Skin
Introduction
The skin is the largest organ in the body, both in weight and surface area. It shows significant regional variation, with the thickest skin being found on the soles of the feet while the thinnest is the delicate skin on the upper and lower eyelids; some of these variations are illustrated and discussed later in this chapter.
The skin is the external body surface and provides protection against a wide variety of external threats, including mechanical, water loss, biological, ultraviolet light and chemical. The most frequent mechanical insult is the frictional and shearing forces experienced by the soles and ventral aspect of the toes in walking and, to a lesser extent the palms and ventral aspects of the fingers during use of the hands. In these areas, skin structure is adapted to resist these shearing forces (see Fig. 9.20).
The skin provides moisture control, providing a barrier against both excessive water loss and wetting. It resists bacterial and fungal invasion; bacteria and fungi do live on the skin surface but cannot penetrate into underlying tissues unless the skin is breached. It is rich in antigen presenting cells (Langerhans cells) and, when breached, an immune response against any foreign antigen is readily initiated.
Skin pigmentation from melanin provides protection against ultraviolet (UV) radiation, and exposure induces increased pigmentation (tanning). Skin has a metabolic function, namely the synthesis of vitamin D3 (cholecalciferol) by the action of UV light on the precursor, 7-dehydrocholesterol. Cholecalciferol is further processed in the liver and kidney to produce the active agent 1,25-dihydroxycholecalciferol, which is important in Ca2+ metabolism and bone formation. Adequate levels of UV exposure are needed to ensure that enough vitamin D is synthesised. Individuals with darker skin (more melanin pigment) require more UV exposure to ensure adequate vitamin D levels.
Skin is important in thermoregulation. Adjustments to blood circulation through skin, particularly extremities (hands, feet and ears), provides for both heat conservation and heat loss. The production of a watery secretion by skin eccrine glands, known as sweat, and its subsequent evaporation, is a major heat-loss mechanism. In humans, body hair is scanty and so provides only minimal heat conservation; however, subcutaneous adipose tissue can provide some heat conservation.
The skin is the largest sensory organ in the body, containing a range of different receptors for touch, pressure, pain and temperature (see Ch. 7). As a regional variation in structure, sensory receptors are most numerous in skin which has the most physical contact with solid objects in the environment, such as soles and palms, fingers and toes (see Fig. 7.24), or with specialised sexual functions.
Hair and nails are specialised components of skin. In humans, hair mostly defines sexual differences, sexual maturity and age; in most other mammals, it provides protection against cold in the form of fur. Nails provide physical support for finger tips and toes and can serve as tools.
The overall appearance of the skin (condition, pigmentation, hair and nails) cannot be underestimated, both clinically as an important indicator of health and in human behaviour.
Rapid repair of injuries to the skin such as lacerations (cuts and tears) and other wounds is critical to minimise infection risks and, while this happens in all tissues, this can rightly be seen as a critical skin function.
Skin Structure
The skin has three main layers:
• The epidermis is a continuously proliferating stratified squamous epithelium which produces a non-living surface layer of the protein keratin, with associated lipid which is in direct contact with the external environment and is constantly shed.
• The dermis consists of fibrous and fibroadipose tissue which supports the epidermis, both physically and metabolically. It contains blood vessels, nerves and sensory receptors.
• The subcutis or hypodermis or panniculus is the layer beneath the dermis and usually consists of adipose tissue with supporting fibrous bands (septa). This layer contains the larger vessels which supply and drain the dermal blood vasculature.
In addition, there are the specialised skin structures and adnexa (appendages) such as nails, hair follicles, sebaceous glands, eccrine (sweat) glands and apocrine glands. The adnexae mainly occupy the dermis and variably the superficial subcutis. They arise as downgrowths from the epidermis into the dermis during embryological development.
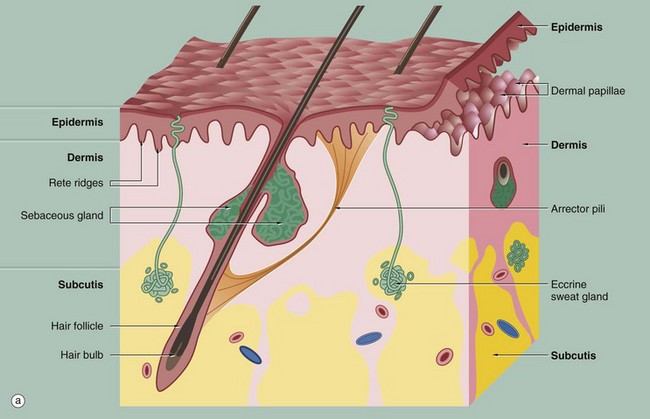
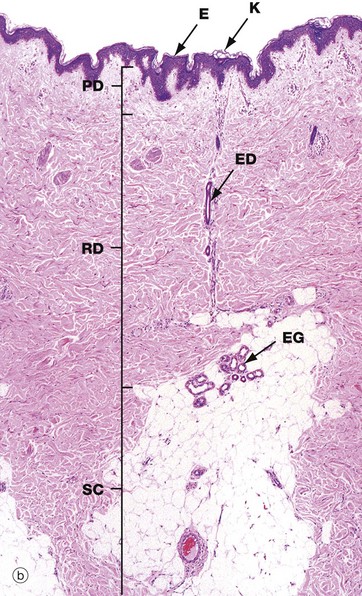
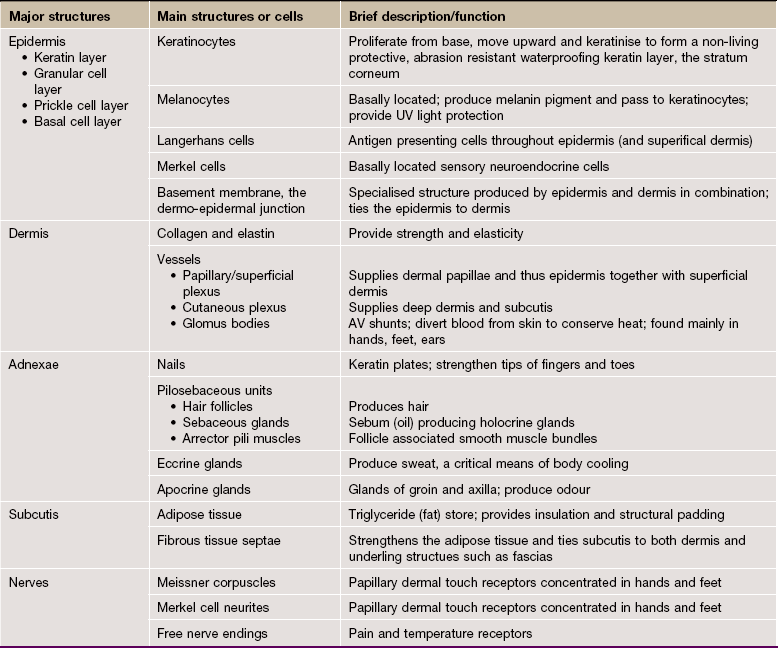
FIG. 9.1 Skin architecture
(a) Diagram (b) H&E (LP)
These illustrations show the basic structure of the skin, with the three component layers: epidermis, dermis and subcutis.
The surface layer in contact with the exterior is the epidermis E, a highly specialised self-regenerating stratified squamous epithelium which produces a non-living surface rich in a protein, keratin K, that is tough and protective and is also partially water resistant (see Ch. 5). The epidermis also contains non-epithelial cells: melanocytes produce melanin pigment to protect against UV light, Langerhans cells act as antigen-presenting cells and induce immune responses to new antigens and Merkel cells act as touch receptors. The epidermis is tightly bound to the underlying dermis by a specialised basement membrane. Additional resistance to frictional shearing force is provided by a series of epidermal downgrowths (rete ridges) which extend into the superficial dermis, with their papillary dermal mirror images projecting upwards (dermal papillae) to provide stronger tethering. These are most developed where exposure to shearing forces is almost constant (e.g. sole, palm).
The dermis immediately adjacent to the epidermis is called the papillary dermis PD; it has relatively fine collagen fibres and contains numerous small blood vessels, sensory nerve endings and sensory structures. The reticular dermis RD is the deeper tough layer of horizontally arranged collagen and elastin fibres with fibroblasts.
The deepest layer is the subcutis SC, also called the panniculus or hypodermis. It is a layer of adipose tissue often compartmentalised by fibrous septa, extending downwards from dermis to the underlying structural connective tissue fascia. The subcutis acts as a shock absorber and thermal insulator as well as a fat store.
The dermis and subcutis contain an assortment of skin adnexa (appendages) such as hair follicles, sebaceous glands, eccrine (sweat) glands EG and ducts ED and, in some areas, apocrine glands.
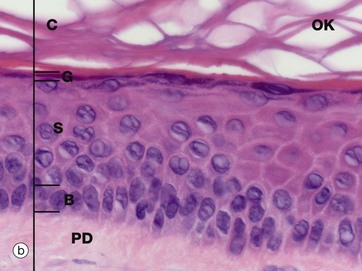
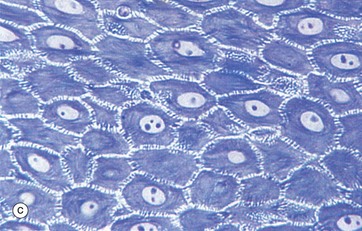
FIG. 9.2 Epidermis
(a) Masson trichrome (HP) (b) H&E (HP) (c) Epoxy resin section, toluidine blue (HP)
Micrographs (a) and (b) show epidermis. The cells of the epidermis are called keratinocytes. The basal layer of keratinocytes (stratum basale) B proliferates continuously with repeated mitotic divisions. This provides cells for a progressive process of displacement towards the surface (upward migration), with associated maturation to renew the other layers. The basal cells are arranged as a single layer of cuboidal or low columnar cells. They are attached to the basement membrane (not seen in these preparations) on their dermal (basal) surface. This basal surface is irregular; the basal cells have a highly indented and folded basal cell membrane with numerous hemi-desmosomes.
Superficially, the basal cells are attached to and mature into the cells of the stratum spinosum S which forms the majority of the epidermis. The stratum spinosum is also known as the prickle cell layer. It is multilayered and composed of polyhedral-shaped keratinocytes with round-oval nuclei, prominent nucleoli and cytoplasm, forming a pavement-like pattern. These cells synthesise cytoplasmic intermediate filaments called cytokeratins which accumulate in aggregates called tonofibrils made up of bundles of tonofilaments. These tonofibrils bind to the numerous desmosomes that form strong contacts between adjacent keratinocytes. In appropriate preparations as in micrograph (c), the desmosome junctions are seen as prickles or spines between the cells, hence the name for this layer.
The keratinocytes mature into the stratum granulosum G or granular layer. Here they acquire dense basophilic, keratohyaline granules which contain proteins rich in sulphur-containing amino acids (cysteine) and proteins such as involucrin which interact with the cytokeratin tonofibrils in the final maturation. The combination of tonofibrils with keratohyaline granule proteins produces keratin, in a process called keratinisation. Progressing towards the surface, the cells lose their nuclei and cytoplasm, becoming flattened interconnected keratin squames (plates/flakes of keratin) which comprise the surface coating of the skin, the stratum corneum C.
These keratin squames connect at their edges, and in transverse sections form a folded basket-weave pattern called orthokeratosis OK. The squames are water repellent, in part because they are coated with lipid-containing anti-wetting agents synthesised during maturation in the granular layer.
Micrograph (a) illustrates skin from a friction-prone area (sole of foot) where keratin production is enhanced. The stratum spinosum has more layers, the granular layer G is thicker and more prominent and the stratum corneum C is thick with dense compact keratin. These changes are a non-specific response to friction.
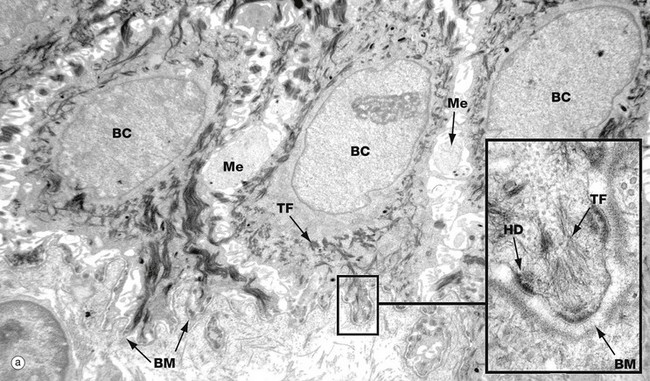
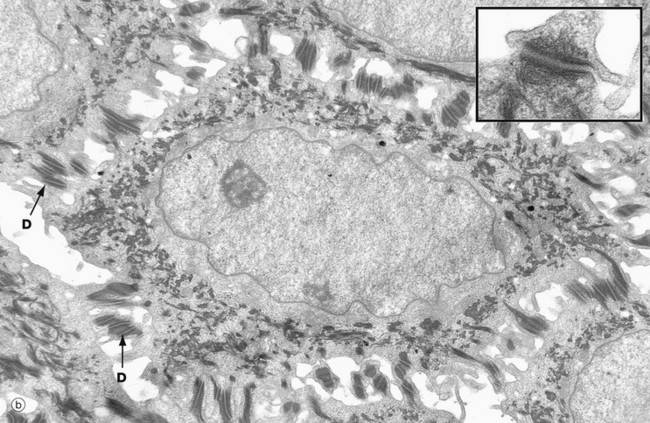
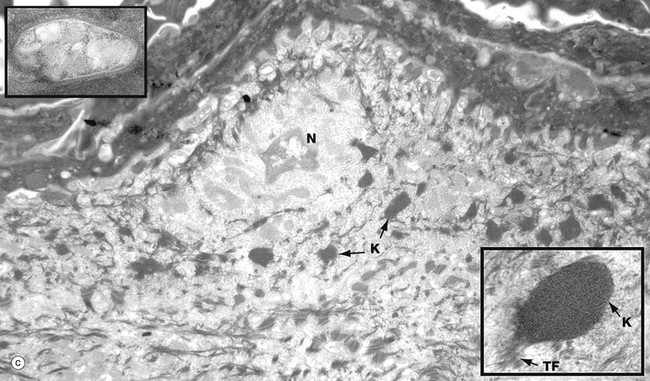
FIG. 9.3 Keratinocytes (illustrations (a) and (b) opposite)
(a) Basal keratinocytes, EM ×10 000, inset ×30 000 (b) Keratinocyte from prickle cell layer, EM ×15 000, inset ×40 000 (c) Keratinocyte from granular layer, EM ×12 000, inset ×50 000
Micrograph (a) shows a row of three basal cells BC sitting on the epidermal basement membrane BM, which can only just be discerned as a convoluted line in the low-magnification main picture. The basal cells are cuboidal with prominent nuclei, nucleoli, and a perinuclear zone containing ribosomes and mitochondria. Tonofibrils TF are present towards the periphery of the cell.
The inset shows the linkage of the basal surface of the cell to the basement membrane BM by hemi-desmosomes HD into which tonofibrils TF are inserted. Basal cells link to each other and to overlying keratinocytes of the prickle cell layer by desmosomes. The pale processes in the spaces between basal cells are cytoplasmic melanocyte processes Me.
Micrograph (b) shows a single keratinocyte from the prickle cell layer. It has abundant electron-dense tonofibrils which extend into the many desmosomes D that link it firmly to adjacent keratinocytes. The inset shows a desmosome linking the cytoplasmic processes of two keratinocytes. Electron-dense tonofibrils from each cell attach to the desmosome.
Micrograph (c) shows a flattening keratinocyte in the granular layer immediately beneath the keratin layer. The cell is keratinising with an ill-defined remnant of the nucleus N. The cytoplasm contains both linear cytokeratin tonofibrils and electron-dense ovoid keratohyaline granules K, one of which is shown merging with tonofibrils TF at higher magnification in the inset bottom right. Also present but not clearly visible in the low-magnification micrograph are lamellated pale-staining ovoid bodies (about 500 nm long) called keratinosomes or Odland bodies; one is shown at higher magnification in the inset top left. They contain a hydrophobic glycolipid which when released coats and binds together the keratin flakes, rendering them relatively water repellent.
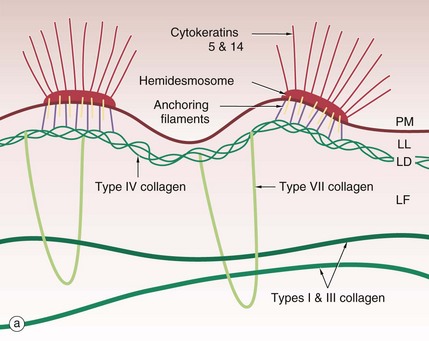
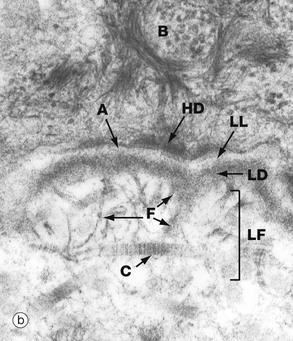
FIG. 9.4 Dermo-epidermal junction
(a) Diagram (b) EM ×64 000
The basement membrane at the junction of epidermis and dermis, known as the dermo-epidermal junction, is a specialised structure which binds epidermis to dermis. The major components are illustrated in the diagram (a).
Cytoplasmic tonofibrils, consisting of cytokeratins 5 and 14, bind to hemidesmosomes in the plasma membrane PM at the base of the basal cells of the epidermis. The hemidesmosomes contain multiple transmembrane and binding proteins including type XVII collagen and integrins. Binding to these on the outside of the plasma membrane are the anchoring filaments, including nidogen-1 and laminins. These correspond to an electron-lucent layer on electron microscopy called the lamina lucida LL. This appears to be the most easily disrupted of the basement membrane layers.
These anchoring filament proteins bind to a type IV collagen layer, type IV collagen being a specialised network/mesh-forming basement membrane collagen. On EM this corresponds to the lamina densa LD, an electron-dense layer.
Type VII collagen is a basement membrane specialised collagen which forms loops extending from the type IV collagen layer around fibres of structural types I and III collagens in the dermis, thereby tethering the basement membrane to the dermis; these are the anchoring fibrils. These form the lamina fibroreticularis LF, a poorly defined layer on EM.
The electron micrograph (b) shows part of a basal cell B, basement membrane layers of lamina lucida LL, lamina densa LD and lamina fibroreticularis LF, in addition to fibrils of type VII collagen F which tie to the type I collagen fibres C in the dermis. There are also finer fibrillin microfilaments which link to dermal elastic fibres. Basal cell hemidesmosomes HD can be seen at the plasma membrane with their fine anchoring protein filaments A which cross the lamina lucida.
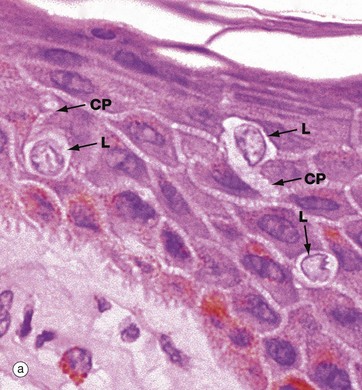
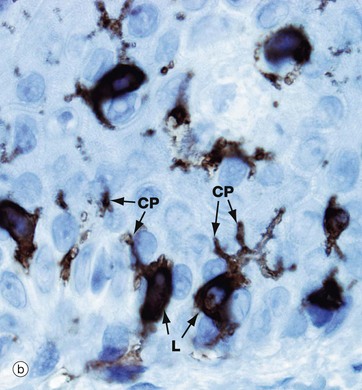
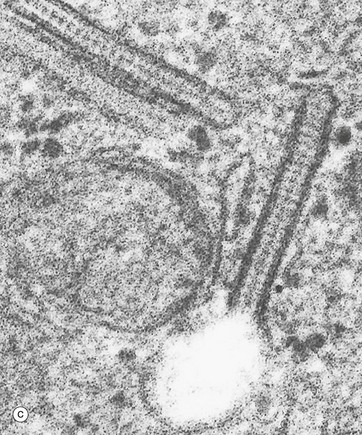
FIG. 9.5 Langerhans cells
(a) H&E (HP) (b) Immunohistochemistry for CD1a (HP) (c) Birbeck granule, EM ×100 000
Langerhans cells are intra-epidermal antigen presenting cells, historically referred to as histiocytes. They are present in all layers of the epidermis but are most easily recognised in the prickle cell layer. They are also present in the upper dermis, particularly around small blood vessels. When stimulated, they migrate to dermis and then via lymphatics to lymph nodes.
Micrograph (a) shows pale-staining Langerhans cells L in the epidermis; they have irregularly lobulated nuclei and almost clear cytoplasm. Cytoplasmic processes CP extend from the cells and insinuate between keratinocytes of all layers. The extensive network of cytoplasmic processes is highlighted in the immunohistochemical preparation shown in (b).
Electron micrograph (c) shows the characteristic cytoplasmic organelle of Langerhans cells, the Birbeck granule, a rod-like structure with regular cross-striations, one end of which frequently distends in a vesicle so that they resemble a tennis racket. They are part of the endosome system. Their exact function is not known.
Melanocytes
Melanocytes produce the pigment melanin which is responsible for skin and hair colour. The pigment exists in various forms from yellowish brown to black and has a protective function against ultraviolet light. Melanin is synthesised from the amino acid tyrosine by melanocytes within specific cytoplasmic organelles called melanosomes. These are transferred to the keratinocytes through a complex network of melanocyte cytoplasmic processes; these processes can be seen in electron micrographs of epidermis, running in the narrow spaces between keratinocytes (Fig. 9.3). Within keratinocytes, melanosomes usually form a cap sitting over the nucleus.
Melanocytes are present as separated individual cells in the basal layer of the epidermis and are more numerous in areas which are more exposed to light. There is no great difference in numbers of melanocytes between white- and dark-skinned people, but they are considerably more synthetically active in darker-skinned people. Melanocytes can be stimulated into producing more melanin by increasing exposure to UV light. This may produce a socially desirable suntan, but forced stimulation of melanocytes has its drawbacks and can be associated with the development of malignant tumours (see textbox opposite).
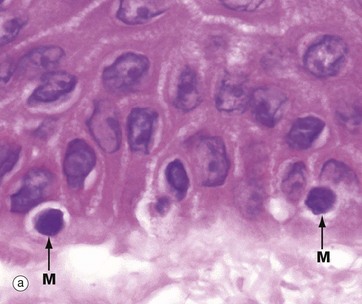
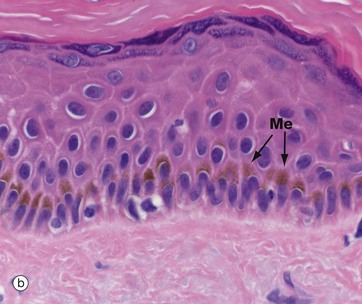
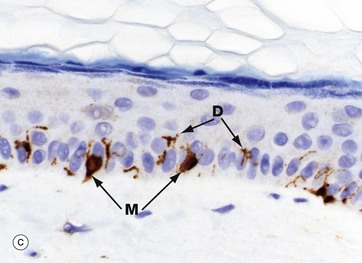
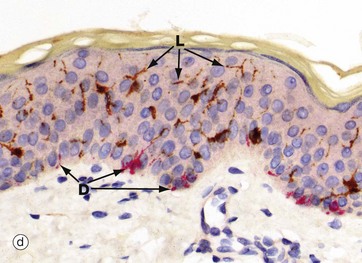
FIG. 9.6 Melanocytes
(a) H&E (HP) (b) H&E, pigmented skin (MP) (c) Immunohistochemistry for melanA (MP) (d) Dual immunohistochemistry for melanA (red) and langerin (brown) (MP)
Micrograph (a) shows normal epidermis with scattered melanocytes M in the basal layer. They appear to have rounded cell bodies with clear cytoplasm, but in fact have multiple fine, branching dendritic processes not seen in the H&E stains. Micrograph (b) demonstrates basal brown melanin pigment Me in dark-coloured skin.
Micrograph (c) is an immunohistochemical stain against a melanocyte antigen (melanA); it shows the globular cell bodies M situated in the basal layer and their branching dendritic processes D extending between keratinocytes. Because of the tortuous routes of these processes between the keratinocytes, their full length is rarely seen, often only short segments in any section. Melanocytes transfer melanosomes to the keratinocytes. These dendritic processes extend the numbers of keratinocytes serviced by each melanocyte.
Micrograph (d) is a dual immunoperoxidase stain with melanocytes (red) and Langerhans cells (brown) showing both cell types and the dendritic processes of melanocytes D and Langerhans cells L.
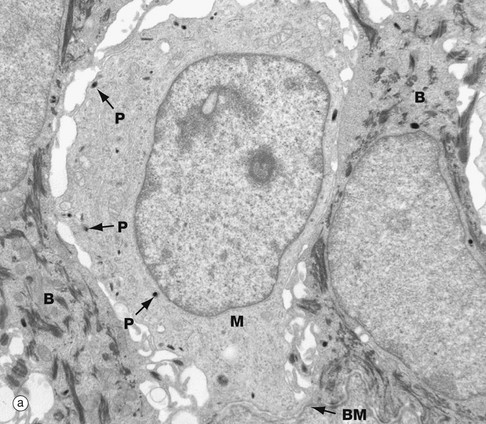
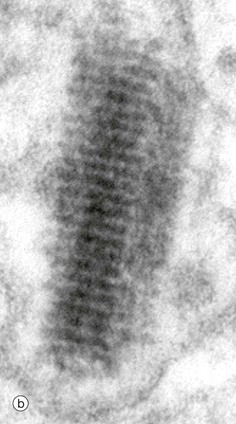
FIG. 9.7 Melanocytes
(a) EM ×15 000 (b) EM ×300 000
At low-power, electron micrograph (a) shows a pale-staining melanocyte M between two tonofibril-containing basal keratinocytes B; all are sitting on an indistinct basement membrane BM. The cytoplasm of the melanocyte has no tonofibrils but contains scanty tiny round or oval dark-staining premelanosomes and melanosomes P responsible for the synthesis of melanin. Tyrosine is converted into dihydroxyphenylalanine (DOPA) and then polymerised into melanin, which later links to protein to form melanoprotein.
The high magnification, electron micrograph (b) shows the ultrastructural features of a premelanosome. It is around or cylindrical electron-dense structure with distinct transverse striations and sometimes faint longitudinal striations. Sometimes, an indistinct surrounding membrane can be seen.
Innervation and Nerve Endings of the Skin
The skin has both an efferent and an afferent nerve supply. The efferent (outgoing from brain) supply consists of non-myelinated fibres from the sympathetic component of the autonomic nervous system. It supplies the blood vessels in the skin and is responsible for vessel diameter and hence blood flow. It also provides a supply to the skin appendages, particularly to arrector pili muscles and the eccrine sweat glands. The afferent nervous system (ingoing to brain) subserves sensation and comprises both myelinated and non-myelinated fibres. It is responsible for transmitting impulses from the various sensory nerve endings to the central nervous system, and with them cutaneous sensation. The sensory nerve endings in the skin are in the form of both free nerve endings and specialised encapsulated nerve endings, the ‘capsules’ being modifications of Schwann cells; these specialised nerve endings in the skin are Meissner, Pacinian and Ruffini corpuscles and are illustrated and discussed in more detail in Ch. 7.
Free nerve endings (see Fig. 7.24) may be myelinated or non-myelinated and are mainly responsible for pain and itch sensations and detecting temperature. They occupy the papillary dermis and send twigs into the epidermis where some of them associate with Merkel cells (see Fig. 9.10); this combination acts as a slowly adapting mechanoreceptor. Free nerve endings also ramify around hair follicles.
Meissner corpuscles (see Fig. 7.24) are rapidly adapting mechanoreceptors responsible for touch sensation. They are particularly prominent in the papillary dermis of the pulps of the fingers and toes and soles and palms.
Pacinian corpuscles (see Fig. 7.25) are responsible for detection of deep pressure and vibration. In the skin, they are usually found deep in the subcutis, singly or in small clusters, being particularly numerous in the palms and soles.
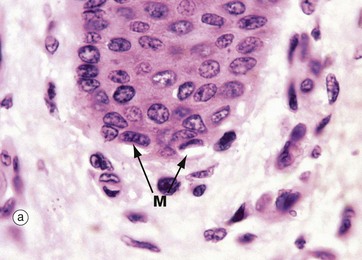
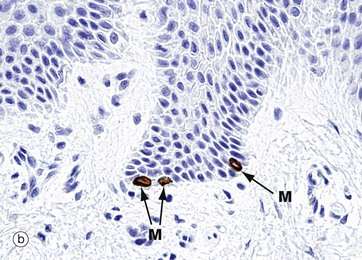
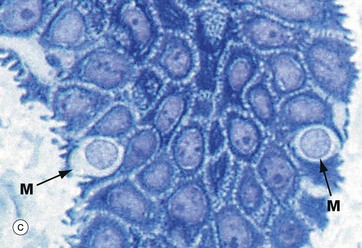
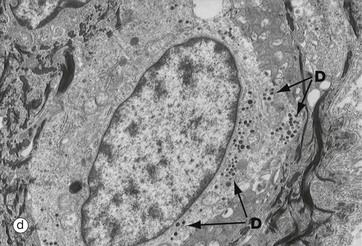
FIG. 9.8 Merkel cell
(a) H&E (MP) (b) Immunohistochemistry for CK20 (MP) (c) Epoxy resin thin section, toluidine blue ×1000 (d) EM ×10 000
Merkel cells are intra-epidermal touch receptors and contain neuroendocrine-type membrane-bound vesicles (dense core granules) in their cytoplasm, particularly near their base where they make synaptic junctions with myelinated sensory nerve twigs in the upper dermis. They are difficult to detect in routine H&E sections.
Micrograph (a) shows two Merkel cells M in the basal layer of epidermis. They are rounded cells with pale-staining cytoplasm. The immunohistochemical preparation in micrograph (b) illustrates three Merkel cells M. Micrograph (c) shows Merkel cells with pale nuclei and cytoplasm, while in electron micrograph (d) the dense core granules D can be seen.
Hair and Nails
Skin appendages first develop in the second trimester of intrauterine development as simple downgrowths of the surface epithelium (epidermis) into the developing subepithelial layers of mesoderm which will eventually become dermis and subcutis. The skin appendages include hair follicles, sebaceous glands, eccrine glands, apocrine glands and nails (fingers and toes).
Hair is produced in follicles in association with sebaceous glands and a smooth muscle bundle (arrector pili); these can be called pilosebaceous follicles or units. Hairs are long, thin, cylindrical shafts composed of keratin; hair shafts have a surface cuticle composed of a single layer of flattened keratin scales. This covers a cortex of keratin forming the bulk of the hair. Large hairs may have a central medulla.
In mammals, the function of hair and fur is thermoregulation, particularly heat conservation. Hair also serves a display function, providing colour and shape. The structure of the hair follicle is complex (Fig. 9.10). Hair growth is cyclical, with three phases: a long phase of active growth (anagen), a short phase of involution (catagen) and a short inactive involuted phase (telogen). The growth cycle of hairs varies from site to site: scalp hair follicles have an anagen growth phase of more than 2 years and a short telogen resting phase of a few months; correspondingly, scalp hair can grow to a great length. Pubic hair, coarse trunk hair, eyelashes and eyebrows have a short growth phase (anagen) and a relatively long resting phase (telogen), thereby limiting hair length at these sites.
In infancy, childhood and in adult females, body hair is fine and soft and is known as vellus, in contrast to the coarser hair of the scalp, which is known as terminal hair. Male sex hormone production at puberty stimulates development of terminal pubic and axillary hair in both sexes and the replacement of vellus hair with terminal hair on the mature male body.
The structure of hair follicles depends on the type of hair being produced: follicles of the scalp and other terminal hairs tend to be long and straight, whereas those which produce fine, downy vellus hairs are relatively short. Curly hair may be produced by curved follicles or follicles in which the hair bulb lies at an angle to the hair shaft.
Contraction of the arrector pili smooth muscle makes the hair stand up, a response called ‘goose flesh’, in a thermoregulation-related response.
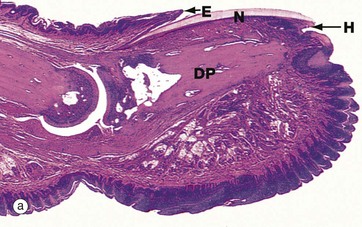
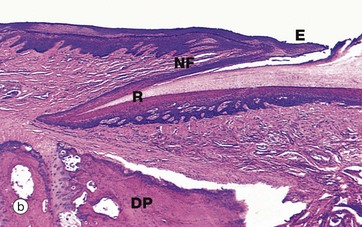
FIG. 9.9 Fingernail, monkey
(a) H&E (LP) (b) H&E (LP)
The dorsal skin surface of the tip of each finger and toe forms a highly specialised appendage, the nail, consisting of a dense keratinised plate, the nail plate N, which rests on a stratified squamous epithelium, the nail bed. The proximal end of the nail, the nail root R, and the underlying nail bed extend deep into the dermis to lie in close apposition to the distal interphalangeal joint. The dermis beneath the nail plate is firmly attached to the periosteum of the distal phalanx DP.
Nail growth occurs by proliferation and differentiation of the epithelium underlying the nail root (known as the nail matrix). The nail plate slides distally over the rest of the nail bed, which does not actively contribute to nail growth. Reflecting its proliferative activity, the nail matrix is thicker than the epithelium of the rest of the nail bed and exhibits pronounced epidermal ridges as seen in micrograph (b); on the surface, the distal part of the nail matrix is marked by the white crescent-shaped lunula at the base of the nail.
The skin overlying the root of the nail is known as the nail fold NF and its highly keratinised free edge is known as the eponychium E. The skin beneath the free end of the nail is known as the hyponychium H.
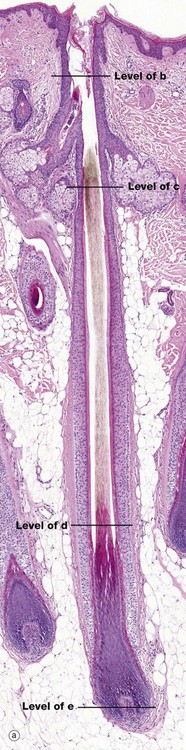
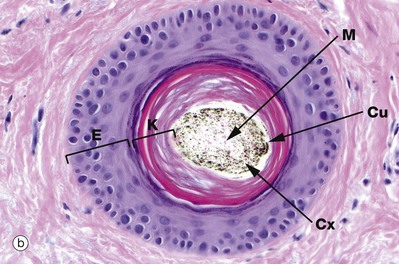
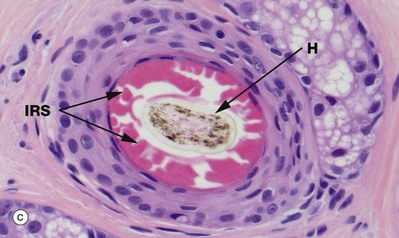
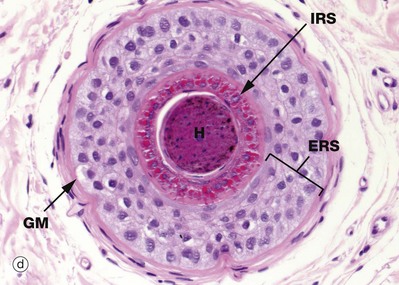
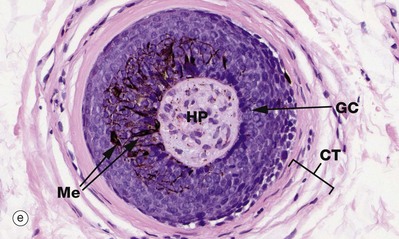
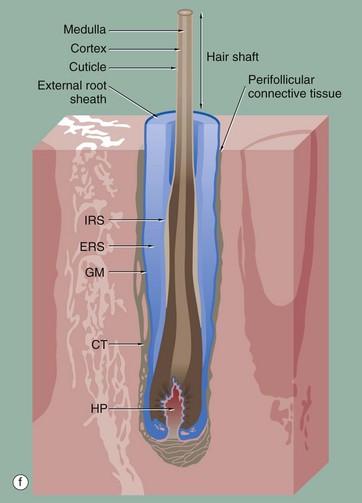
FIG. 9.10 Hair follicle (illustrations (a-e) opposite)
(a) H&E photomontage (LP) (b-e) H&E, TS (HP) (f) Diagram
The hair follicle is a tubular structure formed of specialised peri-follicular connective tissue and epithelium. Micrograph (a) is a full-length image of a terminal scalp hair. At the base, there is a bulbous expansion, the hair bulb, enclosing the hair papilla HP.
During active hair growth, the epithelial cells around the hair papilla proliferate to form the layers of the follicle. In the hair bulb, all the layers start as an indistinguishable proliferative cell mass known as the hair matrix. As they grow, they are pushed towards the skin surface from the hair bulb, and five epithelial layers form. The inner three epithelial layers undergo keratinisation to form the hair shaft (the hair) whilst the outer two layers form an internal and external sheath.
The cells of the innermost layer of the follicle undergo keratinisation to form the medulla M or core of the hair shaft; the medullary layer may not be distinguishable, especially in fine hairs. The medulla is surrounded by a broad, highly keratinised layer, the cortex Cx, which forms the bulk of the hair shaft. A third cell surface layer keratinises to form a hard, thin cuticle Cu on the surface of the hair, consisting of overlapping keratin plates, an arrangement which is said to prevent matting of the hair.
The outer fourth layer of the follicle constitutes the internal root sheath IRS; the cells of this layer become only lightly keratinised and lock with the developing cuticle, keeping the developing hair as a solid unit as it matures. After keratinisation, this layer fractures and fragments, leaving the hair shaft free in the follicle lumen, forming a space into which sebum is secreted around the maturing hair.
The outermost layer, the external root sheath ERS, is the external epithelial layer and merges with epidermis at the sebaceous glands. There is a thick, specialised basement membrane known as the glassy membrane GM and a surrounding specialised perifollicular connective tissue sheath CT.
Micrograph (e) shows a transverse section of the hair bulb, the distended base of the hair follicle, with an invaginated core of connective tissue, the hair papilla HP, containing small blood vessels and myelinated and non-myelinated nerve twigs. This is surrounded by a basal layer of palisaded active germinative cells GC. This germinative epithelium forms the follicle. In active (anagen) growth, the hair bulbs are prominent, with a well-formed hair papilla, and are located in subcutis.
In people with dark-coloured hair, melanocytes Me (see Fig. 9.6) are scattered amongst the proliferating cells of the hair bulb, with melanin being incorporated in the cells which will form the hair shaft. Black, brown and yellow forms of melanin are produced in various combinations and determine final hair colour.
Micrograph (d) is a transverse section through the hair follicle at the level shown by the line. The external root sheath ERS is separated from the connective tissue sheath CT by the glassy membrane GM. Passing inwards, the outermost cells, the external root sheath, have a homogeneous appearance. The internal root sheath IRS is recognised by its prominent, coarse, eosinophilic (keratohyaline) granules. Centrally is the hair shaft H.
In micrograph (c) at the level of sebaceous glands, the hair shaft is mature with a thin, pale-stained cuticle layer Cu which surrounds the pigmented cortex Cx and pale medulla M. The internal root sheath has now keratinised and is fragmenting as it is no longer required to support the developing hair shaft H, while the external root sheath is replaced by epidermis E.
Micrograph (b) is above the sebaceous glands. This part of the follicle is called the follicular infundibulum. It shows the hair shaft surrounded by epidermal-type keratin and epidermis. Note that the hair shaft is out of the plane of section in micrograph (a) at this level.
Each pilosebaceous follicle has an attached small smooth muscle called an arrector pili muscle (see Fig. 9.11).
Skin Glands
The skin has a range of different glands including sebaceous, eccrine and apocrine glands. Sebaceous glands occur in two forms. The majority are associated with hair follicles and develop as lateral protrusions from the hair follicle near the junction between its upper third (follicular infundibulum) and lower two thirds. Sebaceous glands secrete a mixture of lipids called sebum which may provide some waterproofing of the skin surface and hair shafts; the sebum is secreted into the hair follicle (see Fig. 9.11). At some sites in the skin (areolae and nipples, labia minora of vulva, eyelids), the sebaceous glands are independent of hair follicles and open directly onto the skin or mucosal surface.
Each hair follicle has an arrector pili muscle consisting of a bundle of smooth muscle fibres. These insert at one end into the sheath of the follicle just below the sebaceous glands, and at the other end into the dermal papillary area beneath the epidermis. Each hair follicle and its associated arrector pili muscle and sebaceous glands is known as a pilosebaceous unit.
Eccrine glands are found throughout the skin and are essential for thermoregulation through the production of sweat, opening onto the skin surface. Apocrine glands, while similar in architecture to eccrine glands, have a very limited distribution and connect to the follicular infundibulum, the superficial part of pilosebaceous (hair) follicles, and have a possible function in producing odour.
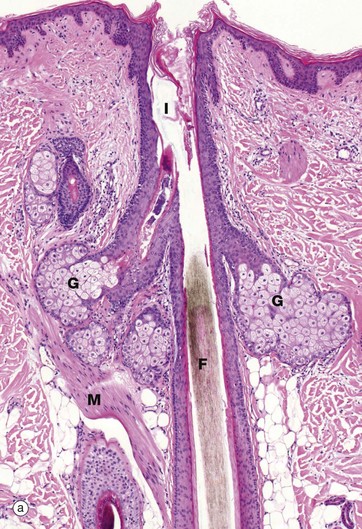
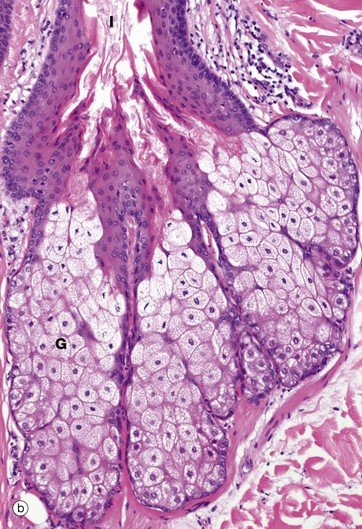
FIG. 9.11 Sebaceous glands and arrector pili
(a) H&E (LP) (b) H&E (MP)
Micrograph (a) illustrates the relationship of a sebaceous gland G and an arrector pili muscle M to a hair follicle F. At about mid-dermis, each hair follicle is surrounded by sebaceous glands which discharge their secretions onto the hair follicle and thence onto the skin surface. The superficial part of the follicle is called the infundibulum I. Sebaceous glands lie within the fibrous sheath surrounding the hair follicle and the glandular epithelium represents an outgrowth of the external root sheath. Arrector pili bind to the follicle just below the sebaceous glands.
More detail of sebaceous gland structure can be seen in micrograph (b). Each sebaceous gland has a branched acinar form, the acini converging upon a short duct which empties into the hair follicle beside the hair shaft. Each acinus consists of a mass of rounded cells packed with lipid-filled vacuoles; during tissue preparation, the lipid is largely removed, leaving clear spaces in the cytoplasm of these cells. Towards the duct, the lipid content of the acinar cells increases and the distended cells degenerate, releasing their contents, sebum, into the duct. This process is known as holocrine secretion (see Ch. 5). Cells lost by holocrine secretion are replaced by mitosis in the basal layer of the acinus.
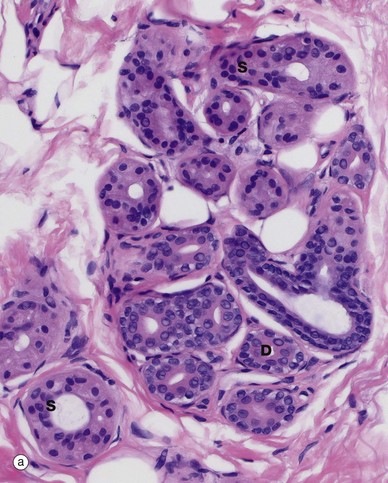
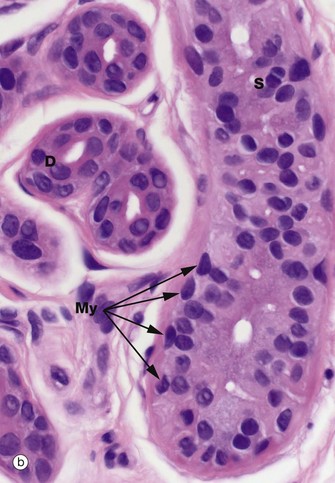
FIG. 9.12 Eccrine sweat glands and ducts
(a) H&E (MP) (b) H&E (HP)
Eccrine glands occur everywhere in the skin and are particularly frequent on the palms, soles, forehead and axillae. They arise as downgrowths from the epidermis during the second trimester of intrauterine life and their function is to secrete sweat. The evaporation of sweat provides a means of lowering body temperature and is an important component of the thermoregulatory system. In addition to water, sweat contains significant quantities of sodium and chloride ions, some other ions, urea and some low molecular weight metabolites.
Histologically, an eccrine gland has two main components. The secretory component is a coiled secretory gland situated in the deep reticular dermis; the secretions formed there are passed into a coiled eccrine duct close to the secretory gland. The duct then becomes straight as it ascends vertically through the dermis towards the skin surface.
The secretory gland component S has an inner layer of large columnar or pyramidal cells with central oval nuclei and pale eosinophilic cytoplasm, interspersed with smaller, rarer darker-staining, cells best identified by a specific stain for mucopolysaccharides such as the PAS reaction. The clear cells secrete the bulk of the watery sweat and the smaller cells secrete a glycoprotein. The lateral walls of the secretory cells show prominent interdigitations which separate in places to form canaliculi that open into the gland lumen (not illustrated). These interdigitations greatly expand the area of the lateral plasma membrane, facilitating ion exchange.
The glands have an attenuated outer layer of contractile myoepithelial cells My, which form a discontinuous layer between the secretory cells and the basement membrane. They are spindle shaped and are arranged with their long axes tangential to the long axis of the coiled tubular gland.
The eccrine ducts D appear darker staining and have an obvious double layer of epithelial cells, the inner layer being larger and more cuboidal with microvilli lining the lumen. The luminal aspect often has a characteristic eosinophilic appearance (sometimes called the cuticle). This is partly due to the presence of a compact layer of circumferentially arranged tonofibrils in the cuboidal duct epithelial cells at the base of the abundant microvilli. The duct epithelium is biochemically active and modifies the composition of sweat, reabsorbing sodium ions.
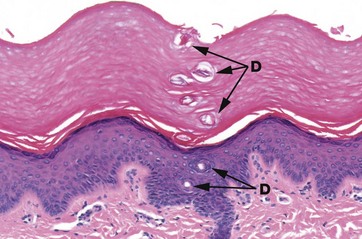
FIG. 9.13 Acrosyringium
H&E (MP)
As it passes through the epidermis, the eccrine duct (here called the acrosyringium) becomes coiled, a feature which is particularly apparent as it passes through the thick epidermis and the overlying keratin of the sole of the foot. Multiple cross-sections of the coiled duct D can be seen in the epidermis, continuing as a structure formed of keratin through the overlying stratum corneum.
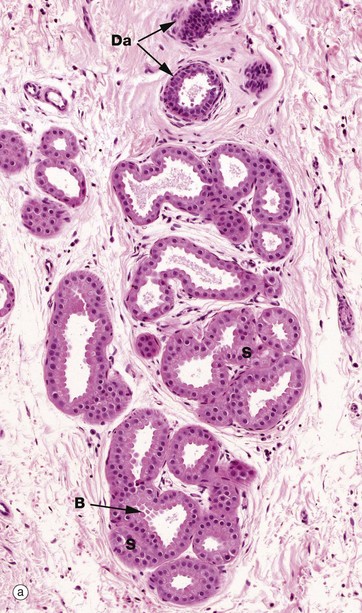
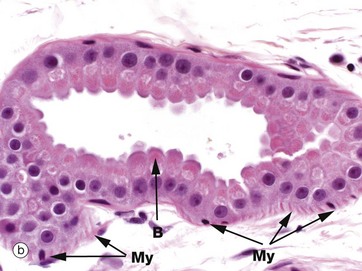
FIG. 9.14 Apocrine glands
(a) H&E (MP) (b) H&E (HP)
Apocrine glands are confined to a few localised areas, mainly in the axilla and groin. The secretory component is located in deep reticular dermis or subcutis and a duct system carries the secretion to be discharged into the upper part of the hair follicle above the sebaceous duct.
Apocrine gland secretions in humans have no defined function but, in other mammals, they are responsible for scent production, used in territory marking and as a sexual attractant. The secretory portion of the gland S is of the coiled tubular type with a widely dilated lumen. The secretory cells are usually low cuboidal with eosinophilic cytoplasm. The budding appearance B of the apical cytoplasm of some cells gave rise to the belief that the mode of secretion was of the apocrine type, but recent evidence suggests that this appearance may be due to a fixation artifact and that the original interpretations were erroneous. Like eccrine sweat glands, apocrine glands have a discontinuous layer of myoepithelial cells My between the base of the secretory cells and the prominent basement membrane. Their duct Da is histologically similar to that of eccrine sweat glands.
Apocrine glands do not become functional until puberty and, in women, undergo cyclical changes under the influence of the hormones of the menstrual cycle.
Dermis and Subcutis
The dermis and the subcutis (also known as the hypodermis or panniculus) are the layers beneath the epidermis. The overall thickness of skin is dependent on the thickness of the dermal and subcutaneous layers; in the eyelids, both layers are very thin and the skin is consequently thin and highly flexible whereas, in the back and buttocks, the dermis is thick and the subcutis variable but usually thick.
The dermis is composed of collagen and elastin fibres and is responsible for the tone and texture of the skin. In the young, the skin is tight because of the quality of the collagen and elastin but, with increasing age, the collagen and elastin in upper dermis progressively degenerate and the skin loses some of its texture and may wrinkle. The dermis also contains the skin appendages, the vascular supply to the skin, and nerves and sensory nerve endings.
The subcutis is predominately composed of adipose tissue, in many areas compartmentalised by vertical fibrous septa running from the deep reticular dermis to the fascial fibrous tissue layer which frequently underlies the subcutis. In areas with terminal hairs, the subcutis contains the deeper parts of anagen hair follicles (e.g. scalp), apocrine glands (e.g. axilla and groin) and eccrine glands (e.g. palms and soles). In parts of the face, the subcutis also contains sheets of skeletal muscle, the muscles of facial expression.
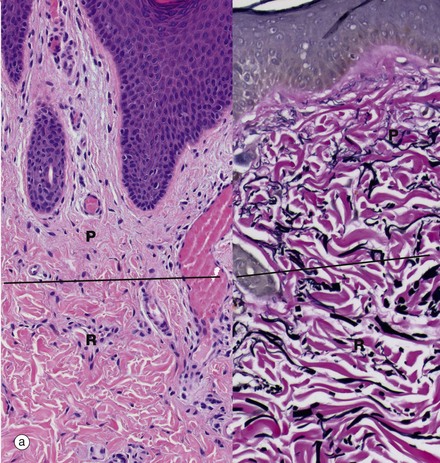
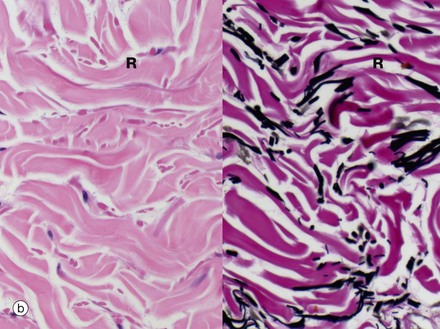
FIG. 9.15 Dermis
(a) Papillary and reticular dermis, H&E and EVG (LP) (b) Reticular dermis, H&E and EVG (HP)
The dermis is composed of bundles of collagen fibres and strands of elastic fibres embedded in scanty amounts of acellular ground substance, together with occasional fibroblasts which synthesise the collagen, elastic fibres and matrix. The dermis contains the vascular supply (see Fig. 9.16) and innervation of the skin and has two layers, a superficial papillary dermis beneath the epidermis and a deeper reticular dermis which borders the subcutis. Micrograph (a) shows the papillary dermis P, which is loose and contains very fine, interlacing collagen and elastic fibres that stain red and black, respectively, in the EVG stain. It contains arterioles, capillary loops and venules, as well as lymphatics and fine nerve twigs from the sensory nerve endings, such as Meissner corpuscles. Beneath the narrow papillary dermis is the reticular dermis R, generally a much thicker layer. The interface may be poorly defined as in these micrographs and has been marked by a line drawn across the images.
High-power micrograph (b) shows the reticular dermis. The collagen bundles and elastic fibres are much thicker than in the papillary dermis. The reticular dermis also contains blood vessels and nerves and the skin appendages. Lymphocytes, mast cells and macrophages are present but are scarce in normal dermis, but increase in number in many skin diseases. The reticular dermis varies greatly in thickness at different sites; it is thickest on the back and thinnest in the eyelids.
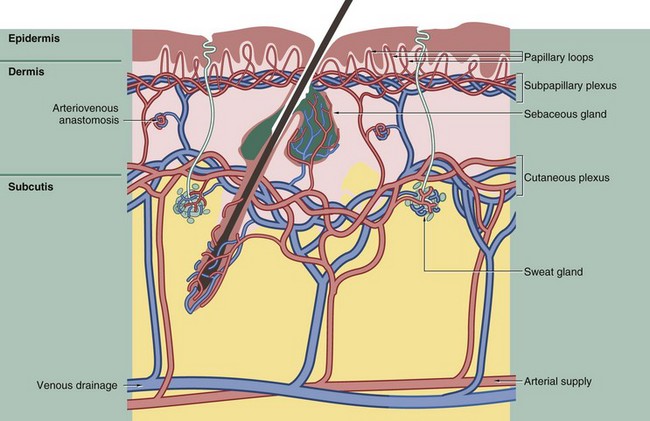
FIG. 9.16 The skin circulation
The circulation of the skin accommodates several different, sometimes conflicting, functional requirements: nutrition of the skin and appendages, increased blood flow to facilitate heat loss in hot conditions and decreased blood flow to minimise heat loss in cold conditions, whilst nevertheless maintaining adequate nutritional flow.
The arteries supplying the skin are located deep in the subcutis, from which they give rise to branches passing upwards to form two plexuses of anastomosing vessels. The deeper plexus lies at the junction of the subcutis and dermis and is known as the cutaneous plexus; the more superficial plexus lies at the junction between papillary and reticular dermis (see Fig. 9.15) and is known as the subpapillary or superficial plexus. This subpapillary plexus supplies the upper aspect of the dermis and gives rise to a capillary loop in each dermal papilla. The cutaneous (deep) plexus supplies the fatty tissue of the subcutis, the deeper aspect of the dermis and the capillary networks which envelop the hair follicles, deep sebaceous glands and sweat glands.
The venous drainage of the skin is arranged into plexuses broadly corresponding to the arterial supply. The skin has a rich lymphatic drainage which forms plexuses corresponding to those of the blood vascular system.
Special shunts called glomus bodies, under nerve control, provide direct arterial-to-venous diversion of blood, particularly in the extremities. This diversion plays an important role in thermoregulation by controlling blood flow to the appropriate parts of the dermis.
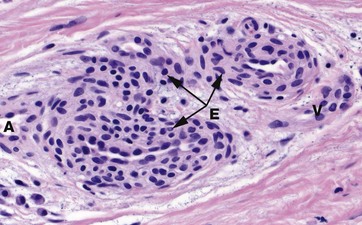
FIG. 9.17 Glomus body
H&E (MP)
In the dermis of the fingertips and other peripheral sites prone to excessive cold, such as the feet and external ear, the skin blood flow is controlled by structures called glomus bodies. The glomus consists of a highly convoluted segment of muscular vessel forming an arteriovenous shunt enveloped by condensed collagenous tissue. In histological section, one or more convolutions of the arterial A and venous V elements of the shunt are usually seen. The wall of the glomus vessel is greatly thickened by numerous smooth muscle cells assuming an epithelioid appearance E. This muscular structure enables controlled diversion of blood from small arteries to veins while dissipating the arterial pressure, thereby protecting the veins from excess pressure.
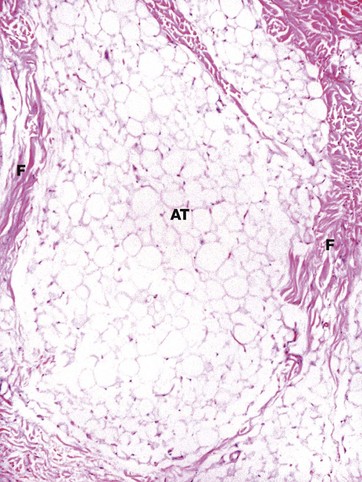
FIG. 9.18 Subcutis
H&E (LP)
This photomicrograph shows the subcutis from the skin of the upper thigh. It is composed of mature adipose tissue AT, partially compartmentalised by collagenous fibrous septa F which pass vertically from the lower reticular dermis. The thickness of the subcutis and the degree of compartmentalisation by fibrous septa varies from site to site. At this site, the subcutis normally contains no hair follicles or apocrine glands.
Examples of Regional Variations
As already mentioned, the skin shows regional variations in different areas of the body. The typical appearances of scalp, sole of foot, and vulval skin are illustrated in the following micrographs.
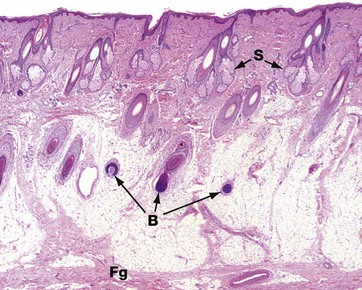
FIG. 9.19 Scalp
H&E (LP)
This low-power photomicrograph shows the full thickness of the skin of the scalp. The dermis is a broad layer containing the upper parts of the abundant hair follicles with their associated sebaceous glands S. The subcutis is similarly broad and contains the deeper parts of the hair follicles, particularly the hair bulbs B. The section does not contain complete pilosebaceous units because they are arranged obliquely; the owner has curly hair. The fibrous tissue layer Fg deep to the subcutis is a layer of fascia, the galea, which slides over the periosteum of the skull.
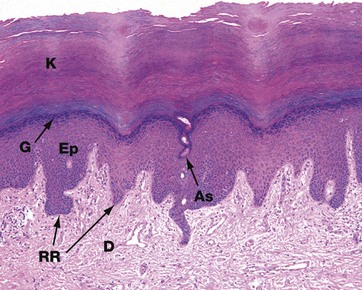
FIG. 9.20 Sole
H&E (LP)
The skin of the soles and palms is glabrous, i.e. completely devoid of hair and hair follicles. Because both are areas subject to regular shearing and frictional forces, the skin is structurally modified to resist these forces. The epidermis Ep is thick, with a prominent granular layer G producing a thick layer of compact keratin K, the stratum corneum. Elongated epidermal rete ridges RR extend into dermis, providing an increased area for attachment of epidermis to dermis D to minimise the risk of separation due to shearing forces during walking. Note the intraepidermal part of a sweat duct, the acrosyringium As, spiralling through epidermis. Sweat glands and nerve endings are numerous at both sites.
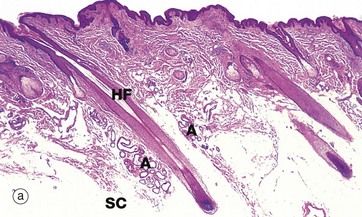
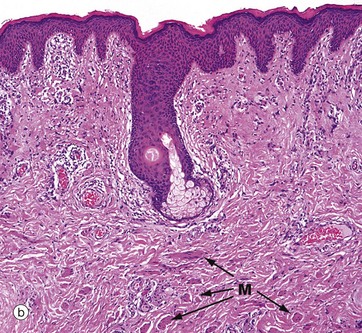
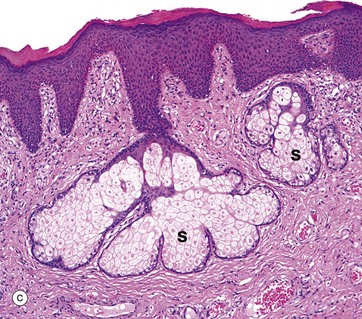
FIG. 9.21 Vulva
(a) Mons pubis, H&E (LP) (b) Labium majus, H&E (LP) (c) Labium minus, H&E (MP)
The vulva comprises the mons pubis, labia majora (singular is labium majus), labia minora (singular is labium minus), and the vestibule with its vestibular glands which mark the junction between vulva and vaginal canal. The vulva is also the site of the opening of the female urethra and the location of the erectile clitoris, the female homologue of the penis. The squamous epithelium of the labia minora is continuous with the squamous epithelium of the lower vagina.
Micrograph (a) shows the mons pubis skin with abundant oblique hair follicles HF (producing curly hair) and some apocrine glands A. This area has a thick pad of subcutaneous adipose tissue SC. The labia majora are longitudinal folds of skin which extend posteriorly from the mons pubis; the outer surface of the fold has the same type of skin as the mons pubis, i.e. with abundant pilosebaceous units, oblique hair follicles and apocrine glands in the subcutis, as shown in (a). On the inner surface, the hair follicles progressively disappear but sebaceous glands persist.
Micrograph (b) shows the inner surface of a labium majus, with the residue of a pilosebaceous unit in the upper dermis and numerous clumps of small smooth muscle fibres M in mid and deep dermis. The labia majora are the female homologue of the male scrotum and the labial muscle fibres are the homologue of the dartos muscle of the scrotum (see Ch. 18).
The labia minora are internal to the labia majora and are thin flaps of skin devoid of hair follicles. Micrograph (c) shows the outer aspect of a labium minus. It has a keratinising stratified squamous epidermis and scattered sebaceous glands S which open directly onto the skin surface, rather than into the necks of hair follicles as they do in hair-bearing skin (see Fig. 9.11). On the inner aspect, the labia minora have a thinner epidermis and keratin layer. The vaginal orifice is one of the sites of a muco-cutaneous junction, where the skin of the labia minora of the vulva meets the mucosa of the vaginal canal (see Ch. 19); similar mucocutaneous junctions exist at the oral, nasal and anal orifices.