▪ Pain is multidimensional; more than one test or measure may be needed in the examination.
▪ Pain may be the cause or the result of other musculoskeletal impairments, so both examination and plan of care should address these relationships.
▪ Identifying the primary source of pain mediation will assist the therapists with developing an appropriate plan of care.
▪ Despite insufficient evidence for the use of specific therapy interventions for pain modulation, patients will seek treatment for their pain.
Pain is defined as an unpleasant sensory and emotional experience associated with actual or potential tissue damage.1 It is the primary reason that patients seek medical attention. As described in Chapter 113, pain mechanisms are complex and multidimensional. The pain experience is unique to each individual patient, thus making the assessment and management of pain challenging to therapists and surgeons.
The Joint Commission on Accreditation of Healthcare Organizations (JCAHO) requires assessment and appropriate management of pain within accredited facilities. Pain is considered the “fifth vital sign” and should be measured regularly along with the other vital signs: blood pressure, heart rate, respiratory rate, and temperature.2 Although not all clinicians treating hand and upper extremity patients work in a JCAHO-accredited institution or clinic, pain assessment is essential in hand rehabilitation. Our initial assessment of pain allows us to select the appropriate intervention and to determine whether a particular intervention has been effective in alleviating pain. Also, if the nature of the pain is not straightforward, clinicians need to determine the extent and location of the pain as a way to determine the underlying cause of the patient’s pain or source of pain mediation.
Pain is usually a symptom associated with musculoskeletal conditions commonly seen in hand rehabilitation, including multiple trauma. It may also be associated with pathology of the cardiovascular, pulmonary, integumentary, neuromuscular, metabolic, and endocrine systems. Pain may occur secondary to impairments in circulation, ventilation, joint integrity, muscle performance, and posture. The presence of pain may result in the loss of motion, decreased muscle performance, decreased sensory discrimination, and edema. Secondary to these impairments, functional limitations are likely to be reported with activities of daily living, work duties, and sports and leisure activities.3 Therefore, the assessment of pain is almost always inclusive of a comprehensive regional examination with medical screening. Furthermore, the management of pain will include strategies to relieve pain as well as interventions to address the impairments that caused the pain or resulted from the presence of pain.
Because of the highly subjective and individual response to pain, pain is one of the most difficult symptoms to examine clinically. Tests and measurements have been developed to quantify pain and to attempt to objectify the pain experience. However, because the patient still provides the information, it still is considered to be “subjective” based on the patient’s perception of his or her pain. There are several individual factors that influence the patient’s pain experience including sex, age, and social, cultural, and genetic factors.4
Nolan5 described five components of pain: physiologic, perceptual, affective, cognitive, and behavioral aspects. Table 114-1 defines each component. The pain behaviors observed in our patients represent the interaction among all five components. Observation of changes in a patient’s behavior, including how he or she reports pain, is the primary way clinicians evaluate the effectiveness of their intervention.
Table 114-1 Five Components of Pain as Described by Nolan5
Component |
Explanation |
Physiologic |
The injury or tissue damage that serves as the source of the noxious stimuli or the source of the pain |
Perceptual |
The sensations conveyed by the nociceptors to the ascending pain pathways of the spinal cord to portions of the brain to alert the individual to respond to the painful stimuli; the brain is made aware of the pain and the individual responds by withdrawing from painful stimuli |
Affective |
Involves the individual’s emotional and psychological state, which can influence how the person responds to pain; the individual’s emotional status also may affect how he or she reports the pain |
Cognitive |
The patient’s knowledge about the cause of the pain; if the individual understands the nature or source of his or her pain, then he or she usually may respond more appropriately to the painful stimuli; lack of information or misinformation can heighten or depress the behavioral response to pain |
Behavioral |
The individual’s expression of the experience of pain, which involves interaction with the other components of pain |
All the established types of assessments for pain are essentially different forms of a common request that each therapist needs to ask of the patient as part of a thorough clinical examination. The request is “Describe your pain.” Pain often is reported as a chief symptom by hand therapy patients; therefore, it is an essential component of a therapist’s examination and intervention.
The reliability of the test can easily be influenced by the testing methodology, the affective and cognitive components of the patient’s pain, and the individual factors that may influence the patient’s pain perception. For example, the first time that a patient comes into the clinic with a fracture, tendon injury, or crush injury for early mobilization, he or she might be apprehensive about performing exercises and report high pain scores. The patient may think that it is too early to begin to exercise and that exercise may be painful or cause tissue damage. However, after the patient has experienced the first session, he or she is more likely to be relaxed during subsequent visits and report lower pain scores. Another example is that many patients with hand injuries or disease processes may undergo a subsequent secondary surgical procedure. Clinical experience has demonstrated that the patient’s reaction to the pain after the secondary procedure is usually less intense than after the primary procedure. The patient has an understanding of the rehabilitation process; therefore, less anxiety and pain behavior are expected.
The threshold at which pain is detected is highly reproducible in different subjects within clinical pain experiments and in the same subject at different time periods. Threshold testing represents the sensory component of pain and determines whether a stimulus is painful. Conversely, pain tolerance is highly variable and correlates well with the affective and cognitive components of pain. Tolerance implies a question about how much pain an individual can take.6 Variables that affect pain tolerance include fatigue, lack of control, stress, and anxiety. During examination, therapists should determine whether any of the variables that modulate pain tolerance are present. Therapy interventions such as patient education about the rehabilitation process and positioning during hours of sleep can be extremely helpful in reducing some of the variables associated with pain tolerance.
As the therapist gathers information about pain, it is necessary to develop a hypothesis about the source of the patient’s pain mediation. Sometimes this is readily determined by the referral diagnosis, but in patients with longstanding pain, therapists often must carefully review the findings to determine the primary source of pain mediation. The International Association for the Study of Pain (IASP) has developed a classification system for pain that includes definitions for pain terms and descriptions of pain syndromes.1 Many of these pain terms are defined in Table 113-1 (online). This established taxonomy enhances communication in the scientific literature and clinic setting. Gifford and Butler7 have used the components of the IASP classification system to promote clinical reasoning among therapists about the primary source of pain mediation in patients with the chief symptom of pain. Understanding the source of the pain mediation allows therapists to effectively assess and manage pain symptoms.
Injured musculoskeletal tissues serve as the source of pain mediation. As discussed in Chapter 113, tissues contain nociceptors that receive chemical, mechanical, or thermal stimulation and initiate impulses along nociceptive fibers (Aδ- and C-fibers) to the dorsal horn. These impulses are cortically registered as pain. This source of pain mediation is most commonly associated with acute inflammation and tissue damage (peripheral sensitization). Nociceptive pain usually relates to acute somatic pain, but it may be present in patients with chronic pain as well. As tissue healing occurs, pain is expected to subside. Common therapy interventions used to modulate pain such as physical agents, orthotic intervention, and graded exercise are usually effective. Patients with traumatic hand injuries or postoperative patients commonly have nociceptive pain.
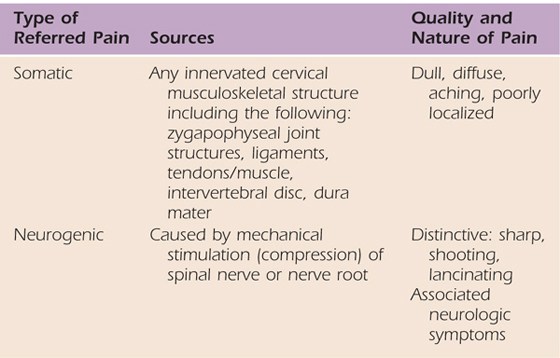
The source of pain is a lesion or dysfunction in the peripheral nervous system.1 Lesions or areas of dysfunction are called abnormal impulse generator sites (AIGs). Pain is mediated by mechanical or chemical stimulation of injured neural tissue (i.e., AIGs).7 Examples of peripheral neurogenic pain in the upper quarter include cervical radicular pain and pain associated with peripheral nerve entrapment, such as carpal tunnel syndrome. Bogduk8 provides a description of the clinical differences associated with somatic versus neurogenic referred pain in the cervical spine. Structures at spinal levels C3 and lower can refer pain to the ipsilateral upper limb. Higher cervical levels refer pain only to the head and neck. Neurogenic referred pain is also associated with neurologic symptoms such as numbness, paresthesias, or weakness.8 These differences, illustrated in Table 114-2, may help the therapist determine whether the primary source of pain mediation is peripheral neurogenic or nociceptive pain, or a combination of the two.
Table 114-2 Referred Pain in the Cervical Spine: Somatic Versus Neurogenic

Pain is mediated by a lesion or dysfunction within the central nervous system (CNS).1 It is theorized that an abnormal sensitivity or discharge of CNS neurons or synapses is associated with pain mechanisms. The nature of these pain symptoms is inconsistent and behaves differently from the peripheral sources of pain. There is usually little or no correlation with stimulus and response. Sudden stabs of unprovoked pain, pain “with a mind of its own,” or pain that comes out of nowhere may occur as a result of abnormal CNS nociceptor activity. Abnormal pain states such as allodynia and hyperalgesia are centrally mediated.9 Common therapy interventions usually are ineffective with this type of pain. Pharmacologic agents and behavior modification are key components to pain modulation in patients with central pain mediation.
The primary source of pain is thought to be a function of the sympathetic nervous system. Both types of complex regional pain syndrome (CRPS), type I (causalgia) and type II (reflex sympathetic dystrophy), are linked to this source of pain mediation.1 Chapter 115 and Chapter 116 discuss the clinical examination, medical management, and appropriate therapist’s intervention for patients with this source of pain mediation.
The source of pain is within the CNS and is related primarily to neurons or pathways concerned with affect or emotion.1 The limbic system is likely to be involved in this source of pain mediation. Other sources of pain mediation can be influenced or enhanced by changes in the patient’s emotional state. This is related to the concept of pain tolerance, as previously discussed. Therapy intervention must include patient education to modify the effect of affective variables on pain output.
Referred pain is pain that occurs at a site remote from the source of the disease or injury. The onset, duration, and perception of pain largely depend on the primary cause of the pain.10 Referred pain occurs as a result of convergence of visceral and peripheral nociceptors on the same common nerve root of the spinal cord11,12 (Fig. 114-1). In the upper quarter, referred pain is generally limited by visceral structures (organs) to the shoulder girdle region (Fig. 114-2). An inflamed, infected, or obstructed heart, spleen, pancreas, or gallbladder may apply direct pressure to the diaphragm and refer pain to the shoulder. The gallbladder is on the right side of the diaphragm and would refer pain to the right shoulder. The heart and spleen would refer pain to the left shoulder. The location of the pancreas allows it to refer pain to either shoulder. The connection here is that the diaphragm is innervated by the phrenic nerve, which comprises cervical nerve roots C3 to C5. The shoulder cutaneous area is also innervated by nerve roots C4 and C5.12
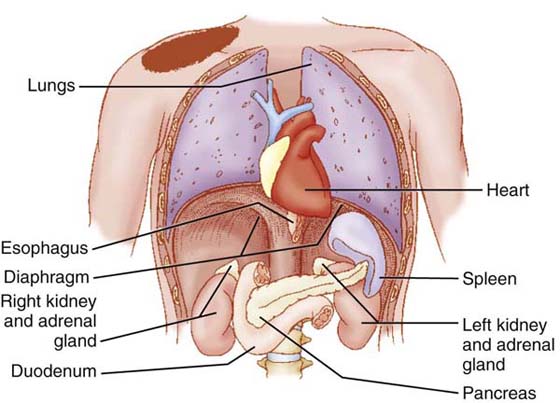
Figure 114-2 Direct pressure from inflamed, infected, or obstructed organs near the diaphragm can refer pain to the ipsilateral shoulder. (From Goodman CC, Snyder TEK, eds. Differential Diagnosis for Physical Therapists: Screening for Referral, 4th ed. St. Louis: Saunders Elsevier; 2007.)
The other common type of referred pain in the upper quarter is radicular or neurogenic as discussed with peripheral neurogenic pain. The nerve roots of C5, C6, and C7 are most frequently involved and may refer pain to the shoulder, elbow, forearm, or hand on the ipsilateral side.8 Additional information on the examination and management of neurogenic referred pain may be found in Chapter 53 and Chapter 55.
The selection of the appropriate measure for pain assessment depends on the aspect of pain the therapist is trying to capture. Table 114-3 classifies the pain measure with the appropriate dimension of pain. Most of the tools described subsequently assess only one dimension of pain. Multidimensional tools would be more helpful because they integrate pain and other symptoms with function.
Table 114-3 Pain Measure Applied to the Appropriate Dimension of Pain
Dimension of Pain |
Pain Measure(s) Used |
Spatial (location) |
Pain drawings or body diagrams |
Intensity (how much) |
Verbal or visual rating scales |
Quality or nature |
Patient interviews |
Temporal |
Pain diary, repeated rating scales or pain drawings |
Functional impairment |
Self-report or specific outcome questionnaires |
The therapist can ask a series of questions regarding the patient’s perception of pain as part of the subjective portion of the clinical examination.13 Questions should elicit information regarding the intensity, quality, temporal aspects, and physical characteristics of pain. Intensity usually is described in terms of the severity of the pain. The quality of the pain may be described as a burning pain or a sharp, stabbing pain. The temporal aspects of pain include whether the pain is constant or intermittent. They also may include the time of day that the pain is better or worse and what activities seem to exacerbate the pain. The physical characteristics of pain refer to the location of the pain and whether the pain is radiating, localized, or diffuse. When conducting the interview, the therapist needs to be careful not to ask leading questions regarding pain. Broad-based questions (e.g., Do you have pain at all times?) also should be avoided. Box 114-1 reviews commonly asked questions during a pain interview.
Box 114-1 Commonly Asked Questions Regarding Pain During Patient Interview
Where do you feel pain? (patient may point to painful areas)
Is your pain deep (within a joint) or superficial?
Is your pain constant or intermittent?
If constant, does it vary in intensity?
If intermittent, when do you have pain?
What is the frequency of the pain? (frequent, occasional)
How long have you had this pain?
Do you have pain now? (during interview)
Describe your pain (throbbing, aching, sharp, dull).
Does the pain move or spread to other areas?
Is pain aggravated by movement?
Is pain aggravated by certain postures?
Can you demonstrate the movement or postures that cause pain?
Do you have stiffness associated with pain?
Do you have pain at night or in the morning?
Does the pain wake you from sleep?
Do you have pain during activity?
Many rating scales have been suggested in the literature as a way to measure the intensity of a patient’s pain.13,14 These rating scales are relatively quick and easy to administer. They can be presented in a verbal or visual format. Rating scales can be used as part of the initial assessment of pain as well as before, during, or after subsequent treatment sessions or procedures. The information obtained from the rating scales has been criticized as being too vague and not providing a true representation of the total pain experience.13 The information gained from the rating scales is momentary and therefore may provide the clinician with limited information regarding the effectiveness of a particular intervention in providing overall relief of pain. To administer a numerical rating scale, the therapist generally asks the patient to assign a number to his or her perception of pain or to “rate the pain” on a scale of 0 to 10, with 0 referring to no pain, and 10 being the worst pain that the patient has ever experienced. The visual analog scale (VAS) has several modifications (Fig. 114-3). Commonly, it includes a 10-cm horizontal or vertical line that represents a range of levels of pain. The line may have no marks or descriptive words except at the ends of the line, which represent no pain at one end and the worst pain possible at the opposite end. Other visual scales may place more word descriptors along the continuum. The patient places a mark on the line to indicate his or her level of pain. A problem that may occur when using the rating scales is that the patient may initially start on the scale near or at the end of the scale, indicating the worst pain, and then the patient’s pain experience becomes worse. In this case, the patient’s response on the scale may exceed the upper limit.

Figure 114-3 Visual analog scales from Schultz upper extremity pain assessment form.(Copyright 1993, Karen Schultz-Johnson.)
The patient or the therapist can fill out a diagram of the human body with both front and back views.13 For the hand-patient population, an enlarged diagram of the upper quarter (Fig. 114-4) may be an appropriate alternative to a full-body diagram. This would allow more detailed information regarding the location, intensity, and quality of the pain to be illustrated specifically in the upper quarter. However, when examining a patient with longstanding pain, we prefer to use a full-body diagram to determine all potential pain complaints. Patients with central sensitization typically report spreading pain or secondary hyperalgesia, so the full-body diagram will allow for assessment of these types of pain mediation. Colored pencils, degrees of shading, or symbols can be used to represent the intensity and the quality of the pain. Numbness and paresthesias associated with the pathologic conditions can be included in this format by using different identification markings such as dotted areas for paresthesias and cross hatches for areas of numbness. Letters such as “E” and “I” can be used to identify the pain as being located externally or internally. These would indicate that the pain is within superficial or deep structures, respectively. Numbers may be used to give a numerical rating to the different areas of pain located on the diagram.
Figure 114-4 Body diagram specifically for upper quarter pain from Schultz upper extremity pain assessment form.(Copyright 1993, Karen Schultz-Johnson.)
Numerous self-report questionnaires have been described in the literature over the past 10 to 15 years. These include quality-of-life questionnaires such as the SF-36,15 region-specific questionnaires such as the Disabilities of the Arm, Shoulder, and Hand (DASH),16 and disease-specific questionnaires such as the Carpal Tunnel Instrument.17 These examples of self-report outcome measures allow correlation to be determined between pain complaints and functional performance. The Michigan Hand Outcomes Questionnaire may be used to determine how the patient feels about his or her pain and function.14,18 Descriptions of many types of questionnaires for the upper extremity are presented elsewhere.14,19 Pain assessment scales within these questionnaires may be more valuable than independent pain assessment tools in determining the outcome of therapy intervention. To learn more about the use of these measures see Chapter 16.
The McGill Pain Questionnaire (Fig. 114-5, online) provides a comprehensive look at the multidimensional aspects of pain. A short form and a long form have been suggested for clinical use. The long form consists of four parts. One part of the questionnaire involves word descriptors that are categorized and ranked with regard to quality and intensity. The patient selects only one word from each group of words. A particular group of words may be omitted if it does not match the patient’s perception of pain. Another part includes a rating scale that could be a modified VAS or a present pain index; this consists of a numerical value being assigned to a descriptive word. The remaining parts include a body diagram and a questionnaire regarding the previously described temporal aspects of pain.14,20
Numerous authors have reported the reliable use of a pressure algometer to quantify the amount of pressure necessary to produce point tenderness as reported by the patient’s report of pain with pressure.21-23 Lower algometer scores would indicate increased point tenderness or pain. Higher pressure tolerance scores would indicate less pain (Fig. 114-6). The pressure algometer may be used to measure hyperalgesia to mechanical stimuli. Primary hyperalgesia such as point tenderness would be examined at the site of injury, and secondary hyperalgesia is examined away from the site of injury. Primary hyperalgesia is related to peripheral nociceptive or neurogenic causes of pain such as inflammation. Secondary hyperalgesia is a sign of central sensitization or altered processing of painful stimuli within the dorsal horn of the spinal cord.
Monofilaments (Von Frey or Semmes–Weinstein) may be used to examine for allodynia, a painful response to a nonpainful stimulus, especially in the distal extremities.1 Allodynia may be seen postoperatively along surgical scars and is commonly referred to as a hypersensitive scar. It is also a common symptom of complex regional pain syndrome. The monofilaments may be applied in a graded manner just as they are used for sensibility testing. However, the response with allodynia will be painful rather than just a positive response to light touch.
After completion of the physical examination and pain assessment, the therapist should apply clinical reasoning skills to establish a hypothesis of the primary source of pain mediation and associated symptoms. As previously stated, pain may be due to other musculoskeletal impairments such as decreased muscle performance or loss of motion or the presence of pain may cause similar impairments. The therapist’s plan of care may need to address these impairments along with direct pain management. Chapters throughout this text provide specific information about pain management for specific clinical conditions. This section of the chapter provides an overview of the interventions used to treat a chief symptom of pain.
With the exception of patient education, the underlying mechanisms for most therapy interventions to decrease pain are based on reducing the inflammatory response in healing tissue, modulating pain via the gating mechanism, acting as a counterirritant or distraction, and releasing endogenous opioids. In addition, interventions that restore function or reduce contributing factors such as decreased motion or muscle weakness may remove the painful irritant or stimulus.
In 1965, Melzack and Wall24 proposed that processing of pain information occurred in the substantia gelatinosa (superficial layers) of the spinal cord. Some type of gating mechanism involved interplay between the large primary afferents (sensory or motor fibers) and the pain fibers (see Fig. 113-5, online) This theory provided some rationale for many clinical observations that were previously unexplained and gave a rationale for new therapies, including transcutaneous electrical nerve stimulation (TENS) of peripheral nerves.11,25,26
Therapists should take the time to explain their hypothesis of why the patient has a chief symptom of pain, including the source of pain mediation. When the patient gains an understanding of his or her pain condition, the therapist is likely to see a reduction in anxiety about the rehabilitation process. Patients need to understand that therapy, especially exercise, is not going to cause more pain or tissue damage. Education should also empower the patient to be an active participant in the plan of care. This may need to be continually emphasized with some patients. If the patient is an active participant, compliance with therapy visits and home programs should be evident. It is important to provide patients with written instructions with pictures for home programs because pain may distract their ability to remember the complete program. Including pictures in home programs is more readily available to therapists today with the explosion of digital cameras and computers in the clinic. Patient education may also include information about graded therapeutic exercise, rest from aggravating activities, ergonomic considerations, modifications of activities of daily living (ADLs), joint protection principles, positioning and postural awareness, and lifestyle changes in the areas of nutrition and exercise. This intervention is an essential component to all patients, regardless of the source of pain mediation.
Patients should be instructed to avoid motions or functional activities that aggravate their pain. This intervention is an essential component to all patients, regardless of the source of pain mediation. It may not be possible to avoid all activities, especially ADLs; therefore, instruction on how to modify these activities may minimize pain. Patients must have a good understanding that constant rest or complete immobilization are detrimental to healing musculoskeletal tissues. Orthoses or limb positioning should be used to offer intermittent rest to painful or injured tissues, but pain-free, controlled range-of-motion exercises should be performed throughout the day.
In cases of acute peripheral nociceptive pain, patients may experience some discomfort during exercise, but it should subside quickly after exercise. Patients and therapists can gauge the vigor of the therapeutic exercise program by observing tissue reactivity. Pain associated with exercise should subside within 30 minutes, edema should not increase, and there should not be a loss of motion if the vigor of exercise is appropriate. If pain, edema, and stiffness increase for a persistent time after exercise, the therapeutic exercise program should be reassessed and downgraded.
Physical agents commonly used for pain modulation are thermal agents and electrotherapeutic techniques to induce electroanalgesia.27,28 Physical agents may also be used to facilitate tissue healing or to control edema, and facilitating either of these indications may also modulate pain. These agents rarely are used in isolation. They may be used in conjunction with therapeutic exercise, orthotic intervention, and patient education.
The hand therapist should consider the physiologic rationale, biophysical properties, proposed mechanisms of pain modulation, and precautions or contraindications for each physical agent to ensure safe and effective use on the hand-injured patient. Selection of a particular modality will depend on the source of pain mediation and other contributing factors such as edema, inflammation, and muscle guarding. In addition, selection may be made through the consideration of potential contraindications or precautions with the injured hand.27,28 Chapter 117 provides additional information on the use of physical agents in hand therapy including pain modulation.
It appears that the pain modulation is short lived for both heat and cold. Despite the lack of data, many clinicians use thermal agents to enhance the patient’s ability to participate, before or after, in therapeutic exercise programs and functional activities. The application of superficial or deep heat may enhance joint movement secondary to the increase in collagen extensibility associated with heating, which may add to the patient’s perception of decreased pain.
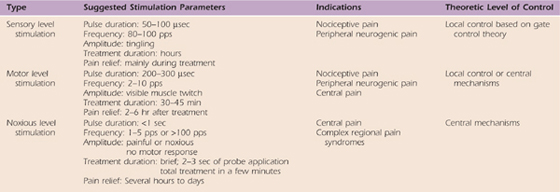
TENS refers to the use of externally applied electrical stimulation with surface electrodes.29 TENS has been commonly used to elicit pain suppression with electrotherapy since Melzack and Wall24 proposed the gate control theory in 1965. Electroanalgesia is a general term to describe the outcome of using electrical stimulation or TENS for pain modulation. Three modes of stimulation have been described to promote electroanalgesia.30 Table 114-4 reviews the common parameters used for each of the levels and the theoretical level of pain modulation. Sensory or motor level stimulation are commonly used to modulate nociceptive or peripheral neurogenic-mediated pain. Noxious level stimulation is used when other methods of pain modulation have failed and in the management of CRPS or centrally mediated pain conditions. Rizk and colleagues31 and Cannon32 suggest that electrotherapy can be integrated into a treatment plan for acute, painful conditions that promotes pain modulation to restore joint range of motion.
Table 114-4 Common Parameters of Electrical Stimulation Used for Pain Modulation
Therapists often use methods of transdermal drug delivery to resolve pain and inflammation.27,28 Iontophoresis and phonophoresis deliver analgesics and anti-inflammatory agents transdermally. These methods are typically used with the painful target tissue, e.g., tendon is readily palpable or superficial such as in a wrist or elbow tendinopathy.
In general, there have been several systematic reviews33-36 that indicate a lack of evidence to support the use of physical agents for pain modulation for any region of the body, but particularly for hand and upper extremity conditions. This author believes that there are several factors that contribute to this lack of evidence. First, it is difficult to design randomized, controlled clinical trials on patients who are referred to therapists. Withholding care to referred patients is not permitted, so therapy studies typically consist of cohort treatment studies in which all groups are treatment groups and there is no control or nontreatment group. Second, comparison of cohort studies remains difficult because investigators do not effectively communicate the treatment parameters and/or methods of application used or there are inconsistencies in the terminology. This is particularly true with studies involving electroanalgesia. Finally, the most difficult problem observed in systematic reviews and meta-analyses on the effectiveness of physical agents is that patients with various sources of pain mediation are compared together. The underlying mechanisms of acute pain are vastly different from chronic, recurrent, or neuropathic pain conditions. This variability regarding the source of pain mediation prevents comparison of the effectiveness of the physical agents used, their treatment parameters, and the methods of application. Additional information related to available evidence is provided in Chapter 117.
Neural tension intervention is particularly important when the primary source of pain mediation is peripheral neurogenic. Butler37 and Elvey38 have introduced therapists to the concept of adverse neural tension. Peripheral nervous system structures are made to move with normal limb movements; however, in the presence of inflammation, normal limb movements may be painful to injured nerves. Physical dysfunction of the nervous system can be examined through specific neural tension tests.37,38 Chapter 118 provides additional information on examination and mobilization of the peripheral nervous system.
It is important to determine the level of irritability of the neural tissues before examining these tissues. The level of irritability will also determine the appropriate intervention. Conditions of high irritability are characterized by the easy provocation of symptoms of neurogenic pain. Once provoked, the symptoms usually are sustained for long periods of time. Strategies to alleviate tension, particularly limb positioning, should be used as should pain-free movement to modulate pain. In the case of low irritability, symptoms are not provoked readily, and recovery occurs more quickly than with high irritability. Patients who demonstrate low irritability should participate in a therapy plan that includes gentle application of neural tension, often called nerve mobilization or gliding.
As pain symptoms are modulated, a graded therapeutic exercise program should be initiated to improve general body conditioning for all patients regardless of the source of pain mediation. Deconditioning is likely to be present in patients who have had a dramatic change in their activity level, especially injured workers.39 Aerobic exercise such as a walking program or cycling will improve the patient’s endurance for functional activities. In the clinic, a treadmill or stationary bike may be used. As tolerated by the injured tissues in the hand and upper limb, general upper extremity strengthening exercises, including scapula stabilization, will enhance muscular endurance. This will improve the patient’s tolerance of functional activity. The specificity of strengthening will depend on the injury, source of pain mediation, and impairments determined from physical examination. Additional information on pain modulation with exercise is described elsewhere.40
Initially, posture retraining may focus on determining a position of comfort (i.e., a position that results in tolerable or minimized pain). Therapists may need to instruct patients in appropriate positions for sleeping, driving, or working. Patients who report sleep difficulty secondary to pain will benefit from education about sleeping postures. Extra pillows for upper limb support in the supine or side-lying position may improve the patient’s ability to sleep. If the patient can improve his or her hours of sleep, pain tolerance may improve because the inability to sleep is one of the variables that affects pain tolerance. A patient’s workstation should be evaluated to determine whether any positions or activities may exacerbate his or her pain. Modifications should be made if awkward postures or limb movements are evident. This is particularly true in patients with sedentary jobs, such as those who primarily use the computer for their work activities.
Posture assessment may reveal common postural faults in patients with upper quarter pain, such as forward head and rounded shoulders. Imbalances between muscle groups may exist as well. Flexibility exercises should be given to stretch tight muscles and strengthening exercises should be performed to strengthen weak muscles.41 Exercises to promote trunk and scapular stability (see Chapter 93) will reduce postural faults and enhance functional performance of the upper limbs. The exercises will need to be integrated to match the patient’s source of pain mediation and tolerance for exercise. The exercises should not aggravate the patient’s pain.
Specific global tactics may be used to promote musculoskeletal tissue healing as well as decrease pain (Fig. 114-7). These lifestyle changes may be beneficial for all sources of pain mediation and tissue healing. The nervous system is highly vascularized and metabolically demanding; therefore, nerves depend on good blood flow to maintain function and health.10 The global tactics focus on lifestyle changes that promote increased blood flow, increased oxygen, and stress-free use of the hand and upper extremity.
In addition to promoting a training effect on the heart, aerobic exercise improves peripheral circulation, which enhances blood flow to the peripheral musculoskeletal tissues and nerves.42 Aerobic exercise training in mice has demonstrated increased collagen production in healing wounds.43 Symptoms associated with carpal tunnel syndrome were reduced in patients who participated in an aerobic exercise program.44 Moderate exercise such as walking and yoga may enhance immune function, decrease inflammation, and stress. Although the full benefits of exercise specific to hand and upper extremity conditions is unknown, patients should be encouraged to participate in a fitness program when it is medically safe for the hand or upper extremity injury. It is recommended that the patient consult with his or her primary care physician before initiating a program on their own.
Conversely, smoking45-48 reduces blood flow, reduces oxygen transport, and decreases collagen production in injured tissues, especially bone,49,50 which leads to delayed healing and other complications. Nicotine decreases vascularization at the site of fracture, causing vasoconstriction and reducing oxygen transport. Nicotine and other toxins in cigarette smoke decrease immune responses by reducing leukocyte activity, which increases the risk of infection.51 There is an increased prevalence of musculoskeletal injuries,52 carpal tunnel syndrome,53-55 and cubital tunnel syndrome56 noted in patients who smoke; therefore, patients should be encouraged to stop using tobacco products including cigarettes and nicotine patches. During the rehabilitation process, patients should also be instructed to eliminate or minimize caffeine products in their diet, including coffee, cola, tea, and chocolate, because caffeine is a known vasoconstrictor just like nicotine. Today, many surgeons educate or require their patients to reduce or cease tobacco and/or caffeine consumption before surgery, even in acute trauma management.
Improving blood flow through exercise and minimizing nicotine and caffeine use are methods to increase oxygenation to injured tissues, but deep-breathing exercises will also increase oxygen intake. Patients should be encouraged to take intentional deep breaths throughout their day. Patients should also be educated to increase their water consumption because drinking water improves tissue hydration and microcirculation.57,58 Oxygen saturation rates in peripheral tissues are affected by tissue hydration. The more hydrated the tissue, the greater the oxygenation to the tissue. This is the primary reason that nerves are highly vascularized. They need high levels of oxygen to meet the metabolic requirements of the nerve tissue.
The last component of the lifestyle changes is to promote stress-free use of the hand and upper limbs. Patients should be instructed to avoid static postures and awkward or painful limb movements. Ergonomic counseling, joint protection principles, and energy conservation strategies should be reviewed with the patient.
Intuitively, these lifestyle changes may be beneficial for all sources of pain mediation, but prospective research studies will help therapists determine whether these changes have an effect on the rehabilitation outcome. These lifestyle changes should also be incorporated into prevention programs for work-related musculoskeletal conditions. Again, future research to evaluate outcome is essential.
Promoting patient responsibility is particularly important for patients with longstanding pain and or affective pain mediation. Patients should be educated, empowered, and encouraged to be active participants in their rehabilitation process. The therapist should help patients identify problems, and the patients should assist in the problem solving. To help promote responsibility, patients may be asked to keep daily journals or pain-activity logs. Comprehensive written home programs will also promote compliance and responsibility. The analogy of teacher and student is appropriate. The therapist teaches the patient what to do, and the patient carries out the plan just as a student completes homework assignments. Therapy visits are used to monitor progress and to assess compliance.
The therapist must consider the source of pain mediation, results of pain assessment, and the proposed mechanism of pain modulation when selecting an intervention and establishing a plan of care. The clinician should form a hypothesis regarding the nature of the pain, the related symptoms, and the expected outcome of treatment. The therapist also should determine how the effectiveness of treatment will be measured, which will establish the tests and measures used to assess pain during the initial examination and follow-up examination. This format should maximize the outcomes of the rehabilitation process for most patients with the chief symptom of pain.
1. Mersky H, Bogduk N, eds. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. 2nd ed Seattle: International Association for the Study of Pain (IASP); 1994.
2. Joint Commission on Accreditation of Healthcare Organizations (JCAHO). New standard for pain assessment and management. PT Magazine. 2001;9:101.
3. American Physical Therapy Association. Guide to physical therapy practice. 2nd ed Phys Ther. 2001;81:9–744.
4. Law LF, George SZ. Individual differences and pain variability. In: Sluka KA, ed. Mechanisms and Management of Pain for the Physical Therapist. Seattle: IASP Press; 2009.
5. Nolan MF. Contemporary perspectives on pain and discomfort. Phys Ther Prac. 1993;2:14.
6. Fields HL. Pain. New York: McGraw-Hill; 1987.
7. Gifford LS, Butler DS. The integration of pain sciences into clinical practice. J Hand Ther. 1997;10:86.
8. Bogduk N. Innervation and pain patterns of the cervical spine. In: Grant R, ed. Physical Therapy of the Cervical and Thoracic Spine. 2nd ed New York: Churchill Livingstone; 1994:65–76.
9. Curatolo M, Arendt-Nielsen L, Petersen-Felix S. Central hypersensitivity in chronic pain: mechanisms and clinical implications. Phys Med Rehabil Clin N Am. 2006;17:287–302.
10. Weisburg J. Pain. In: Heacox B, Andemicael-Mehreteab T, Weisberg J, eds. Physical Agents: A Comprehensive Text for Physical Therapists. Norwalk, Conn: Appleton & Lange; 1994.
11. Basbayn AU, Jessell TM. The perception of pain. In: Kandel ER, Schwartz JH, Jessell TM, eds. Principles of Neural Science. 4th ed New York: Elsevier; 2000.
12. Goodman CC, Snyder TEK. Pain types and viscerogenic pain patterns. In: Goodman CC, Snyder TEK, eds. Differential Diagnosis for Physical Therapists: Screening for Referral. 4th ed St. Louis: Saunders Elsevier; 2007:110–112.
13. Echternach JL. Clinical evaluation of pain. Phys Ther Prac. 1993;2:14.
14. Scudds RA. Pain outcome measures. J Hand Ther. 2001;14:86.
15. Brazier JE, Harper R, Jones NMB, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. Br Med J. 1992;305:160–164.
16. Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (Disabilities of the Arm, Shoulder, and Hand). Am J Ind Med. 1996;29:602–608.
17. Levine DW, Simmons BP, Koris MJ, et al. A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Joint Surg Am. 1993;75:1585–1592.
18. Chung KC, Pillsbury MS, Walters MR, Hayward RA. Reliability and validity testing of the Michigan Outcomes Questionnaire. J Hand Surg. 1998;23A:575–587.
19. Amadio PC. Outcome assessment in hand surgery and hand therapy: an update. J Hand Ther. 2001;14:63.
20. Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1:265.
21. Fischer AA. Pressure threshold meter: its use for quantification of tender spots. Arch Phys Med Rehabil. 1986;67:836.
22. Fischer AA. Pressure algometry over normal muscles: standard values, validity, and reproducibility of pressure threshold. Pain. 1987;30:115.
23. Nirschl RP. Elbow tendinosis/tennis elbow. Clin Sports Med. 1992;11:851.
24. Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971.
25. Cailliet R. Neuroanatomy of pain mechanisms. In: Cailliet R, ed. Pain: Mechanisms and Management. Philadelphia: FA Davis; 1993:1–28.
26. Gersh MR. Transcutaneous electrical nerve stimulation (TENS) for management of pain and sensory pathology. In: Gersh MR, ed. Electrotherapy in Rehabilitation. Philadelphia: FA Davis; 1992.
27. Fedorczyk J. The role of physical agents in modulating pain. J Hand Ther. 1997;10:110.
28. Fedorczyk JM, Michlovitz SL. Pain and limited motion. In: Michlovitz SL, Nolan TP, eds. Modalities for Therapeutic Intervention. 4th ed Philadelphia: FA Davis; 2005:185–206.
29. Mannheimer JS, Lampe GN. Clinical Transcutaneous Electrical Nerve Stimulation. Philadelphia: FA Davis; 1984.
30. Section on Clinical Electrophysiology, American Physical Therapy Association. Electrotherapeutic Terminology in Physical Therapy. Alexandria, VA: APTA Publications; 1990.
31. Rizk TE, Christopher RP, Pinals RS, et al. Adhesive capsulitis (frozen shoulder): a new approach to its management. Arch Phys Med. 1983;64:29–33.
32. Cannon NM. Enhancing flexor tendon glide through tenolysis and hand therapy. J Hand Ther. 1989;3:122.
33. van der Windt D, van der Heijden G, van den Berg S, et al. Ultrasound therapy for musculoskeletal disorders: a systematic review. Pain. 1999;81:257–271.
34. Meyler WJ, deJongste MJ, Rolf CA. Clinical evaluation of pain treatment with electrostimulation: A study of TENS in patients with different pain syndromes. Clin J Pain. 1994;10:22–27.
35. Carroll D, Moore RA, McQuay HJ, et al. Transcutaneous electrical nerve stimulation (TENS) for chronic pain. Cochrane Database Syst Rev. 2004;2.
36. Gam AN, Johannsen F. Ultrasound therapy in musculoskeletal disorders: A meta-analysis. Pain. 1995;63:85–91.
37. Butler DS. The Sensitive Nervous System. Adelaide, Australia: Neuroorthopedic Institute; 2000.
38. Elvey RL. Physical evaluation of the peripheral nervous system in disorders of pain and dysfunction. J Hand Ther. 1997;10:122.
39. Neufer PD. The effect of detraining and reduced training on the physiological adaptations to aerobic exercise training. Sports Med. 1989;8:302.
40. Bement MH. Exercise-induced hypoalgesia: an evidence-based review. In: Sluka KA, ed. Mechanisms and Management of Pain for the Physical Therapist. Seattle: IASP Press; 2009.
41. Novak CB, Mackinnon SE. Repetitive use and static postures: a source of compression and pain. J Hand Ther. 1997;10:151.
42. McArdle WD, Katch FI, Katch VL. Essentials of Exercise Physiology. 2nd ed Philadelphia: Lippincott Williams & Wilkins; 2000.
43. Pynn M, Schafer K, Konstantinides S, et al. Exercise training reduces neointimal growth and stabilizes vascular lesions developing after injury in apolipoprotein e-deficient mice. Circulation. 2004;109:386–392.
44. Nathan PA, Wilcox A, Emerick PS, et al. Effects of an aerobic exercise program on median nerve conduction and symptoms associated with carpal tunnel syndrome. J Occup Environ Med. 2001;43:840–843.
45. Silverstein P. Smoking and wound healing. Am J Med. 1992;93:22S.
46. Trap-Jensen J. Effects of smoking on the heart and peripheral circulation. Am Heart J. 1988;115:263.
47. Zhu BQ, Parmley WW. Hemodynamic and vascular effects of active and passive smoking. Am Heart J. 1995;130:1270.
48. Warner DO. Preoperative smoking cessation, how long is long enough? Anesthesiology. 2005;102:883–884.
49. Castillo RC, Bosse MJ, MacKenzie EJ, et al. Impact of smoking on fracture healing and risk of complications in limb-threatening open tibia fractures. J Orthop Trauma. 2005;19:151–157.
50. Allen G. Cigarette smoking; smoking cessation; reuse of endoscope accessories; conscious sedation. Assoc Perioperative Regist Nurses J. 2005;81:425–428.
51. Kiecolt-Glaser JK, Page GG, Marucha PT, et al. Psychological influences on surgical recovery: perspectives from psychoneuroimmunology. Am Psychol. 1998;53:1209–1218.
52. Porter SE, Hanley EN. The musculoskeletal effects of smoking. J Am Acad Orthop Surg. 2001;9:9.
53. Nathan PA, Meadows KD, Istvan JA. Predictors of carpal tunnel syndrome: an 11-year study of industrial workers. J Hand Surg (Am). 2002;27:644–651.
54. Karpitskaya Y, Novak C, Mackinnon SE. Prevalence of smoking, obesity, diabetes mellitus, and thyroid disease in patients with carpal tunnel syndrome. Ann Plastic Surg. 2002;48:269–273.
55. Tanaka S, Wild DK, Cameron LL. Association of occupational and non-occupational risk factors with the prevalence of self-reported carpal tunnel syndrome in a national survey of the working population. Am J Ind Med. 1997;32:550–556.
56. Richardson JK, Jamieson SC. Cigarette smoking and ulnar mononeuropathy at the elbow. Am J Phys Med Rehabil. 2004;83:730–734.
57. Arkiliç CF, Taguchi A, Sharma N, et al. Supplemental perioperative fluid administration increases tissue oxygen pressure. Surgery. 2003;133:49–55.
58. Wipke-Tevis DD, Williams DA. Effect of oral hydration on skin microcirculation in healthy young and midlife and older adults. Wound Repair Regen. 2007;15:174–185.