Spinal Motor Neurons and Motor Coordination
Motor coordination is the process of linking the contractions of many independent muscles so that they can act synergistically and be controlled as a single unit. This is typically accomplished via the spinal reflexes, which act to coordinate most actions of groups of muscles. Most reflexes at the level of the spinal cord are polysynaptic, which allows for modification of responses by higher centers within the CNS and by local circuits in the spinal cord. Movement of a skeletal muscle is a direct result of stimulation of that muscle by specific controlling elements called the motor units. A motor unit is defined as an alpha motor neuron (spinal motor neuron) and all of the muscle fibers it innervates (Loeb & Ghez, 2000). It is the smallest controllable element of the CNS (Fig. 9-23). Each muscle fiber is innervated by only one alpha motor neuron. However, each motor neuron innervates many muscle fibers. The number of muscle fibers a motor neuron innervates determines that motor neuron’s innervation ratio. All of the muscle fibers innervated by a single motor neuron (a motor unit) respond in an identical manner. The innervation ratio varies between muscles, but is approximately proportional to the size of the muscle and the size of the alpha motor neuron. For example, the gastrocnemius muscle has an innervation ratio of approximately 2000:1 and is innervated by large motor neurons, whereas the small muscles of the hand generally have an innervation ratio of approximately 10:1 and are innervated by relatively small motor neurons. A low innervation ratio indicates finely graded control of muscle force.
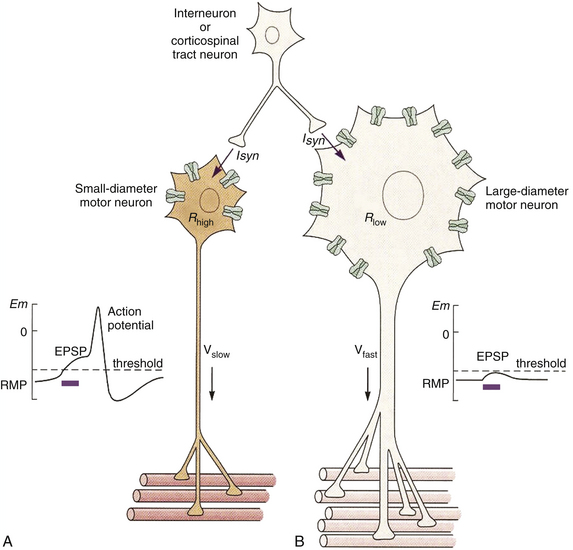
FIG. 9-23 The motor unit. The motor unit consists of the alpha motor neuron and all of the muscle fibers it innervates. A, Small motor neurons generally have a small innervation ratio and are recruited first in the motor neuron pool. After stimulation, the small surface area and relatively high membrane resistance (attributable to fewer leak channels; Rhigh) result in a large synaptic potential that can more readily reach threshold, resulting in an action potential in this case. This action potential then stimulates the small number of muscle fibers to contract. The force generated by these fibers is relatively low, but can usually be maintained indefinitely. B, Large motor neurons generally have a high innervation ratio and are recruited last in the motor neuron pool. After stimulation, the large surface area and low membrane resistance (Rlow) result in a smaller synaptic potential that is usually subthreshold. Therefore summation must occur before this neuron can create an action potential and cause contraction of its muscle fibers. Once recruited, the force generated by the large number of muscle fibers is quite large, but relatively short-lived. (Modified from Loeb GE & Ghez C. [2000]. The motor unit and muscle action. In ER Kandel, JH Schwartz, & TM Jessell [Eds.]. Principles of neural science [4th ed.]. New York: McGraw-Hill.)
Three types of motor neurons can be found in the ventral horn of the spinal cord. One type is the alpha motor neurons (skeletomotor efferents), which are the largest and exclusively innervate skeletal muscle. The alpha motor neurons can be further segregated into three groups based on the size of the alpha motor neuron and the biochemical properties of the muscle fiber they innervate. However, the alpha motor neuron determines the biochemical properties of the muscle tissue. The largest alpha motor neurons have the highest innervation ratios, and innervate muscle tissue that can contract and relax rapidly, but also fatigue rapidly (<1 min). These are known as fast glycolytic or fast fatigable motor units (Rhoades & Tanner, 2003) (see Fig. 9-23, B). The muscle tissue they innervate has few mitochondria, low myoglobin content, high glycogen and glycolytic enzyme content, and high adenosinetriphosphatase (ATPase) activity; can develop the largest forces of muscle tension (up to 80 g); and generally has the greatest cross-sectional area. These muscle fibers can actually function anaerobically for short periods of time because of their glycolytic metabolic pathways. Moderate-size motor units have intermediate innervation ratios, and innervate muscle tissue that has slightly slower contraction times and is resistant to fatigue, meaning the muscles can maintain their force of contraction for many minutes. These are known as the fast oxidative or fast fatigue-resistant motor units (Loeb & Ghez, 2000; Rhoades & Tanner, 2003). The muscle tissue they innervate has many mitochondria, high myoglobin content (which is an O2 chelator), high levels of oxidative and glycolytic enzymes, a powerful myosin ATPase activity, and intermediate force development (20 to 40 g). The smallest alpha motor neurons have the smallest innervation ratios and innervate muscles that contract slowly and precisely, and can maintain their contraction for many hours without fatigue. They are known as oxidative or slow motor units (Loeb & Ghez, 2000; Rhoades & Tanner, 2003) (see Fig. 9-23, A). The muscle tissue these fibers innervate has many mitochondria, many oxidative enzymes, high myoglobin content, and the smallest force development (<20 g). In addition, the firing rate of these slow motor units generally is low, and the motor units have a large hyperpolarizing afterpotential. This afterpotential aids in the summation of action potentials in the muscle, and helps prevent the occurrence of additional action potentials (self-limiting). Individual muscles contain varying proportions of all three sizes of motor units. Their muscle fibers are distributed within the muscle tissue based on their selective metabolic needs. Slow-type fibers typically are more numerous and require the greatest metabolic support, and therefore are located deep in the muscle tissue closest to the blood supply. Fast-type fibers can use glycolysis and function anaerobically, and therefore usually are located peripherally in muscle. The proportion of motor unit types within a muscle corresponds to its functional needs. Postural muscles (e.g., the soleus muscle) have a larger percentage of slow-type compared with fast fatigable-type fibers, whereas strength muscles (e.g., the biceps and gastrocnemius muscles) have a larger proportion of fast-type fibers.
The second and smallest type of motor neuron found in the spinal cord is the gamma motor neuron or fusimotor neuron (Leksell, 1945). The gamma motor neuron exclusively innervates the polar regions of the muscle spindles (see Receptors in the Motor System) and can control their level of sensitivity.
The third type is the beta motor neuron, which innervates both skeletal muscle and the muscle spindles and is known as the skeletofusimotor neuron (Bessou, Emonet-Demand, & Laporte, 1965).
These three types of motor neurons are not segregated within the spinal cord, but rather are mixed together in the ventral horn into groupings called pools. A given motor neuron pool generally innervates one particular muscle. The various motor neuron pools are segregated into longitudinal columns normally extending two to four spinal segments. This arrangement of using motor neuron pools rather than having to stimulate each individual alpha motor neuron simplifies the task of the CNS in controlling movements of muscles. Two major strategies are employed by the CNS to control the force and velocity of contractions in skeletal muscle. First, motor units are recruited or stimulated in a fixed order from weakest (slow-type) to strongest (fast fatigable-type). In this fashion, larger motor neurons are only recruited after a significant increase in the stimulus strength. This phenomenon is called the size principle (Loeb & Ghez, 2000) (see Fig. 9-23). Weak inputs to a motor neuron pool in the ventral horn recruit only the smallest neurons first—those of the slow motor units—which can generate a small but consistent force that can be maintained almost indefinitely. As the input to the motor neuron pool increases, the fast fatigue-resistant motor neurons also are recruited and thereby increase the force of the contraction. Finally, if a large force or rapid contraction is necessary, the input to the motor neuron pool again increases, and the largest motor neurons—those belonging to the fast fatigable motor units—are recruited in addition to all of the other motor units, and the force of the contraction increases to its maximum. Based on the constraints of the size principle, the most numerous motor units (typically the slow fibers) are used first and most often, and are provided with the greatest metabolic support.
The second major strategy employed by the CNS to modulate muscle force or velocity is by altering the frequency of firing of the motor units. This process is known as rate modulation (Loeb & Ghez, 2000). As the CNS increases the firing rate of the motor neuron pool, successive twitches can summate more effectively. This summation results in the muscle contracting and moving the joint to a new position. There is a physiologic range for stimulation of muscle, because of the “low-pass filtering” properties of muscle (action potential generation in muscle is inherently slower than in the CNS). That physiologic range is 8 to 25 Hz. During normal firing of an alpha motor neuron, there is insufficient time for the calcium ions to be completely pumped back into the sarcoplasmic reticulum of the muscle before the next stimulus occurs. This results in a sustained saturation of calcium in the cytoplasm of the muscle, which causes a sustained contraction (tetanus). Stimulation rates less than 8 Hz tend to produce “jerky” types of movements, whereas those greater than 25 Hz simply are ignored by the muscle. Regardless of the frequency of stimuli sent to the muscle, most movements are executed “smoothly” because of the asynchronous firing of the various motor units comprising a muscle and the individual response properties of the individual sarcomeres within a motor unit.
Therefore muscle force, velocity of contraction, and final length of the muscle are not determined by higher centers of the CNS, but rather by the motor neuron pool in the ventral horn of the spinal cord. Muscles simply act like springs, and stimulation of the motor units controls the “stiffness” of the spring and the overall set point around a joint. In the simplest design, the CNS would increase activity to the desired motor unit, which would result in shortening of the contractile element (muscle), which in turn resets the tension and a new equilibrium is reached. Unfortunately, joints are not simple, unopposed hinges. The agonist muscle group is opposed by tendons and antagonist muscles, as well as by ligaments around the joint, the joint capsule, skin, clothing, and any pathology associated with a joint or the muscles that move it. To correctly position the joint, an accurate assessment of both the static (steady-state) and the dynamic (actively contracting) properties of the muscle is essential. This is the responsibility of the muscle receptors.
Receptors in the Motor System
Force and changes in muscle length are dependent on three properties: the initial length of the muscle, the velocity of length change, and the effect of external loads opposing movement. All of these are determined by specialized receptors in muscle tissue known as muscle spindles and Golgi tendon organs (GTOs) (Fig. 9-24, A). The muscle spindles are encapsulated organs ranging from 4 to 10 mm in length. The spindles are fusiform-shaped and contain 2 to 12 specialized muscle fibers known as intrafusal fibers. They are found within the extrafusal (skeletal) muscle fibers in parallel with (surrounded by) the skeletal muscle fibers. The spindles are surrounded by a connective tissue sheath (Hunt, 1990) and are innervated by both sensory and motor neurons.
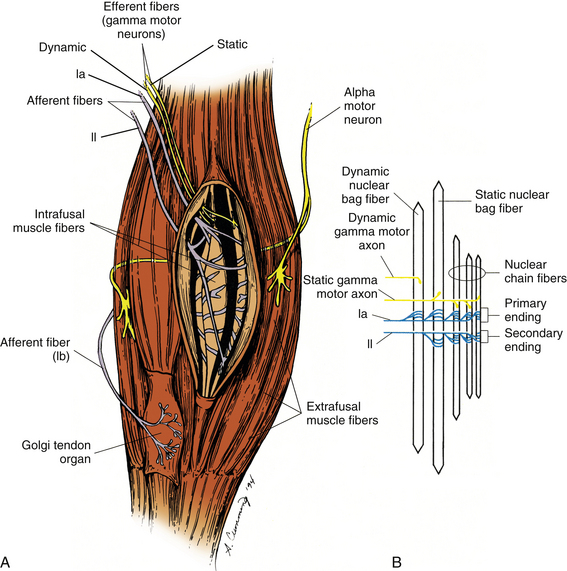
FIG. 9-24 Receptors in the motor system. A, Golgi tendon organs (GTOs) and muscle spindles are two types of specialized receptors associated with skeletal muscle. Extrafusal muscle fibers comprise most of the muscle and are innervated by alpha motor neurons. The GTOs are positioned at the junction between extrafusal muscle fibers and the tendon (in series) and are innervated by only one afferent fiber, the type Ib afferent. Located within the fleshy part of skeletal muscle (in parallel) are the muscle spindles. The spindles are composed of intrafusal fibers innervated by both afferent (types Ia and II) and efferent (gamma motor neuron) fibers. B, Each muscle spindle is composed of three types of intrafusal muscle fibers. The average muscle spindle contains one dynamic nuclear bag fiber, one static nuclear bag fiber, and three or more nuclear chain fibers. A group Ia (dynamic) fiber innervates every intrafusal fiber regardless of the number. A group II (static) afferent fiber innervates the static nuclear bag fiber and all nuclear chain fibers. Each intrafusal fiber also receives motor innervation to the distal (contractile) regions to control its overall length and sensitivity. The dynamic nuclear bag fiber is innervated by dynamic gamma motor axons, whereas the static nuclear bag fiber and all of the nuclear chain fibers are innervated by static gamma motor axons. (Modified from Gordon J & Ghez C. [1991]. Muscle receptors and spinal reflexes: the stretch reflex. In ER Kandel, JH Schwartz, & TM Jessell [Eds.]. Principles of neural science [3rd ed.]. New York: Appleton & Lange.)
Two separate classifications of sensory intrafusal muscle fibers have been identified. They are known as the nuclear chain and nuclear bag fibers (Fig. 9-24, B). The nuclear chain fibers are the smaller of the two, and their nuclei are arranged in a column or row. They are innervated by both type Ia and type II afferent fibers. The nuclear bag muscle fibers are thicker, and their nuclei are clumped together near the center of the fiber. These fibers can be subdivided into two types, based on their physiologic properties. The dynamic nuclear bag fiber is most sensitive to the rate of change of the intrafusal fiber, and is a rapidly adapting type of receptor. It is innervated by only a type Ia afferent fiber. The static nuclear bag fiber is most sensitive to the overall position of the fiber and is innervated by both type Ia and type II afferent fibers (Boyd, 1980).
The physiologic response patterns of the two afferent nerve fibers innervating the muscle spindle differ considerably, and send different types of information back to the spinal cord. The primary afferent fiber is the type Ia, or annulospiral, afferent. These fibers originate from both spindle types (nuclear bag and nuclear chain). This type of afferent fiber encodes information about the dynamic, or actively changing, state of the receptor. These are rapidly adapting types of receptors that are most sensitive to taps, vibration, and small changes in the overall length of the muscle (Fig. 9-25, A). They encode information about the speed (velocity) and position of the muscle during any type of movement (voluntary or involuntary) of the muscle. The secondary fiber is the type II, or flower-spray, afferent. These fibers originate only from the static bag and chain fibers. This type of afferent fiber encodes information about the steady-state position of the receptor. These are slowly adapting types of receptors that send continuous information about overall muscle position (length) back to the spinal cord (see Fig. 9-25, A). Both types of nerve fibers can indirectly alter their sensitivity independently via the gamma motor neurons. The dynamic nuclear bag fiber is innervated by a dynamic gamma motor neuron, whereas the static bag and static chain fibers are innervated by a static gamma motor neuron. This innervation pattern allows the different sensory components to have different levels of sensitivity, based on the predicted outcomes of the current motor task assigned to the extrafusal muscle fibers (Pearson & Gordon, 2000b). For activities that require speed or large forces, the dynamic gamma motor neuron has a relatively greater output compared with the static motor neuron. For tasks that require precise movements or postural adjustments only, the static gamma motor neuron has a relatively greater output than the dynamic motor neuron. This independent control of sensitivity to the static and dynamic sensory components allows the CNS to preset the level of sensitivity for specific types of tasks, and is termed the fusimotor set (Fig. 9-25, B). This mechanism of control also allows the greatest flexibility for a wide range of tasks. In addition to the gamma motor neurons, beta motor neurons (Bessou, Emonet-Demand, & LaPorte, 1965) and sympathetic efferents (Hubbard & Berkoff, 1993) also have been shown to innervate intrafusal fibers. The extent, nature, and clinical relevance of these connections to the muscle spindles are not yet well understood.
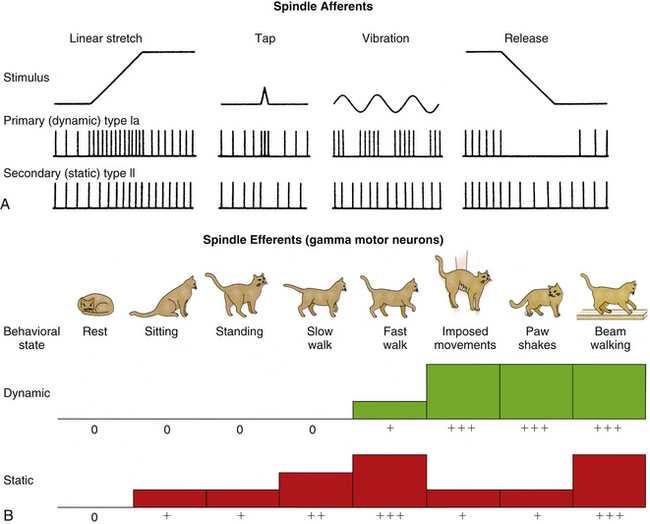
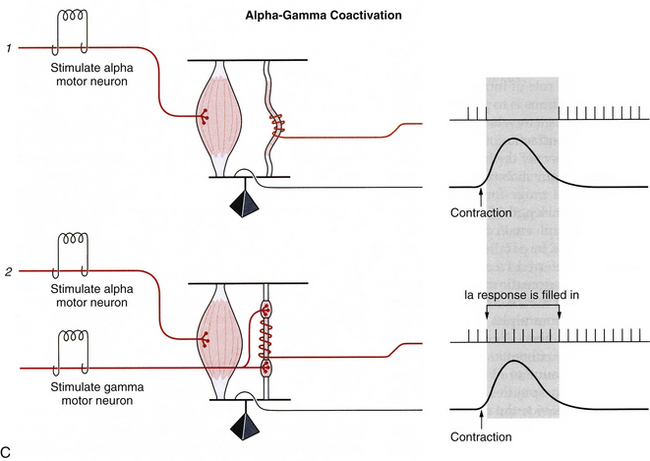
FIG. 9-25 Muscle spindle afferent and efferent functions. A, Comparison of responses from the primary and secondary muscle spindle afferents to various stimuli. The primary receptor (type Ia) is a rapidly adapting receptor that responds to transient stretch of muscle fibers with a burst of action potentials, and release (shortening) of muscle fibers with a dramatic decrease in firing. The secondary receptor (type II) is a slowly adapting receptor that reaches a steady-state firing of action potentials that reflects the overall length of the muscle, but does not respond well to transient changes in muscle length. B, Fusimotor set, or gamma motor neuron activity, can be independently set at different levels, based on different types of behaviors. During activities where muscle length changes occur slowly or predictably (standing, sitting, or walking), only the static gamma motor neurons are activated. During activities in which rapid or unpredictable responses occur (imposed movements, rapid coordinated movements), the dynamic gamma motor neurons are activated. C, During a muscle contraction (1) where only the alpha motor neuron is activated, the muscle shortens, thereby unloading the muscle spindle and resulting in a decrease in its output. Any further reduction in length would be undetectable by the spindle. To prevent this loss of signal (2), both alpha and gamma motor neurons fire during voluntary contractions. The spindle therefore shortens at the same rate as the extrafusal fibers, and the spindle is not unloaded during the contraction. This allows the spindle to “fill in” the missing action potentials and maintain sensitivity during voluntary contractions. This is termed “alpha-gamma coactivation.” (Modified from Gordon J & Ghez C. [1991]. Muscle receptors and spinal reflexes: the stretch reflex. In ER Kandel, JH Schwartz, & TM Jessell [Eds.]. Principles of neural science [3rd ed.]. New York: Appleton & Lange.)
GTOs are found at the junction of the muscle fibers and the tendon (in series with muscle), and not in the tendon proper (Jami, 1992) (see Fig. 9-24, A). The GTOs are encapsulated organs approximately 0.5 mm in length and 0.1 mm in diameter that interface with a discrete number of muscle fibers. The GTO is innervated by a single large afferent fiber (the Ib afferent), which is entwined in the weave of collagen fibers that compose the receptor (see Fig. 9-24, A). The entire GTO is surrounded by a thick lamellar sheath that is continuous with the perineural sheath of the Ib afferent. As weight or tension is placed on the muscle and tendon, the weave of collagen fibers within the GTO compresses the afferent fiber and allows depolarization of the receptor. This allows the GTO fiber to function as a monitor of muscle tension, regardless of whether the extrafusal fibers are being stretched, and provides the CNS with crucial information about muscle tension in the face of fatigue or changing workloads.
Comparison of the output of both the spindles and the GTOs during passive stretch and active contraction highlights the differences in the information these receptors convey to the spinal cord. Passive stretch applied to a muscle results in increased stretch of the intrafusal and extrafusal fibers, and therefore an increase in the output of the receptors (types Ia and II) found in the muscle spindles. In addition, passive stretch increases the tension on the muscle and tendon, and therefore causes an increase in the output of the GTO receptors (type Ib). In contrast, during active contraction of a muscle, the muscle begins to shorten and “unloads” the muscle spindle, resulting in a decrease in the amount of stretch being applied to the receptors (spindles) and a decrease in the output of those receptors. However, the GTO senses a greater tension on the tendon and muscle because of the combined forces of the load and the force of contraction, which results in a further increase in the output of the GTO receptor (type Ib). Because the GTO is in series with the muscle (between the muscle and its tendon), it is incapable of sensing muscle length, but is highly efficient at sensing changes in muscle tension. During passive stretch, much of the force is absorbed by the extrafusal fibers, and the GTO senses only a minimal increase in muscle tension. However, during active contraction both the load and the contractile force of contraction are sensed by the GTO, and combine to dramatically increase the output of this receptor. The intrafusal fibers of the muscle spindle, being parallel with the extrafusal fibers, can accurately detect changes in muscle length, but not tension. During passive stretch, both the intrafusal and extrafusal fibers are stretched by the applied load, and the spindles increase their afferent input into the CNS in direct relation to the amount of force needed to stretch the muscle. However, during active contraction the muscle shortens, and the forces applied to the spindle afferents decrease, resulting in a decreased afferent input into the CNS.
Without a resetting of the sensitivity of the muscle spindle, shortening of the extrafusal fibers could result in the muscle spindle becoming slack and being unable to sense further changes in the length of the muscle, resulting in a loss of position sense (proprioception) (see Fig. 9-25, C). To prevent this loss of proprioceptive information from occurring, the gamma motor neuron system (fusimotor system) functions to keep tension on the muscle spindles during active (voluntary) contraction of muscles. During active contraction of muscle, both the alpha and the gamma motor neurons fire simultaneously. The alpha motor neuron functions to contract the extrafusal fibers, whereas the gamma motor neurons function to contract the intrafusal (muscle spindle) fibers at the same rate or velocity as the extrafusal fibers. This serves to “fill in” the signal that normally would be lost from the muscle spindle and allows it to continue to sense changes in the muscle length that might occur (see Fig. 9-25, C). This process of stimulating both the alpha and the gamma motor neurons is known as alpha-gamma coactivation. In a sense, the gamma motor neuron forces the spindle to “keep pace” with the contraction of the muscle (and the alpha motor neuron), and prevents the loss of proprioception from the muscle receptor.
Based on prior experiences, the gamma motor system controls both the static and the dynamic components of the fusimotor system. These components can be controlled independently of one another, depending on the type of motor function activated (Prochazka et al., 1988). If the movement is a slow, deliberate, or postural type of movement, the output of the static gamma motor neurons is increased by the CNS before and during the movement to ensure good overall position. Because there is little need to increase the dynamic sensitivity during these slow or postural types of movements, the output of the dynamic gamma motor neuron generally is kept low by the CNS. In contrast, if the desired movement requires either great force or velocity, the dynamic gamma motor neuron output is increased by the CNS, and the static component is kept low. For movements that require both postural adjustments and great force or velocity, both components can be increased by the CNS. This variability in the output to the gamma motor neurons, which is based on the expected movement, is called the fusimotor set (see Fig. 9-25, B). The functional role of the gamma efferents is to preserve muscle spindle sensitivity over the wide range of muscle lengths that occur during normal voluntary contractions. In general, the sensitivity is kept quite low to prevent errors in signaling to the muscles. If the sensitivity is too high, corrections occur as the muscle overshoots the expected endpoint. If too low, the muscle “lags behind” the pace of the expected movement. Therefore the sensitivity of the muscle spindle afferents plays a key role in regulating the responsiveness of the muscles based on the planned voluntary movements (Pearson & Gordon, 2000b). These proprioceptive afferent fibers, along with all of the other sensory afferents, help modulate most of the motor actions of the muscles.
Interneurons and Spinal Reflexes
Spinal interneurons, the small excitatory and inhibitory connections within the spinal cord, are the building blocks of all spinal reflexes. The spinal reflexes act to coordinate most actions of groups of muscles, typically across joints, and most spinal reflexes are polysynaptic, which allows modifications of these connections. Most of the spinal reflexes are relatively simple neural circuits. These circuits also are used by descending influences (pathways) to generate and modify more complex motor actions. The actions of the interneurons are restricted to the spinal cord. The types of connections they control are numerous and include divergence of sensory afferents in the dorsal horn, convergence of multiple inputs onto the motor neurons and other interneurons, direct gating of neural circuits (such as the pain pathways; see Chapter 11), indirect gating through presynaptic facilitation and inhibition, activation of reverberating (repeating) circuits, and control of rhythmic pattern generators responsible for complicated motor tasks such as walking (see Figs. 9-26 and 9-27). Most spinal interneurons are mutually inhibitory, meaning that they inhibit one another so that no one circuit becomes dominant in the spinal cord (Floeter, 1999). The inhibitory interneurons coordinate muscle actions around a joint so that agonists, synergists, and antagonists act together as a myotatic unit. The myotatic unit regulates the stiffness of the entire joint (Pearson & Gordon, 2000b). The myotatic or muscle stretch reflex is a useful clinical and physiologic tool to understand how the spinal reflexes are controlled by interneurons. The Ia inhibitory interneuron is used to control this reflex.
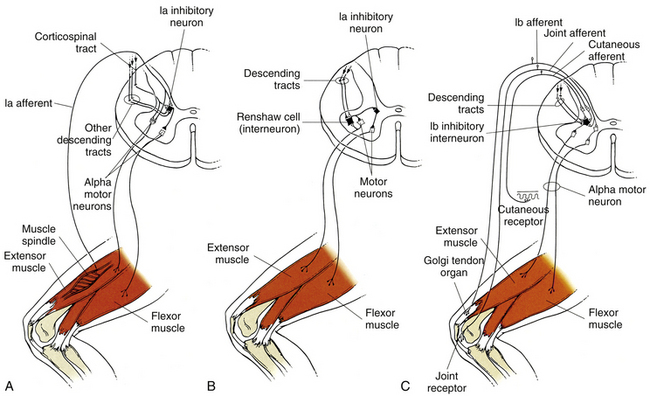
FIG. 9-26 Reflex circuitry in the spinal cord. Inhibitory interneurons that control voluntary and reflexive movements in the ventral horn of the spinal cord. A, The Ia afferent fiber from the spindle enters the dorsal horn and makes direct synaptic connection with an alpha motor neuron that directly innervates that muscle. The Ia afferent also makes a direct synaptic connection with a Ia inhibitory interneuron that inhibits the alpha motor neuron innervating the antagonist. This inhibition allows unopposed movement of the agonist and is called reciprocal inhibition. B, The Renshaw cell is another type of inhibitory interneuron that is stimulated directly from higher cortical areas and/or by a collateral branch of an alpha motor neuron. When stimulated, the Renshaw cell inhibits the agonist (and synergistic) motor neuron pool, thus shortening or reducing the motor neuron’s response. In addition, the Renshaw cell simultaneously inhibits the Ia inhibitory interneuron of the antagonist group, thus disinhibiting (releasing) the antagonist muscle group and initiating cocontraction. Cocontraction serves to stabilize the joint during strenuous activity. C, The Ib inhibitory interneuron is activated polysynaptically by the Ib afferent (GTO), joint receptors, cutaneous receptors, and by descending pathways. The Ib inhibitory interneuron serves to inhibit the motor neuron(s) to the homonymous muscle. It also allows disinhibition of the antagonistic motor neuron pool. The inhibition of the agonist motor neuron pool and indirect stimulation of the antagonist pool allow the affected joint to be released from the tension affecting it. (∆, Excitatory; ▲, inhibitory.)
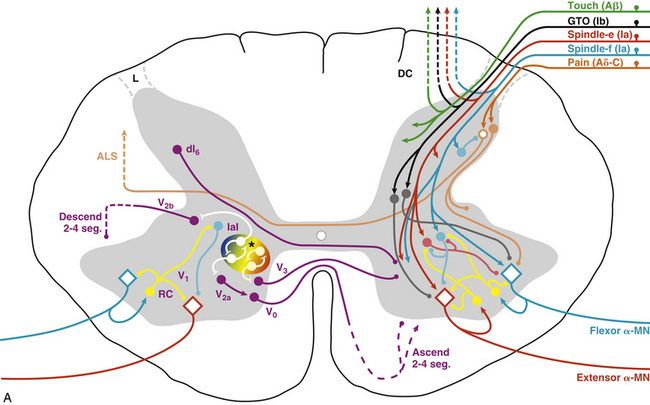
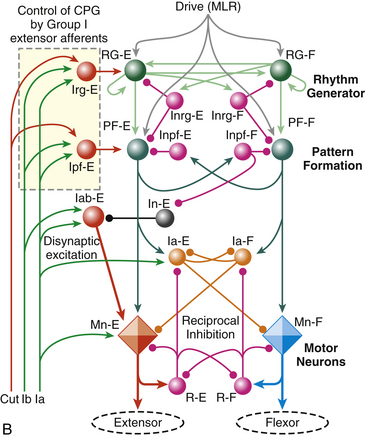
FIG. 9-27 Spinal interneurons, control pathways, and a model of the central pattern generator (CPG) for locomotion. A, Afferent input and the connections related to the ventral horn are shown on the right, and include general sensory input (Aβ fibers, green), GTOs (Ib, black), dynamic spindle afferents (Ia, extensor [red], flexor [blue]), and pain receptors (Aδ and C fibers, orange). Most of these afferents have polysynaptic input to the ventral horn. The Ia fibers have monosynaptic input to the agonist motor neuron and the Ia inhibitory interneuron to the antagonist motor neuron. Intrinsic interneurons related to motor output are shown on the left, and include V0 interneurons projecting contralaterally to influence left-right activity; V1 interneurons (including Renshaw and IaI) projecting ipsilaterally to influence motor neurons directly; V2 interneurons projecting ipsilaterally to influence CPG speed, amplitude, and rhythm; and V3 interneurons projecting both ipsi- and contralaterally to modulate the regularity of the rhythm from the CPG. Most interneurons in the cord are mutually inhibitory. See text for a more detailed explanation. B, Schematic of CPG model by Rybak and McCrea (2006). This three-layer model consists of a rhythm generating layer of extensor (RG-E) and flexor (RG-F) interneurons. Both populations have recurrent excitatory connections, and receive mutually inhibitory input (Inrg cells). The output of the rhythm generator layer projects to a pattern formation layer (PF-E and PF-F), which acts through mutually inhibitory connections (Inpf cells) to sculpt the pattern, which is then output to the alpha motor neurons (Mn-E and Mn-F). The final output of the motor neurons is modulated by Ia inhibitory interneurons (Ia-E and Ia-F) and Renshaw cells (R-E and R-F). Sensory afferents are incorporated into the CPG polysynaptically via interneurons primarily at the rhythm generator (Irg cells) and pattern formation (Ipf cells) areas, and include general cutaneous afferents (type II, Cut), GTOs (Ib), and dynamic spindle afferents (Ia), which also have direct synapses onto the motor neurons and Ia inhibitory interneurons. ALS, Anterolateral system; DC, dorsal column; IaI, Ia inhibitory interneuron; L, Lissauer’s tract; MLR, mesencephalic locomotor region; RC, Renshaw cell; spheres, interneurons; diamonds, motor neurons; →, excitatory;, inhibitory; ∗, CPG kernel. (Reprinted with permission from McCrea & Rybak, 2008.)
The central connections involved in a stretch reflex are relatively simple and well-known. A tendon is tapped, which stretches the intrafusal and extrafusal muscle fibers. The dynamic component of the spindle activates the Ia afferent fibers, which make a direct connection onto the alpha motor neurons that innervate that same muscle. The alpha motor neuron fires, causing that same muscle to contract to “reset” the muscle back to its original position. The spindle afferent often has monosynaptic connections with alpha motor neurons innervating synergistic muscles as well. In addition to the direct connection to the alpha motor neuron (agonist) and synergistic muscles, the spindle afferent also makes direct connections with inhibitory interneurons (Ia inhibitory interneurons) that inhibit the alpha motor neurons of the antagonistic or opposing muscle group (Fig. 9-26, A). This allows the agonist group to contract more freely (with less resistance). Stimulation of the agonist while simultaneously inhibiting the antagonist via the Ia inhibitory interneuron is called reciprocal innervation or reciprocal inhibition. This type of contraction is used also by the CNS for voluntary movements whenever it can accurately gauge the load opposing the movement, such as during isotonic contractions (change in muscle position without change in force or tension) (Fig. 9-28, A). The Ia inhibitory interneuron also receives descending information from the cortex for voluntary movements (corticospinal tract), the brain stem (vestibulospinal and rubrospinal tracts), and cerebellar efferents (i.e., fibers that influence descending neurons), as well as information from other spinal interneurons (Renshaw, Ia inhibitory interneurons) (Floeter, 1999) and extensive connections within the spinal central pattern generators (CPGs) (McCrea & Rybak, 2008).
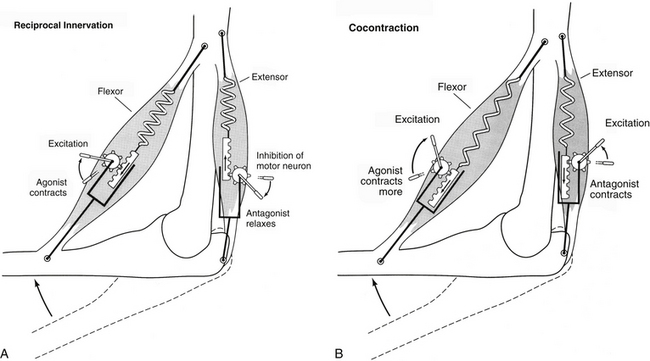
FIG. 9-28 Mechanisms of muscle contraction. Movement around a joint employs one of two strategies: reciprocal innervation or cocontraction. A, During reciprocal innervation, the motor neurons to the agonist group (flexor) are stimulated, while the motor neurons to the antagonist group (extensor) are inhibited. This results in contraction of the flexor and relaxation of the extensor, and the joint flexes. This form of movement is extremely efficient as long as the loads placed on the muscle groups are known precisely. B, During cocontraction, the motor neurons to both agonist and antagonist groups are stimulated simultaneously. Whichever group receives the greater amount of stimulation (in this case the flexor group) is the direction in which the joint will move. The overall effect is an increase in joint stiffness and stability, and allows the nervous system to control both the angle and the overall stiffness of the joint independently. (Modified from Gordon J & Ghez C. [1991]. Muscle receptors and spinal reflexes: the stretch reflex. In ER Kandel, JH Schwartz, & TM Jessell [Eds.]. Principles of neural science [3rd ed.]. New York: Appleton & Lange.)
The Renshaw cell is a second type of inhibitory interneuron found prominently in the spinal cord. The Renshaw cell is excited primarily by collateral branches from alpha motor neurons in the ventral horn. They function to inhibit the alpha motor neuron that activated the Renshaw cell, as well as other neighboring motor neurons in the motor pool (Fig. 9-26, B; Fig. 9-27). This type of inhibition is termed recurrent or feedback inhibition, and tends to decrease or limit the output of the myotatic unit. In addition, the Renshaw cells also inhibit the Ia inhibitory interneuron, which normally inhibits the antagonistic muscle group. By decreasing or removing the inhibition (also termed disinhibition) of the opposing muscle group, the Renshaw cell allows all the muscles around a joint to be activated simultaneously. This concurrent activation of both agonists and antagonists is termed cocontraction, and acts to stabilize the firing rate and counteract large transient changes in joint position during movement (Fig. 9-28, B). The Renshaw cells also receive significant descending input (Davidoff & Hackman, 1991; Floeter, 1999), which modulates the excitability of the Renshaw cells and adjusts the myotatic unit as a whole around a joint. The strength of the recurrent inhibition is quite variable, and is constantly being modulated by the CPG and other interneurons in the spinal cord (Floeter, 1999). These connections are important in ongoing postural adjustments, minor changes in muscle length (Brooks, 1986), and cases in which the precise value of the load affecting the motor pool is unknown or unpredictable.
The Ib inhibitory interneuron is a third type of inhibitory interneuron affecting the motor neurons in the ventral horn. The Ib inhibitory interneurons receive input from GTOs, joint receptors, pain receptors, and general somatic afferents from around the joint affected by the motor neuron pool. All of these afferent fibers converge either directly (monosynaptic) or indirectly (polysynaptic) onto the Ib inhibitory interneuron. The Ib interneuron has a powerful inhibitory connection to the agonist and synergistic muscles of the pool (Fig. 9-26, C; Fig. 9-27). This connection often is called the inverse myotatic reflex, although it is not clinically testable because of the polysynaptic nature of the connections involved. A similar function of this reflex can be observed in the crossed-cord reflex called Phillipson’s reflex, where flexion of one limb causes extrusion of the contralateral limb. In addition to the numerous somatic connections, the Ib inhibitory interneuron also receives significant connections from higher centers (Jami, 1992; Floeters, 1999), the CPG, and other local interneurons. The significant number of convergent connections onto the Ib inhibitory interneuron represents a spinal mechanism that acts to mediate control of movements when integration of different sensory modalities is important, as in guiding limb and hand movements during exploration, allowing precise adjustments of muscle tension once an object is encountered, and preventing damage to the joint during strenuous activity by limiting or terminating agonist activity during maximum contraction.
Using genetic techniques, Jessell and colleagues identified six clusters of distinct interneurons that contribute to the formation of spinal circuitry directly related to CPG function and motoneuronal output (Jessell & Sanes, 2000; Whelan, 2010) (Fig. 9-27, A). The V0D interneurons are GABAergic/glycinergic cells that project across the ventral commissure and ascend two to four levels before presumably synapsing onto Renshaw cells, Ia inhibitory interneurons, and alpha and gamma motoneurons (Lanuza et al., 2004), where they are thought to contribute to the control of left-right alternating activity. The V0V subgroup are glutamatergic (Goulding, 2009) and do not appear to be directly related to locomotor coordination (Lanuza et al., 2004). The V1 class of interneurons are restricted to lamina VII ipsilaterally and are predominantly inhibitory onto the CPG (Sapir et al., 2004; Alvarez et al., 2005), where they appear to control the timing of the rhythm (Goulding, 2009). Renshaw cells and Ia inhibitory interneurons form part of the V1 group. The V2 group of interneurons remain ipsilateral and descend several levels before synapsing. They are subdivided into excitatory (V2a) and inhibitory (V2b) classes (Peng et al., 2007; Al-Mosawie et al., 2007; Lundfald et al., 2007). The V2a interneurons have diverse effects on CPG output, including left-right coupling and speed, amplitude, and regularity of rhythm (Crone et al., 2008, 2009; Whelan, 2010). The V2b interneurons may contribute to the control of reciprocal inhibition between flexors and extensors (Goulding, 2009). The V3 group of interneurons are a mixed class that project both ipsi- and contralaterally (Zhang et al., 2008) and appear to contribute primarily to regularity of rhythm, but not to left-right coordination (Zhang et al., 2008; Whelan, 2010). Finally, a dorsal group of inhibitory interneurons, classified as the dI6 population, have also been identified (Muller et al., 2002). This dorsal group project contralaterally and are thought to control the pattern of locomotion (Goulding, 2009). The central pattern generator (CPG) for many movements is regulated by these and countless other interneurons, including polysynaptic connections from most sensory afferents.
Preprogrammed Movements
Groups of interneurons function together to build most of the preprogrammed or stereotyped movements used in everyday activities. One such circuit is the flexion withdrawal reflex. This reflex is triggered by noxious (damaging) stimuli, and consists of contralateral extension followed by ipsilateral flexion of muscle groups in an attempt to relieve or reduce the noxious stimulus. It involves coordinated contractions of multiple joints using reciprocal innervation and the opposite response in the opposing limb (crossed extension reflex), which provides postural support (typically extension) during withdrawal (typically flexion) of the affected limb. This seemingly simple function actually requires the use of countless excitatory and inhibitory interneurons in the spinal cord to control the timing and regulate the magnitude of the reflexive response. The amplitude and speed of the reflex are determined by the amplitude and location of the sensory input. The flexion withdrawal reflex also is involved in coordinating some voluntary movements. This is accomplished by descending control. The descending control sends collateral connections to spinal interneurons known as flexor reflex afferents.
Highly stereotyped movements such as those just described involve many interneurons, and, like most reflexes, are dependent on stimulus intensity (Pearson & Gordon, 2000b). Cutaneous stimuli elicit complex protective and postural functions. Contraction of specific muscle groups in response to a stimulus at a precise location on the body is termed a “local sign.” Most cutaneous stimuli have a subthreshold effect on motor neuron excitability, and the effects are spatially specific and reciprocal. The type of response elicited depends on the type of stimulus perceived. For example, a stimulus applied to the base of the foot can trigger different circuits and responses based on the strength and pattern of the afferent input into the spinal cord. Stroking the bottom of the foot with a blunt object simulates “slipping” of the foot and elicits plantar flexion, as the toes attempt to “grip” the ground. Light pressure applied to the entire plantar surface of the foot elicits the extensor thrust response (postural adjustment), which allows us to stand. Painful stimuli to the bottom of the foot activate the flexion withdrawal response, which acts to remove the foot from the painful stimulus. Most primary sensory afferent connections and all central connections involve polysynaptic pathways, and modification occurs at local spinal cord connections only. To prevent the various sensory afferents and descending modulatory connections from simultaneously activating multiple or conflicting responses, most pathways are mutually inhibitory. Only one pathway is active at a time, and all other pathways are inhibited or prevented from responding when one circuit is active. In this fashion, the CNS can control most movements and reflexes via differential activation of selected, prewired circuits at the level of the spinal cord.
Muscle Tone and Postural Control Mechanisms
Muscle tone is the resistance of a muscle to active or passive stretch, or the overall stiffness of the muscle. Skeletal muscle has an intrinsic resistance to stretch resulting from the elastic properties of the tendons, connective tissue, and the muscle tissue itself. Therefore muscle behaves much like a spring. Reflexes also function to counteract the active or passive stretch of the muscle tissue via the monosynaptic connections from the spindles to the alpha motor neurons, and work with the elastic components of muscle to resist stretch. Normal muscle tone serves three important functions. First, it assists in maintaining posture, or the resistance of the muscle to the forces of gravity. Muscle tone helps to ensure that the center of gravity is aligned over the base of support. Second, because of a muscle’s inherent ability to act as a spring, it can store energy and release it at a later time. This is particularly important for movements such as walking. When a leg pushes off, some of the stored energy is released and helps propel the leg and body forward, thereby assisting the muscles that normally pull the leg forward. Lastly, because muscles act like springs, they help dampen jerky movements and allow for more “fluidlike” movements of most muscles (Ghez, 1991).
Control of muscle tone is achieved largely through feedback mechanisms. Negative feedback helps to counteract deviations from the desired muscle position. Overall muscle length is chosen by the CNS and regulated by descending connections to the motor neuron pool in the spinal cord. Deviations in the intended position are detected by the muscle spindles and relayed back to the motor neuron pool. Increases in the length of the muscle result in an increased output from the muscle spindle and increased stimulation of the motor neuron pool, which result in an increase in the force of contraction of that muscle to counteract the increase in length. Decreases in muscle length have the opposite effect. Therefore the stretch reflex functions continuously to keep the muscle position as close as possible to the length chosen by the CNS. Two crucial elements of the feedback system are the gain of the system and the loop delay (Gordon & Ghez, 1991). Gain of the system is largely determined by the fusimotor set discussed earlier, and relates to the overall sensitivity of the muscle spindles. The higher the gain (greater sensitivity of spindles), the larger is the reflexive force of contraction by a muscle to counteract a given change in length. Gain can be adjusted by the overall level of fusimotor (intrafusal) activity, by presynaptic modulation of excitatory and inhibitory interneurons (activated by various forms of sensory input), and by direct connections to the motor neuron pool by descending input. The loop delay is the time between the detection of a disturbance or error and the actual compensatory response by the muscle to counteract that error. The loop delay is a sum of the conduction times from the sensory afferents, the motor neurons leading back to the muscle, and the mechanical response from the muscle. The loop delay usually is insignificant when movements are slow. During rapid or extremely precise movements, the loop delay can play a significant role in accurately regulating movement around a joint. Muscle tone also plays a substantial role in most postural control mechanisms.
Postural Control Mechanisms
Posture represents the overall position of the body and limbs relative to one another and their orientation in space. Adjustments to posture should be integrated with voluntary movements to keep the head and body aligned and to “prepare” the body for specific types of voluntary movements. There are three fundamental functions of adjustments to body posture. The first is to support the head and body against gravity and other external forces. The second is to maintain center of body mass aligned and balanced over the base of support. The third is to stabilize the supporting parts of the body while others are being moved. These three functions are achieved by two principal mechanisms: anticipatory feed-forward mechanisms and compensatory feedback mechanisms. Anticipatory feed-forward mechanisms help to predict disturbances by activating preprogrammed (prewired) responses. These responses are modified by experience and their effectiveness improves with practice. These postural adjustments generally occur before voluntary movements are activated. Compensatory feedback mechanisms occur after loss of balance (increased body sway). These responses are automatic and extremely rapid (reflexive in nature), are scalable (size of response depends on size of stimulus), and use stereotyped spatiotemporal organization (muscles closest to loss of balance are activated first) to achieve stable posture. These responses also are continuously refined by experience. Sensory input from visceral, cutaneous, and proprioceptive receptors triggers anticipatory or compensatory responses that typically are automatic, and maintain posture without one’s awareness. Postural control is monitored by three primary neural systems: the proprioceptive system (spindles, GTOs, joint receptors, and general sensory afferents); the vestibular system (both static and dynamic components located in the inner ear); and the visual system. Visual, vestibular, or proprioceptive information alone is not sufficient to trigger an adjustment to posture; there must be a combination of these stimuli to elicit an adjustment (Nashner, 1976). Responses that stabilize posture become facilitated (enhanced) with repeated trials, whereas responses that destabilize posture become adapted (weakened). Responses to sway are shaped by experience and continually adjusted to maintain balance. Most postural adjustments that occur to maintain equilibrium and stabilize the body are activated before the voluntary movements that may destabilize the body are initiated. Postural set is the preparatory state when a specific postural response is selected in advance of a stimulus so the adjustment is executed automatically either before or along with a voluntary movement (Ghez, 1991). Descending influences generated by postural set generally act via spinal interneurons to gate (control) and modulate overall tone and posture at the level of the spinal cord.
Locomotion and Voluntary Movements
Locomotion is a rhythmic behavior that functions to move an animal through space, and is relatively automatic. Most locomotor movements, such as walking or the scratch reflex in dogs, are controlled by groups of spinal interneurons, and these responses can outlast the initiating stimulus (Sherrington, 1947). The spinal interneurons controlling locomotion form complex circuits consisting of excitatory and inhibitory interneurons, and alpha and gamma motor neurons, which can be modulated or modified by higher CNS centers by means of descending pathways. These complicated circuits form the basis of the “rhythmic pattern generators” found in the spinal cord. Spinal animals (experimental animals having a transection of the spinal cord above C2) are actually capable of “walking” (rhythmic stepping), because of the activation of the still intact rhythmic pattern generators found within the spinal cord (Brown, 1911; Grillner & Wallen, 1985). The overall pattern consists of an alternation between contractions of the flexor and extensor muscle groups. The alternating pattern is controlled by a unique combination of excitatory and inhibitory interneurons. These interneurons act synergistically to ensure that only one group of motor neurons (flexors or extensors) is activated at a time in a given limb, and also ensure that the opposing muscle groups are activated in the opposite limb. Walking consists of two distinct phases: the swing phase (foot off of the ground and flexing forward) and the stance phase (foot planted, extended, and bearing weight). The movements controlled by these circuits are timed differentially, and a spatially distributed synergy of muscular contractions exists. These elegantly timed series of muscular contractions are controlled almost exclusively by the interneurons and motor neurons that form the rhythmic pattern generators (Engberg & Lundberg, 1969; Calancie et al., 1994; Belanger et al., 1996; Pearson & Gordon, 2000a). In fact, there are separate pattern generators for each limb (Grillner & Wallen, 1985). The separate pattern generators are interconnected by other local interneurons, and both pattern generators send general information to the postural control systems of the spinal cord via long and short propriospinal interneurons.
Most automatic or patterned movements are also controlled by the spinal cord via central pattern generators. Although the cortex may be responsible for initiating and terminating these rhythmic or patterned movements, the ultimate control mechanisms (circuitry) for these movements take place in the spinal cord. Two theories of CPG function currently exist. The first is the unit oscillator hypothesis (Grillner, 1981), also known as a single-level CPG (McCrea & Rybak, 2008). Although this theory adequately explains basic rhythmic oscillations, it cannot account for non-resetting deletions (spontaneous loss of motor output which does not alter the overall rhythm) or the effects of sensory stimulation that alter phase duration but not the cycle period (McCrea & Rybak, 2008). A two-level CPG (the second theory) can accomplish this, and is illustrated in Figure 9-27, B (Rybak et al., 2006; McCrea & Rybak, 2008). In this model, the CPG contains a rhythm generator with a homogeneous population of both flexor (RG-F) and extensor (RG-E) excitatory interneurons with mutual excitatory interconnections. Reciprocal inhibition between the RG half-centers is mediated by inhibitory interneurons (Inrg-F and Inrg-E). The pattern formation network is similarly organized (PF-F, PF-E, Inrf-F, Inrf-E), but has a lower capacity for rhythmogenesis due to strong inhibitory input from the RG inhibitory interneurons (Inrg-F and Inrg-E, respectively) (Rybak et al., 2006). The pattern formation centers project directly to the motoneurons (alpha and gamma) responsible for flexion (Mn-F) and extension (Mn-E), and the Ia inhibitory interneurons (Ia-F and Ia-E) that provide reciprocal inhibition to the antagonist motoneurons. Output from the motoneurons also stimulates the Renshaw cells (R-F and R-E), which are mutually inhibitory and serve to inhibit the antagonist Ia inhibitory interneurons. An important feature of this model is the ability to differentially regulate locomotor speed at the RG level, and motoneuron activity (amplitude) at the PF level (McCrea & Rybak, 2008). This model can also accommodate most sensory input, including direct activation of motoneurons by Ia spindle afferents, and the polysynaptic input of GTOs (Ib), general somatic afferents, and pain fibers (Cut). In the case of a rhythmic movement, such as walking, the drive (stimulus) for the CPG can originate in the mesencephalic locomotor region (MLR). In addition, during the movements, sensory feedback, particularly from muscle spindles (type Ia directly, type II indirectly), will be influenced by increased gain from changes in fusimotor set (Severin, Orlovsky, & Shik, 1967), thereby resulting in increased responsiveness in the CPG. Thus a relatively simple control signal from the brain stem (mesencephalic locomotor region), modulated in intensity only, can activate locomotion and facilitate changes in speed. However, the pattern itself is controlled by circuits found in the spinal cord. For other movements, the corticospinal tract is the most likely source of input.
Normal locomotion requires multiple levels of neural control to support the body against gravity, maintain normal balance during ongoing movements, and propel it forward. These movements are coordinated by spinal circuits that are influenced by both sensory afferent information and descending control systems, such as the corticospinal, reticulospinal, vestibulospinal, and rubrospinal pathways.
Voluntary Movements
Although a spinal animal can reflexively “walk” (produce rhythmic, alternating movements of the limbs) if supported on a treadmill, it is not capable of balance or goal-directed voluntary movements. These purposeful movements require that an intact motor cortex, basal ganglia, cerebellum, and vestibular system be connected to the spinal cord to initiate the purposeful movements and modulate them. Most daily movements are voluntary in nature, and require that all levels of the CNS, from cerebral cortex to spinal cord, be intact.
There are several key differences between reflexive and voluntary movements. First, with voluntary movements, motor systems can use different strategies in different situations to achieve the same result, a concept termed motor equivalence (Krakauer & Ghez, 2000). Second, the effectiveness of voluntary movements improves with experience and learning; precision increases while variability decreases. Finally, external stimuli need not be present to initiate a voluntary movement. The voluntary system can dissociate the content of the movement (what and how) from the initiation of the movement (when).
Several events must take place before a voluntary movement can be performed. First, there must be identification of an action that is to be performed. Second, a plan of action must be formulated in the cerebral cortex. After these two initial steps have been performed, there must finally be a “GO” signal that results in execution of the planned response. These three steps (identification, planning, and execution) are controlled by distinct regions of the cerebral cortex: the posterior parietal cortex, premotor regions of the frontal cortex (supplementary motor cortex and premotor cortex), and primary motor cortex, respectively. Each of these cortical areas, which controls a different aspect of the overall voluntary movement, is discussed next. In addition, other subcortical areas also can influence motor activity. These include the thalamus, cerebellum, and basal ganglia, but their scope of influence is not discussed here.
The posterior parietal cortex is essentially a sensory integration area that helps develop the plan of action and may contribute to the identification of the task to be performed. This region is critical for integrating visual information on targeted movements and helps to focus attention on salient stimuli. The posterior parietal cortex has a strong motivational component and strong hemispheric specialization. It also receives input from the primary sensory cortex, sensory association areas, and the vestibular system and limbic (motivational) areas of the brain. It is also modulated by states of attention. This area provides the motor regions of the cortex with information about overall body position and sensory inputs and also reflects the subject’s intentions for a specific action.
The premotor areas of the frontal cortex receive strong projections from the posterior parietal cortex, and prepare the motor system for movement. Under optimal conditions, a person can respond (reflexively) to a stimulus in 120 to 150 ms, with proprioceptive responses being the quickest. However, voluntary tasks can take hundreds of milliseconds to initiate. The time it takes to plan or initiate a voluntary response increases linearly with the complexity of the task or number of choices involved. Premotor areas control these more complex actions and also control movements that require a specific sequence of activation for execution (Krakauer & Ghez, 2000). The supplementary motor area of the premotor cortex is important for programming sequences (e.g., orienting the body before executing voluntary movements) and coordinating bilateral movements (such as clapping). The supplementary motor area is also important for mentally rehearsing specific movements and tasks before execution, controlling proximal limb and axial muscles and the initial orientation of body and limbs to a target (coordinating posture and voluntary movement), and responding to instructions for execution of specific actions. Understanding of instructions or cues is normally independent of the time frame for actual execution or initiation of the planned response. Neurons from premotor areas do not control the fine detail of actions to be executed, but rather they are concerned primarily with the global aspects (overall sequence of action) of a motor task and the initiation of the execution command to the primary motor cortex.
Individual neurons in the primary motor cortex code for the force exerted by the muscle, not the direction of movement (Krakauer & Ghez, 2000). Distal muscles (fine control) are represented at more than one site in the primary motor cortex, but this is the only output by the cerebral cortex to these muscles. Small muscles in the hands and the muscles of facial expression do not have concomitant neurons in the other motor areas of the cerebral cortex. The axons of most neurons in the primary motor cortex diverge to influence several motor neuron pools in the spinal cord, with the greatest number of connections being associated with proximal (postural or axial) muscles. In addition, most neurons in the primary motor cortex typically are activated before actual muscle contraction, and they may contribute to the initiation of movement. Direction is encoded by stimulating populations of neurons in the primary motor cortex, rather than by stimulating a single neuron (Krakauer & Ghez, 2000). Individual neurons of this cortical region have a preferred direction or orientation (greatest output), and most respond over a wide range of directions, but with reduced output. Therefore groups of neurons determine the final direction of movement (population vector), whereas individual neurons code for the force or velocity exerted by a particular motor neuron pool in the spinal cord. Output of the primary motor cortex is variable, depending on the task and level of motivation. Input about limb position and speed of movement is updated continuously by means of direct connections from the primary sensory cortex, and indirectly from sensory afferents of the proprioceptive system by way of the thalamus, cerebellum, and basal ganglia. In this way, the primary motor cortex can adjust the output to motor neuron pools in the spinal cord to compensate for changes or disturbances in the planned movement, or to refine the voluntary movement.
By analyzing a relatively simple movement, such as throwing a ball, one can begin to understand how all of the afferent and efferent information being processed by the spinal cord and higher-order systems (e.g., brain stem nuclei, cerebellum, basal ganglia, and cerebral cortex) works together to complete this task (Fig. 9-29). First, the “idea” of throwing the ball has to be formulated somewhere in the cerebral cortex. This idea is relayed, along with all current proprioceptive information (originating primarily from the DC-ML pathways), to the premotor regions of the cortex, where a plan is developed, based on the body’s current position in space. The basal ganglia also aid the premotor regions in developing the appropriate timing and initiation of movements, because of its extensive input from virtually all areas of the cerebral cortex, including sensory, motor, and motivational (limbic) connections. The premotor and supplementary motor cortices then relay this plan for coordinated movements of the legs, arms, trunk, head, and neck to the primary motor cortex. The primary motor cortex encodes force, velocity, and direction to the appropriate motor neurons via the corticospinal pathways to the ventral horn of the appropriate spinal cord segments. In addition, a copy of the planned movements is relayed to the cerebellum by the descending corticospinal tract (termed corollary discharge) (see Fig. 9-29). In the spinal cord, pools of alpha and gamma motor neurons are stimulated by the descending corticospinal tract (primarily lateral corticospinal tract) and cause the appropriate muscles to begin to contract. In addition, the reticulospinal and vestibulospinal pathways relay information for postural adjustments to the spinal cord to compensate for any deviations associated with the ongoing voluntary movement. Since this is a voluntary movement presumably done many times before, the postural adjustments normally occur before the initiation of the voluntary movement to compensate for any possible loss of balance directly attributed by the voluntary movement. Compensation would have been assessed during previous movements, stored in the cerebellum, and recalled automatically by the premotor areas of the cortex from the cerebellum. Continuous assessment of these voluntary (throwing the ball) and involuntary (postural adjustments) movements is monitored by the muscle spindles, GTOs, and joint and skin receptors, and supplemented by visual information. The afferent fibers associated with these receptors, in addition to sending information back to the sensory and motor areas of the cortex via ascending tracts, also can modify the interneurons at the level of the spinal cord (Ia inhibitory interneurons, Renshaw cells, Ib inhibitory interneurons, and CPG interneurons) to ensure that the intended movement proceeds as planned. The primary afferent fibers also relay information to the long and short propriospinal neurons (interneurons) within the spinal cord. The short propriospinal interneurons assess and modify information across multiple joints in either the upper or the lower extremity, whereas the long propriospinal interneurons assess and modify postural (axial) muscles throughout the entire length of the vertebral column and spinal cord to maintain normal balance and posture during the movement. These propriospinal interneurons can directly and indirectly modify the output of the motor neuron pools to alter the ongoing movement. All ascending proprioceptive information traveling in the DC-ML on its way to the thalamus and cortex sends collaterals to the cerebellum (termed reafference) and reticular formation (see Fig. 9-29). The cerebellum acts as a comparator, comparing the planned movement (via corollary discharge) with the actual ongoing movements (via reafference). If the ongoing movements do not match the intended movement, the cerebellum can modify the output of the descending corticospinal pathways via axoaxonic connections and via interneurons at the level of the spinal cord, thus helping to guide the movement and correct the error (termed coordination). The cerebellum also can correct the error by sending a signal via the thalamus to the cerebral cortex to alter the plan. Further modification of these control pathways occurs by other afferents and spinal interneurons, such as pain pathways, which can alter or modify movements after injury or in response to acute pain. The other interneuronal circuits in the spinal cord, such as gating neurons, reverberating circuits, and rhythmic pattern generators, also can work to modify or control specific types of movements based on specific afferent patterns. Therefore ascending and descending tracts, along with extensive influence and modification by the spinal interneurons, work together to produce the movements that a person relies on for most daily activities (Fig. 9-30). Other seemingly simple movements, such as standing (from a seated position), function in much the same way. Although it may not seem like a complicated movement, standing (and sitting) requires much greater control of postural adjustments because of the extreme changes in body mass relative to the base of support. When seated, the base of support is the hips. When standing, the base of support must shift from the hips to the knees and ankles in a coordinated and specific pattern, or balance will be lost. The timing of movements must be specific and ordered, a function uniquely suited to the basal ganglia. The force requirements to overcome gravity and shift the base of support precisely are stored in the cerebellum. In patients with cerebellar or basal ganglia (BG) disorders, this may result in an inability to rise from a seated position without assistance (BG lesions), or errors in how easily the task is accomplished (cerebellar disorders). Voluntary movements of all types, therefore, rely heavily on supraspinal mechanisms and descending pathways to continuously update the spinal cord during these complicated movements.
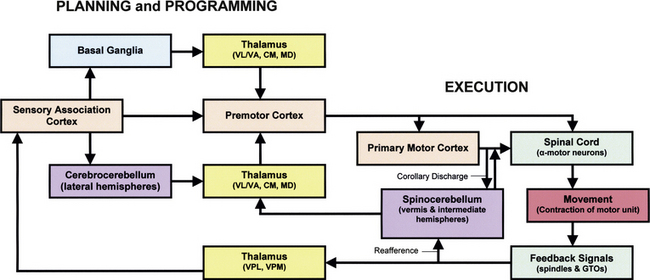
FIG. 9-29 Flowchart of motor system components. This flow diagram shows the extensive integration and levels of control associated with motor function. The motor system is organized both hierarchically and in parallel. All levels of the motor system receive sensory information concerning muscle and joint position via the thalamus (yellow). Motor areas of the cortex (tan) can influence motor neurons in the spinal cord (green) directly and indirectly via the brain stem. The motor system is also influenced by two independent subcortical systems: the basal ganglia (blue) and the cerebellum (purple). The basal ganglia influence only motor planning, whereas the cerebellum influences both planning (cerebrocerebellum) and execution (spinocerebellum) of movements. Both the basal ganglia and the cerebellum act on the cortex via the thalamus.
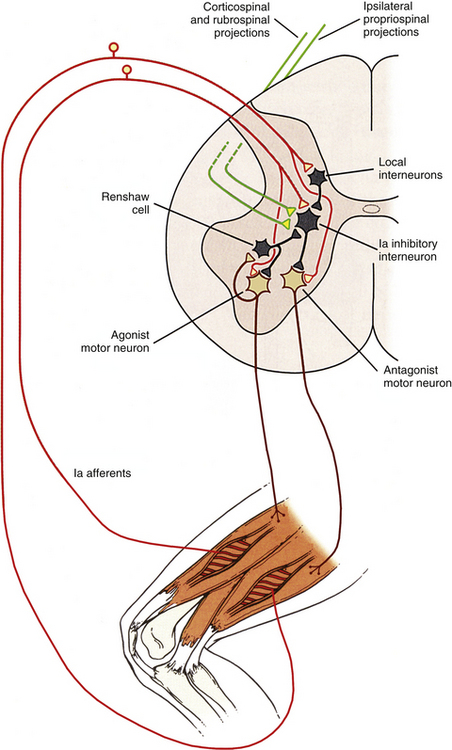
FIG. 9-30 Control of voluntary movements. The control of voluntary movements requires the integration of many different synaptic inputs at the level of the alpha motor neurons in the ventral horn of the spinal cord. By combining information from both ascending (sensory afferent information) and descending tracts (corticospinal, rubrospinal), along with extensive influence and modification by the spinal interneurons and reflex pathways “hardwired” at the level of the spinal cord, a multitude of responses can be generated that work together to produce the movements that we rely on for most of the daily activities we perform. Once a particular pathway is initiated, other competing pathways are mutually inhibited by the spinal interneurons, which results in only one set of instructions being sent to the alpha motor neurons at any one point in time to control movement. This allows the motor neurons to function as a single “myotatic unit” to produce the desired movement.