Chapter 383 Wheezing, Bronchiolitis, andBronchitis
383.1 Wheezing in Infants: Bronchiolitis
Definitions and General Pathophysiology (See also Chapter 365)
A wheeze is a musical and continuous sound that originates from oscillations in narrowed airways. Wheezing is heard mostly on expiration as a result of critical airway obstruction. Wheezing is polyphonic when there is widespread narrowing of the airways, causing various pitches or levels of obstruction to airflow as seen in asthma. Monophonic wheezing refers to a single-pitch sound that is produced in the larger airways during expiration, as in distal tracheomalacia or bronchomalacia. When obstruction occurs in the extrathoracic airways during inspiration, the noise is referred to as stridor.
Infants are prone to wheeze, owing to a differing set of lung mechanics in comparison to older children and adults. The obstruction to flow is affected by the airway caliber and compliance of the infant lung. Resistance to airflow through a tube is inversely related to the radius of the tube to the 4th power. In children <5 yr old, small-caliber peripheral airways can contribute up to 50% of the total airway resistance. Marginal additional narrowing can cause further flow limitation and a subsequent wheeze.
With the very compliant newborn chest wall, the inward pressure produced in expiration subjects the intrathoracic airways to collapse. Flow limitation is further affected in infants by the differences in tracheal cartilage composition and airway smooth muscle tone, causing further increase in airway compliance in comparison to older children. All of these mechanisms combine to make the infant more susceptible to airway collapse, increased resistance, and subsequent wheezing. Many of these conditions are outgrown in the 1st yr of life.
Immunologic and molecular influences can contribute to the infant’s propensity to wheeze. In comparison to older children and adults, infants tend to have higher levels of lymphocytes and neutrophils, rather than mast cells and eosinophils, in bronchoalveolar lavage fluid. The childhood wheezing phenotype has been linked to many early exposures including fetal nutrition, maternal smoking, prenatal and birth maternal complications, prenatal and neonatal exposure to antibiotics, exposure to high levels of environmental allergens, and high infant adiposity. Infections during infancy have been cited as risk factors for later wheezing, including respiratory syncytial virus (RSV), rhinovirus, cytomegalovirus, human metapneumovirus, bocavirus, adenovirus, and Chlamydia pneumoniae.
A variety of inflammatory mediators have also been implicated in the wheezing infant such as histamine, cytokines, leukotrienes, and interleukins. Taken together, these fetal and/or early postnatal exposures can cause a “programming” of the lung that ultimately affects structure and function.
Etiology
Most wheezing in infants is caused by inflammation (generally bronchiolitis), but many other entities can manifest with wheezing (Table 383-1).
Table 383-1 DIFFERENTIAL DIAGNOSIS OF WHEEZING IN INFANCY
INFECTION
Viral
Other
ASTHMA
ANATOMIC ABNORMALITIES
Central Airway Abnormalities
Extrinsic Airway Anomalies Resulting in Airway Compression
Intrinsic Airway Anomalies
Immunodeficiency States
MUCOCILIARY CLEARANCE DISORDERS
ASPIRATION SYNDROMES
OTHER
Acute Bronchiolitis and Inflammation of the Airway
Infection can cause obstruction to flow by internal narrowing of the airways.
Acute bronchiolitis is predominantly a viral disease. RSV is responsible for >50% of cases(Chapter 252). Other agents include parainfluenza (Chapter 251), adenovirus, and Mycoplasma. Emerging pathogens include human metapneumovirus (Chapter 253) and human bocavirus, which may be a primary cause of viral respiratory infection or occur as a co-infection with RSV. There is no evidence of a bacterial cause for bronchiolitis, although bacterial pneumonia is sometimes confused clinically with bronchiolitis, but bronchiolitis is rarely followed by bacterial superinfection. Concurrent infection with viral bronchiolitis and pertussis has been described.
Approximately 75,000-125,000 children <1 yr old are hospitalized annually in the United States due to RSV infection. Increasing rates of hospitalization might reflect increased attendance of infants in daycare centers, changes in criteria for hospital admission, and/or improved survival of premature infants and others at risk for severe RSV-associated disease.
Bronchiolitis is more common in boys, in those who have not been breast-fed, and in those who live in crowded conditions. Risk is higher for infants with young mothers or mothers who smoked during pregnancy. Older family members are a common source of infection; they might only experience minor upper respiratory symptoms (colds). The clinical manifestations of lower respiratory tract illness (LRTI) seen in young infants may be minimal in older patients, in whom bronchiolar edema is better tolerated.
Not all infected infants develop LRTI. Host anatomic and immunologic factors play a significant role in the severity of the clinical syndrome, as does the nature of the viral pathogen. Infants with pre-existent smaller airways and diminished lung function have a more-severe course. In addition, RSV infection incites a complex immune response. Eosinophils degranulate and release eosinophil cationic protein, which is cytotoxic to airway epithelium. Innate immunity plays a significant role and can depend on polymorphisms in toll-like receptor (TLR), interferon (IF), interleukins (IL), and nuclear factor κB (NFκB). Chemokines and cytokines such as tumor necrosis factor α (TNF-α) may be differentially expressed depending on the inciting virus. Co-infection with >1 virus can also alter the clinical manifestations and/or severity of presentation.
Acute bronchiolitis is characterized by bronchiolar obstruction with edema, mucus, and cellular debris. Even minor bronchiolar wall thickening significantly affects airflow because resistance is inversely proportional to the 4th power of the radius of the bronchiolar passage. Resistance in the small air passages is increased during both inspiration and exhalation, but because the radius of an airway is smaller during expiration, the resultant respiratory obstruction leads to early air trapping and overinflation. If obstruction becomes complete, trapped distal air will be resorbed and the child will develop atelectasis.
Hypoxemia is a consequence of ventilation-perfusion mismatch early in the course. With severe obstructive disease and tiring of respiratory effort, hypercapnia can develop.
Chronic infectious causes of wheezing should be considered in infants who seem to fall out of the range of a normal clinical course. Cystic fibrosis is one such entity; suspicion increases in a patient with persistent respiratory symptoms, digital clubbing, malabsorption, failure to thrive, electrolyte abnormalities, or a resistance to bronchodilator treatment(Chapter 395).
Allergy and asthma are important causes of wheezing and probably generate the most questions by the parents of a wheezing infant. Asthma is characterized by airway inflammation, bronchial hyperreactivity, and reversibility of obstruction(Chapter 138). Three identified patterns of infant wheezing are the transient early wheezer, the persistent wheezer, and the late-onset wheezer. Transient early wheezers constituted 19.9% of the general population, and they had wheezing at least once with a lower respiratory infection before the age of 3 yr but never wheezed again. The persistent wheezer constituted 13.7% of the general population, had wheezing episodes before age 3 yr, and were still wheezing at 6 yr of age. The late-onset wheezer constituted 15% of the general population, had no wheezing by 3 yr, but was wheezing by 6 yr. The other 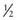 of the children had never wheezed by 6 yr. Of all the infants who wheezed before 3 yr old, almost 60% stopped wheezing by 6 yr.
of the children had never wheezed by 6 yr. Of all the infants who wheezed before 3 yr old, almost 60% stopped wheezing by 6 yr.
Multiple studies have tried to predict which early wheezers will go on to have asthma in later life. Risk factors for persistent wheezing include parental history of asthma and allergies, maternal smoking, persistent rhinitis (apart from acute upper respiratory tract infections), eczema at <1 yr of age, and frequent episodes of wheezing during infancy.
Other Causes
Congenital malformations of the respiratory tract cause wheezing in early infancy. These findings can be diffuse or focal and can be from an external compression or an intrinsic abnormality. External vascular compression includes a vascular ring, in which the trachea and esophagus are surrounded completely by vascular structures, or a vascular sling, in which the trachea and esophagus are not completely encircled(Chapter 426). Cardiovascular causes of wheezing include dilated chambers of the heart including massive cardiomegaly, left atrial enlargement, and dilated pulmonary arteries. Pulmonary edema caused by heart failure can also cause wheezing by lymphatic and bronchial vessel engorgement that leads to obstruction and edema of the bronchioles and further obstruction (Chapter 436).
Foreign body aspiration (Chapter 379) can cause acute or chronic wheezing. It is estimated that 78% of those who die from foreign body aspiration are between 2 mo and 4 yr old. Even in young infants, a foreign body can be ingested if given to the infant by another person such as an older sibling. Infants who have atypical histories or misleading clinical and radiologic findings can receive a misdiagnosis of asthma or another obstructive disorder as inflammation and granulation develop around the foreign body. Esophageal foreign body can transmit pressure to the membranous trachea, causing compromise of the airway lumen.
Gastroesophageal reflux (Chapter 315.1) can cause wheezing with or without direct aspiration into the tracheobronchial tree. Without aspiration, the reflux is thought to trigger a vagal or neural reflex, causing increased airway resistance and airway reactivity. Aspiration from gastroesophageal reflux or from the direct aspiration from oral liquids can also cause wheezing.
Trauma and tumors are much rarer causes of wheezing in infants. Trauma of any type to the tracheobronchial tree can cause an obstruction to airflow. Accidental or nonaccidental aspirations, burns, or scalds of the tracheobronchial tree can cause inflammation of the airways and subsequent wheezing. Any space-occupying lesion either in the lung itself or extrinsic to the lung can cause tracheobronchial compression and obstruction to airflow.
Clinical Manifestations
History and Physical Examination
Initial history of a wheezing infant should include accounts of the recent event including onset, duration, and associated factors (Table 383-2). Birth history includes weeks of gestation, neonatal intensive care unit admission, history of intubation or oxygen requirement, maternal complications including infection with herpes simplex virus (HSV) or HIV, and prenatal smoke exposure. Past medical history includes any comorbid conditions including syndromes or associations. Family history of cystic fibrosis, immunodeficiencies, asthma in a 1st-degree relative, or any other recurrent respiratory conditions in children should be obtained. Social history should include an environmental history including any smokers at home, inside or out, daycare exposure, number of siblings, occupation of inhabitants of the home, pets, tuberculosis exposure, and concerns regarding home environment (e.g., dust mites, construction dust, heating and cooling techniques, mold, cockroaches).
Table 383-2 PERTINENT MEDICAL HISTORY IN THE WHEEZING INFANT
On physical examination, evaluation of the patient’s vital signs with special attention to the respiratory rate and the pulse oximetry reading for oxygen saturation is an important initial step. There should also be a thorough review of the patient’s growth chart for signs of failure to thrive. Wheezing produces an expiratory whistling sound that can be polyphonic or monophonic. Expiratory time may be prolonged. Biphasic wheezing can occur if there is a central, large airway obstruction. The lack of audible wheezing is not reassuring if the infant shows other signs of respiratory distress because complete obstruction to airflow can eliminate the turbulence that causes the sound to resonate. Aeration should be noted and a trial of a bronchodilator may be warranted to evaluate for any change in wheezing after treatment. Listening to breath sounds over the neck helps differentiate upper airway from lower airway sounds. The absence or presence of stridor should be noted and appreciated on inspiration. Signs of respiratory distress include tachypnea, increased respiratory effort, nasal flaring, tracheal tugging, subcostal and intercostal retractions, and excessive use of accessory muscles. In the upper airway, signs of atopy, including boggy turbinates and posterior oropharynx cobblestoning, can be evaluated in older infants. It is also useful to evaluate the skin of the patient for eczema and any significant hemangiomas; midline lesions may be associated with an intrathoracic lesion. Digital clubbing should be noted(Chapter 366).
Acute bronchiolitis is usually preceded by exposure to an older contact with a minor respiratory syndrome within the previous week. The infant 1st develops a mild upper respiratory tract infection with sneezing and clear rhinorrhea. This may be accompanied by diminished appetite and fever of 38.5-39°C (101-102°F), although the temperature can range from subnormal to markedly elevated. Gradually, respiratory distress ensues, with paroxysmal wheezy cough, dyspnea, and irritability. The infant is often tachypneic, which can interfere with feeding. The child does not usually have other systemic complaints, such as diarrhea or vomiting. Apnea may be more prominent than wheezing early in the course of the disease, particularly with very young infants (<2 mo old) or former premature infants.
The physical examination is often dominated by wheezing. The degree of tachypnea does not always correlate with the degree of hypoxemia or hypercarbia, so pulse oximetry and noninvasive determination of carbon dioxide is essential. Work of breathing may be markedly increased, with nasal flaring and retractions. Auscultation might reveal fine crackles or overt wheezes, with prolongation of the expiratory phase of breathing. Barely audible breath sounds suggest very severe disease with nearly complete bronchiolar obstruction. Hyperinflation of the lungs can permit palpation of the liver and spleen.
Diagnostic Evaluation
Initial evaluation depends on likely etiology; a baseline chest radiograph, including posteroanterior and lateral films, is warranted in many cases and for any infant in acute respiratory distress. Infiltrates are most often found in wheezing infants who have a pulse oximetry reading <93%, grunting, decreased breath sounds, prolonged inspiratory to expiratory ratio, and crackles. The chest radiograph may also be useful for evaluating hyperinflation (common in bronchiolitis and viral pneumonia), signs of chronic disease such as bronchiectasis, or a space-occupying lesion causing airway compression. A trial of bronchodilator may be diagnostic as well as therapeutic because these medications can reverse conditions such as bronchiolitis (occasionally) and asthma but will not affect a fixed obstruction. Bronchodilators potentially can worsen a case of wheezing caused by tracheal or bronchial malacia. A sweat test to evaluate for cystic fibrosis and evaluation of baseline immune status are reasonable in infants with recurrent wheezing or complicated courses. Further evaluation such as upper gastrointestinal (GI) contrast x-rays, chest CT, bronchoscopy, infant pulmonary function testing, video swallow study, and pH probe can be considered second-tier diagnostic procedures in complicated patients.
The diagnosis of acute bronchiolitis is clinical, particularly in a previously healthy infant presenting with a first-time wheezing episode during a community outbreak. Chest radiography can reveal hyperinflated lungs with patchy atelectasis. The white blood cell and differential counts are usually normal. Viral testing (polymerase chain reaction, rapid immunofluorescence, or viral culture) is helpful if the diagnosis is uncertain or for epidemiologic purposes. Because concurrent bacterial infection (sepsis, pneumonia, meningitis) is highly unlikely, confirmation of viral bronchiolitis can obviate the need for a sepsis evaluation in a febrile infant and assist with respiratory precautions and isolation if the patient requires hospitalization.
Treatment
Treatment of an infant with wheezing depends on the underlying etiology. Response to bronchodilators is unpredictable, regardless of cause, but suggests a component of bronchial hyperreactivity. It is appropriate to administer albuterol aerosol and objectively observe the response. For children <3 yr of age, it is acceptable to continue to administer inhaled medications through a metered-dose inhaler (MDI) with mask and spacer if a therapeutic benefit is demonstrated. Therapy should be continued in all patients with asthma exacerbations from a viral illness.
The use of ipratropium bromide in this population is controversial, but it appears to be somewhat effective as an adjunct therapy. It is also useful in infants with significant tracheal and bronchial malacia who may be made worse by β2 agonists such as albuterol because of the subsequent decrease in smooth muscle tone.
A trial of inhaled steroids may be warranted in a patient who has responded to multiple courses of oral steroids and who has moderate to severe wheezing or a significant history of atopy including food allergy or eczema. Inhaled corticosteroids are appropriate for maintenance therapy in patients with known reactive airways but are controversial when used for episodic or acute illnesses. Intermittent, high-dose inhaled corticosteroids are not recommended for intermittent wheezing. Early use of inhaled corticosteroids has not been shown to prevent the progression of childhood wheezing or affect the natural history of asthma in children.
Oral steroids are generally reserved for atopic wheezing infants thought to have asthma that is refractory to other medications. Their use in first-time wheezing infants or in infants who do not warrant hospitalization is controversial.
Infants with acute bronchiolitis who are experiencing respiratory distress (hypoxia, inability to take oral feedings, extreme tachypnea) should be hospitalized; risk factors for severe disease include age <12 wk, preterm birth, or underlying comorbidity such as cardiovascular, pulmonary, or immunologic disease. The mainstay of treatment is supportive. Hypoxemic children should receive cool humidified oxygen. Sedatives are to be avoided because they can depress respiratory drive. The infant is sometimes more comfortable if sitting with head and chest elevated at a 30-degree angle with neck extended. The risk of aspiration of oral feedings may be high in infants with bronchiolitis, owing to tachypnea and the increased work of breathing. The infant may be fed through a nasogastric tube. If there is any risk for further respiratory decompensation potentially necessitating tracheal intubation, the infant should not be fed orally but be maintained with parenteral fluids. Frequent suctioning of nasal and oral secretions often provides relief of distress or cyanosis. Suctioning of secretions is an essential part of the treatment of bronchiolitis. Oxygen is definitely indicated in all infants with hypoxia. High-flow nasal cannula therapy can reduce the need for intubation in patients with impending respiratory failure.
A number of agents have been proposed as adjunctive therapies for bronchiolitis. Bronchodilators can produce modest short-term improvement in clinical features. This must be placed in context of potential adverse effects and the lack of any evidence indicating improvement in overall course of the disease. A trial dose of inhaled bronchodilator may be reasonable, with further therapy predicated on response in the individual patient. Corticosteroids, whether parenteral, oral, or inhaled, have been used for bronchiolitis despite conflicting and often negative studies. Corticosteroids are not recommended in previously healthy infants with RSV. Ribavirin, an antiviral agent administered by aerosol, has been used for infants with congenital heart disease or chronic lung disease. There is no convincing evidence of a positive impact on clinically important outcomes such as mortality and duration of hospitalization. Antibiotics have no value unless there is coexisting bacterial infection. Likewise, there is no support for RSV immunoglobulin administration during acute episodes of RSV bronchiolitis in previously healthy children. Combined therapy with nebulized epinephrine and dexamethasone has been used but is not currently recommended. Nebulized hypertonic saline has also been reported to have some benefit.
Prognosis
Infants with acute bronchiolitis are at highest risk for further respiratory compromise in the 1st 48-72 hr after onset of cough and dyspnea; the child may be desperately ill with air hunger, apnea, and respiratory acidosis. The case fatality rate is <1%, with death attributable to apnea, respiratory arrest, or severe dehydration. After this critical period, symptoms can persist. The median duration of symptoms in ambulatory patients is ∼12 days. There is a higher incidence of wheezing and asthma in children with a history of bronchiolitis unexplained by family history or other atopic syndromes. It is unclear whether bronchiolitis incites an immune response that manifests as asthma later or whether those infants have an inherent predilection for asthma that is merely unmasked by their episode of RSV. Approximately 60% of infants who wheeze will stop wheezing.
Prevention
Reduction in the severity and incidence of acute bronchiolitis due to RSV is possible through the administration of pooled hyperimmune RSV intravenous immunoglobulin and palivizumab, an intramuscular monoclonal antibody to the RSV F protein, before and during RSV season. Palivizumab should be considered for infants <2 yr of age with chronic lung disease, a history of prematurity, and some forms of congenital heart disease. Meticulous hand hygiene is the best measure to prevent nosocomial transmission.
Al-Asari K, Sakran M, Davidson BL, et al. Nebulized 5% or 3% hypertonic or 0.9% saline for treating acute bronchiolitis in infants. J Pediatr. 2010;157:630-634.
Amanatidou V, Sourvinos G, Apostolakis S, et al. RANTES promoter gene polymorphisms and susceptibility to severe respiratory syncytial virus-induced bronchiolitis. Pediatr Infect Dis J. 2008;27:38-42.
American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;111:1774-1793.
Bass JL, Gozal D. Oxygen therapy for bronchiolitis. Pediatrics. 2007;119:611.
Corneli HM, Zorc JJ, Mahajan P, et al. A multicenter, randomized, controlled trial of dexamethasone for bronchiolitis. N Engl J Med. 2007;357:331-339.
Everard ML. Acute bronchiolitis and croup. Pediatr Clin N Am. 2009;56:119-133.
Forton JT, Rowlands K, Rockett K, et al. Genetic association study for RSV bronchiolitis in infancy at the 5q31 cytokine cluster. Thorax. 2009;64:345-352.
Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588-598.
Houben ML, Bont L, Wilbrink B, et al. Clinical prediction rule for RSV bronchiolitis in healthy newborns: prognostic birth cohort study. Pediatrics. 2011;127(1):35-41.
Jartti T, Lehtinen P, Vuorinen T, et al. Age and previous wheezing episodes are linked to viral etiology and atopic characteristics. Pediatr Infect Dis J. 2009;28:311-316.
Karr CJ, Demers PA, Koehoorn MW, et al. Influence of ambient air pollutant sources on clinical encounters for infant bronchiolitis. Am J Respir Crit Care Med. 2009;180:995-1001.
Kim CK, Choi J, Kim H, et al. A randomized intervention of montelukast for post-bronchiolitis: Effect on eosinophil degranulation. J Pediatr. 2010;156:749-754.
Koehoorn M, Karr CJ, Demers PA, et al. Descriptive epidemiologic features of bronchiolitis in a population-based cohort. Pediatrics. 2008;122:1196-1203.
McKiernan C, Chua LC, Visintainer PF, et al. High flow nasal cannulae therapy in infants with bronchiolitis. J Pediatr. 2010;156:634-638.
Midulla F, Scagnolari C, Bonci E, et al. Respiratory syncytial virus, human bocavirus and rhinovirus bronchiolitis in infants. Arch Dis Child. 2010;95:35-40.
Milder E, Arnold JC. Human metapneumovirus and human bocavirus in children. Pediatr Res. 2009;65:78R-83R.
Plint AC, Johnson DW, Patel H, et al. Epinephrine and dexamethasone in children with bronchiolitis. N Engl J Med. 2009;360:2079-2089.
Summer A, Coyle D, Mitton C, et al. Cost-effectiveness of epinephrine and dexamethasone in children with bronchiolitis. Pediatrics. 2010;126:623-631.
Wu P, Dupont WD, Griffin MR, et al. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am J Respir Crit Care Med. 2008;178:1123-1129.
Yanney M, Vyas H. The treatment of bronchiolitis. Arch Dis Child. 2008;93:793-798.
Zorc JJ, Hall CB. Bronchiolitis: recent evidence on diagnosis and management. Pediatrics. 2010;125:342-349.
Alm B, Erdes L, Möllborg P, et al. Neonatal antibiotic treatment is a risk factor for early wheezing. Pediatrics. 2008;121(4):697-702.
Bisgaard H, Hermansen MN, Loland L, et al. Intermittent inhaled corticosteroids in infants with episodic wheezing. N Engl J Med. 2006;354(19):1998-2005.
Ducharme FM, Lemire C, Noya FJ, et al. Preemptive use of high-dose fluticasone for virus-induced wheezing in young children. N Engl J Med. 2009;360(4):339-353.
Kaditis AG, Winnie G, Syrogiannopoulos GA. Anti-inflammatory pharmacotherapy for wheezing in preschool children. Pediatr Pulmonol. 2007;42(5):407-420.
Ly NP, Gold DR, Weiss ST, et al. Recurrent wheeze in early childhood and asthma among children at risk for atopy. Pediatrics. 2006;117(6):e1132-e1138.
Murray CS, Woodcock A, Langley SJ, et al. Secondary prevention of asthma by the use of Inhaled Fluticasone propionate in Wheezy INfants (IFWIN): double-blind, randomised, controlled study. IFWIN Study Team. Lancet. 2006;368(9537):754-762.
Panickar J, Lakhanpaul M, Lambert PC, et al. Oral prednisolone for preschool children with acute virus-induced wheezing. N Engl J Med. 2009;360(4):329-338.
Rusconi F, Galassi C, Forastiere F, et al. Maternal complications and procedures in pregnancy and at birth and wheezing phenotypes in children. Am J Respir Crit Care Med. 2007;175(1):16-21.
383.2 Bronchitis
Nonspecific bronchial inflammation is termed bronchitis and occurs in multiple childhood conditions. Acute bronchitis is a syndrome, usually viral in origin, with cough as a prominent feature.
Acute tracheobronchitis is a term used when the trachea is prominently involved. Nasopharyngitis may also be present, and a variety of viral and bacterial agents, such as those causing influenza, pertussis, and diphtheria, may be responsible. Isolation of common bacteria such as pneumococcus, Staphylococcus aureus, and Streptococcus pneumoniae from the sputum might not imply a bacterial cause that requires antibiotic therapy.
Acute Bronchitis
Clinical Manifestations
Acute bronchitis often follows a viral upper respiratory tract infection. It is more common in the winter when respiratory viral syndromes predominate. The tracheobronchial epithelium is invaded by the infectious agent, leading to activation of inflammatory cells and release of cytokines. Constitutional symptoms including fever and malaise follow. The tracheobronchial epithelium can become significantly damaged or hypersensitized, leading to a protracted cough lasting 1-3 wk.
The child 1st presents with nonspecific upper respiratory infectious symptoms, such as rhinitis. Three to 4 days later, a frequent, dry, hacking cough develops, which may or may not be productive. After several days, the sputum can become purulent, indicating leukocyte migration but not necessarily bacterial infection. Many children swallow their sputum, and this can produce emesis. Chest pain may be a prominent complaint in older children and is exacerbated by coughing. The mucus gradually thins, usually within 5-10 days, and then the cough gradually abates. The entire episode usually lasts about 2 wk and seldom >3 wk.
Findings on physical examination vary with the age of the patient and stage of the disease. Early findings are absent or are low-grade fever and upper respiratory signs such as nasopharyngitis, conjunctivitis, and rhinitis. Auscultation of the chest may be unremarkable at this early phase. As the syndrome progresses and cough worsens, breath sounds become coarse, with coarse and fine crackles and scattered high-pitched wheezing. Chest radiographs are normal or can have increased bronchial markings.
The principal objective of the clinician is to exclude pneumonia, which is more likely caused by bacterial agents requiring antibiotic therapy. In adults, absence of abnormality of vital signs (tachycardia, tachypnea, fever) and a normal physical examination of the chest reduce the likelihood of pneumonia.
Differential Diagnosis
Persistent or recurrent symptoms should lead the clinician to consider entities other than acute bronchitis. Many entities manifest with cough as a prominent symptom (Table 383-3).
Table 383-3 DISORDERS WITH COUGH AS A PROMINENT FINDING
| CATEGORY | DIAGNOSES |
|---|---|
| Inflammatory | Asthma |
| Chronic pulmonary processes | |
| Other chronic disease or congenital disorders | |
| Infectious or immune disorders | |
| Acquired | Foreign body aspiration, tracheal or esophageal |
Treatment
There is no specific therapy for acute bronchitis. The disease is self-limited, and antibiotics, although often prescribed, do not hasten improvement. Frequent shifts in position can facilitate pulmonary drainage in infants. Older children are sometimes more comfortable with humidity, but this does not shorten the disease course. Cough suppressants can relieve symptoms but can also increase the risk of suppuration and inspissated secretions and, therefore, should be used judiciously. Antihistamines dry secretions and are not helpful; expectorants are likewise not indicated.
Chronic Bronchitis
Chronic bronchitis is well recognized in adults, formally defined as ≥3 mo of productive cough each year for ≥2 yr. The disease can develop insidiously, with episodes of acute obstruction alternating with quiescent periods. A number of predisposing conditions can lead to progression of airflow obstruction or chronic obstructive pulmonary disease (COPD), with smoking as the major factor (up to 80% of patients have a smoking history). Other conditions include air pollution, occupational exposures, and repeated infections. In children, cystic fibrosis, bronchopulmonary dysplasia, and bronchiectasis must be ruled out.
The applicability of this definition to children is unclear. The existence of chronic bronchitis as a distinct entity in children is controversial. Like adults, however, children with chronic inflammatory diseases or those with toxic exposures can develop damaged pulmonary epithelium. Thus, chronic or recurring cough in children should lead the clinician to search for underlying pulmonary or systemic disorders (see Table 383-3). One proposed entity is persistent or protracted bacterial bronchitis, which may be mistaken for asthma and shares some characteristics with other forms of suppurative lung disease.
Cigarette Smoking and Air Pollution
Exposure to environmental irritants, such as tobacco smoke and air pollution, can incite or aggravate cough. There is a well-established association between tobacco exposure and pulmonary disease, including bronchitis and wheezing. This can occur through cigarette smoking or by exposure to passive smoke. Marijuana smoke is another irritant sometimes overlooked when eliciting a history. There is some evidence that women may be particularly susceptible to long-term pulmonary disease as a consequence of childhood smoking.
A number of pollutants compromise lung development and likely precipitate lung disease, including particulate matter, ozone, acid vapor, and nitrogen dioxide. Because these substances coexist in the atmosphere, the relative contribution of any 1 to pulmonary symptoms is difficult to discern. Proximity to motor vehicle traffic is an important source of these pollutants.
Arnold JC, Singh KK, Spector SA, et al. Human bocavirus: prevalence and clinical spectrum at a children’s hospital. CID. 2006;43:283-288.
Arroll B, Kenealy T. Antibiotics for acute bronchitis. BMJ. 2001;322:939-940.
Chang AB, Redding GJ, Everard ML. Chronic wet cough: protracted bronchitis, chronic suppurative lung disease and bronchiectasis. Pediatr Pulmonol. 2008;43:519-531.
Donnelly D, Critchlow A, Everard ML. Outcomes in children treated for persistent bacterial bronchitis. Thorax. 2007;62:80-84.
Everard M. New respect for old conditions. Pediatr Pulmonol. 2007;42:400-402.
Gauderman WJ, Avol E, Gilliland F, et al. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med. 2004;351:1057-1067.
Gergen PJ, Fowler JA, Maurer KR, et al. The burden of environmental tobacco smoke exposure on the respiratory health of children 2 months through 5 years of age in the United States: Third National Health and Nutrition Examination Survey, 1988 to 1994. Pediatrics. 1998;101:e8.
Gonzales R, Sande MA. Uncomplicated acute bronchitis. Ann Intern Med. 2000;133:981-991.
Irwin RS, Madison JM. The diagnosis and treatment of cough. N Engl J Med. 2000;343:1715-1721.
Kim JJ, Smorodinsky S, Lipsett M, et al. Traffic-related air pollution near busy roads: the East Bay Children’s Respiratory Health Study. Am J Respir Crit Care Med. 2004;170:520-526.
Morgenstern V, Zutavern A, Cyrys J, et al. Atopic diseases, allergic sensitization, and exposure to traffic-related air pollution in children. Am J Respir Crit Care Med. 2008;177:1331-1337.
Morice AH, et al. The diagnosis and management of chronic cough. Eur Respir J. 2004;24:481-492.
Patel BD, Luben RN, Welch AA, et al. Childhood smoking is an independent risk factor for obstructive airways disease in women. Thorax. 2004;59:682-686.
Peters JM, Avol E, Gauderman WJ, et al. A study of twelve Southern California communities with differing levels and types of air pollution: II. Effects on pulmonary function. Am J Respir Crit Care Med. 1999;159:768-775.
Peters JM, Avol E, Navidi W, et al. A study of twelve Southern California communities with differing levels and types of air pollution: I. Prevalence of respiratory morbidity. Am J Respir Crit Care Med. 1999;159:760-767.
Shields MD, Bush A, Everard ML, et al. On behalf of the British Thoracic Society Cough Guideline Group. Recommendations for the assessment and management of cough in children. Thorax. 2008;63:iii1-iii15.
Snow V, Mottur-Pilson C, Gonzales R, et al. Principles of appropriate antibiotic use for treatment of acute bronchitis in adults. Ann Intern Med. 2001;134:518-520.
Terano C, Miura M, Fukuzawa R, et al. Three children with plastic bronchitis associated with 2009 H1N1 influenza virus infection. Pediatr Infect Dis J. 2011;30(1):80-82.