Chapter 26 Alkaloids
Alkaloid-containing plants constitute an extremely varied group both taxonomically and chemically, a basic nitrogen being the only unifying factor for the various classes. For this reason, questions of the physiological role of alkaloids in the plant, their importance in taxonomy, and biogenesis are often most satisfactorily discussed at the level of a particular class of alkaloid. A similar situation pertains with the therapeutic and pharmacological activities of alkaloids. As most alkaloids are extremely toxic, plants containing them do not feature strongly in herbal medicine but they have always been important in the allopathic system where dosage is strictly controlled and in homoeopathy where the dose-rate is so low as to be harmless.
INTRODUCTION
A precise definition of the term ‘alkaloid’ (alkali-like) is somewhat difficult because there is no clear-cut boundary between alkaloids and naturally occurring complex amines. Typical alkaloids are derived from plant sources, they are basic, they contain one or more nitrogen atoms (usually in a heterocyclic ring) and they usually have a marked physiological action on man or other animals. The name ‘proto-alkaloid’ or ‘amino-alkaloid’ is sometimes applied to compounds such as hordenine, ephedrine and colchicine which lack one or more of the properties of typical alkaloids. Other alkaloids, not conforming with the general definition, are those synthetic compounds not found in plants but very closely related to the natural alkaloids (e.g. homatropine). In practice, those substances present in plants and giving the standard qualitative tests outlined below are termed alkaloids, and frequently in plant surveys this evidence alone is used to classify a particular plant as ‘alkaloid-containing’.
HISTORY
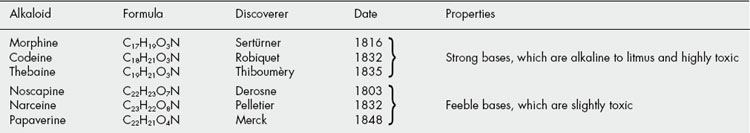
The first isolations of alkaloids in the nineteenth century followed the reintroduction into medicine of a number of alkaloid-containing drugs and were coincidental with the advent of the percolation process for the extraction of drugs. The French apothecary Derosne probably isolated the alkaloid afterwards known as narcotine in 1803 and the Hanoverian apothecary Sertürner further investigated opium and isolated morphine (1806, 1816). Isolation of other alkaloids, particularly by Pelletier and Caventou, rapidly followed; strychnine (1817), emetine (1817), brucine (1819), piperine (1819), caffeine (1819), quinine (1820), colchicine (1820) and coniine (1826). Coniine was the first alkaloid to have its structure established (Schiff, 1870) and to be synthesized (Ladenburg, 1889), but for others, such as colchicine, it was well over a century before the structures were finally elucidated. Modern methods and instrumentation have greatly facilitated these investigations, and it is interesting to note that the yields of ‘minor’ alkaloids, too small for further investigation, isolated by chemists during the first quarter of the last century would now be sufficient, several thousand times over, for a complete structure analysis. In the second half of the twentieth century alkaloids featured strongly in the search for plant drugs with anticancer activity. A notable success was the introduction of Catharanthus alkaloids and paclitaxel into medicine and there is much current interest in other alkaloids having anticancer properties as well as those exhibiting antiaging and antiviral possibilities.
DISTRIBUTION
Some 150 years of alkaloid chemistry had resulted by the mid-1940s in the isolation of about 800 alkaloids; the new technology of the next 50 years increased this figure to the order of 10 000.
True alkaloids are of rare occurrence in lower plants. In the fungi the lysergic acid derivatives and the sulphur-containing alkaloids, e.g. the gliotoxins, are the best known. Among the pteridophytes and gymnosperms the lycopodium, ephedra and Taxus alkaloids have medicinal interest. Alkaloid distribution in the angiosperms is uneven. The dicotyledon orders Salicales, Fagales, Cucurbitales and Oleales at present appear to be alkaloid-free. Alkaloids are commonly found in the orders Centrospermae (Chenopodiaceae), Magnoliales (Lauraceae, Magnoliaceae), Ranunculales (Berberidaceae, Menispermaceae, Ranunculaceae), Papaverales (Papaveraceae, Fumariaceae), Rosales (Leguminosae, subfamily Papilionaceae), Rutales (Rutaceae), Gentiales (Apocynaceae, Loganiaceae, Rubiaceae), Tubiflorae (Boraginaceae, Convolvulaceae, Solanaceae) and Campanulales (Campanulaceae, sub-family Lobelioideae; Compositae, subfamily Senecioneae).
Hegnauer, who has made an intensive study of alkaloid distribution, while recognizing the undoubted potential chemotaxonomic significance of this group, is cautious about its use without due regard to all the other characters of the plant. Nevertheless it continues to be a popular area of research.
Nearly 300 alkaloids belonging to more than 24 classes are known to occur in the skins of amphibians along with other toxins. They include the potent neurotoxic alkaloids of frogs of the genus Phyllobates, which are among some of the most poisonous substances known. Other reptilian alkaloids are strongly antimicrobial. Alkaloids derived from mammals include ones of indole and isoquinoline classes a few are found in both plants and animals.
PROPERTIES
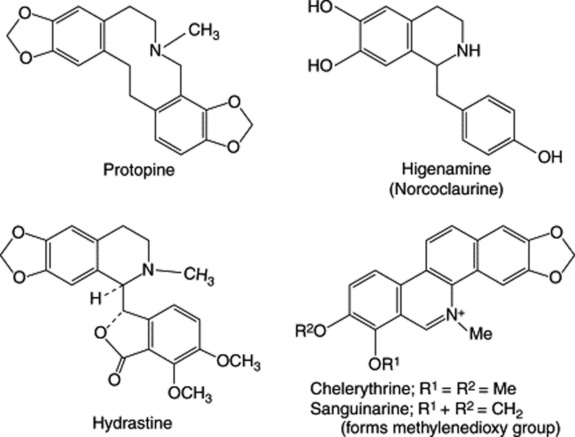
Most alkaloids are well-defined crystalline substances which unite with acids to form salts. In the plant they may exist in the free state, as salts or as N-oxides (see below). In addition to the elements carbon, hydrogen and nitrogen, most alkaloids contain oxygen. A few, such as coniine from hemlock and nicotine from tobacco, are oxygen-free and are liquids. Although coloured alkaloids are relatively rare, they are not unknown; berberine, for example, is yellow and the salts of sanguinarine are copper-red.
A knowledge of the solubility of alkaloids and their salts is of considerable pharmaceutical importance. Not only are alkaloidal substances often administered in solution, but also the differences in solubility between alkaloids and their salts provide methods for the isolation of alkaloids from the plant and their separation from the nonalkaloidal substances also present. While the solubilities of different alkaloids and salts show considerable variation, as might be expected from their extremely varied structure, it is true to say that the free bases are frequently sparingly soluble in water but soluble in water but soluble in organic solvents; with salts the reverse is often the case, these being usually soluble in water but sparingly soluble in organic solvents. For example, strychnine hydrochloride is much more soluble in water than is strychnine base. It will soon be realized that there are many exceptions to the above generalizations, caffeine (base) being readily extracted from tea with water and colchicine being soluble in either acid, neutral or alkaline water. Again, some alkaloidal salts are sparingly soluble—for example, quinine sulphate is only soluble to the extent of 1 part in 1000 parts of water, although 1 part quinine hydrochloride is soluble in less than 1 part of water.
STRUCTURE AND CLASSIFICATION
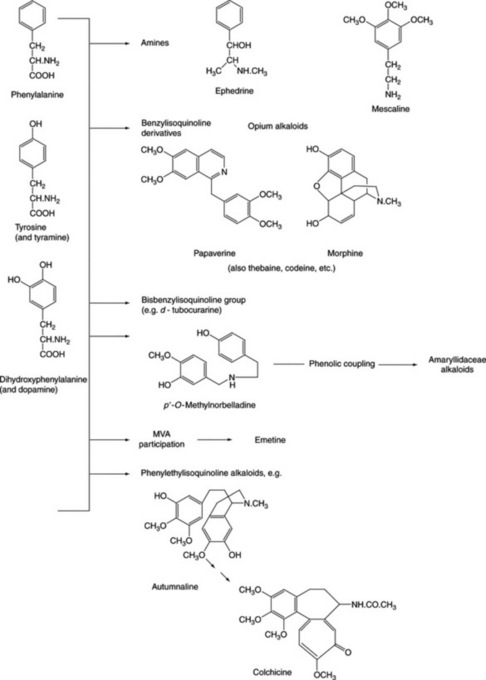
Alkaloids show great variety in their botanical and biochemical origin, in chemical structure and in pharmacological action. Consequently, many different systems of classification are possible. In the arrangement of the well-known drugs which follow later in the chapter, the phytochemical arrangement introduced in the eleventh edition of this book and based on the origin of the alkaloids in relation to the common amino acids has been used. For practical purposes it is useful, therefore, to maintain the well-established classifications based on chemical structures, Fig. 26.1, and Table 26.1 closely follows that used by a number of authors. There are two broad divisions:
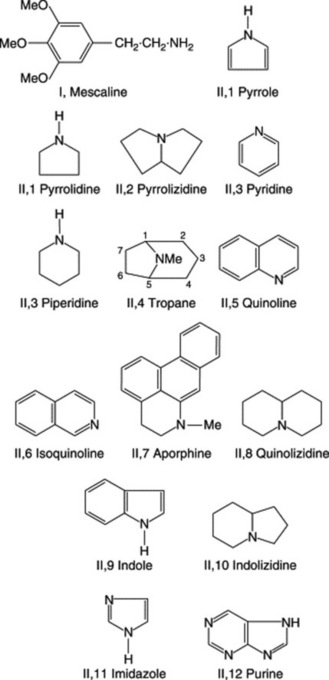
Fig. 26.1 Skeletal structures of alkaloids found in medicinal plants. (Numbers refer to location in Table 26.1).
Table 26.1 Types of alkaloid and their occurrence.
| I. Non-heterocyclic alkaloids | |
| Hordenine or N-methyltyramine | In germinating barley, Hordeum distochon |
| Mescaline, related to tryptamine (see formula) | Lophophora williamsii (Cactaceae) |
| Ephedrine | Ephedra spp. (Ephedraceae) |
| Colchicine (tropolone nucleus with nitrogen in side-chain) | Colchicum spp. and related genera (Liliaceae) |
| Erythromycin (an antibiotic) | Streptomyces erythreus (Bacteriophyta, Actinomycetales) |
| Jurubin (steroid with 3-amino group) | Solanum paniculatum (Solanaceae) |
| Pachysandrine A (steroid with N-containing C-17 side-chain) | Pachysandra terminalis (Buxaceae) |
| Taxol (a modified diterpene pseudo alkaloid) | Taxus brevifolia (Taxaceae) |
| II. Heterocyclic alkaloids | |
| 1. Pyrrole and pyrrolidine | |
| Hygrines | Coca spp. (Erythroxylaceae); often associated with tropane alkaloids of the Solanaceae |
| Stachydrine | Stachys tuberifera (Labiatae), soya bean and other Leguminosae |
| 2. Pyrrolizidine | |
| Symphitine, echimidine | Symphytum spp. |
| Senecionine, seneciphylline, etc. | Senecio spp. |
| 3. Pyridine and piperidine | |
| Trigonelline | Fenugreek (Leguminosae), strophanthus (Apocynaceae), coffee (Rubiaceae) |
| Coniine | Conium maculatum (Umbelliferae) |
| Arecoline | Areca catechu (Palmae) |
| Lobeline | Lobelia spp. (Lobeliaceae) |
| Pelletierine | Punica granatum, the pomegranate (Punicaceae) |
| Nicotine (pyridine + pyrrolidine) | Nicotiana tabacum and other spp. (Solanaceae) |
| Anabasine | Nicotiana glauca; Anabasis aphylla (Chenopodiaceae) |
| Piperine | Piper spp. (Piperaceae) |
| Ricinine | Ricinus communis (Euphorbiaceae) |
| 4. Tropane (piperidine/N-methyl-pyrrolidine) | |
| Hyoscyamine, atropine, hyoscine, meteloidine, etc. | Species of Atropa, Datura, Hyoscyamus, Duboisia, Mandragora and Scopolia (Solanaceae) |
| Calystegines | Convolvulus spp., Ipomoea polpha (Convolvulaceae), some solanaceous spp., Morus spp. (Moraceae) |
| Cocaine | Coca spp. (Erythroxylaceae) |
| Pesudo-pelletierine | Punica granatum (Punicaceae) |
| 5. Quinoline | |
| Quinine, quinidine, cinchonine, cinchonidine | Cinchona spp. (Rubiaceae), Remijia spp. (Rubiaceae) |
| Cusparine | Angostura or cusparia bark, Galipea officinalis (Rutaceae) |
| 6. Isoquinoline | |
| Papaverine, narceine, narcotine | Papaver somniferum (Papaveraceae) |
| Corydaline | Corydalis and Dicentra spp. (Fumariaceae) |
| Hydrastine, berberine | Numerous genera of the Berberidaceae, Ranunculaceae and Papaveraceae |
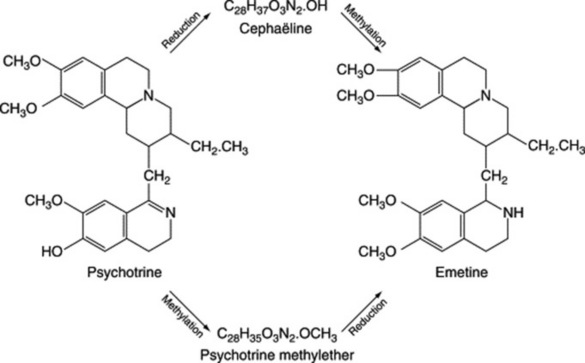
| Emetine, cephaeline | Cephaelis spp. (Rubiaceae) |
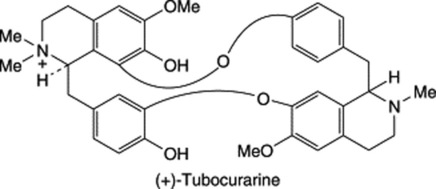
| Tubocurarine | Curare obtained from plants of Menispermaceae |
| Morphine, codeine | Papaver somniferum (Papaveraceae) |
| Erythraline | Erythrina spp. (Leguminosae) |
| Galanthamine | Leucojum aestivum (Amaryllidaceae) |
| 7. Aporphine (reduced isoquinoline/naphthalene) | |
| Boldine | Peumus boldus (Monimiaceae) |
| 8. Quinolizidine | |
| Sparteine, cytisine, lupanine, laburnine | Sometimes called ‘the lupin alkaloids’. Occur particularly in the Leguminosae, subfamily Papilionaceae, e.g. broom. Cytisus scoparius; dyer’s broom, Genista tinctoria; Laburnum and Lupinus spp. |
| 9. Indole or benzopyrrole | |
| Ergometrine, ergotamine | Claviceps spp. (Hypocreaceae) |
| Lysergic acid amide, clavine alkaloids | Rivea corymbosa, Ipomoea violacea (Convolvulaceae) |
| Physostigmine | Physostigma venenosum (Leguminosae) |
| Ajmaline, serpentine, reserpine | Rauwolfia spp. (Apocynaceae) |
| Yohimbine, aspidospermine | Aspidosperma spp. (Apocynaceae) |
| Vinblastine, vincristine | Catharanthus roseus (Apocynaceae) |
| Calabash curare alkaloids | Strychnos spp. (Loganiaceae) |
| Strychnine, brucine | Strychnos spp. (Loganiaceae) |
| 10. Indolizidine | |
| Castanospermine | Castanospermum australe (Leguminosae), Alexa spp. (Leguminosae) |
| Swainsonine | Swainsona spp. (Leguminosae), Loco plants (Leguminosae) |
| 11. Imidazole or glyoxaline | |
| Pilocarpine | Pilocarpus spp. (Rutaceae) |
| 12. Purine (pyrimidine/imidazole) | |
| Caffeine | Tea (Ternstroemiaceae), coffee (Rubiaceae), maté (Aquifoliaceae), guarana (Sapindaceae), cola nuts (Sterculiaceae) |
| Theobromine | Cocoa (Sterculiaceae) |
| 13. Steroidal (some combined as glycosides) | |
| Solanidine (glycoside = solanine) | Shoots of potato (Solanaceae), etc. |
| Veratrum alkamine esters and their glycosides | Veratrum spp. and Schoenocaulon spp. (Liliaceae) |
| Conessine | Holarrhena antidysenterica (Apocynaceae) |
| Funtumine | Funtumia elastica (Apocynaceae) |
| 14. Terpenoid | |
| Aconitine, atisine, lyctonine, etc. | Aconitum and Delphinium spp. (Ranunculaceae) |
The nitrogen of alkaloids
Alkaloids, taken in their broadest sense, may have a nitrogen atom which is primary (mescaline), secondary (ephedrine), tertiary (atropine) or quaternary (one of the N atoms of tubocurarine), and this factor affects the derivatives of the alkaloid which can be prepared and the isolation procedures. In the plant, alkaloids may exist in the free state, as salts or as amine or alkaloid N-oxides.
Alkaloid N-oxides
N-oxidation products of alkaloids, particularly the N-oxides of tertiary alkaloids, are well-known laboratory products, easily prepared from the original base. As early as the 1920s quite extensive pharmacological and toxicological comparisons had been made of common alkaloids such as morphine, strychnine and hyoscyamine and their corresponding N-oxides. Some enthusiasm for the clinical use of N-oxides was engendered by their purported delayed-release properties, low toxicities and low addictive properties compared with the corresponding tertiary alkaloids.
Although the formation of N-oxides and other N-oxidation products of alkaloids in animal systems is well-known, forming part of the wider scheme for the metabolism of amines, the occurrence of such compounds in plants has, until relatively recently, received little attention. This was possibly due to a belief that such compounds represented artefacts arising during the extraction and work-up of tertiary alkaloids. Secondly, because of the high polarity, and water-solubility of alkaloid N-oxides, they were discarded by the normal alkaloid extraction procedures.
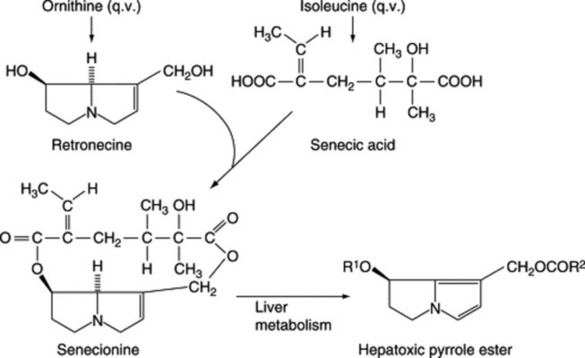
One group of alkaloids known to occur extensively as the natural N-oxides comprises the quinolizidines of the Boraginaceae, Compositae and Papilionaceae; these are alkaloids, including those of Senecio spp., which cause extensive liver damage in animals using plants containing them as fodder. A number of N-oxide alkaloids of the indole series have been isolated from plant materials, and among those of pharmaceutical significance are the simple hallucinogenic indole derivatives of Amanita spp., reserpine, strychnine, and some Mitragyna alkaloids. Fresh Atropa, Datura, Hyoscyamus, Scopolia and Mandragora each contain the two isomeric N-oxides of hyoscyamine.
One of the two possible N-oxides of hyoscine has been isolated from species of the first four genera above. Morphine and codeine N-oxides are natural constituents of the opium poppy latex, and Nicotiana spp. contain two isomeric nicotine N-oxides based on the pyrrolidine nitrogen. Some N-oxides—for example, aspergillic acid and iodinin (1,6-dihydroxyphenazine dioxide)—isolated from microorganisms, possess antibacterial activity.
As with the tertiary alkaloids themselves, there is little evidence to suggest what role the N-oxides may play in the plant’s metabolism. Ontogenetic studies of hyoscyamine N-oxide production in belladonna indicate a dynamic role for the N-oxide with a maximum build-up in the developing fruits. Oxidation–reduction involving N-oxides and tertiary bases is a probability. It has been suggested that N-oxides may be involved in demethylations and their participation in the biosynthesis of benzylisoquinoline alkaloids has also been proposed. The solubility properties of N-oxides could influence the transport of alkaloids both throughout the plant and also within the cell itself.
Tests for alkaloids
Most alkaloids are precipitated from neutral or slightly acid solution by Mayer’s reagent (potassiomercuric iodide solution), by Wagner’s reagent (solution of iodine in potassium iodide), by solution of tannic acid, by Hager’s reagent (a saturated solution of picric acid), or by Dragendorff’s reagent (solution of potassium bismuth iodide). These precipitates may be amorphous or crystalline and are of various colours: cream (Mayer’s), yellow (Hager’s), reddish-brown (Wagner’s and Dragendorff’s). Caffeine and some other alkaloids do not give these precipitates (see below). Care must be taken in the application of these alkaloidal tests, as the reagents also give precipitates with proteins. During the extraction of alkaloids from the plant and subsequent evaporation, some proteins will not be extracted and others will be made insoluble (denatured) by the evaporation process and may be filtered out. If the original extract has been concentrated to low bulk and the alkaloids extracted from an alkaline solution by means of an organic solvent, and then transferred into dilute acid (e.g. tartaric), the latter solution should be protein-free and ready to test for alkaloids.
As mentioned above, caffeine, a purine derivative, does not precipitate like most alkaloids. It is usually detected by mixing with a very small amount of potassium chlorate and a drop of hydrochloric acid, evaporating to dryness and exposing the residue to ammonia vapour. A purple colour is produced with caffeine and other purine derivatives. This is known as the murexide test. Caffeine easily sublimes and may be extracted from tea by heating the broken leaves in a crucible covered with a piece of glass. Colour tests are sometimes useful—for example, the yellow colour given by colchicine with mineral acids or the bluish-violet to red colour given by indole alkaloids when treated with sulphuric acid and p-dimethylaminobenzaldehyde. Other examples will be given under individual drugs.
For the identification of drugs containing known alkaloids, pharmacopoeias commonly employ TLC separations using reference compounds to establish the presence of individual alkaloids. In this respect, some of the alkaloid reagents quoted above are useful for detection of the separated bases.
EXTRACTION OF ALKALOIDS
Extraction methods vary with the scale and purpose of the operation, and with the raw material. For many research purposes chromatography gives both speedy and accurate results. However, if an appreciable quantity of alkaloid is required, one of the following general methods will usually serve.
Process A
The powdered material is moistened with water and mixed with lime which combines with acids, tannins and other phenolic substances and sets free the alkaloids (if they exist in the plant as salts). Extraction is then carried out with organic solvents such as ether or petroleum spirit. The concentrated organic liquid is then shaken with aqueous acid and allowed to separate. Alkaloid salts are now in the aqueous liquid, while many impurities remain behind in the organic liquid.
Process B
The powdered material is extracted with water or aqueous alcohol containing dilute acid. Pigments and other unwanted materials are removed by shaking with chloroform or other organic solvents. The free alkaloids are then precipitated by the addition of excess sodium bicarbonate or ammonia and separated by filtration or by extraction with organic solvents.
Large-scale extractions based on the above principles are sometimes done in the field and the crude mixtures of alkaloids afterwards sent to a factory for separation and purification. This has been done for both cinchona and coca alkaloids in South America and Indonesia, the crude alkaloids then being sent to Europe, USA or Japan for purification. The separation and final purification of a mixture of alkaloids may sometimes be done by fractional precipitation or by fractional crystallization of salts such as oxalates, tartrates or picrates. Chromatographic methods are particularly suitable if the mixture is a complex one and if small quantities of alkaloids will suffice. Supercritical fluid extraction (Chapter 17), although not yet applied to many alkaloids, will probably become of increasing importance for these compounds.
Volatile liquid alkaloids such as nicotine and coniine are most conveniently isolated by distillation. An aqueous extract is made alkaline with caustic soda or sodium carbonate and the alkaloid distilled off in steam. Nicotine is an important insecticide, and large quantities of it are prepared from those parts of the tobacco plant which cannot be used for tobacco manufacture.
Cell, tissue and organ culture
The production of alkaloids using cell, tissue and organ cultures has now been extensively investigated for its commercial potential, as a means of obtaining new alkaloids and for elucidating biosynthetic pathways. These aspects are considered in Chapter 13 and under individual drugs.
FUNCTIONS OF ALKALOIDS IN PLANTS
The characteristic nature of alkaloids and their often very marked pharmacological effects when administered to animals naturally led scientists to speculate on their biological role in the plants in which they occurred. In spite of many suggestions over the years, however, little convincing evidence for their function has been forthcoming. The following points are noteworthy.
Cordell GA, editor. The alkaloids. Chemistry and pharmacology, Vols 41–. London: Academic Press, 1991. This is a continuation of the original Manske and Holmes series started in 1950. Vol. 53. A Brossi as editor appeared in followed by, Vols 23–41, 2000.
Roberts MR, Wink M, editors. Alkaloids. Biochemistry, ecology and medical applications. New York: Plenum, 1998.
Southon IW, Buckingham J. Dictionary of alkaloids. London: Chapman and Hall, 1989.
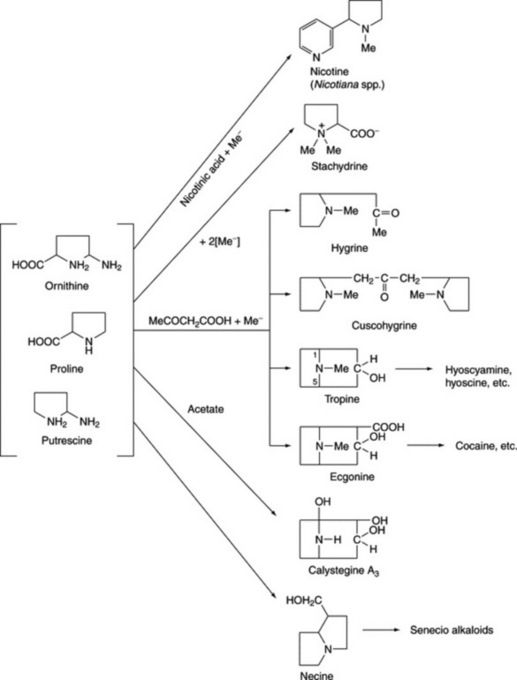
ORNITHINE-DERIVED ALKALOIDS
As indicated in Fig. 26.2, the amino acid ornithine, its decarboxylation product, putrescine, and proline constitute the basic unit of the tropane, ecgonine, nicotine (pyrrolidine ring), necine and stachydrine groups of alkaloids. Biogenetically ornithine is linked to arginine (Fig. 18.13); putrescine can also be formed from arginine without the involvement of ornithine and this has led to problems in the understanding of the stereospecific incorporation, or otherwise, of precursors into particular alkaloids, see below. Pharmaceutically, the tropane group is important.
TROPANE ALKALOIDS
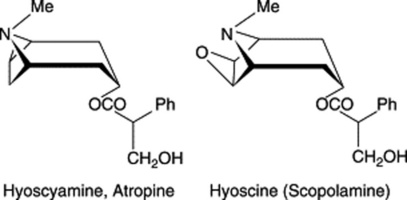
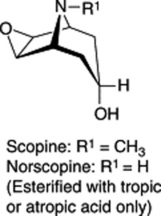
The principal alkaloids of medicinal interest in this group are (−)-hyoscyamine; its more stable racemate atropine, and hyoscine (scopolamine). The compounds are esters and are hydrolysed by heating at 60°C with baryta water; atropine yields tropic acid and tropine; hyoscine gives tropic acid and oscine (scopine is actually formed by
enzymatic hydrolysis but the chemical treatment converts it to the more stable geometric isomer, oscine).
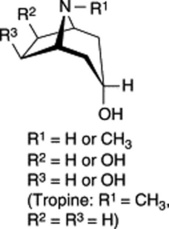
These three specific alkaloids are confined to the Solanaceae, in which some 40 different ester bases of the tropane type have now been discovered; they constitute an interesting chemotaxonomic study within the family. Examples of tropanol esters are given in Table 26.2. Dimeric and trimeric tropanol ester alkaloids involving the dicarboxylic acids mesaconic and itaconic acids are found in Schizanthus. For isolations from S. porrigens see O. Muñoz and S. Cortés, Pharm. Biol., 1998, 36, 162, and from S. hookeri see M. Jordan et al., Phytochemistry, 2006, 67, 570. Other tropane bases occur in the Erythroxylaceae (see cocaine in coca leaves), Convolvulaceae, Dioscoreaceae, Rhizophoraceae, Cruciferae and Euphorbiaceae.
Table 26.2 Examples of ester components of tropane alkaloids of the Solanaceae.
| Genera of pharmaceutical interest | Atropa, Acnistus, Scopolia, Physochlaina, Przewalskia, Hyoscyamus, Physalis, Mandragora, Datura, Solandra, Duboisia, Anthocercis |
| Tropanol components of esters |
 |
| Esterifying acids | Acetic, propionic, isobutyric, isovaleric, 2-methylbutyric, tiglic, nonanoic, tropic, atropic, 2-hydroxy- 3-phenylpropionic, 2,3-dihydroxy-2-phenylpropanoic, p-methoxyphenylacetic, anisic |
Altogether over 200 tropane alkaloids have now been recorded. Semisynthetic derivatives, e.g. hyoscine butylbromide (Buscopan), are of medicinal importance.
BIOGENESIS OF TROPANE ALKALOIDS
As the characteristic alkaloids of the group are esters of hydroxytropanes and various acids (tropic, tiglic, etc.) there are, for each alkaloid, two distinct biogenetic moieties which warrant consideration. Most studies in this connection have utilized various species of Datura because, for a number of reasons, they are one of the most convenient of the Solanaceae with which to work. However, with the advent of isolated root culture techniques the study of alkaloid formation in other genera has become more evident and Japanese workers in particular have employed species of Hyoscyamus and Duboisia with considerable success.
Tropane moiety
The available evidence suggests that the formation of the tropane ring system is generally similar for all Solanaceae studied but there are still apparent variations between species, particularly in the stereospecific incorporation of some precursors.
Early work with isotopes indicated that ornithine and acetate were precursors of the tropane nucleus; later, the incorporation of ornithine was shown to be stereospecific. Hygrine can also serve as a precursor of the tropane ring but is not now considered to lie on the principal pathway. The N-methyl group of the tropane system can be supplied by methionine and can be incorporated at a very early stage of biosynthesis, as demonstrated by the intact incorporation of N-methylornithine into hyoscine and hyoscyamine of Datura metel and D. stramonium. Early involvement of the N-methyl group was reinforced by the isolation in 1981 of naturally occurring δ-N-methylornithine from belladonna plants. Also supporting the stereospecificity of the ornithine incorporation was the work of McGaw and Woolley (Phytochemistry, 1982, 21, 2653) which showed that for D. meteloides the C-2 of hygrine was specifically incorporated into the C-3 of the tropine moiety of the isolated alkaloid. Putrescine (the symmetrical diamine formed by the decarboxylation of ornithine) and its N-methyl derivative also serve as precursors, which, taken in conjunction with the stereospecific incorporation of ornithine, makes it difficult to construct a single pathway for tropane ring formation. A scheme for the biogenesis of the tropane moiety, consistent with the above findings, is shown in Fig. 26.3.
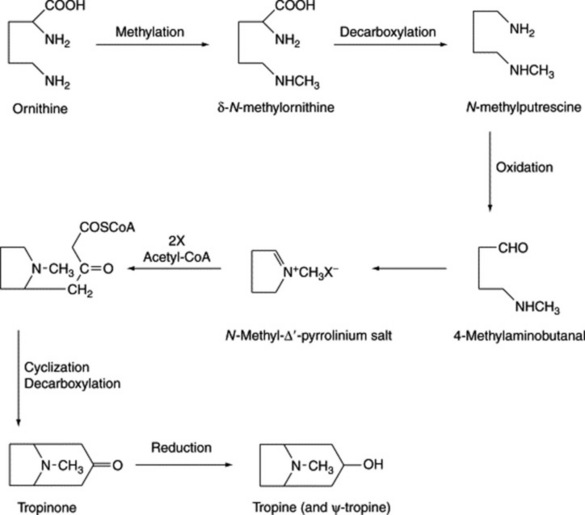
Fig. 26.3 Possible biogenetic routes for tropine and pseudotropine (see text for additional comments).
Studies on the enzyme putrescine N-methyltransferase in cultured roots of Hyoscyamus albus support the role of this enzyme as the first committed enzyme specific to the biosynthesis of tropane alkaloids. (N. Hibi et al., Plant Physiol., 1992, 100, 826.)
It will be observed from Fig. 26.3 that the reduction of tropinone yields both tropine (3α-hydroxytropane) and pseudotropine (3β-hydroxytropane). These reductions are brought about by two independent tropinone reductases (EC 1.1.1.236), often referred to as TR-I and TR-II, which accept NADPH as coenzyme. After considerable research involving principally D. stramonium root cultures both enzymes were separately purified and characterized. Furthermore, cDNA clones coding for the two separate enzymes TR-I and TR-II have been isolated and shown to involve polypeptides containing 272 and 260 amino acids respectively. These clones were expressed in Escherichia coli and the same reductive specificity demonstrated as for the natural TRs isolated from plant material.
As indicated in Table 26.2 for solanaceous alkaloids, hydroxyls and ester groups are also common at C-6 and C-7 (R2 and R3) of the tropane ring system. Current evidence suggests that hydroxylation of these carbons probably occurs after the C-3 hydroxyl has been esterified.
Esterification
The next stage in the biosynthesis of hyoscyamine, the esterification of tropine and tropic acid, has been demonstrated by feeding experiments and isolated enzymes. It was some 40 years ago that Kaçzkowski first recorded the presence of a hyoscyamine esterase in D. stramonium; later, Robins et al. (FEBS Lett., 1991, 292, 293) demonstrated the involvement of two acetyl-CoA-dependent acyltransferases in the respective formation of 3α- and 3β-acetoxytropanes in D. stramonium-transformed root cultures.
Acid moiety
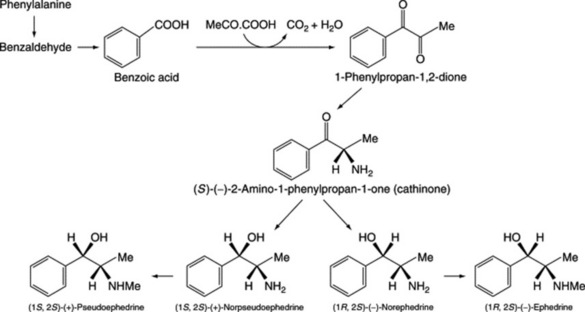
The tropic acid fragment of hyoscine and hyoscyamine is derived from phenylalanine, as is the α-hydroxy-β-phenylpropionic acid (phenyllactic acid) of the tropane alkaloid littorine. The specific incorporations obtained with phenylalanine are given in Fig. 26.4. The sequence involved in the rearrangement of the side-chain in the conversion of phenylalanine to tropic acid has been the subject of longstanding debate. Ansarin and Woolley (Phytochemistry, 1994, 35, 935), by feeding phenyl [1,313C2]lactic acid to D. stramonium and examining the 13C-NMR spectra of the subsequently isolated hyoscine and hyoscyamine, have substantiated the hypothesis that tropic acid is formed by an intramolecular rearrangement of phenyllactate. Furthermore, it has been demonstrated that hyoscyamine is biosynthesized from littorine by a process involving the intramolecular rearrangement of the phenyllactate moiety of the alkaloid. In concordance with this, transformed roots of Datura stramonium will convert exogenously added littorine to hyoscyamine (35% metabolism recorded) but, in contrast, exogenously added hyoscyamine is not metabolized to littorine.
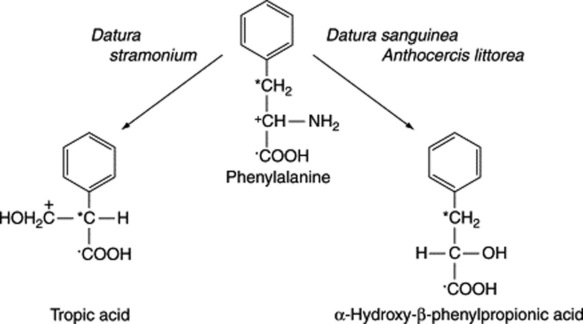
Fig. 26.4 Demonstrated incorporations of phenylalanine into the tropic acid and α-hydroxy-β-phenylpropionic acid (phenyllactic acid) moieties of hyoscyamine and littorine, respectively.
Isoleucine serves as a precursor of the tigloyl and 2-methylbutanoyl moieties of various mono- and di-esters of the hydroxytropanes.
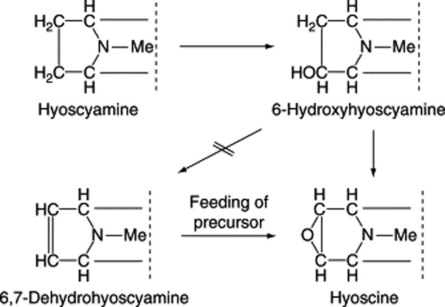
Biogenesis of hyoscine (scopolamine)
Work initiated by Romeike in 1962 showed that hyoscine appeared to be formed in the leaves of D. ferox from hyoscyamine via 6-hydroxyhyoscyamine and 6,7-dehydrohyoscyamine. The former intermediate has been well substantiated, as indicated below, and occurs in quantity in some other genera (Scopolia, Physochlaina, Przewalskia) but the latter, although incorporated into hyoscine when fed as a precursor to D. ferox, has never been isolated from normal plants. Some 25 years later Hashimoto’s group, using Hyoscyamus niger cultured roots, isolated and partially purified the enzyme responsible for the conversion of hyoscyamine to 6-hydroxyhyoscyamine. They used this enzyme to prepare [6-18O]-hydroxyhyoscyamine from hyoscyamine and showed that when the labelled compound, fed to Duboisia myoporoides, was converted to hyoscine the 18O was retained, thus eliminating 6,7-dehydrohyoscyamine from the pathway (Fig. 26.5). For this reaction to proceed the epoxidase enzyme requires 2-oxo-glutarate, ferrous ions and ascorbate as cofactors, together with molecular oxygen.
The elucidation of the above pathway, which has spanned many years, aptly illustrates the value of biotechnology and enzymology in contributing to the resolution of some uncertainties resulting from traditional labelled-precursor experiments.
Ontogenesis
In some plants of the Solanaceae (e.g. belladonna and scopolia) hyoscyamine is the dominant alkaloid throughout the life cycle of the plant. In D. stramonium hyoscyamine is the principal alkaloid at the time of flowering and after, whereas young plants contain principally hyoscine; in many other species of Datura (e.g. D. ferox) hyoscine is the principal alkaloid of the leaves at all times. The relative proportions of hyoscine and hyoscyamine in a particular species not only vary with age of the plant, but also are susceptible to other factors, including day length, light intensity, general climatic conditions, chemical sprays, hormones, debudding and chemical races. Isolated organ cultures of belladonna, stramonium and hyoscyamus indicate that the root is the principal site of alkaloid synthesis; however, secondary modifications of the alkaloids may occur in the aerial parts, for example, the epoxidation of hyoscyamine to give hyoscine, and the formation of meteloidine from the corresponding 3,6-ditigloyl ester.
Griffin WJ, Lin GD. Chemotaxonomy and geographical distribution of tropane alkaloids. Phytochemistry. 2000;53:623-637.
Lounasmaa M, Tamminen T. Cordell GA, editor. The alkaloids. Chemistry and pharmacology, Vol 44. London: Academic Press, 1993.
A review with 484 references listing all known tropane alkaloids
Robins RJ, Walton NJ. The biosynthesis of tropane alkaloids. The alkaloids. Chemistry and pharmacology, 1993.
STRAMONIUM LEAF
Stramonium Leaf BP/EP (Thornapple Leaves; Jimson or Jamestown Weed) consists of the dried leaves or dried leaves and flowering tops of Datura stramonium L. and its varieties (Solanaceae). The drug is required to contain not less than 0.25% of alkaloids calculated as hyoscyamine. The plant is widespread in both the Old and New Worlds. British supplies are derived mainly from the Continent (Germany, France, Hungary, etc.).
Plant
D. Stramonium is a bushy annual attaining a height of about 1.5 m and having a whitish root and numerous rootlets. The erect aerial stem shows dichasial branching with leaf adnation. The stem and branches are round, smooth and green. The flowers are solitary, axillary and short-stalked. They have a sweet scent. Each has a tubular, five-toothed calyx about 4.5 cm long, a white, funnel-shaped corolla about 8 cm long, five stamens and a bicarpellary ovary. The plant flowers in the summer and early autumn. The fruit is originally bilocular but as it matures a false septum arises, except near the apex, so that the mature fruit is almost completely four-celled. The ripe fruit is a thorny capsule about 3–4 cm long. Stramonium seeds (see Fig. 41.6) are dark brown or blackish in colour, reniform in outline and about 3 mm long. The testa is reticulated and finely pitted. A coiled embryo is embedded in an oily endosperm.
D. stramonium var. tatula closely resembles the above; its stems are reddish and the leaves have purplish veins, as also have the lavender-coloured corollas. Varieties of both the above forms occur with spineless capsules.
History
Stramonium was grown in England by Gerarde towards the end of the sixteenth century from seeds obtained from Constantinople. The generic name, Datura, is derived from the name of the poison, dhât, which is prepared from Indian species and was used by the Thugs.
Macroscopical characters
Fresh stramonium leaves or herbarium specimens should first be examined, since the commercial leaves are much shrunken and twisted, and their shape can only be ascertained by careful manipulation after soaking them in water.
The dried leaves are greyish-green in colour, thin, brittle, twisted and often broken. Whole leaves are 8–25 cm long and 7–15 cm wide; they are shortly petiolate, ovate or triangular-ovate in shape, are acuminate at the apex and have a sinuate-dentate margin. They are distinguished from the leaves of the Indian species, D. innoxia, D. metel and D. fastuosa, by the margin, which possesses teeth dividing the sinuses, and by the lateral veins which run into the marginal teeth.
The commercial drug contains occasional flowers and young capsules, which have been described above. The stems are often flattened, longitudinally wrinkled, somewhat hairy and vary in colour from light olive brown (D. stramonium) to purplish-brown (var. tatula). Stramonium has a slight but unpleasant odour, and a bitter taste.
Microscopical characters
A transverse section of a leaf (Fig. 26.6) shows that it has a bifacial structure. Both surfaces are covered with a smooth cuticle and possess both stomata and hairs. Cluster crystals of calcium oxalate are abundant in the mesophyll (Fig. 26.6F, G), and microsphenoidal and prismatic crystals are also found. The stomata are of the anisocytic and anomocytic types. The epidermal cells have wavy walls, particularly those of the lower epidermis. The uniseriate clothing hairs are three- to five-celled, slightly curved, and have thin, warty walls (Fig. 26.6E). The basal cell is usually more than 50 μm long (distinction from D. metel). Small glandular hairs with a one- or two-celled pedicel and others with a two-celled pedicel and an oval head of two to seven cells are also found. If portions of the leaf are cleared with chloral hydrate solution, the abundance of the cluster crystals of calcium oxalate and their distribution with regard to the veins may be noted.
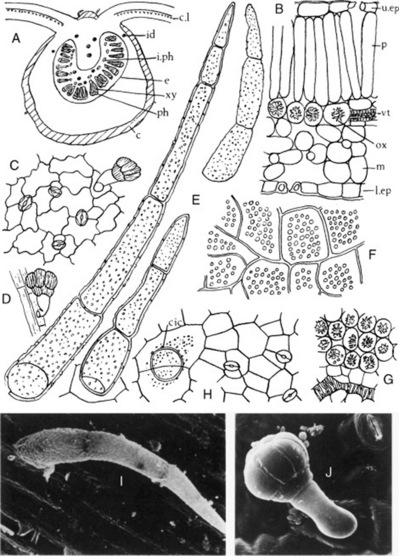
Fig. 26.6 Datura stramonium leaf. A, Transverse sections of midrib (×15); B, transverse section portion of lamina; C, lower epidermis with stomata and glandular trichome; D, glandular trichome over vein; E, clothing trichomes (all ×200); F, arrangement of calcium oxalate crystals in crystal layer, surface view (×50); G, calcium oxalate crystals in cells; H, upper epidermis showing cicatrix and stomata (G and H, ×200). I, J, Scanning electron micrographs of (I) clothing trichome and (J) glandular trichome. c, Collenchyma; cic, cicatrix; c.l, crystal layer; e, endodermis; id, idioblast containing micro-crystals; i.ph, intraxylary phloem; I.ep, lower epidermis with stoma; m, mesophyll; ox, calcium oxalate crystal; p, palisade layer; ph, phloem; u.ep, upper epidermis with stoma; vt, veinlet; xy, xylem.
(Photographs: L. Seed and R. Worsley.)
The midrib shows a bicollateral structure and characteristic subepidermal masses of collenchyma on both surfaces. The xylem forms a strongly curved arc. Sclerenchyma is absent.
Stems are present, but few of these should exceed 5 mm diameter. They possess epidermal hairs up to 800 μm long and have perimedullary phloem. The stem parenchyma contains calcium oxalate similar to that found in the leaf.
Constituents
Stramonium usually contains 0.2–0.45% of alkaloids, the chief of which are hyoscyamine and hyoscine, but a little atropine may be formed from the hyoscyamine by racemization. At the time of collection these alkaloids are usually present in the proportion of about two parts of hyoscyamine to one part of hyoscine, but in young plants hyoscine is the predominant alkaloid. The TLC test for identity given in the BP/EP enables other Datura species containing different proportions of alkaloids to be detected. The larger stems contain little alkaloid and the official drug should contain not more than 3% stem with a diameter exceeding 5 mm. Stramonium seeds contain about 0.2% of mydriatic alkaloids and about 15–30% of fixed oil. The roots contain, in addition to hyoscine and hyoscyamine, ditigloyl esters of 3,6-dihydroxytropane and 3,6,7-trihydroxytropane, respectively, and a higher proportion of alkamines than the aerial portions. For a recent report on the distribution of alkaloids in different organs and in three varieties of D. stramonium, see S. Berkov et al., Fitoterapia, 2006, 77, 179. D. stramonium cell and root cultures have been considerably utilized in biogenetic studies.
Prepared Stramonium BP/EP is the finely powdered drug adjusted to an alkaloid content of 0.23–0.27%.
Allied species
All Datura species examined to date contain those alkaloids found in stramonium, but frequently hyoscine, rather than hyoscyamine, is the principal alkaloid.
Commercial ‘datura leaf’ consists of the dried leaves and flowering tops of D. innoxia and D. metel; it is obtained principally from India. Like those of stramonium, the dried leaves are curled and twisted, but are usually somewhat browner in colour, with entire margins and with differences in venation and trichomes. The leaves contain about 0.5% of alkaloids. Variations in hyoscine and atropine contents in different organs of D. metel during development have been studied (S. Afsharypuor et al., Planta Med., 1995, 61, 383). Over 30 alkaloids have been characterized from D. innoxia by capillary GLC–mass spectrometry. For studies on the anatomy of the leaf of D. metel, see V. C. Anozie, Int. J. Crude Drug Res., 1986, 24, 206; and for the isolation of 3α-anisoyloxytropane see S. Siddiqui et al., J. Nat. Prod., 1986, 49, 511. ‘Datura seeds’ are derived from D. metel and possibly other species. Each seed is light brown in colour and ear-shaped. They are larger and more flattened than stramonium seeds but resemble the latter in internal structure. The alkaloid content, hyoscine with traces of hyoscyamine and atropine, is about 0.2%. D. ferox, a species having very large spines on its capsules, contains as its major alkaloids hyoscine and meteloidine.
The ‘tree-daturas’ constitute Section Brugmansia of the genus; these arboraceous, perennial species are indigenous to South America and are widely cultivated as ornamentals. They produce large, white or coloured trumpet-shaped flowers and pendant unarmed fruits. Some species constitute a potential source of hyoscine (W. C. Evans, Pharm. J., 1990, 244, 651) and D. sanguinea, in particular, has proved a most interesting plant with respect to its wide range of tropane alkaloids and has been cultivated commercially in Ecuador. It yields about 0.8% hyoscine. Plantations have an economically useful life of about 10 years. Chemical races of D. sanguinea are evident, particularly one producing relatively large amounts of 6β-acetoxy-3α-tigloyloxytropane. Various tree datura hybrids developed at Nottingham University, UK, have been used by a number of workers for alkaloid studies involving hairy root and root cultures; as an example see P. Nussbaumer et al., Plant Cell Rep., 1998, 17, 405.
The South American Indians have long cultivated various races of these plants for medicinal and psychotropic use (for a comparison of native assessment of their potency with alkaloid content, see Bristol et al., Lloydia, 1969, 32, 123).
Withanolides (q.v.) have also been recorded in a number of species of the genus; these include various hydroxywithanolides.
Adulteration
Adulterants cited are the leaves of species of Xanthium (Compositae), Carthamus (Compositae) and Chenopodium (Chenopodiaceae), which are, however, easily distinguished from the genuine drug.
Uses
Atropine has a stimulant action on the central nervous system and depresses the nerve endings to the secretory glands and plain muscle. Hyoscine lacks the central stimulant action of atropine; its sedative properties enable it to be used in the control of motion sickness. Hyoscine hydrobromide is employed in preoperative medication, usually with papaveretum, some 30–60 min before the induction of anaesthesia. Atropine and hyoscine are used to a large extent in ophthalmic practice to dilate the pupil of the eye.
HYOSCYAMUS LEAF
Hyoscyamus Leaf (Henbane) BP/EP 2001 consists of the dried leaves or the dried leaves and flowering tops of Hyoscyamus niger (Solanaceae). It is required to contain not less than 0.05% of total alkaloids calculated as hyoscyamine. The pharmacopoeial description refers to petiolate as well as sessile leaves, the first-year biennial leaves being thus admitted. Henbane is no longer cultivated commercially in Britain and supplies are imported from central Europe. The plant is also cultivated in the USA.
Plant
Henbane is a biennial (var. α-biennis) or annual (var. β-annua) plant. It is found wild, chiefly near old buildings, both in the UK and in the rest of Europe, and is widely cultivated. Before examining commercial henbane leaves it is advisable to study growing plants or herbarium specimens. The differences tabulated in Table 26.3 should be noted.
Table 26.3 Comparison of commercial varieties of hyoscyamus.
| First-year biennial | Second-year biennial | Annual |
|---|---|---|
| Stem very short | Stem branched and up to 1.5 m high | Stem simple and about 0.5 m high |
| Leaves in a rosette near the ground. Ovate-lanceolate and petiolate, up to 30 cm long, the lamina being up to 25 cm long. Hairy | Leaves sessile, ovate-oblong to triangular-ovate. 10–20 cm long. Margin deeply dentate or pinnatifid. Very hairy, especially in the neighbourhood of the midrib and veins | Leaves sessile. Smaller than those of the biennial plant, with a less incised margin and fewer hairs |
| Does not normally flower in the first year | Flowers May or June. Corolla yellowish with deep purple veins | Flowers July or August. Corolla paler in colour and less deeply veined |
Henbane flowers have the formula K(5), C(5), A5, G(2). The hairy, five-lobed calyx is persistent. The fruit is a small, two-celled pyxis (see Fig. 41.6B), which contains numerous seeds.
Henbane seeds are dark grey in colour, somewhat reniform in shape and about 1.5 mm long. They have a minutely reticulated testa and an internal structure closely resembling that of stramonium seeds. Henbane seeds contain about 0.06–0.10% of alkaloids (hyoscyamine with a little hyoscine and atropine) together with calystegines (nortropane alkaloids). A number of non-alkaloidal components include various lignanamides (C.-Y. Ma et al., J. Nat. Prod., 2002, 65, 206).
History
Henbane, probably the Continental H. albus, was known to Dioskurides and was used by the ancients. Henbane was used inEngland during the Middle Ages. After a period of disuse in the eighteenth century the drug was restored to the London Pharmacopoeia of 1809, largely owing to the work of Störck.
Collection and preparation
Biennial henbane was the variety traditionally grown in England, but much of the current drug is now of the annual variety or is derived from the allied species H. albus. The germination of henbane seeds is slow and often erratic and may often be assisted by special treatments (e.g. concentrated sulphuric acid, gibberellic acid or splitting of the testa). The plant may be attacked by the potato beetle, and spraying with derris or pyrethrum may be necessary.
The annual plant usually flowers in July or August and the biennial in May or June. The leaves should be dried rapidly, preferably by artificial heat at a temperature of about 40–50°C.
Macroscopical characters
Commercial henbane consists of the leaves and flowering tops described above. The leaves are more or less broken but are characterized by their greyish-green colour, very broad midrib and great hairiness. If not perfectly dry, they are clammy to the touch, owing to the secretion produced by the glandular hairs. The stems are mostly less than 5 mm diameter and are also very hairy. The flowers are compressed or broken but their yellowish corollas with purple veins are often seen in the drug. Henbane has a characteristic, heavy odour and a bitter, slightly acrid taste.
Microscopical characters
A transverse section of a henbane leaf shows a bifacial structure (Fig. 26.7A). Both surfaces have a smooth cuticle, epidermal cells with wavy walls, stomata of both anisocytic and anomocytic types, and a large number of hairs, which are particularly abundant on the midrib and veins. The hairs are up to 500 μm long; some are uniseriate and two to six cells long, while others have a uniseriate stalk and a large, ovoid, glandular head, the cuticle of which is often raised by the secretion (Fig. 26.7E). Similar hairs are found on the stems. The spongy mesophyll contains calcium oxalate, mainly in the form of single and twin prisms, but clusters and microsphenoidal crystals are also present (Fig. 26.7B,D). The broad midrib contains a vascular bundle, distinctly broader than that of stramonium, showing the usual bicollateral arrangement, which is also to be seen in the stems. The mesophyll of the midrib is made up of two thin zones of collenchyma immediately within the epidermi and a ground mass of colourless parenchyma showing large, intercellular air spaces and containing prisms or, occasionally, microsphenoidal crystals of calcium oxalate.
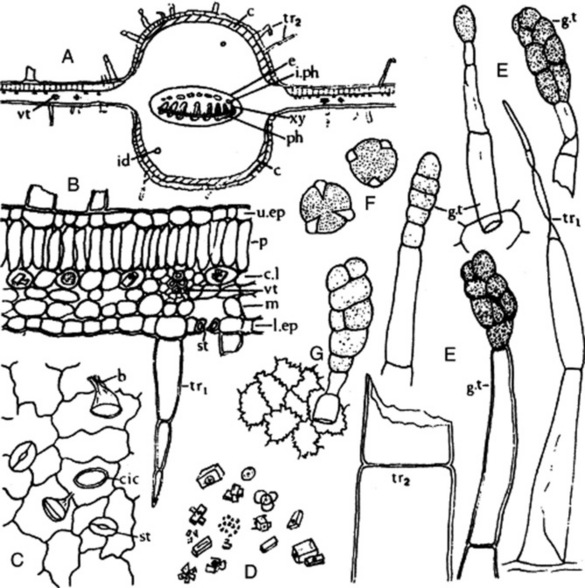
Fig. 26.7 Hyoscyamus niger. A, Transverse section of midrib of leaf (×40); B, transverse section of portion of leaf lamina; C, portion of leaf upper epidermis, surface view; D, calcium oxalate crystals; E, trichomes; F, pollen grains; G, portion of epidermis of corolla with attached glandular trichome (all ×200). b, Base of trichome; c, collenchyma; cic, cicatrix; c.I, crystal layer; e, endodermis; g.t, glandular trichome or portion of; id, idioblast; i.ph, intraxylary phloem; I.ep, lower epidermis; m, mesophyll; p, palisade layer; ph, phloem; st, stoma; tr1, tr2, whole and broken clothing trichomes, respectively; u.ep, upper epidermis; vt, veinlet; xy, xylem.
The calyx possesses trichomes and stomata, as in the leaf. The corolla is glabrous on the inner surface but exhibits trichomes on the outer surface, particularly over the veins (Fig. 26.7G). Those cells of the corolla which contain bluish anthocyanins turn red with chloral hydrate solution. Numerous pollen grains are present, about 50 μm diameter, tricolpate with three wide pores and an irregularly, finely pitted exine (Fig. 26.7F). The testa of the seeds has an epidermis with lignified and wavy anticlinal walls, and sclereids are present in the pericarp.
Constituents
Henbane leaves contain about 0.045–0.14% of alkaloids and yield about 8–12% of acid-insoluble ash (BP/EP 2001 not more than 12%). Hyoscyamine and hyoscine are the principal alkaloids. The petiole appears to contain more alkaloid than the lamina or stem.
Prepared Hyoscyamus BP/EP 2001 is the drug in fine powder adjusted to contain 0.05–0.07% of total alkaloids. It has a ‘loss on drying’ requirement of not more than 5.0%.
Allied drugs
Hyoscyamus albus is grown on the Continent, particularly in France, and in the Indian subcontinent. It has petiolate stem-leaves and the flowers have pale yellow, non-veined corollas. Unlike H. niger, the fruits are barely swollen at the base. Quantitatively and qualitatively its alkaloids appear similar to those of H. niger. It has been used in biogenetic studies (q.v.) and the hairy roots (transformed with Agrobacterium rhizogenes) have been analysed for 7β- and 6β-hydroxyhyoscyamine, littorine, hyoscine and hyoscyamine (M. Sauerwein and K. Shimomura, Phytochemistry, 1991, 30, 3277). In traditional medicine of the Tuscan archipelago the seeds are pressed into the cavities of decayed teeth to obtain pain relief (R. E. Uncini Manganelli and P. E. Tomei, J. Ethnopharmacology, 1999, 65, 181).
Hyoscyamus muticus is indigenous to India and Upper Egypt; it has been introduced into Algiers. For further details, see below.
Indian henbane. Under this name considerable quantities of drug were imported into Britain during World War II. Although H. niger is grown in India and Pakistan, much of the drug came from a closely related plant, H. reticulatus. This contains hyoscine and hyoscyamine and microscopically it is almost identical to H. niger.
Hyoscyamus aureus and H. pusillus are two species which produced hyoscine as the principal alkaloid.
Uses
Henbane resembles belladonna and stramonium in action but is somewhat weaker. The higher relative proportion of hyoscine in the alkaloid mixture makes it less likely to give rise to cerebral excitement than does belladonna. It is often used to relieve spasm of the urinary tract and with strong purgatives to prevent griping.
Hyoscyamus for Homoeopathic Preparations
This is described in the BP/EP Vol. III and consists of the whole, fresh, flowering plant of Hyoscyamus niger L. There is a limit for foreign matter (max. 5%) and a minimal loss on drying at 100–105°C for 2 h of 50%. An assay is described for the Mother Tincture.
Egyptian henbane
Egyptian henbane consists of the dried leaves and flowering tops of Hyoscyamus muticus (Solanaceae). The plant is a perennial about 30–60 cm in height. It is indigenous to desert regions in Egypt, Arabia, Iran, Baluchistan, Sind, western Punjab, and has been introduced into Algiers and is cultivated in southern California. In Egypt it is collected from wild plants by Arab shepherds.
Macroscopical characters
The drug consists of leaves, stems, flowers and fruits. The leaves are usually matted and form a lower proportion of the drug than in the case of European henbane. The leaves are pubescent, pale green to yellowish, rhomboidal or broadly elliptical and up to about 15 cm long. Midrib broad, venation pinnate, margin entire or with about five large teeth on each side. Petiole almost absent or up to 9 cm long. The stems are greyish-yellow, striated, slightly hairy and hollow. The flowers are shortly stalked, with large hairy bracts, a tubular five-toothed calyx and a yellowish-brown corolla which in the dry drug may show deep purple patches. The fruit is a cylindrical pyxidium surrounded by a persistent calyx and containing numerous yellowish-grey to brown seeds. Odour, slightly foetid; taste, bitter and acrid.
Microscopical characters
Egyptian henbane is easily distinguished from H. niger by the numerous branched and unbranched glandular trichomes, which have a one- to four-celled stalk and unicellular heads. Additional characters are the striated cuticle, the prisms of oxalate 45–110 μm, twin prisms and occasional clusters and microsphenoids.
Constituents
Ahmed and Fahmy found about 1.7% of alkaloids in the leaves, 0.5% in the stems and 2.0% in the flowers. The chief alkaloid is hyoscyamine for the isolation of which (as atropine) the plant is principally used. The alkaloidal mixture of plants grown in Afghanistan had the following composition: hyoscyamine 75%, apoatropine 15%, hyoscine 5%, with smaller quantities of noratropine and norhyoscine. A number of non-alkaloidal ketones, an acid and sitosterol have been characterized from plants raised in Lucknow, India. The formation of alkaloids in suspension cell cultures has been widely investigated with variable results; with callus cultures the addition of phenylalanine to the medium produced maximum alkaloid production (3.97%) whereas isoleucine gave the greatest growth (M. K. El-Bahr et al., Fitoterapia, 1997, 68, 423).
BELLADONNA LEAF
Belladonna Leaf BP/EP (Belladonna Herb) consists of the dried leaves and, occasionally fruit-bearing flowering tops of Atropa belladonna L. (Solanaceae); it contains not less than 0.30% of total alkaloids calculated as hyoscyamine. Traditionally the BP drug consisted of all the aerial parts (Belladonna Herb) but under the European requirements there is a limit (3%) of stem with a diameter exceeding 5 mm. The USP, which requires 0.35% alkaloid, also admits A. acuminata (see below) in the Belladonna Leaf monograph.
A. belladonna is cultivated in Europe and the USA.
Plant
The deadly nightshade, A. belladonna, is a perennial herb which attains a height of about 1.5 m. Owing to adnation, the leaves on the upper branches are in pairs, a large leaf and a smaller one.
The flowers appear about the beginning of June. They are solitary, shortly stalked, drooping and about 2.5 cm long. The corolla is campanulate, five-lobed and of a dull purplish colour. The five-lobed calyx is persistent, remaining attached to the purplish-black berry. The latter is bilocular, contains numerous seeds and is about the size of a cherry (see Fig. 41.6C). In the USA the plant is often known as the ‘Poison Black Cherry’, while the German name is ‘Tollkirschen’ (i.e. Mad Cherry). A yellow variety of the plant lacks the anthocyanin pigmentation; the leaves and stems are a yellowish-green and the flowers and berries yellow.
History
Belladonna was probably known to the ancients but it is not clearly recorded until the beginning of the sixteenth century. The leaves were introduced into the London Pharmacopoeia of 1809, but the root was not used in Britain until a liniment prepared from it was introduced by Squire in 1860.
Cultivation, collection and preparation
Belladonna is grown from seed. The leaves are said to be richest in alkaloid at the end of June or in July, and a sunny position is said to give more active leaves than a shady one. Plants about 3 years old are sufficiently large to give a good yield of leaves and, if the roots are being collected, it would seem to be best to replant about every third year (see also ‘Belladonna Root’). Two or more crops of leaves may be collected annually. Leaves left in an imperfectly dry state deteriorate and give off ammonia. They should therefore be dried immediately after collection and be carefully stored. Leaves of a good colour may be obtained by drying in thin layers starting with a moderate heat which is gradually increased to about 60°C and then gradually decreased. Sometimes the leaves are badly attacked by insects and the roots by a fungus.
Macroscopical characters
The drug consists of leaves and the smaller stems, the latter seldom exceeding 5 mm diameter, together with flowers and fruits as described above. If the drug is little broken, the arrangement of the leaves in unequal pairs may be seen. The leaves are dull green or yellowish-green in colour, the upper side being somewhat darker than the lower. Each has a petiole about 0.5–4 cm long and a broadly ovate, slightly decurrent lamina about 5–25 cm long and 2.5–12 cm wide. The margin is entire and the apex acuminate. A few flowers and fruits may be found. If the leaves are broken, the most useful diagnostic characters are the venation and roughness of the surface. The latter is due to the presence of calcium oxalate in certain of the mesophyll cells which causes minute points on the surface of the leaf as the other cells contract more on drying.
Microscopical characters
A transverse section of the leaf of A. belladonna is shown in Fig. 26.8A. It has a bifacial structure. The epidermal cells have wavy walls and a striated cuticle (Fig. 26.8E). Stomata of the characteristic anisocytic type and also some of the anomocytic type are present on both surfaces but are most common on the lower. Hairs are most numerous on young leaves. Some of the hairs are uniseriate, two- to four-celled clothing hairs; others resemble these but have a unicellular glandular head; while a third kind has a short pediceland a multicellular glandular head (Fig. 26.8F). Certain of the cells of the spongy mesophyll are filled with microsphenoidal (‘sandy’) crystals of calcium oxalate (Fig. 26.8B, C). The midrib is convex above and shows the usual bicollateral vascular bundle. A zone of collenchyma underlies both epidermi in the region of the midrib.
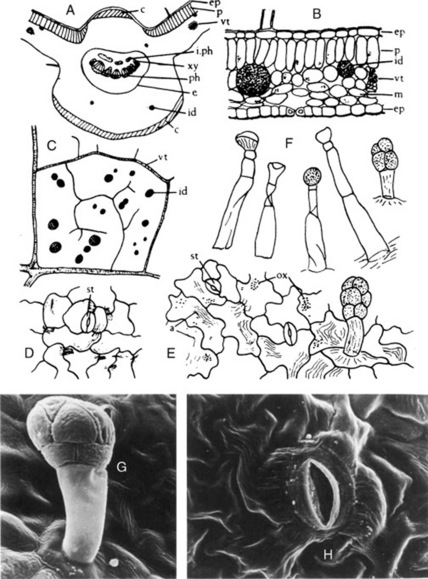
Fig. 26.8 Atropa belladonna leaf. A, Transverse section of midrib (×40); B, transverse section of portion of lamina (×200); C, distribution of idioblasts, surface view of leaf cleared in chloral (×50); D, upper epidermis; E, lower epidermis; F, trichomes (all ×200); G and H, Scanning electron micrographs (G) of glandular trichome and epidermal cells with striated cuticle and (H) stoma and striated cuticle. a, Striations of cuticle; c, collenchyma; e, endodermis; ep, epidermis; id, idioblast containing crystals of calcium oxalate; i.ph, intraxylary phloem; m, mesophyll; ox, calcium oxalate crystals; p, palisade layer; ph, phloem; st, stoma; vt, veinlet; xy, xylem.
(Photographs, L. Seed and R. Worsley.)
Constituents
The drug from A. belladonna contains 0.3–0.60% of alkaloids, the chief of which is hyoscyamine. Small quantities of volatile bases, such as pyridine and N-methylpyrroline, are present, and if not removed during the assay of the drug by heating, increase the titration and appear in the result as hyoscyamine. The leaves also contain a fluorescent substance, β-methylaesculetin (scopoletin), and calcium oxalate. They yield about 14% of ash and not more than 4% of acid-insoluble ash.
Prepared Belladonna Herb is the finely powdered drug adjusted to contain 0.28–0.32% of total alkaloids. Note the ‘loss on drying’ requirement.
Allied drugs
Indian belladonna from A. acuminata Royle ex Lindley differs from that derived from A. belladonna in that its flowers are yellowish-brown and its leaves brownish-green, oblong-elliptical and tapering towards both base and apex. It grows wild in the Himalayan regions of northern India (1800–3400 m) and is cultivated in the Kashmir valley.
Atropa baetica Willk. is a species native to southern Spain and northern Morocco; it produces yellow flowers and black berries and is regarded as an endangered species. R. Zárate et al. have described a rapid in vitro propagation method for the plant, and from hairy root cultures have isolated tigloylpseudotropine—alkaloid not found in the mature plant (Plant Cell Rep., 1999, 18, 418).
Adulterants
Of the numerous recorded adulterants of belladonna leaves, those of Phytolacca decandra (Phytolaccaceae) and Ailanthus glandulosa (Simaroubaceae) are perhaps the most important. In Phytolacca the lamina is denser and less decurrent than in belladonna; the epidermal cells have straight walls, the stomata are of the anomocytic type and some of the mesophyll cells contain bundles of needle-shaped crystals of calcium oxalate. Ailanthus leaves are triangular-ovate, have straight-walled epidermal cells showing a strongly striated cuticle, cluster crystals of calcium oxalate, and on both surfaces white, unicellular clothing hairs which are lignified (Fig. 42.3F).
Belladonna root
Belladonna root consists of the dried roots or rootstock and roots of Atropa belladonna (Solanaceae).
Collection and preparation
Much of the A. belladonna drug is of small size and poor quality. The first-year roots are not profitable to collect from the commercial point of view, although they contain a high proportion of alkaloids. The autumn of the third year would seem to be a suitable time for collection. The roots are dug up, washed, sliced and dried.
Macroscopical characters
Atropa belladonna rapidly develops a large branching root. The aerial stems die back each year and new ones arise independently from the large crown. Dried roots of 3-year-old plants are about 3 cm diameter and roots over 4 cm diameter are exceptional. Most commercial drug is about half this thickness.
The drug is usually cut into short lengths, which are sometimes split longitudinally. The outer surface is a pale greyish-brown. The root breaks with a short fracture and then shows a whitish or, if overheated during drying, brownish interior. A yellowish-green colour in the region of the cambium is often seen.
A transverse section of the bark is non-fibrous and the wood does not show a radiate appearance. The wood consists of scattered groups of vessels, tracheids and fibres which are most abundant near the cambium; there is a central mass of primary xylem (Fig. 41.8G). The extensive parenchyma of bark and wood contains sandy crystals of calcium oxalate and abundant simple and compound starch grains.
The structure gradually changes as the roots pass into rhizome, the wood becoming denser and exhibiting a distinctly radiate structure; the rhizome also shows a distinct pith and internal phloem. The aerial stems found on the upper surface of the crown are hollow.
Constituents
Atropa belladonna root contains about 0.4–0.8% of alkaloids calculated as hyoscyamine.
Samples of belladonna root examined by Kuhn and Schäfer showed 0.3–1.0% of alkaloids, of which 82.8–97.3% was hyoscyamine, 2.7–15.2% atropine, and 0.0–2.6% scopolamine. Capillary GLC–mass spectrometry data revealed the presence of hygrine, hygroline, cuscohygrine, tropinone, tropine, pseudotropine and nine tropanol esters (F. Oprach et al., Planta Med., 1986, 513). Other constituents previously reported include belladonnine together with β-methylaesculetin, calcium oxalate and starch.
A pseudotropine-forming, tropinone reductase (see biogenesis of tropane alkaloids), not entirely similar in chemical and catalytic properties to other samples of the enzyme previously described, has been isolated from transformed belladonna root cultures. The pseudotropine could have implications for the formation of calystegines (q.v). Littorine has been detected in both non-transformed and hairy root-cultures (F. Nakanishi et al., Plant Cell Rep., 1998, 18, 249).
Allied drug
Indian belladonna root from Atropa acuminata (see under ‘Belladonna Leaf’) consists of brownish-grey roots, stolons, rootstock and stem bases. It has been described in detail by Melville. The roots are cylindrical, longitudinally wrinkled, occasionally branched, and 0.5–3 cm diameter. Young roots resemble those of A. belladonna but older ones show concentric zonation of the secondary xylem. The rootstock is 3–9 cm diameter at the top and bears the bases of 4–12 aerial stems. The rootstock, stem bases and stolons all possess a pith which becomes hollow in the stem bases. The constituents are similar to those of European belladonna.
Adulterant
The root of Phytolacca decandra (Phytolaccaceae) is sometimes sliced and mixed with samples of belladonna. It bears little resemblance to belladonna root, but a casual and inexperienced observer might perhaps mistake it for pieces of an old belladonna crown. The transverse section shows a number of concentric cambia, each producing a ring of wood bundles. The parenchyma contains abundant acicular crystals of calcium oxalate.
DUBOISIA LEAVES
Three species of Duboisia are indigenous to Australia and two of these, D. myoporoides and D. leichhardtii, have for over 55 years been a major world source of tropane alkaloids, particularly hyoscine. The third species, D. hopwoodii, contains principally nicotine and related alkaloids and was used by the Australian aborigines for the preparation of ‘pituri’ by mixing powdered leaves with an alkaline wood ash to form a quid which was held in the cheek pouch.
D. myoporoides, discovered by Robert Brown, naturalist to the Flinders expedition of 1802, occurs along the east coast of Australia, where the rainfall exceeds a monthly mean of 5 cm for 11 months of the year and where frosts rarely occur. D. leichhardtii was described by Mueller in 1877 and is named after the explorer Ludwig Leichhardt, who originally collected the plant; it occurs naturally in a limited area of south-east Queensland known locally as the South West Burnett. D. hopwoodii is of wide distribution in Western and Central Australia.
Of the two tropane alkaloid-containing species D. myoporoides is the larger and more densely leaved; both, however, are bushy trees and have the advantage that in one year repeated harvests can often be taken from the same plants. For collection, the small branches are removed, tied in bundles and stood in sheds to dry; the leaves are then easily removed by beating.
In addition to hyoscine and hyoscyamine, minor alkaloids occur in variable amounts and include norhyoscyamine, 6β- hydroxyhyoscyamine, valeroidine, tigloidine, poroidine, isoporoidine, valtropine, 3α-tigloyloxytropane, 3α-acetoxytropane, 3α-nonanoyloxytropane, butropine and apohyoscine. Two discopine esters were identified in 1980 in D. leichhardtii and the greenhouse leaves have yielded calystegines B1, B2, B4, C1, and C2 (A. Kato et al., Phytochemistry, 1997, 45, 425). Other constituents include the triterpenoids ursolic acid and betulonic acid and a number of recently reported aliphatic constituents.
A number of chemical races occur, particularly in D. myoporoides, and include the well-established ‘northern’ and ‘southern’ races which differ in their relative contents of hyoscine and hyoscyamine, and a race which contains nicotine and anabasine as principal bases.
For a number of years, growers have also been cultivating a hybrid of the two species, the origin of which is doubtful, but which Griffin considers may derive from the experimental work of the CSIRO carried out in the early 1950s. Established plantations of the hybrid exhibit no morphological differences and propagation is carried out vegetatively. In a series of experiments on the hybrid, Griffin and Luanratana (J. Nat. Prod., 1980, 43, 552; 1982, 45, 270) have shown that the total alkaloid content of the leaves does not vary throughout the year but there is a decrease in hyoscine content from January to June (summer to autumn) and a gradual increase from June to September; the reverse is true for hyoscyamine. Repeated sprayings of plants with cytokinin solution (which also has a beneficial effect on plant growth), in the form of a sea-weed extract, prevented the hyoscine decline. Such treatment of plants could possibly enhance the hyoscine yield from all-year harvesting. There is evidence (Y. Kitamura et al., Phytochemistry, 1996, 42, 1331) that in the plant the tropic acid moiety of atropine may be recycled.
Addition of putrescine and spermidine to the culture medium of D. myoporoides root cultures has been shown to increase the hyoscine content.
Most of the Australian crop (some 1200 tonnes) is exported to West Germany, Switzerland and Japan for processing. Plantations have also been established in Ecuador.
Scopolia
All species of Scopolia investigated appear to contain tropane alkaloids similar to those found in belladonna (q.v.). Although little used in western Europe, these plants constitute a useful source of hyoscyamine and galenicals in regions where the plant is available locally. Scopolia carniolica is a central and eastern European species somewhat smaller than belladonna. In shape the leaves resemble those of belladonna, although they are more lanceolate and translucent. The cuticle is striated but less markedly so than in belladonna, sandy crystals are less numerous, hairs are rare or absent, and stomata are present on the lower surface only. The fruit, a pyxis, may often be found in the drug. The rhizomes (BPC 1934), which are nearly black in colour and bear numerous depressed stem scars, are used similarly to belladonna root. In addition to hyoscyamine and hyoscine, other alkaloids reported in this species are cuscohygrine, 3α-tigloyloxytropane, pseudotropine and tropine. S. caucasia, S. lurida and S. tangutica all appear to be suitable as sources of hyoscyamine; the last two also contain 6-hydroxyhyoscyamine and an alkaloid named daturamine (anisodine) which is a ‘hydroxyhyoscine’. Both these alkaloids are produced commercially in China. The dried rhizomes of S. japonica (‘Japanese Belladonna Root’) were official in the Japanese Pharmacopoeia 1961; the isolation of steroidal glycosides (scopolosides) has been reported from this species (S. Okamura et al., Chem. Pharm. Bull., 1992, 40, 2981).
Przewalskia tangutica is a related tropane alkaloid-containing plant and is used in Tibetan traditional medicine. The roots have a high content of hyoscyamine with total alkaloids amounting to 1.7–3.8%; 6β-hydroxyhyoscyamine and small amounts of hyoscine are also present.
Mandrake
The true mandrake, Mandragora officinarum, is one of several Mediterranean species. It was well known to Dioskurides (see R. T. Gunther’s English edition of The Greek Herbal of Dioscorides, 1934, Oxford, UK: OUP). B. P. Jackson, in an investigation of the botanical source of the drug, found that the species M. autumnalis is also involved. The leaves and roots were official in France (1818–1883) and in Spain. The roots occur in fusiform or two-branched pieces and their microscopical structure and distinction from belladonna root has been described by Berry and Jackson (Planta Med., 1976, 30, 281). The plant is surrounded with much folklore and superstition and even the collection of the root was formerly accompanied by special rites. The drug, like belladonna, has long been known to contain atropine and the fluorescent substance scopoletin. Recent investigations have established the presence of several other solanaceous alkaloids.
For a review of the isolated constituents of Mandragora spp., including alkaloids, volatile compounds, lipids and related compounds, coumarins and pigments (78 refs), see L. O. Hanus et al., Phytochemistry, 2005, 66, 2408.
COCA LEAF AND COCAINE
Coca leaves are derived from two cultivated shrubs of the Erythroxylaceae, namely Erythroxylum coca Lam. and E. novogranatense (Morris) Hieron. Each comprises two subspecies, as indicated below.
History
Coca leaves have been used in South America as a masticatory from very early times. They were formerly reserved for the sole use of the native chiefs and Incas. Coca was introduced into Europe about 1688 and cocaine was isolated in 1860. By employing the alkaloid in ophthalmic surgery in 1884 Carl Koller was the first to introduce it into clinical practice so heralding the era of modern anaesthetics. For reviews covering both the historical and other aspects of coca see the special issue of The Journal of Ethnopharmacology (1981), Vol. 3: Coca and Cocaine.
Cultivation and collection
These differ depending on geographical source. For Andean coca, plants are raised from seed and cultivated at an altitude of 500–2000 m. Pruning limits the height to about 2 m and traditionally three harvests are collected annually, the first from the pruned twigs, the second in June and the third in November. The leaves are artificially or sun dried and packed into bags. On the other hand, in the Amazon, plants are raised from cuttings, often in jungle clearings and interplanted between other staple crops.
Varieties and characters:
Microscopical characters
A transverse section of a coca leaf shows upper epidermis, palisade parenchyma containing prisms of calcium oxalate, spongy parenchyma and a very characteristic lower papillose epidermis with numerous stomata. The midrib is partly surrounded by an arc of pericyclic fibres, above and below which is a considerable amount of collenchyma. A surface preparation of the lower epidermis shows the papillae as well-marked circles, and numerous stomata (see Fig. 42.2J), each with four subsidiary cells, two of which have their long axes parallel to the pore.
DNA analysis
For a report on the differentiation of the cocaine-producing species and varieties of Erythroxylum using AFLP DNA analysis, see E. L. Johnson et al., Phytochemistry, 2003, 64, 187; 132 Erythroxylum samples were examined.
Constituents
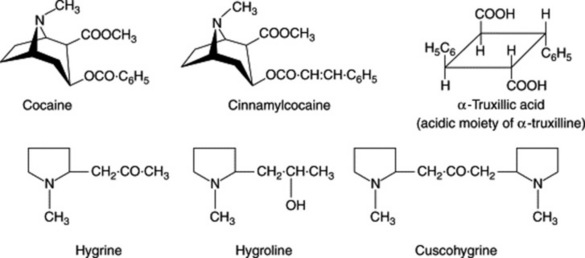
Coca leaves contain about 0.7–1.5% of total alkaloids, of which cocaine, cinnamylcocaine and α-truxilline are the most important (Fig. 26.9). They occur in different proportions in different commercial varieties. Javanese leaves are usually richest in total alkaloids, of which the chief is cinnamylcocaine, while the Bolivian and Peruvian leaves contain less total alkaloid but a higher proportion of cocaine. Other substances isolated from various varieties of the leaves are hygrine, hygroline, cuscohygrine, dihydrocuscohygrine, tropacocaine (3β-benzoyloxytropane), crystalline glycosides and cocatannic acid. 1-Hydroxytropacocaine (free hydroxyl situated at a bridgehead carbon) has been isolated as a major alkaloid of greenhouse-cultivated E. novogranatense var. novogranatense; much lower amounts were detected in var. truxillense and in field cultivated coca from Colombia and Bolivia (J. M. Moore et al., Phytochemistry, 1994, 36, 357).
The leaves also contain essential oil and as early as 1894 Van Romburgh identified methyl salicylate as a component; this was confirmed (13.6%) in a recent study, together with N-methylpyrrole (3.7%) and possibly N,N-dimethylbenzylamine (0.5%) and two dihydrobenzaldehydes (38.9%). The grassy odour of the leaves is explained to a large extent by the presence in the oil of trans-2-hexenal (10.4%) and cis-3-hexen-1-ol (16.1%); no mono- or sesquiterpenes were detected (M. Novák et al., Planta Med., 1987, 53, 113).
Although it had been generally assumed that ecgonine, the basic moiety of the cocaines, was ornithine-derived (Fig. 26.2), the practical demonstration of the incorporation of the usual precursors proved difficult. Then Leete (J. Am. Chem. Soc., 1982, 104, 1403) obtained a significant level of radioactivity in cocaine isolated from Erythroxylum coca, the leaves of which were painted with an aqueous solution of DL-[5-14C]ornithine HCl. The pathway to ecgonine appears to be similar to that for tropine except that the carboxyl is retained and the different stereospecificities need to be accommodated. The benzoyl moiety of cocaine is derived from phenylalanine. For work on the incorporation of labelled 1-methyl-Δ1-pyrrolinium chloride into cuscohygrine, indicating the alkaloid to be a mixture of its meso and optically active diastereomers, see E. Leete et al., Phytochemistry, 1988, 27, 401.
Manufacture of cocaine
The crude alkaloids may be extracted with dilute sulphuric acid or by treatment with lime and petroleum or other organic solvents. Non-alkaloidal matter is roughly separated bytransferring the alkaloids from one solvent to another. The crude alkaloids are obtained in solid form either as free bases by precipitation with alkali, or as hydrochlorides by concentrating an acidified solution.
Pure cocaine is prepared from the leaves, the crude bases or the crude hydrochlorides. The process depends on the fact that cocaine, cinnamylcocaine and α-truxilline are closely related derivatives of ecgonine (Fig. 26.2), which is produced by hydrolysing them with boiling dilute hydrochloric acid.
The ecgonine hydrochloride is purified and converted into the free base. This is benzoylated by interaction with benzoic anhydride and the benzoylecgonine purified. The benzoylecgonine is methylated with methyl iodide and sodium methoxide in methyl alcohol solution, to give methylbenzoylecgonine or cocaine. The latter is converted into the hydrochloride and purified by recrystallization.
Much illicit cocaine is extracted locally in South America and despite the unsophisticated methods employed a high degree of purity can be attained.
In view of the importance of quantitatively determining cocaine and its metabolite, benzoylecgonine, in body fluids, etc., many assays are available for these alkaloids.
Allied species
There are over 200 species of Erythroxylum found throughout the tropical and pantropical regions of the world. Few of the non-cocaine-producing species have been systematically examined but the majority of those that have contain a range of tropane alkaloids (W. C. Evans, J. Ethnopharmacol., 1981, 3, 265 and for Pt. 12 of a further series of papers see P. Christen et al., Phytochemistry, 1995, 38, 1053). Subsequently, other workers have isolated a number of the same alkaloids from other species, together with the characterization of new tropane alkaloids, some named pervilleines and others catuabines. Nortropanols (calystegins, see below) are present in some species. Trimethoxybenzoic acid and trimethoxycinnamic acid commonly occur as esterifying acids. D. Bieri et al. (J. Ethnopharmacology, 2006, 103, 439) have studied the cocaine distribution in 51 species of Erythroxylum from S. America, 28 of which had received no previous phytochemical examination; cocaine was reported for the first time in 14 species, with E. laetevirens having the highest cocaine content of the wild species. It is of interest to note that in this study, the time between collection and analysis of the samples varied from 20 to 25 years.
Calystegines
These relatively new alkaloids are trihydroxy-, tetrahydroxy- or pentahydroxy derivatives of nortropane. They were originally isolated from the roots of the bindweed Calystegia sepium and given the names calystegine A3 (a 1,2,3-trihydroxynortropane) (Fig. 26.2) and calystegine B2 (a 1,2,3,4-tetrahydroxynortropane). By 1998 the structures of nine such alkaloids had been elucidated including the 3-O-β-D-glucopyranoside of calystegine B1. In chemotaxonomic studies of the Convolvulaceae involving GC-MS analyses, T. Schimming et al. (Phytochemistry, 1998, 49, 1989; 2005, 66, 469) record the occurrence of 11 calystegines and the calystegine patterns in 135 species.
Calystegines have also been reported in the Solanaceae including belladonna and hyoscyamus root cultures. R. J. Nash et al., (Phytochemistry, 1993, 34, 1281) found these compounds to be present in the tubers and leaves of potato plants and that these alkaloids can be isolated from certain moths and butterflies, the larvae of which feed on the plant. Other sources are the Brassicaceae, Erythroxylaceae (see ‘Coca’ above) and the Moraceae.
The formation of calystegines in root cultures of Calystegium sepium involves tropinone and pseudotropine as metabolic intermediates (Y. Scholl et al., Phytochemistry, 2003, 62, 325).
Pharmaceutically, interest lies in the calystegines because they are potent inhibitors of glycosidases (R. J. Molyneux et al., Arch. Biochem. Biophysics, 1993, 304, 81) making them possible candidates for the development of antiviral, anticancer and antidiabetic drugs (cf. the trihydroxyindolizidine alkaloids castanospermine and swainsonine and the tetrahydroxypyrrolizidine alkaloid australine).
Tobacco alkaloids
The principal alkaloids of the genus Nicotiana have a pyridine moiety associated with either a pyrrolidine ring (ornithine-derived) or a piperidine ring (lysine-derived). The former group is represented by nicotine (Fig. 26.2) and the latter by anabasine (Fig 26.11).
Although, with the exception indicated below, no drugs are derived from these alkaloids, they have been extensively studied in relation to tobacco manufacture and smoking, and as insecticides (see Chapter 40). Consequently, much is known of their plant biochemistry and genetics of formation.
A pharmaceutical introduction is that of nicotine chewing-gum, nasal spray or patch, intended to help smokers who want to give up smoking but who experience great difficulty in so doing because of their nicotine dependence.
PYRROLIZIDINE ALKALOIDS
Although these alkaloids have at present no great medicinal significance they are important in that they constitute the poisonous hepatotoxic constituents of plants of the genus Senecio (Compositae), well-known for their toxicity to livestock. Some of the alkaloids also show carcinogenic and mutagenic properties and have caused concern in that they occur in small quantities in some herbal products such as comfrey (Boraginaceae) and coltsfoot (Compositae). These alkaloids are known to have an ecological role in some species of butterfly affording protection to some and converting to female flight arrestants in others. In the first demonstration of its kind, the presence of alkaloids on leaf surfaces has been indicated in eight different samples of Senecio jacoboea (K. Vrieling and S. Derridj, Phytochemistry, 2003, 64, 1223). Indicine N-oxide has antitumour properties (q.v.). Australine, recently characterized from the seeds of the leguminous tree Castanospermum australe, is a tetrahydroxypyrrolizidine alkaloid. It was obtained by use of repeated preparative centrifugal TLC. Like the polyhydroxyindolizidine alkaloids it exhibits glycosidase inhibitory activity (for further details see R. J. Molyneux et al., J. Nat. Prod., 1988, 51, 1198). The alkaloids frequently occur as esters, being linked with characteristic mono- or dibasic acids called the necic acids. They are biosynthesized from ornithine via a symmetrical intermediate and labelling experiments have shown the involvement of putrescine and homospermidine. Two molecules of putrescine are required to form one of homospermidine. This pathwayhas been supported by the isolation, partial purification and characterization of the NAD+-dependent enzyme homospermidine synthase, the first pathway-specific enzyme in pyrrolizidine alkaloid biosynthesis (F. Böttcher et al., Phytochemistry, 1993, 32, 679). The components of senecionine are illustrated in Fig. 26.10. The hepatotoxic properties are believed to arise by breakdown of the alkaloids in the liver to strongly alkylating pyrrole esters.
For reports on pyrrolizidine alkaloids see: T. Hartmann and L. Witte (1995), Alkaloids, Chemical and Biological Perspectives (ed. S. W. Pelletier), Vol. 9, New York: Wiley, p. 155. A general review: K. Ndjoko et al., Planta Med., 1999, 65, 562. Determination in Senecio species.
LYSINE-DERIVED ALKALOIDS
As the next homologue to ornithine, lysine and its associated compounds give rise to a number of alkaloids, some of which are analogous to the ornithine group (see Fig. 26.11). The lycopodium alkaloids are also derived from lysine. Although in some cases, such as the quinolizidine lupin alkaloids, lysine is incorporated via a symmetrical precursor, e.g. cadaverine, in the majority of examples (anabasine, sedamine, N-methylpelletierine) the incorporation is asymmetric. In general, for the simple α-substituted piperidines, the C-2 of lysine becomes the point of attachment of the α side-chain.
The wide distribution of these bases throughout the plant kingdom is illustrated by the drugs which follow.
LOBELIA
Lobelia BHP, BP 1988 (Lobelia Herb, Indian Tobacco) consists of the dried aerial parts of Lobelia inflata (Campanulaceae), an annual herb indigenous to the eastern USA and Canada. It is cultivated in the USA and Holland.
History
Lobelia has long been used by the North American Indians. It was recommended for use in asthma by Cutler in 1813 and was introduced to the English medical profession by Reece in 1829.
Cultivation and collection
Lobelia is grown from seed which is sown either in the autumn or in March and April. The plant produces an aerial stem about 50 cm high. It bears alternate leaves 3–8 cm long and pale blue, bilabiate flowers. The inferior ovary develops into an inflated capsule. The plants are cut in August or September, when they bear numerous capsules. After drying, the drug is exported in bales or compressed packets. The seeds are sometimes separated by thrashing.
Macroscopical characters
Up to about 60% (BP, 1988, upper limit) of the drug consists of stems. These are green or purplish, winged and very hairy in the upper part but becoming more rounded and channelled and less hairy below. The pale green leaves are usually more or less broken and are covered with bristly hair. Entire leaves are ovate to ovate-lanceolate in shape. The margin is irregularly serrate-dentate and the teeth bear water-pores. The flowers are rarely seen in the drug. The fruits are 5–8 mm long, ribbed and crowned by the calyx teeth. Each is bilocular and contains numerous oval-oblong, brown reticulated seeds about 0.5–0.7 mm long. The drug has a slightly irritating odour and an acrid taste.
Microscopical characters
The epidermis of the stem is composed of rectangular cells, covered with a striated cuticle and with anticlinal walls clearly pitted, giving a characteristic beaded appearance. The epidermis bears stomata with the pore parallel to the stem axis and large, conical, warty-walled, unicellular hairs up to 600 μm long (Fig. 42.3). The cortex is composed of rounded, thin-walled, chlorophyll-containing parenchyma except in the wings, where the cells are collenchymatous. The endodermis is well-differentiated, the cells clearly showing the Casparian strip. A pericycle composed of small groups of fibres is distinguishable in the lower part of the stem. The phloem is composed of small groups of delicate sieve-tube tissue enclosing the anastomosing latex vessels, readily seen after staining with iodine. The xylem is composed of elongated, thick-walled xylem fibres and spiral and scalariform vessels. The pith is composed of pitted lignified parenchyma.
With the leaf the upper epidermis is composed of straightwalled, papillose cells with the anticlinal walls showing the beaded appearance (Fig. 42.2). The lower epidermal cells have wavy walls, and numerous stomata, without special subsidiary cells, are present. Unicellular covering hairs, like those present on the stem, are borne on both epidermal surfaces. The mesophyll is differentiated into a single-layered palisade tissue and a spongy mesophyll. The mesophyll cells contain small fat crystals. The palisade tissue is interrupted in the midrib and groups of collenchyma occur above and below the midrib bundle. The phloem contains the characteristic latex vessels. Numerous water-pores occur on the upper surface of the marginal teeth.
The surface of the seed is characteristically reticulate. The pollen grains are roughly spherical, 20–30 μm diameter, and show three pores.
Constituents
Lobelia contains about 0.24–0.4% of alkaloids (BP 1988, not less than 0.25% as determined by a standard Stas-Otto procedure) the most important of which is lobeline. This and many related alkaloids including lobelidine, lobelanine, lobelanidine and isolobelanine, have a piperidine nucleus (Fig. 26.12). Others are piperideines (i.e. the ring system is unsaturated).
By analogy with the biosynthesis of coniine (Fig. 26.38), the above alkaloids could be formed from a polyketo acid involving two benzoyl units and acetate. However, the demonstration that phenylalanine can be incorporated by the plant into the lobelia alkaloid lobinaline and into the related sedamine (Sedum acre, Crassulaceae), and the incorporation of lysine into the piperidine ring of lobeline, does not support this hypothesis. A number of Brazilian species contain similar constituents.
Root cultures of L. inflata transformed by Agrobacterium rhizogenes have been shown to produce lobeline at the same concentration as normal pot-grown plants (H. Yonemitsu et al., Plant Cell Rep. 1990, 9, 307).
Uses
Lobelia is used in spasmodic asthma and chronic bronchitis; it is included in some antismoking preparations. In its pharmacological action, lobeline resembles nicotine in having both central and peripheral effects. In toxic doses the drug has a paralytic effect and its continuous use should be avoided.
Indian lobelia
This drug, which is official in India, consists of the dried aerial parts of L. nicotianaefolia, a biennial or perennial herb found in many parts of India at altitudes of 700–2200 m. The leaves and stems are larger than those of the American lobelia and in the form of powder the drug may be distinguished by the trichomes and palisade ratio. Indian lobelia contains not less than 0.8% alkaloids calculated as lobeline.
Pomegranate
The pomegranate, Punica granatum L. Punicaceae, is cultivated thoughout subtropical and tropical regions of the world; some 800 000 metric tons are produced annually, principally for dietary purposes. However, the barks, fruit-rind, flowers and seeds all find medicinal use.
Both stem and root barks are used and occur in curved or channelled pieces about 5–10 cm long and 1–3 cm wide. The outer surface of the stem bark shows longitudinal corky furrows, a few shallow depressions and the bark apothecia of lichens, while that of the root bark shows depressions where the outer layers have exfoliated. The barks are smooth and yellowish on their inner surfaces and break with a short granular fracture. They contain about 0.5–0.9% of volatile liquid alkaloids, the chief of which are pelletierine and pseudopelletierine, together with about 22% of tannin.
Pelletierine tannate, a mixture of the tannates of the alkaloids, was included in the BP 1948 and was used as an anthelminthic with a specific action on tapeworms.
The dried pericarp of the fruit occurs in thin, curved pieces about 1.5 mm thick, some of which bear the remains of the woody calyx or a scar left by the stalk. The outer surface is brownish-yellow or reddish and the inner surfaces bear impressions left by the seeds. A high tannin content (28%) affords its use as an astringent in the treatment of diarrhoea.
The seeds have been studied for their bioactive constituents with new compounds recently reported. Major components are monoacylglycerols, glycerides, sterols, proteins, pectins and sugars (M. Yusuph and J. Mann, Phytochemistry, 1997, 44, 1391; R.-F. Wang et al., J. Nat. Prod., 2004, 67, 2096).
The validity of a seed extract for use in the treatment of diarrhoea, as practised in traditional Indian and Bangladesh medicine, has been experimentally verified (A. K. Das et al., J. Ethnopharm., 1999, 68, 205).
The flowers are used in Chinese medicine; a new polyphenol and six known compounds including maslinic acid (see ‘Olive Leaf’) have been reported (R. Wang et al., Fitoterapia, 2006, 77, 534).
For a study on the antioxidant, antimalarial and antimicrobial tannin-rich fractions, ellagitannins and phenolic acids of pomegranate, see M. K. Reddy et al., Planta Medica, 2007, 73, 461.
N. P. Seeram et al. (see ‘Further reading’) have listed 122 compounds isolated from various organs of the pomegranate; they are classed as ellagitannins and gallotannins, ellagic acid derivatives, catechin and procyanidins, anthocyanins and anthocyanidins, flavonols, organic acids, simple galloyl derivatives, fatty acids and triglycerides, sterols and terpenoids, alkaloids and other compounds.
For studies on the biosynthesis of N-methylpelletierine involving the feeding of 13C-labelled acetoacetate and acetate see Hemscheidt and Spenser (J. Am. Chem. Soc., 1990, 112, 6360). Their results constitute evidence in support of the classical biogenetic concepts regarding the incorporation of acetate into the alkaloid (compare findings for the incorporation of acetate into the tropane skeleton).
The role of pomegranate juices and extracts as nutraceuticals is discussed in Chapter 32: The plant nutraceuticals.
Broom
The broom, Cytisus scoparius (Leguminosae), is a perennial shrub about 1–2 m high. The lower part is woody but the long, straight branches are green and glabrous. The upper parts of the stem bear five prominent, longitudinal ridges. The lower leaves are stalked and consist of three obovate leaflets, but the upper leaves are sessile and usually reduced to a single leaflet.
The flowers are typical of the subfamily Papilionaceae. The fruit is a black, hairy pod about 3–5 cm long.
Broom tops are described in the BHP 1988 and BPC 1949. An aqueous decoction or other extract was used as a mild diuretic. Their chief constituents are quinolizidine alkaloids, including the volatile liquid alkaloid sparteine (Fig. 26.12), a yellow isoflavone scoparin, and flavonoids. The drug has diuretic and cathartic actions but is now little used.
Pepper
Black pepper (Piper nigrum) consists of the dried, unripe fruits of Piper nigrum (Piperaceae), a perennial climbing plant cultivated in the Malay Archipelago, southern India, South America and the West Indies. Large quantities are obtained from Indonesia, Sarawak and Brazil. The structure of the pepper market is extremely complex and highly speculative, with dealers often selling one shipment of pepper several times over on behalf of various principals.
Pepper was known to Theophrastus and other ancient writers. It was the most important spice used in the Middle Ages and was imported into England about ad 1000. The high cost of pepper and other Eastern spices was a big inducement to the Portuguese to find a sea route to India; competition for the spice trade has played a large part in the colonial expansion of European nations.
Pepper is essentially a crop of the wet tropics and is propagated from cuttings (Sarawak) or runners (India). However, it is subject to various diseases which are transmitted by vegetative propagation so that shoot-culture techniques designed for the mass culture of selected disease-free vines have been investigated (V. J. Philip et al., Plant Cell Reps., 1992, 12, 41).
Production
The vines are grown on poles or trees. The inflorescence is a spike of about 20–30 sessile flowers, which develop into sessile fruits (see earlier editions for diagrams). The latter are picked when the lower fruits of the spike turn red. They are then removed from the axis and dried, either in the open air or by artificial heat. The fire-dried spice is most esteemed but the ground spice is usually a blend of different varieties.
White pepper, which is largely used in the East, is also obtained from P. nigrum, but the fruits are allowed to become more completely ripe. After storing them for some days or soaking them in water, the outer part of the pericarp is removed by rubbing and washing and the fruits are dried.
Black pepper fruits are almost globular and 3.5–6 mm diameter. The surface is dark brown or greyish-black and strongly reticulated. The apex shows the remains of the sessile stigmas and a basal scar indicates the point of attachment to the axis. Pepper has an aromatic odour and pungent taste.
Bacterial and fungal contamination of the stored peppers can be reduced by washing and redrying with subsequent maintenance of the moisture content at less than 11%.
In white pepper, owing to the removal of the outer part of the pericarp, the vascular bundles, about 16 in number, run on the outside of the fruit from base to apex.
Constituents
Pepper contains 1–2.5% of volatile oil, 5–9% of the crystalline alkaloids piperine and piperettine, and a resin. The aroma of the spice is due to the volatile oil, which consists largely of terpenes, while the pungency is ascribed to piperine and the resin. Piperine, first isolated in 1819, is also found in the long pepper (1–2%) and in Ashanti pepper, the fruits of Piper guineense. Analyses for the volatile oil are given as β-caryophyllene (21.59–27.70%), limonene (21.06–22.17%), sabinene (8.5–17.6%), β-pinene (9.16–11.08%), α-pinene (5.07–6.18%), myrcene (2.2–2.3%), p-cymeme (0.0–0.18%) and oxygenated constituents (3.39–5.68%); 40 compounds were identified in oil from Sao Tome e Principe (A. P. Martíns et al., Phytochemistry, 1998, 49, 2019).
Pepper was once employed in the treatment of gonorrhoea and chronic bronchitis. Large quantities are used as a condiment.
Long pepper is the dried unripe fruit of Piper retrofractum (P. officinarum) and P. longum, grown in Indonesia, India and the Philippines. The spice consists of whole spikes of small fruits forming a structure about 4 cm long and 6 mm in diameter. Individual fruits show a similar structure to black pepper. For a report on the component alkamides and other constituents see B. Das et al., Planta Med., 1996, 62, 582. Guineesine, a recently isolated alkaloid, is an acyl-CoA:cholesterol acyltransferase inhibitor and has been proposed as an attractive target for the prevention and treatment of hypercholesterolemia and atherosclerosis (S. W. Lee et al., Planta Medica, 2004, 70, 678).
Cubebs or tailed pepper are the dried, full-grown fruits of Piper cubeba, a native of Indonesia, Borneo and Sumatra. The fruits are collected while green and dried in the sun. They were used in Europe as a spice as early as the eleventh century. The spikes of cubebs bear more fruits than those of pepper and become falsely stalked as they mature, owing to an abnormal development of the base of the pericarp. The upper part of the cubeb fruit is globular, 3–6 mm diameter and covered with a greyish-brown, reticulated pericarp, which is prolonged at the base into a straight stalk. Cubebs yield 10–18% of volatile oil containing terpenes and sesquiterpenes, a crystalline inodorous substance (−)-cubebin (formula Table 21.7) and a number of other lignans, a white amorphous substance cubebic acid (1%), and amorphous resin (3%).
The above gives only a token appreciation of the genus as a whole—there are some 700 species of Piper globally of which only about 12% have been studied phytochemically. Nevertheless the literature is extensive and in a review (341 refs) covering the secondary metabolites (V. S. Parmar et al., see ‘Further reading’) nearly 600 constituents are listed. Classes of metabolites considered are alkaloids/amides, propenylphenols, lignans, neolignans, terpenes, steroids, kawa pyrones, piperolides, chalcones, dihydrochalcones, flavones, flavanones and miscellaneous compounds.
Lycopodium
Lycopodium consists of the spores of the clubmoss, Lycopodium clavatum (Lycopodiaceae, Phylum Pteridophyta). Most of the commercial drug is collected in Poland and E. Europe, but Indian and Pakistani supplies are obtained from the Himalayas. The sporangial spikes are cut and dried, and the spores are separated by shaking and then freed from vegetable debris by sieving through four sieves. The drug is exported in sacks, which are usually enclosed in matting.
Lycopodium is a light, yellow, extremely mobile powder without odour or taste. It floats on water without being wetted. The spores are 25–40 μm diameter and have the shape of a three-sided pyramid with a convex base. The surface is covered with polygonal-shaped reticulations which form a projecting ridge at the edge of the spore. Viewed from the apex of the pyramid, the edges of the flat sides form a distinct, triradiate marking. On crushing, yellowish drops of oil exude.
Lycopodium consists about 50% of fixed oil, which consists mainly of the glycerides of lycopodiumoleic acid. The drug also contains about 3% of sugars, phytosterin and alkaloids of the annotine type, which are characteristic of the genus, together with traces of nicotine. The lycopodium alkaloid lycopodine, first reported in 1881, is, like pelletierine, derived from lysine and acetate (for the role of the latter in its biosynthesis in the intact plant see T. Hemscheidt and I. D. Spenser, J. Amer. Chem. Soc., 1993, 115, 2052). For a review of the lycopodium alkaloids see Ayer (Nat. Prod. Rep., 1991, 8, 455).
Adulteration with the pollen of Pinus species, Corylus avellana, Typha latifolia, etc., or with roasted and coloured starches, dextrin, sulphur or inorganic salts, can readily be detected by means of the microscope.
Lycopodium was once used to a limited extent in dusting powders and medicated snuffs, and as a dusting powder for pills. It is employed in quantitative microscopy (see Chapter 43).
PHENYLALANINE-, TYROSINE- AND DIHYDROXYPHENYLALANINE-DERIVED ALKALOIDS
The title compounds and their corresponding decarboxylation products are the precursors of a large number of alkaloids which include simple protoalkaloids, the benzylisoquinolines, phthalideisoquinolines, aporphines and proaporphines, protoberberines, protopines, naphthaphenanthridines, the Erythrina, Amaryllidaceae and ipecacuanha alkaloids and the morphine and rhoeadine-type alkaloids. A group of compounds, first recognized in 1965, is that comprising the phenethylisoquinoline alkaloids; it is from these that colchicine arises. Some of the pharmaceutically more important groups are illustrated in Fig. 26.13.
In addition to the reading matter quoted at the end of the first section of this chapter, see also the following reviews: Isoquinoline alkaloids, H. Guinaudeau and J. Brunetonin Methods in Plant Biochemistry (ed. P. G. Waterman) 1993, Vol 8. London: Academic, p. 373; bisbenzylisoquinoline alkaloids, P. L. Schiff, J. Nat. Prod., 1983, 46, 1; 1987, 50, 529; 1991, 54, 645; dimeric aporphinoid alkaloids, H. Guinaudeau et al., J. Nat. Prod., 1988, 51, 1025; 1994, 57, 1025.
PROTOALKALOIDS
Those alkaloid-like amines which do not have the nitrogen as part of a heterocyclic ring system are often termed protoalkaloids. They are not restricted to any particular class of alkaloids and are often classified according to the amino acids from which they are derived. Some are quaternary bases as, for example, the protoberberine alkaloids which constitute a large group with diverse structures and a wide distributionin nature. Although not prominent in common herbal drugs the alkaloids are being studied for their antibacterial, antimalarial (Chapter 28) and potential genotoxic properties. For reviews with many references see E. V. L. Da-Cunha et al., The Alkaloids, 2005, 62, 1–75; L. Grycova et al., Phytochemistry, 2007, 68, 150.
Ephedra
Various species of Ephedra (Ma-huang) (Ephedraceae) are used as a source of the alkaloids ephedrine and pseudoephedrine, which may also be prepared by synthesis. Among these are the Chinese species Ephedra sinica and E. equisetina and the Indian and Pakistani E. gerardiana, E. intermedia and E. major.
Although ma-huang was known to the Chinese over 5000 years ago and ephedrine was isolated in 1887, it only came into extensive use during the last century.
Collection
The drug is collected in the autumn, this being important, as the amount of alkaloid present shows considerable variation at different seasons. The drug is imported in bales. It consists of slender, more or less broken aerial stems which are woody and usually branch only at the base.
Characters
The stems of the ephedras bear numerous, fine, longitudinal ridges. The leaves are small, connate at the base, and usually in whorls of two (less commonly, whorls of three or four) and decussate.
Constituents
The ephedras contain about 0.5–2.0% of alkaloids. Of the total, ephedrine (and its isomers) forms from 30 to 90%, according to the species. Pseudoephedrine is also present (see Fig. 26.14). The roots also contain a number of macrocyclic alkaloids (ephedradines) and feruloylhistamine which have hypotensive properties.
Biosynthesis of ephedrine and related alkaloids
These alkaloids are formed by a union of a C6–C1 unit and a C2 unit. For many years it has been known that phenylalanine is the originator of the C6–C1 moiety, being converted first to benzaldehyde or benzoic acid. GrueSørensen and Spenser (J. Am. Chem. Soc., 1993, 115, 2052 and references quoted therein) using 13C- and 2H-labelled precursors in feeding experiments with E. gerardiana have shown that benzoic acid combines with the intact CH3CO group of pyruvic acid to form ephedrine and related alkaloids with 1-phenylpropan-1,2-dione and (S)-(−)-2-amino-1-phenylpropan-1-one (cathinone) serving as intermediates. The route is illustrated in Fig. 26.14. 1-Phenylpropan-1,2-dione and cathinone are known constituents of Catha edulis (see below) but the previous non-isolation of cathinone from ephedra is attributed to its high efficiency of incorporation into the nor-alkaloids.
Uses
Ephedrine is used for the relief of asthma and hay fever. Its action is more prolonged than that of adrenaline, and it has the further advantage that it need not be given by injection but may be administered by mouth. In oriental medicine the ephedras are also used as anti-inflammatory drugs and this action is ascribed to an oxazolidone related to ephedrine. In contrast to the herb, which has a sudorific action, the root has been used clinically in China for its antisudorific effect.
Khat or Abyssinian tea
This consists of the fresh leaves of Catha edulis Forsk. (Celastraceae). The plant is cultivated in Abyssinia, in parts of east and southern Africa,and in southern Arabia. There appear to be a number of different varieties of the plant varying in ‘khatamine’ content between 0.1% (Yemen, Madagascar) and 0.5% (Kenya). It is widely employed in African and Arab countries, particularly in the Yemen, for chewing, and its misuse has been surveyed by the WHO. Its traditional use is similar to that of coca in that the fresh leaves, when chewed, have a stimulatory effect with the alleviation of depression and of the sensations of hunger and fatigue. The drug constitutes a problem in the West.
On a dry weight basis the leaves contain about 1.0% of (+)-norpseudoephedrine, and for many years this was thought to be the principle responsible for the stimulant effect of the drug. In 1975 another phenylpropane, (−)-α-aminopropiophenone (cathinone), was isolated at UN laboratories and is considered the principal CNS stimulant of the fresh plant. (−)-Cathinone has pharmacological properties analogous to those of (+)-amphetamine, possessing a similar potency and the same mechanism of action. Many other components (alkaloids, sesquiterpenes, triterpenes, flavonoids, numerous acids as esters), including an essential oil containing about 40 components, have been characterized. Tissue cultures of the plant have been shown to produce quinone-methide triterpenes; these compounds do not appear to be present in the normal plant although they have been reported in several other members of the Celestraceae. Crombie and Whiting have reviewed (64 refs) the alkaloids of khat (Alkaloids, 1990, 39, 139).
BENZYLISOQUINOLINE DERIVATIVES
A number of important drugs come within this heading. Phytochemically the group is often subdivided.
Opium poppy
The opium poppy, Papaver somniferum L., is an annual herb about 50–150 cm in height. The stem and leaves are glaucous. The latter are about 10 cm in length, entire, sessile and amplexicaul. The margin is dentate but varies somewhat in the different varieties. The flowers, which are borne on a slightly hairy peduncle, are solitary, nodding in the bud, and have caducous sepals. They have the floral formula K2, C2 + 2, A∞, G(∞). The unilocular ovary contains numerous ovules attached to parietal placentas. It bears at its apex a flat disc formed by the union of the radiating stigmas. The capsule opens by means of small valves, which are equal in number to the carpels and situated immediately below the stellate stigma.
In addition to numerous garden hybrids, the following varieties are recognized:
P. somniferum var. glabrum Boiss., cultivated in Turkey; flowers purplish but sometimes white; capsule subglobular; stigmata, 10–12; seeds, white to dark violet.
P. somniferum var. album D.C., cultivated in India; flowers and seeds white; capsules more or less egg-shaped, 4–8 cm diameter, no pores under the stigma.
P. somniferum var. nigrum D.C., cultivated in Europe for the seeds, which are slate-coloured and are known as ‘maw seeds’ (probably a corruption of Mohnsamen). The leaves and calyx are glabrous, the flowers violet and the capsules somewhat smaller and more globular than those of the var. album.
P. somniferum var. setigerum D.C., a truly wild form found in southern Europe. The peduncles and leaves are covered with bristly hairs. The leaf lobes are sharply pointed and each terminates in a bristle.
Poppy capsules contain, when ripe, 0.18–0.28% of morphine. Poppy seeds contain only very small quantities of narcotine, papaverine and thebaine in addition to morphine and codeine, all detectable by GC/MS. It has been pointed out that the detection of the former three bases in urine samples may be used to differentiate between poppy seed consumption and the illegal use of morphine or heroin (B. D. Paul et al., Planta Med., 1996, 62, 544). Importantly the seeds also contain 50–60% of a drying oil which is used by artists and also for cooking.
Papaver breeding has received some attention; a morphine-rich strain suitable for mechanical harvesting and a low-morphine variety for seed production have been described. Other strains with little alkaloid, and ones with a higher proportion of codeine, have also been produced.
The red or corn poppy, Papaver rhoeas, was formerly used in pharmacy. The fresh scarlet petals were particularly used as a colouring matter in the form of a syrup and are described in more detail in Chapter 33. They contain the anthocyanidin glucoside mecocyanin, an isomer of the cyanin found in red rose petals. A number of alkaloids are produced (e.g. rhoeadine of the benzyltetrahydroisoquinoline type); they have no morphine-like activity.
OPIUM
Opium (Raw Opium) is the latex obtained by incision from the unripe capsules of Papaver somniferum (Papaveraceae) and dried partly by spontaneous evaporation and partly by artificial heat. It is worked into irregularly shaped masses and is known in commerce as Indian opium. Indian opium is specifically stated because this is a legally available source of the drug. However, a number of countries e.g. Turkey, former USSR and Yugoslavia and Australia (Tasmania) grow considerable quantities of the opium poppy for alkaloid extraction and seed production. For strategic purposes, a relatively small crop is raised in southern England. Much illegal opium is produced in S.E. Asia.
The BP/EP monograph for Raw Opium states that it is intended only as a starting material for the manufacture of galenical preparations (e.g. Tincture of Opium) and is not dispensed as such. It should contain not less than 10% of morphine and not less than 2.0% of codeine. The thebaine content is limited to 3%. The alkaloidal assays are performed by liquid chromatography on the drug dried at 100–105°C.
Prepared Opium BP/EP is raw opium powdered and dried at a temperature not exceeding 70°C. It contains 9.8–10.2% morphine and a minimum 1.0% of codeine; thebaine is limited to 3.0%. The powder may be adjusted to strength by the addition of raw opium or a suitable excipient, which must be recorded on the label.
History
Opium was well known to the ancients. Dioskurides, about AD 77, distinguishes between the latex of the capsules, opos, and an extract of the whole plant, mekonion. The use of opium spread from Asia Minor to Persia, where opium eating became popular, and from there to India and China. However, it was not until the second half of the eighteenth century that opium smoking began to be extensively practised in China and the Far East.
Asia Minor has from very early times been an important source of opium production. In Macedonia cultivation was started as recently as 1865. Persian opium was imported into England from about 1870 to 1955. Opium was cultivated in India during the Middle Ages, and the monopoly of the Mogul Government was taken over first by the East India Company and then by the British Government. Formerly, Indian opiums, being prepared mainly for smoking, were little esteemed for pharmaceutical purposes. However, that now imported is of good quality and constitutes the principal British source for the manufacture of alkaloids.
Production
The plant cultivated in India under licence is P. somniferum var. album. Sowing takes place in November and collection from April to June. The incisions are made in the afternoon with an instrument known as a ‘nushtur’. This bears narrow iron spikes which are drawn down the capsule to produce several longitudinal cuts. The incision must not penetrate into the interior of the capsule or latex will be lost. The latex, which is at first white, rapidly coagulates and turns brown. Early in the morning of the day following the making of the incisions the partly dried latex is scraped off with a trowel-like ‘seetooar’. Each capsule is cut several times at intervals of 2 or 3 days. After collection the latex is placed in a tilted vessel so that the dark fluid (pussewah) which is not required may drain off. By exposure to air the opium acquires a suitable consistency for packing.
Indian opium is exported in 5 kg blocks, packed 12 to a lightweight wooden case to facilitate air transport. Each block is wrapped in grease-proof paper, tied with tape and placed in a polythene bag. The drug has a soft consistency and so arrives as rounded, somewhat flattened, cakes. It contains about 9–12% of morphine. Being difficult to dry and powder because of its plastic nature, Indian opium is less suitable than some other types for the preparation of powdered opium.
Former varieties of legal opium were obtained from Turkey (Turkish Government Monoply Opium) and the former Yugoslavia, and opium is also produced in former Kirghiz SSR and China under government control for national use. Iran and Egypt, former producers, no longer cultivate the plant. Such opiums were very characteristic in appearance and packaging, and illustrations of some different varieties together with tools for production will be found in the 10th edition (1972) of this book. Turkish government opium was until relatively recently much used and consisted of cubical stamped blocks each weighing 2 kg; it was much easier to powder than the Indian drug.
In addition to the above countries, opium has been produced, often only on an experimental scale, in most European countries, the USA, the East Indies and parts of Africa. Experiments have shown that a hot climate is not essential—opium of excellent quality has been produced in Scotland and Norway.
Microscopy
Opium examined under the microscope shows agglomerated latex granules in irregular masses. Other smaller amounts of characteristic material which arise as a result of the preparation process are best seen by examining the residue left after water-extraction of the opium. These particles include occasional spherical pollen grains, fragments of vessels and portions of the epicarp of the capsule the latter showing in surface view polygonal thick-walled cells with a stellate lumen. Pointed trichomes and a few starch grains may be present.
Constituents
Opium contains some 30 alkaloids, which are largely combined with the organic acid meconic acid; the drug also contains sugars, salts (e.g. sulphates), albuminous substances, colouring matters and water. Six principal alkaloids are listed in Table 26.4.
The first group (e.g. morphine) consists of alkaloids which have a phenanthrene nucleus whereas those of the papaverine group have a benzylisoquinoline structure. Some of the less important opium alkaloids (e.g. protopine and hydrocotarnine) are of different structuraltypes. The morphine molecule has both a phenolic and an alcoholic hydroxyl group, and when acetylated forms diacetyl morphine or heroin. Codeine is an ether of morphine (methylmorphine), and other morphine ethers which are used medicinally are ethylmorphine and pholcodine.
New alkaloids continue to be isolated from P. somniferum—a number were recognized during investigations on the biogenesis of morphine and Repasi et al. (Planta Med., 1993, 59, 477) obtained 5′-O-demethylnarcotine during the purification of narcotine from poppy straw; it was also detected in a sample of Indian opium.
Meconic acid, a dibasic acid, is easily detected either in the free state or as a meconate by the formation of a deep red colour on the addition of a solution of ferric chloride. As it is invariably found in opium, its presence has long been used to indicate opium. However, research has shown that some species of Papaver which produce no morphine but other morphinanes may also contain this acid; it may serve as a chemotaxonomic marker for the Papaveraceae. It is notable that the related acid, chelidonic acid, is found in some other members of the Papaveraceae such as in the root of Greater Celandine (q.v).
Papaveretum BP
PapaveretumBP is a mixture of the hydrochlorides of opium alkaloids containing 80.0–88.4% anhydrous morphine HCl, 8.3–9.2% papaverine HCl and 6.6–7.4% codeine HCl. It is assayed by liquid chromatography. Well-known preparations of papaveretum are the trade products Omnopon and Nepenthe which are used mainly for premedication and as analgesics during and after operations. Formerly, Papaveretum (BPC, 1973) also contained the opium alkaloid noscapine but this has now been removed from the preparation on account of its genotoxicity. Noscapine has also been a constituent of many cough mixtures and these have now been withdrawn by the manufacturers.
BIOGENESIS OF THE OPIUM AND RELATED ALKALOIDS
The step-by-step elucidation of the biogenetic pathway of the opium alkaloids constitutes a brilliant chapter in the history of phytochemical research. The principal features of the biosynthesis of thebaine, codeine and morphine as now envisaged are given in Fig. 26.15; a further consideration of the initial stages of this scheme follows later.
In 1910 Winterstein and Trier suggested that there was a structural relationship between the benzylisoquinoline alkaloids and dihydroxyphenylalanine. This was extended by Robinson’s observation that morphine could be derived from these alkaloids by rotation of thetetrahydroisoquinoline residue followed by oxidative ring closure. The validity of such schemes remained untested until the advent of radiochemical techniques, when in 1958–60 experiments with labelled tyrosine, administered to poppy capsules, demonstrated that two molecules of precursor were incorporated into the morphine molecule, in full accord with Robinson’s theory. Further, the intermediate stage was confirmed by the demonstration that norlaudanosoline acted as a more efficient precursor for morphine than did tyrosine and yielded a product labelled as required by the theory. By the cultivation of poppy plants in 14CO2, and by injection of labelled alkaloids into the plant, it was shown that the first major alkaloid formed is thebaine; this is irreversibly converted to codeine and then to morphine.
Many details of the above outline pathway have now been filled in. Theory required that in the oxidative coupling of norlaudanosoline, the hydroxyl groups not involved in the reaction be protected. A base of the type required, in which two of the hydroxyls were methylated, had previously been isolated from another plant (Annona reticulata, Annonaceae, order Magnoliales); this was reticuline. Labelled reticuline and norreticuline both proved to be very efficient precursors of morphine in the poppy, surpassing norlaudanosoline in this respect. Subsequently in 1964 reticuline was found to be a normal, minor component of P. somniferum; this is another instance of the isolation of a natural product from a plant after its presence has been suggested on phytochemical grounds. It also illustrates the point that what may be a principal alkaloid in one plant (reticuline in Annona) is, in another, a transient metabolite, which is essential to some metabolic pathway but which does not accumulate. In support of the theory, when tetrahydropapaverine (all hydroxyl groups methylated) was fed to the opium poppy, negligible incorporation into the alkaloids was obtained. The stages in the conversion of reticuline to thebaine and of thebaine to codeine were demonstrated by the feeding of appropriate labelled intermediates, alkaloids which have since been isolated as minor components of the opium alkaloid mixture.
One sequence of the pathway shown in Fig. 26.15, which has been difficult to establish unequivocally involves the initial stages leading to the formation of (+)-reticuline. Now norcoclaurine (also called higenamine, and a constituent of Annona squamosa, q.v.) is a favoured trihydroxylated precursor. In 1987 the (R)-isomer was shown to be specifically incorporated into thebaine when applied as a labelled precursor to P. somniferum seedlings. Also with cell cultures and plants of Berberis, Peumus, Eschscholtzia and Argemone spp. it was specifically incorporated into protoberberine, aporphine and benzophenanthridine alkaloids (see R. Stadler et al., Phytochemistry, 1989, 28, 1083). The experiments indicated that dopamine and p-hydroxyphenylacetaldehyde (both derived from tyrosine) condense to give norcoclaurine, thus explaining the observed lack of incorporation of DOPA and dopamine into the benzylic portion of reticuline-derived alkaloids. The origin of (+)-reticuline by this route is shown in Fig. 26.16.
A second pathway for the terminal steps in the biosynthesis of morphine has been demonstrated (E. Brochmann-Hanssen, Planta Med., 1984, 50, 343) by using two strains of the opium poppy—a Tasmanian strain known to contain the alkaloid oripavine and the Indra strain. Both species converted labelled oripavine to morphine, and morphinone was also isolated with good incorporation of radioactivity, albeit in small quantity owing to its unstable nature. Codeine and thebaine were not radioactive, demonstrating that the demethylation of the phenolic ether of thebaine is not reversible. This alternative final stage would therefore appear to be thebaine → oripavine → morphinone → morphine (Fig. 26.15).
Two alkaloids which arise as branches of the principal biogenetic pathway are neopine, the presence of which in opium is explained as a reduction product of neopinone, and papaverine, which arises by methylation of norreticuline (Fig. 26.17) followed by dehydrogenation. The presence of some of the other minor alkaloids of opium can be explained by various methylations and dehydrogenations of laudanosoline, reticuline and their nor-derivatives. Various oxidative couplings of reticuline account for other minor alkaloids (e.g. corytuberine and isoboldine).
Role of reticuline in alkaloid biosynthesis
The alkaloids involved in Fig. 26.15 are derived from (−)-reticuline, and the enzymic racemization of reticuline, which is essential for the biosynthesis of the principal opium alkaloids, is very substrate specific. Thus the N-ethyl, 6-ethoxy and 4′-ethoxy analogues of reticuline are completely resistant to racemization. (+)-Reticuline also gives rise to anumber of bases: narcotine (noscopine) and narceine of opium; canadine, berberine and hydrastine of Hydrastis (Berberidaceae); and sinomenine (the enantiomer of the opium alkaloid salutaridine, Fig. 26.15, of Sinomenium acutum, Menispermaceae). With the exception of sinomenine, these alkaloids are termed ‘berberine bridged’ alkaloids and they arise from norlaudanosoline and a one-carbon unit, which is derived from the N-methyl group of (+)-reticuline. The methylenedioxy group of these alkaloids is formed by oxidative cyclization of an o-methoxyphenol function. A scheme indicating the origin of some of these alkaloids is given in Fig. 26.18. For berberine, 13 enzymes are involved in its biosynthesis from two molecules of tyrosine. The enzyme associated with the final reaction, the formation of the methylenedioxy bridge, has now been detected in microsomal preparations from different Ranunculaceae and Berberidaceae cell cultures (M. Rueffer and M. H. Zenk, Phytochemistry, 1994, 36, 1219).
Enzyme studies
As indicated in Chapter 13 cell cultures of P. somniferum do not produce morphine-type alkaloids but accumulate large amounts of sanguinarine and other alkaloids. However, some of the enzymes of the morphinan pathway are present in such cell cultures as shown by the reduction of codeinone to codeine by immobilized cells of P. somniferum and by the isolation from cell cultures of the enzyme which reduces salutaridine to (7S)-salutaridinol. This enzyme has been fully characterized (R. Gerardy and M. H. Zenk, Phytochemistry, 1993, 34, 125) as has 1,2-dehydroreticuline reductase isolated from poppy seedlings. The same group has shown (R. Wilhelm and M. H. Zenk, Phytochemistry, 1997, 46, 701) that the enzyme necessary for the enol cleavage in the conversion of thebaine to neopinone (Fig. 26.15) is present in cell cultures, as evidenced by the formation of a new alkaloid, thebainone, as a result of feeding labelled thebaine to low-thebaine producing P. somniferum cultures. For work on genes encoding (S)-N-methylcoclaurine-5′-hydroxylase and codeinone reductase see F.-C. Huang and T. M. Kutchan, Phytochemistry, 2000, 53, 555.
Tests for opium alkaloids
Tests for morphine and other alkaloids are given in the pharmacopoeias. The solubility of morphine in sodium hydroxide solution is explained by its phenolic nature. Conversely, codeine is precipitated by sodium hydroxide.
Storage
Opium requires careful storage if the morphine content is to be maintained. In the past, Indian opium appears to have suffered more morphine loss during preparation and storage than the other varieties, but when it is dried at 100°C and stored out of contact with air, the loss of morphine is small. Abraham and Rae (1926) ascribe the lossof morphine to a peroxidase which they called opiase. More recently a phenoloxidase which acts on morphine has been isolated from poppy capsules.
Adulteration
Opium has been adulterated with sugary fruits, gum, powdered poppy capsules and other substances too numerous to mention. As far as legitimate commerce is concerned, such adulteration is now pointless because the product is analysed and the price paid is governed by the content of morphine and other alkaloids.
Uses
The alkaloids present in opium in greatest proportion decrease in narcotic properties in the order morphine, codeine, noscopine. Opium and morphine are widely used to relieve pain and are particularly valuable as hypnotics, as, unlike many other hypnotics, they act mainly on the sensory nerve cells of the cerebrum. Codeine is a milder sedative than morphine and is useful for allaying coughing. Both morphine and codeine decrease metabolism, and the latter, particularly before the introduction of insulin, was used for the treatment of diabetes. Opium, while closely resembling morphine, exerts its action more slowly and is therefore preferable in many cases (e.g. in the treatment of diarrhoea). Opium is also used as a diaphoretic. The habitual use of codeine may, in some individuals, produce constipation.
Manufacture of opium alkaloids
The majority of legal opium is used for the isolation of its constituent alkaloids, and in Britain some 90% of the morphine produced is converted into other bases such as codeine, ethylmorphine and pholcodine. In recent years attempts have been made to reduce the illicit traffic in opium either by banning the cultivation of the opium poppy or by cultivation under strict licences. However, two methods by which the opium stage is eliminated are by extraction of the whole poppy capsule and by the use of other species (e.g. P. bracteatum) which do not contain morphine.
Extraction of poppy capsules and straw
The feasibility of extracting poppy straw has long been known and utilized in Europe and recently there has been a world-wide trend towards the extraction of the dried poppy capsules (e.g. in Hungary, former USSR, Tasmania). In a study of poppy capsule drying and storage under commercial conditions in Tasmania it has been shown that kiln-drying of immature capsules (42 days old) at various temperatures (40–100°C) resulted in a loss of morphine content of up to about 11% without effect on codeine and thebaine. However, this morphine loss was significantly less than with the field-dried material. To avoid deterioration of the dried product the moisture content should not exceed 16%. Processes have also been developed in France and in the UK for the harvesting and processing of the green capsule, one technical difficulty being the separation of the seed from the fruit at this stage of development.
Use of morphine-free species of Papaver
The increasing abuse of opiates has stimulated the search for raw materials other than Papaver somniferum which would meet the requirements of the pharmaceutical industry. Thus, plants containing non-addictive thebaine as principal alkaloid could be used for the manufacture of codeine, naloxone (a narcotic antagonist prescribed for babies of heroin addicts) and etorphine (a ‘Bentley’ compound used for sedating large wild animals).
In this respect attention focused on three closely related perennial species of Papaver of the section Oxytona Bernh. of the family, namely, P. bracteatum, P. orientale and P. pseudo-orientale. These are indigenous to the mountainous districts of Iran, eastern Turkey and the Transcaucasian former USSR. Confusion concerning the identity of the characteristic alkaloids for each species had arisen from various factors, including incorrect identification, variations of chromosome number within a species, the existence of chemical races and geographical influences.
Papaver bracteatum
Thebaine is the predominant alkaloid of this species and in a UN-backed programme of large-scale cultivation trials were organized in various countries. High yielding strains were introduced, for example, Ayra II, a race obtained from west Iran in 1974 which gives 3.5% thebaine in the dried capsules. Problems associated with the development were the insufficiency of seed of high-yielding strains and the poorer crops obtained when the plants were removed from their normal environment. However, political decisions also jeopardized the continuation of the programme.
P. bracteatum produces some 27 alkaloids belonging to 10 of the 14 alkaloid groups described for Papaver. The biogenesis of thebaine follows the same pathway as in P. somniferum. Feeding experiments with labelled intermediates have shown that the plant is capable of converting codeinone to codeine but cannot perform either of the demethylations leading to codeine or directly to morphine. Thebaine does not appear to be entirely an end-product and undergoes further metabolism to unknown products (for a report, see H. G. Theuns et al., Phytochemistry, 1985, 24, 581). A more recently described new alkaloid is salutaridine-N-oxide (G. Sariyar et al., Planta Med., 1992, 58, 368).
Papaver orientale
This species is commonly cultivated as the ornamental poppy and a number of alkaloid chemotypes have been described. Generally, oripavine (formed by demethylation of the aromatic ring of thebaine, Fig. 26.15) is the principal alkaloid, but Phillipson et al. (Planta Med., 1981, 43, 261) described one chemotype with mecambridine (a berberine type alkaloid) as principal constituent.
Papaver pseudo-orientale
The plant (2n = 42) is intermediate in many of its characters between those of the above two species and may have arisen as an allohexaploid from them. Phillipson (above reference) divided 16 Turkish samples in three cytological and alkaloid groups. Thirteen samples contained isothebaine, mecambridine and orientalidine as major alkaloids, two contained principally salutaridine and thebaine, and one sample possessed salutaridine as the major component. (For other alkaloids and biogenetic transformations see G. S×zariya et al., Phytochemistry, 1986, 25, 2403.)
(For a comprehensive review of the morphine alkaloids covering general chemistry, biogenesis, occurrence and structure elucidation (391 refs) see C. S×zantay et al., The Alkaloids, 1994, 45, 128.)
BOLDO LEAVES
Boldo leaves BP/EP are derived from Peumus boldus (Monimiaceae); they contain aporphine-type alkaloids, chiefly boldine. The drug has antihepatoxic activity and is described in Chapter 29.
GOLDENSEAL ROOT
Goldenseal root BP/EP, BHP 1990 consists of the dried rhizome and roots of Hydrastis canadensis (Berberidaceae), a small perennial plant indigenous to the woods of eastern Canada and the eastern USA. The wild plants have been exterminated in many districts and the species now has CITES listing (q.v.) for the USA and Canada. Most of the commercial drug is now obtained from cultivated plants grown in America and in Europe. The use of hydrastis, both as a drug and as a dye, was learned by the early European settlers from the Cherokee Indians.
Characters
The drug consists of almost cylindrical rhizomes about 1–5 cm long and 2–10 mm diameter. The rhizomes grow more or less obliquely and bear numerous, short branches, which terminate in cup-shaped scars and bear encircling cataphyllary leaves. Similar scale leaves are found on the rhizome, the outer surface of which is yellowish-brown or greyish-brown. The roots, which originate on the ventral and lateral surfaces, are long and wiry, and in the commercial drug are often broken at a distance of a centimetre or so from the rhizome. The drug breaks with a short, waxy fracture. It has a slight but distinctive odour and bitter taste.
A transverse section of the rhizome shows a fairly thick, yellow or yellowish-brown bark; 12–20 radially elongated, bright yellow wood bundles, separated by wide medullary rays; a large pith.
Constituents
Hydrastis contains the alkaloids hydrastine, berberine, canadine and other minor ones. Commercial samples yield 1.5–4% of hydrastine and 0.5–6.0% of berberine. The latter, as a constituent of an extract of the root, is responsible for activity against multiple drug resistant Mycobacterium tuberculosis (see E. J. Gentry et al., J. Nat. Prod., 1998, 61, 1187)
The BP/EP requires a minimum content of 2–5% (dried drug) for hydrastine and a minimum of 3.0% for berberine. These are determined by liquid chromatography with absorbance measurements at 235 nm using a reference solution of the above alkaloids for comparison. These alkaloids are also identified in the TLC test for the drug using visualization with UV light at 365 nm.
Uses
The use of hydrastis to check uterine haemorrhage, as a bitter stomachic and locally in the treatment of catarrhal conditions of the genito-urinary tract is largely based on empirical observations. Hydrastine hydrochloride and hydrastinine hydrochloride have been used in various forms to control uterine haemorrhage.
FUMITORY
Fumitory BP/EP, BHP consists of the dried aerial parts of Fumaria officinalis L., family Fumariaceae, collected when in flower. It is an annual herb common as a weed and on roadsides throughout most of Europe; it has spread world-wide. The leaves are greyish-green, stalked, each pinnately divided several times giving flattened lanceolate, often toothed segments. The flowers are arranged in racemes with pink, red-tipped petals forming a tubular corolla. Fruits are somewhat flattened achenes, 2.0–2.5 mm, with rough surfaces. Microscopical features of the olive-green powder include leaf epidermi with anomocytic stomata and spherical pollen grains, about 35–40 μm in diameter, with pitted exine and six large pores; also in abundance are features of the stems, flowers and fruits.
Constituents
A range of isoquinoline-type alkaloids includes protoberberines, spirobenzylisoquinolines, benzophenanthridines and indenobenzazepines. Protopine (Fig. 26.19) is the principal alkaloid, the BP/EP requiring a minimum total alkaloid content of 0.40% calculated as this alkaloid. A titrimetric assay is employed, sonication being used for dissolving the plant extract produced in the alkaloid purification procedure.
Other constituents include flavonoids, principally glycosides of quercetin, and acids, including chlorogenic and caffeic acids.
GREATER CELANDINE
Greater celandine BP/EP consists of the dried whole or cut aerial parts of Chelidonium majus L. family Papaveraceae, collected parts of Chelidonium majus L. family Papaveraceae, collected during the flowering period. There is a minimum requirement of 0.6% for total alkaloids expressed as chelidonine.
This perennial herb is widespread throughout Europe, northern Asia and the north-eastern USA, growing along banks, in hedgerows, against walls and in waste areas. The hollow, ribbed stems are up to about 90 cm in height, branched and leafy. When fresh, the stems and leaves exude a milky sap, which turns to orange–red when exposed to the air and is a skin irritant. The leaves are almost pinnate and divided into five to seven ovate to oblong leaflets, the terminal ones often three-lobed; margins are crenately toothed, the upper surfaces glabrous and dark green and the lower somewhat glaucous. The bright yellow flowers, 2–2.5 cm in diameter each have two greenish-yellow sepals and four petals. Stamens are yellow and numerous. Fruit is a capsule 3–5 cm in length, containing black seeds having a white appendage; mature fruits are rarely to be found in the drug.
Microscopic features include: anomocytic stomata on the lower leaflet surfaces, uniseriate possibly fragmented covering trichomes, vascular tissues, associated latex canals with brown contents, corolla fragments with oil droplets, spherical three-pored pollen grains up to 40 μm in diameter.
Constituents
Greater celandine contains up to 4% of alkaloids including α- and β-allocryptopine, berberine (Fig. 28.1), chelerythrine (Fig. 26.19), chelidonine (Fig. 26.18), chelirubine, choline, coptisine, hydroxychelidonine, hydroxysanguinarine, protopine (Fig. 26.19), sanguinarine (Fig. 26.19), sparteine (Fig. 26.12), and others.
The BP/EP TLC test for identity produces a number of unidentified separated components; the assay for total alkaloids is performed on an extract of the drug treated in acid solution with the BP chromotropic, sodium salt reagent and absorbance measurements at 570 nm.
Uses
The drug has many traditional uses based on its reputed anodyne, antispasmodic, caustic, diaphoretic, diuretic, hydragogue, narcotic and purgative properties, Some of these activities have support from pharmacological tests involving particular alkaloids. C. majus is also used in homoeopathic practice and in Chinese medicine.
Annona squamosa (Annonaceae)
Various parts of this plant have featured in the folk medicine of Africa, India and the Far East for the treatment of a number of conditions including heart disorders. The cardioactive effect has been attributed to the alkaloid higenamine (Fig. 26.19) an important precursor of a number of other isoquinolines.
Bloodroot
Bloodroot consists of the dried rhizomes and roots of Sanguinaria canadensis (Papaveraceae), a perennial herb widely distributed in the woods of North America. The drug consists of dark brown, more or less cylindrical pieces of rhizome, 2–7 cm long and 5–15 mm diameter. Some of the pieces are branched and some show numerous wiry roots. The latter, however, are usually broken off in the commercial drug. The rhizome breaks with a short fracture and, if not overheated during drying, shows numerous red dots (secretion cells) distributed throughout the starch-containing parenchyma of the bark and large pith. If dried at too high a temperature, the secretion escapes from its containing cells and the whole section assumes a deep red or brownish-red colour. A ring of small, yellow, fibrovascular bundles lies about 1 mm from the outside. Odour, slight; taste, acrid and bitter. Bloodroot contains the benzophenanthridine alkaloids sanguinarine, chelerythrine (Fig. 26.19), allocryptopine, protopine and dihydrosanguilutine. Sanguinarine and chelerythrine, although themselves colourless, form red and yellow salts, respectively. The drug also contains red resin and starch.
In a report by Rho et al. (Appl. Microbiol. Biotechnol., 1992, 36, 611) S. canadensis cultured cells produced sanguinarine (about 80% of the total alkaloid) together with chelirubine and chelerythrine. In contrast, in the normal rhizome sanguinarine and sanguirubine together account for some 70% of the total alkaloids. (For elicitor studies see G. B. Mahady and C. W. W. Beecher, Phytochemistry, 1994, 37, 415.)
Bloodroot is used mainly in the USA, where it is an ingredient of Compound White Pine Syrup. Sanguinarine, like colchicine, causes the doubling of the chromosomes in cells.
Calumba root
Calumba is the dried, sliced root of Jateorhiza palmata (J. columba) (Menispermaceae), a dioecious climbing plant indigenous to the forests of Mozambique and Madagascar (Malagasy Republic) and other east African countries. It is exported to Europe from Tanzania and the name derives from the fact that it was at one time exported from Colombo (Sri Lanka).
Collection
Attempts to cultivate the drug in various areas do not appear to have been successful and collection is from the wild. The plant possesses a somewhat slender rhizome from which numerous large fusiform roots arise. The older reports state that these are dug up during dry weather (March), the rhizomes are rejected and the roots cut into transverse or oblique slices and dried in the shade. The imported ‘natural calumba’ is frequently washed, brushed and graded, the product being known as ‘washed calumba’.
Macroscopical characters
Calumba occurs in circular or oblique slices. These are usually 2–8 cm diameter and 3–12 mm thick.
The cork is thin, greyish-brown or reddish-brown in colour and longitudinally wrinkled. Within it lies a broad, greenish-yellow zone which extends to the cambium and contains in its outer part isolated sclerenchymatous cells within which are dark-grey, sinuous strands of sieve tissue. The greyish wood, which is separated from the bark by a dark cambium line, shows numerous narrow, radiating lines of yellow vessels separated by abundant parenchyma. The vessels are close together in the region near the cambium and again in the extreme centre of the root, but they are less numerous in the intermediate zone, which therefore shrinks considerably and becomes depressed on drying. Some pieces show two or more concentric zones of wood. The fracture is short and starchy; odour, slight and somewhat musty; taste, bitter.
Calumba frequently contains occasional slices of calumba rhizome. These average about 2–3 cm diameter. The structure is markedly radiate and, owing to its greater woodiness in that region, is not depressed in the centre.
Microscopical characters
The drug is characterized by the sclereids which have unevenly thickened, yellow walls and contain a number of prisms of calcium oxalate, by abundant parenchymatous cells containing starch grains, each grain measuring about 20–85 μm long and having an eccentric, very distinct radiate or cleft hilum, and by the yellow reticulate vessels. The walls of both the sclerenchymatous cells and vessels on treatment with 66% v/v sulphuric acid change colour from yellow to green.
Constituents
Calumba contains about 2–3% of isoquinoline alkaloids, palmatine, jatrorrhizine and columbamine. Bisjatrorrhizine is a quaternary dimeric alkaloid formed by ortho-oxidative coupling of the phenolic group of jatrorrhizine. Other constituents are the non-alkaloidal furanoditerpenes columbin, isocolumbin, palmarin, chasmanthin, jateorin and isojateorin. Some of these occur as glucosides and have been named palmatosides A to G.
Serpentary
Serpentary consists of the dried rhizome and roots of Aristolochia reticulata (Aristolochiaceae). This is known in commerce as Texan or Red River snake-root and is collected in the woods of Texas, Louisiana, Arkansas and Oklahoma.
Macroscopical characters
The drug has a yellowish colour when fresh, becoming brown on keeping. It consists of small rhizomes bearing the remains of subaerial stems and numerous wiry roots. The rhizomes are about 1–2 cm long and 2–3 mm diameter, while the roots are about 10 cm long and 0.2–1.2 mm diameter. The drug contains up to 10% of subaerial stems. Odour, camphoraceous; taste, camphoraceous and bitter.
A transverse section of the rhizomes shows a starchy, eccentric pith (nearer the upper surface of the rhizome than the lower), wedge-shaped, yellowish vascular bundles separated by wide medullary rays, and a narrow bark.
Allied drugs
Virginian snake-root, from Aristolochia serpentaria, was formerly official but its regular importation has now ceased. It closely resembles the Texan drug, but has smaller rhizome and more wiry roots. Indian aristolochia or Indian birthwort consists of the roots and rhizome of Aristolochia indica; it contains aristolochic acid together with other phenanthrene derivatives, an N-glycoside and steroids. A. heterophylla is used in China, and A. constricta, recently investigated (L. Pastrelli et al., J. Nat. Prod., 1997, 60, 1065), is widely employed in folk medicine in S. America. A. clematis (birthwort) is European and has been used both internally and externally. It contains similar constituents to other species.
Constituents
Many species of Aristolochia including A. reticulata contain aristolochic acid and the tumour-inhibiting properties of this compound are of interest. However, in experimental animals it can cause tumour formation and has been associated with cases of renal failure. Aristolochic acid is not alkaloidal, belonging to a small group of naturally occurring nitro-compounds, but is included here because of its direct derivation from isothebaine derivatives in the plant.
(For a review on the structure of the aristolochic acids and their corresponding lactams (aristolactams) see D. B. Mix et al., J. Nat. Prod., 1982, 45, 657 and for the isolation of aristolochic acids I–IV and aristolactams I–III from A. auricularis see P. J. Houghton and M. Ogutveren, Phytochemistry 1991, 30, 253.)
Uses and dangers
Snakeroot has been traditionally employed as an aromatic bitter but other Aristolochia spp. have also been employed in Chinese herbal medicines for various treatments. As from 28 July 1999 an emergency ban on the import, sale and supply of medicinal products containing Aristolochia spp. came into force for the UK. This arose in part from two UK cases of end-stage renal failure in patients using Chinese medicines containing Aristolochia and from many cases of renal failure in Belgium when Aristolochia was substituted for Stephania in a herbal preparation. Apparently in Chinese herbal preparations it is liable to be substituted for other innocuous components such as Stephania, Akebia or Clematis (MCA/CSM, UK, Current Problems in Pharmacovigilance, 1996, 22, 10; see also the report, Pharm. J., 2000, 265, 10).
CURARE
The term ‘curare’ is a generic one applied to various South American arrow poisons. These extracts are made from a number of different plants, particularly members of the Menispermaceae (e.g. Chondrodendron) and the Loganiaceae. From the former, tubocurarine is obtained and the (+)-hydrochloride of this is included in the BP/EP as a muscle relaxant.
Curares from the upper Amazon (Brazil and Peru) seem to be mainly menispermaceous in origin. The original genus Chondrodendron has been divided into two by Barneby and Krukoff (Mem. N. Y. Bot. Gdn, 1971, 22, 1); Chondrodendron itself with three species (Ch. tomentosum, Ch. platyphyllum, Ch. microphyllum) and Curarea with four species (Cu. toxicofera, Cu. candicans, Cu. tecunarum and Cu. cuartecasasii). The principal ones used in curare are Ch. tomentosum (the only plant known to contain tubocurarine), and the first three of the Curarea species. Several other genera of Menispermaceae are known to have been, or to be, ingredients of curares including species of Sciadotenia, Abuta, Telitoxicum and Cissampelos, but little is known about the muscle-relaxant activity, if any, of their alkaloids.
The curares from Guyana, Venezuela and Colombia owe much of their activity to species of Strychnos (Loganiaceae); around 20 species are known to have been incorporated into curares and these include S. toxifera, S. jobertiana, S. peckii and S. guianensis.
(For reviews covering the history, uses, botany and chemistry of curare see N. G. Bisset, J. Ethnopharmacology, 1992, 36, 1; Alkaloids, Chem. Biol. Perspect., 1992, 8, 1.)
History
There is a close botanical relationship between Pareira Brava Radix of the seventeenth- and eighteenth-century pharmacopoeias and the menispermaceous plants yielding curare. In both cases the botanical source of the drugs has long been in doubt. It is now known that Pereira Brava is obtained from two species of Chondrodendron, namely, C. microphyllum, which contains (+)- bebeerine, and C. platyphyllum, which contains (−)-bebeerine. A similar case is the so-called C. tomentosum, which sometimes yields
(+)-tubocurarine and sometimes the less active (−)-tubocurarine. This led King (1948) to write: ‘It seems very probable therefore that two species are involved under the name C. tomentosum’.
Boehm (1895) distinguished three kinds of curare differentiated first by their containers, and second by their different chemical characteristics.
Curare in tins
Much curare has been imported in tins containing about 1 kg of a viscous dark-brown or blackish extract. It has little odour but a very bitter taste.
This drug is similar to the specimen examined by King (1948), who received it from Asher Kates y Cia S.A. of Lima, Peru, together with the leaves of the plant from which it was prepared. These leaves were indistinguishable from those of C. tomentosum and its menispermaceous nature, and similarity to the old tube-curare, was confirmed by the isolation of (+)-tubocurarine chloride and four other alkaloids.
Constituents
(1) Menispermaceous tube-curare and the form now imported in tins contain tubocurarine. From the tin-curare King (1948) isolated (+)-tubocurarine chloride and four non-quaternary bases (isochondrodendrine dimethyl ether, (−)-curine (bebeerine), (+)-chondrocurine and (+)-isochondrodendrine). (2) Loganiaceous calabash-curare derives its activity largely from Strychnos species, particularly S. toxifera. King (1949) has shown that the bark of this species contains 12 crystalline quaternary alkaloids, the toxiferines I–XII, of which two had previously been isolated by Wieland et al. (1937–41). These latter authors examined calabash-curare, probably from Venezuela, and isolated several alkaloids known as C-curarines (C signifies calabash). Toxiferines I and II have been found in calabash-curare.
More recently calabash-curares and Strychnos spp. have been examined by Karrer and his colleagues with the isolation of a large number of new alkaloids. (+)-Tubocurarine is a bisbenzylisoquinoline alkaloid and as such is derived from dopamine, whereas the Loganiaceous curares are indolic and contain C40 compounds of the dimeric strychnine type.
TETRAHYDROISOQUINOLINE MONOTERPENOID ALKALOIDS AND GLYCOSIDES
These alkaloids and alkaloid-glycosides derive from the condensation of dopamine with secologanin (a C10 monoterpene) to give two series of compounds. The best-known examples of their limited occurrence are in species of Cephaëlis (Rubiaceae) and Alangium (Alangiaceae). The former gives ipecacuanha root, and the root, bark, fruits and leaves of A. lamarckii are used in Ayurvedic medicine for the treatment of a number of conditions.
IPECACUANHA
Ipecacuanha (Ipecacuanha Root) of the BP is the dried root or rhizome and root of Cephaëlis ipecacuanha (Brotero) A. Richard (Rubiaceae), known in commerce as Matto Grosso Ipecac. or of Cephaëlis acuminata Karsten, known in commerce as Costa Rica Ipecac. A mixture of both species is also permissible. It should contain a minimum of 2% of ether-soluble alkaloids.
C. ipecacuanha is a shrub 20–40 cm high found over a large area in Brazil, particularly in the moist and shady forests of Matto Grosso and Minãs Geraes; plantations have been established in the Matto Grosso area. It is cultivated to some extent in Malaya, Burma and the Darjeeling Hills of West Bengal. C. acuminata is exported from Colombia, Nicaragua and Costa Rica; Costa Rica is at present the principal source of the drug. However India is now in full production of Costa Rican type root which is of high quality (in excess of 3.5% total alkaloid) and extremely competitive in price; extracts of the Indian root are now being produced and exported.
History
What appears to have been ipecacuanha was mentioned, under the name of Igpecaya, by a Portuguese friar around 1600. It was introduced into Europe in 1672.
Collection and preparation
In the Matto Grosso district of Brazil the drug is collected from wild plants. The collector, using a pointed stick, levers the plant from the ground and, having removed most of the roots, replaces it in the ground, where it usually lives to produce further crops. The roots are dried in the sun or by fires and transported down river to ports such as Rio de Janeiro, Bahia and Pernambuco from which they are exported in bales. Other South American ipecacuanhas are collected in a similar way. The supply of S. American ipecacuanha has been erratic for many years as a result of habitat destruction, overcollection and the uprooting of plants. The high price of the drug has stimulated both cultivation in other areas (see above) and the promotion of cell and root cultures for alkaloid production (see below).
Macroscopical characters
The underground portion consists of thin, horizontal rhizomes from the lower surface of which roots are given off. Some of the latter remain thin, while others develop an abnormally thick bark and become annulated.
The Matto Grosso drug occurs in tortuous pieces up to 15 cm long and 6 mm diameter, but it is usually smaller. The colour of the outer surface varies from a deep brick-red to a very dark brown, the colour being very largely dependent on the type of soil in which the plant has been grown. Most of the roots are more or less annulated externally, and some have a portion of the rhizome attached (Fig. 26.20A), while separate portions of rhizome and non-annulated roots are also found. Generally, the drug of present-day commerce is less markedly annulated than was formerly the case, a fact that points to earlier collection. The ridges are rounded and completely encircle the root; here and there the bark has completely separated from the wood.
Fig. 26.20 Ipecacuanha. A, Cephaëlis ipecacuanha roots with rhizome; B, C. acuminata roots with rhizome (both ×1); C, transverse section of root; D, transverse section of rhizome (both ×4); E, cork cells in surface view; F, starch granules (mounted in cold lactophenol); G, idioblast containing calcium oxalate crystals; H, elements from Schultze maceration of wood (all ×200). a1, Complete annulation of C. ipecacuanha; a2, incomplete annulation of C. acuminata; ck, cork; e, endodermis; f, fibrous cell; id, idioblast containing calcium oxalate; p, pith; pd, phelloderm; ph, phloem; rh, rhizome; tr.v, tracheid vessel; xy, xylem; xy.p, xylem parenchyma.
The root breaks with a short fracture and shows a thick greyish bark and a small, dense wood, but no pith. The rhizomes, on the other hand, have a much thinner bark and a definite pith (Fig. 26.20C, D). The drug has little odour, but is irritating and sternutatory when in fine powder, and has a bitter taste.
The Costa Rica drug (Fig. 26.20B) is exported from Cartagena and Savanilla. The main differences between the Rio and Cartagena drugs are listed in Table 26.5.
Table 26.5 Comparison of ipecacuanha (Cephaëlis) roots.
| C. ipecacuanha | C. acuminata | |
|---|---|---|
| Usual diameter | 1–4 mm | 4–6.5 mm |
| Colour | Brick-red to brown | Greyish-brown |
| Annulations | Very crowded | Less crowded and less projecting |
| Starch | Individual grains up to 15 μm | Individual grains up to 20 μm |
Ipecacuanha stems, although containing the same alkaloids as the roots, usually contain them in smaller proportion. An excessive amount of stem must, therefore, be regarded as an adulteration.
Microscopical characters
A transverse section of the root (Fig. 26.20C) shows a thin, brown cork, the cells of which contain brown, granular material. Within this is a wide secondary cortex (phelloderm), the cells of which are parenchymatous and contain starch, usually in compound grains with from two to eight components, or raphides of calcium oxalate. The individual starch grains are muller-shaped and up to 15 or 20 μm diameter. The phloem is entirely parenchymatous, containing no sclerenchymatous cells or fibres. The compact central mass of xylem is composed of small tracheidal vessels, tracheids, substitutefibres, xylem fibres and xylem parenchyma. Starch is present in the xylem parenchyma and substitute fibres contain starch (Fig. 26.20E–H).
The transverse section of ipecacuanha rhizome (Fig. 26.20D) shows the presence of a ring of xylem and a large pith. The pericycle contains characteristic sclerenchymatous cells. Spiral vessels occur in the protoxylem. The pith is composed of pitted parenchyma which shows some lignification.
Adulterants
At one time other ‘ipecacuanhas’ were regularly imported, the name being applied in South America to a number of different roots which were reputed to have emetic properties. Most of these, briefly described in the 10th edition, are very easily distinguished from the genuine drug and are now rarely imported.
Constituents
Ipecacuanha contains the alkaloids emetine (Pelletier and Magendie, 1817), cephaëline (Paul and Cownley, 1894), psychotrine, psychotrine methylether and emetamine. These are isoquinoline derivatives of a group only known with certainty to occur in the families Alangiaceae, Icacinaceae and Rubiaceae. However, emetine-type alkaloids are not necessarily a characteristic of the genus Cephaëlis as a whole as Solis et al. (Phytochemistry, 1993, 33, 1117) recorded a new indole alkaloid and four other known indole alkaloids from the aerial parts of C. dichroa from Western Panama.
In a review (411 refs) of the ipecacuanha and related bases (T. Fujii and M. Ohba, The Alkaloids, 1998, 51, 271) 39 new alkaloids from ipecacuanha and Alangium are recorded for the 14 years to 1997. For further new alkaloids of A. longiflorum, see for example N. Sakurai et al., Phytochemistry, 2006, 67, 894.
Other constituents of the official drug are monoterpenoid isoquinoline glucosides including ipecoside (Fig. 26.22), alangiside and, reported in a series of papers (1989–), a number of other novel glycosides closely related to the known ipecacuanha alkaloids (A. Itoh et al., Phytochemistry, 2002, 59, 91 and the refs cited therein). The iridoid glucosides sweroside and 7-dehydrologanin together with starch and calcium oxalate are also found in the root. Ipecacuanhin and ipecacuanhic acid, originally designated glycosidal tannins, are now thought to have been impure mixtures of ipecoside and sucrose.
As may be seen from Fig. 26.21, the principal alkaloids are closely related to one another; emetine and psychotrine methylether are nonphenolic, whereas cephaëline and psychotrine are phenolic.
Thus, emetine, which is the alkaloid usually required in medicine, may be prepared by methylating the cephaëline originally present in the drug. These alkaloids may be regarded as being formed in the plant from two phenylethylamine units and a C9 terpenoid precursor. The latter is provided in the plant by secologanin and is incorporated via desacetylisoipecoside into the emetine alkaloids (Fig. 26.22). The glucosidic compound ipecoside is formed from the epimer desacetylipecoside (cf. Fig. 26.22, which illustrates the involvement of secologanin in the formation of some indole alkaloids; in this case, however, only the α-epimer, strictosodine, is formed, but it can serve as a precursor for alkaloids with β-configuration). The isolation by Itoh et al., (Chem. Pharm. Bull., 1994, 17, 1460) of tetrahydroisoquinoline-monoterpenoid glucosides, from Alangium lamarckii fruits, with the same stereo-configuration as desacetylisoipecosides supported the role of the latter as an intermediate in the formation of emetine-type alkaloids. Furthermore cell-free extracts of A. lamarckii have been shown to contain two enzyme activities promoting the condensation of dopamine and secologanin to give the (S)- and (R)-products respectively (W. De-Eknamkul et al., Phytochemistry, 1997, 45, 477).
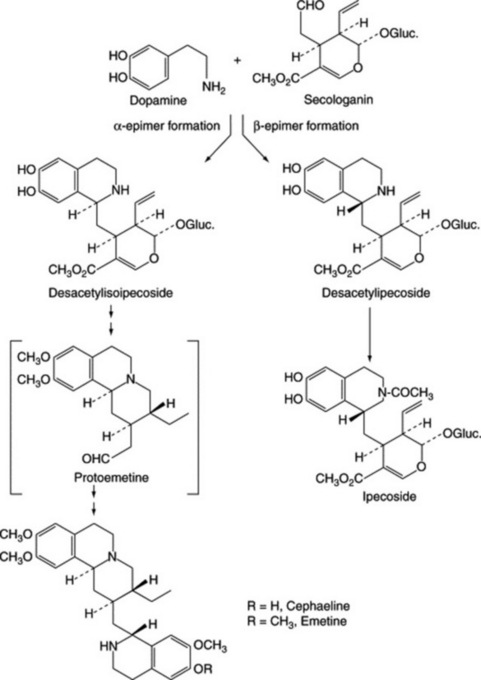
Fig. 26.22 Biosynthetic sequence for the biosynthesis of cephaeline, emetine and the alkaloidal glucoside ipecoside.
Various varieties of ipecacuanha contain different proportions of the principal alkaloids. Thus, the Rio drug, which is now difficult to obtain commercially and is the most esteemed, contains 2–2.4% alkaloids, of which 60–75% is emetine. Those varieties derived from C. acuminata yield 2–3.5% alkaloids, of which emetine may constitute 30–50%.
Cell and root cultures
Production of the ipecacuanha alkaloids by artificial culture would be commercially highly desirable and some research in this area has been reported. The composition of the culture medium and the nature of the added hormones greatly influences alkaloid production but generally root cultures appear to be more satisfactory than callus or suspension cultures. Increased growth of roots is obtained by the induction of hairy roots. In contrast to whole roots, cell cultures produce more cephaëline than emetine, and immobilized cell systems give higher amounts of cephaëline compared with static cell cultures. (For original research papers see K. Yoshimatsu et al., Phytochemistry, 1991, 30, 507; S. Jha et al., ibid., 30, 3999; C. Veeresham et al., ibid., 1994, 35, 947.) The roots of C. ipecacuanha transformed with Agrobacterium rhizogenes grow well in a gamboge medium yielding 112 mg/l of cephaeline and 14 mg/l of emetine after eight weeks of culture (K. Yoshimatsu et al., Planta Med., 2003, 69, 1018).
A simple one-step method for the production of ipecacuanha plants from root cultures has been described (K. Yoshimatsu and K. Shimomura, Plant Cell Rep., 1994, 14, 98).
Test for emetine
Mix 0.5 g of the powdered drug with 20 ml of hydrochloric acid and 5 ml of water; filter, and to 2 ml of the filtrate add 0.01 g of potassium chlorate; if emetine is present, a yellow colour appears, which, on standing for about 1 h, gradually changes to red.
Prepared Ipecacuanha is the drug reduced to a fine powder and adjusted to contain 1.90–2.10% of total alkaloids. It is assayed (BP/EP) by extraction of the alkaloids followed by back-titration of the standarized acid solution with sodium hydroxide.
Uses
Ipecacuanha is used as an expectorant and emetic and in the treatment of amoebic dysentery (Chapter 28). Emetine has a more expectorant and less emetic action than cephaëline, a fact that accounts for the preference shown for the Brazilian drug. In the treatment of amoebic dysentery emetine hydrochloride is frequently given by injection, and emetine and bismuth iodide by mouth. Psychotrine and its O-methyl ether are selective inhibitors of human immunodeficiency virus and their study could lead to the development of therapeutically useful agents (G. J. Tan et al., J. Biol. Chem., 1991, 266, 23529).
Cocillana
Cocillana BP 2001 (Grape Bark, Guapi Bark) is the dried bark of Guarea rusbyi (Meliaceae) and other closely related species. The trees are native to the South American Andes and the bark is collected in Bolivia and Haiti.
Macroscopical characters
The commercial bark which has a slight aromatic odour, occurs in fairly large flattish or curved pieces, up to 60 cm long and 5–20 mm thick. Externally, the cork may be quite extensive and fissured, but the outer layers are missing in some areas and covered by lichen patches in others. The inner surface shows longitudinal striations and is lighter in colour than the grey-brown or orange brown outer tissues.
Microscopical characters
The lignified cork cells occur in bands alternating with layers of lignified parenchyma and sclereids. In the phloem the narrow medullary rays, some cells of which are sclerenchymatous, run between numerous fibre groups, each containing aprism sheath. Many cells contain pigmented contents and a little starch is present. For illustrations showing the macroscopical and microscopical features of the drug, see previous editions.
Constituents
There appears to be no recent work which delineates the active constituents of the drug and it is placed in this position because of its association with ipecacuanha. Arising from work carried out at the end of the nineteenth century, the bark is described as containing 2.3% resins, 2.5% fixed oil, tannin, a small quantity of alkaloid and possibly a glycoside. A study carried out in 1966, which duplicated the earlier methods of extraction and fractionation, gave different results and added credence to the widespread belief that present commercial supplies may not be identical with the earlier ones.
AMARYLLIDACEAE ALKALOIDS
The bulbs of this family are well-known for their toxic properties, at least one fatality in the UK being recorded in 1999 as a result of mistaken consumption of daffodil bulbs for onions.
The alkaloids are derived from one molecule of phenylalanine and one of tyramine and biochemically fall into three series depending on the type of oxidative phenolic coupling undergone by the precursor p′-O-methylnorbelladine (formula Fig. 26.13). These series are represented by the alkaloids haemanthamine (p, p′coupling, lycorine (o, p′ coupling) and galanthamine (o′, p coupling) (Fig. 26.23), all three types often co-occurring in one species. Some 20 genera of the family produce these alkaloids but in the past they have been of minor significance in Western medicine. However Narcissus tazetta var. chinensis,the bulbs of which contain lycorine, pseudolycorine and tazettine, is described in Chinese traditional and herbal remedy texts.
O. Hoshino has reviewed (203 refs) the Amaryllidaceae alkaloids considering ten types plus miscellaneous alkaloids (The Alkaloids, 1998, 51, 323).
Currently there is much interest in galanthamine which has emerged as a useful drug for the treatment of Alzheimer’s disease.
Galanthamine (Galantamine)
This base was first isolated from the Caucasian snowdrop Galanthus woronowi by Russian workers in 1952 and subsequently by other scientists from the common snowdrop G. nivalis. It was rapidly shown to be a metabolite of many species of the family (J. J. Willaman and H.-L. Li, J. Nat. Prod., 1970, 33, 17) from which Leucojum aestivum has emerged as a commercial source with the consequence that wild plants are, like many other medicinal plants, becoming an endangered species.
A. Poulev et al. (Planta Medica, 1993, 59, 442) have used ELISA techniques (Ch. 16) for the quantitative determination of galanthamine in plant materials and obtained yields of from 0.1 to 2.0% for the dried bulbs of L. aestivum with Phaedranassa negistrophylla from Peru giving 7.4%. Various Narcissus cultivars have been examined for the alkaloid and it has been shown that planting depth, planting density, bulb size and flower bud removal did not affect the galanthamine content (R. M. Moraes-Cerdeira et al., Planta Medica, 1997, 63, 472). N. confusus, endemic to Spain, is reported to have the highest galanthamine content of the genus reaching in the emerging bulb a maximum concentration of up to 2.5%, dry weight (S. Lopez et al., Planta Medica, 2003, 69, 1166).
There is a considerable body of literature on the pharmacology of galanthamine (see review by A. L. Harvey, Pharmac. Ther., 1995, 68, 113) and readers will note a possible confusion in nomenclature in a number of the more recent publications by use of the designation ‘galantamine’, a name also employed by the manufacturer to describe the new product mentioned below.
Galanthamine is an acetylcholinesterase inhibitor and as such has been used extensively since the late 1950s in Eastern Europe and the former Soviet Union in anaesthesia as a curare reversal agent. Secondly, the alkaloid also acts centrally by penetrating the blood–brain barrier to serve as a modulator of nicotinic cholinergic receptors, thus augmenting central cholinergic neurotransmission. It is the latter which has invoked investigation into its use in the palliative treatment of Alzheimer’s disease.
Clinical trials with the isolated alkaloid involving patients presenting with mild to moderate symptoms of Alzheimer’s disease have given positive findings for the improvement of memory and intellectual functioning. The drug, as Reminyl®, was granted European marketing approval in July 2000.
Medicinal and aromatic plants – industrial profiles (series ed) Hanks GR, Hardman R, editors. Narcissus and daffodil – the genus Narcissus. Vol 21. CRC Press, Taylor and Francis Group. Boca Raton, FL, 2002.
Heinrich M, Teoh HL. Galanthamine from snowdrop – the development of a modern drug against Alzheimer’s disease from local Caucasian knowledge. J Ethnopharmacology. 2004;92:147-162.