Key Concepts in Analgesic Therapy
THIS chapter presents the key concepts of opioid analgesic administration. Included is a discussion of the recommended approach to managing all types of pain: multimodal analgesia. The research related to preemptive analgesia, accelerated multimodal postoperative rehabilitation, and prevention of persistent postsurgical pain is reviewed. The pros and cons of the various methods for opioid analgesic dosing are described, and an introduction to the concept of patient-controlled analgesia (PCA) and alternative uses of analgesic devices is provided.
Multimodal Analgesia
As discussed in Section III, a multimodal regimen combines drugs with different underlying mechanisms, such as nonopioids, opioids, local anesthetics, and anticonvulsants. This approach allows lower doses of each of the drugs in the treatment plan, which lowers the potential for each to produce adverse effects (Ashburn, Caplan, Carr, et al., 2004; Kim, Kim, Nam, et al., 2008; Marret, Kurdi, Zufferey, et al., 2005; Schug, 2006; Schug, Manopas, 2007; White, 2005). Further, multimodal analgesia can result in comparable or greater pain relief than can be achieved with any single analgesic (Busch, Shore, Bhandari, et al., 2006; Cassinelli, Dean, Garcia, et al., 2008; Huang, Wang, Wang, et al., 2008).
Multimodal analgesia is discussed most often in the context of acute pain treatment; however, pain has multiple underlying mechanisms and is a multifaceted phenomenon, underscoring the importance of using a multimodal approach to manage all types of pain; this should be the rule, rather than the exception (Argoff, Albrecht, Irving, et al., 2009; Kehlet, Wilmore, 2008; Kehlet, Jensen, Woolf, 2006) (see Section I). A sound treatment plan relies on the selection of appropriate analgesics from the opioid, nonopioid, and adjuvant analgesic groups.
WHO Analgesic Ladder for Cancer Pain Relief
Probably the most well-known example of combining analgesics from the nonopioid, opioid, and adjuvant analgesic groups is the World Health Organization (WHO) analgesic ladder (Figure 12-1), which was proposed in the early 1980s as a guide to the management of persistent cancer pain (WHO, 1986; Meldrum, 2005). Still today, it is the clinical model for pain therapy (Ripamonti, Bandieri, 2009). The analgesic ladder focuses on selecting analgesics on the basis of the intensity of the pain using analgesics from each of the analgesic groups and, to some extent, building on previously effective analgesics.
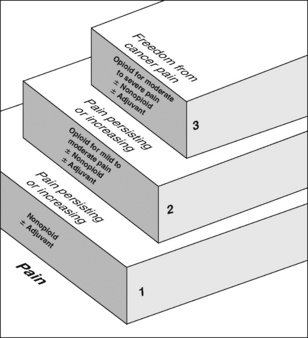
Figure 12-1 WHO three-step analgesic ladder. From World Health Organization (1996). Cancer pain relief, ed 2, Geneva, World Health Organization.
Steps 1, 2, and 3
The three steps of the WHO analgesic ladder address different intensities of pain. However, patients do not always have mild pain at the bottom of the ladder and do not necessarily progress through each of the three levels of pain intensity. Some patients with cancer pain will have moderate to severe pain initially, whereas others may progress directly from mild pain to severe pain. Therefore, treatment of cancer pain does not necessarily begin with step 1, progress to step 2, and follow with step 3 (Eisenberg, Marinangeli, Birkhahn, et al., 2005). If the patient initially has severe pain, step 3 treatment considerations are appropriate (Marinangeli, Ciccozzi, Leonardis, et al., 2004).
Step 1 of the analgesic ladder addresses mild pain by suggesting a nonopioid analgesic, such as acetaminophen or an NSAID, and the possibility of an adjuvant analgesic, particularly if the patient has neuropathic pain. It should be noted, however, that the term adjuvant, when used in this ladder, refers to both the adjuvant analgesics and the adjuvant drugs that are added to analgesics to reduce adverse effects (e.g., laxatives for opioid-induced constipation; see Chapter 19).
If pain is mild to moderate and not relieved by a nonopioid (with or without an adjuvant), step 2 recommends adding an opioid. In other words, the next level of analgesia builds on the previous analgesics. If a nonopioid relieves some but not enough pain, it is continued and an opioid is added. This action, of course, must be predicated on an assessment that indicates the favorable risk:benefit ratio for the continued treatment with the nonopioid drug. Although, in the past, the decision to stop the nonopioid and start an opioid rather than add an opioid to the nonopioid often was considered merely a common mistake, new information about the gastrointestinal (GI) and the cardiovascular (CV) risk of NSAID therapy alters this view. Rather, the decision to use or continue an NSAID cannot be mostly determined by the pain intensity, or the analgesic ladder guideline, but rather, must be decided based on an evaluation of cumulative risk over the remaining time (see Chapter 6).
The clinical usefulness of step 2 is frequently debated (Ripamonti, Bandieri, 2009). An early meta-analysis demonstrated no differences between the safety and efficacy of NSAIDs (step 1 analgesics) and so-called “weak” opioids (step 2 analgesics) (Eisenberg, Berkey, Carr, et al., 1994). Pharmacologically, no difference exists between most of the drugs used on step 2 and those used on step 3. Because the same three groups of analgesics are considered at both steps, the ladder could be reduced to only 2 steps.
In clinical practice, the reason for having step 2 is to assist the clinician in selecting an opioid that may be conventionally preferred for the treatment of moderate to severe pain in the patient who is opioid-naïve, or nearly so. For example, mild to moderate pain is often treated with oral analgesics in a fixed combination of opioid and nonopioid, usually acetaminophen or sometimes aspirin (see Chapter 5). Although the problem with fixed combinations is that the dose of acetaminophen (or other nonopioid) limits the escalation of the opioid dose, the benefit is that it helps the clinician to select a formulation that is generally safe for the patient with very limited opioid exposure (and also allows a potentially more convenient way of combining the nonopioid and opioid). Common examples of opioid/nonopioid fixed combinations are as follows:
• Lortab 5/500 (hydrocodone 5 mg, and acetaminophen 500 mg)
• Percocet (oxycodone 5 mg, and acetaminophen 325 mg)
• Tylenol No. 3 (codeine 30 mg, and acetaminophen 300 mg)
To avoid exceeding the recommended maximum daily dose of 4 g of acetaminophen, the patient cannot take more than 8 tablets per day of those containing 500 mg of acetaminophen or 12 tablets per day of those containing 325 mg of acetaminophen. Recent discussions by the United States Food and Drug Administration (U.S. FDA) encourage clinicians to consider the maximum daily dose of acetaminophen to be 2.6 g, or 8 tablets per day of those formulations containing 325 mg of acetaminophen (U.S. FDA, 2009b; Harris, 2008). This provides more of a safety margin if the patient unintentionally takes other acetaminophen-containing (usually over-the-counter) drugs.
Oxycodone is commonly used in these fixed combinations and is also considered a step 3 drug, a useful opioid for escalating pain. Therefore if a fixed combination of acetaminophen and oxycodone is used at step 2, plain (single-entity) oxycodone may be continued at step 3. Plain acetaminophen can also be continued at appropriate doses.
To avoid step 2 and the need to change opioids or formulations as pain increases, mild to moderate pain may be treated with low doses of plain oxycodone, morphine, or hydromorphone. These mu agonists may be continued throughout the course of therapy because doses of these may be escalated for the relief of increasingly severe pain. Studies have demonstrated the effectiveness of this approach. In 110 opioid-naïve patients with moderate to severe cancer pain, oral morphine at a starting dose of 15 mg/day (10 mg in older adults) followed by titration was found to be well tolerated and effective (Mercadante, Porzio, Ferrera, et al., 2006). A randomized controlled study showed that first-line use of so-called “strong” opioids (e.g., morphine, fentanyl, or methadone) in terminally ill cancer patients resulted in significantly better pain relief and patient satisfaction and necessitated fewer changes in the pain treatment plan than when patients followed the 3-step WHO ladder approach (Marinangeli, Ciccozzi, Leonardis, et al., 2004). Similarly, another study of patients with moderate cancer pain compared the 3-step WHO ladder approach with a modified version that moved patients directly from step 1 to step 3 as pain increased (Maltoni, Scarpi, Modonesi, et al., 2005). Direct progression from step 1 to step 3 resulted in a lower percentage of days with worst pain but were associated with a higher incidence of anorexia and constipation despite laxative treatment. The researchers promoted this approach but underscored the importance of attention to careful management of adverse effects.
Opioid analgesics recommended at step 3 should be available orally and by a variety of other routes of administration so that the opioid need not be changed if the route of administration must change. For example, if a patient taking oral morphine has a temporary episode of nausea and vomiting, morphine may be continued by administering it by other routes, such as rectally or subcutaneously. Many opioids, such as morphine, hydromorphone, and fentanyl, are available by a variety of routes of administration (see Chapter 14).
In much of the world, opioids used at step 3 are either available in a modified-release formulation, or they are long half-life drugs. After dose titration (which in the case of the modified-release drugs often can be accomplished using short-acting formulations), these drugs can be administered with relatively long dosing intervals, which may be more convenient and support adherence to the therapy. For example, morphine and oxycodone have fairly short half-lives—2 to 4 hours (Gutstein, Akil, 2006)—and are available in modified-release formulations that allow dosing every 12 hours.
The existence of active metabolites should be considered when selecting an opioid for long-term therapy. Morphine has active metabolites: morphine-6-glucuronide [M6G] and morphine-3-glucuronide [M3G]. These metabolites accumulate in patients with renal dysfunction and may be associated with toxicity (Johnson, 2007). Hydromorphone (Dilaudid) is a common alternative to morphine, and a modified-release formulation is available in some countries and most recently in the United States. Hydromorphone’s metabolite (hydromorphone-3-glucouronide) can also accumulate in patients with renal dysfunction (Johnson, 2007), but the clinical consequences appear to be limited (Kurella, Bennett, Chertow, 2003). Nonetheless, it has been noted that opioid toxicity can recur when morphine is replaced with hydromorphone in patients with renal dysfunction, and dose should be reduced in such patients (Launay-Vacher, Karie, Fau, et al., 2005). Cautious use of fentanyl in patients with renal dysfunction has been suggested as an alternative when metabolite accumulation is a concern (Dean, 2004; Launay-Vacher, Karie, Fau, et al., 2005) (see Chapter 13 for more on morphine, hydromorphone, and fentanyl).
Other important recommendations that accompany the WHO analgesic ladder are to administer analgesics orally whenever possible and to administer them “by the clock” or around the clock (ATC) to prevent the return of pain.
Effectiveness of the WHO Analgesic Ladder
The use of the WHO ladder in combination with appropriate dosing guidelines is capable of providing adequate pain relief in 70% to 90% of individuals with persistent cancer pain (Hanks, Cherny, Fallon, 2004). This was demonstrated in a 10-year study of 2118 patients, which further indicated that clinically significant pain reduction usually occurred within the first week of treatment (Zech, Grond, Lynch, et al., 1995). Good to satisfactory pain relief was maintained in 88% of the patients over the entire treatment period, and only 12% required invasive procedures for pain relief, such as nerve blocks. Of note is the fact that both opioids and nonopioids were prescribed in 73% of the patients. Also, oral opioid doses reported in this study suggest that the clinicians understood that no ceiling exists on the analgesia of mu agonists because some patients received up to 2400 mg/day of oral morphine. A more recent retrospective study of 3238 patients with advanced cancer reported good pain relief (VAS scores less than 30) in 89% of those following the principles of the WHO analgesic ladder (Bhatnagar, Mishra, Srikanti, et al., 2008). The WHO ladder approach has also been adapted to effectively treat phantom limb pain (Mishra, Bhatnagar, Gupta, et al., 2008) and pain in patients with end-stage renal disease (Salisbury, Game, Al-Shakarchi, et al., 2009).
Preemptive Analgesia for Postoperative Pain Management
In the early 1980s, studies of the spinal cord changes occurring in the context of peripheral afferent input—changes that were termed central sensitization (Woolf, 1983)—generated interest in the therapeutic potential of interventions that could be implemented before tissue injury occurred, in the hope of blocking or reducing this phenomenon (Dahl, Moiniche, 2004; Grape, Tramer, 2007) (see Section I for more on central sensitization). A multimodal approach that includes local anesthetics to block sensory input and NSAIDs and opioids, which act in the periphery and in the CNS, initiated preoperatively and continued intraoperatively and throughout the postoperative course, was suggested as ideal preemptive analgesic treatment (Woolf, Chong, 1993). Since then, numerous studies have investigated a wide variety of agents and techniques in an attempt to show a preemptive analgesic effect (Dahl, Moiniche, 2004; Moiniche, Kehlet, Dahl, 2002). Unfortunately, these studies showed that this approach alone did not result in major benefits postoperatively.
Testing the hypothesis of preemptive analgesia requires comparing the effectiveness of an intervention applied before the surgical incision (experimental group) with the effectiveness of the same or very similar intervention applied only after the surgical incision (control group). The notion that such a simple approach could reduce or possibly prevent postoperative pain stimulated an abundance of research on preemptive analgesia; however, many of the studies had flawed research designs and led to flawed conclusions (Bromley, 2006; Grape, Tramer, 2007; Moiniche, Kehlet, Dahl, 2002; Dahl, Moiniche, 2004). For example, some studies compared preoperative analgesic administration with placebo or no treatment and claimed a preemptive effect when treatment was associated with a subsequent reduction in pain. These and other inaccurate claims of positive results led to an overly optimistic perception of the effectiveness of preemptive analgesia (Grape, Tramer, 2007).
An extensive review of the literature on preemptive analgesia concluded that preoperative (preemptive) administration of systemic opioids did not improve postoperative analgesia (Moiniche, Kehlet, Dahl, 2002). None of the studies reviewed demonstrated a reduction in pain intensity scores in the groups of patients who received preemptive (preincision) analgesia; analysis of the weighted mean differences in pain scores favored the groups who received postoperative (postincision) analgesia. An updated review in 2004 reported the same results (Dahl, Moiniche, 2004).
The general consensus is that preemptive administration of analgesics does not offer major clinical benefits (i.e., consistent immediate postoperative pain relief or reduced need for supplemental analgesia) (Bromley, 2006; Dahl, Moiniche, 2004; Grape, Tramer, 2007; Kelly, Ahmad, Brull, 2001; Moiniche, Kehlet, Dahl, 2002). However, the disappointing research related to preemptive analgesia does not mean that postoperative benefits cannot be realized with aggressive perioperative analgesic interventions. It has been suggested that research and clinical practice should redirect the focus from “preemptive” (timing of a single [most often] conventional intervention) to “protective” analgesia whereby aggressive, sustained multimodal interventions are initiated preoperatively and continued throughout the intraoperative and postoperative periods (Moiniche, Kehlet, Dahl, 2002; Dahl, Moiniche, 2004). Consistent with this approach are the goals of immediate postoperative pain reduction and prevention of prolonged and pathologic pain (Kelly, Ahmad, Brull, 2001). The key underlying pain management principles are to intervene before the onset of pain, use a multimodal approach, and administer analgesics in the proper dose and manner, on time, and for an adequate duration of time (Kelly, Ahmad, Brull, 2001).
Accelerated Multimodal Postoperative Rehabilitation
Advances in the field of pain management have led to more aggressive use of analgesics, but it is unclear if this has resulted in significant improvements in patient outcomes such as the quality of postoperative recovery and long-term function (Liu, Wu, 2007a). An unacceptable number of surgical patients continue to experience delays in recovery, complications, and the need for extended hospital stays (Kehlet, Wilmore, 2008). An extensive review of research (18 meta-analyses, 10 systematic reviews, 8 randomized controlled trials, and 2 observational database articles) revealed that there are insufficient data to show that high-quality postoperative pain management, such as regional analgesia and IV PCA, impacts the incidence and severity of postoperative complications (Liu, Wu, 2007b). The researchers suggested that improvements will depend on the integration of pain control into a comprehensive postoperative rehabilitation program that includes fluid balance and early mobilization and nutrition. For example, a variety of positive outcomes, such as early return of GI function and shortened length of hospital stay, have been observed in patients undergoing major surgery when epidural analgesia is combined in a multimodal postoperative rehabilitation plan (Basse, Billesbolle, Kehlet, 2002; Basse, Hjort Jakobsen, Billesbolle et al., 2000; Brodner, Van Aken, Hertle, et al., 2001; Kehlet, Wilmore, 2004). The use of multimodal strategies that attack the specific physiologic insults of surgery should be considered when developing postoperative treatment plans, particularly for patients who undergo major surgical procedures. For example, the IV administration of hypocaloric dextrose (glucose) 10% in addition to continuous epidural analgesia has been shown to inhibit the catabolic effects of surgery as demonstrated by postoperative suppression of whole-body protein breakdown (Lattermann, Wykes, Eberhart, et al., 2007; Schricker, Meterissian, Wykes et al., 2004).
Patient outcomes have historically been reported as morbidity and mortality data; however, a focus on patient-reported assessments as a subset of morbidity and mortality events may provide unique insight into specific areas that need more intense research and clinical focus (Liu, Wu, 2007a, 2007b). An exhaustive review of the literature evaluated the effect of postoperative analgesia on patient-assessed indicators, which included a variety of aspects of analgesia, presence of adverse effects, health-related quality of life, quality of recovery, and patient satisfaction (Liu, Wu, 2007b). The researchers identified a lack of high-quality data and called for the development of validated tools to measure patient-reported outcomes and well-designed research that examines these as the primary study end points.
Establishing the link between good pain management and improvements in patient outcomes will require changes in the way health care is administered (Kehlet, Wilmore, 2008; Liu, Wu, 2007a, 2007b). Traditional practices in perioperative care, such as prolonged bed rest, withholding oral nutrition for extensive periods, and routine use of tubes and drains, are being increasingly challenged and replaced with evidence-based decision making (Pasero, Belden, 2006). This and other factors have led to the evolution of fast track surgery and enhanced postoperative recovery (Kehlet, Wilmore, 2008). In a review of the literature, Kehlet and Wilmore (2008) describe the evidence that supports key principles of implementing what is referred to as accelerated multimodal postoperative rehabilitation. These are outlined in Box 12-1. Continuous multimodal pain relief is integral to this concept.
Tools that can be used to increase evidence-based perioperative pain management practice patterns are emerging. For example, a novel web-based program called PROSPECT (Procedure Specific Postoperative Pain Management) (http://www.postoppain.org), established by an international team of surgeons and anesthesiologists, posts evidence-based recommendations and algorithms to guide the health care team in decision making with regard to pain management according to specific surgical procedures (Pasero, 2007).
Persistent Postsurgical Pain
As many as 50% of patients undergoing surgical procedures, such as inguinal hernia repair; breast, cardiac, or thoracic surgery; leg amputation; and coronary artery bypass, experience persistent pain; in 2% to 10% of these individuals, the intensity of persistent postsurgical pain is severe (Kehlet, Jensen, Woolf, 2006). A study of 90 women who underwent abdominal hysterectomy pain for noncancer conditions found that 16.7% experienced persistent postoperative pain (Brandsborg, Dueholm, Nikolajsen, et al., 2009). The incidence of persistent post-mastectomy pain is reported to be as high as 65% (Smith, Bourne, Squair, et al., 1999).
Pain following traumatic injury is common as well. A multicenter study conducted in 69 hospitals in 14 states in the United States found that 62.7% of patients (N = 3047) reported injury-related pain at 12 months after a traumatic injury (Rivara, MacKenzie, Jurkovich, et al., 2008). A quarter of patients in an earlier study (N = 397) described pain that interfered with daily activity 7 years following limb-threatening lower extremity trauma; 40% reported high pain intensities (Castillo, MacKenzie, Wegener, et al., 2006).
Further research is needed, but multiple factors are thought to contribute to the likelihood of postsurgical pain, including surgical nerve injury, preexisting pain, and genetic susceptibility. For example, severe pre-amputation pain has long been associated with a higher incidence of phantom limb pain (Bach, Noreng, Tjellden, 1988; Katz, 1997; Nikolajsen, Ilkjaer, Kroner, et al., 1997). A 2-year study of 57 patients who underwent lower extremity amputation revealed that high levels of both pre- amputation pain and acute pain after amputation predicted persistent post-amputation pain (Hanley, Jensen, Smith, et al., 2007). Greater analgesic requirements during the immediate postoperative period following coronary artery bypass surgery predicted persistent pain (multiple anatomic sites) in a study of 736 patients (Taillefer, Carrier, Belisle, et al., 2006). Older patients tend to have a lower risk of developing persistent postsurgical pain than younger patients (Poobalan, Bruce, Smith, et al., 2003; Smith, Bourne, Squair, et al., 1999). For example, one study showed that patients under the age of 40 years old were at increased risk for persistent post-inguinal hernia repair pain (Poobalan, Bruce, King, et al., 2001). Another found that the prevalence of persistent chest and leg pain following cardiac surgery was 55% in patients who were less than 60 years of age and 34% in those over 70 years old (Bruce, Drury, Poobalan, et al., 2003). The reader is referred to an excellent review by Perkins and Kehlet (2000) that includes predictive factors, etiology, and progression of postsurgical pain conditions.
The presence of pain at 3 months after injury was a predictive factor for both the presence and the high severity of persistent pain following major trauma (Rivara, MacKenzie, Jurkovich, et al., 2008). Although the presence of persistent pain varied with age, it was more common in women and in those who had untreated depression before the traumatic injury in this study. Another study found that multiple factors influenced the likelihood of persistent pain 7 years after major lower extremity trauma (Castillo, MacKenzie, Wegener, et al., 2006). These included having less than a high school education, having less than a college education, low self-efficacy for return to usual major activities, a high level of alcohol consumption in the month prior to injury, and high pain intensity, high levels of sleep and rest dysfunction, and elevated levels of depression and anxiety at three months after hospital discharge. Interestingly, those who were treated with opioid analgesics during the first three months after discharge in this study had lower levels of persistent pain at 7 years, underscoring the importance of early initiation of aggressive pain management approaches.
The clinical presentation of persistent postsurgical or post-trauma pain is primarily the patient’s report of the features characteristic of neuropathic pain, such as continuous burning pain and pain beyond the expected time of pain resolution (see Section II for assessment of neuropathic pain). Strategies for preventing these persistent pain states are being investigated, but sustained multimodal pharmacologic approaches that target the underlying mechanisms of neuropathic pain described earlier in this section are recommended (Kehlet, Jensen, Woolf, 2006). See Section I for the underlying mechanisms of the pathology of pain and more on persistent postsurgical pain, and Sections III, IV, and V for discussion of the role of the various analgesics and techniques in the prevention of persistent postsurgical and posttrauma pain.
Around-the-Clock (ATC) Dosing
Two basic principles of providing effective pain management are preventing pain and maintaining a pain rating that allows the patient to accomplish functional or quality of life goals with relative ease (see Section II for goal setting). These may require that the mainstay analgesic be administered on a scheduled ATC basis, rather than PRN (“as needed”), to maintain stable analgesic blood levels. ATC dosing regimens are designed to control baseline pain, defined as the pain the patient reports as being the average pain intensity experienced for 12 hours or more during a 24-hour period (Foley, 2004). In other words, ATC dosing should be considered when pain itself is ATC (continuous) or present for 12 or more hours each day. The use of continuous analgesia prevents the undertreatment of pain in patients who are hesitant to request pain medication and eliminates delays patients encounter waiting for caregivers to prepare and administer pain medication. Another benefit is that patients have been shown to be more adherent with their analgesic regimen when analgesics are prescribed ATC. A 5-week study of oncology outpatients with pain who kept a daily diary to record level of pain and analgesic intake showed that overall adherence rates for those who took their analgesics ATC ranged from 84.5% to 90.8%, compared with 22.2% to 26.6% in those who took them PRN (Miaskowski, Dodd, West, et al., 2001). The researchers pointed out that one might speculate that the reason for lower adherence rates for the PRN regimen was because those patients were experiencing less pain and, therefore, needed less analgesics; however, no significant differences were found in the percentage of patients who reported severe pain (NRS 7 or higher) in the ATC compared with the PRN group.
ATC dosing for continuous pain may be accompanied by provision of additional analgesic doses (called breakthrough doses, supplemental doses, or rescue doses) as needed to relieve pain that exceeds, or breaks through, the ongoing pain (discussed in the following paragraphs). For example, when analgesia is provided orally, ATC dosing often is accomplished with a combination of modified-release opioid given at scheduled times with breakthrough doses of short-acting opioid given if pain breaks through (Cousins, 2007). When invasive routes are used to manage pain, a continuous infusion with PCA boluses or clinician-administered supplemental doses accomplishes the same objectives as this oral approach (see Chapter 17 for more on PCA). Supplemental dosing for breakthrough pain is conventional practice during the management of pain associated with active cancer or other advanced medical illness. It is not considered to be the standard of care in the management of patients with persistent noncancer pain, and in this large and diverse population, the use of this approach requires a separate, careful assessment of potential benefits and burdens (Devulder, Jacobs, Richarz, et al., 2009; Fine, Portenoy, 2007).
Surprisingly, little research has been conducted on the use of ATC dosing. An early cross-over study showed that scheduled ATC dosing of analgesics improved pain relief and mood level compared with on-demand PRN dosing in patients with persistent noncancer pain (Berntzen, Gotestam, 1987). A more recent parallel-design study of medical inpatients with pain from a variety of origins compared ATC scheduled opioid doses with PRN opioid doses and found that those who received ATC doses experienced lower pain intensity ratings (Paice, Noskin, Vanagunas, et al., 2005). As might be expected, a significantly greater percentage of the prescribed opioid was administered when it was given ATC (70.8%) compared with PRN (38%). There were no differences in adverse effects between the two groups. A randomized controlled trial (N = 227) showed that early oral analgesia (first postoperative day) with scheduled 20 mg doses of short-acting morphine every 4 hours and an additional 10 mg dose every 2 hours PRN was safe and effective, producing similar analgesia as IV PCA with a basal rate after intra-abdominal surgery (Pearl, McCauley, Thompson, et al., 2002). An observational, prospective study administered ATC oral short-acting morphine to patients (N = 95) following orthopedic surgery and reported average pain scores were 2.4 at rest and 4.0 during movement in bed (Zaslansky, Eisenberg, Peskin, et al., 2006). Nausea and vomiting, the most common adverse effects, were reported by 22%, and no one required naloxone.
Other research has shown conflicting results. A study of outpatients with pain from bone metastases found no significant differences in average, least, or worst pain intensity in patients taking ATC or PRN opioids, but significantly higher opioid prescriptions and intake (12.4 times more) were reported in patients who took ATC opioids (Miaskowski, Mack, Dodd, et al., 2002). The researchers noted that their findings challenge the accepted principle of treating continuous pain with ATC analgesics but offered a number of possible explanations. These included that the opioid analgesic regimens may not have been effective for the type of pain the patients had (bone pain), analgesic doses were not titrated to optimal effect, and the possibility that comparisons were difficult because the two groups were not receiving the same analgesics (PRN received short-acting opioids and ATC received long-acting opioids). Another study randomized children to receive ATC or PRN acetaminophen plus codeine following tonsillectomy and found no differences in pain intensity ratings or pain relief scores (Sutters, Miaskowski, Holdridge-Zeuner, et al., 2004, 2005). Both groups experienced moderate to severe pain, and similar to the previous study, more analgesic (two times more) was consumed by those in the ATC group. There were no differences in adverse effects, such as nausea and vomiting, sedation, and dizziness, which were described as moderate to severe. An interesting finding was that the parents of children receiving PRN analgesia decreased the dose of analgesic they gave to their children over time despite high pain intensity and poor pain relief.
These studies suggest the need for more research evaluating the best method for analgesic dose administration in a variety of populations and for a variety of types of pain. Until research proves otherwise, prevention of the recurrence of pain with scheduled ATC dosing is recommended for continuous types of pain.
Awakening Patients for Analgesic Administration
Nurses often wonder if patients taking ATC short-acting opioids should be awakened and given pain medication and if they should teach patients in the home setting to wake themselves up during their normal sleep time to take their pain medications to keep pain under control. There is very little research guiding this practice, and none could be found in opioid-naïve patients with acute pain. The consensus expert opinion of the European Association for Palliative Care (EAPC) regarding palliative care patients with moderate to severe pain is to give a double dose of short-acting opioid at bedtime rather than a single dose and awaken the patient for a second dose 4 hours later (Hanks, De Conno, Cherny, et al., 2001). This is based on the assumption that doubling the dose will prolong the duration of analgesia long enough to prevent awakening with pain.
Some research on the efficacy of double dosing vs. usual dosing has been done in patients with cancer pain. A double-blind, randomized, cross-over study that compared the two methods found that average pain, strongest night pain, and sleep quality were slightly better in those who took a double dose compared with those who took a single dose, but the difference was not clinically significant (Dale, Piribauer, Kaasa, et al., 2009). The researchers suggested that the slight difference may have been due to initial higher exposure to morphine’s metabolite M6G (see Chapter 13). An earlier prospective study of palliative care patients found that all pain scores were worse in patients who took a double dose of opioid compared with those who took a single dose at bedtime followed by another dose 4 hours later (Todd, Rees, Gwilliam, et al., 2002). Further, those in the double-dose group required more breakthrough analgesia and experienced more adverse effects.
Double doses of opioids should not be given to opioid-naïve patients, but patients who are opioid tolerant can be told to try double dosing and usual dosing as described above and see which works best to keep their pain under control. Alternatively, the patient who has access to long-acting, modified-release formulations can be switched to one of these in an effort to improve sleep at night. Occasional patients in the ambulatory setting prefer to have a short-acting drug during the day and a dose of a long-acting drug at night; this unconventional practice can be explored on a case-by-case basis. Both opioid-naïve and opioid-tolerant patients with persistent pain in the hospital setting should be awakened to take their pain medication (see Chapter 19 for assessment of sedation and respiratory status before opioid administration). Awakening postoperative patients with moderate-to-severe pain to take their pain medication is especially important during the first 24 to 48 hours of therapy to keep pain under control. Patients should be told that this helps to avoid waking up with severe pain and that if their pain is well controlled, they are more likely to go back to sleep quickly. The patient can transition gradually to PRN dosing and sleeping during the night as pain resolves.
Breakthrough Pain
Breakthrough pain (sometimes called pain flare, episodic pain, or transient pain) is defined as a transitory exacerbation of pain in a patient who has relatively stable and adequately controlled baseline pain (Portenoy, Forbes, Lussier, et al., 2004). The intensity of pain is sometimes included in the definition of breakthrough pain, i.e., a transient increase in pain to greater than moderate intensity occurring on a baseline pain of moderate intensity or less (Foley, 2004). Breakthrough pain is not routinely recognized, evaluated, and treated, and research has revealed a need for standardized methods for diagnosing it (Caraceni, Martini, Zecca, et al., 2004; Mercadante, 2006a, 2006b; Mercadante, Radbruch, Caraceni, et al., 2002) (see Section II for assessment tools).
Like its definition, there is variation in the description of breakthrough pain. Some describe it as occurring spontaneously (not related to activity), rapidly increasing to a high intensity level, and having a short duration (30 to 45 minutes) (de Leon-Casasola, 2008). Others describe subtypes of breakthrough pain, noting that it can have a sudden or gradual onset and can be brief or prolonged; some episodes are spontaneous, and others are associated with an identifiable precipitant (Bennett, Burton, Fishman, et al., 2005a; Payne, 2007). When breakthrough pain is brief and precipitated by a voluntary action, such as movement, it is referred to as incident pain. One study of patients with persistent noncancer pain showed this to be the most common type of breakthrough pain with an identifiable precipitant in 69% of the episodes, and 92% of these were activity-related (Portenoy, Bennett, Rauck, et al., 2006). Incident pain can also be unpredictable and associated with involuntary activity, such as sneezing or coughing. This type of breakthrough pain is also common, with no predictable onset in 45% and sometimes a predictable onset in 31% of the episodes (Portenoy, Bennett, Rauck, et al., 2006). Another subtype, idiopathic pain, is not associated with a known cause and often has a longer duration than incident pain. End-of-dose failure, the last of the subtypes, is characterized by a return of pain before the next analgesic dose is due. The occurrence of increased pain at the end of the scheduled dosing period suggests a need to maintain a higher plasma drug concentration throughout the dosing interval (Portenoy, Forbes, Lussier, et al., 2004). In these cases, the dose of the scheduled analgesic should be increased, or, in some patients, the interval between doses should be shortened, which results ultimately in an increased dose.
The incidence of breakthrough pain in cancer patients is reported to be between 50% and 90% (Portenoy, Bennett, Rauck, et al., 2005). A prospective international survey of patients with cancer pain (N = 1095) in 24 countries reported breakthrough pain in 64.8% of those surveyed (Caraceni, Martini, Zecca, et al., 2004). Most of these studies were performed in inpatient environments or in populations with advanced illness; the prevalence of breakthrough cancer pain in the population of patients undergoing treatment in the community is largely unknown. A longitudinal study (N = 101) of breakthrough pain in advanced cancer patients at home found that on admission to the study, 49% reported breakthrough pain, and 78% reported that the breakthrough pain strongly limited their activity; most had no prescription for breakthrough analgesia (Mercadante, Costanzo, Fusco, et al., 2009). The researchers described breakthrough pain as a dynamic entity dependent on several factors, including analgesic treatment and the course of disease.
Individuals with persistent noncancer pain also may experience a high incidence of breakthrough pain. A telephone questionnaire administered to patients with well-controlled baseline persistent noncancer pain revealed that 74% experienced breakthrough pain (Portenoy, Bennett, Rauck, et al., 2006). The median number of episodes was two per day, and the median time to maximum intensity was 10 minutes with a median duration of 60 minutes. The type of breakthrough pain was somatic (38%), neuropathic (18%), visceral (4%), or mixed (40%). These patients were undergoing treatment at pain clinics, and this prevalence may overstate the rate that exists in the general population.
Although much about the epidemiology of breakthrough pain remains to be elucidated, some early data strongly suggest that, if not addressed adequately, breakthrough pain can have significant negative effects on function and quality of life (Fine, Portenoy, 2007; Mercadante, Villari, Ferrera, et al., 2004; Taylor, Webster, Chun, et al., 2007; Zeppetella, Ribeiro, 2006). One study also indicated that the presence of breakthrough pain in U.S. cancer patients increases the economic burden for patients and the health care system (Portenoy, Bennett, Rauck, et al., 2006).
Treatment of Breakthrough Pain
The routine treatment of breakthrough pain generally is considered to be conventional practice in populations for which opioid therapy is the mainstay for the long-term management of moderate to severe pain—specifically those with active cancer or other types of advanced medical illness. The treatment of breakthrough pain in other populations has yet garnered no consensus, and the prudent approach is to consider this strategy as a separate intervention requiring its own assessment of benefit and burden (Devulder, Jacobs, Richarz, et al., 2009; Fine, Portenoy, 2007).
Addressing the cause of breakthrough pain can eliminate the need for analgesic therapy in some patients (Portenoy, Forbes, Lussier, et al., 2004). For example, surgery, chemotherapy, or radiation therapy in some cancer patients may eliminate the cause. Adjusting the scheduled analgesic regimen, such as by increasing the dose, is another approach that targets cause. Even breakthrough pain that does not appear to be associated with end-of-dose failure (e.g., pain occurring in patients receiving continuous infusions) may be amenable to a dose increase. As a trial, the scheduled analgesic dose should be increased until either acceptable pain relief or intolerable and unmanageable adverse effects supervene (Portenoy, Forbes, Lussier, et al., 2004). If the frequency or intensity of breakthrough pain episodes decline, this change can be continued.
If the decision is made to treat the breakthrough pain specifically, a variety of analgesic interventions may be considered (Portenoy, Forbes, Lussier, et al., 2004). NSAIDs can be used, again based on an analysis of benefit and burden. More often, a short-acting mu agonist opioid is selected and provided on a PRN basis (Mercadante, 2004; Portenoy, Forbes, Lussier et al., 2004). Comparative studies sufficient to guide drug selection and dosing for breakthrough pain are lacking, and guidelines are empirical. Often, a short-acting formulation of the same drug given as a modified-release formulation is selected. For example, short-acting oral morphine is prescribed for breakthrough pain in patients taking modified-release morphine. Although cases have been published that suggest the safety and efficacy of methadone for the treatment of breakthrough pain (Fisher, Stiles, Hagen, 2004), concern about accumulation of this drug with repeated doses suggests that an alternative short half-life opioid drug be selected instead (see Chapter 13). Similarly, a short-acting oral or transmucosal drug usually is prescribed when baseline pain is treated with transdermal fentanyl. (See Chapter 16 for calculation of breakthrough doses and Chapter 18 for patient examples that demonstrate the clinical management of breakthrough pain.)
There is a mismatch between the time-action relationship of oral opioids and the temporal profile of most breakthrough pains. Oral drugs typically have an analgesic onset of at least 20 minutes. Most breakthrough pains reach a maximum intensity in just a few minutes and disappear within an hour. A prospective survey of hospice patients identified this mismatch as a potential barrier to effective management (Zeppetella, 2008). Most patients in this study described their breakthrough pain as sudden and unpredictable, lasting an average of 35 minutes. By the time the oral analgesic was deemed effective, the pain may already have spontaneously resolved. This phenomenon was noted in another study that showed patients often failed to take breakthrough doses when they had breakthrough pain episodes (Davies, Vriens, Kennett, et al., 2008). The mean duration of the breakthrough episodes was 30 minutes. The patients reported that the pain improved before the drug could take effect; most were prescribed an opioid with an onset of 20 to 30 minutes. A perplexing finding was that the most commonly cited explanation for not taking a breakthrough dose in this study was a lack of sufficient pain intensity for breakthrough pain medication even though the breakthrough episodes were described as usually moderate to severe in intensity. The researchers underscored the need for appropriate prescriptions for breakthrough pain and focused education about breakthrough pain for both health care professionals and patients and their caregivers.
Lipophilic transmucosal formulations are undergoing development with the intent of addressing the temporal mismatch by providing a more rapid onset of effect. Oral transmucosal fentanyl (OTFC, Actiq) and buccal fentanyl tablet (Fentora) are both commercially available in many countries, a sublingual fentanyl tablet and a fentanyl nasal spray are available in some countries, and a buccal patch of fentanyl (Onsolis) was recently approved in the United States. Similar formulations of other lipophilic drugs, such as sufentanil, are undergoing development (see Chapter 14 for intranasal and oral transmucosal formulations).
The rapid-onset transmucosal fentanyl formulations have a faster onset of analgesia than the oral formulations; peak effect is substantially earlier, and duration of action is relatively short (Fine, Portenoy, 2007). Patients have reported improvement in breakthrough pain within 15 minutes with OTFC (Mystakidou, Katsouda, Parpa, et al., 2005) and buccal fentanyl (Portenoy, Taylor, Messina, et al., 2006). Oral transmucosal and intranasal sufentanil formulations presumably will have similarly rapid onset (Gardner-Nix, 2001a; Jackson, Ashby, Keech, 2002). A Cochrane Collaboration Review of opioids used for breakthrough cancer pain identified only four randomized controlled trials for analysis, and all four examined the use of OTFC and showed it to be safe and effective (Zeppetella, Ribeiro, 2006). This review underscored the need for more research to evaluate the effectiveness of the opioids used to manage breakthrough pain.
Much is not known about the rapid-onset transmucosal formulations for breakthrough pain. Direct comparisons with oral formulations are very limited, and the extent to which the more rapid onset yields clinically meaningful benefit is uncertain. Presumably, there is a subpopulation that could benefit greatly, but empirical definition of this group has not yet been done. The various formulations (e.g., buccal vs. sublingual vs. intranasal) appear to vary in onset such that the proportion of patients who report meaningful benefit at 5 minutes, 10 minutes, or later points varies by drug; as yet, it is unknown whether these differences will translate into clinical preferences for any formulation or group of formulations. As implied previously, all these formulations have been approved by the regulatory authorities for cancer-related breakthrough pain; there is very little evidence of efficacy in populations with noncancer pain, and the appropriate positioning of these drugs remains a matter of clinical judgment only. Finally, there is concern about the abuse liability of the rapid-onset formulations, but the extent to which these drugs may drive aberrant behavior, relapse into addiction, or diversion now is unknown.
In short, the rapid-onset transmucosal formulations appear to be a therapeutic advance given their likely ability to improve the management of breakthrough pain, at least those with rapid onset. At present, their role relative to conventional oral rescue medication is uncertain and is evolving as studies appear and experience accumulates.
The IV route has also been used to rapidly treat breakthrough pain and optimize the control of baseline pain. In an open-label study, patients (N = 25) with poorly controlled baseline metastatic (bone) cancer pain and movement-related breakthrough pain received rapid IV opioid titration to obtain pain relief (Mercadante, Villari, Ferrera, et al., 2004). The dose was then increased further to challenge the therapeutic ceiling, which was determined by the development of adverse effects rather than pain relief at rest. The daily IV dose was converted to a daily oral dose and administered in modified-release formulation every 8 to 12 hours (mean oral morphine equivalents were 102 mg). This titration approach resulted in significant improvements in movement-related pain. A minority of patients experienced adverse effects that required treatment or decreases in opioid dose. A later open-label study showed similar results in patients with advanced cancer who were administered IV morphine in doses proportional to their ATC regimen (Mercadante, Intravaia, Villari, et al., 2008). The mean IV morphine dose was 12 mg (range of 9 mg to 14 mg), and more than 60% of the patients in this study experienced a > 33% reduction in pain intensity (see Section II for more on meaningful pain relief). There were no adverse events with this approach.
Alternative methods may be necessary when systemic (oral, IV) opioids fail to relieve breakthrough pain. A report of 12 patients with advanced cancer receiving intrathecal analgesia described treatment of breakthrough pain unresponsive to high doses of IV morphine (Mercadante, Arcuri, Ferrera, et al., 2005). The patients were titrated with intrathecal boluses of local anesthetics (levobupivacaine 0.25%, mean, 1.5 mg) or with doses of sublingual ketamine (25 mg), depending on patient preference. Breakthrough pain was achieved for all episodes within 10 minutes by either method. Treatment was well tolerated, but the researchers suggested reserving these alternative methods for select patients and administering them in a setting that can support frequent monitoring and skilled nursing. (See Chapter 15 for more on intraspinal analgesia and Chapter 23 for more on ketamine.)
PRN Dosing
PRN (“as needed”) dosing requires patients to request analgesia. Effective PRN dosing relies on the patient’s active participation. Patient teaching must include reminding patients to “stay on top of pain” and request analgesia before pain is severe and out of control. Obviously crucial to the effective use of PRN dosing is a rapid response to reports of pain and to requests for an analgesic.
In addition to its use for breakthrough pain, PRN dosing of opioid analgesics may be appropriate for other types of pain, such as intermittent pain. It also is useful when initiating opioid analgesic therapy in opioid-naïve patients with moderate to severe persistent pain, especially when pain is escalating rapidly. In these cases, PRN dosing allows for a rapid response to the patient’s need for pain relief while minimizing the chance of overdose. PRN dosing is also helpful when pain is decreasing rapidly (Coyle, Cherny, Portenoy, 1995). When patients with acute pain recover and pain resolves, ATC dosing may be replaced with PRN dosing (Pasero, Portenoy, McCaffery, 1999).
Although PRN dosing may be effective in these scenarios, there is abundant clinical experience suggesting the potential for negative outcomes. A study of outpatient oncology patients with pain who kept a daily diary to record level of pain and analgesic intake showed overall adherence rates for those who took their analgesics ATC ranged from 84.5% to 90.8% compared with 22.2% to 26.6% in those who took them PRN, respectively (Miaskowski, Dodd, West, et al., 2001). When PRN dosing is used in the inpatient setting, patients may be reluctant to ask for pain medication for a variety of reasons (Salmon, Hall, 2001) and request it when pain is severe and out of control despite instructions to stay on top of the pain. After the patient’s request, the nurse must check the record to ensure that enough time has elapsed since the last dose the patient received; then the nurse must obtain and prepare the analgesic, and, if it is an opioid, account for removal of the drug from the drug security system. These activities are time-consuming and result in further loss of pain control.
Research shows that the most vulnerable of patients are at high risk for undertreatment of pain when the PRN approach is used for continuous pain. A study of older adults hospitalized for hip fracture found that most of the analgesics were prescribed for PRN administration and the nurses were unaware that ATC administration of PRN-prescribed analgesics would be preferable for this type of pain; only 22.3% of the patients received ATC analgesic administration of the PRN analgesics, and less than 25% of the minimum morphine equivalents of the opioids prescribed were administered (Titler, Herr, Schilling, et al., 2003). Patients with dementia received significantly less pain medication than those without dementia, and 8 patients with dementia in this study received no opioid at all during the first 72 hours after admission (Ardery, Herr, Hannon, et al., 2003). It is important for nurses to recognize that PRN-prescribed analgesics may be administered ATC within the parameters of the PRN prescription and that this is the preferred dosing method for patients with continuous pain. Because there are so many disadvantages to PRN dosing, the appropriateness of its use should be carefully evaluated in all cases.
Patient-Controlled Analgesia (PCA)
PCA is an interactive method of pain management that permits patients to treat their pain by self-administering doses of analgesics. It has been used to manage all types of pain, most commonly acute pain and less often cancer pain because most cancer pain can be managed with oral opioid analgesics. Procedural sedation has also been delivered via PCA (Lehmann, 2005). Although patients with pain often self-administer their oral analgesics (see Inpatient Oral PCA, later in this chapter), the term PCA is applied usually when dosing opioids by IV, subcutaneous (SC), perineural (i.e., patient-controlled regional analgesia, PCRA), epidural (PCEA), and intranasal routes of administration. Typically, a special infusion pump is used to deliver PCA by most of these routes of administration. In this context, PCA refers to the bolus dose the patient controls when pressing a button on or attached to the pump. PCA can be delivered by two modes: PCA bolus doses with a continuous infusion (basal rate) or PCA bolus doses alone.
The PCA approach recognizes that only the patient can feel the pain and only the patient knows how much analgesic will relieve it (Pasero, McCaffery, 1993; Pasero, Portenoy, McCaffery, 1999). By allowing patients to determine dosing, PCA addresses the significant variations in analgesic requirements between individuals (Grass, 2005; Lehmann, 2005).
PCA is similar to responsive PRN dosing in that it requires patients to recognize that they are experiencing pain and request analgesia (e.g., by pressing a button on a pump to deliver a PCA bolus). The difference between PRN and PCA dosing is that with PCA the patient rather than a caregiver administers the analgesic, so the delay in waiting for a caregiver’s response to the request for analgesia is eliminated. Just as with effective PRN dosing, patients are reminded to “stay on top of the pain” to maintain a steady analgesic level and administer doses before pain is severe and out of control.
Mu agonist opioid analgesics, specifically morphine, hydromorphone, and fentanyl, are the most common analgesics administered by PCA. Frequently, local anesthetics and sometimes the alpha2-adrenergic drug clonidine are added to an opioid for PCEA (see Chapter 15), and local anesthetics are used for PCRA (see Chapter 26).
PCA has been studied extensively, particularly IV PCA in postoperative patients (Gilron, 2008). A meta-analysis of 32 trials comparing opioids via IV PCA with opioids via conventional (intramuscular [IM], IV, SC) methods for postoperative pain management found that IV PCA was associated with improved analgesia, decreased pulmonary complications, and higher patient preference for the method of pain control (Walder, Schafer, Henzi, et al., 2001). A later Cochrane Collaboration Review of 55 randomized controlled studies concluded that IV PCA resulted in better pain control, increased patient satisfaction, and a higher incidence of pruritus compared with conventional methods of pain control (Hudcova, McNicol, Quah, et al., 2006). There were no differences in other adverse effects or length of hospital stay. Patients who used PCA consumed higher opioid doses suggesting that the less effective analgesia from conventional methods may have been related to inadequate dosing. A meta-analysis concluded that IV PCA was associated with higher cumulative morphine consumption at 24 and 48 hours and improved VAS scores compared with nurse-administered analgesia in patients following cardiac surgery (Bainbridge, Martin, Cheng, 2006). One study randomized patients (N = 122) following gynecologic surgery to receive IV hydromorphone by PCA or IV or SC hydromorphone by scheduled nurse-administered doses and reported no differences in pain scores and patient satisfaction (Bell, Shaffer, Schrickel-Feller, 2007). PCA was associated with higher opioid doses, but unlike the previous analysis, this did not result in better pain control. Another randomized controlled trial (N = 93) showed that oral oxycodone plus acetaminophen provided superior pain control with fewer adverse effects compared with IV PCA morphine following cesarean section (Davis, Esposito, Meyer, 2006).
A Cochrane Collaboration Review compared IV PCA with continuous epidural analgesia and found the latter produced superior postoperative pain relief and a higher incidence of pruritus following intra-abdominal surgery, but comparisons of other outcomes were not possible because of a lack of research (Werawatganon, Charuluxananan, 2005). A study (N = 92) comparing continuous peripheral nerve block vs. IV PCA showed a 74% and 35% reduction in postoperative opioid requirement, respectively (Chelly, Greger, Gebhard, et al., 2001). Continuous peripheral nerve block also produced less blood loss, better functional outcomes, fewer complications, and a shorter length of hospital stay than IV PCA (see Chapter 26 for more on continuous peripheral nerve block).
Some have suggested that PCA provides an opportunity for nurses to “distance” themselves from the responsibilities of assessing and managing their patients’ pain (Salmon, Hall, 2001); however, PCA does not absolve nurses from their role as the patient’s primary pain manager. Successful PCA therapy depends on the nurse’s systematic assessment of the patient’s pain, adverse effects, and use of the technology followed by adjustments in the prescription if necessary to optimize pain control (see Chapter 17 for the clinical use of PCA).
Appropriateness of PCA
A number of factors need to be considered in determining whether a patient is a candidate for PCA (Box 12-2). Great care must be taken to ensure that PCA is appropriate, especially the additional cost of a PCA pump and the risks (e.g., infection, programming errors) associated with its use (Macario, 2005).
Clinicians often hesitate to prescribe PCA for children believing that they are too young to understand the concept of PCA and how to use the pump appropriately. However, PCA has been used effectively and safely in developmentally normal children as young as 4 years old (Wellington, Chia, 2009). Although IV PCA has been shown for many years to be safe in older patients (Egbert, Parks, Short, et al., 1990; Gagliese, Gauthier, Macpherson, et al., 2008; Gagliese, Jackson, Ritvo, et al., 2000; Mann, Pouzeratte, Boccara, et al., 2000), clinicians often do not prescribe it for fear of producing confusion in these patients. Although the opioid (by whatever approach it is delivered) can contribute to confusion, the factors that may be responsible are numerous (Bagri, Rico, Ruiz, 2008; Redelmeier, 2007; Sharma, Sieber, Zakriya, et al., 2005; Zakriya, Christmas, Wenz, et al., 2002), and the development of confusion should not be assumed to be related to either the drug or the delivery approach. For example, a study of 333 older (mean age 74) postoperative patients revealed that the presence of postoperative pain and increased intensity of postoperative pain were independent predictors of postoperative delirium (Vaurio, Sands, Wang, et al., 2006). There was also an ordered relationship between the severity of preoperative persistent pain and the risk of postoperative delirium in this study; those with severe preoperative pain were at greater risk than those with moderate preoperative pain. It is important to also note that naloxone administration does not improve postoperative delirium, further suggesting that opioids often are not a primary underlying cause (Redelmeier, 2007) (see Chapter 19 for cognitive effects).
To be considered a candidate for PCA, patients must be able to understand the relationships between pain, pushing the PCA button, and pain relief (Pasero, Portenoy, McCaffery, 1999). In cases when PCA is warranted, patients should not be denied access to this modality simply because of their age. Instead, they should be carefully screened for their cognitive and physical ability to manage their pain by PCA.
It is important that clinicians regularly assess an individual’s ability to self-administer analgesia after PCA is initiated. Patients who are deemed appropriate candidates for the therapy may prove unable to maintain adequate analgesia with this method. Although patient independence in controlling pain has been described as a benefit of PCA, research and clinical experience has shown that not all patients want this responsibility (Salmon, Hall, 2001). Further, it has been noted that a number of factors influence the variation seen in patients’ ability to use PCA successfully (Katz, Buis, Cohen, 2008; Salmon, Hall, 2001). For example, some patients may fear adverse effects or mistrust the technology. Some populations warrant particularly close attention to dosing patterns to help ensure adequate pain relief with PCA. For example, research has shown that older patients tend to self-administer less opioid via PCA than younger patients (Gagliese, Gauthier, Macpherson, et al., 2008; Gagliese, Jackson, Ritvo, et al., 2000). There were no age-related differences in pain intensity in this study. PCA should be discontinued, and alternative methods for managing pain, such as nurse-administered scheduled ATC doses, should be promptly initiated if patients are unable or unwilling to use PCA.
PCA by Proxy
PCA by proxy is the unauthorized administration of a PCA dose by another person. This has the potential to produce significant patient harm because it circumvents an important safeguard of PCA, i.e., the excessively sedated patient will drop the PCA button, thereby preventing further opioid administration and subsequent respiratory depression (Pasero, McCaffery, 2005a). Over the years, there have been reports of the dangers of PCA by proxy. One early report evaluated 3785 patients who received IV PCA and reported 14 critical events, 3 of which involved unauthorized family members pressing the PCA button (Ashburn, Love, Pace, 1994). A review of nearly 6000 patients who had received IV PCA with no basal rate identified unauthorized PCA delivery to sleeping patients by relatives as the cause of 2 of 14 cases of respiratory depression (Sidebotham, Dijkhuizen, Schug, 1997).
These types of reports prompted The Joint Commission (TJC), an independent accrediting body of health care facilities in the United States, to issue a “sentinel event alert” on unauthorized PCA administration. This alert identified 460 PCA-related adverse events over a 5-year period; 15 of these events were the result of unauthorized family or staff members pressing the PCA button (TJC, 2004). Others have echoed concerns regarding this phenomenon (Institute for Safe Medication Practices, 2003a, 2003b).
Given the risks associated with PCA by proxy, TJC now expects to see proof that institutions take steps to minimize the potential for this outcome. These include patient education about the use of PCA prior to initiation of therapy and the use of verbal and written instruction warning against individuals other than the patient pressing the PCA button (Box 12-3). The observation that most PCA by proxy is initiated by well-intentioned family members who want to ensure that their loved one is comfortable underscores the importance of frequent assessment of patients during PCA therapy to identify those who are unable to manage their own pain effectively as well as telling family members to contact staff if they have concerns about the patient’s pain.
Authorized Agent-Controlled Analgesia: Unconventional Use of the PCA Pump
When patients are unable or unwilling to self-administer analgesics, another individual may be authorized to manage the patient’s pain using the PCA technology. For example, family-controlled analgesia (FCA) or caregiver-controlled analgesia (CCA) designates one person to be the patient’s primary pain manager with the responsibility of pressing the PCA button (on the face of the pump or pendant attached to the pump) (Pasero, McCaffery, 1993; Pasero, Portenoy, McCaffery, 1999). With nurse-activated dosing (NAD) (also called nurse-controlled analgesia), the patient’s primary nurse has that responsibility. These methods have collectively been called “authorized agent-controlled analgesia” (AACA) (Wuhrman, Cooney, Dunwoody, et al., 2007) and have been safely and effectively used for many years in patients of all ages. AACA is supported by a position paper with clinical practice recommendations developed by the American Society for Pain Management Nursing (Wuhrman, Cooney, Dunwoody, et al., 2007) and endorsed by other nursing specialty organizations such as the Oncology Nursing Society and the Hospice and Palliative Care Nurses Association (see Boxes 12-3 and 12-4 for guidelines for the use of PCA and AACA).
Although FCA and CCA have long been used in adults (Cohen, Smetzer, 2005; Pasero, McCaffery, 1993; Pasero, Portenoy, McCaffery, 1999), clinical experience and research is most abundant in the use of parent- controlled analgesia, another form of AACA, in pediatric patients (Anghelescu, Burgoyne, Oakes, et al., 2005; Czarnecki, Ferrise, Jastrowski Mano, et al., 2008; Lehr, BeVier, 2003; Monitto, Greenberg, Kost-Byerly, et al., 2000; Voepel-Lewis, Marinkovic, Kostrzewa, et al., 2008). When any of these methods are used, it is particularly important to designate a secondary pain manager to provide respite for the primary pain manager. Alternately, in the hospital setting, NAD may be used during the primary pain manager’s rest periods. However, essential to safe use of these methods is to ensure that only one person is managing the patient’s pain at a time (Pasero, McCaffery, 2005a).
NAD may be used in patients who have no family member or significant other who can manage their pain (Pasero, McCaffery, 2005a). It is ideally suited for critically ill patients who experience significant, continuous pain from surgery or underlying pathology and undergo numerous repetitive, painful procedures. Rarely do these patients meet the cognitive or physical criteria for managing PCA, but the PCA pump can be used to administer a continuous infusion and the nurse can press the PCA button to administer supplemental doses for breakthrough pain and prior to painful procedures (Pasero, McCaffery, 2001; 2005a). This is not only effective and convenient but saves nursing time that would be spent preparing and administering analgesia by conventional methods. In all cases of AACA, the control of analgesia is returned to patients (i.e., PCA) if and as soon as they are able to assume it.
Criteria for determining which patients are candidates for AACA and for selecting a pain manager, policies and procedures, teaching strategies for pain managers and staff, and monitoring guidelines should be developed prior to the use of AACA (see Box 12-4). It is important to note that these methods are not patient controlled, and it is inaccurate and confusing to refer to them as patient controlled (e.g., “PCA by caregiver” or “PCA by nurse”). Attention should be given to insuring that policies and procedures, orders, and patient education material are entitled with the correct name of the therapy (e.g., “caregiver-controlled analgesia” or “nurse-activated dosing”).
Some institutions have adapted PCA equipment for patients who are cognitively able to use PCA but are physically unable to press the PCA button, such as patients with rheumatoid arthritis (Pasero, Portenoy, McCaffery, 1999). One publication described the development of a pneumatic trigger for an 8-year-old child and an 11-year-old child who were unable to activate PCA otherwise because of major burn injuries of both hands and arms (Lehr, BeVier, 2003). The device was activated by pressing the heel of the foot against the trigger. The legal implications of altering infusion devices must be carefully considered before implementing these novel approaches.
Inpatient Oral PCA
A major advantage of using PCA is the elimination of the delay period between the patient’s request for analgesia and the nurse administering it. As mentioned, the use of PCA in the hospital is administered most often by the IV, SC, or epidural routes. Although patients commonly self-administer oral pain medications in the home setting, oral PCA in the hospital setting is a relatively new concept (Kastanias, Snaith, Robinson, 2006; Pasero, Portenoy, McCaffery, 1999). However, it has been used safely and effectively in this setting for the treatment of all types of pain.
Perhaps the first description of inpatient oral PCA was a study of 48 adult patients admitted for orthopedic surgery; 26 patients were allowed to self-administer 5 mg oxycodone + 500 mg acetaminophen (Tylox) from a bedside supply of 25 tablets, and 22 were given the same drug on a PRN basis by the nursing staff (Jones, 1987). There were no significant differences in amount of analgesic the patients took, but older patients took less. Although pain was not formally assessed (common practice in the 1980s), 100% of the patients taking oral PCA stated they would request the same method of analgesic administration in the future. The majority of nurses expressed satisfaction with the method citing benefits such as greater patient independence, improved nurse-patient relationship, and time saved related to the tasks required for conventional opioid drug administration. There were no cases of drug loss or diversion.
Other creative approaches for using oral PCA have been described. One hospital provided selected patients with a Velcro wrist pouch in which one or two doses of opioid analgesic could be stored for PRN self administration (Pasero, Portenoy, McCaffery, 1999). The hospital reported that not one incident of patient noncompliance or loss or diversion of analgesics occurred with the program. Further, the hospital saw a 10% increase in patient satisfaction with oral analgesics with the use of oral PCA. A study of general surgical patients was undertaken to compare this same oral PCA approach (N = 19) with nurse-administered oral analgesia (NAOA) (N = 17) (Riordan, Beam, Okabe-Yamamura, 2004). Despite taking more doses (acetaminophen + hydrocodone), only 65% of the patients in the NAOA group achieved their pain rating goal within 1 hour compared with 93% in the oral PCA group. There were no adverse effects or episodes of missing or diverted analgesics.
A randomized controlled study compared oral PCA and IV PCA in 60 patients following orthopedic surgery (Striebel, Scheitza, Philippi, et al., 1998). After titration to comfort, patients were randomly assigned to self-administer IV doses of morphine via a portable IV PCA device or doses of oral morphine solution via a modified version of the same PCA device. Patients reported comparable pain relief and satisfaction and had a similar low incidence of adverse effects and no respiratory depression. The researchers described oral PCA as “an attractive, simple, inexpensive, and patient-convenient mode of opioid administration for patients who are permitted to drink oral fluids after surgery” (p. 1053).
A Canadian hospital reported success with an oral PCA program for patients after laminectomy or spinal fusion (Kastanias, Snaith, Robinson, 2006). Patients were allowed to keep a single dose of an oral short-acting opioid analgesic at the bedside in a child-resistant container. Patients were told to take the analgesic as needed as often as every 2 hours, record their pain ratings before and after taking a dose, and notify the nurse as soon as a dose was taken so the nurse could replace it with another. There were no reports of diversion or medication loss. A majority (92%) of the patients were satisfied or very satisfied with this method of pain control. Those who were not satisfied cited difficulty completing the flow sheet as the reason.
A mechanical device called the “MOD” (Medication on Demand) may be a solution to system barriers to the use of oral PCA. The MOD is the first device that allows patients access at the bedside to secured oral analgesics when needed for pain (Figure 12-2, A). The device is loaded with a cartridge containing eight doses of the selected analgesic and programmed according to prescribed parameters (dosing frequency [lockout, delay interval]). A green light on the device indicates to the patient that a dose may be accessed. The patient must enter the current pain intensity rating on a large 0 to 10 numerical rating scale and swipe a radio frequency identification wristband over a reader, both located on the face of the device, to request a dose of analgesic (see Figure 12-2, B). If enough time has elapsed since the last dose, the device will allow the patient to take another dose (Figure 12-2, C). The nurse can query the device to obtain and print the patient’s pain ratings and dosing history.
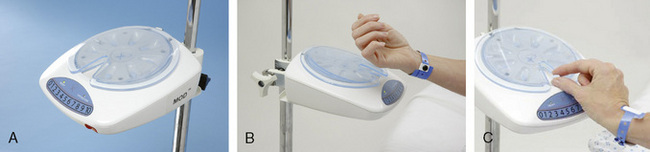
Figure 12-2 A, The MOD (medication on demand) oral PCA device. B, Patient activation of the MOD. C, Patient taking tablet from the MOD. Courtesy Avancen MOD Corporation.
The MOD was evaluated in 20 oncology patients who were anticipated to require PRN analgesics for at least 48 hours (Rosati, Gallagher, Shook, et al., 2007). Eligibility criteria included age 21 years or older, no history of drug abuse, ability to understand and use the oral PCA device, and agreement to maintain security of the device. Physicians prescribed one from a choice of oral opioid analgesics (i.e., hydrocodone/acetaminophen, hydromorphone, morphine, oxycodone, or propoxyphene) and the frequency of PRN administration. The device was loaded with a pharmacy-prepared cartridge containing eight analgesic doses and programmed with the prescribed lockout interval. The device also allowed nurses to override the lockout time to make an immediate dose available if needed. All of the patients (100%) in the study preferred using the device to calling a nurse for each PRN dose and said they would choose to use the device again if rehospitalization was necessary. There were no reports of diversion. Nurses were also surveyed, and 90% stated the device was reliable and easy to query, 88% said it was easy to program, and 98% thought the patient’s pain was better controlled than with the traditional nurse-administered method. Although the pharmacy staff found loading of the device simple, most did not think it saved pharmacy time compared with traditional delivery of medications. The use of commercially-available dose cartridges and refinement of dispensing procedures may help to ease the workload for the pharmacy staff.
Prior to the use of oral PCA, hospitals must establish guidelines for safe use. Box 12-5 provides a framework for the development of an inpatient oral PCA policy and procedure. Form 12-1 provides an example of a pain diary that patients can complete if paper documentation is required.
Conclusion
This chapter has presented some of the basic concepts and principles of pain management and provided suggestions for the appropriate use of PRN and ATC dosing, PCA, and AACA. A working knowledge of the many strategies available for managing pain allows clinicians to individualize the pain treatment plan to meet the patient’s unique needs and capabilities.
