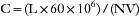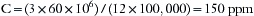Waste Anesthetic Gases and Scavenging Systems
TRACE CONCENTRATIONS OF ANESTHETIC GASES
SOURCES OF ANESTHETIC GAS CONTAMINATION
Adjustable Pressure-Limiting Valve
High- and Intermediate-Pressure Systems of the Anesthesia Workstation
Low-Pressure System of the Anesthesia Machine
OPERATING ROOM VENTILATION SYSTEMS
General Properties of Scavenging Systems
Adjustable Pressure-Limiting and Ventilator Pressure Relief Valves
Scavenging the Anesthesia Ventilator
VOLATILE ANESTHETIC RECLAMATION
ANESTHETIC LEAK DETECTION AND WASTE GAS MANAGEMENT
Testing the High- and Intermediate-Pressure Systems for Leaks
MONITORING TRACE LEVELS OF ANESTHETIC GASES
ARE TRACE CONCENTRATIONS OF WASTE ANESTHETIC GASES HAZARDOUS?
Trace Concentrations of Anesthetic Gases
Concern over trace concentrations of anesthetic gases dates back to 1967, when Vaisman1 reported findings of a survey of 354 anesthesiologists in Russia. All worked in poorly ventilated operating rooms and used nitrous oxide (N2O), halothane, and ether. Of the total, 303 responded to the survey; and of these, 110 were female. Female responders reported 31 pregnancies, 18 of which ended in spontaneous abortion. One pregnancy resulted in a congenitally abnormal child. Vaisman concluded that these problems in pregnancy—as well as other reported effects, such as nausea, irritability, and fatigue—were due to a combination of long-term inhalation of anesthetic vapors, emotional strain, and excessive workload. Although uncontrolled and largely anecdotal, this study drew attention to the possibility that trace concentrations of anesthetics may be harmful. The matter was taken up by investigators in Europe2,3 and in the United States.4-6 Their results, and those from animal studies,7 gave cause for further inquiry.
In 1970, the U.S. Congress passed the Occupational Safety and Health Act,8 the purpose of which was to ensure “safe and healthful working conditions for all men and women in the nation.” The act established the National Institute for Occupational Safety and Health (NIOSH), which was given the responsibility to conduct and fund research in exposure hazards and to recommend safety standards. The act also established the Occupational Safety and Health Administration (OSHA), which, after due procedure, would enact into law and then enforce NIOSH recommended standards.9 NIOSH funded a number of studies, one of which was the National Survey of Occupational Disease Among Operating Room Workers, conducted in conjunction with the American Society of Anesthesiologists (ASA) Ad Hoc Committee on Waste Anesthetic Gases. This study surveyed 49,000 people who were potentially exposed—members of the ASA, American Association of Nurse Anesthetists (AANA), Association of Operating Room Nurses (AORN), Association of Operating Room Technicians (AORT)—and 26,000 unexposed personnel, members of the American Academy of Pediatrics and American Nurses Association.10,11 The results, published in 1974, showed an increased reported incidence among women of spontaneous abortion, liver and kidney disease, and cancer, and a higher incidence of congenital abnormalities in the offspring of exposed women. Among the exposed men, the incidence of cancer did not increase, but that of hepatic disease did. NIOSH sponsored further related studies that included investigation of methods for reducing exposure to waste gases.12 The organization planned to introduce scavenging of waste anesthetic gases and repeat this survey to determine whether scavenging did indeed reduce these adverse health effects and show an association between trace anesthetic gases and disease.
In March 1977, before a repeat survey was commenced, NIOSH published Criteria for a Recommended Standard; Exposure to Waste Anesthetic Gases and Vapors.13 This report estimated that 214,000 workers were potentially exposed to trace concentrations of anesthetic gases on a day-to-day basis. The document reviewed all the available data and found that, although not definitive, the evidence suggested a relationship between health hazards and trace concentrations of anesthetic gases. No cause-and-effect relationship was established, and no safe exposure levels could be identified. However, the document recommended that risks be minimized as much as possible by maintaining “exposures as low as is technically feasible.” The document also recommended measures to reduce exposure and to monitor exposure levels, and it advocated extensive recordkeeping regarding the health of operating room (OR) personnel.
NIOSH recommended environmental limits for the upper boundary of exposure: “Occupational exposure to halogenated anesthetic agents shall be controlled so that no worker is exposed at concentrations greater than 2 ppm [parts per million] of any halogenated anesthetic agent. When such agents are used in combination with N2O, levels of the halogenated agent well below 2 ppm are achievable. In most situations, control of N2O to a time-weighted average (TWA) concentration of 25 ppm during the anesthetic administration period will result in levels of approximately 0.5 ppm of the halogenated agent. Occupational exposure to N2O, when used as the sole anesthetic agent, shall be controlled so that no worker is exposed at TWA concentrations greater than 25 ppm during anesthetic administration. Available data indicate that with current control technology, exposure levels of 50 ppm, and less for N2O, are attainable in dental offices.”
These recommended exposure limits were based on two studies. First, Whitcher and colleagues11 showed that these levels were readily attainable in the OR when certain precautionary measures were taken. Second, Bruce and Bach14 found no decrement in the psychomotor capacities of volunteers exposed for 4 hours at the recommended levels. The newer volatile agents, such as sevoflurane and desflurane, were not available at this time, so exposure limits for these agents have not yet been assessed.
To provide a perspective on how small 1 ppm is, consider that the presence of 25 ppm of N2O in the atmosphere represents a concentration of one four-hundredth of 1% (100% N2O is 1 million ppm [by volume]):
100% N2O = 106 ppm
1% N2O = 10,000 ppm
(1/100) × 1% N2O = 0.01% N2O = 100 ppm
(1/400) × 1% N2O = 0.0025% N2O = 25 ppm
Similarly, 2 ppm and 0.5 ppm of halothane represent a concentration of one five-thousandth and one twenty-thousandth of 1% halothane:
100% halothane = 106 ppm
1% halothane = 10,000 ppm
(1/5000) × 1% halothane = 0.0002% = 2 ppm
(1/20,000) × 1% halothane = 0.00005% = 0.5 ppm
To express this in terms of anesthetic potency, divide the value expressed in parts per million by the minimum alveolar concentration (MAC) of halothane (0.76%) at 1 atmosphere pressure:
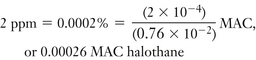
What levels of trace anesthetics may be found in the OR? When no attempt has been made to reduce leakage or to scavenge waste gases, trace gas levels of 400 to 600 ppm of N2O and from 5 to 10 ppm of halogenated agents may be detected.12 Effective scavenging alone can reduce these levels more than 10-fold.
The volume of N2O that must be released into an OR to reach the NIOSH maximum recommended limit of 25 ppm can be calculated by the following equation. Assume the size of an OR is 5 m2 × 4 m. The volume of the OR is therefore given by:
Therefore the NIOSH limit is 25/106. If the OR volume is 100 × 106 mL, this limit is reached by release of 2500 mL (25 × 100) or 2.5 L of N2O.
This calculation assumes uniform mixing of all gases and no ventilation or air conditioning of the OR. If it is assumed that the OR ventilation system produces 12 air changes per hour, in the OR described above, a leakage rate of 2.5 L/5 min or 0.5 L/min N2O would be necessary to maintain the ambient air N2O level at 25 ppm.
In 2000, OSHA revised its recommendations on waste anesthetic gases in the light of current knowledge.15 The revised recommendations are published on the Internet for informational purposes only and are regularly updated as information becomes available. The document is not published in the standard OSHA manual on occupational hazards, however. The recommendations are advisory and have not been promulgated as a standard; rather, they are to be seen as guidelines. OSHA recommends scavenging of waste anesthetic gases in all anesthetizing locations and advocates work practices to reduce trace levels of anesthetic gases in the ambient air. A documented maintenance program should be in place for all anesthetic delivery machines, and an ongoing education program for all personnel to inform them of these recommendations must exist. OSHA recommends a program for monitoring trace anesthetic gases and also recommends a preemployment medical examination for all employees. Each institution also should have a mechanism in place for employees to report any work-related health problems.
Contamination with trace concentrations of anesthetic gases also may occur in the corridors of an OR suite, in the anesthesia workroom, and in the N2O storage area. Poorly ventilated postanesthesia care units (PACUs) also may be contaminated with exhaled anesthetic agents.16
Sources of Anesthetic Gas Contamination
Potential sources of anesthetic gas contamination are the adjustable pressure-limiting (APL) or “pop-off” valve, the high- and low-pressure systems of the anesthesia machine, the anesthesia ventilator, cryosurgery units, and other miscellaneous sources.
Adjustable Pressure-Limiting Valve
The APL valve of the anesthesia breathing circuit is the outlet for waste anesthetic gases during spontaneous or assisted ventilation. Depending on the inflow rate of fresh gas, more than 5 L of gas can exit the circuit through this valve every minute. The effect of such spillage on the level of anesthetic contamination in the OR is given by the following equation:
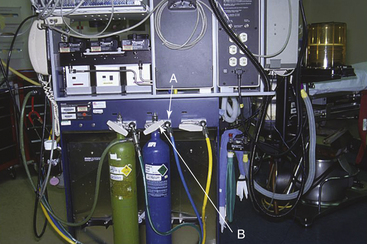
where C is the OR pollutant level in parts per million, L is the pollutant spillage in liters per minute, V is the OR total air volume in liters, and N is the number of air exchanges per hour. For example, if L = 3 L/min, V = 100,000 L, and N = 10 exchanges per hour, then
Very large volumes of anesthetic gas are discharged through the relief valve of nonrebreathing systems, such as the Bain circuit (Mapleson D) or Jackson-Rees (Mapleson E). Without scavenging and proper room ventilation, N2O levels as high as 2000 ppm have been found in the breathing zone of anesthesiologists.17
High- and Intermediate-Pressure Systems of the Anesthesia Workstation
The high- and intermediate-pressure systems of the anesthesia workstation include the N2O central supply pipeline and reserve tanks and the internal piping of the machine that leads to the N2O flowmeter (Fig. 5-1). The pressure in this system can be from 26 to 750 psig; leaks therefore are likely to contribute significantly to the contamination of the OR. Common sources of leaks are defective connectors in the N2O central supply line “quick connects” (see Fig. 5-1) and defective yokes for the N2O reserve tanks. In one OR suite, major high-pressure leaks were detected in 50% of the anesthesia machines.18 When the OR is not being used, background N2O contamination is primarily caused by high-pressure leaks.
Low-Pressure System of the Anesthesia Machine
The low-pressure system of the anesthesia machine includes the N2O flowmeter, the vaporizers, the fresh gas delivery tubing from the anesthesia machine to the breathing circuit (Fig. 5-2, F), the carbon dioxide absorber (Fig. 5-2, E), the breathing hoses (Fig. 5-2, D), the unidirectional valves, the ventilator, and the various components of the waste gas scavenging system. Leaks occur most commonly in the carbon dioxide absorber because of loose screws, worn gaskets, granules of absorbent on the gaskets, and an open petcock. Other leaks may occur as a result of breaks in the rubber and plastic components of the breathing circuit, loose domes on the unidirectional valves (Fig. 5-2, A and B), or a poorly fitting oxygen sensor in the breathing circuit (Fig. 5-2, C). Disposable breathing circuits are particularly prone to leakage, especially those of the swivel type, because of imperfections in the manufacturing process.19 Although vaporizers usually are gas tight, they, too, occasionally leak as a result of loose mounts, defective seals and gaskets, or incompletely closed fill ports.
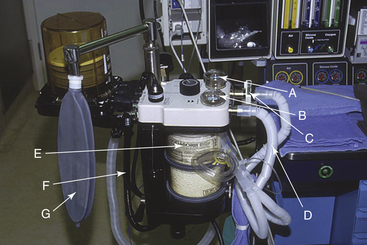
FIGURE 5-2 Sources of leaks in the low-pressure system. A and B, Domes of unidirectional valves. C, Oxygen sensor. D, Breathing circuit hoses. E, Carbon dioxide absorber canister. F, Fresh gas delivery tube. G, Breathing system reservoir bag.
A direct linear relationship exists between the peak pressure in the breathing circuit and the amount of gas that escapes through a leak in the low-pressure system: the higher the pressure, the greater the leak. Despite effective scavenging, as much as 2 L/min may leak from the breathing circuit, increasing the N2O concentration in the air by 100 to 200 ppm.
The scavenging system itself may be a source of leakage. The rubber parts and safety valves may leak anesthetic gases, as may improperly sized hoses, such as 22-mm hoses that have been “adapted” to fit the 19-mm or 30-mm scavenger connections. The system also may be overloaded because of inadequate vacuum or ventilation systems; consequently, waste gases are spilled into the OR atmosphere.
Anesthesia Ventilator
The anesthesia ventilator may be a major source of leakage. Some ventilators leak internally and cause anesthetic gases to mix with the nonscavenged driving gas of the ventilator. In one OR, the N2O level increased from 5 to 80 ppm each time the ventilator was used.
Errors in Anesthesia Technique
With a leak-proofed anesthesia workstation and breathing system and an effective scavenging system, 94% to 99% of all OR anesthetic contamination is caused by errors in anesthesia technique.11 The following are significant errors in technique:
1. Incorrect insufflation techniques.
2. Turning the N2O flowmeter and/or a vaporizer on while the breathing circuit is not connected to the patient causes direct loss of anesthetic gases into room air. This often occurs at the beginning and end of anesthesia administration and during intubation. When the breathing circuit is not connected to the patient, the anesthesiologist should turn off the vaporizer and the gas flows; otherwise, anesthetic-laden gas in the circuit will flow into the room.
3. A poorly fitting face mask, laryngeal mask, or other airway device permits leakage of anesthetic gases around the rim.20
4. An uncuffed tracheal tube that is too small relative to the tracheal diameter or a poorly seated cuffed tracheal tube may allow anesthetic gases to leak and spill into the room air.
5. At the conclusion of surgery, if a deeply anesthetized patient is disconnected from the breathing circuit, relatively high concentrations of anesthetic gases can be exhaled into the room air.
6. Accidental spillage of liquid volatile anesthetic agent while a vaporizer is refilled adds vapor to room air; each milliliter of spilled liquid adds about 200 mL of vapor.
7. Emptying the breathing circuit of anesthetic gases while the circuit is disconnected from the patient spills anesthetic gases directly into the room air.
Cryosurgery
Cryosurgery is used by gynecologists, ophthalmologists, otolaryngologists, and dermatologists and is a major source of OR contamination.21 A jet of 20 to 90 L/min of liquid N2O is used as a surgical tool. The liquid rapidly evaporates and raises the level of N2O in the air. Air contamination with N2O is particularly high when cryosurgery is used in a small, poorly ventilated office.22 These units should be scavenged.
Miscellaneous Sources of Anesthetic Contamination
When a potent volatile anesthetic agent is used during cardiopulmonary bypass, the waste anesthetic vapor is discharged into the room air because scavenging of pump oxygenators affects their performance.23 Diffusion of anesthetic vapors from rubber and plastic goods in the anesthesia workroom is another cause of anesthetic contamination of the atmosphere. As much as 300 mL of anesthetic vapor may be released from a used rubber breathing circuit.11 Cardiopulmonary bypass machines are not sold with a scavenging system. Also, any vaporizer must be added in-house. A scavenging system should be set up from the exit port of the membrane oxygenator if inhaled anesthetic agents are used.24,25
A minor cause of anesthetic contamination is diffusion of anesthetic gases from the surgical wound and the patient’s skin. The concentration of halothane in the air immediately adjacent to the operating field was found to be three to six times higher than in the room air. Higher levels were also found under the surgical drapes, possibly as a result of diffusion of halothane from the patient’s skin.26
An infrequent cause of OR contamination is accidental crossing of the fresh air intake and exhaust ducts of the OR ventilation system. Also, installing the system exhaust port in a position upwind from the fresh air intake port may result in contamination of all ORs supplied by that ventilation system.
Another cause of OR contamination is failure to scavenge the exhaust from a sidestream-sampling capnograph or multigas analyzer. These monitors generally draw from 100 to 300 mL/min of gas via an adapter at the Y-piece in the breathing system. After it has passed through the analyzer, this gas should be directed either to the waste gas scavenging system of the machine or back into the breathing system.
Operating Room Ventilation Systems
The OR ventilation system is an important factor in reducing anesthetic air pollution. Unventilated ORs may have levels of trace anesthetic gases four times as high as those with proper ventilation. The nine components of a typical OR ventilation system are as follows:
1. Fresh air intake from outside
5. Manifold that distributes fresh air to the OR
6. Manifold that collects air from the OR
7. Fresh air inflow port in each OR
8. Exhaust port to the hospital air conditioning system in each OR
The OR fresh air inflow port is located in the ceiling, and the exhaust port is located on an adjacent wall 6 inches above the floor. In general, OR ventilation systems are one of two types: nonrecirculating or recirculating. The following sections describe the various features of each system.
Nonrecirculating Ventilation System
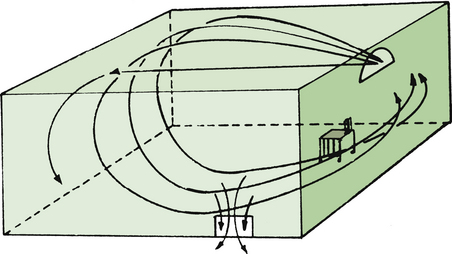
Most ORs are equipped with a nonrecirculating ventilation system (Fig. 5-3). This type of system pumps in fresh air from the outside and removes and discards all stale air. The number of air exchanges per hour varies significantly, even among ORs in the same suite. A survey of one OR suite revealed that the number of air exchanges varied from less than 5 to more than 30 per hour.
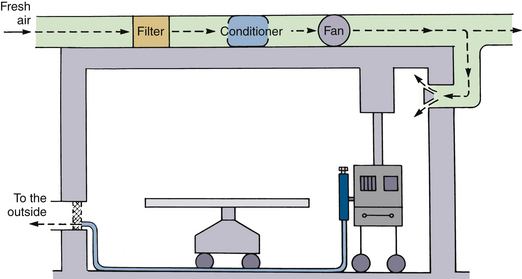
FIGURE 5-3 A nonrecirculating ventilation system serving as a passive disposal route for anesthetic waste gases. (From U.S. Department of Health, Education and Welfare [NIOSH] Publication No. 75-137, Washington, DC, 1975, U.S. Government Printing Office.)
The number of air exchanges per hour is an important determinant of the level of anesthetic contamination in the OR atmosphere. A rate of 10 or more exchanges per hour is recommended. Lower rates might permit creation of hot spots, air pockets highly contaminated by anesthetics; higher rates may create air turbulence that may cause discomfort to OR personnel.
The airflow pattern in the OR and the location of the anesthesia workstation in relation to the airflow also influences the level of anesthetic contamination (Fig. 5-4). When airflow generates floor-to-ceiling eddies, causing extensive air mixing, hot spots are reduced in size and number. On the other hand, hot spots are more likely to form with laminar airflow, which reduces air mixing.
Recirculating Ventilation System
A recirculating ventilation system partially recirculates stale air. Each air exchange consists of part fresh outdoor air and part filtered and conditioned stale air. This is more economical than the nonrecirculating systems because it requires less air conditioning. The recirculating system is particularly popular in locations with extremely hot or cold climates. Because filtering does not cleanse air of anesthetic gases, a recirculating system may contaminate clean ORs by recirculating air from contaminated ORs to clean ORs.
The American Institute of Architects published recommendations for ventilation in ORs and PACUs. New ORs are required to have 15 to 21 air exchanges per hour, of which three must be fresh outside air. For PACUs in use, the minimum number of total air changes is six per hour with a minimum of two air changes per hour of outdoor air.27
Waste Gas Scavenging Systems
Modern anesthesia workstations are factory equipped with scavenging systems. The Joint Commission (TJC; formerly known as The Joint Commission on Accreditation of Healthcare Organizations), requires that all waste anesthetic gases be scavenged using active scavenging methods. Although it is highly unlikely, anesthesia machines not equipped with such systems may still be in use. Some anesthesia personnel discharge the waste anesthesia gases toward the floor, believing that the heavier-than-air anesthetic gases form a layer on the floor and flow out via the OR ventilation system. In reality, waste anesthetic gases are effectively mixed with room air by air eddies created by OR traffic. When the anesthesia machine is equipped with a properly functioning scavenging system, the trace concentrations of waste anesthetic gases and vapors are reduced by 90%.
General Properties of Scavenging Systems
An anesthesia scavenging system collects the waste anesthetic gases from the breathing circuit and discards them. A properly designed and assembled system will not affect the dynamics of the breathing circuit, nor will it affect ventilation and oxygenation of the patient. The American Society for Testing and Materials (ASTM) document F1343-02, last published in 2002, provided standard specifications to serve as guidelines for the manufacturers of equipment that removes excess anesthetic gases from the working environment.28
A typical scavenging system consists of four parts: 1) a relief valve by which gas leaves the circuit, 2) tubing to conduct the gas to a scavenging interface, 3) the interface, and 4) a disposal line. Scavenging systems are classified as either active or passive. In an active scavenging system, a substantial negative pressure (hospital vacuum) is applied to the disposal line connected to the interface, and waste gas is literally sucked away from the interface. In a passive scavenging system, waste gases flow under their own pressure via a wide-bore tube to the OR ventilation exhaust grille.
Adjustable Pressure-Limiting and Ventilator Pressure Relief Valves
When a breathing circuit is in use, the waste anesthetic gases leave the circuit via an APL, or pop-off, valve (Fig. 5-5). The valve is usually spring loaded and requires only minimal positive pressure to open and allow escape of the waste gases from the circuit (see also Chapter 4). The valve has a single exhaust port (Figs. 5-6 and 5-7). To prevent accidental connection to the breathing circuit, this port is usually a 19-mm male fitting with a 1:40 conical shape.
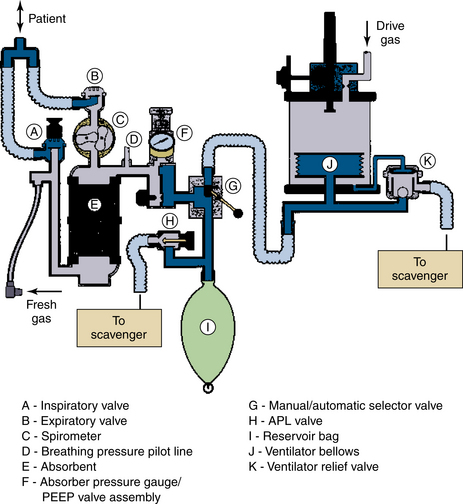
FIGURE 5-5 Points of exit for waste gas from a typical circle system. APL, adjustable pressure-limiting; PEEP, positive end-expiratory pressure. (Courtesy Dräger Medical, Telford, PA.)
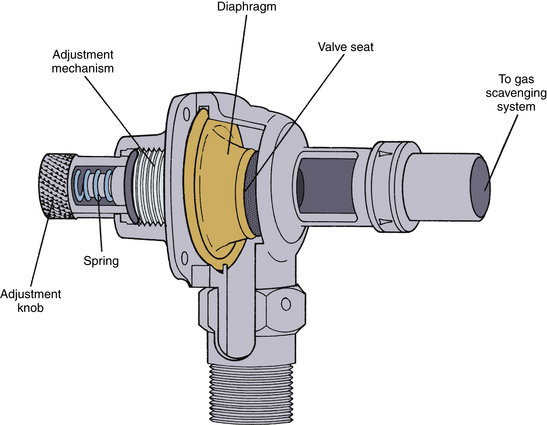
FIGURE 5-6 The Datex-Ohmeda adjustable pressure-limiting valve. (Courtesy GE Healthcare, Waukesha, WI.)
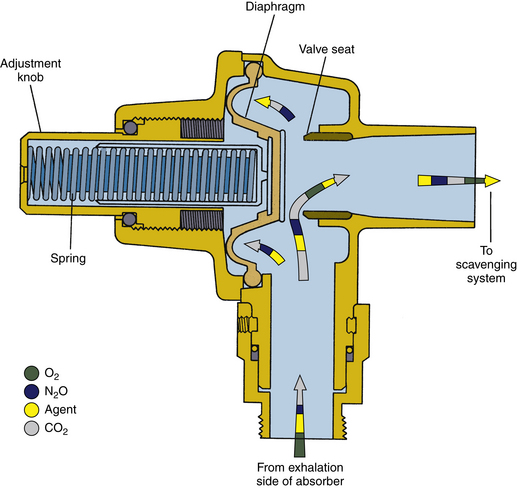
FIGURE 5-7 Schematic of Datex-Ohmeda adjustable pressure-limiting valve. Note that it is a spring-loaded valve in contrast with the needle-valve design used by Dräger Medical. (From Bowie E, Huffman L: The anesthesia machine: essentials for understanding. Madison, WI, 1985, Ohmeda. Reproduced by permission of GE Healthcare, Waukesha, WI.)
When an anesthesia ventilator is in use, the APL valve is out of circuit and the waste anesthetic gases leave the circuit via the ventilator pressure relief (PR) valve (Fig. 5-5). During inspiration, this valve is held closed by positive pressure transmitted from the ventilator driving gas circuit or some other mechanism, as in the case of piston ventilators. The exhaust port of the PR valve is also 19 mm in diameter, as with the APL valve.
Conducting Tubing
The conducting tubing moves the waste anesthetic gases from the APL and PR valve to the scavenging interface. Tubing usually is specified to be of 19 mm or 30 mm diameter to avoid accidental connection to the breathing circuit, and it is made sufficiently rigid to prevent kinking.24
Scavenging Interface
The scavenging interface system is a safety mechanism equipped with relief valves interposed between the breathing circuit and the hospital’s vacuum or ventilation system. A scavenging interface system may contain either a closed or an open reservoir.
Closed-Reservoir Scavenging Interface Systems
A closed-reservoir interface system includes a reservoir bag, which contains exhaled waste anesthetic gases, and spring-loaded valves that prevent the hospital evacuation system from exerting excessively high or low pressures on the breathing circuit (Figs. 5-8 and 5-9). The waste gases can be evacuated from the reservoir bag using either the hospital vacuum or ventilation system.
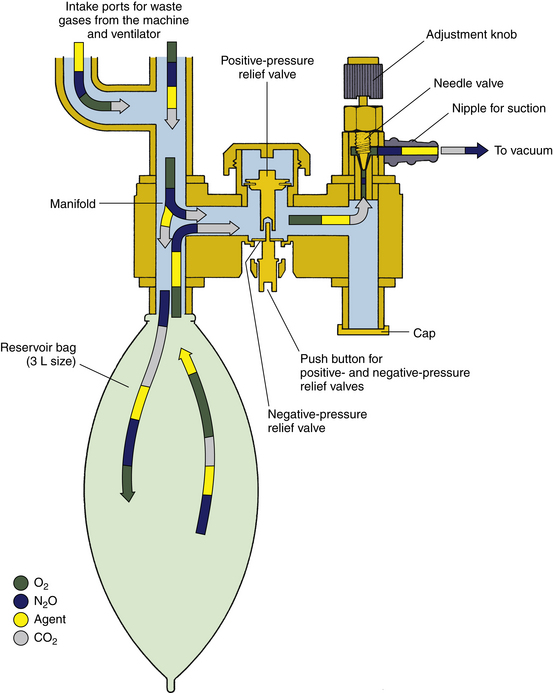
FIGURE 5-8 Datex-Ohmeda closed-reservoir active scavenging safety interface. The negative-pressure relief valve opens at a pressure of −0.25 cm H2O, and the positive-pressure relief valve opens at 4 to 5 cm H2O. (From Bowie E, Huffman L: The anesthesia machine: essentials for understanding. Madison, WI, 1985, Ohmeda. Reproduced by permission of GE Healthcare, Waukesha, WI.)
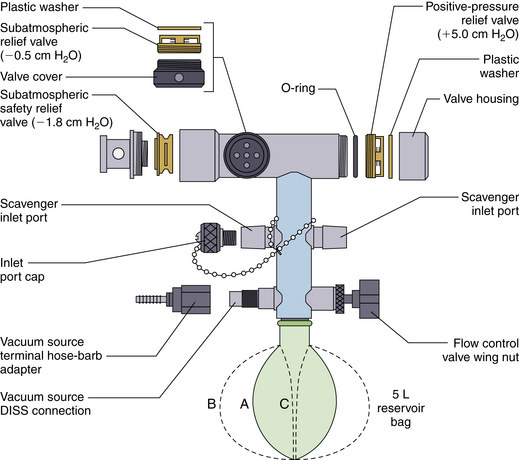
FIGURE 5-9 Schematic of the Dräger scavenging safety interface. This is a closed-reservoir active scavenging system. A, Normal state of distension of the scavenger reservoir bag. B, Overdistended bag as a result of excess delivery or inadequate removal of waste gases from the interface. C, Collapsed position of the bag from application of excessive vacuum to the interface. DISS, diameter index safety system. (Courtesy Dräger Medical, Telford, PA.)
When the hospital vacuum system is used to evacuate waste anesthetic gases, a failure of the system results in excessive pressure buildup in the reservoir, the pop-off valve opens, and the waste anesthetic gases are vented into the room. Such pop-off valves usually open when the positive pressure in the reservoir reaches 5 cm H2O, but some newer systems, such as the Datex-Ohmeda Aisys systems, open at 10 cm H2O.29 In contrast, if the hospital vacuum system generates excessive negative pressure, the negative-pressure relief, or “pop-in,” valve will open and allow room air to be sucked into the reservoir. This avoids application of negative pressure to the breathing circuit.
The Datex-Ohmeda closed-reservoir interface system (see Fig. 5-8) has one negative-pressure relief valve, whereas the Draeger system has two (see Fig. 5-9). The negative-pressure relief valves pop inward when the pressure in the reservoir falls below −0.5 to −1.8 cm H2O.
Figure 5-10 depicts a closed-reservoir interface system evacuated by the hospital ventilation system. No vacuum is connected to the interface, and the needle valve is closed. This is an example of a passive scavenging system.
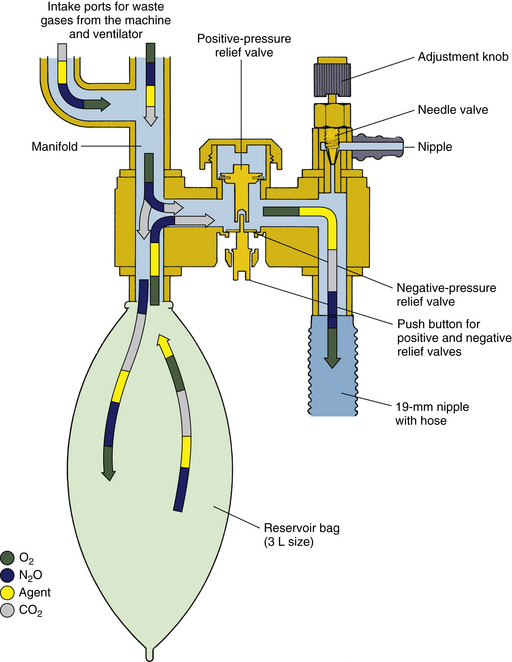
FIGURE 5-10 Datex-Ohmeda closed-reservoir scavenging interface used as a passive system. The vacuum is not connected (compare with Figure 5-9), and gas flows passively to the operating room exhaust ventilation duct. (From Bowie E, Huffman L: The anesthesia machine: essentials for understanding. Madison, WI, 1985, Ohmeda. Courtesy GE Healthcare, Waukesha WI.)
In addition to containing the waste anesthetic gases during exhalation, the reservoir bag of a closed-reservoir scavenging interface provides a visual indication of whether the scavenging system is functioning properly. An overdistended bag indicates a weak vacuum or occluded disposal route. A collapsed bag indicates excessive vacuum. When the scavenging system is operating properly, the bag fills during exhalation and empties during inhalation.
Open-Reservoir Scavenging Interface Systems
An open-reservoir scavenging interface system (Figs. 5-11) is valveless and uses continually open relief ports to avoid positive or negative pressure buildup. In the Draeger open-reservoir interface, the reservoir canister contains the excess waste gas that arrives from the breathing circuit during exhalation, and the gas is removed from the reservoir by the hospital vacuum. Because this type of interface depends on open ports for pressure relief, care must be taken to ensure that the ports remain unoccluded at all times.30
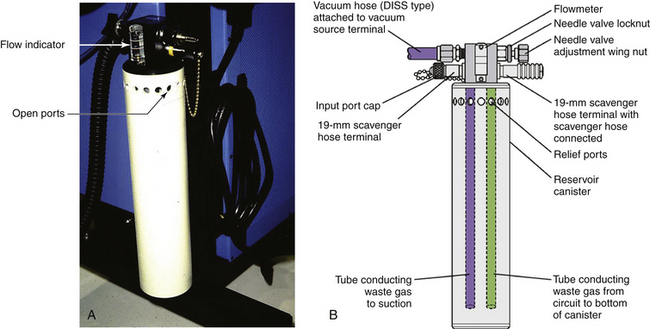
FIGURE 5-11 A, An open-reservoir scavenging system. B, Schematic. DISS, diameter index safety system. (Courtesy Dräger Medical, Telford, PA.)
An open-reservoir scavenging interface incorporates a flowmeter to show that gas is being sucked from the interface into the hospital vacuum disposal system. The Dräger interface has a needle-valve adjustment nut; when the flowmeter ball “floats” between the two line markings, it indicates that gas is being removed from the interface at a rate of 25 L/min (Fig. 5-12). If waste anesthetic gas leaves the breathing system at 3 L/min (fresh gas flow is approximately 3 L/min), the difference in flow of 22 L/min (25 L/min − 3 L/min) is made up by room air entering via the open ports.
A reservoir bag is sometimes incorporated in the open-reservoir systems for the same reasons it is incorporated in closed-reservoir systems.
Disposal Routes
Waste anesthetic gases may be disposed of by active disposal routes, such as wall suction or a dedicated evacuation system, or by passive disposal routes, either the OR ventilation system or a through-the-wall conduit.
Active Disposal Routes
When waste anesthetic gases are disposed of by way of wall suction, the following requirements should be met:
1. The wall suction should be capable of drawing at least 30 L/min of air.
2. The scavenging interface should be equipped with at least one negative-pressure relief valve (closed reservoir) or ports open to the atmosphere (open reservoir).
3. The exhaust port of the wall suction should be at a safe distance from the breathing zone of personnel.
4. Explosive anesthetic gases should not be used (per National Fire Protection Association regulations).
It is preferable to have a separate, dedicated vacuum system for waste anesthetic gases. This is because of two significant disadvantages when the wall suction system is used. First, the strength of the wall suction may not be sufficient to meet the needs of both the anesthesiologist and the surgeon in addition to the needs of the scavenging system. Diverting 20 L/min of air to the scavenging system reduces the intensity of the remaining vacuum by 25%, which may not be adequate to meet the needs of the anesthesiologist. Second, waste anesthetic gases may damage the vacuum system’s machinery.
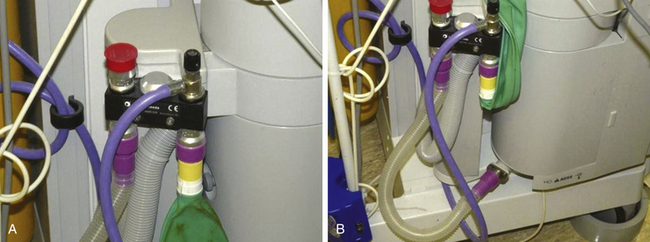
Waste gas scavenging connectors and tubing are purple or yellow, and associated fittings are either 19 mm or 30 mm in diameter (Fig. 5-13, A and B). The waste gas removal tubing that connects the scavenging interface to the vacuum connection on the wall uses diameter index safety system (DISS) fittings to ensure the connection is appropriate.
Passive Disposal Routes
Operating Room Ventilation System
When used for disposal of waste anesthetic gases, the OR ventilation system should be of the nonrecirculating type and must meet the requirements of the American Institute of Architects. The hose leading from the scavenging interface to the ventilation outflow port should be kept off the floor to avoid accidental occlusion. If placed on the floor, the hose should be rigid enough to remain patent under a pressure of 10 kg/cm2 (Fig. 5-14).
Through-the-Wall Disposal
With a passive, through-the-wall disposal system, waste anesthetic gases flow through a duct in the wall, window, ceiling, or floor toward the outside. Because OR ventilation results in a slight positive pressure in the OR with respect to the outside, gases flow naturally into disposal ducts connected to the outside. A potential danger of such a disposal route is occlusion of the exhaust port by ice, nesting birds, or insects. In addition, gusting winds may generate positive pressure at the exhaust port that may interfere with disposal of waste anesthetic gases. These problems can be avoided by directing the exhaust port downward and shielding it with a wire screen. Also, an airflow indicator should be installed in the tubing between the anesthesia machine and the wall to confirm the proper direction of gas flow.
Scavenging the Anesthesia Ventilator
Modern anesthesia ventilators are factory equipped with a disposal system that directs waste anesthetic gases to the anesthesia workstation’s scavenging system. Older anesthesia ventilator designs, however, often discharge waste anesthetic gases directly into the ambient air or do not have a standard 19-mm scavenging connection. Also, older ventilators often are fraught with internal leaks, which mix the anesthetic gases with the ventilator’s driving gas. This significantly increases the volume of gas that must be disposed of with each breath and might therefore overwhelm the capacity of the scavenging system and result in spillage of anesthetic gases.
Scavenging Nonrebreathing (Pediatric) Anesthesia Systems
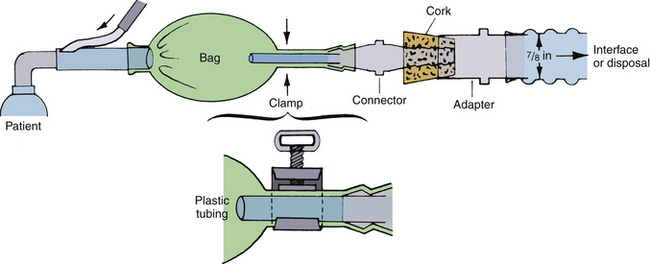
Scavenging a nonrebreathing anesthesia system is best accomplished by connecting its exhalation port to the scavenging system of the anesthesia machine. The pediatric Jackson-Rees system can be scavenged by connecting either the open tail of its breathing bag or of the relief valve (if one is used) directly to the scavenging interface of the anesthesia machine. Vital Signs (Totowa, NJ) manufactures a scavenging relief valve that can be used with all Mapleson systems, including the Jackson-Rees pediatric modification (Fig. 5-15). This relief valve can be easily connected to the scavenging interface of the anesthesia machine.
Scavenging Cryosurgical Units
Newer models of N2O cryosurgical units are equipped with built-in scavenging systems. Older models, however, do not have scavenging systems and spill large amounts of N2O gas into the ambient air. Fitting older units with scavenging systems is strongly recommended.
Scavenging Sidestream-Sampling Gas Analyzers
As previously mentioned, the exhaust from the sidestream-sampling gas analyzers should be conducted via tubing to the waste gas scavenging system or returned to the breathing system for scavenging (Fig. 5-16). Of note, if the multigas analyzer incorporates a paramagnetic oxygen analyzer, the analyzer draws a constant stream, usually about 10 mL/min of room air, to use as a reference. If the monitor exhaust gas is returned to the breathing system, this will include an additional 8 mL/min of nitrogen. In most cases this presents no problem, but if the circle is used as a closed system, nitrogen will accumulate (see also Chapter 8).
Hazards of Scavenging
Scavenging of the anesthesia breathing circuit increases the complexity, and consequently the hazards, of administering anesthesia. If the scavenging interface were mistakenly bypassed or were to malfunction, excessive positive or negative pressure in the scavenging system could be directly transmitted to the breathing circuit. This might cause cardiovascular embarrassment and pulmonary barotrauma to the patient. Excessive negative pressure may be caused by unopposed vacuum, and excessive positive pressure may be caused by occlusion of the connecting tubing (see also Chapter 30).
Scavenging mishaps typically are the result of user error. In one case, a one-way metallic connector was assembled in reverse and obstructed the disposal line, causing excessive positive pressure in the breathing circuit.31 No PR valve had been incorporated into the system. In another case, the perforated connector of a tube-within-a-tube assembly was accidentally replaced with one that had no perforations.32 The unopposed negative pressure in the scavenging system shut off the ventilator’s exhalation valve and caused excessive positive pressure in the breathing circuit, and the patient sustained bilateral pneumothoraces and subcutaneous emphysema. In yet another case, the expiratory breathing hose was mistakenly connected to the scavenging port of the relief valve and caused an abrupt increase in the pressure in the breathing circuit.33 Accidental occlusion of the disposal line by the wheels of the anesthesia machine has also been reported (Fig. 5-17).
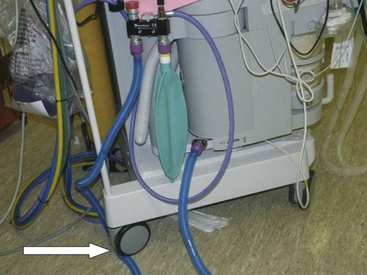
FIGURE 5-17 Occlusion of 19-mm scavenger hose conducting gas from the outlets of the adjustable pressure-limiting and pressure relief valves to the closed-reservoir active scavenging system. Note that the reservoir bag is collapsed. This situation can lead to high pressure in the breathing circuit and barotrauma.
The inherent vulnerability of scavenging systems has been the cause of some mishaps. In one case, the negative-PR valve failed to open despite excessive negative pressure in the system, causing the patient circuit reservoir bag to collapse.34 In another case, ice buildup in the exhaust port of a passive through-the-wall disposal route was apparently the cause of pneumothorax and death in a laboratory animal.35 In yet another case, a plastic bag was sucked in by the perforated connector of an active disposal route,36 which occluded the perforations and led to unopposed negative pressure in the breathing circuit.
Whenever abnormal pressure exists in the breathing circuit, the scavenging system should be checked for possible malfunction. If a malfunction is suspected, the system should be immediately disconnected from the breathing circuit.
In 2004, fires were reported in engineering equipment rooms that house the vacuum pumps used for waste anesthetic gas evacuation.37 In some hospitals, waste gases are not directly vented to the outside but may be vented into machine rooms that have vents that open to the outside. In some anesthesia workstations, the ventilator drive gas also is scavenged. This gas is 100% oxygen in most cases and is added to gas leaving the breathing system. As a result, the environments in these machine rooms may become highly enriched with oxygen gas. One sequela of this has been the production of fires in these spaces outside the OR.37 These sites also may contain equipment or materials, such as petroleum distillates (pumps, oil, or grease) that, in the presence of an oxygen-enriched atmosphere, could be extremely combustible and present a significant fire hazard.
Low-Flow Scavenging Systems
Active waste gas scavenging systems draw large volumes of gas—anesthetic waste plus entrained air in the range of 25 to 75 L/min—from each OR. This requires large and costly vacuum pumps; operating continuously, they incur a high energy cost. Some practitioners disconnect the scavenging interface from the vacuum system when the OR is not in use for long periods, such as overnight and on weekends. Most, however, do not; this results in unnecessary operation of the vacuum pumps, as they suck in room air and waste the fuel and energy needed to power them. Recently, in an effort to reduce the carbon footprint associated with running these pumps, a more efficient, low-flow scavenger interface has been designed and evaluated.38 The Dynamic Gas Scavenging System (DGSS; Anesthetic Gas Reclamation, Nashville, TN; Fig. 5-18) interface is a gas-tight metal container with a 3-L reservoir bag attached to ensure compliance with OSHA recommendations. The design is such that scavenging outflow to the vacuum system remains closed until a pressure of 0.5 cm H2O from the anesthesia workstation exhaust, via the APL or ventilator PR valve, is sensed in the interface enclosure by a sensitive pressure transducer. A solenoid valve then opens and remains open until the internal pressure reaches −0.5 cm H2O, thus emptying the interface reservoir bag. In this way the flow to the vacuum system is continuously titrated according to needs.
The DGSS was evaluated in a suite of four ORs and was placed in regular clinical use for 6 months while data were collected on failures, trace gas exposure, and use of the central vacuum pump for the OR suite. No failures of the DGSS were reported. In an anesthetic technique using fresh gas flows of 2 L/min and ventilator drive gas flows of 6 L/min (drive gas was scavenged), the central vacuum pump duty cycle decreased from 92% before installation of the DGSS to 12% when the ORs were in use and from 92% to 1% when the rooms were not in use. The authors suggest that their novel system will produce energy cost savings and may prolong the life of the vacuum pump. Cost savings are greatest when the DGSS is used with workstations that do not vent ventilator drive gas into the scavenging system and in those that use piston ventilators.
The DGSS is commercially available and compatible with many current anesthesia workstations (Fig. 5-19). An added benefit is that by producing a more concentrated flow of waste gases, technologies designed to recover potent inhaled anesthetic agents from the waste-gas flow are facilitated. Such technologies are likely to become more important because the inhaled anesthetics are greenhouse gases with the potential to increase global warming.39
Volatile Anesthetic Reclamation
An estimated 500,000 gallons of anesthetic agents are used annually in the United States, and this volume is subsequently released into the atmosphere. Reclamation of these agents would be of environmental benefit and also potential financial benefit. A system has been described by which inhaled anesthetic agents could be recovered from a concentrated waste gas stream, such as that produced by the DGSS, by condensation at cryogenic temperatures.40 The recovery rate of undiluted waste anesthetic gas using a condensation temperature of −100° C reportedly would be approximately 98%.41
Anesthetic Leak Detection and Waste Gas Management
The scavenging system removes waste anesthetic gases captured from only the relief valve. Spillage from other sources is not evacuated by the scavenging system and depends solely on the OR’s ventilation system for disposal. As a consequence, trace levels of anesthetic gases in the OR occasionally may be in excess of the levels recommended by NIOSH.
To reduce the level of anesthetic contamination in the OR, the anesthesia workstation should be regularly tested for leaks. Any leaks found should be corrected as soon as possible. Errors in anesthetic technique that lead to spillage of anesthetic gases also should be corrected.
To detect and prevent anesthetic leaks, a waste gas management program should be adopted in every OR suite. Anesthetic equipment should be checked with each use and at specified intervals by a service technician. The results should be documented, and the records should be maintained indefinitely. These records are useful for equipment maintenance follow-up and compliance with recommendations of government agencies and accrediting organizations. The records also can potentially be used for legal defense.
Work Practice Recommendations
Testing the High- and Intermediate-Pressure Systems for Leaks
Testing of the high- and intermediate-pressure systems of the anesthesia machine for leaks begins with the DISS quick connector in the N2O central supply line. The connector is submerged in water or rinsed with liquid soap; the appearance of gas bubbles indicates a leak. Liquid soap also can be used to detect leaks in the yokes of the reserve N2O tanks. Faulty quick connectors should be immediately repaired or replaced, the yokes of the reserve tanks should be tightened, and defective washers should be replaced (Fig. 5-20).
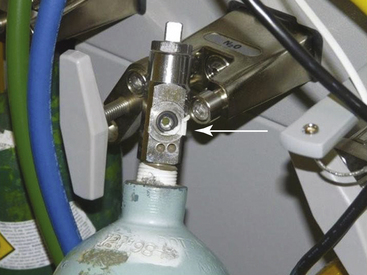
FIGURE 5-20 High-pressure leak caused by a defective washer (arrow) on the yoke of a N2O reserve tank.
Next, the N2O high-pressure system inside the anesthesia machine is tested. The N2O central supply hose is disconnected from the machine, a reserve N2O tank is used to pressurize the N2O system, and the tank is turned off. The N2O system pressure indicated by the N2O pressure gauge is recorded and then rechecked 1 hour later; a significant decrease in pressure suggests a leak inside the machine between the N2O reserve tank and the N2O flowmeter.
In one study, significant high-pressure N2O leaks were detected in 50% of the anesthesia machines.11 After the leaks were corrected, the background N2O level in the OR suite decreased from 19 to 0.2 ppm.
Internal N2O leaks are difficult to correct and should be left to the manufacturer’s maintenance service. A high-pressure N2O system should be serviced by the manufacturer at regular intervals and tested by departmental anesthesia technicians.
Testing the Low-Pressure System for Leaks
The following procedure has been recommended for testing the low-pressure system for leaks:
1. Remove the breathing hoses and bag from the anesthesia breathing circuit.
2. Connect the two unidirectional valves with a short piece of corrugated hose.
3. Close the APL valve tightly, remove the reservoir bag, and occlude the bag mount opening; this minimizes the compliance of the low-pressure system.
4. Slowly turn on the oxygen flowmeter until the breathing circuit pressure gauge indicates 40 cm H2O.
5. Record the rate of oxygen flow necessary to maintain this pressure for 30 seconds. This rate of flow is equal to the low-pressure system’s leak rate. The low-pressure leak rate generally should not exceed 200 mL/min, which would contribute no more than 4 ppm of N2O air pollution to an average-sized OR.
Correcting Errors in the Anesthesia Technique
The following precautions in anesthetic technique significantly reduce the spillage of anesthetic gases:
1. All gases and vaporizers should be turned off when the patient is not connected to the breathing circuit, such as during intubation.
2. The face mask should be carefully selected and firmly held against the patient’s face.
3. The tracheal tube should be carefully selected. Except for pediatric patients, no leak should be allowed around the cuff.
4. Upon conclusion of anesthesia, when the reservoir bag is emptied of anesthetic gases, the breathing circuit should be connected to the patient with the relief valve held wide open. This should direct the contents of the bag into the scavenging system.
5. After completion of surgery, and while still deeply anesthetized, the patient should remain connected to the breathing circuit, breathing 100% oxygen for a few minutes. This will direct the exhaled anesthetic gases to the scavenging system of the anesthesia machine.
6. Vaporizers should be refilled in the evening, when the OR is not being used. This reduces the risk of exposing OR personnel to anesthetic vapors from accidental spillage of volatile anesthetic liquid while the vaporizers are refilled. The careful use of agent-specific filling devices reduces the amount of volatile anesthetic agent spilled (see Chapter 3).
Miscellaneous Preventive Measures
The OR ventilation system should be regularly serviced to ensure optimal function. Hospital engineers should check the filters for cleanliness and the dampers for proper position and balance. If the system is allowed to deteriorate, the number of air exchanges per hour gradually decreases, and the level of anesthetic contamination increases. Also, the function of the hospital vacuum should be checked at regular intervals to ensure adequate negative pressure and flow capacity.
In the PACU, the concentration of N2O in the air is directly related to room ventilation and the number of patients in the unit. If ventilation is maintained at the rate of 500 m3 of fresh air per patient per hour, the mean N2O level in the room is approximately 10 ppm.26
Monitoring Trace Levels of Anesthetic Gases
Monitoring the concentration of trace anesthetic gases may be undertaken to 1) determine whether the leak-detecting and waste anesthetic gas program is effective, 2) uncover occult leaks from unexpected sites, and 3) document compliance with OSHA recommendations.
Monitoring Trace Anesthetic Levels in the Operating Room
The levels of both N2O and halogenated agents in the OR can be determined individually. Infrared (IR) analyzers can measure trace levels of anesthetic agents. These devices are capable of measuring not only N2O, but all the volatile anesthetics in trace concentrations. The MIRAN SapphIRe series of analyzers (Thermo Environmental Instruments, Franklin, MA) measures N2O in the range of 0 to 100 ppm and the halogenated agents in a range of 0 to 30 ppm (Fig. 5-21). Because the level of halogenated agents in the OR atmosphere is closely related to that of N2O, the level of the halogenated agent can be extrapolated from that of N2O, as follows:
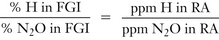
where H is the halogenated agent, FGI is fresh gas inflow, and RA is room air.

FIGURE 5-21 MIRAN SapphIRe infrared spectrophotometer. (Courtesy Thermo Fisher Scientific, Franklin, MA.)
Measurements of trace anesthetic levels are reliable only if the anesthetic gases are evenly distributed in the ambient air. The uniformity of distribution depends on four factors: 1) the number of room air exchanges per hour, 2) the flow pattern of room ventilation, 3) placement of the anesthesia machine in relation to the ventilation flow, and 4) traffic patterns of personnel in the OR. When the number of room air exchanges is greater than 10 per hour, the anesthetic gases are fairly evenly distributed in room air. The air near the ventilation outflow grille represents the mean level of the trace anesthetic gases in the room.22
Optimum reduction of air contamination is achieved by certain control measures that include scavenging and work practices to reduce trace gas levels. Monitoring can determine the efficacy of control measures and leak identification, defects in technique, and documentation of compliance with the recommended standard. OSHA recommends that air sampling for anesthetic gases be conducted every 6 months to measure worker exposures and to check the effectiveness of control measures. Furthermore, OSHA recommends monitoring only the most frequently used agents because proper engineering controls, work practices, and control procedures should reduce all agents proportionately. However, the decision to monitor only selected agents could depend on the frequency of their use as well as the availability of an appropriate analytic method and cost of instrumentation.
The American Society of Anesthesiologists (ASA) emphasizes regular maintenance of equipment and scavenging systems, daily checkout procedures for anesthesia equipment, and education to ensure use of appropriate work practices. The ASA does not consider a routine monitoring program necessary when these actions are being carried out, but rather encourages the use of monitoring when indicated, such as in the event of a known or suspected equipment malfunction. OSHA recommends that monitoring be done either by a knowledgeable person familiar with techniques of sampling or by an industrial hygienist. Monitoring requires both sampling and analysis; the latter may be performed by IR methods or by gas chromatography, either inside or outside the hospital.
Sampling Methods
Two decisions must be made with regard to sampling: where to do it and when to do it. Various methods of sampling are available.
Where to Sample
Monitoring personal exposure by sampling the atmosphere in the face mask area of exposed personnel is not required by NIOSH, although perhaps this method offers the most pertinent information on individual exposure. Sampling in the immediate work area of the most highly exposed person, the anesthesiologist, is recommended. One study has shown that measuring N2O at the level of the anesthesia machine’s shelf correlates well with personal sampling when levels of N2O are below 35 ppm.42 Shelf-level measurement is certainly more practical than personal sampling.
General area sampling is valid if complete air mixing has been previously demonstrated in the OR. In a room where all leakage is under effective control, gas concentrations may be uniform, making this kind of sampling appropriate. This type of sampling may be most appropriate in the empty OR to detect background leakage.
When to Sample
The timing of sampling also is critical to obtain representative exposure concentrations. Three types of temporal sampling are 1) grab sampling, 2) TWA sampling, and 3) continuous sampling.
Temporal Sampling
Grab Sampling
Grab sampling is useful for monitoring steady-state conditions—for example, high-pressure leakage from N2O lines—and to establish baseline N2O levels. With this technique, an air sample from the empty OR is taken for subsequent analysis. Inert containers are available for this purpose. The container is sealed and mailed to the company for analysis of the contents. Delay in obtaining the results is one disadvantage; another is that the value of such sampling is obviously limited because anesthetic leakage tends to be intermittent.
Time-Weighted Average Sampling
The TWA sample may be obtained by continuously pumping ambient air into an inert bag at a constant low rate of flow, usually around 4 L/h. The bag has a capacity of 20 to 30 L, and five-layer aluminized 5-L gas sampling bags also can be used to collect a sample. The resulting concentration in the bag therefore represents an average exposure over the collection period; commercially available TWA gas sampling systems provide TWA concentrations over periods of 1 to 8 hours.
The sampling pump can be mounted on a pole in the OR, and aliquots of a collected TWA sample can be analyzed in-house or mailed in an inert container to a central testing laboratory. The TWA for personal exposure also can be obtained from a battery-powered sampling pump and collection bag worn by the anesthesiologist. Clearly, wearing a bag and pump is inconvenient; however, knowledge of the TWA concentrations is useful for documentation purposes because the recommended standard refers to a TWA concentration of 25 ppm, although the time period for collection is not specified. The OSHA Chemical Information Manual, accessible online, contains current sampling technology for several anesthetic gases.
Nitrous Oxide Time-Weighted Average Sampling
Personal N2O exposures can be determined by using a passive monitor, such as the Vapor-Trak (Kern Medical Products, Farmingdale, NY). These are sometimes called passive dosimeters or diffusive samplers (Fig. 5-22). The dosimeter is a lightweight badge that can be worn by personnel to monitor their individual exposure; its sensitivity is 2 ppm. It can also be mounted on a pole for area sampling. The minimum sampling duration for the dosimeter is 15 minutes; however, it can be used for up to 8 hours of passive sampling. At the end of the sampling/exposure period, the dosimeter is mailed to a laboratory for analysis; the results are sent by e-mail to the individual and/or institution.
Sampling of Halogenated Agents
Three chlorofluorocarbon-based anesthetic agents—halothane, enflurane, and isoflurane—and one fluorocarbon-based agent, desflurane, are listed in the OSHA Chemical Information Manual. The current recommended media sampling for halothane, enflurane, and isoflurane requires an Anasorb tube, and the sample can be taken at a flow rate of 0.5 L/min; total sample volumes not exceeding 12 L are recommended. The current recommended sampling media for desflurane requires an Anasorb 747 tube, and the sample can be taken at a flow rate of 0.05 L/min; total sample volumes not exceeding 3 L are recommended. As with the N2O dosimeters, the tubes are sent to a laboratory for analysis.
Continuous Sampling
The best method for monitoring the OR atmosphere is with the portable IR spectrophotometer, which is capable of continuous sampling (see Fig. 5-21). Because this device offers a continuous readout, it can be used for detection of leaks, demonstration of errors in anesthetic technique, and determination of trace gas levels. Analyzers operate on the principle that most gases possess unique IR absorbance spectra. For example, the IR absorbance spectrum for N2O peaks at a wavelength of around 4.5 μm (see Chapter 8).
IR spectrophotometer trace gas analyzers work by action of a pump that perfuses a cell with OR air. To measure N2O, the IR source generates a light beam that is filtered to pass the IR component at a wavelength of approximately 4.5 μm. The beam is transmitted through the sample cell and is sensed by a detector: the higher the concentration of nitrous oxide, the more IR radiation is absorbed and the less energy is sensed at the detector. The signal is processed and displayed in parts per million N2O, although IR analysis can detect concentrations in parts per 100 million; the sensitivity of the instrument is a function of the path length of the IR beam through the sample of gas, and path lengths of 20 m or more produce very high sensitivity.
Trace concentrations of anesthetics in question are clearly well below the concentrations used for clinical anesthesia. For this reason, the analyzers used to monitor clinically useful concentrations of anesthetic gases, such as the IR gas analyzers used to measure inspired and end-tidal concentrations in the OR, are not sensitive enough to monitor trace concentrations in the atmosphere.
By using a chart recorder connected to the output of an IR trace concentration gas analyzer, a continuous plot of the N2O concentration in room air can be obtained during anesthesia. The value of this type of monitoring is quite obvious. Instantaneous peaks on the chart reflect a high level of leakage of the gas, such as that resulting from adjustment of a face mask. Alternatively, the chart may reflect a leak in some other part of the system. Also, because levels of waste gases do fluctuate, this approach is more reliable than a grab sample for measuring concentrations that may be inhaled by personnel in the OR.
TWA concentrations may be obtained from the IR trace concentration gas analyzer by integrating the output; that is, by determining the area under the time-concentration curve and dividing by the time. This can be done by counting the squares (in a chart record) or with a planimeter. The integration for N2O also can be derived electronically by a relatively inexpensive microcomputer that provides a continuously updated TWA concentration. If a peak concentration suggestive of a leak in the system is sensed, the analyzer, acting as a leak detector, can be used to “sniff out” the source of the leak.
The ideal air monitoring program should measure all anesthetic gases used in the OR. The recommended standard, however, requires monitoring only of the most frequently used agent because following recommended work practices and control procedures will reduce all agents proportionately. Thus N2O can be monitored and act as a tracer for the potent agents that may be administered along with it in some fixed proportion. However, in rooms where N2O is rarely used (i.e., cardiac ORs), this technique is not satisfactory. In these rooms the specific halogenated agents must be monitored.
Biological Exposure
Sonander and colleagues43 attempted to correlate biologic exposure as measured by analysis of urine samples from exposed OR personnel with technical exposure as measured by gas sampling. They studied four anesthesiologists and 25 nurse anesthetists. Urine samples were obtained early in the day, before OR work began, and then 8 hours later during the workday. These personnel also wore personal sampling pumps so that TWA exposure concentrations could be obtained. The N2O concentrations in the gas above the urine in the collection containers, measured by gas chromatography, showed a good correlation (r = 0.97) with technical exposure measurements. The authors suggested that this method of analyzing urine gas provides a useful means of assessing biologic exposure during routine anesthetic work.
Olfaction
Although the anesthesiologist is the ultimate monitor of the patient in the OR, human senses are not very effective at detecting trace concentrations of anesthetics. The olfactory thresholds for N2O and halothane are 10% to 30% (100,000 to 300,000 ppm) and 0.005% to 0.01% (50 to 100 ppm), respectively.44
Are trace concentrations of waste anesthetic gases hazardous?
The United States currently has no formal requirements for monitoring the OR atmosphere for waste anesthetic gases, but recommendations do exist.45,46 Although published in 1977, the limits recommended by NIOSH have not been enacted into law by OSHA, and it is quite likely that they never will be. Such a law has been opposed by both hospitals and anesthesiologists because the recommendations are considered by many to be unreasonable. First, the limits are recommended on the basis of the work of Whitcher and colleagues,11 who showed that these levels were readily achievable. However, their results have not been duplicated.47 In 1979, they reported data to the effect that even when all reduction measures were taken, exposure levels strongly depend on the anesthetic technique used. Thus, when a mask was used, mean levels of 180 ppm of N2O were found. When patients underwent tracheal intubation, mean levels of 16 ppm were achieved. Should all anesthetized patients therefore undergo tracheal intubation so that this arbitrary level can be maintained? Obviously not. Clearly, each technique has its associated degree of atmospheric contamination. An inhalational induction of a pediatric patient would certainly violate the recommended standard, yet it has obvious advantages for the patient. Second, the results of tests of psychomotor activity conducted by Bruce and Bach,13 which also formed a basis for the recommended standard, have not been substantiated by others, all of whom found no effects at much higher trace concentrations of anesthetics.48,49 Third, no “safe” trace level has ever been demonstrated for any of the anesthetics in use.
An even more fundamental issue still remains unresolved. Are trace levels of anesthetics really hazardous? Certainly no cause-and-effect relationship has ever been demonstrated.50,51 The studies to date that have incriminated anesthetics have all been based on questionnaire surveys sent to people who were assumed either to have been exposed to trace anesthetics in the OR, such as members of the ASA or AANA, or those assumed not to have been exposed, such as pediatricians. Such studies are notoriously unreliable; they are open to responder bias, which could explain all the observed differences to date between “exposed” and “unexposed” personnel.
That such bias exists in these studies was very clearly demonstrated by Axelsson and Rylander,52 who studied 655 pregnancies among workers in one Swedish hospital. Following a postal questionnaire survey, they checked the accuracy of the responses they received against the respondents’ medical records. They found that all women who had miscarriages and who worked at sites with exposure to anesthetic gases correctly reported their work sites and miscarriages, whereas one third of all miscarriages by women who were not exposed during pregnancy went unreported in the questionnaire. The authors concluded that their study draws attention to the methodologic difficulties in using questionnaires to study pregnancy outcome and pinpoints the importance of responder bias.
The ASA Ad Hoc Committee on Effects of Trace Anesthetic Agents on Health of Operating Room Personnel retained a group of epidemiologists and statisticians, the Epistat Group, to review all the available data pertaining to health hazards among OR workers. This group reviewed 17 published reports.53 Four were excluded from further consideration because they did not present data on the specific outcomes under evaluation. Five more were excluded because they did not use comparable control groups. Data from two studies among dentists and dental assistants were also excluded because the exposure of these personnel to anesthetic gases substantially differs from that of OR personnel. Thus data from only six studies were considered worthy of scrutiny. The group’s report found that the only health hazard consistently reported among female OR workers was an increased relative risk of spontaneous abortion.
The Epistat Group authors observed that, on the basis of the available data, the relative risk for spontaneous abortion among exposed women is approximately 1.3, or 33% greater than for nonexposed women. The authors further stated that the magnitude of this increase is well within the range that might be due to bias or uncontrolled confounding variables, such as responder bias, and that epidemiologic data currently available are insufficient for developing standards or setting exposure limits. Furthermore, even if this increased relative risk were genuine, it should still be viewed in perspective. For example, maternal smoking of one pack of cigarettes per day increases the spontaneous abortion rate by 80%, and maternal consumption of alcohol may increase the rate by 200%.40,54
The report urged that future studies be “prospective cohort studies, with careful documentation of type, amount and duration of exposure, meticulous and uniform follow-up, and thorough ascertainment and confirmation following predefined criteria of outcome events” to avoid previous methodologic errors.
In the same year, Tannenbaum and Goldberg55 came to a similar conclusion. Their analysis of articles finding adverse health effects from exposure to trace anesthetic gases found that methodologic errors in data collection may have led to inaccuracies in interpretation of the data. They also recommended a prospective study be performed with appropriate epidemiologic methods to avoid bias and confounding variables. Several other reviews have come to the same conclusion.56
Spence and Maran57,58 conducted a prospective study in the United Kingdom. They surveyed 11,500 female medical school graduates younger than 40 years and gathered information on occupational details, work practices, lifestyle, and obstetric and medical history. They found that female anesthesiologists had no greater incidence of infertility compared with other physicians. They also found that the incidence of cancer was unrelated to occupation and that the incidence of spontaneous abortion, and of the development of a congenital abnormality in any infant, was not associated with the occupation of the mother, with working in the OR, or even with the presence of a waste anesthetic gas scavenging system.
Rowland and colleagues59,60 reported an increased incidence of infertility and spontaneous abortion in female personnel working in unscavenged rooms in the dental operatory where N2O was administered; levels of N2O may reach up to 1000 ppm under these circumstances. In any event, no adverse reproductive effects were reported in personnel who worked in areas where scavenging systems for waste anesthetic gases were in place.
In 1999, the ASA published the report of its Task Force on Trace Anesthetic Gases of the ASA Committee on Occupational Health. This informational booklet addresses analysis of the literature, the role of regulatory agencies, scavenging and monitoring equipment, and recommendations.46 A summary of the report appears below.
Risks
Studies have not shown an association between trace levels of anesthetic gases found in scavenged anesthetizing locations and adverse health effects on personnel.
Recommendations
1. Waste anesthetic gases should be scavenged.
2. Appropriate work practices should be used to minimize exposure to waste anesthetic gases.
3. Personnel working in areas where waste anesthetic gases may be present should be educated regarding current studies on health effects of exposure to waste anesthetic gases, appropriate work practices to minimize exposure, and machine check and maintenance procedures.
4. Evidence is insufficient to recommend routine monitoring of trace levels of waste anesthetic gases in the OR and PACU.
5. Evidence is insufficient to recommend routine medical surveillance of personnel exposed to trace concentrations of waste anesthetic gases, although each institution should have a mechanism for employees to report suspected work-related health problems.
To date, trace concentrations of anesthetics have not been satisfactorily proven to be a cause of health problems in OR workers. However, absence of evidence does not constitute evidence of absence; because the possibility of a hazard may exist, measures should be taken to reduce exposure. This recommendation is made by all concerned agencies, including NIOSH, the American Hospital Association, TJC, and the ASA. Exposure limits for N2O have been suggested or agreed upon in some countries, namely The Netherlands (25 ppm) and the United Kingdom, Italy, Sweden, Norway, and Denmark (100 ppm).61-63 The differences illustrate the difficulty in setting standards without adequate data.
The ASA Task Force on Trace Anesthetic Gases of the Committee on Occupational Health of Operating Room Personnel emphasizes that the administration of anesthesia and the safety of the patient are the primary goals, and that atmospheric contamination control must be of secondary concern. This document on waste anesthetic gases emphasizes the use of scavenging and work practices to reduce levels of trace concentrations of waste anesthetic gases and the importance of education. Regular checking and documented maintenance of anesthetic equipment also is recommended. Quality assurance data provide excellent documentation that if the above measures are carried out, levels of trace gases will be below the NIOSH recommendations. In determining adequacy and appropriateness of waste gas scavenging systems, current recommended standards should be reviewed and adopted.
Environmental Concerns
Since the report from the ASA Task Force on Trace Anesthetic Gases of the Committee on Occupational Health of Operating Room Personnel was published in 1999, concern has been growing about the potential global warming effects of chemicals released into the atmosphere. The potent inhaled anesthetic agents isoflurane, sevoflurane, and desflurane are minimally metabolized in the body; once they are released into the atmosphere, they have long lifetimes there.64 Ryan and Nielsen39 have calculated the relative impact of inhaled anesthetics as greenhouse gases and on global warming. They concluded that desflurane has a greater potential impact on global warming than either isoflurane or sevoflurane, and that N2O alone produces a significant greenhouse gas contribution compared with sevoflurane or isoflurane. Their recommendations are to avoid N2O as a carrier gas if possible and to avoid unnecessarily high fresh gas flow rates, especially when using desflurane. They acknowledge that using low fresh gas flow rates requires increased use of CO2 absorbents, the disposal of which introduces other environmental concerns. The ideal solution would be to avoid release of waste anesthetic gases into the atmosphere altogether and to reclaim (recycle) them for subsequent use.
Low-Flow (Fresh Gas) Anesthesia
Most of the anesthetic fresh gas flow eventually finds its way into the atmosphere; minimizing such flow therefore offers both economic and environmental advantages. Low-flow anesthesia means different things to different people. Modern anesthesia workstations and their monitoring features facilitate the use of low flows, but a certain amount of education concerning anesthetic uptake and circuit dynamics is required.65
To help clinicians optimize fresh gas flow settings in real time, one manufacturer (Dräger Medical) has incorporated a Low-Flow Wizard (LFW) into its Narkomed 6000 series and Apollo workstations. The LFW displays the minimum fresh gas flow required to meet patient uptake and system leakage compared with the currently set total fresh gas flow (Fig. 5-23). The minimum fresh gas flow required is continuously computed as the sum of four variables: 1) any leakage between the inspiratory and expiratory ports of the circle system, 2) oxygen uptake, 3) agent uptake, and 4) N2O uptake. Items 2, 3, and 4 are determined from the inspired-expired concentration difference and the patient’s minute ventilation. The information is displayed as two bar graphs: the top graph shows loss of volume from the system, and the bottom shows the current fresh gas flow setting (see Fig. 5-23).

FIGURE 5-23 Dräger Low-Flow Wizard display. The top bar graph shows the loss of volume from the system. The bottom bar graph shows the current fresh gas flow (FGF) setting. If the bottom bar is shorter than the top bar, the display bar is red (too little FGF). If the FGF is less than 1 L/min more than the required flow, then the display bar is green (efficient), as shown. If the FGF is more than 1 L/min more than the required flow, then the display bar is yellow (too much FGF). (Courtesy David Karchner, Dräger Medical, Telford, PA.)
Anesthetic Capture
An alternative solution to both scavenging waste anesthetic gases and protecting the environment is to insert an anesthetic agent–absorbing filter between the anesthesia machine’s waste gas scavenging interface and the hospital waste gas evacuation system in the OR. In 2002, Doyle and colleagues66 reported that silica zeolite was effective at completely removing 1% isoflurane from exhaled gases for periods of up to 8 hours. They concluded that “the technology shows promise in removing isoflurane emitted from anesthesia machine scavenging systems.” This technology is applied in the Deltasorb Anesthetic Collection Service (Blue-Zone Technologies Ltd., Concord, Canada), which uses a portable stainless steel canister that is delivered and exchanged weekly to health care facilities. The canister uses a sievelike filtering matrix (Deltazite) to selectively adsorb each halogenated anesthetic gas—desflurane, sevoflurane, and isoflurane—as waste gas flows through the canister en route to being vented to the atmosphere. Each canister weighs about 5 lb and can adsorb approximately two full bottles of halogenated anesthetics. When the canister is full, it is shipped to the company’s facility, where it is desorbed of contained anesthetic and returned to the hospital for further use (Fig. 5-24). At that time, the reclaimed anesthetic is stored while approval for its reuse is pending (personal communication, Mark Filipovic, Blue-Zone Technologies).
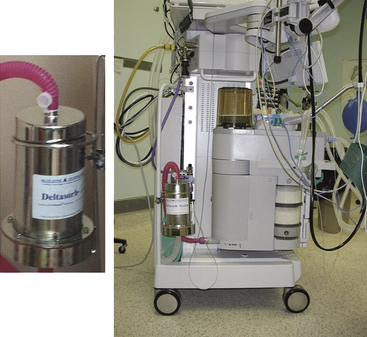
FIGURE 5-24 Left, The Deltasorb stainless steel canister contains Deltazite, an anesthestic agent–adsorbing sieve. Right, Location of the canister is between the waste gas scavenging interface on the anesthesia machine and the tubing connecting to the hospital’s waste gas evacuation system. (Courtesy of Mark Filipovic, Blue-Zone Technologies, Concord, Canada.)
References
1. Vaisman A.I. Working conditions in the operating room and their effect on the health of anesthetists [in German]. Eksp Khir Anestheziol. 1967;12:44–49.
2. Askrog V., Harvald B. Teratogenic effects of inhalation anesthetics. Nord Med. 1970;83(16):498–500.
3. Knill-Jones R.P., Rodrigues L.V., Moir D.D., Spence A.A. Anaesthetic practice and pregnancy: controlled survey of women anaesthetists in the United Kingdom. Lancet. 1972;1:1326–1328.
4. Corbett T.H., Cornell R.G., Lieding K., Endres J.L. Incidence of cancer among Michigan nurse anesthetists. Anesthesiology. 1973;38:260–263.
5. Corbett T.H., Cornell R.G., Endres J.L., Lieding K. Birth defects among children of nurse anesthetists. Anesthesiology. 1974;41:341–344.
6. Cohen E.N., Bellville J.W., Brown B.W., Jr. Anesthesia, pregnancy, and miscarriage: a study of operating room nurses and anesthetists. Anesthesiology. 1971;34:343–347.
7. Fink B.R., Shepard T.H., Blandau R.J. Teratogenic activity of nitrous oxide. Nature. 1967;214:146–148.
8. Congress of the United States. Occupational Safety and Health Act of 1970.
9. All About OSHA. U.S. Department of Labor and Occupational Safety and Health Administration. 2012, OSHA publication 3302.
10. Anonymous: Occupational disease among operating room personnel: a national study. Report of an Ad Hoc Committee on the Effect of Trace Anesthetics on the Health of Operating Room Personnel, American Society of Anesthesiologists. Anesthesiology. 1974;41:321–340.
11. Cohen E.N., Brown B.W., Bruce D.L. Occupational disease among operating room personnel: a national study. Anesthesiology. 1974;41:321–340.
12. Whitcher CE, Piziali R, Sher R, Moffat RJ: Development and evaluation of methods for the elimination of waste anesthetic gases and vapors in hospitals. Cincinatti, 1975, U.S. Department of Health, Education and Welfare, Public Health Service, Centers for Disease Control, NIOSH. Publication No. (NIOSH) 75–137.
13. NIOSH: Criteria for a recommended standard: occupational exposure to waste anesthetic gases and vapors. Bethesda, MD, 1977, US Department of Health, Education, and Welfare, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health.
14. Bruce D.L., Bach M.J. Effects of trace anesthetic gases on behavioural performance of volunteers. Br J Anaesth. 1976;48:871–876.
15. OSHA. Anesthetic Gases: Guidelines for Workplace Exposure. OSHA Directorate of Technical Support and Emergency Management [formerly Directorate of Technical Support]. July 20, 1999 revised May 18, 2000. Accessed 2012 Feb 20 at http://www.osha.gov/dts/osta/anestheticgases.
16. Pfaffli P., Nikki P., Ahlman K. Concentrations of anaesthetic gases in recovery rooms (letter). Br J Anaesth. 1972;44:230.
17. Mehta S., Burton P., Simms J.S. Monitoring of occupational exposure to nitrous oxide. Canad Anaesth Soc J. 1978;25:419–423.
18. Lecky J.H. The mechanical aspects of anesthetic pollution control. Anesth Analg. 1977;56:769–774.
19. Cottrell J.E., Chalon J., Turndorf H. Faulty anesthesia circuits: a source of environmental pollution in the operating room. Anesth Analg. 1977;56:359–362.
20. Hoerauf K.H., Hartmann T., Acimovic S., et al. Waste gas exposure to sevoflurane and nitrous oxide during anaesthesia using the oesophageal-tracheal Combitube small adult. Br J Anaesth. 2001;86:124–126.
21. Wray R.P. A source of non-anesthetic nitrous oxide in operating room air. Anesthesiology. 1980;52(1):88–89.
22. ECRI. Nitrous oxide exhausted from cryosurgical units:. Plymouth, PA: Emergency Care Research Institute; 1979.
23. Blokker-Veldhuis M.J., Rutten P.M., De Hert S.G. Occupational exposure to sevoflurane during cardiopulmonary bypass. Perfusion. 2011;26:383–389.
24. Hoerauf K., Harth M., Wild K., Hobbhahan J. Occupational exposure to desflurane and isoflurane during cardiopulmonary bypass: is the gas outlet of the membrane oxygenator an operating theatre pollution hazard. Br J Anaesth. 1997;78:378–380.
25. Mierdl S., Byhahn C., Abdel-Rahman U. Occupational exposure to inhalational anesthetics during cardiac surgery on cardiopulmonary bypass. Ann Thorac Surg. 2003;75:1924–1927. discussion 1927–1928
26. Berner O. Concentration and elimination of anaesthetic gases in recovery rooms. Acta Anaesth Scand. 1978;22:55–57.
27. American Institute of Architects. Guidelines for construction and equipment of hospitals and medical facilities. Washington, DC: American Institute of Architects; 1992.
28. Standard specification for anesthetic gas scavenging systems: transfer and receiving systems. West Conshohocken, PA: ASTM International; 2002. F1343–02
29. Wabiszewski J., et al. Explore! The Anesthesia System, Aisys. Madison, WI: Datex-Ohmeda; 2005. p 6.6
30. Open reservoir scavenger, operation and maintenance manual. Telford, PA: North American Dräger; 1986.
31. Hamilton R.C., Byrne J. Another cause of gas-scavenging-line obstruction [letter]. Anesthesiology. 1979;51(4):365–366.
32. Abramowitz M., McGill W.A. Hazard of an anesthetic scavenging device [letter]. Anesthesiology. 1979;51:276.
33. Tavakoli M., Habeeb A. Two hazards of gas scavenging. Anesth Analg. 1978;57(2):286–287.
34. Mor Z.F., Stein E.D., Orkin L.R. A possible hazard in the use of a scavenging system. Anesthesiology. 1977;47(3):302–303.
35. Hagerdal M., Lecky J.H. Anesthetic death of an experimental animal related to a scavenging system malfunction. Anesthesiology. 1977;47(6):522–523.
36. Patel K.D., Dalal F.Y. A potential hazard of the Dräger Scavenging Interface System for Wall Suction. Anesth Analg. 1979;58:327–328.
37. Allen M., Lees D.E. Fires in medical vacuum pumps: Do you need to be concerned? ASA Newsletter. 2004;68:22.
38. Barwise J.A., Lancaster L.J., Michaels D. An initial evaluation of a novel anesthetic scavenging interface. Anesth Analg. 2011;113:1064–1067.
39. Ryan S.M., Nielsen C.J. Global warming potential of inhaled anesthetics: application to clinical use. Anesth Analg. 2010;111:92–98.
40. Berry JM: System for removal of halocarbon gas from waste anesthetic gases. U.S. Patent number 6729329, issued May 2004.
41. Berry J.M. Volatile Anesthetic Reclamation: It’s About Time (and Temperature)! Poster presentation. Society for Technology in Anesthesia; 2006.
42. Kaarakka P., Malischke P.R., Kreul J.F. Alternative sites for measuring breathing zone nitrous oxide levels (abstract). Anesthesiology. 1981;55:A139.
43. Sonander H., Stenqvist O., Nilsson K. Nitrous oxide exposure during routine anaesthetic work: measurement of biologic exposure from urine samples and technical exposure by bag sampling. Acta Anaesth Scand. 1985;29(2):203–208.
44. Halsey M.J., Chand S., Dluzewski A.R. Olfactory thresholds: detection of operating room contamination. Br J Anaesth. 1977;49:510–511.
45. Mazze R.I. Waste anesthetic gases and regulatory agencies. Anesthesiology. 1980;52:248–256.
46. American Society of Anesthesiologists Task Force on Trace Anesthetic Gases of the Committee on Occupational Health of Operating Room Personnel: Waste anesthetic gases: information for management in anesthetizing areas and the post anesthesia care unit (PACU). Park Ridge, IL, American Society of Anesthesiologists, 1999.
47. Whitcher C.E., Siukola L.V.M. Occupational exposure, education and sampling methods. Anesthesiology. 1979;51(3):S336.
48. Gambill A.F., McCallum R.N., Henrichs T.F. Psychomotor performance following exposure to trace concentrations of inhalation anesthetics. Anesth Analg. 1979;58(6):475–482.
49. Frankhuizen J.L., Vlek C.A., Burm A.G., Rejger V. Failure to replicate negative effects of trace anaesthetics on mental performance. Br J Anaesth. 1978;50:229–234.
50. Ferstandig L.L. Trace concentrations of anesthetic gases: a critical review of their disease potential. Anesth Analg. 1978;57:328–345.
51. Ferstandig L.L. Trace concentrations of anesthetic gases. Acta Anaesth Scand. 1982;75(Suppl):38–43.
52. Axelsson G., Rylander R. Exposure to anaesthetic gases and spontaneous abortion: response bias in a postal questionnaire. Int J Epidemiol. 1982;11:250–256.
53. Buring J.E., Hennekens C.H., Mayrent S.L., et al. Health experiences of operating room personnel. Anesthesiology. 1985;62:325–330.
54. Hennekens C.H., Colton T., Rosner B., et al. Evaluation of the epidemiologic evidence for occupational hazards of anesthetic gases. Park Ridge, IL: American Society of Anesthesiologists; 1982.
55. Tannenbaum T.N., Goldberg R.J. Exposure to anesthetic gases and reproductive outcome: a review of the epidemiologic literature. J Occup Med. 1985;27:659–668.
56. Ebi K.L., Rice S.A. Reproductive and developmental toxicity of anesthetics in humans. In: Rice S.A., Fish K.J., eds. Anesthetic toxicity. New York: Raven Press; 1994:175–198.
57. Spence A.A. Environmental pollution by inhalation anaesthetics. Br J Anaesth. 1987;59:96–103.
58. Maran N.J., Knill-Jones R.P., Spence A.A. Infertility among female hospital doctors in the UK. Br J Anaesth. 1996;76:581P.
59. Rowland A.S., Baird D.D., Shore D.L., et al. Nitrous oxide and spontaneous abortion in female dental assistants. Am J Epidemiol. 1995;141:531–538.
60. Rowland A.S., Baird D.D., Weinberg C.R., et al. Reduced fertility among women employed as dental assistants exposed to high levels of nitrous oxide. N Engl J Med. 1992;327:993–997.
61. Health Services Advisory Committee. Anaesthetic agents: controlling exposure under COSHH. Suffolk: Health and Safety Executive; 1995.
62. Borm P.J.A., Kant I., Houben G. Monitoring of nitrous oxide in operating rooms: identification of sources and estimation of occupational exposure. J Occup Med. 1990;32:1112–1116.
63. Arbejdstilsynet (The Danish National Institute for Occupational Safety). Grænseværdier for stoffer og materialer. At-anvisning. 1988. Nr.3.1.o.2 (April)
64. Langbein T., Sonntag H., Trapp D., et al. Volatile anaesthetics and the atmosphere: atmospheric lifetimes and atmospheric effects of halothane, enflurane, isoflurane, desflurane and sevoflurane. Br J Anaesth. 1999;82:66–73.
65. Feldman J.M. Managing fresh gas flow to reduce environmental contamination. Anesth Analg. 2012;114(5):1093–1101.
66. Doyle D.J., Byrick R., Filipovic D., Cashin F. Silica zeolite scavenging of exhaled isoflurane: a preliminary report. Can J Anaesth. 2002;49:799–804.