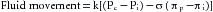8 The Microcirculation and Lymphatics
1. Describe the regulation of regional blood flow by the arterioles.
2. Enumerate the physical and chemical factors that affect the microvessels.
3. Explain the roles of diffusion, filtration, and pinocytosis in transcapillary exchange.
4. Describe the balance between hydrostatic and osmotic forces under normal and abnormal conditions.
The entire circulatory system is geared to supply the body tissues with blood in amounts that are commensurate with their requirements for O2 and nutrients. The system also operates to remove CO2 and other waste products for excretion by the lungs and kidneys. The exchange of gases, water, and solutes between the vascular and interstitial fluid (ISF) compartments occurs mainly across the capillaries. These vessels consist of a single layer of endothelial cells. The arterioles, capillaries, and venules constitute the microcirculation, and blood flow through the microcirculation is regulated by the arterioles, which are also known as the resistance vessels (see Chapter 9). The large arteries serve solely as blood conduits, whereas the veins serve as storage or capacitance vessels as well as blood conduits.
Functional Anatomy
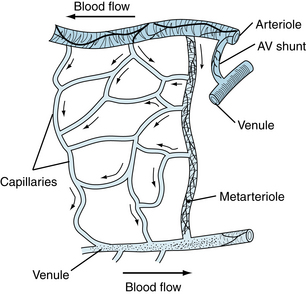
Arterioles are the Stopcocks of the Circulation
The arterioles, which range in diameter from about 5 to 100 µm, have a thick smooth muscle layer, a thin adventitial layer, and an endothelial lining (see Figure 1-2). The arterioles give rise directly to the capillaries (5 to 10 µm in diameter) or in some tissues to metarterioles (10 to 20 µm in diameter), which then give rise to capillaries (Figure 8-1). The metarterioles can serve either as thoroughfare channels to the venules, which bypass the capillary bed, or as conduits to supply the capillary bed. There are often cross-connections between the arterioles and venules as well as in the capillary network. Arterioles that give rise directly to capillaries regulate flow through their cognate capillaries by constriction or dilation. The capillaries form an interconnecting network of tubes of different lengths, with an average length of 0.5 to 1 mm.
Capillaries Permit the Exchange of Water, Solutes, and Gases
Capillary distribution varies from tissue to tissue. In metabolically active tissues, such as cardiac and skeletal muscle and glandular structures, capillaries are numerous. In less active tissues, such as subcutaneous tissue or cartilage, capillary density is low. Also, all capillaries do not have the same diameter. It is necessary for the cells to become temporarily deformed in their passage through these capillaries, because some capillaries have diameters less than those of the erythrocytes. Fortunately, normal red blood cells are quite flexible, and they readily change their shape to conform to that of the small capillaries.
Blood flow in the capillaries is not uniform; it depends chiefly on the contractile state of the arterioles. The average velocity of blood flow in the capillaries is approximately 1 mm per second; however, it can vary from zero to several millimeters per second in the same vessel within a brief period. Such changes in capillary blood flow may be random or they may show rhythmical oscillatory behavior of different frequencies. This behavior is caused by contraction and relaxation (vasomotion) of the precapillary vessels. The vasomotion is partially an intrinsic contractile behavior of the vascular smooth muscle, and it is independent of external input. Furthermore, changes in transmural pressure (intravascular minus extravascular pressure) influence the contractile state of the precapillary vessels. An increase in transmural pressure, whether produced by an increase in venous pressure or by dilation of arterioles, results in contraction of the terminal arterioles at the points of origin of the capillaries. Conversely, a decrease in transmural pressure elicits precapillary vessel relaxation (see myogenic response, Chapter 9).
Reduction of transmural pressure relaxes the terminal arterioles. However, blood flow through the capillaries cannot increase if the reduction in intravascular pressure is caused by severe constriction of the parent vasculature. Large arterioles and metarterioles also exhibit vasomotion. However, in the contraction phase, they usually do not completely occlude the lumen of the vessel and arrest blood flow as may occur when the terminal arterioles contract (Figure 8-2). Thus, flow rate may be altered by contraction and relaxation of small arteries, arterioles, and metarterioles.
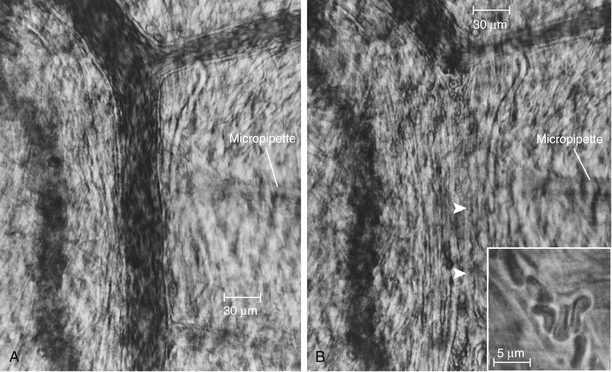
FIGURE 8-2 Arterioles of a hamster cheek pouch before (A) and after (B) injection of norepinephrine. Note the complete closure of the arteriole between the arrowheads and the narrowing of a branch arteriole at the upper right. Inset, Capillary with red blood cells during a period of complete closure of the feeding arteriole. Scale in A and B, 30 µm; in inset, 5 µm.
(Courtesy David N. Damon.)
Because blood flow through the capillaries provides for exchange of gases and solutes between the blood and tissues, the flow has been termed nutritional flow. Conversely, blood flow that bypasses the capillaries in traveling from the arterial to the venous side of the circulation has been termed nonnutritional, or shunt, flow (see Figure 8-1). In some areas of the body (e.g., fingertips and ears), true arteriovenous shunts exist (see Figure 12-1). However, in many tissues, such as muscle, evidence of anatomic shunts is lacking. Nevertheless, nonnutritional flow can occur, and the behavior has been termed physiological shunting of blood flow. This shunting is the result of a greater flow of blood through previously open capillaries, along with either no change or an increase in the number of closed capillaries. In tissues that have metarterioles, shunt flow may be continuous from the arterioles to the venules during low metabolic activity, at which time many precapillary vessels are closed. When metabolic activity rises in such tissues and more precapillary vessels open, blood passing through the metarterioles is readily available for capillary perfusion.
The true capillaries are devoid of smooth muscle and are therefore incapable of active constriction. Nevertheless, the endothelial cells that form the capillary wall contain actin and myosin, and they can alter shape in response to certain chemical stimuli. There is no evidence, however, that changes in endothelial cell shape regulate blood flow through the capillaries. Hence, changes in capillary diameter are passive and are caused by alterations in precapillary and postcapillary resistance.
The Law of Laplace Explains How Capillaries Can Withstand High Intravascular Pressures
The law of Laplace is illustrated in the following comparison of wall tension in a capillary with that in the aorta (Table 8-1). The Laplace equation is:
TABLE 8-1 Vessel Wall Tension in the Aorta and a Capillary
| AORTA | CAPILLARY | |
|---|---|---|
| r (radius) | 1.5 cm | 5 × 10−4 cm |
| h (height of Hg column) | 10 cm Hg | 2.5 cm Hg |
| ρ (density of Hg) | 13.6 g/cm3 | 13.6 g/cm3 |
| g (gravitational acceleration) | 980 cm/s2 | 980 cm/s2 |
| P (pressure) | 10 × 13.6 × 980 = 1.33 × 105 dyne/cm2 | 2.5 × 13.6 x 980 = 3.33 × 104 dyne/cm2 |
| w (wall thickness) | 0.2 cm | 1 × 10−4 cm |
| T = Pr | (1.33 × 105) (1.5) = 2 × 105 dyne/cm | (3.33 × 104) (5 × 10−4) = 16.7 dyne/cm |
| σ (wall stress) = Pr/w | 2 × 105/0.2 = 1 × 106 dyne/cm2 | 16.7/1 × 10−4 = 1.67 × 105 dyne/cm2 |
where T is tension in the vessel wall, ΔP is transmural pressure difference (internal minus external), and r is radius of the vessel.
Wall tension is the force per unit length tangential to the vessel wall. This tension opposes the distending force (ΔPr) that tends to pull apart a theoretical longitudinal slit in the vessel (Figure 8-3). Transmural pressure is essentially equal to intraluminal pressure, because extravascular pressure is usually negligible. The Laplace equation applies to very thin-walled vessels, such as capillaries. Wall thickness must be taken into consideration when the equation is applied to thick-walled vessels such as the aorta. This is done by dividing ΔPr (pressure × radius) by wall thickness (w). The equation now becomes:
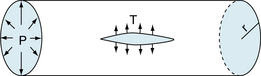
FIGURE 8-3 Diagram of a small blood vessel to illustrate the law of Laplace. T = ΔPr, where ΔP is transmural pressure difference, r is radius of the vessel, and T is wall tension as the force per unit length tangential to the vessel wall, tending to pull apart a theoretical longitudinal slit in the vessel.
Pressure in mm Hg (height of an Hg column) is converted to dynes per square centimeter, according to the following equation:
where h is the height of an Hg column in centimeters, ρ is the density of Hg in g/cm3, g is gravitational acceleration in cm/s2; and (σ)wall stress is force per unit area.
Thus, at normal aortic and capillary pressures, the wall tension of the aorta is about 12,000 times greater than that of the capillary (see Table 8-1). In a person who is standing quietly, capillary pressure in the feet may reach 100 mm Hg. Under such conditions, capillary wall tension increases to 66.5 dynes/cm, a value that is still only one three-thousandths that of the wall tension in the aorta at the same internal pressure. However, σ (wall stress), which takes wall thickness into consideration, is only about tenfold greater in the aorta than in the capillary.
In addition to explaining the ability of capillaries to withstand large internal pressures, the preceding calculations also show that in dilated vessels, wall stress increases even when internal pressure remains constant.
The diameter of the resistance vessels is determined by the balance between the contractile force of the vascular smooth muscle and the distending force produced by the intraluminal pressure. The greater the contractile activity of the vascular smooth muscle of an arteriole, the smaller is its diameter, until the small arterioles are completely occluded. This occlusion is caused by infolding of the endothelium and the consequent trapping of the cells in the vessel. With progressive reduction in the intravascular pressure, vessel diameter decreases, as does tension in the vessel wall. This constitutes the law of Laplace.
The Endothelium Plays an Active Role in Regulating the Microcirculation
For many years, the endothelium was considered to be an inert, single layer of cells that served solely as a passive filter that (1) permitted water and small molecules to pass across the blood vessel wall and (2) retained blood cells and large molecules (proteins) within the vascular compartment. However, the endothelium is now recognized as a source of substances that elicit contraction and relaxation of the vascular smooth muscle (see Figures 8-4 and 9-4).
FIGURE 8-4 Endothelially and nonendothelially mediated vasodilation. Prostacyclin (PGI2) is formed from arachidonic acid (AA) by the action of cyclooxygenase (Cyc-Ox.) and prostacyclin synthase (PGI2 Syn.) in the endothelium and elicits relaxation of the adjacent vascular smooth muscle via increases in cyclic adenosine monophosphate (cAMP). Stimulation of the endothelial cells with acetylcholine (ACh) or other agents (see text) results in the formation and release of an endothelium-derived relaxing factor (EDRF) identified as nitric oxide (NO). The NO stimulates guanylyl cyclase (G Cyc.) to increase cyclic guanosine monophosphate (cGMP) in the vascular smooth muscle to cause relaxation. The vasodilator agent nitroprusside (NP) acts directly on the vascular smooth muscle. Substances such as adenosine, hydrogen ions (H+), CO2, and potassium ions (K+) can arise in the parenchymal tissue and elicit vasodilation by direct action on the vascular smooth muscle (see p. 182). ADP, adenosine diphosphate; L-arg., l-arginine.
As shown in Figure 8-4, prostacyclin (prostaglandin I2, PGI2) can relax vascular smooth muscle via an increase in the cyclic adenosine monophosphate (cAMP) concentration. Prostacyclin is formed in the endothelium from arachidonic acid, and it may be released by the shear stress caused by the pulsatile blood flow. Prostacyclin formation is catalyzed by the enzyme prostacyclin synthase. The primary function of PGI2 is to inhibit platelet adherence to the endothelium and platelet aggregation, thus preventing intravascular clot formation.
CLINICAL BOX
Syphilitic aortic aneurysm (rare because syphilis is now less common) and abdominal aneurysm (caused by atherosclerotic degeneration of the aortic wall) are associated with murmurs caused by the turbulence in the dilated segment of the aorta. The diseased part of the aorta is also under severe stress because of its larger radius and thinner wall. Unless treated, the aneurysm can rupture and cause sudden death. Treatment consists of resection of the aneurysm and replacement with a synthetic polyester fiber (Dacron) graft.
Of far greater importance in endothelially mediated vascular dilation is the formation and release of the endothelium-derived relaxing factor (EDRF) (see Figure 8-4), which has been identified as nitric oxide (NO). Stimulation of the endothelial cells in vivo, in isolated arteries, or in culture by acetylcholine or several other agents (such as adenosine triphosphate [ATP], adenosine diphosphate [ADP], bradykinin, serotonin, substance P, and histamine) produce and release NO. In blood vessels whose endothelium has been removed, these agents do not elicit vasodilation; some, including acetylcholine and ATP, can cause constriction. The NO (synthesized from l-arginine) activates guanylyl cyclase in the vascular smooth muscle. This process raises the cyclic guanosine monophosphate (cGMP) concentration and increases the activity of cGMP-dependent protein kinase (PKG), which in turn activates myosin light-chain phosphatase (MLCP). Myosin light-chain phosphatase induces relaxation by reducing the concentration of phosphorylated myosin regulatory light-chain subunits (MLC20) in vascular smooth muscle (see Figure 9-2). NO release can be stimulated by the shear stress of blood flow on the endothelium, but the physiological role of NO in the local regulation of blood flow remains to be elucidated. The drug nitroprusside also increases cGMP, causing vasodilation. Nitroprusside acts directly on the vascular smooth muscle; its action is not endothelially mediated (see Figure 8-4). Vasodilator agents, such as adenosine, H+, CO2, and K+, may be released from parenchymal tissue and act locally on the resistance vessels (see Figure 8-4).
The endothelium can also synthesize endothelin, a very potent vasoconstrictor peptide (see Figure 9-4A). Endothelin can affect vascular tone and blood pressure in humans, and it may be involved in such pathological states as atherosclerosis, pulmonary hypertension, congestive heart failure, and renal failure.
The Endothelium is at the Center of Flow-Initiated Mechanotransduction
Blood vessels are continuously subjected to cyclic changes of blood pressure and flow. Endothelial cells that form the inner lining of blood vessels are linked with the glycocalyx on their luminal surfaces (see Figure 8-7) and with the basement membrane on their abluminal surfaces (Figure 8-5). These interfaces are important signaling sites for transduction of the mechanical force (shear stress) imparted by the blood, into a signal for the regulation of endothelial cell function. The glycocalyx (composed of proteoglycans and glycoproteins) can extend up to 0.5 µm from the surfaces of endothelial cells. The fibrous network of the glycocalyx serves as a filter at the vessel wall in addition to that of endothelial cells. Also, the glycocalyx serves as a mechanotransducer of shear stress signals to the plasma membrane and cortical cytoskeletons of endothelial cells. For example, shear stress causes flow-mediated release of vasodilators including NO and PGI2 (see Figure 8-4 and Chapter 9). On the one hand, the ability of endothelial cells to release vasodilators is impeded when glycosaminoglycans in the glycocalyx are degraded enzymatically. On the other hand, when flow is laminar, the synthesis of glycocalyx components is increased, becoming a counterforce that sustains the ability of endothelial cells to sense and react to flow patterns.
The basement membrane also functions in mechanotransduction. Normally, the basement membrane underlying endothelial cells is rich in collagen and laminin. Integrins, a family of cell adhesion receptors, are found in the endothelial cell, where they anchor to the cytoskeleton and intracellular signaling molecules. In addition, integrins bind to collagen (α2β1, α1β1) and laminin (α6β1, α6β4) found in the extracellular matrix of the basement membrane. The result of this binding is an endothelial cell phenotype that is slow to proliferate. Injury, such as that produced by turbulent flow, promotes the deposition of fibronectin and fibrinogen in the extracellular matrix, where they bind integrins (α5β1, αvβ3). Thus, by having fibronectin and fibrinogen present to bind other integrins, the endothelial cell expresses a phenotype that proliferates and migrates. The change of matrix structure and composition can trigger a reaction cascade that changes endothelial cell function to initiate inflammation and, eventually, atherosclerosis. Thus, the balance between atheroprotective and atherogenic forces on the endothelial cell can be changed by a transition from laminar to turbulent flow.
The Endothelium Plays a Passive Role in Transcapillary Exchange
Solvent and solute move across the capillary endothelial wall by three processes: diffusion, filtration, and pinocytosis. The permeability of the capillary endothelial membrane is not the same in all body tissues. For example, liver capillaries are quite permeable, and albumin escapes from them at a rate several times greater than that from the less permeable muscle capillaries. Also, permeability is not uniform along the whole capillary; the venous ends are more permeable than the arterial ends, and permeability is greatest in the venules. The greater permeability at the venous ends of the capillaries and in the venules is attributed to the greater number of pores in these regions of the microvessels.
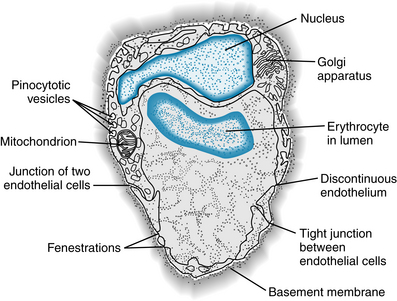
The sites at which filtration occurs have been a controversial subject for many years. Water flows through the capillary endothelial cell membranes through water-selective channels called aquaporins, a large family of intrinsic membrane proteins (28 to 30 kDa) that function as water channels. Each of these pore-forming proteins consists of six transmembrane segments that form a monomer; this structure is incorporated into membranes as homotetramers. The aquaporins are permeable to H2O and other substances (glycerol, urea, Cl−) having diameters of 3.4Å (0.34 nm) or less. Water also flows through apertures (pores) in the endothelial walls of the capillaries (Figures 8-5 and 8-6). Calculations based on the transcapillary movement of small molecules have led to the prediction of capillary pores with diameters of about 4 nm in skeletal and cardiac muscle. In agreement with this prediction, electron microscopy has revealed clefts between adjacent endothelial cells with gaps of about 4 nm (see Figures 8-5 and 8-6). The clefts (pores) are sparse and represent only about 0.02% of the capillary surface area. In cerebral capillaries, there is a blood-brain barrier to many small molecules.
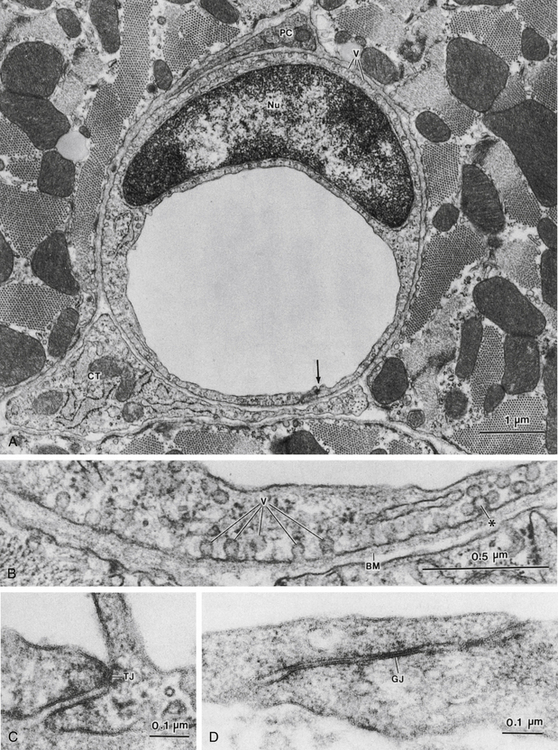
FIGURE 8-6 A, Cross-sectioned capillary in a mouse ventricular wall. The luminal diameter is approximately 4 µm. In this thin section, the capillary wall is formed by a single endothelial cell (Nu, endothelial nucleus), which forms a functional complex (arrow) with itself. The thin pericapillary space is occupied by a pericyte (PC) and a connective tissue (CT) cell (“fibroblast”). Note the numerous endothelial vesicles (V). B, Detail of the endothelial cell in panel A, showing plasmalemmal vesicles (V) attached to the endothelial cell surface. These vesicles are especially prominent in vascular endothelium and are involved in transport of substances across the blood vessel wall. Note the complex alveolar vesicle (∗). BM, basement membrane. C, Junctional complex in a capillary of mouse heart. “Tight” junctions (TJs) typically form in these small blood vessels and appear to consist of fusions between apposed endothelial cell surface membranes. D, Interendothelial junction in a muscular artery of monkey papillary muscle. Although tight junctions similar to those in capillaries are found in these large blood vessels, extensive junctions that resemble gap junctions in the intercalated disks between myocardial cells often appear in arterial endothelium (example shown at GJ).
In addition to clefts, some of the more porous capillaries (e.g., in kidney and intestine) contain fenestrations (see Figure 8-5) that are 20 to 100 nm wide, whereas in other sites (e.g., in the liver) the endothelium is discontinuous (see Figure 8-5). Fenestrations and discontinuous endothelium permit passage of molecules that are too large to pass through the intercellular clefts of the endothelium.
In pathological states such as with tissue inflammation, the enhanced permeability of the endothelium of the venules may be mainly attributed to transcellular pores that develop within the endothelial cells, and not to opening of the interendothelial cell pores.
Diffusion Is the Most Important Means of Water and Solute Transfer Across the Endothelium
Under normal conditions, only about 0.06 mL of water per minute moves back and forth across the capillary wall per 100 g of tissue. The fluid movement is a result of filtration and absorption. However, about 300 mL of water diffuses per minute per 100 g of tissue. The difference is 5000-fold.
When filtration and diffusion are related to blood flow, about 2% of the plasma passing through the capillaries is filtered. In contrast, the diffusion of water is 40 times greater than the rate at which it is brought to the capillaries by blood flow. The transcapillary exchange of solutes is also governed primarily by diffusion. Thus, diffusion is the key factor in promoting the exchange of gases, substrates, and waste products between the capillaries and the tissue cells. However, the net transfer of fluid across the capillary and venule endothelium is achieved mainly by filtration and absorption.
The process of diffusion is described by Fick’s law, as follows:
where J is the quantity of a substance moved per unit time (t), D is the free diffusion coefficient for a particular molecule (the value is inversely related to the square root of the molecular weight), A is the cross-sectional area of the diffusion pathway, and dc/dx is the concentration gradient of the solute.
Fick’s law is also expressed as follows:
where P is the capillary permeability of the substance, S is capillary surface area, Ci is the concentration of the substance inside the capillary, and Co is the concentration of the substance outside the capillary. Hence the PS product provides a convenient expression of available capillary surface, because permeability is rarely altered under physiologic conditions.
Diffusion of Lipid-Insoluble Molecules Is Restricted to the Pores
The mean pore size can be calculated by measurement of the diffusion rate of an uncharged molecule whose free diffusion coefficient is known. Movement of solutes across the endothelium is complex. The movement involves (1) corrections for attraction between solute and solvent molecules, (2) interactions between solute molecules and pore configuration, and (3) the charge on the molecules relative to the charge on the endothelial cells. Solute movement is not simply a question of random thermal movements of molecules down a concentration gradient.
When the movement of solutes is compared with the movement of water across the intact endothelium, solutes exhibit some degree of restriction based on molecular size. Molecules larger than about 60,000 molecular weight (MW) do not penetrate the endothelium, whereas those smaller than 60,000 MW penetrate at a rate that is inversely proportional to their size. This filtering effect appears to be due to the restrictive size of the pore and to a fiber matrix within it. However, the glycocalyx, a 0.5-µm layer lining the luminal side of the endothelium, may also serve as a molecular filter. The glycocalyx is shown in Figure 8-7, where it appears as a clear area between the luminal endothelial membrane and the red blood cell that was moving through the capillary.
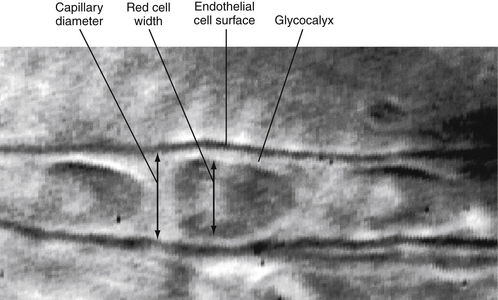
FIGURE 8-7 A single capillary in a hamster cheek pouch showing the glycocalyx. Micrograph was taken during normal blood flow.
(Courtesy of Charmaine Henry.)
For small molecules, such as water, NaCl, urea, and glucose, the capillary pores offer little restriction to diffusion (the reflection coefficient is low). Diffusion is so rapid that the mean concentration gradient across the capillary endothelium is extremely small (see Figure 8-8). The only limit to the net movement of small molecules across the capillary wall is the rate at which blood flow transports the molecules to the capillaries (flow limited).
When transport across the capillary is flow limited, the concentration of a small solute molecule in the blood reaches equilibrium with its concentration in the interstitial fluid (ISF) near the origin of the capillary from the cognate arteriole. If an inert small molecule tracer is infused intra-arterially, its concentration falls to negligible levels near the arterial end of the capillary (Figure 8-8A). If the flow is large, the small molecule tracer will be detectable farther downstream in the capillary. A somewhat larger molecule moves farther along the capillary before it reaches an insignificant concentration in the blood. The number of still larger molecules that enter the arterial end of the capillary and that cannot pass through the capillary pores is the same as the number that leave the venous end of the capillary (Figure 8-8A).
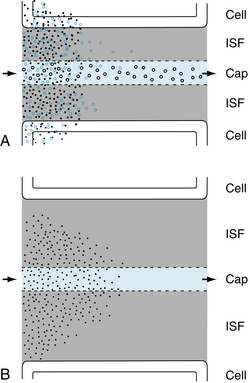
FIGURE 8-8 Flow- and diffusion-limited transport from capillaries (Cap) to tissue. A, Flow-limited transport. The smallest water-soluble inert tracer particles (black dots) reach negligible concentrations after passing only a short distance down the capillary. Larger particles (blue circles) with similar properties travel farther along the capillary before reaching insignificant intracapillary concentrations. Both substances cross the interstitial fluid (ISF) and reach the parenchymal tissue (cell). Because of their size, more of the smaller particles are taken up by the tissue cells. The largest particles (black circles) cannot penetrate the capillary pores and hence do not escape from the capillary lumen except by pinocytotic vesicle transport. An increase in either the volume of blood flow or capillary density increases tissue supply for the diffusible solutes. Note that capillary permeability is greater at the venous end of the capillary (and especially in the venule, not shown) because of the larger number of pores in this region. B, Diffusion-limited transport. When the distance between the capillaries and the parenchymal tissue is large as a result of edema or low capillary density, diffusion becomes a limiting factor in the transport of solutes from capillary to tissue, even at high rates of capillary blood flow.
Diffusion of large molecules across the capillaries becomes a limiting factor (diffusion limited). In other words, capillary permeability to a large molecule solute limits its transport across the capillary wall (Figure 8-8A). The diffusion of small, insoluble lipid molecules is so rapid that diffusion becomes limiting in blood-tissue exchange, when the distances between the capillaries and parenchymal cells are large (e.g., in the presence of tissue edema, or when capillary density is very low; see Figure 8-8B).
Lipid-Soluble Molecules Pass Directly Through the Lipid Membranes of the Endothelium and the Pores
Lipid-soluble molecules move very rapidly between blood and tissue. The degree of lipid solubility (oil-to-water partition coefficient) provides a good index of the ease of transfer of lipid molecules through the endothelium.
O2 and CO2 are both lipid soluble, and they pass readily through the endothelial cells. The O2 supply of normal tissue at rest and during activity is not limited by diffusion or by the number of open capillaries, as indicated by calculations based on (1) the diffusion coefficient for O2, (2) the capillary density and diffusion distances, (3) the blood flow, and (4) the tissue O2 consumption.
Measurements of Po2 and the saturation of blood in the microvessels indicate that, in many tissues, O2 saturation at the entrance of the capillaries has already decreased to about 80% as a result of diffusion of O2 from arterioles and small arteries. Also, CO2 loading and the resultant intravascular shifts in the oxyhemoglobin dissociation curve occur in the precapillary vessels. Hence direct flux of O2 and CO2 occurs between adjacent arterioles, between venules, and possibly between arteries and veins (countercurrent exchange), in addition to gas exchange at the level of the capillaries. This countercurrent exchange of gas represents a diffusional shunt of gas around the capillaries, and at low blood flow rates the supply of O2 to the tissue may be limited.
Capillary Filtration Is Regulated by the Hydrostatic and Osmotic Forces Across the Endothelium
The direction and magnitude of the movement of water across the capillary wall are determined by the algebraic sum of the hydrostatic and osmotic pressures that exist across the membrane. An increase in intracapillary hydrostatic pressure favors movement of fluid from the vessel to the interstitial space. Conversely, an increase in the concentration of osmotically active particles within the vessels favors movement of fluid into the vessels from the interstitial space.
Hydrostatic Forces
The hydrostatic pressure (blood pressure) within the capillaries is not constant, and the forces depend on the arterial pressure, the venous pressure, and the precapillary and postcapillary vessel resistances. An increase in small artery and arterial pressure or in venous pressure elevates capillary hydrostatic pressure, whereas a reduction in each of these pressures has the opposite effect. An increase in arteriolar resistance or closure of arteries reduces capillary pressure, whereas greater resistance in the venules and veins increases the capillary pressure.
Hydrostatic Pressure is the Principal Force in Capillary Filtration
Changes in the venous resistance affect capillary hydrostatic pressure more than do changes in arteriolar resistance. A given change in venous pressure produces a greater effect on the capillary hydrostatic pressure than does the same change in arterial pressure, and about 80% of an increase in venous pressure is transmitted back to the capillaries.
Despite the fact that capillary hydrostatic pressure (Pc) varies from tissue to tissue (even within the same tissue), average values, obtained from many direct measurements in human skin, are about 32 mm Hg at the arterial end of the capillaries and 15 mm Hg at the venous end of the capillaries at the level of the heart (Figure 8-9). When a person stands, the hydrostatic pressure is higher in the legs and lower in the head.
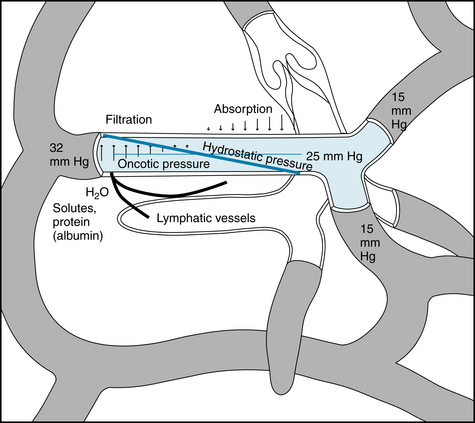
FIGURE 8-9 Schematic representation of the factors responsible for filtration and absorption across the capillary wall as well as the formation of lymph.
Tissue pressure, or more specifically ISF pressure (Pi) outside the capillaries, opposes capillary filtration. It is Pc − Pi that constitutes the hydrostatic driving force for filtration. In the normal (nonedematous) state of the subcutaneous tissue, Pi is close to zero or slightly negative (−1 to −4 mm Hg). Hence Pc represents essentially the hydrostatic driving force.
Osmotic Forces
The key factor that restrains fluid loss from the capillaries is the osmotic pressure of the plasma proteins—usually termed colloid osmotic pressure or oncotic pressure (πp). The total osmotic pressure of plasma is about 6000 mm Hg, whereas the oncotic pressure is only about 25 mm Hg. However, this small oncotic pressure plays an important role in fluid exchange across the capillary wall, because the plasma proteins are essentially confined to the intravascular space. Consequently, the electrolytes that are responsible for the major fraction of plasma osmotic pressure are practically equal in concentration on the two sides of the capillary endothelium. The relative permeability of solute to water influences the actual magnitude of the osmotic pressure. The reflection coefficient (σ) is the relative impediment to the passage of a substance through the capillary wall. The reflection coefficient of water is 0, and that of albumin, to which the endothelium is almost impermeable, is 1. Filterable solutes have reflection coefficients between 0 and 1. Also, different tissues have different reflection coefficients for the same molecule. Therefore, movement of a given solute across the endothelial wall varies with the tissue. The true oncotic pressure (π) is defined by
where σ is the reflection coefficient, R is gas constant, T is absolute temperature, and Ci and Co are solute (albumin) concentrations, respectively, inside and outside the capillary.
Of the plasma proteins, albumin predominates in determining oncotic pressure. The average albumin molecule (69,000 MW) is approximately half the size of the average globulin molecule (150,000 MW). The albumin molecule is present in almost twice the concentration as the globulins (4.5 versus 2.5 g per dL of plasma). Albumin also exerts a greater osmotic force than can be accounted for solely on the basis of the number of molecules dissolved in the plasma. Therefore, it cannot be completely replaced by inert substances of appropriate molecular size, such as dextran. This additional osmotic force becomes disproportionately greater at high concentrations of albumin (as in plasma), and it is weak to absent in dilute solutions of albumin (as in ISF).
The reason for this behavior of albumin is its negative charge at normal blood pH and the attraction and retention of cations (principally Na+) in the vascular compartment (the Gibbs-Donnan effect). Furthermore, albumin binds a small number of Cl− ions, a change that increases its negative charge and, hence, its ability to retain more Na+ inside the capillaries. The small increase in electrolyte concentration of the plasma over that of the ISF produced by the negatively charged albumin enhances its osmotic force to that of an ideal solution containing a solute of 37,000 MW. If albumin did indeed have a molecular weight of 37,000, it would not be retained by the capillary endothelium, because of its small size. Hence, it could not function as a counterforce to capillary hydrostatic pressure. If, however, albumin did not have an enhanced osmotic force, it would require a concentration of about 12 g of albumin per dL of plasma to achieve a plasma oncotic pressure of 25 mm Hg. Such a high albumin concentration would greatly increase blood viscosity as well as the resistance to blood flow through the vascular system.
Some albumin escapes from the capillaries and enters the ISF, where it exerts an osmotic force of up to about 30% of plasma oncotic pressure. This observation of a higher ISF oncotic pressure than previously thought prompted the conclusion that filtration occurs in diminishing amounts along the entire length of the capillary in skin and skeletal muscle during steady-state conditions. Abrupt reductions in capillary hydrostatic pressure can cause transient reabsorption of fluid from the ISF compartment. However, plasma proteins continue to leak out of the capillaries, and this leakage plus the removal of water from the ISF compartment by the lymphatics concentrates the ISF proteins and changes absorption to filtration.
Balance of Hydrostatic and Osmotic Forces
The relationship between hydrostatic pressure and oncotic pressure, and the role of these forces in regulating fluid passage across the capillary endothelium, were expounded by Starling in 1896. This explanation constituted the Starling hypothesis and is expressed by the equation:
where Qf is fluid movement across the capillary wall, Pc is capillary hydrostatic pressure, Pi is ISF hydrostatic pressure, πp is plasma oncotic pressure, πi is ISF oncotic pressure, k is the filtration constant for capillary membrane, and σ is reflection coefficient.
Filtration occurs when the algebraic sum is positive; absorption occurs when it is negative. Originally, it was proposed that filtration occurs at the arterial end of the capillary and that absorption occurs at the venous end, because of the gradient of hydrostatic pressure along the capillary. This may apply for the idealized capillary, as depicted in Figure 8-9, but direct observations have revealed that many capillaries show only filtration, whereas others show only absorption. In some vascular beds (e.g., the renal glomerulus) hydrostatic pressure in the capillary is high enough to result in filtration along the entire length of the capillary. In other vascular beds (e.g., the intestinal mucosa), the hydrostatic and oncotic forces allow absorption along the whole capillary.
As discussed previously, capillary pressure is variable and depends on several factors. The principal factor relates to the contractile state of the precapillary vessels. In the normal steady-state, arterial pressure, venous pressure, postcapillary resistance, ISF hydrostatic and oncotic pressures, and plasma oncotic pressure are relatively constant. A change in precapillary resistance appears to be the determining factor with respect to fluid movement across the vascular wall. The hydrostatic and osmotic forces are nearly in equilibrium along the entire capillary because water moves so quickly across the capillary endothelium. Hence, filtration and absorption in the normal state occur at very small degrees of pressure imbalance across the capillary wall. Only a small percentage (about 2%) of the plasma flowing through the vascular system is filtered. Some is reabsorbed and the rest is returned to the circulating blood via the lymphatic system.
In the lungs, the mean capillary hydrostatic pressure is only 8 to 10 mm Hg, and the plasma oncotic pressure is 25 mm Hg. Also, the lung ISF pressure is approximately 15 mm Hg, and the oncotic pressure of the interstitial fluid is 16 to 20 mg Hg. Thus, the net force favors reabsorption, yet pulmonary lymph is formed, and it consists of fluid that is osmotically drawn out of the capillaries by the plasma protein that escapes through the capillary endothelium.
The Capillary Filtration Coefficient Provides a Method to Estimate the Rate of Fluid Movement Across the Endothelium
The rate of fluid movement (Qf) across the capillary membrane depends not only on the algebraic sum of the hydrostatic and osmotic forces across the endothelium (ΔP), but also on the area of the capillary wall available for filtration (Am), the distance across the capillary wall (Δx), the viscosity of the filtrate (η), and the filtration constant of the membrane (k). These factors may be expressed by the equation:
The dimensions are units of flow per unit of pressure gradient across the capillary wall per unit of capillary surface area. This expression, which describes the flow of fluid through a porous membrane, is essentially Poiseuille’s law for flow through tubes (see p. 125).
In pathological conditions such as left ventricular failure or stenosis of the mitral valve, pulmonary capillary hydrostatic pressure may exceed plasma oncotic pressure. When it does, pulmonary edema, a condition that seriously interferes with gas exchange in the lungs, may occur.
Because the thickness of the capillary wall and the viscosity of the filtrate are relatively constant, they can be included in the filtration constant, k. If the area of the capillary membrane is not known, the rate of filtration can be expressed per unit weight of tissue. Hence the equation can be simplified to:
where kt is the capillary filtration coefficient for a given tissue and the units for Qf are milliliters per minute per 100 g of tissue per millimeter of mercury pressure.
In any given tissue the filtration coefficient per unit area of capillary surface, and hence capillary permeability, is not changed by different physiological conditions, such as arteriolar dilation and capillary distention, or by such adverse conditions as hypoxia, hypercapnia, and reduced pH.
With capillary injury (toxins, severe burns), capillary permeability increases, as indicated by the filtration coefficient, and significant amounts of fluid and protein leak out of the capillaries into the interstitial space. The escaped protein enhances the oncotic pressure of the interstitial fluid, leading to additional fluid loss and dehydration. One of the important therapeutic measures in the treatment of extensive burns is replacement of fluid and plasma proteins.
The filtration coefficient can be used to determine the relative number of open capillaries (total capillary surface area available for filtration in tissue) because capillary permeability is constant under normal conditions. For example, increased metabolic activity of contracting skeletal muscle induces relaxation of precapillary resistance vessels with the opening of more capillaries (capillary recruitment), resulting in an increased filtering surface area.
Disturbances in Hydrostatic-Osmotic Balance
Changes in arterial pressure per se may have little effect on filtration, because the change in pressure may be countered by adjustments of the precapillary resistance vessels (autoregulation; see p. 179), so that hydrostatic pressure in the open capillaries remains the same.
With severe reduction in arterial pressure, as may occur in hemorrhage, there may be arteriolar constriction mediated by the sympathetic nervous system and a fall in venous pressure resulting from the blood loss. These changes lead to a decrease in capillary hydrostatic pressure. Furthermore, the low blood pressure in hemorrhage causes a decrease in blood flow (and hence O2 supply) to the tissue, with the result that vasodilator metabolites accumulate and induce relaxation of arterioles. Precapillary vessel relaxation is also engendered by the reduced transmural pressure (autoregulation, see p. 179). As a consequence of these several factors, absorption predominates over filtration and occurs at a larger capillary surface area. This process is one of the compensatory mechanisms employed by the body to restore blood volume (p. 272).
An increase in venous pressure alone, as occurs in the feet when one changes from the lying to the standing position, would elevate capillary pressure and enhance filtration. However, the increase in transmural pressure causes precapillary vessel closure (myogenic mechanism; see p. 179) so that the capillary filtration coefficient actually decreases. This reduction in capillary surface available for filtration protects against the extravasation of large amounts of fluid into the interstitial space (edema).
With prolonged standing, particularly when associated with some elevation of venous pressure in the legs (such as that caused by pregnancy) or with sustained rises in venous pressure (as seen in congestive heart failure), filtration is greatly enhanced and exceeds the capacity of the lymphatic system to remove the capillary filtrate from the interstitial space (see also Figure 10-25).
A large amount of fluid can move across the capillary wall in a relatively short time. In a normal individual, the filtration coefficient (kt) for the whole body is about 0.0061 mL/min/100 g of tissue/mm Hg. In a 70-kg man, a venous pressure elevation of 10 mm Hg for 10 minutes would increase filtration from capillaries by 342 mL. This would not lead to edema formation, because the fluid is returned to the vascular compartment by the lymphatic vessels. When edema develops, it usually appears in the dependent parts of the body, where the hydrostatic pressure is greatest. However, its location and magnitude are also determined by the type of tissue. Loose tissues, such as the subcutaneous tissue around the eyes or the scrotum, are more prone to collect larger quantities of interstitial fluid than are firm tissues, such as muscle, or encapsulated structures, such as the kidney.
The vascular endothelial growth factors (VEGFs), of which there are at least five, induce angiogenesis. They also elicit vasodilation and, as described originally, increase the endothelial permeability. By causing vasodilation, VEGF permits perfusion of more capillaries, an effect that can be misinterpreted as altered permeability. Nevertheless, in vitro and in vivo studies show VEGF increases capillary permeability. Some of the increased permeability results from opening of tight junctions between endothelial cells and from formation of fenestrations in them. These properties of the VEGFs must be considered in the evaluation of the exciting prospects of VEGFs in promoting angiogenesis in poorly perfused myocardium.
Pinocytosis Enables Large Molecules to Cross the Endothelium
Some transfer of substances across the capillary wall can occur in tiny pinocytotic vesicles (pinocytosis). These vesicles (see Figures 8-5 and 8-6) are formed by a pinching off of the surface membrane. Substances taken up on one side of the capillary wall move by thermal kinetic energy across the endothelial cell and deposit their contents at the other side. The amount of material that can be transported in this way is very small. However, pinocytosis may be responsible for the movement of large lipid-insoluble molecules (30 nm) between blood and interstitial fluid. The number of pinocytotic vesicles in endothelium varies with the tissue (muscle > lung > brain) and increases from the arterial to the venous end of the capillary.
The concentration of the plasma proteins may change in different pathological states and thus alter the osmotic force and movement of fluid across the capillary membrane. The plasma protein concentration is increased in dehydration (e.g., water deprivation, prolonged sweating, severe vomiting, and diarrhea), and water moves by osmotic forces from the tissues to the vascular compartment. In contrast, the plasma protein concentration is reduced in nephrosis (a renal disease in which there is loss of protein in the urine), and edema may occur.
The Lymphatics Return the Fluid and Solutes that Escape through the Endothelium to the Circulating Blood
The terminal vessels of the lymphatic system consist of a widely distributed closed-end network of highly permeable lymph capillaries that resemble blood capillaries. However, they often lack tight junctions between endothelial cells, and they possess fine filaments that anchor them to the surrounding connective tissue. These fine strands distort the lymphatic vessel to open spaces between the endothelial cells and to permit the entrance of protein and large particles that are present in the interstitial fluid. The lymph capillaries drain into larger vessels that finally enter the right and left subclavian veins at their junctions with the respective internal jugular veins. Only cartilage, bone, epithelium, and tissues of the central nervous system are devoid of lymphatic vessels. The plasma capillary filtrate is returned to the circulation by virtue of tissue pressure, facilitated by intermittent skeletal muscle activity, contractions of the lymphatic vessels, and an extensive system of one-way valves. In this respect, they resemble the veins, although even the larger lymphatic vessels have thinner walls than do the corresponding veins, and they contain only a small amount of elastic tissue and smooth muscle.
When either the volume of interstitial fluid exceeds the drainage capacity of the lymphatics or the lymphatic vessels become blocked, as may occur in certain disease states such as elephantiasis (caused by filariasis, a worm infestation), interstitial fluid accumulates (edema) chiefly in the more compliant tissues (e.g., subcutaneous tissue).
The volume of fluid transported through the lymphatics in 24 hours is about equal to an animal’s total plasma volume. The proteins returned by the lymphatics to the blood in a day are about one fourth to one half of the circulating plasma proteins. This is the only means by which protein (albumin) that leaves the vascular compartment can be returned to the blood, because back-diffusion into the capillaries cannot occur against the large albumin concentration gradient. If the protein was not removed by the lymph vessels, it would accumulate in the interstitial fluid. It would then act as an oncotic force to draw fluid from the blood capillaries to produce edema. In addition to returning fluid and protein to the vascular bed, the lymphatic system filters the lymph at the lymph nodes and removes foreign particles such as bacteria. The largest lymphatic vessel, the thoracic duct, in addition to draining the lower extremities, returns protein lost through the permeable liver capillaries. Substances absorbed from the gastrointestinal tract, principally fat in the form of chylomicrons, are delivered to the circulating blood.
Lymph flow varies considerably, being almost nil from resting skeletal muscle, and increasing during exercise in proportion to the degree of muscular activity. It is increased by any mechanism that enhances the rate of blood capillary filtration—for example, increased capillary pressure or permeability or decreased plasma oncotic pressure.
Summary
 Blood flow through the capillaries is chiefly regulated by contraction and relaxation of the arterioles (resistance vessels).
Blood flow through the capillaries is chiefly regulated by contraction and relaxation of the arterioles (resistance vessels).
 The capillaries, which consist of a single layer of endothelial cells, can withstand high transmural pressure by virtue of their small diameter. According to the law of Laplace, T (wall tension) = ΔP (transmural pressure difference) × r (radius of the capillary).
The capillaries, which consist of a single layer of endothelial cells, can withstand high transmural pressure by virtue of their small diameter. According to the law of Laplace, T (wall tension) = ΔP (transmural pressure difference) × r (radius of the capillary).
 The endothelium is the source of an endothelium-derived relaxing factor (EDRF), identified as nitric oxide (NO), and of prostacyclin, both of which relax vascular smooth muscles.
The endothelium is the source of an endothelium-derived relaxing factor (EDRF), identified as nitric oxide (NO), and of prostacyclin, both of which relax vascular smooth muscles.
 Mechanical forces that act on the glycocalyx and on the basement membrane are transduced into signals that modify gene expression of endothelial cells whose morphology and function are changed.
Mechanical forces that act on the glycocalyx and on the basement membrane are transduced into signals that modify gene expression of endothelial cells whose morphology and function are changed.
 Movement of water and small solutes between the vascular and interstitial fluid compartments occurs through capillary pores mainly by diffusion but also by filtration and absorption.
Movement of water and small solutes between the vascular and interstitial fluid compartments occurs through capillary pores mainly by diffusion but also by filtration and absorption.
 Exchange of small lipid-insoluble molecules is flow limited because the rate of diffusion is about 40 times greater than the blood flow in the tissue. The larger the molecule, the slower is its diffusion. Large lipid-insoluble molecules are diffusion limited. Molecules larger than about 70,000 MW are essentially confined to the vascular compartment.
Exchange of small lipid-insoluble molecules is flow limited because the rate of diffusion is about 40 times greater than the blood flow in the tissue. The larger the molecule, the slower is its diffusion. Large lipid-insoluble molecules are diffusion limited. Molecules larger than about 70,000 MW are essentially confined to the vascular compartment.
 Lipid-soluble substances such as CO2 and O2 pass directly through the lipid membranes of the capillary, and the ease of transfer is directly proportional to the degree of lipid solubility of the substance.
Lipid-soluble substances such as CO2 and O2 pass directly through the lipid membranes of the capillary, and the ease of transfer is directly proportional to the degree of lipid solubility of the substance.
 Capillary filtration and absorption are described by the Starling equation:
Capillary filtration and absorption are described by the Starling equation:
where Pc is capillary hydrostatic pressure, Pi is interstitial fluid hydrostatic pressure, πi is interstitial fluid oncotic pressure, πp is plasma oncotic pressure, i is capillary membrane filtration constant and σ is the reflection coefficient. Filtration occurs when the algebraic sum is positive; absorption occurs when it is negative.
 Large molecules can move across the capillary wall in vesicles formed from the lipid membrane of the capillaries by a process called pinocytosis.
Large molecules can move across the capillary wall in vesicles formed from the lipid membrane of the capillaries by a process called pinocytosis.
 Fluid and protein that have escaped from the blood capillaries enter the lymphatic capillaries and are transported via the lymphatic system back to the blood vascular compartment.
Fluid and protein that have escaped from the blood capillaries enter the lymphatic capillaries and are transported via the lymphatic system back to the blood vascular compartment.
Bates D.O. Vascular endothelial growth factors and vascular permeability. Cardiovasc Res. 2010;87:262.
Bendayan M. Morphological and cytochemical aspects of capillary permeability. Microsc Res Tech. 2002;57:327.
Boardman K.C., Swartz M.A. Interstitial flow as a guide for lymphangiogenesis. Circ Res. 2003;92:801.
Chiu J.-J., Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91:327.
Gomes D., Agasse A., Thiébaud P., et al. Aquaporins are multifunctional water and solute transporters highly divergent in living organisms. Biochim Biophys Acta. 2009;1788:1213.
Michel C.C. Microvascular permeability, ultrafiltration, and restricted diffusion. Am J Physiol. 2004;287:H1887.
Michel C.C., Neal C.R. Openings through endothelial cells associated with increased microvascular permeability. Microcirculation. 1999;6:45.
Miserocchi G., Negrini D., Passi A., De Luca G. Development of lung edema: Interstitial fluid dynamics and molecular structure. News Physiol Sci. 2001;16:66.
Schmid-Schonbein G.W. The second valve system in lymphatics. Lymphat Res Biol. 2003;1:25.
Starling E.H. On the absorption of fluids from the connective tissue spaces. J Physiol. 1896;19:312.
Swartz M.A. The physiology of the lymphatic system. Adv Drug Deliv Rev. 2001;50:3.
Tarbell J.M., Weinbaum S., Kamm R.D. Cellular fluid mechanics and mechanotransduction. Annals Biomed Eng. 2005;33:1719.
Tzima E., Irani-Tehrani, Kiosses W.B., et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426.
Welsh D.G., Segal S.S. Endothelial and smooth muscle cell conduction in arterioles controlling blood flow. Am J Physiol. 1998;274:H178.
Xia J., Duling B.R. Patterns of excitation-contraction coupling in arterioles: Dependence on time and concentration. Am J Physiol. 1998;274:H323.
CASE 8-1
History
A 45-year-old man with a long history of alcoholism (averaging a liter of whiskey per day) was admitted to the hospital as an emergency because of vomiting of blood and fainting. In the past few months, he noted progressive anorexia, fatigue, jaundice, generalized itching, and abdominal swelling. Physical examination showed a semicomatose man with pallor, jaundice, and ascites. Blood pressure was 90 mm Hg systolic/40 mm Hg diastolic, heart rate was 100 beats/min, and hematocrit was 35%. Liver function tests indicated severe liver damage. The diagnosis was advanced cirrhosis of the liver. Immediate treatment was transfusion with three units of blood. The following pressures were noted:
Questions
1. The transcapillary pressure responsible for the ascites was (assume the filtration coefficient is 1.0 and the reflection coefficient is 0.7):
2. After lost blood was replaced by transfusion, the treatment for the patient’s condition was:
3. The substance mainly responsible for the oncotic pressure of the patient’s plasma was:
CASE 8-2
History
A 25-year-old man suffered third-degree burns over the upper three quarters of his body in a fire in his home. Several hours elapsed before he reached the hospital. On admission, he was in a shocklike state. Blood pressure was 90 mm Hg systolic/70 mm Hg diastolic, heart rate was 110 mm Hg, and hematocrit was 55%. Blood analysis revealed a sodium level of 145 mEq/L, a potassium level of 4 mEq/L, a chloride level of 105 mEq/L, and an albumin level of 3.5 g/dL.
Questions
1. The most effective treatment is an intravenous infusion of:
2. After several months of treatment with extensive artificial skin grafts, the patient was able to walk and to resume an almost normal life. After prolonged standing, he noted slight ankle swelling but no ecchymoses in his feet. Capillaries in his feet did not rupture when he stood because:
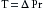 (1)
(1)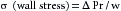 (2)
(2)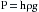 (3)
(3)
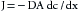 (4)
(4)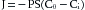 (5)
(5)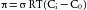 (6)
(6)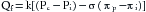 (7)
(7)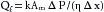 (8)
(8)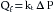 (9)
(9)