Female reproductive system
Introduction
The female reproductive system has six major functions:
• Production of female gametes, the ova, by the process of oogenesis
• Reception of male gametes, the spermatozoa
• Provision of a suitable environment for the fertilisation of ova by spermatozoa
• Provision of an environment for the development of the fetus
• Expulsion of the developed fetus to the external environment
These functions are all integrated by an elegant system of hormonal and nervous mechanisms. The female reproductive system may be divided into three structural units on the basis of function:
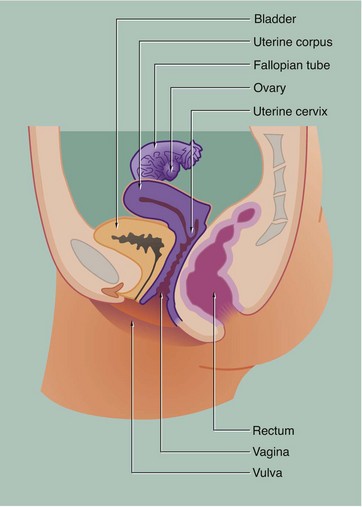
• The ovaries, which are the site of oogenesis, are paired organs lying on either side of the uterus adjacent to the lateral wall of the pelvis. In sexually mature mammals, ova are released by the process of ovulation in a cyclical manner, either seasonally or at regular intervals throughout the year. This cycle is suspended during pregnancy. The ovaries are also endocrine organs, producing the hormones oestrogen and progesterone. Both ovulation and ovarian hormone production are controlled by the cyclical release from the anterior pituitary of the gonadotrophic hormones luteinising hormone (LH) and follicle stimulating hormone (FSH). Oestrogen and progesterone in turn regulate LH and FSH production by feedback mechanisms. Thus, ovulation is coordinated with preparation of the uterus to receive the fertilised ovum.
• The genital tract extends from near the ovaries to an opening at the external surface and provides an environment for reception of male gametes, fertilisation of ova, development of the fetus and expulsion of the fetus at birth. The genital tract begins with a pair of Fallopian tubes, also called oviducts or uterine tubes, which conduct ova from the ovaries to the uterus where fetal development occurs. Fertilisation of ova by spermatozoa occurs within the Fallopian tubes. The uterus is a muscular organ, the mucosal lining of which undergoes cyclical proliferation under the influence of ovarian hormones. This provides a suitable environment for implantation of the fertilised ovum and subsequent development of the placenta. This is the means by which the developing fetus is nourished throughout gestation. At birth (parturition), strong contractions of the muscular uterine wall expel the fetus through the lower part of the uterus, the uterine cervix, into the birth canal or vagina. The vagina is an expansile muscular tube specialised for the reception of the penis during coitus and for the passage of the fetus to the external environment. At the external opening of the vagina there are thick folds of skin, the labia which, along with the clitoris, constitute the vulva.
• The breasts are highly modified apocrine sweat glands which, in the female, develop at puberty and regress at menopause. During pregnancy, the secretory components expand greatly in size and number in preparation for milk production (lactation).
In the non-pregnant state, the female reproductive system undergoes continuous cyclical changes from puberty to menopause. When ovulation is not followed by the implantation of a fertilised ovum, the thickened mucosal lining, the endometrium, degenerates and a new ovulation cycle commences. In humans, the thickened endometrium is shed in a period of bleeding known as menstruation. The first day of bleeding marks the beginning of a new cycle of endometrial proliferation which is known as the menstrual cycle. In humans, the standard menstrual cycle is of 28 days duration, but there is considerable variation among normal individuals. Ovulation usually occurs at the midpoint of the cycle.
In other mammals, the proliferated uterine mucosa is absorbed rather than shed, and the female is receptive to the male only during the period of ovulation, which is known as oestrus (or heat). The remaining part of the cycle is called the dioestrus, and the whole cycle is known as the oestrus cycle.
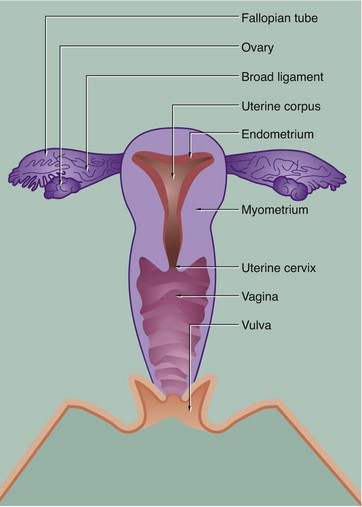
The general anatomy of the female genital tract is illustrated in Figs 19.1 and 19.2.
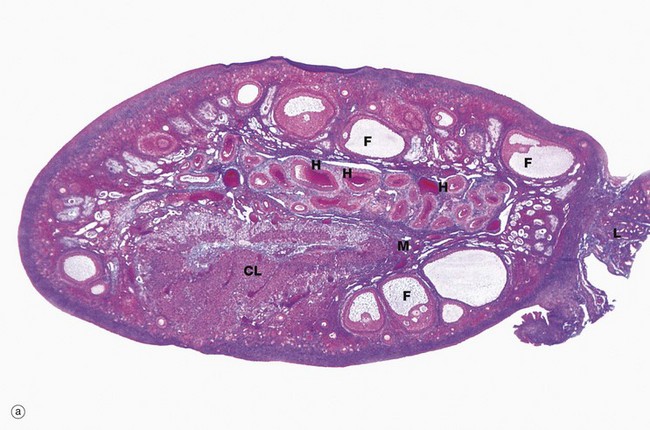
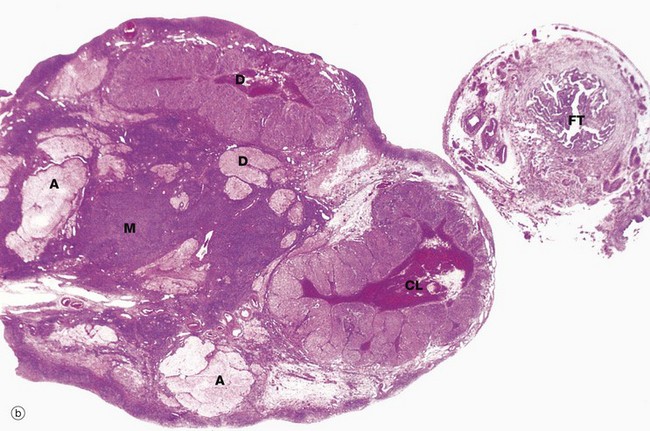
FIG. 19.3 Ovary (illustrations (a) and (b) opposite)
(a) Monkey, Azan (LP) (b) Human, H&E (LP)
The ovaries of all mammals have a similar basic structure. There are, however, considerable variations in accordance with species differences in the ovarian cycle and the stage in the cycle at which the ovary is examined. These micrographs compare the ovarian appearance of the monkey with that of the human.
The ovaries, which are some 3 to 5 cm long in humans, have a flattened ovoid shape. The body of the ovary consists of spindle-shaped cells, fine collagen fibres and ground substance that together constitute the ovarian stroma. The stromal cells resemble fibroblasts, but some contain lipid droplets. Bundles of smooth muscle cells are also scattered throughout the stroma. In the peripheral zone of the stroma, known as the cortex, there are numerous follicles that contain female gametes in various stages of development. In addition, there may also be post-ovulatory follicles of various kinds, namely corpora lutea (responsible for oestrogen and progesterone production, see Fig. 19.8), degenerate and former corpora lutea (corpora albicantes, see Fig. 19.11) and degenerate (atretic) follicles (see Fig. 19.10).
The superficial cortex is more fibrous than the deep cortex and is often called the tunica albuginea. However, unlike the testis, this is not an anatomically distinct capsule. On the surface of the ovary is an epithelial covering, misleadingly called germinal epithelium, which is a continuation of the peritoneum.
In the monkey ovary, numerous follicles F are seen in various sizes and states of development. In contrast, developing follicles are difficult to see in the human ovary (b) at this magnification. An active corpus luteum CL and several degenerating corpora lutea D and corpora albicantes A dominate this human ovary.
The central zone of the ovarian stroma, the medulla M, is highly vascular and contains hilus cells, which are morphologically very similar to Leydig cells of the testis. The ovarian artery (a branch of the aorta) and ovarian branches of the uterine artery form anastomoses in the mesovarium and the broad ligament L. From this arterial plexus, approximately 10 coiled arteries, the helicine arteries H enter the hilum of the ovary, best seen in micrograph (a). Smaller branches form a plexus at the corticomedullary junction, giving rise to straight cortical arterioles that radiate into the cortex. Here, they branch and anastomose to form vascular arcades, giving rise to a rich network of capillaries around the follicles. Venous drainage follows the course of the arterial system, the medullary veins being large and tortuous. Lymphatics arise in the perifollicular stroma, draining to larger vessels which coil around the medullary veins. Innervation of the ovary is by sympathetic fibres that not only supply blood vessels but also terminate on smooth muscle cells in the stroma around the follicles, possibly playing some part in follicular maturation and ovulation. In micrograph (b), the nearby Fallopian tube FT is included in the plane of section.
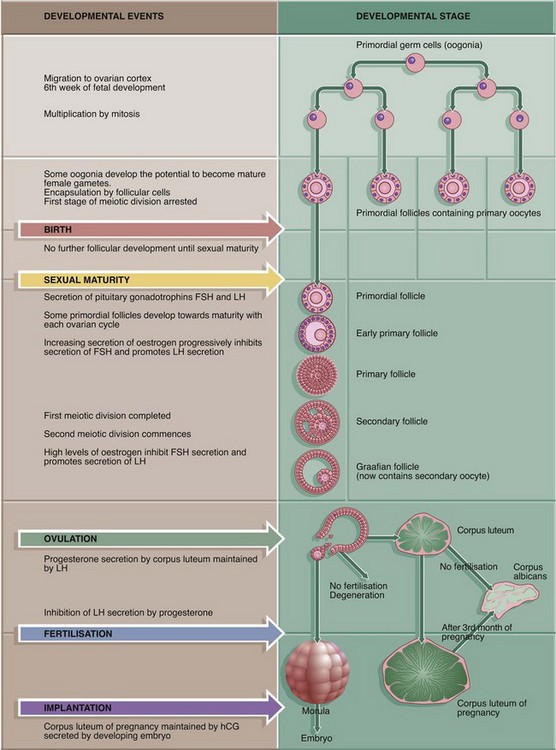
Follicular Development
During early fetal development, primordial germ cells called oogonia migrate into the ovarian cortex where they multiply by mitosis. By the fourth and fifth months of human fetal development, some oogonia enlarge and assume the potential for development into mature gametes. At this stage, they are called primary oocytes and commence the first stage of meiotic division (see Ch. 2). By the seventh month of fetal development, a single layer of flattened follicular cells surrounds the primary oocytes to form primordial follicles, of which there are approximately 500 000 in the human ovary at birth. This encapsulation arrests the first meiotic division and no further development of primordial follicles then occurs until after the female reaches sexual maturity (puberty). The process of meiotic division is only completed during follicular maturation, leading up to ovulation and fertilisation. Thus, all the female germ cells are present at birth, but the process of meiotic division is only completed some 15 to 50 years later! In contrast, in males, meiotic division of germ cells commences only after sexual maturity and formation and maturation of spermatozoa are accomplished within about 70 days (see Ch. 18). Female germ cells may undergo degeneration (atresia) at any stage of follicular maturation.
During each ovarian cycle, a cohort of up to 20 primordial follicles is activated to begin the maturation process. Usually, only one follicle reaches full maturity and undergoes ovulation while the remainder regress before this point. The reason for this apparent wastage is unclear. During maturation, however, the follicles have an endocrine function which may be far beyond the capacity of a single follicle and so the primary purpose of the other follicles may be to act as an endocrine gland.
Follicular maturation involves changes in the oocyte, in the follicular cells and in the surrounding stromal tissue. Follicular maturation is stimulated by FSH (follicle stimulating hormone) secreted by the anterior pituitary gland.
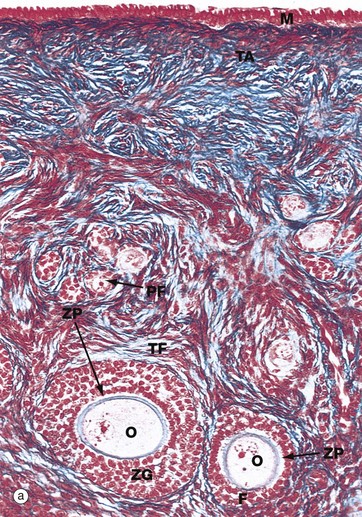
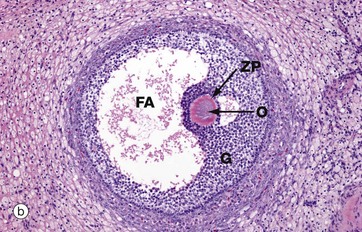
FIG. 19.4 Ovarian cortex
(a) Monkey, Azan (MP) (b) Human, H&E (MP)
Micrograph (a), taken from a monkey, shows the typical appearance of follicles in the ovarian cortex and illustrates several stages in early follicular development.
In the mature ovary, undeveloped follicles exist as primordial follicles PF which are composed of a primary oocyte surrounded by a single layer of flattened follicular cells. The primary oocyte has a large nucleus with dispersed finely granular chromatin, a prominent nucleolus and little cytoplasm.
At the lower right of the field, a primordial follicle has been stimulated, increasing in size to form a primary follicle. Its oocyte O has greatly enlarged and the follicular cells F have multiplied by mitosis and become cuboidal in shape. They are now known as granulosa cells. A thick homogeneous layer of glycoprotein and acid proteoglycans, the zona pellucida ZP, develops between the oocyte and the follicular cells. Both cell types probably contribute to its formation.
With further follicular development, as seen in the large follicle at lower left, the surrounding stromal cells begin to form an organised layer around the follicle called the theca folliculi TF, separated from the granulosa cells by a basement membrane. Theca cells are derived from the fibroblast-like cells of the ovarian stroma. The primary follicle continues to enlarge and the granulosa cells continue to proliferate, forming a layer several cells thick called the zona granulosa ZG.
Note also in this micrograph the fibrous tunica albuginea TA and the single layer of cuboidal or columnar mesothelial cells M on the surface of the ovary. In humans, this mesothelial layer is low cuboidal rather than columnar. This layer is continuous with the mesothelial lining of the peritoneal cavity and was formerly known as the germinal epithelium, based upon the mistaken belief that these cells were the origin of the female germ cells.
Micrograph (b) illustrates a section of human ovary, showing the next stage in follicular maturation. Fluid-filled spaces develop between the granulosa cells G, and these begin to coalesce to form the follicular antrum FA. This is now known as a secondary follicle. The zona pellucida ZP is well seen in this micrograph, but the nucleus of the oocyte O does not lie in this plane of section. Details of the secondary follicle are illustrated at higher magnification in Fig. 19.5.
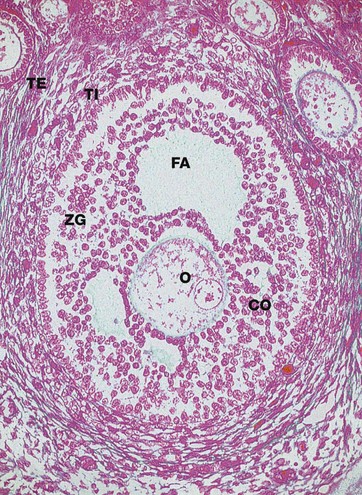
FIG. 19.5 Secondary follicle
Azan (MP)
Primary follicles continue to develop to form secondary follicles and acquire the features seen in this micrograph. By now they are usually situated deeper in the ovarian cortex.
The zona granulosa ZG continues to proliferate, and small fluid-filled spaces appear within it. These fuse to form the follicular antrum FA, in which follicular fluid accumulates. At this stage, the oocyte O has almost reached its full size and becomes situated eccentrically in a thickened area of the granulosa called the cumulus oophorus CO.
At the periphery of the follicle, the theca folliculi has developed two layers, the theca interna TI, comprising several layers of rounded cells, and the less well-defined theca externa TE, consisting of spindle-shaped cells that merge with the surrounding stroma.
The cells of the theca interna have the features of typical steroid-secreting cells (see Fig. 17.16) and produce oestrogen precursors (e.g. androstenedione), oestrogen and, in the preovulatory stage, progesterone. In the ovary, these steroid-secreting cells are often described as luteinised. Follicular hormones promote proliferation of the endometrium in readiness for the implantation of a fertilised ovum.
The theca externa is composed of flattened stromal cells and has no endocrine function. The granulosa cells also produce hormones from the stage of antral formation onwards. Oestrogen is produced from precursors secreted by the theca interna, as well as small amounts of intrafollicular FSH and, at ovulation, the FSH inhibitor inhibin F.
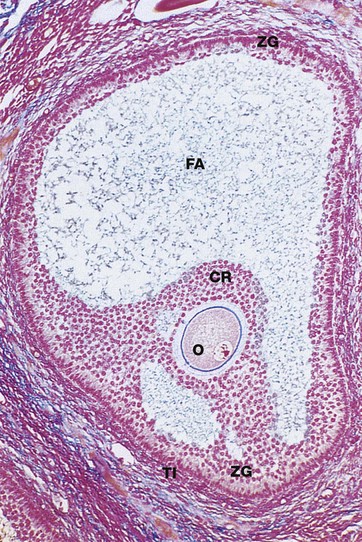
FIG. 19.6 Graafian follicle
Azan (LP)
Approaching maturity, further growth of the oocyte ceases and the first meiotic division is completed just before ovulation. At this stage, the oocyte becomes known as the secondary oocyte and commences the second meiotic division. The first polar body (see Ch. 2), containing very little cytoplasm, remains inconspicuously within the zona pellucida. The follicular antrum FA enlarges markedly and the zona granulosa ZG now forms a layer of even thickness around the periphery of the follicle. The cumulus oophorus diminishes, leaving the oocyte O surrounded by a layer several cells thick, the corona radiata CR, which remains attached to the zona granulosa by thin bridges of cells. Before ovulation, these bridges break down and the oocyte, surrounded by the corona radiata, floats free inside the follicle.
Note the surrounding theca interna TI, consisting of plump luteinised cells. By this stage, the follicle has reached between 1.5 and 2.5 cm in diameter and bulges under the ovarian surface. The overlying surface epithelial cells are flattened and atrophic and the thin intervening stroma becomes degenerate and avascular.
At ovulation, the mature follicle ruptures and the ovum, made up of the secondary oocyte, zona pellucida and corona radiata, is expelled into the peritoneal cavity near the entrance to the Fallopian tube. The second meiotic division of the oocyte is not completed until after penetration of the ovum by a spermatozoon.
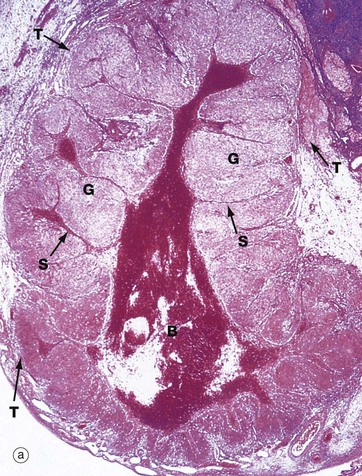
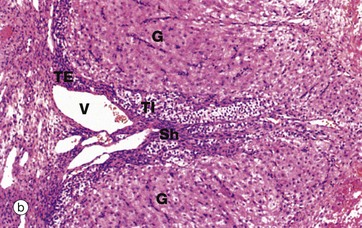
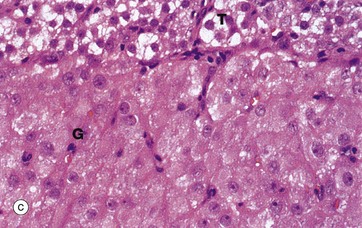
FIG. 19.8 Corpus luteum of menstruation
(a) H&E (LP) (b) H&E (MP) (c) H&E (HP)
Following ovulation, the ruptured follicle collapses and fills with a blood clot to form the corpus luteum of menstruation, which has a brief career as an endocrine organ.
The corpus luteum of menstruation is about the same size as the antecedent ovulatory follicle (i.e. 1.5 to 2.5 cm). Under the influence of luteinising hormone (LH) secreted by the anterior pituitary, granulosa cells increase greatly in size and begin secretion of progesterone. The granulosa cells acquire the characteristics of steroid-secreting cells and are now called granulosa lutein cells. Progesterone promotes the changes in the endometrium that make it ready for implantation of the embryo should fertilisation occur (see Figs 19.15 to 19.19). Thus the cycles of production of oocytes and the preparation of the endometrium (the menstrual cycle) are coordinated by the same set of hormones.
The cells of the theca interna also increase somewhat in size and acquire similar cytoplasmic features to the luteinised granulosa cells. Although interrupted by ovulation, these cells (as well as the granulosa cells) continue to secrete oestrogens which are necessary to maintain the thickened uterine mucosa. These cells become known as theca lutein cells.
The basement membrane between the zona granulosa and the theca interna breaks down and these layers are invaded by capillaries and larger vessels from the theca externa to form a rich vascular network, characteristic of endocrine glands.
Progesterone production by the corpus luteum is dependent on LH from the anterior pituitary, but rising progesterone levels inhibit LH production. Without the continuing stimulus of LH, the corpus luteum cannot be maintained and, 12 to 14 days after ovulation, it regresses, ultimately forming a functionless corpus albicans (see Fig. 19.11). Once the corpus luteum regresses, secretion of both oestrogen and progesterone ceases. Without these hormones, the endometrial lining of the uterus collapses, resulting in the onset of menstruation.
Micrograph (a) shows a corpus luteum of menstruation. In the centre, the remnant of the post-ovulatory blood clot B is seen, surrounded by a broad zone of granulosa lutein cells G, penetrated by septa S containing the larger blood vessels. Peripherally, a thin zone of theca lutein cells T can be seen. Externally, the corpus luteum is bounded by a zone of condensed stromal tissue, representing the theca externa of the antecedent Graafian follicle.
Micrograph (b) shows the margin of a corpus luteum at intermediate magnification. Most of the field is occupied by granulosa lutein cells G, large polygonal cells with abundant pale eosinophilic (pink-stained) cytoplasm and round nuclei. The cytoplasm contains plentiful smooth endoplasmic reticulum, abundant mitochondria, lipid droplets and some lipofuscin, giving the corpus luteum a yellow colour macroscopically.
At the periphery, there are theca cells which also extend in a finger-like extension, forming a sheath Sh around blood vessels V. The theca externa cells TE have darker-stained cytoplasm while the luteinised theca interna cells TI have pale cytoplasm due to their content of lipid droplets.
At high magnification in micrograph (c), granulosa lutein cells G may be compared with theca lutein cells T. The eosinophilic cytoplasm of the granulosa lutein cells contains numerous small lipid droplets which give rise to the vacuolated appearance seen in this preparation. Their larger spherical nuclei contain one or two prominent nucleoli. Theca lutein cells are smaller, with a more densely staining cytoplasm but with larger lipid vacuoles. Their ovoid nucleus has a single large nucleolus. The ultrastructure of the endocrine cells of the corpus luteum is characteristic of all steroid secretory cells (see Fig. 17.16).
As previously described, the granulosa lutein cells secrete progesterone (and a small amount of oestrogen) and the theca lutein cells secrete oestrogen precursors which are converted to oestrogen by the granulosa cells.
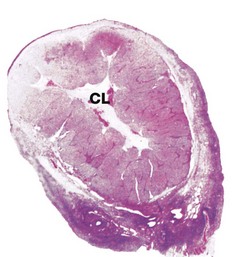
FIG. 19.9 Corpus luteum of pregnancy
H&E (LP)
Implantation of a fertilised ovum in the uterine wall interrupts the integrated ovarian and menstrual cycles. After implantation, a hormone called human chorionic gonadotrophin (hCG) is secreted into the maternal circulation by the developing placenta; hCG has an analogous function to LH and maintains the function of the corpus luteum in secreting oestrogen and progesterone until about the 9th week of pregnancy. After this time, the corpus luteum of pregnancy slowly regresses to form a functionless corpus albicans and the placenta takes over the major role of oestrogen and progesterone secretion until parturition.
This micrograph shows a human ovary during the first trimester of pregnancy. The corpus luteum CL is greatly enlarged and by now occupies most of the ovary. The organisation of the corpus luteum of pregnancy is similar to that of menstruation, but there are some histological changes that are almost specific for the corpus luteum of pregnancy. In particular, the granulosa lutein cells contain hyaline, eosinophilic inclusion bodies that tend to enlarge and then calcify as the pregnancy progresses.
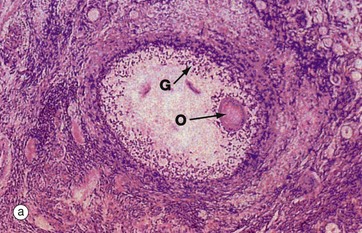
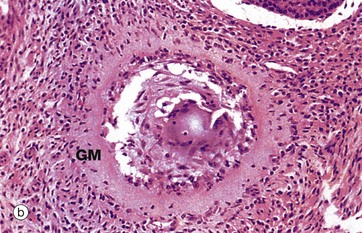
FIG. 19.10 Atretic follicles
(a) H&E (LP) (b) H&E (MP)
The process of follicular atresia (degeneration) may occur at any stage in the development of the ovum. By the sixth month of development, the fetal ovary contains several million primordial follicles yet, by the time of birth, only about half a million remain. Atresia continues until puberty and thereafter through the reproductive years. In addition, with each ovarian cycle approximately 20 follicles begin to mature, usually all but one becoming atretic at some stage before complete maturity.
The histological appearance of atretic follicles varies enormously, depending on the stage of development reached and the progress of atresia. The atretic follicle seen in micrograph (a) is a secondary follicle in early atresia. The oocyte O has degenerated and the granulosa cells G have begun to disaggregate.
Advanced atresia, as seen in micrograph (b), is characterised by gross thickening of the basement membrane between the granulosa cells and the theca interna, forming the so-called glassy membrane GM. Atretic follicles are ultimately replaced completely by collagenous tissue known as the corpus fibrosum. Most corpora fibrosa eventually disappear completely. In the postmenopausal woman, primordial follicles are absent, and the cortex consists of stroma and corpora albicantes only, with no developing follicles. The postmenopausal ovary is smaller than that in premenopausal women.
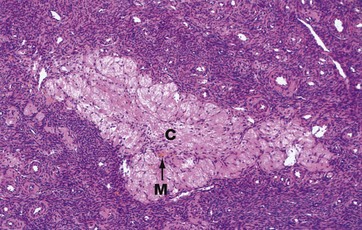
FIG. 19.11 Corpus albicans
H&E (LP)
The corpus albicans C is the inactive fibrous tissue mass that forms following the involution of a corpus luteum. The secretory cells of the degenerate corpus luteum undergo autolysis and are phagocytosed by macrophages M, a few of which, containing cytoplasmic haemosiderin pigment, can be seen here. The vascular supporting tissue regresses to form a relatively acellular collagenous scar containing a few fibroblasts. In the human ovary, corpora albicantes are a dominant feature, increasing in number with age and often appearing to occupy almost the whole ovarian stroma. Most regress completely leaving no trace, otherwise the postmenopausal ovary would contain approximately 500 corpora albicantes.
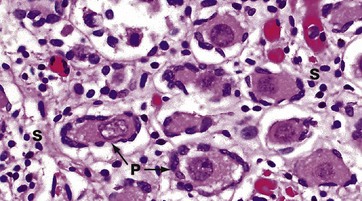
FIG. 19.12 Fetal ovary
H&E (HP)
In this micrograph of ovary from a term fetus, the ovarian cortex is seen to be packed with primordial follicles P. The surrounding stroma S is much more delicate than in an adult woman. These ova are arrested in the first meiotic division and remain so until the onset of puberty signals the waves of maturation of follicles that occur with each cycle in the reproductive years.
The Genital Tract
The genital tract consists of the Fallopian tubes, the uterus and the vagina, all of which have the same basic structure, consisting of a wall of smooth muscle with an inner mucosal lining and an outer layer of loose supporting tissue.
The mucosal and muscular components vary greatly according to their location and functional requirements. The whole tract undergoes cyclical changes under the influence of ovarian hormones which are released during the ovarian cycle. The cyclical changes which occur in the genital tract facilitate the entry of ova into the Fallopian tube, the passage of spermatozoa through the uterine cervix and into the Fallopian tube, the passage of the fertilised ovum into the uterus and the implantation and development of the fertilised ovum in the mucosal lining (endometrium) of the uterus.
Implantation of a fertilised ovum results in secretion of hormones that inhibit the ovarian cycle and produce changes in the genital tract necessary for fetal development and parturition.
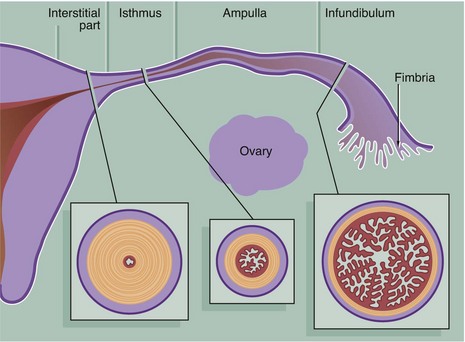
FIG. 19.13 Fallopian tubes
The Fallopian tubes (also called uterine tubes or oviducts) carry ova from the surface of the ovaries to the uterine cavity and are also the site of fertilisation by spermatozoa. The Fallopian tube is shaped like an elongated funnel and is divided anatomically into four parts as shown in the diagram.
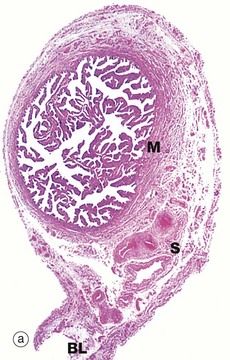
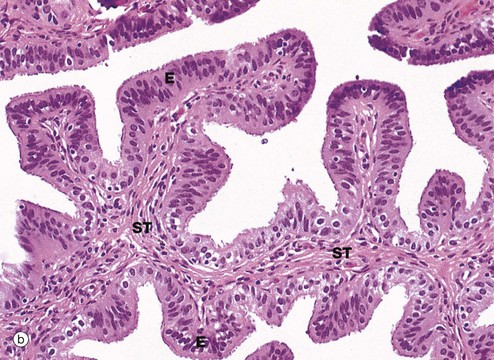
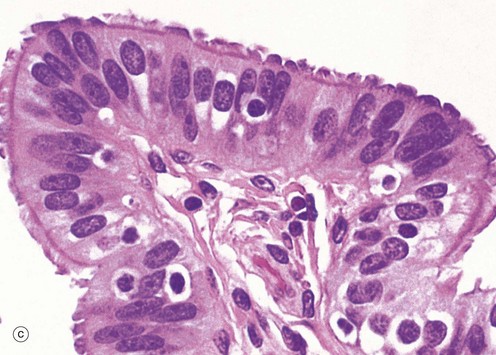
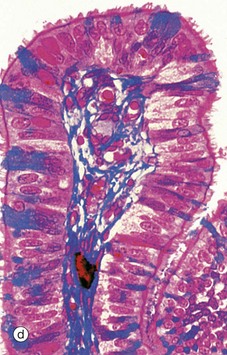
FIG. 19.14 Fallopian tube
(a) H&E (LP) (b) H&E (MP) (c) H&E (HP) (d) Azan (HP)
At the time of ovulation, the infundibulum moves so as to overlie the site of rupture of the Graafian follicle. Finger-like projections called fimbriae extending from the end of the tube envelop the ovulation site and direct the ovum into the tube. Movement of the ovum along the tube is mediated by gentle peristaltic action of the longitudinal and circular smooth muscle layers of the oviduct wall. This is aided by a current of fluid, propelled by the action of the ciliated epithelium lining the tube. The mucosal lining of the Fallopian tube is thrown into a labyrinth of branching longitudinal folds, a feature that is most prominent in the ampulla (a), which is the usual site of fertilisation. Note also in this micrograph, the muscular wall M and the vascular supporting tissue of the serosa S, which is continuous with the broad ligament BL. The serosal layer and broad ligament have a surface lining of mesothelium. The muscular wall has two layers, an inner circular and an outer longitudinal, not discernible at this magnification.
Micrograph (b) focuses on one of the mucosal folds of the ampulla. These have a branching core of vascular supporting tissue ST and are invested by a single layer of tall columnar epithelial cells E.
Micrograph (c) shows the tip of a mucosal fold at high magnification. The columnar cells of the epithelium are of three types: ciliated, non-ciliated secretory and intercalated cells. The non-ciliated cells produce a secretion that is propelled towards the uterus by the wave-like beating of the cilia of the ciliated cells, carrying with it the ovum. This secretion probably also has a role in the nutrition and protection of the ovum. The intercalated cells may be a morphological variant of the secretory cells. The ratio of ciliated to non-ciliated cells and the height of the cells undergo cyclical variations under the influence of ovarian hormones. The ciliated cells are generally shorter than the secretory cells, making the epithelial surface somewhat irregular in outline. Scattered intraepithelial lymphocytes are also present.
Micrograph (d) employs a method that stains the secretory cells blue. Note that the collagen of the supporting tissue core of the mucosal fold is also stained blue.
The Human Menstrual Cycle
The uterus is a flattened pear-shaped organ approximately 7 cm long in the non-pregnant state. Its mucosal lining, the endometrium, provides the environment for fetal development. The thick smooth muscle wall, the myometrium, expands greatly during pregnancy and provides protection for the fetus and a mechanism for the expulsion of the fetus at parturition.
The endometrium is variable in thickness, measuring between 1 and 5 mm at different stages of the menstrual cycle. The myometrium makes up the bulk of the uterus, measuring up to about 20 mm in a woman of reproductive age (see Fig. 19.2).
In women of child-bearing age, the endometrial lining of the uterine cavity consists of a pseudostratified columnar ciliated epithelium forming numerous simple tubular glands, supported by the cellular endometrial stroma. Under the influence of oestrogen and progesterone secreted during the ovarian cycle, the endometrium undergoes regular cyclical changes so as to offer a suitable environment for implantation of a fertilised ovum. These changes are summarised in Fig. 19.15 (overleaf). For successful implantation, the fertilised ovum requires an easily penetrable, highly vascular tissue and an abundant supply of glycogen for nutrition until vascular connections are established with the maternal circulation.
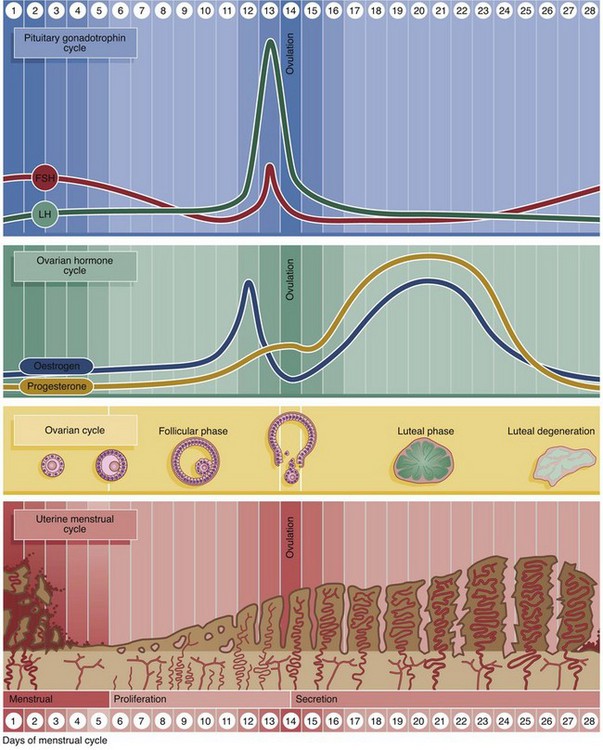
The cycle of changes in the endometrium proceeds through three distinct phases: menstruation, proliferation and secretion. These changes involve both the epithelium and supporting stroma.
• The menstrual phase. The first day of menstruation is, by convention, taken as the first day of the cycle, simply because it is easily identified. This is the phase of endometrial shedding that only occurs if there is failure of fertilisation and/or implantation of the ovum. Progesterone production by the corpus luteum is inhibited by negative feedback on the anterior pituitary, thus suppressing LH release and leading to involution of the corpus luteum. In the absence of progesterone, the endometrium cannot be maintained. Reactivation of FSH secretion initiates a new cycle of follicular development and oestrogen secretion. This, in turn, initiates a new cycle of proliferation of the endometrium from the endometrial remnants of the previous cycle.
• The proliferative phase. The endometrial stroma proliferates, becoming thicker and richly vascularised. The simple tubular glands elongate to form numerous long, coiled glands that begin secretion coincident with ovulation. The proliferative phase is initiated and sustained until ovulation by the increasing production of oestrogens from developing ovarian follicles.
• The secretory phase. Release of progesterone from the corpus luteum after ovulation promotes production of a copious, thick, glycogen-rich secretion by the endometrial glands.
A typical menstrual cycle is 28 days in length, although there is wide variation among normal women. Menstruation lasts on average 5 days. The proliferative phase continues until about the 14th day when ovulation occurs and the secretory phase begins. The secretory phase culminates at the onset of menstruation on about the 28th day.
The endometrium is divided into three histologically and functionally distinct layers. The deepest or basal layer, the stratum basalis, adjacent to the myometrium, undergoes little change during the menstrual cycle and is not shed during menstruation. The broad intermediate layer is characterised by a stroma with a spongy appearance and is called the stratum spongiosum. The thinner superficial layer, which has a compact stromal appearance, is known as the stratum compactum. The compact and spongy layers exhibit dramatic changes throughout the cycle and both are shed during menstruation. These layers are jointly referred to as the stratum functionalis.
The arrangement of the arterial supply of the endometrium has important influences on the menstrual cycle. Branches of the uterine arteries pass through the myometrium and immediately divide into two different types of arteries, straight arteries and spiral arteries. Straight arteries are short and pass a small distance into the endometrium, then bifurcate to form a plexus supplying the stratum basalis. Spiral arteries are long coiled and thick-walled and pass to the surface of the endometrium, giving off numerous branches which give rise to a capillary plexus around the glands and in the stratum compactum. Unlike the straight arteries, the spiral arteries are responsive to the hormonal changes of the menstrual cycle. The withdrawal of progesterone secretion at the end of the cycle causes the spiral arteries to constrict and this precipitates an ischaemic phase that immediately precedes menstruation.
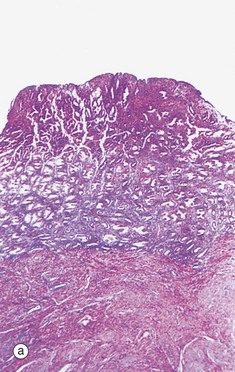
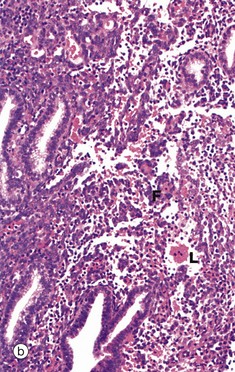
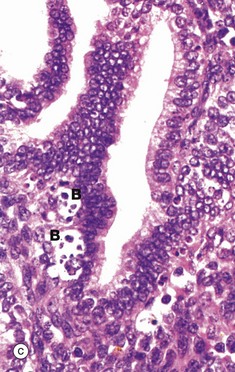
FIG. 19.16 Endometrium, the onset of menstruation
(a) H&E (LP) (b) H&E (MP) (c) H&E (HP)
In the absence of implantation of a fertilised ovum, degeneration of the corpus luteum results in cessation of oestrogen and progesterone secretion. In turn, this initiates spasmodic constriction in the spiral arterioles of the endometrial stratum functionalis F. The resulting ischaemia is initially manifest by degeneration of the superficial layers of the endometrium and leakage of blood L into the stroma. This is illustrated in micrographs (a) and (b). Stromal cells disaggregate and the endometrial glands collapse. These features are indicative of early necrosis of glands and stroma. At high magnification in micrograph (c), nuclear debris of endometrial cells (apoptotic bodies) B can be seen at the onset of menstruation. These cells have died by apoptosis (see Ch. 2).
Further ischaemia leads to degeneration of the whole stratum functionalis, which is progressively shed as menses. Menses is thus composed of blood, necrotic epithelium and stroma. Normally, menstrual blood does not clot due to the local release of inhibitory (anticoagulant) factors and its expulsion is enhanced by uterine contractions. By day 3 to 4 of menstruation, most of the stratum functionalis has been shed and proliferation of the basal layer of the endometrium has begun again.
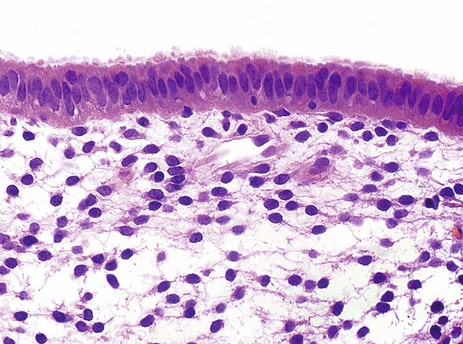
FIG. 19.17 Endometrial surface
H&E (HP)
This micrograph illustrates the surface epithelium of the endometrium, which is tall and columnar in form. Some of the cells bear cilia, the remainder having surface microvilli. Stromal cells have plump, spindle-shaped nuclei and scanty cytoplasm.
This specimen was obtained during the secretory phase of the menstrual cycle at a time when the stroma is quite oedematous. This can be seen in the clear spaces between the spindle-shaped stromal cells.
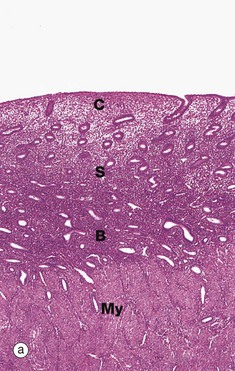
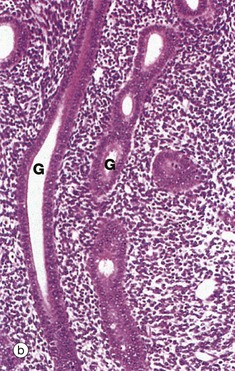
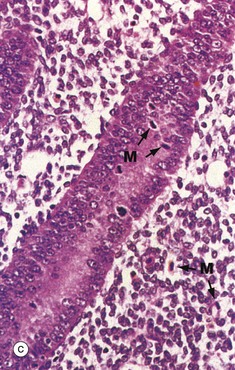
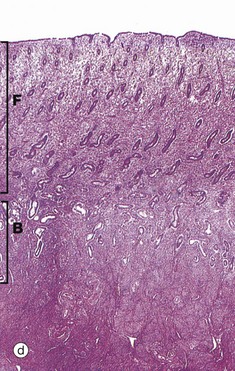
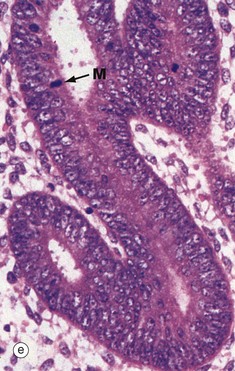
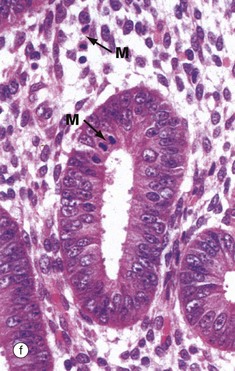
FIG. 19.18 Proliferative endometrium
(a) Early phase, H&E (LP) (b) Early phase, H&E (MP) (c) Early phase, H&E (HP) (d) Late phase, H&E (LP) (e) Late phase, H&E (HP) (f) Late phase, H&E (HP)
Micrograph (a) illustrates early proliferative endometrium at low magnification. The bottom of the field includes part of the muscular wall, the myometrium My. The relatively thin endometrium consists of the stratum basalis B, stratum spongiosum S and stratum compactum C. The glands at this stage are fairly sparse and straight.
As the glands, stroma and vessels proliferate, the endometrium gradually becomes thicker. By day 5 to 6 of the cycle, the surface epithelium has regenerated. During the proliferative phase, the epithelial cells acquire microvilli and cilia as well as the cytoplasmic organelles required for the secretory phase.
At higher magnification in micrograph (b), the straight tubular form of the endometrial glands G can be seen. At very high magnification in micrograph (c), the proliferating glandular epithelium is seen to consist of columnar cells with basally located nuclei exhibiting prominent nucleoli. Mitotic figures M can be seen, both in the epithelium and in the stroma. Note the highly cellular stroma which is almost devoid of collagen fibres.
By the late proliferative stage, shown at low magnification in micrograph (d), the endometrium has doubled in thickness. Note that in contrast to the stratum functionalis F, the appearance of the stratum basalis B is little changed when compared with the early proliferative phase. With further magnification, micrograph (e) shows that the tubular glands are now becoming coiled and more closely packed. At very high magnification in micrograph (f), mitotic figures M are more prevalent in both the glandular epithelium and the supporting stroma. The stroma is also somewhat oedematous at this stage. During the proliferative phase, there is a continuum of change that makes the precise dating of the cycle inaccurate in histological specimens. Lymphocytes and occasional lymphoid aggregates are a normal feature of late proliferative phase endometrium, but plasma cells are abnormal, indicating chronic infection (endometritis).
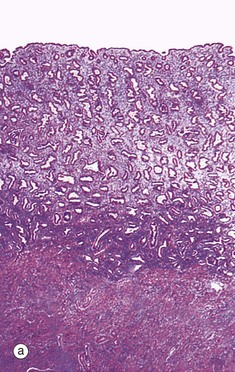
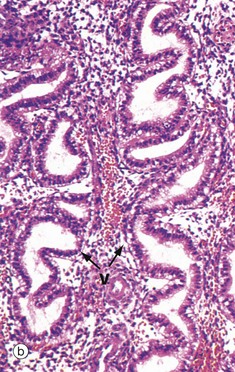
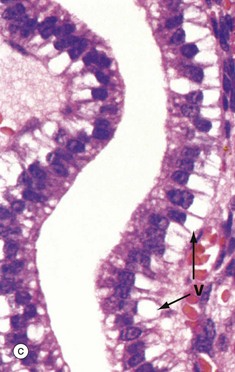
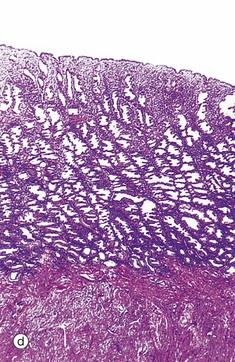
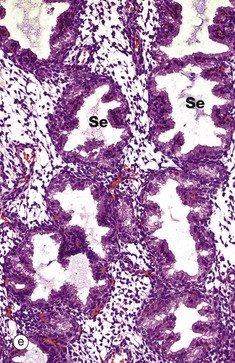
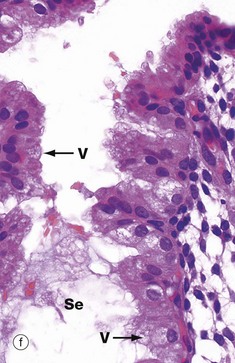
FIG. 19.19 Secretory endometrium
(a) Early phase, H&E (LP) (b) Early phase, H&E (MP) (c) Early phase, H&E (HP) (d) Late phase, H&E (LP) (e) Late phase, H&E (MP) (f) Late phase, H&E (HP)
Ovulation marks the onset of the secretory phase, although endometrial cell division continues for several days. At low magnification in micrograph (a), the coiled appearance of the glands is now more pronounced and the endometrium approaches its maximum thickness.
Under the influence of progesterone, the glandular epithelium is stimulated to synthesise glycogen. Initially, the glycogen accumulates to form vacuoles V in the basal aspect of the cells, thus displacing the nuclei towards the centre of the now tall columnar cells. This basal vacuolation of the cells appears on day 16 and is the characteristic feature of early secretory endometrium as seen at intermediate and high magnification in micrographs (b) and (c), respectively. Glycogen is an important source of nutrition for the fertilised ovum.
The late secretory phase is characterised by a saw-tooth appearance of the glands, containing copious thick glycogen- and glycoprotein-rich secretions Se. This is illustrated at low and intermediate magnification in micrographs (d) and (e), respectively,
At very high magnification in micrograph (f), the cytoplasmic vacuoles V can now be seen on the luminal aspect of the cell, and the nucleus has returned to its basal position. These vacuoles contain glycogen and glycoproteins that are secreted Se into the glandular lumen by apocrine-type secretion. Mitotic figures are absent. The stroma is by now at its most vascular and interstitial fluid begins to accumulate between the stromal cells. Endometrial stromal granulocytes, which are probably large granular lymphocytes, are found in the stroma at this stage. These changes in secretory phase endometrium make more precise dating possible on histological specimens than in the proliferative phase. Such examinations may be helpful in the investigation of infertility.
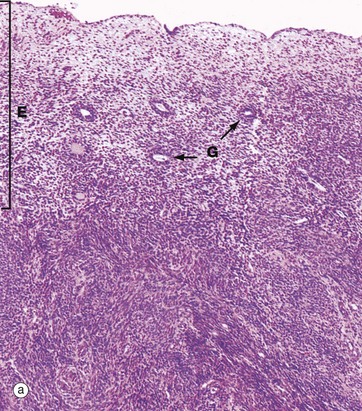
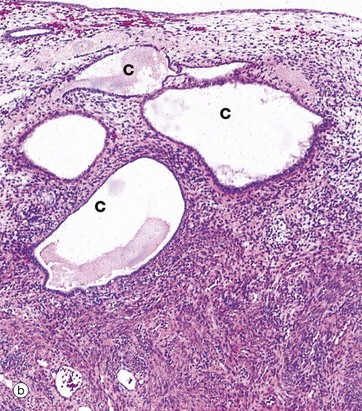
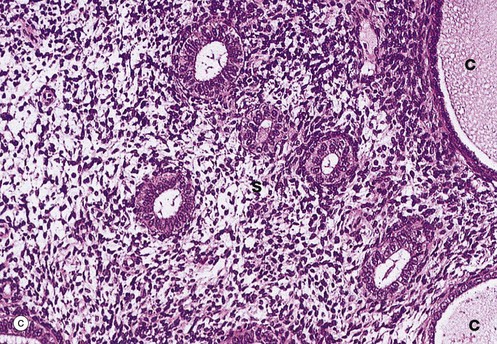
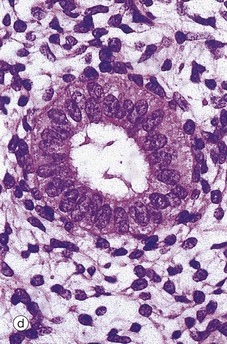
FIG. 19.20 Post-menopausal endometrium
(a) H&E (LP) (b) H&E (LP) (c) H&E (MP) (d) H&E (HP)
After the menopause, the cyclical production of oestrogen and progesterone from the ovaries ceases and the whole genital tract undergoes atrophy. As seen in micrograph (a), the endometrium E is thin, consisting only of the stratum basalis, and the glands G are sparse and inactive.
In some women, the glands become dilated to form cystic spaces C as shown in micrograph (b). The reason for this is unknown, but this appearance is so common as to be considered a normal variant.
At higher magnifications in micrographs (c) and (d), the glandular epithelial cells are cuboidal or low columnar with no mitotic figures or secretory activity. The epithelium which lines cystically dilated glands C, as shown in micrograph (c), is often flattened. The stroma S is much less cellular and contains more collagen fibres than during the reproductive years and no mitotic activity is seen. The myometrium also becomes atrophic after the menopause and the uterus shrinks to about half its former size.
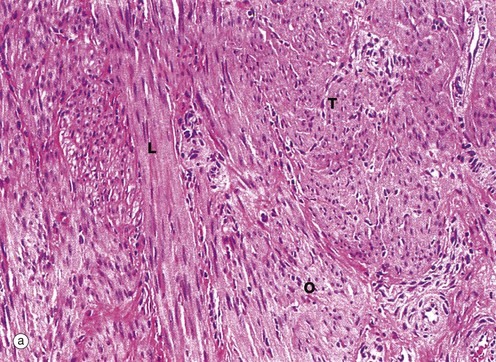
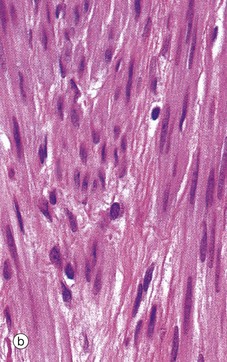
FIG. 19.21 Myometrium
(a) H&E (MP) (b) H&E (HP)
The main bulk of the uterus consists of smooth muscle, the myometrium, which is composed of interlacing bundles of long slender fibres arranged in ill-defined layers. This is readily seen in micrograph (a), which contains bundles of fibres in transverse T, longitudinal L and oblique sections O. Within the muscle, there is a rich network of arteries and veins which are supported by collagenous supporting tissue. Micrograph (b) shows detail of the smooth muscle cells at high magnification, highlighting the closeness with which the muscle fibres are packed.
During pregnancy, in response to increased levels of oestrogens, the myometrium increases greatly in size, mainly by increasing cell size (hypertrophy), although some increase in cell numbers (hyperplasia) due to cell division may also occur.
At parturition, strong contractions of the myometrium are reinforced by the action of the hormone oxytocin, secreted by the posterior pituitary. These contractions expel the fetus from the uterus and also constrict the blood supply to the placenta, thus precipitating its detachment from the uterine wall.
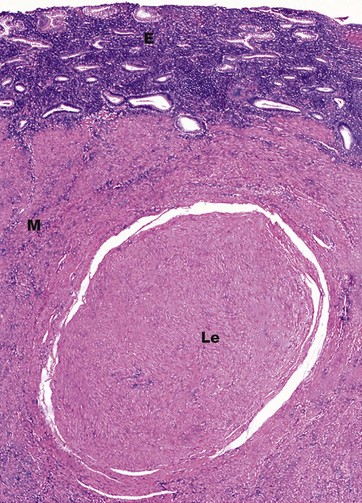
FIG. 19.22 Uterine ‘fibroid’
H&E (LP)
The leiomyoma Le consists of bland smooth muscle fibres which are very like their normal counterparts in appearance. The smooth muscle fibres form whorls and are embedded in a fibrous stroma. The resulting nodule has a very well-circumscribed margin and there is a pseudocapsule of compressed smooth muscle separating it from the normal myometrium M. The endometrium E is seen at the top of this micrograph. Although this example is a small leiomyoma, they may reach considerable size, and examples 15 cm in diameter or larger are not uncommon. Leiomyomas may be described as submucosal, intramural or subserosal, based upon their position within the myometrium. Submucosal leiomyomas occur just beneath the endometrium and may greatly enlarge and distort the endometrial cavity, resulting in very heavy menses (menorrhagia).
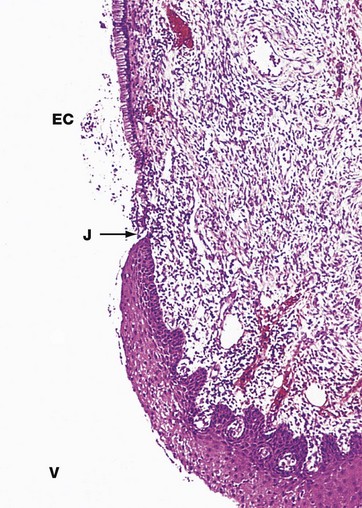
FIG. 19.23 Uterine cervix
H&E (LP)
The uterine cervix protrudes into the upper vagina and contains the endocervical canal, linking the uterine cavity with the vagina. The function of the cervix is to admit spermatozoa to the genital tract at the time when fertilisation is possible, i.e. around the time of ovulation. At other times, including pregnancy, its function is to protect the uterus and upper tract from bacterial invasion. In addition, the cervix must be capable of great dilatation to permit the passage of the fetus during parturition.
As seen in this micrograph, the endocervical canal EC is lined by a single layer of tall columnar mucus-secreting epithelial cells. Where the cervix is exposed to the more hostile environment of the vagina V, the ectocervix, it is lined by thick stratified squamous epithelium as in the vagina and the vulva. The cells of the ectocervix often have clear cytoplasm due to their high glycogen content (not apparent in this specimen).
The junction J between the ecto- and endocervical epithelium is quite abrupt and is normally located at the external os, the point at which the endocervical canal opens into the vagina.
The main bulk of the cervix is composed of tough collagenous tissue containing a little smooth muscle. At the squamocolumnar junction, the cervical stroma is often infiltrated with leucocytes, forming part of the defence against ingress of microorganisms.
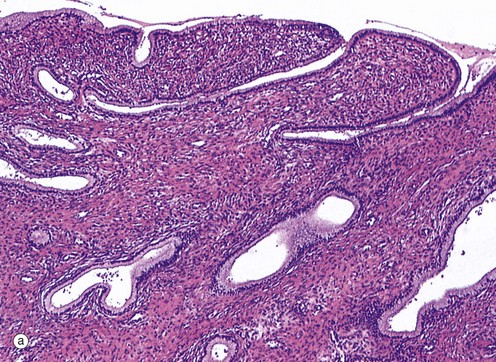
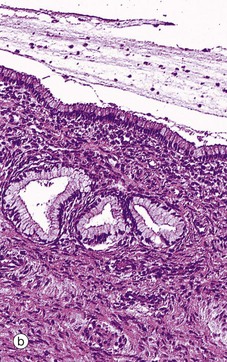
FIG. 19.24 Endocervix
(a) H&E (LP) (b) H&E (MP)
As seen in micrograph (a), the mucus-secreting epithelial lining of the endocervical canal is thrown into deep furrows and tunnels, giving the appearance in two dimensions of branched tubular glands, hence the rather inaccurate term endocervical glands. The columnar mucus-secreting cells lining the ‘glands’ are shown at higher magnification in micrograph (b). Note the leucocytic infiltrate in the superficial stroma and the presence of leucocytes in the endocervical mucus on the surface. Some inflammation is considered to be normal at this site.
During the menstrual cycle, the endocervical epithelium undergoes cyclical changes in secretory activity. In the proliferative phase, rising levels of oestrogen promote secretion of thin, watery mucus which permits the passage of spermatozoa into the uterus around the time of ovulation. Following ovulation, the cervical mucus becomes highly viscid, forming a plug that inhibits the entry of microorganisms (and spermatozoa) from the vagina. This is particularly important should pregnancy occur.
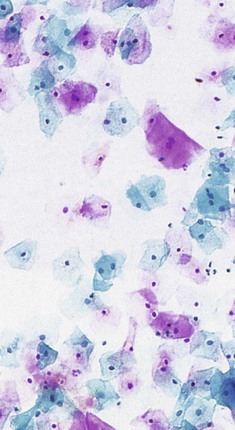
FIG. 19.25 Cervical cytology
Papanicolou (HP)
The cervical stroma is influenced by ovarian hormones, particularly oestrogens, which soften the tissues by reducing collagenous cross-linkages and increasing uptake of water by the ground substance. At its most extreme, this provides the means by which the cervix stretches, thins and dilates in late pregnancy and during parturition. To a much lesser extent, similar changes occur during the normal menstrual cycle. One effect of this is that the volume of the cervical stroma varies during each cycle, causing eversion of the columnar epithelium near the squamocolumnar junction and exposing it to the vaginal environment. This ectropion is known colloquially as ‘cervical erosion’. This induces the growth of stratified squamous epithelium (squamous metaplasia) over the exposed area, considered a normal variant in women of reproductive age. The importance of this transformation zone is that it may undergo malignant change, causing cancer of the cervix.
This area can be studied by scraping cells from the surface using various types of spatula or brush, smearing them on a glass slide and staining them by the Papanicolaou method (the cervical smear or ‘Pap test’). This technique is known as exfoliative cytology and is demonstrated here from a normal healthy cervix. The surface cells of the stratified squamous epithelium have small, contracted nuclei and are stained pink due to the cytoplasmic keratin. Deeper cells have plump nuclei of normal appearance, and the cytoplasm is stained blue/green. An adequate Pap smear should also contain some endocervical cells (demonstrating that the transformation zone has been sampled), as well as cervical mucin and inflammatory cells.
A more recent development of the cervical smear suspends the exfoliated cells in a special alcohol-based fixative medium and then layers them evenly onto a glass slide, a technique known as liquid-based cytology. This gives superior visibility of the cells and improves the ability of the cytologist to see abnormal cells. Various computerised technologies are also becoming available to screen the slides and detect abnormal cells.
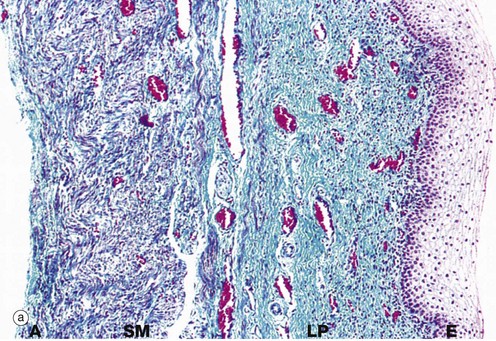
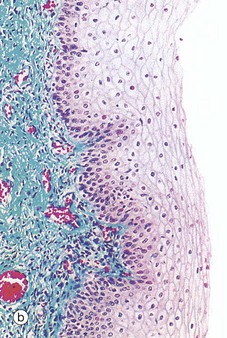
FIG. 19.26 Vagina
(a) Masson trichrome (LP) (b) Masson trichrome (MP)
The wall of the vagina, micrograph (a), consists of a mucosal layer lined by stratified squamous epithelium E, a layer of smooth muscle SM and an outer adventitial layer A. In the relaxed state, the vaginal wall collapses to obliterate the lumen, and the vaginal epithelium is thrown up into folds. The fibrous lamina propria LP contains many elastin fibres, has a rich plexus of small veins and is devoid of glands.
The vagina is lubricated by cervical mucus, a fluid transudate from the rich vascular network of the lamina propria, and mucus secreted by glands of the labia minora. The smooth muscle bundles of the muscular layer are arranged in ill-defined inner circular and outer longitudinal layers. The adventitial layer of the vagina merges with the adventitial layers of the bladder anteriorly and rectum posteriorly.
The combination of a muscular layer and a highly elastic lamina propria and outer adventitia permits the gross distension that occurs during parturition. Conversely, after coitus, involuntary contraction of the smooth muscle layer ensures that a pool of semen remains in the cervical region.
Micrograph (b) illustrates the stratified squamous epithelium that lines the vagina. During the menstrual cycle, this epithelium undergoes cyclical changes in glycogen levels. Throughout the cycle, the superficial cells produce glycogen that is anaerobically metabolised by vaginal commensal bacteria to form lactic acid which inhibits the growth of pathogenic microorganisms.
The Placenta
The placenta is formed from elements of the membranes that surround the developing fetus, as well as the uterine endometrium, and provides the means for physiological exchange between the fetal and maternal circulations. The structure of the placenta varies greatly from one species to another and the following discussion is thus necessarily confined to the human placenta. At various stages during fetal development, the placenta performs a remarkable range of functions until the fetal organs become functional. These include gaseous exchange, excretion, maintenance of homeostasis, hormone secretion, haematopoiesis and hepatic metabolic functions.
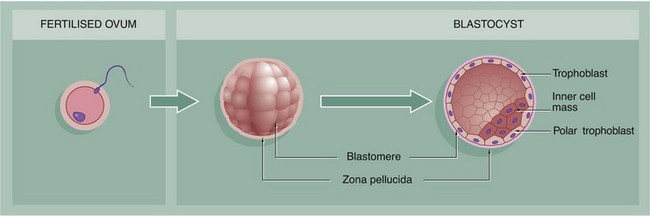
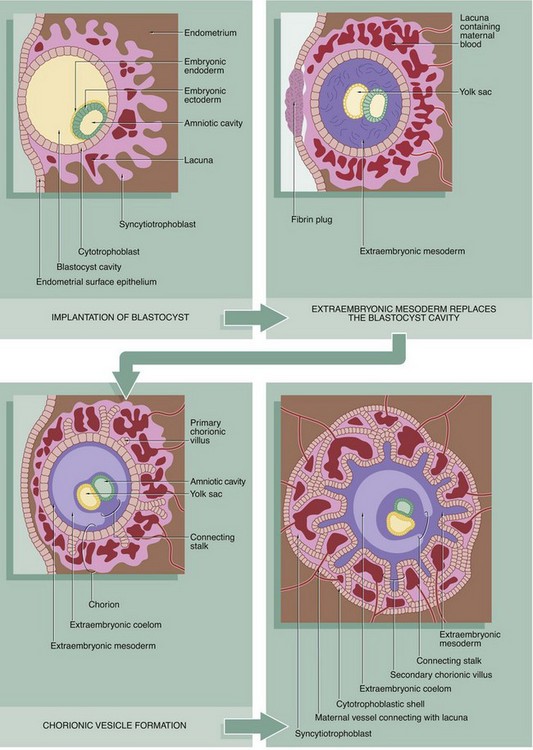
FIG. 19.27 Fertilisation and implantation (illustrations below and opposite)
Within about 24 to 48 hours after ovulation, fertilisation of an ovum by a spermatozoon occurs in the ampulla of the Fallopian tube, with the formation of a zygote. The zona pellucida remains intact (see Fig. 19.4). Within 24 hours, the zygote undergoes its first mitotic cell division, the process continuing until there are some 12 to 16 cells called blastomeres, each with a small portion of the original cytoplasm. The mass, now called a morula (for its resemblance to a mulberry), remains enclosed by the zona pellucida, through which it is nourished by diffusion of oxygen and low molecular weight metabolites from Fallopian tube secretions.
The morula reaches the uterus 2 to 3 days after fertilisation and begins to absorb uterine fluid, forming a central cavity. The blastocyst, as it is now known, consists of a peripheral layer of blastomeres forming the trophoblast, with a mass of cells at one aspect, the polar trophoblast, bulging into the central lumen and known as the inner cell mass. The trophoblast (along with a maternal contribution) eventually gives rise to the placenta while the inner cell mass develops into the embryo. By this time, the blastocyst has grown to about twice the size of the original ovum and the zona pellucida has become quite thin. When the blastocyst has been within the uterine cavity for 2 to 3 days, the zona pellucida disappears and implantation occurs. The polar trophoblast invades the endometrium so that, by the 10th day after conception, the blastocyst is completely buried.
The trophoblast gives rise to two layers, an inner cytotrophoblast layer of mononuclear cells and an outer syncytiotrophoblast layer, formed by fusion of cytotrophoblast cells to form a continuous multinucleate syncytium in which there is no internal cytoplasmic demarcation by plasma membranes. The cytotrophoblast remains as a single layer of cells, whereas the syncytiotrophoblast becomes increasingly broad and develops finger-like projections into the endometrium. A third type of trophoblast known as intermediate trophoblast has histological features intermediate between cytotrophoblast and syncytiotrophoblast and has a major role in invading the endometrium. Within a short time, a sponge-like network of spaces called lacunae develops within the syncytiotrophoblast, initially filled with tissue fluid and uterine secretions. Soon afterwards, invasion by the intermediate trophoblast causes disintegration of endometrial capillaries with leakage of maternal blood into the lacunae. Progressively, the trophoblast envelops maternal capillaries, expanding the lacunar network and establishing an arterial supply and venous drainage system.
By now, the syncytiotrophoblast also secretes a variety of hormones including human chorionic gonadotrophin (hCG), human chorionic somatotrophin (previously human placental lactogen, hPL), oestrogen and progesterone, which are necessary to sustain the endometrial tissues. In the meantime, the blastocyst cavity becomes filled with extraembryonic mesoderm (mesenchyme), which completely surrounds the early embryo, developing from the inner cell mass. The embryo by now comprises plates of embryonic endoderm and ectoderm on either side of which lie the yolk sac and amniotic cavities, enclosed by extraembryonic endoderm and extraembryonic ectoderm, respectively. Subsequently, a cavity forms within the extraembryonic mesoderm; this extraembryonic coelom eventually surrounds the developing embryo, which remains attached to the trophoblast by a connecting stalk of extraembryonic mesoderm. The trophoblast, along with the mesodermal layer remaining beneath it, now constitutes the chorion.
Meanwhile the trabeculae of syncytiotrophoblast and intermediate trophoblast between the lacunae are invaded by columns of cytotrophoblastic cells called primary chorionic villi. These grow out to the periphery and spread out over the interface between the trophoblast and endometrium, forming the cytotrophoblast shell. Extraembryonic mesoderm now invades the primary villi, which thus develop a mesenchymal core, becoming known as secondary chorionic villi.
By about 2 weeks after implantation (i.e. about 24 days after fertilisation), primitive blood vessels begin to develop in the chorionic mesoderm simultaneously with development of the primitive embryonic circulatory system, the embryo now being too large to rely on mere diffusion for its growth and metabolic requirements. When the mesenchymal cores of the villi become vascularised, they become known as tertiary villi (not illustrated).
The form of the placenta is essentially established by the end of the fourth month, after which the placenta grows in diameter, complementing growth in the size of the uterus.
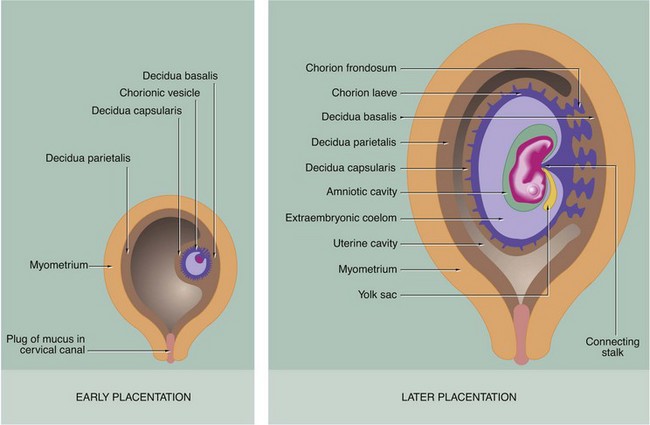
FIG. 19.28 Decidua formation and early placental development (caption opposite, upper)
During the process of implantation, secretion by the syncytiotrophoblast of hCG (which is functionally analogous to luteinising hormone) interrupts the ovarian cycle. This results in growth and proliferation of stromal cells of the endometrial stratum functionalis at the implantation site into large polyhedral decidual cells, a change that has already begun in the late secretory phase. The decidua beneath the developing embryo is known as the decidua basalis and, with the trophoblast, will form the future placenta. The decidua overlying the embryo is known as the decidua capsularis, and the decidual lining of the rest of the uterus is called the decidua parietalis. Ultimately, expansion of the embryo and its enveloping fluid-filled membrane system results in fusion of the capsular and parietal layers of the decidua, with complete obliteration of the uterine cavity.
During the first 2 months of embryological development, the chorion grows fairly uniformly around the whole periphery of the vesicle. From the third month, the chorion in contact with the decidua basalis develops extensive frond-like villous outgrowths into the decidua, becoming known as the chorion frondosum, while the superficial chorion in contact with the decidua capsularis atrophies to become the smooth chorion laeve. Progressively, the chorion frondosum and decidua basalis develop into the flattened placenta and the vessels connecting the chorion to the embryonic circulation become the umbilical cord.
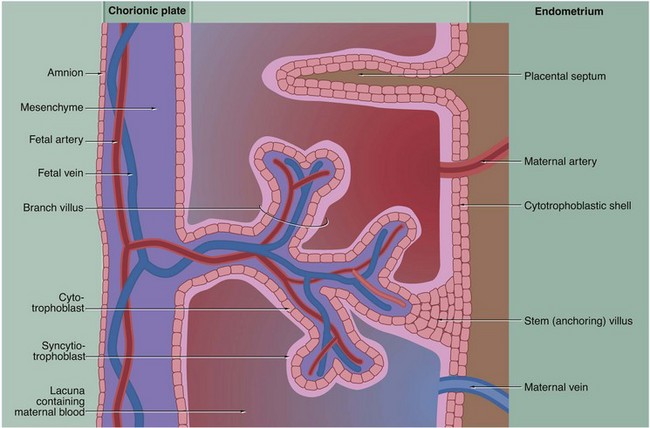
FIG. 19.29 Structure of placental villi (caption opposite, lower)
From the time maternal blood appears within the trophoblastic lacunae, the trabeculae between the lacunae become increasingly robust, with stem villi forming anchorage points with the cytotrophoblastic shell. Side branches grow out into the lacunae, progressively forming a complex villous structure. Each villus has a mesenchymal core containing capillaries served by afferent and efferent fetal blood vessels. Between the villous capillaries and the maternal blood, there is a continuous layer of syncytiotrophoblast supported by a layer of proliferating cytotrophoblast cells. From the fourth month onwards, the cytotrophoblast layer becomes atrophic. As more and more branches are added to the villous tree, the villi become smaller and smaller, and the tissue barrier between fetal capillaries and maternal blood is greatly diminished.
As the placenta develops, the decidua basalis regresses so that all that remains are a number of anastomosing septa of maternal supporting tissue projecting into the cytotrophoblastic shell. When the placenta is shed immediately after childbirth, its maternal surface is seen to be divided into about 20 irregular segments called cotyledons that are demarcated from each other by the positions of the former maternal (placental) septa.
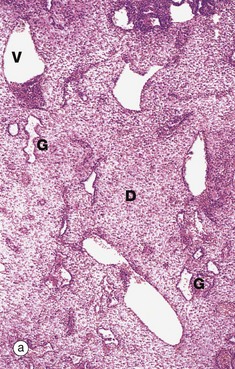
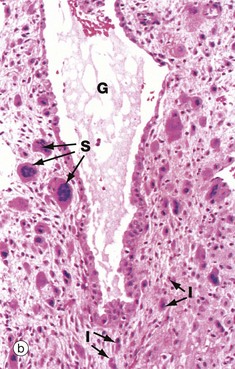
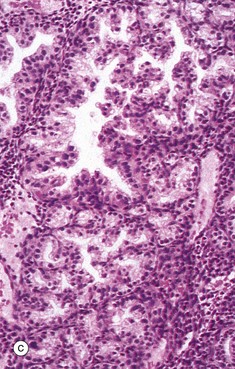
FIG. 19.30 Decidua
(a) H&E (LP) (b) H&E (MP) (c) H&E (HP)
Micrograph (a) illustrates decidual change D in the endometrial stroma. The decidual cells proliferate and enlarge greatly, their cytoplasm staining pink (eosinophilia) due to the presence of numerous mitochondria and intermediate filaments. Dilated blood vessels V and endometrial glands G are apparent.
At higher power in micrograph (b), multinucleated syncytiotrophoblast cells S can be seen infiltrating the decidua. Intermediate trophoblast cells I are actually present in greater numbers than syncytiotrophoblast cells but are less easily identified. In the centre of the field, there is a dilated gland G.
Micrograph (c) shows the deeper part of the endometrium in pregnancy. Here, the decidual reaction is inconspicuous, but the secretory nature of the glands is greatly exaggerated. It is thus often called hypersecretory endometrium. Note the prominent infolding of the glandular epithelium and the vacuolation of the epithelial cell cytoplasm.
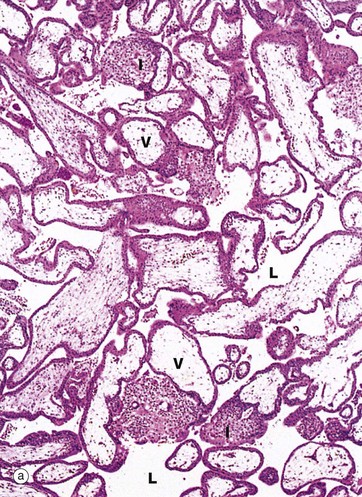
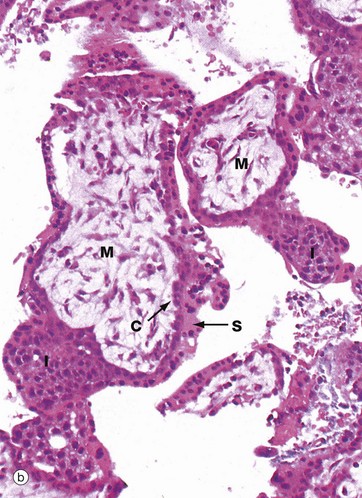
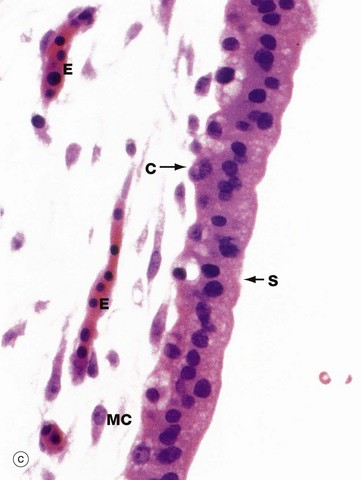
FIG. 19.31 Early placenta
(a) H&E (LP) (b) H&E (MP) (c) H&E (HP)
This series of micrographs illustrates, at increasing magnification, a placenta at about 6 weeks gestational age. Nucleated fetal erythrocytes E, which in humans persist until 9 weeks gestational age, can be seen in the capillary in micrograph (c).
At low magnification in micrograph (a), the main feature is the large numbers of villi V projecting into the lacuna system L that, in vivo, would be filled with maternal blood. Some villi show evidence of branching. Solid cores of cytotrophoblast and intermediate trophoblast I can be seen extending away from the villi to form new branches.
With further magnification in micrograph (b), the villi are seen to have a core of primitive mesenchyme M. The villi are invested by trophoblast, comprising an inner layer of cytotrophoblast cells C and a broader outer syncytiotrophoblast layer S. In some areas, solid buds of trophoblast can be seen forming new branches. The specimen is a little broken up as it is derived from a curettage specimen following incomplete spontaneous abortion.
Micrograph (c) focuses on the margin of a villus at high magnification, the cellular preservation being again less than ideal due to its origin from a spontaneous miscarriage. The syncytiotrophoblast layer S can be distinguished from the single layer of cytotrophoblast cells C, which are smaller. The mesenchymal cells MC are large with extensive branching cytoplasmic processes and the intercellular matrix is myxoid due to its high content of glycosaminoglycans.
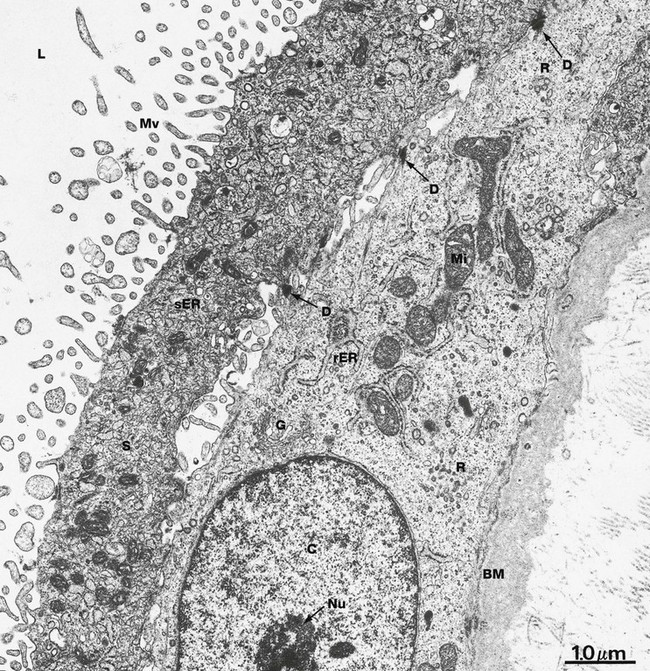
FIG. 19.32 Trophoblast
EM ×16 000
This micrograph shows the general ultrastructural features of the trophoblastic components. These show considerable variation from one region to another and from early to late stages of placental development.
The syncytiotrophoblast S typically presents large numbers of irregular microvilli Mv to the lacunae L, greatly enhancing the surface area for physiological exchange. The plasma membranes of the microvilli incorporate a wide variety of enzymes and receptors involved in membrane transfer processes, as well as receptors for many hormones and growth factors. Microfilaments extend into the microvilli from a cytoskeletal network concentrated immediately below the free surface. Some areas of the syncytiotrophoblast contain rough endoplasmic reticulum while, in other areas, as shown here, smooth endoplasmic reticulum sER predominates, presumably involved in steroid hormone synthesis.
The cytotrophoblast layer C has ultrastructural features of relatively undifferentiated stem cells, exhibiting profiles of rough endoplasmic reticulum rER, a well-defined Golgi apparatus G, relatively few mitochondria Mi and numerous polyribosomes R. The nucleus is typically large with dispersed chromatin and nucleoli Nu. The cytotrophoblast is usually tightly bound to the overlying syncytiotrophoblast by desmosomes D, but in some areas, as in this specimen, spaces can be seen between the cell layers. The reason for this is unclear. Separating the cytotrophoblast from the underlying collagenous stroma is a relatively thick basement membrane BM.
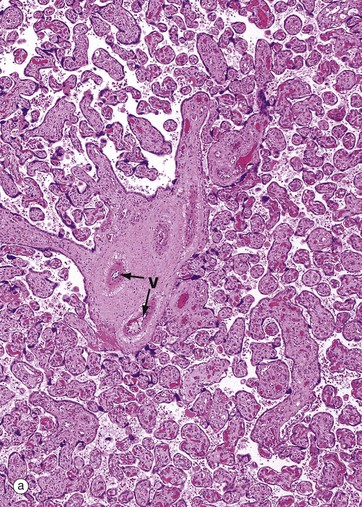
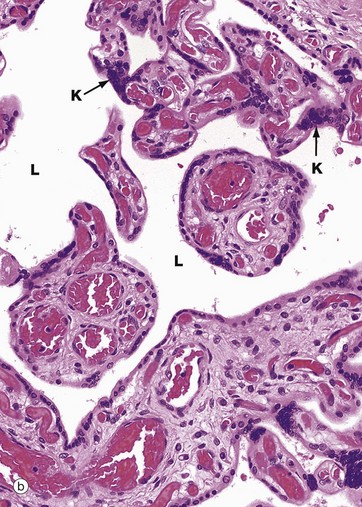
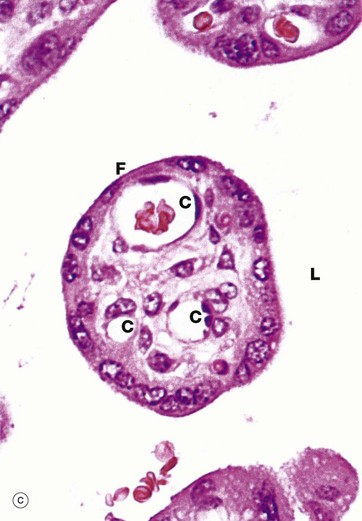
FIG. 19.33 Term placenta
(a) H&E (LP) (b) H&E (MP) (c) H&E (HP)
These micrographs illustrate placenta from a full-term fetus.
At low magnification in micrograph (a), huge numbers of villi can be seen, cut in various planes of section and varying in diameter from large mainstem villi to very small terminal branch villi. Compared with early placenta shown in Fig. 19.31, the villous pattern is much more highly developed and the average villous diameter is much smaller, reflecting the extensive branching growth of the villi as the placenta enlarges. Note the large blood vessels V in the largest villi.
Micrograph (b) demonstrates the branching nature of the villi at higher magnification. Compare the marked vascularity of the villous cores with that of the much earlier placenta in Fig. 19.31 and the greatly increased villous surface area exposed to the lacunae L filled with maternal blood. A feature of the term placenta is the syncytial knot K, where syncytiotrophoblast nuclei are aggregated together in clusters, leaving zones of thin cytoplasm devoid of nuclei between.
Micrograph (c) focuses on a small branch villus and highlights the proximity of blood in fetal capillaries C to maternal blood in the surrounding lacuna L. The trophoblast is reduced to a thin layer of syncytiotrophoblast only and the capillaries tend to be located in the periphery of the core. The diffusion barrier between maternal and fetal circulations comprises five layers: trophoblast, trophoblast basement membrane, villous core supporting tissue, capillary endothelial basement membrane and endothelium. In many cases, fetal capillaries are so close to the trophoblast that their basement membranes fuse F, reducing the diffusion barrier to only three layers.
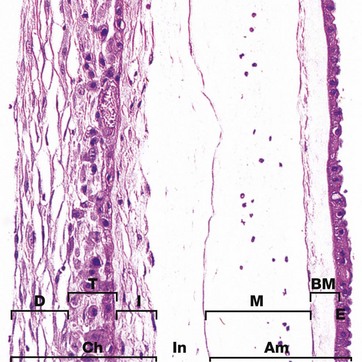
FIG. 19.34 Fetal membranes
H&E (MP)
During early development, the embryo is surrounded by the extraembryonic coelom (see Fig. 19.28), but later this is obliterated as the amniotic cavity expands to surround the fetus. The outer mesenchymal layer of the amnion then comes to lie in contact with (and often fuses with) the inner mesenchymal layer of the chorion, forming the chorioamnion or fetal membranes. The two layers are often difficult to separate from one another at birth.
The amniotic membrane Am comprises a single layer of epithelial cells E derived from extraembryonic ectoderm, resting on a thick basement membrane BM. Beneath this, there is a delicate avascular mesenchymal layer M which is a remnant of the extraembryonic mesoderm. The chorionic membrane Ch consists of three layers. A vascular collagenous inner layer I is also derived from extraembryonic mesoderm. The intermediate zone In, seen here separating it from the amnion, represents the remnant of the extraembryonic coelom and varies greatly in thickness. The trophoblast T of the chorion laeve is represented by the middle layer of eosinophilic epithelial cells, whilst the outermost vascular collagenous layer D is of maternal origin, representing the decidua capsularis (see Fig. 19.28).
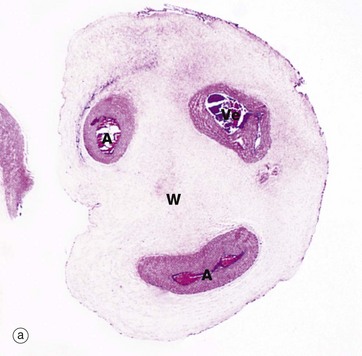
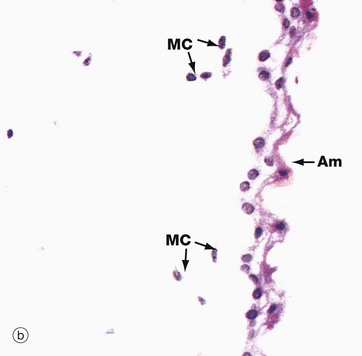
FIG. 19.35 Umbilical cord
(a) H&E (LP) (b) H&E (HP)
The development of the umbilical cord begins with the formation of the extraembryonic coelom which almost surrounds the early embryo and which remains attached to the chorion by the connecting stalk of mesenchyme (see Fig. 19.28). With further embryonic development, the site of attachment of the connecting stalk becomes located ventrally, just caudal to the point where the vitello-intestinal duct connects the yolk sac to the mid-gut. As the embryo grows, the amniotic sac expands greatly, filling the extraembryonic coelom and compressing the vitello-intestinal duct and yolk sac remnant (surrounded by a sleeve of extraembryonic coelom) up against the connecting stalk. These structures ultimately fuse to form the umbilical cord, which now is surrounded by the amnion and amniotic cavity.
By the middle of the fifth month, the remnants of the vitello-intestinal duct, yolk sac and sheath of extraembryonic coelom atrophy and disappear. As seen in micrograph (a), all that remains are two umbilical arteries A and a single umbilical vein Ve, embedded in mesenchyme consisting mainly of ground substance and known as Wharton's jelly W. Mesenchymal cells MC and surface amnion Am are shown at high magnification in micrograph (b). The umbilical arteries convey deoxygenated fetal blood to the placenta while the umbilical vein conveys oxygenated blood back to the fetus.
The Breast
The breasts (mammary glands) are highly modified apocrine sweat glands (see Fig. 9.14) which develop embryologically along two lines, the milk lines, extending from the axillae to the groin. In humans, only one gland develops on each side of the thorax, although accessory breast tissue may be found anywhere along the milk lines.
The breasts of both sexes follow a similar course of development until puberty, after which the female breasts develop under the influence of pituitary, ovarian and other hormones.
Until the menopause, the breasts undergo cyclical changes in activity which are controlled by the hormones of the ovarian cycle. After menopause, the breasts, like the other female reproductive tissues, undergo progressive atrophy and involutional change.
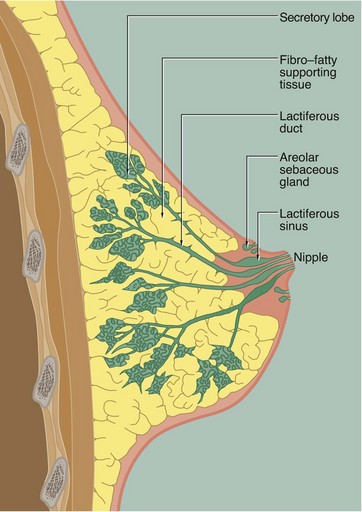
FIG. 19.36 Structure of the breast
This highly schematic diagram illustrates the general organisation of the breast. Each breast consists of 15 to 25 independent units called breast lobes, each consisting of a compound tubulo-acinar gland (see Fig. 5.25). The size of the lobes is quite variable and the bulk of the breast is made up of a few large lobes that connect to the surface. Immediately before opening onto the surface, the duct forms a dilatation called the lactiferous sinus. Smaller lobes end in blind ending ducts that do not reach the nipple surface. The lobes are embedded in a mass of adipose tissue, subdivided by collagenous septa.
The nipple contains bands of smooth muscle, orientated in parallel to the lactiferous ducts and circularly near the base. Contraction of this muscle causes erection of the nipple.
Within each lobe of the breast, the main duct branches repeatedly to form a number of terminal ducts, each of which leads to a lobule consisting of multiple acini. Each terminal duct and its associated lobule is called a terminal duct–lobular unit. The lobules are separated by moderately dense collagenous interlobular tissue, whereas the intralobular supporting tissue surrounding the ducts within each lobule is less collagenous and more vascular.
The skin surrounding the nipple, the areola, is pigmented and contains sebaceous glands which are not associated with hair follicles.
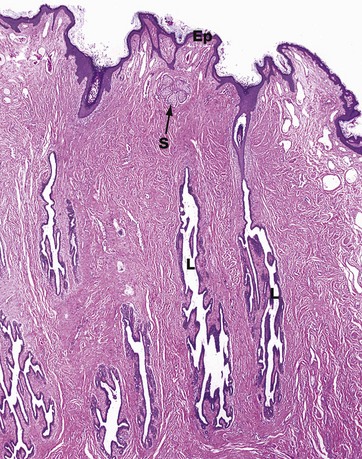
FIG. 19.37 The nipple
H&E (LP)
This low-magnification micrograph of the nipple demonstrates the structure of the lactiferous sinuses and shows their connection to the surface of the skin of the nipple. Several lactiferous sinuses L are seen coursing through the dermis towards the skin surface. Only the lactiferous sinus on the right can be seen connecting to the surface in this micrograph, but this is probably due to a slightly oblique plane of section, rather than indicating blind-ending sinuses.
The undulating surface of the epidermis Ep is seen, and a single sebaceous gland S is also identifiable. The epithelium of the lactiferous sinuses is similar to that of the ducts in the rest of the breast until close to the surface, where the epithelium becomes stratified squamous in type.
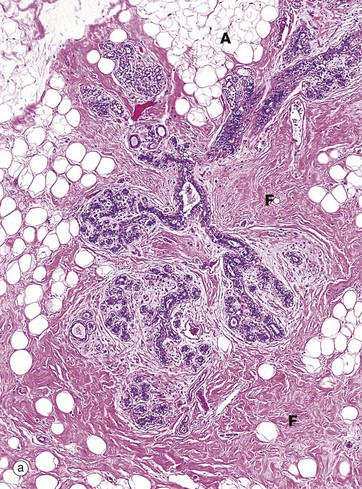
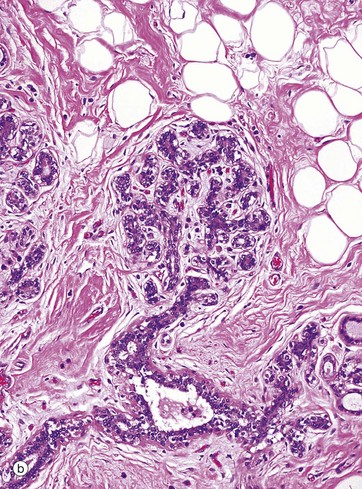
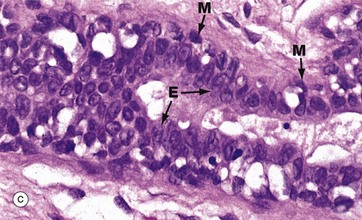
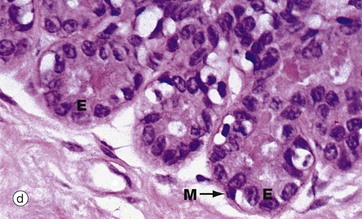
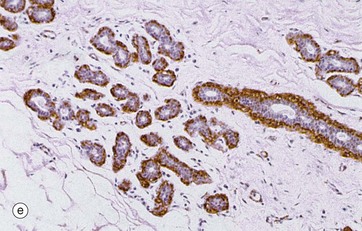
FIG. 19.38 Breast
(a) H&E (LP) (b) H&E (MP) (c) H&E (HP) (d) H&E (HP) (e) Immunohistochemical stain for smooth muscle actin (MP)
These micrographs show breast tissue from a non-pregnant woman of reproductive age. Micrograph (a) shows terminal duct–lobular units (TDLU) at low magnification. The extensive branching duct system is surrounded by relatively dense fibrous interlobular tissue F and adipose tissue A. The interlacing (reticular) arrangement of the coarse collagen of the interlobular tissue is seen at higher magnification in micrograph (b), as is the branching duct system of the lobule.
The breast ducts and acini are lined by two layers of cells, a luminal layer of epithelial cells and a basal layer of flattened myoepithelial cells. In the larger ducts, as shown in micrograph (c), the luminal epithelial cells E are tall columnar in type whereas, in the smaller ducts and acini shown in micrograph (d), the epithelial cells are cuboidal. A discontinuous layer of stellate myoepithelial cells M with pale cytoplasm surrounds the ductal lining cells. In micrograph (e), which uses an immunohistochemical technique to stain the myoepithelial cells for actin, the extent and number of the myoepithelial cells (stained brown) are apparent.
During the reproductive years, the duct epithelium undergoes mild cyclical changes under the influence of ovarian hormones. Early in the cycle, the duct lumina are not clearly evident but, later in the cycle, they become more prominent and may contain an eosinophilic secretion.
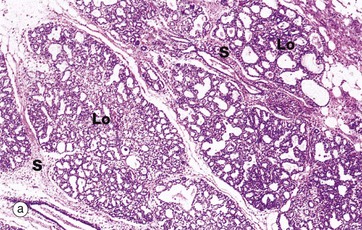
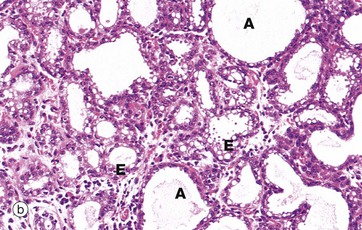
FIG. 19.39 Breast during pregnancy
(a) H&E (LP) (b) H&E (MP)
Under the influence of oestrogens and progesterone produced by the corpus luteum and later by the placenta, the terminal duct epithelium proliferates to form greatly increased numbers of secretory acini. Breast proliferation is also dependent on prolactin, human chorionic somatomammotropin (a prolactin-like hormone produced by the placenta), thyroxine and corticosteroids.
At low magnification in micrograph (a), the breast lobules Lo are seen to have enlarged greatly at the expense of the intralobular tissue and interlobar adipose tissue, although septa S of interlobular tissue still remain. At higher magnification in (b) the acini A are dilated. The lining epithelial cells E vary from cuboidal to low columnar and contain cytoplasmic vacuoles. The intralobular stroma is much less prominent and contains an infiltrate of lymphocytes, eosinophils and plasma cells.
As pregnancy progresses, the acini begin to secrete a protein-rich fluid called colostrum, the accumulation of which dilates the acinar and duct lumina as seen in micrograph (b). Colostrum is the form of breast secretion available during the first few days after birth. It contains a laxative substance and maternal antibodies. Unlike milk, colostrum contains little lipid. Breast secretion is controlled by the hormone prolactin. During pregnancy, prolactin secretion progressively increases, but high levels of circulating oestrogens and progesterone suppress its activity.
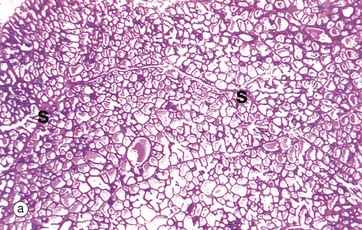
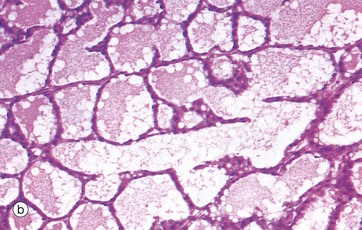
FIG. 19.40 Lactating breast
(a) H&E (LP) (b) H&E (MP)
After parturition, the levels of circulating progesterone and oestrogens, which inhibit milk secretion, fall precipitously. Prolactin stimulates milk production in conjunction with several other hormones.
As seen in micrograph (a), the lactating breast is composed almost entirely of acini distended with milk, the interlobular tissue now being reduced to thin septa S between the lobules. At higher magnification in (b), the acini are filled with an eosinophilic material containing clear vacuoles caused by lipid droplets which have dissolved out during tissue preparation. The epithelial cells are flattened and the acini distended by secretions. However, in different areas, the epithelium may be thicker and the acinar lumina smaller.
Milk production proceeds for as long as suckling continues and can continue for some years after childbirth. A neurohormonal reflex in which nipple stimulation by suckling causes release of prolactin from the anterior pituitary controls the process. A different neurohormonal reflex, also initiated by suckling, causes the release of the hormone oxytocin from the posterior pituitary. Oxytocin causes contraction of the myoepithelial cells which embrace the secretory acini and ducts, thus propelling milk into the lactiferous sinuses (milk ‘let-down’). Withdrawal of the suckling stimulus, and hence the release of pituitary hormones at weaning, results in regression of the lactating breast and resumption of the normal ovarian cycle.
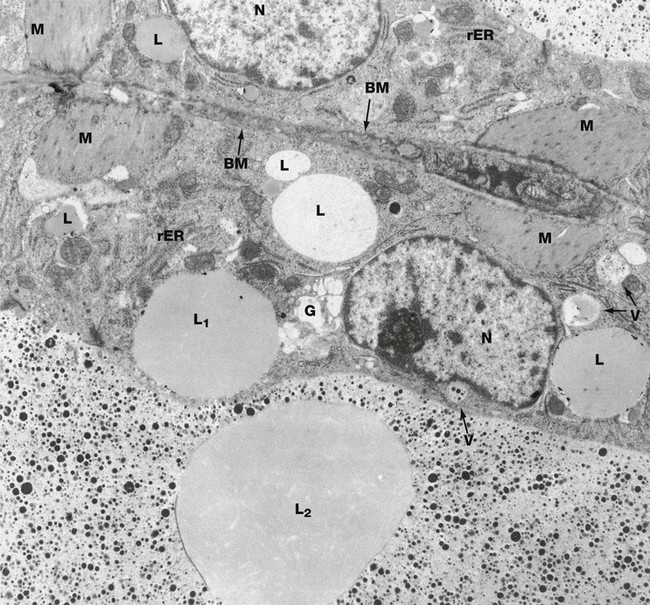
FIG. 19.41 Lactating breast
EM ×9000
This micrograph shows two secretory cells of adjacent acini in a lactating breast. Their nuclei N are large, with prominent nucleoli. Each acinus is bounded by a basement membrane BM, the basement membranes in this example being separated by only a shred of intralobular supporting tissue. Between each basement membrane and the secretory cells are the cytoplasmic processes of myoepithelial cells M, contraction of which expels milk from the gland.
The composition of milk varies somewhat during lactation and even during each suckling episode, but its main constituents are as follows: water (88%), ions (particularly sodium, potassium, chloride, calcium and phosphate), protein (1.5%, mainly lactalbumin and casein), carbohydrate (7%, mainly lactose), lipids (3.5%, mainly triglycerides), vitamins and antibodies (mainly IgA).
Secretion of different components of the milk occurs by different mechanisms. Water and some ions diffuse freely through the apical cell membrane. Proteins are synthesised on the rough endoplasmic reticulum rER, packaged in the Golgi apparatus G and secreted in vacuoles V by exocytosis. Protein in the milk is represented by small electron-dense granules. The Golgi apparatus is extensive and the protein-containing secretory vacuoles also contain a considerable amount of other less electron-dense material, including lactose and calcium.
The cytoplasm of the secretory cells contains lipid droplets L of various sizes which are not bounded by membrane. These contain triglycerides, although whether this is derived directly from blood or synthesised in the secretory cells is uncertain. The lipid is discharged by apocrine secretion, which involves the lipid droplet, surrounding cytoplasm and plasma membrane being cast off into the lumen. A large lipid droplet L1 with thin overlying rim of cytoplasm can be seen in the lower acinus, just prior to secretion. An even larger droplet L2 surrounded by a remnant of cytoplasm and plasma membrane is seen in the lumen close by.
IgA, taken up by receptor-mediated endocytosis at the base of the cell from the bloodstream, is transported across the cell in small membranous vesicles and released by exocytosis into the milk, a process known as transcytosis.
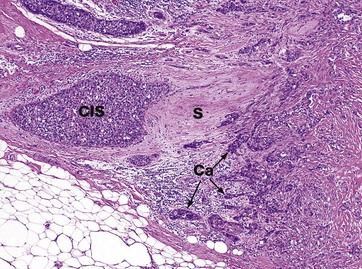
FIG. 19.42 Breast cancer
H&E (MP)
This micrograph shows a medium-power view of an area of breast carcinoma in a middle-aged woman. The cancer is of the commonest type, known as ductal carcinoma, NOS (not otherwise specified). Note how clusters of malignant epithelial cells Ca invade into the normal breast stroma S, destroying it.
In the left side of the field, there is a single expanded duct which appears to be filled and expanded by similar malignant cells. This is an area of ductal carcinoma in situ CIS.
Breast carcinoma in situ often calcifies, allowing the disease to be identified on mammograms. Obviously, the earlier the cancer is identified, the better the chance of a complete cure.
Breast cancer is typically treated by a combination of surgery, hormonal therapy, chemotherapy and radiotherapy, depending upon the type and extent of the tumour.
Review
TABLE 19.1
Review of the female reproductive system
| Part of the female genital tract | Key features | Figure |
| Ovary | Primordial and developing follicles embedded in ovarian stroma Surface covering of epithelium (mesothelium) Corpora lutea and corpora albicantes |
19.43a |
| Fallopian tube | Muscular wall Folded mucosa Ciliated columnar epithelium |
19.43b |
| Uterus | Muscular wall—the myometrium Lining endometrium consisting of glands and stroma, varies with the menstrual cycle |
19.43c |
| Endocervix | Bulk consists of a dense fibromuscular stroma Surface has deep clefts lined by simple columnar mucus-secreting epithelium |
19.43d |
| Ectocervix | Stroma same as for endocervix Stratified squamous non-keratinising surface epithelium |
19.43e |
| Vagina | Fibromuscular wall Stratified squamous non-keratinising surface epithelium |
19.43f |
| Vulva | Stratified squamous epithelium/modified skin (see Ch. 9) | |
| Placenta | Chorionic villi with core of mesenchyme and double surface layer of trophoblast | 19.43g |
| Breast | Stroma consists of adipose tissue with fibrous septa Branching tubulo-acinar glands Glandular epithelium consists of luminal epithelial cells and underlying myoepithelial cells |
19.43h |
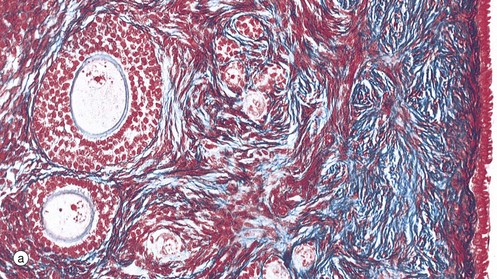
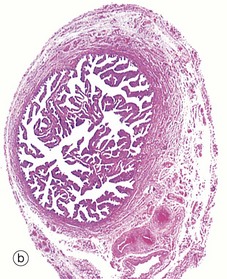
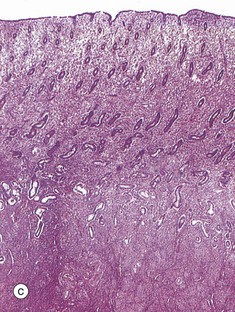
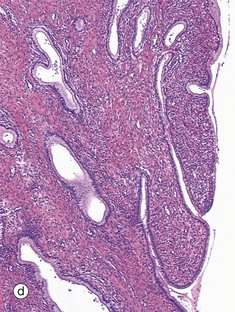
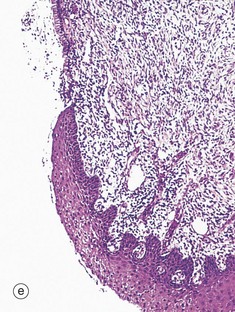
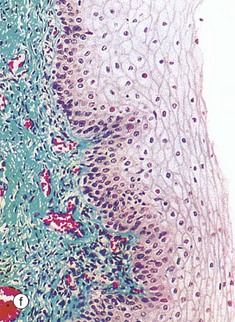
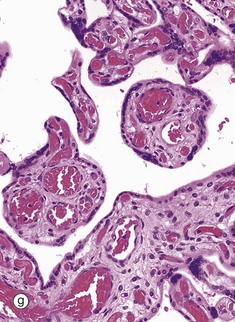
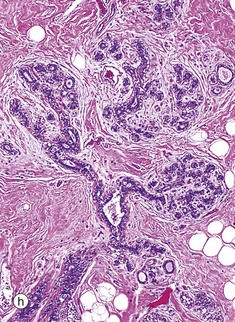
FIG. 19.43 The main components of the female reproductive system (see Table 19.1 opposite)
(a) Azan (MP) (b) H&E (LP) (c) H&E (LP) (d) H&E (MP) (e) H&E (MP) (f) Masson trichrome (MP) (g) H&E (LP) (h) H&E (LP)