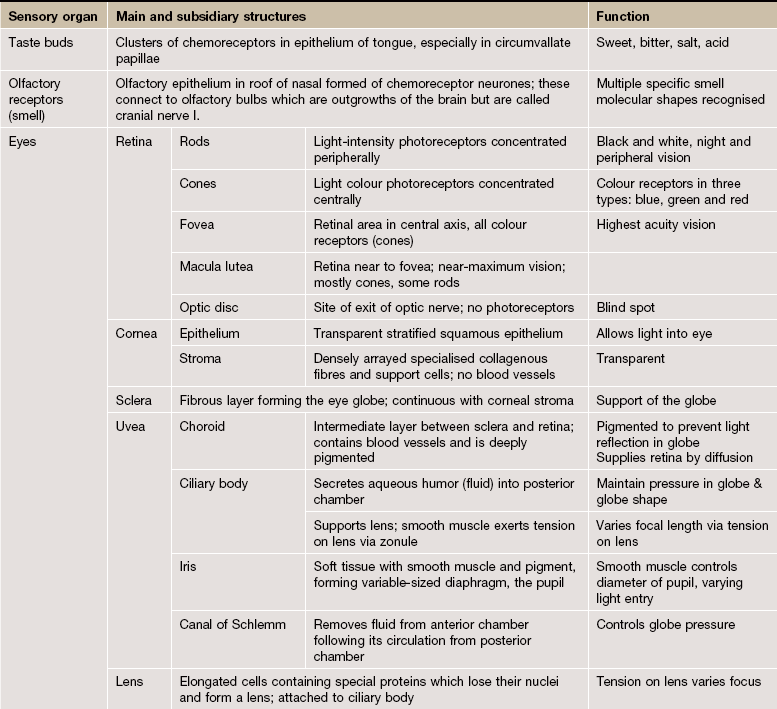
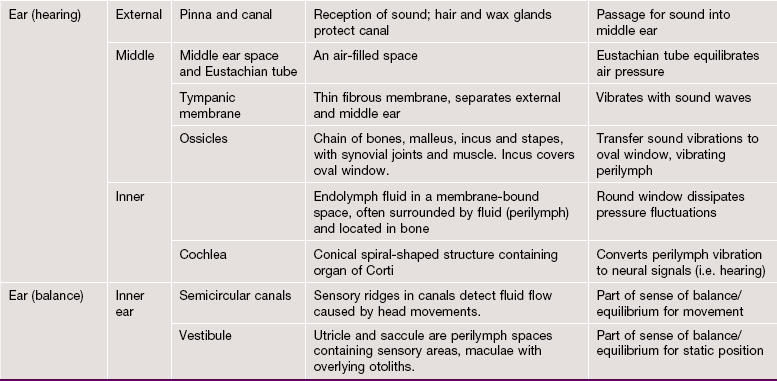
Special sense organs
Introduction
The organs of special sense are sophisticated sensory structures in which the specific neural receptors are incorporated in a non-neural structure which enhances and refines the reception of incoming stimuli. The eye and the audiovestibular apparatus of the ear are the main special sense organs, but the gustatory (taste) and olfactory (smell) receptors are usually also included in this category.
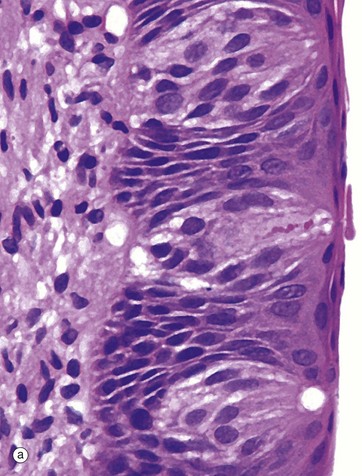
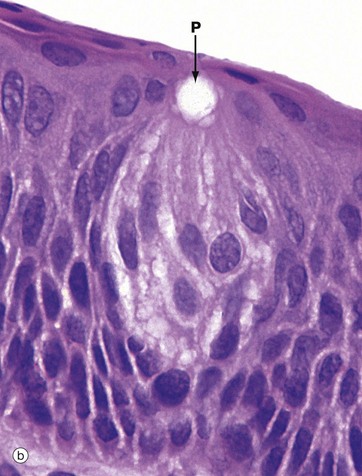
FIG. 21.1 Taste buds
(a) H&E (MP) (b) H&E (HP)
Taste buds, the chemoreceptors for the sense of taste (gustation), are in humans mainly located in the epithelium of the circumvallate papillae of the tongue (see Fig. 13.12), although they are also found scattered in other parts of the tongue, palate, pharynx and epiglottis. In the circumvallate papillae, taste buds face into the deep troughs surrounding the papillae. Serous glands called the glands of von Ebner secrete a serous fluid into the troughs to act as a solvent for taste-provoking substances. The human tongue has approximately 3000 taste buds.
The taste bud is a barrel-shaped organ, occupying the full thickness of the epithelium and opening at the surface via the taste pore P. Each taste bud contains about 50 long, spindle-shaped cells which extend from the basement membrane to the taste pore. Classically, two types of cell are described in the taste bud: light gustatory cells and dark supporting or sustentacular cells. A third cell type, the basal cell, is now generally recognised and may constitute the precursor of one or both of the other cell types. Both gustatory and sustentacular cells have long microvilli extending into the taste pore, which contains a glycoprotein substance, thought to be secreted by the sustentacular cells.
Ultrastructural studies have shown that non-myelinated nerve fibres are associated with both cell types, but there appears to be a more intimate synapse-like relationship between the nerve fibres and the gustatory cells. Although the gustatory cells are thought to be the taste receptors, the sustentacular cells may also serve some receptor function. Like the oral epithelium, all the cells of the taste bud, which represent highly specialised epithelial cells, are renewed continuously, although the gustatory and sustentacular cells are replaced at different rates.
Four taste modalities are recognised: sweet, bitter, acid and salt. Each modality tends to be principally perceived in a specific region of the tongue; however, no structural differences have been demonstrated between taste buds from different areas. The sensations of taste and smell are closely associated, and loss of olfactory sense is accompanied by diminished gustatory perception.
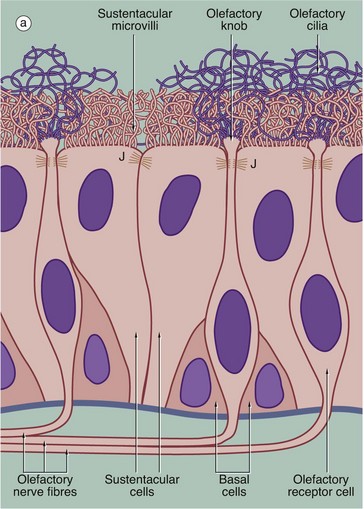
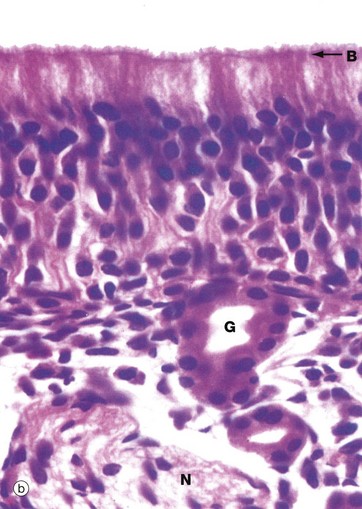
FIG. 21.2 Olfactory receptors
(a) Schematic diagram (b) H&E (HP)
The receptors for the sense of smell are located in a modified form of respiratory epithelium called olfactory epithelium in the nasal cavity; although extensive in some mammals such as the dog, the olfactory epithelium is restricted to a small area in the roof of the nasal cavity in humans. The olfactory epithelium is very tall pseudostratified columnar in form and contains cells of three types: olfactory receptor cells, sustentacular (supporting epithelial) cells and basal epithelial cells.
The olfactory receptor cells are true bipolar neurones (see Fig. 7.2), the cell bodies of which are located in the middle stratum of the olfactory epithelium. A single dendritic process extends from the cell body to the free surface where it terminates as a small swelling, the olfactory knob, which gives rise to about a dozen extremely long modified cilia. These cilia, or olfactory hairs, contain the usual 9 plus 2 arrangement of microtubules in their proximal portion but become thinner distally where they contain variable numbers of single microtubules in different species. The cilia are non-motile and lie flattened against the epithelial surface in the surface mucous layer. The cilia are the sites of interaction between odoriferous substances and the receptor cells. At the basal aspect, each receptor cell gives rise to a single fine non-myelinated axon which penetrates the basement membrane to join the axons of other receptor cells. The bundles of axons pass via about 20 small holes on each side of the cribriform plate of the ethmoid bone to reach the olfactory bulbs of the forebrain where they synapse with second-order sensory neurones.
The supporting or sustentacular cells are elongated with their tapered bases resting on the basement membrane. Many long microvilli extend from their luminal surfaces to form a tangled mat with the cilia of the receptor cells. At the luminal surface, the plasma membranes of the sustentacular and receptor cells are bound together by typical junctional complexes J. The functions of the sustentacular cells are poorly understood, but they probably provide mechanical and physiological support for the receptor cells. The basal cells are small conical cells which appear to be stem cells for both olfactory and sustentacular cells.
In histological section, it is difficult to distinguish individual cell types within the olfactory epithelium; however, the nuclei of sustentacular cells occupy the uppermost stratum, those of the receptor cells the middle stratum and those of the basal cells lie close to the basement membrane. Note the terminal bar B at the luminal surface, representing junctional complexes; note also the fuzzy surface contour representing the tangled meshwork of microvilli and cilia on the surface.
The olfactory epithelium is supported by loose vascular tissue containing bundles of afferent nerve fibres N and numerous serous glands called Bowman's glands G which produce the watery surface secretions in which odoriferous substances are dissolved.
The Eye
The eye is the highly specialised organ of photoreception, a process which involves the conversion of light energy into nerve action potentials. The photoreceptors are modified dendrites of two types of nerve cells, rod cells and cone cells. The rods are integrated into a system which is receptive to light of differing intensity; this is perceived in a form analogous to a black and white photographic image. The cones are of three functional types, receptive to the colours blue, green and red, and constitute a system by which coloured images are seen. The rod and cone receptors and a system of integrating neurones are located in the inner layer of the eye, the retina. The remaining structures of the eye serve to support the retina or to focus images of the visual world upon the retina.
In addition, several accessory structures, namely the eyelids, lacrimal gland and conjunctiva, protect the eye from external damage.
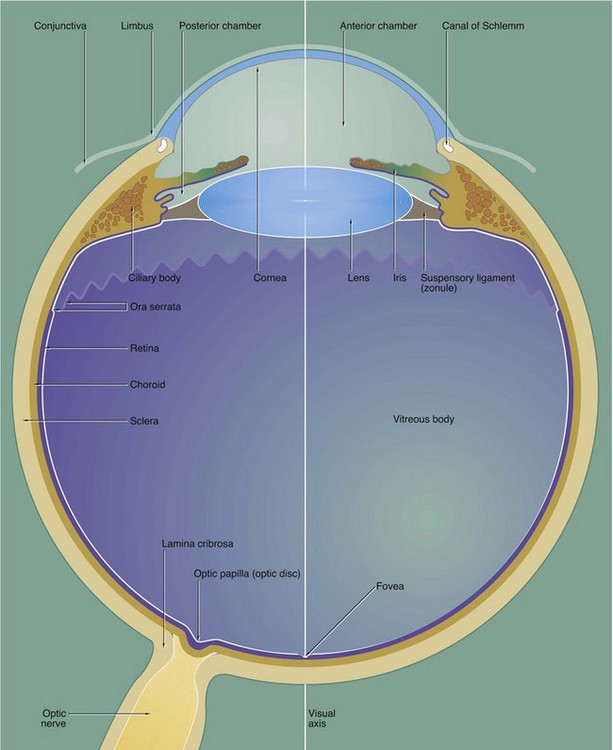
FIG. 21.3 The eye (illustration opposite)
The eye is made up of three basic layers: the outer corneo-scleral layer, the intermediate uveal layer (uveal tract) and the inner retinal layer.
Corneo-scleral layer
The corneo-scleral layer forms a tough fibroelastic capsule which supports the eye. The posterior five-sixths, the sclera, is opaque and provides insertion for the extraocular muscles.
The anterior one-sixth, the cornea, is transparent and has a smaller radius of curvature than the sclera. The cornea is the principal refracting medium of the eye and roughly focuses an image onto the retina; the focusing power of the cornea depends mainly on the radius of curvature of its external surface. The corneo-scleral junction is known as the limbus and is marked internally and externally by a shallow depression. Running from the junction of the cornea and limbus, the surface of the eye is covered by conjunctiva which is reflected into the eyelids.
Uveal layer
The middle layer, the uvea or uveal tract, is a highly vascular layer which is made up of three components: the choroid, the ciliary body and the iris. The choroid lies between the sclera and retina in the posterior five-sixths of the eye. It provides support for the retina and is heavily pigmented, thus absorbing light which has passed through the retina. Anteriorly, the choroid merges with the ciliary body, which is a circumferential thickening of the uvea lying beneath the limbus.
The ciliary body surrounds the coronal equator of the lens and is attached to it by the suspensory ligament or zonule. The lens is a biconvex transparent structure, the shape of which can be varied to provide fine focus of the corneal image upon the retina. The ciliary body contains smooth muscle, the tone of which controls the shape of the lens via the suspensory ligament. The lens, suspensory ligament and ciliary body divide the eye into a large compartment containing a thick gel called the vitreous body and a compartment part in front containing a watery fluid called the aqueous humor.
The iris, the third component of the uvea, forms a diaphragm extending in front of the lens from the ciliary body, so as to incompletely divide the anterior compartment into two chambers; these are known by the terms anterior and posterior chamber. The highly pigmented iris acts as an adjustable diaphragm which regulates the amount of light reaching the retina. The aperture of the iris is called the pupil.
The anterior and posterior chambers contain the aqueous humor, which is secreted into the posterior chamber by the ciliary body and circulated through the pupil to drain into a canal at the angle of the anterior chamber, the canal of Schlemm. The aqueous humor is a source of nutrients for the non-vascular lens and cornea and acts as an optical medium which is non-refractive with respect to the cornea. The pressure of aqueous humor maintains the shape of the cornea.
The large posterior compartment of the eye contains a specialised connective tissue largely composed of a transparent gel known as the vitreous body. The vitreous body supports the lens and retina from within, as well as providing an optical medium which is non-refractive with respect to the lens. In life, the vitreous body contains a canal which extends from the exit of the optic nerve to the posterior surface of the lens; this hyaloid canal represents the course of the hyaloid artery which supplies the vitreous body during embryological development. The vitreous body and hyaloid canal are rarely preserved in histological preparations.
Retinal layer
The photosensitive retina forms the inner lining of most of the posterior compartment of the eye and terminates along a scalloped line, the ora serrata, behind the ciliary body. Anterior to the ora serrata, the retinal layer continues as a non-photosensitive epithelial layer which lines the ciliary body and the posterior surface of the iris.
The visual axis of the eye passes through a depression in the retina called the fovea which is surrounded by a yellow-pigmented zone, the macula lutea. The fovea is the area of greatest visual acuity.
Afferent nerve fibres from the retina converge to form the optic nerve which leaves the eye through a part of the sclera known as the lamina cribrosa. The retina overlying the lamina cribrosa, the optic papilla (optic disc), is devoid of photoreceptors and thus represents a blind spot.
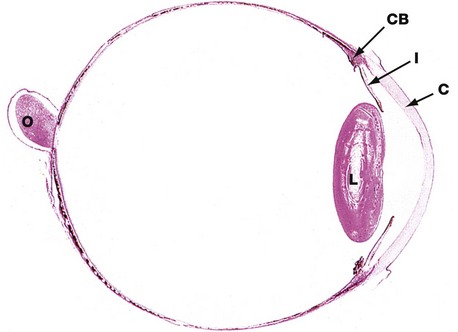
FIG. 21.4 Eye, monkey
H&E (LP)
This horizontal section shows the relative sizes of the components of the eye. At this magnification, the three layers making up the wall of the globe are not readily distinguishable although, in the wall of the posterior compartment, the middle layer (choroid) is recognisable by its high content of pigment.
The other uveal structures, the ciliary body CB and iris I, are readily visible. The lens L has been artefactually distorted during preparation, and the suspensory ligament by which it is attached to the ciliary body is not preserved. Note the relative thickness of the cornea C.
The optic nerve O is seen to penetrate the sclera medial to the visual axis; the fovea is not present in this plane of section.
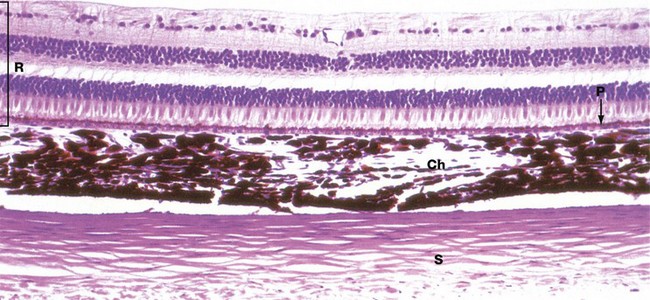
FIG. 21.5 Wall of the eye
H&E (HP)
The three layers of the wall of the eye are illustrated in this micrograph.
The inner photosensitive retina is a multilayered structure, the outermost limit of which is defined by a layer of pigmented epithelial cells, the pigment epithelium P.
The choroid Ch is a layer of loose vascular supporting tissue lying between the sclera S externally and the retina R internally. The choroid and retina are separated by a membrane known as Bruch's membrane which is composed of the basement membranes of the pigmented epithelium of the retina and the endothelium of the choroid capillaries plus intervening layers of collagen and elastin fibres. The blood supply of the uveal layer of the eye is provided by branches of the ophthalmic artery, which penetrates through the sclera. Larger vessels predominate in the superficial aspect of the choroid, with a rich capillary plexus in the deeper aspect providing nourishment for the outer layers of the retina by diffusion across Bruch's membrane. The choroid contains numerous large, heavily pigmented melanocytes which confer the dense pigmentation characteristic of the choroid. The pigment absorbs light rays passing through the retina and prevents interference due to light reflection.
The sclera consists of dense fibroelastic tissue, the fibres of which are arranged in bundles parallel to the surface. This layer contains little ground substance and few fibroblasts. The sclera varies in thickness, being thickest posteriorly and thinnest at the coronal equator of the globe.
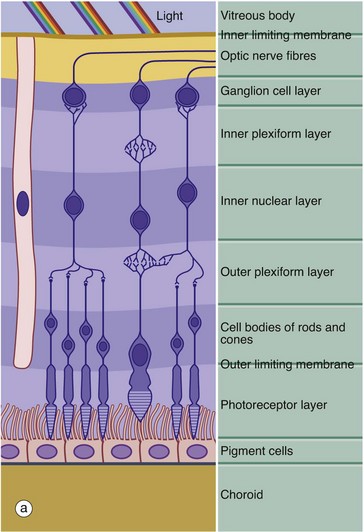
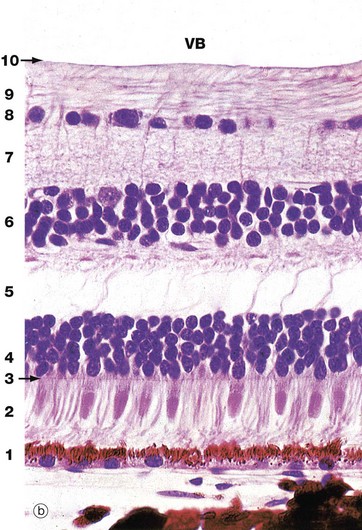
FIG. 21.6 Retina
(a) Schematic diagram (b) H&E (HP)
The retina is made up of three cell types: neurones, pigmented epithelial cells and neurone support cells. The neurones are divided into three functional groups: photoreceptor cells (rod cells and cone cells), the cells of afferent fibres passing in the optic nerve and a group of neurones interposed between the first two types which integrate sensory input from the photoreceptors before transmission to the cerebral cortex. The integrating neurones are further subdivided into three types: bipolar cells, horizontal cells and amacrine cells.
Histologically, the retina is traditionally divided into 10 distinct histological layers, as shown in the micrograph; the distribution of the different cell types being illustrated in a highly schematic manner in the diagram.
The outermost layer (1) consists of the pigmented epithelial cells forming a single layer resting on Bruch's membrane, which separates them from the choroid. The next layer is the photoreceptor layer, made up of the rod and cone processes (2) with a thin eosinophilic structure known as the outer limiting membrane (3) separating them from a layer of densely packed nuclei described as the outer nuclear layer (4). The outer nuclear layer contains the cell bodies of the rod and cone photoreceptors. The almost featureless layer next to this is known as the outer plexiform layer (5) and contains synaptic connections between the short axons of the photoreceptor cells and integrating neurones, the cell bodies of which lie in the inner nuclear layer (6). In the inner plexiform layer (7), the integrating neurones make synaptic connections with dendrites of neurones whose axons form the optic tract. The cell bodies of the optic tract neurones (retinal ganglion cells) comprise the ganglion cell layer (8). Internal to this is the layer of afferent fibres (9) passing towards the optic disc to form the optic nerve. Finally, the inner limiting membrane (10) demarcates the innermost aspect of the retina from the vitreous body VB. Note in the diagram that only bipolar cells are represented in the integrating cell layer; this layer also contains the cell bodies of the horizontal and amacrine cells as illustrated in Fig. 21.8. Note that light impinging on the retina passes through many layers before reaching the photoreceptor cells.
Towards the left of the diagram there is an extremely elongated support cell extending between inner and outer limiting membranes; it has its nucleus in the same layer as the integrating neurones, the inner nuclear layer. These cells, known as Müller cells, are analogous to the neuroglia of the central nervous system and have long cytoplasmic processes which embrace and sometimes even encircle the retinal neurones, filling all the intervening spaces. Müller cells provide structural support and may also mediate the transfer of essential metabolites such as glucose to the retinal neurones.
The outer limiting membrane is not a true membrane but merely represents the line of intercellular junctions between Müller cells and the photoreceptor cells (shown diagrammatically in Fig. 21.7). In contrast, the inner limiting membrane represents the basement membrane of the Müller cells resting on the vitreous body.
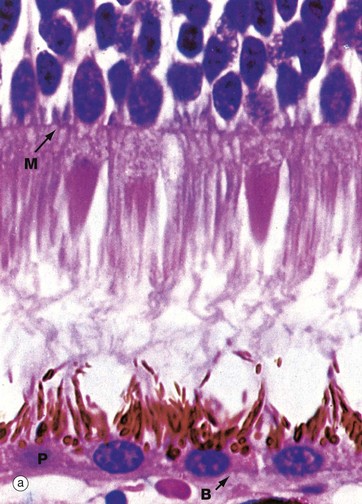
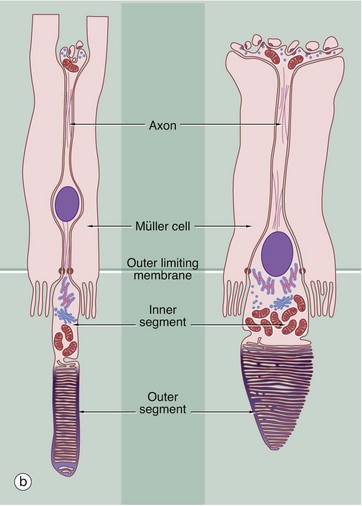
FIG. 21.7 Retinal photoreceptors
(a) H&E (HP) (b) Schematic diagram
The rod and cone photoreceptor layer of the retina is shown at very high magnification in micrograph (a), the cell bodies of the rod and cone cells lying internal to the outer limiting membrane M. Peripherally, the rods and cones mingle with long microvilli extending from the pigmented epithelial cells P.
As shown in the diagram (b), the rod photoreceptors are long slender bipolar cells, the single dendrite of each cell extending beyond the outer limiting membrane as the rod proper. The rod proper consists of inner and outer segments connected by a thin eccentric strand of cytoplasm containing nine microtubule doublets, similar to those of a cilium but without the inner pair of microtubules. The inner segment contains a prominent Golgi apparatus and many mitochondria. The outer segment has a regular cylindrical shape and contains a stack of flattened membranous discs which incorporate the pigment rhodopsin (visual purple). The membranous discs are continuously shed from the end of each rod and phagocytosed by the pigmented epithelial cells. The discs are continuously replaced from the inner part of the outer segment. In essence, the transduction process involves the interaction of light with rhodopsin molecules which promotes a conformational change in the rhodopsin molecule, thus initiating an action potential. The action potential then passes inwards along the dendrite and axon to the layer of integrating neurones.
Cones are similar in basic structure to the rods, but they differ in several details. The outer segment of the cone is a long conical structure, about two-thirds the length of a rod, and contains a similar number of even more flattened membranous discs. Unlike the situation in the rods, however, the disc membrane is continuous with the plasma membrane so that, on one side, the spaces between the discs are continuous with the extracellular environment. The discs are not shed, although the tips of the cones are invested by processes of pigmented epithelial cells. The cones contain visual pigments similar to rhodopsin, receptive to blue, green and red light, and the mechanism of transduction is probably similar. The bodies of the cone cells are generally continuous with the inner segment of the cone proper, without an intervening dendritic process, and the nuclei of cone cells thus form a row immediately deep to the outer limiting membrane.
As seen in micrograph (a), the pigmented epithelial cells are cuboidal in shape with the nuclei located basally towards Bruch's membrane B. Apically, the cells are crammed with melanin granules, numerous mitochondria and lipofuscin, a residual product of phagocytosis (see Fig. 1.25). The pigmented cell microvilli, which are 5 to 7 µm long, extend between the photoreceptors and, with electron microscopy, are seen to contain membranous lamellae similar to those in the rod outer segments; these appear to disintegrate as they pass deeper into the pigmented cells. In addition to phagocytosis, the pigmented epithelial cells provide structural and metabolic support for the rods and cones and also absorb light, thus preventing back reflection.
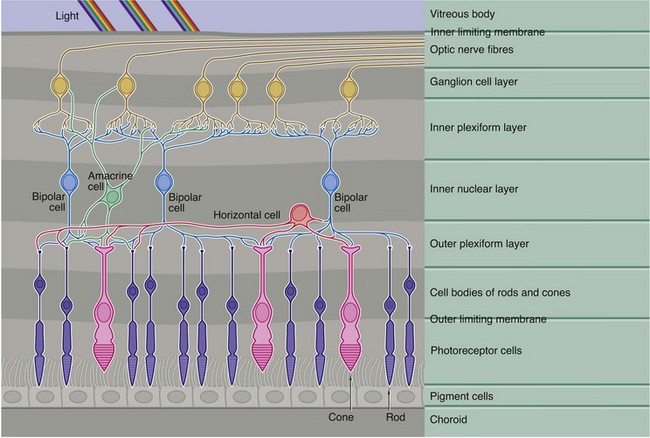
FIG. 21.8 Neuronal interconnections in the retina
This diagram demonstrates the basic pattern of neuronal interconnections between the photoreceptor cells and the afferent neurones of the optic tract. The interneurones consist of three basic cell types, bipolar cells, horizontal cells and amacrine cells, their cell bodies all being located in the inner nuclear layer (along with those of the supporting Müller cells).
Bipolar cells, the most numerous of the integrating neurones, in general make direct connections between one or more photoreceptors and one or more optic tract neurones, as well as with horizontal and amacrine cells. Horizontal cells have several short processes and one long process, the terminal branches of each making lateral connections between adjacent and more distant rods and cones in the outer plexiform layer. Horizontal cells also synapse with the dendrites of bipolar cells. The amacrine cells have numerous dendrites which make connections with bipolar and optic tract neurones in the inner plexiform layer, as well as making occasional feedback connections with photoreceptors in the outer plexiform layer.
As seen in Fig. 21.7(b) opposite, the axons of the rod photoreceptors terminate in spherical processes into which are invaginated their small number of synaptic connections. In contrast, the cone photoreceptors have a flattened pedicle which accommodates hundreds of intercellular contacts.
In all, there are more than 100 million rods and 6 million cones. The cones are particularly dense in the macula and the immediately surrounding area and, in the fovea itself, the photoreceptors are almost exclusively cones. The density of both rods and cones diminishes towards the retinal periphery. The foveal cones have an almost one-to-one relationship with optic tract neurones, giving maximal visual discrimination. There are only about 1 million optic tract neurones and, the more peripheral the photoreceptors, the greater the number of photoreceptors synapsing with each optic tract neurone. This is consistent with the main function of the more peripheral receptors (predominantly rods), which is for determination of light and dark, rather than fine two-point discrimination.
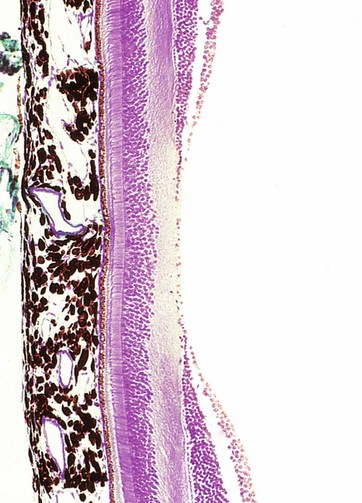
FIG. 21.9 Fovea
Masson trichrome (HP)
The fovea is a conical depression in the retina, corresponding to the point where the visual axis of the cornea and lens meets the retina and lying about 4 mm lateral and slightly inferior to the exit of the optic nerve fibres at the optic disc. Consequently, the fovea is the area subject to the least refractory distortion. To complement this, the foveal retina is modified to obtain the maximum photoreceptor sensitivity and is thus the area of the retina with the greatest visual discrimination; however, its function is poor in conditions of low light intensity. Surrounding the fovea is an ovoid yellow area about 1 mm wide called the macula lutea.
As seen in this micrograph, at the fovea the inner layers of the retina are flattened laterally so as to present the least barrier to light reaching the photoreceptors. Retinal blood vessels are absent at the fovea, as can be readily seen with the ophthalmoscope, and the brownish colour of the choroidal melanin shows through the much attenuated retina. At the fovea, the photoreceptors are almost exclusively cones which are elongated and closely packed (approximately 100 000 cones are contained in the fovea). Neuronal interconnections in the bipolar cell layer provide for a one-to-one ratio of these cones to optic nerve fibres, which means that each foveal photoreceptor is individually represented at the visual cortex.
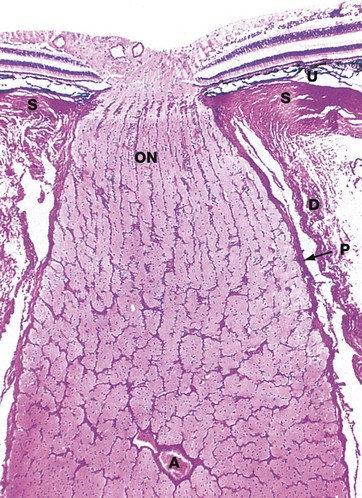
FIG. 21.10 Optic nerve
H&E (LP)
The afferent fibres from the retina converge at a point medial to the fovea, the optic papilla or optic disc, fibres from the lateral quadrants sweeping above and below the macula to avoid the fovea. The fibres then penetrate the sclera S through the lamina cribrosa to form the optic nerve ON. Note the thickness of the optic tract layer overlying the disc and the absence of photoreceptor cells from the optic papilla, which is thus a blind spot on the retina.
In their course across the retina, the afferent fibres are not myelinated as this would obstruct light passing to the photoreceptors. Myelination commences at the optic disc, which imparts the white colour seen with the ophthalmoscope.
The optic nerve and retina develop embryologically as an outgrowth of the primitive forebrain and thus the optic nerve is invested by meninges. The dura mater D becomes continuous with its developmental equivalent, the sclera, while the pia-arachnoid P continues into the eye as the uveal tract U.
The main blood supply of the retina is provided by the central artery of the retina A, a branch of the ophthalmic artery. This divides at the optic disc into four branches supplying the quadrants of the retina. These vessels course within the optic nerve fibre layer, breaking up into a rich capillary network which drains back into a venous system, closely following the course of the arterial supply. The vessels are confined to the optic nerve fibre layer and more superficial layers are dependent on diffusion, the most peripheral retinal layers being supplied likewise from the choroid.
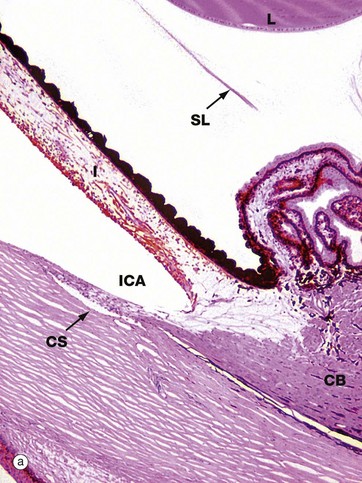
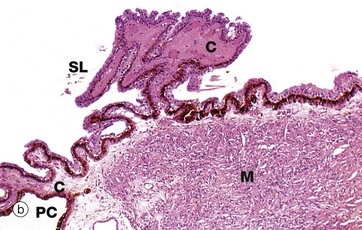
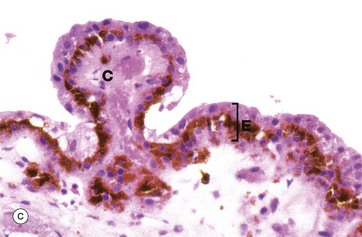
FIG. 21.11 Ciliary body
(a) H&E (LP) (b) H&E (LP) (c) H&E (MP)
The ciliary body is a circumferential structure which bulges into the eye between the ora serrata and the limbus (see Fig. 21.3). As seen in micrograph (a), the ciliary body CB represents the forward continuation of the choroid layer of the uveal tract of the posterior five-sixths of the wall of the eye and, like it, is highly vascular and contains a considerable amount of dark-staining melanin pigment. Anteriorly, it is continuous with the third component of the uveal tract, the iris I, passing in front of the lens L.
As seen in micrograph (c), the ciliary body is lined with a double layer of cuboidal epithelium E. The deep layer is highly pigmented and represents a forward continuation of the pigmented epithelial layer of the retina, while the surface layer, which is not pigmented, is a non-photosensitive forward extension of the receptor layer of the retina.
The ciliary body is attached to the coronal equator of the lens by the suspensory ligaments SL which consist of extremely fine strands composed of the protein fibrillin. Tension in the suspensory ligament tends to flatten the lens which, in the relaxed state, assumes a more globular shape. The bulk of the ciliary body consists of smooth muscle M arranged in such a manner that, when it contracts, tension upon the suspensory ligament is reduced, thus permitting the lens to assume a more convex shape. This mechanism permits fine focusing of images already roughly focused upon the retina by the cornea. The ciliary muscle is innervated by parasympathetic nerve fibres.
From that part of the ciliary body exposed to the angle of the posterior chamber PC, there project a number of branching epithelial folds called ciliary processes C with a supporting tissue core rich in fenestrated capillaries. The ciliary processes are responsible for the continuous production of aqueous humor, which then circulates into the anterior chamber via the pupil. Aqueous humor is continuously reabsorbed into the canal of Schlemm CS seen at the base of the irido-corneal angle of the anterior chamber ICA in micrograph (a).
Aqueous humor is a clear watery fluid, somewhat similar in composition to cerebrospinal fluid and hypotonic with respect to plasma. The production of aqueous humor is an active process, mediated by the two epithelial layers lining the ciliary processes. Balanced rates of secretion and reabsorption of aqueous humor result in the maintenance of a constant intraocular pressure of about 15 mmHg which stabilises the lens and cornea. The flow of aqueous humor also provides for a continuous exchange of metabolites with the cells of the avascular cornea and lens.
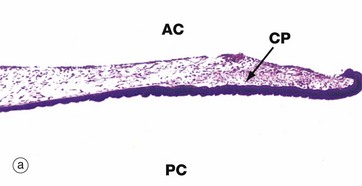
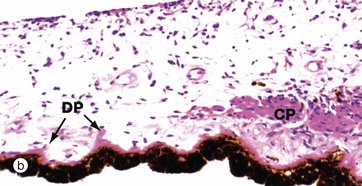
FIG. 21.12 Iris
(a) H&E (LP) (b) H&E (MP)
The iris is the most anterior part of the uveal layer of the eye. It arises from the ciliary body and forms a diaphragm in front of the lens, dividing the anterior compartment of the eye into posterior PC and anterior chambers AC which communicate via the pupil. The pupillary edge of the iris rests on the anterior surface of the lens in life.
The main mass of the iris consists of loose, highly vascular tissue which is pigmented due to the presence of numerous melanocytes scattered in the stroma. The anterior surface of the iris is irregular and consists of a discontinuous layer of fibroblasts and melanocytes; in the fetus, the surface is lined by endothelial cells but these disappear during early childhood. In contrast, the posterior surface is relatively smooth and is lined by epithelium which is derived embryologically as a continuation of the two layers which line the surface of the ciliary body. The surface layer, non-pigmented in the ciliary body, becomes heavily pigmented in the iris such that the individual cells are completely obscured. The deep layer, pigmented in the ciliary body, is transformed in the iris into lightly pigmented myoepithelial cells which constitute the radially orientated dilator pupillae muscle DP of the iris. Even at high magnification (b), these myoepithelial cells are difficult to distinguish.
The constrictor muscle of the pupil (constrictor pupillae) CP consists of a band of circumferentially oriented smooth muscle fibres situated in the stroma near to the free edge of the iris. Like the smooth muscle of the ciliary body, the constrictor pupillae is innervated by the parasympathetic nervous system, whereas the myoepithelial cells of the dilator pupillae are innervated by the sympathetic nervous system.
The colour of the iris depends on the amount of pigment in the stroma, the amount of pigment in the posterior epithelial layer being relatively constant between individuals. Blue eyes contain little pigment, whereas brown eyes have plentiful stromal pigment.
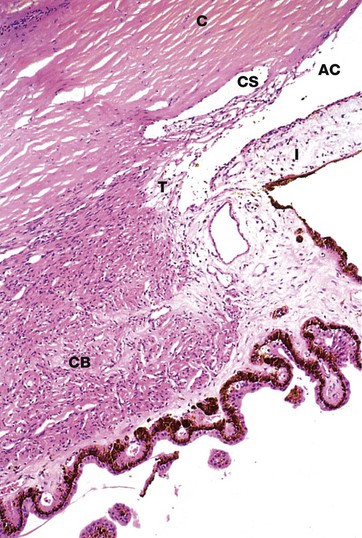
FIG. 21.13 Canal of Schlemm
H&E (MP)
The canal of Schlemm CS is a circumferential canal lined by endothelium which is situated in the inner aspect of the corneal margin C, immediately adjacent to the angle of the anterior chamber AC. At the angle of the anterior chamber, there is a meshwork of fine collagenous trabeculae T lined by endothelium; aqueous humor percolates through the spaces between the trabeculae before reaching the canal of Schlemm. There is no direct communication between the trabecular spaces and the canal of Schlemm and thus reabsorption of aqueous humor involves passage across two layers of endothelium and intervening supporting tissue. The canal of Schlemm drains via minute channels through the sclera into the episcleral venous system, a pressure gradient being maintained to prevent reflux of blood. Note the close relationship between the root of the iris I and the canal of Schlemm. The smooth muscle of the ciliary body CB is easily seen in this micrograph.
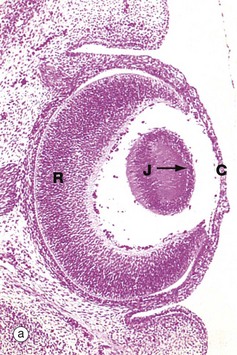
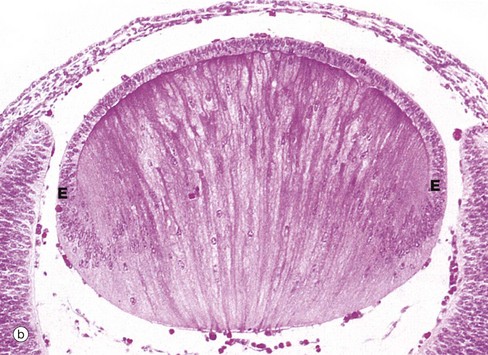
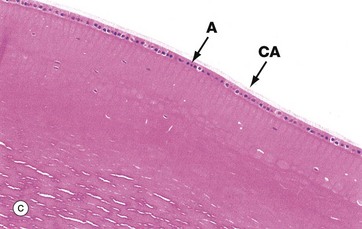
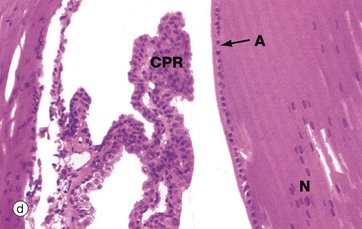
FIG. 21.14 The lens and its development
(a) H&E (MP) (b) H&E (HP) (c) H&E (MP) (d) H&E (MP)
The lens is an elastic biconvex structure which, although transparent and apparently amorphous, is almost entirely composed of living cells. The lens cells are highly modified epithelial cells, derived embryologically from ectoderm which forms a depression, the lens pit, overlying the embryonic optic vesicle.
With further development, the lens pit becomes deeper, its margins fusing to form the lens vesicle which becomes detached from the surface and sinks deeper to become enveloped by the growing optic vesicle; at this stage, the lens vesicle merely consists of a single layer of epithelial cells.
The posterior cells of the lens vesicle now become greatly elongated anteroposteriorly, filling the central cavity of the vesicle. This stage of development is shown in micrograph (a), showing the junction J between posterior and anterior cells of the former lens vesicle. Note the developing cornea C and retina R. The lens cells in the central anteroposterior axis then undergo maturation as seen in micrograph (b), losing their nuclei to become known as lens fibres. Proliferation of the cells at the lens equator E adds further fibres to the central mass, the growth process continuing at a slow rate even into old age.
When fully developed, the lens substance consists of 2000 to 3000 anucleate fibres, each stretching between anterior and posterior poles of the lens. The fibres have the shape of extremely elongated six-sided prisms, the more peripheral fibres curving to follow the anteroposterior surface contour of the lens. The lens fibres are packed with proteins called crystallins and the cell membranes of adjacent fibres are fused, leaving little intervening extracellular substance.
The whole lens is enveloped by a thick epithelial basement membrane forming the lens capsule, which is connected via the suspensory ligament to the ciliary body. The anterior lens surface is covered by a single layer of cuboidal cells which retain their nuclei, this layer merging with the residual proliferative cells at the equatorial margin of the lens. This cell layer lies deep to the capsule and is absent posteriorly.
Micrograph (c) shows part of the mature lens, including the anterior cuboidal epithelium A and lens capsule CA. The lens substance is particularly prone to artefactual distortion during histological preparation. Micrograph (d) shows the equatorial region of the lens and nearby ciliary processes CPR. Note the anterior epithelial layer A and nuclei N in the more recently formed peripheral fibres.
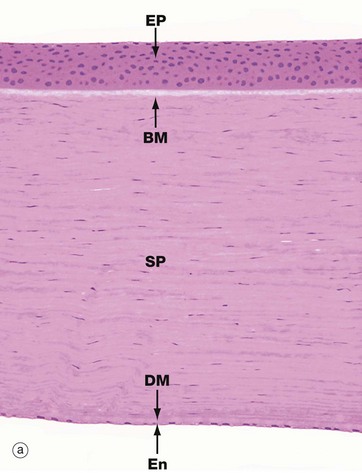
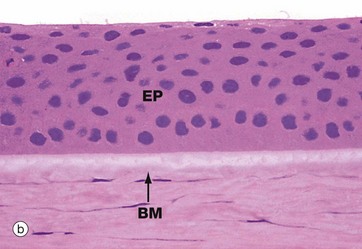
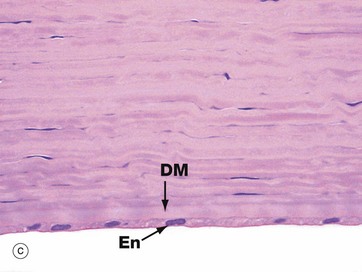
FIG. 21.15 Cornea
(a) H&E (MP) (b) H&E (HP) (c) H&E (HP)
The cornea is the thick transparent portion of the corneo-scleral layer enclosing the anterior one-sixth of the eye. The fixed convexity of the external surface provides the principal mechanism for focusing images upon the retina. Micrograph (a) shows the full thickness of the cornea, while micrographs (b) and (c) show the superficial and deep aspects at higher magnification.
The cornea is an avascular structure consisting of five layers. The outer surface is lined by stratified non-keratinised squamous epithelium EP about six cells thick. This epithelium rests on a thin basal lamina, supported by a thick specialised layer of corneal stroma known as Bowman's membrane BM, which is particularly prominent in humans. The bulk of the cornea, the substantia propria or stroma SP, consists of a highly regular form of dense collagenous tissue forming thin lamellae. Fibroblasts with elongated nuclei and barely visible cytoplasm termed keratocytes are scattered in the ground substance between the lamellae. The inner surface of the cornea is lined by a layer of flattened endothelial cells En which are supported by a very thick basement membrane known as Descemet's membrane DM. The corneal endothelium is highly active in pumping fluid from the substantia propria, preventing excessive hydration which would result in the cornea becoming opaque.
The cornea is sustained by diffusion of metabolites from the aqueous humor and the blood vessels of the limbus; some oxygen is derived directly from the external environment.
There is a rich innervation by free nerve endings, making the cornea very pain sensitive.
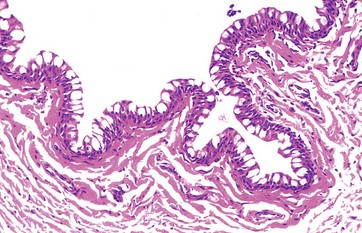
FIG. 21.16 Conjunctiva
H&E (MP)
The conjunctiva is the epithelium which covers the exposed part of the sclera and inner surface of the eyelids. It is stratified columnar in form and, for a stratified epithelium, is unusual in that it contains goblet cells in the surface layers. Melanocytes are found in the basal layer. The conjunctival mucous secretions contribute to the protective layer on the exposed surface of the eye and allow the eyelids to move freely over the eye.
Beneath the conjunctival epithelium is loose vascular supporting tissue.
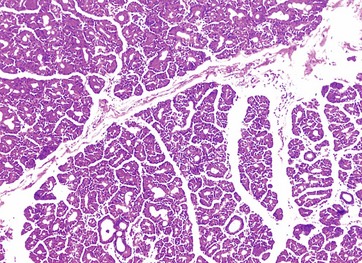
FIG. 21.17 Lacrimal gland
H&E (MP)
The lacrimal gland is responsible for the secretion of tears, a watery fluid containing the antibacterial enzyme lysozyme and electrolytes of similar concentration to plasma.
Histologically, the lacrimal glands are similar to the salivary glands in the lobular structure and compound tubulo-acinar form of the secretory units. The secretory cells have the typical appearance of serous (protein-secreting) cells, with basally located nuclei and strongly stained granular cytoplasm.
Each gland drains via a dozen or more small ducts into the superior fornix. Tears drain to the inner aspect of the eye and then into the nasal cavity via the nasolacrimal duct.
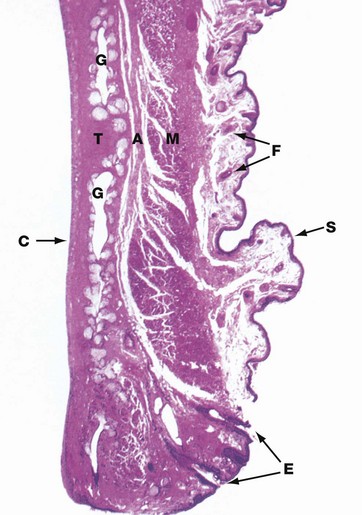
FIG. 21.18 Eyelid
H&E (LP)
Each eyelid consists of a dense fibroelastic plate, the tarsus or tarsal plate T, covered externally by thin highly folded skin S and, on the internal aspect, by smooth conjunctiva C. The skin contains scattered fine hair follicles F, and the underlying supporting tissue is extremely loose and devoid of fat.
Skeletal muscle M of the orbicularis oculi (and levator palpebrae in the upper eyelid) lies immediately superficial to the tarsal plate and is separated from it by a layer of supporting tissue A which, in the upper lid, represents a forward continuation of the sub-aponeurotic layer of the scalp. The clinical importance of this is that blood or inflammatory exudates collecting above the scalp aponeurosis may track forward into the superficial planes of the eyelid; being extremely lax, this area may become markedly swollen. This supporting tissue layer also contains the sensory nerves of the eyelid.
Within the tarsal plate lie some 12 to 30 tarsal (Meibomian) glands G, oriented vertically and opening at the free margin of the eyelid via minute foramina. These glands are modified sebaceous glands, each consisting of a long central duct into which open numerous sebaceous acini. Associated with the eyelashes E are sebaceous glands known as the glands of Zeis and modified apocrine sweat glands known as the glands of Moll. Together, the glands of the eyelid produce an oily layer which is thought to cover the tear layer, thereby preventing evaporation of the tears.
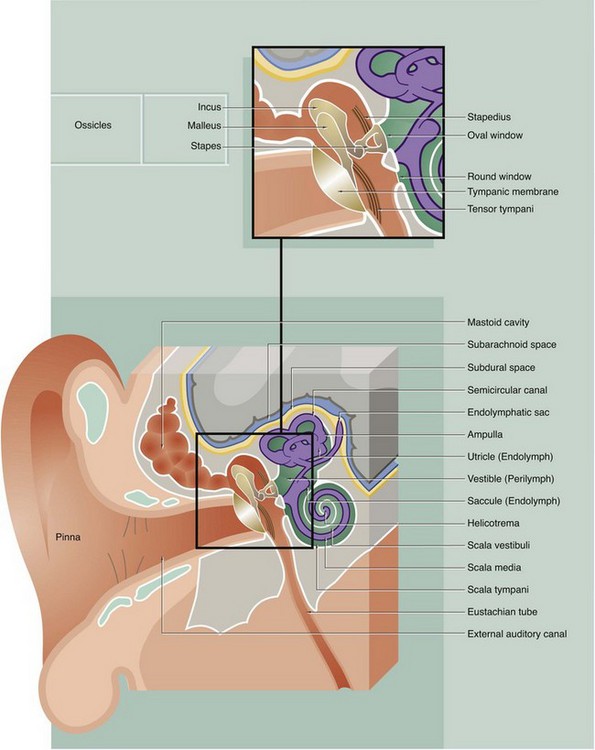
FIG. 21.19 The ear (illustration opposite)
The main structural elements of the vestibulocochlear apparatus are illustrated in this diagram.
External ear
The external ear is responsible for reception of sound waves which are funnelled onto the ear drum (tympanic membrane). It consists of the auricle (pinna), a modified cone-shaped structure composed of elastic cartilage covered by skin, which converges onto the external auditory meatus (canal). Elastic cartilage also forms the wall of the outer third of the canal, while the inner two-thirds of the canal lie in the petrous part of the temporal bone. The canal is lined by hairy skin containing sebaceous glands and modified apocrine glands which secrete a waxy material called cerumen.
Middle ear
The middle ear is an air-filled cavity, the tympanic cavity, located in the petrous temporal bone and separated from the external auditory canal by the tympanic membrane. Sound waves impinging on the tympanic membrane are converted into mechanical vibrations which are then amplified by a system of levers made up of three small bones called ossicles (malleus, incus and stapes) and transmitted to the fluid-filled inner ear cavity. The ossicles articulate with one another via synovial joints and the malleus and incus pivot on tiny ligaments which are attached to the wall of the middle ear cavity. Small slips of muscle, the tensor tympani and stapedius, pass to the midpoint of the tympanic membrane and stapes bone, respectively, and damp down excessive vibrations which might otherwise damage the delicate auditory apparatus. The middle ear cavity communicates anteriorly with the nasopharynx via the auditory (Eustachian) tube, which permits equalisation of pressure changes with the external environment. Posteriorly, the middle ear cavity communicates with numerous interconnected air spaces which lighten the mass of the mastoid part of the temporal bone. The whole of the middle ear and mastoid cavities are lined by simple squamous or cuboidal epithelium.
Internal ear
The internal ear consists of an interconnected fluid-filled membranous labyrinth lying within a labyrinth of spaces of complementary shape in the temporal bone (the osseous labyrinth). The membranous labyrinth is bound down to the walls of the osseous labyrinth in various places but, in the main, is separated from the bony walls by a fluid-filled space. The fluid within the membranous labyrinth is known as endolymph and the fluid in the surrounding perimembranous space is known as perilymph. The perimembranous space is directly connected with the subarachnoid space and, like the latter, is crossed by delicate fibrous strands and lined by squamous epithelium; the perilymphatic fluid is thus similar in composition to cerebrospinal fluid. In contrast, the membranous labyrinth is a closed system with a sac, the endolymphatic sac, lying in the subdural space of the underlying brain. The membranous labyrinth is lined by a simple epithelium except in the endolymphatic sac, where the cells are columnar with morphological features suggesting that this is the site of endolymph absorption.
The osseous labyrinth may be divided into three main areas:
• The vestibule. The central space of the osseous labyrinth is called the vestibule; it gives rise to three semicircular canals posteriorly and to the cochlea anteriorly. The vestibule contains two components of the membranous labyrinth, the utricle and the saccule, which are connected by a short Y-shaped duct from which arises the endolymphatic duct. The walls of the utricle and saccule each contain a specialised area of sensory receptor cells known as a macula (see Fig. 21.27) from which axons pass into the vestibular nerve as part of sensory inputs to maintain equilibrium. Laterally, the vestibule is separated from the middle ear cavity by a thin bony plate containing two fenestrations or windows. The oval window is occluded by the base of the stapes bone and its surrounding annular ligament whereby vibrations are transmitted to the perilymph from the tympanic membrane via the ossicle chain. The round window is closed by a membrane similar to the tympanic membrane and is thus sometimes described as the secondary tympanic membrane. This membrane permits vibrations, which have passed the sensory receptors for sound, to be dissipated.
• The semicircular canals. Three semicircular canals arise from the posterior aspect of the vestibule, two being disposed in vertical planes at right angles to one another and the other in a near-horizontal plane. Within each semicircular canal is a semicircular membranous duct filled with endolymph and continuous at both ends with the utricle; near one end of each semicircular membranous duct is a dilated area called the ampulla. In each ampulla, there is a ridge called the crista ampullaris (see Fig. 21.28) containing sensory receptors with axons converging on the vestibular nerve. Together with the receptors of the maculae of the utricle and saccule, these receptors help maintain balance and equilibrium.
• The cochlea. The cochlea occupies a conical spiral-shaped space in the temporal bone, extending from the anterior aspect of the vestibule. The membranous component of the cochlea arises from the saccule and spirals upwards, with its blind end attached at the apex of the osseous space. The membranous canal is triangular in cross-section and attached to the bony walls of the cochlea in such a manner as to divide the osseous space into three spiral compartments (see Fig. 21.24). The middle compartment, the scala media, contains endolymph and the upper and lower compartments contain perilymph. At the base of the cochlea, the upper perilymph compartment is directly continuous with the perilymph of the vestibule and via this space, called the scala vestibuli, vibrations pass through the perilymph towards the apex of the cochlea. At the apex, the scala vestibuli becomes continuous with the lower perilymphatic space of the cochlear spiral via a minute hole called the helicotrema. This lower space terminates at the secondary tympanic membrane covering the round window and ‘spent’ vibrations are thus dissipated; the lower perilymphatic space is therefore known as the scala tympani. The sensory receptors for sound are located in a spiral-shaped structure known as the organ of Corti, shown in detail in Fig. 21.25.
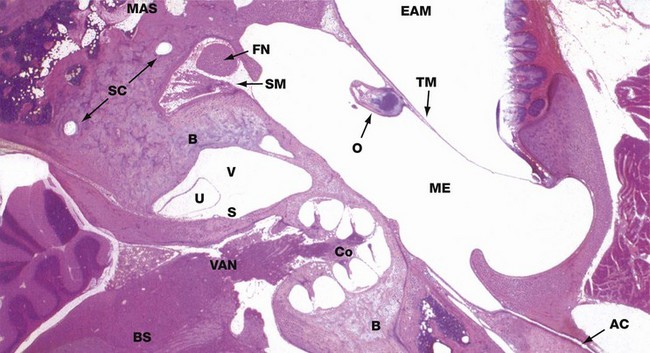
FIG. 21.20 The ear
H&E (LP)
This micrograph shows a horizontal section through the vestibulo-cochlear apparatus which lies within the temporal bone B. The tympanic membrane TM can be seen, stretched between the tympanic plate of the temporal bone anteriorly and the lateral part of the petrous temporal bone posteriorly, dividing the external auditory meatus EAM from the cavity of the middle ear ME. Part of one of the ossicles O, the handle of the malleus, can be seen attached to the inner aspect of the tympanic membrane. From the anterior aspect of the middle ear chamber, the auditory canal (Eustachian tube) AC passes forwards towards the nasopharynx; in the mastoid part of the temporal bone, there are numerous irregular mastoid air spaces MAS.
Near the centre of the field is the vestibule of the inner ear V, containing two delicate membranous structures, the utricle U and saccule S, more anteriorly. Two of the semicircular canals SC can be identified deep in the posterior part of the petrous temporal bone. Immediately posterior to the middle ear cavity, the facial nerve FN is seen in transverse section as it passes inferiorly; just medial to it lies the stapedius muscle SM.
Anterior to the vestibule, the conical spiral of the cochlea Co has been cut in longitudinal section through its central bony axis. From the base of the cochlea, the vestibulo-auditory (vestibulo-cochlear) nerve VAN passes towards the brainstem BS, behind which the cerebellum is easily recognisable.
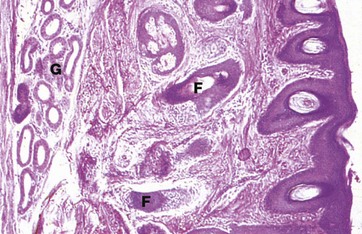
FIG. 21.21 External auditory meatus
H&E (MP)
The external auditory meatus is the canal leading from the auricle to the tympanic membrane. The wall of the outer third is formed by elastic cartilage, whereas the inner two-thirds is formed by the temporal bone. The canal is lined by skin which is devoid of the usual dermal papillae and closely bound down to the underlying cartilage or bone by a dense collagenous dermis. The skin of the outer third (as shown here) has fine hairs and the dermis contains numerous coiled tubular ceruminous glands G which secrete wax (cerumen) and which represent specialised apocrine glands. The ceruminous glands open directly onto the skin surface or into the sebaceous glands associated with hair follicles F. The meatal hairs provide protection from foreign bodies while the cerumen protects the skin of the external meatus from moisture and infection.
The Ear
The ear or vestibulo-cochlear apparatus has the dual sensory function of maintenance of equilibrium and hearing (stato-acoustic system).
Structurally, the system may be divided into three parts, the external ear, the middle ear and the internal ear. The specific sensory receptors for both movement and sound are situated in a membranous structure located in the internal ear, while the external and middle ear are concerned with reception, transmission and amplification of incoming sound waves.
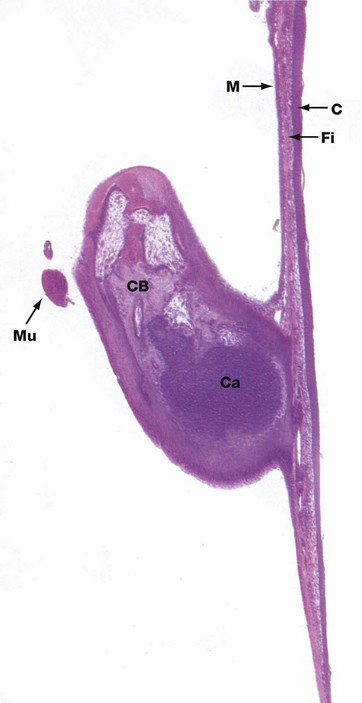
FIG. 21.22 Tympanic membrane and ossicle
H&E (LP)
The tympanic membrane (ear drum) is a thin fibrous membrane separating the external auditory canal from the cavity of the middle ear. With the exception of a small triangular area superiorly, the pars flaccida, the membrane is tense (pars tensa), being firmly attached to the surrounding bone by a fibrocartilaginous ring. The handle of the malleus is attached to the centre of the membrane, the chain of ossicles pulling the membrane slightly inwards.
The tympanic membrane is made up of three layers: an external cuticular layer, an intermediate fibrous layer and an inner mucous layer. The cuticular layer C consists of thin hairless skin, the epidermis being only about 10 cells thick and the basal layer being flat and devoid of the usual epidermal ridges. The thin dermis contains plump fibroblasts and a fine vascular network.
The intermediate fibrous layer Fi consists of an outer layer of fibres radiating from the centre of the membrane towards the circumference and an inner layer of fibres disposed circumferentially at the periphery. These fibres contain a large amount of type II and type III collagen and a small amount of type I collagen, representing a distinct composition especially adapted for the function of the tympanic membrane.
The inner mucous layer M represents a continuation of the modified respiratory-type mucous membrane lining the middle ear cavity, but in this situation it is merely a single layer of cuboidal cells devoid of cilia and goblet cells. The underlying lamina propria is thin with a blood supply separate from that of the dermis of the cuticular layer. A similar modified respiratory-type mucosa invests the ossicles, small muscles and nerves exposed to the middle ear cavity.
The ossicles consist of compact bone CB formed by endochondral ossification, which accounts for the cartilage Ca seen in this specimen from a kitten. Note also the tensor tympani muscle Mu.
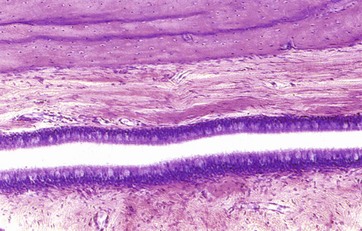
FIG. 21.23 Auditory (Eustachian) canal
H&E (HP)
The auditory canal connects the cavity of the middle ear with the nasopharynx and allows for equalisation of air pressure between the middle ear and the external environment. From the middle ear, the tube first passes through bone, but towards the pharynx, the wall is supported on two sides by cartilage and on the remaining two sides by fibrous tissue.
The tube is lined by typical pseudostratified respiratory epithelium with numerous goblet cells, particularly towards the pharyngeal end. The salpingo-pharyngeus, tensor palati and levator veli palati muscles are connected to the fibrocartilaginous part of the tube, causing it to dilate during swallowing.
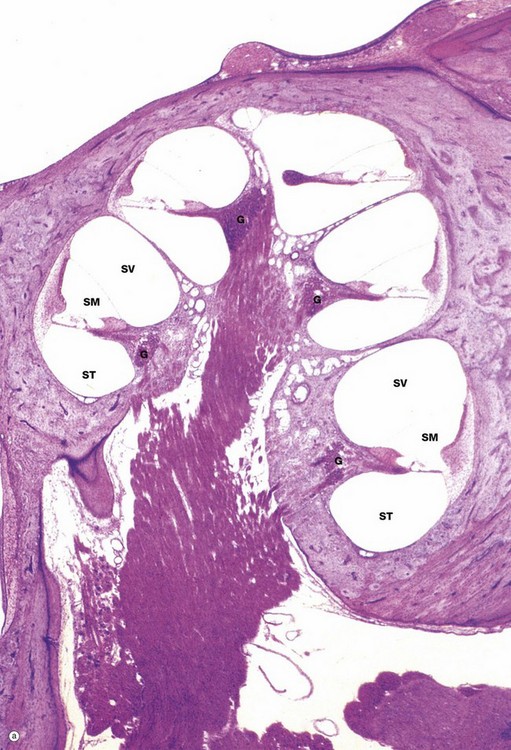
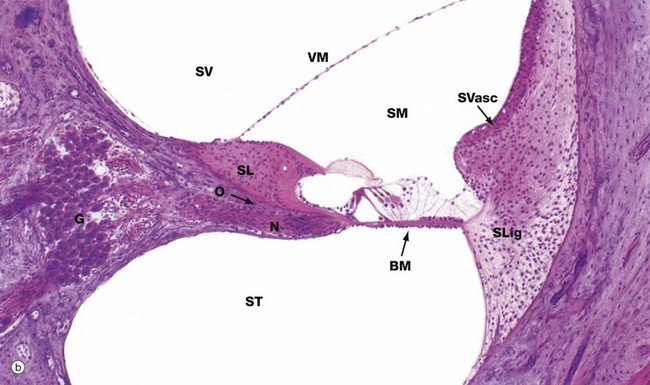
FIG. 21.24 Cochlea (illustration (a) opposite)
(a) H&E (LP) (b) H&E (LP)
The cochlea is the component of the internal ear which contains the auditory sense organ. The conical spiral-shaped form of the cochlea can be visualised in micrograph (a) which shows a cochlea cut in a plane of section which includes its long bony axis. Note that the cavity in the petrous temporal bone is reminiscent of the space inside a conical snail shell. The cochlea has two-and-a-half full turns; in this section, five separate cross-sections of the cochlea can be seen, each turn of the spiral being separated from the next by a thin plate of bone. A corkscrew-like bony structure, the modiolus, forms the central axis of the cochlea.
Each turn of the cochlear canal can be seen to be divided into three compartments, shown at higher magnification in micrograph (b). The central compartment, the scala media SM, is roughly triangular in cross-section, with the apex attached to a spicule of bone spiralling outwards from the modiolus and known as the osseous spiral lamina O. Above the free edge of the osseous spiral lamina is a thickened mass of tissue known as the spiral limbus SL. The base of the scala media is thickened and attached to the outer wall of the cochlea. The membrane making up the walls of the scala media represents that part of the membranous labyrinth extending up into the cochlea from the saccule and the scala media is thus filled with endolymph.
Above the scala media is the scala vestibuli SV, originating in the vestibule near the oval window and the base of the stapes; vibrations are conducted towards the apex of the cochlea in the perilymph of the scala vestibuli. Below the scala media is the perilymphatic space which spirals down from the apex to the secondary tympanic membrane, the scala tympani ST.
The membrane separating the scala media and the scala tympani, known as the basilar membrane BM, supports the organ of Corti which contains the auditory receptor cells; the organ of Corti is described in detail in Fig. 21.25. The cells of the organ of Corti are derived from the simple epithelium lining the membranous labyrinth which, embryologically, is of ectodermal origin. The basilar membrane is composed of fibrous tissue. Axially, it is attached to the osseous spiral lamina and laterally to the spiral ligament SLig, which consists of a marked thickening of the endosteum of the lateral wall of the cochlear canal. The thickened outer wall of the scala media is highly vascular and lined by a stratified epithelium; this area, known as the stria vascularis SVasc, is responsible for maintaining the correct ionic composition of endolymph.
The membrane between the scala media and scala vestibuli, the vestibular (Reissner's) membrane VM, is composed of extremely delicate fibrous tissue lined by simple squamous epithelium on both sides. The scala vestibuli and scala tympani are lined by a simple unspecialised squamous epithelium of mesodermal origin.
In micrograph (b), bundles of afferent nerve fibres N can be seen arising from the base of the organ of Corti and converging towards the spiral ganglion G in the modiolus at the base of the spiral lamina. These ganglion cells represent the cell bodies of bipolar sensory neurones and their proximal axons form the auditory component of the eighth cranial nerve (see Fig. 21.26).
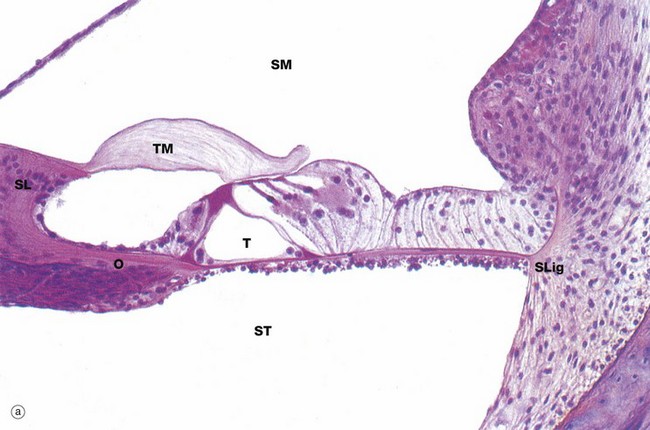
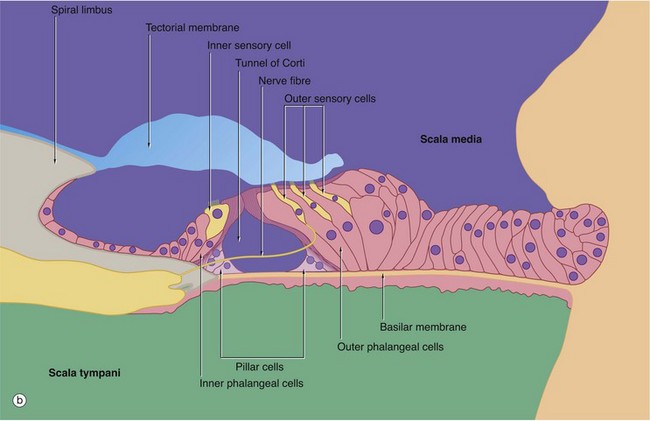
FIG. 21.25 Organ of Corti (illustrations opposite)
(a) H&E (HP) (b) Schematic diagram
The organ of Corti is a highly specialised epithelial structure containing receptor cells which convert (transduce) mechanical energy in the form of vibrations into electrochemical energy, resulting in excitation of auditory sensory receptors.
The organ of Corti lies in the scala media SM, supported on the basilar membrane. The basilar membrane consists of a thin sheet of fibrous tissue stretched between the osseous spiral lamina O of the modiolus and the spiral ligament SLig laterally; its undersurface, exposed to the scala tympani, is lined by a simple epithelium. The basilar membrane is thinnest at the base of the cochlea and becomes progressively thicker as it spirals towards the apex.
The organ of Corti consists of two basic types of cells, sensory (hair) cells and support cells of several different types, including the inner and outer pillar cells and inner and outer phalangeal cells. At the centre of the organ is a triangular-shaped canal, the inner tunnel or tunnel of Corti T, bounded on each side by a single row of tall columnar cells called pillar cells. Each pillar cell contains a dense bundle (pillar) of microtubules and the pillars on either side of the tunnel of Corti converge at the surface and then curve laterally to form a thin, hood-like structure containing small fenestrations. The cell bodies of the pillar cells lie in the acute angles formed by the pillars, and the basilar membrane at the floor of the tunnel.
On the inner aspect of the inner row of pillar cells is a single row of flask-shaped cells, the inner phalangeal cells, which support a single row of inner sensory (hair) cells. The phalangeal cells contain microtubules, some of which support the base of the hair cells while others extend to the free surface around the hair cells. Beyond the outer row of pillar cells there are three to five rows of outer phalangeal cells which support the same number of rows of outer sensory (hair) cells. Cytoplasmic extensions of the phalangeal cells extend to the surface between and around the hair cells and their microtubules support the fenestrated hood-like structure formed by the pillar cells. Through the fenestrations project the free ends of the sensory cells. A variety of other specialised epithelial cells provides the remaining support for the organ of Corti.
The sensory cells are known as hair cells because numerous stereocilia, i.e. very long microvilli (see Fig. 5.15), project from their free ends. The stereocilia are embedded in the surface of the tectorial membrane. As previously described, the spiral ganglion of the cochlea contains bipolar cell bodies of first order sensory neurones. From here, axons pass towards the base of the rows of hair cells, those going to the outer hair cells traversing the tunnel of Corti as shown in the diagram (b). The end of each fibre ramifies into a number of dendrites which make synaptic contact with several hair cells; each sensory cell may synapse with dendrites of several different sensory neurones. In addition, inhibitory neurones arising in the brainstem send fibres which also synapse with the sensory cells and exert a suppressive effect.
From the layer of border cells which cover the spiral limbus SL, there extends a flap-like mass of glycosaminoglycans called the tectorial membrane TM overlying the sensory cells and within which the tips of the stereocilia are embedded.
Function of the organ of Corti
Only an outline of the mechanism of hearing is presented here; molecular details are being discovered at a rapid pace. Sound waves are funnelled into the external auditory meatus and impinge on the tympanic membrane, which vibrates at the appropriate frequency. These vibrations are transmitted to the stapes bone via the malleus and incus and, in the process, their amplitude is enhanced about 10-fold. The base of the stapes, which lies in the oval window, conducts the vibrations into the perilymph of the vestibule of the inner ear and pressure waves pass from here into the scala vestibuli of the cochlea. These pressure waves are probably conducted directly to the endolymph of the scala media across the delicate vestibular membrane, from which vibrations are induced in the basilar membrane upon which rests the organ of Corti. From here, spent vibrations are transmitted into the perilymph of the scala tympani and dissipated at the secondary tympanic membrane over the round window.
The basilar membrane is thinnest at the base of the cochlea and thickest at the apex. It appears that at every point on the spiral, the membrane is ‘tuned’ to vibrate to a particular frequency of sound waves reaching the ear; the overall range of frequencies encompassed is of the order of 11 octaves, with the highest frequencies (pitch) being sensed towards the base of the cochlea and progressively lower frequencies being sensed along the spiral towards the apex. For any given sound frequency, only one specific point of the basilar membrane and organ of Corti is thought to vibrate and thereby activate the appropriate hair cells to initiate afferent sensory impulses which then pass to the auditory cortex of the brain. Deformation of the stereocilia of the hair cells results in either depolarisation or hypopolarisation of the cell membrane which, in turn, excites the sensory nerves which synapse with them.
The sensory input from the cochlea is integrated in the brainstem and auditory cortex, from which efferent suppressor pathways can modulate receptor activity to enhance auditory acuity.
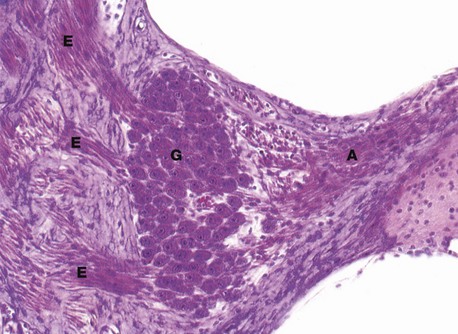
FIG. 21.26 Spiral ganglion
H&E (HP)
The spiral ganglion is a spiral-shaped mass of nerve cell bodies lying in a canal at the extremity of the osseous spiral lamina of the modiolus.
As seen in this micrograph, the ganglion cells G have the typical appearance of somatic ganglion cells (see Fig. 7.20) and represent the cell bodies of bipolar sensory neurones, relaying information from the receptors of the organ of Corti to the brain.
Note the afferent fibres A entering the ganglion from the organ of Corti and numerous bundles of efferent fibres E which pass to the centre of the modiolus to form the cochlear nerve, the auditory component of the eighth cranial nerve; the cochlear nerve is readily seen in Fig. 21.24.
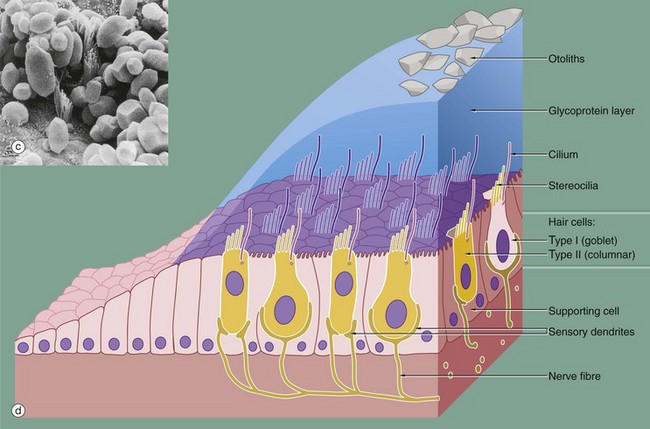
FIG. 21.27 Receptor organs of the saccule and utricle (illustrations opposite)
(a) H&E (HP) (b) H&E (HP) (c) SEM ×5000 (d) Schematic diagram
The saccule and utricle are two dilated regions of the membranous labyrinth, lying within the vestibule of the inner ear and are filled with endolymph. The walls of each are composed of a fibrous membrane which is bound down in places to the periosteum of the vestibule and, in other areas, is attached to the periosteum by fibrous strands, the intervening space being filled with perilymph. Internally, the saccule and utricle are lined by simple cuboidal epithelium but, in each, there is a small region of highly specialised epithelium called the macula, shown in micrographs (a) and (b), containing receptor cells which contribute part of the sensory input to that part of the brain responsible for maintaining balance and equilibrium. The macula of the utricle is oriented at right angles to that of the saccule.
The maculae are made up of two basic cell types, sensory hair cells and support cells. The support cells are tall and columnar with basally located nuclei and microvilli at their free surface. The hair cells lie between the support cells, with their larger nuclei placed more centrally. Each hair cell has a single eccentrically located cilium of typical conformation, often called the kinocilium (see Fig. 5.13), and many stereocilia (long microvilli; see Fig. 5.15) projecting from its surface, hence the name hair cells. The ‘hairs’ are embedded in a thick, gelatinous plaque of glycoprotein, probably secreted by the supporting cells; this is lost during histological preparation. At the surface of the glycoprotein layer is a mass of crystals mainly composed of calcium carbonate and known as otoliths. These are shown in micrograph (c).
There are two different forms of hair cells. Type I hair cells (goblet cells) are bulbous in shape and stain poorly, their nuclei tending to lie at a lower level than those of type II hair cells (columnar cells) which are more slender in shape. The type I hair cells are invested by a meshwork of dendritic processes of afferent sensory neurones, whereas the type II hair cells have only small dendritic processes at their bases. The hair cells also have synaptic connections with modulatory (inhibitory) neurones from the central nervous system.
Function of the maculae
The function of the maculae relates mainly to the maintenance of balance by providing sensory information about the static position of the head in space. This is of particular importance when the eyes are closed or in the dark or under water, and the maculae are consequently more developed in animals other than humans.
When the head is moved from a position of equilibrium, the otolithic membrane tends to move with respect to the receptor cells, causing bending of their stereocilia. When the stereocilia are bent in the direction of the cilium, the receptor cell undergoes excitation and, when the relative movement is in the opposite direction, excitation is inhibited. The orientation of the hair cells in different directions in the maculae causes different hair cells to be stimulated with different positions of the head. The pattern of hair cell stimulation allows the central nervous system to determine the position of the head very accurately with respect to gravity.
The neural pathways of the balance and equilibrium mechanism are complex, and the sensory input from the maculae is integrated with that of other proprioceptors, such as muscle spindles, to elicit reflex responses directed towards the maintenance of postural equilibrium.
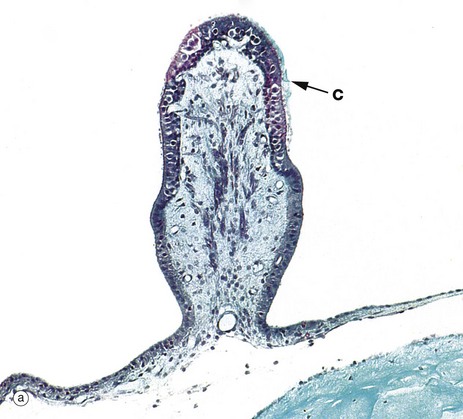
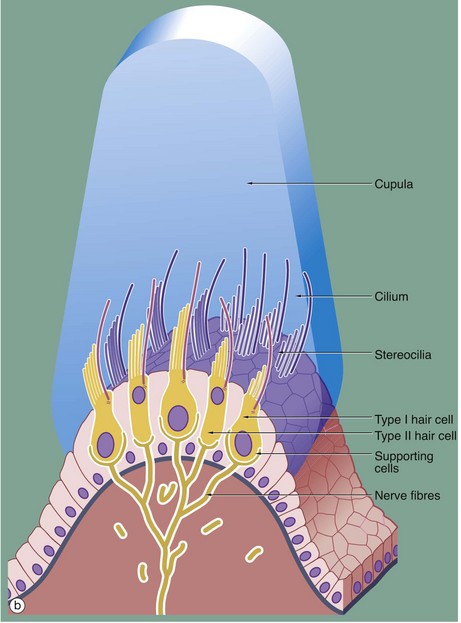
FIG. 21.28 Receptor organs of the semicircular canals
(a) Masson trichrome (MP) (b) Diagram
Three semicircular canals arise from the vestibule of the inner ear, each containing a membranous semicircular duct which opens at both ends into the utricle. At one end of each duct is a dilated portion, the ampulla, which contains a receptor organ called the crista ampullaris, shown in micrograph (a).
Each crista ampullaris is an elongated epithelial structure situated on a ridge of supporting tissue, arising from the membranous wall of the ampulla and oriented at right angles to the direction of flow of the endolymph in the semicircular canal. Structurally, the cristae ampullares bear many similarities to the maculae of the utricle and saccule (see Fig. 21.27). The hair cells are of the same two morphological forms, type I and type II cells, the former being invested by a basket of sensory dendrites and the latter having small dendritic endings at the base only. The hair cells are supported by a single layer of columnar cells which is continuous with the simple cuboidal epithelium lining the rest of the membranous labyrinth.
Like those of the maculae, the hair cells of the cristae have numerous stereocilia and a single kinocilium, the kinocilium being situated at the margin of the cell nearest to the utricle. The stereocilia and the kinocilia of the hair cells are embedded in a ridge of gelatinous glycoprotein which is tall and cone shaped in cross-section, giving rise to the term cupula. In contrast to the macula, the cupula does not contain otolithic crystals. Traces of the cupola C can be seen on the surface of the crista ampullaris in micrograph (a), although most of it has been lost during histological preparation of the specimen.
Function of the crista ampullaris
When the head is moved in the plane of a particular semicircular canal, the inertia of the endolymph acts to deflect the cupula in the opposite direction. The stereocilia of the sensory cells are then deflected towards or away from the cilia, resulting in excitation or inhibition, respectively. In each ear, there are three semicircular canals, two at right angles to each other in vertical planes and one in a near-horizontal plane. Each is paired with a semicircular canal in the other ear, the members of each pair being oriented in parallel. The sensory input from the cristae ampullares mainly concerns changes in the direction and rate of movement of the head. Afferent impulses pass via bipolar sensory neurones with cell bodies in the vestibular ganglion which lies at the base of the internal auditory meatus. Afferent fibres pass via the vestibular part of the eighth cranial nerve to the brainstem, cerebellum and cerebral cortex, where sensory information from various other sources is integrated for the maintenance of balance, position sense and equilibrium.