chapter 69 Conservative Management of Urinary Incontinence
Behavioral and Pelvic Floor Therapy, Urethral and Pelvic Devices
General Considerations
Impact of Urinary Incontinence
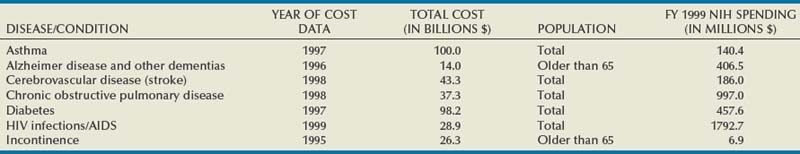
Urinary incontinence (UI) affects people in all strata of society—the young and the old, male and female, rich and poor, all ethnic and racial backgrounds—although women and older individuals bear a disproportionate share of the burden. The impact is enormous. The most recent comprehensive accounting estimated the total annual direct and indirect costs for UI in the United States alone to be $19.5 billion (in year 2000 dollars; Hu et al, 2004). In a 2000 report to Congress, the National Institutes of Health (NIH) estimated the direct cost of UI at $12.5 billion, a figure comparable to other important diseases such as Alzheimer disease, chronic obstructive pulmonary disease, and human immunodeficiency virus infection/acquired immunodeficiency syndrome (Table 69–1). Although medical insurance covers some expenses, patients with UI bear a considerable percentage of the overall disease costs for protective pads, laundry, and other expenses not covered by individual insurance plans. The median out-of-pocket costs to women with weekly or more UI were $186/year (2005 dollars), but those with severe UI spent $372/year and those with very severe UI spent $1148/year (Subak et al, 2007). These costs appear to be increasing rapidly; Medicare expenditures on women with UI increased 57%, adjusted for inflation, between 1992 and 1998 (Anger et al, 2006). As summarized in the Third International Consultation on Incontinence (ICI) Economics Committee (Hu et al, 2005) the consequences of UI lead to serious morbidity, including falls and fractures; urinary tract infections; skin breakdown, including pressure ulcers; and admission to nursing homes. Logically, the presence of lower urinary tract symptoms, including overactive bladder (OAB)/UI symptoms, was found to be associated with approximately 50% increased emergency department visits, hospitalizations, and medical provider visits (Kannan et al, 2009). Despite these figures, and despite markedly increased efforts to inform the public about UI in the past decades, the majority of patients suffer quietly; in one study, fewer than one third of women with bothersome UI in a prepaid health care plan had been diagnosed in the prior 5 years and few had been treated (Kinchen et al, 2007).
The Urologic Diseases in America project has published detailed analyses of expenditures by the U.S. health care system for UI in both men (Stothers et al, 2005) and women (Thom et al, 2005). Among the more interesting findings was a dramatic increase in Medicare expenditures for women from $128.1 to $234.4 million between 1992 and 1998, despite decreasing hospitalizations and length of stay. In men, the prevalence of incontinence was estimated at 17% for those older than 60 years of age (any incontinence over the past 12 months) and the annual expenditures for privately insured male adults with UI were $7702 compared with $3204 for a man without UI. Demographic trends producing increasing numbers of elderly individuals will make this problem a critical challenge to urologists for the coming decades. Those interested in further information are referred to the report of the Epidemiology Committee of the Fourth ICI (Milsom et al, 2009) which provides excellent summaries of prevalence, incidence, remission, and risk factors.
The impact of UI cannot be measured in dollars alone. A “social cancer,” UI impacts every facet—social, physical, sexual, psychological, and medical—of human life at work and at home. Numerous reports continue to document the serious impact of UI and overactive bladder (OAB) on quality of life using high-quality methodology (Coyne et al, 2003, 2004; Avery et al, 2004; Hajjar, 2004). Incontinent patients are more likely to have poor self-esteem, feeling shame and guilt, which may keep them from working effectively and partaking in social activities. UI is strongly associated with depression and vice versa (Steers and Lee, 2001), although the nature of this relationship is not established. Stress urinary incontinence (SUI) restricts the physical activity of patients, many of whom are otherwise healthy young women. The long-term deleterious effects of exercise reduction on health are unclear but may be important. Urgency urinary incontinence (UUI) affects quality of life even more than SUI because of its unpredictable nature. It may lead to loss of sleep as well as the limitations just mentioned. Sexual activity and interpersonal relationships suffer due to UI. Despite this, many and perhaps most individuals still do not seek help for UI for a variety of reasons, including misguided perceptions that UI is a normal consequence of aging and that there is no effective treatment (Shaw, 2001). Even more importantly, expenditures for UI research are a small fraction of that spent on other conditions; NIH-funded UI research is less than 2% than that for stroke or Alzheimer disease (see Table 69–1).
Rationale for Conservative Therapies
This “silent” epidemic was brought to national attention with the 1988 NIH consensus conference (Consensus Conference, 1989) followed by the publication of the first Agency for Health Care Policy and Research (AHCPR) practice guideline on UI in adults in 1992 (Urinary Incontinence Guideline Panel, 1992). Two of the principal recommendations of the guideline relate directly to improving the recognition of UI and validating it as an important medical problem:
A third recommendation of the 1992 AHCPR guideline applies directly to the focus of this chapter:
The rationale for a conservative approach is clear. UI is not an inexorably progressive disease. A moderate delay in surgical therapy does not make such treatment more difficult. As will be amply documented, “conservative” therapies are effective, well tolerated, and safe. In addition, they are preferred by many patients. In the fifth National Association for Continence (NAFC) survey of 130,000 members, 52% of men and 48% of women ranked conservative therapies in general as “most helpful” (NAFC, 1999). Because the impact of UI varies greatly from patient to patient, the patient’s feelings and goals must be taken into consideration in treatment planning. Thus, it is generally appropriate that the least invasive treatment that takes into account patient preferences and offers a reasonable chance for success be used first.
Description: What is “Conservative,” Who is it for?
Traditionally, treatments that are totally reversible have been considered “conservative.” Depending on the reviewer, this may or may not include drugs. However, given the data just presented regarding the costs associated with UI, a more considered assessment is in order. It can no longer be acceptable to call all nonpharmacologic, nonsurgical therapy “conservative” and recommend that such treatments routinely be employed as initial therapy. “Conservative” therapies must be held to the same standards as pharmacologic and surgical treatments. Effectiveness and safety must be determined in the context of patient satisfaction—it does little good to identify a treatment as safe and effective if patients are ultimately not satisfied and go on to “second line” treatments. Ultimately, the clinician and patient need to know the long-term outcomes for continence, adverse events, and cost-effectiveness to develop appropriate treatment algorithms. Ultimately, specific patient factors should be identified that will help predict the likelihood of response to a given therapy. Only then will the precious resources spent on UI be used most effectively.
To this end, levels of evidence and grades of recommendation are used whenever possible and conclusions drawn from systematic literature reviews and the ICI are highlighted. The AHCPR has used specified evidence levels to justify recommendations for the investigation and treatment of a variety of conditions, and the Oxford Centre for Evidence Based Medicine produced a widely accepted adaptation of the work of the AHCPR (Oxford Centre, 2001). The highest level of evidence (level 1) is based on systematic reviews and/or randomized controlled trials. The highest grade of recommendation (grade A) stems from consistent level 1 evidence. When possible these terms are used in the chapter, although there is a great need for further high-quality research and there is a notable lack of long-term follow-up of treatment effect for almost all of the therapies discussed.
Overview
The challenge to the urologist is to understand all of the treatments for UI, to be able to assess the patient efficiently yet thoroughly, and then to construct an appropriate treatment plan with the patient, taking into account the problem and the goals of the individual patient. A review is presented of the basic nonsurgical tools used in treating UI—behavioral therapy, pelvic floor muscle training and biofeedback, external devices, and peripheral electrical and magnetic stimulation. Pharmacologic therapy is discussed in Chapter 68, surgical and injection therapy in Chapters 71 to 74, and neuromodulation in Chapter 70. The detailed evaluation of the incontinent patient is discussed in Chapter 64. Although it is important to rule out serious underlying or associated conditions, invasive testing is rarely required before initiating treatment with the measures discussed here. The focus here is on only that part of the evaluation that directly relates to treatment planning. It is generally sufficient to have a working diagnosis classifying the patient as having stress, urgency, or mixed UI. Patients with symptoms of OAB (the generic term for urgency and frequency with or without UI) are treated in the same manner as those with UUI. Practical algorithms are presented that provide a rational means of applying these varied treatments to an individual patient.
Key Points: General Considerations
The Tools of Conservative Therapy
Behavioral Therapy
Behavioral therapy describes a group of treatments grounded in the concept that the incontinent patient can be educated about his or her condition and develop strategies to minimize or eliminate UI. It is sometimes erroneously reduced to the combination of fluid restriction and timed voiding but is actually a far richer therapy. In fact, one of the main problems with the term behavioral therapy is that it has been used so differently by various practitioners that its meaning has become diluted and vague. There is no one standard protocol or “best” methodology. The shared aims of behavioral therapy are illustrated in Figure 69–1 (Payne, 2000). Although various practitioners may have different emphasis, the different treatment approaches are unified by education about normal urinary tract function. The individual elements of behavioral therapies discussed here are centered on basic educational techniques such as operant learning, which is intended to model activity so as to reproduce normal behavior, in this case urinary continence (Palmer, 2004). All of the individual techniques discussed fall into this category, although pelvic floor muscle training is both a behavioral therapy (education about anatomy and function of the muscles, learning to use the muscles properly to control lower urinary tract function) and a physical therapy (strengthening the muscles to improve function). In any case, education binds the various techniques together and plays the central role in behavioral therapy.
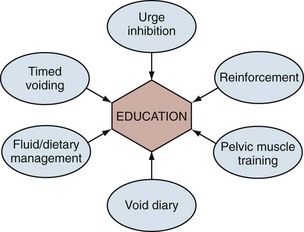
Figure 69–1 Behavioral therapy has many different components, all of which center on patient education. Education leads to understanding of normal lower urinary tract function and the patient’s ability to self-regulate those functions.
Pelvic Floor Education
In a pure form of behavioral therapy there would simply be education about the normal anatomy of the pelvic floor musculature and the function of these muscles in maintaining continence. Pelvic muscle exercises could be explained along with the use of repeated rapid contractions (“quick flicks”) to inhibit urgency by activating the spinal reflex pathway. However, the literature also includes sophisticated biofeedback techniques and treatment by physiotherapists in many trials of behavioral therapy. In most of these cases the purpose of the treatment is aimed at improving muscle strength and control; therefore the detailed discussion of this approach is included in the section on pelvic floor rehabilitation (see later).
Bladder Training/Timed Voiding
The term scheduled voiding is the generic term preferred for describing voiding regimens used for home-dwelling cognitively intact patients as opposed to prompted voiding or toileting, terms properly applied to institutionalized or otherwise dependent patients. The most commonly used technique for patients with OAB and UUI is “bladder training” (“bladder drill,” “bladder retraining”). Bladder training starts a patient voiding on a fixed time interval schedule with the intention that, most of the time, the patient will urinate before experiencing urgency and UI. The interval is gradually increased with clinical improvement. Early practitioners of bladder training first established the effectiveness of intensive inpatient bladder training temporarily supplemented by medications (Frewen, 1978). Next, outpatient treatment was proven to be effective (Elder and Stephenson, 1980; Frewen, 1980). Finally, the durability of response, with 85% initial and 48% three-year response rates was reported (Holmes et al, 1983). Bladder training should always be combined with urge inhibition techniques and is often combined with anticholinergic medical therapy, particularly for more severe cases and for patients with neurogenic bladder. In contrast, “timed voiding” involves having a patient void on a fixed schedule, typically every 2 to 3 hours, and is intended to normalize frequency in a patient with infrequent voiding and/or diminished bladder sensation. This technique can be employed for patients with SUI with the idea that leakage will be less if the bladder is less full when physical stress occurs. It can also be used in a variety of patients with UUI who have a good bladder capacity (the classic example is that of patients with diabetic neurogenic bladder; they do not have proper bladder sensation and thus delay voiding inappropriately).
Although there is no evidence as to the optimal program of bladder training, a common approach is to start at a safe interval based on the patient’s bladder diary and increase the interval by 15 to 30 minutes as the patient achieves continence. The ultimate goal is a comfortable interval between voids with continence—a “retraining” of the bladder. Wilson and colleagues (2005) concluded that if no improvement is made after 3 weeks “the patient should be reevaluated and other treatment options considered.” The ICI authors acknowledge that the quantity of evidence is low but still make a grade A recommendation that “bladder training is recommended as a first line treatment of UI in women” (Hay-Smith et al, 2009). Medical therapy is commonly employed initially, and there are rather limited studies comparing bladder training to anticholinergic medications for detrusor overactivity and UUI. One study demonstrated superior results with the combination of behavioral therapy and anorectal biofeedback in comparison to anticholinergic medication alone (oxybutynin chloride in titrated dosing) in a group of patients with urge and mixed UI (Burgio et al, 1998). Subsequent work by Burgio and colleagues (2000) confirms the clear truth that behavioral and drug therapy are complementary, not competitive treatments; the primary value of this research is to underscore the value of the behavioral component in the treatment plan. Logically, all patients should have behavioral therapy with medications used on an individualized basis. The ICI committee (Hay-Smith et al, 2009) found level 2 evidence that the effect of bladder training may be enhanced by drug therapy and that, for women already taking an antimuscarinic drug, there was no additional benefit from adding brief written instructions on bladder training.
Smoking
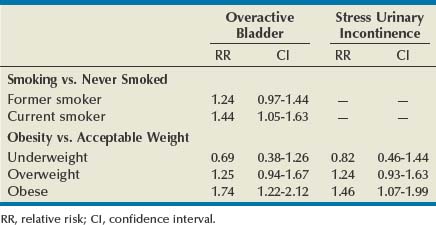
Aside from being a major risk factor for bladder cancer, smoking has been proposed as a risk factor for SUI by increasing coughing episodes and for OAB through bladder irritation from nicotine and toxins excreted in the urine. However, to date, epidemiologic studies of tobacco use have produced inconsistent findings. In women, some studies suggest that smoking increases the risk of UI, or at least severe UI, but others demonstrate no increased risk. A 1-year longitudinal study of 6424 women older than 40 years of age found that current smokers were at higher risk for both SUI and OAB than those who had never smoked, although statistical significance was seen only for OAB (Table 69–2). Former smokers had intermediate risk (Dallosso et al, 2004a). The same group also published a longitudinal study of 4887 men showing no association between smoking and OAB symptoms (Dallosso et al, 2004b). Another population-based study suggested that the risk of UI is limited to subjects with a history of more than 15 pack-years or current consumption of more than 20 cigarettes a day (Hannestad et al, 2003). One group of researchers found that smoking was associated with the severity of UI in women seeking surgical treatment (Richter et al, 2005). As of yet there have been no adequate studies of smoking cessation on prevention or treatment of UI symptoms, and the ICI committee could make no evidence-based recommendation (Hay-Smith et al, 2009). Despite these conflicting results, smoking cessation can logically be recommended as a general health measure, to reduce the risk of bladder cancer, and for those smokers with SUI particularly related to coughing. More research, particularly on the effectiveness of smoking cessation in treating UI and on the relationship between smoking and OAB, would be welcomed.
Caffeine
Caffeine is well known as a nervous system stimulant and has demonstrable effects on detrusor muscle in vivo and in vivo, promoting detrusor overactivity. In one study of community-dwelling elderly women subjects who decreased caffeine and increased fluid intake, increased voiding volumes and fewer accidents were experienced (Tomlinson et al, 1999). High caffeine intake (>400 mg/day average) also correlated with urodynamic detrusor overactivity compared with stress-incontinent women (<200 mg/day average) (Arya et al, 2000). Caffeine has thus been postulated to be a cause of OAB symptoms, and caffeine reduction has been advised for OAB patients. Epidemiologic data are less clear. Reports usually break down consumption by type of beverage rather than actual caffeine consumption as is typically found in smaller studies of dietary intervention. Such small studies have typically shown a correlation between caffeine reduction and reduction of UI (Tomlinson et al, 1999; Bryant et al, 2002). Although larger studies would be helpful, it seems appropriate to recommend restriction, particularly in those patients with very high intake of caffeine. The ICI committee concluded that, “while large cross-sectional surveys indicate no association (level 3 evidence), small clinical trials do suggest that decreasing caffeine intake improves continence (level of evidence 2)” (Hay-Smith et al, 2009).
Fluid Management
Fluid restriction has been advocated in the treatment of both SUI and OAB. The rationale is that abdominal leak pressures appear to be volume dependent; therefore, physical stress occurring at lower bladder volumes both will be less likely to cause UI and will be associated with lower volume loss when leakage does occur. Similarly, OAB is believed to be a volume-driven phenomenon and slower filling promotes bladder compliance and lower pressures. This concept appears to be well accepted, as manifest by a recent U.S. study in which 38% of incontinent women had tried limiting fluids compared with 21% who tried Kegel exercises and 6% using prescription medications (Diokno et al, 2004a). On the other hand, extreme fluid restriction produces concentrated urine, which has been postulated to be a bladder irritant, leading to detrusor overactivity as well as constipation, which can negatively affect bladder function. Indeed, there is conflict regarding fluid management, with some investigators showing improvement with fluid reduction (Swithinbank et al, 2005) and others finding that increasing fluid intake improved UI (Dowd et al, 1996). Hashim and Abrams (2008) studied this controversy using a crossover trial in which patients with OAB were instructed to first decrease fluid intake 25% to 50% below baseline and then to increase intake 25% to 50% above the baseline (or the reverse). A significant improvement in continence was noted with decreasing fluids (and increased UI was noted with increasing fluids). It certainly seems reasonable to obtain a baseline frequency-volume chart and advise those patients with normal to increased fluid intake to try moderately restricting fluid intake. Any potential benefit must be balanced against possible problems with bladder infections and constipation.
The types of fluid intake may also be significant; caffeinated beverages, acidic juices, and alcohol have been suggested to be bladder irritants. Caffeine was discussed earlier. Tea consumption correlated with UI in the EPINCONT study, but alcohol and coffee consumption did not (Hannestad et al, 2003). Although it is possible that caffeine is not the critical component, there is no clear hypothesis as to why tea (which has less caffeine than coffee) would be a relevant dietary factor but not coffee. Unless confirmed in other studies this may turn out to be a statistical aberration. In a population of Chinese women, alcohol consumption correlated with SUI but not UUI (Song et al, 2005), but other studies have shown no link between alcohol use and UI symptoms. Epidemiologic data support a link between consumption of carbonated beverages with both SUI and OAB (Dallosso et al, 2003) with level 2 to 3 evidence. Data on the effects of other beverages are scarce.
Other Dietary Management
A variety of different dietary maneuvers have been recommended for incontinent patients, including avoidance of alcohol, carbonated beverages, acid foods, salt, and others. A detailed study examining dietary factors noted an association between intake of vegetables with lower risk of OAB and intake of fruit with lower risk of SUI (Dallosso et al, 2003). These observations have yet to be reproduced in other populations. A follow-up report (Dallosso et al, 2004a) examined details of the diet in relationship to SUI and found that “intakes of total fat, saturated fatty acids and monounsaturated fatty acids were associated with an increased risk of SUI onset one year later. Of the micronutrients studied, zinc and vitamin B12 were positively associated with SUI onset.” Because these observations are not hypothesis driven, confirmation is mandated before any recommendation can be given. No similar connection was identified with alcohol consumption in the same or other studies, and one study actually suggested a lower risk of OAB in men who were beer drinkers (Dallosso et al, 2004b). Once again, the best advice for incontinent patients would seem to be to follow established guidelines for overall health with moderation in alcohol use and adequate intake of fruits and vegetables.
Obesity/Weight Reduction
In no area of behavioral therapy has there been more advancement than in the recent work delineating obesity as a serious, modifiable, and indeed reversible cause of UI. There has long been an assumption that obesity is linked to UI, particularly SUI, by straining and potentially damaging the supportive structures of the bladder and pelvic organs. However, until recently little has been known about the true risk of obesity or about the effectiveness of weight loss as a therapy for UI.
Epidemiologic data provide persuasive support for a causal association between obesity and UI. The Nurses’ Health Study identified 6,790 women with incident UI over a 2-year period among 35,754 women initially reporting no UI (Townsend et al, 2008a). There were highly significant trends of increasing risk of UI with increasing BMI and waist circumference (P for trend < .001 for both). The same research group showed that not only was obesity a risk for UI but also that weight gain was an independent risk for incident UI (Townsend et al, 2007). Gaining 5 to 10 kg after age 18 increased the risk of developing weekly UI by 44% (OR 1.44, CI 1.05 to 1.97) compared with women who maintained their weight within 2 kg—regardless of the initial weight! Gaining 30 kg increased the risk fourfold. The relationship between obesity, weight gain, and incident UI held for both SUI and UUI. Another powerful study from the United Kingdom followed 1201 women from their birth in 1946 annually from 48 to 54 years (Mishra et al, 2008). At the age of 20, 26, 36, and 43, body mass index (BMI) was positively associated with stress symptoms and severe UI in midlife and there appeared to be a cumulative effect. These relationships existed even after accounting for the effects of aging, childhood enuresis, childbirth characteristics, menopause, educational attainment, and smoking status. Interestingly, these researchers found that BMI was not significantly associated with symptoms of UUI. Finally, in a 1-year longitudinal study of 6424 women older than 40 years of age there was a strong correlation between BMI and the risk of both OAB and SUI (Dallosso et al, 2003) (see Table 69–2). Work in other populations consistently confirms the relationship between obesity and UI (Thom et al, 1997; Brown et al, 1999; Fornell et al, 2004; Larrieu et al, 2004; Melville et al, 2005b). The work in this field provides consistent findings describing a linear relationship between obesity and UI that cumulatively produce conclusive evidence for obesity as an important modifiable risk factor for UI.
More importantly, it is now clear that weight loss is an effective treatment for UI. A prospective randomized controlled trial (RCT) studied 338 overweight and obese women at two centers in the United States (Subak et al, 2009). All had BMI greater than 25 (mean 36) and at least 10 UI episodes per week (>75% with mixed UI). Subjects were randomized to an intensive 6-month weight loss program that included diet, exercise, and behavior modification (226 patients) or to a structured education program (112 patients). Weight loss was moderate (−8 kg vs. −1.6 kg), yet statistically significant decreases were found for all UI (−47% vs. −28%) and SUI (−58% vs. −33%). There was a trend to reduction in UUI (−42% vs. −14%, P = .14). More patients rated themselves to be moderately or very satisfied with the change in UI (75.8% vs. 46.8%, P < .001). These strong results were mirrored in a 2-year open-label trial in the United Kingdom of 64 obese women who were offered a commercially run program of diet and exercise (Rosemary Conley Diet and Fitness Clubs) aiming for weight loss of 5% to 10% over 6 months (Auwad et al, 2008) and by the initial pilot RCT by Subak and colleagues (2005). The high value of bariatric surgery for morbidly obese patients with UI has been clearly documented in four different case series, all showing dramatic reduction in UI episodes (Burgio et al, 2007; Kuruba et al, 2007; Maher et al, 2008, Laungani et al, 2009) (Table 69–3).
In summary, it is clear that obesity is a cause for UI and weight loss is an effective treatment that also provides many other health benefits. Weight loss should be first-line therapy for obese patients with UI, particularly SUI. More research is needed as to the role of obesity and weight loss in patients who are only overweight (BMI 25 to 30) and into the intriguing but less well defined relationship with UUI.
Urge Inhibition
Urgency is the predominant symptom for most patients with OAB and UUI. In many cases, UI has become a reflex as the patient has developed dysfunctional behavior in response to urgency. It is important to break the cycle of rushing to the toilet in response to urgency. The patient is instructed to stop, sit if possible, and use pelvic muscle contractions, distraction, or other techniques until urgency passes. There are no data on use of this practice as an isolated therapy, but it is a critical part of the overall treatment of patients with OAB; patients who learn to delay or overcome urgency may eventually be cured of their condition.
Other Lifestyle Issues
As summarized by Wilson and associates (2005), “there are many other lifestyle interventions suggested either by health care professionals or the lay press for the treatment of UI, including reducing emotional stress, wearing non-restrictive clothing, utilizing a bedside commode, decreasing lower extremity edema, treating allergies and coughs, wearing cotton underwear, and increasing sexual activity. These interventions are, however, all anecdotal in nature.”
It has been shown (level 2 evidence) that stress-induced urine loss can be reduced by positional changes, that is, having a woman cross her legs with coughing, although this was only examined in the acute urodynamic testing situation (Norton and Baker, 1994). The ICI panel (Hay-Smith et al, 2009) concluded that constipation and chronic straining may be a risk factor for the development of UI (level 3 evidence) but that there were no data on the effect of intervention.
There is inadequate knowledge about the relationship of strenuous activity, sports, and work. It has long been known that low-grade SUI is common in female college athletes (Nygaard et al, 1994). However, the long-term clinical significance of this is unknown. Because women are universally advised to restrict activity after surgical treatment for SUI one might assume that the effect of postoperative activity on outcome had been investigated, but there are actually no useful data. One study has examined the association between physical activity and risk of developing UI by analysis of data from incident cases of UI in the Nurses’ Health Study of women aged 54-79 years (Danforth et al, 2007). The authors found that increasing levels of total physical activity (which was mainly walking) were significantly associated with a reduced risk of UI and SUI although not with UUI (Table 69–4). The same researchers examined the dataset to explore the relationship for younger women, age 37 to 54 (Townsend et al, 2008b). They again found that activity was inversely correlated with incident UI and that the protective effect of exercise appeared to be present for both SUI and UUI. Accounting for the independent effect of BMI attenuated the findings, but exercise remained a significant independent factor. Although it is possible that these data may not apply to women engaged in very high level, chronic, strenuous activity, the important lesson is that good health habits clearly correlate with a lower risk of UI.
Table 69–4 Physical Activity and Urinary Incontinence
| Any Incontinence | ||
|---|---|---|
| Cases* | OR† (95% CI) | |
| Physical Activity (MET-hr/wk) | ||
| Quintile 1 | 524 | Referent |
| Quintile 2 | 534 | 1.04 (0.92-1.18) |
| Quintile 3 | 465 | 0.90 (0.79-1.02) |
| Quintile 4 | 434 | 0.85 (0.75-0.98) |
| Quintile 5 | 398 | 0.81 (0.71-0.93) |
| P for trend | <.01 | |
| Walking (MET-hr/wk)‡ | ||
| Quintile 1 | 524 | Referent |
| Quintile 2 | 524 | 1.01 (0.88-1.14) |
| Quintile 3 | 470 | 0.91 (0.80-1.04) |
| Quintile 4 | 463 | 0.90 (0.78-1.04) |
| Quintile 5 | 374 | 0.74 (0.63-0.88) |
| P for trend | <.01 | |
MET, metabolic equivalent task; OR, odds ratio; CI, confidence interval.
* Incontinence cases were defined as leaking urine at least once per week.
† Odds ratios were adjusted for age, race or ethnicity, body mass index, parity, cigarette smoking, and postmenopausal hormone therapy.
‡ Walking analyses were controlled for total activity.
From Danforth KN, Shah AD, Townsend MK, et al. Physical activity and urinary incontinence among healthy, older women. Obstet Gynecol 2007;109(3):721–7.
The ICI committee concluded that “strenuous exercise is likely to unmask the symptoms of SUI during the provocation. There is currently no evidence that strenuous activity causes the condition of UI” and “there is good prospective cohort information suggesting that moderate exercise decreases the incidence of UI in middle aged and older women” (level of evidence II) (Hay-Smith et al, 2009).
Other Health Issues
Increasing attention has been paid to the relationship between diabetes and UI. Data from the Nurses’ Health Study and the Nurses’ Health Study II were examined to study associations between diabetes and UI type in 71,650 women (Danforth et al, 2009). Incident UI cases were identified over a 2-year period. The incidence of at least weekly UI was 5.3% among women without type 2 diabetes and 8.7% among women with diabetes (adjusted odds ratio 1.2; 95% CI 1.0 to 1.3; P = .01). This increase appeared largely explained by significantly greater odds of UUI (OR 1.4; 95% CI 1.0 to 1.9; P = .03). A cross-sectional mail survey of community dwelling women screened women for pelvic floor disorders while diabetes status and other risk factors were obtained from medical record review. Of 3,962 women, 393 (10%) had diabetes. Among women with diabetes, being obese was associated with SUI and OAB (Lawrence et al, 2007). This finding was supported by analysis of an RCT comparing treatment of diabetics with UI using oral hypoglycemics (metformin) versus lifestyle intervention (weight loss and exercise) (Brown et al, 2006). The researchers found that the prevalence of UI was lower in women randomized to lifestyle intervention than those on medication or placebo.
There is a strong relationship between UI and depression. In one study, patients with UI were almost three times more likely to have major depression than those without (6.1% vs. 2.2%) and patients with UI and depression had significantly greater decrements in quality of life and functional status than those with UI alone (Melville et al, 2005a).
A specific investigation into comorbidities and UI was undertaken by a research group in the United Kingdom who note, “The bladder forms part of a sensitive and complex system of the body that operates largely autonomously but remains under voluntary control. As such, it is vulnerable to general disease processes related to ageing. Thus, associations with poor health and obesity could represent pathogenic as well as functional involvement of the bladder.” (McGrother et al, 2006). They used a postal survey of a random sampling of over 19,000 community dwelling women older than 40 years of age with 1-year follow-up surveys. There were strong correlations between poorer health and increased prevalence of both SUI and OAB. There are many interesting findings but one of the most important is that “OAB was independently predicted by poor health … The association with old age, although consistent with other studies, disappeared after controlling for a full range of specific comorbidities, suggesting that the condition is age related rather than age dependent.”
Key Points: The Tools of Conservative Therapy: Behavioral Therapy
Concept of “Therapeutic Package”
It is, of course, logical and appropriate that these various behavioral treatments be used together in a therapeutic package. They are inherently complementary, are completely reversible, and lack significant adverse effects. They also can be (and typically are) combined with the pelvic floor rehabilitation techniques discussed later. Patients who are motivated to avoid surgical or pharmacologic interventions will be inclined to seek out such treatments; the problem remains as to the most efficient way to deliver therapy to the average patient.
Such an approach showed utility in an RCT in which patients were randomized to receive a package of behavioral therapy including individualized counseling about fluid and caffeine intake, quick pelvic floor muscle contraction, voiding frequency, and management of constipation or placed on a waiting list (Kincade et al, 2007). This design provides a good starting point for use in many patients with UI. In addition, Diokno and colleagues (2004b) demonstrated that two simple group sessions were effective in preventing UI. The trial randomized 359 continent women older than age 55 years who were recruited and observed for 1 year. The treatment group received a single 2-hour group session teaching pelvic floor muscle training (PFMT) and bladder training as well as an audiotape on PFMT for reinforcement; the control group was not treated. There was one follow-up office visit with a nurse specialist in 2 to 4 weeks. At the end of 1 year, 56% of the treatment group reported the same or better continence compared with only 41% of controls; 37% of the treatment group reported “absolute continence” compared with 28% of the controls. Several other studies using various combinations of therapies led the ICI group (Wilson et al, 2005) to a level A recommendation that “women with stress, urge, or mixed incontinence should be offered a conservative management program as first line therapy for UI” while acknowledging that there were inadequate data to define the optimal treatment package and that most of the data comes from trials in older women.
Pelvic Floor Rehabilitation
The term pelvic floor rehabilitation should be applied to any treatment intended to increase the strength, bulk, and function of the pelvic floor/levator muscles. Muscle rehabilitation can be achieved through a variety of tools, which can be classified as “basic” or “advanced,” depending on the cost and complexity of the therapy. Basic treatments (Kegel exercises, vaginal cones, and simple home perineometry) are used in the home setting, require no or inexpensive equipment, and can be administered by a relatively unskilled therapist. Advanced treatments are used in an office setting, are administered by a trained therapist, and/or require more sophisticated equipment.
Pelvic Floor Muscle Training
Pelvic floor exercises (PFE) or “Kegels” have been advocated in the treatment of UI since the 1950s (Kegel 1948a, 1948b, 1956). Dr. Kegel taught his patients to forcefully contract the pelvic muscles to treat and prevent postpartum UI. Unfortunately, half of patients are unable to perform a proper contraction with simple instructions (Bump et al, 1991), and up to one fourth will actually promote UI with their efforts. Indiscriminant recommendation of this therapy to all incontinent patients has created a negative bias that PFE are just “something to do” before pharmacologic or definitive surgical treatment. It should also be acknowledged that Dr. Kegel routinely employed a perineometer with his patients in one of the first documented uses of biofeedback in medicine. This implies that Kegel exercises alone should not be considered the standard treatment for pelvic floor rehabilitation.
Currently, PFMT is the currently accepted term, replacing Kegels and PFE. It is defined as “any program of repeated voluntary pelvic floor muscle contractions taught by a health care professional” (Wilson et al, 2005) and is advocated for both prevention and treatment of UI. “Training” is preferred over “exercises” to emphasize the importance of a regimen of repeated exercise over time. The therapy is intended to improve the function of the pelvic floor muscles; whether this happens primarily by increasing the strength, power, and speed and/or improving the timing and coordination of a contraction is not known. Much more is known about the clinical effectiveness of the therapy than the exact physiologic changes that produce the outcomes. With repeated exercise a muscle will develop improved responsiveness that may lead to a faster and/or stronger contraction before any increase in actual bulk. Over longer periods of time the muscle fibers will progressively hypertrophy, producing increased bulk. The ICI committee (Hay-Smith et al, 2009) points out that (1) an increase in strength occurs before visible hypertrophy, (2) early improved strength results from neural adaptation, and (3) hypertrophy begins only after a minimum of 8 weeks and may continue for some years. In any case, muscle bulk is clearly not a prerequisite for improved continence with PFMT, and many studies have failed to show a correlation between improved strength and improved continence.
Although a huge number of studies have investigated PFMT it is challenging to draw clear conclusions for a variety of reasons:
When possible these issues are addressed in the following discussion. The interested reader is strongly encouraged to study the comprehensive review performed by the ICI committee (Hay-Smith et al, 2009).
It is difficult to analyze the literature on PFMT because training is commonly combined with other modalities. The combination can range from general advice about management of UI, specific strategies such as bladder diaries/timed voiding, and biofeedback training. It is important to realize how differently PFMT is applied in research trials and in European practice compared with standard U.S. clinical practice. The definition of PFMT clearly states that it is “taught by a health care professional.” In clinical trials this is always the case; the instructor is typically a skilled physiotherapist who may apply a variety of techniques to promote learning as needed by an individual patient. In U.S. clinical practice a patient is often given only verbal instruction. In more ideal cases the patient will have written instruction after an initial assessment including a pelvic examination and assessment of and instruction in pelvic muscle contraction. Regular follow-up is not an option in most health care settings owing to poor or absent reimbursement. This divergence in care means that results obtained in research trials may be difficult or impossible to obtain in standard clinical practice and that the difference between stand-alone PFMT and PFMT with adjunctive treatments may be much greater in U.S. clinical practice than in research studies. The following discussion of PFMT results is based on the published literature; it remains a challenge for the clinician to translate these somewhat idealized results into clinical practice.
Does PFMT Prevent Urinary Incontinence in Childbearing Women?
PFMT has been used to prevent UI in pregnant women, because pregnancy and vaginal birth are strong risk factors for UI. Three prospective RCTs have investigated this question (Sampselle et al, 1998; Reilly et al, 2002; Mørkved et al, 2003), producing a grade A recommendation that primiparous women “should be offered a supervised and intensive strengthening antepartum PFMT programme to prevent post-partum UI” (Hay-Smith et al, 2009). In all three trials the PFMT group had less postpartum UI; and in one study (Reilly et al, 2002), the effect was still present at 4 years postpartum, although only 100 of 268 patients were available for follow-up. There was no evidence supporting the utility of PFMT in pregnant, previously continent, multiparous women. The evidence reviewed by the ICI group for postpartum PFMT was less compelling, but it was concluded that the evidence also supports a grade C recommendation for supervised intensive PFMT for women after delivery utilizing instruments or delivery of a large infant (≥4000 g) (Hay-Smith et al, 2009).
Is PFMT an Effective Treatment for Urinary Incontinence and Which Patients Are Good Candidates?
The most important question is whether PFMT is effective in the management of established UI and, if so, how to most effectively apply the therapy to the large population of incontinent women. Seventeen RCTs comparing PFMT to no intervention, placebo, sham, or control in childbearing women were summarized by Hay-Smith and colleagues (2009) and are based on the findings of a Cochrane review (Dumoulin et al, 2008). Although the magnitude and duration of the expected effect is not well defined, the overwhelming majority of the trials showed effectiveness of PFMT, leading to a grade A recommendation that “PFMT should be offered, as first line therapy, to all women with stress, urge or mixed incontinence.” This recommendation is, of course, based on the combination of demonstrable effectiveness with essentially no risk to the patient. It does not mandate that all patients undertake a formal course of PFMT before surgical treatment. However, there are as yet no studies that adequately define patient groups who are unlikely to respond to PFMT based on clinical factors such as age, obesity, and so on. In most cases, studies have been too small to examine such predictive factors and there may well be a role for individualized therapeutic recommendations if proper studies could be done. A single trial investigating PFMT for UI in pregnancy did not show efficacy (Woldringh et al, 2007), but this has been criticized owing to lack of specificity about the intervention. The ICI committee specifically points out the need for a large scale, pragmatic trial of PFMT with detailed reporting of long-term (>5 years) follow-up (Hay-Smith et al, 2009). The authors also describe what they believe are the key components of an effective program: (1) it is based on sound principles (specificity, overload, and progression), (2) correct contraction is confirmed before training, and (3) women are supported to maintain treatment adherence (level of evidence 4).
The quality of the data for men with postprostatectomy UI is less robust with too many single-center, underpowered trials. Research has focused on use of pelvic floor therapies to hasten return of continence. A systematic review of the literature concluded that although training may hasten return of continence after surgery it does not affect long-term outcome (MacDonald et al, 2007). Others have found that there is no difference between routine preoperative and postoperative PFMT (Filocamo et al, 2005). In contrast, one RCT found that significantly more men were completely continent 1 year after prostatectomy when performing PFMT with a physiotherapist than without (Overgard et al, 2008). When PFMT is used for established postprostatectomy UI, the results similarly do not establish long-term benefit of therapy, with or without biofeedback. Interested readers are referred to a Cochrane review on the subject (Hunter et al, 2004).
Within these overarching categories it is likely that specific patient subgroups are more or less likely to benefit from PFMT (and probably other conservative techniques). However, studies have not been designed to specifically identify such groups and subgroup analysis of relatively small trials is fraught with error. In one retrospective series of 447 women with SUI, 49% of patients were “successfully treated.” Success appeared to be more likely in patients with milder degrees of UI (not using pads: 67% success; not having daily UI: 63% success; no leakage at first cough: 60% success). Independent risk factors for failure included two or more leaks per day, presence of leakage at first cough, and use of antidepressant/anxiolytic medications (Cammu et al, 2004). Burgio (2004) argues that PFMT is equally effective in treating UI with stress, urge, and mixed UI, whereas Goode (2004) commented that there are as yet no reliable predictors of treatment outcomes.
Other Benefits of PFMT
A small pilot study demonstrated effectiveness of PFMT in symptoms of prolapse and in objective prolapse stage for women with symptomatic stage I or II prolapse (Hagen et al, 2009). If confirmed in larger studies with long-term follow-up this could have a major impact on women’s health.
Implementing PFMT in Clinical Practice
If PFMT is to be accepted as standard therapy, one must also define how to implement training in clinical practice. Many articles have evaluated different regimens and use of ancillary tools, but in many cases quality is lacking. In evaluating the literature it is important to ensure that groups being compared are truly equivalent in all parameters other than that being tested. Key elements include the usual demographic characteristics, the degree of supervision (expertise and contact time), the intensity of the exercise program (number, frequency, and duration of contractions), use of ancillary tools, and, of course, the length of the program and follow-up. The ICI group (Hay-Smith et al, 2009) concluded that:
A representative strengthening program for PFMT used by the author suggests:
In addition to the strengthening program the author recommends use of the “knack” maneuver to promote timing of the contraction coincident to coughing for SUI patients (Miller et al, 2001, 2008) and “quick flicks,” which are short, rapid contractions intended to inhibit urgency for those with OAB symptoms.
Long-Term Results of PFMT
The need to practice PFMT is probably lifelong, and patients should be counseled to integrate exercise into their normal routines. Bo and colleagues (2005) reported a 15-year follow-up of a randomized trial between intensive exercise therapy and home exercises in women with SUI. Ninety percent of the subjects were located and responded to a questionnaire. Half of the women in both groups eventually underwent surgical treatment. It is clear that exercises were not continued (28% exercise at least weekly and 36% never exercise). Although most women in both groups were satisfied with their current condition, “the marked benefit of intensive PFMT seen short-term was not maintained 15 years later.” The author suggests that training one to two times a week at high intensity is enough to maintain muscle strength. A second group was able to query 79 of 123 women with SUI or mixed UI initially treated with PFMT after 8 years. Again, less than 10% continued training throughout the interval (Kondo et al, 2007). Yet 39% of the group considered the treatment to be successful (>50% improvement). On the other hand, Cammu and colleagues (2000) found that 16 of 24 patients (67%) originally satisfied with PFMT remained so after 10 years and only 2 of 24 (8%) went on to surgical therapy. They attributed sustained improvement to patients’ use of a voluntary pelvic muscle contraction (“perineal lock” or “knack”) before physical stress.
PFMT versus Other “Conservative” Modalities
PFMT has also been compared against other therapies, including various forms of vaginal cones, electrical stimulation, bladder training, and pharmacologic therapy. Again, most of the individual trials are relatively underpowered, often differ in the degree of therapist contact/supervision, and have a short follow-up. In addition there is always a selection bias because some women will not accept use of vaginal devices such as cones, biofeedback probes, or stimulators. The following conclusions regarding first-line therapy came from the ICI committee (Hay-Smith et al, 2009):
For women with SUI or mixed UI:
For women with UUI or mixed UI:
Although it is scientifically desirable to understand the relative value of each of these conservative treatments, the practical fact is that these are generally complementary tools. In particular, bladder training and PFMT are useful for all types of UI and require no specialized equipment. Wyman and colleagues (1998) found that adding PFMT to bladder training produced superior short-term results. Another clinical trial demonstrated the feasibility of using a booklet to provide an initial “package” therapy to be used without ongoing supervision (Goode et al, 2003). The standardized booklet was compared with one group receiving office behavioral training and another that used home vaginal electrical stimulation. Episodes of UI were reduced by 52.5%, 68.6%, and 71.9%, respectively. Although the booklet approach was inferior to the more intensive treatment, the results, including a statistically significant improvement in quality of life in all groups, suggest that most cost-effective way to deliver therapy would be to begin with such a booklet, reserving office-based therapy requiring expert clinicians for those not satisfied with the initial results.
Key Points: Pelvic Floor Rehabilitation: Pelvic Floor Muscle Training
Biofeedback
Biofeedback is any method of training a patient to control a bodily function by providing him or her with information about that function. In this case, the patient hopes to gain control of, and strengthen, the pelvic muscles. The tools range from inexpensive vaginal cones to office systems costing thousands of dollars. One must remember, however, that no study has ever demonstrated that a particular device is responsible for restoring continence. On the contrary it is clear that the motivation of the patient and the experience and ability of the therapist are the critical factors for success (Bo et al, 1990a). Published articles in the UI literature typically involve trained physical therapists specializing in UI and pelvic floor problems. In contrast, the biofeedback therapist in the “real world” setting is often a nurse or technician possessing only brief training in the subject. Finally, biofeedback is only a tool, a teaching technique; real improvement in muscle strength and continence is dependent on the patient’s work outside the office. The bottom line with all of these pelvic floor therapies is that no piece of equipment is as important as the effort the patient puts into the program.
Palpation Only
Most of the literature on PFMT assumes at least initial basic evaluation and teaching by digital assessment of a pelvic floor muscle contraction during pelvic examination. This may or may not be performed at all follow-up visits. Although this is clearly an example of biofeedback, in the PFMT literature the term biofeedback is generally reserved for studies using sophisticated electronic or pressure devices to record pelvic floor activity.
Vaginal Cones
Sets of cones sold with varying numbers of cones ranging from 3 to 9, each of different weight, have been marketed for treatment of UI for many years (Fig. 69–2). The patient is instructed to insert a cone in the vagina above the levator muscles and hold it in for 15 to 20 minutes while in the erect position. The concept is that the sensation of the cones slipping down or falling out of the vagina will provide biofeedback to the patient to learn to identify and contract the pelvic muscles. Initially, the patient may only be able to hold the cone at rest for a short time but as she gains strength she will be able to retain the cone with normal activity. She then progresses to the next heaviest cone. This provides additional biofeedback and may encourage the patient to continue with therapy. It is unlikely that this is equivalent to the effect of a skilled therapist, but this approach offers convenience at a very low cost. There are many limitations in the use of cones. Many patients will not use any vaginal device, a substantial group will not even be able to retain the lightest cone, and some patients will retain the cones with use of thigh adductor muscles without proper levator contraction. They are obviously not useful for the incontinent male patient.

Figure 69–2 Small, weighted cones placed in the vagina provide biofeedback when the patient feels the cone slipping out and must tighten the pelvic floor muscles to retain it. They are typically sold in sets of five with progressively greater weight.
(From Payne CK. Advances in nonsurgical treatment of urinary incontinence and overactive bladder. Campbell’s Urology Update 1999;1[1]:6.)
The clinical effectiveness of vaginal cones has been examined in a Cochrane systematic review (Herbison et al, 2004). The authors identified 15 studies involving 1126 women, of whom 466 received cones. However, the trials were small, many were published only as abstracts, and “the quality was hard to judge.” Cones were believed to be better than no treatment but not clearly different from PFMT or electrical stimulation. Hay-Smith and colleagues (2009) concluded that there was grade B evidence that vaginal cones could be offered to women with SUI “who can and are prepared to use them” but cones were inferior to PFMT (grade B). Cones were equally effective to electrical stimulation in four trials reviewed (grade B) and adding cones to PFMT does not appear to offer additional benefit.
Other Biofeedback Techniques
Most modern biofeedback units employ vaginal/anal sensors that display either pressure or electromyographic (EMG) activity on a computer screen for visual biofeedback (Fig. 69–3). In some cases, auditory biofeedback corresponding to the muscle contraction is provided, and this can be beneficial for those patients with visual limitations. Perineometers measure vaginal or anal squeeze pressure (as per Arnold Kegel), are available for about $100, and, in theory, might be more effective in teaching a patient to identify the proper muscles than vaginal cones. In practice, however, pressure-based biofeedback has been criticized because abdominal pressure is also transmitted to the probe (Bo et al, 1990b). A patient may perform a Valsalva maneuver, which is counterproductive, because the pelvic muscles are contracting. The devices are more expensive and have the same problems with patient acceptance as vaginal cones. Pressure-based biofeedback has largely been supplanted by EMG systems, which are more expensive ($250 to $400 and up). These reduce the problem with Valsalva maneuvers but do not guarantee proper muscle isolation because they only register activity of one muscle group. Devices used with vaginal sensors, rectal sensors, and perineal patches are available. Although many randomized controlled trials have been performed examining exercises with and without biofeedback, the results are mixed, and a recent review concluded, “The evidence for firm recommendations to include biofeedback in these conservative strategies is lacking, and further research is needed to clarify the role of biofeedback” (Weatherall, 2000). The ICI review data presented earlier (Hay-Smith et al, 2009) concluded that there is no benefit to adding biofeedback to a good PFMT program.
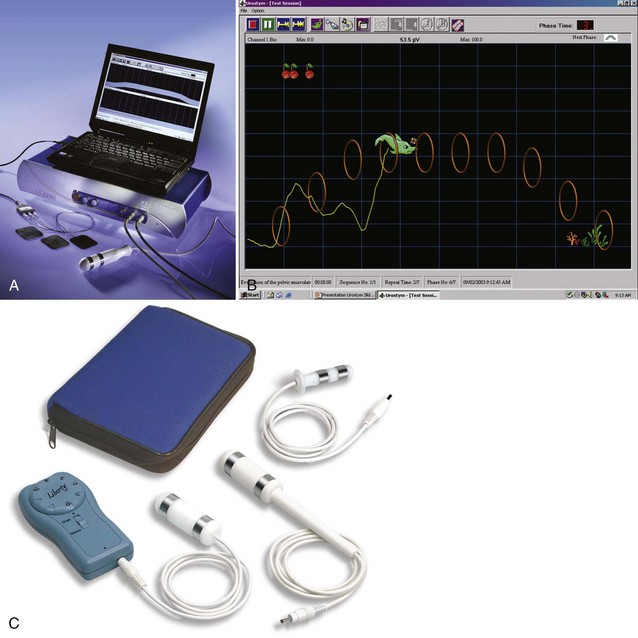
Figure 69–3 Tools for pelvic floor rehabilitation. A, Laptop-based office biofeedback system with probe and patches. B, Animated screen for pediatric biofeedback. C, Typical home electrical stimulation unit.
(A and B, Courtesy of Laborie Corporation, Williston, VT; C, Courtesy of Utah Medical Products, Midvale, UT.)
Peripheral Stimulation
Dissatisfaction with the time, effort, and expertise required for office-based biofeedback generates an interest in passive pelvic floor therapy. The two modalities used in this manner are peripheral electrical stimulation and magnetic stimulation.
Electrical Stimulation
There have been innumerable different devices used to assist and promote rehabilitation of the pelvic floor muscles. The type of stimulation varies by location, current type, waveform, and intensity. High-frequency stimulation (50 to 200 Hz) generally has been used with vaginal or anal electrodes for SUI; the proposed mechanism of action is to directly stimulate the pelvic floor and urethral muscles thereby causing contractions. Exactly how this improves continence—by changing muscle reaction or increasing bulk/strength—is not well documented in the literature. Electrical stimulation can also be used to treat patients with urge or mixed UI. Low-frequency stimulation (5 to 20 Hz) is typically employed, intending to activate inhibitory bladder reflexes and reduce detrusor overactivity. Although most devices employ vaginal or anal electrodes, the effect can also be achieved less invasively with peripheral stimulation using patch electrodes in the perianal or posterior tibial nerve distribution (McGuire et al, 1983; Hasan et al, 1996). Male patients may find this mode of therapy more acceptable than anal electrodes.
The advantage of this modality is that it is dependent only on patient compliance, not effort. The initial expense ($600 to $800) can be partially offset by home administration of treatments. Electrical stimulation can be combined with office biofeedback using the same probe on some systems.
Electrical stimulation has potentially wide applicability and reasonable cost. Unfortunately, the electrical stimulation literature is characterized by case series with small numbers of patients using different devices and widely varying treatment algorithms, few of which provide any follow-up after the initial assessment of response. In addition, the role of dual stimulation for mixed UI has not been adequately evaluated; this treatment would seem to be ideal for such patients using the high frequency for the sphincter and low frequency for the bladder. The various types of protocols for stimulation are well summarized by the ICI committee (Hay-Smith et al, 2009).
The most clinically useful data come from three RCTs using the EMPI product (EMPI Corporation, St. Paul, MN). One study including 121 women with UI found no significant changes in any of the clinical variables examined (frequency, incontinence, subjective improvement) even though active treatment produced an improvement in urodynamics for the subset with detrusor overactivity (Brubaker et al, 1997). In a second study investigating women with SUI, electrical stimulation was demonstrated to be significantly better than sham therapy. Patients were treated with home vaginal stimulation for 15 minutes twice per day for 12 weeks. Leakage episodes decreased from 3.1 to 1.8 (42%) per day, and pad use decreased from 6.2 to 4.1 (34%) per week in the treatment group; there were small, statistically insignificant increases in the placebo group. Importantly, the clinical improvement correlated with improvement in pelvic muscle strength on perineometry (10.6 mm Hg baseline and 15.2 mm Hg at the end of treatment compared with a small decrease in strength in the placebo group) (Sand et al, 1995). The final study examined the optimal treatment regimen; there was no difference in outcome between daily and every-other-day stimulation (Richardson et al, 1996). In a literature review of 361 cumulative patients with UUI, detrusor overactivity, and neurogenic detrusor overactivity, the overall results were 20% dry and another 37% significantly improved using a variety of definitions for outcome (Payne, 1996). The ICI committee concluded that electrical stimulation might be better than no treatment for women with SUI or DO, that low-intensity home electrical stimulation may be superior to maximal stimulation in an office setting, and that adding electrical stimulation does not improve the outcome of a proper PFMT program (all grade C) (Hay-Smith et al, 2009).
The “new” treatment option for peripheral electrical stimulation is peripheral neuromodulation of the posterior tibial nerve using 34-gauge solid needles connected to an external stimulator (PTNS). The idea was initially developed and promoted by Stoller (Payne, 2002). The initial multicenter open-label trial found a 71% response in 53 patients with a variety of lower urinary tract disorders after 12 weekly treatments (Govier et al, 2001). In 2005 a small RCT showed no difference in response between three times per week and weekly treatment (Finazzi Agrò, 2005). The therapy was ultimately approved by the U.S. Food and Drug Administration (FDA) in 2007 as the Urgent PC (Uroplasty, Minnetonka, MN). Since then, further work has shown that the device has considerable promise in the treatment of OAB. Peters and colleagues (2009) compared PTNS with pharmacologic therapy with long-acting tolterodine 4 mg/day in 100 adults with OAB. The results were very similar with near-identical responses in reduction of frequency, nocturia, UUI, and urgency episodes. More subjects reported cure or improvement in OAB symptoms with PTNS than tolterodine (79.5% compared with 54.8%, P = .01). Constipation and dry mouth were significantly less common in the PTNS group, and local adverse effects from PTNS appear to have been minor. A follow-up manuscript reported the durability of PTNS at 1 year (MacDiarmid et al, 2010). Patients initially randomized to PTNS were offered an additional 9 months of therapy but could not use any supplemental anticholinergic medications. Treatments were given at the discretion of the patient and physician. There were 35 responders of the initial 44 patients randomized to PTNS. Thirty-three agreed to enter the continuation trial and 25 continued for 1 year (71% or 56% of those initially assigned to PTNS), and all but 1 continued to self classify as “cured or improved.” Improvement in all voiding diary variables was sustained.
The ICI committee (Hay-Smith et al, 2009) was able to come to several conclusions and recommendations about the use of peripheral electrical stimulation for UI:
Magnetic Stimulation
One commercial device has been available in the United States to treat UI by extracorporeal magnetic stimulation since 1998. The NeoControl (Neotonus Corporation, Marietta, GA) device provides noninvasive, passive stimulation to the pelvic floor. The patient sits in the treatment chair, which emits electromagnetic waves focused on the expected area of the pelvic floor muscles. The recommended treatment session lasts 20 minutes and includes both high- and low-frequency stimulation, regardless of the type of UI. The low-frequency stimulus elicits a pulselike contraction of the levator muscles, whereas the higher frequency produces more of a tetanic contraction. There is some flexibility for the clinician to program the treatment, and the patient adjusts the intensity to comfort level. The potential advantages of this therapy are as follows:
The disadvantage is that the twice-weekly treatments must be administered in the clinician’s office, which is quite inconvenient for many patients in comparison to home electrical stimulation. The stimulation is nonspecific, and magnetic waves are not significantly attenuated by interaction with tissue. Thus, other muscles, nerves, and even the uterus could respond to stimulus, although most patients tolerate treatment well. Although magnetic stimulation is usually thought to act by improving pelvic floor muscle function, stimulation has been shown to suppress detrusor overactivity in the acute setting (McFarlane et al, 1997).
The initial case series suggested that magnetic stimulation might be effective for both SUI and UUI (Galloway et al, 1999; Yokoyama et al, 2004). Subsequent RCTs have had mixed results. Two sham-controlled trials have studied women with urodynamically proven SUI using the NeoControl device. Gilling and colleagues (2009) found that active treatment was not superior to sham therapy in 70 women treated three times per week for 6 weeks. Enrollment in another trial using twice-weekly treatment for 8 weeks was stopped early, having recruited only 48 of 60 patients, owing to poor results and a high dropout rate with adverse events in half of the subjects (Ismail et al, 2009). These results contrast to a case series of women with SUI treated with a similar device, most of whom had a sustained good response for 1 year but then suffered deterioration during the second year (Hoscan et al, 2008). Yet another device developed in Japan was studied in 39 patients with UUI who had failed PFMT (Suzuki et al, 2007). A double-blind crossover design with weekly treatment for 10 weeks was employed. The authors found improvement in UI episodes and urodynamic parameters with active treatment. These results were supported by a long-term follow-up of 48 women with OAB in which 26 of 27 subjects who were considered “cured” at 2 weeks continued to be improved at 24 months (Choe et al, 2007). Another trial using a different type of device (PULSEGEN by Power of Nature, Ft. Myers, FL) included 39 women with urge-predominant mixed UI (But et al, 2005) treated at 18.5 Hz. Those receiving active treatment had statistically significant improvements in several outcome variables, and symptoms improved in 42% with active therapy compared with 23% in the placebo-treatment group. However, this device is worn in a small pocket of specially designed underwear with stimulation used day and night for 2 months.
Thus, the small trials performed to date suggest that extracorporeal magnetic stimulation is not effective for female SUI but may be useful for OAB/UUI. The ICI committee (Hay-Smith et al, 2009) could only conclude that magnetic stimulation was “worthy of further investigation in women with UI.” Several authors have suggested that the therapy might be best used with patients who are unable to perform a pelvic muscle contraction, but this has not been specifically studied. Very limited investigation into the durability of response in patients initially treated successfully suggests that the relapse rate is not unreasonable; whether failures might respond to “booster” therapy is unknown. It is clear that larger-scale trials with pragmatic design are needed before the role of magnetic stimulation can be defined. The most important question is whether any of the advanced pelvic floor rehabilitation techniques provide more consistent or better durability of response than PFMT. Until such data exist, biofeedback, electrical stimulation, and electromagnetic therapy are all potential competitive treatment options to rehabilitate the pelvic floor when PFMT is insufficient.
Key Points: Biofeedback and Peripheral Stimulation
Devices
Vaginal Support (Pessaries) for Stress Urinary Incontinence
Vaginal support prostheses are among the oldest of medical devices. Although primarily used to treat pelvic organ prolapse, these products have been used to treat SUI and there has been an interest in developing devices specifically for SUI in recent years. Older devices, initially developed for lower-stage pelvic organ prolapse, such as the Smith and Hodge pessaries, have been used in treating SUI with limited clinical data as to their effectiveness (Bhatia et al, 1983; Bergman and Bhatia, 1984; Nygaard, 1995). The Incontinence Dish (Milex Corp., Chicago, IL) is another pessary commonly used in treating SUI; although there are no published clinical trials, a urodynamic investigation (Noblett et al, 2008) revealed that the mean Q-tip straining angle without and with the pessary, respectively, was 57.8 (+19.5) and 34.4 (+29.7), the maximum urethral closure pressure was significantly increased by 19.7 cm H2O (P < .001), and 60% of the subjects did not experience any leaking with the device in place. In addition, any of the variety of devices that support the bladder neck have been used to estimate the response to surgical therapy in patients with mixed UI, although the predictive value of the trial is not established.
Advantages of vaginal support devices include:
Disadvantages of vaginal support devices include:
The Introl bladder neck support prosthesis (Fig. 69–4) was the first device specifically approved for SUI in the United States, and it has subsequently been withdrawn from the market. The Introl was a simple device to mechanically support the hypermobile bladder neck. Clinical trials showed good objective relief of SUI without creating emptying difficulty (Davilla and Ostermann, 1994). One problem may have been relative difficulty in fitting because the Introl prosthesis had 25 sizes whereas traditional pessaries have about 10 sizes. There now appear to be three devices in use, but only one is currently available in the United States. All show promising results in small clinical trials.
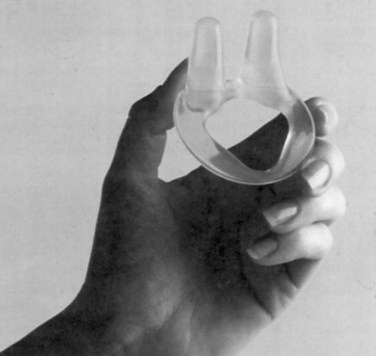
Figure 69–4 The Introl bladder neck support prosthesis.
(Courtesy of UroMed Corporation, Needham, MA.)
The ConTIPI (Fig. 69–5) was developed and is sold in Israel. In contrast to other pessaries, it is disposable and intended for single-daily or as-needed use. It consists of a flexible core with the ends made of resin to provide anchoring. It is covered with a nylon mesh, and a string is attached to the cover for easy removal. This device has been tested in one prospective case series of women with urodynamically proven SUI who were considered surgical candidates (Ziv et al, 2008). There was a 7-day “run in” followed by 28 days using the device. Eighty-five percent of the 60 women tested were considered responders, having achieved 70% or more reduction in home pad testing (P = .01). Improvements in overall quality of life, subjective perception of UI, and satisfaction with the device were observed. There were no changes in flow rates or residual urine, but 67% of subjects experienced some genital tract adverse event, including 25% with pain and 23% with spotting.

Figure 69–5 The ConTIPI Intravaginal Device (ConTIPI LTD, Caesarea, Israel).
(From Ziv E, Stanton SL, Abarbanel J. Efficacy and safety of a novel disposable intravaginal device for treating stress urinary incontinence. Am J Obstet Gyncol 2008;198:594.)
Another device, the “Contiform” (Fig. 69–6), is being used in Australia. It is intended for women with a main complaint of SUI but no pelvic organ prolapse. It comes in four sizes. Studies by Allen and associates (2008) showed that of 73 women invited to participate, 65 enrolled, 52 were successfully fitted (80% of those willing), and 37 completed the study protocol (71% of those fitted, 57% of those willing). At the end of 1 month, 20 (54%) of patients were dry after a 24-hour pad test. Only 8 of 37 (22%) chose to proceed with surgical therapy, whereas the rest were satisfied to continue using the device for a longer term. The patients had relatively mild SUI, with all having less than 25 g of urine loss on the baseline 24-hour pad test. This study points out one of the limitations of using pessaries; patients with prior surgery causing vaginal distortion or introital scarring may not be successfully fit, and those with an overly large genital hiatus may not retain a device.
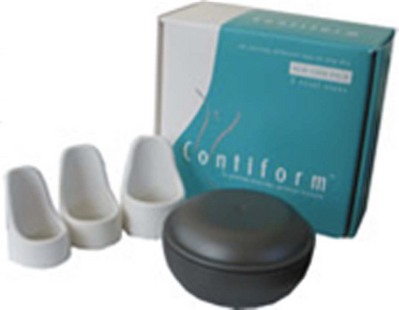
Figure 69–6 The Contiform device (Contiform International, Blacktown, New South Wales, Australia).
(From www.contiform.com.)
The third device, the Uresta (Fig. 69–7), is marketed in the United States and Canada. It was studied in a small group of women with 1-year follow-up (Farrell et al, 2007). Thirty-two women started the trial, and 11 dropped out in the first 2 weeks. Among women successfully fitted at 2 weeks, 16 of 21 (76%, or 50% of the initial cohort) continued using their pessary at 1 year. Weekly UI episodes decreased from 20 to 4 (P = .028) and provocative pad test weight decreased from 20 to 9 g (P = .06).
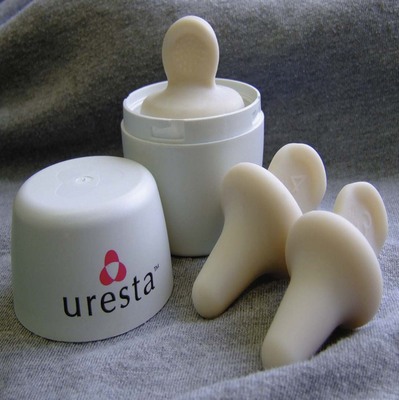
Figure 69–7 The Uresta pessary (EastMed Inc., Halifax, Nova Scotia, Canada).
(From www.uresta.com/sites/default/files/PPP%20Fitting%20Dec%208%2C%202009.pdf.)
Vaginal Support (Pessaries) for Prolapse (Overflow/Bladder Outlet Obstruction/Urgency Urinary Incontinence)
Pessaries have been used for years as a treatment for pelvic organ prolapse and diseases of the uterus (Miller, 1991). In fact, most devices used for SUI in the United States are pessaries primarily designed for pelvic organ prolapse. The Milex Corporation (Chicago, IL) and the Mentor Company (Santa Barbara, CA) offer a full range of pessaries for prolapse and have developed devices intended for SUI alone (Fig. 69–8) or that associated with pelvic organ prolapse (Fig. 69–9). These devices include a “knob” oriented anteriorly in the distal vagina at the level of the proximal urethra to improve closure. Such devices put pressure directly on the urethra and are thus more likely to be obstructive. This design could at least theoretically be useful for the patient with intrinsic sphincter deficiency because there is direct compression of the urethra. No direct comparison has ever been made between any of the various pessaries for any indication. Pessaries used for prolapse only should be fitted with an empty bladder for patient comfort. However, all pessaries intended for use in SUI should be fitted with the bladder at a comfortable fullness. The patient can then cough and strain to get an immediate assessment of effectiveness, and the patient should always urinate adequately before leaving the clinic because obstruction and retention can be a problem.

Figure 69–8 Pessaries available from Milex Corporation, Chicago. All provide varying anatomic support; those with “knobs” apply direct pressure to close the urethra to manage stress incontinence. 1, Incontinence dish with support; 2, Incontinence dish; 3, Hodge with support; 4, Hodge; 5, Incontinence ring; 6, Gehrung with knob; 7, Hodge with support and knob; 8, Cube; 9, Ring with support and knob; 10, Hodge with knob.
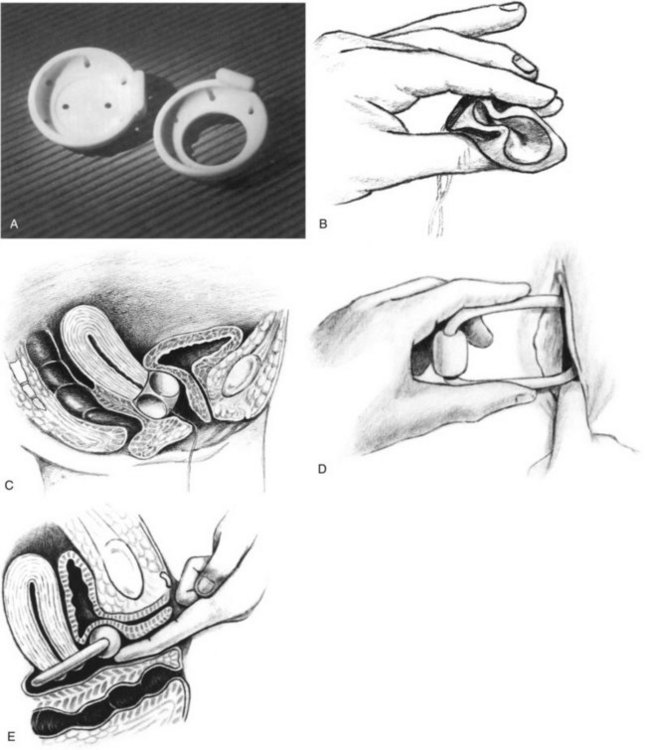
Figure 69–9 A, Dish pessaries, with and without support (available from Mentor Corporation, Santa Barbara, CA), which can be used for stress incontinence. B, Cube pessary is compressed between the thumb and forefingers. If necessary the entering edge can be coated with suitable lubricant. C, Silicone cube in position. D, Ring incontinence pessary being inserted. E, Ring incontinence pessary positioned.
(B to E, Courtesy of Milex Corporation, Chicago, IL.)
Vaginal pessaries have specific roles in diagnosis and treatment of lower urinary tract symptoms in at least three distinct situations:
Prolapse, primarily from the anterior compartment, can impair voiding by mechanically obstructing the urethra through local pressure or by a kinking effect. This same mechanism could prevent clinical manifestations of SUI, and prolapse further diminishes SUI by dissipating abdominal pressure on the bladder neck and proximal urethra. Many authors have advocated using a vaginal pack or pessary test during preoperative evaluation before prolapse surgery (Fianu et al, 1985; Bergman et al, 1988; Ghoneim et al, 1994; Cross, 1998; Hextall et al, 1998; Chaikin et al, 2000; Klutke and Ramos, 2000). The use of a pessary or other means of prolapse reduction before surgery for apical prolapse was studied prospectively in the CARE trial (Colpopexy And urinary Reduction Efforts) by the NIH-sponsored Pelvic Floor Disorders Network. When clinically continent women were demonstrated to leak with stress with prolapse reduction during urodynamics they had a much worse result if no SUI procedure was performed (58% incontinent vs. 32% of those undergoing a Burch suspension) (Brubaker et al, 2003, 2008; Visco et al, 2008). Unfortunately, a negative stress test did not have a good predictive value because 38% of these patients had stress leakage after the prolapse repair. The pessary was the least specific of the five different methods of prolapse reduction tested (although a ring pessary was used that may not be suitable for higher grades of prolapse).
It thus seems reasonable to encourage prolapse patients considering surgery to undergo a trial of a pessary and evaluate the clinical response to the device. If OAB or other lower urinary tract symptoms resolve with the pessary, the patient may elect to use the device either temporarily until a convenient time for surgery or as a long-term solution. Similarly, if symptoms do not resolve after appropriate support with a pessary the patient should be counseled that the symptoms are more likely to persist even after successful repair of the anatomic defect. A pessary trial has an advantage over the packing tests during urodynamics in that the patient can be evaluated chronically and during day-to-day activities. However, many patients with high-grade prolapse cannot retain any pessary and surgical decisions must be made using common sense, urodynamic data, and best clinical judgment. If “de novo” SUI is uncovered, anti-UI surgery is clearly indicated. Even if no SUI is noticed with the pessary, consideration should be given to performing anti-UI surgery, providing the patient empties the bladder normally.
Urethral Meatal Occlusive Devices
At least three devices have been marketed for female SUI that were applied directly to the urethral meatus. The exact mechanism of action for these devices is not clear. The devices generally have a small reservoir or some absorptive capacity. They may create a “dam” to prevent leakage, work through absorption, or increase urethral closure by eliminating “pop off.” The Capsure, Femassist, and Impress devices were approved for marketing, but all have been withdrawn from the market.
These were simple devices that could be more attractive to patients than a pad because they actually prevent leakage rather than simply absorbing the fluid. This would reduce odor and skin irritation. Good effectiveness was demonstrated for mild to moderate SUI, as detailed by Dmochowski (1998):
On the other hand, application was not uniformly easy and patients did not always have resources to help them get through initial problems in utilization. For some types of body habitus it was simply not possible to apply the device. The devices have a limited ability to oppose leakage and were not effective for severe stress leakage or any urge leakage. Finally, local tissue irritation was a minor but common problem. The future role for improved products in this area is unclear.
Urethral Inserts
Sometimes referred to as urethral stents or plugs, the urethral inserts for SUI passively occlude and/or coapt the urethra and must be removed for voiding. The first urethral insert to be approved for marketing by the U.S. FDA, the Reliance (UroMed, Inc., Needham, MA) (Fig. 69–10), has been withdrawn from the market. The second-generation FemSoft stent (Rochester Medical Corporation, Stewartville, MN) (Fig. 69–11) was FDA approved in 1997 and is currently available.
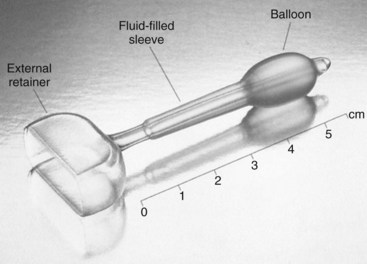
Figure 69–11 FemSoft urethral insert.
(Courtesy of Rochester Medical Corporation, Stewartville, MN.)
The multicenter study that led to the approval of the Reliance device enrolled 255 patients from eight centers. Of these, 135 completed at least 4 months with the device and were considered evaluable. The results were unquestionably good, with 80% of the subjects dry using the device and 95% improved (Staskin et al, 1998). At 1-year follow-up, the efficacy was maintained and there were significant but mostly minor complications, including urinary tract infection (30%), gross hematuria (24%), and local changes on cystoscopy (9%) (Miller and Bavendam, 1996). The device was also studied in a small comparative trial against a novel device, the NEAT, that never came to market. Both were effective against SUI with seemingly reasonable adverse events (Robinson et al, 2003).
In comparison, the one published study of the FemSoft insert was a 5-year, multicenter trial involving 150 women with mean follow-up of 15 months (Sirls et al, 2002). Pad testing and bladder diaries demonstrated efficacy. This device appears to be useful for a wide spectrum of the incontinent population, including those with urgency, low leak point pressure, failed surgery, and advanced age. In contrast, the Reliance was believed to be applicable only for pure SUI; if urgency was present it tended to be exacerbated by the device, resulting in loss of both urine and the device with an involuntary bladder contraction. Adverse events with the FemSoft were again common but not severe, including symptomatic urinary tract infection in 31.3%, mild trauma with insertion in 6.7%, hematuria in 3.3%, and migration in 1.3% of women.
Although urethral inserts are potentially applicable to almost all women with pure SUI and possibly some of those with mixed UI, it must be remembered that the device must be removed and replaced for each void. Despite the new soft, hydrophilic materials, only a small percentage of the SUI population seems to be willing to instrument the urethra to be dry. The highest patient acceptance seems to be among those with very predictable, episodic SUI, such as during sports or dancing. Such patients might use a device several times each week and have complete elimination of leakage. Many of these patients believe that their problem is too mild to undergo a surgical procedure but are happy to have this minimally invasive alternative.
Current practitioners and those who may design and market future devices might be informed by one study of women’s knowledge of anatomy and their attitudes toward devices and genital touching. An Australian team questioned 104 consecutive women with UI using a 10-item questionnaire (Prashar et al, 2000). They found that only 30% of the women felt “quite comfortable” about the concept of touching their genitalia: this attitude was age dependent. Only 21% were quite willing to insert a continence device into their vagina: this attitude varied weakly with age but was significantly affected by previous tampon or diaphragm usage. Only 15% felt very comfortable about placing a continence device onto their urethra, but the likelihood of a positive response to this concept was not at all affected by age and was only slightly more common in previous tampon users. Thus older women are less likely to understand the anatomy of their genitalia or to be comfortable about the idea of exploring it, but age is no barrier to willingness to employ urethral or vaginal continence devices per se.
Urethral Stents
Urethral stents that allow for intermittent voiding through the device have been tested in both men and women with urinary retention/overflow UI. None has ever been approved in the United States. Most of the catheters that have been designed have internal motors that allow the patient to expel urine without removing the device, but one device, the ContiCath (ContiMed Corporation, Minneapolis, MN), works by allowing urine to pass around the catheter through three deep grooves. In contrast to the motorized stents, this device will be effective only for retention due to obstruction with intact detrusor function. In a preliminary trial (Corujo et al, 1999) 33 of 37 patients (90%) with a non-neuropathic cause and retention of 1 week or less were able to establish controlled voiding. The device was ineffective for neurogenic and chronic retention.
Key Points: Devices
Practical Approach to Treatment
General Issues—Patient Goals
Treatment of UI is more challenging and potentially more rewarding than many of the tasks facing the urologist because it requires a true partnership with the patient. In most instances there is no one “best” treatment for the particular problem. Even when there is a superior treatment option, the nature of the disorder—not directly threatening to life and not necessarily progressive—not only allows but also mandates an individualized approach to therapy. The patient’s personal goals should always be the prime concern. A 40-year-old aerobics instructor may be highly motivated to be completely dry, whereas another woman might only want to attend church without fear of leakage; an incontinent man might be much more bothered by getting up three to four times at night than by daytime UI. Brubaker and Shull (2005) point out that it is “the patient’s perspective that drives decisions about further treatment [in quality of life disorders]” in a review describing the term EGGS, which has been coined to facilitate communication about patient-centered treatment outcomes. EGGS—Expectations, Goal setting, Goal achievement, and Satisfaction—describes a process in which the patient’s point of view is explored to develop an individualized treatment plan. This process is equally relevant for medical, surgical, and physical therapies; the importance of the patient perspective in surgical treatment has been demonstrated in prospective clinical trials. In a surgical series, patient satisfaction was moderately correlated to goal achievement but was not related to objective cure of SUI or prolapse (Elkadry et al, 2003), and goal achievement appeared to be durable at longer-term follow-up (Hullfish et al, 2004). Although less is known about expectations and goals as they relate to nonsurgical therapy, a clinician will do well to be aware of the patient’s goals lest he or she lose the patient or find an unsatisfied customer. The very nature of conservative therapies—safe, reversible, and effective—means that most patients will choose these options for first-line treatment. When the treatments are skillfully delivered, most patients will be very satisfied with the results.
Treatment Planning—Data Collection
The clinician needs three primary pieces of information to develop a treatment plan for a given patient: the type of UI, the baseline voiding diary, and an assessment of anatomy with particular emphasis on pelvic floor muscle strength and function. In most cases a clinical diagnosis of stress, urge, or mixed UI is easily made but detailed evaluations are sometimes necessary before beginning treatment. The voiding diary is important for four reasons. First, it is a reasonable substitute for cystometry, because the largest voided volume on a diary has been shown to correlate with the cystometric capacity defined by urodynamic testing (Diokno et al, 1987). Given the high false-negative rate for cystometry and detrusor overactivity, determining a general sense of the bladder’s capacity to store urine is at least as valuable as knowing whether involuntary contractions are present on cystometry. Second, the voiding diary allows the clinician to objectively determine a “safe” voiding interval to begin a program of bladder training. Third, the “baseline” diary becomes the comparison for the clinician to objectively measure change with therapy. Finally, the process of keeping the voiding diary involves the patient in the treatment program, starting the education essential to behavioral therapy. The assessment of baseline pelvic muscle strength is critical because that allows the clinician to intelligently prescribe pelvic floor rehabilitation therapy.
Pelvic floor rehabilitation has a unique role in UI therapy in that all types of UI can benefit and, potentially, be cured. Pelvic floor rehabilitation has long been identified as a treatment for SUI. It has been variously proposed that such treatments increase the resting tension, the contractile force, or the speed of recruitment of the voluntary sphincter. Such changes could increase resting urethral closure and improve the reflex closure that occurs with increases in abdominal pressure. Pelvic floor rehabilitation has been relatively overlooked in treating bladder-related UI. Pelvic floor therapy is theoretically valuable to the patient with OAB in three ways. First, the simple mechanics of voluntary sphincter closure oppose the unstable bladder contraction and prevent or minimize urine leakage. Second, there are inhibitory local reflexes between the pelvic floor muscles and the bladder. At least in theory, increased muscle tone and strength should decrease overactivity. Finally, many patients can use repeated quick, active contractions (“quick flicks”) to inhibit an involuntary contraction once it starts. Thus, improved muscle function can improve continence for the patient with OAB through both passive and active mechanisms. Although pelvic floor therapy is of unquestioned value, it has not been properly appreciated, perhaps owing to confusion about the inappropriate use of the various tools described previously and improper referrals for therapy. A simple assessment of pelvic floor muscle function at physical examination may be useful in triaging patients with UI.
Patients are classified into three groups based on the assessment of pelvic floor muscle strength at baseline examination: (1) those with no or minimal ability to isolate and contract the levator muscles, (2) those who can isolate the correct muscles with poor strength, and (3) those with good pelvic floor muscle strength and isolation on the initial examination. There is no universally accepted objective method of evaluating pelvic muscle strength by digital vaginal examination. It is principally important that the clinician be internally consistent in his or her assessment. The author has used a convention proposed by Laycock (Tries and Eisman, 1995). A 6-point scale is used for contraction strength: 0, absent; 1, flicker; 2, weak; 3, moderate; 4, good; 5, strong. A second score is given to indicate the endurance time in seconds. Romanzi and associates (1999) examined a similar system. Patients can have a maximum score of 9; a score less than 4 correlated with self-reported UI in a small test group. Scores correlated well (r values ~ 0.8) for both intra-rater and inter-rater observations. There was also a reasonable correlation to surface EMG determinations (r values ~ 0.5).
Regardless of which system a clinician chooses to score pelvic muscle strength, it is proposed that patients be stratified into three clinically relevant groups by the physical examination as described earlier. It is possible to assign treatment to patients in the three clinical groups by applying these basic principles. It seems illogical to prescribe home exercises in the first group (inappropriate or absent baseline function), and anecdotal experience suggests that significant improvement is uncommon. These patients need some form of teaching/biofeedback (or passive stimulation) just to get started. Office-based training should be considered to be superior to other forms of biofeedback until proved otherwise. Many of these patients are older and will not accept home therapy. The author’s strategy has been to offer weekly office sessions and convert the patient to a home unit once there is consistent objective and subjective response. Alternatively, passive treatment with peripheral electrical stimulation or magnetic stimulation can be investigated.
The second group of patients demonstrate weak contractions but appropriate isolation and should be good candidates for some form of pelvic muscle rehabilitation; biofeedback has not yet been conclusively proved to be better than PFMT alone in this general population. The lesson learned from biofeedback in SUI is that results are primarily dependent on the motivation of the patient and the skill and enthusiasm of the therapist. The author recommends exercise therapy for most of these patients. They are given written instructions and a follow-up office appointment with a nurse to review their progress. Home therapy with cones or simple EMG/pressure devices is a practical option that is generally well accepted by patients who have a basic knowledge and comfort about their bodies. Definitive clinical trials focusing on this patient population would be welcome.
In the final category (strong baseline pelvic contraction) the value of pelvic floor “rehabilitation” is unclear. If these patients have significant UI there is generally severe urethral incompetence and/or severe bladder dysfunction; and they require more aggressive treatment. The author’s general practice is to instruct the patient in “quick flicks” to inhibit urgency and offer vaginal cones to motivated patients. With these patients the onus is on the clinician to demonstrate effectiveness of more complex and expensive treatment.
Treatment of Female Stress Urinary Incontinence
The two principal treatments of pure female SUI are pelvic floor rehabilitation and surgery. These options have been compared in three trials with similar results. Klaskov and coworkers (1986) randomized 50 consecutive women with SUI to immediate surgery versus five weekly visits with a physical therapist, without complex biofeedback. Ten of 24 (42%) patients treated initially with pelvic floor therapy were satisfied and declined surgery after the training program. Tapp and associates (1989) followed 81 consecutive patients with SUI who were randomized to three groups: PFE, PFE plus electrical stimulation, or immediate surgery. The group treated with pelvic floor therapy again had a 43% satisfaction rate (19/44), although only 4 (9%) were cured of UI. Finally, in a group of 39 women awaiting surgery for SUI, 30 completed 1 month of therapy using vaginal cones for home biofeedback. Seventy percent were considered cured or improved, 11 of 30 chose surgery, and thus 49% of the initial group avoided an operation (Peattie et al, 1988). It thus seems proper to counsel women who might appropriately choose surgery that although surgery is the single most effective treatment for SUI there is a 40% to 50% chance that they can avoid an operation and be satisfied with the outcome by going through PFMT.
In practice, it seems that one could do better than simply giving the same advice to all patients. Certain patients might logically benefit from immediate surgery. These would include those who have significant associated prolapse (beyond the hymenal ring) that may be corrected at the same time, those who are highly motivated to be completely dry or who have high levels of physical stress due to lifestyle or occupation, those with relatively severe SUI, and especially those with good pelvic floor function on initial examination (group 3 mentioned earlier). On the other hand, patients with poorer pelvic floor function might reasonably be expected to have better odds of improvement than those cited earlier; thus a trial of pelvic floor therapy should be strongly encouraged. Those with weak muscles and good isolation may benefit from home pelvic muscle exercises alone, but those with absent voluntary contractions or poor isolation probably absolutely require a therapist or are best treated by immediate use of one of the advanced techniques: biofeedback or pelvic floor stimulation. Even those patients who eventually go on to surgery may be pleased with progress and may have better surgical outcomes because they will be better able to manage postoperative irritative bladder symptoms.
Surgery and pelvic floor therapy are the leading treatments of SUI because they are potentially curative. However, some patients will prefer other options for a variety of reasons. The devices mentioned previously are particularly attractive to the patient who really only needs “PRN therapy,” that is, a patient whose leakage is infrequent but predictable and bothersome. An example would be a patient who only leaks during twice-a-week exercise classes or sports. She may feel that surgery would be too much treatment for such a problem but is not really satisfied with protective pads. Similarly, a patient may really prefer surgery but for personal or financial reasons may need to delay definitive treatment. The devices can provide good palliation of symptoms during the interim. Devices are also, of course, indicated for the nonsurgical patient with severe comorbidities. It is simply uncommon that a patient is too ill to undergo one of the minimally invasive operations yet still is active enough to have significant SUI. When such a patient has significant hypermobility, a vaginal support device may be used. Patients without hypermobility can usually be treated with periurethral injection therapy.
Finally, the role of behavioral therapy in SUI should not be overlooked. Fantyl and associates (1991) demonstrated more than 50% improvement in leakage episodes and volume of urine lost with their program, getting essentially equal results in patients with both SUI and UUI. This is enough improvement to satisfy many patients.
Overactive Bladder and Detrusor-Related Incontinence
Successful treatment of the patient with OAB mandates a complete understanding of the pathophysiology. Although anticholinergic medications are quite effective for most patients with OAB, medical therapy should not be a reflexive initial treatment and should rarely be used in isolation. Abnormal bladder activity is often central to the problem, but it is inappropriate to focus only on the bladder. Dysfunctional behaviors and physical limitations strongly impact and can be the primary cause of OAB symptoms. The patient with OAB with UI almost always has poor pelvic floor muscle function (or the symptoms would be limited to urge frequency without UI). Thus, most patients can benefit from multimodality therapy (Fig. 69–12).
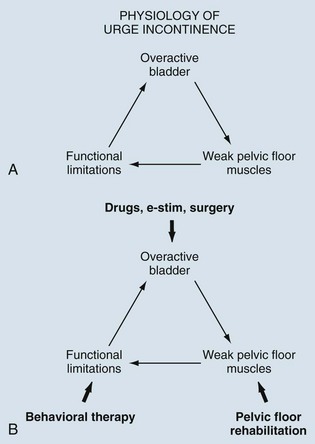
Figure 69–12 A, The clinical manifestation of urgency incontinence is caused not only by bladder overactivity but also by poor pelvic floor muscle function, functional limitations, and dysfunctional behaviors. B, Multimodality treatment is the most effective approach to managing urgency incontinence because each area can be addressed.
Once underlying causes for OAB have been ruled out in the initial history, physical examination, and testing as required, treatment planning should begin with a review of the voiding diary and the patient’s behaviors. The diary reveals the maximum functional bladder capacity, which gives a strong indication as to the severity of the underlying bladder dysfunction. A low functional capacity (<150 to 200 mL) typically correlates with more severe bladder dysfunction, whereas a good functional capacity (>300 mL) indicates better potential bladder function. The clinician may find an unusually large fluid intake to be a factor aggravating the symptoms. A timed voiding regimen is prescribed based on a time interval determined by the patient’s baseline diary. Usually a patient is instructed to void at the longest consistently dry interval. When the patient becomes dry, the interval is increased by 15 to 30 minutes per week until a normal voiding pattern is established. Some patients will benefit from use of a watch with a timing alarm for prompted voiding. Finally, the patient’s response to urge should be reviewed. Inappropriate behaviors such as running to the bathroom must be replaced by proper urge inhibition techniques.
One of the key methods of urge inhibition is use of the pelvic muscle contraction and particularly “quick flicks” of short rapid contractions. The advantage of quick contractions is that the muscles are less likely to fatigue. If the patient can inhibit the urge for about 30 seconds it is likely to pass. Few incontinent patients can hold a maximal contraction that long; therefore, they leak when the muscles fatigue. Some therapists recommend teaching the patient to practice half-strength contractions for the same reason. In any case, pelvic floor therapy will be the appropriate second step for the vast majority of OAB patients who have suboptimal muscle function. The same classification system discussed in the context of SUI is also useful for triage of patients with OAB.
The key question in the treatment of OAB is when to introduce pharmacologic therapy. Unfortunately, most patients have been given drugs as first-line therapy without supplementing treatment with behavioral and pelvic floor therapy. This approach has the tandem problems of exposing many patients to expense and side effects unnecessarily. More importantly, drug therapy is rarely curative alone, whereas the other conservative measures can cure many patients, especially those who start with a reasonable bladder capacity and no underlying neurologic abnormality. Although the newer medications have greatly improved patient acceptance of medical therapy, it still seems appropriate to use drugs selectively and with an eye toward ultimately rehabilitating the bladder and titrating the patient off of medicine. Early use of medication is particularly indicated for patients with a low maximal voided volume on the diary, patients with underlying neurologic disease, and patients who seem uninterested or unable to participate in behavioral techniques. Medications are relatively contraindicated in patients with a very large bladder capacity, medical reasons to avoid anticholinergics, the very old, and the very young. Some clinicians prefer to start anticholinergic therapy on all patients (along with behavioral techniques) as in the original British bladder training protocols (Frewen, 1978; Holmes et al, 1983). This is reasonable because it improves the odds of the patient making an immediate improvement. However, whether drugs are used on all patients or selectively, consideration should be given to the ultimate goal of bladder retraining. Patients who become completely continent with good capacity on the voiding diary probably have approximately a 50% chance of being able to go off medication and stay dry in long-term follow-up (Holmes et al, 1983). Titration off medication should be suggested after 3 to 6 months when drug therapy is successful.
Refractory Overactive Bladder
In a small group of patients, OAB will prove to be refractory to the basic methods described previously. These patients should have further evaluation with sophisticated urodynamic testing and cystoscopy to carefully define the nature of the lower urinary tract dysfunction and to rule out other causes for the symptoms. The patient who truly has refractory detrusor overactivity can be tried on combination medical therapy with a standard anticholinergic plus imipramine. When satisfactory continence is not achieved at the maximal tolerable medical therapy, the remaining options have been sacral neuromodulation or surgical reconstruction (e.g., augmentation, diversion).
Advances with botulinum neurotoxin type A (BoNT/A) injection therapy for detrusor overactivity challenge this algorithm. Although not yet FDA approved for use in the urinary system the drug is commonly used “off label.” Treatments are performed cystoscopically, typically injecting 200 units divided into 20 sites around the bladder. The procedure can be performed on an outpatient basis under local anesthesia or sedation. BoNT/A injections have been proven effective for refractory neurogenic detrusor overactivity in one well-designed randomized controlled trial (Schurch et al, 2005). Fifty-nine patients with multiple sclerosis or spinal cord injury were randomized to receive injections with 200 units of BoNT/A, 300 units of BoNT/A, or a placebo. Dramatic improvements in bladder capacity and continence were seen in both active treatment arms compared with placebo. The gains were maintained for 24 weeks of follow-up. A small prospective randomized trial demonstrated similar results in idiopathic detrusor overactivity (Sahai et al, 2007). More research is needed to define the optimal dose, mode of injection, the length of response, and most importantly the risk for urinary retention and catheterization for those patients who void normally before treatment.
When comparing neuromodulation to BoNT/A injections, the most obvious difference is that the neuromodulation data are almost exclusively from the non-neurogenic population in contradistinction to the neurogenic patients treated with injections. The second key factor is that neuromodulation is FDA approved for UUI, urge-frequency syndrome, and idiopathic urinary retention. BoNT/A injections are not currently FDA approved for any application in the urinary tract. Nevertheless, some patients may prefer a trial of BoNT/A injections, and it would seem reasonable to offer this therapy to a well-informed patient. The advantages of BoNT/A include a lower initial cost, a somewhat simpler procedure, and elimination of the permanent implant (the implant precludes performing magnetic resonance imaging and can complicate travel). The disadvantages of BoNT/A include the need for repeated injections indefinitely over time. Long-term response to neuromodulation is somewhat better established than BoNT/A, but a great deal of research is needed in both areas and a head-to-head study would greatly advance this field.
The Problem of Mixed Incontinence
Mixed UI presents a special challenge to the clinician. These patients have typically been approached by offering a trial of anticholinergic medication first and then operating on those whose disorder does not adequately respond. There are certainly clues that can be used to define the primary component of the UI in at least some of the patients and develop more individualized treatment strategies.
The history can be helpful; the primary problem in mixed UI is often that component (the stress or the urge) that occurred first chronologically. The voiding diary can provide important clues. When OAB is the predominant problem the void volumes should be small both day and night. In contradistinction, if there are small daytime voids with little or no nocturia and a large first morning void the urgency symptoms tend to be a manifestation of SUI, perhaps stimulated as a few drops of urine leak into the proximal urethra with activity and trigger a sense of urgency. Pelvic organ prolapse can have a similar effect. When SUI is suspected to be the primary component, a short-term trial of one of the vaginal support devices may clarify the picture. If the symptoms are relieved by the device the patient may decide to continue using it or feel more confident that surgical intervention will be successful. A pessary trial is doubly important before correcting pelvic organ prolapse (with or without UI). Elevation of a cystocele will unmask occult sphincteric incompetence in approximately 50% of patients who are continent with the prolapse. If an ambulatory trial of a pessary is not performed, then a pessary or vaginal packing may be used during preoperative urodynamic testing as discussed in the pessary section previously. The urethral stent/plug devices are relatively contraindicated in this patient population because clinical experience suggests that they typically aggravate the underlying urge and become totally ineffective.
The patient with mixed UI can usually benefit from each form of conservative treatment: behavioral, pelvic floor, and medical therapies. When medications are prescribed, it is particularly important to obtain voiding records before and during treatment. A patient who is not satisfied with treatment may be shown to have the same number of leaks but a significant improvement in bladder capacity. This would again argue for success with surgical therapy for residual SUI symptoms. Pelvic floor rehabilitation is probably the most important treatment in this patient group, and there may be a special role for electrical stimulation because, at least theoretically, stimulation may specifically improve both urethral and bladder function. The patient uses low- and high-frequency stimulation on alternate days. Any of the other therapies can be combined with stimulation.
Key Points: Practical Approach to Treatment
Conclusion
Urinary incontinence is a pervasive and increasing problem throughout all segments of our society that produces a severe impact on our health care system and patients’ quality of life. The incontinence specialist of the 21st century must be familiar with all types of therapies: conservative/behavioral treatments, medical therapy, and surgical options. There is a great need for quality research that compares various treatment options to determine the initial effectiveness and durability, the adverse effects, and the advantages for specific groups of patients. In the meantime, the therapist must be able to match treatment to the problems and goals of individual patients. Nonsurgical treatments are unquestionably effective and are preferred by many patients. Mastering of all types of therapies will keep the urologist the recognized expert in incontinence. Ignoring nonsurgical therapies will pass the mantle, and the surgery, to other providers.
Auwad W, Steggles P, Bombieri L, et al. Moderate weight loss in obese women with urinary incontinence: a prospective longitudinal study. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(9):1251-1259.
Brown JS, Wing R, Barrett-Connor E, et al. Diabetes Prevention Program Research Group. Lifestyle intervention is associated with lower prevalence of urinary incontinence: the Diabetes Prevention Program. Diabetes Care. 2006;29(2):385-390.
Burgio KL, Locher JL, Goode PS, et al. Combined behavioral and drug therapy for urge incontinence in older women. J Am Geriatr Soc. 2000;48:370-374.
Dallosso HM, McGrother CW, Matthews RJ, et al. The association of diet and other lifestyle factors with overactive bladder and stress incontinence: a longitudinal study in women. BJU Int. 2003;92(1):69.
Danforth KN, Shah AD, Townsend MK, et al. Physical activity and urinary incontinence among healthy, older women. Obstet Gynecol. 2007;109(3):721-727.
Diokno AC, Sampselle CM, Herzog AR, et al. Prevention of urinary incontinence by behavioral modification program: a randomized, controlled trial among older women in the community. J Urol. 2004;171(3):1165-1171.
Fantyl JA, Wyman JF, McLish DK, et al. Efficacy of bladder training in older women with urinary incontinence. JAMA. 1991;265:609-613.
Goode PS, Burgio KL, Locher JL, et al. Effect of behavioral training with or without pelvic floor electrical stimulation on stress incontinence in women: a randomized controlled trial. JAMA. 2003;290(3):345-352.
Hashim H, Abrams P. How should patients with an overactive bladder manipulate their fluid intake? BJU Int. 2008;102(1):62-66.
Hay-Smith J, Berghmans B, Burgio K, et al. Adult conservative management. In Abrams P, Cardozo L, Khoury S, Wein A, editors: Incontinence, 4th ed, Plymouth, UK: Health Publications Ltd, 2009.
Hay-Smith J, Mørkved S, Fairbrother KA, Herbison GP. Pelvic floor muscle training for prevention and treatment of urinary and faecal incontinence in antenatal and postnatal women. Cochrane Database Syst Rev. 2008 Oct 8;(4):CD007471.
Hu TW, Wagner TH, Hawthorne G, et al. Economics of incontinence. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence. Paris: Health Publications, Ltd., 2005.
MacDiarmid SA, Peters KM, Shobeiri SA, et al. Long-term durability of percutaneous tibial nerve stimulation for the treatment of overactive bladder. J Urol. 2010;183(1):234-240.
McGrother CW, Donaldson MM, Hayward T, et al. Leicestershire MRC Incontinence Study Team. Urinary storage symptoms and comorbidities: a prospective population cohort study in middle-aged and older women. Age Ageing. 2006;35(1):16-24.
Milsom I, Altman D, Lapitan MC, et al. Epidemiology of urinary (UI) and faecal (FI) incontinence and pelvic organ prolapse (POP). In Abrams P, Cardozo L, Khoury S, Wein A, editors: Incontinence, 4th ed, Plymouth (UK): Health Publications Ltd, 2009.
Mishra GD, Hardy R, Cardozo L, Kuh D. Body weight through adult life and risk of urinary incontinence in middle-aged women: results from a British prospective cohort. Int J Obes (Lond). 2008;32(9):1415-1422.
Subak LL, Wing R, West DS, et al. PRIDE Investigators. Weight loss to treat urinary incontinence in overweight and obese women. N Engl J Med. 2009;360(5):481-490.
Tomlinson BU, Cougherty MC, Pendergast JF, et al. Dietary caffeine, fluid intake and urinary incontinence in older rural women. Int Urogyn J. 1999;10:22-28.
Townsend MK, Danforth KN, Rosner B, et al. Physical activity and incident urinary incontinence in middle-aged women. J Urol. 2008;179(3):1012-1016. discussion 1016-17
Wyman JF, Fantl JA, McClish DK, Bump RC. Comparative efficacy of behavioral interventions in the management of female urinary incontinence. Am J Obstet Gynecol. 1998;179:999.
Allen WA, Leek H, Izurieta A, Moore KH. Update: the “Contiform” intravaginal device in four sizes for the treatment of stress incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(6):757-761.
Anger JT, Saigal CS, Madison R, et al. Urologic Diseases of America Project. Increasing costs of urinary incontinence among female Medicare beneficiaries. J Urol. 2006;176(1):247-251. discussion 251
Arya LA, Myers DL, Jackson ND. Dietary caffeine intake and the risk for detrusor instability: a case-control study. Obstet Gynecol. 2000;96:85-89.
Auwad W, Steggles P, Bombieri L, et al. Moderate weight loss in obese women with urinary incontinence: a prospective longitudinal study. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(9):1251-1259.
Avery JC, Gill TK, MacLennan AH, et al. The impact of incontinence on health-related quality of life in a South Australian population sample. Aust N Z J Public Health. 2004;28:173-179.
Bergman A, Bhatia NN. Pessary test: simple prognostic test in women with stress urinary incontinence. Urology. 1984;24:109.
Bergman A, Koonings PP, Ballard CA. Predicting postoperative urinary incontinence development in women undergoing operation for genitourinary prolapse. Am J Obstet Gynecol. 1988;158:1171-1175.
Bhatia NN, Bergman A, Gunning JE. Urodynamic effects of a vaginal pessary in women with stress urinary incontinence. Am J Obstet Gynecol. 1983;147:876.
Bo K, Kvarstein B, Hagen R, et al. Pelvic floor muscle exercise for the treatment of female stress urinary incontinence: III. Effects of two different degrees of pelvic floor muscle exercises. Neurourol Urodynam. 1990;9:489-502.
Bo K, Kvarstein B, Hagen R, Larsen S. Pelvic floor muscle exercise for the treatment of female stress urinary incontinence: II. Validity of vaginal pressure measurements of pelvic floor muscle strength and the necessity of supplementary methods for control of correct contraction. Neurourol Urodynam. 1990;9:479-487.
Bo K, Kvarstein B, Nygaard I. Lower urinary tract symptoms and pelvic floor muscle exercise adherence after 15 years. Obstet Gynecol. 2005;105:999-1005.
Brown JS, Grady D, Ouslander JG, et al. Prevalence of urinary incontinence and associated risk factors in postmenopausal women. Heart & Estrogen/Progestin Replacement Study (HERS) Research Group. Obstet Gynecol. 1999;94:66-70.
Brown JS, Wing R, Barrett-Connor E, et al. Diabetes Prevention Program Research Group. Lifestyle intervention is associated with lower prevalence of urinary incontinence: the Diabetes Prevention Program. Diabetes Care. 2006;29(2):385-390.
Brubaker L, Benson JT, Bent A, et al. Transvaginal electrical stimulation for female urinary incontinence. Am J Obstet Gynecol. 1997;177:536-540.
Brubaker L, Cundiff G, Fine P, et al. Pelvic Floor Disorders Network. A randomized trial of colpopexy and urinary reduction efforts (CARE): design and methods. Control Clin Trials. 2003;24(5):629-642.
Brubaker L, Nygaard I, Richter HE, et al. Two-year outcomes after sacrocolpopexy with and without Burch to prevent stress urinary incontinence. Obstet Gynecol. 2008;112(1):49-55.
Brubaker L, Shott S, Tomezsko J, Goldberg RP. Pelvic floor fitness using lay instructors. Obstet Gynecol. 2008;111(6):1298-1304.
Brubaker L, Shull B. EGGS for patient-centered outcomes. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:171-173.
Bryant CM, Dowell CJ, Fairbrother G. Caffeine reduction education to improve urinary symptoms. Br J Nurs. 2002;11:560-565.
Bump RC, Hurt WG, Fantyl JA, Wyman JF. Assessment of Kegel pelvic muscle exercise performance after brief verbal instruction. Am J Obstet Gynecol. 1991;165:322-329.
Burgio KL. Behavioral treatment options for urinary incontinence. Gastroenterology. 2004;126:S82-S89.
Burgio KL, Locher JL, Goode PS, et al. Behavioral vs. drug treatment for urge urinary incontinence in older women. JAMA. 1998;280:1995-2000.
Burgio KL, Locher JL, Goode PS, et al. Combined behavioral and drug therapy for urge incontinence in older women. J Am Geriatr Soc. 2000;48:370-374.
Burgio KL, Richter HE, Clements RH, et al. Changes in urinary and fecal incontinence symptoms with weight loss surgery in morbidly obese women. Obstet Gynecol. 2007;110(5):1034-1040.
But I, Faganelj M, Sostaric A. Functional magnetic stimulation for mixed urinary incontinence. J Urol. 2005;173:1644-1646.
Cammu H, Van Nylen M, Amy JJ. A 10-year follow-up after Kegel pelvic floor muscle exercises for genuine stress incontinence. BJU Int. 2000;85:655-658.
Cammu H, Van Nylen M, Blockeel C, et al. Who will benefit from pelvic floor muscle training for stress urinary incontinence? Am J Obstet Gynecol. 2004;191:1152-1157.
Chaikin DC, Groutz A, Blaivas JG. Predicting the need for anti-incontinence surgery in continent women undergoing repair of severe urogenital prolapse. J Urol. 2000;163:531-534.
Choe JH, Choo MS, Lee KS. Symptom change in women with overactive bladder after extracorporeal magnetic stimulation: a prospective trial. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(8):875-880.
Consensus conference. Urinary incontinence in adults. JAMA. 1989;261:2685-2690.
Corujo M, Badlani GH, Regan JB, et al. A new temporary catheter (Conticath) for the treatment of temporary, reversible, postoperative urinary retention. Urology. 1999;53:1104-1107.
Coyne KS, Payne C, Bhattacharyya SK, et al. The impact of urinary urgency and frequency on health-related quality of life in overactive bladder: results from a national community survey. Value Health. 2004;7:455-463.
Coyne KS, Zhou Z, Thompson C, Versi E. The impact on health-related quality of life of stress, urge and mixed urinary incontinence. BJU Int. 2003;92:731-735.
Cross CA, Cespedes D, McGuire EJ. Treatment results using pubovaginal slings in patients with large cystoceles and stress incontinence. J Urol. 1997;158:431-434. See comment by Zimmern PE in J Urol 1998;160:132–4, with reply
Dallosso H, Matthews R, McGrother C, Donaldson M. Diet as a risk factor for the development of stress urinary incontinence: a longitudinal study in women. Eur J Clin Nutr. 2004;58:920-926.
Dallosso HM, Matthews RJ, McGrother CW, et al. The association of diet and other lifestyle factors with the onset of overactive bladder: a longitudinal study in men. Public Health Nutr. 2004;7:885-891.
Dallosso HM, McGrother CW, Matthews RJ, et al. The association of diet and other lifestyle factors with overactive bladder and stress incontinence: a longitudinal study in women. BJU Int. 2003;92:69.
Danforth KN, Shah AD, Townsend MK, et al. Physical activity and urinary incontinence among healthy, older women. Obstet Gynecol. 2007;109(3):721-727.
Danforth KN, Townsend MK, Curhan GC, et al. Type 2 diabetes mellitus and risk of stress, urge and mixed urinary incontinence. J Urol. 2009;181(1):193-197.
Davilla GW, Ostermann KV. The bladder neck support prosthesis: a nonsurgical approach to stress incontinence in adult women. Am J Obstet Gynecol. 1994;171:206-211.
Diokno AC, Burgio K, Fultz NH, et al. Medical and self-care practices reported by women with urinary incontinence. Am J Manag Care. 2004;10:69-78.
Diokno AC, Sampselle CM, Herzog AR, et al. Prevention of urinary incontinence by behavioral modification program: a randomized, controlled trial among older women in the community. J Urol. 2004;171:1165-1171.
Diokno AC, Wells TJ, Brink CA. Comparison of self-reported voided volume with cystometric bladder capacity. J Urol. 1987;137:698-700.
Dmochowski R. Occlusive and supportive devices. Lecture from panel discussion on Female Urology: Non-surgical Management of Stress Incontinence at 1998 AUA meeting, San Diego, CA. Available on CD-ROM Highlights of the 1998 American Urological Association Annual Meeting. Houston, AUA Office of Education, 1998.
Dowd TT, Campbell JM, Jones JA. Fluid intake and urinary incontinence in older community-dwelling women. J Community Health Nurs. 1996;13:179-186.
Dumoulin C, Hay-Smith J. Pelvic floor muscle training versus no treatment for urinary incontinence in women. A Cochrane systematic review. Eur J Phys Rehabil Med. 2008;44(1):47-63.
Elder DD, Stephenson TP. An assessment of the Frewen regime in the treatment of detrusor dysfunction in females. Br J Urol. 1980;52:467-471.
Elkadry EA, Kenton KS, FitzGerald MP, et al. Patient-selected goals: a new perspective on surgical outcome. Am J Obstet Gynecol. 2003;189:1551-1557. discussion 1557–1558
Fantyl JA, Wyman JF, McLish DK, et al. Efficacy of bladder training in older women with urinary incontinence. JAMA. 1991;265:609-613.
Farrell SA, Baydock S, Amir B, Fanning C. Effectiveness of a new self-positioning pessary for the management of urinary incontinence in women. Am J Obstet Gynecol. 2007;196(5):474. e1–8
Fianu S, Kjaeldgaard A, Larson B. Preoperative screening for latent stress incontinence in women with cystocele. Neurourol Urodyn. 1985;4:3.
Filocamo MT, Li Marzi V, Del Popolo G, et al. Effectiveness of early pelvic floor rehabilitation treatment for post-prostatectomy incontinence. Eur Urol. 2005;48(5):734-738.
Finazzi Agrò E, Campagna A, Sciobica F, et al. Posterior tibial nerve stimulation: is the once-a-week protocol the best option? Minerva Urol Nefrol. 2005;57(2):119-123.
Fornell EU, Wingren G, Kjolhede P. Factors associated with pelvic floor dysfunction with emphasis on urinary and fecal incontinence and genital prolapse: an epidemiological study. Acta Obstet Gynecol Scand. 2004;83:383-389.
Frewen WK. An objective assessment of the unstable bladder of psychosomatic origin. Br J Urol. 1978;50:246-249.
Frewen WK. The management of urgency and frequency of micturition. Br J Urol. 1980;52:367-369.
Galloway NTM, El-Galley RES, Sand PK, et al. Extracorporeal magnetic innervation therapy for stress urinary incontinence. Urology. 1999;53:1108-1111.
Ghoneim GM, Walters F, Lewis V. The value of the vaginal pack test in large cystoceles. J Urol. 1994;152:931-934.
Gilling PJ, Wilson LC, Westenberg AM, et al. A double-blind randomized controlled trial of electromagnetic stimulation of the pelvic floor vs sham therapy in the treatment of women with stress urinary incontinence. BJU Int. 2009;103(10):1386-1390.
Goode PS. Predictors of treatment response to behavioral therapy and pharmacotherapy for urinary incontinence. Gastroenterology. 2004;126(Suppl. 1):S141-S145.
Goode PS, Burgio KL, Locher JL, et al. Effect of behavioral training with or without pelvic floor electrical stimulation on stress incontinence in women: a randomized controlled trial. JAMA. 2003;290:345-352.
Govier FE, Litwiller S, Nitti V, et al. Percutaneous afferent neuromodulation for the refractory overactive bladder: results of a multicenter study. J Urol. 2001;165(4):1193-1198.
Hagen S, Stark D, Glazener C, et al. A randomized controlled trial of pelvic floor muscle training for stages I and II pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(1):45-51.
Hajjar RR. Psychosocial impact of urinary incontinence in the elderly population. Clin Geriatr Med. 2004;20:553-564.
Hannestad YS, Rortveit G, Daltveit AK, Hunskaar S. Are smoking and other lifestyle factors associated with female urinary incontinence? The Norwegian EPINCONT Study. Br J Obstet Gynaecol. 2003;110:247-254.
Hasan ST, Robson WA, Pridie AK, Neal DE. Transcutaneous electrical nerve stimulation and temporary S3 neuromodulation in idiopathic detrusor instability. J Urol. 1996;155:2005-2011.
Hashim H, Abrams P. How should patients with an overactive bladder manipulate their fluid intake? BJU Int. 2008;102(1):62-66.
Hay-Smith J, Berghmans B, Burgio K, et al. Adult conservative management. In Abrams P, Cardozo L, Khoury S, Wein A, editors: Incontinence, 4th ed, Plymouth (UK): Health Publications Ltd, 2009.
Herbison P, Plevnik S, Mantle J. Weighted vaginal cones for urinary incontinence (Cochrane Review). Chichester, UK: John Wiley & Sons; 2004.
Hextall A, Boos K, Cardozo L, et al. Videocystourethrography with a ring pessary in situ: a clinically useful preoperative investigation for continent women with urogenital prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:205-209.
Holmes DM, Stone AR, Barry PR. Bladder training 3 years on. Br J Urol. 1983;55:660-664.
Hoscan MB, Dilmen C, Perk H, et al. Extracorporeal magnetic innervation for the treatment of stress urinary incontinence: results of two-year follow-up. Urol Int. 2008;81(2):167-172.
Hu TW, Wagner TH, Bentkover JD, et al. Costs of urinary incontinence and overactive bladder in the United States: a comparative study. Urology. 2004;63:461-465.
Hu TW, Wagner TH, Hawthorne G, et al. Economics of incontinence. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence. Paris: Health Publications, 2005.
Hullfish KL, Bovbjerg VE, Steers WD. Patient-centered goals for pelvic floor dysfunction surgery: long-term follow-up. Am J Obstet Gynecol. 2004;191:201-205.
Hunter K, Moore K, Glazener CM, et al. Conservative management of post-prostatectomy incontinence. [Cochrane review.]. In: The Cochrane library. Oxford: Update Software; 2004.
Ismail SI, Forward G, Bastin L, et al. Extracorporeal magnetic energy stimulation of pelvic floor muscles for urodynamic stress incontinence of urine in women. J Obstet Gynaecol. 2009;29(1):35-39.
Kannan H, Radican L, Turpin RS, Bolge SC. Burden of illness associated with lower urinary tract symptoms including overactive bladder/urinary incontinence. Urology. 2009;74(1):34-38.
Kegel AH. Progressive resistance exercise in the functional restoration of the perineal muscles. Am J Obstet Gynecol. 1948;56:238-248.
Kegel AH. The non-surgical treatment of genital relaxation. Ann West Med Surg. 1948;5:213.
Kegel AH. Stress incontinence of urine in women: physiologic treatment. J Int Coll Surg. 1956;25:487-499.
Kincade JE, Dougherty MC, Carlson JR, et al. Randomized clinical trial of efficacy of self-monitoring techniques to treat urinary incontinence in women. Neurourol Urodyn. 2007;26(4):507-511.
Kinchen KS, Lee J, Fireman B, et al. The prevalence, burden, and treatment of urinary incontinence among women in a managed care plan. J Womens Health (Larchmt). 2007;16(3):415-422.
Klaskov P, Belving D, Bischoff N, et al. Pelvic floor exercise versus surgery for female urinary stress incontinence. Urol Int. 1986;41:129-132.
Klutke JJ, Ramos S. Urodynamic outcome after surgery for severe prolapse and potential stress incontinence. Am J Obstet Gynecol. 2000;182:1378-1381.
Kondo A, Emoto A, Katoh K, et al. Long-term results of the pelvic floor muscle training for female urinary incontinence: an 8-year transition tree and predictive parameters. Neurourol Urodyn. 2007;26(4):495-501.
Kuruba R, Almahmeed T, Martinez F, et al. Bariatric surgery improves urinary incontinence in morbidly obese individuals. Surg Obes Relat Dis. 2007;3(6):586-590. discussion 590–1
Larrieu S, Peres K, Letenneur L, et al. Relationship between body mass index and different domains of disability in older persons: the 3C study. Int J Obes Relat Metab Disord. 2004;28:1555-1560.
Laungani RG, Seleno N, Carlin AM. Effect of laparoscopic gastric bypass surgery on urinary incontinence in morbidly obese women. Surg Obes Relat Dis. 2009;5(3):334-338.
Lawrence JM, Lukacz ES, Liu IL, et al. Pelvic floor disorders, diabetes, and obesity in women: findings from the Kaiser Permanente Continence Associated Risk Epidemiology Study. Diabetes Care. 2007;30(10):2536-2541.
MacDiarmid SA, Peters KM, Shobeiri SA, et al. Long-term durability of percutaneous tibial nerve stimulation for the treatment of overactive bladder. J Urol. 2010;183(1):234-240.
MacDonald R, Fink HA, Huckabay C, et al. Pelvic floor muscle training to improve urinary incontinence after radical prostatectomy: a systematic review of effectiveness. BJU Int. 2007;100(1):76-81.
Maher JW, Martin Hawver L, Pucci A, et al. Four hundred fifty consecutive laparoscopic Roux-en-Y gastric bypasses with no mortality and declining leak rates and lengths of stay in a bariatric training program. J Am Coll Surg. 2008;206(5):940-944. discussion 944–5
McFarlane JP, Foley SJ, de Winter P, et al. Acute suppression of idiopathic detrusor instability with magnetic stimulation of the sacral nerve roots. Br J Urol. 1997;80(5):734-741.
McGrother CW, Donaldson MM, Hayward T, et al. Leicestershire MRC Incontinence Study Team. Urinary storage symptoms and comorbidities: a prospective population cohort study in middle-aged and older women. Age Ageing. 2006;35(1):16-24.
McGuire EJ, Shi-Chun Z, Horwinski ER, et al. Treatment of motor and sensory detrusor instability by electrical stimulation. J Urol. 1983;129:78-79.
Melville JL, Delaney K, Newton K, Katon W. Incontinence severity and major depression in incontinent women. Obstet Gynecol. 2005;106(3):585-592.
Melville JL, Katon W, Delaney K, Newton K. Urinary incontinence in US women: a population-based study. Arch Intern Med. 2005;165:537-542.
Miller DS. Contemporary use of the pessary. In: Sciarra JJ, editor. Gynecology and obstetrics, vol. 1. Philadelphia: Lippincott-Raven; 1991:1-12. [chapter 39]
Miller JL, Bavendam T. Treatment with the Reliance urinary control insert: one-year experience. J Endourol. 1996;10:287-292.
Miller JM, Perucchini D, Carchidi LT, et al. Pelvic floor muscle contraction during a cough and decreased vesical neck mobility. Obstet Gynecol. 2001;97:255.
Miller JM, Sampselle C, Ashton-Miller J, et al. Clarification and confirmation of the Knack maneuver: the effect of volitional pelvic floor muscle contraction to preempt expected stress incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(6):773-782.
Milsom I, Altman D, Lapitan MC, et al. Epidemiology of urinary (UI) and faecal (FI) incontinence and pelvic organ prolapse (POP). In Abrams P, Cardozo L, Khoury S, Wein A, editors: Incontinence, 4th ed, Plymouth, UK: Health Publications Ltd, 2009.
Mishra GD, Hardy R, Cardozo L, Kuh D. Body weight through adult life and risk of urinary incontinence in middle-aged women: results from a British prospective cohort. Int J Obes (Lond). 2008;32(9):1415-1422.
Mørkved S, Bo K, Schei B, Salvesen KA. Pelvic floor muscle training during pregnancy to prevent urinary incontinence: a single-blind randomized controlled trial. Obstet Gynecol. 2003;101:313-319.
National Association for Continence (NAFC). Consumer focus ’99. Spartanburg [SC]: NAFC; 1999.
National Institutes of Health, Office of Science Policy and Planning, Economic Studies Program. Disease-Specific estimates of direct and indirect costs of illness and NIH support. Fiscal year 2000 update. (Table 1A: Costs of illness and NIH support for selected diseases and conditions). http://ospp.od.nih.gov/pdf/table_1.pdf.
Noblett KL, McKinney A, Lane FL. Effects of the incontinence dish pessary on urethral support and urodynamic parameters. Am J Obstet Gynecol. 2008;198(5):592. e1–5
Norton PA, Baker JE. Postural changes can reduce leakage in women with stress urinary incontinence. Obstet Gynecol. 1994;84:770-774.
Nygaard I. Prevention of exercise incontinence with mechanical devices. J Reprod Med. 1995;40(2):89-94.
Nygaard IE, Thompson FL, Svengalis SL, Albright JP. Urinary incontinence in elite nulliparous athletes. Obstet Gynecol. 1994;84(2):183-187.
Overgård M, Angelsen A, Lydersen S, Mørkved S. Does physiotherapist-guided pelvic floor muscle training reduce urinary incontinence after radical prostatectomy? A randomised controlled trial. Eur Urol. 54(2), 2008. 438–438
Oxford Centre for Evidence Based Medicine. Levels of evidence and grades of recommendation. www.cebm.net/downloads/Oxford_CEBM_Levels_5.rtf.
Palmer MH. Use of health behavior change theories to guide urinary incontinence research. Nurs Res. 2004;53(6 Suppl.):S49-S55.
Payne CK. Electrical stimulation: theory and practice. In: O’Donnell PD, editor. Urinary incontinence. St. Louis: Mosby–Year Book; 1996:287-294.
Payne CK. Behavioral therapy for the overactive bladder. Urology. 2000;55:3-6.
Payne CK. Urinary incontinence: nonsurgical management. In: Walsh PC, Retik AB, Vaughan ED, et al, editors. Campbell’s urology, vol. 2. Philadelphia: WB Saunders; 2002:1069-1091.
Peattie AB, Plevnik S, Stanton SL. Vaginal cones: a conservative method of treating genuine stress incontinence. Br J Obstet Gynaecol. 1988;95:1049-1053.
Peters KM, Macdiarmid SA, Wooldridge LS, et al. Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: results from the overactive bladder innovative therapy trial. J Urol. 2009;182(3):1055-1061.
Prashar S, Simons A, Bryant C, et al. Attitudes to vaginal/urethral touching and device placement in women with urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2000;11(1):4-8.
Reilly ET, Freeman RM, Waterfield MR, et al. Prevention of postpartum stress incontinence in primigravidae with increased bladder neck mobility: a randomised controlled trial of antenatal pelvic floor exercises. Br J Obstet Gynaecol. 2002;109:68-76.
Richardson DA, Miller KL, Siegel SW, et al. Pelvic floor electrical stimulation: a comparison of daily and every-other-day therapy for genuine stress incontinence. Urology. 1996;48:110-118.
Richter HE, Burgio KL, Brubaker L, et al. Urinary Incontinence Treatment Network. Factors associated with incontinence frequency in a surgical cohort of stress incontinent women. Am J Obstet Gynecol. 2005;193(6):2088-2093.
Robinson H, Schulz J, Flood C, Hansen L. A randomized controlled trial of the NEAT expandable tip continence device. Int Urogynecol J Pelvic Floor Dysfunct. 2003;14(3):199-203. discussion 203
Romanzi LJ, Polaneczky M, Glazer HI. Simple test of pelvic muscle contraction during pelvic examination: correlation to surface electromyography. Neurourol Urodyn. 1999;18:603-612.
Sahai A, Khan MS, Dasgupta P. Efficacy of botulinum toxin-A for treating idiopathic detrusor overactivity: results from a single center, randomized, double-blind, placebo controlled trial. J Urol. 2007;177(6):2231-2236.
Sampselle CM, Miller JM, Mims BL, et al. Effect of pelvic muscle exercise on transient incontinence during pregnancy and after birth. Obstet Gynecol. 1998;91:406-412.
Sand PK, Richardson DA, Staskin DR, et al. Pelvic floor electrical stimulation in the treatment of genuine stress incontinence: a multicenter, placebo-controlled trial. Am J Obstet Gynecol. 1995;173:72-79.
Schurch B, de Seze M, Denys P, et al. Botulinum toxin type A is a safe and effective treatment for neurogenic urinary incontinence: results of a single treatment, randomized, placebo controlled 6-month study. J Urol. 2005;174:196-200.
Shaw C. A review of the psychosocial predictors of help-seeking behaviour and impact on quality of life in people with urinary incontinence. J Clin Nurs. 2001;10:15-24.
Sirls LT, Foote JE, Kaufman JM, et al. Long-term results of the FemSoft urethral insert for the management of female stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2002;13:88-95. discussion 95
Song YF, Zhang WJ, Song J, Xu B. Prevalence and risk factors of urinary incontinence in Fuzhou Chinese women. Chin Med J (Engl). 2005;118:887-892.
Staskin D, Bavendam T, Miller J, et al. Effectiveness of a urinary control insert in the management of stress urinary incontinence: early results of a multicenter study. Urology. 1998;47:829-836.
Steers WD, Lee KS. Depression and incontinence. World J Urol. 2001;19:351-357.
Stothers L, Thom D, Calhoun E. Urologic diseases in America project: urinary incontinence in males—demographics and economic burden. J Urol. 2005;173:1302-1308.
Subak L, Van Den Eeden S, Thom D, et al. Reproductive Risks for Incontinence Study at Kaiser Research Group. Urinary incontinence in women: direct costs of routine care. Am J Obstet Gynecol. 2007;197(6):596.
Subak LL, Whitcomb E, Shen H, et al. Weight loss: a novel and effective treatment for urinary incontinence. J Urol. 2005;174:190-195.
Subak LL, Wing R, West DS, et al. PRIDE Investigators. Weight loss to treat urinary incontinence in overweight and obese women. N Engl J Med. 2009;360(5):481-490.
Suzuki T, Yasuda K, Yamanishi T, et al. Randomized, double-blind, sham-controlled evaluation of the effect of functional continuous magnetic stimulation in patients with urgency incontinence. Neurourol Urodyn. 2007;26(6):767-772.
Swithinbank L, Hashim H, Abrams P. The effect of fluid intake on urinary symptoms in women. J Urol. 2005;174:187-189.
Tapp AJS, Hills B, Cardozo LD. Randomised study comparing pelvic floor physiotherapy with the Burch colposuspension. Neurourol Urodynam. 1989;8:356.
Thom DH, Nygaard IE, Calhoun EA. Urologic diseases in America project: urinary incontinence in women—national trends in hospitalizations, office visits, treatment and economic impact. J Urol. 2005;173:1295-1301.
Thom DH, van den Eeden SK, Brown JS. Evaluation of parturition and other reproductive variables as risk factors for urinary incontinence in later life. Obstet Gynecol. 1997;90:983-989.
Tomlinson BU, Cougherty MC, Pendergast JF, et al. Dietary caffeine, fluid intake and urinary incontinence in older rural women. Int Urogyn J. 1999;10:22-28.
Townsend MK, Curhan GC, Resnick NM, Grodstein F. BMI, waist circumference, and incident urinary incontinence in older women. Obesity. 2008;16(4):881-886.
Townsend MK, Danforth KN, Rosner B, et al. Body mass index, weight gain, and incident urinary incontinence in middle-aged women. Obstet Gynecol. 2007;110(2 Pt 1):346-353.
Townsend MK, Danforth KN, Rosner B, et al. Physical activity and incident urinary incontinence in middle-aged women. J Urol. 2008;179(3):1012-1016. discussion 1016–17
Tries J, Eisman E. Urinary incontinence: evaluation and biofeedback treatment. In: Schwartz MS, editor. Biofeedback: practitioner’s guide. New York: Guilford Press, 1995.
Urinary Incontinence Guideline Panel. Urinary incontinence in adults: clinical practice guideline. AHCPR publication No. 92-0038. Rockville, MD: Agency for Health Care Policy and Research, Public Health Service, U.S. Department of Health and Human Services; March 1992.
Visco AG, Brubaker L, Nygaard I, et al. Pelvic Floor Disorders Network. The role of preoperative urodynamic testing in stress-continent women undergoing sacrocolpopexy: the Colpopexy and Urinary Reduction Efforts (CARE) randomized surgical trial. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(5):607-614.
Weatherall M. Biofeedback in urinary incontinence: past, present and future. Curr Opin Obstet Gynecol. 2000;12:411-413.
Wilson PD, Berghmans B, Hagen S. Adult conservative management. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence. Paris: Health Publications Ltd, 2005.
Woldringh C, van den Wijngaart M, Albers-Heitner P, et al. Pelvic floor muscle training is not effective in women with UI in pregnancy: a randomised controlled trial. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(4):383-390.
Wyman JF, Fantl JA, McClish DK, Bump RC. Comparative efficacy of behavioral interventions in the management of female urinary incontinence. Am J Obstet Gynecol. 1998;179:999.
Yokoyama T, Fujita O, Nishiguchi J, et al. Extracorporeal magnetic innervation treatment for urinary incontinence. Int J Urol. 2004;11(8):602-606.
Ziv E, Stanton SL, Abarbanel J. Efficacy and safety of a novel disposable intravaginal device for treating stress urinary incontinence. Am J Obstet Gynecol. 2008;198(5):594. e1–7