Chapter 24 Miscellaneous isoprenoids
In addition to the groups of compounds considered in Chapters 22 and 23, there exists in nature a tremendous range of other isoprenoids, some of which have become of increasing interest as medicinal agents. There are also those plant metabolites of ‘mixed’ biogenetic origin which contain an isoprenoid moiety (e.g. some indole alkaloids, the cannabinoids and chlorophylls) and these are considered in other appropriate chapters.
MONOTERPENES
As illustrated in Figures 18.18 and 18.19, the monoterpenes are derived from the C10 geranyl pyrophosphate and constitute important components of volatile oils. Other examples are given below. Monoterpenoid compounds are reviewed regularly in Natural Product Reports (for coverage of the 1990 literature see D. H. Grayson, ibid., 1994, 11, 225).
IRIDOIDS
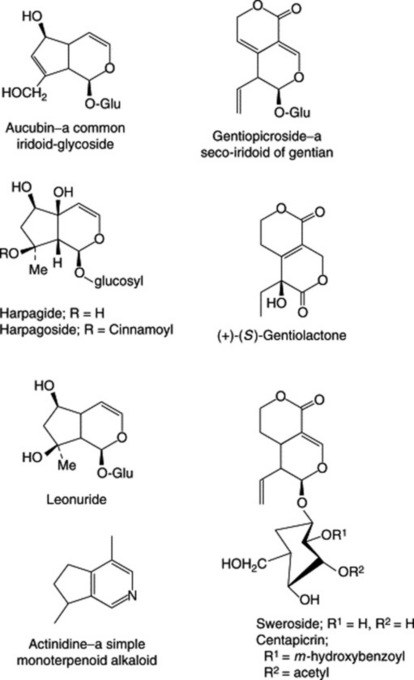
The iridoids are cyclopentan-[c]-pyran monoterpenoids and constitute a group of which the number of known members is constantly increasing. The name derives from Iridomyrmex, a genus of ants which produces these compounds as a defensive secretion. In a series of reviews covering the years up to December 1989 several hundred iridoids, classified originally into 10 groups, have been listed. Junior (Planta Med., 1990, 56, 1) has reviewed (146 refs) the isolation and structure elucidation of these compounds. Most occur as glycosides; some occur free and as bis compounds. There are many seco-iridoids, see secologanin, in which the pyran ring is open, and in a few the pyran ring oxygen is replaced by nitrogen, Fig. 24.1.
For a review covering new naturally occurring iridoids reported during 1994–2005, see B. Dinda et al., Chem. Pharm. Bull., 2007, 54, 159–222; for Part 2 covering the identification of 158 new plant seco-iridoids from 1994 to 2005 and the bioactivity of the two groups, see B. Dinda et al., Chem. Pharm. Bull., 2007, 55, 689–728.
Of pharmaceutical significance is their presence in Valerian, Gentian and Harpagophytum and the involvement of loganin (Chapter 26) as a precursor of the non-indole portion of some alkaloids.
GENTIAN
Gentian (Gentian Root BP, EP, BHP) consists of the dried fermented rhizomes and roots of the yellow gentian, Gentiana lutea L. (Gentianaceae), a perennial herb about 1 m high found in the mountainous districts of central and southern Europe and Turkey. Important districts for its collection are the Pyrenees, the Jura and Vosges Mountains, the Black Forest, former Yugoslavia and the Carpathians.
As it is now a protected plant in some areas, attempts are being made to cultivate it in some EU countries (France, Italy, Germany); for this, the initial selection of plant material is of vital importance.
History
Gentian, possibly not derived from the species now official, was known to Dioskurides and Pliny. The drug was commonly employed during the Middle Ages.
Collection and preparation
When the plants are 2–5 years old, the turf is carefully stripped around each and the rhizomes and roots are dug up. This usually takes place from May to October, collection in the autumn being more difficult on account of the hardness of the soil, although possibly preferable from the medicinal point of view. There is no UK demand for ‘white’ or unfermented gentian, the commercialdrug consisting of ‘red’ or fermented gentian. The method of preparing this varies somewhat in different districts. Usually, the drug is made into heaps, which are allowed to lie on the hillside for some time and may even be covered with earth. After it is washed and cut into suitable lengths the drug is dried, first in the open air and then in sheds. Prepared in this way the drug becomes much darker in colour, loses some of its bitterness and acquires a very distinctive odour.
Macroscopical characters
The plant has a cylindrical rhizome which may attain a diameter of 4 cm and give off roots more than 1 m in length. The crown bears 1–4 aerial stems. The fresh root is whitish and fleshy internally and practically odourless.
The commercial drug consists of simple or branched, cylindrical pieces up to 20 cm long and 1–3 cm diameter. The outer surface is covered with a yellowish-brown cork. The rhizomes are usually of larger diameter than the roots and frequently bear one or more apical buds and encircling leaf scars. On drying, the rhizomes wrinkle transversely, whereas the roots wrinkle longitudinally. The drug is brittle when perfectly dry, but readily absorbs moisture from the air and becomes very tough. It has a characteristic odour and a sweet taste, which later becomes bitter.
Microscopical characters
A transverse section shows an orange–brown bark separated by a darker cambium line from the porous, very indistinctly radiate wood. Only the rhizomes show a pith. More detailed examination shows about 4–6 rows of thin-walled cork cells between which and the cambium is a somewhat thick-walled phelloderm and wide zone of brown, thin-walled parenchyma containing oil globules and minute needles of calcium oxalate. Small groups of soft phloem are seen but phloem fibres are absent.
Examination of the wood and pith shows abundant parenchyma having similar cell contents to those of the bark. The vessels occur either isolated or in small groups and show mainly reticulate or scalariform thickening; a few spiral and annular vessels occur. Groups of soft phloem (‘phloem islands’, ‘interxylary phloem’) occur in the xylem. The drug contains very little starch and no sclerenchymatous cells or fibres.
Allied drugs
The roots of other species of Gentiana (e.g. G. purpurea, G. pannonica and G. punctata) have been imported. They appear to have similar medicinal properties to the official drug but are usually of smaller size. In India the roots of G. kurroa and Picrorhiza kurroa are used as gentian substitutes under the name of ‘kathi roots’. G. kurroa, however is now considered to be a threatened species and shoot multiplication and root formation in in vitro cultures have been studied for its possible propagation. Similarly, work in Japan has focused on the mass production of G. triflora plants by the cultivation of tissue segments in artificial media in the presence of growth hormones. The BP/EP includes a TLC test to differentiate between the official drug and related species.
Adulterants
Adulteration, probably due to careless collection, sometimes occurs. The rhizomes of Rumex alpinus, which give the test for anthraquinone derivatives, have been reported; also a dangerous but easily detected admixture with the rhizomes of Veratrum album.
Constituents
Gentian contains bitter glycosides, alkaloids, yellow colouring matters, sugars, pectin and fixed oil.
The seco-iridoid gentiopicroside (also known as gentiopicrin and gentiamarin; formula see Fig. 24.1) is the principal constituent and was isolated from fresh gentian root in 1862. It occurs to the extent of about 2% and on hydrolysis yields a lactone (gentiogenin) and glucose. A biphenolic acid ester of gentiopicroside, amarogentin, which occurs in small amount (0.025 to 0.05%) has a bitterness value some 5000 times greater than that of gentiopicroside and is therefore an important constituent of the root; other bitters isolated are sweroside and swertiamarin. The isoprenoid gentiolactone has been separated into its enantiomers (Fig. 24.1) by HPLC involving a chiral column (R. Kakuda et al., Chem. Pharm. Bull., 2003, 51, 885) and the same group of workers (J. Toriumi et al., Chem. Pharm. Bull., 2003, 51, 89) has reported on new triterpenes in addition to α-amyrin, β-amyrin and lupeol.
The yellow colour of fermented gentian root is due to xanthones (Chapter 21) and includes gentisin (also known as gentiamarin) (Fig. 21.16), isogentisin and gentioside (a 3β-primeverosidoisogentisin). Gentian also contains gentisic acid (2,5-dihydroxybenzoic acid) and about 0.03% of the alkaloids gentianine and gentialutine, which may be artefacts of the preparation process.
The bitter principles of G. lutea and G. purpurea have been assayed by HPLC and separated preparatively by overpressure layer chromatography. The official BP bitterness value of the root should be not less than 10 000 when determined by comparison with quinine (200 000).
Gentian is rich in sugars, which include the trisaccharide gentianose, the disaccharides gentiobiose and sucrose. During the fermentationprocess these are partially hydrolysed into glucose and fructose. If fermentation is allowed to proceed too far, the hexose sugars are converted into alcohol and carbon dioxide. Gentian should yield 33–40% of water-soluble extractive (BP not less than 33%), but highly fermented root yields much less.
For references to the chemical composition and to the seasonal variations in the content of secondary metabolites, in the aerial parts of G. lutea, see N. Menkovic× et al., Planta Medica, 2000, 66, 178.
Three monoamine oxidase inhibitors have been located in the bark (H. Haraguchi et al., Phytochemistry, 2004, 65, 2255).
Uses
Gentian is used as a bitter tonic. In traditional medicine it has been employed to treat various gastrointestinal conditions, as an anti-inflammatory and wound-healing agent. It is also reported to have choleretic, antioxidative, hepatoprotective and antifungal activities (see A. Mathew et al., Pharm. Biol., 2004, 42, 8).
CENTAURY
Centaury (BP/EP, BHP), family Gentianaceae consists of the dried flowering aerial parts of Centaureum erythraea Rafn, including C. majus and C. suffruticosum.
The biennial plant, some 30 cm in height, is widely distributed throughout Europe, N. America, N. Africa and W. Asia; it is exported from Morocco, Bulgaria and Hungary.
As seen in the dried drug, the hollow stems are yellowish-green with distinct ribs; the sessile leaves, 1–5 cm long, are light green in colour, obovate or spathulate in outline with an entire margin and an obtuse apex; the inflorescence consists of a tubular five-toothed calyx and a joined five-lobed, white-pinkish corolla, five stamens and a cylindrical ovary having parietal placentation and several small brown seeds.
Features of the above are seen in the powder and include fragments of leaf having sinuous epidermal cells with striated cuticles and prisms, occasionally clusters, of calcium oxalate in the mesophyll cells; pollen grains are about 25–30 μm in diameter with three pores and a pitted exine.
The drug has a very bitter taste due to small amounts of seco-iridoid glycosides. Compounds characterized include centapicrin, swertiamarin, sweroside and gentiopicroside. The BP TLC test for identity uses a swertiamarin/rutin test solution. Other constituents include flavonoids (up to 0.4%), methylated xanthone derivatives, traces of pyridine and actinidine alkaloids (Fig. 24.1), triterpenoids and various acids.
Centaury is employed as a bitter, stimulating the appetite and increasing the secretion of bile and gastric juice.
BOGBEAN LEAF
Bogbean Leaf BP/EP, BHP is the dried, entire or fragmented leaf of Menyanthes trifoliata L., family Menyanthaceae. It is a perennial glabrous aquatic or bog plant up to 30 cm in height with leaves and flowers raised above the surface of the water. It grows widely throughout Europe, northern Morocco and N. America. Commercial supplies come largely from central Europe.
The trifoliate leaves have petioles (7–20 cm) with a long, sheathing base. The leaflets are obovate or elliptic with an entire or sometimes sinuous margin and spathulate base. The taste is very bitter and persistent.
Microscopical features include thin-walled sinuous epidermal cells with anomocytic stomata, cuticular striations and characteristic aerenchyma in the leaf lamina and petiole.
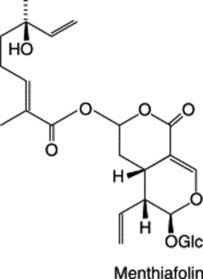
The constituents include: the bitter seco-iridoid glycosides menthiafolin and loganin; flavonoids (hyperin, kaempferol, quercetin, rutin and trifolioside); small amounts of tannin; triterpenes including the betulinic acid derivative menyanthoside which is the principal saponin of the rhizome; phenol-carboxylic acids; coumarins.
Loganin is used as a reference in the BP TLC examination and a minimum of 3000 is specified for the bitterness test.
Bogbean leaf is used for its bitter and diuretic properties and for the treatment of various rheumatic conditions.
PLANTAIN
Three common European plantains are Plantago major L. (common plantain), P. media L. (hoary plantain) and P. lanceolata L.s.l. (ribort plantain, ribwort). They are distributed generally throughout Europe and temperate Asia and have become naturalized in the US and elsewhere; they are common weeds of lawns and cultivated ground. The dried leaves of P. major collected at the time of flowering are included in the BHP 1983 and the leaves and scape of P. lanceolata in the BP/EP (the scape is the leafless ridged pedicel bearing the terminal spike.
The leaves of P. major are 10–30 cm in length, ovate or elliptic, entire or irregularly toothed with the blade abruptly contracting into the long petiole. In the dried drug the leaves are brittle and often folded. P. lanceolata has strongly ribbed, ovate to lanceolate leaves up to 30 cm in length and 4 cm wide with the blade gradually narrowing into the petiole which is about half as long as the blade. The leaf margin is distinctly toothed. The deeply furrowed five- to seven-ribbed scape usually exceeds the leaves in length and terminates in a characteristic spike of bracts and small white flowers, the long stamens of which in the fresh plant are particularly conspicuous.
Both species have similar microscopical features and these, particularly the clothing and glandular trichomes, are fully described in the pharmacopoeias.
Constituents
The constituents appear similar for both species and include the iridoid aucubin and derivatives (Fig. 24.1), flavonoids, e.g. apigenin and luteolin (see Table 21.5), sugars, mucilage and various organic acids. The BP/EP requires a minimum of 1.5% of total o-dihydroxycinnamic acid derivatives expressed as acteoside and detects contamination of the drug with Digitalis lanata leaves by TLC.
VALERIAN ROOT
Valerian consists of the rhizome, stolons and roots of Valeriana officinalis L.s.l. (Valerianaceae), collected in the autumn and dried at a temperature below 40 °C. The plant is a perennial about 1–2 m high. It is obtained from wild and cultivated plants in The Netherlands, Belgium, France, Germany, eastern Europe and Japan. It is also cultivated in the USA. Polyploidy occurs in V. officinalis and there are diploid, tetraploid and octoploid types. British valerian is usually octoploid and central European usually tetraploid.
Cultivation, collection and preparation
Valerian-growing in England has now ceased. Much drug is still produced in Europe, particularly in Holland; trials carried out in 1971–74 at Poznán on light soil showed that propagation by sowing seed, as distinct from the more laborious planting of seedlings, is fully justified.
History
The word ‘Valeriana’ is first met with in writings of the ninth and tenth centuries. The drug is mentioned in Anglo-Saxon works of the eleventh century, and was much esteemed not only for its medicinal properties, but also as a spice and perfume. Spikenard ointment, which was used by the Romans and has long been used in the East, was prepared from young shoots of Nardostachys jatamansi.
Macroscopical characters
The drug consists of yellowish-brown rhizomes, stolons and roots. The rhizomes are erect, 2–4 cm long and 1–2.5 cm wide, and may be entire or sliced. The roots, which are up to 10 cm long and 2 mm diameter, are more or less matted and broken. In some samples of the drug they almost completely envelop the rhizome, while in others they are mainly separated from it. The drug breaks with a short and horny fracture and is whitish or yellowish internally. The development of the characteristic odour during drying and storage results from a breakdown of the unstable valepotriates and the hydrolysis of esters of the oil to give isovaleric acid as a product, see below. The taste is camphoraceous and slightly bitter.
Microscopical characters
A transverse section of the rhizome shows a thin periderm, a large parenchymatous cortex which is rich in starch and an endodermis containing globules of volatile oil. Within a ring of collateral vascular bundles lies a large pith containing scattered groups of sclerenchymatous cells.
A transverse section of a root shows an epidermis bearing papillae and root hairs, and an exodermis containing globules of oil. The cortex and pith, the latter well-developed in old roots, contain starch. The starch is present mainly in compound grains with two to four components, measuring 3–20 μm diameter.
Constituents
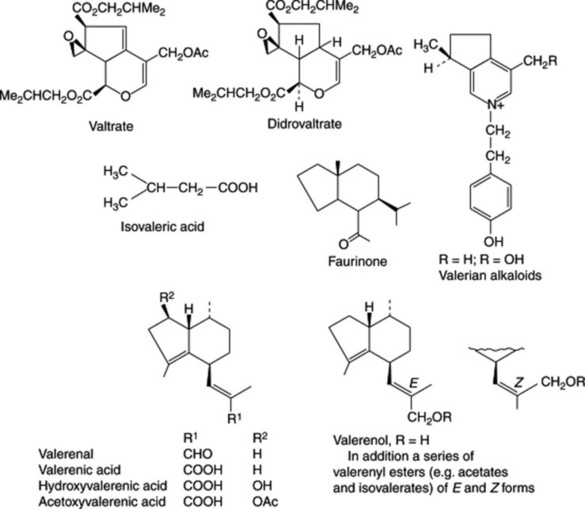
The drug yields about 0.5–1.0% of volatile oil. This contains esters (bornyl isovalerate, bornyl acetate (c. 13.0%), bornyl formate, eugenyl isovalerate, isoeugenyl isovalerate), alcohols, eugenol, terpenes and sesquiterpenes (e.g. valerenal, c. 12%). The latter comprise various acids, esters, alcohols and a ketone (faurinone) some of which are illustrated in the formulae shown (Fig. 24.2).
Also present in the drug are epoxy-iridoid esters called vale-potriates: for example valtrate, didrovaltrate, acevaltrate, and isovaleroyloxyhydroxydidrovaltrate (see formulae).
Valerian also contains alkaloids (0.05–0.1% in the dried root); no structures have been assigned to those (e.g. chatinine and valerine) described in the older literature. Two quaternary alkaloids with a monoterpene structure and which are not identical with those previously isolated have been reported; they are similar to skytanthine and related alkaloids, which occur in widely separated families.
Seasonal variations in the constituents of valerian raised in the Netherlands have been reported. Thus the accumulation of valerenic acid and its derivatives together with valepotriates reached a maximum in February to March whereas the volatile oil remained essentially constant during the period of study. Strains producing 0.9% essential oil and a high content of valerenic acid and derivatives (0.5%) were recognizable (R. Bos et al., Planta Medica, 1998, 64, 143). For the clinical significance of such strains see ‘Action and uses’.
Thirteen valepotriates have been identified in the suspension root culture of V. officinalis; root differentiation promotes production. A new iridoid diester, not present in untransformed roots, has been reported in hairy root cultures of var. sambucifolia which also produce various kessane derivatives, tentatively identified as kessyl alcohol and acetate. (F. Grünicher et al., Phytochemistry, 1995, 38, 103; 40, 142).
Quality control
The pharmacopoeia requires a minimum volatile oil content of 0.5% for the whole drug and 0.3% for the cut drug. There is a minimum requirement for sesquiterpenic acids of 0.17% calculated as valerenic acid and maximum values for total ash (12.0%) and ash insoluble in hydrochlaoric acid (5.0%). Stem bases are limited to 5.0%.
Allied drugs
Indian valerian, which is official in the Indian Pharmacopoeia, consists of the dried rhizome and roots of Valeriana wallichii. It is collected in the Himalayas. The drug consists of yellowish-brown rhizomes, 4–8 cm long and up to 1 cm thick, and a very variable amount of roots up to 7 cm long and 1–2 mm thick. The rhizomes are unbranched and somewhat flattened dorsiventrally. The upper surface bears leaf scars and the lower surface roots or root scars. The rhizome breaks with a short fracture, and the horny interior shows a small dark bark, a well-marked cambium, about 12–15 light-coloured xylem bundles and a dark pith and medullary rays. The odour is valerianaceous and the taste bitter and camphoraceous. The drug contains valepotriates and about 0.3–1.0% of volatile oil containing esters of isovalerenic and formic acids.
Centranthus ruber root (Valerianaceae) also contains a number of the valepotriates of valerian.
Japanese valerian or kesso is obtained from Valeriana angustifolia. It yields as much as 8% of volatile oil, which is, however, not identical with the oil in the European drug.
Action and uses
Valerian is used as a carminative, and as an antispasmodic in hysteria and other nervous disorders. It is often prescribed with bromides or other sedatives. Considerable quantities of valerian are used by the perfumery industry.
Previously, one problem with valerian preparations was their unreliability of action and this undoubtedly arose from both the unstable nature of the active constituents and the genetic variability of the plant material. The situation was not helped by the lack of success in ascetaining the identity of the sedative components. The volatile oil did not appear to account for the entire action of the drug and the alkaloids were also ruled out in this respect. Subsequent characterization and demonstration of activity in the group of compounds termed valepotriates in the late 1960s and early 1970s appeared in part to resolve the situation and interest turned to these compounds. Nevertheless, it had previously been demonstrated that two sesquiterpene components of the oil, valerenic acid and valeranone, were physiologically active. Further, a related species Nardostachys jatamansi, which is used in Asia for the treatment of nervous diseases, was shown to contain valeranone but lacked valepotriates. In 1978 the pharmacological properties of valeranone were confirmed and Japanese workers concluded that the sedative properties of Japanese valerian could be ascribed to this group of compounds. When, therefore, reports on the cytotoxicity of valtrate and didrovaltrate appeared in 1981 and 1982 (although no side-effects of oral administration of valerian in man have been reported), attention switched to races and species of valerian, as well as selective preparations of the drug, which lacked these compounds.
DEVIL’S CLAW (HARPAGOPHYTUM)
Devil’s claw BP/EP consists of the cut and dried tuberous secondary roots of Harpagophytum procumbens D.C. and/or H. zeyheri L. Decne. It contains not less than 1.2% harpagoside calculated with reference to the dried drug.
The plant, which derives its name from the characteristic structure of the fruit, is native to Southern and Eastern Africa and is largely obtained from Namibia, with lesser amounts from S. Africa and Botswana. In 2002, at the height of the drug’s popularity, exports from S. Africa amounted to some 1018 tonnes of dried tubers, representing millions of plants. To avoid extinction of the plant, a proposal was made to add it to the CITES list but in deference to the effect on the economy of rural areas this was withdrawn and efforts were initiated to develop microprogation techniques to solve the problem. For a full review, see ‘Further reading’.
Description
The drug consists of mainly transverse, often fan-shaped slices of the tuberous root with a reddish-brown to dark brown, longitudinally wrinkled, cork. Seen in transverse section the vascular bundles are arranged in radial rows. It is odourless but has a very bitter taste.
Microscopical features of the root include thin-walled yellowish-brown cork cells, thin-walled cells of cortical parenchyma which may contain reddish-brown contents, needles and crystals of calcium oxalate together with the vascular elements. Starch grains are absent.
Constituents
The roots contain iridoid glycosides, flavonoids, various phenolic acids, triterpenes including oleanic and ursolic acids, a quinone (harpagoquinone) and a high concentration of sugars consisting principally of the trisaccharide stachyose (Table 20.1). The principal glycosides are harpagide and its cinnamoyl ester (Fig. 24.1) together with the epoxyiridoid glycoside procumbide. 6-Acetylacteoside and 2,6-diacetylacteoside have been isolated from commercial roots (N. M. Munkombwe, Phytochemistry, 2003, 62, 1231).
The BP/EP includes a TLC test for identification using a solution of harpagoside as reference and a liquid chromatographic assay with methyl cinnamate as an internal standard. The total ash should not exceed 10.0% and the loss on drying not more than 12.0%.
Action and uses
Devil’s claw has a wide reputation for the treatment of rheumatic disease and although the therapeutic contributions of the various constituents have not been unambiguously established, animal tests indicate that the iridoids are involved in the anti-inflammatory and analgesic effects.
SESQUITERPENES
Sesquiterpenes are biogenetically derived from farnesyl pyrophosphate (Figures 18.18 and 18.19) and in structure may be linear, monocyclic or bicyclic. They constitute a very large group of secondary metabolites, some having been shown to be ‘stress compounds’ (q.v.) formed as a result of disease or injury. For many years their presence in certain volatile oils and resins has been recognized.
SESQUITERPENE LACTONES
Over 6000 compounds of this group are known and continue to constitute an active area of research. They are particularly characteristic of the Compositae but also occur sporadically in other families. Not only have they proved of interest from chemical and chemotaxonomic viewpoints, but also many possess antitumour, antileukaemic, cytotoxic and antimicrobial activities. They can be responsible for skin allergies in humans and they also act as insect-feeding deterrents.
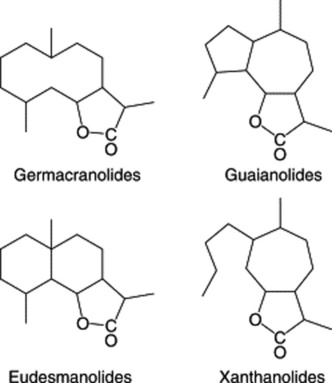
Chemically, the compounds can be classified according to their carbocyclic skeletons; thus, from the germacranolides can be derived the guaianolides, pseudoguaianolides, eudesmanolides, eremophilanolides, xanthanolides, etc. A structural feature of all these compounds, which appears to be associated with much of the biological activity, is the α,β-unsaturated-γ-lactone. As examples see the entries below on ‘Santonica Flowers’, ‘Feverfew’, ‘Chicory’ and ‘Arnica’. Other Compositae which are herbal remedies and contain sesquiterpene lactones are Taraxacum officinale (dandelion), Artemisia absinthium, Cichorium spp., Bidens spp. and Eupatorium spp.
Sesquiterpene lactones of the Umbelliferae are interesting in that the usual skeletal types (germacranolides, guaianolides, etc.) are found but all differ in their stereochemistry from the analogous compounds of the Compositae. It is therefore possible that although the biosynthetic steps in the two families form two parallel series of compounds, the conformation of the trans,trans-farnesyl diphosphate precursor is different in the two cases.
ARNICA FLOWERS
The drug consists of whole or partially broken dried flower-heads of Arnica montana L. (Compositae), a perennial herb with a creeping rhizome. The principal producers are the former Yugoslavia, Spain, Italy and Switzerland where it grows on the lower mountain slopes.
Characters
The receptacle, if present, is about 8 mm in diameter and is slightly convex. It bears pits, corresponding to the position of the flowers, in each of which is a stiff bristle. The involucre consists of two rows of dark-green, hairy, lanceolate bracts about 1 cm in length.
The pistillate, ligulate florets are about 3 cm long. Each consists of a yellow corolla having three teeth and seven to twelve veins, a style and stigma, and a pubescent, dark-brown achene 5 to 7 mm long. The latter is pubescent and glandular and is surmounted by a large, white pappus consisting of very characteristic, barbed bristles. The disc florets resemble the ligulate ones but have a tubular corolla and are hermaphrodite. When examined microscopically, numerous spiny pollen grains and the form of the hairs are seen. Odour, slight but agreeable; taste, bitter and acrid.
Constituents
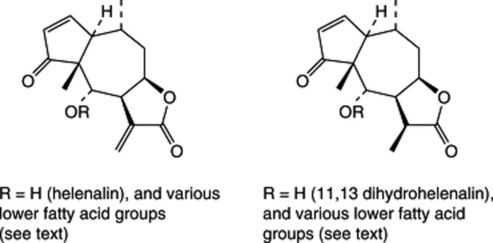
The flowers contain volatile oil (0.5–1.0%), a range of methylated flavones and sesquiterpene lactones of the pseudoguaianolide type which include esters involving acetic acid and various C4 and C5 acids (e.g. isobutyric, 2-methyl butyric, isovaleric, and tiglic acids). The principal active constituents (antirheumatic, antiarthritic, antihyperlipidaemic, respiratory analeptic) are esters of helenalin and 11,13-dihydrohelenalin. The former is characteristic of Eastern European flowers and the latter of Spanish flowers. Other constituents include diterpenes and pyrrolizidine alkaloids (tussilagine and isotussilagine).
J. A. Douglas et al. (Planta Medica, 2004, 70, 166) have studied variations in the sesquiterpene lactone levels throughout the flower-heads; highest levels were recorded for the disc florets (0.872%), lower levels for the ray florets (0.712%) with the flower receptacles (0.354%) and stems (0.028%) the lowest. The total steroidal lactone levels for the drug rose as the flowers matured.
Quality control
The BP/EP 2000 tests include thin-layer chromatography to exclude Calendula officinalis and a liquid chromatography assay to determine total lactone sesquiterpenes (not less than 0.40% expressed as helenalin tiglate).
Allied Drug
Arnica rhizome consists of the dried rhizome and roots of Arnica montana. The rhizome is dark brown in colour, about 2 to 10 cm long, and 2 to 6 mm in diameter. It bears numerous wiry roots and cataphyllary leaves. The transverse section shows a yellowish bark containing oleoresin ducts, a ring of wedge-shaped vascular bundles, and a large pith. The constituents are similar to those of the flowers. About 10 per cent of inulin is also present, but starch is absent.
Uses
Arnica has astringent properties; tinctures and infusions of the dried flower-heads and rhizomes have both been long used as adomestic remedy for the treatment of sprains and bruises. However, neither should be applied to broken skin and treatment should be discontinued should dermatitis develop. In some countries, the use of the drug is subject to legal restrictions.
An arnica gel product was the first ‘traditional herbal medicine’ to be granted registration in the UK under new regulations introduced by the Medicines and Healthcare products Regulatory Agency (Pharm. J., 2006, 277, 566).
FEVERFEW
Feverfew, Tanacetum parthenium (L.) Schultz Bip. [Chrysanthemum parthenium (L.) Bernh.] family Asteraceae/Compositae has a long history as a medicinal plant. It is probably native in S.E. Europe, Asia Minor and the Caucasus but is now established throughout Europe and in N. and S. America, where it is found on roadsides and waste areas.
The plant is a strongly aromatic herb with erect, branching, somewhat downy, stems reaching a height of up to 60 cm. The leaves are pinnate with ovate or oblong segments, pinnatifid and toothed; yellowish-green and pubescent to subglabrous. The numerous flower-heads, 12–22 mm in diameter are long-stalked and form broad terminal corymbs. The involucre is hemispherical with pubescent bracts, white ray florets and yellow disc florets. The fruits are achenes.
Features of the powdered drug include portions of leaf epidermis having a striated cuticle and anomocytic stomata, vascular tissue from the stems and veins, numerous large uniseriate covering trichomes, glandular trichomes, portions of the florets and typical Compositae pollen grains.
Some commercial samples of the drug may be devoid of flowers.
Constituents
In common with many other Compositae, feverfew is phytochemically characterized by the production of sesquiterpene lactones, which can be classified as indicated above. Germacranolides include parthenolide, 3β-hydroxyparthenolide, costunolide, 3β-hydroxycostunolide and others. Chrysanthemin A (canin) and chrysanthemin B are stereoisomers of the guaianolide group, and magnoliolide and others are eudesmanolides. Other constituents are a small amount (up to about 0.07%) of volatile oil containing monoterpenes and sesquiterpenes, tannins and flavonoids.
Feverfew is standardized on its parthenolide content and the BP/EP requires a minimum of 0.2% with reference to the dried drug. The assay involves liquid chromatography of a methanolic extract of the drug using parthenolide as reference compound and absorbance measurements at 220 nm. The assay is particularly important as commercial products vary enormously in parthenolide content, some having none at all. This may be due to chemical races known to exist for the species or to confusion in nomenclature, particularly in the US, where the term ‘feverfew’ may be applied to species other than T. parthenium.
Chicory
Chicory (Cichorium intybus, family Compositae) is indigenous to Europe and is now widespread in northern states of the USA, Canada and parts of Asia; it is widely cultivated. The plant prefers calcareous soils and is easily recognized by its bright blue flowers borne on stiffly erect grooved stems with coriaceous dark-green toothed leaves. In Europe and the USA the root is a traditional herbal remedy and in India the seeds and flowers are also used.
As with some other species of the Compositae the dried roots contain a high proportion (up to 58%) of inulin (q.v.) together with sugars. The coumarins chicoriin, esculetin, esculin, umbelliferone and scopoletin (see Table 21.2) are found in the leaves. Chicory roots also contain various sesquiterpene lactones and glycosides (M. Sato et al., Chem. Pharm. Bull., 1988, 36, 2423); examples from the 13 isolated compounds include cichorioside A (eudesmane type), 8-deoxylactucin (guaiane type) and picriside B (germacrane type). Lactucin and lactucopicrin show antimalarial activity (T. A. Bischoff et al., J. Ethnopharmacology, 2004, 95, 455),
Decoctions of the root are used as a diuretic and to treat liver ailments; the root is also cited as a tonic and laxative. Extracts of the root and root callus culture have been pharmacologically tested, with positive results, for their antihepatotoxic properties. The roasted roots are well-known for their use in coffee mixtures and as a coffee substitute.
The roots of the culinary Cichorium endivia (endive) contain the same constituents as those of C. intybus.
Fish berries
Fish berries or cocculus indicus consists of the dried fruits of Anamirta cocculus (Menispermaceae), a climbing shrub found in south-eastern Asia (particularly the Malabar coast of India) and the East Indies.
As in the other members of the Menispermaceae, the dorsal side of the fruit grows more rapidly than the ventral, with the result that the fruit becomes reniform and the base and apex both lie on the concave side. The pericarp is rough and woody and the cup-shaped seed consists of an oily endosperm surrounding the embryo, which lies with its radicle pointing towards the apex of the fruit. The two cotyledons occupy separate slit-like cavities in the endosperm. The drug has no odour; the pericarp is tasteless, but the seed is intensely bitter.
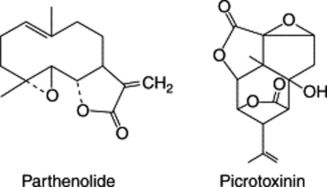
The seed contains about 1.5% of a bitter, crystalline, highly toxic substance, ‘picrotoxin’. This consists of equimolecular proportions of picrotoxinin, C15H16O6, and picrotin, C15H18O7. Picrotoxinin (see formula) is a highly oxygenated sesquiterpene derivative. The seeds also contain about 50% of fat.
Picrotoxin has been official. It is used intravenously in poisoning by barbiturates and other narcotics. Very small quantities of the fruits are sufficient to stupefy fish.
Orris
Orris rhizome is obtained from three species of Iris (Iridaceae), namely I. florentina, found in northern Italy, Iris germanica found in northern Italy, France, central Europe, Morocco and northern India, and Iris pallida, found in Italy (Florence and Lucca) and eastern France. The chief varieties in English commerce are known as Florentine and Veronese. Orris is also produced in Morocco.
Orris root has been used in perfumery from Greek and Roman times. The plants are dug up in August and September, and the peeled rhizomes are dried in the sun for about 5 days either on matting (Florentine) or threaded on cords (Veronese). When dry they are stored for about 3 years in order to develop their full aroma.
Mogadore orris is usually inferior to the European, the rhizome being smaller, darker and less fragrant.
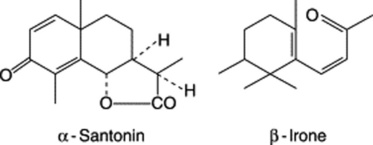
Orris rhizome contains volatile oil which contains irone (see formula below), a substance having an odour of violets. An isomeric substance, ionone, is used as a synthetic violet perfume. Orris also contains starch, calcium oxalate, iridin (a flavone related to rutin), isoflavones, and β-sitosterol and its glycosides.
Powdered orris root is used in dusting powders, while the oil is used in perfumery not only for its delicate odour, but also as a fixative for artificial violet perfumes.
Santonica flowers
Wormseed consists of the dried unexpanded flower-heads of Artemisia cina and other santonin-containing species of Artemisia (Compositae). A. cina is a small plant abundant in Turkestan, where a factory for the extraction of santonin exists at Chimkent. Santonin is prepared from Artemisia species found wild in the Kurran valley in Pakistan, and cultivation in this area has been successfully commenced.
The chief anthelminthic constituent of the drug is the sesquiterpene lactone santonin. It has the structure given below. Wormseed also contains a little volatile oil and a second, crystalline lactone, artemisin, closely related to santonin. The amount of santonin present varies considerably not only in the different species and hybrids, but also at different seasons of the year; Russian workers have reported diurnal variations.
In use wormseed has been replaced by santonin, which is very efficient in its action on roundworms. It has less effect on thread worms and none whatever on Taenia.
Artemisinin
This unusual sesquiterpene lactone possesses an endoperoxide moiety and is a component of the Chinese antimalarial drug Qinghaosu. It has been successful in treating cases of chloroquine-resistant Plasmodium falciparum and particularly cerebral malaria. The increased demand for the drug has led to a supply problem, accompanied by a vast increase in price, giving the WHO concern that the African campaign against malaria would be put in jeopardy. Production of the plant source (sweet Annie) is being increased and it is hoped that a new semi-synthetic bioequivalent artemisinin will lower the cost of treatment (see K. Purcell, HerbalGram, 2006, 69, 24).
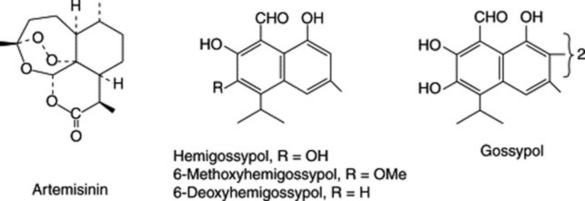
Artemisinin (Fig. 24.3) occurs in the herb Artemisia annua along with smaller amounts of other cadinane-type sesquiterpenes; by 1993 around 16 of these compounds had been isolated from the plant. Various studies on the accumulation of artemisinin during the development of the plant have been reported; some indicate the highest content before flowering, others at full flowering (see J. F. S. Ferreira et al., Planta Med., 1995, 61, 167). K.-L. Chan et al. (Phytochemistry, 1997, 46, 1209–14) report that artemisinin as isolated from A. annua is polymorphic in form. Previously regarded as orthorhombic, the crystals may also be triclinic with the latter possessing a higher dissolution rate.
Callus cultures of Artemisia annua have been reported to produce scopoletin and a triglyceride but no artemisinin. However, shoots differentiated from the callus were comparable with the whole plant (G. D. Brown, J. Nat. Prod., 1994, 57, 975). A suggested pathway for the biosynthesis of artemisinin involves the conversion of a germacranolide to a cadinane-type compound (structure p. 264) and thence through a series of intermediates including artemisinic acid and artemisitene, two sesquiterpenes which have also been isolated from the plant. The structure, biosynthesis and functions of artemisinin have been reviewed (90 refs) by S. Bharel et al. (Fitoterapia, 1996, 67, 387).
Gossypol
Hemigossypol and related aldehydes together with the dimeric gossypol (Fig. 24.3) are sesquiterpene stress compounds found in the subepidermal glands, immature flower buds and seed kernels of the cotton plant (Gossypium spp.). Gossypol was first isolated in 1899, its structure was established in the 1930s and later confirmed by synthesis and spectroscopy.
In addition to having insecticidal and various pharmacological properties, gossypol is of considerable pharmaceutical interest in that in humans it functions as a male antifertility agent. In China it was tested experimentally as a contraceptive with 12 000 men. Work is in progress to reduce possible side-effects and to find alternative systems of delivery. Chinese workers also claim the drug to be active in the therapy of menorrhagia, leiomyoma and endometriosis. Endometrial atrophy occurred in all cases (67 women) and complete recovery of the endometrium was observed within 6 months of the cessation of gossypol treatment.
Inspection of the structural formula of gossypol reveals no chiral centre for the molecule. However, it acquires chirality by the restricted rotation of the bond connecting the two naphthyl moieties and so a pair of atropisomers exist. The compound isolated from the cotton plant was racemic, and this was used in the Chinese clinical trial above;in 1987 the (−)-isomer was shown to be the pharmacologically active principle. By using modern quantitative enantiomorphic separation techniques Cass et al. (Phytochemistry, 1991, 30, 2655) have shown that the gossypol enantiomer ratio appears to be species related. Thus, an excess of (+)-gossypol was found in the seeds of each variety tested of Gossypium arboreum, G. herbaceum (Asiatic cotton) and G. hirsutum (Upland cotton) whereas (−)-gossypol was in excess in each variety of G. barbadense (Egyptian, Tanguis or Pima cotton). Concordant findings have also been reported by other workers (J. W. Jaroszewski et al., Planta Med., 1992, 58, 454).
DITERPENOIDS
The origin of the C20 diterpenoids, involving the mevalonate pathway, was indicated earlier in Fig. 18.2. The group comprises a structurally diverse range involving hundreds of compounds which may be acyclic or possess 1–5 ring systems. They may also be of mixed origin as illustrated by the diterpenoid alkaloids of Taxus and Aconitum.
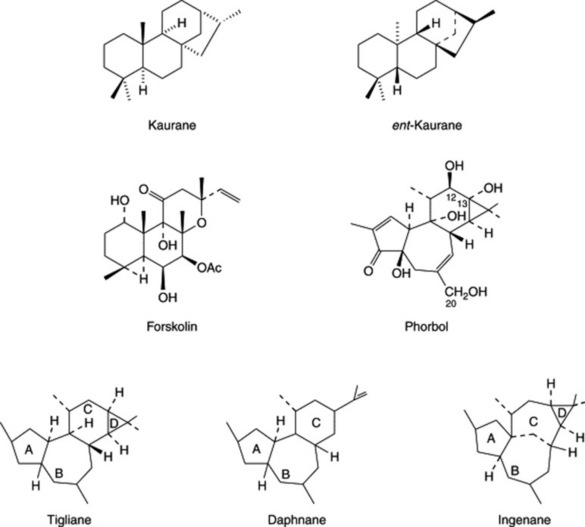
Diterpenoids constitute the active constituents of a number of medicinal plants and are of current interest for their potential as future drugs, either, as isolated from the plant, or as modified derivatives. They include such resin acids as (+)- and (−)-pimaric acid, their isomers, and abietic acid of pine resin. Different stereochemical configurations having the same skeletal structure are also seen in the tetracyclic kaurane and ent-kaurane groups (Fig. 24.4); the latter includes the sweetening agent stevioside (Chapter 33) and the gibberellins. The cytotoxic activity of a number of natural and synthetic ent-kauranes has been studied (S. Rosselli et al., J. Nat. Prod., 2007, 70, 347).
The gibberellins, first obtained from fungi of the genus Gibberella but also found in higher plants, are diterpenoid acids which have a marked effect on growth of seedlings; they are considered in Chapter 12. Phytol, C20H39OH, an unsaturated alcohol, is a component of the chlorophyll molecule. Vitamin K1, an antihaemorrhagic compound, first discovered in plants in 1929, is also a phytol derivative. Vitamin A, a diterpenoid, is referred to below under ‘Carotenes’. Furanoditerpenes constitute the bitter principles of calumba root (q.v.). Teucrium chamaedrys, wall germander, and T. scorodonia, wood sage, family Labiatae, are both used in herbal medicine as diaphoretics and antirheumatics. Besides containing small amounts of volatile oil, flavonoids and tannins, both herbs produce diterpenes of the neoclerodane type. Other diterpenoid derivatives include some of the alkaloids of species of Aconitum (q.v.), Daphne, Delphinium, Garrya, Taxus and Tripterygium. Some diterpenes from Kalmia latifolia (Ericaceae) have antifeedant properties with respect to the gypsy moth.
Forskolin (coleonol; Fig. 24.4) a diterpene isolated by Indian workers from Coleus forskohlii (Labiatae) is the last compound to be formed in the biogenetic sequence of the polyoxygenated diterpenes. Many chemical races of the plant have been revealed and studies on artificial propagation are in progress as, in India, the species is fast becoming extinct owing to large-scale indiscriminate collection. For a pharmacognostical evaluation of the root, see S. K. Srivastava et al., Pharm. Biol., 2002, 40, 129. Preparations of Coleus species have long been used in Hindu and Ayurvedic traditional medicine particularly for the treatment of heart diseases, abdominal colic, etc. Forskolin has been demonstrated to have hypotensive, spasmolytic, cardiotonic and platelet aggregation inhibitory activity; because of its unique adenylate cyclase stimulant activity it is considered a promising drug for the treatment of glaucoma, congestive cardiomyopathy and asthma (see R. A. Vishwakarma et al., Planta Med., 1988, 54, 471.)
The diterpenes of the Euphorbiaceae, e.g. esters of phorbol (Fig. 24.4), and related compounds of other families not only have medicinal potential but are also proving to be useful pharmacological tools; they are described below.
Other drugs containing diterpenes, described in Chapter 21, are agnus castus (rotundifuran and vitexilactone), Java tea (orthosiphols, etc., isopimarone-type diterpenes) and motherwort (labdane-type diterpenes).
Tiglianes, Daphnanes and Ingenanes
These three related groups of diterpenoid compounds (Fig. 24.4) are found in the Euphorbiaceae (e.g. Croton tiglium q.v., Euphorbia spp.) and the Thymelaeaceae (e.g. Daphne, Lasiosiphon, Pimelea and Gnidia spp.). Biologically, they produce intense inflammation on application to the skin and have both tumour-promoting and antitumour activity. Of particular interest are the esters of phorbol (a tigliane derivative). It is the 12,13-diester, 12-O-tetradecanoyl-phorbol-13-acetate, which has been most extensively used in pharmacological investigations although Croton tiglium contains some 10 others. As pharmacological tools they are valuable in that they substitute for diacylglycerol in the activation of the phosphorylating enzyme protein kinase C; the shape of the molecules, with their long-chain ester groupings, seems to match the side-chains on the natural second messenger, diacylglycerol. The 12,13,20-triesters of phorbol are termed ‘cryptic irritants’ because they do not exhibit pro-inflammatory activity on mammalian skin unless the C-20 acyl group is removed by hydrolysis.
Ginkgo
The leaves of ginkgo are obtained from the dioeceous tree Ginkgo biloba (Maidenhair-tree) (Ginkgoaceae), the only extant species of an otherwise fossil family of the pre-Ice Age flora. As the specific name implies the leaves are bilobed, each lobe being triangular in outline with a fine radiating, fan-like venation. The leaf is glabrous, petiolate and has an entire margin. The drupe-like fruits possess a bad-smelling pulp and contain seeds with an edible kernel.
Native to China and Japan but cultivated ornamentally in many temperate regions, the tree has a long medicinal history being recorded as early as 2800 bc in the Chinese literature; traditional Chinese medicine uses mainly seed preparations. It is only relatively recently that the drug has received much attention in the West, where in the USA, for the first 8 months of 1999, ginkgo held its place as top of the herbal mainstream market with retail sales valued at over $100 million (M. Blumenthal, Herbalgram, 1999 (No. 47), p. 64). In Europe it was estimated in 1993 to have an annual turnover of about $500 million (O. Sticher, Planta Medica, 1993, 59, 2). Standardized extracts prepared in France and Germany are much used in Europe for the treatment of circulatory diseases resulting from advancing age. The leaves are official in the BHP 1996 and the BP/EP.
Constituents
From among the many groups of compounds isolated from ginkgo it is the diterpene lactones and flavonoids which have been shown to possess therapeutic activity.
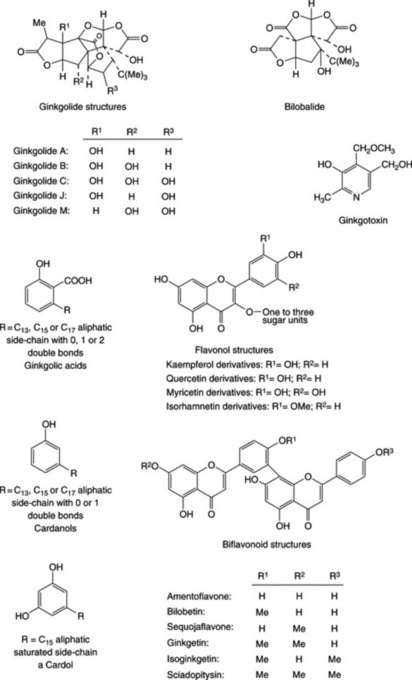
Five diterpene lactones (ginkgolides A, B, C, J, M) have been characterized; these have a cage structure involving a tertiary butyl group and six 5-membered rings including a spiro-nonane system, a tetrahydrofuran moiety and three lactonic groups (Fig. 24.5). These compounds are platelet-activating factor (PAF) antagonists (see Chapter 6) and as they do not react with any other known receptor their effect is very specific. Related to the above, and also possessing a tertiary butyl group, is the sesquiterpene bilobalide; no PAF-antagonist activity has been demonstrated for this compound.
Some 33 flavonoids have now been isolated from the leaves and involve mono-, di- and tri-glycosides of kaempferol, quercetin, myricetin and isorhamnetin derivatives. The tree also synthesizes a number of biflavonoids based on amentoflavone; there has been recent interest in these compounds arising from their antilipoperoxidant, antinecrotic and radical-scavenging properties.
Ginkgolic acids are urushiol-type alkylphenols and occur in quantity in the seed coat and to a much lesser extent in the leaves. They are most noticeably observed in poison ivy (Chapter 39) and are associated with allergic responses, particularly dermatitis. For this reason in 1997 the German Government Commission E limited the ginkgolic acid content of standardized extracts to 5 ppm. Other potentially toxic alkylphenols are the cardanols and cardols (Fig. 24.5). The albumen of the seed also contains neurotoxic 4′-O-methylpyridoxine (ginkgotoxin) and this has been shown by A. Arenz et al. (Planta Medica, 1996, 62, 548) to be present also in the leaves, but in concentrations too low to exert any significant ill-effects in medicines and foods.
Bioproduction
Various investigators have reported considerable fluctuations of terpene concentration in leaves throughout the year, with a maximum in early autumn. With the flavonoids there is a higher concentration of flavonol glycosides in spring leaves and of biflavones in autumn leaves. The age of the tree is an important factor in determining the terpene content of the leaves; those leaves from young trees (10 yr) are the richest source whereas the content is dramatically lowered in leaves of old trees (100–120 yr).
Work by D. J. Carrier et al. (Phytochemistry, 1998, 48, 89) has suggested that terpene trilactone (bilobalide + ginkgolides) synthesis might occur in actively growing tissues such as terminal buds. Ginkgolide B can be produced in cultured cells derived from ginkgo leaves and attempts to maximize yields by optimization of the cultural conditions have been reported (M. H. Jeon et al., Plant Cell Reports, 1995, 14, 501). Whereas terpene production is low in cell cultures, isolated in vitro root cultures accumulate terpenes in concentrations of the same order as those found in the leaves of young trees (J.-P. Balz et al., Planta Medica, 1999, 65, 620).
Evaluation
Commercial extracts are usually standardized for flavonoid glycosides and triterpene lactones together with a limit for ginkgolic acid. Although the marketing of pure ginkgolides has not yet proved feasible there are a number of reported laboratory separations and assays utilizing GC-MS, HPLC-MS, HPLC-RI. N. Fuzzati et al. describe a new HPLC-UV method, not requiring enrichment procedures, for the quantification of ginkgolic acid in extracts (Fitoterapia, 2003, 75, 247). For the crude drug, the BP/EP requires a 0.5% content of flavonoids calculated as flavone glycosides; these are assayed by the liquid chromatography of a hydrolysed acetone extract and spectrophotometric measurements at 370 nm.
Uses
Ginkgo has a traditional use as an antiasthmatic, bronchodilator, and for the treatment of chilblains. Extracts of the leaf containing selected constituents are used especially for improving peripheral and cerebral circulation in those elderly with symptoms of loss of short-term memory, hearing and concentration; it is also claimed that vertigo, headaches, anxiety and apathy are alleviated and positive results have been obtained in trials involving the treatment of dementia and Alzheimer’s disease (see ‘Further reading’).
Ghisalberti EL. The biological activity of naturally occurring kaurane diterpenes. Fitoterapia. 1997;68(4):303-325. Review with 180 references
Singh B, Kaur P, Gopichand RD, Singh, Ahuja PS. Biology and chemistry of Ginkgo biloba. Fitoterapia. 2008;79(6):401-418. Review with 113 references
Van Beek TA, Hardman R, editors. Medicinal and aromatic plants—industrial profiles, Vol 12. Amsterdam: Harwood Academic, 2000. (series ed) Ginkgo biloba
SESTERTERPENES
This relatively recently recognized family of C25 compounds, formed by the addition of a C5 isopentenyl unit to geranylgeranyl diphosphate, is at the moment of limited medicinal interest. Examples are confined principally to the fungi, some marine organisms (e.g. sponges of the genus Ircinia) and insect waxes (e.g. gascardic acid).
TRITERPENOIDS
These C30 constituents are abundant in nature, particularly in resins, and may occur as either esters or glycosides. They may be aliphatic (e.g. the squalene found in animals and in the unsaponifiable matter of many oils such as arachis and olive), tetracyclic or pentacyclic. Tetracyclic ones include the limonoids, the sterols found in wool fat and yeast and the cardioactive glycosides. The triterpenoid saponins, most of which are pentacyclic, are discussed elsewhere.
This is again a very active area of research and is regularly reviewed in Natural Product Reports.
One group of compounds showing a range of interesting biological activity is the quassinoids. These are degradation and rearrangement products of triterpenes and are described under ‘Quassia’ below and in Chapter 27.
Quassia Wood
Quassia (Jamaica Quassia) is the stem wood of Picrasma excelsa (Picroena excelsa or Aeschrion excelsa) (Simaroubaceae), which is known in commerce as Jamaica quassia. The tree, 15–20 m high, grows in the West Indies (Jamaica, Guadeloupe, Martinique, Barbados and St Vincent).
Characters
Quassia occurs in logs, chips or raspings. The logs are of variable length and up to 30 cm diameter (those of Surinam quassia never exceed 10 cm diameter). The logs are covered with a dark grey cork which readily separates from the phloem. The wood is at first whitish but becomes yellow on exposure. It frequently shows blackish markings owing to the presence of a fungus. The logs split readily and the commercial chips, which are cut across the grain, break very readily into smaller fragments. The drug has no odour but an intensely bitter taste.
A small piece of quassia wood should be smoothed and the transverse, radial and tangential surfaces examined with a lens.
A transverse section of quassia shows medullary rays, which are mostly two to five cells wide. The xylem is composed of vessels, wood fibres, and wood parenchyma. The vessels are large (up to 200 μm diameter) and occur singly or in groups of 2–11 which often extend from one medullary ray to the next. Single prisms of calcium oxalate, each 6–30 μm long and enclosed in a delicate membrane, occur scattered in the medullary ray cells and wood parenchyma cells. Starch grains are few; mostly simple, spherical and about 5–15 μm, occasionally two-compound.
Constituents
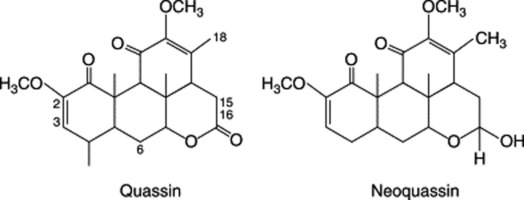
Quassia contains the amaroid (terpenoid) compound quassin, an intensely bitter lactone; also neoquassin, 18-hydroxyquassin, and scopoletin.
The quassins have been traditionally estimated by sensory means (BP, 1973) but Wagner and colleagues (Planta Med., 1980, 38, 204) described three equally effective methods for the quantitative determination of the individual quassinoids. These involve separation by TLC, HPLC and circular chromatography, followed by absorption measurements.
Quassia wood also contains alkaloids, as illustrated by cathine-6-one.
Allied drugs
Surinam quassia is derived from Quassia amara (Simaroubaceae), a shrub growing in the Guianas, northern Brazil and Venezuela. It occurs in smaller billets than those of Jamaica quassia. The medullary rays are only 1 or 2 cells wide but up to 30 cells deep. Calcium oxalate is absent. Cathine-6-one type alkaloids and quassinoids have been isolated from the wood. For a recent phytochemical study see J. A. Dou et al., Int. J. Pharmacognosy, 1996, 34, 349.
A number of Picrasma species produce similar constituents to the above. Three novel C18 quassinoids have been isolated from the leaves of Samadera madagascariensis leaves (P. H. Coombes et al., Phytochemistry, 2005, 66, 2734).
Uses
Quassia is used as a bitter tonic, as an insecticide, and as an enema for the expulsion of thread worms.
Q. amara wood is used in S. American traditional medicine for its stomachic, antiamoebic, antimalarial and antianaemic activity. The commercial ‘quassin’ prepared from Q. amara contains principally quassin and neoquassin and is widely used to give a bitter taste to beverages. It is also used as an insecticide because of its antifeedant properties.
Other quassinoids
Various parts of a number of plants of the family Simaroubaceae have been used in traditional medicine for the treatment of a variety of diseases including cancer, amoebic dysentery and malaria, and research has established that it is the quassinoid (simaroubolide) content of these plants that is responsible for this activity. Such compounds, and in some instances their glycosides, have also been shown to have antileukaemic, antiviral, anti-inflammatory, and (for insects) antifeedant properties. Recent studies are discussed in Chapter 28.
BLACK HOREHOUND
The dried aerial parts of Ballota nigra L. family Labiatae collected during the flowering period are included in the BP/EP and BHP 1996. Dispersed throughout Europe, N. Africa, western Asia, the USA and Australia, the plant is common to roadsides, hedges, etc. and is often regarded as a weed.
Characters of this species, many common to other Labiatae, are the erect square stems, often reddish-brown in the lower parts and up to 100 cm in height; leaves arranged oppositely, 2–5 cm in length, petiolate, rounded to ovate in outline, margin coarsely toothed, surfaces rugose and covered with whitish hairs, venation particularly prominent on the lower surface, depressed on the upper; flowers numerous in whorls in the axils of bracts, calyx about 1 cm in length ten-nervedwith five teeth; corollas purple or more rarely white and two-lipped; fruits consist of four small, three-sided achenes. The plant has an unpleasant odour.
Features of the powdered drug include numerous jointed uniseriate trichomes, various glandular trichomes, predominantly anisocytic but some anomocytic stomata on the lower leaf surface, portions of corolla with a papillose epidermis, pollen grains 25–30 μm in diameter with a smooth exine.
Constituents
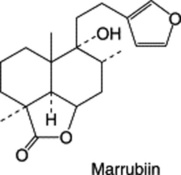
The constituents of black horehound have been extensively studied during the last 35 years, commencing principally with the work of G. Savona and colleagues in 1976, who characterized the diterpenoid content. Marrubiin, recognized for many years as a constituent of white horehound, is present in very small amounts, its derivates ballotinone, ballonigrine, 7α-acetoxymarrubiin, ballotenol and 13-hydroxyballonigrinolide form the major representatives of the group.
Flavonoids include derivatives of luteolin and apigenin.
The BP/EP states a minimum requirement of 1.5% of total ortho-dihydroxycinnamic derivatives for the dried drug expressed as acteoside (verbascoside) (for formula see ‘Mullein’); this also includes apiosyl, xylosyl and arabinosyl derivatives of verbascoside.
WHITE HOREHOUND
White horehound consists of the dried leaves and flowering tops of Marrubium vulgare L., family Labiatae. It is described in the BP/EP, BHP and the Complete German Commission E monographs. The plant is common throughout Europe, including the UK, having become naturalized in many places. Small amounts are obtained commercially for medicinal purposes from S.E. Europe, Morocco, Italy and France. Its principal use is as an expectorant and antispamodic in the treatment of bronchitis and whooping cough; it also possesses choleretic properties.
The active principals appear to be diterpenes. Marrubiin is one such, which, on the opening of its lactone ring, gives marrubinic acid, to which is ascribed the choleretic property of the drug. Related compounds present to a lesser extent are the diterpene alcohols marrubenol and vulgarol. Other constituents are: ubiquitous flavonoids including vitexin, apigenin and luteolin together with their glycosides; the alkaloids betonicine and stachydrine; and a small amount of volatile oil (0.06%) giving the drug its pleasant smell.
The BP/EP gives a TLC test for the drug and requires a minimum content of 0.7% marrubiin determined by liquid chromatography using a solution of marrubiin in methanol as a reference solution with absorption measurements at 217 nm.
TETRATERPENES—CAROTENOIDS
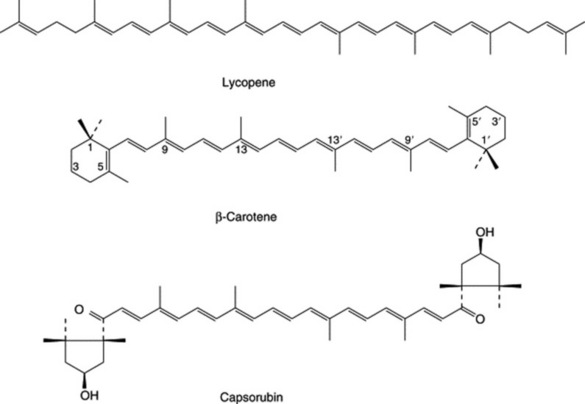
Important among these compounds are the C40 yellow or orange-red carotenoid pigments of which about 500 have been reported. As indicated in Chapter 18 they are formed by the tail to tail union of two molecules of the C20 geranylgeranyl diphosphate to give an acylic intermediate with a cis-configuration of the central double bond. By a change of configuration of the latter to trans and further desaturation of the isoprenoid chain, lycopene, the all-trans pigment of the ripe tomato fruit is formed. The various carotenes and derivatives can be envisaged by cyclization of one or both ends of the lycopene molecule (Fig. 24.6); all are all-trans. Cis-isomers, usually present in extracted carotenoid preparations, are probably artefacts.
In association with chlorophyll, carotenes participate in photosynthesis, but also occur in other non-photosynthetic plant organs such as the carrot and in fungi and bacteria. ‘Carotene’, a mixture of all the carotenes but with β-carotene predominating, was isolated from carrots as early as 1831. Between 1913 and 1915 a fat-soluble growth factor, vitamin A, was recognized to be present in materials such as butter and cod-liver oil and was subsequently shown to be a diterpenoid produced in the livers of animals by enzymic hydrolysis from β-carotene. There are many derivatives of the carotene molecule; some are oxygenated (Table 24.1), some have allenic and acetylenic bonds while others are formed by the loss of a portion of one end of the molecule as with β-citraurin, the characteristic apocarotenoid of citrus fruits. The striking pigments of the red peppers, capsanthin and capsorubin illustrate a contraction of either one (capsanthin) or two (capsorubin) of the usual cyclohexene end groups to a cyclopentane ring.
Table 24.1 Examples of oxygenated carotenoids.
| Carotenoid | Formula | Occurrence |
|---|---|---|
| Bixin | C25H30O4 | Annatto |
| Capsanthin | C40H56O3 | Capsicum spp. |
| Capsorubin | C40H60O4 | Capsicum spp. |
| Crocetin | C20H24O4 | Saffron |
| Crocin | C44H64O24 | Saffron |
| Fucoxanthin | C40H60O6 | Brown algae |
| Lutein | C40H56O2 | Tagetes erecta |
In addition to the pro-vitamin A activity of β-carotene, the carotenoids have more recently come to be recognized as essential for human health not only as antioxidants but also for specific functions such as normal vision and actions favouring the immune system. Thus, in 1997 J. T. Landrum and colleagues (Exper. Eye Res., 65, 57) established that the lutein of the ‘macula lutea’ of the retina of the eye is chemically identical to that giving the colour of marigold flowers (Tagetes erecta, Compositae). This accords with the use of lutein in nutritional supplements as preventatives of age-related macular degeneration and for other health benefits. Importantly, it has been demonstrated (P. Molnar et al., Carotenoid Sci., 2006, 10, 1) that the natural lutein ester, as extracted from marigold, remains virtually stable at gastric pH and body temperature, in contrast to lutein (unesterified), which is degraded by over 60% with the concurrent formation of anhydroluteins and 3′-epilutein. This implies that, for a given dose, relatively more of the ester will reach the intestinal absorption sites than will free lutein.
In 1835 Marquart observed that certain yellow flowers (e.g. buttercup), when treated with strong sulphuric acid, gave dark blue, green or violet colours. This reaction is characteristic of carotenoids and serves as a means of distinguishing them from other natural pigments such as the anthocyanins. The test is best carried out by stratifying an ether or chloroform solution of the carotenoid with 85% sulphuric acid, when a blue colour is formed at the junction of the two layers. Most carotenoids give a blue colour with antimony trichloride in chloroform (Carr–Price test), or a dark-blue colour with concentrated hydrochloric acid containing a little phenol. These tests have been adapted for use as TLC reagents for the identification of relevant crude drugs.
The considerable commercial demand for carotenes has encouraged the development of biotechnological methods for their production. These include mass-culture production from algae, yeast, fungi and recombinant DNA systems. Immobilized enzyme systems for carotenoid production have been studied.
For a concise and informative article (75 refs) on the biological properties of carotenoids, see N. L. Krinsky Pure Appl. Chem., 1994, 66, 1003. See also this book, Chapter 32: The plant nutraceuticals.
POLYTERPENOIDS
Polyterpenes are composed of many isoprene units. Common examples, both having macromolecules of molecular weight over 100 000, are found in indiarubber and gutta-percha. Doubtless the rubber-like substances of many other plants have a similar composition. Chemically pure rubber is cis-1,4-polyisoprene (C5H8)n, although in the natural state other materials are present; its occurrence is confined to the dicotyledons, and the one important commercial source is Hevea brasiliensis. Gutta-percha (see below) is trans-1,4-polyisoprene, and chicle, obtained from Manilkara sapota, contains a mixture of low molecular weight cis- and trans-polyisoprenes. No biological function for polyisoprenes has yet been discovered.
Rubber
A number of species of the families Euphorbiaceae, Apocynaceae, Moraceae, Asclepiadaceae and others produce a latex either in specialized cells or in anastomosing canals (Chapter 42), from which rubber can be prepared.
In Malaysia, Hevea brasiliensis (Euphorbiaceae) is cultivated for commercial use. Tapping is carried out mainly by women in the early morning when the internal latex pressure is highest. Trees are tapped by making an overlapping spiral groove, initially 1.5 m above the ground, with a knife called a jebong. The exuded latex is collected in cups placed at the lower end of the groove. After about 11 years the spiral has reached ground level. Following an initial cleaning of the latex in vats it is coagulated and bleached by treatment with formic acid and a bleaching agent. The latex emerges as blocks which are then passed through a mill 30 times to give thin layers ready for further processing.
Rubber consists of linear chains of about 1500 to 60 000 C5-isoprenoid units linked by cis double bonds (Fig. 18.20C). Compounds initiating the biosynthesis of rubber in H. brasiliensis have been characterized (Y. Tanaka et al., Phytochemistry, 1996, 41, 1501) and possible mechanisms controlling the molecular weights of rubber produced investigated (J. Tangpakdee et al., Phytochemistry, 1996, 42, 353).
Gutta-percha
Gutta-percha is purified, coagulated latex obtained from trees of the genera Palaquium and Payena (Sapotaceae), which are found both wild and cultivated in Malaysia and Indonesia. The method of collection resembles that used for rubber but the latex flows less readily. Depletion of these natural sources has led to the use of Parthenium argentatum (Compositae) for limited production. Gutta-percha differs from rubber in being almost incapable of vulcanization, and in that it becomes plastic when heated to about 45–60 °C. Gutta-percha contains a white, polymerized hydrocarbon gutta, composed of C5-units linked by trans double bonds (Fig. 18.20B); it has fewer units than rubber.
Gutta-percha was used in the form of chloroformic solution as a means of applying drugs to the skin, as gutta-percha tissue for covering moist dressings, and in the manufacture of surgical instruments. The USP/NF (1995) directs that it should be preserved under water in well-closed containers protected from light.