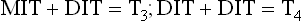Action Of Hormones
Overall regulatory role of the endocrine system was discussed earlier (Chapter 29), emphasizing the working of hormones at cellular and molecular levels, the concept of membrane and intracellular receptors, the nuclear receptors, the second messengers, and their mode of action. The present chapter will focus on arrangement of various endocrine systems, and explain the biosynthesis, chemical nature, and functions of individual hormones. The clinical syndromes arising because of overactivity or underactivity of individual hormones will also be discussed.
By the end of this chapter the student should know:
Arrangement of hypothalamus-pituitary hormone system, chemical nature and effects of the hypothalamic factors, the trophic and non-trophic hormones elaborated by anterior pituitary and the posterior pituitary hormones.
Biosynthesis, chemical nature, and functions of the hormones elaborated by thyroid, adrenals, gastrointestinal tract and gonads; and explain biochemical basis and signs and symptoms of diseases associated with the hormone over- or under-production, e.g. Addison’s disease, Graves’ disease, Cushing’s syndrome, etc.
Several other endocrine systems are also present in the human body, which are not considered in this chapter. Some of these are discussed in previous chapters as part of the physiologic function they control. Thus, the student is referred to Chapter 10 for glucose homeostasis, Chapter 19 for water and electrolyte balance, and Chapter 31 for calcium and phosphorus metabolism.
I Hypothalamus- pituitary System
A significant group of hormones is secreted by peripheral endocrine glands in response to signals from the brain. The brain structures responsible for initiating such signals are the hypothalamus and the pituitary gland (Fig. 30.1 ). Pituitary gland or hypophysis is an oval organ weighing about 0.6 g and is encased in the body cavity of the skull (sella turcica) below the brain. It is demarcated into two parts: the anterior pituitary (adenohypophysis), which accounts for 80% of the gland, and the posterior pituitary (neurohypophysis). The two lobes are connected by pars intermedia, which is rudimentary in humans. Pituitary gland lies below the hypothalamus and is connected with it via the pituitary stalk, which contains a complex array of axons and blood vessels.
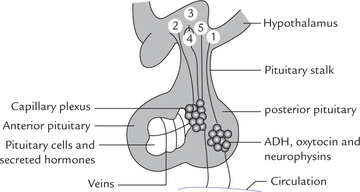
Fig. 30.1 The hypothalamo-pituitary regulatory system. The posterior pituitary hormones are synthesized in supraoptic and paraventricular nuclei (1); and the anterior pituitary releasing/or release-inhibiting hormones in various hypothalamic nuclei (2-5). The posterior pituitary hormones are transported (from hypothalamus) along nerve fibres to posterior pituitary, whereas anterior pituitary hormones travel with portal system to anterior pituitary.
Two connecting pathways are present in the pituitary stalk: (a) a bundle of nerve fibres, leading into the posterior pituitary, and (b) a vascular network, the hypophyseal portal system, joining the anterior pituitary with the hypothalamus. Various hypothalamic hormones, and some pituitary hormones are initially synthesized in hypothalamus and transported to pituitary via these two connecting pathways.
The hormones of posterior pituitary are initially synthesized and packaged in the supraoptic and paraven-tricular nuclei of the hypothalamus, and transported along the nerve fibres to posterior pituitary for storage prior to release in circulation. The hypothalamic release or release-inhibiting hormones are also synthesized in various nuclei of the hypothalamus, but they travel via the portal system to the anterior pituitary, where they elicit the secretion of various trophic or non-trophic hormones. The anterior pituitary hormones act on target endocrine and non-endocrine organs to regulate a broad range of biological functions. Figure 30.2 depicts a bird’s eye view of the hypothalamo-pituitary regulatory system.
II Hypothalamic Hormones
At least seven hormones are secreted by the hypothalamus. Since they are involved with homeostatic mechanisms, their secretion is not constant, rather they are released in bursts (i.e. pulsatile and episodic release). Their secretion is under tight feedback control of the pituitary hormones or the hormones secreted by peripheral endocrine glands. Stimuli from central nervous system also controls secretion of hypothalamic hormones. There are five separate endocrine axes, as shown in Figure 30.2.
Actions of the hypothalamic hormones on their target cells in the pituitary are mediated by (a) calcium phosphoinositide system or (b) via cAMP second messenger system.
Thyrotropin-releasing Hormone (TRH)
It is a modified tripeptide (pyroglutamate-histidine-proline) synthesized as a 26 kDa prohormone. TRH stimulates synthesis and secretion of thyroid-stimulating hormone (TSH) from the thyrotroph cell. It binds to the cell surface receptors that are linked to phospholipase-C. The resulting phosphoinositides stimulate the release of calcium from intracellular storage sites and so lead to exocytosis of TSH. The latter stimulates synthesis and release of the thyroid hormones (T3 and T4).
Corticotropin-releasing Hormone (CRH)
It is a 41 amino acid peptide secreted by the paraventricular nucleus. It acts via the cAMP second messenger system to stimulate release of the pituitary adrenocorticotropic hormone (ACTH). The latter in turn acts on adrenal cortex to stimulate release of adrenocorticosteroids, mainly cortisol.
Arginine Vasopressin (AVP)
It is a nine amino acid peptide, synthesized by both supraoptic and paraventricular nuclei. It elicits an inhibitory effect on pituitary, opposite to that of CRH, by altering the intracellular calciumion channels.
Gonadotropin-releasing Hormone (GnRH)
It is a 20 amino acid peptide synthesized as a 92 amino acid precursor by various hypothalamic nuclei. It has a half-life of about 3 minutes and stimulates the synthesis and secretion of both follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the same gonadotroph cell-type in the anterior pituitary. Its action is mediated via calcium-phosphoinositide system.
Growth Hormone-releasing Hormone (GHRH)
It is a 44 amino acid peptide, synthesized as part of a 108 amino acid prohormone. It binds to its receptor on the pituitary somatotroph cell and triggers both the adenylate cyclase and intracellular calcium-calmodulin system to stimulate GH transcription and release. GH in turns promotes soft tissue and bone growth.
Growth Hormone Release-inhibiting Hormone (GHRIH)
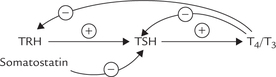
In view of the wide range of action of GH, it is not surprising that an inhibitory hormone also exists, in addition to a stimulatory one to influence the GH secretion. GHRIH, also called somatostatin, is found in two forms—with 14 and 28 amino acids; both are produced from a 116 amino acid gene product. They inhibit GH secretion by lowering intracellular cAMP concentration. Inhibition of TSH secretion is also brought about by somatostatin.
Prolactin Release-inhibiting Factor (PRIF)
Prolactin (PRL) is unique among the pituitary hormones in that it is under predominant inhibitory control from the hypothalamus. Furthermore, the controlling agent (PRIH) is a very simple molecule, dopamine, which works by inhibiting adenylate cyclase. Dopamine inhibits both pro-lactin synthesis and secretion.
Some of these hypothalamic hormones are synthesized in other tissues also. Somatostatin, for example, is found α-cells of pancreas in higher concentrations than in hypothalamus, and it plays an important role in regulating secretion of insulin and glucagon from islet of Langerhans.
III Anterior Pituitary Hormones
Anterior pituitary or adenohypophysis is an endocrine target organ for hypothalamic hormones. The hypothalamic hormones reach pituitary via portal system and induce cause it to release its hormones into the general circulation. By conventional staining three cell types were identified in anterior pituitary: chromophobe, eosinophil and basophil. The cells are now classified by immunological means, and at least five cell types have been identified—somatotrophs secrete GH, lactototrophs secrete PRL, thyrotrophs secrete TSH, gonadotrophs secrete LH and FSH, and corticotrophs secrete ACTH.
The hormones are secreted as either peptides (ACTH, GH, PRL, chorionic somatomammotropin) or glycoproteins (TSH, LH, FSH).
A The Master Gland
Four of the seven pituitary hormones act on target endocrine organs, and are termed as trophic hormones. These are: TSH, ACTH, LH and FSH. The remaining are non-trophic hormones, which act primarily on non-endocrine target tissues or organs. Because of widespread actions of these hormones, the anterior pituitary affects growth, thyroid activity, sexual function, lactation, metabolism of carbohydrate, protein and fat, as well as skin pigmentation (Fig 30.2).
In view of its influence on a vast variety of biochemical processes, pituitary is known as the foremost or master endocrine gland. Accordingly, excess or deficiency of pituitary hormones influence a vast range of functions. Excess is usually due to a pituitary tumour and almost always involves only one hormone. Isolated deficiencies of pituitary hormones occur but are rare, with GH being most common. More commonly, however, the pituitary hormone deficiencies involve more than one, and eventually all the anterior pituitary hormones (panhypopituitarism). There is generalized endocrine dysfunction, and resultant symptoms relate to thyroid or adrenal insufficiency, or there is loss of sexual function.
B Classification
Based on structural characteristics, the anterior pituitary hormones have been traditionally divided in three broad groups:
1. The growth hormone-prolactin-chorionic somatomammotropin group: Growth hormone (GH; somatotropin), prolactin (PRL) and chorionic somatomammotropin (CS; placental lactogen) are protein hormones, exhibiting considerable structural similarities with one another. They are believed to have originated by duplication of a single ancestral gene. The last one (CS) has no definite function in humans.
2. The glycoprotein hormones: Three anterior pituitary hormones, thyroid-stimulating hormone (TSH), follicle-stimulating hormone (FSH), and luteinizing hormone (LH) are glycoprotein in nature. The last two are known as gonadotropins. Human chorionic gonadotropin (hCG), a placental glycoprotein hormone, resembles LH in nature.
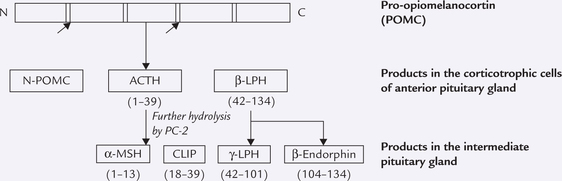
3. The pro-opiomelanocortin peptide family (POMC): It is a 241 amino acid precursor protein (MW 31,000), which is cleaved to yield a number of hormonally active peptides, including the adrenocorticotropic hormone (ACTH), endorphins and melanocyte-stimulating hormone (MSH). ACTH regulates synthesis and secretion of adrenal cortical hormones, endorphins are endogenous analgesics and MSH regulates pigment production by melanocytes.
Growth Hormone (GH)
GH elicits a wide range of actions in regulating growth and intermediary metabolism. Gene for GH is located as a part of the GH-CS gene family in region q 22-24 on the long arm of chromosome 17.
Chemistry
Human growth hormone is a peptide comprising 191 amino acids (MW 21,000) that is synthesized initially as a 29 kDa prohormone. Nearly two-third of GH in circulation is associated with a binding protein that is identical to the extra-cellular domain of the GH receptor. This binding protein prolongs the half-life of GH in plasma (20 minutes). The normal human pituitary contains approximately 10 mg of GH, less than 5% of this is released each day.
Biochemical Functions
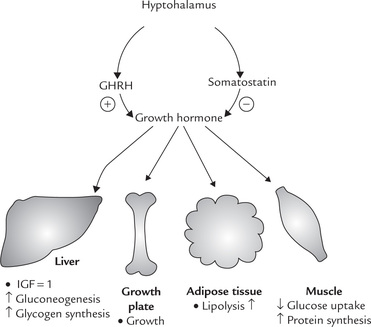
GH is essential for postnatal growth and for normal carbohydrate, lipid, protein and mineral metabolism (Fig. 30.3 ).
1. Effect on growth: GH interacts with its receptor to induce complex series of intracellular events that lead to transcription of many enzymes and peptides, called growth factors or somatomedins, which mediate the growth promoting effects of GH. These compounds are synthesized primarily by the liver under the influence of GH and have strong metabolic and mitogenic effects especially on cartilage, adipose tissue and striated muscle. Two types of growth factors are known: insulin-like growth factor-I (IGF-I) and insulin-like growth factor-II (IGF-II). IGF-I, also known as somatomedin C (formerly sulfation factor), is a 70 amino acid straight-chain basic peptide produced by the liver in response to GH. It is more active than IGF-II, a 67 amino acid peptide, and seems to be directly related to growth. It acts through receptors similar to insulin receptors and is linked to intracellular tyrosine kinase activity.
2. Effect on protein metabolism: GH promotes protein synthesis and cell proliferation in both skeletal and non-skeletal tissues. It increases cellular up-take of amino acids and stimulates transcription. Overall, its growth promoting effects are insulin-like, and tend to turn the nitrogen-equilibrium towards positive nitrogen balance.
3. Effect on lipid and carbohydrate metabolism: Effects of GH on lipid and carbohydrate metabolism are opposite to those of insulin, and not mediated via IGF-I. GH promotes glycogenesis and gluconeogenesis in liver and decreases utilization of glucose in muscles and other tissues. These actions result in increase of blood glucose level, hence GH is a diabetogenic hormone.
GH promotes lipolysis in adipose tissue to elevate the circulating free fatty acid levels. Oxidation of fatty acids in liver is increased and ketogenesis is enhanced, particularly in diabetes.
4. Effect on mineral metabolism: GH increases incorporation of calcium and phosphate in growing bones. This effect is probably mediated via IGF-I and results in increase in bone growth and its mineralization. Incorporation of sodium, magnesium and chloride in bones and of sulphates in cartilage is also increased.
5. Effect on lactation: GH has structural similarity with prolactin, which accounts for its prolactin-like effect of increasing milk production in lactating animals.
6. Others: GH stimulates cellular differentiation and augments actions of other trophic hormones.
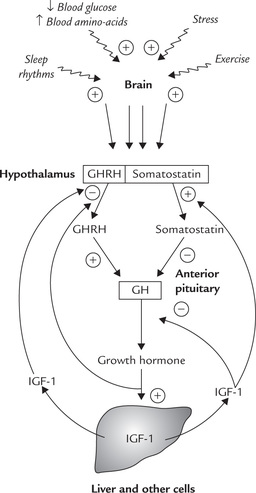
Regulation
GH is controlled by a dual system, namely growth hormone-releasing hormone (GHRH) and growth hormone release-inhibiting hormone (GHRIH), which respectively increases and decreases the release of GH. GHRH, also called somatocrinin, is a peptide with 44 amino acids, and GHRIH or somatostatin is a peptide with 14 amino acids and an S-S bond.
GH secretion is greatly enhanced during sleep when the plasma GH concentration may reach up to 100-fold greater than the baseline. This lends a scientific touch to the proverb “Sleep well and grow well”. Stress, exercise, food intake and obesity also influence GH secretion.
GH inhibits its own secretion via a short feedback loop. IGF-I also causes inhibition of GH by a dual mechanism: (a) it inhibits GHRH secretion, and (b) stimulates somatostatin secretion (Fig. 30.4 ).
Clinical Disorders of GH Secretion
GH deficiency in children results in short stature, while GH excess in children leads to gigantism. In adults, since the epiphyses of the long bones have closed, linear growth is not possible. Therefore, the adult form of GH excess, called acromegaly, manifests by thickening of tissues and a change of facial appearance. GH excess is almost always due to GH-secreting pituitary adenoma, although ectopic GHRH from a pancreatic tumour has been described. GH deficiency may occur as an isolated problem or as part of the general picture of panhypopituitarism.
Prolactin
Chemistry
It is a polypeptide of 199 amino acids (MW 20,000), whose amino acid composition is similar to that of GH (16% sequence homology). It is synthesized in pituitary lactotrophs as a larger prohormone by transcription of a gene located on chromosome 6.
Regulation of PRL Secretion
Secretion of prolactin from anterior pituitary is increased by a hypothalamic hormone, prolactin release factor (PRF). Prolactin release-inhibiting factor (PRIF), most likely dopamine, inhibits prolactin secretion. Dopamine interacts with receptors on pituitary lactotroph (called D2 receptor) to inhibit adenylate cyclase and consequently inhibits both prolactin synthesis and secretion.
Biochemical Functions
In association with other pregnancy-related hormones, prolactin assists initiation and maintenance of lactation, and promotes full development of breast tissue. It binds to its receptor in mammary tissue and stimulates the synthesis of several milk proteins, including lactalbumin.
Thyroid-stimulating Hormone (TSH)
TSH is synthesized in the pituitary thyrotrophs and acts on thyroid gland to increase synthesis and secretion of thyroid hormones. It is also called thyrotrophic hormone or thyrotropin.
Chemistry
TSH is a 28 kDa glycoprotein (MW 30,000). The carbohydrate portion is a complex mixture of acetylated sugars, sialic acid and sulphate. The protein portion is made of non-covalently linked α and β chains. The former comprises 92 amino acids and is identical to the a-chain present in other pituitary glycoprotein hormones (FSH and LH). The β-chain differs in the three hormones and determines biological specificity of each.
Gonadotropins
FSH and LH are called gonadotropins as they stimulate the development and secretory activity of gonads-testis and ovary. Both are secreted in the male and female from the same cell type, the gonadotrophs.
Chemistry
FSH and LH are both glycoproteins with molecular weight of approximately 28 kDa. Each comprises an identical α-subunit (shared with TSH) and a specific β-subunit The β-subunit of both FSH and LH is composed of 115 amino acids and each has two carbohydrate chains.
The gene for FSH β-chain is on chromosome 11, while that for LH β-chain is on chromosome 19. The latter is located close to the gene for the β-subunit of human chorionic gonadotropin (hCG), with which it has considerable homology.
Biochemical Functions
Both LH and FSH activate adenylate cyclase and increase cAMP production, through which their effects are mediated.
In males: LH and FSH act concertedly to promote spermatogenesis in testes.
• LH induces testosterone production by Leydig cells (interstitial cells) of the testes. Testosterone brings about the meiotic divisions necessary for spermatogenesis.
• FSH acts on Sertoli cell of the testes and induces synthesis of several proteins, including androgen-binding protein (ABP) and inhibin. ABP is secreted into the seminiferous tubule lumen where it binds testosterone (or its active form dihydrotestosterone). This ensures high local testosterone concentration which promotes spermatogenesis (Fig. 30.5 ).
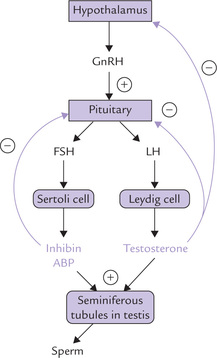
Fig. 30.5 Control of the Hypothalamic-pituitary-gonadal axis (ABP = androgen-binding protein, GnRH = gonadotropin-releasing hormone).
• Inhibin participates in negative feedback loop to inhibit secretion of FSH from anterior pituitary.
In females: FSH and LH have different functions in females, on the basis of which they have been given names. FSH promotes oestradiol synthesis leading to follicular maturation, while LH leads to follicle rupture and oocyte release. Further details are given later (see menstrual cycle).
Regulation
Gonadotropin-releasing hormone (GnRH) regulates the secretion of FSH and LH (Fig. 30.5). The pituitary secretion of these hormone is subject to feedback regulation by the target organ hormones.
• LH is inhibited by the target gland sex hormones, testosterone and oestradiol. The latter inhibits pituitary LH, whereas testosterone inhibits both hypothalamic GnRH and pituitary LH.
Regulation is more complex in females and is linked with menstrual cycle, discussed later.
Human chorionic gonadotropin (hCG) is a placental glycoprotein, structurally similar to LH. It is secreted by fertilized ovum; the secretion begins immediately after implantation. It maintains corpus luteum during pregnancy. Its detection in urine forms the basis for diagnosis of pregnancy.
Adrenocorticotropin (ACTH)
Chemistry
ACTH is formed in pituitary corticotrophs from first 39 amino acids of POMC. POMC is cleaved to release several hormonally active peptides including ACTH (Box 30.1).
Adrenocorticotropin stimulates synthesis and release of glucocorticoids and to some extent mineralocorticoids from the adrenal cortex. The biological activity of ACTH resides in the N-terminal 24 moieties.
Biochemical Functions
ACTH acts via the cAMP second messenger system. The major effects are:
1. Synthesis and release of steroid hormones from the adrenal cortex is induced by ACTH. The conversion of cholesterol to pregnenolone, the first step in steroidogenesis, is enhanced by ACTH. This leads to synthesis of glucocorticoids, and to a lesser extent other steroid hormones as well.
2. Prolonged exposure to high ACTH levels causes lipolysis in adipocytes and increased insulin secretion from the pancreas.
Regulation
The ACTH secretion from pituitary mainly occurs under the influence of
(a) two hypothalamic peptides, corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP), both hypothalamic peptides are under the influence of cerebral factors, and
• CRH is a 41 amino acid peptide secreted by the paraventricular nucleus, which has a stimulatory effect on ACTH secretion. It acts via the cAMP second messenger system.
AVP, is a nine amino acid peptide, which inhibits the ACTH secretion. It is synthesized by both the supraoptic and paraventricular nuclei, and it acts by altering intracellular calcium ion channels (Fig. 30.2).
• The negative feedback occurs by high concentrations of glucocorticoids, particularly cortisol, which inhibits the secretion of ACTH as well as CRH. Further details about this hypothalamo-pituitary-adrenal axis are given in a subsequent section of this chapter (see Fig. 30.11 ).
IV Hormones of Posterior Pituitary
Posterior pituitary is a source of the circulating vasopressin and oxytocin. The principal action of vasopressin is to increase reabsorption of water by the renal tubules, and because of this action it is also known as antidiuretic hormone (ADH). Oxytocin is important in parturition and lactation in females; its role in males is unknown.
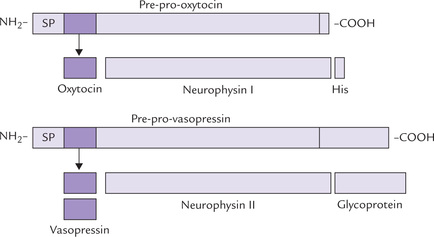
Both the posterior pituitary hormones are non-apep-tides with disulfide bonds, differing from each other by two amino acids only. They are actually synthesized (and packaged) in hypothalamus: oxytocin in supraoptic nucleus and ADH in paraventricular nucleus. From hypo-thalamus they travel as granules down the axons of hypothal-amohypophyseal tract into the posterior pituitary, where they are stored. In hypothalamus, both the hormones are produced as larger precursors: vasopressin as pre-pro-vasopressin and oxytocin as pre-pro-oxytocin (Fig. 30.6 ). The former contains a carrier protein neurophysin II, and likewise pre-pro-oxytocin contains neurophysin I. Upon reaching neurohypophysis they generate ADH and oxytocin by proteolysis.
Oxytocin
Biological activities of oxytocin are linked to parturition (child-birth) and expression of milk from breast. The hormonal release from posterior pituitary occurs in response to nipple stimulation (suckling). As with prolactin, suckling induces oxytocin secretion via the neural pathways. The other stimuli for oxytocin release are vaginal and uterine distention.
Functions
1. Uterine contraction: Oxytocin stimulates contraction of uterine smooth muscles in pregnant women at term. Blood oxytocin levels have been observed to increase just before parturition. Some synthetic analogues of oxytocin such as syntocinon and syntometrine are used to induce labour at term.
2. Milk ejection: Oxytocin stimulates contraction of the myoepithelial cells of breast, leading to milk ejection in response to suckling.
Oxytocin exerts its effects by binding to receptors on myometrial or myoepithelial cell membranes and initiating formation of cAMP or cGMP. The hormone appears to be metabolized by the liver and kidneys or the lactating mammary gland.
Vasopressin
Vasopressin is primarily concerned with the regulation of water balance in the human body. It stimulates water retention to increase blood volume and blood pressure.
Functions
Vasopressin stimulates water absorption from distal tubules and collecting ducts of kidneys. It interacts with specific receptors on epithelial cells, resulting in stimulation of adenylate cyclase and cAMP production. The latter promotes water reabsorption by making the cell membrane permeable to water. This is called facultative reabsorption of water.
Vasopressin helps maintain fluid balance of the body by adjusting the facultative reabsorption of water according to requirements of the body. For instance, when the osmolality of the blood is higher than normal, the osmoreceptors (of hypothalamus) sense the rise in osmolarity and induce vasopressin secretion. In addition, the patient experiences a thirst sensation, although a different set of osmo-receptors is involved in this response. The overall effect is to dilute the blood by increasing blood volume with water.
Another less significant effect of vasopressin is to cause peripheral vasoconstriction resulting in raised blood pressure.
Hypersecretion of ADH: In addition to tonicity and volume, ADH release may also be stimulated by nausea, pain, stress, various infections, and vascular and neoplastic disorders. Such conditions may result in syndrome of inappropriate ADH (SIADH), in which ADH is secreted continuously, resulting in excessive water reabsorption and decreased plasma osmolarity.
Hyposecretion of ADH: Deficiency of ADH, termed diabetes insipidus (DI), results from destruction of the posterior pituitary or hypothalamus secondary to neuro-surgical procedures, tumour, trauma or degenerative processes. Limitation of ADH secretion in DI results in increased water excretion to as high as 10 L/day, the normal being about 1.5 L/day.
Rarely, diabetes insipidus results from hereditary defect in receptor in renal tubules, when it is referred to as hereditary nephrogenic diabetes insipidus. Acquired form of nephrogenic diabetes insipidus is known to result from long term administration of lithium in treatment of depressive illness.
V Hormones of Thyroid Gland
Thyroid gland is the largest endocrine gland in humans, weighting about 20 g. It synthesizes two main hormones, triiodothyronine (T3) and tetraiodothyronine or thyroxine (T4). Calcitonin, a hormone involved in calcium homeostasis, is secreted by special cell types called para-follicular C-cells, or simply C-cells.
T3 and T4 are the only hormones in humans and higher animals that contain organically bound iodine (Fig. 30.7 ). They are necessary both in the long term regulation of the metabolic rate and in the regulation of growth, development and tissue differentiation.
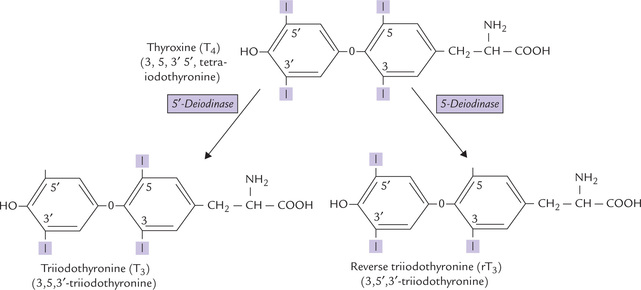
Fig. 30.7 Structures of thyroid hormones. T4, T3, and rT3. Approximately 80% of T4 is converted into equal amounts of T3 and rT3. Thyroxin is synthesized in largest amounts but T3 is biologically more active. rT3 is an inactive form.
A Biosynthesis of Thyroid Hormones
Site of Synthesis
Thyroxine (T4) is produced exclusively by the thyroid gland. T3 is produced by deiodination of thyroxine (Fig. 30.7). This process may occur in the thyroid gland, in target tissues or in other peripheral tissues (e.g. liver and kidneys). Deiodination of thyroxine may also occur at the inner ring to form biologically inactive reverse T3 (r T3).
Substrates for Thyroid Hormones
There are two important substrates for thyroid hormone synthesis: (i) thyroglobulin, the intrinsic substrate, and (ii) elemental iodide, the extrinsic substrate.
1. Thyroglobulin (Tgb): It is a homodimeric glycoprotein (MW 6,669,000) that is synthesized in the rough endoplasmic reticulum of the thyroid follicular cells and secreted (by exocytosis) into the follicular lumen, where it is stored in the extracellular colloid. It contains 134 tyrosyl residues, up to 40 of which become iodinated, but no more than 8 to 10 of these are processed to active hormones.
2. Elemental iodine: The extrinsic substrate iodine, in the form of iodide, is derived from dietary sources, such as drinking water, fish, cereals and iodinated salt. Daily requirement of iodine is 150-200 μg and plasma concentration of iodide is less than 0.2 μg/dL. But thyroid gland readily removes the ion from circulation and concentrates it intracellularly.
Steps in Synthesis
Step 1: Iodide Uptake
The follicular cells in the thyroid gland take up and concentrate iodide against a steep concentration gradient (about 20 : 1). It is an energy requiring step and is rate-limiting for the pathway of thyroid hormone synthesis. The uptake is mediated by a sodium-iodide symporter (an intrinsic membrane protein with approximately 14 transmembrane segments), and is controlled by TSH. From the follicular cell, iodide can enter the lumen of the thyroid follicule by facilitated diffusion.
Antithyroid drugs, such as thiocyanate and perchlorate inhibit iodide uptake (Fig. 30.8 ).
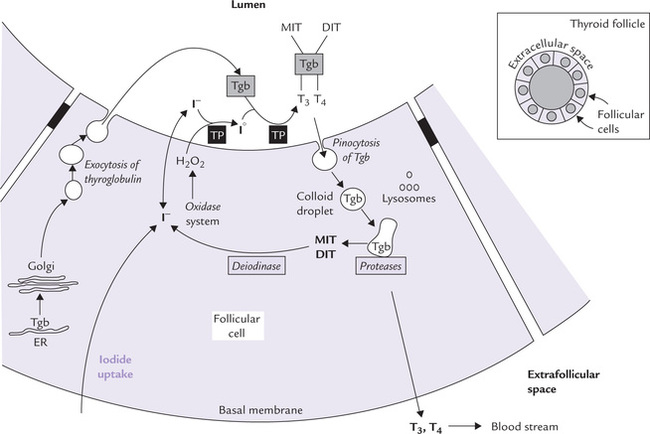
Fig. 30.8 Cellular mechanism for T3 and T4 synthesis and release into blood circulation. The follicular cells produce thyroid hormones, which are then stored in the lumen of the spherical follicle in a material called thyroid colloid. Tgb from these stores is degraded, forming thyroid hormones, which are finally released in blood circulation (Tgb = thyroglobulin, TP = thyroperoxidase).
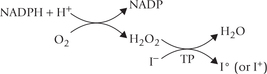
Step 2: Iodide to Iodine Oxidation
The iodide is oxidized by the enzyme thyroperoxidase (TP) to a more reactive form, iodine. The reaction requires hydrogen peroxide, which is supplied by an NADPH-dependent oxidase system, similar to that of leukocytes (Chapter 27).
Thyroid is the only tissue capable of oxidizing iodide (I-) to higher valence states (I+ or Io). The enzyme catalyzing this step, thyroperoxidase is a haem-containing enzyme that is bound to the apical (luminal) surface of the plasma membrane. It is a large tetrameric protein (MW 60,000), and its catalytic domain faces follicular lumen.
Antithyroid drugs such as thiourea, thiouracil and methimazole inhibit oxidation of iodide.
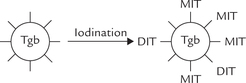
Step 3: Iodination of Tyrosyl Residues in Tgb
Thyroglobulin acts as a precursor for T3 and T4. It contains several tyrosyl groups to which the reactive iodine attaches; the process is referred to as organification of iodine. It requires hydrogen peroxide and catalytic action of thyroperoxidase. The tyrosyl residues are iodinated first at position 3 to form mono-iodo-tyrosine (MIT) and then at position 5 to form di-iodotyrosine (DIT).
Step 4: Coupling Reactions
Coupling of two iodotyrosyl residues results in the formation of a thyroid hormone (iodothyronine). When two DIT residues are thus coupled, formation of a tetra-iodothyronine (thyroxine or T4) residue results. One MIT residue may be coupled with a DIT to form a tri-iodothyronine (T3) residue.
Normally, about 99% of the hormone produced by the thyroid gland is T4.
B Storage and Release of Thyroid Hormones
The thyroid hormones synthesized in the follicular space are still attached to the Tgb molecule. Thus thyroid follicles store an appreciable amount of thyroid hormones, covalently bound to the amino acid sequence of Tgb. The thyroglobulin therefore is considered a storage form of T3 and T4 in the colloid, containing several weeks’ supply of these hormones.
When need for thyroid hormone arises, thyroglobulin enters follicular cells by pinocytosis, and undergoes proteolytic degradation by lysosomal proteases. This releases T3 and T4, which are secreted into blood circulation. The MIT and DIT residues that are also present in the Tgb molecule are deiodinated in the cell, and the released iodide ions are recycled (reutilized for hormone synthesis).
It is important to note that virtually all steps of thyroid hormone biosynthesis are promoted by TSH, including iodide uptake, thyroglobulin synthesis, iodination, coupling, and pinocytosis of thyroglobulin.
C Transport of T3 and T4
Thyroid hormones are transported bound to two specific binding proteins. More than 99% of T3 and 99.9% of T4 thyroxine-binding globulin (TBG; 54 kD) and thyroxine-binding prealbumin (TBPA) or transthyretin. The hormones first bind to TBG, and when it is saturated, to TBPA. Very small amounts are bound with albumin or remain in free form; the latter accounts for all biological actions of thyroid hormones.
T3 binds with proteins with less avidity than T4. About 99% of T3 and 99.9% of T4 are bound to a protein. Therefore, relative concentrations of free, unbound hormones are more balanced, even though the total plasma level of T4 is approximately 50 times higher than that of T3 (80 ng/mL versus 1.5 ng/mL).
The protein binding prevents rapid renal clearance of the hormones and also protects them from enzymatic attack. As a result, their biological half-lives are remarkably long: 6.5 days for T4 and 1.5 days for T3.
D Metabolic Fate of T3 and T4
Approximately 90% of the hormone released from the thyroid gland is T4, but part of it is converted to T3 by deiodination in target tissues by 5’-deiodinase. About two-third of the circulating T3 is derived not directly from the thyroid gland but rather by deiodination of T4 in peripheral tissues, especially the liver and the kidney. T3 is approximately four times more potent than T4, which supports the current theory that T4 is a pro-hormone for T3.
Alternatively, T4 may undergo deiodination in the inner ring to form biologically inactive reverse T3 (Fig. 30.7). Approximately 33% of the thyroxine that is secreted each day produces T3, whereas another 40% produces reverse T3.
The deiodination reactions release iodine that enters plasma and is recycled for new hormone synthesis.
E Regulation of T3 and T4 Synthesis
Synthesis and secretion of thyroid hormones are regulated by hypothalamoanterior pituitary—endocrine axis (Fig. 30.2). The regulation begins with the hypothalamus. TRH, a modified tripeptide is synthesized in the hypothalamus and transported via the portal circulation to the pituitary, where it promotes exocytosis of TSH. The secretion of TSH appears to be regulated by an interplay of negative feedback from circulating free T3 and T4, TRH, and an inhibitory neurotransmitter somatostatin. The negative feedback by thyroid hormones, in fact, occurs at both hypothalamic and pituitary levels. At the pituitary level, free T4 and T3 inhibit TSH secretion by decreasing both the biosynthesis and release of TSH through regulation of gene transcription and TSH glycosylation.
F Biochemical Functions of Thyroid Hormones
The thyroid hormones increase the metabolic activities in nearly all cells of the body. They diffuse through the plasma membrane and interact with the nuclear receptors. The hormone-receptor complex binds to regulator sequences in DNA (hormone responsive elements; HRE) to increase expression of specific genes. The protein thus synthesized mediates effects of the hormone. The major effects are as below:
1. Calorigenic effect or thermogenesis: Thyroid hormones cause uncoupling of oxidative phosphorylation, resulting in heat generation (i.e. calorigenic effect). There is swelling of mitochondria and basal metabolic rate is increased.
2. Metabolic effects: Thyroid hormones increase cellular metabolism. Initially they stimulate transcription and protein synthesis in various tissues, resulting in positive nitrogen balance. Glucose absorption from intestine and its utilization in various tissues is enhanced. Gluconeogenesis in liver and kidney, and lipolysis in adipose tissue are also increased, resulting in increased concentration of glucose and free fatty acids in plasma.
There is induction of the synthesis of 7α-hydroxylase, the regulated step of synthesis of bile acids from cholesterol. This effect may be related to the observed increase of the plasma cholesterol level in patients with hypothyroidism.
2. Physiological effects: Thyroid hormones are required for normal physical growth because they increasesynthesis of structural proteins (may stimulate GHsynthesis). Mental development also requires normallevels of iodothyronines. Other effects include increasein heart rate and cardiac output, rate and depth of respiration, gastrointestinal motility and secretion of digestive juices.
G Clinical Disorders of Thyroid Functions
Insufficient formation of thyroid hormones is known as hypothyroidism and overproduction is known as hyper-thyroidism or thyrotoxicosis. Both are very common in clinical practice affecting almost 3% of the population, and nine times as many women as men are affected. More than 95% of thyroid diseases originate in the thyroid gland, (mostly due to autoimmunity) and the rest are accounted by hypothalamic or pituitary causes.
Hypothyroidism
The commonest cause is failure of the thyroid gland known as primary hypothyroidism. In adults, the cause of primary hypothyroidism is often spontaneous autoimmune disease (Hashimoto’s thyroiditis) or destructive therapy for hyperthyroid states, but some cases are of unknown aetiology. Hypothalamic and pituitary causes of hypothyroidism occur regularly, often as a result of impaired TSH secretion secondary to pressure from an adjacent tumour.
If hypothyroidism occurs in adults, it leads to Myxoedema, with widespread subcutaneous oedema, decreased basal metabolic rate, sluggishness of mental activity and a broad range of non-specific symptoms, outlined in Case 30.1. The biochemical parameters used for specific diagnosis include measurement of serum T3, T4 and TSH. In addition, measurement of serum autoantibodies for thyroglobulin and thyroperoxidase are carried out.
In children, hypothyroidism has serious consequences with severe and irreversible mental deficiency, stunted growth and multiple physical deformities. This condition is called cretinism. Hypothyroidism is treated by oral administration of thyroxine.
Hyperthyroidism
The most common cause of hyperthyroidism is Graves’ disease, which is caused by an IgG auto-antibody known as LATS (long acting thyroid stimulator). It binds with the TSH receptor in the thyroid gland and stimulates them. Toxic multinodular goitre and toxic adenoma are other important causes of hyperthyroidism. Pituitary TSH secreting adenomas are known, but are extremely rare causes of hyperthyroidism.
The clinical features include weight loss, fatigue, weakness, sweating, palpitations, nervousness, etc. The typical biochemical laboratory parameters utilized for diagnosis and monitoring are T3, T4 and TSH estimations (Case 30.2).
Simple or Diffuse Goitre
This results from deficiency of iodine, and the affected patient may suffer from hypothyroidism or may remain euthyroid. Synthesis of T3 and T4 decreases and the TSH release from the pituitary is disinhibited, leading to overstimulation of the thyroid gland by TSH. Hypertrophy and hyperplasia of the follicular cells and accumulation of excess thyroglobulin in the follicular lumen results; this phenomenon is known as simple- or euthyroid-goitre. It is seen in many mountainous areas of the world, where the soil and the plants grown on it are often deficient in iodide. Use of iodized salt is, therefore, recommended in these regions.
VI Hormones of Adrenal Cortex
Adrenal glands lie in relation to the upper poles of the kidneys. Each gland consists of outer cortex and inner medulla, which are embryologically and histologically distinct from each other. The cortex comprises three layers— Zona glomerulosa, Zona fasciculata and Zona reticularis which secrete a number of steroid hormones (> 50). The adrenal medulla is part of the sympathetic nervous system. The medullary cells synthesize and store adrenaline, along with noradrenaline and dopamine.
Chemistry
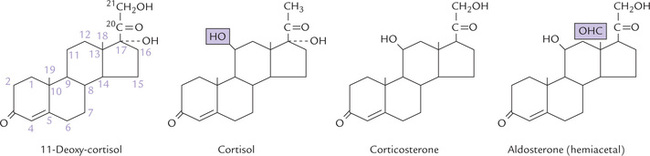
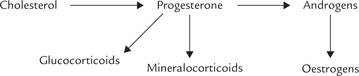
The three zones of adrenal cortex secrete different classes of steroid hormones. Zona glomerulosa, the outermost and the smallest, secretes mineralocorticoids, the C-21 steroids that principally affect water and electrolyte balance. Zona fasciculata, the middle zone produces glucocorticoids (C-21) which mainly affect carbohydrate metabolism, and also lipid and protein metabolism to a smaller extent. Androgens and oestrogens are also produced by this zone, but are quantitatively insignificant. Zona reticularis, the innermost zone, produces androgens (C-19) and oestrogens (C-18), and to a small extent, glucocorticoids also. Structures of some adrenal steroids are illustrated in Figure 30.9 .
Synthesis of Steroid Hormones
All steroid hormones originate from cholesterol (Fig. 30.10). Cholesterol is obtained either from endogenous synthesis or from LDL. On the basis of weight, adrenal cortex (and sex glands), has the highest rates of cholesterol synthesis in the body. Cholesterol esters are also stored abundantly in these cells. Hormonal stimulations by such trophic hormones as ACTH activate a cholesterol esterase that provides a quick supply of cholesterol (from cholesterol esters).
Steps involved in the biosynthesis of hormones or adrenal cortex are as follows:
Step 1: Cholesterol is first acted upon by desmolase, a mitochondrial side chain cleavage enzyme. It cleaves off a 6-carbon unit forming a 21-product, pregnenolone, which is the biosynthetic precursor of all other steroid hormones.
Step 2: Pregnenolone is converted to progesterone by microsomal and cytoplasmic enzymes, e.g. a dehydrogenase and an isomerase.
Step 3: Progesterone is further converted into glucocorticoids and mineralocorticoids by hydroxylations. These reactions are effected by monooxygenases which require cytochrome P-450 as an intermediate electron carrier.
The 17-hydroxylase and 21-hydroxylase are microsomal and 11-hydroxylase is mitochondrial. A summary of all hydroxylation reactions is given in Figure 30.10 .
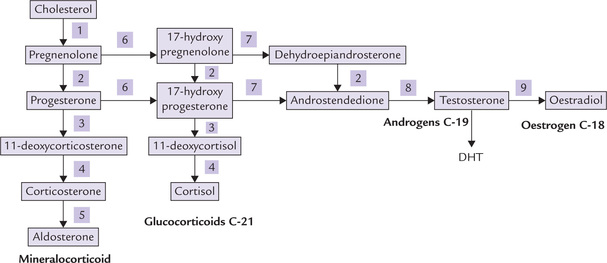
Fig. 30.10 Summary of steroid hormone biosynthesis. (1) Desmolase, (2) 3β-Hydroxysteroid dehydrogenase: Oxosteroid isomerase. (3) 21-Hydroxylase, (4) 11-Hydroxylase, (5) 18-Hydroxylase and 18-Hydroxydehydrogenase (6) 17-Hydroxylase, (7) 17—20 Lyase, (8) 17-Keto-reductase, (9) Aromatase.
Synthesis of all steroid hormones is stimulated by ACTH, which stimulates desmolase so that availability of pregnenolone is increased.
Transport, Metabolism and Excretion
Cortisol is transported by a specific a α1-globulin called cortisol-binding globulin (CBG) or transcortin. It binds approximately 70% of the circulating cortisol, another 20% is bound to albumin and the rest is transported in free form. The free form only is the biologically active fraction. Aldosterone is bound mainly to albumin.
Metabolism of steroid hormones occurs mainly in liver. The double bond in the ring is reduced, the keto group is also reduced, and the reduced compound is conjugated with glucuronic acid and to a smaller extent with sulphate. The conjugated compounds, termed 17-ketosteroids and 17-hydroxy steroids, are excreted in urine. A smaller amount is excreted in faeces.
Biochemical Functions of Adrenal Steroids
The C-21 corticosteroids, glucocorticoids and mineralocorticoids are potent metabolic regulators and immunosuppressants. Many of their biological effects can be understood best as adaptations to sustained stressful situations.
1. Effect on carbohydrate metabolism: As the name glucocorticoids suggests, these hormones have a major role on increasing glucose production by enhancing virtually every step in the gluconeogenesis. In the liver, synthesis of the enzymes of gluconeogenesis (pyruvate carboxylase, PEP carboxykinase, fructose bisphosphatase and glucose 6-phosphatase) is increased. In addition, protein breakdown in skeletal muscle and other extrahepatic tissues is increased, which supplies amino acid precursors for gluconeogenesis. Part of glucose 6-phosphate formed by gluconeogenesis is diverted into glycogen synthesis, and liver glycogen stores are increased.
Tissue uptake and metabolism of glucose is decreased by glucocorticoids. The net effect of increased production and decreased utilization is elevated blood glucose concentration.
2. Effect on lipid metabolism: Glucocorticoids enhance mobilization of adipose tissue triacylglycerols by inducing synthesis of the hormone-sensitive lipase. They also decrease lipogenesis in adipocytes; the net result is hyperlipidaemia.
3. Effect on protein metabolism: Protein and RNA synthesis are stimulated in the liver but inhibited in peripheral tissues (muscle), with protein breakdown products acting as gluconeogenic substrates. The glucocorticoid-induced protein and amino acid catabolism explains negative nitrogen balance in a seriously ill patient (chronic stress), and muscle wasting in any protracted disease.
4. Other effects: Several effects of glucocorticoids are elicited only when these hormones are present in high concentrations. They have a wide range of suppressant effect on humoral and cell-mediated immunity, and thus provide useful form of therapy as anti-inflammatory and anti-allergic agents. It is likely that some of the immunosuppressant effects are important in the control of immune response in normal physiology.
Glucocorticoids also influence heart vasculature, blood pressure, water excretion and electrolyte balance (sodium retention and potassium elimination). They also influence bone turnover through a variety of mechanisms, and the presence of high concentrations of gluco-corticoids for prolonged periods results in osteoporosis. Glucocorticoids can inhibit linear growth and cell division in several tissues. Finally, they decrease synthesis of eicosanoids by inhibiting phospholipase A2, and increase secretion of gastric juice.
Functions of the other C-21 corticosteroids (the mineralocorticoids) are described in a subsequent section.
Mineralocorticoids: The more important effects of mineralocorticoids is to promote tubular reabsorption of sodium ions and to increase secretion of potassium from renal tubular cells into the tubular fluid. The two effects are coupled, i.e. sodium is antiported in exchange for potassium. Mineralocorticoids also promote tubular reabsorption of chloride ions and tubular secretion of hydrogen ions.
Because sodium retention is accompanied by reabsorption of water, it results in expansion of the extracellular fluid volume. Mineralocorticoids also stimulate sodium conservation by the sweat glands and the mucosal cells of the colon, but in normal circumstances these effects are trivial.
Regulation of Synthesis and Secretion of Adrenal Steroids
Adrenocorticotropic hormone (ACTH) alters the rate of biosynthesis and secretion of the glucocorticoid cortisol from the Zona fasciculata and reticularis. ACTH also increases secretion of the adrenal androgens, and aldosterone to a lesser extent.
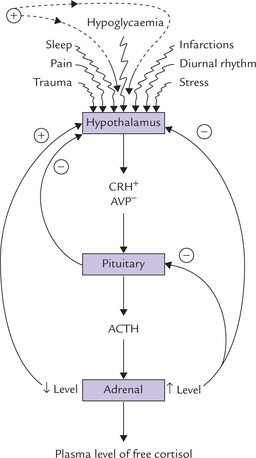
Four major mechanisms appear to control ACTH release and hence cortisol secretion, namely hypothalamic factors, negative feedback, diurnal rhythm and stress. These are illustrated in Figure 30.11.
• Hypothalamic factors, namely CRH and AVP have been discussed earlier.
• Negative feedback operates via inhibition of CRH and ACTH synthesis and secretion by high concentrations of plasma glucocorticoids (long loop). Conversely, reduction in level of plasma cortisol leads to an increased secretion of ACTH. The feedback effect occurs within two time frames: the fast and the slow-feedback.
• Cortisol secretion is controlled by an inherent diurnal rhythmicity of CRH secretion and consequently of ACTH release. The plasma cortisol level is about 10 times higher at 08.00 h than at 24.00 h.
• Stress, such as trauma, pain, apprehension, fever and hypoglycaemia act directly on hypothalamus. Thus, they can override the negative feedback mechanism and the diurnal rhythm to enhance cortisol secretion.
Renin-angiotensin system:
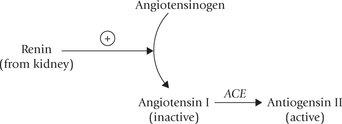
Aldosterone, the most important mineralocorticoid, is primarily controlled by reninangiotensin system (though other factors, including ACTH also regulate aldosterone synthesis). The major stimuli for this system are hypovolaemia, hyponatraemia and renal ischaemia. Specialized cells in the juxtaglomerular apparatus of the nephron sense these changes and respond by secretion of renin, a protease. This enzyme cleaves off a decapeptide from the amino terminal of a plasma protein of liver, termed angiotensinogen. This decapeptide termed angiotensin I is biologically inactive. It is processed to biologically active angiotensin II by angiotensin converting enzyme (ACE), an endothelial enzyme in lung capillaries.
Angiotensin II stimulates aldosterone secretion from the Zona glomerulosa cells. It also has powerful hypertensive effects: it contracts vascular smooth muscle directly and indirectly by enhancing the release of norepinephrine from sympathetic nerve endings. ACE inhibitors (enala-pril, lisinopril) are used in treatment of hypertension.
Finally, angiotensin II is degraded into inactive fragments by angiotensinases released from vascular epithelium.
Dysfunctions of Adrenal Cortex
Decreased hormonal secretions by adrenal gland may result in hormonal deficiency (hypoadrenocorticism) or excess (hyperadrenocorticism).
Hypoadrenocorticism: Hypoadrenocorticism is a rare but life-threatening condition. It may arise in hypotha-lamic-, pituitary-, or adrenal-failure.
Addison’s disease or primary adrenal insufficiency is caused by the destruction of adrenal cortex. The most common causes of Addison’s disease are autoimmune destruction of the gland and tuberculosis in that order. Production of both glucocorticoids and mineralocorticoids is decreased.
• Deficient glucocorticoids production leads to hypoglycaemia between meals. Mobilization of fats is also decreased, resulting in decreased availability of fuels and energy. This leads to weakness and inability to withstand stress.
• Decreased mineralocorticoid production results in renal loss of sodium (so hyponatraemia) and retention of potassium (so hyperkalaemia). Acidosis develops due to decreased excretion ofhydrogen ions. Pathological sodium loss shrinks the extracellular fluid volume, causing hypotension.
Hyperpigmentation is a characteristic feature of Addison’s disease and distinguishes it from adrenal insufficiency secondary to pituitary or hypothalamic disease. It occurs because of loss of negative feedback on pituitary and consequent increased production of ACTH. The structure of this hormone contains part of the amino acid sequence of melanocyte-stimulating hormone. The latter is responsible for skin pigmentation (POMC), and so hyper-pigmentation occurs; common sites are face, neck and dorsum of hands.
Acute adrenal insufficiency is a life-threatening medical emergency and needs prompt treatment with cortisol replacement and fluids.
Hyperadrenocorticism: Hyperfunction of adrenal cortex can result in three distinct syndromes: Cushing’s syndrome, primary aldosteronism and adrenogenital syndrome, which respectively result from excess of cortisol, aldosterone and adrenal androgens.
Cushing’s syndrome: It results from excessive endogenous secretion of cortisol, the sources of which may be classified into ACTH-dependent (pituitary overactivity) and ACTH-independent (primary adrenal disease). Benign adenoma and carcinoma comprise majority of the primary adrenal causes. The clinical features associated with Cushing’s syndrome are summarized in Table 30.1 and laboratory diagnosis is discussed in Chapter 34 (see Case 34.1).
Table 30.1
Clinical features of Cushing’s syndrome
| Feature | Finding in Cushing’s syndrome |
| Fat metabolism | Central obesity, buffalo hump, supraclavicular fat pad. Atherosclerosis, hyperlipidaemia |
| Protein catabolism | Proximal myopathy and muscle wasting. Thin skin, easy bruising, wide purple striae. Poor wound healing |
| Carbohyd rate metabolism | Hyperglycaemia |
| Electrolyte balance | Hypertension, polyuria, lower limb-oedema |
| Androgen excess | Hirsutism, acne, amenorrhoea in females |
| Effect on bone | Osteoporosis. Growth failure in children |
| Neurological | Psychiatric and personality disorders |
The other syndromes of hyperfunction of adrenal cortex are described in Box 30.2.
VII Hormones of Adrenal Medulla
The hormones of adrenal medulla are synthesized from tyrosine. Chemically, they are categorized as catecholamines because their aromatic residue is catechol (Fig. 30.12 ). Several catecholamines are synthesized in the chromaffin cells of the adrenal medulla, of which epinephrine is most predominant. The others, norepinephrine (the term “nor” denotes that the molecule does not contain “R” or methyl group) and dopamine are intermediates in epinephrine biosynthesis. Dopamine acts largely as a brain neurotransmitter, and noradrenaline and adrenaline are released both from chromaffin cells and from peripheral and central neurons.
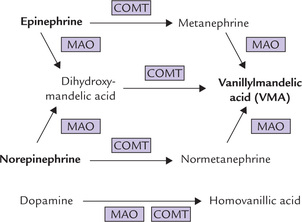
Fig. 30.12 Alternative pathways for enzymatic inactivation of catecholamines (MAO = monoamine oxidase, COMT = catechol-O-methyltransferase).
Synthesis, Storage and Secretion
The synthesis of catecholamines from the common precursor tyrosine tyrosine has been discussed earlier in Chapter 13. Synthesis of adrenaline requires four enzymatic steps, while two and three steps suffice for dopamine and noradrenaline respectively (Fig. 13.23). Apart from adrenals, the catecholamines are synthesized in some extra-adrenal tissues, e.g. brain and sympathetic ganglia. Blood brain barrier is impermeable to norepinephrine and epinephrine, but permeable to dopamine. The dopamine synthesized in adrenals crosses the blood-brain barrier to enter the brain cells where it acts as a neurotransmitter.
Catecholamines are stored in adrenal medulla in the chromaffin granules. Their release is triggered by (a) splanchnic nerve impulses and (b) by other stress hormones, namely corticosteroids.
Metabolism
The enzymatic inactivation of catecholamines occurs both in liver and in target cells. Two enzymes are involved in the process: monoamine oxidase (MAO) and catechol O-methyltransferase (COMT).
• MAO, a copper-containing flavoprotein of the outer mitochondrial membrane, inactivates its substrates by oxidative deamination.
• COMT methylates one of the phenolic hydroxyl groups in its substrates, thereby greatly reducing their biological activities. Its action depends on the presence of S-adenosylmethionine (SAM), a carrier of methyl residues.
Both MAO and COMT have broad specificity and the order in which they act is immaterial (Fig. 30.12) because the end product is same: vanillylmandelic acid (from epinephrine and norepinephrine). Likewise, homovanillic acid is the end product of dopamine catabolism.
Biochemical Functions
Epinephrine is a stress hormone: stress means an environmental insult. Lowering of blood glucose, oxygen deprivation, confinement in a small or crowded quarter or participation in a marathon is to name a few causes of stress. Stress causes release of epinephrine, and to a lesser extent of ACTH and glucocorticoids. It has major effect on
• metabolic pathways (stimulation of glycogenolysis, glyconeogenesis, lipolysis). These effects are mediated via cAMP.
• smooth muscle function (causes relaxation), and on blood pressure (increases), and on blood coagulation (decreases the time of clotting by aggregating platelets).
In response to epinephrine, a sudden extra supply of glucose is delivered to muscle; the heart and lungs work harder to pump oxygen round the circulation; and the body is thus prepared to exert or to defend itself. These are typical features of the “flight or fight” response.
Abnormalities of Catecholamine Production
Pheochromocytomas are the tumours of the adrenal medulla or sympathetic ganglia that produce and release large quantities of catecholamines. Majority of these tumours are located in the adrenal medulla and produce both epinephrine and norepinephrine. Tumours in extra-adrenal locations produce only norepinephrine.
Excessive production of catecholamines causes increased cardiac output and peripheral vasoconstriction, leading to severe hypertension. Diagnosis is made by demonstrating enhanced excretion of vanillylmandelic acid (VMA) and the metanephrines in 24-hour urine.
In neuroblastoma, a pediatric tumour, free dopamine and homovanillic acid are highly elevated in urine. The latter is also increased in the urine of patients with ganglioblastoma.
The Receptors for Epinephrine and Norepinephrine
They are called adrenoreceptors. They are divided into α- or α-receptor classes and subclasses on the basis of their pharmacology. Epinephrine acts on all classes of the receptors, but norepinephrine is more specific for β-receptors. The β -blockers, such as atenolol, are used to treat hypertension and chest pain (angina) in ischaemic heart disease because they antagonize the stimulatory effects of catecholamines on the heart. Non-specific a-blockers have limited use although the more specific α1-blockers such as prazosin and α2-blockers such as clonidine can be used to treat hypertension. Certain subclasses of β -receptors are found in particular tissues; for example, the β2-receptor is present in lung, and β2-receptor agonists, such as salbutamol, are therefore used to produce bronchial dilatation in asthma without stimulating the β1-receptor in the heart.
VIII Gastrointestinal Hormones
A number of peptide hormones are released by specialized cells lining the stomach and small intestine (Fig. 26.1), which play important role in food digestion and absorption. The cells secreting these hormones are not organized into any discrete anatomical structure, but are dispersed throughout the GIT. A large number of hormones have been identified, but the most important ones are gastrin, secretin, cholecystokinin and gastric inhibitory peptide (GIP).
Gastrin
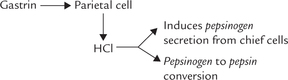
It is a peptide of 17 amino acids (MW 17,000) secreted by the G cells of stomach antrum in response to the ingestion of food. Acetylcholine and vagus nerve also stimulate its secretion. Gastrin migrates via the bloodstream to the fundic region of the stomach, which contains parietal cells and chief cells. The former are induced (by gastrin) to secrete HCl, which induces the chief cells to secrete pepsinogen (proenzyme of pepsin). The same HCl then catalyzes pepsinogen to pepsin conversion by removing of a 42 amino acid peptide from it. Pepsin initiates protein digestion in stomach. Its optimum pH generated by HCl is less than 2.
The effects of gastrin are inhibited by various agents, such as the gastric inhibitory peptide (GIP) and vasoactive intestinal peptide (VIP), produced by small intestine.
Secretin
A small peptide of 27 amino acids (MW 3000), secretin was the first hormone to be discovered. It is secreted by the S cells of the duodenum in response to the acidic gastric contents entering the small intestine. It migrates to the pancreas, where it induces release of a bicarbonate-rich solution into the small intestine via pancreatic duct. Function of bicarbonate is to elevate pH of the acidic gastric contents to 7.0-7.5, which is the optimum pH for action of intestinal enzymes.
Other important functions of secretin are to potentiate action of cholecystokinin on pancreas, and to delay gastric emptying.
Cholecystokinin
Cholecystokinin (CCK) or cholecystokinin-pancreozymin (CCK-PZ) contains 33 amino acids (MW 4000), and is secreted by the mucosa of small intestine in response to entry of the food in the intestinal lumen. Products of protein and lipid digestion, namely peptides, amino acids, mono- or diacylglycerol, fatty acids and glycerol stimulate its secretion.
CCK causes contraction of gall bladder to release bile into the small intestine via the bile duct and causes the release of zymogens by the pancreas. The zymogens are later activated in the small intestine to active enzymes, and participate in food digestion (Chapter 26).
Secretin apparently potentiates the action of CCK on pancreas.
Gastric Inhibitory Peptide (GIP)
GIP is made up of 43 amino acids (MW 5100), and it is secreted by duodenal mucosa in response to carbohydrates and fats entering the duodenum. It inhibits gastric motility and secretion, and also stimulates release of insulin from pancreas. The latter effect explains the observation that insulin level is elevated in plasma before any substantial increase in blood glucose.
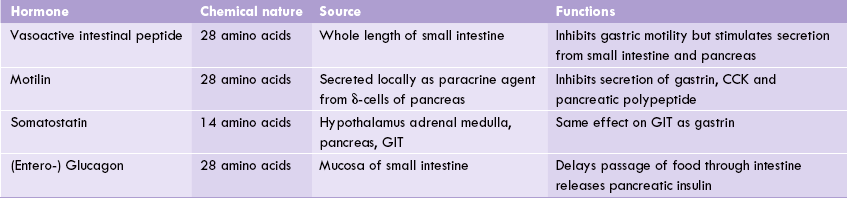
Other gastrointestinal hormones are VIP, motilin, somatostatin and entero glucagon. Their important characteristics are shown in Table 30.2 . They are broadly divided in two homology groups based on their common structural characteristics (Box 30.3).
Pancreatic Hormones
The hormone-secreting, endocrine portion of pancreas is the islet of Langerhans, which contains α, β, δ, and F cells. Insulin and glucagon are perhaps the most influential intermediary metabolism regulating hormones; insulin is released from the β -cells in response to high blood glucose levels (e.g. after a meal) and glucagons from a-cells in response to low blood glucose levels. The δ-cells produce somatostatin and F cells secrete pancreatic polypeptide.
All these pancreatic hormones are peptides and not under any major pituitary, hypothalamic, or neural control.
Insulin
This hormone has profound effect on several aspects of intermediary metabolism, as discussed in appropriate places of Chapter 9 through 15. Its structure, biosynthesis, regulation, and interaction with insulin-receptor were described earlier (Chapter 29) and a detailed account of its role in integration of metabolism and metabolic abnormalities in insulin deficient state (diabetes mellitus) was presented in Chapter 15.
Glucagon
It is a peptide of 29 amino acids (MW 3500), synthesized in a-cells of pancreas, and a small amount originating from the gastrointestinal tract. Major function of glucagon is to maintain blood glucose. It tends to elevate blood glucose by moving glucose from the cells into the bloodstream by stimulating those metabolic processes that give rise to free glucose, e.g. glycogen degradation and gluconeogenesis (see Table 15.2). Glucagon is also associated with enhanced protein degradation. In addition, it stimulates the breakdown of storage fat, whose function is to provide cellular fuel and thus spare glucose. Thus, glucagon is considered as a catabolic hormone.
Regulation of glucagon secretion is affected by blood glucose concentration, being enhanced by hypoglycaemia and inhibited by hyperglycaemia.
Pancreatic Polypeptide
It is a peptide of 36 amino acids, secreted from F-cells of islets of Langerhans in response to protein-rich meal, hypoglycaemia and exercise. It decreases the bicarbonate and protein contents of pancreatic juice.
Motilin
It is a peptide structurally similar to hypothalamic somatostatin, and is secreted locally as a paracrine agent by the δ-cells of pancrease (Table 30.2).
IX Hormones of Gonads
Sex hormones are secreted by gonads (testes in males, ovaries in females) in response to pituitary FSH and LH (Fig. 30.2). In addition, the gonads are also involved in production of germ cells, and this function is influenced by sex hormones.
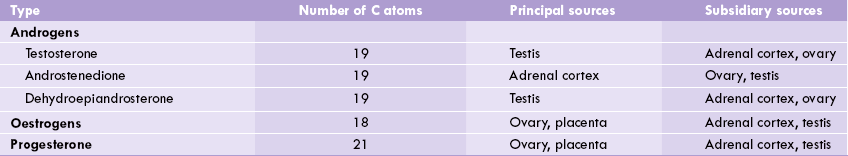
• The ovarian follicular cells secrete C-18 oestrogens, C-19 androgens and C-21 progesterone (Table 30.3 ).
• The Leydig cells of testes secrete testosterone, a C-19 androgen. A small amount of oestrogens are also formed in testes: oestradiol by aromatization of testosterone and oestriol by aromatization of andro-stenedione, a weak androgen produced in adrenal cortex.
• Zona fasciculata and Zona reticularis of adrenal cortex produce weak androgens, which are converted to more potent sex-hormones. The conversion may occur in gonads or in some non-endocrine tissues, such as liver and adipose tissue.
Sex hormones can be divided in three groups (Table 30.3): (a) androgens, the male sex hormones (C-19); (b) Oestrogens, the female sex hormones (C-18); and (c) progesterone (C-21), produced during menstruation and pregnancy.
X Androgens
These are mostly produced by the Leydig cells of testes, though a smaller amount originates from the adrenal glands (Table 30.3). The theca cells at periphery of the ovarian follicles also produce a small amount of androgens.
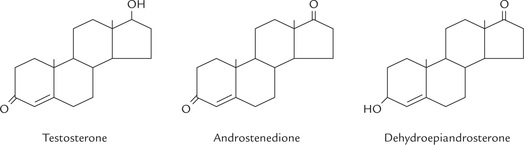
Testosterone is the major androgen synthesized in testes, its plasma level is about 0.7 μg/dL in males and < 0.1 μg/dL in females. Structures of some important androgens are given in Figure 30.13 .
Synthesis
Cholesterol is the biosynthetic precursor of all steroid hormones, including androgens.
Pregnenolone, initially formed from cholesterol, is converted to progesterone by microsomal and cytoplasmic enzymes, which is converted to androstenedione by 17-20 lyase or desmolase (Fig. 30.10). Androstenedione is finally converted to testosterone by a ketoreductase. Alternately, pregnenolone is converted to 17-hydroxy-pregnenolone, which forms testosterone by a similar set of reactions.
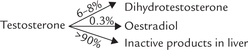
Testosterone is a pro-hormone:
In the target tissues and, to a lesser extent in the testis itself, testosterone is converted to dihydrotestosterone by the enzyme 5-α-reductase. The plasma level of DHT is only about 10% of the testosterone concentration, but DHT is considerably a more potent androgen than testosterone. Therefore, it is a widely acknowledged that testosterone acts as prohormone of the active hormone DHT.
Transport, Metabolism and Excretion
Testosterone circulates in blood in association with two proteins: sex hormone-binding globulin (SHBG) and testosterone-oestrogen-binding globulin (TEBG). Both these proteins are formed in the liver. Only small percentage of testosterone is in the free state, but it is responsible for the biologic activity of the hormone. The liver mainly metabolizes androgens to 17-ketosteroids, which are excreted in urine.
Biochemical Functions
Secretion of androgens is minimal prior to onset of puberty. At puberty, the testes start secreting about 7000 μg of androgens each day into circulation and a further 500 μg originates from the adrenal gland.
Androgens induce development of primary and secondary sexual characteristics and also affects many specific physiological and metabolic activities:
1. Effect on sexual characteristics: Androgens cause growth and development of male internal genitalia. They stimulate differentiation of the wolffian duct system into the epididymus, vas deference and seminal vesicles. These are the primary sexual characteristics. During puberty, testosterone promotes appearance of secondary sexual characteristics, such as deepening of voice and masculine distribution of body hair, libido and potency (erectile functions). It increases secretory activity of sebaceous glands, which may lead to acne.
2. Spermatogenesis: Testosterone, in association with FSH is required for normal spermatogenesis. FSH promotes secretion of ABP. This ensures high local concentration of testosterone, which is necessary for spermatogenesis in seminiferous tubules (Fig. 30.5).
3. Effect on nitrogen balance: Androgens promote RNA synthesis (transcription) and protein synthesis (translation), thereby causing positive nitrogen balance. Stimulation of protein synthesis increases muscle mass ( myotrophic effect) in a wide range of tissues. In this capacity testosterone is the natural anabolic steroid.
4. Effect on carbohydrate and fat metabolism: Production of D-fructose from D-glucose in seminal vesicles is increased by androgens.
The androgens induce synthesis of aldolase, thereby enhancing glycolysis.
5. Effect on electrolytes: Androgens increase renal retention of sodium, chloride and water, though to a much smaller extent than mineralocorticoids.
6. Effect on bones: Androgens promote formation of bone matrix proteins. Calcium deposition is increased because of increased availability of bone matrix. Epiphyseal fusion of long bones is enhanced.
7. Others: Basal metabolic rate is increased by androgens. Erythropoiesis is stimulated through stimulation of erythropoietin secretion.
Mechanism of Action and Regulation
Testosterone binds with the nuclear receptors found in muscle, bone and other tissues where it exerts its influence. The testosterone receptor complex activates specific genes, the protein products of which mediate some (if not all) of the effects of the hormone.
The affinity of DHT for these receptors is much higher than that of testosterone. Thus, testosterone is unique among steroid hormones in that further metabolism, 5α reduction, more than doubles its affinity for androgen receptors (Case 30.2).
Synthesis and secretion of androgens is regulated by hypothalamo-pituitary-gonadal axis, described earlier.
Clinical Disorders of Androgen Secretion
Hypogonadism:
Androgen deficiency may originate from a wide range of disorders of the hypothalamus, pituitary or testes. In hypothalamic disorders, deficient production of GnRH results in delayed puberty, and subnormal FSH, LH, and testosterone. Similar picture is seen in pituitary disorders, and together with hypothalamic disorder they are classified as secondary hypogonadism. In primary hypogonadism, there is failure of testes to produce testosterone.
XI Oestrogens
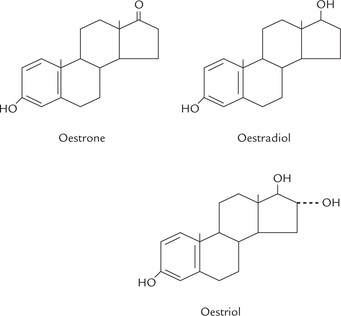
The oestrogens are a family of hormones synthesized in ovarian and extra-ovarian tissues. The three main oestrogens are oestrone, oestradiol and oestriol (Fig. 30.14 ). The first two are predominantly ovarian hormones; and all three are synthesized by placenta. Oestrogens are produced in males also: small quantity comes from testis, but most is produced in adipose tissue, liver, skin, brain and other non-endocrine tissue.
Biosynthesis
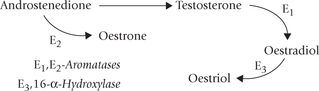
Like other steroid hormones, cholesterol serves as the precursor for synthesis of oestrogens (Fig. 30.10). Oestrogens contain an aromatic ring, which is produced by aromatization (formation of aromatic ring) of androgens-androstenedione and testosterone.
The aromatization reaction is catalyzed by the microsomal enzyme system, aromatase in a complex process that involves three hydroxylation steps, each of which requires O2 and NADPH. Oestradiol is formed if the substrate is testosterone, whereas oestrone production results from aromatization of androstenedione (this occurs in extraovarian tissues).
Oestradiol (E2) is the main oestrogen secreted by the Graafian follicles of ovary, and is biologically the most active form. About 90% of the secreted oestradiol comes from ovaries. Oestrone, a weak oestrogen, is the predominant plasma oestrogen in post-menopausal women. Oestriol is the predominant oestrogen during pregnancy, secreted by the placenta. In non-pregnant women, oestriol is formed in small amounts in the liver from oestradiol and oestrone.
In males significant amounts of oestrogens are produced by peripheral aromatization of androgens. Peripheral aromatization of testosterone to oestradiol accounts for 80% production rate of the latter, and testes account for < 20%.
Transport and Metabolism
Oestrogens are transported in blood bound to SHBG. Affinity of oestradiol for SHBG is less than that of testosterone or DHT. Oestrogens can undergo conjugation with glucuronide or sulphate; the conjugation renders them water soluble so that they can be excreted in urine.
Functions
1. Effect on sexual organs: Oestrogens mediate the growth and development of maternal reproductive organs, especially the gravid uterus. They play important role in maintenance of menstrual cycle. Development of secondary sex characters is also induced by oestrogens: breasts are enlarged and hair develop in pubic and axillary regions.
2. Effect on nitrogen balance: Oestrogens promote transcription and translation, and so nitrogen balance becomes positive under their influence.
3. Effect on bones: Oestrogens enhance osteoblastic activity to promote skeletal growth and calcification, which accounts for growth spurt during puberty. The decalcification and osteoporosis developing in post-menopausal women is accounted for by lack of oestrogens.
4. Effect on electrolytes: Renal retention of sodium, chloride and water is enhanced by oestrogens. This effect is much weaker compared to that of mineralo-corticoids.
5. Cardioprotective effects: Oestrogens lower total cholesterol and LDL, and also increase HDL level, thereby improving lipid profile. This explains low incidence of coronary artery disease and atherosclerosis in women of reproductive age group.
6. Effect on fat deposition: Oestrogens increase fat deposition in adipose tissue, and this may partly explain increased fat deposition (5-10%) in women compared to males of the corresponding age group.
7. Oestrogens activate the enzyme transhydrogenase, which catalyzes transfer of reducing equivalents from NADPH to NAD+. In post-menopausal women this reaction being impaired, the NADPH tends to accumulate and is diverted towards lipogenesis causing weight gain and obesity.
Clinical Disorders of Oestrogen Secretion
1. Primary hypogonadism: This may be due to gonadal agenesis or polycystic ovary, both leading to deficiency in ovarian function.
2. Secondary hypogonadism: This is due to hypothalamic or pituitary disorders. For example, a genetic hypothalamic disorder in children (Kallmann’s syndrome) results in deficient GnRH production, and the affected individuals present with delayed puberty and subnormal FSH, LH and testosterone.
3. Hypergonadism: Early maturation of the normal hypothalamo-pituitary-gonadal axis, an oestradiol-or androgen-secreting cyst or tumour of the ovary or the adrenal gland, or congenital adrenal hyperplasia are the common causes of hypergonadism. They cause precocious puberty in which sexual characteristics and reproductive potential develop prematurely.
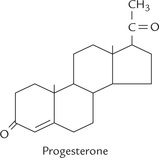
XII Progesterone
Progesterone is a 21-carbon compound, with a steroid nucleus (Fig. 30.15 ). It is synthesized from cholesterol by side chain cleavage, and acts as precursor for steroid hormones (Fig. 30.10). The progesterone released into blood circulation originates from corpus luteum in non-pregnant women and from placenta in pregnancy.
Transport and metabolism: Progesterone is transported in blood bound with a protein, transcortin. The major metabolite of progesterone is a pregnanediol, which is excreted in urine as a glucuronide conjugate.
Biochemical Functions
1. Progesterone promotes secretory changes in the endometrium in the second half of the menstrual cycle (after ovulation), and induces secretion of mucous. This is essential for implantation of the fertilized ovum.
2. Together with oestrogen, progesterone stimulates growth of glandular tissue in breasts.
3. Progesterone has a mild thermogenic effect: it elevates body temperature by 0.5-1°F. This explains mild rise in body temperature with ovulation. The cause of thermogenesis by progesterone is not clearly known.
4. In high doses, progesterone elicits mild mineralocorticoid effect, causing renal retention of sodium, chloride and water.
Regulation of Female Sex Hormones
The hypothalamic GnRH causes release of FSH and LH from anterior pituitary, which induce secretion of oestradiol (Fig. 30.2). The hormones in females are secreted in a cyclic manner during each menstrual cycle.
Menstrual Cycle
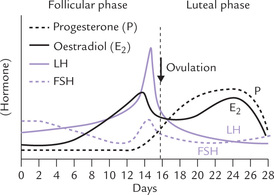
The hormonal changes occurring during a normal menstrual cycle well illustrates how various hormone functions are coordinated to achieve a common cause (Fig. 30.16 ). It is now clear that the pulsatile release of GnRH is ultimately responsible for the control of all hormonal changes in the normal menstrual cycle. A typical cycle has a length of 28 days (varies between 25 and 31 days). It is divided into two phases: the follicular phase (pre-ovulatory) and the luteal phase (post-ovulatory). The length of the post-ovulatory period is remarkably constant at approximately 14 days.
1 Follicular phase
FSH is the dominant hormone of this phase, its level showing progressive rise under the influence of GnRH. FSH acts through its receptors in the ovarian granulose cells and causes the development of ovarian follicles. FSH may also enhance the production of oestradiol (from testosterone) by inducing aromatase. As oestradiol is secreted, FSH secretion falls (by feedback inhibition), and this combination leads to selection of a dominant follicle for further development. Initially several follicles undergo development but only a single (dominant) follicle is selected for further maturation and becomes mature or Graafian follicle.
Development of the dominant follicle continues to progress under the influence of rising oestradiol concentration, and this is turn results in more secretion of oestradiol. When oestradiol level reaches its peak (around midcycle), it causes a positive feedback at the pituitary to initiate LH surge. The LH binds to its receptor on the Graafian follicle and, in tandem with steroid hormones and other factors such as prostaglandins, causes the mature follicle to rupture and release the oocyte. This process is called ovulation. Approximately 14 days are required to produce this mature follicle and to cause its rupture; and these 14 days correspond to the follicular phase of the menstrual cycle.
To summarize, in the follicular phase FSH promotes oestradiol synthesis leading to follicular maturation, while LH causes the final follicular growth and ovulation.
2 Luteal phase
After the ovulation, the Graafian follicle remains in the ovary and undergoes a change and becomes known as the corpus luteum. The latter is the site of steroid secretion from ovary during the luteal phase. It secretes progesterone and lesser amounts of oestradiol; both these hormones sustain the oocyte. Progesterone also serves to prepare the uterus for pregnancy and the lobules of the breast for lactation. Increased level of progesterone may serve in a negative feedback mechanism to decrease the amount of LH released from the anterior pituitary.
If fertilization of the ovum does not occur after ovulation, corpus luteum function declines, resulting in a decrease in progesterone and oestradiol secretion. This brings about vascular changes in the endometrium leading to tissue death and shedding of endometrium, causing menstruation. Fourteen days are required for the process to be completed after ovulation; this period is the luteal phase of the cycle.
The fall in steroid secretion also stimulates FSH secretion to start a new cycle.
Menopause
The menstrual cycle begins at the onset of puberty and continues throughout the active reproductive life. Women normally reach the menopause at the age of 45-50 years, when the cycles cease to occur and this coincides with loss of ovarian function. In post-menopausal women, oestrogen and progesterone levels fall, but because of loss of their negative feedback effect, LH and FSH levels rise. Following with cessation of regular menstrual cycles:
1. Vasomotor symptoms: These are the symptoms related to contraction and dilation of small blood vessels (e.g. flushing), that occur due to oestrogen deficiency.
2. Osteoporosis: It may occur in long-term postmenopausal oestrogen deficiency because of bone loss.
3. Others: Atorphy of the sex tissues and increased risk of cardiovascular disease are the other complications.
Exercises
Essay type questions
1. Explain the arrangement of hypothalamus-pituitary hormone system and discuss function of hypothalamic release and release-inhibiting factors.
2. Discuss the chemical nature, biosynthesis and functions of steroid hormones and explain biochemical basis, and signs and symptoms of diseases associated with their over and underproduction.
3. Discuss chemical and physiologic properties, and actions of the hormones involved in digestion and metabolism of food substances. What controls blood levels of these hormones.
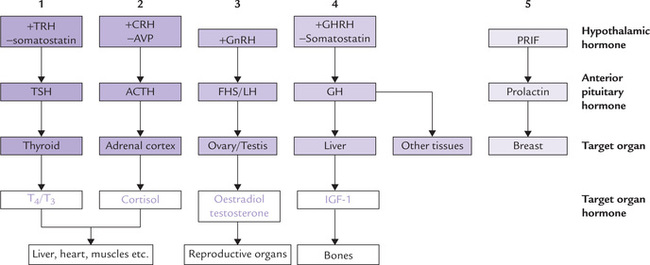
 The release, and in some cases production, of each of the pituitary hormones is under tonic control of at least one hypothalamic release or release-inhibiting hormone.
The release, and in some cases production, of each of the pituitary hormones is under tonic control of at least one hypothalamic release or release-inhibiting hormone.