Oral tissues
Introduction
The digestive process commences in the oral cavity with the ingestion, fragmentation and moistening of food but, in addition to its digestive role, the oral cavity is involved in speech, facial expression, sensory reception and breathing. The major structures of the oral cavity, the lips, teeth, tongue, oral mucosa and the associated salivary glands, participate in all these functions.
Mastication or chewing is the process by which ingested food is made suitable for swallowing. Chewing involves not only coordinated movements of the mandible and the cutting and grinding action of the teeth, but also activity of the lips and tongue, which continually redirect food between the occlusal surfaces of the teeth. The watery component of saliva moistens and lubricates the masticatory process, while salivary mucus helps to bind the food bolus ready for swallowing.
The entire oral cavity is lined by a protective mucous membrane, the oral mucosa, which contains many sensory receptors, including the taste receptors of the tongue. The epithelium of the oral mucosa is of the stratified squamous type, which tends to be keratinised in areas subject to considerable friction such as the palate. The oral epithelium is supported by dense collagenous tissue, the lamina propria. In highly mobile areas such as the soft palate and floor of the mouth, the lamina propria is connected to the underlying muscle by loose submucosal supporting tissue. In contrast, in areas where the oral mucosa overlies bone, such as the hard palate and tooth-bearing ridges, the lamina propria is tightly bound to the periosteum by a relatively dense fibrous submucosa. Throughout the oral mucosa, numerous small accessory salivary glands of both serous and mucous types are distributed in the submucosa.
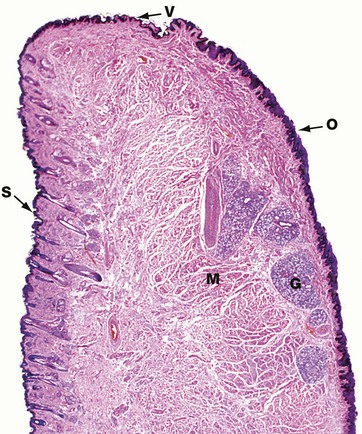
FIG. 13.1 Lip
H&E (LP)
This micrograph illustrates a midline section through a human lower lip, the bulk of which is made up of bundles of circumoral skeletal muscle M seen in transverse section. The external surface of the lip is covered by hair-bearing skin S which passes through a transition zone to merge with the oral mucosa O of the inner surface. The transition zone constitutes the free vermilion border of the lip V, and derives its colour from the richly vascular dermis, which here has only a thin, lightly keratinised epidermal covering. The free border is highly sensitive due to its rich sensory innervation. Since the vermilion border is devoid of sweat and sebaceous glands, it requires continuous moistening by saliva to prevent cracking. The oral mucosa covering the inner surface of the lip has a thick stratified squamous epithelium and the underlying submucosa contains numerous accessory salivary glands G of serous, mucous and mixed seromucous types.
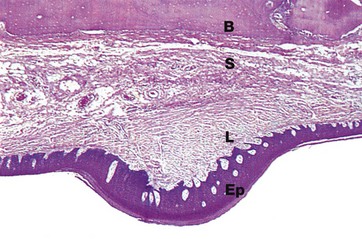
FIG. 13.2 Palatal mucosa, monkey
H&E (LP)
Like the rest of the mouth, the palate is covered by a thick stratified squamous epithelium Ep supported by a tough, densely collagenous lamina propria L. To assist mastication, the palatal mucosa is thrown up into transverse folds or rugae, one of which is shown in this micrograph. The mucosa of the hard palate is bound down to the underlying bone B by relatively dense submucosal tissue S containing a few accessory salivary glands.
In rodents and many other mammals with a coarse diet, the surface epithelium of particularly exposed areas is keratinised for extra protection, as in this specimen taken from a monkey.
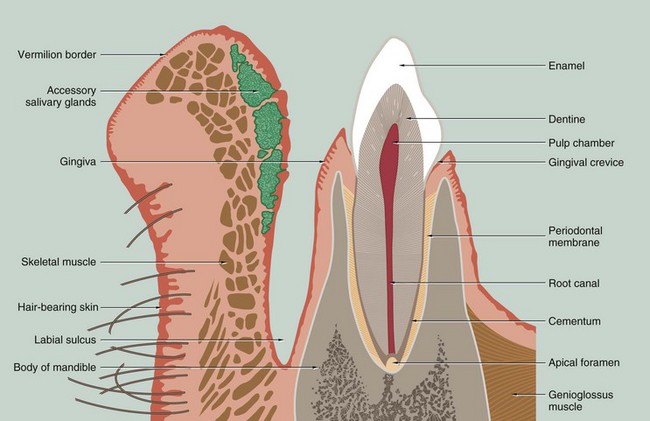
FIG. 13.3 Lip and tooth
This drawing of a section through the lower jaw near the midline illustrates the general arrangement of the lip and a tooth with its supporting structures. Each tooth may be grossly divided into two segments, the crown and the root; the crown is that portion which projects into the oral cavity and is protected by a layer of highly mineralised enamel which covers it entirely. The bulk of the tooth is made up of dentine, a mineralised tissue which has a similar chemical composition to bone. The dentine has a central pulp cavity or chamber containing the dental pulp which consists of specialised supporting tissue containing many sensory nerve fibres. The tooth root is embedded in a bony ridge in the jaw called the alveolar ridge; the tooth socket is known as the alveolus. At the lip or cheek (buccal) aspect of the alveolus, the bony plate is generally thinner than at the tongue (palatal) aspect. The root of the tooth is invested by a thin layer of cementum which is connected to the bone of the socket by a thin fibrous layer called the periodontal ligament or periodontal membrane.
The oral mucosa covering the upper part of the alveolar ridge is called the gingiva and, at the junction of the crown and root of the tooth (the neck of the tooth), the gingiva forms a tight protective cuff around the tooth. The potential space between the gingival cuff and the enamel of the crown is called the gingival crevice. All of the tissues which surround and support the tooth are collectively known as the periodontium.
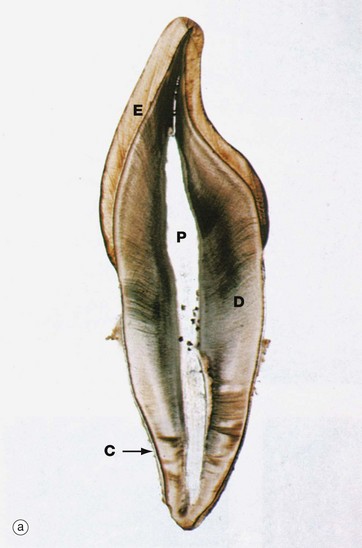
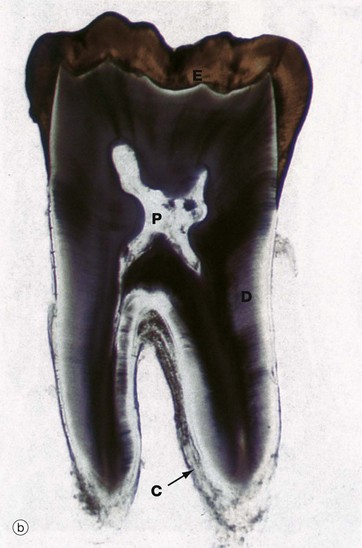

FIG. 13.4 Tooth structure
Undecalcified sections, unstained (a) LP (b) LP (c) (HP)
These undecalcified sections, cut with a diamond wheel, demonstrate the arrangement of the calcified tissues of an upper central incisor tooth, micrograph (a), and a lower molar tooth, micrograph (b). Micrograph (c) demonstrates the tissues of the crown at high magnification.
The dentine D, which forms the bulk of the crown and root, is composed of a calcified organic matrix similar to that of bone. The inorganic component constitutes a somewhat larger proportion of the matrix of dentine than that of bone and exists mainly in the form of hydroxyapatite crystals. Teeth are thus harder than bone. From the pulp cavity P, minute parallel tubules called dentine tubules radiate to the periphery of the dentine.
The crown is covered by enamel E, a translucent substance composed of parallel enamel rods or prisms of highly calcified material, cemented together by an almost equally calcified interprismatic material.
The root is invested by a thin layer of cementum C which is generally thicker towards the apex of the root. The cementum is an amorphous calcified tissue into which the fibres of the periodontal membrane are anchored.
The morphological form of the tooth crown and roots varies considerably in different parts of the mouth; nevertheless, the basic arrangement of the dental tissues is the same in all teeth.
In humans, the primary (deciduous) dentition consists of 20 teeth comprising two incisors, one canine and two molars in each quadrant. These begin to be formed at the age of 6 weeks during fetal development and they erupt between the ages of 6 and 30 months after birth. Between the ages of 6 and 12 years, the deciduous teeth are succeeded by permanent teeth, namely two incisors, one canine and two premolars in each quadrant. Distal to these will develop three permanent molars which have no primary precursors; the first permanent molar erupts at age 6, the second at age 12 and the third (wisdom tooth) at age 17 to 21 years. The sharp points found on the posterior teeth are known as cusps.
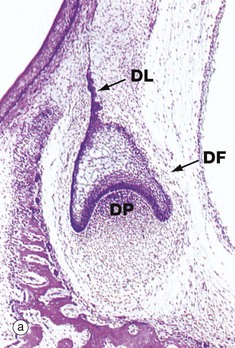
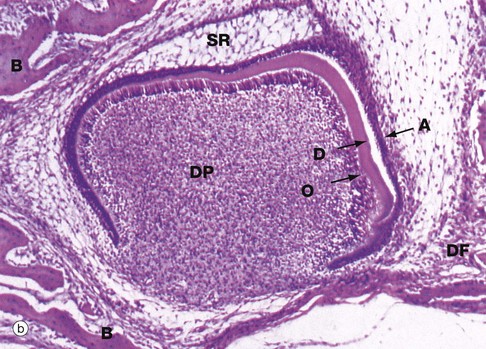
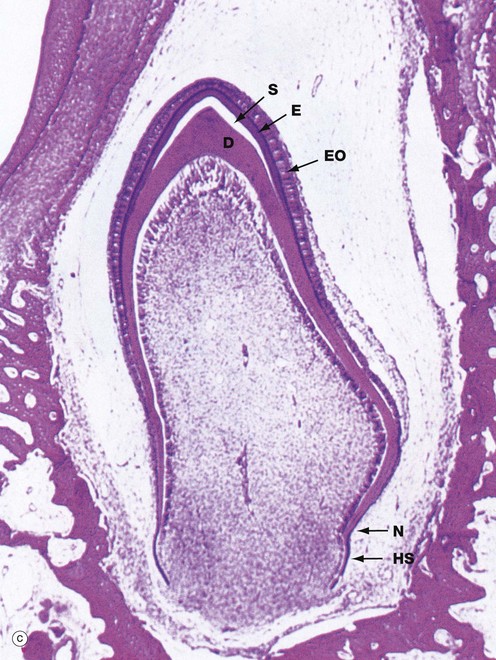
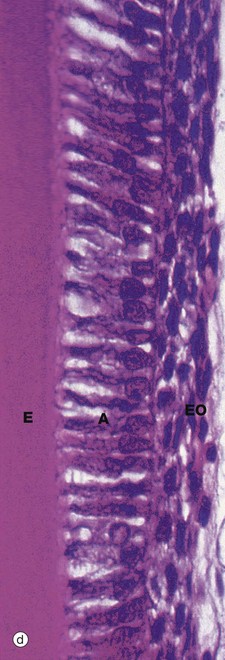
FIG. 13.5 Tooth development (illustrations opposite)
(a) H&E, cap stage (LP) (b) H&E, bell stage (MP) (c) H&E, onset of root development (LP) (d) H&E, ameloblasts (HP)
This series of micrographs illustrates the important stages of tooth development. The tissues of the teeth are derived from two embryological sources. The enamel is of epithelial (ectodermal) origin, while the dentine, cementum, pulp and periodontal ligament are of mesenchymal (mesodermal) origin. The first evidence of tooth development in humans occurs at 6 weeks of fetal life with the proliferation of a horseshoe-shaped epithelial ridge from the basal layer of the primitive oral epithelium into the underlying mesoderm in the position of the future jaws; this is known as the dental lamina. In each quadrant of the mouth, the lamina then develops four globular swellings which will become the enamel organs of the future deciduous central and lateral incisors, canines and first molar teeth. Subsequently, the dental lamina proliferates backwards in each arch, successively giving rise to the enamel organs of the future second deciduous molar and the three permanent molars. The permanent successors of the deciduous teeth will later develop from enamel organs which bud off from the inner aspect of the enamel organs of their deciduous predecessors.
The primitive mesenchyme immediately subjacent to the developing enamel organ proliferates to form a cellular mass, the dental papilla DP. At the same time, the enamel organ becomes progressively cap-shaped, as seen in micrograph (a), enveloping the dental papilla. During the cap stage, the cells lining the concave face of the enamel organ in contact with the dental papilla begin to differentiate into tall columnar cells, ameloblasts, which will be responsible for the production of enamel. This, in turn, induces the differentiation of a layer of columnar odontoblasts, the future dentine-producing cells, in the apical region of the dental papilla. The interface between the differentiating ameloblast and odontoblast layers marks the position and shape of the future junction between enamel and dentine.
As the enamel organ develops further, it assumes a characteristic bell shape as seen in micrograph (b), the free edge of the ‘bell’ proliferating so as to determine the eventual shape of the tooth crown. Meanwhile, the cells of the main bulk of the enamel organ become large and star-shaped, forming the stellate reticulum SR, the extracellular matrix of which is rich in glycosaminoglycans. Between the stellate reticulum and ameloblast layer, two or three layers of flattened cells form the stratum intermedium, while the outer surface of the enamel organ consists of a simple cuboidal epithelium called the external enamel epithelium. By the cap stage of development, the dental lamina DL connecting the enamel organ with the oral mucosa has become fragmented and, around the whole developing bud, a condensation of mesenchyme forms the dental follicle DF which will eventually become the periodontal ligament.
As ameloblasts and odontoblasts differentiate at the tip of the crown, a layer of dentine matrix is progressively laid down between the ameloblast and odontoblast layers. As the odontoblasts retreat, each leaves a long cytoplasmic extension, the odontoblastic process, embedded within the dentine matrix, thereby forming the dentine tubules. Dentine matrix has a similar biochemical composition to that of bone and undergoes calcification in a similar fashion. Deposition of dentine induces the production of enamel by the adjacent ameloblasts. Each retreating ameloblast lays down a column of enamel matrix which then undergoes mineralisation, resulting in the formation of a dense prismatic structure as described below. With the deposition of dentine and enamel, the overlying stellate reticulum atrophies and the enamel organ is much reduced in thickness. These changes are well demonstrated in micrograph (b). A thin layer of dentine D has been laid down by the underlying odontoblastic layer O of the highly cellular dental papilla DP. The ameloblastic layer A is about to lay down enamel in the space next to the dentine; note that in this area, the stellate reticulum has disappeared. Note also the surrounding dental follicle DF and early formation of cancellous bone B.
By the time dentine and enamel formation is well underway at the incisal edge or tips of the cusps (as the case may be), the enamel organ will have fully outlined the shape of the whole tooth crown. This is the case in micrograph (c), the neck of the tooth N marking the junction of crown and root. A thin, densely stained layer of poorly mineralised enamel E can be seen, covered at its external surface by the now much thinner enamel organ EO. The unstained space S between this and the underlying dentine D represents fully mineralised enamel laid down earlier but dissolved away during tissue preparation. Although enamel production is confined to the crown, the rim of the ‘bell’ of the enamel organ nevertheless continues to proliferate, inducing dentine formation and thereby determining the shape of the tooth root. This part of the enamel organ, known as the epithelial sheath of Hertwig HS, disintegrates once the outline of the root is completed. The cementum which later forms on the root surface is derived from the dental follicle. As the dentine of the crown and root are progressively laid down, the dental papilla shrinks and eventually becomes the dental pulp contained within the pulp chamber and root canals.
Growth of the tooth root is one of the principal mechanisms of tooth eruption and root formation is not completed until some time after the crown has fully erupted into the oral cavity.
Micrograph (d) illustrates the characteristic appearance of ameloblasts. Active ameloblasts A are tall columnar epithelial cells which form a single layer apposed to the forming surface of the enamel E. Each ameloblast elaborates a column of organic enamel matrix which undergoes progressive mineralisation by the deposition of calcium phosphate, mainly in the form of hydroxyapatite crystals. Fully formed enamel contains less than 1% organic material and is the hardest and most dense tissue in the body.
Mature enamel consists of highly calcified enamel prisms separated by interprismatic enamel, consisting of similar crystals orientated in a different direction. Each prism extends from the dentino-enamel junction to the enamel surface. The prisms are made up of groups of long, thin, parallel crystallites of hydroxyapatite, covered by a surface layer of organic material. Underlying the ameloblast layer are several layers of cells, also of epithelial origin, which constitute the remainder of the enamel organ EO. As enamel formation progresses, the enamel organ becomes much reduced in thickness compared with earlier stages of its development. At tooth eruption, the enamel organ, including the ameloblasts, degenerates leaving the enamel exposed to the hostile oral environment, completely incapable of regeneration.
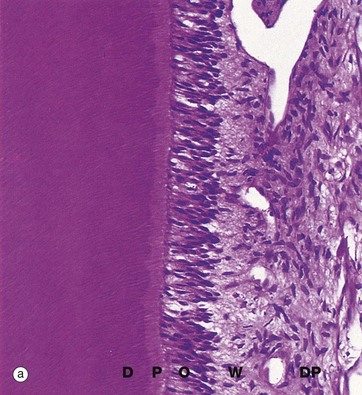
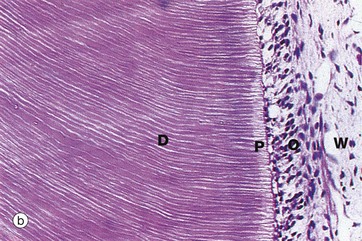
FIG. 13.6 Odontoblasts and dentine
Decalcified sections (a) H&E (MP) (b) H&E (MP)
Dentine, the dense calcified tissue which forms the bulk of the tooth, is broadly similar to bone in composition but is more highly mineralised and thus much harder than bone. The cells responsible for dentine formation, the odontoblasts, differentiate as a single layer of tall columnar cells on the surface of the dental papilla, apposed to the ameloblast layer of the enamel organ. The odontoblasts initiate tooth formation by deposition of organic dentine matrix between the odontoblastic and ameloblastic layers; calcification of this dentine matrix then induces enamel formation by ameloblasts (see Fig. 13.5). Odontoblasts continue to produce dentine which subsequently calcifies. Unlike ameloblasts, each odontoblast leaves behind a slender cytoplasmic extension, the odontoblastic process, within a fine dentine tubule. When dentine formation is complete, the dentine is thus pervaded by parallel odontoblastic processes radiating from the odontoblast layer on the dentinal surface of the reduced dental papilla which now constitutes the dental pulp. After tooth formation is complete, a small amount of less organised secondary dentine continues to be laid down, resulting in the progressive obliteration of the pulp cavity with advancing age.
These micrographs illustrate active odontoblasts O forming a pseudostratified layer of columnar cells at the dentine surface. Parallel dentine tubules containing odontoblastic processes extend through a narrow pale-stained zone of uncalcified dentine matrix called predentine P into the mature dentine D; the dentine tubules are best seen in micrograph (b). Underlying the odontoblastic layer, a relatively acellular layer called the cell-free zone of Weil W gives way to the highly cellular dental pulp DP.
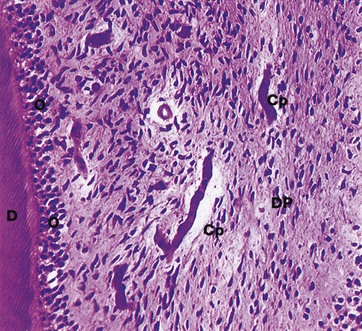
FIG. 13.7 Dental pulp
Decalcified section, H&E (MP)
The dental pulp DP consists of a delicate supporting/connective tissue resembling primitive mesenchyme (see Fig. 4.2); it contains numerous stellate fibroblasts, reticulin fibres, fine collagen fibres and plentiful ground substance. The pulp contains a rich network of thin-walled capillaries Cp supplied by arterioles which enter the pulp canal from the periodontal membrane, usually via one foramen at each root apex. The pulp is also richly innervated by a plexus of myelinated nerve fibres from which fine, non-myelinated branches extend into the odontoblastic layer. Despite the acute sensitivity of dentine, nerve fibres are rarely demonstrable and the mechanism of sensory reception is unknown; it has been suggested that the odontoblastic processes may act as sensory receptors. Odontoblasts O and the edge of the dentine D can also be identified.
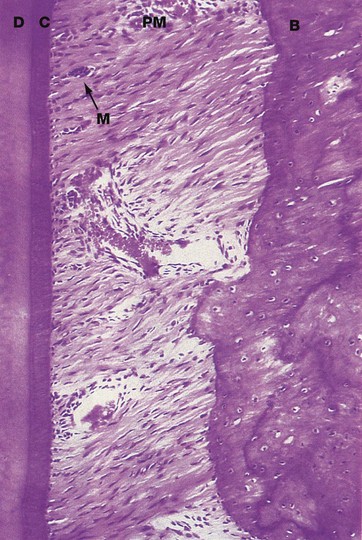
FIG. 13.8 Periodontal membrane and cementum
Decalcified section, H&E (MP)
The periodontal membrane PM forms a thin fibrous attachment between the tooth root and the alveolar bone. The dentine D comprising the root is covered by a thin layer of cementum C which is elaborated by cells called cementocytes, lying on the surface of the cementum. Cementum consists of a dense, calcified organic material, similar to the matrix of bone, and is generally acellular. Towards the root apex, the cementum layer becomes progressively thicker and irregular and cementocytes are often entrapped in lacunae within the cementum.
The periodontal membrane consists of dense collagenous tissue. The collagen fibres, known as Sharpey's fibres, run obliquely downwards from their attachment in the alveolar bone B to their anchorage in the cementum at a more apical position on the root surface. The periodontal membrane thus acts as a sling for the tooth within its socket, permitting slight movements which cushion the impact of chewing. The points of attachment of the collagen fibres in both cementum and bone are in a constant state of reorganisation to accommodate changing functional stresses upon the teeth. Osteoclastic resorption is often seen at one aspect of a tooth socket and complementary osteoblastic deposition at the opposite side, thus indicating bodily movement of the tooth through the bone; this is the mechanism which permits tooth movement during orthodontic treatment.
The periodontal membrane is richly supplied by blood vessels and nerves from the surrounding alveolar bone, the apical region and the gingiva. Small clumps of epithelial cells are often found scattered throughout the periodontal membrane; these cells are remnants of Hertwig's sheath (see Fig. 13.5) and are known as epithelial rests of Malassez M.
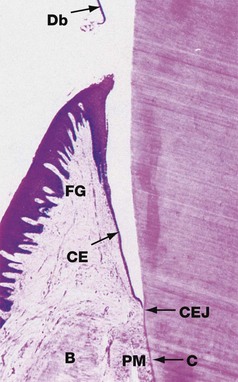
FIG. 13.9 Gingival attachment
Decalcified section, H&E (HP)
This micrograph shows the relationship of the gingiva (gum) to the neck of the tooth. During tissue preparation, the enamel has been completely dissolved from the surface of the crown, but the extent of the outer surface of the enamel can be visualised by shreds of remaining organic debris Db which had been adherent to the tooth surface.
The gingiva may be divided into the attached gingiva, which provides a protective covering to the upper alveolar bone B, and the free gingiva FG, which forms a cuff around the enamel at the neck of the tooth. Between the enamel and the free gingiva is a potential space, the gingival crevice, which extends from the tip of the free gingiva to the cemento-enamel junction CEJ.
The thick stratified squamous epithelium which constitutes the oral aspect of the gingiva undergoes abrupt transition at the tip of the free gingiva to form a thin layer of epithelial cells, tapering to only two or three cells thick at the base of the gingival crevice. This crevicular epithelium CE is easily breached by pathogenic organisms and the underlying supporting tissue is thus frequently infiltrated by lymphocytes and plasma cells. Collagen fibres of the periodontal membrane PM radiate from the cementum C near the cemento-enamel junction into the dense supporting tissue of the free gingiva; these fibres, together with circular fibres surrounding the neck of the tooth, maintain the role of the gingiva as a protective cuff.
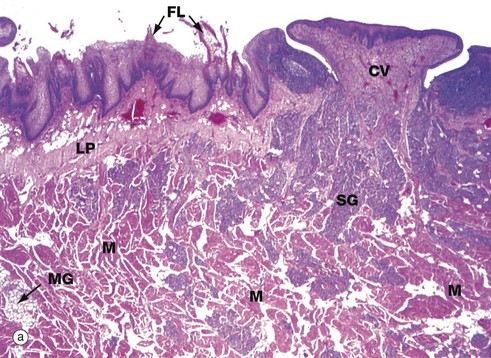
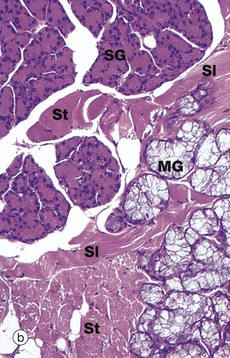
FIG. 13.10 Tongue, anterior two-thirds
(a) H&E (LP) (b) H&E (MP)
The tongue is a muscular organ covered by oral mucosa which is specialised for manipulating food, general sensory reception and the special sensory function of taste. The tongue is also vital for speech.
A V-shaped groove, the sulcus terminalis, demarcates the anterior two-thirds of the tongue from the posterior one-third. The mucosa of the anterior two-thirds is formed into papillae of three types. The most numerous, the filiform papillae, appear as short ‘bristles’ macroscopically. Among them are scattered the small red globular fungiform papillae. Six to fourteen large circumvallate papillae form a row immediately anterior to the sulcus terminalis and these papillae contain most of the taste buds (see Figs 13.12 and 21.1); a circumvallate papilla CV and numerous filiform papillae FL are seen in micrograph (a). Foliate papillae, which are rudimentary in humans, are found in some animal species.
The body of the tongue consists of a mass of interlacing bundles of skeletal muscle fibres M which permit an extensive range of tongue movements. The mucous membrane covering the tongue is firmly bound to the underlying muscle by a dense, collagenous lamina propria LP, which is continuous with the epimysium of the tongue muscle.
Numerous small serous and mucous accessory salivary glands are scattered throughout the muscle and lamina propria of the tongue and are seen at higher magnification in micrograph (b). In these preparations, the serous glands SG are stained strongly, whereas the mucous glands MG are poorly stained. Note bundles of skeletal muscle cut in both transverse St and longitudinal Sl section.
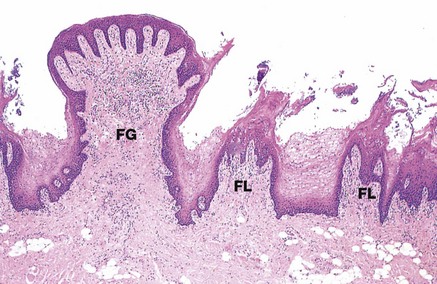
FIG. 13.11 Filiform and fungiform papillae
H&E (LP)
This micrograph illustrates several filiform papillae FL and a fungiform papilla FG. Filiform papillae are the most numerous type and consist of a dense supporting tissue core and a heavily keratinised surface projection. Fungiform papillae have a thin non-keratinised epithelium and a richly vascularised supporting tissue core, giving them a red appearance macroscopically among the more numerous whitish filiform papillae.
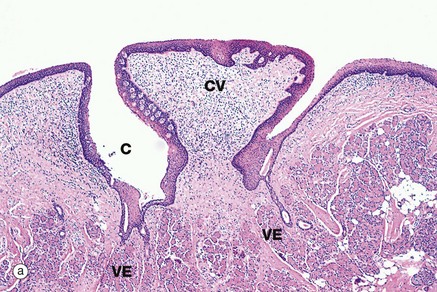
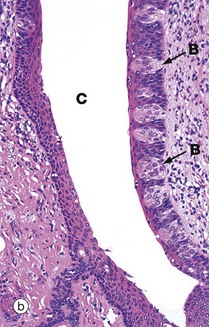
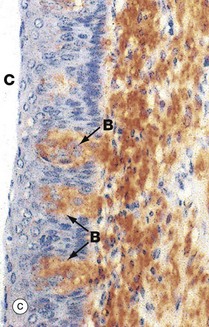
FIG. 13.12 Circumvallate papillae
(a) H&E (LP) (b) H&E (MP) (c) Immunohistochemistry for NSE (MP)
Circumvallate papillae CV are the largest and least common type of papillae on the tongue. They are set into the tongue surface and encircled by a deep cleft C. Aggregations of serous glands called von Ebner glands VE open into the base of the circumvallate clefts, micrograph (a), secreting a watery fluid which dissolves food constituents, thus facilitating taste reception. The stratified squamous epithelium lining the papillary wall of the cleft contains numerous taste buds B as shown in micrograph (b) (see also Fig. 21.1).
Micrograph (c) is stained by the Immunohistochemical method for the enzyme neurone-specific enolase (NSE). This demonstrates the neural nature of the taste buds B and the meshwork of fine axons (stained brown) in the lamina propria underlying the taste buds which subserve taste sensation.
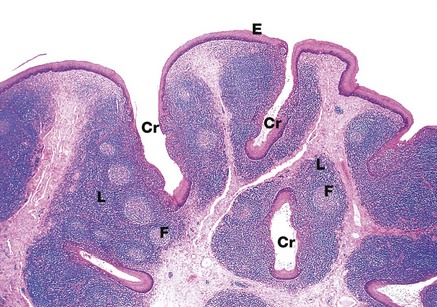
FIG. 13.13 Tongue, posterior third
H&E (LP)
The posterior surface of the tongue has a relatively smooth stratified squamous epithelium E overlying lymphoid tissue L containing lymphoid follicles F. This lymphoid tissue is the lingual tonsil and, with the palatine tonsils and adenoids, completes Waldeyer ring of lymphoid tissue, guarding the entrance to the gastrointestinal and respiratory tracts. Like the palatine tonsils (see Fig. 11.15), epithelial crypts Cr penetrate the lingual tonsil.
Salivary Glands
Saliva is produced by three pairs of major salivary glands, the parotid, submandibular and sublingual glands, and numerous minor accessory glands scattered throughout the oral mucosa. The minor salivary glands secrete continuously and are in general under local control, whereas the major glands mainly secrete in response to parasympathetic activity which is induced by physical, chemical and psychological stimuli. Daily saliva production in humans is 600 to 1500 mL.
Saliva is a hypotonic watery secretion containing variable amounts of mucus, enzymes (principally amylase and the antibacterial enzyme lysozyme), antibodies and inorganic ions. Two types of secretory cells are found in the salivary glands: serous cells and mucous cells. The parotid glands consist almost exclusively of serous cells and produce a thin watery secretion rich in enzymes and antibodies. The sublingual glands have predominantly mucous secretory cells and produce a viscid secretion. The submandibular glands contain both serous and mucous secretory cells and produce a secretion of intermediate consistency. The overall composition of saliva varies according to the degree of activity of each of the major gland types.
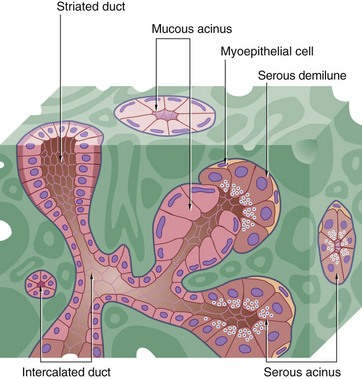
FIG. 13.14 Salivary secretory unit
The salivary secretory unit consists of a terminal branched tubulo-acinar structure composed exclusively of either serous or mucous secretory cells or a mixture of both types. In mixed secretory units where mucous cells predominate, serous cells often form semilunar caps called serous demilunes surrounding the terminal part of the mucous acini. Myoepithelial cells embrace the secretory units, their contraction helping to expel the secretory product.
The terminal secretory units merge to form small intercalated ducts which are also lined by secretory cells. They drain into larger ducts called striated ducts, so named because of their striated appearance by light microscopy. The striations result from the presence of numerous interdigitations of the basal cytoplasmic processes of adjacent columnar lining cells.
The serous cells secrete a fluid isotonic with plasma. In the striated ducts, ions are reabsorbed and secreted to produce hypotonic saliva containing less Na+ and Cl− and more K+ and HCO3− than plasma. The mitochondria which pack the basal processes provide the energy for ion transport.
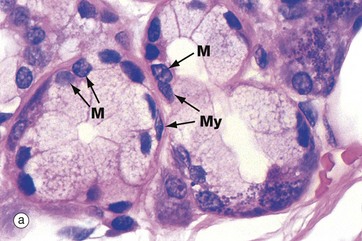
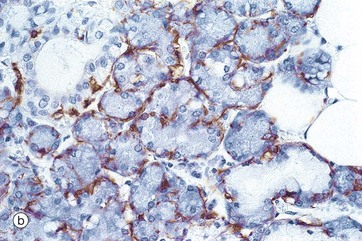
FIG. 13.15 Mucous acinus and myoepithelial cells
(a) H&E (MP) (b) Immunohistochemistry for actin (MP)
In H&E-stained preparations, the mucigen (mucus) granules within the mucous acini are poorly stained. The nuclei of mucous cells M are condensed and are characteristically located at the base of the cell near the basement membrane.
Both serous and mucous acini are embraced by contractile cells called myoepithelial cells which, on contraction, force secretion from the acinar lumen into the duct system. Myoepithelial cells are located between the basal plasma membranes of secretory cells and the basement membrane. These are flattened cells with long processes which extend around the secretory acinus; in section, they can only be recognised by their flattened nuclei lying within the basement membrane around the acinus. Micrograph (a) shows the typical appearance of myoepithelial cells My embracing mucous acini. In micrograph (b), a similar section has been stained using the immunohistochemical technique with an antibody specific for actin, a microfilament characteristic of muscle cells but not usually found in epithelial cells. The myoepithelial cells are stained brown. Myoepithelial cells also show characteristics of epithelial differentiation, including cytoplasmic cytokeratin intermediate filaments.
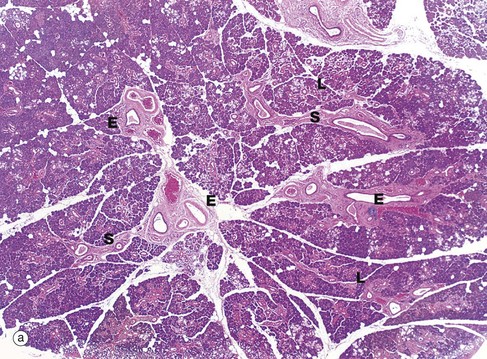
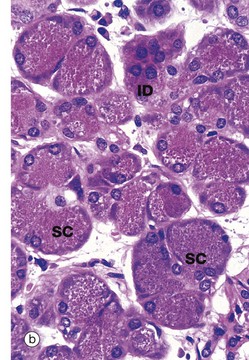
FIG. 13.16 Parotid gland
(a) H&E (LP) (b) Serous acinus and intercalated duct, H&E (MP)
The general architecture of the major salivary glands follows the pattern shown in this micrograph of the parotid gland. The gland is divided into numerous lobules L, each containing many secretory units. Connective tissue septa S radiate between the lobules from an outer capsule and convey blood vessels, nerves and large excretory ducts E. The parotid gland consists mainly of serous secretory units which are darkly stained in H&E preparations. Micrograph (b) shows these serous secretory units at higher power. The serous cells SC have numerous zymogen granules. These are strongly stained cytoplasmic granules containing proteins. Their nuclei are rounded with dispersed chromatin and they usually occupy a more central position within the cell (compared to mucus-secreting cells; see Fig. 13.15). In EM sections (not illustrated), a large Golgi apparatus, prominent rough endoplasmic reticulum and mitochondria are found, in common with other protein-secreting cells (see Fig. 15.15, an EM of a pancreatic secretory cell which is ultrastructurally very similar).
An intercalated duct ID with a lining of cuboidal secretory cells can be seen.
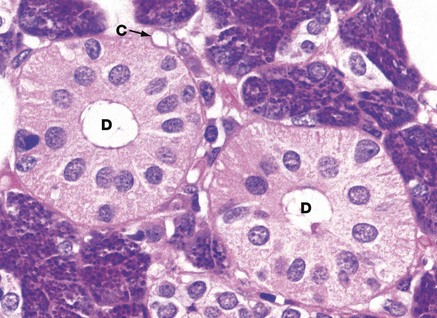
FIG. 13.17 Striated ducts
H&E (HP)
The striated ducts D are lined by tall columnar cells with large nuclei located towards the apex of the cell. The basal cytoplasm appears striated, reflecting the presence of basal interdigitations of cytoplasmic processes of adjacent cells and associated columns of mitochondria. This feature greatly extends the area of membrane available for exchange of water and ions, in a similar fashion to the proximal convoluted tubule of the kidney (see Fig. 16.17). The duct epithelium also secretes lysozyme and immunoglobulin (Ig)A. In predominantly serous salivary glands, the striated ducts are larger than in predominantly mucous glands, a feature associated with the role of the striated duct in modifying isotonic basic saliva to produce hypotonic saliva. The sparse supporting tissue between the secretory acini contains a rich network of capillaries C.
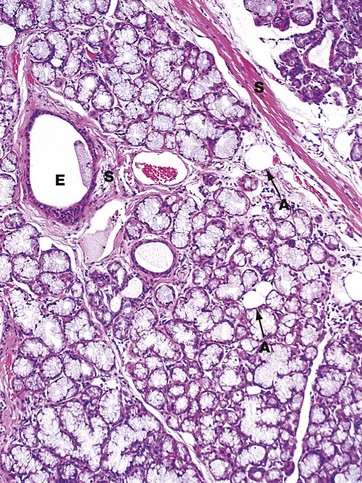
FIG. 13.18 Sublingual gland
H&E (LP)
Mucous acini predominate in the sublingual glands, making them stain very poorly with H&E, in contrast to the serous acini shown in the parotid in Fig. 13.16. A large excretory duct E lined by a stratified cuboidal epithelium is present in the fibrous tissue septum S. The duct is accompanied by blood vessels and nerves. Note also that this gland contains occasional adipocytes A, a feature found in older individuals; the proportion of adipose tissue (fat) in the gland generally increases with increasing age.
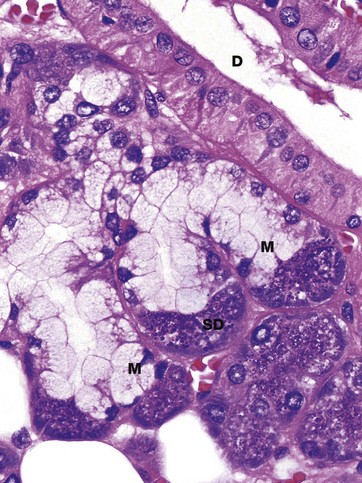
FIG. 13.19 Submandibular gland
H&E (HP)
The submandibular gland consists of a mixture of serous and mucous secretory units which are often found in the form of mixed seromucous secretory units as shown here. However, both pure serous and pure mucous secretory units are also found in the submandibular gland. The mixed secretory units consist of mucous acini M with serous demilunes SD. Running across the corner of the micrograph is a striated duct D cut in longitudinal section.
