Chapter 13 Diseases of the musculoskeletal system
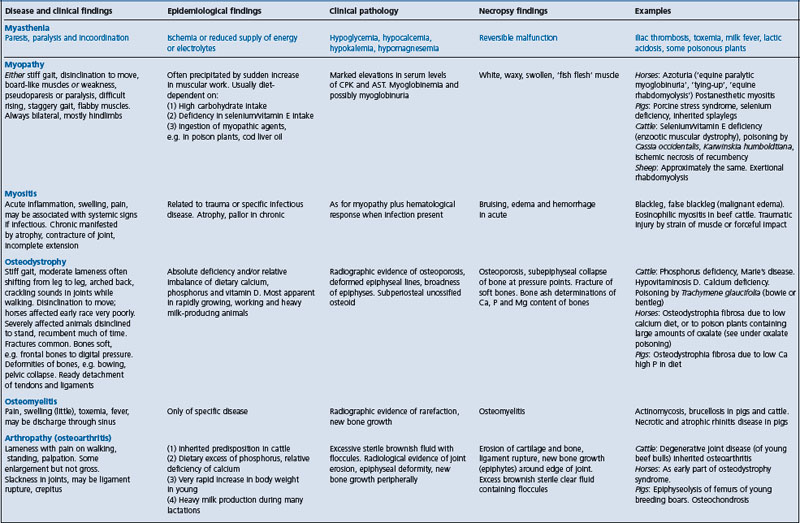
Diseases of the organs of support, including muscles, bones, and joints, have much in common in that the major clinical manifestations of diseases that affect them are lameness, failure of support, insuffciency of movement and deformity. Insuffciency of movement affects all voluntary muscles, including those responsible for respiratory movement and mastication, but lameness and failure of support are manifestations of involvement of the limbs.
Various classifcations of the diseases of the musculoskeletal system, based on clinical, pathological and etiological differences, are in use, but the simplest is that which divides the disease into degenerative and inflammatory types.
• The degenerative diseases of muscles, bones and joints are distinguished as: myopathy, osteodystrophy and arthropathy, respectively
• The inflammatory diseases are myositis, osteomyelitis and arthritis.
Principal manifestations of musculoskeletal disease
LAMENESS
Lameness is an abnormal gait or locomotion characterized by limping (claudication) or not bearing full weight on a leg, usually associated with pain in the musculoskeletal system. Lameness must be distinguished from ataxia, which is an abnormal gait characterized by lack of coordination of muscular action, usually because of a lesion of the central or peripheral nervous system.
Weakness is the inability to maintain a normal posture and gait, usually because of a lesion of muscle or generalized weakness due to an abnormal systemic state such as shock, hypocalcemia, or starvation.
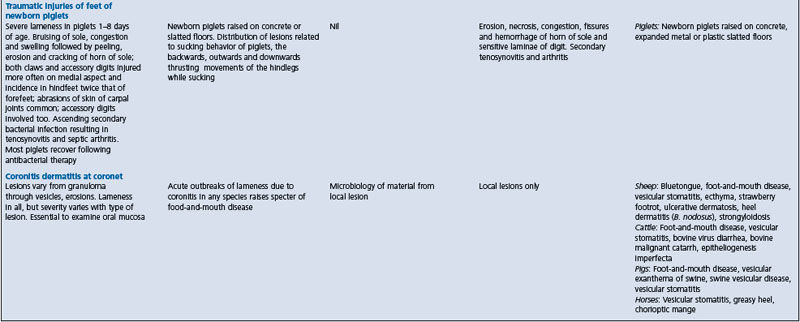
Because of the diffculty inherent in the differentiation of diseases causing lameness, and other abnormalities of gait and posture, a summary is presented in Table 13.1. It does not include lameness in racing horses, which is described in textbooks on lameness in horses, or diseases of the nervous system that interfere with normal movement and posture. These are discussed in Chapter 12.
ABNORMAL POSTURE AND MOVEMENT
As a group, diseases of the musculoskeletal system are characterized by reduced activity in standing up and moving, and the adoption of unusual postures. Abnormal movements include limpness, sagging or stiffness and lack of flexion. Abnormal postures include persistent recumbency, including lateral recumbency. There may be signs of pain on standing, moving or palpation. There is an absence of signs specifcally referable to the nervous system. For example, there are no signs of brain damage and the spinal cord reflexes are present but may be only partly elicitable (the sensory pathway is intact but the motor response may be diminished). Differentiation from diseases of the nervous system and from each other may be aided by specifc biochemical, radiological or hematological fndings that indicate the system involved. Specifc epidemiological fndings may indicate the location of the lesion (which may be secondary) in muscle, bones, or joints, as set out in Table 13.1.
DEFORMITY
Atypical disposition, shape or size of a part of the musculoskeletal system constitutes a deformity. This may occur in a number of ways, and be caused by the following.
Defects of the skeleton
• Dwarfism – inherited miniature calves, achondroplastic dwarves; short legs of inherited congenital osteopetrosis; nutritional defciency of manganese; acorn calves
• Giant stature – inherited prolonged gestation, not really giantism, only large at birth
• Asymmetry – high withers, low pelvis of hyena disease of cattle
• Limbs – complete or partial absence, inherited or sporadic amputates; curvature of limbs in rickets; bowie or bentleg of sheep poisoned by Trachymene sp.
• Head – inherited and sporadic cyclopean deformity; inherited probatocephaly (sheep’s head) of calves; inherited moles, bulldog calves; acquired atrophic rhinitis of pigs.
SPONTANEOUS FRACTURES
Spontaneous fractures occur uncommonly in farm animals and pre-existing diseases are usually present, which include the following:
• Nutritional excess of phosphorus causing osteodystrophia in horses
• Nutritional defciency of calcium causing osteodystrophia in pigs
• Nutritional defciency of phosphorus or vitamin D in ruminants causing rickets and/or osteomalacia; hypervitaminosis A may contribute to this
PAINFUL ASPECTS OF LAMENESS
Musculoskeletal pain can be caused by lacerations and hematomas of muscle, myositis and space-occupying lesions of muscle. Osteomyelitis, fractures, arthritis, joint dislocations, sprains of ligaments and tendons are also obvious causes of severe pain. Among the most painful of injuries are swollen, inflammatory lesions of the limbs caused by deep penetrating injury or in cattle by extension from footrot. Amputation of a claw, laminitis and septic arthritis are in the same category. Ischemia of muscle and generalized muscle tetany, as occurs in electroimmobilization, also appear to cause pain.
Research on the pathophysiology and pharmacology of pain associated with lameness in animals indicates that the thresholds to painful stimuli change in response to pain and this change is seen as an indication of an alteration in nerve function or in nociceptive processing at higher levels. In flocks of sheep with severe lameness due to foot rot, affected sheep had a lower threshold to a mechanical nociceptive stimulus than matched controls and their thresholds remained low when tested 3 months later, after the apparent resolution of the foot lesions.1 Thus hyperalgesia persisted in severely lame sheep for at least 3 months. It is suggested that N-methyl-D-aspartate receptors are involved in the development of this long-term hypersensitivity. Similar fndings have been reported in dairy heifers affected with claw lesions during the peripartum period.2
Relief of musculoskeletal pain
Several aspects about relieving pain in agricultural animals are important. Cost has always been a deterrent to the use of local anesthetics and analgesics but, with changing attitudes, the need to control pain is more apparent. Treatment of the causative lesion is a major priority but the lesion may be painful for varying lengths of time. Relief and the control of pain should be a major consideration. Details on the use of analgesics are presented in Chapter 2.
ECONOMICS OF LAMENESS IN FOOD-PRODUCING ANIMALS
Diseases of the musculoskeletal system and feet that cause lameness cause major economic losses. A survey of the incidence and prevalence of lameness in cattle on 37 dairy farms in the UK in 1989–91 found a mean annual incidence of 54.6 new cases per 100 cows (farm range 11–170%) and a mean annual prevalence of 21% (farm range 2–54%).3 Loss of production occurs because animals that are in pain have diffculty moving around and do not eat and milk normally. Reproductive performance may be reduced because of failure to come into heat normally. The culling rate may be higher than is desirable because so many of the lesions of the feet and legs are incurable. The direct monetary costs for the treatment of lame animals are not high, but the actual treatment of either individual animals or groups of animals is time-consuming and laborious. The condemnation of animals to slaughter because of lesions of the musculoskeletal system also contributes to the total economic loss. When lameness is a herd problem not only are the economic losses increased but clinical management becomes very diffcult.
The epidemiological factors which contribute to lameness include:
• Injuries due to floor surfaces
• Persistently wet, unhygienic ground conditions
• Overcrowding and trampling during transportation and handling
Certain breeds may be more susceptible to diseases of the feet and legs than others. Osteoarthritis occurs most commonly in old animals. Diseases of the legs of dairy cattle occur most commonly at the time of parturition and during the frst 50 days of lactation. Diseases of the feet of dairy cattle occur most commonly in days 50–150 of the lactation period. Often the etiology is complex and a defnitive etiological diagnosis cannot be made. This makes clinical management diffcult and often unrewarding.
EXAMINATION OF THE MUSCULOSKELETAL SYSTEM
The clinical examination of the musculoskeletal system and the feet of farm animals would include the following special examinations.
Analysis of gait and conformation
Inspection of the gait of the animal is necessary to localize the site of lameness. Evaluation of its conformation may provide clues about factors that may contribute to lameness. Details on the examination of farm animals for lameness are available in textbooks on lameness in horses and cattle.
Close physical examination
A close detailed physical examination of the affected area is necessary to localize the lesion. This includes passive movements of limbs to identify fractures, dislocations and pain on movement. Muscles can be palpated for evidence of enlargement, pain, or atrophy.
Radiography
Radiography is useful for the diagnosis of diseases of bones, joints and soft tissue swelling of limbs, which cannot be easily defned by physical examination. Detailed radiographic information about the joint capsule, joint cavity or articular cartilage can be obtained using negative (air), positive or double contrast arthrography. Ultrasonographic imaging can be used to differentiate the pathological changes in the soft tissue structures of digital flexor tendon sheaths of cattle.1
Ultrasonography
Ultrasonography is used extensively in dogs and horses for the visualization of soft tissue structures of the joint. Most veterinary practices have an ultrasound machine that is used for small-animal imaging or transrectal pregnancy diagnosis in cattle and horses.2 Ultrasonography is cheaper, faster and provides important information compared to radiography; it is also less invasive and cheaper than joint fluid aspiration and analysis.
The ultrasonographic anatomy of the elbow, carpal, fetlock, and stifle joints of clinically normal sheep using a 7.5 MHz linear transducer with a stand-off pad has been described.2 The anatomical structures that could be consistently identifed in normal ovine joints included bone, articular cartilage, ligaments and tendons. In sheep with chronic arthritis/synovitis, the gross thickening of the joint capsule is visible as a hyperechoic band up to 20 mm thick.
The ultrasonographic examination of the stifle region in cattle has been described.3 The homogeneously echogenic patellar and collateral ligaments, the combined tendon of the long digital extensor and peroneus tertius muscles, the popliteal tendon, the anechoic articular cartilage of femoral trochlea, the echogenic menisci and the hyperechoic bone surfaces were imaged successfully. The boundaries of the joint pouches became partially identifable only when small amounts of anechoic fluid were present in the medial and lateral femorotibial joint pouches. The main indication for ultrasonography of the bovine stifle is evaluation of acute septic and traumatic disorders of the region, when specifc radiographic signs are often nonspecifc or absent. The cruciate ligaments could not be imaged in live cattle. The cruciate ligaments are identifable in the horse, in which flexion of the hindlimb is a routine procedure necessary for identifcation of these structures.
The ultrasonographic examination of the carpal region in cattle has been described.4 The main indication is the evaluation of septic and traumatic disorders of the carpal joints and tendon sheaths. Each tendon and tendon sheath in carpal region must be scanned separately. The use of a stand-off pad is recommended as it permits adaptation of the rigid transducer to the contours of the carpus. The carpal joint pouches and tendon sheath lumina are not clearly defned in healthy cattle. Thus the ability to image these structures indicates the presence of synovial effusion.
Ultrasonography is a valuable diagnostic aid for septic arthritis. Joint effusion, which is one of the earliest signs of septic arthritis, the accurate location of soft tissue swelling, the extent and character of joint effusion and involvement of concurrent periarticular synovial cavities or other soft tissue structures can be imaged by ultrasonography.5 The ultrasonogram can image the presence of small, hyperechogenic fragments within the joint, appearing very heterogeneous. Normal synovial fluid is anechoic and appears black on the sonogram. A cloudy appearance is usually associated with the presence of pus.6
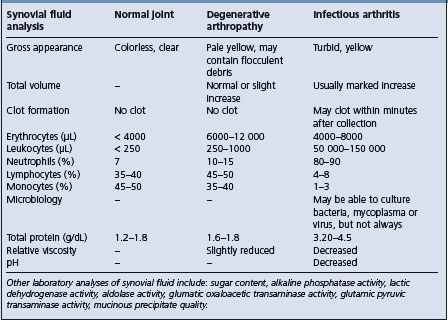
Arthrocentesis
Joint fluid is collected by needle puncture of the joint cavity (arthrocentesis) and examined for the presence of cells, biochemical changes in the joint fluid and the presence of infectious agents. The techniques and application of arthrocentesis for some of the joints commonly sampled in the horse have been reviewed.
Arthroscopy
Special endoscopes are available for inspection of the joint cavity and articular surfaces (arthroscopy). Diagnostic and surgical arthroscopy is now commonplace in specialized equine practice. Surgical arthroscopy is rapidly replacing conventional arthrotomy for the correction of several common surgical conditions of the musculoskeletal system of the horse. Accurate quantifcation of equine carpal lesions is possible when the procedure is performed by an experienced arthroscopist.7 Convalescent time following surgery is decreased and the cosmetic appearance improved compared to arthrotomy. The arthroscopic anatomy of the intercarpal and radiocarpal joints of the horse have been described. A synovial membrane biopsy can be examined histologically and for infectious agents and may yield useful diagnostic information.
Serum biochemistry and enzymology
When disease of bone or muscle is suspected, the serum levels of calcium, phosphorus, alkaline phosphatase and the muscle enzymes creatinine phosphokinase (CPK) and aspartate aminotransferase (AST), also known as serum glutamic oxaloacetic transaminase (SGOT), may be useful. The muscle enzymes are sensitive indicators of muscle cell damage; the serum levels of calcium, phosphorus and alkaline phosphatase are much less sensitive indicators of osteodystrophy.
Nutritional history
Because the most important osteodystrophies and myopathies are nutritional in origin a complete nutritional history must be obtained. This should include an analysis of the feed and determination of the total amount of intake of each nutrient, including the ratio of one nutrient to another in the diet.
Diseases of muscles
MYASTHENIA (SKELETAL MUSCLE ASTHENIA)
The differential diagnosis of paresis, paralysis and incoordination should include a consideration of skeletal muscle weakness unrelated to primary neurogenic hypotonia or to permanent muscle injury, including myopathy and myositis. Most of the syndromes that fall into this group of myasthenia have been described in detail elsewhere in this book and are referred to briefly here only to complete the list of abnormalities of skeletal muscle that affect gait and posture. Unlike myopathy and myositis, they are reversible states.
The common causes of myasthenia in farm animals are:
• Ischemia in iliac thrombosis in the horse and after recumbency in cows with parturient paresis. The end stage is myonecrosis and not reversible
• Metabolic effect on muscle fibers – causes include hypokalemia, hypocalcemia and possibly hypophosphatemia (in parturient paresis of dairy cows), hypomagnesemia (in lactation tetany), hypoglycemia of newborn pigs and lactic acidemia after engorgement on grain
• Toxins – general toxemia is a cause. Also, many plant toxins exert an effect on skeletal muscle activity. Although in most cases the mode of the action of the toxin is unknown, the toxins have been listed as neurotoxins.
MYOPATHY
The term myopathy describes the noninflammatory degeneration of skeletal muscle that is characterized clinically by muscle weakness and pathologically by hyaline degeneration of the muscle fibers. The serum levels of some muscle enzymes are elevated and myoglobinuria is a common accompaniment.
ETIOLOGY AND EPIDEMIOLOGY
The most important myopathies in farm animals are due to nutritional deficiencies of vitamin E and selenium and the effects of unaccustomed exercise. In humans, in contrast, the muscular dystrophies occur as inherited defects of muscle or degenerative lesions caused by interruption of their nerve supply. The skeletal myopathies can be classified into primary and secondary myopathies.
A retrospective analysis of the case records in a veterinary teaching hospital over a 9-year period revealed that the most common myopathy in horses was exercise-associated muscle disorder (69%). The remainder were postexhaustion syndrome (9%), infectious myopathies (10.5%), immunological myopathy (6.0%), nutritional myopathy (4.5%) and hyperkalemic periodic paralysis (1.5%).1
The major causes of myopathy in farm animals and their epidemiological determinants are as follows.
Enzootic nutritional muscular dystrophy
A nutritional deficiency of vitamin E and/or selenium is a common cause in young calves, lambs, foals, and piglets. Factors enhancing or precipitating onset include: rapid growth, highly unsaturated fatty acids in diet and unaccustomed exercise. The disease also occurs in adult horses.
Exertional or postexercise rhabdomyolysis
This is not known to be conditioned by vitamin E (selenium deficiency) and occurs as equine paralytic myoglobinuria (tying-up syndrome, azoturia) in horses after unaccustomed exercise or insufficient training.1 It also occurs in sheep chased by dogs, in cattle after running wildly for several minutes and as capture myopathy during capture of wildlife. An acute myopathy of undetermined etiology occurred in horses at grass in Scotland.2 The horses were not in training, creatine kinase levels were elevated and the urine was dark brown; most of them died and the muscles affected were those of posture and respiration rather than movement.2
Equine polysaccharide storage myopathy is a metabolic disease being recognized with increasing frequency in many breeds of horse.3 It occurs in Quarter-Horse-related breeds and more recently has been recognized in draught horse breeds. It is thought to be due to an inherited metabolic defect affecting carbohydrate metabolism (see Ch. 28).
Metabolic
Hyperkalemic periodic paralysis occurs in certain pedigree lines of North American show Quarter Horses.
Degenerative myopathy
This occurs in newborn calves, sheep and goats affected by Akabane virus infected in utero.
Inherited myopathies
The porcine stress syndrome, which is discussed under that heading, now includes herztod pale, soft, exudative pork encountered at slaughter and malignant hyperthermia following halothane anesthesia. Certain blood types in pigs have been used as predictors of stress susceptibility and malignant hyperthermia in Pietrain pigs is genetically predetermined. Most of these myopathies of pigs thus have an inherited basis and the stress of transportation, overcrowding and handling at slaughter precipitates the lesion and rapid death.
Congenital myopathy of Braunvieh– Brown Swiss calves is thought to be inherited.4 Affected calves become progressively weak and recumbent within 2 weeks of birth.4
Doubling-muscling in cattle and splaylegs of newborn pigs are also considered to be inherited. A dystrophy-like myopathy in a foal has been described and is similar to human muscular dystrophy.5 Dystrophy of the diaphragmatic muscles in adult Meuse– Rhine–Yessel cattle is thought to be inherited. Xanthosis occurs in the skeletal and cardiac muscles of cattle and is characterized grossly by a green iridescence.
Toxic agents
This is caused by poisonous plants, including Cassia occidentalis, Karwinskia humboldtiana, Ixioloena spp., Geigeria spp. and lupins. A special case is enzootic calcinosis of all tissues, especially muscle, and the principal signs are muscular. It is caused by poisoning by Solanum malacoxylon, Tricetum spp., and Cestrum spp.
Ischemia
Ischemic myonecrosis occurs in the thigh muscles of cattle recumbent for about 48 hours or more and is discussed in detail under the heading Downer cow syndrome. Iliac thrombosis in horses is an important cause of ischemic myopathy and has been reported in calves.
Neurogenic
Neurogenic muscular atrophy occurs sporadically due to traumatic injury and subsequent degeneration or complete severance of the nerve supply to skeletal muscle. The myopathy in arthrogryposis associated with the Akabane virus is thought to be due to lesions of the lower motor neurons supplying the affected muscles. It has been suggested that cattle with muscular hypertrophy may be more susceptible to the effects of exercise and the occurrence of acute muscular dystrophy. Suprascapular nerve paralysis in the horse (sweeney) is a traumatic neuropathy resulting from compression of the nerve against the cranial edge of the scapula.
PATHOGENESIS
Primary myopathy
The characteristic change in most cases of primary myopathy varies from hyaline degeneration to coagulative necrosis, affecting particularly the heavy thigh muscles and the muscles of the diaphragm. Myocardial lesions are also commonly associated with the degeneration of skeletal muscle and when severe will cause rapid death within a few hours or days. The visible effects of the lesions are varying degrees of muscle weakness, muscle pain, recumbency, stiff gait, inability to move the limbs and the development of respiratory and circulatory insufficiency.
In primary nutritional muscular dystrophy associated with a deficiency of vitamin E and/or selenium there is lipoperoxidation of the cellular membranes of muscle fibers resulting in degeneration and necrosis. The lesion is present only in muscle fibers and the histological and biochemical changes which occur in the muscle are remarkably similar irrespective of the cause. Variations in the histological lesion occur but indicate variation in the severity and rapidity of onset of the change rather than different causes.
Myoglobinuria
Because of the necrosis of muscle, myoglobin is excreted in the urine and myoglobinuric nephrosis is an important complication, particularly of acute primary myopathy. The degree of myoglobinuria depends on the severity of the lesion, acute cases resulting in marked myoglobinuria, and on the age and species of animal affected. Adult horses with myopathy may liberate large quantities of myoglobin, resulting in dark brown urine. Yearling cattle with myopathy release moderate amounts and the urine may or may not be colored; calves with severe enzootic nutritional muscular dystrophy may have grossly normal urine. In all species the renal threshold of myoglobin is so low that discoloration of the serum does not occur.
Muscle enzymes
An important biochemical manifestation of myopathy is the increased release of muscle cell enzymes that occurs during muscle cell destruction. CPK and serum glutamic oxaloacetate transaminase are both elevated in myopathy and CPK, particularly, is a more specific and reliable indication of acute muscle damage. Increased amounts of creatinine are also released into the urine following myopathy.
Exertional rhabdomyolysis
In exertional rhabdomyolysis in horses there is enhanced glycolysis with depletion of muscle glycogen, the accumulation of large amounts of lactate in muscle and blood and the development of hyaline degeneration of myofibers. Affected muscle fibers are richer in glycogen in the acute stage of ‘tying-up’ than in the late stages, suggesting an increased glycogen storage in the early phase of the disease compared with normal healthy horses. During enforced exercise there is local muscle hypoxia and anaerobic oxidation resulting in the accumulation of lactate and myofibrillar degeneration. The pathogenesis of postanesthetic myositis in horses is uncertain.6 A significant postischemic hyperemia occurs in horses that develop postanesthetic myopathy.6 Postanesthetic recumbency can occur in the horse with polysaccharide storage myopathy.7
Types of muscle fiber affected
In most animals skeletal muscle is composed of a mixture of fibers with different contractile and metabolic characteristics. Fibers with slow contraction times have been called slow twitch or type I fibers and those with fast contraction time are fast twitch or type II. Histochemically, types I and II fibers can be differentiated by staining for myofibrillar ATPase. Type II fibers can be subgrouped into type IIA and IIB on the basis of acid preincubations.8 Several different characteristics of these muscle fibers have been studied in the horse. There are variations in the percentage of each type of fiber present and in composition of muscle fibers dependent on genetic background, age, and stage of training.8 There are also variations in the muscle fibers within one muscle9 and between different muscles.10 The histochemical characteristics of equine muscle fibers have been examined:11,12
• Type I fibers are characterized by strong aerobic capacity, compared with type IIA
• Type IIA fibers are more glycolytic and have strong aerobic and moderate to strong anaerobic capacities
• Type IIB fibers are characterized by a relatively low aerobic and a relatively high anaerobic capacity and are glycolytic.11
The histochemical staining characteristics of normal equine skeletal muscle have been examined and serve as a standard for comparison with data obtained from skeletal muscles with lesions.12
Secondary myopathy due to ischemia
In secondary myopathy due to ischemia there may be multiple focal areas of necrosis, which causes muscle weakness and results in an increase of muscle enzymes in the serum. The degree of regeneration with myofibers depends on the severity of the lesion. Some regeneration occurs but there is considerable tissue replacement. In aortic and iliac thrombosis in calves under 6 months of age the thrombosis results in acute-to-chronic segmental necrosis of some skeletal muscles and coagulation necrosis in others.13
CLINICAL FINDINGS
The nutritional myopathies associated with a deficiency of vitamin E and/or selenium occur most commonly in young growing animals and may occur in outbreak form, particularly in calves and lambs. The details are presented under the heading of vitamin E and selenium deficiency.
Primary myopathy
In general terms, in acute primary myopathy there is a sudden onset of weakness and pseudoparalysis of the affected muscles, causing paresis and recumbency and, in many cases, accompanying respiratory and circulatory insufficiency. The affected animals will usually remain bright and alert but may appear to be in pain. The temperature is usually normal but may be slightly elevated in severe cases of primary myopathy. Cardiac irregularity and tachycardia may be evident, and myoglobinuria occurs in adult horses and yearling cattle. The affected skeletal muscles in acute cases may feel swollen, hard and rubbery but in most cases it is difficult to detect significant abnormality by palpation. Acute cases of primary myopathy may die within 24 hours after the onset of signs.
Acute nutritional myopathy
While acute nutritional myopathy in horses occurs most commonly in foals from birth to 7 months of age, acute dystrophic myodegeneration also occurs in adult horses. There is muscle stiffness and pain, myoglobinuria, edema of the head and neck, recumbency and death in a few days. A special occurrence of myopathy has been recorded in suckling Thoroughbred foals up to 5 months of age. The disease occurs in the spring and summer in foals running at pasture with their dams and is unassociated with excessive exercise. In peracute cases there is a sudden onset of dejection, stiffness, disinclination to move, prostration and death 3–7 days later. Lethargy and stiffness of gait are characteristic of less acute cases. There is also a pronounced swelling and firmness of the subcutaneous tissue at the base of the mane and over the gluteal muscles. There may be excessive salivation, desquamation of lingual epithelium and board-like firmness of the masseter muscles. The foals are unable to suck because of inability to bend their necks. Spontaneous recovery occurs in mild cases but most severely affected foals die.
Severe nutritional myopathy of the masseter muscles in a 6-year-old Quarter Horse stallion has been described.14 The masseter muscles were swollen and painful, and there was exophthalmos and severe chemosis with protrusion of the third eyelids. The mouth could be opened only slightly and masticatory efforts were weak. Serum enzymology supported a diagnosis of nutritional muscular dystrophy, and the concentrations of vitamin E and selenium in the blood and feed were lower than normal.
Tying-up
In tying-up in horses there is a very sudden onset of muscle soreness 10–20 minutes following exercise. There is profuse sweating and the degree of soreness varies from mild, in which the horse moves with a short, shuffling gait, to acute, in which there is a great disinclination to move at all. In severe cases, horses are unable to move their hindlegs, and swelling and rigidity of the croup muscles develops. Myoglobinuria is common.
Postanesthetic myositis
In postanesthetic myositis affected horses experience considerable difficulty during recovery from anesthesia. Recovery is prolonged and when initial attempts are made to stand there is lumbar rigidity, pain and reluctance to bear weight.7 Some affected horses will be able to stand in within several hours if supported in a sling.7 The limbs may be rigid and the muscles firm on palpation. In severe cases the temperature begins to rise – reminiscent of malignant hyperthermia. Other clinical findings include anxiety, tachycardia, profuse sweating, myoglobinuria and tachypnea. Death may occur in 6–12 hours. Euthanasia is the only course for some horses. In the milder form of the syndrome, affected horses are able to stand, but are stiff and in severe pain for a few days.
Exertional rhabdomyolysis
In horses, the clinical findings are variable and range from poor performance to recumbency and death. Signs may be mild and resolve spontaneously within 24 hours or severe and progressive.
The usual presentation is a young (2–5-year-old) female racehorse with recurrent episodes of stiff gait after exercise. The horse does not perform to expectation and displays a short-stepping gait that may be mistaken for lower leg lameness. The horse may be reluctant to move when placed in its stall, be apprehensive and anorexic, and frequently shift its weight. More severely affected horses may be unable to continue to exercise, have hard and painful muscles (usually gluteal muscles), sweat excessively, be apprehensive, refuse to walk and be tachycardic and tachypneic. Affected horses may be hyperthermic. Signs consistent with abdominal pain are present in many severely affected horses. Deep red urine (myoglobinuria) occurs but is not a consistent finding. Severely affected horses may be recumbent and unable to rise.
Many different manifestations of equine polysaccharide storage myopathy occur.3 All manifestations are related to dysfunction, which results in pain, weakness, segmental fiber necrosis, stiffness, spasm, atrophy or any combination of the above. The muscles most severely affected are the powerful rump, thigh and back muscles, including gluteals, semimembranosus, semitendinosus and longissimus.
In exertional rhabdomyolysis in sheep chased by dogs, affected animals are recumbent, cannot stand, appear exhausted and myoglobinuria is common. Death usually follows. A similar clinical picture occurs in cattle that have run wildly for several minutes.
Hyperkalemic periodic paralysis
Initially there is a brief period of myotonia with prolapse of the third eyelid. In severe cases, the horse becomes recumbent and the myotonia is replaced by flaccidity. Sweating occurs, and generalized muscle fasciculations are apparent, with large groups of muscle fibers contracting simultaneously at random. The animal remains bright and alert and responds to noise and painful stimuli. In milder cases, affected horses remain standing and generalized muscle fasciculations are prominent over the neck, shoulder and flank. There is a tendency to stand base-wide. When the horse is asked to move, the limbs may buckle and the animal appears weak. The horse is unable to lift its head, usually will not eat and may yawn repeatedly early in the course of an episode. The serum potassium levels are elevated above normal during the episodes.
Secondary myopathy due to ischemia
In secondary myopathy due to ischemia, e.g. the downer cow syndrome, the affected animal is unable to rise and the affected hindlegs are commonly directed behind the cow in the frogleg attitude. The appetite and mental attitude are usually normal. No abnormality of the muscles can be palpated. With supportive therapy, good bedding and the prevention of further ischemia by frequent rolling of the animal, most cows will recover in a few days.
In calves with aortic and iliac artery thrombosis there is an acute onset of paresis or flaccid paralysis of one or both pelvic limbs.13 Affected limbs are hypothermic and have diminished spinal reflexes and arterial pulse pressures. The diagnosis can be defined using angiography. Affected calves die or are euthanized because treatment is not undertaken.
Neurogenic atrophy
With neurogenic atrophy there is marked loss of total mass of muscle, flaccid paralysis, loss of tendon reflexes and failure of regeneration. When large muscle masses are affected, e.g. quadriceps femoris in femoral nerve paralysis in calves at birth, the animal is unable to bear normal weight on the affected leg.
Dystrophy of the diaphragmatic muscles
In dystrophy of the diaphragmatic muscles in adult Meuse–Rhine–Yessel cattle there is loss of appetite, decreased rumination, decreased eructation and recurrent bloat. The respiratory rate is increased with forced abdominal respirations, forced movement of the nostrils and death from asphyxia in a few weeks.
Severe diaphragmatic necrosis in a horse with degenerative myopathy due to polysaccharide storage myopathy has been described.15 Affected horses may have severe respiratory distress and respiratory acidosis, and do not respond to supportive therapy.
CLINICAL PATHOLOGY
Muscle-derived serum enzymes
The serum levels of the muscle enzymes are characteristically elevated following myopathy due to release of the enzymes from altered muscle cell membranes. Creatine kinase (CK) is a highly specific indication of both myocardial and skeletal muscle degeneration. Plasma CK activity is related to three factors: the amount and rate of CK released from an injured muscle into plasma, its volume of distribution and its rate of elimination.16 CK has a half-life of about 4–6 hours and, following an initial episode of acute myopathy, serum levels of the enzyme may return to normal within 3–4 days if no further muscle degeneration has occurred. Levels of AST are also increased following myopathy but, because the enzyme is present in other tissues such as liver, it is not a reliable indicator of primary muscle tissue degeneration.
Because AST has a longer half-life than CK, the levels of AST may remain elevated for several days following acute myopathy. The daily monitoring of both CK and AST levels should provide an indication of whether active muscle degeneration is occurring. A marked drop in CK levels and a slow decline in AST levels suggests that no further degeneration is occurring whereas a constant elevation of CK suggests active degeneration.
In acute nutritional muscular dystrophy in calves, lambs, and foals the CK levels will increase from normal values of below 100 IU/L to levels ranging from 1000–5000 IU/L and even higher. The levels of CK in calves will increase from a normal of 50 IU/L to approximately 5000 IU/L within a few days after being placed outdoors followed by unconditioned exercise. There is some preliminary investigation into quantification of the amount of skeletal damage in cattle based on the amount of CK activity.16
The measurement of serum levels of glutathione peroxidase is a useful aid in the diagnosis of myopathy due to selenium deficiency.
In downer cows with ischemic necrosis of the thigh muscles, the CK and AST levels will be markedly elevated and will remain elevated if muscle necrosis is progressive in cows that are not well bedded and rolled from side to side several times daily to minimize the degree and extent of ischemic necrosis.
High levels of CK (1000 IU/L and greater) usually indicate acute primary myopathy. Levels from 500–1000 IU/L may be difficult to interpret in animals recumbent for reasons other than primary myopathy. This will necessitate a careful reassessment of the clinical findings, history and epidemiology.
In horses with acute exertional rhabdomyolysis (paralytic myoglobinuria) the CK levels will range from 5000–10000 IU/L. Following vigorous exercise in unconditioned horses, the CK and AST levels will rise as a result of increased cell membrane permeability associated with the hypoxia of muscles subjected to excessive exercise. Lactate dehydrogenase (LDH) has also been used as a biochemical measurement of the degree of physical work done by horses in training. With progressive training in previously unconditioned horses there is no significant change between rest and exercise in the levels of serum CK, AST, and LDH. In horses with postanesthetic myositis the CK levels may exceed 100000 IU/L, the serum calcium is decreased and the serum inorganic phosphorus is increased. In naturally occurring cases of exertional rhabdomyolysis in horses the most consistent acid–base abnormality may be a hypochloremia rather than metabolic acidosis as has been assumed.
Muscle biopsy
Investigation of the structural and biochemical alterations of muscle tissue in myopathy include biopsy techniques that have been described.3,17 Needle biopsies require a specialized Bergstrom muscle biopsy needle, which most practitioners do have on hand. Open biopsy is recommended in order to obtain a strip of muscle. Biopsy of either the semimembranosus or semitendinosus muscles, at a site between the base of the tail and the tuber ischium, provides an adequate sample. Muscle biopsy samples can be processed for either frozen section or routine formalin-fixed, paraffin-embedded sections. The frozen section is considered the gold standard.
Inclusions of periodic-acid–Schiff (PAS)-positive, amylase-resistant complex polysaccharide are abnormal and characteristic findings in muscle of equine polysaccharide storage myopathy.3
Histochemical techniques can be used on muscle biopsies of horses with muscular disease and animals with congenital and inherited myopathies.4
Myoglobinuria
Myoglobinuria is a common finding in adult horses with acute paralytic myoglobinuria but is not a common finding in acute nutritional muscular dystrophy in young farm animals, except perhaps in yearling cattle with acute muscular dystrophy. The myoglobinuria may be clinically detectable as a red or chocolate brown discoloration of the urine. This discoloration can be differentiated from that caused by hemoglobin by spectrographic examination or with the use of orthotoluidine paper strips. Urine becomes dark when myoglobin levels exceed 40 mg/dL of urine. Discoloration of the plasma suggests hemoglobinuria. Both myoglobin and hemoglobin give positive results for the presence of protein in urine. Porphyria causes a similar discoloration although this may not be evident until the urine has been exposed to light for some minutes. The coloration is lighter, pink to red rather than brown, and the urine is negative to the guaiac test and fluoresces with ultraviolet light. Creatinuria accompanies acute myopathy but has not been used routinely as a diagnostic aid.
Electromyography is a special technique for the evaluation of the degree of neurogenic atrophy.
NECROPSY FINDINGS
Affected areas of skeletal muscle have a white, waxy, swollen appearance like fish flesh. Commonly only linear strips of large muscle masses are affected and the distribution of lesions is characteristically bilaterally symmetrical. Histologically the lesion varies from a hyaline degeneration to a severe myonecrosis, with subsequently the disappearance of large groups of muscle fibers and replacement by connective tissue. Calcification of the affected tissue may be present to a mild degree in these cases.
The lesions in exertional rhabdomyolysis in the horse are of a focal distribution and consist of hyaline degeneration with insignificant inflammatory reaction and slight calcification. The degenerative changes affect primarily the fast twitch fibers, which have a low oxidative capacity and are used when the horse trots at very close to its maximum speed.
Most myopathies in farm animals occur in rapidly growing, young animals and are characterized clinically by a sudden onset of acute muscular weakness, and pain often precipitated by unaccustomed exercise. There may be evidence of a dietary deficiency of vitamin and selenium in the case of nutritional muscular dystrophy. A sudden onset of recumbency or stiffness in young farm animals that are bright and alert should arouse suspicion of acute muscular dystrophy. Primary myopathies are not common in adult cattle, sheep or pigs but myopathy secondary to recumbency for other reasons does occur.
Secondary myopathy due to aortic and iliac thrombosis in calves must be differentiated from other common causes of hindlimb paresis including traumatic injury to the spinal cord, spinal cord compression due to vertebral body abscess, nutritional muscular dystrophy, myositis and nerve damage due to trauma of intramuscular injections, and clostridial myositis.14
The exertional myopathies in the horse in training are usually readily obvious. The CK levels are valuable aids to diagnosis. In special circumstances, such as neurogenic myopathy, muscle biopsy and electromyography may be useful additional diagnostic aids. The histological and histochemical staining characteristics of equine muscle have been described and serve as a standard for comparison with abnormal muscle.
Myositis may present a similar syndrome but is usually present as a secondary lesion in a clinically distinguishable primary disease or is accompanied by obvious trauma or toxemia.
TREATMENT
Vitamin E and selenium are indicated for the treatment of nutritional muscular dystrophy and the details are provided under that heading. The treatment of exertional rhabdomyolysis in horses has not been well defined because of the uncertain etiology, but enforced rest and the relief of pain, if necessary, seems logical. Supportive therapy for any case of myopathy, particularly severe cases in which there is persistent recumbency, consists of:
• Liberal quantities of thick bedding
• Removal from solid floors to softer ground
• Frequent turning from side to side to minimize secondary myopathy
• Provision of fluid therapy to prevent myoglobinuric nephrosis
With the exception of the sporadically occurring congenital and inherited myopathies of farm animals, all the nutritional and exertional myopathies are amenable to treatment if it is begun early and if adequate supportive therapy is provided.
In myopathies associated with systemic acidosis the use of a solution of sodium bicarbonate may be indicated. Dietary sodium bicarbonate at the rate of 2% of total dry matter intake has been used for the treatment of exertional rhabdomyolysis in a horse.18 Horses with postanesthetic myositis must be considered as critical care patients for 18–24 hours. Maintenance of adequate renal perfusion is vital. Large quantities of intravenous polyionic balanced electrolyte fluids (50–100 L) must be given over a 24-hour period. Dantrolene sodium at 4 mg/kg body weight (BW) given orally immediately upon recognition of clinical signs is efficacious.
CONTROL
The nutritional myopathies in farm animals can be satisfactorily prevented by the provision of adequate quantities of dietary vitamin E and selenium in the maternal diet during pregnancy or at the strategic times in postnatal life. The prevention of exertional myopathy in the horse depends on a progressive training program and avoidance of sudden unaccustomed exercise in animals that are in good body condition and have been inactive. Similarly, in general terms, the prevention of the porcine stress syndrome will depend on careful handling and transportation techniques combined with genetic selection of resistant pigs.
1 Freestone JF, Carlson GP. Equine Vet J. 1991;23:86.
2 Hosie BD, et al. Vet Rec. 1986;119:444.
3 Valentine BA. Equine Vet Educ. 2003;15:254.
4 Hafner A, et al. J Comp Pathol. 1996;115:23.
5 Sarli G, et al. Vet Rec. 1994;135:156.
6 Serteyn D, et al. Vet Rec. 1988;123:126.
7 Bloom BA, et al. Vet Rec. 1999;144:73.
8 Essen-Gustavsson B, Lindholm A. Equine Vet J. 1985;17:434.
9 Bruce V, Turek RJ. Equine Vet J. 1985;17:317.
10 Van den Hoven R, et al. Am J Vet Res. 1985;46:939.
11 Van den Hoven R, et al. Am J Vet Res. 1985;46:1755.
12 Andrews FM, Spurgeon TL. Am J Vet Res. 1986;47:1843.
13 Morley PS, et al. J Am Vet Med Assoc. 1996;209:130.
14 Step DL, et al. J Am Vet Med Assoc. 1991;198:117.
15 Valentine BA, et al. Can Vet J. 2002;43:614.
16 Lefebvre HP, et al. Am J Vet Res. 1994;55:487.
MYOSITIS
Myositis may arise from direct or indirect trauma to muscle and occurs as part of a syndrome in a number of specific diseases including blackleg, foot-and-mouth disease, bluetongue, ephemeral fever, swine influenza, sarcosporidiosis and trichinosis, although clinical signs of myositis are not usually evident in the latter. Sporadic cases of a localized infectious myositis of skeletal muscles, associated with Escherichia coli, may occur in calves.1 An asymptomatic eosinophilic myositis is not uncommon in beef cattle and may cause economic loss through carcass condemnation. The cause has not been determined.
Acute myositis of limb muscles
This disease is accompanied by severe lameness, swelling, heat and pain on palpation. There may be accompanying toxemia and fever. In chronic myositis there is much wasting of the affected muscles and this is difficult to differentiate clinically from atrophy due to other causes. Biopsy of the muscles may be necessary to confirm the diagnosis.
Injury to the gracilis muscle can cause acute, severe lameness in performance Quarter Horses.2 Horses competing in barrel racing may be susceptible to gracilis muscle injury because the muscle functions to adduct the hind limb. The prognosis is good for returning to athletic use after and an adequate period of muscle healing and mild exercise. However, fibrotic myopathy or muscle atrophy can be a complication of the injury resulting in persistent gait deficits.
In horses traumatic myositis of the posterior thigh muscles may be followed by the formation of fibrous adhesions between the muscles (fibrotic myopathy) and by subsequent calcification of the adhesions (ossifying myopathy). External trauma can result in fibrotic myopathy but it may also be associated with excessive exercise or secondary to intramuscular injections.
Occasionally similar lesions may be seen in the foreleg. The lesions cause a characteristic abnormality of the gait in that the stride is short in extension and the foot is suddenly withdrawn as it is about to reach the ground. The affected area is abnormal on palpation.
An inherited disease of pigs, generalized myositis ossificans, is also characterized by deposition of bone in soft tissues. In traumatic injuries caused by penetration of foreign bodies into muscle masses, ultrasonography may be used to detect fistulous tracts and the foreign bodies.
Extensive damage to or loss of muscle occurs in screwworm and sometimes blowfly infestation, although the latter is more of a cutaneous lesion, and by the injection of necrotizing agents. For example, massive cavities can be induced in the cervical muscles of horses by the intramuscular injection of escharotic iron preparations intended only for slow intravenous injection. Similarly, necrotic lesions can result from the intramuscular injection of infected or irritant substances. Horses are particularly sensitive to tissue injury, or are at least most commonly affected. Some common causes are chloral hydrate, antimicrobials suspended in propylene glycol, and even antimicrobials alone in some horses.
Injection site clostridial infections in horses
Clostridial myositis, myonecrosis, cellulitis, and malignant edema are terms used to describe a syndrome of severe necrotizing soft tissue infection associated with Clostridium spp. Affected horses typically develop peracute emphysematous soft tissue swelling in the region of an injection or wound within hours of the inciting cause. It can occur following the intramuscular or inadvertent perivascular administration of a wide variety of commonly administered drugs.3 In a series of 37 cases, the lesion occurred within 6–72 hours of a soft-tissue injection in most cases and most were in the neck musculature. Aggressive treatment can be associated with a survival rate of up to 81% for cases due to Clostridium perfringens alone; survival rates for other Clostridium spp. are lower. A combination of a high dose of intravenous antibiotic therapy and surgical fenestration and debridement is the recommended approach to treatment.
Injection site lesions in cattle
Muscle lesions associated with injection sites in the cattle industry are a source of major economic loss because of the amount of trim required at slaughter. The presence of injection-site lesions in whole muscle cuts, such as the top sirloin and outside round, limits their use and value. The occurrence of injection-site lesions in muscle is among the top five quality challenges for both beef and dairy market cows and bulls.4 Because injection-site lesions are concealed in muscles and/or are under subcutaneous fat, they are seldom found during fabrication at the packing plant and appear instead during wholesale/retail fabrication or at the consumer level. In 1998, the National Animal Health Monitoring System found that 47% of producers and 37% of veterinarians administered intramuscular injections in the upper or lower rear leg of cows; the need for further educational effort is apparent.
Monitoring the frequency of injection-site lesions allows educational efforts of state and national beef quality assurance programs to evaluate, more definitively, management practices of producers that can be changed to minimize occurrence of these defects. Audits done at abattoirs between 1998 and 2000 in the USA indicate that the frequency of injection-site lesions has decreased but the need remains for educational programs and continued improvements in beef quality assurance practices among beef and dairy cattle producers.4 Historically, most intramuscular injections were given in the gluteals and the biceps femoris muscles, which are prime cuts of beef. Surveys of injection sites in beef cattle in North America have found lesions in a significant percentage of prime cuts of beef.5 Lesions consisting of clear scars and woody calluses are mature and probably originated in calfhood; scars with nodules or cysts are less mature, occurring later in the feeding period. It is now recommended that intramuscular injections be given in the cervical muscles. Reducing the incidence of injection site lesions requires that manufacturers of biological and antibiotic preparations develop less irritating formulations. Products should be formulated for subcutaneous use whenever possible and administered in the neck muscles, which are not prime cuts of beef.
The outcome of an intramuscular injection depends on the nature of the lesion produced. Myodegeneration following intramuscular injections of antibiotics in sheep results in full muscle regeneration within less than 3 weeks.6 Necrosis following the injection results in scar formation with encapsulated debris, which persists for more than a month and leaves persistent scar tissue.
An outbreak of myositis, lameness and recumbency occurred following the injection of water-in-adjuvanted vaccines into the muscles of the left and right hips of near-term pregnant beef cattle.7 Within 24 hours, some cattle were recumbent, some had nonweightbearing lameness and, within 10 days, 50% of the herd developed firm swellings up to 24 cm in vaccination sites. Histologically, granulomatous myositis with intralesional oil was present. The swellings resolved over a period of 6 months. The acute transient lameness was attributed to the use of two irritating biological vaccines in the hip muscles of cows near parturition.
Diseases of bones
OSTEODYSTROPHY
Osteodystrophy is a general term used to describe those diseases of bones in which there is a failure of normal bone development, or abnormal metabolism of bone that is already mature. The major clinical manifestations include distortion and enlargement of the bones, susceptibility to fractures and interference with gait and posture.
ETIOLOGY
The common causes of osteodystrophy in farm animals include the following.
Nutritional causes
Calcium, phosphorus and vitamin D
Absolute deficiencies or imbalances in calcium–phosphorus ratios in diets cause:
• Rickets in young animals, e.g., growing lambs fed a diet rich in wheat bran
• Absolute deficiencies of calcium
• Beef calves on intensive rations with inadequate supplementation1
Osteodystrophia fibrosa in the horse occurs most commonly in animals receiving a diet low in calcium and high in phosphorus.
Osteodystrophia fibrosa in pigs occurs as a sequel to rickets and osteomalacia, which may occur together in young growing pigs that are placed on rations deficient in calcium, phosphorus and vitamin D following weaning.
Other nutritional causes
• Inadequate dietary protein and general undernutrition of cattle and sheep can result in severe osteoporosis and a great increase in ease of fracture
• Chronic parasitism can lead to osteodystrophy in young growing ruminants
• Hypovitaminosis A and hypervitaminosis A can cause osteodystrophic changes in cattle and pigs
• Prolonged feeding of a diet high in calcium to bulls can cause nutritional hypercalcitoninism combined with replacement of trabecular bone in the vertebrae and long bones with compact bone, and neoplasms of the ultimobranchial gland
• Multiple vitamin and mineral deficiencies are recorded as causing osteodystrophy in cattle. The mineral demands of lactation in cattle can result in a decrease in bone mineral content during lactation with a subsequent increase during the dry period.
Chemical agents
• Chronic lead poisoning is reputed to cause osteoporosis in lambs and foals
• Chronic fluorine poisoning causes the characteristic lesions of osteofluorosis, including osteoporosis and exostoses
• Grazing the poisonous plants Setaria sphaceleta, Cenchrus ciliaris, and Panicum maximum var. trichoglume causes osteodystrophia in horses
• Enzootic calcinosis of muscles and other tissues is caused by the ingestion of Solanum malacoxylon, Solanum torvum, Trisetum flavescens (yellow oatgrass), and Cestrum diurnum, which exert a vitamin-D-like activity
• Bowie or bentleg, a disease caused by poisoning with Trachymene glaucifolia, is characterized by extreme outward bowing of the bones of the front limbs.
Inherited and congenital causes
There are many inherited and congenital defects of bones of newborn farm animals, which are described, and discussed in detail in Chapter 34. In summary, these include:
• Achondroplasia and chondrodystrophy in dwarf calves and some cases of prolonged gestation
• Osteogenesis imperfecta in lambs and Charolais cattle. There is marked bone fragility and characteristic changes on radiological examination
• Osteopetrosis in Hereford and Angus calves
• Chondrodystrophy in ‘acorn’ calves
• Inherited exostoses in horses; inherited thicklegs and inherited rickets of pigs, which are well-established entities.
Angular deformities of joints of long bones due to asymmetric growth plate activity are common in foals and are commonly repaired surgically.2 The distal radius and distal metacarpus are most often affected, the distal tibia and metatarsal less commonly. Physiologically immature foals subjected to exercise may develop compression-type fractures of the central or third tarsal bones. Some of these foals are born prematurely or are from a twin pregnancy. Retained cartilage in the distal radial physis of foals 3–70 days of age presents without apparent clinical signs.
Physitis is dysplasia of the growth plate, characterized by an irregular border between the cartilage and the metaphyseal zone of ossification, an increase in the lateromedial diameter of the physis, and distoproximally oriented fissures at the medial aspect of the metaphysis, which originate at the physis. In some cases, these may result in bilateral tibial metaphyseal stress fractures in foals.3
Abnormal modeling of trabecular bone has been recognized in prenatal and neonatal calves.4 Abnormalities included growth retardation lines and lattices, focal retention of primary spongiosa and the persistence of secondary spongiosa. Intrauterine infection with viruses such as bovine virus diarrhea (BVD) may be a causative factor.4
Physical and environmental causes
Moderate osteodystrophy and arthropathy may occur in rapidly growing pigs and cattle raised indoors and fed diets that contain adequate amounts of calcium, phosphorus and vitamin D. Those animals raised on slatted floors or concrete floors are most commonly affected and it is thought that traumatic injury of the epiphyses and condyles of long bones may be predisposing factors in osteochondrosis and arthrosis in the pig (leg weakness) and epiphysitis in cattle. Experimentally raising young calves on metal slatted floors may result in more severe and more numerous lesions of the epiphysis than occurs in calves raised on clay floors. Total confinement rearing of lambs can result in the development of epiphysiolysis and limb deformities. However, the importance of weightbearing injury as a cause of osteodystrophy in farm animals is still uncertain. In most reports of such osteodystrophy, all other known causes have not been eliminated.
Chronic osteodystrophy and arthropathy have been associated with undesirable conformation in the horse.
Vertebral exostoses are not uncommon in old bulls and usually affect the thoracic vertebrae (T2 and T12) and the lumbar vertebrae (L2–L3), which are subjected to increased pressure during the bending of the vertebral columns while copulating. The exostoses occur mainly on the ventral aspects of the vertebrae, fusing them to cause immobility of the region. Fracture of the ossification may occur, resulting in partial displacement of the vertebral column and spinal cord compression. The disease is commonly referred to as spondylitis or vertebral osteochondrosis and also occurs less commonly in adult cows and in pigs. It is suggested that the anulus fibrosus degenerates and that the resulting malfunctioning of the disk allows excessive mobility of the vertebral bodies, resulting in stimulation of new bone formation. A similar lesion occurs commonly in horses and may affect performance, particularly in hurdle races and cross-country events. The initial lesion may be a degeneration of the intervertebral disk.
Some types of growth plate defect occur in young growing foals and these are considered to be traumatic in origin. Failure of chondrogenesis of the growth plate may be the result of crush injuries in heavy, rapidly growing foals with interruption of the vascular supply to the germinal cells of the growth plate. Asymmetric pressures due to abnormal muscle pull or joint laxity may slow growth on the affected side and result in limb angulation.
Femoral fractures occur in newborn calves during the process of assisted traction during birth.5 Laboratory compression of isolated femurs from calves revealed that the fracture configurations and locations are similar to those found in clinical cases associated with forced extraction. The breaking strength of all femurs fell within the magnitude of forces calculated to be created when mechanical devices are used to assist delivery during dystocia. It is suggested that the wedging of the femur in the maternal pelvis and resulting compression during forced extraction accounts for the occurrence of supracondylar fractures of the femur of calves delivered in anterior presentation using mechanical devices in a manner commonly used by veterinarians and farmers.
Tumors
Osteosarcomas are highly malignant tumors of skeletoblastic mesenchyme in which the tumor cells produce osteoid or bone. Osteosarcomas are the most common type of primary bone tumor in animals such as dogs and cats but are rare in horses and cattle. Most tumors of bone in large animals occur in the skull. A periosteal sarcoma on the scapula has been recorded in the horse6 and an osteosarcoma of the mandible in a cow.7
PATHOGENESIS
Osteodystrophy is a general term used to describe those diseases of bones in which there is a failure of normal bone development, or abnormal metabolism of bone that is already mature. There are some species differences in the osteodystrophies that occur with dietary deficiencies of calcium, phosphorus, and vitamin D. Rickets and osteomalacia occur primarily in ruminants, osteodystrophia fibrosa in horses, and all three may occur in pigs.
Rickets
Rickets is a disease of young growing animals in which there is a failure of provisional calcification of the osteoid plus a failure of mineralization of the cartilaginous matrix of developing bone. There is also failure of degeneration of growing cartilage, formation of osteoid on persistent cartilage with irregularity of osteochondral junctions and overgrowth of fibrous tissue in the osteochondral zone. Failure of provisional calcification of cartilage results in an increased depth and width of the epiphyseal plates, particularly of the long bones (humerus, radius and ulna and tibia) and the costal cartilages of the ribs. The uncalcified, and therefore soft, tissues of the metaphyses and epiphyses become distorted under the pressure of weightbearing, which also causes medial or lateral deviation of the shafts of long bones. There is a decreased rate of longitudinal growth of long bones and enlargement of the ends of long bones due to the effects of weight causing flaring of the diaphysis adjacent to the epiphyseal plate. Within the thickened and widened epiphyseal plate there may be hemorrhages and minute fractures of adjacent trabecular bone of the metaphyses. and in chronic cases the hemorrhagic zone may be largely replaced by fibrous tissue. These changes can be seen radiographically as ‘epiphysitis’ and clinically as enlargements of the ends of long bones and costochondral junctions of the ribs. These changes at the epiphyses may result in separation of the epiphysis, which commonly affects the femoral head. The articular cartilages may remain normal or there may be subarticular collapse resulting in grooving and folding of the articular cartilage and ultimately degenerative arthropathy and osteochondrosis. Eruption of the teeth in rickets is irregular and dental attrition is rapid. Growth of the mandibles is retarded and is combined with abnormal dentition. There may be marked malocclusion of the teeth.
Osteomalacia
Osteomalacia is a softening of mature bone due to extensive resorption of mineral deposits in bone and failure of mineralization of newly formed matrix. There is no enlargement of the ends of long bones or distortions of long bones but spontaneous fractures of any bone subjected to weightbearing is common.
Osteodystrophia fibrosa
Osteodystrophia fibrosa may be superimposed on rickets or osteomalacia and occurs in secondary hyperparathyroidism. Diets low in calcium or that contain a relative excess of phosphorus cause secondary hyperparathyroidism. There is extensive resorption of bone and replacement by connective tissue. The disease is best known in the horse and results in swelling of the mandibles, maxillae and frontal bones (the ‘bighead’ syndrome). Spontaneous fracture of long bones and ribs occurs commonly. Radiographically there is extreme porosity of the entire skeleton.
Osteoporosis
Osteoporosis is due to failure or inadequacy of the formation of the organic matrix of bone; the bone becomes porous, light and fragile, and fractures easily. Osteoporosis is uncommon in farm animals and is usually associated with general undernutrition rather than specifically a deficiency of calcium, phosphorus, or vitamin D. Copper deficiency in lambs may result in osteoporosis due to impaired osteoblastic activity. Chronic lead poisoning in lambs also results in osteoporosis due to deficient production of osteoid. In a series of 19 lactating or recently weaned sows with a history of lameness, weakness or paralysis, 10 had osteoporosis and pathological fractures while six had lumbar vertebral osteomyelitis. Bone ash, specific gravity of bone and the cortical to total ratio were significantly reduced in sows with osteoporosis and pathological fractures.
Ovariectomized sheep that are fed a calcium-wasting diet develop osteoporosis, which is being used as a model to study the disease in humans.8
Osteodystrophy of chronic fluorosis
Osteodystrophy of chronic fluorosis is characterized by the development of exostoses on the shafts of long bones due to periosteal hyperostosis. The articular surfaces remain essentially normal but there is severe lameness because of the involvement of the periosteum and encroachment of the osteophytes on the tendons and ligaments.
Congenital defects of bone
These include complete (achondroplasia) and partial (chondrodystrophy) failure of normal development of cartilage. Growth of the cartilage is restricted and disorganized and mineralization is reduced. The affected bones fail to grow, leading to gross deformity, particularly of the bones of the head.
CLINICAL FINDINGS
In general terms there is weakening of the bones due to defective mineralization and osteoporosis, which results in the bending of bones, which probably causes pain and shifting lameness – one of the earliest clinical signs of acquired osteodystrophy. The normal weight and tension stresses cause distortion of the normal axial relationships of the bones, which results in the bowing of long bones. The distortions occur most commonly in young, growing animals. The distal ends of the long bones are commonly enlarged at the level of the epiphyseal plate and circumscribed swellings of the soft tissue around the epiphyses may be prominent, and painful on palpation.
The effects of osteodystrophy on appetite and body weight will depend on the severity of the lesions and their distribution. In the early stages of rickets in calves and pigs the appetite and growth rate may not be grossly affected until the disease is advanced and causes considerable pain. Persistent recumbency due to pain will indirectly affect feed intake unless animals are hand-fed.
Spontaneous fractures occur commonly and usually in mature animals. Common sites for fractures include the long bones of the limbs, pelvic girdle, femoral head, vertebrae, ribs, and transverse processes of the vertebrae. Ordinary hand pressure or moderate restraint of animals with osteomalacia and osteodystrophia fibrosa is often sufficient to cause a fracture. The rib cage tends to become flattened and in the late stages affected animals have a slab-sided appearance of the thorax and abdomen. Separations of tendons from their bony insertions also occur more frequently and cause severe lameness. The osteoporotic state of the bone makes such separations easy. Any muscle group may be affected but, in young cattle in feedlots, separations of the gastrocnemius are the most common. Thickening of the bones may be detectable clinically if the deposition of osteoid or fibrous tissue is excessive, or if exostoses develop as in fluorosis. Compression of the spinal cord or spinal nerves may lead to paresthesia, paresis or paralysis, which may be localized in distribution. Details of the clinical findings in the osteodystrophies caused by nutritional deficiencies are provided in Chapter 30.
Calcinosis of cattle is characterized clinically by chronic wasting, lameness, ectopic calcifications of the cardiovascular system, lungs and kidneys, ulceration of joint cartilage and extensive calcification of bones.
CLINICAL PATHOLOGY
The laboratory analyses that are indicated include the following:
• Serum calcium and phosphorus
• Feed analysis for calcium, phosphorus, vitamin D and other minerals when indicated (such as copper, molybdenum, and fluorine)
• Histopathology of bone biopsy
• Radiographic examination of the skeleton
• Single photon absorptiometry, a safe and noninvasive method for the measurement of bone mineral content, is now available.
Radiographic examination of the affected bones and comparative radiographs of normal bones is indicated when osteodystrophy is suspected. Radiographic examination of slab sections of bone is a sensitive method for detecting abnormalities of trabecular bone in aborted and young calves.4
Serum calcium and phosphorus concentrations in nutritional osteodystrophies may remain within the normal range for long periods and not until the lesions are well advanced will abnormal levels be found. Several successive samplings may be necessary to identify an abnormal trend.
Alkaline phosphatase levels may be increased in the presence of increased bone resorption but this is not a reliable indicator of osteodystrophy. Increased serum levels of alkaline phosphatase may originate from osseous tissues, intestine or liver, but osseous tissue appears to be the major source of activity.
Nutritional history and feed analysis results will often provide the best circumstantial evidence of osteodystrophy.
The definitive diagnosis is best made by a combination of chemical analysis of bone, histopathological examination of bone and radiography. The details for each of the common osteodystrophies are discussed under the appropriate headings.
NECROPSY FINDINGS
The pathological findings vary with the cause, and the details are described under each of the osteodystrophies elsewhere in the book. In general terms, the nutritional osteodystrophies are characterized by bone deformities, bones that may be cut easily with a knife and that bend or break easily with hand pressure and the presence in prolonged cases of degenerative joint disease. In young, growing animals the ends of long bones may be enlarged and the epiphyses may be prominent and circumscribed by periosteal and fibrous tissue thickening. On longitudinal cut sections the cortices may appear thinner than normal and the trabecular bone may have been resorbed, leaving an enlarged marrow cavity. The epiphyseal plate may be increased in depth and width and appear grossly irregular, and small fractures involving the epiphyseal plate and adjacent metaphysis may be present. Separation of epiphyses is common, particularly of the femoral head. The calluses of healed fractures of long bones, ribs, vertebrae and pelvic girdle are common in pigs with osteodystrophy. On histological examination there are varying degrees of severity of rickets in young growing animals and osteomalacia in adult animals, and osteodystrophia fibrosa is possible in both young and adult animals.
In both congenital and acquired osteodystrophy the clinical findings are usually suggestive. There are varying degrees of lameness, stiff gait, long periods of recumbency and failure to perform physical work normally, progressive loss of body weight in some cases and there may be obvious contortions of long bones, ribs, head and vertebral column. The most common cause of osteodystrophy in young growing animals is a dietary deficiency or imbalance of calcium, phosphorus and vitamin D. If the details of the nutritional history are available and if a representative sample of the feed given is analyzed, a clinical diagnosis can be made on the basis of clinical findings, nutritional history and response to treatment. In some cases, osteodystrophy may be due to overfeeding, such as might occur in rapidly growing, large foals.
However, often the nutritional history may indicate that the animals have been receiving adequate quantities of calcium, phosphorus and vitamin D, which necessitates that other less common causes of osteodystrophy be considered. Often the first clue is an unfavorable response to treatment with calcium, phosphorus and vitamin D. Examples include copper deficiency in cattle, leg weakness in swine of uncertain etiology – but perhaps there is weight-bearing trauma and a relative lack of exercise due to confinement – or chemical poisoning such as enzootic calcinosis or fluorosis. These will require laboratory evaluation of serum biochemistry, radiography of affected bones and pathological examination. The presence of bony deformities at birth suggests congenital chondrodystrophy, some cases of which appear to be inherited while some are due to environmental influences.
TREATMENT
The common nutritional osteodystrophies due to a dietary deficiency or imbalance of calcium, phosphorus and vitamin D will usually respond favorably following the oral administration of a suitable source of calcium and phosphorus combined with parenteral injections of vitamin D. The oral administration of dicalcium phosphate, at the rate of three to four times the daily requirement, daily for 6 days followed by a reduction to the daily requirement by the 10th day, combined with one injection of vitamin D at the rate of 10000 IU/kg BW is recommended. Affected animals are placed on a diet that contains the required levels and ratios of calcium, phosphorus, and vitamin D. The oral administration of the calcium and phosphorus will result in increased absorption of the minerals, which will restore depleted skeletal reserves. Calcium absorption is increased in adult animals following a period of calcium deficiency; young animals with high growth requirements absorb and retain calcium in direct relation to intake. General supportive measures include adequate bedding for animals that are recumbent.
The treatment of the osteodystrophies due to causes other than calcium and phosphorus deficiencies depends on the cause. Copper deficiency will respond gradually to copper supplementation. There is no specific treatment for the osteodystrophy associated with leg weakness in pigs and slaughter for salvage is often necessary. Overnutrition in young, rapidly growing foals may require a marked reduction in the total amount of feed made available daily.
Oxytetracycline has been used for the treatment of flexural deformities of the distal interphalangeal joints of young foals.9 It is postulated that oxytetracycline chelates calcium, rendering it unavailable for use for striated muscle contraction. It is considered effective for obtaining a short-term moderate decrease in metacarpophalangeal joint angle in newborn foals. Hemicircumferential periosteal transection and elevation has gained wide acceptance for correction of angular limb deformities in young foals.1
1 Caldow G, et al. Vet Rec. 1995;136:80.
2 Mitten LA, Bertone AL. J Am Vet Med Assoc. 1994;204:717.
3 Frankney RL, et al. J Am Vet Med Assoc. 1994;205:76.
4 O’Connor BP, Doige CE. Am J Vet Res. 1993;57:25.
5 Ferguson JG. Can Vet J. 1994;35:626.
6 Zaruby JF, et al. Can Vet J. 1993;34:742.
7 Plumlee KH, et al. J Am Vet Med Assoc. 1993;202:95.
HYPERTROPHIC PULMONARY OSTEOARTHROPATHY (MARIE’S DISEASE, ACHROPACHIA OSSEA)
Although hypertrophic pulmonary osteoarthropathy is more common in dogs than in the other domestic animals it has been observed in horses,1 cattle and sheep. The disease is characterized by proliferation of the periosteum leading to the formation of periosteal bone, and bilateral symmetrical enlargement of bones, usually the long bones of limbs. The enlargement is quite obvious, and in the early stages is usually painful and often accompanied by local edema. On radiographic examination there is a shaggy periostitis and evidence of periosteal exostosis. The pathogenesis is obscure but the lesion appears to be neurogenic in origin, unilateral vagotomy causing regression of the bony changes. Stiffness of gait and reluctance to move are usually present, and there may be clinical evidence of the pulmonary lesion with which the disease is almost always associated. Such lesions are usually chronic, neoplastic or suppurative processes such as tuberculosis.
The disease is considered to be incurable, unless the thoracic lesion can be removed, and affected animals are usually euthanized. At necropsy the periostitis, exostosis and pulmonary disease are evident. There is no involvement of the joints.
OSTEOMYELITIS
ETIOLOGY AND PATHOGENESIS
Inflammation of bone is uncommon in farm animals except when infection is introduced by traumatic injury or by the hematogenous route. Bacteria can reach bone by any of three routes:
Focal metaphyseal osteomyelitis can occur following open fractures in the horse. Specific diseases that may be accompanied by osteomyelitis include actinomycosis of cattle and brucellosis, atrophic rhinitis and necrotic rhinitis of pigs. Nonspecific, hematogenous infection with other bacteria occurs sporadically and is often associated with omphalitis, abscesses from tail-biting in pigs or infection of castration or docking wounds in lambs. A series of 28 cases of osteomyelitis of the calcaneus of adult horses has been described.1
Foals and calves under 1 month of age and growing cattle 6–12 months of age may be affected by osteomyelitis in one or more bones. The majority of foals with suppurative polyarthritis have a polyosteomyelitis of the bones adjacent to the affected joints. In a series of cases of tarsal osteomyelitis in foals there was usually evidence of infectious arthritis.2 Osteomyelitis of the pubic symphysis associated with Rhodococcus equi in a 2-year-old horse has been described.3 The lameness was localized to the pelvis and was associated with a fever and an inflammatory leukogram.
The infections occur commonly in the metaphysis, physis, and epiphysis, which are sites of bony growth and thus susceptible to blood-borne infections. The metaphyseal blood vessels loop toward the physis and ramify into sinusoids that spread throughout the metaphyseal region. Blood flow through the sinusoids is sluggish and presents an ideal environment for propagation of bacteria. Lesions occur on both sides of the physis in both the metaphysis and the epiphysis. Multiple lesions are common and support the explanation that septic emboli are released from a central focus.
In a series of 445 cattle with bone infection of the appendicular skeleton a distinction was made between hematogenous and post-traumatic orgin (wound/fracture).4 Bone infection was classified into four types according to the site of infection: Type 1 is metaphyseal and/or epiphyseal osteomyelitis close to the growth plate; type 2 is primary subchondral osteomyelitis, mostly accompanied by septic arthritis; type 3 is infectious osteoarthritis with subchondral osteomyelitis, implying that infection in the subchondral bone originates from the infection. Type 4 includes bone infections that cannot be categorized in the other groups. Hematogenous osteomyelitis was 3.2 times more frequent than post-traumatic osteomyelitis. Arcanobacterium (Corynebacterium) pyogenes was the most common etiological agent. About 55% of the affected animals with osseous sequestration had physical evidence of lacerations, contusions, abrasions or puncture wounds from a previous traumatic event.
Hematogenous osteomyelitis in cattle can be of:
• Physeal type, in which an infection generally of metaphyseal bone originates at or near the growth plate, usually affecting the distal metacarpus, metatarsus, radius or tibia5
• Epiphyseal type, in which an infection originates near the junction of the subchondral bone and the immature epiphyseal joint cartilage, most often affecting the distal femoral condyle epiphysis, the patellar and the distal radius.
The epiphyseal osteomyelitises are usually due to infection with Salmonella spp. and are most common in calves under 12 weeks of age. The physeal infections are usually due to A. pyogenes and occur most commonly in cattle over 6 months of age.
Osseous sequestration in cattle
Osseous sequestration is a common orthopedic abnormality in cattle and horses.6 In most cases, the lesions develop in the bones of the distal portion of the limbs. Sequestration is associated with trauma that results in localized cortical ischemia and bacterial invasion secondary to loss of adjacent periosteal and soft-tissue integrity and viability. The soft tissues covering the bones that comprise the distal portions of the limbs fail to provide adequate protection and collateral blood supply to the bone.
Osteomyelitis secondary to trauma
In horses, osteomyelitis is a frequent sequela to wounds of the metacarpal and metatarsal bones and the calcaneus. These bones have limited soft tissue covering, which may predispose them to osteomyelitis following traumatic injury. Similarly, a portion of the lateral aspect of the proximal end of the radius has limited soft-tissue covering. Penetrating and nonpenetrating wounds in this region, therefore, may result in serious consequences even though they may initially appear to be minor. Because lesions may be an extension of septic arthritis, a thorough examination of the wound area is necessary.
Osteomyelitis of the sustentaculum tali in horses has been described.7
Inflammation of bone marrow
Inflammation of bone marrow in animals has been described.8,9 Acute inflammation commonly accompanies bacterial sepsis, resulting in either multifocal microabscesses or perivascular infiltrates of neutrophils, fibrin, edema, and hemorrhage. The most common abnormality associated with fibrinous inflammation is disseminated intravascular coagulopathy. Discrete granulomas may occur in the marrow of animals with systemic mycotic disease, idiopathic granulomatous disease and serous atrophy of fat.
CLINICAL FINDINGS
The common clinical findings of osteomyelitis include:
• Generalized soft tissue swelling and inflammation
• Pain on palpation of the affected area
• Secondary muscle atrophy of the affected limb.1
Erosion of bone occurs and pus discharges into surrounding tissues, causing a cellulitis or phlegmon, and to the exterior through sinuses, which persist for long periods. The affected bone is often swollen and may fracture easily because of weakening of its structure. When the bones of the jaw are involved, the teeth are often shed and this, together with pain and the distortion of the jaw, interferes with prehension and mastication. Involvement of vertebral bodies may lead to the secondary involvement of the meninges and the development of paralysis. Lameness and local swelling are the major manifestations of involvement of the limb bones.
Most osseous sequestra in cattle are associated with the bones of the extremities, most commonly the third metacarpal or metatarsal bone. Cattle 6 months to 2 years of age are most likely to have a sequestrum compared with animals less than 6 months of age.6
The lesions are typically destructive of bone and cause severe pain and lameness. Those associated with Salmonella spp. are characteristic radiographically in foals and calves. A. pyogenes, Corynebacterium spp., and E. coli may also be causative agents. Affected animals are very lame and the origin of the lameness may not be obvious. A painful, discrete soft-tissue swelling over the ends of the long bones is often the first indication. The lameness characteristically persists in spite of medical therapy and the animal may become lame in two or more limbs and spend long periods recumbent.
Osteomyelitis affecting the cervical vertebrae, usually the fourth to sixth vertebra, causing a typical syndrome of abnormal posture and difficulty with ambulation. Initially there is a stumbling gait, which then becomes stiff and restricted and with a reluctance to bend the neck. Soon the animal has difficulty eating off the ground and must kneel to graze pasture. At this stage there is obvious atrophy of the cervical muscles and pain can be elicited by deep, forceful compression of the vertebrae with the fists. There is no response to treatment and at necropsy there is irreparable osteomyelitis of the vertebral body and compression of the cervical spinal cord. Radiological examination is usually confirmatory.
Cervicothoracic vertebral osteomyelitis in calves between 2 and 9 weeks of age is characterized by difficulty in rising with a tendency to knuckle or kneel on the forelimbs, which are hypotonic and hyporeflexic. Pain can be elicited on manipulation of the neck. The lesion usually involves one or more of the vertebrae from C6–T1.8 Salmonella dublin is commonly isolated from the vertebral lesion.
CLINICAL PATHOLOGY
The lesions are characteristically centered at the growth and extend into both metaphysis and epiphysis. Culture of the inflammatory exudate and necrotic sequestra removed surgically is necessary to determine the species of bacteria and their antimicrobial sensitivity.6 Samples of bone obtained at surgery provide the most accurate culture results compared to specimens obtained from the draining sinuses, which may yield a mixed flora. Specimens should consist of sequestra and soft tissues immediately adjacent to bone thought to be infected. Special transport media are desirable for optimum culture results. Anaerobic bacteria are frequently associated with osteomyelitis and should be considered when submitting samples for culture.
NECROPSY FINDINGS
At necropsy the osteomyelitis may not be obvious unless the bones are opened longitudinally and the cut surfaces of the metaphysis and epiphysis are examined.
A differential diagnosis for a destructive lesion in the end of a long bone of a foal or calf would include: a healing fracture, traumatic periostitis or osteitis, bone tumor, nutritional osteodystrophy and infection of the bone due to external trauma, fracture, extension from adjacent infection or hematogenous spread. The absence of equal pathological involvement in the comparable parts of long bones and the young age of the animal will usually suggest infection of bone. The pathological features of multiple bone infection in foals are described.
TREATMENT
Despite advances in antimicrobial therapy and refined diagnostic techniques, the clinical management of osteomyelitis is difficult. Medical therapy alone is rarely completely successful because of the poor vascularity of the affected solid bone and the inaccessibility of the infection. In cases of long-term infection or those with extensive bone necrosis, surgery is generally recommended to remove sequestra, devitalized tissue and sinus tracts that are harboring large numbers of bacteria.1,6 Good results are obtained when the affected bone is removed and the affected area is irrigated daily through a temporary drainage tube.
In septic physitis, the implantation of homologous cancellous bone grafts following debridement of necrotic bone, and the application of a walking cast for 4–5 weeks and antimicrobial therapy for 2 weeks was highly successful.10 Absolute asepsis is required for successful application of a bone graft and, after debridement of the necrotic bone, the cavity is flushed with saline and ampicillin.
Antimicrobials are an integral part of the treatment and selection of the most appropriate drug should be based on identification of the organism. Parenteral antimicrobial therapy should be continued for 4–6 weeks following surgical curettage. However, in a series of osteomyelitis of the calcaneus of adult horses, there was no difference in the survival rate of animals between those treated surgically and those treated conservatively.1 Prolonged antibiotic therapy can be successful for the treatment of osteomyelitis of the proximal end of the radius in the horse.11
Most anaerobic bacteria associated with osteomyelitis are sensitive to penicillin and the cephalosporins, but some species of Bacteroides fragilis and Bacteroides asaccharolyticus and other species of Bacteroides are known to produce beta-lactamases, which can inactivate penicillin and cephalosporin. Metronidazole and clindamycin will penetrate bone and can be considered.
1 MacDonald MH, et al. J Am Vet Med Assoc. 1989;194:1317.
2 Firth EC, Goodegebuure SA. Vet Q. 1988;10:99.
3 Clark-Price SC, et al. J Am Vet Med Assoc. 2003;222:969.
4 Verschooten F, et al. Vet Radiol Ultrasound. 2000;41:250.
5 Firth EC, et al. Vet Rec. 1987;120:148.
6 Valentino LW, et al. J Am Vet Med Assoc. 2000;217:376.
7 Hand DR, et al. J Am Vet Med Assoc. 2001;219:341.
8 Healy AM, et al. Vet J. 1997;154:227.
9 Weiss DJ, et al. Vet Clin Pathol. 1992;21:79.
Diseases of joints
ARTHROPATHY (OSTEOARTHROPATHY, DEGENERATIVE JOINT DISEASE)
The terms osteoarthropathy and degenerative joint disease are used here to describe noninflammatory lesions of the articular surfaces of joints characterized by:
• Degeneration and erosion of articular cartilage
• Eburnation of subchondral bones
• Hypertrophy of bone surrounding the articular cartilage resulting in lipping and spur formation at the joint margins.
Osteochondrosis is a degeneration of both the deep layers of the articular cartilage and the epiphyseal plate – a defect in endochondral ossification – which occurs in pigs and horses and is similar to the well-recognized disease in dogs.
ETIOLOGY AND EPIDEMIOLOGY
The etiology is not clear but in most of the commonly occurring cases the lesions are considered to be multifactorial and perhaps secondary to conformational defects resulting in excessive joint laxity, acute traumatic injury of a joint, the normal aging process and nutritional deficiencies. The etiological information is primarily circumstantial and some of the epidemiological observations that have been associated with osteoarthritis of farm animals are outlined here.
Nutritional causes
• Secondary to, or associated with, rickets, osteomalacia, bowie and osteodystrophia fibrosa
• Coxofemoral arthropathy in dairy cattle associated with aphosphorosis
• Copper deficiency thought to be related to enlargement of limb joints in foals on pasture and pigs fed experimental copper-deficient diets
• Experimental diets deficient in manganese or magnesium causing arthropathy and joint deformity in some calves
Steroid-induced
The intra-articular injection or prolonged parenteral administration of corticosteroids in horses may lead to degenerative joint disease.
Biomechanical trauma
• Acute traumatic injury, e.g. injury to joint surfaces, menisci and ligaments, especially the cruciate ligaments of the stifle joints of breeding bulls, may lead to chronic progressive osteoarthritis. Injuries to the femorotibial ligaments of horses can predispose to osteoarthropathy of the stifle joint
• Repeated subacute trauma to joint surfaces can lead to degenerative arthropathy. This is common in young racehorses in training, which may have their joint surfaces and surrounding tissues made susceptible to injury because of conformational defects and subtle deficiencies of calcium and phosphorus. Hard running surfaces may also contribute to the onset
• Trauma caused by movement is suspected of contributing to the erosive lesions on the articular surfaces of some horses affected by enzootic incoordination, the intervertebral joints of caudal thoracic and cranial lumbar vertebrae of old bulls with spondylitis, and the condition of bulls with inherited spasticity. Coxofemoral osteoarthritis may occur in aged horses with joint instability and in calves with hip dysplasia.
Growth rate, body size, and genetic predisposition
Degenerative coxofemoral arthropathy occurs in young beef bulls as early as 9 months of age. A congenital shallow acetabulum may predispose. It may be secondary to hip dysplasia, but in some cases there is no evidence of this. The large, weightbearing joints subjected to the greatest movement and concussion appear to be most susceptible. Rapidly growing bull calves appear to be most susceptible and some of them have an inherited susceptibility.
Osteochondrosis
Osteochondrosis is an important cause of lameness in horses. It is usually seen in young rapidly growing animals, and affects males more commonly than females. The predilection sites of osteochondrosis in the horse and their general order of incidence are hock, stifle, shoulder, fetlock, and cervical spine. The stifle, hock, and shoulder joints are more commonly affected, but many other joints may also be affected, including the metatarsal and metacarpal bones and rarely the acetabula of young foals.
The epidemiology, heritability and body measurements and clinical findings of osteochondrosis of hock and fetlock joints in Standardbred trotters have been examined.1 The incidence of the disease is high in the Swedish Standardbred population and well developed by the age of 1.5 years. The incidence of osteochondrosis is higher in horses born later in the foaling season than earlier and the incidence was related to body size: affected horses were taller at the withers and had a greater circumference of the carpus.1 This suggests that differences in body size at birth and the first few months of the foal’s life are of major importance in the development of osteochondrosis. The heritability estimates of osteochondrosis in the hock and fetlock joints of 753 Standardbred trotters 6–21 months of age was 0.52 and 0.21, respectively.2
Aging process
Degenerative arthropathy in aged dairy cows and bulls may be a manifestation of the normal aging process. Osteochondrosis, degenerative joint disease and vertebral osteophysis occur in middle-aged bulls.
Osteoarthrosis of the antebranchial joint of riding horses has been described.3 Affected animals were aged mares that developed osteoarthrosis and ankylosis. The cause was unknown.
Conformation and intensive animal production
Osteoarthropathy occurs in rapidly growing cattle and pigs raised in confinement on hard, usually concrete, floors and with minimal exercise. Osteochondrosis in feedlot cattle may be associated with a high-caloric diet and rapid growth rate. It is thought that weight-bearing trauma in these rapidly growing animals is sufficient to cause degenerative lesions of certain joints, especially in animals with a skeletal conformation that results in abnormal stress on certain weightbearing condyles of long bones.
In a series of 42 cases of stifle lameness in cattle, 18 had evidence of subchondral bone cyst and ranged in age from 6–18 months. It is suggested that the subchondral bone cyst is an indicator of osteochondrosis. In a series of osteochondrosis in cattle, male, purebred cattle of a mean age of 21 months were affected.4
Osteochondrosis may occur in rapidly growing bull beef calves fed a diet lacking adequate calcium, sodium, copper and vitamins A, D, and E,5,6 grazing beef cattle on improved native pasture in which a common ancestral sire and gender (all males) may have been contributing factors.7 Severe osteochondrosis of multiple joints but with remarkable changes in the humeral head and glenoid of both shoulder joints in 10-month-old beef calves has been described.8
Osteochondrosis similar to that seen in pigs has been recorded in purebred Suffolk lambs raised in a system designed to produce rapidly growing, high-value rams.9 The disease has been recorded in a single pedigree Suffolk ram.10
Osteochondrosis and arthrosis are considered to be major causes of ‘leg weakness’ in rapidly growing pigs.11 Restricting the energy intake appears to decrease the prevalence and severity of osteochondrosis when gilts are examined at 100 kg. The prevalence and severity of osteochondrosis in growing pigs is probably not related to floor type.11 Recent work has shown a significant relationship between body conformation and the presence of joint lesions. Pigs with a narrow lumbar region, broad hams and a large relative width between the stifle joints were highly susceptible to poor locomotor ability due to lesions in the elbow and stifle joints, the lumbar intervertebral joints and the hip joint.
This excellent work represents real progress in understanding the relationship between skeletal conformation and bone and joint lesions. It is postulated that inherited weakness of muscle, ligaments, cartilage and exterior joint conformation results in local overloading in the joint and the development of osteochondrosis and arthrosis. Some breeds, such as the Duroc, have more problems of structure and movement in the front legs than in the rear legs, but osteochondrosis is not responsible for the leg weakness. Osteochondrosis has been recorded in wild boar–Swedish Yorkshire crossbred pigs in which the growth rate was low.12
Osteoarthrosis of the distal tarsal joints of the horse (bone spavin)
Osteoarthrosis of the distal tarsal joints (hock), commonly known as bone spavin, is common in Icelandic horses and strongly related to age.13,14 In Icelandic horses aged 6–12 years and used for riding, the prevalence of radiographic signs of osteoarthrosis in the distal tarsus increased from 18% in horses 6 years of age up to 54% in 12-year-old horses. The age onset of radiographic signs reflect a predisposition to bone spavin indicating a trait with medium–high heritability.13 There is a high prevalence of chondronecrosis in young Icelandic horses, indicating an early onset and slow progression of disease.14 The disease is the most common cause of culling due to disease in riding horses in the age group 7–17 years.15
PATHOGENESIS
The details of the pathogenesis of degenerative joint disease have been reviewed.16 A brief review of the structure and biochemistry of the normal articular joint will serve as background for understanding the pathogenesis of osteoarthropathy.17
Articular cartilage is a tissue consisting of chondrocytes scattered in a matrix of collagen fibers and an amorphous intercellular substance containing proteoglycans. Articular cartilage contains no nerves, is avascular and has a high matrix-to-cell ratio. The chondrocytes are the only living matter in cartilage, produce the fine strands of collagen and are engaged in protein and proteoglycan synthesis. The matrix of the cartilage consists of water-soluble proteoglycans interspersed with collagen fibers, which are arranged in parallel rows superficially and crisscross rows closer to the calcified layer. This enables the cartilage to withstand shearing stresses superficially and compression more deeply.
The proteoglycans are glycosaminoglycan–protein complexes, bound by a link glycoprotein to a linear hyaluronic acid molecule. The glycosaminoglycans in articular cartilage are chondroitin 4-sulfate, chondroitin 6-sulfate and keratan sulfate. About 75% of the proteoglycans exist on aggregates that protect them from degradation and, because of their high water content, form large polyanionic complexes that have considerable elastic resistance to compression.
Nutrition of the articular cartilage is provided via the synovial fluid and is dependent on the capillary flow to the synovial membrane. Nutrients flow through the synovial fluid and diffuse through the cartilage to the chondrocytes. Proteoglycans are synthesized by the chondrocytes and secreted to the cell exterior. Proteoglycans are also degraded intracellularly by lysosomes. The normal equilibrium between anabolism and catabolism is maintained by several different low-molecular-weight proteins. When the equilibrium is disturbed and shifts toward catabolism, degeneration occurs.
Primary osteoarthropathy
This is due to normal aging processes and ordinary joint usage. The initial lesions occur in the superficial layers of the articular cartilages where, with increasing age, there is loss of the normal resilience of the cartilage, a lowering of the chondroitin sulfate content and reduction in the permeability of the cartilaginous matrix, which results in progressive degeneration of the articular cartilage. There is grooving of the articular cartilage, eburnation of subchondral bone and secondary hypertrophy of marginal cartilage and bone, with the formation of pearl-like osteophytes. In experimentally induced arthritis in the horse the major changes include synovitis, increased synovial effusion and superficial fibrillation with chondrocyte necrosis in the articular cartilage. These are comparable to the early changes in naturally occurring degenerative joint disease.
Secondary osteoarthropathy
This appears to be initiated by injuries or congenital conformational defects that create greater shearing stresses on particular points, in contrast to the intermittent compressive stresses typical of ordinary weightbearing. These irregular stresses result in cartilaginous erosion, increased density of subchondral bone at points of physical stress and proliferation of bone and cartilage at the articular margins.
Following acute trauma, the initial changes are often characterized by acute synovitis and capsulitis. As a result of the inflammatory response, leukocytes, prostaglandins, lysosomal enzymes and hyaluronidase enter the synovial fluid, which becomes less viscous and affects the nutrition of the cartilage. There is some evidence of immune complexes associated with collagen-type-specific antibodies in horses with secondary osteoarthritis. Cytokines can be detected in the synovial fluid after racing in horses with degenerative joint disease.18 The cartilage matrix undergoes a variety of changes, possibly because of chondrocyte damage with lysosomal enzyme release, or to collagen fiber injury. There is an increase in water content and loss of orientation of the collagen fibers. Proteoglycans are lost and, while increased chondrocyte activity synthesizes proteoglycans, they are of lower molecular weight and altered glycosaminoglycan composition. This leads to loss of elasticity and surface integrity of the cartilage, resulting in increased friction, blistering and ulceration. There is additional lysosomal enzyme release from the chondrocytes, resulting in matrix destruction and further proteoglycan destruction. The degrading enzymes enter the altered matrix and cause further degradation.
The first stage of matrix degradation involves discoloration, softening and blistering of the tangential layer of the cartilage surface, a process known as early fibrillation. As the fissuring extends to the radial layer, microfractures occur, with loss of cartilage fragments (detritus) into the synovial fluid. As the cartilage is destroyed the underlying bone is exposed and becomes sclerotic. Bony proliferation occurs in the floor of the cartilage lesions, while at the joint margins osteophyte formation occurs. The pathogenesis of degenerative joint disease indicates that the ideal treatment would be the use of a substance that would promote synthesis of matrix components and retard catabolic processes.
The major proteoglycan in cartilage is a high-molecular-weight aggrecan that contains chondroitin sulfate and keratin sulfate chains located on specific regions of the core protein. These macromolecules are continuously released into the synovial fluid during normal cartilage matrix metabolism. Cartilage proteoglycans are degraded early in the course of joint disease and released from the cartilage into the synovial fluid, where they can be identified.19
In horses with degenerative joint disease, proteoglycan fragments – glycosaminoglycans – have been determined in equine synovial fluid as indicators of cartilage metabolism in various types of arthritides.19 The presence of high-molecular-weight proteoglycans and high concentrations of hyaluronate in horses with various arthritides – acute or chronic traumatic arthritis, intra-articular fracture and infectious arthritis, with and without abnormal radiographic and/or arthroscopic findings – compared with control joints has been investigated.19
The intra-articular injection of corticosteroids depresses chondrocyte metabolism, alters the biochemical composition and causes morphological changes in the articular cartilage, which remains biochemically and metabolically impaired for several or more weeks.
In femoral–tibial osteoarthrosis of bulls the secondary degenerative joint lesions are due to rupture of the attachments of the lateral meniscus resulting in mechanical instability in the joint with unusual mechanical stresses on the articular cartilage leading to degeneration. The cranial cruciate ligament becomes progressively worn and eventually ruptures, resulting in loss of all joint stability and the development of gross arthrosis. In cattle with severe degenerative joint disease of the coxofemoral joints, an acetabular osseous bulla may develop at the cranial margin of the obturator foramen.
Osteochondrosis
Osteochondrosis (dyschondroplasia) is characterized by disturbance of the normal differentiation of the cells in the growing cartilage. Both the metaphyseal growth plate (the growth zone of the diaphysis) and immature joint cartilage (the growth zone of the epiphysis) are affected. The loss of normal differentiation of the cartilage cells results in failure of provisional calcification of the matrix and endochondral ossification ceases. Degeneration and necrosis of blood vessels in cartilage canals results in ischemia of an area of growing cartilage followed by chondrocyte degeneration and death. The initial lesion occurs in growing cartilage and dyschondroplasia is a more appropriate term. The primary lesion of osteochondrosis directly affects the differentiation and maturation of the cartilage cells and the surrounding matrix that are destined to become replaced by bone. This can occur at the two sites of endochondral ossification in long bones – the articular/epiphyseal cartilage complex and the metaphyseal growth plate. In osteochondrosis, the capillary buds fail to penetrate the distal region of the hypertrophic zone, which leads to a failure of the final stages of cartilage maturation and modification of the surrounding matrix. These changes lead to retention and thickening of cartilage with subsequent weakening of the articular/epiphyseal cartilage complex.
Typical lesions in the horse involve extensive cartilaginous and subchondral bone degeneration with flap formation and, ultimately, loose pieces in the joint. This is usually referred to as osteochondritis dissecans and is associated with synovial effusion and varying degrees of synovitis. Osteochondral fracture associated with severe pathological changes to the subchondral bone occurs most commonly on the trochlear ridges and the lateral or medial malleoli of the hock. In some instances, cartilage damage weakens underlying bone and causes a bone cyst to form, usually at a site of biomechanical stress or weightbearing.11
It is suggested that osteochondrosis lesions in horses develop prior to 7 months of age and that ischemic necrosis of cartilage secondary to a defect in vascular supply is an important factor in the pathogenesis of the disease in horses.20 An osteochondrotic lesion in the metaphyseal growth plate may disturb growth to such a degree that the whole shape of the bone is altered. Epiphyseolysis may also occur. Osteochondrosis of joint cartilage may lead to osteochondritis dissecans and secondary osteoarthrosis. The lesion may heal and only the sequelae are present once the period of growth is over.
In rapidly growing pigs raised in confinement with minimal exercise, osteochondrosis and arthrosis are seen as degeneration of the deep layer of the articular cartilage and adjacent subchondral bone with degenerative lesions of the epiphyseal plate. Lesions in the epiphyseal plate may result in epiphysiolysis, which occurs most commonly in the femoral head. The typical lesions are usually symmetrical and commonly involve the elbow, stifle and hip joints and the distal epiphyseal plate of the ulna. Lesions also occur in the intervertebral articulations. The lesions are common in pigs when they are examined at slaughter (90–100 kg BW) and there may have been no evidence of clinical abnormality or a proportion of the pigs with severe lesions may have been affected with the leg-weakness syndrome. Osteochondrosis and Erysipelothrix rhusiopathiae are the most common causes of nonsuppurative joint disease of pigs examined at the abattoir. Thus not all lesions are clinical.
CLINICAL FINDINGS
The major clinical characteristic is a chronic lameness that becomes progressively worse over a long period of time and does not usually respond to treatment. The disease is insidious and generally not clinically apparent in the early stages. A common clinical history is that the affected animal becomes progressively more lame over a period of weeks and months and prefers long periods of recumbency. The lesion may develop slowly over a period of weeks and months during the convalescent stages of an acute traumatic injury to the joint when recovery is expected but the animal continues to be lame. Young breeding bulls in the early stages of coxofemoral arthropathy may be reluctant to perform the breeding act and yet appear to have sufficient libido. One of the first clinical abnormalities of osteochondrosis and epiphyseolysis in young breeding boars may be inability to mount the sow – impotentia coeundi.
There is usually difficulty in flexing affected joints normally, which results in a stiff and stilted gait. In cattle confined to stanchions one of the earliest and persistent signs is shifting of weight from limb to limb. In dairy cattle, as the lesions become more painful, there is a decline in appetite and milk production, prolonged recumbency and considerable difficulty in rising from the recumbent state. In the early stages there may be an apparent remission of the lameness, but relapses are common. The bony prominences of the joint eventually appear more prominent than normal, which is due to disuse muscle atrophy of the affected limbs. Distension of the joint capsule is not a characteristic, as it is in an infectious or suppurative arthritis. The joint capsule of palpable joints is usually not painful on palpation. Passive flexion of affected joints may be painful and it may be possible to elicit crepitus due to detached pieces of cartilage and bone and osteophytes surrounding the articular cartilage. However, crepitus is most common in the large movable joints, such as the stifle, and commonly in osteoarthropathy secondary to acute traumatic injury of the meniscus and cranial cruciate ligament of the joint.
Epiphysiolysis of the head of the femur occurs in young pigs from 5 months to 1 year of age. There is usually a history of slight to moderate lameness, sudden in onset and affecting one or both hindlimbs. The onset of lameness may coincide with some physical activity such as breeding, farrowing or transportation. The lameness is progressive and in about 7–10 days the animal is unable to use its hindlegs. Crepitus may be audible on circumduction of the affected limb and radiography may reveal the separation.
In leg weakness associated with osteochondrosis and arthrosis of pigs the common clinical findings are hyperflexion of the carpus, limb bowing, adduction of both forelegs at the level of the carpus, hyperextension of the fore and hind phalanges and anterior curvature of the tarsus. Locomotory dysfunction involves primarily the hindlegs. There is pronounced swaying of the hindquarters, and crossing the hindlegs with each step, which makes the pig appear incoordinated.
Osteochondrosis in cattle is characterized by chronic long-standing lameness, either with or without joint effusion.4 Joint fluid analysis is usually normal or indicates nonseptic inflammation. The stifle joint is most commonly affected followed by the hock joint. In osteochondrosis in young growing bulls there is reluctance to move, stiffness, enlargement of the ends of long bones and a straightened joint. While there may be clinical evidence of lameness in less than 40% of affected cattle, radiographically, 88% of the lesions are bilateral.4
Osteochondrosis in the horse is characterized by a wide range of clinical signs and in some cases lesions are not accompanied by clinical signs. The most common sign of osteochondrosis is a nonpainful distension of an affected joint. In foals under 6 months of age, a tendency to spend more time lying down is common. This is accompanied by joint swelling, stiffness and difficulty keeping up with the other animals in the group. An upright conformation of the limbs may also be present. In yearlings or older animals the common clinical signs are stiffness of joints, flexion responses and varying degrees of lameness.
In the horse with osteochondrosis of the shoulder joint there is intermittent lameness, characterized by a swinging leg, shoulder lameness with pain elicited by extension, flexion or abduction of the limb. Secondary joint disease is also a common finding. In a retrospective study of osteochondrosis dissecans in 21 horses, affected animals were 8 months to 5 years of age. The usual age of onset of clinical abnormalities was 18–24 months. The common presenting complaints included joint effusion and lameness of either gradual or sudden onset. The prevalence was higher in males than in females.
CLINICAL PATHOLOGY
Joint fluid
The changes in the synovial fluid of joints affected with degenerative arthropathy are usually unremarkable and can be distinguished from the changes in infectious arthritis. A summary of the laboratory evaluation of synovial fluid in diseases of the joints is set out in Table 13–2. The isolation of an infectious agent from the synovial fluid of a diseased joint suggests the presence of an infectious arthritis but failure to isolate an organism must not be interpreted as the presence of a noninfectious arthritis. In well advanced cases of infectious arthritis the number of organisms may be small or they have been phagocytosed by neutrophils in the joint fluid.
Total protein concentration and viscosity of synovial fluid of horses can be determined. Normal values are available21 and the concentration and molecular weight distribution of hyaluronate in synovial fluid from clinically normal horses and horses with diseased joints have been compared.19,22 Synovial fluid viscosity is reduced in horses with infectious and chronic arthritides and with radiographic evidence of cartilage degeneration. The synovial fluid hyaluronate concentration can be used as a diagnostic marker for chronic traumatic arthritis. However, high-molecular-weight proteoglycans or other markers in the synovial fluid cannot be used for diagnosing or monitoring degenerative joint disease.19
Hematology and serum biochemistry should be combined with appropriate hematology and serum biochemistry where indicated. The concentration of hyaluronic acid in synovial fluid can be determined using an assay technique. The determination of serum calcium and phosphorus may reveal the existence of a dietary deficiency or imbalance of minerals.
Radiography
Radiography of the hock joints in a craniomedial–caudolateral oblique view and of the fetlock joints in lateromedial view are standard techniques for the diagnosis of osteochondrosis in the horse. Those joints with abnormal radiographs may be radiographed from additional perspectives. Horses with bony fragments or defects at the cranial edge of the intermediate ridge of the distal aspect of the tibia or defects at the lateral trochlea of the talus can be classified as having osteochondrosis.2 The radiographic progression of femoropatellar osteochondrosis in horses under 1 year of age at the onset of clinical signs has been examined.22 The full extent of the radiographic lesions may take several weeks to develop.
Arthroscopy
Arthroscopic examination and surgery of affected joints of horses with osteochondrosis can provide considerably more information than is possible from clinical and radiographic examination alone.23
NECROPSY FINDINGS
In degenerative joint disease the joint cartilage is thin or patchily absent and polished subchondral bone is evident. The articular surfaces are irregular and sometimes folded. Exposed bone may be extensively eroded and osteophytes (small bony excrescences, like pearls) may be present on the nonarticular parts of the joint on the circumference of the articular cartilage. The synovial fluid is usually only slightly increased in volume and appears amber-colored. Menisci, intra-articular, cartilages and ligaments may be entirely absent and there may be areas of calcification in the joint capsule and cartilages free in the synovium. When the stifle is affected, fractures of the head of the tibia occur commonly, usually a chip of the lateral condyle having become separated. In such cases, fractures of the lateral condyle of the distal end of the femur may follow. With either of these fractures, lameness is extreme and the animal may often refuse to rise.
The radiographic and pathological findings of femoral–tibial osteoarthrosis in bulls is described. When the hip joint of bulls is affected, the head of the femur becomes smaller and more flattened than normal, the acetabulum is shallower and the round ligament is usually ruptured. The pathology of coxofemoral arthropathy is young bulls is described.
The pathological changes in experimentally induced osteoarthritis in the horse are similar to the early changes of naturally occurring degenerative joint disease.
In osteochondrosis there is splitting and invagination of articular cartilage, loss of articular cartilage, chip fractures of condyles, exposed and collapsed subchondral bone, osteophyte formation around the circumference of the articular cartilage and loose pieces of cartilage in the joint. In the epiphyseal plates (e.g., the distal ulna in pigs with leg weakness) the cartilage is uneven and thickened with hemorrhage, fibrous tissue, collapse of bone tissue in the metaphysis and epiphyseal separation. Complete separation of the epiphysis occurs most commonly at the head of the femur. The ultrastructural appearance of normal epiphyseal cartilage of the articular–epiphyseal cartilage complex in growing swine has been examined and serves as a standard for comparison with the lesions in affected pigs. The lesions may be present in pigs at an early age as part of the usual growth pattern of cartilages.
In equine osteochondrosis (dyschondroplasia), the histological lesions can be divided into two groups.24 In one group there are accumulations of small rounded chondrocytes, areas of necrosis and chondrocyte clusters. In the second group, there are alterations in the appearance of the mineralized matrix, areas of necrosis, chondrocyte clusters and an alteration in type VI collagen immunoreactivity within the chondrocyte clusters.
TREATMENT
The treatment of arthropathy depends largely upon correction of the cause, but in most cases the lesions are progressive and irreparable and food-producing animals should be slaughtered for salvage. Tarsal degenerative joint disease in cattle has been treated with intra-articular injections of corticosteroids and has provided temporary relief from pain and discomfort. However, the corticosteroids do not promote healing of the joint and their use in arthropathy may actually accelerate erosion of articular cartilage, loss of joint sensation and the development of ‘steroid arthropathy’. Large doses of acetylsalicylic acid may be given to reduce pain in animals that are kept for breeding purposes.
Osteoarthropathy is characterized clinically by a chronic lameness that becomes progressively worse and usually does not respond to treatment. The gait is stiff, there is disuse muscle atrophy, the bony prominences of the joint are more apparent but usually there is no marked distension and pain of the joint capsule, as in infectious arthritis. Examination of synovial fluid may aid in differentiation from infectious arthritis.
Radiographically there is erosion of articular cartilage, sclerosis of subchondral bone and periarticular accumulations of osteophytes. In the early stages of the disease in large animals, radiographic changes may not be visible and repeated examinations may be necessary. The radiographic changes of osteochondrosis in the shoulder joint of the horse consist of:
The literature on the medical management of osteoarthritis in the horse has been reviewed.25 There are many choices available for controlling inflammation in osteoarthritis. Treatment is symptomatic and largely nonspecific.
Nonsteroidal anti-inflammatory agents
Several nonsteroidal anti-inflammatory drugs (NSAIDs), such as phenylbutazone, flunixin meglumine, ketoprofen, naproxen, and carprofen, are available treatment options. Each has associated toxicities. They are now the most commonly used drugs because of their analgesic, antipyretic and anti-inflammatory properties.25 They inhibit some component of the enzyme system that converts arachidonic acid into prostaglandins and thromboxanes. All cells, including chondrocytes and synoviocytes, possess arachidonic acid as a fatty acid constituent of phospholipids. Once released, arachidonic acid is oxidized by either cyclooxygenase (COX) or 5-lipooxygenase. COX oxidation leads to prostaglandin production, while lipoxygenase oxidation leads to leukotriene formation. The effect of NSAIDs is primarily from inhibiting COX, which blocks arachidonic acid conversion to prostaglandin.
Intra-articular steroids
Various steroidal formulations for intra-articular administration are available and correct dosage, frequency of administration, indications and toxicity are factors to consider for each drug. They include methylprednisolone acetate, betametasone, and triamcinolone acetonide.25
Chondroprotective agents
Various chondroprotective drugs such as hyaluronic acid, polysulfated glycosaminoglycan, and oral glucosamine-chondroitin sulphate are also used to control inflammation and provide viscosupplementation.25
There is a notable lack of treatment information based on randomized, blinded placebo-controlled clinical trials in the horse to identify the efficacy of therapeutic agents for both symptomatic and disease-modifying activity in degenerative joint disease.25 Until there are validated outcome measures that can be used practically in clinical trials, there will always be uncertainty about whether these therapeutic agents have any real disease-modifying action.26
Hyaluronic acid
The changes in the synovia following the intra-articular injection of sodium hyaluronate into normal equine joints and after arthrotomy and experimental cartilage damage have been examined, but in general the results are inconclusive.
Polysulfated glycosaminoglycans
Polysulfated glycosaminoglycans have been reported to induce articular cartilage matrix synthesis and to decrease matrix degradation.27 Experimentally, intra-articular injections of polysulfated glycosaminoglycan provides some protection against chemically induced articular cartilage damage but not against physical defects of articular cartilage in the horse. The polysulfated glycosaminoglycans inhibit lysosomal enzymes and neutral proteases. The allotransplantation of synovial fluid into the joints of horses with arthropathies has been examined. A survey of the use of polysulfated glycosaminoglycans by equine practitioners for the treatment of lameness in horses found that the drug is moderately effective overall and is considered most beneficial in the treatment of subacute degenerative joint disease.28 Its efficacy for incipient and chronic forms of degenerative joint disease is considered comparable to that of sodium hyaluronate.
The prevention of further trauma should be assured and possible nutritional causes corrected. The treatment of active disease, particularly in soft tissues, that is contributing to articular degeneration includes rest, immobilization, physical therapy, intra-articular injections of corticosteroids, NSAIDs, joint lavage and intra-articular injection of sodium hyaluronate, all of which have been used with variable success.29
Other treatments
Surgical therapy includes curettage of articular cartilage, removal of osteophytes and surgical arthrodesis.29 In a retrospective study of stifle lameness in 42 cattle admitted to two veterinary teaching hospitals over a period of 6 years, 18 had radiographic evidence of subchondral bone cyst without radiographic evidence of degenerative joint disease. The prognosis in those with a subchondral bone cyst was favorable, 75% returning to their intended function, while in septic arthritis only 22% returned to normal.
Chemical arthrodesis using the intra-articular injections of monoiodoacetate (MIA) has been described as an alternative to surgical arthrodesis for the treatment of degenerative joint disease of the distal tarsal joints.30 MIA causes an increase in intracellular concentration of adenosine triphosphate resulting in inhibition of glycolysis and cell deaths. It causes dose-dependent cartilage degeneration characterized by cartilage fibrillations, chondrocyte death and glycosaminoglycan and proteoglycan depletion. MIA produces reliable radiographic and histological ankylosis of the distal tarsal joints. Resolution of the lameness required 12 months and occasionally longer. Soundness was achieved in 82% and 85% of horses at 12 and 24 months, respectively. Complications of the injections were uncommon and were probably related to peri-articular injection or leakage of MIA, or to use of higher concentrations or volumes. Postinjection pain was marked in a small number of horses but was transient and managed effectively with analgesic drugs. The procedure is controversial.31 Some clinicians argue that arthrodesis should only be used where lameness is localized to the tarsometatarsal and centrodistal joints with objective means such as local analgesic techniques, and when other more conservative treatments have failed.
CONTROL AND PREVENTION
Prevention of osteoarthropathy will depend upon recognition and elimination of the predisposing causes: provision of an adequate diet and the avoidance of overnutrition; regular exercise for confined animals; the provision of suitable flooring to minimize persistent concussion and the use of breeding stock that have a body conformation that does not predispose to joint lesions.
1 Sandgen B, et al. Equine Vet J Suppl. 1993;16:31. 38, 48
2 Grondahl AM, Dolvik NI. J Am Vet Med Assoc. 1993;203:101.
3 Magnusson LE, Ekman S. Acta Vet Scand. 2001;42:429.
4 Trostle SS, et al. J Am Vet Med Assoc. 1997;211:1566.
5 Davies IH, et al. Cattle Pract. 1998;4:243.
6 Davies IH, Munro R. Vet Rec. 1999;145:232.
7 Hill BD, et al. Aust Vet J. 1998;76:171.
8 Scott PR, et al. Vet Rec. 2000;147:608.
9 Scott CA, et al. Vet Rec. 1996;139:165.
10 Doherty ML, et al. Vet Rec. 1996;138:137.
11 Ray CS, et al. Equine Vet J. 1996;28:225.
12 Uhlorn H, et al. Acta Vet Scand. 1995;36:41.
13 Arnason T, Bjornsdottir S. Livestock Prod Sci. 2003;79:285.
14 Bjornsdottir S, et al. Equine Vet J. 2004;36:5.
15 Bjornsdottir S, et al. Acta Vet Scand. 2003;44:161.
16 Clyne MJ. Equine Vet J. 1987;19:15.
17 Palmer JL, Bertone AL. Equine Vet J. 1994;26:263.
18 Billinghurst RC, et al. Equine Vet J. 1995;27:208.
19 Tulamo RM, et al. Am J Vet Res. 1996;57:932.
20 Carlson CS, et al. Vet Pathol. 1995;32:641.
21 Tulamo RM, et al. Am J Vet Res. 1994;55:710.
22 Davbareiner RM, et al. Vet Surg. 1993;22:515.
23 McIlwraith CW. Equine Vet J Suppl. 1993;16:27.
24 Henson FMD, et al. Vet J. 1997;154:53.
25 Goodrich LR, Nixon AJ. Vet J. 2006;171:51.
26 Clegg PD. Vet J. 2006;171:9.
27 Todhunter RJ, Lust G. J Am Vet Med Assoc. 1994;204:1245.
28 Caron JP, Kaneene JB. J Am Vet Med Assoc. 1996;209:1564.
29 McIlwraith CW. J Am Vet Med Assoc. 1982;180:239.
ARTHRITIS AND SYNOVITIS
Inflammation of the synovial membrane and articular surfaces as a result of infection occurs commonly in farm animals. It is characterized by varying degrees of lameness and a warm and swollen painful joint. The synovial fluid is usually abnormal, containing an increased leukocyte count and the pathogens causing the arthritis. The arthritis may be severe enough to cause systemic illness, and in some cases a draining sinus tract may occur.
ETIOLOGY AND EPIDEMIOLOGY
Specific bacterial infections of the joints are most common in newborn farm animals, in which localization of infection occurs in joints following bacteremia or septicemia. Surveys of Thoroughbred studs have shown that the incidence of infectious arthritis is higher in foals with other perinatal abnormalities and in which the ingestion of colostrum was delayed for more than 4 hours after birth. Calves with hypogammaglobulinemia are particularly susceptible to bacteremia and meningitis, ophthalmitis and arthritis. Some of the important infectious causes of arthritis are as follows.
Lambs
Piglets
• Streptococci, Lancefield groups C, E, and L
• E. rhusiopathiae in pigs of any age. Up to 65% of joints of pigs at slaughter are affected and up to 80% of the farms from which the pigs come do not vaccinate for erysipelas. Mortality in preweaning groups of pigs may affect 18% of litters, 3.3% of the piglets with a herd mortality of 1.5%
• In a 4-year period in a swine research station, 9411 piglets were born alive and 9.8% were treated for lameness.1 About 75% of the cases were observed in piglets under 3 weeks of age. The incidence of lameness was much higher in piglets born from sows of parity 3 (11.4%) compared to piglets born to sows of parity 4–7 (8%).
Cattle
• Histophilus somni is a cause of synovitis
• Mycoplasma agalactia var. bovis is a common cause of synovitis, arthritis and pneumonia in young feedlot cattle
• Mycoplasma bovigenitalium may cause mastitis in cows, with some animals developing arthritis
• Mycoplasma mycoides may cause arthritis in calves vaccinated with the organism against contagious bovine pleuropneumonia. Calves already sensitive to the organism develop an immediate-type allergic reaction of the synovial membrane
• Brucella abortus: occasional cows with brucellosis develop an arthrodial synovitis
• Some cases of ephemeral fever have a sterile arthritis
• BVD virus in young bulls, rarely
• Idiopathic septic arthritis in dairy heifers. The etiology is unknown
• Septic arthritis of the proximal interphalangeal (pastern) joint in cattle due to perforating wounds.2 A. pyogenes is the most common cause in cattle.
Horses
• Septic arthritis after penetrating wounds, intra-articular injection of corticosteroids, and surgery; young foals under 6 months of age usually associated with a septicemia; adult horses without a known etiology
• In a series of 34 cases of monoarticular infectious arthritis in adult horses admitted to a veterinary teaching hospital over a period of 10 years, 16 had a penetrating wound over the joint, four had a puncture wound of the sole and in five the infection was iatrogenic (three had received intra-articular corticosteroids, one had received intra-articular anesthesia and one had sepsis after a purulent thrombophlebitis).3 In nine cases, no cause could be determined
• Spread to the joints from generalized strangles
• Rare cases of non-erosive polysynovitis in a horse, possibly immunological and immune-mediated polysynovitis in foals4
• Acedosporium prolificans, a newly recognized opportunistic fungus, has been associated with an incurable arthritis and osteomyelitis in a mature horse.5
All species
• Traumatic perforation of the joint capsule
• Spread from surrounding tissues, e.g. footrot to interphalangeal joints in cattle and pigs, interdigital abscess in sheep
• Hematogenous spread from suppurative lesions commonly in udder, uterus, diaphragmatic abscess, infected navel or tail, castration wound.
PATHOGENESIS
In infectious arthritis that is hematogenous in origin there is usually a synovitis initially, followed by changes in the articular cartilages and sometimes bone. With almost any systemic infection there may be localization of the infectious agent in the synovial membrane and joint cavity. The synovial membrane is inflamed and edematous, and there are varying degrees of villous hypertrophy and deposition of fibrin. Bacteria colonize in synovial membranes, which makes treatment difficult. The synovitis causes distension of the joint capsule with fluid and the joint is painful and warm. Successful treatment and elimination of infection at this early stage of synovitis will minimize changes in articular cartilage and bone and healing will result. A progressive infectious synovitis commonly results in pannus formation between articular surfaces with erosion of articular cartilage, infection of subchondral bone and osteomyelitis. In the chronic stages there is extensive granulation tissue formation, chronic synovitis and degenerative joint disease with osteophyte formation, and ankylosis is possible. Depending on the organism, the arthritis may be suppurative or serofibrinous. Suppurative arthritis is particularly destructive of cartilage and bone and commonly there is rupture of the joint capsule. In foals with septic arthritis there may be a concurrent polyosteomyelitis, usually in either the epiphysis and/or the metaphysis of the long bones.
Experimental infectious arthritis in calves
Septic arthritis induced by E. coli is a reliable and reproducible model of infectious arthritis in laboratory animals, horses and calves.6 The inoculation of E. coli into the tarsal joint of newborn colostrum-fed calves resulted in septic arthritis in all calves. Clinical signs of septic arthritis appeared on day 2 after infection and persisted until day for all calves. E. coli was cultured from synovial fluid on day 2 for one calf and until day 4 for five other calves. Polymerase chain reaction (PCR) for E. coli was positive in the synovial fluid of all calves. Synovial fluid neutrophil and white blood cell counts were increased on days 2–4. All bacterial cultures were negative on day 8, although clinicopathological signs of inflammation persisted until day 20. Rapid recovery occurred within 1 week when an appropriate treatment was begun early in the course of the disease.
Foals with septicemia
Septicemic foals may develop infectious arthritis and a concurrent polyosteomyelitis because of the patency of transphyseal vessels in the newborn foal; this allows spread of infection across the physes with the development of lesions in the metaphysis, epiphysis and adjacent to the articular cartilage. The syndrome is classified according to the location of the lesions:
• A foal with S-type septic arthritis–osteomyelitis has synovitis without macroscopic evidence of osteomyelitis
• Foals with E-type osteomyelitis have osteomyelitis of the epiphysis at the subchondral bone–cartilage junction
• Those with P-type have osteomyelitis directly adjacent to the physis
• The same joint may have a single type or any combination of types but most foals with the S-type have concurrent bone lesions.
Horses
Septic arthritis has been reproduced experimentally in horses and the sequential synovial fluid changes monitored. Following intra-articular inoculation of S. aureus, clinical signs are evident as early as 8 hours after infection. A high and persistent neutrophilia is one of the earliest and most accurate diagnostic abnormalities. The total white blood cell count rises within 12–24 hours to a mean value of 100 × 109/L. Total protein also increases. Synovial fluid acidosis also occurs in infectious arthritis, which may interfere with the antibacterial activity of some antimicrobials. In experimental arthritis, the synovial pH declined from a mean value of 7.43 to 7.12. Bacteria could be detected in 40% of the smears of infected synovial fluid samples and primary cultures of the fluid were positive in 70%. The intra-articular inoculation of E. coli into horses induces a reliable, reproducible and controlled model of infectious arthritis consistent with the naturally occurring disease and has been used to evaluate the efficacy of gentamicin for treatment. The injection of E. coli lipopolysaccharide into various joints of horses can cause clinical signs of endotoxemia, and the synovial fluid total nucleated cell count and total protein are linearly responsive in increases in endotoxin.7
Endothelin (ET)-1, a 21-amino-acid polypeptide, is locally synthesized in the joints of horses with various forms types of joint disease.8 It induces a potent and sustained vasoconstriction. Synovial fluid concentrations of ET-1 varies among horses with joint disease, with higher concentrations in animals with joint sepsis suggesting a pathogenetic role in septic arthritis.
Synovial fluid in infectious arthritis in the horse may contain the proteolytic enzymes collagenase and caseinase which may derive from both synovial cells and neutrophils.9 These enzymes are involved in the degradation of connective tissue and loss of cartilage matrix. Lavage of affective joints is intended to remove these enzymes.
Infectious arthritis may occur following traumatic injury to a joint but the pathogenesis is obscure. Traumatic injury of the joint capsule resulting in edema and inflammation may allow latent organisms to localize, proliferate and initiate an arthritis.
CLINICAL FINDINGS
Inflammation of the synovial membrane causes pain and lameness in the affected limb, sometimes to the point that the animal will not put it to the ground. Pain and heat are usually detectable on palpation and passive movement of the joint is resented. The joint may be swollen but the degree will depend on the type of infection. Pyogenic bacteria cause the greatest degree of swelling and may result in rupture of the joint capsule. Some enlargement of the epiphysis is usual and this may be the only enlargement in nonpyogenic infections, particularly that associated with E. insidiosa.
Fever, inappetence to anorexia, endotoxemia, loss of body weight and discomfort may occur in animals with only one severely affected joint or when several joints are less severely affected.
In many of the neonatal infections there will also be an accompanying omphalophlebitis and evidence of lesions in other organs, particularly the liver, endocardium and meninges. Arthritis in older animals may also be accompanied by signs of inflammation of the serous membranes and endocardium when the infection is the result of hematogenous localization.
The joints most commonly involved are the hock, stifle and knee but infection of the fetlock, interphalangeal and intervertebral joints is not uncommon. In chronic cases there may by physical impairment of joint movement because of fibrous thickening of the joint capsule, periarticular ossification and rarely ankylosis of the joints. Crepitus may be detectable in joints where much erosion has occurred.
In newborn and young animals, involvement of several joints is common. The joints may become inflamed simultaneously or serially. Lameness is often so severe that affected foals lie down in lateral recumbency most of the time and may have to be assisted to rise. Decubitus ulcerations due to prolonged recumbency are common. The gait may be so impaired as to suggest ataxia of central origin.
The prognosis in cases of advanced septic arthritis is poor. Neglected animals may die or have to be destroyed because of open joints or pressure sores. The subsequent development of chronic arthritis and ankylosis may greatly impede locomotion and interfere with the usefulness of the animal.
CLINICAL PATHOLOGY
Arthrocentesis
Aspiration of joint fluid for culture and analysis is necessary for a definitive diagnosis. Careful disinfection of the skin and the use of sterile equipment is essential to avoid the introduction of further infection.
Analysis of joint fluid
Total and differential cell count, total protein concentration and specific gravity are determined.
In infectious arthritis the volume of joint fluid is increased and the total leukocyte count is increased, with a high percentage (80–90%) of neutrophils. The severity of infectious arthritis may be manifested systemically by a leukocytosis with a marked regenerative left shift. In degenerative joint disease, the volume may be normal or only slightly increased and the total and differential leukocyte count may be manifested within the normal range. In traumatic arthritis there may be a marked increase in the number of erythrocytes. Special biochemical examinations of joint fluid are available that measure for viscosity, strength of the mucin clot and concentrations of certain enzymes. The laboratory findings in examination of the joint fluid are summarized in Table 13.2. The synovial fluid analysis of 130 cases in cattle compared the characteristics of animals with infectious and noninfectious arthritis.10
Culture of joint fluid
Joint fluid must be cultured for aerobic and anaerobic bacteria and on specific media when Mycoplasma sp. is suspected. It is often difficult to isolate bacteria from purulent synovial fluid. The rates of recovery of organisms vary from 40–75%. In one study of suspected infectious arthritis in 64 horses admitted to a veterinary teaching hospital over a period of 8.5 years, positive cultures were obtained from 55% of the joints sampled.11 The most common organisms were S. aureus, E. coli and Pseudomonas aeruginosa, accounting for more than half the isolates obtained.
There is no single test that is reliable for the diagnosis of septic arthritis. Failure to isolate organisms on culture does not exclude the a bacterial cause, and organisms are often not observed in synovial fluid smears. Poor collection, storage and laboratory techniques, prior administration of antibiotics or partial success of the immune system in containing the infection may explain the failure to detect organisms. Arthrocentesis should be done before antibiotics are given and a blood culture bottle should be inoculated immediately, a Gram stain made and culture for anaerobes included.12 Positive cultures from synovial fluid can be expected in only about 65% of cases.
A biopsy sample of synovial membrane may be more reliable than synovial fluid for culture but there is little evidence based on comparative evaluations to support such a claim. PCR has been examined in in-vitro studies to detect selected bacterial species in joint fluid compared with microbial culture.13 The benefits would include rapid and accurate diagnosis infectious arthritis, ability to detect bacteria in synovial fluid in the presence of antimicrobial drugs and diagnosis of infectious arthritis when culture results are inconclusive. However, initial studies found no difference between microbial culture and PCR analyses.
Serology of joint fluid
Serological tests may be of value in determining the presence of specific infections with Mycobacterium mycoides, Salmonella spp., Brucella spp., and E. insidiosa. Radiographic examination may aid in the detection of joint lesions and can be used to differentiate between inflammatory and degenerative changes. In foals with arthritis and suspected osteomyelitis there may be radiographic evidence of osteolysis of the metaphysis or epiphysis.
Radiography
Radiography of the affected joint will often reveal the nature and severity of the lesions. Typical radiographic findings of septic arthritis include osteolytic lesions of the articular cartilage, increased width of intra-articular joint space, and soft tissue swelling. Osteomyelitic changes are seen in some cases. Because radiographic changes usually appear after 2–3 weeks when destruction of subchondral bone has become extensive, it may be necessary to take a series of radiographs several days apart before lesions are detectable.
Ultrasonography
Arthrosonography is an effective, fast and noninvasive complement to traditional diagnostic techniques for comprehensive evaluation of the pathology of joints of cattle.14 Distension of the joint cavities can be imaged; assessing echogenicity, acoustic enhancement and ultrasonographic character of the exudate correlates well with findings by arthrocentesis, arthrotomy or at necropsy. Joint effusion, which is the earliest indication of septic arthritis, can usually be detected with ultrasound by an experienced operator in the early stages. The synovial membrane, synovial fluid, ligaments, tendons and periarticular soft tissue, only inadequately imaged by radiography, can be imaged with ultrasonography. In advanced septic arthritis, ultrasonography provides accurate information on the location of the soft-tissue swelling, the extent and character of the joint effusion and involvement of concurrent periarticular synovial cavities.
Arthroscopy
Endoscopy is now used widely to define joint abnormalities more clearly and to gain access to the joint cavity as an aid in the treatment of septic arthritis.15
NECROPSY FINDINGS
The nature of the lesions varies with the causative organism. The synovial membrane is thickened and roughened and there is inflammation and erosion of the articular cartilage. There is usually an increase in the amount of synovial fluid present, varying from a thin, clear, serous, brownish fluid through a thicker, serofibrinous fluid to pus. There may be some inflammation of the periarticular tissues in acute cases and proliferation of the synovial membrane in chronic cases. In the latter, plaques of inspissated necrotic material and fibrin may be floating free in the synovial fluid. Infectious arthritis due to A. pyogenes is characterized by extensive erosion and destruction of articular cartilage and extensive suppuration. There may be a primary omphalophlebitis in newborn animals and metastatic abscesses may be present in other organs.
Infectious arthritis is characterized clinically by swollen joints which are painful and warm to touch, and lameness of varying degrees of severity. The volume of joint fluid is usually markedly increased and the leukocyte count is increased with a high percentage of neutrophils. In the early stages of synovitis and in chronic nonsuppurative arthritis, the joint may not be visibly enlarged and careful examination by palpation may be necessary to reveal abnormalities of the joint capsule. Lameness is common, however, even though only slight in some cases, and should arouse suspicion of the possibility of arthritis.
• The diseases of the musculoskeletal system that cause lameness and stiffness of gait include:
• Diseases of the nervous system, especially the peripheral nerves and spinal cord, may be confused with arthritis unless the joints are examined carefully
• Some severe cases of polyarthritis may cause recumbency that may be erroneously attributed to the nervous system.
Degenerative joint disease is characterized by an insidious onset of moderate lameness and stiffness of gait that becomes progressively worse over several weeks. The joint capsule is usually not grossly enlarged and not painful, and there is usually no systemic reaction. The total leukocyte count in the joint fluid is only slightly increased and the differential count may be normal. Chronic arthritis is often difficult to differentiate clinically from degenerative joint disease. Chronic arthritis is more common in young animals than in older animals such as rapidly growing yearling bulls, adult bulls, aged dairy cows and horses, in which degenerative arthropathy is most common. A sudden onset of acute lameness and marked swelling of a joint with severe pain suggests an infectious arthritis or traumatic injury to the joint. Marked swelling of several joints suggests infectious polyarthritis.
Osteodystrophy is characterized by:
• Lameness and stiffness of gait
• Usually an absence of joint capsule abnormalities
• Enlargements and deformities of the long bones in growing animals
• A number of animals may be affected at about the same time.
Radiography may reveal the abnormal bones and the nutritional history may explain the cause.
Degenerative myopathy causes acute lameness, a stiff and trembling gait, often leading to recumbency and absence of joint or bone involvement.
Traumatic sprains of tendons or ligaments and fractures of the epiphyses may cause lameness and local pain and, when they involve periarticular tissues, may be difficult to differentiate from arthritis.
Arthritis is never present at birth and apparent fixation of the joints should arouse suspicion of a congenital anomaly. The differentiation between arthritis and diseases of the peripheral nerves or spinal cord, both of which can cause lameness and/or recumbency, may be difficult if the arthritis is not clinically obvious. Diseases of the peripheral nerves cause lameness due to flaccid paralysis and neurogenic atrophy. Lesions of the spinal cord usually result in weakness of the hindlimbs, weak or absent withdrawal reflexes and loss of skin sensation.
TREATMENT
Parenteral antimicrobials
Acute septic arthritis should be treated as an emergency to avoid irreversible changes in the joint. The conservative approach is the use of antimicrobials given parenterally daily for several days and up to a few weeks in some cases. The selection of the drug of choice will depend on the suspected cause of the arthritis. The antimicrobial sensitivities of bacterial isolates from horses with septic arthritis/synovitis or osteomyelitis after fracture repair vary widely. A combination of cephalosporin and amikacin is recommended before culture and sensitivity results are available.
The antimicrobials that perfuse into the joint in therapeutic concentrations include the natural and synthetic penicillins, tetracycline, trimethoprim-potentiated sulfonamides, neomycin, gentamicin, and kanamycin.
Cloxacillin, methicillin, or penicillin have been used successfully for the treatment of staphylococcal septic arthritis in the horse.
Amphotericin B given intravenously daily for up to 30 days combined with joint drainage has been used for the treatment of Candida sp. arthritis in the horse.16
The relative efficacies of antimicrobials administered parenterally versus by intra-articular injections has been uncertain. Trimethoprim–sulfadiazine, given to calves parenterally, results in therapeutic concentrations of the drug in the synovial fluid of calves and penetrability was not enhanced nor restricted by experimental joint inflammation. Oxytetracycline and penicillin given parenterally readily penetrate the synovial membrane of both normal neonatal calves and those with experimental arthritis. Since peak synovial joint fluid levels of oxytetracycline and penicillin exceeded the minimum inhibitory concentrations for organisms such as A. pyogenes, the use of parenteral antimicrobials for the treatment of infectious arthritis in calves is appropriate. Ceftiofur at 1 mg/kg BW intravenously every 12 hours for 20 days, along with joint lavage, was successful in treating experimental septic arthritis associated with E. coli.6 The duration of antibiotic therapy is empirical; 3 weeks is recommended. Cephapirin administered parenterally to normal calves or those with arthritis resulted in synovial fluid levels approximately 30% of serum levels. The use of ampicillin trihydrate in calves with suppurative arthritis, at a dose of 10 mg/kg BW intramuscularly, resulted in a peak serum concentration of 2.5 μg/mL, 2 hours after injection; the highest concentration in normal synovial fluid was 3.5 μg/mL at 4 hours and the highest concentration in suppurative synovial fluid was 2.7 μg/mL at 2 hours.
Marbofloxacin at 4 mg/kg BW intramuscularly daily for 10 days was effective for the treatment of infectious arthritis in calves.17 Amoxicillin at 40 mg/kg BW intravenously is effective for the treatment of infectious joint disease in horses.18
The administration of trimethoprim– sulfadiazine at 30 mg/kg BW orally once daily to horses with experimentally induced S. aureus arthritis was ineffective in maintaining adequate levels of both drugs in infected synovial fluid. The use of the same drug at 30 mg/kg BW orally given every 12 hours was effective in maintaining therapeutic concentrations of both drugs in the serum and in the joint fluid.
In piglets at 2 weeks of age, streptococcal arthritis is most likely and it will respond quickly to penicillin given parenterally. Likewise, acute arthritis associated with erysipelas in pigs will respond beneficially if treated early before there is pannus formation.
Synovitis due to Histophilus somni infection responds quickly to systemic treatment. However, in other specific types of infectious arthritis the response is poor and recovery, if it does occur, requires several days or a week. Mycoplasmal arthritis in cattle is relatively nonresponsive to treatment and affected cattle may be lame for up to several weeks before improvement occurs and complete recovery may not occur. Chronic arthritis due to infection of pigs with E. insidiosa will commonly develop into a rheumatoid-like arthritis and be refractory to treatment.
Failure to respond to conservative therapy has been attributed to:
• Inadequate concentrations of antimicrobials achieved in the joint cavity
• Presence of excessive amounts of exudate and fibrin in the joint making the infectious agent inaccessible to the antimicrobial
• The development of rheumatoid-like arthritis, which is chronic and progressive.
It is often not possible to determine which situation is responsible.
If conservative treatment is not providing sufficient improvement and the value of the animal warrants extended therapy, a joint sample should be obtained for culture and sensitivity. The most suitable antimicrobial may then be given parenterally and/or by intra-articular injection. Strict asepsis is necessary to avoid introduction of further infection.
Intra-articular antimicrobials
The combined intra-articular and intravenous administration of gentamicin to normal horses can result in concentrations 10–100 times greater than after intravenous administration alone. In addition, gentamicin concentration in synovial fluid remained above the minimum inhibitory concentration for many common equine bacterial pathogens for at least 24 hours after treatment. The intra-articular administration of gentamicin is advantageous for the treatment of infectious arthritis in animals in which the systemic administration of the drug may be contraindicated, especially in the presence of impaired renal function or endotoxemia. Continuous infusion of gentamicin into the tarsocrural joint of horses for 5 days is an acceptable method of treating septic arthritis.19
Antimicrobial-impregnated polymethylmethacrylate beads have been used for the treatment of orthopedic infections involving bone, synovial structures and other soft tissues.20-22 The antimicrobials diffuse from the beads in a bimodal fashion. There is a rapid release of 5–45% of the total amount of antimicrobial within the first 24 hours after implantation and then a sustained elution that persists for weeks to months, depending on the antimicrobial used. For effective diffusion, the antimicrobials must be water-soluble, heat-stable and available in powder form. Aminoglycosides and the cephalosporins have been incorporated most commonly into the beads.
Regional limb perfusion with antimicrobials has been used for the treatment of experimentally induced septic arthritis. The antimicrobial is infused under pressure to a selected region of the limbs through the venous system. The concentration of the antimicrobial in the septic synovial fluid will usually exceed those obtained by intravenous administration. However, there are insufficient data available to evaluate the procedure in naturally occurring cases. Therapeutic concentrations of cefazolin are achieved in the synovial fluid of clinically normal cows when injected intravenously distal to a tourniquet and the technique could be used as an alternative to systemic administration of antimicrobials to provide adequate concentrations in a joint cavity.
Lavage of joint
Drainage of the affected joint and through-and-through lavage of the joint is also desirable along with the systemic administration of antimicrobials. Aspiration and distension–irrigation of the joint cavity using polyionic electrolyte solutions buffered to 7.4 is recommended.6 The irrigation removes exudates and lysozymes that destroy articular cartilage. A through-and-through lavage system may also be used with drainage tubes. General or local anesthesia should be provided. The distended joint is identified by palpation, the hair is clipped short and the skin is prepared with appropriate surgical disinfection. A 2 cm 16-gauge needle is inserted into the joint cavity, avoiding direct contact with the bones of the joint. A second needle is inserted into the joint as far as possible from the first needle to cause any fluid perfused into the joint to pass through as much of the joint cavity as possible. 0.5–1 L of Ringer’s solution warmed to 37°C is flushed through the joint using a hand-pumped pressure bag to keep a steady fluid flow into the joint.6
Arthroscopy
Arthroscopy provides excellent visualization of most parts of an affected joint and can be used to access the joint for the treatment of septic arthritis.15 The endoscope can be used to explore and debride the affected joint during the same intervention. Purulent exudate can be removed and necrotic areas within the synovial membrane can be debrided.
Surgical drainage and arthrotomy
Failure to respond to parenteral and intra-articular medication may require surgical opening of the joint capsule, careful debridement and excision of synovium and infected cartilage and bone. This may be followed by daily irrigation of the joint cavity with antimicrobials and saline. A lavage system can be established and the joint cavity infused with an antimicrobial and saline daily for several days.23 Arthrotomy with lavage was more effective in eliminating joint infections by providing better drainage than arthroscopy, synovectomy and lavage. However, with arthrotomy the risk of ascending bacterial contamination is greater and the major difficulty is to eliminate the infection from the joint and incision site.24 Infected sequestra and osteomyelitis of subchondral bone will prevent proper healing. Curettage of septic physeal lesions in foals may be necessary.
Open drainage and intra-articular and parenteral antimicrobials has been used to treat persistent or severe septic arthritis/tenosynovitis. While joint lavage through needles is still effective in many horses with acute infectious arthritis or tenosynovitis, in those with chronic or recurring septic arthritis, open drainage is indicated to remove the inflammatory exudate from the synovial space. Infected synovial structures are drained through a small (3 cm) arthrotomy incision left open and protected by a sterile bandage. Joint lavage using antimicrobials is done daily and parenteral antimicrobials are given intensively.
Septic pedal arthritis in cattle may be treated successfully by the creation of a drainage tract to promote adequate drainage. In cattle with septic arthritis of the digit, placement of a wooden block under the unaffected digit decreases weightbearing on the affected digit and provides for earlier, less painful ambulation.25
Arthrodesis or artificial ankylosis
Surgical arthrodesis can be used for the treatment of chronic septic arthritis in horses and calves.26,27
Septic arthritis of the distal interphalangeal joint is a common complication of diseases of the feet of cattle. Facilitated ankylosis of the joint is a satisfactory alternative to amputation of the affected digit in valuable breeding animals.28 In a series of 12 cases of septic arthritis of the distal interphalangeal joint treated by use of facilitated ankylosis, the success rate was 100%.
Physical therapy
The local application of heat, by hot fomentations or other physical means, is laborious but, if practiced frequently and vigorously, will reduce the pain and local swelling. Analgesics are recommended if there is prolonged recumbency. Persistent recumbency is one of the problems in the treatment of arthritis, particularly in foals. The animal spends little time feeding or sucking and loses much condition. Compression necrosis over bony prominences is a common complication and requires vigorous preventive measures.
Anti-inflammatory agents
NSAIDs are used parenterally to decrease the inflammatory response and to provide analgesia. In experimental synovitis in the horse, similar to septic arthritis, phenylbutazone was more effective than ketoprofen in reducing lameness, joint temperature, synovial fluid volume and synovial fluid prostaglandin.29
Prognosis for survival and athletic use in horses with septic arthritis
The factors affecting the prognosis for survival and athletic use in 93 foals treated for septic arthritis have been examined.12 The femoropatellar and tarsocrural joints were most commonly affected. Osteomyelitis or degenerative joint disease were detected in 59% of the foals. Failure of transfer of passive immunity, pneumonia and enteritis were common. Treatment consisted of lavage, lavage and arthroscopic debridement with or without partial synovectomy, or lavage and arthrotomy to debride infected bone and parenteral antibiotics. Seventy-five foals survived and were discharged from hospital, and approximately one-third raced. Isolation of Salmonella from synovial fluid was associated with an unfavorable prognosis for survival, and multisystemic disease was associated with an unfavorable prognosis for survival and ability to race. The key to successful outcome for septic arthritis is rapid diagnosis and initiation of treatment.
In a series of 507 horses treated for joint disease at one equine hospital during a period of 7 years, the risk factors affecting discharge from the hospital, of ever being sound, or of being alive after a 3-month followup were examined;30 58% of foals, 78% of yearlings and 94% of racing adults were discharged. Foals with a less severe lameness, duration of less than 1 day and infectious arthritis had increased odds of discharge.
CONTROL
The control of infectious arthritis is of major importance in newborn farm animals. The early ingestion of adequate quantities of good-quality colostrum and a clean environment for the neonate are necessary. The prophylactic use of antimicrobials may be considered to reduce incidence. Some of the infectious arthritides associated with specific diseases can be controlled through immunization programs. For example, vaccination of piglets at 6–8 weeks of age will provide protection against both the septicemic and arthritic forms of erysipelas.
1 Zoric M, et al. Vet Rec. 2003;153:323.
2 Kofler J. Berl Munch Tierarztl Wschr. 1995;108:281.
3 Peremans K, et al. J Equine Vet Sci. 1991;11:27.
4 Gagnon H, et al. J Vet Pharmacol Ther. 1994;17:31.
5 Swerczek TW, et al. J Am Vet Med Assoc. 2001;218:1800.
6 Francoz D, et al. J Vet Intern Med. 2005;19:336.
7 Palmer JL, Bertone AL. Equine Vet J. 1994;26:492.
8 De la Calle J, et al. Am J Vet Res. 2002;63:1648.
9 Spiers S, et al. Equine Vet J. 1994;26:48.
10 Rohde C, et al. Vet Surg. 2000;4:341.
11 Madison JB, et al. J Am Vet Med Assoc. 1991;198:1655.
12 Steel CM, et al. J Am Vet Med Assoc. 1999;215:973.
13 Crabill MR, et al. Vet Surg. 1996;25:195.
14 Kofler J. Br Vet J. 1996;152:683.
15 Munroe GA, Cauvin ER. Aust Vet J. 1994;150:439.
16 Madison JB, et al. J Am Vet Med Assoc. 1995;206:328.
17 Grandemange E, et al. Ir Vet J. 2002;55:237.
18 Errecalde JO, et al. J Vet Pharmacol Ther. 2001;24:1.
19 Lescum TB, et al. Am J Vet Res. 2002;63:683.
20 Streppa HK, et al. J Am Vet Med Assoc. 2001;219:40.
21 Sayegh AI. Compend Contin Educ Pract Vet. 2003;25:788.
22 Trostle SS, et al. J Am Vet Med Assoc. 1996;208:404.
23 McClure SR, et al. J Am Vet Med Assoc. 1993;202:973.
24 Bertone AL, et al. Am J Vet Res. 1992;53:585.
25 Guard C. Proc Am Assoc Bovine Pract. 2000;35:21.
26 Riley CB, Farrow CS. Aust Vet J. 1998;39:438.
27 Groom LJ, et al. Can Vet J. 2000;41:117.
28 Desrochers A, et al. J Am Vet Med Assoc. 1995;206:1923.
Congenital defects of muscles, bones, and joints
Defects of the musculoskeletal system are among the most common congenital abnormalities in farm animals. In cattle 476 such defects are listed. Many of them are lethal, and most of the remainder are life-threatening because of interference with grazing or the prehension of food. Many of them occur in combinations so that single defects are uncommon. For example, most axial skeletal defects and cleft palates occur in calves that already have arthrogryposis.
Because of the very large volume of literature involved it is not possible to deal with all the recorded defects here, and the text is limited to those defects that are thought to be of general importance. Whether or not they are inherited or have an environmental cause is often not known so that an etiological classification is not very effective. Nor is an anatomical or pathological classification, so we are reduced to a classification based on abnormal function.
FIXATION OF JOINTS
Because arthrogryposis, which has been used to convey the description of joint fixation, strictly means fixation in flexion, the term congenital articular rigidity has been introduced. The immobilization of the joint may be due to lack of extensibility of muscles, tendons, ligaments or other tissues around the joint, or to deformity of articular surfaces, or theoretically to fusion between the bones at the articular surface. Muscle contracture, which is the principal cause of joint fixation, has been produced experimentally, and occurs naturally, as a result of primary muscle atrophy or of atrophy resulting from denervation. Articular surface deformity is usually associated with gross deformity of the limb bones and is usually identifiable but the principal problem in the diagnosis of congenital articular rigidity is to determine what the pathogenesis might have been and, beyond that, what was the specific cause.
Congenital fixation of joints can be caused by some well known entities, as follows.
Cattle
• Hereditary congenital articular rigidity (HCAR) with cleft palate in Charolais
• HCAR with normal palates in Friesians, Danish Reds, Swedish, Shorthorns
Environmentally induced congenital articular rigidity caused by:
Foals
• ‘Contracted’ foals having congenital axial and appendicular contractures of joints in the us, cause unknown, not thought to be inherited. Deformities include torticollis, scoliosis, thinning of ventral abdominal wall, sometimes accompanied by eventration, asymmetry of the skull, flexion contracture in distal limb joints
• Congenital articular rigidity also occurs in foals from mares fed on hybrid Sudan grass pastures
• Sporadic cases of congenital joint deformity occur in foals and calves. They are manifested usually by excessive flexion of the metacarpophalangeal joints causing affected animals to ‘knuckle’ at the fetlocks and sometimes walk on the anterior aspect of the pastern. A similar defect occurs in the hindlegs. Many mild cases recover spontaneously but surgical treatment may be required in badly affected animals. The cause in these sporadic cases in unknown and necropsy examination fails to reveal lesions other than excessive flexion of the joints caused by shortening of the flexor tendons. Rarely such fixations are associated with spina bifida or absence of ventral horn cells of the spinal cord.
HYPERMOBILITY OF JOINTS
This is recorded as an inherited defect in Jersey cattle. Affected animals are unable to rise or stand because of the lack of fixation of limb joints. The joints and limbs are usually all affected simultaneously and are so flexible that the limbs can be tied in knots. Causes include:
WEAKNESS OF SKELETAL MUSCLES
A number of sporadic myopathies are recorded in cattle and sheep. Causes have not been determined in most of them. Splayleg in pigs has been well described and occurs in most countries.
CONGENITAL HYPERPLASIA OF MYOFIBER
There is only one identified state; it is the inherited form of doppelender, double muscling or culard of cattle, described in Chapter 35. The principal cause of the bulging muscles is an increase in the number of myofibers in the muscle.
OBVIOUS ABSENCE OR DEFORMITY OF SPECIFIC PARTS OF THE MUSCULOSKELETAL SYSTEM
A number of these defects are known to be inherited and are dealt with in Chapter 34. They include:
• Achondroplastic dwarfism, inherited miniature calves, bulldog calves
• Umbilical, scrotal hernia, cryptorchidism
• Tail deformity (kinking), taillessness
• Reduced phalanges, including hemimelia (individual bones missing), amputates (entire limbs missing), vestigial limbs (all parts present but limbs miniaturized). Amputates in outbreak form are recorded in cattle and produced experimentally by irradiation injury of sows, cows and ewes during early pregnancy. Inherited arachnomyelia (spidery limbs) of calves
• Congenital thickleg of pigs, osteopetrosis of calves, muscular hypertrophy of calves
• Cyclopian deformity. Inherited form associated with prolonged gestation. Toxic form associated with ingestion of Veratrum californicum
• Displaced molar teeth, mandibular prognathism. Agnathia in lambs takes a variety of forms, including complete absence of lower jaw and tongue.