Cell cycle and replication
Introduction
The development of a single fertilised egg cell to form a complex multicellular organism involves cellular replication, growth and progressive specialisation (differentiation) for a variety of functions. The fertilised egg (zygote) divides by a process known as mitosis to produce two genetically identical daughter cells, each of which divides to produce two more daughter cells and so on. Some of these daughter cells progressively specialise and eventually produce the terminally differentiated cells of mature tissues, such as muscle or skin cells. Most tissues, however, retain a population of relatively undifferentiated cells (stem cells) that are able to divide and replace the differentiated cell population as required. The interval between mitotic divisions is known as the cell cycle. All body cells divide by mitosis except for male and female germ cells, which divide by meiosis to produce gametes (see Fig. 2.6).
In the fully developed organism, the terminally differentiated cells of some tissues, such as the neurones of the nervous system, lose the ability to undergo mitosis. In contrast, the cells of certain other tissues, such as the stem cells of gut and skin, undergo continuous cycles of mitotic division throughout the lifespan of the organism, replacing cells lost during normal wear and tear. Between these extremes are cells such as liver cells that do not normally divide but retain the capacity to undergo mitosis should the need arise (facultative dividers). Cell division is tightly controlled to meet the needs of the organism; uncontrolled cell division is one of the features of cancer.
Cell division and differentiation are balanced by cell death, both during the development and growth of the immature organism and in the mature adult. In these circumstances, cell death occurs by a mechanism known as apoptosis (see Fig. 2.7).
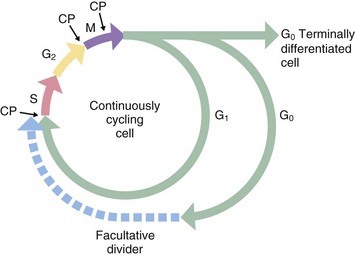
FIG. 2.1 The cell cycle
Historically, only two phases of the cell cycle were recognised: a relatively short mitotic phase (M phase) and a non-dividing phase (interphase), which usually occupies most of the life cycle of the cell. However, there is a discrete period during interphase when nuclear DNA is replicated; this phase, described as the synthesis or S phase, is completed some time before the onset of mitosis. Thus interphase may be divided into three separate phases. Between the end of the M phase and the beginning of the S phase is the first gap or G1 phase; this is usually much longer than the other phases of the cell cycle. During the G1 phase, cells differentiate and perform their specialised functions as part of the whole tissue. The interval between the end of the S phase and the beginning of the M phase, the second gap or G2 phase, is relatively short and is the period in which cells prepare for mitotic division.
Stem cells in some tissues progress continually through the cell cycle to accommodate tissue growth or cell turnover, whereas terminally differentiated cells leave the cell cycle after the M phase and enter a state of continuous differentiated function designated as G0 phase. Facultative dividers enter the G0 phase but retain the capacity to re-enter the cell cycle when suitably stimulated. Liver cells are a prominent example of facultative dividers with differentiated hepatocytes acting as stem cells in cases of massive liver injury. Some liver cells appear to enter a protracted G2 phase in which they perform their normal differentiated functions despite the presence of a duplicated complement of DNA. These cells may be seen histologically as binucleate cells.
In general, the S, G and M phases of the cell cycle are relatively constant in duration, each taking up to several hours to complete, whereas the G1 phase is highly variable, in some cases lasting for several days or weeks. M phase typically lasts about 1 hour, while S phase takes 10 to 12 hours to complete. The G0 phase may last for the entire lifespan of the organism.
During mitosis, various checkpoints CP prevent progression to the next phase before the previous one is completed. For example, the metaphase checkpoint prevents progression to anaphase before all the chromosomes are properly connected to the mitotic spindle and lined up at the cell equator; this prevents unequal distribution of the chromosomes to the two daughter cells.
Mitosis
Division of somatic cells (all body cells except for the germ cells) occurs in two phases. Firstly, the chromosomes duplicated in S phase are distributed equally between the two potential daughter cells; this process is known as mitosis. Secondly, the dividing cell is cleaved into genetically identical daughter cells by cytoplasmic division or cytokinesis. Although mitosis is always equal and symmetrical, cytokinesis may, in some situations, result in the formation of two daughter cells with grossly unequal amounts of cytoplasm or cytoplasmic organelles. In other circumstances, mitosis may occur in the absence of cytokinesis, as in the formation of binucleate and multinucleate cells.
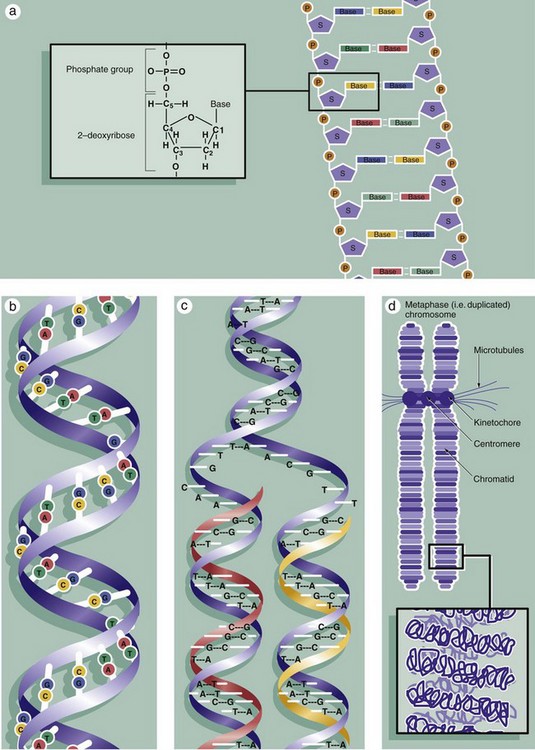
FIG. 2.2 Chromosomes during mitosis (illustrations opposite)
The nuclei of all somatic cells of an individual contain the same fixed complement of deoxyribonucleic acid (DNA), a quantity called the genome. The DNA is arranged into chromosomes, with each species having a set number. DNA is a very large molecular weight polymer consisting of many deoxyribonucleotides with a double-stranded structure. Each strand consists of a backbone of alternating deoxyribose S and phosphate P moieties. Each deoxyribose unit is covalently bound to a purine or pyrimidine base, which is in turn non-covalently linked to a complementary base on the other strand, thus linking the strands together. The bases are of four types, adenine A, cytosine C, thymine T and guanine G, with adenine only linking to thymine and cytosine only linking to guanine, thus making each strand complementary to the other, diagram (a). Linked in this way, the strands assume a double helical conformation around a common axis, the internucleotide phosphodiester bonds running in opposite directions (i.e. antiparallel) and the planes of the linked bases lying at right angles to the axis, diagram (b). The sequence of bases in either strand of the DNA molecule forms the genetic code for the individual. The bases are read in groups of three called codons, each coding for one amino acid. In human cells, there are 46 chromosomes (the diploid number) comprising 22 homologous pairs, the autosomes, and 2 sex chromosomes, either XX in the female or XY in the male. The members of each pair of autosomes have the same length of DNA and code for the same proteins.
Histologically, individual chromosomes are not visible within the cell nucleus during interphase. During S phase, each chromosome is duplicated, as shown in diagram (c). The resulting identical chromosomes, now known as sister chromatids, remain attached to one another at a point called the centromere and become even more tightly coiled and condensed when they may be visualised with the light microscope (see Figs 2.3 and 2.4). Accurate reproduction of the DNA strand is vital and multiple complex mechanisms exist to prevent mistakes (mutations) from occurring.
The extremely long DNA molecule making up each chromosome binds to a range of histone proteins that hold the chromosome in a supercoiled and folded conformation, compact enough to be accommodated within the nucleus (not illustrated). Thus the 2 nm–diameter double helix is coiled and packed through several orders of three-dimensional complexity to form an elongated structure some 300 nm in diameter and very much shorter in length than the otherwise uncoiled molecule would be. This is the form in which the chromosome is structured during the G1 and G0 phases of the cell cycle, during which gene transcription (the prerequisite for protein synthesis) occurs. Histone proteins are also reduplicated during S phase so that the sister chromatids will have their own complement of histones.
Diagram (d) shows further detail of the structure of mitotic chromosomes and their supercoiled three-dimensional structure. Note the position of the kinetochore (see Fig. 2.3) that provides attachment for the microtubules of the cell spindle during cell division and seems also to control the progression of mitosis. Examination of the chromosomes of dividing cells, karyotyping, can give diagnostic information about the chromosomal complement of an individual or of a malignant tumour (see Fig. 2.5).
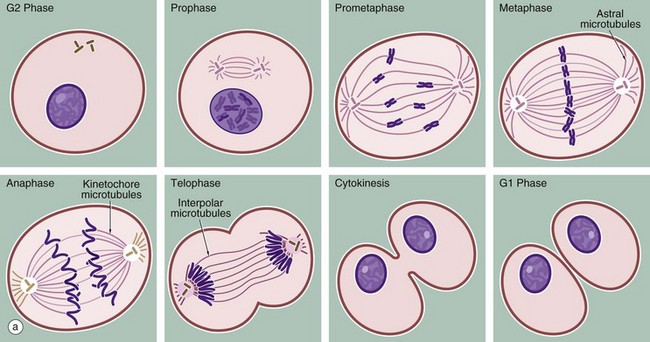
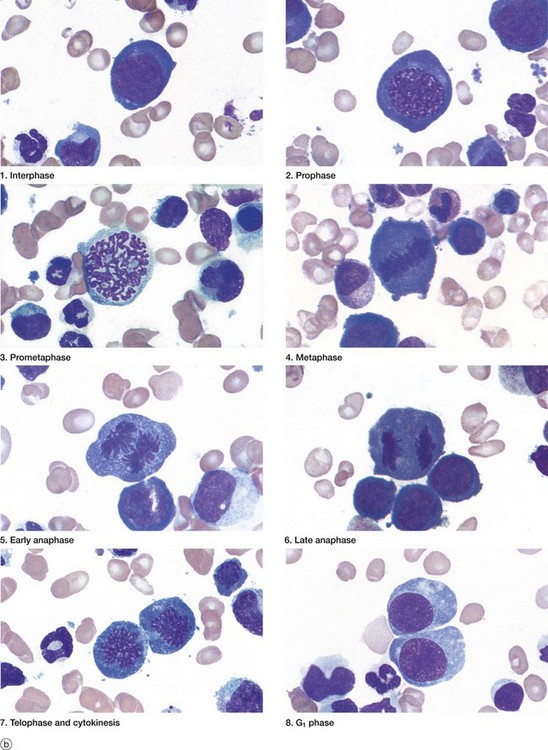
FIG. 2.3 Mitosis (illustration (b) opposite)
(a) Schematic diagram (b) Mitotic series Giemsa (HP)
The series of micrographs shown opposite illustrates the mitotic process in actively dividing immature blood cells from a smear preparation of human bone marrow. Mitosis is a continuous process that is traditionally divided into five phases, prophase, prometaphase, metaphase, anaphase and telophase, each stage being readily recognisable with the light microscope. Cell division requires the presence of a structure called the mitotic apparatus, which comprises a spindle of longitudinally arranged microtubules extending between a pair of centrioles (see Figs 1.17 and 1.18) at each pole of the dividing cell. The mitotic apparatus is visible within the cytoplasm only during M phase as it disaggregates shortly after completion of mitosis.
• Prophase. The beginning of this stage of mitosis is defined as the moment when the chromosomes (already duplicated during the preceding S phase) first become visible within the nucleus. As prophase continues, the chromosomes become increasingly condensed and shortened and the nucleolus disappears. During prophase, the microfilaments and microtubules of the cytoskeleton disaggregate into their protein subunits. The centrosome has already divided during the preceding interphase and, in prophase, the two pairs of centrioles migrate towards opposite poles of the cell while simultaneously a spindle of microtubules is formed between them (interpolar microtubules).
• Prometaphase. Dissolution of the nuclear envelope marks the beginning of prometaphase. The mitotic spindle then moves into the nuclear area and each duplicated chromosome becomes attached at a site called the kinetochore to another group of microtubules of the mitotic spindle (kinetochore or chromosome microtubules). The kinetochore is a DNA and protein structure on each duplicated chromosome, located at the centromere, the structure which binds the duplicated chromosomes (chromatids) together (see also diagram (d), Fig. 2.2). Other microtubules attach the chromosome arms to the spindle.
• Metaphase. The chromosomes are then dragged to the plane of the spindle equator, known as the equatorial or metaphase plate. The kinetochore also controls entry of the cell into anaphase so that the process of mitosis does not progress until all chromatid pairs are aligned at the cell equator. This is called the metaphase checkpoint and prevents the formation of daughter cells with unequal numbers of chromosomes.
• Anaphase. The splitting of the centromere marks this stage of mitosis. The mitotic spindle becomes lengthened by the action of the motor protein kinesin 5 on the interpolar microtubules; meanwhile astral microtubules, joining the centrosome to the cell cortex (the area underlying the plasma membrane), shorten. The centrioles are thus pulled apart and the chromatids of each duplicated chromosome drawn to opposite ends of the cell, thus achieving an exact division of the duplicated genetic material. By the end of anaphase, two groups of identical chromosomes are clustered at opposite poles of the cell.
• Telophase. During the final phase of mitosis, the chromosomes begin to uncoil and to regain their interphase conformation. The nuclear envelope reassembles and nucleoli again become apparent.
• Cytokinesis. The plane of cytoplasmic division is usually defined by the position of the spindle equator, thus producing two cells of equal size. The plasma membrane around the spindle equator becomes indented to form a circumferential furrow around the cell, the cleavage furrow, which progressively constricts the cell until it is cleaved into two daughter cells. A ring of microfilaments is present just beneath the surface of the cleavage furrow and cytokinesis occurs as a result of contraction of this filamentous ring. In early G1 phase, the mitotic spindle disaggregates and, in many cell types, the single pair of centrioles begins to duplicate in preparation for the next mitotic division.
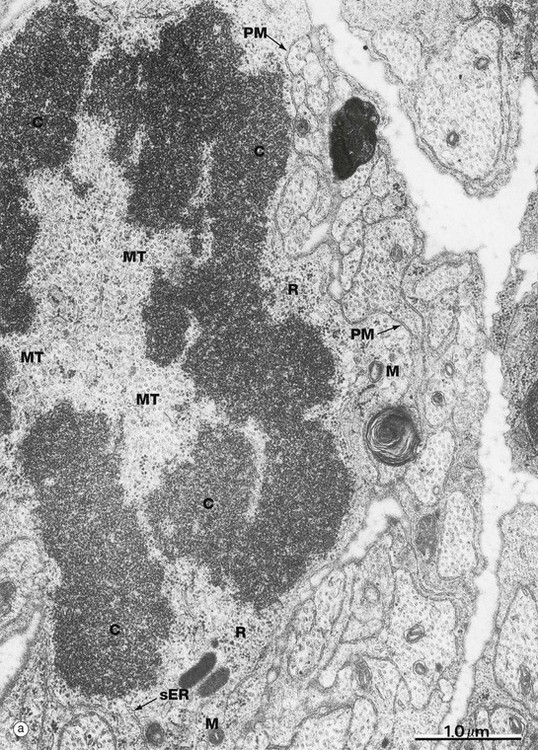
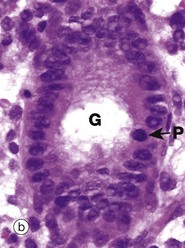
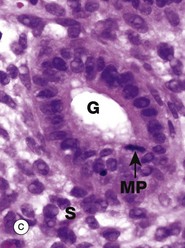
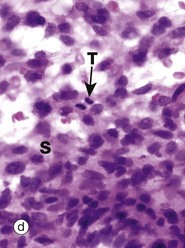
FIG. 2.4 Mitotic figures in tissue sections
(a) EM ×30 000 (b) H&E (HP) (c) H&E (HP) (d) H&E (HP)
Micrograph (a) shows an electron microscopic view of part of a dividing cell, a Schwann cell in the developing nervous system. The plasma membrane PM is evident, but the nuclear membrane has disintegrated and the chromatin C has spread out into the cytoplasm. Mitochondria M, smooth endoplasmic reticulum sER and ribosomes R are seen at the periphery. In the centre of the chromatin, there are numerous microtubules MT cut in cross-section, representing part of the spindle apparatus. These features suggest that this cell is in anaphase, with the plane of section through one end of the dividing nuclear material at right angles to the spindle axis. Micrographs (b), (c) and (d) show the typical appearance of mitotic figures in normal tissue sections at high magnification. All three are of endometrium (see Ch. 19). In contrast to smear preparations where the entire cell is visualised, the process of preparing a tissue section cuts through the cell in a random plane; thus it is more difficult to recognise mitotic figures with certainty in tissue sections. In the proliferative phase of the menstrual cycle, both the epithelial cells of the glands G and the stromal cells S of the endometrium undergo frequent cell division by mitosis. In micrograph (b), a glandular epithelial cell is seen in prometaphase P, with the duplicated chromosomes forming a roughly circular tangle and the nuclear membrane dispersed. In (c) an epithelial cell exhibits a metaphase plate MP, with the chromosomes lined up at the equator of the cell. In micrograph (d) a stromal cell is in telophase T, with the chromatids separated and moving towards the poles of the spindle.
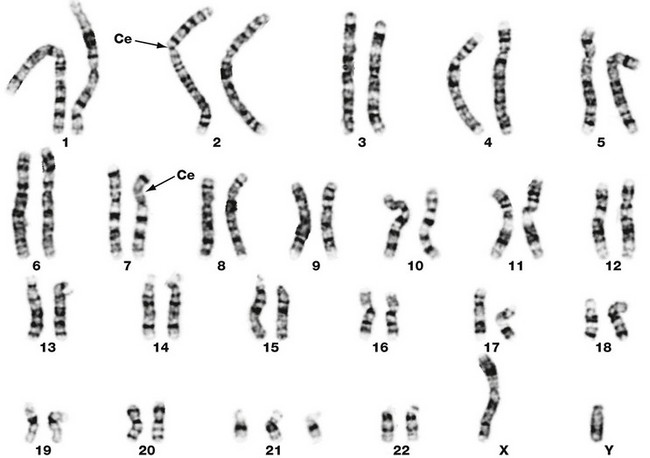
FIG. 2.5 Human karyotype
Leishman (HP)
This micrograph illustrates the chromosomes of a human cell grown in vitro and harvested at the onset of mitosis. The cell has been disrupted and the chromosomes stained to reveal their characteristic cross-banding pattern. This approach allows the investigator to identify chromosomes 1, 2 etc. At this stage the chromosomes are duplicated and the two chromatids (not visible at this magnification) are joined at the centromere Ce. Each member of a homologous pair of mitotic chromosomes is identical in length, centromere location and banding pattern. Study of the chromosomes in this fashion, karyotyping, can reveal structural and numerical chromosomal abnormalities, known as cytogenetic abnormalities. In this particular case there are three copies of chromosome 21, i.e. this is a trisomy 21 karyotype. All the other chromosomes are normal. There is one X and one Y chromosome demonstrating that the fetus is male.
Meiosis
In dividing somatic cells, cell division (mitosis) results in the formation of two daughter cells, each one genetically identical to the mother cell. Somatic cells contain a full complement of chromosomes (the diploid number) which function as homologous pairs, one member of each pair deriving from the father and one from the mother. The process of sexual reproduction involves the production by meiosis of specialised male and female cells called gametes; meiotic cell division is thus also called gametogenesis. Each gamete contains the haploid number of chromosomes (23 in humans), i.e. one from each homologous pair. When the male and female gametes fuse at fertilisation to form a zygote, the diploid number of chromosomes (46 in humans) is restored. The member of each chromosome pair assigned to a particular gamete is entirely random, so that a particular gamete contains a mix of chromosomes from the mother and father of the individual forming the gamete. This mixing of chromosomes contributes to the genetic diversity of the next generation. Further genetic diversity, and therefore possible evolutionary advantage, is added by the mechanism of crossing over (see below). The process of meiosis involves many of the mechanisms and control systems that regulate mitosis. The process of meiosis is outlined below and compared to mitosis in Fig. 2.6 opposite.
• Before meiosis can begin, the chromosomes are duplicated as for mitosis (meiotic S phase).
• This is immediately followed by crossing over of the chromatids, so that genetic information is exchanged between the two chromosomes of the homologous pair. As one of each pair of chromosomes is derived from the father and one from the mother, crossing over mixes up these paternally and maternally derived alleles (alternative forms of the same gene) so that the haploid gamete ends up with only one of each chromosome pair, but each individual chromosome includes alleles from each parent (see Fig. 2.5). The mechanism of crossing over is called chiasma formation. This phase is known as prophase I. As well as generating great genetic diversity, this mixing up of genes explains the conviction of many teenagers that they must be adopted.
• The first meiotic division then proceeds, involving separation of the homologous pairs of chromosomes, each one consisting of a pair of chromatids still joined together at the centromere (metaphase I and anaphase I). This separation of chromosomes is carried out by a microtubules spindle, as in mitosis. Thus at the end of the first meiotic division, each daughter cell contains a half complement of duplicated chromosomes, one from each homologous pair of chromosomes.
• The second meiotic division involves splitting of the chromatids by pulling apart the centromeres (metaphase II). The chromatids then migrate to opposite poles of the spindle (anaphase II, telophase II).
Thus, meiotic cell division of a single diploid germ cell gives rise to four haploid gametes. In the male, each of the four gametes undergoes morphological development into a mature spermatozoon. In the female, unequal distribution of the cytoplasm results in one gamete gaining almost all the cytoplasm from the mother cell, while the other three acquire almost none; the large gamete matures to form an ovum and the other three, called polar bodies, degenerate.
During both the first and second meiotic divisions, the cell passes through stages that have many similar features to prophase, metaphase, anaphase and telophase of mitosis. Unlike mitosis, however, the process of meiotic cell division can be suspended for a considerable length of time. For example, in the development of the human female gamete, the germ cells enter prophase of the first meiotic division during the fifth month of fetal life and then remain suspended until some time after sexual maturity; the first meiotic division is thus suspended for between 12 and 50 years!
The primitive germ cells of the male, the spermatogonia, are present only in small numbers in the male gonads before sexual maturity. After this, spermatogonia multiply continuously by mitosis to provide a supply of cells which then undergo meiosis to form male gametes. In contrast, the germ cells of the female, called oogonia, multiply by mitosis only during early fetal development, thereby producing a fixed complement of cells with the potential to undergo gametogenesis.
Chromosomes are not the only source of genes in the germ cells. Mitochondria also contain DNA that codes for some intrinsic mitochondrial proteins required for energy production. Because the spermatozoa shed their mitochondria at the time of fertilisation, only maternal mitochondrial genes are passed on to the offspring. A number of inherited diseases are known to be transmitted through mitochondrial DNA.
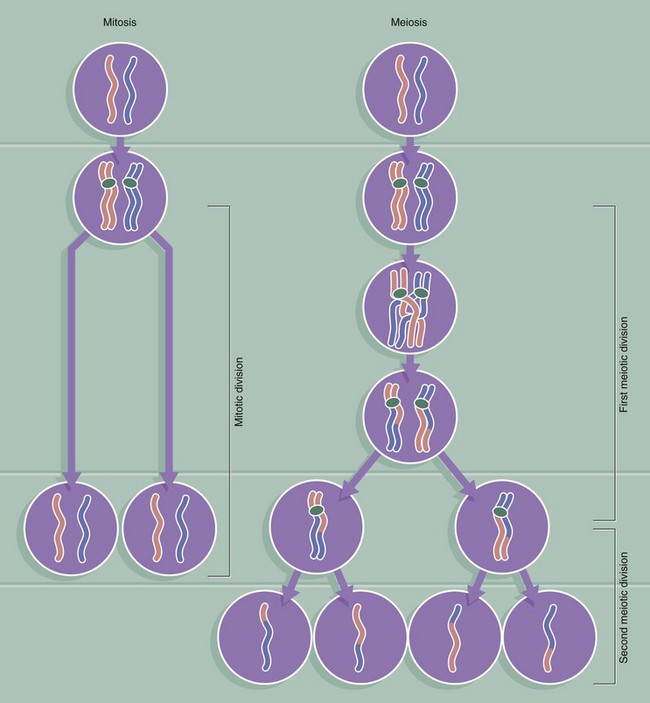
FIG. 2.6 Comparison of mitosis and meiosis
This diagram compares the behaviour of each homologous pair of chromosomes during mitosis and meiosis. For simplicity, the progress of only one homologous pair is represented here. The key differences between the two forms of cell division are as follows:
• Meiosis involves one reduplication of the chromosomes followed by two sequential cell divisions. Thus a diploid cell produces four haploid germ cells (gametes).
• Crossing over occurs only in meiosis, to rearrange alleles such that every gamete is genetically different. In contrast, the products of mitosis are genetically identical.
Programmed Cell Death
Programmed cell death is an essential part of normal fetal development, growth of juveniles and control of cell numbers in adults, where it exactly balances cell division. The most common and best understood method of programmed cell death is apoptosis, which also occurs in certain pathological conditions. Apoptosis has characteristic microscopic features and is a highly controlled and ordered mechanism that removes cells in a way that causes minimal disruption to the surrounding tissue. Apoptosis is brought about by different mechanisms than those causing necrosis, a mode of cell and tissue death that occurs only in pathological conditions. A well-known example of necrosis is myocardial infarction, where heart muscle dies from lack of oxygen due to blockage of a coronary artery. Apoptosis is an active process requiring the expenditure of energy, while necrosis is characterised by the inability of cells to produce the energy (ATP) required to maintain homeostasis.
Control of apoptosis is very finely balanced and a wide variety of triggers may initiate the process. The signal for apoptosis may be the binding of an external signal molecule to a membrane receptor (the ‘death receptor’ known as Fas) or may arise from intracellular signals such as DNA damage, leading to the release of the enzyme cytochrome c from the mitochondria into the cytoplasm. Within the cell, many regulatory proteins control apoptosis, including members of the bcl-2 and inhibitors of apoptosis (IAP) families. The end result of all these various signals is a common mechanism known as the caspase cascade. The activated enzymes of the caspase cascade cleave cellular proteins such as the lamins of the nucleus and activate additional enzymes such as DNAase to cleave DNA.
Examples of apoptosis include:
• Some cell types have a preset limited lifetime and inevitably undergo apoptosis as part of their life cycle (e.g. epithelial cells in the skin or the lining of the gastrointestinal tract).
• Other cells are triggered to destroy themselves if they behave in ways which are potentially harmful; for example, developing T lymphocytes that are capable of reacting to normal body components are triggered to self-destruct in the thymus, a process known as clonal deletion (see Ch. 11). Failure of clonal deletion may lead to autoimmune disorders such as autoimmune thyroiditis or pernicious anaemia.
• During development certain cells are programmed to die by apoptosis; for example, in humans the webs between the fingers and toes disappear and the tadpole loses its tail as it matures into a frog.
• Certain tissues in adults grow and regress in a cyclical fashion, such as the growth of ovarian follicles before ovulation in females, followed by regression of the corpus luteum by apoptosis to form a corpus albicans (see Ch. 19).
• Apoptosis is a common mode of cell death of abnormal cells, such as those infected by viruses or with genetic mutations. Failure of apoptosis may be as important in cancer as unrestricted cell division.
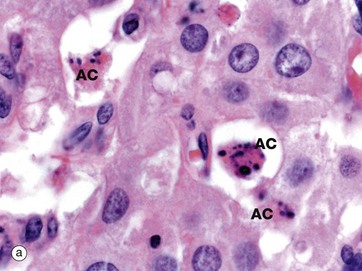
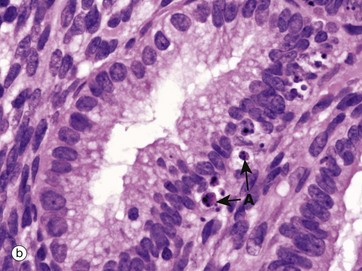
FIG. 2.7 Apoptosis in normal tissues
(a) H&E (HP) (b) H&E (HP)
These two micrographs illustrate the typical features of apoptotic cells in normal tissues. Micrograph (a) is a corpus luteum, formed from an ovarian follicle after discharge of an ovum (see Ch. 19). If the ovum is not fertilised, the corpus luteum will involute, a process that involves progressive death of its constituent cells, leaving a fibrotic scar known as a corpus albicans. In this micrograph several apoptotic cells AC can be identified by their condensed nuclei and eosinophilic cytoplasm.
Micrograph (b) shows a later stage of apoptosis in epithelial cells of endometrial glands at the beginning of menstruation (see Ch. 19). Several cells have undergone apoptosis and reached the stage of forming easily identified apoptotic bodies A. The apoptotic bodies have been phagocytosed by adjacent cells, which are themselves about to undergo the same process as the superficial part of the endometrium is shed.
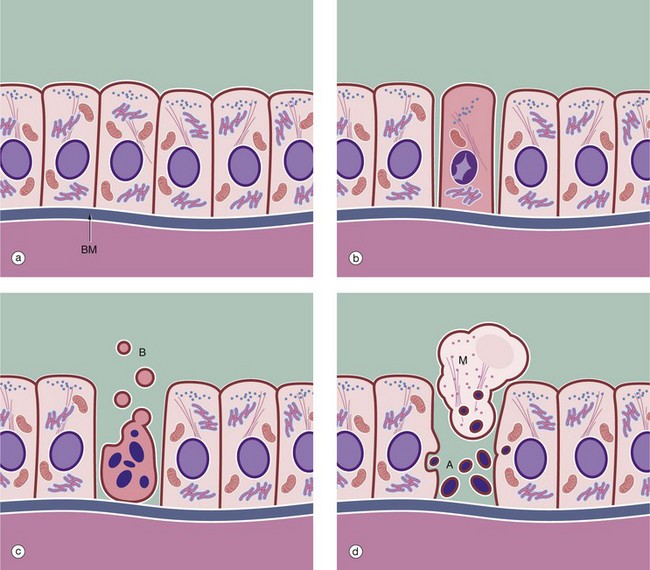
FIG. 2.8 The mechanism of apoptosis
Although a variety of extrinsic and intrinsic triggers may initiate apoptosis, at the molecular level the final common pathway is the activation of the caspase cascade. Caspases are a set of enzymes found in inactive form in all cells. When the first in the series is activated, by cleaving off a short peptide sequence it is then able to activate the next enzyme in the series and so on. Because each enzyme is able to activate many copies of the next enzyme, the reaction is greatly amplified. This enzyme cascade mechanism is also seen in other situations requiring a rapid but controlled response, such as the blood clotting mechanism (the coagulation cascade) and the complement cascade.
The process of apoptosis is shown in this diagram of simple columnar epithelial cells resting on a basement membrane BM. When a normal cell (a) receives a signal to initiate apoptosis, the characteristic change by light microscopy (b) is condensation of the nuclear chromatin (pyknosis) to form one or more dark-staining masses found against the nuclear membrane. At the same time, the cell shrinks away from its neighbours, with loss of cell-cell contacts and increasing eosinophilia (pink staining) of the cytoplasm. The cytoplasmic organelles are still preserved at this stage. As the process continues (c), the nuclear material breaks into fragments (karyorrhexis). This is accompanied by dissolution of the nuclear membrane. Cytoplasmic blebs B break away from the cell surface, and eventually the entire cell breaks up (karyolysis) (d) to form membrane-bound fragments. Some of the cell fragments contain nuclear material and are known as apoptotic bodies A. These apoptotic bodies may be phagocytosed by adjacent cells or by tissue macrophages M, scavenger cells derived from the bone marrow and found in virtually every tissue in the body.
In some circumstances, part of the cell remains as a normal tissue component after apoptosis. For instance, in the skin, epithelial cells undergo apoptosis as part of their normal life cycle, but for some considerable time after the nucleus has disappeared, the cell cytoplasm filled with keratin intermediate filaments remains as an anucleate ‘squame’ to form a waterproof coating on the surface of the skin (see Ch. 9).
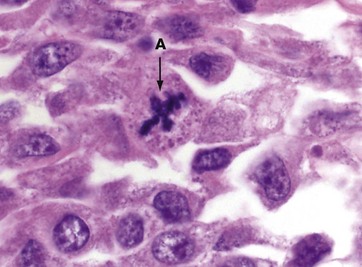
FIG 2.9 Abnormal mitotic figure
H&E (HP)
This micrograph of a malignant tumour of the skin contains an abnormal mitotic figure A. The cell is in metaphase, but rather than a metaphase plate with two sets of chromatids and two spindles, the cell has produced four sets of chromatids and four spindles, a quadripolar mitosis. Such abnormalities are frequently seen in malignant tumours and are virtually never found in normal tissues and benign tumours; thus an abnormal mitotic figure can be a helpful clue in diagnostic pathology.
Review
TABLE 2.1
Review of cell cycle and replication
| Process | Definition | Key features |
| The cell cycle | The period of time it takes for a cell to complete one cell division | May last a few hours in certain continuously dividing cells May last years in stable, terminally differentiated cells Consists of gap phase 1 (G1), synthesis phase (S), gap phase 2 (G2) and mitosis phase (M) Some cells enter G0 phase, a prolonged non-dividing phase. |
| Mitosis | Cell division of somatic cells | Results in two diploid daughter cells Phases are prophase, prometaphase, metaphase, anaphase, telophase and cytokinesis |
| Meiosis | Division of germ cells to produce zygotes | Results in four haploid cells—four spermatogonia in males and one oogonium and three polar bodies in females Crossing over during meiosis results in greatly increased genetic diversity in offspring. |
| Apoptosis | Controlled cell death to remove unnecessary or potentially harmful cells | Various internal or external receptors may trigger apoptosis. Cell death occurs by activation of the caspase cascade. Histologically, apoptotic bodies may be seen. Cell death occurs and the cell is removed without disruption or damage to the adjacent tissue. |