Chapter 31 Vitamins and hormones
Both vitamins and hormones constitute a range of many different types of organic molecule which are essential to the proper functioning of the human organism. Their absence or depletion gives rise to deficiency diseases, and, particularly with hormones, an excess can also be harmful. Vitamins are obtained largely from the diet, whereas hormones are manufactured in the body.
VITAMINS
Vitamins, formerly known as ‘accessory food factors’, are present in many animal and vegetable foods. Their absence from the diet causes deficiency diseases such as scurvy, beri-beri, rickets and night blindness. The value of citrus juices in the treatment of scurvy was realized in the eighteenth century. Systematic feeding experiments began about 1873 and much work on the subject was done by Gowland Hopkins from 1906 to 1912. Fraser and Stanton established that beri-beri was produced in people living mainly on polished rice, who could be cured if ‘rice-polishings’, the outer part of the grain removed in making polished rice, were added to the diet. In 1911 Funk coined the name ‘vitamine’ now usually spelt vitamin, for the active fraction of rice-polishings.
The existence of vitamin A was proved in 1915 and other letters were applied to later vitamins discovered. Many vitamins have since been proved to be extremely complex mixtures, and one now speaks, for example, of the vitamin B complex, components of which can be referred to as B1, B2, etc., or by their chemical or other names. As the chemical nature of the vitamins has been discovered and vitamin complexes have been resolved into their constituents, there is an increasing tendency in the scientific and medical literature to discard the term ‘vitamin’ with its associated letter (and number) in favour of the chemical name for the material under consideration (see, for example, the BP monographs on Hydroxocobalamin, Riboflavine and Thiamine Hydrochloride). However, in the lay literature the original vitamin terminology persists and pharmacists need to be familiar with this. Some vitamins have as yet no proved role in the treatment of human diseases but others are valuable items of the materia medica. A large number of different pharmacopoeial and proprietary vitamin preparations are available but with a well-balanced diet the normal individual should require no vitamin supplementation (Table 31.1). However, people on a strict vegetarian diet who eat no eggs or dairy produce need a supplement of vitamin B12; and alcoholics need vitamin B1, which is required for the complete metabolism of ethanol. Other groups, such as narcotic drug users, whose diet is generally inadequate are also prone to vitamin deficiency. Need for vitamins is still great in many underdeveloped countries. Notwithstanding the above, the consumption by the general public of vitamin preparations is enormous and this is one of the larger areas of the pharmaceutical industry. Numerous publications on healthy foods and promotional leaflets ensure that these substances are universally recognized.
Table 31.1 Sources of vitamins.
| Vitamin | Alternative names | Distribution |
|---|---|---|
| A (A1, A2) | Anti-infective or antixerophthalmic vitamin, retinol | Fish livers (cod, halibut, shark, etc.) and other animal fats. Plants contain proto-vitamin A, the vitamin precursors (e.g. α-, β- and γ-carotene) and cryptoxanthine; these are converted to vitamin A in liver |
| B1 | Aneurine, thiamine | Rice polishings, cereal germ, animal organs, yeast or prepared synthetically |
| B2 | Riboflavine | Widely distributed in both plants and animals; bacteria, yeasts and other fungi, cereal grains and many fruits |
| B3 | Niacin, nicotinic acid, nicotinamide, niacinamide, pellagra-preventing or PP vitamin | Milk, eggs, liver, yeast, malted barley, or may be prepared by fermentation |
| B5 | Pantothenic acid | Yeast, liver, red meat, chicken, milk, mushrooms, beans, bananas, nuts, avocados, potatoes |
| B6 | Pyridoxine, pyridoxine hydrochloride | Prepared synthetically but present in many foodstuffs, including yeast, liver, red meat, fish, yoghurt, bananas, cabbage, wholegrains |
| B9 | Folic acid, folacin, vitamin M | Yeast, liver, green plants, wholemeal bread, oranges, nuts |
| B12 | Cyanocobalamin, megaloblastic anaemia vitamin | From livers or from the metabolic products of microorganisms such as Streptomyces griseus |
| C | Ascorbic acid | Fruits, particularly citrus fruits, tomatoes, potatoes, capsicums; raw vegetables; or made synthetically |
| D2 | Antirachitic vitamin; calciferol, ergocalciferol | Calciferol is produced by irradiation of ergosterol |
| D3 | Cholecalciferol | Formed by irradiation of cholesterol. It is found in fish-liver oils (e.g. cod, halibut) and in human skin following exposure to sunlight |
| E | Tocopherols, alpha tocopheryl acetate | Embryos of cereals (wheat and maize germ oils); other vegetable oils (palm, olive, etc.); fresh vegetables, nuts, eggs, butter |
| H | Biotin (two forms), coenzyme R | Yeast, peanuts, chocolate, carrots, liver, kidney, eggs |
| K1 | Phytomenadione, coagulation factor, antihaemorrhagic vitamin | From plants (e.g. alfalfa, lucerne, tomatoes, etc.); or by synthesis. Abundant in the human intestine, where it is synthesized by intestinal bacteria |
| P | Permeability factor (significance now doubtful) | Flavonoids derived especially from Citrus, Ruta, Sophora and other genera |
| Ubiquinone 10 | Ubidecanenone; coenzyme Q10. Has been referred to as Vitamin Q10 | A coenzyme found in liver; also in other metabolic tissues of plants and animals |
It will be noted in Table 31.1 that a number of gaps appear in the naming of the vitamins and this is because some substances once regarded as vitamins (e.g. vitamin F and a number of the B group) are of indefinite character or have been reclassified as essential nutritional factors.
Chemically, vitamins vary from very simple compounds to very complex ones. They belong to no one chemical type. Vitamin A has already been mentioned under ‘Diterpene compounds’; vitamin C has affinity with the sugars, being the enolic form of 3-oxo-L- gulofuranolactone; B12, which first became official in 1963, has a very complex molecule. Several forms of vitamin D occur. Vitamin K1 is 2-methyl-3-phytyl-1,4-naphthoquinone. As might be expected from these wide variations in structure, vitamins differ from one another in physical properties such as solubility. They have been traditionally classified according to their water-solubility and fat-solubility properties and this division is still useful. In the main, the water-soluble vitamins are non-toxic and can be consumed in large doses without harm; they also remain in the body for a relatively short time. Conversely, the fat-soluble vitamins are more toxic in large doses and are stored in the fatty reserves of organs of the body for long periods of time. The solubilities also determine the type of food products in which the two groups occur, e.g. fatty dairy products as opposed to plant juices.
FAT-SOLUBLE VITAMINS
Vitamin A (A1; A2)
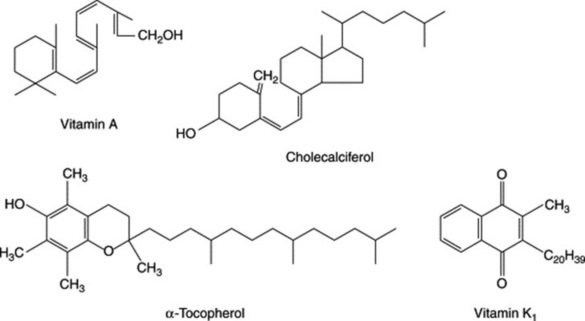
Vitamin A is found as such only in the animal kingdom and is particularly abundant in fish-liver oils. The preparation of cod-liver oil is described below. Vitamin A occurs in three or more forms termed vitamers. Vitamin A1, retinol (see Fig. 31.1), is an alcohol and retinal is its corresponding aldehyde. Vitamin A2, dehydroretinal, has a second unsaturated bond in the ring system and also occurs as the aldehyde dehydroretinol. The carotenes (see Chapter 24) are C40 compounds found in the plant kingdom and are converted to vitamin A in the small intestine and other organs. Although the formulae of the carotenes might suggest that each molecule would give rise to two molecules of vitamin A, the successive oxidations of the molecule in fact give rise to only one molecule of the vitamin. Infants and young children have only a limited capacity to effect this conversion and true carnivores (e.g. cats) and invertebrate animals are unable to utilize carotene in this respect.
Vitamin A is decomposed by exposure to light and may be assayed in fish-liver oils and other preparations by ultraviolet absorption and spectrophotometry.
Vitamin A is essential for the normal functioning of the body epithelia and the retina. Deficiency is indicated by night blindness and by a drying and crusting of the mucous membranes.
Vitamin D
The compounds comprising this group have antirachitic activity and are individually designated D2–D6; they are formed by the opening of ring B of a steroidal provitamin. Vitamin D3 (cholecalciferol, see Fig. 31.1) is the only member to occur naturally in higher animals and is formed photochemically from 7-dehydro-cholesterol by the sun’s irradiation of the skin. Vitamin D2 (calciferol, ergocalciferol) differs from D3 in having an unsaturated side-chain. D4, D5 and D6 are produced artificially by the irradiation of 22-dihydroergosterol, 7-dehydrositosterol and 2-dehydrostigmasterol respectively. These vitamins are relatively stable and preparations containing them are assayed (BP/EP) by liquid chromatography using, as a standard, a preparation of crystalline vitamin D3.
Vitamin D regulates the calcium and phosphorus balance in the body by direct action on phosphorus metabolism. It promotes calciumabsorption and is an essential factor in bone formation (a deficiency causes rickets). Excessive doses of the vitamin should be avoided.
Vitamin E
Contained in this group are a number of tocopherols, prefixed α-, β-, γ-, etc, which are of wide occurrence in plants, being particularly abundant in the germ oil of cereals. For the preparation of vitamin products the cereal embryos are conveniently separated during the manufacture of the appropriate starches; α- (see Fig. 31.1), β- and γ-tocopherols are among those found in the germ of wheat, barley and rye, whereas others are found in soya beans, ground nuts and maize. Oats contains some five different tocopherols. The various tocopherols differ in the methylation patterns of the ring system. Virgin Wheat-germ Oil and Refined Wheat-germ Oil are included in the BP/EP; also seven monographs based on derivatives of the racemic and RRR-α-tocopherols. These are evaluated by gas chromatography.
Discovered in 1922, vitamin E is a powerful antioxidant and has an important role in the preservation of the well-being of cells, for slowing their ageing effects and in counteracting the harmful aspects of toxins in the blood and lungs. It may assist protection of the cardiovascular system by preventing blood–lipid peroxidation with the subsequent formation of sticky deposits. Traditionally the vitamin has been associated with the improvement of fertility.
A normal diet supplies adequate amounts of the vitamin; deficiency leads to the destruction of red blood cells with resultant anaemia. It may be added to cod-liver oil (q.v.).
Vitamin K (phytomenadione, phylloquinone)
This vitamin occurs in several natural forms. Vitamin K1 (Fig. 31.1) is found in many plant sources and has a C20 side-chain with one unsaturated linkage. K2, originally prepared from decaying fish, has a polyunsaturated isoprenoid side-chain which is of variable length. These compounds, termed menaquinones (MK), are produced by bacteria and, as an example, MK-8 refers to a menaquinone produced by Escherichia coli with 8 isoprene units and 40 carbon atoms in the side chain. (For the biogenesis of these compounds, see R. Bentley and R. Meganathan, J. Nat. Prod., 1983, 46, 44.) The formation of phylloquinone in green plants has received less attention; chorismic acid (q.v.) and 2-succinylbenzoic acid are probable intermediates. Similar compounds with vitamin K activity have been synthesized.
Vitamin K is a necessary factor in the blood-clotting process; it acts indirectly by activating those substances which are necessary for the conversion of prothrombin to thrombin. In healthy individuals it is possible that the intestinal flora provides an adequate supply of the vitamin. Deficiency symptoms are prolonged bleeding and excessive bruising.
COD-LIVER OIL
Medicinal cod-liver oil is a fixed triglyceride oil prepared from the fresh liver of the cod, Gadus morhua L. and other species of Gadus (family Gadidae) under conditions which give a palatable oil containing a due proportion of vitamins. To comply with European requirements, two oils (Type A and Type B) are described in the BP. Both have identical standards for vitamin contents but the former has a limit test governing secondary oxidation of the oil (see standardization). The Type B oil is the principal commercial product. In Western Europe the principal producers and suppliers of the raw material are now Norway and Iceland with much of the crude oil coming to the UK for subsequent refining and processing. (Note: the production of fish-liver oils should not be confused with that of fish-body oils; some tonnage of the latter is produced in the UK but more of the requirement is satisfied by imported material).
History
Cod-liver oil was exported from Norway during the Middle Ages but it appears to have been used solely for non-medical purposes. Its introduction into medicine was largely due to Dr Samuel Kay, a physician at Manchester Infirmary from 1752 to 1784. The original method of preparation was the ‘rotting process’, in which the livers were allowed to rot in barrels and the oil rising to the surface was skimmed off. The more modern ‘steaming process’ was introduced about 1850.
Collection and extraction
The following account is based largely on information supplied by Seven Seas Health Care Ltd., leading refiners and processors of cod-liver oil worldwide.
The cod livers, which contain about 50% oil, are removed immediately the fish are boarded and transferred to steamers in which the oil is released from the tissue, or stored in chilled conditions for later processing at a shore station. All this takes place mainly on Norwegian and Icelandic vessels. On arrival in port the oil is stored in land-based tanks prior to bulk shipment to the UK for refining and processing, although some preliminary refining of oils is now conducted at the extraction plants in Norway and Iceland.
Preparation
The principal stages in the preparation of the medicinal oil are (1) refining of the crude oil, (2) drying, (3) winterization, (4) deodorization, (5) standardization for vitamin content.
Refining. Quality and flavour of cod-liver oil are improved by refining under air-free conditions to avoid oxidation; at Marfleet, UK, this is carried out in a continuous, automatic, hermetic refining plant consisting of a battery of mixers linked to centrifuges. The crude oil is rapidly heated to 77°C in a heat exchanger and passed to disc-type mixers, where controlled addition of an aqueous reagent takes place which removes impurities and causes further dissolution of the small amount of liver tissue present. Oil and water phases are separated in a hermetic separator (centrifuge: 7000 r.p.m.) without contact with air. The refined oil is then mixed with water, reheated and the separation process is repeated in a second and third set of centrifuges.
Drying. Drying is effected in a vacuum drying tower which continuously evaporates any small amount of residual water and discharges a clear, bright, highly refined oil. The plant can refine 50–60 tonnes of oil per day.
Winterization. All medicinal oil and veterinary oils are cooled to about 0°C, which causes stearin (triglycerides with a higher saturated fatty acid content) to separate. The solid is removed by cold filtration and a polyunsaturated (enriched) product is left. Photographs illustrating the above processes can be found in the 14th edition of this book.
Deodorization. Final deodorization is achieved by steaming under vacuum which removes about 0.02% of aldehydic and ketonic impurities, and once again protects the oil from oxidation. This process establishes the palatable flavour of the finished oil.
Standardization. The medicinal oil is finally standardized for vitamin content by blending. The BP/EP oil is required to contain in 1 g, 600 to 2500 Units of vitamin A and 60 to 250 International Units of vitamin D3. The former is assessed by the HPLC method of the Pharmacopoeia, the Unit being equivalent to 0.344 mg of all-trans-vitamin A acetate or 0.3 mg of the corresponding alcohol. The determination of the vitamin D content requires two chromatographic procedures—the first for purification of the solution under test and the second for the separation of ergocalciferol and cholecalciferol. Ergocalciferol EPCRS is used as an internal standard and peak heights or areas are measured.
The fatty acid composition of the oil is determined by gas chromatography and limits are given for 15 individual acids classified as saturated, mono-unsaturated and poly-unsaturated fatty acids.
As mentioned above, the BP/EP includes Type A and Type B oils; both have identical vitamin-content requirements but Type A has, in addition, an anisidine value of  30.0. The latter represents a limit of aldehydes and ketones produced by secondary oxidation of the oil. For determination, the oil is reacted with anisidine in glacial acetic acid and the yellow–brown colour produced measured at 350 nm (anisidines are methyl ethers of o- and p-aminophenol).
30.0. The latter represents a limit of aldehydes and ketones produced by secondary oxidation of the oil. For determination, the oil is reacted with anisidine in glacial acetic acid and the yellow–brown colour produced measured at 350 nm (anisidines are methyl ethers of o- and p-aminophenol).
It is now common practice to add some vitamin E to cod-liver oil (often as dl-α-tocopheryl acetate) to assist in the in vivo protection against reduction of the user’s vitamin E status, owing to higher intake of polyunsaturates.
Storage
The oil should be kept in well-filled airtight containers, protected from light and in a cool place. The addition of small amounts (0.01%) of certain antioxidants (e.g. dodecyl gallate, octyl gallate) is permitted.
Characters
Medicinal cod-liver oil is a very pale yellow liquid with only a slightly fishy odour and taste. The acid value should not exceed 2.0 but varies with age. The iodine value, as may be inferred from the constituents, is high (150–180). In contrast to halibut-liver oil, the unsaponifiable matter is low (1.5%).
Constituents
The medicinal properties of cod-liver oil are mainly due to vitamin A and vitamins of the D group. The main antirachitic activity appears to be due to D3 (cholecalciferol). The oil consists of glycerides of unsaturated (about 85%) and saturated (about 15%) acids. In the unsaturated group the acids possess 14, 16, 18, 20 or 22 carbon atoms, and up to 6 ethylenic linkings; in the ω-3 series eicosapentaenoic acid (C20:5) and decosahexaenoic acid (C22:6) are pre-eminent with smaller amounts of docosapentaenoic acid (C22:5) (see Fatty acids, Chapter 19 for explanation of nomenclature). Evidence is increasing that these polyunsaturated acids are significant for human health. The saturated acids include myristic acid (C14:0), palmitic acid (C16:0) and traces of stearic acid (C18:0).
Uses
Cod-liver oil is still widely used in underdeveloped countries for the prevention and cure of rickets. In Europe and the USA its use has changed somewhat as in addition to its traditional use as a vitamin supplement it now finds application in the relief of rheumatic pains and joint and muscle stiffness. Cod-liver oil has the established activity of reducing blood cholesterol levels and affording protection against cardiovascular disease (see also Chapter 6). It has extensive veterinary use.
Allied drugs
Halibut-liver Oil BP is a fixed oil obtained from the livers of the halibut, Hippoglossus vulgaris (Pleuronectidae). It is a pale golden-yellow liquid containing relatively large amounts of vitamins A and D, assayed spectrophotometrically. The standard for unsaponifiable matter is not less than 7.0%. It is used for the same purposes as cod-liver oil but in proportionately smaller doses, often in capsule form diluted with a vegetable oil to achieve specific vitamin potencies. Many other fish-liver oils resemble cod-liver oil, and shark-liver oil, Oleum Selachoidei, is included in the Indian Pharmacopoeia.
WATER-SOLUBLE VITAMINS
Vitamin B1 (thiamine, aneurine)
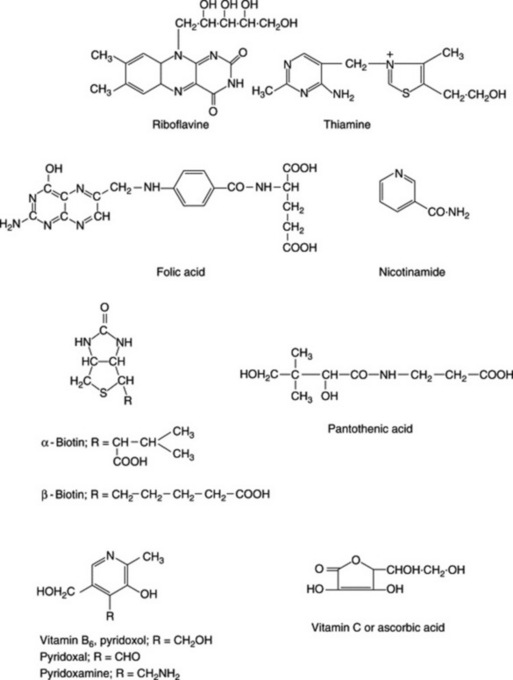
The vitamin B1 molecule is comprised of a pyrimidine and a thiazole unit connected by a methylene bridge (Fig. 31.2). It is official (BP/EP) as the hydrochloride and nitrate and is widely available from plant and animal sources (Table 31.1). In plants it is biosynthesized in the leaves and transported to the roots where it acts as a growth factor. Animals accumulate either the pyrophosphate (cocarboxylase) or a protein-magnesium complex.
Vitamin B1 in food is destroyed by boiling and its preparations should be protected from light. The BP/EP assay for the hydrochloride and nitrate is by non-aqueous titration.
In the body, carbohydrate metabolism and the normal functioning of the nervous system are dependent on adequate supplies of the vitamin. Severe deficiency causes beri-beri and was classically observed when people whose staple diet was whole ground rice were converted to polished rice. Initially symptoms of deficiency include loss of appetite, muscular atrophy and mental disturbances.
Vitamin B2 (riboflavine, lactoflavine)
Vitamin B2 is built up from a ribose and an isoalloxazine residue, the name riboflavin(e) being derived from the sugar component and the intense yellow fluorescence of its aqueous solution. It is of wide occurrence in nature and constitutes a component of the flavin coenzyme systems. Synthesis by microorganisms of the intestinal flora of humans can result in a higher excretion in the faeces of vitamin B2 than is actually present in the diet. The vitamin is unstable to light and strong alkalis and should be stored in a well-closed container.It is assayed (BP/EP) by measurement of the absorbance of a solution of the acetate at 444 nm.
Deficiency in humans is rarely encountered; symptoms include a cracking of the corners of the mouth, dermatitis and conjunctivitis.
Pantothenic acid (vitamin B3 or B5)
This compound (Fig. 31.2) is a component of coenzyme A (q.v.). Deficiency symptoms are not well-defined and differ appreciably with different species of animal.
Vitamin B6 (pyridoxine)
Pyridoxol (Fig. 31.2), pyridoxal and pyridoxamine are three forms of the vitamin. The first is found in large quantity in plant sources and the other two in animal tissues. In man, B6 is synthesized by microorganisms of the large gut, but how much of this is utilized appears uncertain.
The vitamin participates in an important coenzyme system in protein synthesis and is involved in fat metabolism. It has been tested for various disorders of the body and is indicated by the BP/EP for the treatment of sideroblastic anaemias. Although not medically proven, many women appear to derive beneficial effects from large doses of the vitamin taken to combat premenstrual tension. Deficiency symptoms, which are rare in humans, resemble those for other B vitamins and include convulsions, polyneuritis and skin disease.
Pyridoxine Hydrochloride of the BP/EP is assayed by non-aqueous titration; it should be stored protected from light.
Nicotinamide (vitamin B7, vitamin PP) and nicotinic acid (niacin)
These compounds (Fig. 31.2) are found, principally as the amide, in a variety of foods and are manufactured in the body, with the aid of other B vitamins, from tryptophan. Nicotinamide is a component of a number of coenzymes (Chapter 18) which play an important role in the primary metabolism of the cell.
The classical deficiency disease associated with the vitamin is pellagra but other supplementary factors involving a lack of other B vitamins, an unbalanced diet, and exposure to the sun are also involved. Symptoms of deficiency are skin inflammation, diarrhoea and delirium. Nicotinic acid has a vasodilatory effect.
The vitamin is stable in foodstuffs; nicotinic acid should be protected from light and nicotinamide should be stored in well-closed containers. The BP/EP assay utilizes non-aqueous titration (nicotinamide) and acid–base titration (nicotinic acid).
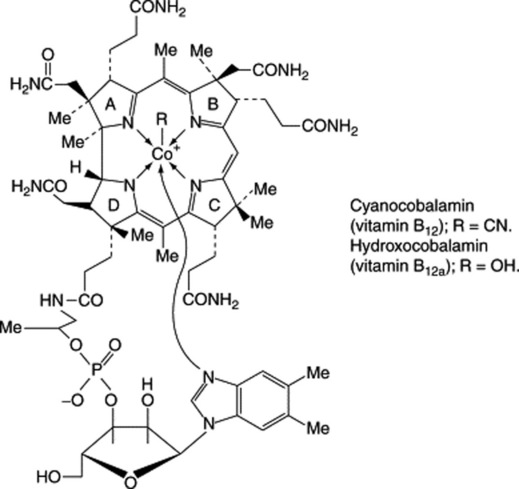
Vitamin B12 (cyanocobalamin)
This vitamin is not found in plants or yeasts but occurs in meat, in particularly large quantities in livers and kidneys. In the serum it is largely combined with serum globulins. B12 is also produced by a number of microorganisms (e.g. species of Streptomyces and Bacillus) and these are used for the commercial production of the vitamin. The molecule is a porphyrin derivative complexed with cobalt and linked to a nucleotide. As a natural complexed porphyrin derivative it may be compared with chlorophyll (Mg2+) and haemoglobin (Fe2+), which together have been described as ‘pigments of life’. The vitamin complex exists in a number of forms designated B12 (cyanocobalamin), B12a (hydroxocobalamin), B12b etc., the term cobalamin being restricted to those members having 5,6-dimethylbenzimidazole as the basic portion of the nucleotide. Hydroxocobalamin (B12a) has, in alkaline solution, a hydroxy group instead of the cyanide ion of B12. Cyanocobalamin BP/EP is assayed by absorbance measurements at 361 nm and hydroxocobalamine, official as the acetate, chloride and sulphate by measurements at 351 nm.
Nearly 40 years after the structure elucidation of vitamin B12 by the late Dorothy Hodgkin in 1956, the biosynthetic pathway for this compound was finally established in what Battersby has described as the Everest of biosynthetic problems [for an account (19 refs) see L. R. Milgrom, Chemistry in Britain, 1994, 31, 923].
In the body, vitamin B12 is involved with the metabolism of amino acids particularly the methylation of homocysteine to give methionine (see Fig. 18.15) and the breakdown of other amino acids.
Vitamins B12 and B12a are used for the treatment of pernicious anaemia; they are best given by injection and replace the former treatment with raw liver and liver extracts. Hydroxocobalamin binds more strongly with the serum proteins than does B12 and so has a longer period of action.
Cyanocobalamin (57Co) and (58Co) are radioactive forms of the vitamin used in diagnostic tests for pernicious anaemia; they have radioactive half-lives of 271.7 days and 70.8 days respectively, and are prepared by cultivating suitable microorganisms on a medium containing the radioactive cobaltous ions. Owing to its long radioactive half-life (5.27 years), cyanocobalamin (60Co) should not be used in this test.
Folic acid (folacin, vitamin Bc, vitamin M, factor V)
Folic acid refers to pteroylmonoglutamic acid (Fig. 31.2), as distinct from the tri- and heptaglutamic acids also found in this group of substances. The structure of the original vitamin-like material isolated from spinach leaves in 1941 and named ‘folic acid’ is not known. In the body, folic acid is necessary for cell division and for the normal production of red blood cells. With normal diets, deficiency is rare, but supplementation may be required during pregnancy and as a result of taking oral contraceptives. Lack of the vitamin produces diarrhoea, loss of weight and megaloblastic anaemia. The last two symptoms resemble those of vitamin B12 deficiency and correct diagnosis and avoidance of self-medication is essential.
Vitamin C (ascorbic acid)
Ascorbic acid (Fig. 31.2) is prepared synthetically or by extraction from plant materials such as rose hips, blackcurrants and the juice of citrus fruits (q.v.). One of the richest sources appears to be the fruit of an edible Combretaceous tree, Terminalia ferdinandiana, found along the north-west coast of Australia. The edible fruits contain some 2300–3150 mg ascorbic acid per 100 g of edible fruit, a figure two to three times higher than that for rose hips. In the plant ascorbic acid is biosynthesized from D-glucose and a pathway involving fructose, mannose and galactose derivatives has also been proposed (G. L. Wheeler et al., Nature, 1998, 393, 365; see also F. A. Loewus, Phytochemistry, 1999, 52, 193).
Vitamin C is essential for the normal functioning of living cells and is involved in many enzymic reactions. It is required for the development of cartilage, teeth and bones, for wound healing and for aiding the absorption of iron from the intestine. Gross deficiency causes scurvy; early signs of a lack of the vitamin in individuals are muscular weakness, tiredness, reduced resistance to infection and easy bruising. Large doses of vitamin C have been tested for the prevention of the common cold, but without significant success.
The pharmacopoeial assay involves titration with 0.05 M iodine solution with starch as indicator.
The reducing and associated antioxidant properties of vitamin C are utilized in the food industry and in the formulation of some pharmaceutical preparations.
Biotin (vitamin H)
Biotin occurs in so-called α- and β-forms, which differ in their side-chain structure (Fig. 31.2). In the body, these substances, in some instances, operate with other water-soluble vitamins and enzymes and are required for digestion and carbohydrate metabolism. Large quantities are produced by the intestinal microorganisms and deficiency conditions such as dermatitis are rare.
The pharmacopoeia illustrates the β form, which is assayed by potentiometric titration with 0.1 M tetrabutylammonium hydroxide. Various possible impurities, largely involving the structure of the side-chain, are listed. Tests include TLC and IR spectrometry.
Ubiquinone (ubidecarenone, coenzyme Q10)
In the mitochondria of plants and animals this coenzyme is involved in electron transport. It may act as a free radical scavenger and function as an antioxidant and membrane stabilizer. For patients with cardiovascular disorders it is regarded as a useful addition to orthodox treatment but a double-blind study involving 46 patients failed to give a positive result; for a brief report see Pharm. J., 1999, 263, 848. It is sold to the general public as a popular food supplement for protection against heart and gum disease and for maintaining general well-being.
DOG ROSE (ROSE HIPS)
Dog rose consists of the incompletely dried, almost ripe hips, with the achenes removed, of various species of Rosa (Rosaceae) including the common dog roses (R. canina L.), downy-leaved roses (R. villosa L.) and ‘alpine rose’ (R. pendulina L.). The hips should be collected between the period when they just begin to change colour and when they are fully red, and used for the preparation of galenicals as soon as possible.
The hip is an aggregate fruit formed from the apocarpous gynacecium of a single flower. Fruits of different species of Rosa naturally vary in size and shape. That of R. canina is urn-shaped, almost 2 cm long, bright red and glossy when ripe. As the commercial drug, it occurs as broken fragments of the fleshy, hollow receptacle, strongly wrinkled on the convex surface and bristly on the inner. The upper end of the receptacle bears the scars of the five fallen sepals. A characteristic feature of the powder is the large unicellular trichomes up to 2 mm in length which arise from lignified cells of the inner epidermis.
Rose hips are used for their vitamin content containing 0.1–1.0% ascorbic acid (Vitamin C) (Fig. 31.2) and smaller amounts of vitamin A, aneurine, riboflavine and nicotinic acid. The BP/EP requires a minimum 0.3% ascorbic acid for the dried drug, which is determined spectrophotometrically.
The syrup, prepared from the fresh fruit, is unstable and loses up to 50% of its ascorbic acid within 6 months.
BLACKCURRANT
The BP material requires little description and consists of the fresh ripe fruits of Ribes nigrum L. (Grossulariaceae, but often included in the Saxifragaceae) together with their pedicels and rachides. The plants are commonly cultivated in most temperate regions. The fruits contain various acids (e.g. citric and malic), pectin, colouring matter and ascorbic acid. The ascorbic acid content varies from 100 to 300 mg 100 g−1. They are used for the preparation of Black Currant Syrup and in some lozenges. The leaves are used in Europe as a traditional treatment for rheumatic diseases; the active constituents may be prodelphinidin oligomers. Other species of Ribes, for example, the gooseberry (R. grossularia) and red currant (R. rubrum), have also been used in medicine.
Citrus juices
Lemon juice is produced on a large scale in many lemon-growing countries. The fruits yield about 30% of juice, which may be packed at natural strength or after concentration. Large quantities are used for citric acid manufacture. Lemon juice is used for its vitamin C content, but orange juice is richer in this vitamin and is more suited to infant feeding. Decitrated orange and lemon juices are used for making vitamin C concentrates. Vitamin C, or ascorbic acid, may be prepared from other vegetable sources (e.g. the ripe fruits of Capsicum annuum) or made synthetically.
Dried yeast
Dried yeast consists of the cells of a suitable strain of Saccharomyces cerevisiae (Order Protoascales, Saccharomycetaceae) dried so as to preserve the vitamins present.
Collection and preparation
Yeast is produced by growing the parent cells in a liquid containing sugars and nitrogenous compounds. Distillers’ or bakers’ compressed yeast is separated from the medium by the use of filter presses and is a by-product in the manufacture of alcoholic liquors. However, yeast may be the sole product of a yeast factory. Compressed yeast contains about 70% of moisture and is converted into dried yeast by heating at a temperature not exceeding 30°C until the moisture content is reduced to below 9%.
Characters
Dried yeast occurs as a pale buff powder. Under the microscope it shows spherical, elliptical or ovate cells up to 8 μm long, some showing budding. They are transparent and have a cell wall enclosing a granular protoplasm in which are one or two glycogen vacuoles. The nucleus exists as a small mass near the centre of the cell and cannot usually be seen without the use of a special staining procedure. Yeast should contain no starchy material.
Constituents
Important constituents of yeast are the vitamins of the B group (aneurine, nicotinic acid, riboflavine, folic acid and B12). It also contains about 46% of protein, 36% of carbohydrates (particularly glycogen), fats, sterols and enzymes (the zymase complex, glycogenase, invertase, maltase and emulsin).
Uses
Yeast is used in the treatment of furunculosis and as a source of the B vitamins. It is a rich source of biologically complete protein and is used in the manufacture of nucleic acid. In addition to the yeast described above, Torula yeast, derived from Candida utilis (Cryptococcaceae), is used. It contains about 45% of protein and is rich in vitamins.
In molecular genetics S. cerevisiae has been utilized as a suitable organism for the overexpression of active enzymes of other plants and of animals (e.g. hirudin of the medicinal leech).
HORMONES
Some textbooks of pharmacognosy include endocrine organs and hormones; others do not. The pharmacy student usually acquires knowledge of these partly in pharmacology, pharmaceutical chemistry and pharmaceutics. The brief account which follows may form a useful starting point.
Hormones, or ‘chemical messengers’, are substances secreted by the endocrine or ductless glands of animals. Until recently it was fashionable to deride the therapeutic use of animal products; for example, livers from various animals by the ancient Egyptians and toad-skins by the ancient Chinese. Research has since shown that such materials often contain therapeutically valuable substances. This is especially true of the ductless glands, whose function was a mystery to men such as Galen. An early example of the rational use of endocrine organs was the employment of hog testis by Magnus in the thirteenth century for male impotence. For a long period it was known that abnormalities of the thyroid produced myxoedema and cretinism, and when in 1891 Horsley showed that such patients benefited from the administration by mouth of animal thyroid glands, the modern period of organotherapy started. Suprarenal extracts were introduced about 1894 and two thyroid preparations (a dry powder and a solution) were included in the BP 1898. The practice of using the glands or more or less crude preparations of them (organotherapy) has gradually been displaced bythe use of their active principles (hormone therapy). With a knowledge of their chemical structure some hormones can now be best made synthetically. Adrenaline was first used in 1901 and became official in 1914. Thyroxin was isolated in 1915, was synthesized in 1928 and has gradually replaced the use of thyroid glands. In Britain, on grounds of safety and in the light of the more reliable alternatives available, the licensing authority removed all thyroid extract products from the market from October 1982. One of the most notable advances was the discovery of insulin by Banting and Best in 1921, which revolutionized the treatment of diabetes; the hormone became official in the BP 1932. In another big step forward, insulin has continued in the forefront of pharmaceutical development in that human insulin is now produced by microorganisms which have been engineered to contain the necessary human genetic material for hormone production. The first sex hormones were isolated from urine in 1931; testosterone became official in 1948 and testosterone implants in 1963. Oral contraception greatly increased the demand for substances of this class (see Chapter 24).
Hormones, like vitamins, are chemically a diverse class (Table 31.2). Some are related to the polypeptides and proteins, while others are steroidal. The preparation and purification of hormones such as insulin from natural sources at first presented difficult technical problems. Chemists also had formidable tasks in determining structures and evolving methods for synthesis.
Table 31.2 Distribution of hormones.
| Hormones | Nature and occurrence |
|---|---|
| Gonadotropins | Water-soluble glycoproteins. Pituitary glands of man, horse, sheep and pig |
| Corticotropins | Polypeptides of pituitary glands |
| Thyrotropin | Protein combined with carbohydrate. Pituitary gland |
| Oxytocin and vasopressin | Octapeptides. Pituitary gland |
| Thyroxin | An iodine-containing compound. Thyroid gland |
| Adrenalin | (−)-α-3,4-Dihydroxy-phenyl-β-methyl aminoethanol. Suprarenal gland or prepared synthetically |
| Insulin | Molecule contains two unbranched polypeptide chains linked by two disulphide bridges. Islets of Langerhans of pancreas |
| Corticosteroids | From adrenal cortex from which over 40 steroids, many having hormonal activity, have been isolated. Examples: cortisone, aldosterone. Many others have been prepared synthetically |
| Oestrogens | Steroidal female sex hormones. From pregnant mares or human urine, hog ovaries, etc. |
| Androgens | Steroidal male sex hormones (e.g. testosterone and androsterone). From urine or by partial synthesis |
Phyto-oestrogens
are non-steroidal plant substances of flavonoid constitution exhibiting oestrogenic properties. They have recently received considerable press and scientific attention and are described in Chapter 21p. 252.