Metabolism of Carbohydrates II: Secondary Pathways and Regulation of Blood Glucose Level
The mainline metabolic pathways of glucose utilization generate ATP energy and fuel molecules. In addition to such pathways, there are other minor (or secondary) pathways taken by glucose, which are specialized for synthesis of glycolipids, glycoproteins and other special products needed by the cells. Two such pathways are pentose phosphate pathway, which leads to formation of ribose 5-phosphate and NADPH; and uronic acid pathway, which generates D-glucuronate and ascorbic acid. Rate of flux of metabolic intermediates through the secondary pathways is much less compared to that through the mainline pathways.
Significance of certain other minor metabolic pathways that metabolize sugars other than glucose, such as fructose, galactose, mannose, or the disaccharides and the polysaccharides is also outlined this chapter. Maintenance of blood glucose level within the normal range has been discussed.
After going through this chapter, the student should be able to understand:
• Reactions of pentose phosphate pathway and uronic acid pathway, and their significance.
• Minor pathways of metabolism of carbohydrates other than glucose.
• Metabolic significance and clinical consequences of enhanced rate of sorbitol pathway in diabetes mellitus.
• The interplay of various factors that are responsible for maintenance of normal blood glucose levels.
I Pentose Phosphate Pathway
The pentose phosphate pathway also called hexose monophosphate (HMP) shunt or phosphogluconate pathway is required for provision of five-carbon sugars and NADPH. Activity of this cytosolic pathway is minimal in muscle and brain, where almost all of the glucose is degraded by glycolysis. However, it is highly active in liver, adipose tissue, adrenal cortex and lactating (but not the nonlactating) mammary gland. These tissues are involved in synthesis of cholesterol and fatty acids, the processes dependent on NADPH. Because NADPH is important for the antioxidant defenses of the body, the pentose phosphate pathway is well developed in cells that are exposed to a high oxygen partial pressure, such as erythrocytes and cornea of the eye. It accounts for about 60% of the total oxygen consumption in cornea.
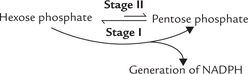
The pathway consists of three irreversible oxidative reactions (Stage I), followed by a series of reversible sugarphosphate interconversions (i.e. non-oxidative reactions Stage II). An overview is presented below.
The Stage I, irreversible oxidative reactions result in formation pentose phosphates, while a series of reversible non-oxidative interconversions take place in the Stage II, which convert these pentose phosphates into glycolytic intermediates.
A Specialized Products Generated by Pentose Phosphate Pathway
1. Reduced nicotinamide adenine dinucleotide phosphate (NADPH) serves as a biochemical reductant in a number of reactions. Such reductive reactions form important steps of several biosynthetic pathways, such as steroidogenesis and lipogenesis. Therefore, rate of the pentose phosphate pathway is high in tissues that synthesize steroids and fatty acids.
NADPH generation through HMP shunt is essential for maintaining integrity of the erythrocyte membranes because glutathione reduction is brought about by NADPH (Case 10.1). These functions are discussed in detail later in this chapter.
2. Ribose 5-phosphate is an important constituent of the purine and pyrimidine nucleotides, that perform a variety of important functions. It is an essential structural component of various coenzymes such as coenzyme-A, FAD and NAD+. Ribose 5-phosphate can be reconverted to hexose phosphates through reversible sugarphosphate interconversions by the non-oxidative reactions of the pentose phosphate pathway. This provides an important mechanism for the metabolism of five carbon sugars.
B Reactions of Pentose Phosphate Pathway
Stage I: Oxidative Phase
The oxidative reactions of Stage I bring about conversion of glucose 6-phosphate to ribulose 5-phosphate (Fig. 10.1 ). Two NADPH molecules are also generated during this reaction sequence comprising of three irreversible oxidative reactions. The reactions are shown in Figure 10.2 .
Dehydrogenation of glucose 6-phosphate
Glucose 6-phosphate is irreversibly converted to 6- phosphogluconolactone by the enzyme glucose 6- phosphate dehydrogenase. NADP+ as a co-enzyme is specific for this reaction. NADP+ acts as an acceptor of the reducing equivalents (i.e. a pair of hydride) that are removed from the glucose 6-phosphate molecule and gets converted to NADPH.
This step is the regulatory step of the pathway. NADPH acts as a negative modulator, causing competitive inhibition of glucose 6-phosphate dehydrogenase (G6PD).
Hydrolysis of 6-phosphopgluconolactone
6-Phosphogluconolactone is converted to 6-phosphogluconate by addition of a water molecule. This reaction is catalyzed by the enzyme gluconolactonase and is irreversible, like the previous step.
Formation of ribulose 5-phosphate
Oxidative decarboxylation of 6-phosphogluconate, catalyzed by 6-phosphogluconate dehydrogenase, then yields the ketose sugar, ribulose 5-phosphate, plus a molecule of NADPH.
The non-equilibrium nature of the Stage I reactions enables the cell to maintain a cytoplasmic ratio of NADPH : NADP + = 100. Interestingly, the ratio of NADH : NAD + in the cytoplasm is nearly the inverse, less than 0.01. For this reason the cells employ NADPH rather than NADH whenever a strong reducing agent is required.
The ribulose 5-phosphate, the end product of the Stage I, is convertible to other pentose phosphates: xylulose 5-phosphate and ribose 5-phosphate. The enzymes for these reactions are:
Stage II: Non-oxidative Interconversion Phase
The pentose phosphates generated in Stage I, ribose 5- phosphate, xylulose 5-phosphate and ribulose 5-phosphate, are converted to glycolytic intermediates in the second stage of HMP (Fig. 10.3 ). This stage comprises a series of non-oxidative reversible interconversions, as noted earlier. The enzymes of this stage are transketolase and transaldose, which catalyze the following three reactions:
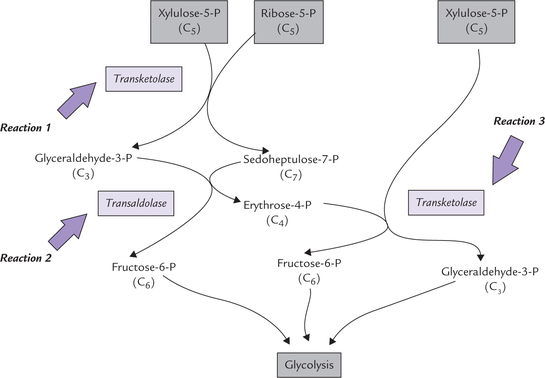
Fig. 10.3 The non-oxidative reactions of pentose phosphate pathway. The transketolase transfers a 2-carbon unit, and the transaldolase causes transfer of a 3-carbon unit.
Reaction 1
This reaction is catalyzed by transketolase, which transfers a two carbon unit from a ketose sugar to an aldose sugar. When the two-carbon unit is transferred from a xylulose 5-phosphate (a 5-C ketose) to ribose 5-phosphate (a 5-C aldose), formation of a seven-carbon sugar (sedoheptulose phosphate) and a three carbon-sugar (glyceraldehyde 3-phosphate) results.
Thiamine pyrophosphate, a coenzyme for transketolase, serves as transient carrier of the two carbon units in this reaction.
Reaction 2
This is catalyzed by transaldolase, which transfers a threecarbon unit from the sedoheptulose γ-phosphate to glyceraldehyde 3-phosphate. This results in the formation of a erythrose 4-phosphate and fructose 6-phosphate.
Reaction 3
This is again catalyzed by transketolase. The tetrose phosphate (erythrose 4-phosphate) generated in the previous step, accepts a two-carbon unit from a new pentose phosphate (xylulose 5-phosphate) molecule to form a fructose 6-phosphate and glyceraldehyde 3-phosphate.
Net result of these reactions is formation of two hexose phosphates (fructose 6-phosphate) and a triose phosphate (glyceraldehyde 3-phosphate), which directly enter the glycolytic sequence.
Thus, the non-oxidative interconversions (of the Stage II) permit conversion of the pentoses (generated in Stage I) into intermediates of glycolysis. This establishes a link between the pentoses and the other metabolizable sugars, thereby integrating the HMP shunt pathway with glycolysis.
A summary of reactions of the pentose phosphate pathway, as shown in Figures 10.2 and 10.3 can be written as:
C Regulation
The regulatory enzymes of the pentose phosphate pathway are glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase. Synthesis of both the enzymes is induced by insulin. Thus, in fed state, their intracellular concentrations rise, leading to enhanced oxidation of glucose through this pathway, and conversely in starvation and diabetes mellitus, the pathway is suppressed.
Activity of glucose 6-phosphate dehydrogenase is competitively inhibited by NADPH, as mentioned earlier. It is the ratio of NADPH : NADP + that determines the overall rate of the pathway. Under normal circumstances, the ratio of NADPH/NADP+ is sufficiently high (up to 100), which keeps the activity of G6PD inhibited. However, when demand for NADPH increases, its utilization also increases resulting in the fall of the intracellular NADPH concentration. This removes the inhibition on the enzyme activity. Consequently, this reaction is speeded up, resulting in enhanced production of the required NADPH.
D Functions
The pentose phosphate pathway is a multifunctional pathway, unique in generation of two important products: pentoses and NADPH.
• Pentoses: The most important pentose is ribose 5-phosphate, which is required for a number of biosynthetic and other functions, as mentioned earlier in this chapter.
• NADPH: It has the same redox potential as NADH, but the functions of the two coenzymes are different. Whereas NAD+ collects hydrogen from catabolic substrates for transfer to respiratory chain, NADPH is required for reductive biosynthesis and several other functions:
Reductive biosynthesis
The synthesis of fatty acids and cholesterol from acetyl CoA require reductive power of NADPH.
Antioxidant role
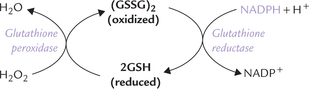
NADPH is necessary for the maintenance of a reducing environment inside the cell. This is necessary for avoiding damage to unsaturated fatty acids, proteins and DNA by peroxides or other oxidizing agents, to which the cell is continuously exposed. NADPH plays a key role in antioxidant defenses by converting the oxidized glutathione into the reduced glutathione, which is protective:
As diagrammed above, the reduced glutathione (GSH) detoxifies hydrogen peroxide; the enzyme glutathione peroxidase catalyzes this reaction. However, GSH is oxidized during the reaction, and must be regenerated to continue detoxification. NADPH participates in the reaction responsible for the regeneration of reduced glutathione from the oxidized one; glutathione reductase catalyzes this reaction.
Phagocytosis (literally “cell eating”)
It is the engulfment of solid particles into the cell, such as granulocytes, monocytes and macrophages. These cells participate in our immune system by eating up and destroying infectious microorganisms (Chapter 33). NADPH is required for production of superoxide anion radicals by macrophages, which have bactericidal activity (Case 27.1).
Metabolism of xenobiotics
Lipophilic xenobiotics are metabolized to water-soluble products by hydroxylation in the microsomal cytochrome P450 system; the process requires NADPH (Chapter 15).
Special function in erythrocytes
NADPH is especially important to prevent oxidative damage to the RBC membrane and intracellular proteins in erythrocytes because the cell cannot replace these by new synthesis during its 120-day lifespan. NADPH maintains the antioxidant defenses by converting the oxidized, dimeric glutathione into the protective reduced form. This restores the level of reduced glutathione, which is oxidized while detoxifying peroxides and other oxidizing agents. Thus, requirement of NADPH is relatively higher in erythrocytes. The extra demand is met by a slight modification in the HMPshunt, as follows.
HMP-shunt can be Modified
Based on demand of the tissue, the HMP shunt is modified as below:
(a) Extra demand for NADPH: In erythrocytes requirement for NADPH is relatively more, which is met as below:
• Conversion of the pentoses to hexoses through the reversible Stage II reactions is favoured.
• The hexose phosphates so formed are then funneled into another cycle of oxidative reactions to produce more of NADPH.
This establishes a cyclic pathway, with each of these cycles generating 2 NADPH molecules.
(b) Extra demand for pentoses: Demand for pentoses is greater than that for NADPH in some tissues. The nonoxidative reactions can provide the ribose 5-phosphate molecules directly from fructose 6-phosphate via the Stage II reactions in such tissues.
Glucose 6-phosphate Dehydrogenase Deficiency
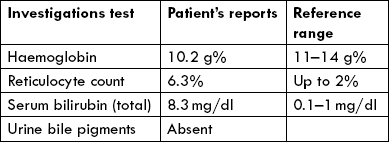
Some persons have a genetic defect in this enzyme, typically yielding an unstable enzyme, that has shorter halflife in the RBC, or an enzyme that is unusually sensitive to inhibition by NADPH. In either case, insufficient flux of the HMP-shunt and so decreased production of NADPH results, and the cell’s ability to recycle GSSG to GSH is impaired. Drug-induced oxidative stress leads to lysis of RBCs (haemolysis); haemolytic anaemia is the obvious consequence (Case 10.1).
Wernicke-Korsakoff Syndrome
Thiamine deficiency in alcoholics does not cause beriberi but Wernicke-Korsakoff syndrome. Reduced activity of the thiamine-dependent transketolase enzyme occurs in this condition resulting is impairment of HMP shunt.
This condition is diagnosed by clinical features that include mental derangements, delirium and motor incoordination, which may progress to chronic stage (called Korsakoff psychosis) characterized by amnestic syndrome. Early diagnosis and immediate treatment is essential, because brain damage in this condition is irreversible.
II Uronic Acid Pathway
Uronic acid pathway is a source of glucuronic acid. It also produces ascorbic acid in some animals. Glucuronic acid is a useful product required in the synthesis of mucopolysaccharides, detoxification of some drugs and for conjugation of bilirubin and steroid hormones. The unutilized glucuronic acid (glucuronate) is converted to xylulose 5-phosphate, which is metabolized via the pentose phosphate pathway.
A Reactions of the Pathway
Glucose is first converted to UDP-glucose by a series of reactions, as discussed earlier in glycogenesis. The UDP glucose further proceeds as below:
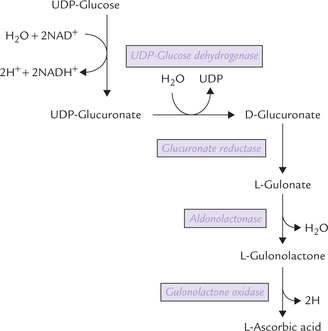
1. The glucose portion of the UDP-glucose is enzymatically oxidized, resulting in formation of UDP-glucuronate. The reaction is catalyzed by the enzyme UDP-glucose dehydrogenase. UDP-glucuronate is the metabolically active compound, as discussed later.
2. From the UDP-glucuronate release of D-glucuronate occurs (Fig. 10.4 ).
3. D-Glucuronate is reduced by the NADPH-dependent enzyme glucuronate reductase to form L-gulonate. Glucuronate contains an acid carboxylate group at C-6, whereas gulonate contains this group at C-1.
4. L-Gulonate then loses a water molecule to form L-gulonolactone. The enzyme is aldonolactonase.
5. Removal of a pair of hydrogen atoms from the L-gulonolactone by the enzyme gulonolactone oxidase yields L-ascorbate or vitamin C.
This pathway is used by all plants and animals for the synthesis of vitamin C. However, some primates, such as humans, monkeys, guinea pigs, as well as some birds and fishes lost the capability to do so during evolution. This is because of genetic deficiency of the enzyme gulonolactone oxidase, which causes conversion ofL-gulonolactone to L-ascorbic acid. It appears that the capacity to synthesize the ascorbic acid was lost by these species because of a mutation, which was not lethal. These species require vitamin C in diet.
B UDP-Glucuronate is Metabolically Active Compound
UDP-Glucuronate can readily denote the glucuronate residue for the following functions:
1. To detoxify foreign compounds or drugs. During detoxification, the glucuronate residues are covalently attached to these substances. Since glucuronate residues are strongly polar, their attachment imparts polar character to these compounds, thus making possible their renal excretion. Bilirubin and steroid hormones are also rendered more polar for excretion in this manner.
2. To synthesize the acid mucopolysaccharides, such as hyaluronic acid and heparin, which contain glucuronic acid as an essential component (Chapter 2).
C Alternate Route for Oxidation of L-Gulonate
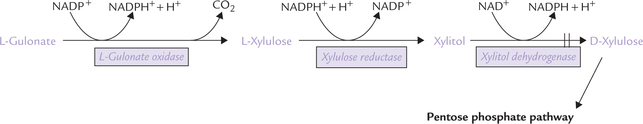
• The L-gulonate (produced from unutilized D- glucuronate) is converted to a pentose sugar, L-xylulose, by oxidative decarboxylation.
• L-xylulose is then converted to its D-isomer, D- xylulose (via an intermediate xylitol).
• D-xylulose is then phosphorylated at the expense of an ATP. The xylulose 5-phosphate so formed is an intermediate of the pentose phosphate pathway (Fig. 10.5 ).
Thus, the uronic acid pathway is connected with the pentose phosphate pathway.
Essential Pentosuria
In the rare hereditary disease termed “essential pentosuria”, the enzyme that catalyzes reduction of L-xylulose to xylitol is deficient. As a result, excretion of significant amounts of L-xylulose in the urine occurs.
Effect of Drugs
Certain drugs increase the flux of intermediates through the uronic acid pathway and thereby enhance generation of the products of the pathway. In rats, synthesis of ascorbic acid is enhanced by these drugs. Barbitol, aminoantipyrine and chlorobutanol are some examples. These drugs increase excretion of L-xylulose in the pentosuric patients.
III Metabolism of Other Sugars
The metabolic pathways of other monosaccharides, disaccharides and polysaccharides, referred to as the feeder pathway, have been described in the previous chapter. Though these pathways appear quantitatively insignificant, they play an important supplementary role.
IV Sorbitol Pathway
Sorbitol pathway also known as polyol pathway, causes conversion of glucose to fructose via sorbitol. The pathway is greatly enhanced in uncontrolled diabetes mellitus, and is implicated in progression of chronic diabetic complications. It comprises of two sequential reactions, as shown below.
The first step involves conversion of glucose to sorbitol by the enzyme aldose reductase. This enzyme is present in a significant concentration in those cell types that do not require action of insulin for the transport of glucose across the cell membrane (e.g. insulin unresponsive cells). Some examples are epithelial cells of lens, Schwann cells of peripheral nerves, seminal vesicle cells and papillae of kidneys. The second enzyme of the sorbitol pathway, i.e. sorbitol dehydrogenase, is present in liver and seminal vesicle cells. It can oxidize sorbitol to fructose. Fructose is preferred over glucose for supplying energy in sperm cells because of the presence of this enzyme.
Sorbitol Pathway in Diabetes Mellitus
Normally, flux of metabolic intermediates through the sorbitol pathway is not significant because aldose reductase has a high Km (i.e. low affinity) for glucose. Therefore, glucose is taken up by this pathway in small amount, the rest being rapidly phosphorylated to glucose 6-phosphate. However, when blood glucose concentration is markedly elevated, such as in uncontrolled diabetes mellitus, large quantities of glucose enters the insulin-unresponsive cells. Large concentration of glucose substrate overcomes the effect of low affinity (of aldose reductase for glucose) and pushes the equilibrium of the first reaction towards sorbitol formation. Consequently, large quantities of sorbitol is produced in prolonged hyperglycaemia.
Sorbitol cannot be further metabolized in lens and nerve cells (because sorbitol dehydrogenase has either low activity or is absent in these tissues) and cannot diffuse out of the cell easily. Therefore, concentration of sorbitol rapidly builds up, exerting an osmotic effect, which is responsible for many of the physiologic and pathologic alterations associated with diabetes. These alterations lead to development of cataract, peripheral neuropathy, retinopathy, and vascular problems (see Chapter 15).
V Regulation of Blood Glucose Level
Normally the blood glucose level is kept within the range of 60–100 mg/dL in fasting state. The fasting state here implies that the there has been no food intake for the last 12–16 hours. After a meal, glucose is absorbed from intestines and blood glucose level starts rising, reaching a maximum in about one hour. In a normal individual, the maximum value remains less than 1.5 times the fasting value.
Thus, the blood glucose level does not rise above 140 mg/dL, or fall below about 60 mg/dL. This limit is not crossed in a normal subject even though he may take food at certain fixed times and fast between the meals. Several mechanisms of blood glucose regulation remain constantly in operation for this purpose. It is hazardous for the body if above-stated limits are crossed. For example:
• A fall below about 60 mg/dL will deprive the nervous tissue of adequate energy, because under normal circumstances, nerve tissue is heavily dependent on glucose for its energy needs (Case 10.2).
• Conversely, a rise above 180 mg/dL (called renal threshold) will result in urinary elimination of glucose since the amount of glucose filtered by the glomeruli will exceed the amount which can be reabsorbed by the tubules.
Concentration of blood glucose is determined by a balance between the amount of glucose that pours into the blood circulation and the amount that leaves it. Various sources of glucose generation and utilization are shown in Figure 10.6 .
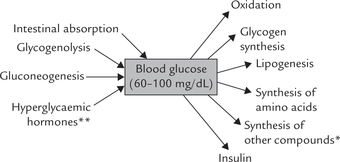
Fig. 10.6 Sources of glucose generation and utilization. (*These include lactose, ribose, mucopolysaccharides, glycolipids, glycoproteins. They are synthesized from glucose or its metabolites. **Hyperglycaemic hormones are glucagon, growth hormone, thyroxine, epinephrine, glucocorticoids, etc.).
A regulated interplay of these factors ensures blood glucose regulation. Liver and endocrine glands play a vital role in this.
A Role of Liver
Liver is capable of utilizing large amounts of glucose that is delivered to it at high concentration (in portal blood) during and following meals. This is because:
High permeability of hepatocytes to glucose
Liver cells are highly permeable to glucose because high-capacity, low-affinity (Km > 10 mmol/L) glucose transporter, GLUT-2, are located on surface of hepatocytes.
Rapid utilization of the internalized glucose
Liver is also rich in glucokinase, an enzyme that is specific for glucose and converts it into glucose 6-phosphate. As discussed earlier, glucokinase is inducible by continued consumption of high-carbohydrate diet, not subject to product inhibition. These properties permit glucokinase to efficiently phosphorylate the intracellular glucose and force it into all of the major pathways of glucose metabolism.
Most of the glucose is channeled into glycogen, providing a carbohydrate reserve for maintenance of blood glucose during the post-absorptive state. The glucose 6- phosphate in liver, beyond that needed to replenish glycogen reserves, is funneled into glycolysis, both for energy production and for conversion into triacylglycerols, which are exported for storage in adipose tissue.
B Role of Endocrines
Several hormones participate in the regulation of blood glucose levels. All except insulin tend to raise blood glucose level, hence termed hyperglycaemic or diabetogenic hormones (Fig. 10.6). Insulin is the only hormone that brings down the blood glucose level.
Role of Glucagon
It is a peptide hormone secreted by the α-cells in endocrine pancreas that is released in response to a decreased blood glucose level. It causes rapid stimulation of glucoseproducing pathways (including hepatic glycogenolysis and gluconeogenesis), and depresses glycogen synthesis and glycolysis, thereby contributing to the hyperglycaemic effect during fasting.
Role of Glucocorticoids
Glucocorticoids cause stimulation of gluconeogenesis during chronic stress by causing induction of the key enzymes, especially PEP carboxykinase. Other effects are: induction of fructose 1,6 bisphosphatase, glucose 6-phosphatase and amino transferases; stimulation of release of amino acids and lactate from muscles, which act as precursors for gluconeogenesis. However, overall effect of glucocorticoids is relatively slow.
Role of Catecholamines
The catecholamines – epinephrine and norepinephrine, are released during acute stress (physical exercise and cold exposure) and psychological emergencies from adrenal medulla. They induce slight elevation of cAMP level to increase glycogenolysis in liver and muscles. Liver contributes to increased level of blood glucose, whereas muscles generate lactate for gluconeogenesis. Other effects are:
Role of Growth Hormone
It inhibits glycolysis (inhibits PFK) and mobilizes fatty acids from adipose tissue stores.
Role of Insulin
It is a peptide from pancreatic (3-cells, released in response to elevation of blood glucose level. Its plasma concentration is 10 times higher after a carbohydrate rich meal than during fasting. It reduces the cAMP level in the liver, probably by activating a cAMP degrading phosphodiesterase, which means that it opposes the effects of glucagon and catecholamines. It stimulates several glucose-utilizing pathways, and inhibits the glucose-generating pathways. Details are given in Chapter 15 (see Table 15.2).
An interplay of various factors maintains blood glucose in different metabolic states: for example, in fasting state, glucose does not enter blood circulation through intestinal absorption, but some amount leaves it to enter tissues for utilization. The blood glucose level, therefore, tends to fall. An excessive fall is prevented by the following metabolic changes in tissues:
1. Instead of glucose, tissues start using alternate fuels, such as fatty acids and ketone bodies. Glucose is spared for utilization by erythrocytes and brain (Chapter 15).
2. Glycogen synthesis in the liver and muscle stops, whereas glycogen breakdown is stimulated. These adaptations are brought about by increased secretion of glucogen and catecholamines, and decreased secretion of insulin. The glucose produced by way of hepatic glycogenolysis is poured into the blood circulation.
3. Formation of glucose in liver occurs by way of stimulation of gluconeogenesis also. For this purpose, the protein degradation in muscles is enhanced and the amino acids so released are transported to liver where they serve as substrates for gluconeogenesis. Glucocorticoids play a key role in this process.
4. In the adipose tissue, lipolysis is stimulated, resulting in excessive generation of fatty acids.
Thus, adipose tissue becomes a source of alternate fuel molecules (e.g. free fatty acids).
These factors ensure that the blood glucose does not fall steeply during fasting and starvation. Likewise, in the fed state, metabolic changes occur to prevent excessive rise of blood glucose concentration. Failure of the regulatory mechanisms occurs in some pathological conditions, such as diabetes mellitus, hyperpituitarism, hyperthyroidism, Cushing’s syndrome, severe liver diseases, etc. Further details about these are given in Chapter 30.
Exercises
Essay type questions
1. Describe the pentose phosphate pathway of glucose metabolism, giving names of enzymes and coenzymes involved. What are the functions of this pathway?
2. Explain the relationship between the integrity of erythrocyte membrane to the operation of HMP shunt pathway. How is the demand for the extra NADPH in red cells met?
3. What is glutathione and what role does it play in the human body? Why does it fail to prevent the haemolytic anaemia in defi ciency of glucose 6-phosphate dehydrogenase?
4. Describe the role of various factors involved in maintenance of normal blood glucose level.

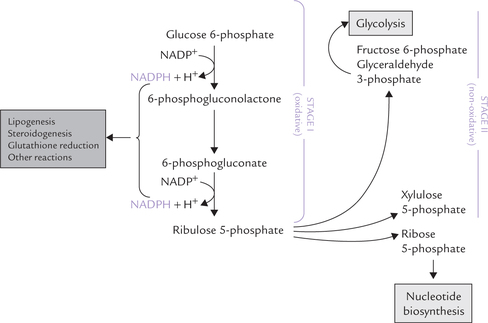
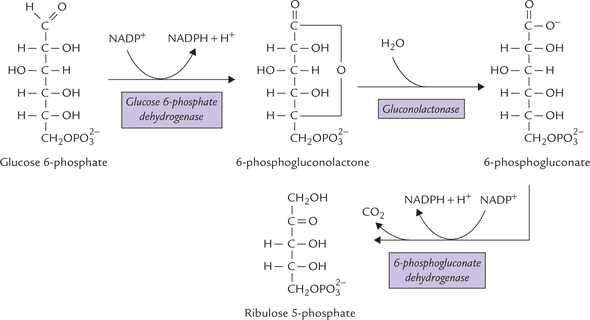
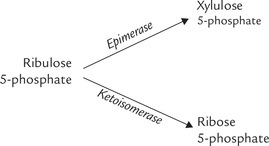
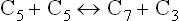
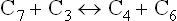
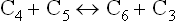
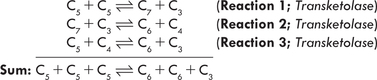

 The 1st phase of HMP shunt is the oxidative pathway in which glucose 6-phosphate is oxidized and decarboxylated to produce two NADPH, a carbon dioxide and a ribulose 5-phosphate. The interconversion phase consists of reversible rearrangements of the phosphorylated pentoses. It links ribulose 5-phosphte to the glycolytic pathway.
The 1st phase of HMP shunt is the oxidative pathway in which glucose 6-phosphate is oxidized and decarboxylated to produce two NADPH, a carbon dioxide and a ribulose 5-phosphate. The interconversion phase consists of reversible rearrangements of the phosphorylated pentoses. It links ribulose 5-phosphte to the glycolytic pathway.