The Immune Response
Introduction
The immune system has evolved to produce a coordinated response to protect the host from, and remove, ongoing infection
Key to this is the ability to distinguish self from nonself, whilst attempting to maintain the homeostasis of the body. Immunity can be categorized as being either innate (nonspecific) or adaptive (acquired/specific). Inappropriate responses to either self or nonself can result in immune-mediated conditions such as autoimmunity or hypersensitivity. In addition, the importance of a healthy and effective immune response can be seen in individuals who have one of the many immunodeficiency states. They can present with a rainge of ailments, from minor recurring infections to life-threatening illnesses, depending on severity of condition. The immune system often seems too complicated. In effect the complex collection of cells and molecules has evolved to eliminate the almost infinite infectious organisms we might meet, whilst causing minimal damage to self, and ultimately resolving. Key to this is the ability to recognize the pathogen. The following sections will describe how the mechanisms involved in innate and adaptive responses differ.
Innate immune response
When activated, the innate response is seen as an inflammatory response
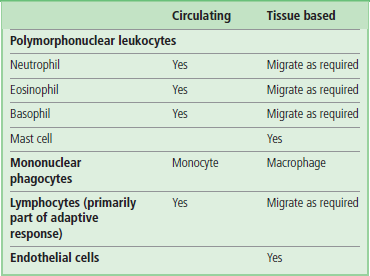
Innate immunity is the body's immediate response and first line of defense. The innate immune response protects an organism from attack, using physicochemical barriers, such as the skin and mucosal epithelia, and their associated secreted products, e.g. sweat, mucus and acid. It is often referred to as ‘natural’ or ‘nonspecific’, as it appears to be pre-existing. When activated, the innate response is often seen as an inflammatory response. Inflammation is the body's response to injury or tissue damage. Its purpose is to limit, and then repair, the damage brought about by any injurious agent. It involves the interaction of the microvasculature, circulating blood cells, other immune cells in the tissues, and their secreted effector molecules. Endothelial activation, increased vascular permeability and vasodilation allow the normally circulating leukocytes to migrate into tissue where, along with other tissue resident immune cells, they mount an effective and rapid response to try to eliminate the pathogen (Table 38.1). This will often include release of toxic mediators and phagocytosis, a process first described over 100 years ago by Mechnikov, who observed cells ‘eating’ the pathogens.
Inflammatory mediators contribute to the immune response
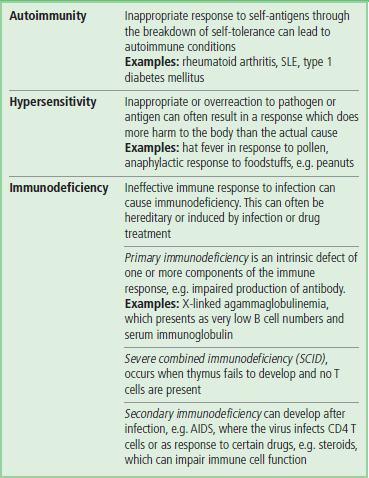
Innate immune cells, when activated, can synthesize and secrete a wide variety of different soluble chemical substances termed inflammatory mediators. Some may be directly toxic to the pathogen, whilst others (cytokines) may be released in an attempt to signal to, recruit, and activate other immune cells, in order to help in the response. The liver also produces a number of these mediators, present in the blood, including acute phase reactants such as C-reactive protein (CRP) (Chapter 4) and components of the complement system described below (Fig. 38.1).
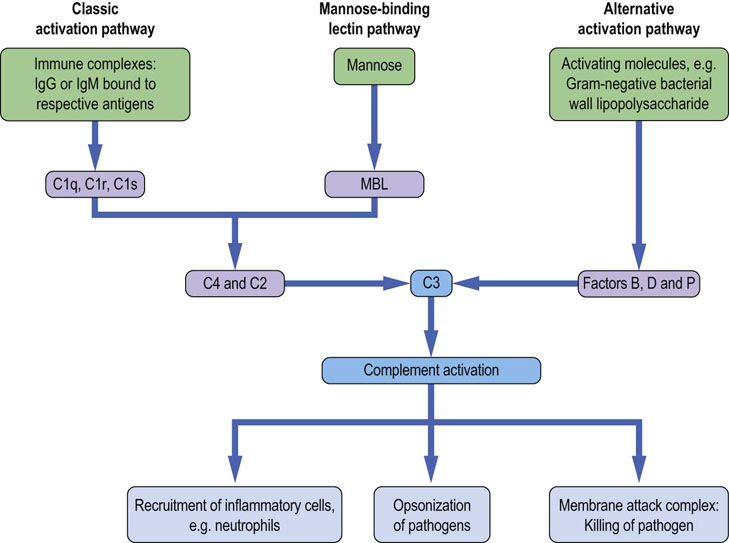
Fig. 38.1 The complement cascade.
Activating stimuli include surfaces that trigger complement activation, and to which the activated component can attach itself. Activation of complement recruits innate cells during early phase of an immune response. The late polymeric macromolecule (the membrane attack complex) can insert itself into the activating surface (the cell wall in the case of bacteria), breaching its integrity and causing osmotic lysis. MBL, mannose-binding lectin.
Cytokines
Cytokines are soluble mediators of inflammatory and immune responses
Cytokines are produced by a variety of cell types, including those of the innate and adaptive immune response. Covering a large number and different families, cytokines are small (usually less than 20 kDa) peptides or glycoproteins active at concentrations between 10−9 and 10−15 mol/L. In general, macrophages are often their main producers during innate responses, and T cells during adaptive responses. However, many cell types, including all the cells of the immune system as well others, such as fibroblasts, epithelial cells and adipocytes, can secrete cytokines. By interacting with specific receptors on the surfaces of their target cells, the large number of cytokines now identified exhibit many effects. The majority act locally to their site of production (paracrine action) or on the very cells that produced them (autocrine action). A few, however, are capable of acting on cells more distant from their site of production (endocrine action). The cytokine network displays both redundancy and pleiotropy, with several having overlapping effects and an ability to act on numerous cell types. Cytokines have been grouped into subfamilies, based on structure and function, as discussed briefly below. Cytokine receptors are not restricted to cells of the immune system, being found widespread on disparate cell types. For more detail on cytokine signaling, see Chapter 40.
Cytokines may be classified into families by their principal effect:
 Colony-stimulating factors: as the name suggests, these are involved in the development and differentiation of immune cells from bone marrow precursors.
Colony-stimulating factors: as the name suggests, these are involved in the development and differentiation of immune cells from bone marrow precursors.
 Interferons (IFNs): while IFN-α and IFN-β have a role in inhibiting viral replication, IFN-γ regulates immune responses. The latter is made primarily by T cells and activates macrophages.
Interferons (IFNs): while IFN-α and IFN-β have a role in inhibiting viral replication, IFN-γ regulates immune responses. The latter is made primarily by T cells and activates macrophages.
 Interleukins (ILs): currently there are in excess of 30 interleukins recognized, participating in regulating both innate and adaptive immune responses. They are made by a number of immune (and other) cell types, as the name suggests, the principal mode of action is communication between leukocytes.
Interleukins (ILs): currently there are in excess of 30 interleukins recognized, participating in regulating both innate and adaptive immune responses. They are made by a number of immune (and other) cell types, as the name suggests, the principal mode of action is communication between leukocytes.
 Tumor necrosis factor (TNF) family: this is a mixed collection of cytokines whose effects range from promoting inflammation (TNF-α and TNF-β) to stimulating osteoclasts and bone resorption (osteoprotegerin, OPG).
Tumor necrosis factor (TNF) family: this is a mixed collection of cytokines whose effects range from promoting inflammation (TNF-α and TNF-β) to stimulating osteoclasts and bone resorption (osteoprotegerin, OPG).
 Chemokines: these are a family of cytokines that bring about chemokinesis – movement in response to chemical stimuli. Interest has increased dramatically in the receptors for these mediators since some appear to act as co-receptors for infection (in particular HIV infection of CD4+ T lymphocytes).
Chemokines: these are a family of cytokines that bring about chemokinesis – movement in response to chemical stimuli. Interest has increased dramatically in the receptors for these mediators since some appear to act as co-receptors for infection (in particular HIV infection of CD4+ T lymphocytes).
Previously it was fashionable to describe cytokines as either pro- or anti-inflammatory. It is now clear that this can be confusing given their pleiotropic effects. Comparing cytokine production in innate and adaptive responses and their cells of origin can now be considered.
 During innate responses macrophages, dendritic cells and natural killer (NK) cells are major producers of TNF-α, IL-1, IL-6, IL-8 and many chemokines, IL-12, IL-15 and IL-18, IFN-γ (NK cells). These can all be thought of as important intercellular communicators, inducing inflammation and immune responses.
During innate responses macrophages, dendritic cells and natural killer (NK) cells are major producers of TNF-α, IL-1, IL-6, IL-8 and many chemokines, IL-12, IL-15 and IL-18, IFN-γ (NK cells). These can all be thought of as important intercellular communicators, inducing inflammation and immune responses.
 If an adaptive or cell-mediated response develops, T cells, especially CD4+ T cells, become a major producer of cytokines. Their effects generally either promote or control further responses and they include IL-2, IL-4, IL-5, IL-10, IL-13, IL-17, IL-22, and TGF-β.
If an adaptive or cell-mediated response develops, T cells, especially CD4+ T cells, become a major producer of cytokines. Their effects generally either promote or control further responses and they include IL-2, IL-4, IL-5, IL-10, IL-13, IL-17, IL-22, and TGF-β.
The complement system
Complement is activated in a series of sequential steps
The complement system, consisting of a series of proteins, both circulating and cell-membrane-bound, plays an important role in antimicrobial host defense. There are three pathways of complement activation. As part of the innate response, and in the absence of antibody, the alternative and lectin pathways activate complement during infection. For example, recognition of lipopolysaccharide found on Gram-negative bacterial cell walls will trigger the alternative pathway and mannose and other particular carbohydrates found in the cell wall of fungi, bacteria and viruses will trigger the lectin pathway. In addition, antibody produced during the adaptive response to infection can bind microbial antigens and activate complement via the classic pathway. The sequential activation of the cascade by proteolytic cleavage results in an autoamplifying response, producing a number of effector molecules involved in elimination of the microbial infection, as shown in Figure 38.1. The different pathways converge to produce a common outcome, whereby the late components combine with each other to form a multimolecular complex that can breach the integrity of the surface of infecting organisms by insertion into their membrane (the membrane attack complex, MAC). Fragments produced as a result of the complement cascade have distinct biological activities, which include the facilitation of phagocytosis (termed opsonization), the attraction of cells (chemotaxis), and stimulating the degranulation of immune cells (anaphylatoxin activity).
Cells participating in the innate response
Neutrophils and monocytes are recruited to sites of infection
Neutrophils and monocytes which are normally found circulating in the bloodstream are recruited to sites of infection by the process of extravasation. Through the interaction of receptors on the phagocyte and counter-ligands on vascular endothelium, cells attach, arrest and move from the circulation to the infected tissue. Neutrophils are the most abundant leukocytes in the bloodstream, numbering 4000–10,000/mm3. This increases rapidly during infection through recruitment from the bone marrow, and numbers often reach 20,000/mm3. Neutrophils are generally the first cells to respond to infection, phagocytosing microbes in the circulation and moving rapidly into infected tissue. They are short-lived (normally a few hours to days) and die rapidly after reaching the tissue and exerting their effect by the process of apoptosis (Chapter 42).
Monocytes transform into macrophages, which are‘the dustbin of the immune response’
Monocytes are found in much lower numbers within the blood, 500–1000/mm3, and, by contrast with neutrophils, are longer-lived. Similarly to neutrophils, they can also migrate into tissue, and on doing so they differentiate into macrophages. Macrophages have a number of key functions, including phagocytosis of infecting microbes, antigen presentation and general removal of dying or damaged host cells. Indeed, the macrophage has often been termed the ‘dustbin of the immune response’. Most organs of the body and connective tissue have resident macrophages, whose job it is to survey their environment for signs of infection. Their rapid identification of infection with the resultant release of numerous cytokines and chemokines, initiates the inflammatory response.
Neutrophils and macrophages recognize attacking microbes through their receptors
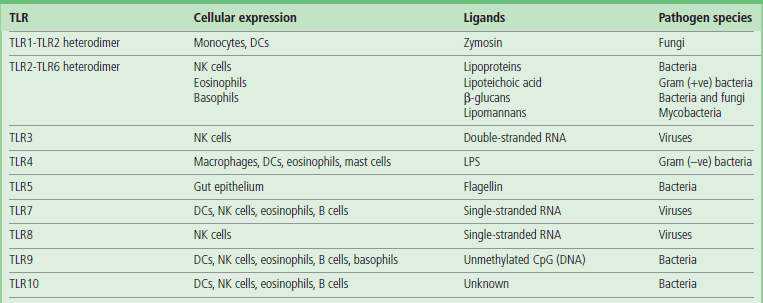
In order to mount an efficient response to infection, neutrophils and macrophages must realize that the body is under attack. They do so through a number of germline-encoded cell surface and intracellular receptors which, unlike the receptors used by cells of the adaptive immune response, are not produced by somatic recombination of their genes (Table 38.2). As a result, the response elicited by such receptors is amnesic, meaning that the cells will respond similarly on reinfection. The receptors involved in microbial recognition, and often termed pattern recognition receptors (PRR), identify structures that are shared by various microbes, and which are generally not present on host cells, such as nucleic acids, lipids, sugars, proteins, or a combination of molecules. Often the structures recognized by these receptors, called pathogen-associated molecular patterns (PAMPs), are conserved structural features required by the pathogen for survival or infectivity, and are generally common to particular microbial families.
Table 38.2
Comparison of antigen receptors of innate and adaptive immunity
| Receptor characteristic | Innate immunity | Adaptive immunity |
| Triggers an immediate response | Yes | No |
| Receptors germline encoded | Yes | No |
| Specificity same across lineage | Yes | No |
| Broad spectrum of recognition | Yes | No |
| Encoded by multiple gene segments | No | Yes |
| Gene rearrangement occurs | No | Yes |
| Each receptor has unique specificity | No | Yes |
There are four main categories of pattern recognition receptors, classified according to location and function
The first is mannose-binding lectin (MBL), which is actually not a cell-associated molecule, but rather a free circulating plasma protein, which on recognizing and binding to pathogen PAMP can activate the complement cascade via the lectin pathway (Fig. 38.1). The remaining are either surface-bound receptors that promote phagocytic function of the cell, or membrane-bound signaling receptors that found either on the outer cell membrane of the cell or within the cytoplasm.
Receptors are used by neutrophils and macrophages to promote phagocytosis
Microbes can be coated by soluble mediators of the immune response, such as complement components or antibodies, in the process termed opsonization. This makes the phagocytic process of microbial uptake by neutrophils and macrophages more efficient. Surface receptors used by neutrophils and macrophages to promote phagocytosis and killing of microbes include the mannose and scavenger receptors, complement receptors and Fc receptors. Mannose and scavenger receptors allow direct microbial recognition by phagocytes, whilst the complement and Fc receptors which recognize the opsonized microbe coated with complement components and antibodies, respectively, will also enhance phagocytic activity (Fig. 38.2).
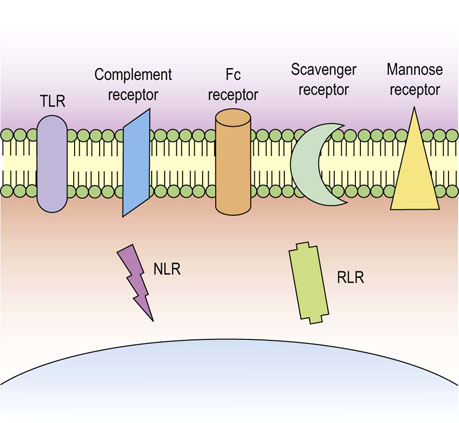
Fig. 38.2 Phagocytes utilize numerous receptors to detect pathogens.
Cells of the innate immune system express numerous receptor types, both on the cell membrane and intracellularly, to detect pathogen and initiate an effective immune response. TLR, toll-like receptor, NLR, NOD-like receptor, RLR, RIG-1-like receptor.
Signaling receptors are used by innate immune cells to trigger many of their functions
One of the best characterized signaling PRR families is the evolutionary conserved Toll-like receptor (TLR) system in mammals, named after a homologous receptor system used by the Drosophila fruit fly for protection from infection. In man, there are 10 expressed TLR genes (13 in mice), their products forming homo- or heterodimers with other family members, thus increasing the repertoire for recognition. TLR4, for example, has been shown to be the receptor recognizing lipopolysaccharide (LPS) found on the surface of Gram-negative bacteria such as E. coli but not present on mammalian cells. The effects of TLR activation can vary depending on need, and may include increased cytokine/chemokine production, enhanced phagocytosis, upregulation of costimulatory molecules on cell surface, cell migration and, if appropriate, processing and presentation of pathogen antigens to T cells in order to activate an adaptive immune response. Table 38.3 summarizes TLR function and cellular distribution. TLRs can be expressed either on external cell membrane or on intracellular vesicles and function primarily to recognize extracellular pathogens. Whilst certain intracellular TLRs (TLR3/7/9) can detect viral RNAs and DNAs, they interact primarily with extracellular pathogen products entering by the endocytic pathway.
NOD-like receptors are located in the cytoplasm
Other pattern recognition receptors including the more recently described NOD-like receptors (NLR), are located within the cytoplasm. They act as intracellular sensors, ultimately triggering the NFκB pathway, which results in similar responses to those activated following TLR engagement. In the presence of certain invading pathogenic stimuli (bacterial, fungal, or viral), TLRs and NLRs cooperate, activating a cytoplasmic multiprotein complex called the inflammasome. The resulting caspase-1 activation leads to the processing and release of mature forms of pro-inflammatory cytokines, including IL-1 and IL-18.
A final family of intracellular signaling PRRs, the RIG-1-like receptors (RLR), which detect viral RNAs and stimulate antiviral responses through the production of interferon (Fig. 38.2).
Activation of PRRs such as TLR/NLR not only induce efficient innate responses to deal with microbial infections but also may induce responses, which in turn lead to the full activation of the adaptive immune response.
Adaptive immune response
Specificity of the response is achieved through receptors that recognize antigen
Adaptive immune responses are brought into play if our innate defenses are unsuccessful, e.g. due to the persistence of the triggering agent. The response is initiated when the lymphocytes recognize components of the infectious agent. The infectious agents are called antigens and their binding to receptors on lymphocytes triggers an adaptive response. Receptors on B cells and T cells differ and see quite different forms of antigen.
Thymic education and self-tolerance help distinguish between self and nonself
Crucial for successful adaptive responses is the ability to distinguish between self and nonself. The immune system does this through the processes of thymic education and self-tolerance. This ensures that appropriate immune responses to infection develop. Failure of this process and inappropriate activation of the immune response by self-antigens can result in developing autoimmunity, presenting as e.g. rheumatoid arthritis or systemic lupus erythematosus.
Adaptive immune response needs time to develop and remembers what it sees
When an adaptive immune response is first initiated, relatively few cells and components are likely to be available that could react specifically with any chosen antigenic substance. There is a delay while these increase to a level which can ensure elimination of the antigen, or at least reduce the antigen to a level that would be manageable by the innate immune response.
The adaptive immune response employs a mechanism to remember a specific encounter, so that if the same foreign antigen is encountered again, it can be dealt with more quickly and effectively. This is called immunologic memory. Thus, in comparison to innate immunity, the adaptive response exhibits both specificity for and memory to the foreign or nonself antigen.
Cells primarily responsible for adaptive response are the lymphocytes
Adaptive immunity is mediated, similarly to the innate response, by cellular and humoral elements. The cells primarily responsible are the lymphocytes. There are two major types of lymphocytes circulating in blood and present within the lymphoid tissues:
 T cells, which are responsible for a number of cellular responses.
T cells, which are responsible for a number of cellular responses.
 B cells, which are responsible for humoral responses, i.e. antibody production.
B cells, which are responsible for humoral responses, i.e. antibody production.
In addition to the lymphocytes, professional antigen-presenting cells (APCs) are required for the initial efficient activation of the T lymphocytes.
T and B lymphocytes
T and B lymphocytes carry particular collections of cell surface markers that can assist in assigning their lineage
The effector cells primarily involved in the adaptive immune response are the T and B lymphocytes. In total, lymphocytes are present in the peripheral blood at 1.3–4.0 × 109/L. Of these, approximately 50–70% are T cells and 10–20% are B cells.
A third population termed ‘natural killer’ (NK) cells, so-called because they demonstrate the ability to kill neoplastic or virally infected cells without prior exposure or sensitization. They are atypical lymphocytes and are generally considered to be part of the innate response. Identification of T and B cells is based on immunophenotypic or functional studies. They carry particular collections of cell surface markers that can assist in assigning their lineage. The distinction between T and B cells is most easily made with reference to their antigen receptors (Fig. 38.3).
Fig. 38.3 Similarity in structure of T and B cell antigen receptors.
The receptors used by T cells and B cells to detect antigen share structural similarity. V, variable regions; C, constant regions.
Insert new para:The surface markers on lymphocytes and other immune cells are classified according to the cluster of differentiation (CD) system based on antibody-binding characteristics of these molecules.
B and T lymphocytes are activated by binding of antigen and by costimulatory molecules
The antigen recognition receptor on the B cell is a surface immunoglobulin termed ‘sIg’. On binding to its antigen, it brings about the cell's activation, and subsequent proliferation and differentiation. In addition to sIg, B, cells express several other markers, the best characterized of which include CD19, CD20 and the major histocompatibility complex (MHC) class II molecules.
The T cell antigen receptor is termed the T cell receptor (TCR) and it is complexed with CD3
Two other CD markers whose expression appears to be mutually exclusive on T cells are the CD4 and CD8, and they are useful in further categorizing the T cell function.
NK cells are currently identified by the expression of the combination of CD16 and CD56
These markers are often used in flow cytometric technology using fluorescent monoclonal antibodies to identify cell types.
Another group of surface molecules, the co-stimulatory molecules, are found on the surface of T and B cells
Following exposure to antigen, CD28 on T cells will bind CD80/CD86 on the APC, while CD40 on B cells will bind CD40L on T cells, resulting in full activation. Without such co-stimulation, T and B cells would not be fully activated following exposure to antigen and could become anergic, i.e. nonresponsive.
Molecules involved in antigen recognition
Antigen is recognized by specific receptors on T and B cells
The ability to recognize the enormous number of possible antigenic configurations is achieved by differences in amino acid sequence of these receptors, which gives rise to differences in their shape or conformation. The antigen and its specific receptor have a ‘hand-in-glove’ relationship. Both T and B cell antigen receptors show marked variability in the sequence of amino acids that come into contact with the antigen, while other parts of these molecules are relatively constant with regard to their amino acid sequences.
Unlike the antigen receptors found on innate cells, which are germline encoded, the receptors found on the T and B cells are generated by random recombination of receptor genes during cell maturation. These antigen recognition receptors are clonally distributed. As a result, each clone will exhibit unique specificity for a particular antigen, thus generating the enormous pool of cells capable of responding to all antigens.
As mentioned previously, T and B cells differ in what they recognize as ‘foreign’. The sIg antigen receptor found on B cells is capable of recognizing macromolecules (proteins, polysaccharides, lipids, etc.), whereas T cell receptors recognize small peptides of proteins previously processed by the APC.
Although the number of T and B cell clones, each recognizing different antigens, is enormous, the engagement by appropriate antigen will generally induce a similar response, i.e. signal transduction. This may lead to full cell activation resulting, for B cells, in antibody production, and for T cells, in the proliferation and promotion of the cellular adaptive immune response.
The T cell antigen receptor
The T cell antigen receptor (TCR) resembles the binding portion of an immunoglobulin molecule
The TCR is a heterodimer made up of two nonidentical polypeptide chains termed α and β (see Fig. 38.3). In addition, a small unique T cell population found primarily within the gut expresses alternative TCRs, their chains being termed γ and δ. Each chain of the TCR comprises two domains – one constant and one variable amino acid sequence. The antigen-binding site of the TCR is in the cleft formed by the adjoining single N-terminal variable domains of the constituent α (Vα) or β (Vβ) chains. The effector function of the constant domain in each of the antigen receptor chains is signal transduction. The two chains come into close contact via the covalent bonds between the variable domains and noncovalent hydrophobic interactions between the opposing faces of the constant domains. Structurally, the TCR resembles the binding portion of an immunoglobulin molecule, the antigen receptor found on B cells, but it is quite distinct, being the result of different gene products.
Major histocompatibility complex
The MHC is responsible for how T cells ‘see’ an antigen against a background of self
For an immune response to be initiated, antigen cannot simply bind to the nearest T cell but must be ‘formally’ presented to the immune system. This occurs when APCs express processed antigenic peptides bound within grooves of MHC molecules on their cell surface. The MHC class I and II molecules also provide a differential mechanism for processing antigens that originate from within cells, e.g. viruses, and those that arise from the extracellular environment, e.g. many bacterial antigens. The different class MHC molecules lead such antigens through different pathways to interact with the immune system, in particular with the T cells, on the basis that each will be better dealt with by differing effector systems: class I leads to CD8+ T-cytotoxic responses, and class II instructs CD4+ helper T to provide appropriate help to B cells for an antibody-mediated response.
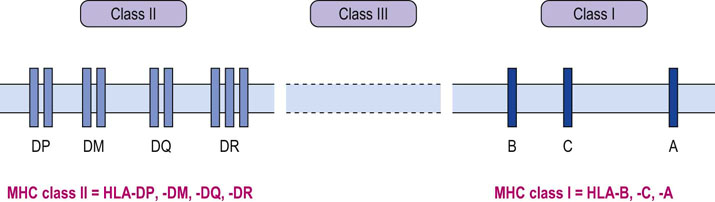
The MHC complex of genes is grouped into three regions, termed class I, II and III
The MHC complex of genes is found on the short arm of chromosome 6 and is grouped into three regions, termed class I, II and III, with the same nomenclature being applied to the respective polypeptide products (Fig. 38.4). Key to the success of the adaptive immune response is the polygenic and polymorphic nature of the MHC. By this we mean that there are various different MHC class I and II genes, and for any one, multiple variants, or alleles, can be expressed. Class I and II molecules are directly involved with immune recognition and cellular interactions, whereas class III molecules are involved in the inflammatory response by coding for soluble mediators, including complement components of the innate response and TNF.
MHC class I genes are organized into several loci, the most important of which are those termed HLA-A, HLA-B and HLA-C
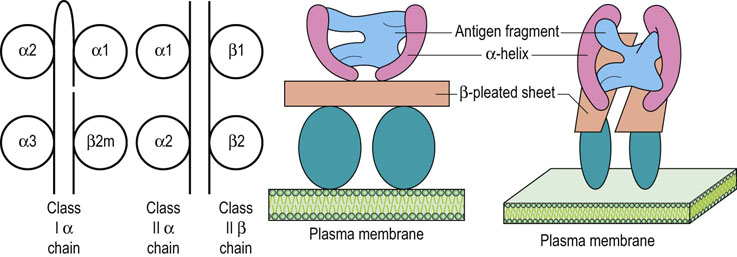
Alleles are transmitted and expressed in Mendelian codominant fashion. Owing to their closeness on the chromosome, they are inherited en bloc as parts of a haplotype and are expressed on the surface of all nucleated cells. The α-chains they encode have three domains, one of which is structurally similar to those found in immunoglobulin molecules, but the other two show significant differences. The α-chains combine with β2-microglobulin to give rise to a functional class I molecule (Fig. 38.5).
Class II genes are HLA-DR, HLA-DQ, HLA-DM and HLA-DP
The class II subregion genes, termed HLA-DR, HLA-DQ, HLA-DM and HLA-DP, are organized into α- and β-loci, giving rise to α- and β-polypeptide chains, respectively. Both are of approximately the same molecular weight and combine to form a heterodimer with a tertiary structure similar to a class I molecule, with a peptide groove into which the processed antigenic fragment is inserted during antigen presentation (Fig 38.5). Unlike class I expression, class II is far more restricted, being expressed mainly on professional APCs, such as dendritic cells, as well as on macrophages and B cells.
Many (currently in excess of 1000) allelic variants can be identified in each of the loci associated with antigen presentation. There are six major loci, each having between 10 and 60 functionally recognizable alleles, and as each parent passes on one set or haplotype on each chromosome, it is easy to appreciate that the likelihood of another individual in the same species having an identical set is remote.
The B cell antigen receptor
The B cell antigen receptor (BCR) is a membrane form of the immunoglobulin molecules found circulating in serum
Immunoglobulins are Y-shaped molecules made up of four polypeptide chains (Chapter 4, Fig. 4.5) – a pair of heavy chains each of approximate molecular weight 150 kDa and a pair of light chains each of approximate molecular weight 23 kDa. The arms interact with antigen and their structure is based on immunoglobulin domains with constant and variable sequences of amino acids in both the heavy and the light chains. It is the variably sequenced amino-terminal domains of both the heavy and the light chains that form a pocket that constitutes the antigen-binding site; the ‘fragment antigen binding’ (Fab) portion sits at the end of the arms (Fig. 4.5). The remaining relatively constant amino acid sequence domains of the chains are termed constant heavy (CH) or constant light (CL) and form the stem (Fc portion) that has a number of functions, including binding complement components and binding to Fc receptors on various leukocytes including macrophages, NK cells, neutrophils, mast cells and B cells.
There is an almost infinite range of possibilities for antibody specificities
The receptor repertoire, which most likely has in excess of 1011 different specificities, occurs as a result of the process whereby the various genes are involved in making the molecule combine. The variable region of a light chain is the product of two different genes (V = variable, and J = joining). This in turn combines with the gene product for the constant region, giving the complete transcribed and translated light chain protein. For the heavy chain, the level of complexity is increased with the addition of the D (diversity) gene product, forming part of the variable area in addition to V and J gene segments. Again these will combine to the C region segments, but for heavy chains multiple C genes products make up the completed protein. Multiple copies of each of the gene segments in germline DNA, which are in turn used randomly, as well as the possibility for polymorphisms in individuals, result in the almost infinite range of possibilities for antibody specificities. Mature B cells have the capacity to accumulate small point mutations in the DNA encoding the heavy and light chains of immunoglobulin, termed somatic hypermutations, which add further variation to specificity of the recognition process.
Lymphoid tissues
Primary (central) lymphoid tissues
Lymphocytes originating from common bone marrow-derived hematopoietic stem cells are initially found within primary lymphoid tissue, where they undergo early development and differentiation.
Maturation of most B cells occurs within the bone marrow
Initially, progenitor B cells rearrange their immunoglobulin genes. They do so in an antigen-independent process by interacting with stromal cells within the bone marrow. The resulting immature B cell expresses surface IgM as an antigen receptor. If they interact too strongly with environmental antigens at this stage they are removed by the process of negative selection, thus limiting the chance of autoreactivity. Following exit into the periphery, the B cells will express both surface IgM and IgD and can be activated by antigen engagement. These cells will proliferate, some becoming antibody-secreting plasma cells and others long-lived memory cells.
T lymphocyte progenitors travel to the thymus where they develop into T lymphocytes
The thymus is a multilobed structure found in the midline of the body just above the heart. At the macroscopic level, there is an outer cortex and an inner medullary area within each lobule. T cell development progresses in the thymus as the immature T cells migrate from the cortex to the medulla. The immature T cells interact with thymic epithelia and dendritic cells. These cells are thought to be responsible for the processes of positive and negative selection that take place as part of the ‘thymic education of T cells’. During this processes the T cells are assessed for their ability to interact with self-MHC and, if appropriate, they receive survival signals. Cells which show excessive reactivity to self, receive signals leading to their deletion whilst still within the thymus. This removes autoreactive cells, which, if released into the periphery, could potentially induce autoimmunity. The development of both early T and B cells in the primary lymphoid tissues is independent of extrinsic antigen stimulation.
Secondary (peripheral) lymphoid tissues
The secondary lymphoid tissues comprise lymph nodes, spleen, and the mucosa-associated lymphoid tissues (MALT)
These tissues are functionally organized throughout the body and have in common a degree of compartmentalization, with specific areas for T cells and B cells, and areas of overlap where they interact and respond to antigen. It is at these sites that immune reactions actually develop. For example, on exiting the thymus, the naïve T cells will recirculate via the bloodstream, and enter the lymph nodes by appropriate upregulation of adhesion molecules and chemokine receptors, which allows them to localize in the T cell areas of the tissue.
Within the lymph node, the T cell area is the paracortex and the B cell area are the follicular areas of the medulla
Here follicular structures of two types can be found: the unstimulated primary follicle, and stimulated secondary follicles, characterized by the presence of germinal centers. Lymph, which drains from the tissues to the lymph nodes will carry antigens, which in turn can be sampled by the APCs for presentation to the lymphocytes. In addition, peripheral APCs which have encountered pathogen will have migrated to their nearest draining lymph node, processing the antigen on the way in the hope of activating the T cell with appropriate specificity. On activation, the T cell will again alter chemokine receptor expression and leave the lymph node to recirculate to the site of infection where it can induce an effector response.
The spleen, contains nonlymphoid tissue (the red pulp) as well as lymphoid areas, the white pulp
Within the white pulp, follicular B cell areas are evident and the T cell areas lie between them in the interfollicular space. The spleen is used by the immune response for the presentation of blood-borne antigens.
MALT comprises the lymphoid elements adjacent to the mucosal surfaces
They are found at the entrance to the respiratory tract and gut, and include the tonsils and adenoids. Further down the digestive tract, unencapsulated aggregates of lymphoid cells referred to as Peyer's patches are found, overlaid by specialized areas of epithelium for sampling the antigenic environment. Similar to the lymph nodes and spleen, these tissues are important for initial antigen sampling and presentation, in particular for antigens which enter the body through a breach of the epithelium or via the gut.
Antigen-presenting cells
APCs are specialized cells which display microbial antigens on their surface to allow T cell activation
Dendritic cells are the major APC and are found throughout the body. The skin and different organs have their resident population of such cells. Dendritic cells can migrate throughout the body from tissue to circulation, and en route may enter the specialized secondary lymphoid organs such as lymph nodes (where they may activate lymphocytes for an adaptive response). On uptake of antigen, APCs can process and re-express it, in the context of the MHC, on the cell surface, to allow presentation to the T cell. Dendritic cells are termed ‘professional APC’, as in addition to being able to present the antigen, they also possess a number of other cell surface molecules, e.g. CD80/86, which provide the additional signals, so-called ‘co-stimulation’. These signals are required by a naïve T cell for complete activation. In addition, they may release certain cytokines e.g. IL-12, which will influence T cell activation and differentiation. Other cells which also are capable of presenting antigens, and hence can be considered APCs, include macrophages and B cells.
Adhesion molecules
Adhesion molecules mediate adhesion between cells
Cellular interactions during an immune response are dependent on the expression of the molecules and ligands that mediate adhesion between cells or between cells and the extracellular matrix. These are termed ‘adhesion molecules’. They are found on a wide variety of cell types, not only cells of the immune system but also, for instance, on vascular endothelium (Chapter 18). A major determinant of their expression is the prevailing cytokine environment and the surrounding connective tissue matrix. Typically, they are transmembrane glycoproteins. They deliver intracellular signals, and during immune responses are primarily involved in promoting cell–cell interactions and cell migration. The migration includes the movement of innate cells from blood to tissue during infection, as well as aiding lymphocytes to enter and leave lymph nodes as they circulate, looking for activation signals resulting from antigen presentation in these peripheral organs. Adhesion molecules involved in immunity are grouped into three major families:
 Integrins. These are heterodimeric proteins expressed on leukocytes, such as lymphocyte function-associated antigen 1 (LFA-1), or the macrophage adhesion molecule 1 (MAC-1).
Integrins. These are heterodimeric proteins expressed on leukocytes, such as lymphocyte function-associated antigen 1 (LFA-1), or the macrophage adhesion molecule 1 (MAC-1).
 Immunoglobulin supergene family adhesion molecules. These are often expressed on endothelial cells, e.g. intercellular adhesion molecule 1 (ICAM-1;CD54), or platelet/cell adhesion molecule 1 (PECAM-1; CD31).
Immunoglobulin supergene family adhesion molecules. These are often expressed on endothelial cells, e.g. intercellular adhesion molecule 1 (ICAM-1;CD54), or platelet/cell adhesion molecule 1 (PECAM-1; CD31).
 Selectins. They are expressed on leukocytes and endothelial cells, e.g. L-selectin or P-selectin.
Selectins. They are expressed on leukocytes and endothelial cells, e.g. L-selectin or P-selectin.
 Mucin-like vascular addressins. These are often found on leukocytes and endothelium. They bind selectins.
Mucin-like vascular addressins. These are often found on leukocytes and endothelium. They bind selectins.
Reaction with, the response to, and the elimination of, antigens
On binding to the antigen, the cell differentiates into progeny with an effector function or a memory function
On successful antigen binding, the activated lymphocyte undergoes repeated division or proliferation. Differentiation follows, which can lead to either the development of an effector function or the generation of memory.
Clonal selection creates clones of identical cells with unique antigen specificity
Clonal selection is the process whereby the immune response creates clones of identical cells, each clone having unique antigen specificity. With this clonal repertoire, the antigen determines which specific lymphocyte will be activated. The process of antigen drainage and lymphocyte recirculation to the peripheral lymphoid tissue ensures that antigen is inspected by many lymphocytes and can select for proliferation and differentiation the cell that bears a specific and reciprocal antigen receptor. Clonal selection ensures not only an adequate number of effector cells to deal with the threat at the time of initial stimulation but also a suitable number of part-primed memory cells that will be able to complete their activation more rapidly on subsequent antigen exposure. Figure 38.6 shows events for B cells, but T cells also undergo a similar process, resulting in proliferating clones of primed effector cells, and memory cells being generated for subsequent responses.
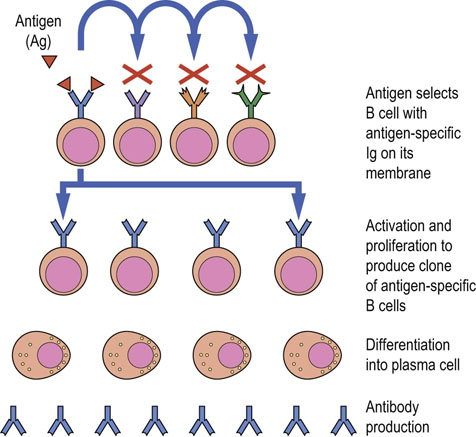
Fig. 38.6 Clonal selection in B cells.
Antigen-specific (Ag-specific) surface immunoglobulin (sIg) on the B cell membrane has a shape reciprocal to the antigen. Antigen-immunoglobulin binding leads to activation and proliferation to produce a clone of antigen-specific B cells. Each member of the specifically activated clone then undergoes differentiation into a plasma cell, which produces and secretes large quantities of a single homogeneous immunoglobulin, with specificity identical to the sIg that triggered the response in the first instance.
Immunologic memory distinguishes the adaptive immune response from the innate response
How immunologic memory is generated is still the subject of much research. On re-exposure to the same antigen, the adaptive immune response, due to the reactivation of long-lived memory cells, mounts a more rapid and more effective response, compared to the primary response. The long-lasting protection offered by vaccination is a result of immunologic memory. There are clear differences between the way naïve and memory lymphocytes respond to antigen. For example naïve and effector cells are relatively short-lived but memory lymphocytes persist for years and as a result often give lifelong protection after initial exposure. Additionally, there are more memory cells compared to naïve cells specific for the same antigen.
Adaptive response is an integrated response
Adaptive immune response is mediated by cellular and humoral elements, T cells being considered responsible for cellular immunity and B cells for humoral immunity. It is important to consider the adaptive response as being an integrated response, not occurring in isolation. For example many of the events and functions of T cells will impact on how efficient B cells respond and make appropriate antibodies. Similarly, B cells can in turn activate T cells. Interestingly, this integrated response reflects back on innate immunity too. For example, the cells of the innate immune response have evolved to be more efficient when antibodies have been produced, and may respond to cytokines released by the lymphocytes.
T cell response
Distinct populations of T cells exist. All T cells, once they have left the thymus, express either CD4 or CD8 on their surface. This phenotypic distinction also has major consequences for effector function: CD4+ T cells are often called T helper cells (TH), whilst CD8+ cells are cytotoxic T cells (CTL). TH cells can be further subdivided. They were originally divided into TH1 and TH2 cells, but in recent years evidence has shown this to be too simplistic. There is much current interest in the subset being termed TH17 due to their release of the cytokine IL-17. T cells are also responsible for regulating the activities of the other arms of the immune response. They achieve this by direct cell–cell contact or by the secretion of soluble mediators that interact with the relevant cells, i.e. other T cells, B cells, cells involved in the innate immune response such as macrophages, or cells of other tissues. The different subsets of T cells are shown schematically in Figure 38.7.
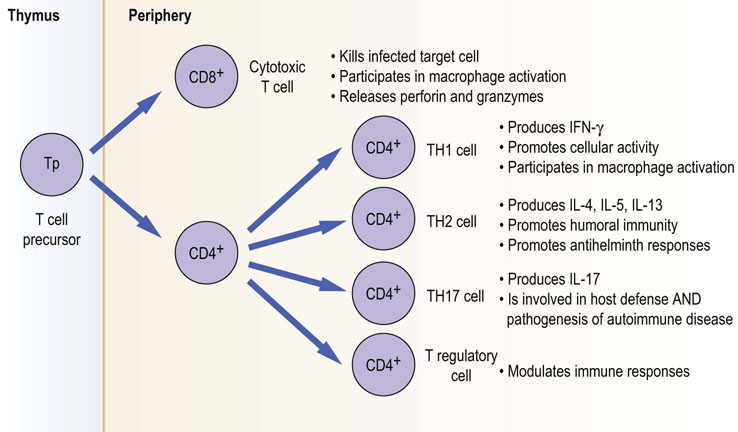
Fig. 38.7 Functional T cell subsets.
T cell precursor cells within the thymus develop into cells with different effector functions.
T helper cell subsets: TH1/TH2, TH17 and T regulatory (Treg)
The effector functions of CD4+ cells appear to be largely in ‘helping’ other immune responses. We have said above that T cells need to be presented with antigen in the context of MHC on the surface of an APC: CD4+ T cells this is done by MHC II molecules.
These T cells see antigen peptides which have been processed by the APC and expressed within the binding grooves of the MHC molecule. They also receive co-stimulatory signals when receptors on the T cell surface bind to counter-ligands on the surface of the APC. An example of this is the interaction between CD28 on the T cell and CD80/86 on the APC. Once activated, the T cell will differentiate, proliferate and perform appropriate effector functions.
TH1/TH2 cells
The subdivision into TH1 and TH2 subsets was originally made on the basis of their apparent function. TH1 cells appeared to function as promoting cellular responses. Once activated, they could release IFN-γ, which in turn further promotes macrophage activity. In addition, they may release TNF-α, which, through endothelial activation and subsequent upregulation of adhesion molecules and chemokines, would promote further leukocyte recruitment. In addition, they may provide help to B cells, enhancing antibody production.
TH2 cells help cellular responses in a different way. They too appear to help B cells make antibody, and produce IgE through their release of IL-4. They also preferentially stimulate eosinophil-driven inflammation through IL-5 production. Together these promote the major antihelminth response. By releasing IL-4 and IL-13, TH2 cells limit the TH1 activation of macrophages, and similarly TH1 products will inhibit TH2 responses. Therefore the effector functions of TH cells appear to be determined by the cytokine environment they produce and the response required. Finally expression of distinct transcription factors appears to be crucial in driving either TH1 or TH2 development with T-bet transcription factor being preferentially expressed by TH1 cells and GATA-3 is by the TH2 subset.
TH17 cells
Recently there has been much interest in another subset of TH cell which does not fit with the original TH1/TH2 paradigm. The TH17 cell was originally identified in animal models of a number of autoimmune diseases, including multiple sclerosis, rheumatoid arthritis and inflammatory bowel disease. Understanding the potential role of this subset, particularly in human disease, has been a major research focus. It is known that in the presence of IL-6 and TGF-β, but the absence of IL-4 and IL-12, the default will be for developing CD4 T cells to become TH17. They will also require IL-21, itself a T cell product, and IL-23, produced by APCs. TH17 cells also appear to make IL-22. Clearly this is a complicated network and the finer points are still under investigation. Whilst the understanding from animal models has implicated TH17 in driving the pathology of several autoimmune diseases, it would appear that the natural, physiologic role for TH17 may be in host defense against certain bacterial pathogens, including Klebsiella pneumoniae and fungi such as Candida albicans. The IL-17 and IL-22 they produce likely act on local stromal and epithelial cells of infected tissue, promoting local production of chemokines (IL-8), which, in turn, recruits innate effector cells such as neutrophils. This again shows the close interaction between innate and adaptive responses for effective immunity.
T regulatory cells
Originally cells which can control a cell-mediated response were identified as ‘suppressor cells’. However, the original description of a CD8+ cell providing this function is no longer considered valid. The cell types now studied are the so-called T regulatory cells (Treg). This appears to be a heterogeneous group. The most studied is a CD4+ T cell which appears to be able to control the action of other immune cells through a combination of soluble mediator release (IL-10 and TGF-β) and direct cell–cell contact. These cells are thought to also express the transcription factor FoxP3, which appears to be crucial for T regulatory cell development. As discussed earlier, the thymus plays an important role in deleting autoreactive T cells before they exit into the periphery by the mechanism of central tolerance. This process is not 100% efficient, and it is now recognized that T regulatory cells play an important role in the process of peripheral tolerance (i.e. the holding in check cells within the circulation, which if allowed would be autoreactive and cause autoimmunity). The translation to clinical applications of preventing autoimmune disease is currently under investigation. The potential of the TReg cells may also be applicable to induce tolerance to organ grafts.
CD8+ cytotoxic T cells (CTL) kill infected cells
The other major population of T cells based on their surface expression of CD8 is known as the cytotoxic T cell. Their role is primarily to kill infected cells (e.g. by virus). CD8+ T cells recognize peptides of the antigen associated with MHC I on the surface of the infected cell. By doing this, they are able to limit infection. It is likely that initially, naïve CD8+ T cells require help from CD4+ T cells, as well as being activated by APCs to become effector cells. Once in the periphery, effector CTLs will become activated when they encounter virally infected cells. They will bind tightly to the infected cell using adhesion molecules: the main method of killing infected cells is by the calcium-dependent release of serine proteases known as granzymes, and perforins proteins capable of perforating cell membranes, from their granules. The result of delivery of these enzymes to the infected cell is activation of caspase-driven apoptosis (Chapter 42). Apoptotic cells are subsequently removed by innate phagocytic cells such as macrophages – the ‘dustbin’.
The adaptive humoral immune response
Humoral immune response is characterized by the release of antibodies from fully matured plasma B lymphocytes
Humoral or antibody-mediated specific immunity is directed at extracellular infection, especially bacteria and their products, extracellular parasites, and also at the extracellular phase of viral infection. Antibodies also play a major role in the immunopathogenesis of many autoimmune or aberrant responses resulting in hypersensitivity. The humoral immune response is characterized by the release of antibodies from fully matured plasma cells of the B lymphocyte lineage. As antibodies recognize many types of molecules, including polysaccharides and lipids, this response is particularly efficient against extracellular pathogens. The antibody binding to structural surface components of microbes blocks the adhesion of these bacteria or viruses, and prevents the harmful effects of their toxins in a process termed neutralization. However, simple antibody binding, in most situations, will not guarantee elimination of the antigen. To promote the response, the non-antigen-binding fragment of the molecule (Fc portion) is able to activate other components of the innate system, through complement activation or by binding to receptors on phagocytes. The diversity of effector functions is achieved by the genetic recombinations of the heavy- and light-chain genes, as described previously.
B cell subsets are involved in the humoral immune response
Similar to the cellular response, which is mediated by a number of T cell subsets, the humoral response uses distinct B cell subsets. As noted earlier, T cells, particularly the TH cells, interact with B cells both directly and indirectly via cell surface receptors and cytokines, respectively. This happens to such a degree that effective B cell responses are often described as being T cell-dependent. The B cells termed B-2 are found in the follicles of the secondary lymphoid organs. They typically respond to protein antigens and produce the high-affinity antibodies typical of effective humoral responses. Within the marginal zone of the spleen, there is another population of B cells, which typically responds to polysaccharide antigens delivered via the bloodstream. They tend to secrete IgM but can class-switch to produce IgG. Another population, termed B-1, which express similar receptors, account for around 5% of all B cells, is found in mucosal tissue and the peritoneum. They express surface IgM as their antigen receptor and little surface IgD (the pattern opposite to that of the classical B-2 follicular B cells). B-1 cells predominantly show an IgM response, typically to nonprotein antigens, undergo little somatic hypermutation and exhibit little memory development.
Antibodies illustrate the capability of the immune system for diversity
For T cell-dependent responses, re-exposure to antigen will induce a secondary antibody response. The higher levels of the produced antibody will have increased affinity and avidity for the particular antigen, as a result of the processes of heavy-chain class switching and affinity maturation. The normal human immune system is capable of producing a limitless number of highly specific antibodies with the ability to recognize any and all nonself elements with which it comes into contact. Failure of effective immune response control can result in the production of antibodies against self-antigens, termed ‘autoantibodies’, and these are characteristic of a number of autoimmune diseases, including, among others, systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA).
The terms antibody, gamma globulin and immunoglobulin are synonymous
Five classes of immunoglobulin are recognized – IgG, IgA, IgM, IgD, and IgE – with subclasses being recognized for IgG (IgG1, 2, 3, and 4) and for IgA (1 and 2). When studied at the individual molecular level, no other proteins show such amino acid sequence variation between individual members of the same class or subclass. This is most evident in the N-terminal domains of both heavy and light chains which are responsible for the antigen recognition portion of the molecule. Antibodies are capable of discriminating between the molecules that characterize the outer capsular coverings of differing bacterial species which may vary by a single amino acid or a monosaccharide residue. This is a consequence of the dimensions of the area recognized by the antibody molecule being 10–20 Å (10−10m), and thus being capable of being influenced by the alteration in three-dimensional conformation brought about by the change of a single residue.
Antibodies are good examples of how function is intimately related to structure
Antibodies (immunoglobulins) are Y-shaped molecules (see Fig. 4.5). The ends of the arms interact specifically with the pathogen (antigen) and the stem provides additional or effector functionality. This secondary or effector function endows the antibody with an ability to initiate immune responses that help eliminate the pathogen to which it is directed. An example of this is the activation of complement. The effector functions of antibodies are summarized in Table 38.4.
Table 38.4
The effector functions of antibodies
| Type | Functions |
| lgG | Neutralization Opsonization for neutrophils and macrophages Passive immunity for fetus via transplacental passage Complement activation via classic pathway Antibody-dependent, cell-mediated cytotoxicity Natural killer function: cell killing of antibody-bound cells achieved by the receptors for the Fc portion Major isotype used in a secondary antibody response |
| lgA | Defense of mucosal surfaces; the most predominant immunoglobulin produced by MALT Neutralization |
| lgM | Neutralization Most effective classic complement pathway activator Predominant isotype in primary antibody responses |
| lgD | Possible role in signal transduction and B cell maturation Significance of circulating IgD is undefined |
| lgE | Major role is defense of mucosal surfaces against multicellular microorganisms |
Activation of the complement system is one of the most important antibody functions
Activation of the complement system (see Fig. 38.1) is one of the most important antibody effector functions in the adaptive immune response. This is achieved by using a set of components termed the ‘classic activation pathway’, which comprise C1q, C1r, C1s, C4, and C-2. Sequential activation of these components leads to the activation of the pivotal and critically important C-3 component, which is an absolute requirement for full complement activation. Once this is achieved, the terminal membrane attack complex, which comprises the components C5, C6, C7, C8, and C9, is activated. This complex eventually generates the polymeric ring structure that inserts into the cell membrane of bacteria and is responsible for cell lysis. This classic pathway is triggered by C1q binding an IgG or IgM that is already bound to its specific antigen.
Two other pathways of activation exist, both constituting parts of the nonspecific immune response; they are probably older in evolutionary terms and have been described above.
Figure 38.8 summarizes the key elements of the adaptive immune response, showing the interrelationships between cellular and humoral components.
Vaccination
Vaccination has been probably the single most beneficial application developed to harness the immune response
The process of vaccination illustrates well the interactions of the humoral and cellular arms of the adaptive immune response, and the features that characterize it best – specificity and memory. On first encounter with antigen, the immune system and antigen interact to select lymphocytes with the receptors specific for that antigen. These undergo activation, proliferation and differentiation into effector memory cells, a process that may take up to 14 days to complete (Fig. 38.8). However, the process of memory cell generation now leaves a population of cells semi-primed for that specific antigen. On subsequent exposure, the response is more rapid in view of the partly activated state of the memory cells. It is also more effective as a consequence of a degree of maturation of the response, due to the differentiation of the lymphocytes that has already taken place.
With reference to antibody responses, the primary challenge elicits a predominantly IgM response. On subsequent challenge, the B lymphocytes undergo further maturation, differentiation and isotype switching through ‘help’ from appropriate T cells, and more rapidly produce a predominantly IgG response. This provides additional effector functions to that obtained with just IgM. It is this heightened and more specific response that can reduce both the severity and the duration of any damage caused by the offending antigen.
Autoimmunity is normally prevented by thymic education; a breakdown in the processes involved may lead to autoimmune disease
While the immune system's activities are mostly beneficial, there are several situations in which they can have deleterious effects. These are best considered as aberrations of the quality, quantity or direction of the response.
One particular aspect of these disorders, that of autoimmunity (self-reactivity), is avoided by the processes of central tolerance (during thymic education), and peripheral tolerance which induces clonal deletion and anergy. The self-reactive clones are eliminated or rendered impotent either through deletion within the thymus, or by being controlled by T regulatory cells in the periphery. These mechanisms can be seen as a multilayered fail-safe strategy. Should these processes break down or be circumvented, the resulting state of self-reactivity and the inflammatory damage constitutes autoimmune disease.
The form of autoimmune disease is determined by the target antigen and the form of the immune response. At its simplest, reactions against ubiquitous antigens lead to what are termed nonorgan-specific autoimmune diseases. On the other hand, reactions to unique components of individual tissues, organs or systems lead to organ-specific disease. The former are best exemplified by systemic lupus erythematosus (SLE), in which the apparent target antigens are components common to all nuclei. Damage is seen in several tissues, including the skin, joints, kidneys, and nervous system. These diseases, and the others mentioned in Table 38.5, are the focus of the disciplines of clinical immunology and immunopathology. More information can be found in the books cited in the Further Reading below.
Summary
 Integrated immune response to nonself or altered-self elements (antigens) is made up of a number of components. Some of these show unique specificity for the particular stimulating antigen(s) and comprise the specific or adaptive immune response, whilst others recognize pathogen signatures and comprise the nonspecific or innate immune response.
Integrated immune response to nonself or altered-self elements (antigens) is made up of a number of components. Some of these show unique specificity for the particular stimulating antigen(s) and comprise the specific or adaptive immune response, whilst others recognize pathogen signatures and comprise the nonspecific or innate immune response.
 The innate response represents the first-line response and is present in all eukaryotes. The cells and soluble mediators involved are primarily those associated with the processes of inflammation and vascular activation.
The innate response represents the first-line response and is present in all eukaryotes. The cells and soluble mediators involved are primarily those associated with the processes of inflammation and vascular activation.
 The adaptive response is more refined and usually invoked only in the face of either failure or continued stimulation of the innate response. The cells responsible for the adaptive immune response are the T and B lymphocytes. The specificity they show for the inciting antigen is achieved via the use of specific antigen receptors, expressed on their cell surface and clonal expansion.
The adaptive response is more refined and usually invoked only in the face of either failure or continued stimulation of the innate response. The cells responsible for the adaptive immune response are the T and B lymphocytes. The specificity they show for the inciting antigen is achieved via the use of specific antigen receptors, expressed on their cell surface and clonal expansion.
 T cells recognize processed antigen via their antigen receptors, interacting with antigen presented by MHC-bearing cells. This leads to the secretion of additional cytokines and the generation of effector functions such as T cell help and T cell-mediated cytotoxicity, brought about by the T helper and T cytotoxic subsets, respectively. Historically, T cell responses have been termed the cellular immune response. A distinct CD4+ subset of T cells is termed ‘T regulatory cells’, as they function to control adaptive responses and in part prevent autoreactivity by the immune response.
T cells recognize processed antigen via their antigen receptors, interacting with antigen presented by MHC-bearing cells. This leads to the secretion of additional cytokines and the generation of effector functions such as T cell help and T cell-mediated cytotoxicity, brought about by the T helper and T cytotoxic subsets, respectively. Historically, T cell responses have been termed the cellular immune response. A distinct CD4+ subset of T cells is termed ‘T regulatory cells’, as they function to control adaptive responses and in part prevent autoreactivity by the immune response.
 B cells recognize native antigen and secrete proteins, termed antibodies, which can bind directly to the antigen. Historically, B cells and their antibody products have been termed the humoral immune response.
B cells recognize native antigen and secrete proteins, termed antibodies, which can bind directly to the antigen. Historically, B cells and their antibody products have been termed the humoral immune response.
 Both T and B cells and their products are able to recruit and utilize components of the innate response in a more effective and targeted manner, with the aim of eliminating or eradicating the antigen.
Both T and B cells and their products are able to recruit and utilize components of the innate response in a more effective and targeted manner, with the aim of eliminating or eradicating the antigen.
 In addition to demonstrating specificity, the adaptive immune response also demonstrates another critically important characteristic not seen with the innate response: the memory for its encounter with antigen. The benefit of this is that, on subsequent contact with the same antigen, a heightened and more efficient response will lead to a quicker removal of the causative agent, hopefully with less tissue damage than on first encounter.
In addition to demonstrating specificity, the adaptive immune response also demonstrates another critically important characteristic not seen with the innate response: the memory for its encounter with antigen. The benefit of this is that, on subsequent contact with the same antigen, a heightened and more efficient response will lead to a quicker removal of the causative agent, hopefully with less tissue damage than on first encounter.
Abbas, AK, Lichtman, AH. cellular and molecular immunology, ed 7. Philadelphia, PA: Saunders Elsevier; 2012.
Chapel, H, Heaney, M, Misbah, S, et al. Essentials of clinical immunology, ed 5. Oxford: Blackwell; 2006.
Kumar, H, Kawai, T, Akira, S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011; 30:16–34.
Liu, Z, Davidson, A. Taming lupus – a new understanding of pathogenesis is leading to clinical advances. Nature Med. 2012; 18:871–882.
MacPherson, G, Austyn, J. Exploring immunology. Hoboken, NJ: Wiley-Blackwell; 2012.
Murphy, K. Janeway's immunobiology, ed 8. London: Garland; 2011.
Shevach, EM. Biological functions of regulatory T cells. Adv Immunol. 2011; 112:137–176.