Development of the Spine and Spinal Cord
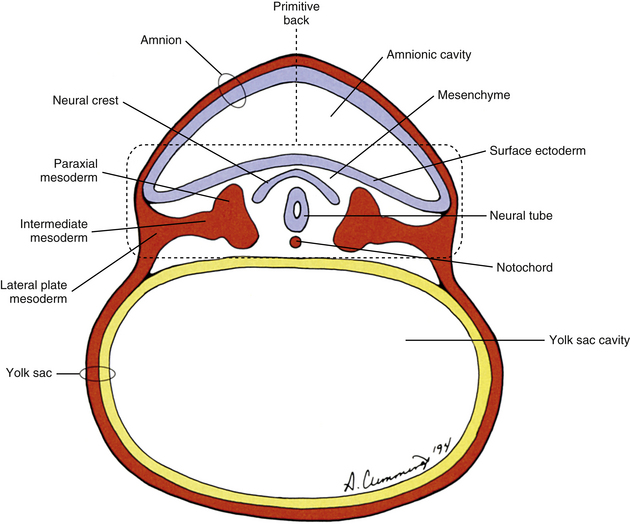
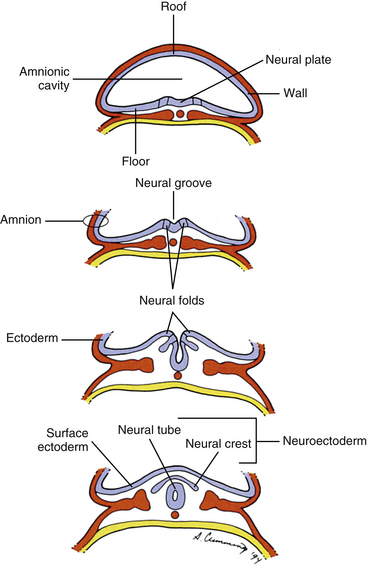
The human back can be identified as early as the end of the third week of development when the embryo consists of a disc of cells between the floor of the amnionic cavity and the roof of the yolk sac cavity (Fig. 12-1). Because the embryo is a flattened disc of cells, no complete body wall (i.e., no sides or front) exists, only the back (Brash, 1951b). Even at this early point, all the primordia that comprise the definitive back are present: the surface ectoderm, neural tube, neural crest, notochord, and paraxial mesoderm. The posterior boundary of the back is the surface ectoderm flooring the amnionic cavity, and its anterior boundary is the endoderm roofing the yolk sac cavity.
Blastocyst
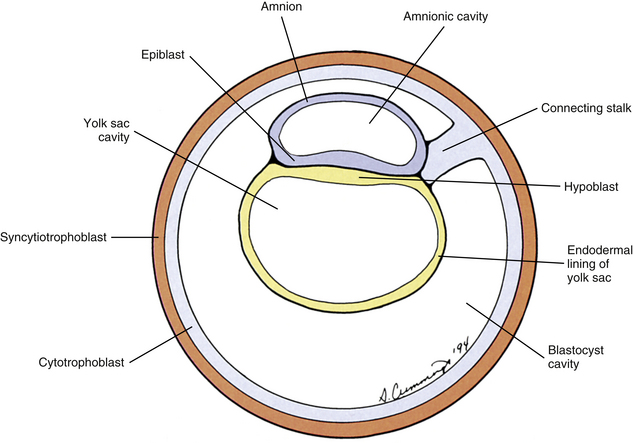
The human begins as the fertilized ovum, which cleaves itself into smaller and smaller embryonic cells known as blastomeres, each carrying all the genetic information needed to build any tissue or organ in the body (Standring et al., 2008). These cells at first form a solid sphere, but rearrangement and continued cell division produce a hollow sphere known as the blastocyst (Patten, 1964). The blastocyst is a single cell layer thick except at one pole, where the cells form into a disc that is two cell layers thick (Brash, 1951b) (Fig. 12-2, A). This disc is called the inner cell mass to distinguish it from all the other cells, which are referred to collectively as the trophoblast (Mossman, 1987). The hole in the center of the blastocyst is known as the blastocyst cavity.
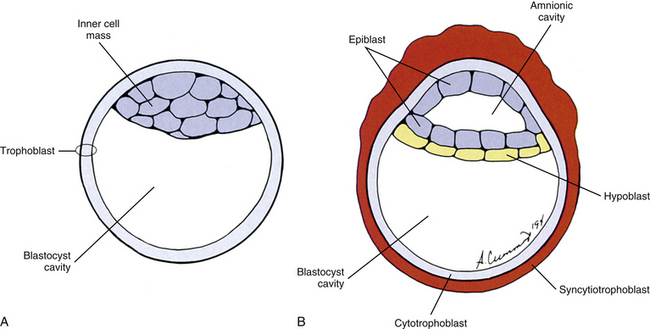
FIG. 12-2 Cross section of the blastocyst. A, At the beginning of the second embryonic week. B, In the middle of the second embryonic week.
The inner cell mass and the trophoblast have different fates. The former goes on to form the embryo proper. The latter can be likened to embryonic scaffolding that eventually is dismantled or discarded. Before that happens, trophoblast cells proliferate by mitosis until they are several cell layers thick. The innermost layer of the trophoblast still lines the blastocyst cavity. It remains cellular and is called the cytotrophoblast (Baxter, 1953). The cells in the outermost layer begin to lose their cell membranes, thus forming a syncytium known as the syncytiotrophoblast (Standring et al., 2008) (Fig. 12-2, B). This syncytium releases proteolytic enzymes that enable the blastocyst to digest its way into the lining of the uterus, called the endometrium. This process is known as implantation and usually is completed by the end of the second embryonic week. The implanted blastocyst does not leave the uterine wall until it bursts out in the form of a newborn (Beck et al., 1985).
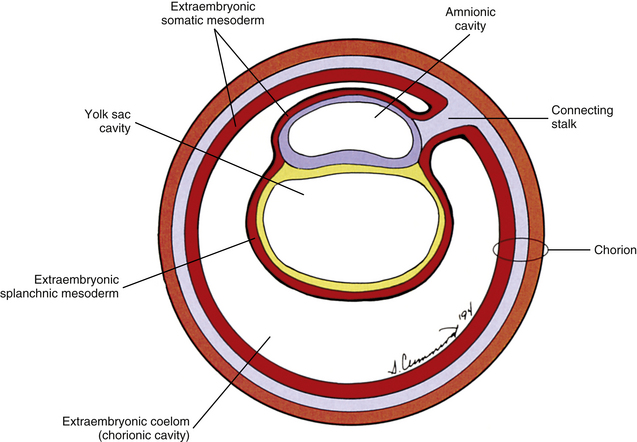
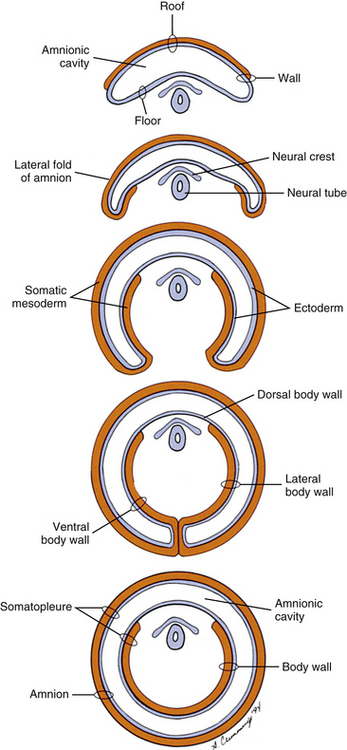
Also during the second week of development, the inner cell mass has been changing (Standring et al., 2008). A space, the first sign of the amnionic (amniotic) cavity, has appeared within it (see Fig. 12-2, B). The amnionic cavity can be thought of as being enclosed by walls, a roof, and a floor (Langebartel, 1977). The roof and walls become the amnion. The floor becomes the dorsal surface of the embryo and later transforms into the outer layer of the skin on the newborn’s back (Sadler, 2010). By this time the inner cell mass is attached to the cytotrophoblast by only a narrow bridge of cells that is called the connecting stalk (Fig. 12-3). The connecting stalk marks the tail or caudal end of the embryo just as the amnion marks its dorsal surface. A close observer would note that the cells in the inner cell mass have grouped themselves into two layers (see Fig. 12-2, A). One layer floors the amnionic cavity and is called the epiblast. The other layer roofs the blastocyst cavity and is called the hypoblast (Moore & Persaud, 2008). The hypoblast develops into a layer of cells that eventually lines the blastocyst cavity. The blastocyst cavity then is known as the primitive yolk sac even though it contains a negligible amount of yolk (Scammon, 1953) (see Fig. 12-3).
The yolk sac cells next bud off other cells that deposit themselves on the outside of the yolk sac, where they constitute a layer called extraembryonic mesoderm (Moore & Persaud, 2008) (Fig. 12-4). The triple layer, consisting of the cytotrophoblast sandwiched between the syncytiotrophoblast and extraembryonic mesoderm, is called the chorion and is the embryonic portion of the placenta (Goss, 1966). That means the cavity inside the chorion actually corresponds to the previous blastocyst cavity or primitive yolk sac (Sadler, 2010). To recognize the changes that have occurred (e.g., the imminent appearance of the secondary or definitive yolk sac and extraembryonic mesoderm), this cavity is renamed the extraembryonic coelom and is also called the chorionic cavity (Goss, 1966) (see Fig. 12-4).
Gastrulation and Development of the Notochord
At the beginning of the third week of development, a narrow groove forms on the caudal portion of the dorsal surface of the epiblast known as the primitive streak (Hamilton & Mossman, 1972) (Fig. 12-5). Epiblast cells begin to migrate toward the primitive streak. Once there, the cells detach from the epiblast and invaginate deep to it through the primitive streak. Once invaginated, the cells move in all directions. Some of these cells displace the hypoblast and form the endoderm (Sadler, 2010). The endoderm eventually will form the lining of the respiratory and gastrointestinal tracts. Other invaginating cells form a new layer of cells between the epiblast and the newly formed endoderm called the intraembryonic mesoderm. The cells remaining in the epiblast become the ectoderm. The ectoderm, mesoderm, and endoderm are the three germ layers from which the entire embryo will differentiate. The process of forming these germ layers and creating a trilaminar embryonic disc is known as gastrulation.
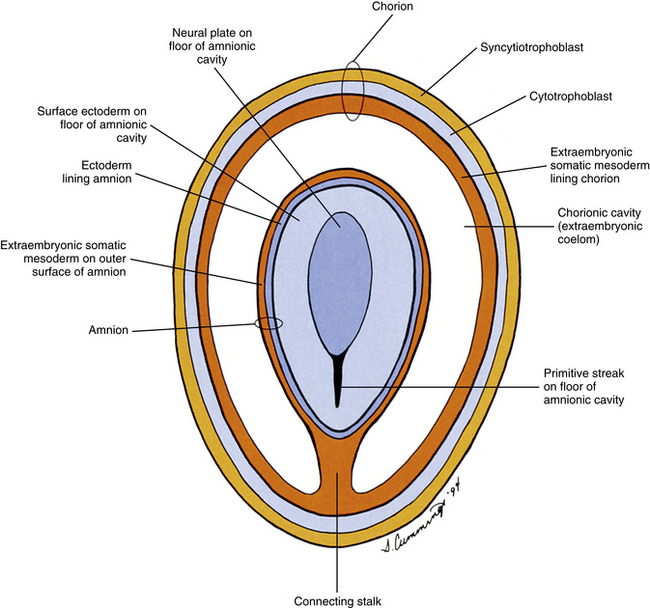
FIG. 12-5 Frontal section through chorion and amnion during third embryonic week revealing a view of the floor of the amnionic cavity as would be seen by an observer positioned on the roof of that cavity.
Some of the invaginated epiblast cells moving superiorly in the midline toward the future head turn into a structure known as the notochord (Fig. 12-6; see also Fig. 12-1). The notochord is a relatively rigid rod of cells that marks the longitudinal axis of the embryonic body and induces the nervous system to develop. The vertebral column later occupies this site, and by then the notochord has turned into the part of the intervertebral disc known as the nucleus pulposus.
Neurulation
Also during the third week of development, the notochord induces the ectoderm to form a thickening along its length in the midline called the neural plate, which is the primordium of the entire nervous system (Arey, 1965). The cells of the neural plate are known as the neuroectoderm. A groove forms, which runs the length of the neural plate, with two folds flanking it. This neural groove deepens and sinks below the surface. The neural folds close over it, thus forming a hollow tube of neuroectoderm called the neural tube (Scothorne, 1976) (Fig. 12-7). The neural tube is the primordium of the brain and spinal cord, and the process of its formation is called neurulation. Some of the morphogenetic processes and molecular mechanisms underlying neurulation are beginning to be understood, mostly from studies of the mouse model (Greene & Copp, 2009). Other neuroectoderm cells invaginate along with the neural tube but remain apart from it (Romanes, 1972a, b). They are collectively known as the neural crest (see Fig. 12-7) and give rise, among other things, to many important components of the nervous system, including all sensory neurons, all postganglionic autonomic neurons, and all ganglia, both sensory and motor (Scothorne, 1976). The rest of the ectodermal cells, which are not part of the neural plate, are destined mostly to form the epidermis (the surface layer of the skin), and are appropriately named surface ectoderm.
Somite Development
The mesoderm lying just lateral to the notochord parallels the long axis of the embryo and is called the paraxial mesoderm. Beginning during the third embryonic week, the paraxial mesoderm subdivides into blocks of cells known as somites, which are major contributors to the muscles, bones, and the dermis of the body. These form sequentially along the craniocaudal axis of the embryo. The fundamentals of these processes are beginning to be understood, and they appear to be under the control of multiple signaling gradients involving Wnt, fibroblast growth factor (FGF) and retinoic acid pathways and Hox gene expression (Aulehla & Pourquie, 2010). Just lateral to the somites on both sides of the embryo, other mesodermal cells have formed into a structure called intermediate mesoderm. The intermediate mesoderm eventually will develop into the majority of the genitourinary system (Fig. 12-8; see also Fig. 12-1). Lateral to the intermediate mesoderm is the lateral mesoderm, which will, along with the overlying ectoderm, form the lateral and ventral parts of the body wall.
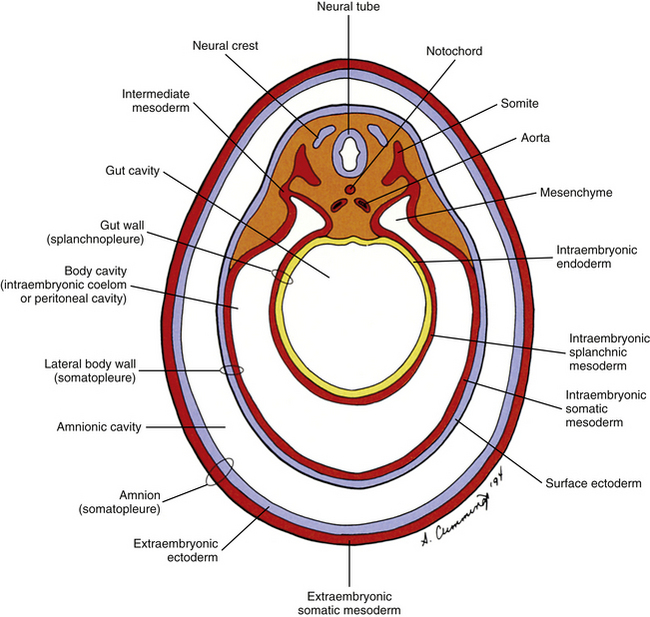
FIG. 12-8 Cross section through the two concentric tubes that run the length of the embryonic body at the end of the third embryonic week: the inner primitive gut tube (splanchnopleure) and the outer body wall tube (somatopleure). The space between them becomes the body cavity.
The somites are staging areas from which mesoderm cells deploy to new locations (Sadler, 2010). The cells of the ventromedial portion of the somite will migrate toward the notochord and neural tube. These cells eventually produce hard tissues, such as bone, cartilage, and ligaments, so the part of the somite from which they are derived is appropriately called the sclerotome (Harrison, 1972) (Fig. 12-9).
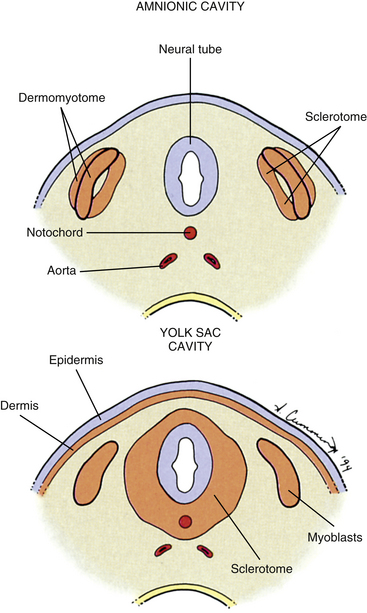
FIG. 12-9 Cross section through two stages in somite differentiation: before (top) and after (bottom) dermis and sclerotome cells have migrated.
The rest of the somite is called the dermomyotome or epithelial plate of the somite. Traditionally, once myoblasts could be identified in the somite, termed the myotome, the remainder of the somite was called the dermatome, which developed into the dermis of the skin (Beck et al., 1985) (see Fig. 12-9). It is now believed that the superficial portion of the somite, the region of the traditionally defined dermatome, contributes a significant amount of muscle precursor cells as it elongates through the body wall (Standring et al., 2008). Also, the only dermis that appears to be derived exclusively from somites may be that covering the epaxial musculature, which is a much more restricted distribution than implied by the term dermatome. The remainder of the dermomyotome forms myoblasts that will eventually form skeletal muscles (Fischman, 1972) (see the following).
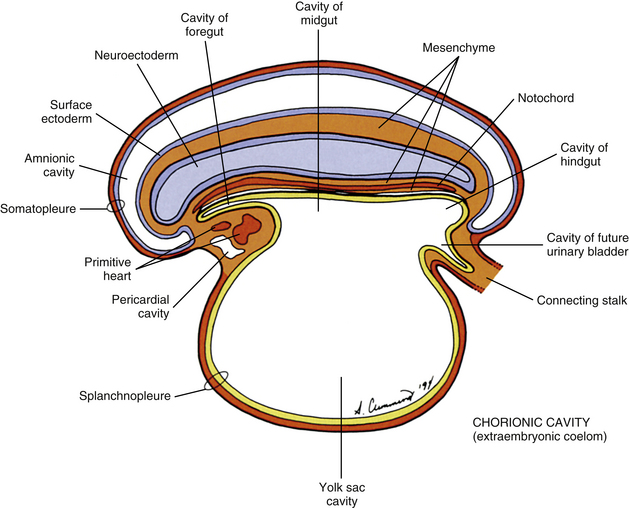
During the next several weeks, the amnion undergoes a series of folds that create the embryo’s body wall and define the lumen of the embryonic gut (Fig. 12-10). The embryo’s body cut in cross section at this time appears as two concentric rings. The inner ring is the splanchnopleure; the outer is the somatopleure (Goss, 1966) (see Fig. 12-8). The outer ring actually has the shape of a jeweler’s signet ring. Tucked close together inside the signet part are the neural tube, neural crest, notochord, somites, intermediate mesoderm, and mesenchyme (see Fig. 12-8). The signet part corresponds to what is variously called the back, the posterior body wall, and the posterior abdominal or posterior thoracic wall (Callander, 1939; Grant, 1952; Davenport, 1966).
Vertebral Development
Vertebral development can be conveniently divided into four stages. The notochordal stage is first. The notochord not only forms the original basis for the development of the vertebrae by defining the axis of the embryo but also forms some of the cells of the intervertebral disc. The second stage is the mesenchymal, or blastemal, stage. Mesenchyme is the connective tissue of the embryo that is, at least in the case of the vertebrae, derived from paraxial mesoderm. Each vertebra is formed from a mesenchymal model. The third stage is the cartilage stage, in which the vertebral mesenchyme is replaced by hyaline cartilage. The osseous stage is last, in which the hyaline cartilage is replaced by bone. Therefore each vertebra is formed by the process of endochondral ossification.
Cells of the sclerotome migrate medially from the somite to surround the neural tube and notochord (Breathnach, 1958). Instead of forming a continuous tunnel of cells, they form a series of discrete blocks of mesenchyme (Standring et al., 2008). Those cells ventral to the neural tube that specifically surround the notochord are known as the centrum. The cells dorsolateral to the neural tube are called the neural arch (O’Rahilly, 1986) (Fig. 12-11). Each neural arch forms a pedicle, which projects ventromedially toward the centrum, and a lamina, which projects dorsomedially toward the corresponding lamina from the other side. The laminae eventually fuse and expand to form the spinous process. Cranial and caudal projections of the neural arch form articular processes, which will develop into the zygapophysial joints with adjacent vertebrae. A lateral projection of the neural arch forms the true transverse process. Projecting anterolaterally from the neural arch is the costal element, which expands to meet the tip of the transverse process. The patterning of the mesenchyme into presumptive vertebrae appears to be controlled by a complicated interplay of mechanisms that involve transcription factors, such as those encoded by Hox genes, and signaling molecules, like those involved in Gdf11, FGF, Wnt, and retinoic acid signaling (Mallo, Vinagre, & Carapuco, 2009).
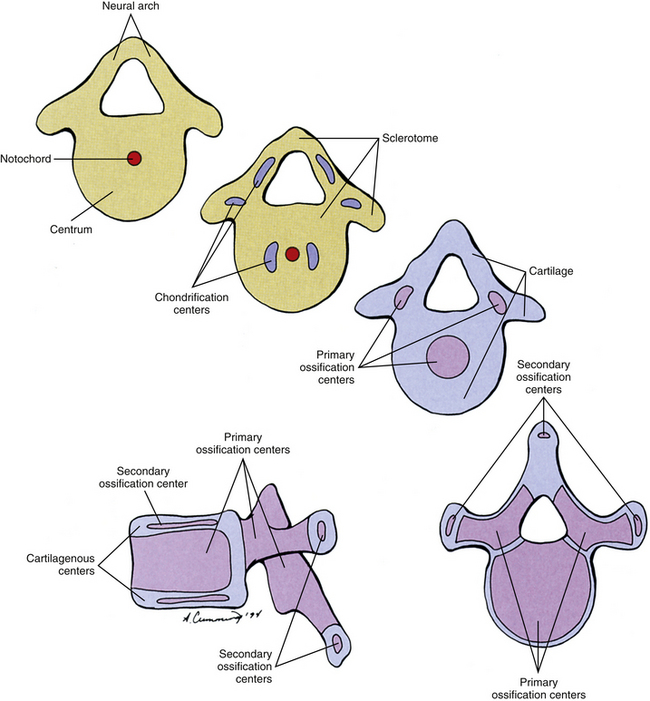
FIG. 12-11 Four cross-sectional views and a lateral view of a vertebra composed at first almost entirely of sclerotome cells (tan); these cells are gradually replaced by cartilage (blue), which is almost entirely replaced by bone (purple).
For years the accepted view has been that the sclerotome cells form a centrum derived from two contiguous somites (Beck et al., 1985). This dual origin accounts for the staggered position of myotomes and centra (Sadler, 2010). This accepted view was challenged by Verbout (1985). However, experimental support for the accepted view also has been adduced (Bagnall et al., 1987). Most evidence still suggests that approximately the cranial half of a centrum is derived from the caudal portion of the somite above, whereas the caudal half of a centrum is derived from the cranial portion of the somite below (Standring et al., 2008) (Fig. 12-12).
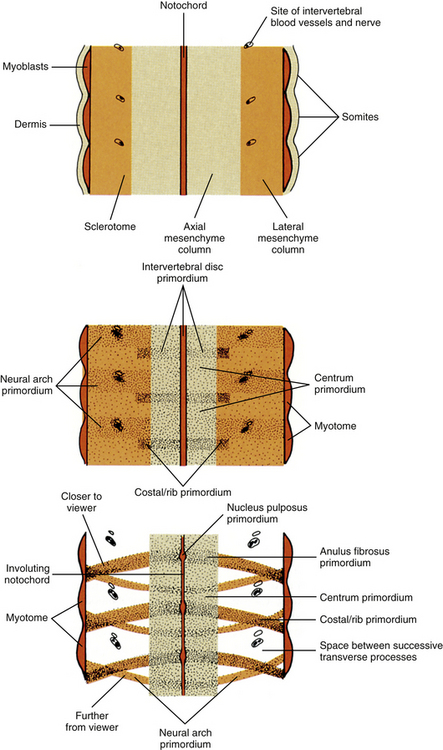
FIG. 12-12 Frontal section through notochord and somites depicting three stages in the formation of intervertebral discs and vertebrae. Cells migrate from the sclerotome to surround the notochord, but cell density is not the same everywhere along the notochordal length. Cell condensations mark the sites where intervertebral discs will appear. The vertebral centra will develop in the spaces between cell condensations. Other sclerotome cells located dorsolaterally form condensations that mark the sites where neural arches will appear. The spinal nerve and intervertebral blood vessels appear in the spaces between the developing neural arches. Other sclerotome cells located laterally condense next to the disc at the site where the costal element will appear.
Regardless of their origin, by the end of the second embryonic month, some of the mesenchymal cells of the vertebrae change into chondroblasts and begin producing an extracellular cartilaginous matrix (Hall, 1978). There are typically two chondrification centers in each centrum, located on either side of the notochord. These rapidly replace the notochord in the region of the centrum with cartilage to form a single mass of cartilage (Standring et al., 2008) (see Fig. 12-11). Also, each side of the neural arch develops at least one chondrification center.
By the end of the embryonic period, primary ossification centers appear in the cartilaginous vertebra. These usually appear in a cranial to caudal pattern, beginning in the upper cervical region by approximately week 8 and reaching the sacral area by week 22 (Standring et al., 2008). Typically two primary ossifications centers form in the centrum (ventral and dorsal), which rapidly fuse into one center (Moore & Persaud, 2008). There is also a primary ossification center that develops in each side of the neural arch (O’Rahilly & Muller, 1992) (see Fig. 12-11). By birth, each vertebra consists of three bony segments connected by hyaline cartilage: the centrum and each half of the neural arch. The cartilages between the centrum and each neural arch actually are located within the region that will become the adult vertebral body. This region is known as the neurocentral synchondrosis (or junction) and usually fuses together into compact bone by age 6 (Maat et al., 1996). Therefore the terms vertebral body and centrum are not equivalent. Likewise the terms neural arch and vertebral arch are not synonymous. The vertebral body encompasses all of the centrum and a portion of each neural arch. The vertebral arch is only a portion of each neural arch (Trotter & Peterson, 1966).
At approximately the time of puberty, secondary ossification centers appear in the remaining cartilage (Sinclair, 1972) (see Fig. 12-11). Typically each vertebra develops five secondary ossification centers: one for the tip of each transverse process, one for the tip of the spinous process, and two anular apophyses (outdated term: anular epiphyses)—one on the superior and one on the inferior rim of the vertebral body. A vertebra can increase in height and diameter before and after birth by employing intrinsic bone-depositing mechanisms around the circumference, or periosteum, and at the ends (Bogduk, 2005). Also, there can be some variation in the distribution, and sometimes even number, of ossification centers from vertebra to vertebra, accounting for some of their individual variation (Inkster, 1951).
Although each vertebra has costal elements, the eventual fates of these elements vary by region of the spine. In the cervical spine, the costal elements eventually form most of the adult transverse process, including the portions that form the ventral and dorsolateral boundaries of the foramen of the transverse process. Only the portion of the adult transverse process that forms the dorsomedial border of the foramen of the transverse process develops from the embryonic transverse process. In the thoracic region, the costal elements reach their maximal length and form the ribs. In the lumbar spine, the costal elements form the anterior and lateral portions of the adult transverse processes. The embryonic transverse process forms only the dorsomedial portion of the adult lumbar transverse process, which includes the accessory process (Robertson, 1966).
Atypical Vertebrae (Developmentally)
There are several vertebrae that have atypical patterns in their development. These include the atlas, axis, seventh cervical, lumbars, sacrum, and coccyx.
Atlas
In the mesenchyme stage, most of the cells of the posterior aspect of the centrum of the first cervical vertebra actually become associated with the superior aspect of the cells of the centrum of the second cervical vertebra. This detachment of cells accounts for the lack of a vertebral body in the atlas and gives this bone its characteristic ring shape. These “extra” cells will become the odontoid process of the axis. The remaining cells of the anterior aspect of the centrum of the atlas will become the anterior arch of that bone. The atlas usually is ossified from three ossification centers. There is one ossification center for each lateral mass. Also, a third ossification center forms for the anterior arch, but this center does not usually develop until after the time of birth (Pharaoh, 1956).
Axis
Most of the mesenchyme cells from the posterior aspect of the centrum of the atlas become associated with the superior aspect of the centrum of the axis. These cells will eventually become the odontoid process, although there is recent evidence that the odontoid process is not derived solely from the centrum of the atlas (David et al., 1998). The axis usually has five primary and two secondary ossification centers. The centrum and neural arches ossify in the typical manner (one primary center for each side of the neural arch and one for the vertebral body). At the beginning of the third trimester of pregnancy, two ossification centers appear laterally in the base of the cartilaginous odontoid process. A thin piece of cartilage may separate the base of the odontoid process and the body of the axis. This rudimentary disc ossifies slowly and may not completely disappear until the later teenage years. A secondary ossification center, known as the ossiculum terminale, forms in the apex of the odontoid process at approximately age 3 to 6 and unites with the rest of the odontoid process by approximately age 12. The last secondary ossification center is a typical anular apophysis associated with the inferior aspect of the body of the axis.
Seventh Cervical
The seventh cervical vertebra has the typical ossification centers with addition of separate secondary ossification centers for the costal processes. These appear at approximately the sixth fetal month and unite with the rest of the vertebra at approximately age 6 years. These may remain separate from the rest of the vertebra and form cervical ribs. Occasionally separate ossification centers for the costal processes can be found for the sixth, fifth, and even fourth cervical vertebrae (Standring et al., 2008).
Lumbars
Generally, the lumbar vertebrae ossify in the typical manner. In addition, there is usually a secondary ossification center that forms for each mamillary process.
Sacrum
The sacrum begins as five vertebral segments that eventually fuse into a single bone. Primary ossification centers for the centrum and neural arches of each sacral vertebra appear between the tenth and twelfth weeks of development. The neural arches and centra begin uniting during the second year. This process begins in the lower segments and is completed in the upper segments by the sixth year. The neural arches from both sides are usually fused by approximately the eighth year. Primary ossification centers also appear for the costal elements of the upper three (and sometimes more) segments superior and lateral to the pelvic sacral foramina during the third trimester of pregnancy. The costal elements fuse with the corresponding neural arches during the second to fifth years and to the centra by approximately the eighth year. Laterally the conjoined neural arches and costal elements are separated from the segments above and below by epiphyseal cartilages. These epiphyseal cartilages are associated with the auricular surfaces. Around the time of puberty, various secondary ossification centers may form that are associated with the upper and lower aspects of the centra, spinous tubercles, transverse tubercles, and costal elements. The centers for the costal elements complete the ossification process for the auricular surfaces by age 20 (Pharaoh, 1956).
The bodies of the sacral vertebrae during early life are separated by intervertebral fibrocartilages. The margins of the bodies begin to fuse around age 18, beginning inferiorly. By the mid-twenties, the sacrum usually is a single, solid bone, although vestiges of the intervertebral fibrocartilages may persist (Standing et al., 2008).
Coccyx
Each segment of the coccyx ossifies from a single primary ossification center. The center for the first segment appears soon after the time of birth. Occasionally there are separate ossification centers that appear for the coccygeal cornua at approximately this same time (Standring et al., 2008). The ossification centers for the remaining coccygeal segments appear, from superior to inferior, variably during childhood and puberty, so that all of them have usually appeared by age 20. The segments gradually fuse with one another so that by age 30 the coccyx usually is a solid bone. The coccyx may variably fuse with the sacrum in later life.
Intervertebral Discs and Other Joints
Although the mesenchyme of the sclerotome replaces the notochordal tissue to form the centra of the vertebrae, the notochordal tissue located between the developing vertebrae actually expands to form the presumptive nucleus pulposus of the intervertebral discs (see Figs. 12-9 and 12-12). This expanded notochordal tissue is surrounded by sclerotomal mesenchyme called the perichordal disc. The perichordal disc will eventually form the anulus fibrosus and cartilaginous end plates. Around the sixth fetal month, the cells of the notochord begin to degenerate, being replaced by cells of the surrounding mesenchyme. This process continues so that all of the notochordal cells have disappeared by the end of the second decade of life (Standring et al., 2008). Therefore the only structures in the adult that are of notochordal origin are possibly some noncellular matrices of the nucleus pulposus, although recent lineage mapping studies suggest that the nucleus pulposus may retain some notochordal cells throughout life (Risbud, Schaer, & Shapiro, 2010).
The zygapophysial joints develop in a similar manner to other synovial joints from the mesenchyme of the sclerotome (Bogduk, 2005). The mesenchyme between the presumptive articular processes condenses around the sixth fetal week. Around the periphery of this region, the mesenchyme differentiates into the capsular and flaval ligaments. Centrally the mesenchymal cells disappear to form the synovial cavity by a process of apoptosis. The mesenchyme lining the inside of the fibrous capsule eventually forms the synovial membrane.
The other ligaments associated with the spine (Walmsley, 1972), including the sacroiliac ligaments (Salsabili & Hogg, 1991), also develop as condensations of mesenchyme of the sclerotome. In the appropriate intervertebral regions, this mesenchyme condenses and forms dense regular collagenous connective tissue that becomes the various named ligaments.
Developmental Anomalies
Because the development of the vertebrae is a complicated process, it should not be surprising that developmental anomalies are common occurrences. Many of these do not pose any problems and are never detected or are incidental findings. On the other hand, some of these can have a profound impact on the health and lifestyle of the individual. Many of these conditions are discussed in further detail in other chapters. References to these chapters appear where appropriate.
One of the more common etiologies for certain developmental anomalies (e.g., block vertebra) is alteration in the segmentation processes. The genetic control of these processes is beginning to be understood (Giampietro et al., 2009). Also, a variety of skeletal dysplasias and syndromes can have an effect on the normal development of the spine, leading to an assortment of spinal deformities (Song & Maher, 2007).
Klippel-Feil Syndrome
Klippel-Feil syndrome is a developmental anomaly of the cervical spine in which there is fusion or malformation of multiple vertebrae. There also may be absence of one or more cervical vertebrae. The head of a person with this problem appears low between the shoulders, and the neck usually appears too short. Commonly there is fusion of the lower cervical vertebrae into one large, misshapen bone, which reduces cervical ranges of motion.
Basilar Impression (Platybasia)
Basilar impression is mainly a developmental defect in the occiput in which it appears to have been pushed superiorly by the cervical spine. Frequently there is an accompanying malformation of one or both of the upper cervical vertebrae. The atlas may be ankylosed to the occiput. Because the occipital condyles and the squama of the occiput are misshapen, the foramen magnum commonly is relatively small in diameter and distorted. Also, the odontoid process can project unusually high into the area of the foramen magnum. These latter two malformations can lead to profound neurologic insult to the lower portion of the brain stem or upper region of the spinal cord. (See Chapter 5 for additional details.)
Occipital Vertebra
This is a condition in which there appears to be an occipital vertebra. There are two main forms of this anomaly. The first is a true occipital vertebra in which a rudimentary vertebra forms, almost always attached to the inferior aspect of the occiput. Usually a normal atlas is found below this malformation. This anomaly is thought to represent a vestige of the proatlas seen in primitive vertebrates and results from the improper incorporation of the occipital sclerotomes (Pharaoh, 1956).
The second form of this anomaly is an atlanto-occipital fusion; this is sometimes termed occipitalization. In this case, the articular processes of the atlas and the occipital condyles are ankylosed. Other than this bony attachment of the two bones, both may be normal in appearance, although the atlas may be somewhat misshapen.
Detached Apex of Odontoid Process
This anomaly represents a nonunion of the apical region of the odontoid process with the rest of that process (see Figs. 13-10 and 13-13). There appears to be a small fragment of bone just above the odontoid process, which is commonly known as a terminal ossicle (ossiculum terminale, ossiculum terminalis). The rest of the odontoid process appears somewhat shortened and blunted. This ossicle usually is embedded in the apical odontoid ligament. (See Chapter 13 for additional information.)
Os Odontoideum
Sometimes there is a nonunion of the odontoid process with the body of the axis (see Figs. 13-10 and 13-12). If this happens, the ununited ossicle is termed an os odontoideum. This can lead to a relatively unstable upper cervical region, although there usually is a soft tissue connection between the ossicle and the body of the axis. Trauma in the region that otherwise would not present much of a problem can lead to catastrophic consequences with this anomaly. (See Chapters 5 and 13 for additional information.)
Congenital Absence of the Odontoid Process
Obviously this rare anomaly can be dangerous. This condition represents a failure of mesenchymal or other (e.g., ossification) developmental centers of the odontoid process. Usually compensatory ankylosis in the region can be found, which may be the result of the increased mobility and instability in the region.
Accessory Ribs
Accessory ribs, usually rudimentary, are an overgrowth of the costal elements of either lumbar or cervical vertebrae (Fig. 12-13). Lumbar ribs are much more common than cervical ribs (Moore & Persaud, 2008), and usually cause no clinical problems. Cervical ribs may be involved in neurovascular insults in the region of the thoracic inlet, which are known commonly in clinical circles as a type of thoracic outlet syndrome. (See Superior Thoracic Aperture [Thoracic Inlet] in Chapter 6.)
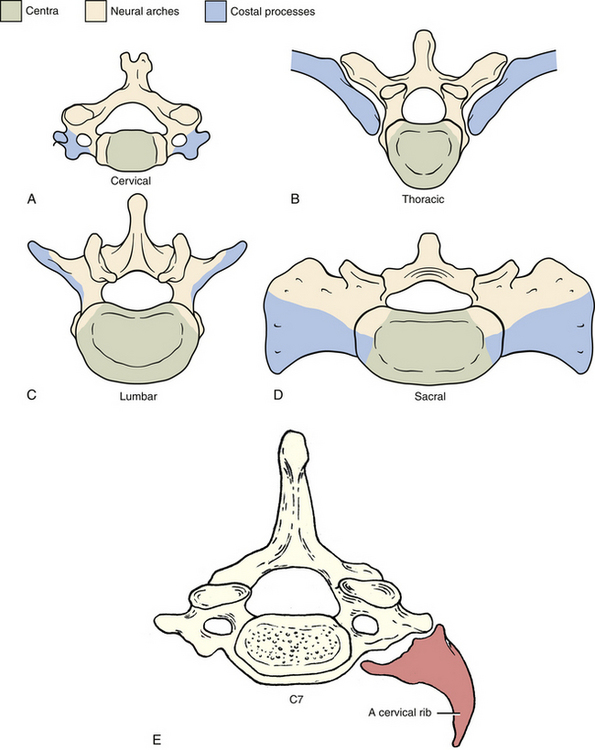
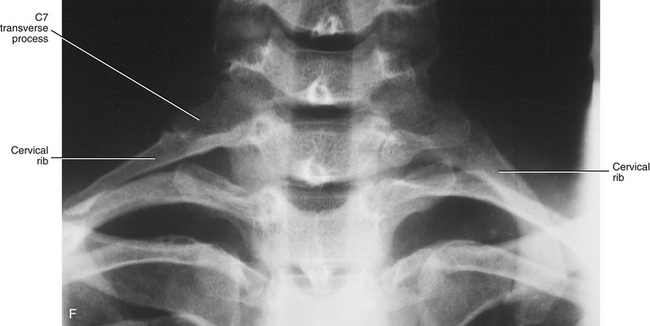
FIG. 12-13 Costal processes, neural arches, and centra of the, A, cervical (notice each costal process here includes all but the most proximal part of the posterior root of the transverse process); B, thoracic (which normally become ribs); C, lumbar; and, D, sacral regions of the vertebral column. E, Illustration showing a cervical rib. (E, Modified from Mathers LH. [1996]. Clinical anatomy principles. St Louis: Mosby.) (E, Modified from Mathers LH. [1996]. Clinical anatomy principles. St Louis: Mosby.) F, Anteroposterior x-ray showing bilateral cervical ribs. Sizes of cervical ribs vary considerably. A cervical rib may, but does not always, articulate with the first thoracic rib or the sternum. When the cervical rib does not have an osseous anterior attachment, a fibrous band may extend from the anterior tip of the rib to either the first thoracic rib or the sternum (see Cervical Ribs in Chapter 5 for further details).
Hemivertebra
When only either the right or the left half of a vertebral body develops, it results in the anomaly known as hemivertebra, or cuneiform vertebra, and usually occurs in the thoracic spine (Fig. 12-14, A and B). The problem presumably occurs in the cartilage stage of development or earlier. Because there are two chondrification centers for the centrum, right and left, it is hypothesized that one of these fails to develop (Rothman & Simeone, 1992). Usually the half of the vertebral body that is present becomes deformed because of weight bearing such that it is triangular in shape (when seen from in front, as in an anteroposterior [AP] plain film radiograph), with the apex directed medially. This deformation results in an obvious scoliosis that usually undergoes degenerative changes relatively early in life. Frequently two hemivertebrae can be found several segments away from each other. The two hemivertebrae are formed from opposite halves of their respective developmental centers. Thus they compensate for one another, decreasing the severity of the scoliosis.
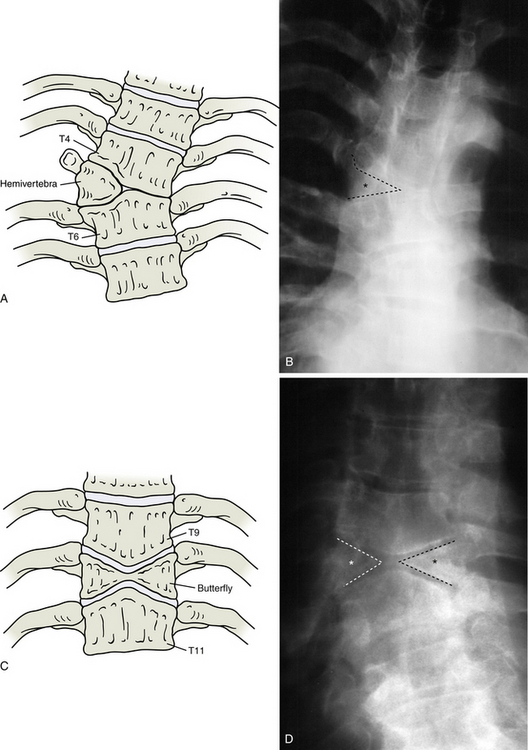
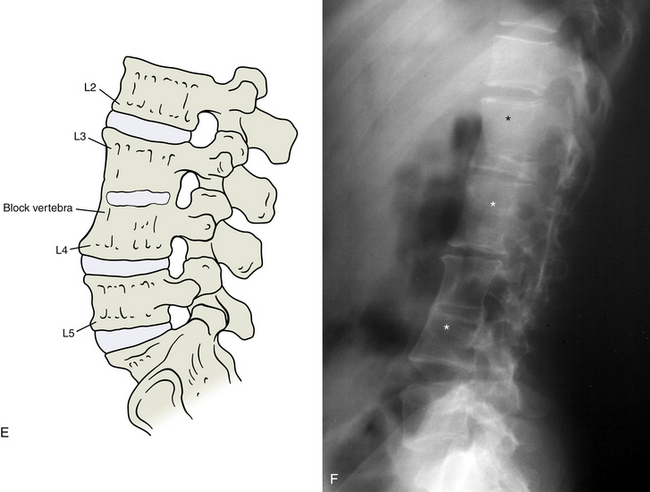
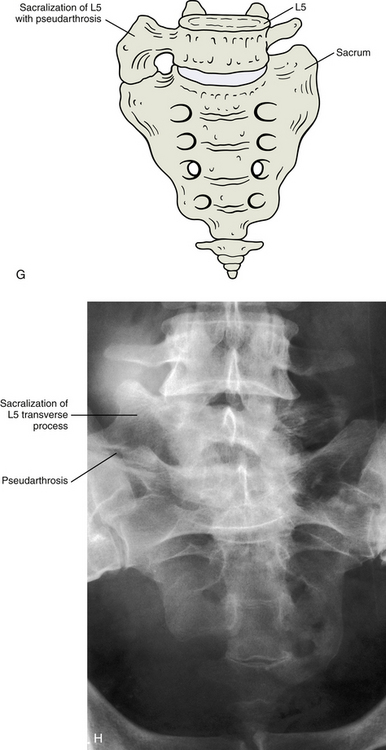
FIG. 12-14 Illustrations (left) and x-rays (right) showing many of the most common developmental anomalies. A and B, Hemivertebra (outline and asterisk on the anteroposterior thoracic x-ray of B). C and D, Butterfly vertebra (outlines and asterisks on the anteroposterior thoracic x-ray of D show that this butterfly vertebra is completely bisected in the midline). E and F, Block vertebra (lumbar region, notice the three consecutive block vertebrae indicated by the asterisks in F).
G and H, Anteroposterior view and x-ray, respectively, of the transitional segment between L5 and the sacrum (sacralization) forming an accessory joint (pseudarthrosis). (X-rays courtesy Dr. Jeff Rich.)Butterfly Vertebra
This is a deformity in which both halves of the vertebral body have formed but have not united properly in the midline (Fig. 12-14, C and D). Each half is usually wedge-shaped, such that each is not as tall in the midline as it is laterally. When viewed in an AP radiograph, the vertebral body has a shape that is reminiscent of a butterfly, hence the name of the deformity. The developmental problem presumably happens in the chondrification stage, or earlier, because of an imperfect union of the regions of the centrum formed by the side-by-side chondrification centers. This anomaly usually occurs in the thoracic spine and may cause spinal curvatures: hyperkyphosis, scoliosis, or both.
Block Vertebra
This anomaly represents a nonsegmentation of the somites (Fig. 12-14, E and F). It results in the formation of a vertebra that is actually two adjacent vertebrae that are fused together into a single bone. The vertebral body is twice as high as normal. The vertebral arches usually are also fused together, although not always completely. This anomaly is most frequent in the lumbar spine, but can occur anywhere in the spine and may involve additional segments. It may result in a kyphotic deformity because of the tendency for overdevelopment of the posterior aspect of the vertebral body and underdevelopment of the anterior aspect.
Lumbosacral Transitional Segment
This is a general term for two types of developmental anomalies that have an incidence of 5% (Nicholson, Roberts, & Williams, 1988) to more than 12% in the general population (Bron, van Royen, & Wuisman, 2007; Tague, 2011). One is when the L5 vertebra overdevelops, especially its transverse processes, and appears similar to the sacral segments. This specific anomaly is called a sacralization of L5 and is not too uncommon (Fig. 12-14, G and H). It may happen either unilaterally or bilaterally. This overdevelopment may go so far as to result in the actual fusion of L5 with S1. This fusion involves one or both transverse processes. Sometimes L5 may completely fuse with S1 to form a sacrum that is truly composed of six segments. This type of anomaly, especially the types in which there is bilateral asymmetry, could cause altered biomechanics in the region. (See Chapter 7 for additional information.)
The other type of transitional segment in this region is when the S1 segment develops in such a way as to be more similar to a lumbar vertebra than a sacral segment. This usually is termed a lumbarization of S1. It is not as common as a sacralization of L5. This independence of S1 may go so far as to result in six true lumbar vertebrae. This type of anomaly causes resultant changes in the structure of the sacroiliac joint.
Spina Bifida
Spina bifida is the result of nonunion of the right and left halves of the neural arches (Fig. 12-15, A-E). Unfortunately, it also may involve the underlying neural tissue, and, if so, is one of the most serious vertebral anomalies. If no neural tissue is involved in the defect, it is termed spina bifida occulta, because there are rarely any clinical manifestations. This type of anomaly occurs most commonly in the lumbosacral region. In fact, the sacral hiatus may be considered a physiologic spina bifida. The second most common location is C1, in which case it is called agenesis of the atlas (see Chapter 13). Spina bifida occulta may be found in nearly 10% of the otherwise normal population (Sadler, 2010). In these cases, the separation between the halves of the vertebral arch usually is not wide, is filled with connective tissue, and is covered with skin. Sometimes a tuft of hair may be found over the defect, especially in the lumbosacral region.
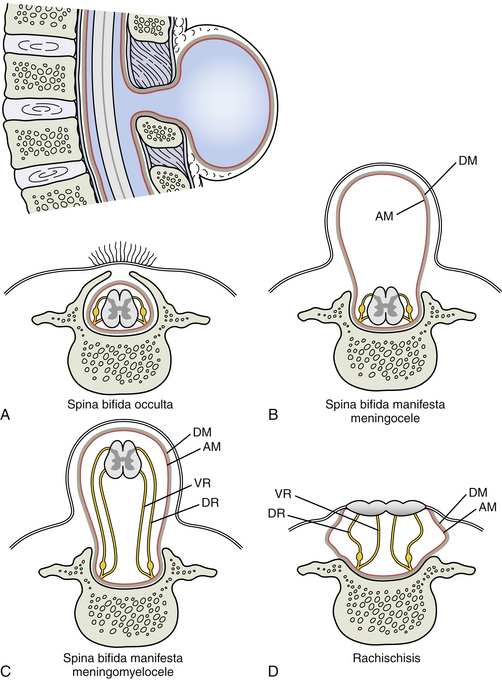
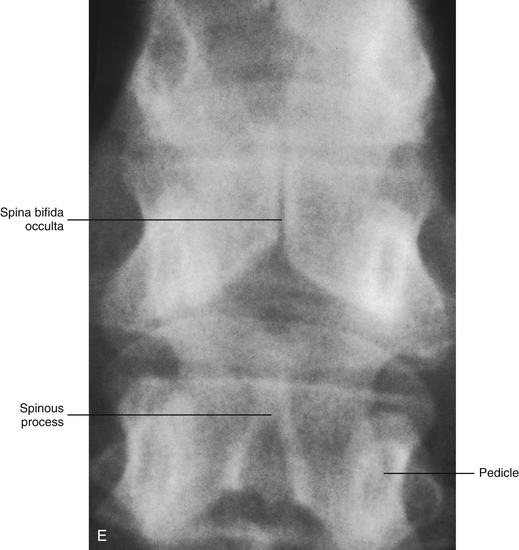
FIG. 12-15 Spina bifida. The orientation illustration in the upper left of the figure is of a midsagittal section through T11-L2 in a newborn with spina bifida manifesta (meningocele) at the level of T12. Parts A through D show the different types of spina bifida in horizontal sections through T12. A, Spina bifida occulta. B and C, Spina bifida manifesta showing, B, meningocele and, C, meningomyelocele. D, Rachischisis. Notice the neural tissue is completely exposed to the external environment in this latter condition. See text for further details on each of these types of spina bifida. AM, Arachnoid mater; DM, dura mater; DR, dorsal root; VR, ventral root.
E, X-ray showing spina bifida occulta of a thoracic vertebra. (X-ray courtesy Dr. Jeffrey Rich.)
If the developmental defect involves not only osseous structures but also the underlying neural tissue, it is termed spina bifida cystica or manifesta. These actually represent a form of neural tube defect and may occur in approximately 1/1000 births (Sadler, 2010). In these cases, neural tissue protrudes through the defect in the vertebral arch to form a cystlike sac. If only fluid-filled meninges protrude through the vertebral defect, it is termed meningocele. If true neural tissue also protrudes through the defect, it is known as meningomyelocele. Rarely the neural folds do not fuse properly with each other. Instead they fuse with the overlying surface ectoderm, resulting in a mass of flattened neural tissue. This is known as myeloschisis or rachischisis. Usually spina bifida cystica is found in the lumbosacral region, but it may be associated with anencephaly when it is found in the cervical region. Spina bifida cystica usually results in severe neurologic deficits.
Axial Muscle Development
While the sclerotome is starting to form the vertebrae, the dermomyotome is also continuing to develop. Cells along the craniomedial edge of the dermomyotome elongate in a cranial to caudal direction and are collectively called the myotome. These cells will form the epaxial muscles, which are dorsolateral to the vertebrae. The myotome of one somite soon encounters the myotomes cephalic and caudal to it. The cells of the myotome are postmitotic myoblasts that soon lose their individual identities and develop into the deep muscles of the back (Lockhart, 1951). The deep back muscles also are called the intrinsic or “true” muscles of the back and include the erector spinae and transversospinalis groups, two splenius muscles, and suboccipital muscles. Other deep back muscles that are epaxial in nature are the interspinales, levators costarum, and medial portions of the intertransversarii. These muscles are all innervated by the dorsal rami of spinal nerves (Joseph, 1976) (see Figs. 4-2 to 4-4).
Hypaxial Muscles
The cells of the ventrolateral edge of the dermomyotome at the levels of the limbs will migrate into the developing limb buds to form the limb musculature. The remainder of the cells of the dermomyotome will grow into the flank region of the trunk to form the hypaxial musculature (Langebartel, 1977). All of the hypaxial muscles, and also the limb muscles, are innervated by ventral rami of spinal nerves (Sadler, 2010).
The myoblasts from adjacent somites fuse, losing their individual identities, and form the muscles of the thoracic and abdominal body wall (Standring et al., 2008). These include many muscles that can be seen from the back, such as the intercostal, lateral portions of the intertransversarius, subcostal, the two serratus posterior, and the external abdominal oblique muscles (Sinclair, 1972). Myoblasts move ventromedially toward the vertebral column, where they join with myoblasts from adjacent somites to form muscles located at the sides and front of the vertebral column (Lockhart, 1951). These include the three scalene, two longus (colli and capitis), two psoas, and quadratus lumborum muscles (Snell, 1975). Other myoblasts form the two muscular sheets that roof and floor the abdominopelvic cavity, the thoracoabdominal and pelvic diaphragms (Arey, 1965; Moore & Persaud, 2008), both of which intersect the posterior body wall. Still other myoblasts move posteriorly to form the muscles that hold the scapula to the vertebral column or ribs (Corliss, 1976). These muscles include the two rhomboid, levator scapulae, and serratus anterior, all of which are innervated by ventral rami that have been channeled through the brachial plexus (Leeson & Leeson, 1972) (see Fig. 4-1).
The scapula is somewhat of an intermediary between the bones visible from the back (spine and ribs) and the bone that extends from the elbow to the shoulder (humerus) (Breathnach, 1958). The shoulder muscles that connect the scapula to the humerus include the supraspinatus, infraspinatus, teres major, teres minor, subscapularis, and deltoid (Romanes, 1976). All but the subscapularis can be seen from behind (Crafts, 1966), but the gross anatomist does not regard them as back muscles, even though the lay person might (Hollinshead, 1982). These muscles develop from the ventrolateral edge of the dermomyotome and consequently are innervated by ventral rami of spinal nerves through the brachial plexus (Langebartel, 1977). However, one muscle connects the humerus directly to the posterior aspect of the trunk, largely bypassing the scapula (Sinclair, 1972). This muscle is the latissimus dorsi, and its origin can be traced back to this same region of the dermomyotome. It is also innervated by ventral rami through the brachial plexus (Lockhart, 1951).
The mentioned hypaxial muscles that migrated toward the back can be regarded as comprising the extrinsic muscles of the back, just as the derivatives of the true myotome compose the intrinsic musculature of the back (Mortenson & Pettersen, 1966). It is interesting to note that the muscles that began more dorsally in the somite, the epaxial muscles, eventually form the deep back muscles. The hypaxial muscles, even though they began more ventrally in the somite, migrate dorsally and eventually come to overlie the epaxial muscles, becoming the superficial back muscles (Scothorne, 1976). The extrinsic muscles, with one exception (trapezius), are innervated by ventral rami; the intrinsic muscles, with no exception, are innervated by dorsal rami (Lockhart, 1951).
Trapezius Muscle
One superficial, or extrinsic, back muscle may not be derived from the somites, but overlies those that do (Zuckerman, 1961). This muscle, the trapezius, may derive from the mesoderm located in the transition zone between the pharyngeal (branchial) arches and cervical somites (Lockhart, 1951). Its motor innervation comes from the same cranial nerve that innervates the most caudal branchial arches, the (spinal) accessory or eleventh, and its sensory innervation may come from cervical spinal nerves (Standring et al., 2008). However, some disagreement exists about how to interpret this dual innervation (Hollinshead, 1982).
Neural Development in the Back
The caudal portion of the neural tube will form the spinal cord or spinal medulla. Some neuroectodermal cells of the neural tube differentiate into macroglioblasts and others into neuroblasts (Clark, 1951). The latter soon take on the characteristics of a neuron-to-be (Standring et al., 2008). A blob of protoplasm, the future nerve cell body, contains the chromosomes. The cytoplasm streams out into fingerlike projections that become axons and dendrites (Sadler, 2010). Sometimes the cytoplasmic processes merely connect one side of the neural tube with the other, never leaving the tube (Noback & Demarest, 1975). At other times, one cytoplasmic process travels down or up the spinal cord, or even to the brain stem itself, whereas other processes remain at the same level of the neural tube at which they originated (Standring et al., 2008).
A cytoplasmic process of one neuroblast usually travels with others having a common origin and destination (Standring et al., 2008). Such a bundle of cytoplasmic processes inside the neural tube is called a tract (Kiernan, 2008). The cytoplasmic processes traveling together originate from a group of nerve cell bodies in the primitive spinal cord and, for example, may terminate in the thalamus. These cytoplasmic processes form the spinothalamic tract (Goss, 1966). Sometimes the cytoplasmic process from a neuroblast leaves the spinal cord altogether (Durward, 1951) and, with many others, forms an aggregate known as the ventral root of the spinal nerve (Goss, 1966). An individual cytoplasmic process of this type finds its way to a particular region of a particular skeletal muscle. Along the way it has passed through a spinal nerve, the dorsal or ventral ramus of the spinal nerve, perhaps through a nerve plexus, and finally through a peripheral nerve (Larsell, 1953).
The derivatives of the neural tube (i.e., brain and spinal cord) are ensheathed in connective tissue layers termed the meninges. The innermost layer, which is intimately adhered to the surface of the central nervous system, is a thin layer of loose connective tissue called the pia mater. It is still uncertain as to whether the pia traces back to mesoderm or to neural crest (Standring et al., 2008). A similar uncertainty exists about the origin of the arachnoid mater, which faces the pia mater across the subarachnoid space (Carlson, 1981). It is generally agreed that the outermost meninx, the dura mater, derives from mesoderm (Standring et al., 2008). The spinal nerve roots also are ensheathed by meninges, mostly in the form of the dural root sleeves, which blend with the epineurium of the peripheral nerve.
Spinal Nerve Roots
Two cytoplasmic processes issue forth in opposite directions from most of the clusters of neural crest cells that are not destined to become autonomic neurons. One process travels dorsomedially into the neural tube, where as an axon (central process) it terminates in the region that becomes the dorsal horn of the gray matter (see Chapters 3 and 9). The central process synapses with neuroblasts derived from the neural tube in the dorsal horn. The central processes also can ascend the dorsal aspect of the spinal cord to form the dorsal columns (see Chapter 9). The second cytoplasmic process travels ventrolaterally, where it enters a spinal nerve as a dendrite (peripheral process). The cytoplasmic process going dorsomedially and the one traveling ventrolaterally, as well as the nerve cell body to which they are both attached, are said to lie in the dorsal root of the spinal nerve. Such a dorsal root contains many other neuroblasts with the same shape and spatial configuration, and all their nerve cell bodies together form the spinal ganglion, also known as the dorsal root ganglion (Clark, 1951). This is a sensory ganglion, and no synapses occur within it. The dorsal root is called a sensory root because the current consensus holds that it contains only fibers of afferent neuroblasts derived from the neural crest (Scothorne, 1976). The consensus also holds that the ventral root is a motor root and contains only axons of efferent neuroblasts derived from the neural tube (Cohen et al., 1992). That means that the spinal nerve formed by the intersection of the sensory and motor roots is a mixed nerve, and that the cell bodies of the nerve fibers are derived from both neural tube and neural crest (Clark, 1976) (see Fig. 3-12).
The dorsal and ventral roots are attached to the neural tube at one end and converge to form the spinal nerve at the other end (Clark, 1976). In the early embryo, the roots on one side are not substantially different in length from the roots on the opposite side and from one spinal level to another (Francis & Voneida, 1966). However, these relationships change with time (Sadler, 2010).
Each dorsal and corresponding ventral root grows laterally toward the paraxial mesoderm before the vertebrae have formed (Beck et al., 1985). Both eventually become trapped between the vertebra formed cephalic and the vertebra formed caudal to them (Hollinshead & Rosse, 1985) (see Fig. 12-12). When first formed, all these roots at all spinal levels have approximately the same length, and they all extend laterally from the neural tube at approximately the same angle, nearly 90 degrees. However, when these roots become trapped they do not all remain the same length and do not all continue to make the same angle with the neural tube (Cohen et al., 1992). The dorsal and ventral roots that are trapped between a pair of vertebral primordia must elongate when the neural tube stays fixed in place because the vertebral primordia seem to be moving caudally. The vertebral primordia trapping cervical and thoracic roots move caudally the least, if at all, and thus their nerve roots elongate the least (Romanes, 1972a, b). By contrast, the vertebral primordia trapping lumbar and sacral roots move caudally the most, which means their nerve roots elongate the most (Snell, 1981) (see Fig. 3-8). The angle the elongated roots make with the neural tube also has been reduced less than 90 degrees to an acute angle, and the more elongated the root, the more acute the angle (Clark, 1976). The longest roots have had their angle reduced to 0 degrees, so they hang down vertically, paralleling the neural tube itself (Parke, 1992b). Early anatomists thought the dozens of nerve roots hanging down together caudal to the neural tube resembled a horse’s tail, thus the name in Latin, cauda equina (Hollinshead, 1982) (see Fig. 3-11).
Autonomic Nervous System
Other neuroblasts are located elsewhere in the gray matter of the neural tube, in an area known as the lateral horn (Romanes, 1972a). Each neuroblast sends one of its cytoplasmic processes into the closest ventral root, not heading for skeletal muscle but rather toward nonskeletal muscle or glands (or both) (Sadler, 2010). Each process can change course, leave the spinal nerve it has entered, and travel to a nearby structure known as a sympathetic chain ganglion (Goss, 1966) (see Fig. 10-7). In so doing, the processes create a bridgelike structure called a white ramus communicans. It is known as white because this ramus consists mostly of myelinated nerve fibers (Ellis, 1983).
These neuroblasts, stretching from the lateral horn (the future intermediolateral cell column) to a sympathetic chain ganglion, are myelinated and are called preganglionic neuroblasts (Romanes, 1972a, b). They function as motor neurons, which means the white ramus is comprised of axons derived from the neural tube. As a rule, white rami are found attached only to the T1 through L2 or L3 spinal nerves (Crafts, 1966). The lateral horn corresponds to the nerve cell bodies, and the white ramus corresponds to the axons of the same neuroblasts, or preganglionic sympathetic neuroblasts (Durward, 1951). Above and below these spinal levels, there is no lateral horn, no white rami exist, and the ventral roots do not contain myelinated axons of preganglionic sympathetic neuroblasts (Bruce & Walmsley, 1939).
Some axons of preganglionic neuroblasts cross over a white ramus to a sympathetic chain ganglion but do not terminate within the ganglion (Francis & Voneida, 1966). Instead, they pass through the ganglion and continue up the sympathetic chain or down the chain to a sympathetic chain ganglion at a different spinal level, where they terminate (Langebartel, 1977). Inside they synapse with neuroblasts known as postganglionic sympathetics (Goss, 1966). Some axons of preganglionic neuroblasts reach sympathetic chain ganglia by way of white rami, but they do not terminate in any sympathetic chain ganglion (Goss, 1966). Instead, these preganglionic axons pass through the sympathetic chain ganglion without synapsing and continue on their way to a sympathetic ganglion in the abdominal cavity, where they terminate (Francis & Voneida, 1966). These are called splanchnic nerves (see Chapter 10). Some preganglionic neuroblasts have nerve cell bodies located in the gray matter of the sacral region of the neural tube and are classified as parasympathetic. Their axons travel long distances and terminate in parasympathetic ganglia located close to the pelvic organs they innervate (Scothorne, 1976). They synapse inside these parasympathetic ganglia with postganglionic parasympathetic neuroblasts located within or close to the organs (Romanes, 1972b).
All sympathetic and parasympathetic postganglionic neuroblasts, as well as the ganglia they help to form, derive from the neural crest (O’Rahilly & Muller, 1992). These motor ganglia are made up of the nerve cell bodies of postganglionic neuroblasts that migrated from the original site of the neural crest (i.e., dorsolateral to the neural tube) to the site of the ganglion. Most, if not all, postganglionic neuroblasts in a sympathetic chain ganglion send their cytoplasmic processes out of the ganglion to the closest spinal nerve and, in so doing, form a bridge called a gray ramus communicans. It is termed gray (as opposed to white) because this ramus is made up of unmyelinated nerve fibers (Romanes, 1972a). Once in the spinal nerve, the postganglionic axons twist their way through the branches of the spinal nerves. These include dorsal and ventral rami, nerve plexuses, when present, and peripheral nerves (Woodburne, 1983). The postganglionic axons terminate in the smooth muscle of blood vessels or in glands (Hollinshead & Rosse, 1985).
Although all spinal nerves have gray rami communicantes, sometimes the axons of postganglionic sympathetic neuroblasts shun the gray rami and are not distributed by a spinal nerve (Larsell, 1953). Instead, they travel directly to the organ they innervate (Durward, 1951). This is the case with the heart, in which the axons of postganglionic sympathetic neuroblasts constitute the cardiac sympathetic nerves (Francis & Voneida, 1966). The nerve cell bodies for these postganglionic neuroblasts lie in cervical sympathetic chain ganglia (Durward, 1951).
Vascular Development in the Back
While all the mentioned embryonic and fetal events have been taking place, the ubiquitous mesoderm has been constructing the cardiovascular system (Brash, 1951a). On the surface of the yolk sac, reddish spots develop, called blood islands. The redness is caused by hemoglobin in erythroblasts that appear inside these blood islands as they become hollow and confluent with each other. Other vascular plexuses are formed in the same way throughout the embryo, but certain channels begin to predominate while others disappear (Yoffey, 1976). Two large channels located ventral to the foregut eventually become the mesodermal rudiments of the heart. This primitive heart drains into vascular channels that have derived from the mesoderm that lies on either side of the nearby pharyngeal arches (Patten, 1953). These paired channels carry blood dorsally away from the heart to the left and right dorsal aortae, and they are called aortic arches (Yoffey, 1976) or aortic arch arteries (Moore & Persaud, 2008).
The blood entering the paired dorsal aortae moves into all the branches from this pair of arteries, which are the largest of the embryonic body. The paired branches going to the back have been called segmental arteries (Hollinshead, 1982). The capillaries of the back return that blood to segmental veins that empty into paired anterior and posterior cardinal veins (Brash, 1951a). The anterior and posterior cardinals on each side empty into a common cardinal vein, and then two of these empty into the primitive heart (Sadler, 2010). This arrangement results in a complete circulatory pathway, but restructuring of this vascular pattern commences almost immediately (Hollinshead & Rosse, 1985).
Arteries
The aortic arches largely disappear, except for the third, fourth, and sixth pairs; those that remain are modified (Oelrich, 1966). The third pair is used to help form the carotid artery system (Walls, 1972). One component of that system, the external carotid artery, serves the back through two of its branches—the ascending pharyngeal and occipital arteries (Standring et al., 2008).
The fourth pair of aortic arches also helps shunt blood toward the back by contributing to the formation of the subclavian artery and arch of the aorta. The subclavian artery sends blood through many of its branches to the back (Oelrich, 1966). A list of these branches includes the vertebral artery, branches of the costocervical trunk (the deep cervical and highest intercostal arteries), and branches of the thyrocervical trunk (the ascending cervical, transverse cervical, and suprascapular arteries) (Romanes, 1966).
As development proceeds, the two dorsal aortae fuse into a single aorta, located caudally, and the paired segmental arteries branching from this single aorta proceed to designations that vary, depending on the body region they serve. These segmental branches include intercostals, subcostals, and lumbars; all these arteries send branches to the back (Standring et al., 2008). Those paired segmental arteries supplying paired organs embedded retroperitoneally in the embryo’s posterior body wall are named after the organ they serve (e.g., renal, adrenal, and gonadal) (Brash, 1951a).
Before attenuation of the single dorsal aorta into an unpaired terminal artery known as the median sacral, the dorsal aorta gives rise to a pair of umbilical arteries that help to form the common iliac trunk on each side (Walls, 1972). The segmental arteries called the fifth lumbars also may help to form this trunk (Standring et al., 2008). One artery that branches from the common iliac trunk is the internal iliac artery, and it gives rise to two arteries that serve the back: the iliolumbar and lateral sacral (Parke, 1992a).
The aortic arches and dorsal aorta send branches to the embryo’s intervertebral foramina (IVFs) (Standring et al., 2008). By the time arterial differentiation is complete, the arteries supplying branches to the IVFs have undergone changes that vary according to the spinal level (Woodburne, 1983). In the cervical region, they branch from the ascending cervical, deep cervical, and primarily the vertebral arteries; in the thoracic region, they branch from the posterior intercostals and subcostal arteries; and in the abdominal region, they branch from the lumbar, iliolumbar, lateral sacral, and median sacral arteries (Oelrich, 1966). They rebranch once inside the IVF (Hollinshead, 1982), and in the process, intersect three arteries that run the length of the spinal cord: the single anterior spinal and the two posterior spinal arteries (see Chapter 3).
The anterior spinal artery had formed near the juncture of the brain and spinal cord by the union of a branch from one vertebral artery with a branch from the other vertebral artery to form an artery that extends caudally on the cord’s anterior aspect (Yoffey, 1976) (see Fig. 3-15). Each posterior spinal artery had branched from a vertebral and sometimes posterior inferior cerebellar artery. The posterior spinal artery extends caudally on the posterior aspect of the spinal cord (Walls, 1972). The posterior inferior cerebellar arteries were themselves branches from the vertebral arteries, which branch from the subclavian arteries (Goss, 1966). Thus the blood supply for the spinal cord originates from within the cranium by way of the vertebral arteries and, periodically all along the spine’s length, by way of feeder arteries (i.e., tributaries of arteries that enter IVFs) (Parke, 1992a). Chapter 3 discusses the arterial supply of the spinal cord in further detail.
The previous account related how the embryo constructs mesodermal channels delivering blood to those back muscles that are called intrinsic, deep, or true. The embryo develops different channels to the other back muscles that have been designated extrinsic or superficial. These latter arteries branch from the subclavian or axillary and include the following: transverse cervical, suprascapular, scapular circumflex, posterior humeral circumflex, and thoracodorsal (Crafts, 1966). Their synonymy and variability is complex (Goss, 1966). Mesoderm also has formed channels returning blood from these superficial back muscles, and often these veins retrace the route of the arteries and also bear the same names (Hollinshead & Rosse, 1985). Their variability is even greater than that of the arteries (Brash, 1951a).
Veins
While all the arterial development has been happening, the mesoderm in the epidural space surrounding the spinal cord has been forming a network of veins (Romanes, 1976) (see Fig. 3-17). This plexus lies between the meninges and the vertebrae and is known as the internal (epidural) vertebral venous plexus. This plexus receives blood from the spinal cord and the vertebrae and channels it into the intervertebral veins (Walls, 1972). These veins exit the IVFs and ventral sacral foramina (Woodburne, 1983).
Outside the foramina, the intervertebral veins drain into veins called segmental by some (Hollinshead, 1982) and intersegmental by others (Brash, 1951a). These segmental/intersegmental veins receive specific names according to their spinal level (e.g., vertebrals, intercostals, lumbars, lateral sacrals) (Brash, 1951a). These veins carry the blood back to the heart by different routes. The vertebral veins drain into the brachiocephalic veins, which pass the blood to the superior vena cava (Romanes, 1976). The intercostal veins drain into the azygos system of veins (Gosling et al., 1990), and this variable system also drains the blood to the superior vena cava. The superior vena cava empties into the right atrium of the heart (Romanes, 1976).
The lumbar veins are even more variable in their drainage pattern (Brash, 1951a). The first and second lumbar veins drain into a vein called the ascending lumbar, which in turn drains into the subcostal vein or directly into the azygos (right side) veins or hemiazygos veins (left side). The subcostal vein drains into the azygos system, and the venous blood continues on to the heart by way of the superior vena cava (Oelrich, 1966). The third and fourth lumbar veins are tributaries to the inferior vena cava, which empties into the right atrium of the heart (Brash, 1951a). The fifth lumbar vein drains into the iliolumbar vein. The iliolumbar vein drains into the common iliac vein, itself a tributary of the inferior vena cava, which empties into the heart (Romanes, 1976). The intervertebral veins exiting from the ventral sacral foramina drain into the lateral sacral veins (Oelrich, 1966). From there the blood passes to the internal iliac vein, then to the common iliac vein, and finally into the inferior vena cava, which drains into the right atrium of the heart (Goss, 1966). These lower segmental veins frequently drain into the ascending lumbar vein as well.
The intervertebral veins draining into veins in the neck, thorax, and abdomen have a common pattern. In each case they intersect a vein running lengthwise. In the cervical region the intervertebral veins are linked vertically by the vertebral vein, because they are all tributaries to it (Brash, 1951a). In the thoracic region they are connected to the vertically running azygos system of veins (Goss, 1966). In the abdominal region they drain to the ascending lumbar vein (Romanes, 1976). In the pelvic region they are linked vertically by the lateral sacral vein (Brash, 1951a). At least one authority has regarded the azygos vein as a cephalic continuation of the ascending lumbar vein (Goss, 1966).
When the mesoderm constructed a venous plexus inside the vertebral canal, it also constructed one on the outside—the external vertebral venous plexus (Parke, 1992). This external plexus of veins drains the soft and hard tissues contiguous to it (Oelrich, 1966). This external plexus also anastomoses with the internal plexus of veins inside the vertebral canal (Hollinshead, 1982). Both venous plexuses drain into the segmental veins (Goss, 1966). (The latter includes the posterior intercostal, lumbar, and lateral sacral veins [Walls, 1972].) Thus the segmental veins receive blood from the vertebrae and the soft tissues that envelop them by way of the external vertebral venous plexus (O’Rahilly, 1986). All the blood from the internal and external plexuses is added to the blood already in the segmental veins, blood that was returning from the deep muscles and skin of the back (Romanes, 1976). The blood next flows from these segmental veins into various definitive veins that the fetus has fashioned out of various precursor veins of the early embryo (Brash, 1951a).
The definitive vein that the blood from segmental veins in the cervical region ultimately drains into is known as the superior vena cava (Oelrich, 1966). It is derived from the right common cardinal vein and a part of the right anterior cardinal vein (Patten, 1953). Blood from segmental veins in the thoracic region drains into the azygos system of veins: the azygos, hemiazygos, and accessory hemiazygos veins (Walls, 1972). This azygos system forms from the embryo’s posterior cardinal veins (Goss, 1966). According to Patten (1953), the embryo’s supracardinal veins also may contribute. Blood from segmental veins in the lower lumbar and sacral regions drains into the ascending lumbar veins or the inferior vena cava, which forms from the posterior cardinal, subcardinal, and supracardinal veins of the embryo (Patten, 1953). Blood from the embryonic gonads, kidneys, and adrenal glands is delivered to the inferior vena cava by veins named after the organ they serve. They are all derived from the subcardinal veins of the embryo (Standring et al., 2008).
Virtually all the tissues comprising the bulk of the posterior body wall are derived from mesoderm (e.g., the thoracic duct and its lymphatic tributaries and the various fasciae: superficial, deep, endothoracic, transversalis, and thoracolumbar) (Goss, 1966). However, the neural elements (e.g., the lumbosacral plexus) develop from ectoderm (Larsell, 1953).
Space limitations and other considerations do not allow discussion of investigative studies on the development of frog, chick, and mouse embryos as related to the human embryo (Ballard, 1964; Rugh, 1964). However, the reader has been provided a substantial amount of background information with which to continue the study of the enormous amount of information available in the field of human development (Rothman & Simeone, 1992). Readers requiring more depth and breadth than given in this chapter are recommended to consult the scholarly treatises of Parke (1992a,b) and O’Rahilly and Muller (1992), both authorities in the field. They fill in the lacunae, sort through the controversies, and are reliable guides to primary sources in the literature. Also, some of the genetic control of development has been investigated recently (Moore & Persaud, 2008; Sadler, 2010). The reader also is encouraged to search the primary literature for the latest advances in this exciting field.
References
Arey, L.B. Developmental anatomy, 7th ed. Philadelphia: WB Saunders; 1965.
Aulehla, A., Pourquie, O. Signaling gradients during paraxial mesoderm development. Cold Spring Harb Perspect Biol. 2010;2(2):a000869.
Bagnall, K., et al. Some experimental data to support the theory of resegmentation in vertebral formations. Anat Rec. 1987;218:1A–12A.
Ballard, W.W. Comparative embryology. New York: Ronald Press; 1964.
Baxter, J.S. Frazer's manual of embryology. London: Baillière, Tindall & Cox; 1953.
Beck, F., et al. Human embryology, 2nd ed. Oxford, UK: Blackwell; 1985.
Bogduk, N. Clinical anatomy of the lumbar spine, 4th ed. London: Churchill Livingstone; 2005.
Brash, J.C. Blood vascular and lymphatic systems. In Brash J.C., ed.: Cunningham's textbook of anatomy, 9th ed., London: Oxford University Press, 1951.
Brash, J.C. Human embryology. In Brash J.C., ed.: Cunningham's textbook of anatomy, 9th ed., London: Oxford University Press, 1951.
Breathnach, A.S. Frazer's anatomy of the human skeleton, 5th ed. London: Churchill Livingstone; 1958.
Bron, J.L., van Royen, B.H., Wuisman, P.I. The clinical significance of lumbosacral transitional anomalies. Acta Orthop Belg. 2007;73(6):687–695.
Bruce, J., Walmsley, R. Beesly and Johnston's manual of surgical anatomy. London: Oxford University Press; 1939.
Callander, C.L. Surgical anatomy, 2nd ed. Philadelphia: WB Saunders; 1939.
Carlson, B.M. Patten's foundations of embryology, 4th ed. New York: McGraw-Hill; 1981.
Clark, W.E. Central nervous system. In Brash J.C., ed.: Cunningham's textbook of anatomy, 9th ed., London: Oxford University Press, 1951.
Clark, W.E. Central nervous system. In Hamilton W.J., ed.: Textbook of human anatomy, 2nd ed., St Louis: Mosby, 1976.
Cohen, M.S., et al. Anatomy of the spinal nerve roots in the lumbar and lower thoracic spine. In Rothman R.R., Simeone F.A., eds.: The spine, 3rd ed., Philadelphia: WB Saunders, 1992.
Corliss, C.E. Patten's human embryology. New York: McGraw-Hill; 1976.
Crafts, R.C. A textbook of human anatomy. New York: Ronald Press; 1966.
Davenport, H.A. Introduction and topographic anatomy. In Anson B.J., ed.: Morris' human anatomy, 12th ed., New York: McGraw-Hill, 1966.
David, K.M., et al. Cartilaginous development of the human craniovertebral junction as visualized by a new three-dimensional computer reconstruction technique. J Anat. 1998;192(Pt 2):269–277.
Durward, A. Peripheral nervous system. In Brash J.C., ed.: Cunningham's textbook of anatomy, 9th ed., London: Oxford University Press, 1951.
Ellis, H. Clinical anatomy, 7th ed. Oxford: Blackwell; 1983.
Fischman, D.A. Development of striated muscle. In Bourne G.H., ed.: The structure and function of muscle, 2nd ed., New York: Academic Press, 1972.
Francis, C.C., Voneida, J.J. The nervous system. In Anson B.J., ed.: Morris' human anatomy, 12th ed., New York: McGraw-Hill, 1966.
Giampietro, P.F., et al. Progress in the understanding of the genetic etiology of vertebral segmentation disorders in humans. Ann N Y Acad Sci. 2009;1151:38–67.
Gosling, J.A., et al. Human anatomy, 2nd ed. London: Gower Medical Publishing; 1990.
Goss, C.M. Gray's anatomy of the human body, 28th ed. Philadelphia: Lea & Febiger; 1966.
Grant, J.C.B. A method of anatomy, 5th ed. Baltimore: Williams & Wilkins; 1952.
Greene, N.D., Copp, A.J. Development of the vertebrate central nervous system: formation of the neural tube. Prenat Diagn. 2009;29(4):303–311.
Hall, B.K. Developmental and cellular skeletal biology. New York: Academic Press; 1978.
Hamilton, W.J., Mossman, H.W. Human embryology, 4th ed. Baltimore: Williams & Wilkins; 1972.
Harrison, R.G. Introduction to human embryology. In Romanes G.J., ed.: Cunningham's textbook of anatomy, 11th ed., London: Oxford University Press, 1972.
Hollinshead, W.H. The back and limbs. Anatomy for surgeons, 3rd ed. Philadelphia: Harper & Row; 1982.
Hollinshead, W.H., Rosse, C. Textbook of anatomy, 4th ed. Philadelphia: Harper & Row; 1985.
Inkster, R.G. Osteology. In Brash J.C., ed.: Cunningham's textbook of anatomy, 9th ed., London: Oxford University Press, 1951.
Joseph, J. Locomotor system. In Hamilton W.J., ed.: Textbook of human anatomy, 2nd ed., St Louis: Mosby, 1976.
Kiernan, J.A. Barr's the human nervous system, 9th ed. Philadelphia: JB Lippincott; 2008.
Langebartel, D.A. The anatomical primer. Baltimore: University Park Press; 1977.
Larsell, O. The nervous system. In Schaeffer J.P., ed.: Morris' human anatomy, 11th ed., New York: McGraw-Hill, 1953.
Leeson, C.R., Leeson, T.S. Human structure. Philadelphia: WB Saunders; 1972.
Lockhart, R.D. Myology. In Brash J.C., ed.: Cunningham's textbook of anatomy, 9th ed., London: Oxford University Press, 1951.
Mallo, M., Vinagre, T., Carapuco, M. The road to the vertebral formula. Int J Dev Biol. 2009;53:1469–1481.
Maat, G.J.R., et al. Postnatal development and structure of the neurocentral junction. Its relevance for spinal surgery. Spine. 1996;21(6):661–666.
Moore, K.L., Persaud, T.V.N. The developing human, 8th ed. Philadelphia: WB Saunders; 2008.
Mortenson, O., Pettersen, J.C. The musculature. In Anson B.J., ed.: Morris' human anatomy, 12th ed., New York: McGraw-Hill, 1966.
Mossman, H.W. Vertebrate fetal membranes. New Brunswick, NJ: Rutgers University Press; 1987.
Nicholson, A.A., Roberts, G.M., Williams, L.A. The measured height of the lumbosacral disc in patients with and without transitional vertebrae. Br J Radiol. 1988;61:454–455.
Noback, C.R., Demarest, R.J. The human nervous system, 2nd ed. New York: McGraw-Hill; 1975.
Oelrich, T.M. The cardiovascular system: arteries and veins. In Anson B.J., ed.: Morris' human anatomy, 12th ed., New York: McGraw-Hill, 1966.
O'Rahilly, R. Gardner-Gray-O'Rahilly anatomy: a regional study of human structure, 5th ed. Philadelphia: WB Saunders; 1986.
O'Rahilly, R., Muller, F. Human embryology and teratology. New York: John Wiley & Sons; 1992.
Parke, W.W. Applied anatomy of the spine. In Rothman R.R., Simeone F.A., eds.: The spine, 3rd ed., Philadelphia: WB Saunders, 1992.
Parke, W.W. Development of the spine. In Rothman R.R., Simeone F.A., eds.: The spine, 3rd ed., Philadelphia: WB Saunders, 1992.
Patten, B.M. The cardiovascular system. In Schaeffer J.P., ed.: Morris' human anatomy, 11th ed., New York: McGraw-Hill, 1953.
Patten, B.M. Foundations of embryology, 2nd ed. New York: McGraw-Hill; 1964.
Pharaoh, D.O. Chiropractic orthopedy. Davenport, Iowa: Palmer School of Chiropractic; 1956.
Risbud, M.Y., Schaer, T.P., Shapiro, I.M. Toward an understanding of the role of notochordal cells in the adult intervertebral disc: from discord to accord. Dev Dyn. 2010;239(8):2141–2148.
Robertson, G.G. Developmental anatomy. In Anson B.J., ed.: Morris' human anatomy, 12th ed., New York: McGraw-Hill, 1966.
Romanes, G.J. The central nervous system. In Romanes G.J., ed.: Cunningham's textbook of anatomy, 11th ed., London: Oxford University Press, 1972.
Romanes, G.J. The peripheral nervous system. In Romanes G.J., ed.: Cunningham's textbook of anatomy, 11th ed., London: Oxford University Press, 1972.
Romanes, G.J. Cunningham's manual of practical anatomy, 14th ed. London: Oxford University Press; 1976.
Rothman, R.R., Simeone, F.A. The spine, 3rd ed. Philadelphia: WB Saunders; 1992.
Rugh, R. Vertebrate embryology. New York: Harcourt, Brace, & World; 1964.
Sadler, T.W. Langman's medical embryology, 11th ed. Baltimore: Williams & Wilkins; 2010.
Salsabili, N., Hogg, D.A. Development of the human sacroiliac joint. Clin Anat. 1991;4(2):199–208.
Scammon, R.E. Developmental anatomy. In Schaeffer J.P., ed.: Morris' human anatomy, 11th ed., New York: McGraw-Hill, 1953.
Scothorne, R.J. Peripheral nervous system. In Hamilton W.J., ed.: Textbook of human anatomy, 2nd ed., St Louis: Mosby, 1976.
Sinclair, D.C. Muscles and fasciae. In Romanes G.J., ed.: Cunningham's textbook of anatomy, 11th ed., London: Oxford University Press, 1972.
Snell, R.S. Clinical embryology for medical students, 2nd ed. Boston: Little, Brown; 1975.
Snell, R.S. Clinical anatomy for medical students, 2nd ed. Boston: Little, Brown; 1981.
Song, D., Maher, C.O. Spinal disorders associated with skeletal dysplasias and syndromes. Neurosurg Clin N Am. 2007;18(3):499–514.
Standring, S., et al. Gray's anatomy: the anatomical basis of clinical practice, 40th ed. Edinburgh: Churchill Livingstone; 2008.
Tague, R.G. Sacralization is not associated with elongated cervical costal process and cervical rib. Clin Anat. 2011;24:209–217.
Trotter, M., Peterson, R.R. Osteology. In Anson B.J., ed.: Morris' human anatomy, 12th ed., New York: McGraw-Hill, 1966.
Verbout, A.J. The development of the vertebral column. Adv Anat Embryo Cell Biol. 1985;90:1–122.
Walls, E.W. The blood vascular and lymphatic systems. In Romanes G.J., ed.: Cunningham's textbook of anatomy, 11th ed., London: Oxford University Press, 1972.
Walmsley, R. Joints. In Romanes G.J., ed.: Cunningham's textbook of anatomy, 11th ed., London: Oxford University Press, 1972.
Woodburne, R.T. Essentials of human anatomy, 7th ed. New York: Oxford University Press; 1983.
Yoffey, J.M. Cardiovascular system. In Hamilton W.J., ed.: Textbook of human anatomy, 2nd ed., St Louis: Mosby, 1976.
Zuckerman, S. A new system of anatomy. London: Oxford University Press; 1961.