CHAPTER 34 Endocrine health
At the completion of this chapter and with some further reading, students should be able to:
• Describe the locations and basic anatomy of each endocrine gland and the factors which control hormone synthesis and release from them
• Define and explain the actions of the hormones secreted by each endocrine gland
• Identify and describe the disorders associated with each endocrine gland
• Discuss the pathophysiological influences associated with endocrine gland and hormone disorders
• Explain the effects, signs or symptoms of endocrine disorders
• Understand the rationale behind the diagnostic tests that may be used when assessing endocrine function
• Assist in the planning and implementation of nursing care for the client with an endocrine disorder
The endocrine system is comprised of a number of small glands which secrete hormones, chemical messengers which aid in maintaining homeostasis in the body. Hormones are involved in physiological processes such as metabolism, growth, fluid balance and reproduction. Over- or under-production of these hormones can have widespread effects because of the close relationship between the endocrine and other systems in the body. An understanding of the function of these hormones, and the effects of hormone imbalances, gives healthcare professionals the ability to interpret dysfunction and to care for individuals with an endocrine disorder.
I was 35 weeks pregnant and, as usual, I came to the end of my day feeling tired, irritable, nauseous and emotional; all normal pregnancy symptoms! But that night was different. I was experiencing heart palpitations and the nausea and vomiting was worse than usual. I started to shake and despite thinking everything was most probably fine, I felt I should go to the ER department of our local hospital just to make sure. To my surprise, the nurses admitted me and I began undergoing immediate tests.
During that night, they discovered that the calcium levels in my blood were very high and the staff told me that this was most likely the result of a tumour on one or more of my parathyroid glands. The endocrinologists, together with the obstetrics team, decided to deliver my baby the next day by caesarean section.
Two weeks after the birth, I underwent an MRI scan to locate the tumour and two weeks later I was operated on. The following few days were spent in hospital, and I had nurses and doctors giving me 2–3 blood tests daily to ensure that my calcium levels were coming down.
Days later, now at home and recovering from the operation, I began to experience severe numbing and a ‘pins and needles’ type feeling in my hands and feet and around my mouth. I woke during the night with major tingling in my arms, legs and face. Within minutes of waking I was almost completely paralysed, my face contorted and my hands spasming. Speaking became impossible. I no longer had control over any of my movement. My husband woke to find me unable to speak and with my muscles locked, and I was rushed to hospital in an ambulance. My calcium levels had plummeted too low and as a result my muscular system had been majorly affected. It was like nothing I had ever experienced! I was absolutely terrified! Once at the hospital, the nurses set me up with a calcium drip and within 2 hours my body was back to normal. I then went into a deep sleep for several hours, much like a person after a severe epileptic fit. I was completely exhausted.
Today, I am feeling 100% and take just one calcium tablet to keep my levels in check. I still have 3 monthly checkups with my endocrinologist. Who would ever have thought calcium plays such an important role in our bodies?
STRUCTURE AND FUNCTION OF THE ENDOCRINE SYSTEM
Endocrine glands and hormones
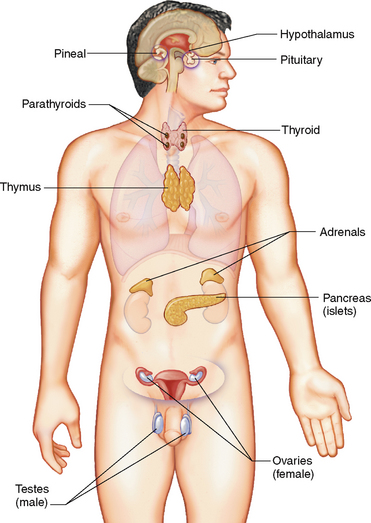
The endocrine system is a cellular communication system involving the secretion of hormones by glands. A hormone is a chemical regulator that integrates and coordinates cellular activities. Once secreted, hormones are transported to areas of the body where they stimulate a specific cellular activity in a target organ (Patton & Thibodeau 2010). Figure 34.1 illustrates the major endocrine glands and their location. Hormones are either steroids or proteins, and despite being circulated widely by the blood, only target cells with specific receptors have the ability to respond to a specific hormone. Binding to these receptors initiates a response that may take seconds or days: compared to the nervous system, the effects of hormones are much more prolonged (Marieb & Hoehn 2010).
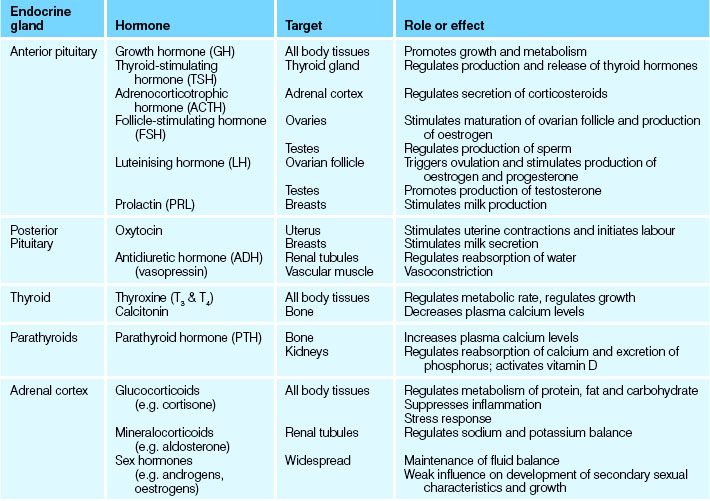
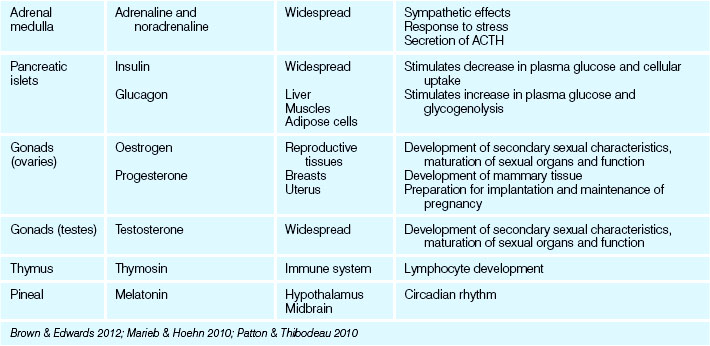
The effects of many hormones are widespread and diverse, while others are quite tissue specific. The major bodily processes controlled by the endocrine system are: reproduction; growth and development; maintenance of fluid electrolytes; maintenance of blood nutrients; and cellular metabolism and energy regulation (Marieb & Hoehn 2010). Most hormones are continuously secreted at a rate determined by stimulation of the gland that releases them. Endocrine gland secretion may be enhanced or depressed by blood-borne stimuli, neural input or other hormones: some hormones can exert control over other endocrine glands and other hormones by influencing their action, metabolism, synthesis and transport. The activation or inhibition of endocrine glands relies on negative feedback to maintain homeostatic levels of hormones and their effects (Patton & Thibodeau 2010). Disruption of this homeostasis presents as endocrine and hormone imbalances and pathologies. Table 34.1 lists all the major hormones, their effects and the endocrine glands responsible for their production and release.
The pituitary gland
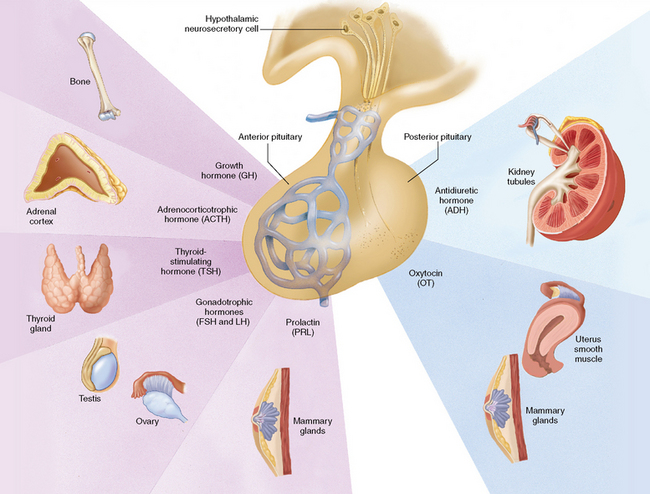
The pituitary gland, or hypophysis, is about the size of a pea and is positioned at the base of the brain in a depression in the sphenoid bone. The pituitary gland (Fig 34.2) is often referred to as the ‘master gland’ of the endocrine system (Marieb & Hoehn 2010). This term reflects not only the number of hormones it secretes, but the influence of many of these hormones in controlling other endocrine tissues as well. It lies just beneath the hypothalamus, to which it is physically connected by a stalk containing blood vessels and nervous tissue. It has two functional lobes: the anterior lobe comprised of glandular secretory tissue and the posterior lobe which is nervous tissue. Each lobe performs specific functions which reflect their embryological origin.
The anterior lobe or adenohypophysis produces and releases:
• Growth hormone (GH), which is a metabolic hormone concerned with body growth, particularly that of skeletal muscles and long bones
• Thyrotrophic hormone (TH), also referred to as thyroid-stimulating hormone (TSH), which is responsible for controlling the growth and activity of the thyroid gland
• Adrenocorticotrophic hormone (ACTH), which regulates the activity of the cortex of the adrenal glands in hormone production
• Prolactin (PRL), also referred to as lactogenic hormone, which stimulates the mammary glands to secrete milk after the birth of a baby
• Gonadotrophic hormones, which control the development and functions of the ovaries and testes. These hormones are:
The posterior lobe receives, stores and releases the following hormones which are synthesised in the hypothalamus:
• Oxytocin, which stimulates uterine contraction and the initiation of childbirth, and causes the ducts of the mammary glands to contract and expel breast milk
• Antidiuretic hormone (ADH), which stimulates the kidney tubules to reabsorb water, resulting in decreased excretion of water in the urine (Marieb & Hoehn 2010).
TSH, ACTH, FSH and LH may all be considered as trophic hormones in that they affect the activity and secretion of other hormones from other endocrine glands. To this effect, alterations in pituitary gland function or health have widespread consequences (Patton & Thibodeau 2010).
The pineal gland
The pineal gland is a small cone-shaped structure situated in the roof of the third ventricle of the brain. The endocrine function of the pineal gland is unknown but it is believed to secrete the hormone melatonin, which appears to rise and fall during the course of the day and night and is believed to establish the body’s day–night wake–sleep pattern (Marieb & Hoehn 2010). A rare neoplasm of the pineal gland (pinealoma) may obstruct the flow of cerebrospinal fluid, causing hydrocephalus (Farrell & Dempsey 2011).
The thymus
The thymus gland is positioned in the upper thorax posterior to the sternum. It is large in infants and children, reaching its maximum size at puberty, then decreasing in size and function throughout adulthood (Patton & Thibodeau 2010). The thymus produces a hormone called thymosin, which promotes the growth of lymphoid tissue in the body and aids in the body’s immune response. While endocrine dysfunction of the thymus gland is uncommon, congenital thymic hypoplasia causes deficient cellular immunity, making the individual more susceptible to infections.
The thyroid gland
The thyroid gland lies in the anterior neck just inferior to the larynx. It consists of two lateral lobes positioned either side of the trachea. The lobes are joined together by a central mass called the isthmus, which lies anteriorly across the trachea. The thyroid gland is covered by a connective tissue capsule that forms trabeculae, which then pass inwards and divide the gland into lobes. It is richly vascularised, which presents surgeons with a tricky and dangerous task. The thyroid gland is the largest pure endocrine gland in the body, and produces two main hormones: thyroid hormone and calcitonin (Patton & Thibodeau 2010).
Thyroid hormone (TH) is actually two iodine-containing amine hormones: thyroxine (T4) and triiodothyroxine (T3). Thyroid hormone contains large quantities of iodine which is derived from the diet and stored in the thyroid gland, and is produced by the follicles of the thyroid gland. Thyroid hormone largely controls cellular metabolism, thereby affecting virtually every cell in the human body. In doing so, TH influences:
• Growth and physical development
• Neural and mental development
Calcitonin, also referred to as thyrocalcitonin, is produced by the parafollicular cells of the thyroid gland, and decreases blood calcium levels by stimulating calcium uptake and deposition in the bones. In humans, the relative role of calcitonin is not important: clients who have had thyroid surgery or removal are not adversely affected by the loss of calcitonin secretion. However, individuals with skeletal disorders such as Paget’s disease may be administered calcitonin therapeutically (Marieb & Hoehn 2010).
The parathyroid glands
The parathyroids are four small glands embedded in the posterior surface of each lobe of the thyroid gland. The parathyroid glands produce parathyroid hormone (PTH), also referred to as parathormone, which is primarily responsible for the regulation of blood calcium and phosphate levels. Parathormone release is triggered by falling blood calcium levels and is antagonistic to calcitonin: it promotes release into the blood of calcium and phosphate from bone; enhances reabsorption of calcium and excretion of phosphate by the kidneys; and also promotes the activation by the kidney of vitamin D, which assists in calcium absorption by the gut (Marieb & Hoehn 2010).
The pancreas
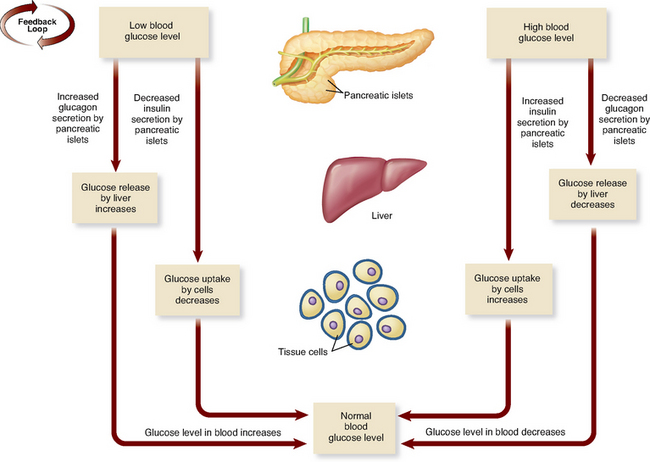
The pancreas is largely an exocrine gland, secreting enzyme-rich digestive juices destined for the small intestine (see Ch 30). Scattered among these cells are the pancreatic islets, small clusters of endocrine cells formerly referred to as the islets of Langerhans. They produce two hormones, insulin from beta (β) cells and glucagon from alpha (α) cells, which both help to regulate blood glucose level (Patton & Thibodeau 2010).
Insulin and glucagon are antagonistic hormones, and their general effects and relationship are illustrated in Figure 34.3. Insulin promotes hypoglycaemia, which happens when the pancreas detects high or elevated glucose levels (the presence of some amino acids and fatty acids also stimulate or increase insulin release). The major effects of insulin are to lower blood glucose by increasing the uptake of glucose into the cells and reducing the release of glucose from the liver. Insulin also promotes the conversion of glucose to fatty acids within the liver, and generally stimulates protein synthesis. Insulin is essential for the use of glucose by the cells of the body; without it, most cells would be unable to access or utilise glucose for energy (Marieb & Hoehn 2010).
Glucagon in contrast promotes hyperglycaemia. It is released in response to a reduction or decrease in blood glucose level. Its output is reduced in the presence of raised blood glucose and insulin and thus acts as an antagonist of insulin. Glucagon raises blood glucose levels by stimulating the breakdown of glycogen in the liver (glycogenolysis), inhibiting glycogen synthesis and enhancing the production (gluconeogenesis) and release of glucose (Patton & Thibodeau 2010).
The adrenal glands
The paired adrenal (suprarenal) glands are located on top of each kidney. Each gland is surrounded by a capsule and contains an outer cortex and an inner medulla. The cortex and medulla synthesise different hormones and, just like the pituitary gland, reflect different glandular and neural embryological origins (Marieb & Hoehn 2010).
The adrenal cortex
The cortex is divided structurally and functionally into three zones. Each zone is responsible for the secretion of a different class of steroid hormones, collectively called corticosteroids. The adrenal cortex, and therefore the corticosteroids, are under the influence of ACTH released by the anterior pituitary gland (Patton & Thibodeau 2010).
The following are examples of hormones synthesised and released by the adrenal cortex:
• Mineralocorticoids: mostly aldosterone, which is concerned with the reabsorption of sodium ions and the elimination of potassium ions in the kidneys. Aldosterone thus helps regulate both water and electrolyte balance in body fluids
• Glucocorticoids: which include cortisone, hydrocortisone (cortisol) and corticosterone and are concerned with:
Given their role in glucose metabolism, glucocorticoids are often referred to as hyperglycaemic hormones
• Gonadocorticoids: (sex hormones—androgens and oestrogens) are concerned with the development of secondary sexual characteristics and the functioning of the reproductive organs. Both male and female hormones are produced by the adrenal cortex, regardless of gender. These gonadocorticoids released by the adrenal cortex are relatively weak and in low concentration compared to the hormones released by the gonads (Marieb & Hoehn 2010).
The adrenal medulla
The medulla, or inner portion of the adrenal glands, produces two similar hormones referred to as catecholamines: adrenaline (also referred to as epinephrine) and noradrenaline (also referred to as norepinephrine). When the medulla is stimulated by neurons of the sympathetic nervous system, the two hormones are released into the bloodstream. The physiological responses to the medullary hormones mimic those of the sympathetic nervous system, which are:
• Cardiac and skeletal vasodilation; visceral and dermal vasoconstriction
• Increase in heart rate and contractility; increase in blood pressure
• Dilation of the bronchial tubes
• Reduction of peristalsis in the digestive system
• Stimulation of the liver to release more glucose into the bloodstream
Adrenaline prepares the body for fight or flight, and its secretion is increased when the person feels threatened or experiences strong emotions such as fear, anger or excitement, thereby helping the body to cope with a stressful situation (Marieb & Hoehn 2010).
The gonads
The female gonads are the ovaries and the male gonads are the testes (see Ch 35). The female and male gonads produce sex hormones identical to those produced by the adrenal cortex, but in greater concentrations and potency. During and after puberty, the gonads assume the major role in reproductive hormone production.
The hormones produced by the ovaries are oestrogens and progesterone. In females, oestrogens influence development of the reproductive organs and the secondary sexual characteristics. Oestrogen also promotes oogenesis and ovulation, and stimulates the lining of the uterus to thicken in preparation for a fertilised ovum to develop. Progesterone promotes the final thickening of the uterine lining and inhibits uterine contraction, enabling implantation of the fertilised ovum. Progesterone also inhibits ovulation during pregnancy and stimulates the mammary glands to produce milk.
The testes produce the androgen testosterone, which stimulates the development of male characteristics and the growth and function of the reproductive organs. Testosterone and androgens generally masculinise the human body, irrespective of gonadal sex (Patton & Thibodeau 2010).
ENDOCRINE DISORDERS
Disorders of the pituitary gland
Abnormalities of the pituitary gland are generally associated with hyper- or hyposecretion of its products. Diseases of the pituitary gland, diseases of the hypothalamus, radiation therapy to the head or neck, trauma or tumour interfere with pituitary function and thus the function of other trophic glands like the thyroid, adrenals and gonads. Therefore pituitary problems potentially affect the homeostasis of other hormones and their effects are widespread. Clients with anterior pituitary disorders require nursing interventions to assist them to cope with the physical and emotional changes and also to prevent complications involving other organs (Farrell & Dempsey 2011).
Hyperfunction of the pituitary gland commonly results from a tumour, which may create pressure on cerebral structures and cause neurological manifestations, such as severe headaches and visual disturbances, or excessive secretion of pituitary hormones, with consequent increased stimulation of the target organs (Brown & Edwards 2012; Patton & Thibodeau 2010). In adults, hypersecretion of the anterior pituitary gland most commonly involves ACTH or GH and results in Cushing’s disease and syndrome or acromegaly, respectively. Hypersecretion of prolactin is also common. Diseases of the posterior pituitary are rare: hypersecretion of the posterior pituitary is most commonly associated with the syndrome of inappropriate antidiuretic hormone (SIADH). A number of inherited syndromes exist which involve tumours of more than one endocrine gland. These are collectively known as multiple endocrine neoplasia syndromes (MEN) (Australian and New Zealand Endocrine Surgeons 2011a).
Gigantism
Disturbances to GH release are chronic progressive disorders marked by hormonal dysfunction and skeletal overgrowth, which most commonly can appear in two forms: gigantism and acromegaly. Hypersecretion of GH in childhood results in gigantism, and begins before epiphyseal closure (Marieb & Hoehn 2010). Children with gigantism may grow up to 15 cm a year, reach a height of 2.4 m, exhibit slow sexual development and may have slow mental development. Radiation therapy, surgical removal of the tumour and drug therapy may be used to decrease secretion of the growth hormone and slow down the excessive growth pattern (Monahan et al 2007; Craft et al 2011). With this treatment, prognosis for the individual with gigantism is generally good (Brown & Edwards 2012).
Acromegaly
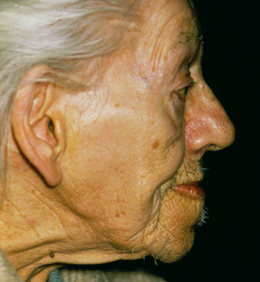
Acromegaly is the manifestation of GH hypersecretion and occurs in adulthood after epiphyseal closure. It is characterised by atrophy of skeletal muscle and formation of new bone and cartilage. The long bones are unable to grow in length, but the smaller bones of the hands, feet and face grow, giving the individual a characteristic appearance, with an enlarged lower jaw, bulging forehead and thickened ears and nose (Fig 34.4). The extremities become elongated and enlarged, resulting in large hands and feet (Patton & Thibodeau 2010). Thickening of the tongue may cause the voice to sound deep and hollow, associated with slurred speech. Acromegaly is a chronic disfiguring disease that often shortens life expectancy, as it may lead to respiratory, cerebrovascular and congestive heart diseases (Monahan et al 2007). Surgical intervention to remove the pituitary tumour may lead to hypopituitarism or recur (Australian Pituitary Foundation 2011).
Prolactinoma
The most common tumour resulting from hypersecretion is prolactinoma (Australian Pituitary Foundation 2008). Hypersecretion of prolactin often results in gynaecomastia and galactorrhoea (breast swelling and milk production) in both males and non-breastfeeding females, and may be accompanied by menstrual or libido alterations. Evidence suggests that despite around 10% of the population possessing microprolactinomas, only 0.1% will be clinically affected each year in Australia (Australian Pituitary Foundation 2008).
SIADH
The syndrome of inappropriate antidiuretic hormone (SIADH) includes excessive ADH secretion from the posterior pituitary, but it may also be non-endocrine in origin. Individuals with pulmonary problems such as carcinoma, severe pneumonia and other lung dysfunctions often also suffer from SIADH (Craft et al 2011). As a result, a consideration of pulmonary health is important in the nursing diagnosis and management of SIADH.
Individuals with this disorder display enhanced renal water retention which leads to imbalances in sodium balance and blood osmolarity. Hyponatraemia is induced due to the relative increases in water, and is common in hospitalised patients. For example, one-quarter of the residents aged over 65 in an Australian geriatric facility had hyponatraemia, with half of these caused by SIADH (Anpalahan 2001).
Hyponatraemia suppresses the release of renin from the kidneys, which in turn regulates aldosterone release. The result is a loss of sodium in the urine. Diagnoses of SIADH include low serum osmolarity and sodium and urine hyperosmolarity; the symptoms are determined by the extent of severity of the continued ADH release.
Restricting and monitoring fluid intake, as well as the possible administration of diuretics, may reduce the underlying cause (Brown & Edwards 2008). Careful fluid restrictions for clients with SIADH of 0.5–1 L daily are suggested to allow sodium levels to increase slowly. Likewise, monitoring urine output and quality, weight and blood chemistries are indicated for individuals at risk (Monahan et al 2007).
Hypofunction of the anterior pituitary gland can also result from a tumour in the gland, secondary to trauma of the hypothalamus or infection (Monahan et al 2007). Hypopituitarism results in an absence or deficiency of GH and gonadotrophins and is marked by growth retardation, which in children causes dwarfism and delay of puberty. It can present in three forms, which share the characteristics of delayed or retarded growth, and decreased mentation and sexual development: Fröhlich’s syndrome, commonly caused by an anterior pituitary tumour; dwarfism, which results from hyposecretion of growth hormone and is characterised by proportionately small individuals; and Simmonds’ disease, atrophy of the anterior lobe of the pituitary gland, which is a rare disorder with widespread effects (Farrell & Dempsey 2011).
Treatment of hypopituitarism commonly involves hormone replacement of the target gland hormone (Australian Pituitary Foundation 2011; Endocrinology Expert Group 2009). Constant monitoring and adjusting of hormone levels is required to attain optimal results.
Diabetes insipidus
Diabetes insipidus is a rare condition characterised by a deficiency of ADH and may be of neurogenic (posterior lobe damage, resulting from head trauma, tumour or surgical or irradiative interventions) or nephrogenic in origin (kidneys failing to respond to adequate levels of ADH) (Craft et al 2011). Without ADH, the individual exhibits polyuria, passing vast quantities of dilute, colourless urine (volumes vary, but may be as high as 20 L daily), the osmolarity and specific gravity of which is very low. To compensate for the dehydration from the excessive fluid loss, the individual experiences polydipsia (excessive thirst, especially for cold drinks) and drinks large amounts of fluid (Farrell & Dempsey 2011).
Diagnostic procedures centre on fluid management. A fluid deprivation test in which the patient is unable to concentrate urine or continually passes large volumes is indicative of diabetes insipidus. This test is based on the principle that withholding fluid for several hours stimulates secretion of ADH. Other procedures include measurement of plasma ADH and osmolarity, or IV infusion of hypertonic saline (Craft et al 2011; Farrell & Dempsey 2011). Treatment for individuals with diabetes insipidus involves medication as opposed to limiting fluid intake, because the high volume of fluid loss through urine is independent of fluid intake. The replacement of ADH with synthetic analogues such as vasopressin or desmopressin has a good prognosis (Endocrinology Expert Group 2009; Monahan et al 2007). During the acute stage of this condition, clients need supportive care in relation to their polyuria and increased need for fluids.
Clinical Scenario Box 34.1
You are a nurse and you have just begun your shift. Your first client is a 27-year-old male who shows no abnormalities during a routine physical examination and whose vital signs are all within range. He tells you that he cycles regularly and tries to keep fit. He has had blood tests and has had a urine sample taken for urinalysis earlier in the day. While you are reading his information and flicking through his charts, you ask the client how he has been feeling. He tells you that recently he has been drinking a lot and urinating frequently.
You ask the client whether he has been eating differently as well as drinking a lot, and he tells you that he has made little change to his diet.
‘Would you say that you eat a lot of sweets or cakes or sugar?’ you ask.
‘No more than normal,’ he replies.
You check the blood serum tests and find that fasting glucose is normal. You also look at the urinalysis results and find they are negative for glucose.
• What is the rationale behind asking about diet and sugar intake, and do the blood and urine results confirm or rule out your original assumption?
‘I’m always drinking and going to the toilet,’ he says. ‘It’s really interrupting my work.’
You suspect that there may be an imbalance in the amount of antidiuretic hormone (ADH), as this is a major hormone of water balance in the body.
• What are the two major endocrine disorders associated with imbalances in ADH levels?
• What are the typical signs or symptoms of these disorders?
‘What do you think it could be?’ he asks.
You explain that the blood and urine glucose levels indicate that it probably isn’t diabetes mellitus, but you’re not sure, so you make a closer inspection of the chart and results. The blood tests also indicate that the client’s plasma osmolality is high, with sodium levels more than 145 mmol/L.
You check the corresponding information on the urinalysis results and find that the previous nurse noted a low specific gravity (1.001) and that the urine was pale and almost colourless. The volume was considerable.
‘It looks like you passed a lot of weak urine for your test,’ you say to the client.
‘Exactly! That’s what I told my doctor and the other nurse this morning,’ he replies.
• Given what you know about the blood and urinalysis results, which disorder of ADH do you think your client may be suffering from?
• Was there anything in his history that confirms your conclusion?
• Do you think the information that your client provided while you were chatting to him assisted your conclusion?
During your rounds you revisit this client and ask him how he’s going.
‘Fine, but I’m thirsty again,’ he says.
You ask him if he has had a drink recently and he tells you that he can’t, as he has been asked to undertake a ‘fluid deprivation test’ and an MRI of his head.
Diagnostic tests
Pituitary function may be assessed by estimating the level of trophic hormones and by measuring the response to stimulation or suppression tests (Brown & Edwards 2008). The most common tests for evaluating anterior pituitary function are the serum growth hormone test and the insulin intolerance test. The insulin intolerance test, also called the growth hormone stimulation test, provides information about the secretion of growth hormone and ACTH. Investigations may also include x-ray, magnetic resonance imaging (MRI) or computerised tomography (CT) scan of the skull (Farrell & Dempsey 2011). Testing posterior pituitary function includes the water-deprivation test, which is performed when the client’s symptoms indicate diabetes insipidus.
Disorders of the thyroid gland
Hyperthyroidism
Hyperthyroidism is one of the more common endocrine disorders and about 2% of Australian women will experience some hyperthyroidism (Queensland Health 2008a). Hyperthyroidism may be caused by many factors, such as thyroiditis or hypersecretion of TSH. An increase in the secretion of the thyroid hormones T3 and T4 affects the basal metabolic rate, as well as the functions of other body systems. The manifestations of hyperfunctioning are generally related to increased metabolic processes, and exhibit characteristic signs and symptoms which reflect heightened metabolism or adrenergic stimulation, and are sometimes collectively referred to as thyrotoxicosis. The presenting condition is often nervousness. The condition can also be caused by genetic or immunological factors (Michels & Eisenbarth 2007), thyroid gland nodules or chronic inflammation of the thyroid gland (Craft et al 2011). Table 34.2 lists the most common signs and symptoms of hyperthyroidism and hypothyroidism.
Table 34.2 Some common signs and symptoms of hyperthyroidism (including Graves’ disease) and hypothyroidism
| Hyperthyroidism | Hypothyroidism |
|---|---|
| Nervousness, hyperexcitability and irritability | Apathy, fatigue, low mental energy |
| Cardiac palpitations and arrhythmias, tachycardia and increased systolic blood pressure | Bradycardia, oedema |
| Poor heat tolerance | Cold intolerance, lowered metabolic rate |
| Alterations (usually increase) in appetite and dietary intake | Cold dry skin |
| Weight loss and possible diarrhoea | Weight gain, constipation |
| Possible exophthalmos and goitre | Goitre, coarse facial features, myxoedema (advanced), impaired speech |
Graves’ disease
An autoimmune disorder, Graves’ disease is the most common hyperthyroid disease (Craft et al 2011; Farrell & Dempsey 2011; Thyroid Australia 2007). Elevated levels of circulating TSH receptor antibodies stimulate the thyroid gland, leading to the production and release of high concentrations of thyroid hormones and potential glandular hypertrophy. Graves’ disease more commonly affects young women, and is eight times more prevalent in females (Farrell & Dempsey 2011) (see Clinical Scenario Box 34.2). It may appear after a stressful episode, illness or shock. Symptoms include all the characteristics of hyperthyroidism: tachycardia, nervousness, hyperactivity and excitability. The individual will have a voracious appetite but loses weight and can be quite underweight. Diarrhoea is common, as peristaltic rate increases. Excessive sweating and extreme thirst are also common, because of the increased metabolic rate. One outstanding characteristic of Graves’ disease is that of exophthalmos, or protrusion of the eyeballs, which is due to oedema in the tissues behind the eye, and does not resolve even when the hyperthyroidism is corrected (Craft et al 2011) (Fig 34.5). A serious complication of Graves’ disease is thyroid crisis (or storm), which is discussed in Clinical Interest Box 34.1.
Clinical Scenario Box 34.2
I was always a very fit and active person until I started to notice that my heart rate would increase rapidly when simply walking up stairs and I would become hot and sweaty for no reason and unable to sleep. Then one day a few minutes into a jog I had heart palpitations and I was completely out of breath: I was terrified. My doctor tested my blood pressure and my heart for a murmur and I had blood tests for a whole range of things. While waiting for the results I lost 4 kg in just 3 days! This coincided with an enormous increase in appetite—I was eating 5 meals a day!
My blood test results showed I had Graves’ disease. I had never even heard of it before. And neither had anyone in my family.
After being diagnosed with Graves’ disease it took months of cycling from hyperactive to underactive while adjusting my medication until I felt ‘normal’ again. Some days I would be hot, sweaty and intolerant to heat, have an increased appetite, lose weight and be unable to sleep. Then I would just as quickly be cold and tired; I would feel depressed and I would put on weight.
Susie, 33-year-old fitness instructor
Susie just started getting really restless and hyperactive. I told her it was because of all the exercise she was doing, but it didn’t make sense. I couldn’t even sleep in the same bed! She would toss and turn and kick off the doona.
When she started eating two breakfasts every morning and chocolate every night, and she was losing weight, we knew it was something more than just nerves.
The problem is that Susie’s radioactive treatment worked too well—now she has to take thyroid hormone replacement for the rest of her life.
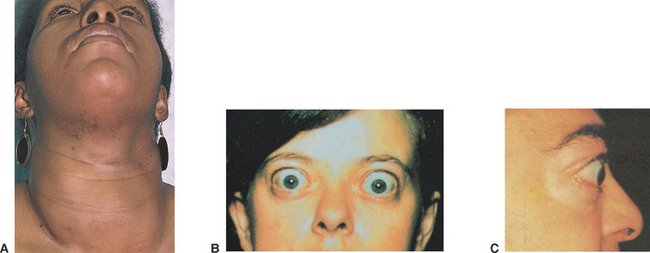
Figure 34.5 Exophthalmos and/or goitre
(Patton & Thibodeau 2012:574. Images: A Swartz MH: Textbook of physical diagnosis, 4th edn, Philadelphia, 2002, Saunders. B Stein HA, Slatt BJ, Stein RM: The ophthalmic assistant: fundamentals and clinical practice, 7th edn, Philadelphia, 2000, Mosby. C Swartz MH: Textbook of physical diagnosis history and examination, 5th edn, Philadelphia, 2006, Saunders)
CLINICAL INTEREST BOX 34.1 Thyroid crisis (thyroid storm or thyrotoxicosis)
This situation is a sudden, severe form of hyperthyroidism, and usually occurs in a person already with advanced hyperthyroidism. It is typically characterised by high fever, severe tachycardia, tachypnoea and an altered mental state: all symptoms of excessive thyroid hormone. An individual presenting with thyroid crisis requires immediate attention and supportive nursing care, as it is considered a medical emergency and is life threatening. It can be instigated by emotional stress, infection, trauma, cardiovascular disease or following thyroid surgery. Management involves lowering body temperature, providing sufficient oxygen and IV fluids containing carbohydrate and interfering with thyroid hormone release.
Goitre
An enlarged thyroid gland (goitre) may be the result of thyroiditis, neoplasia, hyper- or hypoactivity. Goitre can cause local problems by exerting pressure on the trachea, which may produce dysphagia or respiratory distress (Monahan et al 2007). Simple goitre (non-toxic goitre) is due to an iodine deficiency that causes a compensatory enlargement of the thyroid gland in an attempt to maintain thyroid hormone production. Introduction of iodised salt has reduced the incidence in Australia and New Zealand, and in most other western countries (Farrell & Dempsey 2011), although localised iodine deficiency still persist in Australia (Australian Thyroid Foundation 2009; Endocrinology Expert Group 2009). Exophthalmic goitre occurs in hyperthyroidism (Fig 34.5).
Hypothyroidism
Hypothyroidism is the most common disorder of the thyroid and occurs more commonly in women (6–10%) (Endocrinology Expert Group 2009; Queensland Health 2008b). Hypothyroidism is characterised by a decreased secretion of thyroid hormones and may be congenital or it may develop later in life. Cretinism is the congenital form of hypothyroidism and typically results from absence or underdevelopment of the thyroid gland or from severe maternal iodine deficiency during pregnancy. The manifestations of cretinism are related to a marked depression of metabolic processes and progressive mental impairment. Typically the infant is overweight, lethargic, has dry thick skin, coarse features, a broad flat nose and protruding tongue and abdomen (Marieb & Hoehn 2010). If the disease goes unchecked, sexual organs fail to develop and muscle growth is retarded. Treatment is started as soon as diagnosis is made and involves lifelong thyroid hormone replacement therapy (HRT). Hypothyroidism can also result from surgical intervention used to correct hyperthyroidism.
The most common natural cause of hypothyroidism is an autoimmune disorder, Hashimoto’s disease. Symptoms of hypothyroidism are the opposite of those of hyperthyroidism (see Table 34.2). The individual is fatigued, drowsy and sensitive to cold temperatures, gains excessive weight, has thin nails and brittle hair, decreased pulse and respiratory rates and irregular menstruation (Monahan et al 2007) (Fig 34.6). Advanced hypothyroidism may produce personality and cognitive changes characteristic of other mental dysfunctions such as dementia or depression. Depression has been linked to, both causatively and symptomatically, and misdiagnosed as hypothyroidism (Chueire et al 2007; Krausz et al 2007). Diagnosis of hypothyroidism may be difficult due to the nonspecific nature of the symptoms, which are often diverse and atypical (Endocrinology Expert Group 2009). Despite this, hypothyroidism responds well to HRT and symptoms may disappear after a few months of treatment.
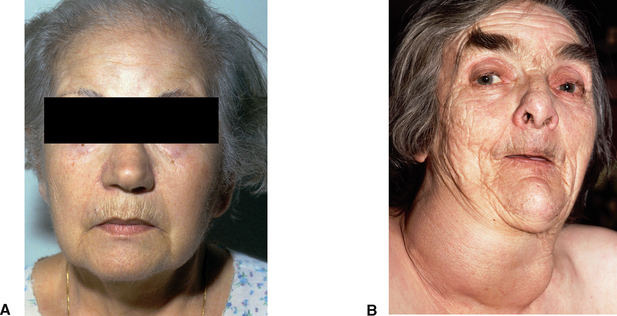
Figure 34.6 Myxoedema (A) and hypothyroidism (B)
(A Talley NJ & O’Connor S (2009) Clinical Examination, 6th edn. Churchill Livingstone, Elsevier Australia, Fig 10.8; B Dr P. Marazzi/Science Photo Library
Myxoedema is the characteristic sign of severe or prolonged hypothyroidism. The name refers to oedema in the dermis of the skin, especially around the eyes, hands and feet (Brown & Edwards 2012) (Fig 34.6). Myxoedema and myxoedema coma however—the decompensated state of severe hypothyroidism in which a patient may lose consciousness—if left untreated may lead to death. Maintenance of vital functions and monitoring for increasing severity of signs and symptoms is a major nursing intervention (Monahan et al 2007).
Diagnostic tests
A combination of tests is generally performed to evaluate thyroid function, including direct tests of thyroid function, tests that measure concentration and binding of the thyroid hormones and tests involving the use of scanning or imaging techniques (LeMone et al 2011). Blood tests are performed to measure serum hormone levels. The best screening test of thyroid function is the measurement of serum TSH concentrations, due to its sensitivity. This may be augmented by assessing serum free or bound T3 and T4. Radioactive iodine tests, such as the radioactive iodine uptake test, evaluate thyroid function by measuring the amount of orally ingested iodine that accumulates in the thyroid gland over a time regimen (Brown & Edwards 2012). Thyroid scanning is visualisation of the thyroid gland after administration of a radioisotope. Thyroid ultrasonography helps evaluate thyroid structure and differentiation between a cyst and a tumour on the thyroid gland. If an individual is to have thyroid tests, the nurse must ascertain medications taken by the person (especially if they contain iodine) (Farrell & Dempsey 2011).
Most people with classic hyperthyroidism rarely need hospitalisation. Critically ill individuals, those with extreme manifestations of thyrotoxicosis plus a significant concurrent illness, require inpatient acute care on a medical unit. Management of hyperthyroidism involves assisting the individual and their family to manage the symptoms associated with hyperthyroidism until it is controlled with medication (hormone replacement therapy is the preferred option) or surgery if indicated. Surgical intervention may involve a subtotal thyroidectomy, which removes part of the thyroid gland, leaving enough gland to produce adequate thyroid hormone, or a total thyroidectomy may be performed if the gland is cancerous (Monahan et al 2007). A person who has had a total thyroidectomy requires long-term hormone replacement (Australian and New Zealand Endocrine Surgeons 2011b).
The nurse should assess the nutritional and emotional status of a client with hyper- and hypothyroidism, and manage appropriately. The nurse should also reassure the client that the emotional reactions, especially with hypothyroidism, are normal for that condition (Farrell & Dempsey 2011).
Disorders of the parathyroid glands
Hyperparathyroidism
Hyperparathyroidism results in an overproduction of PTH. This condition can be caused by hereditary factors, tumours or enlargement of the glands. It is more common in women, and in patients above 60 years of age (Suliburk & Perrier 2007). Secondary hyperthyroidism can be caused by renal disease or other calcium perturbations such as osteomalacia or rickets. Excessive PTH production leads to hypercalcaemia (excessive calcium in the blood) as the hormone stimulates resorption of calcium from bone, the kidney and gut (Marieb & Hoehn 2010). As the calcium is pulled from the bones, individuals with this condition display manifestations that are widespread and include symptoms such as backache, bone curvature and pathological fractures from bone weakness (Farrell & Dempsey 2011). Renal stones may develop because of the excess calcium output in the urine, especially in conjunction with metabolic acidosis and alkaline urine. The digestive system increases absorption of calcium, causing abdominal pain, vomiting and constipation. Hypercalcaemia leads to hyperactivity of muscles, causing impaired neuromuscular coordination and cardiac arrhythmias.
Hypoparathyroidism
Hypoparathyroidism is relatively uncommon and is indicated by deficiency in PTH secretion. Causes may include familial hypoparathyroidism, autoimmune disorders or, most commonly, surgical removal of parathyroid glands in an effort to treat other thyroid disorders (Monahan et al 2007). Low or lack of circulating PTH causes hypocalcaemia and hyperphosphataemia (high phosphate levels in the blood), resulting in neuromuscular symptoms ranging from paraesthesia to tetany. Manifestations include muscle spasm, anxiety, hyperreflexia and laryngeal spasm (Marieb & Hoehn 2010).
Tetany results from hyperexcitability of nerves and skeletal muscles because of low calcium blood levels. It begins with tingling in the fingertips, toes and around the mouth (Brown & Edwards 2012). The tingling increases and produces muscle tension and spasms, with consequent adduction of the thumbs, wrists, elbows and toes (carpopedal spasm). The tetany associated with hypoparathyroidism mainly affects the face and hands, causing uncontrolled contraction of these muscles.
Diagnostic tests
The general diagnosis of both hyper- and hypoparathyroidism is often difficult due to the vague symptoms of pain or fatigue. Tests used to assess parathyroid function therefore include estimation of serum calcium and serum phosphorus levels (LeMone et al 2011). Elevated serum calcium alone is not definitive as blood calcium may be influenced by diet and medications also. A 24-hour urine collection may be performed to measure the total urine calcium, as hyperparathyroidism causes increased excretion of calcium in the urine, and hypoparathyroidism causes decreased excretion of calcium. Scans or x-rays to ascertain bone changes and antibody parathyroid tests may also be implemented. Testing for hypocalcaemia and hypoparathyroidism involves checking for a positive Chvostek’s sign (facial muscle spasm occurring when the facial nerve is tapped) and a positive Trousseau’s sign (hand muscle spasm when pressure is applied to the nerves and vessels of the upper arm) (Farrell & Dempsey 2011; Monahan et al 2007).
Treatment of hyperparathyroidism is directed at the cause and often results in good prognosis for the client. Surgical removal of tumours or removal of the parathyroid glands may be necessary, leaving half of one parathyroid gland, which is all that is required to maintain normal PTH levels. Other treatments include hydration or diuretic therapy to force increased excretion of calcium by the kidneys, and decreasing calcium intake in the diet (Farrell & Dempsey 2011).
An important aspect of the nursing care is the client education and management of dietary calcium and phosphate intake. Treatment of hypoparathyroidism with calcium supplements and vitamin D, which controls absorption of calcium in the gastrointestinal tract, gives a good prognosis (McCance & Huether 2010). Parathyroid autotransplantation, where the patient’s parathyroid glands are replaced during a thyroidectomy, is being implemented more often in Australia and New Zealand (Ebrahimi et al 2009). Following thyroidectomy, hypocalcaemia and tetany may occur. In this instance the client is infused calcium intravenously. Parenteral PTH may also be administered.
Disorders of the adrenal glands
Disorders of the adrenal cortex—hyperadrenalism
Hyperadrenalism is usually confined to the cortex of the gland. It may be caused by neoplasia or hyperplasia, or it may be iatrogenic, that is, a condition caused by medical treatment or procedures. Other factors resulting in hyperfunctioning of the cortex include tumours or hyperplasia of the pituitary gland, whereby the negative feedback control of the adrenal cortex by ACTH is disturbed. Manifestations of hyperfunctioning are generally related to an excess of glucocorticoids, mineralocorticoids and sex hormones. Three major disorders can result.
Cushing’s syndrome
Cushing’s syndrome is a collection of endocrine disorders due to excess corticosteroids (Brown & Edwards 2012). This condition may result from excessive use of corticosteroids, hypersecretion of pituitary ACTH (Cushing’s disease) or a tumour of the adrenal glands or pituitary. Manifestations of this condition are related to hyperglycaemia, abnormal distribution of lipids and protein wasting. Classic symptoms include obesity of the trunk with a pad of fat across the shoulders (buffalo hump), a moon face, striae on the breasts, abdomen and legs, muscle weakness, osteoporosis and skin fragility (Craft et al 2011; Farrell & Dempsey 2011; Monahan et al 2007) (Fig 34.7). Persistent hyperglycaemia may develop into diabetes mellitus. Elevated cortisol levels exacerbate catecholamine effects and, as a result, hypertension is a common feature. This may lead further to atherosclerosis, ischaemic heart disease and nephrosclerosis.
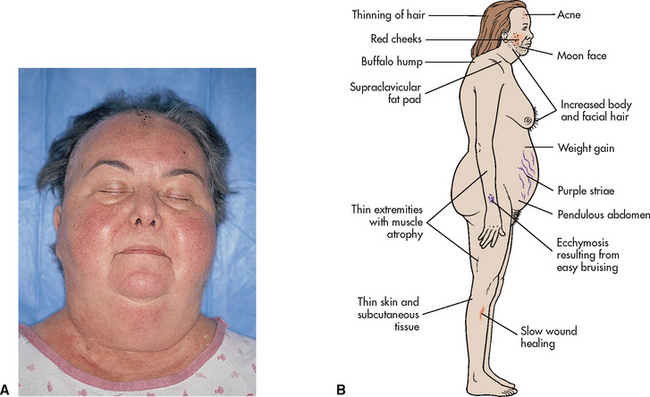
Figure 34.7 Cushing’s syndrome
(A Seidel HM, Ball JW, Dains JE et al (2006) Mosby’s Guide to Physical Examination, 6th edn. Mosby, St Louis, Fig 50-11; B Lewis SL, Ruff Dirksen S, Heitkemper MM et al (2011) Medical-Surgical Nursing, 8th edn. Mosby, St Louis, Fig 50-12)
Women are more likely to develop Cushing’s syndrome, and excess androgen production causes virilisation in females of all ages (Farrell & Dempsey 2011). Male traits such as hirsutism and voice deepening and breast atrophy may occur. A complication of Cushing’s syndrome is the individual’s decreased immune response and increased susceptibility to infection (see Clinical Interest Box 34.2).
CLINICAL INTEREST BOX 34.2 Cortisone, cortisol and Cushing’s syndrome
Cortisone is a therapy used to treat inflammatory conditions such as arthritis because of its anti-inflammatory properties. It does not cure the disease but gives individuals pain relief enabling them to continue their daily lives with as much normality as possible. Problems arise, however, because cortisone and other glucocorticoids like cortisol also depress the body’s immune system. Hypersecretion, long-term use or abuse and over-administration of cortisone or cortisol may lead to the development of Cushing’s syndrome, so the nurse needs to monitor these clients for symptoms of this syndrome. Additionally, due to the enhanced anti-inflammatory response, infections become overwhelmingly severe before producing recognisable symptoms. The nurse should also therefore protect the individual or patient from infection. Sadly, without treatment, almost half of the individuals with Cushing’s syndrome die within 5 years of onset due, in part, to complications with infection and hypertension.
Primary aldosteronism
Primary aldosteronism, also known as Conn’s syndrome, is usually caused by an adrenal tumour and is the result of hypersecretion of the mineralocorticoid aldosterone (Craft et al 2011). Conn’s syndrome leads to impaired reabsorption of sodium and water in the renal tubules, resulting in hypertension, hypokalaemia, muscle weakness, polyuria, polydipsia, metabolic acidosis and neuromuscular dysfunction (Brown & Edwards 2012). Given the high incidence of hypertension in Australia and New Zealand (see Ch 23), alterations of aldosterone function should be of consideration and interest in the nursing assessment.
Androgenital syndrome
Hypersecretion of androgens may result in androgenital syndrome, especially in prepubertal children. Excessive secretion of androgens may cause contradictions in secondary sexual characteristics. For example, female children are seen with excessive hair growth on the legs, chest and abdomen, an enlarged clitoris, a deepened voice and amenorrhoea (features exhibited also by individuals suffering Cushing’s syndrome). Male children may experience precocious puberty (premature sexual development) and gynaecomastia (breast development) (Congenital Adrenal Hyperplasia Support Group Australia nd).
Disorders of the adrenal cortex—hypoadrenalism
Addison’s disease
Hypofunctioning of the adrenal cortex may be caused by primary insufficiency, resulting in a condition known as Addison’s disease, or adrenocortical insufficiency. This is a rare condition thought to be due to an autoimmune reaction (Michels & Eisenbarth 2007), infection, acquired immunodeficiency syndrome (AIDS), cancer or adrenal gland atrophy. Up to 90% of the cortex can be destroyed before the symptoms become evident; as a result, the disease is often advanced before it is diagnosed (Australian Addison’s Disease Association 2006; Brown & Edwards 2012). The manifestations are related to a deficiency of all corticosteroids. The lack of mineralocorticoids depletes sodium, which leads to diarrhoea, dehydration, weakness, weight loss and hypotension. The lack of glucocorticoids affects blood sugar levels, leading to hypoglycaemia (Marieb & Hoehn 2010). An increased ACTH level, due to disturbed negative feedback control, may lead to brown pigmentation or bronzing of the skin and mucous membranes, affecting the palms, elbows, scars, skin folds and areolae (Australian Addison’s Disease Association 2006). Addison’s disease may also have profound effects on an individual’s mental state, with individuals exhibiting depression, confusion or apathy (Brown & Edwards 2012; Monahan et al 2007).
Congenital adrenal hyperplasia (CAH) is often classified together with Addison’s as general adrenal insufficiency. Similar to Addison’s disease, cortisol and aldosterone are either partially or not produced. The condition may be classified as ‘classic’ (congenital, a genetic defect in steroid synthesis) or ‘late-onset’ (early adult onset). Growth and adrenal problems may develop, as for other adrenal insufficiencies (Endocrinology Expert Group 2009). In New Zealand CAH is part of routine screening for newborns and about one in 10 000 children are born with CAH (Congenital Adrenal Hyperplasia Support Group New Zealand nd). Treatment centres on replacement of the corticoids with analogues such as dexamethasone and fludrocortisone.
Disorders of the adrenal medulla
Phaeochromocytoma
Phaeochromocytoma is a generally benign tumour of the medulla or of the sympathetic chain, which causes over-secretion of adrenaline and noradrenaline. Incidence peaks in middle age and is also high in familial members, and may occur as part of multiple endocrine neoplasia (MEN) (Farrell & Dempsey 2011). The resultant increase in sympathetic activity manifests as episodes of hypertension, tachycardia, chest pain, pallor, sweating, headaches, anxiety, tremor, nausea and vomiting and frequency of urination. These symptoms can cause life-threatening scenarios for the individual, so early diagnosis and treatment is imperative (Monahan et al 2007).
Diagnostic tests
Typically, blood and urine tests are performed that enable measurement of the levels of adrenal cortical hormones. Blood tests determine the level of serum aldosterone, serum cortisol or serum catecholamines (LeMone et al 2011). These levels aid in the diagnosis of adrenal function; for example, serum cortisol levels are increased in Cushing’s syndrome, while decreased serum cortisol levels are seen in Addison’s disease. A 24-hour urine collection may be performed to estimate the total daily free cortisol, aldosterone or catecholamine levels. A CT scan or suprarenal venography may also be used to aid in the diagnosis of tumours.
Surgical removal of the tumour or the dysfunctional adrenal gland (adrenalectomy) may correct Cushing’s syndrome for the individual (LeMone et al 2011). Lifetime hormone therapy to replace the cortical hormones is then required. Clinical Interest Box 34.2 provides an insight into cortisone therapy. Nurses should decrease risk of injury and infection, encourage rest and activity, promote skin care and stimulate mental activity for patients or clients with Cushing’s syndrome. Treatment and nursing care for Addison’s disease and CAH includes corticosteroid therapy, and the assessment and management of fluid levels, signs of shock and careful monitoring of symptoms (Craft et al 2011; LeMone et al 2011).
Measurements of blood and urine for catecholamines are the most direct and conclusive tests for disorders of the adrenal medulla like phaeochromocytoma. Likewise, the presence of hypertension, headache, hypermetabolism and hyperglycaemia indicates phaeochromocytoma. Imaging studies may also be carried out to identify the location of the suspected tumour, which is preferably removed surgically. Treatment and nursing management procedures include those for high blood pressure, high blood glucose and tachycardia, and HRT is often indicated following surgery (Brown & Edwards 2012; Monahan et al 2007).
Disorder of the gonads
Hypergonadism and hypogonadism
Gonadal disorders may reflect interruptions in the normal control by the pituitary, a tumour or congenital syndromes such as Turner’s or Klinefelter’s (Endocrinology Expert Group 2009; Craft et al 2011). Hypergonadism, as opposed to hypersecretion of gonadotrophins by the pituitary gland or androgenital syndrome, results from increased gonadal hormone production before puberty and leads to precocious sexual development and enhanced sexual characteristics in both sexes. Causes of hypergonadism are primarily unknown but some cases have been caused by ovarian tumours and testicular tumours (Funnel et al 2008). Treatment for both sexes involves removal or radiation of the tumours, and hormone therapy to suppress or counteract the effects of the sex hormone.
Hypogonadism results from decreased sex hormone production by the age of normal puberty. Due to the control of gonad function by the pituitary and adrenals, hypogonadism prior to puberty reflects abnormalities of these endocrine glands. Treatment with testosterone or oestrogen is usually effective (Endocrinology Expert Group 2009).
Endocrine deficiencies of the gonads in the adult female, for example, are implicated in two common disorders: osteoporosis and polycystic ovary syndrome (PCOS). This reflects the strong interrelationship between endocrine organs and hormones in the human body. Osteoporosis and PCOS are discussed further in Clinical Interest Boxes 34.3 and 34.4 respectively.
CLINICAL INTEREST BOX 34.3 Osteoporosis and oestrogen
Osteoporosis is characterised by an imbalance between bone formation and bone resorption, where bone cells called osteoclasts digest bone faster than it can be deposited. Nurses and clients usually become aware of osteoporosis only when the weakened bones break and clients are admitted as a result of fractures. While osteoporosis is strongly linked to calcium intake, calcium metabolism is controlled by the parathyroid glands and PTH. Likewise, the appearance or acceleration of bone weakening with the onset of menopause in females is closely related to the levels of oestrogen: oestrogen secreted by the ovaries actually exerts inhibition over the osteoclasts, preventing them from digesting too much bone and calcium. Decreasing levels of oestrogen postmenopause may therefore contribute to osteoporosis. The use of hormone replacement therapy (HRT) and dietary supplements helps to address this endocrine imbalance.
CLINICAL INTEREST BOX 34.4 Polycystic Ovarian (Ovary) Syndrome (PCOS)
Despite the misleading name, polycystic ovarian (ovary) syndrome (PCOS) is a hormonal disorder in females in which excessive androgen production is triggered by inappropriate secretion of gonadotrophins. This is perhaps better reflected by the alternative name ‘hyperandrogen anovulation syndrome’. Surprisingly it is the most common endocrine disturbance affecting women, affecting from 5% to 12% of reproductive age women in Australia.
PCOS develops as a result of ovarian hypersecretion of androgens, with an overall result of increasing testosterone levels. Hyperinsulinaemia is thought to stimulate this hypersecretion of androgens. Elevated levels of androgens lead to elevated levels of oestrogen and testosterone, which trigger an imbalance in the pituitary gonadotrophins FSH and especially LH.
Clinical signs or symptoms of PCOS reflect the interruption of ovulation and the elevated male hormone levels. Various signs or symptoms exhibited by individuals mirror those of other endocrine disorders (especially those of the thyroid, adrenal cortex and gonads generally), thus nursing assessment of PCOS may be difficult. Manifestations include dysmenorrhoea or amenorrhea, hirsutism or androgenic alopecia, obesity and infertility. Women with PCOS also appear to be at greater risk of developing hyperglycaemia, cardiovascular disease and type 2 diabetes mellitus later in life.
(Craft et al 2011; Endocrinology Expert Group 2009; Polycystic Ovary Association of Australia 2011)
Disorders of the pancreas
Diabetes mellitus
Diabetes mellitus (DM) is a group of metabolic disorders characterised primarily by abnormal glucose metabolism, with the key feature of elevated blood glucose (hyperglycaemia). Diabetes mellitus affects a great number of the Australian and New Zealand population, with more than 7% of the Australian population over 25 having diabetes (Australian Institute of Health and Welfare 2008, 2009). In New Zealand, over 120 000 people suffer from diabetes (New Zealand Ministry of Health 2007), although current estimates suggest as many as 270 000 (Diabetes New Zealand 2011). Type 2 diabetes mellitus is more common than type 1, with over 95% of the population having type 2. Incidences and death rates in both Australia and New Zealand are higher for males than females, and both Indigenous Australians and Māori populations are almost twice as likely to be diagnosed (Australian Institute of Health and Welfare 2008, 2009; New Zealand Ministry of Health 2007). Such is the increasing prevalence in diabetes worldwide that diabetes nursing is becoming more specialised, with distinct nursing career and competency frameworks being developed (Davis et al 2008).
Diabetes mellitus is a disorder of glucose regulation characterised by abnormal metabolism of carbohydrate, protein and fat (Farrell & Dempsey 2011). A feature of the disorder is some degree of hyperglycaemia. Diabetes mellitus results from hyposecretion or hypoactivity of insulin, intolerance to insulin (hyperinsulinaemia) or from the production of substances antagonistic to insulin, which cause it to be ineffective. It may be caused by genetic or hereditary factors, pancreatic disorders or by disorders of other endocrine glands such as the pituitary or adrenal glands (recall that these endocrine glands secrete hormones that affect glucose metabolism). It is also thought to be autoimmune in origin: the destruction of β cells by the body may be a response to molecular mimicry by a pathogen, or due to possible diabetes susceptibility genes (Craft et al 2011; Farrell & Dempsey 2011; Marieb & Hoehn 2010).
A deficiency of insulin causes changes in the metabolism of glucose, which results in numerous physiological effects (Fig 34.3). In the liver glycolytic enzymes are inhibited, gluconeogenic enzymes are activated and amino acids are used to form glucose. Consequently, additional glucose is liberated into the bloodstream. Without insulin, glucose is unable to cross the cell membranes into muscle and fat cells, and hyperglycaemia ensues (Marieb & Hoehn 2010). The blood glucose level eventually exceeds the renal threshold, and the excess glucose is excreted in the urine (glycosuria). The most significant effect of elevated blood glucose level is dehydration of the tissue cells because the increased osmotic pressure in the extracellular fluids causes osmotic transfer of water out of the cells. In addition, the loss of glucose via the urine causes diuresis (polyuria), because of the osmotic effect of glucose in the renal tubules. The result is extreme thirst, or polydipsia (Monahan et al 2007).
Gestational diabetes is one form of the disorder and appears only during pregnancy (about 5% of pregnancies) (AIHW 2010; Diabetes Australia 2011b), and is characterised by impaired glucose tolerance. A woman who has had gestational diabetes runs a greater risk (50–60%) of developing diabetes mellitus later on in life (AIHW 2010; Craft et al 2011). Diabetes mellitus itself is classified into two types: type 1, or insulin-dependent diabetes mellitus (IDDM); and type 2, or non-insulin-dependent diabetes mellitus (NIDDM). A third minor type, pancreatic or type 3c diabetes (Hardt et al 2008), relates to exocrine insufficiency and may contribute to diabetes mellitus. It is believed therefore to be generally un- or misdiagnosed. The diagnosis of diabetes mellitus is confirmed, among other tests, by the presence of an elevated blood glucose level. The manifestations of diabetes mellitus vary according to the type.
Type 1 diabetes mellitus
Type 1 diabetes mellitus is characterised by the genetic or autoimmune destruction or deficiency of the pancreatic β cells that produce insulin and usually progresses so quickly it is often diagnosed in childhood, hence its previous name of juvenile-onset diabetes (Farrell & Dempsey 2011) (see Clinical Scenario Box 34.3). It can occur, however, at any age from infancy up to about age 40. There is a sudden onset of signs and symptoms related to hyperglycaemia, which includes weight loss, polyuria and polydipsia (related to osmotic diuresis), fatigue and the presence of glucose and ketones in the urine (glycosuria and ketonuria) (Craft et al 2011). Hyperglycaemia and dehydration accelerate the development of metabolic acidosis and, if untreated, can lead to coma and death.
Clinical Scenario Box 34.3
I was dreaming that I was drinking an ice cold drink of water, it was great, so cool then I woke up. Oh no, it’s happening again! What is going on with me? I’m just so thirsty all the time. The teachers are getting really tired of me leaving the classroom heaps of times to go to the toilet. The thing is I’m not mucking around. I really do need to go. Plus, I’m so tired now, the thought of doing physical education at school freaks me out and there is no way I want to try out for the netball team, I’m just too tired. God, I’m scared, what if I’m sick? I’m at the fridge getting a cold drink. It’s 4 am. I turn to go back to bed and there is Mum. I just start crying.
I knew something was wrong with Emma, and had so many times thought it was just part of growing up—you know, hormonal changes and all that goes with an adolescent girl. I had discussed her continual thirst and tiredness with my friends and none of them seemed to have experienced the same problems with their girls. Now, this person who is a diabetes educator is trying to tell me Emma has diabetes! I don’t believe it! She is my little girl, I feel as if my world is falling apart. I know I have to be strong for Emma but I faint at the sight of blood and the nurse is talking about injections and blood tests. I just don’t understand—what have I done wrong?
As a diabetes educator I am often faced with this situation when adolescents are diagnosed with type I diabetes. The experiences often involve parents feeling they have failed the child and done something wrong, and the child often feels as if their life is now virtually over. As these individuals are going to be dealing with diabetes now for life, an extensive education including family support is paramount to their continuing holistic health. That is where I come in.
Type 2 diabetes mellitus
Type 2 diabetes mellitus is a hyperglycaemia despite the availability of insulin (insulin resistance at the cell) and is more likely to occur after age 40. The signs and symptoms develop slowly and the individual is generally obese: obesity and metabolic syndrome are significant risk factors and often precursors for type 2 diabetes mellitus (Craft et al 2011). Type 2 diabetes mellitus may often go undetected, as the signs and symptoms of fatigue, polyuria and polydipsia can be quite insignificant. Diagnosis occurs when the individual presents with another disease, which is a common complication of diabetes mellitus (McDowell et al 2007). The individual with diabetes mellitus is prone to a range of complications, especially if the blood glucose level is uncontrolled. Complications include:
• Hyperosmolar nonketotic hyperglycaemia
• Cardiovascular and peripheral vascular disease
• Hypoglycaemia (imbalance of food, exercise and medications).
It is these complications that the nurse may need to assess and monitor. The maintenance of blood glucose levels are achieved by a balance between blood-borne stimuli, nervous input (e.g. the sympathetic nervous system) and hormonal influences. As such, there are a number of influences on blood glucose levels that the nurse should take into consideration.
Complications of diabetes mellitus
Complications may develop rapidly or progress insidiously over years before they become evident. The two main complications that occur because of uncontrolled blood glucose levels are hypoglycaemia and hyperglycaemia.
Hypoglycaemia
Hypoglycaemia, sometimes referred to as insulin shock, is an abnormally low blood glucose level (3.5–4.0 mmol/L) (Diabetes Australia 2011c) and may be caused by excessive insulin, delayed or decreased food intake, excess physical activity and carbohydrate use (Farrell & Dempsey 2011). Hypoglycaemia may develop when glucose levels drop rapidly and early manifestations are hunger, pallor, sweating, tachycardia, yawning, nausea, blurred vision, slurred speech, confusion or irrational behaviour (Brown & Edwards 2012). If the hypoglycaemia is not corrected rapidly, the person will become comatose and, if the coma is prolonged, permanent brain damage or death may occur (Farrell & Dempsey 2011).
Management and intervention
Mild symptoms can often be reversed by ingesting a simple carbohydrate, such as fruit juice or honey. People with type 1 diabetes should ensure that they always have ready access to some form of carbohydrate that is easily absorbed, such as barley sugar or jelly beans (Farrell & Dempsey 2011). If the person is unable to ingest a carbohydrate substance, intravenous administration of glucose (up to 50%) (Endocrinology Expert Group 2009) or the hormone glucagon will be necessary. Glucagon can also be administered subcutaneously or intramuscularly. The Somogyi effect is a complication that may follow a hypoglycaemic episode or administration of too much insulin, and results in rebound hyperglycaemia (McDowell et al 2007). Management of this phenomenon involves lowering the insulin dosage (Craft et al 2011).
Hyperglycaemia
Hyperglycaemia is an abnormally high blood glucose level and is a clinical feature of diabetes. It may be caused by low insulin levels, incorrect diet high in carbohydrate, lack of physical activity or stress (with infection being a common stressor). Glucose and lipids are not able to enter target cells, depriving them of nutrients. This is turn stimulates increased hunger and food intake (polyphagia). Together, polyphagia, polyuria and polydipsia are the three main symptoms of hyperglycaemia (Craft et al 2011; Monahan et al 2007). If hyperglycaemia is not corrected, metabolic acidosis occurs, producing vasodilatation and hypotension. Severe acidosis, if untreated, results in coma and death. Hyperglycaemia develops gradually over a period of hours or days, and the early manifestations are thirst, anorexia, nausea, vomiting, abdominal pain, muscle cramps, polyuria and drowsiness. Deep rapid respirations (Kussmaul’s breathing) develop in an attempt to control acidosis (Brown & Edwards 2012). Dehydration and electrolyte imbalance occur as a result of osmotic diuresis.
Ketones, produced by the liver as fat, are broken down instead of glucose (diabetic ketoacidosis), glucose is excreted in the urine and the breath smells of ketones (sweet fruity odour). Diabetic ketoacidosis (DKA) develops primarily in individuals with type 1 diabetes mellitus, and in the absolute deficiency of insulin. As a result of altered liver metabolism, acidosis ensues, which may lead to coma or death (LeMone et al 2011). Advanced diabetic ketoacidosis is an emergency requiring nursing and medical care. Similarly, hyperglycaemic hyperosmolar state (HHS) occurs in people with type 2 diabetes and is characterised by higher than normal blood osmolarity, greatly elevated blood glucose and altered levels of consciousness. It is a serious medical emergency with a slow onset (Farrell & Dempsey 2011; LeMone et al 2011).
Management and intervention
Management of hyperglycaemia focuses on correcting the fluid and electrolyte imbalances and lowering blood glucose levels to normal. Intravenous fluids such as normal saline are administered together with intravenous short-acting insulin. After the initial decrease in blood glucose level, the insulin dosage is reduced. This allows the blood glucose levels to return to normal more slowly, and prevents the development of hypoglycaemia. If the client becomes comatose as a result of hyperglycaemia and ketoacidosis, care is directed at preventing the complications of unconsciousness (Brown & Edwards 2012; McDowell et al 2007).
Long-term complications
Cardiovascular diseases
Atherosclerosis is a relatively common complication of diabetes mellitus, with coronary artery disease being the major complication. Myocardial infarction, cerebrovascular accident and renal failure are among the major causes of death in people with diabetes (AIHW 2008). Peripheral vascular disease is often widespread, causing manifestations of peripheral ischaemia—cold lower extremities, intermittent claudication, diminished or absent pulses, ulcers, infection and gangrene. Hypertension is also a common complication of diabetes mellitus (AIHW 2008). It affects 20–60% of all individuals and is a major risk factor for cardiovascular disease and microvascular complications such as neuropathy and retinopathy (LeMone et al 2011).
Neuropathy
Neuropathy (inflammation and degeneration of the peripheral nerves) gives rise to disturbances of sensation such as tingling and numbness, particularly in the lower limbs (Farrell & Dempsey 2011). Another form of neuropathy is characterised by a decreased sense of position (proprioceptive disturbances) and diminished sensation to touch, pain and temperature. These sensory deficits increase the possibility of injury and increase the chance of injuries being unnoticed by the individual. Peripheral motor involvement results in muscle weakness and atrophy, which may lead to deformities, particularly of the feet (Charcot’s arthropathy) (Farrell & Dempsey 2011). Early identification of peripheral neuropathy is an important part of preventative foot care (Endocrinology Expert Group 2009).
Retinopathy
Retinopathy is a disorder of retinal blood vessels, characterised by haemorrhages and leakage of blood and serum into the retina. Repeated vitreous haemorrhage may result in retinal detachment or blindness. Glaucoma and cataracts may also develop in the presence of diabetes mellitus. Individuals should consider consulting or be referred to an ophthalmologist or optometrist (Farrell & Dempsey 2011).
Nephropathy
Nephropathy, or kidney disease, may occur over a period of many years, and few signs or symptoms occur until the presence of blood proteins in the urine (microalbuminuria), uraemia and oedema develop (Farrell & Dempsey 2011; LeMone et al 2011). Diabetic nephropathy is more likely to develop if diabetes is poorly controlled. Renal function is gradually impaired by glomerulosclerosis, and it may progress to chronic renal failure. Preventing and treating any condition that may impair renal function, such as urinary tract infections or hypertension, reduces the development of diabetic nephropathy (McDowell et al 2007).
Sexual dysfunction
Impotence occurs in about half of all men with diabetes (LeMone et al 2011). In addition to the psychological causes of impotence, the condition is thought to be caused by damage to the nerve endings as a result of diabetic neuropathy, where the nerves controlling reflex blood flow to the penis are affected by persistently altered blood glucose levels. Controlling blood glucose levels may therefore help to prevent or alleviate the problem of impotence associated with diabetes. Possible complications in women include decreased vaginal lubrication and libido, and increased urinary tract infections and vaginitis (LeMone et al 2011).
Diagnostic tests
Laboratory evaluation of the pancreatic islets is mainly concerned with levels of glucose in the blood and urine. Urine is typically examined for the presence of glucose and ketones (see Ch 29). As a result of increased lipolysis, excess ketone bodies accumulate and are excreted in the urine. Urine may also be tested for the presence of protein (microalbuminuria), which may detect the early onset nephropathy. Blood tests measure the body’s use of glucose and include:
• Glucose tolerance test. This test evaluates glucose absorption after its oral or intravenous administration. The individual fasts overnight before the administration of glucose; glucose levels are then measured at intervals to diagnose diabetes mellitus
• Fasting serum glucose test. Also referred to as fasting blood or plasma glucose (FBG, FPG), this test measures glucose level after the individual has fasted for an 8-hour period; the blood glucose level is then measured. In diabetes mellitus, the absence or deficiency of insulin allows persistently high serum glucose levels above 7.8 mmol/L (LeMone et al 2011)
• Two-hour postprandial serum glucose. This test provides information about the body’s utilisation and disposal of glucose after a meal. An elevated blood glucose level is indicative of diabetes mellitus
• Tolbutamide tolerance test. An intravenous injection of the drug tolbutamide usually stimulates the secretion of insulin. Normally, after administration of the drug, the serum glucose level will fall rapidly and return to normal baseline levels in 3 hours. In diabetes mellitus there is a slow initial drop and a prolonged time to return to pre-set levels
• Glycosylated haemoglobin (HbA1c) test. This test is performed to review the control of diabetes mellitus over the previous 2–3 months. Results reflect the average glucose level attached to haemoglobin within erythrocytes rather than the serum glucose level, and the timeline reflects the average longevity of the erythrocyte. In diabetes mellitus it may be elevated to three times the normal level
(Brown & Edwards 2012; LeMone et al 2011; McDowell et al 2007).
Cultural considerations: diabetes in the Māori population
In New Zealand, groups at risk of developing type 2 diabetes include not only those who are overweight, have familial history of the disease or who have a reduced physical activity, but also those of Asian, Māori or Polynesian descent (Diabetes New Zealand 2008a). The incidence of diabetes mellitus, particularly type 2, is both higher in Māori than Pākehā (European or white New Zealanders), and is expected to rise more quickly in the future. Currently about 1 in 32 New Zealand Pākehā adults has known diabetes, whereas 1 in 12 Māori and 1 in 12 Pacific Island adults have known diabetes (Diabetes New Zealand 2008a). In 2020 it is expected that 1 in 22 Pākehā and 1 in 6 Māori and Pacific Island adults will have known diabetes.
A contributing factor is the body size and weight status of Māori in New Zealand. A recent New Zealand Health Survey found that body size indicators (body mass index—BMI) for Māori male and female children and adults were consistently higher than those for non-Māori. The only exception was for males over 15 years where non-Māori males had a higher BMI than Māori. However, significantly more Māori men over 15 years were obese compared to non-Māori (New Zealand Ministry of Health 2010).
Associated with this increased prevalence and incidence is an increased risk of developing secondary complications, particularly renal and lower limb complications. According to the New Zealand Ministry of Health, renal failure is nine times more prevalent in Māori with concurrent diabetes compared to non-Māori. Likewise lower limb amputations are four times more likely in Māori (New Zealand Ministry of Health 2010). A descriptive analysis of Māori health status data is available in the form of Māori Health Chart books (Tatau Kahukura 2010 and Tatau Kura Tangata 2011) (New Zealand Ministry of Health 2011).
As a population group, Māori have on average the poorest health status of any ethnic group in New Zealand. The New Zealand Government and the Ministry of Health have made it a key priority to reduce the health inequalities that affect Māori and to close the gap in disease prevention and education between Māori and non-Māori. As a result, the Ministry of Health has developed Māori health strategies (He Korowai Oranga) and Health action plans (Whakatātaka Tuarua) which aim to set directions for Māori health development and to implement programs, policies and interventions (New Zealand Ministry of Health 2011). Table 34.3 is a summary of major endocrine imbalances.
Table 34.3 Summary of major endocrine imbalances
| Disorder | Gland or hormone implicated | Homeostatic imbalance |
|---|---|---|
| Acromegaly | Pituitary—GH | Hypersecretion in adults |
| Cushing’s disease | Pituitary—ACTH | Hypersecretion |
| Diabetes insipidus | Pituitary—ADH | Hyposecretion or insensitivity to |
| Gigantism | Pituitary—GH | Hypersecretion in children |
| Pituitary dwarfsm | Pituitary—GH | Hyposecretion in children |
| SIADH (syndrome of inappropriate ADH secretion) | Pituitary—ADH | Hypersecretion |
| Cretinism | Thyroid—TH | Hyposecretion during development |
| Graves’ disease | Thyroid—TH; or TSH from pituitary | Hypersecretion |
| Hypothyroidism: Hashimoto’s disease, myxoedema | Thyroid—TH | Hyposecretion |
| Hyperparathyroidism | Parathyroid—PTH | Hypersecretion |
| Hypoparathyroidism | Parathyroid—PTH | Hyposecretion |
| Diabetes mellitus—Type 1 | Pancreas—insulin | Hyposecretion/Hypoactivity |
| Diabetes mellitus—Type 2 | Pancreas—insulin | Resistance or insensitivity to |
| Cushing’s syndrome | Adrenal cortex—corticosteroids | Hypersecretion or overuse |
| Hypoadrenalism—Addison’s disease | Adrenal cortex or ACTH from pituitary—corticosteroids | Hyposecretion |
| Primary aldosteronism—Conn’s syndrome | Adrenal cortex or ACTH from pituitary—aldosterone | Hypersecretion |
| Phaeochromocytoma | Adrenal medulla—catecholamines | Hypersecretion |
| Osteoporosis | Ovary—oestrogen | Postmenopausal hyposecretion |
| Polycystic ovarian syndrome | Ovary—numerous | Hypersecretion of androgens |
CARE OF THE CLIENT WITH AN ENDOCRINE DISORDER
Because of the complexity of the endocrine system and its interrelation with other body systems, dysfunction of any endocrine gland can have widespread effects. The possibility of an endocrine cause needs to be kept in mind in a wide range of medical emergencies. Most endocrine disorders result from hyper- or hypoactivity of the gland or tissue. The manifestations and effects resulting from a glandular disorder therefore relate to the hormone the affected gland secretes.
Effects of endocrine dysfunction
Endocrine dysfunction has widespread effects, and the medical management and nursing care depends on the diagnosis of a specific disorder. When care is being planned, the nurse must consider the effects that the disorder may have on the individual, and any issues the individual may be coping with. While individual treatments and care suggestions have been listed for a number of conditions already discussed, below are major nursing care considerations.
A major change in lifestyle
As a result of an endocrine imbalance, the individual may have to depend on lifelong medication, dietary and fluid intake modifications, reductions in activity or exposure to stressors, and potentially irreversible physical and physiological changes. In addition, individuals may experience sexual dysfunction, an often neglected complication of endocrine disorders (Bhasin et al 2007). For example, individuals with diabetes mellitus may have to depend on lifelong medication and dietary alterations, and a lifelong commitment to monitoring blood glucose. All clients with chronic conditions should also be encouraged to wear a medical alert bracelet.
The prospect of facing major surgery
Removal of the thyroid gland may be indicated in the presence of hyperthyroidism, goitre or carcinoma of a gland. Likewise, adrenalectomy may be the preferred intervention with adrenal gland tumour or hypersecretion of corticosteroids or catecholamines. A similar approach may be taken, in addition to medication, for pituitary tumours. Clients facing major surgery should be educated and counselled on the preparation for surgery, the procedure and care required postoperatively.
Possible postoperative changes
Surgical intervention may result in the balance of hormones shifting in the opposite direction. For example, thyroidectomy for hyperthyroidism or hyperparathyroidism may result in hypofunction of the gland after removal of much of the tissue. Individuals should be educated in the likelihood of having hypofunction of the gland and the reliance on medication to address these changes.
Alterations in nutritional status
Weight gain often occurs in hypothyroidism, whereas weight loss is common in people with type 1 diabetes mellitus. Nutritional status may also be affected if the individual is experiencing anorexia, nausea or vomiting. Hyperthyroidism is associated with increased cellular metabolism and individuals often experience rapid weight loss: management of calorific intake is therefore a concern. In addition, iodine intake may need to be restricted or increased in patients with goitre or thyrotoxicosis.
Nursing care plans should also consider the reduction or management of dietary intake of calcium for clients with parathyroid imbalances. Diabetes mellitus obviously requires the management of glucose intake by health professionals and educators, and most especially by the patient.
Alterations in fluid and electrolyte balance
Excessive loss of fluid (polyuria) may occur in diabetes mellitus or posterior pituitary gland disorders such as diabetes insipidus. Loss of fluid may also occur if the individual has diarrhoea, as may be present in disorders such as hyperthyroidism and Addison’s disease. To address polyuria, the increased thirst (polydipsia) and fluid intake of clients with diabetes mellitus and diabetes insipidus should be considered.
Conversely, retention of fluid may occur in disorders such as Cushing’s syndrome, the oedema associated with hypothyroidism (and myxoedema) and SIADH. Careful fluid restrictions for clients with SIADH are suggested to allow sodium levels to increase slowly. Where the effects of aldosterone are compromised or enhanced (e.g. primary aldosteronism), electrolyte intake (especially of sodium) as well as fluids should be considered.
Management of discomfort
Many endocrine disorders result in problems related to body temperature regulation. A person with hypothyroidism is very sensitive to cold, whereas an individual with hyperthyroidism is commonly intolerant to heat. Other factors that may cause discomfort include pruritus associated with hyperthyroidism or diabetes mellitus, and joint pain associated with acromegaly. Anxiety and nervousness associated with certain disorders such as hyperthyroidism, or hypertension and headache with phaeochromocytoma, may also cause discomfort as the individual is restless and finds it difficult to relax. Apathy and difficulty in concentration may occur in hypothyroidism.
Calcium imbalances potentially affect nervous and muscular function, and individuals with hyper- and hypoparathyroidism may experience anxiety, uncomfortable nervous tingling and muscle spasms, or backache associated with calcium resorption from bones.
Reduced independence
Extreme fatigue often accompanies endocrine disorders, and the individual may require assistance to carry out activities of daily living. Certain disorders (e.g. Cushing’s syndrome or hyperparathyroidism) may be accompanied by alterations in mental status, learning and memory (McCance & Huether 2010). Altered states of awareness or consciousness (hypothyroidism, decreases in fluid electrolyte levels) will also reduce the individual’s level of independence.
Hypertension and arteriosclerosis associated with disturbances to glucose and lipid metabolism (e.g. diabetes mellitus, Cushing’s syndrome) may also restrict clients to less exertive activities and a greater reliance on assistance.
Altered body image
Clients may experience both physical and mental alterations to body image and subsequent changes in behaviour to address these concerns. Healthcare professionals should be prepared to explain the manifestations to the client, and to consult and counsel the client in the event of changes in self-esteem or behaviour associated with physical changes.
Obvious changes in physical appearance occur with Cushing’s syndrome (e.g. buffalo hump, moon face), and exophthalmos and possible goitre associated with hyperthyroidism. Clients may also exhibit changes in height and appearance with gigantism and acromegaly. Hirsutism associated with adrenal, pituitary or gonadal dysfunction may severely affect the self-esteem of clients (especially females), and children suffering from androgenital syndrome are especially likely to suffer from psychological effects of an altered body image. Individuals with Addison’s disease may experience increased skin pigmentation (bronzing).
The plan of care that the nurse designs for a client should specifically address the need for comfort, psychological support, and maintenance of nutritional and fluid status. Clients should also be given information regarding professional support and self-help groups and should be encouraged to contact these organisations for further support and information. A list of organisations and websites is provided in online resources.
Care of the client with diabetes mellitus
The main aims of management of the client with diabetes mellitus are to maintain the blood glucose level within an acceptable range and to implement measures to assist the person to live as normal a life as possible. Diabetes can be controlled at present, but not cured; management is therefore aimed at controlling it and preventing complications. Care of a client with diabetes involves regulating the blood glucose level by diet, exercise and insulin or oral hypoglycaemic agents, preventing infection and monitoring blood and urine glucose levels. The overall aim for the individual is self-management of the disorder, which makes education of the client in all aspects of care an essential part of management.
Diet, nutrition and energy requirements
Diet is an important aspect in control of both type 1 and type 2 diabetes mellitus. A dietitian works with the client to develop a well-balanced, healthy food intake. It is essential that the client gains understanding of the principles and can then modify and adapt the diet to meet demands experienced during illness, injury, emotional stress or major changes in lifestyle. The aims of the diet for a person with diabetes are to:
• Improve health and prevent or delay complications
• Provide for normal growth rate in children
• Provide a balanced dietary intake
• Attain and maintain a desirable body weight
• Meet energy requirements and
• Maintain the level of blood glucose within the normal range to prevent hypoglycaemia and hyperglycaemia.
Normal or average blood glucose is around 5 mmol/L (4–6 mmol/L fasting), with a range of 3.5–8 mmol/L. Fasting plasma glucose in a non-diabetic individual is usually less than 6.1 mmol/L, and a repeated fasting level of at least 7.0 mmol/L is indicative of hyperglycaemia (LeMone et al 2011). The Heart and Diabetes Institute of Australia (Heart and Diabetes Institute of Australia 2011) recommend goals for dietary and nutrition management.
Alcohol should be limited, as not only is it high in sugar and may cause adverse reactions to some medications used in the treatment of diabetes mellitus, it may also mask hypoglycaemia (Endocrinology Expert Group 2009). The dietitian will supply the individual with instructions for planning nutritious and varied meals within the prescribed limitations. The importance of regular meals and snacks is emphasised, particularly an evening snack to prevent nocturnal hypoglycaemia. The diet is continually evaluated and adjusted as required. When the individual is in hospital it is important that the nurse observes the amount of each meal eaten because if nutrients are not all consumed the person may experience hypoglycaemia (Brown & Edwards 2012; Craft et al 2011; Farrell & Dempsey 2011).
Exercise
The amount of exercise required by the client should be estimated and planned to balance with the dietary intake and insulin (Farrell & Dempsey 2011). A well-planned program of regular exercise that is adjusted as necessary is beneficial in weight control, in its physiological and psychological effects and in terms of blood glucose control. Regular gentle exercise stimulates the uptake of glucose by muscle cells, lowering blood glucose levels and increasing the absorption of injected insulin. For these reasons, exercise can increase the likelihood of hypoglycaemia. To prevent hypoglycaemia after exercise, the individual needs to either increase the nutritional intake or decrease the insulin dosage. Education is essential for the client to gain understanding to correctly balance the level of exercise with dietary intake and insulin dosage under varying conditions. Exercise also has the benefits of decreasing cholesterol and triglycerides, and reducing the risk of cardiovascular disease (LeMone et al 2011). Strenuous exercise, however, can trigger the release of hyperglycaemic hormones, such as adrenaline and glucagon, so should be avoided (Brown & Edwards 2008).
Insulin
As insulin is essential for the normal metabolism of glucose, it is a major factor involved in the regulation of blood glucose levels. Many types of insulin are available and these may be rapid onset-fast acting, short-, intermediate- or long-acting, or mixed (Diabetes Australia 2011d). The different types of insulin vary in the time of onset and the duration of action, and are selected according to the time of day, activity and nutritional intake. As soon as possible after diagnosis, the client is given instruction on insulin administration and care for the equipment required. Insulin may be administered via syringe and needle (now less commonly used), specialised pens or by pump (CSII—continuous subcutaneous insulin infusion) (Diabetes Australia—Vic 2010; LeMone et al 2011; McDowell et al 2007). Clients are also instructed on rotating injection sites around the abdomen and thighs to prevent tissue atrophy or hypertrophy at regularly used injection sites. The abdomen provides the fastest and most consistent rate of absorption and is an accessible site for the person to use (Figs 34.8 and 34.9).
Figure 34.8 Insulin injection sites
(Lewis SL, Ruff Dirksen S, Heitkemper MM et al (2011) Medical-Surgical Nursing, 8th edn. Mosby, St Louis, Fig 49-5)
Short-acting insulin may also be delivered continuously at a basal rate subcutaneously by a CSII pump, with dosages entirely self-determined and monitored by the individual. Clients choosing this insulin regimen need to be motivated, and educated by a nurse or diabetes educator (Australian Diabetes Educator Association 2011b). Insulin pumps and associated equipment are subsidised in Australia by the NDSS (National Diabetes Services Scheme) (Endocrinology Expert Group 2009; Diabetes Australia–NDSS 2011).
Oral hypoglycaemic agents
Oral hypoglycaemic agents may be prescribed in the management of type 2 diabetes mellitus unresponsive to diet alone. The medications used in the treatment of diabetes mellitus act in a variety of ways, by stimulating the pancreas to release insulin (secretogogues), reducing insulin resistance (sensitisers), the promotion of glucose use or delaying glucose absorption across the gut (Endocrinology Expert Group 2009).
Hygiene and preventing infection
People with diabetes mellitus are susceptible to infection as a result of impaired body defence mechanisms and elevated glucose providing an environment for bacterial growth (Craft et al 2011). The person with diabetes is particularly at risk of developing urinary tract infections, fungal infections such as Candida and infected skin lesions (Brown & Edwards 2012). To minimise the risk of infection the person should understand the importance of good control of blood glucose levels, of maintaining a high standard of personal hygiene and of avoiding unnecessary exposure to infection (LeMone et al 2011). The skin should be kept clean and free from excessive irritation, friction or pressure. All injuries to, or infections of, the skin, however minor, should be cleaned with a mild antiseptic solution and covered with a dressing (McDowell et al 2007).
Skin lesions of the feet commonly occur in people with poorly controlled diabetes mellitus (Heart and Diabetes Institute of Australia 2011). Such lesions result from, or are exacerbated by, impaired circulation and peripheral neuropathy (Craft et al 2011). If a minor injury or lesion is undetected or untreated, ulceration and infection may develop (Fig 34.10). Between 50% and 75% of lower extremity amputations are performed on people with diabetes (Farrell & Dempsey 2011). The nurse should also consider the lesion or aberration to be of different origin: melanomas of the feet have been misdiagnosed as diabetic foot ulcers (Rogers et al 2007; Yesil et al 2007). Care of the feet and prevention of foot lesions is therefore of prime importance and national guidelines on the prevention, identification and management of foot complications in diabetes are available (Heart and Diabetes Institute of Australia 2011).
Guidelines for foot care in individuals with diabetes
• Wear well-fitting socks or stockings, and shoes. Socks should be made from natural fibre (e.g. wool or cotton) with care taken to avoid hosiery with seams under the feet
• Practise daily cleansing, drying and inspection of the feet. A mild soap is used and the feet should not be soaked for long periods. After washing, the feet should be patted dry rather than rubbed. Dry feet may be gently massaged with lanolin or oil to prevent fissures
• Consult a podiatrist if corns or calluses need attention
• Trim the toenails straight across, to avoid damage to the surrounding skin
• Keep the feet warm but avoid placing them close to heating devices, to prevent possible skin damage. If an electric blanket is used to warm the bed, it should be turned off before the person gets into bed
• Avoid walking barefooted or on hot surfaces
• Perform foot and leg exercises to increase circulation
• Avoid constrictive clothing (e.g. socks with elasticised tops) and crossing the legs, both of which may reduce blood flow
• Seek immediate attention from the podiatrist or medical officer if there is any injury, colour change, infection or discharge of pus or blood
(Craft et al 2011; Diabetes Australia 2011e; Farrell & Dempsey 2011; LeMone et al 2011; McDowell et al 2007).
Monitoring blood glucose levels
Blood glucose monitoring is an accurate method of assessing the effectiveness of diet, exercise levels and medication therapy and to gain information regarding a client’s current blood glucose level. Blood glucose monitoring is usually performed by obtaining a drop of capillary blood, which is applied to a reagent strip and read by one of the many types of glucometers available on the market. Most meters available currently show acceptable reading precision, and errors are clinically insignificant (Cohen at al 2006). A digital display of the glucose level is provided, or the colour of the strip may be compared with a standard, either visually or with a reflectance meter. A variety of blood glucose monitoring machines are available, including ones with automatic finger-pricking, which run either on batteries or from mains power (Fig 34.11).
Figure 34.11 Blood glucose meter and monitoring
(A, B, C: © Miriam Doerr/Shutterstock; D: © Lisa S./Shutterstock)
The aim of self blood glucose monitoring is to assist clients with diabetes to assume more independence in managing their condition. Each person using blood glucose monitoring receives individual instruction in the technique, from a diabetes health educator, for example (Australian Diabetes Educators Association nd, 1). People with diabetes are advised on the appropriate times at which to perform the test and are shown how to make suitable adjustments to their insulin dose if the blood glucose levels are above or below those recommended. If the nurse is required to perform blood glucose monitoring they should also receive instruction in the technique and should become familiar with the manufacturer’s instructions accompanying each monitoring device (see Procedural Guideline 34.1).
Procedural Guideline 34.1 Measuring blood glucose
Diabetes Australia—Vic 2010; Farrell & Dempsey 2011; McDowell et al 2007; Monahan et al 2007; LeMone et al 2011
| Review and carry out the standard steps for all nursing procedures/interventions |
| Action | Rationale |
|---|---|
| Explain to client what is happening throughout procedure | Promotes participation in care and understanding of health status |
| Gather all necessary equipment before starting procedure (glucometer, reagent strips, warm washcloth, paper towel, sharps container, disposable gloves, goggles) | Prevents hunting for supplies when ready to perform test |
| Provide privacy for client if possible and appropriate | Promotes client comfort |
| Select appropriate puncture site in consultation with client | Ensures free flow of blood after puncture |
| Dilate the capillaries by applying a warm compress | Promotes vasodilation |
| Assist client to wash hands with soap and water then dry them | Promotes skin cleansing |
| Ensure that reagent strip and glucometer are calibrated—check the code on the test strip vial | Some machines must be calibrated so that an accurate reading is obtained. Code on test strip must match code entered into the glucose meter |
| Check expiry date for reagent strip | Ensures accurate reading is obtained |
| Choose a vascular area for puncture site. In stable adults, select lateral side of finger; be sure to avoid central tip of finger, which has more dense nerve supply. Hold area to be punctured in a dependent position while gently massaging finger towards puncture site | Increases blood flow to area before puncture |
| Position lancet correctly (to the side of the finger) and pierce the skin sharply and quickly | Ensures proper skin penetration |
| Correctly apply the blood to the reagent patch on the strip | Ensures proper coverage of test pad on reagent strip |
| Apply correct pressure to the puncture site | Promotes haemostasis |
| Place the strip in the glucometer, according to the manufacturer’s instructions | Strip must be inserted correctly to obtain an accurate reading |
| Read the digital result from the glucometer or compare colour change on the strip with the standardised colour chart on the container (older models) | Each meter has a specifed time for reading glucose level (5–30 seconds, usually less than 1 minute) |
| Check puncture site for any bleeding before leaving client and apply a band aid to the puncture wound if required | Can be a source of discomfort for the client. Prevents further bleeding and protects wound |
| Perform hand hygiene after procedure and dispose of contaminated equipment, sharps and gloves appropriately | Reduces risk of transmission of infection |
| Record results accurately on chart and in correct location in client’s history. Share the result with the client and then report the observation to the RN | Keeps client informed. Documentation provides a record of the reading |
Although not as accurate or desirable, blood glucose and ketone analysis may be augmented by urinalysis, performed by either a nurse or the client at home. Glycosuria is also indicative of other disorders such as Cushing’s syndrome. As a result, information on the effects of treatments or severity of other endocrine disorders may be provided by the specific gravity, presence of ketones or pH recorded on urinalysis strips. For further information on urinalysis, see Chapter 29.
Education
The person with diabetes mellitus has to learn to accept the condition and the resulting lifestyle changes that need to occur. They need to gain confidence in managing the condition, which requires learning a considerable amount of medical information and technical skills. A well-planned education program helps alleviate anxiety, creates autonomy in management and results in well-managed clients. Involving family members and close friends in the education program also assists the individual. The aims of an education program are that the individual will:
• Accept the condition and understand the importance of good diabetic control
• Acquire the technical skills needed to monitor blood glucose levels and administer medications
• Understand the importance of diet, exercise and medications in maintaining blood glucose levels
• Recognise disturbances of blood glucose levels and the development of complications.
A person with diabetes mellitus should always carry a form of diabetic alert identification with them, such as a card, bracelet or medallion. This ensures that appropriate care will be administered if a medical emergency occurs. Clients suffering from diabetes mellitus or at risk may be referred by their health professional to the Australian Government National Diabetes Services Scheme for access to subsidised products to assist in home blood testing (Australian Diabetes Educators Association nd, 2; Diabetes Australia–NDSS 2011). In New Zealand blood glucose meters are often provided free of charge to clients with a community services card (Diabetes New Zealand 2008b).
Summary
The endocrine system is made up of a number of glands that secrete chemical substances called hormones directly into the bloodstream. Each hormone has a specific purpose in the body, and their effects are often wide-ranging and prolonged. The endocrine system influences major bodily processes as diverse as growth, sexual maturation, blood calcium and fluid balance. All glands and hormones work in concert to maintain homeostasis.
Hormone imbalances and endocrine dysfunction may result from autoimmune reactions, trauma, tumours and genetic disorders, among other factors. More common or recognisable endocrine health problems include Graves’ disease, Cushing’s syndrome, acromegaly and diabetes mellitus. Diagnostic tests to assess gland function include estimating serum hormone and metabolite levels, urine tests, scanning and ultrasonography. A number of disorders are characterised by definitive signs and symptoms and nursing intervention and care varies according to the specific disorder. The nursing care is designed to support and help clients meet all their needs.
Diabetes mellitus is specifically a disorder of blood glucose regulation. Management of diabetes mellitus includes monitoring diet and blood glucose, activity levels, use of insulin and the prevention of complications.
1. One of your clients, a 47-year-old male, presents with hypertension (160/101 mmHg), tachycardia and increased respiratory rate. Given what you know about the endocrine system, which hormones might be responsible for these signs? Is there more than one endocrine gland or imbalance that might be responsible for his condition?
2. One of your clients, a 38-year-old mother of two, has been admitted with a possible adrenal tumour. Considering the role of the adrenal gland, what possible endocrine disorders might the patient suffer from? What might be their physical manifestations, and how can you best explain to the client what she might be experiencing?
3. Louise, 46, is an active community care worker who has arrived at a medical clinic for assessment, concerned by the gradual onset of several symptoms. She complains of weight gain. She also conveys to you a feeling of generally ‘slowing down’, describing symptoms such as constipation, tiredness, an impaired memory and feeling cold despite the warm spring evenings. She speaks slowly, occasionally slurring her words.
On examination, you observe a bradycardia of 52 beats per minute, and note the client’s dry skin, oedematous eyelids and enlarged tongue. What endocrine disorder is Louise likely to be suffering from?
Louise is started on medication with the aim of normalising her thyroxine levels. If these oral thyroid medications cause her to become hyperthyroid, what signs and symptoms would you educate her to be aware of?
What further education will Louise require to help her cope with the diagnosis?
4. Paul is an obese, 54-year-old man with a four-year history of type 2 diabetes mellitus. Despite his best efforts, he tells you that he is finding it difficult to alter his diet and activity levels. His doctor has recently told him to take extra care with his feet. He asks you, a nurse, why this is important. What do you say to him about skin care in people with poorly controlled diabetes mellitus?
1. Describe how the pituitary gland regulates the thyroid and adrenal glands, and the gonads.
2. Describe gigantism. Does gigantism occur before or after epiphyseal closure? How does this differ to acromegaly?
3. What is the function of antidiuretic hormone? Blood tests of one of your clients shows they are suffering from hyponatraemia, serum hypo-osmolarity and urine hyperosmolarity. What sort of endocrine imbalance might be occurring? Which gland do you think is responsible?
4. Explain the difference between diabetes insipidus and diabetes mellitus. A dysfunction of which endocrine glands might be responsible for these conditions?
5. How do you account for the hyperactivity, tachycardia and nervousness of Graves’ disease?
6. List three (3) situations that could instigate a thyroid storm or thyroid crisis.
7. The parathyroid glands secrete parathormone (PTH). What is the action of this hormone? What is the name and source of the hormone which is antagonistic to PTH? Where else do we find hormones with opposite actions?
8. Describe the main functions of glucocorticoids such as cortisol. What possible health problems may develop with prolonged secretion or administration of cortisol?
9. Explain how problems with the adrenal cortex might manifest in body fluid imbalances.
10. How is it that the adrenal medulla has an identical, but more prolonged, influence on your body than the sympathetic nervous system?
11. What sort of signs or symptoms would you expect a female client with an excess of androgens to exhibit?
12. Describe the two types of diabetes mellitus. Is diabetes necessarily a problem with the pancreas, or a product of the pancreas? Explain.
13. Describe the significance of finding a high level of ketones in the urine of an individual with type 1 diabetes mellitus.
14. Where is the best location on a person’s finger to perform a blood glucose test and why?
References and Recommended Reading
Anpalahan M. Chronic idiopathic hyponatremia in older people due to syndrome of inappropriate antidiuretic hormone secretion (SIADH) possibly related to aging. Journal of the American Geriatrics Society. 2001;49:788–792.
Australian Addison’s Disease Association. What is Addison’s? Online. Available: www.addisons.org.au/core.htm, 2006.
Australian and New Zealand Endocrine Surgeons. Multiple endocrine neoplasia syndromes. Online. Available: www.endocrinesurgeons.org.au/multiple-endocrine-neoplasia, 2011.
Australian and New Zealand Endocrine Surgeons. Thyroid gland. Online. Available: www.endocrinesurgeons.org.au/thyroid-gland, 2011.
Australian Diabetes Educators Association. (nd, 1) Diabetes Educators. Online. Available: www.adea.com.au/main/diabeteseducators.
Australian Diabetes Educators Association. (nd, 2) Resource Links. Online. Available: www.adea.com.au/main/livingwithdiabetes/resourcelinks.
Australian Institute of Health and Welfare (AIHW) (2008) Diabetes: Australian Facts 2008. Diabetes series no. 8. Cat. No CVD 40. AIHW, Canberra
Australian Institute of Health and Welfare (AIHW). Diabetes Prevalence in Australia: An Assessment of National Data Sources. Cat. No CVD 46. AIHW, Canberra. Online. Available: www.aihw.gov.au/publication-detail/?id=6442468288, 2009.
Australian Institute of Health and Welfare (AIHW) (2010) Diabetes in Pregnancy: Its Impact on Australian Women and Their Babies. Diabetes series no. 14. Cat. No CVD 52. AIHW, Canberra
Australian Pituitary Foundation. Prolactinoma. Online. Available: www.pituitary.asn.au/ThePituitaryGland/ConditionsIntroduction/Prolactinoma.aspx, 2008.
Australian Pituitary Foundation. Non functioning adenoma. Online. Available: www.pituitary.asn.au/ThePituitaryGland/ConditionsIntroduction/NonFunctioningTumours.aspx, 2011.
Australian Thyroid Foundation. Iodine: the Newest Ingredient in Bread. Online. Available: www.thyroidfoundation.com.au/news/latest-news, 2009.
Brown D, Edwards H. Lewis’s Medical-Surgical Nursing, 3rd edn. Sydney: Elsevier, 2012.
Bhasin S, Enzlin P, Coviello A, et al. Sexual dysfunction in men and women with endocrine disorders. The Lancet. 2007;369(9561):597–611.
Chueire VB, Romaldini JH, Ward LS. Subclinical hypothyroidism increases the risk for depression in the elderly. Archives of Gerontology and Geriatrics. 2007;44(1):21–28.
Cohen M, Boyle E, Delaney C, et al. A comparison of blood glucose meters in Australia. Diabetes Research and Clinical Practice. 2006;71:113–118.
Congenital Adrenal Hyperplasia Support Group Australia. (nd) What is CAH? Online. Available: www.cah.org.au/v2/index.php?option=com_content&view=article&id=46&Itemid=34.
Congenital Adrenal Hyperplasia Support Group New Zealand. (nd) About Congenital Adrenal Hyperplasia. Online. Available: www.cah.org.nz/about_cah.
Craft J, Gordon C, Tiziani A. Understanding Pathophysiology. Sydney: Elsevier, 2011.
Davis R, Turner E, Hicks D, et al. Developing an integrated career and competency framework for diabetes nursing. Journal of Clinical Nursing. 2008;17:168–174.
Diabetes Australia. Gestational Diabetes. Online. Available: www.diabetesaustralia.com.au/living-with-diabetes/gestational-diabetes/, 2011.
Diabetes Australia. Hypoglycaemia. Online. Available: www.diabetesaustralia.com.au/en/Understanding-Diabetes/What-is-Diabetes/Hypoglycaemia/, 2011.
Diabetes Australia. Insulin. Online. Available: www.diabetesaustralia.com.au/en/Living-with-Diabetes/Type-1-Diabetes/Managing-Type-1-Diabetes/Insulin/, 2011.
Diabetes Australia. Diabetes and your feet. Online. Available: www.diabetesaustralia.com.au/en/Living-with-Diabetes/Mind--Body/Diabetes--Your-Feet/, 2011.
Diabetes Australia. Blood glucose monitoring. Online. Available: www.diabetesaustralia.com.au/en/Living-with-Diabetes/Type-1-Diabetes/Managing-Type-1-Diabetes/Blood-Glucose-Monitoring/, 2011.
Diabetes Australia—NDSS. National Diabetes Services Scheme. Online. Available: www.diabetesaustralia.com.au/en/NDSS/, 2011.
Diabetes Australia—Vic. Talking diabetes no. 04: Blood glucose monitoring. Online. Available: www.diabetesvic.org.au/images/stories/04BGlucose_2010_2.pdf, 2010.
Diabetes New Zealand. Diabetes Fact Sheet 2008. Online. Available: www.diabetes.org.nz/resources/facts_and_figures, 2008.
Diabetes New Zealand. Home Blood Glucose Testing. Online. Available: www.diabetes.org.nz/living_with_diabetes/type_1_diabetes/home_blood_glucose_testing, 2008.
Diabetes New Zealand. About Diabetes. Online. Available: www.diabetes.org.nz/about_diabetes, 2011.
Ebrahimi H, Edhouse P, Lundgren CI, et al. Does autoimmune thyroid disease affect parathyroid autotransplantation and survival? ANZ Journal of Surgery. 2009;79:383–385.
Endocrinology Expert Group. Therapeutic Guidelines: Endocrinology, Version 4. Melbourne: Therapeutic Guidelines Limited, 2009.
Farrell M, Dempsey J. Smeltzer and Bare’s Textbook of Medical-Surgical Nursing, 2nd edn. Philadelphia: Lippincott Williams & Wilkins, 2011.
Hardt PD, Brendel MD, Kloer HU, et al. Is pancreatic diabetes (type 3c diabetes) underdiagnosed and misdiagnosed? Diabetes Care. 2008;31(2):S165–S169.
Heart and Diabetes Institute of Australia. National Evidence-Based Guideline on Prevention, Identification and Management of Foot Complications. Online. Available: http://t2dgr.bakeridi.edu.au/LinkClick.aspx?fileticket=Z_vePysE8Ng%3d&tabid=760&mid=1629, 2011.
Krausz Y, Freedman N, Lester H, et al. Brain SPECT study of common ground between hypothyroidism and depression. International Journal of Neuropsychopharmacology. 2007;10:99–106.
LeMone P, Burke K, Dwyer T, et al. Medical-Surgical Nursing: Critical Thinking in Client Care. Pearson, Frenchs Forest, NSW, 2011.
Marieb EN, Hoehn K. Human Anatomy and Physiology, 8th edn. San Francisco: Pearson, 2010.
McCance KL, Huether SE. Pathophysiology: The Biologic Basis for Disease in Adults and Children, 6th edn. Sydney: Elsevier, 2010.
McDowell JRS, Brown FJ, Matthews DM. Diabetes: a Handbook for the Primary Healthcare Worker. Edinburgh: Churchill Livingstone, 2007.
Michels AW, Eisenbarth GS. Immunologic endocrine disorders. Journal of Allergy and Clinical Immunology. 2007;125(2):S226–S237.
Monahan FD, Sands JK, Neighbours M, et al. Phipps’ Medical-Surgical Nursing: Health and Illness Perspectives, 8th edn. St Louis: Mosby, 2007.
New Zealand Ministry of Health. Diabetes Surveillance: Population-Based Estimates and Projections for New Zealand, 2001–2011. Public Health Intelligence Occasional Bulletin No 46. Online. Available: www.moh.govt.nz/moh.nsf/pagesmh/6796/$File/diabetes-suveillance-population-estimates-projections-2001-2011.pdf, 2007.
New Zealand Ministry of Health. Māori Health: Health Status Indicators—Diabetes. Online. Available: www.maorihealth.govt.nz/moh.nsf/indexma/diabetes, 2010.
New Zealand Ministry of Health. Māori Health: Statistics. Online. Available: www.maorihealth.govt.nz/moh.nsf/menuma/Statistics, 2011.
Osteoporosis Australia. What Is Osteoporosis? Online. Available: www.osteoporosis.org.au/about/about-osteoporosis/what-is-osteoporosis/, 2011.
Patton KT, Thibodeau GA. Anatomy and Physiology, 7th edn. St Louis: Mosby, 2010.
Patton KT, Thibodeau GA. Anatomy and Physiology, 8th edn. Mosby Elsevier: St Louis, 2012.
Polycystic Ovary Association Australia. What is PCOS? Online. Available: http://main.posaa.asn.au/index.php/what-is-pcos, 2011.
Queensland Health. Hyperthyroidism. Online. Available: http://access.health.qld.gov.au/hid/NutritionalDietandHormonalHealth/ThyroidProblems/hyperthyroidism_ap.asp, 2008.
Queensland Health. Hypothyroidism. Online. Available: http://access.health.qld.gov.au/hid/NutritionalDietandHormonalHealth/ThyroidProblems/hypothyroidism_ap.asp, 2008.
Rogers LC, Armstrong DG, Boulton AJM, et al. Malignant melanoma misdiagnosed as a diabetic foot ulcer. Diabetes Care. 2007;30(2):444–445.
Santamaria N, Carville K, Ellis I, et al. The effectiveness of digital imaging and remote expert wound consultation on healing rates in chronic lower leg ulcers in the Kimberley region of Western Australia. Prim Intention. 2004;12(2):62–70.
Stone MA, Camosso-Stefinovic J, Wilkinson J, et al. Incorrect and incomplete coding and classification of diabetes: a systematic review. Diabetic Medicine. 2010;27:491–497.
Suliburk JW, Perrier ND. Primary hyperparathyroidism. The Oncologist. 2007;12:644–653.
Thibodeau GA, Patton KT. The Human Body in Health and Disease, 5th edn. St Louis: Mosby, 2010.
Thyroid Australia. Thyroid Basics. Online. Available: www.thyroid.org.au/Download/Flyer_2007.2_Basic.pdf, 2007.
Yesil S, Demir T, Akinci B, et al. Amelanotic melanoma misdiagnosed as a diabetic foot ulcer. Journal of Diabetes and its Complications. 2007;21(5):335–337.
Australasian Paediatric Endocrine Group, www.apeg.org.au.
Australian Addison’s Disease Association, www.addisons.org.au.
Australian and New Zealand Bone and Mineral Society, www.anzbms.org.au.
Australian and New Zealand Endocrine Surgeons, www.endocrinesurgeons.org.au.
Australian Bureau of Statistics, www.abs.gov.au.
Australian Diabetes Educators Association, www.adea.com.au/.
Australian Diabetes Society, www.diabetessociety.com.au.
Australian Institute of Health and Welfare, www.aihw.gov.au.
Australian Pituitary Foundation, www.pituitary.asn.au.
Australian Thyroid Foundation, www.thyroidfoundation.com.au.
Clinical Practice Guidelines, www.clinicalguidelines.gov.au.
Congenital Adrenal Hyperplasia Support Group New Zealand, www.cah.org.nz.
Diabetes Australia, www.diabetesaustralia.com.au.
Diabetes New Zealand, www.diabetes.org.nz.
Endocrine Society of Australia, www.endocrinesociety.org.au.
ENSA—The Endocrine Nurse’s Society of Australasia, www.ensa.org.au.
Heart and Diabetes Institute of Australia—Baker IDI, www.bakeridi.edu.au/.
National Institute of Clinical Studies—National Health and Medical Research Council, www.nhmrc.gov.au/nics.
New Zealand Addison’s Network, www.addisons.org.nz.
New Zealand Ministry of Health, www.moh.govt.nz/diabetes.
New Zealand Society of Endocrinology, http://nzse.rsnz.org.
Osteoporosis Australia, www.osteoporosis.org.au.
Osteoporosis New Zealand, www.bones.org.nz.
Phaeochromocytoma and Paraganglioma Research Support Organisation, www.pressor.org.
Polycystic Ovary Association of Australia, http://main.possa.asn.au.
Thyroid Australia, www.thyroid.org.au.