Chapter Ten Pain assessment: the fifth vital sign
INTRODUCTION
The International Association for the Study of Pain (IASP) defines pain as ‘an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage’ (Merskey and Bogduk, 1994). Over forty years ago, McCaffery introduced the often quoted definition of ‘… pain is what the person says it is and exists whenever he or she says it does’ (McCaffery, 1968). The recognition that pain is more than a physiological manifestation and that the persons experiencing pain are the most reliable sources (or ‘gold-standard’) for understanding their pain experience, has shaped the way pain is assessed clinically. In addition, the more recent emphasis on recording pain intensity as ‘the fifth vital sign’ has had as its aim, increasing awareness of the importance of pain assessment (JCAHO and NPC, 2001).
The purpose of pain assessment is to, where possible, establish patients’ perception of their experience of pain, identify how pain interferes with physical and psychosocial wellbeing and provide a baseline for decisions about pharmacological and nonpharmacological treatment, self-management and response to treatment.
NEUROANATOMICAL PATHWAY
Pain is a highly complex and subjective experience that originates from the central (CNS) or peripheral nervous system (PNS) or both. Specialised nerve endings called nociceptors are designed to detect painful sensations from the periphery and transmit them to the central nervous system. Nociceptors are located within the skin; connective tissue; muscle; and the thoracic, abdominal and pelvic viscera. These nociceptors can be stimulated directly by trauma or injury or secondarily by chemical mediators that are released from the site of tissue damage.
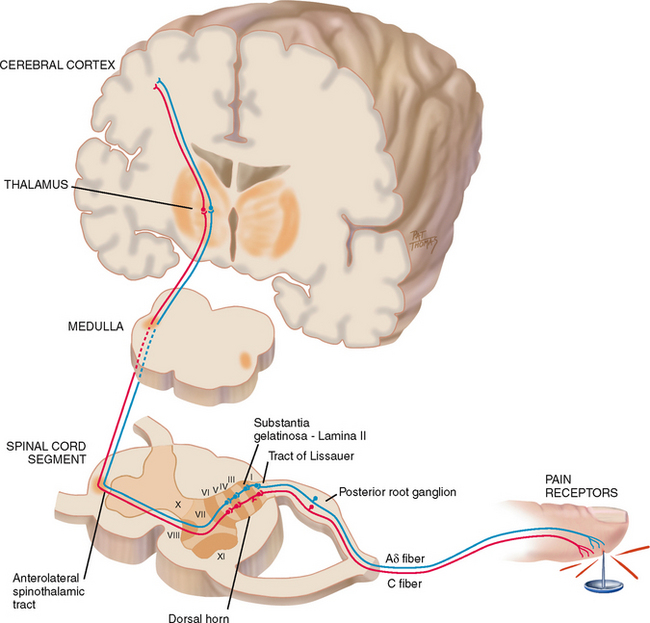
Nociceptors carry the pain signal to the central nervous system by two primary sensory (or afferent) fibres: A-delta and C-fibres (Fig 10.1). A-delta fibres are myelinated and larger in diameter, and they transmit the pain signal rapidly, known as ‘first pain’, to the CNS. Very localised, short-term and sharp sensations result from A-delta fibre stimulation. In contrast, C-fibres are unmyelinated and smaller, and they transmit the signal more slowly known as ‘second pain’. Sensations are diffuse and burning or aching and they persist after the initial injury. C-fibre stimulation produces constant pain.
Peripheral sensory A-delta and C-fibres enter the spinal cord by posterior nerve roots within the dorsal horn by the tract of Lissauer. The fibres synapse with interneurons located within a specified area of the cord called the substantia gelatinosa. A cross section shows that the grey matter of the spinal cord is divided into a series of consecutively numbered laminae (layers of nerve cells) (see Fig 10.1). The substantia gelatinosa is lamina II, which receives sensory input from various areas of the body. The pain signals then cross over to the other side of the spinal cord and ascend to the brain via the anterolateral spinothalamic tract. Pain sensation is located in the thalamus, and axons from this area project into the limbic system, where the emotional reaction to pain occurs, and to the cerebral cortex where the perception and interpretation (location and type) of pain takes place.
NOCICEPTION
Nociception is the term used to describe how noxious stimuli are typically perceived as pain. Nociception can be divided into four phases: (1) transduction, (2) transmission, (3) perception and (4) modulation (Fig 10.2).
Initially, the first phase of transduction occurs when a noxious stimulus in the form of traumatic or chemical injury, burn, incision or tumour takes place in the periphery. The periphery includes the skin, as well as somatic and visceral structures. These injured tissues then release a variety of chemicals, including substance P, histamine, prostaglandins, serotonin and bradykinin. These chemicals are neurotransmitters that propagate a pain message, or action potential, along sensory afferent nerve fibres to the spinal cord. These nerve fibres terminate in the dorsal horn of the spinal cord. Because the initial afferent fibres stop in the dorsal horn, a second set of neurotransmitters carry the pain impulse across the synaptic cleft to the dorsal horn neurons. These neurotransmitters include substance P, glutamate and adenosine triphosphate (ATP).
In the second phase, known as transmission, the pain impulse moves from the level of the spinal cord to the brain. Within the spinal cord, at the site of the synaptic cleft are opioid receptors that can block this pain signalling with endogenous or exogenous opioids. However, if left uninterrupted, the pain impulse moves to the brain via various ascending fibres within the spinothalamic tract that terminate in the brain stem and thalamus. Once the pain impulse moves through the thalamus, the message is dispersed to higher cortical areas via mechanisms that are not clearly understood at this time.
The third phase, perception, indicates the conscious awareness of a painful sensation. Cortical structures such as the limbic system account for the emotional response to pain, and somatosensory areas can characterise the sensation. Only when the noxious stimuli are interpreted in these higher cortical structures can this sensation be identified as pain.
Lastly, the pain message is inhibited through the phase of modulation. Descending pathways from the brain stem to the spinal cord produce a third set of neurotransmitters that slow down or impede the pain impulse, producing an analgesic effect. These neurotransmitters include serotonin; norepinephrine; neurotensin; gamma-aminobutyric acid (B) (GABA(B)); and our own endogenous opioids, β-endorphins, encephalins and dynorphins.
NOCICEPTIVE SOURCES OF PAIN
Nociceptive pain arises from stimulation of superficial or deep tissue pain receptors. Visceral pain originates from the larger interior organs (i.e. kidneys, stomach, intestines, gallbladder, pancreas). The pain can stem from direct injury to the organ, or from stretching of the organ from tumour, ischaemia, distension or severe contraction. The pain impulse is transmitted by ascending nerve fibres along with nerve fibres of the autonomic nervous system. That is why visceral pain often presents along with autonomic responses such as vomiting, nausea, pallor and diaphoresis.
Somatic pain can be classified as superficial or deep. Superficial or cutaneous somatic pain is derived from injury to the skin surface and subcutaneous tissues. Deep somatic pain comes from injury as a result of pressure, trauma or ischaemia to blood vessels, joints, tendons, muscles and bone.
In general, somatic pain is well localised with surrounding tenderness and is characterised as sharp, stinging or burning. Whereas visceral pain is poorly localised, dull cramping or colicky in nature accompanied by local or referred tenderness.
Pain that is felt at a particular site but originates from another location is termed referred pain. Both sites are innervated by the same spinal nerve, and it is difficult for the brain to differentiate the point of origin. Referred pain may originate from visceral or somatic structures. Various structures maintain their same embryonic innervations. For example, an inflamed appendix in the right lower quadrant of the abdomen may have referred pain in the periumbilical area. It is useful to have knowledge of areas of referred pain for diagnostic purposes (see Table 19.2).
NEUROGENIC SOURCES OF PAIN
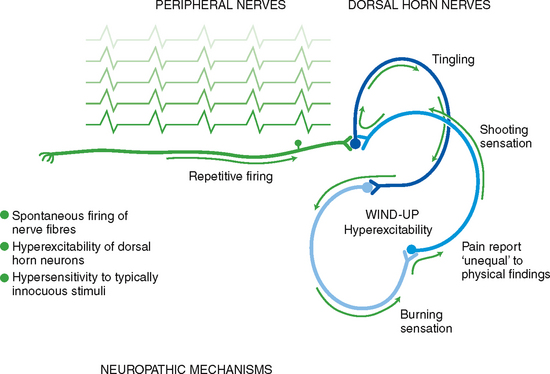
Disturbances of the peripheral or central nervous system can produce pain in the absence of nociceptor stimulation by trauma or disease. Two forms of neurogenic pain are neuropathic pain and central pain. Neuropathic pain indicates a type of pain that does not adhere to the typical and rather predictable phases inherent in nociceptive pain. It occurs as a result of actual nerve cell or axonal damage due to inflammation, trauma (including surgery) or degenerative disease. It is most commonly a chronic pain, but can present as acute pain after trauma or surgery. A proposed mechanism is that injury to peripheral neurons can result in spontaneous and repetitive firing of nerve fibres, almost seizure-like in activity (Fig 10.3). Neuropathic pain may be sustained centrally in a phenomenon known as neuronal ‘wind-up’. Within the dorsal horn of the spinal cord, neurons are thought to be transformed into a hyperexcitable state. This amplified central sensitisation to pain results in a state where a stimulus that normally would not produce pain does (allodynia) and a patient may exhibit an exaggerated pain response to a painful stimulus (hyperalgesia). The abnormal processing of the neuropathic pain impulse can be continued by the peripheral or central nervous system.
Neuropathic pain is difficult to assess and treat. Pain is often perceived long after the site of injury heals. Pain is sustained on a neurochemical level that cannot be identified by X-ray, computerised axial tomography (CAT scan), or magnetic resonance imaging (MRI); electromyography and nerve-conduction studies are needed (Mendell and Sahenk, 2003).
Factors that may be associated with neuropathic pain are a history of events associated with a high risk of nerve injury, such as amputations, hernia repairs or chest wall procedures (Gray, 2008). Neuropathic pain is characterised by pain descriptors such as burning, shooting or stabbing; lack of precipitating factors for the pain occurence; and the presence of unpleasant abnormal sensations (dysaesthesias) such as hyperalgesia (increased response to painful stimulus), allodynia (pain response to stimulus that does not normally produce pain response) or hypoaesthesia. There may be regional autonomic changes, such as change in colour or temperature, or phantom sensations (Macintyre et al, 2010).
Central pain is pain initiated or caused by a primary lesion or dysfunction in the central nervous system that includes the brain, brainstem and spinal cord. Central pain is often referred to as a syndrome and can be caused by stroke, multiple sclerosis, tumours or Parkinson’s disease among other neurological conditions. The extent of pain is usually related to the cause of the CNS injury or damage and is usually made worse by touch and temperature changes.
TYPES OF PAIN (BY DURATION)
Pain can be classified by its duration. The duration can provide information on possible underlying mechanisms and thus inform treatment decisions. Pain is divided into acute or chronic categories. Acute pain is short term and self-limiting, often follows a predictable trajectory and dissipates after an injury heals. Acute pain serves a self-protective purpose; acute pain warns the individual of actual or potential tissue damage. Acute pain may also present as recurrent pain as occurs with migraines or the menstrual cycle (National Pain Summit Initiative (NPSI), 2010) and in these circumstances these types of pain may be referred to as chronic pain conditions (Macintosh and Elson, 2008). Acute pain may progress to chronic pain. The transition of acute pain to chronic pain is sometimes called the ‘sub-acute phase’ and refers to the pain that occurs after tissue healing and that persists up to the 6 months that is the defining duration for chronic pain (NPSI, 2010).
In contrast, chronic (or persistent) pain is diagnosed when the pain continues for 6 months or longer. Chronic pain can be further divided into cancer related and chronic noncancer pain (non-malignant pain). Cancer-related pain often parallels the pathology created by the tumour cells. The pain is induced by tissue necrosis or stretching of an organ by the growing tumour. The pain fluctuates within the course of the disease. Chronic noncancer pain is often associated with musculoskeletal conditions, such as arthritis, low back conditions or fibromyalgia. Chronic pain originates from abnormal processing of pain fibres in peripheral or central sites and not, uncommonly, the source of the pain is unknown.
Chronic noncancer pain does not stop when the injury heals. It persists after the predicted trajectory associated with injury. Chronic pain outlasts its protective purpose, and the level of pain intensity does not correspond with the physical findings. Chronic pain may not respond readily to therapy and, unfortunately, many patients with chronic pain are not believed and are often labelled as malingerers, attention seekers, drug seekers and so forth (Henschke et al, 2009; Shaw and Lee, 2010). In Australia, it is estimated that chronic pain affects the quality of life of 1 in 5 adults (Blyth et al, 2001) and as such is a significant problem that has an estimated annual cost of $34.3 billion in terms of both financial costs and loss of healthy life (MBF, 2007).
DEVELOPMENTAL CONSIDERATIONS
Early childhood
Infants have the same capacity for pain as adults; however, during the postnatal period, there is functional and structural immaturity in the nociceptive pathways that affects the pattern of activity in the infants’ central nervous system (Fitzgerald and Walker, 2009). This immaturity means that there is less discrimination between noxious and non-noxious stimuli. In addition, inhibitory networks and neurotransmitters are in insufficient supply in early development (Fitzgerald, 1987). Therefore the neonate, contrary to popular belief, is rendered more sensitive to painful stimuli than the older child.
Preverbal infants are at high risk for undertreatment of pain because of persistent myths and beliefs that infants have lower sensitivity to pain or do not remember pain (Schultz et al, 2009; Shrestha-Ranjit and Manias, 2010). Repetitive and poorly controlled pain in infants (daily heel sticks, venepunctures) can result in long-term adverse consequences such as neurodevelopmental problems, poor weight gain and learning disabilities (Anand, 2000; Walker et al, 2009).
Late adult (65+ years)
The ageing population and advances in anaesthetic and surgical techniques have resulted in an increase in the age of patients undergoing major surgery (Kojima and Narita, 2006). In addition, older people are more likely to have chronic pain conditions. The most common pain-producing conditions for ageing adults include pathologies such as arthritis, osteoarthritis, osteoporosis, peripheral vascular disease, cancer, peripheral neuropathies, angina and chronic constipation. According to the National Health and Medical Research Council (NHMRC) acute pain guidelines (Macintyre et al, 2010), there are many factors that combine to make effective pain management difficult in older adults. These include the higher incidence of comorbid conditions and concurrent medications, age-related changes in physiology, pharmacodynamics and pharmacokinetics and altered responses to pain.
Although pain is a common experience among individuals 65 years of age and older, it is not a normal process of ageing. People over the age of 65 report more chronic pain and demonstrate a decreased tolerance to experimental pain than do people under the age of 65 (Cole et al, 2010). Pain indicates pathology or injury. Pain should never be considered something to tolerate or accept in one’s later years. Unfortunately, many clinicians and older adults wrongfully assume that pain should be expected in ageing, which leads to less aggressive treatment. Older adults have additional fears about becoming dependent, undergoing invasive procedures, taking pain medications and having a financial burden.
Altered cognitive function may present a challenge for pain assessment within this group, but the somatosensory cortex is generally unaffected by dementia of the Alzheimer’s type, therefore sensory discrimination is preserved in cognitively intact and impaired adults (Cole et al, 2010). Because the limbic system is affected by Alzheimer’s disease, current research focuses on how the person interprets and reports these pain messages (Benedetti et al, 2006; Buffum et al, 2001). The challenge for accurate pain assessment in older adults with altered cognitive function is the decreased reporting associated with diminished memory and communication difficulties requiring focused assessment strategies to detect and measure pain in this group of patients (McAuliffe et al, 2008).
Gender differences
Gender differences are influenced by societal expectations, hormones and genetic make-up. Hormonal changes are found to have strong influences on pain sensitivity for women. Women are two to three times more likely to experience migraines during childbearing years, are more sensitive to pain during the premenstrual period and are six times more likely to have fibromyalgia (Fillingim, 2000). Gender differences in reported pain intensity, frequency of pain conditions and reported pain coping strategies have been identified in children and adolescents from the age of 8 onwards (Hechler et al, 2009). With recent findings from the Human Genome Project, genetic differences between both sexes may account for the differences in pain perception (Mogil, 2002). A pain gene exists, which helps to explain why some people feel more/less pain even with the same stimulus. Efforts are being made to tailor pharmacological agents to improve pain treatment based upon genetic sequencing.
CULTURAL AND SOCIAL CONSIDERATIONS
Please review the cultural variations in Chapter 4. Patient race and ethnicity has been shown to be a factor in reported disparities in effective pain treatment. Studies in the US have shown that African-American and Hispanic patients are less likely to receive opioid analgesics than Caucasian patients and this finding is consistent within a variety of settings including emergency departments (Cintron and Morrison, 2006; Pletcher et al, 2008).
The need to understand different cultures when assessing and managing pain extends beyond just accounting for language. Culture influences individuals’ (including health professionals) beliefs, attitudes, expectations and behaviours. These factors affect how pain is interpreted, how pain is expressed and individuals’ pain-relief seeking behaviours. For example, individuals from cultures that value stoicism tend to avoid vocalising (moaning or screaming) when in pain whereas other cultural groups may be more expressive (Narayan, 2010).
In Australia, as in many other countries, there is significant cultural and ethnic diversity. The NHMRC identify that disparities in the assessment and effectiveness of pain treatment exist across cultural groups; however, pain assessment and treatment should be individualised to avoid cultural stereotyping that can lead to false assumptions about pain responses and its management (Macintyre et al, 2010).
The pain experience of Aboriginal and Torres Strait Islander peoples has received very little research attention. Indigenous peoples are a heterogeneous group. This heterogeneity is evident in language, links to traditional cultural beliefs and links to the land, and their understanding of western medicine (Macintyre et al, 2010). Pain assessment and management of Indigenous patients is often performed by non-Indigenous nurses. Poor understanding of their cultures in relation to pain experiences can lead to inadequate pain management. Current pain assessment tools may have limited utility. According to Fenwick (2006), the use of a numerical rating scale of 0 to 10 is likely to produce inaccurate pain assessment as some Indigenous languages do not have a conceptual recognition of certain numbers. Pain behaviours and language that may be unique to Indigenous people such as averting their eyes or turning their head away or feigning sleep can be misinterpreted and pain may be undertreated.
SUBJECTIVE DATA
Pain is a highly subjective and personal experience, hence the subjective report is the most reliable indicator of pain. The assessment of pain includes a thorough history, physical examination and an evaluation of associated functional impairment (Macintyre et al, 2010).
THE MEASUREMENT OF PAIN—PAIN ASSESSMENT TOOLS
Assessment of pain varies in complexity depending on the context of pain and the ability of patients to communicate their pain. In situations of acute pain and pain as a symptom of trauma or disease (Breivik et al, 2008), assessment of the presence, intensity (at rest and with movement) and location of pain allows characterisation of pain and evaluation of the effectiveness of treatment. Assessment of chronic pain (both cancer and noncancer pain) is more challenging and requires the assessment of the qualitative aspects of pain and its impact on function. In situations where patients cannot communicate their pain because of altered conscious state, impaired cognition, language or developmental aspects, pain assessment needs to be modified to encompass behavioural and physiological manifestations of pain.
Pain is multidimensional in scope, encompassing physical, affective and functional domains. Various assessment tools have been developed to capture either a single dimension (i.e. intensity) or provide information about multiple (objective and subjective) dimensions of pain. Selection of a pain assessment tool is based upon its purpose, time involved in administration and the patient’s ability to comprehend and complete the tool. First, teach patients how to use each tool, with practice sessions to strengthen the validity and reliability of the response. Enlarge the print when appropriate for individuals with impaired vision. The printed language should be translated to the native language of the patient.
Multidimensional tools
Multidimensional pain assessment tools are more useful for chronic pain conditions, persistent pain (Grimmer-Somers et al, 2009) or particularly problematic acute pain problems. These tools provide more information about the characteristics of pain and its impact on the individual. A few examples include the Initial Pain Assessment, the Brief Pain Inventory and the McGill Questionnaire.
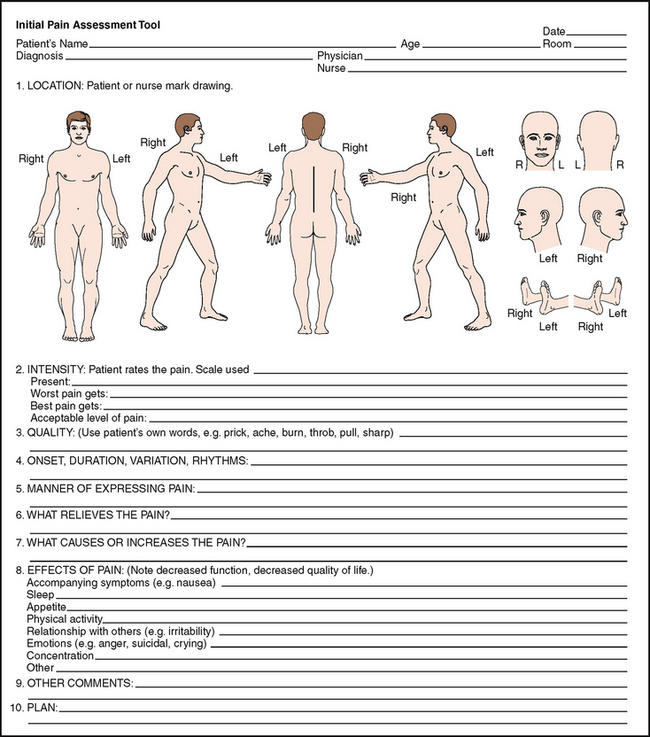
In the Initial Pain Assessment (McCaffery and Pasero, 1999), the clinician asks the patient to answer eight questions concerning location, duration, quality, intensity and aggravating/relieving factors. Further, the clinician adds questions about manner of expressing pain and the effects of pain that impairs quality of life (Fig 10.4).
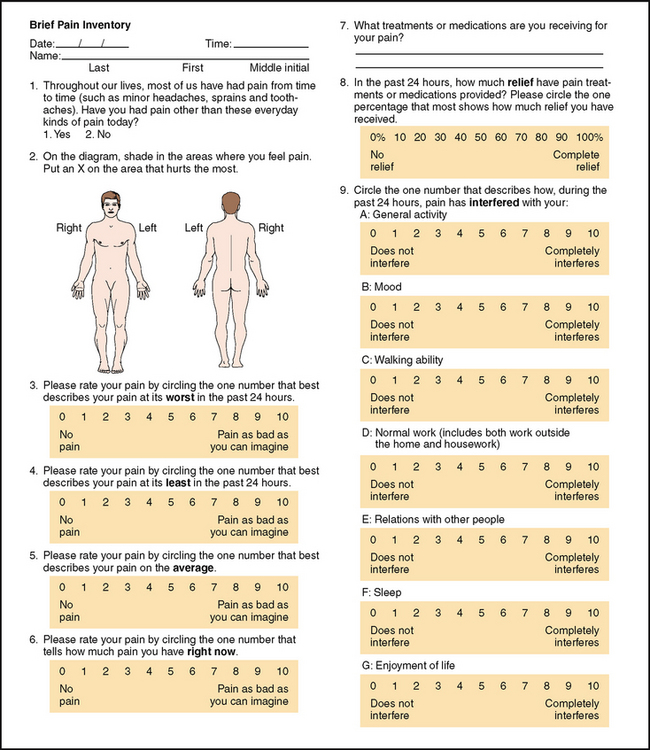
The Brief Pain Inventory (Daut and Cleeland, 1982) asks the patient to rate the pain within the past 24 hours using graduated scales (0–10) with respect to its impact on areas such as mood, walking ability and sleep (Fig 10.5). The Short-Form McGill Pain Questionnaire (Melzack, 1987, not pictured) asks the patient to rank a list of descriptors in terms of their intensity and to give an overall intensity rating to their pain.
Unidimensional tools
Pain rating scales are unidimensional and are intended to reflect pain intensity. They come in various forms. Pain rating scales can indicate baseline intensity, track changes due to changing disease state or recovery and give some degree of evaluation of treatment.
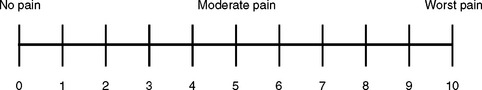
Numeric rating scales, such as the Numerical Rating scale (NRS), can be administered verbally or visually along a vertical or horizontal line (Fig 10.6). Typically patients are asked to choose a number that rates the level of pain, with 0 being no pain and the highest anchor, 10, indicating the worst pain.
An alternative to the NRS is to use a categorical scale such as the simple Verbal Descriptor Scale (VDS) where words are used to describe the magnitude of pain. Words that describe different levels of pain intensity, such as no pain, mild pain, moderate pain and severe pain, can then be converted to numeric scores (e.g. 0, 2, 5, 8, 10). Older adults may find the NRS abstract and have difficulty responding, especially with a fluctuating chronic pain experience and will therefore often respond to scales such as the VDS in which words are selected rather than numbers. Again, it is essential to teach the person how to use the scale to enhance accuracy. There also needs to be awareness that personal, linguistic and cultural differences may affect interpretation of the descriptor words (Scott and McDonald, 2008).
Essentially, the choice of tool should align with the policy and practice guidelines of an organisation so that documentation of pain intensity scores is consistent and interpreted accurately by other clinicians.
INFANTS AND CHILDREN
Assessment of pain in infants and children requires the use of age- and context-appropriate assessment tools. It is recommended that behavioural and physiological signs are evaluated in conjunction with a child’s self-report of pain (Macintyre et al, 2010).
Because infants are ‘preverbal and incapable of self report’, pain assessment is dependent exclusively upon behavioural and physiological cues. Refer to the Objective Data section below. It is important to underscore the understanding that infants do feel pain.
Children 2 years of age can report pain and point to its location. They cannot rate pain intensity at this developmental level. It is helpful to ask the parent or caregiver what words their child uses to report pain (e.g. ouch). Be aware that some children will try to be ‘grown up and brave’ and often deny having pain in the presence of a stranger or if they are fearful of receiving a ‘needle’. Rating scales can be introduced at 3 to 4 years of age (Wong and Baker, 2005). The Wong-Baker Scale is one example of a faces pain scale (FPS). The child is given an explanation that each face is a person who feels happy because they have some or a lot of pain. The child is asked to choose a face that shows ‘how much hurt you have now’ (Fig 10.7). Similarly, the Oucher Scale (Beyer, 1983) has six photographs of young boys’ faces with different expressions of pain, ranked on a 0–5 scale of increasing intensity. The child is asked to point at the face that best matches their own hurt/pain. You may use Oucher Scale variations for girls and diverse ethnic groups. The advantages are that the FPS has been validated in children (Bosenberg et al, 2003), is simple to administer and can be converted to a numerical value for charting.
OBJECTIVE DATA
| Preparation | Equipment needed |
|---|---|
| The physical exam process can help you understand the nature of the pain. Consider whether this is an acute or chronic condition. Recall that physical findings may not always support the patient’s pain complaints, chronic pain syndromes. Pain should not be discounted when objective physical evidence is not found. Based on the patient’s pain report, make every reduce or eliminate the pain with appropriate analgesic and nonpharmacological intervention. According to the American Pain Society (1992: 3): | |
| In cases in which the cause of acute pain is uncertain, establishing a diagnosis is a priority, but symptomatic treatment of pain should be given while the investigation is proceeding. With occasional exceptions (e.g. the initial examination of the patient with an acute condition of the abdomen), it is rarely justified to defer analgesia until a diagnosis is made. In fact, a comfortable patient is better able to cooperate with diagnostic procedures. |
| Procedures and normal findings | Abnormal findings and clinical alerts |
|---|---|
| GENERAL APPEARANCE | |
| Identify pain-related features through observation of alteration in mobility or guarding. Observe for changes in facial expression, posture, gait or mood. | For example, abdominal pain may result in a hunched posture; appearance of tiredness or grimacing; uneven gait such as limping, which may indicate site of injury. |
| Vital sign changes may include tachycardia, high or low blood pressure, increased respiratory rate, pallor. | |
| THE LIMBS AND JOINTS | |
| Note the size and contour of the joint. Measure circumference of the involved joint for comparison with baseline. Check active or passive range of motion (see complete technique, Ch 15). Joint motion normally causes no tenderness, pain or crepitation. | |
| THE MUSCLES AND SKIN | |
| Inspect the skin and tissues for colour, swelling, temperature and any masses or deformity. | Bruising, lesions, open wounds, tissue damage, atrophy, bulging, change in hair distribution, heat or cold and pallor or redness. |
| To assess for changes in sensation, ask the person to close his or her eyes. Test the person’s ability to perceive sensation by breaking a tongue blade in two lengthwise. Lightly press the sharp and blunted ends on the skin in a random fashion and ask the patient to identify it as sharp or dull (see Fig 22.29). This test will help you identify location and extent of altered sensation. | Absent pain sensation (analgesia); increased pain sensation (hyperalgesia); or if a severe pain sensation is evoked with a stimulus that does not normally induce pain (e.g. the blunt end of the tongue blade, cotton ball, clothing) (allodynia). |
| THE ABDOMEN | |
| Observe for contour and symmetry. Palpate superficially for muscle guarding. Note any areas of referred pain (see Table 19.2). | Swelling, bulging, herniation, inflammation and muscle guarding. Observe face during palpation. Leave any reported area of tenderness until last so as not to cause guarding or unnecessary discomfort. Observe patient’s face and nonverbal responses, such as drawing up of legs, guarding, groaning while palpating. |
| FUNCTIONAL IMPACT OF PAIN | |
Measure pain intensity scores on movement and with deep breathing and coughing. Can use the Functional Activity Scale (FAS) the purpose of which is to assess whether a patient can undertake a certain activity (Scott and McDonald, 2008).
|
The FAS has a simple three-level ranked categorical score: A–No limitation (the patient is able to undertake the activity without limitation due to pain) (pain intensity score typically 0–3), B–Mild limitation (the patient is able to undertake the activity but experiences moderate to severe pain) (pain intensity score is typically 4–10), C–Signifcant limitation (the patient is unable to complete the activity due to pain or pain treatment-related side effects independent of pain intensity scores). |
Table 10.1 lists physiological changes resulting from poorly controlled pain.
TABLE 10.1 Physiological changes from poorly controlled pain
| Pain is not a benign symptom. Poorly controlled acute and chronic pain have a negative impact on physiological systems. | |
|---|---|
| Physiological system | Acute pain responses |
| Cardiac | |
| Pulmonary | |
| Gastrointestinal | |
| Renal | |
| Musculoskeletal | |
| Endocrine | Increased adrenergic activity |
| Central nervous system | |
| Immune | |
| Poorly controlled chronic pain | |
NONVERBAL BEHAVIOURS OF PAIN
When the individual cannot verbally communicate the pain, you can (to a limited extent) identify pain using behavioural cues. Recall that individuals react to painful stimuli with a wide variety of behaviours. Behaviours are influenced by a wide variety of factors, including the nature of the pain (acute versus chronic), age and cultural and gender expectations.
Acute pain behaviours
Because acute pain involves autonomic responses and has a protective purpose, individuals experiencing moderate to intense levels of pain may exhibit the following behaviours: guarding, grimacing, vocalisations such as moaning, agitation, restlessness, stillness, diaphoresis or change in vital signs. This list of behaviours is not exhaustive because they should not be used exclusively to deny or confirm the presence of pain. For example, in a postoperative patient, pulse and blood pressure can be altered by fluid volume, medications and blood loss.
Chronic pain behaviours
Persons with chronic pain often live with the experience for months and years. One cannot function physiologically and go on with life in a repetitive state of grimacing, diaphoresis, guarding, etc. The person adapts over time, and clinicians cannot look for or anticipate the same acute pain behaviours to exist in order to confirm a pain diagnosis.
Chronic pain behaviours have even more variability than acute pain behaviours. Persons with chronic pain typically try to give little indication that they are in pain and therefore are at higher risk for under-detection (Fig 10.8). Behaviours that have been associated with chronic pain include bracing, rubbing, diminished activity, sighing and change in appetite. Whenever possible it is best to ask the person how they act or behave when in pain. Chronic pain behaviours, such as being with other people, movement, exercise, prayer, sleeping or inactivity, underscore the more subtle, less anticipated ways in which persons behave when they are experiencing chronic pain. Sleeping is one way persons behave in response to chronic pain in order to self distract. Unfortunately, clinical staff may inadvertently interpret this behaviour as ‘comfort’ and do not follow up with an appropriate pharmacological intervention.
There are many misconceptions held by clinicians and carers about chronic pain that can influence how pain is assessed and treated. These misconceptions include over-emphasis on the contributing role of psychological factors on pain experience, lack of knowledge regarding the risk of addiction to pain medications and misunderstandings about the meanings of pain tolerance (Shaw and Lee, 2010).
DEVELOPMENTAL CONSIDERATIONS
Infants
Most pain research on infants has focused on acute, procedural pain. We have a limited understanding of how to assess chronic pain in the infant. At this time, there is no one assessment tool that adequately identifies pain in the infant. Using a multidimensional approach for the whole infant is encouraged. Changes in facial activity and body movements may help assess pain. Crying can be described in terms of its presence or absence, duration and amplitude or pitch. Much effort and time is spent on decoding facial expressions (e.g. taut tongue, bulging brow, eye squeeze, nasolabial furrow), which may be difficult for the general practitioner to carry out in a busy clinical setting.
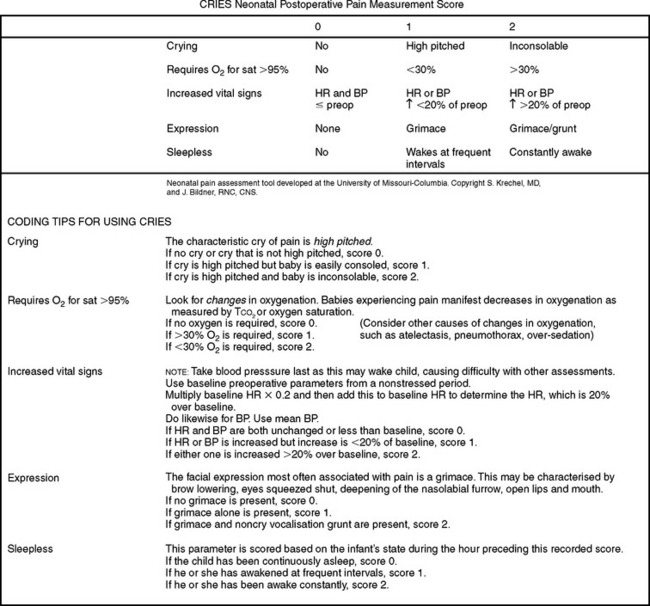
One tool that has been developed for postoperative pain in preterm and term neonates is the CRIES developed by Krechel and Bildner (1995). It measures physiological and behavioural indicators on a 3-point scale (Fig 10.9).
Because the sympathetic nervous system is engaged particularly in acute episodes of pain, physiological changes take place that may indicate the presence of pain. These include sweating, increases in blood pressure and heart rate, vomiting, nausea and changes in oxygen saturation. However, like the adult, these physiological changes cannot be used exclusively to confirm or deny pain because of other factors such as stress, medications and fluid changes.
Note that these measures target acute pain. No biological markers have been identified for long-term chronic pain in infants or children. Therefore evaluate the whole individual. Look for changes in temperament, expression and activity. If a procedure or disease process is known to induce pain in adults (e.g. circumcision, surgery, sickle cell disease, cancer), it will induce pain in the infant or child.
Late adult (65+ years)
Although pain should not be considered a ‘normal’ part of ageing, it is prevalent. When the older adult reports a history of conditions such as osteoarthritis, peripheral vascular disease, cancer, osteoporosis, angina or chronic constipation, be alert and anticipate a pain problem. Older adults will often deny having pain for fear of dependency, further testing or invasive procedures, cost and fear of taking pain killers or becoming a drug addict. During the interview you must establish an empathic and caring rapport to gain trust.
When you look for behavioural cues, look at changes in functional status. Observe for changes in dressing, walking, toileting or involvement in activities. A slowness and rigidity may develop and fatigue may occur. Look for a sudden onset of acute confusion, which may indicate poorly controlled pain. However, you will need to rule out other competing explanations such as infection or adverse reaction from medications.
Adults with cognitive impairment and disability
Self-assessment pain scales can be used reliably in most patients with mild-to-moderate cognitive impairment (Pautex et al, 2005) although dementia and delirium can limit a person’s ability to report pain. The person with cognitive impairment may report pain, but may not be able to describe the pain, discern variations in pain, recall severity of pain or alert others about their pain. When this is the case, then observation of pain behaviours is an important component of pain assessment. Behaviours such as restlessness, frowning and grimacing or sounds such as grunting or groaning can be used to assess pain but may not always be valid indicators of pain in nonverbal adults (Farrell et al, 1996). A number of observational pain assessment scales have been developed and used in patients with varying degrees of dementia. A commonly used scale in Australia is the Abbey Pain Scale (Abbey et al, 2004) (Fig 10.10). This scale is a one-page assessment tool where the presence and severity of six observable cues (vocalisation, facial expression, change in body language, behavioural change, physiological change and physical changes) are scored to provide a total pain score.
DOCUMENTATION AND CRITICAL THINKING
FOCUSED ASSESSMENT: CLINICAL CASE STUDY
BP is an 85-year-old, Italian-Australian female with a 20-year history of osteoarthritis.
Subjective
BP reports increased pain and stiffiness in her hips and knees for the past month. Denies radiation of pain. Denies tingling or numbness in lower extremities.
Having difficulty getting in and out of the bath and dressing herself. Describes pain as aching, with ‘good and bad days’. Becomes frustrated when asked to rate her pain intensity. Replies ‘I don’t know what number to give; it hurts a lot, on and off’. Takes Panadol Osteo, two tablets, when the pain ‘really gets bad’, with some degree of relief. Restricts her activities, such as walking to the local shops, because she ‘hurts too much’.
POSTSURGICAL NEUROPATHIC PAIN
The International Association for the Study of Pain defines neuropathic pain as ‘pain due to a primary lesion or dysfunction of the peripheral or central nervous system’. Neuropathic pain continues beyond the normal healing period after an injury. It is estimated that 260 000 Australians and 50 000 New Zealanders may have neuropathic pain (approximately 1.3% of each population). Posttraumatic neuropathic pain is due to long-term neurobiological changes that occur within hours of acute injury. These changes follow nerve injury where there is generalised hyperexcitability, spinal cord changes and central sensitisation.
Neuropathic pain is characterised by burning and paroxysms (shooting or stabbing pain occurring spontaneously). There is the presence of allodynia (pain from a non-noxious stimulus), hyperalgesia (lowered pain threshold and exaggerated response to painful stimuli), hyperpathia (an explosive pain response), paraesthesias (abnormal non-painful sensations such as tingling), referred pain and abnormal pain radiation. Persistent postsurgical neuropathic pain is a mostly unrecognised clinical problem. Prevention is the best approach and this is through recognition of risk factors, use of multi-modal analgesia and continued analgesia well into the postoperative period.
Abbey J, Piller N, De Bellis A. The Abbey Pain Scale: a 1-minute numerical indicator for people with end-stage dementia. Int J Palliat Nurs. 2004;10(1):6–13.
American Pain Society (APS). Principles of analgesic use in the treatment of acute and cancer pain, 3rd edn. Glenview, Ill: Author, 1992.
Anand KJS. Effects of perinatal pain and stress. Prog Brain Res. 2000;122:117–119.
Benedetti F, Arduino C, Costa S. Loss of expectation-related mechanisms in Alzheimer’s disease makes analgesic therapies less effective. Pain. 2006;121(1–2):133–144.
Beyer JE. The oucher: a user’s manual and technical report. Evanston, Ill: Judson, 1983.
Blyth FM, March LM, Brnabic AJ, et al. Chronic pain in Australia: A prevalence study. Pain. 2001;89:127–134.
Bosenberg A, Thomas J, Lopez T, et al. Validation of a six-graded faces scale for evaluation of postoperative pain in children. Paediatr Anaesth. 2003;13:708–713.
Breivik H, Borchgrevink PC, Allen SM, et al. Assessment of pain. British Journal of Anaesthesia. 2008;101(1):17–24.
Buffum MD, Miaskowski C, Sands L, et al. A pilot study of the relationship between discomfort and agitation in patients with dementia. Geriatr Nurs. 2001;22(2):80–85.
Cintron A, Morrison RS. Pain and ethnicity in the United States: A systematic review. J Palliat Med. 2006;9(6):1454–1473.
Cole LJ, Farrell MJ, Gibson SJ, et al. Age-related differences in pain sensitivity and regional brain activity evoked by noxious pressure. Neurobiology of Aging. 2010;31:494–503.
Daut RL, Cleeland CS. The prevalence and severity of pain in cancer. Cancer. 1982;50:1913–1918.
Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–121.
Farrell MJ, Katz B, Helme RD. The impact of dementia on the pain experience. Pain. 1996;67(1):7–15.
Fenwick C. Assessing pain across the cultural gap: Central Australian Indigenous people’s pain assessment. Contemporary Nurse. 2006;22(2):218–227.
Fillingim RB. Sex, gender and pain. Seattle: IASP Press, 2000.
Fitzgerald M. Pain and analgesia in neonates. Trends in neurosciences. 1987;19(9):344–346.
Fitzgerald M, Walker SM. Infant pain management: a developmental neurobiological approach. Nat Clin Pract Neurol. 2009;5(1):35–50.
Gray P. Acute neuropathic pain: diagnosis and treatment. Current Opinion in Anaesthesiology. 2008;21(5):590–595.
Grimmer-Somers K, Vipond N, Kumar S, et al. A review and critique of assessment instruments for patients with persistent pain. Journal of Pain Research. 2009;2:21–47.
Hechler T, Chalkiadis GA, Hasan C, et al. Sex differences in pain intensity in adolescents suffering from cancer: differences in pain memories? J Pain. 2009;10(6):586–593.
Henschke N, Maher CG, Refshauge KM, et al. Characteristics of patients with acute low back pain presenting to primary care in Australia. Clinical Journal of Pain. 2009;25(1):5–11.
International Association for the Study of Pain. Pain measurement in children. Pain Clinical Updates. 1995;1(3):2.
Joint Commission on Accreditation of Healthcare Organizations (JCAHO) and National Pharmaceutical Council (NPC). 2001: Pain: current understanding of assessment, management and treatments. Available at www.jcaho.org/news+room/health+care+issues/pm+monographs.htm.
Kojima Y, Narita M. Postoperative outcomes among elderly patients after general anaesthesia. Acta Anaesthesiol Scand. 2006;50(1):19–25.
Krechel SW, Bildner J. CRIES: a new neonatal postoperative pain measurement score. Initial testing of validity and reliability. Paediatr Anaesth. 1995;5(1):53–61.
Macintosh C, Elson S. Chronic pain: clinical features, assessment and treatment. Nursing Standard. 2008;23(5):48–56.
Macintyre PE, Schug SA, Scott DA, et al. Working Group of the Australian and New Zealand College of Anaesthetists and Faculty of Pain Medicine (2010), Acute Pain Management: Scientific Evidence, 3rd edn. Melbourne: ANZCA & FPM, 2010.
Manterola C, Astudillo P, Losada H: Analgesia in patients with acute abdominal pain, Cochrane Database Syst Rev (3):CD005660.
MBF Foundation. The high price of pain: the economic impact of persistent pain in Australia. Available at http://www.mbf.com.au/MBF/About%20MBF/Forms/MBF_Foundation_the_price_of_pain.pdf, 2007.
McAuliffe L, Nay R, O’Donnell M, et al. Pain assessment in older people with dementia: literature review. Journal of Advanced Nursing. 2008;65(1):2–10.
McCaffery M. Nursing practice theories related to cognition, bodily pain, and main-environment interactions. Los Angeles: University of Los Angeles, 1968.
McCaffery M, Pasero C. Pain: clinical manual, 2nd edn. St Louis: Mosby, 1999.
Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–197.
Mendell JR, Sahenk Z. Painful sensory neuropathy. N Engl J Med. 2003;348(13):1243–1255.
Merskey H, Bogduk N. Classification of chronic pain, IASP taskforce on taxonomy. Seattle: IASP Press, 1994.
Mogil JS, Pain genetics: pre- and post-genomic findings. International Association for the Study of Pain Technical Corner Newsletter, 2002;2:3–6
Narayan MC. CE test 2.9 hours: culture’s effects on pain assessment and management. American Journal of Nursing. 2010;110(4):38–47.
National Pain Summit Initiative, 2010: National Pain Strategy. Pain management for all Australians. Available at www.painsummit.org.au.
Pautex S, Herrmann F, Le Louis P. Feasibility and reliability of four pain self-assessment scales and correlation with an observational rating scale in hospitalised elderly demented patients. J Gerontol A Biol Sci Med Sci. 2005;60(4):524–529.
Pletcher MJ, Kertesz SG, Kohn MA. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA. 2008;299(1):70–78.
Schultz M, Loughran-Fowlds A, Spence K. Neonatal pain: A comparison of the beliefs and practices of junior doctors and current best evidence. Journal of Paediatrics and Child Health. 2009;46:23–28.
Scott DA, McDonald WM, Assessment, measurement and history. Macintyre PE, Rowbotham D, Walker S. Textbook of clinical pain management; acute pain, 2nd edn., London: Hodder Arnold, 2008.
Shaw S, Lee A. Student nurses’ misconceptions of adults with chronic nonmalignant pain. Pain Management Nursing. 2010;11(1):2–14.
Shrestha-Ranjit JM, Manias E. Pain assessment and management practice in children following surgery of the lower limb. Journal of Clinical Nursing. 2010;19:118–128.
Walker SM, Franck LS, Fitzgerald M, et al. Long-term impact of neonatal intensive care and surgery on somatosensory perception in children born extremely preterm. Pain. 2009;141(1–2):79–87.
Wong D, Baker C. Reference manual for the Wong-Baker faces pain rating scale. Tulsa: Wong and Baker, 2005.