Fluid Analysis Studies
Reasons for Performing Fluid Analysis
Procedural Care for Fluid Analysis
Overview
Reasons For Performing Fluid Analysis
Body fluid analysis can provide a significant amount of information concerning diseases that affect a patient. Normal body fluids can provide information concerning the body's hormonal status (cervical mucus test) and fertility (semen analysis, Sims-Huhner test). Cerebrospinal fluid (CSF) analysis (obtained by lumbar puncture) can provide significant data concerning diseases involving the CNS (brain and spinal cord). The normal collection of fluid that surrounds a fetus during pregnancy can be aspirated to gain information about the present and future health of the child and mother.
Abnormal accumulations of fluid (effusions) can be aspirated from the body to gain information about the disease process that caused the fluid to develop. Effusions can occur nearly anywhere in the body. Their presence is abnormal. In this chapter we discuss effusions within the pericardium, pleura, peritoneum, and joints. Effusions are classified as a transudate or an exudate. The purpose of this classification is to categorize possible diagnoses. In general, exudates are caused by inflammatory, infectious, or neoplastic diseases. Transudates are generally caused by venous engorgement, hypoproteinemia, or fluid overload.
Other body fluids are analyzed to indicate specific disease such as cystic fibrosis (sweat electrolytes or pancreatic enzymes). The secretion of these body fluids is stimulated to obtain enough fluid for analysis.
Most body fluids are not easily obtained. Usually a cavity of the body must be invaded to obtain the fluids for analysis. A needle is used for aspiration of fluid from the subarachnoid space of the central nervous system (CNS) (lumbar puncture), uterus (amniocentesis), pericardium (pericardiocentesis), pleura (thoracentesis), peritoneum (paracentesis), or joint (arthrocentesis). This aspiration must be done under complete and ensured sterile technique to avoid the introduction of infection to the body cavity. The quantity aspirated can vary from 20 mL to 5 L, depending on the location and original volume of the fluid. Testing of the fluid should be performed immediately to prevent inaccurate results caused by cellular or chemical deterioration. If testing cannot be done immediately, guidelines for preservation should be closely followed. Usually the fluid is evaluated for gross appearance, color, odor, red and white cell counts and differential, albumen and protein content, glucose and lactic dehydrogenase (LDH) levels, cytology, fungi, tuberculosis, and bacteria (culture or Gram stain). Other tests may be performed, depending on the specifics of the fluid or the suspected disease.
Not only is the aspiration of fluid helpful diagnostically, but it is often helpful therapeutically. The aspiration of fluid from the pleura often improves ventilation and oxygenation. Aspiration of fluid from the peritoneum often relieves pressure and allows the patient to breathe more easily and eat more comfortably. Joint fluid aspiration may improve joint function. Pericardial fluid aspiration improves diastolic filling and cardiac output. Furthermore, therapeutic drugs (steroids or antibiotics) or diagnostic contrast materials (for x-ray evaluation) can be injected through the aspirating needle.
Although some other body fluids do not require aspiration, care must still be applied to obtaining and transporting the fluid properly (semen analysis, cervical mucus, sweat electrolytes, and pancreatic enzymes). One must be aware that the evaluation of some body fluids may be very important as criminal legal evidence (Sims-Huhner test in rape cases). It is extremely important that cross-contamination of fluid samples be prevented. It is possible to cross-contaminate specimens merely by failing to change gloves or by labeling specimens improperly.
Procedural Care for Fluid Analysis
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Obtain informed consent for this procedure.
Obtain informed consent for this procedure.
![]() Tell the patient that no fasting is necessary unless heavy sedation or an operative procedure is used to obtain the fluid.
Tell the patient that no fasting is necessary unless heavy sedation or an operative procedure is used to obtain the fluid.
• Have the patient urinate or empty the bladder before the test to avoid inadvertent puncture of the bladder during paracentesis or hip joint aspiration.
During
• The patient is positioned in a manner designed to make the fluid most accessible to the aspirating needle.
• Aspirating techniques are always performed under sterile conditions.
• When obtaining semen or cervical mucus, penile or vaginal preparation is contraindicated.
• With aspiration techniques a variable amount of fluid is aspirated. Small volumes are aspirated into a syringe. For larger volumes the aspirating needle is attached to a plastic tubing. The other end of the tubing is placed in the collection receptacle (usually a container with a pressurized vacuum).
• If medications are to be administered, a syringe containing the preparation is attached to the needle and the drug is injected.
• To compare fluid levels to blood level and to calculate ratios, blood is simultaneously drawn for glucose, albumin, total protein, LDH, and so on.
After
• All tests performed on fluid should be performed immediately to avoid false results because of chemical or cellular deterioration.
• Place a small bandage over the needle site after aspiration is performed.
• Label the specimen with the patient's name, date, source of fluid, and diagnosis.
• Send the specimen promptly to the laboratory.
• Observe the puncture site for bleeding, continued drainage, or signs of infection if aspiration is performed.
• Monitor vital signs for evidence of hemodynamic changes if large volumes of fluid are withdrawn.
• Write any recent antibiotic therapy on the microbiology laboratory requisition slip.
• Place the patient is a position designed to minimize further leakage of fluid from an aspiration site.
• Monitor the patient and educate the patient about signs of potential complications.
Potential Complications of Fluid Analysis Testing
The complications associated with fluid analysis are those of aspirating fluid for analysis. In general, they include the following:
• Injury to an organ by penetration with the aspirating needle
• Bleeding into the fluid space as a result of blood vessel penetration during aspiration
• Reflex bradycardia and hypotension because of the patient's anxiety about the procedure
• Infection of the soft tissue around the needle aspiration site
• Infection of the remaining fluid within the fluid space
• Seeding of the aspirating needle tract with tumor when malignant effusion exists
• Persistent leakage of effusion fluid after withdrawal of the aspirating needle
Reporting Results
In most instances, fluid is obtained by a physician. The laboratory tests are performed by technologists and are usually reported the same day. Cytologic study results are interpreted by a pathologist and are reported after several days. Culture and sensitivity reports also take several days.
Amniocentesis (Amniotic Fluid Analysis)
Normal Findings

Indications
Amniocentesis is performed on women to gather information about the fetus. Fetal maturity, fetal distress, and risk for respiratory distress syndrome can be assessed. Genetic and chromosomal abnormalities can be identified. Maternal-fetal Rh incompatibility can be diagnosed. The sex of the child can be ascertained. This is important for a mother carrying a sex-linked gene. Neural tube defects can also be recognized. The test is performed on mothers whose pregnancies are considered to be high risk. These may include diabetic mothers, very obese mothers, older mothers (over 35 to 40 years) especially if there is a family history of trisomy 21, mothers with repeated spontaneous abortions, mothers whose prior children have genetic defects, and mothers in a couple in which either the mother or the father is a carrier for genetic defects. This test is also done on women who have an abnormal obstetric ultrasound.
Test Explanation
Amniocentesis involves the placement of a needle through the patient's abdominal and uterine walls into the amniotic cavity to withdraw fluid for analysis. Studying amniotic fluid is vitally important in assessing the following:
1. Fetal maturity status, especially pulmonary maturity (when early delivery is preferred). Fetal maturity is determined by analysis of the amniotic fluid in the following manner:
a. Lecithin and sphingomyelin (L/S ratio). The measurement of the ratio of the lipids L/S ratio has emerged as the standard criterion test to evaluate fetal lung maturity. Lecithin is the major constituent of surfactant, an important substance required for alveolar ventilation. If surfactant is insufficient, the alveoli collapse during expiration. This results in atelectasis and respiratory distress syndrome (RDS), which is a major cause of death in immature babies. In the immature fetal lung, the sphingomyelin concentration in amniotic fluid is higher than the lecithin concentration. At 35 weeks of gestation, the concentration of lecithin rapidly increases, whereas the sphingomyelin concentration decreases. An L/S ratio of 2:1 (3:1 in mothers with diabetes) or greater is a highly reliable indication that the fetal lung, and therefore the fetus, is mature. In such a case the infant would be unlikely to develop RDS after birth. As the L/S ratio decreases, the risk of RDS increases.
Unfortunately, the L/S ratio assay involves a long and labor-intensive thin layer chromatography separation of the lipids. An alternative test is an assay based on fluorescence depolarization, implemented on the TDx fluorescence polarimeter and is called TDx Fetal Lung Maturity (FLM) test. This test, which yields the ratio of surfactant to albumin (S/A ratio), is quite sensitive.
FLM results are less affected by other factors such as contaminated blood or meconium. A fluorescent phospholipid analogue (C6-NBD-PC) is added to amniotic fluid and its fluorescence polarization is measured with a TDx fluorescence polarimeter. Polarization values decrease during gestation in parallel with maturation of the pulmonary surfactant system. Polarization value can be used to predict the probability that a fetus will develop respiratory distress syndrome following birth. Infrared (IR) spectroscopy offers an alternative method to detect and quantitate the key surfactants. The infrared spectrum of amniotic fluid shows strong absorptions from protein such as albumin when compared with the surfactant lipids contributing subtle absorption differences to the overall profile.
b. Phosphatidylglycerol (PG). This is a minor component (about 10%) of lung surfactant phospholipids. However, because PG is synthesized almost entirely by mature lung alveolar cells, it is a good indicator of lung maturity. Because PG appears late in gestation, this test indicates a more mature surfactant than that found in the L/S ratio described previously. In healthy pregnant women, PG appears in amniotic fluid after 35 weeks of gestation, and levels gradually increase until term. An advantage of the PG assay is that it is not affected by contamination of amniotic fluid by blood or meconium. These two contaminants cause false-positive and false-negative results for the L/S ratio evaluation. In addition, the presence of PG in the amniotic fluid in the vagina after the membranes are ruptured indicates a low risk for RDS of the newborn. The simultaneous determination of the L/S ratio and the presence of PG is an excellent method of assessing fetal maturity based on pulmonary surfactant.
c. Lamellar body count. This newer test to determine fetal maturity is also based on the presence of surfactant. Lamellar bodies are concentrically layered structures produced by type II pneumocytes. On cross section, these small (about 3 μm) structures look like an onion. These lamellar bodies represent the storage form of pulmonary surfactant. Because lamellar bodies and platelets are indistinguishable to cell counters, the lamellar body count is obtained by analyzing the amniotic fluid with a cell counter and recording the platelet count. Lamellar body results are calculated in units of particle density per microliter of amniotic fluid. Some researchers have recommended cutoffs of 30,000/μL and 10,000/μL to predict low and high risk for RDS, respectively. If the count is greater than 30,000/μL, the negative predictive value for RDS is 100% (i.e., there is a 100% chance that the infant's lungs are mature enough to not experience RDS). If the lamellar body count is less than 10,000/μL, the probability of RDS is high (67%). Values between 10,000/μL and 30,000/mcL represent intermediate risk for RDS. At this time, not enough information is available on lamellar body count in diabetics to advocate its use in this high-risk group. There are several advantages of lamellar body counts. First, they are faster, more precise, and more objective, and they require less amniotic fluid than phospholipid analysis. Second, test results are not invalidated by the presence of blood or meconium. Third, the instrumentation required for this test is readily available, thus allowing it to be performed in all laboratories.
d. Microviscosity. Microvisocity in lipid aggregates is dependent on the L/S ratio and the degree of saturation of fatty acid side chains. The pattern of change of amniotic fluid microviscosity during gestation parallels the expected development of the surfactant system. Amniotic fluid microviscosity is high during early gestation and abruptly and sequentially decreases between the 28th and 36th week of gestation. The measurements are an accurate reflection of the development of the surfactant system and thereby fetal lung maturity. With the development of more accurate testing such as FLM as described above, this testing is no longer routinely performed and is included here more for recent historical value.
2. Sex of the fetus. Sons of mothers who are known to be carriers of X-linked recessive traits have a 50:50 risk of inheritance. It is important to note that amniocentesis is not done to determine the sex of the child just out of interest.
3. Genetic and chromosomal aberrations, such as hemophilia, Down syndrome, and galactosemia. Genetic and chromosomal studies performed on cells aspirated within the amniotic fluid can indicate the gender of the fetus (important in sex-linked diseases such as hemophilia) or many genetic and chromosomal aberrations (e.g., trisomy 21). (See Laboratory Genetics, p. 1104).
4. Fetal status affected by Rh isoimmunization. Mothers with Rh isoimmunization have a series of amniocentesis procedures during the second half of pregnancy to assess the level of bilirubin pigment in the amniotic fluid. The quantity of bilirubin is used to assess the severity of hemolytic anemia in Rh-sensitized pregnancy. The higher the amount of bilirubin, the lower is the amount of fetal hemoglobin. Amniocentesis is usually initiated at 24 to 25 weeks. This allows assessment of the severity of the disease and the status of the fetus. Early delivery or blood transfusion may be indicated. It is important to take into consideration the volume of amniotic fluid because bilirubin concentration will be affected by total fluid volume.
5. Hereditary metabolic disorders, such as cystic fibrosis.
6. Anatomic abnormalities, such as neural tube closure defects (myelomeningocele, anencephaly, spina bifida). Increased levels of alpha-fetoprotein (AFP) in the amniotic fluid may indicate a neural crest abnormality (p. 54). Decreased levels of AFP may be associated with increased risk of trisomy 21.
7. Fetal distress, detected by meconium staining of the amniotic fluid. This is caused by relaxation of the anal sphincter. In this case the normally colorless and pale, straw-colored amniotic fluid may be tinged with green. Other color changes may also indicate fetal distress. For example, a yellow discoloration may indicate a blood incompatibility. A yellow-brown opaque appearance may indicate intrauterine death. A red color indicates blood contamination from either the mother or the fetus.
Amniocentesis may be done on the premise that elective abortion could be performed if the fetus is severely defective. Chorionic villus sampling (CVS) may be even better than amniocentesis for karyotyping and genetic analysis. CVS can be performed earlier in the pregnancy than can amniocentesis. (The earliest one can obtain amniotic fluid is at about 12 to 14 weeks.) Thus with CVS a decision can be made concerning abortion much earlier in the pregnancy than with amniocentesis.
The timing of the amniocentesis varies according to the clinical circumstances. With advanced maternal age and if chromosomal or genetic aberrations are suspected, the test should be done early enough to allow a safe abortion. If information on fetal maturity is sought, performing the study during or after the thirty-fifth week of gestation is best. Placental localization by ultrasonography (see p. 887) should be done before amniocentesis to avoid the needle passing into the placenta, possibly interrupting the placenta, and inducing bleeding or abortion.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient. Allay any fears and allow the patient to verbalize her concerns.
Explain the procedure to the patient. Allay any fears and allow the patient to verbalize her concerns.
• Obtain an informed consent from the patient and her partner.
![]() Tell the patient that no food or fluid is restricted.
Tell the patient that no food or fluid is restricted.
• Evaluate the mother's blood pressure and the fetal heart rate.
• Follow instructions regarding emptying the bladder, which depend on gestational age. Before 20 weeks of gestation, the bladder may be kept full to support the uterus. After 20 weeks, the bladder may be emptied to minimize the chance of puncture.
• Localize the placenta by ultrasound examination before the study to permit selection of a site that will avoid placental puncture.
During
• Place the patient in the supine position.
• Note the following procedural steps:
1. The skin overlying the chosen site (often determined by obstetric ultrasonography) is prepared and usually anesthetized locally.
2. A needle with a stylet is inserted through the midabdominal wall and directed at an angle toward the middle of the uterine cavity (Figure 5-1).
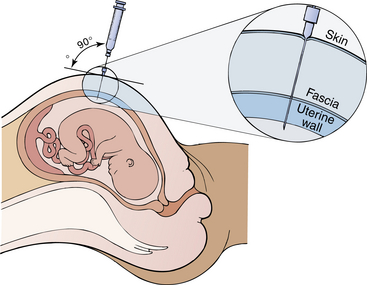
Figure 5-1 Amniocentesis. Ultrasound scanning is usually used to determine the placental site and to locate a pocket of amniotic fluid. The needle is then inserted. Three levels of resistance are felt as the needle penetrates the skin, fascia, and uterine wall. When the needle is placed within the uterine cavity, amniotic fluid is withdrawn.
3. The stylet is then removed and a sterile plastic syringe attached.
4. After 5 to 10 mL of amniotic fluid is withdrawn, the needle is removed. (This fluid volume is replaced by newly formed amniotic fluid within 3 to 4 hours after the procedure.)
5. The specimen is placed in a light-resistant container to prevent breakdown of bilirubin.
6. The site is covered with an adhesive bandage.
7. If the amniotic fluid is bloody, the physician must determine whether the blood is maternal or fetal in origin. Kleihauer-Böetke stain will stain fetal cells pink. Meconium in the fluid is usually associated with a compromised fetus.
• Amniotic fluid volume is calculated by injecting a known concentration of solute (such as para-aminohippuric acid [PAH]) into the amniotic fluid to distribute throughout the amniotic fluid. Amniotic fluid is then withdrawn, and the PAH concentration is determined.
• Note that this procedure is performed by a physician and takes approximately 20 to 30 minutes.
![]() Tell the patient that the discomfort associated with amniocentesis is usually described as a mild uterine cramping that occurs when the needle contacts the uterus. Some women may complain of a “pulling” sensation as the amniotic fluid is withdrawn.
Tell the patient that the discomfort associated with amniocentesis is usually described as a mild uterine cramping that occurs when the needle contacts the uterus. Some women may complain of a “pulling” sensation as the amniotic fluid is withdrawn.
• Remember that many women are extremely anxious during this procedure.
After
• Place amniotic fluid in a sterile, siliconized glass container and transport it to a special chemistry laboratory for analysis. Sometimes the specimen may be sent by air mail to another commercial laboratory for genetic and other testing.
![]() Inform the patient that the results of this study are usually not available for over 1 week.
Inform the patient that the results of this study are usually not available for over 1 week.
• For women who have Rh-negative blood, administer RhoGAM because of the risk of immunization from the fetal blood.
• Assess the fetal heart rate after the test to detect any ill effects related to the procedure. Compare this value with the preprocedural baseline value.
![]() If the patient felt dizzy or nauseated during the procedure, instruct her to lie on her left side for several minutes before leaving the examining room.
If the patient felt dizzy or nauseated during the procedure, instruct her to lie on her left side for several minutes before leaving the examining room.
• Observe the puncture site for bleeding or other drainage.
![]() Instruct the patient to call her physician if she has any amniotic fluid loss, bleeding, temperature elevation, abdominal pain or cramping, fetal hyperactivity, or unusual fetal lethargy.
Instruct the patient to call her physician if she has any amniotic fluid loss, bleeding, temperature elevation, abdominal pain or cramping, fetal hyperactivity, or unusual fetal lethargy.
Test Results and Clinical Significance
Hemolytic disease of the newborn: This may be apparent as increased bilirubin in the amniotic fluid. The fetal hemolysis causes free heme to form. This is then catabolized to bilirubin.
Rh isoimmunization: A rising anti-Rh antibody titer in an Rh-negative woman would indicate potential for erythroblastosis fetalis (Rh-positive fetus). The higher the bilirubin in the amniotic fluid, the greater is the risk to the fetus.
Neural tube closure defects (e.g., myelomeningocele, anencephaly, spina bifida),
Abdominal wall closure defects (e.g., gastroschisis, omphalocele),
An elevated AFP level most commonly indicates neural tube defects. However, other closing defects (e.g., abdominal wall) can occur. Neoplasms associated with neural tube defects may also be associated with increased AFP levels. Blood levels of AFP are also increased with these abnormalities.
Meconium staining: This is evidence of fetal distress and is noted as greenish staining of the amniotic fluid.
Immature fetal lungs: This may occur with premature labor, maternal hypertension, or placental injuries. The risk of RDS increases as evidence of fetal lung immaturity increases. Fetal lung maturity is diminished in diabetic mothers. This is also noted in hydrops fetalis.
Hereditary metabolic disorders (e.g., cystic fibrosis, Tay-Sachs disease, galactosemia),
Genetic or chromosomal aberrations (e.g., sickle cell anemia, thalassemia, Down syndrome),
Sex-linked disorders (e.g., hemophilia):
The genetic defects of many diseases can be recognized through gene recognition and karyotyping. Other genetic defects causing metabolic disorders can be recognized by the results of protein analysis of the amniotic fluid.
Polyhydramnios: This occurs in patients who have diabetes. When polyhydramnios (>2000 mL) is present, the risk of congenital aberrations increases significantly.
Oligohydramnios: This is recognized as less than 300 mL of amniotic fluid at 25 weeks' gestation. It is associated with fetal renal diseases. Near term, it is associated with early membrane rupture, intrauterine growth restriction, or significant postterm pregnancy.
Related Tests
Chorionic Villus Sampling (CVS) (p. 1088). This is a test whereby the chorionic placental tissue (which has the same genetic material as the fetus) is tested for genetic analysis and karyotyping. This is a rapid and accurate method of determining genetic defects. CVS can be performed earlier in pregnancy than can amniocentesis.
Maternal Screen Testing, (p. 354). This is a series of screening tests that can identify fetal distress and chromosomal abnormalities.
Fetoscopy (p. 612). This is another method of obtaining fetal tissue for genetic and maturity testing.
Obstetric Ultrasound (p. 887). Significant fetal disease and evidence of fetal distress can be detected on ultrasound examination. If findings are abnormal, amniocentesis is indicated.
Amyloid Beta Protein Precursor, Soluble (sBPP)
Indications
This test is performed on patients who become increasingly demented and confused. It is a test used to help diagnose Alzheimer disease (AD) and other forms of senile dementia.
Test Explanation
Amyloid protein is a 42-amino-acid peptide that is broken off of a larger amyloid pre-cursor protein (beta APP). These beta amyloid proteins have been shown to be neurotrophic and neuroprotective. Beta amyloid is deposited on the brain in the form of plaques in patients with AD. It has been discovered that these plaques contain damaged nerve cells in a compacted core of beta amyloid protein. As a result of this deposition, levels of beta amyloid are decreased in the cerebrospinal fluid of patients with AD and other forms of dementia. Research has demonstrated the diagnostic potential of this biochemical marker for AD.
Ongoing research has also focused on using cerebrospinal fluid (CSF) levels of tau protein as another biochemical marker for AD. Neurofibrillary tangles, also noted in the brains of patients with AD, are composed primarily of hyperphosphorylated tau. There is a general consensus that CSF levels of tau are significantly increased in patients with AD as compared with healthy control subjects and patients with non-AD neurologic disease. These tests require a CSF sample obtained by lumbar puncture (p. 651).
At this time, there is little or no consensus on the use of screening tests for diagnosing early AD. This is due to lack of sensitivity and specificity and sufficient normative data. However, there is consensus that using a combination of early neuropsychologic changes and biomarkers will facilitate making the diagnosis of prodromal AD earlier than current criteria for probable AD allow.
Recently, PET scanning with amyloid imaging (p. 823) has shown promise for the diagnosis of AD. Pittsburgh Agent B (PIB) appears to reliably detect brain amyloid due to the accumulation of A beta 42 within plaques. Studies so far have revealed high levels of amyloid retention in the brain at prodromal stages of AD and the possibility of discriminating AD from other dementia disorders by scanning with PIB. The PET scans using PIB as the imaging agent have shown a dramatically different amyloid deposition pattern in AD versus normal brains. Since amyloid accumulation is one of the earliest signs of AD, early diagnosis may be facilitated by identifying amyloid early in the disease progression, perhaps before symptoms emerge.
Anti-amyloid beta precursor protein antibody can be identified in brain tissue by immunohistochemistry and is diagnostic for AD.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
• Refer to the instructions for a lumbar puncture and CSF examination (p. 651).
Arthrocentesis With Synovial Fluid Analysis (Synovial Fluid Analysis, Joint Aspiration)
Indications
Arthrocentesis is performed to establish the diagnosis of joint infection, arthritis, crystal-induced arthritis (gout and pseudogout), synovitis, or neoplasms involving the joint. This procedure is also used to identify the cause of joint inflammation or effusion, to monitor chronic arthritic diseases, and to inject antiinflammatory medications (usually corticosteroids) into a joint space.
Test Explanation
Arthrocentesis is performed by inserting a sterile needle into the joint space of the involved joint to obtain synovial fluid for analysis. Synovial fluid is a liquid found in small amounts within the joints. Aspiration (withdrawal of the fluid) may be performed on any major joint, such as the knee, shoulder, hip, elbow, wrist, or ankle.
The fluid sample is examined microscopically and chemically. A gram stain and culture of the fluid is usually performed. Normal joint fluid is clear, straw colored, and quite viscous because of the hyaluronic acid, which acts as a lubricant. Viscosity is reduced in patients with inflammatory arthritis. Viscosity can be roughly estimated by forcing some synovial fluid from a syringe. Fluid of normal viscosity forms a “string” more than 5 cm long; fluid of low viscosity as seen in inflammation drips in a manner similar to water.
The mucin clot test correlates with the viscosity and is an estimation of hyaluronic acid-protein complex integrity. This test is performed by adding acetic acid to joint fluid. The formation of a tight, ropy clot indicates qualitatively good mucin and the presence of adequate molecules of intact hyaluronic acid. Hyaluronic acid can be directly quantified by Enzyme Linked Immunoabsorbent Assay. The mucin clot is poor in quality and quantity in the presence of an inflammatory joint disease, such as rheumatoid arthritis (RA). By itself, synovial fluid should not spontaneously form a fibrin clot (clot without the addition of acetic acid) because normal joint fluid does not contain fibrinogen. If, however, bleeding into the joint (from trauma or injury) has occurred, the synovial fluid will clot.
The synovial fluid glucose value is usually within 10 mL/dL of the fasting serum glucose value. For proper interpretation the synovial fluid glucose and serum glucose samples should be drawn simultaneously after the patient has fasted for 6 hours. The synovial fluid glucose level falls with increasing severity of inflammation. Although lowest in septic arthritis (the synovial fluid glucose value may be less than 50% of the serum glucose value), a low synovial glucose level also may be seen in patients with rheumatoid arthritis. The synovial fluid is also tested for protein, uric acid, and lactate levels. Increased uric acid levels indicate gout. Increased protein and lactate levels indicate bacterial infection or inflammation.
Cell counts are also performed on the synovial fluid. Normally the joint fluid contains less than 200 WBCs/mm3 and 2000 RBCs/mL. An increased WBC count with a high percentage of neutrophils (over 75%) supports the diagnosis of acute bacterial infectious arthritis. Leukocytes can also occur in other conditions, such as acute gouty arthritis and rheumatoid arthritis. The differential white cell count, however, will indicate monocytosis or lymphocytosis with these later-mentioned diseases.
Bacterial and fungal cultures are usually requested and performed when infection is suspected. The administration of antibiotics prior to arthrocentesis may diminish growth of bacteria from synovial fluid cultures and confound results. Smears for acid-fast stains for tubercle bacilli are also performed on the synovial fluid. Synovial fluid is also examined under polarized light for the presence of crystals, which permits differential diagnosis between gout and pseudogout. (The calcium pyrophosphate dihydrate crystals of pseudogout are birefringent [blue on red background] when examined with a polarized light microscope.)
The synovial fluid is also analyzed for complement levels (p. 172). Complement levels are decreased in patients with systemic lupus erythematosus, rheumatoid arthritis, or other immunologic arthritis. These decreased joint complement levels are caused by consumption of the complement induced by the antigen-antibody immune complexes within the joint cavity.
One of the most important tests routinely performed on synovial fluid is the microscopic examination for crystals. For example, urate crystals indicate gouty arthritis. Calcium pyrophosphate crystals are found in pseudogout. Cholesterol crystals occur in rheumatoid arthritis.
A physician performs this procedure in an office or at the patient's bedside in approximately 10 minutes. The only discomfort associated with this test is from the injection of the local anesthetic. The joint-space pain may worsen after fluid aspiration, especially in patients with acute arthritis. The administration of steroids is also associated with pain for as much as 2 days after the injection.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
• Obtain an informed consent if this is the institution's policy.
![]() Keep the patient on nothing by mouth (NPO) status after midnight on the day of the test. This is done to prevent alterations of the chemical determinations (e.g., glucose) that may be performed with the study. However, this study may be done more conveniently in a physician's office without the patient fasting.
Keep the patient on nothing by mouth (NPO) status after midnight on the day of the test. This is done to prevent alterations of the chemical determinations (e.g., glucose) that may be performed with the study. However, this study may be done more conveniently in a physician's office without the patient fasting.
During
• Have the patient lie on his or her back with the joint fully extended.
• Note the following procedural steps:
1. The skin is locally anesthetized to minimize pain.
2. The area is aseptically cleansed, and a needle is inserted through the skin and into the joint space.
3. Fluid is obtained for analysis. The joint area sometimes may be wrapped with an elastic bandage to compress free fluid within a certain area, thereby ensuring maximal collection of fluid.
4. If a corticosteroid or other medications (e.g., antibiotics) are to be administered, a syringe containing the steroid preparation is attached to the needle and the drug is injected.
5. The needle is removed, and a pressure dressing may be applied to the site.
6. Sometimes a peripheral venous blood sample is taken to compare chemical tests on the blood with chemical studies on the synovial fluid.
After
• Assess the joint for any pain, fever, or swelling, which may indicate infection.
• Apply ice to decrease pain and swelling.
• Keep a pressure dressing on the joint to avoid re-collection of joint fluid or development of a hematoma.
![]() Tell the patient to avoid strenuous use of the joint for the next several days.
Tell the patient to avoid strenuous use of the joint for the next several days.
Test Results and Clinical Significance
This can be the result of penetrating trauma or blood-borne infection resulting from bacteremia. One would expect to see a red, warm, swollen, and painful joint. The joint fluid would be expected to have a reduced glucose level, increased levels of WBCs, protein, and lactate (because of the lactate produced by the bacteria). Gram stains and cultures (p. 704) may identify the offending organism.
Degenerative arthritis (osteoarthritis): Degenerative changes involving the joint space may be caused by excess nongouty crystals within the joint space and cartilage. The course is usually chronic and without acute flare-up. Nonsteroidal antiinflammatory drugs are usually helpful.
Synovitis: This can be inflammatory or infectious. The synovial membrane is the tissue surrounding the joint space.
Neoplasm: Synovial, cartilaginous, and bony tumors (benign and malignant) can begin in the joint. Protein levels can be expected to be elevated. Microscopy may reveal malignant cells.
Joint effusion: Joint effusion (fluid in the joint) causes the joint to be swollen. The fluid is obtained to determine the source of the effusion.
Autoimmune or collagen-vascular diseases can be associated with immunogenic arthritis. One may expect a reduced complement level and increased levels of WBCs and protein.
Crystal-induced arthritis occurs when urate (gout) or calcium pyrophosphate (pseudogout) is deposited into the joint-surrounding structures and joint surface cartilage. Inflammation follows, and arthritis occurs. In time, cartilage destruction occurs.
Trauma: When a joint is injured, a joint effusion may develop. This is usually a transudate. However, if a ligament or cartilage is torn, bleeding may occur within the joint.
Related Test
Arthroscopy (p. 583). This is an endoscopic procedure designed to directly view the joint space and to provide access to the joint for surgical treatment of disease and injury.
Breast Cyst and Nipple Discharge Fluid Analysis
Indications
These two tests are used to attempt to make the diagnosis of cancer within breast cysts or to exclude the diagnosis of breast cancer as a cause of persistent nipple discharge.
Test Explanation
Fluid from breast cysts or nipple discharge can be examined cytologically for evidence of cancer cells. Most simple cysts (cysts that contain fluid and no tissue—as recognized by ultrasound, p. 871) are benign. The exceptions are if the aspirated fluid is bloody, the cyst repeatedly recurs after aspirations, or if the cyst does not completely collapse after aspiration. The contents of these simple cysts should be sent for cytologic examination. A complex cyst (one that contains some tissue) can be cancerous (cystic adenocarcinoma of the breast) and its contents should also be aspirated and examined microscopically. The cyst aspiration can be directed by palpation of the doctor or by ultrasound.
Cytologic examination of nipple discharge is not terribly reliable in the identification of cancer. Nearly all nonbloody nipple discharge comes from benign pathology. Only 10% to 12% of bloody discharges are related to breast cancer. Of that small percentage, less than half can be detected by a cytologic examination of the nipple discharge. Cellular deterioration can be misinterpreted as atypical or suspicious cytologic changes. This may cause an unnecessary breast biopsy.
Potential Complications
• Infection in the breast as a result of the needle aspiration
• Pneumothorax as a result of the needle penetrating a thin chest wall in attempting to aspirate a cyst in the posterior portion of the breast
• Hematoma in the breast as a result of intraglandular bleeding from a blood vessel penetrated by the aspirating needle
Procedure and Patient Care
Before
• Because cyst aspiration may cause intraglandular bleeding that may temporarily distort mammography, a bilateral mammogram may be performed before cyst aspiration.
![]() Inform the patient of the proposed procedure.
Inform the patient of the proposed procedure.
![]() Allay the patient's concern about anticipated pain related to cyst aspiration. Only a very-small-bore needle is used. If a larger-bore needle is required, local anesthetic is used first.
Allay the patient's concern about anticipated pain related to cyst aspiration. Only a very-small-bore needle is used. If a larger-bore needle is required, local anesthetic is used first.
During
Nipple Discharge
• Note the following procedure:
1. Express the nipple discharge from the breast.
2. Smear the discharge onto a clean microscope slide as for a Pap test.
3. The cells are immediately fixed either by immersing the slide in equal parts of 95% alcohol and ether or by using a commercial spray (e.g., Aqua Net hair spray). The secretions must be fixed before drying because drying will distort the cells and make interpretation difficult. This fixing process kills most infectious organisms so that the specimen is less infectious to the personnel who handle the specimen.
4. The slide is labeled with the patient's name, date of birth, date of test, and site of the lesion.
Cyst Aspiration
• Note the following procedure:
1. While the patient is in the supine position, the cyst is identified by palpation or by ultrasound guidance.
2. The skin overlying the cyst is prepared in a sterile manner.
3. If a 25-gauge needle is to be used for aspiration, no local anesthetic is required. If, however, the fluid is suspected to be thick, a 20-gauge needle is used. In this circumstance, local anesthetic is infiltrated into the skin.
4. The needle is inserted through the skin and into the cyst. Fluid is aspirated until the cyst is completely collapsed.
5. The fluid is injected into a fixative solution (Carbowax) and appropriately labeled as described previously.
After
• Pressure is applied to the aspiration site. An adhesive bandage is applied.
![]() The patient should be informed that it is not uncommon to develop an ecchymosis in the area of the breast where the aspiration was performed.
The patient should be informed that it is not uncommon to develop an ecchymosis in the area of the breast where the aspiration was performed.
![]() Allay the patient's fears, stating that if clear cyst fluid was obtained, the lesion is most certainly benign.
Allay the patient's fears, stating that if clear cyst fluid was obtained, the lesion is most certainly benign.
Test Results and Clinical Significance
As indicated previously, cystic adenocarcinoma of the breast is very rare. When clear fluid is obtained and the cyst collapses completely, the cyst is considered to be benign.
Intraductal papilloma: This is a common cause of breast discharge. Intraductal papillomas are benign, and no treatment is required unless the discharge is copious.
Breast Ductal Lavage
Indications
This test is performed on women who are at increased risk for developing breast cancer and would make a decision to accept treatment designed to diminish that risk if atypical (premalignant) cells were found in their ducts.
Test Explanation
The theory behind ductal lavage is that by washing out exfoliated cells from a few breast ducts, the risk of developing breast cancer in the near future can be assessed. If atypical cells are obtained, the risk of developing breast cancer in the next decade may be as high as 4 to 10 times normal. Once that risk is identified, the patient may choose to attempt to alter that risk by using chemopreventive medications (such as selective estrogen receptor modulators) or surgery.
Initially, it was hoped that ductal lavage would identify ductal carcinoma of the breast at its earliest stages. The results of several large studies did not support that fact. Its use has now been limited to women who have been found to be at a statistically higher personal risk for breast cancer by breast cancer risk models. These statistical models are based on age of menarche, age of first pregnancy, prior breast surgery, family history, and history of atypical changes in previous breast biopsies. In women found to be at increased risk, many would like more data before they decide to take a medication designed to reduce those risks. If they were found to have atypical cells in the lavage, most would choose to take the medication. If no atypical cells were found, they may choose just close observation.
There are still no data to confirm that the findings do accurately reflect a true risk for breast cancer. Furthermore, there are no data to indicate what a negative lavage means.
Procedure and Patient Care
During
• Note the following procedural steps:
1. Prior to suction, the breast is massaged for a few minutes.
2. A suction apparatus is applied to the nipple area. Ducts that reveal fluid with the suction are then chosen for cannulation.
3. A tiny catheter is gently placed into the nipple and the duct is lavage with 5 to 10 mL of saline.
4. The effluent is then collected in a small tube and sent for cytology.
5. The procedure is then repeated for other ducts that produced fluid with nipple suction. A separate catheter is used for each duct.
6. The sites for each cannulated duct are recorded on a grid representing the nipple for future reference.
• This procedure is performed by a surgeon in the office in approximately 30 minutes. There is minimal to moderate discomfort associated with the nipple suction, duct cannulation, and lavage.
Test Results and Clinical Significance
Atypical cells: Atypical cells indicate that the patient is at an increased risk for developing breast cancer and should consider cancer preventive therapy.
Ductal cancer cells: Identification of cancer cells presents a very perplexing problem because the location of the cancer often cannot be determined thereby precluding conservative simple excision for treatment. It is prudent to confirm the presence of malignant cells through a second cytopathologic opinion.
Related Tests
Mammography (p. 1043). This is an x-ray study of the breast that has proved to be a very accurate method of screening and diagnosing breast cancer.
Ductoscopy (p. 603). This test provides an endoscopic view of the breast ducts.
Magnetic Resonance Imaging (MRI) of the Breast (p. 1106). This is a very sensitive method of breast imaging.
Fetal Fibronectin (fFN)
Indications
To help predict preterm delivery, some doctors now suggest that women with symptoms of preterm labor be screened for the presence of fetal fibronectin (fFN). The presence of fFN in the cervicovaginal secretions of symptomatic women during weeks 22 through 34 of gestation indicates an increased risk of preterm delivery. However, the absence of fFN is a more reliable predictor that the pregnancy will continue for at least another 2 weeks.
Test Explanation
Fibronectin may help with implantation of the fertilized egg into the uterine lining. Normally, fibronectin cannot be identified in vaginal secretions after 22 weeks of pregnancy. However, concentrations are very high in the amniotic fluid. If fibronectin is identified in vaginal secretions after 24 weeks, the patient is at high risk for preterm (premature) delivery within the next 2 weeks. Its use is limited to women whose membranes are intact and cervix dilatation of less than 3 cm in women with signs and symptoms of labor.
A negative fFN test result is a highly reliable predictor that delivery will not occur within the next 2 weeks. A positive result is a less reliable predictor of preterm labor: there is still a fair chance that the pregnancy will continue for at least another 2 weeks. The greatest value of the fFN test is the high level of reliability of a negative test result. A negative test result reassures medical providers and expectant parents that the risk of preterm delivery is currently low, and helps reduce the need for medical interventions. A positive fFN result, although less reliable, allows doctors and patients to take preventive measures to delay labor for as long as possible, by hospitalization and/or administering labor-suppressing (tocolytic) medications.
The American College of Obstetricians and Gynecologists (ACOG) currently does not recommend the test for routine screening, as its use has not been shown to be clinically effective in predicting preterm labor in low-risk, asymptomatic pregnancies. This test can be done at the bedside in a few minutes. This quick assay has been shown to be highly concordant with the original enzyme-linked immunosorbent assay (ELISA), which required 48 hours to obtain a result.
Procedure and Patient Care
During
• Note the following procedural steps:
1. The patient is placed in the lithotomy position.
2. A vaginal speculum is inserted to expose the cervix.
3. Vaginal secretions are collected from the posterior vagina and paracervical area with a Dacron swab that comes with the fibronectin laboratory kit.
4. The slide is labeled with the patient's name, age, estimated date of confinement.
![]() Tell the patient that no discomfort, except for insertion of the speculum, is associated with this procedure.
Tell the patient that no discomfort, except for insertion of the speculum, is associated with this procedure.
• Note that this procedure is performed by a physician or other licensed health care provider in several minutes.
After
![]() Inform the patient that usually the result will be available the next day.
Inform the patient that usually the result will be available the next day.
![]() Educate the patient of the signs of preterm labor: cramps, vaginal bleeding, uterine contractions, pelvic pressure, or the rupture of membranes.
Educate the patient of the signs of preterm labor: cramps, vaginal bleeding, uterine contractions, pelvic pressure, or the rupture of membranes.
![]() Encourage the patient to express concerns regarding the plans for preterm delivery.
Encourage the patient to express concerns regarding the plans for preterm delivery.
Human Papillomavirus (HPV Test, HPV DNA Testing)
Indications
An HPV test is performed to identify genital HPV infection in a woman with an abnormal Pap test.
Test Explanation
HPV is a small, nonenveloped, double-stranded, circular deoxyribonucleic acid (DNA) tumor virus, classified in the genus Papillomavirus of the Papovaviridae family of viruses. More than 100 distinct types of HPV have been identified that infect the genital areas, throat, and mouth of males and females. Approximately 50 of these infect the epithelial membranes of the anogenital tract of women. HPV DNA incorporates itself into the cervical cell genome, promoting its effects through activation of oncogenes and suppression of host cell immune response. HPV protein products prevent DNA repair and programmed cell death, which can lead to instability and unchecked cell growth.
HPV infects the genital epithelium and is spread via skin-to-skin contact. Some strains of HPV cause genital warts, but HPV infections often produce no signs or symptoms. As a result, infected persons are frequently unaware that they are carriers, and transmission occurs unknowingly.
Genital HPV strains are divided into two groups (low and high risk) based on their oncogenic potential and ability to induce viral-associated tumors. Low-risk strains (HPV 6, 11, 42, 43, and 44) are associated with condylomata genital warts and low-grade cervical changes, such as mild dysplasia. Lesions caused by low-risk HPV infection have a high likelihood of regression and little potential for progression, and are considered of no or low oncogenic risk. High-risk strains (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) are associated with intraepithelial neoplasia and are more likely to progress to severe lesions and cervical cancer.
A clear causal relationship has been established between HPV infection and cervical cancer (99% of cervical cancers are related particularly to types 6, 11, 16, and 18). HPV is found in almost all cases of cervical malignancies world-wide. Of the high-risk HPV strains, HPV 16 and 18 are the most carcinogenic and most prevalent. HPV 16 is the predominant strain in almost all regions of the world, with the exception of Southeast Asia, where HPV 18 has the highest prevalence. High-grade cervical intraepithelial lesions are most commonly associated with HPV 16 and 18, yet these strains are also frequently found to be the causative factor in minor lesions and mild dysplasia. The latency period between initial HPV exposure and development of cervical cancer may be months or years. Although rapid progression is possible, average time from initial infection to manifestation of invasive cervical cancer is estimated at about 15 years. Women who have normal Pap test results and no HPV infection are at very low risk (0.2%) for developing cervical cancer. Women who have an abnormal Pap test and a positive HPV test are at higher risk (6% to 7% or greater) for developing cervical cancer.
Gardasil is a vaccine that will guard against HPV 6, 11, 16, and 18. The Centers for Disease Control and Prevention (CDC) recommends Gardasil for all girls and boys 11 or 12 years of age. The vaccine is also recommended in young men and women 13 through 26 years of age who have not already received the vaccine or have not completed all booster shots. Gardasil is given as an intramuscular injection in a series of three shots. Second and third boosters are provided at 2 months and 6 months after the first.
The HPV test is now performed routinely on most women but particularly those who have an abnormal Pap test. Pap test results such as "atypical squamous cells of undetermined significance (ASC-US)" or "low-grade squamous intraepithelial lesion" often prompt a routine HPV test. The most commonly used test is the Hybrid Capture II (HC II) DNA assay. It uses ribonucleic acid (RNA) probes in a modified enzyme-linked immunosorbent assay (ELISA) platform to identify the presence or absence of 13 strains of "high-risk" HPV DNA. Another commonly performed method of HPV testing uses nucleic acid probe/polymerase chain reaction.
Numerous sources indicate that more than 60% of women with an abnormal Pap test will test positive for high-risk HPV. If the HPV test is positive, the woman should undergo colposcopy or repeat cytology to look for a more serious cervical lesion such as cancer. It is well known that HPV infection in younger women is more prevalent and will often spontaneously regress, particularly in those under the age of 30. In contrast, persistent high-risk infection peaks in women over 30. As a result, some physicians recommend that HPV testing be reserved for clinical use in the evaluation of women over the age of 30 to 35 or for younger women with ASC-US with a negative Pap test. Most recent studies have suggested that HPV testing is more sensitive than Pap testing in the detection of serious cervical disease.
HPV testing is typically included as a part of regular screening with a Pap test in these women. There is increasing clinical evidence to suggest that HPV DNA screen with cytology triage (Pap/ThinPrep [p. 743], if positive) is more accurate than conventional cervical cancer screening using Pap/ThinPrep alone. Most cervical cancer is associated with HPV 16 and 18, which occur at earlier ages. Once a woman has been vaccinated with Gardasil (which includes HPV 16 and 18 protection), cervical cancer screening may be delayed. (See Table 5-1 for cervical screening.)
TABLE 5-1
American Cancer Society Recommendations for Cervical Screening
| Population - by Age | Recommended Screening |
| <21 | No screening |
| 21-29 | Pap/Thin Prep alone every 3 years |
| 30-65 | HPV and Pap/Thin Prep co-testing every 5 years |
| >65 | No screening following adequate negative prior screening |
| After hysterectomy | No screening |
| HPV vaccinated | Follow above recommendations (? Delay screening for 3-5 years) |
Several clinical professional societies have made recommendations as to the appropriate use of high-risk HPV testing. HPV high risk (oncogenic) testing is suggested for women who are:
• 30 to 65 (without any prior cervical abnormalities). They may extend the interval between screens to 5 years if they use HPV tests in conjunction with the Pap test. The HPV test should not be used in younger women because many of them will have HPV infection that they will naturally clear without treatment.
• 30 years and older with a prior positive test for low-risk HPV
• 30 years and older with atypical cells of undetermined significance (ASC-US)
• Over 21 and have atypical squamous cells of undetermined significance
• Postmenopausal and have ASC-US or a low-grade squamous intraepithelial lesion. Women over 65 should not be screened with Pap or HPV, as long as they have had consistently normal Pap tests and are not at high risk for cervical cancer.
• Any age and have atypical glandular cells or high-grade squamous intraepithelial lesion after colposcopy
HPV cannot be cultured. It cannot be identified easily on routine histology. Molecular testing is the most effective way of identifying HPV. The hybrid capture to test is a liquid/solid phase signal amplification with multiple RNA probes directed against the genomic sequence of 13 high-risk HPV types, including 16 and 18. When combined with the specimen, HPV DNA/RNA hybrids form. Anti-hybrid antibodies can be identified by a particular label.
HPV HR assay is another essay that can be automated but is associated with significant HPV cross-hybridization. Using similar techniques and HPV 16/18 assay is specifically designed for the detection of those particular HPV types. Both use Invader Chemistry Technology. With in situ hybridization procedures the HPV can be directly associated with a particular high-risk histologic cervical lesion. PCR methods allow for target amplification of HPV DNA. Different methods for the detection of the amplified sequence exist.
Procedure and Patient Care
Before
![]() Explain the procedure for Pap test (p. 743).
Explain the procedure for Pap test (p. 743).
![]() Instruct the patient not to douche or bathe in a tub during the 24 hours before the Pap test. (Some physicians prefer that patients refrain from sexual intercourse for 24 to 48 hours before the test.)
Instruct the patient not to douche or bathe in a tub during the 24 hours before the Pap test. (Some physicians prefer that patients refrain from sexual intercourse for 24 to 48 hours before the test.)
![]() Instruct the patient to empty her bladder before the examination.
Instruct the patient to empty her bladder before the examination.
![]() Instruct the patient to reschedule testing if she is menstruating.
Instruct the patient to reschedule testing if she is menstruating.
During
Note the following procedural steps:
1. The patient is placed in the lithotomy position as for a Pap test.
2. With the use of either a cytology brush or a wooden spatula, a cervical mucus specimen is obtained by placing the instrument into the cervical os and rotating 3 to 5 times in clockwise and counterclockwise directions.
3. After specimen collection, rotate the broomlike device or spatula and cytobrush several times in the collection vial to remove the specimen. Firmly cap the vial and discard the collection devices.
4. Affix a patient identification label to the vial.
5. Seal the vial and place in a plastic specimen bag along with a properly completed cytology requisition form, and send to the laboratory.
• Specimens for HPV can be obtained in two ways. Reflex testing uses the residual cell suspension from liquid-based cytology from the original Pap test. A second sample can also be obtained at the time of the original Pap test or during a second procedure. The cervical specimen is then placed into a transport medium in a separate tube for HPV testing.
• Note that a Pap test is obtained by a nurse or a physician in approximately 10 minutes.
![]() Tell the patient that no discomfort, except for insertion of the speculum, is associated with this procedure.
Tell the patient that no discomfort, except for insertion of the speculum, is associated with this procedure.
Test Results And Clinical Significance
HPV infection: These women should consider more aggressive cervical cancer screening.
Related Test
Papanicolaou Test (p. 743). This is a commonly performed screening test for cervical/uterine cancer that is performed at the same time the specimen is obtained for HPV testing.
Lumbar Puncture and Cerebrospinal Fluid Examination (LP and CSF Examination, Spinal Tap, Spinal Puncture, Cerebrospinal Fluid Analysis)
Normal Findings
Indications
This examination may assist in the diagnosis of primary or metastatic brain or spinal cord neoplasm, cerebral hemorrhage, meningitis, encephalitis, degenerative brain disease, autoimmune diseases involving the central nervous system (CNS), neurosyphilis, and demyelinating disorders (e.g., multiple sclerosis, acute demyelinating polyneuropathy).
Test Explanation
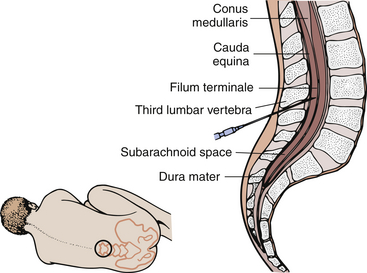
By placing a needle in the subarachnoid space of the spinal column (Figure 5-2), one can measure the pressure of that space and obtain CSF for examination and diagnosis. Lumbar puncture may also be used to inject therapeutic or diagnostic agents and to administer spinal anesthetics. Furthermore, lumbar puncture may be used to reduce intracranial pressure in patients with normal pressure hydrocephalus with pseudotumor cerebri.
CSF is made by selective secretion from the plasma by the choroid plexus (a group of small blood vessels) in the ventricles of the brain. There are three membranes surrounding the brain and spinal cord. From inner to outer, they are the pia mater, arachnoid, and dura mater. The CSF exists within the space between the pia mater and the arachnoid (called the subarachnoid space). This fluid (about 150 to 200 mL) bathes and protects the brain and spinal cord. The fluid acts as a shock absorber when head or back trauma or sudden change in position occurs. The CSF transports nutrients and clears metabolic wastes. Because the CSF is made from plasma, its constituents are about the same as plasma. Chloride levels are higher, however. Blood constituents of larger molecular size cannot be secreted by the choroid plexus (blood-brain barrier).
Examination of the CSF includes evaluation for the presence of blood, bacteria, and malignant cells, as well as quantification of the amount of glucose and protein present. Color is noted, and various other tests, such as a serologic test for syphilis (p. 473), are performed.
Occasionally, lumbar puncture is contraindicated because of nearby infection or suspected spinal canal CSF blockage.
Pressure
By attaching a sterile manometer to the needle used for LP, the pressure within the subarachnoid space can be measured. A pressure of 20 cm H2O or above is considered abnormal and indicative of increased spinal pressure. Because the subarachnoid space surrounding the brain is freely connected to the subarachnoid space of the spinal cord, any increase in intracranial pressure will be directly reflected as an increase at the lumbar site. Tumors, infection, hydrocephalus, and intracranial bleeding can cause increased intracranial and spinal pressure. If it is suspected that this normal connection is obstructed by tumor or postinfection scarring, a Queckenstedt-Stookey test is performed (see “Procedure and Patient Care”) to document that. Intracranial pressure is related to the volume of CSF fluid, which is determined by the homeostatic balance between production and resorption of CSF. Also, because the cranial venous sinuses are connected to the jugular veins, obstruction of those veins or of the superior vena cava will increase intracranial pressure.
Decreased pressure is noted in hypovolemia (dehydration or shock). A chronic leakage of CSF through a previous LP site, or through a nasal sinus fracture with a dura tear, is associated with reduced pressures.
Pressures are routinely measured at the beginning and the end of an LP. If there is a significant difference in these values, one must suspect a spinal cord obstruction (tumor). In these instances, a small amount of CSF exists below the tumor. Removal of a large percentage of that fluid will drastically reduce the pressure. Large differences in opening and closing pressures are also seen in patients with hydrocephalus. If high opening pressures are noted, normal volumes of CSF should not be removed to prevent the risk of cerebellar herniation. One must be aware that a child who is crying and holding the breath may have transient elevations of pressure that reduce as the child relaxes.
Color
Normal CSF is clear and colorless. Xanthochromia (usually refers to a yellow tinge) is commonly used to indicate an abnormal color of CSF. Color differences can occur with hyperbilirubinemia, hypercarotenemia, melanoma, or elevated protein levels.
A cloudy appearance may indicate an increase in the WBC count or protein level. Normally CSF contains no blood. A red tinge to the CSF indicates the presence of blood. Blood may be present because of bleeding into the subarachnoid space or because the needle used in the LP has inadvertently penetrated a blood vessel on the way into the subarachnoid space. These causes of bleeding must be differentiated because it is important to identify and document a subarachnoid bleed (Table 5-2).
TABLE 5-2
Differential Diagnosis of Causes of Blood in the Cerebrospinal Fluid (CSF)
| Traumatic Puncture | Subarachnoid Bleeding | |
| CSF pressure | Low | High |
| Duration of bleeding | Decreases when CSF is withdrawn | No change in color when CSF is withdrawn |
| Clotting | Present | Absent |
| Repeat lumbar puncture | Not bloody | Bloody |
| Centrifugation | Clear fluid | Xanthochromia |
With a “traumatic puncture” the blood within the CSF will clot. No clotting occurs in a patient with subarachnoid hemorrhage. Also, with a traumatic tap the fluid clears toward the end of the procedure when successive CSF samples are obtained. This clearing does not occur with a subarachnoid hemorrhage.
Blood
Blood within the CSF indicates cerebral hemorrhage into the subarachnoid space or a “traumatic tap” as just described.
Cells
The number of red blood cells (RBCs) is merely an indication of the amount of blood present within the CSF. Except for a few lymphocytes, the presence of white blood cells (WBCs) in the CSF is abnormal (Table 5-3). The presence of polymorphonuclear leukocytes (neutrophils) is indicative of bacterial meningitis or cerebral abscess. When mononuclear leukocytes are present, viral or tubercular meningitis or encephalitis is suspected. Leukemia or other primary or metastatic malignant tumors may cause elevated WBCs. Pleocytosis is a term used to indicate turbidity of CSF because of an increased number of cells within the fluid. WBCs can be present in the CSF as a result of a “traumatic tap,” where the spinal needle hits a blood vessel while the spinal tap is being performed. However, more than 1 WBC per 500 RBCs is considered pathologic and can indicate infection such as meningitis.
TABLE 5-3
Causes of Leukocytes in the Cerebrospinal Fluid
| Cell Type | Infection | Other Diseases |
| Neutrophils | Bacterial meningitis Tubercular meningitis Cerebral abscess |
Subarachnoid bleeding Tumor |
| Lymphocytes or plasma cells | Viral, tubercular, fungal, syphilitic meningitis | Multiple sclerosis Guillain-Barré syndrome |
| Eosinophils | Parasitic meningitis | Allergic reaction to radiopaque dyes |
| Macrophages | Tubercular, fungal meningitis | Hemorrhage, brain infarction |
Culture and Sensitivity
Most of the organisms that cause meningitis or brain abscess can be cultured from the CSF. Organisms found also may include atypical bacteria, fungi, or Mycobacterium tuberculosis. A Gram stain (p. 704) of the CSF may give the clinician preliminary information about the causative infectious agent. This may allow appropriate antibiotic therapy to be initiated before the 24 to 72 hours necessary to complete the culture and sensitivity report.
There are microorganisms that are viable but cannot be grown in culture. There are also viruses and parasites associated with meningitis and brain abscesses that are not detected by traditional bacterial culture techniques.
The most common causes of meningitis include Haemophilus influenzae (in children) and Neisseria or Streptococcus in adults.
Protein
Normally very little protein is found in CSF because protein is a large molecule that does not cross the blood-brain barrier. The proportion of albumin to globulin is normally higher in CSF than in blood plasma (p. 424) because albumin is smaller than globulin and therefore can pass more easily through the blood-brain barrier. The amount of protein is usually lower in CSF obtained from the cisternal puncture and even lower still with a ventricular puncture, compared with the CSF obtained from an LP. Disease processes, however, can alter the permeability of the blood-brain barrier, allowing protein to leak into the CSF. Examples of diseases that may be associated with a more permeable blood-brain barrier include infectious or inflammatory processes such as meningitis, encephalitis, or myelitis. Furthermore, CNS tumors may produce and secrete protein into the CSF. Obstruction of CSF flow in the spinal canal caused by tumors or a disk is also associated with high protein counts because normal CSF circulation and resorption are impaired by the obstruction.
CSF protein electrophoresis is very important in the diagnosis of CNS diseases. Patients with multiple sclerosis, neurosyphilis, or other immunogenic degenerative central neurologic disease have elevated immunoglobulins in their CSF. Normally, less than 12% of the total protein consists of gamma globulin. An increase in the CSF level of immunoglobulin G (IgG), an increase in the ratio of IgG to other proteins (e.g., albumin), and the detection of oligoclonal gamma globulin bands are highly suggestive of inflammatory and autoimmune diseases of the CNS, especially multiple sclerosis (MS). Myelin basic protein, a component of myelin (the substance that surrounds normal nerve tissue) can be elevated when demyelinating diseases (such as MS or amyotrophic lateral sclerosis) occur. This protein, detected by radioimmunoassay (RIA) of the CSF, can be used to monitor the course of these deteriorating diseases.
Because albumin and prealbumin are not made in the CNS, increased levels of these specific proteins indicate increased permeability of the blood-brain barrier (as discussed previously).
Glucose
The glucose level is decreased when bacteria, inflammatory cells, or tumor cells are present. A blood sample for glucose (p. 253) is usually drawn before the spinal tap is performed. A CSF glucose level less than 60% of the blood glucose level may indicate meningitis or neoplasm.
Chloride
The chloride concentration in CSF may be decreased in patients with meningeal infections, tubercular meningitis, and conditions of low blood chloride levels. An increase in the chloride level in CSF is not neurologically significant; it correlates with the blood levels of chloride (p. 152). CSF is not routinely evaluated for chloride; this test is done only if specifically requested.
Lactic Dehydrogenase
Quantification of lactic dehydrogenase (LDH) (specifically, fractions 4 and 5; p. 329) is helpful in diagnosing bacterial meningitis. The source of LDH is the neutrophils that fight the invading bacteria. When the LDH level is elevated, infection or inflammation is suspected. The elevated WBC count associated with CNS leukemia is also associated with elevated LDH levels. The nerve tissue in the CNS is also high in LDH (isoenzymes 1 and 2). Therefore disease directly affecting the brain or spinal cord (e.g., stroke) is associated with elevated LDH levels.
Lactic Acid
Elevated levels indicate anaerobic metabolism associated with decreased oxygenation of the brain. The CSF lactic acid level is increased in both bacterial and fungal meningitis but not in viral meningitis. The lactic acid level is also increased when the CSF glucose level is very low or the CSF WBC count is elevated. Because lactic acid does not readily pass through the blood-brain barrier, elevated blood lactate levels are not reflected in the CSF. Chronic cerebral hypoxemia or cerebral ischemia (hypoxic encephalopathy) is associated with elevated CSF lactic acid levels. Lactic acid levels can also be increased in patients with some forms of mitochondrial diseases that affect the CNS.
Cytology
Examination of cells found in the CSF can determine if they are malignant. Tumors in the CNS may shed cells from their surface. These cells can float freely in CSF. Their presence suggests neoplasm as the cause of any neurologic symptoms.
Tumor Markers
Increased levels of tumor markers such as carcinoembryonic antigen, alpha-fetoprotein, or human chorionic gonadotropin may indicate metastatic tumor.
Serology for Syphilis
Latent syphilis is diagnosed by performing one of many available serologic tests on CSF. These include the following:
• The Venereal Disease Research Laboratory (VDRL) test (p. 473)
• The fluorescent treponemal antibody (FTA) test (p. 473): The FTA test is considered to be the most sensitive and specific. When test results are positive, the diagnosis of neurosyphilis is made and appropriate antibiotic therapy is initiated.
Glutamine
The CSF can be evaluated for the presence of glutamine. Elevated glutamine levels are helpful in the detection and evaluation of hepatic encephalopathy and hepatic coma. The glutamine is made by increased levels of ammonia, which are commonly associated with liver failure. (See discussion of serum ammonia on p. 59.) Levels of glutamine are also often increased in patients with Reye syndrome.
C-Reactive Protein
As noted on p. 184, C-reactive protein (CRP) is a nonspecific, acute-phase reactant used in the diagnosis of bacterial infections and inflammatory disorders. Elevated CSF levels of CRP have been useful in the diagnosis of bacterial meningitis. Failure to find elevated CSF levels of CRP appears to be strong evidence against bacterial meningitis. Some research studies have shown that CSF levels of CRP have been valuable in distinguishing bacterial meningitis from viral meningitis, tuberculosis meningitis, febrile convulsions, and other central nervous system disorders. Serum levels of CRP (see p. 184) are more frequently used in the diagnosis of bacterial meningitis.
LP is performed by a physician in approximately 20 minutes. This procedure is described as uncomfortable or painful by most patients. Some patients complain of feeling pressure from the needle. Some patients complain of a shooting pain in their legs.
Contraindications
• Patients with increased intracranial pressure: The LP may induce cerebral or cerebellar herniation through the foramen magnum.
• Patients who have severe degenerative vertebral joint disease: It is very difficult to pass the needle through the degenerated arthritic interspinal space.
• Patients with infection near the LP site: Meningitis can result from contamination of CSF with infected material.
• Patients receiving anticoagulation drugs because of the risk for epidural hematoma.
Potential Complications
• Persistent CSF leak, causing severe headache
• Introduction of bacteria into CSF, causing suppurative meningitis
• Herniation of the brain through the tentorium cerebelli or herniation of the cerebellum through the foramen magnum: In patients with increased intracranial pressure, the quick reduction of pressure in the spinal column by release through the LP may induce herniation of the brain. This can cause compression of the brainstem, which may result in deterioration of the patient's neurologic status and death. In adults, especially, most clinicians will obtain a computed tomography (CT) scan of the head before performing lumbar puncture to identify intracranial abnormalities and thus avoid the risk of brain herniation.
• Inadvertent puncture of the spinal cord, caused by inappropriately high puncture of the spinal canal
• Puncture of the aorta or vena cava, causing serious retroperitoneal hemorrhage
Procedure and Patient Care
Before
![]() Explain the procedure to the patient. Many patients have misconceptions regarding LP. Allay the patient's fears and allow time to verbalize concerns.
Explain the procedure to the patient. Many patients have misconceptions regarding LP. Allay the patient's fears and allow time to verbalize concerns.
• Obtain informed consent if required by the institution.
• Perform a baseline neurologic assessment of the legs by assessing the patient's strength, sensation, and movement.
![]() Tell the patient that no fasting or sedation is required.
Tell the patient that no fasting or sedation is required.
![]() Instruct the patient to empty the bladder and bowels before the procedure.
Instruct the patient to empty the bladder and bowels before the procedure.
![]() Explain to the patient that he or she must lie very still throughout this procedure. Movement may cause traumatic injury. Encourage the patient to relax and take deep, slow breaths with the mouth open.
Explain to the patient that he or she must lie very still throughout this procedure. Movement may cause traumatic injury. Encourage the patient to relax and take deep, slow breaths with the mouth open.
During
• Note the following procedural steps:
1. This study is a sterile procedure that can be easily performed at the bedside. The patient is usually placed in the lateral decubitus (fetal) position (see Figure 5-2).
2. The patient is instructed to clasp the hands on the knees to maintain this position. Someone usually helps the patient maintain this position. (A sitting position also may be used.)
3. A local anesthetic is injected into the skin and subcutaneous tissues after the site has been aseptically cleaned.
4. A spinal needle containing an inner obturator is placed through the skin and into the spinal canal.
5. The subarachnoid space is entered.
6. The insert (obturator) is removed, and CSF can be seen slowly dripping from the needle.
7. The needle is attached to a sterile manometer, and the pressure (opening pressure) is recorded.
8. Before the pressure reading is taken, the patient is asked to relax and straighten the legs to reduce the intraabdominal pressure, which causes an increase in CSF pressure.
9. Three sterile test tubes are filled with 5 to 10 mL of CSF. Usually the first tube is sent for chemical and immunologic testing because these results are not affected by any blood if a “traumatic tap” occurs. The second may be sent for culture, and the third is used for microscopic examination.
• Note that if blockage in CSF circulation in the spinal subarachnoid space is suspected, a Queckenstedt-Stookey test may be performed. For this test the jugular vein is occluded either manually by digital pressure or by a medium-sized blood pressure cuff inflated to approximately 20 mm Hg. Within 10 seconds after jugular occlusion, CSF pressure should increase 15 to 40 cm H2O and then promptly return to normal within 10 seconds after release of the pressure. A sluggish rise or fall of CSF pressure suggests partial blockage of CSF circulation. No rise after 10 seconds suggests a complete obstruction within the spinal canal.
After
• Apply digital pressure and an adhesive dressing to the puncture site.
• Place the patient in the prone position with a pillow under the abdomen to increase the intraabdominal pressure, which will indirectly increase the pressure in the tissues surrounding the spinal cord. This retards continued CSF flow from the spinal canal.
• All testing of the CSF is ordered stat to diminish the false results that may occur because of cellular deterioration, and so on.
![]() Encourage the patient to drink increased amounts of fluid with a straw to replace the CSF removed during the lumbar puncture. Drinking with a straw will enable the patient to keep the head flat.
Encourage the patient to drink increased amounts of fluid with a straw to replace the CSF removed during the lumbar puncture. Drinking with a straw will enable the patient to keep the head flat.
• Usually keep the patient in a reclining position for up to 12 hours to avoid the discomfort of potential postpuncture spinal headache. Allow the patient to turn from side to side as long as the head is not raised.
• Label and number the specimen jars appropriately and deliver them to the laboratory immediately after the test. Refrigeration will alter test results. A delay between collection time and testing can invalidate results, especially cell counts.
• Assess the patient for numbness, tingling, and decreased movement of the extremities; pain at the injection site; drainage of blood or CSF at the injection site; and the ability to void. Notify the physician of any unusual findings.
Test Results and Clinical Significance
The CSF can be expected to be turbid, to contain malignant cells, and to have elevated protein and LDH levels.
Multiple sclerosis and other demyelinating diseases:
The CSF of these patients may be turbid; it may contain increased protein levels (including myelin basic protein) and oligoclonal bands of proteins; and it may be associated with elevated LDH levels.
Neurosyphilis: Not only do these patients have elevated protein levels, increased turbidity, and increased LDH levels in their CSF, but immunologic testing is also positive.
Related Tests
Glucose (p. 253). This test is concomitantly performed with the LP to assess the CSF glucose level.
Serum Protein (p. 424). Along with the LP, measurement of serum protein levels is helpful in the calculation of many formulas involved in the evaluation of CSF.
Amyloid Beta Protein Precursor (p. 639). This test is performed on CSF to diagnose Alzheimer's disease.
Pancreatic Enzymes (Pancreatic Secretory Test, Amylase, Lipase, Trypsin, Chymotrypsin)
Indications
This is a corroborative test used in the evaluation of cystic fibrosis (CF) and pancreatitis (acute and chronic). This test is indicated in children with recurrent respiratory tract infections, malabsorption syndromes, or failure to thrive.
Test Explanation
CF is an inherited disease characterized by abnormal secretion by exocrine glands within the bronchi, small intestines, pancreatic ducts, bile ducts, and skin (sweat glands). Because of this abnormal exocrine secretion, children with cystic fibrosis develop mucus plugs that obstruct their pancreatic ducts that can lead to significant malabsorption, steatorrhea, and diarrhea. The pancreatic enzymes (e.g., amylase [p. 61], lipase [p. 339], trypsin, and chymotrypsin) cannot be expelled into the duodenum and therefore are either completely absent or present only in diminished quantities within the duodenal aspirate. For the same reasons, bicarbonate and other neutralizing fluids cannot be secreted from the pancreas. In this test, secretin and pancreozymin are used to stimulate pancreatic secretion of these enzymes and bicarbonate into the duodenum. The duodenal contents are then aspirated and examined for pH, bicarbonate, and pancreatic enzyme levels. Amylase is the most frequently measured enzyme. Diminished values are suggestive of cystic fibrosis. Pancreatic enzyme testing is not diagnostic of cystic fibrosis, but is an excellent screening test especially in the newborn with meconium ileus. Genetic testing is required for definitive diagnosis of cystic fibrosis.
Trypsinogen, another pancreatic exocrine enzyme, is measured in the serum as trypsin-like immunoreactivity. This test is used to support the diagnosis of chronic pancreatitis. Levels diminish as pancreatic exocrine function becomes increasingly impaired.
When any of these pancreatic enzymes are measured in the serum, they can reflect acute inflammation of the pancreas. Like amylase and lipase, trypsin, chymotrypsin, and trypsin-like immunoreactivity are increased with acute pancreatic inflammation. Likewise, in patients with burned-out chronic pancreatitis, serum measurements of these pancreatic enzymes are low.
Trypsinogen has two isoenzymes that are excreted in the urine. Trypsinogen-1 is rapidly reabsorbed in the kidneys. Trypsinogen-2, however, is not well reabsorbed by the kidneys and concentrations will increase in the urine during acute pancreatitis.
A physician obtains the duodenal contents in approximately 2 hours in the x-ray department. Discomfort and gagging may occur during placement of the Dreiling tube. The pancreatic enzymes are then measured and serially diluted for quantification.
Procedure and Patient Care
During
• Note the following procedural steps:
1. With the use of fluoroscopy, a Dreiling tube is passed through the patient's nose and into the stomach.
2. The distal lumen of the tube is placed within the duodenum.
3. The proximal lumen of the tube is placed within the stomach.
4. Both lumens are aspirated. The gastric lumen is continually aspirated to avoid contamination of the gastric contents in the duodenum aspirate.
5. A control specimen of the duodenal juices is collected for 20 minutes.
6. The patient is tested for sensitivity to secretin and pancreozymin by low-dose intradermal injection.
7. If no sensitivity is present, these hormones are administered intravenously (IV). Secretin can be expected to stimulate pancreatic water and bicarbonate secretion. Pancreozymin can be expected to stimulate pancreatic enzyme (lipase, amylase, trypsin, and chymotrypsin) secretion.
8. Four duodenal aspirates are collected at 20-minute intervals and placed in the specimen container.
9. Each specimen is analyzed for pH, volume, bicarbonate, and amylase levels.
Test Results and Clinical Significance
 Decreased Levels
Decreased Levels
Cystic fibrosis: These patients do not have adequate levels of exocrinic pancreatic enzymes or bicarbonates because of mucus plugging of the small pancreatic duct tributaries.
Sprue: The pathophysiology of this observation is not definitely known. It is thought that patients with sprue have a damaged intestinal mucosa. As a result, they do not have a normal stimulatory response to secrete secretin and pancreozymin. Therefore the pancreas is chronically under stimulated. When these hormones are administered, the pancreas can respond to a slight degree, but not as much as normal because of the previous prolonged periods of absence of stimulation.
Chronic pancreatitis: These patients do not have adequate levels of exocrinic pancreatic enzymes or bicarbonates because of pancreatic acinar cell destruction.
Related Tests
Sweat Electrolytes (p. 678). This is the definitive test used in the diagnosis of cystic fibrosis. Skin sweat is analyzed for sodium and chloride content.
Genetic Testing (p. 1093). This is another confirmatory test for cystic fibrosis or hereditary pancreatitis.
Amylase (p. 61), Lipase (p. 339). When measured in the serum, these pancreatic enzymes will be increased in patients with acute pancreatitis.
Paracentesis (Peritoneal Fluid Analysis, Abdominal Paracentesis, Ascitic Fluid Cytology, Peritoneal Tap)
Normal Findings
Indications
Paracentesis is performed on patients who have unexplained ascites to determine the cause. It is an important part of evaluating the patient with multiple trauma to rule out abdominal trauma. Paracentesis is also performed to relieve the intraabdominal pressure that accumulates with large-volume ascites.
Test Explanation
Paracentesis is an invasive procedure entailing the insertion of a needle into the peritoneal cavity for removal of ascitic fluid. The peritoneum is defined as the space between the visceral peritoneum (thin membrane covering all the abdominal organs) and the parietal peritoneum (thin membrane covering the inside of the abdominal wall). Within the peritoneal membrane is an intricate network of capillary and lymphatic vessels. Fluid is constantly being secreted by the peritoneal membranes and constantly being reabsorbed by those same membranes. If secretion is increased or reabsorption blocked, buildup of peritoneal fluid (ascites) will develop.
Peritoneal fluid is removed for diagnostic and therapeutic purposes. Diagnostically paracentesis is performed to obtain and analyze fluid to determine the cause of the peritoneal effusion. Peritoneal fluid is classified as transudate or exudate (Table 5-4). This is an important differentiation and is very helpful in determining the cause of the effusion. Transudates are most frequently caused by congestive heart failure, cirrhosis, nephrotic syndrome, myxedema, peritoneal dialysis, hypoproteinemia, and acute glomerulonephritis. Exudates are most often found in infectious or neoplastic conditions. However, collagen-vascular disease, pulmonary infarction, gastrointestinal diseases, trauma, and drug hypersensitivity also may cause an exudative effusion.
TABLE 5-4
Differentiation Between Transudate and Exudate
| Transudate | Exudate | |
| Total protein fluid/serum ratio | <0.5 | >0.5 |
| Total protein level | <3 g/dL | >3 g/dL |
| LDH fluid/serum ratio | <0.6 | >0.6 |
| Albumin gradient | <1.1 | >1.1 |
| Serum – Fluid = Albumin gradient | ||
| Specific gravity | >1.015 | >1.015 |
| Clotting | None | Present |
| WBCs | <300/μL | >500/μL |
| Differential | Mononuclear | Neutrophils |
| Glucose | Equal to serum | <60 mg/dL |
| Serum – Fluid = Glucose difference | <30 mg/dL | >30 mg/dL |
| Appearance | Clear, thin fluid | Cloudy, viscous |
| Etiology | Cirrhosis, nephrosis, heart failure, low protein | Infection, inflammation, malignancy, collagen-vascular diseases |
Therapeutically, this procedure is done to remove large amounts of ascitic fluid from the abdominal cavity. These patients usually experience transient relief of symptoms (shortness of breath, distention, and early satiety) because of the fluid within the abdominal cavity.
The peritoneal fluid is usually evaluated for gross appearance, RBCs, WBCs, protein, glucose, amylase, ammonia, alkaline phosphatase, lactate dehydrogenase (LDH), cytology, bacteria, fungi, and other tests such as CEA levels. Each is discussed separately. Urea and creatinine may be measured if there is a question that the fluid may represent urine from a perforated bladder.
Gross Appearance
Transudative peritoneal fluid may be clear, serous, or light yellow, especially in patients with hepatic cirrhosis. Milk-colored peritoneal fluid may result from the escape of chyle from blocked abdominal or thoracic lymphatic ducts. Conditions that may cause lymphatic blockage include lymphoma, carcinoma, and tuberculosis involving the abdominal or thoracic lymph nodes. The triglyceride value in a chylous effusion exceeds 110 mg/dL.
Cloudy or turbid fluid may result from inflammatory or infectious conditions such as peritonitis, pancreatitis, and appendicitis. Bloody fluid may be the result of a traumatic tap (the aspirating needle penetrates a blood vessel), intraabdominal bleeding, tumor, or hemorrhagic pancreatitis. Bile-stained, green fluid may result from a ruptured gallbladder, acute pancreatitis, or perforated intestines.
Cell Counts
Normally, no RBCs should be present. The presence of RBCs may indicate neoplasms, tuberculosis, or intraabdominal bleeding. Increased WBC counts may be seen with peritonitis, cirrhosis, and tuberculosis.
Protein Count
Total protein levels greater than 3 g/dL are characteristic of exudates, whereas transudates usually have a protein content of less than 3 g/dL. It is now thought that the albumin gradient between serum and ascitic fluid can differentiate better between the transudate and exudate nature of ascites than can the total protein content. This gradient is obtained by subtracting the ascitic albumin value from the serum albumin value. Values of 1.1 g/dL or more suggest a transudate, which is usually caused by portal hypertension due to cirrhosis. Values less than 1.1 g/dL suggest an exudate but will not differentiate the potential cause of the exudate (malignancy from infection or inflammation).
Because there is significant overlap in protein values differentiating transudate from exudate, the total protein ratio (fluid/serum) has been considered to be a more accurate criterion. A total protein ratio of fluid to serum of greater than 0.5 is considered to indicate an exudate.
Glucose
Usually peritoneal glucose levels approximate serum glucose levels. Decreased levels may indicate tuberculous or bacterial peritonitis or peritoneal carcinomatosis.
Amylase
Increased amylase levels may be seen in patients with pancreatic trauma, pancreatic pseudocyst, acute pancreatitis, and intestinal necrosis, perforation, or strangulation. In these diseases, the amylase level is usually less than 1.5 times higher than serum levels.
Ammonia
High ammonia levels occur in ruptured or strangulated intestines and also with a ruptured appendix or ulcer.
Alkaline Phosphatase
Levels of alkaline phosphatase are greatly increased in infarcted or strangulated intestines.
Lactic Dehydrogenase
A peritoneal fluid/serum LDH ratio of greater than 0.6 is typical of an exudate. An exudate is identified with a higher degree of accuracy if the peritoneal fluid/serum protein ratio is greater than 0.5 and the peritoneal fluid/serum LDH ratio is greater than 0.6.
Cytology
A cytologic study is performed to detect tumors. The tumors most often seen are ovarian, pancreatic, colon, and gastric. The interpretation of cytologic changes requires that the pathologist have considerable experience in cytology. It can be difficult to differentiate malignancy from severely inflammatory mesothelial cells. In general, malignant cells tend to clump together and to have a high nucleus/cytoplasm ratio, prominent and multiple nucleoli, and unevenly distributed chromatin.
Cytologic examination of the fluid is improved by spinning down a large volume of fluid and examining the sediment. A large number of cells can be seen and compared with each other.
Bacteria
Usually the fluid is cultured and the antibiotic sensitivities are determined. Gram stains (p. 704) are often performed.
Gram Stain and Bacteriologic Culture
The presence of bacteria may indicate a ruptured intestine, primary peritonitis, or infections such as appendicitis, pancreatitis, or tuberculosis. Culture and gram stains are used to assist in the identification of the organisms that may be involved in the infection. Microbial cultures may provide information concerning possible antibiotic sensitivity or resistance. (See p. 704 for a more thorough discussion of Gram stain, cultures, and sensitivity.) These tests are routinely performed to diagnose bacterial peritonitis. If possible, these tests should be done before initiation of antibiotic therapy.
Carcinoembryonic Antigen
Elevated peritoneal fluid levels for CEA are associated with abdominal malignancy, usually arising from the GI tract.
Paracentesis is performed by a physician at the patient's bedside, in a procedure room, or in the physician's office in less than 30 minutes. Usually the volume removed is limited to about 4 L at any one time to avoid hypovolemia if the fluid is rapidly reaccumulated. Although local anesthetics eliminate pain at the insertion site, the patient may feel a pressure-like pain as the needle is inserted.
Potential Complications
• Hypovolemia if a large volume of peritoneal fluid was removed and the fluid reaccumulates, with the fluid coming from the intravascular volume
• Hepatic coma in a patient with chronic liver disease
• Seeding of the needle tract with tumor cells when malignant ascites exists
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
• Obtain informed consent for this procedure.
![]() Tell the patient that no fasting or sedation is necessary.
Tell the patient that no fasting or sedation is necessary.
![]() Have the patient urinate or empty the bladder before the test to avoid inadvertent puncture of the bladder with the aspirating needle.
Have the patient urinate or empty the bladder before the test to avoid inadvertent puncture of the bladder with the aspirating needle.
During
• Note the following procedural steps:
1. Position the patient in a high-Fowler position in bed.
2. Paracentesis is performed under strict sterile technique. A paracentesis tray usually contains all necessary supplies.
3. The needle insertion site is aseptically cleansed and anesthetized locally.
4. A scalpel may be used to make a stab wound into the peritoneal cavity approximately 1 to 2 inches below the umbilicus.
5. A trocar, cannula, or needle is threaded through the incision.
6. A piece of plastic tubing is attached to the cannula. The other end of the tubing is placed in the collection receptacle (usually a container with a pressurized vacuum).
After
• All tests performed on peritoneal fluid should be performed immediately to avoid false results related to chemical or cellular deterioration.
• Place a small bandage over the needle site.
• Label the specimen with the patient's name, date, source of fluid, and diagnosis.
• Send the specimen to the laboratory promptly.
• Observe the puncture site for bleeding, continued drainage, or signs of inflammation.
• Measure the abdominal girth and weight of the patient; compare with baseline values.
• Monitor vital signs frequently for evidence of hemodynamic changes. Watch for signs of hypotension if a large volume of fluid was removed.
• Note any recent antibiotic therapy on the laboratory requisition slip.
• Because of the high protein content of ascitic fluid, albumin infusions may be ordered after paracentesis to compensate for protein loss. Monitor serum protein and electrolyte (especially sodium) levels.
• Occasionally ascitic fluid continues to leak out of the needle track after removal of the needle. A suture can stop that. If this is unsuccessful, a collection bag should be applied to the skin to allow for measurement of the volume of fluid loss.
Test Results and Clinical Significance
Exudate
Lymphoma: These tumors can involve the lymph nodes of the chest and abdomen. Reabsorption of fluid cannot occur, and chylous effusion develops.
Carcinoma: When cancer involves the peritoneal membranes, reabsorption of fluid is diminished. Furthermore, the tumors (especially ovarian) can secrete large volumes of fluid. Ascites develops.
Transudate
The capillary vessels experience an increased portal venous drainage pressure. Reabsorption is diminished and fluid accumulates.
The nephrotic syndrome is characterized by renal albumin wasting. This and other forms of hypoproteinemia are associated with decreased intravascular oncotic pressure. The fluid tends to leak out of the intravascular space into the peritoneum.
Congestive heart failure: The venous drainage of peritoneum is diminished by the right heart failure that exists and causes increased venous pressures. Peritoneal fluid accumulates.
Pericardiocentesis
Indications
Pericardiocentesis is performed to determine the cause of an unexplained pericardial effusion. It is also performed to relieve the intrapericardial pressure that accumulates with a large volume of fluid and inhibits diastolic filling.
Test Explanation
Pericardiocentesis, which involves the aspiration of fluid from the pericardial sac with a needle, may be performed for therapeutic and diagnostic purposes. Therapeutically the test is performed to relieve cardiac tamponade by removing blood or fluid to improve diastolic filling. Diagnostically pericardiocentesis is performed to remove a sample of pericardial fluid for laboratory examination to determine the cause of the fluid accumulation. This is similar to the evaluation described for pleural fluid on p. 681.
A physician usually performs this procedure in the cardiac catheterization laboratory, operating room, or emergency room in approximately 10 to 20 minutes. This procedure is associated with very little discomfort. Most patients feel pressure when the needle is inserted into the pericardial sac.
Potential Complications
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
• Obtain informed consent for this procedure.
• Restrict fluid and food intake for at least 4 to 6 hours (if this is an elective procedure).
• Obtain intravenous (IV) access for infusion of fluids and cardiac medications if required.
• Administer pretest medication. Atropine may be given to prevent the vasovagal reflex of bradycardia and hypotension.
During
• Note the following procedural steps:
1. The patient is placed in the supine position.
2. An area in the fifth to sixth intercostal space at the left sternal margin (or subxyphoid) is prepared and draped. Alternatively the subxyphoid space is used for access to the pericardium.
3. After a local anesthetic is administered, a pericardiocentesis needle is placed on a 50-mL syringe and introduced into the pericardial sac (Figure 5-3).
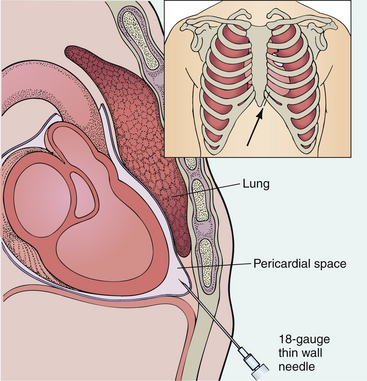
Figure 5-3 Pericardiocentesis using subxiphoid route for aspiration of pericardial fluid. The 18-gauge needle is introduced at 30- to 40-degree angle.
4. An electrocardiographic lead is often attached by a clip to the needle to identify any ST-segment elevations, which may indicate penetration into the epicardium. Echocardiography may be used for guidance of the needle.
5. Pericardial fluid is aspirated and placed in multiple specimen containers.
6. Some patients who have recurring cardiac tamponade may require placement of an indwelling pericardial catheter for continuous draining for 1 to 3 days. Occasionally a surgical pericardial window (excision of a small portion of the pericardium) is necessary to prevent recurrent effusions.
7. With certain types of pericarditis, medications (e.g., antibiotics, antineoplastic drugs, corticosteroids) may be instilled during pericardiocentesis to diminish the risk of recurrent effusions.
After
• Closely monitor the patient's vital signs. An increased temperature may indicate infection. Pericardial bleeding would be marked by hypotension or pulsus paradoxus (abnormal decrease in systolic blood pressure during inspiration).
• Label and number the specimen tubes that contain the pericardial fluid and deliver them to the appropriate laboratories for examination. Note the following possibilities:
1. Usually the fluid is taken to the chemistry laboratory, where the color, turbidity, glucose, albumin, protein, and lactic dehydrogenase levels are obtained. (See the discussion of thoracentesis on p. 681.)
2. A tube of blood often goes to the hematology laboratory, where red and white blood cells are evaluated. (See the discussion of thoracentesis on p. 681.)
3. The bacteriology laboratory performs routine cultures, Gram stains, fungal studies, and acid-fast stains.
4. When malignancy is suspected, the fluid should be sent for cytologic examination.
• All tests performed on pericardial fluid should be performed immediately to avoid false results caused by chemical or cellular deterioration.
• Apply a sterile dressing to the catheter if one has been left for continuing pericardial drainage.
• Establish a closed system if continued pericardial drainage is required. This is usually performed via the straight drainage method.
• Note that to minimize infection, pericardial catheters, if used, are usually removed after 2 days, although there are exceptions. After the sutures are cut and the catheter is removed, apply a sterile dressing to the puncture site.
Test Results and Clinical Significance
Pericarditis: Pericarditis can occur as a sequela to myocardial infarction; myocarditis; viral, bacterial, or tuberculous infections; or collagen-vascular diseases. The fluid is usually an exudate. (See the discussion of thoracentesis on p. 681.)
The nephrotic syndrome is characterized by renal albumin wasting. This and other forms of hypoproteinemia are associated with decreased intravascular oncotic pressure. The fluid tends to leak out of the intravascular space into the peritoneum. This fluid is usually a transudate.
Congestive heart failure: Normally a small amount of fluid exists within the pericardial space. Fluid is constantly secreted and reabsorbed by the pericardium. If venous pressure of the pericardium is increased as a result of passive congestion of the pericardium associated with congestive heart failure, fluid will accumulate.
Metastatic cancer: Neoplasms affecting the pericardium primarily (mesothelioma) or secondarily (breast, lung, ovarian, lymphoma) secrete excess volume of fluid into the pleural space. This fluid is an exudate.
Blunt or penetrating cardiac trauma,
Rupture of ventricular aneurysm:
These events cause sudden accumulation of blood within the closed pericardial space. As a result, diastolic filling is diminished and cardiac output diminishes. Immediate treatment is required if the patient is to survive.
Collagen-vascular disease: Patients with these autoimmune diseases can develop an inflammatory pericardial effusion. Usually the effusion develops slowly, allowing enough time for anatomic and functional compensatory changes. Sometimes, however, the effusion is acute enough or large enough to require pericardiocentesis.
Related Tests
Glucose, Lactic Dehydrogenase (LDH), Protein, and Amylase (p. 253, 329, 424, and 61, respectively). These blood tests are performed concomitantly to assist in the evaluation of the peritoneal fluid.
Chest X-Ray (p. 1014). This is an important part of identifying a pericardial effusion. Furthermore, a chest x-ray should be routinely performed on completion of pericardiocentesis to ensure that a pneumothorax has not iatrogenically occurred.
Electrocardiography (p. 544). This test is simultaneously performed to indicate location of the aspirating needle.
Computed Tomography (CT) of the Chest (p. 1029). This test can accurately assess the volume of fluid in the pericardium.
Semen Analysis (Sperm Count, Sperm Examination, Seminal Cytology, Semen Examination)
Normal findings
Indications
Semen analysis is used to evaluate the quality of sperm, to evaluate an infertile couple, and to document the adequacy of operative vasectomy.
Test Explanation
Semen production depends on the function of the testicles; semen analysis is a measure of testicular function. Gonadotropin-releasing hormone (Gn-RH) is secreted by the hypothalamus in response to decreased levels of testosterone. Gn-RH stimulates the pituitary to produce follicle-stimulating hormone (FSH) and luteinizing hormone (LH, also called interstitial cell–stimulating hormone). The FSH stimulates the Sertoli cell growth within the seminiferous tubules (location of sperm production). LH stimulates the Leydig cells to produce testosterone, which in turn stimulates the seminiferous tubules to produce sperm. Inadequate sperm production can be the result of primary gonadal failure (because of age, genetic cause [Klinefelter syndrome], infection, radiation, or surgical orchiectomy) or secondary gonadal failure (because of pituitary diseases). These forms of gonadal failure can be differentiated by measuring LH and FSH levels. In primary gonadal failure, LH and FSH levels are increased. In secondary gonadal failure, they are decreased. Stimulation tests using Gn-RH agonists such as leuprolide acetate clomiphene, or human chorionic gonadotropin are also used in the differentiation. Men with aspermia (no sperm) or oligospermia (<20 million/mL) should be evaluated endocrinologically for pituitary, thyroid, or testicular aberrations.
Semen analysis is one of the most important aspects of the fertility workup because the cause of a couple's inability to conceive often lies with the man. After 2 to 3 days of sexual abstinence, semen is collected and examined for volume, sperm count, motility, and morphology.
The freshly collected semen is first measured for volume. After liquefaction of the white, gelatinous ejaculate, a sperm count is done. Men with very low or very high counts likely are infertile. The motility of the sperm is then evaluated; at least 50% should show progressive motility. Morphology is studied by staining a semen preparation and calculating the number of normal versus abnormal sperm forms. The semen specimen is consideral abnormal if greater than 70% of the sperm have abnormal forms.
More exhaustive semen analysis for male infertility may include a sperm penetration assay (SPA), a multistep laboratory test that offers a biologic assessment of several aspects of human sperm fertilizing ability. Hyaluronan binding assay (HBA) is a qualitative assay used to determine the maturity of sperm in a fresh semen sample. The assay is based on the ability of mature, but not immature, sperm to bind to hyaluronan, the main mucopolysaccharide of the egg matrix and a component of human follicular fluid. Hyaluronan-binding capacity is acquired late in the sperm maturation process; immature sperm lack this ability. Therefore a low level of sperm binding to hyaluronan suggests that there is a low proportion of mature sperm in the sample. Similar to the sperm penetration assay, it has been suggested that the HBA assay may be used to determine the need for an intracytoplasmic sperm injection procedure as part of an assisted reproductive technique.
Aside from the conventional parameters of sperm quality such as concentration, motility, and morphology, sperm DNA integrity is a potential cause of idiopathic male infertility. Although sperm with fragmented DNA may be able to fertilize oocytes, subsequent embryo and fetal development may be impaired. DNA fragmentation in sperm increases with age. Therefore impaired DNA integrity may be an increasing infertility factor among older couples. Available flow cytometry tests of DNA integrity include the sperm chromatin structure assay test and the sperm DNA fragmentation assay (SDFA) test. The sperm specimen is considered abnormal if more than 70% of the sperm have abnormal forms.
A single sperm analysis, especially if it indicates infertility, is inconclusive because sperm count varies from day to day. A semen analysis should be done at least twice and possibly a third time, 3 weeks apart. A normal semen analysis alone does not accurately assess the male factor unless the effect of the partner's cervical secretion on sperm survival is also determined. (See the discussion of the Sims-Huhner test on p. 676.) In addition to its value in infertility workups, semen analysis is also helpful in documenting adequate sterilization after a vasectomy. It is usually performed 6 weeks after the surgery. If any sperm are seen, the adequacy of the vasectomy must be suspect.
Interfering Factors
![]() Drugs that may cause decreased sperm counts include antineoplastic agents (e.g., methotrexate), cimetidine, estrogens, and methyltestosterone.
Drugs that may cause decreased sperm counts include antineoplastic agents (e.g., methotrexate), cimetidine, estrogens, and methyltestosterone.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Instruct the patient to abstain from sexual activity for 2 to 3 days before collecting the specimen. Prolonged abstinence before the collection should be discouraged, because the quality of the sperm cells, and especially their motility, may diminish.
Instruct the patient to abstain from sexual activity for 2 to 3 days before collecting the specimen. Prolonged abstinence before the collection should be discouraged, because the quality of the sperm cells, and especially their motility, may diminish.
• Give the patient the proper container for the semen collection.
![]() Instruct the patient to avoid alcoholic beverages for several days before the collection.
Instruct the patient to avoid alcoholic beverages for several days before the collection.
![]() For evaluation of the adequacy of vasectomy, the patient should ejaculate once or twice before the day of examination to clear the distal portion of the vas deferens.
For evaluation of the adequacy of vasectomy, the patient should ejaculate once or twice before the day of examination to clear the distal portion of the vas deferens.
During
• Note that semen is best collected by ejaculation into a clean container. For best results the specimen should be collected in the physician's office or laboratory by masturbation.
• Note that less satisfactory specimens can be obtained in the patient's home by coitus interruptus or masturbation. Note the following procedural steps:
Test Results and Clinical Significance
Infertility: One of the most common causes of infertility is inadequate sperm production.
Vasectomy (obstruction of vas deferens): Semen analysis is necessary before vasectomy can be considered to be adequate.
Orchitis: This is usually caused by a virus (varicella) or rarely by a bacterium.
Testicular failure: This can be congenital (Klinefelter syndrome) or acquired (e.g., infection). Usually, with acquired forms of testicular failure, sperm are present but in low quantities. With congenital forms of testicular failure, no sperm are seen.
A common finding in sperm analysis is called “stress pattern.” This is said to exist when greater than 20% of the sperm have abnormal appearance and sperm counts are low. The stress pattern indicates presence of a varicocele or recent febrile illness.
Pituitary pathologic condition (adenoma, infarction): This causes hypospermia because of reduced or absent LH and FSH levels. As a result, spermatogenesis does not occur.
Related Tests
Antispermatozoal Antibody (p. 96). These antibodies can exist in the blood of males and destroy sperm quality. Furthermore, these antibodies can be produced by women and exist in the cervical mucus, making fertility difficult.
Sims-Huhner (p. 676). The Sims-Huhner test consists of a postcoital examination of the cervical mucus to measure the ability of the sperm to penetrate the mucus and maintain motility. It is used in the diagnostic workup of infertility. This analysis is also helpful in documenting cases of suspected rape by testing the vaginal and cervical secretions for sperm.
Luteinizing Hormone and Follicle-Stimulating Hormone Assay (p. 348). These hormones are useful in determining the pituitary effect on gonadal function and spermatogenesis.
Sexual Assault Testing
Indications
This testing is used to obtain evidence of a recent sexual assault and to obtain specimens to identify sexually transmitted diseases.
Text Explanation
The sexual assault victim needs to have psycho-emotional support, treatment of any physical injuries, and accurate and reliable evidentiary testing. Nearly all acute care centers have protocols in place that provide that care to victims of sexual assault. Furthermore, in most circumstances, there are nurses specifically trained in obtaining the appropriate specimens. These nurses know the importance of following the chain of evidence protocols to ensure that evidence is admissible in court.
While being provided with emotional support and assistance, the patient is first interviewed in a nonjudgmental manner. A thorough gynecologic history is obtained. A brief summary of the assault (if there was vaginal, oral, or anal penetration) and timing of the assault is important. After 72 hours, very little evidence persists. It is important to ascertain if the victim changed clothing, showered, or used a douche before coming to the hospital. These will affect the presence of evidence. The general demeanor of the patient, status of the clothing, and physical maturation assessment is documented.
The victim's clothes are removed and separately placed in a paper bag for possible deoxyribonucleic acid (DNA) sources of the victim's or assailant's body parts. Plastic bags are not used because bacteria may grow in them and can destroy DNA. Photographs of all injuries should be obtained, if possible. The victim is then examined for signs of external and internal injuries. A pelvic examination is then performed. A “sexual assault evidence collection kit” is now most commonly used to obtain all the needed specimens. The directions must be carefully followed to ensure that any and all evidence is obtained and is useful toward identification and conviction of any perpetrator (Box 5-1).
Vaginal secretions are obtained for sperm (see p. 671), or other cells from the assailant. Acid phosphatase (see p. 25) or prostate specific antigen (PSA) (see p. 420) are also obtained using this specimen. Cervical secretions are obtained for sexually transmitted disease (STD) (p. 756) testing. These anatomic areas along with the anorectal area are swabbed per directions in the kit. In the male victim, penile and anorectal areas are swabbed. Pubic hair is obtained by combing or plucking. STD testing would include syphilis (p. 473), trichomoniasis (p. 759), gonorrhea (p. 756), and chlamydia (p. 722). Later, blood testing for human immune deficiency virus (HIV) (p. 297) and pregnancy (p. 304) is obtained.
Next, blood specimens are obtained for DNA testing per the testing kit directions—usually an EDTA containing tube (lavender topped). More blood or urine may also be collected for evidence of mind altering drugs/alcohol or for serologic evidence of STDs. After this testing, a more detailed examination of the vagina, cervix, and rectum are performed using a Wood lamp to more easily identify saliva or sperm from the assailant. These areas are examined for subtle injuries from forced penetration. Two methods used to identify these injuries are the toludine blue dye test and use of a colposcope (see p. 595). The toludine blue dye test can also be used to identify recent or healed genital or anorectal injuries. A 1% aqueous solution is applied to the area of concern and washed off with a lubricant (e.g., K-Y Jelly) or a 1% acetic acid solution. Injured mucosa will retain the dye and become more apparent to the naked eye. Finally, the fingernails are scraped underneath because they may potentially contain tissue from the assailant. On completion of the examination, the victim is usually interviewed by the police for further investigation.
Unless medically contraindicated, all victims should be offered antimicrobial therapy to prevent STDs. The following combination of drugs is used in many hospitals: ciprofloxacin 250 mg PO stat dose; doxycycline 100 mg bid for 7 days; and metronidazole 2 g stat. The use of antiretroviral drugs in the prevention of HIV transmission may be recommended and the current guideline for postexposure prophylaxis following needle stick injuries should be used. It may also be advisable to offer victims a hepatitis B vaccination or hepatitis B immunoglobulin as the disease may be fatal. Victims who are at risk for HIV infection should also be given counseling on HIV/acquired immunodeficiency syndrome (AIDS).
A pregnancy test should be done before any treatment or drugs are prescribed. If there is a risk of pregnancy, the victims should be offered postcoital contraception if the rape occurred less than 72 hours before examination by the health worker. If it occurred more than 72 hours but less than 7 days before the examination, an intrauterine contraceptive device may be used to prevent pregnancy. Pregnancy testing may be repeated in the succeeding week after the rape.
Interfering Factors
• Delays in examination after the alleged attack diminish the possibilities of identifying meaningful evidence.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient and provide emotional support.
Explain the procedure to the patient and provide emotional support.
• Obtain consent to treat the patient or family.
• Notify any family members the patient would like to be present during the examination.
• Assess the patient's emotional condition and determine if the victim is able to undergo sexual assault testing.
During
• Obtain a thorough history as described previously.
• Use the SAPS Sexual Assault Evidence Collection Kit (SAECK) or similar test kit exactly as described to maintain the chain of evidence (see Box 5-1).
• Properly handle the kit specimens to maintain the chain of custody.
• Refrigerate all samples containing biological evidentiary material such as DNA to prevent putrefaction (decomposition).
• It is important to carefully examine all area of the body to help corroborate the victims's version of the alleged events.
Test Results and Clinical Significance
The psychological effects of a sexual assault in either sex is overwhelming. These patients do best if referred for psychological support. Often the conviction of the offender lessens the fear of the victim. Furthermore, observing just punishment of the offender may hasten healing. It is important to accurately obtain evidence so that all data are admissible and useful to law enforcement agencies.
Sims-Huhner (Postcoital, Postcoital Cervical Mucus, Cervical Mucus Sperm Penetration)
Indications
The Sims-Huhner test consists of a postcoital examination of the cervical mucus to measure the ability of the sperm to penetrate the mucus and maintain motility. It is invaluable in the evaluation of infertility.
Test Explanation
This study evaluates interaction between the sperm and the cervical mucus. It also measures the quality of the cervical mucus. This test can determine the effect of vaginal and cervical secretions on the activity of the sperm. This procedure is performed only after a previously performed semen analysis has been determined to be normal.
This test is performed during the middle of the ovulatory cycle because at this time the secretions should be optimal for sperm penetration and survival. During ovulation the quantity of cervical mucus is maximal, whereas the viscosity is minimal, thus facilitating sperm penetration. The endocervical mucus sample is examined for color, viscosity, and tenacity (spinnbarkeit). The fresh specimen is then spread on a clean glass slide and examined for the presence of sperm. Estimates of the total number and of the number of motile sperm per high-power field are reported. Normally, 6 to 20 active sperm cells should be seen in each microscopic high-power field; if the sperm are present but not active, the cervical environment is unsuitable (e.g., abnormal pH) for their survival. After the specimen has dried on the glass slide, the mucus can be examined for ferning to demonstrate estrogen effect. The Sims-Huhner study is invaluable in fertility examinations; however, it is not a substitute for the semen analysis. If the results of the Sims-Huhner test are less than optimal, the test is usually repeated during the same or the next ovulatory cycle.
This analysis is also helpful in documenting cases of suspected rape by testing the vaginal and cervical secretions for sperm. This procedure is performed by a physician in approximately 5 minutes. The only discomfort associated with this study is insertion of the speculum.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Inform the patient that basal body temperature recordings should be used to indicate ovulation.
Inform the patient that basal body temperature recordings should be used to indicate ovulation.
![]() Tell the patient that no vaginal lubrication, douching, or bathing is permitted until after the vaginal cervical examination, because these factors will alter the cervical mucus.
Tell the patient that no vaginal lubrication, douching, or bathing is permitted until after the vaginal cervical examination, because these factors will alter the cervical mucus.
![]() Inform the patient that this study should be performed after 3 days of male sexual abstinence.
Inform the patient that this study should be performed after 3 days of male sexual abstinence.
![]() Instruct the patient to remain in bed for 10 to 15 minutes after coitus to ensure cervical exposure to the semen. After this rest period, the patient should report to her physician for examination of her cervical mucus within 2 hours after coitus.
Instruct the patient to remain in bed for 10 to 15 minutes after coitus to ensure cervical exposure to the semen. After this rest period, the patient should report to her physician for examination of her cervical mucus within 2 hours after coitus.
Test Results and Clinical Significance
Infertility: This test determines the capability of the sperm to function and exist in an environment outside the male urethra. It is the final determinant of the adequacy of sperm. Although semen analysis may indicate an adequate sperm count, etc., if the sperm cannot function within the vaginal or cervical environment, fertility is improbable.
Suspected rape: The demonstration of sperm within vaginal secretions indicates that intercourse has occurred.
Related Tests
Semen Analysis (p. 671). Semen analysis is used to evaluate the quality of sperm, to evaluate an infertile couple, and to document the adequacy of operative vasectomy.
Antispermatozoal Antibody Test (p. 96). These antibodies can exist in the blood of males and destroy sperm quality. Furthermore, these antibodies can be produced by women and exist in the cervical mucus, making fertility difficult.
Luteinizing Hormone and Follicle-Stimulating Hormone Assay (p. 348). These hormones are useful in determining the pituitary effect on gonadal function and spermatogenesis.
Sweat Electrolytes (Iontophoretic Sweat)
Indications
This test is used to diagnose cystic fibrosis. The sweat electrolytes test is indicated in children with recurrent respiratory tract infections, chronic cough, early onset asthma, malabsorption syndromes, late passage of meconium stool, or failure to thrive. This test is also used to screen for the disease in children or siblings of cystic fibrosis (CF) patients.
Test Explanation
Patients with cystic fibrosis have increased sodium and chloride contents in their sweat. That forms the basis of this test, which is both sensitive and specific for CF. CF is an inherited disease (autosomal recessive) characterized by abnormal secretion by exocrine glands within the bronchi, small intestines, pancreatic ducts, bile ducts, and skin (sweat glands). Sweat, induced by electrical current (pilocarpine iontophoresis), is collected, and its sodium and chloride contents are measured. The degree of abnormality is no indication of the severity of cystic fibrosis; it merely indicates that the patient has the disease.
Patients with CF have a mutation in the CF transmembrane conductance regulator (CFTR) gene. This gene encodes the synthesis of a protein that serves as a channel through which chloride enters and leaves the cells. A mutation in this gene alters the cell's capability to regulate the chloride (and as a result, sodium) transport. Normally, at the base of a sweat gland, sodium and chloride concentrations are very high. As the sweat moves closer to the skin surface, chloride is transported through the lining cells out of the sweat. Sodium follows. By the time the sweat comes to the surface, nearly all of the chloride and sodium has been removed. In patients with CF, the transport of these ions does not occur. The sweat, therefore, has high concentrations of sodium and chloride. Almost all patients with CF have sweat sodium and chloride contents two to five times greater than normal values. In patients with suspicious clinical manifestations, these levels are diagnostic of CF.
Abnormal sweat test results can also occur in patients with glycogen storage diseases, adrenal hypofunction, and G-6-PD deficiency.
The sweat test is not reliable during the first few weeks of life. High serum concentrations of immunoreactive trypsin may be a better test for this age-group. An experienced technologist performs the sweat test in approximately 90 minutes in the laboratory or at the patient's bedside. A small electrical current is experienced during the test, but this is not painful. Generally there is no discomfort or pain associated with this test.
Interfering Factors
• In a cold room, sweating is inhibited. The room should be warmed or the child covered to maintain body heat.
• Dehydration is associated with reduced volume of sweat and increased concentration of sodium and chloride. The test results are not accurate.
• Values in pubertal adolescents may vary significantly and are not accurate.
Procedure and Patient Care
During
• Note the following procedural steps:
1. For iontophoresis, a low-level electrical current is applied to the test area (the thigh in infants, the forearm in older children) (Figure 5-4).
2. The positive electrode is covered by gauze and saturated with pilocarpine hydrochloride, a stimulating drug that induces sweating.
3. The negative electrode is covered by gauze saturated with a bicarbonate solution.
4. The electrical current is allowed to flow for 5 to 12 minutes.
5. The electrodes are removed, and the arm is washed with distilled water.
6. Paper disks are placed over the test site with the use of clean, dry forceps.
7. These disks are covered with paraffin to obtain an airtight seal, preventing evaporation of sweat.
8. After 1 hour the paraffin is removed. The paper disks are transferred immediately by forceps to a weighing jar and sent for sodium and chloride analysis.
A screening test may be done to detect sweat chloride levels. For screening, a test paper containing silver nitrate is pressed against the child's hand for several seconds. The test is positive when the excess chloride combines with the silver nitrate to form a white powder (silver chloride) on the paper. That is, the child with CF will leave a “heavy” handprint on the paper. A positive screening test is usually validated by iontophoresis.
Test Results and Clinical Significance
Cystic fibrosis: Normally, the sweat produced at the bottom of a sweat duct is rich in chloride and sodium. As the fluid traverses the duct leading to the outer skin level, the chloride (followed by sodium) escapes the lumen through the epithelial cells, leaving only water behind. In patients with CF, the epithelial lining cells of the ducts of sweat glands fail to take up the electrolytes efficiently from the lumen. The sweat at the skin level is therefore high in sodium and chloride.
Related Tests
Pancreatic Enzymes (p. 660). This is a test whereby pancreatic efflux is measured for amylase and other components. It is a corroborative test for CF.
Genetic Testing (p. 1093). Identification of the cystic fibrosis transmembrane conductance regulator (CFTR) gene is another confirmatory test for the disease. It is more commonly used to identify carriers of the gene that causes CF.
Thoracentesis and Pleural Fluid Analysis (Pleural Tap)
Normal Findings
Indications
Thoracentesis is performed to determine the cause of an unexplained pleural effusion. It is also performed to relieve the intrathoracic pressure that accumulates with a large volume of fluid and inhibits respiration.
Test Explanation
Thoracentesis is an invasive procedure that entails insertion of a needle into the pleural space for removal of fluid (or rarely, air) (Figure 5-5). The pleural space is defined as the space between the visceral pleura (thin membrane covering the lungs) and the parietal pleura (thin membrane covering the inside of the thoracic cavity). Within the peritoneal membrane is an intricate network of capillary and lymphatic vessels. Fluid is constantly being secreted by the pleural membranes and constantly being reabsorbed by those same membranes. If secretion is increased or reabsorption blocked, pleural fluid will develop.
Pleural fluid is removed for diagnostic and therapeutic purposes. Therapeutically, it is done to relieve pain, dyspnea, and other symptoms of pleural pressure. Removal of this fluid also permits better radiographic visualization of the lung.
Diagnostically, thoracentesis is performed to obtain and analyze fluid to determine the cause of the pleural effusion. Pleural fluid is classified as transudate or exudate. This is an important distinction and is very helpful in determining the cause of the effusion. See Table 5-4 (p. 663) for differentiation between transudate and exudate. Transudates are most frequently caused by congestive heart failure, cirrhosis, nephrotic syndrome, and hypoproteinemia. Exudates are most often found in inflammatory, infectious, or neoplastic conditions. However, collagen-vascular disease, pulmonary infarction, trauma, and drug hypersensitivity also may cause an exudative effusion.
A decubitus chest x-ray film (p. 1014) is obtained before thoracentesis to ensure that the pleural fluid is mobile and accessible to a needle placed within the pleural space.
Pleural fluid is usually evaluated for gross appearance; cell counts; protein, triglyceride, LDH, glucose, and amylase levels; Gram stain and microbial cultures (for example) Mycobacterium tuberculosis and fungi; cytology; CEA levels; and sometimes for other specific tests. Each is discussed separately.
Gross Appearance
The color, optical density, and viscosity are noted as the pleural fluid appears in the aspirating syringe. Transudative pleural fluid may be clear, serous, and light yellow, especially in patients with hepatic cirrhosis. Milk-colored pleural fluid may result from the escape of chyle from blocked thoracic lymphatic ducts. An opalescent, pearly fluid is characteristic of chylothorax (chyle in the pleural cavity). Conditions that may cause lymphatic blockage include lymphoma, carcinoma, and tuberculosis involving the thoracic lymph nodes. The triglyceride value in a chylous effusion exceeds 110 mg/dL.
Cloudy or turbid fluid may result from inflammatory or infectious conditions such as empyema. Empyema is characterized by the presence of a foul odor and thick, pus-like fluid. Bloody fluid may be the result of a traumatic tap (the aspirating needle penetrates a blood vessel), intrathoracic bleeding, or tumor.
Cell Counts
The white blood cells (WBCs) and differential counts are determined. A WBC count exceeding 1000/mL is suggestive of an exudate. The predominance of polymorphonuclear leukocytes usually is an indication of an acute inflammatory condition (e.g., pneumonia, pulmonary infarction, early tuberculosis effusion). When more than 50% of the WBCs are small lymphocytes, the effusion is usually caused by tuberculosis or tumor. Normally, no red blood cells (RBCs) should be present. The presence of RBCs may indicate neoplasms, tuberculosis, or intrathoracic bleeding.
Protein Content
Total protein levels greater than 3 g/dL are characteristic of exudates, whereas transudates usually have a protein content of less than 3 g/dL. It is now thought that the albumin gradient between serum and pleural fluid can differentiate better between the transudate and exudate nature of pleural fluid than can the total protein content. This gradient is obtained by subtracting the pleural albumin value from the serum albumin value. Values of 1.1 g/dL or more suggest a transudate. Values of less than 1.1 g/dL suggest an exudate but will not differentiate the potential cause of the exudate (malignancy from infection or inflammation).
Because there is significant overlap in protein values differentiating transudate from exudate, the total protein ratio (fluid/serum) has been considered to be a more accurate criterion. A total protein ratio of fluid to serum of greater than 0.5 is considered to indicate an exudate.
Lactic Dehydrogenase
A pleural fluid/serum LDH ratio of greater than 0.6 is typical of an exudate. An exudate is identified with a high degree of accuracy if the pleural fluid/serum protein ratio is greater than 0.5 and the pleural fluid/serum LDH ratio is greater than 0.6.
Glucose
Usually pleural glucose levels approximate serum levels. Low values appear to be a combination of glycolysis by the extra cells within an exudate and impairment of glucose diffusion because of damage to the pleural membrane. Values of less than 60 mg/dL also indicate exudate.
Amylase
In a malignant effusion, the amylase concentration is slightly elevated. Amylase levels above the normal range for serum or two times the serum level are seen when the effusion is caused by pancreatitis or rupture of the esophagus associated with leakage of salivary amylase into the chest cavity.
Triglyceride
Measurement of triglyceride levels is an important part of identifying chylous effusions. These effusions are usually produced by obstruction or transection of the lymphatic system caused by lymphoma, neoplasm, trauma, or recent surgery. The triglyceride value in a chylous effusion exceeds 110 mg/dL.
Gram Stain and Bacteriologic Culture
Culture and Gram stains are routinely performed when bacterial pneumonia or empyema is a possible cause of the effusion. These tests identify the organisms involved in the infection and also provide information concerning antibiotic sensitivity. (See p. 704 for a more thorough discussion of Gram stain, cultures, and sensitivity.) If possible, these tests should be done before initiation of antibiotic therapy.
Cultures for Mycobacterium tuberculosis and Fungus
Tuberculosis is less often a cause for pleural effusion in the United States today than it was in the past (although its incidence is now on the rise, especially among immuno-suppressed patients). Fungus may be a cause of pulmonary effusion in patients with compromised immunologic defenses. (See p. 768 for more information about tuberculosis culture techniques.)
Cytology
A cytologic study is performed to detect tumors. It is positive in approximately 50% to 60% of patients with malignant effusions. Breast and lung are the two most frequent tumors; lymphoma is the third. The interpretation of cytologic changes requires that the pathologist have considerable experience in cytology. It can be difficult to differentiate malignancy from severe inflammatory mesothelial cells. In general, malignant cells tend to clump together and have a high nucleus/cytoplasm ratio, prominent and multiple nucleoli, and unevenly distributed chromatin.
Cytologic examination of the fluid is improved by spinning down a large volume of fluid and examining the sediment. A large number of cells can be seen and compared with each other. A cytologic study is performed to detect tumor cells.
Carcinoembryonic Antigen
Pleural fluid CEA levels are elevated in various malignant (gastrointestinal [GI], breast) conditions.
Special Tests
The pH of pleural fluid is usually 7.4 or greater. The pH is typically less than 7.2 when empyema is present. The pH may be 7.2 to 7.4 in tuberculosis or malignancy. In some instances the rheumatoid factor (p. 454) and the complement levels (p. 172) are also measured in pleural fluid. Pleural fluid antinuclear antibody (ANA) levels and the pleural fluid/serum ANA ratio are often used to evaluate pleural effusion secondary to systemic lupus erythematosus.
Thoracentesis is performed by a physician at the patient's bedside, in a procedure room, or in the physician's office in less than 30 minutes. Although local anesthetics eliminate pain at the insertion site, the patient may feel a pressure-like pain when the pleura is entered and the fluid is removed.
Potential Complications
• Pneumothorax caused by puncture of the lung or entry of air into the pleural space through the aspirating needle
• Intrapleural bleeding because of puncture of a blood vessel
• Hemoptysis caused by needle puncture of a pulmonary vessel
• Reflex bradycardia and hypotension
• Seeding of the needle track with tumor when malignant pleural effusion exists
• Empyema caused by infection delivered by the aspirating needle
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
• Obtain informed consent for this procedure.
![]() Tell the patient that no fasting or sedation is necessary.
Tell the patient that no fasting or sedation is necessary.
![]() Inform the patient that movement or coughing should be minimized to avoid inadvertent needle damage to the lung or pleura during the procedure.
Inform the patient that movement or coughing should be minimized to avoid inadvertent needle damage to the lung or pleura during the procedure.
• Administer a cough suppressant before the procedure if the patient has a troublesome cough.
• Note that an x-ray film or ultrasound scan is often used to assist in location of the fluid. Fluoroscopic examination also may be used.
During
• Note the following procedural steps:
1. The patient is usually placed in an upright position with the arms and shoulders raised and supported on a padded overhead table. This position spreads the ribs and enlarges the intercostal space for insertion of the needle.
2. Patients who cannot sit upright are placed in a side-lying position on the unaffected side with the side to be tapped uppermost.
3. The thoracentesis is performed under strict sterile technique.
4. The needle insertion site, which is determined by percussion, auscultation, and examination of a chest radiograph film, ultrasound scan, or fluoroscopy, is aseptically cleansed and anesthetized locally.
5. The needle is positioned in the pleural space, and the fluid is withdrawn with a syringe and a three-way stopcock. Most thoracentesis kits now use a blunt-tip soft catheter over the needle. The needle is withdrawn and the soft Silastic catheter is left in place. The fluid is aspirated. The use of these soft catheters has greatly diminished the incidence of pneumothorax as a complication of this procedure.
6. Various mechanisms to stabilize the pleural needle or catheter are available to secure the needle depth during the fluid collection.
7. A short polyethylene catheter may be inserted into the pleural space for fluid aspiration; this decreases the risk of puncturing the visceral pleura and inducing a pneumothorax.
8. Also, large volumes of fluid may be collected by connecting the catheter to a gravity-drainage system.
• Monitor the patient's pulse for reflex bradycardia and evaluate the patient for diaphoresis and the feeling of faintness during the procedure.
After
• Place a small bandage over the needle site. Usually, turn the patient on the unaffected side for 1 hour to allow the pleural puncture site to heal.
• Label the specimen with the patient's name, date, source of fluid, and diagnosis. Send the specimen promptly to the laboratory.
• All tests done on pleural fluid should be performed immediately to avoid false results caused by chemical or cellular deterioration.
• Obtain a chest x-ray study as indicated to check for pneumothorax.
• Monitor the patient's vital signs.
• Observe the patient for coughing or expectoration of blood (hemoptysis), which may indicate trauma to the lung.
• Evaluate the patient for signs and symptoms of pneumothorax, tension pneumothorax, subcutaneous emphysema, and pyogenic infection (e.g., tachypnea, dyspnea, diminished breath sounds, anxiety, restlessness, fever).
• Assess the patient's lung sounds for diminished breath sounds, which could be a sign of pneumothorax.
![]() If the patient has no complaints of dyspnea, normal activity usually can be resumed 1 hour after the procedure.
If the patient has no complaints of dyspnea, normal activity usually can be resumed 1 hour after the procedure.
Test Results and Clinical Significance
Exudate
Empyema is most often the result of pneumonia. Occasionally, however, it can follow surgery, pleuritis, or trauma.
Tuberculosis effusion: This is usually a bloody effusion that is the result of the primary tuberculous infection of the lung and pleura.
Pancreatitis: This pleural effusion is most often a "sympathetic" effusion in response to the inflammatory process below the diaphragm.
Ruptured esophagus: The pleural fluid can occur as a result of a free communication of the ruptured esophagus with the pleural cavity. The pleura covering the mediastinum usually prevents this free communication, and the fluid is a "sympathetic" reaction to the mediastinal infection. The fluid, however, subsequently becomes infected and acts as an empyema.
Tumors: Neoplasms affecting the pleura primarily (mesothelioma) or secondarily (breast, lung, ovarian) secrete excess volumes of fluid into the pleural space.
Lymphoma: The tumor infiltrates the lymph nodes through which the thoracic lymphatic ducts flow. As a result, the lymph fluid is not reabsorbed and collects as a chylous effusion within the pleural space (chylothorax).
Pulmonary infarction: This bloody effusion is also a "sympathetic" effusion in response to the necrosis of lung tissue following a pulmonary embolus.
Collagen-vascular disease: Rheumatoid arthritis, systemic lupus erythematosus
Drug hypersensitivity: An immunogenic pleuritis and subsequent effusion may be the sequelae of autoimmune diseases or drug hypersensitivities as indicated previously.
Transudate
With increased venous pressure that results from either portal vein hypertension or passive congestion from congestive heart failure, pleural fluid is not absorbed. As a result, pleural fluid accumulates.
The nephrotic syndrome is characterized by renal albumin wasting. This and other forms of hypoproteinemia are associated with decreased intravascular oncotic pressure. The fluid tends to leak out of the intravascular space into the pleural space.
Trauma: Injury to the thorax, lungs, or great blood vessels can cause bleeding into the pleural space (hemothorax).
Related Tests
Glucose, Lactic Dehydrogenase (LDH), Protein, and Amylase (pp. 253, 329, 424, and 61, respectively). These blood tests are performed concomitantly to assist in the evaluation of the peritoneal fluid.
Chest X-Ray (p. 1014). This is an important part of identifying a pleural effusion. Furthermore, a chest x-ray should be routinely performed on completion of thoracentesis to ensure that a pneumothorax has not iatrogenically occurred.
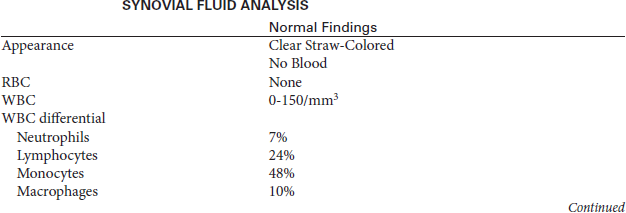
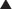 Increased Levels
Increased Levels