; R. Gebhardt, Planta Med., 2000, 66, 99).
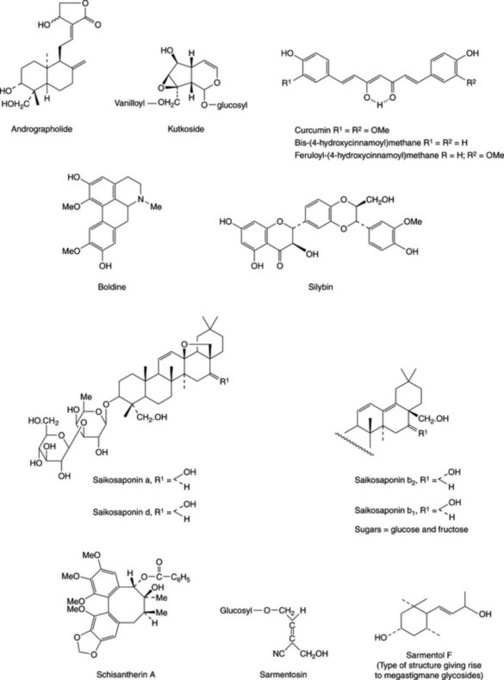
Handa and his group (Fitoterapia, 1991, 62, 229) reported that about 170 phytoconstituents isolated from 110 plants belonging to 55 families were stated to possess liver-protective activity; about 600 commercial herbal formulations with claimed hepatoprotective activity are being marketed world-wide. The active constituents elucidated to date involve a wide range of components including terpenoids, curcuminoids, lignoids, flavonoids, cyanogenetic glycosides etc., and some examples are given in Fig. 29.1. The terminal events in the attack on the liver by carbon tetrachloride involve the production of a highly reactive radical leading to lipid oxidation and the inhibition of the calcium pump of the microsome giving rise to liver lesions. Glycyrrhizin, glycyrrhetinic acid (Table 23.5) and wuweizisu C (Table 21.7) and gomisin A (lignoid constituents of Schizandra chinensis fruits) exert their activity as antioxidants. Similarly, the patented flavonoid extract (Kolaviron) of Garcinia kola seeds for the treatment of hepatic disorder has been shown to have antioxidant and scavenging properties E. O. Farombie et al., Pharm. Biol., 2002, 40, 107. Based on lignan content, high pressure liquid chromatography has proved useful for the identification and differentiation of samples of Schisandra chinensis and S. sphenanthera collected from different regions of China (Zhu Min et al., Chromatographia, 2007, 66, 125).
A number of plant drugs used for treating biliary disorders are cholagogues (they promote the flow of bile). Herbalists prescribe such drugs either singly or more commonly as mixtures; a cholagogue tea, for example, may consist of a mixture of Peppermint leaves 50.0% (principal cholagogue), Melissa leaves 20.0% (sedative adjuvant), Fennel fruits 20.0% (complementary carminative), Frangula bark 10.0% (gentle laxative).
In Europe the most widely used plant hepatoprotective agent is silymarin, a purified extract from Silybum marianum. Silymarin is also used as a standard against which to test other drugs. This and other antihepatotoxic and cholagogue drugs are listed below.
Silybum marianum
This plant, syn. Carduus marianus (Compositae) is one of the milk-thistles. Indigenous to the Mediterranean region, it has been introduced to most areas of Europe, North and South America and Southern Australia. In addition to cultivation as a medicinal crop the plant is grown as an annual or biennial for its attractive foliage. The glabrous leaves are dark green, oblong sinuate-lobed or pinnatifid, with spiny margins. White veins give the leaves, which initially form a flat rosette, a diffusely mottled appearance. The terminal heads which appear from July to September are also spiny with deep violet and slightly fragrant flowers. The achenes, 6–7 mm in length and transversely wrinkled, are dark in colour, grey-flecked with a yellow ring near the apex. Attached to the achene is a long white pappus.
G. Ram et al. (Fitoterapia, 2005, 76, 143) studied 15 accessions of the plant and suggest that an improved crop (seed yield/plant, number of capsules/plant) could be obtained by direct selection.
As a bitter the milk-thistle has a long history but more recently it has become recognized in Germany and continental Europe as a most effective liver remedy, particularly in those forms of hepatitis affecting the liver parenchyma.
Milk-thistle fruit
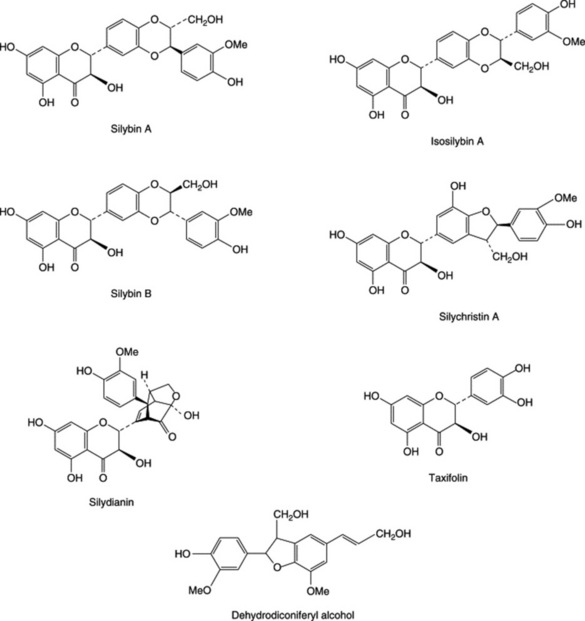
BP/EP, BHP 1996 consists of the mature fruit, devoid of pappus, of Silybum marianum L. Gaertner. Several so-called flavanolignans with marked hepatoprotective properties have been isolated from the fruits. A mixture of these, termed silymarin, is available commercially as a dried, purified and standarized extract. Principal components of the extract are three pairs of diastereoisomers: silybin (silibinin) A and silybin (silibinin) B, isosilybin (isosilibinin) A and isosilybin (isosilibinin) B and, silychristin (silicristin) and silychristin (silicristin) B; see Fig. 29.2 for formulae. Other related constituents are silandrin, 3-deoxysilychristin, silymonin and silydianin. A new compound, silyamandin, has recently been isolated from incubated tincture of milk thistle; it may arise from the degradation of silydianin (Fig. 29.2) (S. L. Mackinnon et al., Planta Medica, 2007, 73, 1214). The literature nomenclature for these compounds can be confusing as recommended international proprietary names (in brackets above) may also be used and both nomenclatures still appear to be used by authors. For isolation and structural reports on the above see DY-W. Lee and Y. Liu, J. Nat. Prod., 2003, 66, 1171, 1632; W. A. Smith et al., Planta Med., 2005, 71, 877. The levels of these flavonolignans vary markedly in samples of S. marianum from different sources; they are formed by various couplings of the flavonoid taxifolin and the lignan precursor coniferyl alcohol. Silymarin, in addition to the above flavonolignans, contains 20–30% of polyphenolic compounds. The scavenging (antioxidant) properties of the silymarin components have been tested against phenylglyoxylic ketyl radicals (F. Sersen et al., Fitoterapia, 2006, 77, 525).
Other non-specific constituents reported for the fruits involve flavonoids including taxifolin, sterols, dehydrodiconiferyl alcohol and fixed oil based principally on linoleic and oleic acids.
Silybin-like metabolites have been isolated from cell suspension cultures and although, to date, no glycosides of silybin have been reported in the plant, silybin-7-O-β-D-glucopyranoside can be produced by cell cultures of Papaver somniferum var. setigerum with added silybin (V. Kren et al., Phytochemistry, 1998, 47, 217.
The BP/EP test for milk-thistle fruit identifies silybin, silychristin and taxifolin and the liquid chromatography assay involves separation of the flavonolignans, summation of their peak areas and calculation of their total content in the drug by use of a reference solution of the same constituents; absorbance measurements are made at 282 nm.
Apart from the established use of silymarin as an antihepatotoxic agent, recent research together with clinical studies, have shown the flavonolignans to have anticancer properties (see Chapter 27).
For a 1995 extensive review of the drug (211 refs) see P. Morazzoni and E. Bombardelli, Fitoterapia, 1995, 66, 3.
Cynaria scolymus (Asteraceae/Compositae)
The leaves of the globe artichoke have been described in Chapter 19, the drug being used principally as a cholagogue (stimulation of bile production in the liver and promotion of emptying of the gall bladder and bile ducts). There has been more recent interest in the hepatoprotective properties of the plant and significant antioxidative activity has been demonstrated involving chlorogenic acid and cynarin; other constituents are, however, also implicated. For further details and references, see J. Barnes et al., Herbal Medicines, 3rd ed., 2007, p. 67. Pharmaceutical Press, London.
Peumus boldus
Boldo leaves (BP/EP; BHP 1996) are collected from the small tree Peumus boldus Molina (Monimiaceae) indigenous to Chile. The leaves are up to about 8 cm long, of coriaceous texture with a strong camphoraceous lemony odour owing to the presence of volatile oil (2%) containing ascaridole, linalool, p-cymene and cineole. E. Miraldi et al. (Fitoterapia, 1996, 67, 227) identified 46 components of the oil, 22 of which were recorded for the first time in P. boldus. The active constituents have been shown by laboratory testing to be alkaloids of the aporphine type (1–3%) the chief of which is boldine (Fig. 29.1).Boldine is reported to be a potent scavenger of hydroxyl, lipid and alkyl peroxyl radicals and experimental evidence supports its cytoprotective and anti-inflammatory properties (M. Gotteland et al., Planta Medica, 1997, 63, 311).
Studies on the genetic variation of the essential oil and alkaloid composition of the drug (H. Vogel et al., Planta Medica, 1999, 65, 90) indicated no significant differences in boldine concentration in samples from North, Central and South Chile; highest values for ascaridole were in northern collections and for p-cymene in the southern ones. There appeared to be considerable potential for the selection of superior strains. The BP requirement for volatile oil is not more than 2.0% (whole drug) and 1.5% (fragmented drug), and for alkaloid content (as boldine), not less than 0.1%. Reticuline and flavonoid glycosides have also been recorded. The bark, which contains a higher content of alkaloids (6–10%), is exported from Chile for the commercial production of boldine.
The leaves have a stimulant action on the liver and diuretic properties. An extract of the total alkaloids has a greater activity than boldine itself. The drug is also used in proprietary slimming preparations.
Taraxacum officinale root (Compositae). Syn: Dandelion root
Although now obsolete as a drug in the allopathic system of medicine dandelion root has maintained its importance in herbal medicine in which it is used as a hepatic stimulant, diuretic, tonic and antirheumatic. Commercial supplies of the cultivated drug come principally from Eastern Europe.
The drug consists of the dried vertical rhizome and tap-root which pass imperceptibly into one another. Up to 30 cm long when fresh and2.5 cm diameter, it is frequently branched with an apical crown bearing brownish hairs. The rhizome has a ring of vascular bundles and a pith; the root has a central yellow wood. Concentric zones of latex vessels occur in the thick bark. Dandelion root contains up to 25% of inulin and other polysaccharides, sesquiterpene lactones including taraxacoside, triterpenes including taraxerol, taraxol and B-amyrin, various acids including caffeic and p-hydroxyphenylacetic acids, and carotenoid yellow colouring matter.
In addition to its use in medicine the dandelion root is useful botanically for the demonstration of latex vessels (Fig. 42.7) and inulin (Fig. 42.1). The leaf has similar medicinal uses. Flavonoids, cinnamic acids and coumarins from different plant tissues and medicinal preparations have been recorded (C. A. Williams et al., Phytochemistry, 1996, 42, 121). For a review of the phytochemistry and pharmacology of the drug (over 90 refs + formulae), see K. Schultz et al., J. Ethnopharmacol. 2006, 107, 313.
Other drugs used to treat liver ailments as exemplified by the BHP (1983, 1991) and R. F. Weiss (1988, Herbal Medicine Ab Arcanum, Gothenberg, Sweden; Beaconsfield Publishers Ltd, Beaconsfield, UK), are listed below:
Euonymus atropurpureus (Celastraceae)
Bark is used in combination with other drugs for the treatment of gall bladder and liver disorders; it is essentially a cholagogue.
Fumaria officinalis (Fumariaceae), syn. Common fumitory
The whole herb, which also features in a number of commercial Indian preparations, is used for liver disorders; it contains isoquinoline alkaloids including canadine, dicentrine, fumaricine and sanguinarine.
Hydrastis canadensis (Ranunculaceae)
The root is employed in atonic dyspepsia with hepatic symptoms. A fuller description of the drug is given in Chapter 26.
Juglans cinerea (Juglandaceae), syn. White walnut, Butternut
The inner bark contains naphthoquinones including juglone together with fixed oil, volatile oil and tannins. Extracts are used for various conditions including use as a cholagogue.
Veronicastrum virginicum (Scrophulariaceae), syn. Black root
The bulk of modern research into the testing of plant antihepatotoxic drugs has focused on those that are used in the Asian and Oriental systems of medicine in which these medicines have an important role. On the Indian market there are available many patent polyherbal preparations involving a variety of combinations of Indian herbs (for details of the components of these preparations see A. Sharma et al., Fitoterapia, 1991, 62, 229). In many instances the liver protective properties ascribed to these long-established drugs have been corroborated by clinical and animal testing. For this group of drugs more modern work appears to have been reported on the Asian and Oriental drugs than on the corresponding plants used in the traditional European system. Research from other geographical areas is now appearing in the literature. A few notes on those which have received considerable attention are given below.
Andrographis paniculata (Acanthaceae)
This plant is found throughout the plains of India and all parts have been extensively used in Unani and Ayurvedic medicine; it is also utilized in Chinese medicine. The leaf juice is a household remedy for many ailments of the alimentary tract. Sharma et al. (loc. cit.) find this plant to be one of the most active ingredients of the Indian polyherbal preparations used to treat liver ailments. A number of researchers have described the isolation of flavonoids, xanthones, sesquiterpenes, lactones and other groups of compound from the plant. (M. K. Reddy et al., Phytochemistry, 2003, 62, 1271; V. K. Dua et al., J. Ethnopharmacol., 2004, 95, 247; W. Li et al., Chem. Pharm. Bull., 2007, 55, 455). More than 20 diterpenoids and not less than 10 flavonoids are known). The active antihepatotoxic principle is probably the diterpene lactone andrographolide (Fig. 29.1), which has been shown to protect against alcohol- and carbon tetrachloride-induced hepatic microsomal lipid peroxidation. For a report on the antiallergic activity of andrographolide and neoandrographolide see P. P. Gupta et al., Pharm. Biol., 1998, 36, 72. In a systematic review of the safety and efficacy of the drug for the treatment of uncomplicated upper respiratory tract infections, J. T. Coon and E. Ernst record adverse effects as mild and infrequent with preliminary evidence for a protective effect for the above condition (Planta Medica, 2004, 70, 293).
Aralia elata (Araliaceae)
A Japanese patent (Chem. Abs., 1991, 115, 132150) exists for the isolation from this plant of pentacyclic triterpenoid saponins for the treatment of liver disorders.
Boerhavia diffusa (Nyctaginaceae)
A plant with uses in the Ayurvedic system, including the treatment of jaundice; recently, it has also been shown to be a source of phytoecdysones. A number of pharmacological actions have been demonstrated for plant extracts including inhibition of increased serum aminotransferase activity in arthritic animals and an increase in liver ATP phosphohydrolase activity. A study on the whole-plant extract indicated hepatoprotective activity in CCl4-induced hepatotoxicity in rats; the extract is considered to be a safe and potent antihepatotoxic. A number of compounds have been isolated from the roots and hepatoprotective properties demonstrated; the most active roots were those collected in May (Lucknow) (A. K. S. Rawat et al., J. Ethnopharm., 1997, 56, 61).
Bupleurum falcatum roots (Umbelliferae)
This is one of the most important drugs in kampo, the traditional medicine of Japan, and in Chinese medicine. It is used for the treatment of chronic hepatitis, nephrosis syndrome and auto-immune diseases. A kampo formula which has shown promise in clinical trials for the treatment of chronic hepatitis B infection consists of a mixture of Bupleurum falcatum, licorice roots, Panax ginseng, Scutellaria baicalensis, Zizyphus jujuba, ginger root and Pinellia ternata (R. Reichert, Quart. Rev. Nat. Med., 1997 (Summer), 103; thro’ HerbalGram, 1998, No 43, 20).
The roots contain a group of oleanene saponins called saikosaponins a, b1–4, c, d and f (Fig. 29.1) and in addition a coumarin, several flavonoid derivatives, polyacetylenes, and other common constituents. Minor components include three saikogenins, two polyhydroxy sterols, a trihydroxy fatty acid, a lignan and a simple chromone designated saikochromone. The total pharmacological activity of the roots is not entirely accounted for by the saikosaponin content and H. Yamanda et al. (Planta Med., 1991, 57, 555) have pointed out that the root polysaccharides have a potent antiulcer effect against HCl/ethanol-induced ulcerogenesis in mice.
Saikosaponins a and d are more active on the liver enzymes than b1, b2 and c. The former pair also have the stronger haemolytic and anti-inflammatory properties. Hashimoto et al. (Planta Med., 1985,51, 401) have shown that the saikosaponins enhance the activity of low doses of corticosterone with respect to the induction of liver tyrosine aminotransferase. (For summaries of the pharmacological effects, see the above paper and K. Ohuchi et al., Planta Med., 1985, 51, 208.)
Saikosaponins have been produced by cell culture and by root culture; in the former, the dried cells are recorded as containing 0.26% saikosaponin d, similar to that found in the normal roots and in the latter this saponin occurred in high concentrations in 3-month-old adventitious roots derived from callus.
In experiments in which plants were cultivated from seven collections of seeds from different habitats in Japan it was shown that the species was polymorphic with respect to the saikosaponin contents of the roots.
It appears that a number of drugs are sold on the Far East markets under the name Bupleurum Root. For Taiwanese material Bupleurum falcatum contains the highest saikosaponin content followed by the indigenous B. kaoi.
For further information on the botany, cultivation, chemistry, pharmacology, clinical applications and patents for plants of the genus see S.-L. Pan (ed.), R. Hardman (series ed.) 2006 Traditional Herbal medicines for Modern Times (Vol 7): Bupleurium Species. CRC Press/Taylor and Francis Group, Boca Raton, Fl.
Eclipta alba (Compositae)
This species is a common annual weed found throughout India and elsewhere at altitudes up to 2000 m. In India it is a common component of polyherbal mixtures used for the treatment of liver disorders. The juice of the leaves is used as a hepatic tonic and deobstruent. The herb is reported to contain an alkaloid principle (ecliptine), lactones (e.g. wedelolactone) and resin. DNA-damaging steroidal alkaloids have been reported in material obtained from the Surinam rain forest (M. S. Abdel-Kader et al., J. Nat. Prod., 1998, 61, 1202).
Opuntia fuliginosa, prickly pear cactus (Cactaceae)
This cactus is used traditionally in Mexico for the treatment of oral diabetes mellitus. Research has demonstrated its value for human subjects and a purified extract, precluding a dominant role for dietary fibre, can achieve control of experimentally induced diabetes in rats. The active constituent(s) have not been identified. (A. Trejo-González et al., J. Ethnopharm., 1996, 55, 27).
Picrorrhiza kurroa (Scrophulariaceae)
There are two species only of this genus, both perennial herbs and found in the North-western Himalayan region at 3000–4000 m. P. kurroa rhizome (kutaki in Hindi) is used for liver ailments and as a cholagogue in Indian remedies. In 1965 clinical studies on the drug by Chaturvedi and colleagues indicated that, in patients with infective hepatitis and jaundice, there was a rapid fall in serum bilirubin levels towards the normal range and a quicker recovery with no untoward effects. In 1969 extracts of P. kurroa were shown to exert hepatoprotective activity in rats against CCl4-induced toxicity and in 1971 a hydrocholagogue activity was demonstrated. As indicated by the references given below the drug is still under intensive investigation.
The rhizome contains iridoid glycosides which have been named picroside I, picroside II, kutkoside (Fig. 29.1) and pikoroside. Various mixtures of these glycosides have been shown to possess hepato-protective properties. For references to the above and reports on the protective action of ‘Picroliv’ (a product containing about 60% picroside I and kutkoside, 1:1.5) against hepatotoxic agents see R. A. Asari et al., J. Ethnopharm., 1991, 34, 61; P. K. S. Visen et al., Phytotherapy Res., 1991, 5, 224; S. Srivastava et al., Fitoterapia, 1996, 67, 252; Qi Jia et al., J. Nat. Prod., 1999, 62, 901; R. Ananden and T. Devaki, Fitoterapia, 1999, 70, 54; K. L. Joy and R. Kuttan, J. Ethnopharm., 1999, 67, 143; B. Saraswat et al., J. Ethnopharm., 1999, 66, 263. A US patent exists for the extraction of these pharmacologically active compounds (Chem. Abs., 1992, 117, 220069).
Sedum sarmentosum (Crassulaceae)
A number of Sedum species have found use in Chinese medicine. In 1982 Chinese scientists reported the isolation of a new cyanogenetic glycoside, sarmentosin (Fig. 29.1), from plants of the genus; this compound significantly lowered the serum glutamic-pyruvic transaminase (SGPT) level of patients with chronic viral hepatitis. A considerable number of various megastigmanes and their glycosides based on the type of structure illustrated by sarmentol F (Fig. 29.1) have recently been reported in the whole plant (T. Morikawa et al., Chem. Pharm. Bull., 2007, 55, 435; M. Yoshikawa et al., J. Nat. Prod., 2007, 74, 575).
Schisandra chinensis (Magnoliaceae)
The fruits and seeds of this East Asian liana and other species of the genus are used in Chinese medicine to treat a chronic persistent type of hepatitis. In addition to its antihepatotoxic activity, the drug has been shown to have anticancer, antioxidant and physical performance improvement properties; clinical effectiveness has yet to be established. The active constituents are various biphenyl octenoid lignans; compounds isolated and characterized include wuweizisu C (Table 21.7), wuweizichun B, schisantherin A (Fig. 29.1), B, C and D which all produce a lowering of the level of SGPT. The structure–activity relationships of these lignans as PAF antagonists has been studied (I. S. Lee et al., Biol. Pharm. Bull., 1999, 22, 265). The herb Wu Wei Zi is becoming familiar in Western health food stores as a general tonic, being sold either singly or as a component of a mixture.
For a review of the botany, chemistry and pharmacology of Schisandra chinensis see J. L. Hancke et al., Fitoterapia, 1999, 70, 451; for an overview of Russian research and medicinal uses see A. Panossian and G. Wikman, J. Ethnopharm., 2008, 118, 183.
Other species of Schisandra recently investigated for their biologically active constituents, chiefly lignans and triterpenoids, include S. sphaerandra and S. propinqua; for a report on the Thai species S. verruculosa, and further references, see R. Wilairat et al., Pharm. Biol., 2006, 44, 411. The composition and biological activity of different extracts from S. chinensis and S. spheranthera have been studied (C. Huyke et al., Planta Medica, 2007, 73, 1116).
Turmeric
Turmeric (Curcuma) is the dried rhizome of Curcuma longa (Zingiberaceae), cultivated in India, West Pakistan, China and Malaya. The primary and secondary rhizomes are dug up, steamed or boiled, and dried.
The primary rhizomes are ovate or pear-shaped and are known as ‘bulb’ or ‘round’ turmeric, while the more cylindrical secondary, lateral rhizomes are about 4–7 cm long and 1–1.5 cm wide. The latter are known as ‘fingers’ and contain more yellow colouring matter than the bulb variety. Turmeric has an aromatic odour and a warm somewhat bitter taste.
Turmeric contains about 5% of diaryl heptanoid colouring materials known as curcuminoids, the chief of which is curcumin (diferuloylmethane), together with smaller quantities of dicaffeoylmethane and caffeoylferuloylmethane (Fig. 29.1). Dihydrocurcumin was reported in 1980. The volatile oil (about 5%) contains sesquiterpenes (e.g. zingiberene, 25%), sesquiterpene alcohols and ketones, and monoterpenes. A detailed study of the distribution of components of the oil in various organs of the plant has been reported (R. P. Bansal et al., Pharm. Biol., 2002, 40, 384). Enzymes associated with the biosynthesis of curcuminoids in turmeric and ginerols in ginger have been identified (M. del C. Ramirez-Ahumada et al., Phytochemistry, 2006, 67, 2017).
In a series of papers Japanese workers reported on the characterization of the constituents of a polysaccharide fraction of the drug, which has a marked immunological activity. The acid glycans designated ukonan A, B and C show remarkable reticuloendothelial system (RES) potentiating properties. Ukonan A, for example, is composed of L-arabinose, D-xylose, D-galactose, D-glucose, L-rhamnose and D-galacturonic acid in addition to small amounts of peptide moieties. Ukonan D, a neutral polysaccharide, also shows RES-potentiating activity as indicated in a carbon clearance test; it has an estimated molecular mass of 28 000 and is composed of L-arabinose, D-galactose, D-glucose and D-mannose in the molar ratio 1:1:12:0.2, plus a small peptide fraction. (For further details and references concerning these polysaccharides see R. Gonda et al., Chem. Pharm. Bull., 1992, 40, 185.)
The rhizomes also contain free arabinose (about 1.0%), fructose (12%) and glucose (2%). Abundant zingiberaceous starch grains, about 30–60 μm long and often gelatinized, are present.
Turmeric is used in Indian and Oriental medicine as an aromatic stomachic and diuretic as well as a treatment for jaundice and hepatitis. The choleretic activity of curcumin was described in 1956–7 and in vitro experiments have now demonstrated the strong antihepatotoxic action of the curcuminoids. For a review of the pharmacology of the drug see H. P. T. Ammom and M. A. Wahl, Planta Med., 1991, 57, 1.
Large quantities of turmeric are used in the preparation of curries and sauces. Paper impregnated with an alcoholic extract of turmeric is used as a test for boric acid and borates.
Javanese turmeric
The rhizome of this plant, Curcuma xanthorrhiza, is described in the BP/EP. As the name implies it is obtained from Indonesia with smaller quantities coming from India. Its constituents are similar in chemical nature and pharmacological properties to those of C. longa.
The official requirement for volatile oil content is not less than 5%, one sesquiterpene component (xanthorrhizol) being characteristic for the species. Dicinnamoyl methane derivatives ( 0.1%), expressed as curcumin, are assayed by absorbance measurement at 530 nm.
0.1%), expressed as curcumin, are assayed by absorbance measurement at 530 nm.
Curcuma zedoaria rhizome (zedoary) finds medicinal use in the East as a carminative and digestive stimulant and in the treatment of colds and infections. It occurs as circular slices of rhizome resembling bulb turmeric and contains many types of sesquiterpenoids. An active curcuminoid, demethoxycurcumin, has been isolated by bioassay-directed fractionation (W.-J. Syn et al., J. Nat. Prod., 1998, 61, 1531). The antimicrobial properties of both C. zedoaria and C. malanarica have been demonstrated (B. Wilson et al., J. Ethnopharmacol., 2005, 99, 147).
C. wenyujin is a Chinese medicinal plant, the constituents of which exhibit antitumour activity. Among other constituents the essential oil contains a novel superoxidized sesquiterpene named wenjine.
Wendelia calendulaceae (Compositae)
This herb is ascribed medicinal properties similar to those of Eclipta spp, and may be used as a single plant remedy in Asian medicine. An alcoholic extract of the plant has been shown to possess hepatoprotective activity.
Other plants for which positive antihepatotoxic properties have been recorded include: Aeginetia indica (Orobanchaceae); Artemesia capillaris buds, A. maritima (Compositae); Atractylodes sp., rhizome (Compositae); Berberis spp. (Berberidaceae); Citrus limon fruits (Rutaceae); Glycyrrhiza spp. (Leguminosae), principally glycyrrhetinic acid and glycyrrhizin (q.v.); Mallotus japonicus (Euphorbiaceae); Panax ginseng roots (Araliaceae) (q.v.); Phyllanthus niruri (Euphorbiaceae); Plumbago zeylanica (Plumbaginaceae); Salvia plebeia (Labiatae); Swertia japonica (Gentianaceae); Tephrosia purpurea (Leguminosae); Tetrapanax papyriferum leaves (Araliaceae); Uncaria gambir (Rubiaceae); Withania somnifera (Solanaceae).
There is obviously much further scope for the clinical testing of these drugs to ascertain whether toxicity levels and side-effects of the constituents would permit them to be recognized in Western medicine.
PLANTS WITH ORAL HYPOGLYCAEMIC ACTIVITY
Diabetes arises from a deficient production of insulin by the β-cells of the pancreatic islets. The endocrine hormone operates at various sites throughout the body regulating carbohydrate, triglyceride and protein metabolism and controlling entry of glucose into the blood. Insufficient insulin results in hyperglycaemia and the symptoms of diabetes, namely, an excess of sugar in the blood and urine, hunger, thirst and a gradual loss of weight. The disease is estimated to affect 4–5% of the population and patients are generally classified as either insulin-dependent diabetics (type 1) or non-insulin-dependent diabetics (type 2). A third type, malnutrition-related diabetes mellitus, affects young people in poor tropical countries and is associated with a history of nutritional deficiency. The type 1 group includes all diabetic children, the majority of those under 40 years of age and a few over 40 years. These individuals lack the functional β-cells necessary to synthesize the hormone and insulin therapy is the only satisfactory treatment. Type 2 diabetics, constituting some 75% of the diabetic population, have functional pancreatic β-cells but there is, nevertheless, a deficiency in insulin production; patients are those in which the disease has usually manifested after the age of 40.
In many cases the type 2 condition can be controlled by a suitable diet and exercise but if this is not successful treatment with oral hypoglycaemics in conjunction with a suitable dietary regimen may prove satisfactory. These drugs act in a variety of ways: (1) by stimulating the β-cells to produce insulin; (2) by decreasing gluconeogenesis and increasing peripheral utilization of glucose—success is still dependent on some limited production of insulin by the pancreas; (3) retardation of carbohydrate absorption from the gut resulting in a reduction of excessive postprandial plasma-glucose concentration. In Western medicine these three aspects are effectively accommodated by the respective use of (1) sulphonylureas, (2) biguanides and (3) Guar gum.
It is important however to appreciate that there are also many plants and plant extracts which possess marked hypoglycaemic activity. From ancient times such materials have been used for the treatment of diabetes mellitus and still find extensive use in traditional medicine world-wide. Apart from their importance per se these plants represent possible sources of new drugs which may act in ways additional to those indicated above; as long-established traditional remedies they may be less toxic than some previously untried medicaments. As emphasized in Chapter 36, traditional cures are much used by immigrants who seek advice from their own healers and it is important that medical staff at hospitals recognize that such patients may be taking drugs about which little is known in orthodox medicine. Diabetic patients taking such traditional oral hypoglycaemics present a particular problem.
There have been several comprehensive reviews covering plant hypoglycaemics. Oliver-Bever and Zahnd (Quart. J. Crude Drug Res., 1979, 17, 139) reviewed the literature up to 1978 and Ivorra et al. (J. Ethnopharm., 1989, 27, 243, c. 165 refs) covered the subsequent 10 years and have tabulated both constituents and plant species. Atta-ur-Rahman and Zaman’s review (J. Ethnopharm., 1989, 26, 1, 383 refs) of the published literature on the antidiabetic activity of some 343 plants covers the period 1907–88 andtheir Table illustrates well the world-wide usage of these plants; the chemical structures of over 40 hypoglycaemic phytochemicals are given. Handa et al. (Fitoterapia, 1989, 60, 195) reviewed (260 refs) about 150 plants reported to have hypoglycaemic activity and, where available, have incorporated information on active principles. A. Andrade-Cetto and M. Heinrich have recorded 306 species of Mexican plants traditionally used for their hypoglycaemic effect in the treatment of diabetes; seven of these spp. are discussed in some detail highlighting current knowledge and the enormous gaps regarding toxicity, pharmacokinetics and metabolism of the active constituents (J. Ethnopharmacol., 2005, 99, 325). See also ‘Further reading’ for more recent reviews.
Methods of studying oral hypoglycaemics include: clinical trials involving both normal human volunteers and type 2 diabetics; effect on glucose levels of normal animals (rabbits, rats, mice and dogs have been used); measurement of the reduction of blood sugar levels of animals having glucose-induced hyperglycaemia, and also with alloxan- and streptozotocin-induced diabetic animals. Such tests do not evaluate toxicity, and specifically, a number of drugs which are hepatotoxic and affect liver enzymes involved in gluconeogenesis will lower the blood sugar level, thus giving false-positive results. An example of this concerns the unripe fruits of the akee tree, Blighia sapida (Sapindaceae), which contain the toxic principles hypoglycin A and B. These cyclopropanoid amino acids (formula Table 39.1) have marked hypoglycaemic properties and act on the liver by blocking the oxidation of fatty acids. The resulting depletion of liver glycogen leads to a lowering of the blood sugar level. In the West Indies green akee fruits are eaten parboiled, the cooking water being discarded, but over the years many fatalities appear to have occurred in Jamaica as a result of the consumption of uncooked fruits.
The wide range of structures of those plant constituents which appear to be the active hypoglycaemic principles suggests different sites of action within the body. Polysaccharides feature prominently in antidiabetic plants and they include the glycans of Aconitum carmichaelii roots (a traditional oriental drug), Ephedra distachya (the oriental crude drug Mao), Gymnema sylvestre leaves (an Ayurvedic drug used to control blood sugar levels in diabetics), Lithospermum erythrorhizin (q.v.), Panax ginseng and P. quinquefolium (q.v.) and the non-sucrose portion of the juice of stems of Saccharum officinarum. The traditional use of various Dioscorea species in oriental medicine appears justified by demonstration of the hypoglycaemic activity of their polysaccharides in animal tests. In Mexican traditional medicine one of the most important antidiabetic remedies is a root decoction of Psacalium decompositum (syn. Cacalia decomposita), Compositae. F. J. Alarcon-Aguilar et al. (J. Ethnopharm., 2000, 69, 207) have found the hypoglycaemic effect on healthy mice to lie, not in the known sesquiterpenes (cacalol, cacalone, maturin), but in two polysaccharide fractions of the freeze-dried water decoction of the roots. Some polysaccharide seaweed extracts have been shown to be active. Plant mucilages with similar pharmacological properties include those from some members of the Malvaceae (Abelmoschus spp., Althaea spp.) and Plantago (Plantaginaceae). Generally, deacetylation of mucilages enhances activity.
Some flavonoids, phenols and related compounds have been shown to have hypoglycaemic activity. One such compound is (−)-epicatechin (formula, Fig. 21.7), which is contained in the bark of the tree Pterocarpus marsupium (Leguminosae). As a drug it is popular with Ayurvedic physicians for the treatment of diabetes mellitus and has been the subject of a number of clinical trials in India. The heart-wood is similarly used and beakers turned from the wood are filled with water and allowed to stand overnight to give ‘Beeja wood water’. This cold infusion is stated to maintain the blood sugar of diabetics at normal concentrations until treatment is withdrawn. The wood contains pterostilbene (Fig. 29.3) the positive activity of which has been confirmed. The dried juice of this tree is the source of Malabar kino.
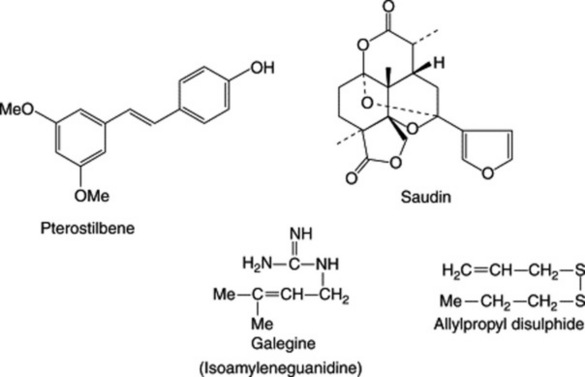
Fig. 29.3 Phytochemicals having oral hypoglycaemic activity illustrated by a phenol, a diterpene, a guanidine derivative and a disulphide. For other groups of active compounds see text.
Steroid-containing plants known to exhibit antidiabetic activity include the barks of various species of Ficus, the roots of ginseng [the ginsenosides (steroids) as well as the pannaxosides (glycans, above) have been shown to be active in many animal tests], fenugreek, and the fruits and seeds of various Cucurbitaceae. The last includes Momordica charantia or karela fruit, extracts of which are commonly used on the Indian subcontinent for the treatment of diabetes. The seeds of the related M. cochinchinensis (Sprengel seeds) contain two active glycosides as tested on streptozotocin-induced diabetic rats. Both glycosides have oleanolic acid as aglycone; one involves glucose and the other glucose and arabinose. Balanites aegyptica (Zygophyllaceae) finds a variety of uses as a folk medicine in many countries of Africa. In Egypt the fruits, after removal of the epicarp, are used as an oral antidiabetic. Aqueous extracts have been shown to exhibit a pronounced effect by oral administration to streptozotocin-induced diabetic mice. The fruits contain steroidal saponins which are active as a recombinant mixture but not singly. The saponins of B. aegyptica are also molluscicidal (q.v.). The genus Ajuga contains steroidal withanolides (q.v.) and A. bracteosa and A. iva are used in Pakistan and N. Africa respectively for the treatment of diabetes. The active constituents are stated to potentiate the effects of insulin.
In a series of papers Mossa and associates reported on a diterpenoid compound named saudin (Fig. 29.3) with a novel 6,7-secolabdane skeleton (Int. J. Crude Drug Res., 1990, 28, 163 and references cited therein). Saudin was isolated from the euphorbiaceous plant Cluytia richardiana which grows in the mountainous regions of western and southern Saudi Arabia. It has marked hypoglycaemic effects and its use in the treatment of diabetes mellitus has been patented (US).
Comparatively speaking, the number of alkaloid-containing plants reported to have antidiabetic properties appears to be small. Coptis chinensis (Ranunculaceae), a Chinese medicinal herb, contains berberine, and active simpler nitrogen compounds are found in Capsicum, Allium, Lathyrus, Lepidium and Galega. Galega officinalis (Leguminosae), syn. Goat’s Rue, is a herb native to S.E. Europe but introduced elsewhere including Britain. An erect glabrous perennial up to 150 cm high, it has leaves composed of 13 or 15 oblong or oblong-ovate, mucronate or marginate leaflets with white, lilac or pinkish papilionate flowers. It is included in the BHP (1983) and the active principle is galegine (isoamyleneguanidine) (Fig. 29.3), which shows hypoglycaemic activity when tested on alloxan-diabetic rats. Other constituents are 4-hydroxygalegine, peganine, saponins and flavonoids. Peganine and related alkaloids are well-known as constituents of Peganum harmala (Zygophyllaceae) seeds which are themselves used in some areas for treating diabetes. However, these seeds have other marked pharmacological actions and on the basis of their toxicity to rats M. M. Al-Zaid et al. (Int. J. Pharmacognosy, 1991, 29, 81) have suggested that their use should be discouraged.
A plant, the various parts of which have been used in antidiabetic preparations in Europe and India, is Syzygium cumini (Myrtaceae) syn. Eugenia jambolana. The dried fruits are described in the BHP (1983) and may be prescribed with Galega (see above). Constituents of the seed include phenols, tannins, essential oil in small quantity, an alkaloid and glycoside, and triterpenes. The hypoglycaemic action of the fruit extract seeds (M. R. M. Rafiullah et al., Pharm. Biol., 2006, 44, 95), and seed kernels (K. Ravi et al., Pharm. Biol., 2003, 41, 578) have been experimentally verified. Likewise for the seed kernels of the fever nut, Caesalpinia bonducella, (S. Parameshwar et al., Pharm. Biol., 2002, 40, 590).
A number of complex indole alkaloids of the Apocynaceae have been shown to exhibit hypoglycaemic activity with animal models and include those of Rauwolfia, Vinca and Catharanthus. It is of interest that Handa records (loc. cit.) that the Madagascan periwinkle C. roseus, now used as a source of anticancer alkaloids was, during World War II, used as a tea by diabetic natives of the Philippine Islands and apparently served as a successful substitute for the then unavailable insulin.
The onion (Allium cepa) and garlic (A. sativum) (Liliaceae) have long been used in traditional medicine but have recently been a source of much interest because of their antithrombitic, hypolipidaemic, hypoglycaemic, hypotensive, diaphoretic, expectorant and antibiotic medicinal properties. Their hypoglycaemic action derives from disulphides such as allicin (diallyldisulphide oxide) and allylpropyldisulphide (Fig. 29.3) both of which have been shown to be active in animals and humans. It has been envisaged that, by virtue of their thiol groups, these disulphides act as sparing agents for insulin by competing with it for inactivating compounds.
The inorganic materials such as potassium, zinc, calcium, manganese and small amounts of chromium found in plants are believed to have a beneficial effect in the treatment of diabetes mellitus. In preliminary studies using the oral glucose tolerance test, A. Kar et al. (J. Ethnopharmacol., 1999, 64, 179) investigated the inorganic constituents of 30 medicinal plants which are used in the traditional treatment of hyperglycaemia in the form of their ash. The results included: Syzygium cumini (Eugenia jambolana) seed and Momordica chirantia afforded ashes having a superior action to the organic plant extract; Ficus glomeratus and Gymnema sylvestre showed activity for both the organic and inorganic samples; Vinca rosea and Zingiber officinale had greater activity in the organic state than as ashes.
There are also numerous plants that by traditional experience and pharmacological tests have proven hypoglycaemic activity but for which the chemical constitution is lacking. One such example concerns the leaves of Globularia alypum (Globulariaceae) used in Morocco for the treatment of diabetes and shown to have hypoglycaemic activity in rats. Enhanced peripheral metabolism of glucose and increased insulin release has been advanced to explain its pharmacological activity (F. Skim et al., Fitoterapia, 1999, 70, 382). A similar situation exists for the flowers of Punica granatum which are also recommended in Unani medicine for the treatment of diabetes, see N. A. Jafri et al., J. Ethnopharmacol., 2000, 70, 309. Viscum album (mistletoe) leaves are widely used in Nigerian folkloric medicine for the treatment of diabetes and positive hypoglycaemic properties have been reported using alloxan-induced diabetic animals (F. C. Ohiri et al., Pharm. Biol., 2003, 41, 184). In similar experiments, positive results have been obtained with an ethanolic leaf extract of Nymphaea stellata, a plant used traditionally in Indian medicine to treat a variety of conditions including diabetes (S. P. Dhanabal et al., Fitoterapia, 2007, 78, 288). The traditional use in Indian medicine of Curculigo orchioides tubers (family Hypoxidaceae) for the treatment of diabetes has also been supported by experiment (V. Makhavan et al., Pharm. Biol., 2007, 45, 18).
The above examples serve to show that there is potential scope for the use of oral plant antidiabetics in medicine. However it must be remembered that with experimental models the most pronounced hypoglycaemic dose is not necessarily achieved with the normal recommended therapeutic dose of starting plant material. In most cases further toxicity studies must be undertaken and clinical trials and stability of the plant extract generally require further study. Meanwhile, diabetic patients hoping to find a ‘natural’ cure for their condition should be dissuaded from embarking on unsuperivsed herbal treatment to the neglect of their diet and insulin therapy. High acetone levels or diabetic polyneuritis which may arise by inadequate therapy then require much skill and time to remedy.
Li WL, Zhen HC, Bukuru J, De Kimpe N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. Journal of Ethnopharmacology. 2004;92(1):1-21. Over 80 plants considered, chemical formulae, over 200 references
Mukherjee PK, Maiti K, Mukherjee K, Houghton PJ. Leads from Indian medicinal plants with hypoglycemic potentials. Journal of Ethnopharmacology. 2006;106(1):1-28. Over 60 spp. listed, alkaloids, imidazoline compounds, polysaccharides, flavonoids, dietary fibres, saponins, ferulic acid
Soumyanath A, Hardman R, editors (series ed) Traditional herbal medicine for modern times: antidiabetic plants. CRC/Taylor and Francis Group, Boca Raton, FL. 42-page table of plants, chapters covering polysaccharides, saponins and polyphenols, 2006;527 pp.