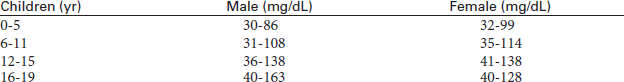Thrombosis Indicators (Fibrin Monomers [Fibrin Degradation Products (FDPs)], Fibrin Split Products [FSPs], Fibrinopeptide A [FPA], Prothrombin Fragment [F1+2])
Indications
Identification of FDPs, FPA, and F1+2 is mostly used to document that fibrin clot formation and, therefore, thrombosis is occurring. These tests support the diagnosis of disseminated intravascular coagulation (DIC). They also provide an indication about the effectiveness of anticoagulation therapy. Finally, they are used to support the diagnosis and follow treatment for hypercoagulable states.
Test Explanation
F1+2 is liberated when prothrombin is converted to thrombin in reaction 4 of secondary hemostasis (see Figure 2-12, p. 167). These fragments are primarily used to indicate thrombosis. Significantly increased F1+2 levels are also noted in patients with leukemia, severe liver disease, and after myocardial infarction. Patients with elevated F1+2 concentration before the beginning of heparin therapy show decreases after 1 day of therapy. For patients in the stable phase of oral anticoagulant therapy decreasing F1+2 concentrations are noted with increasing INR values. Thus F1+2 determination is particularly helpful in monitoring anticoagulant therapy.
FPA is made up of two small peptide chains removed from the N-terminal segment of the alpha chains of fibrinogen during its conversion to fibrin. It is released into the bloodstream by that reaction during the blood coagulation process and is therefore a measure of thrombosis.
Measurement of FDPs provides a direct indication of the activity of the fibrinolytic system. The fibrinolytic system plays an important role in balancing clot formation and clot dissolution. Clot formation stimulates the activation of three major activators of the fibrinolytic system. These in turn act on plasminogen, which was previously absorbed into the clot, to form plasmin. Plasmin degenerates the fibrin polymer of the clot into fragments called FDPs (X, D, E, Y). These degradation products are usually cleared by macrophages. If present in increased quantities, they can have an anticoagulant effect by inhibiting fibrinogen conversion to fibrin and by interrupting fibrin polymerization to tighten the clot.
When present in large amounts, FDPs indicate increased fibrinolysis, as occurs in thrombotic states. The thrombosis stimulates the activation of the fibrinolytic system. Other diseases can secondarily activate the fibrinolytic system and elevate FDP levels. These may include extensive malignancy, tissue necrosis, and gram-negative sepsis. Thrombolytic therapy used in myocardial infarction (MI), for example, is associated with increased FDPs. Streptokinase or urokinase stimulates the conversion of plasminogen to plasmin. The plasmin splits the fibrinogen polymer into FDPs, as discussed above.
These products of hemostasis and fibrinolysis may also be elevated in patients with extensive malignancy, tissue necrosis, and gram-negative sepsis. For discussion of D-dimer fibrin degradation products, see p. 202.
Interfering Factors
• Traumatic venipunctures may increase FPA levels.
• Surgery or massive trauma is associated with increased levels of these indicators because of the thrombosis that is instigated by surgery.
• Menstruation may be associated with increased FDP levels.
• The presence of rheumatoid factor may give falsely high levels.
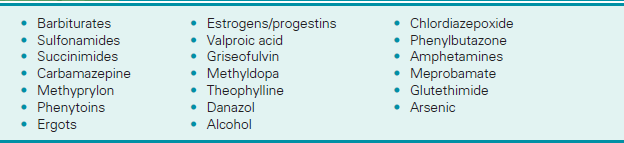
![]() Drugs that may cause increased levels include barbiturates, heparin, streptokinase, and urokinase.
Drugs that may cause increased levels include barbiturates, heparin, streptokinase, and urokinase.
![]() Drugs that may cause decreased indicator levels include warfarin and other oral anticoagulants.
Drugs that may cause decreased indicator levels include warfarin and other oral anticoagulants.
Procedure and Patient Care
During
• Draw the sample before initiating heparin therapy.
• Collect a venous blood sample in a small, blue-top tube or in the colored tube designated by the laboratory.
• Avoid excessive agitation of the blood sample.
• Note that it is best to place the blood on ice and take it immediately to the hematology laboratory.
• List on the laboratory slip any drugs that may cause elevated levels.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Disseminated intravascular coagulation (DIC),
Deficiency in protein S and C: The “protein C–protein S” system is an important inhibitor of coagulation. With deficiencies in these proteins, thrombosis proceeds without inhibition.
Antithrombin III deficiency: Antithrombin III complexes with activated coagulation proteins and blocks their biologic activity. Even mild reductions in this protein are therefore associated with marked increased thrombosis.
Related Test
Disseminated Intravascular Coagulation (DIC) Screening (p. 210). This is a description of commonly used tests to diagnose DIC.
Thyroglobulin (Tg, Thyrogen-Stimulated Thyroglobulin)
Normal Findings
| Age | Male (ng/mL) | Female (ng/mL) |
| 0-11 months | 0.6-5.5 | 0.5-5.5 |
| 1-11 years | 0.6-50.1 | 0.5-52.1 |
| 12 years and older | 0.5-53.0 | 0.5-43.0 |
Test Explanation
Tg is the protein precursor of thyroid hormone and is made by normal well-differentiated benign thyroid cells or thyroid cancer cells. Because Tg is normally only made by thyroid cells, it serves a useful readout for the presence or absence of thyroid cells especially after thyroid cancer surgery. In the treatment of well-differentiated thyroid cancers, it is important to remove as much thyroid tissue as possible so that adjunctive radioactive iodine treatment will not go to residual thyroid gland tissue in the neck, but will go instead to any metastatic thyroid cells. If postoperative Tg levels are low, very little thyroid tissue remains.
Tg is also used as a “tumor marker” in these postoperative patients. Tg is a marker of disease activity and the volume of thyroid tumor. Ideally, the Tg levels will be low (<2 ng/mL) or undetectable after treatment (usually surgery followed by radioactive iodine). Rising levels herald tumor recurrence and progression. Although Tg levels may be elevated in patients with thyroid cancer, a large number of benign thyroid conditions may also be associated with elevated levels of Tg. Therefore an increased Tg alone in a patient is not a sensitive or specific test for the diagnosis of thyroid cancer. Simply examining the thyroid or carrying out a thyroid biopsy can produce significant elevations in the circulating blood level of Tg. Similarly, patients with thyroid inflammation can have very high levels of Tg. Some patients with antithyroglobulin antibodies (see p. 102) may have inaccurate Tg levels.
After thyroidectomy, thyroid hormone replacement is required for normal metabolic function. Because of thyroid hormone replacement therapy, thyroid-stimulating hormone (TSH) levels are usually very low and endogenous stimulation of any residual thyroid cells is minimal in these patients. As a result, Tg and thyroid endogenous thyroid hormones are low. Until recently, in order to stimulate Tg production in these patients for cancer surveillance testing, thyroid hormone was temporarily discontinued for as much as 6 weeks until the body was depleted of any thyroid hormone. TSH was then maximally stimulated and was able to stimulate the production of Tg from any thyroid cells. If there were any functioning thyroid cancer cells, Tg would be elevated. During the time of thyroid hormone withdrawal, the patient was very uncomfortable, lethargic, tired, and slow.
Thyrogen-stimulated testing has eliminated the need for withdrawal of thyroid hormone medications and provides a safe and effective method to elevate TSH levels so that even minimal levels of Tg can be detected. This allows patients to undergo periodic thyroid cancer follow-up evaluation while avoiding the often debilitating side effects of hypothyroidism caused by withdrawal of hormone medication. Thyrogen is a highly purified recombinant source of human thyroid-stimulating hormone. Thyrogen raises serum TSH levels and thereby stimulates Tg production. Normal thyroid remnant and well-differentiated thyroid tumors display a greater (>10-fold) serum Tg response to TSH stimulation. If Thyrogen-stimulated Tg levels are elevated after thyroid surgery, either a significant amount of normal thyroid gland was left in the neck or metastatic disease exists. If Thyrogen-stimulated Tg levels are elevated after postoperative therapeutic 131I (given to destroy any residual thyroid tissue in the neck), metastatic disease certainly exists and will require treatment.
Thyrogen stimulation is also used for patients undergoing 131I whole body scanning for metastatic thyroid cancer. Like Tg testing, in the past these patients had to withdraw from their thyroid hormone replacement medicine so that their endogenous TSH levels would rise, stimulate any metastatic thyroid cancer cells to pick up 131I, and be detected on a nuclear scan of the body. Now with the use of Thyrogen, the ill effects of hormone withdrawal are not experienced.
Interfering Factors
• Tg levels are decreased in less well-differentiated thyroid cancers.
• Thyrogen stimulation of Tg levels is less in patients whose tumors do not have TSH receptors or whose tumors cannot make Tg.
• Tg autoantibodies cause either underestimation or overestimation of serum Tg measurements made by immunometric assay (IMA) and radioimmunoassay (RIA) methods, respectively.
Procedure and Patient Care
During
• Collect a venous blood sample in a gold-top (serum separator) tube.
• If Thyrogen stimulation is to be used:
1. Administer Thyrogen intramuscularly to the buttock every 24 hours for two or three doses.
2. Collect blood in a gold-top (serum separator) tube in 3 days.
1. The nuclear medicine technologist will administer radioiodine 24 hours following the final Thyrogen injection.
2. Scanning is usually performed 48 hours after radioiodine administration. Whole-body images are acquired for a minimum of 30 minutes and/or should contain a minimum of 140,000 counts.
3. Scanning times for single (spot) images of body regions may be obtained.
Related Test
Antithyroglobulin Antibody (p. 102). Although used primarily to identify patients with thyroiditis, the presence of these antibodies can affect Tg test results.
Thyroid-Stimulating Hormone (TSH, Thyrotropin)
Indications
This test is used to diagnose primary hypothyroidism and to differentiate it from secondary (pituitary) and tertiary (hypothalamus) hypothyroidism.
Test Explanation
The TSH (also called thyrotropin) concentration aids in differentiating primary and secondary hypothyroidism. Pituitary TSH secretion is stimulated by hypothalamic thyroid-releasing hormone (TRH). Low levels of triiodothyronine (T3) and thyroxine (T4) are the underlying stimuli for TRH and TSH. Therefore a compensatory elevation of TRH and TSH occurs in patients with primary hypothyroid states, such as surgical or radioactive thyroid ablation; in patients with burned-out thyroiditis, thyroid agenesis, idiopathic hypothyroidism, or congenital cretinism; or in patients taking antithyroid medications.
In secondary or tertiary hypothyroidism the function of the pituitary or hypothalamus gland, respectively, is faulty as a result of tumor, trauma, or infarction. Therefore TRH and TSH cannot be secreted, and plasma levels of these hormones are near zero despite the stimulation that occurs with low T3 and T4 levels.
The TRH Stimulation Test is sometimes used to stimulate low levels of TSH to identify primary from secondary hypothyroidism in cases in which TSH is low. However, this test is not commonly used because extremely low levels of TSH can now be identified with the use of immunoassays.
The TSH test is used to monitor exogenous thyroid replacement or suppression as well. The goal of thyroid replacement therapy is to provide an adequate amount of thyroid medication so that TSH secretion is in the “low normal range,” indicating a euthyroid state. The goal of thyroid suppression is to completely suppress the thyroid gland and TSH secretion by providing excessive thyroid medication. This treatment is used to diminish the size of a thyroid goiter. The dose of medication is given to keep the TSH level less than 2 for replacement. Even lower TSH levels are preferred if thyroid suppression is the clinical goal.
This test is also used to detect primary hypothyroidism in newborns with low screening T4 levels. TSH and T4 levels are frequently measured to differentiate pituitary and thyroid dysfunction. A decreased T4 and normal or elevated TSH level can indicate a thyroid disorder. A decreased T4 with a decreased TSH level can indicate a pituitary disorder.
Interfering Factors
• Recent radioisotope administration may affect test results.
• Severe illness may cause decreased TSH levels.
• There is a diurnal variation in TSH levels. Basal levels occur around 10 AM and highest levels (about two to three times basal levels) occur around 10 PM.
![]() Drugs that may cause increased levels include antithyroid medications, lithium, potassium iodide, and TSH injection.
Drugs that may cause increased levels include antithyroid medications, lithium, potassium iodide, and TSH injection.
![]() Drugs that may cause decreased levels include aspirin, heparin, nonsteroidal antiarthritics dopamine, steroids, and T3.
Drugs that may cause decreased levels include aspirin, heparin, nonsteroidal antiarthritics dopamine, steroids, and T3.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Primary hypothyroidism (thyroid dysfunction),
In these diseases, inadequate thyroid hormone levels act as a potent stimulant for the release of TSH from the anterior pituitary. TSH levels rise. In some cases, however, TSH may be diminished.
Pituitary TSH-secreting tumor: This is very rare, but when it occurs, TSH levels are increased.
 Decreased Levels
Decreased Levels
Secondary hypothyroidism (pituitary or hypothalamus dysfunction): Diseases of the hypothalamus diminish the capability of the hypothalamus to secrete TRH, which is the major factor that determines TSH production and secretion. Diseases of the pituitary diminish pituitary production of TSH.
Hyperthyroidism: Increased levels of thyroid hormones inhibit the release of TSH.
Suppressive doses of thyroid medication: When thyroid medication (e.g., Synthroid) is administered (usually to shrink a goiter), TSH levels fall because of inhibition by the thyroid medication.
Factitious hyperthyroidism: These patients take thyroid medication without prescription. These medications act to inhibit TSH production.
Related Tests
Thyroid-Stimulating Immunoglobulins (p. 491). LATS and other thyroid-stimulating immunoglobulins are used to support the diagnosis of Graves disease, especially when the diagnosis is complex.
Thyrotropin-Releasing Hormone Stimulation Test (p. 492). This test assists in the evaluation of patients with hyperthyroidism and hypothyroidism. It is especially helpful in the differential diagnosis of hypothyroidism.
Thyroid-Stimulating Hormone Stimulation (see following test). This test is also used to differentiate primary from secondary (and tertiary) hypothyroidism.
Thyroxine-Binding Globulin (p. 495). This is a measure of TBG, the major thyroid hormone protein carrier. It is used in the evaluation of patients who have abnormal total T4 and T3 levels. When performed concurrently with the T4/T3 test, T4 and T3 levels can more easily be interpreted.
Thyroxine, Total (p. 497). This is one of the first tests done for assessing thyroid function. It is used to diagnose thyroid function and to monitor replacement and suppressive therapy.
Triiodothyronine (p. 506). T3 is used to evaluate thyroid function. It is mostly used to diagnose hyperthyroidism. It is also used to monitor thyroid replacement and suppressive therapy.
Thyroxine Index, Free. This test is used to evaluate thyroid function. It corrects for changes in thyroid hormone-binding serum proteins that can affect total T4 levels. It is used to diagnose hyperthyroidism and hypothyroidism.
Thyroxine, Free (p. 497). The FT4 is used to evaluate thyroid function in patients who may have protein abnormalities that could affect total T4 levels. It is used to diagnose thyroid function and to monitor replacement and suppressive therapy.
Antithyroglobulin Antibody (p. 102). This test is used primarily in the differential diagnosis of thyroid diseases, such as Hashimoto thyroiditis and chronic lymphocytic thyroiditis (in children).
Thyroid-Stimulating Hormone Stimulation (TSH Stimulation)
Test Explanation
The TSH stimulation test is used to differentiate primary (thyroid) hypothyroidism and secondary (hypothalamic-pituitary) hypothyroidism. Normal people and patients with hypothalamic-pituitary hypothyroidism are capable of increasing thyroid function when exogenous TSH is given. Patients with primary hypothyroidism because of disease in the thyroid, however, are not; their thyroid gland is inadequate and cannot function no matter how much stimulation it receives. Patients with less than a 10% increase in radioactive iodine uptake (RAIU) or less than a 1.5 mcg/dL rise in thyroxine (T4) are considered to have primary hypothyroidism. If the hypothyroidism is caused by inadequate pituitary secretion of TSH or hypothalamic secretion of thyroid-releasing hormone (TRH), the RAIU should increase at least 10% and the T4 level should rise 1.5 mcg/dL or more. This is characteristic of secondary hypothyroidism.
Procedure and Patient Care
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Primary hypothyroidism (thyroid dysfunction),
In these diseases the thyroid is unable to increase T4 levels or RAIU no matter how significant the stimulation, because the disease involves the thyroid itself.
Secondary hypothyroidism (pituitary or hypothalamus dysfunction): The thyroid is capable of producing T4 and RAIU, but the pituitary/hypothalamic stimulation is inadequate for appropriate stimulation of those functions. When TSH is administered, T4 and RAIU increase significantly.
Related Tests
Long-Acting Thyroid Stimulator (p. 491). LATS and other thyroid-stimulating immunoglobulins are used to support the diagnosis of Graves disease, especially when the diagnosis is complex.
Thyrotropin-Releasing Hormone Stimulation Test (p. 492). This test assists in the evaluation of patients with hyperthyroidism and hypothyroidism. It is especially helpful in the differential diagnosis of hypothyroidism.
Thyroid-Stimulating Hormone (p. 486). This test is used to diagnose primary hypothyroidism and to differentiate it from secondary (pituitary) and tertiary (hypothalamus) hypothyroidism.
Thyroxine-Binding Globulin (p. 495). This is a measure of TBG, the major thyroid hormone protein carrier. It is used in the evaluation of patients who have abnormal total T4 and T3 levels. When performed concurrently with a T4/T3 test, the T4 and T3 levels can more easily be interpreted.
Thyroxine, Total (p. 497). This is one of the first tests done for assessing thyroid function. It is used to diagnose thyroid function and to monitor replacement and suppressive therapy.
Triiodothyronine (p. 506). T3 is used to evaluate thyroid function. It is used primarily to diagnose hyperthyroidism. It is also used to monitor thyroid replacement and suppressive therapy.
Thyroxine Index, Free. This test is used to evaluate thyroid function. It corrects for changes in thyroid hormone-binding serum proteins that can affect total T4 levels. It is used to diagnose hyperthyroidism and hypothyroidism.
Thyroxine, Free (p. 497). The FT4 is used to evaluate thyroid function in patients who may have protein abnormalities that could affect total T4 levels. It is used to diagnose thyroid function and to monitor replacement and suppressive therapy.
Antithyroglobulin Antibody (p. 102). This test is primarily used in the differential diagnosis of thyroid diseases, such as Hashimoto thyroiditis and chronic lymphocytic thyroiditis (in children).
Thyroid-Stimulating Immunoglobulins (TSI, Long-Acting Thyroid Stimulator [LATS], Thyroid-Binding Inhibitory Immunoglobulin [TBII], Thyrotropin Receptor Antibody)
Indications
These are used to support the diagnosis of Graves disease, especially when the diagnosis is complex.
Test Explanation
Thyroid-stimulating immunoglobulins (TSI) represent a group of immunoglobulin-G (IgG) antibodies directed against the thyroid cell receptor for thyroid-stimulating hormone (TSH) and are associated with autoimmune thyroid disease states such as chronic thyroiditis and Graves disease. These autoantibodies bind and transactivate the TSH receptors (TSHRs). This instigates stimulation of the thyroid gland independent of the normal feedback–regulated thyroid-stimulating hormone (TSH) stimulation. This, in turn will stimulate the release of thyroid hormones from the thyroid cells. Some patients with Graves disease also have TSHR-blocking antibodies, which do not transactivate the TSHR. The balance between TSI and TSHR-blocking antibodies, as well as their individual titers, are felt to be determinants of Graves disease severity.
The use of these antibodies is helpful in the evaluation of patients for whom the diagnosis of Graves disease is confused by conflicting data (such as subclinical Graves hyperthyroidism or euthyroid patients with ophthalmopathy). In these cases, the antibodies help determine and support the diagnosis of Graves disease.
The effect of these antibodies on the thyroid may be long lasting, and titers do not decrease until nearly 1 year after successful treatment of the thyroid disease. However, measurement of these antibodies may be helpful in identifying remission or relapse of Graves disease after treatment. Because TSI can cross the placenta, they may be found in neonates whose mothers have Graves disease. These infants experience hyperthyroidism for as long as 4 to 8 months. This syndrome must be identified and treated early.
TSI and TSHR antibodies can be measured individually. Other antibodies associated with autoimmune thyroid diseases include thyroglobulin antibodies (p. 102) and antithyroid peroxidase antibodies (p. 104).
Related Tests
Thyrotropin-Releasing Hormone Stimulation Test (see following test). This test assists in the evaluation of patients with hyperthyroidism and hypothyroidism. It is especially helpful in the differential diagnosis of hypothyroidism.
Thyroid-Stimulating Hormone (p. 486). This test is used to diagnose primary hypothyroidism and to differentiate it from secondary (pituitary) and tertiary (hypothalamus) hypothyroidism.
Thyroid-Stimulating Hormone (TSH) Stimulation (p. 489). This test is also used to differentiate primary and secondary (and tertiary) hypothyroidism.
Thyroxine-Binding Globulin (p. 495). This is a measure of thyroxine-binding globulin (TBG), the major thyroid hormone protein carrier. It is used in the evaluation of patients who have abnormal total T4 and T3 levels. When done concurrently with a T4/T3 test, one can more easily interpret the T4 and T3 levels.
Thyroxine, Total (p. 497). This is one of the first tests done in assessing thyroid function. It is used to diagnose thyroid function and to monitor replacement and suppressive therapy.
Triiodothyronine (p. 506). T3 is used to evaluate thyroid function. It is mostly used to diagnose hyperthyroidism. It is also used to monitor thyroid replacement and suppressive medical therapy.
Thyroxine, Free (p. 497). The FT4 is used to evaluate thyroid function in patients who may have protein abnormalities that could affect total T4 levels. It is used to diagnose thyroid function and to monitor replacement and suppressive therapy.
Antithyroglobulin Antibody (p. 102). This test is primarily used in the differential diagnosis of thyroid diseases such as Hashimoto thyroiditis and chronic lymphocytic thyroiditis (in children).
Thyrotropin-Releasing Hormone Stimulation Test (TRH Stimulation Test, Thyrotropin-Releasing Factor Stimulation Test [TRF Stimulation Test])
Normal Findings
Prompt rise in serum thyroid-stimulating hormone (TSH) level to approximately twice the baseline value in 30 minutes after an intravenous (IV) bolus of TRH
| Clinical Disease | Baseline Thyroid-Stimulating Hormone (μU/mL) | Stimulated TSH∗ |
| Euthyroid | <10 | >2 |
| Hyperthyroid | <10 | <2 |
| Primary hypothyroid (thyroid) | >10 | >2 |
| Secondary hypothyroid (pituitary) | <10 | <2 |
| Tertiary hypothyroid (hypothalamus) | <10 | >2 |
∗Stimulated TSH (times the baseline) is measured 30 minutes after the IV injection of thyrotropin-releasing hormone.
Indications
This test assists in the evaluation of patients with hyperthyroidism and hypothyroidism. It is especially helpful in the differential diagnosis of hypothyroidism.
Test Explanation
The TRH stimulation test assesses the anterior pituitary gland via its secretion of TSH in response to an IV injection of TRH. After the TRH injection the normally functioning pituitary gland should secrete TSH (and prolactin). In hyperthyroidism, either a slight increase or no increase in the TSH level is seen, because pituitary TSH production is suppressed by the inhibitory effect of excess circulating thyroxine (T4) and triiodothyronine (T3) on the pituitary gland. A normal result is considered reliable evidence for excluding the diagnosis of thyrotoxicosis. Since the development of a very sensitive radioimmunoassay for TSH, the TRH stimulation test is no longer required to diagnose hyperthyroidism. However, it still has a role in the evaluation of pituitary deficiency.
In addition to assessing the responsiveness of the anterior pituitary gland, this test aids in the detection of primary, secondary, and tertiary hypothyroidism. In primary hypothyroidism (thyroid gland failure) the increase in the TSH level is two or more times the normal result. With secondary hypothyroidism (anterior pituitary failure), no TSH response occurs. Tertiary hypothyroidism (hypothalamic failure) may be diagnosed by a delayed rise in the TSH level. Multiple injections of TRH may be needed to induce the appropriate TSH response in this case.
The TRH stimulation test also may be useful in differentiating primary depression, manic-depressive psychiatric illness, and secondary types of depression. In primary depression the TSH response is blunted in most patients, whereas patients with other types of depression have a normal TRH-induced TSH response.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Hyperthyroidism: Because the pituitary is already maximally suppressed by the high levels of T3 and T4, pituitary response to TRH will be blunted and baseline levels will be less than double.
Primary hypothyroidism (thyroid disease): Because the TSH is already stimulated by the lack of T3 and T4 stimulation will be maximized by the TRH and stimulated TSH will be more than double the baseline.
Secondary hypothyroidism (pituitary disease): Because the diseased pituitary is unable to produce TSH, no matter how significant the stimulation, TSH will not double after TRH stimulation.
Tertiary hypothyroidism (hypothalamus): The pituitary is functioning normally. If TRH is provided exogenously, the pituitary will respond normally and produce twice the TSH level.
Psychiatric primary depression: In primary depression the TSH response is blunted in most patients, whereas patients with other types of depression have a normal TRH-induced TSH response.
Related Tests
Thyroid-Stimulating Hormone (p. 486). This test is used to diagnose primary hypothyroidism and to differentiate it from secondary (pituitary) and tertiary (hypothalamus) hypothyroidism.
Thyroid-Stimulating Hormone Stimulation (p. 489). This test is also used to differentiate primary and secondary (and tertiary) hypothyroidism.
Thyroxine-Binding Globulin (p. 495). This is a measure of TBG, the major thyroid hormone protein carrier. It is used in the evaluation of patients who have abnormal total T4 and T3 levels. When performed concurrently with a T4/T3 test, the T4 and T3 levels can be more easily interpreted.
Thyroxine, Total (p. 497). This is one of the first tests done for assessing thyroid function. It is used to diagnose thyroid function and to monitor replacement and suppressive therapy.
Triiodothyronine (p. 506). T3 is used to evaluate thyroid function. It is primarily used to diagnose hyperthyroidism. It is also used to monitor thyroid replacement and suppressive therapy.
Thyroxine Index, Free. This test is used to evaluate thyroid function. It corrects for changes in thyroid hormone-binding serum proteins that can affect total T4 levels. It is used to diagnose hyperthyroidism and hypothyroidism.
Thyroxine, Free (p. 497). The FT4 is used to evaluate thyroid function in patients who may have protein abnormalities that could affect total T4 levels. It is used to diagnose thyroid function and to monitor replacement and suppressive therapy.
Long-Acting Thyroid Stimulator (p. 491). LATS and other thyroid-stimulating immunoglobulins are used to support the diagnosis of Graves disease, especially when the diagnosis is complex.
Antithyroglobulin Antibody (p. 102). This test is used primarily in the differential diagnosis of thyroid diseases, such as Hashimoto thyroiditis and chronic lymphocytic thyroiditis (in children).
Thyroxine-Binding Globulin (TBG, Thyroid-Binding Globulin)
Indications
This is a measure of TBG, the major thyroid hormone protein carrier. It is used in the evaluation of patients who have abnormal total T4 and T3 levels. When performed concurrently with a T4/T3 test, the T4 and T3 levels can be more easily interpreted.
Test Explanation
Assays of T4 and T3 are a measure of total T4/T3 levels. That is, they are a measure of bound and unbound thyroid hormones. Most of these hormones are bound to TBG. The unbound or “free T4/T3” is the metabolically active hormone. Certain illnesses are associated with elevated or decreased TBG levels. With increased TBG levels, more T4 and T3 is bound to that protein. Less free, metabolically active T4/T3 is available. TSH is stimulated to produce higher levels of T4 and T3 to compensate. T4 and T3 levels increase but do not cause hyperthyroidism, because the increase is merely a compensation for the increased TBG. When total T4 is elevated, one must ascertain whether that elevation is due to an elevation in TBG or a real elevation in T4 alone associated with hyperthyroidism. There are other indirect measurements of TBG, including thyroid hormone-binding ratio (THBR).
The most common causes of elevated TBG are pregnancy, hormone replacement therapy, or use of oral contraceptives. Elevated TBG is also present in some cases of porphyria and in infectious hepatitis. Decreased TBG is commonly associated with other causes of hypoproteinemia (e.g., nephrotic syndrome, gastrointestinal [GI] malabsorption, malnutrition).
Interfering Factors
• Previous administration of diagnostic radioisotopes may confound test results, if TBG is measured by radioimmunoassay (RIA).
![]() Drugs that increase TBG include estrogens, methadone, oral contraceptives, and tamoxifen.
Drugs that increase TBG include estrogens, methadone, oral contraceptives, and tamoxifen.
![]() Drugs that decrease TBG include androgens, danazol, phenytoin, propranolol, and steroids.
Drugs that decrease TBG include androgens, danazol, phenytoin, propranolol, and steroids.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Pregnancy (and estrogen-replacement therapy, estrogen-producing tumors): All proteins, including TBG, are increased with increased estrogen levels.
Infectious hepatitis: The pathophysiology of this observation is not well known.
Genetic increase of TBG: Rarely a patient will have a genetic variation that causes elevated TBG.
Acute intermittent porphyria: The pathophysiology of this observation is not well known.
Related Tests
Long-Acting Thyroid Stimulator (p. 491). LATS and other thyroid-stimulating immunoglobulins are used to support the diagnosis of Graves disease, especially when the diagnosis is complex.
Thyrotropin-Releasing Hormone (p. 492). This test assists in the evaluation of patients with hyperthyroidism and hypothyroidism. It is especially helpful in the differential diagnosis of hypothyroidism.
Thyroid-Stimulating Hormone (p. 486). This test is used to diagnose primary hypothyroidism and to differentiate it from secondary (pituitary) and tertiary (hypothalamus) hypothyroidism.
Thyroid-Stimulating Hormone Stimulation (p. 489). This test is also used to differentiate primary and secondary (and tertiary) hypothyroidism.
Thyroxine, Total (p. 497). This is one of the first tests done for assessing thyroid function. It is used to diagnose thyroid function and to monitor replacement and suppressive therapy.
Triiodothyronine (p. 506). T3 is used to evaluate thyroid function. It is mostly used to diagnose hyperthyroidism. It is also used to monitor thyroid replacement and suppressive medical therapy.
Thyroxine, Free (see following test). The FT4 is used to evaluate thyroid function in patients who may have protein abnormalities that could affect total T4 levels. It is used to diagnose thyroid function and to monitor replacement and suppressive therapy.
Antithyroglobulin Antibody (p. 102). This test is used primarily in the differential diagnosis of thyroid diseases, such as Hashimoto thyroiditis and chronic lymphocytic thyroiditis (in children).
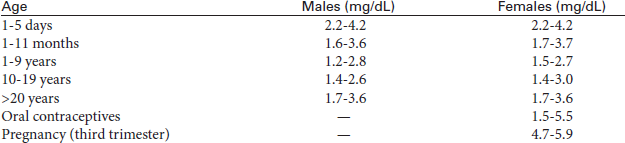
Thyroxine, Total and Free (T4, Thyroxine Screen, FT4)
Normal Findings
Indications
Thyroxine tests are used to determine thyroid function. Greater than normal levels indicate hyperthyroid states, and subnormal values are seen in hypothyroid states. T4 and TSH are used to monitor thyroid replacement and suppressive therapy.
Test Explanation
Thyroid hormones are produced when tyrosine incorporates organic iodine to form monoiodotyrosine. This complex picks up iodine and becomes diiodotyrosine. Two diiodotyrosines combine to form tetraiodothyronine (also called T4 thyroid hormone). If a diiodotyrosine combines with a monoiodotyrosine, triiodothyronine (p. 506) (also called T3 thyroid hormone) is formed. T4 makes up nearly 90% of what we call thyroid hormone. T3 makes up less than 10% of thyroid hormone. Nearly all of T4 and T3 is bound to protein. Thyroxine-binding globulin (TBG) binds most of T3 and T4. Albumin and prealbumin bind the rest. Total T4 measurement consists of both the bound and unbound fractions. Free T4 is a measure of unbound metabolically active T4. Thyroid hormones regulate a number of developmental, metabolic, and neural activities throughout the body. Thyrotropin-releasing hormone (TRH) is secreted in the hypothalamus. This stimulates the anterior pituitary to secrete thyrotropin (thyroid-stimulating hormone [TSH]). TSH stimulates the thyroid to secrete thyroid hormone. The increased levels of T3 and T4 inhibit further production of TRH.
Abnormalities in protein levels can have a significant effect on the results of the total T4. Pregnancy and hormone replacement therapy increase TBG and cause T4 to be falsely elevated, suggesting that hyperthyroidism exists when in fact the patient is euthyroid. If the free T4 is measured in these patients, it would be normal, indicating that free T4 is a more accurate indicator of thyroid function than total T4. In cases in which TBG is reduced (e.g., hypoproteinemia), the total T4 is likewise reduced, suggesting hypothyroidism. Measurement of free T4 would indicate normal levels and thereby discount the abnormal total T4 as merely a result of the reduced TBG and not as a result of hypothyroidism.
Free thyroxine (FT4) is measured using an automated, competitive, chemiluminescent immunoassay. Total thyroxine is measured by immunoenzymatic assay.
Interfering Factors
• Neonates have higher free T4 levels than older children and adults.
• Prior use of iodinated radioisotopes or iodinated contrast can alter test results.
• Pregnancy causes increased total T4 levels.
![]() Drugs that increase free T4 levels include aspirin, danazol, heparin, and propranolol.
Drugs that increase free T4 levels include aspirin, danazol, heparin, and propranolol.
![]() Drugs that decrease free T4 levels include furosemide, methadone, phenytoins, and rifampicin.
Drugs that decrease free T4 levels include furosemide, methadone, phenytoins, and rifampicin.
![]() Exogenously administered thyroxine causes increased free T4 results.
Exogenously administered thyroxine causes increased free T4 results.
![]() Drugs that may cause increased total T4 levels include clofibrate, estrogens, heroin, methadone, and oral contraceptives.
Drugs that may cause increased total T4 levels include clofibrate, estrogens, heroin, methadone, and oral contraceptives.
![]() Drugs that may cause decreased T4 levels include anabolic steroids, androgens, antithyroid drugs (e.g., propylthiouracil), lithium, phenytoin, and propranolol.
Drugs that may cause decreased T4 levels include anabolic steroids, androgens, antithyroid drugs (e.g., propylthiouracil), lithium, phenytoin, and propranolol.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Evaluate the patient's medication history.
Evaluate the patient's medication history.
![]() If indicated, instruct the patient to stop exogenous T4 medication 1 month before testing.
If indicated, instruct the patient to stop exogenous T4 medication 1 month before testing.
![]() Tell the patient that no fasting is required.
Tell the patient that no fasting is required.
![]() Explain to parents that newborns should be screened before discharge (regardless of age), because of the consequences of delayed diagnosis.
Explain to parents that newborns should be screened before discharge (regardless of age), because of the consequences of delayed diagnosis.
• Note that the optimal collection time is 2 to 4 days after birth.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Primary hyperthyroid states (e.g., Graves disease, Plummer disease, toxic thyroid adenoma): The thyroid produces increased T4 despite lack of TSH stimulation.
Acute thyroiditis: The thyroid secretes increased T4 during the acute inflammatory stages of thyroiditis (e.g., Hashimoto thyroiditis). However, in the latter stages the thyroid may become burned out and the patient may develop hypothyroidism.
Familial dysalbuminemic hyperthyroxinemia: These patients have a genetically defective form of albumin that binds T4 unusually tightly. As a result, the bound portion of T4 increases. The patient is not hyperthyroid because the protein-bound T4 is not metabolically active.
Factitious hyperthyroidism: Patients who self-administer T4 will have elevated levels. Many patients believe they will feel more energetic or will lose weight faster if they take T4.
Struma ovarii: Ectopic thyroid tissue in the ovary or anywhere can produce excess T4.
TBG increase (e.g., as occurs in pregnancy, hepatitis, congenital hyperproteinemia): Because the T4 assay measures total bound and unbound T4 any condition associated with elevated TBG will cause an elevation of T4.
 Decreased Levels
Decreased Levels
Hypothyroid states (e.g., cretinism, surgical ablation, myxedema): The thyroid in these diseases cannot produce an adequate amount of T4 despite the stimulation provided.
Pituitary insufficiency: The pituitary produces an insufficient amount of thyrotropin. As a result, the thyroid is not stimulated to produce T4.
Hypothalamic failure: The hypothalamus produces an insufficient amount of TRH. As a result, the pituitary does not produce thyrotropin, and the thyroid is not stimulated to produce T4.
Protein malnutrition and other protein-depleted states (e.g., nephrotic syndrome): With a reduced protein source, TBG and albumin decrease. Because T4 assay measures hormone bound to these proteins, T4 can be expected to be reduced.
Iodine insufficiency: Iodine is the basic raw material for T4. Without iodine, T4 cannot be produced. With the introduction of iodide in most table salts, iodine insufficiency is rare in the United States.
Nonthyroid illnesses (e.g., renal failure, Cushing disease, cirrhosis, surgery, advanced cancer): The pathophysiology of these observations is not well known. It may be in part because of a depletion of thyroid-binding proteins associated with severe medical illnesses.
Related Tests
Thyroid-Stimulating Immunoglobulins (p. 491). This and other thyroid-stimulating immunoglobulins are used to support the diagnosis of Graves disease, especially when the diagnosis is complex.
Thyrotropin-Releasing Hormone (p. 492). This test assists in the evaluation of patients with hyperthyroidism and hypothyroidism. It is especially helpful in the differential diagnosis of hypothyroidism.
Thyroid-Stimulating Hormone (p. 486). This test is used to diagnose primary hypothyroidism and to differentiate it from secondary (pituitary) and tertiary (hypothalamus) hypothyroidism. The free thyroxine value, combined with the TSH value, gives a more accurate picture of the thyroid status in patients with abnormal thyroid-binding globulin levels.
Thyroid-Stimulating Hormone Stimulation (p. 489). This test is also used to differentiate primary and secondary (and tertiary) hypothyroidism.
Thyroxine-Binding Globulin (p. 495). This is a measure of TBG, the major thyroid hormone protein carrier. It is used in the evaluation of patients who have abnormal total T4 and T3 levels. When performed concurrently with a T4/T3 test, the T4 and T3 levels can be more easily interpreted.
Triiodothyronine (p. 506). T3 is used to evaluate thyroid function. It is primarily used to diagnose hyperthyroidism. It is also used to monitor thyroid replacement and suppressive therapy.
Antithyroglobulin Antibody (p. 102). This test is primarily used in the differential diagnosis of thyroid diseases, such as Hashimoto thyroiditis and chronic lymphocytic thyroiditis (in children).
Toxoplasmosis Antibody Titer
Indications
These serologic tests are used to diagnose acute toxoplasmosis in immunosuppressed patients, pregnant women, and newborn infants. Immunity obtained from prior infection (e.g., fetal infection) is also determined by this test.
Test Explanation
Toxoplasmosis is a protozoan disease caused by Toxoplasma gondii, which is found in humans and many animals (especially cats). Humans become infected by eating poorly cooked or raw meat. Exposure to feces of cats or other infected material can cause infection. Infected humans are most often asymptomatic. When symptoms occur, this disease is characterized by CNS lesions, which may lead to blindness, brain damage, and death. The condition may occur congenitally or some time after birth. Because approximately 25% to 70% of the adult population have been exposed to toxoplasmosis as determined by positive antibody titers, the Centers for Disease Control and Prevention (CDC) recommends that pregnant women be serologically tested for this disease. Again, most acutely infected pregnant women are asymptomatic, and the best way to diagnose infection is by antibody testing.
The presence of antibodies before pregnancy indicates prior exposure and chronic asymptomatic infection. The presence of these antibodies probably ensures protection against congenital toxoplasmosis in the child. Fetal infection occurs if the mother acquires toxoplasmosis after conception and passes it to the fetus through the placenta. Repeat testing of pregnant patients with low or negative titers may be done before the twentieth week and before delivery to identify antibody converters and determine appropriate therapy (e.g., therapeutic abortion at 20 weeks, treatment during the remainder of the pregnancy, or treatment of the newborn).
Hydrocephaly, microcephaly, chronic retinitis, and convulsions are complications of congenital toxoplasmosis. Congenital toxoplasmosis is diagnosed when the antibody levels are persistently elevated or a rising titer is found in the infant 2 to 3 months after birth.
The term TORCH (toxoplasmosis, other, rubella, cytomegalovirus, herpes) has been applied to maternal infections with recognized detrimental effects on the fetus. TORCH testing refers to the testing for IgG (indicating past infection) and IgM (indicating recent infection) antibodies to the particular infectious agents as described. Included in the category of other are infections such as syphilis. All of these tests are discussed separately:
Herpesvirus, p. 731
Because of the difficulty in growing Toxoplasma in culture, the best way to diagnose this disease is by serologic testing. A commonly used test is the indirect fluorescent antibody test. With this technique, immunoglobulin (Ig)M and IgG can be detected in sum or separately. IgM rises about 1 week after inoculation, peaks in about 2 to 3 months, and declines to undetectable levels in about 1 year. IgG begins to rise about 2 weeks after inoculation, peaks in about 2 to 3 months, and declines to low but persistent levels in about 6 months. Low titers of IgG especially indicate past infections and protection from passing acute infection to an unborn child. High or rapidly rising titers of either IgM or IgG indicate acute infection in the adult or newborn infant. Hemagglutination is another more easily performed method of detecting IgG antibodies to toxoplasmosis. This is often used to screen new mothers. Enzyme-linked immunosorbent assay (ELISA) and radioimmunoassay (RIA) are other techniques to identify antibodies.
Elevated IgM antibodies, IgG titers greater than 1:1000, or a fourfold rise in IgG antibodies indicates an acute Toxoplasma infection. Low but significant titers of IgG indicate past infection. High, nonrising titers indicate acute infection more than 3 to 12 months before testing.
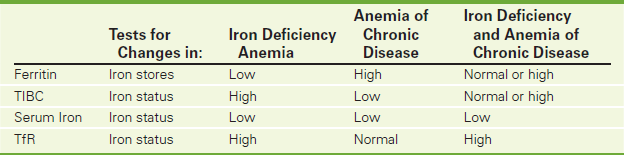
Transferrin Receptor Assay (TfR)
Indications
Serum transferrin receptor (TfR) concentration is used to differentiate iron deficiency anemia from the anemia of chronic disease (ACD) or other “iron low” anemias—particularly in children.
Test Explanation
Both iron metabolism and transport are altered in chronic and critical illness. Differentiation of the ACD (also called anemia of inflammation or anemia of aging) from iron deficiency anemia may be difficult, and the results of conventional laboratory assessment of iron stores may not be definitive. The most valuable iron store marker in distinguishing these two entities is the TfR concentration.
TfR is a cell surface protein found on most cells and especially those with a high requirement for iron, such as immature erythroid and malignant cells. Its function is to internalize absorbed iron into target cells. TfR is increased when erythropoiesis is enhanced (such as often occurs in iron deficiency). The concentration of cell surface–transferrin receptor is carefully regulated by transferrin receptor mRNA, according to the internal iron content of the cell and its individual iron requirements. Iron-deficient cells contain increased numbers of receptors, while receptor numbers are downregulated in iron-replete cells.
An increased mean TfR concentration is noted in patients with iron deficiency anemia as compared with patients with anemia secondary to chronic critical illnesses. TfR is also useful in distinguishing iron deficiency anemia from situations that are commonly encountered in childhood, adolescence, and during pregnancy when iron stores are uniformly low to absent. In these situations, iron-deficient erythropoiesis is not necessarily present, and TfR levels are not elevated. Finally, in situations in which iron deficiency anemia coexists with anemia of chronic disease, transferrin receptor concentrations increase secondary to the underlying iron deficiency, thus avoiding the need for a bone marrow examination.
In general, to increase sensitivity and specificity, the measurement of serum soluble transferrin receptor should be performed in combination with other tests of iron status, including ferritin, TIBC, and serum iron (Table 2-46). Calculation of the ratio of transferrin receptor to log ferritin concentration provides an even higher sensitivity and specificity for the detection of Fe deficiency.
The principal method for measurement of soluble transferrin receptor (TfR) is immunoturbidimetry using a commercially available clinical analyzer. Latex-bound anti-TfR antibodies react with the antigen in the sample to form an antigen-antibody complex. Following agglutination, this is measured turbidimetrically.
Interfering Factors
• Individuals who live at high altitudes have a reference range that extends 6% higher than the upper level of this reference interval.
• Results are related to ethnicity. Individuals of African descent can be expected to have higher levels.
![]() Drugs that may cause increased TfR levels include recombinant human erythropoietins.
Drugs that may cause increased TfR levels include recombinant human erythropoietins.
Triglycerides (TGs)
Indications
TGs identify the risk of developing coronary heart disease (CHD). This test is part of a lipid profile that includes the measurement of cholesterol and lipoproteins. This test is also performed on patients with suspected fat metabolism disorders.
Test Explanation
TGs are a form of fat in the bloodstream. They are transported by very-low-density lipoproteins (VLDLs) and low-density lipoproteins (LDLs). TGs are produced in the liver using glycerol and other fatty acids as building blocks. TGs act as a storage source for energy. When TG levels in the blood are high, TGs are deposited in the fatty tissues. TGs constitute most of the fat in the body and are a part of a lipid profile that also evaluates cholesterol and lipoprotein. A lipid profile is performed to assess the risk of coronary and vascular disease.
Interfering Factors
• Ingestion of fatty meals may cause elevated TG levels.
• Ingestion of alcohol may cause elevated levels of TG by increasing the production of VLDL.
• Pregnancy may cause increased levels.
![]() Drugs that may cause increased TG levels include cholestyramine, estrogens, and oral contraceptives.
Drugs that may cause increased TG levels include cholestyramine, estrogens, and oral contraceptives.
![]() Drugs that may cause decreased levels include ascorbic acid, asparaginase, clofibrate, colestipol, fibrates, and statins.
Drugs that may cause decreased levels include ascorbic acid, asparaginase, clofibrate, colestipol, fibrates, and statins.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Instruct the patient to fast for 12 to 14 hours before the test. Only water is permitted.
Instruct the patient to fast for 12 to 14 hours before the test. Only water is permitted.
![]() Tell the patient not to drink alcohol for 24 hours before the test.
Tell the patient not to drink alcohol for 24 hours before the test.
![]() Inform the patient that dietary indiscretion for as much as 2 weeks before this test will influence results.
Inform the patient that dietary indiscretion for as much as 2 weeks before this test will influence results.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Glycogen storage disease (von Gierke disease): VLDL (TG-carrying proteins) synthesis is increased, whereas catabolism is decreased. TG levels in the blood increase.
Familial hypertriglyceridemia: This is a genetic predisposition to elevated TGs.
Apoprotein C-II deficiency: This congenital disease is associated with lipoprotein lipase deficiency. TGs accumulate.
Hyperlipidemias: As lipids in the blood increase, so does TG, the major blood lipid.
Hypothyroidism: Catabolism of TG is diminished.
High-carbohydrate diet: Excess carbohydrates are converted into TG and blood levels of TG rise.
Poorly controlled diabetes: Diabetics have an increased synthesis of TG-carrying VLDL and a decreased catabolism of the same. Therefore TG blood levels increase.
Nephrotic syndrome: The loss of proteins diminishes the plasma oncotic pressures. This appears to stimulate hepatic lipoprotein synthesis of VLDL and LDL. Also, lipoprotein disposal is possibly diminished.
Chronic renal failure: Insulin levels are high in these patients, because insulin is excreted by the kidney. Insulin increases lipogenesis and causes TG levels to increase. Also, these patients have a deficiency in lipoprotein lipase that clears the blood of TG.
 Decreased Levels
Decreased Levels
Malabsorption syndrome: These patients have a malabsorption of fat from the diet. As TG is the major component of dietary fat, TG levels can be expected to fall in light of poor gastrointestinal (GI) absorption.
Abetalipoproteinemia: Not only do these patients have a malabsorption of fat, but they also have a defective synthesis of apoprotein B (TG-carrying lipoproteins). TG blood levels are low.
Malnutrition: These patients have diminished fat in the diet. As TG is the major component of dietary fat, TG levels can be expected to fall.
Hyperthyroidism: The catabolism of VLDL, the main TG-carrying lipoprotein, is increased. Therefore, TG blood levels diminish.
Related Tests
Cholesterol (p. 154). This is a measure of total cholesterol in the blood. It is a part of the lipid profile.
Lipoprotein (HDL, VLDL, and LDL) (p. 342). These proteins play an important role in the transport of lipids in the bloodstream. They, too, have been used in the assessment of risk for coronary heart disease.
Triiodothyronine (Total T3 Radioimmunoassay [T3 by RIA], Free T3)
Normal Findings
| 1-3 days | 100-740 ng/dL |
| 1-11 months | 105-245 ng/dL |
| 1-5 years | 105-270 ng/dL |
| 6-10 years | 95-240 ng/dL |
| 11-15 years | 80-215 ng/dL |
| 16-20 years | 80-210 ng/dL |
| 20-50 years | 70-205 ng/dL or 1.2-3.4 nmol/L (SI units) |
| >50 years | 40-180 ng/dL or 0.6-2.8 nmol/L (SI units) |
Indications
T3 is used to evaluate thyroid function. It is used primarily to diagnose hyperthyroidism. It is also used to monitor thyroid replacement and suppressive therapy.
Test Explanation
Thyroid hormones are produced when tyrosine incorporates organic iodine to form a monoiodotyrosine. This complex picks up another iodine and becomes diiodotyrosine. Two diiodotyrosines combine to form tetraiodothyronine (also called T4 thyroid hormone). If a diiodotyrosine combines with a monoiodotyrosine, triiodothyronine (also called T3 thyroid hormone) is formed. A large proportion of T3 is formed in the liver by conversion of T4 to T3. As with the T4 test, the serum T3 test is an accurate indicator of thyroid function. T3 is less stable than T4 because it is much less tightly bound to serum proteins than T4. Only about 7% to 10% of thyroid hormone is composed of T3. And 70% of that T3 is bound to proteins (thyroxine-binding globulin [TBG] and albumin). Only minute quantities are unbound or “free.” It is the free T3 that is metabolically active. Furthermore, measurement of free T3 is not subject to the effects that alterations of serum proteins have on the total T3, which is described in this test. This test measures the total bound and unbound (free) T3. Generally, when the T3 level is below normal, the patient is in a hypothyroid state.
Other severe non-thyroid diseases can decrease T3 levels by diminishing the conversion of T4 to T3 in the liver. This makes T3 levels less useful in indicating hypothyroid states. Furthermore, there is considerable overlap between hypothyroid states and normal thyroid function. Because of this, T3 levels are used primarily to assist in the diagnosis of hyperthyroid states. An elevated T3 indicates hyperthyroidism, especially when T4 is also elevated. In a rare form of hyperthyroidism called “T3 toxicosis,” T4 is normal and T3 is elevated.
In the hypothalamus, thyrotropin-releasing hormone (TRH) is secreted. This stimulates the anterior pituitary to secrete thyrotropin (thyroid-stimulating hormone [TSH]). TSH stimulates the thyroid to secrete thyroid hormone. The increased levels of T3 and T4 inhibit further production of TRH.
This test is performed by direct dialysis extraction of both bound T3 and free T3 and is measured by RIA. This test is not the same as the T3 uptake test and should not be confused with it.
Interfering Factors
• Radioisotope administration before the test may alter the results, if this test is performed by RIA methods.
• Total T3 values are increased in pregnancy, because serum proteins are increased at that time. Free T3, however, is not affected by protein levels.
![]() Drugs that may cause increased levels include estrogen, methadone, and oral contraceptives.
Drugs that may cause increased levels include estrogen, methadone, and oral contraceptives.
![]() Drugs that may cause decreased levels include anabolic steroids, androgens, phenytoin (Dilantin), propranolol (Inderal), reserpine, and salicylates (high dose).
Drugs that may cause decreased levels include anabolic steroids, androgens, phenytoin (Dilantin), propranolol (Inderal), reserpine, and salicylates (high dose).
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Primary hyperthyroid states (e.g., Graves disease, Plummer disease, toxic thyroid adenoma): The thyroid produces increased T3 despite lack of TSH stimulation.
Acute thyroiditis: The thyroid secretes increased T3 during the acute inflammatory stages of thyroiditis (e.g., Hashimoto thyroiditis). However, in the latter stages the thyroid may become burned out and the patient may develop hypothyroidism.
Factitious hyperthyroidism: Patients who self-administer T3 will have elevated levels. Many patients believe they will feel more energetic or will lose weight faster if they take T3.
Struma ovarii: Ectopic thyroid tissue in the ovary or anywhere can produce excess T3.
TBG increase (e.g., as occurs in pregnancy, hepatitis, congenital hyperproteinemia): Because T3 assay measures total bound and unbound T3, any condition associated with elevated TBG will cause elevation of T3. Free T3 will not be elevated, however.
 Decreased Levels
Decreased Levels
Hypothyroid states (e.g., cretinism, surgical ablation, myxedema): The thyroid in these diseases cannot produce an adequate amount of T3 despite the stimulation provided.
Pituitary insufficiency: The pituitary produces an insufficient amount of thyrotropin. As a result, the thyroid is not stimulated to produce T3.
Hypothalamic failure: The hypothalamus produces an insufficient amount of TRH. As a result, the pituitary does not produce thyrotropin, and the thyroid is not stimulated to produce T3.
Protein malnutrition and other protein-depleted states (e.g., nephrotic syndrome): With a reduced protein source, TBG and albumin decrease. Because the T3 assay measures hormones bound to these proteins, T3 can be expected to be reduced. Free T3 levels will be unaffected by serum protein changes.
Iodine insufficiency: Iodine is the basic raw material for T3. Without iodine, T4 cannot be produced. With the introduction of iodide in most table salts, iodine insufficiency has become rare in the United States.
Nonthyroid illnesses (e.g., renal failure, Cushing disease, cirrhosis, surgery, advanced cancer): The pathophysiology of these observations is not well known. It may be, in part, because of a depletion of thyroxine-binding proteins, which is associated with severe medical illnesses. T3 is more significantly affected by these diseases than is T4.
Hepatic diseases: Because a large proportion of T3 is made by conversion of T4 in the liver, severe liver dysfunction may affect T3 levels. Often, however, other peripheral tissues take over T3 synthesis by T4 conversion.
Related Tests
Long-Acting Thyroid Stimulator (LATS) (p. 491). This and other thyroid-stimulating immunoglobulins are used to support the diagnosis of Graves disease, especially when the diagnosis is complex.
Thyrotropin-Releasing Hormone Stimulation Test (p. 492). This test assists in the evaluation of patients with hyperthyroidism and hypothyroidism. It is especially helpful in the differential diagnosis of hypothyroidism.
Thyroid-Stimulating Hormone (p. 486). This test is used to diagnose primary hypothyroidism and to differentiate it from secondary (pituitary) and tertiary (hypothalamus) hypothyroidism.
Thyroid-Stimulating Hormone (TSH) Stimulation (p. 489). This test is also used to differentiate primary and secondary (and tertiary) hypothyroidism.
Thyroxine-Binding Globulin (p. 495). This is a measure of TBG, the major thyroid hormone protein carrier. It is used in the evaluation of patients who have abnormal total T4 and T3 levels. When performed concurrently with a T4/T3 test, the T4 and T3 levels can be more easily interpreted.
Thyroxine, Total (p. 497). This is one of the first tests done for assessing thyroid function. It is used to diagnose thyroid function and to monitor replacement and suppressive therapy.
Thyroxine, Free (p. 497). The FT4 is used to evaluate thyroid function in patients who may have protein abnormalities that could affect total T4 levels. It is used to diagnose thyroid function and to monitor replacement and suppressive therapy.
Antithyroglobulin Antibody (p. 102). This test is primarily used for the differential diagnosis of thyroid diseases, such as Hashimoto thyroiditis and chronic lymphocytic thyroiditis (in children).
Troponins (Cardiac-Specific Troponin T [cTnT], Cardiac-Specific Troponin I [cTnI])
Indications
This test is performed on patients with chest pain to determine if the pain is caused by cardiac ischemia. It is a specific indicator of cardiac muscle injury. It is also helpful in predicting the possibility of future cardiac events.
Test Explanation
Cardiac troponins are biochemical markers for cardiac disease. This test is used to assist in the evaluation of patients with suspected acute coronary ischemic syndromes. In addition to improving the diagnosis of acute ischemic disorders, troponins are also valuable for early risk stratification in patients with unstable angina. They can be used to predict the likelihood of future cardiac events.
Troponins are proteins that exist in skeletal and cardiac muscle that regulate the calcium-dependent interaction of myosin with actin for the muscle contractile apparatus. Cardiac troponins can be separated from skeletal troponins by the use of monoclonal antibodies or enzyme-linked immunosorbent assay (ELISA). There are two cardiac-specific troponins: cardiac troponin T (cTnT), and cardiac troponin I (cTnI).
Because of their extraordinarily high specificity for myocardial cell injury, cardiac troponins are very helpful in the evaluation of patients with chest pain. Their use is similar to that of creatine phosphokinase MB (CPK-MB) (see p. 186). However, there are several advantages that cardiac troponins have over CPK-MB. Cardiac troponins are more specific for cardiac muscle injury. CPK-MB can be elevated with severe skeletal muscle injury, with brain or lung injury, or in renal failure. Cardiac troponins will nearly always be normal in noncardiac muscle diseases. Cardiac troponins become elevated sooner and remain elevated longer than CPK-MB. This expands the time window of opportunity for diagnosis and thrombolytic treatment of myocardial injury. Finally, cTnT and cTnI are more sensitive to muscle injury than CPK-MB. That is most important in evaluating patients with chest pain.
Cardiac troponins become elevated as early as 2-3 hours after myocardial injury. Typically 2-3 sets of troponins over the course of a day are required to indicate myocardial infarction. Levels of cTnI may remain elevated for 7 to 10 days after myocardial infarction, and cTnT levels may remain elevated for up to 10 to 14 days. Measurement of these troponins is preferable to measurement of LDH (see p. 329) and its isoenzymes in patients who seek medical attention more than 24 to 48 hours after the onset of symptoms. However, if reinfarction is considered, troponins are not helpful because they could be elevated just from the first ischemic event. Each cardiac monitor has its specific use depending on the time from onset of chest pain to the time of presentation to the hospital.
Troponins can be detected by monoclonal antibody immunoassay; by ELISA; and most recently, by monoclonal “sandwich” antibody qualitative testing. The test results using the first two laboratory techniques listed are available after about 2 hours. The “sandwich” technique is performed at the bedside in about 20 minutes and is read visually much like a glucometer. This fast turnaround time for this blood test is extremely useful. The earlier myocardial injury is detected, the more rapidly treatment directed toward revascularization can begin. The earlier revascularization occurs, the less myocardial muscle is injured.
Cardiac troponins are used in the following cardiac clinical situations:
1. Evaluation of patient with unstable angina. These patients can be separated into two groups based on cardiac troponins. If cardiac troponin levels are normal, no myocardial injury has occurred, and there will be no lasting cardiac dysfunction. If cardiac troponin levels are elevated, muscle injury has occurred. Thrombolytic therapy may be indicated because this latter group is at great risk for a subsequent cardiac event (infarction or sudden death).
2. Detection of reperfusion associated with coronary recanalization. A “washout” or second peak of cardiac troponin levels accurately indicates reperfusion by way of recanalization or coronary angioplasty.
3. Estimation of MI size. Late (4 weeks) cardiac troponin levels are inversely related to left ventricular ejection fraction. These late elevations in cardiac troponins are related to degradation of the contractile apparatus.
4. Detection of perioperative MI. The use of CPK-MB determinations in the diagnosis of MI after surgery is difficult because of the frequent increase of this enzyme associated with skeletal muscle injury during surgery. Cardiac troponins are not affected by skeletal muscle injury.
5. Evaluation of the severity of pulmonary emboli. Elevated levels may indicate more severe disease and the need for thrombolytic therapy.
6. Congestive heart failure—persistently elevated tropinins indicate continued ventricular strain.
Elevations of troponin T do not in and of themselves indicate the presence of an ischemic mechanism. Many other disease states are associated with elevations of troponin T via mechanisms different from those that cause injury in patients with acute coronary syndromes. These include cardiac trauma (e.g., contusion ablation or pacing), congestive heart failure, hypertension, hypotension (often with arrhythmias), pulmonary embolism, renal failure, and myocarditis.
Procedure and Patient Care
During
• Collect a venous blood sample in a yellow-top (serum separator) tube. This is usually done initially and 12 hours later followed by daily testing for 3 to 5 days and possibly weekly for 5 to 6 weeks.
• Rotate the venipuncture sites.
• Record the exact time and date of venipuncture on each laboratory slip. This aids in the interpretation of the temporal pattern of enzyme elevations.
• If a qualitative immunoassay is to be done at the bedside, whole blood is obtained in a micropipette and placed in the sample well of the testing device. A red or purple color in the “read” zone indicates that 0.2 ng/mL or more cardiac troponin is present in the patient's blood.
Related Tests
Creatine Phosphokinase MB (p. 186). Elevation of CPK-MB on this blood test is closely linked to myocardial muscle. It is elevated early in myocardial injury. Its usefulness is limited in patients who have had chest pain for more than 24 hours.
Myoglobin (p. 365). This protein is a nonspecific indicator of cardiac disease. However, it is also elevated with skeletal muscle disease or trauma.
Electrocardiography (p. 544). This is the electrodiagnostic test most commonly used to detect myocardial injury and infarction.
Urea Nitrogen, Blood (Blood Urea Nitrogen [BUN], Serum Urea Nitrogen)
Indications
BUN is an indirect and rough measurement of renal function and glomerular filtration rate (if normal liver function exists). It is also a measurement of liver function. It is performed on patients undergoing routine laboratory testing. It is usually performed as a part of a multiphasic automated testing process.
Test Explanation
The BUN measures the amount of urea nitrogen in the blood. Urea is formed in the liver as the end product of protein metabolism and digestion. During ingestion, protein is broken down into amino acids. In the liver these amino acids are catabolized and free ammonia is formed. The ammonia molecules are combined to form urea, which is then deposited in the blood and transported to the kidneys for excretion. Therefore the BUN is directly related to the metabolic function of the liver and the excretory function of the kidney. It serves as an index of the function of these organs. Patients who have elevated BUN levels are said to have azotemia or be azotemic.
Nearly all renal diseases cause an inadequate excretion of urea, which causes the blood concentration to rise above normal. If the disease is unilateral, however, the unaffected kidney can compensate for the diseased kidney and the BUN may not become elevated. The BUN also increases in conditions other than primary renal disease. Prerenal azotemia refers to elevation of the BUN as a result of pathologic conditions that affect urea nitrogen accumulation before it gets to the kidney. Examples of prerenal azotemia include shock, dehydration, congestive heart failure, and excessive protein catabolism. Another example of prerenal azotemia is gastrointestinal bleeding that causes variable and sometimes significant blood in the intestinal tract. The proteins in the blood and blood cells are digested to urea. As the marked increase in intestinal urea is absorbed, the BUN can be expected to increase, sometimes significantly. Postrenal azotemia refers to pathologic conditions that affect urea nitrogen accumulation after it gets to the kidney. Examples of this include ureteral and urethral obstruction.
Finally, the synthesis of urea depends on the liver. Patients with severe primary liver disease will have a decreased BUN. With combined liver and renal disease (as in hepatorenal syndrome), the BUN can be normal because poor hepatic functioning results in decreased formation of urea and is not an indicator that renal excretory function is adequate.
The BUN is interpreted in conjunction with the creatinine test. These tests are referred to as “renal function studies.” The BUN/creatinine ratio is a good measurement of kidney and liver function. The normal adult range is 6 to 25, with 15.5 being the optimal value.
Interfering Factors
• Changes in protein intake may affect BUN levels. Low-protein diets will decrease BUN if caloric intake is maintained with carbohydrates. High-protein diets or alimentary tube feeding is associated with elevated BUN levels.
• To some degree, muscle mass determines BUN levels. Women and children tend to have lower BUN levels than men.
• Advanced pregnancy may cause increased levels as a result of high protein metabolism.
• Gastrointestinal bleeding can cause increased BUN levels.
• Overhydration and underhydration will affect levels. Overhydrated patients tend to dilute the BUN and have lower levels. Dehydrated patients tend to concentrate BUN and have higher levels.
![]() Drugs that may cause increased BUN levels include allopurinol, aminoglycosides, cephalosporins, chloral hydrate, cisplatin, furosemide, guanethidine, indomethacin, methotrexate, methyldopa, nephrotoxic drugs (e.g., aspirin, amphotericin B, bacitracin, carbamazepine, colistin, gentamicin, methicillin, neomycin, penicillamine, polymyxin B, probenecid, vancomycin), propranolol, rifampin, spironolactone, tetracyclines, thiazide diuretics, and triamterene.
Drugs that may cause increased BUN levels include allopurinol, aminoglycosides, cephalosporins, chloral hydrate, cisplatin, furosemide, guanethidine, indomethacin, methotrexate, methyldopa, nephrotoxic drugs (e.g., aspirin, amphotericin B, bacitracin, carbamazepine, colistin, gentamicin, methicillin, neomycin, penicillamine, polymyxin B, probenecid, vancomycin), propranolol, rifampin, spironolactone, tetracyclines, thiazide diuretics, and triamterene.
![]() Drugs that may cause decreased levels include chloramphenicol and streptomycin.
Drugs that may cause decreased levels include chloramphenicol and streptomycin.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Prerenal Causes
With reduced blood volume, renal blood flow is diminished. Therefore renal excretion of BUN is decreased and BUN levels rise.
With reduced cardiac function, renal blood flow is diminished. Therefore renal excretion of BUN is decreased and BUN levels rise.
Excessive protein ingestion (alimentary tube feeding):
Blood or feeding supplements overload the gut with protein. Urea is formed at a higher rate and BUN accumulates.
As protein is broken down to amino acids at an accelerated rate, urea is formed at a higher rate and BUN accumulates.
Sepsis: For a host of reasons, renal blood flow and primary renal function are reduced. BUN levels rise.
 Decreased Levels
Decreased Levels
Liver failure: BUN is made in the liver from urea. Reduced liver function is associated with reduced BUN levels.
Overhydration because of fluid overload syndrome of inappropriate antidiuretic hormone secretion (SIADH): BUN is diluted by fluid overload.
Negative nitrogen balance (e.g., malnutrition, malabsorption): With protein depletion, urea production is reduced and therefore BUN is reduced.
Pregnancy: Early pregnancy is associated with increased water retention and BUN dilution.
Nephrotic syndrome: This syndrome is associated with protein loss in the urine. With protein depletion, BUN is reduced.
Uric Acid, Blood
Normal Findings
Test Explanation
Uric acid is a nitrogenous compound that is a product of purine (a deoxyribonucleic acid [DNA] building block) catabolism. Uric acid is excreted to a large degree by the kidney and to a smaller degree by the intestinal tract. When uric acid levels are elevated (hyperuricemia), the patient may have gout. Gout is a common metabolic disorder characterized by chronic hyperuricemia, defined as serum urate greater than 6.8 mg/dL (>0.360 mmol/L). At this level, uric acid concentrations exceed the physiologic saturation threshold and monosodium urate crystals may be deposited in the joints and soft tissues. Gout may be managed through urate-lowering therapy with the goal of treatment being uric acid less than 6 mg/dL or less than 0.357 mmol/L.
Causes of hyperuricemia can be overproduction or decreased excretion of uric acid (e.g., kidney failure). Overproduction of uric acid may occur in patients with a catabolic enzyme deficiency that stimulates purine metabolism or in patients with cancer in whom purine and DNA turnover is great. Other causes of hyperuricemia may include alcoholism, leukemia, metastatic cancer, multiple myeloma, hyperlipoproteinemia, diabetes mellitus, renal failure, stress, lead poisoning, and dehydration caused by diuretic therapy. Ketoacids (as occur in diabetic or alcoholic ketoacidosis) may compete with uric acid for tubular excretion and may cause decreased uric acid excretion. Many causes of hyperuricemia are undefined and therefore labeled as idiopathic.
Interfering Factors
• Stress may cause increased uric acid levels.
• X-ray contrast agents increase uric acid excretion and may cause decreased levels.
• High-protein infusion (especially glycine), as in total parental nutrition, may cause increased uric acid, which is a breakdown product of glycine.
![]() Drugs that may cause increased levels include alcohol, ascorbic acid, aspirin (low dose), caffeine, cisplatin, diazoxide, epinephrine, ethambutol, levodopa, methyldopa (Aldomet), nicotinic acid, phenothiazines, and theophylline.
Drugs that may cause increased levels include alcohol, ascorbic acid, aspirin (low dose), caffeine, cisplatin, diazoxide, epinephrine, ethambutol, levodopa, methyldopa (Aldomet), nicotinic acid, phenothiazines, and theophylline.
![]() Drugs that may cause decreased levels include allopurinol, aspirin (high dose), azathioprine (Imuran), clofibrate, corticosteroids, diuretics, estrogens, glucose infusions, guaifenesin, mannitol, probenecid, and warfarin.
Drugs that may cause decreased levels include allopurinol, aspirin (high dose), azathioprine (Imuran), clofibrate, corticosteroids, diuretics, estrogens, glucose infusions, guaifenesin, mannitol, probenecid, and warfarin.
Test Results and Clinical Significance
 Increased Levels (Hyperuricemia)
Increased Levels (Hyperuricemia)
Increased Production of Uric Acid
Increased ingestion of purines: Nucleic acid content is high in such foods as liver, sweetbreads, kidney, and anchovies.
Genetic inborn error in purine metabolism: The most common is an X-linked disorder that causes an increase in an enzyme that produces increased synthesis of purines and therefore an increased amount of purine breakdown products, including uric acid. A second type of genetic error is a deficiency of an enzyme that produces ribonucleic acid (RNA) and DNA from building blocks of those substances. With a deficiency of these enzymes, these building blocks accumulate and are broken down to uric acid, which is then present in high levels in the blood.
Rapid cell destruction associated with rapidly growing cancers (with high cell turnover) and especially after chemotherapy for rapidly growing tumors causes the cells to lyse and spill their nucleic acids into the bloodstream. These free nucleic acids are converted to uric acid in the liver. Levels of uric acid increase.
Hemolysis: The nucleic acid in the RBC and adenosine triphosphate (ATP) in the RBC are spilled into the bloodstream when hemolysis occurs. These free nucleic acids are converted to uric acid in the liver. Levels of uric acid increase.
Rhabdomyolysis (e.g., heavy exercise, burns, crush injury, epileptic seizure, myocardial infarction): Muscle cell lysis leads to excessive muscle ATP (uric acid is a breakdown product of adenosine) in the blood. Uric acid levels increase.
Decreased Excretion of Uric Acid
Idiopathic: This is the most common cause of hyperuricemia. For unknown reasons, these patients have reduced uric acid clearance in the kidney. As a result, uric acid accumulates in the blood. Patients with gout excrete less than half the uric acid in their urine as normal persons.
Chronic renal disease: The pathophysiology regarding why these individuals cannot excrete uric acid in appropriate quantities is not known for sure. It may be because of decreased glomerular filtration only, but other mechanisms seem to be at work here.
Acidosis (ketotic [diabetic or starvation] or lactic): Decreased renal tubular secretion of uric acid in the urine causes reduced excretion of uric acid. Furthermore, ketoacids (as occur in diabetic or alcoholic ketoacidosis) may compete with uric acid for tubular excretion and may cause decreased uric acid excretion. Uric acid levels increase in the blood.
Alcoholism: Alcohol consumption causes accelerated breakdown of ATP in the liver, which increases uric acid production. The chronic acidosis from excessive alcohol ingestion decreases renal tubular secretion of uric acid into the urine. Both lead to hyperuricemia.
Shock or chronic blood volume depletion states: The increased tubular reabsorption of water and electrolytes causes increased tubular reabsorption of uric acid.
 Decreased Levels
Decreased Levels
Related Test
Uric Acid, Urine (p. 954). This test is used to evaluate uric acid levels in the urine and is used in the evaluation of patients with nephrolithiasis.
Uroporphyrinogen-1-Synthase
Indications
This test is used to identify persons at risk for porphyria. It is also used to diagnose porphyria in the acute and latent stages.
Test Explanation
Porphyria is a group of genetic disorders characterized by an accumulation of porphyrin products in the liver or RBC. Liver porphyrias are much more common. Symptoms of liver porphyrias include abdominal pain, neuromuscular signs and symptoms, constipation, and occasionally psychotic behavior. This group of disorders results from enzymatic deficiencies in the synthesis of heme (a portion of hemoglobin). Acute intermittent porphyria (AIP) is the most common form of liver porphyria; this is caused by a deficiency in uroporphyrinogen-1-synthase (also called porphobilinogen deaminase). This enzyme is necessary for erythroid cells to make heme.
Most patients with AIP have no symptoms (latent phase) until the acute phase is precipitated by surgery, infection, a low-calorie diet, or certain drugs (Box 2-20). The acute phase is highlighted by symptoms of abdominal and muscular pain, nausea, vomiting, hypertension, mental symptoms (anxiety, insomnia, hallucinations, paranoia), sensory loss, and urinary retention. Hemolytic anemia also may occur with these acute attacks. These acute symptoms are associated with increased serum and urine levels of porphyrin precursors (see urine tests for aminolevulinic acid, porphyrins, and porphobilinogens).
This enzyme is significantly reduced during the acute and latent phases of this disorder. It is important to identify this disease process, because acute bouts of porphyria occasionally may be fatal. The acute phase can be avoided by controlling factors that can precipitate the acute symptoms.
Procedure and Patient Care
Related Tests
Delta-Aminolevulinic Acid, Urine (p. 922). This test is used to diagnose porphyria. It is also used in the evaluation of children with subclinical lead poisoning.
Porphyrins and Porphobilinogens, Urine (p. 940). This is a quantitative measurement of porphyrins and porphobilinogen in the urine. This test helps define the porphyrin pattern that can classify the type of porphyria.
Vitamin B12 and Methylmalonic Acid (MMA)
Indications
This test measures the amount of vitamin B12 (Cyanocobalamin) in the blood. It is used to identify the cause of megaloblastic anemia and to evaluate malnourished patients.
Test Explanation
Vitamin B12 is necessary for conversion of the inactive form of folate to the active form. This reaction is vital for the synthesis of nucleic acids and amino acids. This is most notable in the formation and function of red blood cells (RBCs). Vitamin B12 deficiency, like folic acid deficiency, causes anemia. The RBCs formed in light of these deficiencies become large megaloblastic RBCs. These RBCs cannot conform to the size of small capillaries. Instead they fracture and hemolyze. The shortened life span ultimately leads to anemia. RBCs are not the only blood cells affected. Other marrow cells are also affected—causing, for example, giant segmented neutrophils and large nucleated platelets. It may take 6 to 18 months of vitamin B12 depletion before anemia develops.
Meats, eggs, and dairy products are the main source of vitamin B12. In the stomach, gastric acid detaches vitamin B12 from its binding proteins. Intrinsic factor (IF), necessary for vitamin B12 absorption in the small intestine, is made in the stomach mucosa. Without IF, vitamin B12 cannot be absorbed. Deficiency of IF is the most common cause of vitamin B12 deficiency (pernicious anemia [PA]). The next most common cause of vitamin B12 deficiency is lack of gastric acid to separate the ingested vitamin B12 from its binding proteins. A third cause of vitamin B12 deficiency is malabsorption caused by diseases of the small terminal ileum.
Serum B12 is a measurement of recent B12 ingestion. More prolonged B12 deficiency is better and more easily measured by urinary methylmalonic acid (MMA) measurement. Elevated serum MMA levels and urinary excretion of MMA are direct measures of tissue vitamin B12 activity. The active form of B12 is essential in the intracellular conversion of L-methylmalonyl coenzyme A (MMA CoA) to succinyl CoA. Without B12, MMA CoA metabolism is diverted to make large quantities of MMA. MMA is then excreted by the kidneys. MMA testing is the most sensitive test for vitamin B12 deficiency.
With the exception of vitamin D, most other vitamins are not commonly measured (Box 2-21).
Test Results and Clinical Significance
 Decreased Levels
Decreased Levels
Pernicious anemia: Intrinsic factor, necessary for vitamin B12 absorption, is deficient.
Malabsorption syndromes (e.g., inflammatory bowel disease, sprue, Crohn disease): Absorption of vitamin B12 is inadequate.
Intestinal worm infestation: Competition for vitamin B12 in the gut leaves very little vitamin B12 for absorption.
Intrinsic factor, necessary for vitamin B12 absorption, is deficient, because the mucosal gastric cells necessary for production of IF are absent.
Resection of terminal ileum: Vitamin B12 is absorbed at the terminal portion of the ileum. Without that piece of intestine, vitamin B12 cannot be absorbed.
Achlorhydria: Gastric acid is necessary to separate vitamin B12 from binding proteins. Without gastric acid, vitamin B12 stays bound and cannot be absorbed from the intestine.
Pregnancy: Vitamin B12 deficiency in pregnancy is probably caused by a combination of inadequate intake and increased demand placed by the fetus on the maternal source of folic acid.
Related Tests
Folic Acid (p. 242). This is a measurement of serum folic acid level. Folic acid should always be determined when vitamin B12 levels are measured. The clinical symptoms of either deficiency are the same.
Complete Blood Cell Count (CBC) (p. 174). This is performed routinely and can identify megaloblastic anemia.
Vitamin D (25-Hydroxy Vitamin D2 and D3; 1,25-Dihydroxyvitamin D [1,25(OH)2D])
Indications
Vitamin D levels are used to ensure that postmenopausal women have adequate vitamin D levels to absorb dietary calcium. Because of the increased number of research studies investigating the role of vitamin D in osteoporosis and cancer prevention, more and more patients are having this blood test.
Test Explanation
Vitamin D is a fat-soluble vitamin. The two major forms of vitamin D are vitamin D2 (or ergocalciferol) and vitamin D3 (or cholecalciferol). The term vitamin D also refers to the “hydroxy-” metabolites of these substances. Vitamin D2 is provided by dietary sources. Because only fish is naturally rich in vitamin D, most of the vitamin D2 intake in the industrialized world is from fortified products including milk, soy milk, and breakfast cereals or supplements.
Vitamin D3 is produced in skin exposed to sunlight, specifically ultraviolet B (UVB) radiation. In this scenario, 7-dehydrocholesterol reacts with UVB ultraviolet light at wavelengths between 270 to 300 nm to produce vitamin D3. These wavelengths are present in sunlight at sea level when the UV index is greater than 3. These wavelengths occur on a daily basis within the tropics, daily during the spring and summer seasons in temperate regions, and almost never within the arctic circles. Adequate amounts of vitamin D3 can be made in the skin after only 10 to 15 minutes of sun exposure at least 2 times per week to the face, arms, hands, or back without sunscreen. Melanin functions as a light filter in the skin. Individuals with higher skin melanin content require more time in sunlight to produce the same amount of vitamin D as individuals with lower melanin content.
Once vitamin D is produced in the skin or consumed in food, it is converted in the liver and kidney to form 1,25-dihydroxyvitamin D (1,25[OH]2D), the physiologically active form of vitamin D. Following this conversion, the hormonally active form of vitamin D is released into the circulation. After binding to a carrier protein in the plasma, vitamin D–binding protein (VDBP), it is transported to various target organs. The hormonally active form of vitamin D mediates its biologic effects by binding to the vitamin D receptor (VDR), which is principally located in the nuclei of target cells. The binding of D3 to the VDR allows the VDR to act as a transcription factor that modulates the gene expression of transport proteins (such as TRPV6 and calbindin), which encourage calcium absorption in the intestine. VDR activation in the intestine, bone, kidney, and parathyroid gland cells leads to the maintenance of calcium and phosphorus levels in the blood.
Vitamin D regulates the calcium and phosphorus levels in the blood by promoting their absorption from food in the intestines, and by promoting reabsorption of calcium in the kidneys. This enables normal mineralization of bone needed for bone growth and bone remodeling.
Vitamin D inhibits parathyroid hormone secretion from the parathyroid gland. Vitamin D promotes the immune system by increasing phagocytosis, antitumor activity, and other immunomodulatory functions.
Vitamin D deficiency can result from inadequate dietary intake, inadequate sunlight exposure, malabsorption syndromes, liver or kidney disorders, or by a number of metabolic hereditary disorders. Deficiency results in impaired bone mineralization and leads to bone softening diseases (rickets in children and osteomalacia in adults). Vitamin D deficiency may also contribute to the development of osteoporosis.
VDR is thought to be involved in cell proliferation/apoptosis and cell differentiation. This may have some influence on the recent observations that vitamin D deficiencies are associated with cancers in the colon, breast, and pancreas. Several recent reports indicate a beneficial correlation between vitamin D intake and prevention of cancer. Vitamin D deficiency is associated with an increase in high blood pressure and cardiovascular risk. Vitamin D also affects the immune system through VDR expressed in monocytes and activated T and B cells.
Vitamin D levels can be measured in the blood. Usually 25 hydroxy D2 and D3 are measured and added to obtain the total 25 hydroxy D level. Therapy is based on the measurement of total hydroxy D levels. Levels below 20 ng/mL indicate a vitamin D deficiency. D levels between 20 and 30 ng/mL suggest insufficiency. Optimal levels are greater than 30 ng/mL (Table 2-47). Dietary Guidelines for Americans recommend that older adults, people with dark skin, and those exposed to insufficient ultraviolet radiation (i.e., sunlight) consume extra vitamin D from vitamin D–fortified foods (such as milk) and/or supplements. Fish liver oils and eggs are naturally high in vitamin D.
TABLE 2-47
Clinical Features and Associated Vitamin D Blood Levels
| ng/mL | Clinical Features |
| <11 | Associated with vitamin D deficiency and rickets in infants and young children |
| <10-15 | Generally considered inadequate for bone and overall health in healthy individuals |
| ≥30 | Proposed by some as desirable for overall health and disease prevention, although a recent government-sponsored expert panel concluded that insufficient data are available to support these higher levels. |
| Consistently >200 | Considered potentially toxic, leading to hypercalcemia and hyperphosphatemia, although human data are limited. In an animal model, concentrations ≤400 ng/mL (≤1000 nmol/L) demonstrated no toxicity. |
Vitamin D requirements increase with age, while the ability of skin to convert 7-dehydrocholesterol to D3 decreases. At the same time, the ability of the kidneys to convert D2 to its active form also decreases with age, prompting the need for increased D supplementation in elderly individuals (Table 2-48). Others particularly at risk for D deficiency include:
• Breastfed infants because human milk alone does not have adequate D levels
• People with limited sun exposure, such as homebound individuals and people living in northern latitudes (such as New England and Alaska)
• Women who wear long robes and head coverings for religious reasons
• People with occupations that prevent sun exposure
• Individuals with a body mass index (BMI) ≥30 because D2 is trapped in the subcutaneous fat and cannot get into the bloodstream
• Individuals who have a reduced ability to absorb dietary fat because, as a fat-soluble vitamin, vitamin D requires some dietary fat in the gut for absorption
• Patients with liver or renal disease because they cannot convert vitamin D to its active metabolic forms
Vitamin D toxicity can cause nonspecific symptoms such as nausea, vomiting, poor appetite, constipation, weakness, weight loss, confusion, and heart rhythm abnormalities (associated with hypercalcemia). The use of the supplements calcium and vitamin D by postmenopausal women to decrease the risk of osteoporosis has been associated with a 17% increase in the risk of kidney stones.
Interfering factors
![]() Corticosteroid drugs can decrease vitamin D levels by reducing calcium absorption.
Corticosteroid drugs can decrease vitamin D levels by reducing calcium absorption.
![]() The weight-loss drug, orlistat, and the cholesterol-lowering drug, cholestyramine, can decrease vitamin D levels by reducing the absorption of vitamin D and other fat-soluble vitamins.
The weight-loss drug, orlistat, and the cholesterol-lowering drug, cholestyramine, can decrease vitamin D levels by reducing the absorption of vitamin D and other fat-soluble vitamins.
![]() Barbiturates and phenytoin decrease vitamin D levels by increasing hepatic metabolism of vitamin D to inactive compounds.
Barbiturates and phenytoin decrease vitamin D levels by increasing hepatic metabolism of vitamin D to inactive compounds.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Williams syndrome (WS): This is a rare genetic disorder characterized by mild to moderate mental retardation or learning difficulties, a distinctive facial appearance, and a unique personality that combines over-friendliness and high levels of empathy with anxiety. The most significant medical problem associated with WS is cardiovascular disease caused by narrowed arteries. WS is also associated with elevated blood calcium and vitamin D levels in infancy.
Excess dietary supplements: With increased oral ingestion of vitamin D, blood levels can rise to toxic levels.
 Decreased Levels
Decreased Levels
Vitamin D encourages the absorption of calcium from the intestines. Bone matrix formation depends on adequate levels of calcium.
Gastrointestinal malabsorption syndromes: Vitamin D is a fat-soluble vitamin that will not be absorbed in diseases of maldigestion or malabsorption.
Diseases affecting the metabolic function of these organs will inhibit the conversion of vitamin D to its active form, 1,25 dihydroxyvitamin D.
Familial hypophosphatemic rickets (X-linked hypophosphatemic rickets): This is a disease caused by a mutation in the PHEX gene on the X chromosome. These patients experience high levels of phosphaturia that is resistant to vitamin D therapy.
Acute inflammatory disease: Because inflammation leads to the increased conversion of 25 hydroxy D into 1,25 hydroxy D, 25 hydroxy D (total) will be decreased.
Inadequate dietary intake: With decreased oral ingestion of vitamin D, blood levels can fall to insufficient or deficient levels.
Inadequate exposure to sunlight: With decreased exposure to adequate sunlight, endogenous production of vitamin D levels can fall to insufficient or deficient levels.
Related Tests
Calcium (p. 138). This test is used to evaluate parathyroid function and calcium metabolism by measuring the calcium in the blood.
Bone Mineral Density (p. 1002).This test determines bone mineral content and density to diagnosis osteoporosis.
Phosphorus (p. 391). This test assists in the interpretation of studies investigating parathyroid and calcium abnormalities.
West Nile Virus Testing
Indications
Testing for West Nile virus is indicated when the flu like symptoms occur in an area in which the virus exists. In other areas, testing is only performed when the disease has progressed to one of the more complicated syndromes as discussed below.
Test Explanation
West Nile virus (WNV) is a RNA virus of the Flavivirus family. Reservoir hosts include birds (especially crows and jays) and farm animals (particularly horses). The vector is the common household mosquito, which carries the virus from the hosts to humans. WNV is not transmitted from human to human. Before 1999, this disease was mostly limited to the African continent. Now, every state in America has reported cases of the disease. It is most common during peak mosquito season (July through October).
Common symptoms of this infection are flu like and include fever, lethargy, headache, neck/body aches, and a skin rash. This disease can progress to encephalitis, aseptic meningitis, and an atypical form of Guillain Barré acute flaccid paralysis.
Front-line testing measures IgM antibodies to flaviviruses and is not specific to WNV. This antibody is measurable by enzyme-linked immunosorbent assay (ELISA) or indirect immunofluorescent antibody assay about 10 days after symptoms start in nearly all patients. If the front-line test for IgM is positive and the symptoms fulfill the Centers for Disease Control and prevention (CDC) criteria, the diagnosis of WNV can be made and treatment altered. This is especially true if the person lives or has traveled to an area that is known to harbor WNV.
If the rapid front-line testing is positive, confirmatory tests may be carried out (especially in areas in which WNV has not been previously known to exist). This testing is more important for public health officials and researchers. Confirmatory tests may include:
• A second IgM serology on convalescing serum 3 to 4 weeks later. A fourfold rise would be confirmatory.
• Direct detection of WNV RNA by nucleic acid amplification testing (NAT) (Plague Reduction Neutralization test performed by the CDC)
• Detection of IgM West Nile virus antibodies in the cerebrospinal fluid
Unfortunately, the sensitivity of the PCR testing is low; therefore negative PCR testing does not exclude West Nile virus infection. WNV can be transmitted through donated blood or blood components. For that reason, in some centers WNV testing kits for WNV antibodies are routinely performed on all donated blood.
Because arboviruses (viruses transmitted by mosquitoes and ticks) are closely related and exhibit serologic cross-reactivity, sometimes it may be epidemiologically important to attempt to pinpoint the infecting virus by conducting cross-neutralization tests using an appropriate battery of closely related viruses (e.g., California, St. Louis, Eastern equine, and Western equine). WNV diseases can be prevented by applying insect repellent containing DEET to exposed skin and clothing.
Procedure and Patient Care
During
• Blood: Obtain a venous blood sample in a red-top tube.
• CSF: During lumbar puncture (see p. 651), 1 to 2 mL is reserved in a sterile tube until bacteriologic specimens are found to be negative. Then, the reserved specimen is sent out for testing.
White Blood Cell Count and Differential Count (WBC and Differential, Leukocyte Count, Neutrophil Count, Lymphocyte Count, Monocyte Count, Eosinophil Count, Basophil Count)
Indications
The measurement of the total and differential WBC count is a part of all routine laboratory diagnostic evaluations. It is especially helpful in the evaluation of the patient with infection, neoplasm, allergy, or immunosuppression (Box 2-22).
Test Explanation
The WBC count has two components. The first is a count of the total number of WBCs (leukocytes) in 1 mm3 of peripheral venous blood. The other component, the differential count, measures the percentage of each type of leukocyte present in the same specimen. An increase in the percentage of one type of leukocyte means a decrease in the percentage of another. Neutrophils and lymphocytes make up 75% to 90% of the total leukocytes. These leukocyte types can be identified easily by their morphology on a peripheral blood smear (see p. 710) or by automated counters. The total leukocyte count has a wide range of normal values, but many diseases may induce abnormal values.
An increased total WBC count (leukocytosis, WBC count >10,000) usually indicates infection, inflammation, tissue necrosis, or leukemic neoplasia. Trauma or stress, either emotional or physical, may increase the WBC count. In some infections, especially sepsis, the WBC count may be extremely high and reach levels associated with leukemia. This is called a “leukemoid” reaction and quickly resolves as the infection is successfully treated.
A decreased total WBC count (leukopenia; WBC count <4000) occurs in many forms of bone marrow failure (e.g., following antineoplastic chemotherapy or radiation therapy, marrow infiltrative diseases, overwhelming infections, dietary deficiencies, autoimmune diseases).
The major function of WBCs is to fight infection and react against foreign bodies or tissues. Five types of WBCs may easily be identified on a routine blood smear. These cells, in order of frequency, include neutrophils, lymphocytes, monocytes, eosinophils, and basophils. All of these WBCs arise from the same “pluripotent” stem cell within the bone marrow as the RBC (Figure 2-30). Beyond this origin, however, each cell line differentiates separately. Most mature WBCs are then deposited into the circulating blood.
White blood cells are divided into granulocytes and nongranulocytes. Granulocytes include neutrophils, basophils, and eosinophils. Because of their multilobed nuclei neutrophils are sometimes referred to as polymorphonuclear leukocytes (PMNs or “polys”). The normal ranges for absolute counts depend on age, sex, and ethnicity. For example, normal range for absolute neutrophils for adult African American males is 1400 to 7000 cells/microliter.
The most common granulocyte, neutrophils, are produced in 7 to 14 days, and exist in the circulation for only 6 hours. The primary function of the neutrophil is phagocytosis (killing and digestion of bacterial microorganisms). Acute bacterial infections and trauma stimulate neutrophil production, resulting in an increased WBC count. When neutrophil production is significantly stimulated, early immature forms of neutrophils often enter the circulation. These immature forms are called band or stab cells. This occurrence, referred to as a “shift to the left” in WBC production, is indicative of an ongoing acute bacterial infection.
Basophils (also called mast cells) and especially eosinophils are involved in the allergic reaction. They are capable of phagocytosis of antigen-antibody complexes. As the allergic response diminishes, the eosinophil count decreases. Eosinophils and basophils do not respond to bacterial or viral infections. The cytoplasm of basophils contains heparin, histamine, and serotonin. These cells infiltrate the tissue (e.g., hive in the skin) involved in the allergic reaction and serve to further the inflammatory reaction. Parasitic infestations also are capable of stimulating the production of these cells.
Nongranulocytes (mononuclear cells) include lymphocytes and monocytes (the count also includes histiocytes). They have no cytoplasmic granules and have a small, single, rounded nuclei. Lymphocytes are divided into two types: T cells (mature in the thymus) and B cells (mature in the bone marrow). T cells are involved primarily with cellular-type immune reactions, whereas B cells participate in humoral immunity (antibody production). T cells are the killer cells, suppressor cells, and the T4 helper cells (see lymphocyte immunophenotyping on p. 147). The primary function of lymphocytes is to fight chronic bacterial infection and acute viral infections. The differential count does not separate the T and B cells but rather counts the combination of the two.
Monocytes are phagocytic cells capable of fighting bacteria similar to the way neutrophils do. Through phagocytosis, they remove necrotic debris and microorganisms from the blood. The monocytes produce interferon, which is the body's endogenous immunostimulant. Monocytes can be produced more rapidly, however, and can spend a longer time in the circulation than the neutrophils.
The WBC and differential count are routinely measured as part of the complete blood cell count (see p. 174) (Figure 2-31). Serial WBC counts and differential counts have both diagnostic and prognostic value. For example, a persistent increase in the WBC count (and particularly the neutrophils) may indicate worsening of an infectious process (e.g., appendicitis). A reduction in WBC count to the normal range from a previously elevated range indicates resolution of an infection. A dramatic decrease in the WBC count below the normal range may indicate marrow failure. In patients receiving chemotherapy, a reduced WBC count may contraindicate further chemotherapy.

Figure 2-31 Medical technologist conducting microscopic examination of a blood smear after the automated Beckman-Coulter CBC analyzer indicated a population of abnormal white blood cells.
The absolute count is calculated by multiplying the differential count (%) by the total WBC count. For example, the absolute neutrophil count (ANC) is helpful in determining the patient's real risk for infection. It is calculated by multiplying the WBC count by the percent of neutrophils and percent of bands, that is:
If the ANC is below 1000, the patient may need to be placed in protective isolation as he or she could be severely immunocompromised (see Box 2-22, p. 527) and is at great risk for infection.
Interfering Factors
• Eating, physical activity, and stress may cause an increased WBC count and alter the differential values.
• Pregnancy (final month) and labor may be associated with increased WBC levels.
• Patients who have had a splenectomy have a persistent mild to moderate elevation of WBC counts.
• The WBC count tends to be lower in the morning and higher in the late afternoon.
• The WBC count tends to be age related. Normal newborns and infants tend to have higher WBC counts than adults. It is not uncommon for the elderly to fail to respond to infection by the absence of leukocytosis. In fact, the elderly may not develop an increased WBC count even in the face of a severe bacterial infection.
![]() Drugs that may cause increased WBC levels include adrenaline, allopurinol, aspirin, chloroform, epinephrine, heparin, quinine, steroids, and triamterene (Dyrenium).
Drugs that may cause increased WBC levels include adrenaline, allopurinol, aspirin, chloroform, epinephrine, heparin, quinine, steroids, and triamterene (Dyrenium).
![]() Drugs that may cause decreased WBC levels include antibiotics, anticonvulsants, antihistamines, antimetabolites, antithyroid drugs, arsenicals, barbiturates, chemotherapeutic agents, diuretics, and sulfonamides.
Drugs that may cause decreased WBC levels include antibiotics, anticonvulsants, antihistamines, antimetabolites, antithyroid drugs, arsenicals, barbiturates, chemotherapeutic agents, diuretics, and sulfonamides.
Test Results and Clinical Significance
 Increased WBC Count (Leukocytosis)
Increased WBC Count (Leukocytosis)
Infection: WBCs are integral to initiating and maintaining the body's defense mechanism against infection.
Leukemic neoplasia or other myeloproliferative disorders: These neoplastic cells are produced by the marrow and are released into the bloodstream.
Other malignancy: Advanced non-marrow cancers (e.g., lung) are associated with leukocytosis. The pathophysiology of this observation is not defined.
Trauma, stress, or hemorrhage: The WBC count is probably under hormonal influence (e.g., epinephrine). However, the pathophysiology of this observation is not defined.
The pathophysiology of these observations is complex, including the recognition of necrotic or normal tissue as “foreign” so that a WBC response is instituted.
Dehydration: Not only is dehydration a stress that, by itself, increases the WBC count, but also by virtue of hemoconcentration, the WBC count increases.
Thyroid storm: The WBC count is probably influenced by thyroid hormones. Marked increases in these hormones could be associated with an increased WBC count.
 Decreased WBC Count (Leukopenia)
Decreased WBC Count (Leukopenia)
Drug toxicity (e.g., cytotoxic chemotherapy; see also drugs that decrease the WBC count),
Dietary deficiency (e.g., vitamin B12, iron deficiency),
Bone marrow infiltration (e.g., myelofibrosis):
The above are associated with all different forms of bone marrow failure whereby WBC production is reduced.
Autoimmune disease: The pathophysiology of this observation is not known.
Hypersplenism: The spleen more aggressively extracts WBCs from the bloodstream.
Related Tests
Lymphocyte Immunophenotyping (p. 147). This test is used to detect the progressive depletion of CD4 T lymphocytes, which is associated with an increased likelihood of clinical complications from acquired immunodeficiency syndrome (AIDS). Test results can indicate if an AIDS patient is at risk for developing opportunistic infections.
Peripheral Blood Smear (p. 710). This is a direct microscopic analysis of the cellular components of the blood.
D-Xylose Absorption (Xylose Tolerance)
Indications
This test is used to evaluate the absorptive capability of the intestines. It is used in the evaluation of patients with suspected malabsorption.
Test Explanation
D-Xylose is a monosaccharide that is easily absorbed by the normal intestine. In patients with malabsorption, intestinal D-xylose absorption is diminished, and as a result, blood levels and urine excretion will be reduced. D-Xylose is the monosaccharide chosen for the test because it is not metabolized by the body. Serum levels directly reflect intestinal absorption.
This monosaccharide is also used because absorption does not require pancreatic or biliary exocrine function. Its absorption is directly determined by the absorptive function of the small intestine. This test is used to differentiate diarrhea caused by maldigestion (pancreatic/biliary dysfunction) and diarrhea caused by malabsorption (sprue, Whipple disease, Crohn disease). It is also used to quantitate the degree of malabsorption to monitor therapy.
In this test the patient is asked to drink a fluid containing a prescribed amount of D-xylose. Blood and urine levels are subsequently evaluated. Excellent gastrointestinal (GI) absorption is documented by high blood levels and good urine excretion of D-xylose. Poor intestinal absorption is marked by low blood levels and urine excretion.
Interfering Factors
• Patients with abnormal kidney function, because they may not be able to excrete the xylose. The urine measurement for D-xylose should not be performed, and the interpretation should be based on the blood test results only.
![]() Drugs that may affect test results include aspirin, atropine, and indomethacin.
Drugs that may affect test results include aspirin, atropine, and indomethacin.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Instruct the adult patient to fast for 8 hours before testing. Water should be encouraged, however.
Instruct the adult patient to fast for 8 hours before testing. Water should be encouraged, however.
![]() Tell the pediatric patient or the parents that the patient should fast for at least 4 hours before testing.
Tell the pediatric patient or the parents that the patient should fast for at least 4 hours before testing.
During
• Collect a venous blood sample in a red-top tube before the patient ingests the D-xylose.
• Collect a first-voided morning urine specimen and send it to the laboratory.
![]() Ask the patient to take the prescribed dose of D-xylose dissolved in 8 ounces of water. Record the time of ingestion.
Ask the patient to take the prescribed dose of D-xylose dissolved in 8 ounces of water. Record the time of ingestion.
• Calibrate pediatric doses according to the patient's body weight.
• Repeat venipunctures to obtain blood in exactly 2 hours for an adult and 1 hour for a child.
• Collect urine for a designated time, usually 5 hours, in a dark bottle. Refrigerate the urine during the collection period.
• Observe the patient for nausea, vomiting, and diarrhea, which may occur as side effects of D-xylose ingestion.
![]() Instruct the patient to remain in a restful position. Intense physical activity may alter the digestive process and affect the test results.
Instruct the patient to remain in a restful position. Intense physical activity may alter the digestive process and affect the test results.
Test Results and Clinical Significance
 Decreased Levels
Decreased Levels
Malabsorption caused by sprue, lymphatic obstruction, enteropathy (e.g., radiation), Crohn disease, or Whipple disease: The D-xylose is not absorbed in these patients; therefore blood and urine levels are not as normally expected.
Short-bowel syndrome: Because of the lack of absorptive surface, absorption of D-xylose does not occur. Therefore blood and urine levels are not as normally expected.
Related Test
Small Bowel Follow-Through (p. 1064). This is an x-ray test that visualizes the mucosa of the small intestine. Patients with Crohn disease and other malabsorption syndromes may have obvious abnormal findings.
Zinc Protoporphyrin (ZPP)
Test Explanation
ZPP is used in screening for iron deficiency anemia or lead poisoning. It is also used in monitoring the treatment/interventions of chronic lead poisoning. ZPP is found in red blood cells when heme production is inhibited by lead toxicity. Lead prevents iron, but not zinc, from attaching to the protoporphyrin. Or, if there is iron deficiency, instead of incorporating a ferrous ion to form heme, protoporphyrin (the immediate precursor of heme) incorporates a zinc ion, forming ZPP. In addition to lead poisoning and iron deficiency, zinc protoporphyrin levels can be elevated as the result of a number of other conditions (e.g., sickle cell anemia). Because of this lack of specificity, ZPP is not commonly used as a screening test for lead poisoning.
The fluorescent properties of ZPP in intact red cells allow the ZPP/heme molar ratio to be measured quickly, at low cost, and in a small sample volume. However, it is more commonly measured using a hematofluorometer, which is able to measure the ZPP/heme ratio.