Nuclear Scanning
Overview
Reasons for Performing Nuclear Medicine Studies
With the administration of a radiopharmaceutical and subsequent detection of the photons emitted from a particular organ, anatomic and functional abnormalities of various body areas can be detected. Nuclear medicine studies do not identify the specific cause (disease) of the abnormality. They provide supportive information to be used in conjunction with other diagnostic modalities. There are many indications for nuclear scanning, some of which are listed below:
1. To stage cancer by detecting metastasis (PET scan)—or to test specific organs such as the bone (bone scan), liver (liver scan), or brain (brain scan)
2. To diagnose acute and chronic cholecystitis (gallbladder scan)
3. To detect cerebral pathologic conditions (brain scan)
4. To evaluate gastric emptying (gastric emptying scan)
5. To localize sites of gastrointestinal (GI) bleeding (GI bleeding scan)
6. To diagnose pulmonary embolism (lung scan)
7. To determine perfusion, structure, and function of the kidneys (renal scan) or heart (cardiac scan)
8. To evaluate thyroid nodules (thyroid scan)
9. To evaluate testicular swelling and pain (scrotal scan)
10. To evaluate cardiac function and coronary artery patency
The radionuclides used in diagnostic medicine are artificially produced by either a nuclear reactor or a charged particle accelerator (cyclotron) by irradiating the nuclei and causing them to be unstable. Because of this instability, the nucleus of the radionuclide atom emits radioactive particles (photons in the gamma radiation range). The radionuclides used in nuclear scanning have short half-lives, which refers to the time required for 50% of the radioactive atoms to undergo decay. Technetium-99m (99mTc) is used extensively in nuclear scanning because its half-life is 6 hours and it emits low levels of gamma rays. Other commonly used radionuclides include gallium, thallium, and iodine.
To get to the desired organ, radionuclides are combined with a transport molecule. This combination of radionuclide and transport molecule is called a radiopharmaceutical. A radiopharmaceutical is the compound, labeled with the radionuclide that is administered to the patient and localized in the organ to be studied. For most nuclear scans, radiopharmaceuticals are given intravenously. Less commonly used methods of administration include the oral and inhalation routes. Radiopharmaceuticals concentrate in target organs by various mechanisms. For example, some labeled compounds, such as iodohippurate sodium 131I (Hippuran 131I), are cleared from the blood and excreted by the kidneys. Some phosphate compounds concentrate in the bone and infarcted tissue. Lung function can be studied by imaging the distribution of inhaled gases and aerosols. Other radiopharmaceuticals (such as FDG) are selectively taken up by cancers.
After the radioisotope concentrates in the desired area, it emits gamma rays. The area is scanned with a gamma camera or a scintillation scanner that detects and records the emission of gamma rays. With each gamma ray detected, a light particle is emitted from the scintillation scanner. A computer translates these light readings into a two-dimensional image or scan (scintigram) that is printed in various shades of gray. Using multiple scanners, a three-dimensional image (SPECT) can be obtained. Scintigrams can now be produced in color. The shades of gray or color show the distribution of the radionuclide in the organ. When superimposed on a baseline computerized tomogram (PET/CT), accurate anatomy can be created. Hot spots are areas of increased uptake, and cold spots are areas with decreased uptake of the radionuclide. Normally the uptake of the radionuclide in an organ is diffuse and homogeneous. Hot and cold spots may mean different things on different scans. For example, a cold spot identified in the liver, spleen, or brain would indicate tumor, abscess, or some other space-occupying lesion. A cold spot detected on a thallium scan of the heart would not be suggestive of tumor but rather indicates an area of ischemia or infarction. On bone scan, hot spots may indicate areas of osteoblastic activity surrounding tumor. Arthritis or fracture may also be evident as hot spots. The scanning usually takes place in the nuclear medicine department.
Scanning can be static, which means that the patient and the camera are held in one position until an image is completed. Often the patient is rotated into another position for a static image of another view of the same organ. Dynamic scanning can also be performed and allows one to evaluate the blood flow to a certain organ, such as the brain or the liver. Single-photon emission computed tomography (SPECT) is a technique in which a gamma camera is serially placed at multiple angles around the entire circumference of the patient. With this method, three-dimensional images can be obtained of the organ to be studied. Increased sensitivity is obtained. Positron emission tomography (PET) scanning can demonstrate anatomic, functional, and biochemical abnormalities in an organ.
Although nuclear scanning includes a risk of radiation for the patient, the risk associated with most radionuclides is much less than that of an x-ray study. The half-lives of the radioisotopes are short, resulting in minimal radiation contamination by way of fecal and urine wastes. Unless the benefit outweighs the risk, nuclear scans are contraindicated in pregnant women and nursing mothers because of the risk of injury to the fetus or infant. To help protect patients and others, patients should take some precautions for 12 hours after injection of radionuclides. Whenever possible, a toilet should be used, rather than a urinal. The toilet should be flushed several times after each use. Spilled urine should be cleaned up completely. After each voiding or fecal elimination, patients should thoroughly wash their hands. All urine- or fecal-soiled clothes should be washed separately.
Procedural Care for Nuclear Scans
Before
![]() Explain the procedure to the patient. Assure the patient that radiation exposure is limited and minimal.
Explain the procedure to the patient. Assure the patient that radiation exposure is limited and minimal.
• Assess for an allergy to the radiopharmaceutical (especially when iodine is used).
• Note whether the patient has had any recent exposure to radionuclides. The previous study could interfere with the interpretation of the current study.
• Record the patient's age and current weight. This information is sometimes used to calculate the amount of radioactive substance needed.
• Many of the scanning procedures do not require any preparation. However, a few have special requirements. For example, in bone scanning the patient is encouraged to drink several glasses of water between the time of the injection of the isotope and the actual scanning.
• For some studies, blocking agents may need to be given to prevent other organs from taking up the isotope. For example, Lugol iodine solution may be needed to protect the thyroid gland from iodine-tagged radioisotopes. Potassium chloride may be used during a brain scan to prevent an inordinate amount of technetium uptake by the choroid plexus, which would simulate a pathologic condition.
During
• Most radionuclides are injected intravenously. Often the patient is encouraged to drink water between administration of the rad7ioisotope and the scanning. Radionuclides can also be given orally (gastric emptying scan) or by inhalation (ventilation scan).
• The area is scanned at the designated time period. The delay between administration of the radionuclide and scanning depends on the length of time required for the specific organ or tissue to take up the radionuclide and concentrate it. The patient must lie still during the scanning. Scans are usually repeated over a period that may extend from 1 hour to 3 days. The patient returns to the nuclear medicine department for each scanning.
After
![]() Assure the patient that only tracer doses of radioisotopes have been used and that no precautions against radioactive exposure are necessary.
Assure the patient that only tracer doses of radioisotopes have been used and that no precautions against radioactive exposure are necessary.
• Although the amount of radionuclide excreted in the urine is very low, rubber gloves are sometimes recommended if the urine must be handled. Some doctors may advise the patient to flush the toilet several times after voiding.
![]() Encourage the patient to drink extra fluids to aid in excretion of the isotope from the body.
Encourage the patient to drink extra fluids to aid in excretion of the isotope from the body.
• If the isotope was injected intravenously, inspect the site for signs of infection, bruising, or hematoma.
Bone Scan
Indications
The bone scan is used to identify metastatic cancer involving the bone. It is often performed on cancer patients as a routine part of staging before and after treatment. To a lesser degree, bone scanning is used to identify pathologic bone conditions that cannot be identified on plain films of the bone (e.g., osteomyelitis, hairline fractures).
Test Explanation
The bone scan permits examination of the skeleton by a scanning camera after intravenous (IV) injection of a radionuclide material. Usually technetium-99m (99mTc) is the radionuclide utilized. After injection of the 99mTc, the radiopharmaceutical is taken up by the bone. Gamma rays are emitted from the 99mTc through the body and are detected by a scintillation scanner. The scintillation scanner emits light with each photon it receives from the gamma ray. When these light patterns are arranged in a spatial order, a realistic image of the bones is apparent.
The degree of radionuclide uptake is related to the metabolism of the bone. Normally a uniform concentration should be seen throughout the bones of the body. There is symmetric distribution of activity throughout the skeletal system in healthy adults. Urinary bladder activity, faint renal activity, and minimal soft-tissue activity are also normally present. An increased uptake of isotope is abnormal and may represent tumor, arthritis, fracture, degenerative bone and joint changes, osteomyelitis, bone necrosis, osteodystrophy, or Paget disease (Figure 8-1). These areas of concentrated radionuclide uptake are often called hot spots and are detectable months before an ordinary x-ray film can reveal the pathologic condition. Hot spots occur because new bone growth is usually stimulated around areas of abnormality. If a pathologic condition exists and there is no new bone formation around the lesion, the scan will not pick up the abnormality. Increased uptake of radionuclide is also seen in the normal physiologic active epiphyses of children (growth plates).
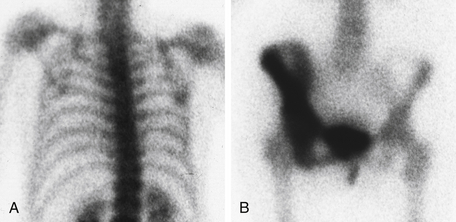
Figure 8-1 Bone scan. A, Upper body. B, Lower body. There is normal uptake of radionuclide in the bones of the upper body. In the lower body view, the right iliac, ischium, and pubic bones are associated with diffuse increased uptake of radionuclide, consistent with Paget disease.
The major reason a bone scan is performed is to detect metastatic cancer to the bone. All malignancies capable of metastasis may reach the bone, especially those of the prostate, breast, lung, kidney, urinary bladder, and thyroid gland. Bone scans are also useful in staging primary bone tumors such as osteogenic sarcomas and Ewing sarcoma. Bone scans may be serially repeated to monitor tumor response to antineoplastic therapy.
Bone scans also provide valuable information for the evaluation of patients with trauma or unexplained pain. Bone scanning is much more sensitive than routine x-ray films in detecting small and difficult-to-find fractures, especially in the spine, ribs, face, and small bones of the extremities. Bone scans are used to determine the age of a fracture as well. If a fracture line is seen on a plain x-ray film and the uptake around that fracture is not increased on a bone scan, the injury is said to be an “old” fracture, exceeding several months in age.
Although the bone scan is extremely sensitive, unfortunately it is not very specific. Fractures, infections, tumors, and arthritic changes all appear similar in this scan. When plain films fail to identify the classic findings of bone infection (osteomyelitis), bone scans are helpful.
A three-phase bone scan may be performed if inflammation (arthritis) or infection (osteomyelitis, septic arthritis) is suspected. In a three-phase bone scan, imaging is performed at three different times after injection of the radionuclide. Early uptake of the radionuclide would indicate infection or inflammation rather than neoplasm. Uptake of the radionuclide on delayed imaging that had not been present on early imaging would indicate neoplasm.
When the metastasis process is diffuse, virtually all of the radiotracer is concentrated in the skeleton, with little or no activity in the soft tissues or urinary tract. The resulting pattern, which is characterized by excellent bone detail, is frequently referred to as a “superscan.” A superscan may also be associated with metabolic bone diseases such as Paget disease, renal osteodystrophy, or osteomalacia. Unlike in metastatic disease, however, the uptake in metabolic bone disease is more uniform in appearance and extends into the distal appendicular skeleton. Intense calvarial uptake disproportionate to that in the remainder of the skeleton is another feature of a metabolic superscan.
The bone scan is performed by a nuclear medicine technologist in 30 to 60 minutes. It is interpreted by a physician trained in nuclear medicine imaging. The injection of the radioisotope causes slight discomfort. There may be some pain caused by lying on the hard scanning table for an hour. In many circumstances, magnetic resonance imaging (MRI) is used in place of bone scans. It is more specific in indicating disease pathology.
Contraindications
• Patients who are pregnant, unless the benefits outweigh the risk of fetal injury
• Patients who are lactating, because of the risk of contaminating maternal milk
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Assure patients they will not be exposed to large amounts of radioactivity because only tracer doses of the isotope are used.
Assure patients they will not be exposed to large amounts of radioactivity because only tracer doses of the isotope are used.
![]() Tell the patient that no fasting or sedation is required.
Tell the patient that no fasting or sedation is required.
![]() Inform the patient that the injection of the radioisotope may cause slight discomfort, nausea, or vomiting.
Inform the patient that the injection of the radioisotope may cause slight discomfort, nausea, or vomiting.
During
• Note the following procedural steps:
1. The patient receives an IV injection of an isotope, usually methylene diphosphate (MDP) or hydroxymethylene diphosphate (HDP) in a peripheral vein.
2. The patient is encouraged to drink several glasses of water between the time of radioisotope injection and the scanning. This facilitates renal clearance of any circulating tracer not picked up by the bone. The waiting period before scanning is approximately 2 to 3 hours.
3. The patient is instructed to urinate to eliminate any tracer that is in the bladder because it may block the view of the underlying pelvic bones.
4. The patient is positioned in the supine position on the scanning table in the nuclear medicine department (Figure 8-2).
Figure 8-2 Patient undergoing nuclear bone scan. The scintigraphy camera is on the lower part of the body.
5. A scintillation camera is placed over the patient's body and records the radiation emitted by the skeleton.
6. This information is translated into a two- or three-dimensional view of the skeleton, which is then visualized on film.
7. The patient may be repositioned in the prone and lateral positions during the test.
Test Results and Clinical Significance
Primary or metastatic tumors of the bone: These can be singular or multiple. It is difficult to specifically diagnose tumor. Serial scans may help.
Fracture: Increased uptake in the bone of a patient with anatomic pain is very suggestive of a fracture missed on routine plain films.
Osteomyelitis: Small islands of increased uptake within the bone of a patient with a compatible clinical history indicates infection.
Bone necrosis: Decreased uptake (cold spot) may be present if there is not new bone growth surrounding the area of bone necrosis.
Brain Scan (Cisternogram, Cerebral Blood Flow, DaT Scan)
Indications
The usefulness of nuclear brain scan is narrow when compared to CT, MRI, and PET scans of the brain. The cost of these newer scans sometimes precludes their utilization and nuclear brain scanning may be preferably used. This test can be used to identify pathologic conditions (tumor, infarction, infection) involving the cortex. It is used for patients with headaches, epilepsy, and other neurologic symptoms. The nuclear cerebral blood flow brain scan is used to support the diagnosis of cerebral brain death.
Test Explanation
The brain scan permits examination of the brain by a scanning camera after intravenous (IV) injection of a radionuclide material (Figure 8-3). A technetium-99m (99mTc) radionuclide, such as hexamethylpropyleneamine (Tc-HMPAO) or ethyl cysteinate dimer (Tc-ECD), bicisate, or Neurolite, is most commonly used.
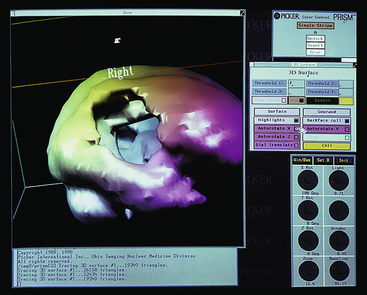
Figure 8-3 Image produced by radionuclide scan. This particular image demonstrates a deficit in cerebral blood flow caused by an arteriovenous malformation.
Primarily, nuclear brain scan is used to indicate complete and irreversible cessation of brain function (brain death). This determination, when combined with appropriate clinical data, allows for cessation of medical therapy and opportunity for the harvest of potential donor organs. With brain death, there is complete absence of blood perfusion to the brain. In cerebral blood flow scanning, one normally sees an early “arterial visualization phase” followed by a “blood pool phase” in which the venous sinuses but not the brain tissue are seen. In severe brain damage or death, there is usually asymmetric or no blood flow noted on the angiographic phase and an abnormal blood pool phase.
The brain scan can also be used to indicate cerebral vascular occlusion or stenosis. With the use of Diamox (acetazolamide), an accurate assessment of local cerebral blood flow can be determined. Diamox is carbonic anhydrase inhibitor that results in the elevation of PCO2 in the bloodstream. Normally this causes dilatation of the cerebral blood vessels. If asymmetric blood flow is noted after Diamox injection, cerebral vascular occlusion or stenosis can be suspected.
The brain scan can also document successful therapeutic disruption of the normal blood-brain barrier to inject chemotherapeutic agents into localized brain tumors. Furthermore this scan has been used in the evaluation of patients with seizure disorders, psychiatric disease, and dementia. Although not nearly as accurate as an MRI or PET scan, the nuclear brain scan is able to identify primary brain neoplasms (e.g., gliomas, astrocytomas primary lymphoma) and metastatic tumors. Because sometimes nuclear medicine can indicate tissue viability, nuclear brain scanning is used to differentiate radiation necrosis from recurrent brain viable tumor.
Brain scans are also used to investigate the ventricular system (cisternogram) of the central nervous system. Normal pressure hydrocephalus and ventricular shunt dysfunction can be identified and located. Cisternogram may be performed by injecting radioactive material into the subarachnoid space and then taking serial scans of the head. These scans are useful in evaluating ventricular size and patency of the cerebrospinal fluid (CSF) pathways and reabsorption. Because only a small amount of CSF enters the ventricles, their uptake of radioactive material normally should be minimal. Blocks in the CSF pathways may prevent this reabsorption, however; thus large amounts of isotopes may appear in the ventricles. A cisternogram may also be used to evaluate CSF leakage (e.g., into the nasal sinuses) in patients with recurrent meningitis and to evaluate hydrocephalus.
The technique of single-photon emission computed tomography (SPECT) has significantly improved the quality of brain scanning. With SPECT scanning, the radionuclide is injected and the scintillation cameras are placed to receive images from multiple angles (around the circumference of the head). This technique greatly increases the usefulness of nuclear brain scanning. In general, CT scans, MRI scans, and carotid duplex scans have replaced the brain scan in diagnostic neurology. However, a host of traumatic, inherited, and acquired diseases can be identified with nuclear brain scanning.
A SPECT brain scan using isoflurane I123 (also known as phenyltropane) can be helpful in the diagnosis of Parkinson disease. This test is often referred to as a DaT scan. Patients with Parkinson's disease experience degeneration of presynaptic dopamine neurotransmitter cells first in the basal ganglia of the brain and then other parts of the brain. Isoflurane tags these neurons with I123. In a healthy brain, isoflurane I123 is seen concentrated in the basal ganglia. This is demonstrated as hot spots. In these parts of the brain in which dopamine cells should be remain dark on the brain SPECT scan, Parkinson disease is suspected. These changes may be subtle. There are commonly identified patterns that can separate out Parkinson disease from other forms of brain deterioration or aging.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
• Administer blocking agents as ordered before scanning. For example, potassium chloride prevents an inordinate amount of technetium uptake by the choroid plexus, which would simulate a pathologic cerebral condition. Similar solutions (e.g., potassium iodine, Lugol iodine solution) may be given orally to block thyroid uptake. Blocking agents are not necessary with the use of 99mTc diethylenetriamine pentaacetic acid.
• Check for allergy to iodine if an iodinated solution will be used.
• Consider having a sedative ordered for agitated patients.
![]() Tell the patient that no discomfort is associated with this study other than the peripheral IV puncture required for injection of the radioisotope.
Tell the patient that no discomfort is associated with this study other than the peripheral IV puncture required for injection of the radioisotope.
During
• Note the following procedural steps:
1. After administration of the radioisotope, the patient is placed in the supine, lateral, and prone positions while a counter is placed over the head (Figure 8-4).
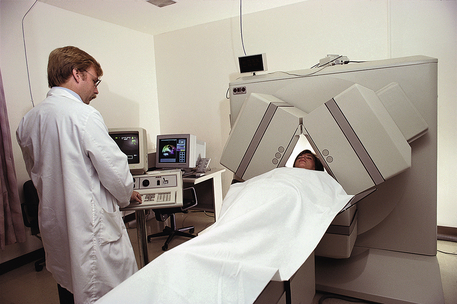
Figure 8-4 Patient positioned for a radionuclide scan of the brain. In this diagnostic study, a small amount of radioactive material crosses the blood-brain barrier to produce an image. This study is known as single-photon emission computed tomography (SPECT).
2. The radioisotope counts are anatomically displayed and photographed while the patient remains very still.
3. When cerebral flow studies are performed, the counter is immediately placed over the head.
4. The counts are anatomically recorded in timed sequence to follow the isotope during its first flow through the brain.
5. Another scan is obtained 30 minutes to 2 hours later for identification of pathologic tissues.
• Note that this study is performed by a technologist in the nuclear medicine department in approximately 35 to 45 minutes.
After
![]() Assure the patient that the radioactive material is usually excreted from the body within 6 to 24 hours.
Assure the patient that the radioactive material is usually excreted from the body within 6 to 24 hours.
• Because only tracer doses of radioisotopes are used, remember that no precautions need to be taken to prevent radioactive exposure to other personnel or family present.
![]() Encourage the patient to drink fluids to aid in the excretion of the isotope from the body.
Encourage the patient to drink fluids to aid in the excretion of the isotope from the body.
Test Results and Clinical Significance
Cerebral death: This is noted by asymmetric or absence of cerebral blood flow when associated with other clinical indications of death.
Cerebral vascular stenosis/occlusion: Depending on the timing after the incident, affected areas will demonstrate perfusion changes.
Seizure disorder: The use of this test for this indication is limited because of the need to withdraw from anti-seizure medication.
Dementia: Alzheimer disease can be differentiated from other neurodegenerative diseases using nuclear scanning especially when combined with PET scanning.
Cerebral neoplasm: When using radionuclide such as FDG, tumors are obvious by enhancement of radionuclide within the tumor. Primary lymphomas have unique nuclear features that suggest their diagnosis. Metastatic lesions are often multiple and associated with focal increased nuclear activity.
Brain infection and abscess: Hypometabolic areas may represent abscess. Increased activity may be noted with acute infection.
CSF leakage: The most common site of leakage of CSF is into the nasal cavity. This can be the result of tumor or infection.
Hydrocephalus: This is evident on cisternogram. Ventricular shunt dysfunction becomes obvious by lack of nuclear flow.
Related Tests
Computed Tomography (CT) Scan of the Brain (p. 1026). This test is very accurate in identifying pathologic conditions of the brain. X-rays are directed to the brain from multiple circumferential angles and then gathered to produce multiple images of the brain.
Magnetic Resonance Imaging (MRI) Scan of the Brain (p. 1106). This test does not use x-rays but rather detects electromagnetic differences among the brain tissues. It visualizes the brain from similar angles as the CT scan, producing an accurate image.
PET scan (p. 821). This nuclear spatial scan is particularly useful to identify neoplasm involved in the central nervous system.
Breast Scintigraphy (Breast Scan, Breast-Specific Gamma Imaging [BSGI], Scintimammography, Miraluma Scan, Breast Scintigraphy with Breast-Specific γ-Camera (BSGC)
Normal Findings
Negative: Minimal, symmetric, bilateral, and uniform breast uptake equal to soft-tissue uptake
Indications
This test is used to identify breast cancer, especially in young women with dense breasts in whom the accuracy of mammography is diminished.
Test Explanation
Nuclear scans of the breast, using technetium (99mTc)-labeled sestamibi or tetrofosmin as a radiotracer, are used to identify breast cancer in patients whose dense breast tissue precludes accurate evaluation by conventional mammography. To conduct BSGI, patients are given an intravenous injection with a small dose of a tracing agent (Technetium 99mTc) that emits gamma rays. The radioisotope is transported by passive diffusion into the cell and is sequestered within the mitochondria. Thus cancer cells that usually contain a large number of mitochondria will show an increased uptake of 99mTc as compared with noncancerous cells. BSGI is a functional scan that indicates physiologic behavior of cells. Cancerous areas show up as “hot spots” on breast specific specialized high-resolution, small field-of-view gamma cameras. The cameras are compact and maneuverable and they can be placed close to the chest to image deep within the breast.
This test has also been used as an adjunct in patients with an indeterminate mammogram abnormality and in women with indeterminate palpable breast masses. However this scan may miss as many as 10% to 15% of cancers, and the false-positive rate is about 15% to 25%. Areas of benign cellular hyperplasia also trap the radiotracer. Because cellular hyperplasia is a common finding in the breast just before menses, imaging at this time in the menstrual cycle should be avoided.
Breast nuclear scans will not replace the role of mammography in breast imaging. Nor will they ever be an effective screening tool for the early detection of breast cancer among large populations. Other technologies currently used for similar post-mammography evaluation include ultrasound (p. 871) and magnetic resonance imaging (MRI) (p. 1106). Each of these technologies has its advantages and limitations. Ultrasound is well tolerated, it does not use ionizing radiation or require intravenous contrast administration, and it is able to identify small lesions in dense breast tissue. MRI of the breast offers accuracy similar to ultrasound and BSGI. However, MRI is not suitable for many patients, such as women with pacemakers, who are claustrophobic, and who cannot lie prone for the required length of the exam. BSGI is not without limitations; it is limited by its inability to reliably image cancers smaller than 1 cm.
Procedure and Patient Care
Before
During
Note the following procedural steps:
1. The patient may be positioned in the supine, prone, or sitting position.
2. Twenty millicuries of 99mTc sestamibi is injected intravenously into the arm contralateral to the suspicious breast.
3. Imaging begins a few minutes after injection. A scintillator camera is placed over the breast and records the radiation emitted.
4. This information is translated into a two-dimensional view of the breast, which is then visualized on film.
5. These images are compared with surrounding soft-tissue readings.
After
![]() Tell the patient that because only tracer doses of radioisotope are used, no precautions need to be taken to prevent radioactive exposure to other personnel or family present.
Tell the patient that because only tracer doses of radioisotope are used, no precautions need to be taken to prevent radioactive exposure to other personnel or family present.
![]() Assure the patient that the radioactive substance is usually excreted from the body within 6 to 24 hours.
Assure the patient that the radioactive substance is usually excreted from the body within 6 to 24 hours.
![]() Encourage the patient to drink fluids to aid in the excretion of the radioactive substance.
Encourage the patient to drink fluids to aid in the excretion of the radioactive substance.
Related Tests
Mammography (p. 1043). This is an x-ray image of the breasts. It is generally the most accurate screening and diagnostic test to identify breast cancer.
Breast Ultrasonography (p. 871). This ultrasound technique is also used to identify and characterize breast pathologic conditions. This test is especially useful to identify breast cysts.
MRI of the Breast (p. 1106). This magnetic resonance imaging of the breast using gadolinium is both an anatomic and functional scan of the breast tissue.
Cardiac Nuclear Scan (Myocardial Perfusion Scan, Myocardial Perfusion Imaging, Myocardial Scan, Cardiac Scan, Heart Scan, Thallium Scan, MUGA Scan, Isonitrile Scan, Sestamibi Cardiac Scan, Cardiac Flow Studies, and Nuclear Stress Test)
Normal Findings
Heterogeneous uptake radionuclide throughout the myocardium of the left ventricle
Left ventricular end diastolic volume ≤70 ML
Left ventricular end systolic volume ≤25 ML
Left ventricular ejection fraction >50%
Test Explanation
See Table 8-1 for an overview of cardiac nuclear scanning. A cardiac perfusion scan measures the coronary blood flow at rest and during exercise. It is often used to evaluate the cause of chest pain. It may be done after a coronary ischemic event to evaluate coronary patency or heart muscle function.
In this test, radionuclide is injected intravenously into the patient. Myocardial perfusion images are then obtained while the patient is lying down under a single-photon emission computed tomography (SPECT) camera that generates a picture of the radioactivity coming from the heart. This scan can be performed at rest or with exercise such as treadmill or bicycling (myocardial nuclear stress testing). Medications may be administered that duplicate exercise stress testing. Vasodilators (dipyridamole, adenosine and Regadenoson) or chronotropic agents (dobutamine) are commonly used. Regadenoson is the most recent A2A adenosine receptor agonist that instigates coronary vasodilatation. It is associated with fewer side effects (e.g., heart block, bronchospasm) and can be injected more quickly.
Although the initial radioisotope used was thallium (thus the name thallium scan), technetium agents such as tetrofosmin and sestamibi (isonitrile) are more commonly used today. The uptake of these agents is proportional to the myocardial coronary flow (Figure 8-5). At rest, a coronary stenosis must exceed 90% of the normal diameter before blood flow is impaired enough to see it on the perfusion scan. With exercise stress testing, however, stenosis of 50% becomes obvious. Often stenosis or coronary obstruction is noted by a normal resting perfusion scan followed by stress perfusion scan that demonstrates cold spots compatible with decreased coronary perfusion. Myocardial perfusion scans can be synchronized by gating the images with the cardiac cycle and thereby allowing the visualization and evaluation of cardiac muscle function. The contractility of the muscle wall can be evaluated at the same time. Prior muscle injury is demonstrated by reduced muscle wall motion. Most often, nuclear myocardial scans include both perfusion and gated wall motion images. Cardiac ejection fraction, the end-systolic volume of the left ventricle can be calculated.
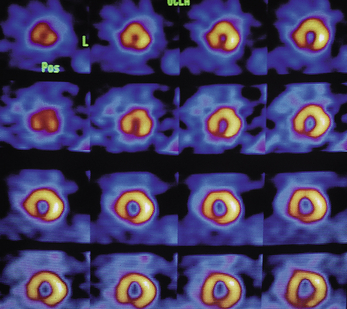
Figure 8-5 Thallium-201 scintigraphy produces a series of images of blood flow and tissue perfusion.
Cardiac nuclear stress testing is more accurate than echocardiography stress testing (p. 877) or radiographic stress ventriculography (p. 1008). The nuclear myocardial scan is the best initial imaging study for the detection of myocardial ischemia; however, stress echocardiography is performed more often because it is more readily available and many cardiologists are better trained in echocardiography and are more comfortable with echocardiography. The assessment of myocardial perfusion and function using PET and hybrid positron emission tomography (PET)/CT imaging (p. 822) is becoming more available as the cost of the technology decreases and as positron-emitting radiopharmaceuticals become more available. Myocardial PET scanning provides better cardiac and coronary imaging.
Cardiac nuclear imaging when gated to the cardiac cycle (Multi Gated Acquisition Scan [MUGA], gated blood pool scan) can provide an accurate measure of ventricular function through the calculation of the ventricular ejection fraction (Figure 8-6). In this scan the patient's red blood cells are tagged with technetium. Red blood cell binding with technetium can be performed in vivo or in vitro. In vivo techniques are more convenient and less time-consuming but in vitro labeling is more efficient, especially in patients who have large indwelling venous access.
Ventricular volumes can be calculated and used to accurately calculate the amount of blood that is ejected from the ventricle with each contraction (ejection fraction). This is used in the initial assessment of cardiac function and subsequently to monitor therapy designed to improve cardiac function. Patients with cardiomyopathies (ischemic, infiltrative, inflammatory), cardiac transplant, or drug-induced cardiac muscle toxicity (from doxorubicin or Herceptin) require frequent evaluation of ventricular ejection fraction.
This test is usually performed in a few hours in the nuclear medicine department by a technologist and interpreted by a nuclear medicine physician. Delayed images may be required 24 hours later.
Interfering Factors
• Cardiac flow studies can be altered by excessive alterations in chest pressure (as exists with excessive crying in children).
• Recent nuclear scans (e.g., thyroid or bone scan)
![]() Drugs, such as long-acting nitrates, may only temporarily improve coronary perfusion and cardiac function.
Drugs, such as long-acting nitrates, may only temporarily improve coronary perfusion and cardiac function.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Instruct the patient that a short fasting period may be required, especially when using sestamibi or tetrofosmin.
Instruct the patient that a short fasting period may be required, especially when using sestamibi or tetrofosmin.
![]() Tell the patient that the only discomfort associated with this test is the venipuncture required for injection of the radioisotope.
Tell the patient that the only discomfort associated with this test is the venipuncture required for injection of the radioisotope.
• Be sure all jewelry is removed from the chest wall.
• Obtain a consent form if stress testing is to be performed.
During
• Take the patient to the nuclear medicine department. Depending on the type of nuclear myocardial scan, each scanning protocol is different.
• Note the following general procedural steps:
1. One or more intravenous (IV) injection of radionuclide material is performed.
3. Depending on the radionuclide used, scanning is performed 15 minutes to 4 hours later.
4. A SPECT camera is placed at the level of the precordium.
5. If a single gamma camera is used, the patient is placed in a supine position (Figure 8-7), and then may be repositioned to the lateral position and/or in the right and left oblique positions. In some departments, the detector can be rotated around the patient, who remains in the supine position.
6. The gamma ray scanner records the image of the heart, and an image is immediately developed.
7. For an exercise stress test, additional radionuclide is injected during exercise when the patient reaches a maximum heart rate. The patient then lies on a table, and scanning is done. A repeat scan may be done 3 to 4 hours later.
8. If an isonitrile stress test is needed, the radionuclide material is injected and a scan performed 30 to 60 minutes later for the resting phase. Four hours later, cardiac stress testing is done. After a second injection, scanning is repeated.
• Note that myocardial scans are usually performed in less than 30 minutes by a nuclear medicine technician.
• If nuclear cardiac stress testing is performed, follow routine protocol described on p. 540.
After
![]() Inform the patient that because only tracer doses of radioisotopes are used, no precautions need to be taken against radioactive exposure to personnel or family.
Inform the patient that because only tracer doses of radioisotopes are used, no precautions need to be taken against radioactive exposure to personnel or family.
![]() Instruct the patient to drink fluids to aid in the excretion of the radioactive substance.
Instruct the patient to drink fluids to aid in the excretion of the radioactive substance.
• Apply pressure or a pressure dressing to the venipuncture site.
• Assess the venipuncture site for bleeding.
• If stress testing was performed, evaluate the patient's vital signs at frequent intervals (as indicated).
Test Results and Clinical Significance
Coronary artery occlusive disease: This diagnosis can be made when comparing a resting scan or during a cardiac stress nuclear scan. The manner with which this abnormality becomes evident depends on the radionuclide used.
Decreased myocardial function associated with ischemia, myocarditis, cardiomyopathy, or congestive heart failure: These diseases, affecting the myocardium, are evident as hypokinesia of the cardiac wall. Infarcted areas have little or no wall motion. Paradoxical motion may be noted.
Decreased cardiac output: Many coronary, myocardial, and valvular diseases are associated with reduced cardiac output. A reduced ejection fraction is an indirect measurement of cardiac output. Often a reduced ejection fraction is the first sign of those diseases.
Related Tests
Cardiac Stress Testing (p. 540). In this test, stress is provided to maximize the cardiac function. The heart is then often imaged with nuclear scanning to see the effect of the stress.
Cardiac Catheterization (p. 1008). This test provides similar images through the use of radio-opaque dyes injected through catheters placed in and around the heart.
Echocardiography (p. 877). This is an ultrasound directed image of the cardiac muscle and chambers.
Gallbladder Nuclear Scanning (Hepatobiliary Scintigraphy, Hepatobiliary Imaging, Biliary Tract Radionuclide Scan, Cholescintigraphy, DISIDA Scanning, HIDA Scanning, IDA Gallbladder Scanning)
Normal Findings
Gallbladder, common bile duct, and duodenum visualize within 60 minutes after radionuclide injection. (This confirms patency of the cystic and common bile ducts.)
Indications
Cholescintigraphy is valuable in evaluating patients for suspected gallbladder disease. The primary use of this study is to diagnose acute cholecystitis in patients who have acute right upper quadrant abdominal pain. When gallbladder ejection fraction is calculated, chronic cholecystitis can be diagnosed. This study is also used to assist in the diagnosis of extrahepatic biliary obstruction.
Test Explanation
Through the use of iminodiacetic acid analogues (IDAs) labeled with technetium-99m (99mTc), the biliary tract can be evaluated in a safe, accurate, and noninvasive manner. These radionuclide compounds are extracted by the liver and excreted into the bile. Gamma rays are emitted from the 99mTc in the bile through the body and are detected by a scintillation camera. The scintillation camera emits light with each photon it receives from the gamma ray. When these light patterns are arranged in a spatial order, a realistic image of the biliary tree is apparent.
Failure to visualize the gallbladder 60 to 120 minutes after injection of the radionuclide is virtually diagnostic of an obstruction of the cystic duct, which instigates the pathophysiology of acute cholecystitis. Delayed filling of the gallbladder is associated with chronic or acalculous cholecystitis. This procedure is also helpful in diagnosing biliary duct obstructions. The identification of the radionuclide in the biliary tree but not in the bowel is diagnostic of common bile duct obstruction.
This procedure is superior to oral cholecystography, intravenous (IV) cholangiography, ultrasonography, and computed tomography (CT) of the gallbladder in the detection of cholecystitis (Table 8-2). Also, with cholescintigraphy, gallbladder function can be numerically determined by calculating the capability of the gallbladder to eject its contents. It is believed that an ejection fraction below 35% indicates primary gallbladder disease. To a large degree, abdominal ultrasound (p. 866) has replaced this test in the diagnosis of acute cholecystitis.
Occasionally, morphine sulfate is given intravenously during nuclear scanning. The morphine causes increased ampullary contraction. Not only can this reproduce the patient's symptoms of biliary colic, but it also serves to force the bile containing the radionuclide into the gallbladder. If no radionuclide is seen in the gallbladder with the use of morphine within 15 to 60 minutes, the diagnosis of acute cholecystitis is nearly certain. This greatly decreases the scanning time because without morphine it requires 4 hours to obtain a definitive diagnosis of acute cholecystitis.
A nuclear medicine technologist performs this study in 1 to 4 hours in the nuclear medicine department. A physician trained in interpretation of diagnostic nuclear medicine interprets the test in a few minutes. The only discomfort associated with this procedure is the IV injection of radionuclide.
Procedure and Patient Care
Before
![]() Assure the patient that he or she will not be exposed to large amounts of radioactivity.
Assure the patient that he or she will not be exposed to large amounts of radioactivity.
![]() Instruct the patient to fast for 2 to 4 hours before the test.
Instruct the patient to fast for 2 to 4 hours before the test.
![]() Avoid morphine or Demerol administration for 4 to 12 hours before the scan.
Avoid morphine or Demerol administration for 4 to 12 hours before the scan.
During
• Note the following procedural steps:
1. After IV administration of a 99mTc-labeled IDA (e.g., DISIDA, PIPIDA, HIDA), the right upper quadrant of the abdomen is scanned.
2. Serial images are obtained over 1 hour.
3. Subsequent images can be obtained at 15- to 30-minute intervals.
4. If the gallbladder, common bile duct, or duodenum is not visualized within 60 minutes after injection, delayed images are obtained up to 4 hours later.
5. Images are recorded on film.
6. When an ejection fraction is to be determined, the patient is given a fatty meal or cholecystokinin is administered to evaluate emptying of the gallbladder. The gallbladder is continually scanned to measure the percentage of isotope ejected.
Test Results and Clinical Significance
Acute cholecystitis: No visualization of the gallbladder will be seen because a gallstone is stuck in the cystic duct, causing acute cholecystitis. The rest of the biliary tree is visualized.
Delayed visualization of the gallbladder is seen after several hours. The gallbladder ejection fraction is below 35%. The pathophysiology of cystic duct syndrome is not well known.
Common bile duct obstruction secondary to gallstones, tumor, or stricture: This is evident when the radionuclide is seen in a large bile duct but not in the bowel. Obstruction of the bile duct must be present.
Gallium Scan
Indications
Gallium becomes concentrated in areas of the body where white blood cells (WBCs) tend to congregate (areas of tumor, infection, and inflammation). It is used to stage gallium-avid tumors (those that attract high concentrations of gallium; e.g., lymphomas, lung cancer). It is used to locate infection or inflammation in patients with fever of unknown origin. Finally, it is used to monitor response to treatment of infection, inflammation, or tumor.
Test Explanation
A gallium scan of the total body is usually performed 24, 48, and 72 hours after an intravenous (IV) injection of radioactive gallium. Most commonly, however, a single scan is performed 2 to 4 days after injection of the gallium. Gallium is a radionuclide that is concentrated in areas of inflammation and infection, by abscesses, and by benign and malignant tumors. Not all types of tumors, however, will concentrate gallium. Lymphomas are particularly gallium avid. Other tumors that can be detected by a gallium scan include sarcomas, hepatomas, and carcinomas of the gastrointestinal (GI) tract, kidney, uterus, stomach, and testicle.
This test is useful in detecting metastatic tumor, especially lymphoma, even when other diagnostic imaging tests are normal. To a large degree, PET scans (p. 821) have replaced the use of gallium scans for the identification of malignancy. The gallium scan also is useful in demonstrating a source of infection in patients with a fever of unknown origin. Gallium can be used to identify noninfectious inflammation within the body in patients who have an elevated sedimentation rate. Unfortunately, this test is not specific enough to differentiate among tumor, infection, inflammation, and abscess. Although a gallium scan is better able to detect sites of chronic inflammation, PET scans are more commonly used to identify areas of acute infection.
Some organs (liver, spleen, bone, colon) normally retain gallium. Therefore a normal total-body gallium scan study would demonstrate some uptake in these organs, but this uptake is much less concentrated than in pathologic areas (e.g., tumor, inflammation).
Another method of scanning is called SPECT (single-photon emission computed tomography). With SPECT scanning, the patient lies supine on the table surrounded by a donut-like gantry. The photon detection camera rotates around the patient to obtain photon counts from 360 degrees. This provides a more detailed image.
A nuclear medicine technologist performs each scan in approximately 30 to 60 minutes. Repeated scanning is required. Repeated injections are not necessary. The test results are interpreted by a physician trained in nuclear medicine and are usually available 72 hours after the injection. No pain or discomfort is associated with this procedure other than the IV injection. However, it occasionally can be uncomfortable to lie still on a hard table for the duration required.
Procedure and Patient Care
During
• Note the following procedural steps:
1. The unsedated patient is injected with gallium.
2. A total-body scan may be performed 4 to 6 hours later by slowly passing a scintillation camera over the body.
3. The images provided by the scintillation camera are recorded on film.
4. Additional scans are usually taken 24, 48, and 72 hours later.
5. During the scanning process the patient is positioned in the supine position.
Gastric Emptying Scan
Normal Findings
Normal values are determined by type and quantity of radiolabeled ingested food.
| Time | Lower Normal Limits | Upper Normal Limits |
| 0 minutes | ||
| 30 minutes | 70% | |
| 1 hour | 30% | 90% |
| 2 hours | 60% | |
| 3 hours | 30% | |
| 4 hours | 10% |
Values lower than normal represent abnormally fast gastric emptying. Values higher than upper limits represent delayed gastric emptying.
Indications
This scan is used to determine the rate of gastric emptying. It is used to diagnose gastroparesis or gastric obstruction in patients who have postcibal nausea, vomiting, bloating, early satiety, belching, or abdominal pain.
Test Explanation
In this study the patient ingests a solid or liquid “test meal” containing a radionuclide such as technetium (Tc). The stomach is then scanned until gastric emptying is complete (Figure 8-8). This study is used to assess the stomach's ability to empty solids or liquids and to evaluate disorders that may cause a delay in gastric emptying, such as obstruction (caused by peptic ulcers or gastric malignancies) and gastroparesis. This scan is also useful in determining the patency of a gastrointestinal (GI) surgical anastomosis.
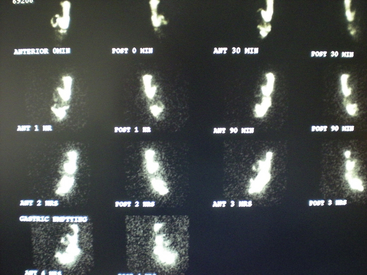
Figure 8-8 Emptying scan. Nuclear content are noted initially in the stomach and duodenum. As time progresses (from 0 minutes on top to 4 hours on bottom), only a portion of the contents within the stomach empty into the small intestine. At 4 hours, 22% of the radionuclide remains in the stomach. Delayed gastric emptying is apparent.
This procedure lasts approximately 4 hours, depending on the gastric emptying time. The test is interpreted by a nuclear medicine physician. Results are available the same day. There is no discomfort associated with the test.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Assure the patient that no pain is associated with this study.
Assure the patient that no pain is associated with this study.
![]() Inform the patient that only a small dose of nuclear material is ingested. Reassure the patient that this is a safe dose.
Inform the patient that only a small dose of nuclear material is ingested. Reassure the patient that this is a safe dose.
![]() Instruct the patient to keep on nothing by mouth (NPO) status after midnight on the day of the test.
Instruct the patient to keep on nothing by mouth (NPO) status after midnight on the day of the test.
![]() Tell the diabetic patient not to take insulin or oral medications before testing because they will be fasting until the next meal.
Tell the diabetic patient not to take insulin or oral medications before testing because they will be fasting until the next meal.
![]() Tell the patient that smoking is prohibited on the day of examination because exposure to tobacco can inhibit gastric emptying.
Tell the patient that smoking is prohibited on the day of examination because exposure to tobacco can inhibit gastric emptying.
During
• Note the following procedural steps:
1. In the nuclear medicine department the patient is asked to ingest a test meal. In the solid-emptying study the patient eats scrambled egg whites containing Tc.
In the liquid-emptying study the patient drinks orange juice or water containing technetium-99m diethylenetriamine pentaacetic acid (DTPA) or indium-111 DTPA.
2. After ingestion of the test meal the patient lies supine under a gamma camera that records gastric images. Images are obtained for 2 minutes every 30 to 60 minutes until gastric emptying is complete. This may take several hours, although each particular timed scan takes only a few minutes.
• With the use of computer calculations of timed images, the rate of gastric emptying can be determined.
Test Results and Clinical Significance
Gastric obstruction caused by gastric ulcer or cancer: Tumors located at the gastric outlet can obstruct or delay gastric emptying. Ulcers, particularly in the duodenum, can cause edema and scarring, which also can cause delay in gastric emptying. The scan, although not specific regarding the cause of the obstruction, will demonstrate prolonged gastric emptying.
Nonfunctioning GI anastomosis: Postoperative edema is suspected to be the cause of delayed gastric emptying after gastric surgery. Gastroparesis also may play a role.
Gastroparesis: The muscle function required for gastric emptying can be affected by nerve damage caused by diabetes or other neuropathies. There may be endocrine factors (gastrin related) affecting gastric emptying. This process is not uncommon after prolonged periods of gastric obstruction. Here, again, the gastric emptying scan will be prolonged.
Gastroesophageal Reflux Scan (GE Reflux Scan, Aspiration Scan)
Indications
This scan is performed on patients who complain of heartburn, reflux of food, water brash (sour taste in the mouth), aspiration, or paroxysmal nocturnal dyspnea (from nocturnal aspiration). It can detect gastroesophageal reflux and/or aspiration.
Test Explanation
GE reflux scans are used to evaluate patients with symptoms of heartburn, regurgitation, vomiting, and dysphagia. Also, these scans are used to evaluate the medical or surgical treatment of patients with GE reflux. Finally, aspiration scans may be used to detect aspiration of gastric contents into the lungs and to evaluate swallowing function.
This procedure is performed in the nuclear medicine department in approximately 30 minutes. There is no discomfort associated with this test.
Procedure and Patient Care
During
GE Reflux Scan
1. The patient is placed in the supine position and asked to swallow 100 to 150 mL of a tracer cocktail (e.g., orange juice, diluted hydrochloric acid, and technetium-99m–labeled colloid).
2. Images are immediately taken of the patient's esophageal area.
3. The patient is asked to assume other positions to determine whether GE reflux occurs and, if so, in what position.
4. A large abdominal binder that contains an air-inflatable cuff is placed on the patient's abdomen. This is insufflated to increase abdominal pressure.
5. Images are again taken over the esophageal area to determine if any GE reflux occurs.
Aspiration Scan
1. This scan may be performed by adding a radionuclide to the patient's evening meal and keeping the patient in the supine position until the next morning.
2. Images are made over the lung fields to detect esophagotracheal aspiration of the tracer.
3. In infants being evaluated for chalasia, the tracer is added to the feeding or formula. Nuclear tracer films are then taken over the next hour, with delayed films as needed.
After
• With the use of computer calculations based on the images of the scans, the severity and percentage of reflux can be calculated.
![]() Assure the patient that he or she has ingested only a small dose of nuclear material. No radiation precautions need to be taken against the patient or his or her body secretions.
Assure the patient that he or she has ingested only a small dose of nuclear material. No radiation precautions need to be taken against the patient or his or her body secretions.
Test Results and Clinical Significance
Gastroesophageal reflux: The radionuclide can be seen to reflux from the stomach into the esophagus. This should diminish or disappear with successful medical or surgical treatment.
Pulmonary aspiration: This can be the result of severe gastroesophageal reflux or the result of faulty swallowing function.
Related Test
Gastric Emptying Scan (p. 801). This is a similarly performed scan designed to identify delayed gastric emptying, which can contribute to gastroesophageal reflux.
Gastrointestinal Bleeding Scan (Abdominal Scintigraphy, GI Scintigraphy)
Test Explanation
The GI bleeding scan is a test used to localize the site of bleeding in patients who are having active GI hemorrhage. The scan also can be used in patients who have suspected intraabdominal (nongastrointestinal) hemorrhage from an unknown source. Localization of the source of GI or other bleeding can be quite difficult. When surgery is required under these circumstances, it is difficult, cumbersome, and prolonged. The surgeon may have extreme difficulty finding the source of bleeding. The bleeding scan helps localize the bleeding for the surgeon.
Box 8-1 provides an overview of the diagnostic procedures used in evaluating GI bleeding. Many of these studies have limitations that warrant the use of the GI bleeding scan. For example, endoscopy has proved to be extremely useful in determining the source of intestinal bleeding; however, endoscopy is not helpful if the source is within the small intestine or the colon. Although colonoscopy allows excellent visualization of the colon when it is cleared out, it is extremely difficult to see when acute, active intestinal bleeding is occurring. Arteriography has three limitations in its evaluation of GI bleeding. First, arteriography can determine the site of bleeding, but the rate of bleeding must exceed 0.5 mL/min for detection. Second, if GI bleeding is intermittent, the results of the arteriogram can be falsely negative. Third, arteriography visualizes only the blood vessels to the small bowel, right colon, and transverse colon through a superior mesenteric angiogram. If the left colon and sigmoid vessels are to be visualized (most bleeding comes from these areas), an inferior mesenteric angiogram must be requested. This is more difficult to perform.
The GI bleeding scan has several advantages over arteriography. The GI bleeding scan can detect bleeding if the rate is in excess of 0.05 mL/min. Also, with the use of technetium-labeled RBCs, delayed films (as long as 24 hours) can be obtained to indicate the site of an intermittent or extremely slow intestinal bleed.
A GI scintigram is much more sensitive in locating the site of GI bleeding; however, it is not very specific in pinpointing the site or the cause of bleeding. Usually, when the results of a GI scintigram are positive, the exact source of bleeding cannot be localized any more accurately than indicating the affected quadrant of the abdomen (e.g., right upper, left lower). This test is usually performed by injecting sulfur colloid labeled with technetium-99m (99mTc) or 99mTc-labeled red blood cells (RBCs) into the patient. If the patient is bleeding at a rate in excess of 0.05 mL/min, pooling of the radionuclide will ultimately be detected in the abnormal segment of the intestine. Few false-positive results occur. Again, it is important to recognize that the test will only localize the bleeding; it will not indicate the exact pathologic condition causing the bleeding. With this test result, if surgery is required, the surgeon is directed to the abnormal area and hopefully can detect and resect the pathologic bleeding source.
It is important to realize that this test can take at least 1 to 4 hours to obtain useful information. Unstable patients should not leave the intensive care environment for that long. Furthermore, the unstable patient may need to go to surgery in minutes and the surgeon may not have the luxury of taking several hours to determine the region of active bleeding.
Contraindications
• Patients who are pregnant or lactating unless the benefits outweigh the risk or damage to the fetus or newborn
• Medically unstable patients whose stay in the nuclear medicine department may be risky
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
• Assess the patient's vital signs to ensure that they are stable for the patient's transfer to and from the nuclear medicine department.
• Accompany the patient to the nuclear medicine department if vital signs are questionably stable.
![]() Assure the patient that only a small amount of nuclear material will be administered.
Assure the patient that only a small amount of nuclear material will be administered.
![]() Instruct the patient to notify the nuclear medicine technologist if he or she has a bowel movement during the test. Blood in the GI tract can act as a cathartic.
Instruct the patient to notify the nuclear medicine technologist if he or she has a bowel movement during the test. Blood in the GI tract can act as a cathartic.
![]() Inform the patient that no pretest preparation is required.
Inform the patient that no pretest preparation is required.
![]() Instruct the nuclear medicine technologist to notify the nurse of all bloody bowel movements that occur while the patient is in the nuclear medicine department.
Instruct the nuclear medicine technologist to notify the nurse of all bloody bowel movements that occur while the patient is in the nuclear medicine department.
![]() Tell the patient that the only discomfort associated with this study is the injection of the radioisotope.
Tell the patient that the only discomfort associated with this study is the injection of the radioisotope.
During
• Note the following procedural steps:
1. Ten millicuries of freshly prepared 99mTc-labeled sulfur colloid is administered to the patient intravenously. If 99mTc-labeled RBCs are to be used, 3 to 5 mL of the patient's own blood is combined with the 99mTc and reinjected into the patient.
2. Immediately after administration of the radionuclide, the patient is placed under a scintillation camera.
3. Multiple images of the abdomen are obtained at short intervals (5 to 15 minutes). Delayed films may be performed as late as 6 to 24 hours later to detect slow, intermittent, or chronic bleeding. The scintigrams are recorded on film.
4. Detection of radionuclide in the abdomen indicates the site of bleeding. If no bleeding sites are noted in the first hour, the scan may be repeated at hourly intervals for as long as 24 hours.
• Note that areas of the bowel hidden by the liver or spleen may not be adequately evaluated by this procedure. Also, the rectum cannot be easily evaluated because other pelvic structures (e.g., the bladder) obstruct the view. If the initial study is negative and subsequent films give evidence of active bleeding, a repeat scan may be performed.
• Note that this test is usually performed in approximately 20 to 30 minutes if Tc-sulfur colloid is used by a technologist in the nuclear medicine department. The scan may take longer if Tc-labeled red blood cells are used.
Test Results and Clinical Significance
Related Tests
Arteriography (p. 988). This is a radiographic study used to evaluate the patient with GI bleeding at a rate greater than 1 mL/min.
Esophagogastroduodenoscopy (p. 608) and Colonoscopy (p. 591). These endoscopic tests can be very helpful in identifying the source of GI blood. In some cases endoscopic therapies can be used to stop the bleeding.
Liver/Spleen Scanning (Liver Scanning)
Indications
This test allows for visualization of the liver and spleen. It is indicated in patients with cancer to rule out metastatic tumor to the liver. It is a routine part of tumor staging. It is also indicated in patients with primary tumors (hepatomas) or in patients with cirrhosis who are at high risk for the development of primary hepatomas. Patients with abnormal liver enzymes will also have their liver visualized. Liver scanning is used to monitor liver diseases and response to therapy.
Test Explanation
This radionuclide procedure is used to outline and detect structural changes of the liver and spleen. A radionuclide, usually technetium-99m (99mTc)-labeled sulfur colloid, is administered intravenously. Later, a scintillation camera is placed over the right upper and left upper quadrants of the patient's abdomen. This records the distribution of the radioactive particles emitted from the liver and spleen. Images are obtained that are comparable to the gamma ray emission and are recorded digitally or on an analog film.
Because the scan can demonstrate only filling defects greater than 2 cm in diameter, false-negative results may occur in patients with space-occupying lesions (e.g., tumors, cysts, granulomas, abscesses) smaller than 2 cm. The scan may be incorrectly interpreted as positive for filling defects in patients with cirrhosis because of the distortion of the patient's liver parenchyma. The liver scan can detect tumors, cysts, granulomas, abscesses, and diffuse infiltrative processes affecting the liver (e.g., amyloidosis, sarcoidosis).
When a liver filling defect is observed, the most common cause is a benign hemangioma. This can be differentiated from tumor with the use of Tc-labeled red blood cells (RBCs). The patient's own RBCs are labeled with Tc and reinjected into the patient. Immediate uptake of the radionuclide by the filling defect is suggestive of a hemangioma, for which no therapy is usually required.
In general, computed tomography (CT) scans and magnetic resonance imaging (MRI) scans have replaced the liver scan in diagnostics. Single-photon emission computed tomography (SPECT) has significantly improved the quality and accuracy of liver scanning. With SPECT scanning the radionuclide is injected and the scintillation camera is placed to receive images from multiple angles (around the circumference of the liver). This greatly increases the usefulness of nuclear liver scanning. With the use of radioactive carbon, nitrogen, fluorine, or oxygen, anatomic and biochemical changes can be visualized within the liver. This method of liver scanning is called positron emission tomography (PET) scanning (p. 821).
The liver scan can also identify portal hypertension. Normally, most of the radionuclide administered during a liver scan is taken up by the liver. If the liver-to-spleen ratio is reversed (i.e., the spleen takes up more of the radionuclide), reversal of hepatic blood flow exists as a result of portal hypertension.
Splenic hematoma, abscess, cyst, tumor, infarction, and infiltrate processes such as granulomas can be detected. SPECT scanning can also be used to improve visualization of the spleen.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Tell the patient that no fasting or premedication is required.
Tell the patient that no fasting or premedication is required.
![]() Assure the patient that he or she will not be exposed to large amounts of radiation because only tracer doses of isotopes are used.
Assure the patient that he or she will not be exposed to large amounts of radiation because only tracer doses of isotopes are used.
![]() Tell the patient that the only discomfort associated with this procedure is the IV injection of the radionuclide.
Tell the patient that the only discomfort associated with this procedure is the IV injection of the radionuclide.
During
• Note the following procedural steps:
1. The patient is taken to the nuclear medicine department, where the radionuclide is administered intravenously. (For inpatients a nuclear medicine technologist may administer the radionuclide at the bedside.)
2. Thirty minutes after injection, a gamma ray detector is placed over the right upper quadrant of the patient's abdomen.
3. The patient is placed in supine and prone positions as the camera rotates around the patient (Figure 8-9) so that all surfaces of the liver can be visualized.
4. The radionuclide image is recorded digitally or on an analog film.
• Note that this procedure is performed by a trained technologist in approximately 1 hour. A physician trained in nuclear medicine interprets the results.
Test Results and Clinical Significance
Primary or metastatic tumor of the liver or spleen,
Abscess of the liver or spleen,
Hematoma of the liver or spleen,
Lacerations of the liver or spleen: The organ can be seen to be fractured, with a hematoma within the laceration.
Infiltrative processes (e.g., sarcoidosis, amyloidosis, tuberculosis, or granuloma of the liver or spleen),
These diseases are apparent as diffuse irregularity in the uptake of the radionuclide within the liver or spleen.
Portal hypertension: There is reversal of the normal liver/spleen ratio of uptake of the radionuclide. Usually the liver takes up most of the radionuclide. In portal hypertension, with reversal of hepatic portal blood flow, the spleen takes up more of the radionuclide.
Accessory spleen: The radionuclide aggregates in extrasplenic sites. This is very helpful to the surgeon who is planning a splenectomy and removal of all spleen tissue for patients with autoimmune thrombocytopenia or hemolytic anemia.
Splenic infarction: This is evident as a localized space-filling defect within the spleen in a patient with sudden onset of left upper quadrant pain.
Related Tests
Computed Tomography (CT) Scan of the Liver and Spleen (p. 1020). This is probably a more accurate test for the evaluation of these organs. However, to make the diagnosis of hemangioma, liver scanning with autologous RBCs labeled with Tc is superior in accuracy to a CT scan.
Magnetic Resonance Imaging (MRI) Scan of the Liver and Spleen (p. 1106). This is considered to be more accurate than the nuclear scans; however, it is more difficult to obtain and more expensive.
Lung Scan (Ventilation/Perfusion Scanning [VPS], V/Q Scan)
Indications
The lung scan is very helpful in making the diagnosis of pulmonary embolism (PE). It is easily and rapidly performed on patients who have sudden onset of noncardiac chest pain or shortness of breath. It is often performed on patients who have unexplained tachycardia or hypoxemia (Box 8-2).
Test Explanation
This nuclear medicine procedure is used to identify defects in blood perfusion of the lung in patients with suspected PE. Blood flow to the lungs is evaluated using a macroaggregated albumin (MAA) tagged with technetium (Tc), which is injected into the patient's peripheral vein. Because the diameter of the radionuclide aggregates is larger than that of the pulmonary capillaries, the aggregates become temporarily lodged in the pulmonary vasculature. A scintillation camera detects the gamma rays from within the lung microvasculature. With the use of light conversion a realistic image of the lung is obtained on film.
A homogeneous uptake of particles that fills the entire pulmonary vasculature conclusively rules out PE. If a defect in an otherwise smooth and diffusely homogeneous pattern is seen, a perfusion abnormality exists (Figure 8-10). This can indicate PE. Unfortunately, many other serious pulmonary parenchymal lesions (e.g., pneumonia, pleural fluid, emphysematous bullae) also cause a defect in pulmonary blood perfusion. Therefore, although the scan may be sensitive, it is not specific because many different pathologic conditions can cause the same abnormal results.
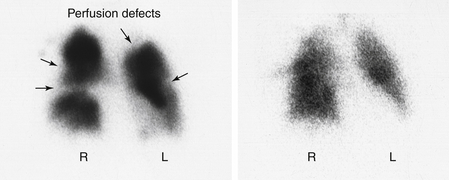
Figure 8-10 Lung scan. A, Perfusion. B, Ventilation. There are multiple perfusion defects noted on the perfusion lung scan. However, the uptake of radionuclide on the ventilation scan is normal. The combination of findings is because of pulmonary emboli.
The chest x-ray film aids in the interpretation of the perfusion scan because a defect on the perfusion scan seen in the same area as a pulmonary parenchymal abnormality on the chest x-ray film does not indicate PE. Rather, the defect may represent pneumonia, atelectasis, effusion, and so on. When a perfusion defect occurs in an area of the lung that is normal on a chest x-ray study, however, PE is very likely.
Specificity of a perfusion scan also can be enhanced by the concomitant performance of a ventilation lung scan, which detects parenchymal abnormalities in ventilation (e.g., pneumonia, pleural fluid, emphysematous bullae). The ventilation scan reflects the patency of the pulmonary airways using xenon gas or technetium (Tc) diethylenetriamine pentaacetic acid (DTPA) as an aerosol. When vascular obstruction (embolism) is present on a perfusion scan, ventilation scans will demonstrate a normal wash-in and a normal wash-out of radioactivity from the embolized lung area. If parenchymal disease (e.g., pneumonia) is responsible for the perfusion abnormality, however, wash-in or wash-out will be abnormal. Therefore the “mismatch” of perfusion and ventilation is characteristic of embolic disorders, whereas the “match” is indicative of parenchymal disease. When ventilation and perfusion scans are performed synchronously, this is called a ventilation/perfusion (V/Q) scan.
Most nuclear physicians place the lung scan results in one of several categories: negative for PE, low probability of PE, high probability of PE, or positive for PE.
With the increased availability of rapid access spatial CT scanning of the chest (see p. 1029), the diagnosis of PE is now more easily made with CT scanning of the chest using CT angiography. CT angiography is faster and more accurate than ventilation/perfusion lung scans and is less invasive than pulmonary angiography.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
• Obtain informed consent if required by the institution.
![]() Assure the patient that he or she will not be exposed to large amounts of radioactivity because only tracer doses of isotopes are used.
Assure the patient that he or she will not be exposed to large amounts of radioactivity because only tracer doses of isotopes are used.
![]() Tell the patient that no fasting is required.
Tell the patient that no fasting is required.
• Note that a recent (within the last 24 to 48 hours) chest x-ray film should be obtained.
![]() Instruct the patient to remove jewelry around the chest area.
Instruct the patient to remove jewelry around the chest area.
![]() Tell the patient that no discomfort is associated with this test other than the peripheral venipuncture.
Tell the patient that no discomfort is associated with this test other than the peripheral venipuncture.
During
• The unsedated, nonfasting patient with suspected PE is taken to the nuclear medicine department (Figure 8-11).
Ventilation Scan
1. The patient breathes through a closed-system face mask with a mouthpiece. The radionuclide tracer is then administered into the system.
2. Tc DTPA images are usually obtained before perfusion images and require patient cooperation with deep breathing and appropriate use of breathing equipment to prevent contamination.
Perfusion Scan
1. The patient is given a peripheral intravenous (IV) injection of radionuclide-tagged MAA.
2. While the patient lies in the appropriate position, a gamma ray detector is passed over the patient and records radionuclide uptake.
3. The patient is placed in the supine position with the camera rotating around the patient. This allows for anterior, posterior, and lateral and oblique views, respectively.
4. The results are interpreted by a physician trained in diagnostic nuclear medicine.
• Note that this test is usually performed by a technologist in approximately 30 minutes.
Test Results and Clinical Significance
Related Tests
Computed Tomography (CT) Scan of the Lung (p. 1029). This has now become the preferred test to diagnose PE.
Arterial Blood Gases (p. 109). Hypoxemia is the hallmark of PE.
Electrocardiography (p. 544). Although the EKG is usually normal with PE, right heart strain can be identified with large acute PE.
Chest X-Ray (p. 1014). Although PEs are not evident on the plain chest x-ray film, identification of parenchymal abnormalities is important to accurately interpret the perfusion lung scan.
D-dimer (p. 202). This test is used to identify intravascular clotting. It is an excellent screening test for pulmonary embolism.
Meckel Diverticulum Nuclear Scan
Indications
This scan is designed to identify a Meckel diverticulum that contains ectopic gastric mucosa. It is indicated in patients who have recurrent lower abdominal pain or in pediatric or young adult patients who have occult gastrointestinal (GI) bleeding.
Test Explanation
Meckel diverticulum is the most common congenital abnormality of the intestinal tract. It is a persistent remnant of the omphalomesenteric tract. The diverticulum usually occurs in the ileum, approximately 2 feet proximal to the ileocecal valve. Approximately 20% to 25% of Meckel diverticula are lined internally by ectopic gastric mucosa. This gastric mucosa can secrete acid and cause ulceration of the intestinal mucosa nearby. Bleeding, inflammation, and intussusception are other potential complications of this congenital abnormality. The majority of these complications occur by 2 years of age.
Both normal gastric mucosa within the stomach and ectopic gastric mucosa in Meckel diverticulum concentrate technetium-99m pertechnetate. When this radionuclide is injected intravenously, it is concentrated in the ectopic gastric mucosa of Meckel diverticulum. One can then expect to see a hot spot in the right lower quadrant of the abdomen at about the same time as the normal stomach mucosa is visualized. This is a very sensitive and specific test for this congenital abnormality.
It is possible that Meckel diverticulum is present but contains no ectopic gastric mucosa within. Usually this is not symptomatic. No concentration of radionuclide will occur within the diverticulum. This test is not helpful in these cases.
Other conditions can simulate a hot spot compatible with Meckel diverticulum containing ectopic gastric mucosa. Usually these are associated with inflammatory processes within the abdomen (e.g., appendicitis, Crohn disease, or ectopic pregnancy).
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Advise the patient to refrain from eating or drinking anything for 6 to 12 hours before the examination.
Advise the patient to refrain from eating or drinking anything for 6 to 12 hours before the examination.
• A histamine H2-receptor antagonist is usually given for 1 to 2 days before the scan. This blocks secretion of the radionuclide from the ectopic gastric mucosa and improves visualization of Meckel diverticulum.
![]() Inform the patient that there is no pain associated with this test.
Inform the patient that there is no pain associated with this test.
During
• The patient lies in a supine position, and a large-view nuclear detector camera is placed over the patient's abdomen to identify concentration of nuclear material after intravenous (IV) injection.
• Images are taken at 5-minute intervals for 1 hour.
• Patients may be asked to lie on their left side to minimize the excretion of the radionuclide from the normal stomach because that would flood the intestine with radionuclide and preclude visualization of Meckel diverticulum.
• Occasionally glucagon is provided to prolong intestinal transit time and avoid downstream contamination with the radionuclide.
• Occasionally gastrin is given to increase the uptake of the radionuclide by the ectopic gastric mucosa.
Test Results and Clinical Significance
Increased uptake in the right lower quadrant: This is compatible with Meckel diverticula containing ectopic gastric mucosa. As indicated above, if the diverticulum does not contain ectopic gastric mucosa, the test will not be positive. Furthermore, one must be aware that other inflammatory diseases can cause false-positive results.
Octreotide Scan (Carcinoid Nuclear Scan, MIBG Scintigraphy Neuroendocrine Nuclear Scan)
Indications
Octreotide scans are used to identify and localize neuroendocrine primary and metastatic tumors. This scan is indicated in patients with known neuroendocrine tumors (e.g., carcinoid tumors and gastrinomas). It is used preoperatively to direct the surgeon to primary and metastatic tumors. This scan is also used to monitor therapy of these tumors.
Test Explanation
Octreotide scan is a specific example of nuclear peptide scanning that is increasingly being used to identify neoplasms by their altered state of physiology. Using peptides for which tumors have an increased uptake because of cellular membrane receptors or idiosyncratic physiology (glycolysis, proliferation, angiogenesis, or oxidation) will allow anatomic localization of many previously hidden tumors. These molecular imaging techniques can also provide information regarding the effect of anticancer therapy on tumor growth and survival.
Most neuroendocrine cells have a somatostatin receptor on the cellular membrane. Neuroendocrine tumors retain these receptors. Octreotide is an analogue of somatostatin. When combined with a radiopharmaceutical (such as indium-111 DTPA), the radiolabeled octreotide will attach to the somatostatin receptors of the neuroendocrine tumor cells. With the use of a scintillation camera the uptake can be observed and localized. In pediatrics, metaiodobenzylguanidine (MIBG) is used more frequently than octreotide as the radioisotope for identification of neuroendocrine tumors.
In patients with known neuroendocrine tumors, this test is used to direct the surgeon to the primary and metastatic sites within the body (especially the abdomen). This test is also used in the surveillance of patients who have been or are being treated for these neuroendocrine tumors. When this test is used as a monitor of disease, recurrence or progression can be identified quite easily and accurately. The liver, however, is more difficult to evaluate with octreotide scanning. The use of single-photon emission computed tomography (SPECT) imaging improves the sensitivity of this test. Many different types of hormone-producing tumors can be detected by this scan. Most notable include carcinoid, gastrinoma, insulinoma, glucagonoma, pheochromocytoma, and small cell lung cancer. Other abnormalities can pick up octreotide, including granulomatous infections (such as sarcoidosis or tuberculosis), rheumatoid arthritis, and nonhormonal cancers (breast, lymphoma, and non–small cell lung cancers).
The imaging procedure is performed by a trained technologist in approximately 30 minutes. A physician trained in nuclear medicine interprets the results. The only discomfort associated with this procedure is the intravenous (IV) injection of the radionuclide.
Contraindications
• Patients who are pregnant or lactating, unless the benefit outweighs the risk of damage to the fetus or infant
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Tell the patient that no fasting or premedication is required.
Tell the patient that no fasting or premedication is required.
![]() Assure the patient that he or she will not be exposed to large amounts of radiation because only tracer doses of isotopes are used.
Assure the patient that he or she will not be exposed to large amounts of radiation because only tracer doses of isotopes are used.
• If an iodinated radionuclide is to be used, ensure that the patient does not have an allergy to iodine.
• If an iodinated radionuclide is to be used, administer 5 drops of Lugol iodine solution daily for 3 days. This will block uptake of the radionuclide by the thyroid gland.
• If the patient has been receiving octreotide as a form of antineoplastic treatment, this must be discontinued for 2 weeks before scanning.
During
• Note the following procedural steps:
1. The patient is taken to the nuclear medicine department, where the radionuclide is administered intravenously. (For inpatients, a nuclear medicine technologist may administer the radionuclide at the bedside.)
2. One hour after injection, a gamma camera is successively placed over the entire body.
3. The patient is placed in supine, lateral, and prone positions so that all surfaces can be visualized.
4. The radionuclide image is recorded on film. SPECT images may also be performed.
5. After 4 hours the patient is given a strong laxative to clear the octreotide from the bowel.
6. Repeat scanning is again performed at 2, 4, 24, and 48 hours after administration of the octreotide.
Test Results and Clinical Significance
Carcinoid tumors: This tumor consists of neuroendocrine argentaffin cells that have somatostatin receptors. They usually arise from the appendix, small bowel, or colon. However, any organ can be the primary site of a carcinoid tumor.
Neuroendocrine tumors: Neuroendocrine tumors and other tumors as listed above can take up octreotide.
Granulomatous infections such as sarcoidosis and tuberculosis: The pathophysiology of this observation is not well understood.
Parathyroid Scan (Parathyroid Scintigraphy)
Indications
This test is used to locate the parathyroid glands before surgery. It also indicates the cause of the hyperparathyroidism.
Test Explanation
Hypercalcemia can be caused by hyperparathyroidism. Parathyroid hyperplasia, adenoma, or cancer can cause hyperparathyroidism. It is important for the surgeon planning resection of the parathyroid abnormality to know how many parathyroid glands are involved and their locations. Preoperative parathyroid scanning is the most accurate method of providing this information. Parathyroid hyperplasia causes enlargement of all four parathyroid glands. A parathyroid adenoma or cancer, however, causes enlargement of only one parathyroid gland and suppression (decrease in size) of the other three glands. Based on the parathyroid scan, the surgeon will know whether to suspect disease in one or all four of the glands.
Parathyroids are located most commonly on the lateral borders of the thyroid lobes—two on each side. However, parathyroid anatomic location varies considerably, and they may be located anywhere from the upper neck to the lower mediastinum. Parathyroid scanning is also done immediately before surgery to help the surgeon identify the parathyroid glands and particularly the pathologic glands. In this test, the scan is performed on the parathyroid glands as described previously. In the operating room, the surgeon scans the entire anterior neck with a hand-held gamma ray detector. Increased counts are noted in the regions where the parathyroids are located.
Scanning is now done more frequently in newly diagnosed patients. In some centers, scanning is reserved for those patients in whom an initial neck exploration failed to identify all four parathyroid glands and the hypercalcemia persisted after the operation.
There are two methods of parathyroid scanning. The first is the single tracer double phase (STDP) test using technetium-99m (99mTc) sestamibi or 99mTc tetrofosmin. With this method, the patient is injected with the sestamibi tracer. Images are obtained at 15 minutes and 3 hours. The tracer initially lights up both the thyroid and the parathyroid glands. At 3 hours, however, the tracer is washed out of all normal endocrine tissue and remains only in the pathologic parathyroid tissue.
The second method is the dual isotope subtraction test in which 99mTc pertechnetate or iodine 123 (123I) is administered. Only the thyroid gland takes up either of these two later tracers. Scanning is performed. 99mTc sestamibi is then administered and is taken up by both the thyroid and the parathyroid glands. Scanning is repeated and that first image is then “subtracted” from the second image leaving an image of only the parathyroid glands.
Interfering Factors
• Patient movement can inhibit the quality of imaging, especially when subtraction scanning is performed.
• Recent administration of x-ray contrast agents can alter test results.
![]() Iodine-containing foods or drugs (including cough medicines) can affect test results.
Iodine-containing foods or drugs (including cough medicines) can affect test results.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Tell the patient that fasting is usually not required. Check with the laboratory.
Tell the patient that fasting is usually not required. Check with the laboratory.
• Check the patient for allergies to iodine.
![]() Instruct the patient about medications and food that need to be restricted for weeks before the test (e.g., thyroid drugs, medications or food containing iodine).
Instruct the patient about medications and food that need to be restricted for weeks before the test (e.g., thyroid drugs, medications or food containing iodine).
• Obtain a history concerning recent contrast x-ray studies, nuclear scanning, or intake of any thyroid-suppressive or antithyroid drugs.
![]() Tell the patient that no discomfort is associated with this test.
Tell the patient that no discomfort is associated with this test.
During
There are two methods of parathyroid scanning. Note the following procedural steps:
STDP Method (Single Tracer Double Phase)
1. Technetium-99m sestamibi or tetrofosmin is injected intravenously.
2. At 15 minutes and 3 hours, the patient is placed in a supine position, a detector is passed over the neck and upper chest area, and the radioactive counts are recorded and displayed.
3. Initially the tracer lights up both the thyroid and parathyroid glands. At 3 hours, the tracer remains only in the pathologic parathyroid tissue.
Dual Isotope Subtraction Test
1. Technetium-99m pertechnetate is injected intravenously. Iodine 123 may be administered orally instead.
2. At 15 minutes after IV injection (or 3 to 4 hours after oral administration) the patient is placed in a supine position, a detector is passed over the neck and upper chest area, and the radioactive counts are recorded and displayed. Only the thyroid gland takes up these tracers.
3. Technetium-99m sestamibi is then injected intravenously and imaging is repeated.
4. With the computer, subtraction images are obtained, leaving only the parathyroid gland images.
![]() Tell the patient that no discomfort is associated with this study.
Tell the patient that no discomfort is associated with this study.
• A nuclear medicine technologist or a physician in the nuclear medicine department performs this procedure. The duration of the test is approximately 30 minutes. Scanning can be repeated several hours later for the STDP method.
Test Results and Clinical Significance
Parathyroid adenoma, carcinoma, or hyperplasia: Adenomas and cancers of the parathyroid gland usually involve only one gland. Hyperplasia of the parathyroids usually involves all four glands. All must be found at the time of surgery. Parathyroid scan provides the location of those glands for the surgeon.
Aberrantly placed parathyroid tissue in the upper neck, thyroid gland, or mediastinum: The information provided by this scan can identify abnormally malpositioned parathyroid tissue. This is invaluable to the surgeon when this information is known preoperatively.
Positron Emission Tomography (PET Scan)
Indications
PET scanning is used in many areas of medicine, most commonly for evaluation of the heart and brain. It is also commonly used in many aspects of oncology.
Test Explanation
In PET scanning, radioactive chemicals are administered to the patient. These chemicals are used in the normal metabolic process of the cells of the particular organ being imaged. Positrons emitted from the radioactive chemicals in the organ are sensed by a series of detectors positioned around the patient. Positron counts are received by these detectors and—with the combination of computed tomography—the positron emissions are recorded into a high-resolution three-dimensional image indicating a particular metabolic process in a specific anatomic site (Figure 8-12). Therefore PET provides images representing not only anatomy but also physiology. Like CT scans, MRI merely produces images of the body's anatomy or structure—not its metabolism. In most disease states, physiologic changes precede anatomic changes. Still in other disease states, such as Alzheimer disease, no anatomic changes occur—yet PET can identify classic physiologic changes that are diagnostic of the disease. Depending on the particular radionuclide used, PET can demonstrate the glucose metabolism, oxygenation, blood flow, and tissue perfusion of any specific area. Pathologic conditions are recognized and diagnosed by alterations in the normal metabolic process.
Figure 8-12 Positron emission tomography (PET). Clinical setting for PET. Shown are the Siemens ECAT scanner gantry and patient bed.
Certain radioactive chemical compounds provide specific information depending on the information required and the organ being evaluated. A cyclotron is used to create the radioactive chemical. Radioactive oxygen is used to make radioactive water (H215O). This is used to evaluate blood flow and tissue perfusion of an organ.
Radioactive fluorine is applied to a glucose analog and called fluorodeoxyglucose (FDG). Because most cells use glucose as an energy source, FDG is particularly useful in concentrating in regions of high metabolic activity of a particular organ. Radioactive carbon-labeled glucose is also useful for this purpose. Radioactive nitrogen is used in radioactive ammonia, which can be used in evaluating the liver. Other applications of radionuclides are listed in Table 8-3. PET scanning is becoming more widely applied and commonly used as research continues. Its greatest use thus far has been in the fields of neurology, cardiology, and oncology.
TABLE 8-3
Radionuclides Used in PET Scanning
| Radionuclide | Application |
| Carbon-11 | Cerebral, cardiac, pulmonary perfusion |
| Detection of myocardial infarction | |
| Cerebral function | |
| Nitrogen-13 | Cerebral and cardiac perfusion |
| Pulmonary inhalation | |
| Liver function | |
| Oxygen-15 | Cerebral perfusion and oxygen utilization |
| Fluorine-18 | Cerebral function and glucose metabolism |
| Gallium-68 | Cerebral perfusion |
| Lymphoreticular function |
In many centers, PET images can be superimposed with computed tomography (CT) or magnetic resonance imaging (MRI) to produce an anatomically accurate image showing the physiology/metabolism of the organ imaged. With newer units, PET/CT imaging can be performed by the same machine (Figure 8-13). This is called PET/CT image fusion or PET/CT co-registration. These composite views, which allow the information from two different studies to be digitally correlated and superimposed onto one image, lead to more precise information and accurate diagnoses. The CT images are acquired with the use of iodine contrast. In less than 60 minutes after the FDG is administered, the PET scan is performed in the same unit. The images are imposed on each other. The combined PET/CT scans provide images that pinpoint the location of abnormal metabolic activity within the body.
Neurology
Most brain imaging is performed with FDG. The brain uses glucose as its sole metabolic fuel. Pathologic areas of the brain that are more metabolically active (such as cancers) more avidly take up FDG than do normal areas. Because of the high physiologic rate at which glucose is metabolized by normal brain tissue, the detectability of tumors with only modest increases in glucose metabolism, such as low-grade tumors and, in some cases, recurrent tumors, is difficult with FDG. Another radioactive marker that is being used is 3,4-dihydroxy-6-18F-fluoro-L-phenylalanine (18F-FDOPA). This seems to improve visibility of low-grade brain tumors.
Epilepsy, Parkinson disease, and Huntington disease are identified as localized areas of increased metabolic activity indicating rapid nerve firing. Brain trauma resulting in a hematoma or bleeding is evident as decreased metabolic activity in the area of trauma. Stroke can also be identified and its extent determined. With the use of radioactive water (H215O), brain blood flow can be determined. Areas of decreased blood flow take up less (H215O) than normal areas and represent areas at risk for stroke.
Alzheimer disease can be recognized by identifying hypometabolism in multiple areas of the brain (temporal and parietal lobe) as scanning is performed during cognitive exercises. PET scanning with amyloid imaging using radioactive markers such as Pittsburgh agent compound B (PiB), flutemetamol, or fluorine-18 has been very helpful in identifying amyloid protein precursors (p. 639) in the brain. These agents bind to the beta-amyloid plaques that are increased in patients with Alzheimer disease. A negative PET scan with amyloid imaging eliminates the possibility of Alzheimer disease in a patient with cognitive impairment. Because other neurologic conditions (especially in elderly people) are also associated with amyloid neuritic plaques, a positive scan does not certainly establish the diagnosis of Alzheimer disease.
Cardiology
PET scans of the heart can show decreased blood flow, indicating coronary artery occlusive disease. PET scans are also used when cardiac muscle function is reduced. A PET scan can indicate whether the dysfunction arises from reversible ischemic muscle that would benefit from revascularization or from muscle tissue that is no longer viable. In the former case, surgical revascularization should be considered. In the latter case, revascularization would not be beneficial.
Oncology
The most commonly used agent in oncology is FDG because increased glucose metabolism is so prevalent in malignant tumors when compared to normal or benign pathologic tissue. PET can be used to visualize rapidly growing tumors and indicate their anatomic location. It is used to determine tumor response to therapy, identify recurrence of tumor after surgical removal, and differentiate tumor from other pathologic conditions (e.g., infection). PET is particularly helpful in identifying regional and metastatic spread for a particular tumor (Figures 8-14 [head and neck] and 8-15 [chest]). PET is more accurate in oncologic staging than CT scan. Its sensitivity exceeds 95% with a specificity of over 80%. In lung cancer, for example, if the FDG fails to concentrate in any area other than the primary tumor, no spread is suspected and the patient is considered an ideal candidate for surgery. PET has also been particularly useful for identifying metastasis from lung, melanoma, breast, pancreas, colon, lymphoma, and brain cancers.
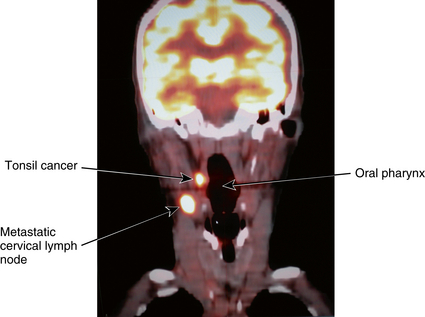
Figure 8-14 PET scan of the head and neck. Avid uptake of normal brain is noted. See two hotspots noted in the neck representing primary tonsil cancer and metastatic cervical lymph node.
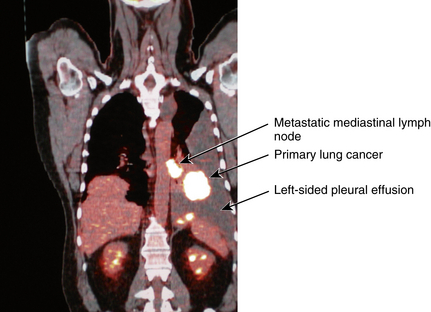
Figure 8-15 PET scan of the chest. Note two hotspots representing a primary lung cancer and multiple mediastinal lymph node metastasis. A large pleural effusion is noted.
Rapidly growing tumors are associated with a high metabolic rate and will therefore concentrate FDG particularly well. The amount of uptake of FDG is measured by the Standardized Uptake Value (SUV)—the amount of uptake of FDG in tumor compared to the normal tissue in that same area. SUV helps to distinguish between benign and malignant lesions—the higher the SUV, the more likely the tumor is malignant.
When the SUV is greater than the “cutoff value” (as determined by each institution), cancer rather than a benign pathologic condition is suspected. PET scanning is particularly helpful in the evaluation of solitary pulmonary nodules. CT scans and chest x-rays are inadequate to distinguish benign from malignant lesions. PET scanning can accurately provide that information over 75% of the time.
Bone
A PET/CT scan with a sodium fluoride F18 injection (18F NaF) scans the entire skeletal system and produces high-resolution images of the bones. These images are used to detect areas of abnormal bone growth associated with tumors. This test is more accurate than conventional nuclear bone scans. The PET/CT scan of the bone is particularly helpful for patients with prostate or breast cancer. The uptake of 18F NaF in the skeleton reflects sites of increased blood flow and bone remodeling associated with bone injury or metastatic disease. A bone PET/CT scan's high-resolution images and its ability to scan the entire skeleton make it very helpful in detecting bone disease.
Small parts PET scans are being used with increasing frequency for foot inflammatory pathology. PET mammography or positron emission mammography (PEM) is seeing growing use as a tool for diagnostic breast imaging. PEM holds the promise of improving sensitivity and specificity of routine mammography (see p. 1043).
Interfering Factors
• Recent use (within 24 hours) of caffeine, alcohol, or tobacco may affect test results.
• Ingestion of a small- to moderate-sized meal can cause a marked uptake of FDG in the gut and muscles, thereby leaving little or no radionuclide to be taken up by tumor. This can cause a false-negative result.
• Anxiety can cause increased uptake in multiple areas (e.g., neck, upper mediastinum) of the body. If the patient is anxious, sedatives can be administered 30 minutes before testing. However, these could interfere with PET scanning of the brain if cognitive activities will be used to measure changes in brain activity.
• Mild to moderate exercise can instigate marked uptake of FDG in the muscles thereby leaving little or no radionuclide to be taken up by tumor. This causes a false-negative result.
• The liver and spleen avidly take up FDG. Therefore those organs are difficult to evaluate on PET imaging.
• FDG is excreted by the urinary system. As a result, the bladder may obscure areas of increased uptake in the pelvis.
• Uptake of FDG can occur in the lymph node basin draining the site of the FDG injection. If PET is being done to stage tumors that could metastasize to those lymph nodes, inject the FDG on the contralateral side.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
• Obtain informed consent if required by the institution.
![]() Inform the patient that he or she may have an intravenous (IV) line inserted.
Inform the patient that he or she may have an intravenous (IV) line inserted.
![]() Inform the patient that he or she may need to restrict food or fluids for 4 hours on the day of the test. The patient should refrain from alcohol, caffeine, and tobacco for 24 hours.
Inform the patient that he or she may need to restrict food or fluids for 4 hours on the day of the test. The patient should refrain from alcohol, caffeine, and tobacco for 24 hours.
![]() Instruct diabetic patients to take their pretest dose of insulin at a meal 3 to 4 hours before the test.
Instruct diabetic patients to take their pretest dose of insulin at a meal 3 to 4 hours before the test.
![]() Tell the patient that no sedatives or tranquilizers should be taken, because he or she may need to perform certain mental activities during the brain PET scan.
Tell the patient that no sedatives or tranquilizers should be taken, because he or she may need to perform certain mental activities during the brain PET scan.
![]() Tell the patient to empty the bladder before the test for comfort. A Foley catheter may be inserted for PET scanning of the pelvic region.
Tell the patient to empty the bladder before the test for comfort. A Foley catheter may be inserted for PET scanning of the pelvic region.
![]() Tell the patient that the only discomfort associated with this study is insertion of the IV line.
Tell the patient that the only discomfort associated with this study is insertion of the IV line.
• Depending on the organ being evaluated, specific protocols exist for the examination.
During
• Note the following procedural steps:
1. The patient is positioned in a comfortable, reclining chair.
2. The radioactive material can be infused through an IV line or inhaled as a radioactive gas.
3. The gamma rays that penetrate the tissues are recorded outside the body by a circular array of detectors and are displayed by a computer.
4. If the brain is being scanned, the patient may be asked to perform different cognitive activities (e.g., reciting the Pledge of Allegiance) to measure changes in brain activity during reasoning or remembering.
5. Extraneous auditory and visual stimuli are minimized by a blindfold and ear plugs.
6. If the chest is being scanned, instruct the patient to breathe in a shallow manner until the middle of the chest is reached. Then ask the patient to hold the breath after expiration until the middle of the abdomen is reached. This will improve visibility of the chest anatomy.
• Note that a physician performs this procedure with a trained technologist in approximately 40 to 90 minutes.
Test Results and Clinical Significance
Areas of ischemia or infarction are associated with decreased flow and decreased glucose metabolism. This is indicated by hypoconcentration of FDG.
Cerebrovascular accident (stroke): These areas are evident as decreased blood flow and metabolism.
Specific areas of decreased metabolism are noted in classic regions of the brain (temporal and parietal lobes).
Malignant tumor: Malignancy is associated with increased glucose metabolism in rapidly dividing cells. This is indicated by concentration of FDG in levels exceeding SUV cutoff points.
Related Tests
Computed Tomography (CT) Scanning (p. 1020). This uses a technique that recognizes differences in density coefficients to visualize different organs and tissues. Unlike PET, a CT scan cannot provide information concerning metabolism or function.
Single-Photon Emission Computed Tomography (SPECT) Scanning (p. 780). This is another form of radionuclear imaging that can provide three-dimensional anatomic and perfusion images but lacks the capability to indicate metabolism.
Magnetic Resonance Imaging (MRI) (p. 1106). This provides a picture of normal and pathologic anatomy by temporarily altering the magnetic field of the cells in the area to be evaluated.
ProstaScint Scan (Radioimmunoscintigraphy [RIS])
Test Explanation
By using a radionuclide that is able to attach to prostate cancer cells only, metastatic prostate cancer outside the prostate gland can be easily identified. In this scan, mouse monoclonal antibody (capromab) directed against prostate specific membrane antigen (PSMA) is tagged with indium111. PSMA, located in the cytoplasm of prostate cancer (or transitional cell urogenital cancer), is detected on radionuclide scan images. Disease outside the prostate (e.g., retroperitoneum, liver, lung, bone) indicates metastatic prostate cancer. Other radionuclides, such as technetium labeled red blood cells, can be used, but the images provide less accurate results.
This scan is helpful in staging newly diagnosed prostate cancer patients who are at high risk for metastatic disease to the lymph nodes or other organs. This test can also be used to identify recurrent or metastatic disease after curative therapy. This test can document completeness of anti-prostate cancer therapy. Finally this scan helps elucidate abnormalities that may be noted on other diagnostic imaging tests, such as CT scan (especially in patients with prior prostate cancer).
The ProstaScint scan is usually performed in conjunction with other diagnostic testing, such as CT scan, PET scan, or ultrasound. Because of cost and labor intensity, this test is not used routinely for prostate screening.
This test takes about 30 minutes (per day for as many as 5 days) and is performed by a nuclear medicine technologist. It is interpreted by a nuclear medicine physician. There is no discomfort associated with this test other than an intravenous injection.
Procedure and Patient Care
Before
During
• After proper identification, the patient is injected with the radiolabeled monoclonal antibody.
• Initial images are obtained 30 minutes after injection. Images are repeated over as many as 5 days.
• The patient is asked to lie on a padded table during imaging.
• A scintigraphy camera is placed over the anterior or posterior surface of the chest, abdomen, and pelvis. Approximately 10 minutes are required for each view.
• The patient may be asked to return the following day or the day after that for repeated images.
• Little or no discomfort is associated with this procedure.
• The procedure takes approximately 1 hour each day over a period of 1 to 5 days.
• This procedure is performed in the nuclear medicine department.
Related Tests
Prostate Specific Antigen (PSA) (p. 420). This is a screening test for prostate cancer.
CT Scan of the Abdomen and Pelvis (p. 1020). This test is able to identify abnormalities compatible with metastatic prostate cancer. Test results are less specific for prostate cancer.
PET/CT Scan (p. 822). This test is able to identify abnormalities compatible with metastatic prostate cancer. Test results are less specific for prostate cancer.
Renal Scanning (Kidney Scan, Radiorenography, Renography, Radionuclide Renal Imaging, Nuclear Imaging of the Kidney, DSMA Renal Scan, DTPA Renal Scan, Captopril Renal Scan)
Indications
Renal scans are used to indicate the perfusion, function, and structure of the kidneys. They are also used to indicate the presence of obstruction or renovascular hypertension. Because this study uses no iodinated dyes, it is safe to use on patients who have iodine allergies or compromised renal function. Renal scans are used to monitor renal function in patients with known renal disease. This scan also plays a large part of the diagnosis of renal transplant rejection.
Test Explanation
This nuclear medicine procedure provides visualization of the urinary tract after intravenous administration of a radioisotope. The radioactive material is detected by a scintillation camera, which can detect the gamma rays emitted by the radionuclide in the kidney. The scintillation camera information can be translated into light and thereby create a realistic image of the renal structure. That information is collated by a computer, and the amount of gamma ray emission per unit of time can be calculated to determine renal function, vascular insufficiency, or renal obstruction. Scans do not interfere with the normal physiologic process of the kidney. The resultant image (scan) indicates distribution of the radionuclide within the kidney and ureters.
There are several different types of renal scans, depending on what information is needed (Table 8-4). Different isotopes may be more suitable for different scans, based on the manner in which the kidney handles the radioisotope.
TABLE 8-4
| Types | Purpose | Examples of Findings |
| Blood flow (perfusion) | Evaluates blood flow to each kidney | Renal artery stenosis, renovascular hypertension, transplant rejection, hypervascular tumors |
| Structural | Identifies structural abnormalities | Tumor, cyst, abscess, congenital disorders, malposition or absence, horseshoe-shaped kidney |
| Function (renogram) | Evaluates function by uptake and excretion of radioisotopes | Glomerulonephritis, decreased blood supply, transplant rejection, renal failure |
| Hypertension | Detects presence and source of renal hypertension | Renal artery stenosis, vascular obstruction |
| Obstruction | Identifies outflow obstruction | Renal pelvis obstruction, ureter obstruction, bladder outlet obstruction |
Renal Blood Flow (Perfusion) Scan
This type of renal scan is used to evaluate the blood flow to each kidney. It is used to identify renal artery stenosis, renovascular hypertension, and rejection of renal transplant. Also, it is used to demonstrate hypervascular lesions (renal cell carcinoma) in the kidney.
The basic test is performed by rapid IV injection of the radionuclide (usually technetium-99m diethylenetriamine pentaacetic acid [99mTc DTPA], 99mTc disodium monomethane arsenate [DSMA], or iodohippurate sodium 131I) while the patient is positioned under the scintigraphy camera. Computers collate the data obtained by the camera and create a curve of gamma activity per unit of time. Each kidney is compared to the opposite kidney and to the aorta. Decreased gamma activity is noted in the kidney with arterial stenosis or renovascular hypertension. Decreased activity relative to the aorta is noted in a transplanted kidney that is experiencing rejection. Increased gamma activity is noted in the kidney that contains a hypervascular tumor (cancer).
Renal Structural Scan
This type of renal scan is performed to outline the structure of the kidney to identify a pathologic condition that may alter normal anatomic structure (e.g., tumor, cyst, abscess). Congenital disorders (e.g., hypoplasia or aplasia of the kidney, malposition of the kidney) can also be detected. Also, information following renal transplants can be obtained with this scan. A filling defect in the renal parenchyma may indicate a tumor, cyst, abscess, or infarction. Horseshoe-shaped kidney, pelvic kidney, or absence of a kidney may be evident. Anatomic alterations in the parenchymal distribution of tracer may indicate transplant rejection.
99mTc DTPA or 99mTc DSMA can be used for this scan. DSMA is particularly good because it is rapidly taken up by the kidney but excreted very slowly, allowing good visualization of the renal structure.
Renal Function Scan (Renogram)
Renal function can be determined by documenting the capability of the kidney to take up a particular radioisotope and excrete it. A well-functioning kidney can be expected to rapidly assimilate the isotope and then excrete the same isotope. A poorly functioning kidney will not be able to take up the isotope rapidly or excrete it in a timely manner. Each radioactive tracer is handled by the kidney in a different manner. Different renal functions can be tested according to which isotope is used:
In this study the dose of radionuclide is determined by calculation based on the body weight or surface area. The patient is placed under the scintigraphy camera. The radioisotope is injected, and a computer analyzes the data obtained from the camera. Activity per unit of time equals the function of the kidney, which is plotted on graph paper. This is called a renogram curve (isotope renography). The function tested depends on the radioisotope being used. Disappearance of the isotope is also plotted as part of that same curve and is a measurement of excretory function of the kidney. The curves are plotted, and their shapes can be compared to expected normal values and to the opposite kidney. Furthermore, renal function can be monitored by serially repeating this test and comparing results. Renal function can be noted to be improved or deteriorating, depending on serial comparisons of the curves.
The kidney with diminished renal function (e.g., glomerulonephritis) or decreased blood supply can be expected to not have rapid uptake of activity and rapid disappearance (excretion) of the radionuclide. The curve will be much flatter. This can also be seen in rejection after transplantation. Impending renal failure can be identified with this scan.
Renal Hypertension Scan
This scan is used to determine the presence and the source of renovascular hypertension. This scan usually uses an angiotensin-converting enzyme (ACE) inhibitor (such as captopril).
The captopril scan (captopril renography/scintigraphy) determines the functional significance of a renal artery or arteriole stenosis. After the administration of captopril, the glomerular filtration rate (GFR) in a kidney with a partial vascular obstruction is reduced despite the preservation of renal plasma flow. The GFR in the contralateral kidney is maintained. This would be demonstrated as delayed radioactivity in the affected kidney after injection of a radionuclide. These scans may predict the response of the blood pressure to medical treatment, angioplasty, or surgery.
Renal Obstruction Scan
This scan is performed to identify obstruction of the outflow tract of the kidney because of obstruction of the renal pelvis, ureter, or bladder outlet. In this study the radionuclide is rapidly injected while the patient is under the scintigraphy camera. Activity is measured and plotted per unit of time. After about 10 minutes, a diuretic (Lasix) is administered. The radionuclide in the unobstructed kidney can be seen to rapidly wash out (be excreted) from the kidney. A slow excretion without a wash-out is seen in an obstructed but still functioning kidney. Furthermore, when the collecting system does become visible, it is observed to be dilated.
Often several of these scans are combined to obtain the maximum amount of information about the renal system. A triple renal study may use all of these techniques to evaluate renal blood perfusion, structure, and excretion.
Renal scans are superior to other testing in determining renal function, identifying renal infarction, monitoring renovascular hypertension, and identifying primary renal diseases and transplant rejection. This radionuclear scan is also helpful in the evaluation of the following:
• Arterial atherosclerosis or trauma; the renal uptake of the radionucleated material will be delayed or absent on the affected side or sides
• Pathologic renal or ureteral conditions in patients who cannot have IV pyelography (IVP) (p. 1057) because of dye allergies or poor renal function
• Renal tumors, abscesses, or cysts in patients who may have an allergy to iodine; these appear as cold spots because of the nonfunctioning tissue
• Renal or ureteral disease in patients whose renal function is already poor and who would be at risk for further reduction in function if iodinated dye were to be administered
For anatomic abnormalities, tumors, or cysts, ultrasound (p. 866), computed tomography (p. 1020), or MRI (p. 1106) scans are preferable and more accurate.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
• Do not schedule a renal scan within 24 hours after an IVP. The iodinated dye may temporarily diminish renal function.
![]() Assure the patient that he or she will not be exposed to large amounts of radioactivity because only tracer doses of isotopes are used.
Assure the patient that he or she will not be exposed to large amounts of radioactivity because only tracer doses of isotopes are used.
![]() Remind the patient to void before the scan.
Remind the patient to void before the scan.
![]() Tell the patient that no sedation or fasting is required but that good hydration is essential.
Tell the patient that no sedation or fasting is required but that good hydration is essential.
![]() Instruct the patient to drink two to three glasses of water before the scan.
Instruct the patient to drink two to three glasses of water before the scan.
![]() Tell the patient that no pain or discomfort is associated with this procedure.
Tell the patient that no pain or discomfort is associated with this procedure.
![]() Inform the patient that he or she must lie still during this study.
Inform the patient that he or she must lie still during this study.
During
• Note the following procedural steps:
1. The unsedated, nonfasting patient is taken to the nuclear medicine department.
2. A peripheral IV injection of radionuclide is given. It takes only minutes for the radioisotopes to be concentrated in the kidneys.
3. While the patient assumes a prone or sitting position, a gamma ray scintigraphy camera is passed over the kidney area and records the radioactive uptake on film.
4. For a Lasix renal scan or a diuretic renal scan, the patient is imaged with DTPA. Images are obtained for 10 to 20 minutes, then Lasix is administered intravenously, and images are obtained for another 20 minutes.
5. For the captopril renal scan, the patient is scanned after the administration of an ACE inhibitor, such as captopril.
6. Scans may be repeated at different intervals after the initial isotope injection. For the renal blood flow and the renal function scans, scanning is started immediately after the injection.
7. For structural renal scans the patient is asked to lie still for the entire time of the scan (30 minutes).
• Note that the duration of this test varies from 1 to 4 hours, depending on the specific information required. Perfusion scans are done in approximately 20 minutes and functional scans in less than 1 hour. Static structure scans require 20 minutes to 4 hours for completion.
• Note that this study is performed by a nuclear medicine technologist or physician.
Test Results and Clinical Significance
Urinary obstruction: This is obvious on a renal obstruction scan. After diuresis the obstructed kidney fails to demonstrate excretion (wash-out) of the radionuclide. Prolonged obstruction ultimately leads to total loss of function of the obstructed kidney. That kidney will not light up after injection of a radionuclide.
Renovascular hypertension: The renal hypertension scan is performed after administration of captopril. The time for the affected kidney to light up is significantly prolonged.
Renal infarction: This can be seen as a wedge-shaped defect on the structural scan and perhaps as decreased blood flow on the perfusion scan.
Renal arterial atherosclerosis: This is evident as delayed light-up on the perfusion scan. The plotted curve is flatter than normal.
If significant enough to affect renal function, these diseases are evident on the renal function scans. The affected kidney does not light up as quickly as normal. The plotted curves of function per unit of time are flatter than normal. No uptake is seen with absence of renal function.
Congenital abnormalities such as renal aplasia, hypoplasia, and malposition: These abnormalities are evident on the renal structural scan.
Renal trauma: With arterial injury, the renal blood flow scan will demonstrate prolonged or no visualization of the affected kidney. The renal structural scan may demonstrate a laceration of the kidney with extravasation of radionuclide out of the renal capsule.
Transplant rejection: This is apparent with many of the scans described in this section. With rejection of a transplanted kidney, one may see reduced blood flow to the transplanted kidney, reduced function of the transplanted kidney, and/or alterations in renal structure of the transplanted kidney.
Related Tests
Intravenous Pyelography (IVP) (p. 1057). This is an x-ray examination of the kidneys and lower urologic tract. With the use of iodinated IV contrast medium, this test can also provide information about renal function, blood flow, and structure. Renal obstruction can also be demonstrated.
Computed Tomography (CT) (p. 1020). This scan is an x-ray study utilizing the technology of computed tomography. This test can provide information about renal function, blood flow, and structure. Also, renal obstruction can be demonstrated.
Salivary Gland Nuclear Imaging (Parotid Gland Nuclear Imaging)
Indications
This test is used to evaluate patients with xerostomia (dry mouth), salivary gland pain, tumors, or possible parotid duct obstruction.
Test Explanation
The ability of the epithelial cells of the salivary glands to transport large pertechnetate from the blood and to secrete it into the saliva provides the principle for imaging the salivary glands. The functional capabilities, structural integrity, and location of the glands can be assessed. Most usually, the parotid gland alone is visualized. Occasionally, the submandibular glands can be seen.
By following the radionuclide immediately after injection, blood flow can be evaluated. Because this blood flow comes from the cerebral arteries, this test is a measure of the patency of those vessels. Tumors have increased blood flow that can be identified during this part of the study. Patients with acute inflammation will also have increase blood flow during the early stages of the test.
In about 10 to 20 minutes after injection, gland function becomes obvious by uptake of the nuclide into the gland. This uptake is usually compared to the thyroid, which is visualized at the same time. Function will be diminished in patients with severe inflammation or autoimmune diseases, such as Sjögren syndrome. Five to 10 minutes later, one should see secretion of nuclear material into the mouth. Salivary calculi will impede excretion and wash-out of the radionuclide because of obstruction of the excretory duct.
Wash-out demonstrates complete salivary gland excretion. Usually the patient is asked to suck on a lemon to encourage rapid wash-out. Static lateral pictures of the salivary glands can demonstrate tumors or cysts. Most commonly the parotid gland is affected by tumors, and usually they are benign. In neoplasm of the salivary glands, wash-out is slow (i.e., the tumor may remain “hot” [retain radionuclide]) for longer periods of time. Nearly 50% of the benign tumors are hot. A cold tumor (does not take up radionuclide as well as “cold” surrounding tissue) is common in malignant tumors.
Procedure and Patient Care
During
• Note the following procedure steps:
1. Tc-99m pertechnetate is injected into the antecubital vein.
2. Dynamic images are obtained immediately by placing the detector over the facial area. Radioactive counts are recorded and displayed.
3. Repeat images are obtained every 3 to 5 minutes for total of 15 to 20 minutes.
4. A salivary gland stimulant is administered following completion of static images. Either lemon juice or a lemon slice should be swished in the mouth and then expectorated.
5. “Wash-out” images are obtained 5 to 10 minutes after the salivary gland stimulant. The thyroid gland is included for reference/comparison.
• This procedure is performed by a nuclear medicine technologist or a physician in the nuclear medicine department in approximately 35 to 45 minutes.
Scrotal Nuclear Imaging (Scrotal Scan, Testicular Imaging)
Indications
Scrotal imaging is helpful in the diagnosis of patients with a sudden onset of unilateral testicular swelling and pain. Scrotal imaging can differentiate unilateral testicular torsion from other causes of testicular pain (e.g., acute epididymitis, torsion of the testicular appendage, orchitis, strangulated hernia, testicular hemorrhage). This test is not used frequently because scrotal ultrasound can reliably provide the same information more rapidly and more cheaply.
Test Explanation
Testicular torsion is a surgical emergency requiring prompt surgical exploration to salvage the involved testicle. To provide immediate surgical care, the surgeon must differentiate the condition from other causes of painful testicular swelling that do not require surgery. Use of radionuclide scrotal imaging enables the surgeon to diagnose testicular torsion. This study is usually performed on an emergency basis and in the nuclear medicine department.
The patient is positioned under the gamma camera with the scrotum supported between the abducted thighs. Technetium-99m (99mTc) pertechnetate is administered, and a dynamic radionuclide nuclear angiogram is obtained. Static images are obtained immediately afterward. An area of decreased perfusion corresponding to the involved testes indicates a high probability of torsion of the testicle. If the clinically involved testis is normally perfused or hypervascular, a disease other than torsion of the testicle (as described earlier) exists.
Procedure and Patient Care
During
• The patient is placed on a padded table in the supine position.
• The patient's legs are abducted, and the testicles are supported with tape or a lead shield. The penis is taped to the lower abdomen.
• A small intravenous (IV) injection of 99mTc pertechnetate is administered.
• Radionuclide imaging is then immediately performed over both testicles. Both dynamic and static images are obtained.
Related Test
Scrotal Ultrasonography (p. 893). This is now the preferred test to indicate torsion of the testicle. It is more rapidly performed and most accurate.
Sentinel Lymph Node Biopsy (SLNB, Lymphoscintigraphy)
Indications
Lymphoscintigraphy is used to identify the “sentinel” lymph node—the one most likely to contain metastasis from a nearby primary tumor. It is used to map the lymphatic drainage of a primary cancer so that surgery can be directed for diagnostic and possibly therapeutic resection of lymph nodes. It is primarily used in breast cancer and melanoma.
Test Explanation
With this procedure, the first (sentinel) lymph node in line to catch metastatic tumor cells from a primary tumor is identified and biopsied. To stage most breast or melanoma cancers, a lymph node draining the primary site must be evaluated microscopically. With the use of SLNB, the first lymph node in the chain of lymph nodes can be identified and biopsied. If results are negative, as is the case in most patients with small tumors, the rest of the axillary lymph contents can be safely assumed to be free of tumor and are not removed. This saves women from the potential complications associated with a full lymph node dissection including arm swelling, cellulitis, postoperative pain, and reduced range of motion. Furthermore this test can identify unusual locations for lymph node metastasis that would not normally be identified by the surgeon.
To summarize the procedure, a tracer (isosulfan blue dye or technetium [99mTc] sulfur colloid) is injected into the skin or tissue near the tumor. If technetium is used, a lymphoscintigraphy scan is performed about 1 to 2 hours after the injections. There are specific protocols depending on the lymph node basin being studied and on the primary tumor site. Lymph nodes that take up the radionuclide are the sentinel lymph nodes that the surgeon will identify and remove. In the operating room, using a hand-held gamma detector, the surgeon is able to identify the region of maximum radioactivity. These are removed and sent to the pathologists for immediate evaluation. If isosulfan or methylene blue dye is injected, a stained lymphatic vessel is identified by the surgeon in the subcutaneous tissue and followed to the first blue-colored node. The sentinel lymph nodes are the blue, or “hot,” nodes closest to the primary tumor.
If the sentinel lymph node is negative on frozen section or imprint cytology (touch prep) pathologic study, the lymph node dissection procedure is not required. If the sentinel lymph node is positive, a full lymph node dissection may be performed. In some instances light microscopy may be negative but subsequent immunohistochemical staining may indicate the presence of cancer in the node. The sentinel lymph node can also be evaluated right in the operating room by using molecular assays for epithelial cell specific components such as cytokeratin or mammaglobin.
This test is quickly becoming an important part of the standard treatment for breast and melanoma cancer surgery. The only discomfort associated with the test is the preoperative injections required around the tumor. The technetium injection and subsequent scanning are usually performed in the nuclear medicine department. When isosulfan blue is used as the lymph node tracer, the injection is administered in the operating room under anesthesia.
Nuclear lymphoscintigraphy is also used to evaluate the lymph node status in patients with Hodgkin disease and other lymphomas. Patients with chronic lymphedema of an extremity may also be evaluated by lymphoscintigraphy.
Procedure and Patient Care
During
Note the following procedural steps:
1. The patient is taken to the nuclear medicine department, where the radionuclide is injected around the tumor.
2. The site of lymph node drainage is then scanned immediately and 1 to 24 hours later.
3. Lymph node uptake is reported to the surgeon.
4. In the operating room, a handheld gamma detector locates “hot” areas of radionuclide uptake in the lymph node–bearing area. The most proximal “hot” node is excised as the sentinel node.
1. In the operating room, 4 to 5 mL of isosulfan blue dye is injected around the tumor.
2. After 5 to 9 minutes, a small incision is made overlying the lymph node–bearing area and the proximal blue lymph node is removed as the sentinel lymph node.
• If the sentinel lymph node is negative for tumor, the lymph node dissection procedure is discontinued. If the sentinel lymph node is positive, a complete lymph node dissection may be performed.
After
![]() Inform the patient that no precautions are required if technetium is used because the radionuclide dose is minimal.
Inform the patient that no precautions are required if technetium is used because the radionuclide dose is minimal.
• If isosulfan blue dye is used, the patient's skin may develop a transient blue hue (looking almost like severe cyanosis). This will dissipate over the next 6 hours.
![]() Warn the patient that the urine will have a blue tinge as a result of the isosulfan blue dye injection.
Warn the patient that the urine will have a blue tinge as a result of the isosulfan blue dye injection.
• Observe the patient for signs of allergy (rare) caused by the blue dye injection.
Thyroid Scanning (Thyroid Scintiscan)
Indications
This test is used to visualize the thyroid gland when disease of the thyroid is suspected. It is particularly useful in the evaluation of patients with a suspected thyroid nodule. With thyroid nuclear scanning the nodule can be classified and more appropriately treated.
Test Explanation
Thyroid scanning allows the size, shape, position, and physiologic function of the thyroid gland to be determined with the use of radionuclear scanning. A radioactive substance such as technetium-99m (99mTc) or Iodine131 is given to the patient to visualize the thyroid gland. A scintigraphy camera is passed over the neck area, and an image is recorded (Figure 8-16).
Thyroid nodules are easily detected by this technique. Nodules are classified as functioning (warm/hot) or nonfunctioning (cold) depending on the amount of radionuclide taken up by the nodule (Figure 8-17). A functioning nodule could represent a benign adenoma or a localized toxic goiter. A nonfunctioning nodule may represent a cyst, carcinoma, nonfunctioning adenoma or goiter, lymphoma, or localized area of thyroiditis.
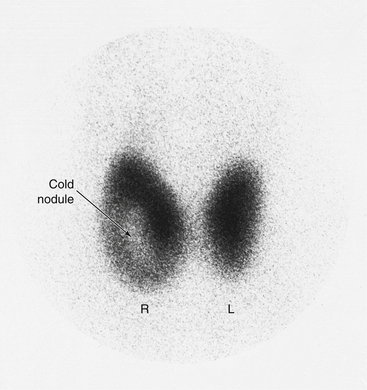
Figure 8-17 Thyroid scan. Note the cold nodule in the right (larger) lobe of the thyroid gland. This finding is consistent with tumor, cyst, or goiter.
Scanning is useful in patients with the following clinical conditions:
2. Thyroid nodule. Thyroid cancers are usually nonfunctioning (cold) nodules.
3. Hyperthyroidism. Scanning will assist in differentiating Graves disease (diffusely enlarged hyperfunctioning thyroid gland) from Plummer disease (nodular hyperfunctioning gland).
4. Metastatic tumors without a known primary site. A normal scan excludes the thyroid gland as a possible primary site.
Another form of thyroid scan is called the whole-body thyroid scan. This scan is performed on patients who have previously had a thyroid cancer treated. Iodine131 is administered orally, and the entire body is scanned to look for metastatic thyroid tissue. A hot spot would indicate recurrent tumor. Before this test can be performed, all of the thyroid tissue in the neck must be either surgically excised or ablated with radioactive 131I. If the patient is receiving thyroid replacement therapy, the thyroid medicine must be discontinued at least 6 weeks before testing. This makes any metastatic thyroid tissue, particularly iodine, avid. A high thyroid-stimulating hormone blood level ensures that any thyroid cancer tissue will take up the administered radioactive iodine. This test is performed routinely (every 1 to 2 years) on patients who have had a thyroid cancer larger than 1 cm. Smaller cancers are unlikely to metastasize. Much of the procedure is similar to thyroid scanning.
Interfering Factors
• Iodine-containing foods affect results because the iodine may saturate all of the iodine-binding sites and very little iodine tracer will be taken up by the thyroid. Also, if large quantities of iodine are ingested, the thyroid may shut down and even Tc tracer will not be taken up by the thyroid.
• Recent administration of x-ray contrast agents affects results because these agents contain large quantities of iodine. For the reasons described above, contrast agents should be avoided before thyroid scanning.
![]() Drugs that may affect test results include cough medicines, multiple vitamins, some oral contraceptives, and thyroid drugs.
Drugs that may affect test results include cough medicines, multiple vitamins, some oral contraceptives, and thyroid drugs.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
• Check the patient for allergies to iodine.
![]() Instruct the patient about medications that need to be restricted for 6 weeks before the test (e.g., thyroid drugs, medications containing iodine).
Instruct the patient about medications that need to be restricted for 6 weeks before the test (e.g., thyroid drugs, medications containing iodine).
• Obtain a history concerning previous contrast x-ray studies, nuclear scanning, or intake of any thyroid-suppressive or antithyroid drugs.
![]() Tell the patient that fasting is usually not required. Check with the laboratory.
Tell the patient that fasting is usually not required. Check with the laboratory.
![]() Tell the patient that no discomfort is associated with this study.
Tell the patient that no discomfort is associated with this study.
During
• Note the following procedural steps:
1. A standard dose of radioactive iodine is usually given to the patient by mouth. The capsule is tasteless.
2. Scanning is usually performed 24 hours later. If intravenous technetium is used, scanning may be performed 2 hours later.
3. At the designated time, the patient is placed in a supine position and a scintigraphy camera is placed over the thyroid area.
• Note that this study is performed by a nuclear medicine technologist in less than 30 minutes.
Test Results and Clinical Significance
Adenoma: This may be evident as a hot nodule if it is functioning or a cold nodule if it is not functioning.
Toxic and nontoxic goiter: The toxic goiter will be apparent as a hot nodule. The nontoxic goiter will be a cold nodule.
Graves disease: This disease is evident on thyroid scan as diffuse increased uptake of radionuclide involving the entire thyroid gland.
Plummer disease: This disease produces a single or multiple nodular areas of increased uptake.
In general, hyperthyroid patients have increased uptake of radionuclide, and hypothyroid patients have reduced uptake.
Hashimoto disease: This often is apparent as mottled uptake of radionuclide.
Related Tests
Thyroid Ultrasonography (p. 895). This is an important part of evaluation of the thyroid gland. In general, all solid nodules on ultrasound that are cold on thyroid scan should be considered suggestive of cancer.
Triiodothyronine (T3), Thyroxine (T4), Thyroid-Stimulating Hormone (TSH) (pp. 506, 497, and 486, respectively). These tests are the most common methods by which thyroid function is measured. They are more accurate than radioactive iodine uptake and thyroid scanning. No radioactive material needs to be administered to the patient. These tests should be a part of every thyroid evaluation.
Computed Tomography (CT) Scan of the Neck (p. 1029). With CT scan, the thyroid nodule can be more accurately located and its characteristics evaluated for malignancy.
Total Blood Volume (TBV, Red Blood Cell [RBC] Volume)
Indications
Total blood volume measurement may be useful in the following clinical circumstances:
1. Congestive heart failure: The actual amount of fluid overload can be calculated and diuresis can be more appropriately determined.
2. Presurgery: The patient's fluid status can be accurately determined, as can RBC status.
3. Acutely ill patients: There are often large fluid shifts in these patients and TBV may help in guiding IV fluid replacement.
4. Azotemia: Measurement of TBV will indicate if azotemia is prerenal (hypovolemia) or primary renal.
5. Hypertension: TBV may indicate plasma volume overload versus vascular constriction.
6. Anemia: TBV and RBC volumes can indicate accurately the extent of anemia that otherwise could be affected by fluid status, etc.
Test Explanation
Measurement of total blood volume is an accurate indicator of true plasma (liquid components of blood) measurement. Based on the patient's height, weight, gender, and body composition, a TBV can determine whether the measured volumes are normal, high, or low compared with what would be ideal for the particular patient. The report indicates actual volumes for TBV and RBCs that deviate from normal.
To maintain blood volume within a normal range, the kidneys regulate the amount of water and sodium lost into the urine. For example, if excessive water and sodium are ingested, the kidneys normally respond by excreting more water and sodium into the urine. This auto adjustment is mediated through the renin-angiotensin-aldosterone system. Both angiotensin and aldosterone, although by different mechanisms, stimulate distal tubular sodium reabsorption and decrease sodium and water loss by the kidney and thereby adjust blood volume. Another important hormone in regulating blood volume is vasopressin (antidiuretic hormone [ADH]). This hormone is released by the posterior pituitary. One of its actions is to stimulate water reabsorption in the collecting duct of the kidney, thereby decreasing water loss and increasing blood volume. Blood volume affects cardiac output and blood pressure.
Radioiodine labeled albumin is injected intravenously. Blood is withdrawn every 5 minutes for five samples. The radioactivity is counted and compared with what would be considered normal. A lower amount of the radioactivity in the sample indicates a higher plasma volume. The hematocrit is then used to derive the red cell volume. The total blood volume is obtained by adding the plasma volume and the red cell volume.
Related Tests
Hematocrit (p. 277). This is a measure of the volume of RBCs in the blood. This measurement is inversely related to the amount of intravascular fluid, given that the RBCs are normal in size.
WBC Scan (Inflammatory Scan)
Indications
This scan is used to identify and localize occult inflammation or infection. It is used for patients who have a fever of unknown origin, suspected osteomyelitis, or inflammatory bowel disease. It is used to indicate whether or not an abnormal mass (e.g., a pancreatic pseudocyst) is infected.
Test Explanation
This test is based on the fact that WBCs are attracted to areas of infection or inflammation. When the patient has a suspected infection or inflammation, yet the site cannot be localized, the injection of radiolabeled WBCs may identify and localize that area of inflammation or infection. Appropriate treatment can then be performed. This is especially helpful in patients who have a fever of unknown origin, suspected occult intraabdominal infection, or suspected (yet radiographically unapparent) osteomyelitis. The scan can differentiate infectious from noninfectious processes. Areas of noninfectious inflammation (e.g., inflammatory bowel disease) also take up the radiolabeled WBCs.
This scan requires drawing about 40 to 50 mL of blood from the patient, separating out the WBCs, labeling the WBCs with technetium or indium, and reinjecting them into the patient. Four to 24 hours later, imaging of the whole body may show an area of increased radioactivity suggestive of accumulation of the radiolabeled WBCs in an area of infection or inflammation.
The imaging procedure is performed by a trained technologist in approximately 30 minutes. A physician trained in nuclear medicine interprets the results. The only discomfort associated with this procedure is the intravenous (IV) injection of the radionuclide.
Procedure and Patient Care
During
• Note the following procedural steps:
1. Approximately 40 to 50 mL of blood is withdrawn from the patient, and the WBCs are extracted from the rest of the blood cells. This is usually done by centrifugation. With leukopenia, the WBC count is so low that separating them out from the other blood cellular components would be very difficult. In these instances, donor WBCs are used instead of autologous WBCs. Donor WBCs are also used for human immunodeficiency virus (HIV) positive patients to minimize the risk to laboratory workers.
2. The WBCs are suspended in saline and tagged with technetium-99m (99mTc) or indium-111 (111In) lipid-soluble product.
3. The tagged WBCs are reinjected into the patient.
4. In 4, 24, and 48 hours after injection, a gamma camera is placed over the body.
5. The patient is placed in supine, lateral, and prone positions so that all surfaces of the body can be visualized.
6. The radionuclide image is recorded digitally on a computer monitor and on film.
Test Results and Clinical Significance
Infection (abscess, osteomyelitis, or poststernotomy infections): The WBCs are localized to the area of infection and show up as increased radionuclear uptake (hot spot).
Inflammation (e.g., inflammatory bowel disease, arthritis): Like infection, areas of noninfectious inflammation attract the radiolabeled WBCs.
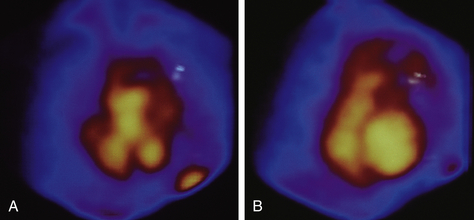
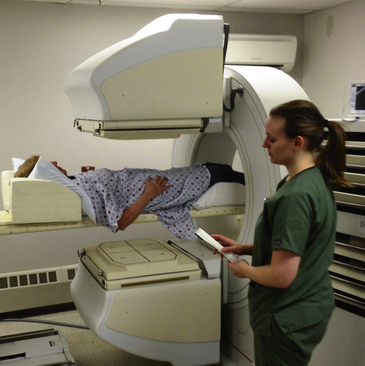
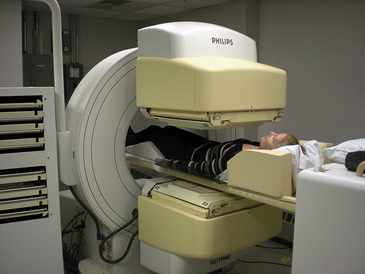
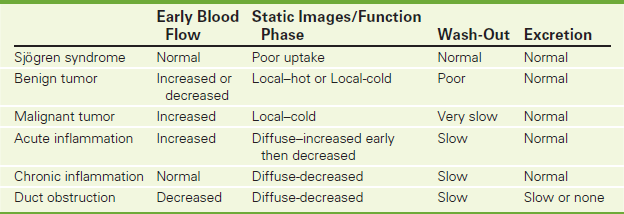
 Increased Testicular Blood Flow
Increased Testicular Blood Flow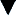 Decreased Testicular Blood Flow
Decreased Testicular Blood Flow