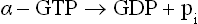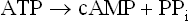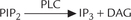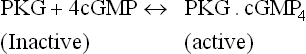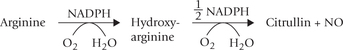Biochemical Endocrinology
Hormones are chemical messengers, secreted by cells of specialized tissues called endocrine glands, and transported by blood to stimulate specific functions of distant tissues or organs. They play key role in the intercellular communication and coordination of responses. Together with nervous and immune systems, the hormones are important constituents of the signalling system of the body that mediates interactions between various tissues. It is essential to have such interactions between different tissues and organs, each of which is especially designed for performing its specialized function. The discovery of existence of hormones, or chemical messengers (as they were originally called), stems from experiments of Bayliss and Starling (1902). These workers demonstrated that injection of the duodenal mucosa extract into the blood stream stimulated flow of pancreatic juice. (The factors present in the duodenal mucosa must have reached the pancreas through blood).
The word hormone is of Greek origin, which means “to arouse to activity”. By the classic definition, a hormone is synthesized in one tissue, secreted in the circulatory system and transported as mobile messenger to control metabolic and biological activities in the target cells. However, this definition is too restrictive and it is now well established that some hormones can act on the adjacent cells in a given tissue (paracrine function) as well as on the cells in which they are synthesized (autocrine function). Examples: interleukin-2 is autocrine hormone for it is being produced by T cells and stimulates proliferation of T cells; and prostaglandins have paracrine function since they act on nearby cells. In this chapter, general characteristics and mode of action of various classes of hormones are described.
After going through this chapter, the student should be able to understand:
Chemical diversity, biosynthesis, and transport of hormones to the target tissues; their interaction with receptors on target cells, concepts of signal transduction (receptor-effector coupling); and biochemical basis for development of disease in case of defect in receptors or in events following hormone-receptor interaction.
Working of hormone at cellular and molecular levels, concept of membrane and intracellular receptors, and various types of second messengers and their mode of action.
The above concepts will be illustrated with the help of clinical cases.
I General Characteristics of Hormone Systems
Hormones are chemically diverse. They transmit messages or chemical signals (i.e. endocrine signalling), which can control almost any aspect of cellular functions and is exquisitely suited for orchestration of physiological responses throughout the body. Molecular basis of action of hormones is different for various groups of hormones. It is interesting to note that all hormones act by regulating pre-existing processes; none of the known hormones is an enzyme or a coenzyme.
A Chemical Diversity of Hormones
Substances of diverse origin and chemical nature may serve as hormones. Based on their chemical nature, the hormones have been categorized into the following four groups:
1. Lipid hormones: Most lipid hormones are derived from cholesterol, such as adrenocortical hormones, sex hormones and calcitriol. Others are derived from arachidonic acid, such as prostaglandins.
The cholesterol-derived hormones contain a steroid nucleus and are lipophilic in nature; they readily traverse the cell membrane of their target cells and interact with the cytoplasmic receptors.
2. Amino acid hormones: These hormones are produced by enzymatic modification of an amino acid molecule. For example, both epinephrine and thyroxine are derived from tyrosine molecule.
3. Peptide and protein hormones: These hormones are made up of amino acids, joined by peptide bonds. Smallest of them is thyrotropin releasing hormone (TRH), a hypothalamic-releasing factor, which consists of only three amino acids. Other examples include antidiuretic hormone (9 amino acids), glucagon (29 amino acids), parathormone (84 amino acids), and growth hormone (191 amino acids).
4. Glycoprotein hormones: A glycoprotein hormone consists of peptide chain to which carbohydrate moieties are covalently attached. The latter are necessary for the biological activity of these hormones. Examples include pituitary hormones (TSH, LH and FSH), and chorionic gonadotropin (hCG) of placental origin.
B Biosynthesis
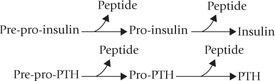
Biosynthetic mechanisms for hormones are diverse. Some hormones are initially synthesized as large precursor proteins which are converted to the biologically active forms by removal of specific peptide sequences. Insulin (MW 5500), for example, is initially synthesized as an inactive precursor, pre-pro-insulin (MW 11,500). A sequential removal of two peptide sequences results in production of an insulin molecule. Likewise, parathormone (PTH), an 84 amino acid peptide, is formed from the 115 amino acid precursor, pre-pro-parathormone by successive removal of two peptide segments.
Perhaps the most exaggerated example is that of thyroxine, a single amino acid hormone, which is processed from a 115 amino acid glycoprotein precursor, thyroglobulin.
Conversion of each of the precursor proteins mentioned above to its activated form takes place in the endocrine gland of its origin only. For example, insulin is synthesized (from pre-proinsulin) in (β-cells of the pancreas, and parathormone (PTH) is produced from the precursor pre-pro-PTH in the parathyroid glands. Other precursor molecules are activated in distant peripheral tissues. Peripheral activation of androgen precursors (dehydroepiandrosterone and androstenedione) of adrenal cortex to potent androgens (testosterone and dihydrotestosterone) is an example. Similarly, 25-hydroxy-cholecalciferol is modified to the active form, calcitriol by 1-hydroxylation in the renal tubules (Chapter 18). Other hormones like glucocorticoids and mineralocorticoids, secreted from the adrenal cortex, do not need any modification; they are synthesized and secreted in the final form only.
Biosynthesis of Insulin
Like several other peptide hormones that are processed from larger precursor molecules, insulin is also synthesized from a precursor. The synthesis begins in the rough endoplasmic reticulum (RER). The precursor, called pre-pro-insulin, is a single chain peptide with 103 amino acids (Fig. 29.1 ). Production of mature insulin of 51 amino acid from this larger precursor involves the following sequence of events:
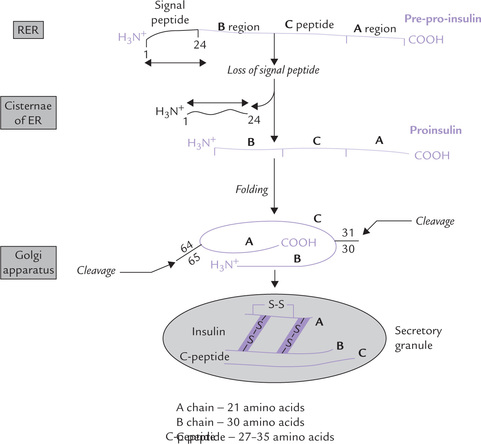
Fig. 29.1 Synthesis of insulin from pre-pro-insulin precursor by removal of 2 peptide fragments: the signal peptide sequence and the C-peptide. Signal peptide consisting of the first 24 amino acids at the amino terminus is cleaved by a peptidase, yielding pro-insulin. The C-peptide is removed by an enzyme in secretory granule, yielding insulin (ER = endoplasmic reticulum).
• Removal of a hydrophobic amino acid sequence of 24 amino acids, called the signal peptide or the leader sequence, from the N-terminus of pre-pro-insulin. Occurs first it is removed by signal peptidase in the ER immediately after translation.
• The remaining structure, known as proinsulin, consists of the insulin sequence interspersed by a connecting C-peptide (C for connecting). C-peptide is required for proper folding of the prohormone and formation of correct disulphide bonds. Its structure is far less conserved than that of insulin. Its length varies from 27-35 amino acids (therefore, proinsulin may contain 78 to 86 amino acids).
• At the final stage of insulin synthesis, proinsulin is cleaved at paired-basic amino acid residues to yield the hormonally inactive C-peptide fragment, and active fragment, the insulin.
• Both are packaged into secretory vesicle and are released from the cell.
C-peptide is released in an amount equimolar to insulin. This is exploited in the clinical laboratories to assess the (β-cell function in diabetic patients under insulin treatment. Although the circulating insulin of these patients comes in part from their own pancreas and in part from therapeutically injected insulin, the circulating C-peptide level is directly proportion to the insulin release from the patient’s β-cells.
C Transport
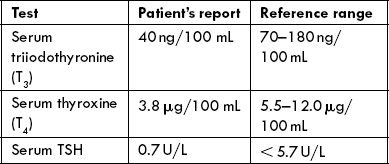
The peptide hormones circulate in the blood in free form, unbound to any transport protein. This is due to their polar (hydrophilic) nature. In contrast, the steroid hormones and the thyroid hormones are predominantly hydrophobic in nature and therefore, cannot circulate in blood entirely in an unbound form. They are mostly transported to their site of action by carrier proteins, where they exert their action and are inactivated by further metabolism. Relatively smaller percentage of the total circulating hormone is left in the free form (Table 29.1 ). The thyroid hormones are bound with two specific carrier proteins: thyroxine-binding globulin (TBG) and thyroxine-binding pre-albumin (TBPA), which together bind nearly all the T3 (tri-iodothyronine) and T4 (thyroxine) in plasma.
Table 29.1
Carrier proteins regulating delivery and biological half-life of the hormone
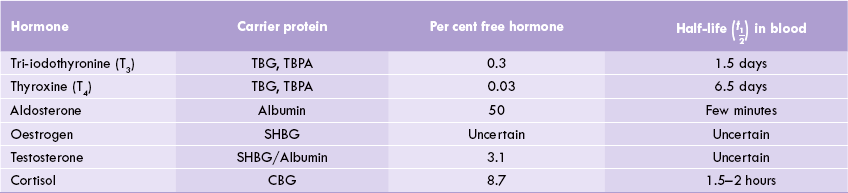
TBG = thyroxine-binding globulin, TBPA = thyroxine-binding prealbumin, SHBG = sex hormone-binding globulin, CBG = cortisol binding globulin (half-life of peptide hormones is less than a minute).
In addition to playing a key role in hormone transport, the carrier protein also prevents rapid clearance of the hormone and thereby, prolongs its half-life. Because of this, the half-life of the steroids and thyroid hormones, which mostly exist in bound states, is much longer than that of the peptide hormones which is less than 30 minutes (Table 29.1). The avidity of binding also determines the half-life. T4, which binds the carrier proteins more tightly than T3, has a longer half-life compared to that of T3.
Only the free form of a hormone, and not the bound form, is able to interact with the cellular receptors. Therefore, it is the free form that is responsible for inducing metabolic and biological effects in target cells. The bound form merely acts as a circulating reservoir, releasing the free hormone in the immediate vicinity of the target tissue, as per cellular requirements. Therefore, the ratio of free: bound forms, and not the total circulating hormone concentration, is true reflection of the observed biological activity. For example, in case of aldosterone, which is the most potent mineralocorticoid, high free: bound ratio is observed. This high ratio among other factors, accounts for its high biological activity.
D Target Tissue Concept
The physiological and biochemical effects of a given hormone are elicited only in a specific tissue, known as its target tissue. A target tissue has specific receptors with which the hormone interacts. The hormone-receptor interaction triggers a series of events that subsequently lead to elicitation of the biological effects of the hormone. For example:
• Target tissue for thyroid-stimulating hormone (TSH) is the thyroid gland, where this hormone stimulates synthesis and secretion of the iodothyronines (T3 and T4).
• Similarly, adrenal cortex, the target tissue for ACTH, responds to ACTH by increasing steroidogenesis.
• Other hormones (e.g. insulin, growth hormone, and cortisol) have more than one target tissues, including liver, muscle and adipose tissue, where they influence a variety of metabolic processes.
More recently, as the knowledge about the hormone-receptor interaction has increased, the definition of target tissue has been expanded. Presently it includes all such tissues which have cellular receptors for the given hormone, irrespective of whether the biological response is elicited or not. For instance, endothelium is the target tissue for insulin receptors on endothelial cells, even though the latter are unresponsive to this hormone.
E Feedback Concept
Blood levels of the target tissue hormones (e.g. thyroid gland) have an effect (mostly inhibitory) on the secretary activities of either the hypothalamus or pituitary. An elaborate system of feedback effects, termed the short feedback loop and long feedback loop exist, which relies on a precise signalling, and it helps to maintain the circulating plasma concentration of the hormones to the required levels. Such feedback mechanism is best illustrated by the hypothalamo-pituitary-target gland axis (Fig. 29.2 ).
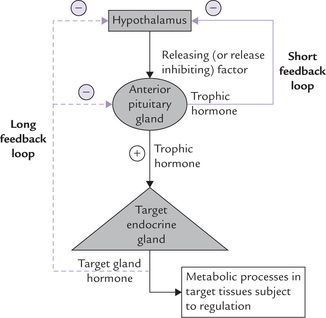
Fig. 29.2 Hypothalamic-pituitary-target gland axis in negative feedback; indicates negative feedback effect.
• The hypothalamic-releasing factor stimulates the release of the anterior pituitary hormone (called trophic hormone).
• The trophic hormone enhances the synthesis and/or secretion of the hormone from the target gland.
• The blood level of the target gland hormone thereby rises and exerts inhibitory effect (i.e. negative feedback effect) on the anterior pituitary and the hypothalamic secretions.
• The net result of these inhibitions is that secretion of the trophic pituitary hormone decreases. Diminished level of the pituitary hormone decreases stimulus for the target gland, so that secretion of the target gland hormone also falls.
In this way, a self-regulatory loop is formed referred to as long feedback loop (Fig. 29.2). The pituitary hormone may also directly cause feedback inhibition of the hypothalamic secretion (i.e. short feedback loop). Defect in this axis can disturb the normal regulation and cause hormonal disorders (Case 29.1).
In some cases, the feedback modulator of a given hormone may be a metabolite, the circulating level of which is controlled by the hormone itself. For example, raised blood glucose concentration is the most potent (positive) modulator of insulin synthesis and secretion from the (β-cells of pancreas. Similarly, decreased serum calcium is a potent stimulus for parathormone (PTH), which in turn mobilizes calcium from the bony reservoir to restore the serum calcium levels.
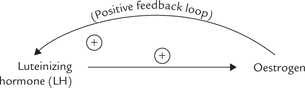
Not all Feedbacks are Negative
In addition to the negative feedback mechanisms, those for positive feedback modulation are also known. A classic example is stimulation for release of luteinizing hormone (form anterior pituitary) by increased plasma levels of oestrogen. It is noteworthy that oestrogen is itself released from ovarian follicles in response to the luteinizing hormone (LH).
The oestrogen-mediated release of LH secretion is called LH surge, which induces ovulation during menstrual cycle (Chapter 30).
Failure of feedback mechanisms may disturb the precisely controlled systems described above. These may result in a disease, as exemplified in Case 29.2.
Central nervous system also exerts important influence on these mechanisms and may even override the effects of the feedback regulations. In fact, cascades of signals that start from external or internal environment are first transmitted to the CNS. These may involve components of the limbic system such as hippocampus and amygdala. These structures innervate the hypothalamus in a specific region, which responds by liberating hypo-thalamic-releasing factors.
II Hormone Receptors
A General Characteristics
Receptors are cell-associated recognition molecules that play a crucial role in the hormone action.
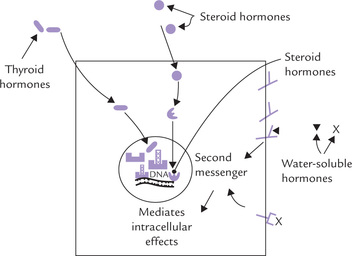
• The receptors for water-soluble hormones (peptides, proteins, or glycoproteins) are present on the cell surface. Interaction of hormones with receptors stimulates certain molecules namely second messengers, which mediate biochemical functions intracellularly.
• The receptors for lipophilic hormones such as the steroids and the thyroid hormones are located intracellularly (Fig. 29.3 ).
The lipophilic hormones readily traverse the cell membrane because of their hydrophobic nature and enter the cell. Intracellularly, they interact with specific receptors located either in the cytosol (e.g. steroid hormones) or within the nucleus (e.g. thyroid hormones) to form hormone-receptor complexes. The latter serves as the intracellular messengers, through which biochemical functions are mediated.
Thousands of receptor molecules may be present in a single cell. They bind the hormone with a high affinity. The interaction is highly specific, involving electrostatic and hydrophobic interactions, and is reversible in most cases.
B Receptor-effector Coupling
The hormone-receptor interaction has a unique feature, i.e. Receptor-effector coupling, implying that occupancy of a receptor with its specific ligand molecule influences several intracellular events. This is because of the two distinct functional domains present in a receptor molecule: the binding domain and the coupling domain. The binding domain recognizes and binds the hormone; the signal is transmitted (signal transduction) through the coupling domain to the intracellular proteins, usually enzymes. The activity and/or intracellular concentration of an enzyme changes in response to the signal; and it is through such change that the effect of the hormone is mediated.
A classic example is the hormone, epinephrine, which binds with the extracellular binding domain of the receptor, located on the cell surface of the hepatocyte. Following signal transduction, adenylate cyclase, an intracellular enzyme, is activated. This enzyme influences several metabolic pathways by triggering a series of events discussed later in this Chapter. Thus, the hormone may influence several intracellular events even without entering the cell.
C Regulation of Receptors
Sensitivity of the target tissue to the hormone is determined by number of receptors on the target cell, and the affinity of these receptors for the respective ligand is important in this regard. Both these factors are subject to regulation. Prolonged exposure to an elevated concentration of the hormone results in decreased sensitivity of the target tissue to the hormone (desensitization). Desensitization involves two mechanisms: down regulation and covalent modulation of the receptors.
Down Regulation
This implies internal sequestration of the receptors, so that fewer receptors are available on the cell surface for interaction with the agonist. Moreover, the internalized receptors get segregated from other components of the response system, resulting in decreased response of the target tissue. Down regulation is a reversible process; removal of the agonist results in return of the receptors onto the cell surface (Case 15.1).
Covalent Modulation
This involves phosphorylation of the receptors which causes uncoupling of the binding and the signal transmitting functions of the receptors. This impairs the response of the target tissue.
A few hormones, such as prolactin and angiotensin, “up regulate” their own receptors, thus amplifying the target tissue sensitivity. Such increase in the number of receptors occurs within minutes and serves as an efficient way of regulating the biological response elicited by the hormone.
D Structure of Receptors
Purification and characterization of receptors have been difficult. This is because it is difficult to obtain the requisite amount of receptors for such analysis. With development of recombinant DNA technology, this difficulty has been overcome. Samples of adequate size can be obtained by recombinant techniques and subjected to analysis. Structures of various hormone receptors are being elucidated in this way. Structure of the acetylcholine receptor, for instance, has been determined by molecular cloning techniques and is known to consist of four peptide sub-units. The steroid hormone receptors have also been shown to be proteins; some of these are single chain proteins, while others are heterodimers. Details about insulin receptors—an outcome of extensive research in recent years—are known.
Insulin Receptor
Insulin binds with a specific receptor type in target tissues. The receptor is a membrane protein with an unusual enzymatic activity. It is a tetramer, consisting of two alpha subunits and two beta subunits linked by disulphide bonds. The beta subunits possess tyrosine kinase activity while the alpha subunits provide binding site for insulin.
1. Binding of insulin to the alpha subunits occurs in the extracellular domain, as shown in Figure 29.4 . This switches on the tyrosine kinase activity of the beta subunits. The above stimulation causes phosphorylation of protein in intracellular domain on tyrosine side chains. (Phosphorylation of tyrosine is in contrast to that of hormone stimulated protein kinases A and C; the latter phosphorylates serine and threonine residues on their substrates.)
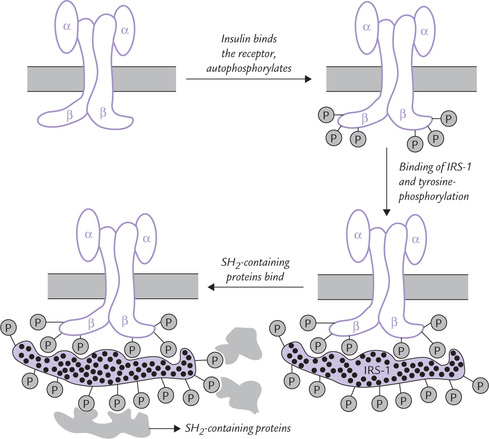
Fig. 29.4 The insulin receptor ( indicates phosphoryl group, IRS-1 = insulin receptor substrate-1, SH = src homology).
indicates phosphoryl group, IRS-1 = insulin receptor substrate-1, SH = src homology).
2. The substrate specificity of the insulin receptors is rather unusual: after ligand binding, the receptors tend to aggregate in the lipid bilayer of membrane and phosphorylate one another. This activity is called autophosphorylation.
3. The next event after autophosphorylation is binding of the receptor to 131-kD phosphoprotein, called IRS-1 (insulin receptor substrate 1), which becomes extensively phosphorylated on approximately 20 tyrosine residues (Fig. 29.4).
4. These phosphotyrosine residues on IRS-1 serve as the docking sites for the SH2-containing proteins, meaning that a number of proteins having a specialized domain, the SH2 domain (SH for src homology), bind with the tyrosine-phosphorylated sites on IRS-1.
5. Though the signalling pathways are still poorly known beyond this stage the following changes in SH2-containing proteins have been defined:
• Some of the SH2-containing proteins get tyrosine phosphorylated by IRS-1, and then participate in multistep phosphorylation cascades (one protein phosphorylating another, and so on). These cascades will eventually alter the activities of enzymes of various pathways, e.g. glycogen and fatty acid metabolism.
• Some other effector proteins are recruited to the plasma membrane, where they facilitate glucose transport. Few such transport proteins are normally located on the cell membrane. Binding of insulin to the receptor initiates a rapid mobilization of intracellular stores of such transporter to the plasma membrane.
E Hormone Receptors and Diseases
Abnormalities of hormone receptors may impair hormone action, which may cause various receptor related diseases (Table 29.2 ). Based on the nature of abnormality of the receptor, these diseases are divided into three categories:
Table 29.2
Diseases related to abnormalities of hormone receptors
| Disease | Receptor | Defect |
| Vitamin D resistant rickets; type II | Calcitriol | Receptor deficiency |
| Congenital nephrogenic diabetes insipidus | ADH | Receptor deficiency |
| Pseudo-hypoparathyroidism | PTH | Receptor deficiency |
| Acanthosis nigricans | Insulin | Antibody (IgG) blocks hormone-receptor interaction |
| Graves’ disease | TSH | Antibody stimulates TSH receptor |
| Myasthenia gravis | Acetylcholine | Antibody enhances turnover of acetylcholine receptors |
| Asthma | β-Adrenergic | Antibody blocks β-adrenergic binding |
| Non-insulin dependent diabetes mellitus | Insulin | Decreased receptors on target cells |
| Obesity | Insulin | Decreased receptors on target cells |
ADH = antidiuretic hormone; PTH = parathormone; TSH = thyroid stimulating hormone.
1. The first category includes the diseases in which binding of the hormone and the receptor is impaired; in some cases, no binding has been detected. Diseases developing due to defective receptors for calcitriol (vitamin D resistant rickets, type II), ADH (congenital nephrogenic diabetes insipidus), PTH (pseudohypo-parathyroidism) and fall into this category.
2. In the second category, antibodies of IgG class, directed against a specific hormone receptor, have been detected. Interaction of these antibodies with the receptor may have varying effects on the hormone action. The antibodies may
(a) block the action of the hormone (acanthosis nigricans with insulin resistance),
3. In the third category, the receptor regulation is defective. For example, in patients with non-insulin dependent diabetes mellitus and obesity, down regulation of the insulin receptors occurs. The target cell insensitivity and glucose intolerance develop in these patients, in spite of elevated plasma insulin levels. With weight reduction, the receptors return to the cell surface resulting in improvement of the target tissue response (Case 15.1).
In some cases, more than one-receptor defect may be present. For example, in acanthosis nigricans, the patient is mostly obese. Therefore, in addition to the primary receptor defect (insulin-receptor binding decreased), down regulation of receptors also occurs. Some clinical cases discussed in this chapter illustrate as to how a variety of hormonal disorders can develop because of defective receptors (see Cases 29.3 to 29.5).
III Mechanism of Action of Hormones
Hormones have been classified in two major groups based on their mechanisms of action (Table 29.3 ). The mechanism of action depends upon the location of the hormone receptor and the nature of the signal transmitted following the hormone-receptor interaction.
Table 29.3
Classification of hormones based on mechanism of action
| Group I: Hormones that bind to intracellular receptors | |
| Ia. Cytosolic receptors | |
| Glucocorticoids | |
| Mineralocorticoids | Oestrogens |
| Progestins | Calcitriol |
| Ib. Nuclear receptors | |
| Thyroxine (T4) | |
| Triiodothyronine (T3) | |
| Group II: Hormones that bind to receptors located on cell surface | |
| IIa. Second messenger is cAMP | |
| Human chorionic gonadotropin (hCG) | Calcitonin |
| Luteinizing hormone (LH) | Glucagon |
| Follicular-stimulating hormone (FSH) | Lipotropin (LPH) |
| Antidiuretic hormone (ADH) | Opioids |
| β-Adrenergic catecholamines | Parathormone (PTH) |
| α2-Adrenergic catecholamines | Somatostatin |
| Thyroid-stimulating hormone (TSH) | Angiotensin II |
| Melanocyte-stimulating hormone (MSH) | |
| Adrenocorticotrophic hormone (ACTH) | |
| Corticotropin-releasing hormone (CRH) | |
| IIb. Second messenger is cGMP | |
| ANF (atrial natriuretic factor) | |
| NO (nitric oxide) | |
| IIc. Second messenger is calcium or phosphatidylinositides or both | |
| Gonadotropin-releasing hormone (GnRH) | Angiotensin II |
| Thyrotropin-releasing hormone (TRH) | Vasopressin |
| Acetylcholine (muscarinic) | Gastrin |
| α-Adrenergic catecholamines | Cholecystokinin |
| IId. The intracellular messenger is a protein kinase or phosphatase cascade | |
| Insulin | Growth hormone (GH) |
| Epidermal growth factor (EGF) | Prolactin (PRL) |
| Nerve growth factor (NGF) | Oxytocin |
| Fibroblast growth factor (FGF) | Erythropoietin |
| Chorionic somatomammotropin (CS) | |
| Insulin-like growth factors (IgF-I, IGF-II) |
Group I
This group comprises the lipophilic hormones that are derived from cholesterol and the thyroid hormones (exceptions—T3 and T4). These hormones interact with cytoplasmic or nuclear receptors respectively. The hormone-receptor complex itself acts as an intracellular messenger and directly influences the gene expression (Fig. 29.3).
Group II
This group comprises the peptide, protein, and glycoprotein hormones. These hormones bind with the surface receptors, located on the plasma membrane of the target cells. The hormone-receptor interaction transmits a signal across the membrane, that results in elevation of the intra-cellular level of an intermediary molecule, the so called second messenger (the hormone itself is the first messenger). The second messenger acts as a signal-conducting molecule, through which the biological effects of a hormone are mediated.
Depending on the chemical nature of the second messenger generated, group II hormones are further divided into three subgroups (IIa, IIb and IIc):
• Group IIa hormones employ cAMP as the second messenger (Table 29.3). The concept of second messenger was introduced with the discovery of cAMP by Sutherland, who reported raised intracellular cAMP levels following binding of epinephrine with plasma membrane of pigeon erythrocytes.
• The second messenger for the group IIb hormones is cGMP and those for the group IIc hormones is calcium or phosphatidylinositides (or both).
• Hormones of group IId employ some multistep phosphorylation cascade that has not been fully identified/settled.
Some hormones use different secondary messengers in different tissues. Action of PTH, for example, is mediated via cAMP in renal cells and phosphoinositide/Ca2+ in bone cells. Likewise, vasopressin also employs two different messengers in renal cells and muscle cells.
The intracellular events that follow the hormone-receptor interaction play a role of vital significance in eliciting the biological effects of the hormone.
A Mechanism of Action of Group I Hormones
Sterol-derived Hormones
The hormones of this subgroup readily diffuse through plasma membrane of the target cells and encounter specific, high-affinity cytosolic receptors (Fig. 29.3). Formation of hormone-receptor complex is followed by change in its conformation and surface charge, which results in activation of the complex. The activated hormone-receptor complex is able to selectively bind specific regions of DNA (called hormone responsive elements) and enhance transcription of specific genes.
Why is the receptor able to bind with specific DNA sequences only after its interaction with the hormone? The reason is that the unstimulated receptor normally resides in the cytoplasm complexed to cytoplasmic proteins that mask its DNA-binding domain. After the hormone binding, the receptor releases its cytoplasmic-binding proteins, and the DNA-binding domain is thereby exposed. Together with the bound hormone, the activated receptor is thus able to bind the hormone responsive elements (HRE).
Direct action of the hormone-receptor complex upon various cell organelles and membranes have also been reported. In addition, some hormones (glucocorticoids) effect post-translational processing of some proteins. However, of all these actions, the most predominant one is on gene transcription. Further details about molecular mechanisms involved in the process are given in Chapter 24.
Iodothyronine Hormones
These hormones also change expression of specific genes, like the sterol-derived hormones. However, they do not associate with the cytoplasmic receptors. Rather, they associate with certain high affinity receptors present within the nucleus. Thus, receptors for these hormones are also ligand-binding transcription factors (Fig. 29.3).
B Mechanism of Action of Group II Hormones
Hormones of this group are considered first messengers, and their intracellular effects are elicited through mediator molecules termed second messengers. The hormones bind with cell surface receptors, which are integral membrane glycoproteins, having three functional domains:
1. The extracellular domain that binds the hormone.
2. One or more transmembrane a-helices that penetrate the lipid bilayer.
3. Intracellular domain that is coupled with an effector mechanism.
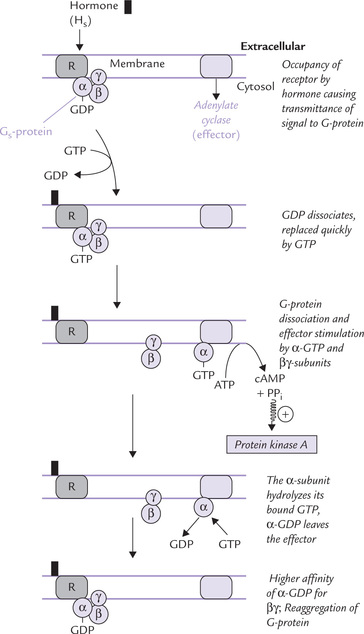
The hormone receptor interaction transmits signal across the cell membrane to target proteins by the following sequence of events (Fig. 29.5 ):
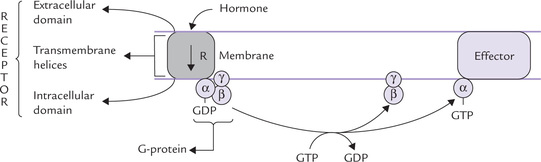
Fig. 29.5 The G-protein signalling. In the resting state the complete G-protein (ap^) is associated with the receptor (R) and GDP. Binding of hormone to the receptor causes cleavage of the G-proteins and the products dissociate from the receptor to reach their target proteins (effectors) whose properties they affect by allosteric mechanism.
• Binding of hormone with the extracellular domain of the receptor induces conformational changes in the receptor.
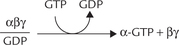
• These changes are transmitted to the transmembrane α-helices, from where they reach the intracellular domain, which is associated with a guanine nucleotide-binding regulatory protein or G-protein. It is a trimeric complex of three subunits (α β and γ) associated with GDP in its inactivated state.
• The conformational change in the intracellular domain of the receptor activates G protein allosterically. The activation involves GDP-GTP exchange, and dissociation of G-proteins into βγ and α-GTP subunits.
• Both these products bind to target proteins in plasma membrane, called effectors. This results in allosteric modulation of the effector.
The best known effectors are second messenger synthesizing enzymes, such as adenylate cyclase, and phospholipase C, guanylate cyclase, etc. These enzymes, when activated, generate second messengers which serve as mediators of the hormone action. The commonest second messengers are:
cAMP as Second Messenger (Group IIa Hormones)
The series of events that lead to activation of adenylate cyclase and consequent generation of cAMP are discussed in brief below (Fig. 29.6 ):
1. The hormone (H) binds to its receptor (R) and the signal is transmitted to the G-protein: a trimer of α-subunit (47 D), (β-subunit (37 kD) and γ-subunit (7 kD). The β- and γ-subunits are tightly associated with each other; the α-subunit binds only loosely with βγ.
2. The G-protein responds to the signal from the H-R complex by undergoing a conformational change, which greatly reduces affinity of the α-subunit for GDP. The GDP, therefore, dissociates and is replaced quickly by GTP.
3. Once GTP is bound, it induces dissociation of the trimeric complex into α-GTP subunit and βγ complex.
α-GTP, called active-α, is a potent stimulator of adenylate cyclase.
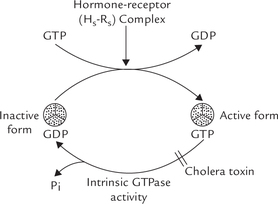
4. Action of the active-α is self-limiting. This is because of the intrinsic GTPase activity of the active-α, that hydrolyzes the bound GTP to GDP and inorganic phosphate.
5. The α-GDP is biologically inactive. It dissociates from adenylate cyclase, and thereby ceases to activate it. Further, it reassociates with the βγ subunits to form the trimeric (αsβγ) complex again.
The α-GTP (also called active αs) stimulates adenylate cyclase. Some isoforms of adenylate cyclase are stimulated by βγ-subunits. The stimulated enzyme generates cAMP, as discussed in the following section.
Action of cAMP: An Overview
The adenylate cyclase, stimulated by the hormone stimulated G-protein, acts on ATP:
The inorganic pyrophosphate is immediately hydrolyzed by pyrophosphatases, making the reaction irreversible.
The cAMP activates an intracellular enzyme called cAMP-dependent protein kinase A (PKA; A is the designation for cAMP).
How does cAMP cause activation of protein kinase A? The mechanism is discussed below.
Protein kinase consists of four subunits: two catalytic and two regulatory (Fig. 29.7 ). Its subunit structure is R2C2. In absence of cAMP, the C and R are bound together and the enzyme is inactive in this form. Four cAMP molecular bind to the inactive protein kinase and cause dissociation of R and C subunits.
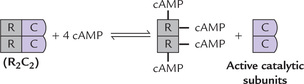
Fig. 29.7 Binding of 2 molecules of cAMP to each R-subunit results in conformational changes that lead to dissociation of (cAMP2-R2) dimer from the C-subunit. The monomeric, active C-subunits, then proceed to phosphorylate serine and threonine residues in target enzymes. C = catalytic subunits, R = regulatory (inhibitory) subunits.
The catalytic C subunits phosphorylate a variety of protein substrates in the cytoplasm and the nucleus. These include several metabolic and regulatory enzymes. (Table 29.4 ), which ultimately cause biochemical responses.
Table 29.4
Intracellular substrates for protein kinase A
| Intracellular protein | Tissue | Effect of phosphorylation |
| Glycogen synthase | L, M | Inhibition |
| Phosphorylase kinase | L, M | Stimulation |
| Pyruvate kinase | L | Inhibition |
| Hormone sensitive lipase | A | Stimulation |
| Acetyl CoA carboxylase | L | Inhibition |
| Phenylalanine hydroxylase | L | Stimulation |
| Inhibitor-1 | L | Stimulation |
L = liver; M = muscle; A = adipose tissue.
The cAMP Cascade AMplifies Hormonal Stimulus
Protein kinase A is fully activated at submicromolar concentration of cAMP, so that it is very sensitive to small changes in adenylate cyclase activity. The hormonal response is amplified following receptor binding because a single receptor can generate several active-a molecules. Each active-a acts on adenylate cyclase long enough to cause synthesis of hundreds of cAMP molecules, and so on.
The Inhibitory G-Protein (Gi)
Some hormones (Hi) inhibit the adenylate cyclase activity (Fig. 29.8 ). The effect is mediated through a different receptor type (Ri) and an inhibitory G-protein complex (Gi). This complex consists of three subunits (αi βγ); the structure of βγ-subunits of Gi is similar to those of the stimulatory G-protein, but α-subunit of Gi (αi) is different with a molecular weight of 45,000 (Recall that the α-subunit of the stimulatory G-protein is αs, having molecular weight of 47,000). The hormone-receptor (Hi-Ri) interaction results in dissociation of the complex in two parts: αi-GTP and βγ. The former has a negative effect on the activity of adenylate cyclase and therefore on cAMP production. This explains fall in intracellular cAMP level by certain hormones (Hi), such as α2-adrenergics, opioids, somatostatin and angiotensin II (Table 29.5 )
Table 29.5
Hormones that use cAMP as the intracellular messenger may be stimulatory (Hs) or inhibitory (Hi) for adenylate cyclase system. The stimulatory hormones increase the intracellular cAMP concentration, and the inhibitory ones decrease it
| Hs | Hi |
| Human chorionic gonadotropin (hCG) | Acetylcholins |
| Luteinizing hormone (LH) | α2-adrenergies |
| Follicular-stimulating hormone (FSH) | Somatostatin |
| Antidiuretic hormone (ADH) | Angiotensin II |
| (β-Adrenergic catecholamines | |
| Thyroid-stimulating hormone (TSH) | |
| Melanocyte-stimulating hormone (MSH) | |
| Adrenocorticotrophic hormone (ACTH) | |
| Corticotropin-releasing hormone (CRH) | |
| Calcitonin Glucagon Lipotropin (LPH) | |
| Parathormone (PTH) |
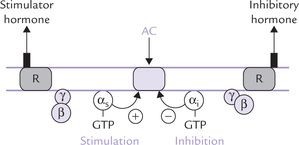
Fig. 29.8 Regulation of adenylate cyclase by the stimulatory and inhibitory hormones acting via stimulatory G-protein (Gs) and inhibitory G-protein (Gi), respectively. Most cells contain both Gs-linked receptor (Rs) and Gi-linked receptors (Ri). The actual activity of adenylate cyclase depends on the balance between the stimulatory and the inhibitory hormones (AC = adenylate cyclase).
Abnormalities of the hormone-G protein-adenylate cyclase axis may result in impaired action of hormones, as exemplified in Case 29.4.
Other Types of G-proteins
Humans have approximately 20 different G-protein α-subunits, five different β-subunits and 10 β-subunits. These subunits are expressed in different combinations in different cell types. According to structure and function of their a-subunit, four types of G-proteins have been recognized.
The first two—Gs-proteins and Gi-proteins—which stimulate and inhibit adenylate cyclase have been already discussed. The other two groups are as follows:
• Gq proteins stimulate a phosphatidylinositol-specific phospholipase C (Fig. 29.9 ). The latter forms the second messengers inositol triphosphate and diacylglycerol (discussed later).
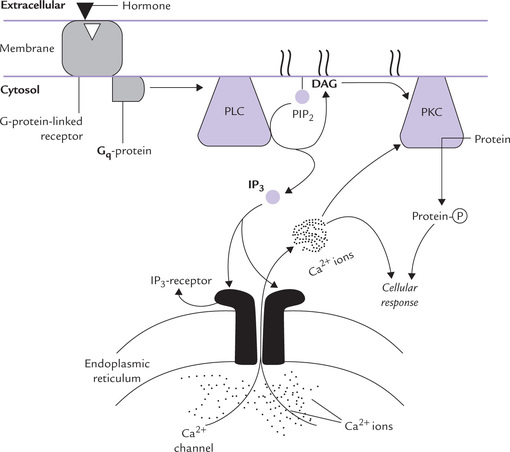
Fig. 29.9 The phosphatidylinositol 4, 5-bisphosphate (PIP2), a membrane phospholipid, is hydrolyzed by phospholipase C (PLC) to generate two second messengers: inositol triphosphate (IP3) and diacylglycerol (DAG). IP3 is released into cytosol where it mobilizes intracellular stores of calcium; and DAG, a membrane anchored second messenger, activates a family of signalling enzymes known as protein kinase C (PKC).
Modification of G-protein by Some Bacterial Toxins
The stimulatory (Gs) as well as the inhibitory (Gi) G-proteins are targets of some bacterial toxins: the Gs for the cholera toxin, and the Gi for the pertussis toxin.
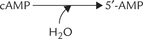
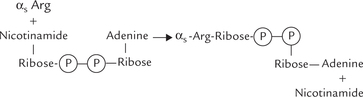
Cholera is an intestinal infection caused by Vibrio cholerae. The offending bacterium produces a lethal toxin that produces severe diarrhoea, which is often fatal. This enterotoxin binds with a ganglioside Gm1 on the surface of intestinal mucosal cells initially, and then one of its subunits enters the intestinal mucosal cell, where it acts as an enzyme. Intracellularly, this subunit brings about covalent modification of the α -subunit of the Gs-protein. The covalent modulation it causes is referred to as ADP-ribosylation:
Activity of the ADP-ribosylated α-subunit is selectively modified as below:
1. It loses its GTPase activity.
2. It retains capability of binding GTP, thereby getting activated itself and stimulating adenylate cyclase.
Thus, the α-subunit gets activated normally, but remains trapped in this (activated) form only. The protein is “turned on” but is “locked” in the activated form, having lost its “turn off” switch. Persistent stimulation of adenylate cyclase follows so that excessive cAMP is generated. Flooding of the cell with cAMP is the natural consequence. This causes profuse secretion of water and electrolytes, resulting in the dreaded diarrhoea.
The pertussis toxin modifies the αi-subunit of the Gi by covalent modulation. It causes ADP-ribosylation of this subunit, which makes Gi unable to inhibit the adenylate cyclase. Consequently, the latter is “locked” in an activated form, and so the cell is flooded with cAMP. Bordetella pertussis, the bacterium which produces pertussis toxin, is the cause of whooping cough.
Phosphatidylinositides/Calcium System as Second Messenger (Group IIc Hormones)
This system generates two intracellular second messengers: inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG). The hormones of group IIc (and some neurotransmitters) that act through this system are shown in Table 29.3. They bind to a cell-surface receptor, and the receptor-hormone complex activates a G-protein of the Gq family (Fig. 29.9). The latter activates a membrane bound enzyme phospholipase C (PLC), which acts on a variety of substrates, present on the inner leaflet of the plasma membrane. Phosphatidyl inositol 4, 5-bisphosphate (PIP2) is the most important of such substrates because its cleavage forms two intracellular messengers, IP3 and DAG.
Inositol 1,4,5-Triphosphate (IP3)
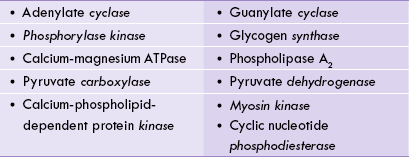
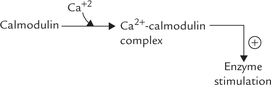
This water soluble second messenger is generated at the plasma membrane initially. It diffuses through the cytoplasm and binds to the IP3 receptor on the endoplasmic reticulum. This binding releases intracellular calcium stores. The Ca2+ in turn influences a variety of biochemical processes, particularly through the mediation of calmodulin (Table 29.6 ).
Diacylglycerol (DAG)
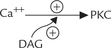
DAG is non-polar and so remains membrane associated, where it functions as a second messenger, activating protein-kinase-C (PKC). The latter translocates from the cytosol to the membrane and gets activated by DAG. This activation requires ionic calcium (hence the C in designation), and DAG greatly increases affinity of PKC for calcium.
The activated PKC in turn phosphorylates a wide range of target proteins (e.g. HMG CoA reductase, tyrosine hydroxylase, and several signal transduction molecules), resulting in altering their functional status. Interestingly, PKC can phosphorylate the same protein targets as PKA but, whereas PKC generally phosphorylates the protein at serine residues, PKA usually phosphorylates threonine residues.
It is evident from the given discussion that both second messengers (DAG and IP3) act synergistically. DAG activates PKC by increasing its affinity for calcium, and calcium is made available by IP3.
Activation of the phospholipase C system also causes influx of calcium (form ECF), to cause further elevation of intracellular calcium concentration. It does so by increasing cell permeability so that calcium moves from high concentration in extracellular space (1-2 mmol/L) into cell (0.1-10 μmol/L).
Calmodulin is a calcium-binding and calcium sensing protein, which is an important mediator of intracellular effect of Ca2+. It has four binding sites for calcium. Occupancy of these sites with calcium activates the calmodulin by inducing alterations in its conformation. The activated calcium-calmodulin complex has ability to interact with a number of enzymes; the interaction changes activities of these enzymes (Table 29.6).
Detailed studies on the enzyme glycogen phosphorylase have shown that the enzyme consists of four subunits (α, β, γ, δ), and calmodulin itself constitutes one of the four subunits (δ). Binding of calcium to the calmodulin subunit alters its conformation, which in turn alters conformation of the enzyme protein and hence its activity.
Though calcium (and cAMP) primarily acts by modifying activities of target enzymes, it may also regulate their synthesis (Box 29.1).
cGMP as Second Messenger (Group IIb Hormones)
cGMP is an important intracellular messenger in retinal cells and some non-retinal cells. Steps involved in the synthesis and degradation resemble the corresponding steps of cAMP. It is generated from GTP by the enzyme guanylate cyclase (GC), and degraded by the enzyme phosphodiesterase (PDE), which terminates its action.
The chief action of cGMP in the non-retinal cells is to activate protein kinase G (action of cGMP in retinal cells has been explained in Chapter 18).
Activation of PKG occurs by allosteric mechanism. PKG is a serine/threonine protein kinase. It mediates most of the cGMP effects by phosphorylating a number of intracellular proteins.
cGMP can also act directly by binding to ion-channels, or by altering activities of some phosphodiesterases. However, physiological substrates for cGMP are largely unknown.
In contrast to adenylate cyclase, an integral membrane enzyme, the GC exists in two forms: the membrane bound form and the cytoplasmic form.
1. Membrane bound GC: This form of GC is actually the intracellular domain of a receptor for the peptide, atrial natriuretic factor (ANF). The receptor contains an extracellular ligand-binding domain, a single transmembrane helix, and an intracellular domain that acts as guanylate cyclase, all on the same polypeptide. The intracellular GC domain requires binding of ANF to the extracellular domain for its catalytic activity (Box 29.2). Once activated, it generates cGMP that activates the protein kinase to mediate most of the cGMP effects, as discussed earlier.
2. Cytoplasmic GC: The principal GC of the vascular smooth muscles is the soluble cytoplasmic form. It is not activated by the ANF, but by nitric oxide; and generates cGMP, which mediates vasodilatation.
Signalling by Nitric Oxide (NO)
Nitric oxide causes vasodilatation and is an important regulator of blood pressure. It acts by stimulating the cytoplasmic GC, thereby increasing cGMP. NO is unique intracellular messenger (Box 29.3) for being membrane soluble. It readily diffuses to nearby cells and increases cGMP level in them. Such a phenomenon occurs in vascular endothelial cells and the nearby smooth muscles: NO synthesized in the endothelium diffuses into the smooth muscle cells where it increases cGMP. The latter relaxes smooth muscles to cause vasodilatation.
NO is synthesized by the enzyme, NO synthase in the endothelial cells from one of the nitrogen molecules in the side chain of arginine.
The reaction sequence involves NADPH and the products include NO, citrulline and NADP+. Activity of NO synthase is stimulated by calcium-calmodulin. Therefore, the agents that elevate cytoplasmic calcium concentration increase the NO synthase activity.
The chemical agents that generate NO during their metabolism relax smooth muscles. This forms the basis for their use in treatment of angina pectoris and other vascular disorders by some NO generating agents, such as nitroprusside and nitroglycerine. Physiological agents that increase intracellular calcium concentration also relax smooth muscle by the same mechanism (i.e. by stimulating NO synthesis). Histamine, acetylcholine, bradykinin, thrombin and ATP are some examples of such agents. On the other hand, the agents that decrease NO generation by inhibiting NO synthase cause elevation of blood pressure. These observations amply illustrate that NO is an important regulator of blood pressure under ordinary circumstances.
Protein Kinase o Phosphatase Cascade as Intracellular Messenger
Hormones of this group, such as insulin, and a variety of growth factors, interact with receptors whose C-terminal intracellular domains have tyrosine kinase activity. After binding of ligand to the extracellular domain, the intracellular domain of these receptors cause auto-phosphorylation of proteins on tyrosine side chains (Fig 29.10 ). This is termed as intrinsic ligand activated tyrosine kinase activity. Phosphorylation at tyrosine side chains initiates a multistep phosphorylation cascade that may involve several protein kinases, phosphatases and other regulatory proteins. This leads to generation of several gene products, which mediate the hormone action. Like other intracellular signals, activities of these protein kinases must be turned off after they have delivered their message. The off switch is provided by tyrosine phosphatases, enzymes that dephosphorylate phosphotyrosyl residues. Defect in these events may lead to diseases (Case 30.5).
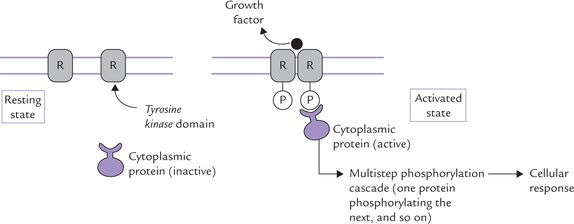
Fig. 29.10 Receptor tyrosine kinase activation. Growth factor binding turns on the tyrosine kinase activity in the intracellular domain, resulting in its (auto-) phosphorylation. The autophosphorylation enables the intracellular domain to bind cytoplasmic proteins, which get tyrosine phosphorylated, and initiate a phosphorylation cascade, that ultimately leads to cellular response (R = receptor).
IV Neurotransmitters
Like hormones, neurotransmitters are also important extracellular messengers. They bring about transmittance of a targeted message from a neuron to the responding cells. A neurotransmitter is released by a neuron at synapse to effect another post-synaptic neuron or organ (e.g. heart, lungs). Its effect is confined to the synapse and, unlike hormones, it is into released into the bloodstream. In addition to amino acids and their derivatives (described in Chapter 4), other compounds that may serve as neurotransmitters are peptides, purines, gases (e.g. NO), etc.
• • Peptides: A number of physiologically active peptides, including the enkephalins, cholecystokinin, octapeptide, vasoactive intestinal peptide (VIP) somatostatin, substance P (an undecapeptide in primary afferents and the basal forebrain) are well-established neurotransmitters.
• Purines, such as ATP and adenosine in CNS and sympathetic nerves.
• Nitric oxide, which is derived from arginine in CNS and genitourinary system.
• Miscellaneous, such as acetylcholine that acts in parasympathetic nerves and CNS.
Exercises
Essay type questions
1. What do you understand by second messenger concept in hormone action?
2. Discuss the second messenger mechanism of G-proteins in signal transduction.
3. Give schematic diagram of insulin receptor, mention its important properties and discuss its functional significance.
4. Describe the mechanism of action of epinephrine at molecular level.
5. Describe the activity of each component of the signalling pathways based on adenylate cyclase, receptor tyrosine kinases and phosphoinositides.
 The peptide hormones circulate free (unbound), whereas the steroid and thyroid hormones are mostly bound with carrier proteins. The latter act as circulating reservoir of hormone, and also prolong its half-life.
The peptide hormones circulate free (unbound), whereas the steroid and thyroid hormones are mostly bound with carrier proteins. The latter act as circulating reservoir of hormone, and also prolong its half-life.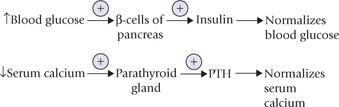
 = thyroid hormone receptor,
= thyroid hormone receptor,  = steroid hormone receptor,
= steroid hormone receptor,  and
and