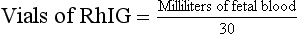Erythropoietin (EPO)
Indications
Erythropoietin (EPO) is used to assist in differentiating the cause of anemia or polycythemia.
Test Explanation
Erythropoietin is a glycoprotein hormone produced in the peritubular interstitial cells located in the inner cortex of the kidney. In response to decreased oxygen sensed by these renal cells and perhaps the carotid body cells, the production of EPO is increased. EPO stimulates the bone marrow to increase red blood cell (RBC) production. This improves oxygenation in the kidney, and the stimulus for EPO is reduced. This feedback mechanism is very sensitive to minimal persistent changes in oxygen levels. In patients with normal renal function, EPO levels are inversely proportional to the hemoglobin concentration.
As a hormone, EPO is often administered to patients who experience anemia as a result of chemotherapy. Occasionally, athletes abuse this hormone to improve oxygen carrying capacity and thereby improve performance.
EPO testing is performed to assist in the differential diagnosis of patients with anemia and polycythemia. EPO is elevated in patients who have a low hemoglobin because of failure of marrow production or RBC destruction (iron-deficiency or hemolytic anemia, respectively). The anemia results in reduced oxygen in the kidneys and EPO production is stimulated. However, although patients with renal diseases (or bilateral nephrectomy) are anemic, they do not have elevated EPO levels. The peritubular renal cells are damaged by renal disease. EPO levels fall and these patients experience anemia.
Patients who have polycythemia as an appropriate response to hypoxemia have elevated EPO levels. Yet patients who have malignant polycythemia vera may have reduced EPO levels. Some renal cell or adrenal carcinomas can produce elevated EPO levels that are unresponsive to the normal feedback inhibitory mechanisms.
Estrogen Fraction (Estriol Excretion, Estradiol, Estrone)
Indications
Estrogen measurements are used to evaluate sexual maturity, menstrual problems, and fertility problems in females. This test is also used in the evaluation of males with gynecomastia or feminization syndromes. In pregnant women it is used to indicate fetal-placental health. In patients with estrogen-producing tumors it can be used as a tumor marker.
Test Explanation
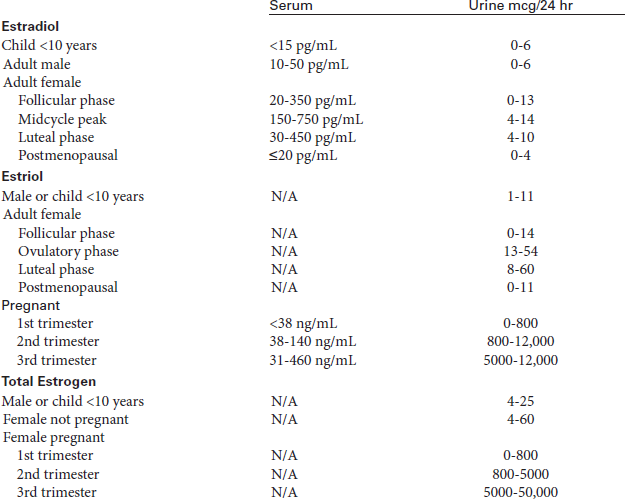
There are three major estrogens. E2 (estradiol) is predominantly produced in the ovary. In females there is a feedback mechanism for the secretion of E2. Low levels of E2 stimulate the hypothalamus to produce gonadotropin-releasing factors. These hormone factors stimulate the pituitary to produce follicle-stimulating hormone (FSH) and luteinizing hormone (LH). These two hormones stimulate the ovary to produce E2, which peaks during the ovulatory phase of the menstrual cycle. This hormone is measured most often to evaluate menstrual and fertility problems, menopausal status, sexual maturity, gynecomastia, and feminization syndromes or as a tumor marker for patients with certain ovarian tumors.
E1 (estrone) is also secreted by the ovary, but most is converted from androstenedione in peripheral tissues. Estrone is a more potent estrogen than estriol but is less potent than estradiol. Estrone is the major circulating estrogen after menopause.
E3 (estriol) is the major estrogen in the pregnant female. Serial urine and blood studies of estriol excretion provide an objective assessment of placental function and fetal normality in high-risk pregnancies. Excretion of estriol increases around the eighth week of gestation and continues to rise until shortly before delivery. Estriol is produced in the placenta from estrogen precursors, which are made by the fetal adrenal gland and liver. The measurement of excreted estriol is an important index of fetal well-being. Rising values indicate an adequately functioning fetoplacental unit. Decreasing values suggest fetoplacental deterioration (failing pregnancy, dysmaturity, preeclampsia/eclampsia, complicated diabetes mellitus, anencephaly, fetal death) and require prompt reassessment of the pregnancy. If the estriol levels fall, early delivery of the fetus may be indicated.
Serial studies usually begin at approximately 28 to 30 weeks of gestation and are then repeated weekly. The frequency of these estriol determinations can be increased as needed to evaluate a high-risk pregnancy. Collection may be done daily. Although the first collection is the baseline value, all collection results are compared with previous ones, because decreasing values suggest fetal deterioration. Some physicians use an average of three previous values as a control value.
Estriol excretion studies can be done using 24-hour urine tests or blood studies. Because urinary creatinine excretion is relatively constant, creatinine clearance is often simultaneously tested to assess the adequacy of the 24-hour urine collection for estriol. A serially increasing estriol/creatinine ratio is a favorable sign in pregnancy. Plasma estriol determinations also can be used to evaluate the fetoplacental unit. These studies can conveniently and rapidly assess the quantity of free estriol in the plasma by radioimmunoassay (RIA). The plasma collected by venipuncture is an accurate reflection of the current status of the placenta and fetus. The advantage of the plasma estriol determination is that it is more easily obtained than a 24-hour urine specimen and is less affected by medications. All the estrogens can be measured by gas chromatography, but immunoassay techniques are more accurate and less affected by drugs or birth control pills.
Unfortunately only severe placental distress will decrease urinary estriol sufficiently to reliably predict fetoplacental stress. Furthermore, plasma and urinary estriol levels are normally associated with a significant daily variation, which may confuse serial results. Maternal illnesses, such as hypertension, preeclampsia, anemia, and impaired renal function, can also factitiously decrease urinary estriol levels. Because these problems create a high number of false-positive and false-negative findings, most clinicians now use nonstress fetal monitoring (p. 569) to indicate fetal-placental health.
Interfering Factors
• Recent administration of radioisotopes may alter test results if RIA methods are used.
• Glycosuria and urinary tract infections (UTIs) can increase urine estriol levels.
![]() Drugs that may increase levels include adrenocorticosteroids, ampicillin, estrogen-containing drugs, phenothiazines, and tetracyclines.
Drugs that may increase levels include adrenocorticosteroids, ampicillin, estrogen-containing drugs, phenothiazines, and tetracyclines.
Procedure and Patient Care
During
24-Hour Urine
![]() Instruct the patient to begin the 24-hour urine collection after voiding. Discard the initial specimen and start the 24-hour collection at that point.
Instruct the patient to begin the 24-hour urine collection after voiding. Discard the initial specimen and start the 24-hour collection at that point.
![]() Collect all urine passed during the next 24 hours. Make sure the patient knows where to store the urine container.
Collect all urine passed during the next 24 hours. Make sure the patient knows where to store the urine container.
• Keep the specimen on ice or refrigerated during the 24-hour collection period.
• Indicate the starting time on the urine container and laboratory slip.
• Post the hours for the urine collection in a prominent place to prevent accidentally discarding the specimen.
![]() Instruct the patient to void before defecating so that the urine is not contaminated by feces.
Instruct the patient to void before defecating so that the urine is not contaminated by feces.
![]() Remind the patient not to put toilet paper in the collection container.
Remind the patient not to put toilet paper in the collection container.
![]() Encourage the patient to drink fluids during the 24 hours.
Encourage the patient to drink fluids during the 24 hours.
• Collect the last specimen as close as possible to the end of the 24-hour period. Add this urine to the collection.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Feminization syndromes: Estrogens are increased in these syndromes for a variety of reasons. The male begins to develop female secondary sex characteristics.
Precocious puberty: Children who develop secondary sexual characteristics at an abnormally early age often have a genetic defect in adrenal cortisol metabolism. As a result, large amounts of sex steroid precursors accumulate and are converted to estrogens by the ovary. This causes precocious secondary sexual changes.
Gonadal tumors (e.g., granulosa thecal cell tumors) secrete estrogens. The higher the levels, the greater the tumor burden. In these instances estrogen can act as a tumor marker that can be used to monitor the disease.
Normal pregnancy: E3 is the main estrogen elevated during pregnancy, although E1 and E2 are also elevated. Multiple pregnancies are associated with particularly high levels of E3.
Estrogens are catabolized, in part, by the liver. If liver function is deficient, estrogens and their precursors accumulate. Adult feminization can result.
Hyperthyroidism: An estrogen-related increase in the production of thyroid-binding globulin produces an elevation of serum total T4.
 Decreased Levels
Decreased Levels
A failing pregnancy is associated with reduced placental production of E3: Any disease that causes fetal distress, dysmaturity, Rh isoimmunization, preeclampsia/eclampsia, anencephaly, or fetal death will be associated with reduced E3 levels.
Turner syndrome: This syndrome is seen in females who are missing one X chromosome. They have gonadal dysgenesis to varying degrees.
Primary and secondary hypogonadism,
Diseases affecting the organs involved in the synthesis of sex hormones anywhere in the hypothalamus/pituitary/gonadal axis will be associated with reduced estrogen levels.
Menopause: With normal age-related ovarian failure, estrogen (especially E1) levels decline.
Anorexia nervosa: Reduction in fat intake reduces sterol precursors available for estrogen synthesis.
Ethanol (Ethyl Alcohol, Blood Alcohol, Blood EtOH)
Test Explanation
Ethanol depresses the central nervous system and may cause reduced alertness, coma, and death. Proper collection, handling, and storage of blood alcohol are important for medicolegal cases involving sobriety testing. Legal testing must be done by specially trained people and must have a strict chain-of-custody (a paper trail that records sample movement and handling).
Samples tested for legal purposes may include blood, breath, urine, and/or saliva. The blood test is the specimen of choice. Blood is taken from a peripheral vein in living patients and from the aorta in cadavers. Results are given as mg/dL, g/100 mL or as a percentage. Each represents the same amount of alcohol. Blood alcohol concentrations (BACs) >80 mg/dL (0.08%) may cause flushing, slowing of reflexes, and impaired visual activity. Depression of the CNS occurs with BACs >0.1%, and fatalities are reported with levels >0.4%. BACs >0.1% can cause hypotension, although this is rare. This is especially important to recognize in the trauma patient in shock. Persons with BACs <0.05% are not considered under the influence of alcohol. Levels >0.05% to 0.10% are considered to be illegal and definite evidence of intoxication in most states. The American Medical Association says that a person can become impaired when the blood alcohol level hits 0.05%.
For legal purposes, when outside of a laboratory or hospital, taking a blood sample for later analysis in the laboratory is not practical or efficient. Breath testing is the most common test performed on automobile drivers. It uses the tail end sample of breath from deep in the lungs and uses a conversion factor to estimate the amount of alcohol in the blood. Blood alcohol testing may be ordered to confirm or refute findings, and/or ordered as an alternative to breath testing. Alcohol that a person drinks shows up in the breath because it gets absorbed from the intestinal tract and into the bloodstream. The alcohol is not metabolized on first pass through the liver. As the blood goes through the lungs, some of the volatile alcohol moves across the alveolar membranes and is exhaled. Conversion tables are available to calculate blood levels based on alcohol levels identified in the various nonblood specimens.
Urine testing may also be performed as an alternative to blood. Usually a patient collects and discards a urine sample and then collects a second sample 20 to 30 minutes later. Saliva alcohol testing is not as widely used, but may be used as an alternate screening test. Alcohol stays in the saliva for 6 to 12 hours. Finally hair testing is used but represents a more chronic use of alcohol.
Interfering Factors
• Elevated blood ketones (as with diabetic ketoacidosis) can cause false elevation of blood and breath test results.
• Bacteria in the urine of diabetic patients with glucosuria can metabolize the glucose to alcohol.
• Alcohols other than ethanol (e.g., isopropyl [rubbing alcohol] or methanol [grain alcohol]) will also cause testing to be positive.
![]() The use of alcohol-based mouthwash or cough syrup may cause false-positives on a breath test.
The use of alcohol-based mouthwash or cough syrup may cause false-positives on a breath test.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
• Follow the institution's protocol if the specimen will be used for legal purposes.
![]() Patients should be advised of their legal rights. Sometimes this is best done by a law enforcement officer. The alcohol level may be used as evidence for later court proceedings.
Patients should be advised of their legal rights. Sometimes this is best done by a law enforcement officer. The alcohol level may be used as evidence for later court proceedings.
During
• Use a povidone-iodine wipe or peroxide instead of an alcohol wipe for cleansing the venipuncture site.
• Collect a venous blood sample in a gray-top or red-top tube according to the agency's protocol.
• If a gastric or urine specimen is indicated, approximately 20 to 50 mL of fluid is necessary.
• Breath samples for analysis are taken at the end of expiration after a deep inspiration.
After
• Apply pressure or a pressure dressing to the venipuncture site.
• Assess the venipuncture site for bleeding.
• Follow the agency's protocol regarding specimen collection.
• The exact time of specimen collection should be indicated. Also, signatures of the collector and a witness may be needed in some instances for legal evidence.
Factor V-Leiden (FVL, Mutation Analysis)
Test Explanation
Factor V is an important factor in reaction 4 (common pathway) of normal hemostasis (see p. 163). The term factor V-Leiden refers to an inherited abnormal form of factor V in which there is a specific glutamine-to-arginine substitution at nucleotide 1691 in the gene for factor V. That genetic mutation causes a single amino acid replacement (Arg506 Gln) at one of three cleavage sites in the factor V molecule. The endogenous anticoagulant, protein C (see p. 432) normally is able to break down factor V at one of these cleavage sites. However, protein C cannot inactivate this same cleavage site on factor V-Leiden. FVL is therefore inactivated at a rate approximately ten times slower than normal factor V and persists longer in the circulation. This results in increased thrombin generation and a mild hypercoagulable state reflected by elevated levels of prothrombin fragment F1+2 and other activated coagulation markers.
Individuals heterozygous for the factor V-Leiden mutation have a slightly increased risk for venous thrombosis. Homozygous individuals have a much greater thrombotic risk (e.g., deep vein thrombosis [DVT], arterial thrombosis, or pulmonary embolism).
Individuals who are candidates for FVL testing include patients who have:
• Experienced a thrombotic event without any predisposing factors
• A strong family history of thrombotic events
• Experienced a thrombotic event before 30 years of age
• Experienced DVT during pregnancy or while taking birth control pills
• Had venous thrombosis at unusual sites (e.g., cerebral, mesenteric, portal, or hepatic veins)
Factor V-Leiden is the most common hereditary blood coagulation disorder in the United States. It is present in 5% of the Caucasian population and 1.2% of the African-American population. Only about 10% of patients who have FVL will experience a thrombotic event.
Testing for FVL is sometimes preceded by a screening coagulation test called the activated protein C (APC) resistance test. This is a test to identify resistance of factor V to activated protein C (APC). Protein C (see p. 432), in the presence of its cofactors thrombomodulin and thrombin, is enzymatically cleaved to its active form, activated protein C. APC is an important natural anticoagulant (to balance coagulation) that functions by inactivating the critical coagulation factors fVa and fVIIIa. In thrombotic patients (many of whom have FVL), those factors will be resistant to deactivation when exposed to APC. Pregnancy and reactive causes of increased factor VIII can also be associated with APC resistance.
APC resistance testing is performed on citrated plasma (blue tops) from thrombotic patients with a normal activated partial thromboplastin time (aPTT) before anticoagulant therapy. Briefly, a standard aPTT test (see p. 383) is performed in the absence and then in the presence of commercially available activated protein C. In the normal response, the aPTT is prolonged in the presence of APC due to the anticoagulant action of this protein. An abnormality is detected by failure to prolong the aPTT caused by “resistance to APC.” The results are reported as a ratio of the APC-aPTT/aPTT with a normal result greater than 2.0. Patients with the lowest APC ratios appear to be homozygous for the abnormal factor V molecule while heterozygotes appear to have ratios intermediate between the normal range and homozygote levels.
If APC resistance is identified, the patient then may choose to undergo mutation testing by DNA analysis of the F5 gene, which encodes the factor V protein. Polymerase chain reaction/restriction enzyme digestion/gel electrophoresis or fluorescent direct mutation testing methods are commonly used. This testing should be accompanied by professional genetic counseling for the patient and family members.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
• If the patient is receiving heparin by intermittent injection, plan to draw the blood specimen for the aPTT 30 minutes to 1 hour before the next dose of heparin.
• If the patient is having FVL mutational analysis, anticoagulants will not interfere with testing.
Test Results and Clinical Significance
APC resistance: These patients most probably have FVL, but other forms of thrombophilia (predisposition to thrombotic events) can cause APC resistance.
Homozygous: These patients have received a FVL gene from each parent and have a thrombotic risk that exceeds 80 times that of the normal population.
Heterozygous: These patients have received a FVL gene from one parent and normal factor V from the other. These individuals have a thrombotic risk for about 10 times that of the normal population.
Febrile Antibodies (Febrile Agglutinins)
Test Explanation
Febrile antibodies are used to support the diagnosis and monitoring of infectious diseases such as salmonellosis, rickettsial diseases, brucellosis, and tularemia. Neoplastic diseases, such as leukemias and lymphomas, are also associated with febrile agglutinins. Appropriate antibiotic treatment of the infectious agent is associated with a drop in the titer/activity of febrile antibodies. Screening testing (EIA) is first performed and reported in dilution. If positive (>1:80), disease-specific antibodies are then quantified by immunofluorescence assay. This test is nonspecific and insensitive. More specific testing for these infective agents provides more sensitive and specific laboratory testing.
Salmonella and acute brucellosis along with the spotted group of rickettsial agents (R. rickettsii causing Rocky Mountain spotted fever; R. akari causing Rickettsialpox; and R. conorii causing Boutonneuse fever) can be identified. The typhus fever group of rickettsial agents (R. typhi causing endemic or murine typhus; R. prowazekii causing epidemic typhus; and Brill-Zinsser disease caused by reactivation of latent R. prowazekii) can also be quantified.
IgM reactivity usually indicates an acute infection. However, IgM reactivity, in the absence of IgG reactivity, may represent a false-positive reaction. Recent infection should be confirmed by demonstrating either IgG seroconversion or a fourfold or greater increase in IgG titer when acute and convalescent sera are tested in parallel.
Temperature regulation is important for the performance of these tests. Under no circumstances should the febrile agglutinin be heated before delivery to the laboratory.
Ferritin
Test Explanation
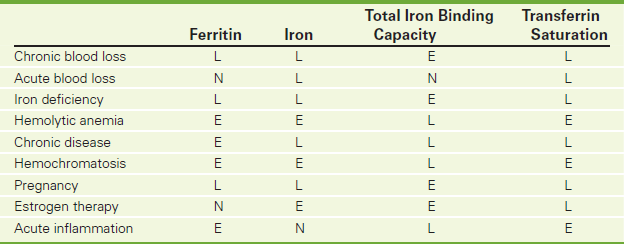
The serum ferritin study is a good indicator of available iron stores in the body. Ferritin, the major iron-storage protein, is normally present in the serum in concentrations directly related to iron storage. In normal patients, 1 ng/mL of serum ferritin corresponds to approximately 8 mg of stored iron. Ferritin levels rise persistently in males and postmenopausal females. In premenopausal females, levels stay about the same. Decreases in ferritin levels indicate a decrease in iron storage associated with iron-deficiency anemia. A ferritin level of below 10 mg/100 mL is diagnostic of iron-deficiency anemia. A decrease in serum ferritin level often precedes other signs of iron deficiency, such as decreased iron levels or changes in red blood cell (RBC) size, color, and number. Only when protein depletion is severe can ferritin be decreased by malnutrition. Increased levels are a sign of iron excess, as seen in hemochromatosis, hemosiderosis, iron poisoning, or recent blood transfusions. Increases in ferritin are also noted in patients with megaloblastic anemia, hemolytic anemia, and chronic hepatitis. Furthermore, ferritin is factitiously elevated in patients with chronic disease states such as neoplasm, alcoholism, uremia, collagen diseases, or chronic liver diseases. The ferritin test is also used in patients with chronic renal failure to monitor iron stores.
A limitation of this study is that ferritin also can act as an acute-phase reactant protein and may be elevated in conditions not reflecting iron stores (e.g., acute inflammatory diseases, infections, metastatic cancer, lymphomas). Elevations in ferritin occur 1 to 2 days after onset of the acute illness and the level peaks at 3 to 5 days. If iron deficiency coexists in patients with these diseases, it may not be recognized because the levels of ferritin would be factitiously elevated by the concurrent disease.
When combined with the serum iron level and total iron-binding capacity (TIBC), this test is useful in differentiating and classifying anemias. For example, in patients with iron deficiency anemia the ferritin, iron, and transferrin saturation levels are low, whereas the TIBC and transferrin levels are high (Table 2-24). Ferritin is measured by either radioimmunoassay or enzyme immunoassay.
Interfering Factors
• Recent transfusions or recent ingestion of a meal containing a high iron content (red meats) may cause elevated ferritin levels. The iron that is ingested stimulates ferritin production to store the increased serum iron.
• Recent administration of a radionuclide can cause abnormal levels if testing is performed by radioimmunoassay.
• Hemolytic diseases may be associated with an artificially high iron content. Iron is freed from the hemoglobin that is released from the hemolyzed RBCs. Ferritin synthesis is increased to store the increased serum iron.
• Acute and chronic inflammatory conditions and Gaucher disease can falsely increase ferritin levels.
• Disorders of excessive iron storage (e.g., hemochromatosis, hemosiderosis) are associated with high ferritin levels. Ferritin synthesis is increased to store the increased serum iron.
• Iron-deficient menstruating women may have decreased ferritin levels, because their iron stores are generally low as a result of monthly menses.
![]() Iron preparations may increase ferritin levels. Ferritin synthesis is increased to store the increased serum iron.
Iron preparations may increase ferritin levels. Ferritin synthesis is increased to store the increased serum iron.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
RBCs in anemias lyse and release iron into the bloodstream. Ferritin production is stimulated to store the excess free iron.
Alcoholic/inflammatory hepatocellular disease,
Because ferritin is an acute-phase reactant protein, its production is increased with acute diseases.
Chronic illnesses such as leukemias, cirrhosis, chronic hepatitis, or collagen-vascular diseases: The pathophysiology of this observation is not known.
 Decreased Levels
Decreased Levels
Iron-deficiency anemia: When iron stores are decreased, less ferritin is required. Levels diminish.
Severe protein deficiency: Ferritin is a protein. In severely depleted persons, ferritin synthesis is reduced.
Hemodialysis: Iron stores can be reduced by dialysis. Decreased iron stores require less ferritin. Levels diminish.
Related Tests
Iron Level (p. 322). This is a direct measurement of bound iron in the blood.
Total Iron-Binding Capacity (p. 322). TIBC is a measurement of all proteins available for binding mobile iron. Transferrin represents the largest quantity of iron-binding proteins.
Transferrin (p. 322). Transferrin represents the largest quantity of iron-binding proteins.
Transferrin Receptor Assay (p. 502). This is used to differentiate iron deficiency from other anemias.
Fetal Hemoglobin Testing (Kleihauer-Betke Test)
Indications
This test is performed on pregnant women to determine the presence of and quantify the amount of fetal-maternal hemorrhage.
Test Explanation
Fetal hemoglobin may be present in the mother's blood because of fetal-maternal hemorrhage (FMH), which causes leakage of fetal cells into the maternal circulation. When large volumes of fetal blood are lost in this way, serious and potentially fatal neonatal outcomes can result. Massive FMH may be the cause of around 1 in every 50 stillbirths. No historical or clinical features allow antecedent identification of those in whom FMH may be the cause of an intrauterine death. Therefore a large proportion of patients with FMH will continue to remain undetected.
Leakage of fetal RBCs can begin anytime after the mid-first trimester. It presumably results from a breach in the integrity of the placental circulation. As pregnancy continues, more and more women will show evidence of fetal RBCs in their circulation so that by term about 50% will have detectable fetal cells. Most of these, however, are the result of very small leaks. The total fetal blood volume lost in this way is 2 mL or less in 96% to 98% of pregnancies. Small leaks are not implicated in intrauterine death.
Risk factors correlated with the increasing risk for massive FMH include maternal trauma, placental abruption, placental tumors, third trimester amniocentesis, fetal hydrops, pale fetal organs, antecedent sinusoidal fetal heart tracing, and twinning. Having one or more of these features should be an indication for fetal hemoglobin testing.
The standard method of detecting FMH is the Kleihauer-Betke test. This takes advantage of the differential resistance of fetal hemoglobin to acid. A standard blood smear is prepared from mother's blood. An acid bath is then used that removes all adult hemoglobin but does not remove fetal hemoglobin. Subsequent staining makes fetal cells (containing fetal hemoglobin) rose pink, although the mother's cells are only seen as “ghosts.” A large number of cells (e.g., 5000) are counted under the microscope and the ratio of fetal to maternal cells is generated.
The flow cytometric method for HbF determination offers several advantages over the traditional Kleihauer-Betke method. This more objective method has been shown to improve sensitivity, precision, and linearity over traditional methods.
FMH becomes of even greater significance when the mother is Rh negative as this is the mechanism through which Rh sensitization could develop if the fetus has paternal Rh-positive blood cells. If this is known to exist, RhoGAM (RhIG, Rh immunoglobulin) antibodies directed to Rh-positive fetal cells are given to the pregnant mother (at about 28 weeks of pregnancy, and within 72 hours after a birth, miscarriage, abortion, or amniocentesis). RhoGAM is often administered if any invasive procedure is performed on the Rh-negative mother where she may be exposed to the Rh-positive fetal blood. The RhoGAM antibodies kill the fetal RBCs in the maternal bloodstream before the mother has an opportunity to develop any antibodies to fetal Rh-positive RBCs. This precludes more aggressive anti-fetal RBC occurrences in the near or remote future. By determination of the amount and volume of fetal blood loss, a dose of Rhogam can be calculated using the following formula:
This test is often performed on women who have delivered a stillborn baby to see if FMH was a potential cause of fetal death.
Test Explanation and Clinical Significance
 Increased Levels
Increased Levels
Feto-maternal hemorrhage (FMH): Fetal, placental, or maternal pathology can result in leakage of fetal cells into the maternal bloodstream.
Hereditary persistence of fetal hemoglobin: With any hemoglobinopathy, fetal hemoglobin is often continually made in RBCs as a compensatory mechanism to insure good tissue oxygenation. This will be identified through this test and may give the false sense of FMH.
Intrachorionic thrombi: Placental thrombosis causes a breakdown in maternal/fetal membrane barrier. Fetal cells can cross over into the maternal circulation.
Fetal Scalp Blood pH
Test Explanation
Measurement of fetal scalp blood pH provides valuable information on fetal acid-base status. This test is useful for diagnosing fetal distress.
Although the oxygen partial pressure (PO2), carbon dioxide partial pressure (PCO2), and bicarbonate ion (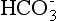 ) concentration can be measured with the fetal scalp blood sample, the pH is the most useful clinically. The pH normally ranges from 7.25 to 7.35 during labor; a mild decline within the normal range is noted with contractions and as labor progresses.
) concentration can be measured with the fetal scalp blood sample, the pH is the most useful clinically. The pH normally ranges from 7.25 to 7.35 during labor; a mild decline within the normal range is noted with contractions and as labor progresses.
Fetal hypoxia causes anaerobic glycolysis, resulting in excess production of lactic acid. This causes an increase in hydrogen ion concentration (acidosis) and a decrease in pH. Acidosis reflects the effect of hypoxia on cellular metabolism. A high correlation exists between low pH levels and low Apgar scores.
Fetal oxygen saturation can be measured by oximetry, see p. 1114.
Procedure and Patient Care
During
• Note the following procedural steps:
1. Amnioscopy is performed with the mother in the lithotomy position.
2. The cervix is dilated, and the endoscope (amnioscope) is introduced into the cervical canal.
3. The fetal scalp is cleansed with an antiseptic and dried with a sterile cotton ball.
4. A small amount of petroleum jelly is applied to the fetal scalp to cause droplets of fetal blood to bead.
5. After the skin on the scalp is pierced with a small metal blade, beaded droplets of blood are collected in long, heparinized capillary tubes.
6. The tube is sealed with wax and placed on ice to retard cellular respiration, which can alter the pH.
7. The physician performing the procedure applies firm pressure to the puncture site to retard bleeding.
• Note that this study is performed by a physician in approximately 10 to 15 minutes.
![]() Tell the patient that she may be uncomfortable during the cervical dilation.
Tell the patient that she may be uncomfortable during the cervical dilation.
Related Test
Arterial Blood Gases (p. 109). This test provides valuable information in assessing and managing a patient's respiratory (ventilation) and metabolic (renal) acid-base and electrolyte homeostasis. It is also used to assess adequacy of oxygenation.
Fetal Oxygen Saturation (p. 1114). This is useful in monitoring fetal well-being during labor and delivery.
Fibrinogen (Factor I, Quantitative Fibrinogen)
Indications
Fibrinogen is used primarily to aid in the diagnosis of suspected bleeding disorders. This testing is used to detect increased or decreased fibrinogen (factor I) concentration of acquired or congenital origin. It is also used for monitoring severity and treatment of disseminated intravascular coagulation and fibrinolysis.
Test Explanation
Fibrinogen is essential to the blood-clotting mechanism. It is part of the “common pathway” (fourth reaction) in the coagulation system. Fibrinogen is converted to fibrin by the action of thrombin during the coagulation process. Fibrinogen, which is produced by the liver, is also an acute-phase reactant protein. It rises sharply during tissue inflammation or tissue necrosis.
High levels of fibrinogen have been associated with an increased risk for coronary heart disease (CHD), stroke, myocardial infarction (MI), and peripheral arterial disease. Reduced levels can be seen in patients with liver disease, malnourished states, and consumptive coagulopathies (e.g., disseminated intravascular coagulation [DIC]). Large-volume blood transfusions are also associated with low levels, because banked blood does not contain fibrinogen. Reduced levels of fibrinogen will cause prolonged prothrombin (PT) and partial thromboplastin (PTT) times. Electromagnetic mechanical clot or viscosity detection is the most commonly performed laboratory method used in quantification.
Interfering Factors
• Blood transfusions within the past month may affect test results.
• Diets rich in omega-3 and omega-6 fatty acids reduce fibrinogen levels.
![]() Drugs that may cause increased levels include estrogens and oral contraceptives.
Drugs that may cause increased levels include estrogens and oral contraceptives.
![]() Drugs that may cause decreased levels include anabolic steroids, androgens, asparaginase, phenobarbital, streptokinase, urokinase, and valproic acid.
Drugs that may cause decreased levels include anabolic steroids, androgens, asparaginase, phenobarbital, streptokinase, urokinase, and valproic acid.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
 Decreased Levels
Decreased Levels
Liver disease (hepatitis, cirrhosis): Fibrinogen is not made in adequate volume.
Consumptive coagulopathy (DIC),
Congenital afibrinogenemia: A genetic defect precludes the synthesis of fibrinogen.
Large-volume blood transfusion: Fibrinogen does not exist in normal levels in banked blood. The more that is transfused, the more the native fibrinogen is diluted.
Related Tests
Prothrombin Time (PT) (p. 434). The PT is used to evaluate the adequacy of the extrinsic system and common pathway in the clotting mechanism.
Partial Thromboplastin Time (PTT) (p. 383). This test is used to evaluate the intrinsic system and the common pathway of clot formation. It is most commonly used to monitor heparin therapy.
Coagulating Factor Concentration (p. 163). This is a quantitative measurement of specific coagulation factors.
Thrombosis Indicators (p. 482). These tests are most commonly used to support the diagnosis of DIC.
Folic Acid (Folate)
Indications
This test quantifies the folate level in the blood. It is used in patients who have megaloblastic anemia. It is also used to assess nutritional status, especially in alcoholics.
Test Explanation
Folic acid, one of the B vitamins, is necessary for normal function of red blood cells (RBCs) and white blood cells (WBCs). It is needed for the adequate synthesis of certain purines and pyrimidines, which are precursors of deoxyribonucleic acid (DNA). It is also used in the synthesis of several amino acids. Vitamin B12 is necessary for conversion of inactive 5-methyltetrahydrofolate to the active tetrahydrofolate, the active form of folate. As with vitamin B12, the folate level depends on adequate dietary ingestion and normal intestinal absorption of this vitamin.
The finding of a low serum folate means that the patient's recent diet has been subnormal in folate content and/or that recent absorption of folate has been subnormal. In time, folate levels will also drop in the tissues. Tissue folate is best tested by determining the content of folate in RBCs. A low RBC folate can mean either that there is tissue folate depletion due to folate deficiency requiring folate therapy, or alternatively, that the patient has primary vitamin B12 (see p. 518) deficiency blocking the ability of cells to take up folate. In the latter case, the proper therapy would be with vitamin B12 rather than with folic acid. For these reasons it is advisable to determine RBC folate in addition to serum folate.
Folic acid blood levels are performed to assess folate availability in pregnancy, to evaluate hemolytic disorders, and to detect anemia caused by folic acid deficiency (in which the RBCs are abnormally large, causing a megaloblastic anemia). These RBCs have a shortened life span and impaired oxygen-carrying capacity. If low, RBC folate is measured.
The main causes of folic acid deficiency include dietary deficiency (usually in the alcoholic patient), malabsorption syndrome, pregnancy, and certain anticonvulsant drugs. Decreased folic acid levels are seen in patients with folic acid deficiency anemia (megaloblastic anemia), hemolytic anemia, malnutrition, malabsorption syndrome, malignancy, liver disease, sprue, and celiac disease. Some drugs (e.g., anticonvulsants, antimalarials, alcohol, aminopterin, methotrexate) are folic acid antagonists and interfere with nucleic acid synthesis.
Elevated levels of folic acid may be seen in patients with pernicious anemia. Because there is not an adequate amount of vitamin B12 in these patients to metabolize folic acid, levels of folate rise in pernicious anemia. The folic acid test should be done in conjunction with tests for vitamin B12 levels.
The folate test is often part of the workup in alcoholic patients to assess nutritional status. Folate must be depleted for at least 5 months before megaloblastic anemia occurs.
Radioimmunoassay (RIA), enzyme immunoassay (EIA), quantitative chemiluminescent immunoassay, or automated competitive-binding receptor assay techniques are commonly used methods of folic acid measurement.
Interfering Factors
• A folate-deficient patient who has received a blood transfusion may have a falsely normal result.
• Because RIA is the method of choice for folic acid determination, radionuclide administration should be avoided for at least 24 hours.
![]() Drugs that may cause decreased folic acid levels include alcohol, aminopterin, aminosalicylic acid, ampicillin, antimalarials, chloramphenicol, erythromycin, estrogens, methotrexate, oral contraceptives, penicillin derivatives, phenobarbital, phenytoin, and tetracyclines.
Drugs that may cause decreased folic acid levels include alcohol, aminopterin, aminosalicylic acid, ampicillin, antimalarials, chloramphenicol, erythromycin, estrogens, methotrexate, oral contraceptives, penicillin derivatives, phenobarbital, phenytoin, and tetracyclines.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Pernicious anemia: When there is an inadequate amount of vitamin B12 to metabolize folic acid, levels of folate rise.
Vegetarianism: Increased ingestion of folate-containing vegetables can lead to increased levels of folic acid.
Recent massive blood transfusion: Folate in the hemolyzed RBCs of banked blood can falsely raise serum folate levels.
 Decreased Levels
Decreased Levels
Malnutrition: Inadequate intake of folic acid is the most common cause of folate deficiency. This is most common in alcoholics. Alcohol also reduces folic acid absorption.
Malabsorption syndrome (e.g., sprue, celiac disease): Folic acid, like vitamin B12, is absorbed in the small intestine. In malabsorption, folic acid is not absorbed. Serum and tissue levels decline.
Pregnancy: Folic acid deficiency in pregnancy probably results from a combination of inadequate intake and increased demand placed by the fetus on the maternal source of folic acid.
Galectin-3 (GAL-3)
Test Explanation
Heart failure progresses primarily by dilatation of the ventricular cardiac chamber through remodeling in fibrosis as a response to cardiac injury and/or overload. Galectin-3 (GAL-3) is a biomarker that appears to be actively involved in both the inflammatory and fibrotic pathways involved in remodeling. GAL-3 is a carbohydrate-binding lectin whose expression is associated with inflammatory cells, including macrophages, neutrophils, and mast cells. GAL-3 has been linked to cardiovascular physiologic processes, including myofibroblast proliferation, tissue repair, and cardiac remodeling in the setting of heart failure. Concentrations of GAL-3 have been used to predict adverse remodeling after a variety of cardiac insults.
Elevated GAL-3 results indicate an increased risk for adverse outcomes. Elevated levels are associated with increased risk of mortality and prolongation of the symptoms associated with congestive heart failure. Unlike natriuretic peptides, such as beta natriuretic peptides (BNP) (p. 367), GAL-3 is not useful in the diagnosis of heart failure. Testing was performed by quantitative 2-site manual enzyme-linked immunosorbent assay (ELISA).
Interfering Factors
• Hemolysis increases GAL-3 levels.
• Heterophil antibodies (p. 363) increase GAL-3 levels.
Related Tests
Chest X-Ray (p. 1014). This test may demonstrate an enlarged heart, commonly associated with congestive heart failure. Pulmonary edema can also be noted.
Echocardiography (p. 877). This test is accurate in determining cardiac remodeling and enlarged cardiac ventricles.
Beta Natriuretic Peptides (p. 367). Natriuretic peptides are used to identify and stratify patients with congestive heart failure (CHF).
Gamma-Glutamyl Transpeptidase (GGTP, g-GTP, Gamma-Glutamyl Transferase [GGT])
Indications
This is a sensitive indicator of hepatobiliary disease. It is also used as an indicator of heavy and chronic alcohol use.
Test Explanation
The enzyme GGTP participates in the transfer of amino acids and peptides across the cellular membrane and possibly participates in glutathione metabolism. The highest concentrations of this enzyme are found in the liver and biliary tract. Lesser concentrations are found in the kidney, spleen, heart, intestine, brain, and prostate gland. Men may have higher GGTP levels than women because of the additional levels in the prostate. Very small amounts have been detected in endothelial cells of capillaries. This test is used to detect liver cell dysfunction, and it is highly accurate in indicating even the slightest degree of cholestasis. This is the most sensitive liver enzyme for detecting biliary obstruction, cholangitis, or cholecystitis. As with leucine aminopeptidase and 5'-nucleotidase, the elevation of GGTP generally parallels that of alkaline phosphatase; however, GGTP is more sensitive. Also, as with 5'-nucleotidase and leucine aminopeptidase, GGTP is not increased in bone diseases as is alkaline phosphatase. A normal GGTP level with an elevated alkaline phosphatase level would imply skeletal disease. An elevated GGTP and elevated alkaline phosphatase level would imply hepatobiliary disease. GGTP is also not elevated in childhood or pregnancy as alkaline phosphatase (ALP) usually is.
Another important clinical value of GGTP is that it can detect chronic alcohol ingestion. It is, therefore, very useful in the screening and evaluation of alcoholic patients. GGTP is elevated in approximately 75% of patients who chronically drink alcohol.
Why this enzyme is elevated after an acute myocardial infarction (AMI) is not clear. It may represent the associated hepatic insult (if elevation occurs in the first 7 days) or the proliferation of capillary endothelial cells in the granulation tissue that replaces the infarcted myocardium. The elevation usually occurs 1 to 2 weeks after infarction.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Liver diseases (e.g., hepatitis, cirrhosis, hepatic necrosis, hepatic tumor or metastasis, hepatotoxic drugs, cholestasis, jaundice): Liver and biliary cells contain GGTP. When injured or diseased, these cells lyse and the GGTP leaks into the bloodstream.
Myocardial infarction (MI): The pathophysiology is not clear. It may be associated with hepatic insult or the proliferation of capillary endothelial cells in the granulation tissue that replaces the infarcted myocardium.
Alcohol ingestion: The pathophysiology is not clear. It may be associated with hepatic insult.
Pancreatic diseases (e.g., pancreatitis, cancer of the pancreas): Pancreatic cells contain GGTP. When injured or diseased, these cells lyse and the GGTP leaks into the bloodstream.
Epstein-Barr virus (EBV) (infectious mononucleosis), cytomegalovirus infections, and Reye syndrome: The pathophysiology is not clear. It may be associated with subclinical hepatitis that can occur with these infections.
Related Tests
Alanine Aminotransferase (ALT) (p. 39). This liver enzyme is elevated in hepatocellular disease.
Alkaline Phosphatase (ALP) (p. 47). This test is used to detect and monitor diseases of the liver or bone.
Aspartate Aminotransferase (AST) (p. 119). This liver enzyme is elevated in hepatocellular disease.
5'-Nucleotidase (p. 376). This liver enzyme is elevated in diseases affecting the biliary tree.
Creatine Phosphokinase (CPK) (p. 186). This enzyme is similar to AST and exists predominantly in the heart and the skeletal muscle.
Lactic Dehydrogenase (LDH) (p. 329). This intracellular enzyme is used to support the diagnosis of injury or disease involving the heart, liver, red blood cells, kidneys, skeletal muscle, brain, and lungs.
Leucine Aminopeptidase (LAP) (p. 337). This enzyme is specific to the hepatobiliary system. Diseases affecting that system will cause elevation of this enzyme.
Gastrin
Indications
This test is used in the evaluation of patients with peptic ulcers to diagnose Zollinger-Ellison (ZE) syndrome or G-cell hyperplasia.
Test Explanation
Gastrin is a hormone produced by the G cells located in the distal part of the stomach (antrum). Gastrin is a potent stimulator of gastric acid. In normal gastric physiology an alkaline environment (created by food or antacids) stimulates the release of gastrin. Gastrin then stimulates the parietal cells of the stomach to secrete gastric acid. The pH environment in the stomach is thereby reduced. By negative feedback, this low-pH environment suppresses further gastrin secretion.
ZE syndrome (gastrin-producing pancreatic tumor) and G-cell hyperplasia (overfunctioning of G cells in the distal stomach) are associated with high serum gastrin levels. Patients with these tumors have aggressive peptic ulcer disease. Unlike the patient with routine peptic ulcers, the patient with ZE syndrome or G-cell hyperplasia has a high incidence of complicated and recurrent peptic ulcers. It is important to identify this latter group of patients to institute more appropriate, aggressive medical and surgical therapy. The serum gastrin level will be normal in patients with routine peptic ulcers and greatly elevated in patients with ZE syndrome or G-cell hyperplasia.
It is important to note, however, that patients who are taking antacid peptic ulcer medicines, have had peptic ulcer surgery, or have atrophic gastritis will have a high serum gastrin level (in response to alkalinity in the stomach). However, levels usually are not as high as in patients with ZE syndrome or G-cell hyperplasia.
Not all patients with ZE syndrome exhibit increased levels of serum gastrin. Some may have “top” normal gastrin levels, which makes these patients difficult to differentiate from patients with routine peptic ulcer disease. ZE syndrome or G-cell hyperplasia can be diagnosed in these “top” normal patients by gastrin stimulation tests using calcium or secretin. Patients with these diseases will have greatly increased serum gastrin levels associated with the infusion of these drugs.
Interfering Factors
• Peptic ulcer surgery creates a persistent alkaline environment, which is the strongest stimulant to gastrin.
• Ingestion of high-protein food can result in an increase in serum gastrin two to five times the normal level.
![]() Diabetic patients taking insulin may have falsely elevated levels in response to hypoglycemia.
Diabetic patients taking insulin may have falsely elevated levels in response to hypoglycemia.
![]() Drugs that may increase serum gastrin include antacids and H2-blocking agents (e.g., esomeprazole, lansoprazole, omeprazole, pantoprazole, rabeprazole). These medications create an alkaline environment, which is the strongest stimulant to gastrin.
Drugs that may increase serum gastrin include antacids and H2-blocking agents (e.g., esomeprazole, lansoprazole, omeprazole, pantoprazole, rabeprazole). These medications create an alkaline environment, which is the strongest stimulant to gastrin.
![]() Calcium or insulin can increase gastrin levels by acting as a gastrin stimulant.
Calcium or insulin can increase gastrin levels by acting as a gastrin stimulant.
![]() Other drugs that may increase gastrin levels include catecholamines and caffeine.
Other drugs that may increase gastrin levels include catecholamines and caffeine.
![]() Drugs that may decrease levels include anticholinergics and tricyclic antidepressants.
Drugs that may decrease levels include anticholinergics and tricyclic antidepressants.
Procedure and Patient Care
During
• Collect a venous blood sample in a red-top tube.
• For the calcium infusion test, administer calcium gluconate intravenously. A preinfusion serum gastrin level is then compared with specimens taken every 30 minutes for 4 hours.
• For the secretin test, administer secretin intravenously. Preinjection and postinjection serum gastrin levels are taken at 15-minute intervals for 1 hour after injection.
• Indicate on the laboratory slip any drugs that may affect test results.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
ZE syndrome: This syndrome is associated with a pancreatic islet cell gastrin-producing tumor.
G-cell hyperplasia: The G cells in the antrum of the stomach are hyperplastic and produce increased amounts of gastrin.
An achlorhydric alkaline environment exists in these illnesses. This is a strong stimulant to gastrin secretion.
Gastric carcinoma: Cancer of the stomach usually exists in an achlorhydric alkaline environment. This is a strong stimulant to gastrin secretion.
Chronic renal failure: Gastrin is metabolized by the kidney. Without adequate kidney function, gastrin levels increase.
Pyloric obstruction or gastric outlet obstruction: The stomach becomes distended. Gastric distention is a potent stimulant to gastrin production.
Retained antrum after gastric surgery: Antral tissue mistakenly left on the duodenal stump after gastric resection is constantly bathed in duodenal alkaline juices. This is a strong stimulant to gastrin secretion.
Gliadin Antibodies (Endomysial Antibodies, Tissue Transglutaminase Antibodies)
Normal Findings
| Age | Normal | |
| Gliadin IgA/IgG | 0-2 years 3 years and older |
<20 EU <25 EU |
| Endomysial IgA | All ages | Negative |
| Tissue transglutaminase IgA | All ages | <20 EU |
Indications
This test is used to diagnose celiac disease and sprue by identifying antibodies to gliadin and gluten in affected patients.
Test Explanation
Gliadin and gluten are proteins found in wheat and wheat products. Patients with celiac disease cannot tolerate ingestion of these proteins or any products containing wheat. These proteins are toxic to the mucosa of the small intestine and cause characteristic pathologic lesions. These patients experience severe intestinal malabsorption symptoms. The only treatment is for the patient to abstain from wheat and wheat-containing products.
When an affected patient ingests wheat-containing foods, gluten and gliadin build up in the intestinal mucosa. These gliadin and gluten proteins (and their metabolites) cause direct mucosal damage. Furthermore, IgA immunoglobulins (antigliadin, antiendomysial, and antitissue transglutaminase [tTG-ab]) are made, appearing in the gut mucosa and in the serum of severely affected patients. The identification of these antibodies in the blood of patients with malabsorption is helpful in supporting the diagnosis of celiac sprue or dermatitis herpetiformis. However, a definitive diagnosis of celiac disease can be made only when a patient with malabsorption is found to have the pathologic intestinal lesions characteristic of celiac disease. Also, the patient's symptoms must be improved with a gluten-free diet. Both are needed for the diagnosis. Because of the high specificity of endomyosial antibodies (EMA) for celiac disease, the test may obviate the need for multiple small bowel biopsies to verify the diagnosis. This may be particularly advantageous in the pediatric population, including the evaluation of children with failure to thrive.
In patients with known celiac disease, these antibodies can be used to monitor disease status and dietary compliance. Furthermore, these antibodies identify successful treatment, as they will become negative in patients on a gluten-free diet.
Glucagon
Indications
This is a direct measurement of glucagon in the blood. It is used to diagnose a glucagonoma. It is also useful in the evaluation of some diabetic patients. Finally, pancreatic function can be investigated with the use of this test.
Test Explanation
Glucagon is a hormone secreted by the alpha cells of the pancreatic islets of Langerhans. Glucagon is secreted in response to hypoglycemia and increases the blood glucose by breaking down glycogen to glucose in the liver. It also increases glucose in other tissues by inhibiting passage of glucose into cells and by encouraging efflux of glucose from the cell. Glucagon oxidizes triglycerides to fatty acids and glycerol that forms glucose. As serum glucose levels rise in the blood, glucagon is inhibited by a negative feedback mechanism.
Elevated glucagon levels may indicate the diagnosis of a glucagonoma (i.e., an alpha islet cell neoplasm). Glucagon deficiency occurs with extensive pancreatic resection or with burned-out pancreatitis. Arginine is a potent stimulator of glucagon. If the glucagon levels fail to rise even with arginine infusion, the diagnosis of glucagon deficiency as a result of pancreatic insufficiency is confirmed.
Normally glucagon decreases after ingestion of a carbohydrate-loaded meal through an elaborate negative feedback mechanism. This does not occur in patients with diabetes. Furthermore, in the insulin-dependent diabetic, glucagon stimulation caused by hypoglycemia does not occur. Arginine stimulation is performed to differentiate pancreatic insufficiency and diabetes. The diabetic will have an exaggerated elevation of glucagon with arginine administration. In pancreatic insufficiency, glucagon is not stimulated with arginine. In diabetic patients, hypoglycemia fails to stimulate glucagon release, as occurs in a nondiabetic person.
Because glucagon is thought to be metabolized by the kidneys, renal failure is associated with high glucagon levels and, as a result, high glucose levels. When rejection of a transplanted kidney occurs, one of the first signs of rejection may be increased serum glucose levels. Quantitative radioimmunoassay is commonly used for quantification of glucagon levels.
Interfering Factors
• Test results may be invalidated if a patient has undergone a radioactive scan within the previous 48 hours and glucagon is measured by radioimmunoassay (RIA). Administration of radionuclides can affect results.
• Levels may be elevated after prolonged fasting, stress, or moderate to intense exercise.
![]() Drugs that may cause increased levels include some amino acids (e.g., arginine), cholecystokinin, danazol, gastrin, glucocorticoids, insulin, nifedipine, and sympathomimetic amines.
Drugs that may cause increased levels include some amino acids (e.g., arginine), cholecystokinin, danazol, gastrin, glucocorticoids, insulin, nifedipine, and sympathomimetic amines.
![]() Drugs that may cause decreased levels include atenolol, propranolol, and secretin.
Drugs that may cause decreased levels include atenolol, propranolol, and secretin.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Familial hyperglucagonemia: There is a genetic defect that causes a predominance of a glucagon precursor.
Glucagonoma: There are several syndromes, including the more common multiple endocrine neoplasia, that are associated with glucagonomas.
Diabetes mellitus (DM): Inappropriate elevations in glucagon levels in hyperglycemic type I diabetic patients indicate that paradoxical glucagon release may contribute to disease severity.
Chronic renal failure: Glucagon is metabolized by the kidney. With loss of that function, glucagon and glucose levels rise.
Severe stress, including infection, burns, surgery, and acute hypoglycemia: Stress stimulates catecholamine release. This in turn stimulates glucagon secretion.
Acromegaly: Growth hormone is a stimulator of glucagon.
Hyperlipidemia: The pathophysiology of this observation is not well established.
Acute pancreatitis: The contents of the pancreatic cells (including glucagon) are spilled into the bloodstream as they are injured during the inflammation.
Pheochromocytoma: Catecholamines are potent stimulators to glucagon secretion.
 Decreased Levels
Decreased Levels
Idiopathic glucagon deficiency: The pathophysiology of this process is not well understood. An autoantibody process may be the cause.
Diabetes mellitus (DM): In diabetic patients, low glucagon levels (undetectable or in the lower quartile of the normal range) in the presence of hypoglycemia indicate impairment of hypoglycemic counter-regulation. These patients may be particularly prone to recurrent hypoglycemia.
The chronically diseased pancreas cannot produce glucagon.
Postpancreatectomy: In the absence of pancreatic tissue, glucagon secretion will not occur.
Cancer of pancreas: Pancreatic tissue destroyed by tumor will not secrete glucagon.
Glucose, Blood (Blood Sugar, Fasting Blood Sugar [FBS])
Normal Findings
Cord: 45-96 mg/dL or 2.5-5.3 mmo1/L (SI units)
Premature infant: 20-60 mg/dL or 1.1-3.3 mmo1/L
Neonate: 30-60 mg/dL or 1.7-3.3 mmo1/L
Infant: 40-90 mg/dL or 2.2-5.0 mmo1/L
Indications
This test is a direct measurement of the blood glucose level. It is most commonly used in the evaluation of diabetic patients.
Test Explanation
Through an elaborate feedback mechanism, glucose levels are controlled by insulin and glucagon. Glucose levels are low in the fasting state. In response, glucagon, which is made in the alpha cells of the pancreatic islets of Langerhans, is secreted. Glucagon breaks glycogen down to glucose in the liver and glucose levels rise. If the fasting persists, protein and fatty acids are broken down under glucagon stimulation. Glucose levels continue to rise.
Glucose levels are elevated after eating. Insulin, which is made in the beta cells of the pancreatic islets of Langerhans, is secreted. Insulin attaches to insulin receptors in muscle, liver, and fatty cells, in which it drives glucose into these target cells to be metabolized to glycogen, amino acids, and fatty acids. Blood glucose levels diminish. Many other hormones (e.g., adrenocorticosteroids, adrenocorticotropic hormone [ACTH], epinephrine, growth, thyroxine) can also affect glucose metabolism.
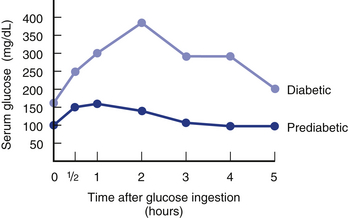
The serum glucose test is helpful in diagnosing many metabolic diseases. Serum glucose levels must be evaluated according to the time of day they are performed. For example, a glucose level of 135 mg/dL may be abnormal if the patient is in the fasting state, but this level would be within normal limits if the patient had eaten a meal within the last hour. Glycosylated hemoglobin (p. 266) is now being performed more frequently to identify diabetes because this blood test represents blood sugar levels over the last 120 days. That being said, the diagnosis of diabetes should be confirmed with a repeat of the same tests initially performed but on a different day to guard against laboratory error.
In general, true glucose elevations indicate diabetes mellitus (DM), however, there are many other possible causes of hyperglycemia. Similarly, hypoglycemia has many causes. The most common cause is inadvertent insulin overdose in patients with brittle diabetes. If diabetes is suspected based on elevated fasting blood levels, a glycosylated hemoglobin or glucose tolerance test can be performed.
Glucose determinations must be performed frequently in new diabetic patients to monitor closely the insulin dosage to be administered. Finger stick blood glucose determinations are usually performed before meals and at bedtime. Results are compared with a sliding-scale insulin chart ordered by the physician to provide coverage with subcutaneous regular insulin.
For diabetic patients who experience recurrent episodes of severe hypoglycemia or who require more than three doses of insulin per day, minimally invasive glucose monitoring is available. A small, sterile, disposable glucose-sensing device is inserted into the subcutaneous tissue (usually the arm). This sensor measures the change in glucose in the interstitial fluid. This information is recorded in a small beeper-sized monitor for 3 to 4 days. The monitor is taken to the doctor's office, where it is connected to a standard personal computer. Specialized software then downloads the stored information and a more effective insulin regimen can be developed.
Interfering Factors
• Many forms of stress (e.g., trauma, general anesthesia, infection, burns, myocardial infarction [MI]) can cause increased serum glucose levels.
• Caffeine may cause increased levels.
• Many pregnant women experience some degree of glucose intolerance. If significant, it is called gestational diabetes.
![]() Most intravenous (IV) fluids contain dextrose, which is quickly converted to glucose. Most patients receiving IV fluids will have increased glucose levels.
Most intravenous (IV) fluids contain dextrose, which is quickly converted to glucose. Most patients receiving IV fluids will have increased glucose levels.
![]() Drugs that may cause increased levels include antidepressants (tricyclics), antipsychotics, beta-adrenergic blocking agents, corticosteroids, cyclosporins, IV dextrose infusion, dextrothyroxine, diazoxide, diuretics, epinephrine, estrogens, glucagon, isoniazid, lithium, niacin, phenothiazines, phenytoin, salicylates (acute toxicity), and triamterene.
Drugs that may cause increased levels include antidepressants (tricyclics), antipsychotics, beta-adrenergic blocking agents, corticosteroids, cyclosporins, IV dextrose infusion, dextrothyroxine, diazoxide, diuretics, epinephrine, estrogens, glucagon, isoniazid, lithium, niacin, phenothiazines, phenytoin, salicylates (acute toxicity), and triamterene.
![]() Drugs that may cause decreased levels include acetaminophen, alcohol, alpha-glucosidase inhibitors, anabolic steroids, biguanides, clofibrate, disopyramide, gemfibrozil, incretin mimetics, insulin, monoamine oxidase inhibitors, meglitinides, pentamidine, propranolol, sulfonylureas, and thiazolidinediones.
Drugs that may cause decreased levels include acetaminophen, alcohol, alpha-glucosidase inhibitors, anabolic steroids, biguanides, clofibrate, disopyramide, gemfibrozil, incretin mimetics, insulin, monoamine oxidase inhibitors, meglitinides, pentamidine, propranolol, sulfonylureas, and thiazolidinediones.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() For fasting blood sugar determination, the patient must fast for 8 hours. Water is permitted.
For fasting blood sugar determination, the patient must fast for 8 hours. Water is permitted.
![]() To prevent starvation, which may artificially raise the glucose levels, the patient should not fast longer than 8 hours.
To prevent starvation, which may artificially raise the glucose levels, the patient should not fast longer than 8 hours.
• Withhold insulin or oral hypoglycemics until after blood is obtained.
During
• Collect a venous blood sample in a red-top or gray-top tube.
• Glucose levels can also be evaluated by a finger stick blood test using either a visually read test or a reflectance meter. The advantage of the visually read test is that it does not require an expensive machine. However, the patient must be able to visually interpret the color of the reagent strip. Using reflectance meters (e.g., Glucometer, Accu Check bG, Stat Tek) improves the accuracy of the blood glucose determination.
Test Results and Clinical Significance
 Increased Levels (Hyperglycemia)
Increased Levels (Hyperglycemia)
Diabetes mellitus (DM): This disease is defined by glucose intolerance and hyperglycemia. A discussion of the many possible etiologies is beyond the scope of this manual.
Acute stress response: Severe stress, including infection, burns, and surgery, stimulates catecholamine release. This in turn stimulates glucagon secretion, which causes hyperglycemia.
Cushing syndrome: Blood cortisol levels are high. This in turn causes hyperglycemia.
Pheochromocytoma: Catecholamine stimulates glucagon secretion, which causes hyperglycemia.
Chronic renal failure: Glucagon is metabolized by the kidney. With loss of that function, glucagon and glucose levels rise.
Glucagonoma: Glucagon is autonomously secreted, causing hyperglycemia.
Acute pancreatitis: The contents of the pancreatic cells (including glucagon) are spilled into the bloodstream as the cells are injured during the inflammation. The glucagon causes hyperglycemia.
Diuretic therapy: Certain diuretics cause hyperglycemia.
Corticosteroid therapy: Cortisol causes hyperglycemia.
Acromegaly: Growth hormone stimulates glucagon, which causes hyperglycemia.
 Decreased Levels (Hypoglycemia)
Decreased Levels (Hypoglycemia)
Insulinoma: Insulin is autonomously produced without regard to biofeedback mechanisms.
Hypothyroidism: Thyroid hormones affect glucose metabolism. With diminished levels of this hormone, glucose levels fall.
Hypopituitarism: Many pituitary hormones (adrenocorticotropic hormone [ACTH], growth hormone) affect glucose metabolism. With diminished levels of these hormones, glucose levels fall.
Addison disease: Cortisol affects glucose metabolism. With diminished levels of this hormone, glucose levels fall.
Extensive liver disease: Most glucose metabolism occurs in the liver. With decreased liver function, glucose levels decrease.
Insulin overdose: This is the most common cause of hypoglycemia. Insulin is administered at too high of a dose (especially in brittle diabetes) and glucose levels fall.
Starvation: With decreased carbohydrate ingestion, glucose levels diminish.
Related Tests
Diabetes Mellitus (DM) Autoantibody Panel (p. 206). This test is used in the evaluation of insulin resistance. It is also used to identify type I diabetes and patients with a suspected allergy to insulin. This antibody panel is also used in surveillance of patients who have received pancreatic islet cell transplants.
Glucose, Urine (p. 924). Testing for glucose in the urine is a part of routine urinalysis. If present, glucose in the urine reflects the degree of glucose elevation in the blood. Urine glucose tests are also used to monitor the effectiveness of therapy for DM.
Glycosylated Hemoglobin (p. 266). This is an accurate method of indicating average glucose levels over the past 100 to 120 days before the test.
Glucose Tolerance (p. 261). This is a test of a patient's capability to handle a glucose load.
Glucose, Postprandial (p. 257). This is a timed glucose measurement after a carbohydrate meal.
Glucagon (p. 251). This is a direct measurement of glucagon, which acts to increase glucose in the blood.
Insulin Assay (p. 315). This is a direct measurement of insulin, which acts to decrease glucose in the blood.
Glucose, Postprandial (2-Hour Postprandial Glucose [2-Hour PPG], 2-Hour Postprandial Blood Sugar, 1-Hour Glucose Screen for Gestational Diabetes Mellitus, O'Sullivan Test)
Indications
The 2-hour PPG test is a measurement of the amount of glucose in the patient's blood 2 hours after a meal is ingested (postprandial). It is used to diagnose diabetes mellitus (DM).
Test Explanation
For this study, a meal acts as a glucose challenge to the body's metabolism. Insulin is normally secreted immediately after a meal in response to the elevated blood glucose level, causing the level to return to the premeal range within 2 hours. In patients with diabetes the glucose level usually is still elevated 2 hours after the meal. The PPG is an easily performed screening test for DM. If the results are greater than 140 and less than 200 mg/dL, a glucose tolerance test may be performed to confirm the diagnosis. If the 2-hour PPG is greater than 200 mg/dL, the diagnosis of DM is confirmed. Also, a glucose tolerance or glycosylated hemoglobin test can be performed to corroborate and better evaluate the disease.
The 1-hour glucose screen is used to detect gestational DM, which is the most common medical complication of pregnancy. Gestational diabetes is a carbohydrate intolerance first recognized during pregnancy and affects 3% to 8% of pregnant women, with up to half of these women developing overt diabetes later in life. The detection and treatment of gestational diabetes may reduce the risk for several adverse perinatal outcomes (e.g., excessive fetal growth and birth trauma, fetal death, neonatal morbidity).
Screening for gestational diabetes is performed with a 50-100 g oral glucose load followed by a glucose level determination 1 hour later. This is called the O'Sullivan test. Screening is done between 24 and 28 weeks of gestation. However, patients with risk factors such as a previous history of gestational diabetes may benefit from earlier screening. Patients whose serum glucose level equals or exceeds 140 mg/dL may be evaluated by a 3-hour glucose tolerance test.
Interfering Factors
• Stress can increase glucose levels through the catecholamine effect of increasing serum glucose.
• If the patient eats a small snack or eats candy during the 2-hour interval, glucose levels will be falsely elevated.
• If the patient is not able to eat the entire test meal or vomits some or all of the meal, levels will be falsely decreased.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
• Usually a fasting blood glucose is done before the meal is given. This acts as a baseline glucose level (see p. 253).
![]() For the 2-hour PPG, instruct the patient to fast for 12 hours before testing. Instruct the patient to eat the entire meal (with at least 75 g of carbohydrates) and then not to eat anything else until the blood is drawn 2 hours later.
For the 2-hour PPG, instruct the patient to fast for 12 hours before testing. Instruct the patient to eat the entire meal (with at least 75 g of carbohydrates) and then not to eat anything else until the blood is drawn 2 hours later.
• For the 1-hour glucose screen for gestational diabetes, give the fasting or nonfasting patient a 50-g oral glucose load.
![]() Instruct the patient not to smoke during the test.
Instruct the patient not to smoke during the test.
![]() Inform the patient that he or she should rest during the 1- or 2-hour interval, because exercise can increase glucose levels.
Inform the patient that he or she should rest during the 1- or 2-hour interval, because exercise can increase glucose levels.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Diabetes mellitus (DM): This disease is defined by glucose intolerance and hyperglycemia. A discussion of the many possible etiologies is beyond the scope of this manual.
Gestational diabetes mellitus (DM): This disease is defined by glucose intolerance and hyperglycemia during pregnancy.
Malnutrition: Malnourished patients have very poor glucose tolerance when they start to eat. The pathophysiology and theories of this observation are not well defined and are multiple.
Hyperthyroidism: Thyroid hormone is an ancillary hormone that affects glucose metabolism and acts to increase glucose levels.
Acute stress response: Severe stress, including infection, burns, and surgery, stimulates catecholamine release. This in turn stimulates glucagon secretion, which causes hyperglycemia.
Cushing syndrome: Blood cortisol levels are high. This in turn causes hyperglycemia.
Pheochromocytoma: Catecholamine stimulates glucagon secretion, which causes hyperglycemia.
Chronic renal failure: Glucagon is metabolized by the kidney. With loss of kidney function, glucagon and glucose levels rise.
Glucagonoma: Glucagon is autonomously secreted, causing hyperglycemia.
Diuretic therapy: Certain diuretics cause hyperglycemia.
Corticosteroid therapy: Cortisol causes hyperglycemia.
Acromegaly: Growth hormone stimulates glucagon, which causes hyperglycemia.
Extensive liver disease: Most glucose metabolism occurs in the liver. With decreased function of the liver, glucose levels decrease.
 Decreased Levels
Decreased Levels
Insulinoma: Insulin is autonomously produced without regard to biofeedback mechanisms.
Hypothyroidism: Thyroid hormone affects glucose metabolism. With diminished levels of this hormone, glucose levels fall.
Hypopituitarism: Many pituitary hormones (adrenocorticotropic hormone [ACTH], growth hormone) affect glucose metabolism. With diminished levels of these hormones, glucose levels fall.
Addison disease: Cortisol affects glucose metabolism. With diminished levels of this hormone, glucose levels fall.
Insulin overdose: This is the most common cause of hypoglycemia. Insulin is administered at too high of a dose (especially in brittle diabetes), and glucose levels fall.
Malabsorption or maldigestion: The test meal is not absorbed and glucose levels do not increase.
Related Tests
Glucose, Blood (p. 253). This test is a direct measurement of the blood glucose level. It is most commonly the initial test in the evaluation of diabetic patients.
Glycosylated Hemoglobin (p. 266). This is an accurate method of indicating glucose tolerance in the recent past.
Glucose Tolerance (p. 261). This is a test of a patient's capability to handle a glucose load.
Glucose-6-Phosphate Dehydrogenase (G6PD Screen, G6PD Quantification, Glucose-6-Phosphate Dehydrogenase Deficiency [G-6-PD] DNA Sequencing)
Indications
This test is used to identify G-6-PD deficiency in patients who have developed hemolysis after taking certain oxidizing drugs. It is especially useful in males of certain ethnic populations who are susceptible to this genetic defect.
Test Explanation
G-6-PD is an enzyme used in glucose metabolism. G-6-PD deficiency causes precipitation of oxidized (forms Heinz bodies) hemoglobin. This may result in hemolysis of variable severity. This disease is a sex-linked, recessive trait carried on the X chromosome. The full effect of this genetic defect is not seen if the normal gene is present on a second X chromosome to oppose the genetic defect. In males there is no second X gene and the genetic defect is unopposed. Affected males inherit this abnormal gene from their mothers, who are usually asymptomatic. In these males the disease is most severe.
In rare cases when females have the defective gene on both X chromosomes, the disease is equally severe. Most commonly, however, women act as a “carrier” of the gene in that they have the defective gene on one X chromosome and have a normal gene for G-6-PD on the other X chromosome. These women have variable expressions of the disease from no symptoms to moderate symptoms if under a significant degree of stimulation. Most mutations identified are classified according to the following scheme: Class I—severe enzyme deficiency with chronic non-spherocytic hemolytic anemia; Class II—severe enzyme deficiency with <10% normal activity; Class III—mild to moderate enzyme deficiency (10%-60% normal activity); and Class IV—very mild to almost normal enzyme activity (>60% normal activity with no clinical consequences).
In the United States, G-6-PD is found mainly in African Americans. About 10% to 15% of that population is affected by the disease. Also, those of Mediterranean descent (Italians, Greeks, Sephardic Jews) are at risk for the genetic defect.
G-6-PD is an important enzyme involved in the pentose pathway of glucose metabolism that produces NADPH in the RBC. Most of the glucose in a young RBC is metabolized to produce NADH by the Embden-Meyerhof pathway. As the RBC ages, the pentose pathway becomes the dominant pathway of glucose metabolism. The NADPH in turn maintains the supply of reduced glutathione, which is used to neutralize free oxidative radicals that damage the RBC. Glutathione protects the RBC against potentially destructive oxidizing states such as infection, pharmacologic agents, or certain foods (e.g., fava beans). If NADPH is present through the action of G-6-PD, the RBC is not susceptible to oxidizing agents. When the reductive effects of glutathione are deficient, as in patients with G-6-PD deficiency, proteins precipitate on the RBC membrane, leading to RBC destruction and hemolysis. Patients with severe G-6-PD deficiency can have chronic or intermittent hemolysis. Persons with mild deficiency do not have hemolysis without exposure to the oxidizing drugs or oxidizing events. With the administration of an oxidizing drug, hemolysis can start as early as the first day and usually by the fourth day. The most common oxidizing drugs known to precipitate hemolysis and anemia in G-6-PD deficiency are antimalarials, sulfa drugs, nitrofurantoin, aspirin, and phenacetin (Box 2-10). Acute bacterial or viral or acidosis can also precipitate a hemolytic process in these patients.
This test is used to diagnose G-6-PD deficiency in suspected individuals. The quantification is used as a screening test. There are several different testing methods available for G-6-PD screening and testing. G-6-PD enzyme assay direct quantitation is most definitive.
Glucose-6-phosphate dehydrogenase deficiency (G6PD) DNA sequencing by polymerase chain reaction/sequencing can most accurately make the diagnosis of this disease. Combined with quantification, the disease course can be accurately determined. As in all genetic diseases, pretesting counseling and informed consent are recommended.
Procedure and Patient Care
After
• Apply pressure or a pressure dressing to the venipuncture site.
• Assess the venipuncture site for bleeding.
![]() If the test indicates a G-6-PD deficiency, give the patient a list of drugs that may precipitate hemolysis. Instruct patients with the Mediterranean type of this disease not to eat fava beans. Teach patients to read labels on any over-the-counter (OTC) drugs for the presence of agents (e.g., aspirin, phenacetin) that may cause hemolytic anemia.
If the test indicates a G-6-PD deficiency, give the patient a list of drugs that may precipitate hemolysis. Instruct patients with the Mediterranean type of this disease not to eat fava beans. Teach patients to read labels on any over-the-counter (OTC) drugs for the presence of agents (e.g., aspirin, phenacetin) that may cause hemolytic anemia.
Glucose Tolerance (GT, Oral Glucose Tolerance [OGT])
Indications
This test is used to assist in the diagnosis of diabetes mellitus (DM). It is also used in the evaluation of patients with hypoglycemia.
Test Explanation
In the past, The National Diabetes Data Group (NDDG) has defined criteria sufficient for the diagnosis of diabetes mellitus (Table 2-25). These include any one the following:
TABLE 2-25
National Diabetes Data Group's Reclassification of Diabetic Patients
| NDDG Classification | Diagnosis∗ | Old Classification |
| Diabetes mellitus (insulin and noninsulin dependent) | Unequivocal signs/symptoms FBC >126 more than once; GT at 2 hours >200 more than once | Overt diabetes |
| Impaired GT | Blood glucose between 140 and 200 mg/dL 2 hours after oral glucose load | Latent (chemical) diabetes |
| Previous abnormality of glucose tolerance | Normal GT test but previous abnormal GT test | Subclinical diabetes |
| Potential abnormality of GT | Genetic predisposition to diabetes (family history) | Prediabetes |
| Gestational diabetes mellitus | Onset of unequivocal diabetes or GT test results exceeding pregnancy criteria† | Diabetes of pregnancy |
GT, Glucose tolerance; FBG, fasting blood glucose.
∗Glucose levels are recorded in mg/100 mL.
†Onset or recognition of criteria during pregnancy but not before.
1. Sufficient clinical symptoms (polydipsia, polyuria, ketonuria, weight loss) plus random blood glucose >200 mg/dL.
2. Elevated FBG >126 mg/dL on more than one occasion.
3. A 2-hour blood glucose >200 mg/dL during oral GT testing.
These criteria should be reconfirmed by repeat testing on a different day in the absence of unequivocal hyperglycemia and metabolic decompensation. A diagnosis of diabetes mellitus could be made on the basis of the results from two tests—fasting blood glucose (p. 253) and GTT—performed on separate days that are close in time. The American Diabetes Association (ADA), the International Diabetes Federation, and the European Association for the Study of Diabetes all indicate that two abnormal glycosylated hemoglobin assays (p. 266) should be used whenever possible instead of the fasting glucose and GTT.
The GT test, then, is used when diabetes is suspected (retinopathy, neuropathy, diabetic-type renal diseases), but the criteria for the diagnosis cannot be met without the data obtained by the GT test. This test is not part of routine screening for diabetes. The GT test may be used for the following:
• Patients with a family history of diabetes
• Patients who are massively obese
• Patients with a history of recurrent infections
• Patients with delayed healing of wounds (especially on the lower legs or feet)
• Women who have a history of stillbirths, premature births, or large babies
• Patients who have transient glycosuria or hyperglycemia during pregnancy or following myocardial infarction (MI), surgery, or stress
In the GT test, the patient's ability to tolerate a standard oral glucose load (75 g) is evaluated by obtaining serum and urine specimens for glucose level determinations before glucose administration and then at 30 minutes, 1 hour, 2 hours, 3 hours, and sometimes 4 hours after the administration. Normally there is a rapid insulin response to the ingestion of a large oral glucose load. This response peaks in 30 to 60 minutes and returns to normal in about 3 hours. Patients with an appropriate insulin response are able to tolerate the dose quite easily, with only a minimal and transient rise in serum glucose levels within 1 to 2 hours after ingestion. Glucose will not spill over into the urine in normal patients.
Patients with diabetes will not be able to tolerate this load. As a result, their serum glucose levels will be greatly elevated from 1 to 5 hours (Figure 2-17). Also, glucose can be detected in their urine.
Gestational diabetes also can be diagnosed by the GT test. Generally the diagnosis of diabetes can be made if two or more of the results exceed the following:
The American Diabetes Association recommends that pregnant women who have not previously had an abnormal GT result should be screened between 24 and 28 weeks of gestation with a 50-g dose of glucose. This is called the O'Sullivan test. A glucose level of 130-140 mg/100 mL one hour later should be followed by a 3-hour GTT.
GI absorption can vary among individuals. For that reason, some centers prefer to administer an intravenous (IV) glucose load rather than depend on gastrointestinal (GI) absorption. Also, occasionally a patient is unable to tolerate the oral glucose load (e.g., patients with prior gastrectomy, short-bowel syndrome, malabsorption). In these instances an intravenous glucose tolerance (IV GT) test can be performed by administering the glucose load intravenously. The values for the IV GT test differ slightly from those of the oral GT test because IV glucose is absorbed faster.
Glucose intolerance also may exist in patients with oversecretion of hormones that have an ancillary affect on glucose, such as patients with Cushing syndrome, pheochromocytoma, acromegaly, aldosteronism, or hyperthyroidism. Patients with chronic renal failure, acute pancreatitis, myxedema, type IV lipoproteinemia, infection, or cirrhosis can also have an abnormal GT test. Certain drugs, as mentioned below, can cause abnormal GT results.
The GT test is also used to evaluate patients with hypoglycemia. This hypoglycemia may occur as late as 5 hours after the initial glucose load.
Interfering Factors
• Smoking during the testing period stimulates glucose production because of the nicotine.
• Stress (e.g., from surgery, infection) can increase glucose levels.
• Exercise during the test can affect glucose levels.
• Fasting or reduced caloric intake before the GT test can cause glucose intolerance.
![]() Drugs that may cause glucose intolerance include antihypertensives, antiinflammatory drugs, aspirin, beta blockers, furosemide, nicotine, oral contraceptives, phenothiazines, psychiatric drugs, steroids, and thiazide diuretics.
Drugs that may cause glucose intolerance include antihypertensives, antiinflammatory drugs, aspirin, beta blockers, furosemide, nicotine, oral contraceptives, phenothiazines, psychiatric drugs, steroids, and thiazide diuretics.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Educate the patient about the importance of having adequate food intake with adequate carbohydrates (150 g) for at least 3 days before the test.
Educate the patient about the importance of having adequate food intake with adequate carbohydrates (150 g) for at least 3 days before the test.
![]() Instruct the patient to fast for 12 hours before the test.
Instruct the patient to fast for 12 hours before the test.
![]() Instruct the patient to discontinue drugs (including tobacco) that could interfere with test results.
Instruct the patient to discontinue drugs (including tobacco) that could interfere with test results.
![]() Give the patient written instructions explaining the pretest dietary requirements.
Give the patient written instructions explaining the pretest dietary requirements.
• Obtain the patient's weight to determine the appropriate glucose loading dose (especially in children).
During
• Obtain fasting blood and urine specimens.
• Administer the prescribed oral glucose solution, usually 75 g of glucose or dextrose for nonpregnant patients or 100 g for pregnant patients. There are several commercial preparations available. The glucose load may be diluted in as much as 300 mL of lemon juice/water mixture.
• Give pediatric patients a carbohydrate load based on their body weight.
![]() Instruct the patient to ingest the entire glucose load.
Instruct the patient to ingest the entire glucose load.
![]() Tell the patient that he or she cannot eat anything until the test is completed. However, encourage the patient to drink water. No other liquids should be taken during the testing period.
Tell the patient that he or she cannot eat anything until the test is completed. However, encourage the patient to drink water. No other liquids should be taken during the testing period.
![]() Inform the patient that tobacco, coffee, and tea are not allowed, because they cause physiologic stimulation.
Inform the patient that tobacco, coffee, and tea are not allowed, because they cause physiologic stimulation.
• Collect a venous blood sample in a gray-top tube at 30 and 60 minutes and at hourly periods.
• Collect urine specimens at hourly periods.
• During the testing period, assess the patient for reactions such as dizziness, sweating, weakness, and giddiness. (These are usually transient.) If the symptoms are persistent, obtain a serum glucose level.
• For the IV GT test, administer the glucose load intravenously over 3 to 4 minutes.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Diabetes mellitus (DM): This disease is defined by glucose intolerance and hyperglycemia. A discussion of the many possible etiologies is beyond the scope of this manual.
Acute stress response: Severe stress, including infection, burns, and surgery, stimulates catecholamine release. This in turn stimulates glucagon secretion, which causes hyperglycemia and glucose intolerance.
Cushing syndrome: Blood cortisol levels are high. This causes hyperglycemia and glucose intolerance.
Pheochromocytoma: Catecholamines stimulate glucagon secretion, which causes hyperglycemia and glucose intolerance.
Chronic renal failure: Glucagon is metabolized by the kidney. With loss of that function, glucagon and glucose levels rise.
Glucagonoma: Glucagon is autonomously secreted, causing hyperglycemia.
Acute pancreatitis: The contents of the pancreatic cells (including glucagon) are spilled into the bloodstream as the cells are injured during the inflammation. The glucagon causes hyperglycemia.
Diuretic therapy: Certain diuretics cause hyperglycemia.
Corticosteroid therapy: Cortisol causes hyperglycemia and glucose intolerance.
Acromegaly: Growth hormone stimulates glucagon, which causes hyperglycemia and glucose intolerance.
Myxedema: Usually these patients have a flat GT curve, but they may have a “diabetic curve.”
Somogyi response to hypoglycemia: This is a reactive hyperglycemia following hypoglycemia that may occur from an exaggerated insulin response to the glucose load.
After gastrectomy: These patients can dump most of the glucose load into the small intestines in just minutes because the normal pylorus is absent. This can cause rapid absorption of glucose into the bloodstream and cause a false elevation in glucose level during the early part of the test.
Related Tests
Diabetes Mellitus (DM) Autoantibody Panel (p. 206). This test is used in the evaluation of insulin resistance. It is also used to identify type 1 diabetes and patients with a suspected allergy to insulin. This antibody panel is also used in surveillance of patients who have received pancreatic islet cell transplants.
Glucose, Blood (p. 253). This is the primary screening test to diagnose diabetes.
Glucose, Urine (p. 924). Testing for glucose in the urine is a part of routine urinalysis. If present, it reflects the degree of glucose elevation in the blood. Urine glucose tests are also used to monitor the effectiveness of therapy for diabetes mellitus.
Glycosylated Hemoglobin (p. 266). This is an accurate method of indicating glucose tolerance in the recent past.
Glucagon (p. 251). This is a direct measurement of glucagon, which acts to increase glucose in the blood.
Insulin Assay (p. 315). This is a direct measurement of insulin, which acts to decrease glucose in the blood.
Glycosylated Hemoglobin (GHb, GHB, Glycohemoglobin, Hemoglobin A1c [HbA1c], Diabetic Control Index, Glycated Protein)
Indications
This test is used to diagnose and monitor diabetes treatment. It measures the amount of HbA1c in the blood and provides an accurate long-term index of the patient's average blood glucose level.
Test Explanation
In adults about 98% of the hemoglobin in the red blood cell (RBC) is hemoglobin A. About 7% of hemoglobin A consists of a type of hemoglobin (HbA1) that can combine strongly with glucose in a process called glycosylation. When glycosylation occurs, it is not easily reversible.
HbA1 is actually made up of three components: A1a, A1b, and A1C. HbA1c is the component that combines most strongly with glucose. Therefore, HgA1c is the most accurate measurement because it contains the majority of glycosylated hemoglobin. If the total HbA1 is measured, its value is 2% to 4% higher than the HbA1c component.
As the RBC circulates, it combines its HbA1 with some of the glucose in the bloodstream to form glycohemoglobin (GHb). The amount of GHb depends on the amount of glucose available in the bloodstream over the RBC's 120-day life span. Therefore determination of the GHb value reflects the average blood sugar level for the 100- to 120-day period before the test. The more glucose the RBC is exposed to, the greater the GHb percentage. One important advantage of this test is that the sample can be drawn at any time, because it is not affected by short-term variations (e.g., food intake, exercise, stress, hypoglycemic agents, patient cooperation). It is also possible for very high short-term blood glucose levels to cause an elevation of GHb. Usually, however, the degree of glucose elevation results not from a transient high level but from a persistent, moderate elevation over the entire life of the RBC.
Like GHb, the glucose can nonenzymatically bind to proteins in proportion to the mean blood glucose level. The glycated protein is stable until the degradation of the protein. Recall that the average life span of an RBC (and the GHb within) is 120 days. The GHb, therefore, may not reflect more recent changes in glucose levels. Because the turnover rate of proteins is much faster than that of hemoglobin, the measurement of serum glycated proteins (such as glycated albumin) or fructosamine provides more recent information about glucose levels. Glycated proteins reflect an average blood glucose level of the past 15 to 20 days. Although an initial single glycated protein result may not separate good glucose control from poor control, serial testing provides a much better indication of glucose control.
The GHb or glycated proteins tests are particularly beneficial for the following:
1. Evaluating the success of diabetic treatment and patient compliance.
2. Comparing and contrasting the success of past and new forms of diabetic therapy.
3. Determining the duration of hyperglycemia in patients with newly diagnosed diabetes.
4. Providing a sensitive estimate of glucose imbalance in patients with mild diabetes.
5. Individualizing diabetic control regimens.
6. Providing a sense of reward for many patients when the test shows achievement of good diabetic control.
7. Evaluating the diabetic patient whose glucose levels change significantly from day to day (brittle diabetes).
8. Differentiating short-term hyperglycemia in nondiabetics (e.g., recent stress or myocardial infarction [MI]) and diabetics (in whom the glucose has been persistently elevated).
9. Differentiating short-term hyperglycemia in nondiabetics (e.g., recent stress or myocardial infarction [MI]) and diabetics (in whom the glucose has been persistently elevated).
A diagnosis of diabetes mellitus can be made on the basis of the results from two tests—fasting blood glucose (p. 253) and GTT—performed on separate days that are close in time. The American Diabetes Association (ADA), the International Diabetes Federation, and the European Association for the Study of Diabetes all indicate that two abnormal GHb assays should be used whenever possible instead of the fasting glucose and GTT.
By a relatively simple calculation, GHb can be correlated accurately with the daily mean plasma glucose (MPG) level, the average glucose level throughout the day. This has been very helpful for diabetics and their health care professionals in determining and evaluating daily glucose goals. There is a linear relationship between A1c (GHb) and PG:
with a Pearson correlation coefficient (r) of 0.82. Each 1% change in GHb represents a change of approximately 35 mg/dL MPG or 2 mmol/L (Table 2-26). At present there are several ongoing studies designed to identify and document the accuracy of simple mathematical equations design to easily convert GHb to MPG.
TABLE 2-26
Correlation Between GHb and MPG
| A1c (%) | Approximate Mean Plasma Glucose (mg/dL) | Interpretation |
| 4 | 65 | Nondiabetic range |
| 5 | 100 | Nondiabetic range |
| 6 | 135 | Nondiabetic range |
| 7 | 170 | ADA target |
| 8 | 205 | Action suggested |
The results of the glycosylated hemoglobin assay will be inaccurate in patients with conditions that involve high red blood cell turnover (e.g., hemolytic anemia) or who have had recent blood transfusions.
Interfering Factors
• Hemoglobinopathies can affect results, because the quantity of hemoglobin A (and, as a result, HbA1) varies considerably in these diseases.
• Falsely elevated values occur when the RBC life span is lengthened because the HbA1 has a longer period available for glycosylation.
• Abnormally low levels of proteins may falsely indicate normal glycated fructosamine levels despite the reality of high glucose levels.
![]() Ascorbic acid may cause falsely low levels of glycated fructosamine.
Ascorbic acid may cause falsely low levels of glycated fructosamine.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Newly diagnosed diabetic patient: This test is not used to diagnose new diabetics because the range of “normal” is so broad; it is best used to assess glycemic control during treatment.
Poorly controlled diabetic patient,
Nondiabetic hyperglycemia (e.g., acute stress response, Cushing syndrome, pheochromocytoma, glucagonoma, corticosteroid therapy, acromegaly):
Patients with these illnesses tend to have persistently elevated glucose levels that cause an elevated GHb and glycated proteins.
Patients with splenectomy: In these patients RBC survival is prolonged. More time for hemoglobin glycosylation is available. GHb and glycated proteins levels increase.
Pregnancy: In some women with gestational diabetes or prediabetes, persistently high levels of glucose occur that cause elevated GHb and glycated proteins levels.
 Decreased Levels
Decreased Levels
Related Tests
Glucose, Blood (p. 253). This is the primary screening test for diabetes.
Glucose, Urine (p. 924). Testing for glucose in the urine is a part of routine urinalysis. If glucose is present in the urine, it reflects the degree of glucose elevation in the blood. Urine glucose tests are also used to monitor the effectiveness of therapy for diabetes mellitus (DM).
Glucose Tolerance (p. 261). This is a test of a patient's capability to handle a glucose load.
Glucose, Postprandial (p. 257). This is a timed glucose measurement after a carbohydrate meal.
Glucagon (p. 251). This is a direct measurement of glucagon, which acts to increase glucose in the blood.
Insulin Assay (p. 315). This is a direct measurement of insulin, which acts to decrease glucose in the blood.
Growth Hormone (GH, Human Growth Hormone [HGH], Somatotropin Hormone [SH])
Indications
This test is used to identify GH deficiency in adolescents with short stature, delayed sexual maturity, or other growth deficiencies. It is also used to document the diagnosis of GH excess in patients with gigantism or acromegaly. GH is used to identify and follow patients with ectopic growth hormone produced by neoplasms. Finally, it is often used as a screening test for pituitary hypofunction.
Test Explanation
GH, or somatotropin, is secreted by the acidophil cells in the anterior pituitary gland and plays a central role in modulating growth from birth until the end of puberty. There is an elaborate feedback mechanism associated with the secretion of GH. The hypothalamus secretes growth hormone–releasing hormone, which stimulates GH release from the pituitary. GH exerts its effects on many tissues through a group of peptides called somatomedins. The most commonly tested somatomedin is somatomedin C (p. 317), which is produced by the liver and has a major effect on cartilage. High levels of somatomedins stimulate the production of somatostatin from the hypothalamus. Somatostatin inhibits further secretion of GH from the pituitary. GH is secreted during sleep, exercise, and ingestion of protein and in response to hypoglycemia.
In the total absence of GH, linear growth occurs at one half to one third of the normal rate. GH also plays a role in the control of body anabolism throughout life by increasing protein synthesis, increasing the breakdown of fatty acids in adipose tissue, and increasing the blood glucose level.
If GH secretion is insufficient during childhood, limited growth and dwarfism may result. Also, a delay in sexual maturity may occur in adolescents with reduced GH levels. Conversely, overproduction of GH during childhood results in gigantism, with the person reaching nearly 7 to 8 feet in height. An excess of GH during childhood (after closure of long bone end plates) results in acromegaly, which is characterized by an increase in bone thickness and width but no increase in height.
GH tests are also used to confirm hypopituitarism or hyperpituitarism. GH assay is the most widely used test for GH deficiency or excess. Because GH secretion is episodic, random assays for GH are not adequate determinants of GH deficiency. Normal GH levels overlap significantly with deficient levels. Low GH levels may indicate deficiency or may be normal for certain individuals at certain times of the day. To negate time variables in GH testing, GH should be drawn 60 to 90 minutes after deep sleep. Levels increase during sleep. Also, strenuous exercise can be performed for 30 minutes in an effort to stimulate GH production. GH levels drawn at the end of the exercise period are expected to be maximal. These two methods are helpful in evaluating GH deficiency.
To negate the common variations in GH secretion, screening for insulin-like growth factor (IGF-1) or somatomedin C provides a more accurate reflection of the mean plasma concentration of GH. If the IGF-1 is normal, the patient virtually never has acromegaly. IGF-1 is not helpful in evaluating patients with GH deficiency because levels are affected by nutritional status, liver and thyroid function, and age. These proteins are not affected by the time of day or food intake as is GH, because they circulate bound to proteins that are durable and long lasting.
A GH stimulation test (p. 272) can be performed to evaluate the body's ability to produce GH in cases of suspected GH deficiency. The growth hormone suppression test is used to identify gigantism in children or acromegaly in the adult. If GH can be suppressed to less than 2 ng/mL, neither of these conditions exists. The most commonly used suppression test is the oral glucose tolerance test (p. 261). Through a rise in glucose, GH normally is suppressed. In acromegalic patients, only a slight or no decrease in GH occurs.
Interfering Factors
• Random measurements of GH are not adequate determinants of GH deficiency, because hormone secretion is episodic.
• A radioactive scan performed within the week before the test may affect test results if levels are determined by radioimmunoassay (RIA).
• GH secretion is increased by stress, exercise, diet, and low blood glucose levels.
![]() Drugs that may cause increased levels include amphetamines, arginine, dopamine, estrogens, glucagon, histamine, insulin, levodopa, methyldopa, and nicotinic acid.
Drugs that may cause increased levels include amphetamines, arginine, dopamine, estrogens, glucagon, histamine, insulin, levodopa, methyldopa, and nicotinic acid.
![]() Drugs that may cause decreased levels include corticosteroids and phenothiazines.
Drugs that may cause decreased levels include corticosteroids and phenothiazines.
Procedure and Patient Care
After
• Apply pressure or a pressure dressing to the venipuncture site.
• Assess the venipuncture site for bleeding.
• Indicate the patient's fasting status and the time the blood is collected on the laboratory slip. Include the patient's recent activity (e.g., sleeping, walking, eating).
• Because the half-life of GH is only 20 to 25 minutes, send the blood to the laboratory immediately after collection.
Test Results and Clinical Significance
 Decreased Levels
Decreased Levels
GH is produced in the pituitary. Diseases, tumors, ischemia, or trauma to the pituitary or hypothalamus causes GH deficiency.
Dwarfism: This is a result of GH deficiency in children.
Hyperglycemia: Elevated glucose levels inhibit GH secretion.
Failure to thrive: This is a result of GH deficiency in infants.
Delayed sexual maturity: This is a result of GH deficiency in adolescents.
Related Tests
Growth Hormone (GH) Stimulation (p. 272). This is a test designed to stimulate the secretion of GH. It is required to accurately diagnose GH deficiency.
Somatomedin C (p. 317). Somatomedin C levels parallel GH levels. However, they are not affected by the many factors that cause significant variations in GH results.
Growth Hormone Stimulation (GH Provocation, Insulin Tolerance [IT], Arginine)
Indications
The GH stimulation test is used to identify patients who are suspected of having GH deficiency. A normal patient can have low GH levels, but if GH is still low after GH stimulation, the diagnosis can be more accurately made.
Test Explanation
Because GH secretion is episodic, random measurement of plasma GH is not adequate to make the diagnosis of GH deficiency. To diagnose GH deficiency, GH stimulation tests are indicated. One of the most reliable GH stimulators is insulin-induced hypoglycemia, in which the blood glucose declines to less than 40 mg/dL. Other GH stimulants include vigorous exercise and drugs (e.g., arginine, glucagon, levodopa, clonidine). Glucagon is more widely used for GH stimulation because of safety concerns with insulin-induced hypoglycemia.
Pituitary GH deficiency cannot be diagnosed by identifying a deficiency of GH to just one stimulant, because as many as 20% of normal patients will fail to respond to the stimulant. Therefore a double-stimulated test is usually performed: arginine infusion is followed by insulin-induced hypoglycemia. Arginine is an amino acid that stimulates GH secretion; hypoglycemia also stimulates GH secretion. A GH concentration over 10 mg/L after stimulation effectively excludes GH deficiencies. Hypothyroidism should be excluded before GH stimulation testing.
GH also can be stimulated by vigorous exercise. This may entail running or stair-climbing for 20 minutes. Blood samples of GH are obtained at 0, 20, and 40 minutes. GH-releasing factor can also be used to stimulate GH. At present the best method of identifying patients deficient in GH is a positive stimulation test followed by a positive response to a therapeutic GH trial. GH deficiency is also suspected when bone age, as determined by x-ray films of the long bones (see p. 1006), indicates delayed growth according to chronologic age.
Only minor discomfort is associated with this test and results from the insertion of the intravenous (IV) line and the hypoglycemic response induced by the insulin injection. This may include postural hypotension, somnolence, diaphoresis, and nervousness. This procedure is usually performed by a nurse under physician supervision. This test takes approximately 2 hours to perform.
Contraindications
• Patients with epilepsy, because seizures can be induced by the hypoglycemia
• Patients with cerebrovascular disease, because hypoglycemia may induce stroke
• Patients with myocardial infarction (MI), because the stress associated with the hypoglycemia may cause angina or an MI
• Patients with low basal plasma cortisol levels, because they cannot respond to or compensate for the hypoglycemia
Procedure and Patient Care
During
• Note the following procedural steps:
1. A saline lock IV line is inserted for the administration of medications and the withdrawal of frequent blood samples.
2. Baseline blood levels are obtained for GH, glucose, and cortisol.
3. Venous samples for GH are obtained 0, 60, and 90 minutes after injection of arginine and/or insulin or glucagon.
4. Blood glucose levels are monitored at 15- to 30-minute intervals with the glucometer. The blood sugar should drop to less than 40 mg/dL for effective measurement of GH reserve.
• Monitor the patient for signs of hypoglycemia, postural hypotension, somnolence, diaphoresis, and nervousness.
• Ice chips are often given during the test for patient comfort.
After
• Observe the venipuncture site for bleeding.
![]() Inform the patient and family that results may not be available for approximately 7 days. Some laboratories run GH tests only once a week.
Inform the patient and family that results may not be available for approximately 7 days. Some laboratories run GH tests only once a week.
• After the test, give the patient cookies and punch or an IV glucose infusion.
• Send the blood to the laboratory immediately after collection, because the half-life of growth hormone is only 20 to 25 minutes.