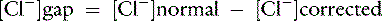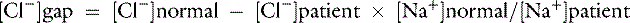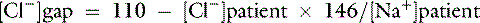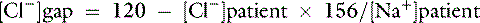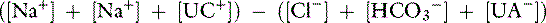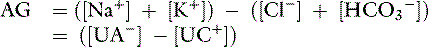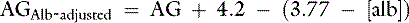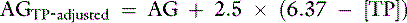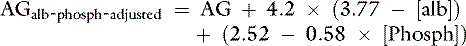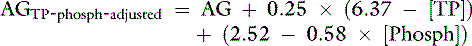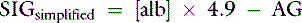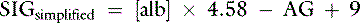Chapter 12 Mixed Acid-Base Disorders
A mixed acid-base disturbance is characterized by the presence of two or more separate primary acid-base abnormalities occurring in the same patient. An acid-base disturbance is said to be simple if it is limited to the primary disturbance and the expected compensatory response. Box 12-1 shows a classification of mixed acid-base disorders.
Recognition of a mixed acid-base disorder is important from a diagnostic and a therapeutic point of view. It permits early detection of complications (e.g., the presence of metabolic acidosis and respiratory alkalosis in a dog with parvovirus gastroenteritis may indicate sepsis), provides orientation for treatment (e.g., NaHCO3 is contraindicated in the majority of patients with metabolic acidosis and respiratory acidosis), and allows detection of complications associated with therapy (e.g., a patient with chronic respiratory acidosis that develops metabolic alkalosis after treatment with diuretics experiences further compromise of ventilation by the metabolic process).
Patients with long-standing conditions may have a simple acid-base disorders. Those patients are at higher risk of developing a mixed acid-base disturbance when the disease progresses, when they develop complications, or when they are treated with drugs that interfere with acid-base status (e.g., furosemide). Box 12-2 shows examples of potential causes of such preexisting conditions.
Box 12-2 Examples of Potential Preexisting Disease Process Associated with Chronic Acid-Base Disorders*
Respiratory Acidosis
* These patients are at higher risk for mixed acid-base disorders.
In approaching mixed acid-base disturbances, a proper understanding of the terms acidosis, alkalosis, acidemia, and alkalemia is crucial. Acidosis and alkalosis refer to the pathophysiologic processes that cause net accumulation of acid or alkali in the body, whereas acidemia and alkalemia refer specifically to the pH of extracellular fluid. In acidemia, the extracellular fluid pH is less than normal and the [H+] is higher than normal. In alkalemia, the extracellular fluid pH is higher than normal and the [H+] is lower than normal. For example, a patient with chronic respiratory alkalosis may have a blood pH value that is within the normal range. Such a patient has alkalosis but does not have alkalemia.
Compensation
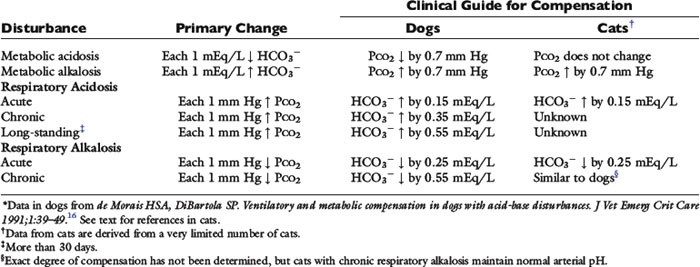
The definition of a simple acid-base disturbance includes both the primary process causing changes in Pco2 or [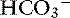 ] and the compensatory mechanisms affecting these measurements. A primary increase or decrease in one component (e.g., Pco2 or [
] and the compensatory mechanisms affecting these measurements. A primary increase or decrease in one component (e.g., Pco2 or [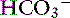 ]) is associated with a predictable compensatory change in the same direction in the other component (Table 12-1). Lack of appropriate compensation is evidence of a mixed acid-base disorder. Unfortunately, the magnitude of expected compensation in a given clinical situation is not known with certainty, and data in dogs have been derived mainly from experiments using normal dogs16 (Table 12-2). Compensatory rules for cats should be used with caution because values are derived from a limited number of normal cats with experimentally induced acid-base disorders. The reader is referred to Chapters 9, 10, and 11 for further discussion of compensation.
]) is associated with a predictable compensatory change in the same direction in the other component (Table 12-1). Lack of appropriate compensation is evidence of a mixed acid-base disorder. Unfortunately, the magnitude of expected compensation in a given clinical situation is not known with certainty, and data in dogs have been derived mainly from experiments using normal dogs16 (Table 12-2). Compensatory rules for cats should be used with caution because values are derived from a limited number of normal cats with experimentally induced acid-base disorders. The reader is referred to Chapters 9, 10, and 11 for further discussion of compensation.
Table 12-1 Primary and Secondary Changes in Simple Acid-Base Disorders
| Disorders | Primary Change | Compensatory Response |
|---|---|---|
| Metabolic acidosis | ↓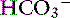 |
↓ Pco2 |
| Metabolic alkalosis | ↑ 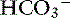 |
↑ Pco2 |
| Respiratory acidosis | ↑ Pco2 | ↑ 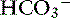 |
| Respiratory alkalosis | ↓ Pco2 | ↓ 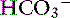 |
Respiratory compensation in metabolic processes
Metabolic acidosis is characterized by an increase in [H+], a decrease in serum [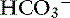 ] and blood pH, and a secondary decrease in Pco2 as a result of secondary hyperventilation. The expected decrease in Pco2 in dogs with metabolic acidosis may be estimated as 0.7 mm Hg for each 1-mEq/L decrease in [
] and blood pH, and a secondary decrease in Pco2 as a result of secondary hyperventilation. The expected decrease in Pco2 in dogs with metabolic acidosis may be estimated as 0.7 mm Hg for each 1-mEq/L decrease in [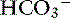 ].16 Cats with experimentally induced metabolic acidosis consistently show a lack of ventilatory compensation. In one study in which cats were chronically fed a diet containing NH4Cl, significant decreases in pH and [
].16 Cats with experimentally induced metabolic acidosis consistently show a lack of ventilatory compensation. In one study in which cats were chronically fed a diet containing NH4Cl, significant decreases in pH and [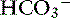 ] were observed, but there was no change in Pco2.9 Similar results were obtained in another study also adding NH4Cl to the diet31 and with dietary phosphoric acid supplementation.19 Contrary to what happens in dogs and humans, the feline kidney apparently is unable to adapt to metabolic acidosis and does not increase production of ammonia or glucose from glutamine during acidosis.31 Based on these studies, cats may not compensate for metabolic acidosis to the same extent (if at all) as do dogs and humans. Thus formulas for dogs or humans should not be extrapolated for use in cats. The clinical finding of metabolic acidosis and normal Pco2 in a cat should not be interpreted as evidence of a mixed process until more data are available about respiratory compensation in cats.
] were observed, but there was no change in Pco2.9 Similar results were obtained in another study also adding NH4Cl to the diet31 and with dietary phosphoric acid supplementation.19 Contrary to what happens in dogs and humans, the feline kidney apparently is unable to adapt to metabolic acidosis and does not increase production of ammonia or glucose from glutamine during acidosis.31 Based on these studies, cats may not compensate for metabolic acidosis to the same extent (if at all) as do dogs and humans. Thus formulas for dogs or humans should not be extrapolated for use in cats. The clinical finding of metabolic acidosis and normal Pco2 in a cat should not be interpreted as evidence of a mixed process until more data are available about respiratory compensation in cats.
Metabolic alkalosis is characterized by a decrease in [H+], an increase in serum [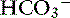 ] and blood pH, and a secondary increase in Pco2 as a result of compensatory hypoventilation. As a rule of thumb, a 1.0-mEq/L increase in plasma [
] and blood pH, and a secondary increase in Pco2 as a result of compensatory hypoventilation. As a rule of thumb, a 1.0-mEq/L increase in plasma [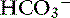 ] is expected to be associated with an adaptive 0.7-mm Hg increase in Pco2 in dogs with metabolic alkalosis.16 Little is known about respiratory compensation in cats with metabolic alkalosis. In one study with 12- to 14-week-old kittens made alkalotic by selective dietary chloride depletion, a 1.0-mEq/L increase in plasma [
] is expected to be associated with an adaptive 0.7-mm Hg increase in Pco2 in dogs with metabolic alkalosis.16 Little is known about respiratory compensation in cats with metabolic alkalosis. In one study with 12- to 14-week-old kittens made alkalotic by selective dietary chloride depletion, a 1.0-mEq/L increase in plasma [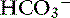 ] concentration was associated with a 0.7-mm Hg increase in Pco2.62 This value is remarkably similar to that observed in humans and dogs, but care should be exercised when extrapolating data from normal kittens to sick adult cats.
] concentration was associated with a 0.7-mm Hg increase in Pco2.62 This value is remarkably similar to that observed in humans and dogs, but care should be exercised when extrapolating data from normal kittens to sick adult cats.
Time is an important consideration when assessing compensation. Even in the experimental setting in which sudden changes in [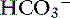 ] can be achieved, the respiratory response to acute metabolic acidosis in dogs occurs slowly, and it often takes 17 to 24 hours for maximal respiratory compensation to develop.16 Thus using the formulas within the first 24 hours of onset of metabolic acidosis may lead to an underestimation of the ventilatory response and the erroneous assumption that a mixed metabolic and respiratory disorder is present.
] can be achieved, the respiratory response to acute metabolic acidosis in dogs occurs slowly, and it often takes 17 to 24 hours for maximal respiratory compensation to develop.16 Thus using the formulas within the first 24 hours of onset of metabolic acidosis may lead to an underestimation of the ventilatory response and the erroneous assumption that a mixed metabolic and respiratory disorder is present.
Metabolic compensation in respiratory processes
Respiratory acidosis is that acid-base disorder resulting from a primary increase in carbon dioxide tension (Pco2) in the blood. It is synonymous with primary hypercapnia and is characterized by increased Pco2, increased [H+], decreased pH, and a compensatory increase in [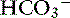 ] in blood. Respiratory alkalosis is that acid-base disorder resulting from a primary decrease in Pco2 in the blood. It is synonymous with the term primary hypocapnia and is characterized by decreased Pco2, decreased [H+], increased pH, and a compensatory decrease in [
] in blood. Respiratory alkalosis is that acid-base disorder resulting from a primary decrease in Pco2 in the blood. It is synonymous with the term primary hypocapnia and is characterized by decreased Pco2, decreased [H+], increased pH, and a compensatory decrease in [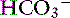 ] in blood.
] in blood.
Adaptive changes in plasma [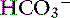 ] occur in two phases. In respiratory acidosis, the first phase represents titration of nonbicarbonate buffers, whereas in respiratory alkalosis, the first phase represents release of H+ from nonbicarbonate buffers within cells. This response is completed within 15 minutes (see Chapter 11). The second phase reflects renal adaptation and consists of increased net acid excretion and increased
] occur in two phases. In respiratory acidosis, the first phase represents titration of nonbicarbonate buffers, whereas in respiratory alkalosis, the first phase represents release of H+ from nonbicarbonate buffers within cells. This response is completed within 15 minutes (see Chapter 11). The second phase reflects renal adaptation and consists of increased net acid excretion and increased 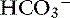 reabsorption (decreased Cl− reabsorption) in respiratory acidosis and a decrease in net acid excretion in respiratory alkalosis. Experimentally, renal adaptation requires 2 to 5 days for a chronic steady state to be established.21,46,51
reabsorption (decreased Cl− reabsorption) in respiratory acidosis and a decrease in net acid excretion in respiratory alkalosis. Experimentally, renal adaptation requires 2 to 5 days for a chronic steady state to be established.21,46,51
During acute respiratory acidosis, a compensatory increase of 0.15 mEq/L in [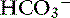 ] for each 1-mm Hg increase in Pco2 should be expected in dogs.16 There is a lack of data for compensation in cats with acute respiratory acid-base disorders, but values appear to be similar to those observed in dogs. In anesthetized, artificially ventilated cats made hypercapnic by exposure to increasing CO2 levels, the average compensatory increase in [
] for each 1-mm Hg increase in Pco2 should be expected in dogs.16 There is a lack of data for compensation in cats with acute respiratory acid-base disorders, but values appear to be similar to those observed in dogs. In anesthetized, artificially ventilated cats made hypercapnic by exposure to increasing CO2 levels, the average compensatory increase in [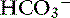 ] was 0.07 to 0.1 mEq/L for each 1-mm Hg increase in Pco2.24,54 In three awake cats exposed to an FIco2 of 4%,53 [
] was 0.07 to 0.1 mEq/L for each 1-mm Hg increase in Pco2.24,54 In three awake cats exposed to an FIco2 of 4%,53 [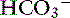 ] increased 0.16 mEq/L for each 1-mm Hg increase in Pco2, a value very similar to the one observed in dogs. During acute respiratory alkalosis, a compensatory decrease of 0.25 mEq/L in [
] increased 0.16 mEq/L for each 1-mm Hg increase in Pco2, a value very similar to the one observed in dogs. During acute respiratory alkalosis, a compensatory decrease of 0.25 mEq/L in [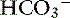 ] for each 1-mm Hg decrease in Pco2 should be expected in dogs.16 Compensation to hyperventilation has only been studied in anesthetized cats. The [
] for each 1-mm Hg decrease in Pco2 should be expected in dogs.16 Compensation to hyperventilation has only been studied in anesthetized cats. The [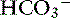 ] decreased an average 0.26 mEq/L for each 1-mm Hg decrease in Pco2, a value similar to that obtained in dogs.24
] decreased an average 0.26 mEq/L for each 1-mm Hg decrease in Pco2, a value similar to that obtained in dogs.24
In dogs with chronic respiratory alkalosis, a decrease of 0.55 mEq/L in [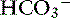 ] is expected for each 1-mm Hg decrease in Pco2.2,16 It is interesting to note that even in severe chronic respiratory alkalosis, the pH usually is normal. However, the normalization of pH in a clinical setting may take longer than 5 to 7 days. In humans with sustained respiratory alkalosis, the pH may not return to normal for 2 or more weeks.40 Cats chronically exposed to a hypoxic environment (FIo2 = 10%) for 28 days also were able to maintain a normal arterial pH.4 Expected compensation in cats cannot be inferred from this study, but based on the ability to maintain a normal pH, it may be reasonable to assume that cats can compensate for chronic respiratory alkalosis as well as dogs and humans. In dogs with chronic respiratory acidosis, serum [
] is expected for each 1-mm Hg decrease in Pco2.2,16 It is interesting to note that even in severe chronic respiratory alkalosis, the pH usually is normal. However, the normalization of pH in a clinical setting may take longer than 5 to 7 days. In humans with sustained respiratory alkalosis, the pH may not return to normal for 2 or more weeks.40 Cats chronically exposed to a hypoxic environment (FIo2 = 10%) for 28 days also were able to maintain a normal arterial pH.4 Expected compensation in cats cannot be inferred from this study, but based on the ability to maintain a normal pH, it may be reasonable to assume that cats can compensate for chronic respiratory alkalosis as well as dogs and humans. In dogs with chronic respiratory acidosis, serum [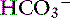 ] increases 0.35 mEq/L for each 1-mm Hg increase in Pco2.16 Similar rules have been used in humans with chronic respiratory acidosis, but these rules have been shown to work well in unstable, but not in stable, patients with long-standing respiratory acidosis.35 In this latter group of patients, a 0.51-mEq/L increase in [
] increases 0.35 mEq/L for each 1-mm Hg increase in Pco2.16 Similar rules have been used in humans with chronic respiratory acidosis, but these rules have been shown to work well in unstable, but not in stable, patients with long-standing respiratory acidosis.35 In this latter group of patients, a 0.51-mEq/L increase in [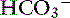 ] is expected for each 1-mm Hg increase in Pco2.35 Thus arterial pH appears to remain near reference ranges in human patients with long-standing respiratory acidosis.3 Similar results have been observed in dogs with chronic respiratory acidosis and no other identifiable reason for increased [
] is expected for each 1-mm Hg increase in Pco2.35 Thus arterial pH appears to remain near reference ranges in human patients with long-standing respiratory acidosis.3 Similar results have been observed in dogs with chronic respiratory acidosis and no other identifiable reason for increased [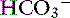 ] concentration other than renal compensation.22,25 Increases of 0.4525 to 0.57 mEq/L22 [
] concentration other than renal compensation.22,25 Increases of 0.4525 to 0.57 mEq/L22 [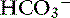 ] for each 1-mm Hg increase in Pco2 have been observed in dogs with chronic respiratory acidosis, suggesting that renal compensation in dogs with long-standing respiratory acidosis may return arterial pH to normal in stable patients.
] for each 1-mm Hg increase in Pco2 have been observed in dogs with chronic respiratory acidosis, suggesting that renal compensation in dogs with long-standing respiratory acidosis may return arterial pH to normal in stable patients.
Clinical approach
The first step is a careful history to search for clues that may lead the clinician to suspect the presence of acid-base disorders, followed by a complete physical examination. Urinalysis, routine serum chemistries, and electrolyte concentrations are useful, but confirmation of a mixed acid-base disorder requires blood gas analysis. After identifying the primary acid-base disorder (respiratory or metabolic), the expected compensation of the opposing parameter [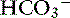 ] in a respiratory process; Pco2 in a metabolic process) should be calculated using the formulas in Table 12-2. A mixed acid-base disorder should be suspected when inappropriate compensation for the primary disorder is demonstrated. Compensation is said to be inappropriate if a patient’s Pco2 differs from expected Pco2 by more than 2 mm Hg in a primary metabolic process or if a patient’s [
] in a respiratory process; Pco2 in a metabolic process) should be calculated using the formulas in Table 12-2. A mixed acid-base disorder should be suspected when inappropriate compensation for the primary disorder is demonstrated. Compensation is said to be inappropriate if a patient’s Pco2 differs from expected Pco2 by more than 2 mm Hg in a primary metabolic process or if a patient’s [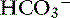 ] differs from the expected [
] differs from the expected [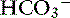 ] by more than 2 mEq/L in a respiratory acid-base disorder.2,16
] by more than 2 mEq/L in a respiratory acid-base disorder.2,16
An example illustrates how compensation can be estimated. Consider a dog with diarrhea caused by a parvovirus infection with the following arterial blood gas results: pH = 7.35, [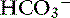 ] = 13 mEq/L, and Pco2 = 24 mm Hg. The pH in the low normal range with decreased [
] = 13 mEq/L, and Pco2 = 24 mm Hg. The pH in the low normal range with decreased [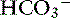 ] indicates that the primary process is a metabolic acidosis. The expected compensation is estimated assuming Pco2 = 36 mm Hg and [
] indicates that the primary process is a metabolic acidosis. The expected compensation is estimated assuming Pco2 = 36 mm Hg and [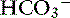 ] = 21 mEq/L as midpoint values. The change in [
] = 21 mEq/L as midpoint values. The change in [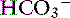 ] (
] (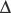 [
[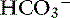 ]) is:
]) is:
Knowing that for each mEq/L decrease in [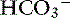 ] in a metabolic acidosis, Pco2 decreases 0.7 mm Hg (see Table 12-2), the expected compensatory change in Pco2 is estimated as:
] in a metabolic acidosis, Pco2 decreases 0.7 mm Hg (see Table 12-2), the expected compensatory change in Pco2 is estimated as:
where
Because the expected compensation has an error margin of ±2,
This patient has a Pco2 (24 mm Hg) that is more than 2 mm Hg lower than the minimal value for the expected Pco2 (28.4 mm Hg), indicating the presence of respiratory alkalosis in addition to metabolic acidosis. A similar line of thinking can be applied to calculate the expected compensation in other primary acid-base disorders. Some guidelines for adequate use of compensatory rules from Table 12-2 are expressed in Box 12-3. Some useful guidelines for quickly detecting mixed acid-base disorders in selected patients are shown in Box 12-4, whereas potential technical problems that may lead to misdiagnosing a mixed acid-base disorder are shown in Box 12-5.
Box 12-3 Guidelines for Adequate Use of Compensatory Rules from Table 12-2
* Exceptions: chronic respiratory alkalosis (>14 days), and potentially long-standing respiratory acidosis (>30 days).
Box 12-4 Guidelines for Quickly Detecting a Mixed Process
Quick Diagnosis of Mixed Disorders
Pco2 and [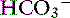 ] changing in opposite directions
] changing in opposite directions
Presence of a normal pH (with abnormal Pco2 and/or [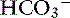 ])*
])*
A pH change in a direction opposite to that predicted for the known primary disorder
* Exceptions: chronic respiratory alkalosis (>14 days), and potentially long-standing respiratory acidosis (>30 days).
Evaluation of the metabolic component of the acid-base disorder
Metabolic alkalosis can result from an increase in the strong ion difference (SID) caused by hypochloremia or by decrease in the concentration of total plasma weak acids [Atot] caused by hypoalbuminemia. Metabolic acidosis can be caused by a decrease in SID as a result of hyperchloremia or increased concentration of other strong anions (e.g., lactate, sulfate, β-hydroxybutyrate), or by an increase in [Atot] as a result of hyperphosphatemia. See Chapter 13 for further discussion of the role of albumin and phosphate in acid-base disorders.
Chloride Changes
Chloride is the most important extracellular strong anion. Increases in chloride lead to metabolic acidosis by decreasing SID, whereas decreases in chloride cause metabolic alkalosis by increasing SID. Therefore plasma [Cl−] and [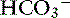 ] have a tendency to change in opposite directions in hypochloremic alkalosis and hyperchloremic acidosis. The contribution of [Cl−] to changes in base excess (BE) and [
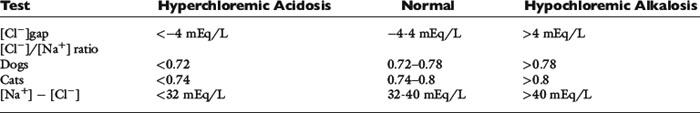
] have a tendency to change in opposite directions in hypochloremic alkalosis and hyperchloremic acidosis. The contribution of [Cl−] to changes in base excess (BE) and [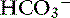 ] can be estimated by calculating the chloride gap, the chloride/sodium ratio, and the sodium-chloride difference (Table 12-3).
] can be estimated by calculating the chloride gap, the chloride/sodium ratio, and the sodium-chloride difference (Table 12-3).
Chloride gap is calculated as:
or
Normal values may vary among laboratories, but using midpoint values from Chapter 4, chloride gap can be estimated for dogs as:
Values greater than 4 mEq/L are associated with hypochloremic alkalosis, whereas values less than −4 mEq/L are associated with hyperchloremic acidosis. A shorter way to evaluate chloride contribution is to use the chloride/sodium ratio.18 Reference values have not been adequately established for dogs and cats, but experience with limited number of cases suggests that values greater than 0.78 in dogs and more than 0.80 in cats are associated with hyperchloremic metabolic acidosis, whereas values less than 0.72 in dogs and less than 0.74 in cats are associated with hypochloremic alkalosis. Whenever sodium concentration is normal, the difference between the sodium and chloride concentrations ([Na+] − [Cl−]) can be used. Normally, [Na+] − [Cl−] is approximately 36 mEq/L in dogs and cats. Values greater than 40 mEq/L are an indication of hypochloremic alkalosis, whereas values less than 32 mEq/L are associated with hyperchloremic acidosis.15
It is always important to remember that the renal adaptation to respiratory disorders is accomplished by changing SID by varying the amount of chloride or bicarbonate that is reabsorbed with sodium. Thus in chronic respiratory acidosis, there is a compensatory hypochloremic alkalosis, whereas in chronic respiratory alkalosis, there is a compensatory hyperchloremic acidosis. In fact, all change in bicarbonate concentration can be explained by the changes in chloride during chronic respiratory acidosis.3
Increase in Unmeasured Anions
Unlike chloride, most other strong anions (e.g., ketoanions, lactate, anions of renal failure) are not routinely measured and need to be estimated. Three methods combining blood gas results with electrolyte and protein data will be considered here: anion gap (AG), BE algorithm, and strong ion gap (SIG). The AG is further discussed in Chapters 9 and 10, whereas the BE algorithm and the SIG are further discussed in Chapter 13.
The anion gap is a helpful tool in the differentiation between hyperchloremic and high-AG metabolic acidoses. Chemically, there is no AG because the law of electroneutrality must be maintained. The AG is the difference between the unmeasured anions (UA−) and unmeasured cations (UC+). Following the electroneutrality law, we obtain:
or
Thus every time there is an increase in [Cl−] or [UA−], [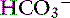 ] decreases to maintain electroneutrality. The AG estimates all unmeasured anions, making no distinction between unmeasured strong anions (e.g., lactate, ketoanions) that can change pH and weak anions (e.g., negatively-charged phosphate ions and proteins) that do not affect pH or [
] decreases to maintain electroneutrality. The AG estimates all unmeasured anions, making no distinction between unmeasured strong anions (e.g., lactate, ketoanions) that can change pH and weak anions (e.g., negatively-charged phosphate ions and proteins) that do not affect pH or [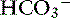 ]. In acidosis resulting from a decrease in SID caused by an increase in [Cl−], [
]. In acidosis resulting from a decrease in SID caused by an increase in [Cl−], [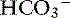 ] decreases and the difference ([UA−] − [UC+]), and consequently the AG remain constant (hyperchloremic or normal AG acidosis). When the SID decreases because of an increase in an unmeasured strong anion (e.g., lactate), [
] decreases and the difference ([UA−] − [UC+]), and consequently the AG remain constant (hyperchloremic or normal AG acidosis). When the SID decreases because of an increase in an unmeasured strong anion (e.g., lactate), [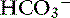 ] decreases, [Cl−] is unchanged, and the difference ([UA−] − [UC+]) increases; thus the AG also increases (normochloremic or high-AG acidosis).
] decreases, [Cl−] is unchanged, and the difference ([UA−] − [UC+]) increases; thus the AG also increases (normochloremic or high-AG acidosis).
Except for some relatively uncommon circumstances, an increase in the AG implies an accumulation of organic acids in the body.40 Unfortunately, the AG is not very sensitive in detecting increases in unmeasured strong anions, especially in lactic acidosis. In addition, the AG in normal dogs and cats is mostly a result of the net negative charge of proteins and thus is heavily influenced by protein concentration, especially albumin.12,36 In fact, hypoalbuminemia probably is the only important cause of a decrease in the AG. At plasma pH of 7.4 in dogs, each decrease of 1 g/dL in albumin concentration is associated with a decrease of 4.1 mEq/L in the AG, whereas each decrease of 1 g/dL in total protein concentration is associated with a decrease of 2.5 mEq/L in the AG.12 Similar data are not available for cats.
Because many critically ill patients with increased unmeasured strong anions also have hypoalbuminemia, the AG may be artificially normal because of the decrease in [UA−] resulting from hypoalbuminemia. The AG can be corrected for changes in protein concentration in dogs by using the following formulas12:
or
where [alb] is albumin concentration in g/dL and [TP] is total protein concentration in g/dL.
Although the contribution of serum phosphate concentration to the AG is negligible in normal dogs and cats, hyperphosphatemia also can increase the AG in the absence of an increase in strong unmeasured anions. The AG can be adjusted for an increase in phosphate concentration by expressing phosphate in mEq/L (see Chapter 7) and assuming plasma pH to be 7.4 as:
where [Phosph] is the concentration of phosphorus in milligrams per deciliter.
The base excess algorithm is another method to estimate unmeasured strong ions that has been adapted for use in dogs and cats14 and applied in clinical cases.17,30,59 It accounts first for the effects of changes in free water, chloride, protein, and phosphate concentrations in the BE. Any remaining BE is attributed to the presence of unmeasured strong anions. Formulas to use with the BE algorithm are presented in Chapter 13 (see Box 13-4). Values less than −5 mmol/L are suggestive of an increase in unmeasured strong anions.14 The BE algorithm is a useful clinical tool despite a few shortcomings. There are theoretical limitations in extrapolating traditional BE calculations for use in dogs and cats. In addition, protein influence on BE is estimated based on data for human albumin, which behaves differently than canine12 and feline albumin.36
The strong ion gap is the difference between all unmeasured strong anion charges and all unmeasured strong cation charges.11 The SIG has been simplified (SIGsimplified) to be estimated based on [Atot], the total concentration of nonvolatile weak acids in plasma (see Chapter 13).11 Albumin is used to estimate [Atot] in the SIGsimplified because albumin is the most important buffer in plasma. Assuming a plasma pH of 7.4, SIGsimplified can be calculated in dogs as12:
In cats, at a plasma pH of 7.35, SIGsimplified is estimated as36:
Increase in unmeasured strong anions is suspected whenever SIGsimplified is less than −5 mEq/L. In patients with hyperphosphatemia, however, AG should be corrected for the presence of hyperphosphatemia [AGphosph-adjusted = AG + (2.52 − 0.58 × [Phosph])] before calculating SIGsimplified. The SIGsimplified has not been adequately tested in dogs and cats, but its derivation is sound, and it is superior to the AG for detecting increases in unmeasured strong anions in horses.11
A stepwise approach should be followed in all patients with suspected mixed acid-base disorders (Figure 12-1):
1. Perform electrolyte and blood gas analysis.
2. Determine the pH and the nature of the primary disorder.
3. Calculate the expected compensation: Is it a simple or mixed disorder?
4. Calculate the chloride contribution to metabolic disorder ([Cl−]gap, [Cl−]/[Na+] ratio, [Cl−] − [Na+]; see Table 12-3).
5. Estimate the concentration of the unmeasured strong anions (AG, BE algorithm, or SIGsimplified).
6. Compare the chloride contribution with the presence of unmeasured strong anions: Is there a mixed metabolic disorder? (Table 12-4)
7. Consider other laboratory data (e.g., creatinine, glucose, and so on).
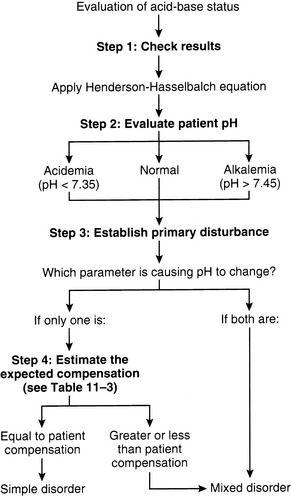
Figure 12-1 Algorithm for evaluation of acid-base status in patients with suspected mixed acid-base disorders.
Table 12-4 Evaluation of Mixed Metabolic Disorders
| Chloride Contribution* | Unmeasured Strong Anion Contribution | |
|---|---|---|
| NORMAL AG or | AG or | |
| SIGsimplified*−5 mEq/L or | SIGsimplified<−5 mEq/L or | |
| UAstrong*−5 mmol/L | UAstrong<−5 mmol/L | |
| ↓ [Cl−]gap, | Hyperchloremic acidosis | Hyperchloremic acidosis and ↑ unmeasured strong anion acidosis |
| ↓ [Cl−]/[Na+] or | ||
| ↓ [Na+] − [Cl−] | ||
| Normal [Cl−]gap, | Normal | ↑ Unmeasured strong anion acidosis |
| Normal [Cl−]/[Na+] or Normal | ||
| [Na+] − [Cl−] | ||
| ↑ [Cl−]gap, | Hypochloremic alkalosis | Hypochloremic alkalosis and ↑ unmeasured strong anion acidosis |
| ↑ [Cl−]/[Na+] or | ||
| ↑ [Na+] − [Cl−] | ||
AG, Anion gap; SIGsimplified, simplified strong ion gap; UAstrong, unmeasured strong anions estimated using the base excess algorithm; [Cl−]gap, chloride gap; [Cl−]/[Na+], chloride to sodium ratio; [Na+] − [Cl−], sodium to chloride difference.
* Metabolic compensation in chronic respiratory acid-base disturbances can also change chloride concentration.
Mixed acid-base disturbances
Disorders with neutralizing effects on pH
Patients with mixed disorders comprised of primary problems with an offsetting effect on pH may have a normal, low, or high pH. When pH is abnormal, however, because of the counterbalancing effect of the second primary disorder, changes tend not to be pronounced. Box 12-6 shows examples of potential causes of counterbalancing mixed acid-base disorders.
Box 12-6 Examples of Potential Causes of Counterbalancing Mixed Acid-Base Disorders
Mixed Respiratory and Metabolic Disorders
Respiratory Alkalosis and Metabolic Acidosis
Hypoadrenocorticism-like syndrome in dogs with gastrointestinal disease
Liver disease (RTA and impaired metabolism of lactate)
Pulmonary edema with hypoxemia or low cardiac output
Parvovirus gastroenteritis and sepsis
Severe exercise Acute tumor lysis syndrome
Respiratory Acidosis and Metabolic Alkalosis
This combination is an uncommon clinical situation, and in human medicine it usually occurs in patients with chronic lung disease who develop vomiting or who are treated with diuretics.6 It may occur in acute situations in dogs with gastric dilatation-volvulus that can present with metabolic alkalosis caused by loss of gastric acid and respiratory acidosis resulting from diaphragmatic compression caused by the distended stomach.2 The Pco2 and [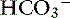 ] are high, and the pH tends to be normal or only slightly abnormal. It is important to remember that dogs with long-standing respiratory acidosis can have normal arterial pH.22 When the mixed disorder is confirmed, treatment should be directed at correcting the most life-threatening underlying disease process first. No therapy is necessary to correct pH if pH is normal or near normal. Patients with chronic pulmonary disease that have hypoxemia and hypercapnia are at greater risk from metabolic alkalosis than are others because superimposition of metabolic alkalosis can further reduce ventilation and lead to worsening of hypoxemia.49 Therefore metabolic alkalosis should not be overlooked if the patient has a chronic lung disease.
] are high, and the pH tends to be normal or only slightly abnormal. It is important to remember that dogs with long-standing respiratory acidosis can have normal arterial pH.22 When the mixed disorder is confirmed, treatment should be directed at correcting the most life-threatening underlying disease process first. No therapy is necessary to correct pH if pH is normal or near normal. Patients with chronic pulmonary disease that have hypoxemia and hypercapnia are at greater risk from metabolic alkalosis than are others because superimposition of metabolic alkalosis can further reduce ventilation and lead to worsening of hypoxemia.49 Therefore metabolic alkalosis should not be overlooked if the patient has a chronic lung disease.
Respiratory Alkalosis and Metabolic Acidosis
Many clinical situations can lead to this mixed disorder, usually with high-AG metabolic acidosis. These patients have low Pco2 and low [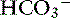 ], and their pH tends to be nearly normal. It is important to remember that chronic respiratory alkalosis is a simple acid-base disturbance, and affected patients can have a normal pH. Thus in the presence of a normal pH, low Pco2, and low [
], and their pH tends to be nearly normal. It is important to remember that chronic respiratory alkalosis is a simple acid-base disturbance, and affected patients can have a normal pH. Thus in the presence of a normal pH, low Pco2, and low [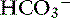 ], the clinician must decide whether the patient has simple respiratory alkalosis or metabolic acidosis associated with respiratory alkalosis. In this situation, the history can provide important clues. The presence of hypoxemia with increased hematocrit suggests chronic respiratory alkalosis. An increase in unmeasured strong anions is helpful because the majority of metabolic acidoses associated with respiratory alkalosis are normochloremic, whereas compensation for chronic respiratory alkalosis is characterized by corrected hyperchloremia.
], the clinician must decide whether the patient has simple respiratory alkalosis or metabolic acidosis associated with respiratory alkalosis. In this situation, the history can provide important clues. The presence of hypoxemia with increased hematocrit suggests chronic respiratory alkalosis. An increase in unmeasured strong anions is helpful because the majority of metabolic acidoses associated with respiratory alkalosis are normochloremic, whereas compensation for chronic respiratory alkalosis is characterized by corrected hyperchloremia.
Diseases associated with metabolic acidosis and respiratory alkalosis are shown in Box 12-6. In some conditions such as sepsis, patients initially may have respiratory alkalosis; metabolic acidosis (usually caused by lactic acidosis) only develops later.23,26 Sepsis complicating any disease known to cause metabolic acidosis can result in a metabolic acidosis superimposed on respiratory alkalosis. Exercise also can cause a mixed disorder that begins with respiratory alkalosis. In mild exercise (35% of maximal O2 consumption), mild respiratory alkalosis occurs.38 When dogs are maximally exercised, lactic acidosis is superimposed on the initial respiratory alkalosis.27,38,45 Dogs with heat stroke also initially have respiratory alkalosis and later develop mixed respiratory alkalosis and metabolic acidosis.50 Salicylate toxicity in dogs and cats causes hyperventilation initially, but metabolic acidosis then develops.42 The hyperventilation associated with salicylate toxicity is caused by central stimulation, and only a small portion of the hyperventilation can be attributed to hyperthermia.52 In human patients with salicylate intoxication, metabolic acidosis is caused by accumulation of organic acids, including lactate and ketoacids.49 This also may be true in small animals.42
Gastric dilatation-volvulus complex has been associated with respiratory alkalosis and metabolic acidosis in dogs37 in which the respiratory alkalosis may be the result of pain,37 sepsis,2 or restriction of pulmonary expansion. Patients with liver disease may develop a wide variety of acid-base disturbances. Hyperventilation is common and appears to be multifactorial.8 Metabolic acidosis also has been associated with liver disease in dogs.13 Human patients with cirrhosis demonstrate enhanced proximal renal tubular sodium reabsorption that may limit distal H+ secretion5 and lead to hyperchloremic acidosis. Type B lactic acidosis also can develop in patients with liver failure because liver disease can decrease liver uptake and metabolism of lactate.40 Distal renal tubular acidosis has been associated with hepatic lipidosis in a cat with normal AG acidosis.7
Special considerations apply to cardiopulmonary resuscitation. Arterial blood gases may indicate respiratory alkalosis because gas exchange is occurring in blood that traverses the pulmonary circulation. Mixed venous Pco2 has been shown to be significantly higher than arterial Pco2 during cardiopulmonary resuscitation in dogs.32 In this setting, arterial values reflect the adequacy of ventilatory support, whereas mixed venous values may correlate better with tissue pH.58
In patients with mixed metabolic acidosis and respiratory alkalosis, pH tends to be normal, and specific treatment to correct pH usually is not necessary. Treatment should be directed at the underlying causes of the metabolic acidosis and respiratory alkalosis.
Metabolic Acidosis and Metabolic Alkalosis
This mixed disorder usually is seen in patients with long-standing, high-AG metabolic acidosis (e.g., chronic renal failure, uncomplicated ketoacidosis) that begin vomiting and develop hypochloremic alkalosis. Because albumin is a weak acid, a decrease in albumin concentration is associated with metabolic alkalosis. Superimposition of hypoalbuminemia on chronic metabolic acidosis also can lead to this mixed acid-base disorder. Alternatively, this mixed metabolic disorder can begin as metabolic alkalosis with subsequent development of severe volume depletion resulting in hypoperfusion and lactic acidosis. Depending on the relative severity of the two opposing disorders, pH and [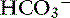 ] can be increased, normal, or decreased. Recognition of both disturbances in this setting is very important because treatment of one without attention to the other permits the unattended abnormality to emerge unopposed. Information in Table 12-4 can be used to help diagnose mixed metabolic acidosis and metabolic alkalosis. Mixed hyperchloremic metabolic acidosis and hypochloremic metabolic alkalosis can theoretically coexist (e.g., patients with vomiting and diarrhea), but because these disturbances have offsetting effects on [Cl−] and [
] can be increased, normal, or decreased. Recognition of both disturbances in this setting is very important because treatment of one without attention to the other permits the unattended abnormality to emerge unopposed. Information in Table 12-4 can be used to help diagnose mixed metabolic acidosis and metabolic alkalosis. Mixed hyperchloremic metabolic acidosis and hypochloremic metabolic alkalosis can theoretically coexist (e.g., patients with vomiting and diarrhea), but because these disturbances have offsetting effects on [Cl−] and [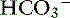 ], only the prevailing disorder can be identified. Diseases associated with mixed metabolic acidosis and metabolic alkalosis are shown in Box 12-6. The pH usually is normal in these settings, and treatment of stable patients should be directed at resolving the underlying disease processes. Patients with lactic acidosis and severe volume depletion need more aggressive therapy.
], only the prevailing disorder can be identified. Diseases associated with mixed metabolic acidosis and metabolic alkalosis are shown in Box 12-6. The pH usually is normal in these settings, and treatment of stable patients should be directed at resolving the underlying disease processes. Patients with lactic acidosis and severe volume depletion need more aggressive therapy.
Disorders with additive effects on pH
Mixed disorders composed of primary problems with an additive effect on pH always have abnormal pH. Depending on the combination of primary problems, the pH can be dangerously high or low and requires immediate attention. Box 12-7 shows examples of potential causes of additive mixed acid-base disorders.
Box 12-7 Examples of Potential Causes of Additive Mixed Acid-Base Disorders
Mixed Respiratory and Metabolic Disorders
Respiratory Acidosis and Metabolic Acidosis
Hypoadrenocorticism-like syndrome in dogs with gastrointestinal disease
Thoracic trauma with hypovolemic shock
Low cardiac output heart failure with pulmonary edema
Advanced septic shock (![]() /
/![]() mismatch)
mismatch)
Gastrointestinal endoscopy*
Venom of the scorpion Leiurus quinquestriatus
Neurotoxic poisons and metabolic conditions disrupting neuromuscular junction function
Respiratory Alkalosis and Metabolic Alkalosis
Respiratory Acidosis and Metabolic Acidosis
This combination of acid-base disturbances may occur in a variety of settings usually in patients with acute severe respiratory compromise (e.g., thoracic trauma, pulmonary edema, cardiopulmonary arrest, acute neuromuscular junctional disruption such as with toxic or metabolic or junctionopathies) that also have lactic acidosis as a result of hypoxemia, shock, or poor cardiac output (see Box 12-7). Thus metabolic acidosis usually is caused by an increase in unmeasured strong ions. There is an additive effect lowering the pH because the normal compensation for metabolic acidosis is impaired because of pulmonary disease. The [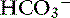 ] is low; Pco2 is normal or high; and the resultant pH can be dangerously low.
] is low; Pco2 is normal or high; and the resultant pH can be dangerously low.
Dogs, cats, and human patients with cardiopulmonary arrest typically develop lactic acidosis as a result of low cardiac output and hypoventilation.32,39,58 During resuscitation, however, arterial blood gases may indicate a normal pH with mixed metabolic acidosis and respiratory alkalosis and not reflect the ongoing marked reduction in mixed venous and tissue pH. Mixed venous blood should be used for analysis in this setting.58 In addition to being better for assessing global tissue perfusion and cardiac output, venous pH and Pco2 will change earlier and to a greater extent than arterial values during periods of circulatory insufficiency.44 Patients with pulmonary edema may develop hypoxemia and lactic acidosis.57 The situation is worse in patients in which pulmonary edema is secondary to heart failure. Low cardiac output compromises tissue perfusion, worsening the lactic acidosis.40 Dogs in septic shock usually demonstrate respiratory alkalosis and metabolic acidosis. Later in the course of the disease process, however, patients may develop respiratory acidosis because of ventilation-perfusion (![]() /
/![]() ) mismatch.23,26 Dogs with gastric dilation-volvulus complex also can present with metabolic acidosis caused by lactic acidosis and respiratory acidosis resulting from diaphragmatic compression by the distended stomach.37
) mismatch.23,26 Dogs with gastric dilation-volvulus complex also can present with metabolic acidosis caused by lactic acidosis and respiratory acidosis resulting from diaphragmatic compression by the distended stomach.37
A recent study evaluated the acid-base balance of neonatal dogs over the first hour following birth under normal birthing conditions, following dystocia and following birthing assisted by oxytocin administration. It was shown that independent of how the dogs were born, all had respiratory and metabolic acidosis 5 minutes following birth. The ecbolic effect of oxytocin aggravated the metabolic component of the acidosis compared with the other two birthing groups. Regardless of the conditions of birth, the hypercapnia resolved within 1 hour, but the metabolic component (reflected in the base deficit) persisted. It was concluded that at birth a mixed respiratory metabolic acidosis is present and that adverse events during birthing aggravate acid-base balance.32a
Systemic pH is very low in patients with combined metabolic and respiratory acidosis, and specific therapy must be initiated quickly.6 In those patients in which lactic acidosis is the cause of metabolic acidosis, tissue hypoxia is the most likely underlying cause, and therapeutic measures should be taken to augment oxygen delivery to the tissues and to reestablish cardiac output.33 Patients should be artificially ventilated if necessary. This will reduce Pco2 and increase pH. Sodium bicarbonate is not indicated to treat patients with metabolic acidosis that also have respiratory acidosis because they cannot excrete the CO2 generated by NaHCO3 administration. The CO2 will diffuse into the cells and further decrease intracellular pH. Sodium bicarbonate may be considered in ventilated patients with [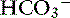 ] less than 5 mEq/L because at this concentration even a small decrease in serum bicarbonate is associated with a large decrease in serum pH.20 In this situation, small titrated doses of NaHCO3 are used as a temporizing measure to maintain [
] less than 5 mEq/L because at this concentration even a small decrease in serum bicarbonate is associated with a large decrease in serum pH.20 In this situation, small titrated doses of NaHCO3 are used as a temporizing measure to maintain [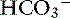 ] greater than 5 mEq/L while attempts to improve oxygenation are continued. (See Chapter 10 for further discussion of lactic acidosis.)
] greater than 5 mEq/L while attempts to improve oxygenation are continued. (See Chapter 10 for further discussion of lactic acidosis.)
Respiratory Alkalosis and Metabolic Alkalosis
This mixed disorder is commonly present in human patients with hepatic failure or in those with congestive heart failure and pulmonary edema who are treated with diuretics. These patients have low Pco2, high [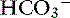 ], and high pH, and their alkalemia may be severe. Similar clinical conditions also occur in small animal medicine (see Box 12-7), but severe alkalemia is not common. Mixed respiratory and metabolic alkalosis was not observed in a study of 20 dogs with alkalemia identified from 962 dogs in which blood gas analysis was performed.48 In dogs with experimental metabolic alkalosis, superimposition of chronic respiratory alkalosis causes a decrease in [
], and high pH, and their alkalemia may be severe. Similar clinical conditions also occur in small animal medicine (see Box 12-7), but severe alkalemia is not common. Mixed respiratory and metabolic alkalosis was not observed in a study of 20 dogs with alkalemia identified from 962 dogs in which blood gas analysis was performed.48 In dogs with experimental metabolic alkalosis, superimposition of chronic respiratory alkalosis causes a decrease in [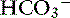 ] sufficient not only to prevent development of significant alkalemia but also to offset entirely the effect of hypocapnia on plasma [H+].34 In dogs, mixed metabolic alkalosis and respiratory alkalosis are more common in patients with chronic respiratory disease placed on diuretics. Severe alkalemia is only likely to occur in dogs with long-standing respiratory acidosis and a compensatory increase in [
] sufficient not only to prevent development of significant alkalemia but also to offset entirely the effect of hypocapnia on plasma [H+].34 In dogs, mixed metabolic alkalosis and respiratory alkalosis are more common in patients with chronic respiratory disease placed on diuretics. Severe alkalemia is only likely to occur in dogs with long-standing respiratory acidosis and a compensatory increase in [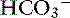 ] that are placed on a ventilator. This maneuver acutely lowers Pco2, whereas [
] that are placed on a ventilator. This maneuver acutely lowers Pco2, whereas [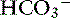 ] remains high for approximately 24 hours.22 Severe alkalemia also was observed in dogs with severe canine babesiosis caused by Babesia canis rossi.30
] remains high for approximately 24 hours.22 Severe alkalemia also was observed in dogs with severe canine babesiosis caused by Babesia canis rossi.30
Because most patients with this mixed disorder have metabolic alkalosis superimposed on chronic respiratory alkalosis, therapy usually is directed at correcting the metabolic alkalosis. In addition, compensation for simple chronic respiratory alkalosis is so effective that the pH is usually normal. Therefore correction of the metabolic alkalosis will be associated with normalization of pH even if the chronic respiratory alkalosis cannot be treated. The goal of treatment in metabolic alkalosis is to replace the chloride deficit while providing sufficient potassium and sodium to replace existing deficits. Dehydrated patients should be rehydrated accordingly. Definitive treatment of the underlying disease process prevents recurrence of the metabolic alkalosis.
Hyperchloremic and High-AG Metabolic Acidoses
This mixed disorder usually is seen in patients with renal failure, in the resolving phase of ketoacidosis, or in patients with high-AG acidosis that develop diarrhea or receive fluid therapy (see Box 12-7). The pH and [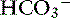 ] are low, and the diagnosis is suggested by an increase in unmeasured anions and a chloride gap of less than −4 mEq/L (see Table 12-4).
] are low, and the diagnosis is suggested by an increase in unmeasured anions and a chloride gap of less than −4 mEq/L (see Table 12-4).
Human patients with chronic renal failure (serum creatinine concentration of 2 to 4 mg/dL) initially develop hyperchloremic acidosis. With progression of the disease (serum creatinine concentration of 4 to 14 mg/dL), metabolic acidosis progresses, but the further decrease in total CO2 is associated with an increase in unmeasured strong ions (e.g., sulfate, acetate) and hyperphosphatemia, whereas hyperchloremia remains unchanged.60 However, human patients with advanced renal failure sometimes may have a simple acid-base disorder, either hyperchloremic or high-AG acidosis.47,56 Patients with diabetes mellitus may have a mixed high-AG and hyperchloremic acidosis because of development of diarrhea or in the resolving phase of the ketoacidotic crisis.47,56 Hyperchloremia in the recovery phase develops for at least three reasons: (1) large volumes of saline are administered; (2) KCl is infused in large doses; and (3) ketones are lost in the urine and NaCl is reabsorbed by the kidneys.40 As discussed earlier, human patients with chronic hepatic disease may have enhanced proximal renal tubular sodium reabsorption that may limit distal H+ secretion.5 This may lead to hyperchloremic acidosis, decreased lactate metabolism, and development of a high-AG acidosis. Severe canine babesiosis caused by B. canis rossi also has been shown to cause this combination of disturbances.30 The treatment in mixed hyperchloremic and high-AG acidoses should be directed at the primary disorders responsible for metabolic acidosis. Treatment with NaHCO3 may be necessary in selected patients with low pH and severe corrected hyperchloremia or renal failure. Limitations of NaHCO3 treatment for lactic acidosis were discussed earlier. Sodium bicarbonate is not indicated in diabetic patients even if the pH is less than 7.0.43,55
Mixed High-AG Metabolic Acidosis
Two different causes of high-AG metabolic acidosis may coexist in the same patient, and this usually is a result of lactic or uremic acidosis superimposed on another cause of high-AG acidosis. The pH and [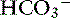 ] are low in affected patients with increased unmeasured ions and normal chloride gap (see Table 12-4). It is not possible to differentiate between simple and mixed high-AG metabolic acidosis if only blood gases and serum electrolytes are assessed. Serum creatinine concentration, blood urea nitrogen (BUN), and plasma lactate concentration must be measured to confirm the presence of this mixed disorder.40
] are low in affected patients with increased unmeasured ions and normal chloride gap (see Table 12-4). It is not possible to differentiate between simple and mixed high-AG metabolic acidosis if only blood gases and serum electrolytes are assessed. Serum creatinine concentration, blood urea nitrogen (BUN), and plasma lactate concentration must be measured to confirm the presence of this mixed disorder.40
Patients with ketoacidosis may develop lactic acidosis because of decreased tissue perfusion or impaired lactate use caused by decreased insulin activity. In this circumstance, lactic acidosis promotes conversion of acetoacetate to β-hydroxybutyrate, which does not react with nitroprusside in the urinalysis dipstrip reagent pad, thereby masking the ketoacidosis.40 It has been suggested that adding a few drops of hydrogen peroxide to the urine specimen would nonenzymatically convert β-hydroxybutyrate to acetoacetate, which then would be detected by the nitroprusside reagent.41 However, this method has been shown to be ineffective in converting β-hydroxybutyrate to acetoacetate in dogs.10
Treatment in this mixed disorder should be directed toward resolving the primary disorder causing metabolic acidosis and toward stabilizing the patient. The use and limitations of NaHCO3 in lactic acidosis, uremic acidosis, and ketoacidosis have been discussed previously. Patients with severe acidosis (pH, <7.1) and renal failure may benefit from small, titrated doses of NaHCO3.
Mixed Hyperchloremic Metabolic Acidosis
This is a very rare disorder in veterinary medicine because the only clinical situation that commonly causes hyperchloremic acidosis is diarrhea. The pH and [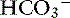 ] are decreased in these patients, and the AG is normal with corrected hyperchloremia (see Table 12-4). Fluid therapy with lactated Ringer’s solution or 0.9% NaCl solution with or without KCl supplementation is a common cause of hyperchloremic acidosis in hospitalized patients. In patients with a preexisting hyperchloremic acidosis, fluid therapy will induce a mixed hyperchloremic metabolic acidosis. Parenteral nutrition in patients with diarrhea also could cause a mixed hyperchloremic acidosis because of the addition of cationic amino acids (e.g., lysine HCl, arginine HCl). Treatment should be directed toward resolving the primary disease responsible for the acidosis. Treatment with NaHCO3 is safer in hyperchloremic acidoses and should be used if the pH is less than 7.10 or the [
] are decreased in these patients, and the AG is normal with corrected hyperchloremia (see Table 12-4). Fluid therapy with lactated Ringer’s solution or 0.9% NaCl solution with or without KCl supplementation is a common cause of hyperchloremic acidosis in hospitalized patients. In patients with a preexisting hyperchloremic acidosis, fluid therapy will induce a mixed hyperchloremic metabolic acidosis. Parenteral nutrition in patients with diarrhea also could cause a mixed hyperchloremic acidosis because of the addition of cationic amino acids (e.g., lysine HCl, arginine HCl). Treatment should be directed toward resolving the primary disease responsible for the acidosis. Treatment with NaHCO3 is safer in hyperchloremic acidoses and should be used if the pH is less than 7.10 or the [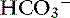 ] is less than 10 mEq/L. The potential causes of mixed metabolic disorders are summarized in Box 12-7.
] is less than 10 mEq/L. The potential causes of mixed metabolic disorders are summarized in Box 12-7.
Triple disorders
Metabolic Acidosis, Metabolic Alkalosis, and Respiratory Acidosis or Alkalosis
Triple disorders occur whenever a respiratory disturbance complicates a mixed metabolic acidosis and metabolic alkalosis. The pH and [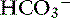 ] may be normal, decreased, or increased, and Pco2 is higher than expected when the mixed metabolic disturbance is complicated by respiratory acidosis and lower than expected when it is complicated by respiratory alkalosis. Patients with low-output heart failure treated with diuretics may develop lactic acidosis and hypochloremic alkalosis. If such a patient develops interstitial pulmonary edema, there is a decrease in compliance, and stimulation of ventilation causes Pco2 to decrease and respiratory alkalosis to develop.1 With increasing severity of the edema, hypoventilation with respiratory acidosis may occur.1 However, dogs have good collateral ventilation, and hypercapnia occurs only in fulminant pulmonary edema.57
] may be normal, decreased, or increased, and Pco2 is higher than expected when the mixed metabolic disturbance is complicated by respiratory acidosis and lower than expected when it is complicated by respiratory alkalosis. Patients with low-output heart failure treated with diuretics may develop lactic acidosis and hypochloremic alkalosis. If such a patient develops interstitial pulmonary edema, there is a decrease in compliance, and stimulation of ventilation causes Pco2 to decrease and respiratory alkalosis to develop.1 With increasing severity of the edema, hypoventilation with respiratory acidosis may occur.1 However, dogs have good collateral ventilation, and hypercapnia occurs only in fulminant pulmonary edema.57
Patients with gastric dilatation-volvulus can have metabolic alkalosis and lactic acidosis.29,37,61 These patients also can develop respiratory alkalosis as a result of a pain-induced increase in ventilation37 or sepsis.2 Respiratory acidosis also can develop if ventilation is impaired by a grossly overdistended stomach.37 Severe babesiosis in dogs infected with B. canis rossi also can cause triple disorders with respiratory alkalosis as a result of a systemic inflammatory response syndrome (in a sepsis-like state), lactic acidosis, and hyperchloremic acidosis.30 It is not known why dogs with this disease develop hyperchloremic acidosis. Other potential causes of triple disorders are outlined in Box 12-8. The treatment of triple disorders should be directed at stabilizing the patient’s clinical condition and resolving the underlying disease process. In the majority of these cases, the metabolic acidosis is caused by lactic acid accumulation. Therefore the principles discussed under mixed respiratory acidosis with lactic acidosis are valid here.
Treatment
When treating a patient with a mixed disorder, always prioritize the order in which the abnormalities are managed:
1. Treat the most life-threatening disorder that the body cannot address itself first (e.g., decompress the dilated stomach of a patient with gastric dilatation-volvulus while aggressively supporting intravascular volume before trying to manipulate ventilatory volume or rate or correct electrolyte disturbances; manage the metabolic components [carbohydrate and fluid abnormalities] of the acid-base abnormalities in the patient with diabetic ketoacidosis before concerning yourself with the respiratory abnormalities). Correcting these priority disorders will allow the body the opportunity to address the lesser abnormalities itself.
2. Treat the most treatable disorder next.
3. Direct manipulation of blood pH is rarely required and more often than not may be contraindicated.
4. Take into consideration the systemic pH of the patient. For example, if a dog has a pH of 7.35 and [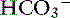 ] of 12 mEq/L, no attempts should be made to correct this relatively normal pH. The exception to the rule is the patient with [
] of 12 mEq/L, no attempts should be made to correct this relatively normal pH. The exception to the rule is the patient with [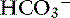 ] of 5 mEq/L or less. In these patients, a small decrease in [
] of 5 mEq/L or less. In these patients, a small decrease in [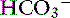 ] is associated with a large decrease in pH.
] is associated with a large decrease in pH.
5. Do not overlook the second disorder. The effect that treating one disorder has on the second disorder must be anticipated, and both processes should be assessed simultaneously.
The potential complications of treatment also should be anticipated (e.g., overshoot metabolic alkalosis after NaHCO3 treatment), and iatrogenic mixed acid-base disorders should be avoided (e.g., administration of drugs that suppress ventilation in patients with metabolic acidosis). The reader is referred to chapters on the individual acid-base disorders for further discussion of treatment (see Chapters 10 and 11). However, bear in mind that mixed disturbances that cause additive effects on pH (e.g., respiratory and metabolic acidosis) require more aggressive therapy than those with neutralizing effects (e.g., respiratory alkalosis and metabolic acidosis).
1 Aberman A., Fulop M. The metabolic and respiratory acidosis of acute pulmonary edema. Ann Intern Med. 1972;76:173-178.
2 Adams L.G., Polzin D.J. Mixed acid-base disorders. Vet Clin North Am Small Anim Pract. 1989;19:307-326.
3 Alfaro V., Torras R., Ibáñez J., et al. A physical-chemical analysis of the acid-base response to chronic obstructive pulmonary disease. Can J Physiol Pharmacol. 1996;74:1229-1235.
4 Barnard P., Andronikou S., Pokorski N., et al. Time-dependent effect of hypoxia on carotid body chemosensory function. J Appl Physiol. 1987;63:685-691.
5 Better O., Goldschmid Z., Chaimowitz C., et al. Defect in urinary acidification in cirrhosis. Arch Intern Med. 1972;130:77-82.
6 Bia M., Thier S.O. Mixed acid-base disturbances: a clinical approach. Med Clin North Am. 1981;65:347-361.
7 Brown S.A., Spyridakis L.K., Crowell W.A. Distal renal tubular acidosis and hepatic lipidosis in a cat. J Am Vet Med Assoc. 1986;189:1350-1352.
8 Center S.A. Pathophysiology and laboratory diagnosis of liver disease. In: Ettinger S.J., editor. Textbook of veterinary internal medicine: diseases of dog and cat. 3rd ed. Philadelphia: WB Saunders; 1989:1421-1478.
9 Ching S.V., Fettman M.J., Hamar D.W., et al. The effect of chronic dietary acidification using ammonium chloride on acid-base and mineral metabolism in the adult cat. J Nutr. 1989;119:902-915.
10 Christopher M., Pereira J., Brigmon R., et al. Automated determination of β-hydroxybutyrate for the assessment of ketoacidosis. Proc Am Coll Vet Intern Med. New Orleans, La. 1991:903.
11 Constable P.D., Hinchcliff K.W., Muir W.W. Comparison of anion gap and strong ion gap as predictors of unmeasured strong ion concentration in plasma and serum from horses. Am J Vet Res. 1998;59:881-887.
12 Constable P.D., Stämpfli H.R. Experimental determination of net protein charge and Atot and Ka of nonvolatile buffers in canine plasma. J Vet Intern Med. 2005;19:507-514.
13 Cornelius L.M., Rawlings C.A. Arterial blood gas and acid-base values in dogs with various diseases and signs of disease. J Am Vet Med Assoc. 1981;178:992-995.
14 de Morais H.S.A. A nontraditional approach to acid-base disorders. In: DiBartola S.P., editor. Fluid therapy in small animal practice. Philadelphia: WB Saunders; 1992:297-320.
15 de Morais H.S.A. Mixed acid-base disorders. In: DiBartola S.P., editor. Fluid therapy in small animal practice. 2nd ed. Philadelphia: WB Saunders; 2000:251-261.
16 de Morais H.S.A., DiBartola S.P. Ventilatory and metabolic compensation in dogs with acid-base disturbances. J Vet Emerg Crit Care. 1991;1:39-49.
17 DiBartola S.P., de Morais H.S.A. Appendix: clinical cases. In: DiBartola S.P., editor. Fluid therapy in small animal practice. Philadelphia: WB Saunders; 1992:599-688.
18 Durward A., Skellett S., Mayer S., et al. The value of the chloride:sodium ratio in differentiating the aetiology of metabolic acidosis. Intensive Care Med. 2001;27:828-835.
19 Fettman M.J., Coble J.M., Hamar D.W., et al. Effect of dietary phosphoric acid supplementation on acid-base balance and mineral and bone metabolism in adult cats. Am J Vet Res. 1992;53:2125-2135.
20 Gauthier P.M., Szerlip H.M. Metabolic acidosis in the intensive care unit. Crit Care Clin. 2002;18:298-308.
21 Gennari F.J., Goldstein M.B., Schwartz W. The nature of the renal adaptation to chronic hypocapnia. J Clin Invest. 1972;51:1722-1730.
22 Goodman L.A., de Morais H.A.S. Unpublished observation. 2005.
23 Goodwin J.-K., Schaer M. Septic shock. Vet Clin North Am Small Anim Pract. 1990;19:1239-1258.
24 Hampson N.B., Jöbsis-VandlerVliet F.F., Piantadosi C.A. Skeletal muscle oxygen availability during respiratory acid-base disturbances in cats. Respir Physiol. 1987;70:143-158.
25 Hara Y., Nezu Y., Harada Y., et al. Secondary chronic respiratory acidosis in a dog following the cervical cord compression by an intradural glioma. J Vet Med Sci. 2002;64:863-866.
26 Hauptman J.G., Tvedten H. Osmolal and anion gaps in dogs with acute endotoxic shock. Am J Vet Res. 1986;47:1617-1619.
27 Ilkiw J.E., Davis P.E., Church D.B. Hematological, biochemical, blood-gas, and acid-base values in greyhounds before and after exercise. Am J Vet Res. 1989;50:583-586.
28 Jergens A.E., Riedesel D.H., Ries P.A., et al. Cardiopulmonary responses in healthy dogs during endoscopic examination of the gastrointestinal tract. Am J Vet Res. 1995;56:215-220.
29 Kagan K.G., Schaer M. Gastric dilatation and volvulus in a dog-a case justifying electrolyte and acid-base assessment. J Am Vet Med Assoc. 1983;183:703-705.
30 Leisewitz A.L., Jacobson L.S., de Morais H.S.A., et al. The mixed acid-base disturbances of severe canine babesiosis. J Vet Intern Med. 2001;15:445-452.
31 Lemieux G., Lemieux C., Duplessis S., et al. Metabolic characteristics of cat kidney: failure to adapt to metabolic acidosis. Am J Physiol. 1990;259:R277-R281.
32 Lippert A.C., Evans A.T., White B.C., et al. The effect of resuscitation technique and pre-arrest state of oxygenation on blood-gas values during cardiopulmonary resuscitation in dogs. Vet Surg. 1988;17:283-290.
32a Lúcio C.F., Silva L.C., Rodrigues J.A., et al. Acid-base changes in canine neonates following normal birth or dystocia. Reprod Domest Anim. 2009;44(Suppl. 2):208-210.
33 Madias N.E. Lactic acidosis. Kidney Int. 1986;29:752-774.
34 Madias N.E., Cohen J.J., Adrogué H.J. Influence of acute and chronic respiratory alkalosis on preexisting chronic metabolic alkalosis. Am J Physiol. 1990;258:F479-F485.
35 Martinu T., Menzies D., Dial S. Re-evaluation of acid-base prediction rules in patients with chronic respiratory acidosis. Can Respir J. 2003;19:311-315.
36 McCullough S.M., Constable P.D. Calculation of the total plasma concentration of nonvolatile weak acids and the effective dissociation constant of nonvolatile buffers in plasma for use in the strong ion approach to acid-base balance in cats. Am J Vet Res. 2003;64:1047-1051.
37 Muir W.W.III. Acid-base and electrolyte disturbances in dogs with gastric dilatation-volvulus. J Am Vet Med Assoc. 1982;181:229-231.
38 Musch T.I., Friedman D.B., Haidet G.C., et al. Arterial blood gases and acid-base status of dogs during graded dynamic exercise. J Appl Physiol. 1986;61:1914-1919.
39 Nakakimura K., Fleischer J.E., Drummond J.C., et al. Glucose administration before cardiac arrest worsens neurologic outcome in cats. Anesthesiology. 1990;72:1005-1011.
40 Narins R.G., Emmett M. Simple and mixed acid-base disorders: a practical approach. Medicine. 1980;59:161-187.
41 Narins R.G., Jones E.R., Stom M.C., et al. Diagnostic strategies in disorders of fluid, electrolyte and acid-base abnormalities. Am J Med. 1982;72:46-52.
42 Oehme F.W. Aspirin and acetaminophen. In: Kirk R.W., editor. Current veterinary therapy IX. Philadelphia: WB Saunders; 1986:188-190.
43 Okuda Y., Adrogue H.J., Field J.B., et al. Counterproductive effects of sodium bicarbonate in diabetic ketoacidosis. J Clin Endocrinol Metab. 1996;81:314-320.
44 Oropello J.M., Manasia A., Hannon E., et al. Continuous fiberoptic arterial and venous blood gas monitoring in hemorrhagic shock. Chest. 1996;109:1049-1055.
45 Pieschl R.L., Toll P.W., Leith D.E., et al. Acid-base changes in the running greyhound: contributing variables. J Appl Physiol. 1992;73:2297-2304.
46 Polak A., Haynie G.D., Hays R.M., et al. Effects of chronic hypercapnia on electrolyte and acid-base equilibrium. I. Adaptation. J Clin Invest. 1961;40:1223-1237.
47 Ray S., Piraino B., Chong T.K., et al. Acid excretion and serum electrolyte patterns in patients with advanced chronic renal failure. Miner Electrolyte Metab. 1990;16:355-361.
48 Robinson E.P., Hardy R.M. Clinical signs, diagnosis, and treatment of alkalemia in dogs: 20 cases (1982-1984). J Am Vet Med Assoc. 1988;7:943-949.
49 Rose B.D. Clinical physiology of acid-base and electrolyte disorders, 3rd ed. New York: McGraw-Hill; 1989.
50 Schall W.D. Heat stroke. In: Kirk R.W., editor. Current veterinary therapy VII. Philadelphia: WB Saunders; 1980:195-197.
51 Schwartz W.B., Brackett N.C., Cohen J.J. The response of extracellular hydrogen ion concentration to graded degrees of chronic hypercapnia: the physiologic limits of defense of pH. J Clin Invest. 1965;44:291-301.
52 Silva P.R., Fonseca-Costa A., Zin W.A., et al. Respiratory and acid-base parameters during salicylic intoxication in dogs. Braz J Med Biol Res. 1986;19:279-286.
53 Szlyk P.C., Jennings B.D. Effects of hypercapnia on variability of normal respiratory behavior in awake cats. Am J Physiol. 1987;252:R538-R547.
54 Torbati D., Mokashi A., Lahiri S. Effects of acute hyperbaric oxygenation on respiratory control in cats. J Appl Physiol. 1989;67:2351-2355.
55 Viallon A., Zeni F., Lafond P., et al. Does bicarbonate therapy improve the management of severe diabetic ketoacidosis? Crit Care Med. 1999;27:2690-2693.
56 Wallia R., Greenberg A., Piraino B., et al. Serum electrolyte patterns in end-stage renal disease. Am J Kidney Dis. 1986;8:98-104.
57 Ware W.A., Bonagura J.D. Pulmonary edema. In: Fox P.R., editor. Canine and feline cardiology. New York: Churchill Livingstone; 1988:205-217.
58 Weil M.H., Rackow E.C., Trevino R., et al. Difference in acid-base state between venous and arterial blood during cardiopulmonary resuscitation. N Engl J Med. 1986;315:153-156.
59 Whitehair K.J., Haskins S.C., Whitehair J.G., et al. Clinical application of quantitative acid-base chemistry. J Vet Intern Med. 1995;9:1-11.
60 Widmer B., Gerhardt R.E., Harrington J.T., et al. Serum electrolyte and acid-base composition: the influence of graded degrees of chronic renal failure. Arch Intern Med. 1979;139:1099-1102.
61 Wingfield W.E., Twedt D.C., Moore R.W., et al. Acid-base and electrolyte values in dogs with acute gastric dilatation-volvulus. J Am Vet Med Assoc. 1982;180:1070-1072.
62 Yu S., Morris J.G. Chloride requirement of kittens for growth is less than current recommendations. J Nutr. 1999;129:1909-1914.
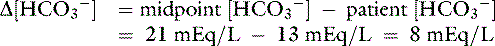
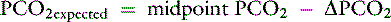
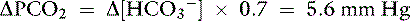
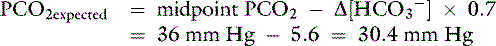
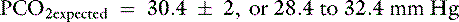
 ] and P
] and P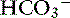 ]
]