The Lumbar Region
Lumbar Lordosis and Characteristics of Typical Lumbar Vertebrae,
Developmental Considerations and the Lumbar Curve (Lordosis),
Unique Aspects of the Lumbar Vertebrae
Ligaments and Intervertebral Discs of the Lumbar Region
Lumbar Anterior Longitudinal Ligament
Lumbar Posterior Longitudinal Ligament
The lumbar portion of the vertebral column has the ideal structure to simultaneously optimize the functions of mobility and stability (Putz & Müller-Gerbl, 1996). This region of the spine is sturdy and is designed to carry the weight of the head, neck, trunk, and upper extremities. However, pain in the lumbar region is one of the most common complaints of individuals, experienced by approximately 80% of the population at some time in their lives (Nachemson, 1976; Jonsson, 2000). The estimated annual cost for low back pain claims in the United States in 1996 was $12.2 billion (Dagenais, Caro, & Haldeman, 2008) and is undoubtedly greater than that today. The total annual costs of treatment, loss of productivity, and resulting disability have been estimated to be more than $28 billion in the United States (Rizzo, Abbott, & Berger, 1998). Low back pain is the most common complaint of patients who are seen at clinics that deal primarily with musculoskeletal conditions. Low back pain of mechanical origin is the most frequent subtype found in this group (Cramer et al., 1992). The most common sources of low back pain are the lumbar zygapophysial joints (Z joints) and intervertebral discs (IVDs) (Bogduk, 1985).
Much of the reason for the high incidence of low back pain is probably related to humans being bipedal. Being able to walk on the hind limbs is accompanied by increased freedom of movement and increased ability to interact with the environment, other species, and other members of the same species. Animals that walk on the hind legs (primarily humans) can normally turn their heads with relative ease to look around to both the left and right sides. They also have the ability to use their hands for an almost infinite number of tasks without having to be concerned about using their upper extremities to help maintain balance.
However, the ability to walk on the lower extremities (the bipedal stance) has one significant drawback: Increased stress is placed on the spine. The weight of the body is concentrated on a smaller region compared with quadrupeds. The weight of the human trunk is completely supported by the lower extremities and lumbar spine during standing and it is completely absorbed by the lumbar spine and sacroiliac joints during sitting. Therefore the lumbar region, sacrum, and sacroiliac joints are susceptible to more problems than are encountered by four-legged animals. These problems can be divided into three types of lumbar disorders as well as sacroiliac joint difficulties:
1. Problems with the lumbar region:
a. The Z joints (facet joints; see Figs. 7-3 through 7-6). Increased weight borne by these joints can be a direct cause of back pain. These joints also are susceptible to arthritic changes (e.g., osteoarthritis; arthritis associated with wear and tear).
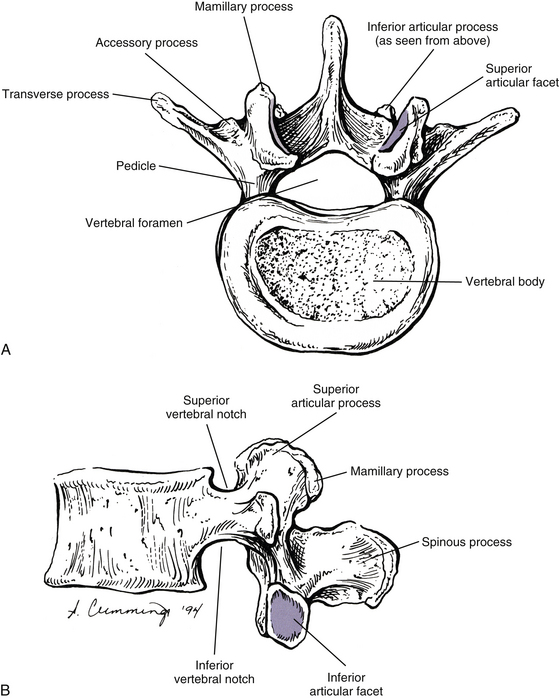
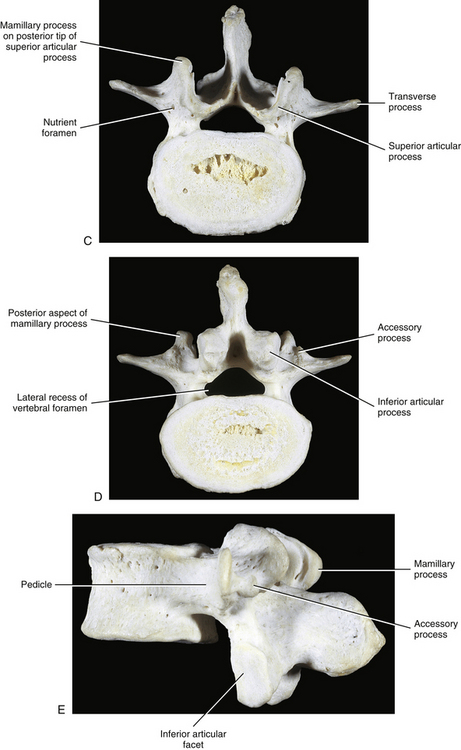
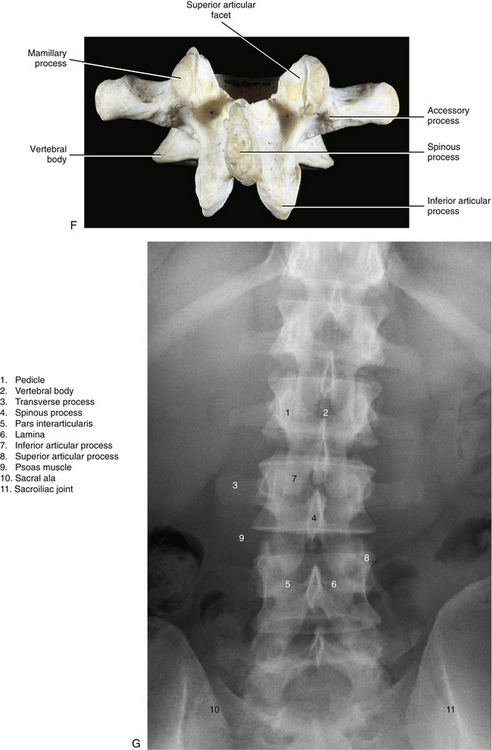
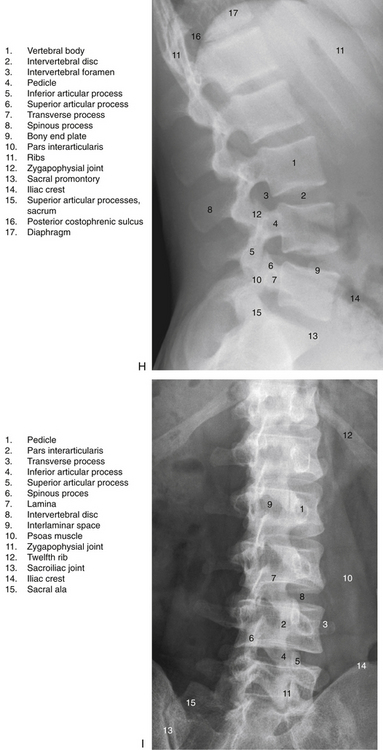
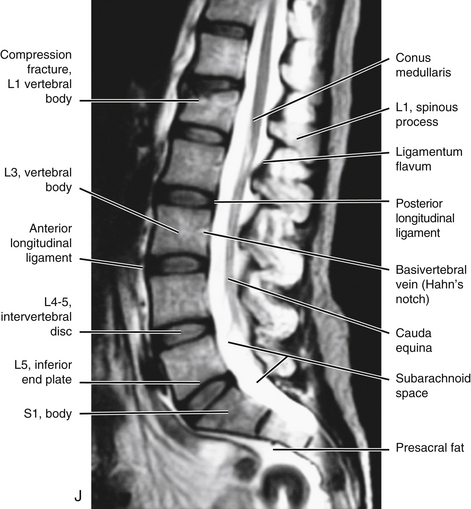
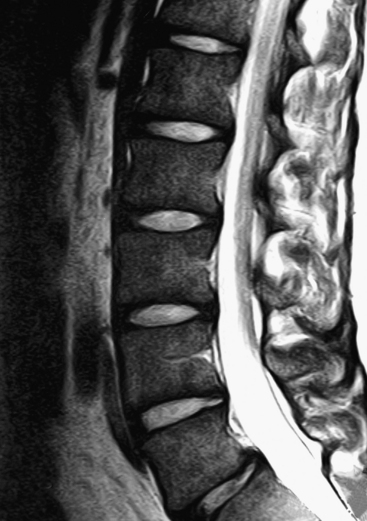
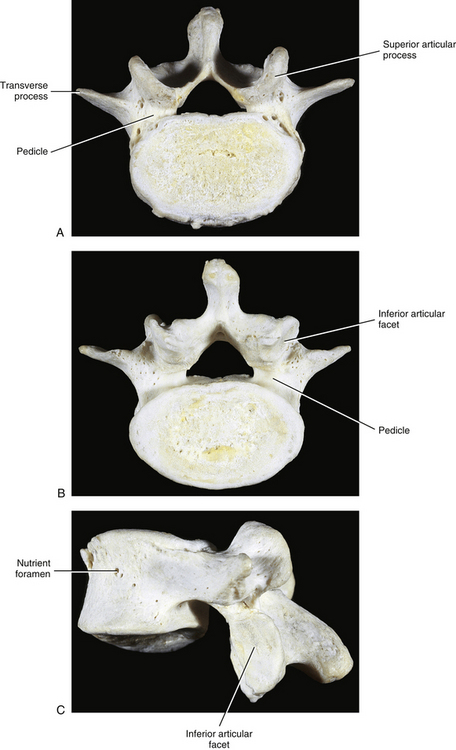
FIG. 7-2 Typical lumbar vertebra. A and C, Superior view. B and E, Lateral (slightly oblique) view. D, Inferior view. F, Posterior view. Notice in C that the superior articular process of this typical vertebra is concave posteriorly; also notice the labeled nutrient foramen (one of many) located at the junction of the pedicle and transverse process. Notice in B and E the superior vertebral notch located above the pedicle. G-I, Lumbar spine x-rays: anterior-posterior (G), lateral (H), and oblique (I) views. J, Midsagittal lumbar MRI scan (T2-weighted). (G-J, Courtesy Dr. William Bogar, National University of Health Sciences, Lombard, IL.)
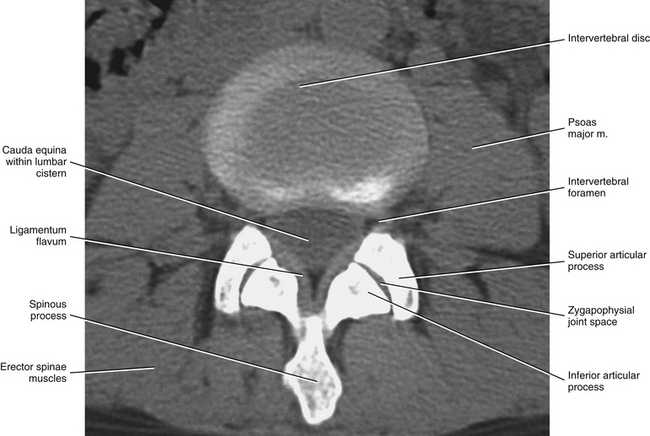
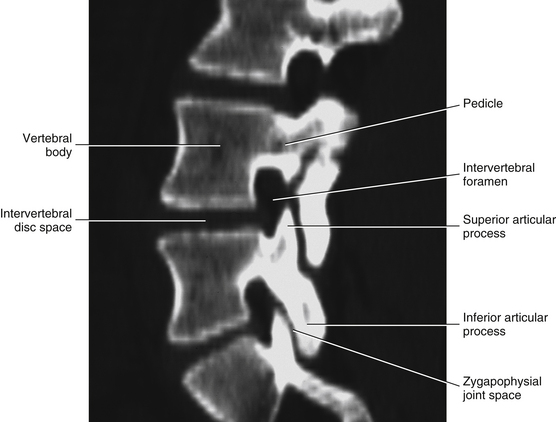
FIG. 7-3 Horizontal computed tomography scan showing the orientation of the lumbar Z joints. Notice that the left and right superior articular processes face posteriorly and medially. (Image courtesy Dr. Dennis Skogsbergh.)
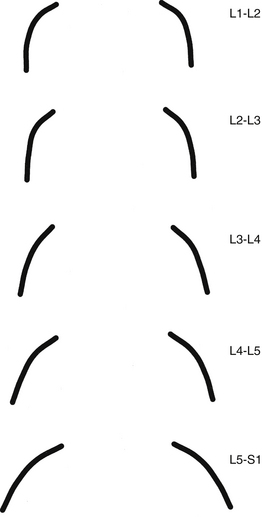
FIG. 7-4 Orientation of lumbar Z joints. Notice the changes from L1-2 to L5-S1. (From Taylor JR & Twomey LT. [1986]. Age changes in lumbar zygapophyseal joints: observations on structure and function. Spine, 11, 739-745.)
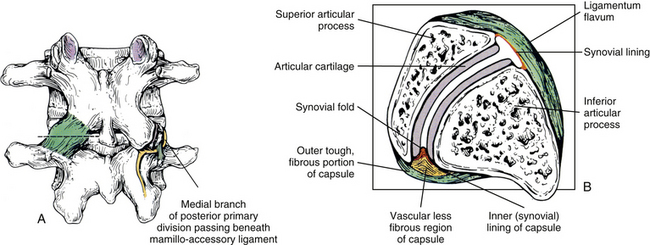
FIG. 7-5 The lumbar Z joints. A, Joint capsule (left). Mamillo-accessory ligament and its relationship to the medial branch of the posterior primary division (dorsal ramus) of the spinal nerve (right). B, Cross section through the left Z joint at the level of the dashed line shown in A. Notice the ligamentum flavum anteriorly and the Z joint capsule posteriorly. The lateral aspect of the capsule blends with the articular cartilage, and the medial aspect extends for a considerable distance along the posterior aspect of the inferior articular process. Also notice that a synovial fold can be seen extending into the joint space.
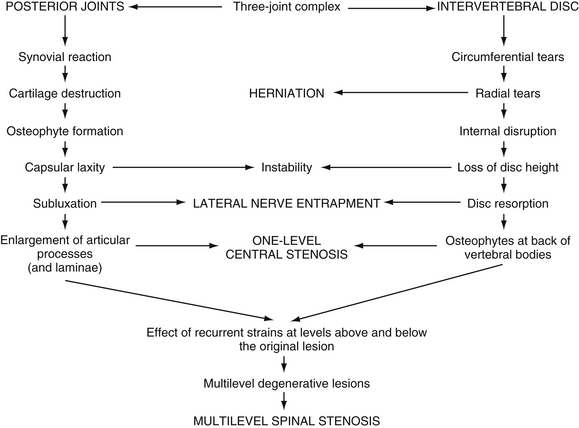
FIG. 7-6 The process of Z joint and disc degeneration and the interrelation between the two. (From Kirkaldy-Willis WH et al. [1978]. Pathology and pathogenesis of lumbar spondylosis and stenosis. Spine, 3, 319-328.)
b. The intervertebral discs (IVDs) absorb most of the increased stress received by the low back in bipeds. The discs may bulge or protrude, and by doing so compress the spinal nerves that exit behind them (see Figs. 7-25, 7-26, 11-10, and 11-11). Such protrusion can result in back pain that also has a sharp radiation pattern into the thigh and sometimes into the leg and foot. This type of pain is frequently described as feeling like a “bolt of lightning” or a “hot poker” (see Chapter 11). The IVDs may undergo degeneration also. This narrows the space between the vertebrae, which may result in arthritic changes and additional pressure on the Z joints (see Chapters 2 and 14). The discs themselves are supplied by sensory nerves and therefore can be a direct source of back pain (i.e., they do not have to compress neural elements to cause back pain).
Note: The lumbosacral region, between L5 and the sacrum, receives the brunt of the biomechanical stress of the biped spine. The lumbosacral joints (interbody joint and left and right Z joints between L5 and the sacrum) are a prime source of low back pain. In addition to the biomechanical stresses, the opening for the spinal nerve at this level is the smallest in the lumbar region, making it particularly vulnerable to IVD protrusions and compression from other sources.
c. The muscles of the low back in bipeds are needed to hold the spine erect (erector spinae muscles; see Chapter 4). Therefore when they are required to carry increased loads (this sometimes includes the added weight of a protruding abdomen), these muscles can be torn (strained).
2. The sacroiliac joints are the joints between the sacrum and the left and right ilia. The weight carried in the upright posture also can result in damage to the sacroiliac joint, another source of low back pain (see Chapter 8).
The lumbar region has been the focus of extensive high-quality research because of its clinical importance. Numerous descriptive, quantitative, and experimental studies have been completed on this area of the spine. This chapter concentrates on the unique characteristics of the lumbar vertebrae and the ligamentous, neural, and vascular elements of the lumbar region. It also includes the most pertinent results of research in an attempt to explain clearly the most important and clinically relevant idiosyncrasies of this intriguing area of the spine.
All the lumbar vertebrae are considered to be typical, although the fifth lumbar vertebra is unique. This chapter presents the typical characteristics of lumbar vertebrae, the lumbar vertebral canal, and the intervertebral foramina (IVFs). A description of the unique characteristics of L5 and the lumbosacral articulation follows. The ranges of motion of the lumbar region are included also. The chapter concludes with a discussion of the nerves, vessels, and related viscera of the lumbar region.
Lumbar Lordosis and Characteristics of Typical Lumbar Vertebrae
Developmental Considerations and the Lumbar Curve (Lordosis)
The development of lumbar vertebrae is similar to the development of typical vertebrae in other regions of the spine (see Chapter 12). Two additional secondary centers of ossification on each vertebra are unique to the lumbar region. This brings the total number of secondary centers of ossification per lumbar vertebra to seven. These additional centers are located on the posterior aspect of the superior articular processes and develop into the mamillary processes.
Between 2 and 16 years of age, the lumbar vertebrae grow twice as fast as the thoracic vertebrae. Because the anteroposterior curves of these two regions face in opposite directions (thoracic kyphosis versus lumbar lordosis), the posterior elements of thoracic vertebrae probably grow faster than their vertebral bodies; the reverse (lumbar vertebral bodies grow faster than their posterior elements) is true in the lumbar region (Clarke et al., 1985).
Normally the lumbar lordosis is more prominent than the cervical lordosis. The lumbar lordosis extends from T12 to the L5 IVD, and the greatest portion of the curve occurs between L3 and L5 (Fig. 7-1). The lordosis is greater in the standing than the seated positions (De Carvalho et al., 2010). The lumbar lordosis is created by the increased height of the anterior aspect of the L4 and L5 lumbar vertebral bodies and all of the lumbar IVDs, with the discs contributing more to the lordosis than the increased height of the vertebral bodies.
Either an increase or a decrease of the lumbar lordosis may contribute to low back pain (Mosner et al., 1989). This has sparked an interest in measurement of the lumbar curve; as a result, the lumbar curve has been measured in a variety of ways. One method, developed by Mosner and colleagues (1989), used measurements from lateral x-ray films taken with the patient in the supine position. A line was drawn across the superior vertebral end plate of L2 and another across the superior aspect of the sacral body. These two lines were continued posteriorly until they intersected, and the angle between them was measured. Using this method, angles of 47 and 43 degrees were found to be normal for women and men, respectively. This is in agreement with the values given by other authors (Standring et al., 2008).
Another relatively common method of measuring the lumbar lordosis calls for passing a line across the superior aspect of the body of L1 and another such line across the body of the first sacral segment, and then measuring the angle formed by the intersection of the continuation of the two lines. However, care must be taken when making such measurements. Normally the first lumbar vertebral body is slightly taller anteriorly than posteriorly; however, a relatively frequent anomaly is for the first lumbar vertebral body to be wedge-shaped, so that it is shorter anteriorly than posteriorly. Such wedging of the vertebra being used as a landmark for measurement can cause significant variation of measurements of the lumbar lordosis (Worril & Peterson, 1997).
In the past, many clinicians incorrectly assumed that the lumbar lordosis in the black population was greater than that in the white population, but this has been found to be incorrect; the lordosis is appromimately the same in both races (Mosner et al., 1989).
Clinical Considerations
Certain individuals develop a painful degenerative lumbar kyphosis that is characterized by disc and vertebral body wedging and weakness of lumbar paraspinal muscles. This condition may be related to a prolonged working posture in flexion. For example, a higher incidence of this condition has been found in Japanese dairy farmers when compared with Japanese field farmers. The working posture of the former group calls for long periods of forward flexion while squatting. This posture is maintained for the majority of each working day throughout the workers’ careers, which can last for many decades (Takemitsu et al., 1988). Such prolonged flexion is thought to cause remodeling of the vertebral bodies of the lumbar region so that they are shorter anteriorly than posteriorly.
The lumbar lordosis is often significantly increased in achondroplasia. This can lead to a marked compensatory thoracic (thoracolumbar) kyphosis, which can be severe in some cases (Giglio et al., 1988).
A high incidence of scoliosis (64%) exists in the lumbar region of individuals with Marfan syndrome. Other lumbar vertebral anomalies associated with Marfan syndrome include a high incidence of transitional lumbosacral segments (18%), concavity of both the superior and inferior aspects of the vertebral bodies (biconcavity), and longer than normal transverse processes (Tallroth et al., 1995).
Vertebral Bodies
When viewed from above, the vertebral bodies of the lumbar spine are large and kidney-shaped with the concavity facing posteriorly (Fig. 7-2). However, L5 is more elliptical in shape. In addition, the inferior and superior bony end plates of adjacent vertebrae sharing the same IVD are similar in size and shape. Although the lumbar vertebral bodies of males generally have greater dimensions than those of females, the shapes of the vertebral bodies are similar (Hall et al., 1998). The superior surfaces of the vertebral bodies possess small elevations along their posterior rim. These represent remnants of the uncinate processes of the cervical region. The inferior surfaces of the vertebral bodies have two small notches along their posterior rim. These notches correspond to the uncinate-like elevations of the vertebra below. These elevations and notches have been used as landmarks on x-ray films as a means for evaluating normal and abnormal movement between adjacent lumbar segments (Dupuis et al., 1985).
The volume of the lumbar vertebral bodies (average 35 cm3; range 19.7 to 61.5 cm3) is greater in males than females and increases from L1 to L4, with L5 having a smaller volume than L4 (Limthongkul et al., 2010). The vertebral bodies are wider from side to side (lateral width) than from front to back. The L4 and L5 vertebral bodies are taller in front (anteriorly) than behind. The reverse is true for L1 and L2, although the difference in heights is less, and the anterior and posterior heights of L3 are approximately the same (Masharawi et al., 2008). Therefore as mentioned, the L4 and L5 vertebral bodies are partially responsible for the creation and maintenance of the lumbar lordosis, which is most pronounced from L3 to L5.
Normally, the lateral width of the lumbar vertebrae increases from L1 to L3. L4 and L5 are somewhat variable in width (Standring et al., 2008). Ericksen (1976, 1978) found that the L1-4 vertebral bodies (he did not study L5) became wider from side to side with age. Also, he noted a decrease in height of the vertebral body’s anterior aspect, corresponding with an increase in its lateral width, in both males and females. Further, the increase in lateral width in males was accompanied by a corresponding decrease in height of the vertebral body’s posterior aspect (Erickson, 1976, 1978). Masharawi and colleagues (2008) found a “left lateral wedging” of L1, L2, and L5, with the superior-inferior height of the right side of these vertebrae being greater than that of the left side. The wedging was more common in females than in males. See Chapter 6, Vertebral Bodies for further details on lateral wedging of vertebral bodies.
Up to 90% of the compressive loads received by the vertebral bodies are carried by the inner cancellous (trabecular) bone (Silva, Keaveny, & Hayes, 1997); however, this may be an overestimation (Cao, Grimm, & Yang, 2001). The remaining 10% (or more) of the compressive loads are carried by the thin outer cortical shell of the vertebral bodies (Silva, Keaveny, & Hayes, 1997). However, with the bone loss associated with osteoporosis a higher percentage of the compressive loads is carried by the outer cortical shell of bone (Cao, Grimm, & Yang, 2001).
Perhaps somewhat paradoxically, the bony end plates of the vertebral bodies are particularly important in resisting compressive loads placed on the spine. Oxland and colleagues (2003) found that removal of the bony end plates decreased the ability of the vertebral bodies to resist compression fracture (2003) found that removal of the bony end plates decreased the ability of the vertebral bodies to resist compression fracture by 66%. The end plates of the lumbar region are weakest in the center, and strongest posterolaterally. In addition, the inferior end plates are stronger than the superior ones, and the sacral end plate is similar in strength to the inferior lumbar bony end plates (Grant, Oxland, & Dvorak, 2001).
The trabeculae of vertebral bodies adapt to the external loads placed on them (Bian et al., 2011); however, this trabecular “plasticity” diminishes significantly after the age of 50 years (Cvijanovic et al., 2004). The decreased trabecular plasticity (along with decreased trabecular size) could plausibly contribute to vertebral body compression fractures in the elderly populations.
The blood supply to the lumbar vertebral bodies is extensive and complex (Bogduk, 2005). Each lumbar segmental artery gives off up to 20 primary periosteal arteries as it courses across the anterolateral aspect of the vertebral body. The posterior aspect is supplied by many branches of the anterior vertebral canal artery. The anterior vertebral canal artery refers to the anterior branch of the artery that passes through the IVF. The artery that passes through the IVF sometimes is known as the spinal ramus of the (lumbar) segmental artery.
The end branches of periosteal arteries form a ring around both the superior and inferior margins of the vertebral body. These rings are known as the metaphyseal anastomoses (superior and inferior). They not only help to supply the metaphyseal region of the vertebral body, but also send penetrating branches (metaphyseal arteries) to the region of the vertebral end plate (Bogduk, 2005). A dense capillary network is associated with the superior and inferior vertebral end plates. This network receives contributions from the metaphyseal and nutrient arteries.
Nutrient arteries also arise from the anterior vertebral canal arteries. The nutrient arteries enter the center of the posterior aspect of the vertebral body, pass deep within the substance of the cancellous bone of the vertebral body’s center, and then form superior (ascending) and inferior (descending) branches. In addition to giving off periosteal arteries, the lumbar segmental arteries also send branches that enter the cancellous bone of the anterior and lateral aspects of the vertebral bodies. These branches enter along the superior-to-inferior midpoint of the vertebral bodies. Known as the equatorial arteries, these vessels are similar to the nutrient arteries in that they also give rise to ascending and descending branches deep within the substance of the vertebral bodies (Bogduk, 2005). See Chapter 2 and Figure 2-3 for further details on the blood supply to and venous drainage of typical vertebrae.
The lumbar vertebral bodies serve as attachment sites for several structures. Table 7-1 lists those structures that attach to the lumbar vertebral bodies.
Table 7-1
Attachments to Lumbar Vertebral Bodies
| Region | Structure(s) Attached |
| Anterior surface | Anterior longitudinal ligament on superior and inferior borders |
| Posterior surface | Posterior longitudinal ligament on superior and inferior borders |
| Lateral surface | Crura of diaphragm (anterolateral surface of left L1 and L2 and right L1, L2, and L3) Origin of psoas major muscle (posterolateral aspect of superior and inferior surface of all lumbars); series of tendinous arches between vertebral attachments of psoas major muscle creates concave openings between arches and vertebral bodies, allowing for passage of segmental arteries, veins, and gray communicating rami of sympathetic chain |
From Standring S et al. (2008). Gray’s anatomy: the anatomical basis of clinical practice (40th ed.). Edinburgh: Churchill Livingstone.
Clinical Considerations
Osteophytes of the lumbar vertebral bodies can be large. They are much more prominent laterally than anteriorly (the opposite of what is commonly found in the thoracic region), and usually they are completely absent anteriorly at the L3-4 and L4-5 levels. This seems to be associated with the pulsing action of the abdominal aorta and proximal portions of the common iliac arteries (Edelson & Nathan, 1988).
Fractures of the secondary centers of ossification associated with the superior and inferior vertebral end plates, the ring apophyses (also known as anular apophyses; see Chapters 2 and 12), have been reported. These fractures are rare but occur most frequently during adolescence. The signs and symptoms of apophyseal ring fractures resemble those of IVD protrusions. Such fractures may be unnoticed on conventional x-ray films. Sagittally reformatted computed tomography (CT) is currently the imaging modality that shows these fractures to best advantage (Thiel, Clements, & Cassidy, 1992).
Pedicles
The pedicles of the lumbar spine are short but strong (see Fig. 7-2). They attach lower on the vertebral bodies than the pedicles of the thoracic region, but higher than those of the cervical region. Therefore each lumbar vertebra has a superior vertebral notch that is less distinct than that of the cervical region. On the other hand, the inferior vertebral notch of lumbar vertebrae is prominent.
The size of the pedicles, like the size of many spinal structures, can vary among individuals of different ethnic backgrounds. Such normal ethnic variations should be kept in mind when evaluating x-ray films and CT or magnetic resonance imaging (MRI) scans. For example, the width and length of the pedicles of individuals of East Indian descent are less than those of whites (Mitra, Datir, & Jadhav, 2002; Acharya, Dorje, & Srivastava, 2010).
The role of the pedicles in the transfer of loads is discussed in Chapter 2. More study is needed to confirm the role played by the pedicles in the transfer of loads in the upper lumbar region (Pal et al., 1988). However, the trabecular pattern of the L4 and L5 pedicles seems to indicate that most loads placed on these vertebrae may be transferred from the vertebral bodies to the region of the posterior arch, specifically to the pars interarticularis (see Laminae).
Transverse Processes
Each transverse process (TP) (left and right) of a typical lumbar vertebra projects posterolaterally from the junction of the pedicle and the lamina of the same side (see Fig. 7-2). It lies in front of (anterior to) the articular processes and behind (posterior to) the lumbar IVF.
The lumbar TPs are quite long, the TPs of L3 being the longest. As with other components of lumbar vertebrae, the left and right transverse processes are asymmetric in length, with the left transverse process being longer than the right (same as the thoracic region). In addition, the pedicles of males are larger than those of females (Masharawi & Salame, 2011). The distance between the apices of the left and right TPs is much greater on L1 than T12. This distance increases on L2 and is the greatest in the entire spine on L3. The intertransverse distance between the left and right L4 TPs is smaller than that of L3 and is even smaller for L5. The lumbar TPs are flat and thin from front to back. They are also narrower from superior to inferior than their thoracic counterparts. They possess neither articular facets (as do thoracic TPs) nor foramina of the transverse processes (as do cervical TPs). The anterior aspects of the lumbar TPs also are known as the costal elements, and they occasionally develop into ribs. This happens most frequently at L1.
The lateral aspect of the anterior surface of the lumbar TPs is creased by a ridge that runs from superior to inferior. This ridge is created by the anterior layer of the thoracolumbar fascia. The middle layer of the thoracolumbar fascia attaches to the apex of the TPs. Table 7-2 lists structures that attach to the lumbar TPs.
Table 7-2
Attachments to Lumbar Transverse Processes
| Region | Structure(s) Attached |
| Anterior surface | Psoas major and quadratus lumborum muscles Anterior layer of thoracolumbar fascia (separates psoas major and quadratus lumborum muscles) Medial and lateral arcuate ligaments (lumbocostal arches) (L1) |
| Apex | Middle layer of thoracolumbar fascia Iliolumbar ligament (L5, occasionally L4) |
| Superior border | Lateral intertransversarius muscle |
| Inferior border | Lateral intertransversarius muscle |
| Posterior surface | Deep back muscles (longissimus thoracis) |
From Standring S et al. (2008). Gray’s anatomy: the anatomical basis of clinical practice (40th ed.). Edinburgh: Churchill Livingstone.
Accessory Processes
The accessory processes are unique to the lumbar spine. Each accessory process projects posteriorly from the junction of the posterior and inferior aspect of the TP with the corresponding lamina. These processes serve as attachment sites for the longissimus thoracis muscles (lumbar fibers) and the medial intertransversarii lumborum muscles (Standring et al., 2008). (See Figure 4-5, B, for the attachment of the medial intertransversarii lumborum muscles to the accessory processes.)
Articular Processes
Left and right superior articular processes are formed on every vertebrae of the lumbar spine (see Fig. 7-2). Each superior articular process possesses a hyaline cartilage–lined superior articular facet that is oriented in a vertical plane. That is, these facets are not angled to the vertical plane like the superior articular facets of the cervical and thoracic regions.
All the lumbar superior articular facets face posteriorly and medially. The articular surface of a typical superior articular facet can be gently curved with the concavity facing medially (Figs. 7-3 and 7-4), or the articular surface can be angled abruptly. When the articular surface is angled abruptly, two relatively distinct articulating surfaces are formed. One surface faces posteriorly and forms almost a 90-degree angle with the second surface, which faces medially. As with the curved facet, the concavity faces posteriorly and medially. In either case (curved or angled articular surface), the shape conforms to the inferior articular facet of the vertebra above. The hyaline cartilage of the central region of the superior articular facet (the area of greatest concavity) increases in thickness with age, probably because this region receives much of the load during flexion of the spine (Taylor & Twomey, 1986). Also, the articular processes may fracture as a result of age-related degeneration (Kirkaldy-Willis et al., 1978).
The orientation of the superior articular facets varies from one vertebral level to another (see Fig. 7-4). A line passed across each superior articular facet, on transverse CT scans, shows that the L4 superior facets (and therefore the L3-4 Z joints) are more sagittally oriented than the L5 facets. Also, the S1 superior facets (and therefore the L5-S1 Z joints) are more coronally oriented than the L4 and L5 facets (Van Schaik, Verbiest, & Van Schaik, 1985). (See Zygapophysial Joints for further detail on the orientation of the superior and inferior articular facets.) The angle of the L4-L5 Z joint (i.e., the angle of the superior articular facets) has been found to become more sagittally oriented with age, which may explain the higher incidence of degenerative spondylolisthesis (forward slippage of L4 on L5) at this level in individuals more than 60 years of age (Wang & Yang, 2009). The left and right articular facets are usually symmetrical in orientation (unlike the thoracic facets) (Masharawi et al., 2004) with the exception of the facets that form the L5-S1 articulation where left to right asymmetry (tropism) occurs rather frequently. However, the right facets of L1-L4 are slightly longer from superior to inferior than the left facets (Masharawi et al., 2005).
The mamillary processes (see Fig. 7-2), which project posteriorly from the superior articular processes of lumbar vertebrae, are unique to the lumbar spine. Each mamillary process is a small rounded mound of variable size. Some are almost indistinguishable, whereas others are relatively prominent. The mamillary processes serve as attachment sites for the multifidi lumborum muscles.
Mamillo-Accessory Ligament
A mamillo-accessory ligament (Bogduk, 1981; Lippitt, 1984) is found between the mamillary and accessory processes on the left and right sides of each lumbar vertebra. Occasionally one or more of these ligaments may ossify in the lower lumbar levels (L3-5). The incidence of ossification increases in frequency from L3 to L5. Ossification of these ligaments at L5 occurs at a frequency of 10% (Bogduk, 1981) to 26% (Maigne, Maigne, & Guervin-Surville, 1991). Maigne and colleagues (1991) studied 203 lumbar spines and found that ossification of this ligament at L5 occurred twice as often on the left. They offered no reason to explain why the ligament ossified more frequently on this side. However, they believed the ossification was the result of osteoarthritis, because they found no evidence of ossification on the spines of children and young adults. These authors also stated that an ossified mamillo-accessory ligament could occasionally be seen on standard lumbar x-ray films.
The mamillo-accessory ligament has been described as a tough, fibrous band that may represent tendinous fibers of origin of the lumbar multifidi muscles or fibers of insertion of the longissimus thoracis pars lumborum muscle (Bogduk, 1981). Regardless of its precise structure, the reported purpose of the mamillo-accessory “ligament” is to hold the medial branch of the dorsal ramus of the above spinal nerve (e.g., the L2 medial branch is associated with the L3 mamillo-accessory ligament) against (a) the bone between the base of the superior articular process and the base of the transverse process and (b) the Z joint, which is slightly more medial (Fig. 7-5). The medial branch of the dorsal ramus gives off articular branches to the capsule of the Z joint as it passes deep to the mamillo-accessory ligament. The medial branch then continues medially across the vertebral lamina to reach the multifidus muscle (Bogduk, 1981). Therefore the medial branch of the dorsal ramus (posterior primary division) is held within a small osteofibrous canal along the posterior arch of a lumbar vertebra. The medial branch could possibly become irritated or even entrapped within this tunnel, which would result in low back pain. However, more investigation is necessary to determine if such irritation or entrapment occurs and, if so, its frequency (Bogduk, 1981; Maigne, Maigne, & Guerin-Surville, 1991).
Inferior Articular Processes
The inferior articular processes of lumbar vertebrae are convex anteriorly and laterally. They possess inferior articular facets that cover their anterolateral surface. As with the superior articular facets, the inferior ones vary in shape. Even though articular processes vary from one vertebral level to another, and even from one side to another, superior and inferior articulating processes of one Z joint conform to one another. This conformation is such that each inferior articular facet usually fits remarkably well into the posterior and medial concavity of the adjoining superior articular facet. The exception to this is that the inferior articular processes and their facets are less vertically oriented and angle slightly anteriorly to the frontal plane. This orientation (“disharmony” with the superior articular processes) likely enhances motion of the lumbar region (Masharawi et al., 2004).
Zygapophysial Joints
The Z joints have been positively identified as a source of back pain (Mooney & Robertson, 1976; Yamashita et al., 1990; Bogduk, 1992; Dreyfuss & Dreyer, 2001). In addition, Rauschning (1987) states that these joints “display typical degenerative and reparative changes which are known to cause osteoarthritic pain in peripheral synovial joints.” Therefore the lumbar Z joints are highly significant clinically. Because these joints are discussed in detail in Chapter 2, this section focuses on the unique and clinically significant aspects of the lumbar Z joints.
The lumbar Z joints are considered to be complex synovial joints oriented in the vertical plane (Standring et al., 2008). They are fashioned according to the shape of the superior and inferior articular facets (see previous discussion). Therefore the lumbar Z joints are concave posteriorly and even have been described as being biplanar in orientation (Taylor & Twomey, 1986). That is, they have a coronally oriented, posterior-facing, anteromedial component and a large, sagittally oriented, medial-facing, posterolateral component. Taylor and Twomey (1986) state that the lumbar Z joints are coronally oriented in children and that the large sagittal component develops as the individual matures. The sagittal component limits rotation, whereas the coronal component limits flexion. More specifically, the shape of the lumbar Z joints allows a large amount of flexion to occur in the lumbar region, yet the size of the lumbar Z joints is what eventually limits flexion at the end of the normal range of motion. Therefore the long contact surfaces between the coronal component of the superior articular processes and the adjacent inferior articular processes finally limit flexion by “restraining the forward translational component of flexion” (Taylor & Twomey, 1986).
Even though the size of the Z joints eventually limits flexion, approximately 60 degrees of flexion is able to occur in the lumbar region before the bony restraints of the lumbar articular processes prevent further movement. However, the size and shape of the Z joints dramatically limit rotation. By limiting rotation, the Z joints protect the IVDs from rotational injury. During rotation of the lumbar region, distraction (or gapping) occurs between adjacent lumbar articular facets (superior facet of vertebra below and inferior facet of vertebra above) on the side of rotation. For example, right rotation results in gapping of the facets on the right side. Also during rotation, the two opposing facets of the opposite side are pressed together. This causes them to act as a fulcrum for the distracting facets on the side of rotation (Paris, 1983; Cramer et al., 2003). Side posture spinal adjusting, performed by chiropractors and other health practitioners, also causes gapping of the Z joints. The gapping action produced by such adjustive procedures is thought to break-up fibrous adhesions that develop in Z joints that are moving less than normal (are hypomobile) (Cramer et al., 2000, 2002, 2003, 2010).
Extension of the lumbar region is limited by the inferior articular process on each side of a lumbar vertebra contacting the junction between the lamina and superior articular process of the vertebra below. This junction between the lamina and superior articular process is known as the pars interarticularis.
Variation of Zygapophysial Joint Size and Shape
A considerable degree of variation exists between individual Z joints at different lumbar levels and between the left and right Z joints at the same vertebral level. The shapes range from a slight and gentle curve, concave posteriorly; to a pronounced, dramatic, and posteriorly concave curve; and in some cases to a joint in which the posterior and medial components face one another at an angle of nearly 90 degrees. Generally, the Z joints of the upper lumbar levels are more sagittally oriented than those of the lower lumbar levels (see Fig. 7-4). This makes the lower lumbar joints more susceptible to recurrent rotational strain (Kirkaldy-Willis et al., 1978). However, Z joint osteoarthritis is more prevalent in lower lumbar (L3-L4, L4-L5, and L5-S1) Z joints (particularly at L4-L5) when they are more sagittally oriented (Kalichman et al., 2009).
Biomechanical Considerations
The articular facets do not absorb any of the compressive forces of the spine when humans are sitting erect or standing in a slightly flexed posture. The IVD and vertebral bodies absorb almost all the compressive loads under these conditions. However, when a person stands erect (slight extension), the facets resist approximately 16% (range, 3% to 25%) of the compressive forces between vertebrae. Disc degeneration may lead to increased stress on the Z joints, causing them to resist up to approximately 47% of the loads. This results in pain, not from the articular cartilage, but usually from pressure on the subchondral bone of the articular processes, entrapment of soft tissue between the articular facets, or added strain on the articular capsules (Dunlop, Adams, & Hutton, 1984; Yang & King, 1984; Hutton, 1990). In addition, beyond the limit of normal physiologic flexion, the inferior articular process is forced over the superior articular process, and the compressive loads carried by the facets again increase from those carried in the neutral position (Shirazi-Adl & Drouin, 1987).
The superior and inferior articular processes of the Z joints also resist anterior shear forces. The articular processes carry approximately one third of the anterior shear forces during forward flexion, whereas the IVDs carry approximately two thirds of the shear loads (when the load is applied suddenly). However, because of the viscoelasticity of the IVD, if a shear load is applied slowly, the vertebra above creeps further forward than when shear loads are applied rapidly. Thus the shear forces on the articular processes are increased with slowly applied and long-lasting loads in forward flexion (e.g., bending forward for long periods to weed a garden).
Articular Capsules
An articular capsule covers the posterior aspect of each lumbar Z joint (see Fig. 7-5), and the ligamentum flavum covers the anteromedial aspect of the Z joint. The articular capsule is tough, possesses a rich sensory innervation, and is well vascularized (Giles & Taylor, 1987; Ashton et al., 1992).
The bundles of collagen fibers that form the thick outer fibrous layer of a typical lumbar Z joint capsule are oriented in different directions, depending on whether the fibers are located in the superior, middle, or inferior aspect of the capsule. The superior fibers attach laterally to the groove that is located between the mamillary process and posterior ridge of the superior articular process. From here the fibers of the superior capsule course medially across the superior joint recess, attaching to the medial aspect of the laminae of the superior-most vertebrae forming the Z joint. Fibers of the middle part of the Z joint capsule course more horizontally (lateral to medial), beginning from their attachment to the posterior aspect of the mamillary process (on the posterior aspect of the superior articular process) (Yamashita et al., 1996). These fibers then cross the Z joint gap and attach to the medial part of the lamina of the suprajacent vertebra. The fibers just described extend a considerable distance medially and become continuous with the lateral fibers of the interspinous ligament. Finally, the fibers of the more inferior aspect of the capsule course from superomedial to inferolateral, covering the joint space and the inferior end of the inferior Z joint recess. The superomedial fibers attach to the medial and inferior part of the lamina. The bundles of collagen fibers of this inferior aspect of the capsule are longer and thicker than those of the superior aspect of the capsule (Yamashita et al., 1996).
The orientation of the fibers within the tough outer layer of the capsule helps to limit forward flexion (Paris, 1983). The medial-to-lateral arrangement of the fibers in the central part of the Z joint may tend to stabilize the joint during axial rotation, while allowing movement in flexion and extension (Yamashita et al., 1996). The contribution of the lumbar Z joint capsule in stabilizing and perhaps even in helping to limit motion during axial rotation also has been supported by biochemical studies that show evidence of increased capsular resistance to tension in regions of the capsule that would receive the greatest stretch during this motion (Boszczyk et al., 2001). The collagen fibers of the superior and middle aspects of the Z joint are shorter than those of the inferior aspect of the capsule and are thought to be under more tensile stretch than the inferior aspect of the capsule. The posterosuperomedial-most corner of the capsule (just inferior to the superior Z joint recess) is thought to be the area of the capsule that receives the highest tensile stretch (Yamashita et al., 1996). In fact, Yamashita and colleagues (1996) stated that the lumbar Z joint capsules undergo “extensive stretch under physiological loads.”
The collagen fibers of the inferior aspect of the lumbar Z joint capsules are thicker and more heavily innervated than those found in the superior and middle aspects of the capsules. This may be because the inferior aspects of the capsule can be compressed (therefore requiring increased thickness), and even pinched (thus resulting in pain from additional nociceptive nerve endings), during full extension of the lumbar region (Yamashita et al., 1996).
Laterally, fibers of each articular capsule frequently are continuous with the articular cartilage lining the superior articular facet. A gradual transition occurs from the fibrous tissue of the capsule to fibrocartilage and finally to the hyaline cartilage of the superior articular facet (Taylor & Twomey, 1986).
Each capsule has a relatively large superior and inferior recess that extends away from the joint. The capsular fibers (or fibers of the ligamentum flavum, anteriorly) surrounding these recesses are relatively thin and loose, and there may be openings through which neurovascular bundles enter the recesses (Taylor & Twomey, 1986). Paris (1983) states that effusion within the Z joint may enter the superior recess and as little as 0.5 ml of effusion may cause the superior recess to enter the anteriorly located IVF. Once in the IVF, this expanded superior capsular recess may compress the dorsal root ganglion or the exiting spinal nerve, resulting in radiculopathy (see Chapter 11 for a full discussion of radiculopathy). Such a distinct protrusion of the superior recess of the Z joint capsule constitutes an example of a large Z joint synovial cyst (Xu et al., 1991; Bougie, Franco, & Segil, 1996).
Xu and colleagues (1991), in a study of 50 pairs of lumbar Z joints from an elderly population, found that the capsules varied greatly in thickness and regularity. They found that the capsules were “irregularly thickened, amorphous, and calcified in 22 cases.” In addition, they found that in most subjects the synovium of the joint space extended 1 to 2 mm outside the boundaries of the joint in one or more locations. These synovial extensions, which could be considered to be small synovial cysts, maintained communication with the joint space, and were found on both the anterior and posterior aspects of the joint. Anteriorly the spaces most often extended along the posterior border of the ligamentum flavum (64% of Z joints). Sometimes a synovial joint extension extended directly into the ligamentum flavum (8% of cases). Posteriorly the synovial cysts usually extended either laterally along the superior articular process (7% of cases) or medially along the inferior articular process (29%). Extensions were found on both the inferior and superior articular processes in 41% of the Z joints, and no synovial extensions were found in 23% (7% of individuals) of the Z joints. Xu and colleagues (1991) believed that the synovial joint extensions (synovial cysts) probably were more common in the older population (e.g., the specimens in their study) than in younger age groups. They stated that their findings may explain why Z joint arthrography (visualization of the Z joint on x-rays after injection with radiopaque dye) could be successful even when the joint space was not entered with the injecting needle (i.e., the needle could enter a synovial extension).
In another study, Xu, Haughton, and Carrera (1990) visualized the synovial joint extensions (cysts) with magnetic resonance imaging (MRI). Their positive identification of these synovial joint extensions was aided with the injection of paramagnetic contrast medium (gadodiamide, Winthrop Pharmaceuticals). Their imaging was performed on dissected spines using small surface coils, long acquisition times, and thin slice thicknesses. Therefore clear delineation of these extensions is probably still beyond the resolving capabilities of MRI scans obtained in a typical clinical setting. However, Xu and colleagues (1991) thought their results helped to explain the variable appearance of the facet joints on MRI scans. They stated that “the inhomogeneity detected at MR imaging and computerized tomography in the ligamentum flavum near the facet joint most likely represents extension of the joint capsule between the ligamentum flavum and the articular processes…” (Xu et al., 1991).
The superior and inferior recesses are filled with fibrofatty pads. These pads are well vascularized and are lined with a synovial membrane. They also protrude a significant distance into the superior and inferior aspects of the Z joint. Engel and Bogduk (1982) state that adipose tissue pads probably develop from undifferentiated mesenchyme of the embryologic Z joint. Furthermore, they note that in certain instances, mechanical stress to the joint may cause an adipose tissue pad to undergo fibrous metaplasia, which results in the formation of a large fibroadipose meniscoid (an adipose tissue pad with a fibrous tip composed of dense connective tissue, to be differentiated from the normal synovial folds described in Ch 2). The authors believed that both the adipose tissue pads and the normally found synovial folds, or meniscoids, “play some form of normal functional role” (see Fig. 7-5).
In addition, “fringes” of synovium extend from the capsule to the region between the articular facets. These fringes fill the small region in which the facets do not form a complete union.
Sometimes a fatty synovial fold develops a relatively long fibrous tip that extends a considerable distance between the joint surfaces, where it may become compressed (Taylor & Twomey, 1986). Such protruding synovial folds (again, different from the normal synovial folds) have been associated with the early stages of degeneration (Rauschning, 1987). Rauschning (1987) typically found hemarthrosis and effusion into the Z joints when the meniscal folds of this type were torn or “nipped” between the joint surfaces.
Occasionally the aging process causes a piece of articular cartilage to break from the superior or inferior articular facet. The piece usually breaks along the posterior aspect of the Z joint, parallel to the joint space. However, the attachment to the articular capsule usually is maintained. The result is the presence of a large, fibrocartilaginous meniscoid inclusion within the joint. Taylor and Twomey (1986) reported that the formation of this type of meniscoid is partially caused by the regular pulling action on the posterior articular capsule by the multifidi muscles that originate from these capsules. The attachment of the capsule to the periphery of the articular cartilage may then result in the cartilage tears found to run parallel with the joint surface. Again, the torn cartilage fragment is capable of developing into a Z joint meniscoid, which can become interposed between the two surfaces of the joint. The authors also noted that the posterior aspect of the Z joint may open when the multifidi and other deep back muscles relax, allowing the meniscoids and other joint inclusions to bow into the joint space and become entrapped (Kos, Hert, & Sevcik, 2002). The result of this type of entrapment could be (a) loss of motion (locked back), resulting from the cartilage being lodged between two opposing joint surfaces; (b) pain, because the meniscoids remain attached and therefore might put traction on the pain-sensitive joint capsule; or (c) both decreased motion and pain.
As described in Chapter 2, the Z joint articular capsule receives sensory innervation from the medial branch of the posterior primary division of three consecutive spinal nerves. The sensory receptors in the Z joint capsule are both free nerve endings (for nociception) and mechanoreceptors (for proprioception). Consequently, the Z joint capsules not only are potential sources of low back pain (i.e., are potential “pain generators”) but also are likely to play a role in lumbar proprioception (Ianuzzi, Pickar, & Khalsa, 2011). Using nerve tracing techniques in rats, Suseki and colleagues (1997) found that some sensory neurons innervating the lower lumbar Z joint capsules arise from the L1 and L2 dorsal root ganglia. These sensory fibers course through the lumbar sympathetic chain and then through gray communicating rami to reach the lower lumbar Z joints. These authors felt that this innervation of lower lumbar spinal tissues by sensory nerves arising from upper lumbar dorsal root ganglia might help to explain the clinical finding that pathology of lower lumbar spinal tissues sometimes can be described by patients as referring to regions innervated by higher lumbar segments (e.g., radiation into the inguinal region from a painful L5-S1 Z joint).
The pain-sensitive articular capsule and the synovial lining of the ligamentum flavum of each lumbar Z joint normally are held out of the joint by three structures. First, the multifidus lumborum muscles take part of their origin (several originate from each vertebra) from the posterior aspect of each articular capsule (Taylor & Twomey, 1986). These muscles pull the capsule out of the joint’s posterior aspect. Second, the ligamentum flavum pulls the synovium out of the joint’s anterior aspect. Third, when the joint surfaces are compressed, the Z joint synovial folds push the capsule out of the joint (Paris, 1983). If either of the two mechanisms associated with the articular capsule fails to function properly, the result could be painful pinching of the capsule, with subsequent acute low back pain and muscle tightness (spasm).
In addition, recall that the Z joint folds possess nociceptive fibers (Giles, 1987, 1988; Giles & Taylor, 1987). Consequently, entrapment within a Z joint of these innervated folds may be a primary source of back pain and muscle tightness (spasm) even without traction of the capsule. Chapter 2 describes Z joint synovial folds and other Z joint inclusions in further detail.
Narrowing of the IVD space results in increased pressure on the articulating surfaces of the Z joint. This pressure is further increased with extension of the lumbar region. Disc space narrowing also causes or increases the severity of impingement of synovial folds. Therefore increased pressure on the subchondral bone of the articular processes and increased impingement of articular tissues (soft tissue “nipped” between the facets) may be two causes of back pain in patients with decreased height of one or more IVDs (Dunlop, Adams, & Hutton, 1984).
Zygapophysial Joint Degeneration
A strong correlation has been found between increasing age and degeneration of the Z joints (Peterson, Boulton, & Wood, 2000). Kirkaldy-Willis and colleagues (1978) stated that such changes include inflammatory reaction of the synovial lining of the Z joint, changes of the articular cartilage, development of loose bodies in the Z joint, and laxity of the joint capsule. All these result in joint instability. Changes of the Z joints (left and right) of any given vertebral level frequently are accompanied by degenerative changes in the IVD of the same level (Kirkaldy-Willis et al., 1978; Peterson, Boulton, & Wood, 2000). Disc degeneration leads to increased rotational instability of the Z joints, resulting in further degeneration of these structures. This process of degenerative changes of the IVDs leading to degeneration of the Z joints also has been supported with computer models of spine function (Goel et al., 1993). The Z joint degenerative changes usually take the form of increased bone formation (e.g., arthrosis, spur formation), which can compress the exiting spinal nerve (Epstein et al., 1973; Rauschning, 1987). Much less frequently, degenerative change manifests as erosion of the superior articular process (Kirkaldy-Willis et al., 1978). This can lead to degenerative spondylolisthesis. Figure 7-6 shows the process of Z joint and disc degeneration and the interrelation between the two.
Individuals with low back pain secondary to trauma have been found to have more advanced degeneration of the Z joints than low back pain patients who do not experience trauma, although no difference between pain and disability has been found between these two groups of patients. In addition, as low back pain increases, the number of lumbar Z joints showing signs of degeneration and the severity of the degeneration also increase (Peterson, Boulton, & Wood, 2000).
The articular cartilage lining of the lumbar Z joints usually become irregular with age (Taylor & Twomey, 1986). The cartilage of the posterior aspect of the joint frequently is worn thin or even may be completely absent. The articular cartilage of the anterior aspect of the joint usually remains thick but may show many fissures (known as cartilaginous fibrillation) that extend from the articular surface of the cartilage deep to the attachment of the cartilage to the subchondral bone. These types of articular cartilage changes often are more pronounced at the most superior and inferior aspects of the joint (Taylor & Twomey, 1986).
As mentioned, osteophytes (or bony spurs) often develop with age on the superior and inferior articular processes. Frequently this occurs along the periphery of the Z joint along the attachment sites of the ligamentum flavum or the articular capsule. The osteophytes most often develop at the attachment site of the ligamentum flavum on the anteromedial aspect of the superior articular process and extend into the posterolateral aspect of the vertebral canal and also into the IVF. Taylor and Twomey (1986) found that fat-filled synovial pads developed in the region of osteophytes associated with the Z joints. These pads formed a cushion between the osteophytes and inferior articular process. The authors also occasionally found that a prominent pad had developed within the inferior recess of the Z joint. This pad was found to lie between the tip of the inferior articular process and the lamina at the base of the subjacent vertebra’s superior articular process. This is where the inferior articular process contacts the lamina during extension of the lumbar spine. This fat pad often was found to become thickened and sclerotic with age, probably in response to the mechanical stimulation of the inferior articular process.
Laminae
The laminae in the lumbar region are broad and thick but do not completely overlap one another. Therefore in contrast to the thoracic region, a distinct space exists between the laminae of adjacent lumbar vertebrae in a dried preparation. This space allows relatively easy access to the spinal subarachnoid space and is used in many diagnostic and therapeutic procedures.
As with other components of lumbar vertebrae, the left and right laminae are asymmetric in superior-to-inferior length (height), with the right laminae being longer in this dimension than the left (same as in the thoracic region). In addition, the superior-to-inferior laminae height of males is larger than that of females (Masharawi & Salame, 2011). Each lumbar lamina can be divided into superior and inferior portions. The superior part is curved and smooth on its inner surface, whereas the inferior part has a rough inner surface for attachment of the ligamentum flavum. Also, the inferior part of the lamina forms a buttress for the inferior articular process (Van Schaik, Verbiest, & Van Schaik, 1985) (see Fig. 7-2, F).
Pars Interarticularis
The region of the lumbar lamina located between the superior and inferior articular processes is known as the pars interarticularis. This region can be fractured rather easily, a condition known as spondylolysis. As mentioned, at the levels of L4 and L5 loads probably are transferred from the vertebral bodies to the pars interarticularis by means of the pedicles. As the trabeculae in the L4 and L5 pedicles pass posteriorly, they are most highly concentrated where they extend into the pars interarticularis. Because trabeculae develop along the lines of greatest stress, the trabecular arrangement leading to the pars interarticularis of the L4 and L5 pedicles has been used to explain the frequency of spondylolysis at these levels.
As a result of bilateral spondylolysis, the vertebral body, pedicles, TPs, and superior articular processes can displace anteriorly. This anterior displacement is known as spondylolisthesis. Spondylolysis and spondylolisthesis are most common at L5. However, they may occur at any lumbar level. (The subsection entitled Spondylolysis and Spondylolisthesis of the section Fifth Lumbar Vertebra found later in this chapter describes these conditions in more detail.)
Lumbar Vertebral Foramen and Vertebral Canal
The vertebral foramina in the lumbar region generally are triangular in shape, although they are somewhat more rounded in the upper lumbar vertebrae and more triangular, or trefoil, in the lower lumbar vertebrae. The triangular shape of the vertebral foramina of the middle and lower lumbar vertebrae is reminiscent of the shape of these openings in the cervical region; however, the lumbar foramina are smaller than those of the cervical region. On the other hand, the lumbar vertebral foramina are larger and more triangular than the rounded foramina of the thoracic region.
The maximum midsagittal diameter in the horizontal plane of the lumbar vertebral foramina is reached by the age of 4 years. Therefore if development is delayed in early childhood, “catch-up growth” does not occur for this dimension. However, the distance between the pedicles (interpedicular distance) increases until adulthood. Thus the lumbosacral vertebral canal becomes more trefoil in appearance with age, reaching its adult dimensions in the early to mid-twenties (Papp, Porter, & Aspden, 1994).
The size of the lumbar vertebral canal ranges from 12 to 20 mm in its anteroposterior dimension at the midsagittal plane. The transverse diameter ranges from 18 to 27 mm, and averages 23.2 to 24.4 mm (Dommisse & Louw, 1990; Ebraheim et al., 1997b). Flexion of the lumbar region significantly increases the size of the vertebral canal at the level of the IVDs (both cross-sectional area and midsagittal diameter) and lumbar extension significantly decreases canal size (Inufusa et al., 1996; Schmid et al., 1999; Madsen et al., 2008). Stenosis has been defined as a narrowing below the lowest value of the range of normal (Dommisse & Louw, 1990). Because of the clinical significance of the vertebral canal, Table 7-3 has been included as a ready source of the dimensions of this region. However, care must be taken when using published values of morphometric measurements of anatomic structures to make clinical decisions. For example, the midsagittal diameter of the vertebral foramina is smaller in Asians than in Europeans or North Americans (You-Lu & Wu, 1988; Lee et al., 1995).
Table 7-3
Dimensions of the Lumbar Vertebral Foramina (Vertebral Canal)∗
| Dimension | Size Range (mm)† |
| Anteroposterior (in midsagittal plane) | 12–20 |
| Transverse (interpedicular distance) | 18–27 |
∗Dimensions below the lowest value indicate spinal (vertebral) canal stenosis (Dommisse & Louw, 1990). A typical vertebral foramen is rather triangular (trefoil) in shape. However, the upper lumbar vertebral foramina are more rounded than the lower lumbar foramina. L1 is the most rounded, and each succeeding lumbar vertebra becomes increasingly triangular, with L5 the most dramatically trefoil of all.
†Dimensions of lumbar vertebral foramina usually are smaller than those of the cervical region but larger than those of the thoracic region.
Lumbar epidural adipose tissue has been found to be histologically different from adipose tissue in other regions of the body (e.g., subcutaneous adipose tissue). Epidural fat is less solid than subcutaneous fat. The adipocytes are almost identical in size and shape throughout epidural adipose tissue. In addition, epidural adipose tissue has less connective tissue within it and the connective tissue present forms “gliding planes” separated by distinct “slits.” The adipocytes of subcutaneous fat are irregular and varied in shape, there is much more connective tissue, and no gliding planes separated by slits are found. The epidural fat in fetuses completely surrounds the dura mater and is evenly distributed from superior to inferior in the lumbar vertebral canal. However, in the adult spine, the lumbar epidural fat is found almost exclusively along the posterior aspect of the dura mater and is most prominent at the level of the IVDs. The epidural fat is isolated into these “pads” by thin connective tissue membranes that are located on the anterior surface of the adipose tissue. These thin connective tissue membranes lie against the posterior surface of the spinal dura mater, and attach laterally to the lateral aspects of the left and right ligamenta flava and intervertebral foramina, and attach superiorly and inferiorly to the suprajacent and subjacent laminae, respectively. Each adipose tissue pad is connected by a small connective tissue pedicle to the posterior midline. Therefore in horizontal section, the pads are triangular in shape with their apex pointing posteriorly and their base resting anteriorly against the posterior spinal dura mater. The size (volume) of the pads increases from the L1-2 to the L4-5 level. These anatomic features suggest a functional role of the epidural adipose tissue—a role designed to aid in the smooth passage of the dura mater during flexion and extension movements across the bony and ligamentous structures of the posterior aspect of the vertebral canal (Beaujeux et al., 1997).
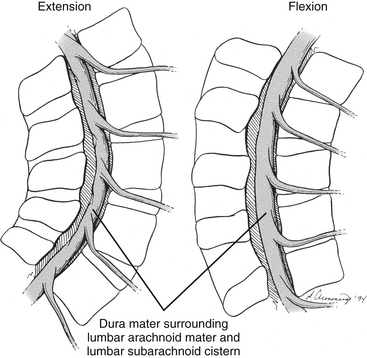
It should be recalled that the conus medullaris of the spinal cord extends to about the L1 to L2 vertebrae in adults (approximately S1 in fetuses, L3 in neonates, and L1 to L2 within the first few months to 2 years of life) (Wilson & Prince, 1989; Macdonald et al., 1999; Malas et al., 2000). (See Chapter 3 for a full discussion of this topic.) Inferior to the level of the conus medullaris the vertebral canal (Figs. 7-7 and 7-8) contains the cauda equina. The cauda equina is bathed in the cerebrospinal fluid of the subarachnoid space. The subarachnoid space in the lumbar vertebral canal is large compared with that in the cervical and thoracic regions. Because of its size, the subarachnoid space below the level of the conus medullaris (L1 to L2 vertebral foramen) is known as the lumbar cistern. Also within the lumbar vertebral canal are the meninges: pia mater, attached to rootlets; arachnoid; and dura mater, which surrounds the arachnoid and to which the arachnoid is closely applied. In addition, adipose tissue, vessels, and nerves are located within the epidural space of the vertebral canal. Clinicians who specialize in diagnostic imaging frequently use the term thecal sac when collectively referring to the dura mater, arachnoid, and subarachnoid space within the vertebral canal. This term is not restricted to the lumbar region and is used when referring to these structures in the cervical and thoracic regions as well.
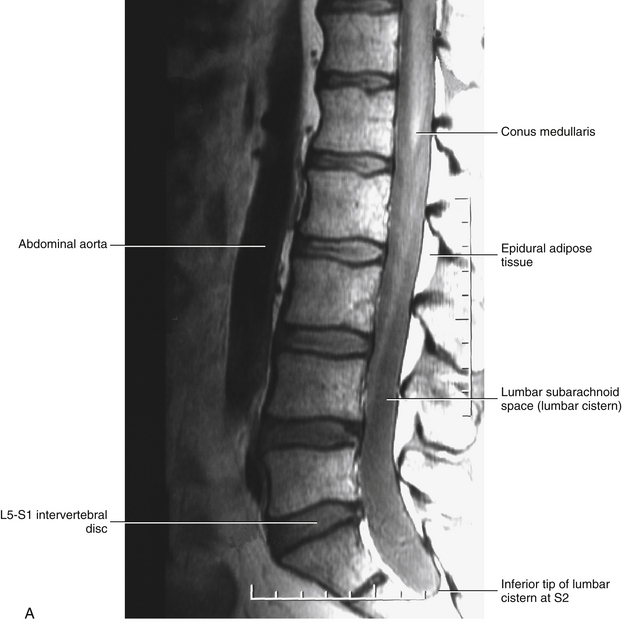
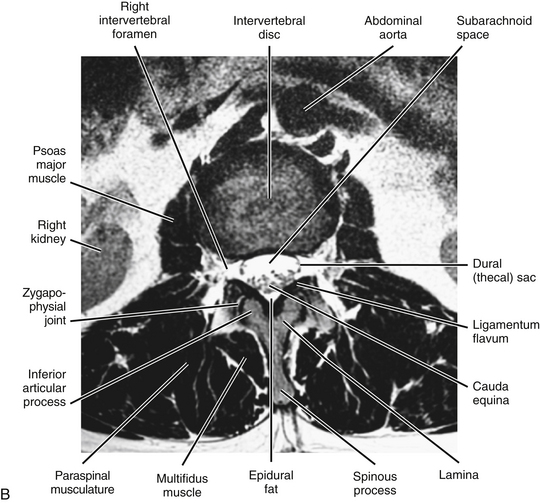
FIG. 7-7 A, Midsagittal plane MR scan of the lumbar region. (A, Courtesy Dr. Dennis Skogsbergh.)B, Axial (horizontal) plane MR scan of the lumbar region at the L1-L2 intervertebral disc level. The T2-weighted image shows the high-signal cerebrospinal fluid in the subarachnoid space to best advantage. (B, Courtesy Dr. William Bogar, National University of Health Sciences, Lombard, IL.)
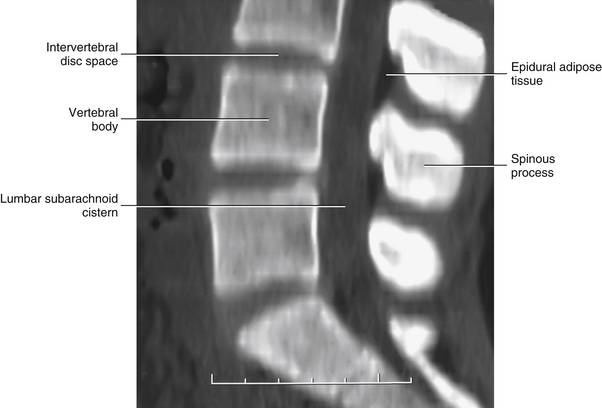
FIG. 7-8 Horizontal computed tomography images digitally reformatted to produce this midsagittal plane view of the lumbar vertebral canal. (Image courtesy Dr. Dennis Skogsbergh.)
Tethered cord syndrome: The conus medullaris extends further inferiorly than normal in a condition known as “tethered cord syndrome.” In the fully developed spine this condition is caused by either a thickened filum terminale or adhesions connecting the filum terminale to adjacent tissues (Marchiori & Firth, 1996; Salbacak et al., 2000). Normally the filum terminale stretches when experiencing tension, as with the tension that occurs during flexion of the cervical region (Lew, Morrow, & Lew, 1994). However, with infiltration of adipose tissue, or the formation of a lipoma within the filum terminale, the filum can lose its resilience and can even shorten. Although fat infiltration of the filum terminale is found in approximately 5% of individuals, only a small percentage of these have a tethered spinal cord. If the filum terminale becomes hardened or shortened, a traction injury (e.g., a hyperflexion injury of the cervical region) can place abnormal tensile forces on the conus medullaris and the lower lumbar spinal cord segments. Such stretching of the lower spinal cord results in low back pain accompanied by a variety of motor and sensory signs and symptoms in the lower extremities. MRI is the imaging modality of choice for tethered cord syndrome. MRI shows the conus medullaris extending further inferiorly than normal with infiltration of adipose tissue into the filum terminale, or a lipoma in the filum terminale, if tethered cord syndrome is present (Marchiori & Firth, 1996). See Chapter 3 for additional information on this very clinically relevant condition.
Dural Attachments within the Vertebral Canal
The dura mater of the lumbar spine has a series of attachments to neighboring vertebrae and ligaments. These attachments are found at each segmental level and are usually located at the level of the IVD (Rauschning, 1987). They have been called the dural attachment complex (Dupuis, 1988), meningovertebral ligaments (Bashline, Bilott, & Ellis, 1996), or Hoffmann ligaments (Rauschning, 1987; Dupuis, 1988). A centrally placed set of connective tissue bands attaches the anterior aspect of the dura mater to the posterior aspect of the lumbar vertebral bodies and the posterior longitudinal ligament. This set of bands has been called midline Hoffmann (meningovertebral) ligaments (Dupuis, 1988). These are the most numerous and the most robust of the meningovertebral ligaments (Bashline, Bilott, & Ellis, 1996). A second set attaches the anterior and lateral aspects of the dura mater to the lateral, flared extension of the posterior longitudinal ligament, which is attached to the IVD (Fig. 7-9). These bands have been called the lateral Hoffmann (meningovertebral) ligaments (Dupuis, 1988), and are less common and less robust than the midline meningovertebral ligaments (Bashline, Bilott, & Ellis, 1996). A third set of connective tissue bands attaches the exiting dural root sleeves with the inferior pedicles forming the IVFs. These are known as the lateral root ligaments, and may act to limit medial and superior mobility of the nerve root. These meningovertebral ligaments are the least common (i.e., not found at all lumbar vertebral levels) and least robust, but are still found in all specimens (Bashline, Bilott, & Ellis, 1996) (see Connective Tissue Attachments of the Dura to the Borders of the Intervertebral Foramina later in this chapter). These three types of meningovertebral ligaments are variable in distribution, size, and length (varying in length from a few millimeters to as long as 2.5 cm) (Bashline, Bilott, & Ellis, 1996). Posterior Hoffmann (meningovertebral) ligaments also have been identified. They are much less common than the others and course from the posterior aspect of the dura mater to the ligamentum flavum and the posterior aspects of the neural canal (Bashline, Bilott, & Ellis, 1996). A single fibrous band (most common), or pair of fibrous bands, has been identified passing between the posterior aspect of the dura, at the level of the superior aspect of the superior articular process of S1, and the ligamentum flavum at the L5-S1 vertebral level. The ligamentous band(s) was (were) found in 10 of 14 (71%) lumbar spine surgery patients (Solaroglu, Okutan, & Beskonakli, 2011).
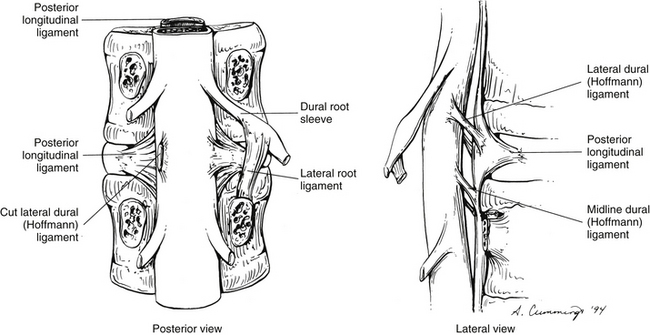
FIG. 7-9 Attachments of the dura mater to surrounding structures. (Modified from Dupuis PR. [1988]. The anatomy of the lumbosacral spine. In W Kirkaldy-Willis (Ed.). Managing low back pain (2nd ed.). New York: Churchill Livingstone.)
Although meningovertebral ligaments have been identified in the thoracic and cervical regions, they are much less common and substantive than those of the lumbosacral region (Scapinelli, 1990; Bashline, Bilott, & Ellis, 1996). However, sturdy attachments of the cervical dura mater to the ligamentum nuchae and rectus capitis posterior minor muscle have been found (see Chapter 5).
It should be recalled that irritation of the anterior aspect of the lumbar spinal dura mater has been found to result in pain felt in the midline, radiating into the low back and superior aspect of the buttock (Edgar & Ghadially, 1976). This pattern of referral is also seen in irritation of the posterior longitudinal ligament (see Ligaments and Intervertebral Discs of the Lumbar Region). Bashline and colleagues (1996) speculated that with bulging or protrusion of the IVD, the presence of midline and lateral meningovertebral ligaments would cause traction of the dura mater and the related nerve roots, increasing back and lower extremity pain. They later verified that a bulging IVD can cause traction of the dura mater by means of meningovertebral ligaments (Bashline, Bilott, & Ellis, 1996). They further speculated that the absence of meningovertebral ligaments at some levels in some individuals may be one reason why disc bulges of the same size in two individuals, or at two different levels in the same individual, may present with dramatically different signs and symptoms. The abundance of meningovertebral ligaments in the lumbar region, coupled with the higher incidence of disc bulging and protrusion in this region, would make the lumbar region the most susceptible to this phenomenon. Bashline and colleagues (1996) further speculated that the greater number of anterior meningovertebral ligaments in the lumbar region may result from the greater volume of the lumbar vertebral canal, allowing for more ligaments of greater size. Another possible reason given was to provide additional stabilization of the dural sac in the more inferiorly located lumbar region than in other regions of the vertebral column (attachment of the dura to the bones of the cranial vault stabilizes the dura superiorly). The authors also hypothesized that these ligaments would pull the dural sac anteriorly with spondylolisthesis resulting in pain in this condition (Bashline, Bilott, & Ellis, 1996). In addition, calcification of some of these ligaments has been noted. Such calcification could potentially cause mechanical irritation of the anterior aspect of the dura mater and the underlying nerve roots (Spencer, Irwin, & Miller, 1983; Bashline, Bilott, & Ellis, 1996). In addition, Spencer and colleagues (1983) believed that the commonly seen “cap” of bone present along the posterior aspect of lumbar vertebral bodies could result from the attachment of meningovertebral ligaments causing a type of “traction spur.”
Spinal Canal Stenosis
Narrowing of the vertebral canal (spinal canal) is most often known as spinal canal stenosis, or, simply, spinal stenosis. Many variations of this condition exist in the lumbar region, several of which are discussed in this section. The possible pathologic condition that can result from spinal canal stenosis and that is common to all the variations is compression of one or more of the nerves that course through the vertebral canal. Such compression can lead to pain and dysfunction, probably caused by ischemia of the entrapped nerves (Lancourt, Glenn, & Wiltse, 1979). Lumbar spinal canal stenosis affects the nerves of the cauda equina or the dorsal and ventral roots as they leave the vertebral canal and enter IVFs.
Spinal stenosis is usually characterized by diffuse and bilateral low back pain accompanied by radicular pain (see Chapter 11) that radiates along the posterior aspect of one or both thighs and extends to or below the knees. In addition to low back and radicular pain, a patient with spinal stenosis usually experiences weakness of the lower extremities that occurs after prolonged standing with the lumbar region in extension or after walking (neurogenic claudication) (Amundsen et al., 1995). Takahashi and colleagues (1995) and Takahashi, Shima, and Porter (1999) measured epidural pressure by means of epidural transducers in patients with spinal stenosis. The epidural pressures were found to be low when patients were sitting or lying down and were high when patients were standing. Flexion decreased epidural pressure and extension increased it. The epidural pressure was found to be the highest during extension while standing. The increased pressures also were positively associated with neurologic symptoms of cauda equina compression.
Subdivisions of spinal stenosis include lateral recess stenosis and foraminal (intervertebral foraminal) stenosis. The exiting nerve roots travel through the narrower lateral aspect of the vertebral canal, known as the lateral recess, before entering the IVF. As the roots pass through this region of the vertebral canal, pressure may be placed on them. This is known as lateral recess stenosis. Another type of stenosis includes narrowing of the IVF. This condition is known as foraminal stenosis.
Causes: Spinal canal stenosis may be congenital in nature (Verbiest, 1954; Arnoldi et al., 1976). That is, some people are born with a “tight tube,” and their vertebral canal remains narrow throughout their lives. Spinal canal stenosis also can be the result of degenerative changes. The most common causes of degenerative spinal stenosis include arthrosis (increased bone formation) of the medial aspect of the Z joint, especially of the superior articular process. Thickening (hypertrophy) of the ligamentum flavum also can be a degenerative cause of spinal canal stenosis (Arnoldi et al., 1976; Liyang et al., 1989).
Other causes of spinal canal stenosis include spondylolisthesis, Paget’s disease, fluorosis, degenerative changes after trauma (Kirkaldy-Willis et al., 1978), and iatrogenic (physician-induced) causes. The latter category includes complication after laminectomy, spinal fusion, and chemonucleolysis (Arnoldi et al., 1976). Box 7-1 lists possible causes of spinal stenosis.
Any combination of congenital and degenerative causes of spinal canal stenosis may be present at one time. Because the contents of the lumbar vertebral canal are more frequently affected by disc protrusions than the contents of the cervical and thoracic regions (Clarke et al., 1985), a protruding or bulging IVD can increase the severity of signs and symptoms in a patient who has spinal stenosis. Arnoldi and colleagues (1976), who developed a classification system for this condition, have included IVD protrusion in their system of nomenclature for spinal canal stenosis (e.g., congenital stenosis with IVD protrusion, degenerative stenosis with IVD protrusion).
Also important in the development of signs and symptoms of spinal canal stenosis is the ratio between the size of the neural elements within the lumbar vertebral canal (the cauda equina) and the dimensions of the vertebral canal (Liyang et al., 1989). A person with relatively narrow vertebral foramina may be free of symptoms if the sizes of the roots constituting the cauda equina and the surrounding meninges are proportionately small. On the other hand, if a person has a normal-sized vertebral canal and one or more enlarged nerve roots, as can occur with a schwannoma of the cauda equina (Caputo & Cusimano, 1997), compression of many or all of the nerve roots of the cauda equina can occur. Conversely, if the vertebral canal is narrow (either congenitally or secondary to pathologic conditions or degeneration) and the roots composing the cauda equina are of normal size, signs and symptoms of spinal stenosis can occur. Two possible results of a normal-sized cauda equina within a narrow vertebral canal are the conditions known as neurogenic claudication and redundant nerve roots.
Neurogenic claudication: Neurogenic claudication is pain, paresthesias, and weakness in one or both lower extremities that is aggravated by walking, and is the result of compression of the cauda equina at more than one vertebral level. Compression of the cauda equina at more than one level leads to venous pooling and stasis within the veins draining the cauda equina between the compression sites. The resulting increased venous pressure is thought to lead to a lack of sustained dilation of the arterioles to the cauda equina after exercise (walking), which in turn results in decreased motor nerve function (similar to that found in an entrapment neuropathy such as carpal tunnel syndrome) and subsequent weakness of the lower extremity muscles supplied by the affected nerves (Porter, 1996).
Men experience neurogenic claudication more often than women, and the condition is most common after the age of 50 years. Those affected often have a congenitally narrow vertebral canal that is also affected by degenerative changes (e.g., osteophytes extending from the posterior aspect of the vertebral bodies and also from the articular processes). Neurogenic claudication can be difficult to differentiate from claudication caused by peripheral vascular disease (intermittent claudication), and the two conditions frequently are found together. Standard x-rays can identify a narrow vertebral canal, and advanced imaging procedures (e.g., CT, MRI, and CT-myelography) can assess the extent of degeneration and determine the number of stenotic vertebral levels.
Redundant nerve roots: Redundant nerve roots refers to roots of the cauda equina that bend, curve frequently (undulate within the vertebral canal), or buckle during their course through the vertebral canal. The buckling can be quite severe, blocking the flow of radiopaque dye on myelography. The roots in some cases appear to form dramatic loops (redundancies) when viewed during spinal surgery (Tsuji et al., 1985). The redundancies usually occur rather high in the lumbar spinal canal. Degenerative spinal stenosis is thought to be the usual cause of this condition.
Redundant nerve roots once were considered a rare occurrence, but they may occur more frequently than previously expected. Tsuji and colleagues (1985) found this condition in 45% of a series of 117 consecutive cadavers without a recorded history of low back or leg pain. They also found that “22 of 56 patients (39%) had obvious redundant nerve roots, which indicates that this condition is a rather common abnormality in degenerative spinal stenosis.”
Tsuji and colleagues (1985) presented a hypothesis of the progression of redundant nerve roots, which is summarized in Box 7-2. Their hypothesis began with the finding that the vertebral column decreases in superior-to-inferior length with age (an average of 14 mm). Shortening of the vertebral canal would force the roots of the cauda equina to become somewhat redundant, causing them to fill the subarachnoid space (thecal space) more completely. Posterior spondylosis (osteophytes) from the vertebral bodies, or other constrictions within the vertebral canal, could then more easily rub against the roots during movement. (Their study showed that considerable movement of the roots probably occurs during flexion-extension excursions of the spine.) The pressure from spondylosis (or other compressive elements) over many years could result in a friction neuritis, which would enlarge the nerve roots. The friction neuritis was thought to result in the large redundant roots seen in several specimens. During walking and standing (extension), increased pressure is placed on the nerve roots (Fig. 7-10), which would cause ischemia of the neural elements. Nerve root ischemia results in the signs and symptoms of intermittent claudication (pain and weakness in the lower extremities during standing and walking), which frequently are associated with spinal stenosis and redundant nerve roots. An average conduction velocity of 50% below normal values was found in cauda equina roots of individuals with redundant nerve roots. Tsuji and colleagues (1985) believed that such neurologic changes were probably permanent.
Ischemia during stenosis: As mentioned, stenosis of the vertebral canal has been implicated as a possible cause of ischemia to the roots of the cauda equina (Lancourt, Glenn, & Wiltse, 1979; Tsuji et al., 1985; Dommisse & Louw, 1990). This ischemia probably occurs in the roots’ “vulnerable region” of vascularity. The roots that form the cauda equina receive their blood supply (vasa nervorum) distally from radicular arteries and also proximally from the arterial anastomosis (vasa corona) surrounding the conus medullaris (see Chapter 3 for a full discussion of the blood supply to the nerve roots of the cauda equina). The proximal and distal vessels form an anastomosis at about the junction of the proximal and middle thirds of the cauda equina roots. This has been called the “critical zone” of vascularity and represents a region where the roots are vulnerable to compression (Dommisse & Louw, 1990). In addition, compression of the neural elements at two contiguous vertebral segments (two-point compression), which is relatively common, can also lead to a lack of blood flow to the nerve roots located between the two areas of compression (Parke, 2005). Consequently, compression in either the critical zone of vascularity or the two-point compression along neural elements of the cauda equina can result in neural ischemia causing the symptoms and signs usually associated with spinal stenosis (see the following discussion).
Symptoms: The symptoms of spinal canal stenosis usually include pain radiating from the lumbar region into the lower extremities, occasionally inferior to the knee. The symptoms usually are posture dependent and are made worse by standing or walking for variable periods of time. Flexion of the lumbar region usually relieves the pain.
It should be recalled that flexion of the lumbar region significantly increases the size of the vertebral canal (both cross-sectional area and midsagittal diameter) and extension significantly decreases canal size (Inufusa et al., 1996). In addition, Liyang and colleagues (1989) found that the volume of the dural sac (subarachnoid space), as studied in 10 cadavers, increased by 3.5 to 6.0 ml during excursion from full extension to full flexion. These changes were found to be highly significant (p < 0.001). The sagittal diameter of the dural sac (subarachnoid space), as measured from myelograms of the cadaveric spines, also was found to increase significantly during flexion; the greatest increase occurred at the level of L5. Also, the length of the lumbar vertebral (spinal) canal was found to increase by an average of 19.4 mm during flexion. The epidural pressure measurements of Takahashi and colleagues (1995) and Takahashi, Shima, and Porter (1999) discussed previously support all of these findings. These results help to explain the clinical findings that flexion generally relieves the symptoms of spinal canal stenosis (see Fig. 7-10).
Because extension of the lumbar region is accompanied by broadening of the cauda equina, slackening of the ligamenta flava, bulging of the IVDs into the vertebral (spinal) canal, and narrowing of the IVF, one can understand how extension of the lumbar region can increase the symptoms of spinal canal stenosis (see Fig. 7-10). Therefore therapeutic interventions that increase flexion and reduce extension are indicated in patients with this condition (Liyang et al., 1989). Such interventions include exercises that increase tone of abdominal muscles, weight reduction if indicated, and adjustive (manipulative) procedures that promote flexion (Kirk & Lawrence, 1985; Cassidy & Kirkaldy-Willis, 1988; Cox, 2011; Bergmann, Peterson, & Lawrence, 1993). If stenosis is severe, positive effects from manipulation may be more difficult to achieve. “Nevertheless, it can be helpful in some patients and is worth a try in the early management of this syndrome” (Cassidy & Kirkaldy-Willis, 1988). Several authors have reported positive results from wearing a brace to keep the lumbar spine in flexion. Liyang and colleagues (1989) suggested that spinal stenosis be treated by surgical decompression (laminectomy) of the spinal (vertebral) canal, followed by fixation of the spine in flexion. Interestingly, Kikuchi and colleagues (1984) found that infiltration of a single nerve root with local anesthetic usually extinguished the symptoms of cauda equina claudication secondary to spinal stenosis. This seems to be contrary to the widely held belief that neurogenic claudication is the result of compression of the entire cauda equina.
Spinous Processes
The spinous processes of lumbar vertebrae are broad from superior to inferior, are narrow from side to side, and project directly posteriorly. They are, more or less, flat and rectangular in shape. Their posteroinferior ridge is thickened for the attachment of ligaments and muscles.
The lumbar spinous processes have been found to undergo morphologic changes after age 40, reaching the highest incidence of change in persons older than age 60 (Scapinelli, 1989). The most common change is the addition of bone along the posterior aspect of the spinous processes, which may increase their anteroposterior length by as much as 1 cm or more. The greatest increase in length is usually at L3. Frequently the added bone presents a sharp, spurlike margin, usually on the posterosuperior aspect of the spinous process. A smaller increase in the superior-to-inferior dimension usually occurs simultaneously with the anteroposterior change. Occasionally the spinous processes touch one another in the neutral position. This is known as “kissing spines,” or Baastrup’s syndrome, and can be a source of localized pain.
These changes in the size of the lumbar spinous processes are created by replacement of ligamentous tissue of the supraspinous and interspinous ligaments and the related fibrous tissue below L3 (the supraspinous ligament usually does not extend below L3) with fibrocartilage and eventually bone. Scapinelli (1989) believes these changes are associated with decreased movement as one ages, increased lumbar lordosis, and traction from ligaments and tendons of muscles. The greatest increase in bone is in individuals with degenerative changes of the vertebral bodies and Z joints (degenerative spondyloarthrosis), especially those with diffuse idiopathic skeletal hyperostosis (DISH, or Forestier’s disease). With the exception of DISH, the changes are believed to increase the lever arm of the erector spinae muscles, helping with the maintenance of an erect posture (Scapinelli, 1989).
Table 7-4 lists those structures that normally attach to the lumbar spinous processes.
Table 7-4
Attachments to Lumbar Spinous Processes
| Type | Structure(s) Attached |
| Ligaments | Thoracolumbar fascia (posterior lamella) Supraspinous and interspinous |
| Muscles | Deep back muscles (spinalis thoracis, multifidus, interspinalis) |
From Standring S et al. (2008). Gray’s anatomy: the anatomical basis of clinical practice (40th ed.). Edinburgh: Churchill Livingstone.
Lumbar Intervertebral Foramina and Nerve Root Canals
The bony and ligamentous canals called the intervertebral foramina (singular, foramen) are described in Chapter 2. However, several features of the lumbar IVFs are unique. In addition, these regions have been the subject of extensive descriptive and clinical investigation. The relationship between the lumbosacral nerve roots and their surrounding tissues is important in the proper diagnosis of low back pain and pain radiating into the lower extremity (Hasue et al., 1983). This section therefore focuses on the unique aspects of the anatomy of the lumbar IVFs, the pertinent conclusions of previous and current studies of the IVF, and the clinical relevance of this fascinating area.
Many features of the region of the lumbar IVFs are different from those of the rest of the spine because of the unique characteristics of the lumbar and sacral spinal nerves (Fig. 7-11). Because the spinal cord ends at generally the level of L1 to L2, the lumbar and sacral dorsal and ventral roots must descend, sometimes for a considerable distance, within the subarachnoid space of the lumbar vertebral canal. This region of subarachnoid space is known as the lumbar cistern. The exiting nerves (dorsal and ventral rootlets or roots) leave the lumbar cistern by entering a sleeve of dura mater. This usually occurs slightly inferior to the level of the IVD at the level above the IVF that the roots eventually will occupy. For example, the L4 roots enter their dural sleeve just beneath the L3-4 disc and then course inferiorly and laterally to exit the L4-5 IVF. More specifically, on leaving the subarachnoid space of the lumbar cistern, the exiting dorsal and ventral roots pass at an oblique inferior and lateral angle while retaining a rather substantial and very distinct covering of dura mater. This covering, known as the dural root sleeve, surrounds the neural elements and their accompanying radicular arteries and veins until they leave the confines of the IVF (see Fig. 2-15). The largest lumbar dural root sleeve (proximal to the dorsal root ganglion) is L5 (5.6 mm diameter) and the smallest is L1 (3.5 mm diameter) (Torun et al., 2008).
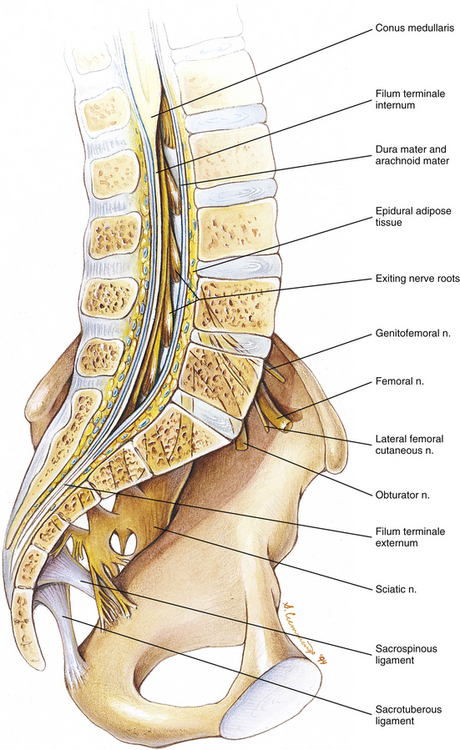
FIG. 7-11 Midsagittal view of the spine showing nerve roots traversing the lumbar vertebral canal, exiting the intervertebral foramina, and the anterior primary divisions (vertebral rami) forming the lumbar and sacral plexuses.
Frequently the dorsal and ventral rootlets that arise from the spinal cord do not all unite to form dorsal and ventral roots until they are well within the dural root sleeve (Rauschning, 1987; Dupuis, 1988). In addition, the dorsal and ventral roots combine to form the spinal nerve while within the distal aspect of the funnel-shaped dural root sleeve. This latter union occurs at the level of the IVF. The exiting spinal nerve has been found to be larger than the combined size of the individual dorsal and ventral roots (dePeretti et al., 1989). On reaching the lateral edge of the IVF, the dural root sleeve becomes continuous with the epineurium of the spinal nerve.
Many authors (Vital et al., 1983; Bose & Balasubramaniam, 1984; Rauschning, 1987; Lee, Rauschning, & Glenn, 1988) have described the region beginning at the exit of the neural elements from the lumbar subarachnoid cistern and continuing to the lateral edge of the IVF as having significant clinical importance. Perhaps because of the clinical significance of this region, several terms have been used to describe it, including lumbar radicular canal (Vital et al., 1983), nerve root canal, or simply root canal (Rauschning, 1987) (Fig. 7-12). The term nerve root canal (NRC) is used in the following paragraphs when discussing the course of the dural root sleeve and its contents, and the term IVF is used to describe the terminal part of the NRC that lies between the pedicles of two adjacent vertebrae.

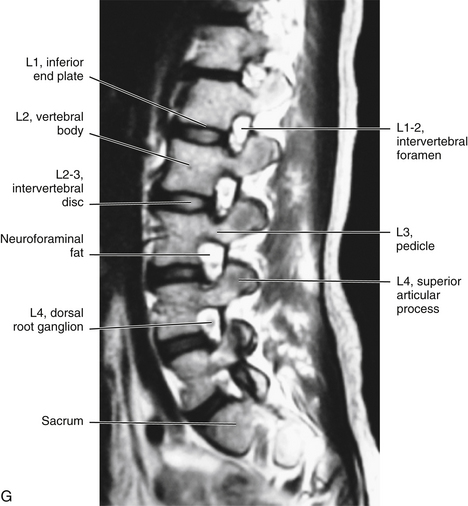
FIG. 7-12 A–C, Regions of the intervertebral foramen (IVF) as described by various authors. A, The nerve root canal (NRC) as the course of the dural root sleeve and its contents (yellow and red regions combined). Other terms used when referring to this general region are lumbar radicular canal (Vital et al., 1983) and root canal (Rauschning, 1987). The IVF is shown in red as the terminal part of the NRC, located between the pedicles of two adjacent vertebrae. This also represents the classic anatomic description of the IVF. B, Regions of the NRC (lumbar radicular canal) as described by Vital and colleagues (1983). Notice the retrodiscal portion in blue. This is the portion of the NRC that lies posterior to the intervertebral disc (IVD) superior to the level that the spinal nerve eventually exits. The parapedicular portion of the NRC (green) is the region medial to the pedicle. Vital and colleagues (1983) refer to the region occupied by the uniting dorsal and ventral roots and the exiting spinal nerve as the IVF proper (red). Table 7-8 describes the borders of the retrodiscal, parapedicular, and IVF proper subdivisions of the NRC. C, Divisions of the NRC (root canal) as described by Rauschning (1987) and Lee and colleagues (1988). The entrance zone (pink) is the region that begins as the dorsal and ventral roots enter the dural root sleeve and extends through the lateral recess. Other authors use lateral recess, lateral canal, subarticular gutter, or lateral nerve canal when referring to the entrance zone. The middle zone, or pedicle zone (blue), is the region of the NRC located between the two adjacent pedicles that comprise an IVF. This area was described in panels A and B as the IVF proper (Vital et al., 1983). The term exit zone, or foramen proper, is used by Rauschning (1987) and Lee and colleagues (1988) to refer to the almost two-dimensional opening (“doorway”) formed by a parasagittal plane passed along the lateral edge of the two pedicles that help to form an IVF. The exit zone is shown in red in panel C. D, Lateral view of the lumbar IVFs. The specific dimensions of the lumbar IVFs are given in Chapter 2 (see Tables 2-6 and 2-7 and Figs. 2-20 through 2-22). E, Lateral view of two IVFs. The structures that normally traverse this region have been drawn in the more superior IVF. The most common locations of the transforaminal ligaments also are shown traversing this IVF. F, Parasagittal section through the fourth lumbar IVF. A typical lumbar IVF is sometimes described as being shaped like an “inverted teardrop” or an “inverted pear” when viewed from the side. (E and F, Courtesy National University of Health Sciences, Lombard, IL.)G, Parasagittal MRI (T2-weighted) of the lumbar region showing the IVFs. (G, courtesy Dr. William Bogar,National University of Health Sciences, Lombard, IL.)
Within the dural root sleeve an interneural complex of fibrous connections (Dupuis, 1988) anchors the neural elements (rootlets and roots) to the surrounding dura mater of the NRC. More specifically, these connections course from the dura and inner arachnoid mater of the sleeve to the pia mater surrounding the rootlets and roots. Recall that farther laterally the root sleeve unites with the spinal nerve to form its epineurium.
Connective Tissue Attachments of the Dura to the Borders of the Intervertebral Foramina
The external surface of the dural root sleeve is usually attached by one or more connective tissue bands to the inferior pedicle of the IVF (see Fig. 7-9). This connective tissue attachment is called the lateral root ligament (Dupuis, 1988). It limits the medial and upward mobility of the root sleeve and its contents (Rauschning, 1987). Fibrous connections from the lateral aspect of the IVF to the exiting spinal nerve have also been identified (Hasue et al., 1983; dePeretti et al., 1989). Grimes and colleagues (2000) found four connective tissue attachments from the dural root sleeve to the adjacent borders of the IVF at all lumbar levels. One such attachment passed posteriorly to the lateral-most aspect of the ligamentum flavum as it blended with the Z joint capsule. Another connective tissue attachment was found to extend superiorly to the pedicle above, and a third formed a mirror image to the second by extending inferiorly to the pedicle below. Finally, another connective tissue attachment extended anteriorly to the posterior aspect of the IVD (Grimes, Massie, & Garfin, 2000). An appropriate term for these connective tissue attachments would seem to be “intraforaminal meningovertebral ligaments,” after Bashline and colleagues (1996), who used the term meningovertebral ligaments to refer to Hoffmann’s ligaments connecting the dura mater to surrounding tissues within the vertebral (spinal) canal. These intraforaminal structures were found to stabilize the neural structures within the IVF and to resist considerable traction applied to the spinal nerve and anterior primary division distal to the connective tissue attachments (dePeretti et al., 1989; Grimes, Massie, & Garfin, 2000). Because the fibrous attachments have been found to give considerable resistance to traction of the anterior primary divisions, they serve to spare the lumbar roots from traction injuries. The dural root sleeve also provides resistance to traction. In fact, the dural root sleeve ruptures before avulsion of the rootlets from the conus medullaris occurs. These resistive forces, when combined with the fact that the rootlets and roots forming the spinal nerves are of excess length within the dura mater, indicate that the IVF seems to provide an almost insurmountable protective barrier to traction forces placed on the exiting spinal nerves (dePeretti et al., 1989).
Entrapment of the Neural Elements
Unfortunately, the exiting neural elements are not as well protected from pressure injuries as they are from traction injuries. Compression, or entrapment, of neural elements as they pass through the NRC or the IVF is of extreme clinical importance (Lancourt, Glenn, & Wiltse, 1979). Also a detailed understanding of this region is essential because treatment may differ depending on the cause of entrapment. The clinician should attempt to localize a lesion as precisely as possible to determine the structure causing the problem and to identify the specific neural elements being affected (Rauschning, 1987). These neural elements include the dorsal and ventral roots and their union as the spinal nerve. Causes of such compression include degenerative changes of the superior articular processes and posterior vertebral bodies, protrusion of the IVD, and pressure from the superior pedicle of the IVF (McNab, 1971; Hasue et al., 1983; Vital et al., 1983) (see Chapter 11).
Occasionally osteophytes from the Z joints and vertebral bodies can become so large that they can almost completely divide the IVF into two smaller foramina, one on top of the other (Kirkaldy-Willis et al., 1978). Such changes may result in entrapment of the exiting spinal nerve. However, osteophytes of the superior articular processes forming the Z joints cause venous obstruction of the intervertebral veins more often than compression of nerves in the IVF. This leads to “compression, congestion, and resultant dilation of foraminal veins.” The venous changes are also associated with intraneural and perineural fibrosis, focal demyelination, and edema of the nerve roots. These neural changes probably result from ischemia secondary to poor venous return from the nerve roots upstream to the venous obstruction (Hoyland, Freemont, & Jayson, 1989).
Because of the clinical importance of the NRCs and the IVFs, a more accurate anatomic description is necessary.
Anatomy of the Nerve Root Canals
The sizes of the NRCs vary considerably from the upper to lower lumbar segments. They are smaller in length at the level of L1 and L2 because, after exiting the lumbar cistern, the L1 and L2 nerves course almost directly laterally to reach the IVF. This led Crock (1981) to state that the concept of a nerve root canal at L1 and L2 is useless, because the beginning of the dural root sleeve lies against the inferior and medial aspect of the IVF’s upper pedicle. Therefore no true dural “canal” exists for these nerve roots.
Table 7-5 lists the obliquities and lengths of the lumbar NRCs. Other authors (e.g., Ebraheim et al., 1997b) have found a more superior-to-inferior course of the NRCs (i.e., lower obliquity values than those presented in the table). The NRCs become progressively longer from L1 to S1 as the dural root sleeves generally exit at a more oblique inferior angle (i.e., lower obliquity values in Table 7-5). Therefore the NRCs of L5 and S1 are the longest in the lumbar region and the most susceptible to damage from pathologic conditions of surrounding structures (Crock, 1981). Tables 7-6 and 7-7 describe the relationships of the L5 and S1 NRCs, respectively. Many, if not most, of the types of borders described in Table 7-6 for the L5 NRC also apply to the L3 and L4 NRCs as well.
Table 7-5
Obliquity and Length of the Lumbar Nerve Root Canals
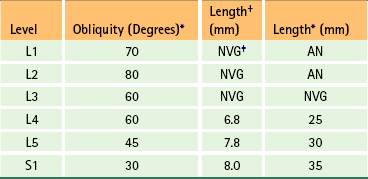
∗According to Bose and Balasubramaniam (1984); obliquity given in degrees from a sagittal plane (low values indicate a more inferior course, high values indicate a more lateral course).
†According to Vital et al. (1983) (see Fig. 7-12).
‡AN, Almost nonexistent; NVG, no value given.
Table 7-6
Relationships of the L5 Nerve Root Canal
| Surface | Relationship |
| Origin | L4-5 intervertebral disc (sometimes does not begin this far superiorly, in which case begins at L5 vertebral body) |
| Medial surface | Lateral aspect of S1 nerve root canal |
| Lateral surface | Medial aspect of pedicle of L5, then enters L5 intervertebral foramina |
| Anterior surface∗ | Posterior aspect of L5 vertebral body |
| Posterior surface† | L5-S1 Z joint and overlying ligamentum flavum |
| Other relationships | Surrounded by epidural adipose tissue, and small arteries, veins, and lymphatics (very small) |
†Posterior relationships vary considerably depending on the length of the nerve roots and the orientation of the L5 lamina, which changes with a change in the angle between L5 and the sacrum (Crock, 1981).
From Bose K & Balasubramaniam P. (1984). Nerve root canals of the lumbar spine. Spine, 9, 16-18.
Table 7-7
Relationships of the S1 Nerve Root Canal
| Surface | Relationship |
| Origin | Medial aspect of L5 pedicle |
| Medial surface | Lateral aspect of dural sac |
| Lateral surface | L5 nerve root, then L5-S1 intervertebral foramina, then S1 pedicle, then enters S1 intervertebral foramen |
| Anterior surface | Posterior aspect of L5 vertebral body, then L5 intervertebral disc, then posterior aspect of S1 vertebral body |
| Posterior surface∗ | Bony ridge of anterior aspect of L5 lamina (formed by attachment of ligamentum flavum), then ligamentum flavum, then anteromedial aspect of S1 superior articular process |
| Other relationships | Surrounded by epidural adipose tissue, and small arteries, veins, and lymphatics (very small) |
∗Posterior relationships vary considerably depending on the length of the nerve roots and the orientation of the L5 lamina, which changes with a change in the angle between L5 and the sacrum (Crock, 1981).
From Crock HV. (1981). Normal and pathological anatomy of the lumbar spinal nerve root canals. J Bone Joint Surg, 63, 487-490; and Vital JM et al. (1983). Anatomy of the lumbar radicular canal. Anat Clin, 5, 141-151.
All the exiting rootlets or roots course over an IVD either just before entering their dural root sleeve (L1 and L2) or, in the case of L3-S1, directly in the region where they enter the dural root sleeve. However, only the S1 dural root sleeve (and contents) passes completely over an IVD (the L5 disc). The S1 NRC passes through a movable, narrow opening between the L5 disc anteriorly and the L5-S1 ligamentum flavum posteriorly. Therefore the S1 nerve is exposed to possible compression both anteriorly (disc protrusion) and posteriorly (ligamentum flavum bulging or buckling, hypertrophy of superior articular process of sacrum) in this region (Rauschning, 1987).
Anomalies of the Neural Elements and the Dural Root Sleeves
Anomalies of the rootlets as they come off of the spinal cord are common. Such anomalies occur much more often with the dorsal rootlets than the ventral rootlets (Kikuchi et al., 1984). The anomalies take the form of rootlets of one spinal cord segment passing to the root of the nerve below or above. Such anomalies could conceivably result in the perception of radicular symptoms different from the normal pain pattern.
Anomalies of the roots making up the cauda equina while within the lumbar cistern also are quite common. These usually consist of nerve bundles from one root passing to a neighboring root. The clinical implications of this type of anomaly are the same as those just discussed for anomalous rootlets.
Congenital anomalies associated with the dural root sleeves also occur with some frequency. Hasue and colleagues (1983) found anomalies in 5 of the 59 cadavers (8.5%) in their study. Several types of anomalies have been identified (Hasue et al., 1983; Neidre & MacNab, 1983; Kikuchi et al., 1984; Aota et al., 1997; Bogduk, 2005) (Fig. 7-13). The first type is known as conjoined nerve roots and is the most common type of dural sleeve anomaly (see Fig. 7-13, C). With this anomaly, roots of adjacent spinal cord segments share the same dural root sleeve for a short distance. The roots then separate into their own dural root sleeve and exit the appropriate IVF. Conjoined nerve roots cause one set of dorsal and ventral roots (or both sets) to take a tortuous course, possibly making them more susceptible to traction across a bulging disc or narrow NRC.
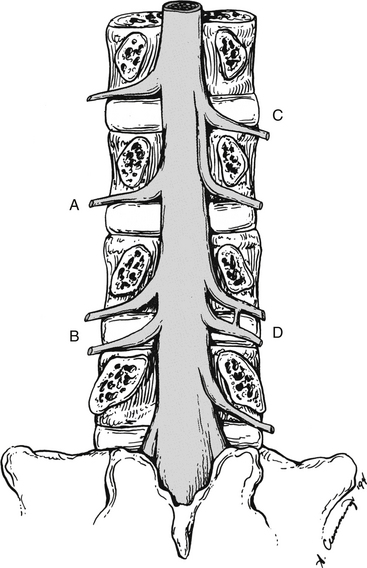
FIG. 7-13 Posterior view of the vertebral canal showing anomalies of dural root sleeves. A, The dural root sleeve is normal. Notice that it is exiting the upper region of the intervertebral foramen (IVF). B, The anomaly shows the roots of two distinct spinal cord levels, within their appropriate root sleeves, exiting the same IVF. This leaves an adjacent IVF (in this case, the IVF below) devoid of exiting roots. C, The anomaly shows conjoined nerve roots. In this case the roots of adjacent spinal cord segments share the same dural root sleeve for a short distance. The roots then separate into their own dural root sleeve and exit the appropriate IVF. Conjoined nerve roots cause one of the roots (or both) to take a rather tortuous course. D, The anomaly is a variation of B. The anomaly shows additional dorsal and ventral roots, and the dural root sleeve covering these roots passing through the same IVF with the neural and dural structures that normally pass through that IVF. Therefore all the IVFs on the right side of the illustration have roots within them, and one IVF has two pairs of roots passing through it. Also notice that another type of anomaly is shown here. A communicating branch, surrounded by its own dural sheath, is seen passing from one dural sleeve to its neighbor, before either exits the IVF. B-D, All these types of anomalies can have significant clinical implications (see text). (Modified from Bogduk N. [2005]. Clinical anatomy of the lumbar spine (4th ed.). London: Churchill Livingstone.)
The second type of root sleeve anomaly is the most dramatic. The dorsal and ventral roots of two distinct spinal cord levels, within their appropriate root sleeves, exit the same IVF, leaving the adjacent IVF devoid of exiting roots (see Fig. 7-13, B). In a variation of this anomaly, an additional dorsal and ventral root and their covering dural root sleeve pass through the same IVF with the neural and dural structures that normally pass through the IVF. In this instance, all of the IVFs have roots within them, and one IVF has two pairs of roots passing through it (see Fig. 7-13, D). The nerves within an IVF containing more than one set of roots in these types of anomalies are more susceptible to entrapment. Entrapment of two sets of roots within one IVF might lead to radicular symptoms of more than one dermatome (see Chapters 9 and 11).
In the third type of dural root sleeve anomaly, a communicating branch, surrounded by its own dural sheath, passes from one NRC to its neighbor before either primary root exits its normal IVF. The result of this anomaly could be similar to that of roots sending connecting branches to neighboring roots, causing dispersion of radicular symptoms to include an adjacent segment (e.g., L5 compression may result in some pain or paresthesia in the distribution of L4). However, because it runs within its own dural root sleeve, the neural elements within the “bridging sleeve” are more vulnerable to compression either when they are inside a lateral recess or as they pass behind an IVD. This type of anomaly also can occur in conjunction with the variation of the second type of anomaly mentioned. In this case a communicating branch is found between two dural root sleeves as they both exit the same IVF (communicating branch shown in Fig. 7-13, D).
Anomalies of the nerve roots and dural sleeves can be a sole cause of radicular symptoms. They also can augment radicular symptoms from other causes (Hasue et al., 1983). Even though the incidence of symptoms as a result of such NRC anomalies is thought to be rare, they should be kept in mind when patients have unusual distribution patterns of radicular pain (Bogduk, 2005).
Terminology Associated with the Nerve Root Canals and the Intervertebral Foramina
Terminology with regard to the exiting roots can be confusing. Some authors (Vital et al., 1983) describe the beginning of the NRC (that portion posterior to the IVD directly above the level of the IVF of exit) as the retrodiscal portion of the NRC (see Fig. 7-12, B). As the nerve continues to descend more laterally in what is described by many as the lateral recess, the nerve lies medial to the pedicle that forms the upper border of the IVF that the nerve roots eventually exit. This region is sometimes called the parapedicular portion of the NRC (Vital et al., 1983). The portion of the IVF occupied by the uniting dorsal and ventral roots and the exiting spinal nerve is called the IVF proper. Table 7-8 describes the borders of the retrodiscal, parapedicular, and IVF proper subdivisions of the NRC. This table is included to help clarify the regions where the nerve roots and (mixed) spinal nerve are most vulnerable to various types of pathologic conditions.
Table 7-8
Relationships of the Various Regions of a Nerve Root Canal
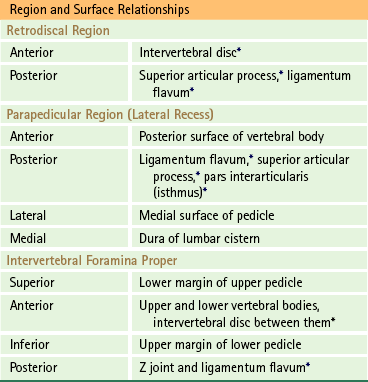
∗Indicates relationships that are of key clinical importance.
Moving from the upper to the lower lumbar region, the parapedicular portion (lateral recess) of the NRC widens in the transverse plane (i.e., left to right), becomes shorter from top to bottom, and becomes narrower from anterior to posterior (average, 12 mm at L2 and 8 mm at L5) (Vital et al., 1983); therefore this part of the NRC becomes more like a true lateral recess, or gutter, as one descends the lumbar spine.
The position of the entire lumbar region affects the NRC and the neural elements (Vital et al., 1983). The anteroposterior dimension of the retrodiscal space narrows in the upright posture. This is because of slight posterior bulging of the IVD and slight anterior buckling of the ligamentum flavum. During flexion of the lumbar region, the neural elements become stretched and pressed against the anterior walls of the retrodiscal and parapedicular spaces. Also, the IVF proper increases in height and width during flexion. Extension of the lumbar region results in slackening of the neural elements. They also move against the posterior wall of the lateral recess during extension. In addition, the IVF becomes significantly shorter from superior to inferior and anterior to posterior during extension (Mayoux-Benhamou et al., 1989).
A different set of terms was introduced by Rauschning (1987) and Lee and colleagues (1988) (see Fig. 7-12, C). These authors used the term entrance zone when referring to the region that begins as the roots enter the dural root sleeve and continues through the lateral recess. Other authors use the terms lateral recess, lateral canal, subarticular gutter, or lateral nerve canal when referring to this region (Rauschning, 1987). This area corresponds to the combination of the retrodiscal and parapedicular regions previously described. The most common cause of stenosis in the entrance zone is hypertrophic osteoarthritis of the Z joint, usually of the superior articular process. Other causes include congenital variations of the Z joints or a congenitally short pedicle. Also, a bulging anulus fibrosus (e.g., L3 anulus compressing the L4 nerve) or osteophytic spurs from the superior vertebral end plate coursing along the anulus could compress the neural elements in the entrance zone.
Rauschning (1987) and Lee and colleagues (1988) use the term midzone or pedicle zone when referring to the region of the NRC located between the two adjacent pedicles that constitute an IVF (see Fig. 7-12, C). This area was described previously as the IVF proper (Vital et al., 1983) (see Fig. 7-12, A). The dorsal root (spinal) ganglion and ventral root are located within this region and can be compressed here. Because of its large size, the dorsal root ganglion is more susceptible to minor compression than the ventral root. Osteophyte formation along the ligamentum flavum (anterior to the pars interarticularis) and hypertrophy of fibrous tissue along a fracture of the pars interarticularis were cited by Lee and colleagues (1988) as being the most common causes of stenosis in the middle zone. They also believed that clinicians may overlook stenosis of this region because the middle zone is difficult to evaluate with diagnostic imaging procedures. They stated that neural entrapment of the middle zone may result in symptoms of activity-related, intermittent, neurogenic claudication. They also noted that some patients progress until they experience constant pain and diminished sensation even during times of rest. These unprovoked resting symptoms may be caused by spontaneous action potentials arising from compressed or entrapped ganglionic cells (Lee et al., 1988).
The term exit zone, or foramen proper, was used by Rauschning (1987) and Lee and colleagues (1988) to refer to the almost two-dimensional opening (“doorway”) formed by a parasagittal plane passed along the lateral edge of the two pedicles that help to form an IVF (see Fig. 7-12, C). The most common causes of stenosis in this region were cited as “hypertrophic osteoarthritic changes of the facet joints (Z joints) with subluxation and osteophytic ridge formation along the superior margin of the disc” (Lee, Rauschning, & Glenn, 1988).
Clinical Conditions Related to the Nerve Root Canals
Narrowing of the IVD, particularly at the L5-S1 level, causes narrowing of the IVF at the same level (L5-S1 IVF in this case) and also narrowing of the NRC of the nerve exiting at the IVF below (the S1 NRC in this case). Crock (1981) stated that the remaining anulus fibrosus of the disc may bulge posteriorly, bringing the posterior longitudinal ligament along with it. The superior articular facet of the segment below (S1) moves superiorly and anteriorly, again compressing the NRC (S1). The combination of posterior anulus bulge along with anterior displacement of the superior articular facet of the vertebra below can result in dramatic narrowing of the NRC that runs between these two structures (Rauschning, 1987). Osteophyte (bony spur) formation is fairly common, both from the vertebral body along the attachment of the anulus fibrosus and from the superior articular process along the attachment of the ligamentum flavum. These spurs can further compress the neural and vascular elements of the NRC (Rauschning, 1987). In addition, a bony ridge may develop along the anterior and inferior surface of the lamina. This can compress the more medial NRC (that of the nerve exiting at the IVF of the level below, S1 in this instance) and also the lateral and distal (inferior) aspect of the NRC of the nerve exiting at the same level (in this case, L5).
The L5 NRC is related to both the L4-5 (at its origin) and the L5-S1 (at its exit) IVDs. Therefore with suspected entrapment of the L5 nerve, a differentiation between compression from an L4 or L5 disc bulge or protrusion, or from another structure along the L5 NRC, must be made (Bose & Balasubramaniam, 1984).
Congenital hypertrophy of the L5-S1 articular processes and facets may cause localized obstruction of the L5-S1 IVF. Osteoarthritis of the L5-S1 facets may cause the same condition, but usually such arthritis tends to cause obstruction more medially, affecting the descending S1 NRC (Crock, 1981).
Intervertebral Foramina Proper
When viewed from the side, the lumbar IVFs face laterally. A typical lumbar IVF is sometimes described as being shaped like an inverted teardrop or an inverted pear (Fig. 7-14). The specific dimensions of the lumbar IVFs are given in Chapter 2 (see Tables 2-6 and 2-7).
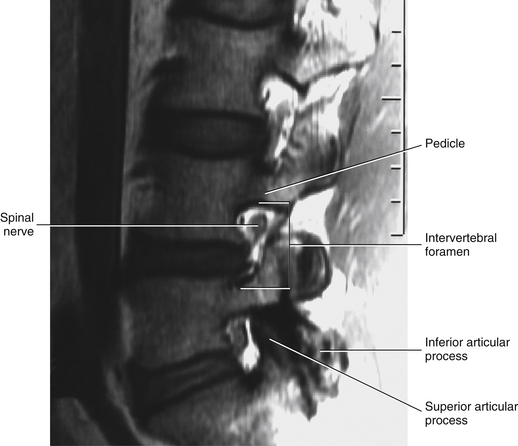
FIG. 7-14 Parasagittal plane magnetic resonance imaging scan of the lumbar region. Note the prominent intervertebral foramina. (Image courtesy Dr. Dennis Skogsbergh.)
The spinal nerve is located in the upper third of each lumbar IVF. As it enters the IVF, the spinal nerve is close to the medial and inferior aspect of the superior pedicle that forms the upper boundary of the IVF (Crock, 1981). Here the nerve is accompanied by a branch (or sometimes branches) of the lumbar segmental artery; by the superior intervertebral (segmental, or pedicle) veins, which connect the external and internal vertebral venous plexuses; and by the sinuvertebral nerve (Rauschning, 1987). The spinal nerve occupies approximately one third of the IVF in the lumbar region. However, the spinal nerve can be as close as 0.4 mm (range, 0.4 to 0.8 mm) to the bony anterior and posterior IVF borders (Giles, 1994). This allows crowding by the articular facets during extension (Bose & Balasubramaniam, 1984; Rauschning, 1987). The inferior aspect of the IVF is usually narrowed to a slit by the anulus fibrosus, which normally bulges slightly posteriorly. The inferior aspect of the IVF is also narrowed by the posteriorly located ligamentum flavum. The inferior intervertebral (segmental, discal) veins usually lie in this narrow space. As with the superior intervertebral (pedicle) veins, the inferior veins also unite the internal (epidural) vertebral venous plexus with the external vertebral venous plexus and the ascending lumbar vein.
The lateral borders of the IVFs are covered with an incomplete layer of transforaminal fascia (Paz-Fumagalli & Haughton, 1993). This fascia condenses in several locations at each IVF to form the accessory ligaments of the IVF (see Chapter 2). An exiting spinal nerve could be affected by these structures as it leaves the IVF (Bachop & Janse, 1983; Bachop & Ro, 1984; Bose & Balasubramaniam, 1984; Rauschning, 1987; Bakkum & Mestan, 1994; Qian, Qin, & Zheng, 2011). In addition to compression by accessory ligaments or transforaminal fascia, Rauschning (1987) states that lateral disc herniations and bony structures such as the TPs (usually of L5, but at higher lumbar levels on rare occasions) “may compress, kink, or constrict the lumbar nerves beyond the foraminal outlet.” The author calls this region the extraforaminal region or the postcanal zone.
Also, the lateral borders of the L1 through L4 IVFs are associated with the origin of the psoas major muscle. In fact, because of the posterior origin of the psoas major muscle from the front of the TPs and its anterior origin from the lumbar vertebral bodies and IVDs, the psoas major almost completely surrounds the lateral opening of the first four lumbar IVFs. Therefore the anterior primary divisions (ventral rami), by necessity, run through the substance of the psoas major muscle, frequently uniting with neighboring ventral rami within the muscle to form the branches of the lumbar plexus. In addition to the protection given by the dural root sleeve and the meningovertebral (Hoffmann) ligaments (see previous discussion and Fig. 7-9), the psoas major muscle may provide some protection for the dorsal and ventral roots during traction of the peripheral nerves of the lumbar plexus (dePeretti et al., 1989). Such traction may occur as a result of hyperflexion or hyperextension of the lower extremity.
The boundaries of the IVF can be imaged well with both MRI and CT (Cramer et al., 1994) (Fig. 7-15; see also Fig. 7-14). Occasionally ossification of the superior attachment of the ligamentum flavum results in foraminal spurs, which can be seen on CT. These spurs are considered to be normal variants; may project well into the IVF, posterior to the dorsal root ganglion; are frequently bilateral; and are usually asymptomatic (Helms & Sims, 1986).
Changes in Dimensions of the Intervertebral Foramina
Flexion of the lumbar region has been shown to significantly increase the height and width of the IVFs by separating the pedicles (increases height), by decreasing the normal bulging of the anulus fibrosus of the IVD (increases IVF width), and by decreasing the thickness of the ligamentum flavum (increases IVF width) (Fujiwara et al., 2001). Extension significantly decreases the height and width of the IVFs and increases pressure within the IVF (Morishita et al., 2006) by having the opposite effects as flexion (Fujiwara et al., 2001). Flexion increases the cross-sectional sagittal area of the IVF from the neutral position by 12%, whereas extension decreases the area of the IVF from the neutral position by 15% (Inufusa et al., 1996); other investigators have found even greater changes in cross-sectional IVF area (up to 44%) during flexion and extension (Schmid et al., 1999). Distraction (traction) has been found to increase IVF size in cadavers (Schlegel et al., 1994), and compression decreases the height of the IVFs (Nowicki et al., 1990). Titanium cages (BAK Interbody Fusion System) implanted in the IVDs during interbody fusion spine surgery also have been shown to increase IVF volume at L4-5 and L5-S1 (Chen et al., 1995). Many of the pathologic conditions described as affecting the NRC, such as anterior osteophytes on the superior articular processes of the Z joints, also can diminish the dimensions of the IVF proper (Bailey & Casamajor, 1911; Giles, 1994). In addition, recall that the IVD forms a part of the anterior border of the IVF. Because of this, a far lateral IVD prolapse can cause a significant decrease in the anterior-to-posterior dimension of the IVF, resulting in a dramatic change of shape of the spinal nerve in the IVF and the development of fibrotic adhesions in and around the spinal nerve (Hadley, 1948). In addition, a decrease in disc height also results in a decrease of the vertical dimension of the IVF (Crock, 1981; Cinotti et al., 2002). In fact, a decrease in IVF height of only 4 mm, when combined with lumbar extension, can cause compression of the dorsal root ganglion or the spinal nerve within the IVF (Revel et al., 1988). This is most likely because the dorsal root ganglion and spinal nerve normally can be as close as 0.4 mm to the bony anterior and posterior borders of the IVFs (Giles, 1994). Cinotti and colleagues (2002) found that a decrease in the sagittal diameter of the vertebral (spinal) canal was related to a decreased width of the IVF at the same level (usually resulting from decreased length of the pedicles). This relationship of decreased spinal canal size with decreased IVF size; along with the close relationship of disc degeneration, acquired spinal canal stenosis, IVF stenosis, and decreased IVF height, led Cinotti and colleagues (2002) to conclude that IVF stenosis should be suspected whenever spinal canal stenosis is present. In a related study, Morishita and colleagues (2006) found increased L5-S1 IVF pressures during lumbar extension. They then combined IVF pressure measurements with electrophysiologic recordings taken during spinal surgery and concluded that a double compression of the L5 nerve roots occurred at (1) the vertebral canal and (2) the IVF, during extension in patients with lumbar spinal stenosis. For these reasons, having access to the normal size of the lumbar IVFs as they appear on MRI scans can be clinically useful. (These values are presented in Chapter 2 in the section Intervertebral Foramen, Tables 2-6 and 2-7.)
L5 Nerve Root Canal and L5 Intervertebral Foramina
The anatomy of the L5 NRC is unique, and because of this the L5 roots and nerve are susceptible to compression in many different locations. This canal is longer and runs at a more oblique anterior angle than the rest of the lumbar NRCs. Also, the lateral recess of the L5 vertebra is the deepest laterally and often the narrowest from anterior to posterior of the entire spine. This narrow lateral recess may lead to compression of the L5 nerve in some instances (Rauschning, 1987). Hasue and colleagues (1983) found histologic evidence of compression (intraneural fibrosis) of the L5 dorsal root. Compression occurred between the superior articular process of S1 and the posterior aspect of the vertebral body of L5 (Hasue et al., 1983).
After leaving the lateral recess, the L5 roots and their dural root sleeve continue along the L5 NRC by wrapping around the posterior and lateral aspect of the L5 vertebral body. The roots continue around the posterolateral aspect of the L5 IVD and unite to form the spinal nerve. The L5 nerve, which is the largest of the lumbar nerves, exits the lateral border of the L5 NRC (the IVF proper), which is the smallest IVF of the lumbar spine (Olsewski et al., 1991; Cramer et al., 2003). This makes the L5 nerve particularly susceptible to compression within its IVF.
The anterior primary division (APD) of L5 is given off at the most lateral aspect of the IVF and then passes along a depression on the front of the sacral ala. The APD frequently is bounded anterosuperiorly in this region by the corporotransverse ligament (Bachop & Janse, 1983; Bachop & Ro, 1984; Rauschning, 1987). The corporotransverse ligament passes from the vertebral body and IVD of L5 to the TP of L5. Several investigators (Nathan, Weizenbluth, & Halperin, 1982; Olsewski et al., 1991; Briggs & Chandraraj, 1995) report that the inferior band of the iliolumbar ligament, known as the lumbosacral ligament (LSL), consistently courses from the vertebral body and TP of L5 to the ala of the sacrum (see Fig. 7-23). The descriptions of the corporotransverse ligament by Bachop and Janse (1983) and Bachop and Ro (1984) are consistent with what Nathan and colleagues (1982) consider to be the fibers of origin of the LSL. This indicates that the fibers of origin of the LSL are much more substantial than those fibers found more inferiorly and laterally, and that frequently a distinct tough, fibrous band, the corporotransverse ligament, is formed in the region of origin of the LSL.
After passing beneath the corporotransverse ligament and the LSL, the APD of L5 continues inferiorly (Nathan, Weizenbluth, & Halperin, 1982; Bachop & Janse, 1983; Bachop & Ro, 1984; Olsewski et al., 1991). Therefore the corporotransverse ligament of Bachop and the LSL significantly extend the osteoligamentous canal of the L5 NRC, and the most inferior aspect of the LSL forms the anterior and inferior boundary of the L5 osteoligamentous canal. Nathan and colleagues (1982) state that the gray communicating ramus from the sympathetic chain to the APD of L5 pierces the LSL. Previous studies had shown that, throughout the spine, osteophytes from vertebral bodies frequently exert pressure on the sympathetic trunk, rami communicantes, and spinal nerves. This was found to be particularly true with the neural elements of L5 (Nathan, Weizenbluth, & Halperin, 1982). Finally, just inferior to the LSL, a branch of the APD of L4 joins the APD of L5 to form the lumbosacral trunk.
The unique characteristics of the L5-S1 NRC, the IVF proper, and the lateral osteoligamentous canal of the L5 APD make the L5 nerve “extremely vulnerable to compression by any of the structures forming the tunnel. A tight LSL, osteophytes on the border of the L5-S1 disc or a combination of the two may impinge on the nerve and compress it against the ala of the sacrum” (Nathan, Weizenbluth, & Halperin, 1982). Olsewski and colleagues (1991) reported that the APD of L5 was observed to be compressed by the LSL in 11% of the 102 cadaveric specimens they studied by gross dissection. Histologic evidence of compression (e.g., perineurial and endoneurial fibrosis, peripheral thinning of myelin sheaths, and shift to a smaller fiber diameter) was shown in 3% of L5 APD specimens studied by Olsewski and colleagues (1991), and in 9% of the specimens studied by Briggs and Chandraraj (1995). Osteophytes extending posterolaterally from both the inferior surface of the L5 body and the upper border of the body of the sacrum were found to narrow the L5 osteoligamentous canal further in 1 of 59 spines (2%) studied by Olsewski and colleagues (1991). This was found to contribute to compression of the APD of L5. Nathan and colleagues (1982) found such osteophytes “frequently” and also noted that the L5 nerve was “very often” entrapped or compressed to some degree by osteophytes or the LSL while passing through the osteoligamentous tunnel. Briggs and Chandraraj (1995) found that IVD narrowing also contributed to compression of the L5 APD; they felt that the reason for this may be the addition of fibers to the LSL in response to instability in the region after IVD degeneration.
Compression of the L5 APD by the corporotransverse ligament or the LSL could result in pain along the distribution of L5 nerves to include the L5 dermatome (lateral aspect of the leg distally to the great toe [Floman & Mirovsky, 1990]) and possible loss of motor function of the muscles primarily innervated by the L5 APD (motor paresis of the extensor hallucis longus muscle is found in 5% to 11% of patients with L5 nerve root compression [Jönsson & Stromqvist, 1995]). Referred pain may be experienced in the lumbar region (see Chapter 11), although this has not been fully documented. Myelography, discography, standard CT scans, and transverse plane MRI scans would all be negative (Olsewski et al., 1991). Far lateral parasagittal MRI scans (farther lateral than standard MRI protocols) may show the relationship between the L5 nerve and the corporotransverse ligament and the LSL (Nowicki & Haughton, 1992). Perhaps far lateral parasagittal MRI would be a useful diagnostic procedure for patients exhibiting L5 dermatomal and motor symptoms and signs when other imaging modalities do not reveal a possible cause of entrapment (see Fig. 2-25). Further investigation in this region is warranted. In the meantime, “in the patient presenting with L5 root signs, if the myelogram, discogram, and CT scan do not reveal any defect, then the possibility of extraforaminal compression must be considered as a possible source of the clinical signs” (Olsewski et al., 1991).
Because of the unique anatomy of this region, several distinct conditions, besides those already described, can affect the L5 nerve roots, the L5 spinal nerve, or the APD of L5. For example, because the neural elements of L5 are related to the L5 IVD for a relatively long distance, far lateral L5 IVD protrusion (lateral to the IVF) may affect the L5 spinal nerve or the APD of L5. Another example of the unique vulnerability of the L5 neural elements is spondylolisthesis after spondylolysis (see Fig. 7-17). This condition may result in compression of the L5 nerve along its course from behind the L5 disc to the nerve’s lateral relation with the corporotransverse ligament and the L5 TP, both of which lie above the nerve (Bachop & Janse, 1983; Bachop & Ro, 1984; Rauschning, 1987).
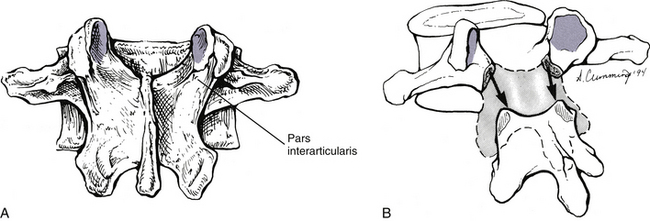
FIG. 7-17 A, Posterior view of a typical lumbar vertebra. B, Bilateral fracture of the pars interarticularis (spondylolysis) and separation (spondylolisthesis) of the region anterior to the pars interarticularis from the remainder of the posterior arch.
A 20% or greater anterior shift of a spondylolisthetic L5 vertebra may result in compression of the APD of L5 between the TP of L5 and the sacral ala (Wiltse et al., 1984). Such compression is usually unilateral but occasionally may be bilateral. Also, both asymmetric degeneration of the L5 IVD and degenerative lumbar scoliosis result in lateral tilting and rotation of L5. As with spondylolisthesis, this also can result in compression of the L5 nerve between the TP of L5 and the sacral ala. Such far lateral compressions of the L5 nerve have been called the “far-out syndrome (Wiltse et al., 1984) of Wiltse” (Dommisse & Louw, 1990). Wiltse and colleagues (1984) stated that the lateral entrapment caused by either degenerative scoliosis or asymmetric disc degeneration probably was most common in elderly patients, whereas lateral entrapment as a result of spondylolisthesis was found most frequently in a somewhat younger group of adult patients. The authors also stated that far lateral entrapment occasionally could occur at levels higher than L5 and may be accompanied by the radiographic findings of marked scoliosis with closely approximated TPs of two adjacent vertebrae. CT was found to be the imaging modality of choice to view the region of entrapment. A “wide window” setting for the CT scan was necessary to view the laterally placed TPs. CT images reformatted in the coronal plane were particularly useful in evaluating the relationship between the L5 TPs and the sacral ala (Wiltse et al., 1984).
Nathan and colleagues (1982) found that branches of the iliolumbar artery and relatively large veins always accompanied the APD of L5 beneath the LSL. Using examples of neuralgias and pareses of cranial nerves caused by compression of these nerves by adjacent arteries, they hypothesized that likewise the APD of L5 could become entrapped within its osteoligamentous tunnel by branches of the iliolumbar artery and accompanying veins. This would be particularly feasible when the APD of L5 already was partially compressed within a narrow tunnel or by osteophytes extending posterolaterally from the inferior border of the L5 body.
Unique Aspects of the Lumbar Vertebrae
The L5 vertebra, its relationship with the sacrum, and the soft tissue elements in between are some of the most clinically relevant anatomic structures of the spine. Pain in this region is extremely common (Cramer et al., 1992); therefore an accurate working knowledge of the L5-S1 region is essential for those treating patients with low back and leg pain. The anatomy of this region is subject to much variation. Nathan and colleagues (1982) stated that “such skeletal variations are accompanied by changes and adjustments of the related soft tissues, including the nerves and vessels.” Therefore clinically relevant variations frequently accompany the normal anatomy of this area.
The L5 vertebral body is the largest of the entire spine (Fig. 7-16). It is taller anteriorly than posteriorly, which contributes to the increase in the lower lumbar lordosis (the lower lumbar lordosis is frequently called the lumbosacral angle). The spinous process of L5 is the smallest of all those of the lumbar vertebrae. It projects inferiorly, and its posterior aspect is more rounded in appearance than the rest of the lumbar spinous processes (see Fig. 7-16). The TPs of L5 are much wider from anterior to posterior and from superior to inferior than those of the rest of the lumbar spine. They originate from the entire lateral aspect of the pedicles, and their origin continues posteriorly to the adjacent lamina (see Fig. 7-16). However, the TPs do not extend as far laterally as other lumbar TPs (see Transverse Processes). The lateral aspect of the TPs of L5 also angle slightly superiorly, with the angulation beginning at about the midpoint of each of the two (left and right) processes.
Spondylolysis and Spondylolisthesis
Spondylolysis is a defect of the lamina (Fig. 7-17) between the superior and inferior articular processes. This region is known as the pars interarticularis. Controversy exists as to whether this defect is most frequently caused by trauma or if it is hereditary (Schwab, Farcy, & Roye, 1997). Causes of pars (isthmic) defects include lytic or stress fractures of the pars interarticularis (probably the most common cause), elongated but intact pars (not spondylolysis), and acute fracture of the pars (Day, 1991). The lesions range from subtle hairline fractures to the large gaps seen in spondylolisthesis (Ward et al., 2007). The fifth lumbar vertebra is the segment most vulnerable to acute pars fracture (Ebraheim et al., 1997a). Athletes whose sport requires them to rapidly alternate between flexion and extension of the lumbar region are commonly afflicted with acute fractures of the pars interarticularis (McGill, 1997). Bilateral spondylolysis may result in forward slippage of that portion of the vertebra located anterior to the laminar defect (i.e., vertebral body, pedicles, TPs, superior articular processes). This leaves the inferior articular processes, a portion of the laminae, and the spinous process behind. Such forward displacement of the structures anterior to the spondylolysis is known as spondylolisthesis and is found in 5% to 6% of lumbar spines (Standring et al., 2008; Herman, Pizzutillo, & Cavalier, 2003) (see Fig. 7-17). Although most common at L5, spondylolisthesis occurs with some frequency at L4 and may be seen at any level of the lumbar spine. Other causes of spondylolisthesis include (a) improper formation of the posterior vertebral arch, known as the dysplastic type of spondylolisthesis; (b) degeneration and subsequent erosion of the superior articular process; (c) fracture of part of the posterior arch other than the pars interarticularis (e.g., pedicle fracture); and (d) pathologic conditions of the bone forming the posterior arch (e.g., Paget’s disease).
Even though spondylolysis is probably most often due to acute or stress fracture, there is also a genetic predisposition, with a 30% to 70% prevalence in family members of individuals diagnosed with spondylolysis (Faingold et al., 2004). The genetic component is supported by the findings that both an increased sacral kyphosis and an increased slope of the superior S1 bony end plate are associated with L5-S1 spondylolisthesis in children and adolescents (Wang et al., 2008). (See Chapter 8 section titled The Sacrum for additional information.) In addition, the distance between the lateral aspect of the left and right inferior articular processes is narrower in individuals with L5 spondylolysis. This distance normally increases from L4 to L5. When the distance does not increase (most likely a genetically inherited trait), the inferior articular process of L4 and the superior articular process of S1 have a “pincher effect” on the pars interarticularis of L5 during activities that involve hyperextension (e.g., gymnastics and swimming using butterfly and breast-strokes). This pincher effect predisposes these indivduals to L5 spondylolysis (Ward et al., 2007).
Sometimes the pars defect fills with connective tissue. The connective tissue attachment has been termed the spondylolysis ligament (Wiltse, 1962) and contains several zones of fibrous and cartilaginous tissues (Nordström et al., 1994). Einstein and colleagues (1994) found that this “ligament” was innervated by nerves that met the criteria of pain-sensitive (nociceptive) fibers (e.g., immunoreactivity to calcitonin gene–related peptide [CGRP] and vasoactive intestinal peptide), indicating that deformation of the ligament during spondylolisthesis could result in low back pain. Of course, several other tissues (e.g., IVD, muscles, ligaments) that are stretched because of spondylolisthesis also could generate pain as well (Einstein et al., 1994).
Frequently a “rounding defect” occurs on the anterosuperior aspect of the vertebral segment, or usually the sacrum, below the level of spondylolisthesis. This convex, curving defect is thought to increase the slippage of the spondylolisthetic segment. The rounding defect may be due to damage of the anular apophysis caused by pressure from the spondylolisthesis above (Higashino et al., 2007).
Standard x-ray films and CT remain the imaging modalities of choice for visualizing spondylolysis. However, parasagittal MRI does help to reveal a defect of the pars, which appears as a decrease in signal intensity perpendicular to the plane of the articular facets on these images (Grenier et al., 1989a). Occasionally, rather than the development of a spondylolisthesis, the posterior segment created by bilateral spondylolysis moves further posteriorly. When such posterior displacement occurs, it can frequently be identified on sagittal plane MRI scans (Ulmer et al., 1995).
Spondylolisthesis is graded by the degree to which the affected vertebral body is anteriorly displaced in relation to the vertebral (or sacral) body located immediately inferior to it. For example, a 25% spondylolisthesis represents forward displacement of a vertebral body (measured at the posterior and inferior border of the vertebral body) along one fourth of the length of the vertebral body (or sacral body) directly inferior to it. Spondylolisthesis also can be graded on a scale of 1 to 5 (Meyerding classification), with each grade representing up to 25% of anterior slippage. A grade 5 (original classification only went to grade 4) describes a vertebral body that has been displaced completely off of the vertebra (or usually, the sacrum) directly beneath it, a condition known as spondyloptosis (Maxfield, 2010).
Although spondylolysis with spondylolisthesis has been implicated as a cause of spinal canal (specifically, lateral recess) stenosis at the level of the pars interarticularis defect, Liyang and colleagues (1989) reported an increase in vertebral canal dimensions at the level of spondylolisthesis in 1 cadaveric spine, which was included in their study of 10 normal spines. Spondylolisthesis at L5 also may result in entrapment of the L5 nerve as it passes laterally, in front of the pars interarticularis, to exit the L5 IVF. The entrapment is caused by the portion of the pars located above the fracture site. This is the part of the pars that is displaced anteriorly with the vertebral body and pedicles. By moving anteriorly, it can compress the L5 nerve (Kirkaldy-Willis et al., 1978).
Degenerative spondylolisthesis (DS) is a condition in which the superior articular processes undergo erosion with age rather than the usual increase in bone formation that frequently occurs in these processes with age. This erosion results in the vertebrae above (usually L4, the next most common is L3) moving anteriorly, bringing its posterior arch with it. The shape and orientation of the superior articular processes and facets also appear to play a role in DS. First, sagittally oriented facets are associated with DS (Kalichman et al., 2009). Second, the cephalad aspects of the superior articular processes are more sagittally oriented and the caudad aspects of the same facets are more coronally oriented in not only the DS segment but also the adjacent lumbar segments. This unique orientation and shape of the articular facets throughout the lower lumbar spine in individuals with DS indicates that there may be a genetic predisposition to the development of this condition (Toyone et al., 2009).
The amount of anterior slippage (olisthesis) with DS ranges from 8% to 43% (Postacchini & Perugia, 1991). The consequence of this anterior slippage of the entire vertebra, including the posterior arch, is spinal canal stenosis with possible compromise of the cauda equina (Kirkaldy-Willis et al., 1978). As mentioned, most frequently L4 moves anteriorly on L5, and the inferior articular processes of L4 entrap the L5 and S1 nerve roots against the posterior aspect of the vertebral body of L5 (Dommisse & Louw, 1990).
At the opposite end of the clinical spectrum for the pars interarticularis: the pars can occasionally enlarge as a result of degeneration. This is significant at the level of L5 because it can lead to entrapment of the S1 nerve as it courses along the lateral aspect of the vertebral canal (lateral recess).
Lumbosacral Articulation
The lumbosacral articulation is actually composed of several articulations between L5 and the sacrum. It consists of two components: the joining, by the fifth lumbar IVD, of the inferior aspect of the L5 body with the body of the S1 segment; and the joints between the left and right inferior articular processes of L5 and the superior articular processes of the sacrum. These latter joints are not nearly as curved as are the Z joints of the rest of the lumbar spine. The plane of articulation of the lumbosacral Z joints is subject to much variation, ranging from 20 to 90 degrees to the sagittal plane (average, 40 to 60 degrees). Frequently asymmetry, known as tropism, exists between the left and right L5-S1 Z joints. Tropism may be a cause of premature degeneration and pain (Farfan, Huberdeau, & Dubow, 1972; Lippitt, 1984), with increased degenerative changes of the articular cartilage having been found on the side of the superior and inferior articular processes that are more closely oriented along the sagittal plane (Giles, 1987). However, the clinical significance of tropism remains a matter of controversy. For example, no association has been found between asymmetric facets and prolapsed IVD (Vanharanta et al., 1993; Boden et al., 1996) or degenerative spondylolisthesis (Boden et al., 1996; Kalichman et al., 2009); however, an association has been found between both of those clinical entities and bilateral sagittally oriented facets. This positive association holds true for sagittally oriented facets at both L4-L5 and L5-S1 (Boden et al., 1996).
The L5-S1 IVD is typically narrower than the IVDs of the rest of the lumbar region (Nicholson, Roberts, & Williams, 1988). This may contribute to the IVF at this level being smaller than those of the rest of the lumbar spine. It should be recalled that the spinal nerve at this level is the largest lumbar spinal nerve. Therefore more than one third of the L5-S1 IVF is filled by the spinal nerve. Even though the IVD and IVF are smaller in this region, the L5-S1 articulation is by far the most movable of all the lumbar joints (5 degrees of unilateral rotation, 3 degrees of lateral bending, 10 degrees of flexion, 10 degrees of extension). These factors, along with the others discussed in the previous section devoted to the L5 NRC, make the L5 roots and spinal nerve vulnerable to compression as they traverse the L5 NRC.
The intraarticular space of the left and right lumbosacral Z joints usually is wider than those of the remainder of the lumbar region. A recess normally exists along the inferomedial edge of the lumbosacral Z joints. This recess has been shown to be filled with a large intraarticular synovial fold, which is primarily composed of adipose tissue. Another intraarticular synovial protrusion usually projects into the superomedial aspect of the L5-S1 Z joint (Giles & Taylor, 1987). These synovial folds are susceptible to entrapment between the apposing L5-S1 articular facets and are a likely source of low back pain and subsequent muscle tightness. Gentle, well-controlled spinal manipulation to open the facets and allow an entrapped synovial fold to be pulled out of the joint by its attachment to the joint capsule has been suggested as the treatment of choice for this condition (Giles & Taylor, 1987).
A transitional segment between the lumbar spine and sacrum is found in approximately 5% of the population (Nicholson, Roberts, & Williams, 1988), but may be higher (Tague, 2011). This takes the form of either a lumbarization of the S1 segment or, more frequently, a sacralization of the L5 vertebra. Sacralization refers to elongation of the TPs of L5 with varying degrees of fusion or articulation with either the sacral ala or the iliac crest. Tague (2011) found the incidence of sacralization to be be 11%. The union between L5 and the sacrum may be bilateral, but usually it is only unilateral (see Fig. 12-14, G and H). The L5-S1 IVD in cases of sacralization is normally significantly thinner than that of typical L5-S1 segments (Nicholson, Roberts, & Williams, 1988; Hsieh et al., 2000). It is also usually devoid of nuclear material, and therefore usually does not undergo pathologic change or degeneration to the degree seen in discs above the sacralized segment (Nicholson, Roberts, & Williams, 1988). Sacralization is also associated with decreased superior-inferior height of the “true sacrum” (Mahato, 2010).