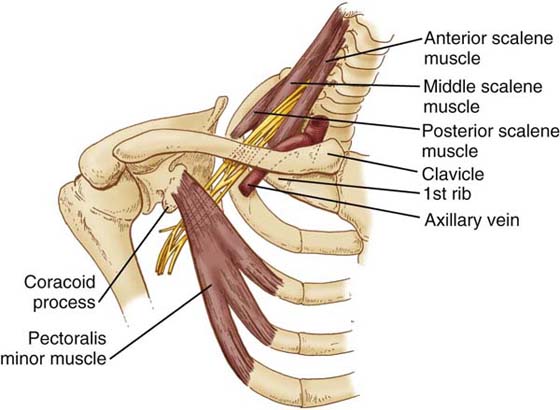
Figure 55-1 Illustration of the anatomic relationships of the thoracic inlet. (From Pratt N. Clinical Musculoskeletal Anatomy. Philadelphia: JB Lippincott, 1991.)
▪ There are two major types for the therapist to consider, compressive and entrapment.
▪ Appropriate therapist classification of patients into one of two types is imperative to providing appropriate treatment.
▪ Therapist management requires a comprehensive evaluation to determine the level and tissue involvement.
▪ Therapy is guided by patient classification and three phases of intervention: control, restorative and rehabilitative.
This chapter focuses on painful conditions in the upper extremity related to the brachial plexus, otherwise known as brachial plexus neuropathies (BPN). Other peripheral neuropathies of the involved upper extremity and cervical spine pathology can complicate the diagnosis. The existence of one type of BPN is referred to as thoracic outlet syndrome (TOS) and remains controversial.1,2 As this pain syndrome becomes more ingrained within the central nervous system, accompanying alterations in the neural biomechanics of the brachial plexus and peripheral nerves occur, making diagnosis and treatment more difficult. It is the intent of this chapter to communicate a clearer understanding of brachial plexopathy and its varied manifestations, allowing the clinician to develop a logical sequence of evaluation, assessment, and treatment.
Two major types of brachial plexopathy are discussed. The first, compressive brachial plexus neuropathy (CBPN), is classically described as TOS. This implies that compression on the neurovascular structures is occurring as they pass through the thoracic inlet as a result of a reduction in the diameter of this potential space. The mechanism for this compression could be anatomic anomalies,3-5 muscular hypertrophy or adaptive shortening of surrounding fascia, or space-occupying lesions.5 Postural dysfunction is a major component of both types of brachial plexopathies.6,7 Alterations in posture, especially longstanding ones, may result in the narrowing of spaces necessary for the neurovascular structures to freely traverse the thoracic inlet. Longstanding forward head posture can potentially create space limitations in shape and size secondary to adaptive shortening of tissues of the scalene triangle, costoclavicular, or axillary interval.
The second type of brachial plexopathy, brachial plexus entrapment syndrome (BPES), is less understood. The term may often be used synonymously with the diagnosis of neurogenic TOS (NTOS). More recently this has been referred to as cervical brachial pain syndrome (CBPS). A result of a traction injury to the brachial plexus,8,9 whiplash injuries,10 or other compressive neuropathies in the upper extremity,11-14 BPES impairs neural tissue mobility and tolerance to tension as a result of intraneural or extraneural fibrosis from direct trauma,15 local pathology within the cervical or thoracic spine,10,16 or longstanding compression or overuse. This limitation in the nerve’s adaptability or compliance is referred to as adverse neural tension (ANT),17,18 or, more recently, neural tension dysfunction (NTD).19 As a result of NTD movement of the patient’s involved extremity beyond the limits of the nervous system’s compliance creates abnormal tension in the peripheral nervous system (traction) further exacerbating the patient’s symptoms.
Several anatomic relationships are important to the therapist’s evaluation and management of BPN. The first relationship is at the level of the cervical spine between the exiting nerve roots of the brachial plexus and the prevertebral fascia.20 The trunks of the brachial plexus enter the thoracic inlet through the scalene triangle. An important distinguishing feature at this level is the relationship of the subclavian artery as it accompanies the trunks of the brachial plexus through the scalene triangle. In comparison, the subclavian vein enters the thoracic inlet anterior to the scalene triangle. Clinically this may explain why patients present with neurogenic and classic arterial symptoms without venous symptoms (Fig. 55-1).

Figure 55-1 Illustration of the anatomic relationships of the thoracic inlet. (From Pratt N. Clinical Musculoskeletal Anatomy. Philadelphia: JB Lippincott, 1991.)
Moving laterally, the neurovascular bundles converge and traverse the costoclavicular interval, inferior to the clavicle and superior to the first rib. Continuing laterally, the neurovascular bundle enters the upper extremity through the axilla. At the costoclavicular interval, the patient may present with neurologic symptoms related to the anterior and posterior divisions of the brachial plexus. At the axilla the symptoms may follow a cord distribution. Figure 55-1 further illustrates these relationships. Clinically, each of these could present with differing neurologic complaints and pain distribution. The final relationship to consider is muscular. Machleder and colleagues21 and Sanders and coworkers22 described histologic changes of the scalene muscles in patients presenting with a diagnosis of BPN. These changes include increased type I collagen and type II muscle fiber atrophy within the scalene muscles. These histologic changes support the theory that longstanding cervicobrachial pain may be an underlying cause and pathology for BPN. The increased percentage of connective tissue within the scalene muscles compared with normal muscle tissue may indicate a “stiffening” of the scalene triangle, resulting in a decrease in compliance of these muscles, placing the neurovascular structures at greater risk.
There are three potential spaces for CBPN or the development of BPES within the thoracic inlet. The first and most medial space is the interscalene triangle located within the boundaries of the posterior cervical triangle. The presence of a prefixed or postfixed brachial plexus along with other anatomic anomalies may add to poor neurovascular mobility and tension attenuation. Injury to the shoulder girdle or repetitive trauma may lead to symptoms and pathology. As previously discussed, the second potential space is the costoclavicular interval. The third potential space moving laterally is the axillary interval. In this area of the anterior structures, the deltopectoral fascia, pectoralis minor, and coracoid have all been implicated as potential sources of compression of the neurovascular structures.23
BPN occurs more often in women, usually between the fourth and the sixth decades of life.24 Brachial plexopathies have also been associated with a history of cervical,10 thoracic,25 or shoulder trauma16,26; arthritis16; bad posture; and repetitive motion disorders.27 Brachial plexopathies can include symptoms that are related to the venous, arterial, neurologic, or autonomic systems.28 The symptoms associated with multiple system involvement are often extremely variable, making the diagnosis of CBPN or BPES predominantly a clinical diagnosis made by a process of exclusion rather than specific objective signs or diagnostic tests. Therefore careful and meticulous evaluation and hypothesis formulation are necessary to identify the potential causes of the multiple problems that may coexist in many of these patients.
The diagnosis and classification of these patients continues to remain controversial. Arguments have been put forth to support and refute the existence of brachial plexopathy, especially those labeled as TOS.1,2 The classification and diagnosis of brachial plexopathy centers on four types, based on symptoms: vascular–arterial, vascular–venous, true (specific) neurogenic, and false (nonspecific) neurogenic TOS.29 The problem is that the term TOS is often used as a diagnosis for other neuropathologies that include the brachial plexus. This confused diagnostic scheme makes it difficult for the treating clinician to develop an appropriate treatment strategy. Either way, brachial plexopathy needs to be considered as upper quarter neuropathic pain. This requires the therapist to have a clear understanding of pain. The reader is referred to Chapter 113 for further information on pain mechanisms and Chapter 114 for additional information on pain assessment and management.
Cuetter and Bartoszek29 classified thoracic outlet and brachial plexopathy into four categories. They identified two vascular components, arterial and venous, and believed that these were undisputed because diagnostic tests are available to confirm occlusion or changes of either vascular system. In addition, specific clinical examination techniques, discussed later in this chapter, may further support the presence of vascular involvement. The remaining two classification categories are neurogenic. Sanders and associates also identified three types: arterial, venous, and neurogenic.30 These have been identified as true (nondisputed) or false (disputed) NTOS.31,32 Four criteria for true NTOS are (1) the presence of a cervical rib on radiograph, (2) intrinsic wasting of the hand, (3) sensory changes, and (4) pain or paresthesia over the lower trunk distribution implicating lower trunk involvement.31,32 LeForestier and colleagues31 also included a fifth criterion: positive electrodiagnostic findings. However NTOS may also exhibit symptoms related to the upper plexus.33 Approximately 5% of neurovasculopathies within the thoracic inlet are true neurogenic or vascular.32,34 The remaining 95% are classified as false NTOS32,34 and may be more appropriately classified as BPES as will be discussed later. Within this classification, false NTOS symptoms are identical to those found in true NTOS; however; these cases lack the four criteria. Ribbe and coworkers35 developed a TOS index of signs and symptoms in an attempt to establish clear criteria for the diagnosis of TOS. The index included positive symptoms provoked by arm elevation, paresthesia over the ulnar nerve or lower trunk distribution, tenderness of the brachial plexus over the supraclavicular fossa, and a positive Roos’ test. The lack of a standardized classification system clouds the identification or diagnosis of BPN and hinders the development of a logical treatment approach.
As a result of this lack of consensus, I found it necessary to develop a clinical classification dividing brachial plexopathies into two major types. The contrast between these two types is found in Table 55-1. The first type of brachial plexopathy is classic TOS or which I classify as CBPN, a compressive vasculopathy or neuropathy of the brachial plexus. Classic TOS, as described in the literature, has six identifiable components. (1) Posture appears to play a role in the patient’s symptoms. (2) The onset of discomfort is usually described as insidious with transient symptoms. (3) These symptoms are usually associated with extremity position, posture, or particular motions described by the patient such as overhead work or extended periods of static upper extremity positioning. (4) After the offending posture or activity is corrected, symptoms usually subside. These same symptoms may be transiently provoked during treatment. (5) The TOS provocative tests, discussed later, may be more reliable in identifying the potential anatomic interval of compression. (6) Finally, most of these patients present with minimal resting pain, minimal sleep disturbance, low pain scores (verbal reporting or visual analog pain scales), rapid recovery when symptoms are provoked, less mechanical tissue sensitivity to physical examination, with rapid resolution of symptoms once the offending clinical examination is terminated, and the knowledge needed to relieve their symptoms, indicating low tissue irritability.
Table 55-1 Therapist’s Clinical Classification Criteria
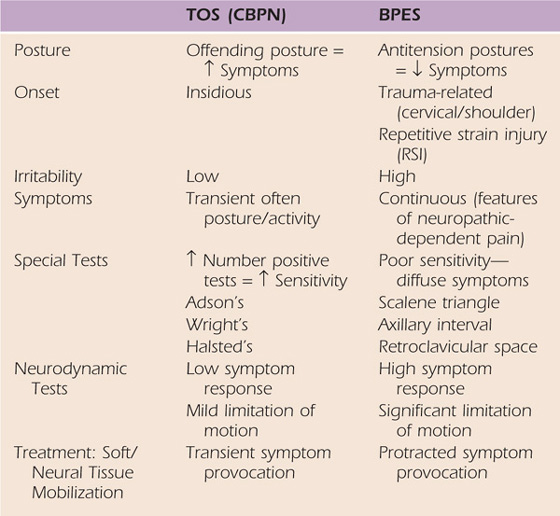
CBPN, compressive brachial plexus neuropathy; BPES, brachial plexus entrapment syndrome; TOS, thoracic outlet syndrome.
The second type of brachial plexopathy is brachial plexus entrapment syndrome (BPES), often associated with trauma8,9 that involves either a traction injury directly or indirectly to the brachial plexus, or local soft tissue inflammation resulting in a compromise of adequate blood flow to the brachial plexus or intraneural or extraneural fibrosis. This fibrosis compromises brachial plexus neural excursion and its ability to attenuate tensile forces placed across the plexus from upper limb or combined cervical motion. These patients typically report delayed onset of their intractable pain that can occur several days, weeks, or months after their injury.36 It is theorized that the delay in onset of the symptoms is explained by the normal course of biologic healing and the development of neuropathic pain. Mature scar formation eventually compresses the neurovascular structures or limits brachial plexus mobility, creating neural tension dysfunction. Under these conditions, upper quarter motion results in repetitive traction to the neural tissues and development of symptoms. In these patients, the reliability of the TOS provocative tests for determining the interval of involvement is poor and may easily provoke symptoms by placing traction on the neural or surrounding tissues, creating a false positive result. Treatment that might be used for the classic TOS patient may provoke symptoms in patients with BPES at the time of treatment, or the response may be delayed by several hours to a day. Often these patients report a significant increase in symptoms at the next follow-up appointment. Finally, the tissue response in patients with BPES tends to be much more irritable. Symptoms are easily provoked with minimal movement of the upper quarter, and patients often report spontaneous bursts of pain and other features of neuropathic pain. The reader is referred to Chapters 113 and 118 for further discussions on neurogenic pain. In addition, additional concomitant dysfunction, such as myofascial trigger points, shoulder pathology, or cervicothoracic spine involvement, may accompany the brachial plexus symptoms.
This theory of differentiating TOS from BPES was explored by Jordan and associates in the development of a Cervical Brachial Symptom Questionnaire.14 They identified several factors that predicted responsiveness to treatment in a group of 85 patients treated for TOS and compared the TOS treatment-responsive group (N = 59) and nonresponsive group (N = 26). The authors identified this latter group as suffering from treatment-resistant cervical brachial pain syndrome (CBPS). The results indicated the TOS treatment-responsive group was less likely to have comorbidities, fewer surgeries, fewer widespread sensory symptoms, and was less likely to have weakness extending beyond the lower trunk distribution. In comparison, the CBPS group had sensory and strength complaints extending beyond the lower trunk distribution, greater history of non-TOS-related surgeries, and greater comorbidities, such as complex regional pain syndrome and fibromyalgia.14
In addition to classifying these cases, it is also essential for the therapist to consider differential diagnoses of other potential conditions that may mimic the symptoms associated with brachial plexopathy. Major ones are listed in Box 55-1. Myofascial trigger points, as indicated by Travell and Simons,37 can mimic the distribution of brachial plexopathy involvement. Table 55-2 contains common myofascial trigger points that may mimic brachial plexopathy symptomatology.37 Glenohumeral joint pathology or dysfunction may also provoke symptoms that are similar in nature to the brachial plexopathies referred to as the dead arm syndrome.38 As reported by Upton and McComas39 and others,39-41 the presence of double- or multiple-crush syndromes may also disguise the involvement of the brachial plexus. Eurroll and Hurst,42 MacKinnon,43 and Seror44 reported that these associated double crushes could include carpal tunnel syndrome, ulnar nerve involvement, or anterior interosseus syndrome.45 The presence of these disorders and others may help explain treatment failure. Therapists must also consider visceral causes such as an apical lung tumor encroaching on the brachial plexus or coronary pathology.
Table 55-2 Common Myofascial Trigger Points
Muscle |
Area of Referral |
Trapezius |
Face and interscapular region |
Scalene |
Posterolateral arm/radial three digits |
Supraspinatus |
Lateral arm/forearm |
Infraspinatus |
Lateral arm/forearm and radial half of hand |
Latissimus dorsi |
Posteromedial arm, forearm, and ulnar half to hand |
Pectoralis |
Anterior shoulder, medial arm, and ulnar two digits |
Subscapularis |
Posteromedial arm and wrist |
Serratus |
Medial arm, forearm, and ulnar half of hand/digits |
To classify an upper quarter compression neuropathy, a careful and thorough examination is essential. This examination starts with a detailed inquiry about the mechanism of the problem and the specific distribution and qualitative attributes of the patient’s symptoms. As previously discussed, the distribution of symptoms can vary greatly. The history also provides insightful information about particular positions, postures, or activities that relieve, accentuate, or aggravate the symptoms. This helps the therapist determine the tissue or anatomic space involved. Figure 55-2 is an example of the presentation of symptoms related to the upper and lower trunks of the brachial plexus. The neurogenic symptoms are classically distributed over the lower trunk46,47 but may also include the upper trunk,47 middle trunk,5,47,48 and cords of the brachial plexus.23 With upper trunk plexopathies (C5-C6 distribution), the pain may tend to be more proximal in nature. This proximal pain may be distributed over the anterior and lateral aspect of the cervical region, portions of the face, and the scapular and interscapular region of the involved side.49 Distal paresthesia and pain may be distributed over what appears to be the median or ulnar nerve distribution (or both) or the C5-C6 dermatomal region. In contrast, lower trunk (C8-T1) plexopathy symptoms of pain and paresthesia are distributed mostly distal. The paresthesia may be located over the medial aspect of the arm, the forearm, and the ulnar aspect of the hand, appearing to be ulnar nerve-related. If involvement of the brachial plexus occurs at a more lateral position, such as the division or cord level, the variability of the symptom distribution may be even more pronounced. The use of a body diagram to represent symptom distribution may provide further insight, to which the Cervical Brachial Symptom Questionnaire previously alluded.14
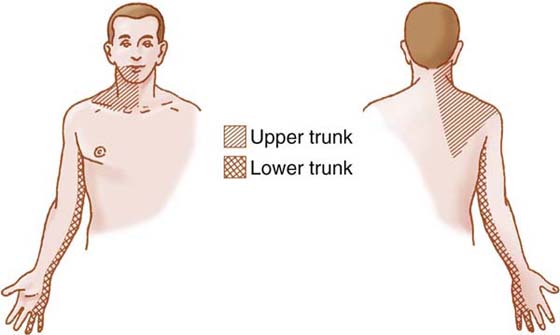
Figure 55-2 Illustration of the distribution of symptoms that may involve the upper trunk or lower trunk of the brachial plexus.
Taking the patient history also includes investigating past injuries or medical problems to determine prior upper quarter trauma or symptoms (e.g., a previous motor vehicle accident with cervical spine injury,10 or blunt trauma directly over the superior aspect of the shoulder and upper trapezius region). Prior injury might suggest preexisting mobility problems of the plexus. The medical history also provides valuable information about contributory medical conditions such as diabetes mellitus, hyperthyroidism or hypothyroidism, arthritis, or other systemic neurologic disease. It is also important to note the length of time the symptoms have been present. There is a tendency for secondary tissue dysfunctions to develop the longer the symptoms have persisted; knowing the duration of symptoms assists in developing an accurate prognosis. In general, symptoms that are more diffuse14 or have persisted for a longer period require extended therapy and are less likely to completely resolve.50 Specific questioning about occupational or avocational activities that could compromise the neurovascular structures within the thoracic inlet is also essential.
Neurogenic Symptoms. Information regarding symptom distribution and previous history leads to more specific questions about the qualitative nature of the symptoms. Symptoms with neurogenic features involve the motor, sensory, or autonomic nervous system, such as specific muscle performance deficits, alterations in sensibility, and vasomotor instability. These may be intertwined in the pain as associated sensory disturbances of paresthesia and numbness over the same distribution. Accompanying these early neurologic symptoms may be autonomic nervous system complaints such as hyperhidrosis and burning pain over the same distribution. Raskin and coworkers51 and others28 reported that headache was present in 26 of 30 patients diagnosed with TOS. Validation of the diagnosis was based on relief of symptoms after first rib resection. Utilizing an isokinetic testing technique, Ozcakar and associates52 were able to confirm and quantify the weakness and fatigue often reported by the patients.
Late neurogenic symptoms can include complaints of pain; sensory changes; and paresthesia distributed over the posterior lateral cervical region, anterior shoulder, and posterior lateral aspects of the humerus.1,46,47 More evident intrinsic muscular weakness of the hand with lower trunk involvement, reflex changes, and actual sensory loss6 may be present. These sensory changes may also manifest with pencil pointing of the digits for the involved nerve distribution. The most common complaints are listed in Box 55-2.
Box 55-2 Common Symptomatology of Brachial Plexus Neuropathies
Shoulder/arm pain: Intermittent (TOS) or neuropathic features (BPES)
Shoulder/arm paresthesia: Intermittent (TOS) or continuous (BPES)
Pain/paresthesia with lifting/carrying: Brachial plexus traction
BPES, brachial plexus entrapment syndrome; TOS, thoracic outlet syndrome.
Compiled from Ide J, Kataoka Y, Kamago M, et al. Compression and stretching of the brachial plexus in thoracic outlet syndrome: Correlation between neuroradiographic findings and symptoms and signs produced by provocation manoeuvres. J Hand Surg. 2003;28-B:218–223; and from Ozcakar L, Inanici F, Kaymak B, et al. Quantification of the weakness and fatigue in thoracic outlet syndrome with isokinetic measurements. Brit J Sports Med. 2005;39:178–181.
Vascular Symptoms. Venous symptoms include reports of distal edema, especially after activity and pain described as a dull ache over a nonspecific distribution. The patient may also report a sensation of heaviness in the involved extremity.46,47 With more significant venous involvement, cyanosis may also be present. Arterial symptoms can include descriptions of fatigue, ischemia-like pain, coldness in the distal part of the extremity, and Raynaud’s phenomenon.46,47 The complaint of ischemic pain may be diffuse or specific to a localized area over the distal extremity. Although rarely seen, late arterial signs could include distal thrombosis or embolization with ischemia changes.53 Clinical signs of vascular involvement include loss or a decrease in the quality of distal pulses when performing provocative stress tests; vascular involvement may also be detected with an arteriogram.
Diagnostic tests can also be of assistance. Radiographs may indicate the presence of a cervical rib, other bony conditions, or a prominent C7 transverse process, which may suggest the presence of a rudimentary fibrous band that has the potential of occupying space in the scalene triangle. Arteriograms may indicate a possible blockage of subclavian or axillary vessels. The use of somatosensory evoked potentials,51,54 nerve conduction velocities of the medial antebrachial cutaneous nerve, and electromyography studies are also helpful.31,54,55 All of these tests may provide additional information about the location and degree of the neuropathology and can rule out the presence of double- or multiple-crush syndromes.
The use of imaging techniques has been found to be more predictive when combined with the provocative testing positions. Gillard and colleagues confirmed vascular changes when visualized with sonography in 48 patients diagnosed with TOS, utilizing MRI in 29 patients and 12 healthy individuals as controls.56 Demirbag and associates determined there was a significant difference in MRI findings in the patient group when comparing neutral to provocative test positions and a significant difference in the positional change values in MRI between the groups.57 Although further studies are needed to demonstrate imaging’s usefulness, these studies show promise in bringing more objectivity to the diagnosis of CBPN (TOS).
While obtaining the history from the patient, the therapist should observe the patient’s standing and sitting postures. The therapist should look for any cervical asymmetry, thoracic kyphotic changes, or accessory breathing patterns. Examples of cervical postures are demonstrated in Figure 55-3. As is evident from these photos, observing the posture strictly in the sagittal plane may result in incorrect or insufficient information. Figure 55-3B demonstrates the classic forward-head and rounded-shoulder posture in the sagittal plane, with a flattened upper thoracic kyphosis commonly seen in CBPN. In the frontal plane (Fig. 55-3A) cervical asymmetry is evident with rotation and lateral flexion of the cervical spine toward the affected side, accompanied by increased upper trapezius muscle tone. Through this observation alone, the therapist is able to hypothesize the patient’s level of irritability. This posture demonstrates the patient’s effort to decrease the tension on the brachial plexus by elevating the scapula via contraction of the upper trapezius and levator scapulae and rotating and laterally flexing the cervical spine toward the involved side. The therapist must also inspect for the presence edema in the supraclavicular fossa and any atrophy and trophic, temperature, or color changes in the extremity. The position of the upper extremity should also be noted to determine whether the patient is using distal joints to reduce neural tension by maintaining the elbow in flexion, the forearm in neutral, and the wrist and digits in flexion.58,59 These components may exist separately or in combination, and each may vary in the amount it contributes to the patient’s position and pain.
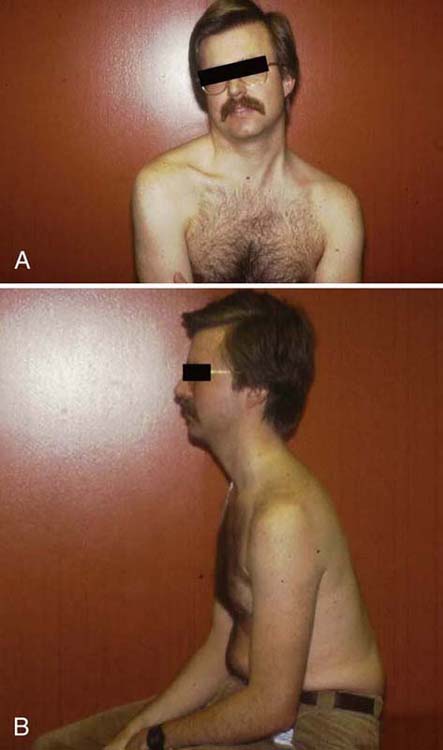
Figure 55-3 Photograph of common cervical posture seen in patients with brachial plexus neuropathy. A, Frontal plane. B, Sagittal plane. Note how examining in one plane only may not give the therapist the complete picture.
Details of the upper quarter screen are presented in Chapter 10, but in regard to upper quarter neuropathies, the cervical spine’s active range of motion (ROM) is assessed to determine limitations in motion and the presence of mechanical spine pain, which may be provoking the patient’s pain. Special tests, including Spurling’s test for foraminal encroachment60 and the vertebral artery test, are executed to determine nerve root or vascular involvement.61 Myotome scanning and reflex testing provide further information on neural conduction. Tinel’s test in the supraclavicular fossa, in the axilla, and along the peripheral nerves allows identification of neural hyperalgesia or other peripheral nerve pathology in the upper extremity. Ide and associates reported that in 111 patients diagnosed with combined compression and stretch TOS, 103 patients had a positive Tinel’s sign over the supraclavicular fossa.62 Finally, the supraclavicular fossa and axilla should be auscultated for the presence of a bruit.
Careful sensory evaluation is undertaken using vibrometry and monofilament cutaneous pressure sensation testing. These threshold tests are reported to be more sensitive than other forms of sensibility testing for early detection of peripheral neuropathies.7,63 Sensory evaluation should be carried out to investigate: (1) dermatomal distribution, to rule out possible cervical root involvement; (2) peripheral nerve distribution, to rule out the possible local peripheral nerve compressive neuropathies; and (3) sensory disturbance related to the brachial plexus and its divisions. On completion of the general upper quarter screen, assessment for active motion dysfunction is undertaken. This process is explained in Chapter 118. The purpose of active dysfunction testing is to determine whether imparting tension on the peripheral nervous system in various locations alters active motion of the cervical spine or upper extremity. The presence of active motion dysfunction assists the therapist in identifying the nervous system’s role in the presenting complaints.
Specific provocative tests, described later in this chapter, are carried out only after the therapist has taken an adequate history and completed an upper quarter screening to develop an initial hypothesis about the level of irritability. These provocative positions can potentially place adverse tension on the peripheral nervous system or brachial plexus, exacerbating the patient’s symptoms and creating false positive results. These special tests were originally designed to determine the integrity of the vascular system and the brachial plexus.
Numerous authors have questioned the specificity, sensitivity, and reliability of these tests. Most of these studies examined asymptomatic subjects as to the frequency of positive tests defined as diminished or lost pulse. None of these studies compared normal subjects with a patient population or considered provocation of symptoms. Falconer and Weddell64 examined the specificity and sensitivity of the costoclavicular maneuver in four case studies—three vascular and one neurogenic—that had a positive costoclavicular maneuver. They confirmed the involvement of the costoclavicular interval surgically. In 100 normal subjects, 50 males and 50 females 19 to 47 years of age, the costoclavicular maneuver was positive for pulse changes in 25 males and 29 females. In 50% of the males and 60% of the females, either a positive Adson’s or costoclavicular maneuver was obtained.64 In contrast, Adson65 found 9 males and 11 females had a decrease or obliteration of pulse performing his provocative maneuver. In 1980, Gergoudis and Barnes investigated the reliability and validity of the provocative maneuvers. The authors used photoplethysmography to measure the changes in vascular status that occurred with Adson’s, costoclavicular, and Wright’s test in 130 normal subjects. They determined that 60% had an abnormal finding with at least one test, 27% with two tests, and less than 7% had an abnormal finding when all three tests were performed.66 It should be noted that the provocation of any kind of symptoms from a neurogenic standpoint was not measured. In 1987, Warrens and Heaton examined the validity of these provocative maneuvers by determining the frequency of false positive results and the role of photoplethysmography. In 64 normal volunteers, they found 17% were reporting some symptoms. They determined that complete obliteration of the pulse occurred in 58% of the population with at least one test, and 30% had bilateral findings. The incidence of positive findings was 27% for the costoclavicular maneuver, 15% for Adson’s test, and 14% for Wright’s test. Using photoplethysmography, at least one test was positive in 39% of the subjects. In only 2% of the population were all three tests positive.67
In determining the prevalence of a positive elevated arm stress test (EAST) or Wright’s maneuver, Costigan and Wilbourn used two groups: 24 normal subjects and 65 patients diagnosed with carpal tunnel syndrome (CTS). They determined that the EAST was positive for 92% of the CTS patients and 74% of the controls.68 Novak and coworkers reported that in 65 of 115 patients with possible TOS, the EAST was positive in 94% of the 65 patients and in 100% of the 65 patients with confirmed TOS when direct pressure over the supraclavicular region was combined with the EAST. A positive result was symptom production, but not necessarily the exact symptoms reported by the patient.27 In 1995, Rayan and Jensen studied the prevalence of a positive response for three provocative maneuvers in a typical patient population of 100. The subjects were divided into two groups: those younger than 40 and those older than 40. They reported that 87 of 100 subjects had at least one positive test for vascular signs and that 41 of 100 had at least one positive test for a neurogenic response.69 Plewa and Delinger examined 50 healthy subjects and reported changes in pulse in 11% for both Adson’s and costoclavicular maneuver, 62% for Wright’s test, and 21% for supraclavicular pressure. Provocation of symptoms (pain or paresthesia) was positive for 11% with Adson’s maneuver, 15% with costoclavicular maneuver, and 36% with Wright’s test. They concluded that as the number of positive maneuvers increased in each subject, the specificity improved because only six subjects had all three tests positive.70 In 1999, Toomingas and colleagues examined the position of abduction/external rotation among male industrial and office workers. They determined the positive prevalence value was 24% of the population in 1987 and 15% in 1992. Distal symptoms were positive in 12% to 20% of the population, whereas proximal symptoms were present in 5% to 6%. It was their opinion that the symptoms of numbness in the hands had the highest specificity and sensitivity associated with decreased sensitivity to touch.71
Most recently the accuracy of the special tests has been examined using MRI and ultrasonography. Demirbag and associates, using MRI, measured various space size and anatomic relationships of the thoracic inlet and confirmed that the provocative tests of Adson, hyperabduction (Wright), and Halsted (costoclavicular) resulted in significant alteration of these measurements in a group of 29 patients. There was also a significant difference noted between the patient group and the control group of 12 for the Halsted and hyperabduction test.57 Gillard and associates reported mean sensitivity and specificity values of 72% and 53%, respectively, for the three primary tests alluded to earlier using ultrasonography. They also determined that combining more than one test improved sensitivity.56 Finally Ide and coworkers, using neuroradiographic techniques to examine 150 patients diagnosed with compressive, mixed compressive/stretch, and stretch TOS, determined that the “stretch test” (axial distraction of the upper extremity in neutral) and 90 degrees of abduction/external rotation position (Wright) resulted in greater sensitivity for the compression and mixed compression/stretch group of patients.62
The original proponents of provocative tests used them to delineate the location of compression of the neurovascular structures in the thoracic inlet. In the case of Adson, the proposed test implicated compromise at the scalene triangle. This test, described by Adson and Coffey in 1927,3 involves cervical rotation and extension to the tested side with the upper extremities supported in the patient’s lap. This is followed by a deep inspirational breath, which is held for 30 seconds while the examiner palpates for changes in the radial pulse. Obliteration or diminution of the pulse is a positive test. As discussed previously, the importance of the pulse remains in question.66 Of equal or greater importance is symptom provocation reported by the patient.6 The clinician is also reminded that this position may stress the contralateral scalene triangle and indirectly provoke symptoms.
The stress hyperabduction test (Wright’s test) described by Wright23 in 1945 implicates the axillary interval. This test is performed in two steps as the patient sits comfortably positioned with the cervical spine in neutral. The arm is passively positioned in 90 degrees of adduction and 90 degrees of external rotation for up to 1 minute while the clinician monitors the patient’s symptoms and the quality of the radial pulse. The maneuver may implicate the subclavian vessels and plexus as it is stressed across the coracoid pectoralis loop. A positive test is loss of pulse and implicates the axillary interval. When this test was performed on 150 normal young adults, 83.3% had obliteration of their radial pulse on the right and 82% on the left.23
Halsted4 initially described, and Falconer and Weddell further researched and described the costoclavicular maneuver or military brace position for stressing the costoclavicular interval.64 This test is performed with the patient in the sitting position while the clinician helps position the patient into scapular protraction, elevation, retraction, and depression. The patient holds this position for 30 seconds. The patient’s arms remain comfortably supported on the thighs while the examiner simultaneously monitors for any pulse changes. The test is positive when radial pulse changes occur or symptoms are provoked.
In 1966, Roos and Owens47 described a provocative maneuver that uses exercise stress and positioning. No specific anatomic interval is tested. The patient sits in a neutral position, humerus abducted to 90 degrees, full external rotation, and elbows flexed to 90 degrees. The patient then performs repetitive finger flexion and extension that can be continued for up to 3 minutes. The examiner monitors for any evidence of dropping of the extremities, indicating possible fatigue and arterial compromise. The therapist also observes the color of the distal extremity, comparing left to right. According to Roos, this test stresses all three intervals and places the arterial, venous, and nervous system in tension. The test is considered positive when the patient is unable to maintain the elevation for 3 minutes because of fatigue or pain. Examples of these four maneuvers are depicted in Figure 55-4.
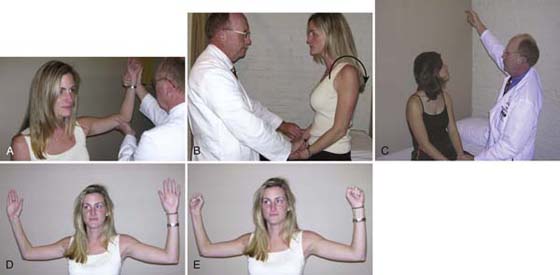
Figure 55-4 The four commonly applied tests for thoracic outlet syndrome: A, Wright’s. B, Costoclavicular. C, Adson’s. D, E, Roos’. The breath is held only for the Adson’s test.
Smith,72 Lindgren,73,74 Elvey,8 and Butler and Gifford75 hypothesized three additional tests to stress the neurovascular structures through the thoracic inlet. Smith described the stress hyperextension position,72 which potentially implicates all three intervals and is nonspecific for vascular or neural involvement. A positive test is a change in pulse and provocation of symptoms. In 1992, Lindgren and colleagues74 described the cervical rotation/lateral flexion test, designed to assess the elevated position of the first rib in patients presenting with brachialgia (TOS). They examined the reproducibility of this test by comparing it with the cineradiography for first rib position. In 23 symptomatic patients, the test was positive for restricted cervical motion. First described by Elvey8 and refined by Butler and Gifford,75,76 the upper limb tension test systematically places the neurovascular structures of the upper extremities into segmental tension. A positive response is indicated by the provocation of symptoms and motion limitations. The test should be performed on the noninvolved side first to determine each patient’s normal response. See Chapter 118 for further definitions and an explanation of the correct application of this test.
The pectoralis minor and the deltopectoral fascia should be observed for tightness with the patient in a supine position, noting shoulder height and symmetry in the frontal plane. The therapist can also examine for pectoralis minor tightness. Standing at the patient’s side facing the patient’s head, the therapist places his or her inside hand on the inferior angle of the thoracic cavity and then passively forward flexes the shoulder on the same side. The therapist palpates the inferior costal angle to determine whether any anterior and superior movement occurs. This movement is the result of pectoralis minor tightness as the coracoid process translates superiorly and posteriorly and is illustrated in Figure 55-5. An alternative method to evaluate pectoralis minor tightness is to measure the relative difference in resting height from the surface of the plinth to the acromioclavicular joint, comparing the involved and uninvolved side when observing the patient from the head in the supine position. An examination for active myofascial trigger points as described by Travell and Simons37 is performed to ascertain whether the pain or symptoms are related to the myofascial trigger points mimicking brachial plexus pathology. The most common myofascial trigger points capable of this are listed in Table 55-2. Finally, examination should be undertaken for any evidence of sternoclavicular joint tenderness or muscle spasm of the cervical and shoulder girdle region. This is done to differentiate tissue involvement of the cervical region and upper quarter, which may help support or refute the hypothesis of BPN.
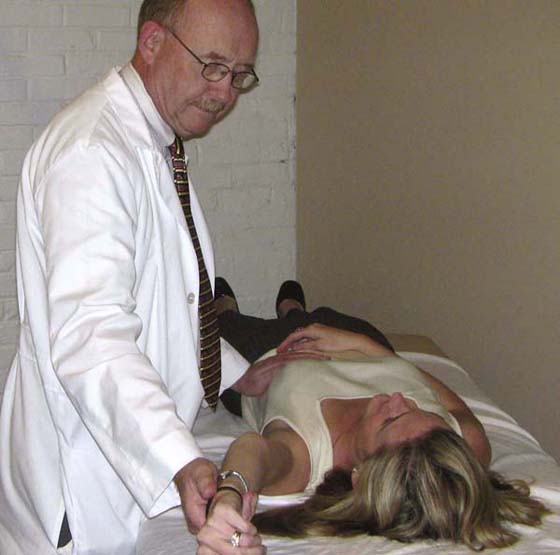
Figure 55-5 Photograph of testing for pectoralis minor tightness. If the pectoralis is tight, the costal inferior angle will translate anteriorly into the examiner’s palpating hand.
The therapist is likely to encounter different opinions about the role of conservative care for the management of TOS or BPES. Much of the discrepancy that surrounds the implementation of conservative care is related to controversies regarding the existence of this entity and the criteria used to verify the diagnosis. Peet and coworkers77 reported one of the first descriptions for conservative management for TOS in 1956. Treatment techniques for the 55 patients in their study included moist heat, massage, shoulder elevator strengthening, pectoralis strengthening, and postural correction exercises. They reported improvement in 70.9% of the patients; 20 patients improved in 3 to 28 days, and 13 patients improved in 4 to 12 weeks. In 1968, Urschel and associates, using a conservative approach of moist heat, active motion, shoulder elevation strengthening, and cervical traction, reported their treatment was “effective” for 50% of the 120 patients in the study. Treatment duration varied from 3 months to several years.78 In 1974, Dale and Lewis described a conservative treatment program consisting of shoulder girdle strengthening and medication. They stated that 63% of the 150 extremities “did well.”79 McGough and colleagues studied a large population of 1300 patients. Treatment consisted of shoulder girdle strengthening, postural correction, moist heat, massage, and medication; only 9.4% required surgery. The average treatment time was 7.2 months, ranging from 2 months to 2 years.80 Woods described a treatment program including medication, exercise, and transcutaneous electrical nerve stimulation (TENS). Of the 109 patients in the study, 50% obtained relief within 9 months mean treatment time; TENS was found effective in 40 of the 109 patients.81 The major weaknesses with each of these studies were a lack of consistent criteria for the diagnosis and that only descriptive outcomes were reported.
In 1979, Smith described a treatment protocol composed of orthopedic manual techniques to increase flexibility of the thoracic inlet, flexibility exercises, and behavioral and postural modification. A significant decrease in symptoms was obtained in 75% of the 20 patients. The mean treatment time was 10 visits, with a range of 1 to 14.72 In 1984, Walsh re-created this treatment approach using inclusion criteria of insidious onset of symptoms, no history of trauma, and two or more of the provocative maneuvers positive for pulse changes and symptom provocation. Symptoms were predominantly transient paresthesia and pain. Asymptomatic relief was obtained in 68.5% of 16 patients involving 19 extremities; 10.5% reported moderate relief, 5.2% reported temporary relief, and 15.8% reported no relief. Surgery was performed in three of the six patients with moderate or no relief. There was one reoccurrence that was relieved after 14 additional visits.82
Other conservative approaches have been tested. Ingesson and coworkers described a physiotherapeutic method of treatment for CBPN (TOS). Treatment included general and specific components. General components were patient education, avoidance of provocative postures and activities, and ergonomic intervention. Specific components were relaxation for involved muscles; breathing exercises and training; stretching of shortened muscles; postural training, including coordination for anterior and posterior postural muscles; specific mobilization of the cervical and thoracic spine; and a home exercise program. A “positive effect” was achieved in 50% of the patients (63 of 125); 45 of the remaining 62 patients required surgery.83 In 1990, Sucher presented four case studies in which patients were treated with myofascial release techniques as the primary tactic. In all four cases symptoms were “markedly improved.” The particular techniques were not described in detail. However, it appears that contract–relax, spray and stretch, and vigorous stretching of involved musculature or surrounding fascia were discussed.84 In 1995, Novak and associates reported on 42 patients treated conservatively for TOS. Treatment included education about pathophysiology, avoidance of offending positions, and postural awareness. Therapy treatment incorporated postural correction, pain control, stretching and therapeutic exercise, aerobic conditioning, and a home exercise program. Symptomatic improvement was obtained in 25 patients, 10 were unchanged, and 7 worsened. Poor overall outcome was related to obesity, worker’s compensation, and concomitant double-crush injury (carpal or cubital tunnel syndrome). Arm and hand pain was significantly improved in patients who did not have these concomitant problems.85 In 1997, Lindgren reported on 139 patients treated with a therapeutic model of scapula ROM, upper cervical spine-normalizing exercises (chin tucks while standing against the wall), resisted cervical forward flexion, rotation and extension to normalize first rib function, and stretching the anterior cervical spine and levator muscles. At the time of discharge from the hospital, 88.1% of the patients were satisfied with the outcome and improved impairment.73
Cramer described a reconditioning program for athletes to decrease the rate of recurrence of injury-induced brachial plexus neuropraxia. The program included 4 weeks of conservative management and 8 weeks of progressive reconditioning consisting of cervical strengthening three times per week and cervical mobilization and modified shoulder strengthening two times per week. No specific patient data were presented to support this approach.86
Numerous other authors have also described conservative approaches. The concept of adverse neural tension (ANT),18,75 or neural tension dysfunction (NTD),19 provides additional causes and treatment approaches to be considered in the treatment of BPN patients. The recent concept of ANT or NTD is what led me to the development of the classification system of CBPN and BPES. Several authors have examined the effect of treatment of BPES (cervical brachial pain syndrome). Allison and coworkers in a single-blind, randomized controlled trial of 30 patients and a control group compared the effects of two different manual therapy techniques: cervical and neural mobilization versus indirect manual techniques of articular structures of the glenohumeral joint and thoracic spine. They reported improved pain intensity, pain quality scores, and functional disability levels in the manual therapy groups. The neural mobilization group also had significantly lowered visual analog scale (VAS).11 In 2003 Coppieters and associates published their findings comparing two intervention groups: cervical contralateral lateral glide mobilization versus therapeutic ultrasound in 20 patients with neurogenic CBPS. They reported a significant reduction in pain perception, a decrease in shoulder girdle elevation (superior scapulae elevation), and increased ROM (elbow extension) with the upper limb neural tension test in the manual therapy group.13 In a quasiexperimental study of 50 patients, Hanif and colleagues treated NTOS patients with a combination of strengthening exercises of the paraspinal and scapular muscles along with stretching of the scalene, sternocleidomastoid, and pectoralis muscles, performed once a day, 4 days a week, for 6 months. Thirty-one of the patients had a full or marked recovery, 16 partial improvement, and 6 no change or worse.87 Landry and associates compared the long-term functional outcomes in patients with NTOS treated surgically and conservatively. The conservative intervention was physical therapy; however, no specific interventions were described. The patients undergoing surgery missed more work; however, there was no significant difference in number of subjects that returned to work, symptom severity, or change in symptomatic status since onset. They concluded that first rib resection did not improve functional status.88
Variability in the duration of the various conservative treatment strategies relates to the lack of diagnostic criteria and specific treatment approaches. Guidelines for treatment duration are based on patient symptom response and the therapist’s physical examination. In general, (1) the longer the duration of symptoms, the longer conservative care may be necessary; (2) multiple-system involvement such as glenohumeral joint pathology, myofascial trigger points, or double-crush syndromes may necessitate longer-term conservative measures; (3) conservative care may take longer for the patient with BPES than for those that have the classically described TOS (CBPN); (4) social, medical, emotional and occupational factors play an important role in the patient’s response to conservative care; and (5) the presence of multiple- or double-crush syndrome may require treatment to address the cervical spine, distal pathology, and BPN.
Multisystem involvement is often present in patients diagnosed with TOS and BPES. Treatment programs are developed from information obtained from the evaluation and assessment. There is no recipe or cookbook approach for treating these patients, especially when their conditions are highly complex. Whether the problem is CBPN (TOS) or BPES, the purpose of the control phase of treatment is to decrease and control the patient’s symptoms. In the more complex cases, it may be useful to formulate a problem list for each identified impairment or tissue dysfunction. This list may include bursitis or specific tendinitis of the shoulder; adhesive capsulitis; active myofascial trigger points; mechanical, cervical, or thoracic spine pain; double or multiple crushes; and other soft tissue conditions. The therapist must keep in mind that a double- or multiple-crush syndrome involvement may necessitate treatment for a more distal peripheral neuropathy.24,39,42 The problem list should be prioritized based on the overall goal of the control phase. Therefore the brachial plexus component of the patient’s symptomatology may in fact be the last issue addressed in the intervention plan. Initially, attempting to manage the brachial plexus component may only exacerbate the patient’s symptoms and result in failure. Also imperative in this phase is identifying activities, positions, and treatments that exacerbate or relieve the patient’s symptoms. The therapist’s understanding of the level of tissue irritability and methods of relief is essential for progressing to restorative phase.
The restorative phase of conservative management commences only after control and comfort have been achieved. The patient should have a minimal level of resting pain and no sleep disturbance. During this phase, treatment of tissues that create impairments such as limited motion or provoke the symptoms directly related to the compression or entrapment of the brachial plexus and its accompanying neurovascular structures may be initiated. Treatment may transiently exacerbate the patient’s complaints; however, this should not last beyond the treatment session. To ensure that it does not, the therapist must have command of the methods necessary to regain symptom control. Postural awareness and correction are also being initiated during this phase.
The final phase, the rehabilitative phase, involves conditioning and strengthening the muscles necessary to maintain postural correction, rehabilitate weakened muscles in the extremity, and increase activity tolerance and endurance. Postural correction is carried over into activities of daily living and occupational situations that may lead to NTD situations. Functional and occupational activities are addressed via patient education and ergonomic intervention.
Patient Education and Behavior Modification. In general, the treatment approach for classic CBPN (TOS) and the more complex case of BPES can be combined based on the primary goal of symptom control and relief. Although the duration of treatment varies greatly for each of the two conditions, the BPES patient is likely to require more time. Control-phase treatment centers on behavior modification, postural correction and awareness, and the development of a diaphragmatic breathing pattern. Behavior modification addresses factors contributing to symptoms, quality of life, and occupational or avocational activities. Behavior modification may include instructing the patient in appropriate positioning of the upper extremity at rest to avoid placing tension across the brachial plexus and accompanying vascular structures. Passive shoulder elevation corrects the depressive traction component on the brachial plexus and has been beneficial in decreasing symptoms. Positioning suggestions include using the opposite, noninvolved extremity to support the involved extremity in the brachial plexus slack position, as demonstrated in Figure 55-6, or resting the affected extremity on the armrest of a chair or a pillow on the lap. While standing, the patient can obtain additional relief from tension by placing her hand in a coat pocket or by supporting it on her belt. Resting the affected arm on the armrest of a chair or pillow for 30 minutes before bedtime may also aid in decreasing symptoms before sleeping. Obtaining adequate rest after strenuous activities is important for relaxation of any muscular components involved. It is beneficial during the initial phases of treatment to have the patient refrain from engaging in strenuous aerobic activities, which may create exertional breathing. This increases accessory muscle activity and potentially compromises the neurovascular structures throughout the thoracic inlet.

Figure 55-6 Brachial plexus slack position; note the posture of the head and cervical spine favoring the involved side.
Activities that aggravate the patient’s symptoms are identified during history taking. Many times, the patient is unaware of similar activities that include the same exacerbating movements. The patient should be educated to avoid all activities and motions that exacerbate symptoms during the initial phase of treatment. This educational process continues throughout the entire course of care. The patient should avoid any pressure over the thoracic inlet. Additional padding that increases the surface area can diminish pressure from automobile shoulder-restraint straps. Women should wear a strapless bra or use additional padding to increase the strap surface area. Finally, the patient should avoid carrying heavy objects, including handbags, with the affected extremity. These activities increase shoulder depression and traction on the neurovascular structures.
Proper sleep positioning is often important to obtaining symptom control and avoiding sleep disturbance. Sleep positions should place the affected upper extremity in a position that minimizes tension on the brachial plexus and its neurovascular structures, the cervical spine, and distal peripheral nerve tissues. Examples of sleep positions are demonstrated in Figure 55-7. The position should maintain the spine in neutral and support the upper extremity, avoiding tension on the neurovascular structures in the thoracic inlet. Finding a helpful sleep position may require nothing more than changing the side of the bed the patient sleeps on.
Postural Education. The postural educational process should be integrated into daily activities and occupational situations. Eliminating offending resting postures such as the forward-head and rounded-shoulder posture or upper extremity over head positions during work is an important component of this education. Modifications in the workplace may be necessary to achieve these goals. It is important to respect a longstanding offending posture and the accompanying tissue adaptations that result from it. Therefore reestablishment of a balanced posture and stability of the upper quarter during use requires patience. Postural awareness and correction is only taken seriously by the patient when the therapist presents these concepts appropriately. It is necessary to spend time at each treatment session discussing and working on posture and breathing. A proper balance of stretching and strengthening exercises is necessary to permit postural correction. Overcorrecting to an established “textbook” posture often leads to further exacerbation of symptoms. Because most of these patients are 40 years of age or older, these posture abnormalities result in longstanding adaptive tissue changes. These soft tissue adaptations occur within the fascia, muscle, articular structures, and neurovascular structures. Postural correction should also take into account the nervous system to avoid additional adverse neural tension on the plexus and its accompanying nerve roots and peripheral nerves. It may also be helpful to involve family members in the postural correction process and teach them to recognize abnormal breathing patterns to increase patient awareness. A low-impact, tolerable aerobic exercise program to encourage large segmental and muscle group activity is implemented during this stage as well. As reported by Novak and colleagues,85 many of these patients have body weight issues and compromised fitness status.
Proper Breathing Patterns. Altered breathing patterns at rest and during activity are common in patients with BPN. Accessory breathing patterns using the scalene, intercostal, and pectoralis minor muscles often occur when patients are focused and statically postured. All of these structures affect the path of the neurovascular structures as they progress laterally through the thoracic inlet. This accessory breathing pattern is identified by shallow breaths with increased cephalic excursion and a decrease in circumferential expansion of the thoracic cavity. This same breathing pattern may be evident during activities such as playing an instrument, working at a computer, reading, or writing. Patient education and instruction about these aberrant breathing patterns is essential in developing a diaphragmatic breathing pattern. Diaphragmatic breathing requires instruction by the therapist to help the patient reestablish the diaphragm as the major muscle responsible for breathing. Diaphragmatic breathing allows for accessory muscle relaxation and improved excursion of the thoracic cavity. Patient command of this is beneficial when progressing into the latter phases of treatment that incorporate diaphragmatic breathing into manual treatment techniques and the home program.
Additional Considerations. Treatment of BPES patients involves two additional considerations. It is imperative the therapist recognize the level of neuropathic pain involved and identify the extent to which each of the five clinical pain patterns described by Gifford and Butler89 is involved: (1) peripheral nociceptive, (2) peripheral neurogenic, (3) central nervous system-related, (4) sympathetic nervous system-related, and (5) affective–motivational. Although one or two of these may dominate the patient’s complaints, all five may be present, further complicating the problem. Through identification of these different pain patterns, a more direct treatment approach is established and the prognosis is improved. Patients with predominant central nervous system, sympathetic nervous system, or affective–motivational pain patterns are more difficult to treat, and their outcomes are often less successful. Often, it is necessary for a therapist to accept that complete resolution of a patient’s symptoms is unrealistic. The goal in this particular group of patients may be to improve the quality of the patient’s life by controlling symptoms and obtaining greater pain-free environmental ROM, thus allowing the patient to use the upper extremity in a more functional and comfortable manner.
During this time, identified secondary problems may be treated directly, for example, addressing active myofascial trigger points that are referring symptoms, providing pain modulation through modalities and exercise, or treating double- or multiple-crush situations. It is also appropriate to implement large-amplitude motions using all extremities through a low-impact aerobic program or using general nerve mobilization techniques via the spine and lower extremities. See Chapter 118 for further information and direction.
Restorative Phase. The primary purpose of the restorative phase of treatment is to “restore” or reverse soft tissue dysfunction identified during the evaluation. This phase of treatment is initiated only after comfort and symptom control have been achieved. In classic compressive TOS, soft tissue mobilization described by Smith72 may be instituted. The goals of these manual techniques are to improve flexibility of the associated tissues, restore normal tissue resting lengths, and assist in restoring normal posture. Addressing these problems may increase the size of the potential compression intervals and minimize neurovascular compression.84,90 Soft tissue mobilization includes addressing joint and soft tissue mobility of the acromioclavicular and sternoclavicular joints and scapulothoracic articulation as necessary. In addition, it is appropriate to improve first rib position,73 mobility, and cervical spine function. These techniques also address adaptively shortened muscles such as the pectoralis group and the scalene muscles via deep massage and stretch while avoiding brachial plexus tension. Brachial plexus gliding or peripheral nerve mobilization may be instituted. Examples of these techniques are demonstrated in Figure 55-8.
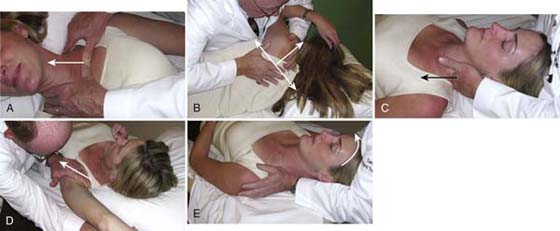
Figure 55-8 Manual techniques that can be used to improve the flexibility of the thoracic inlet. A, Sternoclavicular joint. B, Scapular-thoracic articulation. C, First rib. D, Pectoralis minor tightness. E, Scalene stretch.
It is beyond the scope of this chapter to discuss the specifics of peripheral nerve mobilization. See Chapter 118 for additional information. In general, the purpose of peripheral nerve mobilization is to restore normal neurophysiology and neurobiomechanics, thereby improving neural compliance and excursion.50 It is theorized that alleviating intraneural and extraneural compression results in improved vascular function and axoplasmic flow.75,76 This is accomplished by using components of the upper and lower limb tension tests to restore neural mobility. However, therapists are advised to obtain further information regarding these techniques and their appropriate use before proceeding with peripheral nerve mobilization techniques. Use of these techniques, especially during the early portions of the first two phases, without symptom control could significantly exacerbate the patient’s symptoms.
A home exercise program is instituted during the restorative phase treatment. Originally described by Peet and coworkers77 and subsequently modified by numerous other authors, the home program aims to improve the flexibility of the entire thoracic inlet region and its accompanying neurovascular structures. The following examples are not intended to be all-inclusive; other exercises may be more appropriate for a particular patient. Scalene stretching is preferably done in a supine position to minimize cervical muscle activity while maximizing stretch. Cervical protraction and retraction or “axial extension” exercises50 can help eliminate the forward-head and rounded-shoulder posture and reestablish proprioceptive input for proper cervical spine positioning. The therapist is reminded to correct any occipital rotation and restore lumbar posture. Incorporating diaphragmatic breathing into the exercise program assists the patient in habituating appropriate breathing patterns and is a gentle way of adding restorative forces to the involved tissues and indirectly mobilizing the brachial plexus.91 Diaphragmatic breathing can be combined with scalene, deltopectoral fascia, and pectoralis minor stretching. For example the patient performs shoulder forward flexion to a subthreshold position of symptom onset, and then uses diaphragmatic breathing to stretch the pectoralis minor by using the diminishing circumference of the lower thoracic cavity on exhalation to stretch the pectoralis minor. Pectoralis stretching can also be accomplished with a corner stretch or while the patient stands with arms elevated at 90 degrees in a doorway. Scapulothoracic flexibility exercises can be performed to improve soft tissue and brachial plexus flexibility and scapular motor control. In addition, scapulothoracic stabilization techniques such as quadruped positioning or using a therapeutic ball may help restore optimal scapulothoracic motor control. Cervical spine flexibility may be improved with active ROM, contract–relax, and a host of other techniques. Appropriate nerve gliding exercises may be instituted in conjunction with these techniques or as separate exercises. Examples of some home exercises are demonstrated in Figure 55-9.
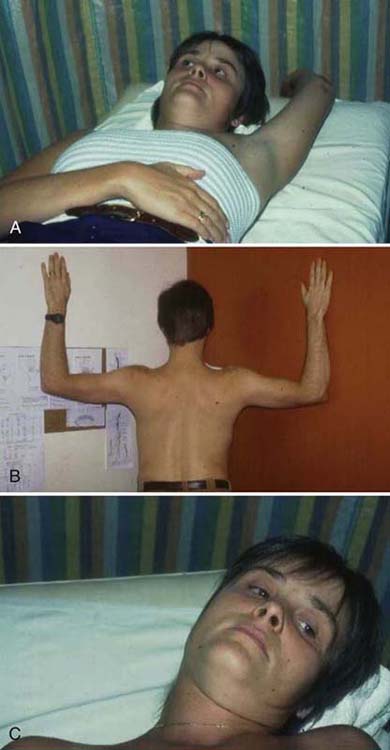
Figure 55-9 A, Diaphragmatic breathing combined with humeral forward flexion for pectoralis tightness. B, Corner stretching for pectoralis group and deltopectoral fascia tightness. C, Scalene stretching.
In patients classified with BPES these exercises may need to be modified to avoid placing tension on the neurovascular plexus and its accompanying peripheral nerves. Treatment for previously identified secondary problems continues. During this stage, the therapist must be mindful of any active motion dysfunction resulting from neurogenic involvement to avoid the end-ranges of motion, which may lead to adverse tension and exacerbation of pain. If symptom control has been achieved, specific nerve gliding techniques for the brachial plexus may be instituted. Because of the longstanding nature of the problems faced by this patient population, a home program is imperative and may be more important than specific hands-on techniques performed in the clinic. It is only over an extended period that the adaptive tissue changes can be corrected and balances between the soft tissues and the neurovascular structures can be restored. At no time during this phase should any treatment or home exercise program provoke more than mild transient symptoms. As previously alluded to, most of these patients present with neuropathic pain, and aggressive handling or too rapid a progression in therapy may provoke their initial level of tissue irritability.
Compressive Brachial Plexus Neuropathy. The primary strategies of this phase for patients with compressive BPN are intended to increase overall aerobic capacity and fitness, improve postural muscle imbalances, and institute workplace modifications, while continuing to emphasize posture awareness and breathing. In addition, a balanced nutritional program is encouraged to assist overweight patients; excess weight is a problem because obesity has been associated with TOS.85 Strengthening of postural muscles and incorporating scapular motor control continues to support the emphasis on postural awareness. It is sometimes unrealistic to expect that a longstanding postural fault or habit can be totally overcome through these techniques. It is realistic to expect the patient to reverse the offending posture as often as possible throughout the day. Rehabilitation exercises should also address work conditioning, be work task specific, and include modifications that help avoid inappropriate stresses across the brachial plexus region. In conjunction with this, the continued application of posture and diaphragmatic breathing in the workplace should be addressed. The patient’s awareness of these latter two components often begins to fail, resulting in continuance or recurrence of symptoms. Finally, during the rehabilitative phase of treatment for CBPN, continuous reassessment of patients’ subjective, objective, functional, and vocational status should be performed to address any final issues before discharge.
Brachial Plexus Entrapment Syndrome. These same strategies may also be instituted in patients with BPES. The return to, or institution of, any strengthening exercises involving the upper extremities should be approached with caution to avoid exacerbation or recurrence of the patient’s symptoms by placing inappropriate tension on the nervous system. In addition, specific nerve mobilization techniques for localized and secondary involvement of the peripheral nervous system should be implemented and previous nerve mobilization techniques continued as needed. This may require periodic visits to the clinic to appropriately modify or progress the exercise prescription. It is also important during this phase for the therapist to reevaluate expectations of recovery for the patient. It has been my unfortunate experience on several occasions, on achieving a 60% to 70% improvement, that further attempts to improve upper extremity conditioning resulted in exacerbation of the patient’s initial complaints. This necessitated a return to the initial phase of treatment to regain control before progressing again through the remaining two stages. The goals and expected outcome established by the therapist and patient may have to be tempered by the reality that 100% alleviation of symptoms may not be possible. Therefore educating patients about acceptable levels of activity tolerance and the positions that exacerbate their symptoms becomes extremely important in allowing continued daily activities and occupational tasks.
The following case example may help exemplify the treatment approach for brachial plexus neuropathy patients:
A 29-year-old woman was involved in a motor vehicle accident 18 months before she was referred to our clinic with the diagnosis of “brachial plexus neuropathy.” Three physicians and two physical therapists provided previous treatment without any significant improvement. Previous therapy included palliative modalities and a rigorous conditioning program. Despite this intervention, her symptoms progressively worsened. Objectively, the patient presented with three active myofascial trigger points, restricted cervical spine motion, glenohumeral joint limitations, and evidence of brachial plexus neural tension dysfunction. The patient also had positive active and passive motion dysfunction (neurodynamic testing) of the involved extremity, indicating NTD.
Control-phase treatment was directed toward the patient’s myofascial trigger points and employed appropriate techniques to deactivate the trigger points and interventions to eliminate brachial neurovascular tension and compression. This was achieved by placing the patient in the brachial plexus slack position (see Fig. 55-6). From this position, localized stretching of the involved trigger points was performed, minimizing stimulation of the sensitized peripheral nervous system. Restricted cervical ROM was managed with an active ROM program and manual mobilization techniques. At this point, the BPN components of her problem were not specifically addressed.
Progression into the restorative phase was initiated once the active myofascial trigger points were deactivated; cervical spine motion improved, and symptoms were controlled. Interventions now included soft tissue mobilization and peripheral nerve mobilization (gliding). These techniques continued until the patient no longer demonstrated evidence of ANT signs, negative provocative TOS maneuvers, inactive myofascial trigger points, and symmetrical cervical motion. Although complete subjective relief was not attained, the patient returned to work and functional activities she had been avoiding before treatment. During the rehabilitative phase she was instructed in additional postural strengthening exercises. Postural and breathing awareness and symptom avoidance education continued. Figure 55-10 contrasts the pretreatment and post-treatment findings for this patient.
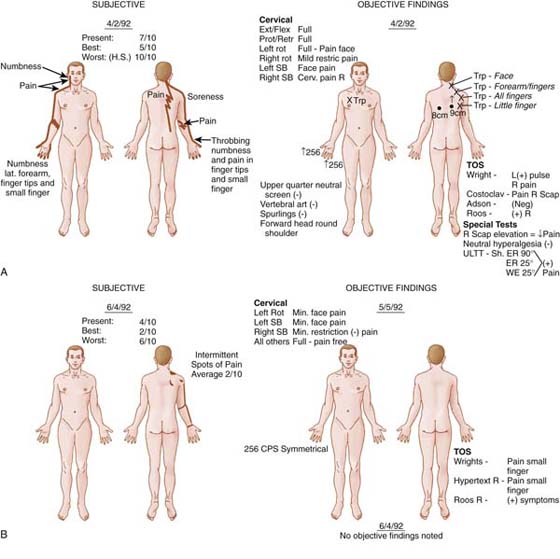
Figure 55-10 Actual patient diagrams of pretreatment and post-treatment findings for the patient in the case example. A, Pretreatment subjective pain diagram. B, Post-treatment subjective pain diagram.
The treatment of patients with upper quarter (brachial plexus) neuropathy, especially those presenting with long-term neuropathic pain, can be a complex and challenging problem for the occupational or physical therapist. Literature supports the use of conservative care as the preferred initial approach with these patients suffering with CBPN or BPES. Many of these patients present with additional secondary system complaints, including active myofascial trigger points, primary or secondary glenohumeral pathology, cervical pathology, and more distal peripheral neuropathies in the form of double or multiple crush. For this reason, treatment of these patients is rarely straightforward and is usually complex.
Treatment requires that the therapist understand the pathologic mechanism contributing to the patient’s complaints. This understanding, which is obtained only through a thorough examination performed by the therapist, is extremely important in appropriately classifying these patients’ conditions into one of two types: CBPN or BPES. Treatment should initially address the issues of comfort and symptom control. Gradual progression through the three phases of treatment requires that the therapist be alert to the patient’s symptom responses throughout the course of care. The therapist must also appreciate that tissue maladaptations occur over a number of years, especially when there was a strong postural and concomitant repetitive nature to their symptoms. In these particular cases, treatment requires patience and understanding that this is an ongoing process, best addressed through compliance with the home exercise prescription, postural awareness, and correction of abnormal breathing patterns. Finally, treatment progression requires the therapist to proceed in a very gentle, systematic, and controlled manner with minimal symptom response. Proper education of the patient for behavior modification, exercise compliance, symptom control, and postural awareness is requisite for optimal outcomes.
1. Roos DB. Thoracic outlet syndrome is under diagnosed [Comment]. Muscle Nerve. 1999;22:126–129.
2. Wilbourn AJ. Thoracic outlet syndrome is over diagnosed [Comment]. Muscle Nerve. 1999;22:130–136.
3. Adson A, Coffey JR. Cervical rib: a method of anterior approach for relief of symptoms by division of the scalenus anticus. Ann Surg. 1927;85:839–857.
4. Halsted WS. An experimental study of circumscribed dilation of an artery immediately distal to a partially occluding band and its bearing on the dilation of the subclavian artery observed in certain cases of cervical rib. J Exp Med. 1916;24:271.
5. Willshire WH. Supernumerary first rib clinical records. Lancet. 1860;2:633.
6. Leffert R. Thoracic outlet syndrome. Hand Clin. 1992;8:285–297.
7. Szabo R, Gelberman RH, Williamson RV, et al. Vibratory sensory testing in acute peripheral nerve compression. J Hand Surg [Am]. 1984;9A:1104–1109.
8. Elvey R. Brachial plexus tension test and the pathoanatomical origin of arm pain. In: Glasgow E, Tavomey L, eds. Aspects of Manipulative Therapy. Melbourne, Australia: Lincoln Institute of Health Sciences; 1979:105–109.
9. Elvey R. Treatment of arm pain associated with abnormal brachial plexus tension. Aust J Physiother. 1986;32:225–230.
10. Ide M, Ide J, Yamaga M, Takagi K. Symptoms and signs of irritation of the brachial plexus in whiplash injuries. J Bone Joint Surg. 2001;83-B:226–229.
11. Allison G, Nagy B, Hall T. A randomized clinical trial of manual therapy for cervico-brachial pain syndrome—a pilot study. Man Ther. 2002;7:95–102.
12. Cohen M, Arroyo JF, Champion GD, Browne CD. In search of the pathogenesis of refractory cervicobrachial pain syndrome. Med J Aust. 1992;156:432–436.
13. Coppieters M, Stappaerts KH, Wouters LL, Janssens K. Aberrant protective force generation during neural provocative testing and the effect of treatment in patients with neurogenic cervicobrachial pain. J Manipulative Physiol Ther. 2003;26:99–106.
14. Jordan S, Ahn S, Gelabert H. Differentiation of thoracic outlet syndrome from treatment-resistant cervical brachial pain syndromes: development and utilization of a questionnaire, clinical examination and ultrasound evaluation. Pain Physician. 2007;10:441–452.
15. Capistrant T. Thoracic outlet syndrome in whiplash injury. Ann Surg. 1977;185:175–178.
16. Weinberg H, Nathan H, Magora F, et al. Arthritis of the first costovertebral joint as a cause of thoracic outlet syndrome. Clin Orthop. 1972;86:159–163.
17. Butler D. Adverse mechanical tension in the nervous system: a model for assessment and treatment. Aus J Physiother. 1989;35:237–238.
18. Shacklock M. Positive upper limb tension test in a case of surgically proven neuropathy: analysis and validity. Man Ther. 1996;1:154–161.
19. Shacklock M. Improving application of neurodynamic (neural tension) testing and treatments: a message to researchers and clinicians. Man Ther. 2005;10:175–179.
20. Pratt N. Clinical Musculoskeletal Anatomy. Philadelphia: J. B. Lippincott; 1991.
21. Machleder H, Moll F, Verity A. The anterior scalene muscle in thoracic outlet compression syndrome: histochemical and morphometric studies. Arch Surg. 1986;121:1141–1144.
22. Sanders RJ, Jackson CG, Banchero N, Pearce WH. Scalene muscle abnormalities in traumatic thoracic outlet syndrome. Am J Surg. 1990;159:231–236.
23. Wright IS. The neurovascular syndrome produced by hyperabduction of the arm. Am Heart J. 1945;29:1–19.
24. Thomas G, Jones TW, Stavney LS, et al. Thoracic outlet syndrome. Am Surg. 1978;78:483–495.
25. Conroy J, Schneiders A. The T4 syndrome. Man Ther. 2005;10:292–296.
26. Mulder D, Greenwood F, Brooks C. Posttraumatic thoracic outlet syndrome. J Trauma. 1981;13:706–714.
27. Novak CB, Mackinnon SE, Patterson GA. Evaluation of patients with thoracic outlet syndrome. J Hand Surg [Am]. 1993;18A:292–299.
28. Ozdemir O, Ozcakar L. Thoracic outlet syndrome: another cause for unilateral palmar hyperhidrosis. Clin Rheumatol. 2007;26:1375–1376.
29. Cuetter AC, Bartoszek DM. The thoracic outlet syndrome: controversies, overdiagnosis, overtreatment, and recommendations for management. Muscle Nerve. 1989;12:410–419.
30. Sanders R, Hammond S, Rao N. Diagnosis of thoracic outlet syndrome. J Vasc Surg. 2007;46:601–604.
31. LeForestier N, Moulonguet A, Maisonobe T, et al. True neurogenic thoracic outlet syndrome: electrophysiological diagnosis in six cases. Muscle Nerve. 1998;21:1129–1134.
32. Urschel J, Hamred SM, Grewal R. Neurogenic thoracic outlet syndrome. Postgrad Med J. 1994;70:785–789.
33. Brantigan C, Roos D. Etiology of neurogenic thoracic outlet syndrome. Hand Clin. 2004;20:17–22.
34. Sobey A, Grewal RP, Hutchison KJ, Urschel JD. Investigation of non specific thoracic outlet syndrome. J Cardiovasc Surg (Torino). 1993;34:343–345.
35. Ribbe EB, Lindgren SHS, Norgren LEH. Clinical diagnosis of thoracic outlet syndrome—evaluation of patients with cervico-brachial symptoms. Man Med. 1986;2:282–285.
36. Brantigan C, Roos D. Diagnosing thoracic outlet syndrome. Hand Clin. 2004;20:27–36.
37. Travell J, Simons D. Myofascial Pain and Dysfunction, The Triggerpoint Manual. Baltimore: Williams & Wilkins; 1983.
38. Leffert R, Cumley G. The relationship between dead arm syndrome and thoracic outlet syndrome. Clin Orthop. 1987;223:20–31.
39. Upton A, McComas A. The double crush in nerve entrapment syndrome. Lancet. 1973;2:359–362.
40. Urschel H, Razzuk MA, Wood RE, et al. Objective diagnosis (ulnar nerve conduction velocity) and current therapy of the thoracic outlet syndrome. Ann Thorac Surg. 1979;12:608–620.
41. Wood V, Biondi J. Double-crush nerve compression in thoracic-outlet syndrome. J Bone Joint Surg. 1990;72-A:85–87.
42. Eurroll R, Hurst L. The relationship of thoracic outlet syndrome and carpal tunnel syndrome. Clin Orthop. 1982;164:149–153.
43. MacKinnon S. Double and multiple “crush” syndromes: double and multiple entrapment neuropathies. Hand Clin. 1992;8:369–390.
44. Seror P. Symptoms of thoracic outlet syndrome in women with carpal tunnel syndrome. Clin Neurophysiol. 2005;116:2324–2329.
45. Schollen W, Degreef I, DeSmet L. Kiloh-Nevin syndrome: a compression neuropathy or brachial plexus neuritis? Acta Orthop Belg. 2007;73:315–318.
46. Kelly T. Thoracic outlet syndrome current concepts of treatment. Ann Surg. 1979;190:657–662.
47. Roos D, Owens C. Thoracic outlet syndrome. Arch Surg. 1966;93:71–74.
48. Williams H, Carpenter N. Surgical treatment of the thoracic outlet compression syndrome. Arch Surg. 1978;113:850–852.
49. Jamieson WG, Chinnick B. Thoracic outlet syndrome: fact or fancy? A review of 409 consecutive patients who underwent operation. Can J Surg. 1996;39:321–326.
50. Butler D. The Sensitive Nervous System. Adelaide, Australia: Noigroup Publications; 2000.
51. Raskin NH, Howard MW, Ehrenfeld WK. Headache as the leading symptom of the thoracic outlet syndrome. Headache. 1985;25:208–210.
52. Ozcakar L, Inanici F, Kaymak B, et al. Quantification of the weakness and fatigue in thoracic outlet syndrome with isokinetic measurements. Br J Sports Med. 2005;39:178–181.
53. Casey R, Richards S, O’Donohoe M. Exercise induced critical ischaemia of the upper limb secondary to a cervical rib. Br J Sports Med. 2003;37:455–456.
54. Komanetsky RM, Novak CB, Mackinnon SE, et al. Somatosensory evoked potentials fail to diagnosis thoracic outlet syndrome. J Hand Surg [Am]. 1996;21A:662–666.
55. Yiannikas C, Walsh JC. Somatosensory evoked responses in the diagnosis of thoracic outlet syndrome. J Neurol Neurosurg Psychiatry. 1983;46:234–240.
56. Gillard J, Maryse Pérez-Cousin, Éric Hachulla, et al. Diagnosing thoracic outlet syndrome: contribution of provocative tests, ultrasonagraphy, electrophysiololgy, and helical computed tomography in 48 patients. Joint Bone Spine. 2001;68:416–424.
57. Demirbag D, Unlu E, Ozdemir F, et al. The relationship between magnetic resonance imaging findings and postural maneuver and physical examination tests in patients with thoracic outlet syndrome: results of a double-blind, controlled study. Arch Phys Med Rehabil. 2007;88:844–851.
58. Pollach W. Surgical anatomy of the thoracic outlet syndrome. Surg Gynecol Obstet. 1980;150:97–103.
59. Quinter J. Stretch induced cervicobrachial pain syndrome. Aust J Physiother. 1990;36:99–103.
60. Spurling RG, Scoville WB. Lateral rupture of the cervical intervertebral discs: a common cause of shoulder and arm pain. Surg Gynecol Obstet. 1944;78:350–358.
61. Magee D. Orthopedic Physical Assessment. 3rd ed Philadelphia: W.B. Saunders Company; 1997.
62. Ide J, Kataoka Y, Yamaga M, et al. Compressions and stretching of the brachial plexus in thoracic outlet syndrome: correlation between neuroradiographic findings and symptoms and signs produced by provocations manoeuvres. J Hand Surg [Br]. 2003;28-B:218–223.
63. Szabo R, Gelberman R, Dimick M. Sensibility testing in patients with carpal tunnel syndrome. J Bone Joint Surg. 1984;66A:60–64.
64. Falconer MA, Weddell G. Costoclavicular compression of the subclavian artery and vein: relation to the scalenus anticus syndrome. Lancet. 1943;2:539–544.
65. Adson AW. Cervical ribs: symptoms, differential diagnosis, and indications for section of the insertion of the scalenus anticus muscle. J Int Coll Surg. 1951;14:546–558.
66. Gergoudis R, Barnes R. Thoracic outlet arterial compression: prevalence in normals. Angiology. 1980;1980:538–541.
67. Warrens A, Heaton J. Thoracic outlet compression syndrome: the lack of reliability of its clinical assessment. Ann R Coll Surg Engl. 1987;69:203–204.
68. Costigan DA, Wilbourn AJ. The elevated arm stress test: specificity in the diagnosis of thoracic outlet syndrome [Abstract]. Neurology. 1985;35(Suppl 1):74–75.
69. Rayan G, Jensen C. Thoracic outlet syndrome: provocative examination maneuvers in a typical population. J Shoulder Elbow Surg. 1995;4:113–117.
70. Plewa M, Delinger M. The false-positive rate of thoracic outlet syndrome shoulder maneuvers in healthy subjects. Acad Emerg Med. 1998;5:337–342.
71. Toomingas A, Nilsson T, Hagberg M, Lundström R. Predictive aspects of the abduction external rotation test among male industrial and office workers. Am J Ind Med. 1999;35:32–42.
72. Smith K. The thoracic outlet syndrome: a protocol of treatment. J Orthop Sports Phys Ther. 1979;1:89–99.
73. Lindgren K-A. Conservative treatment of thoracic outlet syndrome: a 2-year follow-up. Arch Phys Med Rehabil. 1997;78:373–378.
74. Lindgren K-A, Leino E, Manninen H. Cervical rotation lateral flexion test in brachialigia. Arch Phys Med Rehabil. 1992;73:735–737.
75. Butler D, Gifford L. The concept of adverse mechanical tension in the nervous system, part 1: testing for “dural tension. Physiotherapy. 1989;75:622–629.
76. Butler D, Gifford L. The concept of adverse mechanical tension in the nervous system, part 2: examination and treatment. Physiotherapy. 1989;75:629–636.
77. Peet RM, Hendriksen JD, Anderson TP, et al. Thoracic outlet syndrome: evaluation of a therapeutic exercise program. in Staff Meeting of The Mayo Clinic. 1956. Rochester.
78. Urschel H, Paulson B, McNamara J. Thoracic outlet syndrome. Ann Surg. 1968;6:1–9.
79. Dale W, Lewis M. Management of thoracic outlet syndrome. Ann Surg. 1975;181:575–585.
80. McGough E, Pearce M, Byme J. Management of thoracic outlet syndrome. J Cardiovasc Surg (Torino). 1979;77:169–172.
81. Woods S. Thoracic outlet syndrome. West J Med. 1978;128:9–12.
82. Walsh MT. Therapist management of thoracic outlet syndrome. J Hand Ther. 1994.7.
83. Ingesson E, Ribbe E, Norgren L. Thoracic outlet syndrome: evaluation of a physiotherapeutical method. Man Med. 1986;2:86–88.
84. Sucher B. Thoracic outlet syndrome—a myofascial variant: part 2. treatment. J Am Osteopath Assoc. 1990;90:810–823.
85. Novak C, Collins D, Mackinnon S. Outcome following conservative management of thoracic outlet syndrome. J Hand Surg [Am]. 1995;20A:542–548.
86. Cramer C. A reconditioning program to lower the recurrence rate of brachial plexus neuropraxia in collegiate football players. J Athl Train. 1999;34:390–396.
87. Hanif S, Tassadaq N, Rathore MF, et al. Role of therapeutic exercises in neurogenic thoracic outlet syndrome. J Ayub Med Coll Abbottabad. 2007;19:85–88.
88. Landry G, Moneta GL, Taylor LM Jr, et al. Long-term functional outcome of neurogenic thoracic outlet syndrome in surgically and conservatively treated patients. J Vasc Surg. 2001;33:312–319.
89. Gifford L, Butler D. The integration of pain sciences into clinical practice. J Hand Ther. 1997;10:86–95.
90. Sucher BM. Thoracic outlet syndrome—a myofascial variant: part 1. Pathology and diagnosis. J Am Osteopath Assoc. 1990;90:686–704.
91. Greening J, Dilley A, Lynn B. In vivo study of nerve movement and mechanosensitivity of the median nerve in whiplash and non-specific arm pain patients. Pain. 2005;115:248–253.