Pancreatobiliary FISH Testing
Test Explanation
It is sometimes difficult to differentiate benign bile duct strictures from early pancreatobiliary cancer. When a stricture is identified on an endoscopic retrograde cholangiopancreatography (ERCP, p. 605), cancer must be considered as a possible cause. If an obvious cancer is not seen at the time of ERCP, a brush is repeatedly swept along the bile duct to obtain duct surface cells for conventional cytology to identify cancer cells. In conventional cytology, the brushing specimens are placed on a slide and stained with a PAP stain. Slides are then interpreted by a cytopathologist to determine whether they show features that are positive for malignancy, suspicious for malignancy, atypical (meaning there are cells that are not normal but cannot be definitely ascribed to a neoplastic process), or negative for malignancy.
With the use of fluorescence in situ hybridization (FISH) testing, three chromosome enumeration probes and a gene-specific probe to P16 tumor suppressor gene are able to determine if more than one pair of chromosomes or P16 genes exists in the cells obtained from the brushings of the bile duct during ERCP. If extra copies of two or more of the chromosomes or P16 genes are evident, the cells are considered to be polysomic, which indicates a high chance of malignancy. Based on conventional cytology, FISH testing, and other clinical data, the likelihood of cancer can be calculated.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
• Obtain informed consent from the patient.
• Keep the patient NPO as of midnight the day of the test.
• Follow the procedure for ERCP (p. 605).
Related Tests
ERCP (p. 605). With upper GI endoscopic techniques, the pancreatobiliary tree can be accessed for pancreatic biliary FISH testing.
Papanicolaou Test (Pap Test, Pap Smear, Cytologic Test for Cancer, Liquid-Based Cervical Cytology [LBCC], ThinPrep)
Indications
Pap test are the mainstay of screening for cancer of the vagina, cervix, and uterus. They are routinely performed on women older than 21 years or on younger women who are sexually active.
Test Explanation
A Pap test is taken to detect neoplastic cells in cervical and vaginal secretions. This test is based on the fact that normal cells and abnormal cervical and endometrial neoplastic cells are shed into the cervical and vaginal secretions. By examining these secretions microscopically, one can detect early cellular changes associated with infection, premalignant conditions, or an existing malignant condition. The Pap test is 95% accurate in detecting cervical carcinoma; however, its accuracy in the detection of endometrial carcinoma is only approximately 40%.
The Bethesda System for reporting cervical and vaginal cytologic diagnoses was developed and revised by the National Cancer Institute to minimize discrepancy in result reporting and create a standardized framework for reporting results that were clinically useful. A proper patient history is essential to the successful interpretation of cytological specimens and is a regulatory requirement associated with Pap testing. This reporting system was updated in 2001 and includes evaluation of the following five components (Box 7-1):
1. Adequacy of specimen—An indication of the adequacy of the specimen is provided here. The specimen either has enough cells that can be evaluated or does not.
2. General categorization (optional)—This is a quick summary of the cellular findings that allows the clinician to triage results readily.
3. Interpretation/Result—This is a report of the cytopathologist's interpretation of the cells examined. It is not a diagnosis because other diagnostic data may be required to make a diagnosis.
a. Negative for intraepithelial lesion or malignancy—includes infections such as those caused by Trichomonas or Candida or reactive changes from an intrauterine device (IUD) or radiation therapy.
b. Epithelial cell abnormalities—These range from atypical to cancer for both the squamous and glandular cancer lines.
4. Automated review and ancillary testing (where appropriate)—This is reported if slides are scanned by automated computer systems (see p. 745). Also the use of any ancillary molecular tests such as human papillomavirus (HPV) (see p. 745) should be specified here.
5. Educational notes (optional)—Here comments are written regarding the significance of the cytology results, or recommendations for further diagnosis are provided.
A more common method of Pap test specimen collection is liquid-based cervical cytology (LBCC [more commonly called ThinPrep]). With this technique, the specimen obtained from the cervix is placed into a preservative solution instead of smearing it onto a slide as is done during conventional Pap smear testing (CPT). Any blood cells and debris are then isolated by centrifuge, leaving only cervical cells. A thin film of the residuum is then placed on a slide to be evaluated. The specimen can be “split” into two parts. The first is evaluated for cytopathology. In the event that cytologic abnormalities of undetermined significance are found that could be better elucidated with further testing, the cells in the second “split” specimen are used for that testing (to avoid having to obtain another cervical sample). For example, if cellular changes are found that may be related to HPV, the second “split” specimen is tested by real-time PCR for HPV DNA (see p. 648). HPV has been implicated as the cause of more than 95% of cervical cancers.
When compared with CPT, ThinPrep has a significantly greater percentage of satisfactory specimens for Pap testing. A significantly greater percentage of low-grade squamous intraepithelial lesion (LSIL) and high-grade squamous intraepithelial lesion (HSIL) Pap test results were reported using ThinPrep compared with the CPT. The predictive value of a positive ThinPrep test (93.9%) was similar to that for a positive CPT (87.8%) when compared with histology results.
Automated Pap test readings are increasingly being used because the volume of screening Pap tests exceeds the ability of the cytopathologists to spend enough time to accurately interpret the slides. Automation is especially accurate when performed on ThinPrep specimens. The ThinPrep Imaging System, for example, integrates automated imaging with screening by cytotechnologists to identify fields that contain potentially relevant cellular abnormalities. If the cytotechnologist identifies significant abnormalities, the slide is directly examined under a microscope. ThinPrep has replaced Pap testing because with ThinPrep the application of cells to the glass slide is standardized; cells are distributed evenly on the slide; mucus, blood, and inflammatory cells are reduced; fixation is effective and even; higher rates of serious cervical pathology are detected; and the material is less often considered inadequate for interpretation. A slightly different and less expensive technique called the PapSpin uses a special brush placed in a collection device and centrifuged to provide a cellular concentrate. The cellular concentrate is then examined microscopically.
Like screening for all cancers, as more studies become available, guidelines change. Furthermore, different medical professional societies may differ on certain aspects of PAP guidelines. Not only has the U.S. Preventive Services Task Force (USPSTF) recommended that the HPV test (p. 648) is appropriate for some women as part of routine cervical cancer screening, but it has also changed its recommendations as follows.
• Women aged 21 to 65 should get Pap tests no more than every 3 years. Previous guidelines, issued in 2003, recommended that women be screened “at least” every 3 years, allowing for annual screens.
• Women aged 30 to 65 may extend the interval between screens to 5 years if they use HPV tests in conjunction with the Pap test. The HPV test should not be used in younger women because many of them will have HPV infection that they will naturally clear without treatment.
• Women under 21 should not be screened for cervical cancer, regardless of sexual history. Previous advice recommended that women begin cervical cancer screening within 3 years of becoming sexually active.
• Women over 65 should not be screened as long as they have had consistently normal Pap tests and are not at high risk for cervical cancer.
The guidelines apply to healthy women who do not have abnormal Pap tests. They do not apply to women who have a history of cervical cancer.
Contraindications
• Patients menstruating, because this can alter test interpretation
• Patients with known vaginal infections, because the infections can create cellular changes that may be misinterpreted as precancerous
Interfering Factors
• A delay in fixing a specimen allows the cells to dry, destroys effectiveness of the stain, and makes cytologic interpretation difficult.
• Using lubricating jelly on the speculum can alter the specimen.
• Douching and tub bathing before testing may wash away cellular deposits and interfere with the test results.
• Menstrual flow may alter test results. The best time to perform a Pap test is 2 weeks after the start of the last menses.
• Infections may interfere with hormonal cytology.
![]() Drugs such as digitalis and tetracycline may alter the test results by affecting the squamous epithelium.
Drugs such as digitalis and tetracycline may alter the test results by affecting the squamous epithelium.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Instruct the patient not to douche or tub bathe during the 24 hours before the Pap test. (Some physicians prefer that the patients refrain from sexual intercourse for 24 to 48 hours before the test.)
Instruct the patient not to douche or tub bathe during the 24 hours before the Pap test. (Some physicians prefer that the patients refrain from sexual intercourse for 24 to 48 hours before the test.)
![]() Instruct the patient to empty her bladder before the examination. A full bladder inhibits complete palpation of pelvic structures.
Instruct the patient to empty her bladder before the examination. A full bladder inhibits complete palpation of pelvic structures.
During
Note the following procedural steps:
1. The patient is placed in the lithotomy position.
2. A vaginal speculum is inserted to expose the cervix.
3. Material is collected from the cervical canal by rotating a cotton swab moistened with saline or a wooden (plastic for LBCC) spatula within the cervical canal and in the squamocolumnar junction (Figure 7-5). If a maturation index is requested for hormonal information, the smear is taken off the vaginal wall. Care is taken to exclude the cervix.
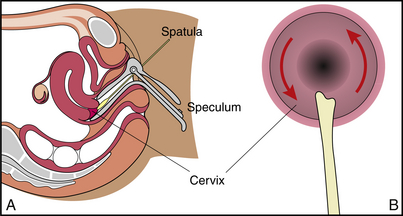
Figure 7-5 Papanicolaou (Pap) test. A, Cross-sectional view of the process of obtaining a cervical specimen. B, Cervix is scraped with bifid end of a spatula to obtain Pap test.
4. The cells are immediately wiped across a clean glass slide and fixed either by immersing the slide in equal parts of 95% alcohol and ether or by using a commercial spray (e.g., Aqua Net hairspray). The secretions must be fixed before drying, because drying will distort the cells and make interpretation difficult. Furthermore, this fixing process kills any infectious organisms so that the specimen is less infectious to the personnel who handle the specimen.
5. The slide is labeled with the patient's name, age, parity, and date of her last menstrual period. If this is not done, the specimen is considered unsatisfactory for interpretation.
6. If LBCC is performed, the cervical specimen is placed in the fixative preservative solution. Once placed in this solution, cells can be evaluated anytime within the next 3 weeks (if kept frozen).
7. The patient's medication history (e.g., oral contraceptives) and the reason for the examination should be written on the laboratory request form.
After
• If the Pap test has induced some bleeding, provide the patient with a perineal napkin.
• Note that once in the laboratory, the Pap test slide is stained and reviewed microscopically by the pathologist. Several computer programs are now able to recognize abnormal cells and classify them. These programs are used to assist the pathologist in screening particular cellular smears.
![]() Inform the patient that usually she will be notified of the test results by her physician only if further evaluation is needed.
Inform the patient that usually she will be notified of the test results by her physician only if further evaluation is needed.
Test Results and Clinical Significance
Cancer: The diagnosis of malignancy can be made only on biopsy of the tumor. All patients with suspicious Pap test must be more thoroughly examined with colposcopy, cone biopsy, and/or dilation and curettage.
Sexually transmitted diseases,
Many of these infectious diseases cause cellular changes on Pap tests. Culture of these organisms, however, is required to make the diagnosis.
Infertility: Lack of estrogenic effect noted on vaginal Pap tests may indicate ovarian failure in a woman of usual menstrual age.
Pleural Biopsy
Indications
This test is indicated when the pleural fluid obtained by thoracentesis (p. 681) is exudative fluid, which suggests infection, neoplasm, or tuberculosis. The pleural biopsy is indicated to distinguish among these disease processes. It is also performed when chest imaging indicates a pleural-based tumor, reaction, or thickening.
Test Explanation
Pleural biopsy is the removal of pleural tissue for histologic examination. Pleural biopsy is usually performed by a percutaneous needle biopsy. It also can be performed via thoracoscopy, which is done by inserting a scope into the pleural space for inspection and biopsy of the pleura (see Thoracoscopy, p. 627). Pleural tissue also may be obtained by an open pleural biopsy, which involves a limited thoracotomy and requires general anesthesia. For this procedure a small intercostal incision is made and the biopsy of the pleura is done under direct observation. The advantage of these open procedures is that a larger piece of pleura can be obtained.
Percutaneous needle biopsies are usually performed by a physician at the patient's bedside, in a special procedure room, or in the physician's office in approximately 30 minutes. Because of the local anesthetic, little discomfort is associated with this procedure. Open biopsies are done in the operating room.
Procedure and Patient Care
During
• Note the following procedural steps for percutaneous needle biopsy:
1. This procedure is usually performed with the patient in a sitting position with his or her shoulders and arms elevated and supported by a padded overhead table.
2. After the presence of the fluid has been determined by the thoracentesis technique, the skin overlying the biopsy site is anesthetized and pierced with a scalpel blade.
3. A needle is inserted with a cannula until fluid is removed. (Some fluid is left in the pleural space after the thoracentesis to make the biopsy easier.)
4. The inner needle is removed, and a blunt-tipped, hooked biopsy trocar, attached to a three-way stopcock, is inserted into the cannula.
5. The patient is instructed to exhale all air and then perform the Valsalva maneuver to prevent air from entering the pleural space.
6. The cannula and biopsy trocar are withdrawn while the hook catches the parietal wall and takes a specimen with its cutting edge.
7. Usually three biopsy specimens are taken from different sites at the same session.
8. The specimens are placed in a fixative solution and sent to the laboratory immediately.
9. After the specimens are taken, additional parietal fluid can be removed.
After
• Apply an adhesive bandage to the biopsy site.
• Note that a chest x-ray film is usually taken to detect the potential complication of pneumothorax.
• Observe the patient for signs of respiratory distress (e.g., shortness of breath, diminished breath sounds) on the side of the biopsy.
• Observe the patient's vital signs frequently for evidence of bleeding (increased pulse rate, decreased blood pressure).
• Ensure that the biopsy specimen is sent to the laboratory immediately.
Test Results and Clinical Significance
Neoplasm: Pleural tumors can be primary (mesothelioma) or metastatic (breast, lung, ovarian, gastrointestinal, etc.). These tumors are often associated with a pleural effusion.
Infection: Lung and pleural space infections can cause thickened pleura and pleural effusions (empyema). Most infections can be identified on Gram stains and cultures of the pleural fluid. However, some infections cannot be identified without tissue for culture or tissue for other forms of identification. This is especially true for the unusual infections occurring in immunocompromised patients (Pneumocystis jiroveci).
Progesterone Receptor Assay (PR Assay, PRA, PgR)
Indications
Progesterone receptor assay is performed on breast cancer tissue to indicate sensitivity to hormone manipulative therapy and to indicate prognosis of breast cancer.
Test Explanation
The PR assay is used in determining the prognosis and treatment of breast cancer and, to a lesser degree, other cancers. These assays help determine whether a tumor is likely to respond to endocrine therapy. The test is done on breast cancer specimens when a primary or recurrent cancer is identified; it is usually done in conjunction with estrogen receptor (ER) assay (see p. 728) to increase the predictability of a tumor response to hormone therapy. Breast tumors tend to be PR positive in postmenopausal women more often than in premenopausal women. PR-positive tumors are suspected to be associated with a better prognosis than PR-negative tumors. Tumor response rates to hormonal manipulation are found to be potentiated if the ER assay is positive. Response rates are as follows:
The most commonly used laboratory method provides accurate information on paraffin-embedded tissue or fixed slides using immunohistochemical staining for PR proteins. Positive reactivity by immunohistochemistry is observed in the nuclei of the tumor cells. Only a small portion of the tissue is required for testing. Results are usually available in less than 1 week. Only the cancerous tissue is evaluated for PR receptors.
Other tumors (such as ovarian, melanoma, uterine, or pancreatic) are occasionally studied for ER and PR assay. This is mostly done within clinical trials.
Procedure and Patient Care
Before
• Prepare the patient for breast biopsy according to routine protocol.
• Record the menstrual status of the patient.
• Record any exogenous hormone the patient may have used during the last 2 months.
![]() Instruct the patient to discontinue exogenous hormone therapy before breast biopsy. This is done in consultation with the physician.
Instruct the patient to discontinue exogenous hormone therapy before breast biopsy. This is done in consultation with the physician.
Related Tests
Estrogen Receptor Assay (p. 728). Like PR assay, this test predicts the likelihood of tumor response to endocrine manipulative therapy.
Breast Cancer Genomics (p. 1086). This test is used as a prognosticator indicating the risk of recurrent breast cancer. It is also a powerful predictor of benefit from hormone therapy or chemotherapy.
Renal Biopsy (Kidney Biopsy)
Indications
Renal biopsy is performed for the following purposes:
1. To diagnose the cause of renal disease (e.g., poststreptococcal glomerulonephritis, Goodpasture syndrome, lupus nephritis)
2. To detect primary and metastatic malignancy of the kidney in patients who may not be candidates for surgery
3. To evaluate kidney transplantation rejection, which enables the physician to determine the appropriate dose of immunosuppressive drugs
Test Explanation
Biopsy of the kidney affords microscopic examination of renal tissue. Renal biopsy is most often obtained percutaneously (Figure 7-6). During this procedure a needle is inserted through the skin and into the kidney to obtain a sample of kidney tissue. The biopsy needle is more accurately placed when guided with CT scan, ultrasonography, or fluoroscopy. These visualization techniques allow more precise localization of the desired kidney tissue. This procedure is performed by a physician in approximately 10 to 30 minutes. The biopsy is uncomfortable, but only minimally if enough lidocaine is used.
Occasionally, open renal biopsy is performed. This involves an incision through the flank and dissection to expose the kidney surgically.
Contraindications
• Patients with coagulation disorders, because of the risk of excessive bleeding
• Patients with operable kidney tumors, because tumor cells may be disseminated during the procedure
• Patients with hydronephrosis, because the enlarged renal pelvis can be easily entered and cause a persistent urine leak requiring surgical repair
• Patients with urinary tract infections, because the needle insertion may disseminate the active infection throughout the retroperitoneum
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
• Ensure that written informed consent for this procedure is obtained by the physician.
![]() Keep the patient on nothing by mouth (NPO) status after midnight on the day of the test in the event that bleeding or inadvertent puncture of an abdominal organ necessitates surgical intervention.
Keep the patient on nothing by mouth (NPO) status after midnight on the day of the test in the event that bleeding or inadvertent puncture of an abdominal organ necessitates surgical intervention.
• Assess the patient's coagulation studies (platelet count, prothrombin time, partial thromboplastin time).
• Check the patient's hemoglobin and hematocrit values.
• Note that the patient's blood may need to be typed and crossmatched in case of severe hemorrhage requiring transfusions.
![]() Tell the patient that no sedative is required.
Tell the patient that no sedative is required.
• Note that the needle biopsy may be done at the bedside.
• If CT scan or ultrasound guidance is to be used, note that the needle biopsy is performed in the radiology or ultrasonography department.
During
• Note the following procedural steps:
1. The patient is placed in a prone position with a sandbag or pillow under the abdomen to straighten the spine.
2. Under sterile conditions, the skin overlying the kidney is infiltrated with a local anesthetic (lidocaine).
3. While the patient holds his or her breath to stop kidney motion, the physician inserts the biopsy needle into the kidney and takes a specimen.
4. After this procedure is completed, the needle is removed and pressure is applied to the site for approximately 20 minutes.
After
![]() Turn the patient on his or her back and have the patient stay in bed for approximately 24 hours.
Turn the patient on his or her back and have the patient stay in bed for approximately 24 hours.
• Check the patient's vital signs, puncture site, and hematocrit values frequently during the 24-hour period.
![]() Instruct the patient to avoid any activity that increases abdominal venous pressure (e.g., coughing).
Instruct the patient to avoid any activity that increases abdominal venous pressure (e.g., coughing).
• Assess the patient for signs and symptoms of hemorrhage (e.g., decrease in blood pressure, increase in pulse rate, pallor, backache, flank pain, shoulder pain, light-headedness).
• Evaluate the patient's abdomen for signs of bowel or liver penetration (e.g., abdominal pain and tenderness, abdominal muscle guarding and rigidity, decreased bowel sounds).
• Inspect all urine specimens for gross hematuria. Usually the patient's urine will contain blood initially, but this generally will not continue after the first 24 hours. Urine samples may be placed in consecutive chronologic order to facilitate comparison for evaluation of hematuria. This is referred to as “rack,” or serial, urine samples.
![]() Encourage the patient to drink large amounts of fluid to prevent clot formation and urine retention.
Encourage the patient to drink large amounts of fluid to prevent clot formation and urine retention.
• Obtain blood for hemoglobin and hematocrit level determination after the biopsy to assess for active bleeding. One lavender-top tube of blood is needed.
![]() Instruct the patient to avoid strenuous exercise (e.g., heavy lifting, contact sports, horseback riding) or any activity that could cause jolting of the kidney for at least 2 weeks.
Instruct the patient to avoid strenuous exercise (e.g., heavy lifting, contact sports, horseback riding) or any activity that could cause jolting of the kidney for at least 2 weeks.
![]() Teach the patient the signs and symptoms of renal hemorrhage, and instruct him or her to call the physician if any of these symptoms occur.
Teach the patient the signs and symptoms of renal hemorrhage, and instruct him or her to call the physician if any of these symptoms occur.
![]() Instruct the patient to report burning on urination or any temperature elevations. These could indicate a urinary tract infection.
Instruct the patient to report burning on urination or any temperature elevations. These could indicate a urinary tract infection.
Test Results and Clinical Significance
Renal disease (e.g., poststreptococcal conditions, Goodpasture syndrome, lupus nephritis): These primary diseases of the kidney have classic histologic appearances. It is important to document the type of renal disease to ensure proper therapy. Immunofluorescent stains are often applied to the tissue to identify renal disease of immunologic origin (e.g., Goodpasture disease).
Primary and metastatic malignancy of the kidney: The most common cancers of the kidney are primary renal cell carcinomas. It is dangerous to perform a biopsy of this tumor because it is quite vascular. Furthermore, the biopsy could cause tumor studding along the needle track. However, in cases of metastatic disease, or if medical conditions preclude surgery, tissue can be obtained by kidney biopsy.
Rejection of kidney transplant: This is the definitive manner in which rejection is diagnosed. If the problem is caught early enough, the immunosuppressive medication regimen can be altered to stop the rejection process.
SARS Viral Testing
Test Explanation
SARS has now killed more than 100 people and infected some 2600 in 20 countries. A coronavirus causes SARS. China's southern Guangdong province, which includes Hong Kong, is believed to be the source of the virus, which has about an 8- to 10-day incubation period. Symptoms are similar to any pneumonia (fever, chills, and cough). The diagnosis should be suspected in a symptomatic patient who lives in or has traveled to an area where there has been documented transmission of the illness. Routine testing for the SARS virus is not conducted unless a cluster of cases develops and health officials are able to rule out all other infectious agents.
There are three tests that are currently available. These include:
1. Enzyme-Linked Immunosorbent Assay (ELISA)—This test detects antibodies to Coronavirus. The test identifies antibodies 20 days after the start of symptoms. That means it cannot be used to detect cases in the early stage of illness.
2. Immunofluorescence Assay (IFA)—This method detects SARS antibodies as early as 10 days after infection, but it is a complex and relatively slow test that requires growing the virus in the laboratory.
3. Reverse-Transcription Polymerase Chain Reaction (RT-PCR)—This molecular test detects the SARS virus by amplifying ribonucleic acid (RNA) genetic information from a cultured sample by a RT-PCR. It is good at detecting early stages of the infection, and results can be available in 2 days.
The diagnosis can only be made with positive test results in the following situations with:
• One specimen tested on two occasions using the original clinical specimen on each occasion
• Two clinical specimens from different sources (e.g., nasopharyngeal and stool)
• Two clinical specimens collected from the same source on two different days (e.g., two nasopharyngeal aspirates)
Eight of the following types of respiratory specimens may be collected for viral and/or bacterial diagnostics: (1) nasopharyngeal wash/aspirates, (2) nasopharyngeal swabs, (3) oropharyngeal swabs, (4) bronchioalveolar lavage, (5) tracheal aspirate, (6) pleural fluid tap, (7) sputum, and (8) postmortem tissue. Nasopharyngeal wash/aspirates are the specimen of choice for detection of most respiratory viruses.
Serum and blood (plasma) should be collected early in the illness for RT-PCR testing. The reliability of RT-PCR testing performed on blood specimens decreases as the illness progresses. Both acute and convalescent serum specimens should be collected for antibody testing. To confirm or rule out SARS-CoV infection, it is important to collect convalescent serum specimens more than 28 days after the onset of illness.
A virus culture to isolate SARS coronavirus is available but takes a few days for results. The capability to isolate and cultivate the virus is particularly important for epidemiologists and researchers.
Procedure and Patient Care
During
• To obtain a nasopharyngeal wash/aspirate, have the patient sit with the head tilted slightly backward. Instill 1 mL to 1.5 mL of nonbacteriostatic saline (pH 7.0) into one nostril. Flush a plastic catheter or tubing with 2 mL to 3 mL of saline. Insert the tubing into the nostril. Aspirate nasopharyngeal secretions. Repeat this procedure for the other nostril. Collect the specimens in sterile vials.
• To obtain a nasopharyngeal or oropharyngeal swabs, use only sterile Dacron or rayon swabs with plastic shafts. Do not use a cotton swab or swabs with wooden sticks, as they may contain substances that inactivate some viruses and inhibit PCR testing. Insert the swab into the nostril. Leave the swab in place for a few seconds to absorb secretions. Swab both nostrils. (For oropharyngeal culture—swab the posterior pharynx and tonsillar areas, avoiding the tongue.)
• To collect sputum, educate the patient about the difference between sputum and oral secretions. Have the patient rinse the mouth with water and then expectorate deep cough sputum directly into a sterile screw-cap sputum collection cup or sterile dry container.
• To collect blood, collect 5 mL to 10 mL of whole blood in a serum separator tube for serum RT-PCR testing or for ELISA antibody testing. Collect 5 mL to 10 mL of blood in an EDTA (purple-top) tube for plasma testing.
Sexually Transmitted Disease Testing (STD Culture, Culture of Cervix, Urethra, and Anus)
Indications
These cultures and smears are performed for patients who have a vaginal discharge, pelvic pain, urethritis, or penile discharge and are at risk for STDs.
Test Explanation
In the United States and other countries, some STDs (see Table 7-2, p. 759) have become epidemic, whereas others have become increasingly frequent. In this test discussion, we will concentrate on three common STD organisms: Chlamydia trachomatis (also discussed on p. 722), Neisseria gonorrhoeae, and Trichomonas vaginalis. Whereas trichomonas often does not cause any symptoms, these organisms can cause urethritis, vaginitis, endometritis, pelvic inflammatory disease, pharyngitis, proctitis, epididymitis, prostatitis, and salpingitis. Children born of infected mothers may develop conjunctivitis, pneumonia, neonatal blindness, neonatal neurologic injury, and even death.
Specimens for STD infections are obtained on men and women with suggestive symptoms. If the result is positive, sexual partners should be evaluated and treated. Cervical cultures/swabs are usually done for women; urethral swabs and cultures are done for men. Rectal and throat cultures are performed in persons who have engaged in anal and oral intercourse. Because rectal gonorrhea accompanies genital gonorrhea in a high percentage of women, rectal cultures are recommended in all women with suspected gonorrhea. Performing STD testing is also part of the prenatal workup. If the STD culture is positive, treatment during pregnancy can prevent possible fetal complications (e.g., ophthalmia neonatorum) and maternal complications. Rectal and orogastric specimens should be performed on the neonates of infected mothers.
Gram stains of smears or cultures should be taken before a patient begins antibiotic therapy. Cultures for gonorrhea use a special medium such as Thayer-Martin, designed for the cultivation of Neisseria gonorrhoeae.
Although cultures are used as the definitive test for documentation of STD infections, nucleic acid (RNA/DNA) probes (Nucleic Acid Testing, NAT) are most commonly used for identification of these STD organisms. Each suspected organism must be specifically requested. Some laboratories do routine “genital cultures” that include gonorrhea, beta streptococcus, and Gardnerella investigations. T. vaginalis can be diagnosed via a wet mount, in which “corkscrew” motility is observed. Newer methods, such as rapid antigen testing (similar to Strept Screen, p. 765) and transcription-mediated amplification, have even greater sensitivity, but are not in widespread use. The presence of T. vaginalis can also be diagnosed by PCR. Specimens obtained in a Pap test/ThinPrep can also be used for testing.
STD cultures and smears are obtained by a physician or nurse in several minutes. Very little discomfort is associated with these procedures.
Interfering Factors
Procedure and Patient Care
During
Cervical Culture
1. The female patient is told to refrain from douching and tub bathing before the cervical culture.
2. The patient is placed in the lithotomy position, and a moistened, unlubricated vaginal speculum is inserted to expose the cervix (see Figure 7-5, A, on p. 747).
3. Excess cervical mucus is removed with a cotton ball held in a ring forceps.
4. A sterile cotton-tipped swab is inserted into the endocervical canal and moved from side to side to obtain the specimen.
5. The swab is placed in sterile saline or a transporting fluid obtained from the laboratory. The specimen should be plated as soon as possible. The specimen should not be refrigerated.
Anal Canal Culture
1. An anal culture of the female or male patient is taken by inserting a sterile, cotton-tipped swab approximately 1 inch into the anal canal (Figure 7-7).
Urethral Culture
1. The urethral specimen should be obtained from the male patient before he voids. Voiding within 1 hour before collection washes secretions out of the urethra, making fewer organisms available for culture. The best time to obtain the specimen is before the first morning micturition.
2. A culture is taken by inserting a sterile swab gently into the anterior urethra (Figure 7-8).
3. Place the male patient in the supine position to prevent falling if vasovagal syncope occurs during introduction of the cotton swab or wire loop into the urethra.
4. The patient is observed for hypotension, bradycardia, pallor, sweating, nausea, and weakness.
5. In the male, prostatic massage may increase the chances of obtaining positive cultures.
Urine Culture
Obtain the first voided specimen in the female. (Urine cultures for STD are not helpful in males.) A small quantity of urine is placed in the transporting fluid or sterile empty container obtained from the laboratory.
Pap Test ThinPrep (See p. 743)
After
• Place the swabs for gonorrhea in a Thayer-Martin medium and roll them from side to side.
• Label and send the culture bottle to the microbiology laboratory.
• Transport the specimen to the laboratory as soon as possible.
• Handle all specimens as though they were capable of transmitting disease.
• Do not refrigerate the specimen.
• Mark the laboratory slip with the collection time, date, source of specimen, patient's age, current antibiotic therapy, and clinical diagnosis.
![]() Advise the patient to avoid intercourse and all sexual contact until test results are available.
Advise the patient to avoid intercourse and all sexual contact until test results are available.
![]() If the culture results are positive, tell the patient to receive treatment and have sexual partners evaluated.
If the culture results are positive, tell the patient to receive treatment and have sexual partners evaluated.
• Note that repeat cultures should be taken after completion of treatment to evaluate therapy.
Test Results and Clinical Significance
Chlamydia (p. 722),
These and other STDs can be identified by these and other diagnostic tests (Table 7-2).
TABLE 7-2
Sexually Transmitted Diseases (STDs) and Methods of Diagnosis
| Disease | Method of Diagnosis |
| Gonorrhea | Cervical, urethral, anal, oropharyngeal cultures |
| Chlamydia Lymphogranuloma venereum C. trachomatis |
Cervical and urethral cultures, serology, DNA probe testing |
| Herpes genitalis | Culture from lesion, serology |
| Syphilis | Serology, fluid cultures (CNS), darkfield slide |
| Hepatitis | Serology, nucleic acid testing |
| HIV | Serologic, virologic, nucleic acid testing |
| Trichomonas vaginalis | Cervical and urethral cultures, urine, ThinPrep PAP, serology, nucleic amplification tests |
| Candida | Wet mount, fungal culture |
| Gardnerella vaginalis | Cervical, urethral, anal cultures |
Skin Biopsy (Cutaneous Immunofluorescence Biopsy, Skin Biopsy Antibodies, Skin Immunohistopathology, Direct Immunofluorescence Antibody Test)
Indications
Testing of inflamed skin or mucosa is performed to evaluate and diagnose immunologically mediated dermatitis, such as pemphigoid, pemphigus, bullosa acquisita and bullous lupus erythematosus. It is indicated when an immunologic source for a skin rash is suspected.
Test Explanation
Autoimmune skin diseases are associated with autoantibodies in the skin and serum. Either can be tested (see Antiscleroderma Antibody, p. 93, and Indirect Immunofluorescence Antibody, p. 197). Direct (testing for antibodies in the skin) immunofluorescence antibody (IFA) is most specific and diagnostic. Furthermore, skin/mucosal histology is reported. For this study, a tissue specimen in or around the skin/mucosal lesion is obtained and evaluated by routine histology and by IFA methods. Deposition of human immunoglobulins (IgG, IgA, or IgM), complement C3, or fibrinogen components are determined. This test is also used to confirm the histopathology of skin lesions and monitor the results of treatment. Indirect (testing for IFA detected antibodies in the serum) immunofluorescence (IF) testing may be diagnostic when histologic or direct IFA studies are only suggestive, nonspecific, or negative.
Procedure and Patient Care
During
• The skin area used for biopsy is anesthetized locally to minimize discomfort. The area chosen for biopsy depends on the disease suspected. For some diseases, the skin lesion is tested. For others, the margin or nearby skin is tested.
• A 4-mm punch biopsy or elliptical tissue excision is obtained.
Test Results and Clinical Significance
Systemic lupus erythematosus (SLE),
Lupus erythematosus is associated with acute, subacute, and chronic skin lesions. All are associated with deposits of immunoglobulins and complement in the epidermal basement membrane zone. The acute changes are represented by the butterfly rash of SLE. Subacute changes of SLE are represented by photosensitive ulcers. The chronic changes of discoid lupus are scale-like changes about the neck and face. Once the scale is removed, an ulcer remains until healing and scarring occur.
These immunologic dermatitis diseases are highlighted clinically as subepidermal blistering of skin, usually in older adults.
Dermatitis herpetiformis: This urticarial immunologic dermatitis is often associated with gluten-sensitive enteropathy.
Related Tests
Antiscleroderma Antibody (p. 93). This antibody is diagnostic for cutaneous manifestations of scleroderma.
Indirect Immunofluorescence Antibody (p. 197). This test determines the presence of IgG, IgA, and IgM autoantibodies in the serum. This is helpful in the diagnosis of autoimmune skin diseases if the direct IFA skin biopsy is not definitive.
Sputum Culture and Sensitivity (Sputum C&S, Sputum Culture, and Gram stain)
Indications
Sputum culture is indicated in any patient with a persistent productive cough, fever, hemoptysis, or a chest x-ray picture compatible with a pulmonary infection. This test is used to diagnose pneumonia, bronchiectasis, bronchitis, or pulmonary abscess. Bacterium, fungus, or virus can be cultured.
Test Explanation
Sputum cultures are obtained to determine the presence of pathogenic bacteria in patients with respiratory infections, such as pneumonia. A Gram stain is the first step in the microbiologic analysis of sputum. Through sputum staining, bacteria are classified as gram positive or gram negative. This may be used to guide drug therapy until the C&S report is complete. The sputum sample is then applied to a series of bacterial culture plates. The bacteria that grow on those plates 1 to 3 days later are then identified. Determinations of bacterial sensitivity to various antibiotics (also called drug sensitivity testing) are performed to identify the most appropriate antimicrobial drug therapy. This is done by observing a ring of growth inhibition around an antibiotic disk in the culture medium. Sputum for C&S should be collected before antimicrobial therapy is initiated, unless the test is being performed to evaluate the effectiveness of medications already being given. Preliminary reports are usually available in 24 hours. Cultures require at least 48 hours for completion. Sputum cultures for fungus (e.g., Pneumocystis) and Mycobacterium tuberculosis take 6 to 8 weeks.
Procedure and Patient Care
Before
![]() Explain the procedure for sputum collection to the patient.
Explain the procedure for sputum collection to the patient.
![]() Remind the patient that sputum must be coughed up from the lungs and that saliva is not sputum (Figure 7-9).
Remind the patient that sputum must be coughed up from the lungs and that saliva is not sputum (Figure 7-9).
Figure 7-9 Collection of sputum specimen. The specimen should be representative of pulmonary secretions—not saliva.
• Hold antibiotics until after the sputum has been collected.
• If an elective specimen is to be obtained, give the patient a sterile sputum container on the night before the sputum is to be collected so that the morning specimen may be obtained on arising.
![]() Instruct the patient to rinse out his or her mouth with water before the sputum collection to decrease contamination of the sputum by particles in the oropharynx. Antiseptic mouthwash, however, is to be avoided.
Instruct the patient to rinse out his or her mouth with water before the sputum collection to decrease contamination of the sputum by particles in the oropharynx. Antiseptic mouthwash, however, is to be avoided.
During
• Note that sputum specimens are best taken when the patient awakes in the morning before eating or drinking.
• Collect at least 1 teaspoon of sputum in a sterile sputum container.
• Usually obtain sputum by having the patient cough after taking several deep breaths.
• If the patient is unable to produce a sputum specimen, stimulate coughing by lowering the head of the patient's bed or giving the patient an aerosol administration of a warm, hypertonic solution.
• Note that other methods to collect sputum include endotracheal aspiration, fiberoptic bronchoscopy, and transtracheal aspiration.
Test Results and Clinical Significance
Bacterial infection (e.g., pneumonia),
Atypical bacterial infection (e.g., tuberculosis),
Sputum that is obtained for the above-listed types of organisms is plated on several types of culture media to grow the organisms that could grow in the pulmonary tree. Some of these organisms are quite difficult to grow in the laboratory and require great attention to detail to effectively grow these pathogens and demonstrate disease.
Related Test
Tuberculosis Culture (p. 768). This is the only manner in which the diagnosis of tuberculosis (TB) can be made with certainty. When TB is grown from the culture of a specimen, the diagnosis of TB can be made and treatment based on drug sensitivities can be started.
Sputum Cytology
Indications
Sputum for cytologic examination is indicated for any patient in whom the diagnosis of cancer of the lung is considered. Bronchoscopy and percutaneous lung biopsy have supplanted the need for sputum cytology to a great degree. Now its greatest use is in patients who have abnormal chest x-ray film results, productive cough, and nothing visible on bronchoscopy. It is also used to monitor smokers who have had some atypical changes on prior examination of the lower respiratory tract.
Test Explanation
Tumors within the pulmonary system frequently slough cells into the sputum. When the sputum is gathered, the cells are examined. If the cytologic examination indicates malignant cells, a lung tumor exists within the mucosa of the trachea, bronchi, and lungs. If only normal epithelial cells are observed, either no malignancy exists or any existing tumor is not shedding cells at that time. Therefore a positive test indicates malignancy; a negative test means nothing. The test is more likely to be positive on smokers who have a chronic productive cough and/or hemoptysis. Furthermore, the more specimens obtained, the greater the accuracy of the test. This test is rarely performed, now that tissue for biopsy can be easily obtained by bronchoscopic biopsy (p. 587).
Interfering Factors
• False-negative findings can occur as a result of poor cytologic preparation or inadequate specimen acquisition. Interpretation of cytologic changes is difficult, but most pathologists who have had experience with cytology maintain good accuracy. This is usually monitored by quality assurance studies within the pathology department.
Procedure and Patient Care
Before
![]() Explain the procedure for sputum collection to the patient.
Explain the procedure for sputum collection to the patient.
![]() Remind the patient that sputum must be coughed up from the lungs and that saliva is not sputum.
Remind the patient that sputum must be coughed up from the lungs and that saliva is not sputum.
• Give the patient a sterile sputum container the night before the sputum is to be collected so that the morning specimen may be obtained on arising.
![]() Instruct the patient to rinse out her or his mouth with water to decrease contamination of the sputum by particles in the oropharynx.
Instruct the patient to rinse out her or his mouth with water to decrease contamination of the sputum by particles in the oropharynx.
During
• Note that sputum specimens are best collected when the patient awakes in the morning.
• Collect at least 1 teaspoon of sputum in the sterile sputum container. The container may or may not contain alcohol as an immediate fixative—this varies according to the laboratory. Certainly if a 24-hour specimen is requested, alcohol must be within the container to diminish cellular deterioration during the collection period.
• Usually obtain sputum by having the patient cough after taking several deep breaths.
• If the patient is unable to produce a sputum specimen, stimulate coughing by lowering the head of the patient's bed or with aerosol administration of a warm hypertonic solution.
• Note that other methods to collect sputum include endotracheal aspiration, fiberoptic bronchoscopy, and transtracheal aspiration. Bronchial brushings can obtain excellent specimens for cytologic examination. A brush is placed through the bronchoscope and wiped on the bronchial mucosa. It is then withdrawn back into its sheath and wiped on a dry slide, which is immediately fixed.
• Usually collect sputum for cytologic examination once daily on 3 successive days. The first morning specimen is the best.
Test Results and Clinical Significance
Malignancies: Malignancies of the trachea, bronchus, and lung can be detected. Marked changes in the nuclear/cytoplasmic ratio, size of the cell, and differentiation of the cell indicate suspicious changes. It is now thought that cellular changes progress from benign, normal-looking cells to metaplastic cells, to atypical cells, to frankly cancerous cells. The cytologic report may indicate the cells are somewhere within that spectrum. The cells may be labeled benign, abnormal, suspicious, or definitely cancer. The epithelial cells of the lower respiratory system seem to make those progressive changes as the number of years the person smokes increases.
Benign cellular changes: This is most commonly related to infection (bronchiectasis), exposure (asbestosis), or viral pneumonitis.
Asthma: These patients often have an increased number of eosinophils within their sputum.
Related Test
Bronchoscopy (p. 587). This is an endoscopic test through which pulmonary lavage can be performed and specimens for cytologic examination can be obtained.
Throat and Nose Cultures
Indications
A throat or nose culture is obtained to diagnose bacterial, viral, gonococcal, or candidal pharyngitis. It is indicated on patients who complain of a sore throat, have a fever of unknown cause, or may be chronic carriers of recurrent infection. Nose cultures are used to identify acute nasal and/or sinus infections and to identify carriers of pathogenic bacteria.
Test Explanation
Because the throat is normally colonized by many organisms, culture of this area serves only to isolate and identify a few particular pathogens (e.g., streptococci, meningococci, gonococci, Bordetella pertussis, Corynebacterium diphtheriae). Recognition of these organisms requires treatment. Streptococci are most often sought, because a beta-hemolytic streptococcal pharyngitis (Figure 7-10) may be followed by rheumatic fever or glomerulonephritis. This type of streptococcal infection most frequently affects children between the ages of 3 and 15 years. Therefore all children with a sore throat and fever should have a throat culture done to attempt to identify streptococcal infections. In adults, however, fewer than 5% of patients with pharyngitis have a streptococcal infection. Therefore throat cultures in adults are indicated only when the patient has severe or recurrent sore throat, often associated with fever and palpable lymphadenopathy. These adults often have a history of previous streptococcal infections.
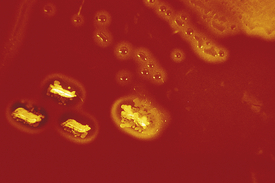
Figure 7-10 Blood agar plate showing beta-hemolytic colonies (clear zone around colony) of group A streptococci, the organism that causes bacterial pharyngitis.
Because Streptococcal pharyngitis remains an important cause of morbidity and is one of the leading reasons for physician visits, it is essential to focus on this organism specifically with throat and nose cultures. Although there are clinical algorithms to assess the probability that pharyngitis is caused by Streptococcus pyogenes, the diagnosis of streptococcal pharyngitis cannot be made on clinical grounds alone. Same-day testing by Rapid Antigen Detection Test (strept screen) is an important strategy to reduce unnecessary antibiotic use. With these strept screen kits, the streptococcus organism can be identified directly from the swab specimen. The streptococcus is chemically or enzymatically extracted from the swab specimen. It is then tested with the antisera containing the antibodies to group A streptococcus antigen. ELISA (chromatographic immunoassay) are used to detect group A streptococcus. This is a qualitative study with positive and negative results being color coded (in most kits). Obviously some kits perform better than others. False-negative results may occur with any test method if the specimen contains small numbers of streptococci (early in the course of the infection). Therefore a good throat swab is crucial. All antigen-negative swabs should have the negative result confirmed by culture. The rapid serologic tests can be performed in about 15 minutes in any lab or in most physicians' offices that treat children. The final culture report takes at least 2 days.
Infections by group A streptococci are unique because they can be followed by a serious complication (e.g., rheumatic fever, scarlet fever, or glomerulonephritis). Serologic tests (p. 470) are used primarily to determine if a previous group A Streptococcus infection (e.g., pharyngitis, pyodermia, pneumonia) has occurred and is the cause of a post-streptococcal disease. These post-streptococcal diseases occur following the infection and after a period of latency during which the patient is asymptomatic. The latency period for glomerulonephritis is approximately 10 days, and for rheumatic fever is about 20 days.
Antibodies (e.g., anti-streptolysin O and antideoxyribonuclease) are directed against streptococcal extracellular products that are primarily enzymatic proteins. Serial rising titers of these antibodies over several weeks, followed by a slow fall in titers, are more supportive than a single titer in the diagnosis of a previous streptococcal infection. The highest incidence of positive results is during the third week after the onset of acute symptoms of the poststreptococcal disease. By 6 months, only about 30% of patients have abnormal titers. By 12 months, levels return to normal.
A routine throat culture uses several different types of culture media (chocolate, streptococcus-specific, and other agar) to grow various bacteria. When a specific streptococcus culture is requested or if the strept screen is negative, the specimen is plated on streptococcus-specific agar only. All cultures should be performed before antibiotic therapy is initiated. Otherwise, the antibiotic may interrupt the growth of the organism in the laboratory. More often than not, however, the physician will want to institute antibiotic therapy before the culture results are reported. In these instances, a Gram stain of the specimen smeared on a slide is most helpful and can be reported in less than 10 minutes. All forms of bacteria are grossly classified as gram-positive (blue staining) or gram-negative (red staining). Knowledge of the shape of the organism (e.g., spherical [coccus], rod-shaped [bacillus]) also can be very helpful in the tentative identification of the infecting organism. With knowledge of the Gram stain results, the physician can institute a reasonable antibiotic regimen based on past experience regarding the organism's possible identity and sensitivity. Most organisms take approximately 24 hours to grow in the laboratory, and a preliminary report can be given at that time. Occasionally, a period of 48 to 72 hours is required for growth and identification of the organism. Cultures may be repeated on completion of appropriate antibiotic therapy to identify resolution of the infection.
Nasal and pharyngeal cultures are often done to screen for infections and carrier states caused by various other organisms such as Staphylococcus aureus, Haemophilus influenzae, Neisseria meningitidis, respiratory syncytial virus (RSV), and viruses containing rhinitis. Health care workers in the operating room and newborn nursery may have these cultures performed to screen potential sources of spread once an outbreak occurs in a hospital setting. These cultures are also used to detect infection in elderly and debilitated patients.
Procedure and Patient Care
During
• Obtain a throat culture by depressing the tongue with a wooden tongue blade and touching the posterior wall of the throat (Figure 7-11) and areas of inflammation, exudation, or ulceration with a sterile cotton swab. Two swabs are preferred. Growth of streptococcus from both swabs is more accurate, and the second swab can also be used in the strept screen. Avoid touching any other part of the mouth. Place the swabs in a sterile container.
• Obtain a nasal culture by gently raising the tip of the nose and inserting a flexible swab into the nares. Rotate the swab against the side of the nares. Remove the swab and place it in an appropriate culture tube.
• Obtain a pharyngeal culture by gently raising the tip of the nose and inserting a flexible swab along the bottom of the nares. Guide this swab until it reaches the posterior pharynx. Rotate the swab to obtain secretions and then remove it. Place the swab in an appropriate culture tube.
• Wear gloves and handle the specimen as if it were capable of transmitting disease.
• Place the swab in a sterile container and send it to the microbiology laboratory within 30 minutes.
• Note the following special considerations for specimen collection in young children:
Test Results and Clinical Significance
Acute pharyngitis: Throat cultures are used to identify pathogenic bacteria such as streptococci, Corynebacterium diphtheriae, gonococci, Bordetella pertussis, Neisseria, and staphylococci. Candida, and Bordetella infections can also be identified.
Tonsillar infections: These infections can be identified and their source determined if the swab is applied adequately to the tonsillar areas.
Chronic nasal carriers of bacteria: Some people are chronic carriers of bacterial diseases that can initiate an infection when transferred to others. These people may carry staphylococci, streptococci, influenza, or respiratory syncytial virus.
Related Test
Streptococcus Serologic Testing (p. 470). This antibody panel is used to identify previous Group A streptococcus infections in a patient who is considered to have a post-streptococcus disease such as rheumatic fever or glomerulonephritis.
Tuberculosis Culture (TB Culture, BACTEC Method, Polymerase Chain Reaction, AFB Smear)
Indications
TB culture is indicated in any patient with a persistent productive cough, night sweats, anorexia, weight loss, fever, and hemoptysis. This diagnosis should be especially considered in high-risk patients, such as those who are immunocompromised, have alcoholism, or have had a recent exposure to TB.
Test Explanation
The diagnosis of TB can be made only by identification and culture of Mycobacterium tuberculosis in the specimen. (See p. 1126 for other TB testing.) Conventional culture techniques for growth, identification, and susceptibility testing of acid-fast mycobacterium take 4 to 6 weeks. Because the patient suspected of having TB cannot be isolated from society for that duration, the disease may spread to many other people while the patient is waiting for the diagnosis. With the resurgence and increasing incidence of TB in the U.S. population (especially among immunocompromised patients with acquired immunodeficiency syndrome [AIDS]), newer, more rapid culture techniques have been identified and are now being utilized.
The BACTEC method is a radiometric culture technique in which the growth medium for culturing mycobacteria is supplanted with a substrate labeled with radioactive carbon (14C). This substrate is used by mycobacteria, and during metabolism, radioactive carbon dioxide (14CO2) is produced from the substrate. The 14CO2 is detected quantitatively by counting the radioactivity with the Becton Dickinson Diagnostic Instrument System. The rate and amount of CO2 produced is directly proportional to the rate and amount of growth occurring on the medium. With this technique, very small quantities of 14CO2 can be detected. This permits quick identification of mycobacterial growth. This technique is used not only to isolate mycobacteria from clinical specimens but also to differentiate M. tuberculosis complex from other mycobacteria and for antimicrobial susceptibility testing.
Polymerase chain reaction (PCR) genotyping has been developed. With the addition of a deoxyribonucleic acid (DNA) polymerase, genetic chromosomal parts can be multiplied. This allows amplification of genomes, which then can be detected by genetic DNA probes. With genotyping, M. tuberculosis can be identified in as little as 36 to 48 hours. With this reduction in diagnostic time, treatment can be started earlier. It is anticipated that the spread of TB therefore will be greatly reduced. The average detection time, however, is longer for extrapulmonary specimens than for sputum specimens. The time for identification is greatly reduced when numerous mycobacteria are present. Generally organisms in specimens from patients already receiving antituberculosis treatment take longer to grow.
After identification and growth of mycobacteria, antibiotic susceptibility testing is performed to identify the most effective antimycobacterial drugs. The culture can be performed on sputum, body fluids, cerebrospinal fluid (CSF), and even biopsy tissue specimens.
When tuberculosis is suspected, a sputum smear for acid-fast bacillus (AFB) can be obtained. After taking up a dye, such as fuchsin, M. tuberculosis is not decolorized by acid alcohol (i.e., it is acid-fast). It is seen under the microscope as a red, rod-shaped organism. If this bacillus is seen, the patient may have active TB. Other specimens, such as cerebrospinal fluid, tissue, and synovial fluid, may be used. AFB is also used to monitor treatment for TB. If after adequate therapy (2 months) the sputum still contains AFB (even though the culture may be negative because of anti-TB drugs), treatment failure should be considered.
Procedure and Patient Care
During
• For sputum, obtain an early morning specimen. It is best to induce sputum production with an ultrasonic or nebulizing device.
• Collect three to five early morning specimens. All specimens must contain mycobacteria to make the diagnosis of TB.
• For urine collection, obtain three to five single, clean-voided specimens early in the morning.
• Note that swabs, intestinal washings, and biopsy specimens should be transported to the laboratory immediately for preparation.
• Follow the institution's policy for universal specimen handling. Staff should wear an N95 respirator mask when in contact with the patient. Ideally, the patient should be placed in a negative pressure room.
• Note the following procedural steps:
1. Once the specimen is received by the laboratory, a decontamination process is applied to it to kill all non-mycobacteria. The specimen is then cultured in the appropriate medium.
2. With the rapid growth techniques, the specimen is evaluated every 24 hours.
3. When cultural growth is considered adequate, the organisms are stained for acid-fast bacilli and identified (p. 706).
4. With DNA genetic probes, the Mycobacterium species is identified.
5. At this point, if M. tuberculosis is present, the report will read “culture is positive for mycobacteria.” If the species has been identified, this also will be reported.
6. Drug-susceptibility testing then will be carried out and subsequently reported.
Test Results and Clinical Significance
Atypical mycobacterial nontuberculous disease: These organisms require special medium plates to grow. Any fluid or tissue can be used as a culture specimen. If the lungs are considered to be the sight of infection, sputum or pleural fluid is used. If the kidneys are suspected to be involved, urine should be tested. Others specimens include abdominal fluid, stomach aspirate, and bone tissue.
Related Tests
Acid-Fast Bacilli Smear (p. 706). This smear is used to support the diagnosis of TB because it cannot, by itself, indicate the diagnosis of TB. The smear (usually of sputum) is also used to monitor treatment for TB.
Tuberculin Skin Testing (p. 1126). Purified protein derivative is administered intradermally to test for prior exposure to TB. Skin testing cannot indicate active or dormant TB. Positive results imply nothing more than previous exposure.
Chest X-Ray (p. 1014). Because TB is usually infective to the lungs due to inhalation of airborne infectious material, the chest x-ray examination often demonstrates the results (Ghon complex) of the acute granulomatous infection.
Interferon Gamma Release Assay (QuantiFERON-TB Gold) (see following test). The IGRA is a whole-blood test for use as an aid in diagnosing Mycobacterium tuberculosis infection. It is a measure of the patient's cell–mediated immune response to TB infection.
Tuberculosis Testing (TB Testing, Interferon Gamma Release Assay [IGRA], QuantiFERON-TB Gold [QFT, QFT-G, TB Gold Test, TB Blood Test], Nucleic Acid Amplification for TB [NAAT], TB Antibody)
Normal Findings
| IGRA Result | Interpretation |
| Positive | Mycobacterium tuberculosis infection likely |
| Negative | Mycobacterium tuberculosis infection unlikely, but cannot be excluded. If TB disease is highly suspected, a negative result does not rule out infection. False-negative results may be seen in immunocompromised patients. |
| Indeterminate | Test not interpretable. Collection of a new specimen for testing is recommended if clinically indicated. |
Indications
These tests are used to diagnose active TB infection in patients recently exposed to or suspected to have TB infection.
Test Explanation
Interferon gamma release assays (IGRA) (e.g., QuantiFERON-TB), NAA tests, and serologic TB testing are used to diagnose active TB infection. The gold standard for making the diagnosis of active TB is the TB culture (p. 768). However, it takes 2 to 6 weeks to obtain results. Identifying acid-fast bacilli in a smear (AFB smear) (p. 706) of the body fluid (usually sputum) is a rapid method of identifying TB in 24 hours. Unfortunately, AFB is not very sensitive or specific. It is often positive in non-tuberculosis mycobacterial diseases. The IGRA is a whole-blood test used in diagnosing Mycobacterium tuberculosis infection. The NAAT is a rapid and accurate test of sputum and is used as corroborative information in the diagnosis of TB. Serology testing on blood is also a rapid test used to identify active TB disease infection (Table 7-3).
TABLE 7-3
CDC Recommendations for Initial Sputum Specimens for TB Diagnosis
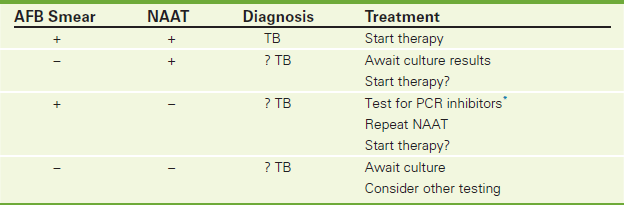
∗Inhibitors may include blood, fabrics, tissues, excess salts, ionic detergents, sarkosyl, ethanol, isopropanol, and phenol.
The value of decreasing the time it takes to make the diagnosis of TB is significant. With an earlier laboratory confirmation of TB disease, treatment can be initiated earlier, patient outcome can be improved, opportunities to interrupt transmission of the disease can be increased, and more effective public health interventions can be instigated. For those reasons, there has been increasing clinical interest in establishing the diagnosis of TB as early as possible. IGRA TB antibody testing and NAA can provide rapid confirmation of TB infection. However, these tests cannot indicate anti-TB drug sensitivities.
The diagnosis of active or latent TB still requires additional testing (including a chest x-ray, sputum smear, and culture). IGRA is a diagnostic aid that measures a component of cell-mediated immune reactivity to M. tuberculosis, much like the tuberculin skin testing (TST) (p. 1126). The TST is performed by injecting a small amount of tuberculin purified protein derivative (PPD) into the forearm and examining the injection site at 48 to 72 hours after administration. This skin test assesses an in vivo delayed-type hypersensitivity immune response to a polyvalent antigenic mixture in PPD. Unlike TST, however, IGRA can be performed on patients with prior bacille Calmette-Guérin (BCG) vaccination without causing a hypersensitivity response. Furthermore, compared with TST, IGRA results are not subject to reader bias and error. Like TST, false negatives can occur in anergic patients.
IGRA cannot differentiate active from latent TB infection. Its use in latent infection is being studied. Because IGRA uses TB-specific antigens as compared with TST that uses nonspecific PPD antigens, IGRA is more accurate and specific. IGRA results are available in 24 hours. Because this is an in vitro test that never exposes the patient to its antigenic proteins, IGRA never generates a “booster” false-positive response. Finally, the elimination of a second patient visit for skin reading makes IGRA an attractive alternative to TST. IGRA can be used for serial surveillance testing up to 12 months after a negative PPD if the initial IGRA is negative.
IGRA and NAAT are used in the same patient population as TST. These include contact investigations, evaluation of recent immigrants, and sequential-testing surveillance programs for infection control, such as those for health care workers.
The IGRA is an ELISA test in which blood samples are mixed with synthetic PPD protein derivative antigens (ESAT-6, TB7.7, and CFP-10). After incubation of the blood with these antigens for 16 to 24 hours, interferon-gamma (IFN-γ) from T-cell lymphocytes is measured. If the patient's T-cell lymphocytes are sensitized to Mycobacterium tuberculosis complex organisms (i.e., Mycobacterium tuberculosis, Mycobacterium bovis, Mycobacterium africanum, Mycobacterium microti, and Mycobacterium canetti), the T-cell lymphocytes in their blood that recognize specific mycobacterial antigens and their lymphocytes will release large quantities of IFN-γ in response to contact with the TB antigens.
The IGRA may be falsely negative in patients with advanced TB because of a suppressed IFN response. The IGRA (unlike some skin tests) does not test for anergy and may be inaccurately negative in immunosuppressed patients. The sensitivity and rate of indeterminate results using IGRA is diminished in immunocompromised persons with HIV infection, AIDS, current treatment with immunosuppressive drugs, selected hematologic disorders, and certain malignancies. These conditions or treatments are known or suspected to decrease responsiveness to the TST, and they might also decrease production of IFN-γ in the IGRA assay. As with a negative TST result, negative IGRA results alone might be insufficient to exclude tuberculosis infection in these persons.
NAAT is designed to identify TB complex DNA in a body fluid (bronchoalveolar lavage, bronchial washing, sputum, stool, pleural/abdominal fluid, tissue, or urine sample). This test provides a rapid result in 24 hours. Like the described rapid tests, NAAT cannot indicate active infection from a previously treated TB infection. NAAT is now standard practice in the United States to aid in the initial diagnosis of patients suspected to have TB. The Centers for Disease Control has indicated that NAAT should be performed on at least one respiratory specimen for each patient with signs and symptoms of pulmonary TB for whom a diagnosis of TB is being considered but is not yet been established and for whom the test results would alter case management.
TB antibody serology is designed to identify IgG antibodies to TB mycobacteria in patients with active TB infections. This blood test can be used in previously vaccinated BCG patients. It is particularly useful in evaluating the effectiveness of anti-TB therapy and documenting a response to therapy. Like IGRA, serology may not be positive in immunocompromised patients, making its use in HIV-infected patients less helpful.
Interfering Factors
• Heterophile (e.g., human antimouse) antibodies in serum or plasma of certain individuals are known to cause interference with immunoassays. These antibodies from other inflammatory conditions may interfere with specific responses to ESAT-6, CFP-10, or TB7.7 peptides, leading to indeterminate and unreliable results.
• A false-negative interferon gamma release assay (IGRA) result can be caused by the stage of infection (i.e., specimen obtained before the development of cellular immune response), comorbid conditions that affect immune function, or other individual immunologic factors.
Procedure and Patient Care
During
• Collect 1 mL whole blood in each of three lab-specified collection tubes. The accuracy of the IGRA is dependent on the proper collection and incubation of the blood specimen. Blood should fill the tube as close to the 1-mL mark as possible. Underfilling or overfilling the tubes outside the 0.8- to 1.2-mL range may lead to erroneous results.
• Immediately following collection, each specimen tube must be shaken vigorously by shaking the tube up and down 10 times to ensure that the entire inner surface of the tube has been coated with blood. This distributes the stimulating antigens, allowing optimal processing and presentation of the antigens to T cells, which causes release of IFN-γ.
• For NAAT testing, 1 to 3 mL of sputum or body fluid is required. This should be refrigerated in a screw cap sterile container.
After
• Apply pressure or a pressure dressing to the venipuncture site.
• Incubate the blood tubes upright at 37° C for 16 to 24 hours (within 16 hours of collection).
![]() If the patient's results are positive, educate him or her about the necessary follow-up studies, such as chest radiograph and sputum cultures.
If the patient's results are positive, educate him or her about the necessary follow-up studies, such as chest radiograph and sputum cultures.
Virus Testing
Test Explanation
Viral infections are the most common infections affecting children and adults. Viruses are subdivided by the nuclear material they contain (ribonucleic acid [RNA] or deoxyribonucleic acid [DNA]). Infections from viruses are often indistinguishable from bacterial infections. Testing for a virus is indicated when a person with viral symptoms lives or has traveled to an area harboring the virus. Testing is done in the clinical setting when a patient has severe symptoms contributing to significant morbidity. Testing is also performed for epidemiologic reasons to identify a viral outbreak and its extent. Finally, testing can indicate immunity after exposure to the virus or a vaccination.
Viral testing is performed by identifying:
1. Antibodies to a specific antigen in the blood or in other body fluids (e.g., mononucleosis or Epstein-Barr)
2. Antigen parts of the virus by Reverse Transcription Polymerase Chain Reaction (RT-PCR) (e.g., respiratory syncytial or influenza A or B) or Nucleic Acid Amplification Tests (NAAT) (e.g., Dengue)
Epstein-Barr (p. 217), hepatitis (p. 286), respiratory syncytial, herpes, parainfluenza, HIV (p. 297), Dengue fever, Coxsackie, choriomeningitis, mumps, West Nile (p. 524), arbovirus, equine, cytomegalovirus (p. 200), rubella (p. 457), and influenza A, B are some of the viruses that have highest clinical priorities today.
Most viral infections have common symptoms that are flu-like and include fever, lethargy, headache, and neck/body aches. Certain patients (including infants, the elderly, the immunocompromised, and those with impaired lung function) are at risk for serious complications. Once the diagnosis is confirmed, where possible, aggressive antiviral treatment can be instigated and isolation can be carried out.
Front-line testing measures IgM or IgG antibodies to the virus may or may not be specific to that particular virus. If the front-line testing is positive, confirmatory tests may be carried out. This testing is important for public health officials and researchers. Test results may not correlate with cultures or viral load. Yet for other viral diseases, serology acts as the confirmatory test.
Viral RNA/DNA can be detected by RT-PCR (or in combination with NAAT) in serum (Dengue), or respiratory secretions, including upper and lower respiratory specimens (RSV or influenza). Nasopharyngeal swabs or aspirates are the preferred specimen types. This is particularly useful for suspected infections by influenza A, influenza B, and RSV. Molecular detection of influenza A and B RNA is also available. The sensitivity of the assay is very dependent upon the quality of the specimen submitted. Tracheal aspirates are not acceptable for testing because of the viscous nature of these specimens. Test accuracy depends on viral load in the specimen. Unlike serology, this testing is very specific for the suspected virus. RT PCR viral testing is often performed as panel testing, for example, respiratory virus panel. This panel, typically performed on a nasopharyngeal swab or bronchial mucus specimen, is able to detect 12 viruses/viral subtypes (adenovirus, rhinovirus, human meta-pneumovirus, RSV types A/B, parainfluenza types 1 to 3, influenza B, influenza A [with subtypes H1 and H3]). Viral RNA/DNA testing has advantages that make this testing preferable (Box 7-2).
Growth and identification of the virus in culture from a patient specimen provides a definitive diagnosis when positive. The ability to isolate a virus in culture depends on many aspects of the culture process. The first is determining the correct specimen for culture. That depends on the organ involved and the type of virus suspected (Table 7-4). Timing is important. Viral load is always greatest in the early stages of the disease. Cultures obtained in the first few days after symptoms begin offer the best chance of identifying the infective culture. Using the correct culture medium is essential. In general, the culture medium used to grow the viral culture is a tissue/cell culture. Different viruses vary greatly in their ability to grow in specific cell cultures. Viral cultures take 3 to 7 days to be reported. Culture results are not an indication of viral load, severity of disease, or disease progression/response to therapy.
TABLE 7-4
Specimen Culture for Common Viruses and Diseases
| Common Virus | Specimens | Disease |
| Adenovirus | Throat culture | Influenza |
| Influenza | Bronchoscopic aspiration | Pneumonia |
| Respiratory syncytial | Nose culture | Pharyngitis |
| Rhinovirus | — | Common cold |
| Rubella | Throat culture | Skin rash |
| Rubeola | Skin vesicle | Zoster |
| Coxsackie | — | Hand, foot, and mouth |
| Varicella | — | Chickenpox |
| Arbovirus | Throat culture | Meningitis |
| Enterovirus | Cerebrospinal fluid (CSF) | Encephalitis |
| Herpes | Blood | |
| Cytomegalovirus | Urine, sputum, mouth | CMV infection |
| Parvovirus | Stool | Skin rash |
| Adenovirus | Blood | Arthropathy |
| Sputum | Upper respiratory infection | |
| Influenza A or B | Throat culture | Flu syndrome |
| Epstein-Barr | Blood | Mononucleosis Epstein-Barr–associated disease |
Interfering Factors
• Inadequate specimen, timing, or choice of culture medium will cause false-negative tests.
• The use of a cotton swab or wooden applicator for specimen collection may destroy the virus.
Procedure and Patient Care
During
• If blood is the specimen, obtain a venous blood sample in the appropriate tube as determined by the laboratory.
• For other body fluid samples, such as nasopharyngeal or sputum, follow the guidelines below:
1. Use a closed specimen system to obtain and transport the specimen to the laboratory.
2. Transport the specimen immediately to the laboratory. Viruses in specimens quickly lose their vitality.
3. Place samples on ice if delivery to the laboratory is not immediate.
4. Small volume specimens (e.g., tissue aspirates) are often best transported in a liquid medium. If bacterial cultures are to be performed, use sterile saline solution for transfer.
Related Tests
Throat and Nose Cultures (p. 765). This test is obtained to diagnose bacterial, viral, gonococcal, or candidal pharyngitis.
Specific discussions are included for each of the following viruses:
Wound and Soft-Tissue Culture and Sensitivity (C&S)
Indications
A wound or soft tissue culture is indicated when a wound or soft tissue has signs of infection (redness, warmth, swelling, and pain). In a postoperative patient with a persistent fever of unknown origin, a wound may be probed and cultured even if the signs of infection are not present. Any spontaneous drainage from a wound or soft tissue should be cultured to document infection for treatment and drug sensitivities and to document the appropriateness of skin and wound isolation precautions.
Test Explanation
Wound cultures are obtained to determine the presence of pathogens in patients with suspected wound infections. Wound infections are most often caused by pus-forming organisms. All cultures should be performed before antibiotic therapy is initiated. Otherwise, the antibiotic may interrupt the growth of the organism in the laboratory. More often than not, however, the physician will want to institute antibiotic therapy after the wound is cultured but before the culture results are reported. In these instances, a Gram stain of the specimen smeared on a slide is most helpful and can be reported in less than 10 minutes. All forms of bacteria are grossly classified as gram-positive (blue staining) or gram-negative (red staining). Knowledge of the shape of the organism (e.g., spherical, rod shaped) also may be very helpful in the tentative identification of the infecting organism. With knowledge of the Gram stain results, the physician can institute a reasonable antibiotic regimen based on past experience regarding the organism's possible identity. Most organisms require approximately 24 hours to grow in the laboratory, and a preliminary report can be given at that time. Occasionally 48 to 72 hours are required for growth and identification of the organism. Cultures may be repeated after appropriate antibiotic therapy to assess for complete resolution of the infection.
It is important to recognize that many wound infections contain more than one organism. Multiple organisms may grow on culture. Deep-space wounds, wounds containing necrotic debris or gas, and postoperative wounds commonly contain aerobic and anaerobic bacteria. These different types of organisms require different culture media and conditions for growth.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
• Assemble all equipment (Figure 7-12).
Figure 7-12 Equipment for collection of specimens from an open wound or decubitus ulcer. The specimen obtained with red-tipped applicators is immediately placed into its protective tube. The specimen is adequate for aerobic and anaerobic cultures. Large-volume fluid specimens may be placed in the blue-tipped container. However, this type of container must be transported immediately to the lab if anaerobic cultures are to be added to aerobic cultures.
During
• Aseptically place a sterile cotton swab into the pus of the patient's wound, and then place the swab into a sterile, covered test tube. (Culturing specimens from the skin edge is much less accurate than culturing the suppurative material.)
• If an anaerobic organism is suspected, obtain an anaerobic culture tube from the microbiology laboratory. The specimen is best obtained by aspirating a closed wound and directly transferring the pus to the anaerobic culture tube.
• If wound cultures are to be obtained on a patient requiring wound irrigation, obtain the culture before the wound is irrigated.
• If any antibiotic ointment or solution has been previously applied, remove it with sterile water or saline before obtaining the culture several hours later.
• Handle all specimens carefully. These specimens are capable of transmitting disease.
• Indicate on the laboratory slip any medications the patient may be taking that could affect test results.
After
• Transport the specimen to the laboratory immediately after testing (within no more than 30 minutes).
• Notify the physician of any positive results so that appropriate antibiotic therapy can be initiated.
• Note that if pus was observed in the wound or soft tissue, the patient should be immediately placed on skin and wound isolative precautions. The culture report documenting the infection does not need to be received before appropriate protective precautions are instituted.
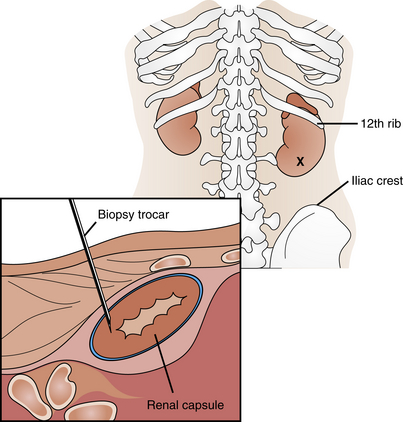
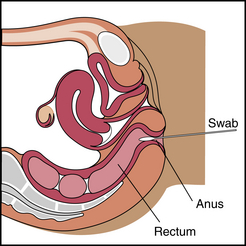
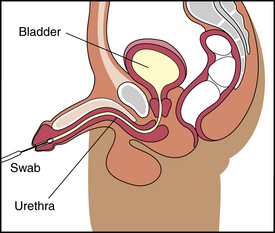
 Increased Levels
Increased Levels