General Anatomy of the Spinal Cord
The purpose of this chapter is to describe in detail the gross anatomy of the external spinal cord, its coverings, and its vasculature. To help the reader acquire a general appreciation of the spinal cord as a complete entity, this chapter also provides a cursory description of the organization and physiology of the internal aspect of the spinal cord. Subsequent chapters expand on the generalities presented here. The intimate relationship of the spinal cord to the vertebral column makes the anatomy of the spinal cord extremely important to those who treat disorders of the spine.
Overview of Spinal Cord Organization
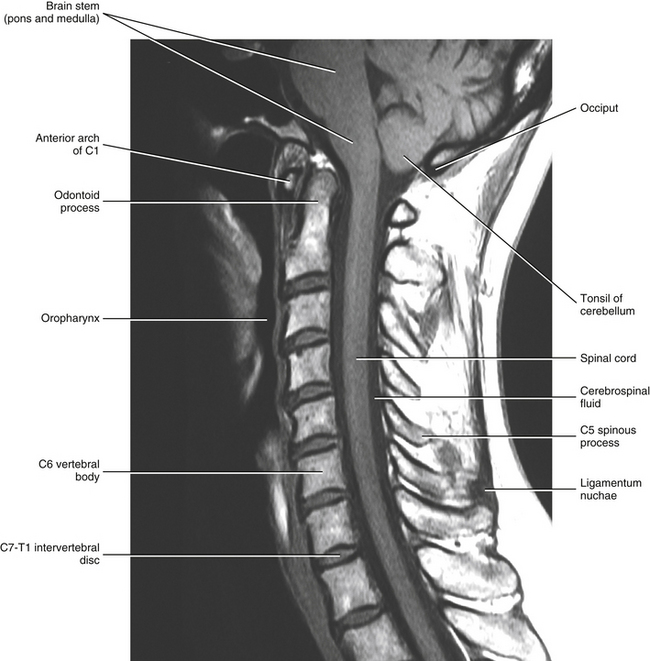
The spinal cord, which is located in the vertebral (spinal) canal, is continuous cranially with the medulla oblongata of the brain stem and terminates caudally with a tapered inferior end. The cord is well protected by the vertebrae and the ligaments associated with the vertebrae. In addition to these bones and ligaments, cerebrospinal fluid (CSF) and a group of membranes, collectively called the meninges, also provide protection. The spinal cord does not lie immediately adjacent to the bone and ligaments but is separated from them by fluid, meninges, fat, and a venous plexus (Fig. 3-1, A and B).
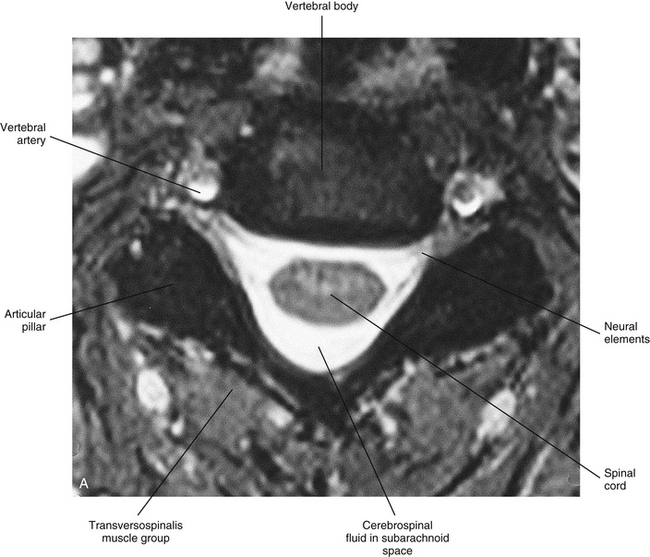
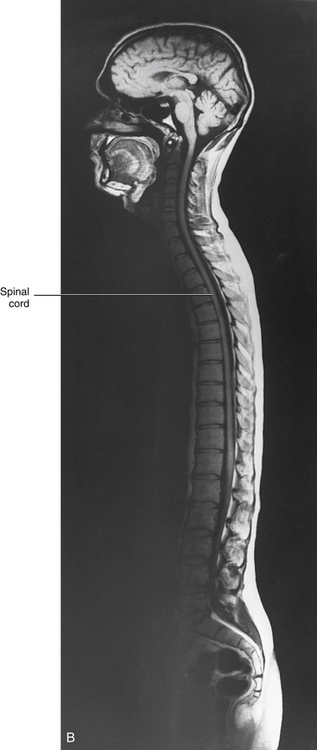
FIG. 3-1 A, Magnetic resonance image (MRI) showing a horizontal section of the cervical spinal cord within the vertebral canal. B, Midsagittal MRI demonstrating the entire spinal cord within the vertebral canal. (B, From Nolte J. [2002]. The human brain: an introduction to its functional anatomy [5th ed.]. St Louis: Mosby.)
The spinal cord and brain develop from the same embryologic structure, the neural tube, and together they form the central nervous system (CNS). One end of the neural tube becomes encased in the skull, whereas the remainder of the neural tube becomes encased in the vertebral column. Size alone suggests that the higher centers constituting the brain process information more thoroughly and more intricately than the processing that occurs in the spinal cord. For the CNS to respond to the environment, it requires input from structures peripheral to the CNS and, in turn, a means to send output to structures called effectors. The sensory input begins in peripheral receptors found throughout the entire body in skin, muscles, tendons, joints, and viscera. These receptors send electrical currents (action potentials) toward the spinal cord of the CNS via the nerves that compose the peripheral nervous system (PNS). The sensory receptors respond to general sensory information such as pain, temperature, touch (including the submodalities of vibration and pressure), or proprioception (awareness of body position and movement). The PNS also is used when the CNS sends output to the body’s effectors, which are the smooth muscle, cardiac muscle, skeletal muscle, and glands of the body. Thus the PNS consists of the body’s peripheral nerves and is the means by which the CNS communicates with its surrounding environment.
Within a peripheral nerve, the individual fibers are classified as part of either the somatic division of the nervous system or the visceral division of the nervous system. Somatic fibers (both sensory/afferent and motor/efferent) innervate skin, joints, tendons, and skeletal muscle located in the extremities and body wall. Visceral (or autonomic) fibers carry sensory/afferent information from the viscera to the CNS and provide motor/efferent control of smooth muscle, cardiac muscle, and glands. Twelve pairs of these peripheral nerves are associated with the brain (10 of which are attached to the brain stem) and innervate structures primarily in the head. These nerves, called cranial nerves, also convey special sense information such as hearing, vision, smell, and taste. In the context of this chapter, another group of peripheral nerves is more pertinent. These 31 pairs of nerves attach to the spinal cord; communicate with structures primarily located in the neck, trunk, and extremities; and are called the spinal nerves. In general, once input reaches the spinal cord via the spinal nerves, spinal cord neurons integrate and modulate the information and then send information back to the periphery as a motor response (i.e., reflexive, postural, or voluntary). In addition, such input to the spinal cord may result in the transmission of sensory input to higher brain centers for further processing. The higher centers then may transmit information down to the spinal cord neurons, which in turn relay it to the periphery, again via spinal nerves (Fig. 3-2).
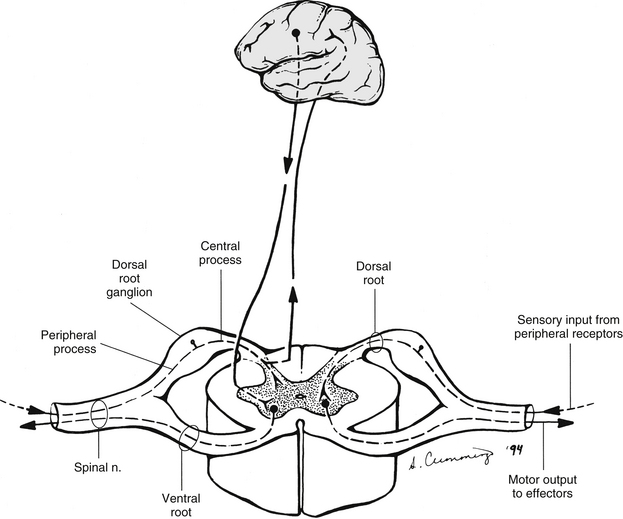
FIG. 3-2 General functions of the spinal cord. Right, Two-way communication between the spinal cord and the periphery. Left, Information within the cord traveling to and from higher centers in the brain.
A spinal nerve is formed by the merger of two roots within the intervertebral foramen (IVF). One root, called the dorsal root, contains fibers that convey sensory information. The cell bodies of these sensory fibers are located in a reddish oval strucure called the dorsal root ganglion. The cell bodies are not found in the spinal cord because they developed from the neural crest (see Chapter 12). Each sensory neuron of the PNS is pseudounipolar because two processes diverge from one common stem (see Fig. 3-2). One of the processes is called the peripheral process and is continuous with a peripheral receptor. The other process is the central process, which courses in the dorsal root and enters the spinal cord (CNS). The dorsal root contains fibers of various diameters and conduction velocities that convey all types of sensory information. For example, some fibers in the dorsal root convey cutaneous sensory information from a specific area of skin called a dermatome. As the dorsal root approaches the spinal cord within the vertebral canal, it divides into approximately six to eight dorsal rootlets, or filaments. These rootlets attach in a vertical row to the cord’s dorsolateral sulcus. The other root, which helps form a spinal nerve, is called the ventral root and contains fibers that convey motor information to the body’s effectors, that is, all muscle tissue and glands. The cell bodies of these axons are located in the spinal cord. The axons emerge from the cord’s ventrolateral sulcus as ventral rootlets and unite to form one ventral root. Near the point of attachment of a dorsal or ventral rootlet to the spinal cord is the transition between the CNS and PNS. This CNS-PNS transition zone is a segment of the rootlet containing both CNS and PNS tissue. Each fiber coursing in a dorsal and ventral rootlet will cross a transition zone (TZ). Microscopically, the TZ consists of a peripheral PNS component overlying an axial CNS component. The TZ has a dome-shaped apex with a convex surface projecting in a distal direction. CNS fibers comprise the center of the dome, which is surrounded by an outer layer of astrocytes. The astrocytic processes project into the endoneurium of the peripheral nerve and intertwine with Schwann cells. Axons of the root fibers must pass through the network of astrocytic processes (Standring et al., 2008). Within the IVF the dorsal and ventral roots form the spinal nerve, which subsequently divides into its two major components: the dorsal and ventral rami (also known as the posterior primary division and anterior primary division, respectively) (Fig. 3-3).

FIG. 3-3 Components and somatic branches of a typical spinal nerve. The dorsal and ventral roots unite within the intervertebral foramen to form a spinal nerve. The spinal nerve branches into a dorsal ramus and a ventral ramus. Each ramus contains motor (red) and sensory (blue) fibers. The sensory neurons are pseudounipolar. Note: Sympathetic fibers are not shown.
From this description, it can be seen that the spinal nerve and nerves formed distal to it (including the dorsal ramus and the ventral ramus) are mixed because they contain fibers conveying sensory input and fibers conveying motor output. However, proximal to the IVF, sensory and motor information is segregated in the fibers forming separate dorsal and ventral roots, respectively. This segregation of dorsal and ventral root fibers, and therefore of root function, was discussed and demonstrated by Bell and Magendie in the early 1800s and later was called the law of separation of function of spinal roots (law of Bell and Magendie) (Coggeshall, 1980). However, data do exist suggesting that the ventral roots of animals and humans contain some unmyelinated afferent fibers. It appears that the fibers (peripheral processes), the cell bodies of which are located in the dorsal root ganglion, travel into the ventral root and then loop back out and course toward the periphery (Coggeshall, Coulter, & Willis, 1973; Risling & Hildebrand, 1982; Risling et al., 1984; Azerad et al., 1986; Ko et al., 2009).
At the level of the skull’s foramen magnum, the spinal cord becomes continuous with the medulla oblongata of the brain stem (Fig. 3-4). Although in cross section it is impossible to delineate the exact beginning of the cord and the end of the brain stem, grossly the beginning of the cord is easily distinguished by the definitive presence of the skull and vertebrae. As discussed in Chapter 12, a period during development occurs when the spinal cord extends the length of the vertebral column. However, while the vertebral column continues to develop in length, the spinal cord lags behind so that it eventually occupies the upper two thirds of the vertebral (spinal) canal (Fig. 3-1, B). It has been reported that at birth the cord ends at approximately the level of the L3 vertebra. Malas and colleagues (2000) used ultrasonography on full-term neonates (40 ± 2 weeks) and found the termination level of the cord to range anywhere from the L1-2 disc space to the L2-3 disc space. The spinal cord terminated at the L2-3 disc space in 50% (7 out of 14) of the neonates. In adults, because of continued greater growth of the vertebral column, the spinal cord ends approximately at the level of the L1 vertebra. In some individuals, however, the spinal cord may end as high as the disc between the T11 and T12 vertebrae or as low as the L3 vertebra (see Meninges and Table 3-1 for further description). At about the level of the caudal part of the T12 vertebral body, the cord tapers to a cone, which is known as the conus medullaris (Fig. 3-5, B). The overall length of the spinal cord to the inferior tip of the conus medullaris is approximately 42 cm in an average-sized woman and 45 cm in an average-sized man. The spinal cord’s weight is approximately 30 to 35 g. Remember that in most individuals the spinal cord does not extend inferior to the L2 vertebra. Therefore a lesion such as a herniated disc or trauma occurring below the L2 vertebra does not directly affect the spinal cord in most individuals.
Table 3-1
Termination Level of the Conus Medullaris as Determined by Magnetic Resonance Imaging Scans
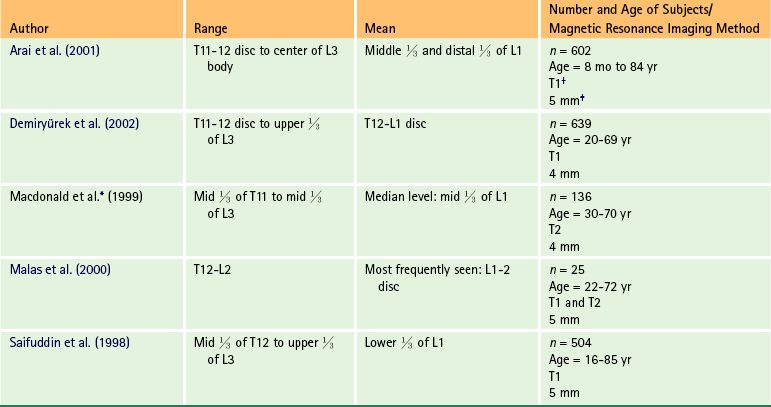
∗Macdonald and colleagues (1999) did not use the disc spaces as a possible point of termination. The intervertebral disc (IVD) was considered to be a part of the vertebral level above or below based on whether the conus ended at the upper or lower half of the disc (e.g., conus ending at upper part of the L1-2 IVD considered to end at L1 level).
†T1 and T2, T1- and T2-weighted images, respectively.
‡Slice thickness in millimeters.
External Morphology
The external surface of the spinal cord is not a smooth surface but instead shows grooves of various depths called sulci and fissures. (When discussing cord anatomy, understand that the terms dorsal and ventral can be used interchangeably with posterior and anterior, respectively.) The spinal cord’s dorsal surface includes a midline dorsal median sulcus, right and left dorsal intermediate sulci (located from the midthoracic cord region superiorly), and right and left dorsolateral sulci. The cord’s ventral surface includes a midline ventral median fissure (approximately 3 mm deep) and right and left ventrolateral sulci. When inspecting the cord’s external surface, the dorsal and ventral rootlets are readily apparent, and the outward attachment to the cord of the paired (left and right) dorsal rootlets and paired ventral rootlets that serve one pair of spinal nerves defines one spinal cord segment (Fig. 3-6; see also Fig. 3-11, C).
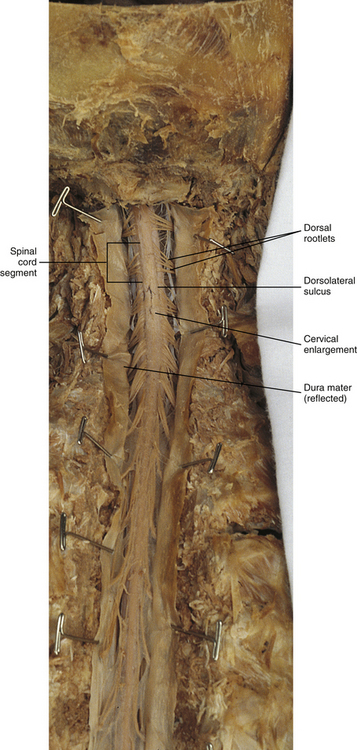
FIG. 3-6 Dorsal view of the spinal cord. The cord segments are delineated by the attachment of rootlets to the spinal cord.
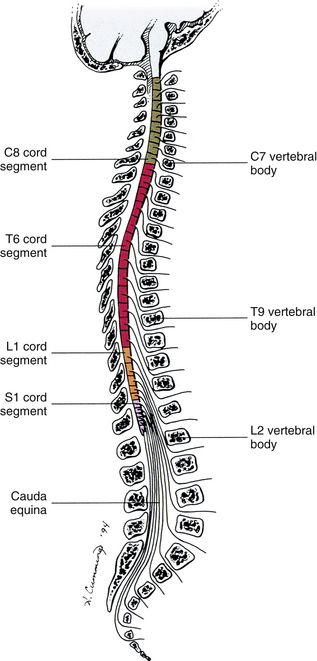
Therefore 1 pair of spinal nerves is associated with 1 cord segment; because 31 pairs of spinal nerves exist, there are also 31 spinal cord segments. These cord segments are numbered similarly to the numbering of the spinal nerves: 8 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 1 coccygeal cord segment. (Note that the first seven cervical nerves exit the IVF above their corresponding vertebra, and the remaining nerves exit below their corresponding vertebra. This allows one more cervical spinal nerve than cervical vertebrae.) Therefore the coccygeal segment is found at the very tip of the conus medullaris, which, as mentioned, is usually at the approximate level of the L1 vertebra (Fig. 3-7). This means that cord segments are not necessarily located at the same level as their corresponding vertebrae (Fig. 3-8). The relationship between cord segments and vertebral levels is always an approximation because cord length may vary among individuals.
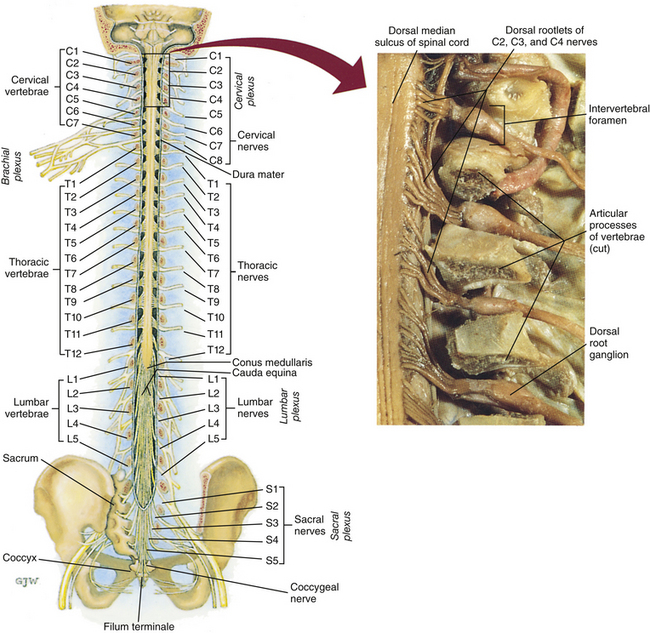
FIG. 3-7 Dorsal view of the external morphology of the spinal cord within the vertebral canal and the spinal nerves exiting within the intervertebral foramina. The tip of the cord (conus medullaris) is located approximately at the level of the L1 vertebra in this figure (see Table 3-1). The roots of the lumbar and sacral cord segments form the cauda equina, which is found in the lumbar cistern. The inset shows the cervical region, the dorsal rootlets, and the location of the dorsal root ganglia in the exposed intervertebral foramina. (From Thibodeau GA & Patton KT. [2003]. Anatomy & physiology [5th ed.]. St Louis: Mosby.)
The spinous processes (SPs) of vertebrae serve as landmarks for identifying the approximate levels of cord segments; but in doing so the relationship of the vertebral body to the SP must be remembered. Therefore, in general, cervical SPs correspond to the succeeding cord segment, upper thoracic SPs correspond to cord segments two levels below, and lower thoracic SPs correspond to cord segments three levels below (Standring et al., 2008). For example, the C5 SP is at the level of the C6 cord segment; the T3 SP is at the level of the T5 cord segment; and the T10 SP is at the level of the L1 cord segment. The L1 through coccygeal cord segments, which include the segments responsible for the innervation of the lower extremities (i.e., L1-S3), are found at approximately the levels of the T10-L1 vertebral SPs. This anatomic relationship of cord segment to vertebra is important to remember for clinical reasons. For example, a patient with a fractured L1 vertebra does not experience the same lower extremity lesion signs and symptoms as a patient with a fractured T10 vertebra, because a T10 fracture typically injures upper lumbar segments and an L1 fracture injures the lower sacral and coccygeal segments. Although the spinal cord ends approximately at the L1 vertebra, each root that corresponds to a cord segment forms a spinal nerve and exits at its corresponding IVF. This includes the IVFs below the L2 vertebra. Therefore the rootlets and roots of the more inferior cord segments are longer and descend to their respective IVFs at a more oblique angle than the rootlets and roots of cervical segments, which are shorter and located almost at right angles to the spinal cord. The lumbosacral roots, therefore, become the longest and most oblique. The collection of these elongated lumbosacral roots coursing inferiorly to their corresponding IVFs is called the cauda equina (see Figs. 3-7, 3-8, and 3-11, B) because of its resemblance to a horse’s tail (the literal English translation of the term in Latin).
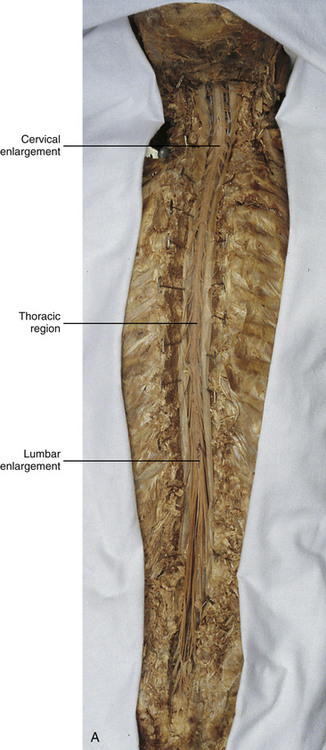
In addition to the sulci and fissures, another anatomic characteristic seen on gross inspection of the spinal cord is the presence of two enlarged areas. One area is the cervical enlargement seen in cord segments C3 to T2. These cord segments include the segments that are responsible for the input from and output to the upper extremities. The other cord enlargement is the lumbar enlargement, which is visible from segments L1 to S3. These segments are responsible for the input from and output to the lower extremities. Because many more structures must be innervated in the extremities than the trunk, it is necessary to have more neuron cell bodies in the cord, and thus these two regions are enlarged (Standring et al., 2008) (Fig. 3-9; see also Figs. 3-5, A, and 3-6).
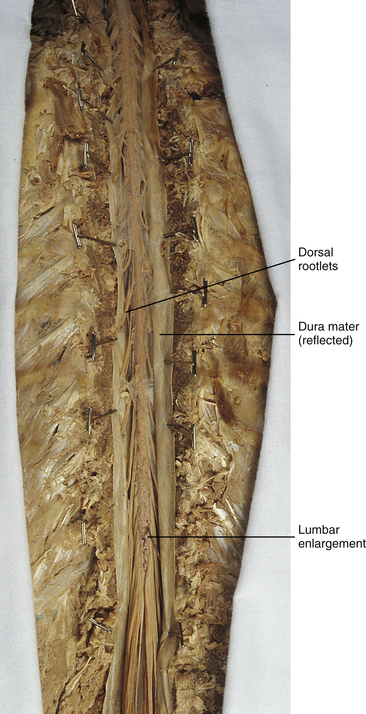
FIG. 3-9 Dorsal view of the spinal cord showing the lumbar enlargement. Cervical enlargement is shown in Figures 3-5, A and Figure 3-6.
Meninges
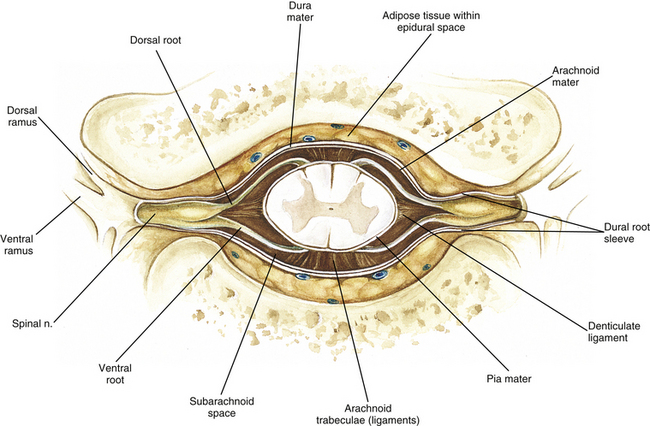
Surrounding and providing protection and support to the spinal cord is a group of three membranes that are collectively called the meninges. The meninges surrounding the spinal cord are a continuation of the meninges surrounding the brain and consist of the dura mater (pachymeninx) and the leptomeninges, the latter being composed of the arachnoid mater and pia mater (Figs. 3-10, 3-11, and 3-12). During development, mesenchyme surrounding the neural tube thickens to form the primordial meninx. The outermost layer thickens and becomes the dura mater. The thin, innermost layer becomes infiltrated with neural crest cells and forms the leptomeninges. CSF fills spaces that coalesce (the future subarachnoid space) within, and subsequently separate the leptomeninges into two layers—the arachnoid mater and the pia mater (Moore & Persaud, 1998) (see the following text). As they separate, tiny strands remain and can still be identified in the adult subarachnoid space (Figs. 3-10 and 3-12). These strands are sometimes called arachnoid trabeculae or ligaments and are composed of a collagenous core surrounded by leptomeningeal cells (Standring et al., 2008). They may become concentrated and form septa in the vertebral subarachnoid space.
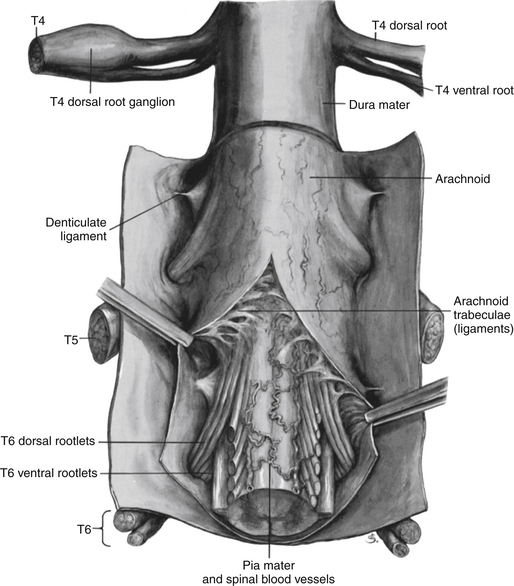
FIG. 3-10 The meninges surrounding the spinal cord. Notice the denticulate ligament attaching to the dura mater and anchoring the spinal cord. (From Mettler FA. [1948]. Neuroanatomy [2nd ed.]. St Louis: Mosby.)
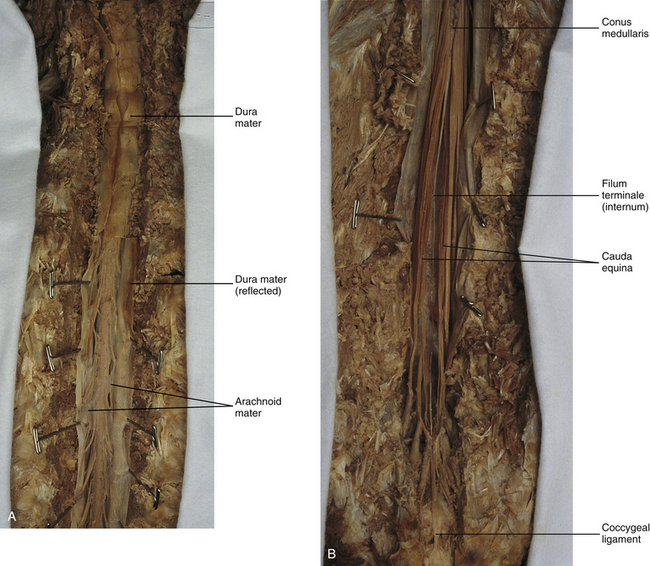
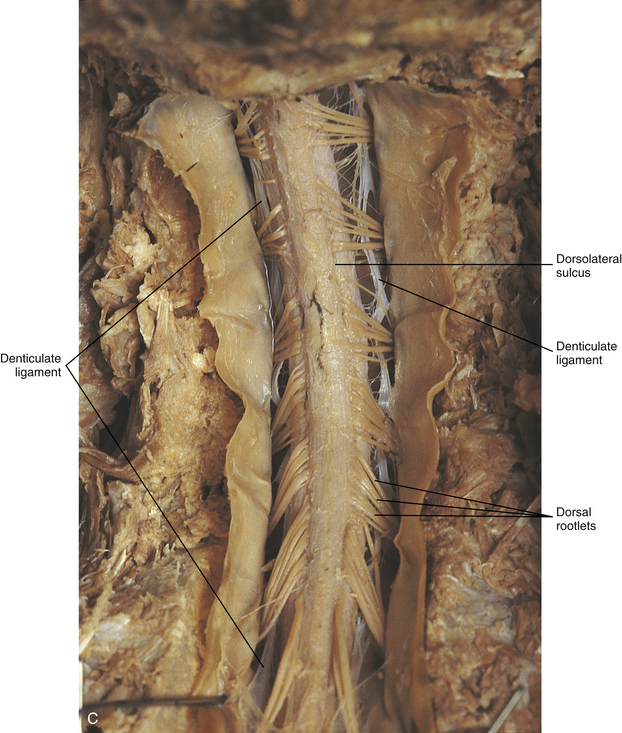
FIG. 3-11 Dorsal view of the spinal cord showing the meninges. A, Cervical and thoracic regions. B, Lumbar cistern and its contents. C, Cervical cord segments.
The fully developed dura mater (pachymeninx) of the cord is the tough, outermost membrane and is a continuation of the inner layer or meningeal layer of dura mater surrounding the brain. It is separated from the vertebrae by the epidural space, which contains epidural fat, loose connective tissue, an extensive internal vertebral venous plexus, and fibrous bands called meningovertebral ligaments (see Chapter 2). The spinal dura mater attaches to the edge of the foramen magnum, the posterior aspect of the C2-3 vertebral bodies, and the posterior longitudinal ligaments (Standring et al., 2008) by fibrous bands of tissue. This elongated dural sac is held in place to the borders of the vertebral canal by the filum terminale externum and many other thickenings of connective tissue that vary in type in different regions of the vertebral canal (Barbaix et al., 1996; Fricke, Andres, & Von During, 2001; Dean & Mitchell, 2002; Humphreys et al., 2003). These connective tissue attachments to the dura mater are discussed in detail in Chapters 5 and 7.
The recurrent meningeal nerve (or sinuvertebral nerve of von Luschka), which is formed outside the IVF and reenters the vertebral canal, provides significant innervation to the anterior aspect of the spinal dura mater. Although a few nerves sparsely innervate the posterolateral dura mater, the posteromedial region appears to have no innervation, which may explain why a patient feels no pain when the dura mater is pierced during a lumbar puncture (Groen, Baljet, & Drukker, 1988).
The dura mater has been analyzed microscopically and has been found to be composed of outer and inner parts. The outer portion contains layers of fibroblasts, collagen fiber bundles (providing tensile strength and protection), and some elastic fibers. The latter afford flexibility for mechanical changes during movements and postural adjustments. The fibers are not arranged in a longitudinal or parallel fashion but are oriented in a variety of directions (Haines, Harkey, & Al-Mefty, 1993; Vandenabeele, Creemers, & Lambrichts, 1996; Reina et al., 1997, 1998; Fricke, Andres, & Von During, 2001).
The inner portion of the dura mater lies adjacent to the arachnoid mater and consists of layers of cells called dural border, subdural, or neurothelial cells. These flattened cells are described as being sinuous with interdigitating processes that create extracellular spaces and few intercellular junctions (Haines, Harkey, & Al-Mefty, 1993; Vandenabeele, Creemers, & Lambrichts, 1996; Reina et al., 1998; Fricke, Andres, & Von During, 2001; Reina et al., 2002).
Deep to the dura mater is the arachnoid mater, which is a vascular, delicate, and loosely arranged membrane (see Figs. 3-10 and 3-11, A). The outer portion consists of layers of flattened cells called arachnoid barrier cells. This multilayered structure is impermeable to CSF because it forms an anatomic and functional barrier between the dural blood supply and CSF in the subarachnoid space. Underneath the barrier cells is an extensive intermediate layer that is attached to the overlying arachnoid layer. This intermediate layer forms sheets that are highly perforated and have a lacelike appearance. Ultrastructurally, the intermediate layer is seen to consist of a core of collagen coated by leptomeningeal cells. This is similar to the structure of trabeculae in the cranial subarachnoid space. These sheets, which are most evident on the dorsal aspect, lie over the surface of the spinal cord. They laterally envelope spinal nerve roots and arteries and gradually disappear. This layer also develops into compact regions that form ligaments (also called trabeculae). Dorsal ligaments form a discontinuous series of connections from the outer arachnoid layer to the spinal cord, and dorsolateral ligaments extend from the outer layer to the dorsal roots. Although the ventral intermediate layer is less extensive, it forms ventral ligaments that have a similar arrangement to the dorsal ligaments. The intermediate layer may function as a baffle to help regulate CSF flow within the subarachnoid space. It may also be the site of considerable fibrosis from inflammation in the spinal subarachnoid space, leading to the complications of chronic arachnoiditis (Weller, 2005; Standring et al., 2008).
Numerous studies (Haines, Harkey, & Al-Mefty, 1993; Vandenabeele, Creemers, & Lambrichts, 1996; Reina et al., 1998; Nolte, 2002; Reina et al., 2002) suggest that there is continuity between the inner surface of the dura mater and the arachnoid barrier cell layer such that there is no naturally occurring subdural space. Because the cellular characteristics of the dural border (neurothelial) cell layer create a structurally weak plane and an area of low resistance, disruption of this layer during surgery or by trauma, for example, can create an artificial subdural space. However, Hugh (2010) studied myelograms in which an oily contrast medium (Myodil) had been inadvertently injected into the subdural space instead of the subarachnoid space during lumbar puncture procedures. Based on the predictability, speed, and ease in which the oil traveled, Hugh suggested that a well-defined physiologic subdural space did exist. He speculated that the subdural space may be a lymphatic space or reservoir because it is connected to perineural lymphatic channels surrounding major peripheral nerves and to the central lymphatic system. The subdural space may also function as a major passageway in the reabsorption of CSF in humans when in an erect stance and also as a conduit for the spreading of metastatic tumors and pathologic organisms.
Both the dura and the arachnoid (collectively forming the dural sac, or thecal sac) have typically been described as extending to the lower border of the S2 vertebra, well below the end of the spinal cord (conus medullaris). However, Macdonald and colleagues (1999) studied magnetic resonance imaging (MRI) scans of the lumbosacral region of 136 adults and found that the level of termination ranged from the upper border of S1 to the upper border of S4, with the median level being the middle one third of S2. They also noticed a gender difference in the termination level, with the median level for males being the upper one third of S2, and the middle one third of S2 the median level for females. This is supported by the work of Hansasuta and colleagues (1999).
In addition to typically extending inferiorly to the S2 level, the dura and arachnoid also extend laterally and invest the nerve roots in a manner similar to a coat sleeve, as the roots travel distally toward the IVF to form their spinal nerve (see Fig. 3-12). At that point the dura blends in with the epineurial connective tissue surrounding the newly formed spinal nerve, whereas the arachnoid merges with the perineurium (Hewitt, 1970; Snell, 2001; FitzGerald & Folan-Curran, 2002; Standring et al., 2008).
The subarachnoid space is under the arachnoid (see Fig. 3-12). This space is filled with CSF. At various locations throughout the CNS, the subarachnoid space becomes enlarged, forming regions known as cisterns. The subarachnoid space inferior to the conus medullaris is such an enlargement and is called the lumbar cistern (see Figs. 3-7 and 3-11, B). At this level the lumbar cistern contains not only CSF but also the cauda equina and filum terminale (see the following discussion). CSF is actively secreted, via various transport mechanisms, by the choroid plexus. The choroid plexus is specialized tissue located in the ventricles within the brain. Although CSF is often compared with plasma, its ionic composition is different; therefore CSF is not considered to be an ultrafiltrate of blood. CSF has numerous functions: it provides buoyancy and protection for the CNS against mechanical trauma; it provides a route for the removal of products of neuronal metabolism (sometimes referred to as “acting as a large metabolic sink”) (Benarroch et al., 1999; Nolte, 2002); it provides and regulates a stable chemical microenvironment to ensure normal functioning of neurons and glial cells; and it is a route by which neuroactive hormones may travel through the nervous system. Such neuroactive hormones include hypothalamic hormones that bind to distant target cells in the brain (Laterra & Goldstein, 2000) and pineal secretions traveling to the pituitary gland (Snell, 2001; Nolte, 2002).
The CSF flows inferiorly in one direction within the ventricles located in the brain. At a level just rostral to the foramen magnum, most CSF leaves the most caudal (fourth) ventricle, enters the subarachnoid space, and flows superiorly over the brain and inferiorly around the spinal cord. In addition, a very small amount of CSF remains within the central canal of the cord. The CSF in the subarachnoid space gradually and slowly flows inferiorly into the lumbar cistern and then makes its way back superiorly. Pulsation of large spinal arteries within the subarachnoid space, respiratory movements, movements of the vertebral column, changing of body positions, and intrathoracic and intraabdominal pressure changes contribute to the flow of the CSF. Some CSF surrounding the cord flows superiorly and into the subarachnoid space surrounding the brain. Because of a pressure gradient, this CSF eventually flows from the subarachnoid space through arachnoid granulations (i.e., multiple villi that serve as one-way valves) and into the venous sinuses of the cranial dura mater. The CSF around the spinal cord also is absorbed through arachnoid villi that penetrate the dural root sleeves in the region of the dorsal root ganglia and project into small spinal veins leaving the IVF. It has been suggested that 25% of CSF reabsorption occurs through spinal arachnoid villi (Afifi & Bergman, 1998; Laterra & Goldstein, 2000; FitzGerald & Folan-Curran, 2002; Johnston & Papaiconomou, 2002; Nolte, 2002; Pollay, 2010). Emptying into the venous system completes the one-way circulation of the CSF. Human and animal studies also suggest that CSF drains into lymphatic vessels and subsequently into regional lymph nodes (Boulton et al., 1996; Miura, Kato, & von Ludinghausen, 1998; Fricke, Andres, & Von During, 2001; Snell, 2001; Johnston & Papaiconomou, 2002; Pollay, 2010).
The innermost membrane of the meninges is called the pia mater. This layer consists of one to two layers of flattened cells that are joined by desmosomes and gap junctions. They are continuous with the leptomeningeal cells of the ligaments of the arachnoid intermediate layer. Separating this layer from the neural tissue of the external surface of the spinal cord (the astrocytic glia limitans) is the subpial space. This space contains a substantial amount of collagen fiber bundles, fibroblast-like cells, and blood vessels, such as the anterior spinal artery. The pia mater invests the spinal cord and forms a fold within the ventral median fissure. It also surrounds the arteries, rootlets, and roots as they course into the IVF. In addition to acting as a separation between the subarachnoid and subpial spaces, it has been suggested that the pia mater surrounding the brain may serve as a regulatory interface between the two spaces by means of the pinocytotic action of the pial cells (Standring et al., 2008).
Two specializations of pia mater that function to stabilize the spinal cord within the vertebral canal are the left and right denticulate ligaments and filum terminale. Each denticulate ligament is a collagenous, triangular-shaped serrated ribbon coursing the length of the cord. It consists of a medial border that is continuous with the subpial connective tissue of the spinal cord, and a lateral apical border that attaches to the dura mater in an even distribution at approximately 21 points on each side along the cord’s length. Because of its location, the denticulate ligament forms a shelf within the vertebral canal between the dorsal and ventral roots (see Figs. 3-10, 3-11, C, and 3-12) and divides the canal into anterior and posterior compartments. Superiorly the ligament attaches to the dura mater above the lateral rim of the foramen magnum and behind the hypoglossal nerve. The ligament lies between the anteriorly placed vertebral artery (which separates the ligament from the first cervical ventral root) and the ascending spinal root of the spinal accessory nerve. The most inferior portion arises from the conus medullaris and descends as a narrow oblique band to attach laterally to the dura mater lying between the roots of the exiting T12 and L1 spinal nerves. Cineradiography has shown that the form and position of the denticulate ligaments may change during movements of the spine (Standring et al., 2008). In the cervical region, the lateral apical attachments of the left and right denticulate ligaments attach to the dura further away from the exiting nerve roots than those of the lower thoracic region (Kershner & Binhammer, 2002). Tubbs and colleagues (2001) dissected 12 cadavers and described the morphology of the ligament in detail. They described the lateral apices of most ligaments as consisting of superior and inferior prongs that were approximately 1 mm in length. These two pronged ligaments were most prominent in the cervical and upper thoracic regions; the lower thoracic ligaments were not pronged. The lateral apical attachments in the cervical region were found to be thicker than those found in the thoracic and lumbar regions. Based on gross inspection and electron microscopy of 56 cadavers, Kershner and Binhammer (2002) suggested that developmental remnants of the lateral apex of denticulate ligaments formed “intrathecal ligaments” that were associated with the cauda equina. An average of 18 such ligaments per cadaver were found within the lumbar cistern. These dense collagenous intrathecal ligaments were covered with a thin layer of leptomeningeal cells and varied in thickness (0.13 to 0.35 µm) and length (3 mm to 3.5 cm). Some of the ligaments randomly connected dorsal nerve roots of the cauda equina to the dura mater, and occasionally the ligaments joined dorsal and ventral roots. These intrathecal ligaments are thought to be derived from the denticulate ligaments because the spinal cord ascends relative to the rest of the vertebral column during development. The clinical significance of these structures is unknown.
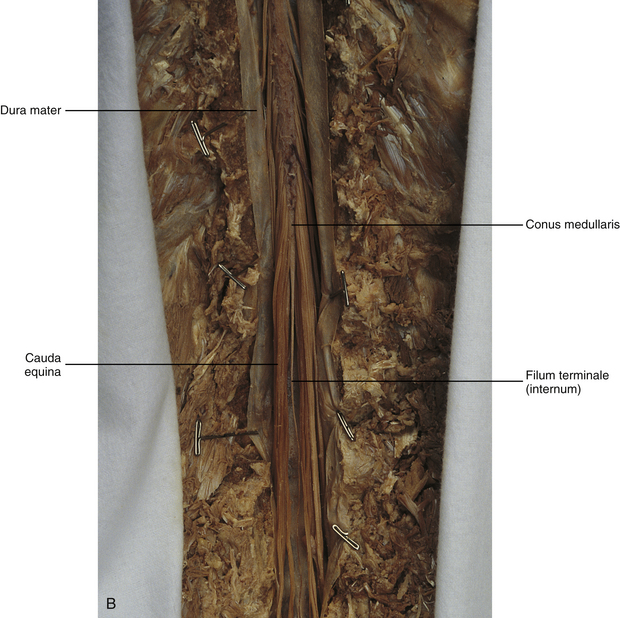
The other special component of pia mater is a bluish-white structure called the filum terminale. This slender filament consists of glial cells and ependyma (ependymal cells line the central canal of the spinal cord), and is covered by pia mater. The first 5 to 6 mm of the filum terminale includes a central canal. The filum extends approximately 20 cm from the tip of the conus medullaris within the lumbar cistern to the dorsum of the coccyx, where it blends into the connective tissue covering this bone (see Fig. 3-11, B). The portion of the filum terminale between the conus medullaris and the inferior tip of the dural sac is known as the filum terminale internum (approximately 15 cm long). Because the filum terminale pierces the dura and arachnoid at the S2 level on its way to the dorsum of the coccyx (thereby exiting the lumbar cistern), the filum terminale picks up two additional layers (dura and arachnoid); thus from S2 to the coccyx, it is usually called the coccygeal ligament (filum terminale externum). A few strands of nerve fibers adhere to the upper part of the filum. It has been suggested that these are likely to be rudimentary roots of the second and third coccygeal spinal nerves (Standring et al., 2008). The filum terminale has been described as a viscoelastic band that functions to “fixate, stabilize, and buffer the distal cord from normal and abnormal cephalic and caudal traction” (Bui, Tubbs, & Oakes, 2007) and to allow minimal movement of the conus medullaris during flexion and extension of the spine.
The filum terminale may seem to be an unassuming remnant but it does have clinical significance in its contribution to a condition known as tethered cord syndrome (TCS). Originally the term “tethered spinal cord” described patients with an abnormally low conus medullaris that was “tethered or anchored” by a thickened (≥2 mm in diameter) filum. The definition of a tethered cord has expanded and is now associated with many disorders including a wide range of occult spinal dysraphisms (OSDs), trauma, infection, and neoplasm (Bui, Tubbs, & Oakes, 2007). TCS can be seen in children because of a congenital closed neural tube defect. In this developmental situation the filum terminale becomes abnormally thick or infiltrated with fat and its viscoelasticity is lost or diminished. This places tension, traction, and undue stress on the caudal cord that interferes with and prevents the cord’s normal ascent, resulting in a low-lying conus medullaris and TCS (Afifi & Bergman, 1998; Pinto et al., 2002). Of all spinal disorders seen by pediatric neurosurgeons, TCS is the most common. A study (Lad et al., 2007) examining data from 9733 patients with TCS who had undergone surgery between 1993 and 2002 showed 71% were 17 or younger. Data indicated TCS had occurred in adult patients also: 18.6% in 18 to 44 year olds, 8.6% in 45 to 64 year olds, and 1.8% in adults older than 65. The clinical presentation of patients with TCS includes a variety of signs and symptoms. The most common presentation frequently includes cutaneous features associated with OSD such as lumbosacral hypertrichosis, cutaneous capillary hemangiomas, midline subcutaneous lipomas, and lumbosacral skin appendages. Other signs and symptoms include neurogenic bladder with the development of incontinence or urinary tract infection, leg or foot weakness, numbness and/or spasticity, unequal leg or foot lengths, deformities of the feet and spine, and nondermatomal back and leg pain (Bui, Tubbs, & Oakes, 2007). In the case of TCS occurring in adults, a subclinical degree of spinal cord traction is present. The traction can become clinically apparent when abrupt cord traction occurs because of sudden flexion of the vertebral canal (e.g., the abdominal flexion movements and trauma resulting from motor vehicle accidents) (Pinto et al., 2002). The sudden traction leads to neurologic deficits caused by anatomic and metabolic changes in the spinal cord. The most common neurologic deficit is pain in the lower back and pain radiating into the lower limbs. This pain is exacerbated by physical activity involving flexion and extension of the trunk. Other findings include sensory deficits in the lower extremities, lower extremity deformities, musculoskeletal deformities such as scoliosis, and urinary incontinence. Adults who present with these types of deficits, but are asymptomatic as children, often are misdiagnosed with “failed back syndrome” or other unrelated problems of the spine (Yamada & Lonser, 2000). The radiologic criteria that have been established to aid in diagnosing this condition are a filum terminale that has a diameter greater than 2 mm or a spinal cord that terminates lower than the L2 or L3 vertebral body levels, and a conus medullaris displaced posteriorly with the filum in contact with the dural sac at or near the L5 lamina (Yamada & Lonser, 2000; Pinto et al., 2002). However, because of considerable anatomic variation of the conus medullaris and filum terminale, the patient’s history, examination, and radiologic findings are important in diagnosing TCS. The surgical treatment for TCS is to perform a laminectomy at the lumbosacral junction, incise the dura and open the arachnoid, and then section the filum and untether the cord. Care must be taken to not cut any spinal roots (Bui, Tubbs, & Oakes, 2007). (See Chapter 7 for additional information on tethered cord syndrome in the adult.)
The vast majority (89%) of fila fuse on the dorsal midline of the dura, with 11% fusing to the left or right of the midline. The level of fusion varies anywhere from the lower L5 vertebral body to the upper S3 body. The majority (approximately 85%) of fila fuse at or below S1, and approximately 15% fuse above the S1 level. Forty-four percent of the time the fusion is at the same level as the termination of the dural sac (Hansasuta, Tubbs, & Oakes, 1999). The work of Pinto and colleagues (2002) supports these findings. The diameter of the filum terminale decreases in a superior to inferior direction; the mean thickness at the midpoint of the filum is 0.76 ± 0.39 mm and the mean thickness at the initial point of origin is 1.38 ± 0.56 mm.
In addition to the variation in the anatomy of the filum and its relationship with the dural sac, anatomic variations of the conus medullaris are still being documented. The accepted view was that the mean level of termination of the conus medullaris was at the level of the L1-2 intervertebral disc (Reimann & Anson, 1944). Many recent MRI (Table 3-1) (Saifuddin, Burnett, & White, 1998; Macdonald et al., 1999; Malas et al., 2000; Arai et al., 2001; Demiryürek et al., 2002) and cadaveric (Gatonga et al., 2010) studies indicate that the conus medullaris usually extends to somewhere between the superior aspect of the vertebral body of L1 to the inferior aspect of the body of L2 (range = middle third of T11 to lower third of L3 vertebral bodies), although there is considerable variation among the studies. The discrepancies in the data among various investigators may result from racial differences, differences in statistical analysis, the presence of transitional lumbosacral vertebra, and the limitations of cadaveric and MRI studies (Choi, Carroll, & Abrahams, 1996; Saifuddin, Burnett, & White, 1998; Macdonald et al., 1999; Demiryürek et al., 2002; Gatonga et al., 2010).
The majority of studies also indicate there is no significant difference in level of termination of the conus medullaris between males and females (Saifuddin, Burnett, & White, 1998; Macdonald et al., 1999; Arai et al., 2001; Gatonga et al., 2010). One exception to this is the study of Demiryürek and colleagues (2002) who reported that the termination level in females was lower than that in males (L1-2 IVD versus T12-L1 IVD, respectively).
In addition, there appears to be no difference in termination levels of the conus medullaris with increasing age (Saifuddin, Burnett, & White, 1998; Arai et al., 2001; Demiryürek et al., 2002), although Arai and colleagues (2001) reported a more caudal distribution of the termination of the conus medullaris in children less than 11 years of age.
The conus medullaris generally is centrally located within the lumbar subarachnoid cistern; however, it slants ventrally 10% of the time and dorsally 30% of the time (Arai et al., 2001).
Knowing the vertebral level in which the majority of spinal cords terminate and being aware that the sites of termination vary among individuals (middle third of T11 to lower third of L3 vertebral bodies) is important. For example, neurologic deficits seen in patients who have experienced vertebral fractures, especially burst fractures, and osteoporotic vertebral collapse at the thoracolumbar level differ depending on the location of the conus. Also, noting variations is important when an invasive procedure such as a lumbar puncture (spinal tap) is performed. In this procedure, a long needle is inserted in the midline between adjacent lower lumbar vertebrae (L3-4 or L4-5) and into the lumbar cistern. Because the cauda equina is floating in the CSF, the roots usually are avoided by the needle. Once the needle is inserted, agents can be injected into the region for diagnostic imaging and anesthetic purposes. For example, in myelography, a radiopaque iodinated contrast medium is injected to outline the spinal cord and roots. Anesthetics occasionally may be injected into the subarachnoid space (spinal anesthesia) for abdominal, pelvic, or lower extremity surgery. Hamandi and colleagues (2002) and Reynolds (2001) reported on a total of 12 cases in which spinal anesthesia alone or in combination with epidural anesthesia was used on 8 patients undergoing obstetric operations and 4 undergoing surgical operations. Direct damage from needle insertion to the cord was seen on MRI scans in these patients, resulting in long-term neurologic deficits in the lower extremity such as pain, muscle atrophy weakness, footdrop, decreased sensation, and absent ankle muscle stretch reflexes. Because of the seriousness of these deficits, the authors of both studies indicate the importance of inserting the needle in the interspace of lower lumbar vertebrae.
The volume, pressure, and contents of CSF also are clinically relevant; for example, although not done routinely, the removal and analysis of typically 5 to 15 ml of CSF by means of lumbar puncture, when indicated, can be used as an important neurodiagnostic test. The total volume of CSF ranges from 80 to 150 ml, and CSF is produced sufficiently to replace itself four to five times daily. Notice that there is a dramatic variation in CSF volume in different individuals (Hogan et al., 1996). In addition, the volume of CSF has been found to decrease in relatively obese individuals as a result of increased abdominal pressure (a condition seen in obese and pregnant individuals). This decrease was greater at the levels of the IVFs, which suggested that CSF was displaced by the inward movement of soft tissue from the IVF into the vertebral canal. A study by Edsbagge and colleagues (2011) looked at MRI images of 22 (11 men, 11 women) healthy (no symptoms or signs of neurologic or spinal morbidity) individuals ages 64 to 76 years. The total CSF volume in the spinal canal and also the volume in the cervical, thoracic, and lumbosacral regions were measured. The mean spinal CSF volume was 81 ml with a large range of between 52 and 103 ml. The mean volume (and range, both in milliliters) in the cervical, thoracic, and lumbosacral regions was 19 (range 14-27), 37 (range 22-57), and 25 (range 11-38), respectively. There was considerable variability of the lumbosacral CSF volume compared to other studies that may have been attributable to methodology and/or age of the subjects. These data may be useful for generating information for the analysis of CSF biomarkers, for the administering of intrathecal drugs, and for understanding the CSF dynamics relative to common neurologic disorders of the elderly such as normal pressure hydrocephalus.
CSF pressure ranges from 80 to 180 mm H2O and is measured on a patient lying in a curled, lateral recumbent position. CSF is normally clear, colorless, and slightly alkaline. It contains approximately six white blood cells (WBCs), usually lymphocytes, per milliliter and no red blood cells (RBCs). As with plasma, it includes sodium, potassium, magnesium, and chloride ions. It also contains glucose and protein, but the concentrations are substantially less than those in plasma.
CSF volume may become altered as a result of a pathologic state. The Monro-Kellie hypothesis states that the sum of brain tissue, intracranial blood, and CSF volumes is constant, and that if one of these volumes increases, the other volumes must compensate because the bony confines do not. If no compensatory readjustment occurs, intracranial pressure (ICP) increases. Interference in CSF circulation in the cranium by a space-occupying lesion such as a tumor or hematoma can increase CSF pressure. If an increase in ICP is suspected, a lumbar puncture (spinal tap) is contraindicated. Removal of CSF in such cases could produce a vacuum and cause herniation of the cerebellum into the foramen magnum, with serious consequences, including death. Increased CSF pressure also can cause swelling of the optic disc (papilledema) of the retina. Because the retina can be observed easily by an ophthalmoscope, papilledema could contraindicate the performance of a lumbar puncture. Although the condition of increased intracranial pressure presents very serious clinical sequelae, the Monro-Kellie hypothesis also is applicable to a decrease in intracranial pressure and reduced CSF volume. The necessary compensation that occurs is to affect the intracranial blood volume, resulting in intracranial (usually venous) hyperemia and changes in the cranium that can be seen on MRI scans. A decrease in CSF volume also results in changes in the spinal canal, such as an engorgement of the epidural venous plexus that compensates for a moderate degree of collapse of the dura mater, which is also observed on MRI scans (Mokri, 2001).
In addition to pressure changes, the appearance, cell content, levels of gamma globulins (antibodies), and protein and glucose concentrations in CSF may be altered in patients with some pathologic conditions. For example, in bacterial meningitis (inflammation of the leptomeninges) the CSF is cloudy, pressure is increased, WBC count is elevated, protein concentration is increased, and glucose concentration is decreased (Daube et al., 1986). The CSF in a patient with a subarachnoid hemorrhage appears cloudy, and RBCs are present. Therefore the alterations of certain characteristics of the CSF become useful in diagnosing certain pathologic conditions.
Internal Organization of the Spinal Cord
Having discussed the external morphology of the spinal cord and its coverings, the internal morphology is now described to provide an understanding of spinal cord anatomy in its entirety. In Chapter 9, a more detailed description of the internal aspect of the spinal cord and its functions is given.
The overall internal organization of the spinal cord can be observed in a transverse or horizontal cross section. This view shows the spinal cord as being clearly divided into a butterfly- or H-shaped central area of gray matter and a peripheral area of white myelinated axons.
Gray Matter
Each half of the H-shaped gray matter consists of a dorsal horn, ventral horn, and intermediate area between the horns (Fig. 3-13). In thoracic segments the intermediate zone includes a lateral horn. The crossbar of the gray matter unites the two laterally placed halves and is called the gray commissure. The gray commissure can be divided into dorsal and ventral components, based on their relationship to the central canal. The gray commissure is thinnest in the thoracic segments and thickest in the conus medullaris and contains two longitudinal veins. Coursing through the dorsal aspect of the commissure are transverse myelinated axons that are sometimes called the dorsal white commissure (Standring et al., 2008). The gray commissure also contains the central canal. This canal is the remnant of the lumen of the embryologic neural tube and is a continuation of the fourth ventricle of the brain stem. Lined by a single layer of ependymal cells, it extends the length of the spinal cord and continues into the filum terminale for approximately 5 to 6 mm. Within the conus medullaris it expands into a triangular-shaped, 8- to 10-mm-long structure called the terminal ventricle. The location of the canal relative to the midpoint of the spinal cord varies among the regions of the cord. It is slightly ventral in the cervical and thoracic segments, central in the segments of the lumbar enlargement, and dorsal in the conus medullaris. Immediately adjacent to the canal lies the substantia gelatinosa centralis, which is a region containing a few neurons, neuroglia, and a reticulum of fibers. Coursing through this area are processes radiating from the basal aspects of the ependymal cells lining the canal. Peripheral to the substantia gelatinosa centralis is the gray commissure (Williams et al., 1995).
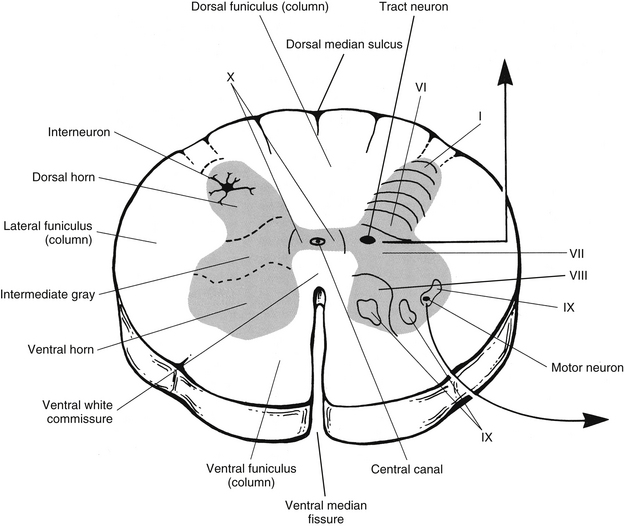
FIG. 3-13 Cross section of the spinal cord. Left, Organization of gray matter into regions. Right, Lamination of the gray matter. Motor and tract neurons and interneurons also are shown.
The central canal is frequently involved with cavitary lesions of the spinal cord, such as syringomyelia. The pathogenesis of this condition, which is a dilation of the central canal causing a syrinx, appears to be related to a hydrodynamic mechanism relating to the CSF. Studies on kaolin-induced hydrocephalic animals indicated that the distending forces of a downward movement of CSF from ventricles in the brain into the central canal caused spinal cord cavitation including central canal distention and disruption into the cord parenchyma (Yasui et al., 1999).
After birth the central canal becomes progressively occluded (typically with ependymal cells) as one ages. Histologic studies of the canal indicate that ependymal cell deterioration during the aging process contributes to the canal occlusion. The ependymal cell changes result in glial bundle formation, proliferation of ependymal cells and astrocytes, formation of ependymal rosettes or microcanals, proliferation of subependymal gliovascular buds, and intracanalicular gliosis (Milhorat, Kotzen, & Anzil, 1994; Yasui et al., 1999). Interestingly, Milhorat and colleagues (1994) suggest that central canal stenosis is an acquired pathologic lesion rather than a degenerative process related to aging. They postulate that the cause is recurring episodes of ependymitis caused by exposure to common viral infections throughout a lifetime.
Using a computerized three-dimensional histologic study on a small sample of spinal cords, Storer and colleagues (1998) demonstrated a variety of anatomic features of the central canal. They observed forking within the lower conus medullaris near the terminal ventricle with outpouchings of each fork of the canal into the filum terminale. They also noticed an extension of ependymal cells from the lumen of the canal to the surface of the pia mater that suggested a possible functional communication between the canal and the subarachnoid space. The ependymal lining of the canal was also in close proximity to the pial surface throughout most of the length of the extrusion of the central canal into the filum terminale. This region was found to have openings into the subarachnoid space at two levels within the caudal filum. The researchers postulated that this connection may provide a physiologically important fluid communication that may play a role in the “sink” function of the canal. Other data also suggest that the canal functions like a “sink” based on its capability of clearing substances such as vital dyes, horseradish peroxidase, and cellular elements from cord parenchyma (Milhorat, Kotzen, & Anzil, 1994). However, Yasui and colleagues (1999) believe that this proposed function of the human central canal is insignificant after birth. They found that the patency rate of the central canal was 100% in infants younger than 1 year of age. A marked decrease was seen in the percentage of individuals with patent central canals in the second decade, and by the fourth decade all levels were occluded except in the cervical cord, where the patency rate remained high. The canals were occluded in the vast majority of all the segments after the seventh decade. The T6 and L5-S2 levels occluded earliest, and the upper cervical segments were the last to occlude. The progressive nature and segment location of the canal stenosis most likely affect the clinical presentation of syringomyelia, and, in fact, Yasui and colleagues (1999) suggest that because the canal is obliterated, it is not involved in the development of the adult form of syringomyelia.
The large, dense gray area of the cord (gray matter) consists of neurons, primarily cell bodies; neuroglia; and capillaries. Microscopically this region appears to be a tangle of neuron processes and their synapses and neuroglial processes, all of which form the neuropil. This network forms the cord’s amazingly complex circuitry. The neurons of the gray matter consist of four general types: motor, tract, interneuron, and propriospinal. The larger motor and tract neurons, which have long axons (1 m or more in length), are sometimes called Golgi type I cells. Interneurons and propriospinal neurons, which have shorter axons, may be called Golgi type II cells. These are the more numerous of the two (Carpenter, 1991; Williams et al., 1995; Kiernan, 2009). The neurons are not distributed equally throughout the gray matter but instead are found in clusters.
Axons of motor neuron cell bodies leave the spinal cord, enter the ventral root, and ultimately innervate the body’s effector tissues—that is, skeletal muscle, smooth muscle, cardiac muscle, and glands. Skeletal muscle is innervated by α (alpha) and γ (gamma) motor neurons. Smooth muscle, cardiac muscle, and glands are innervated by autonomic motor fibers. Motor neuron cell bodies are located in either the ventral horn or the intermediate gray area and are densely covered with presynaptic terminals of axons from higher centers, incoming sensory afferent fibers, propriospinal neurons, and interneurons. It is believed that each of the large α-motor neurons that innervates a skeletal muscle may have at least 20,000 synapses on its surface (Davidoff & Hackman, 1991; Kiernan, 2009).
Axons of tract neurons in the gray matter emerge from the gray matter and ascend in the white matter to higher centers. These axons help to form the ascending tracts of the cord’s white matter. The cell bodies of these neurons are located primarily in the dorsal horn and intermediate gray area.
The third type of neuron is the interneuron. Interneurons constitute the vast majority of the neuronal population, and their cell bodies are found in all parts of the gray matter. They conduct the important “business” of the CNS by forming complex connections. Although various types of interneurons exist, their processes are all relatively short. They usually are located within the limits of one cord segment, although the axons may include collateral branches that terminate both contralaterally and ipsilaterally. The interneurons receive input from each other, incoming sensory afferents, propriospinal neurons, and descending fibers from higher centers. Through their elaborate synaptic circuitry, the interneurons transform this input and disseminate it to other neurons, including the motor neurons. For example, some interneurons play a crucial role in motor control by their actions in motor reflexes involving peripheral proprioceptive input from neuromuscular spindles and Golgi tendon organs (see Chapter 9).
Other “motor” interneurons called Renshaw cells provide a negative feedback mechanism to adjacent motor neurons. This intricate circuitry may be necessary for synchronizing events such as the force, rate, timing, and coordination of contraction of muscle antagonists, synergists, and agonists that must occur in the complex motor activities performed by humans. In addition, other interneurons located in the dorsal horn are involved in the circuitry that modifies and edits pain input conveyed into the dorsal horn by afferent fibers (see Chapter 9).
The fourth type of neuron located in the gray matter is the propriospinal neuron. This neuron’s axon leaves the gray matter at one level, ascends or descends, and terminates in the gray matter at a different cord level. The majority of the axons are located in the white matter immediately adjacent to the gray matter (fasciculus proprius), although some axons are spread diffusely within the white matter funiculi. The classification of propriospinal neurons is based on the length of their axons. Short propriospinal neurons travel ipsilaterally over a distance of approximately six to eight segments; intermediate neurons course ipsilaterally more than eight segments but less than the entire length of the cord; and long propriospinal neurons project the length of the cord, descending bilaterally and ascending contralaterally.
These neurons allow communication to occur among cord segments for the coordination of skeletal muscle contraction and regulation of autonomic functions such as sudomotor (to sweat glands) and vasomotor activities, and bladder and bowel control (Standring et al., 2008).
As mentioned, in cross section the gray matter resembles a butterfly- or H-shaped area. Each half is subdivided into a dorsal horn, an intermediate area that in certain segments includes a lateral horn, and a ventral horn (see Fig. 3-13). The dorsal horn functions as a receiving area for both descending information from higher centers and sensory afferents from the dorsal roots. The cell bodies of the sensory afferents are located in the dorsal root ganglia. The sensory afferents bring information from receptors in the skin (exteroceptors); muscles, tendons, and joints (proprioceptors, i.e., mechanoreceptors for proprioception); and the viscera (interoceptors). These afferent fibers synapse on interneurons, propriospinal neurons, and tract neurons, depending on the type of information carried and the resulting action needed.
The intermediate region, which is actually the central core of each half of the gray matter, receives proprioceptive input from sensory afferents, input from the dorsal horn, and descending input from higher centers, thus becoming an area in which interaction of sensory and descending input can occur. In general, the intermediate area comprises interneurons, tract neurons, propriospinal neurons, and the cell bodies of neurons innervating smooth muscle, cardiac muscle, and glands. These cell bodies are located in cord segments T1 to L2-3 and constitute the lateral horn.
The ventral horn of the gray matter includes interneurons, propriospinal neurons, and the cell bodies of motor neurons. The axons of these motor neurons, also called α- and γ-motor neurons or anterior horn cells, leave via the ventral roots to innervate skeletal muscle. Descending tracts from the brain, axons from propriospinal neurons in other cord segments, intrasegmental interneurons, and primary afferents from mechanoreceptors (proprioceptors) involved with monosynaptic muscle stretch reflexes terminate in this region of ventral gray matter.
As is the case throughout the nervous system, neurons communicate with each other and form circuits within the gray matter of the spinal cord by means of synapses and chemical substances. A synapse is the junction between two neurons. The average neuron is thought to have as many as 10,000 synapses on its surface and to give rise to approximately 1000 synaptic connections (Kandel & Siegelbaum, 2000). The chemical substances called neuromediators are released at the synapse from the terminal of a presynaptic neuron. These mediators cross the synaptic cleft and bind to receptors on the postsynaptic neuron, causing either a depolarization or hyperpolarization of the cell membrane (via neurotransmitters), or a structural or functional change in the neuron (via neuromodulators). Through use of techniques such as autoradiography and immunohistochemistry, much new information concerning the neural circuitry of the spinal cord is emerging. By using antibody labeling techniques, neuromediators have been localized in neuronal cell bodies of the gray matter and in axon terminals of neurons, including descending fibers and primary afferent fibers. Examples of neuromediators that have been found in the dorsal horn are enkephalins, somatostatin, substance P, cholecystokinin, dynorphin, γ-aminobutyric acid (GABA), glycine, glutamate, and calcitonin gene–related peptide (CGRP). The intermediate gray matter and ventral horn include neuromediators such as cholecystokinin, enkephalins, serotonin, vasoactive intestinal polypeptide (VIP), glycine, GABA, and CGRP. These chemicals bind to specific receptors, some of which also have been located through labeling techniques. Examples of receptors located in various regions of the gray matter include opiate receptors, muscarinic cholinergic receptors, and receptors for GABA, CGRP, and thyrotropin-releasing hormone (Schoenen, 1991; Willis & Coggeshall, 1991).
In addition to the types of neurons comprising the spinal cord gray matter, each half is also described microscopically by its longitudinal laminar cytoarchitectural pattern. These longitudinal laminae contain combinations of motor, tract, propriospinal, and interneurons. In some instances, neuron cell bodies of one of the four types may form a cluster, called a nucleus (i.e., an aggregation of neuron cell bodies found within the CNS). The neurons of each nucleus are similar morphologically, and the axons have a common termination and function. Numerous nuclei have been identified in the gray matter. Some are located in all cord segments, whereas others are limited to specific cord segments.
The laminar cytoarchitectural organization of the spinal cord gray matter was proposed by Rexed in 1952. Studying the size, density, staining characteristics, and connections of the neurons in feline spinal cords, he identified 10 layers within the gray matter. This has since been accepted as standard in human spinal cords as well. Rexed’s gray matter laminae proceed sequentially from dorsal to ventral (see Fig. 3-13). Lamina I is the tip of the dorsal horn, and lamina IX is in the ventral horn. Lamina X corresponds to the gray commissures (dorsal and ventral) surrounding the central canal. Each lamina contains specific types of neurons and, in some cases, nuclei, which indicate a function for that layer of cells. The neuronal populations of each lamina and the functional significance of the laminae, relative to the connections between the cord and brain and between the CNS and the periphery, are described in more detail in Chapter 9.
White Matter
A cross section of the spinal cord demonstrates that peripheral to the gray matter of the cord is a well-defined area of white matter (see Fig. 3-13). White matter includes a longitudinal arrangement of predominantly myelinated axons (which gives the area the white appearance), neuroglia, and blood vessels. The white matter is divided into three major areas called columns or funiculi. The dorsal funiculus or column is located between the dorsal horns, the lateral funiculus or column between each dorsal and ventral horn, and the ventral funiculus or column between the ventral horns (see Fig. 3-13). The white area connecting the two halves of the cord and surrounding the gray commissure forms the dorsal and ventral white commissures. The dorsal white commissure is small. The ventral commissural area includes clinically important decussating axons of pain and temperature tract neurons. The neuronal cell bodies of the axons that course in the funiculi are found in various locations. The cell bodies of axons that interconnect cord segments or ascend to the brain are located in the cord’s gray matter or dorsal root ganglia. The cell bodies of axons that descend in the spinal cord to synapse in the gray matter are located in the brain.
The long ascending and descending axons are not randomly mixed together but are organized into bundles called tracts or fasciculi (see Chapter 9, Fig. 9-8). The axons of each tract or fasciculus share a common origin and convey similar information to a common destination. For example, axons that are conveying impulses for pain and temperature are found in an anterolateral position in the cord. Although the tracts are well organized, they still overlap somewhat, and boundaries are often arbitrary. The axons that compose the funiculi vary in diameter (Standring et al., 2008). Makino and colleagues (1996) studied the morphometry of the C7 cord segment in seven adult cadavers. They found that myelinated axons in the dorsal funiculus (DF) (location of the dorsal columns), the marginal zone of the lateral funiculus (M-LF) (location of the spinocerebellar tracts), and the dorsal half of the lateral funiculus (LF) (location of the lateral corticospinal tract) consist of small-diameter fibers (<5 µm in the DF and <7 µm in the M-LF and LF) and large-diameter fibers (>5 µm in the DF and >7 µm in the M-LF and LF). The fiber density was greatest in the LF, followed by the DF, which was denser than the M-LF. There was considerable variation among the specimens concerning the density of small-diameter fibers, but not of large-diameter fibers. The density of small-diameter fibers appears to determine the total myelinated fiber density. A large variation also was noted among specimens relative to the total area of all funiculi (ventral, lateral, and dorsal) and the hemilateral area of the cord. In thick cords (large cross-sectional area) the absolute number of large-diameter fibers was greater than in the thin cords (small cross-sectional areas). Research on peripheral nerve fibers suggests that ischemia or compression selectively injures large-diameter fibers. Therefore it is possible that a thin cord with a small cross-sectional area in which the absolute number of large-diameter fibers is less could be more susceptible to neural injury caused by chronic compressive myelopathy (Makino et al., 1996).
Regional Characteristics
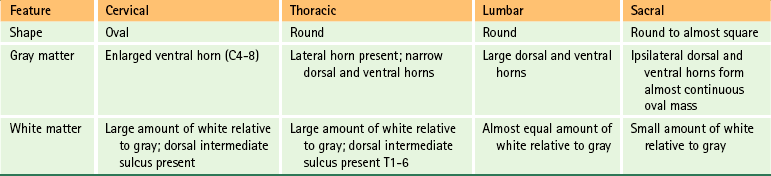
Although the characteristics of gray and white matter are generally the same in all cord segments, some identifying features are seen in cross section that distinguish the cervical, thoracic, lumbar, and sacral regions of the spinal cord (Fig. 3-14 and Table 3-2). For example, differences exist in the appearance and amount of white matter because of the presence and absence of certain tracts at different levels such that the volume of white matter increases as cord segments progress cranially. Also, the gray matter changes its appearance because of regional differences in the number of autonomic motor neuron cell bodies (the axons of which innervate smooth muscle, cardiac muscle, and glands) and somatic sensory and motor neuron cell bodies associated with the innervation of the extremities and trunk. Because the density of sensory receptors is greater in the extremities than in the trunk, more neurons are needed to process this information, which results in a larger dorsal horn. Also, the extremities have more muscles than the trunk, so there are more somatic motor neurons and associated interneuronal pools to regulate limb movement, which results in a larger ventral horn.
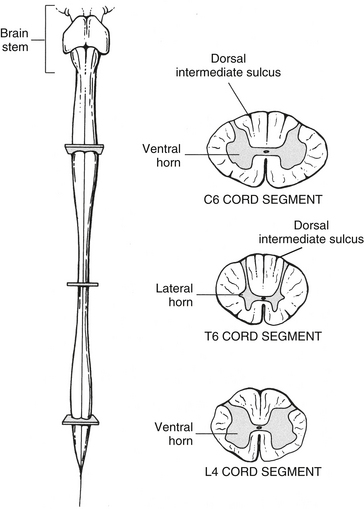
FIG. 3-14 Cross sections of the spinal cord at cervical, thoracic, and lumbar levels showing regional characteristics of gray and white matter.
Numerous investigators have measured the human spinal cord using cadaveric specimens and also using computed tomography (CT) myelography and MRI techniques on patients (Elliott, 1945; Nordqvist, 1964; Fujiwara et al., 1988; Carpenter, 1991; Schoenen, 1991; Choi, Carroll, & Abrahams, 1996; Kameyama, Hashizume, & Sobue, 1996; Fountas et al., 1998). The specific data for measurements of individual cord segments in these studies differ significantly. This may result from the variability in cord size among individuals or the techniques and methodologies used, which are inherently different. Because of this, the regional characteristics of the cord are described in more general terms (see the preceding references for detailed descriptions). However, for reference purposes, all of the sagittal and transverse diameters of the cord are in the range of 5 to 15 mm.
Kameyama and colleagues (1996) measured the transverse (side-to-side) and sagittal (dorsal-to-ventral) diameters and the cross-sectional area of the entire normal cadaveric cord. Their data indicate that the transverse diameter increases from C2 (10.5 ± 0.8 mm), peaks at approximately C6 (12.6 ± 0.7 mm), and then dramatically decreases to the T2 level (9.2 ± 0.6 mm). In the thoracic segments there was a gradual decrease, with the smallest diameter being at the T8 and T9 segments (7.4 ± 0.5 mm). At this point, the diameter increases until it peaks again, but to a lesser degree, at L4 (8.7 ± 0.4 mm). It then decreases until the S3 level (5.2 ± 0.7 mm). The sagittal diameter gradually decreases from C2 (6.4 ± 0.4 mm) to the thoracic levels T3-9, where it plateaus (5.0 ± 0.5 mm). From here the sagittal diameter increases, forming a small peak at L4 (6.4 ± 0.6 mm), and then decreases until the S3 level (4.0 ± 0.4 mm). The cross-sectional area measurements mirror the measurements of the transverse diameters in that the cross-sectional area is largest at C6 (58.5 ± 7.2 mm2), decreases and plateaus, and then peaks again at L4 (43.4 ± 5.1 mm2).
Cervical cord segments are nearly circular in shape in the upper region but become oval shaped in the middle and lower segments (as well as the T1 segment). At almost all levels, the transverse diameter is greater than the sagittal diameter and the increased transverse diameter forms the cervical enlargement (Kameyama, Hashizume, & Sobue, 1996). Because all ascending and descending axons to and from the brain must traverse the cervical region, the amount of white matter is greater here than in other regions. The increase in white matter content, rather than gray matter, is the basis for the cervical enlargement (Kameyama, Hashizume, & Sobue, 1996). However, the gray matter does contribute to this enlargement. The gray matter cross-sectional area is greatest in the C7 segment and the ventral horn of gray matter found in the segments forming the cervical enlargement bulges laterally because of the increased number of interneurons and motor neuron cell bodies. The axons of these motor neurons innervate upper extremity skeletal muscles (see Fig. 3-14).
Thoracic segments are most easily distinguished by their small amount of gray matter relative to white matter. Because the thoracic segments are not involved with innervating the muscles of the extremities, the lateral enlargement of the ventral horn is absent. Compared with cervical segments, both the transverse and the sagittal diameters of the thoracic spinal cord are smaller. One distinguishing feature of the thoracic segments is the presence of a lateral horn. This horn is located on the lateral aspect of the intermediate gray matter and is the residence of sympathetic neuronal cell bodies, the axons of which innervate smooth muscle, cardiac muscle, and glands. In addition, from midthoracic levels superiorly, the dorsal funiculus includes a dorsal intermediate sulcus (see Fig. 3-14).
Lumbar segments are distinguished by their nearly round appearance. Although they contain relatively less white matter than cervical regions, the dorsal and ventral horns are very large. The ventral horns of the lumbar segments are involved with the innervation of lower extremity skeletal muscles, and the additional neuron cell bodies for the motor axons are located laterally in those ventral horns. Therefore this lumbar enlargement (also including the S1-3 segments) is the result of an increase in gray matter (not white matter), and is formed by an increase in both sagittal and transverse diameters (Kameyama, Hashizume, & Sobue, 1996). The cross-sectional area of gray matter is greatest in the L5 segment. Although the T12 and L1 cord segments are indistinguishable, the large dorsal and ventral horns make lumbar segments easy to identify compared with cervical and thoracic segments (see Fig. 3-14).
Sacral segments are recognized by their predominance of gray matter and relatively small amount of white matter. The short and wide shape of the dorsal horns is also characteristic of these segments. Interestingly, although cervical and lumbar segments have large transverse and sagittal diameters, the thoracic segments are the greatest in length (superior to inferior) and the sacral the shortest. The average superior-to-inferior dimensions of various cord segments are cervical, 13 mm; midthoracic, 26 mm; lumbar, 15 mm; and sacral, 5 mm (Schoenen, 1991).
Advances in imaging techniques allow greater precision in identifying neuroradiographic anatomic structures. Having an understanding of the morphologic characteristics of the normal human spinal cord provides accuracy in interpreting imaging findings, diagnosing various cord pathologies such as degenerative syndromes and compressive cervical myelopathies, and providing treatment (Fujiwara et al., 1988; Hackney, 1992; Kameyama, Hashizume, & Sobue, 1996). Table 3-2 summarizes the regional characteristics of the spinal cord.
Arterial Blood Supply of the Spinal Cord
The spinal cord is vascularized by branches of the vertebral artery and branches of segmental vessels. The vertebral artery, a branch of the subclavian artery, courses superiorly through the foramina of the transverse processes of the upper six cervical vertebrae to enter the posterior cranial fossa via the foramen magnum (see Chapter 5 for further details). Within the posterior fossa of the cranial cavity, the anterior spinal artery (ASA) is formed. This artery is typically described as being formed from a small ramus from each of the vertebral arteries anastomosing in a Y-shaped configuration (Fig. 3-15, A). However, studies of the rami and the ASA (Turnbull, Brieg, & Hassler, 1966; Gövsa et al., 1996; Santos-Franco et al., 2006; Er, Fraser, & Lanzino, 2008) have shown that there is a great amount of variability in the branching patterns and morphology of the rami. In general, three types of variations can be found in the formation of the ASA. The first is by the fusion of two bilateral rami, the second is the presence of the ASA arising from one ramus, and the third is the occurrence of two separate ASAs. Although Gövsa and colleagues (1996) determined that the ASA originated from two rami 75% of the time, Santos-Franco and colleagues (2006) and Er and colleagues (2008) found that this presentation occurred approximately only 30% of the time. In addition, the fusion of the two rami was shown to occur at various distances along the medulla oblongata and as far inferiorly as the C5 cord segment. While in the posterior cranial fossa, branches of the rami as well as small branches of the ASA help to supply the anterior and inferior aspect of the medulla oblongata. Understanding the variations of these vascular structures is important in the planning and execution of surgical and endovascular procedures in the region of the foramen magnum, ventral side of the medulla, and cervicomedullary junction. The anterior spinal artery continues inferiorly through the foramen magnum within the pia mater covering the ventral median fissure of the spinal cord. This artery is usually straight, tapering as it courses inferiorly in the midline until it becomes extremely small, and often barely evident, just superior to the level of the artery of Adamkiewicz usually between the T8-L3 cord segments (see Anterior Radiculomedullary Arteries) (Gillilan, 1958; Schoenen, 1991). It may alter to one side as the anterior radiculomedullary arteries anastomose with it. The diameter of the anterior spinal artery varies from approximately 0.2 to 0.5 mm in the cervical and thoracic cord segments to 0.5 to 0.8 mm in the lumbar cord. Its diameter has been reported in a preliminary study to be approximately 0.47 mm above and 1.12 mm below its anastomosis with the artery of Adamkiewicz (Biglioli et al., 2000).
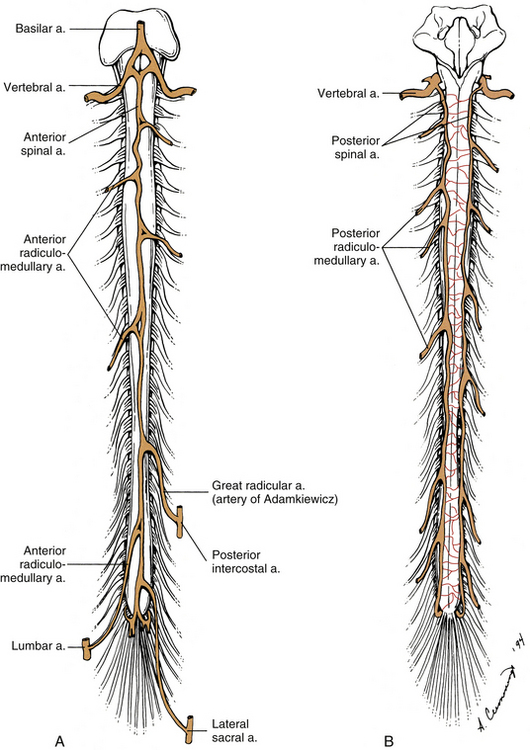
FIG. 3-15 Arterial blood supply of the spinal cord showing spinal and radiculomedullary arteries. A, Anterior view. B, Posterior view.
Also branching from each vertebral artery, and less frequently from the posterior inferior cerebellar artery, is the posterior spinal artery (Fig. 3-15, B). Each posterior spinal artery supplies the dorsolateral region of the caudal medulla oblongata. As it continues on the spinal cord, each artery forms two longitudinally irregular, anastomotic channels that course inferiorly on both sides of the dorsal rootlet attachment to the spinal cord. The medial channel is larger than the lateral (Schoenen, 1991). The two anastomotic channels are interconnected across the midline by numerous small vessels forming a plexiform network of small arteries that contribute to the pial plexus on the cord’s posterior surface.
At the level of the conus medullaris, a loop or basket is formed at the anastomosis of the anterior spinal artery with the two posterior spinal arteries (Lazorthes et al., 1971). At this location the artery of the filum terminale branches off and courses on the filum’s ventral surface (Djindjian et al., 1988).
Although the spinal arteries originate in the cranial cavity, segmental vessels, which help to supply blood to the cord, originate outside the vertebral column. The segmental arteries in the cervical region include branches of the cervical part of the vertebral artery, which vascularize most of the cervical cord, and branches of the ascending and deep cervical arteries. The latter arteries originate from the subclavian artery’s thyrocervical and costocervical trunks, respectively. Segmental branches that help to supply the rest of the cord arise from the posterior intercostal and lumbar arteries, which are branches of the aorta, and the lateral sacral arteries, which are branches of the internal iliac artery. These vessels provide spinal branches (spinal rami) that enter the vertebral canal through the IVF; vascularize the meninges, ligaments, osseous structures, roots, and rootlets (see Chapter 2); and reinforce the spinal arteries.
As each of the 31 pairs (Lazorthes et al., 1971) of spinal branches of the segmental spinal arteries enters its respective IVF, it divides into three branches. Anterior and posterior branches vascularize the dura mater, ligaments, and osseous tissue of the vertebral canal (see Chapter 2). The third branch, called the neurospinal artery (of Kadyi) (Schoenen, 1991), courses with the spinal nerve and divides into anterior and posterior branches that give off branches that either supply the roots or anastomose with the spinal arteries associated with the cord. Those small (<0.2 mm diameter) branches that vascularize the roots and their meningeal coverings are called radicular arteries. Each anterior and posterior radicular artery also supplies small branches to its neighboring root. The posterior radicular arteries also supply branches to the dorsal root ganglion. These small branches enter the ganglion at both the proximal and the distal poles and supply the ganglion cells. A dense capillary bed is formed by branches coursing parallel to the long axis of the ganglion. On the surface, branches of the polar arteries form a delicate network of vessels (the periganglionic plexus) that communicates with the deeper capillaries (Parke & Whalen, 2002).
The other (larger) branches of the neurospinal artery vary in number, and they anastomose with and reinforce the anterior or posterior spinal arteries, thus enhancing the circulation of the cord. Unfortunately, there is no consensus as to the name of these vessels. Some authors (Zhang et al., 1995; Bowen & Pattany, 1999; Krauss, 1999; Milen et al., 1999; Greathouse, Halle, & Dalley, 2001) consider these vessels to be separate branches and call them medullary arteries (the term medulla is a derivative of the Latin word for marrow and refers to the spinal cord). These authors believe that the medullary arteries contribute minimally or not at all to the vascularization of the roots. Others (Brockstein, Johns, & Gewertz, 1994; Lu et al., 1996; Tator & Koyanagi, 1997; Lo et al., 2002; White & El-Khoury, 2002) refer to the vessels that supply nerve roots and rootlets and then continue medially to reinforce the anterior or posterior spinal arteries as radiculomedullary arteries, because they are considered to be radicular branches that continue to anastomose with spinal arteries (Brockstein, Johns, & Gewertz, 1994) (see Figs. 3-15 and 3-16). This latter nomenclature is used in this text. Usually the anterior and posterior radiculomedullary arteries do not reach their respective spinal arteries at the same segmental level (Gillilan, 1958; Turnbull, Brieg, & Hassler, 1966).
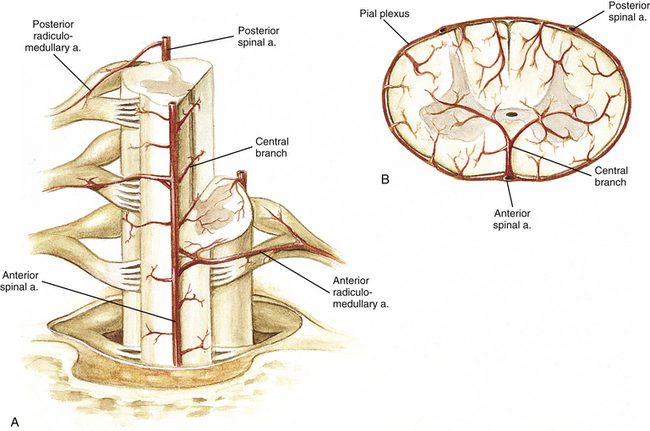
FIG. 3-16 Cross section of the spinal cord showing the arterial blood supply. A, Radiculomedullary arteries reinforce the spinal arteries. The anterior spinal artery gives off central branches. B, Note the spinal cord areas vascularized by the anterior spinal artery and by the posterior spinal arteries.
Anterior Radiculomedullary Arteries
Anterior radiculomedullary (feeder) arteries (defined here as those that reach the anterior spinal artery) vary in number from 5 to 10. Because the anterior spinal artery sufficiently supplies the first two or three cervical segments, the anterior radiculomedullary arteries of the vertebral, ascending and deep cervical, and highest intercostal arteries in general vascularize the lower cervical and upper thoracic segments, that is, the cervical enlargement (Lazorthes et al., 1971). Turnbull and colleagues (1966) conducted microdissection and microangiographic studies on the C3 to T1 cord segments of 43 cadavers. They discovered a range of one to six anterior radiculomedullary arteries, which were found as often on the left as on the right sides. Of the 43 spinal cords studied, 13 had a total of only 2 anterior radiculomedullary arteries, and another 13 had a total of 4 arteries reinforcing the cord. However, 39 of 43 had at least 1 anterior radiculomedullary artery at the C7 or C8 segment.
Anterior radiculomedullary arteries from the posterior intercostal and lumbar arteries are found primarily on the left side (Turnbull, 1973; Carpenter, 1991), possibly because the aorta is on the left. Although the thoracic cord segments are the longest, usually just one to four anterior radiculomedullary arteries are present. As an anterior radiculomedullary artery approaches the ventral median fissure, it often sends a branch into the pial plexus on the cord’s lateral side. It then bifurcates, usually branching gently up and sharply down before uniting with the anterior spinal artery. If both right and left anterior radiculomedullary arteries join the anterior spinal artery at the same level, the anastomosis becomes diamond shaped (Turnbull, Brieg, & Hassler, 1966).
The largest anterior radiculomedullary artery was first described in 1882 by Albert Adamkiewicz and subsequently named the artery of Adamkiewicz. It is also called the arteria radicularis magna of Adamkiewicz, great anterior medullary artery, segmental medullary artery, great radicular artery, arteria radiculo-medullaris, or the artery of the lumbar enlargement because of its area of distribution (Turnbull, 1973; Zhang et al., 1995; Bowen & Pattany, 1999; Krauss, 1999; Milen et al., 1999; Pawlina & Maciejewska, 2002). Although most anterior radiculomedullary arteries range from 0.2 to 0.8 mm in diameter, the diameter of the artery of Adamkiewicz is typically larger. Various reports describe it to range from 0.5 to 1.49 mm (Turnbull, 1973; Alleyne et al., 1998; Koshino et al., 1999; Biglioli et al., 2000). Generally the artery enters the vertebral canal most frequently (68% to 80% of the time) on the left side (Turnbull, 1973; Alleyne et al., 1998; Koshino et al., 1999; Biglioli et al., 2000) as a branch of a posterior intercostal or lumbar artery within the range of lower thoracic and upper lumbar levels (i.e., from T8 to L3) (Turnbull, 1973; Alleyne et al., 1998; Koshino et al., 1999; Biglioli et al., 2000). It also has been located with a midthoracic T5 to T8 root (15%), in which case a lower lumbar anterior radiculomedullary artery is present and is called the artery of the conus medullaris. Lo and colleagues (2002) reported an anatomic variation of the artery of Adamkiewicz (3 cases out of 4000 angiographies) in which the fourth lumbar artery supplied the anterior spinal artery of the conus medullaris. Before reaching the anterior spinal artery, the artery of Adamkiewicz supplements the posterior spinal artery by providing an anastomotic branch. When it reaches the anterior spinal artery, the artery of Adamkiewicz makes an abrupt caudal (“hairpin” in appearance) turn, dividing into a large descending and a small ascending branch (Lazorthes et al., 1971; Turnbull, 1973; Bowen & Pattany, 1999; Biglioli et al., 2000). This artery is the predominant source of blood to the lower two thirds or one half of the cord (deGroot & Chusid, 1988; Zhang et al., 1995; Standring et al., 2008). Zhang and colleagues (1995) calculated that the arterial resistance would be 14.8 times greater in the anterior spinal artery above the anastomosis with the artery of Adamkiewicz as compared to below it. This puts the lower thoracic cord, which relies on its blood supply from the artery of Adamkiewicz flowing in a cephalad direction, at a higher risk of ischemic damage than lumbar segments, if the artery of Adamkiewicz is compromised.
Because severe neurologic deficits can occur when the blood supply to the spinal cord is compromised (see anterior spinal artery syndrome under Arterial Supply of the Internal Cord), the location of this artery has important clinical relevance. In a cadaveric microsurgical anatomic study, Alleyne and colleagues (1998) described the location of the artery of Adamkiewicz within the IVF. The artery was located at the rostral or middle region, ventral and slightly rostral to the union of the dorsal root ganglion and ventral root (Alleyne et al., 1998). Another study (Murthy, Maus, & Behrns, 2011) examined spinal angiograms to assess the intraforaminal location of the artery of Adamkiewicz within the neural foramen in order to provide safer placement of a needle for thoracic and lumbar transforaminal epidural steroid injections. It has been suggested that during the injections neurologic deficits have occurred by occlusion of the artery by induced arterial vasospasm, direct needle injury with thrombosis, or arterial embolization with particulate steroids. Using the spinal angiograms, 120 arteries were identified: 83% were located on the left and 17% were located on the right; all were found between T2 and L3 with 92% located between T8 and L1; the highest incidence was 28% at the T10 level. The distribution of the locations of 113 arteries in neural foramina was determined at the mid-pedicular plane of the foramen. The results showed that 97% of the time the artery of Adamkiewicz was located in the upper half of the foramen; 88% were found in the upper third of the foramen; the middle third of the foramen was the location of 9%; and 2% were located in the lower third of the foramen. No arteries were found in the inferior one fifth of the foramen. It was suggested that the safest site of needle placement for an epidural injection at L3 and above is in an inferior and slightly posterior location within the foramen relative to the exiting spinal nerve. In another study (Charles et al., 2011), 100 spinal angiographies of patients who had undergone spinal surgery were reviewed in order to describe the topography of the artery of Adamkiewicz and analyze the relevance for anterior surgical approaches of the thoracolumbar spine. Spinal angiograms are routinely performed preoperatively on patients undergoing anterior or transforaminal surgery of the thoracolumbar spine. Knowledge of the topography of the artery of Adamkiewicz would allow surgeons to modify the surgical approach or surgical technique in order to avoid possible damage to the artery. The results of the 96 angiograms clearly indicating the artery of Adamkiewicz showed comparable data to previous studies—that is, the artery was always located between T8 and L3; 50% of the arteries were at the T9 or T10 level; 75% entered on the left side; and an additional radiculomedullary artery was present 43% of the time. In this study, this information resulted in modifications of the surgical technique in 13% of the patients.
Posterior Radiculomedullary Arteries
Posterior radiculomedullary arteries (defined here as those that reach the posterior spinal arteries) vary in number from 10 to 23 and outnumber the anterior radiculomedullary arteries. They range in diameter from 0.2 to 0.5 mm (Turnbull, 1973), which generally is smaller than an average anterior radiculomedullary artery, and they are largest in the cervical and lumbar cord segments (Bowen & Pattany, 1999). Turnbull and colleagues (1966) showed that in the C3 to T1 segments of 43 cadavers the number of posterior radiculomedullary arteries ranged from 0 to 8. Only two or three posterior radiculomedullary arteries were found in 75% of the cadavers, and of those, most were found coursing with lower cervical roots. No relationship seemed to exist between anterior and posterior radiculomedullary arteries concerning their number and position. Although posterior radiculomedullary arteries often enter on the left side, they are less prone to do so than the anterior radiculomedullary arteries (Carpenter, 1991). Also, as it nears the posterior spinal artery, which it reinforces, the posterior radiculomedullary artery often forms a small branch to the pial plexus on the cord’s lateral side.
Vascularization of the Lumbosacral Nerve Roots
The vascularization of the long lumbosacral roots (cauda equina) found within the thecal sac is of interest in that the roots have a dual blood supply. There are no radiculomedullary arterial branches arising along the length of the root. Instead, and as mentioned previously, the radicular arteries give off branches to the distal segments of the roots. The proximal segments of dorsal roots are supplied by direct radicular branches of the posterior spinal artery and the proximal segments of ventral roots are supplied by radicular branches from the anterior vasa corona of the cord (Parke, Gammel, & Rothman, 1981). The diameter of these longitudinally arranged radicular branches (proximal and distal) is maintained until they anatomose with each other in the middle of the fascicles of the roots. These arteries give off inter- and intrafascicular branches (Parke & Watanabe, 1985). Also, they often form large arteriovenous (AV) anastomoses throughout the length of the root. In addition to the vasculature, the CSF serves as an additional nutritional source for the lumbosacral roots. The nerve roots are bathed in CSF, which allows a metabolic exchange to occur between the two. The roots are surrounded by a refined gauzelike layer of pia that allows nutrients to be easily exchanged between CSF and the roots. By comparing the uptake of a labeled isotope injected intravenously into the subarachnoid space of pigs, it was shown that of the actual amount entering the root tissue, 35% came from a vasculature route and 58% was contributed by CSF (Rydevik et al., 1990). Compromising the blood supply as well as the CSF percolation of nutrients into the roots can cause edema and venous congestion, resulting in an inflamed and hypersensitive root segment that generates pain in response to further injury. It has been speculated that in the case of a single compression on a root the AV shunts would provide a mechanism for the roots to maintain their vascular supply and allow for some compensation for a focal compression. Therefore, it seems likely that compressions in two locations along a long root would isolate a root segment from its vascular supply (although it may retain some nutritional benefit from the CSF) and produce symptoms indicative of metabolic deprivation of the nerve root. Radicular veins also supply the roots (see Venous Drainage of the Spinal Cord) although their vascular pattern of distribution is different than the arterial pattern—that is, there are fewer veins, they do not travel with their corresponding artery, and they tend to be found in a more central and deeper location in the root. The radicular veins also have proximal and distal components (Parke & Watanabe, 1985). Because of the thin wall of the radicular veins and their position in the root, studies suggest that veins and venous flow are more susceptible to pathologic mechanical stresses and inflammation associated with degenerative conditions of the lower spine such as compressive injuries. This may result in edema and venous congestion when roots are compromised and subsequent alterations in nerve root function (Parke, 2005).
Arterial Supply of the Internal Cord
Having discussed the location of the spinal arteries on the external surface of the spinal cord, it is pertinent to elaborate on the vascularization of the underlying nervous tissue. The spinal cord tissue is vascularized by branches of the anterior spinal artery, posterior spinal anastomotic channels, and their interconnecting vessels. The unpaired anterior spinal artery that lies in the ventral median fissure periodically gives off a single branch, which is called the central, or sulcal, branch (Fig. 3-16). Compared with the distance between central branches in the cervical cord, the distance is greater between these branches in thoracic segments and is less between branches in lumbar and sacral segments (Hassler, 1966). The central branch emerges at right angles in the lumbar and sacral segments, but arises at an acute superior or inferior angle in the cervical and thoracic cord (Hassler, 1966; Turnbull, 1973). Its length is approximately 4.5 mm, and the central branch courses deep into the fissure, alternately turning to the left or right.
In addition, the central branches are more numerous and larger in the cervical and lumbar regions and less frequent in the thoracic region (Gillilan, 1958; Turnbull, 1973; Carpenter, 1991; Kiernan, 2009). In the human cord these arteries number between 250 and 300 (Gillilan, 1958). The anterior spinal artery produces 5 to 8 central branches per centimeter in the cervical region, 2 to 6 branches per centimeter in the thoracic region, and 5 to 12 branches per centimeter in the lumbar and sacral regions (Turnbull, 1973). The widest average diameter of the central branch is in the lumbar region (0.23 mm), followed by the cervical (0.21 mm), upper sacral (0.20 mm), and thoracic (0.14 mm) regions. While the branches of the central arteries vascularize the cord, they extend superiorly and inferiorly and overlap each other, particularly in the lumbar and sacral segments. Tator and Koyanagi (1997) studied a small sample of cervical cords and determined that the overlap was such that each segment of gray matter was vascularized by capillaries from two to three central arteries.
In addition to the vascularization of the deep tissue, an interconnecting plexus of arteriolar size vessels called the pial peripheral plexus, or vasa corona, is located in the pia mater encircling the spinal cord. The dorsal and ventral aspects of the pial plexus are formed from small branches of the posterior and anterior spinal arteries, respectively. The lateral aspects of the pial plexus are formed by branches from both spinal arteries and occasionally from small branches of the radicular (radiculomedullary) arteries (Gillilan, 1958; Turnbull, 1973; Carpenter, 1991; Kiernan, 2009). The pial plexus vascularizes a band of white matter on the periphery of the spinal cord. Much overlap occurs between the distributions of the central arteries and the peripheral plexus. When one considers all branches, the anterior spinal artery vascularizes nearly the ventral two thirds of the cross-sectional area of the spinal cord. This area includes the ventral horn, lateral horn, central gray matter, base of the dorsal horn, and the majority of the ventral and lateral funiculi. The posterior spinal artery, in conjunction with the pial plexus, vascularizes the remainder of the cord. This includes the bulk of the dorsal horn and dorsal funiculus and outer rim of the lateral and ventral funiculi (Tator & Koyanagi, 1997; Afifi & Bergman, 1998; Bowen & Pattany, 1999; Krauss, 1999; Nolte, 2002) (Fig. 3-16, B). In the C3 to T1 segments, the top of the dorsal horn is particularly well vascularized (Turnbull, Brieg, & Hassler, 1966).
When one studies the details of the arterial supply, it is apparent that at any given level of the spinal cord, a direct relationship exists between the size of the anterior spinal artery and radiculomedullary artery and the amount of gray matter, as seen in cross section (Gillilan, 1958). Because gray matter has a higher metabolic rate than white matter (Gillilan, 1958) and includes the regions of highest neuronal density, the capillaries are denser in gray matter, especially around the top of the dorsal horn and the ventral horn cells, than in white matter. In addition, studies suggest that because there are more neurons in the cervical and lumbosacral regions and these neurons have a high metabolic rate because they innervate the extremities, these levels contain a richer capillary bed than found in the midthoracic region (Duggal & Lach, 2002). Concerning the white matter of the cord, the dorsal and lateral white funiculi have a more extensive capillary supply than the ventral white funiculus (Turnbull, 1973).
The distribution of the anterior and posterior spinal arteries to the cross-sectional area of the spinal cord is clinically important and much research has been devoted to this topic. Any interruption of the vascular supply to the spinal cord (aorta, segmental arteries, radiculomedullary branches, spinal arteries, and intrinsic vessels) can cause ischemia and cell necrosis, resulting in serious functional deficits.
Because the spinal arteries alone are unable to supply the spinal cord sufficiently, the radiculomedullary supply is critical. In the middle to upper thoracic spinal cord segments, especially around T4-6, reinforcing radiculomedullary arteries originating from posterior intercostal segmental arteries are scarce; therefore this area has been described as a watershed zone of ischemic vulnerability. For example, ischemia can develop if one radiculomedullary artery is occluded and another is insufficient. Duggal and Lach (2002) studied 66 human spinal cords demonstrating global ischemic changes resulting from cardiac arrest or a severe hypotensive episode. In all cases, histologic changes were seen in the gray matter (in the large neurons in the ventral horn and in Clarke’s nucleus of the low thoracic and lumbar segments), but only in the most severe cases was a narrow rim of white matter affected. In addition, their data showed that the area that most commonly revealed ischemic change was the lumbosacral region (69.7%). The thoracic cord was affected only 7.6% of the time and always in association with another cord region. It is possible that the large number of metabolically active neurons in the ventral horn of the lumbosacral region explains these results, even though the thoracic cord is called the watershed zone based on the anatomic studies of its regional blood supply.
Because the artery of Adamkiewicz is typically the only artery to supply a significant amount of blood to the lumbosacral cord, it is of supreme clinical, surgical, and radiologic importance. Unfortunately, the blood flow in this artery, and in any radiculomedullary artery, can be directly or indirectly disrupted for various reasons, including the following: during surgical treatment of descending thoracic and thoracoabdominal aortic aneurysms (Alleyne et al., 1998; Koshino et al., 1999; Biglioli et al., 2000; FitzGerald & Folan-Curran, 2002); thoracic aortic dissection causing an expanding blood clot in the aortic wall (Snell, 2001); leaking aneurysm putting direct pressure on the lumbar arteries (Snell, 2001); acute aortic dissection (which commonly presents as an abrupt onset of pain localized to the chest, neck, or back with additional signs of systemic distress) (Patel, Curtis, & Weiner, 2002); elective coronary artery bypass grafting resulting in microembolization of atherosclerotic plaques or cholesterol emboli that then enter a segmental artery (Geyer, Maik, & Pillai, 2002); hereditary protein S deficiency (a congenital deficiency in coagulation inhibitors) (Ramelli et al., 2001); and postural compression of the second right lumbar artery by the diaphragmatic crus (Rogopoulos et al., 2000). Also, any obstruction to venous return resulting in spinal cord edema (FitzGerald & Folan-Curran, 2002), and any operation in which severe hypotension occurs (Snell, 2001), can compromise blood flow to the spinal cord, resulting in loss of cord function.
Although less common, direct occlusion of the anterior spinal artery may occur from such circumstances as thrombus or embolus formation, herniated disc compression, and tumor development. Such occlusion results in spinal cord ischemia (Gillilan, 1958; Turnbull, 1973; Morris & Phil, 1989). Lesions affecting the anterior spinal arterial region of distribution are more common than those affecting the posterior spinal arterial region (Gillilan, 1958; Daube et al., 1986).
The consequences of an infarct are devastating in terms of the loss of function. Damage is to the areas that house cell bodies of motor neurons (ventral horn), descending motor pathways (lateral white matter), and the ascending pain and temperature pathways (ventrolateral white matter) (see Chapter 9). In these cases the posterior spinal arterial distribution to the dorsal one third of the spinal cord, which includes the pathway for vibration, position sense, and discriminatory touch (dorsal white funiculus), is left intact. Several cases in the late 1800s and early 1900s established that a lesion causing insufficient vascularization to the area of distribution of the anterior spinal artery could frequently and clearly be diagnosed clinically (Gillilan, 1958). This lesion has subsequently been called the anterior spinal artery syndrome and presents with sudden bilateral loss of pain and temperature sense below the level of the lesion with a preservation of vibratory and position sense (dissociated sensory loss), motor deficits (usually paraplegia) below the level of the lesion, and loss of bladder and bowel control. Painful dysesthesia develops in some patients approximately 6 to 8 months after the onset of symptoms. This phenomenon is explained either by the misinterpretation of sensory input by the brain, resulting from the imbalance created between the lesioned ventrolateral white matter and normal dorsal white matter, or by a sparing of the spinoreticulothalamic tract, which allows nociceptive impulses to pass to the reticular formation and thalamus (Afifi & Bergman, 1998).
Venous Drainage of the Spinal Cord
The venous system found within the vertebral canal consists of an epidural, or internal, vertebral venous plexus, located in the epidural space (see Chapter 2), and also an irregular venous plexus lying on the cord. This entire venous system is devoid of valves and anastomoses freely with itself. Please see Chapter 2 for a full description of the internal and external vertebral venous plexuses.
The venous system of the cord is a tortuous plexus that consists of six variable longitudinal veins: three anterior and three posterior (Fig. 3-17, A). The anteromedian vein is located deep in the ventral median fissure and has an average diameter ranging between 0.5 and 1.5 mm. It is a single vessel in the lumbar region but is accompanied by two anterolateral veins in the thoracic and cervical segments (Bowen & Pattany, 1999). These anterior venous channels receive blood from the neural tissue via sulcal veins and subsequently drain into anterior radiculomedullary veins. (Note: The nomenclature used for these vessels is inconsistent [Brockstein, Johns, & Gewertz, 1994; Bowen & Pattany, 1999; Krauss, 1999]. This text uses radiculomedullary to identify the veins draining the cord [which are variable in number] and roots [which are present at every level].) The anterior radiculomedullary vessels range in number from 10 to 20 (Bowen & Pattany, 1999), and empty into the epidural venous plexus. In the lumbar region, there may be one large anterior radiculomedullary vein, the vena radicularis magna (Carpenter, 1991; Brockstein, Johns, & Gewertz, 1994; Bowen & Pattany, 1999; Krauss, 1999), which usually courses with one of the left nerve roots located between the T11 and L3 spinal cord segments. The posterior aspect of the cord has a similar venous pattern. A midline posteromedian vein is the dominant vessel in the lumbar area with two posterolateral veins accompanying it in other regions. These drain the dorsal funiculus, dorsal gray horns, and their adjacent lateral white matter. These posteromedian and posterolateral veins in turn become tributaries of posterior radiculomedullary veins, which number the same as, or more than, the anterior radiculomedullary veins (Bowen & Pattany, 1999). The posterior radiculomedullary veins also empty into the epidural venous plexus. At the level of the dorsal root ganglion, the posterior radiculomedullary vein receives blood from the parenchyma of the dorsal root ganglion via a periganglionic venous plexus located on the surface of the ganglion. Because this plexus lies immediately underneath the dura, an external force could compress the dura onto the underlying plexus and impede venous flow. This would alter the permeability of the vessel wall and lead to endoneurial edema. The edema would press the venous plexus against its overlying covering of dura, resulting in chronic venostasis. If this condition continued it could lead to increased sensitivity, endoneurial fibrosis, and ectopic firing of the ganglion neurons (Parke & Whalen, 2002). A large posterior radiculomedullary vein also is found in the lumbar region, and usually is seen accompanying a L1 or L2 root. Using MR angiography images, the intersection of either an anterior or a posterior radiculomedullary vein with a spinal vein (e.g., anteromedian vein) can be identified by its “coat hook” appearance, which distinguishes it from the “hairpin” look of the artery of Adamkiewicz (Bowen & Pattany, 1999). As also seen in the arterial system, an encompassing venous vasa corona interconnects the six longitudinal veins.
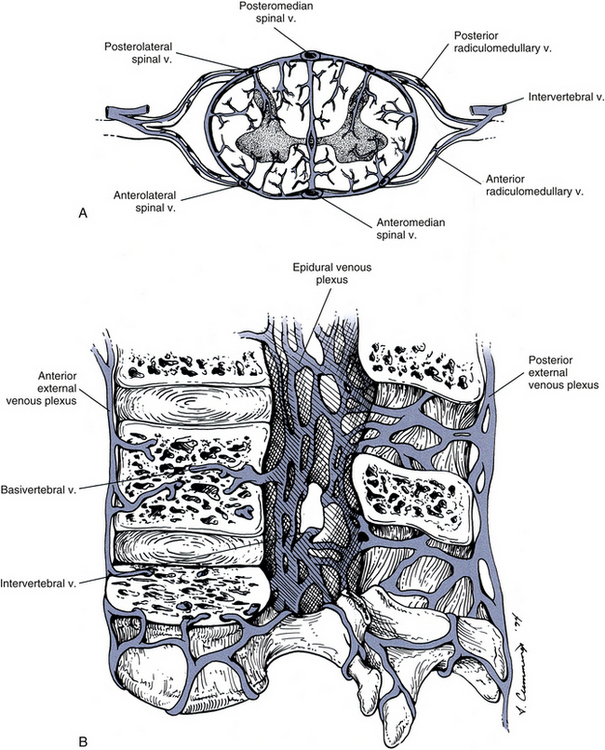
FIG. 3-17 Venous drainage of the spinal cord. A, Cross section of the spinal cord. B, Median view of the vertebral canal. The spinal cord has been removed to show the epidural venous plexus.
The epidural (internal vertebral) venous plexus, which drains blood not only from the cord but also from bone and red bone marrow, consists of several anterior and posterior longitudinal and interconnecting vessels. At the level of the foramen magnum, it forms a dense network that communicates with vertebral veins. In addition, it is continuous with the dural venous sinuses (occipital and sigmoid) and venous channels (basilar plexus, venous plexus of the hypoglossal canal, and the emissary veins of the condyles [Standring et al., 2008]) within the skull (see Chapter 2). This plexus also drains into intervertebral veins in the IVFs and also into another longitudinally arranged plexus, the external vertebral venous plexus (Fig. 3-17, B). From intervertebral veins, venous blood drains into segmental veins such as the vertebral, intercostal, lumbar, and lateral sacral veins (Standring et al., 2008). These segmental veins lie outside the vertebral column. Chapter 2 provides a full description of the external and internal vertebral venous plexuses.
A metastatic tumor in the epidural space may damage the cord by impeding venous return and cause vasogenic edema (Grant et al., 1991). Also, because the internal vertebral venous plexus lacks valves, blood flow through the intervertebral veins (which communicate with segmental veins) is allowed to be reversed. This provides the opportunity for cancer to metastasize from such areas as the prostate, lung, breast, and thyroid gland to the brain and vertebral bodies (FitzGerald & Folan-Curran, 2002; Standring et al., 2008) (see Chapter 2).
References
Afifi, A.K., Bergman, R.A. Functional neuroanatomy: text and atlas. St Louis: McGraw-Hill; 1998.
Alleyne, C.H., Jr., et al. Microsurgical anatomy of the artery of Adamkiewicz and its segmental artery. J Neurosurg. 1998;89:791–795.
Arai, Y., et al. Magnetic resonance imaging observation of the conus medullaris. Bull Hosp Jt Dis. 2001;60:10–12.
Azerad, J., et al. Afferent fibres in cat ventral roots: electrophysiological and histological evidence. J Physiol. 1986;379:229–243.
Barbaix, E., et al. Anterior sacrodural attachments: Trolard's ligaments revisited. Man Ther. 1996;2:88–91.
Benarroch, E.E., et al. Medical neurosciences: an approach to anatomy, pathology, and physiology by systems and levels, 4th ed. Baltimore: Lippincott Williams & Wilkins; 1999.
Biglioli, P., et al. The anterior spinal artery: the main arterial supply of the human spinal cord: a preliminary anatomic study. J Thorac Cardiovasc Surg. 2000;119:376–379.
Boulton, M., et al. Drainage of CSF through lymphatic pathways and arachnoid villi in sheep: measurement of 125I-albumin clearance. Neuropathol Appl Neurobiol. 1996;22:325–333.
Bowen, B.C., Pattany, P.M. Vascular anatomy and disorders of the lumbar spine and spinal cord. Magn Reson Imaging Clin North Am. 1999;7:555–571.
Brockstein, B., Johns, L., Gewertz, B.L. Blood supply to the spinal cord: anatomic and physiologic correlations. Ann Vasc Surg. 1994;8:394–399.
Bui, C.J., Tubbs, R.S., Oakes, W.J. Tethered cord syndrome in children: a review. Neurosurg Focus. 2007;23(2):E2.
Carpenter, M.B. Core text of neuroanatomy, 4th ed. Baltimore: Williams & Wilkins; 1991.
Charles, Y.P., et al. Relevance of the anatomical location of the Adamkiewicz artery in spine surgery. Surg Radiol Anat. 2011;33:3–9.
Choi, D., Carroll, N., Abrahams, P. Spinal cord diameters in cadaveric specimens and magnetic resonance scans, to assess embalming artefacts. Surg Radiol Anat. 1996;18:133–135.
Coggeshall, R.E. Law of separation of function of the spinal roots. Physiol Rev. 1980;60:716–755.
Coggeshall, R.E., Coulter, J.D., Willis, W.D., Jr. Unmyelinated fibers in the ventral root. Brain Res. 1973;57:229–233.
Daube, J.R., et al. Medical neurosciences, 2nd ed. Boston: Little, Brown; 1986.
Davidoff, R.A., Hackman, J.C. Aspects of spinal cord structure and reflex function. Neurol Clin. 1991;9:533–550.
Dean, N.A., Mitchell, B.S. Anatomic relation between the nuchal ligment (ligamentum nuchae) and the spinal dura mater in the craniocervical region. Clin Anat. 2002;15:182–185.
deGroot, J., Chusid, J.G. Correlative neuroanatomy, 20th ed. East Norwalk, Conn: Appleton & Lange; 1988.
Demiryürek, D., et al. MR imaging determination of the normal level of conus medullaris. Clin Imag. 2002;26:375–377.
Djindjian, M., et al. The normal vascularization of the intradural filum terminale in man. Surg Radiol Anat. 1988;10:201–209.
Duggal, N., Lach, B. Selective vulnerability of the lumbosacral spinal cord after cardiac arrest and hypotension. Stroke. 2002;33:116–126.
Edsbagge, M., et al. Spinal cerebrospinal fluid volume in healthy elderly individuals. Clin Anat. 2011;24:733–740.
Elliott, H.C. Cross-sectional diameters and areas of the human spinal cord. Anat Rec. 1945;93:287–293.
Er, U., Fraser, K., Lanzino, G. The anterior spinal artery origin: a microanatomical study. Spinal Cord. 2008;46:45–49.
FitzGerald, M.J.T., Folan-Curran, J. Clinical neuroanatomy and related neuroscience, 4th ed. Edinburgh: WB Saunders; 2002.
Fountas, K.N., et al. Cervical spinal cord: smaller than considered? Spine. 1998;23:1513–1516.
Fricke, B., Andres, K.H., Von During, M. Nerve fibers innervating the cranial and spinal meninges: morphology of nerve fiber terminals and their structural integration. Microsc Res Tech. 2001;53:96–105.
Fujiwara, K., et al. Morphometry of the cervical spinal cord and its relation to pathology in cases with compression myelopathy. Spine. 1988;13:1212–1216.
Gatonga, P., Ogeng'o, J.A., Awori, K.O. Spinal cord termination in adult Africans: relationship with intercristal line and the transumbilical plane. Clin Anat. 2010;23:563–565.
Geyer, T.E., Maik, M.J., Pillai, R. Anterior spinal artery syndrome after elective coronary artery bypass grafting. Ann Thorac Surg. 2002;73:1971–1973.
Gillilan, L.A. The arterial blood supply of the human spinal cord. J Comp Neurol. 1958;110:75–103.
Gövsa, F., et al. Origin of the anterior spinal artery. Surg Radiol Anat. 1996;18:189–193.
Grant, R., et al. Changes in intracranial CSF volume after lumbar puncture and their relationship to post-LP headache. J Neurol Neurosurg Psychiatry. 1991;54:440–442.
Greathouse, D.G., Halle, J.S., Dalley, A.F., II. Blood supply to the spinal cord. Phys Ther. 2001;81:1264–1265.
Groen, G.J., Baljet, B., Drukker, J. The innervation of the spinal dura mater: anatomy and clinical implications. Acta Neurochir (Wien). 1988;92:39–46.
Hackney, D.B. Normal anatomy. Top Magn Reson Imaging. 1992;4:1–6.
Haines, D.E., Harkey, H.L., Al-Mefty, O. The “subdural” space: a new look at an outdated concept. Neurosurgery. 1993;32:111–120.
Hamandi, K., et al. Irreversible damage to the spinal cord following spinal anesthesia. Neurology. 2002;59:624–626.
Hansasuta, A., Tubbs, R.S., Oakes, W.J. Filum terminale fusion and dural sac termination: study in 27 cadavers. Pediatr Neurosurg. 1999;30:176–179.
Hassler, O. Blood supply to the human spinal cord. Arch Neurol. 1966;15:302–307.
Hewitt, W. The intervertebral foramen. Physiotherapy. 1970;56:332–336.
Hogan, Q.H., et al. Magnetic resonance imaging of cerebrospinal fluid volume and the influence of body habitus and abdominal pressure. Anesthesiology. 1996;84:1341–1349.
Hugh, A.E. The subdural space of the spine: a lymphatic sink? Myodil's last message. Clin Anat. 2010;23:829–839.
Humphreys, B.K., et al. Investigation of connective tissue attachments to the cervical spinal dura mater. Clin Anat. 2003;16:152–159.
Johnston, M., Papaiconomou, C. Cerebrospinal fluid transport: a lymphatic perspective. News Physiol Sci. 2002;17:227–230.
Kameyama, T., Hashizume, Y., Sobue, G. Morphologic features of the normal human cadaveric spinal cord. Spine. 1996;21:1285–1290.
Kandel, E.R., Siegelbaum, S.A. Overview of synaptic transmission. In Kandel E.R., Schwartz J.H., Jessell T.M., eds.: Principles of neural science, 4th ed., St Louis: McGraw-Hill, 2000.
Kershner, D., Binhammer, R. Lumbar intrathecal ligaments. Clin Anat. 2002;15:82–87.
Kiernan, J.A. Barr's the human nervous system, 9th ed. Philadelphia: Lippincott Williams & Wilkins; 2009.
Ko, H.-Y., et al. Unmyelinated fibers in human spinal ventral roots: C4 to S2. Spinal Cord. 2009;47:286–289.
Koshino, T., et al. Does the Adamkiewicz artery originate from the larger segmental arteries? J Thorac Cardiovasc Surg. 1999;117:898–905.
Krauss, W.E. Vascular anatomy of the spinal cord. Neurosurg Clin North Am. 1999;10:9–15.
Lad, S.P., et al. Tethered cord syndrome: nationwide inpatient complications and outcomes. Neurosurg Focus. 2007;23(2):E3.
Laterra, J., Goldstein, G.W. Ventricular organization of cerebrospinal fluid: blood-brain barrier, brain edema, and hydrocephalus. In Kandel E.R., Schwartz J.H., Jessell T.M., eds.: Principles of neural science, 4th ed., St Louis: McGraw-Hill, 2000.
Lazorthes, G., et al. Arterial vascularization of the spinal cord. J Neurosurg. 1971;35:253–262.
Lo, D., et al. Unusual origin of the artery of Adamkiewicz from the fourth lumbar artery. Neuroradiology. 2002;44:153–157.
Lu, J., et al. Vulnerability of great medullary artery. Spine. 1996;21:1852–1855.
Macdonald, A., et al. Level of termination of the spinal cord and the dural sac: a magnetic resonance study. Clin Anat. 1999;12:149–152.
Makino, M., et al. Morphometric study of myelinated fibers in human cervical spinal cord white matter. Spine. 1996;21(9):1010–1016.
Malas, M.A., et al. The relationship between the lumbosacral enlargement and the conus medullaris during the period of fetal development and adulthood. Surg Radiol Anat. 2000;22:163–168.
Milen, M.T., et al. Albert Adamkiewicz, 1850–1921: his artery and its significance for the retroperitoneal surgeon. World J Urol. 1999;17:168–170.
Milhorat, T.H., Kotzen, R.M., Anzil, A.P. Stenosis of central canal of spinal cord in man: incidence and pathological findings in 232 autopsy cases. J Neurosurg. 1994;80:716–722.
Miura, M., Kato, S., von Ludinghausen, M. Lymphatic drainage of the cerebrospinal fluid from monkey spinal meninges with special reference to the distribution of the epidural lymphatics. Arch Histol Cytol. 1998;61:277–286.
Mokri, B. The Monro-Kellie hypothesis. Neurology. 2001;56:1746–1748.
Moore, K.L., Persaud, T.V.N. The developing human: clinically oriented embryology, 6th ed. Philadelphia: WB Saunders; 1998.
Morris, J.H., Phil, D. The nervous system. In Cotran R.S., Kumar V., Robbins S.L., eds.: Robbins pathologic basis of disease, 4th ed., Philadelphia: WB Saunders, 1989.
Murthy, N.S., Maus, T.P., Behrns, C.L. Intraforaminal location of the great anterior radiculomedullary artery (artery of Adamkiewicz): a retrospective review. Pain Medicine. 2010;11:1756–1764.
Nolte, J. The human brain: an introduction to its functional anatomy, 5th ed. St Louis: Mosby; 2002.
Nordqvist, L. The sagittal diameter of the spinal cord and subarachnoid space in different age groups. A roentgenographic post-mortem study. Acta Radiol. 1964;227:1–96.
Parke, W.W. Role of epidural and radicular veins in chronic back pain and radiulopathy. In Kambin P., ed.: Arthoscopic and endoscopic spinal surgery text and atlas, 2nd ed., Totowa, NJ: Humana Press, 2005.
Parke, W.W., Gammell, K., Rothman, R.H. Arterial vascularization of the cauda equina. J Bone Joint Surg. 1981;63A:53–62.
Parke, W.W., Watanabe, R. The intrinsic vasculature of the lumbosacral spinal nerve roots. Spine. 1985;10:508–515.
Parke, W.W., Whalen, J.L. The vascular pattern of the human dorsal root ganglion and its probable bearing on a compartment syndrome. Spine. 2002;27:347–352.
Patel, N.M., Curtis, R., Weiner, B.K. Aortic dissection presenting as an acute cauda equina syndrome: a case report. J Bone Joint Surg Am. 2002;84A:1430–1432.
Pawlina, W., Maciejewska, I. Albert Wojciech Adamkiewicz, 1850–1921. Clin Anat. 2002;15:318–320.
Pinto, F.C.G., et al. Anatomic study of the filum terminale and its correlations with the tethered cord syndrome. Neurosurgery. 2002;51:725–730.
Pollay, M. The function and structure of the cerebrospinal fluid outflow system. Cerebrospinal Fluid Res. 2010;7:1–20.
Ramelli, G.P., et al. Anterior spinal artery syndrome in an adolescent with protein S deficiency. J Child Neurol. 2001;16:134–135.
Reimann, A.F., Anson, B.J. Vertebral level of termination of the spinal cord with report of a case of sacral cord. Anat Rec. 1944;88:127–138.
Reina, M.A., et al. New perspectives in the microscopic structure of human dura mater in the dorsolumbar region. Reg Anesth. 1997;22:161–166.
Reina, M.A., et al. Does the subdural space exist? Rev Esp Anestesiol Reanim. 1998;45:367–376.
Reina, M.A., et al. The origin of the spinal subdural space: ultrastructure findings. Anesth Analg. 2002;94:991–995.
Rexed, B. The cytoarchitectonic organization of the spinal cord in the cat. J Comp Neurol. 1952;96:415–495.
Reynolds, F. Damage to the conus medullaris following spinal anaesthesia. Anaesthesia. 2001;56:238–247.
Risling, M., et al. Electron microscopic and immunohistochemical evidence that unmyelinated ventral root axons make U-turns or enter the spinal pia mater. J Comp Neurol. 1984;225:53–63.
Risling, M., Hildebrand, C. Occurrence of unmyelinated axon profiles at distal, middle and proximal levels in the ventral root L7 of cats and kittens. J Neurol Sci. 1982;56:219–231.
Rogopoulos, A., et al. Lumbar artery compression by the diaphragmatic crus: a new etiology for spinal cord ischemia. Ann Neurol. 2000;48:261–264.
Rydevik, B., et al. Diffusion from the cerebrospinal fluid as a nutritional pathway for spinal nerve roots. Acta Physiol Scand. 1990;138:247–248.
Saifuddin, A., Burnett, S.J.D., White, J. A magnetic resonance imaging study. Spine. 1998;23:1452–1456.
Santos-Franco, J., et al. Microsurgical considerations of the anterior spinal and the anterior-ventral spinal arteries. Acta Neurochir (Wien). 2006;148:329–338.
Schoenen, J. Clinical anatomy of the spinal cord. Neurol Clin. 1991;9:503–532.
Snell, R.S. Clinical neuroanatomy for medical students, 5th ed. Baltimore: Lippincott Williams & Wilkins; 2001.
Standring, S., et al. Gray's anatomy: the anatomical basis of clinical practice, 40th ed. Edinburgh: Churchill Livingstone; 2008.
Storer, K.P., et al. The central canal of the human spinal cord: a computerised 3-D study. J Anat. 1998;192:565–572.
Tator, C.H., Koyanagi, I. Vascular mechanisms in the pathophysiology of human spinal cord injury. J Neurosurg. 1997;86:483–492.
Tubbs, R.S., et al. The denticulate ligament: anatomy and functional significance. J Neurosurg Spine. 2001;94:271–275.
Turnbull, I.M. Blood supply of the spinal cord: normal and pathological considerations. Clin Neurosurg. 1973;20:56–84.
Turnbull, I.M., Brieg, A., Hassler, O. Blood supply of cervical spinal cord in man. J Neurosurg. 1966;24:951–966.
Vandenabeele, F., Creemers, J., Lambrichts, I. Ultrastructure of the human spinal arachnoid mater and dura mater. J Anat. 1996;189:417–430.
Weller, R.O. Microscopic morphology and histology of the human meninges. Morphologie. 2005;89:22–34.
White, M.L., El-Khoury, G.Y. Neurovascular injuries of the spinal cord. Eur J Rad. 2002;42:117–126.
Williams, P.L., et al. Gray's anatomy, 38th ed. Edinburgh: Churchill Livingstone; 1995.
Willis, W.D., Jr., Coggeshall, R.E. Sensory mechanisms of the spinal cord, 2nd ed. New York: Plenum Press; 1991.
Yamada, S., Lonser, R.R. Adult tethered cord syndrome. J Spin Disord. 2000;13:319–323.
Yasui, K., et al. Age-related morphologic changes of the central canal of the human spinal cord. Acta Neuropathol. 1999;97:253–259.
Zhang, T., et al. The size of the anterior spinal artery in relation to the arteria medullaris magna anterior in humans. Clin Anat. 1995;8:347–351.