Computed Tomography, Chest (Chest CT Scan; Helical/Spiral CT Scan, Chest)
Indications
This test is used to more thoroughly evaluate suspected disease in the chest. Questionable or vague abnormalities on the routine chest x-ray can be more thoroughly evaluated with CT scanning of the chest.
Test Explanation
CT of the chest is a noninvasive yet accurate radiographic procedure for diagnosing and evaluating pathologic conditions such as tumors, nodules, hematomas, parenchymal coin lesions, cysts, abscesses, pleural effusion, and enlarged lymph nodes affecting the lungs and mediastinum. Tumors and cysts of the pleura and fractures of the ribs can also be seen. When an intravenous (IV) contrast material is given, vascular structures can be identified and a diagnosis of aortic or other vascular abnormality can be made. With oral contrast material, the esophagus and upper gastrointestinal (GI) structures can be evaluated for tumor and other conditions. CT provides a cross-sectional view of the chest and is especially useful in detecting small differences in tissue density, demonstrating lesions that cannot be seen with conventional radiography and tomography. The mediastinal structures can be visualized in a manner that cannot be equaled with conventional x-ray films and tomographic scans.
The x-ray image results from using a body scanner (x-ray tube in a circular gantry) to deliver x-rays through the patient's chest at many different angles. The variation in density of each tissue allows for variable penetration of the x-rays. Each density is given a numeric value called a coefficient, which is digitally computed into shades of gray. This is then displayed digitally on a computer monitor as a photograph of the anatomic area sectioned by the x-rays.
The CT scan continuously obtains images as the patient is passed through the gantry. With multidetector CT (MDCT) technology, much more image data can be obtained as the patient is passed through the CT gantry. With the use of multiple collimators (and multiple banks of detectors), large data images can be obtained in a very short period of time. The entire chest can be scanned in less than 30 seconds with one breath hold. The “slices” are very thin (1 to 5 mm). With thin slices and rapid accession, breathing and motion distortion are minimized. This produces faster and more accurate images. This is particularly helpful in scanning uncooperative adults and children.
With this CT study 200 to 500 individual images can be obtained. Volume imaging with three-dimensional (3-D) real-time display of the volume of data allows the interpreter to visualize and analyze the data in three dimensions. 2-D and 3-D reconstructions of data can provide very accurate images of the heart (see p. 1032), lungs, chest wall, pleura, esophagus, great vessels, and soft tissue in a few seconds allowing the radiologists to see these structures from multiple views and directions. Utilizing this technology, virtual bronchoscopy and virtual esophagoscopy will increasingly be used in place of their invasive counterparts.
Spiral CT scan is considered the preferred study to identify pulmonary emboli (CT pulmonary arteriography). It can be performed easily and rapidly. CT scanning of the heart (see p. 1032) is able to identify tiny calcifications in the coronary arteries. This finding is indicative of increased risk for an ischemic event. Pulmonary nodules are particularly well evaluated with this rapid form of CT scanning because breathing misrepresentations are eliminated.
With the use of 3-D volumetric imaging, a 3-D perspective can now be added to the organs or tumors that are imaged. This provides data for virtual angiography.
This procedure is performed by a radiologist in less than 10 minutes. If dye is administered, the procedure time may be doubled because CT scanning is done before and after administration of the contrast dye. The only discomfort associated with this study is from lying still on a hard table and from the peripheral venipuncture. Mild nausea is common when contrast dye is used, and an emesis basin should be readily available. Some patients may experience a salty taste, flushing, and warmth during the dye injection.
Contraindications
• Patients who are allergic to iodinated dye or shellfish
• Patients who are claustrophobic
• Patients who are pregnant, unless the benefits outweigh the risks
• Patients whose vital signs are unstable
• Patients who are very obese (usually over 300 pounds), because the CT table cannot support the weight
Potential Complications
• For potential complications for allergies to iodinated dye, see p. 985.
• Acute renal failure from dye infusion: Adequate hydration beforehand may reduce the likelihood of this complication (see Box 12-2, p. 984).
• Hypoglycemia or acidosis may occur in patients who are taking metformin (Glucophage) and receive iodine dye. The metformin should be held on the day of testing to prevent this complication.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient. Cooperation is necessary because the patient must lie still during the procedure.
Explain the procedure to the patient. Cooperation is necessary because the patient must lie still during the procedure.
• Obtain informed consent if required by the institution.
• For assessment of allergy to iodinated dye, see p. 985.
![]() Show the patient a picture of the CT machine and encourage verbalization of concerns about claustrophobia. Most patients who are mildly claustrophobic can tolerate this study after appropriate premedication with antianxiety drugs.
Show the patient a picture of the CT machine and encourage verbalization of concerns about claustrophobia. Most patients who are mildly claustrophobic can tolerate this study after appropriate premedication with antianxiety drugs.
• Keep the patient NPO for 4 hours before the test in the event that contrast dye is administered.
During
• Note the following procedural steps:
1. The patient is taken to the radiology department and asked to remain motionless in a supine position. Any motion will cause blurring and streaking of the final scan. This problem is eliminated with the use of helical scanning. Data acquisition is so rapid that the entire study can be performed in less than 30 seconds. Motion and breath holding are not a problem.
2. An encircling x-ray camera (body scanner) takes pictures at varying intervals and levels over the chest area. Monitor equipment allows immediate display, and the image is recorded on x-ray film.
3. Very often, IV dye is administered to enhance the chest image, and the x-ray studies are repeated.
After
![]() Encourage patients who received dye injection to increase their fluid intake, because the dye is excreted by the kidneys and causes diuresis.
Encourage patients who received dye injection to increase their fluid intake, because the dye is excreted by the kidneys and causes diuresis.
• Evaluate the patient for delayed reaction to the dye (e.g., dyspnea, rashes, tachycardia, hives). This usually occurs 2 to 6 hours after the test. Treat with antihistamines or steroids.
Test Results and Clinical Significance
Lung
Lung tumor (primary or metastatic): This is evident as soft-tissue masses in the lung fields.
Pneumonia: Increased lucency in the lung field indicates pneumonia or atelectatic lung.
Pleural effusion: Fluid in the chest wall is evident as increased lucency outside the lung fields, particularly in the costophrenic margins.
Chronic obstructive pulmonary disease: Increased lung space is classic for chronic obstructive pulmonary disease (COPD).
Atelectasis: Collapse of pulmonary alveoli is evident as white patches or lines in the lung fields.
Tuberculosis (TB): Usually in the upper lobes, chronic TB and other granulomatous diseases are usually associated with calcification.
Lung abscess: Lung abscess is evident as a lung mass with a hollow (radiolucent) center. Sometimes fungus grows inside the abscess.
Pleuritis: A thickened pleura indicates pleuritis, which is from a viral, bacterial, neoplastic, or other cause.
Chest Wall
Primary tumor masses of the bony thorax and chest wall soft tissue, they are evident as masses arising from those areas of the chest.
Fracture (ribs or thoracic spine): This is usually associated with other chest trauma.
Metastatic tumor to bony thorax: Osteolytic (dark) or osteoblastic (white) nodules can be seen in the bony thorax. Breast, prostate, kidneys, and lungs are among the most common cancers to metastasize to the bones in this region.
Mediastinum
Aortic calcinosis: Evident as white lines indicating the walls of the calcified aorta.
Enlarged lymph nodes: Central-occurring masses in the mediastinum indicate enlarged lymph nodes, usually of a neoplastic etiology.
Dilated aorta: This is indicative of aneurysm. Dissection, if present, is obvious on CT scans of the chest.
Metastatic tumor to mediastinum: Esophageal and upper stomach cancers may metastasize to the mediastinal lymph nodes.
Perforation of esophagus (spontaneous [Boerhaave syndrome] or iatrogenic [following esophageal dilation]): Meglumine diatrizoate (Gastrografin) that was previously ingested will be seen free in the mediastinum.
Related Test
Chest X-Ray (p. 1014). This is a routine part of every thorough evaluation of the cardiopulmonary system. Although not so accurate as CT, it provides a tremendous amount of information easily.
Computed Tomography, Heart (Coronary CT Angiography, Coronary Calcium Score)
Indications
The exact role of CT of the heart has not been clearly delineated. However, it holds great promise in providing information about the patency of the coronary vessels in patients who have chest pain.
Test Explanation
With the developments in low-dose x-ray multidetector CT (MDCT) technology, much data can be obtained about the heart and coronary vessels. This test is being used to help stratify patients according to risks of future cardiac events, instigate preventive medicinal interventions (such as statin drugs), monitor progression of coronary vascular disease and effects of statin drugs, evaluate chest pain, and indicate the need for stress testing or coronary angiography.
MDCT produces fast and accurate images of the heart. With the use of multiple collimators (and multiple banks of detectors—usually 4 to 64), large data images can be obtained in a very short period of time. The entire heart can be scanned in 10 seconds with one breath hold. The “slices” are very thin (1 to 5 mm). With thin slices and rapid accession, breathing and motion distortion are minimized.
With advances in software technology, two- and three-dimensional reconstructions of data can provide very accurate images of the heart and coronary vessels in a few seconds, allowing radiologists to see these structures from multiple directions (Figure 12-15). Furthermore, with shorter scanning times, intravenous contrast effect can be greater while using less contrast volume. The newest MDCT scanners allow routine cardiac gating that synchronizes the scanning with each heartbeat, thereby eliminating further motion distortion.
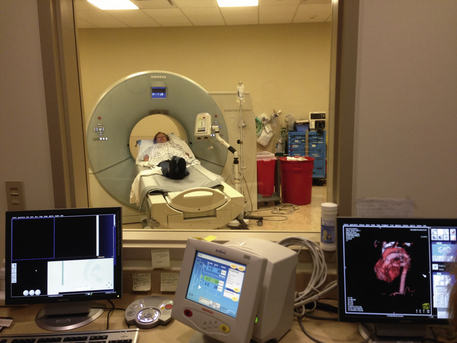
Figure 12-15 CT scan of the heart from the technologist's observer station. Note computer-generated image of heart and great vessels, lower right corner.
Calcified atheromatous plaques can be seen and quantified (calcium score) with the use of MDCT. The assessment of coronary artery calcification has received considerable attention with respect to its potential role in the early detection of subclinical atherosclerosis and in the diagnostic workup of coronary artery disease. Coronary calcium is a surrogate marker for coronary atherosclerotic plaque. In the coronary arteries, calcifications occur almost exclusively in the context of atherosclerotic changes. Within a coronary vessel or larger segment of the vessel, the amount of coronary calcium correlates moderately closely with the extent of atherosclerotic plaque burden. On the other hand, not every serious atherosclerotic coronary plaque is calcified. However, in the vast majority of patients with acute coronary syndromes, coronary calcium can be detected, and the amount of calcium in these patients is substantially greater than in matched control subjects without coronary artery disease.
The Agatston score has most frequently been used to quantify the amount of coronary calcium in CT. The distribution of calcification scores in populations of individuals without known heart disease has been studied extensively. From those data we know that the amount of calcification increases with age. Men develop calcifications about 10 to 15 years earlier than women. Furthermore, in the majority of asymptomatic men over 55 years of age and women over 65 years of age, calcification can be detected. These data have been used to create tables that compare the amount of calcium of an individual to a group of people of similar age and gender (percentiles). See Table 12-4 for categorizing absolute Agatston scores.
It is well established that individuals with Agatston scores above 400 have an increased occurrence of coronary procedures (bypass, stent placement, angioplasty) and events (myocardial infarction [MI] and cardiac death) within 2 to 5 years after the test. Individuals with very high Agatston scores (over 1000) have a 20% chance of suffering an MI or cardiac death within a year. Even among elderly patients (over 70 years), who frequently have calcification, an Agatston score above 400 is associated with a higher risk of death.
Variability of the Agatston score can be high for patients with small amounts of calcium but is lower for higher calcium scores. There is a variability of about 20%. Excessively high calcium scores can inhibit the visualization of the coronary arteries. Therefore, when calcium scores are excessively high, injection of radiopaque dye is not performed and coronary CT cannot be carried out.
MDCT can directly and accurately visualize the coronary artery lumen after intravenous injection of a contrast agent (coronary CT angiography). Regular and low heart rates are a prerequisite for reliable visualization of the coronary arteries. Hence, most centers have proposed the administration of a short-acting beta blocker or a calcium-channel blocker before scanning if the heart rate exceeds 60 to 70 beats/min. The use of sublingual nitroglycerin is also recommended to achieve coronary vasodilatation and maximize image quality.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient. The patient's cooperation is necessary because he or she must lie still during the procedure.
Explain the procedure to the patient. The patient's cooperation is necessary because he or she must lie still during the procedure.
• Obtain informed consent if required by the institution.
• Assess the patient for allergies to iodinated dye or shellfish.
• Assess the patient's vital signs. If the heart rate exceeds protocol levels, administer a rapid-acting beta blocker or ACE inhibitor per protocol orders.
![]() Show the patient a picture of the CT machine and encourage the patient to verbalize concerns regarding claustrophobia. Most patients who are mildly claustrophobic can tolerate this study after appropriate premedication with antianxiety drugs.
Show the patient a picture of the CT machine and encourage the patient to verbalize concerns regarding claustrophobia. Most patients who are mildly claustrophobic can tolerate this study after appropriate premedication with antianxiety drugs.
During
• Note the following procedure for the cardiac CT scan:
1. The patient is taken to the CT department and asked to remain motionless in a supine position because any motion will cause blurring and streaking of the final picture.
2. EKG leads are applied to synchronize the EKG signal to the image data (gating).
3. An encircling x-ray camera (body scanner) takes pictures at varying intervals and levels over the heart while the patient holds his or her breath (for about 10 seconds).
4. A nonenhanced scan is performed first for calcium scoring.
5. If the calcium scoring is below threshold levels of the protocol, intravenous (IV) dye is rapidly administered through a large-bore IV catheter, and the scan is repeated.
6. A fast-acting nitrate (usually nitroglycerin) is administered to maximize coronary dilatation.
• Note that a radiologist or cardiologist performs this procedure in about 20 minutes.
![]() Tell the patient that the discomforts associated with this study include lying still on a hard table and peripheral venipuncture.
Tell the patient that the discomforts associated with this study include lying still on a hard table and peripheral venipuncture.
• Nausea is a common sensation when contrast dye is used. An emesis basin should be readily available.
• Some patients may experience a salty taste, flushing, and warmth during the dye injection.
After
![]() Encourage patients to increase their fluid intake because the dye is excreted by the kidneys and causes diuresis.
Encourage patients to increase their fluid intake because the dye is excreted by the kidneys and causes diuresis.
• See p. 985 for appropriate interventions concerning the care of patients with iodine allergy.
![]() Tell the patient that a headache from the nitroglycerin is not uncommon.
Tell the patient that a headache from the nitroglycerin is not uncommon.
Test Results and Clinical Significance
Coronary vascular congenital anomalies:
The coronary vessels can be visualized completely and any obstruction or anatomic variation is obvious.
Aortic aneurysm or dissection,
These functional abnormalities are obvious by demonstrating anatomic alterations of the normal heart muscle/valvular motion during a cardiac cycle.
Related Tests
Cardiac Catheterization (p. 1008). This test is used to visualize the heart chambers, arteries, and great vessels. It is used most often to evaluate chest pain and to locate the region of coronary occlusion in patients with coronary occlusive disease.
Cardiac Nuclear Scan (p. 791). This test is used to detect myocardial ischemia, infarction, cardiac wall dysfunction, and decreased ejection fraction. It is commonly used as the imaging method portion of cardiac stress testing to detect ischemia.
Cystography (Cystourethrography, Voiding, Cystography, Voiding Cystourethrography)
Indications
Cystography enables radiographic visualization of the bladder. It is useful in patients with hematuria, recurrent urinary tract infections (UTIs), and suspected bladder trauma.
Test Explanation
Filling the bladder with contrast material provides visualization of the bladder for radiographic study. Either fluoroscopic or x-ray films demonstrate bladder filling and collapse after emptying. Filling defects or shadows in the bladder indicate primary bladder tumors. Extrinsic compression or distortion of the bladder is seen with pelvic tumor (e.g., rectal, cervical) or hematoma (secondary to pelvic bone fractures). Extravasation of the dye is seen with traumatic rupture, perforation, and fistula of the bladder. Vesicoureteral reflux (abnormal backflow of urine from bladder to ureters), which can cause persistent or recurrent pyelonephritis, also may be demonstrated during cystography. Although the bladder is visualized during intravenous pyelography (IVP) (p. 1057), primary pathologic bladder conditions are best studied by means of cystography.
A radiologist performs the study in approximately 15 to 30 minutes. This test is moderately uncomfortable if bladder catheterization is required.
Procedure and Patient Care
During
• Note the following procedural steps:
1. The patient is taken to the radiology department and placed in a supine or lithotomy position.
2. Unless a catheter is already present, one is placed.
3. Approximately 300 mL (much less for children, based on weight) of air or radiopaque dye is injected through the catheter into the bladder, and the catheter is clamped.
5. If the patient is able to void, the catheter is removed and the patient is asked to urinate while films are taken of the bladder and urethra (voiding cystourethrogram) (Figure 12-16).
• Ensure that in male patients a lead shield is placed over the testes to prevent irradiation of the gonads.
• The ovaries in female patients cannot be shielded without blocking bladder visualization. Ensure that female patients are not pregnant.
Test Results and Clinical Significance
Bladder tumor: Primary cancers of the bladder are evident as filling defects (radiolucent shadow) in the bladder.
Pelvic tumor or hematoma: Any mass that distorts the pelvic anatomy is seen as external compression of the dye-filled bladder.
Bladder trauma: Laceration or perforation of the bladder is evident by the finding of dye outside the bladder. This is usually best demonstrated on the postvoid film.
Vesicoureteral reflux: Reflux of urine or dye from the bladder into the ureter is obvious with distention of the bladder with dye.
Hysterosalpingography (Uterotubography, Uterosalpingography, Hysterogram)
Indications
This test is part of a workup for infertility. The result can indicate patency or obstruction of the fallopian tubes.
Test Explanation
In hysterosalpingography, the uterine cavity and fallopian tubes are visualized radiographically after the injection of contrast material through the cervix. Uterine tumors, intrauterine adhesions, and developmental anomalies can be seen. Tubal obstruction of the fallopian tubes caused by internal scarring, tumor, infection, or kinking also can be detected. A possible therapeutic effect of this test is that passage of dye through the tubes may clear mucous plugs, straighten kinked tubes, or break up adhesions. This test also may be used to document adequacy of surgical tubal ligation. Its main purpose is in the evaluation of infertility to see if there is any obstruction of the fallopian tubes.
This procedure is performed by a physician in approximately 15 to 30 minutes. The patient may feel occasional, transient menstrual-type cramping and may have shoulder pain caused by subphrenic irritation from the dye as it leaks into the peritoneal cavity.
Contraindications
• Patients with infections of the vagina, cervix, or fallopian tubes, because of risk of extending the infection
• Patients with uterine bleeding, because contrast material may enter the open blood vessels. Further, clots may be pushed out of the uterus and into the fallopian tubes, causing obstruction.
• Patients who are pregnant, because contrast material may induce abortion
Procedure and Patient Care
Before
![]() Explain the procedure to the patient. Ask the patient when she had her last menstrual period. If pregnancy is suspected, the test is not performed.
Explain the procedure to the patient. Ask the patient when she had her last menstrual period. If pregnancy is suspected, the test is not performed.
• Obtain informed consent if required by the institution.
• Assess the patient for allergy to iodine dye or shellfish.
![]() Instruct the patient to take laxatives the night before the test, if ordered.
Instruct the patient to take laxatives the night before the test, if ordered.
• Administer enemas or suppositories on the morning of the test, if ordered.
• Administer sedatives (e.g., midazolam [Versed]) or antispasmodics, if ordered, before the test.
![]() Tell the patient that no food or fluid restrictions are needed.
Tell the patient that no food or fluid restrictions are needed.
During
• Note the following procedural steps:
1. A plain x-ray film of the abdomen is often obtained before the test to ensure that preparation adequately eliminated gastrointestinal gas and feces.
2. After voiding, the patient is placed on the fluoroscopy table in the lithotomy position.
3. A speculum is inserted into the vagina, and the cervix is visualized and cleansed.
4. Contrast material is injected during fluoroscopy, and x-ray films are obtained.
5. More dye is injected so that the entire upper genital tract (uterus and fallopian tubes) can be filled.
6. This test can be considered satisfactorily performed only if the uterus and the tubes are distended to their maximal capacity or fluid flows through the fallopian tubes.
After
![]() Inform the patient that a vaginal discharge (sometimes bloody) may be present for 1 or 2 days after the test. A perineal pad should be worn.
Inform the patient that a vaginal discharge (sometimes bloody) may be present for 1 or 2 days after the test. A perineal pad should be worn.
• Evaluate the patient for delayed reaction to dye (e.g., dyspnea, rash, tachycardia, hives). Treat symptoms with antihistamines or steroids.
![]() Inform the patient that cramping and dizziness may occur after the study.
Inform the patient that cramping and dizziness may occur after the study.
![]() Evaluate the patient for signs and symptoms of infection (e.g., fever, increased pulse rate, pain). Instruct the patient to call her physician and report these symptoms if they occur.
Evaluate the patient for signs and symptoms of infection (e.g., fever, increased pulse rate, pain). Instruct the patient to call her physician and report these symptoms if they occur.
Test Results and Clinical Significance
Uterine tumor (e.g., leiomyoma, cancer) or polyps: Filling defects in the uterus may indicate tumor.
Developmental anomaly of the uterus (e.g., uterus bicornis): The anatomy of the uterus can be well visualized and evaluated.
Intrauterine adhesions: Usually from previous infection, these adhesions within the uterus can cause infertility.
Uterine fistula: Usually traumatic (iatrogenic [e.g., during dilation and curettage]), a fistula is evident as extravasation of dye from the uterus.
Obstruction, kinking, or twisting of the fallopian tubes secondary to adhesions: This is indicated by stenosis or complete obstruction. Fertility is unlikely unless tubal patency is reestablished.
Extrauterine pregnancy: Early tubal pregnancy can be demonstrated with this study, but there are better and easier ways to determine tubal pregnancy (e.g., CT of the pelvis [p. 1020]).
Tumor of the fallopian tubes: Tumors are evident as tubal filling defects.
Kidney, Ureter, and Bladder X-Ray (KUB, Flat Plate of the Abdomen, Plain Film of the Abdomen, Scout Film)
Indications
This screening x-ray strategy is used to rapidly evaluate the abdomen in patients with abdominal pain or trauma. It can demonstrate pathologic conditions of the urinary or GI system.
Test Explanation
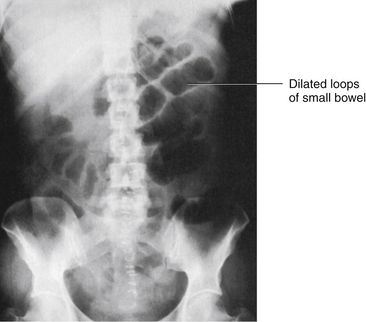
The KUB is an unenhanced image of the abdomen. It is often referred to as a plain film or scout film. The KUB is similar to the supine view on an obstruction series (see p. 1051) and can be performed to demonstrate the size, shape, location, and any malformations of the kidneys and bladder. The KUB can also be used to identify calculi in these organs and in the ureters. This is often one of the first studies done to diagnose other intraabdominal diseases, such as intestinal obstruction, soft-tissue masses, and a ruptured viscus (Figure 12-17). The KUB is useful in detecting abnormal accumulations of gas within the GI tract and identifying ascites. No contrast medium is used for this study.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Tell the patient that no fasting or sedation is required.
Tell the patient that no fasting or sedation is required.
• Schedule this study before any barium studies.
• In male patient the testicles should be shielded with a lead apron to prevent their irradiation.
• In female patients, the ovaries cannot be shielded because of their proximity to the kidneys, ureters, and bladder.
![]() Tell the patient that no discomfort is associated with this study.
Tell the patient that no discomfort is associated with this study.
During
• In the radiology department, the patient is placed in the supine position. X-ray films are obtained of the patient's abdomen (Figure 12-18).
• Note that the KUB is performed by a radiologic technologist in a few minutes, and is interpreted by a radiologist.
Test Results and Clinical Significance
Calculi: A calcified stone in the area of the KUB where the ureters would be is indicative of a ureteral calculi. Nearly 80% of ureteral stones can be seen on KUB.
Abnormal accumulation of bowel gas: Abnormal accumulation of bowel gas can indicate intestinal obstruction or paralytic ileus.
Ascites: The classic “ground glass” appearance of the entire abdomen on the KUB films indicates peritoneal effusion.
Soft-tissue masses: Large soft-tissue masses can be seen surprisingly well on this plain film without use of any contrast material.
Ruptured viscus: Free air (i.e., air outside the bowel but inside the abdomen) is indicative of a perforated viscus.
Congenital anomalies (e.g., location, size, and number of kidneys): Because the kidneys can be well visualized with KUB, anomalies are fairly easily detected.
Organomegaly or bladder distention: An enlarged liver or spleen is seen as a large soft-tissue mass in the right or left upper quadrant, respectively. A large soft-tissue mass in the midline or pelvis is usually a distended bladder.
Related Test
Obstruction Series (p. 1051). This study includes a KUB as part of the series of plain film x-rays obtained to evaluate abdominal pain.
Mammography (Mammogram, Digital Mammography)
Normal Findings
Breast Imaging Reporting and Database System (BI-RADS®)
Category 2: Benign findings noted
Category 3: Probably benign findings: short-term follow-up is suggested
Category 4: Suspicious findings: further evaluation is indicated
Category 5: Cancer is highly suspected
Category 6: Known breast cancer
Category 0: Abnormality noted for which more imaging is recommended
Indications
Mammography enables detection of breast cancers, benign tumors, and cysts before they are even palpable. Mammography can be performed for screening (patients without any breast symptom) or diagnostic (patients with breast symptoms, such as a breast lump, pain, nipple discharge, or asymmetry) purposes.
Screening Mammography Guidelines
There are varying guidelines from multiple organizations regarding screening mammography.
National Institute of Health
• Women age 40 and older should have mammograms every 1 to 2 years.
• Women or men who are at higher-than-average risk of breast cancer should talk with their health care providers about whether to have mammograms before age 40 and how often to have them. Those at higher risk would include women with:
• Personal history of breast cancer
• Family history—A woman's chance of developing breast cancer increases if her mother, sister, and/or daughter have a history of breast cancer (especially if they were diagnosed before age 50).
• Certain breast changes on biopsy—A diagnosis of atypical hyperplasia (a non-cancerous condition in which cells have abnormal features and are increased in number) or lobular carcinoma in situ (LCIS) (abnormal cells found in the lobules of the breast) increases a woman's risk of breast cancer. Women who have had two or more breast biopsies for other benign conditions also have an increased chance of developing breast cancer. This increased risk is a result of the condition that led to the biopsy, and not the biopsy itself.
• Genetic alterations (changes)—Specific alterations in BRCA1, BRCA2 (see p. 1086).
• Radiation therapy (“x-ray therapy”)—Women who had radiation therapy to the chest (including the breasts) before age 30 are at an increased risk of developing breast cancer throughout their lives. This includes women treated for Hodgkin lymphoma.
When to Stop Screening
As long as a woman is in reasonably good health and would be a candidate for treatment, she should continue to be screened with mammography. However, if an individual has an estimated life expectancy of less than 5 to 7 years, severe functional limitations, and/or multiple co-morbidities likely to limit life expectancy, it may be appropriate to consider cessation of screening. Chronologic age alone should not be the reason for the cessation of regular screening. That being said, there is insufficient evidence that mammogram screening is effective for women age 75 and older.
Test Explanation
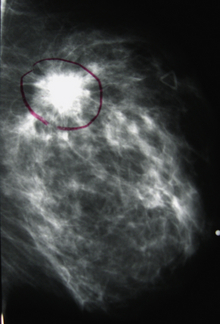
Mammography is an x-ray examination of the breast. Careful interpretation of these x-rays can identify cancers (Figure 12-19). In many cases, breast cancers can be detected before they become palpable. It is believed that early detection of breast cancer may improve patient survival. Radiographic signs of breast cancer include fine, stippled, clustered calcifications (white specks on the breast x-ray films); a poorly defined, spiculated mass; asymmetric density; and skin thickening.
Although mammography is not a substitute for breast biopsy, results are reliable and accurate when interpreted by a skilled radiologist. The detection rate for breast cancer with mammography is greater than 85%. This means that less than 15% of breast cancers are missed at mammography. Cancers that are missed are in areas of the breast that are not well imaged by the x-ray (e.g., the high axillary tail of the breast), are in women with very dense breast tissue, or are too small to identify. Nearly 70% of breast cancers are not palpable and are detected only with mammography. Mammography also can detect other diseases of the breast, such as acute suppurative mastitis, abscess, fibrocystic changes, cysts, benign tumors (e.g., fibroadenoma), and intraglandular lymph nodes.
A woman receives minimal radiation exposure during a mammography (about 0.5 rad per view). Females younger than age 25 are most susceptible to the neoplastic effects of ionizing radiation. Therefore, mammography is rarely recommended in young women. Most mammograms include two views of each breast (in the cranial to caudal dimension and in the medial to lateral dimension). It is important to inform the woman that “callbacks” are not uncommon. If the radiologist sees something that should be more thoroughly evaluated with magnified views, deeper views, or ultrasound, the patient may be “called back” for further testing.
Mammograms can be performed using analog x-rays films or utilizing digital technology (digital mammography). Digital images are viewed on a computer monitor, allowing the radiologist to manipulate the contrast and brightness of the images so as to miss fewer cancers. Portions of the breast image can be magnified. Results of mammography can only suggest a diagnosis of breast cancer. The diagnosis of cancer must be confirmed with microscopic histologic review of a biopsy specimen.
Mammography is performed by a certified radiologic technologist in approximately 10 minutes. The x-ray films are interpreted by an accredited radiologist. Moderate discomfort is associated with mammography. This is caused by the pressure required to compress the breast tissue while the x-ray films are obtained. In patients with tender breasts, this may be painful. The ACR also accredits the mammography machine for quality of picture and accuracy of x-ray dose. The ACR has recommended a standardized method for reporting of mammogram results. This is described under Normal Results.
Mammography can also be used to locate a mammographically identified (i.e., not palpable) lesion for biopsy. One method is preoperative mammogram localization of a previously identified abnormality, followed by open biopsy. For this procedure, the patient is taken to the mammography room. A grid printed on transparent adhesive material is attached to the breast containing the abnormality. Craniocaudal and direct lateral views are obtained. With the grid still in place, the radiologist, using the coordinates on the grid, numbs the skin and places a needle into the abnormal area. A wire is then disengaged through the needle into the breast. Repeated mammograms are obtained. The patient is then taken to the operating room. After appropriate anesthesia, an incision is made along the wire, to the abnormal tissue, which is then removed for biopsy. All localizing wire can be placed in a suspicious area of the breast for biopsy by use of stereotactic images (see the following) or by ultrasound (p. 871).
Nonoperative needle biopsy with a stereotactic biopsy device is the least invasive manner of obtaining tissue from a nonpalpable mammographic abnormality. For this procedure the patient is placed prone on a specialized table. Through a hole in the table, the breast is placed in a mammography machine under the table. The mammogram is connected to a computer that can identify the exact location of the mammographic abnormality (Figure 12-20). The machine positions the biopsy device in alignment with the lesion. When fired, the biopsy equipment is in the center of the lesion, and specimens are obtained for biopsy. No surgery or sutures are required. Only minimal pain is experienced, because a breast in compression has little skin pain sensation.
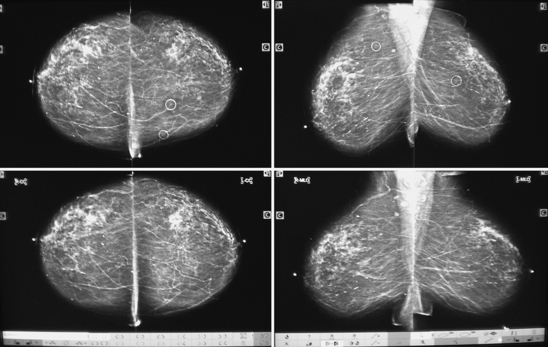
Figure 12-20 The mammography film is placed on the digitizer, and coordinates of the breast lesion are determined and displayed. These coordinates guide the needle to the precise location of the lesion, and the aspirate is drawn for biopsy.
Breast tomography (3-D mammography) using multiple mammogram views through different thicknesses of the breast tissue is rapidly becoming a method of interest for the diagnosis of disease of the breast. Unfortunately, these radiographic techniques are too expensive to provide to a large population of non-symptomatic women in screening.
There are multiple other methods of breast imaging. Mammography, however, is the most accurate testing when considering cost-effectiveness and efficiency. MRI of the breast (p. 1106) is more accurate than mammography, but far more expensive and labor intensive. Because of that, it is not applied as a screening modality for most women. However, MRIs are very helpful in the identification of cancer in dense-breasted women. Breast nuclear scintigraphy is also available for breast imaging. Like the MRI, breast scintigraphy is far more expensive and labor intensive than mammography. Radiation exposure with some radionuclides can be quite high. Diagnostic accuracy in comparison with other forms of breast imaging has yet to be determined.
Interfering Factors
• Talcum powder and antiperspirants give the impression of calcifications within the breast.
• Jewelry worn around the neck can preclude total visualization of the breast.
• Breast augmentation implants can inhibit total visualization of the breast. However, the implants can be displaced so the native breast tissue can be imaged.
• Previous breast surgery can distort mammographic findings.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Inform the patient that some discomfort may be experienced during breast compression. Compression allows better visualization of the breast tissue. Assure the patient that the breast will not be harmed by compression. Premenopausal women with very sensitive breasts can choose to schedule their mammogram 1 to 2 weeks after their menses to reduce any discomfort caused by compression required for the mammogram.
Inform the patient that some discomfort may be experienced during breast compression. Compression allows better visualization of the breast tissue. Assure the patient that the breast will not be harmed by compression. Premenopausal women with very sensitive breasts can choose to schedule their mammogram 1 to 2 weeks after their menses to reduce any discomfort caused by compression required for the mammogram.
![]() Tell the patient that no fasting is required.
Tell the patient that no fasting is required.
![]() Explain to the patient that a minimal radiation dose will be used during the test.
Explain to the patient that a minimal radiation dose will be used during the test.
![]() Instruct the patient to disrobe above the waist and put on an x-ray gown.
Instruct the patient to disrobe above the waist and put on an x-ray gown.
![]() Instruct the patient to report the location of any symptom or lump she may have noted.
Instruct the patient to report the location of any symptom or lump she may have noted.
• Markers will be placed on any skin bump that may be interpreted to an abnormality on the x-ray image.
During
• Note the following procedural steps:
1. The patient is taken to the radiology department and stands in front of a mammogram machine.
2. One breast is placed on the x-ray plate.
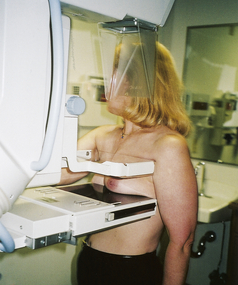
3. The x-ray cone is brought down on top of the breast to compress it gently between the broadened cone and the x-ray plate (Figure 12-21).
4. The x-ray film is exposed, for a craniocaudal view.
5. The x-ray plate is turned about 45 degrees medially and placed on the inner aspect of the breast.
6. The broadened cone is brought in medially and again gently compresses the breast. A mediolateral view is obtained.
7. Occasionally direct lateral (90-degree) or magnified spot views are obtained to more clearly visualize an area of suspicion. Elongated or small cones are applied to the x-ray tube to enhance visualization of a specific area of the breast.
After
![]() Take the opportunity to instruct the patient in breast self-examination.
Take the opportunity to instruct the patient in breast self-examination.
![]() Support the patient in her concerns if additional views are required. It is always frightening if further views are required. Usually these additional views include spot magnified views, which allow the radiologist to better visualize an area of the breast.
Support the patient in her concerns if additional views are required. It is always frightening if further views are required. Usually these additional views include spot magnified views, which allow the radiologist to better visualize an area of the breast.
Test Results and Clinical Significance
Breast cancer: This can be evident as a radiodense (white) stellate or spiculated mass, a cluster of calcifications, or vague asymmetric radiodensity. When cancer invades the skin, the skin will appear thickened. Also, the nipple can appear inverted if a subareolar cancer exists.
Benign tumor (e.g., fibroadenoma): Benign tumors are usually well-rounded masses with discrete borders. Sometimes fibroadenomas can degenerate, and calcifications can develop within. Colloid or medullary cancers can appear similarly well rounded.
Breast cyst: Cysts are seen as well-rounded masses with discrete borders. Ultrasound of the breast demonstrates the cysts to be fluid filled.
Fibrocystic disease: This is the most common breast finding. Nearly every women has some degree of fibrocystic disease. On mammograms, this is seen as a vague asymmetric radiodensity (white). It can also be evident as calcifications.
Related Tests
Ultrasound of the Breast (p. 871). An important adjunct to mammography, ultrasound can differentiate between cystic and solid lesions.
Magnetic Resonance Imaging (MRI) of the Breast (p. 1106). Although a more accurate test for breast cancer, its high rate of false positives makes it too expensive for screening.
Myelography (Myelogram, CT Myelography)
Indications
Myelography provides radiographic visualization of the subarachnoid space of the spinal canal. The cord, nerve roots, and surrounding meninges can be seen. This test is indicated in patients with severe back pain or localized neurologic signs that suggest narrowing of the spinal canal (e.g., herniated lumbar disk).
Test Explanation
By placing radiopaque dye into the subarachnoid space of the spinal canal, the contents of the canal can be fluoroscopically outlined. Cord tumors, meningeal tumors, metastatic spinal tumors, herniated intervertebral disks, and arthritic bone spurs can be readily detected with this study. These lesions appear as spinal canal narrowing or as varying degrees of obstruction to the flow of the dye column within the canal. The entire canal (from lumbar to cervical areas) can be examined. Because this test is usually performed with lumbar puncture (LP; see p. 651), all the potential complications of that procedure exist.
Several different contrast materials can be used for myelography. In general, non-ionic low osmolar radiopaque dyes such as iohexol (Omnipaque) or iopamidol (Isovue) are associated with a significantly lower risk of CNS toxicity than some of the oil-based or heavier radiopaque contrast materials. The water-soluble contrast is absorbed by the blood excreted by the kidneys. Oil-based contrast media stays in the subarachnoid space much longer.
After injection of contrast material into the subarachnoid spinal space, images are obtained. These images can be obtained by simple x-ray images of the spine from multiple directions. More commonly additional images are obtained with computed tomography (CT myelography). MRI of the spine (p. 1106) is the preferred modality to image the spine and its contents. Myelography is more time-consuming and invasive than MRI. Plain film or CT myelography is reserved for those patients who do not have access to an MRI or for equivocal cases.
After the procedure, the patient's head and thorax should be elevated 30 to 50 degrees for approximately 6 to 8 hours to reduce upward dispersion of the dye and to prevent contact of the water-soluble agent with the cerebral meninges, which could precipitate a seizure. Bed rest may be ordered for up to 6 hours.
Contraindications
Potential Complications
• Hypoglycemia or acidosis in patients who are taking metformin (Glucophage) and receive iodine dye
• For potential complications to iodinated dye, see p. 985.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
• Ensure that the physician has obtained written, informed consent for this procedure.
• For assessment of allergy to iodinated dye, see p. 985.
![]() Explain to the patient that he or she must lie very still during the procedure.
Explain to the patient that he or she must lie very still during the procedure.
• Food and fluid restrictions vary according to the type of dye used. Check with the radiology department for specific restrictions.
![]() Inform the patient that he or she will be tilted into an upside-down position on the table so that the dye can properly fill the spinal canal and provide adequate visualization of the desired area.
Inform the patient that he or she will be tilted into an upside-down position on the table so that the dye can properly fill the spinal canal and provide adequate visualization of the desired area.
During
• Note the following procedural steps:
1. Lumbar puncture (see p. 651) or cisternal puncture is performed.
2. A 15-mL sample of CSF is withdrawn, and 15 mL or more of radiopaque dye is injected into the spinal canal.
3. The patient is placed prone on the tilt table, with the head tilted down.
4. Representative x-ray images are obtained.
5. After myelography is performed, the needle is removed and a dressing is applied.
After
• Note that nursing interventions after the procedure depend on the type of contrast agent used.
• See lumbar puncture (p. 651) for appropriate “after” care.
Test Results and Clinical Significance
Spinal cord tumors (e.g., astrocytoma, neurofibroma, meningioma): These are seen as radiolucent filling defects in the column of radiopaque dye in the canal.
Metastatic spinal tumor: Extrinsic spinal tumors (usually metastatic) are evident as extrinsic radiolucent filling defects in the column of radiopaque dye in the canal.
Cervical ankylosing spondylosis,
Arthritic lumbar stenosis from arthritic bone spurs:
These bony changes can compress the spinal canal and are evident as distortion of the cord or nerve roots.
Herniated intravertebral disk: A herniated disk acts as external compression on the spinal cord or the nerve root. The most common areas of disk herniation are L4-L5 and L5-S1.
Avulsion of nerve roots: Traumatic avulsion of the nerve root can cause profound neurologic changes.
Cysts: Cysts of the cord or meninges surrounding the cord are evident as extrinsic radiolucent filling defects in the column of radiopaque dye in the canal.
Related Test
Magnetic Resonance Imaging (MRI) (p. 1106). This test is more accurate than myelography for viewing the spinal cord and its surrounding structures.
Obstruction Series
Test Explanation
The obstruction series is a group of x-ray images of the abdomen in patients with suspected bowel obstruction, paralytic ileus, perforated viscus, abdominal abscess, kidney stones, appendicitis, or foreign body ingestion. The series of films usually consists of at least two x-ray studies. The first is an erect abdominal film, which should include visualization of both diaphragms. The film is examined for evidence of free air under either diaphragm, which is pathognomonic for perforated viscus. This view is also used to detect air-fluid levels within the intestine; the presence of an air-fluid level is compatible with bowel obstruction or paralytic ileus. Occasionally, patients are too ill to stand erect. In this case, an x-ray film can be taken with the patient in the left lateral decubitus position. If free air is present, it will be seen between the liver and the right side of the abdominal wall. Air fluid levels also can be detected.
The second view in the obstruction series is usually a supine abdominal x-ray study, similar to the kidney, ureter, and bladder (KUB) study (p. 1040). An abdominal abscess may be seen as a cluster of tiny bubbles within a localized area. A calcification within the ureter could indicate a kidney ureteral stone. A small calcification in the right lower quadrant in a patient with pain in this quadrant may be an appendicolith. A gas-filled, distended bowel is compatible with bowel obstruction or paralytic ileus.
The obstruction series can also be used to monitor the clinical course of gastrointestinal (GI) disease. For example, repeated obstruction series in patients with partial small bowel obstruction or paralytic ileus can indicate clinical worsening or improvement.
Frequently, a cross-table lateral view of the abdomen is included in an obstruction series, to detect abdominal aorta calcification, which often occurs in older patients. The calcification represents the anterior wall of the aorta. If an aortic aneurysm exists, this calcification will be seen to protrude from the spine.
The supine abdominal x-ray study can be used as a scout image before performing GI or abdominal contrast material-enhanced x-ray studies (e.g., barium enema [p. 994] or intravenous pyelography (IVP) [p. 1057]), to ensure nothing is obstructing adequate visualization of what needs to be studied.
The obstruction series is performed in minutes in the radiology department by a radiologic technologist; however, it can be performed at the bedside with a portable x-ray machine. A radiologist interprets the films. No discomfort is associated with the study.
Test Results and Clinical Significance
Abdominal aortic calcification or abdominal aortic aneurysm: Abdominal aortic aneurysm is evident by calcification in the anterior wall of the aorta, displaced significantly anterior from the vertebrae.
Calculi: A calcified stone in the area where the ureters would be is indicative of ureteral calculi. This finding requires further supportive evidence by means of IVP (p. 1057). Appendicolithiasis (stone in the appendix) is suspected when a patient with right lower quadrant abdominal pain is seen to have a stone in that quadrant.
Abdominal accumulation of bowel gas: Abnormal accumulations of bowel gas can indicate intestinal obstruction or paralytic ileus.
Ascites: The classic ground-glass appearance of the entire abdomen on the supine abdominal film indicates peritoneal effusion.
Soft-tissue masses: Large soft-tissue masses or abscesses can be seen surprisingly well on this plain film x-ray, without contrast material enhancement.
Ruptured viscus: Free air (i.e., air outside the bowel but inside the abdomen) is indicative of a perforated viscus.
Congenital anomalies in the location, size, and number of kidneys: Because the kidneys are well seen, abnormalities of these features are easily detected.
Organomegaly or bladder distention: An enlarged liver or spleen is seen as a large soft-tissue mass in the right or left upper quadrant, respectively. A large soft-tissue mass in the midline or pelvis is usually a distended bladder.
Foreign body: A bullet or other solid object is obvious on x-ray films. A surgical sponge or instrument left during surgery is also visible.
Related Test
Kidney, Ureter, and Bladder (KUB) X-Ray (p. 1040). This is only one component of the obstruction series and therefore does not contribute as much information as does an obstruction series.
Percutaneous Transhepatic Cholangiography (PTC, PTHC)
Indications
This procedure allows visualization of the bile ducts and sometimes the pancreatic duct. Patients with jaundice can be evaluated for tumors, gallstones, and other diseases.
Test Explanation
By passing a needle through the liver and into an intrahepatic bile duct, iodinated dye can be injected directly into the biliary system. The intrahepatic and extrahepatic biliary ducts, and occasionally the gallbladder, can be visualized and studied for partial or total obstruction from gallstones, benign strictures, malignant tumors, congenital cysts, and anatomic variations. This is especially helpful in patients with jaundice. If the jaundice is a result of extrahepatic obstruction, a catheter can be left in the bile duct and used for external drainage of bile. Furthermore, a stent can be placed across a stricture to decompress the biliary system internally.
PTC and endoscopic retrograde cholangiopancreatography (ERCP, p. 605) are the only methods available to visualize the biliary tree in patients with jaundice. ERCP is used more frequently because of its lower complication rate. PTC, however, is the only way to visualize the biliary tree after most gastric surgery. Occasionally (if the pancreatic duct and the common bile duct are from a common channel), part or all of the pancreatic duct can be filled with dye from the same injection. See Table 12-5 for a list of diagnostic tests to visualize the pancreatobiliary system, along with their advantages and disadvantages.
TABLE 12-5
Diagnostic Tests to Visualize the Pancreatobiliary System
| Test | Advantages | Disadvantages |
| Intravenous cholangiography | Easy to perform | Poor visualization of ducts |
| Oral cholecystography | Easy to perform | Visualizes only the gallbladder |
| ERCP | Good visualization of the pancreas and bile ducts; able to decompress the biliary system | Difficult to perform; complications possible |
| PTC | Good visualization of the ducts; able to decompress the biliary system | Difficult to perform; complications possible |
| Nuclear radioscintigraphy | Easy to perform | Poor visualization of the biliary system; not specific as to disease |
PTC is performed by a radiologist in approximately 1 hour, during which time the patient must lie still. Abdominal pain may be felt for several hours after the test. Occasionally the patient also may have right shoulder-top pain because of diaphragmatic irritation of leaking bile or blood.
Potential Complications
• For potential complications of iodinated dye, see p. 985.
• Peritonitis caused by bile extravasation from the liver after the needle has been removed
• Bleeding caused by inadvertent puncture of a large hepatic blood vessel
• Sepsis and cholangitis from injection of the dye into an already infected and obstructed bile duct: The pressure of injection pushes the bacteria into the bloodstream, causing bacteremia.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
• For assessment of allergy to iodinated dye, see p. 985.
• Type and cross-match the patient's blood. The patient may bleed and require a transfusion or surgery.
• Verify that results of coagulation studies are within the normal range.
• Keep the patient on nothing by mouth (NPO) status after midnight on the day of the test. A laxative may be ordered.
• Premedicate the patient as indicated, usually with atropine and meperidine.
During
• Note the following procedural steps:
1. The patient is placed supine on an x-ray table in the radiology department.
2. The abdominal wall or lower chest wall (over the liver) is anesthetized with lidocaine (Xylocaine).
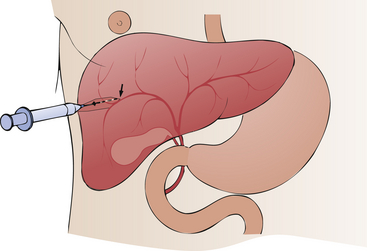
3. With the use of fluoroscopic monitoring, the needle is advanced through the skin and into the liver (Figure 12-22).
4. When bile flows freely out from the liver through the needle, radiographic dye is injected.
5. X-ray images are obtained immediately.
6. If an obstruction is found, a catheter or stent is placed over a guide wire and left temporarily in the biliary tract to establish drainage and decompression of the biliary tract.
After
• Keep the patient on bed rest for several hours.
• Observe the patient for hemorrhage or bile leakage. A small amount of bleeding is normal.
• Keep the patient NPO for a few hours after the test in the event intraabdominal bleeding or bile extravasation develops that requires surgery.
• Repeatedly assess the patient's vital signs for evidence of hemorrhage.
• Assess the patient for signs of bacteremia or sepsis.
• If a catheter is left in the biliary tract, establish a sterile, closed drainage system.
• Withhold high doses of pain medications that may blunt the abdominal signs associated with hemorrhage or bile extravasation.
Test Results and Clinical Significance
Tumors, strictures, or gallstones of the hepatic or common bile duct: These diseases cause partial or complete obstruction of the biliary tree. Tumors usually cause long strictures. Benign strictures are more likely to cause short segment narrowing. Gallstones usually are evident as rounded radiolucent (dark) filling defects in the bile duct. When they obstruct the bile duct, the obstruction is seen as a soft convex cutoff of the bile duct.
These conditions are due to inflammatory or fibrotic changes around the bile ducts; this leads to a long stricture in most of the biliary tree and its radicals.
Cysts of the common bile duct: These congenital outpouchings of the bile duct may vary in size from tiny and barely noticeable to large and voluminous. The pressure surrounding these cysts can obstruct the normal portion of the bile duct in the closed space of the right upper quadrant of the abdomen.
Tumors, strictures, inflammation, or true or pseudocysts of the pancreatic duct: If the pancreatic duct is visualized, these abnormalities can be identified. Pancreatic tumors are evident as long strictures. Postinflammatory strictures are usually short segment narrowing. Neoplastic cysts may be connected to the main pancreatic duct and fill with dye. Pseudocysts, caused by pancreatic duct disruption that follows severe pancreatitis, nearly always connect with the main pancreatic duct and therefore fill with dye.
Anatomic biliary or pancreatic duct variations: Duplications, aberrant entry of the pancreatobiliary ducts into the intestine, and other anomalies can be identified.
Related Test
Endoscopic Retrograde Cholangiopancreatography (ERCP) (p. 605). This is the preferred method of visualizing the pancreatobiliary tree in the patient with jaundice.
Pyelography (Intravenous Pyelography [IVP], Excretory Urography [EUG], Intravenous Urography [IUG, IVU], Retrograde Pyelography, Antegrade Pyelography)
Indications
The test has been mostly replaced by CT scan (p. 1020) because the accuracy of the CT scan is better than IVP. Nevertheless, IVP is still indicated for patients with:
• Pain compatible with urinary stones
• Proposed pelvic surgery to locate the ureters
Test Explanation
Pyelography is an x-ray study that uses radiopaque contrast material to visualize the kidneys, renal pelvis, ureters, and bladder. The contrast can be injected intravenously (IVP), through a catheter placed into the ureter (retrograde pyelography), or through a catheter placed into the proximal renal collecting system (antegrade pyelography). IVP testing is not performed as frequently as it was several years ago.
For IVP, dye is injected intravenously, filtered out at the kidney by the glomeruli, and then passed through the renal tubules. X-ray films taken at set intervals over the next 30 minutes will show passage of the dye material through the kidneys and ureters and into the bladder. If the artery leading to one of the kidneys is blocked, the dye cannot enter that kidney or part thereof and it will not be visualized. If the artery is partially blocked, the length of time required for the appearance of the contrast material will be prolonged.
With primary glomerular disease (e.g., glomerulonephritis), the glomerular filtrate is reduced, which causes a reduction in the quantity of dye filtered. Therefore it requires more time for kidney visualization. Defects in dye filling of the kidney can indicate renal tumors or cysts.
If the obstruction of the ureter has been of sufficient duration, the collecting system proximal to the obstruction will be dilated (hydronephrosis). Retroperitoneal and pelvic tumors, aneurysms, and enlarged lymph nodes also can produce extrinsic compression and distortions of the opacified collecting system.
IVP can be used to assess the effect of trauma on the urinary system. Renal hematomas distort the renal contour. Renal artery laceration is suggested by non-opacification of one kidney. Laceration of the kidneys, pelvis, ureters, or bladder often causes urine leaks, which are identified by dye extravasation from the urinary system. Furthermore, IVP can assess a patient for congenital absence or malposition of the kidneys. Horseshoe kidneys (connection of the two kidneys), double ureters, and pelvic kidneys are typical congenital abnormalities.
Retrograde pyelography refers to radiographic visualization of the urinary tract through ureteral catheterization and the injection of contrast material. The ureters are catheterized during cystoscopy. A radiopaque material is injected into the ureters, and x-ray films are taken. This test can be performed even if the patient has an allergy to IV contrast dye, because none of the dye injected into the ureters is absorbed.
Retrograde pyelography is helpful in radiographically examining the ureters in patients when visualization with intravenous pyelography is inadequate or contraindicated. When a ureter is obstructed, IVP will visualize only the ureter proximal to the obstruction, if at all. To visualize the distal part of the ureter, retrograde pyelography is necessary. Also, in patients with unilateral renal disease, the involved kidney and collecting system are not visualized because renal function is so poor. As a result, no dye will be filtered into the collecting system (during IVP) by the nonfunctioning kidney. To rule out ureteral obstruction as a cause of the unilateral kidney disease, retrograde pyelography must be done.
Antegrade pyelography provides visualization of the renal pelvis for accurate placement of nephrostomy tubes. This study is used to identify the upper collecting system in an obstructed kidney and used as a map for accurate percutaneous placement of a nephrostomy tube. This is performed on patients who have an obstruction of the ureter and hydronephrosis. With this procedure, the renal pelvis is identified with CT imaging or ultrasound. A needle is placed into the pelvis. Radio-opaque dye is then injected and the entire upper renal collecting system is demonstrated by obtaining x-rays in rapid succession. Proper positioning for the nephrostomy is then decided based on these images.
Contraindications
• Patients who are allergic to shellfish or iodinated dyes
• Patients who are severely dehydrated, because this can cause renal shutdown and failure (Geriatric patients are particularly vulnerable.)
• Patients with renal insufficiency, as evidenced by a blood urea nitrogen value greater than 40 mg/dL, because the iodinated nephrotoxic dye can worsen kidney function
• Patients with multiple myeloma, because the iodinated nephrotoxic dye can worsen renal function
• Patients who are pregnant, unless the benefits outweigh the risks of radiation exposure to the fetus
Potential Complications
• Infiltration of contrast dye
• Renal failure. This occurs most often in elderly patients who are chronically dehydrated before the dye injection.
• Hypoglycemia or acidosis may occur in patients who are taking metformin (Glucophage) and receive iodine dye.
• Hemorrhage at the needle puncture site during antegrade pyelography, because the kidney is highly vascular
• Complications associated with retrograde pyelography include:
Interfering Factors
• Fecal material, gas, or barium in the bowel may obscure visualization of the renal system.
• Abnormal renal function studies may prevent adequate visualization of the urinary tract.
• Retained barium from previous studies may obscure visualization. Studies using barium (e.g., barium enema) should be scheduled after an IVP.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient. Inform the patient that several x-ray films will be taken over 30 minutes.
Explain the procedure to the patient. Inform the patient that several x-ray films will be taken over 30 minutes.
• Obtain informed consent if required by the institution.
• Check the patient for allergies to iodinated dye and shellfish.
• Give the patient a laxative (e.g., castor oil) or a cathartic, as ordered, the evening before the test.
![]() Inform the patient of the required food and fluid restrictions. Some institutions prefer abstinence from solid foods for 8 hours before testing. Some allow a clear-liquid breakfast on the test day.
Inform the patient of the required food and fluid restrictions. Some institutions prefer abstinence from solid foods for 8 hours before testing. Some allow a clear-liquid breakfast on the test day.
• Ensure adequate hydration for the patient (IV or oral) before and after the test to avoid dye-induced renal failure.
• Note that pediatric patients will have decreased fasting times, as ordered on an individual basis.
• Note that elderly and debilitated patients should have fasting times indicated specifically for them.
• Note that patients receiving high rates of IV fluids may have infusion rates decreased for several hours before the study to increase the concentration of the dye within the urinary system.
• Assess the patient's blood urea nitrogen and creatinine levels. Abnormal renal function could deteriorate as a result of the dye injection.
• Schedule any barium studies after completion of the IVP.
• Give the patient an enema or suppository on the morning of the study, if ordered.
• If the antegrade or retrograde pyelography will be performed with the patient under general anesthesia, follow routine general anesthesia precautions. Keep the patient NPO after midnight on the day of the test. Fluids may be given intravenously.
During
Intravenous Pyelography
1. The patient is taken to the radiology department and placed in the supine position.
2. A plain film of the abdomen (KUB) is taken to ensure that no residual stool obscures visualization of the renal system. This also screens for calculi in the renal collecting system.
3. Skin testing for iodine allergy is often done.
4. A peripheral IV line is started (if not in place), and a contrast dye (e.g., Hypaque, Renografin) is given.
5. X-ray films are taken at specific times, usually at 1, 5, 10, 15, 20, and 30 minutes and sometimes longer, to follow the course of the dye from the cortex of the kidney to the bladder.
6. The patient is taken to the bathroom and asked to void.
7. A postvoiding film is taken to visualize the empty bladder.
![]() Inform the patient that the dye injection often causes a transitory flushing of the face, a feeling of warmth, a salty taste in the mouth, or even transient nausea. Initial IV needle placement and lying on a hard x-ray table are the only other discomforts associated with IVP.
Inform the patient that the dye injection often causes a transitory flushing of the face, a feeling of warmth, a salty taste in the mouth, or even transient nausea. Initial IV needle placement and lying on a hard x-ray table are the only other discomforts associated with IVP.
Retrograde Pyelography
1. The ureteral catheters are passed into the ureters by means of cystoscopy (see p. 598).
2. Radiopaque contrast material (Hypaque or Renografin) is injected into the ureteral catheters, and x-ray films are taken.
3. The entire ureter and renal pelvis are demonstrated.
4. As the catheters are withdrawn, more dye is injected, and more x-ray films are taken to visualize the complete outline of the ureters.
5. A delayed film is often performed to assess the emptying capabilities of the ureter. This is usually done about 5 minutes after the last injection.
6. If obstruction is noted, a stent may be left in the ureter so that the ureter can drain.
![]() Inform the patient that antegrade or retrograde pyelography is uncomfortable. If awake, the patient will feel pressure and an urge to void.
Inform the patient that antegrade or retrograde pyelography is uncomfortable. If awake, the patient will feel pressure and an urge to void.
Antegrade Pyelography
1. The renal pelvis is localized by means of ultrasound.
2. Under local anesthesia, a thin-walled needle is advanced into the lumen of the renal pelvis.
3. Contrast material is injected and x-ray films in posteroanterior (PA), oblique, and anteroposterior (AP) views are obtained.
4. The nephrostomy tube is placed over guide wires and its position is affirmed by repeating the x-rays.
After
![]() Maintain on adequate oral or IV hydration for several hours after pyelography to counteract fluid depletion caused by the test preparation. Encourage fluid intake.
Maintain on adequate oral or IV hydration for several hours after pyelography to counteract fluid depletion caused by the test preparation. Encourage fluid intake.
![]() Assess the patient's urinary output. A decreased output may be an indication of renal failure. Instruct the patient to report a decreased output.
Assess the patient's urinary output. A decreased output may be an indication of renal failure. Instruct the patient to report a decreased output.
• Evaluate elderly and debilitated patients for weakness because of the combination of fasting and catharsis necessary for test preparation. Instruct these patients to ambulate only with assistance.
• Note the color of the urine; a pink tinge is typically present. Report bright red blood or clots to the physician.
• See p. 985 for appropriate interventions concerning care for patients with iodine allergy.
Test Results and Clinical Significance
Pyelonephritis or glomerulonephritis: Primary renal disease usually is evident as reduced opacification of the kidney with dye. This is because it takes a long time for enough dye to be filtered to the renal system to opacify the kidney.
Kidney tumor (benign or malignant),
Cyst or polycystic disease of the kidney:
These are usually evident as a radiolucent (dark) filling defect in the kidney parenchyma. Ultrasound of the kidney (p. 866) is diagnostic for cysts.
Congenital abnormality of the urologic tract: Congenital anomalies may include absence of a kidney or altered shape, size, or location of a kidney. The collecting system can be duplicated, with more than one ureter per kidney. The bladder can be divided by a congenital septum into two small bladders.
Renal or ureteral calculi: Calculi (stones) are evident as radiolucent filling defects that can obstruct the ureters. This is most evident on retrograde pyelography.
Trauma to the kidneys, ureters, or bladder: Injury may be evident as leakage of dye from the injured organ. Hematomas are seen as filling defects or radiolucent shadows.
Tumor of the collecting system: This can partially or completely obstruct the collecting system.
Hydronephrosis: This condition is due to prolonged obstruction of the collecting system distal to the hydronephrotic area. The distal ureter is best evaluated by retrograde pyelography.
Extrinsic compression of the collecting system (e.g., caused by tumor, aneurysm): Nonurologic tumors or masses can distort or obstruct the ureters or bladder.
Bladder tumor: This is seen as a radiolucent (dark shadow) filling defect in the bladder.
Prostate enlargement: This is evident as an extrinsic protrusion into the base of the bladder and inadequate emptying of the bladder because of outlet obstruction.
Related Tests
Computed Tomography (CT) of the Abdomen (p. 1020). Allows better visualization of the kidneys, ureters, and bladder.
Cystography (p. 1036). Study of the bladder after placement of dye directly into the bladder. With this study, the dye is more concentrated, and more information concerning the bladder can be obtained.
Sialography
Test Explanation
Sialography is an x-ray procedure used to examine the salivary ducts (parotid, submaxillary, submandibular, sublingual) and related glandular structures after injection of a contrast medium into the desired duct. The procedure is used to detect calculi, strictures, tumors, or inflammatory disease in patients with pain, tenderness, or swelling in these areas. Computed tomography (CT) of the salivary ducts is more reliable for detection of salivary parenchymal tumors or inflammation. Sialography is effective for ductule calculi or strictures.
A radiologist performs this procedure in the radiology department in less than 30 minutes. The patient may feel slight pressure as the contrast medium is injected into the ducts.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient. The thought of dye injection in the mouth is frightening to many patients. Provide emotional support.
Explain the procedure to the patient. The thought of dye injection in the mouth is frightening to many patients. Provide emotional support.
• Obtain informed consent if required by the institution.
![]() Instruct the patient to remove jewelry, hairpins, and dentures, which could obscure x-ray visualization.
Instruct the patient to remove jewelry, hairpins, and dentures, which could obscure x-ray visualization.
![]() Instruct the patient to rinse the mouth with an antiseptic solution to reduce the possibility of introducing bacteria into the ductal structures.
Instruct the patient to rinse the mouth with an antiseptic solution to reduce the possibility of introducing bacteria into the ductal structures.
During
• Note the following procedural steps:
1. X-ray studies are taken before the dye injection to ensure that radiopaque stones are not present, which could prevent the contrast material from entering the ducts.
2. The patient is placed supine on an x-ray table.
3. The contrast medium is injected directly into the desired orifice through a cannula or special tiny catheter.
4. X-ray films are obtained with the patient in various positions.
5. The patient is given a sour substance (e.g., lemon juice) orally to stimulate salivary excretion of the dye.
6. Another set of x-ray studies is obtained to evaluate ductal drainage.
Test Results and Clinical Significance
Tumor: Most tumors of the salivary glands are benign, and in the parotid gland. These tumors are demonstrated as radiolucent filling defects or distortion of the glandular ductules within the gland.
Inflammatory disease: Sialitis (especially parotitis) can result from viral illnesses (e.g., mumps) or bacterial infections.
Skull X-Ray
Indications
This x-ray study is used to evaluate the skull and paranasal sinuses for trauma or disease.
Test Explanation
An x-ray film of the skull provides visualization of the bones making up the skull, the nasal sinuses, and any central nervous system (CNS) calcification. This study is indicated when a pathologic condition is suspected in any of these structures.
Skull fractures are easily seen as abnormal radiolucent lines in an otherwise radiopaque skull bone (Figure 12-23). Metastatic tumors of the skull can easily be seen as radiolucent spots on an otherwise normal film. Opacification of the nasal sinuses may indicate sinusitis, hemorrhage, or tumor.
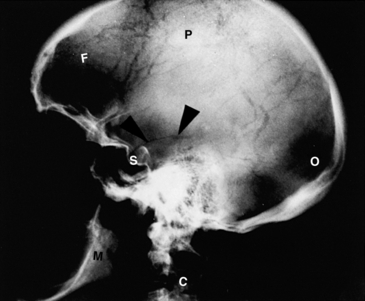
Figure 12-23 Skull x-ray, lateral view. Pointers indicate fracture line in temporal bone. C, Cervical vertabra; F, frontal bone; M, mandible; O, occipital bone; P, parietal bone; S, Sella turcica.
Located in the middle of the brain, the pineal gland is thought to regulate biorhythms in mammals. This gland may become calcified after puberty. When calcified, the pineal gland is a useful marker and allows the midline of the brain to be easily identified on skull x-ray films. Conditions such as unilateral hematoma or tumor will cause a shift of the midline structures (and the calcified pineal gland) to the side opposite the site of the pathologic condition. Simple skull x-ray films therefore allow easy detection of these unilateral, space-occupying lesions.
The sella turcica is the bony structure surrounding and protecting the pituitary gland. Tumors of the pituitary gland may cause an increase in size or erosion of the sella turcica. These changes can be detected on skull x-ray films.
Most head trauma (or instances where skull injury is suspected) is evaluated with a CT scan of the brain. However, skull x-ray is still the best method of determining skull bone suture lines for the evaluation of children with abnormal head shape/size.
Procedure and Patient Care
Before
During
• The patient is taken to the radiology department and placed on an x-ray table. Axial (submentovertical), half-axial (Towne), posteroanterior, and lateral views of the skull are usually obtained (Figure 12-24).
Figure 12-24 Patient positioned for a skull x-ray study (Towne view—anteroposterior projection with posterior view). A lead apron is placed on the patient to prevent unnecessary exposure to radiation.
• A radiologic technologist obtains the skull films in a few minutes.
Test Results and Clinical Significance
Skull fracture: This is seen as a radiolucent line in the skull. The normal bone sutures (where the bones have grown together during normal growth and development) can look like fractures to the untrained eye.
Metastatic tumor: Breast cancer, Paget disease, myeloma, and many other tumors can metastasize to the skull. Metastatic tumor can be osteolytic (radiolucent) or osteoblastic (radiopaque).
Sinusitis: Swelling and mucus in the sinuses are evident as increased density on the x-ray film. Air-fluid levels may be evident also.
When unilateral, these abnormalities cause shift of midline structures (including the calcified pineal gland) to the opposite side.
Congenital anomaly: Many anomalies in the normal growth and development of the skull are obvious.
Related Test
Computed Tomography (CT) Scan of the Brain (p. 1026). This test can be used to evaluate the skull. Its main purpose is to evaluate the brain.
Small Bowel Follow-Through (SBF, Small Bowel Enema)
Indications
This contrast-enhanced x-ray study of the small intestines is most often used to identify and determine the cause of small bowel obstruction. It is also used to identify tumors, strictures, inflammation, and other congenital or acquired diseases of the small intestine.
Test Explanation
The SBF study is performed to identify abnormalities in the small bowel. Usually, the patient is asked to drink barium; in patients who cannot drink, barium can be injected through a nasogastric tube. X-ray films are then obtained at timed intervals (usually 30 minutes to 1 hour) to follow the progression of barium through the small bowel. Significant delays in barium transit time may occur as a result of both benign and malignant forms of partial obstruction or diminished intestinal motility (ileus). On the other hand, the flow of barium is faster in patients who have hypermotility of the small bowel (e.g., malabsorption syndromes). Failure of the progression through the small bowel can be seen in patients with complete mechanical small bowel obstruction. Furthermore, SBF series are helpful in identifying and defining the anatomy of small bowel fistulas (abnormal connections between the small bowel and other abdominal organs or skin). Strictures related to Crohn disease or radiation are also evident with SBF.
A more accurate radiographic evaluation of the small intestine is enabled by the small bowel enema. Barium is injected into a tube previously placed in the small bowel. This small bowel enema provides better visualization of the entire small bowel, because the barium is not diluted by gastric and duodenal juices, as when the patient drinks barium. This test is especially useful in the evaluation of partial small bowel obstruction of unknown cause. Tumors, ulcers, and small bowel fistulas are more easily identified and defined with the enema.
Table 12-6 lists x-ray studies used to visualize the gastrointestinal (GI) tract. Note that this procedure is performed by a radiologist in the radiology department in approximately 30 minutes. Inform the patient that this test is not uncomfortable.
Contraindications
• Patients with a complete small bowel obstruction: The introduction of barium into an obstructed bowel may create a stonelike impaction; however, this is extremely rare.
• Patients with a suspected perforated viscus: Barium should not be used in this situation because it may cause persistent and recurrent infections if it leaks out of the bowel. Meglumine diatrizoate (Gastrografin), a water-soluble contrast medium, can be used if perforation is suspected. However, it becomes diluted rapidly, minimizing the accuracy of the SBF with this contrast medium.
• Patients with unstable vital signs: These patients should be closely supervised during the time required for this study.
Interfering Factors
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Instruct the patient not to eat anything for at least 8 hours before the test. Usually, keep the patient NPO after midnight on the day of the test.
Instruct the patient not to eat anything for at least 8 hours before the test. Usually, keep the patient NPO after midnight on the day of the test.
![]() Inform the patient that the SBF series may take several hours. Suggest that the patient bring reading material or some paperwork to occupy the time.
Inform the patient that the SBF series may take several hours. Suggest that the patient bring reading material or some paperwork to occupy the time.
• Accompany the patient to the radiology department if vital signs are not stable.
• Arrange for transportation of the hospitalized patient back to the nursing unit between serial films.
During
• Note the following procedural steps:
1. A specially prepared drink containing barium sulfate is mixed as a milkshake, which the patient drinks through a straw.
2. Usually, an upper GI series is performed concomitantly (see p. 1072).
3. Barium flow is followed through the upper GI tract by means of fluoroscopy.
4. At frequent intervals (15 to 60 minutes), repeated x-ray films are obtained to follow the flow of barium through the small intestine until barium is seen flowing into the right colon. This usually takes 60 to 120 minutes, but in patients with delayed progression of the barium, the test may take as long as 24 hours to complete.
Test Results and Clinical Significance
Small bowel tumor: This may be evident as partial or complete small bowel obstruction. Usually, however, tumors are seen as filling defects in the column of barium in the small bowel.
Small bowel obstruction: Adhesions are the most common cause of small bowel obstruction, followed by extrinsic tumor, hernia, and stricture from inflammatory bowel disease in adults. Hernias, intussusception, malrotation, bowel atresia, and volvulus are most common in children. Small bowel obstruction can be partial or complete. With complete obstruction, the barium column does not progress past the area of obstruction. With partial obstruction, the barium passes the area of obstruction, but abnormally slowly.
Inflammatory small bowel disease (e.g., Crohn disease): Inflammatory bowel disease usually is apparent as a stricture causing partial small bowel obstruction.
Malabsorption syndromes (e.g., Whipple disease, sprue): These are usually evident as rapid transit of contrast material through the small bowel.
Congenital or acquired anatomic anomaly (e.g., malrotation): Malrotation usually causes the ligament of Treitz (junction of the duodenum and jejunum) to be in the right lower quadrant instead of its normal location in the left upper quadrant. Short bowel syndrome related to surgical small bowel bypass or resection is evident as rapid transit of barium through the small bowel.
Congenital abnormalities (e.g., small bowel atresia, duplication, Meckel diverticulum): Bowel atresia or duplication can be noted as bowel obstruction in children. Meckel diverticulum is evident as an outpouching of the ileum. It may not be apparent until adulthood and may never cause symptoms.
Small bowel intussusception: An upper segment of bowel becomes invaginated (swallowed up) into a lower segment. This usually causes bowel obstruction and is most common in children.
Small bowel perforation: When contrast leaks out of the intestine, perforation is present.
Radiation enteritis: This becomes evident years after radiation therapy. It is demonstrated as a stricture of one or more segments of the small bowel.
Related Test
Barium Enema (p. 994). This test is contrast material–enhanced x-ray study of the colon. Often the distal small bowel is visualized.
Spinal X-Rays (Cervical, Thoracic, Lumbar, Sacral, or Coccygeal X-Ray Studies)
Test Explanation
Spinal x-ray studies may be performed to evaluate any area of the spine. They usually include anteroposterior, lateral, and oblique views of these structures. These x-ray films are often obtained to assess back or neck pain, degenerative arthritic changes, traumatic fractures, tumor metastasis, spondylosis (degenerative disease of the spinal structures), and spondylolisthesis (slipping of one vertebral disk over another). Cervical spine x-ray studies are routinely performed in cases of multiple trauma to ensure absence of fracture before the patient is moved or the neck is manipulated. However, CT scanning of the cervical vertebrae is increasingly becoming the standard of practice to ensure that there is no cervical fracture. Spinal radiographs are helpful in evaluating for spinal alignment abnormalities (e.g., kyphosis [Figure 12-25], scoliosis). MRI is another very accurate method of evaluating the spine.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Instruct the patient to remove any metal objects covering the area to be visualized.
Instruct the patient to remove any metal objects covering the area to be visualized.
• Immobilize the patient if a spinal fracture is suspected. Apply a neck brace if a cervical spine fracture is suspected.
![]() Tell the patient that no fasting or sedation is required; however, if a fracture is suspected, the patient may be kept on nothing by mouth (NPO) status.
Tell the patient that no fasting or sedation is required; however, if a fracture is suspected, the patient may be kept on nothing by mouth (NPO) status.
During
• Note that the patient is placed on an x-ray table. Anterior, posterior, lateral, and oblique x-ray films are obtained of the desired area of the spinal cord. The same views can be obtained with the patient in the standing position.
• A radiologic technologist obtains spinal x-ray films in a few minutes.
![]() Tell the patient that no discomfort is associated with this study.
Tell the patient that no discomfort is associated with this study.
Test Results and Clinical Significance
Degenerative arthritis changes: Bone destruction or spurring is seen in patients with degenerative arthritic changes of the spinal joints.
Traumatic or pathologic fracture:
The cervical and lumbar portions of the spine are most frequently injured. Any portion, however, can be affected by metastatic neoplasm (e.g., myeloma, Paget disease, breast or lung cancer). Bone metastasis can lead to fracture without a traumatic event.
Suspected spinal osteomyelitis: This test is helpful in detecting the infection.
Swallowing Examination (Videofluoroscopy Swallowing Examination)
Test Explanation
Problems in swallowing may result from local structural diseases (e.g., tumors, upper esophageal diverticula, inflammation, extrinsic compression of the upper gastrointestinal [GI] tract) or surgery to the oropharyngeal tract. Motility disorders of the upper GI tract (e.g., Zenker diverticulum) and neurologic disorders (e.g., stroke syndrome, Parkinson disease, neuropathies) also may cause difficulty in swallowing. Videofluoroscopy of the swallowing function allows the speech pathologist to delineate more clearly the exact pathologic condition in the swallowing mechanism; this leads to determining the most appropriate treatment and teaching the patient proper swallowing technique.
This test is performed by asking the patient to swallow barium or a barium-containing meal. With videofluoroscopy, the act of swallowing is visualized and documented. Structural abnormalities and functional impairment can be identified easily with the slow-framed progression and reversal possible with videofluoroscopy. This test is similar to barium swallow (p. 999), but finer details of swallowing can be evaluated with videofluoroscopy.
Procedure and Patient Care
During
• In the radiology department, the patient is asked to swallow a barium-containing meal. The consistency of the meal (e.g., liquid, semi-soft [e.g., applesauce], or solid [e.g., a tea biscuit]) will be determined by the speech therapist and radiologist, to simulate foods to which the patient is to be initially reintroduced. While the patient swallows, videofluoroscopy is recorded in both the lateral and the anterior positions.
• The video is repeatedly examined forward and backward by the radiologist and by the speech pathologist.
Test Results and Clinical Significance
Related Test
Barium Swallow (p. 999). This test is similar to videofluoroscopy except that multiple still x-ray films are obtained and the entire swallowing mechanism cannot be fully evaluated.
T-Tube and Operative Cholangiography
Normal Findings
Normal common bile duct with no dilatation or filling defects
Good runoff of dye through the ampulla of Vater into the duodenum
Indications
Cholangiography provides visualization of the bile ducts during and after surgery. It is most commonly used to identify common bile duct stones.
Test Explanation
In operative cholangiography, the common bile duct is directly injected with radiopaque material through the cystic duct. This is usually performed during cholecystectomy. Stones appear as radiolucent shadows. Gallstones, tumors, or strictures cause partial or total obstruction of the flow of dye into the duodenum. Visualization of the biliary duct structures enables the surgeon to see the surgical anatomy of the biliary tree. This reduces the possibility of inadvertent common bile duct injury during cholecystectomy. If common duct stones are demonstrated during operative cholangiography, a common duct exploration is performed. Some surgeons perform operative cholangiography in all patients who undergo cholecystectomy. Other surgeons use specific indications for operative cholangiography, including the following:
T-tube cholangiography is performed postoperatively following T-tube placement during a common duct exploration. Its main purpose is to detect retained common bile duct stones and demonstrate good flow of contrast dye into the duodenum. This test is performed, usually 5 to 10 days after surgery, with use of a T-shaped rubber tube placed in the common bile duct at surgery. If no stones are evident and there is good runoff of bile into the duodenum, the T-tube can be removed. If there are residual stones, the tube tract can be used to extract the stones.
Procedure and Patient Care
During
Operative Cholangiogram
1. Performed through catheterization of the cystic duct during cholecystectomy.
2. Alternatively, a needle or catheter is placed in the common bile duct.
3. The dye is injected directly into the common bile duct.
4. X-ray films are obtained while the patient is on the operating table and are immediately reviewed by the surgeon.
T-Tube Cholangiogram
1. The patient is taken to the radiology department.
2. A sterile dye solution is injected into the T-tube previously placed by the surgeon.
3. X-ray images are obtained of the right upper quadrant of the abdomen with the patient placed in various positions.
• A radiologist or surgeon performs these procedures in approximately 10 minutes.
![]() Tell the patient that no discomfort is associated with these studies.
Tell the patient that no discomfort is associated with these studies.
Test Results and Clinical Significance
Common bile duct stones: These appear as radiolucent rounded filling defects in the column of dye within the common bile duct.
Anatomic variations: Many types of bile duct congenital anatomic variations can exist and are demonstrated at cholangiography.
Bile duct cysts: Though rare, these cysts appear as small to large outpouchings of the bile ducts.
Stricture or tumor obstructing the common bile duct: Tumors of the bile duct (cholangiocarcinoma) or extrinsic tumors (e.g., pancreas, colon) can partially or completely obstruct the bile duct. Benign inflammatory or posttraumatic strictures also can cause varying degrees of obstruction.
Bile duct surgical trauma: Ligation or laceration of the bile duct is obvious at the time of surgery with the use of cholangiography.
Related Tests
Endoscopic Retrograde Cholangiopancreatography (ERCP) (p. 605). This is an endoscopic procedure performed to radiographically visualize the bile and pancreatic ducts.
Percutaneous Transhepatic Cholangiography (PTC) (p. 1053). This procedure provides visualization of the bile ducts by percutaneous access to the biliary tree through the liver.
Upper Gastrointestinal Tract X-Ray (Upper GI Series, UGI)
Normal Findings
Normal size, contour, patency, filling, positioning, and transit of barium through the lower esophagus, stomach, and upper duodenum
Indications
This contrast-enhanced x-ray study provides visualization of the mucosa of the esophageal, gastric, and duodenum lumens. It is indicated in patients with upper abdominal pain, dyspepsia, dysphagia, early satiety, or suspected gastroduodenal obstruction.
Test Explanation
The upper GI study consists of a series of x-ray images of the lower esophagus, stomach, and duodenum, usually using barium sulfate as the contrast medium. When there is concern for leakage of x-ray contrast through a perforation of the GI tract, however, meglumine diatrizoate (Gastrografin), a water-soluble contrast medium, is used. This test can be performed in conjunction with a barium swallow (p. 999) or small bowel (p. 1064) series, which can precede or succeed the upper GI study, respectively.
The purpose of this examination is to detect ulcerations, tumors, inflammation, or anatomic malposition (e.g., hiatal hernia) of upper GI organs, and obstruction in the upper GI tract. The patient is asked to drink a beverage containing barium. As the contrast agent descends, the lower esophagus is examined for position, patency, and filling defects (e.g., tumors, scarring, varices). As the barium enters the stomach, the gastric wall is examined for benign or malignant ulcerations, filling defects (most often in cancer), and anatomic abnormalities (e.g., hiatal hernia). The patient is placed in a flat or head-down position, and the gastroesophageal area is examined for evidence of gastroesophageal reflux of barium.
As the contrast agent leaves the stomach, patency of the pyloric channel and the duodenum is evaluated. Benign peptic ulceration is the most common pathologic condition affecting these areas. Extrinsic compression caused by tumors, cysts, or enlarged pathologic organs (e.g., liver) near the stomach also can be identified based on anatomic distortion of the outline of the upper GI tract.
A radiologist performs this procedure in approximately 30 minutes. The patient may be uncomfortable lying on the hard x-ray table and may occasionally experience a sensation of bloating or nausea during the test.
Contraindications
• Patients with complete bowel obstruction
• Patients with suspected upper GI perforation: Water-soluble Gastrografin should be used instead of barium.
• Patients with unstable vital signs: These patients should be supervised during the time required for this test.
• Patients who are uncooperative, because of the necessity of frequent position changes
Interfering Factors
• Previously administered barium: This may block visualization of the upper GI tract.
• Incapacitated patient: Such patients cannot assume the multiple positions required for the study.
• Food and fluid in the stomach: They give the false impression of filling defects in the stomach, precluding adequate evaluation of the gastric mucosa.
• Obtundation: These patients cannot safely drink the barium.
Procedure and Patient Care
During
• Note the following procedural steps:
1. The patient is asked to drink approximately 16 ounces of barium sulfate. This is a chalky substance usually suspended in milkshake form and drunk through a straw (Figure 12-26). Usually, the drink is flavored to increase palatability.
2. After drinking the barium, the patient is moved through several position changes (e.g., prone, supine, lateral) to promote filling of the entire upper GI tract.
3. Films are taken at the discretion of the radiologist as the flow of barium is observed fluoroscopically.
4. The flow of barium is followed through the lower esophagus, stomach, and duodenum.
5. Several x-ray images are obtained throughout the course of the test.
6. For an air-contrast upper GI study, the patient is asked to rapidly swallow carbonated powder. This creates CO2 in the stomach, providing air contrast to the barium within the stomach and increased visualization of the gastric mucosa.
After
![]() Inform the patient that if meglumine diatrizoate (Gastrografin) was used, significant diarrhea may develop. This contrast agent is an osmotic cathartic.
Inform the patient that if meglumine diatrizoate (Gastrografin) was used, significant diarrhea may develop. This contrast agent is an osmotic cathartic.
![]() Instruct the patient to use a cathartic (e.g., milk of magnesia) if barium sulfate was used as the contrast medium. Water absorption may cause the barium to harden and create a fecal impaction if catharsis is not carried out.
Instruct the patient to use a cathartic (e.g., milk of magnesia) if barium sulfate was used as the contrast medium. Water absorption may cause the barium to harden and create a fecal impaction if catharsis is not carried out.
![]() Instruct the patient to note the stools to ensure that all of the barium has been removed. The stools should return to normal color after the barium is completely expelled, which may take as long as a day and a half.
Instruct the patient to note the stools to ensure that all of the barium has been removed. The stools should return to normal color after the barium is completely expelled, which may take as long as a day and a half.
Test Results and Clinical Significance
Esophageal cancer: Most esophageal cancers occur in the lower esophagus. They are seen as a stricture or complete obstruction of the barium column.
Esophageal varices: Serpiginous filling defects indicate esophageal varices.
Hiatal hernia: There are two types of hiatal hernias. With a sliding hiatal hernia, the esophagogastric (EG) junction and upper stomach are in the chest. With the rolling type, the EG junction is normal but the fundus of the stomach rolls up into the chest. This latter type can become incarcerated and perforate. Surgical repair is required when this condition is identified.
Diverticula: These can be in the upper esophagus (Zenker) and due to spasm of the cricopharyngeus muscle (upper esophageal sphincter), or in the lower esophagus (epiphrenic) and due to paraesophageal infection.
Gastric cancer: Cancers can be evident as large polypoid filling defects within the stomach or as ulcerative masses in the wall of the stomach.
Gastric inflammatory disease (e.g., Ménétrier disease): Thickened rugae or gastric folds are classic for chronic inflammatory changes of the stomach.
Benign gastric tumor (e.g., leiomyoma): Tumors can be small polypoid masses in the stomach or giant tumors that distort the entire upper abdomen.
Extrinsic compression by pancreatic pseudocyst, cysts, pancreatic tumors, or hepatomegaly: Masses in the upper abdomen can distort the stomach. Because the stomach is a large sack, it is unusual for that structure to be obstructed by extrinsic compression.
Perforation of the esophagus, stomach, or duodenum: Perforation is obvious when contrast material is evident outside the esophagus, stomach, or duodenum.
Congenital abnormalities (e.g., duodenal web, pancreatic rest, malrotation syndrome): These are congenital abnormalities that commonly cause duodenal obstruction in infants.
Gastric ulcer (benign or malignant): Malignancy can be ulcerative. Benign ulcers (stress or peptic) can also develop.
Duodenal ulcer: Most duodenal ulcers are peptic ulcers. They are most commonly seen in the first portion of the duodenum, called the bulb.
Duodenal cancer: This is very rare and usually is seen as a filling defect in the column of barium within the duodenum.
Duodenal diverticulum: Not uncommon, these outpouchings rarely cause symptoms.
Related Test
Esophagogastroduodenoscopy (EGD) (p. 608). This endoscopic procedure provides better visualization of the upper GI organs. Further, biopsy can be performed with this procedure.
Venography (Phlebography, Venogram)
Indications
This contrast-enhanced x-ray study of the venous system of the lower or upper extremity is used to identify obstruction or thrombosis of the venous system in patients with a swollen arm or leg.
Test Explanation
Venography is an x-ray study designed to identify and locate thrombi in the venous system (most commonly in the extremities). Dye is injected into the venous system of the affected extremity. X-ray films are then obtained at timed intervals to visualize the venous system. Obstruction to the flow of dye or a filling defect within the dye-filled vein indicates thrombosis. Positive study results accurately confirm the diagnosis of venous thrombosis; however, negative results—although not so accurate—make the diagnosis of venous thrombosis unlikely. Often both extremities are studied, even when only one leg is suspected to contain deep-vein thrombosis. The normal extremity is used for comparison with the involved extremity. Venography is more accurate than venous Doppler (p. 900) for thrombi in veins below the knee or in the femoral veins. Venography is also performed in the upper extremities to evaluate the more proximal axillary, subclavian, and innominate veins in patients with a swollen arm or hand.
A radiologist performs this study in approximately 30 to 90 minutes. Venous catheterization is only as uncomfortable as a needlestick or a small incision in the foot.The dye may cause the patient to feel a warm flush (although not so severe as with arteriography). Inform the patient that mild degrees of nausea, vomiting, or skin itching also may occasionally occur.
Contraindications
• Patients with severe edema of the legs, making venous access for dye injection impossible
• Patients who are uncooperative
• Patients who are allergic to iodinated dye or shellfish
• Patients with renal failure, because iodinated dye is nephrotoxic
Potential Complications
• For potential complications of iodinated dye, see p. 985.
• Renal failure, especially in elderly persons with chronic dehydration or mild renal failure (see Box 12-2, p. 984).
• Subcutaneous infiltration of the dye, causing cellulitis and pain
• Venous thrombophlebitis caused by the dye
• Bacteremia caused by a break in sterile technique
• Venous embolism caused by dislodgement of a deep-vein clot, induced by the dye injection
• Lactic acidosis may occur in patients who are taking metformin (Glucophage) and receive iodine dye. The metformin should be held on the day of the test.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
• Obtained informed consent if required for this procedure.
• For assessment of allergy to iodinated dye, see p. 985.
• If needed, provide appropriate pain medication so the patient is able to lie still during the procedure.
• Ensure that the patient is appropriately hydrated before testing. Injection of the iodinated contrast material may cause renal failure, especially in the elderly.
During
• Note the following procedural steps:
1. The patient is taken to the radiology department and placed supine on the x-ray table.
2. Catheterization of a superficial vein on the foot is performed. This may require a surgical cutdown.
3. An iodinated, radiopaque dye is injected into the vein.
4. X-ray films are obtained to follow the course of the dye up the leg.
5. Frequently, a tourniquet is placed on the leg to prevent filling of the superficial saphenous vein. All of the dye, therefore, goes to the deep venous system, which contains the most clinically significant thrombosis that can embolize.
After
• Continue appropriate fluid administration to prevent dehydration caused by the diuretic action of the dye.
• Observe the puncture site for infection, cellulitis, or bleeding.
• Assess the patient's vital signs for signs of bacteremia (e.g., fever, tachycardia, chills).
• Evaluate the patient for signs of allergic reaction (e.g., rash, chills, fever, irritability). Treat with antihistamines or steroids.
Related Tests
Venous Doppler flow studies (p. 900). With the use of ultrasound, blood flow within the vein can be evaluated. Obstruction to flow is easily noted. This test is less invasive, equally accurate (above the knee), and associated with less risk than venography.
Venous Plethysmography. This is a manometric study of the venous system of the extremity. It enables identification of venous occlusion.