Calcium And Phosphate: Metabolism And Regulation
Calcium and phosphate are the most abundant minerals in bones where they exist in form of crystal lattice. Nearly 99% of the total body calcium and 85% of the total body phosphate are present in these crystals of hydroxyapatite; the remainder are distributed in soft tissues, teeth and extracellular fluid (ECF). The plasma pool of these elements is relatively smaller (Ca = 9–11.5 mg/dL and phosphorous, largely as 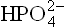 amounts to 2.7–4.5 mg/dL), but a multitude of cell and organ functions are dependent on the tight control of their concentrations in plasma. Under normal circumstances, small amounts of calcium, and presumably phosphate, are exchanged daily between bone and plasma as a result of constant bone remodeling, i.e. coupled processes of bone resorption by osteoclasts and bone formation by osteoblasts. The exchange process is part of a complex homeostatic mechanism which utilizes bone as a reservoir of calcium when deficiency exists, and as a store of calcium when the body is replete. It requires interplay of a number of regulatory factors, including hormones such as parathormone, calcitonin and calcitriol.
amounts to 2.7–4.5 mg/dL), but a multitude of cell and organ functions are dependent on the tight control of their concentrations in plasma. Under normal circumstances, small amounts of calcium, and presumably phosphate, are exchanged daily between bone and plasma as a result of constant bone remodeling, i.e. coupled processes of bone resorption by osteoclasts and bone formation by osteoblasts. The exchange process is part of a complex homeostatic mechanism which utilizes bone as a reservoir of calcium when deficiency exists, and as a store of calcium when the body is replete. It requires interplay of a number of regulatory factors, including hormones such as parathormone, calcitonin and calcitriol.
Calcium and phosphate homeostasis and various other aspects of metabolism of these minerals are described in this chapter. After going through the chapter, the student should be able to understand:
I Calcium
Of all nutritionally important minerals, calcium occurs in largest amount in the human body: a 70 kg adult male contains 1.0–1.4 kg (25–33 g/kg of fat-free tissue) of calcium. Over 99% of the total body calcium is present in bones and teeth, and about 1% in various body fluids. Extracellular calcium represents physiologically active fraction being involved in a number of critical functions.
A Nutritional Requirement and Sources
Daily dietary requirement of calcium is about 400–500 mg. This replaces the daily loss of 300–400 mg calcium in urine and an additional loss in faeces and sweat. In the growing age group (12–20 years), about 1200 mg per day is required.
It has been recommended that the daily intake of calcium in the post-menopausal women must be higher (around 1500 mg) since they are at risk of developing osteoporosis, a condition characterized by loss of bone organic matrix as well as progressive demineralization.
Several other factors influence the dietary requirement. For example, vitamin D is required for the optimal utilization of calcium, and therefore, adequate supply of this vitamin decreases the dietary requirement of calcium. Excess dietary proteins, on the other hand, may upset calcium balance by causing rapid excretion of this element. Exercise increases efficiency of calcium utilization. The calcium balance studies carried out in Peruvian Indians, who have extensive exposure to sunlight, perform adequate exercise and subsist on low protein, vegetarian diet, indicate a need of only 300-400 mg of calcium per day.
B Functions
In Bone
Calcium contributes enormously to the physical strength of bones (and teeth). Within the bone matrix, type I collagen is the major protein (90%) and calcium-rich crystals of hydroxyapatite [Ca10(PO4)6(OH)2] are found on the collagen fibres. The crystals are very small (less than 0.1 nm long) and are surrounded by a hydration shell of water. Smaller amount of the bone calcium is present in form of calcium phosphate and calcium carbonate and some other calcium salts. Together, all these minerals constitute about 50% of the total skeletal mass; the rest is made up of organic elements.
The calcium stores in bones are in a dynamic equilibrium with the surrounding extracellular fluid. As much as 700 mg of calcium may leave or enter the bones each day. Thus, bones serve as prime reservoir of body calcium. Deposition of calcium in bones depends on serum concentration of calcium (and phosphate). When 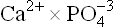 ion product rises beyond a limit value, calcification is promoted. A value above 70 mg/dL reflects tendency of soft tissue calcification, and below 20 mg/dL, it reflects defect of bone mineralization.
ion product rises beyond a limit value, calcification is promoted. A value above 70 mg/dL reflects tendency of soft tissue calcification, and below 20 mg/dL, it reflects defect of bone mineralization.
In Ionic Form
The calcium present in the body fluids, though extremely small in amount compared to that in bones, mediates a large number of physiological functions, as discussed here:
1. Muscle contraction: Calcium mediates excitation and contraction of muscle fibres. Contraction of striated muscles requires binding of calcium to troponin on thin filaments. The calcium-troponin interaction enhances reaction between actin and myosin, which triggers muscle contraction. In addition, calcium stimulates ATPase.
Smooth muscles do not have troponin. Their contraction is also calcium-dependent, but the calcium-sensing protein in smooth muscle is calmodulin, and not troponin.
2. Blood coagulation: Coagulation of blood occurs through a cascade of reactions, most of which require calcium.
3. Nerve excitability: Excitability and conductivity of nerves depends upon a number of cations, including calcium. A raised plasma calcium level decreases and a low plasma calcium level increases the excitability of nerves.
4. Activation of enzymes: Calcium causes direct activation of a number of enzymes, such as succinate dehydrogenase, ATPase and pancreatic lipase. Intracellularly, it interacts with calmodulin, a calcium-binding regulatory protein, and the calcium-calmodulin complex activates certain enzymes (Table 29.6).
As Intracellular Messenger
Cytoplasmic calcium is an important intracellular signal. It is referred to as a “second messenger” because it mediates cellular response to a wide range of stimuli in a manner analogous to cAMP. For example, it acts as a second messenger for epinephrine or glucagon in hepatic glycogenolysis. Some authors consider calcium as the second messenger in phosphoinositide system, and as a third messenger for some hormones, such as ADH (Chapter 29).
Other Functions
1. Neuromuscular transmission: Neuromuscular transmission occurs through release of acetylcholine from the motor end plate, which requires calcium.
2. Membrane integrity and permeability: Transport of a number of substances across the membranous barrier is influenced by calcium.
3. Action on heart: Cardiac muscles are dependent on calcium for the generation of rhythmic impulses. Increased calcium concentration increases myocardial contractility and vice versa.
4. Secretory processes: Secretion of water-soluble products by exocytosis is triggered by calcium. Examples include the release of zymogens by pancreas, insulin from ß-cells, histamine from mast cells and neurotransmitters from nerve terminals. Endocytosis-exocytosis, cell motility and other such processes mediated via microtubules-microfilaments are also regulated by calcium.
C Metabolism
Absorption
Dietary calcium is absorbed in the duodenum and proximal jejunum through mediation of an intestinal calcium-binding protein (CBP). This protein transfers the luminal calcium across the intestinal mucosal cell by an energy dependent active process.
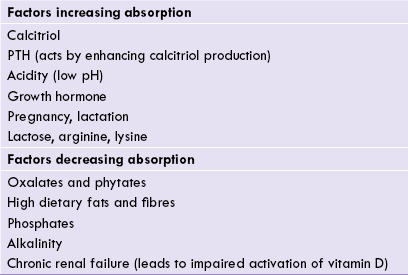
Factors promoting absorption
Synthesis of CBP is enhanced by 1,25-dihydroxycholecalciferol, i.e. calcitriol, the activated form of vitamin D. This accounts for stimulation of intestinal calcium absorption by calcitriol.
Table 31.1 lists various factors that increase calcium absorption.
Factors inhibiting absorption
Calcium absorption is inhibited by certain compounds such as oxalates, phytates, and phosphates. These compounds form insoluble calcium salts. The undigested dietary fats also impair calcium absorption by forming insoluble calcium soaps. Dietary fibres also interfere with the absorption. Thus, a large part of dietary calcium is not absorbed and is eliminated in faeces.
Plasma Calcium
Following absorption, calcium pours into blood plasma. Total plasma calcium is 9–11 mg/dL, and it exists in three forms:
1. Ionized calcium: It is the biologically active fraction of the calcium in plasma. Maintenance of its concentration within tight limits (4.5–5.0 mg/dL) is required for nerve function, membrane permeability, muscle contraction and glandular secretion.
2. Protein bound calcium: The majority of the remaining calcium is mainly bound to negatively charged albumin.
3. Calcium complexed to substances such as citrate and phosphate: It constitutes a small fraction (10% of total).
Excretion
Calcium is ultimately excreted through the following routes:
1. A large amount (—200 mg/day) is secreted into the intestinal lumen and lost in faeces.
2. About 300–400 mg/day is lost in urine; the kidneys start filtering calcium when the plasma levels exceed 7.0 mg%.
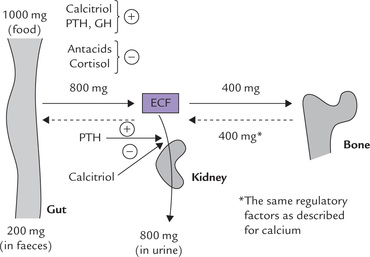
Fecal calcium excretion varies widely in response to diet, whereas elimination through other routes remains relatively constant. Various aspects of calcium metabolism and the factors regulating them are shown in Figure 31.1 .
D Regulation of Serum Calcium Levels
Calcium homeostasis is modulated by hormones. Parathyroid hormone, calcitriol and calcitonin are the most important calcium regulators; others, such as thyroxine, growth hormone, and oestrogen play a relatively minor role.
PTH and calcitriol have hypercalcaemic effect, whereas calcitonin decreases the serum calcium levels. Their actions are interlinked: PTH enhances production of calcitriol
Parathyroid Hormone (Parathormone; PTH)
Source
PTH is produced by four small glands having a total weight of 0.05-0.30 g. Parathyroid glands can be found anywhere in the neck or upper mediastinum but are usually located just behind the posterior thyroid capsule.
Structure of PTH
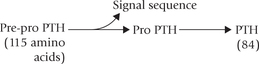
Parathormone (PTH) is an 84-amino acid single chain peptide (MW 9500). Biological activity of the hormone resides in the N-terminal portion of the molecule: PTH 1-34 possesses full biological activity.
Synthesis and Secretion of PTH
Parathormone is secreted as a 115-amino acid precursor molecule, the pre-pro-parathormone. It is converted to proparathormone by removal of a 25-amino acid sequence, the so called hydrophobic signal sequence from the N-terminal. Pro-parathormone is transported to the Golgi apparatus where enzymatic removal of another 6-amino acid sequence from the N-terminal occurs, to generate the parathormone.
Transport and Metabolism
Like other peptide hormones, PTH has a short half-life (< 20 min), because it does not bind with any transport protein in the blood circulation. It is quickly removed by the liver and the kidneys and is subjected to proteolytic degradation in these tissues. Kupffer cells are especially active in this process. Specific fragments of the PTH (PTH 34–84, and PTH 37–84) are generated in the liver; the role of kidneys may be to remove and excrete these fragments.
(A number of proteolytic enzymes, including cathepsin B and D have been identified in the parathyroid tissue itself.)
Mechanism of Action
PTH interacts with specific receptors (MW 70,000) located on the target cell surface. The receptors appear to be identical in bones and kidneys, but they are linked with different intracellular messenger systems.
Renal cells
The hormone-receptor interaction activates adenylate cyclase to generate cAMP.
This in turn initiates a signaling cascade, which promotes phosphorylation of proteins (by kinases) and finally brings about biological actions.
Osteoblasts
The hormone-receptor interaction acts through the second messangers (IP3 and DAG) to cause release of calcium from the intracellular stores. Interaction of calcium with certain intracellular enzymes and proteins results in alteration in properties of the latter, and this brings about biological effects of PTH (Box 31.1).
Biological Effects
Target tissues of the PTH are bone, kidney and small intestine. PTH exerts a direct effect on bone and kidneys, whereas its effect upon the intestine is mediated via calcitriol.
Effect on bones
PTH acts on both osteoblasts, which build bones, and osteoclasts, which destroy bones (i.e. bone resorption). Initially, PTH interacts with the cell surface receptors on the osteoblasts and triggers a series of events that cause mobilization of calcium from bones (by activating a calcium pump on cell membrane; Box 31.1). Calcium release into the blood circulation is accompanied by release of phosphate as well, because the two are present together in the hydroxyapatite crystals. Uptake of calcium and phosphate by bone is also decreased by PTH, resulting in hypercalcaemia and hyperphosphataemia. The above effects take place almost instantaneously, and are termed short-term effects.
These are followed by the long-term effects that begin about 12 hours later. These involve differentiation of the precursor cells into the osteoclasts. Osteoclasts cause dissolution of the bone matrix, resulting in release of various constituents including the organic matrix elements, calcium and phosphate. This process is called bone resorption and it results in decalcification and deminer-alization of bones. Recent evidence suggests that intra-cellular synthesis of the enzymes of bone resorption is stimulated by the PTH.
Effect on kidneys
PTH increases calcium reabsorption from the distal convoluted tubules (Table 31.2 ). However, due to its hypercalcaemic effect, more calcium load is presented to the kidneys than can be reabsorbed. The excess is excreted in the urine (i.e. hypercalciuric effect; Case 31.1).
Table 31.2
Control of calcium and phosphate metabolism
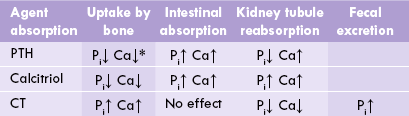
*PTH elicits both short-term and long-term effects on bones (see text). PTH = parathormone, CT = calcitonin.
PTH increases renal clearance of phosphate by inhibiting its reabsorption in the proximal convoluted tubules. This results in increased urinary excretion of phosphate, i.e. phosphaturia. The phosphaturic effect of PTH overrides its hyperphosphataemic effect, so that there is net fall in serum phosphate levels by PTH.
Effect on intestine
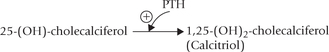
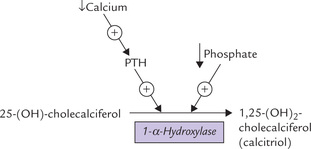
Effect of PTH on intestine is mediated via calcitriol. PTH increases calcitriol generation from 25-hydroxycholecalciferol by activating the renal tubular enzyme 1-α -hydroxylase (Fig. 31.2 ). Calcitriol then enhances calcium absorption from intestine.
Calcitriol
Calcitriol increases calcium absorption from the small intestine by intestinal transcription and translation of a specific gene that encodes for a calcium-binding protein (Chapter 18). The latter binds the available calcium in the intestinal mucosal cells and transfers it across the intestinal mucosa.
Together with PTH, calcitriol decreases calcium uptake by bone and enhances bone resorption by osteoclasts. These effects increase serum calcium (and phosphate) levels.
Effects of calcitriol on other target tissues are summarized in Table 31.2. Low calcitriol causes abnormal mineralization of newly formed osteoid as a result of low calcium and phosphate availability, which results in rickets among children, and osteomalacia in adults.
Calcitonin
Source and Structure
Calcitonin (CT) is a 32-amino acid peptide, with one disulfide bond and a characteristic N-terminal loop. It is synthesized and secreted mainly by the parafollicular C-cells of the thyroid, and to a lesser extent, by parathyroid gland and thymus.
Secretion
Secretion of calcitonin is inversely related to that of PTH. As in case of PTH, secretion of calcitonin is controlled by the ECF ionized calcium (and probably magnesium) concentration, but the stimulus for the hormone release is hypercalcaemia. This is in contrast with PTH where hypocalcaemia is the stimulus for the release. CT secretion increases linearly when calcium concentration is between 9.5 mg/dL and 15 mg/dL.
Glucagon and pentagastrin are potent CT secretagogues. The pentagastrin is used in the provocative test for medullary thyroid cancer, a malignant tumour of the calcitonin-secreting parafollicular c-cells.
Biological Effects
The precise role played by the CT is still unclear. It appears to prevent hypercalcaemia by decreasing the serum calcium.
Action of calcitonin on the bones is opposite to that of the PTH; it inhibits osteoclast-mediated bone resorption and, therefore, decreases the release of calcium and phosphate. It promotes the phosphate influx into the bone cells from blood circulation with a concomitant fall in extrusion of calcium. All these actions result in hypocalcaemia and hypophosphataemia, and account for effectiveness of CT in treating the hypercalcaemia associated with cancer. These actions are independent of PTH, probably because the target cells for CT (wherein it elevates the cAMP levels) are not same as the ones on which the PTH acts.
Steroid and Peptide Hormones
Several hormones for which the primary action is not related to calcium regulation, directly or indirectly affect calcium homeostasis.
1. Thyroid hormones stimulate osteoclast-mediated resorption of bone to cause hypercalcaemia.
2. Adrenal and gonadal steroids, particularly oestrogen sin women and testosterone in men, have important regulatory effects, increasing osteoblast and decreasing osteoclast function. They also decrease renal calcium (and phosphate) excretion and PTH function.
3. Growth hormone has anabolic effects on bone, mediated by insulin-like growth factors (IGF-I and IGF-II). Growth hormone increases the urinary excretion of calcium (while decreasing that of phosphate).
Self-regulatory Loop in Calcium Homeostasis
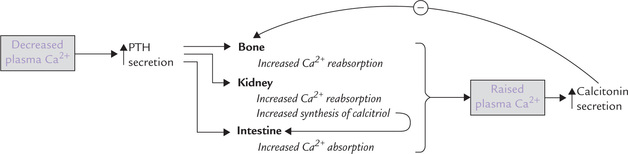
The circulating (unbound) calcium plays a role in calcium homeostasis via self-regulatory loop. For instance, hypocalcaemia induces PTH secretion, which elicits its various actions on bone, intestine and kidneys. As a result serum calcium level rises to normal. The self-regulatory loop is illustrated in Figure 31.3 .
E Disorders of Calcium Metabolism
The factors discussed so far in this section ensure meticulous regulation of serum calcium level within a narrow range. However, alteration of serum calcium levels— hypercalcaemia or hypocalcaemia—may occur in a num ber of conditions.
Hypercalcaemia
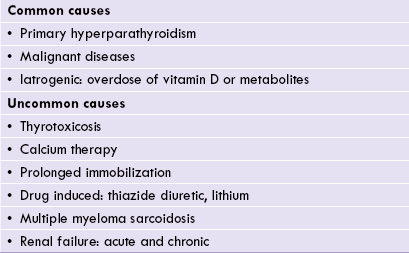
The commonest causes of hypercalcaemia are primary hyperparathyroidism and hypercalcaemia of malignancy Inappropriate dosage of vitamin D or metabolites, e.g. in the treatment of hypoparathyroidism or renal disease also causes hypercalcaemia (Table 31.3 ).
Hypocalcaemia
Many acute and chronic illnesses lead to a decrease in serum albumin, which in turn decreases serum total calcium concentration (Chapter 5). Other important causes are:
1. Hypoparathyroidism: Idiopathic, after neck surgery, or occasionally due to anticonvulsant therapy.
2. Vitamin D deficiency: It may occur due to dietary deficiency, malabsorption, or inadequate exposure to sunlight.
3. Renal disease: The diseased kidney fails to synthesize calcitriol.
4. PTH resistance: In pseudohypoparathyroidism, the hormone is secreted but there is failure of target tissue receptors to respond to the hormone. Hypomagnesaemia also causes PTH resistance.
5. Rarer causes: Malignancy, acute pancreatitis, sarcoidosis, multiple myeloma and thyrotoxicosis.
Osteoporosis
A chronic dietary deficiency of calcium accelerates the net loss of bone mass after the age of about 35 years. This process, called bone resorption, can ultimately lead to osteoporosis. Most common sufferers are the postmenopausal women. Osteoporosis is characterized by frequent bone fractures, which are a major cause of disability among the elderly people.
The risk of fracture in postmenopausal osteoporosis is treated by increasing the bone mass through oestrogen replacement and by calcium supplementation. Most often this treatment is given in combination with vitamin D. It is recommended that the current average calcium intake (approximately 500 mg/day) be supplemented with an additional 1000 mg/day calcium in these women. Milk and milk products are recommended since they are rich sources of the vitamin. Regular exercise also tends to increase the bone mass.
II Phosphorus
Total amount of phosphorus in a 70 kg adult is about 1000 g, of which, about 85% is present as hydroxyapatite in bones. About 10% is found in muscles and bones in association with proteins, carbohydrates and lipids. The rest is distributed in various compounds in the extracellular fluid (ECF) and the intracellular fluid (ICF).
Serum phosphate concentration of adults ranges from 2.4 mg/dL to 4.5 mg/dL. In children, it is higher because of higher bone turnover.
A Nutritional Requirement
An average daily intake of 1 g is sufficient to meet the body needs. Being a component of a number of biomolecules, phosphorus is present in a variety of foods of both plant and animal origin. Leafy vegetables, milk, meat, egg and cereals are especially rich sources.
B Functions
Phosphorus plays important role in intracellular as well as extracellular fluids.
Role in ICF
1. Component of biomolecules: Phosphorus (as phosphate) is present in a vast array of biomolecules such as nucleic acids, nucleotides, phospholipids, and some proteins.
2. The high-energy compounds (e.g. nucleoside phosphates like ATP, GTP, CTP, etc.) and several coenzymes (NAD+, NADP+, FAD, FMN) also contain phosphate.
3. Phosphate buffer is an important intracellular buffer.
4. Phosphorylated intermediates play important role in energy metabolism (Chapter 9).
5. Activation of several proteins and enzymes occurs by phosphorylation.
C Metabolism
Most of the dietary phosphate (70–80%) is absorbed in gut; the absorption is maximum when the dietary Ca : P ratio lies between 1 : 2 and 2 : 1. The absorption requires mediation of calcitriol. PTH also increases the phosphate absorption mainly by enhancing calcitriol production. Excessive use of antacids decreases absorption of dietary phosphates. This is because heavy metal ions present in antacids (Mg2+, Al3+ and Ca2+) bind phosphate ions to form insoluble salts. Other factors that influence intestinal absorption are shown in Figure 31.4 .
In serum, phosphate circulates in free ionic form (40%) or in a complex form (50%) with cations, such as, Ca2+, Mg2+, K+ or Na+. It is deposited in bones as hydroxyapatite; the process is mainly regulated by calcitriol. Excretion of phosphate occurs mainly through the renal route. About 90% of the circulating phosphate is filtered in glomeruli, and its further fate depends on action of calcitriol and parathormone on renal tubules. Calcitriol enhances the renal tubular reabsorption, but parathormone diminishes it. Thus, calcitriol has phosphate retaining effect, which contrasts with the phosphaturic effect of PTH (Table 31.2). Effect of PTH is far more potent than calcitriol. In all, 85–95% of the filtered phosphate is reabsorbed in renal tubules and the rest is excreted, which amounts to about 800 mg per day. About 200 mg phosphate is eliminated in faeces each day (Fig. 31.4).
D Regulation of Serum Phosphate Levels
A complex interplay of various hormones, such as PTH, calcitriol and calcitonin keeps plasma phosphate levels within the narrow range of 4.5–5.0 mg/dL (Table 31.2). It is increased in hyperparathyroidism and decreased in hypoparathyroidism, rickets and renal distress.
E Deficiency
Excessive excretion through kidneys or decreased absorption from intestine is the major cause of phosphate depletion. Dietary deficiency is extremely rare due to wide distribution of phosphate in a variety of foodstuffs.
Effect of phosphate depletion is most pronounced on skeletal system. Decreased mineralization of bones leading to rickets-like symptoms may occur (Case 31.2). In addition, abnormalities of erythrocytes, leukocytes and platelets may occur.
F Toxicity
Renal failure, both acute and chronic, may result in phosphate retention, thereby leading to phosphate toxicity. There is a concomitant fall of circulating calcitriol level due to inhibitory effect of phosphate on the calcitriol production. Because calcitriol has hypercalcaemic effect, serum calcium levels tend to fall in such cases.
Treatment of phosphate toxicity involves oral administration of antacids, which bind phosphate in intestinal lumen and thereby inhibit its absorption. As the serum phosphate levels are lowered, a concomitant rise in calcitriol also occurs. The latter then promotes calcium absorption from the intestine to normalize the circulating calcium levels.
 The hydroxyapatite crystals contribute significantly to the strength of bones. The bone matrix consists of fibrils of collagen to which these crystals are attached in a regular fashion. Between the fibrils is the ground substance; a mixture of proteins, glycoproteins and proteoglycans.
The hydroxyapatite crystals contribute significantly to the strength of bones. The bone matrix consists of fibrils of collagen to which these crystals are attached in a regular fashion. Between the fibrils is the ground substance; a mixture of proteins, glycoproteins and proteoglycans.