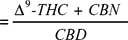Chapter 39 Hallucinogenic, allergenic, teratogenic and other toxic plants
The plants included in this chapter are toxic species which, although finding little use in modern medicine, are, because of the pharmacological effects which they produce, of considerable interest to pharmacognosists.
HALLUCINOGENS
Most cultures of man, from earliest times, have had recourse to some form of narcotic, often hallucinogenic, drug. These hallucinogens, often derived from plants, have frequently been used within a religious context. In recent years peyote, Indian hemp and lysergic acid derivatives have received much attention, but there are many other similar drugs used by local populations whose existence and use are still being investigated by ethnobotanists. In this respect, Professor R. E. Schultes (1915–2001) made extensive studies of such plants in South America and has emphasized the great need for recording the wealth of knowledge possessed by native tribes on narcotic plants before the activities of these peoples are overcome by ‘civilization’.
With the exception of cannabis, the principal known hallucinogenic plants contain alkaloids related to the neurophysiological transmitters noradrenaline and 5-hydroxytryptamine (serotonin).
FUNGI
Some of the poisonous fungi when taken orally produce hallucinations; these include toadstools of the genera Amanita, Psilocybe and Conocybe.
The Amanitas
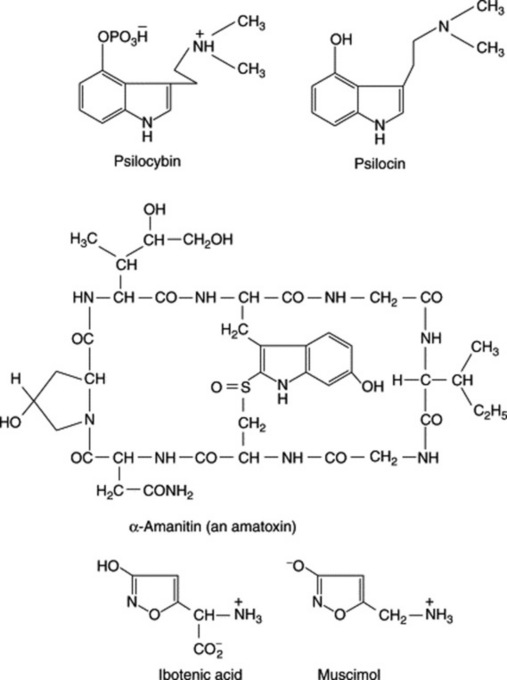
A number of Amanita species, in addition to promoting hallucinogenic effects, are extremely toxic. The appearance of the serious symptoms is considerably delayed (particularly with amatoxins, formula Fig. 39.1) after ingestion, by which time effective treatment becomes difficult. Three classes of toxins are recognized in the genus—tryptamines (e.g. bufotenine), cyclic peptides (phallotoxins and amatoxins) and isoxazole alkaloids (e.g. ibotenic acid, formula Fig. 39.1). The three classes of compound appear to be restricted to certain specific sections of the genus.
The fly agaric
The fly agaric (Amanita muscaria) is readily distinguished by its red or orange cap, often covered with white flecks. It contains a mixture of isoxazole alkaloids ibotenic acid and muscimol. Polysaccharides and a carboxymethylated derivative of the fungus have been shown to possess antitumour activity (T. Kiho et al., Biol. Pharm. Bull., 1994, 17, 1460). The pigments (betalains) of the fungus, also found in the Caryophyllales, are formed from tyrosine and the rapid development of pigment formation in A. muscaria has given an ideal system for isolating the enzymes involved (L. A. Mueller et al., Phytochemistry, 1996, 42, 1511; 1997, 44, 567).
The pharmacological effects appear within an hour or so of ingestion, with an initial period of excitation followed by muscular twitches, a slowed pulse rate, impaired breathing, delirium and coma; however, ingestion of the fungus is rarely fatal. The mushroom has a traditional use as an inebriant in regions of Siberia; one hypothesis suggests it to be the soma of the Rig Veda. The panther cap, A. pantherina, contains similar principles including pantherine (5-aminomethyl-3-hydroxyisoxazole), a flycidal alkaloid. A branched (1→3)-β-D-glucose isolated from an alkaline extract of the fungus exhibited significant activity in mice.
Hallucinogenic Mexican mushrooms
A number of small toadstools—particularly species of Psilocybe (P. mexicana), Conocybe (C. cyanopus) and Stropharia—constitute the Mexican hallucinogenic mushrooms (teonanacatl, ‘flesh of the gods’, much revered by the Aztecs). The onset of symptoms after ingestion of the fungi is rapid, and includes inability to concentrate and the occurrence of hallucinations. The active constituents are the tryptamine derivatives psilocybin and psilocin, compounds related to serotonin. These compounds are also found in similar toadstools (e.g. Psilocybe and Paneolus, Copelandia, Gymnopilus, Inocybe, Panneolina, Pluteus and Stropharia spp.) which are found in temperate regions. In Britain the ‘liberty cap’, Psilocybe semilanceata, a small toadstool common on lawns and parkland, and in Australia P. subaeruginosa both contain psilocybin. What is claimed to be the highest proportion of psilocin contained in any mushroom (3.3%, dry weight) was reported in Psilocybe cubensis; for a review of the mushroom alkaloids (567 refs), see R. Autkowiak et al., Alkaloids, 1991, 40, 189); for the concise large-scale synthesis of psilocin and psilocybin, see O. Shirota et al., J. Nat. Prod., 2003, 66, 885.
LYSERGIC ACID DERIVATIVES
The hallucinogenic properties of lysergic acid, and, in particular, the diethylamide derivative (LSD), are well-known. This acid forms the non-peptide portion of a number of ergot alkaloids (q.v.) and can also be produced by suitable cultivation of the fungus in liquid culture. It was with some surprise that lysergic acid was also found as a component of some convolvulaceous seeds (species of Ipomoea, Rivea and Argyreia), and as far as is known at present they constitute the only higher plants containing ergot-type alkaloids.
Morning Glory seeds
In the sixteenth century the Spaniards in Mexico reported the use of sacred hallucinogenic seeds known as ‘ololiuqui’. The climbing plant from which they were obtained was subsequently identified as Rivea corymbosa. Closely related in constituents and action are the seeds of Ipomoea tricolor (I. violacea) and those of various species of Argyreia. The name ‘Morning Glory’ is applied to Ipomoea tricolor but also to a number of other species (e.g. to I. purpurea and to the Japanese Morning Glory, I. hederacea). The trade names of species of Ipomoea are endlessly mixed. The seeds of the above-mentioned Ipomoea hederacea have long been used in the East as a purgative and were formerly official in the British Pharmacopoeia under the name ‘kaladana’ or ‘pharbitis seeds’.
PEYOTE
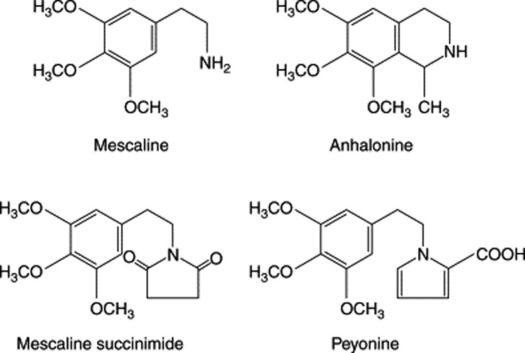
Certain cacti are of pharmaceutical and pharmacological interest, as they contain protoalkaloids, some of which have marked hallucinogenic properties. One of these is the cactus Lophophora williamsii which has long been used by Mexican Indians. It is known as peyotl, anhalonium or mescal buttons. The latter name is derived from the cactus stems, which are cut into slices about 20–50 mm in diameter. An interesting report (H. R. El-Seedi et al., J. Ethnopharm., 2005, 101, 238) sheds more light on its possible use by native N. Americans some 5700 years ago: two specimens from the Witte Museum in San Antonio were radiocarbon-dated to 3780–3600 BCE and, on analysis (TLC and GC-MS), gave alkaloids (2%) in which mescaline was identified, making these the oldest examples of a plant drug to yield a major bioactive compound. The chief active constituent is the alkaloid mescaline. The chemistry dates back to 1888, in which year Lewin isolated anhalonine, a crystalline tetrahydroisoquinoline alkaloid. By 1973 some 56 alkaloids had been characterized from the cactus and these could be classified as (1) mono-, di- and trioxygenated phenethylamines and their amides; (2) tetrahydroisoquinoline alkaloids and their amides; (3) phenethylamine conjugates with Kreb’s cycle acids; (4) pyrrole derivates. Examples of these groups are given in Fig. 39.2. The alkaloids can arise in the plant from dopamine, and grafting experiments involving Trichocereus pachanoi (‘San Pedro’) (a mescaline-producing species) and T. spachianus (no mescaline) have indicated that biosynthesis of the hallucinogen is confined to the aerial parts.
INDIAN HEMP
The Indian hemp plant was originally considered as a distinct species but came to be regarded as a variety of Cannabis sativa, the common European hemp, which thus exhibited a variety of ecotypes giving rise to differing cannabinoid mixtures. Subsequently (in 1974), a case was presented by American taxonomists for the recognition of three distinct species. C. sativa, C. indica and C. ruderalis. Other botanists have proposed sub-species of C. sativa.
The plant is found wild in India, Bangladesh and Pakistan. The drug consists of the dried flowering and fruiting tops of the pistillate plants from which no resin has been removed. Limited cultivation is permitted in some countries. The drug has been produced in East Africa, South Africa, Tripoli, Asia Minor and USA. Large confiscations of illicit cannabis and its preparations continue to be made in most countries. In temperate climates large quantities of hemp are grown for the stem fibre and for the seeds, which yield 30–35% of a drying oil.
History
Hemp has been cultivated for its seeds and fibres from a very remote period, but the narcotic properties are usually not marked in plants grown in temperate regions, and even in India an active drug can only be grown in certain districts. The drug is mentioned in early Hindu and Chinese works on medicine, and its use slowly spread through Persia to the Arabs. It was used by the Mohammedan sect known as the Hashishin or Assassins, who came into contact with the Crusaders in the eleventh and twelfth centuries. The drug attracted the attention of Europeans at the time of Napoleon’s Egyptian expedition.
Hemp products
Three main type of narcotic product are produced.
In America and Europe the product used by addicts is known as marihuana, in north Africa as kief, in South Africa as dagga, and in Arabia and Egypt as hashish.
Production of ganja
This is legally only produced by a few licensed growers in Bengal, Mysore and Madras. The seed is sown in rows about 1.3 m apart and male plants are eliminated as soon as they can be recognized. The resinous tops, largely of unfertilized female plants, are cut about 5 months after sowing and pressed into cakes. The yield is about 120 kg per acre.
Macroscopical characters
The flat- or Bombay-ganja occurs in agglutinated flattened masses of a dull green or greenish-brown colour. The resin is no longer sticky but hard and brittle; the odour, which is very marked in the fresh drug, is faint. The drug has a slightly bitter taste. Here and there ovoid hemp seeds may be picked out. Before further examination the drug should be soaked in successive quantities of alcohol to remove the resin and then softened in water.
The lower digitate leaves of the plant are seldom found in the drug. The thin, longitudinally furrowed stems bear simple or lobed, stipulate bracts. These subtend the bracteoles, enclosing the pistillate flowers. The bracts are stipulate and the lamina may be simple or three-lobed. The bracteole enclosing each flower is simple. The perigone enveloping the lower part of the ovary and the two reddish-brown stigmas can be seen with a lens.
Microscopical characters
The resin is secreted by numerous glandular hairs, 130–250 μm long (see Figs 39.3; 42.5). The head is usually eight-celled and the pedicel multiseriate or unicellular. To differentiate these from similar trichomes (e.g. those of the Labiatae) specificstains can be used. These include Fast Blue Salt B and, as described by Corrigan and Lynch in 1978, a reagent consisting of vanillin in ethanolic sulphuric acid which stains the cannabis glands a deep reddish-purple. It is possible to analyse individual trichomes by GLC by which means it has been shown that the glands represent a dynamic system in the cannabinoid synthetic activity of the plant. In addition, sessile glands (Fig. 39.3), abundant conical, curved, unicellular hairs, are also found, many having cystoliths of calcium carbonate in their enlarged bases (see Figs 39.3; 42.3); however, these cystolith hairs are not confined solely to the genus Cannabis. Cluster crystals of calcium oxalate are abundant, particularly in the bracteoles.
Constituents
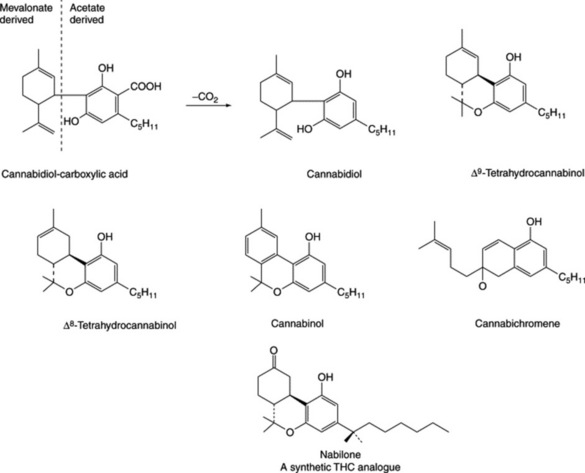
The narcotic resin is a brown, amorphous semisolid; soluble in alcohol, ether and carbon disulphide. It contains over 60 compounds (cannabinoids) all composed of an aromatic portion (C11 or C12), theoretically derivable from six acetate units, and an isoprenoid component (C10). They appear to form a natural group of C21 terpenophenolics of unique occurrence. Some principal components are cannabinol, tetrahydrocannabinol (THC), cannabidiol (CBD), cannabidiol-carboxylic acid, cannabigerol and cannabichromene (Fig. 39.4). Cannabinodiol is the aromatic analogue of cannabidiol. Cannabigerol precedes Δ9-THC in the biosynthetic pathway and is incorporated, by the plant, into the latter and other neutral cannabinoids. The identification of phloroglucinol β-D-glucoside in the shoot laticifer exudate of C. sativa, and phloroglucinol as a prominent component, and the only phenol, in the glandular trichomes suggests that it may have an important role in the in vivo enzymatically regulated biosynthesis of the cannabinoids (C. T. Hammond and P. G. Mahlberg, Phytochemistry, 1994, 37, 755).
Cannabipinol, isolated in 1967, contains a bicyclic monoterpene moiety in addition to the acetate-derived portion. Cannabidivarin, described in 1969, is a cannabidiol homologue with a 5-propyl-resorcinol moiety. In view of the importance of the detection of Indian hemp, a number of gas and thin-layer chromatographic techniques coupled with mass spectrometry have been developed for the separation of these substances.
Δ9-THC is the principal psychoactive constituent; Δ8-THC is almost as active but is only present in the plant in small amounts; cannabinol is less potent; although lacking psychotropic properties cannabidiol has anticonvulsant and possible analgesic effects. Cannabichromene may enhance THC activity and has antifungal, antimicrobial and anti-inflammatory activity; for enzyme studies related to its formation see S. Morimoto et al., Phytochemistry, 1998, 49, 1525.
The plant also contains a small quantity of laevorotatory volatile oil (about 30 components) containing terpenes and a sesquiterpene (cannibene); the bases choline, trigonelline, spermidine and an alkaloid cannabisativine; flavonoid O-glycosides of both vitexin and orientin; and calcium carbonate. It yields about 15% of ash and 10–18% of alcoholic extract.
Resin production
The study of resin production in cannabis continues to attract considerable attention. In practice, two varieties of Cannabis sativa are recognized: one produces fibre and the other resin. However, there is still no unanimity of opinion on the generic status of resin production, a situation which makes difficult a realistic legal definition of the narcotic drug. Cannabinoid production does not appear to be directly dependent on the presence of chlorophyll, and both green shoots and completely white shoots (as from a sport) will continue synthesis in the dark. Some evidence indicates that the plant’s ability to produce resin is governed mainly by the environment; thus, progeny of seeds of European fibre-producing plants when grown in Egypt reverted to resin-producing plants in a matter of a few years and, conversely, seeds from resin-producing plants of the Middle East failed to produce abundant narcotic resin when grown in temperate Europe. These observations have been supported by work at the phytotron near Paris, which suggests that cannabis has, from its early growth stages, the chemical capacity to become either fibrous or resinous, depending on the climate. Nevertheless, it is possible to raise resin-containing plants in temperate regions and plants raised in the UK from overseas seed-stock (Morocco, Sri Lanka, Zambia) for a number of generations broadly retained the cannabinoid content typical of the source countries but tetrahydro-cannabinolic acid (THCA) consistently predominated over THC, the ratio THCA/THC being 17 compared with 2 in plants from the original areas (J. E. Pitts et al., J. Pharm. Pharmacol., 1992, 44, 947).
Apart from ability to produce resin per se, it appears certain that resin-producing plants do exist as chemical races. The principal chemotypes recognized are those involving a preponderance of Δ9-THC, CBD or cannabigerol together with others having various ratios of THC/CBD. There may also be a variation in resin content between male and female plants; again, this may be an inherited feature or may be differing climatic responses of the male and female. A study of wild-growing plants of cannabis collected in northern India at different altitudes and locations showed that these, too, showed great variations in the proportions of cannabinoids present. Possibly, a complete range of genetic types covering the transition from the fibrous form to the various resin types will emerge, with climatic factors also influencing the chemical products of any one type.
All the above factors obviously contribute to the very variable narcotic action of different samples of the drug.
Cannabis evaluation
The many factors above which determine the cannabinoid composition mean that care must be taken in ascertaining the chemical phenotype of a plant. The general view that cannabis preparations can be evaluated on their Δ9-THC content neglects other active components, and in attempts to classify cannabis on the basis of its narcotic/fibre content a number of systems, some very complex, have been devised. A relatively simple relationship introduced by Waller is based on the combined Δ9-THC and cannabinol (CBN) in relation to cannabidiol (CBD).
A sample with a value greater than 1 = a drug type of cannabis; a sample with a value less than 1 = a fibre type.
High potency grades of marihuana (>20% Δ9-THC dry wt.) are reportedly available on the illicit drug market and from one such sample S. A. Ahmed et al. have characterized eleven new cannabinoid esters (J. Nat. Prod., 2008, 71, 536).
Uses
Medicinal properties of cannabis were recognized some 5000 years ago. In the mid-nineteenth century it was used in Europe as a hypnotic, anticonvulsant, analgesic, antianxiety and antitussive agent and was still official in the BPC 1949, together with the extract and tincture. Over many years it fell into disuse in human and veterinary medicine, and because of its narcotic properties importation into many countries became illegal. Promising results on the use of Δ9-THC (dronabinol) for the relief of nausea and vomiting caused by cancer chemotherapy led to its use in the USA as an antiemetic. It is also employed to stimulate the appetite of AIDS patients. It can be prescribed in the UK under licence on a named-patient basis. Sativex is a relatively new Canadian product containing a mixture of THC and CBD. It is a spray not licensed for production in the UK but can be prescribed under Home Office licence for specific patients. Nabilone (Fig. 39.4), a synthetic cannabinoid antiemetic, is described in the British National Formulary; it is recommended to be administered in a hospital setting under close observation.
Cannabis also appears to have value in the relief of the symptoms of multiple sclerosis and other neurological disorders. The debate on its clinical usefulness continues.
Subject to Medicines Control Agency approval, Phase II trials by GW Pharmaceuticals involving MS sufferers and patients with other neurological disorders were proposed (Report, Pharm. J., 1999, 263, 811).
Addiction has been common in many parts of Asia for more than 1000 years but only in recent years has the problem become world-wide.
Amar BA. Cannabinoids in medicine. A review of their therapeutic potential. J Ethnopharmacology. 2006;105(1–2):1-25. A review with over 170 refs
British Medical Association. Therapeutic uses of cannabis. Amsterdam, The Netherlands: Harwood Academic Publishers, 1997.
Medicinal and aromatic plants – industrial profiles (series ed) Brown DT, Hardman R, editors. Cannabis the genus Cannabis. Vol 4. CRC Press, Taylor and Francis Group. Boca Raton, FL, 1998 904 references
Mechoulam R, editor. Cannabinoids as therapeutics – milestones in drug therapy. Birkhauser Verlag, Basel, 2005. 282 references
OTHER HIGHER PLANTS
Apocynaceae
Iboga root (Tabernanthe iboga), an African narcotic, contains alkaloids of the indole group. The alkaloid ibogaine has received attention as a possible antiaddictive drug (P. Popik and P. Skolnick, The Alkaloids, 1999, 52, 197).
Labiatae
Salvia divinorum: the leaves have long been used in Mazatec shamanistic divination ceremonies in the region of Oaxaca, Mexico. R. G. Wasson, working in the 1950s and 1960s, reported and identified this unusual intoxicant plant, which he believed to represent the sacred Aztec narcotic called pipilzintzintli. He stated that the hallucinogenic effects were similar to, but shorter and less striking than, those of the Mexican narcotic mushrooms (q.v.). Later, the ethnobotanist Daniel Siebert described the hallucinogenic effects of a minute amount of the active constituent, salvinorin A, as ‘awesome and frightening’.
The active constituents comprise a considerable number of neoclerodane diterpenes, including salvinorins A–I, salvinicins A and B and others. Salvinorin A is a κ-opioid selective agonist and salvinicin A is a partial κ-opioid agonist; salvinicin B is the first known neoclerodane μ-opioid antagonist (T. A. Munro and M. A. Rizzacasa, J. Nat. Prod., 2003, 66, 703; A. K. Bigham et al., J. Nat. Prod., 66, 1242; O. Shirota et al., J. Nat. Prod., 2006, 69, 1782).
The use of S. divinorum (‘magic mint’, ‘Sally D’) as an hallucinogen has now spread globally and its possession in Australia, Italy, Sweden and four American States is illegal. For a current overview, see G. Vince, New Scientist, 2006, Sept. 30, 44; and for a review update on the pharmacology and analytical methodology of the plant and salvinorin, A. O. Grundmann et al., Planta Medica, 2007, 73, 1039.
Leguminosae
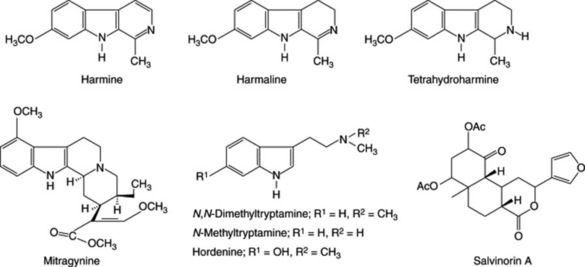
The beans of Anadenanthera peregrina are used in northern South America for the preparation of snuff. A root decoction of Mimosa hostilis is used in east Brazil. Both contain tryptamines. M. ophthalmocentra root is used similarly and contains N,N-dimethyltryptamine, N-methyltryptamine and hordenine (L. M. Batista et al., Pharm. Biol., 1999, 37, 50).
Malpighiaceae
A number of Banisteriopsis species, e.g. B. caapi, a forest liana, are used as a snuff or beverage in the Amazon basin. Such species are reportedly psychoactive and contain tryptamine derivatives and the simple β-carboline alkaloids harmine, harmaline and tetrahydroharmine (Fig. 39.5).
Ayahuasca (hoasca) is an Amazonian ritual decoction made by boiling together a mixture of B. caapi and Psychotria viridis (Rubiaceae) the latter containing the psychedelic compound N,N-dimethyltryptamine (DMT) (Fig. 39.5). Modern research involving human volunteers has demonstrated the subtlety of this shamanistic preparation in which DMT consumed orally exerts its full effect by the build-up of 5-hydroxytryptamine made possible by the monoamine oxidase inhibitory properties of the Banisteriopsis alkaloids. It would appear that the combination of these two drugs produces a pharmacological response equivalent to that observed in acute psychotic unmedicated patients. For details of the above research see A. B. Pomilio et al., J. Ethnopharmacology, 1999, 65, 29; J. C. Callaway et al., ibid., 1999, 65, 243.
Ayahuasca is banned in the USA but in 2006 the Supreme Court unanimously ruled that a small religious sect could continue to import and utilize a hallucinogenic tea central to its ritual ceremonies (see report by C. Cavaliere and M. Blumenthal, HerbalGram, 2006, 17, 62).
Myristicaceae
Nutmeg has received attention as a psychotropic agent and this action may possibly arise from the myristicin and elemicin components; the formal relationship of these compounds to the amphetamines (some of which exert hallucinogenic effects) is of interest. Dimeric phenylpropanoids are also found in the seeds.
Virola spp. are also of the family Myristicaceae. They yield a blood-red, bark resin which is used by Indian tribes of the Amazon region for the preparation of hallucinogenic snuffs. They contain various tryptamines. Virola sebifera, used in Venezuela, contains the well-known psychotomimetic N,N-dimethyltryptamine (DMT) and also 5-hydroxy-DMT, 5-methoxy-DMT and 2-methyl-1,2,3,4-tetrahydro-β-carboline. V. multinerva also contains diarylpropanoids similar to those found in nutmeg.
Besides nutmeg, other essential oils that contain elemicin are those of parsley (Petroselinum sativum) and elemi-tree (Canarium commune) (M. DeVincenzi et al., Fitoterapia, 2004, 75, 615).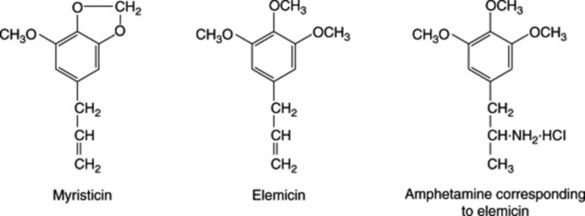
Rubiaceae
Mitragyna speciosa leaves (q.v.) contain the alkaloid mitragynine, which binds to the same opioid receptor in the brain as morphine, codeine and diamorphine. The leaves (kraton) are widely available in the US, where it is used for its energizing and euphoric effects. It is an illegal substance in some S.E. Asian countries and in Australia. For a survey, see G. Vince under Salvia divinorum above.
Zygophyllaceae
Peganum harmala produces a range of harmine alkaloids and the seeds are recognized in India for their psychoactive properties.
African hallucinogens
For ethnopharmacological notes on genera including Alchornea, Monadenium, Mostuea and Voacanga see P. A. G. M. De Smet, J. Ethnopharm., 1996, 50, 141.
The so-called Iboga alkaloids, which possess a catharanthine-type structure, are found in a number of African plants and are used locally as a stimulant. Apparently, a global medical subculture has arisen in which the alkaloid ibogaine is used principally to alleviate the symptoms of opioid withdrawal (K. R. Alper et al., J. Ethnopharm., 2008, 115(1), 9–24).
NATURAL ALLERGENS
A large number of plant and animal materials give rise to allergic reactions in certain individuals. The allergenic material is transmitted by direct skin contact, by airborne pollens, smoke and dried plant particles, and on the coats of domestic animals. Once a person has been sensitized to a particular allergen, subsequent exposure to the materials produces an antigen–antibody reaction which results in the liberation of histamine or histamine-like compounds which in turn cause the allergic symptoms. Allergies are commonly manifested as hay fever, asthma and dermatitis. Desensitization is often possible once the specific cause has been established, and a considerable number of allergenic extracts are now available for diagnostic and prophylactic treatments. As fatal anaphylactic reactions are possible, desensitization using allergenic extracts should be carried out only in situations where full cardiorespiratory resuscitation facilities are available. The following allergens are well-known.
Pollens
Responsible for seasonal hay fever, which may progress to chronic asthma. Pollen counts of the atmosphere are regularly recorded and published. Grass pollens form the highest proportion of the total count and may constitute 62% of the total count during June and July. In London, for these months, daily counts of 200 m−3 are not uncommon. Pollen counts of 50, and as low as 10, will produce discomfort in susceptible individuals. Common grasses involved include timothy (Phleum pratensis), cocksfoot (Dactylis glomerata) and perennial rye (Lolium perenne). The pollen of nettle (Urtica dioica) is second in importance to the grasses in this connection in the UK. It occurs in the air throughout the summer, reaching a peak in late June to July. Other relevant pollens are those of the plantain (Plantago spp.) and mugwort (Artemesia vulgaris). In the USA pollens of the ragweeds (Ambrosia spp.) are important. Tree pollens are contained in the atmosphere in the spring; they are not as common as allergens as those of grasses, the ones of most clinical importance being from the birch and plane trees.
Spores
A number of common moulds produce spores which cause rhinitis and asthma in sensitive individuals. They are often responsible for those conditions which extend beyond the normal pollen season and up to the beginning of frosts. Moulds flourish in damp conditions where organic decay is progressing, and peak sporulation occurs during hot, dry conditions when the atmosphere may become heavily contaminated. In the UK the spores of Cladosporium herbarum and Sporobolomyces roseus cause the most trouble. Exposure to lycopodium spores (q.v.) has caused allergic reactions varying from dermatitis to severe asthma attacks. There are also reports of spores causing adhesions on serous surfaces and foreign-body granulomas in soft tissues. This could have implications for the use of lycopodium powder as a dusting powder for non-lubricated condoms.
Rhus (Toxicodendron) spp
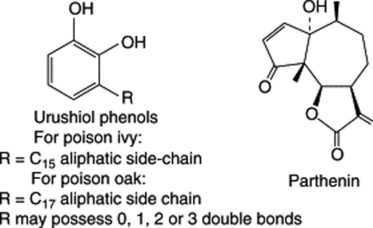
Rhus radicans (poison ivy), R. toxicodendron (poison oak), R. diversiloba (Pacific poison oak) and R. vernix (poison sumach, poison elder) (Anacardiaceae) contain contactant allergens which produce severe dermatitis associated with watery blisters which burst and quickly spread across the skin. The allergens are contained in the plant sap and are easily transmitted (on clothing, hands, animal fur and even as the result of bush fires). These compounds are known as urushiols and belong to a class of alkenyl polyphenols found in the Anacardiaceae. They constitute an interesting chemotaxonomic group and are an example of the use of a starter other than acetyl-CoA in fatty-acid synthesis by which C2 units derived from malonate are added to an unsaturated fatty acid; subsequent cyclization forms, as one example, the urushiols. With the exception of the cultivated sumach, which does not appear to be troublesome, these plants are not found in Britain, but they constitute a considerable hazard in the USA, where poison ivy, in particular, is particularly widespread as a woody vine. Lacquer, used in the sixteenth and seventeenth centuries for producing an oriental-type finish on furniture, was derived from R. vernicifera, and its use constituted an industrial hazard for the craftsmen. Similar compounds to the above have been isolated from the fruit pulp of Ginkgo biloba and from the glandular trichomes of annual Phacelia spp. (Hydrophyllaceae) of the Californian Mojave desert. The dermatitic action of these compounds is consistent with oxidation of the allergen to a quinone which then binds covalently to a protein nucleophile giving an antigenic complex.
Sesquiterpene lactones
These compounds (see Chapter 24), obtained from members of the Compositae, Lauraceae and Magnoliaceae and from the liverwort Frullania (Jubulaceae), are a major class of substances causing allergic contact dermatitis in man. The presence of an α-methylene group, exocyclic to the γ-lactone, appears to be the principal immunochemical requisite for activity. The compounds are illustrated, from the many, by the pseudoguaianolide parthenin, obtained from the plant Parthenium hysterophorus, an aggressive weed causing public health problems in parts of India. Some individuals are sensitive to feverfew and others to chrysanthemums. Two other plant species which can give rise to allergic reactions are the common rue (Ruta graveolens) and the indoor ornamental ‘dumb cane’ (Dieffenbachia seguine, Araceae). In the latter instance it would appear that the irritant substances are introduced into the body tissues by abrasion, through punctures caused by acicular crystals of calcium oxalate contained in idioblasts.
TERATOGENS OF HIGHER PLANTS
Teratogenic substances, when ingested by the mother, can cause abnormalities in the developing fetus; thalidomide represents the tragic example of a synthetic drug having such undetected properties at the time of its use. Such substances undoubtedly occur also in plants, but no species has been shown as having been responsible for malformations in humans. That the possibility exists is demonstrated by the teratogenic effects of certain plants when incorporated into animal fodder.
Teratogens usually, but not invariably, act during a short, relatively early period of the gestation cycle, so that when the abnormalities become apparent in the offspring the causative plant may have disappeared from the fodder source. The range of plant constituents known to have teratogenic effects, albeit often demonstrated using only laboratory animals and large doses not normally experienced by humans, includes 14 different groups of alkaloids, coumarins, lignans, macrolides, nitriles, terpenoids, toxic amino acids and unidentified compounds of many plants. As with the hallucinogens, the majority of teratogens contain nitrogen; for some examples see Table 39.1.
Table 39.1 Teratogens of higher plants.
| Plant source | Constituents | Notes |
|---|---|---|
| Senecio spp. (Compositae) | Pyrrolizidine alkaloids: lasiocarpine, retrorsine | Possible teratogenic effects in rats and in utero deaths of calves |
| Indigofera spicata (Leguminosae) |
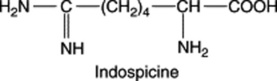 |
Cleft palate and embryo lethality in rats. Possible malformations in domestic livestock. Indospicine teratogenesis |
| Nicotiana spp. (Solanaceae), Lobelia spp. (Campanulaceae) | Pyridine alkaloids | Probably responsible for some skeletal deformations in pigs but effect not positively attributable to alkaloids |
| Blighia sapida (Akee) fruits and seeds (Sapindaceae) |
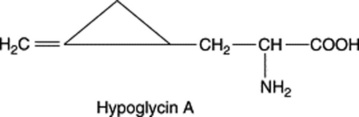 |
Hypoglycin A is known to be hypoglycaemic in humans and teratogenic in rats; it is twice as toxic as its peptide derivative hypoglycin B |
| Leucaena leucocephala; Mimosa spp. (Leguminosae) |
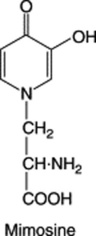 |
Large quantities toxic to livestock. Teratogenic effects demonstrated in pigs and rats |
| Locoplants e.g. Astragalus lentiginosus (Leguminosae) | Unknown | Contains osteolathyrogens (substances ingested by young through mother’s milk), and teratogens characterized by causing excessive flexure of carpal joints or contracted tendons |
| Lupins e.g. Lupinus sericeus (Leguminosae) | Quinolizidine alkaloids, e.g. cytisine, anagyrine | Teratogenic effect results in crooked calf disease |
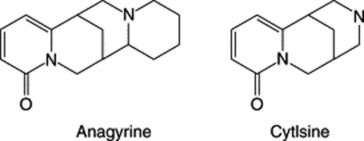 |
||
| Conium maculatum (Umbelliferae) | Coniine | Alkaloid teratogenic, shown to produce crooked calf disease |
| Veratrum californicum (Liliaceae) | Many steroidal alkaloids | Teratogenic effect causes cyclopian and related cephalic malformations in lambs. The three active alkaloids have a fused furanopiperidine ring E/F arrangement as in cyclopamine |
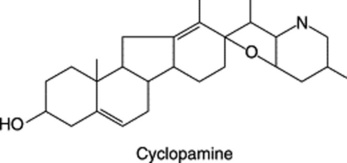 |
OTHER TOXIC PLANTS
In addition to those plants mentioned in this chapter and those of medicinal significance considered in Chapters 19–26, there remain many others with poisonous properties. Such plants are generally of local importance, and it is desirable that the pharmacist should have some knowledge of those found in his own locality, be familiar with those characters by which the plant can be identified and be aware of the antidotes required for the treatment of poisoning.
Cases of poisoning of humans by higher plants are most likely to occur with children and to involve those plants that produce attractive berries (e.g. belladonna, cotoneaster), seeds (e.g. laburnum) eaten for green peas, and those which may be introduced into the mouth for other reasons (e.g. the hollow stems of hemlock used as pea-shooters). Mistaken identity occasionally leads to fatalities and this is particularly so in the case of members of the Umbelliferae. Cases include a fatality in Maine, USA from consumption of Cicuta maculata (spotted cowbane) in mistake for ginseng. The poisonous principle present in Cicuta spp. is a C17-polyunsaturated alcohol named cicutoxin. Some eleven such polyacetylenes have been isolated from C. virosa (water-hemlock) and using a model neurone, U. Wittstock et al. have suggested that the toxic property of cicutoxin could be due to a prolonged neuronal action potential (Planta Medica, 1997, 63, 120). In Holland, four people were poisoned by Oenanthe crocata (hemlock water dropwort) consumed in mistake for celery (these people probably survived because the roots were boiled before eating). This plant its widespread in marshy and damp woodland areas of Europe; its roots have an odour and taste resembling parsnips or celery and the poisonous principle is oenanthetoxin, isomeric with cicutoxin.
The poisoning of livestock by plants is relatively common, particularly in extensive grazing areas where there is no attempt to control weeds. Poisonous plants may be consumed by animals because the plants happen to be growing among the fodder or were collected and dried with hay. In the latter case some unstable poisonous constituents may disappear with drying and storage. In poor seasons animals may forage and consume plants which they would not normally eat. During the dry summer of 1976 numerous cows died near Crediton, UK after eating O. crocata.
Some widespread poisonous plants owe their properties to the presence of hepatotoxic pyrrolizidine alkaloids (see Fig. 26.10). These include the Senecio spp. (ragworts) and members of the tribe Eupatorieae of the Compositae. Several commonly used herbs containing small quantities of these alkaloids include comfrey, Russian comfrey, coltsfoot and petasites. Human fatalities have been reported in relation to the herbal use of Senecio longibobus in the USA. In the UK, a voluntary agreement by herbal medicine suppliers not to supply senecio products is now being followed with proposed legislation by the Medicines and Healthcare products Regulatory Agency to implement a ban (Pharm. J., 2007, 278, 448).
Another group of compounds which has been shown to promote liver cancer in rats is that containing safrole and other alkenylbenzene derivatives. Although these are weak carcinogens and concentrations in products for human consumption never approach the toxic levels observed in laboratory tests, the desirability of their use has been questioned. Oils in which they occur include sassafras, Brazilian sassafras, star-anise, nutmeg, cinnamon, camphor (natural), calamus, tarragon, basil and ylang-ylang. M. De Vincenzi et al. have considered the specific occurrence of methyleugenol as a component of aromatic plants (Fitoterapia, 2000, 71, 216).
Other carcinogens or cocarcinogens discussed elsewhere are the betel quid and tigliane and daphnane derivatives and related diterpenes.
The fruits of Blighia sapida have been mentioned elsewhere in connection with teratogenesis (Table 39.1) and hypoglycaemia (Chapter 29). The unripe fruits present an exceptional hazard for young children in the areas in which the trees grow. In 1998 an epidemic of fatal encephalopathy involved 29 preschool children in Burkina Faso, W. Africa (H. A. Meda et al., Lancet, 1999, 353, 536) and a similar explanation was suggested for an epidemic involving more than 100 children in the Ivory Coast in 1984 and for poisonings in Jamaica. The unripe fruits contain the highest hypoglycin A content but nevertheless the ripe fruits also need to be par-boiled before consumption. As in all cases of poisoning, rapid identification of the poison is essential if the most effective treatment is to be given. Often, the material available for identification is scant and rarely sufficient to enable identification by means of standard methods based on a flora; this reinforces a need for knowledge of the local poisonous plants and the characters of their various morphological parts.
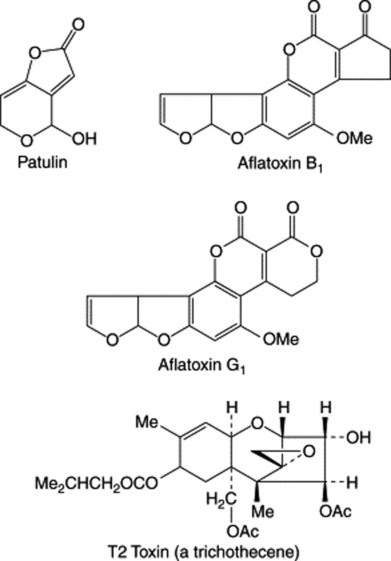
Fungi have a geographically universal potential as toxic agents, and the significance of their active constituents, mycotoxins, is only now coming to be fully appreciated. Well-established are the poisonous principles of various Basidiomycetes which may be confused with the edible mushroom and toadstools. Also, the infection of rye with the ergot fungus resulting in the disease St Anthony’s Fire, which, although now rare, still occasionally occurs in rye-eating countries. The polyketide patulin is produced by species of Penicillium and is often present in mouldy apples; it was originally studied for its antibiotic properties but proved also to be a potent carcinogen.
The more widespread danger of mycotoxins became apparent in World War II, when the consumption of mouldy grain in Russia led to thousands of fatalities. The mycotoxins produced by various Aspergillus spp. (e.g. A. flavus, A. parasiticus) are termed aflatoxins, all having a coumarin nucleus fused to a bifuran unit and possessing in addition a pentenone ring (B series) or a six-membered lactone (G series). Examples are given in Fig. 39.6.
The above moulds are particularly likely to occur in oil-seed meals and in cereals; they have been circumstantially implicated in the deaths of children in a number of countries. Animal tests have shown the aflatoxins to be potentially harmful in a number of respects—as potent toxins, as carcinogens, as teratogens and as mutagens. In Britain these compounds came to prominence in 1959 when great numbers of turkeys and chickens in East Anglia rapidly succumbed to their toxicity after being fed contaminated groundnut meal imported from S. America. The recognition of the serious health hazards to humans and animals of aflatoxins in foods has led to the legal imposition in the UK of a limit of 10 μg kg−1 aflatoxin B1 in nuts and nut products for human consumption, and an EU guideline for a limit of 20 μg kg−1 aflatoxin B1 for animal feedstuffs. Methods of assay for these compounds have been developed in recent years and include HPLC and TLC (slow and cumbersome) and various RIA and EIA techniques.
Another condition arising from consumption of overwintered mouldy cereals, and reported to occur principally in Russia, is alimentary toxic aleukia; other manifestations include weight loss, skin inflammation and death. The responsible organisms (largely Fusarium and Trichothecium spp.) produce active constituents termed trichothecenes of which about 172 had been reported by 1981 (see J. F. Grove, Nat. Prod. Rep., 1993, 10, 429). These compounds are tricyclic sesquiterpenes and the majority are exemplified by the structure of T2 toxin (Fig. 39.6). However, some 67 compounds are macrocyclic in structure and it is interesting that they also occur in two toxic Brazilian species of Baccharis (Compositae), a finding which was originally incorrectly attributed to fungal infection (B. B. Jarvis et al., J. Nat. Prod., 1988, 51, 736; Phytochemistry, 1991, 30, 789). Matossian of the University of Maryland considers that F. tricinctum was the probable fungal food contaminant causing ‘putrid malignant fever’, a great child-killer of the early eighteenth century. These compounds also achieved notoriety as alleged agents of chemical warfare (‘yellow rain’).
Crude drugs, unless sterilized, are often grossly contaminated with mould spores, and if such drugs are to be consumed as such (as distinct from use for the isolation of active constituents), then it is important that they are free of dangerous mycotoxins. A Polish survey of 246 crude drug samples found only two (salvia leaves and tormentilla rhizome) which contained Aspergillus spp. producing aflatoxin B1. Nevertheless, it is a situation that requires monitoring.
(translator)Frohn D, Pfander HJ, Alford I. Poisonous plants: a handbook for doctors, pharmacists, toxicologists, biologists and veterinarians. An appendix includes information concerning N. American plants. 2nd edn. Timber Press. Portland, OR, 2005.
Dictionary of plant toxins. In: Harborne JB, Baxter H, Moss GP, editors. A number of books on poisonous plants, now mostly out of print but useful if accessible, are given in the 14th edition of this book. Chichester, UK: John Wiley; 1996:532.