Cements
CHARACTERISTIC PROPERTIES OF DENTAL CEMENTS
A variety of cements have been used in dentistry through the years to retain single restorations or prostheses in a fixed position within the mouth. In addition, cements can be used for specialized purposes in the restorative, endodontic, orthodontic, periodontic, and surgical fields of dentistry, such as in high-strength bases beneath direct restorations and endodontic sealers, and to cement orthodontic bands and brackets.
In the last 20 years, there has been an explosion of different types of dental cements that have become available to practicing dentists, many of them tailored for specialized types of restorations. In fact, there are so many products to choose from that the task can become confusing. This chapter will describe the composition, properties, manipulation, and indications for use of three broad categories of cements—water-based, resin-based and oil-based permanent and temporary cements.
In general, water-based cements rely on an acid-base setting reaction and are usually acidic during cementation. The cements in this category generally either do not bond at all or have a low bond to tooth structure, but they are easy to use, some release fluoride, and most have a relatively low film thickness.
Resin-based cements have a chemistry based on resin composites and have higher bond strengths to tooth structure when bonded with adhesives. Some products also contain monomers or use primers that enable bonding to many dental alloys and to ceramics. In general, these types of cements have stronger mechanical properties than the water- or oil-based cements, but the cementation (bonding) process is more technique sensitive than in other categories of cements.
Oil-based cements are primarily used for luting provisional restorations. Most contain eugenol, but some oil-based cements are eugenol-free. These cements have a higher film thickness than permanent cements and much lower mechanical properties.
Although members of the profession are not in unanimous agreement regarding the purposes of each cement or the necessity for all the types, they are available to the dental profession and have been employed principally in the ways listed. Box 20-1 shows a classification of dental cements based on their chief chemical ingredients and application.
CHARACTERISTIC PROPERTIES OF DENTAL CEMENTS
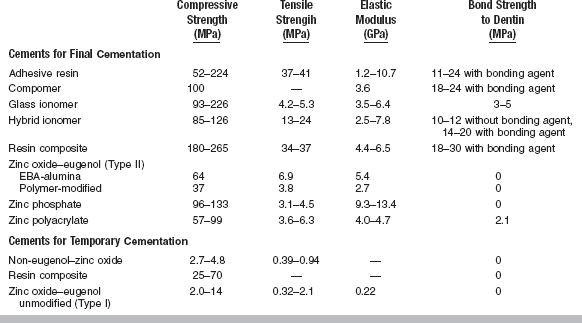
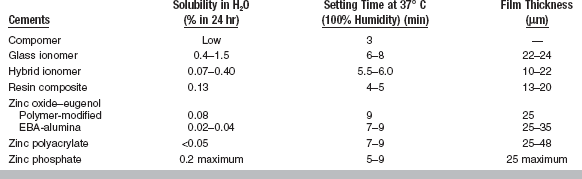
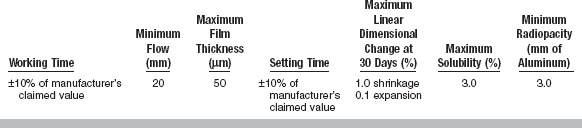
All cements exhibit certain properties during setting. The more important properties are included in ANSI/ADA Specification No. 96 (ISO 9917) for dental water-based cements. A summary of these requirements is given in Table 20-1. Selected mechanical and physical properties of all types of luting cements are listed in Tables 20-2 and 20-3.
TABLE 20-1
Specification Requirenments for Dental Water-Based Cements
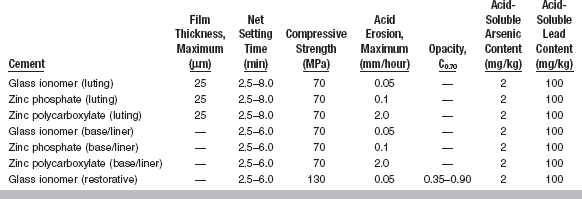
Modified from ANSI/ADA Specification No. 96 for dental water-based cements.
FILM THICKNESS AND CONSISTENCY
The film thickness of the cement greatly determines the adaptation of the restoration to the tooth. The retention may also be influenced by the film thickness. ANSI/ADA Specification No. 96 has requirements for cements designed for the seating of precision appliances. The maximum film thickness is 25 μm. The heavier the consistency, the greater the film thickness (Fig. 20-1) and the less complete the seating of the restoration. The ultimate film thickness that a well-mixed, nongranular cement attains depends first on the particle size of the powder, the concentration of the powder in the liquid, the viscosity of the liquid, and the consistency of the cement. The film thickness also varies with the amount of force and the manner in which this force is applied to a restoration during cementation. The type of restoration being cemented influences the ease of the cement escaping from around the margins of the restoration. A full-crown cast alloy presents the greatest problem in obtaining maximum displacement of the cement.
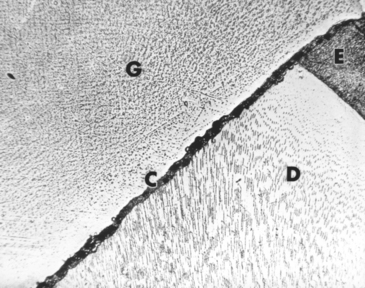
FIGURE 20-1 Cement film between gold inlay and tooth. G, Gold inlay; C, cement; D, dentin; E, enamel.
Obviously, the consistency of the cement to be used in the cementation of a casting is critical. For example, in a powder/liquid type of cement, an increased amount of powder incorporated into the liquid will increase the consistency of the cement mass. Heavier-than-normal inlay-seating consistencies of cement are more difficult to express from under a restoration, and incomplete seating of the inlay or crown may result from their use. The operator must frequently test each mass as the end of the mixing time is approached.
VISCOSITY
The consistency of cements can be quantified by measuring viscosity. Increases in temperature and time have both been shown to increase the viscosity of some cements. The rapid increase in viscosity with time demonstrates the need for prompt cementation after the completion of mixing to take advantage of the lower viscosity of the cement. Delays in cementation can result in considerably larger values of film thickness and insufficient seating of the restoration. For resin cements, viscosity can vary widely, and in fact, some products come with a choice of different viscosities.
SETTING TIME
Of equal importance to the viscosity of the cement is its setting time. A sufficient period of time must be available after mixing to seat and finally adapt the margins of a restoration, to seat and adjust a series of orthodontic bands, or to properly contour a base or provisional restoration. Adequate working time is expressed by net setting time, which, as determined by ANSI/ADA Specification No. 96 and based on a luting consistency, is between 2.5 and 8 minutes at a body temperature of 37° C. The setting time test measures the time at which the cement is sufficiently hard to resist indentation by a standard indenter. The net setting time should occur within 2.5 to 8 minutes so that final finishing procedures associated with the restoration can occur. The first 60 to 90 seconds are consumed by mixing the cement, so the net setting time is the time elapsed between the end of mixing and the time of setting, as measured by resistance to a standard indentor. One advantage of light-cured resin cements is the fact that there is virtually an unlimited setting time with low ambient lighting prior to light-curing. Table 20-3 shows approximate setting times of many representative cements.
STRENGTH
ANSI/ADA Specification No. 96 (ISO 9917) stipulates that the standard luting consistency of a dental cement must exhibit a minimum 24-hour compressive strength of 70 MPa. The compressive strength of many types of cements are shown in Table 20-2.
SOLUBILITY
Solubility in water and oral fluids is also an important consideration in cement properties. In general, water-based cements are more soluble than resin- or oil-based cements. ANSI/ADA Specification No. 96 allows a maximum rate of acid erosion, which is variable for the different types of cements, when a cement is subjected to lactic acid erosion by an impinging jet technique. Table 20-1 specifies maximum acid erosion levels for three water-based cements. This specification also sets limits on the acid-soluble arsenic content and lead content (see Table 20-1).
WATER-BASED CEMENTS
Composition
Glass ionomer cements are usually supplied as a powder and a liquid. Several products are encapsulated. The liquid typically is a 47.5% solution of 2:1 polyacrylic acid/itaconic acid copolymer (average molecular weight 10,000) in water. The itaconic acid reduces the viscosity of the liquid and inhibits gelation caused by intermolecular hydrogen bonding; D(+) tartaric acid (5%, the optically active isomer) in the liquid serves as an accelerator by facilitating the extraction of ions from the glass powder.
The powder of glass ionomer cement is a calcium fluoroaluminosilicate glass with a formula of
The nominal composition of the glass is listed in Table 20-4. The maximum grain size of the powder appears to be between 13 and 19 μm. The powder is described as an ion-leachable glass that is susceptible to acid attack when the Si/Al atomic ratio is less than 2:1. Barium glass or zinc oxide may be added to some powders to provide radiopacity.
TABLE 20-4
Nominal Composition of Calcium Fluoroaluminosilicate Glass Used in Powder of Glass lonomer Cement

Adapted from Prosser HJ, Richards CP, Wilson AD: J Biomed Mater Res 16:431, 1982.
In some products the polyacrylic acid is coated on the powder. The liquids of these products may be water or a dilute solution of tartaric acid in water.
Setting Reaction
The setting reaction is an acid-base reaction between the acidic polyelectrolyte and the aluminosilicate glass, as diagrammed here:
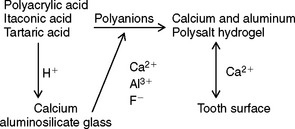
The polyacid attacks the glass to release cations and fluoride ions. These ions, probably metal fluoride complexes, react with the polyanions to form a salt gel matrix. The Al3+ ions appear to be site bound, resulting in a matrix resistant to flow, unlike the zinc polyacrylate matrix. During the initial setting reaction in the first 3 hours, calcium ions react with the polycarboxylate chains. Subsequently, the trivalent aluminum ions react for at least 48 hours. Between 20% and 30% of the glass is decomposed by proton attack. The fluoride and phosphate ions form insoluble salts and complexes. The sodium ions form a silica gel. The structure of the fully set cement is a composite of glass particles surrounded by silica gel in a matrix of polyanions cross-linked by ionic bridges. Within the matrix are small particles of silica gel containing fluoride crystallites.
Glass ionomer cements bond chemically to enamel and dentin during the setting process. The mechanism of bonding appears to involve an ionic interaction with calcium and phosphate ions from the surface of the enamel or dentin. Bonding is more effective with a cleaned surface provided the cleansing process does not remove an excessive amount of calcium ions. Treating dentin with an acidic conditioner followed by a dilute solution of ferric chloride improves the bonding. The cleansing agent removes the smear layer of dentin while the Fe3+ ions are deposited and increase the ionic interaction between the cement and dentin.
Manipulation
Glass ionomer cements mixed with the more viscous carboxylic acid liquids have a powder/liquid ratio of 1.3:1 to 1.35:1, whereas those mixed with water or a liquid with a consistency like that of water have a powder/liquid ratio of 3.3:1 to 3.4:1. The powder and liquid are dispensed onto a paper or glass slab. The powder is divided into two equal portions. The first portion is incorporated into the liquid with a stiff spatula before the second portion is added. The mixing time is 30 to 60 seconds. Encapsulated products are typically mixed for 10 seconds in a mechanical mixer and dispensed directly onto the tooth and restoration. The cement must be used immediately because the working time after mixing is about 2 minutes at room temperature (23° C). An extension of the working time to 9 minutes can be achieved by mixing on a cold slab (3° C), but because a reduction in compressive strength and in the modulus of elasticity is observed, this technique is not recommended. Do not use the cement once a “skin” forms on the surface or when the viscosity increases noticeably.
Glass ionomer cements are very sensitive to contact with water during setting. The field must be isolated completely. Once the cement has achieved its initial set (about 7 minutes), coat the cement margins with the coating agent supplied with the cement.
Properties
Requirements of ANSI/ADA Specification No. 96 (ISO 9917) for glass ionomer cements used as cements, bases, and restorative materials are given in Table 20-1. As shown in Table 20-3, the film thickness of glass ionomer cements is suitable for cementation, slightly less than 25 μm. Glass ionomer cements set within 6 to 8 minutes from the start of mixing. The setting can be slowed when the cement is mixed on a cold slab, but this technique has an adverse effect on the strength.
The 24-hour compressive strength of glass ionomer cements ranges from 90 to 230 MPa. Values of tensile strength are much lower than for resin-based cements because of the brittle nature of glass ionomers. Glass ionomer cements demonstrate brittle failure in the diametral compression test. The elastic modulus of glass ionomer cements is comparable to composite resin and resin-modified glass ionomer cements. The rigidity of glass ionomer cement is improved by the glass particles and the ionic nature of the bonding between polymer chains.
The compressive strength of glass ionomer cements increases between 24 hours and 1 year. A glass ionomer cement formulated as a filling material showed an increase from 160 to 280 MPa over this period. The strength of glass ionomer cements improves more rapidly when the cement is isolated from moisture during its early life.
Glass ionomer cements bond to dentin with values of tensile bond strength reported between 1 and 3 MPa. The bond strength of glass ionomer cements to dentin is somewhat lower than that of zinc polyacrylate cements, perhaps because of the sensitivity of glass ionomer cements to moisture during setting. The bond strength has been improved by treating the dentin with an acidic conditioner followed by an application of a dilute aqueous solution of FeCl3. Glass ionomer cements bond well to enamel, stainless steel, tin oxide–plated platinum, and gold alloy.
Values of solubility of glass ionomer cements as measured in water are substantially higher than those measured for other cements (see Table 20-3). However, when these cements are tested in acid (0.001 N lactic acid), the values are quite low compared with values for other water-based cements. The rankings determined by solubility tests in acid correlate well with clinical evaluations.
Biological evaluations of glass ionomer cements have been done by tissue culture and animal tests. The culture cells showed a weaker reaction to glass ionomer cement than to zinc oxide–eugenol (ZOE) or zinc polyacrylate cements. Pulp tissue reactions of monkeys tested in vivo showed no difference between glass ionomer and ZOE cements. These reactions have been described as mild.
Glass ionomer luting cements may cause prolonged hypersensitivity, varying from mild to severe. Microleakage has been suggested as an explanation, but a recent study showed no increase in bacterial counts 56 days after cementation of crowns with a glass ionomer cement. These cements may be bacteriostatic or bactericidal, however, because of fluoride release. Good isolation appears essential when glass ionomer cements are used. The use of the proper powder/liquid ratio and the application of a calcium hydroxide base in areas closest to the pulp are recommended.
Applications
Glass ionomer cements are used primarily for permanent cementation, as a base, and as a Class 5 filling material (see Chapter 8). The cement has been evaluated as a pit and fissure sealant and as an endodontic sealer. The sensitivity of the cement to moisture and desiccation may minimize its use in these latter applications. Glass ionomer cements are being used clinically for cementation of orthodontic bands because of their ability to minimize decalcification of enamel by means of fluoride release during orthodontic treatment.
RESIN-MODIFIED GLASS IONOMER CEMENT
Self-cured and light-cured resin-modified glass ionomers (or hybrid ionomers) are available as a powder-liquid, paste-paste, or encapsulated unit dose ampules for cementation. Resin-modified glass ionomers are also used as restorative materials (described in Chapter 8).
Composition
One self-cured resin-modified glass ionomer cement powder contains a radiopaque, fluoroaluminosilicate glass and a microencapsulated potassium persulfate and ascorbic acid catalyst system. The liquid is an aqueous solution of polycarboxylic acid modified with pendant methacrylate groups. It also contains 2-hydroxyethylmethacrylate (HEMA) and tartaric acid. Another self-cured cement contains a mixture of fluoroaluminosilicate and borosilicate glasses in the powder. Its liquid is a complex monomer containing carboxylic acid groups that can undergo an acid-base reaction with glass and vinyl groups that can polymerize when chemically activated. One light-cured resin-modified glass ionomer cement contains fluoroaluminosilicate glass in the powder and a copolymer of acrylic and maleic acids, HEMA, water, camphorquinone, and an activator in the liquid.
Setting Reaction
The setting reaction of resin-modified glass ionomer cement comprises two different mechanisms. The first is an acid-base reaction as described in the previous section. The second mechanism is either a light-cured or self-cured polymerization reaction of the pendant methacrylate groups. Therefore, two types of bonding to tooth structure occur—an ionic bond and a hybrid layer bond.
Manipulation
The powder is fluffed before dispensing. The liquid is dispensed by keeping the vial vertical to the mixing pad. For one product, the powder/liquid ratio is 1.6 g of powder to 1.0 g of liquid, and the powder is incorporated into the liquid within 30 seconds to give a mousse-like consistency. The working time is 2.5 minutes. The cement is applied to a clean, dry tooth that is not desiccated. Some products recommend the use of a conditioner for enhanced bonding to dentin. No coating agent is needed. HEMA is a known contact allergen; therefore, use of protective gloves and a no-touch technique are recommended.
Properties
Requirements for light-activated cements, which are water-based and set by multiple reactions, including an acid-base reaction and polymerization (Type I), and by cements that set only after light-activation (Type II), are described by ANSI/ADA Specification No. 96 (ISO 9917, Part 2). Properties for liners and bases and restoratives are given in Table 20-5.
TABLE 20-5
Specification Requirements for Light-activated Dental Water-based Cements
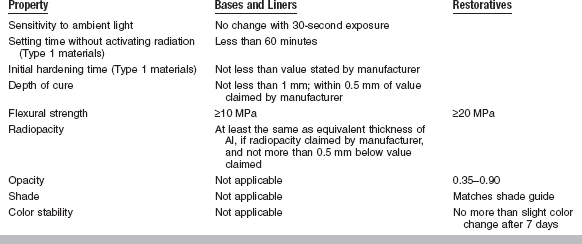
Modified from ANSI/ADA Specification No. 96 for dental water-based cements—Part 2: Light-activated cements. Type 1 cements are light cured but also set in absence of activating light; Type 2 cements require light activation.
The compressive and tensile strengths of resin-modified glass ionomer cements are similar to those of glass ionomer cements (see Table 20-2). The fracture toughness is higher than that of other water-based cements but lower than composite cements. The bond strength to moist dentin ranges from 10 to 14 MPa and is much higher than that of most water-based cements. Resin-modified glass ionomer cements have very low solubility when tested by lactic acid erosion. Water sorption is higher than for resin cements. Delayed fracture of ceramic restorations cemented with resin-modified glass ionomer cements has been reported. Recently, some resin-modified glass ionomer cements have been modified to have less water sorption. Fluoride release and rechargeability are similar to glass ionomer cements. The early pH is about 3.5 and gradually rises. Clinical experience indicates minimal postoperative sensitivity.
Applications
Self-cured resin-modified glass ionomer cements are indicated for permanent cementation of ceramic-metal crowns; bridges; metal inlays, onlays, and crowns; postcementation; and luting of orthodontic appliances. Additional uses include adhesive liners for amalgam, bases, provisional restorations, and cementation of specific ceramic restorations. Light-cured resin-modified glass ionomer cements are used primarily for liners and bases. Restorative applications of light-cured resin-modified glass ionomer cements are discussed in Chapter 8. One light-cured product is recommended for direct bonding of orthodontic brackets and bands.
ZINC POLYACRYLATE CEMENT
Zinc polyacrylate cements (or zinc polycarboxylate) are supplied as a powder and a liquid or as a powder that is mixed with water. The liquid is a water solution of polyacrylic acid, the formula being:
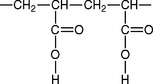
Most commercial liquids are supplied as a 32% to 42% solution of polyacrylic acid, having a molecular weight of 25,000 to 50,000. The manufacturer controls the viscosity of the cement liquid by varying the molecular weight of the polymer or by adjusting the pH by adding sodium hydroxide. Itaconic and tartaric acids may be present to stabilize the liquid, which can gel on extended storage.
The cement powder is essentially zinc oxide and magnesium oxide that have been sintered and ground to reduce the reactivity of the zinc oxide. The cement powder that is mixed with water contains 15% to 18% polyacrylic acid coated on the oxide particles.
Setting Reaction
The set cement is a zinc polyacrylate ionic gel matrix that unites unreacted zinc oxide particles. The gel is bound to the polyanion chains by electrostatic interactions rather than by stronger specific ion binding. The matrix appears to be amorphous. The setting reaction can be retarded by a cool environment or accelerated by a warm environment.
Manipulation
The cements supplied with the polyacrylic acid in the liquid are usually mixed at a powder/liquid ratio of 1:1 to 2:1. The mixed cement is pseudoplastic; that is, the viscosity decreases as the shear rate increases, or in other terms, the flow increases as spatulation increases or as force is placed on the material. The correct consistency is found in a mix that is viscous but that will flow back under its own weight when drawn up with a spatula.
Dispense the liquid immediately before mixing to prevent evaporation of water and subsequent thickening. A nonabsorptive surface, such as a glass slab or treated paper, will keep all the liquid available for the reaction and facilitate spatulation. Mix polyacrylate cements within 30 to 60 seconds, with half to all of the powder incorporated at once to provide the maximum length of working time (typically 2.5 to 6 minutes). Extend the working time to 10 to 15 minutes by mixing on a glass slab chilled to 4° C. The strength of the mixed cement is not compromised by this technique. Some manufacturers supply the cement as a capsulated powder-liquid system for mixing in a mechanical mixer.
Polyacrylate cements have been used to cement metal inlays and crowns and to make bases. Apply the cement to clean cavity walls that are well isolated in a dry field. Use the mixed cement only as long as it appears glossy on the surface. Once the surface becomes dull, the cement develops stringiness and the film thickness becomes too great to seat a casting completely.
Properties
ANSI/ADA Specification No. 96 establishes maximum values of setting time, film thickness, acid erosion, and arsenic and lead content, and a minimum value of compressive strength for zinc polyacrylate cement. A summary of these requirements is given in Table 20-1.
As shown in Table 20-3, setting of the zinc polyacrylate cements usually occurs within 7 to 9 minutes from the start of mixing.
The film thickness of zinc polyacrylate cement is the highest of any of the water-based cements and therefore is sometimes difficult to completely seat a retentive, well-fitting restoration.
The compressive strength test excludes materials that have a compressive strength of less than 70 MPa. Clinical studies have shown that cements of this strength or greater will satisfactorily retain cast alloys with a good fit.
The 24-hour compressive strength of polyacrylate cements for luting is lower than any other water-based cements (see Table 20-2). However, zinc polyacrylate cement is higher than zinc phosphate cement in tensile strength. In the diametral compression test, zinc polyacrylate cement specimens deform somewhat before breaking. The deformation results in a higher load before fracture is recorded than would occur if brittle fracture occurred. The modulus of elasticity of the zinc polyacrylate cements is about one third that of the zinc phosphate cements mixed to a luting consistency.
An interesting feature of polyacrylate cement is its bonding to enamel and dentin, which is attributed to the ability of the carboxylate groups in the polymer molecule to chelate to calcium. The bond strength to enamel has been reported to be from 3.4 to 13 MPa, and the bond strength to dentin has been found to be 2.1 MPa. Optimum bonding, however, requires cleaned tooth surfaces. The bonding of the polyacrylate cements to gold casting alloy is likewise highly dependent on surface preparation. Sandblasting or electrolytic etching of the gold alloy surface is necessary to achieve optimum bonding. Clinical studies have not demonstrated improved retention of crowns and bridges as a result of cementation with polyacrylate cements, however.
Because of the adhesion of polyacrylate cements to enamel, the cements were used at one time for direct bonding of orthodontic brackets to teeth. Presently, direct bonding is accomplished with resin cements.
Solubility in water at 1 day varies from 0.12% to 0.25% for typical zinc polyacrylate cements. One cement tested increased in solubility from 0.25% at 1 day to 0.60% at 1 month in water. Other cements were not affected by longer-term storage. ANSI/ADA Specification No. 96 specifies the maximum rate of acid erosion of zinc polyacrylate cements as 2.0 mm/hour.
The zinc polyacrylate cements show a linear contraction when setting at 37° C. The amount of contraction varies from 1% for a wet specimen at 1 day to 6% for a dry specimen at 14 days.
Zinc polyacrylate cements are slightly more acidic than zinc phosphate cements when first mixed, but the acid is only weakly dissociated, and penetration of the high-molecular-weight polymer molecules toward pulpal tissue is minimal. Histological reactions to polyacrylate cements appear similar to those of ZOE cements, although the production of reparative dentin under the polyacrylate cements is more evident.
ZINC PHOSPHATE CEMENT
Zinc phosphate cement is a traditional crown and bridge cement used for alloy restorations. It is supplied as a powder and a liquid, both of which are carefully compounded to react with one another during mixing to develop a mass of cement possessing desirable physical characteristics.
Composition
Powder: The principal ingredient of the zinc phosphate cement powder is zinc oxide. Magnesium oxide, silicon dioxide, bismuth trioxide, and other minor ingredients are used in some products to alter the working characteristics and final properties of the mixed cement. A typical formulation of a zinc phosphate cement powder and liquid is shown in Table 20-6. The magnesium oxide, usually in quantities of about 10%, is added to the zinc oxide to reduce the temperature of the calcination process. The silicon dioxide is an inactive filler in the powder and during manufacture aids in the calcination process. Although the bismuth trioxide is believed to impart a smoothness to the freshly mixed cement mass, in large amounts it may also lengthen the setting time. Tannin fluoride may be added to provide a source of fluoride ions in some products.
TABLE 20-6
Typical Composition of Zinc Phosphate Cement Powder and Liquid

Adapted from Paffenbarger GC, Sweeney WT, Issacs A: J Am Dent Assoc 20:1960, 1933.
The ingredients of the powder are heated together at temperatures ranging from 1000°to 1300° C for 4 to 8 hours or longer, depending on the temperature. Calcination results in a fused or sintered mass. The mass is then ground and pulverized to a fine powder, which is sieved to recover selected particle sizes. The degree of calcination, fineness of particle size, and composition determine the reactivity of the powder with the liquid.
Liquid: Zinc phosphate cement liquids are produced by adding aluminum and sometimes zinc, or their compounds, to a solution of orthophosphoric acid. Although the original acid solution contains about 85% phosphoric acid and is a syrupy fluid, the resulting cement liquid usually contains about one-third water, as shown in Table 20-6. The partial neutralization of the phosphoric acid by the aluminum and zinc tempers the reactivity of the liquid and is described as buffering. This reduced rate of reaction helps establish a smooth, nongranular, workable cement mass during the mixing procedure. The zinc phosphate cement liquid is adjusted by both partial neutralizing or buffering and dilution so it reacts with its powder to produce a cement mass with proper setting time and mechanical qualities.
Setting Reaction
When an excess of zinc phosphate cement powder is brought into contact with the liquid to begin the cement mix, wetting occurs and a chemical reaction is initiated. The surface of the alkaline powder is dissolved by the acid liquid, resulting in an exothermic reaction.
The set zinc phosphate cement is essentially a hydrated amorphous network of zinc phosphate that surrounds incompletely dissolved particles of zinc oxide. This amorphous phase is extremely porous. There is no evidence that the magnesium oxide present in the powder reacts with the phosphoric acid.
Manipulation
The manner in which the reaction between the zinc phosphate cement powder and liquid is permitted to occur determines to a large extent the working characteristics and properties of the cement mass. Incorporate the proper amount of powder into the liquid slowly on a cool slab (about 21° C) to attain the desired consistency of cement.
A properly cooled, thick glass slab will dissipate the heat of the reaction. Should a rapid reaction occur, ample working time would not be available for proper manipulation of the cement before hardening or setting occurs. The mixing slab temperature should be low enough to effectively cool the cement mass but must not be below the dew point. A temperature of 18° to 24° C is indicated when room humidity permits.
The amount of powder that can be incorporated into a given quantity of liquid greatly determines the properties of the mixed mass of cement. Because an increase in the ratio of powder to liquid generally provides more desirable properties, incorporate as much powder as possible to obtain a particular consistency.
By initially incorporating small portions of powder into the liquid, minimal heat is liberated and easily dissipated. The heat of the reaction is most effectively dissipated when the cement is mixed over a large area of the cooled slab. Use a relatively long, narrow-bladed stainless steel spatula to spread the cement across this large area to control the temperature of the mass and its setting time.
During the neutralization of the liquid by the powder, the temperature of the mixing site is inversely proportional to the time consumed in mixing. Thus, if a large volume of powder is carried to the liquid all at once rather than spatulated over a large area of the slab for a sufficient time, the temperature at the site of the reaction becomes higher. This temperature rise speeds the reaction and hinders control over the consistency. In this case the consistency of the mass is achieved by the rapid approach of the initial setting rather than by the establishment of a higher powder/liquid ratio under more ideal mixing conditions.
During the middle of the mixing period, larger amounts of powder may be incorporated to further saturate the liquid with the newly forming complex zinc phosphates. The quantity of unreacted acid is less at this time because of the prior neutralization gained from initially adding small increments of powder. The amount of heat liberated will likewise be less, and it can be dissipated adequately by the cooled slab.
Finally, smaller increments of powder are again incorporated, so the desired ultimate consistency of the cement is not exceeded. Thus, the mixing procedure begins and ends with small increments, first to achieve slow neutralization of the liquid with the attendant control of the reaction and last to gain a critical consistency.
Depending on the product, 60 to 90 seconds of mixing appears adequate to accomplish a proper zinc phosphate cementing mass. When the mixing time is unduly long, the cementing mass may be ultimately weakened by the breaking down of the matrix because it tends to form and bind the undissolved powder particles together.
Properties
Zinc phosphate cements exhibit certain properties during setting and when hardened. The more important of the properties are included in ANSI/ADA Specification No. 96 (ISO 9917) for dental water-based cements. A summary of these requirements is given in Table 20-1.
Selected properties of a typical zinc phosphate cement and of other luting materials are listed in Tables 20-2 and 20-3.
Two consistencies for zinc phosphate cement, termed luting and base, are in general use. A third consistency, which lies midway between the inlay seating and the cement base, is used for the retention of orthodontic bands and has been termed a band-seating consistency.
The luting consistency of zinc phosphate cement is used to retain alloy restorations. The final consistency will be fluid, yet the cement will string up from the slab on the spatula about 2 to 3 cm as the spatula is lifted away from the mass.
A heavy, puttylike consistency of zinc phosphate cement can be used as a thermal and chemical insulating barrier over thin dentin and as a high-strength base. The cement base or filling consistency is achieved when using a powder/liquid ratio higher than that used for the luting or band-seating consistency.
Although the viscosity of zinc phosphate cement is very low, delays in cementation can result in considerably larger values of film thickness and insufficient seating of the restoration.
Several factors influence the setting time and other properties of zinc phosphate cement (Table 20-7). Although the manufacturer initially adjusts the setting time, for example, improper handling of the powder and liquid can greatly modify the setting time. Anything that tends to speed the rate of reaction will shorten the setting time.
TABLE 20-7
Effects of Manipulative Variables on Selected Properties of Zinc Phosphate Cement
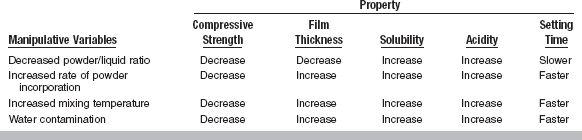
*Water contamination should not be confused with water incorporated in the frozen slab method.
From Craig RG, Powers JM, Wataha JC: Dental materials: properties and manipulation, ed 8, St Louis, 2004, Mosby.
The strength of zinc phosphate cement is influenced by the initial powder and liquid composition, the powder/liquid ratio and manner of mixing, and the handling of the cement during its placement. The compressive strength of zinc phosphate cement develops rapidly, with the luting consistency reaching at least two thirds of its final strength within 1 hour.
A proper mixing technique ensures a higher powder/liquid ratio for the consistency of cement desired, increasing the compressive strength of the cement mass. A cement base consistency, when properly achieved, offers greater strength than the luting consistency does. The maximum powder/liquid ratio is reached, however, when further amounts of powder do not increase the strength but may in fact decrease it because of the presence of excess unattached powder. For this reason a mass of mixed cement should evenly incorporate the powder throughout the mix.
Wear, abrasion, and attack of food decomposition products accelerate the disintegration of zinc phosphate cements within the oral cavity. Greater resistance to solution and disintegration is obtained by increasing the powder/liquid ratio. A thicker mix of cement therefore exhibits less solubility and disintegration than a thinner mix.
The difference between the resistance to abrasive and chemical attack intraorally and the passive resistance to solution and disintegration in distilled water causes a different clinical observation between zinc phosphate and other cements. This lack of correlation has been demonstrated in a clinical study in which the order of solubility of cements tested in the mouth was, from least to most soluble, glass ionomer, zinc phosphate, ZOE reinforced with ethoxybenzoic acid, and polyacrylate cements. Laboratory tests in distilled water indicate that glass ionomer cements are the most soluble and that polyacrylate, ZOE, and zinc phosphate cements are the least soluble.
During the formation of zinc phosphate cement, the union of the zinc oxide powder with the phosphoric acid liquid is accompanied by a change in pH. In the early manipulative stage this increase in pH is relatively rapid, with a standard mix reaching a pH of 4.2 within 3 minutes after mixing is started. At the end of 1 hour this value increases to about 6 and is nearly neutral at 48 hours.
Investigation has shown that the initial acidity of zinc phosphate cement at the time of placement into a tooth may excite a pulpal response, especially where only a thin layer of dentin exists between the cement and the pulp. In a normal, healthy tooth this response may be entirely reversible, whereas in a tooth whose pulp has already been put under stress from other trauma the response may be irreversible, with pulp death ensuing. When zinc phosphate cement is to be used, exercise precaution in a deep cavity to protect the nearby pulp tissue from further trauma by the initial acidity of the cement. Such precautions include the use of resinous, film-forming, cavity varnishes; calcium hydroxide and zinc oxide suspensions; ZOE or calcium hydroxide bases; and more recently, dentin bonding agents.
Gross decalcifications occasionally observed after the removal of orthodontic bands that have been retained in place with zinc phosphate cement are most probably attributable to the loss of the luting material between the band and the tooth, resulting in a favorable environment for bacterial action.
RESIN-BASED CEMENTS
COMPOSITES AND ADHESIVE RESINS
Cements based on resin composites are now used for cementation of crowns and conventional bridges; for bonding of esthetic ceramic and laboratoryprocessed composite single restorations and resin-bonded bridges to teeth; and for direct bonding of orthodontic brackets to enamel. Recently, composite cements have been developed for cementation of provisional restorations.
ISO 4049 for polymer-based filling, restorative, and luting materials (ANSI/ADA No. 27) describes the following three classes of composite cements:
Property requirements based on ISO 4049 can be summarized as follows:
Class 1, 2, 3: film thickness, maximum, 50 μm
Class 1, 3: working time, minimum, 60 seconds
Class 1, 3: setting time, maximum, 10 minutes
Class 2: depth of cure, minimum, 0.5 mm (opaque), 1.5 mm (others)
Cementation of Alloy Crowns and Bridges
Resin cements based on methyl methacrylate have been available since 1952 for use in cementation of inlays, crowns, and appliances. In the early 1970s, resin composite was introduced as a crown and bridge cement. Now, cementation of traditional alloy-based restorations is being accomplished with self-cured resin cement more frequently than ever before.
Composition and Setting Reaction: Self-cured, composite cements are typically hand- or automixed two-paste systems. One major component is a diacrylate oligomer diluted with lower-molecular-weight dimethacrylate monomers. The other major component is silanated silica or glass. The initiator-accelerator system is peroxide-amine.
One adhesive resin cement is a self-cured, powder-liquid system formulated with methacryloxyethylphenyl phosphate or 4-methacryloxyethyl-trimellitic anhydride (4-META). The 4-META cement is formulated with methyl methacrylate monomer and acrylic resin filler and is catalyzed by tributylborane. Another adhesive resin cement is a phosphonate cement supplied as a two-paste system, containing Bis-GMA (bis-glycidyldimethacrylate) resin and silanated quartz filler. The phosphonate molecule is very sensitive to oxygen, so a gel is provided to coat the margins of a restoration until setting has occurred. The phosphate end of the phosphonate reacts with calcium of the tooth or with a metal oxide. The double-bonded ends of both 4-META and phosphonate cements react with other double bonds when available. Setting of resin cements results from self- or light-cured polymerization of carbon-carbon double bonds.
Properties: Some properties of composite and adhesive resin cements are listed in Tables 20-2 and 20-3. A comparison of bond strengths of adhesive and conventional resin cements is given in Table 20-8. Adhesive resin cements have superior bonding to Type IV gold alloys. Other studies have shown that adhesive resin cements also bond to stainless steel, titanium, ceramic, and composite.
TABLE 20-8
Bond Strengths (MPa) of Adhesive and Conventional Resin Composite Cements to Various Substances
| Adhesive Resin Composite Cement | Conventional Resin Composite Cement (with Adhesive) | |
| Dentin | 18 | 19 |
| Ceramic | 23 | 26 |
| Low-gold alloy | 27 | 18 |
From O’Keefe KL, Trajtenberg CP, Powers JM: J Dent Res 79:436, 2000.
Applications: Adhesive resin cements and resin cements in conjunction with bonding agents are being used as cements for all types of alloy crowns, bridges, posts, and cores. Bond strengths of 14 MPa have been reported for silica-treated posts cemented with 4-META resin cement in extracted teeth. For one type of phosphonate-based adhesive resin cement, bond strengths of 30 MPa to stainless steel, 37 MPa to titanium, and 32 MPa to ceramic post materials have been reported.
Adhesive resin cements have also been used to bond denture teeth to acrylic dentures and metal removable partial denture frameworks.
New self-adhesive, self-curing, automixed resin cements are available for cementation of all types of restorations without the need for a separate bonding agent.
Bonding of Esthetic Restorations
The bonding of all-ceramic, tooth-colored crowns, veneers, inlays, and onlays became popular in the late 1980s when dentin bonding became predictable. Smear layer removal (total etch adhesive systems) or dissolution (self-etching adhesive systems) followed by infiltration of intertubar and intratubular dentin with hydrophilic monomers have made it possible to obtain long-term, predictable bonds of composite and ceramic to tooth structure (see Chapter 10). Dual-cured resin composite cements are ideal for bonding feldspathic, pressed leucite, or CAD/CAM-prepared leucite ceramic restorations or laboratory-processed composite inlays.
Light-cured composite cements are useful for bonding thin ceramic veneers where achieving adequate depth of cure is not a problem. Light-cured resin cements can be more color stable than dual- or self-cured cements. However, it has been shown that light transmission through porcelain can be as little as 2% to 3%, with a porcelain thicknesses ranging from 0.5 to 2 mm. Therefore, increasing the polymerization time may be prudent to ensure adequate polymerization.
Composition: Composite cements are microfilled or hybrid composites formulated primarily from Bis-GMA or urethane dimethacrylate resins and fumed silica or glass fillers (20% to 75% by weight) or both. Dual-cured cements come in a base-catalyst form and must be mixed before use. New automixed and dispensed dual-cured resin cements are easy to obtain the correct proportions of each. Light-cured composites are photoinitiated in the presence of a camphorquinone-amine system. They consist of a base paste only, with no mixing necessary, and usually provide a wide selection of shades, tints, and opaquers for anterior use.
Manipulation: Proper manipulation using careful isolation technique is critical to the success of a bonded ceramic or indirect composite restoration. Cementation with resin cement includes a series of critical steps.
For ceramic, the internal surface of the restoration must be etched with hydrofluoric acid gel and silanated. The tooth should be cleaned with pumice, isolated, and etched before placing the adhesive. Next, the prepared internal surface of the ceramic should be wet with unfilled resin, and the cement mixed (if dual-curing resin is used) and placed inside the restoration. After the restoration is completely seated, the cement is gently wiped from the margins, and the restoration is light-cured.
Figure 20-2, A, shows a patient with malposed and slightly discolored teeth Nos. 7 and 8 due to a traumatic accident. Figure 20-2, B, shows teeth Nos. 7 to 10 prepared to receive porcelain veneers. Figure 20-2, C, shows the postoperative veneer case for teeth Nos. 7 to 10. Adhesive bonding using resin cements have allowed for a much more conservative restoration of damaged anterior teeth compared to conventional cementation techniques that were available 20 years ago.
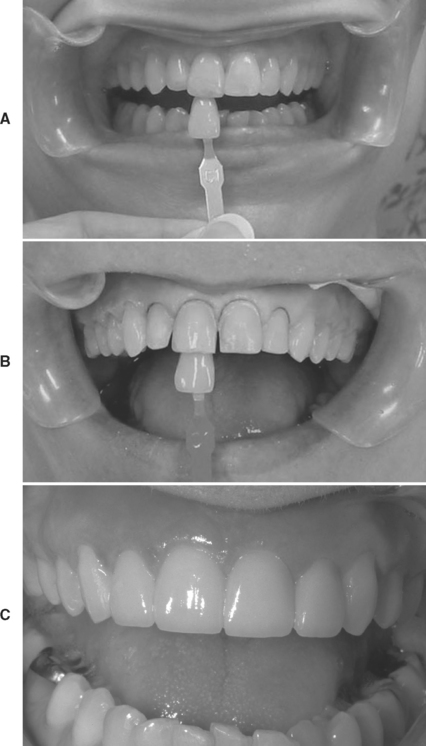
FIGURE 20-2 A, Post-trauma injury patient; teeth Nos. 7 and 8 are endodontically treated, tooth No. 8 has super-erupted, and teeth Nos. 7 to 10 have been chipped and built up with composite. B, Prepared teeth Nos. 7 to 10 with shade tab. C, Post-cementation of porcelain veneers for teeth Nos. 7 to 10.
The cementation procedure for indirect resin restorations is similar to cementation of ceramic. To prepare the internal surface of indirect resin, some manufacturers suggest applying silane because of the high percentage of glass filler, and some recommend placing an unfilled resin “activator” to wet the surface of the polymerized resin prior to placing the resin cement. All other aspects of the bonding process are the same for both substrates. Based on in vitro studies, resin cements bond well to postcured composite inlays. A summary of clinical tips for cementation of ceramic and indirect composite restorations with resin cements is shown in Box 20-2.
Properties: Compressive strengths of dual- and light-cured resin composite cements have been reported from 180 to 265 MPa (see Table 20-2). Viscosity has been measured subjectively to range from low to high. Film thicknesses on vented crowns range from 13 to 20 μm. The cements are radiopaque for use in the posterior portion of the mouth.
Some resin cements include glycerin-based, water-soluble try-in pastes to observe the color match before cementation. Research has shown that some try-in pastes match the color of the cured cement more closely than others. It has also been shown that rinsing the try-in paste from the restoration before cementation does not impair the bond strength of the final cemented restoration.
Studies have shown that there is the potential for incompatibility between adhesives and composites based on curing mode differences and on other chemical incompatibilities. Some adhesives may produce an acidic bonding surface, which can cause an incomplete polymerization of the resin cement at the bonding surface. It is prudent to use similar curing modes for adhesive and composite and to always follow recommendations by the manufacturer.
Cementation of Provisional Restorations
Composite cements for cementation of interim restorations were introduced in the 1990s. These cements are paste-paste systems, which can be dual- or light-cured. They are useful for cementation of interim restorations in the esthetic zone of the mouth, because they are tooth-colored and fairly translucent. They are easy to clean, and some release fluoride. Resin cementation of provisionals is useful when the final cement will also be resin because there is no eugenol present to potentially impair polymerization of the final cement.
Composite cements used for cementation of provisional restorations (25 to 70 MPa) have a substantially lower compressive strength than composite cements used for permanent cementation (180 to 265 MPa).
Resin-Metal Bonding
Bonding composite to the metal framework of a bridge and denture acrylic to a partial denture framework can be improved by the use of silica coatings. Presently there are three processes for applying silica to either noble or base-metal alloys. One method applies pyrogenic silica using a propane flame. Other methods use heat in an oven or ceramic blasting to coat the restoration or appliance. Bond strengths of composites to silica-coated Au-Pd or Ni-Cr-Be alloys range from 16 to 22 MPa. Silica coating of noble alloys eliminates the need for tin plating these alloys to improve adhesion of composites. The bond strength of denture acrylics to Ni-Cr-Be alloys range from 7 to 23 MPa when the alloy is treated with a silica coating or primed with adhesive resin cement. Recently, metal primers based on thiophosphate chemistry have been introduced as a treatment for resin-metal bonding.
Bonding of Orthodontic Brackets
Resin cements were evaluated for direct bonding of orthodontic brackets (without bands) in the late 1960s. Advances in acid etching of enamel substantially increased the popularity of the technique in the mid-1970s. Now resin composite cements have completely replaced acrylic resin cements for orthodontic bonding. Self-etching adhesives used in conjunction with resin composite cements have replaced the routine use of phosphoric acid to prepare the enamel surface for bonding. Composite cements are used with metal, plastic, or ceramic orthodontic brackets.
Composition and Setting: Composite cements are formulated from various diacrylate oligomers diluted with lower-molecular-weight dimethacrylate monomers and fillers of silica, glass, or colloidal silica. The highly filled cements typically contain silanated inorganic particles (more than 60% by weight). The slightly filled cements contain 28% colloidal silica. The initiator-accelerator systems of these composite cements depend on the mode of initiating the polymerization. Amine-cured systems include conventional two-paste products and one-step products. The more popular light-cured systems are polymerized by visible light.
Manipulation: Traditionally, the success of composite cements used for direct and indirect bonding was highly dependent on proper isolation and acid etching of the enamel. The acid-etching technique involved etching the tooth for 15 to 30 seconds with a solution of phosphoric acid, followed by rinsing and drying. If the enamel is contaminated, re-etching of the tooth was necessary. Acid etching of enamel is discussed in greater detail in Chapter 10. Recently, commercial self-etching adhesives have become available as an alternative to phosphoric acid etching for orthodontic bonding to enamel. These acidic primers are applied without rinsing, followed by placement of the composite cement and bracket.
Two-paste composite cements require mixing for 20 to 30 seconds before they are applied to the enamel and bracket base. A primer such as methyl methacrylate monomer in a solvent usually must be applied to a plastic bracket base. Sometimes a sealant formulated from an unfilled diacrylate is applied initially to the acid-etched enamel. The two-paste cements set several minutes after mixing.
The one-step (no-mix) cements require no mixing. A priming liquid is applied to the etched enamel, and the paste is applied to the bracket base. A plastic bracket may require a bracket primer. Polymerization is initiated when the bracket is placed on the primed tooth. The effect of film thickness on the polymerization of these cements has been investigated. Generally, there is a decrease in tensile bond strength as the thickness of one-step cements increases. Failures are characterized by incomplete polymerization of the resin. Bond strength of one-step cements decreases if the primer is exposed to a simulated oral environment for a minute or more, so bases should be placed promptly after the primer is applied to the teeth.
Light-cured composite cements are single-paste systems that require no mixing. The resin is applied to the tooth and bracket base, and polymerization is activated by the light source. A sealant may be used for bonding to the teeth, and a primer may be required for bonding to a plastic bracket. Recently, high-intensity, light-curing units (plasma-arc, LED) have been used in orthodontics to save time.
Properties: Two important properties of composite cements for orthodontic bonding are esthetics and bond strength to tooth structure and the bracket base.
Changes in color of composite cements can result from staining or from the formation of colored reaction products. After accelerated aging or exposure to a tea stain, the cements were darker and more chromatic. Exposure to the tea stain caused a greater change in color than the aging test.
The bond strength of composite cements to tooth structure appears clinically adequate if proper isolation and manipulative techniques are followed. Bonding to tooth structure results from the resin matrix penetrating into the etched areas of enamel.
Bonding to orthodontic bracket bases depends on the type of bracket base (metal, plastic, or ceramic) and the type of cement (hybrid ionomer, highly filled composite, or slightly filled composite), as shown in Table 20-9. Failures typically occur at the cement-base interface or, less often, within the cement or base. Bonding to plastic bases appears to be chemical, whereas bonding to metal and ceramic bases is mechanical. Failures at the cement-metal base interface are initiated at areas of stress concentration in the metal base, such as weld spots or damaged mesh (Fig. 20-3). Plastic brackets tend to fail at the wings rather than debonding in laboratory testing. Failure at the interface of the cement-ceramic bracket is influenced by the amount of penetration of resin into the retention areas of the base.
TABLE 20-9
Effect of Types of Cement and Bracket Base on Bond Strength (MPa) of Direct-Bonding Cements
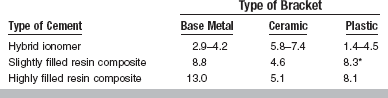
*With bracket primer.
Adapted from Buzzitta VAJ, Hallgren SE, Powers JM: Am J Orthod 81:87, 1982; de Pulido LG, Powers JM: Am J Orthod 83:124, 1983; and Blalock KA, Powers JM: Am J Orthod Dentofac Orthop 107:596, 1995.
FIGURE 20-3 Scanning electron photomicrograph of direct-bonding metal mesh base damaged by spot welds. (From Dickinson PT, Powers JM: Am J Orthod 78:630, 1980.)
Improved metal bases, including photographically etched and grooved types, have been tested with surface treatments such as silanation, etching, and activation. Etching of the grooved base was most effective in improving the bond strength. Studies of the new alumina and glass-ceramic brackets indicate that high bond strengths can be attained. Most clinical failures are attributed to breakage of the wings of the ceramic brackets.
Reconditioning of metal bases by thermal treatment, chemical treatment, and grinding with a green stone have been evaluated. Reconditioning caused a 20% to 56% decrease in bond strength of several composite cements to a mesh metal base.
COMPOMERS
Compomer cement is a resin-based cement indicated for cementation of cast alloy crowns and bridges, ceramic-metal crowns and bridges, and cast gold inlays and onlays. Cementation of all-ceramic crowns, inlays, onlays, and veneers, with some exceptions, is contraindicated. The cement should not be used as a core or filling material. Compomers are also known as poly acid- modified composites. A compomer cement was recently introduced for orthodontic bonding.
Composition
The cement powder contains strontium aluminum fluorosilicate glass, sodium fluoride, and self- and light-cured initiators. The liquid contains polymerizable methacrylate/carboxylic acid monomer, multifunctional acrylate/phosphate monomer, diacrylate monomer, and water.
Setting Reaction
Setting is the result of self- and light-cured polymerization. Once the cement comes into contact with oral fluids, an acid-base reaction may occur. The carboxylic acid groups contribute to the adhesive capability of the cement.
Manipulation
Dry the tooth to be cemented but do not desiccate. Tumble the powder before dispensing. Mix the powder and liquid rapidly for 30 seconds. Place the mixed cement in the crown only and then seat the crown. A gel state is reached after 1 minute, at which time the excess cement is removed with floss and a scaler. Light-cure the exposed margins to stabilize the restoration. Setting occurs 3 minutes after start of mix. Once set, compomer cement is very hard.
Properties
Compomer cement has high values of retention, bond strength, compressive strength, flexural strength, and fracture toughness (see Table 20-2). The cement has low solubility and sustained fluoride release.
OIL-BASED CEMENTS
ZINC OXIDE–EUGENOL AND NONEUGENOL CEMENTS
When certain types of zinc oxide are mixed with eugenol, the mix sets to a hard cement that is compatible with both the hard and soft tissues of the mouth. Cements of this type have been used extensively since the 1890s. Simple mixtures of these two materials do not have great strength when compared with water- or resin-based cements, and their use has been limited to situations in which strength is not important. Quite early it was found that they had a sedative effect on exposed dentin. For many years, ZOE cements have been used as provisional restorations, soft tissue packs in oral surgery and periodontics, and root canal sealers. Because eugenol acts as an inhibitor for free-radical polymerized materials, select other materials for provisional restorations when bonding of the permanent restoration is anticipated.
Noneugenol–zinc oxide cements are also available for temporary cementation. These cements are suitable for patients sensitive to eugenol.
Composition
A typical formula for a ZOE cement compounded as a provisional filling material is shown in Table 20-10. The powder is mainly zinc oxide, with added white rosin to reduce the brittleness of the set cement, zinc stearate as a plasticizer, and zinc acetate to improve the strength of the cement. The liquid is eugenol with olive oil as a plasticizer. Two compositional changes have been used to increase the strength of the cement for luting purposes. In one, methyl methacrylate polymer is added to the powder, and in the other, alumina (Al2O3) is added to the powder and ethoxybenzoic acid (EBA) to the liquid.
TABLE 20-10
Formula for a Typical Zinc Oxide–Eugenol Temporary Filling Cement

Adapted from Wallace DA, Hansen HL: J Am Dent Assoc 26:1536, 1933.
A typical polymer-reinforced cement has 80% zinc oxide and 20% poly(methyl methacrylate) in the powder and eugenol in the liquid. These cements are sufficiently strong for final cementation of fixed prostheses and are used also as cement bases and provisional restorations. A typical EBA-alumina-reinforced ZOE cement contains 70% zinc oxide and 30% alumina by weight in the powder. In some cases, rosin and copolymers may be added to reduce the brittleness and film thickness and improve the mixing qualities. The liquid of the EBA-alumina-reinforced cements contains 62.5% ortho-EBA by weight and 37.5% eugenol by weight. The compressive strength of a typical EBA cement is shown in Table 20-2.
The noneugenol–zinc oxide cements typically contain an aromatic oil and zinc oxide. Other ingredients may include olive oil, petroleum jelly, oleic acid, and beeswax.
Setting Reaction
The setting of ZOE cements is a chelation reaction in which an amorphous, zinc eugenolate is formed. In the setting reaction, shown at the end of this paragraph, two molecules of eugenol react with ZnO in the presence of water to form the chelate, zinc eugenolate. Excess zinc oxide is always used, so the set material consists of a matrix of amorphous zinc eugenolate that binds the unreacted zinc oxide particles together. The setting reaction is accelerated by increases in temperature or humidity. EBA also forms a chelate with zinc oxide, and its presence allows some crystalline zinc eugenolate to form, which provides additional strength. The reaction is not measurably exothermic, and the presence of moisture is essential for setting to occur.

Manipulation: Dispensing and Mixing Procedures
The unmodified ZOE and noneugenol–zinc oxide cements are typically two-paste systems. Equal lengths of each paste are dispensed and mixed to a uniform color.
For some cements used for temporary cementation or for provisional restorations, powder is often incorporated into a dispensed amount of liquid until a suitable consistency is achieved for the operation at hand. The dentist makes this determination from experience. A considerable amount of powder can be incorporated into the liquid by heavy spatulation with a stiff spatula. In general, the more powder incorporated, the stronger the cement and the more viscous the mixed cement.
Cements intended for final cementation of restorations carry manufacturers’ directions and measuring devices that are important to use. Because of the deceptive flow qualities of these cements, adding powder until the operator feels the mix is of suitable consistency for cementing a restoration will lead to a cement deficient in powder and a lowered strength in the set cement.
Characteristic Properties
The variety of compositions of the ZOE cements and the many uses to which they are applied make it difficult to write a specification for these cements. ANSI/ADA Specification No. 30 (ISO 3107) for dental ZOE cements and zinc oxide noneugenol cements gives standards for temporary cements, permanent cements, filling materials and bases, and cavity liners. It sets requirements for the general characteristics of the powders, liquids, and pastes used in these cements and for the important physical properties of setting time, compressive strength, disintegration, film thickness, and acid-soluble arsenic content, where these are applicable. The limiting values established for these properties for each type of cement are shown in Table 20-11.
TABLE 20-11
Specification Requirements for Zinc Oxide–Eugenol and Zinc Oxide–Non-Eugenol Cements
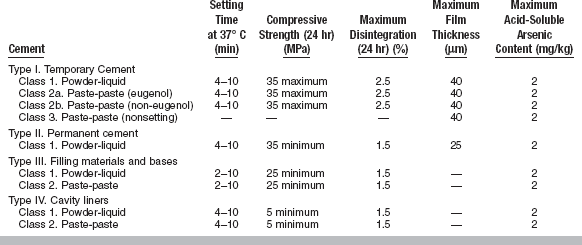
Modified from ANSI/ADA Specification No. 30 for dental zinc oxide–eugenol cements and zinc oxide–non-eugenol cements, 2000.
Film Thickness: Film thickness is an important factor in the complete seating of restorations at the time of cementation. The film thickness should be not more than 25 μm for cements used for permanent cementation and not more than 40 μm for cements used for temporary cementation, as determined by the specification test. This requirement is not applied to cements used for purposes other than cementation.
Setting Time: For all except two of the cements, a range of setting times from 4 to 10 minutes is required. For cements intended for filling materials and bases, the preference of some operators for a faster setting cement is recognized in the specification by extending the lower end of the range to 2 minutes.
Compressive Strength: A maximum value of 35 MPa is required for cements intended for temporary cementation. A minimum of 35 MPa is required for cements intended for permanent cementation and 25 MPa for filling materials and bases. Lining materials are required to have a minimum compressive strength of 5 MPa. Clinical studies have shown that for temporary cementing of restorations a variety of cements with compressive strengths varying from 1.4 to 21 MPa is desirable. The strength of a cement for temporary cementation is selected in relation to the retentive characteristics of the restoration and the expected problems of removing the restoration when the time arrives.
Disintegration: The disintegration of cement is generally regarded as less critical for cements used as provisional restorations or for temporary cementation. This is reflected in the maximum specification values for disintegration in 24 hours. A maximum value of 2.5% is acceptable for provisional cementing materials, but a value of 1.5% is required for the other cements. The test used is the amount of disintegration, measured by weight loss, which occurs in a disk of the cement immersed in distilled water for 24 hours. There is no close correlation between this test and the clinical behavior of these cements, and the test serves only to compare cements of known clinical acceptability with other products. It is a useful monitoring method for evaluating new products and ensuring quality control of manufacturing processes.
Applications
A range of ZOE and modified ZOE cements are suitable for many uses in restorative dentistry, and the practitioner should become familiar with each type and its application.
Temporary Cementation: Unmodified ZOE cements are also used as luting materials for provisional crowns and temporary cementation of metal restorations in crown and bridge prosthodontics. Laboratory studies have shown that the retention of metal restorations with unmodified cements is proportional to the compressive strength of the cements. A clinical study of the use of various unmodified cements for luting provisional crowns indicated that a cement with a compressive strength of 15 to 24 MPa was the most appropriate cement, based on (1) retention, (2) taste, (3) ease of removal, and (4) ease of cleaning. Another clinical study indicated that an unmodified ZOE cement with a compressive strength of 6.9 MPa was the most commonly used material for the temporary cementation of complete crown and bridge restorations.
The noneugenol–zinc oxide cements do not adhere as well to preformed metal crowns as the eugenol-containing cements, and they are slower setting. The noneugenol cements, however, do not soften provisional acrylic crowns.
Provisional Restorations: The EBA-alumina-modified cements have been tried as provisional restorations based on their physical properties. Clinical studies showed these cements were handled easily and had improved carvability, which prevented chipping during trimming, and that symptomatic teeth without pulp exposure showed no symptoms. The EBA-alumina-modified cements, despite their low solubility in water, disintegrated and wore excessively in the mouth. A thick mix, 2.6 g/0.4 mL of polymer-modified ZOE, was more serviceable than the EBA-alumina-modified type, and although some chipping was observed at the margins, all provisional restorations of ZOE were serviceable for 2 to 10 months of observation.
Bases: Materials having a compressive strength of 5.5 to 39 MPa are used as a cement base, and the strength reaches a maximum in about 12 to 15 minutes. The ZOE cements have the advantage that the thermal insulating properties of the cements are excellent and are approximately the same as those for human dentin. Bases are discussed later in this chapter.
Endodontic Sealers: Endodontic ZOE preparations have been used as a root canal sealer alone and with gutta-percha. There are two major groups of products based on ZOE cements—conventional and therapeutic sealers.
Composition and Setting: Conventional sealers are generally based on the formulas of Grossman or Rickert, as summarized in Table 20-12. The setting reaction occurs between zinc oxide and eugenol. Resins improve the mixing characteristics and retard setting. Radiopacity is improved by adding barium or bismuth salts or silver powder. The conventional sealers are used with gutta-percha points.
Therapeutic sealers are usually used without a core material and are formulated with ingredients such as iodoform, paraformaldehyde, or trioxymethylene, which may have therapeutic value. The use of these sealers is controversial.
ANSI/ADA Specification No. 57 (ISO 6876) covers materials used in endodontics within the tooth to seal the root canal space. The physical properties specified include working time, flow, film thickness, setting time, dimensional change following setting, solubility, and radiopacity. A summary of these requirements is given in Table 20-13.
Viscosity: The ability of a sealer to penetrate into irregularities and accessory canals has been termed flow, though viscosity is a more correct term. Viscosity has been shown by several different tests to decrease during the mixing procedure (an example of shear thinning). Values of viscosity range from 8 to 680,000 cp (centipoise).
Setting Time: The setting time of cements is measured by a penetration test and ranges from 15 minutes to 12 hours at mouth temperature. Sealers set much more rapidly at mouth temperature than at room temperature.
Film Thickness: Film thickness, measured as directed by ANSI/ADA Specification No. 57, is influenced by viscosity, setting time, and the size of filler particles in the cement. Lower values of film thickness are considered desirable for condensation of gutta-percha. Values range from 80 to 500 μm, depending on the testing load.
Compressive Strength: The strength of a sealer is an indication of its ability to support tooth structure weakened by the cleaning of the canal and its durability. Values range from 8 to 50 MPa.
Solubility: Solubility is an undesirable characteristic in a root canal sealer, because the process of dissolution can cause the sealer to release components that may be biologically incompatible. Solubility has been measured in water, and typically values range from 0.1% to 3.5%.
Radiopacity: Radiopacity is desirable, and a minimum value has been established at the equivalent of 3 mm of aluminum. Values of radiolucency range from 0.10 to 0.98 among various sealers and 0.78 for gutta-percha. These are relative values with lower numbers for more radiopaque materials.
Dimensional Change: Most root canal sealers shrink as a result of setting. This shrinkage affects the integrity of the bond between the sealer and the tooth or core material. Values of volume loss after 90 days in a capillary tube range from 0.7% to 5.0%.
Biological Properties: These properties have been studied by in vivo and in vitro tests of HeLa cells, human skin fibroblasts, and bovine pulp tissue; endodontic fillings in dogs, monkeys, and rats; and implants in tibias and in subcutaneous connective tissues of animals. Conventional ZOE sealers generally elicit mild to moderate reactions, whereas several of the therapeutic sealers elicit severe reactions.
Periodontal Management: Another variation of the ZOE cements has been necessitated by the special requirements imposed in the management of gingival tissues. This group of cements is used in two ways: (1) to mechanically displace soft tissue, and (2) to dress soft tissues after surgery. When these cements are used as a mechanical tissue pack, a thin consistency is incorporated into cotton fibers that are placed into the gingival sulcus. As a surgical dressing, this preparation affords greater comfort to the patient during eating, obtunds the tissue affected by surgery, promotes epithelial growth, and helps prevent the overgrowth of granulation tissue.
The setting times of these ZOE surgical cements must be quite long to facilitate the mixing of rather large quantities and to permit the proper placement and contouring of the dressing. Usually no accelerator is added to these materials. On placement of the packs in the mouth, the moisture and increased temperature tend to hasten the setting reaction. When mixed to a proper consistency, the cement must be soft enough to permit placement and contouring with gentle pressures, and yet firm enough to maintain the desired form.
These formulations generally have greater quantities of mineral, peanut, or almond oil present to increase plasticity over that of filling cements. Cotton fibers are often added to increase the strength and durability. In addition to the normal ingredients (zinc oxide, rosin, and eugenol), tannic acid is often added as a hemostatic agent and to decelerate the setting reaction. Aromatic oils and coloring agents may be incorporated to improve the taste and color of the dressing. Chlorhexidine has also been added as an antibacterial agent.
CAVITY VARNISHES
A cavity varnish is used to provide a barrier against the passage of irritants from cements or other restorative materials and to reduce the penetration of oral fluids at the restoration-tooth interface into the underlying dentin. Varnishes help reduce postoperative sensitivity when applied to dentinal surfaces under newly placed restorations. Cavity varnishes are rapidly being replaced by bonding agents. Cavity varnishes have no bond to tooth structure and should not be used with cements that use adhesives to increase bond strength both to tooth structure and to the restoration. Most commonly, cavity varnishes are used with zinc phosphate cement.
COMPOSITION
A cavity varnish is a solution of one or more resins from natural gums, synthetic resins, or rosin. The solvents that may be used to dissolve these materials are chloroform, alcohol, acetone, benzene, toluene, ethyl acetate, and amyl acetate. Medicinal agents such as chlorobutanol, thymol, and eugenol also have been added. The volatile solvents evaporate quickly when the varnish is applied to the prepared tooth surface, thus leaving a thin resin film. The addition of fluoride to cavity varnish has not been established as effective.
MANIPULATION
Varnish solutions are usually applied by means of a small cotton pledget. Use a gentle stream of air for drying, but take care to avoid forming ridges. Add a new layer only to a previously dried one. Two thin layers have been found to be more protective than one heavy layer. To prevent contamination of the cavity varnish, use a new cotton pledget for each application. Tightly cap varnish solutions immediately after use to minimize loss of solvent. Most varnishes are supplied with a separate bottle of pure solvent. This solvent may be used to keep the varnish from becoming too thick. Replace the loss from evaporation by adding solvent to keep the bottle at least half full by diluting the contents with the solvent.
PROPERTIES
Cavity varnishes reduce but do not prevent the passage of constituents of the phosphoric acid cements into underlying dentin. Variations in results and the mere reduction rather than the prevention of the passage of acid appear to be a result of pinpoint holes in the varnish film formed during volatilization of the organic solvent. Greater continuity of the dried varnish film is possible by the use of successive layers of thin varnish; this technique is more effective than using just one layer of thicker varnish.
Thin films of resinous cavity varnishes significantly reduce leakage around the margins and walls of metallic restorations. Although the effect of the cavity varnishes is not completely known, it can be hypothesized that this reduction of fluid penetration around cavity margins would minimize postoperative sensitivity.
Varnishes neither possess mechanical strength nor provide thermal insulation because of inadequate film thickness. Values of film thickness have been measured at between 1 and 40 μm for different commercial varnishes. Contact angles of varnishes on dentin range from 53 to 106 degrees. Improved integrity of a varnish film might be achieved by improvement in the spreading of the varnish on the tooth surface.
APPLICATIONS
Cavity varnishes are indicated for use on dentinal surfaces to minimize the penetration of acid from zinc phosphate cements. Cavity varnishes appear also to retard the penetration of discolored corrosion products from dental amalgam into dentin. Varnishes are not used under composite restorations, because bonding agents effectively seal the dentin tubules.
CAVITY LINERS
Cavity liners are two-paste calcium hydroxide or ZOE cements that set to a hard mass when mixed. These cements are commonly referred to as liners, intermediary bases, or pulp-capping agents (calcium hydroxide products only). Glass ionomer, resin-modified glass ionomer (hybrid ionomer), and compomer bases are discussed in the section on high-strength bases.
COMPOSITION AND CHEMISTRY OF SETTING
The base paste of a typical product contains calcium tungstate, tribasic calcium phosphate, and zinc oxide in glycol salicylate. The catalyst paste contains calcium hydroxide, zinc oxide, and zinc stearate in ethylene toluene sulfonamide. The ingredients responsible for setting are calcium hydroxide and a salicylate, which react to form an amorphous calcium disalicylate. Fillers such as calcium tungstate or barium sulfate provide radiopacity.
A light-cured calcium hydroxide liner consists of calcium hydroxide and barium sulfate dispersed in a urethane dimethacrylate resin.
Zinc Oxide and Eugenol Bases
These liners are nonmodified (Type IV) ZOE cements as described in ANSI/ADA Specification No. 30 (ISO 3107). They are typically two-paste systems in which the zinc oxide and eugenol are formulated with inert oils and fillers. The cement sets to a hard mass when mixed. The setting reaction is accelerated by moisture and an increase in temperature.
MANIPULATION
Calcium hydroxide and ZOE cavity liners are both supplied as two-paste systems. Equal lengths of the different-colored pastes are dispensed on a paper pad and then mixed to a uniform color.
PROPERTIES
Calcium hydroxide cements are used for lining deep cavities or for direct pulp capping. The antibacterial action of calcium hydroxide makes these cements useful in indirect pulp-capping procedures involving carious dentin. ZOE cements are used in deep cavities to retard penetration of acids and reduce possible discomfort to the pulp. Root canal sealers containing ZOE and brands that contain calcium hydroxide are used in endodontics.
Calcium Hydroxide Liners
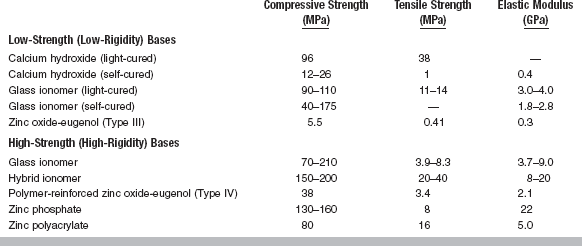
The important properties of these products are mechanical and thermal properties, solubility, and pH. Research has also shown that these products can create secondary dentin bridges when applied to direct pulp exposures and are commonly used in these instances. Calcium hydroxide (self-cured) liners have low values of tensile strength and compressive strength, or elastic modulus, compared with high-strength bases (Table 20-14). Although setting times vary between 2.5 and 5.5 minutes, compressive strengths of these cements continue to increase over a 24-hour period. For a group of five commercial products, compressive strengths ranged from 6.5 to 14.3 MPa at 10 minutes to from 9.8 to 26.8 MPa at 24 hours. The low elastic modulus of calcium hydroxide cavity liners restricts their usage to areas not critical to the support of restorations. Mechanical support should be provided by sound dentin or by a high-strength base. Calcium hydroxide liners are, however, considered strong enough to support the forces of condensation of amalgam.
Calcium hydroxide liners may provide some thermal insulation to the pulp if used in sufficiently thick layers. A thickness greater than 0.5 mm is not suggested. Practically, thermal protection should be provided by the overlying high-strength base or composite restoration.
The solubility of calcium hydroxide bases has been measured in several solvents for various periods of immersion and has been found to be significant. One product that was resistant to dissolution in water and in acid disintegrated when exposed to ether. Some solubility of the calcium hydroxide is necessary to achieve its therapeutic properties, although an optimum value is not known. Clearly the use of acid-etching procedures and varnish in the presence of calcium hydroxide liners must be done with care. Over a long term, some calcium hydroxide products seem to “disappear” from the cavity. The cause of this dissolution is unclear, but some products have been reformulated in an attempt to minimize the problem.
The pH of commercial products has been measured at between 9.2 and 11.7. Free calcium hydroxide in excess of that necessary to form the calcium disalicylate stimulates secondary dentin in proximity to the pulp and shows antibacterial activity. In today’s dental offices, calcium hydroxide liners are used often in direct pulp capping, but to line deep dentin, other materials such as light-cured resin-modified glass ionomer bases have become more popular because of their fluoride release, decreased solubility, and stronger mechanical properties.
Zinc Oxide and Eugenol Liners
ANSI/ADA Specification No. 30 (ISO 3107) requirements for ZOE liners are listed in Table 20-11. The mechanical properties of these cements are compared with those of calcium hydroxide liners and high-strength bases (see Table 20-14). ZOE products tend to be weaker and less rigid than calcium hydroxide pastes. Because the liner is used in thin layers, it provides little thermal insulation. The eugenol has a sedative (obtundent) effect on the pulp. The liner should not be used when a composite is to be placed, because eugenol can inhibit polymerization of the bonding agent and the composite.
HIGH-STRENGTH BASES
High-strength bases are used to provide thermal protection for the pulp and mechanical support for a restoration. Bases are usually prepared from a base or secondary consistency (higher powder/liquid ratio) of zinc phosphate, zinc polyacrylate, glass ionomer, resin-modified glass ionomer, or compomer cements. A polymer-reinforced ZOE base is also available.
Recently, self-cured and light-cured glass ionomer and resin-modified glass ionomer cements have become available as cavity liners and high-strength bases. The cavity liners typically flow more readily than the high-strength bases and are less rigid, as shown in Table 20-14. Light-cured glass ionomers are water-based cements but have a unique chemistry of setting that involves both an acid-base reaction between carboxylate ions and the glass particles and a light-accelerated polymerization of dimethacrylate oligomers. Most of the light-cured liners are premixed pastes or powder-liquid systems mixed by hand, but one recent light-cured liner is encapsulated. The composition and setting reaction of the resin-modified glass ionomers used as bases are identical to those of the luting cements discussed earlier in this chapter.
PROPERTIES
The tensile strength, compressive strength, and elastic modulus of five types of high-strength bases are compared in Table 20-14. The “base” consistency of these cements result in higher values of strength and elastic modulus than values obtained from mixes of a primary (luting) consistency (see Table 20-2). Zinc phosphate cements are the most rigid of the five types, whereas the polymer-modified ZOE cements (Type III) have the lowest properties. ANSI/ADA Specification No. 96 specifies the net setting time, minimum compressive strength, and maximum values of acid erosion and acid-soluble arsenic and lead content for zinc phosphate, zinc polyacrylate, and glass ionomer bases, as listed in Table 20-1.
The ability of a cement base to support a restoration during function has been studied by stress analysis techniques, as described in Chapter 4. The thickness and elastic modulus of the base affect the deflection of the base and restoration. Mismatches in the moduli of the base and restorative material can cause tensile stresses at the cement-restoration interface that can lead to failure of either material. Studies have recommended that a zinc phosphate base be used to support an amalgam restoration, whereas zinc phosphate, glass ionomer, or zinc polyacrylate may be used to support a Class 1 resin composite restoration.
The glass ionomer lining cements and bases are unique in their ability to bond to dentin. Bond strengths measured in tension vary from 2.0 to 4.9 MPa. These cements also can be etched to provide additional retention to a composite restoration (the so-called sandwich technique). Glass ionomer lining cements and bases release fluoride ions and are radiopaque.
The thermal conductivity and diffusivity of dental cements (see Table 3-3 and Box 3-2) are similar to those of enamel and dentin. Thus cement bases provide thermal protection to the pulp if used in a sufficiently thick layer (>0.5 mm).
American Dental Association Council on Scientific Affairs. Products of excellence. J Am Dent Assoc (suppl). 1998;129:1.
Barakat, MM, Powers, JM. In vitro bond strength of cements to treated teeth. Aust Dent J. 1986;31:415.
Craig, RG, Peyton, FA. Thermal conductivity of tooth structure, dental cements, and amalgam. J Dent Res. 1961;40:411.
Craig, RG, Peyton, FA, Johnson, DW. Compressive properties of enamel, dental cements, and gold. J Dent Res. 1961;40:936.
Dennison, JB, Powers, JM. A review of dental cements used for permanent retention of restorations. I. Composition and manipulation. J Mich Dent Assoc. 1974;56:116.
Diaz-Arnold, AM, Vargas, MA, Haselton, DR. Current status of luting agents for fixed prosthodontics. J Prosthet Dent. 1999;81:135.
Donovan, TE, Cho, GC. Contemporary evaluation of dental cements. Compendium. 1999;20:197.
McCabe, JF, Wilson, HJ. The use of differential scanning calorimetry for the evaluation of dental materials. I. Cements, cavity lining materials and anterior restorative materials. J Oral Rehabil. 1980;7:103.
Mesu, FP. Degradation of luting cements measured in vitro. J Dent Res. 1982;61:655.
Mitchem, JC, Gronas, DG. Clinical evaluation of cement solubility. J Prosthet Dent. 1978;40:453.
Myers, ML, Staffanou, RS, Hembree, JH, Jr., et al. Marginal leakage of contemporary cementing agents. J Prosthet Dent. 1983;50:513.
Norman, RD, Swartz, ML, Phillips, RW. Studies on film thickness, solubility, and marginal leakage of dental cements. J Dent Res. 1963;42:950.
Norman, RD, Swartz, ML, Phillips, RW. Direct pH determinations of setting cements. I. A test method and the effects of storage time and media. J Dent Res. 1966;45:136.
Norman, RD, Swartz, ML, Phillips, RW. Direct pH determinations of setting cements. II. The effects of prolonged storage time, powder/liquid ratio, temperature, and dentin. J Dent Res. 1966;45:1214.
Oilo, G, Espevik, S. Stress/strain behavior of some dental luting cements. Acta Odontol Scand. 1978;36:45.
Osborne, JW, Swartz, ML, Goodacre, CJ, et al. A method for assessing the clinical solubility and disintegration of luting cements. J Prosthet Dent. 1978;40:413.
Paddon, JM, Wilson, AD. Stress relaxation studies on dental materials. I. Dental cements. J Dent. 1976;4:183.
Phillips, LJ, Schnell, RJ, Phillips, RW. Measurement of electric conductivity of dental cement. III. Effect of increased contact area and thickness; values for resin, calcium hydroxide, zinc oxide-eugenol. J Dent Res. 1955;34:597.
Powers, JM, Craig, RG. A review of the composition and properties of endodontic filling materials. J Mich Dent Assoc. 1979;61:523.
Powers, JM, Farah, JW, Craig, RG. Modulus of elasticity and strength properties of dental cements. J Am Dent Assoc. 1976;92:588.
Richter, WA, Ueno, H. Clinical evaluation of dental cement durability. J Prosthet Dent. 1975;33:294.
Rosenstiel, SF, Land, MF, Crispin, BJ. Dental luting agents: a review of the current literature. J Prosthet Dent. 1998;80:280.
Tanizaki, K, Inoue, K. Effect of tannin-fluoride preparation on the reduction of secondary caries. J Osaka Univ Dent Sch. 1979;19:129.
Vermilyea, S, Powers, JM, Craig, RG. Rotational viscometry of a zinc phosphate and a zinc polyacrylate cement. J Dent Res. 1977;56:762.
Walls, AW, McCabe, JF, Murray, JJ. An erosion test for dental cements. J Dent Res. 1985;64:1100.
Watts, DC, Smith, R. Thermal diffusion in some polyelectrolyte dental cements: the effects of powder/liquid ratio. J Oral Rehabil. 1984;11:285.
Wilson, AD. The chemistry of dental cements. Chem Soc Rev. 1978;7:265.
Cavity Varnishes, Liners, and Bases
Bryant, RW, Wing, G. A simulated clinical appraisal of base materials for amalgam restorations. Aust Dent J. 1976;21:322.
Chong, WF, Swartz, ML, Phillips, RW. Displacement of cement bases by amalgam condensation. J Am Dent Assoc. 1967;74:97.
Costa, CAS, Mesas, AN, Hebling, J. Pulp response to direct capping with an adhesive system. Am J Dent. 2000;13:81.
Farah, JW, Hood, JAA, Craig, RG. Effects of cement bases on the stresses in amalgam restorations. J Dent Res. 1975;54:10.
Farah, JW, Powers, JM, Dennison, JB, et al. Effects of cement bases on the stresses and deflections in composite restorations. J Dent Res. 1976;55:115.
Fisher, FJ. The effect of three proprietary lining materials on micro-organisms in carious dentin. Br Dent J. 1977;143:231.
Gordon, SM. Gum copal solution for cavity lining and varnish. J Am Dent Assoc. 1936;23:2374.
Gourley, JM, Rose, DE. Comparison of three cavity base materials under amalgam restorations. J Can Dent Assoc. 1972;38:406.
Hilton, TJ. Cavity sealers, liners, and bases: Current philosophies and indications for use. Oper Dent. 1996;21:134.
Leinfelder, KF. Changing restorative traditions: The use of bases and liners. J Am Dent Assoc. 1994;125:65.
McComb, D. Comparison of physical properties of commercial calcium hydroxide lining cements. J Am Dent Assoc. 1983;107:610.
Plant, GC, Wilson, HJ. Early strengths of lining materials. Br Dent J. 1970;129:269.
Soremark, R, Hedin, M, Rojmyr, R. Studies on incorporation of fluoride in a cavity liner (varnish). Odontol Rev. 1969;20:189.
Swartz, ML, Phillips, RW, Norman, RD, et al. Role of cavity varnishes and bases in the penetration of cement constituents through tooth structure. J Prosthet Dent. 1966;16:963.
Glass Ionomer and Resin-Modified Glass Ionomer Cements
Barry, TI, Clinton, DJ, Wilson, AD. The structure of a glass-ionomer cement and its relationship to the setting process. J Dent Res. 1979;58:1072.
Berry, EA, III., The clinical uses of glass ionomer cements. Hardin, JF, eds. Clark’s clinical dentistry, 4. Philadelphia: JB Lippincott, 1993. (Chap. 20A)
Berry, EA, III., Powers, JM. Bond strength of glass ionomers to coronal and radicular dentin. Oper Dent. 1994;19:122.
Council on Dental Materials, Instruments, and Equipment. Biocompatibility and postoperative sensitivity. J Am Dent Assoc. 1988;116:767.
Crisp, S, Kent, BE, Lewis, BG, et al. Glass ionomer cement formulations. II. The synthesis of novel polycarboxylic acids. J Dent Res. 1980;59:1055.
Crisp, S, Lewis, BG, Wilson, AD. Characterization of glass-ionomer cements. I. Long term hardness and compressive strength. J Dent. 1976;4:162.
Crisp, S, Lewis, BG, Wilson, AD. Characterization of glass-ionomer cements. 5. The effect of the tartaric acid concentration in the liquid component. J Dent. 1979;7:304.
Crisp, S, Lewis, BG, Wilson, AD. Characterization of glass-ionomer cements. 6. A study of erosion and water absorption in both neutral and acidic media. J Dent. 1980;8:68.
Crisp, S, Pringuer, MA, Wardleworth, D, et al. Reactions in glass ionomer cements. II. An infrared spectroscopic study. J Dent Res. 1974;53:1414.
Crisp, S, Wilson, AD. Reactions in glass ionomer cements. I. Decomposition of the powder. J Dent Res. 1974;53:1408.
Crisp, S, Wilson, AD. Reactions in glass ionomer cements. III. The precipitation reaction. J Dent Res. 1974;53:1420.
Farah, JW, Powers, JM. Traditional cements. Dent Advis. 2001;18(6):1.
Finger, W. Evaluation of glass ionomer luting cements. Scand J Dent Res. 1983;91:143.
Fitzgerald, M, Heys, RJ, Heys, DR, et al. An evaluation of a glass ionomer luting agent: bacterial leakage. J Am Dent Assoc. 1987;114:783.
Forss, H. Release of fluoride and other elements from light-cured glass ionomers in neutral and acidic conditions. J Dent Res. 1993;72:1257.
Forsten, L. Fluoride release from a glass ionomer cement. Scand J Dent Res. 1977;85:503.
Friedl, K-H, Powers, JM, Hiller, K-A. Influence of different factors on bond strength of hybrid ionomers. Oper Dent. 1995;20:74.
Hunt PR, editor: The next generation, Proceedings of the 2nd International Symposium on Glass Ionomers, Philadelphia, 1994.
Johnson, GH, Herbert, AH, Powers, JM. Changes in properties of glass-ionomer luting cements with time. Oper Dent. 1988;13:191.
Kawahara, H, Imanishi, Y, Oshima, H. Biological evaluation on glass ionomer cement. J Dent Res. 1979;58:1080.
Maldonado, A, Swartz, ML, Phillips, RW. An in vitro study of certain properties of a glass ionomer cement. J Am Dent Assoc. 1978;96:785.
Mitra, SB, Li, MY, Culler, SR. Setting reaction of Vitrebond light cure glass-ionomer liner/base, 130. In Watts DC, Setcos JC: Proceedings of the Conference on Setting Mechanisms of Dental Materials. Trans Acad Dent Mater. 1992;5(2):175.
Negm, MM, Beech, DR, Grant, AA. An evaluation of mechanical and adhesive properties of polycarboxylate and glass ionomer cements. J Oral Rehabil. 1982;9:161.
Nicholson, JW. The setting of glass-polyalkenoate (“glass-ionomer”) cements, 113. In Watts DC, Setcos JC: Proceedings of the Conference on Setting Mechanisms of Dental Materials. Trans Acad Dent Mater. 5(2), 1992.
Oilo, G. Bond strength of new ionomer cements to dentin. Scand J Dent Res. 1981;89:344.
Prosser, HJ, Richards, CP, Wilson, AD. NMR spectroscopy of dental materials. II. The role of tartaric acid in glass-ionomer cements. Biomed Mater Res J. 1982;16:431.
Ryan, MD, Powers, JM, Johnson, GH. Properties of glass ionomer luting cements. J Mich Dent Assoc. 1985;67:17.
Shalabi, HS, Asmussen, E, Jorgensen, KD. Increased bonding of a glass-ionomer cement to dentin by means of FeCl3. Scand J Dent Res. 1981;89:348.
Wilson, AD. Resin-modified glass-ionomer cements. Int J Prosthodont. 1990;3:425.
Wilson, AD, Crisp, S, McLean, JW. Experimental luting agents based on the glass ionomer cements. Br Dent J. 1977;142:117.
Wilson, AD, Crisp, S, Prosser, HJ, et al. Aluminosilicate glasses for polyelectrolyte cements. I&EC Prod Res & Develop. 1980;19:263.
Balderamos, LP, O’Keefe, KL, Powers, JM. Color accuracy of resin cements and try-in paste. Int J Prosthodont. 1997;10:111.
Blackman, R, Barghi, N, Duke, E. Influence of ceramic thickness on the polymerization of light-cured resin cement. J Prosthet Dent. 1990;63:295.
Blalock, KA, Powers, JM. Retention capacity of the bracket bases of new esthetic orthodontic brackets. Am J Orthod Dentofac Orthop. 1995;107:596.
Buzzitta, VAM, Hallgren, SE, Powers, JM. Bond strength of orthodontic direct-bonding cement-bracket systems as studied in vitro. Am J Orthod. 1982;81:87.
Chan, KC, Boyer, DB. Curing light-activated composite cement through porcelain. J Dent Res. 1989;68:476.
Cochran, D, O’Keefe, KL, Turner, DT, et al. Bond strength of orthodontic composite cement to treated porcelain. Am J Orthod Dentofac Orthop. 1997;111:297.
DeSchepper, EJ, Tate, WH, Powers, JM. Bond strength of resin cements to microfilled composites. Am J Dent. 1993;6:235.
Dickinson, PT, Powers, JM. Evaluation of fourteen direct-bonding orthodontic bases. Am J Orthod. 1980;78:630.
Evans, LB, Powers, JM. Factors affecting in vitro bond strength of no-mix orthodontic cements. Am J Orthod. 1985;87:508.
Farah, JW, Powers, JM. Resin cements. Dent Advis. 2003;20(2):1.
Farah, JW, Powers, JM. Adhesive resin cements. Dent Advis. 2005;22(8):1.
Faust, JB, Grego, GN, Fan, PL, et al. Penetration coefficient, tensile strength, and bond strength of thirteen direct bonding orthodontic cements. Am J Orthod. 1978;73:512.
Jordan, RE, Suzuki, M, Sills, PS, et al. Temporary fixed partial dentures fabricated by means of the acid-etch resin technique: a report of 86 cases followed for up to three years. J Am Dent Assoc. 1978;96:994.
Livaditis, GJ, Thompson, VP. Etched castings: an improved retentive mechanism for resin-bonded retainers. J Prosthet Dent. 1982;47:52.
Mennemeyer, VA, Neuman, P, Powers, JM. Bonding of hybrid ionomers and resin cements to modified orthodontic band materials. Am J Orthod Dentofac Orthop. 1999;115:143.
Miller, BH, Nakajima, H, Powers, JM, et al. Bond strength between cements and metals used for endodontic posts. Dent Mater. 1998;14:312.
Noie, F, O’Keefe, KL, Powers, JM. Color stability of resin cements after accelerated aging. Int J Prosthodont. 1995;8:51.
O’Keefe, KL, Miller, BH, Powers, JM. In vitro tensile bond strength of adhesive cements to new post materials. Int J Prosthodont. 2000;13:47.
O’Keefe, KL, Powers, JM. Light-cured resin cements for cementation of esthetic restorations. J Esthet Dent. 1990;2:129.
O’Keefe, KL, Pease, PL, Herrin, HK. Variables affecting the spectral transmittance of light through porcelain veneer samples. J Prosthet Dent. 1991;66:434.
O’Keefe, KL, Powers, JM, McGuckin, RS, et al. In vitro bond strength of silica-coated metal posts in roots of teeth. Int J Prosthodont. 1992;5:373.
Powers, JM. Adhesive resin cements. Shigaku. 1991;79:1140.
Powers, JM, Kim, H-B, Turner, DS. Orthodontic adhesives and bond strength testing. Semin Orthod. 1997;3:147.
de Pulido, LG, Powers, JM. Bond strength of orthodontic direct-bonding cement-plastic bracket systems in vitro. Am J Orthod. 1983;83:124.
Rabchinsky, DS, Powers, JM. Color stability and stain resistance of direct-bonding orthodontic cements. Am J Orthod. 1979;76:170.
Rochette, AL. Attachment of a splint to enamel of lower anterior teeth. J Prosthet Dent. 1973;30:418.
Siomka, LV, Powers, JM. In vitro bond strength of treated direct-bonding metal bases. Am J Orthod. 1985;88:133.
Tate, WH, DeSchepper, EJ, Powers, JM. Bond strength of resin cements to a hybrid composite. Am J Dent. 1993;6:195.
Wright, WL, Powers, JM. In vitro tensile bond strength of reconditioned brackets. Am J Orthod. 1985;87:247.
Zinc Oxide–Eugenol and Zinc Oxide–Noneugenol Cements
Brauer, GM. A review of zinc oxide–eugenol type filling materials and cements. Rev Belg Med Dent. 1965;20:323.
Brauer, GM, McLaughlin, R, Huget, EF. Aluminum oxide as a reinforcing agent for zinc oxide–eugenol-o-ethoxy-benzoic acid cements. J Dent Res. 1968;47:622.
Civjan, S, Brauer, GM. Physical properties of cements, based on zinc oxide, hydrogenated rosin, o-ethoxybenzoic acid, and eugenol. J Dent Res. 1964;43:281.
Civjan, S, Brauer, GM. Clinical behavior of oethoxy-benzoic acid-eugenol-oxide cements. J Dent Res. 1965;44:80.
Civjan, S, Huget, EF, Wolfhard, G, et al. Characterization of zinc oxide-eugenol cements reinforced with acrylic resin. J Dent Res. 1972;51:107.
El-Tahawi, HM, Craig, RG. Thermal analysis of zinc oxide–eugenol cements during setting. J Dent Res. 1971;50:430.
Farah, JW, Powers, JM. Temporary cements. Dent Advis. 2005;22(6):1.
Gilson, TD, Myers, GE. Clinical studies of dental cements. I. Five zinc oxide–eugenol cements. J Dent Res. 1968;47:737.
Gilson, TD, Myers, GE. Clinical studies of dental cements. II. Further investigation of two zinc oxide-eugenol cements for temporary restorations. J Dent Res. 1969;48:366.
Gilson, TD, Myers, GE. Clinical studies of dental cements. III. Seven zinc oxide–eugenol cements used for temporarily cementing completed restorations. J Dent Res. 1970;49:14.
Gilson, TD, Myers, GE. Clinical studies of dental cements. IV. A preliminary study of a zinc oxide–eugenol cement for final cementation. J Dent Res. 1970;49:75.
Grossman, LI. Physical properties of root canal cements. J Endod. 1976;2:166.
Harvey, W, Petch, NJ. Acceleration of the setting of zinc oxide cements. Br Dent J. 1946;80:1.
Higginbotham, TL. A comparative study of the physical properties of five commonly used root canal sealers. Oral Surg Oral Med Oral Pathol. 1967;24:89.
McComb, D, Smith, DC. Comparison of physical properties of polycarboxylate-based and conventional root canal sealers. J Endod. 1976;2:228.
Molnar, EJ, Skinner, EW. Study of zinc oxide–rosin cements. I. Some variables which affect the hardening time. J Am Dent Assoc. 1942;29:744.
Nielsen, TH. Sealing ability of chelate root filling cements. Part I. Device for measuring linear changes of setting chelate cements. J Endod. 1980;6:731.
Powers, JM, Craig, RG. A review of the composition and properties of endodontic filling materials. J Mich Dent Assoc. 1979;61:523.
Silvey, RG, Myers, GE. Clinical studies of dental cements. V. Recall evaluation of restorations cemented with a zinc oxide–eugenol cement and a zinc phosphate cement. J Dent Res. 1976;55:289.
Silvey, RG, Myers, GE. Clinical studies of dental cements. VI. A study of zinc phosphate, EBA reinforced zinc oxide—eugenol and polyacrylic acid cements as luting agents in fixed prostheses. J Dent Res. 1977;56:1215.
Silvey, RG, Myers, GE. Clinical studies of dental cements. VII. A study of bridge retainers luted with three different dental cements. J Dent Res. 1978;57:703.
Vermilyea, SG, Huget, EF, DeSimon, LB. Extrusion rheometry of fluid materials. J Dent Res. 1979;58:1691.
Wilson, AD, Mesley, RJ. Chemical nature of cementing matrixes of cements formed from zinc oxide and 2-ethoxy benzoic acid-eugenol liquids. J Dent Res. 1974;53:146.
Crisp, S, Jennings, MA, Wilson, AD. A study of temperature changes occurring in the setting dental cements. J Oral Rehabil. 1978;5:139.
Kendziar, GM, Leinfelder, KF, Hershey, HG. The effect of cold temperature mixing on the properties of zinc phosphate cement. Angle Orthod. 1976;46:345.
Mitchem, JC, Gronas, DG. Clinical evaluation of cement solubility. J Prosthet Dent. 1978;40:453.
Muhler JC (Indiana University Foundation, Bloomington, IN): Dental cement. Canadian Patent 912,026 (Cl 6-36), Oct 17, 1972; Appl July 20, 1970, 23 pp.
Servais, GE, Cartz, L. Structure of zinc phosphate dental cement. J Dent Res. 1971;50:613.
Wilson, AD. Specification test for the solubility and disintegration of dental cements: a critical evaluation. J Dent Res. 1976;55:721.
Ady, AB, Fairhurst, CW. Bond strength of two types of cements to gold casting alloy. J Prosthet Dent. 1973;29:217.
Bertenshaw, BW, Combe, EC. Studies on polycarboxylate and related cements. I. Analysis of cement liquids. J Dent. 1972;1:13.
Bertenshaw, BW, Combe, EC. Studies on polycarboxylate and related cements. II. Analysis of cement powders. J Dent. 1972;1:65.
Brannstrom, M, Nyborg, H. Pulpal reaction to polycarboxylate and zinc phosphate cements used with inlays in deep cavity preparations. J Am Dent Assoc. 1977;94:308.
Chamberlain, BB, Powers, JM. Physical and mechanical properties of three zinc polyacrylate dental cements. J Mich Dent Assoc. 1976;58:494.
Crisp, S, Lewis, BG, Wilson, AD. Zinc polycarboxy-late cements: a chemical study of erosion and its relationship to molecular structure. J Dent Res. 1976;55:299.
Crisp, S, Prosser, HJ, Wilson, AD. An infra-red spectroscopic study of cement formation between metal oxides and aqueous solutions of poly(acrylic acid). J Mater Sci. 1976;11:36.
Jendresen, MD, Trowbridge, HO. Biological and physical properties of a zinc polycarboxylate cement. J Prosthet Dent. 1972;28:264.
Jurecic A (Pennwalt Corporation): Acrylic acid copolymers in dental cements, Canadian Patent 909,414, Sept 5, 1972.
McLean, JW. Polycarboxylate cements—five years’ experience in general practice. Br Dent J. 1972;132:9.
Mizrahi, E, Smith, DC. The bond strength of a zinc polycarboxylate cement. Br Dent J. 1969;127:410.
Oilo, G. Linear dimensional changes during setting of two polycarboxylate cements. J Oral Rehabil. 1976;3:161.
Plant, CB. The effect of polycarboxylate cement on the dental pulp—a study. Br Dent J. 1970;129:424.
Powers, JM, Johnson, ZG, Craig, RG. Physical and mechanical properties of zinc polyacrylate dental cements. J Am Dent Assoc. 1974;88:380.
Smith, DC. A new dental cement. Br Dent J. 1968;125:381.
Truelove, EL, Mitchell, DG, Phillips, RW. Biologic evaluation of a carboxylate cement. J Dent Res. 1971;50:166.