Circulatory system
Introduction
The circulatory system mediates continuous movement of all body fluids, its principal functions being the transport of oxygen and nutrients to the tissues as well as transport of carbon dioxide and metabolic waste products from the tissues. The circulatory system is also involved in temperature regulation and the distribution of molecules (e.g. hormones) and cells (e.g. those of the immune system). The circulatory system has two functional components: the blood vascular system and the lymph vascular system.
The blood circulatory system comprises a circuit of vessels through which blood flow is initiated by continuous action of a central muscular pump, the heart. The arterial system provides a distribution network to the peripheral microcirculation, the capillaries and postcapillary venules, the main sites of interchange of gas and metabolite molecules between the tissues and the blood. The venous system carries blood from the capillary system back to the heart.
The lymph vascular system is a network of drainage vessels for returning excess extravascular fluid, the lymph, to the blood circulatory system and for transporting lymph to the lymph nodes for immunological screening (see Ch. 11). The lymphatic system has no central pump but there is an intrinsic pumping system effected by contractile smooth muscle fibres in the lymph vessel walls, combined with a valve system preventing backflow.
The whole circulatory system has a common basic structure:
• An inner lining, the tunica intima, comprising a single layer of extremely flattened epithelial cells called endothelial cells supported by a basement membrane and delicate collagenous tissue.
• An intermediate predominantly muscular layer, the tunica media.
• An outer supporting tissue layer called the tunica adventitia.
The tissues of the thick walls of large vessels (e.g. aorta) cannot be sustained by diffusion of oxygen and nutrients from their lumina, and are supplied by small arteries (vasa vasorum) which run in the tunica adventitia and send arterioles and capillaries into the tunica media.
The muscular content exhibits the greatest variation from one part of the system to another. For example, it is totally absent in capillaries but comprises almost the whole mass of the heart. Blood flow is predominantly influenced by variation in activity of the muscular tissue.
The Heart
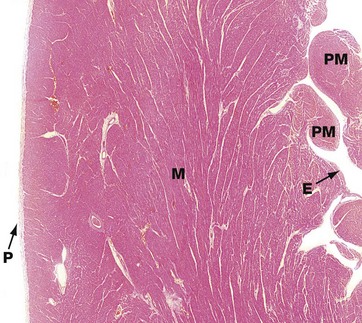
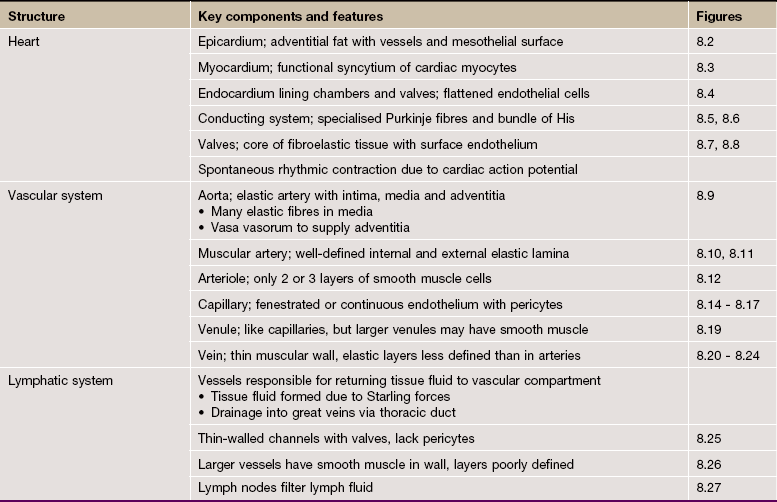
FIG. 8.1 Heart: left ventricular wall
H&E (LP)
This low-power micrograph shows the three basic layers of the heart wall, in this case the left ventricle.
The tunica intima equivalent of the heart is the endocardium E, normally a thin layer in a ventricle. This is lined by a single layer of flattened endothelial cells, as is the case elsewhere in the circulatory system.
The tunica media equivalent is the myocardium M, made up of cardiac-type muscle (see Ch. 6). In the left ventricle, this layer is very prominent due to its role in pumping oxygenated blood throughout the systemic circulation, but it is less thick in the right ventricle and in the atria which operate at much lower pressures. Note the origins of the papillary muscles PM, extensions of the myocardium which protrude into the left ventricular cavity and provide attachment points of the chordae tendinae which tether the cusps of the atrio-ventricular valves.
The equivalent of the tunica adventitia is the epicardium or visceral pericardium P, usually a thin layer (as here) but, in some areas, containing adipose tissue (see Fig. 8.2a). The coronary arteries run within the epicardial fat.
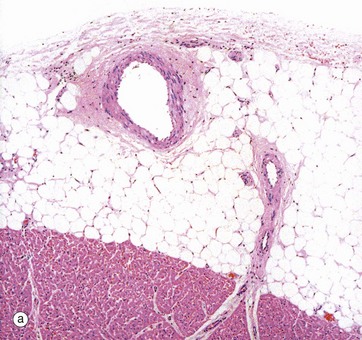
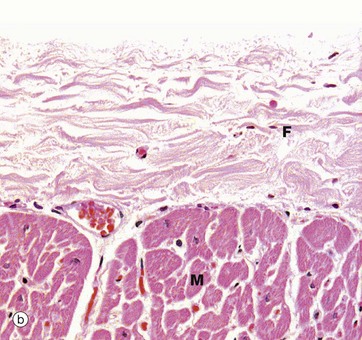
FIG. 8.2 Heart: epicardium (visceral pericardium)
(a) H&E (MP) (b) H&E (HP)
The constant layer of the epicardium is a dense sheet of fibrocollagenous tissue F which also contains elastic fibres. On its outer surface, there is a flat monolayer of mesothelial cells Me (not clearly seen here). These cells are responsible for secretion of lubricating fluid. Micrograph (a) shows an area where the epicardium contains a large branch of the coronary artery CA, with a smaller branch penetrating the myocardium M. Note that in areas containing artery branches, there is a variable layer of adipose tissue A. Micrograph (b) shows the appearance of the epicardium over most of the heart surface, where the fibrocollagenous layer F lies directly on the myocardium M without intervening adipose tissue.
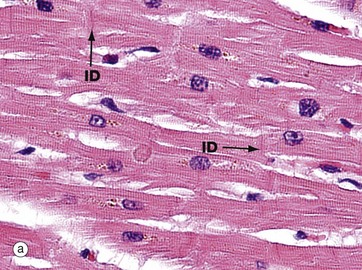
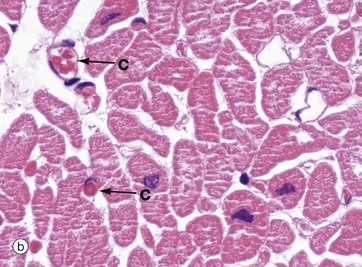
FIG. 8.3 Myocardium
(a) H&E, LS (HP) (b) H&E, TS (HP)
In longitudinal section (a), cardiac muscle fibres form an interconnecting network, joined to each other by intercalated discs ID. These specialised intercellular junctions provide both mechanical and electrophysiological coupling, allowing the cardiac myocytes to act as a functional syncytium. The cells possess central nuclei and regular cytoplasmic cross-striations. The intercalated discs and cross-striations can be clearly seen using special methods such as the immunohistochemical technique for α-B crystallin and in thin resin sections stained with toluidine blue (see Fig. 6.24).
In transverse section in micrograph (b), the extensive and intimate capillary network C between the myocardial fibres is easily seen. The vessels in this section are distended with red blood cells (see also Fig. 6.21). This high level of vascularity is a reflection of the high and constant oxygen demand of the myocardium, particularly in the left ventricle which is shown in these two pictures.
Further structural details of the cardiac muscle of the myocardium are given in Ch. 6.
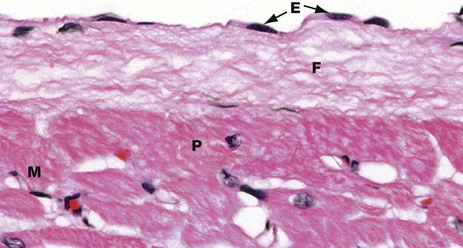
FIG. 8.4 Endocardium
H&E (HP)
The endocardium has a surface layer of flattened endothelial cells E. The endothelium is supported by a layer of fibrous connective tissue F containing variable amounts of elastic tissue. This merges into the collagen fibres surrounding adjacent cardiac muscle cells M, as well as the larger Purkinje fibres P (see Fig. 8.6).
The endocardium shown here is from the wall of the left ventricle. The endocardium of the atria is much thicker than this and includes more elastic fibres.
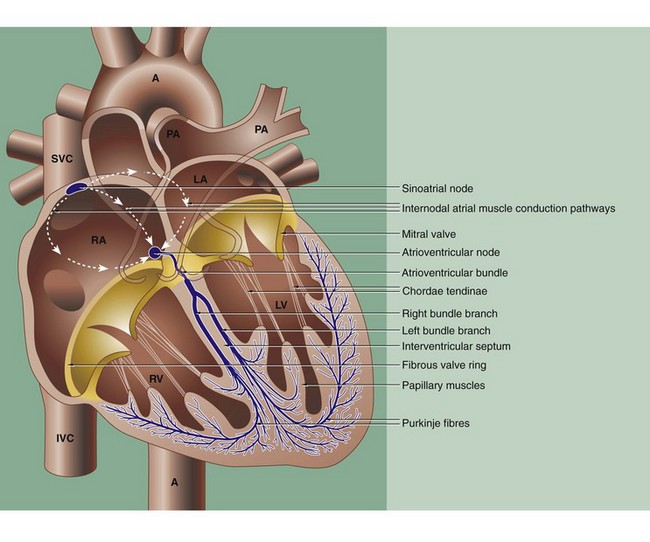
FIG. 8.5 The conducting system of the heart
The coordinated contraction of the heart is largely effected by a specialised conducting system of modified cardiac muscle fibres. The initial impulse originates spontaneously in the sino-atrial node, situated in the right atrial wall near the entry of the superior vena cava SVC. The impulse rate is controlled by the autonomic nervous system.
The impulse passes through the muscle of the atria RA and LA, causing them to contract, and reaches the atrioventricular node in the medial wall of the right atrium just above the tricuspid valve ring at the base of the interatrial septum. Both the sinoatrial and atrioventricular nodes are irregular meshworks of very small specialised myocardial fibres, with electrochemical stimuli being transmitted via gap junctions. The nodal fibres are embedded in collagenous fibrous tissue which contains blood vessels and many autonomic nerve fibres.
From the atrioventricular node, the impulse is passed along a specialised bundle of conducting fibres, the atrioventricular bundle (of His), which initially divides into right and left bundle branches that then (halfway down the interventricular septum) become Purkinje fibres which run immediately beneath the endocardium before penetrating the myocardium (see Figs 8.4 and 8.6).
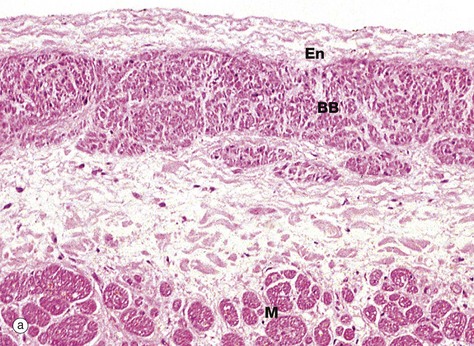
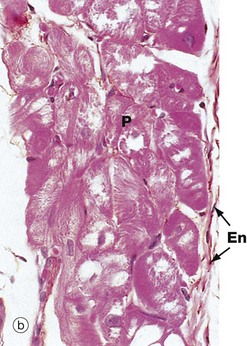
FIG. 8.6 Heart
(a) Bundle branch, H&E (MP) (b) Purkinje fibres, H&E (HP)
Micrograph (a) shows the left branch bundle of conducting fibres BB running in the interventricular septum, just beneath the endocardium En lining the left ventricular cavity. At this level, the conducting fibres are separated from the myocardial fibres M of the septum by a layer of fibrous tissue. The conducting fibres are specialised cardiac muscle fibres and contain comparatively few myofibrils, which are mainly located beneath the cell membrane, but abundant glycogen granules and mitochondria. This makes these fibres paler staining than normal myocardial fibres by most stains.
Micrograph (b) shows the distal extension of the branch bundle, with the Purkinje fibres P beneath the thin endocardium En. These fibres are larger than cardiac muscle fibres and have a pale-staining central area with most of the red-staining myofibrils around the periphery of the cell. Unlike myocardial fibres, Purkinje and other conducting fibres have no T tubule system and connect with each other by desmosomes and gap junctions, rather than intercalated discs.
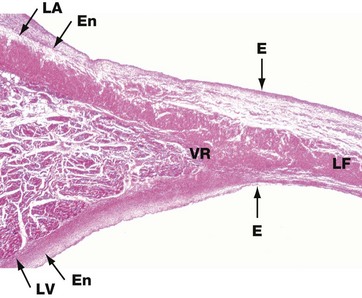
FIG. 8.7 Heart valve
H&E (LP)
The heart valves consist of leaflets of fibroelastic tissue. The surfaces are covered by a thin layer of endothelium E which is continuous with that lining the heart chambers and great vessels. This low-power micrograph shows the left atrioventricular valve (the mitral valve), arising at the junction of the walls of the left atrium LA and left ventricle LV.
The fibroelastic layer of the endocardium En condenses to form the valve ring VR, and from this arises the central fibroelastic sheet of the valve, the lamina fibrosa LF.
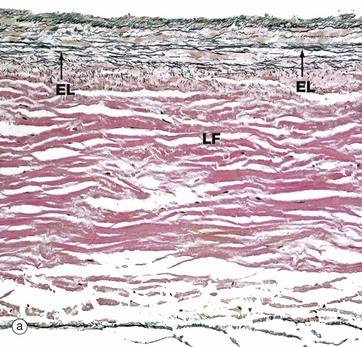
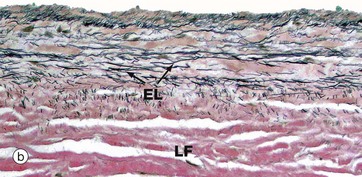
FIG. 8.8 Heart valve
(a) Elastic van Gieson (LP) (b) Elastic van Gieson (MP)
The valves are sheets of fibroelastic tissue covered on both sides by endocardium. There is a dense central plate of collagen (the lamina fibrosa LF) containing scattered elastic fibres (black in this stain) as shown in micrograph (a) at low magnification. In the left atrioventricular valves (as here), there is a distinct elastic lamina (EL) towards the atrial surface in micrograph (b) and the collagen (red staining here) is particularly prominent on the ventricular surface where the chordae tendinae are attached.
The Arterial System
The function of the arterial system is to distribute blood from the heart to capillary beds throughout the body. The cyclical pumping action of the heart produces a pulsatile blood flow in the arterial system. With each contraction of the ventricles (systole), blood is forced into the arterial system causing expansion of the arterial walls; subsequent recoil of the arterial walls assists in maintenance of arterial blood pressure between ventricular beats (diastole). This expansion and recoil is a function of elastic tissue within the walls of the arteries.
The flow of blood to various organs and tissues may be regulated by varying the diameter of the distributing vessels. This function is performed by the circumferentially disposed smooth muscle of vessel walls and is principally under the control of the sympathetic nervous system and adrenal medullary hormones.
The walls of the arterial vessels conform to the general three-layered structure of the circulatory system but are characterised by the presence of considerable elastin and the smooth muscle wall is thick relative to the diameter of the lumen. There are three main types of vessel in the arterial system:
• Elastic arteries. These comprise the major distribution vessels and include the aorta, the innominate (brachiocephalic trunk), common carotid and subclavian arteries and most of the large pulmonary arterial vessels.
• Muscular arteries. These are the main distributing branches of the arterial tree, such as the radial, femoral, coronary and cerebral arteries.
• Arterioles. These are the terminal branches of the arterial tree which supply the capillary beds.
There is a gradual transition in structure and function between the three types of arterial vessel rather than an abrupt demarcation. In general, the amount of elastic tissue decreases as the vessels become smaller and the smooth muscle component assumes relatively greater prominence.
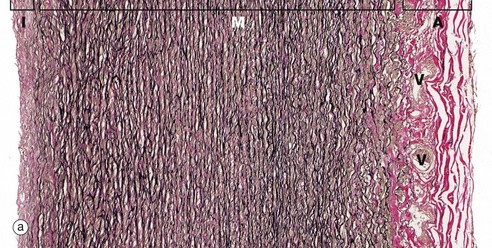
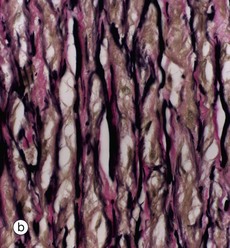
FIG. 8.9 Elastic artery: aorta
(a) Elastic van Gieson (LP) (b) Elastic van Gieson (HP)
The highly elastic nature of the aortic wall is demonstrated in these preparations in which the elastic fibres are stained brownish-black. In micrograph (a), the three basic layers of the wall can be seen: the narrow tunica intima I, the broad tunica media M and the tunica adventitia A.
The tunica intima consists of a single layer of flattened endothelial cells (not seen at this magnification) supported by a layer of collagenous tissue rich in elastin disposed in the form of both fibres and discontinuous sheets. The subendothelial supporting tissue contains scattered fibroblasts and other cells with ultrastructural features akin to smooth muscle cells and known as myointimal cells. Both cell types are probably involved in elaboration of the extracellular constituents. The myointimal cells are not invested by basement membrane and are thus not epithelial (myoepithelial) in nature. With increasing age, the myointimal cells accumulate lipid and the intima progressively thickens. If this process continues, atherosclerosis will develop.
The tunica media is particularly broad and extremely elastic. At high magnification in (b), it is seen to consist of concentric fenestrated sheets of elastin (stained black) separated by collagenous tissue (stained reddish-brown) and smooth muscle fibres (stained yellow). As seen in micrograph (a), the collagenous tunica adventitia (stained reddish-brown) contains small vasa vasorum V which also penetrate the outer half of the tunica media.
Blood flow within elastic arteries is highly pulsatile. With advancing age, the arterial system becomes less elastic, thereby increasing peripheral resistance and thus arterial blood pressure.
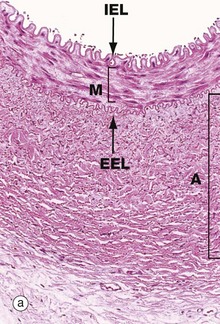
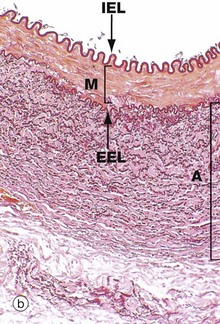
FIG. 8.10 Muscular artery
(a) H&E (MP) (b) Elastic van Gieson (MP)
In muscular arteries, the elastic tissue is largely concentrated as two well-defined elastic sheets. One sheet is the internal elastic lamina IEL between the tunica intima and the tunica media. The less prominent and more variable external elastic lamina EEL lies between the tunica media M and the adventitia. The tunica intima is usually a very thin layer, not visible at low magnification, and the tunica media M is composed of concentrically arranged smooth muscle fibres with scanty elastic fibres between them. The tunica adventitia A is of variable thickness and is composed of collagen and a variable amount of elastic tissue. In larger muscular arteries, this layer may contain prominent vasa vasorum.
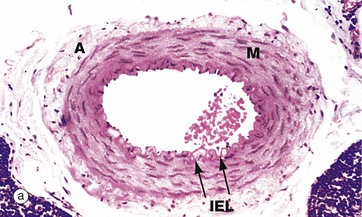
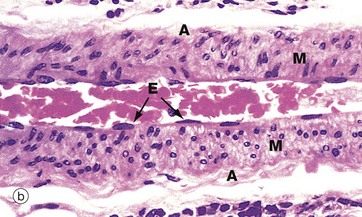
FIG. 8.11 Small muscular artery
(a) H&E, TS (MP) (b) H&E, LS (HP)
The diameter of a small muscular artery is approximately 0.5 to 2 mm and a thin but distinct internal elastic lamina is present, but there is usually little or no external elastic lamina. The tunica media has 3 to 10 concentric layers of smooth muscle cells and contains almost no elastic fibres. Micrograph (a) shows an artery in transverse section. The distinction between the tunica media M and adventitia A is obvious. The internal elastic lamina IEL can just be distinguished as a densely staining wavy line. Micrograph (b) is a smaller artery at higher magnification. The nuclei of the intimal endothelial cells E are visible, but the elastic lamina has largely disappeared.
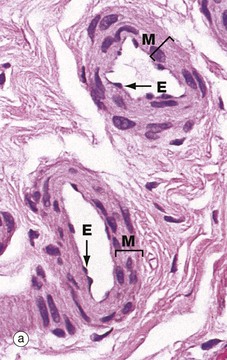
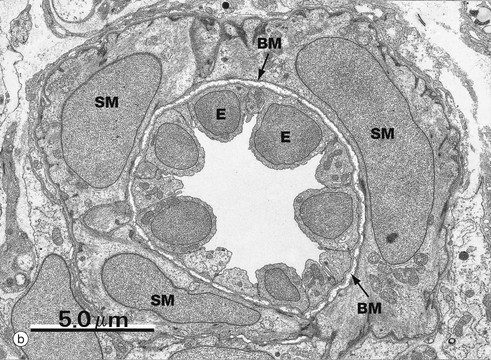
FIG. 8.12 Arterioles
(a) Large arteriole H&E, TS (MP) (b) Small arteriole, EM ×5250, TS
Small muscular arteries merge into large arterioles, which eventually become small arterioles. These transitions are gradual with no sharp demarcations and involve loss of the internal elastic lamina and progressive reduction of the number of muscle layers in the media. Micrograph (a) shows two large arterioles, with a thin intima lined by endothelial cells E and a tunica media M comprising only 2 to 3 layers of muscle. The adventitia is thin and merges imperceptibly with surrounding supporting collagenous fibrous tissue.
Micrograph (b) is an electron micrograph of a small arteriole, with a single layer of smooth muscle cells SM separated from endothelium E by basement membrane BM. The endothelium is prominent because the arteriole is constricted.
The Microcirculation
The microcirculation is that part of the circulatory system concerned with the exchange of gases, fluids, nutrients and metabolic waste products. Exchange occurs mainly within the capillaries, extremely thin-walled vessels forming an interconnected network. Blood flow within the capillary bed is controlled by the arterioles and muscular sphincters at the arteriolar-capillary junctions called precapillary sphincters. The capillaries drain into a series of vessels of increasing diameter, namely postcapillary venules, collecting venules and small muscular venules which make up the venous component of the microcirculation.
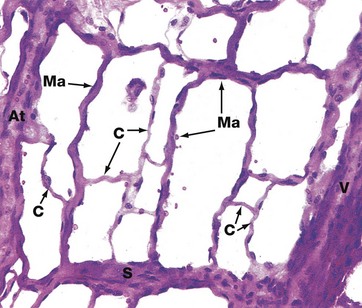
FIG. 8.13 The microcirculation, mesenteric spread
H&E (MP)
This image demonstrates a network of anastomosing capillaries between an arteriole At and a venule V. The capillary network comprises small-diameter capillaries C with a single layer of endothelial cells and basement membrane, as well as larger-diameter capillaries known as metarterioles Ma. These are characterised by a discontinuous outer layer of smooth muscle cells. Small capillaries arise from both arterioles and metarterioles.
At the origin of each capillary, there is a sphincter mechanism, the precapillary sphincter, which is involved in regulation of blood flow. There is also a direct wide-diameter link between the arteriole and venule, an arteriovenous shunt S. Metarterioles also form direct communications between arterioles and venules. Contraction of the smooth muscle of shunts and metarterioles directs blood through the network of small capillaries. Thus arterioles, metarterioles, precapillary sphincters and arteriovenous shunts regulate blood flow in the microcirculation. The smooth muscle activity of these vessels is modulated by the autonomic nervous system and by circulating hormones (e.g. adrenal catecholamines).
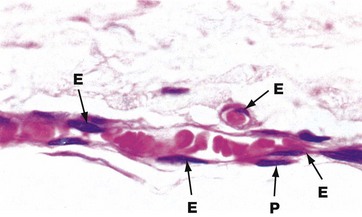
FIG. 8.14 Capillaries
H&E (HP)
The vessels seen here in longitudinal and transverse section illustrate the characteristic features of capillaries. A single layer of flattened endothelial cells lines the capillary lumen. The thin layer of cytoplasm is difficult to resolve by light microscopy. The flattened endothelial cell nuclei E bulge into the capillary lumen. In longitudinal section, the nuclei appear elongated, whereas in transverse section they appear more rounded. Muscular and adventitial layers are absent. Occasional flattened cells called pericytes P embrace the capillary endothelial cells and may have a contractile function. Note that the diameter of capillaries is similar to that of the red blood cells contained within them.
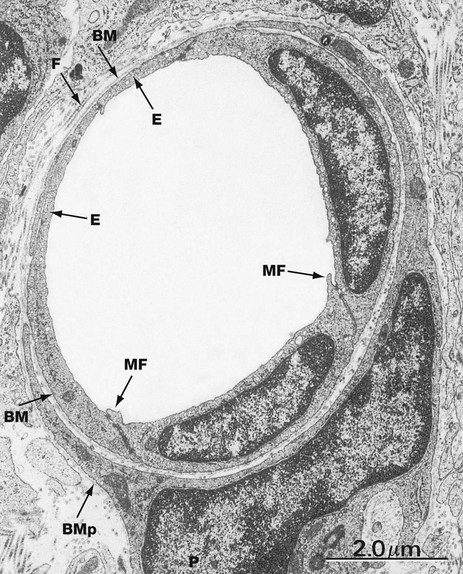
FIG. 8.15 Capillary, continuous endothelium type
EM ×12 000
This electron micrograph illustrates the ultrastructure of capillaries of the continuous endothelium type, the type found in most tissues. Endothelial cells E encircle the capillary lumen, their plasma membranes approximating one another very closely and bound together by scattered tight junctions of the fascia occludens type (see Fig. 5.11). Small cytoplasmic flaps called marginal folds MF extend across the intercellular junctions at the luminal surface. The capillary endothelium is supported by a thin basement membrane BM and adjacent collagen fibrils F. A pericyte P embraces the capillary and is supported by its own basement membrane BMp.
Exchange between the lumen of the continuous-type capillary and the surrounding tissues is believed to occur in three ways. Passive diffusion through the endothelial cell cytoplasm mediates exchange of gases, ions and low molecular weight metabolites. Proteins and some lipids are transported by pinocytotic vesicles (see Ch. 1). White blood cells pass through the intercellular space between the endothelial cells, in some way negotiating the endothelial intercellular junctions. Some researchers maintain that the intercellular spaces also permit molecular transport. In capillaries of the continuous endothelial type, the basement membrane is thought to present little barrier to exchange between capillaries and surrounding tissues.
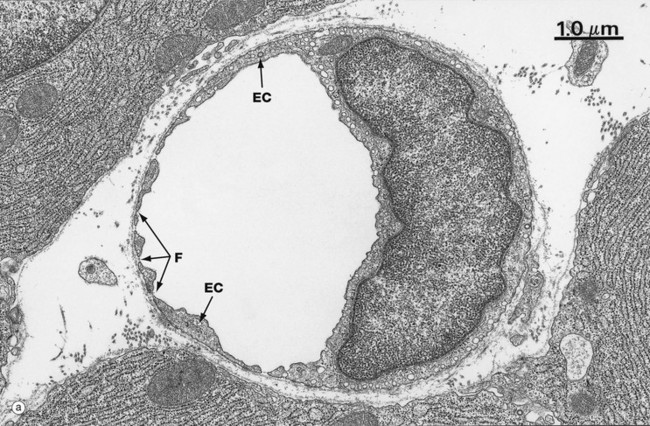
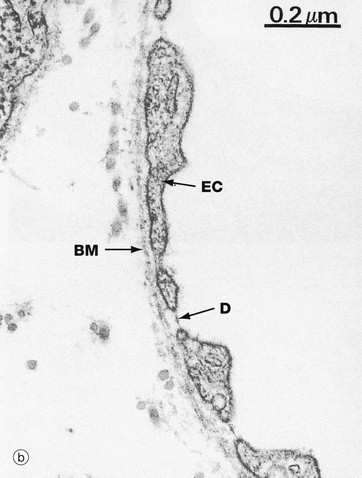
FIG. 8.16 Capillary, fenestrated endothelium type
(a) Whole capillary, EM ×15 000, TS (b) Very high power view of capillary fenestrations, EM ×60 000
Fenestrated capillaries are found in some tissues where there is extensive molecular exchange with the blood. Such tissues include the small intestine, endocrine glands and kidney.
At low magnification in image (a), fenestrations F appear as pores through attenuated areas of the endothelial cytoplasm EC; however, only a small proportion of these areas are fenestrated. At high magnification in image (b), the fenestrations appear to be traversed by a thin electron-dense line D which may constitute a diaphragm. The biochemical and functional nature of this is not understood. Fenestrated capillaries without a diaphragm are found in the glomeruli of the kidney (see Fig. 16.14).
The permeability of fenestrated capillaries is much greater than that of continuous endothelium-type capillaries. Molecular labelling techniques have demonstrated that fenestrations permit rapid passage of macromolecules smaller than plasma proteins from the lumina of fenestrated capillaries into surrounding tissues.
Like continuous endothelium-type capillaries, all fenestrated capillaries are supported by a basement membrane BM which is continuous across the fenestrations. However, the endothelium of the sinusoids in the bone marrow, spleen and liver has large fenestrations without diaphragms and, in these sites, the underlying basement membrane is discontinuous. Pericytes are rarely found in association with fenestrated capillaries.
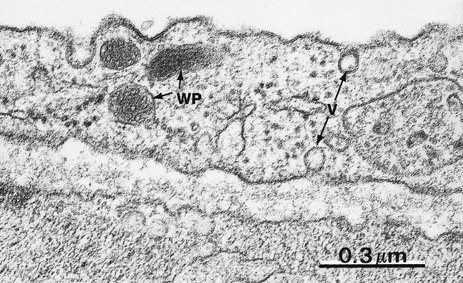
FIG. 8.17 Endothelial cell
EM ×68 000
Endothelial cells are flat polygonal cells which are connected to each other by junctional complexes. They have numerous pinocytotic vesicles V and specialised membrane-bound organelles called Weibel-Palade bodies WP which store von Willebrand factor.
Endothelial cells have a range of metabolic functions (see box), many concerned with the fine control of blood coagulation and thrombosis, as well as regulating local control of blood vessel constriction/dilatation and changes in vessel wall permeability. Endothelial cell damage may lead to pathological thrombosis or haemorrhage, or exudation of some components of blood into the extravascular tissues.
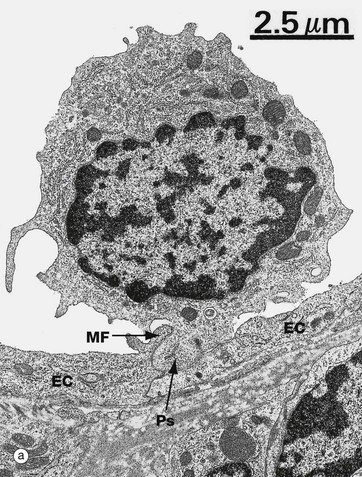
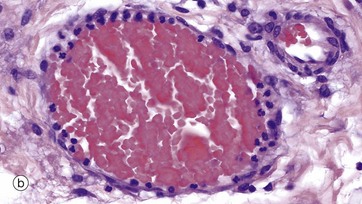
FIG. 8.18 Cell migration from the microcirculation
(a) EM ×6000 (b) H&E (HP)
Fluids and cells pass from the circulation into the tissues in the microcirculation, mainly capillaries and postcapillary venules. The electron micrograph (a) shows a lymphocyte in the process of migration through the wall of a postcapillary venule (see Fig. 8.19). Its pseudopodium Ps has lifted the marginal fold MF at the contact point of two endothelial cells EC. In contrast to capillaries, intercellular junctional complexes are relatively uncommon between endothelial cells in postcapillary venules, and this facilitates leucocyte emigration.
Micrograph (b) shows a markedly dilated postcapillary venule in an area of tissue damage. Neutrophils in the circulation have migrated to the periphery of the erythrocyte stream and have become attached to the endothelial cell surface (margination) prior to emigration into the tissues.
The Venous System
The systemic venous system is a low-pressure component of the blood circulatory system which is responsible for carrying blood from the capillary networks back to the right atrium of the heart.
The force impelling the blood towards the heart, often against gravity, is a combination of contraction of the smooth muscle of the vein wall and external compression of veins by contraction of skeletal muscles, particularly in the lower limbs. Backflow of blood is prevented by valves, particularly in small and medium-sized veins. These valves are derived from the intima of the vessel. Valve failure in the veins of the legs is the basis for the development of the common condition known as varicose veins.
The structure of the venous system conforms to the general three-layered arrangement elsewhere in the circulatory system, but the elastic and muscular components are much less prominent features. A major part of the total blood volume is contained within the venous system.
Variations in relative blood volume, for example due to dilation of capillary beds or haemorrhage, may be compensated for by changes in the capacity of the venous system. These changes are mediated by contraction or relaxation of the smooth muscle in the tunica media. This controls the luminal diameter of muscular venules and veins.
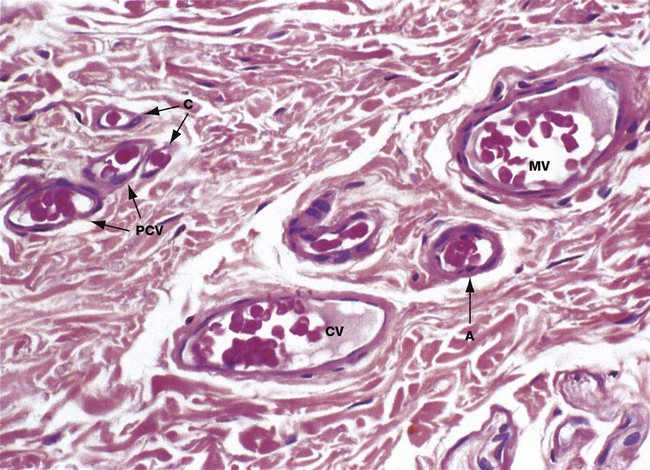
FIG. 8.19 Postcapillary, collecting and muscular venules
H&E (HP)
The capillaries drain into a series of thin-walled vessels which form the first part of the venous system. Postcapillary venules PCV are the smallest of these vessels and are formed by confluence of several capillaries C. Postcapillary venules have a similar structure to large capillaries, with an endothelium and pericytes but no smooth muscle layer. Blood flow in postcapillary venules is sluggish and it appears that these vessels are the main site of migration of white cells into and out of the circulation.
Postcapillary venules drain into collecting venules CV which are structurally similar but larger, with more surrounding pericytes. Collecting venules drain into vessels of increasing diameter which eventually acquire a wall of smooth muscle cells two or three layers thick; at this stage the vessels are called muscular venules MV. This micrograph also shows a small arteriole A with only a single layer of smooth muscle cells in the wall. Its wall structure is similar to that of muscular venules, but the lumen is considerably smaller.
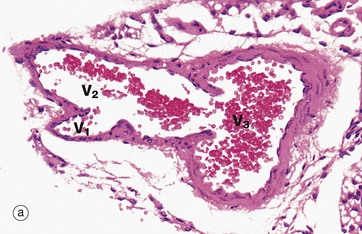
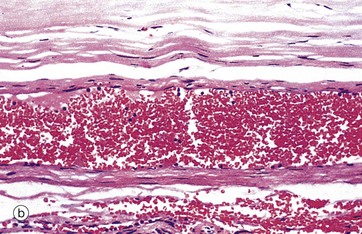
FIG. 8.20 Muscular venules and small veins
(a) H&E (MP) (b) H&E (MP)
Micrograph (a) illustrates the confluence of a small muscular venule V1 with a larger muscular venule V2 which then joins a small vein V3 cut in transverse section. Note the valve at the junction of the large venule and vein. Muscular venules are characterised by a clearly defined intimal layer devoid of elastic fibres and a tunica media consisting of one or two layers of smooth muscle fibres. Veins are characterised by a thicker muscular wall and a poorly developed internal elastic lamina. Note that the tunica adventitia of these vessels is continuous with the surrounding collagenous supporting tissue.
Micrograph (b) shows a small vein cut in longitudinal section and fixed whilst still distended with blood. The wall of the vein consists of two to three layers of smooth muscle fibres. Note the wide diameter of the lumen relative to the thickness of the wall.
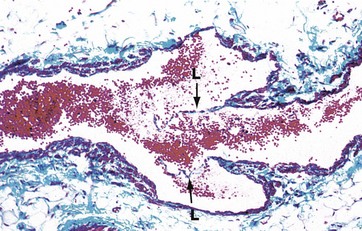
FIG. 8.21 Vein with valve
Masson trichrome (MP)
This micrograph demonstrates a valve in a small vein. The valve consists of delicate semilunar projections of the tunica intima of the vein wall. These projections are composed of a layer of fibroelastic tissue which is lined on both sides by endothelium.
Each valve usually consists of two leaflets L, the free edges of which project in the direction of blood flow. These serve to prevent backflow of blood due to the effects of gravity. Valves only occur in veins which are more than 2 mm in diameter, particularly those draining the extremities.
Varicose veins are abnormal dilatations of superficial veins which typically occur in the lower legs. These form due to incompetence of valves in the leg veins.
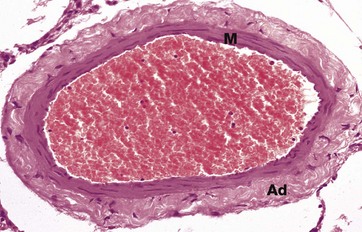
FIG. 8.22 Medium-sized vein
H&E (MP)
This micrograph shows a medium-sized vein which is distended with red blood cells. The tunica intima consists of little more than the endothelial cell layer supported by a very narrow band of supporting intimal fibrous tissue. The intima is difficult to discern in this micrograph. The tunica media M is thin when compared with that of an equivalent-sized artery (compare with Fig. 8.11a) and consists of only 2 to 4 layers of smooth muscle fibres. These are arranged in a circumferential fashion.
In veins, the tunica adventitia Ad is usually the thickest layer of the vessel wall. The adventitia is composed of collagenous fibrous tissue and the collagen fibres usually run in a predominantly longitudinal direction.
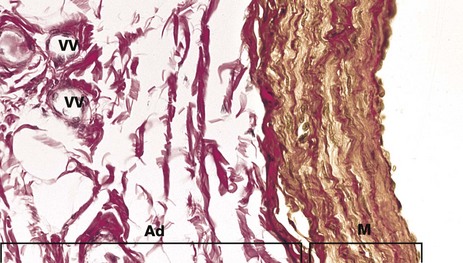
FIG. 8.23 Large muscular vein
Elastic van Gieson (HP)
Large veins such as the femoral and renal veins again have a very narrow tunica intima, but the media M is more substantial, consisting of several layers of smooth muscle (stained yellow in this stain), separated by layers of collagenous connective tissue (red) and scanty elastic fibres (black).
The tunica adventitia Ad is broad and is composed of collagen (red) and contains numerous vasa vasorum VV.
Elastic fibres are particularly prominent at the junction between media and adventitia, but there are no distinct elastic laminae as there are in arteries.
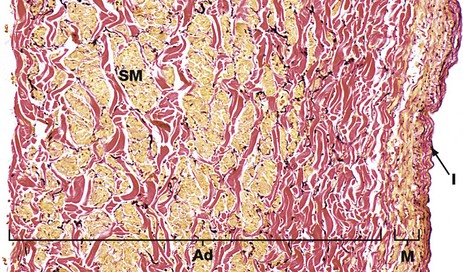
FIG. 8.24 Inferior vena cava
Elastic van Gieson (MP)
The superior and inferior venae cavae are the largest veins in the body and return deoxygenated blood from all areas of the body (except the lungs) to the right atrium of the heart. They have the thickest walls of all veins, comprising a distinct intima I of fibroelastic tissue, a narrow tunica media M composed of mainly circular smooth muscle, beneath which is a thick adventitia Ad composed of collagen (red) and thick bundles of longitudinally arranged smooth muscle fibres (yellow) SM. There are elastic fibres (black) scattered throughout the wall and, in some areas, there is a variable internal elastic lamina between intima and media.
The Lymph Vascular System
The lymph vascular system drains excess fluid, the lymph, from extracellular spaces and returns it to the blood vascular system. Lymph is formed in the following manner. At the arterial end of blood capillaries, the hydrostatic pressure of blood exceeds the colloidal osmotic pressure exerted by plasma proteins. Water and electrolytes therefore move out of capillaries and into the extracellular space. Some plasma proteins also leak out through the endothelial wall. At the venous end of blood capillaries, the pressure relationships are reversed and fluid tends to be drawn back into the blood vascular system. In this way, about 2% of plasma passing through the capillary bed is exchanged with the extracellular tissue fluid. The rate of tissue fluid formation at the arterial end of capillaries generally exceeds the re-uptake of fluid at the venous end. The excess fluid, lymph, is drained by a system of lymph capillaries which converge to form progressively larger-diameter lymphatic vessels.
As lymphatics get larger, they acquire smooth muscle cells in their walls and these contribute to the movement of lymph by pumping it onwards, the valves preventing backflow. Lymph eventually passes into much larger ducts (the thoracic and right lymphatic ducts) which empty lymph into the blood circulation at the confluence of the internal jugular and subclavian veins of both sides. These large ducts have a substantial muscle layer with longitudinal and circular layers, but the layers are poorly demarcated.
Along the course of the larger lymphatic vessels, there are aggregations of lymphoid tissues called lymph nodes where lymph is sampled for the presence of foreign material (antigen) and where activated cells of the immune system and antibodies join the general circulation (see Ch. 11). Lymphatic vessels are found in all tissues except the central nervous system, cartilage, bone, bone marrow, thymus, placenta, cornea and teeth.
Lymphatic capillaries differ from blood capillaries in several respects which reflect the greater permeability of lymphatic capillaries. In particular, the endothelial cell cytoplasm of lymphatics is extremely thin, the basement membrane is rudimentary or absent and there are no pericytes. Fine collagenous filaments known as anchoring filaments link the endothelium to the surrounding supporting tissue, preventing collapse of the lymphatic lumen.
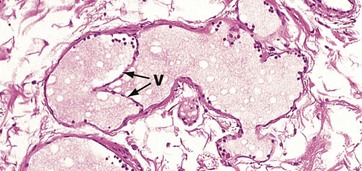
FIG. 8.25 Valve of a lymphatic vessel
H&E (LP)
A characteristic feature of the lymphatic system is the presence of numerous delicate valves within small and medium-sized vessels.
The structure of these valves V is similar to that of valves in the venous system, but the supporting tissue core includes only some reticulin fibres and a little ground substance. Note the presence of light pink–stained proteinaceous lymph fluid in the channels, with scattered lymphocytes around the periphery.
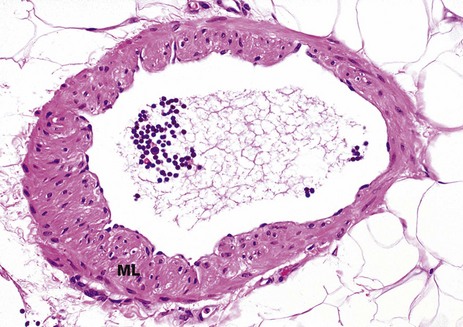
FIG. 8.26 Medium-sized lymphatic vessel
H&E (MP)
Fig. 8.25 shows small lymphatic vessels containing only a very small amount of smooth muscle in their walls. As lymphatic channels become larger, the muscle layer ML becomes thicker and its contraction makes a greater contribution to the movement of lymph along the vessel. Backflow of lymph fluid is prevented by valves (not illustrated here).
The muscle layers are most prominent in the largest lymphatic vessels which drain into the venous system (the thoracic duct and right lymphatic duct).
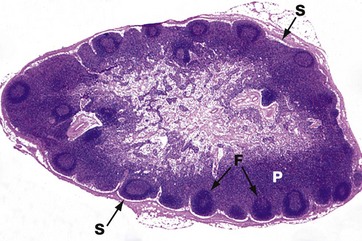
FIG. 8.27 Normal lymph node
H&E (LP)
This micrograph illustrates a normal lymph node (see also Ch. 11), the site of filtration of lymph fluid.
Afferent lymphatic channels enter the convexity of the lymph node, and fluid drains into the peripheral subcapsular sinus S. The sinuses of the lymph node are lined by macrophages, and these cells filter out any particulate debris from the fluid. The fluid percolates through the sinuses and emerges via an efferent lymphatic channel at the hilum of the node (not shown here).
Note the presence of follicles F in the cortex of the lymph node, the site of B-cell activation. The intervening paracortical area P is the main site of T cells within the lymph node.