Urinary system
Introduction
The principal function of the urinary system is the maintenance of water, electrolyte and acid-base homeostasis, which requires that any input into the system is balanced by an equivalent output. The kidney provides the mechanism by which excess water and electrolytes are eliminated from the body, while the ureters, bladder and urethra form the storage and outflow tract. A second major function of the urinary system is the excretion of many toxic metabolic waste products, particularly the nitrogenous molecules urea and creatinine, compounds that can conveniently be excreted dissolved in water. The end product of these processes is urine. Since all body fluids are maintained in dynamic equilibrium with one another by the circulatory system, any adjustment in the composition of the blood results in similar changes in the other fluid compartments of the body. Thus regulation of the osmotic concentration of blood plasma by the kidneys (osmoregulation) ensures the osmotic regulation of all other body fluids. The third major function of the kidney is the maintenance of normal blood pressure.
The functional and structural unit of the kidney, the nephron, consists of a renal corpuscle (including the glomerulus) plus a long, folded renal tubule. The human kidney contains approximately 1 million nephrons that perform the functions of osmoregulation and excretion by the following processes:
• Filtration in the glomerulus of most small molecules from blood plasma to form an ultrafiltrate of plasma
• Selective reabsorption in the tubule of most of the water and some other molecules from the ultrafiltrate, leaving behind excess and waste materials to be excreted
• Secretion in the tubule of some excretory products directly from blood into the urine
• Maintenance of the acid-base balance by selective secretion by the tubule of H+ ions into the urine
The kidney also has hormonal and metabolic functions:
• Renin, synthesised in the kidney, is a component of the renin-angiotensin-aldosterone mechanism that controls blood pressure.
• Erythropoietin, synthesised in the kidney, stimulates the production of erythrocytes in the bone marrow and thus regulates the oxygen-carrying capacity of the blood.
• Vitamin D, which regulates calcium balance, is converted to an active form in the kidney.
FIG. 16.1 The urinary system
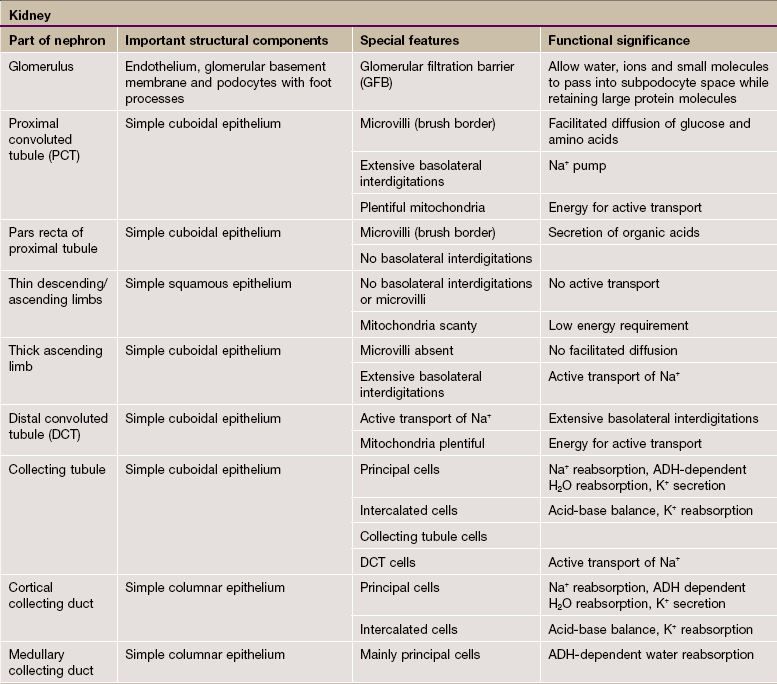
The urinary system comprises two kidneys, two ureters, a bladder and a urethra. Urine is produced in the kidneys and flows down the ureters to the bladder where it is stored until voided via the urethra. No further modification of the urine takes place after it leaves the kidneys. The kidneys and ureters are found in the retroperitoneum, while the urinary bladder is in the anterior part of the pelvis.
Blood is supplied to each kidney by the renal arteries, which arise from the aorta. One or more renal veins drains the blood from each kidney to the inferior vena cava. The total blood volume of the body is circulated through the kidneys about 300 times each day.
FIG. 16.2 Kidney
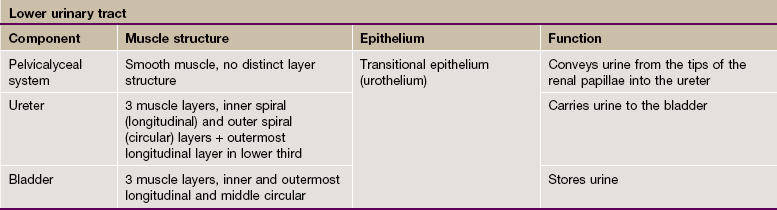
The kidney is a bean-shaped organ lying in the upper retroperitoneal area and oriented with the concave surface directed medially. In adults, the kidney measures 10 to 12 cm. The hilum is the site of entry and exit of the renal blood vessels and the ureter.
The archetypal kidney of lower mammals consists of a single lobe made up of a medullary pyramid (actually cone-shaped), the base of which is enveloped by the cortex containing the renal corpuscles and the proximal and distal parts of the tubules. Nephrons arise in the cortex, loop down into the medulla and return to the cortex. From here they drain into collecting ducts that descend again into the medulla to discharge urine from the apex of the medullary pyramid. The apical part of the pyramid (known as the renal papilla) is enveloped by a funnel-shaped renal pelvis, which represents the dilated proximal part of the ureter.
The human kidney is made up of 10 to 18 lobes. In the adult, the cortical components of the lobes are fused so that the cortex forms a continuous smooth outer zone which extends down between the pyramids. The renal medulla is made up of multiple medullary pyramids, separated by medullary extensions of the cortex. Each renal papilla is surrounded by a branch of the renal pelvis called a calyx; the whole urinary collecting system within the kidney being described as the pelvicalyceal system. The space between the branches of the pelvicalyceal system is filled with fatty supporting tissue and is known as the renal sinus.
The kidney is invested by a tough fibrous capsule which is surrounded by a thick layer of perinephric fat that is in turn encased in a delicate condensation of connective tissue known as Gerota's fascia. The fat around the kidney cushions it against trauma.
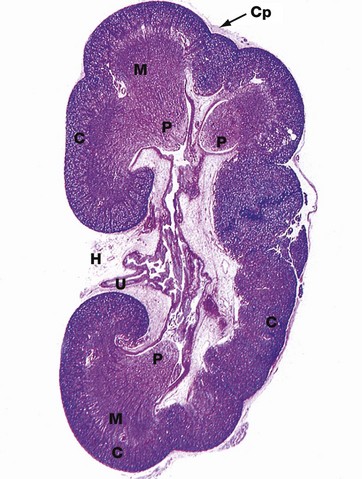
FIG. 16.3 Kidney
H&E (LP)
This micrograph of a kidney from a stillborn child illustrates at low power the features of the kidney described in Fig. 16.2. The kidney of a baby has been chosen as it is small enough to section and photograph in its entirety. Furthermore its convex surface is irregular, reflecting the development of the many lobes making up the organ. In histological section, only a single plane through the pelvicalyceal system can be visualised. This plane of section includes the axes of three lobes, the papilla P of each one projecting into the central pelvicalyceal space; this drains into the ureter U that leaves the kidney via the hilum H.
The darker-stained cortex C can be clearly differentiated from the paler-stained medulla M. The cortex contains large numbers of tiny spheroidal structures, the developing renal corpuscles (see Fig. 16.8). The medullary pyramids are characterised by the numerous tubules converging towards the tips of the renal papillae. Note the continuity of the cortex throughout the outer zone of the kidney and the cortical extension between the two medullary pyramids at the top of the field. The fibrous capsule Cp of the kidney is continuous at the hilum with fatty supporting tissue, which packs the space (known as the renal sinus) between the hilar structures. The renal artery and vein also pass through the hilum but are not seen in this plane of section.
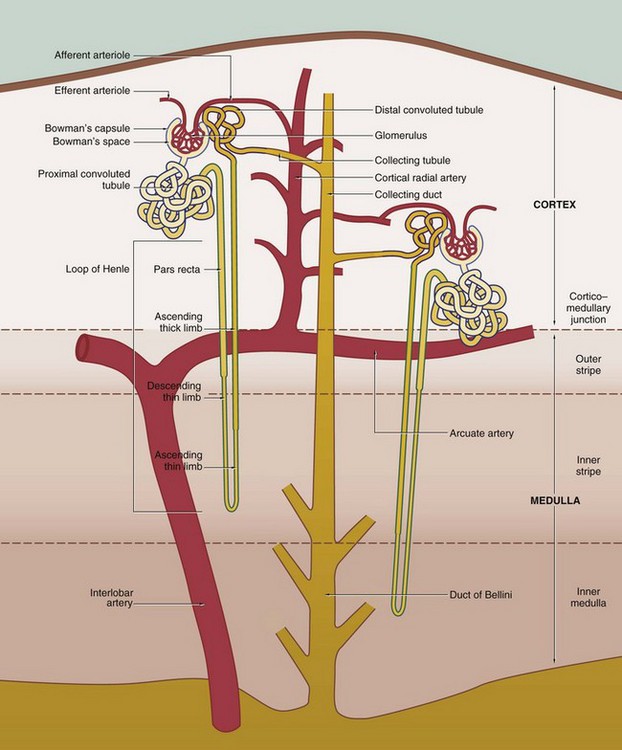
FIG. 16.4 Basic organisation of the nephron, collecting system and renal vasculature (caption continues opposite)
The nephron, the functional unit of the kidney, consists of two major components, the renal corpuscle and the renal tubule. A normal adult kidney contains between 0.6 and 1.2 × 106 nephrons.
Renal corpuscle
The renal corpuscle is responsible for the filtration of plasma and is a combination of two structures, Bowman's capsule and the glomerulus.
Bowman's capsule consists of a single layer of flattened cells resting on a basement membrane; it is derived from the distended blind end of the renal tubule. The glomerulus is a globular network of anastomosing capillaries which invaginates Bowman's capsule (see Fig. 16.7). Thus the capillary loops of the glomerulus are invested by the visceral layer of Bowman's capsule, a highly specialised layer of epithelial cells called podocytes (see Fig. 16.12). The visceral layer is reflected around the vascular stalk of the glomerulus to become continuous with the parietal layer that constitutes Bowman's capsule proper. The space between the two layers is known as Bowman's space and is continuous with the lumen of the renal tubule; the parietal epithelium of Bowman's capsule is continuous with the epithelium lining the renal tubule.
In the renal corpuscle, water and low molecular weight constituents of plasma are filtered from the glomerular capillaries into Bowman's space to form the glomerular ultrafiltrate, which then passes into the renal tubule. Thus the filtration barrier between the capillary lumen and Bowman's space consists of the capillary endothelium, the podocyte layer and their common basement membrane known as the glomerular basement membrane (see Fig. 16.14); these three components are sometimes called the glomerular filtration barrier.
The afferent arteriole, which supplies the glomerulus, and the efferent arteriole, which drains it, enter and leave the corpuscle at the vascular pole that is usually situated opposite the entrance to the renal tubule, the urinary pole (see Fig. 16.8).
Renal tubule
The renal tubule extends from Bowman's capsule to its junction with a collecting duct. The renal tubule is up to 55 mm long in humans and is lined by a single layer of epithelial cells. The primary function of the renal tubule is the selective reabsorption of water, inorganic ions and other molecules from the glomerular filtrate. In addition, some inorganic ions are secreted directly from blood into the lumen of the tubule. In humans, glomerular filtrate is produced at a steady rate of approximately 120 mL/min; of this, all but about 1 mL is reabsorbed by the renal tubules, giving a normal rate of urine production of around 1mL/min. The renal tubule has a convoluted shape and has four distinct zones, each of which has a different role in tubular function and a corresponding difference in histological appearance.
1. The proximal convoluted tubule (PCT) is the most convoluted section of the tubule and is responsible for the reabsorption of approximately 65% of the ions and water of the glomerular filtrate. PCTs are confined to the renal cortex and make up the greater part of its bulk.
2. The loop of Henle includes the distal straight part of the proximal tubule, the pars recta, the thin descending and ascending limbs and the thick ascending limb. The difference between these parts is due to differences in the epithelium. The thin segments of the loop of Henle dip down into the medulla where they form a hairpin bend. The length of the loop of Henle varies from short to long, depending on the location of the renal corpuscle of the particular nephron. The corpuscles of short-looped nephrons tend to be located in the superficial and midcortical regions, the loops extending very little beyond the corticomedullary junction. Long-looped nephrons are mainly associated with juxtamedullary corpuscles; a small proportion of long loops almost reach the tips of the renal papillae, but successively greater numbers turn back at higher levels as necessitated by the tapering shape of the medullary pyramids. The limbs of the loop of Henle are closely associated with parallel wide capillary loops, the vasa recta (not shown in this diagram), which arise from the efferent arterioles of glomeruli located near the corticomedullary junction. The vasa recta descend into the medulla then loop back on themselves to drain into veins at the junction of the medulla and cortex. The main function of the loops of Henle is to generate a high osmotic pressure in the extracellular fluid of the renal medulla; the mechanism by which this is achieved is known as the counter-current multiplier system (see Fig. 16.26). In some animals, the loop of Henle plays a major role in reabsorption of water from the glomerular filtrate back into the circulation via the vasa recta; however, this function is of lesser importance in the human kidney. The medulla can be divided into different zones according to the components of the loop of Henle that are present: the inner medulla contains only thin limbs of the loop of Henle, the inner stripe of the outer medulla contains thick descending limbs as well as thin limbs and the outer stripe of the outer medulla contains thick ascending limbs as well as thick descending limbs and thin limbs.
3. The distal convoluted tubule (DCT) is a continuation of the thick limb of the loop of Henle after its return to the cortex. Shorter and less convoluted than the PCT, the DCT is responsible for reabsorption of sodium ions, an active process controlled by the adrenocortical hormone aldosterone. Sodium reabsorption is coupled with the secretion of hydrogen or potassium ions into the DCT, the secretion of hydrogen ions resulting in a net loss of acid from the body.
4. The collecting tubule is the straight terminal portion of the nephron, several collecting tubules converging to form a collecting duct. The collecting ducts descend through the cortex in parallel bundles called medullary rays (see Fig. 16.5), progressively merging in the medulla to form the large ducts of Bellini, which open at the tips of the renal papillae to discharge urine into the pelvicalyceal system. The collecting tubules and ducts are not normally permeable to water. However, in the presence of antidiuretic hormone (ADH) secreted by the posterior pituitary, the collecting tubules and ducts become permeable to water. Thus the high osmotic pressure generated by the counter-current multiplier system into the interstitial tissues of the medulla removes water that is returned to the general circulation via the vasa recta. The loops of Henle and ADH thus provide a mechanism for the production of urine that is hypertonic with respect to plasma.
Renal vasculature
In most cases, each kidney is supplied by a single renal artery which divides in the hilum into two main branches; in some individuals, however, there are two or even more renal arteries that derive directly from the aorta. Each of these gives rise to several interlobar arteries which ascend between the pyramids to the corticomedullary junction. Here they branch to form the arcuate arteries, which run in an arc-like course parallel to the capsule of the kidney. The arcuate arteries give rise to numerous interlobular (cortical radial) arteries that radiate towards the capsule, branching to form the afferent arterioles of the glomeruli.
As previously described, the vasa recta form a continuation of the efferent arterioles of juxtamedullary glomeruli and form the microcirculation of the renal medulla. The efferent arterioles of the rest of the cortex divide to form the plexus of capillaries that surround the tubules of the renal cortex. The cortical and medullary capillaries drain via cortical radial (interlobular) veins to arcuate veins at the cortico-medullary junction and thence to the renal vein.
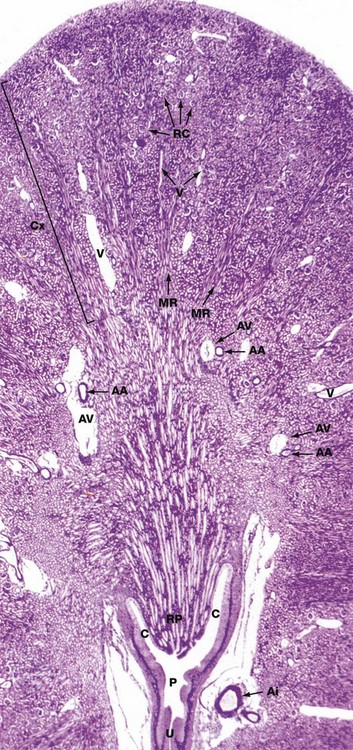
FIG. 16.5 Kidney, monkey
Jones methenamine silver H&E (LP)
The basic geography of the kidney can be seen in this unilobar kidney that has been sectioned through the axis of the medullary pyramid. Note the cup-shaped calyx C surrounding the renal papilla RP. The calyces fuse to form the pelvis P that in turn leads to the ureter U.
In the cortex Cx, numerous renal corpuscles RC (200 µm in diameter) are just visible at this magnification. The corpuscles tend to be arranged in parallel rows at right angles to the capsule, separated by interlobular arteries from which they derive their blood supply. Interlobular arteries are too narrow to be identified at this magnification, but a number of their accompanying thin-walled interlobular veins V are easily seen.
Most of the cortical parenchyma surrounding the renal corpuscles consists of proximal and distal convoluted tubules. From the cortex, medullary rays MR course towards the medulla; they consist of collecting tubules and ducts draining nephrons located high in the cortex. The collecting ducts merge in the medulla to form the larger ducts of Bellini that converge towards the tip of the renal papilla. Although not visible at this magnification, long loops of Henle dip into the medulla between and parallel with the collecting ducts. The long, straight vasa recta also dip down into the medulla alongside the loops of Henle; these vessels, too small to be seen at this magnification, absorb water from the loops of Henle and collecting ducts.
The corticomedullary junction is marked by several arcuate arteries AA and their associated thin-walled arcuate veins AV. Note a large interlobar branch of the renal artery Ai in the hilar supporting tissue.
The Renal Cortex
The renal cortex is easily identified even at low magnification by the presence of renal corpuscles, which are absent in the renal medulla. However, the bulk of the cortex is occupied by the proximal and distal convoluted tubules. The arcuate arteries and veins help to demarcate the cortex from the medulla.
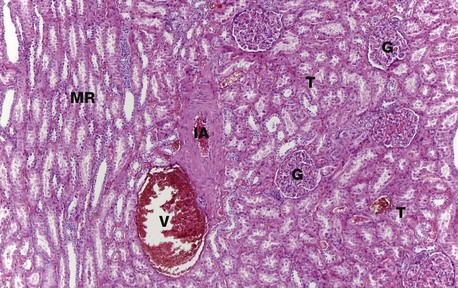
FIG. 16.6 Renal cortex
H&E (MP)
At higher magnification, the renal corpuscles are dense rounded structures, the glomeruli G, surrounded by narrow Bowman's spaces, normally filled with plasma ultrafiltrate and only just visible at this magnification The tubules T fill the bulk of the parenchyma between the corpuscles. The cortex consists mainly of proximal convoluted tubules lined by more eosinophilic epithelial cells, with smaller numbers of distal convoluted tubules and collecting tubules. At the left side of the micrograph, part of a medullary ray MR composed of collecting tubules is easily identified. An interlobular artery IA and vein V are also easily identified.
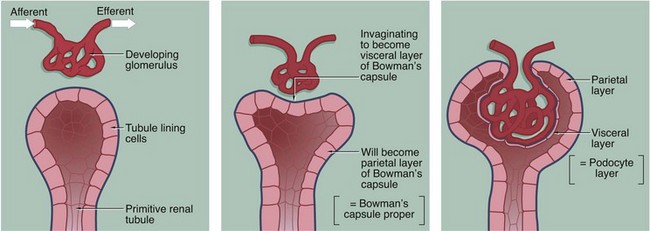
FIG. 16.7 Development of the renal corpuscle
The three-dimensional structure of the renal corpuscle can be clarified by studying its development. The renal tubules develop from the embryological metanephros as blind-ended tubes consisting of a single layer of cuboidal epithelium. The ends of the tubules dilate and become invaginated by a tiny mass of mesoderm tissue that differentiates to form the glomerulus. The layer of invaginated epithelium flattens and differentiates into podocytes that become closely applied to the outer surfaces of glomerular capillaries (the visceral layer of Bowman's capsule). Most of the intervening tissue disappears so that the basement membranes of glomerular endothelial cells and podocytes effectively fuse, forming the glomerular basement membrane. A small amount of tissue remains to support the capillary loops and differentiates to form the mesangium. Where the mesangium stretches between the capillary loops, its urinary surface is invested by podocyte cytoplasm with underlying basement membrane.
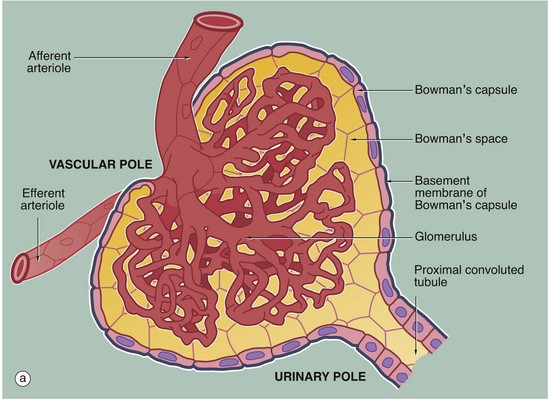
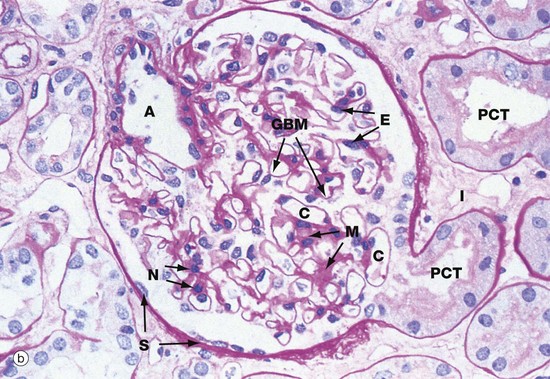
FIG. 16.8 Renal corpuscle
(a) Schematic diagram (b) PAS (HP)
The main structural features of the renal corpuscle are demonstrated in diagram (a). The relatively wide-diameter afferent arteriole enters Bowman's capsule at the vascular pole of the renal corpuscle and then branches to form an anastomosing network of glomerular capillaries, each major branch giving rise to a lobule. The glomerulus is thus suspended in Bowman's space from the vascular pole. The spaces between the capillary loops in each glomerular lobule are filled by mesangium which contains mesangial cells (not shown).
The efferent vessel draining the glomerulus is unusual in that it has the structure of an arteriole and is thus called the efferent arteriole (not venule). The efferent arteriole is of smaller diameter than the afferent arteriole, and a pressure gradient is thus maintained that drives the filtration of plasma into Bowman's space.
The layer of podocytes investing the glomerular capillaries (visceral epithelial cells) is not shown in this diagram. At the vascular pole, the podocyte layer is reflected to become continuous with the parietal epithelial cells of Bowman's capsule, which in turn becomes continuous with the first part of the renal tubule, the proximal convoluted tubule.
The renal corpuscle in micrograph (b) has been sectioned through the vascular pole and shows the afferent arteriole A entering the glomerulus. The efferent arteriole is not seen in this plane of section. At the urinary pole, the start of the proximal convoluted tubule PCT is seen. Other proximal convoluted tubules can be seen cut in various planes of section embedded in the renal interstitium I. Glomerular capillaries C are cut in transverse, longitudinal and oblique sections. The numerous nuclei in the glomerulus are those of capillary endothelial cells, mesangial cells and podocytes.
The PAS stain picks out the glomerular basement membrane GBM and the mesangium M, which consists of basement membrane–like material. Mesangial cells are found embedded within the mesangium, but only their nuclei N can be discerned at this magnification. The capillary lumina are lined by endothelial cells, again only identifiable by their nuclei E.
Note the flattened nuclei of the parietal epithelial cells S lining Bowman's capsule. This squamous epithelium is continuous with the epithelium of the proximal convoluted tubule and undergoes an abrupt transition to cuboidal form at the urinary pole. The basement membrane of Bowman's capsule is a thick basement membrane which is highlighted by the PAS stain; it is most likely synthesised by the overlying epithelial cells. Bowman's capsule is a permeability barrier preventing escape of the plasma ultrafiltrate into the interstitium. The epithelial cells are connected by tight junctions and also contribute to the permeability barrier.
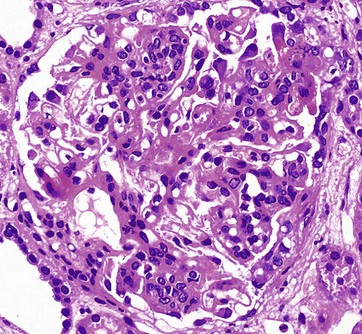
FIG. 16.9 Glomerulonephritis
H&E (HP)
This glomerulus is from a patient with glomerulonephritis. Compare this glomerulus with the normal one in Fig. 16.8. Note how the glomerulus seems full of cells and is virtually solid. The glomerular capillary loops are partially obstructed by a mixture of activated endothelial cells and mesangial cells. Less obvious with this staining method is the thickening of the glomerular basement membrane. This patient has the autoimmune disease systemic lupus erythematosus (SLE), and the histological changes in the glomerulus result from the deposition of immune complexes and the response of the intrinsic glomerular cells to these immune complexes. The immune complexes consist of antibodies and antigen, such as double-stranded DNA, a normal body component. The clinical symptoms and signs of lupus nephritis include haematuria, proteinuria including nephrotic syndrome, hypertension and, in some cases, eventual chronic renal failure.
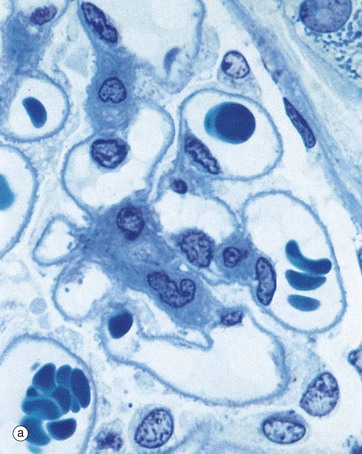
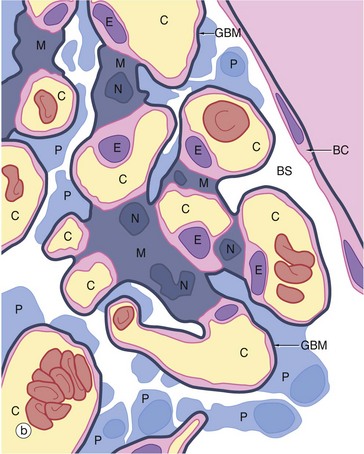
FIG. 16.10 Glomerulus
(a) Thin epoxy resin section, toluidine blue (HP) (b) Schematic diagram
Using resin-embedding techniques it is possible to cut thin sections (approximately 0.5 to 1.0 µm thick) which permit much greater resolution at high magnification.
In this preparation, the glomerular capillaries C, some of which contain erythrocytes, are defined by the prominent glomerular basement membranes GBM. Occasional capillary endothelial cell nuclei E are seen bulging into the capillary lumina. The mesangium M consists of material similar to basement membrane and contains mesangial cells, identifiable by their nuclei N. Mesangial cells, which have features resembling smooth muscle cells, are contractile and are thus able to modify the diameter of the glomerular capillaries in response to vasoactive substances, some of which they themselves produce. Thus mesangial cells have an important role in the control of capillary flow in the glomerulus. They also secrete the mesangial matrix and have a phagocytic function. The mesangium is separated from the capillary lumen only by a thin layer of fenestrated endothelial cell cytoplasm, the basement membrane of which merges with the mesangial matrix. Thus particulate matter from blood may pass into the mesangium where it can be phagocytosed and degraded by mesangial cells. The podocytes and their basement membrane invest the outer surface of the mesangium.
Podocytes P also invest the capillary loops exposed to Bowman's space BS. The podocytes have extensive branching pale-stained cytoplasm and large, round pale-stained nuclei. Note the nuclei of two squamous cells of Bowman's capsule BC.
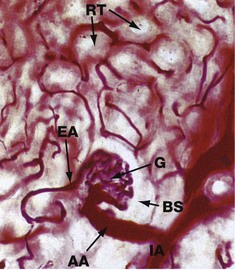
FIG. 16.11 Blood supply of the glomerulus
Carmine-gelatine perfused (MP)
This section is from a kidney that has been perfused with a red dye in order to demonstrate the renal blood supply; the nephrons remain unstained.
An interlobular artery IA can be seen branching to form the afferent arteriole AA of a glomerulus G. The efferent arteriole EA leaving the glomerulus is of much smaller diameter than the afferent arteriole, an arrangement which maintains pressure within glomerular capillaries necessary for blood plasma to be filtered into Bowman's space BS. Blood pressure within the glomerulus is controlled by variation of the diameter of the afferent and efferent arterioles, a function shared by podocytes and mesangial cells.
In the superficial and midcortex as shown here, efferent arterioles give rise to a network of capillaries, the peritubular capillaries, which surround the renal tubules RT; towards the medulla, efferent arterioles give rise to the vasa recta. Molecules reabsorbed from glomerular filtrate are returned to the general circulation via this capillary network which drains into the renal venous system.
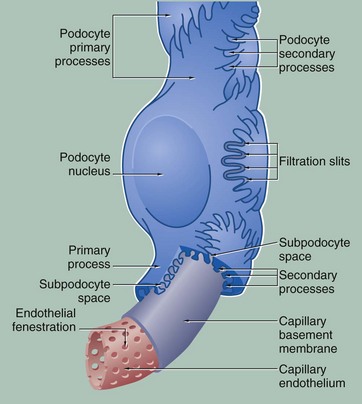
FIG. 16.12 The glomerular filter
During filtration of plasma from glomerular capillaries into the renal tubule, the filtrate passes through at least four layers of the glomerular filtration barrier (GFB): capillary endothelium, glomerular basement membrane, the podocyte foot processes with slit diaphragms and the subpodocyte space. All four contribute to the filtration process.
The capillary endothelium contains numerous large round fenestrations (60-100 nm in diameter) which occupy 20% to 50% of the endothelial surface area. It was previously thought that the fenestrations do not exhibit diaphragms as in fenestrated capillaries elsewhere in the body (see Fig. 8.16). The endothelial cells, including their fenestrations, are covered on the capillary side by a thick glycocalyx (220-400 nm) composed of glycoproteins, glycosaminoglycans and sialoglycoproteins. This glycocalyx contributes to the filtration barrier.
The glomerular basement membrane (approximately 350 nm in adults) is much thicker than other basement membranes and appears to be produced by both capillary endothelial cells and podocytes. As with basement membranes elsewhere (see Ch. 4), it consists of a feltwork of type IV collagen, structural glycoproteins (fibronectin and laminin) and proteoglycans rich in heparan sulphate, the interstices of this highly cross-linked structure being occupied by water molecules. By electron microscopy, the glomerular basement membrane consists of three layers, a dense central layer, the lamina densa with a thinner electron-lucent layer on either side of it, the lamina rara interna under the endothelium and the lamina rara externa supporting the podocytes. Both laminae rarae are negatively charged.
The podocytes have long cytoplasmic extensions called primary processes that embrace the capillaries, giving rise to short secondary foot processes (pedicels), which interdigitate with those of other primary processes. The secondary foot processes are directly applied to the lamina rara externa and bound to it by fine filaments. The gaps between adjacent secondary foot processes, known as filtration slits, are of uniform width (40 nm) and are bridged by slit diaphragms. The slit diaphragm is composed of a single layer of the transmembrane protein, nephrin, whose extracellular domains from adjacent foot processes link together rather in the manner of a zip. A glycocalyx rich in negatively charged podocalyxin covers the urinary surface of the podocytes, including the slit diaphragms. The intracellular component of nephrin is bound to the actin cytoskeleton of the podocyte and it has been suggested that the slit diaphragm is in fact a modified tight junction.
The subpodocyte space (SPS), the space between the podocyte foot processes and the podocyte cell bodies, has been recently shown to be a restricted space that comprises a fourth component of the GFB. The SPS covers approximately 60% of the glomerular capillary surface. Ultrafiltrate in this confined space can only leave it via the subpodocyte space exit pore, which leads into the interpodocyte space between the podocyte cell bodies and finally into Bowman's space proper. The SPS has higher hydrostatic pressure than Bowman's space, and it is thought that the podocytes, by altering the size of the SPS and the exit pore, are able to regulate filtration though the GBM.
As mentioned above, all layers contribute to the selective filtration barrier. Clinical evidence demonstrates that free haemoglobin (MW 65 000) and smaller molecules pass freely through the glomerular filter, whereas albumin (MW 68 000) and larger molecules are retained. For macromolecules, three factors determine permeability: charge, size and configuration. Negatively charged (anionic) molecules are blocked by the negatively charged endothelial cell coat and laminae rarae of the basement membrane, while the meshwork of the lamina densa of the basement membrane discriminates on the basis of molecular size and configuration. The slit diaphragm restricts the passage of any large molecules, but its main role is in controlling water flow, which is also held back by the colloidal osmotic pressure of retained albumin and other large molecules.
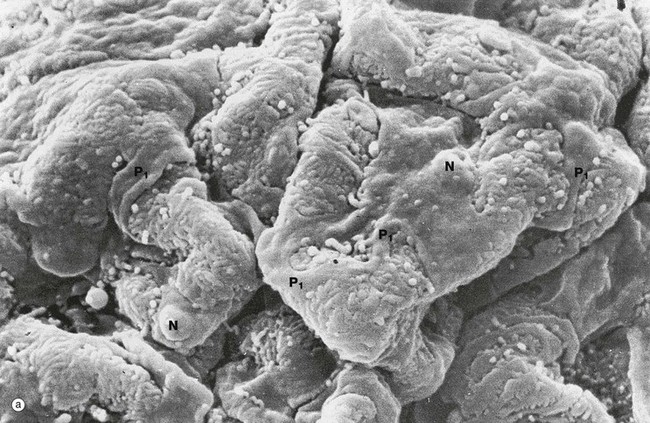
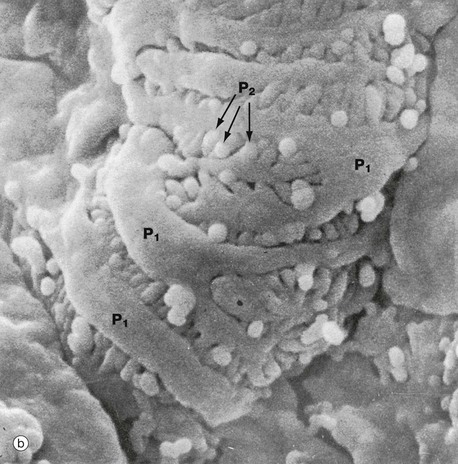
FIG. 16.13 Glomerulus
(a) SEM ×1500 (b) SEM ×6000
Scanning electron microscopy readily demonstrates the three-dimensional relationships of podocytes and their processes that extend like octopus tentacles over the whole surface of the glomerulus.
Micrograph (a) shows part of a glomerular capillary tuft. The capillaries are enveloped by podocytes which have large flattened cell bodies and bulging nuclei N. Each podocyte has several long primary processes P1 that embrace one or more capillaries. Each primary process has numerous secondary foot processes (pedicels) which rest on the lamina rara externa of the glomerular basement membrane.
At higher magnification in micrograph (b), the secondary foot processes P2 can be seen as extensions of the large primary processes P1. The secondary foot processes interdigitate with those of other primary processes, separated by filtration slits of uniform width.
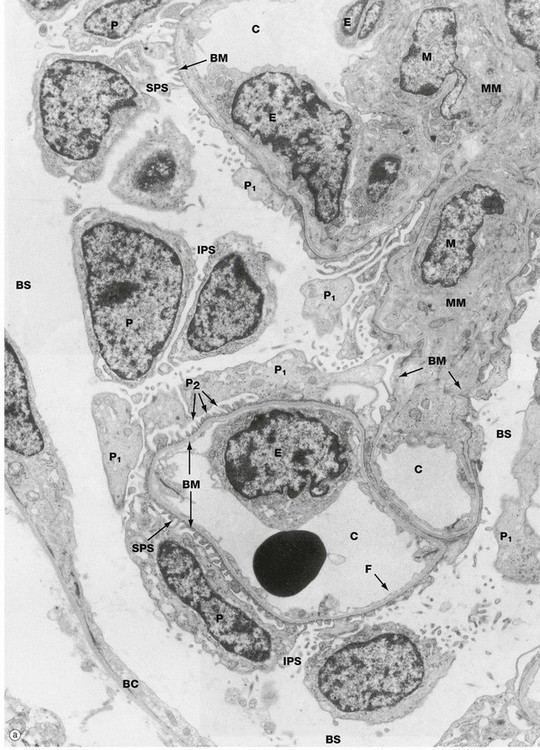
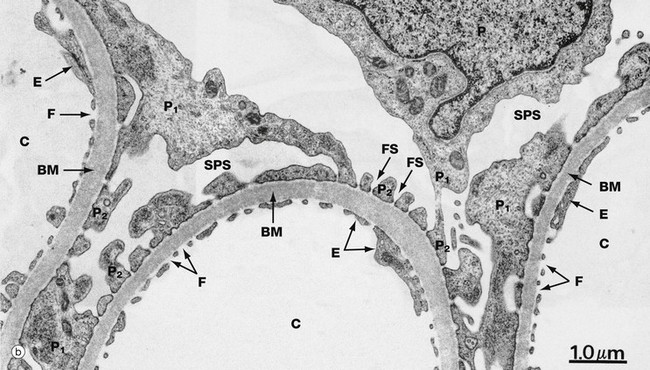
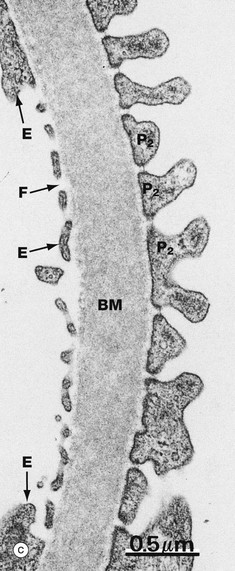
FIG. 16.14 Glomerulus (illustration (a) opposite)
(a) EM ×4800 (b) EM ×14 000 (c) EM ×30 000
When examining both light and electron microscope specimens of glomeruli, the podocytes, endothelial cells and mesangium are identified most easily by tracing out the glomerular basement membrane. Micrograph (a) shows several capillary loops C lined by a thin layer of fenestrated endothelial cytoplasm. The endothelial cell nuclei E can be seen bulging into the capillary lumina. The capillary endothelial fenestrations F are better seen at higher magnification in micrographs (b) and (c). The nuclei of several podocytes P can be seen, their primary processes P1 giving rise to numerous secondary foot processes P2 that rest on the glomerular basement membrane BM. At right midfield a branched mesangial stalk comprising mesangial cells M and mesangial matrix MM provides support for the capillary loops. The mesangium is separated from the capillary lumen only by the cytoplasm of the endothelial cells, while the podocytes and their basement membrane continue around the mesangial stalk, separating it from Bowman's space. Part of Bowman's capsule BC is seen at the periphery, consisting of a squamous epithelial cell and underlying basement membrane. The subpodocyte space SPS and interpodocyte space IPS are easily identified, although the subpodocyte space exit pore is not seen. At the periphery of the glomerulus, Bowman's space BS is delineated by the podocyte cell bodies on one side and the parietal epithelial cells on the other.
Micrograph (b) shows three glomerular capillaries C lined by attenuated endothelial cytoplasm E with wide fenestrations F. A podocyte P extends several primary processes P1 onto the capillaries, these in turn giving rise to multiple secondary foot processes P2 separated by filtration slits FS. The subpodocyte space SPS can again be identified. The glomerular basement membrane BM separates the podocytes and capillary endothelium. The thickness of the basement membrane appears variable, but this is due to the slightly oblique plane of section; the basement membranes are in fact of uniform width.
At even higher magnification in micrograph (c), three of the components of the glomerular filter are seen. The fenestrated capillary endothelium E is closely applied to the luminal surface of the glomerular basement membrane BM; on the opposite side are podocyte secondary foot processes P2, separated by filtration slits of uniform width and bridged by the slit diaphragms. Part of the subpodocyte SPS space is seen, but the podocyte cell body which delimits the subpodocyte space is not apparent The wide central lamina densa of the glomerular basement membrane can be seen bordered on each side by a narrow lamina rara. The glycocalyces of the endothelial cells and podocytes are not apparent in these micrographs; special fixation and processing techniques are required to demonstrate them.
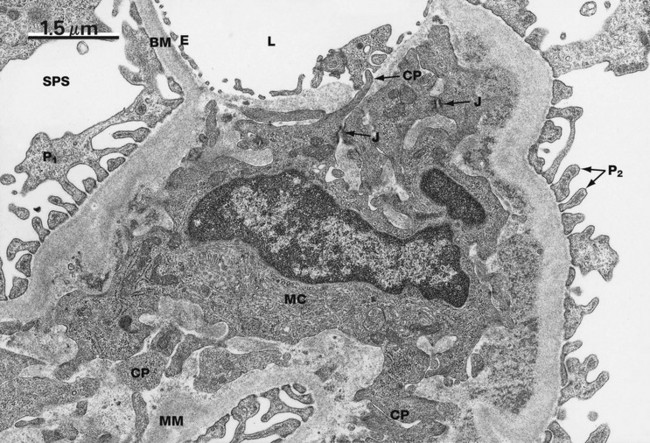
FIG. 16.15 The mesangium, rat
EM ×14 000
This electron micrograph shows an area of mesangium along with part of a glomerular capillary lumen L. The capillary lumen is lined by the delicate fenestrated endothelium E. Podocyte primary processes P1 and secondary foot processes P2 are easily seen, as well as subpodocyte space SPS. The mesangium is composed of fibrillary basement membrane-like material MM within which is embedded a mesangial cell MC. Mesangial cells have long cytoplasmic processes CP that ramify through the mesangium and form cell junctions with the processes of other mesangial cells. Several of these cell junctions J can be seen in this field. Thus the mesangial cells form a network supporting the glomerular capillaries.
One function of mesangial cells is the secretion of mesangial matrix; they also secrete vasoactive factors and cytokines and phagocytose particles such as immune complexes from the blood. The majority of mesangial cells have a well-developed filamentous cytoskeleton rich in actin and are thought to be specialised pericytes, but a small proportion of these cells display phagocytic characteristics such as the expression of surface Fc and C3 receptors. Mesangial cells with this phagocytic phenotype are able to phagocytose and destroy large particles such as immune complexes taken up from the blood.
This micrograph demonstrates the close relationship between the mesangial and endothelial cells. These are not separated from each other by a basement membrane and in fact lie within the same basement membrane–bound compartment. Conceptually it might be helpful to consider the mesangium as a modified segment of the glomerular capillary wall. Also in this electron micrograph the morphological difference between the glomerular basement membrane BM and the mesangial matrix MM is apparent; this reflects the differences in chemical composition between the two. The main structural components of mesangial matrix are type IV collagen, laminin, fibronectin and proteoglycans such as decorin and biglycan.
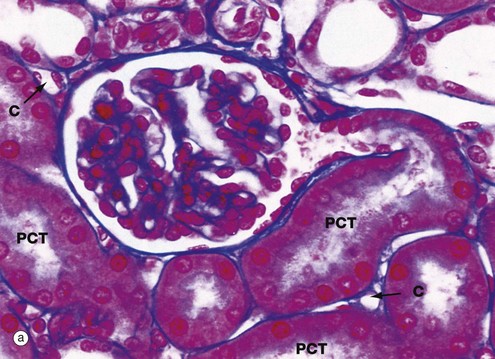
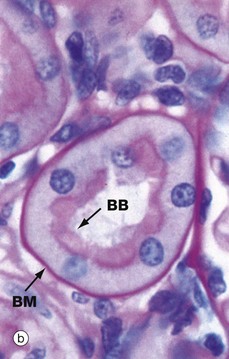
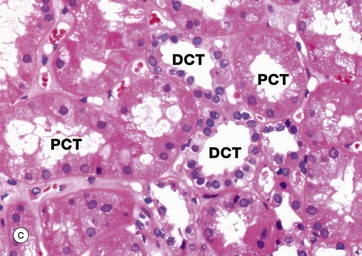
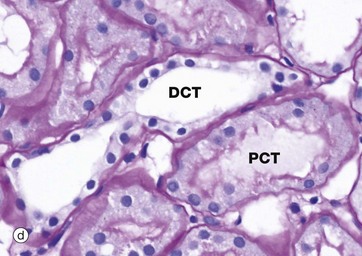
FIG. 16.16 Proximal and distal convoluted tubules
(a) PCT, Azan (HP) (b) PCT, PAS (HP) (c) DCT, H&E (HP) (d) DCT, PAS (HP)
These micrographs compare the appearances of the proximal and distal convoluted tubules. The intervening loop of Henle is discussed in Fig. 16.19. The proximal convoluted tubule (PCT) is a coiled tube measuring approximately 14 mm in length and random sections of PCT thus occupy most of the renal cortex. Approximately 65% of the glomerular filtrate is reabsorbed from the PCT, a function reflected in the structure of the epithelial lining.
Micrograph (a) shows a proximal convoluted tubule PCT arising from a renal corpuscle; convolutions of the PCT are also seen in longitudinal, oblique and transverse sections. The simple cuboidal epithelium has a prominent blue-stained brush border of tall microvilli, increasing the surface area of the plasma membrane some 20-fold. The cytoplasm of PCT epithelial cells stains intensely due to a high content of organelles, principally mitochondria. Basement membranes stain blue by this technique, thus highlighting the tubular and glomerular basement membranes and that of Bowman's capsule.
The PAS staining method has been used in micrograph (b) to demonstrate the prominent brush border BB projecting into the lumen of the PCT. The brush border is PAS-positive, since the surfaces of the microvilli are coated with a prominent glycocalyx (see Fig. 1.2). Like those elsewhere, the basement membrane BM supporting the tubular epithelium is strongly PAS-positive. In both micrographs, note that the epithelial cells of the PCT have round nuclei with prominent nucleoli.
A rich network of peritubular capillaries C arising from the efferent arteriole of the glomerulus (see Fig. 16.11) surrounds the proximal tubules and returns molecules reabsorbed from the glomerular filtrate back into the general circulation.
The distal tubule is a continuation of the thick ascending limb of the loop of Henle after its return to the cortex and forms the third segment of the renal tubule. Distal tubules are thus found within the cortex among the proximal convoluted tubules. The first part of the distal tubule forms the macula densa (see Fig. 16.18) while the remainder makes up the distal convoluted tubule (DCT). In the DCT, sodium ions are reabsorbed from the tubular fluid, with one hydrogen or potassium ion being secreted in exchange. This adjustment of acid-base balance is controlled by the hormone aldosterone secreted by the adrenal cortex (see Fig. 16.18).
As seen in micrograph (c), distal convoluted tubules DCT may be differentiated from proximal convoluted tubules PCT by the absence of a brush border, a larger more clearly defined lumen, more nuclei per cross-section (since DCT cells are smaller than PCT cells) and paler cytoplasm (due to fewer organelles). In addition, sections of DCT are less numerous than sections of PCT, since the DCT is much shorter than the PCT. In micrograph (d) the prominent brush border of the proximal convoluted tubule PCT is contrasted with the lack of brush border in the distal convoluted tubule DCT.
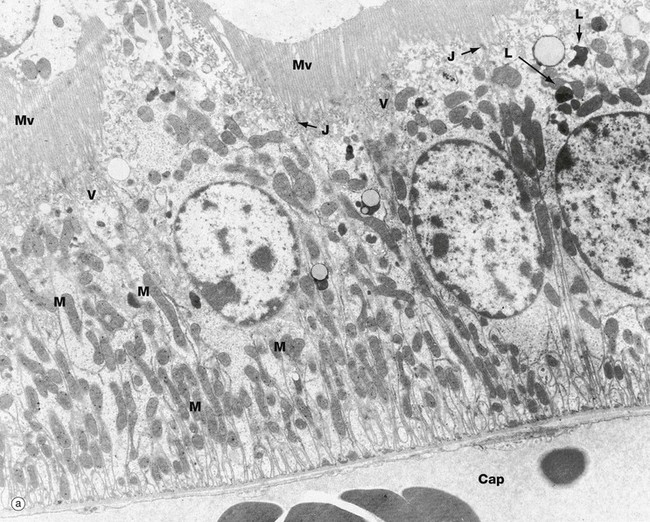
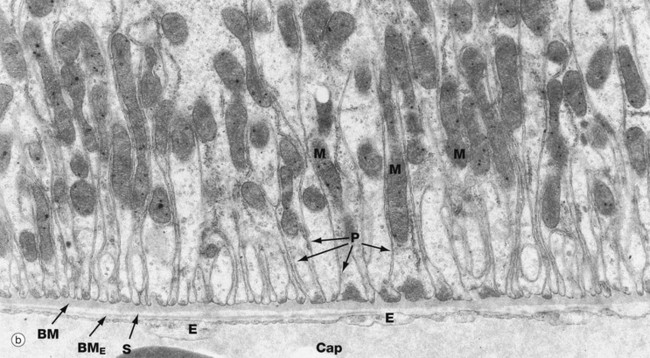
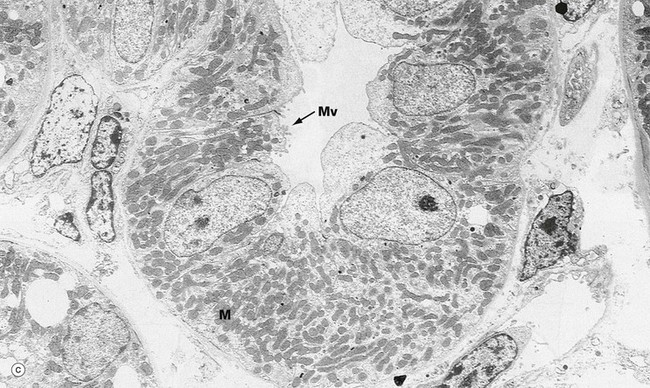
FIG. 16.17 Proximal and distal convoluted tubules (illustrations (a) and (b) opposite)
(a) PCT, EM ×10 000 (b) PCT, EM ×19 000 (c) DCT, EM ×5000
These electron micrographs compare the ultrastructure of the proximal and distal convoluted tubules. Micrograph (a) of the proximal tubule reveals profuse tall microvilli Mv constituting the brush border seen with light microscopy. The cytoplasm immediately beneath the brush border contains many pinocytotic vesicles V (that are just visible at this magnification) and lysosomes L, both of which are involved in reabsorption and degradation of small amounts of protein that have leaked through the glomerular filter. Reabsorbed solutes are transported into surrounding peritubular capillaries Cap, with attenuated endothelium E resting on a very thin basement membrane BME; note the narrow intervening supporting tissue layer S in micrograph (b).
The epithelial cells of the PCT form multiple lateral processes P (micrograph b) which interdigitate with each other to form a complex lateral intercellular space, with a plasma membrane area equivalent to the luminal plasma membrane. The lateral intercellular space is separated from the lumen of the PCT by a ring of junctional complexes J near the luminal surface. The mitochondria M in these processes are elongated and arranged at right angles to the basement membrane BM. These mitochondria supply ATP for the active transport of Na+ by the Na+-K+ ATPase (sodium pump) located in the basolateral plasma membrane. Thus active transport of Na+ occurs across the plasma membrane into the lateral intercellular space. This active transport of Na+ out of the cell is accompanied by facilitated transport into the cells of Na+, glucose and amino acids by means of transport proteins found in the membrane of the brush border. Almost 100% of the filtered glucose and amino acids is reabsorbed by the PCT.
The distal convoluted tubule (c) has many ultrastructural features in common with the proximal convoluted tubule, in particular the lateral cell interdigitations and large numbers of mitochondria M. The basolateral plasma membrane contains the Na+-K+ ATPase which drives active transport of sodium ions. The most striking difference is that the DCT lacks a brush border, having only a few irregular microvilli Mv at the luminal surface. The DCT cells have less cytoplasm than those of the PCT, although the nucleus is of about the same size and consequently occupies much more of the cell. The nuclei of the DCT cells lie close to the luminal surface and tend to bulge into the lumen; the overlying cytoplasm is devoid of mitochondria but contains large numbers of tiny pinocytotic vesicles (not seen at this magnification).
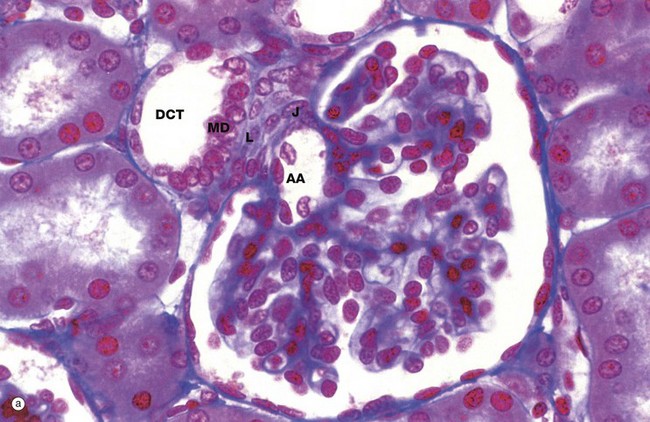
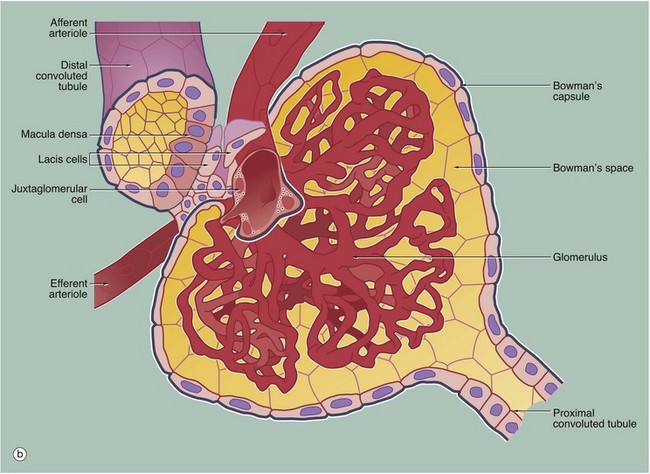
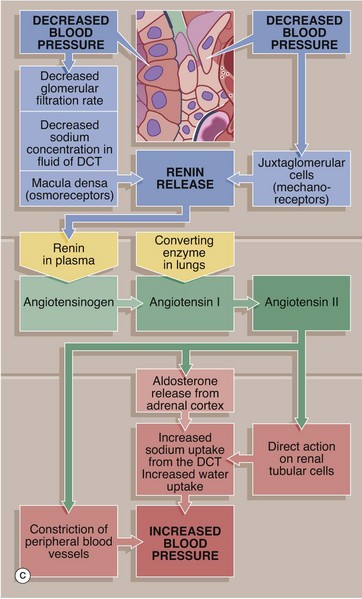
FIG. 16.18 Juxtaglomerular apparatus (illustrations (a) and (b) opposite)
(a) Azan (HP) (b) Diagram (c) Control of blood pressure
The juxtaglomerular apparatus (JGA) is a specialisation of the glomerular afferent arteriole AA and the distal convoluted tubule DCT of the same nephron and is involved in the regulation of systemic blood pressure via the renin-angiotensin-aldosterone system (RAAS). The juxtaglomerular apparatus is made up of three components: the macula densa of the DCT, renin-secreting juxtaglomerular cells of the afferent arteriole and extraglomerular mesangial cells.
• Macula densa. On returning to the cortex from the renal medulla, the ascending thick limb of the loop of Henle becomes the first part of the distal tubule and comes to lie in the angle between the afferent and efferent arterioles at the vascular pole of the glomerulus. The macula densa MD is an area of closely packed, specialised DCT epithelial cells where the DCT abuts the vascular pole of the glomerulus. Compared with other DCT lining cells, the cells of the macula densa are taller and have larger more prominent nuclei situated towards the luminal surface. Mitochondria are scattered throughout the cytoplasm, and Na+ pump activity is absent. The basement membrane between the macula and underlying cells is extremely thin. The cells of the macula densa are sensitive to the concentration of sodium ions in the fluid within the DCT; a decrease in systemic blood pressure results in decreased production of glomerular filtrate and hence decreased concentration of sodium ions in the distal tubular fluid.
• Juxtaglomerular cells. Juxtaglomerular cells J are modified smooth muscle cells of the wall of the afferent arteriole, forming a cluster around it just before it enters the glomerulus. Juxtaglomerular cell cytoplasm contains immature and mature membrane-bound granules of the enzyme renin.
• Extraglomerular mesangial cells. Also called Goormaghtigh cells or lacis cells L, these cells form a conical mass, the apex of which is continuous with the mesangium of the glomerulus; laterally it is bounded by the afferent and efferent arterioles, and its base abuts the macula densa. The lacis cells are flat and elongated, with extensive fine cytoplasmic processes extending from their ends and surrounded by a network (‘lacis’) of mesangial material. These cells participate in the tubuloglomerular feedback mechanism by which changes in Na+ concentration at the macula densa give rise to signals that directly control glomerular blood flow. The extraglomerular mesangial cells are thought to be responsible for transmission of a signal arising in the macula densa to the intraglomerular mesangial cells, which then contract or relax to make the capillary loops narrower or wider.
Role of the JGA in the control of blood pressure
The juxtaglomerular apparatus is believed to act as both a baroreceptor and a chemoreceptor, controlling systemic blood pressure by the secretion of renin by the juxtaglomerular cells. The juxtaglomerular cells are suitably placed to monitor systemic blood pressure, with a fall in blood pressure resulting in renin secretion. Reduction in blood pressure results in reduced glomerular filtration and consequently a lower concentration of sodium ions in the DCT.
Acting as chemoreceptors, the cells of the macula densa in some way then promote renin secretion. Renin diffuses into the bloodstream, catalysing the conversion of angiotensinogen, an α2-globulin synthesised by the liver, into the decapeptide angiotensin I. In the lungs, angiotensin converting enzyme (ACE) cleaves two amino acids from angiotensin I to form angiotensin II, which is a potent vasoconstrictor.
Angiotensin II raises blood pressure in three ways: constriction of peripheral blood vessels, release of aldosterone from the adrenal cortex and via a direct effect on the renal tubules, where it promotes the reabsorption of sodium ions (and therefore water) from the DCT, thus expanding the plasma volume and increasing blood pressure. As mentioned above, the tubuloglomerular feedback mechanism is also thought to operate at a local level to control glomerular blood flow and therefore indirectly influencing systemic blood pressure.
The Renal Medulla
The renal medulla consists of closely packed tubules of two types: the loop of Henle and the collecting tubules and ducts, as well as the vasa recta. The loop of Henle is a continuation of the proximal convoluted tubule. It dips down into the medulla, where it loops back on itself and returns to the cortex to its own renal corpuscle, becoming the first part of the distal convoluted tubule.
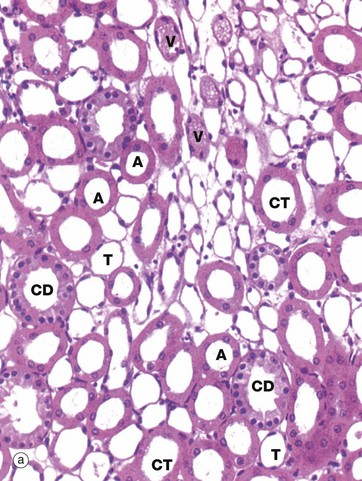
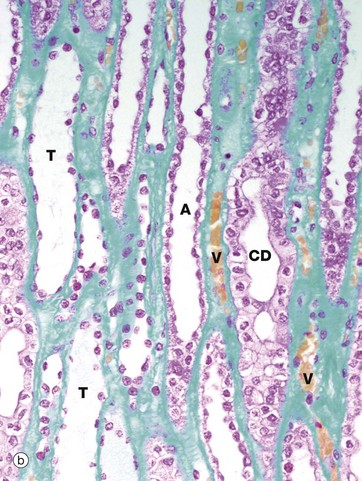
FIG. 16.19 Loop of Henle
(a) H&E, TS (HP) (b) EMSB, LS (HP)
The loop of Henle is made up of four parts:
The thick descending limb is the second, straight part of the proximal tubule that extends down into the outer medulla. There is an abrupt transition to the thin descending limb, which loops down into the medulla for a variable distance. The thin limbs of juxtamedullary nephrons extend down to the inner medulla before turning back on themselves, while those in the outer cortex only extend a short way into the medulla. After the hairpin bend, the tubule becomes the thin ascending limb for a short distance before abruptly changing into the thick ascending limb. Thus the thin descending limb is longer than the thin ascending limb.
The thin limbs T have a simple squamous epithelium and may be differentiated from the vasa recta V by the absence of erythrocytes and their regular rounded shape in transverse section. Erythrocytes, stained orange by this staining method, are easily seen in the vasa recta in micrograph (b). The thick ascending limbs A are lined by low cuboidal epithelium and are also round in cross-section. Neither thick nor thin limbs of the loop of Henle have a brush border. Collecting tubules CT have a similar epithelial lining to the ascending limbs but are wider and less regular in shape. The collecting ducts CD are easily recognised by their large diameter and pale stained columnar epithelial lining.
The function of the loop of Henle is to produce an increasing osmotic gradient from the cortex to the tip of the renal papilla by the counter-current multiplier mechanism (see Fig. 16.26). In brief, the parts of the loop of Henle with a thick (cuboidal) epithelium participate in active transport of various ions and molecules out of the lumen and into the interstitium. On the other hand, the thin limbs are lined by a flattened squamous epithelium with little capacity for active transport. The thin descending limb allows free diffusion of H2O but is fairly impermeable to NaCl, while the thin ascending limb is permeable to NaCl but not to H2O. The vasa recta take up water from the medullary interstitium and return it to the general circulation.
As the urine flows into the thick ascending limb, active transport of NaCl again occurs, and this correlates with the appearances of the epithelium. Here the cuboidal epithelium exhibits basolateral processes that interdigitate with each other, forming an extensive basolateral intercellular space in a similar manner to the PCT. This active transport process is fuelled by ATP produced by the many mitochondria found in these processes. The thick ascending limb is also impermeable to water. Tamm-Horsfall protein is a unique glycoprotein produced only by the epithelium of the thick ascending limb. Tamm-Horsfall protein has protective functions, including binding to certain types of Escherichia coli to prevent the bacteria adhering to the renal tubular epithelium and prevention of the formation of renal calculi.
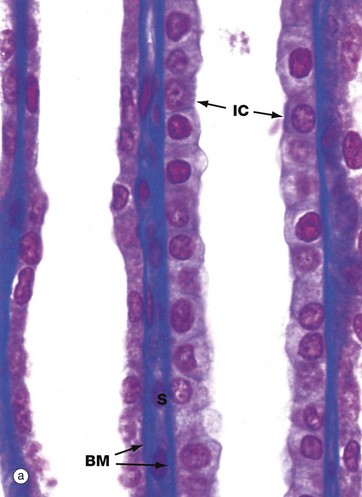
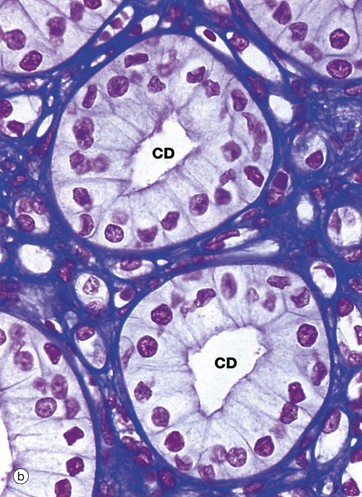
FIG. 16.20 Collecting tubules and ducts
(a) Azan, LS (HP) (b) Azan, TS (HP)
The collecting or connecting tubule joins the distal convoluted tubule to the collecting duct. Several collecting tubules merge to form each collecting duct. The collecting tubules and ducts descend in the medullary rays (see Fig. 16.5) towards the renal medulla where they progressively merge to form the large ducts of Bellini which drain urine from the tip of the renal papilla into the pelvicalyceal system.
The collecting tubules and ducts concentrate urine by passive reabsorption of water into the medullary interstitium following the osmotic gradient created by the counter-current multiplier system of the loops of Henle (see Fig. 16.26). The vasa recta return this water to the general circulation. The amount of water reabsorbed is controlled by antidiuretic hormone (ADH, vasopressin) secreted by the posterior pituitary in response to dehydration. ADH acts by increasing the permeability to water of the collecting tubule and ducts, resulting in retention of water by the body and the production of hypertonic urine. Conversely, ADH secretion is inhibited by water overload and an increased volume of hypotonic urine is thus produced. The collecting tubules and ducts are also the final site of H+, K+, Na+ and HCO3− secretion and/or reabsorption to achieve homeostasis; these functions are modified and controlled by the RAAS and local paracrine factors such as kallikrein.
The simple low columnar epithelium of the collecting ducts consists of two cell types, principal cells and intercalated cells. Principal cells have pale cytoplasm with scanty organelles and short microvilli. These cells have prominent infoldings of the basolateral plasma membrane but no lateral interdigitations. Principal cells actively reabsorb Na+ and secrete K+, as well as reabsorbing water. Intercalated cells have darker cytoplasm due to the content of multiple mitochondria, polyribosomes and membrane-bound vesicles. These cells secrete H+ and reabsorb bicarbonate and are thus important in acid-base homeostasis. The number of intercalated cells varies between different parts of the collecting duct and they are virtually absent in the inner medullary segment.
The collecting tubules are lined by a mixture of DCT cells, collecting tubule cells, principal cells and intercalated cells. Overall, the epithelium is cuboidal and becomes increasingly tall distally until it merges with the columnar epithelium of the collecting duct.
Micrograph (a) illustrates two collecting tubules in the renal cortex, the tubule on the left being more proximal and the tubule on the right more distal, as shown by the flatter cuboidal lining of the former. The majority of the lining cells are relatively poorly stained. The different cell types cannot be differentiated by light microscopy, except for a small number of dark intercalated cells IC with surface microvilli. Note the blue-stained tubular basement membranes BM and narrow intervening supporting tissue S, mainly occupied by capillaries.
Micrograph (b) is from the renal medulla and illustrates two collecting ducts CD surrounded by loops of Henle and vasa recta that cannot be readily distinguished from one another. In the medullary portion of the collecting ducts, principal cells are predominant and no intercalated cells can be seen in this section.
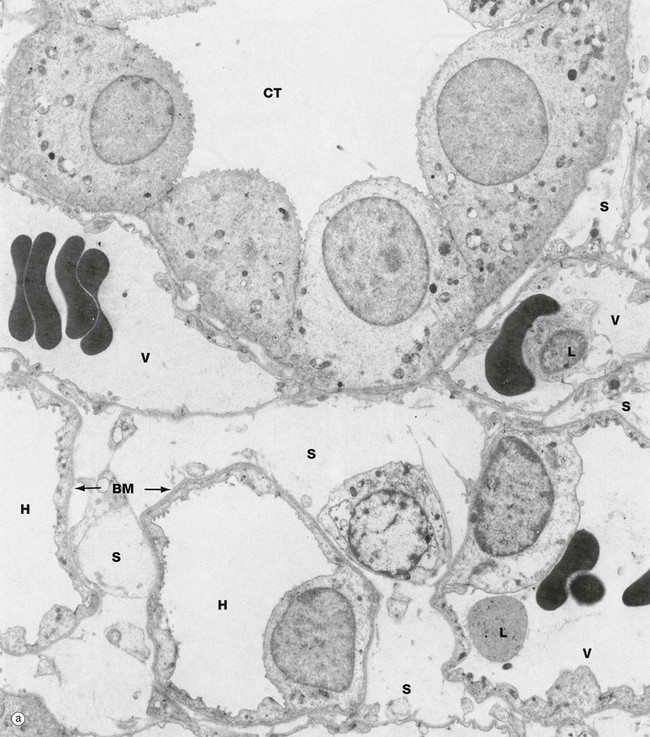
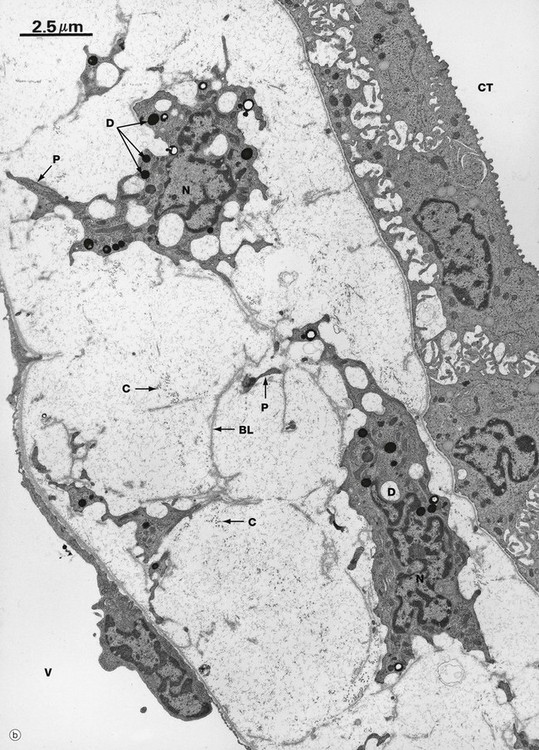
FIG. 16.21 Renal medulla, rat (illustration (b) opposite)
(a) EM ×4000 (b) EM ×8000
Micrograph (a), a transverse section of the outer medulla, illustrates the ultrastructural features of a collecting tubule CT, thin loops of Henle H and vasa recta V. Lying between the vasa recta and nephrons is the delicate interstitial supporting tissue S containing a little collagen along with renal interstitial medullary cells (RIMC).
The collecting tubule in this section is lined mainly by principal cells whose basal mitochondria, associated with infoldings of the plasma membrane, can just be identified at this power and are seen clearly in micrograph (b) in the collecting tubule CT in the right upper corner. The cells of the thin limbs of loops of Henle are similar to capillary endothelial cells in structure, most of the wall consisting of a thin irregular layer of cytoplasm with a few very short luminal microvilli and the nucleus bulging into the lumen. The epithelium is supported by a thin basement membrane BM. The vasa recta can only be readily distinguished from the thin limbs by their content of erythrocytes, occasional leucocytes L and precipitated plasma proteins.
The interstitium of the inner medulla in some species, including humans, contains unusual cells called renal interstitial medullary cells. These are illustrated in micrograph (b), a longitudinal section of the medulla, where the cell bodies of two such cells are identifiable by their nuclei N. These cells have plentiful lipid droplets D within the cytoplasm and long cytoplasmic processes P that form a network throughout the loose supporting tissue containing collagen fibrils C that fills the intervening space. Interestingly, there are also fragments of redundant basal lamina BL in the supporting tissue, implying that these RIMC may change their position over time. These cells are often arranged at right angles to the collecting tubules CT and vasa recta V. The function of these cells is not yet clear, but they may be involved in the production of prostaglandins and/or hormones that regulate blood pressure.
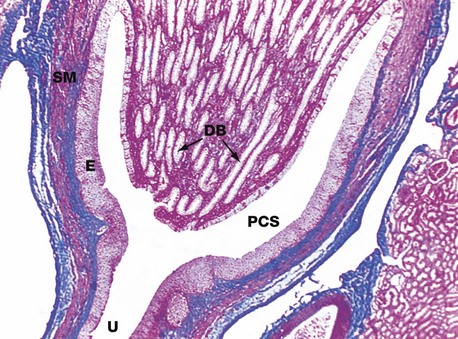
FIG. 16.22 Renal papilla, monkey
Azan (LP)
The renal papilla forms the apex of the medullary pyramid, where it projects into the pelvicalyceal system PCS. Ducts of Bellini DB, the largest of the collecting ducts, converge to drain urine through a number of holes (cribriform area) at the tip of the papilla. Between the ducts are the longest loops of Henle and vasa recta (not visible at this magnification). This papilla is a simple papilla, but at the poles of the human kidney, the papillae are often fused to form complex papillae.
The pelvicalyceal system represents the proximal end of the ureter U and, as such, is lined by typical urinary (transitional) epithelium E. The wall of the pelvis contains smooth muscle SM, continuous with that of the ureter.
The Lower Urinary Tract
The lower urinary tract includes the renal pelvis and calyces, the ureters, the urinary bladder and the urethra. The lower urinary tract is specialised for the storage and excretion of urine at a convenient time; no further modification of the urine is possible after it leaves the renal medulla.
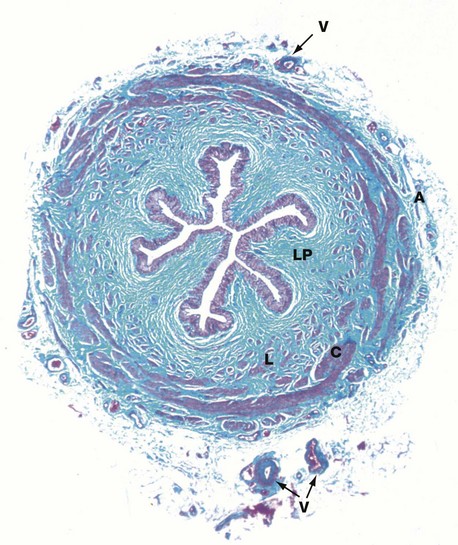
FIG. 16.23 Ureter
Masson trichrome (LP)
The ureters are muscular tubes that carry urine from the kidneys to the bladder. Urine is transported from the pelvicalyceal system as a bolus, propelled by peristaltic action of the ureteric wall. The wall of the ureter contains two layers of smooth muscle, arranged as an inner elongated spiral but traditionally known as the longitudinal layer L and an outer tight spiral traditionally described as the circular layer C. Another outer longitudinal layer is present in the lower third of the ureter. However, in reality the three layers are often difficult to distinguish from each other.
The lumen of the ureter is lined by transitional epithelium (urothelium) which is thrown up into folds in the relaxed state, allowing the ureter to dilate during the passage of a bolus of urine. Beneath the epithelium is a broad collagenous lamina propria LP, the collagen fibres of which are stained greenish-blue in this preparation. Surrounding the muscular wall is a loose collagenous adventitia A containing blood vessels V, lymphatics and nerves.
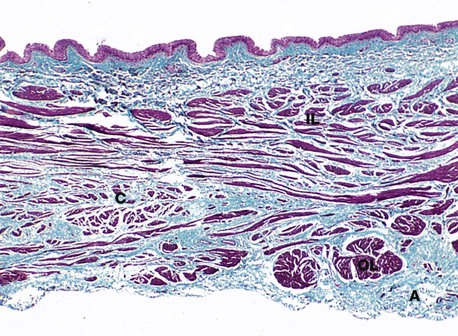
FIG. 16.24 Bladder
Masson trichrome (LP)
The urinary bladder serves as a urine store in which urine can be held until a convenient time and place for its excretion (micturition). The general structure of the bladder wall resembles that of the lower third of the ureters. The wall of the bladder consists of three loosely arranged layers of smooth muscle and elastic fibres that contract during micturition. Note the inner longitudinal IL, outer circular C and outermost longitudinal OL layers of smooth muscle; together the three layers are called the detrusor muscle. As in the ureter, the layers are often difficult to distinguish. The transitional epithelium lining the bladder is thrown into many folds in the relaxed state. A delicate, often incomplete muscularis mucosa (not identifiable at this magnification) separates the lamina propria from the submucosa in some but not all individuals. The outer adventitial coat A contains arteries, veins and lymphatics.
The urethra, the final conducting portion of the urinary tract, is discussed as part of the male reproductive tract in Ch. 18.
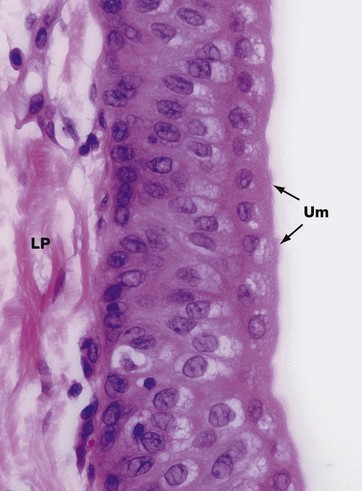
FIG. 16.25 Transitional epithelium
H&E (HP)
Transitional epithelium, also called urothelium, is found only within the conducting passages of the urinary system, for which it is especially adapted. The epithelium is stratified, comprising three to six layers of cells, the number of layers being greatest when the epithelium is least distended at the time of fixation.
The cells of the basal layer are compact and cuboidal in form, while those of the intermediate layers are more columnar, with their nuclei orientated at right angles to the basement membrane. The surface cells are called umbrella Um or dome cells and have unique features that allow them to maintain the impermeability of the epithelium to urine, even when at full stretch. This permeability barrier also prevents water from being drawn through the epithelium into hypertonic urine. The umbrella cells are large and ovoid with round nuclei and plentiful eosinophilic cytoplasm; some surface cells are binucleate (not illustrated). The surface outline has a characteristic scalloped appearance and the superficial cytoplasm is fuzzy, indistinct and more intensely stained than the rest of the cytoplasm.
Ultrastructural studies have revealed that much of the surface plasma membrane consists of thickened inflexible plaques, often called asymmetrical unit membrane, interspersed with narrow zones of normal membrane. These normal areas act as ‘hinges’, allowing sections of the membrane to fold inwards somewhat like a concertina, forming deep clefts and stacks of flattened plasma membrane segments, inappropriately called fusiform vesicles. This structure allows the umbrella cells to expand greatly and quickly when the bladder is distended and the epithelium is at full stretch. Plentiful junctional complexes between the cells maintain the cohesion of adjacent cells. These features of the urothelium allow it to store chemically toxic urine in considerable volumes for quite long periods of time without damage to the tissues.
Urinary epithelium rests on a basement membrane that is often too thin to be resolved by light microscopy. The loose lamina propria LP is seen underlying the epithelium.
Review
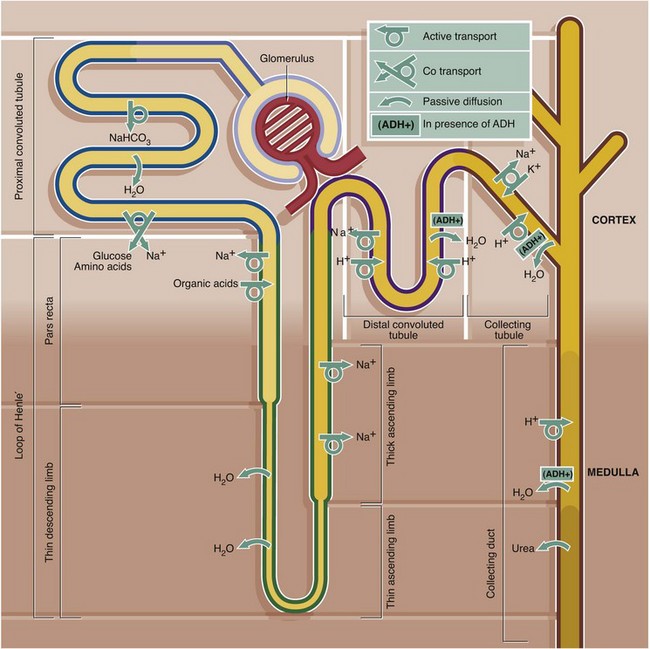
FIG. 16.26 Summary of major activities of different parts of the renal tubule
The function of the renal tubule is to transform an ultrafiltrate of plasma into a concentrated solution of waste products such as urea, creatinine, excess H+ and K+ and many other substances. At the same time, the tubule conserves essential water, Na+, bicarbonate, amino acids, glucose and low molecular weight proteins. This complex procedure is carried out by a variety of mechanisms in different segments of the tubule, including active transport, co-transport, passive diffusion, facilitated diffusion (see Ch. 1) and differential permeability of different parts of the tubule.
The ability of the tubule to produce concentrated urine is dependent on the high osmolarity of the renal medulla, which is created by the unique structure of the loops of Henle and vasa recta dipping down into the medulla. This is known as the counter-current multiplier mechanism. In the presence of ADH, which renders the collecting tubule and duct permeable to water, the high osmolarity of the interstitium of the renal medulla draws water passively out of the tubule and into the medulla where it is carried away by the vasa recta. The counter-current multiplier mechanism is set up by the ability of the thick ascending limb of the loop of Henle to pump large amounts of NaCl into the interstitium against a concentration gradient while remaining impermeable to water. The thin descending limb is permeable to water but not NaCl, and water is reabsorbed into the medulla, resulting in hyperosmolar urine reaching the hairpin bend of the loop. This water, however, is removed by the vasa recta. The hyperosmolarity of the medulla is also partly due to the high concentrations of urea resulting from passive diffusion of urea from the medullary collecting duct into the interstitium along its concentration gradient.
This diagram outlines the major movements of solutes and water into and out of the different parts of the renal tubule. For further detail of these processes the reader is referred to current physiology texts.