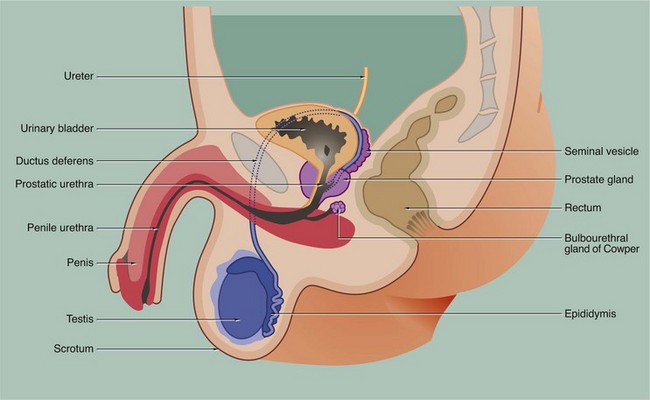
Male reproductive system
Introduction
The male reproductive system is responsible for the production of spermatozoa and their delivery into the female reproductive tract and may be divided into four major functional components:
• The testes or male gonads, paired organs lying in the scrotal sac, are responsible for production of the male gametes, spermatozoa, and secretion of male sex hormones, principally testosterone.
• A system of ducts consisting of ductuli efferentes, epididymis, ductus (vas) deferens and ejaculatory duct collects, stores and carries spermatozoa from each testis. The ejaculatory ducts converge on the urethra, from which spermatozoa are expelled into the female reproductive tract during copulation.
• Two exocrine glands, the paired seminal vesicles and the single prostate gland, secrete a nutritive and lubricating fluid medium called seminal fluid in which spermatozoa are conveyed to the female reproductive tract. Semen, the fluid expelled during ejaculation, consists of seminal fluid and spermatozoa, plus some desquamated duct-lining cells.
• The penis is the organ of copulation. A pair of small accessory glands, the bulbourethral glands of Cowper, secrete a fluid which lubricates the urethra for the passage of semen during ejaculation.
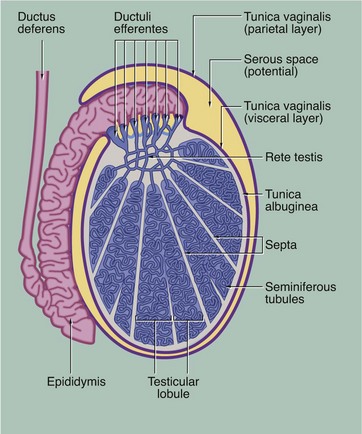
FIG. 18.2 Testis
During embryological development, each testis with the first part of its duct system, blood vessels, lymphatics and nerves descends from the posterior wall of the peritoneal cavity to the scrotum. During migration, the testis carries with it an investing layer of peritoneum so that in the scrotum the testis is almost completely surrounded by a double layer of mesothelium, enclosing a potential space. This double lining is called the tunica vaginalis and, like the pleura, consists of visceral and parietal layers, separated by a thin layer of serous fluid. The fluid is secreted by the mesothelial cells and acts as a lubricant, allowing the testis to move freely in the scrotal sac. The visceral layer of the tunica vaginalis rests on the capsule of the testis, the tunica albuginea, which gives rise to numerous incomplete collagenous septa. These divide the testis into about 250 testicular lobules. Within each lobule, there are one to four highly convoluted tubes, the seminiferous tubules, in which spermatozoa are produced. The seminiferous tubules converge upon a plexus of channels, the rete testis. From the rete testis, 15 to 20 small ducts called the ductuli efferentes carry spermatozoa to the extremely tortuous first part of the ductus deferens, which is known as the epididymis.
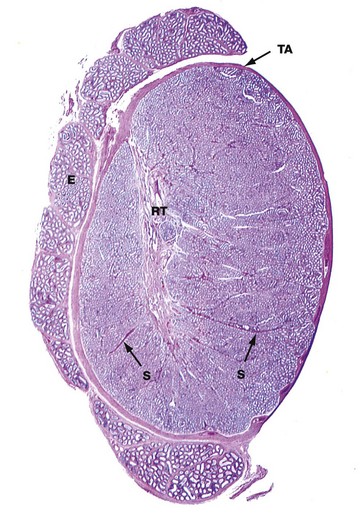
FIG. 18.3 Testis, monkey
H&E (LP)
This micrograph illustrates the macroscopic features of a testis; cut in the sagittal plane, it shows the relationship to the epididymis E, which lies on its posterior aspect. The testis is packed with coiled seminiferous tubules which can just be seen in various planes of section at this magnification. Groups of up to four seminiferous tubules are segregated into testicular lobules by fine interlobular septa S.
The dense fibrous capsule which invests the testis, and which is continuous with many of the interlobular septa, is called the tunica albuginea TA. It contains fibroblasts and abundant myofibroblasts and smooth muscle cells, particularly in the posterior aspect close to the rete testis, which subject the seminiferous tissue to rhythmic contractions. Scattered Leydig cells are also found within the tunica albuginea. The deepest layer of the tunica albuginea consists of loose connective tissue containing blood and lymphatic vessels, sometimes called the tunica vasculosa.
Spermatozoa pass from the seminiferous tubules into the rete testis RT, which is connected to the epididymis via the ductuli efferentes at the upper posterior pole of the testis; the ductuli are not included in the plane of this section. The epididymis is a tightly coiled tube which forms a compact mass extending down the whole length of the posterior surface of the testis and is the major site of storage of newly formed spermatozoa. At the lower pole of the testis, the epididymal tube becomes continuous with the relatively straight ductus (vas) deferens, not seen in this section.
Gametogenesis
In all somatic cells, cell division (mitosis) results in the formation of two daughter cells, each one genetically identical to the mother cell. Somatic cells contain a full complement of chromosomes (the diploid number) which function as homologous pairs (see Ch. 2). The process of sexual reproduction involves the fusion of specialised male and female cells called gametes to form a zygote, which has the diploid number of chromosomes. Each gamete contains only half the diploid number of chromosomes, one representative of each pair; this half complement of chromosomes is known as the haploid number.
The production of haploid cells involves a unique form of cell division called meiosis, which occurs only in the germ cells of the gonads during the formation of gametes; meiotic cell division is thus also called gametogenesis. Meiosis involves two cell division cycles, of which only the first is preceded by duplication of chromosomes (see Ch. 2). Thus, meiotic cell division of a single diploid germ cell gives rise to four haploid gametes. In the male, each of the four gametes undergoes morphological development into a mature spermatozoon. In contrast, in the female, unequal distribution of the cytoplasm during meiosis results in one gamete gaining almost all the cytoplasm from the mother cell, while the other three acquire almost no cytoplasm; the large gamete matures to form an ovum and the other three, called polar bodies, degenerate.
The primitive germ cells of the male, the spermatogonia, are present only in small numbers in the male gonads before sexual maturity. After puberty, spermatogonia multiply continuously by mitosis to provide a supply of cells which then undergo meiosis to form male gametes. In contrast, the germ cells of the female, called oogonia, multiply by mitosis only during early fetal development, thereby producing a fixed complement of cells with the potential to undergo gametogenesis. Gametogenesis in the female is discussed more fully in Ch. 19. The production of male gametes is called spermatogenesis and the subsequent development of the male gamete into a motile spermatozoon is called spermiogenesis, the whole process taking approximately 70 days; both these processes occur within the testes, although final maturation of spermatozoa occurs in the epididymis.
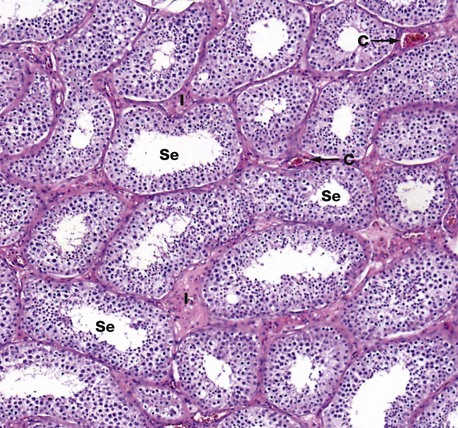
FIG. 18.4 Seminiferous tubules
H&E (MP)
This micrograph illustrates seminiferous tubules cut in various planes of section. The seminiferous tubules are highly convoluted and are lined by:
• Germ cells in various stages of spermatogenesis and spermiogenesis, which are collectively referred to as the spermatogenic series
• Non–germ cells, called Sertoli cells, which support and nourish the developing spermatozoa are also found within the seminiferous tubules.
• In the interstitial spaces between the tubules, endocrine cells called Leydig cells are found either singly or in groups in the supporting tissue.
In this micrograph of normal testis at medium power, note the seminiferous tubules Se cut in various planes of section, giving round and ovoid profiles. Between the seminiferous tubules the interstitium I contains Leydig cells (which cannot be discerned at this magnification) and small capillaries C. Larger arteries and veins are found in the fibrous septa that divide the organ into lobules.
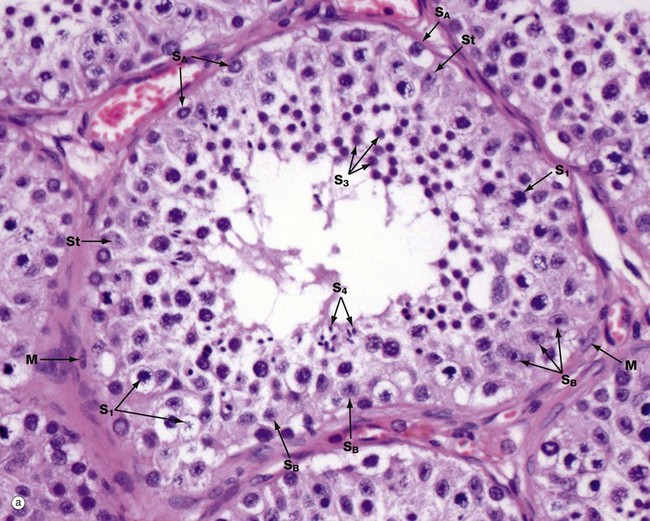
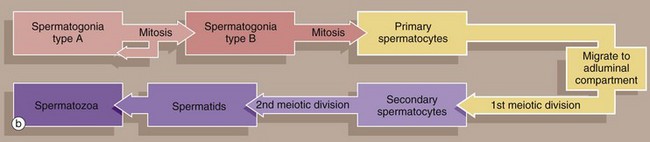
FIG. 18.5 Seminiferous tubule
(a) H&E (HP) (b) Diagram
Micrograph (a) illustrates an adult seminiferous tubule cut in transverse section. The processes of spermatogenesis and spermiogenesis are synchronised, with waves of activity occurring sequentially along the length of each tubule. Thus in a single cross-section of a tubule, not all development phases will be represented (b).
The undifferentiated diploid germ cells, found in the basal compartment of the seminiferous tubule, are called type A spermatogonia. These go through several cycles of mitosis to produce further type A spermatogonia, which maintain the germ cell pool, and type B spermatogonia, which are committed to production of spermatozoa. Spermatogonia type A SA are characterised by a large round or oval nucleus with condensed chromatin; peripheral nucleoli and a nuclear vacuole may be prominent. Spermatogonia type B SB have dispersed chromatin, central nucleoli, and no nuclear vacuole. Both types of spermatogonia have sparse poorly stained cytoplasm.
Type B spermatogonia undergo further mitotic divisions to produce primary spermatocytes. These migrate to the adluminal compartment of the seminiferous tubule before commencing the first meiotic division. Primary spermatocytes S1 are readily recognised by their copious cytoplasm and large nuclei containing coarse clumps or thin threads of chromatin; dividing cells may be seen. In humans, the first meiotic division cycle takes approximately 3 weeks to complete, after which time the daughter cells become known as secondary spermatocytes. The smaller secondary spermatocytes rapidly undergo the second meiotic division and are therefore seldom seen.
The gametes thus produced, called spermatids S3, then proceed through the long maturation process known as spermiogenesis to become recognisable as spermatozoa. During this process, the nuclei of the spermatids assume the small pointed form of spermatozoa S4 (see Fig. 18.7). Examination of different sections of the tubules of a normal testis shows about half the spermatogenic cells to be in the late spermatid stage.
During the developmental process, the cells of the spermatogenic series are supported by Sertoli cells St, whose nuclei are usually found towards the basement membrane of the seminiferous tubule. The Sertoli cell nucleus is typically triangular or ovoid in shape with a prominent nucleolus and dispersed chromatin.
The basal layer of germinal cells is supported by a basement membrane which is surrounded by a lamina propria containing several layers of spindle-shaped myofibroblasts M and fibroblasts.
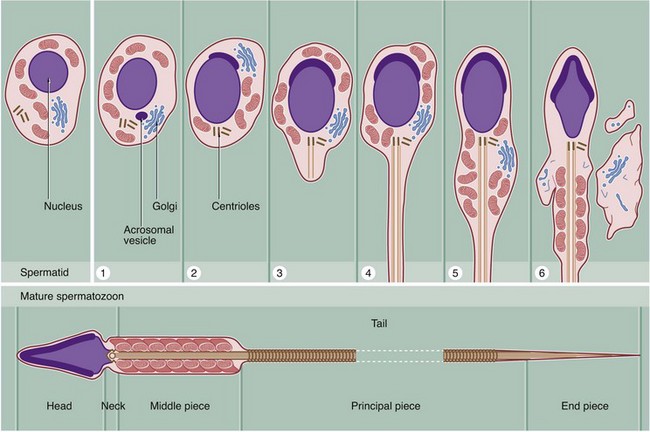
FIG. 18.6 Spermiogenesis
Spermiogenesis is the process by which spermatids, the gametes produced by meiotic division, are transformed into motile mature spermatozoa. This involves the following major stages:
1. The Golgi apparatus elaborates a large membrane bound vesicle, the acrosomal vesicle, which accumulates carbohydrates and hydrolytic enzymes.
2. The acrosomal vesicle becomes applied to one pole of the progressively elongating nucleus to form a structure known as the acrosomal head cap.
3. Meanwhile, both centrioles migrate to the end of the cell opposite to the acrosomal head cap; the centriole aligned parallel to the long axis of the nucleus elongates to form a flagellum which has a basic structure similar to that of the cilium (see Fig. 5.13).
4. As the flagellum elongates, nine coarse fibrils, which may contain contractile proteins, become arranged longitudinally around the core of the flagellum. Further rib-like fibrils then become disposed circumferentially around the whole flagellum.
5. The cytoplasm migrates to surround the first part of the flagellum. The remainder of the flagellum appears to project from the cell but in fact remains surrounded by plasma membrane. This migration of cytoplasm thus concentrates mitochondria in the flagellar region.
6. As the flagellum elongates, excess cytoplasm is phagocytosed by the enveloping Sertoli cell prior to release of the spermatid into the lumen, a process called spermiation.
The mitochondria become arranged in a helical manner around the fibrils which surround the first part of the flagellum.
The structure of fully formed spermatozoa varies in detail from species to species but conforms to the basic structure seen in this diagram of a human spermatozoon.
Throughout the entire developmental process from spermatogonia to spermatozoa, hundreds of spermatids remain connected to one another by narrow cytoplasmic bridges which only break down upon release of spermatozoa into the lumen of the seminiferous tubule. This explains the synchronous development of spermatozoa at any one part of the tubule.
Sertoli cells are important in the regulation of spermatogenesis and spermiogenesis. Sertoli cells form tight junctions with each other as well as with the developing germ cells. It is well established that high concentrations of androgen hormones, secreted by Leydig cells of the testicular interstitium (see Fig. 18.10), are essential for production and maturation of spermatogenic cells. Sertoli cells secrete an androgen-binding protein which transports testosterone and dihydrotestosterone to the lumen of the seminiferous tubule. These hormones are also necessary for function of the epithelium of the rete testis and epididymis; production of this binding protein is believed to be dependent on the pituitary gonadotrophin follicle stimulating hormone (FSH).
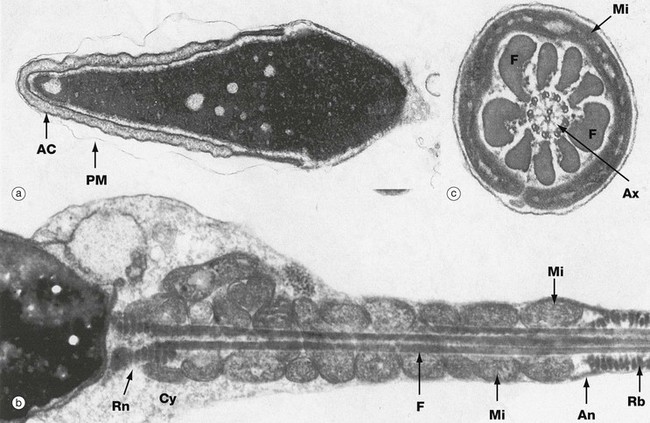
FIG. 18.7 Spermatozoa
(a) Head, LS, EM ×14 000 (b) Neck (middle piece and principal piece), LS, EM ×17 000 (c) Middle piece, TS, EM ×48 000
The ultrastructural features of human spermatozoa are shown in these micrographs. The spermatozoon is an extremely elongated cell (about 65 µm long) consisting of three main components: the head, neck and tail. The tail is subdivided into three segments: the middle piece, principal piece and end piece (see Fig. 18.6).
The head is the most variable structure between different mammalian species. In humans the head is about 7 µm long and has a flattened pear shape. As seen in micrograph (a), the nucleus, which occupies most of the head, is composed of very condensed chromatin; in humans, this contains a variable number of areas of dispersed chromatin called nuclear vacuoles. Surrounding the anterior two-thirds of the nucleus is the acrosomal cap AC, a flattened membrane-bound vesicle containing a range of glycoproteins and a variety of hydrolytic enzymes, principally hyaluronidase; the enzymes disaggregate the cells of the corona radiata and dissolve the zona pellucida during fertilisation (see Ch. 19). Note the plasma membrane PM, which has become partially separated during preparation.
The neck is a very short segment connecting the head with the tail. It contains vestiges of the centrioles, one of which gives rise to the axoneme Ax of the flagellum, which is seen in micrograph (c). The axoneme has the standard ‘nine plus two’ arrangement of microtubule doublets seen in cilia (see Fig. 5.13). The axoneme of the neck is surrounded by several condensed fibrous rings Rn seen in micrograph (b). In human spermatozoa, a significant amount of cytoplasm Cy often remains in the neck region.
The middle piece, the first part of the tail, is about the same length as the head and consists of the flagellar axoneme surrounded by nine coarse (outer dense) fibres F arranged longitudinally. External to this core, elongated mitochondria Mi are arranged in a tightly packed helix providing the energy required for flagellar movement. A fibrous thickening beneath the plasma membrane, called the annulus An, prevents the mitochondria from slipping into the principal piece. The principal piece, which constitutes most of the tail length, consists of a central core comprising the axoneme and the nine coarse fibres continuing from the middle piece. Surrounding this core are numerous fibrous ribs Rb arranged in a circular manner and seen in micrograph (b). Two of the longitudinal fibrils of the core are fused with the surrounding ribs so as to form dorsal and ventral columns extending throughout the length of the principal piece (not illustrated). This arrangement divides the principal piece longitudinally into two functional compartments, one containing three coarse fibrils and the other containing four. Little is known of the mechanism of flagellar motion, but this asymmetry may account for the more powerful stroke of the tail in one direction, the so-called power stroke; this can easily be observed in fresh live preparations of spermatozoa viewed with the light microscope. The end piece, not shown in these micrographs, is merely a short, tapering portion of the tail containing the axoneme only.
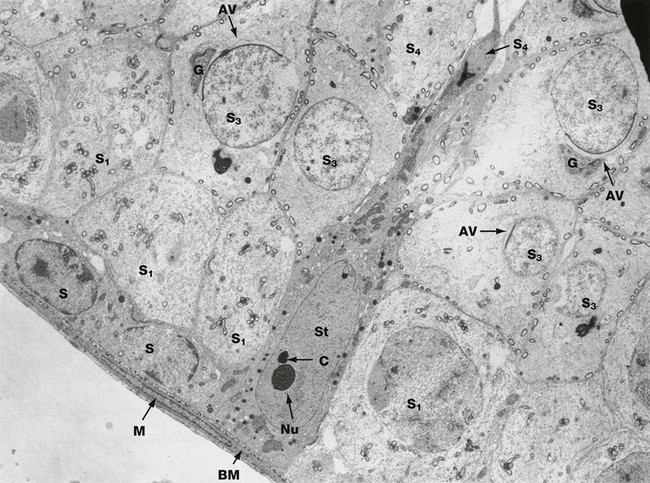
FIG. 18.8 Sertoli cell
EM ×3400
The intimate relationship of a Sertoli cell St to cells of the spermatogenic series is demonstrated in this electron micrograph.
The Sertoli cell rests on the basement membrane BM of the seminiferous tubule and its cytoplasm extends to the lumen of the tubule. Sertoli cells have an extensive cytoplasm which ramifies throughout the whole germinal epithelium, enclosing all the cells of the spermatogenic series. The cytoplasmic outline of the Sertoli cell is thus highly irregular and constantly changing to permit the progressive movement of developing spermatozoa towards the luminal surface. The oval nucleus of the Sertoli cell is characteristically orientated at right angles to the basement membrane and often exhibits a deep indentation. A prominent nucleolus Nu is a constant feature and dense chromatin bodies C are often associated with the nucleolus. The cytoplasm contains a moderate number of mitochondria, lipid droplets and a small amount of rough endoplasmic reticulum. Plentiful smooth endoplasmic reticulum is also present, as well as lamellar protein arrays known as Charcot-Bottcher crystals (not shown in this cell).
Sertoli cells are bound to one another by junctional complexes containing extensive tight junctions (see Ch. 5). The junctional complex is located towards the basal layer of the spermatogenic epithelium so as to divide the tubule into basal and adluminal compartments. The latter contains the spermatids, which are thus isolated by a blood-testis barrier. The Sertoli cells mediate all metabolic exchange with the systemic compartment. The function of this barrier is to prevent exposure of gametes, which are antigenically different from somatic cells, to the immune system, thus preventing an autoimmune response. Sertoli cells have multiple functions including:
• Secretion of factors which regulate spermatogenesis and spermiogenesis
• Secretion of factors which regulate the function of Leydig cells and peritubular cells
A variety of cells of the spermatogenic series are seen in this micrograph. Spermatogonia S rest upon the basement membrane BM, beneath which is a myofibroblast M. Above the germ cell layer, primary spermatocytes S1 are seen; secondary spermatocytes are short-lived and therefore rarely seen. Spermatids S3 in different phases of spermiogenesis are seen in upper layers; these cells have developing acrosomal vesicles AV elaborated by a large Golgi apparatus G (see Fig. 18.6). At the luminal surface, the Sertoli cell partly envelops the head of an almost fully formed spermatozoon S4.
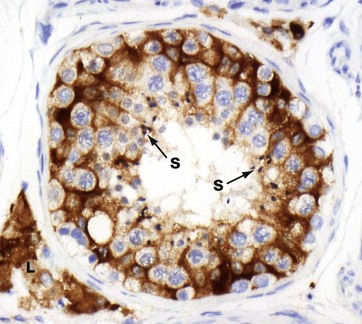
FIG. 18.9 Sertoli cells (HP)
Immunohistochemical method for inhibin
Sertoli cells can be difficult to identify in standard H&E-stained sections. However, this micrograph demonstrates the extensive cytoplasm of the Sertoli cells as they ramify around the cells of the spermatogenic series. The immunohistochemical stain uses an antibody directed against inhibin, a product of Sertoli and Leydig cells, and so the cytoplasm of both of these cell types is strongly stained brown; note the cluster of Leydig cells L in the interstitium. The nuclei are not stained. Note the maturing spermatozoa S towards the lumen of the seminiferous tubules.
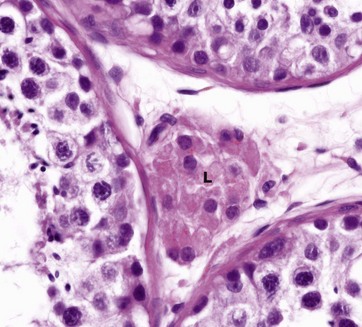
FIG. 18.10 Interstitial (Leydig) cells of the testis
H&E (HP)
Leydig cells L, the principal cell type found in the interstitial supporting tissue between the seminiferous tubules, synthesise and secrete the male sex hormones and other non-steroid substances. They occur singly or in clumps and are embedded in the rich plexus of blood and lymph capillaries which surrounds the seminiferous tubules. The nucleus is round with dispersed chromatin and one or two nucleoli at the periphery. The extensive eosinophilic cytoplasm contains variable numbers of lipid vacuoles and, seen by electron microscopy, closely resembles the steroid-secreting cells of the adrenal cortex (see Fig. 17.16). In humans (and wild bush rats) but no other species, Leydig cells also contain elongated cytoplasmic crystals of Reinke which are large enough to be seen with light microscopy when suitably stained; these crystals are found only in adults but their function is unknown.
Testosterone is the main hormone secreted by Leydig cells. Testosterone is not only responsible for the development of male secondary sexual characteristics at puberty but is also essential for the continued function of the seminiferous epithelium. The secretory activity of Leydig cells is controlled by the pituitary gonadotrophic hormone luteinising hormone, sometimes called interstitial cell stimulating hormone (ICSH) in the male.
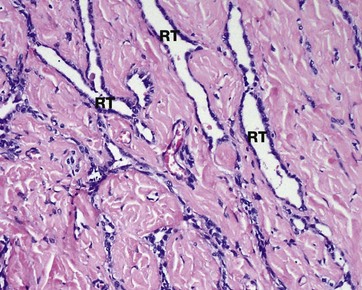
FIG. 18.11 Rete testis
H&E (LP)
The seminiferous tubules converge upon the mediastinum testis, which consists of a plexiform arrangement of channels, the rete testis RT, surrounded by highly vascular collagenous supporting tissue containing myoid cells. The rete testis is lined by a single layer of low cuboidal epithelial cells with surface microvilli and a single cilium.
Myoid cell contraction helps to mix the spermatozoa and move them towards the epididymis. The lining epithelium reabsorbs protein and potassium from the seminal fluid. Ciliary activity is presumed to aid the progress of spermatozoa, which do not become motile until after maturation is completed in the epididymis.
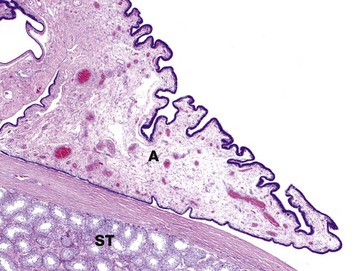
FIG 18.12 Appendix testis
H&E (LP)
The appendix testis (hydatid of Morgagni) is a tiny tag of tissue protruding from the antero-superior aspect of the testis. It is important because it may undergo torsion leading to loss of blood supply and necrosis (infarction), an extremely painful process. The appendix testis is a remnant of the müllerian duct and is present in approximately 80% of men.
The appendix testis A has a fibrovascular core and a cuboidal to columnar surface epithelium which may be ciliated (not visible at this magnification). Note the seminiferous tubules ST in the adjacent testis.
Other appendages in this area are the less common appendix epididymis, the vas aberrans (organ of Haller) and the paradidymis (organ of Giraldes).
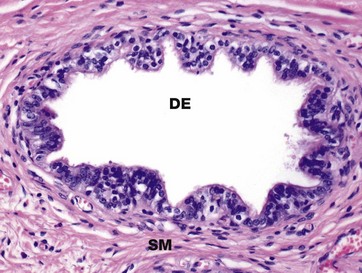
FIG. 18.13 Ductulus efferens
H&E (MP)
The rete testis drains into the head of the epididymis via some 15 to 20 convoluted ducts, the ductuli efferentes DE. The ductuli are lined by a single layer of epithelial cells, some of which are tall columnar and ciliated and others which are short and non-ciliated; both cell types often contain a brown pigment of unknown composition. Ciliary action in the ductuli propels the still non-motile spermatozoa towards the epididymis. The non-ciliated cells reabsorb some of the fluid produced by the testis. Basal cells, which do not reach the lumen, are also present and probably act as stem cells. A thin band of circularly arranged smooth muscle SM surrounds each ductulus and aids propulsion of the spermatozoa towards the epididymis.
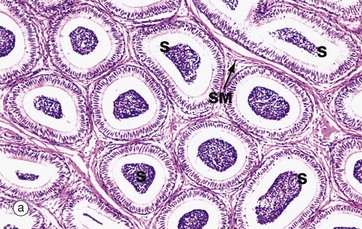
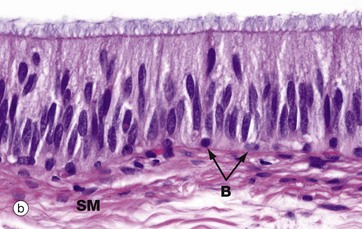
FIG. 18.14 Epididymis
(a) H&E (LP) (b) H&E (HP)
The epididymis is a long, extremely convoluted duct extending down the posterior aspect of the testis to the lower pole where it becomes the ductus (vas) deferens. The epididymis consists of a head at the upper pole of the testis, a body lying along the posterior margin and a tail at the lower pole of the testis. The major function of the epididymis is the accumulation, storage and maturation of spermatozoa S; in the epididymis, the spermatozoa develop motility.
The epididymis is a tube of smooth muscle lined by a pseudostratified epithelium. From the proximal to the distal end of the epididymis, the muscular wall increases from a single circular layer SM, as in these micrographs, to three layers organised in the same manner as in the ductus deferens (see Fig. 18.15). Proximally, the smooth muscle exhibits slow, rhythmic contractility which gently moves spermatozoa towards the ductus deferens. Distally, the smooth muscle is richly innervated by the sympathetic nervous system, which produces intense contractions of the lower part of the epididymis during ejaculation.
The epithelial lining of the epididymis exhibits a gradual transition from a tall pseudostratified columnar form in the head, as seen in micrograph (b), to a shorter pseudostratified form at the tail. The principal cells of the epididymal epithelium bear tufts of very long microvilli, inappropriately called stereocilia (see Fig. 5.15), which are thought to be involved in absorption of an excess of fluid accompanying the spermatozoa from the testis. The ultrastructure of the cells strongly suggests an additional secretory function but the nature of epididymal secretory products, if any, remains unknown. Basal cells B are prominent at the base of the epithelium.
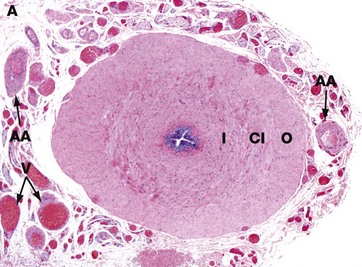
FIG. 18.15 Vas deferens
H&E (LP)
The vas (or ductus) deferens, which conducts spermatozoa from the epididymis to the urethra, is a thick-walled muscular tube consisting of inner I and outer O longitudinal layers and a thick intermediate circular layer CI. Like the distal part of the epididymis, the vas deferens is innervated by the sympathetic nervous system, producing strong peristaltic contractions to expel its contents into the urethra during ejaculation.
The vas deferens is lined by a pseudostratified columnar epithelium similar to that of the epididymis (see Fig. 18.14); the epithelial lining and its supporting lamina propria are thrown into longitudinal folds, permitting expansion of the duct during ejaculation. The dilated distal portion of each vas deferens, known as the ampulla, receives a short duct draining the seminal vesicle, thus forming the short ejaculatory duct; the ejaculatory ducts from each side converge to join the urethra as it passes through the prostate gland. In this low-power micrograph, the adipose tissue A, arteries AA and veins V that accompany the vas are easily seen; together these structures comprise the spermatic cord.
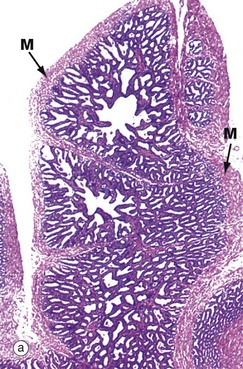
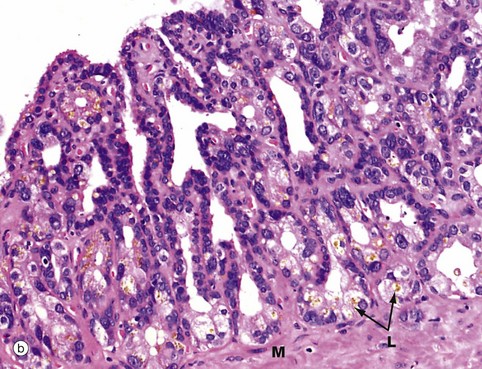
FIG. 18.16 Seminal vesicle
(a) H&E (LP) (b) H&E (HP)
Each seminal vesicle is a complex glandular diverticulum of the associated ductus deferens. Between them, the seminal vesicles secrete 50–70% of the total volume of seminal fluid, most of the rest being secreted by the prostate gland. The lumen of each seminal vesicle is highly irregular and recessed, giving a honeycomb appearance at low magnification.
The epithelial lining is usually of a pseudostratified tall columnar type and consists of secretory cells with lipid droplets in the cytoplasm, giving it a foamy appearance. The seminal vesicles produce a yellowish viscid alkaline fluid containing a wide range of substances, including fructose, fibrinogen, vitamin C and prostaglandins. The epithelial cells often contain brown lipofuscin granules L and characteristically have rather variable nuclear shape and size. Both of these features are seen in micrograph (b). Although not thought to store spermatozoa, seminal vesicles are often seen to contain spermatozoa which have probably entered by reflux from the ampulla. The prominent muscular wall M is arranged into inner circular and outer longitudinal layers and is supplied by the sympathetic nervous system; during ejaculation, muscle contraction forces secretions from the seminal vesicles into the urethra via the ampullae.
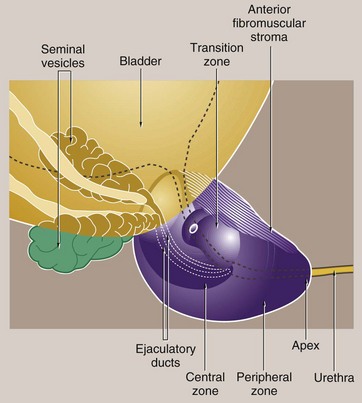
FIG. 18.17 Prostate gland
The prostate gland, which in young adults is about the size of a walnut, surrounds the bladder neck and the first part of the urethra, known as the prostatic urethra. The urethra courses through the prostate to become the membranous urethra at the apex of the prostate. In the substance of the gland, the urethra merges with the ejaculatory ducts and at this point angles anteriorly.
The prostate consists of branched tubulo-acinar glands, embedded in a fibromuscular stroma. There is a partial capsule enclosing the posterior and lateral aspects of the prostate but the anterior and apical surfaces are bounded by the anterior fibromuscular stroma, a part of the gland consisting, as the name implies, only of collagenous stroma and muscle fibres.
In the past, the prostate was described as consisting of a number of ill-defined lobes. However, this terminology has been replaced by the concept of prostate zones and the gland is now described as consisting of four zones of unequal size:
• The transition zone surrounds the proximal prostatic urethra and comprises about 5% of the glandular tissue.
• The central zone (20%) surrounds the ejaculatory ducts.
• The peripheral zone makes up the bulk of the gland (approximately 70%).
• The anterior fibromuscular stroma contains no glandular tissue and lies anteriorly.
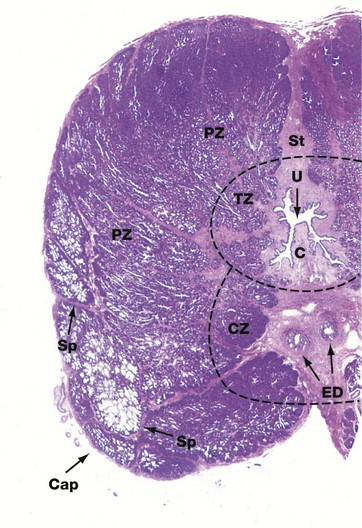
FIG. 18.18 Prostate gland, dog
H&E (LP)
This low-power view of the prostate of a dog shows the general architectural features of the gland. The urethra U lies centrally, surrounded by a fibrous stroma St. The ejaculatory ducts ED also lie in this central stroma as they course towards their junction with the prostatic urethra. The zones of the prostate are not clearly demarcated from each other anatomically. Partial fibrous septa Sp separate the gland into lobules. The transition zone TZ surrounds the first part of the prostatic urethra. The central zone CZ lies posterior to the transition zone and encircles the ejaculatory ducts ED. The peripheral zone PZ makes up the main bulk of the gland. The ducts of the peripheral zone glands empty into the posterolateral recesses of the urethra on either side of the verumontanum (urethral crest) C.
The different zones of the prostate are important because they tend to be the sites of different disease processes. Most cases of carcinoma of the prostate arise in the peripheral zone, while the transition zone harbours almost all cases of benign nodular hyperplasia (see below).
At this power the anterior fibromuscular stroma appears continuous with the capsule Cap and its content of muscle fibres cannot be discerned.
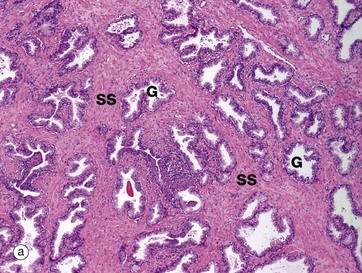
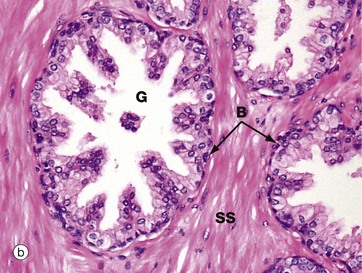
FIG. 18.19 Prostate gland
(a) H&E (LP) (b) H&E (HP)
The prostate gland is composed of glands and stroma. The supporting stroma SS is a mixture of collagenous fibrous tissue and smooth muscle fibres, which are best seen at higher magnification in micrograph (b). The glands G show a convoluted pattern with the epithelium thrown up into folds, sometimes into almost a papillary pattern.
The secretory product of the prostate, which makes up about 30–50% the seminal fluid volume, is a thin liquid rich in citric acid and proteolytic enzymes, including fibrinolysins, which liquefies the coagulated semen after it has been deposited in the vagina. Inspissated secretions may accumulate in some glands to form spherical concretions (corpora amylacea) which increase in number with age and may become calcified (not seen in this example).
Micrograph (b), taken at higher magnification, shows the detail of the epithelium of the prostate glands. The main epithelial cell type is the tall columnar secretory cell with prominent round basal nuclei and pale-staining cytoplasm. There is also a scanty population of small, flat, basal cells at the base of the gland in contact with the basement membrane. The basal cells are easily seen in this micrograph. These cells act as stem cells and may become quite prominent in prostatic hyperplasia.
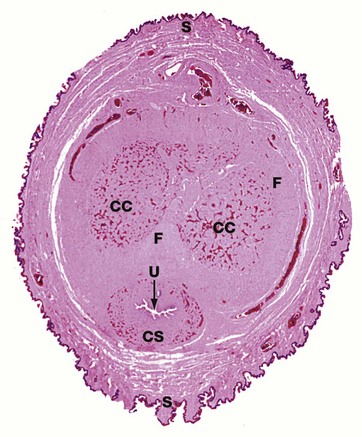
FIG. 18.20 Penis
H&E (LP)
This transverse section of the human penis shows the arrangement of the erectile tissues, which exist in the form of three columns. The two dorsal columns are called the corpora cavernosa CC and the single ventral column is the corpus spongiosum CS through which runs the penile urethra U. At its distal end, the corpus spongiosum expands to form the glans penis. The erectile corpora are enclosed within and separated by a fibrocollagenous capsule F. The erectile centre of the penis is enclosed in a sheath of skin S to which it is connected by a loose subcutis containing prominent blood vessels.
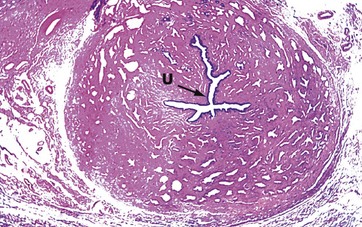
FIG. 18.21 Corpus spongiosum
H&E (LP)
The corpus spongiosum is composed of erectile tissue, large irregular interconnected vascular channels with fibrocollagenous stroma between; the stroma contains some smooth muscle fibres. Running through the centre of the corpus spongiosum is the penile urethra U. Small paraurethral mucus glands open into the urethra.
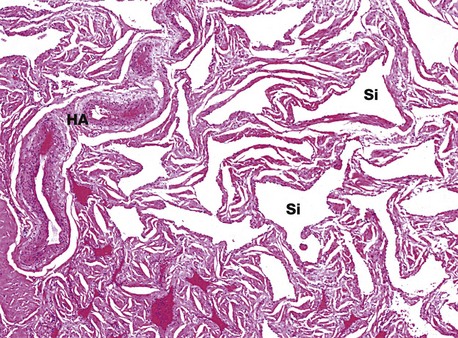
FIG. 18.22 Penile erectile tissue
H&E (LP)
The vascular sinuses Si of the cavernous bodies of the penis are supplied by numerous anastomosing thick-walled arteries and arterioles called helicine arteries HA, since they follow a spiral course in the flaccid state. Blood drains from the sinuses via veins which lie immediately beneath the dense fibroelastic tissue investing the cavernous bodies. During erection, dilatation of the helicine arteries, mediated by the parasympathetic nervous system, results in engorgement of the vascular sinuses, which enlarge, compressing and restricting venous outflow. The process is enhanced by relaxation of smooth muscle cells in the trabeculae of the cavernous bodies.
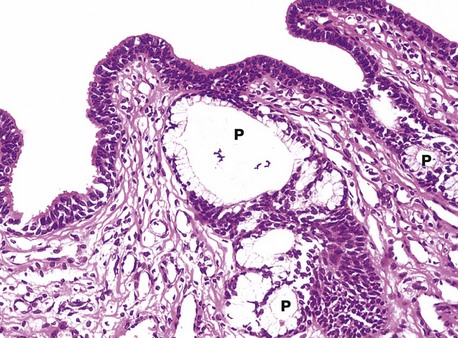
FIG. 18.23 Penile urethra
H&E (HP)
Apart from the prostatic urethra, which is lined by transitional epithelium, the male urethra is lined by stratified or pseudostratified columnar epithelium, although small areas of stratified squamous epithelium may also be found in human adult males. The external opening (urethral meatus) is lined by stratified squamous epithelium which becomes continuous with the epithelium of the glans.
The urethra is lubricated by mucous secretions from the para-urethral glands P and the bulbo-urethral glands of Cowper (see Fig. 18.1), which have a similar but more discrete organisation.
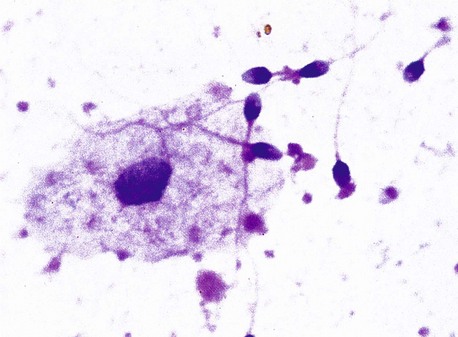
FIG. 18.24 Semen
H&E (HP)
Semen, the product of ejaculation, consists of spermatozoa and seminal fluid, which is derived principally from the seminal vesicles and prostate gland. The volume of each human ejaculate is about 3.5 mL, containing from 50 to 150 × 106 spermatozoa per mL. In normal fertile human males, up to 25% of the ejaculated spermatozoa are abnormal or degenerate forms. By the time of ejaculation, spermatozoa have matured and acquired the property of motility; nevertheless, they remain incapable of fertilising an ovum until after undergoing a process called capacitation within the female genital tract.