Epithelial tissues
Introduction
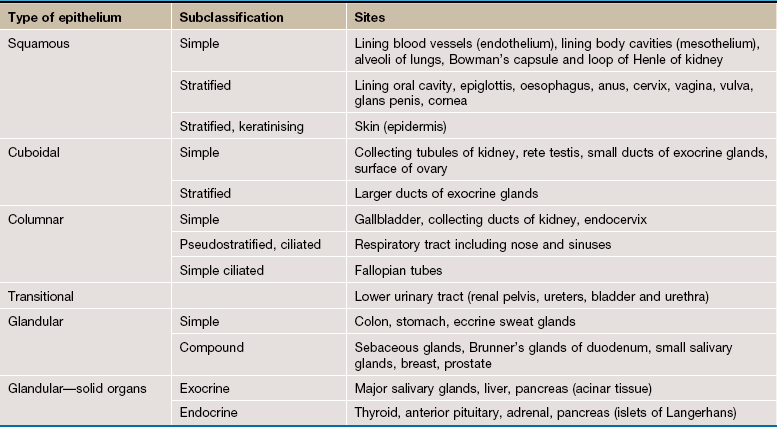
The epithelia (singular: epithelium) are a diverse group of tissues that include both surface epithelia and solid organs. Surface epithelia cover or line all body surfaces, cavities and tubes and form the interface between different biological compartments. For instance, the epidermis of the skin is exposed to the external environment and the epithelial lining of the gastrointestinal tract is exposed to partially digested food and bacteria in the lumen of the gut. Functions of epithelia include: forming a protective barrier, regulation of the exchange of molecules between compartments (selective diffusion and absorption) and synthesis and secretion of glandular products. Many of these major functions may be exhibited at a single epithelial surface. For example, the epithelial lining of the small intestine is primarily involved in absorption of the products of digestion, but the epithelium also protects itself from noxious intestinal contents by secreting a surface coating of mucus. Epithelial cells are characterised by the production of keratin intermediate filaments (see Ch. 1), and this can be used to recognise epithelial cells using immunohistochemistry, a technique often used in diagnostic histopathology to classify difficult malignant tumours (see Appendix 2).
Surface epithelia form continuous sheets comprising one or more layers of cells. Epithelial cells are bound to adjacent cells by a variety of cell junctions that provide physical strength and mediate exchange of information and metabolites. All epithelia are supported by a basement membrane (see Ch. 4) which separates the epithelium from underlying supporting tissues. Thus epithelial cells are polarised, with one side facing the basement membrane and underlying supporting tissues (the basal surface) and the other facing outwards (the apical surface).
Blood vessels never cross epithelial basement membranes, so epithelia depend on the diffusion of oxygen and metabolites from adjacent supporting tissues.
Classification of Surface Epithelia
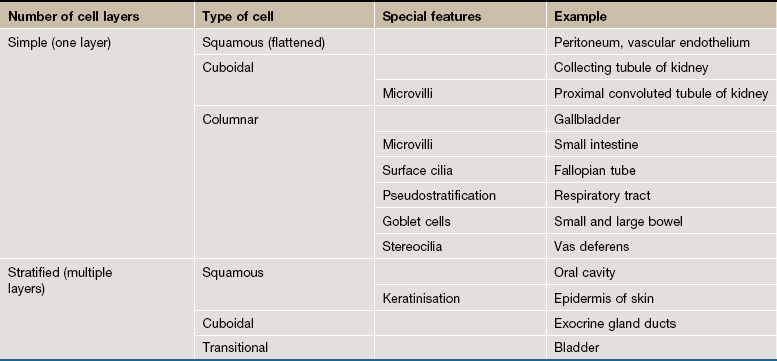
Surface epithelia are traditionally classified according to three morphological characteristics: number of cell layers, type of cell (profile perpendicular to basement membrane) and special features. Special features include adaptations such as cilia or goblet cells that may be characteristic of particular sites (e.g. the epithelium of the upper respiratory tract is a ciliated pseudostratified columnar epithelium).
Glandular Epithelia
Epithelium that is primarily involved in secretion is often arranged into structures called glands. Glands are merely invaginations of epithelial surfaces which are formed during embryonic development by proliferation of epithelium into the underlying tissues. For example, glandular epithelium is characteristic of the lining of much of the gastrointestinal tract.
However, some solid organs are composed largely of epithelial cells with a supporting tissue framework. Some of these organs are connected to the surface epithelium of the gastrointestinal tract by a branching system of ducts and belong to the category of exocrine glands (e.g. salivary glands). Endocrine glands on the other hand have lost their connection to the epithelial surface from which they developed and release their secretions directly into the blood (e.g. thyroid gland). Most of the solid epithelial organs such as liver, pancreas and thyroid are described in detail in the relevant organ system chapter and only a few examples are described here.
Simple Epithelia
Simple epithelia are defined as surface epithelia consisting of a single layer of cells. Simple epithelia are almost always found at interfaces involved in selective diffusion, absorption and/or secretion. They provide little protection against mechanical abrasion and thus are not found on surfaces subject to such stresses. The cells comprising simple epithelia range in shape from flattened to tall columnar, depending on their function. For example, flattened simple epithelia are ideally suited to diffusion and are therefore found in the air sacs of the lung (alveoli), the lining of blood vessels (endothelium) and lining body cavities (mesothelium). In contrast, highly active epithelial cells, such as the cells lining the small intestine, are generally tall since they must accommodate the appropriate organelles. Simple epithelia may exhibit a variety of surface specialisations, such as microvilli and cilia, which facilitate their specific surface functions.
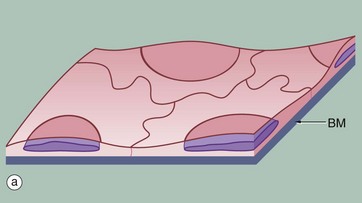
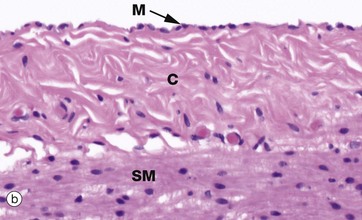
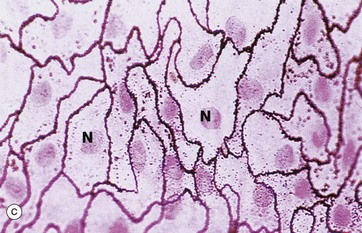
FIG. 5.1 Simple squamous epithelium
(a) Diagram (b) H&E (HP) (c) Spread preparation, silver method/neutral red (HP)
Simple squamous epithelium is composed of flattened, irregularly shaped cells forming a continuous surface that is sometimes called pavemented epithelium; the term squamous derives from the comparison of the cells to the scales of a fish. Like all epithelia, this delicate lining is supported by an underlying basement membrane BM as shown diagrammatically.
Simple squamous epithelium is found lining surfaces involved in passive transport (diffusion) of either gases (as in the lungs) or fluids (as in the walls of blood capillaries). Simple squamous epithelium also forms the delicate lining of the pleural, pericardial and peritoneal cavities where it allows passage of tissue fluid into and out of these cavities. Although these cells appear simple in form they have a wide variety of important roles.
Micrograph (b) shows a mesothelium (peritoneum) covering the surface of the appendix and illustrates the typical appearance of simple squamous epithelium in section. The mesothelial lining cells M are so flattened that they can only be recognised by their nuclei, which bulge on the surface. The supporting basement membrane is thin and, in H&E stained preparations, has similar staining properties to the underlying collagenous supporting tissue C; hence it cannot be seen in this micrograph. Deeper in the wall of the appendix, the smooth muscle SM of the muscularis propria can be identified.
In the preparation used in micrograph (c), the mesothelial lining of the peritoneal cavity has been stripped from the underlying tissues and spread onto a slide, thus permitting a surface view of simple squamous epithelium. The intercellular substance has been stained with silver thereby outlining the closely interdigitating and highly irregular cell boundaries. The nuclei N are stained a slightly darker pink.
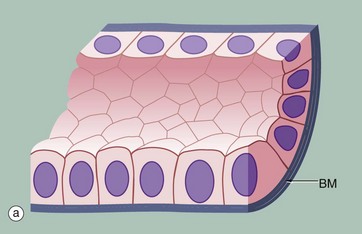
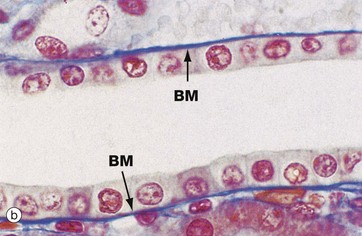
FIG. 5.2 Simple cuboidal epithelium
(a) Diagram (b) Azan (HP)
Simple cuboidal epithelium represents an intermediate form between simple squamous and simple columnar epithelium; the distinction between tall cuboidal and low columnar is often arbitrary and is of descriptive value only. In the section perpendicular to the basement membrane BM, the epithelial cells appear square, leading to its traditional description as cuboidal epithelium; on surface view, however, the cells are actually polygonal in shape. The nucleus is usually round and located in the centre of the cell.
Simple cuboidal epithelium usually lines small ducts and tubules that may have excretory, secretory or absorptive functions; examples are the collecting tubules of the kidney and the small excretory ducts of the salivary glands and pancreas.
Micrograph (b) shows the cells lining a collecting tubule in the kidney. Although the boundaries between individual cells are indistinct, the nuclear shape provides an approximate indication of the cell size and shape. The underlying basement membrane BM appears as a prominent blue line with the Azan staining method, in contrast to basement membranes stained with the standard H&E stain (see Fig. 5.3b) that are generally indistinguishable.
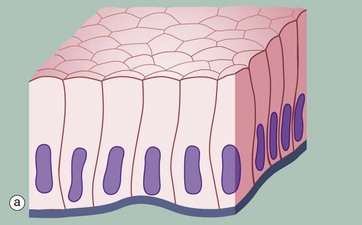
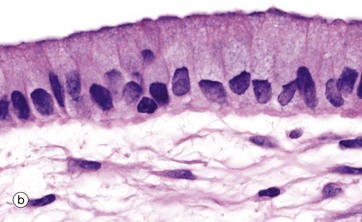
FIG. 5.3 Simple columnar epithelium
(a) Diagram (b) H&E (HP)
Simple columnar epithelium is similar to simple cuboidal epithelium except that the cells are taller and appear columnar in sections perpendicular to the basement membrane. The height of the cells may vary from low to tall columnar, depending on the site and/or degree of functional activity. The nuclei are elongated and may be located towards the base, the centre or occasionally the apex of the cytoplasm; this is known as the polarity of the nucleus. Simple columnar epithelium is found on absorptive surfaces such as in the small intestine, as well as at secretory surfaces such as that of the stomach.
Micrograph (b) shows simple columnar epithelium taken from the endocervix where it has the function of secreting mucus. Note the typically basally located nuclei.
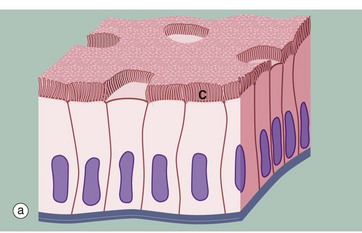
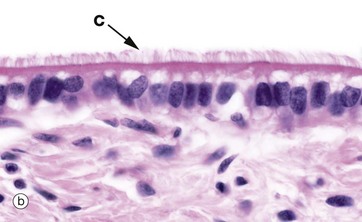
FIG. 5.4 Simple columnar ciliated epithelium
(a) Diagram (b) H&E (HP)
Some simple columnar epithelia have surface cilia C on the majority of the cells (see also Fig. 5.13). Among the ciliated cells are scattered non-ciliated cells that usually have a secretory function.
Cilia are much larger than microvilli (see Fig. 5.14) and are readily visible with the light microscope. Each cilium consists of a finger-like projection of the plasma membrane, its cytoplasm containing modified microtubules. Each cell may have up to 300 cilia that beat in a wave-like manner, synchronised with the adjacent cells. The waving motion of the cilia propels fluid or minute particles over the epithelial surface. Simple columnar ciliated epithelium is found mainly in the female reproductive tract. Micrograph (b), taken from the Fallopian tube (oviduct), shows one of its numerous folds covered by simple columnar ciliated epithelium. The predominant cell type in this epithelium is tall columnar and ciliated, the nuclei being located towards the midzone of the cells. The less numerous blue-stained cells with basally located nuclei are not ciliated and have a secretory function. Ciliary action facilitates transport of the ovum from the ovary towards the uterus.
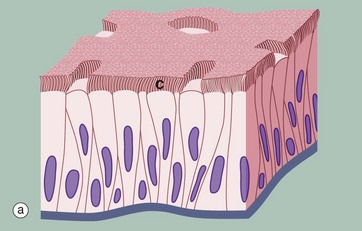
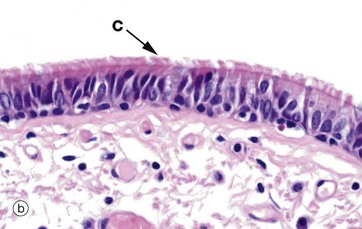
FIG. 5.5 Pseudostratified columnar ciliated epithelium
(a) Diagram (b) H&E (MP)
Another variant of simple columnar epithelium is described in which the majority of cells are also usually ciliated C. The term pseudostratified is derived from the appearance of this epithelium in section, which conveys the erroneous impression that there is more than one layer of cells. In fact, this is a true simple epithelium, since all the cells rest on the basement membrane. The nuclei of these cells, however, are disposed at different levels, thus creating the illusion of cellular stratification. Scattered stem cells (see Ch. 2) are found throughout the epithelium; these generally are devoid of cilia (i.e. less differentiated) and do not extend to the luminal surface.
Pseudostratified columnar ciliated epithelium may be distinguished from true stratified epithelia by two characteristics. Firstly, the individual cells of the pseudostratified epithelium exhibit polarity, with nuclei being mainly confined to the basal two-thirds of the epithelium. Secondly, cilia are never present on true stratified epithelia.
Pseudostratified epithelium is almost exclusively confined to the airways of the respiratory system in mammals and is therefore often referred to as respiratory epithelium. Micrograph (b) illustrates the lining of a bronchus. In the respiratory tract, the cilia propel a surface layer of mucus containing entrapped particles towards the pharynx in what is often described as the mucociliary escalator. The mucus is secreted by nonciliated goblet cells found amongst the ciliated cells (not seen in this micrograph, see Figs 5.16 and 5.17).
Stratified Epithelia
Stratified epithelium is defined as epithelium consisting of two or more layers of cells. Stratified epithelia have mainly a protective function and the degree and nature of the stratification are related to the kinds of physical stresses to which the surface is exposed. In general, stratified epithelia are poorly suited for absorption and secretion by virtue of their thickness, although some stratified surfaces are moderately permeable to water and other small molecules. The classification of stratified epithelia is based on the shape and structure of the surface cells, since cells of the basal layer are usually cuboidal in shape. Transitional epithelium is a stratified epithelium found only in the urinary outflow tract, with special features to make it waterproof as well as expansile.
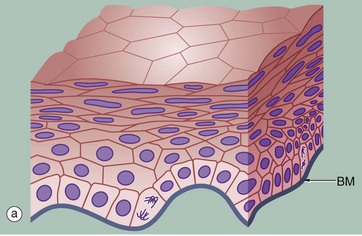
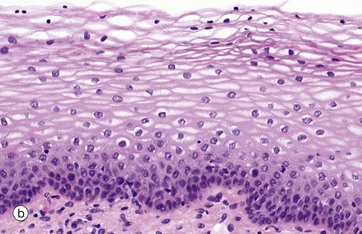
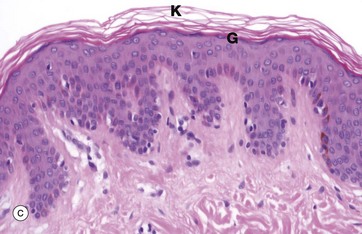
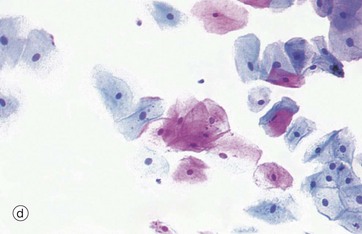
FIG. 5.6 Stratified squamous epithelium
(a) Diagram (b) H&E (HP) (c) H&E (MP) (d) Papanicolaou (HP)
Stratified squamous epithelium consists of a variable number of cell layers that exhibit maturation from a cuboidal basal layer to a flattened surface layer. The basal cells which are adherent to the underlying basement membrane include continuously dividing stem cells, their offspring migrating towards the surface where they are ultimately shed as anucleate squames. Stratified squamous epithelium is adapted to withstand abrasion, with plentiful cell junctions and a prominent intermediate filament (keratin) cytoskeleton. This type of epithelium lines the oral cavity, pharynx, oesophagus, anal canal, uterine cervix and vagina, sites which are subject to mechanical abrasion but which are kept moist by glandular secretions, such as the salivary glands of the mouth.
The epithelium in micrograph (b) is from the uterine cervix. Note the cuboidal basal layer and the maturation through the large polygonal cells of the intermediate layers to the flattened superficial squamous cells. The cytoplasm in these cells often appears clear due to the glycogen content.
Keratinising stratified squamous epithelium (c) constitutes the epithelial surface of the skin (the epidermis) and is adapted to withstand the constant abrasion and desiccation to which the body surface is exposed. During maturation, the epithelial cells accumulate keratin intermediate filaments which are cross-linked with proteins such as involucrin and loricrin in a process called keratinisation (or cornification). This results in the formation of a tough, non-living surface layer (stratum corneum) consisting of a compacted cross-linked keratin matrix K interspersed with specialised lipids (see Ch. 9). The underlying granular cell layer G consists of epithelial cells with extensive tight junctions, forming a waterproof barrier. The nuclei of the maturing epithelial cells become progressively condensed (pyknotic) and eventually disappear along with the other cellular organelles. Keratinisation may be induced in normally non-keratinising stratified squamous epithelium such as that of the oral cavity when exposed to excessive abrasion (e.g. poorly-fitting false teeth).
Micrograph (d) shows a smear made from normal cells scraped from the uterine cervix as it projects into the vagina. The degenerate, scaly superficial cells stain pink with this staining method, while the living cells from deeper layers stain blue. This is the basis of the well-known ‘Pap smear’ which examines cytological preparations of cervical cells for pre-cancerous changes.
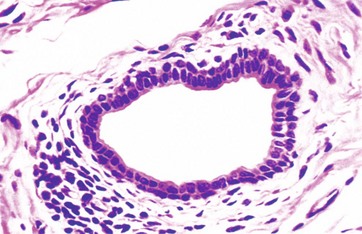
FIG. 5.7 Stratified cuboidal epithelium
H&E (HP)
Stratified cuboidal epithelium is a thin, stratified epithelium that usually consists of only two or three layers of cuboidal cells. This type of epithelium is usually confined to the lining of the larger excretory ducts of exocrine glands such as the salivary glands. Stratified cuboidal epithelium is probably not involved in significant absorptive or secretory activity but merely provides a more robust lining than would be afforded by a simple epithelium.
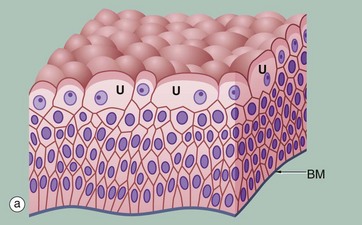
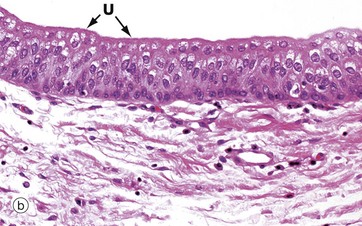
FIG. 5.8 Transitional epithelium
(a) Diagram (b) H&E (HP)
Transitional epithelium (or urothelium) is a form of stratified epithelium found only in the urinary tract in mammals, where it is highly specialised to accommodate a great degree of stretch and to withstand the toxicity of urine. This epithelial type is so named because it has some features intermediate (transitional) between stratified cuboidal and stratified squamous epithelia. In the non-distended state, transitional epithelium appears to be about four to five cell layers thick. The basal cells are roughly cuboidal, the intermediate cells are polygonal and the surface cells (umbrella or dome cells U) are large and rounded and may contain two nuclei. In the stretched state, transitional epithelium often appears only two or three cells thick (although the actual number of layers remains constant) and the intermediate and surface layers are extremely flattened.
Micrograph (b) shows the appearance of transitional epithelium from the lining of a non-distended bladder. The shape and apparent size of the basal and intermediate cells vary considerably depending on the degree of distension, but the cells of the surface layer usually retain characteristic features. Firstly, the surface umbrella cells are large and pale stained with a scalloped surface outline often overlapping two or more of the underlying cells. Secondly, the luminal surface of the cells appears thickened and more densely stained.
Membrane Specialisations of Epithelia
The plasma membranes of epithelial cells exhibit a variety of specialised structures that allow them to perform their function as a barrier with selective permeability. In some cases, the epithelial barrier is very impermeable, such as the transitional epithelium of the bladder, while other epithelia, such as the lining of the small intestine or the convoluted tubules of the kidney, promote movement of selected ions and molecules across the epithelium.
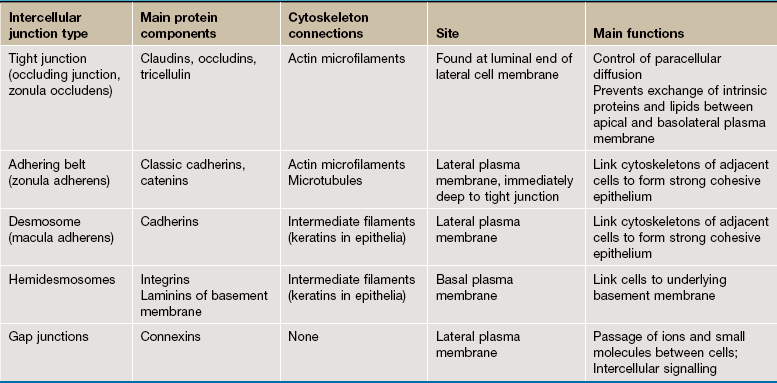
• Intercellular surfaces. The adjacent or lateral surfaces of epithelial cells are linked by cell junctions so that the epithelium forms a continuous, cohesive layer. Cell junctions also operate as communication channels governing such functions as growth and cell division. The various types of cell junction are composed of transmembrane proteins that interact with similar proteins on adjacent cells and are linked to intracellular structures on the cytoplasmic side. Adhering junctions and communicating junctions are not exclusive to epithelia and are also present in cardiac and visceral muscle where they appear to serve similar functions. The main features of intercellular junctions are summarised in Table 5.2.
• Luminal Surfaces. The luminal or apical surfaces of epithelial cells may incorporate three main types of specialisation: cilia, microvilli and stereocilia. Cilia are hair-like organelles that are easily resolved by light microscopy. In contrast, microvilli are shorter projections of the plasma membrane that cannot be individually resolved with the light microscope. A single cell may have thousands of microvilli or only a few. Stereocilia are extremely long microvilli usually found only singly or in small numbers.
• Basal Surfaces. The interface between all epithelia and underlying supporting tissues is marked by a non-cellular structure known as the basement membrane (see Ch. 4) that provides structural support for the epithelium and constitutes a selective barrier to the passage of materials between epithelium and supporting tissue. Hemidesmosomes, a variant of desmosomes, bind the base of the cell to the underlying basement membrane by linking to the cell's intermediate filament network.
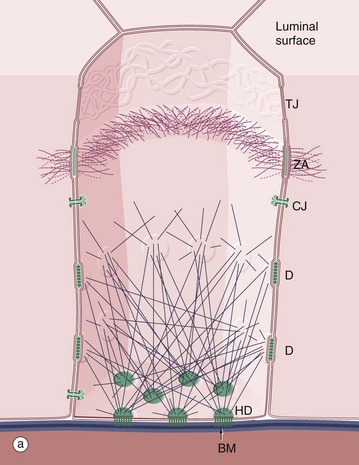
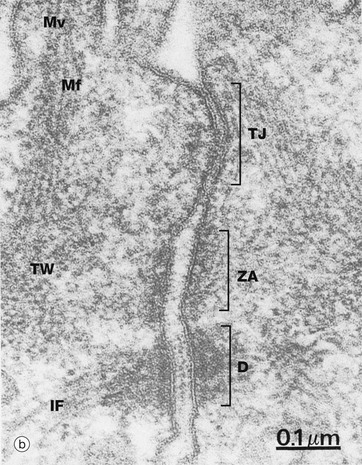
FIG. 5.9 Intercellular junctions
(a) Schematic diagram (b) Junctional complex, EM ×125 000
The diagram (a) outlines the three-dimensional organisation of the various intercellular junctions and their interaction with the cytoskeleton. In simple cuboidal and simple columnar epithelia, a junctional complex encircles each cell, sealing the intercellular spaces and holding the cells tightly together. Also illustrated are the basement membrane BM with associated hemidesmosomes HD.
As seen in micrograph (b) of intestinal columnar epithelium, the junctional complex at the luminal end of the lateral plasma membrane is made up of three components: a tight junction TJ (zonula occludens), an adhering belt (zonula adherens) ZA and a row of desmosomes D. The bases of microvilli Mv covering the surface of the small intestinal lining cells can be identified. Each microvillus contains a core of actin microfilaments Mf which insert into the terminal web TW (see Fig. 5.11). Actin microfilaments are anchored to the zonula adherens, and keratin intermediate filaments IF bind to desmosomes.
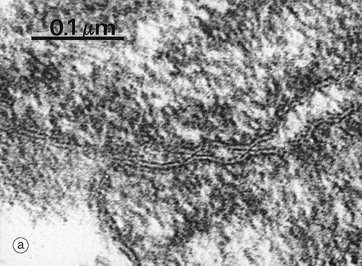
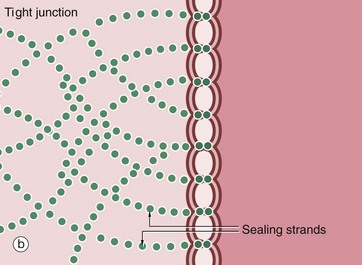
FIG. 5.10 Tight junctions
(a) EM ×190 000 (b) Schematic diagram
The tight junction (occluding junction or zonula occludens) forms a collar around each cell immediately beneath the apical surface, blocking passage of luminal contents between cells and also lateral movement of plasma membrane proteins and lipids in the plane of the membrane between the apical and the basolateral plasma membrane. As seen in this electron micrograph, the outer electron-dense layers of opposing cell membranes come extremely close together and, in places, appear to fuse completely. At the molecular level, members of the transmembrane protein families, the claudins and occludins, form tight links between adjacent cells. Claudins allow passage of selected cations between cells, acting as ion channels (the paracellular pathway). The presence of different claudin molecules in different epithelia explains the variability in permeability between epithelia. Tricelullin is also present at junctions where three cells meet. Multiple aggregates of tight junction proteins form a branching network known as sealing strands. On the cytoplasmic side of the plasma membrane, the tight junctions are linked to the actin cytoskeleton.
Structurally similar but discontinuous strips of tight junction, called fascia occludens, are found between the endothelial cells lining blood vessels, except in the vessels of the brain where they are of the continuous (zonula occludens) type.
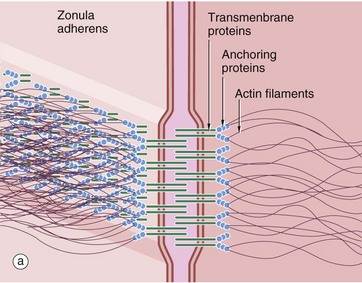
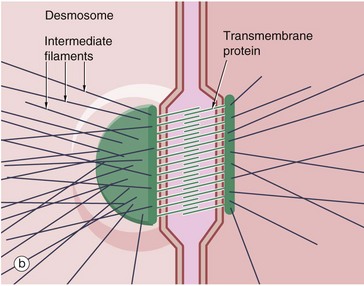
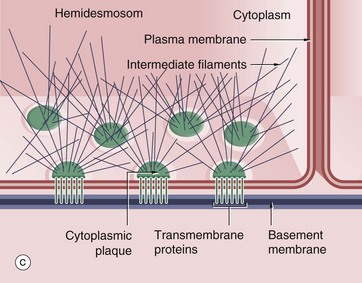
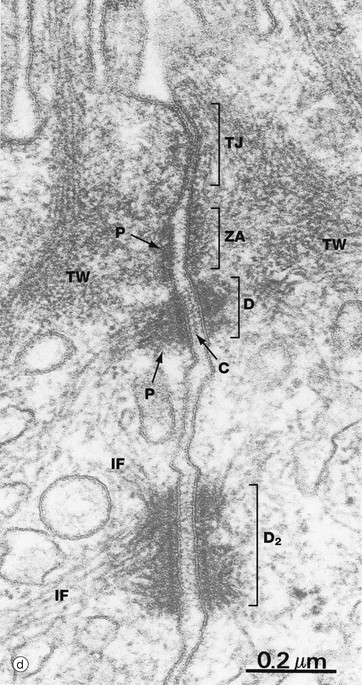
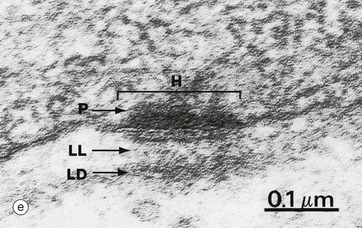
FIG. 5.11 Adhering junctions (caption continues opposite)
(a-c) Diagrams (d) EM ×95 000 (e) EM ×150 000
Adhering junctions provide anchorage points for cytoskeletal elements, linking the cytoskeletons of individual cells into a strong transcellular network. These junctions are of three types; the adhesion belt (zonula adherens) and the desmosome (macula adherens) link adjacent cells, and the hemidesmosome links the cell to the underlying basement membrane. The zonula adherens forms a single continuous band lying deep to the tight junction at the luminal end of the lateral plasma membranes of columnar epithelium. Deep to the zonula adherens there is ring of desmosomes, the third component of the junctional complex. Larger desmosomes are also scattered over the intercellular surfaces of all epithelial cells. Adhering junctions consist of three components: transmembrane proteins bind to similar proteins on adjacent cells (zonula adherens and desmosomes) or to extracellular matrix (hemidesmosomes), and anchoring proteins on the cytoplasmic side of the junction that link the transmembrane proteins to the third component, the cytoskeleton.
Zonula adherens (a) consist of cadherin transmembrane proteins linked to intracellular catenins and actin microfilaments. The cadherins (mainly E-cadherin) span the plasma membranes of the cells and bind to identical cadherins on adjacent cells. The cytoplasmic tails of the cadherins bind to anchor proteins (catenins, vinculin and α-actinin) which in turn bind to actin microfilaments. The intracellular component of the zonula adherens can be seen in (d) as a small electron-dense plaque P on the cytoplasmic side of the plasma membrane.
Desmosomes (b) employ different members of the cadherin superfamily as transmembrane proteins. The overlapping segments of the cadherin molecules in the intercellular space form an electron-dense line C. On the cytoplasmic side, anchoring proteins (desmoplakin and plakoglobin) bind to intermediate filaments IF forming a prominent electron-dense plaque P. Desmosome numbers are greatest in stratified squamous epithelia that have to withstand the greatest friction.
Hemidesmosomes (c) are modified desmosomes that are found at the basal surface of the cell. In this case, the transmembrane proteins are integrins, the extracellular components of which bind to extracellular laminins in the basement membrane (see Ch. 4). The intracellular component of the integrins binds to the anchor protein plectin and thus to the intermediate filament keratin. Again the intracellular component can be seen as an electron-dense plaque P.
Micrograph (d) from the intestinal lining illustrates a junctional complex comprising tight junction TJ, zonula adherens ZA and desmosome D. At a deeper level, a larger desmosome D2 is seen. Note the small electron-dense plaque P of the zonula adherens and the larger plaques P of the desmosomes. The electron-dense line created by overlapping cadherin molecules C is also visible in the desmosomes. The terminal web TW of the surface microvilli is also evident.
Micrograph (e) illustrates a hemidesmosome H along the basal plasma membrane of an epithelial cell. On the cytoplasmic aspect of the plasma membrane is the protein plaque P. The underlying lamina densa LD is thickened and more electron-dense than usual, as is the lamina lucida LL which contains an electron-dense line. This appearance is the result of binding of the extracellular component of the integrins to the laminins of the basement membrane.
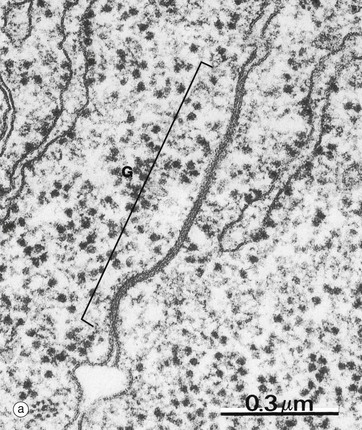
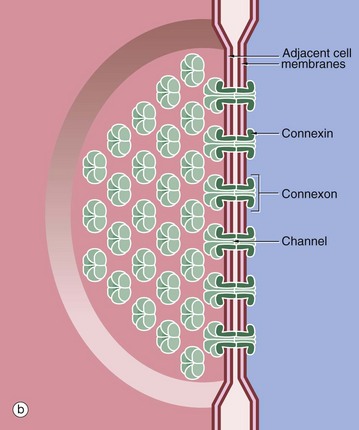
FIG. 5.12 Gap junctions
(a) EM ×80 000 (b) Diagram
Communicating or gap junctions are broad patches where adjacent plasma membranes are closely opposed, leaving a narrow intervening gap 2 to 4 nm in diameter. A gap junction G is demonstrated in micrograph (a) taken from intestinal epithelium.
As seen in diagram (b), each gap junction contains numerous transmembrane channels (connexons) that permit the passage of inorganic ions and other small molecules (approximately 1.5 nm in diameter) from the cytoplasm of one cell to another. Large molecules and negatively charged ions are denied access. Gap junctions are thought to be important in the control of growth, development, cell recognition and differentiation. Gap junctions also provide the means of electrical coupling of visceral and cardiac muscle cells, permitting synchronous contraction.
Each connexon is made up of six transmembrane proteins known as connexins. Each connexon aligns with a connexon of a neighbouring cell to form a direct channel between the two cells. There are more than 20 different connexin proteins in humans and these form specific connexons in different tissues with specificity for different molecules and ions. Connexons may be opened or closed depending on the intracellular concentration of calcium ions, the pH or on extracellular signals. For instance, the neurotransmitter dopamine closes gap junctions between certain nerve cells in the retina. A rise in intracellular calcium concentration, a feature of cell death, also closes connexons and this mechanism appears to provide a means of sealing off apoptotic cells and their potentially noxious contents from adjacent viable cells.
Communicating junctions are more numerous in embryonic epithelia, where they appear to be involved in exchange of chemical messengers, as well as in cell recognition, differentiation and control of cell position. They are also probably involved in the passage of nutrients from cells deep in the epithelium (adjacent to supporting tissues and blood vessels) to cells more remote from the nutritional supply.
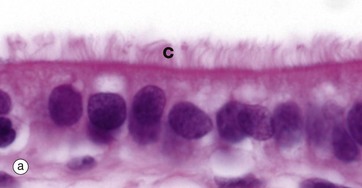
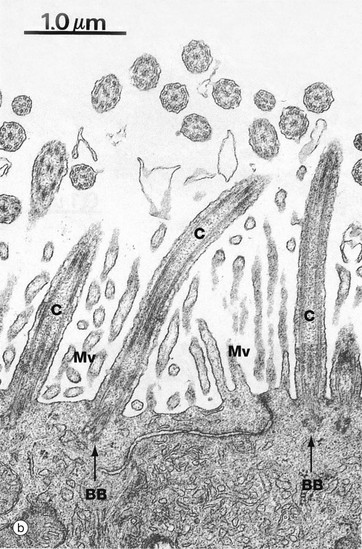
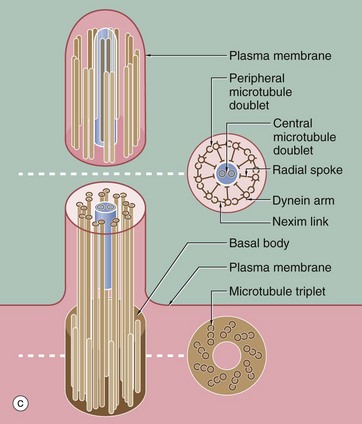
FIG. 5.13 Cilia
(a) H&E (HP) (b) EM ×20 000 (c) Schematic diagram
There are two types of cilia: motile and non-motile. Motile cilia project from the apical surfaces of certain epithelial cells, notably in the respiratory and female reproductive tracts. These cilia beat with a wave-like synchronous rhythm, propelling surface films of mucus or fluid in a consistent direction over the epithelial surface. In the airways, mucus traps debris from inspired air and the cilia move the mucus towards the throat where it is swallowed, thus keeping the airways clear. In the Fallopian tubes, ciliary action propels the ovum from the ovary to the uterus. In the ventricles of the brain, the cilia of ependymal cells move the cerebrospinal fluid. Cilia are up to 10 µm long (up to half the height of the cell). The flagellum of spermatozoa is a modified cilium (see Ch. 18).
A single epithelial cell may have up to 300 cilia, usually of similar length. A single non-motile cilium is found on most mammalian cells and is often called the primary cilium or sensory cilium; as suggested by the name, these cilia function as sensors for mechanical and chemical signals.
Micrographs (a) and (b) show ciliated cells from the Fallopian tube. Cilia C are readily visible at the apical surface of the cell by light microscopy. In micrograph (b), the proximal parts of three cilia C are seen in longitudinal section and, more superficially, the tips of a number of others otherwise lying outside the plane of section. Small surface microvilli Mv are seen between the cilia. Each cilium is bounded by plasma membrane and, as shown in (c), contains a central core called the axoneme. In motile cilia, the axoneme consists of 20 microtubules arranged as a central doublet surrounded by nine peripheral doublets. The peripheral doublets are linked by a protein called nexin and radial spokes extend towards the central doublet. In non-motile cilia, the central doublet, nexin links and radial spokes are absent. At the base of the cilium, the microtubule doublets are continuous with the basal body, consisting of nine microtubule triplets. Basal bodies have a very similar structure to the centriole. Each peripheral doublet of the cilium axoneme is continuous with the two inner microtubules of the corresponding triplet of the basal body. The basal bodies BB are easily seen in (b).
Each doublet consists of one complete microtubule closely applied to a second incomplete C-shaped tubule. From each complete tubule, pairs of ‘arms’ consisting of the protein dynein, a motor protein, extend towards the incomplete tubule of the adjacent doublet. Ciliary action results from bending of the doublets first in one direction and then in the other and is fuelled by dynein-catalysed conversion of ATP to ADP.
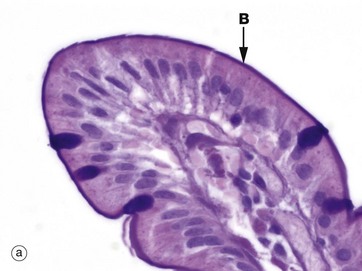
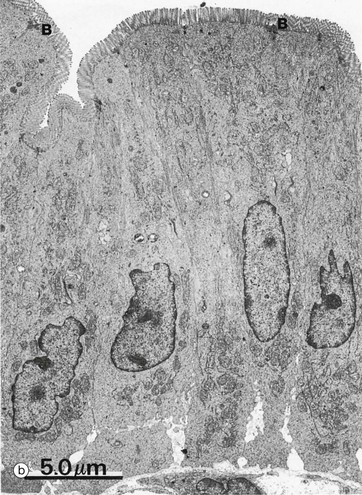
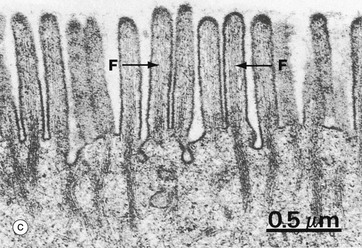
FIG. 5.14 Microvilli
(a) PAS (HP) (b) EM ×4000 (c) EM ×30 000
Microvilli are minute finger-like projections of the plasma membrane found in many epithelia, particularly those specialised for absorption, where their presence may increase the surface area as much as 30-fold. Most epithelia have only a small number of irregular microvilli. However, in the small intestine and proximal renal tubules, the epithelial cells have up to 3000 regular microvilli at the apical surface of each cell, and these can be seen with the light microscope as striated or brush borders (see also Figs 14.25 and 16.17). Micrographs (a) and (b) illustrate the typical features of microvilli constituting the brush border B of cells lining the small intestine. Microvilli are only 0.5 to 1 µm in length and are thus very short in relation to the size of the cell, in contrast with cilia. Individual microvilli are too small to be resolved by light microscopy, as can be seen in micrograph (a) which shows the tip of an intestinal villus. The microvilli can only be seen as a magenta-stained band on the surface of the epithelium. Note also the scattered goblet cells containing magenta-stained mucus which fills the apical part of the cell (see also Fig. 5.16).
As seen at higher magnification in micrograph (c), the cytoplasmic core of each microvillus contains parallel bundles of actin microfilaments F which insert into the terminal web, a specialisation of the actin cytoskeleton lying immediately beneath the cell surface. The actin filaments are tightly packed in a hexagonal array and held together by actin binding proteins such as villin. At the periphery of the cell, the terminal web is anchored to the zonula adherens (see Fig. 5.11). At the tip of the microvillus, the filaments attach to an electron-dense part of the plasma membrane. The microfilaments maintain stability of microvilli and may also mediate some contraction and elongation of the microvilli.
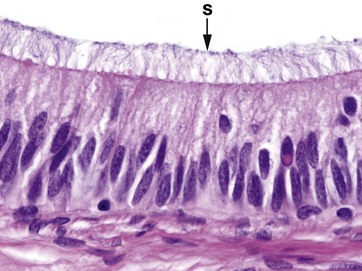
FIG. 5.15 Stereocilia
H&E (HP)
Stereocilia are long microvilli (1.5 to 5.5 µm), readily visible with light microscopy; they are found in the epididymis (shown in this micrograph, see also Ch. 18) and also in the middle ear (see Ch. 21). Originally these structures were thought to be an unusual form of cilia and were termed ‘stereocilia’; however, electron microscopy has shown that they have an actin microfilament skeleton similar to that of microvilli. Stereocilia S are thought to facilitate absorptive processes in the epididymis, but the reason for their unusual form is not known.
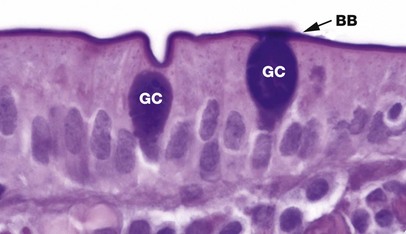
FIG. 5.16 Goblet cell
PAS (HP)
Goblet cells are modified columnar epithelial cells that synthesise and secrete mucus. They are scattered amongst the cells of many simple epithelial linings, particularly those of the respiratory and gastrointestinal tracts and are named for their resemblance to drinking goblets. Goblet cells are terminally differentiated and do not divide.
The distended apical cytoplasm contains a dense aggregation of mucigen granules which, when released by exocytosis, combine with water to form the viscid secretion called mucus. Mucigen is composed of a mixture of neutral and acidic glycoproteins (mucopolysaccharides) and therefore can be readily demonstrated by the PAS method, which stains carbohydrates magenta. The ‘stem’ of the goblet cell is occupied by a condensed basal nucleus and is crammed with other organelles involved in mucin synthesis.
In this example from the lining of the small intestine showing two goblet cells GC, note the tall columnar nature of the surrounding absorptive cells. The PAS-positive brush border BB is composed partly of membrane-bound mucins on the numerous microvilli, which characterise small intestine absorptive cells, and a surface layer of free mucin (see Figs 1.2 and 5.14).
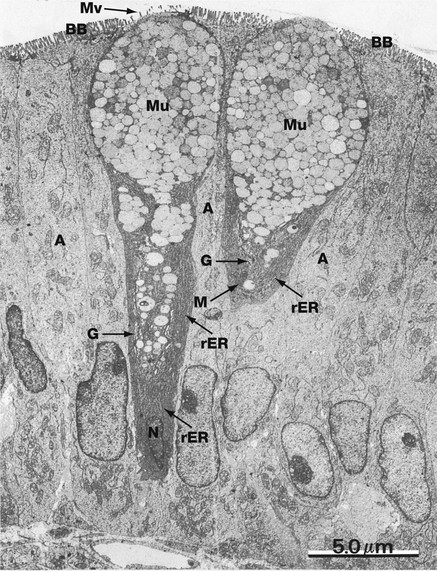
FIG. 5.17 Goblet cell
EM ×5000
This micrograph shows two goblet cells among columnar absorptive cells A (or enterocytes) of the small intestine. The nucleus of the goblet cell on the right is outside the plane of section, the nucleus N of the other being typically highly condensed (see also Fig. 14.23 showing a goblet cell in horizontal section). The cytoplasm is packed with rough endoplasmic reticulum rER and a few mitochondria M. A prominent Golgi apparatus G is found in the supranuclear region, although it is barely visible at this magnification.
The protein component of mucigen is synthesised by the rough endoplasmic reticulum and passed to the Golgi apparatus where it is combined with carbohydrate and packaged into membrane-bound secretory granules containing mucigen Mu. Goblet cells secrete at a steady basal rate and may be stimulated by local irritation to release their entire mucigen contents. Sparse microvilli Mv are seen at the surface of the goblet cell. Note the microvilli forming the brush border BB of the absorptive cells.
Mucus has a variety of functions. In the upper gastrointestinal tract, it protects the intestinal lining cells from autodigestion, whilst in the lower tract, it lubricates the passage of faeces. In the respiratory tract it protects the lining from drying, contributes to the humidification of inspired air and acts as a sticky surface trap for fine dust particles and microorganisms. Goblet cells also secrete various anti-microbial factors.
Exocrine Glands
As discussed earlier in this chapter, epithelial cells are the major component of all the glands of the body. The simplest glands can be easily recognised as an invagination of a surface epithelium. However, increasingly complex glandular structures have evolved over time, and some of the most elaborate have lost contact with the epithelial surface completely. Thus there are two major subdivisions in the classification of glands: exocrine glands, which release their contents onto an epithelial surface either directly or via a duct, and endocrine glands, which have no duct system but by releasing their secretions into the bloodstream can act on distant tissues. Endocrine glands are dealt with briefly at the end of this chapter and in much more detail in Ch. 17.
This section deals with exocrine glands, which vary from microscopic, such as sweat glands of the skin, to large solid organs such as the liver, weighing approximately 1.2 kg. The duct system of the liver ramifies throughout the solid gland and empties its secretions (bile) into the duodenum. In contrast, the simple tubular glands (crypts) of the large bowel (see also Ch. 14) consist entirely of the secretory component and empty directly onto the surface of the bowel. Indeed, the simplest exocrine glands of all are single mucus-secreting cells such as goblet cells.
Exocrine glands may be subclassified according to the morphology and the means of secretion of the gland.
Exocrine glands can be divided into the secretory component and the duct:
• The duct system may be unbranched (simple gland) or branched (compound gland).
• The secretory component may be tubular or acinar (roughly spherical).
• Both types of secretory component may also be coiled or branched.
• Almost any combination of duct and secretory component may occur (see Figs 5.18 to 5.25).
Secretion from exocrine glands may occur in one of three ways:
• Merocrine (eccrine) secretion involves the process of exocytosis and is the most common form of secretion; proteins are usually the major secretory product.
• Apocrine secretion involves the discharge of free, unbroken, membrane-bound vesicles containing secretory product; this is an unusual mode of secretion and applies to lipid secretory products in the breasts and some sweat glands.
• Holocrine secretion involves the discharge of whole secretory cells, with subsequent disintegration of the cells to release the secretory product. Holocrine secretion occurs principally in sebaceous glands.
In general, all glands have a continuous basal rate of secretion which is modulated by nervous and hormonal influences. The secretory portions of some exocrine glands are surrounded by contractile cells that lie between the secretory cells and the basement membrane. The contractile mechanism of these cells is similar to that of muscle cells and has given rise to the term myoepithelial cells, as these cells share characteristics of both epithelial and muscle cells.
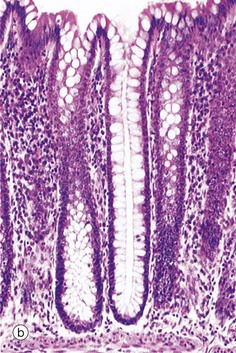
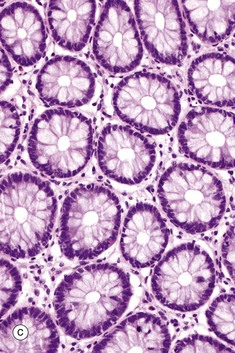
FIG. 5.18 Simple tubular glands
(a) Diagram (b) H&E (LP) (c) H&E (MP)
This example of simple tubular glands is taken from the large intestine. This type of gland has a single, straight tubular lumen into which the secretory products are discharged. In this example, secretory cells line the entire duct; the secretory cells are goblet cells. The glands are shown in longitudinal section in micrograph (b) and in transverse section in (c), which emphasizes the regular arrangement of the glands and the large number of mucus-secreting goblet cells in the epithelium. At other sites, mucus is secreted by columnar cells that do not have the classic goblet shape but nonetheless function in a similar manner.

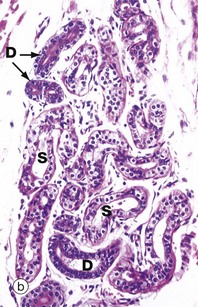
FIG. 5.19 Simple coiled tubular glands
(a) Diagram (b) H&E (LP)
Sweat glands are almost the only example of simple coiled tubular glands. Each consists of a single tube that is tightly coiled in three dimensions; portions of the gland are thus seen in various planes of section. Sweat glands have a terminal secretory portion S lined by simple cuboidal epithelium, which gives way to a non-secretory (excretory) duct D lined by stratified cuboidal epithelium.
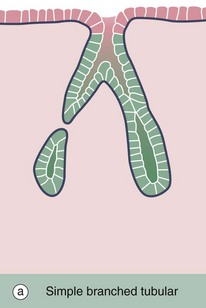
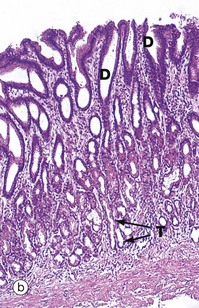
FIG. 5.20 Simple branched tubular glands
(a) Diagram (b) H&E (LP)
Simple branched tubular glands are found mainly in the stomach. The mucus-secreting glands of the pyloric part of the stomach are shown in this example. Each gland consists of several tubular secretory portions T, which converge onto a single unbranched duct D of wider diameter. Mucus-secreting cells also line the duct but, unlike those of the large intestine (see Fig. 5.18), these mucus cells do not have a goblet shape.
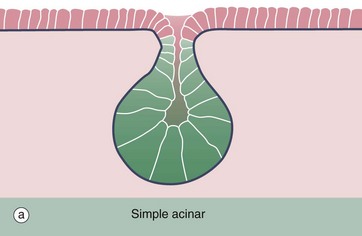
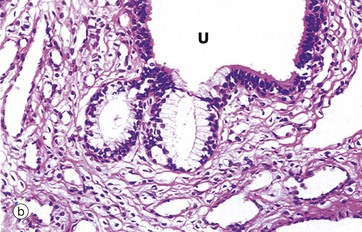
FIG. 5.21 Simple acinar glands
(a) Diagram (b) H&E (LP)
Simple acinar glands occur in the form of pockets in epithelial surfaces and are lined by secretory cells. In this example of the mucus-secreting glands of the penile urethra, the secretory cells are pale stained compared to the non-secretory cells lining the urethra U. Note that the term acinus can be used to describe any rounded exocrine secretory unit.
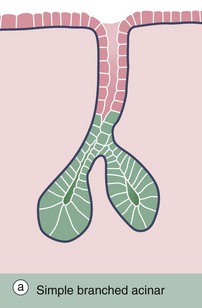
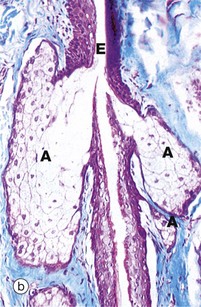
FIG. 5.22 Simple branched acinar gland
(a) Diagram (b) Masson trichrome (LP)
Sebaceous glands provide a good example of simple branched acinar glands. Each gland consists of several secretory acini A that empty into a single excretory duct; the excretory duct E is formed by the stratified epithelium surrounding the hair shaft. The mode of secretion of sebaceous glands is holocrine, i.e. the secretory product, sebum, accumulates within the secretory cells and is discharged by degeneration of the cells.
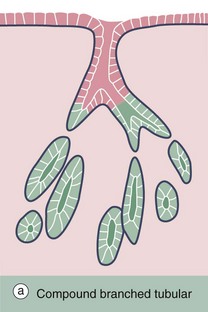
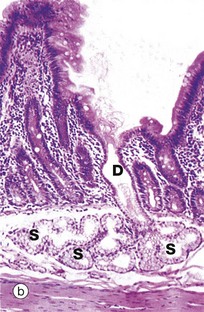
FIG. 5.23 Compound branched tubular gland
(a) Diagram (b) H&E (LP)
Brunner's glands of the duodenum, as shown in this example, are described as compound branched tubular glands. Although difficult to visualise here, the duct system D is branched, thus defining the glands as compound glands, and the secretory portions S have a tubular form which is branched and coiled.
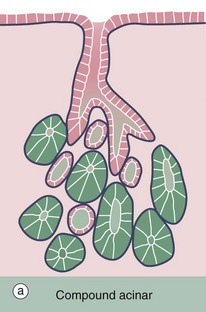
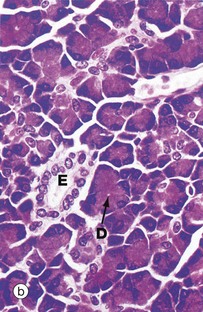
FIG. 5.24 Compound acinar gland
(a) Diagram (b) Chrome alum haematoxylin/phloxine (MP)
Compound acinar glands are those in which the secretory units are acinar in form and drain into a branched duct system. The pancreas shown in this micrograph consists of numerous acini, each of which drains into a minute duct. These minute ducts D, which are just discernible in the centre of some acini, drain into a system of branched excretory ducts E of increasing diameter which are lined by simple cuboidal epithelium.
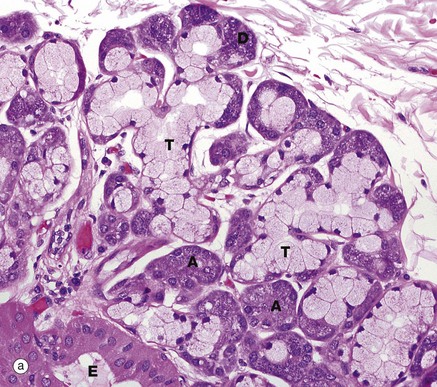
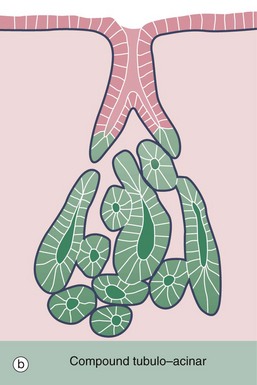
FIG. 5.25 Compound tubulo-acinar gland
H&E (MP)
Compound tubulo-acinar glands have three types of secretory units: branched tubular, branched acinar and branched tubular with acinar end-pieces called demilunes. The submandibular salivary gland shown here is the classic example. It contains two types of secretory cells, mucus-secreting cells and serous cells; the former are pale but the latter, which have a protein-rich secretion (digestive enzymes), stain strongly due to their prominent content of rough endoplasmic reticulum. Generally, the mucous cells form tubular components T, whereas the serous cells form acinar components A and demilunes D. Part of an excretory duct E is also seen in the lower left corner of the micrograph.
Endocrine Glands
As described earlier, endocrine (or ductless) glands release their secretions directly into the bloodstream rather than via a duct. Endocrine glands are the source of many of the body's chemical messengers, hormones, that act at a distance from their source. For example, insulin secreted by the pancreas (see Ch. 17) acts on muscle and adipose tissue throughout the body to control the metabolism of glucose. Other hormones may act only on a single tissue; thus thyroid-stimulating hormone (TSH) secreted by the pituitary gland is widely disseminated in the blood, but only the thyroid gland has the necessary receptors to respond.
Endocrine glands are very varied in their size, location and appearance and are described in more detail in Ch. 17.
• Many are solid organs, but some consist of widely distributed single cells.
• Most endocrine glands release more than one hormone product.
• Several endocrine glands consist of more than one type of secretory cell.
• The pancreas is both an endocrine and an exocrine gland because it contains nests of endocrine cells (islets of Langerhans) embedded in a large exocrine gland, the exocrine pancreas (see Ch. 15).
• In general, secretion of hormones by endocrine glands is controlled by metabolic factors (e.g. blood glucose levels), the secretion of other hormones (e.g. TSH controls secretion of thyroxine) and the nervous system (e.g. secretion of adrenaline by the adrenal medulla) or a mixture of all these factors.
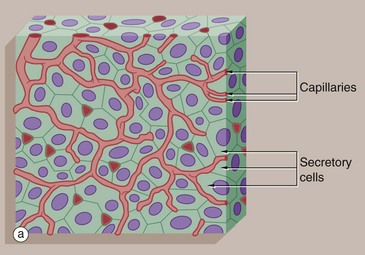
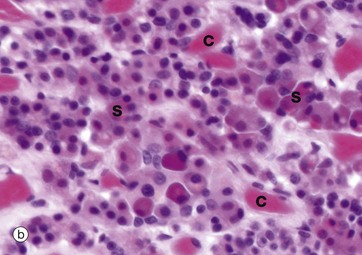
FIG. 5.26 Endocrine gland
(a) Diagram (b) H&E (MP)
Most endocrine glands consist of clusters or cords of secretory cells surrounded by a rich network of small blood vessels. Each cluster of endocrine cells is surrounded by a basement membrane, reflecting its epithelial origin. Endocrine cells release hormones into the intercellular spaces, from which they diffuse rapidly into surrounding blood vessels and from there throughout the body.
Micrograph (b) of the anterior pituitary gland shows the typical features of endocrine glands. The secretory cells S are arranged in cords and clusters and are surrounded by delicate supporting tissue containing a rich network of broad capillaries C. The basement membrane surrounding each group of endocrine cells is not visible at this magnification. Like many other endocrine glands, the secretory cells of the pituitary are of several different types; in this case, the majority are acidophilic (red stained), while some stain blue (basophilic) and some stain very little (chromophobes).
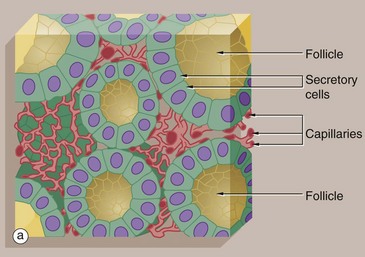
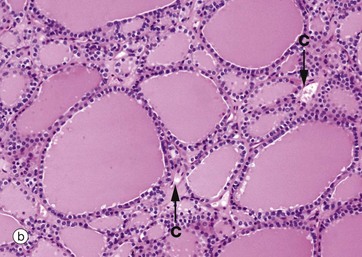
FIG. 5.27 Follicular endocrine gland
(a) Diagram (b) H&E (LP)
The thyroid gland is an unusual endocrine gland in that it stores hormone (thyroxine) within roughly spherical cavities enclosed by the secretory cells; these units are called follicles. Secretion of stored hormone involves reabsorption of hormone from the follicular lumen, release into the surrounding interstitial spaces, and then diffusion into the rich capillary network that embraces each follicle.
Micrograph (b) shows typical thyroid follicles F. The secretory cells lining the follicles are of flattened cuboidal shape, although they may vary from low cuboidal to tall columnar depending on the state of activity of the gland. Stored thyroxine is bound to a glycoprotein (thyroglobulin), which is strongly eosinophilic and fills the centre of the follicle. The relatively sparse interfollicular supporting tissue is mainly occupied by capillaries C (see also Fig. 17.6).