12 Special Circulations
1. Describe the regulation of cutaneous blood flow and its role in maintaining a constant body temperature.
2. Indicate the relative importance of the local and neural factors in adjustments of skeletal muscle blood flow at rest and during exercise.
3. Describe the regulation of cerebral blood flow.
4. Explain the regulation of the pulmonary circulation.
5. Describe the characteristics of the renal circulation.
6. Relate the intestinal and hepatic components of the splanchnic circulation.
7. Explain the changes in the fetal circulation that occur at birth.
Previous chapters describe how the heart and vessels function to provide the tissues of the body with O2 and nutrients and how CO2 and waste products are removed. This chapter on special circulations is included because the circulations of the various body organs differ to some degree with respect to their function and regulation.
Cutaneous Circulation
The O2 and nutrient requirements of the skin are relatively small. Unlike most other body tissues, the supply of these essential materials is not the chief governing factor in the regulation of cutaneous blood flow. The primary function of the cutaneous circulation is to maintain a constant body temperature. Consequently, the skin shows wide fluctuations in blood flow, depending on the need for loss or conservation of body heat. Mechanisms responsible for alterations in skin blood flow are primarily activated by changes in ambient and internal body temperatures.
Skin Blood Flow Is Regulated Mainly by the Sympathetic Nervous System
There are essentially two types of resistance vessels in skin: arterioles and arteriovenous (AV) anastomoses. The arterioles are similar to those found elsewhere in the body. AV anastomoses shunt blood from the arterioles to the venules and venous plexuses; hence they bypass the capillary bed. The anastomoses are found primarily in the fingertips, palms of the hands, toes, soles of the feet, ears, nose, and lips. The anastomoses differ morphologically from the arterioles, in that they are either short, straight vessels, or long, coiled vessels about 20 to 40 µm in lumen diameter. The thick muscular walls are richly supplied with nerve fibers (Figure 12-1). These vessels are almost exclusively under sympathetic neural control, and they become maximally dilated when their nerve supply is interrupted.
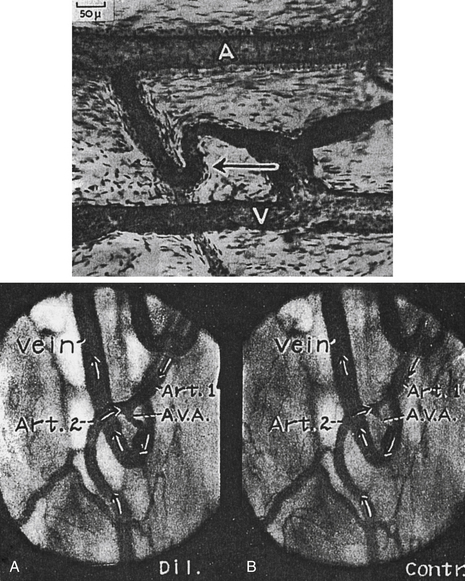
FIGURE 12-1 Top, Arteriovenous (AV) anastomosis in the human ear injected with Berlin blue. The walls of the AV anastomosis in the fingertips are thicker and more cellular. Arrow points to AV anastomosis. A, Artery; V, Vein. Bottom, Two frames from a motion picture record of the same relatively large AV anastomosis (A.V.A.) in a stable rabbit ear chamber installed 3.5 months previously. On this day the lumen of the A.V.A. measured 51 µm dilated and 5 µm contracted at its narrowest point. A, A.V.A. dilated; B, A.V.A. contracted. Art., artery.
(Top illustration from Prichard MML, Daniel PM: Arteriovenous anastomoses in the human external ear. J Anat 90:309, 1956; bottom illustration from Clark ER, Clark EL: Am J Anat 54:229, 1934.)
Conversely, reflex stimulation of the sympathetic fibers to these vessels may cause them to constrict to the point of obliteration of the vascular lumen. Although AV anastomoses do not exhibit basal tone (tonic activity of the vascular smooth muscle that is independent of innervation), they are highly sensitive to vasoconstrictor agents such as epinephrine and norepinephrine. Furthermore, AV anastomoses do not appear to be under metabolic control, and they fail to show reactive hyperemia or autoregulation of blood flow.Thus the regulation of blood flow through anastomotic channels is governed principally by the nervous system, in response to reflex activation by temperature receptors or by higher centers of the central nervous system.
In contrast to AV anastomoses, the resistance vessels in the skin exhibit some basal tone. These resistance vessels are under dual control of the sympathetic nervous system and local regulatory factors, in much the same manner as are resistance vessels in other vascular beds. However, in the case of skin, neural control plays a more important role than local factors. Stimulation of sympathetic nerve fibers to skin blood vessels (arteries, veins, and arterioles) induces vasoconstriction, and severance of the sympathetic nerves induces vasodilation. With chronic denervation of the cutaneous blood vessels, the degree of tone that existed before denervation is gradually regained over a period of several weeks. This is accomplished by an enhancement of basal tone that compensates for the degree of tone previously contributed by sympathetic nerve fiber activity. Epinephrine and norepinephrine elicit only vasoconstriction in cutaneous vessels. Denervation of the skin vessels enhances sensitivity to circulating catecholamines (denervation hypersensitivity).
Parasympathetic vasodilator nerve fibers do not innervate the cutaneous blood vessels. However, stimulation of the sweat glands, which are innervated by cholinergic fibers of the sympathetic nervous system, dilates the resistance vessels in the skin. Sweat contains an enzyme that acts on a protein moiety in the tissue fluid to produce bradykinin, a polypeptide with potent vasodilator properties. Bradykinin, formed in the tissue, acts locally to dilate the arterioles and increase blood flow to the skin.
The skin vessels in certain regions, particularly the head, neck, shoulders, and upper chest, are influenced by higher centers of the central nervous system. Blushing, in response to embarrassment or anger, and blanching, as with fear or anxiety, are examples of cerebral inhibition and stimulation, respectively, of the sympathetic nerve fibers to the affected regions.
In contrast to AV anastomoses in the skin, the cutaneous resistance vessels show autoregulation of blood flow and reactive hyperemia. If the arterial inflow to a limb is stopped with an inflated blood pressure cuff for a brief period, the skin becomes very red below the point of vascular occlusion when the cuff is deflated. This increased cutaneous blood flow (reactive hyperemia) is also manifested by distention of the superficial veins in the erythematous extremity. Autoregulation of blood flow in the skin is best explained by a myogenic mechanism (see p. 179).
Ambient Temperature and Body Temperature Play Important Roles in the Regulation of Skin Blood Flow
The primary function of the skin is to preserve the internal milieu and to protect it from adverse changes in the environment. The vasculature of the skin is chiefly influenced by environmental temperature because ambient temperature is a very important external variable with which the body must contend. Exposure to cold elicits a generalized cutaneous vasoconstriction that is most pronounced in the hands and feet. This response is principally mediated by the nervous system. Arrest of the circulation to a hand with a pressure cuff, and immersion of that hand in cold water, results in vasoconstriction in the skin of the other extremities that are exposed to room temperature. With the circulation to the chilled hand intact, the reflex vasoconstriction is caused in part by the cooled blood that returns to the general circulation. This cooling stimulates the temperature-regulating center in the anterior hypothalamus. Direct cooling of this region of the brain produces cutaneous vasoconstriction.
The skin vessels of the cooled hand also show a direct response to cold. Moderate cooling or exposure for brief periods to severe cold (0° C to 15° C) causes constriction of the resistance and capacitance vessels, including AV anastomoses. However, prolonged exposure of the hand to severe cold has a secondary vasodilator effect. Prompt vasoconstriction and severe pain are elicited by immersion of the hand in water near 0° C, but the responses are soon followed by dilation of the skin vessels, reddening of the immersed part, and alleviation of the pain. With continued immersion of the hand, alternating periods of constriction and dilation occur, but the skin temperature rarely drops as low as it did in response to the initial vasoconstriction.
Prolonged severe cold causes tissue damage. The rosy faces of people working or playing in a cold environment are examples of cold vasodilation. However, the blood flow through the skin of the face may be greatly reduced, despite the flushed appearance. The red color of the slowly flowing blood is in large measure the result of reduced O2 uptake by the cold skin and by the cold-induced shift to the left of the oxyhemoglobin dissociation curve (see Figure 1-5).
Direct application of heat to the skin produces not only local vasodilation of resistance and capacitance vessels and AV anastomoses but also reflex dilation in other parts of the body. The local effect is independent of the vascular nerve supply, whereas the reflex vasodilation is a combination of anterior hypothalamic stimulation by the returning warmed blood and of stimulation of the receptors in the heated part. However, evidence of a reflex from peripheral temperature receptors is not as definitive for warm stimulation as it is for cold stimulation.
The close proximity of the major arteries and veins to each other permits considerable heat exchange (countercurrent) between artery and vein. Cold blood that flows toward the heart in veins from a cooled hand takes up heat from adjacent arteries. This results in warming of the venous blood and cooling of the arterial blood. Heat exchange is in the opposite direction with exposure of the extremity to heat. Thus, heat conservation is enhanced and heat gain is minimized during exposure of extremities to cold and warm environments, respectively.
The fingers (and sometimes the toes) of some individuals are very sensitive to cold. When exposed to cold, the arteries and arterioles to the hands constrict, producing ischemia of the fingers characterized by blanching of the skin, which is associated with tingling, numbness, and pain. The blanching is followed by cyanosis and later by redness as the arterial spasm subsides. This condition, called Raynaud disease, is of unknown cause and occurs most frequently in young women.
Skin Color Depends on the Volume and Flow of Blood in the Skin and on the Amount of O2 Bound to Hemoglobin
The color of the skin is determined in large part by pigment, in all but very dark skin. The degree of pallor or ruddiness is mainly a function of the amount of blood in the skin. With little blood in the venous plexus, the skin appears pale. However, with moderate to large quantities of blood in the venous plexus, the skin displays color. Whether this color is bright red, blue, or some shade between is determined by the degree of oxygenation of the blood in the subcutaneous vessels. As examples, a combination of vasoconstriction and reduced hemoglobin can produce an ashen gray color of the skin, and a combination of venous engorgement and reduced hemoglobin can produce a dark purple hue. Skin color provides little information about the rate of cutaneous blood flow. Rapid blood flow and pale skin may coexist when the AV anastomoses are open, and slow blood flow and red skin when the extremity is exposed to cold.
Skeletal Muscle Circulation
The rate of blood flow in skeletal muscle varies directly with the contractile activity of the tissue and the type of muscle. Blood flow and capillary density in red, slow-twitch, highly oxidative muscles are greater than in white, fast-twitch, slowly oxidative muscle. In resting muscle, the precapillary arterioles exhibit asynchronous, intermittent contractions and relaxations. Hence, at any given moment, a large fraction of the capillary bed is not perfused. Consequently, total blood flow through quiescent skeletal muscle is low (1.4 to 4.5 mL/min/100 g). During exercise, the resistance vessels relax and muscle blood flow may increase manyfold (up to about 20 times the resting level); the magnitude of the increase depends largely on the severity of the exercise.
Regulation of Skeletal Muscle Circulation
As with all tissues, physical factors such as arterial pressure, tissue pressure, and blood viscosity influence muscle blood flow. However, another physical factor operates during exercise; that is, the squeezing effect of the active muscle on the vessels. When the contractions are intermittent, inflow is restricted, and venous outflow is enhanced during each brief contraction (Figure 12-2). The presence of the venous valves prevents backflow of blood in the veins between contractions, thus aiding the forward propulsion of the blood (see also p. 218). With strong, sustained contractions, the vascular bed can be compressed to the point at which blood flow actually ceases temporarily.
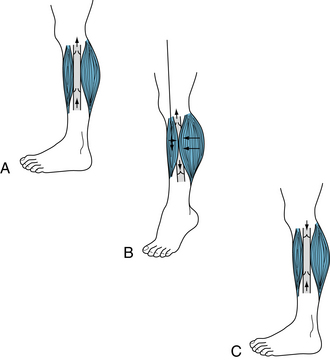
FIGURE 12-2 Action of the muscle pump in venous return from the legs. A, Standing at rest, the venous valves are open and blood flows upward toward the heart by virtue of the pressure generated by the heart and transmitted through the capillaries to the veins from the arterial side of the vascular system (vis a tergo). B, Contraction of the muscle compresses the vein so that the increased pressure in the vein drives blood toward the thorax through the upper valve and closes the lower valve in the uncompressed segment of the vein just below the point of muscular compression. C, Immediately after muscle relaxation, the pressure in the previously compressed venous segment falls, and the reversed pressure gradient causes the upper valve to close. The valve below the previously compressed segment opens because pressure below it exceeds that above it, and the segment fills with blood from the foot. As blood flow continues from the foot, the pressure in the previously compressed segment rises. When it exceeds the pressure above the upper valve, this valve opens, and continuous flow occurs as in A.
Neural Factors
Although the resistance vessels of muscle possess a high degree of basal tone, they also display tone attributable to continuous low-frequency activity in the sympathetic vasoconstrictor nerve fibers. The basal frequency of firing in the sympathetic vasoconstrictor fibers is quite low (about 1 to 2 per second), and maximal vasoconstriction is observed at frequencies as low as 8 to 10 per second. Stimulation of the sympathetic nerve fibers to skeletal muscle elicits vasoconstriction that is caused by norepinephrine release at the fiber endings. Skeletal muscle vessels have α1-adrenergic receptors (vasoconstriction) and β2-adrenergic receptors (vasodilation). Intraarterial injection of norepinephrine elicits only vasoconstriction (α1-adrenergic receptor activation), whereas low doses of epinephrine produce vasodilation (β2-adrenergic receptor activation) in muscle, and large doses cause vasoconstriction (α1-adrenergic receptor activation). Thus, the type and amount of catecholamine determine the resultant vascular effect (see Chapter 9 and Figure 9-4).
Baroreceptors reflexes greatly influence the tonic activity of the sympathetic nerves. An increase in carotid sinus pressure results in dilation of the muscle vascular bed, and a decrease in carotid sinus pressure elicits vasoconstriction (Figure 12-3). When the existing sympathetic constrictor tone is high, as in the experiment illustrated in Figure 12-3, the decrease in blood flow associated with common carotid artery occlusion is small, but the increase after the release of occlusion is large. The vasodilation produced by baroreceptor stimulation is caused by inhibition of sympathetic vasoconstrictor activity. Because muscle is the major body component on the basis of mass and thereby represents the largest vascular bed, the participation of its resistance vessels in vascular reflexes plays an important role in maintaining a constant arterial blood pressure.
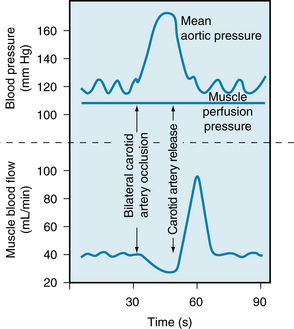
FIGURE 12-3 Evidence for participation of the muscle vascular bed in vasoconstriction and vasodilation mediated by the carotid sinus baroreceptors after common carotid artery occlusion and release. In this preparation the sciatic and femoral nerves constituted the only direct connection between the hind leg muscle mass and the rest of the dog. The muscle was perfused by blood at a constant pressure that was completely independent of the animal’s arterial pressure.
(Redrawn from Jones RD, Berne RM: Vasodilation in skeletal muscle. Am J Physiol 204:461, 1963.)
When the valves of the superficial leg veins are incompetent, as may occur with pregnancy, thrombophlebitis, or obesity, the veins become dilated and tortuous. Such varicose veins can be treated by surgical removal, by injection of sclerosing solutions, or merely by the use of elastic stockings.
A comparison of the vasoconstrictor and vasodilator effects of the sympathetic nerves to blood vessels of muscle and skin is summarized in Figure 12-4. Note the lower basal tone of the skin vessels, their greater constrictor response, and the absence of active cutaneous vasodilation.
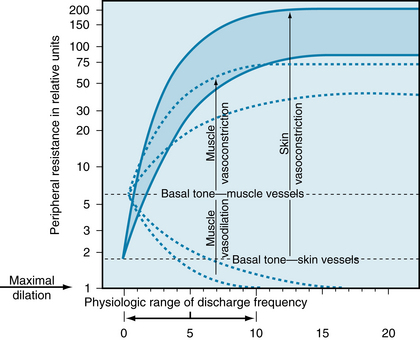
FIGURE 12-4 Basal tone and the range of response of the resistance vessels in muscle (dashed lines) and skin (shaded area) to stimulation and section of the sympathetic nerves. Peripheral resistance is plotted on a logarithmic scale.
(Redrawn from Celander O, Folkow B: A comparison of the sympathetic vasomotor fibre control of the vessels within the skin and the muscles. Acta Physiol Scand 29:241, 1953.)
Local Factors
Whether neural or local factors predominate in the regulation of skeletal muscle blood flow depends on the activity of the muscle. Neural factors predominate in resting muscle, and neurogenic vasoconstrictor tone is superimposed on the nonneural basal tone (see Figure 12-4). This is evidenced by the facts that cutting the sympathetic nerves or administration of α-adrenergic receptor antagonists to muscle abolishes the neural component of vascular tone and unmasks the intrinsic basal tone of the blood vessels. During exercise however, some (but not necessarily all) muscles must contract and perform work. The blood flow to the working skeletal muscles must increase greatly in order to meet their greater metabolic needs. As a consequence of their increased metabolism, the working muscles produce more metabolites, and these metabolic factors, which are vasodilatory, override the intrinsic sympathetic vasoconstriction and allow greatly increased local blood flow. Thus, working, but not nonworking skeletal muscles (which are not producing increased metabolites) receive an increased blood flow. Local factors underlying this vasodilation (see Chapter 9) in skeletal muscle involve the action of metabolites (e.g., H+, K+, prostaglandins, adenosine triphosphate [ATP], CO2, and nitric oxide [NO]). The rhythmic contraction of exercising muscle provides a physical component to the local factors regulating blood flow. In summary, neural and local blood flow–regulating mechanisms oppose each other, and during muscle contraction the local vasodilator mechanism overrides the neural vasoconstrictor mechanism. Nevertheless, tonic sympathetic nerve activity persists during exercise, in part via reflexes initiated at carotid chemoreceptors. This sympathetic nerve activity, which produces vasoconstriction only in the nonworking muscles, limits the extent of metabolite-induced vasodilation and, together with the action of the muscle pump, is essential to sustain mean arterial pressure.
CLINICAL BOX
Disease of the arterial walls can lead to obstruction of the arteries and symptoms, called intermittent claudication when it occurs in the legs. The symptoms consist of leg pain that occurs during walking or climbing stairs and is relieved by rest. The disease, called thromboangiitis obliterans, is seen most frequently in men who are smokers. With minimal walking, the resistance vessels become maximally dilated by local metabolite release; when the O2 demand of the muscles increase with further walking, blood flow cannot increase sufficiently to meet the muscle needs for O2, and pain caused by muscle ischemia results.
Cerebral Circulation
Blood reaches the brain through the internal carotid and vertebral arteries. The latter vessels join to form the basilar artery, which, in conjunction with branches of the internal carotid arteries, forms the circle of Willis. A unique feature of the cerebral circulation is that it all lies within a rigid structure, the cranium. Because intracranial contents are incompressible, any increase in arterial inflow, as with arteriolar dilation, must be associated with a comparable increase in venous outflow. The volume of blood and extravascular fluid varies considerably in most tissues. In the brain, the volume of blood and of extravascular fluid is relatively constant; changes in either of these fluid volumes must be accompanied by a reciprocal change in the other. Unlike the rate of blood flow in most other organs, the rate of total cerebral blood flow is held within a relatively narrow range; in humans it averages 55 mL/min per 100 g of brain.
Local Factors Predominate in the Regulation of Cerebral Blood Flow
At rest, the brain consumes 20% of total body O2 and 25% of total body glucose. Of the various body tissues, the brain is the least tolerant of ischemia. Interruption of cerebral blood flow for as little as 5 seconds results in loss of consciousness. Ischemia that lasts just a few minutes may cause irreversible tissue damage. Fortunately, regulation of the cerebral circulation is primarily under the direction of the brain itself. Local regulatory mechanisms and reflexes that originate in the brain tend to maintain a relatively constant cerebral circulation in the presence of such adverse effects as sympathetic vasomotor nerve activity, circulating humoral vasoactive agents, and changes in arterial blood pressure. Under certain conditions, the brain also regulates its blood flow by initiating changes in systemic blood pressure.
Neural Factors
The extrinsic innervation of cerebral (pial) vessels consists of components of the autonomic nervous system. Cervical sympathetic nerve fibers that accompany the internal carotid and vertebral arteries into the cranial cavity innervate the cerebral vessels. Relative to other vascular beds, sympathetic control of the cerebral vessels is weak and the contractile state of the cerebrovascular smooth muscle depends primarily on local metabolic factors. The density of α1-adrenergic receptors is less than in other vascular beds. The cerebral vessels are not innervated appreciably by sympathetic vasodilator nerves, but the vessels do receive parasympathetic fibers from the facial nerve. These parasympathetic fibers produce a slight vasodilation on stimulation.
Local Factors
Generally, total cerebral blood flow is constant. Cerebral blood flow autoregulation involves interplay among myogenic, metabolic and neural mechanisms much as described for peripheral vessels (see Chapter 9). However, regional cortical blood flow is associated with regional neural activity, an example of intrinsic innervation. For example, movement of one hand results in increased blood flow only in the hand area of the contralateral sensorimotor and premotor cortex. Also, talking, reading, and other stimuli to the cerebral cortex are associated with increased blood flow in the appropriate regions of the contralateral cortex (Figure 12-5). Stimulation of the retina with flashes of light increases blood flow only in the visual cortex. Glucose uptake also corresponds with regional cortical neuronal activity. For example, when the retina is stimulated by light, uptake of 14C-2-deoxyglucose is enhanced in the visual cortex.
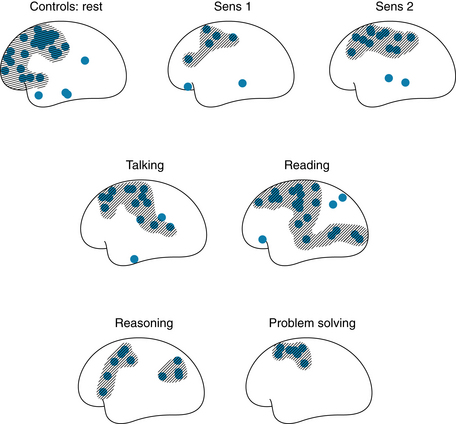
FIGURE 12-5 Effects of different stimuli on regional blood flow in the contralateral human cerebral cortex. Sens 1, low-intensity electrical stimulation of the hand; Sens 2, high-intensity electrical stimulation of the hand (pain).
(Redrawn from Ingvar DH: Functional landscapes of the dominant hemisphere. Brain Res 107:181, 1976.)
Production of vasoactive compounds is thought to link increased neuronal activity to greater uptake of O2 and glucose. Astrocytes participate in this through a phenomenon termed “neurovascular coupling.” At one pole, astrocytes surround presynaptic and postsynaptic neurons at synapses. At the other pole, astrocytes converge upon vascular smooth muscle and endothelial cells of cerebral vessels. When this neurovascular unit is activated by the neurotransmitter glutamate, astrocytes produce inositol triphosphate (IP3), which releases Ca++ that, in turn, activates K+ channels. The released K+ (0.5-10 mM) causes hyperpolarization of smooth muscle by activating the Na-K pump and increasing the conductance of inwardly rectifying K+ channels. The hyperpolarization reduces Ca++ entry in vascular smooth muscle. With respect to K+, stimuli such as hypoxia, electrical stimulation of the brain, and seizures elicit rapid increases in cerebral blood flow, and they are associated with increases in perivascular K+. The increments in K+ are similar to those that produce pial arteriolar dilation when K+ is applied topically to these vessels. When extracellular K+ exceeds 15 mM, smooth muscle cells depolarize, and Ca++ entry is increased to cause contraction and vasoconstriction. Thus, K+ has a dual effect on smooth muscle function that derives from its actions on K+ conductance and the K+ concentration gradient.
The cerebral vessels (pial and parenchymal) are very sensitive to CO2 tension. Increases in arterial blood CO2 tension (Paco2) elicit marked cerebral vasodilation; inhalation of 7% CO2 results in a twofold increment in cerebral blood flow. Similarly, decreases in Paco2, such as those elicited by hyperventilation, produce a decrease in cerebral blood flow. Carbon dioxide evokes changes in arteriolar resistance by altering perivascular pH (and probably also by altering intracellular vascular smooth muscle pH). By changing Paco2 and bicarbonate concentration independently, some researchers have demonstrated that pial vessel diameter and pH (and presumably blood flow) are inversely related, regardless of the level of the Paco2. Acidosis initiates a marked vasodilation of brain arterioles. The vasodilation is mediated by a very localized release of Ca++ from the endoplasmic reticulum (Ca “sparks”). This local Ca++ signal activates large-conductance K+ channels (BKCa); the ensuing hyperpolarization stabilizes the vascular smooth muscle cell and opposes vasoconstriction.
Carbon dioxide can diffuse to the vascular smooth muscle from the brain tissue or from the lumen of the vessels, whereas H+ in the blood is prevented from reaching the arteriolar smooth muscle by the blood-brain barrier. Hence the cerebral vessels dilate when the H+ concentration of the cerebrospinal fluid is increased. However, the vessels show only minimal dilation in response to an increase in the H+ concentration in the arterial blood. Chemical regulation of cerebral blood flow by Paco2 is impaired in human subjects with endothelial dysfunction (diabetes, hypertension); the relative roles of H+ and NO in response to changes in Paco2 are not clear. However, in humans with normal endothelial cell function, inhibition of NO production has no effect on pressure-dependent autoregulation.
Adenosine levels of the brain rise with ischemia, hypoxemia, hypotension, hypocapnia, electrical stimulation of the brain, and induced seizures. Topically applied adenosine is a potent dilator of the pial arterioles. In short, any intervention that either reduces the O2 supply to the brain or increases the O2 requirements of the brain results in rapid (within 5 seconds) formation of adenosine in the cerebral tissue. Unlike pH or K+ concentration, the adenosine concentration of the brain increases with initiation of the stimulus, and it remains elevated throughout the period of O2 imbalance. The adenosine released into the cerebrospinal fluid during conditions associated with inadequate brain O2 supply is available to the brain tissue for reincorporation into cerebral tissue adenine nucleotides.
Three factors—pH, K+, and adenosine—may act in concert to adjust the cerebral blood flow to the metabolic activity of the brain. The cerebral circulation shows reactive hyperemia and excellent autoregulation when the pressures are between about 60 and 160 mm Hg. Mean arterial pressures below 60 mm Hg result in reduced cerebral blood flow and syncope, whereas mean pressures above 160 may lead to increased permeability of the blood-brain barrier and cerebral edema. Autoregulation of cerebral blood flow is abolished by hypercapnia and any other potent vasodilator. None of the candidates for metabolic regulation of cerebral blood flow has been shown to be responsible for this phenomenon. Hence autoregulation of cerebral blood flow is probably attributable to a myogenic mechanism, although experimental proof is still lacking.
Elevation of intracranial pressure results in an increase in systemic blood pressure. This response, called Cushing’ phenomenon, is apparently caused by ischemic stimulation of vasomotor regions in the medulla. It helps maintain cerebral blood flow in such conditions as expanding intracranial tumors.
The Pulmonary and Systemic Circulations are in Series with Each Other
Under steady-state conditions, the total pulmonary and systemic blood flows are virtually identical (see Chapter 10). Despite this similarity in the rate of blood flow, the anatomic, hemodynamic, and physiological characteristics of these two sections of the cardiovascular system differ substantially.
Functional Anatomy
Pulmonary Vasculature
The pulmonary vascular system is a low-resistance network of highly distensible vessels. The main pulmonary artery is much shorter than the aorta. The walls of the pulmonary artery and its branches are much thinner than the walls of the aorta, and they contain less smooth muscle and elastin. Unlike systemic arterioles, which have very thick walls composed mainly of circularly arranged smooth muscle, pulmonary arterioles are thin and contain little smooth muscle. The pulmonary arterioles do not have the same capacity for vasoconstriction as do their counterparts in the systemic circulation. The pulmonary venules and veins are also very thin and possess little smooth muscle.
The pulmonary capillaries differ markedly from the systemic capillaries. Whereas the systemic capillaries constitute an interconnecting network of tubular vessels, the pulmonary capillaries are aligned so that the blood flows in thin sheets between adjacent alveoli (Figure 12-6). Hence the capillary blood is exposed optimally to the alveolar gases. The total surface area for exchange between alveoli and blood is about 50 to 70 m2. Only thin layers of vascular and alveolar endothelium separate the blood and alveolar gas. The thickness of the sheets of blood between adjacent alveoli depends on the intravascular and intraalveolar pressures. Ordinarily, the width of an interalveolar sheet of blood is about equal to the diameter of a red blood cell (see Figure 12-6). During pulmonary vascular congestion, as when the left atrial pressure is elevated, the width of the sheet may increase severalfold. Conversely, when local alveolar pressure exceeds the adjacent capillary pressure, the capillaries may collapse and blood will not flow to those alveoli. Hydrostatic factors participate in this phenomenon, particularly with respect to the regional distribution of blood flow to the lungs, as described in the next section.
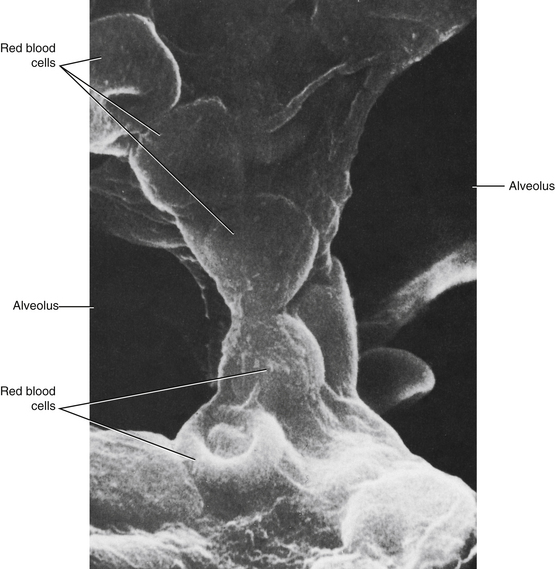
FIGURE 12-6 Scanning electron micrograph of mouse lung to show an interalveolar septum. Note that the membranes that separate an alveolus from a capillary are so thin that the shapes of the erythrocytes in the capillary can easily be discerned.
(From Greenwood MF, Holland P: The mammalian respiratory tract surface. A scanning electron microscopic study. Lab Invest 27:296, 1972.)
Bronchial Vasculature
The bronchial arteries are branches of the thoracic aorta. These arteries and their branches, down to the arterioles, have the structural characteristics of most systemic arteries. Hence they have much thicker walls and more smooth muscles than do the pulmonary arterial vessels of equivalent caliber. The bronchial vessels supply blood to the tracheobronchial tree down to the terminal bronchioles.
CLINICAL BOX
Sustained abridgment of the pulmonary arterial blood supply to a lung, after pulmonary embolism, for example, usually increases the precapillary (arterial) communications between vessels of the systemic and pulmonary circuits. Certain inflammatory and degenerative pulmonary diseases, such as emphysema, are often associated with increased bronchial blood flow, and significant admixtures of blood take place between the two systems.
Pulmonary Hemodynamics
The bronchial veins drain partly into the pulmonary venous system, and partly into the azygos veins, which are a part of the systemic venous system. The bronchial circulation normally constitutes about 1% of the cardiac output. Therefore the fraction of the bronchial blood flow that returns to the left atrium (via the pulmonary veins) rather than to the right atrium (via the azygos veins) constitutes at most 1% of the venous return to the heart. This small quantity of bronchial venous blood, plus a small amount of coronary venous blood that drains directly into the left atrium or left ventricle, “contaminates” the pulmonary venous blood, which is ordinarily fully saturated with O2. Hence, the aortic blood is very slightly desaturated. This small quantity of venous drainage directly into the left side of the heart also accounts for the fact that, under true steady-state conditions, the output of the left ventricle slightly exceeds that of the right ventricle.
CLINICAL BOX
The mean left atrial pressure is an index of the left ventricular filling pressure. To determine whether a patient suffers from left heart failure, it is desirable to measure the left atrial pressure directly, a difficult process. However, a flexible, balloon-tipped catheter can easily be guided into the pulmonary artery. If the catheter is advanced until the tip is wedged into a small branch of the pulmonary artery, the pulmonary artery wedge pressure serves as a useful estimate of the pressure in the left atrium. The wedged catheter halts flow in the small vessels distal to the catheter. These vessels then serve as an extension of the catheter and thereby allow the catheter to communicate with the pulmonary veins and left atrium.
Pressures in the Pulmonary Circulation
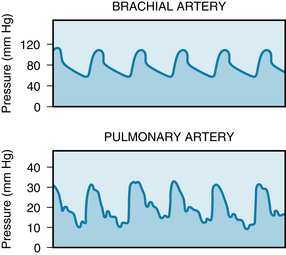
In normal individuals, the average systolic and diastolic pressures in the pulmonary artery are about 25 and 10 mm Hg, respectively, and the mean pressure is about 15 mm Hg (Figure 12-7). These pressures are much lower than those in systemic arteries because the pulmonary vascular resistance is only about one tenth the resistance of the systemic vascular bed. The mean pressure in the left atrium is normally about 5 mm Hg, and so the total pulmonary arteriovenous pressure gradient is only about 10 mm Hg. The mean hydrostatic pressure in the pulmonary capillaries lies between the pulmonary arterial and pulmonary venous values but somewhat closer to the latter.
Pulmonary Blood Flow
Under steady-state conditions the pulmonary and systemic blood flows are equal, except for the small disparity contributed by the bronchial circulation. Gravity affects the regional distribution of blood flow in the lungs because of the low pressures in the pulmonary blood vessels and their great distensibility.
Three distinct flow patterns may be found at different hydrostatic levels in the lung, as illustrated in Figure 12-8. Consider that the pulmonary artery delivers blood at a steady pressure of 15 mm Hg, and that pulmonary venous pressure remains constant at 5 mm Hg. In those pulmonary arterial and venous branches that are 13 cm below (zone C) the hydrostatic level of the main pulmonary vessels, the respective pressures will be 10 mm Hg, which is equivalent to 13 cm of blood greater than those in the main vessels, by virtue of the gravitational effects. Conversely, in pulmonary arterial and venous branches that are 13 cm above (zone A) the main vessels, the respective pressures will be 10 mm Hg less than those in the main vessels. At the same hydrostatic level (zone B) as the main vessels, the respective pressures in the branches will be approximately equal to those in the main vessels.
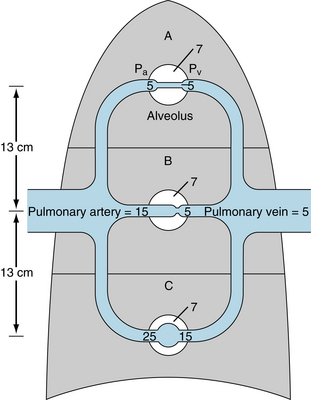
FIGURE 12-8 Schematic representation of the three types of flow regimens that might exist in the pulmonary circulation. In zone A, alveolar pressure exceeds intravascular pressures. Pulmonary capillaries in this zone will not be perfused. In zone B, alveolar pressure is intermediate between pulmonary arterial and venous pressures. Pulmonary capillaries will flutter between the open and closed states. In zone C, intravascular pressures exceed alveolar pressure. The pulmonary capillaries are always open, but the flow resistances in individual vessels vary with the hydrostatic pressure in the vessel. Pa, pulmonary artery pressure; Pv, pulmonary vein pressure; alveolar pressure is 7 mm Hg in all zones. All pressures are in mm Hg.
Consider that the alveolar pressure equals 7 mm Hg in all alveoli. Such an alveolar pressure might exist in an individual who is receiving positive-pressure ventilation. In zone A in Figure 12-8, the alveolar pressure would exceed the local arterial and venous pressures. The pulmonary capillary pressures lie between the pressures of the arteries and veins, and therefore alveolar pressure would also exceed capillary pressure. Capillaries lying between adjacent alveoli would collapse. Those alveoli would not be perfused, and gas would not be exchanged.
Ordinarily, the mean pressure in the alveoli is atmospheric. Therefore the conditions depicted in zone A in Figure 12-8 do not ordinarily prevail in any region of the lungs. In hypovolemic shock, however, the mean pulmonary artery pressure is often very low. Therefore, vascular pressures in the lung apices might be subatmospheric. The atmospheric pressure in the alveoli would then compress the apical capillaries, so that virtually no blood would flow to that zone from the pulmonary circulation. However, the bronchial circulation, which operates at much higher pressures, would be unaffected.
In zone B in Figure 12-8, the alveolar pressure lies between the local arterial and venous pressures. Again, if alveolar pressure equals 7 mm Hg, a capillary in that region will flutter between the open and closed states. When the capillary is open, blood will flow through it, and the capillary pressure will decrease progressively from the arterial to the venous end. The pressure at the venous end will be less than the alveolar pressure, and therefore the capillary will collapse at that end. When flow ceases, the arterial and capillary pressures will equalize at a given hydrostatic level. Thus the capillary pressure will quickly rise to that in the small local arteries, which exceeds the prevailing alveolar pressure. The capillary will then be forced open. With the restitution of flow, however, the pressure will drop along the length of the capillary. As the pressure at the venous end drops below the ambient alveolar pressure, the capillary will again close. Hence the capillary will flutter between the open and closed states.
The critical pressure gradient for flow in zone B is the arterioalveolar pressure difference, not the arteriovenous pressure difference, as it is for most vessels in the body. As long as venous pressure is less than alveolar pressure, venous pressure does not influence the flow. Such a flow condition is called a waterfall effect because the height of a waterfall has no influence on the flow.
In zone C in Figure 12-8, the arterial and venous pressures both exceed the alveolar pressure. Hence the pressure everywhere along the capillary exceeds the alveolar pressure, and the capillary remains permanently open. In this zone, the flow is determined by the arteriovenous pressure gradient, and the resistance may be calculated by the hydraulic analog of Ohm’s law.
The large and small pulmonary vessels, including the capillaries, are very distensible, as previously noted. The pressure difference that determines the caliber of a distensible tube is the transmural pressure; that is, the difference between the internal and external pressures. In an erect individual, the intravascular pressures in the lungs increase from apex to base. The transmural pressures increase accordingly, and the diameter of the pulmonary vessels increases from apex to base. Because resistance to flow varies inversely with vessel caliber, in zone C resistance decreases and flow increases in the apex-to-base direction. Such predicted changes in flow have been verified in humans.
Regulation of the Pulmonary Circulation
The total volume of blood pumped by the heart passes through the pulmonary circulation. Therefore the various cardiac and vascular factors that determine cardiac output in general also determine total pulmonary blood flow. These factors have been discussed in Chapter 10.
The autonomic nervous system innervates the pulmonary blood vessels. Although the small pulmonary vessels contain little smooth muscle, small changes in smooth muscle tone may alter vascular resistance substantially because the pressures in the pulmonary circulation are so low. Baroreceptor stimulation can dilate the pulmonary resistance vessels reflexly. Conversely, peripheral chemoreceptor stimulation constricts the pulmonary vessels; however, the importance of such neural regulation remains to be established.
Hypoxia has the most important influence on pulmonary vasomotor tone. Acute or chronic hypoxia increases pulmonary vascular resistance (Figure 12-9). Regional reductions in alveolar O2 tension constrict the nearby arterioles. This response helps maintain an optimal ventilation-perfusion ratio. The O2 tension in poorly ventilated alveoli approaches the Po2 in the pulmonary arterial blood. Blood flowing by such alveoli is not well oxygenated, and therefore the O2 tension of the blood returning to the left atrium decreases. Arteriolar vasoconstriction reduces the blood flow to poorly ventilated alveoli and thereby reduces the contamination of the pulmonary venous blood with poorly oxygenated blood. Thus this mechanism shunts pulmonary blood flow from the poorly ventilated regions to the better ventilated regions of the lungs and thereby improves the O2 saturation of the systemic arterial blood.
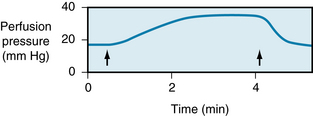
FIGURE 12-9 Effect of hypoxia on vascular resistance of an isolated rat lung. The lung was perfused with blood at a constant flow. When the O2 tension of the inspired air was reduced (between the arrows), the pulmonary resistance vessels constricted, as indicated by the substantial rise in perfusion pressure.
(Modified from Grover RF, Wagner WW Jr, Mcmurtry IF, et al: Pulmonary circulation. In Shepherd JT Abboud FM, editors: Handbook of physiology, section 2: the cardiovascular system-peripheral circulation and organ blood flow, vol III, Bethesda, Md, 1983, American Physiological Society.)
The mechanism by which hypoxia raises pulmonary vascular resistance has received much attention. Isolated pulmonary artery smooth muscle cells have a “sensor” for O2 because they contract in hypoxic conditions. The O2 sensor is not known but is suspected to be mitochondria. The intact pulmonary artery is more sensitive to hypoxia than is the isolated cell. This greater sensitivity depends on the endothelium; the endothelial cells are thought to amplify rather than initiate the vasoconstrictor effect of hypoxia. Electrophysiological studies of pulmonary vascular smooth muscle cells indicate that hypoxia inhibits an outward K+ current. The resulting depolarization of the cell membrane augments the influx of Ca++ into the smooth muscle cells and thereby induces contraction. The mechanism for the contraction involves the calcium-calmodulin system that causes phosphorylation of myosin light chains (see Figure 9-2). These results suggest that in an intact pulmonary blood vessel, hypoxia would lead to vasoconstriction. Chronic hypoxia results in proliferation of vascular smooth muscle, which thickens the blood vessel wall and culminates in pulmonary hypertension. Again, increased Ca++ entry is thought to be involved, because sustained elevation of cytosolic Ca++ activates a signal transduction process to activate genes involved in regulation of cell division.
The Renal Circulation Affects the Cardiac Output
Anatomy
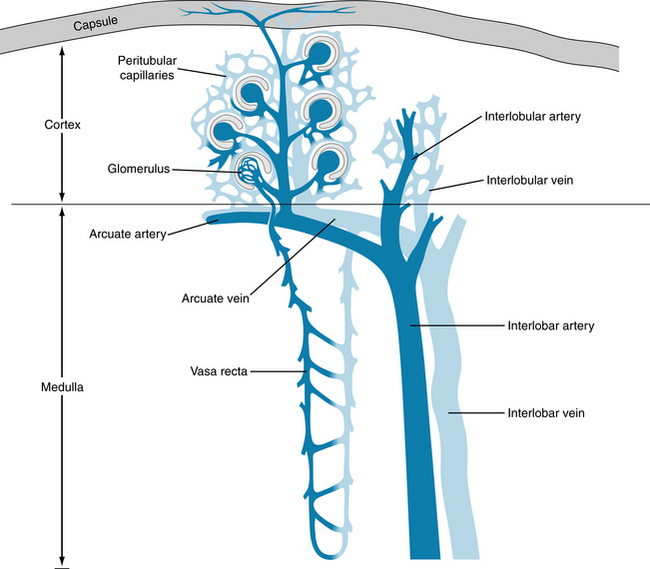
The primary branches of the renal artery divide into a number of interlobar arteries. These interlobar branches (Figure 12-10) proceed radially from the hilus to the corticomedullary junction, which lies between the adjacent medullary pyramids. As an interlobar artery approaches the corticomedullary junction, it branches into a number of arcuate arteries. These travel in various directions over the bases of the adjacent medullary pyramids, in the zone between the cortex and the medulla. The arcuate branches that arise from adjacent interlobar arteries do not interconnect. Hence occlusion of an interlobar artery destroys a pyramid-shaped region of the kidney.
From the arcuate arteries, a number of interlobular branches travel toward the capsular surface of the kidney (see Figure 12-10). The afferent arterioles to the glomeruli are branches of these interlobular arteries. Each human kidney has approximately 1 million glomeruli. The afferent arteriole to each glomerulus divides into several vessels that form discrete capillary loops (Figure 12-11). The proximal and distal limbs of each loop are interconnected by many smaller capillaries. The distal limbs of each capillary loop within a glomerulus rejoin to form the efferent arteriole. The diameter of these arterioles is usually less than that of the afferent arteriole. The entire glomerular capillary tuft is enveloped by Bowman capsule, which collects the glomerular filtrate.
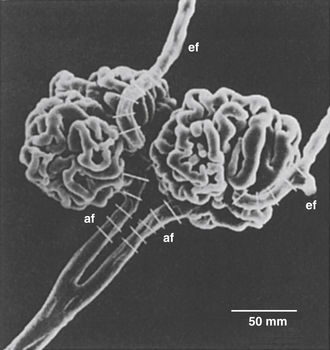
FIGURE 12-11 Scanning electron micrograph of the mammalian glomerulus. An interlobular artery branches with two afferent arterioles (af) proceeding into the glomerular tuft and emerging in efferent arterioles (ef). The afferent arterioles are 15 to 20 µm in diameter.
(From Kimura K, Hirata Y, Nanba S et al: Effects of atrial natriuretic peptide on renal arterioles: morphometric analysis using microvascular casts. Am J Physiol 259:F936, 1990.)
The efferent arterioles divide into another capillary network, the peritubular capillaries (Figure 12-12). The architecture of the peritubular capillary network varies, depending on whether the efferent arteriole arises from glomeruli close to the medullary border (juxtamedullary glomeruli) or from glomeruli in the more peripheral regions of the renal cortex (see Figure 12-10).
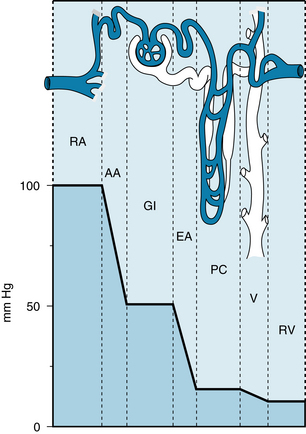
FIGURE 12-12 Intravascular pressures in the renal artery (RA), afferent arterioles (AA), glomerular capillaries (GI), efferent arterioles (EA), peritubular capillaries (PC), venules (V), and renal vein (RV).
(From Frohnert PP: Renal blood flow. In Knox FG, editor: Textbook of Renal Pathophysiology, Hagerstown, MD, 1978, Harper & Row. Based on data from Brenner BM: The dynamics of glomerular ultrafiltration in the rat. J Clin Invest 50:1776, 1971.)
Capillaries that originate from the regular cortical glomeruli surround relatively short renal tubules, which are located almost entirely in the renal cortex. The capillary networks of neighboring cortical nephrons communicate freely with one another. Most of the capillaries arising from the efferent arterioles of juxtamedullary glomeruli form long hairpin loops (vasa recta) that accompany the loops of Henle deep into the renal medulla. Sometimes these loops extend to the tips of the renal papillae (see Figure 12-10). The vasa recta participate in the countercurrent exchange system, which is responsible for concentrating the urine. In general, the renal venous system parallels the arterial distribution to the renal tissues.
Renal Hemodynamics
Segmental Resistance
The pressure drops only slightly in the interlobar, arcuate, and interlobular arteries: the main preglomerular resistance resides in the afferent arterioles (see Figure 12-12). The pressure in the glomerular capillaries is normally about 50 to 60 mm Hg. Thus the net balance of forces across the capillary wall favors the outward filtration of plasma water along the entire length of the capillary loop. The filtration coefficient of the glomerular capillaries exceeds that of most other capillaries in the body. Hence about 20% of the plasma water that enters the glomerular capillaries is filtered into Bowman capsule. The greatest hydraulic resistance in the renal circulation is in the efferent arterioles. Consequently, the pressure in the peritubular capillaries is normally about 10 to 20 mm Hg. Such pressures favor net reabsorption of the large quantities of fluid that pass from the renal tubules to the interstitial spaces of the kidney. The peritubular capillaries are also considerably more permeable than most of the other capillaries in the body.
Renal Blood Flow
The weight of the kidneys constitutes only about 0.5% of total body weight, yet the kidneys receive about 20% of cardiac output. Most of this rich blood supply perfuses the renal cortex; and blood flow per unit weight to the inner medulla and papillae is only about one tenth that to the cortex.Nevertheless, these medullary tissues receive as much blood per unit weight as does the brain.
The kidney has one of the highest metabolic rates in the body. However, the large renal blood flow is not essential to subserve the high metabolic rate. The kidney extracts less than 10% of the O2 present in the renal arterial blood. Therefore the renal blood flow is at least ten times greater than that needed to deliver the required O2 and nutrients. The very high renal blood flow is important because it delivers large volumes of blood to the glomeruli for the process of ultrafiltration.
The Renal Circulation Is Regulated by Intrinsic Mechanisms
Autoregulation
Renal blood flow tends to remain constant despite fluctuations in arterial perfusion pressure. In the experiment illustrated in Figure 12-13, for example, arterial pressure was suddenly raised from 140 to 190 mm Hg. This stepwise change in pressure rapidly increased the renal blood flow from 135 to 165 mL/min. The rise in renal blood flow was transitory, however, and flow returned close to the control level in less than 1 minute. Over a pressure range of about 75 to 170 mm Hg, the steady-state level of renal blood flow is relatively insensitive to changes in arterial pressure (Figure 12-14). Beyond this range, however, renal blood flow varies directly with perfusion pressure.
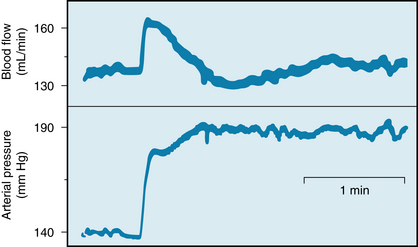
FIGURE 12-13 Changes in renal blood flow evoked by a sudden increase in arterial perfusion pressure from 140 to 190 mm Hg in an isolated dog kidney. The kidney was perfused from a peripheral artery of another dog.
(Modified from Semple SJG, deWardener HE: Effect of increased renal venous pressure on circulatory autoregulation of isolated dog kidneys. Circ Res 7:643, 1959.)
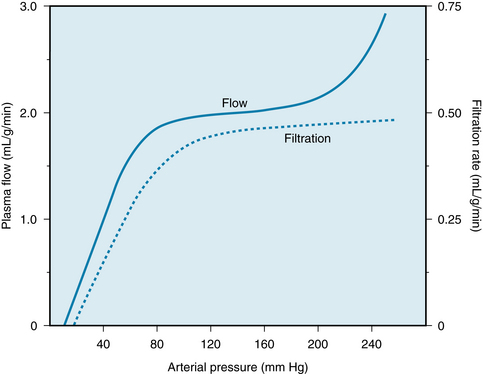
FIGURE 12-14 Renal plasma flow and glomerular filtration rate as functions of arterial perfusion pressure in an anesthetized dog.
(Adapted from Shipley RE, Study RS: Changes in renal blood flow, extraction of inulin, glomerular filtration rate, tissue pressure and urine flow with acute alterations of renal artery blood pressure. Am J Physiol 167:676, 1951.)
This ability of the renal blood flow to remain constant despite fluctuations in perfusion pressure is a process intrinsic to the kidney itself. The steady flow has been demonstrated even in isolated kidney preparations (see Figure 12-13). Concomitant measurement of glomerular filtration rate (GFR) reveals that the tendency for GFR to be autoregulated is equally pronounced (see Figure 12-14). Therefore the resistance change induced by an alteration in perfusion pressure must occur mainly in the afferent arteriole. As the perfusion pressure is raised, for example, afferent arteriolar constriction not only limits the increase in renal blood flow but also restricts the rise in glomerular capillary pressure and the concomitant increment in GFR.
Renal autoregulation has been studied extensively, but its mechanism remains controversial. The two principal mediators appear to be the myogenic mechanism and tubuloglomerular feedback. The myogenic mechanism is the intrinsic property of vascular smooth muscle to contract in response to a stretching force, such as an increase in transmural pressure (see Chapter 9). Tubuloglomerular feedback involves a feedback loop, in which a change in the flow of renal tubular fluid is detected by the macula densa of the juxtaglomerular apparatus, and the juxtaglomerular apparatus then sends a signal to the afferent arterioles to restore basal levels of renal blood flow and GFR.
The precise nature of the signal that regulates the caliber of the afferent arterioles is controversial. Many vasoactive substances, such as prostaglandins, catecholamines, adenosine, NO, and the components of the renin-angiotensin system, may participate in the process of tubuloglomerular feedback. The juxtaglomerular apparatus is the source of one of the principal humoral mechanisms, the renin-angiotensin system, which is involved in the regulation of blood volume and blood pressure. In response to various types of signals, such as a reduction in GFR, the juxtaglomerular apparatus releases the enzyme renin. This enzyme acts on a substrate, angiotensinogen, which circulates in the blood, to release the decapeptide angiotensin I. This peptide is then split by a converting enzyme found on endothelial cell surfaces mainly in the lungs and kidney, to form an octapeptide, angiotensin II. This peptide not only is a potent arteriolar vasoconstrictor but also affects many other vital functions, such as the regulation of certain renal tubular transport processes and the release of aldosterone, an adrenocortical hormone that affects renal excretion of Na+ and water.
Neural Regulation
Stimulation of the renal sympathetic nerves decreases renal blood flow substantially but reduces GFR only slightly. The neural activity constricts the afferent and efferent arterioles and the proximal segments of the vasa recta. Presumably the reduction of renal blood flow exceeds the reduction of GFR, because the postglomerular constriction exceeds the preglomerular constriction.
In resting subjects, the basal level of renal sympathetic tone is low; abolition of that tone scarcely affects renal blood flow. The arterial baroreceptor reflexes influence the renal vasculature only slightly. Activation of low-pressure vascular receptors elicits much larger reflex effects on the renal circulation. A reduction in left atrial pressure, for example, increases renal nerve activity and renal vascular resistance greatly. Emotional reactions such as anxiety, fear, and rage also curtail renal blood flow dramatically.
The Splanchnic Circulation Provides Blood Flow to the Gastrointestinal Tract, Liver, Spleen, and Pancreas
Several features distinguish the splanchnic circulation, the most noteworthy being that two large capillary beds are partially in series with each other. The small splanchnic arterial branches supply the capillary beds in the gastrointestinal tract, spleen, and pancreas. From these capillary beds, the venous blood ultimately flows into the portal vein, which normally provides most of the blood supply to the liver. However, the hepatic artery also supplies blood to the liver.
Intestinal Circulation
Anatomy
The gastrointestinal tract is supplied by the celiac, superior mesenteric, and inferior mesenteric arteries. The superior mesenteric artery is the largest of all the aortic branches and carries more than 10% of the cardiac output. Small mesenteric arteries form an extensive vascular network in the submucosa (Figure 12-15). Their branches penetrate the longitudinal and circular muscle layers, and they give rise to third- and fourth-order arterioles. Some third-order arterioles in the submucosa become the main arterioles to the tips of the villi.
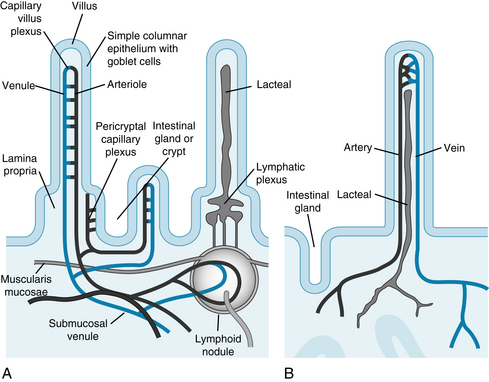
FIGURE 12-15 Diagrammatic view of the microcirculation of the small intestine. A, Arterioles in the villus and in the crypt give rise to capillaries; blood flows to venules that eventually enter the portal circulation. B, Lymphatic vessels (lacteals) are found in the villus and form a plexus at the base of the villus where lymphoid nodules are located.
(Redrawn from Kierszenbaum A: Histology and cell biology: an introduction to pathology, Philadelphia, Mosby, 2002.)
The direction of the blood flow in the capillaries and venules in a villus is opposite to that in the main arteriole (Figure 12-16). This arrangement constitutes a countercurrent exchange system. An effective countercurrent multiplier in the villus facilitates the absorption of Na+ and water. The countercurrent exchange also permits diffusion of O2 from arterioles to venules. At low flow rates, a substantial fraction of the O2 may be shunted from arterioles to venules near the base of the villus. Thus the supply of O2 to the mucosal cells at the tip of the villus may be curtailed. When intestinal blood flow is reduced, the shunting of O2 is exaggerated, possibly causing extensive necrosis of the intestinal villi.
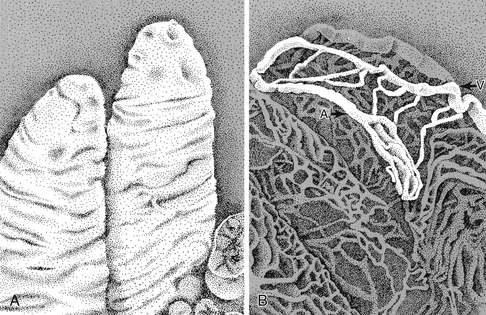
FIGURE 12-16 Scanning electron micrographs of rabbit intestinal villi (A) and corrosion cast of the microcirculation in the villus (B). A, arteriole; V, venule.
(From Gannon BJ, Gore RW, Rogers PAW: Is there an anatomical basis for a vascular counter-current mechanism in rabbit and human intestinal villi? Biomed Res 2(Suppl):235, 1981.)
Neural Regulation
The neural control of the mesenteric circulation is almost exclusively sympathetic. Increased sympathetic activity constricts the mesenteric arterioles, precapillary sphincters, and capacitance vessels. These responses are mediated by α1-adrenergic receptors, which are dominant in the mesenteric circulation; β2-adrenergic receptors are also present. Infusion of a β-adrenergic receptor agonist, such as isoproterenol, causes vasodilation.
In response to fighting, or to artificial stimulation of the hypothalamic “defense” area, vasoconstriction becomes pronounced in the mesenteric vascular bed. This shifts blood flow from the temporarily less important intestinal circulation to the more crucial skeletal muscles, heart, and brain.
Autoregulation
Autoregulation in the intestinal circulation is not as well developed as it is in certain other vascular beds, such as those in the brain and kidney. The principal mechanism responsible for autoregulation is metabolic, although a myogenic mechanism probably also participates (see Chapter 9). The adenosine concentration in the mesenteric venous blood rises fourfold after brief arterial occlusion. Adenosine is a potent vasodilator in the mesenteric vascular bed, and it may be the principal metabolic mediator of autoregulation. However, K+ and altered osmolality may also contribute to the overall response.
The O2 consumption of the small intestine is more rigorously controlled than is the blood flow. In one series of experiments, the O2 uptake of the small intestine remained constant when arterial perfusion pressure was varied between 30 and 125 mm Hg.
Functional Hyperemia
Food ingestion increases intestinal blood flow. The secretion of certain gastrointestinal hormones contributes to this hyperemia. Gastrin and cholecystokinin augment intestinal blood flow, and these hormones are secreted when food is ingested. The absorption of food affects intestinal blood flow. Undigested food has no vasoactive influence, whereas several products of digestion are potent vasodilators. Among the various constituents of chyme, the principal mediators of mesenteric hyperemia are glucose and fatty acids.
Hepatic Circulation
Anatomy
The blood flow to the liver is normally about 25% of cardiac output. The flow is derived from two sources: the portal vein and the hepatic artery. Ordinarily, the portal vein provides about three fourths of the blood flow. The portal venous blood has already passed through the gastrointestinal capillary bed, and therefore much of the O2 has already been extracted. The hepatic artery delivers the remaining one fourth of the blood, which is fully saturated with O2. Hence about three fourths of the O2 used by the liver is derived from the hepatic arterial blood.
The small branches of the portal vein and hepatic artery give rise to terminal portal venules and hepatic arterioles (Figure 12-17). These terminal vessels enter the hepatic acinus (the functional unit of the liver) at its center. Blood flows from these terminal vessels into the sinusoids, which constitute the capillary network of the liver. The sinusoids radiate toward the periphery of the acinus, where they connect with the terminal hepatic venules. Blood from these terminal venules drains into progressively larger branches of the hepatic veins, which are tributaries of the inferior vena cava.
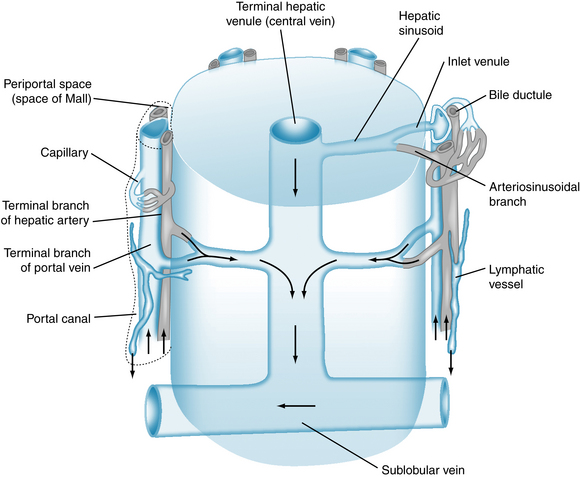
FIGURE 12-17 Microcirculation in a hepatic acinus. The arrows indicate the direction of blood flow from the hepatic artery and portal vein to the hepatic sinusoids. The admixture of blood flows through the terminal hepatic venule and then into the sublobular vein.
(Redrawn from Ross MH, Pawling W: Histology: A text and atlas: with correlated cell and molecular biology, Philadelphia, Lippincott William & Wilkins, 2006.)
Hemodynamics
The mean blood pressure in the portal vein is about 10 mm Hg, and that in the hepatic artery is about 90 mm Hg. The resistance of the vessels upstream to the hepatic sinusoids is considerably greater than is that of the downstream vessels. Consequently, the pressure in the sinusoids is only 2 or 3 mm Hg greater than that in the hepatic veins and inferior vena cava.
The ratio of presinusoidal resistance to postsinusoidal resistance in the liver is much greater than the ratio of precapillary resistance to postcapillary resistance in almost any other vascular bed. Hence, drugs and other interventions that alter the presinusoidal resistance usually affect the pressure in the sinusoids only slightly. Such changes in presinusoidal resistance have little effect on the fluid exchange across the sinusoidal wall. Conversely, changes in hepatic venous (and in central venous) pressure are transmitted almost quantitatively to the hepatic sinusoids and profoundly affect the transsinusoidal exchange of fluids.
Regulation of Blood Flow
Blood flows in the portal venous and hepatic arterial systems vary reciprocally. When blood flow is curtailed in one system, the flow increases in the other system. However, the ensuing increase in flow in one system usually does not fully compensate for the initiating reduction in flow in the other system. The portal venous system does not autoregulate. As portal venous pressure and flow are raised, resistance either remains constant or decreases. However, the hepatic arterial system displays autoregulation.
The liver tends to maintain a constant O2 consumption because the extraction of O2 from the hepatic blood is very efficient. As the rate of O2 delivery to the liver is varied, the liver compensates with an appropriate change in the fraction of O2 that is extracted from the blood. This extraction is facilitated by the distinct separation of the presinusoidal vessels at the acinar center from the postsinusoidal vessels at the periphery of the acinus (see Figure 12-17). The substantial distance between these types of vessels prevents a countercurrent exchange of O2, contrary to the condition that exists in an intestinal villus (see Figure 12-16).
CLINICAL BOX
When central venous pressure is elevated, as in congestive heart failure, large quantities of plasma water transude from the liver into the peritoneal cavity; such a fluid accumulation in the abdomen is known as ascites. Extensive fibrosis of the liver, as in the various types of hepatic cirrhosis, leads to a pronounced increase in hepatic vascular resistance, which raises the pressure substantially in the portal venous system. The consequent increase in capillary hydrostatic pressure throughout the splanchnic circulation also leads to extensive fluid transudation into the abdominal cavity (i.e., ascites). Furthermore, the pressure may rise substantially in other veins that anastomose with the portal vein. A noteworthy example is that of the esophageal veins. These veins may enlarge considerably to form esophageal varices, which may rupture and thereby lead to severe, and frequently fatal, internal bleeding. To obviate these grave problems associated with elevated portal venous pressure, an anastomosis (portacaval shunt) is often created surgically between the portal vein and the inferior vena cava to decrease portal venous pressure.
The sympathetic nerves constrict the presinusoidal resistance vessels in the portal venous and hepatic arterial systems. Neural effects on the capacitance vessels are more important, however. The liver contains about 15% of the total blood volume of the body. Under appropriate conditions, such as in response to hemorrhage, about half of the hepatic blood volume can be rapidly expelled by virtue of a constriction of the capacitance vessels. Hence the liver constitutes an important blood reservoir in humans. In certain other species, such as the dog, the spleen is a more important blood reservoir.
Fetal Circulation
The circulation in the fetus displays various differences from that in the postnatal infant. The fetal lungs are functionally inactive, and the fetus depends completely on the placenta for O2 and nutrient supply. Oxygenated fetal blood from the placenta passes through the umbilical vein to the liver. Approximately half passes through the liver, and the remainder bypasses the liver and reaches the inferior vena cava through the ductus venosus (Figure 12-18). In the inferior vena cava, blood from the ductus venosus joins blood returning from the lower trunk and extremities, and this combined stream is in turn joined by blood from the liver through the hepatic veins.
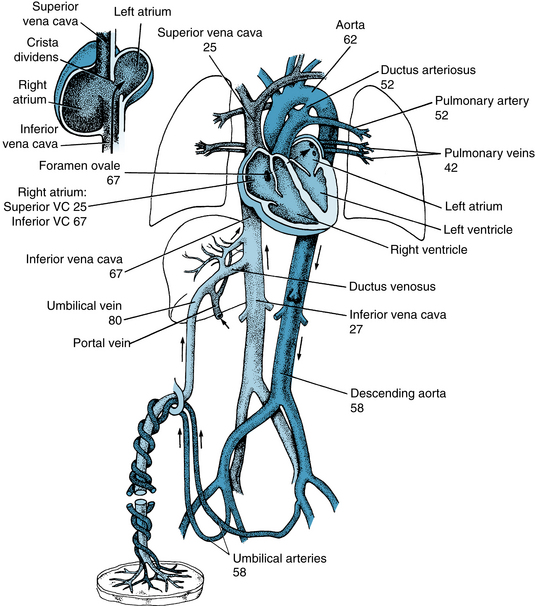
FIGURE 12-18 Schematic diagram of the fetal circulation. The numbers represent the percentages of O2 saturation of the blood flowing in the indicated blood vessels. The inset at upper left illustrates the direction of flow of a major portion of the inferior vena cava (VC) blood through the foramen ovale to the left atrium.
(Values for O2 saturations are from Dawes GS, Mott JC, Widdicombe JG: The foetal circulation in the lamb. J Physiol 126:563, 1954.)
The streams of blood tend to maintain their identity in the inferior vena cava, and they are divided into two streams of unequal size by the edge of the interatrial septum (crista dividens). The larger stream, which is mainly blood from the umbilical vein, is shunted to the left atrium through the foramen ovale, which lies between the inferior vena cava and the left atrium (see inset, Figure 12-18). The other stream passes into the right atrium, where it is joined by superior vena caval blood that returns from the upper parts of the body and by blood from the myocardium.
Unlike in the adult, in whom the right and left ventricles pump in series, the ventricles in the fetus operate essentially in parallel. Only one tenth of right ventricular output goes through the lungs, because of the large pulmonary resistance. Fetal oxygenation is derived from gas exchange in the placenta; O2 tension in the alveoli is low. The low O2 tension causes tonic pulmonary artery vasoconstriction. The hypoxia also results in dilation of the ductus arteriosus. This dilation shunts the remaining blood from the pulmonary artery to the aorta, at a point that is distal to the origins of the arteries to the head and upper extremities (see Figure 12-18). Blood flows from pulmonary artery to the aorta because the pulmonary resistance is great and the diameter of the ductus arteriosus is as large as that of the descending aorta.
The large volume of blood coming through the foramen ovale into the left atrium is joined by blood returning from the lungs. The blood from these sources is pumped out by the left ventricle into the aorta. Most of the blood in the ascending aorta goes to the head, upper thorax, and arms; the remainder joins blood from the ductus arteriosus and supplies the rest of the body and the placenta. The amount of blood pumped by the left ventricle is about half that pumped by the right ventricle. The major fraction of the blood that passes down the descending aorta comes from the ductus arteriosus and right ventricle, and it flows by way of the two umbilical arteries to the placenta.
Figure 12-18 illustrates the O2 saturations of the blood at various points of the fetal circulation. Fetal blood leaving the placenta is 80% saturated. However, the O2 saturation of the blood passing through the foramen ovale is reduced to 67% by mixing with the desaturated blood returning from the lower part of the body and the liver. Addition of the desaturated blood from the lungs reduces the O2 saturation of left ventricular blood to 62%, which is the level of saturation of the blood that reaches the head and upper extremities.
The blood in the right ventricle, a mixture of desaturated superior vena caval blood, coronary venous blood, and inferior vena caval blood, is only 52% saturated with O2. When the major portion of this blood traverses the ductus arteriosus and joins that pumped out by the left ventricle, the resulting O2 saturation of blood traveling to the lower part of the body and back to the placenta is 58%. Thus, the tissues that receive blood of the highest O2 saturation are the liver, heart, and upper parts of the body, including the head.
At the placenta, the chorionic villi dip into the maternal sinuses, and the O2, CO2, nutrients, and metabolic waste products are exchanged across the membranes. The barrier to exchange is quite large, and the equilibrium of Po2 between the two circulations is not reached at normal rates of blood flow. Therefore the O2 tension of the fetal blood leaving the placenta is very low. Were it not true that fetal hemoglobin has a greater affinity for O2 than does adult hemoglobin, the fetus would not receive an adequate O2 supply. The fetal oxyhemoglobin dissociation curve is shifted to the left, so that at equal pressures of O2, fetal blood carries significantly more O2 than does maternal blood. In early fetal life, the high cardiac glycogen levels that prevail may protect the heart from acute periods of hypoxia. The glycogen levels decrease in late fetal life and reach adult levels by term.
If the mother is subjected to hypoxia, the reduced blood O2 tension is reflected in the fetus by tachycardia and an increase in blood flow through the umbilical vessels. If the hypoxia persists or if flow through the umbilical vessels is impaired, fetal distress occurs and is first manifested as bradycardia.
Changes in the Circulatory System at Birth
The umbilical vessels have thick muscular walls that are very reactive to trauma, tension, sympathomimetic amines, bradykinin, angiotensin, and changes in Po2. In animals in which the umbilical cord is not tied, hemorrhage of the newborn is prevented by constriction of these large vessels in response to various stimuli. Closure of the umbilical vessels increases the total peripheral resistance and the blood pressure. When blood flow ceases through the umbilical vein, the ductus venosus closes. This structure is a thick-walled vessel with a muscular sphincter. The event that closes the ductus venosus is still unknown.
The asphyxia that starts with constriction or clamping of the umbilical vessels, together with the cooling of the body, activates the respiratory center of the newborn infant. As the lungs fill with air, pulmonary vascular resistance decreases to about one tenth of the value that exists before lung expansion. This resistance change is not caused by the presence of O2 in the lungs, because the change is just as great if the lungs are filled with nitrogen. However, filling the lungs with liquid does not reduce pulmonary vascular resistance.
The left atrial pressure is raised above the pressure in the inferior vena cava and right atrium by (1) the decrease in pulmonary resistance, with the resulting large flow of blood through the lungs to the left atrium; (2) the reduction of flow to the right atrium caused by occlusion of the umbilical vein; and (3) the increased resistance to left ventricular output produced by occlusion of the umbilical arteries. This reversal of the pressure gradient across the atria abruptly closes the valve over the foramen ovale, and the septal leaflets fuse over several days.
With the decrease in pulmonary vascular resistance, the pressure in the pulmonary artery falls to about one half its previous level (to about 35 mm Hg). This change in pressure, coupled with a slight increase in aortic pressure, reverses the flow of blood through the ductus arteriosus. However, within several minutes the large ductus arteriosus begins to constrict. This response produces turbulent flow, which is manifested as a murmur in the newborn. Constriction of the ductus arteriosus is progressive, and it is usually complete within 1 to 2 days after birth. Closure of the ductus arteriosus appears to be initiated by (1) the high O2 tension of the arterial blood that passes through it and (2) the pulmonary ventilation with O2 that closes the ductus. Furthermore, ventilation with air low in O2 opens this shunt vessel. Whether O2 acts directly on the ductus or through the release of a vasoconstrictor substance is not known.
The ductus arteriosus occasionally fails to close after birth. This failure constitutes a congenital cardiovascular abnormality that is amenable to surgical correction.
At birth the walls of the two ventricles are approximately of the same thickness, with a possibly slight preponderance of the right ventricle. Also, in the newborn the muscle layer of the pulmonary arterioles is thick, a feature that is partly responsible for the high pulmonary vascular resistance of the fetus. After birth the thickness of the walls of the right ventricle diminishes, as does the muscle layer of the pulmonary arterioles; the left ventricular walls become thicker. These changes are progressive over a period of weeks after birth.
Summary
Skin Circulation
 Most of the resistance vessels in the skin are under dual control of the sympathetic nervous system and local vasodilator metabolites, but the arteriovenous anastomoses found in the hands, feet, and face are solely under neural control.
Most of the resistance vessels in the skin are under dual control of the sympathetic nervous system and local vasodilator metabolites, but the arteriovenous anastomoses found in the hands, feet, and face are solely under neural control.
 The main function of skin blood vessels is to aid in the regulation of body temperature by constricting to conserve heat and dilating to lose heat.
The main function of skin blood vessels is to aid in the regulation of body temperature by constricting to conserve heat and dilating to lose heat.
 Skin blood vessels dilate directly and reflexly in response to heat and constrict directly and reflexly in response to cold.
Skin blood vessels dilate directly and reflexly in response to heat and constrict directly and reflexly in response to cold.
Cerebral Circulation
 Cerebral blood flow is predominantly regulated by metabolic factors, especially H+, CO2, K+, and adenosine.
Cerebral blood flow is predominantly regulated by metabolic factors, especially H+, CO2, K+, and adenosine.
 Increased regional cerebral activity produced by stimuli such as touch, pain, hand motion, talking, reading, reasoning, and problem solving is associated with enhanced blood flow in the activated areas of the contralateral cerebral cortex.
Increased regional cerebral activity produced by stimuli such as touch, pain, hand motion, talking, reading, reasoning, and problem solving is associated with enhanced blood flow in the activated areas of the contralateral cerebral cortex.
Pulmonary Circulation
 The pulmonary circulation consists of the pulmonary vasculature, whose function is to promote the exchange of O2 and CO2 across the pulmonary capillaries, and the bronchial vasculature, whose function is to deliver O2 and nutrients to the airways.
The pulmonary circulation consists of the pulmonary vasculature, whose function is to promote the exchange of O2 and CO2 across the pulmonary capillaries, and the bronchial vasculature, whose function is to deliver O2 and nutrients to the airways.
 The resistance of the pulmonary vasculature is low. The distribution of blood flow to different regions of the lungs is affected by the body’s position in space because of the low pulmonary vascular pressures.
The resistance of the pulmonary vasculature is low. The distribution of blood flow to different regions of the lungs is affected by the body’s position in space because of the low pulmonary vascular pressures.
 The smooth muscles in the pulmonary arterioles constrict in response to hypoxia. This response tends to shift blood flow to the well-ventilated alveoli and away from the poorly ventilated alveoli.
The smooth muscles in the pulmonary arterioles constrict in response to hypoxia. This response tends to shift blood flow to the well-ventilated alveoli and away from the poorly ventilated alveoli.
Renal Circulation
 Renal blood flow is very high (about 20% of cardiac output), and the chief resistance to blood flow in the kidneys resides in the afferent and efferent arterioles.
Renal blood flow is very high (about 20% of cardiac output), and the chief resistance to blood flow in the kidneys resides in the afferent and efferent arterioles.
 Glomerular capillary pressure is high (about 50 to 60 mm Hg), and the glomerular capillaries are very permeable to water; these two factors favor the filtration of large quantities of water from the blood to Bowman’ capsule.
Glomerular capillary pressure is high (about 50 to 60 mm Hg), and the glomerular capillaries are very permeable to water; these two factors favor the filtration of large quantities of water from the blood to Bowman’ capsule.
Hepatic Circulation
 The liver receives about 25% of cardiac output; about three fourths of this comes via the portal vein and about one fourth via the hepatic artery. When flow is diminished in either the portal or hepatic system, flow in the other system usually increases, but not proportionately.
The liver receives about 25% of cardiac output; about three fourths of this comes via the portal vein and about one fourth via the hepatic artery. When flow is diminished in either the portal or hepatic system, flow in the other system usually increases, but not proportionately.
 The liver tends to maintain a constant O2 consumption, in part because its mechanism for extracting O2 from the blood is so efficient.
The liver tends to maintain a constant O2 consumption, in part because its mechanism for extracting O2 from the blood is so efficient.
 The liver normally contains about 15% of total blood volume. It serves as an important blood reservoir for the body.
The liver normally contains about 15% of total blood volume. It serves as an important blood reservoir for the body.
Fetal Circulation
 In the fetus a large percentage of right atrial blood passes through the foramen ovale to the left atrium, and a large percentage of pulmonary artery blood passes through the ductus arteriosus to the aorta.
In the fetus a large percentage of right atrial blood passes through the foramen ovale to the left atrium, and a large percentage of pulmonary artery blood passes through the ductus arteriosus to the aorta.
 At birth the umbilical vessels, ductus venosus, and ductus arteriosus close by contraction of their muscle layers. The reduction in pulmonary vascular resistance caused by lung inflation is the main factor that reverses the pressure gradient between the atria, thereby closing the foramen ovale.
At birth the umbilical vessels, ductus venosus, and ductus arteriosus close by contraction of their muscle layers. The reduction in pulmonary vascular resistance caused by lung inflation is the main factor that reverses the pressure gradient between the atria, thereby closing the foramen ovale.
Ainslie P.N., Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1473.
Baschat A.A., Gembruch U., Harman C.R. Coronary blood flow in fetuses with intrauterine growth restriction. J Perinat Med. 1998;26:143.
Dabertrand F., Nelson M.T., Brayden J.E. Acidosis dilates brain parenchymal arterioles by conversion of calcium waves to sparks to activate BK channels. Circ Res. 2012;110:285.
Delp M.D., O’Leary D.S. Integrative control of the skeletal muscle microcirculation in the maintenance of arterial pressure during exercise. J Appl Physiol. 2004;97:1112.
Faraci F.M., Heistad D.D. Regulation of the cerebral circulation: Role of endothelium and potassium channels. Physiol Rev. 1998;78:53–97.
Granger D.N., Kvietys P.R., Korthuis R.J., et al, Microcirculation of the intestinal mucosa. Schultz S.G., ed. Handbook of Physiology, Section 6: The Gastrointestinal System-Motility and Circulation, vol I. Bethesda, Md: American Physiological Society, 1989.
Greenway C.V., Lautt W.W., Hepatic circulation. Schultz S.G., ed. Handbook of Physiology, Section 6: The Gastrointestinal System-Motility and Circulation, vol I. Bethesda, Md: American Physiological Society, 1989.
Kiserud T., Acharya G. The fetal circulation. Prenat Diagn. 2004;24:1049.
Mauban J.R., Remillard C.V., Yuan J.X. Hypoxic pulmonary vasoconstriction: Role of ion channels. J Appl Physiol. 2005;98:415.
Mortensen S.P., González-Alonso J., Bune L.T., et al. ATP-induced vasodilation and purinergic receptors in the human leg: roles of nitric oxide, prostaglandins, and adenosine. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1140.
Rychik J. Fetal cardiovascular physiology. Pediatr Cardiol. 2004;25:201.
Stamler J.S., Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev. 2001;81:209.
Stickland M.K., Miller J.D., Smith C.A., Dempsey J.A. Carotid chemoreceptor modulation of regional blood flow distribution during exercise in health and chronic heart failure. Circ Res. 2007;100:1371.
Straub S.V., Nelson M.T. Astrocytic calcium signaling: The information currency coupling neuronal activity to the cerebral microcirculation. Tr Cardiovasc Med. 2007;17:183.
Thomas G.D., Segal S.S. Neural control of muscle blood flow during exercise. J Appl Physiol. 2004;97:731.
Toda N., Ayajiki K., Okamura T. Cerebral blood flow regulation by nitric oxide: recent advances. Pharmacol Rev. 2009;61:62.
Waypa G.B., Schumacker P.T. Hypoxic pulmonary vasoconstriction: Redox events in oxygen sensing. J Appl Physiol. 2005;98:404.
CASE 12-1
History
A 53-year-old man has consumed substantial amounts of alcohol over the past 3 decades. For the past 2 or 3 years, he has noticed that his belt size has progressively increased and that his abdomen is distended. His physician was able to evoke a fluid wave across the patient’s abdomen by tapping it. The physician made the diagnosis of hepatic cirrhosis, which is associated with extensive fibrosis of the liver.
CASE 12-2
History
A 6-year-old boy was referred by his family physician to a pediatric cardiologist because of chronic fatigue, effort intolerance, and a heart murmur. On physical examination, the boy appeared slightly small for his age, and had normal skin color, no clubbing of the fingers, and a harsh murmur throughout systole that was heard best in the fourth intercostal space to the left of the sternum but extending over the entire precordium. An x-ray revealed an enlarged heart, especially the right ventricle. An ear oximeter showed normal oxygenation of arterial blood. Hematocrit was normal. Cardiac catheterization data were as follows:
CASE 12-3
History
A 55-year-old man visits his family physician and presents with a chief complaint of pain in his left leg when he walks about 2 blocks from his home to the grocery store. The pain subsides when he rests for a few minutes but it returns when he walks home. He has hypertension for which he takes amlodipine and a diuretic. He had a mild heart attack 4 years ago. Since that time, he has had occasional angina, which is treated with oral nitrates. Although counseled to stop smoking, he continues his one pack per day habit. He has a resting blood pressure of 140/95 mm Hg and an ankle-brachial index (ABI) of 0.5 in the left leg and of 1.1 in the right leg (see Chapter 7). He is diagnosed with intermittent claudication due to peripheral vascular disease in the left leg.