CHAPTER 22 The Role of Dental Calculus and Other Local Predisposing Factors
The primary cause of gingival inflammation is bacterial plaque. Other predisposing factors include calculus, faulty restorations, complications associated with orthodontic therapy, self-inflicted injuries, use of tobacco, and others. These will be discussed in turn.
Calculus
Calculus consists of mineralized bacterial plaque that forms on the surfaces of natural teeth and dental prostheses.
Supragingival and Subgingival Calculus
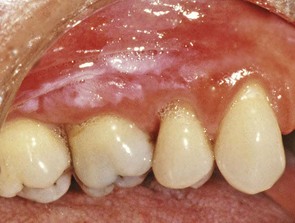
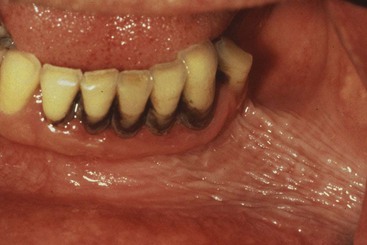
Supragingival calculus is located coronal to the gingival margin and therefore is visible in the oral cavity. It is usually white or whitish yellow in color, hard with claylike consistency, and easily detached from the tooth surface. After removal, it may rapidly recur, especially in the lingual area of the mandibular incisors. The color is influenced by contact with such substances as tobacco and food pigments. It may localize on a single tooth or group of teeth, or it may be generalized throughout the mouth.
The two most common locations for supragingival calculus to develop are the buccal surfaces of the maxillary molars (Figure 22-1) and the lingual surfaces of the mandibular anterior teeth (Figure 22-2).32 Saliva from the parotid gland flows over the facial surfaces of upper molars via the parotid duct, whereas the submandibular duct and lingual duct empty onto the lingual surfaces of the lower incisors from the submaxillary and sublingual glands, respectively. In extreme cases, calculus may form a bridgelike structure over the interdental papilla of adjacent teeth or cover the occlusal surface of teeth lacking functional antagonists.
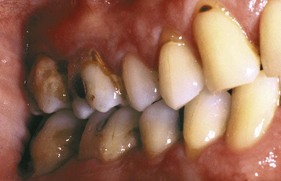
Figure 22-1 Supragingival calculus is depicted on the buccal surfaces of maxillary molars adjacent to orifice for parotid duct.
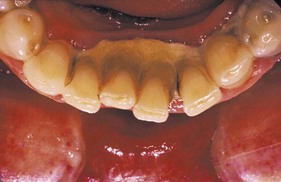
Figure 22-2 Extensive supragingival calculus is present on the lingual surfaces of lower anterior teeth.
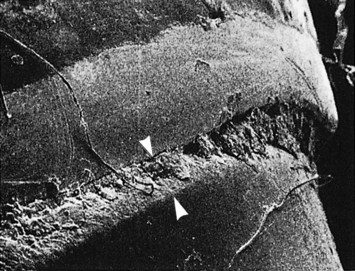
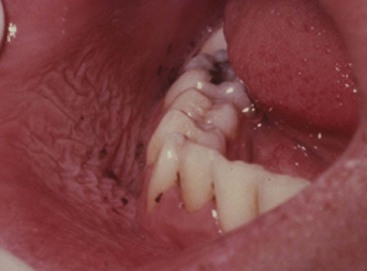
Subgingival calculus is located below the crest of the marginal gingiva and therefore is not visible on routine clinical examination. The location and extent of subgingival calculus may be evaluated by careful tactile perception with a delicate dental instrument such as an explorer. Clerehugh et al29 used a World Health Organization #621 probe to detect and score subgingival calculus. Subsequently, these teeth were extracted and visually scored for subgingival calculus. An agreement of 80% was found between these two scoring methods. Subgingival calculus is typically hard and dense and frequently appears dark brown or greenish black in color (Figure 22-3) and is firmly attached to the tooth surface. Supragingival calculus and subgingival calculus generally occur together, but one may be present without the other. Microscopic studies demonstrate that deposits of subgingival calculus usually extend nearly to the base of periodontal pockets in chronic periodontitis but do not reach the junctional epithelium.
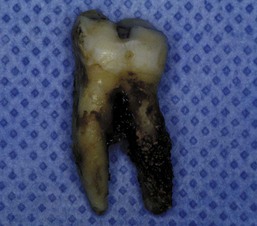
Figure 22-3 Dark pigmented deposits of subgingival calculus are shown on the distal root of an extracted lower molar.
When the gingival tissues recede, subgingival calculus becomes exposed and is therefore reclassified as supragingival (Figure 22-4). Thus supragingival calculus can be composed of both supragingival calculus and previous subgingival calculus. A reduction in gingival inflammation and probing depths with a gain in clinical attachment can be observed after the removal of subgingival plaque and calculus (Figure 22-5) (see Chapter 45).
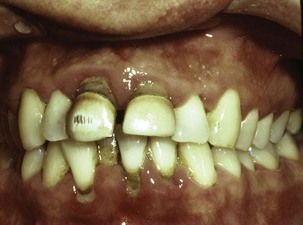
Figure 22-4 A 31-year-old white male is shown with extensive supragingival and subgingival calculus deposits throughout his dentition.
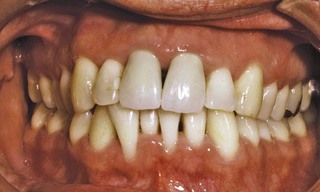
Figure 22-5 The same patient shown in Figure 22-4, 1 year after receiving thorough scaling and root planing to remove supragingival and subgingival calculus deposits, followed by restorative care. Note the substantial reduction in gingival inflammation.
Prevalence
Anerud and co-workers4 observed the periodontal status of a group of Sri Lankan tea laborers and a group of Norwegian academicians for a 15-year period. The Norwegian population had ready access to preventive dental care throughout their lives, whereas the Sri Lankan tea laborers did not. The formation of supragingival calculus was observed early in life in the Sri Lankan individuals, probably shortly after the teeth erupted. The first areas to exhibit calculus deposits were the facial aspects of maxillary molars and the lingual surfaces of mandibular incisors. Deposition of supragingival calculus continued as individuals aged, reaching a maximal calculus score around 25 to 30 years of age. At this time, most of the teeth were covered by calculus, although the facial surfaces had less calculus than the lingual or palatal surfaces. Calculus accumulation appeared to be symmetric, and by age 45 only a few teeth, typically the premolars, were without calculus. Subgingival calculus appeared first either independently or on the interproximal aspects of areas where supragingival calculus already existed.4 By age 30, all surfaces of all teeth had subgingival calculus without any pattern of predilection.
The Norwegian academicians received oral hygiene instructions and frequent preventive dental care throughout their lives. The Norwegians exhibited a marked reduction in the accumulation of calculus as compared with the Sri Lankan group. However, despite the fact that 80% of teenagers formed supragingival calculus on the facial surfaces of the upper molars and the lingual surfaces of lower incisors, no additional calculus formation occurred on other teeth, nor did it increase with age.4
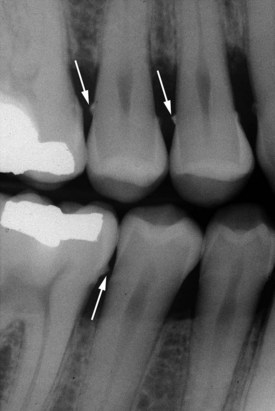
Both supragingival calculus and subgingival calculus may be seen on radiographs (see Chapter 31). Highly calcified interproximal calculus deposits are readily detectable as radiopaque projections that protrude into the interdental spaces in Figure 22-6. However, the sensitivity level of detecting calculus by radiographs is inconsistent.23 The location of calculus does not indicate the bottom of the periodontal pocket because the most apical plaque is not sufficiently calcified to be visible on radiographs.
Composition
Inorganic Content
Supragingival calculus consists of inorganic (70% to 90%50) and organic components. The major inorganic proportions of calculus have been reported as approximately 76% calcium phosphate, Ca3(PO4)2; 3% calcium carbonate, CaCO3; and traces of magnesium phosphate, Mg3(PO4)2, and other metals.169 The percentage of inorganic constituents in calculus is similar to that in other calcified tissues of the body. The principal inorganic components have been reported as approximately 39% calcium, 19% phosphorus, 2% carbon dioxide, and 1% magnesium and trace amounts of sodium, zinc, strontium, bromine, copper, manganese, tungsten, gold, aluminum, silicon, iron, and fluorine.107
At least two-thirds of the inorganic component is crystalline in structure.85 The four main crystal forms and their approximate percentages are as follows: hydroxyapatite 58%, magnesium whitlockite 21%, octacalcium phosphate 12%, and brushite 9%.
Generally, two or more crystal forms are typically found in a sample of calculus. Hydroxyapatite and octacalcium phosphate are detected most frequently (i.e., in 97% to 100% of all supragingival calculus) and constitute the bulk of the specimen. Brushite is more common in the mandibular anterior region and magnesium whitlockite in the posterior areas. The incidence of the four crystal forms varies with the age of the deposit.15
Organic Content
The organic component of calculus consists of a mixture of protein-polysaccharide complexes, desquamated epithelial cells, leukocytes, and various types of microorganisms.94
Between 1.9% and 9.1% of the organic component is carbohydrate, which consists of galactose, glucose, rhamnose, mannose, glucuronic acid, galactosamine, and sometimes arabinose, galacturonic acid, and glucosamine.87,93,154 All of these organic components are present in salivary glycoprotein, with the exception of arabinose and rhamnose. Salivary proteins account for 5.9% to 8.2% of the organic component of calculus and include most amino acids. Lipids account for 0.2% of the organic content in the form of neutral fats, free fatty acids, cholesterol, cholesterol esters, and phospholipids.88
The composition of subgingival calculus is similar to that of supragingival calculus, with some differences. It has the same hydroxyapatite content, more magnesium whitlockite, and less brushite and octacalcium phosphate.136,158 The ratio of calcium to phosphate is higher subgingivally, and the sodium content increases with the depth of periodontal pockets.89 These altered compositions may be attributed to the origin of subgingival calculus being plasma, whereas supragingival calculus is partially composed of saliva constituents. Salivary proteins present in supragingival calculus are not found subgingivally.10 Dental calculus, salivary duct calculus, and calcified dental tissues are similar in inorganic composition.
Attachment to the Tooth Surface
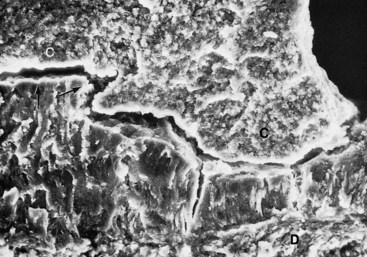
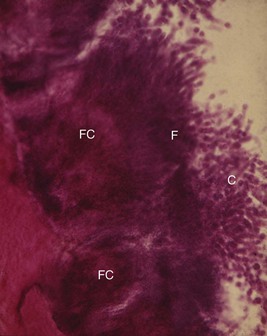
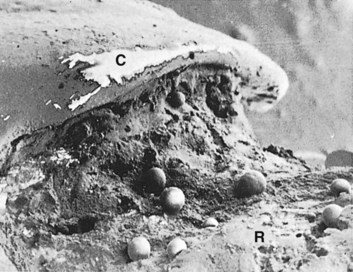
Differences in the manner in which calculus is attached to the tooth surface affect the relative ease or difficulty encountered in its removal. Four modes of attachment have been described.81,138,145,173 Attachment by means of an organic pellicle on cementum is depicted in Figure 22-7, and attachment on enamel is shown in Figure 22-8. Mechanical locking into surface irregularities, such as caries lesions or resorption lacunae, is illustrated in Figure 22-9. The fourth mode of attachment consists of close adaptation of the undersurface of calculus to depressions or gently sloping mounds of the unaltered cementum surface,154 as shown in Figure 22-10, and penetration of bacterial calculus into cementum, as shown in Figures 22-11 and 22-12.
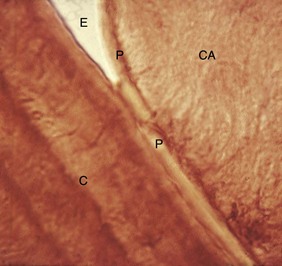
Figure 22-7 Calculus attached to pellicle on enamel surface and cementum. An enamel void (E) has been created in the preparation of the specimen. CA, Calculus; P, pellicle; C, cementum.
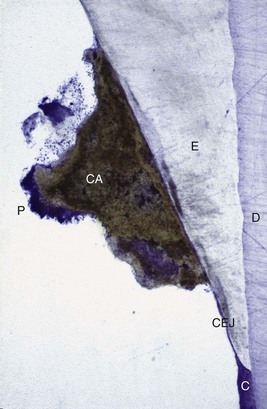
Figure 22-8 Non-decalcified specimen with calculus (CA) attached to enamel (E) surface just coronal to cementoenamel junction (CEJ). Note plaque (P) on the surface of the calculus; dentin (D) and cementum (C) are identified.
(Courtesy Dr. Michael Rohrer, Minneapolis.)
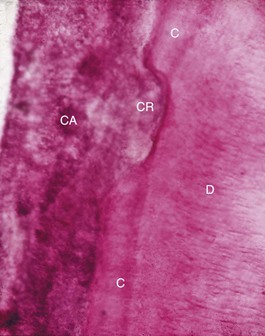
Figure 22-9 Calculus (CA) attached to a cemental resorption area (CR) with cementum (C) adjacent to dentin (D).
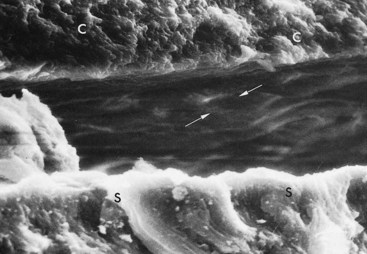
Figure 22-10 Undersurface of subgingival calculus (C) previously attached to the cementum surface (S). Note impression of cementum mounds in calculus (arrows).
(Courtesy Dr. John Sottosanti, La Jolla, CA.)
Formation
Calculus is mineralized dental plaque. The soft plaque is hardened by the precipitation of mineral salts, which usually starts between the first and fourteenth days of plaque formation. Calcification has been reported to occur in as little as 4 to 8 hours.159 Calcifying plaques may become 50% mineralized in 2 days and 60% to 90% mineralized in 12 days.106,139,149 All plaque does not necessarily undergo calcification. Early plaque contains a small amount of inorganic material, which increases as the plaque develops into calculus. Plaque that does not develop into calculus reaches a plateau of maximal mineral content within 2 days.140 Microorganisms are not always essential in calculus formation because calculus occurs readily in germ-free rodents.57
Saliva is the source of mineralization for supragingival calculus, whereas the serum transudate called gingival crevicular fluid furnishes the minerals for subgingival calculus.69,156 The calcium concentration/content in plaque is 2 to 20 times that found in saliva.15 Early plaque of heavy calculus formers contains more calcium, three times more phosphorus, and less potassium than that of noncalculus formers, suggesting that phosphorus may be more critical than calcium in plaque mineralization.94 Calcification entails the binding of calcium ions to the carbohydrate-protein complexes of the organic matrix and the precipitation of crystalline calcium phosphate salts.92 Crystals form initially in the intercellular matrix and on the bacterial surfaces and finally within the bacteria.51,174
Calcification of supragingival plaque and in the attached component of subgingival plaque begins along the inner surface adjacent to the tooth. Separate foci of calcification increase in size and coalesce to form solid masses of calculus (see Figure 22-12). Calcification may be accompanied by alterations in the bacterial content and staining qualities of the plaque. As calcification progresses, the number of filamentous bacteria increases and foci of calcification change from basophilic to eosinophilic. There is a reduction in the staining intensity of groups exhibiting a positive periodic acid–Schiff reaction.
Sulfhydryl and amino groups also are reduced and instead stain with toluidine blue, which is initially orthochromatic but becomes metachromatic and disappears.165 Calculus is formed in layers, which are often separated by a thin cuticle that becomes embedded in the calculus as calcification progresses.96
The initiation of calcification and the rate of calculus accumulation vary among individuals, between tooth variety in the same dentition, and at different times in the same person.107,161 On the basis of these differences, persons may be classified as heavy, moderate, or slight calculus formers or as noncalculus formers. The average daily increment in calculus formers is from 0.10% to 0.15% of dry weight calculus.149,161 Calculus formations continue until it reaches a maximum, after which it may be reduced in amount. The time required to reach the maximal level has been reported between 10 weeks30 and 6 months.163
The decline from maximal calculus accumulation, referred to as reversal phenomenon, may be explained by the vulnerability of bulky calculus to mechanical wear from food and from the cheeks, lips, and tongue.
Theories Regarding the Mineralization of Calculus
The theoretical mechanisms by which plaque becomes mineralized can be stratified into two categories.108
Role of Microorganisms in the Mineralization of Calculus
Mineralization of plaque generally starts extracellularly around both gram-positive and gram-negative organisms but may also start intracellularly.83 Filamentous organisms, diphtheroids, and Bacterionema and Veillonella species have the ability to form intracellular apatite crystals (see Figure 22-12). Mineralization spreads until the matrix and bacteria are calcified.51,175
Bacterial plaque may actively participate in the mineralization of calculus by forming phosphatases, which changes the pH of the plaque and induces mineralization,38,92 but the prevalent opinion is that these bacteria are only passively involved51,131,168 and are simply calcified with other plaque components. The occurrence of calculus-like deposits in germ-free animals supports this opinion.57 However, other experiments suggest that transmissible factors are involved in calculus formation and that penicillin in the diets of some of these animals reduces calculus formation.9
Etiologic Significance
Distinguishing between the effects of calculus and plaque on the gingiva is difficult because calculus is always covered with a nonmineralized layer of plaque.140 A positive correlation between the presence of calculus and the prevalence of gingivitis exists,126 but this correlation is not as great as that between plaque and gingivitis.53 The initiation of periodontal disease in young people is closely related to plaque accumulation, whereas calculus accumulation is more prevalent in chronic periodontitis found in older adults.53,86
The incidence of calculus, gingivitis, and periodontal disease increases with age. It is extremely rare to find periodontal pockets in adults without at least some subgingival calculus being present, although the subgingival calculus may be of microscopic proportions.
Calculus does not contribute directly to gingival inflammation but provides a fixed nidus for the continued accumulation of plaque and its retention in close proximity of the gingiva (Figure 22-13). Subgingival calculus is likely to be the product rather than the cause of periodontal pockets. Plaque initiates gingival inflammation, which leads to pocket formation, and the pocket in turn provides a sheltered area for plaque and bacterial accumulation. The increased flow of gingival fluid associated with gingival inflammation provides the minerals that mineralize the continually accumulating plaque resulting in the formation of subgingival calculus (Figure 22-14). Over a 6-year period, Albandar et al observed 156 teenagers with aggressive periodontitis.2 They noted that areas with detectable subgingival calculus at the initiation of the study were much more likely to experience loss of periodontal attachment than sites that did not initially exhibit subgingival calculus.
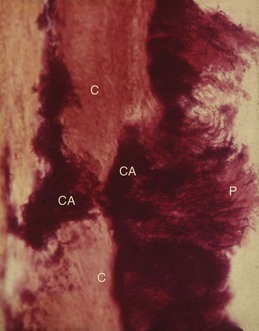
Figure 22-13 Calculus (CA) penetrates the tooth surface and is embedded within the cementum (C). Note plaque (P) attached to the calculus.
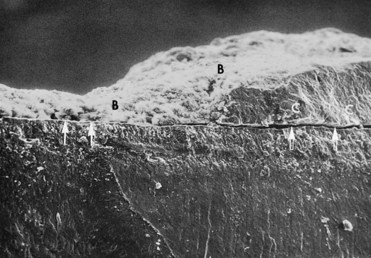
Figure 22-14 Scanning electron microscope view of an extracted human tooth showing a cross-section of subgingival calculus (C) separated (arrows) from the cemental surface during processing of specimen. Note bacteria (B) attached to calculus and cemental surfaces.
(Courtesy Dr. John Sottosanti, La Jolla, CA.)
Although the bacterial plaque that coats the teeth is the main etiologic factor in the development of periodontal disease, the removal of subgingival plaque and calculus constitute the cornerstone of periodontal therapy. Calculus plays an important role in maintaining and accentuating periodontal disease by keeping plaque in close contact with the gingival tissue and creating areas where plaque removal is impossible. Therefore the clinician must not only possess the clinical skill to remove the plaque and calculus but also must be very conscientious about performing this task.
Materia Alba, Food Debris, and Dental Stains
Materia alba is an accumulation of microorganisms, desquamated epithelial cells, leukocytes, and a mixture of salivary proteins and lipids, with few or no food particles, and it lacks the regular internal pattern observed in plaque.141 It is a yellow or grayish-white, soft, sticky deposit and is somewhat less adherent than dental plaque. The irritating effect of materia alba on the gingiva is caused by bacteria and their products.
Most food debris is rapidly liquefied by bacterial enzymes and cleared from the oral cavity by salivary flow and the mechanical action of the tongue, cheeks, and lips. The rate of clearance from the oral cavity varies with the type of food and the individual. Aqueous solutions are typically cleared within 15 minutes, whereas sticky foods may adhere for more than 1 hour.84,162 Dental plaque is not a derivative of food debris, nor is food debris an important cause of gingivitis.37,73
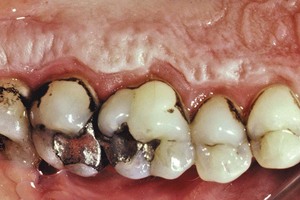
Pigmented deposits on the tooth surface are called dental stains. Stains are primarily an aesthetic problem and do not cause inflammation of the gingiva. The use of tobacco products (Figure 22-15), coffee, tea, certain mouthrinses, and pigments in foods can contribute to stain formation.90,155
Other Predisposing Factors
Iatrogenic Factors
Deficiencies in the quality of dental restorations or prostheses are contributing factors to gingival inflammation and periodontal destruction. Inadequate dental procedures that contribute to the deterioration of the periodontal tissues are referred to as iatrogenic factors. Iatrogenic endodontic complications that can adversely affect the periodontium include root perforations, vertical root fractures, and endodontic failures that may necessitate tooth extraction.172,173
Characteristics of dental restorations and removable partial dentures that are important to the maintenance of periodontal health include the location of the gingival margin for the restoration, the space between the margin of the restoration and the unprepared tooth, the contour of restorations, the occlusion, materials used in the restoration, the restorative procedure itself, and the design of the removable partial denture. These characteristics are described in this chapter as they relate to the etiology of periodontal disease. A more comprehensive review with special emphasis on the interrelationship between restorative procedures and the periodontal status is presented in Chapter 66.
Margins of Restorations
Overhanging margins of dental restorations contribute to the development of periodontal disease by (1) changing the ecologic balance of the gingival sulcus to an area that favors the growth of disease-associated organisms (predominately gram-negative anaerobic species) at the expense of the health-associated organisms (predominately gram-positive facultative species)84 and (2) inhibiting the patient’s access to remove accumulated plaque.
The frequency of overhanging margins on proximal restorations varies in different studies from 16.5% to 75%.17,49,68,115 A highly significant statistical relationship has been reported between marginal defects and reduced bone height.17,58,68 Removal of overhangs permits more effective control of plaque, resulting in a reduction of gingival inflammation and a small increase in radiographic alveolar bone support52,61,152 (Figures 22-16 and 22-17).
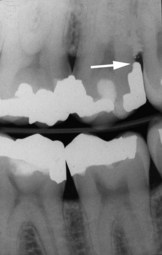
Figure 22-16 Radiograph of amalgam overhang on the distal surface of maxillary second molar that is a source of plaque retention and gingival irritation.
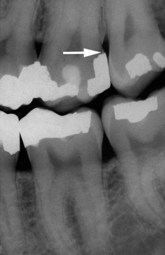
Figure 22-17 Radiograph of the same patient shown in Figure 22-16 after the excessive amalgam has been removed.
The location of the gingival margin of a restoration is directly related to the health status of adjacent periodontal tissues.150 Numerous studies have shown a positive correlation between margins located apical to the marginal gingiva and the presence of gingival inflammation.49,65,72,129,152 Subgingival margins are associated with large amounts of plaque, more severe gingivitis, and deeper pockets. Even high-quality restorations, if placed subgingivally, will increase plaque accumulation, gingival inflammation,* and the rate of gingival fluid flow.11,54 Margins placed at the level of the gingival crest induce less severe inflammation, whereas supragingival margins are associated with a degree of periodontal health similar to that seen with nonrestored interproximal surfaces.44,150
Roughness in the subgingival area is considered to be a major contributing factor to plaque buildup and subsequent gingival inflammation.150 The subgingival zone is composed of the margin of the restoration, the luting material, and the prepared, as well as the unprepared tooth surface. Sources of marginal roughness include grooves and scratches in the surface of acrylic resin, porcelain, or gold restorations (Figure 22-18); separation of the restoration margin and luting material from the cervical finish line, thereby exposing the rough surface of the prepared tooth (Figure 22-19); dissolution and disintegration of the luting material between the preparation and the restoration, leaving a space (Figure 22-20); and inadequate marginal fit of the restoration.150 Subgingival margins typically have a gap of 20 to 40 µm between the margin of the restoration and the unprepared tooth.148 Colonization of this gap by bacterial plaque undoubtedly contributes to the detrimental effect of margins placed in a subgingival environment.

Figure 22-18 A, Polished gold alloy crown demonstrates surface scratches. B, Gold alloy crown that had been in the mouth for several years has scratches filled with deposits.
(From Silness J: Dent Clin North Am 24:317, 1980.)
Contours/Open Contacts
Overcontoured crowns and restorations tend to accumulate plaque and possibly prevent the self-cleaning mechanisms of the adjacent cheek, lips, and tongue6,79,104,171 (Figures 22-21 and 22-22). Restorations that fail to reestablish adequate interproximal embrasure spaces are associated with papillary inflammation. Undercontoured crowns that lack a protective height of contour do not retain as much plaque as overcontoured crowns and therefore may not be as detrimental during mastication as once thought.171
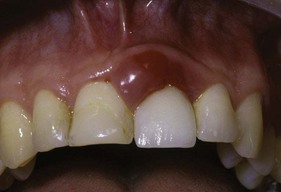
Figure 22-21 Inflamed marginal and papillary gingiva adjacent to an overcontoured porcelain–fused-to-metal crown on the maxillary left central.
The contour of the occlusal surface as established by the marginal ridges and related developmental grooves normally serves to deflect food away from the interproximal spaces. The optimal cervico-occlusal location for a posterior contact is at the longest mesiodistal diameter of the tooth, which is generally just apical to the crest of the marginal ridge. The integrity and location of the proximal contacts along with the contour of the marginal ridges and developmental grooves typically prevent interproximal food impaction. Food impaction is the forceful wedging of food into the periodontium by occlusal forces. As the teeth wear down, their originally convex proximal surfaces become flattened and the wedging effect of the opposing cusp is exaggerated. Cusps that tend to forcibly wedge food into interproximal embrasures are known as plunger cusps. The interproximal plunger cusp effect may also be observed when missing teeth are not replaced and the relationship between proximal contacts of adjacent teeth is altered. An intact proximal contact precludes the forceful wedging of food into the interproximal embrasure space, whereas a light or open contact is conducive to impaction.
The classic analysis of the factors leading to food impaction was made by Hirschfeld,63 who recognized the following factors: uneven occlusal wear, opening of the contact point as a result of loss of proximal support or from extrusion, congenital morphologic abnormalities, and improperly constructed restorations.
The presence of the previously mentioned abnormalities does not necessarily lead to food impaction and periodontal disease. A study of interproximal contacts and marginal ridge relationships84 in three groups of periodontally healthy males revealed that 0.7% to 76% of the proximal contacts were defective and 33.5% of adjacent marginal ridges were uneven.114 However, greater probing depth and loss of clinical attachment have been reported for sites that exhibited both an open contact and food impaction as compared with contralateral control sites without open contacts or food impaction.70 Excessive anterior overbite is a common cause of food impaction on the lingual surfaces of the maxillary anterior teeth and the facial surfaces of the opposing mandibular teeth. These areas may be exemplified by attachment loss with gingival recession.
Materials
In general, restorative materials are not in themselves injurious to the periodontal tissues.6,75 One exception to this may be self-curing acrylics166 (Figure 22-23).
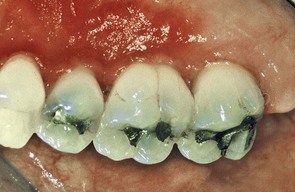
Figure 22-23 Inflamed palatal gingiva associated with a maxillary provisional acrylic partial denture. Note the substantial difference in color of the inflamed gingiva adjacent to the premolars and first molar compared with the gingiva adjacent to the second molar.
Plaque that forms at the margins of restorations is similar to that found on adjacent nonrestored tooth surfaces. The composition of plaque formed on all types of restorative materials is similar, with the exception of that formed on silicate.109 Although surface textures of restorative materials differ in their capacity to retain plaque,170 all can be adequately cleaned if they are polished and accessible to methods of oral hygiene.110,137 The undersurface of pontics in fixed bridges should barely touch the mucosa. Access for oral hygiene is inhibited with excessive pontic to tissue contact, thereby contributing to plaque accumulation that will cause gingival inflammation and possibly formation of pseudopockets.43,150
Design of Removable Partial Dentures
Several investigations have shown that after the insertion of partial dentures, the mobility of the abutment teeth, gingival inflammation, and periodontal pocket formation all increase.16,25,144 This is because partial dentures favor the accumulation of plaque, particularly if they cover the gingival tissue. Partial dentures that are worn during both night and day induce more plaque formation than those worn only during the daytime.16 These observations emphasize the need for careful and personalized oral hygiene instruction to avoid harmful effects of partial dentures on the remaining teeth and periodontium.11 The presence of removable partial dentures induces not only quantitative changes in dental plaque47 but also qualitative changes, promoting the emergence of spirochetal microorganisms.48
Restorative Dentistry Procedures
The use of rubber dam clamps, matrix bands, and burs in such a manner as to lacerate the gingiva results in varying degrees of mechanical trauma and inflammation. Although such transient injuries generally undergo repair, they are needless sources of discomfort to the patient. Forceful packing of a gingival retraction cord into the sulcus to prepare subgingival margins on a tooth or for the purpose of obtaining an impression may mechanically injure the periodontium and leave behind impacted debris capable of causing a foreign body reaction.
Malocclusion
Irregular alignment of teeth as found in cases of malocclusion may make plaque control more difficult. Several authors have found a positive correlation between crowding and periodontal disease,24,117,157 whereas other investigators did not find any correlation.46 Uneven marginal ridges of contiguous posterior teeth have been found to have a low correlation with pocket depth, loss of attachment, plaque, calculus, and gingival inflammation.77 Roots of teeth that are prominent in the arch (Figures 22-24 and 22-25), such as in a buccal or lingual version, or that are associated with a high frenal attachment and small quantities of attached gingiva frequently exhibit recession.1,101
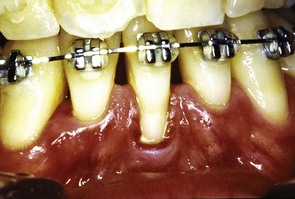
Figure 22-24 Lower incisor showing a prominent root with gingival recession and lack of attached gingiva.
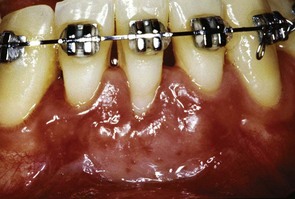
Figure 22-25 The same patient as shown in Figure 22-24 after placement of a soft tissue graft to gain attached gingiva and treat the gingival recession.
Failure to replace missing posterior teeth may have adverse consequences on the periodontal support for the remaining teeth.27 The following hypothetical scenario illustrates the possible ramifications of not replacing a missing posterior tooth. When the mandibular first molar is extracted, the initial change is a mesial drifting and tilting of the mandibular second and third molars with extrusion of the maxillary first molar. As the mandibular second molar tips mesially, its distal cusps extrude and act as plungers. The distal cusps of the mandibular second molar wedge between the maxillary first and second molars and open the contact by deflecting the maxillary second molar distally. Subsequently, food impaction may occur and be accompanied by gingival inflammation with eventual loss of the interproximal bone between the maxillary first and second molars. The preceding example does not occur in all cases where mandibular first molars are not replaced. However, drifting and tilting of the remaining teeth with an accompanying alteration of the proximal contacts is generally a consequence of not replacing posterior teeth that have been extracted.
Tongue thrusting exerts excessive lateral pressure on the anterior teeth, which may result in spreading and tilting of the anterior teeth (Figures 22-26 and 22-27). Tongue thrusting is an important contributing factor in tooth migration and the development of an anterior open bite.26 Mouth breathing may be observed in association with a habit of tongue thrusting and an anterior open bite. Marginal and papillary gingivitis is frequently encountered in the maxillary anterior sextant in cases involving an anterior open bite with mouth breathing. However, the role of mouth breathing as a local etiologic factor is unclear because conflicting evidence has been reported.3,66,67,157
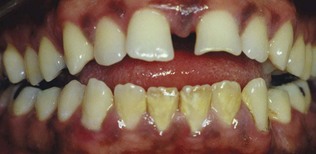
Figure 22-26 Anterior open bite with flared incisors as observed in association with a habit of tongue thrusting.
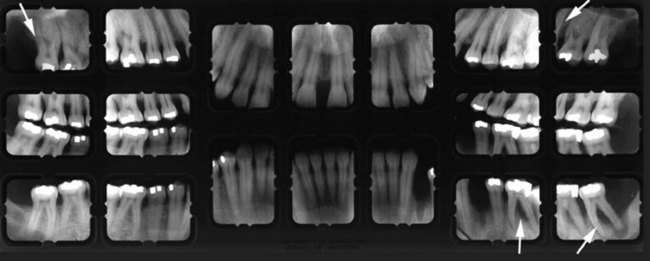
Figure 22-27 Radiographs of patient shown in Figure 22-26 with anterior open bite. Note severe periodontal destruction (arrows) in molar regions.
Restorations that do not conform to the occlusal pattern of the mouth result in occlusal disharmonies that may cause injury to the supporting periodontal tissues. Teeth with deeper initial probing depths, worse prognoses, and greater mobility have been observed for teeth with occlusal discrepancies than teeth without initial occlusal discrepancies. 60,112 Histologic features of the periodontium for a tooth subjected to traumatic occlusion include a widened subcrestal periodontal ligament space, a reduction in collagen content of the oblique and horizontal fibers, an increase in vascularity and leukocyte infiltration, and an increase in the number of osteoclasts on bordering alveolar bone.13 However, these observations are generally apical and separate from the bacterial-induced inflammation that occurs at the base of the sulcus. On the basis of current human trials, it is still impossible to definitively answer the question, “Does occlusal trauma modify the progression of periodontal attachment loss associated with periodontal inflammation?”147 (See Chapter 49 for a more detailed explanation of periodontal trauma from occlusion and the periodontal response to external forces.)
Periodontal Complications Associated with Orthodontic Therapy
Orthodontic therapy may affect the periodontium by favoring plaque retention, by directly injuring the gingiva as a result of overextended bands, and by creating excessive forces, unfavorable forces, or both on the tooth and supporting structures.
Plaque Retention and Composition
Orthodontic appliances not only tend to retain bacterial plaque and food debris, resulting in gingivitis (Figure 22-28), but also are capable of modifying the gingival ecosystem. An increase in Prevotella melaninogenica, Prevotella intermedia, and Actinomyces odontolyticus and a decrease in the proportion of facultative microorganisms was detected in the gingival sulcus after the placement of orthodontic bands.35 More recently, Aggregatibacter actinomycetemcomitans was found in at least one site in 85% of children wearing orthodontic appliances.116 In contrast, only 15% of unbanded control subjects were positive for A. actinomycetemcomitans.
Gingival Trauma and Alveolar Bone Height
Orthodontic treatment is often started soon after eruption of the permanent teeth, when the junctional epithelium is still adherent to the enamel surface. Orthodontic bands should not be forcefully placed beyond the level of attachment because this will detach the gingiva from the tooth and result in apical proliferation of the junctional epithelium with an increased incidence of gingival recession.118
The mean alveolar bone loss per patient for adolescents who underwent 2 years of orthodontic care during a 5-year observation period ranged between 0.1 and 0.5 mm.19 This small magnitude of alveolar bone loss was also noted in the control group and therefore considered to be of little clinical significance. However, the degree of bone loss during adult orthodontic care may be higher than that observed in adolescents,91 especially if the periodontal condition is not treated before initiating orthodontic therapy. Therefore orthodontic treatment should not be commenced in the presence of uncontrolled periodontal disease.
Tissue Response to Orthodontic Forces
Orthodontic tooth movement is possible because the periodontal tissues are responsive to externally applied forces.128,143 Alveolar bone is remodeled by osteoclasts inducing bone resorption in areas of pressure and osteoblasts forming bone in areas of tension. Although moderate orthodontic forces ordinarily result in bone remodeling and repair, excessive force may produce necrosis of the periodontal ligament and adjacent alveolar bone.121-123 Excessive orthodontic forces also increase the risk of apical root resorption.21,22 The prevalence of severe root resorption, as indicated by resorption of more than one third of the root length, during orthodontic therapy in adolescents has been reported at 3%.71 The incidence of moderate to severe root resorption for incisors among adults ranging in age from 20 to 45 years has been reported as 2% pretreatment and 24.5% posttreatment.91 Risk factors associated with root resorption during orthodontic treatment include duration of treatment, magnitude of the force applied, direction of the tooth movement, and continuous versus intermittent application of forces120 (Figures 22-29 to 22-31). It is important to avoid excessive force and/or too rapid of tooth movement in orthodontic treatment.
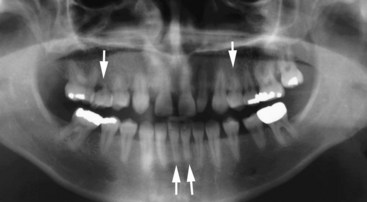
Figure 22-29 Panoramic radiograph illustrating a limited degree of pretreatment root resorption (arrows) existed before orthodontic care was initiated.
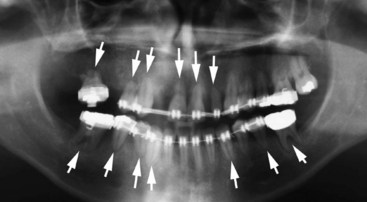
Figure 22-30 Panoramic radiograph of the same patient as shown in Figure 22-29 after 4 years of intermittent orthodontic treatment. Note that several roots have undergone severe resorption (arrows) during orthodontic care.
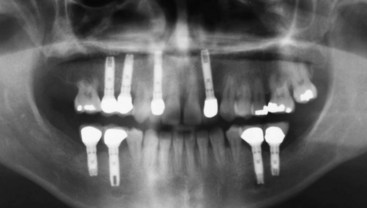
Figure 22-31 Panoramic radiograph of the same patient as depicted in Figure 22-30. Note the teeth that had developed extensive root resorption with accompanying hypermobility have been extracted and replaced by implant supported crowns.
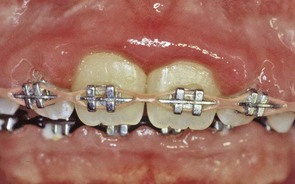
The use of elastics to close a diastema may result in severe attachment loss with possible tooth loss as the elastics migrate apically along the root (Figures 22-32 and 22-33). Surgical exposure of impacted teeth and orthodontic-assisted eruption has the potential to compromise the periodontal attachment on adjacent teeth (Figures 22-34 and 22-35). However, the majority of impacted teeth that are surgically exposed and aided in their eruption by orthodontic treatment subsequently exhibited >90% of their attachment intact.62
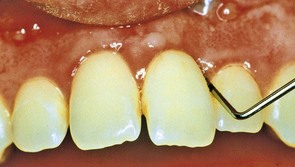
Figure 22-32 Maxillary central incisors in which an elastic ligature was used to close a midline diastema. Note inflamed gingiva and deep probing depths.
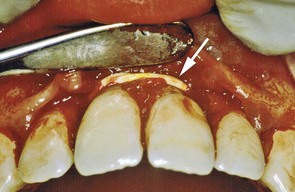
Figure 22-33 The same patient as shown in Figure 22-32 with a full-thickness mucoperiosteal flap reflected to expose the elastic ligature and angular intrabony defects around the central incisors.
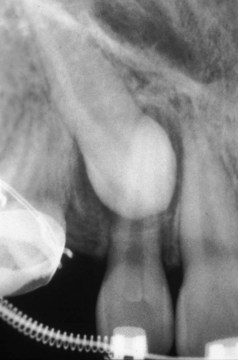
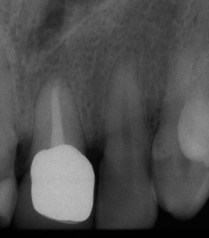
Figure 22-34 Radiograph of an impacted maxillary canine that required surgical exposure and orthodontic assistance to erupt.
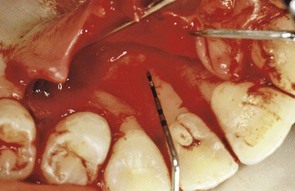
Figure 22-35 Same patient shown in Figure 22-34 with palatal flap reflected to reveal bony dehiscence on the maxillary lateral incisor after orthodontic therapy.
It has been reported that the dentoalveolar gingival fibers that are located within the marginal and attached gingiva are stretched when teeth are rotated during orthodontic therapy.36 Surgical severing or removal of these gingival fibers in combination with a brief period of retention may reduce the incidence of relapse after orthodontic treatment intended to realign rotated teeth.20,102
Extraction of Impacted Third Molars
Numerous clinical studies have reported that the extraction of impacted third molars often results in the creation of vertical defects distal to the second molars.7 This iatrogenic effect is unrelated to flap design28 and appears to occur more often when third molars are extracted in individuals older than 25 years.7,80,99 Other factors that appear to play a role in the development of lesions on the distal surface of second molars, particularly in those older than 25 years, include the presence of visible plaque, bleeding on probing, root resorption in the contact area between second and third molars, presence of a pathologically widened follicle, inclination of the third molar, and close proximity of the third molar to the second molar (Figure 22-36).80 Other potential iatrogenic adverse consequences of removal of third molars include permanent paresthesia (numbness of the lip, tongue, and cheek), which occurs at a frequency of approximately 1 in 100,000 among wisdom teeth removed in the United States.45
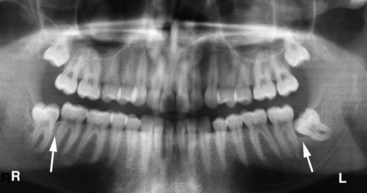
Figure 22-36 Panoramic radiograph illustrating a mesial impacted lower left third molar with a widened follicle and no apparent bone on the distal interproximal surface of the second molar. Alternatively, the lower right third molar is vertically impacted and exhibits interproximal bone distal to the second molar and mesial to the third molar.
Habits and Self-Inflicted Injuries
Patients might not be aware of their self-inflicted injurious habits that may be important to the initiation and progression of their periodontal disease. Mechanical forms of trauma can stem from improper use of a toothbrush, wedging of toothpicks between the teeth, application of fingernail pressure against the gingiva, pizza burns, and others18 (Figure 22-37). Sources of chemical irritation include topical applications of caustic medications such as aspirin or cocaine, allergic reactions to toothpaste and chewing gum, use of chewing tobacco, and concentrated mouthrinses.146 Accidental and iatrogenic gingival injuries may be caused by a variety of chemical, physical, and thermal sources, yet they are generally self-limiting. Iatrogenic injuries are often acute, whereas factitious injuries tend to be more chronic in nature.127
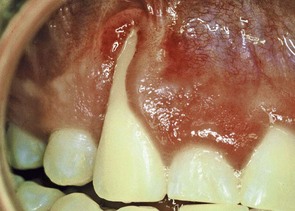
Figure 22-37 Gingival recession on a maxillary canine caused by self-inflicted trauma from the patient’s fingernail.
Trauma Associated with Oral Jewelry
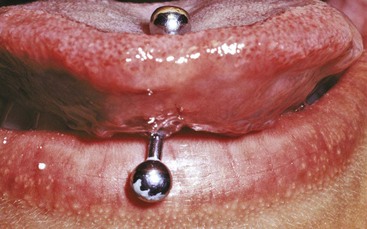
The use of piercing jewelry in the lip or tongue has become more common recently among teenagers and young adults (Figure 22-38). Whittle and Lamden170 surveyed 62 dentists and found that 97% had seen patients with either lip- or tongue-piercing jewelry within the previous 12 months. The incidence of lingual recession with pocket formation (Figure 22-39) and radiographic evidence of bone loss (Figure 22-40) was 50% among subjects with a mean age of 22 years who wore lingual “barbells” for 2 or more years.34 Chipped lower anterior teeth were noted in 47% of the patients who wore tongue-piercing jewelry for 4 years or more. Patients need to be informed of the risks of wearing oral jewelry and cautioned against such practices.
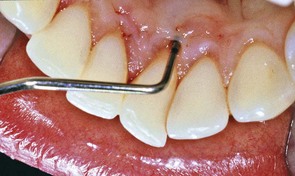
Figure 22-39 Probing depth of 8 mm with 10 mm clinical attachment loss on lingual surface of lower central incisor adjacent to oral jewelry in pierced tongue. Central incisor was found to be vital.
(Courtesy Dr. Leonidas Batas, Minneapolis.)
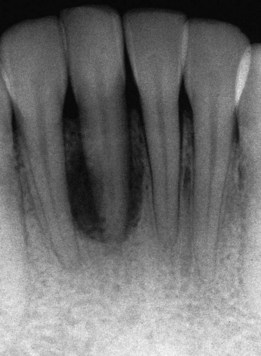
Figure 22-40 Radiograph of lower incisor shown in Figure 22-39 depicting bone loss associated with tongue pierced by oral jewelry.
Toothbrush Trauma
Abrasions of the gingiva, as well as alterations in tooth structure, may result from aggressive brushing in a horizontal or rotary fashion. The deleterious effect of excessively forceful brushing is accentuated when highly abrasive dentifrices are used. The gingival changes attributable to toothbrush trauma may be acute or chronic. The acute changes vary in their appearance and duration from scuffing of the epithelial surface to denudation of the underlying connective tissue with the formation of a painful gingival ulcer (Figure 22-41). Diffuse erythema and denudation of the attached gingiva throughout the mouth may be a striking result of overzealous brushing. Signs of acute gingival abrasion are frequently noted when the patient first uses a new brush. Puncture lesions may be produced when heavy pressure is applied to firm bristles that are aligned perpendicular to the surface of the gingiva. A forcibly embedded toothbrush bristle may be retained in the gingiva and cause an acute gingival abscess.
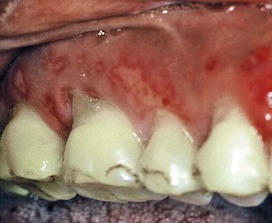
Figure 22-41 Overzealous use of a toothbrush resulted in denudation of the gingival epithelial surface and exposure of the underlying connective tissue as a painful ulcer.
Chronic toothbrush trauma results in gingival recession with denudation of the root surface. Interproximal attachment loss is generally a consequence of bacteria-induced periodontitis, whereas buccal and lingual attachment loss is frequently the result of toothbrush abrasion.153 Improper use of dental floss may result in lacerations of the interdental papilla.
Chemical Irritation
Acute gingival inflammation may be caused by chemical irritation resulting from either sensitivity or nonspecific tissue injury. In allergic inflammatory states, the gingival changes range from simple erythema to painful vesicle formation and ulceration. Severe reactions to ordinarily innocuous mouthwashes, dentifrices, or denture materials are often explainable on this basis.
Acute inflammation with ulceration may be produced by the nonspecific injurious effect of chemicals on the gingival tissues. The indiscriminate use of strong mouthwashes, topical application of corrosive drugs such as aspirin (Figure 22-42) or cocaine, and accidental contact with drugs such as phenol or silver nitrate are common examples of chemicals that cause irritation of the gingiva. A histologic view of an aspirin-induced chemical burn shows vacuoles with serous exudates and an inflammatory infiltrate in the connective tissue (Figure 22-43).
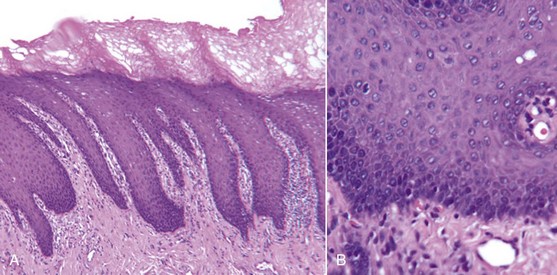
Smokeless Tobacco
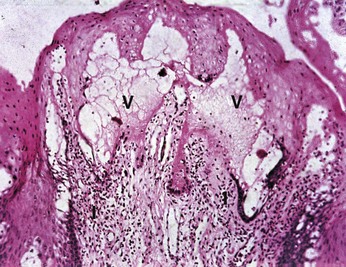
Snuff and chewing tobacco constitute the two main forms of smokeless tobacco. Snuff is a fine cut form of tobacco available as loosely packed or in small sachets. Chewing tobacco is a more coarse-cut available in the form of loose leaves, a solid block, or plug and as a twist of dried leaves. Chewing tobacco is typically placed in the mandibular buccal vestibule for several hours, during which time saliva and dilute tobacco is periodically expectorated.167 The nicotine uptake of smokeless tobacco is similar to smoking cigarettes in that consumption of a 34-g container of smokeless tobacco is approximately equal to 1.5 packs of cigarettes.55 Many professional baseball players use chewing tobacco. Perceived benefits of chewing tobacco are those derived from nicotine, including improved mental alertness and diminished reaction time, muscle relaxation, and reduced anxiety and appetite.56,132 A 1990 survey of 1109 professional baseball players in the United States reported 39% of the players used smokeless tobacco with 46% of the users exhibiting leukoplakia within the gingiva and/or mucosa (Figure 22-44).40 Histologic features of oral leukoplakia associated with smokeless tobacco include (1) chevronlike pattern of hyperkeratosis with focal areas of inflammation and (2) hyperplasia in the basal cell layer (Figure 22-45). Increased incidence of gingival recession, cervical root abrasion, and root caries have been reported with smokeless tobacco (Figure 22-46).113,133,134,160 The incidence of gingival recession among adolescents who use smokeless tobacco has been reported as 42% compared to 17% among nonusers.31,100,101,103 The NHANES III epidemiologic study investigated the adverse effect of smokeless tobacco on the periodontium and found double the incidence of severe periodontitis (odds ratio 2.1; 95% CI 1.2 to 3.7) among 12,932 adults who used smokeless tobacco but never smoked cigarettes.41 However, Bergstrom et al found similar incidences of severe periodontitis among both smokeless tobacco users and nonusers.12 It can be concluded that the use of smokeless tobacco is associated with at least localized gingival recession, clinical attachment loss, leukoplakia, and possibly enhanced susceptibility to severe periodontitis.
Radiation Therapy
Radiation therapy has cytotoxic effects on both normal cells and malignant cells. A typical total dose of radiation for head and neck tumors is in the range of 5000 to 8000 cGy (cGy = 1 rad) or equivalent to 50 to 80 Sieverts (Sv).119 The total dose of radiation is generally given in partial incremental doses referred to as fractionation. Fractionation helps minimize the adverse effects of the radiation while maximizing the death rate for the tumor cells.42 Fractionated doses are typically administered in the range of 100 to 1000 cGy, or 1 to 10 Sv, per week.
Radiation treatment induces an obliterative endarteritis that results in soft tissue ischemia and fibrosis while irradiated bone becomes hypovascular and hypoxic.97 Adverse affects of head and neck radiation therapy include dermatitis and mucositis of the irradiated area, as well as muscle fibrosis and trismus, which may restrict access to the oral cavity.135 The mucositis typically develops 5 to 7 days after radiation therapy is initiated. The severity of the mucositis can be reduced by asking the patient to avoid secondary sources of irritation, such as smoking, alcohol, and spicy foods, to the mucous membrane. Use of a chlorhexidine digluconate mouthrinse may help reduce the mucositis.125 However, most chlorhexidine mouthrinses currently available in the U.S. have a high alcohol content that may act as an astringent, which dehydrates the mucosa, thereby intensifying the pain. Saliva production is permanently impaired when salivary glands that are located within the portal of radiation receive ≥6000 cGy or 60 Sv.98 Xerostomia results in greater plaque accumulation and a reduced buffering capacity by saliva. The use of effective oral hygiene, professional dental prophylaxis cleanings, fluoride applications, and frequent dental examinations are essential to control caries and periodontal disease. The use of customized trays appears to be a more effective method of fluoride application as compared with the toothbrush.76
Periodontal attachment loss and tooth loss has been reported to be greater in cancer patients who were treated with high-dose unilateral radiation as compared with the nonradiated control side of the dentition.39 Patients diagnosed with oral cancer that require radiation therapy should ideally be assessed for dental needs (mucositis, xerostomia, faulty restorations, periapical lesion, coronal and cervical decay, and periodontal status) before initiating radiation treatment.59 Treatment and prevention of trismus, oral fungal infections, odontogenic infections, osteoradionecrosis, decay, and periodontal disease is critical to minimize oral morbidity for these patients. Dental and periodontal infections have the potential to be a severe risk for a patient who has been treated with head and neck radiation. The risk of osteoradionecrosis for oncology patients can be minimized by evaluating their oral status, providing dental care, and allowing time for tissue repair before the commencement of radiotherapy.78
Oral conditions that increased the risk of osteoradionecrosis for patients about to undergo radiation therapy for oral malignancies include >5 mm periodontal probing depths, >40% dental plaque score, and >60% alveolar bone loss.74 Nonrestorable teeth and/or teeth with significant periodontal problems should be extracted before radiation therapy to reduce the risk of postradiotherapy osteoradionecrosis.78 (See Chapter 37 for more detail on the periodontal management of medically compromised patients.)
The risk of osteoradionecrosis must be evaluated before performing atraumatic extractions or limited periodontal surgical procedures in previously irradiated sites. Therefore the dentist may choose to consult with the patient’s oncologist before initiating therapy. A recent randomized multicenter clinical trial questions the merit of using hyperbaric oxygen (HBO) therapy to treat osteoradionecrosis, since at 1-year after treatment only 19% of test subjects responded to HBO versus 32% of placebo subjects.5 Administration of the combination of pentoxifylline with vitamin E as antioxidants currently shows the greatest promise for revascularization and treatment of osteoradionecrosis sites.33
![]() Science Transfer
Science Transfer
Calculus plays a significant role in periodontal inflammation primarily because it provides an ideal surface for bacterial plaque accumulation. One of the universal goals for treatment of gingivitis and periodontitis is the complete removal of all calculus, particularly subgingival calculus. The presence of residual subgingival calculus after treatment predisposes the patient to ongoing periodontal destruction. The removal of calculus embedded into cementum with root planing is one of the most difficult procedures in periodontal therapy and requires special skills that clinicians are only able to develop with repeated procedures over time. Exposing subgingival calculus with surgical approaches increases the possibility of calculus removed compared to nonsurgical root planing. Therefore the cornerstone of all periodontal flap techniques is commitment to root planing, even though the patient may have previously had nonsurgical or initial therapy.
Other predisposing factors for periodontitis, such as restorative inadequacies and poorly designed removable partial dentures, are significant primarily because they increase the accumulation of bacterial dental plaque. Loss of even one tooth can lead to a plethora of positional changes of adjacent and opposing teeth with an increasing risk of periodontal breakdown caused by open contacts, plunger cusps, and occlusal instability. Thus it is good clinical procedure to replace missing teeth with implants or fixed bridges to maintain periodontal health of the remaining dentition.
1 Addy M, Dummer P, Hunter M, et al. A study of the association of fraenal attachment, lip coverage and vestibular depth with plaque and gingivitis. J Periodontol. 1987;58:752.
2 Albandar J, Kingman A, Brown L, et al. Gingival inflammation and subgingival calculus as determinants of disease progression in early-onset periodontitis. J Clin Periodontol. 1998;25:231.
3 Alexander A. Habitual mouth breathing and its effect on gingival health. Parodontologie. 1970;24:49.
4 Anerud A, Löe H, Boysen H. The natural history and clinical course of calculus formation in man. J Clin Periodontol. 1991;18:160.
5 Annane D, Depondt J, Aubert P, et al. Hyperbaric oxygen therapy for radionecrosis of the jaw: a randomized, placebo-controlled, double-blind trial from the ORN96 study group. I Clin Oncol. 2004;22:4893-4900.
6 App G. Effect of silicate, amalgam and cast gold on the gingiva. J Prosthet Dent. 1961;11:522.
7 Ash M, Costich E, Hayward J. A study of periodontal hazards of third molars. J Periodontol. 1962;33:209.
8 Baer P, Burstone M. Esterase activity associated with formation of deposits on teeth. Oral Surg. 1959;12:1147.
9 Baer P, Keyes P, White C. Studies on experimental calculus formation in the rat. XII. On the transmissibility of factors affecting dental calculus. J Periodontol. 1968;39:86.
10 Baumhammers A, Stallard R. A method for the labeling of certain constituents in the organic matrix of dental calculus. J Dent Res. 1966;45:1568.
11 Bergman B, Hugoson A, Olsson C. Periodontal and prosthetic conditions in patients treated with removable partial dentures and artificial crowns. Acta Odontol Scand. 1971;29:621.
12 Bergstrom J, Keilani H, Lundholm C, et al. Smokeless tobacco (snuff) use and periodontal bone loss. J Clin Periodontol. 2006;33:549-554.
13 Biancu S, Ericsson I, Lindhe J. Periodontal ligament tissue reactions to trauma and gingival inflammation. An experimental study in beagle dogs. J Clin Periodontol. 1995;22:772.
14 Bibby B. The formation of salivary calculus. Dent Cosmos. 1935;77:668.
15 Birkeland J, Jorkjend L. The effect of chewing apples on dental plaque and food debris. Commun Dent Oral Epidemiol. 1974;2:161.
16 Bissada N, Ibrahim S, Barsoum W. Gingival response to various types of removable partial dentures. J Periodontol. 1974;45:651.
17 Bjorn AL, Bjorn H, Grcovic B. Marginal fit of restorations and its relation to periodontal bone level. Odont Revy. 1969;20:311.
18 Blanton P, Hurt W, Largent M. Oral factitious injuries. J Periodontol. 1977;48:33.
19 Bondemark L. Interdental bone changes after orthodontic treatment: a 5-year longitudinal study. Am J Orthod Dentofac Orthop. 1998;114:25.
20 Brain W. The effect of surgical transsection of free gingival fibers on the regression of orthodontically rotated teeth in the dog. Am J Orthod. 1969;55:50.
21 Brezniak N, Wasserstein A. Root resorption after orthodontic treatment: Part 1. Literature review. Am J Orthod Dentofac Orthop. 1993;103:62.
22 Brezniak N, Wasserstein A. Root resorption after orthodontic treatment: Part 2. Literature review. Am J Orthod Dentofac Orthop. 1993;103:138.
23 Buchanan S, Jenderseck R, Granet M, et al. Radiographic detection of dental calculus. J Periodontol. 1987;58:747.
24 Buckley L. The relationship between malocclusion and periodontal disease. J Periodontol. 1972;43:415.
25 Carlsson G, Hedegard B, Koivumaa K. Studies in partial dental prosthesis. IV. Final results of a four-year longitudinal investigation of dentogingivally supported partial dentures. Acta Odontol Scand. 1965;23:443.
26 Carranza F, Sr Carraro J. El empuje lingual como factor traumatizante en periodoncia. Rev Asoc Odontol Argent. 1959;47:105.
27 Chaikin B. Anterior periodontal destruction due to the loss of one or more unreplaced molars. Dent Items Int. 1939;61:17.
28 Chin Quee T, Gosselin D, Millar E, et al. Surgical removal of the fully impacted mandibular third molar. The influence of flap design and alveolar bone height. J Periodontol. 1985;56:625.
29 Clerehugh V, Abdeia R, Hull P. The effect of subgingival calculus on the validity of clinical probing measurements. J Dentistry. 1996;24:329.
30 Conroy C, Sturzenberger O. The rate of calculus formation in adults. J Periodontol. 1968;39:142.
31 Creath C, Cutter G, Bradley D, et al. Oral leukoplakia and adolescent smokeless tobacco use. Oral Surg Oral Med Oral Pathol. 1991;72:35.
32 Dawes C. Recent research on calculus. N Zealand Dent J. 1998;94:60.
33 Delanian S, Lefaix JL. Current management for late normal tissue injury: radiation-induced fibrosis and necrosis. Semin Radiat Oncol. 2007;17:99-107.
34 De Moor RJ, et al. Dental and oral complications of lip and tongue piercings. Br Dent J. 2005;199:506-509.
35 Diamanti-Kipioti A, Gusberti F, Lang N. Clinical and microbiological effects of fixed orthodontic appliances. J Clin Periodontol. 1987;14:326.
36 Edwards J. A study of the periodontium during orthodontic rotation of teeth. Am J Orthod. 1968;54:441.
37 Egelberg J. Local effect of diet on plaque formation and development of gingivitis in dogs. III. Effect of frequency of meals and tube feeding. Odontol Revy. 1965;16:50.
38 Ennever J. Microbiologic mineralization: a calcifiable cell-free extract from a calcifiable microorganism. J Dent Res. 1983;41:1383.
39 Epstein J, Lunn R, Le N, et al. Periodontal attachment loss in patients after head and neck radiation therapy. Oral Surg Oral Med Oral Pathol. 1998;86:673.
40 Ernster VL, Grady DG, Greene JC, et al. Smokeless tobacco use and health effects among baseball players. JAMA. 1990;264:218-224.
41 Fisher MA, Taylor GW, Tilashalski KR. Smokeless tobacco and severe active periodontal disease, NHANES III. J Dent Res. 2005;84:705-710.
42 Fletcher G. The role of irradiation in the management of squamous-cell carcinomas of the mouth and throat. Head Neck Surg. 1979;1:441.
43 Flemmig T, Sorensen J, Newman M, et al. Gingival enhancement in fixed prosthodontics. Part II. Microbiologic findings. J Prosthet Dent. 1991;65:365.
44 Flores-de-Jacoby L, Zafiropoulos G, Ciancio S. The effect of crown margin location on plaque and periodontal health. Int J Periodont Restor Dent. 1989;9:197.
45 Friedman JW. The prophylactic extraction of third molars: a public health hazard. Am J Public Health. 2007;97:1554-1559.
46 Geiger A, Wasserman B, Turgeon L. Relationship of occlusion and periodontal disease. VIII. Relationship of crowding and spacing to periodontal destruction and gingival inflammation. J Periodontol. 1974;45:43.
47 Ghamrawy E. Quantitative changes in dental plaque formation related to removable partial dentures. J Oral Rehabil. 1976;3:115.
48 Ghamrawy E. Qualitative changes in dental plaque formation related to removable partial dentures. J Oral Rehabil. 1979;6:183.
49 Gilmore N, Sheiham A. Overhanging dental restorations and periodontal disease. J Periodontol. 1971;42:8.
50 Glock G, Murray M. Chemical investigation of salivary calculus. J Dent Res. 1938;17:257.
51 Gonzales F, Sognnaes R. Electromicroscopy of dental calculus. Science. 1960;131:156.
52 Gorzo I, Newman H, Strahan J. Amalgam restoration, plaque removal and periodontal health. J Clin Periodontol. 1979;6:98.
53 Greene J. Oral hygiene and periodontal disease. Am J Public Health. 1963;53:913.
54 Grossi S, Genco R, Machtei E, et al. Assessment of risk for periodontal disease. II Risk indicators for alveolar bone loss. J Periodontol. 1995;66:23.
55 Guggenheimer J. Implications of smokeless tobacco use in athletes. Dent Clin North Am. 1991;35:797-808.
56 Guggenheimer J. Tobacco and athletics. Update and current status. Dent Clin North Am. 2000;44:179-187.
57 Gustafsson B, Krasse B. Dental calculus in germ free rats. Acta Odontol Scand. 1962;20:135.
58 Hakkarainen K, Ainamo J. Influence of overhanging posterior tooth restorations of alveolar bone height in adults. J Clin Periodontol. 1980;7:114.
59 Hancock PJ, et al. Oral and dental management related to radiation therapy for head and neck cancer. J Can Dent Assoc. 2003;69:585-590.
60 Harrel SK, Nunn ME. The effect of occlusal discrepancies on periodontitis. II. Relationship of occlusal treatment to the progression of periodontal disease. J Periodontol. 2001;72:495-505.
61 Highfield J, Powell R. Effects of removal of posterior overhanging metallic margins of restorations upon the periodontal tissues. J Clin Periodontol. 1978;5:169.
62 Hinrichs J, ElDeeb M, Waite D, et al. Periodontal evaluation of canines erupted through grafted alveolar cleft defects. J Oral Maxillofac Surg. 1984;42:717.
63 Hirschfeld I. Food impaction. J Am Dent Assoc. 1930;17:1504.
64 Hodge H, Leung S. Calculus formation. J Periodontol. 1950;21:211.
65 Huttner G. Follow-up study of crowns and abutments with regard to the crown edge and the marginal periodontium. Dtsch Zahnarztl Z. 1971;26:724.
66 Jacobson L. Mouth breathing and gingivitis. J Periodont Res. 1973;8:269.
67 Jacobson L, Linder-Aronson S. Crowding and gingivitis: a comparison between mouth breathers and non-mouth breathers. Scand J Dent Res. 1972;80:500.
68 Jeffcoat M, Howell T. Alveolar bone destruction due to overhanging amalgam in periodontal disease. J Periodontol. 1980;51:599.
69 Jenkins G. The physiology of the mouth. Oxford: Blackwell Scientific Publications; 1966.
70 Jernberg G, Bakdash B, Keenan K. Relationship between proximal tooth open contact and periodontal disease. J Periodontol. 1983;54:529.
71 Kaley J, Phillips C. Factors related to root resorption in edgewise practice. Angle Orthod. 1991;61:125.
72 Karlsen K. Gingival reactions to dental restorations. Acta Odontol Scand. 1970;28:895.
73 Kashket S, Zhang J, Niederman R. Gingival inflammation induced by food and short-chain carboxylic acids. J Dent Res. 1998;77:412.
74 Katsura K, Sasai K, Sato K, Saito M, Hoshina H, Hayashi T. Relationship between oral health status and development of osteoradionecrosis of the mandible: a retrospective longitudinal study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:731-738.
75 Kawakara H, Yamagani A, Nakamura MJr. Biological testing of dental materials by means of tissue culture. Int Dent J. 1968;18:443.
76 Keene H, Fleming T, Toth B. Cariogenic microflora in patients with Hodgkin’s disease before and after mantle field radiotherapy. Oral Surg Oral Med Oral Pathol. 1994;78:577.
77 Kepic T, O’Leary T. Role of marginal ridge relationships as an etiologic factor in periodontal disease. J Periodontol. 1978;49:570.
78 Koga DH, Salvajoli JV, Alves FA. Dental extractions and radiotherapy in head and neck oncology: review of the literature. Oral Dis. 2008;14:40-44.
79 Koivumaa K, Wennstrom A. A histological investigation of the changes in gingival margins adjacent to gold crowns. Odont T. 1960;68:373.
80 Kugelberg C. Third molar surgery. Curr Opin Dent. 1992;2:9.
81 Kupczyk L, Conroy M. The attachment of calculus to root planed surfaces. Periodontics. 1968;6:78.
82 Lang N, Kiel R, Anderhalden K. Clinical and microbiological effect of subgingival restorations with overhanging or clinically perfect margins. J Clin Periodontol. 1983;10:563.
83 Leach S, Saxton C. An electron microscopic study of the acquired pellicle and plaque formed on the enamel of human incisors. Arch Oral Biol. 1966;11:1081.
84 Leon A. Amalgam restorations and periodontal disease. Br Dent J. 1976;140:377.
85 Leung S, Jensen A. Factors controlling the deposition of calculus. Int Dent J. 1958;8:613.
86 Lilienthal B, Amerena V, Gregory G. An epidemiological study of chronic periodontal disease. Arch Oral Biol. 1965;10:553.
87 Little M, Bowman L, Casciani C, et al. The composition of dental calculus. III. Supragingival calculus. The amino acid and saccharide component. Arch Oral Biol. 1966;11:385.
88 Little M, Bowman L, Dirksen T. The lipids of supragingival calculus. J Dent Res. 1964;43:836.
89 Little M, Hazen S. Dental calculus composition. 2. Subgingival calculus: ash, calcium, phosphorus, and sodium. J Dent Res. 1964;43:645.
90 Löe H, Schiott C. The effect of mouth rinses and topical application of chlorhexidine on the development of dental plaque and gingivitis in man. J Periodont Res. 1970;5:79.
91 Lupi J, Handelman C, Sadowsky C. Prevalence and severity of apical root resorption and alveolar bone loss in orthodontically treated adults. Am J Orthod Dentofac Orthop. 1996;109:28.
92 Mandel I. Calculus formation. The role of bacteria and mucoprotein. Dent Clin North Am. 1960;4:731.
93 Mandel I. Histochemical and biochemical aspects of calculus formation. Periodontics. 1963;1:43.
94 Mandel I. Biochemical aspects of calculus formation. J Periodont Res. 1969;4(suppl):7.
95 Mandel I, Levy B, Wasserman B. Histochemistry of calculus formation. J Periodontol. 1957;28:132.
96 Manly R. A structureless recurrent deposit on teeth. J Dent Res. 1973;22:479.
97 Marciani R, Ownby H. Osteoradionecrosis of the jaw. J Oral Maxillofac Surg. 1986;44:218.
98 Markitziu A, Zafiropoulos G, Tsalkis L, et al. Gingival health and salivary function in head and neck-irradiated patients. A five year follow-up. Oral Surg Oral Med Oral Pathol. 1992;73:427.
99 Marmary Y, Brayer L, Tzukert A, et al. Alveolar bone repair following extraction of impacted mandibular third molars. Oral Surg Oral Med Oral Pathol. 1986;61:324.
100 Martin G, Brown J, Eifler C, et al. Oral leukoplakia status six weeks after cessation of smokeless tobacco use. J Amer Dent Assoc. 1999;130:945.
101 Miller P. Root coverage with the free gingival graft. Factors associated with incomplete coverage. J Periodontol. 1987;58:674.
102 Moffett B. Remodeling changes of the facial sutures, periodontal and temporomandibular joints produced by orthodontic forces in Rhesus monkeys. Bull Pac Coast Soc Ortho. 1969;44:46.
103 Monten U, Wennstrom JL, Ramberg P. Periodontal conditions in male adolescents using smokeless tobacco (moist snuff). J Clin Periodontol. 2006;33:863-868.
104 Morris M. Artificial crown contours and gingival health. J Prosthet Dent. 1962;12:1146.
105 Mueller H. The effect of artificial crown margins on the periodontal conditions in a group of periodontally supervised patients treated with fixed bridges. J Clin Periodontol. 1986;13:97.
106 Mühlemann H, Schroeder H. Dynamics of supragingival calculus formation. Adv Oral Biol. 1964;1:175.
107 Mühler J, Ennever J. Occurrence of calculus through several successive periods in a selected group of subjects. J Periodontol. 1962;33:22.
108 Mukherjee S. Formation and prevention of supragingival calculus. J Periodont Res. 1968;3(suppl 2):1.
109 Neuman W, Neuman M. The chemical dynamics of bone mineral. Chicago: University of Chicago Press; 1958.
110 Norman R, Mehia R, Swartz M, et al. Effect of restorative materials on plaque composition. J Dent Res. 1972;51:1596.
111 Normann W, Regolati B, Renggli H. Gingival reaction to well-fitted subgingival proximal gold inlays. J Clin Periodontol. 1974;1:120.
112 Nunn ME, Harrel SK. The effect of occlusal discrepancies on periodontitis. I. Relationship of initial occlusal discrepancies to initial clinical parameters. J Periodontol. 2001;72:485-494.
113 Offenbacher S, Weathers DR. Effects of smokeless tobacco on the periodontal, mucosal and caries status of adolescent males. J Oral Pathol. 1985;14:169-181.
114 O’Leary T, Badell M, Bloomer R. Interproximal contact and marginal ridge relationships in periodontally healthy young males classified as to orthodontic status. J Periodontol. 1975;46:6.
115 Pack A, Coxhead L, McDonald B. The prevalence of overhanging margins in posterior amalgam restorations and periodontal consequences. J Clin Periodontol. 1990;17:145.
116 Paolantonio M, Girolamo G, Pedrazzoli V, et al. Occurrence of Actinobacillus actinomycetemcomitans in patients wearing orthodontic appliances. A cross-sectional study. J Clin Periodontol. 1996;23:112.
117 Paunio K. The role of malocclusion and crowding in the development of periodontal disease. Int Dent J. 1973;23:420.
118 Pearson L. Gingival height of lower central incisors, orthodontically treated and untreated. Angle Orthod. 1968;38:337.
119 Peterson D, D’Ambrosio J. Nonsurgical management of head and neck cancer patients. Dent Clin North Am. 1994;38:425.
120 Pizzo G, Licata ME, Guiglia R, Giuliana G. Root resorption and orthodontic treatment. Review of the literature. Minerva Stomatol. 2007;56:31-44.
121 Polson A, Reed BE. Long-term effect of orthodontic treatment on crestal alveolar bone levels. J Periodontol. 1984;55:28.
122 Polson A, Adams R, Zander H. Osseous repair in the presence of active tooth hypermobility. J Clin Periodontol. 1983;10:370.
123 Polson A. The relative importance of plaque and occlusion in periodontal disease. J Clin Periodontol. 1986;13:923.
124 Prinz H. The origin of salivary calculus. Dent Cosmos. 1921;63:231. 369, 503, 619
125 Raether D, Walker P, Bostrum B, et al. Effectiveness of oral chlorhexidine for reducing stomatitis in a pediatric bone marrow transplant population. Pediatr Dent. 1989;11:37.
126 Ramfjord S. The periodontal status of boys 11 to 17 years old in Bombay, India. J Periodontol. 1961;32:237.
127 Rawal SY, Claman LJ, Kalmar JR, Tatakis DN. Traumatic lesions of the gingiva: a case series. J Periodontol. 2004;75:762-769.
128 Reitan K. Tissue changes following experimental tooth movement as related to the time factor. Dent Record. 1953;73:559.
129 Renggli H. The influence of subgingival proximal filling borders on the degree of inflammation of the adjacent gingiva. A clinical study. Schweiz Monatsschr Zahnheilkd. 1974;84:181.
130 Renggli H, Regolati B. Gingival inflammation and plaque accumulation by well-adapted supragingival and subgingival proximal restorations. Helv Odontol Acta. 1972;16:99.
131 Rizzo A, Martin G, Scott D, et al. Mineralization of bacteria. Science. 1962;135:439.
132 Robertson PB, DeRouen TA, Ernster V, et al. Smokeless tobacco use: how it affects the performance of major league baseball players. J Am Dent Assoc. 1995;126:1115-1121.
133 Robertson PB, Walsh M, Greene J, et al. Periodontal effects associated with the use of smokeless tobacco. J Periodontol. 1990;61:438-443.
134 Robertson PB, Walsh MM, Greene JC. Oral effects of smokeless tobacco use by professional baseball players. Adv Dent Res. 1997;11:307-312.
135 Rothwell B. Prevention and treatment of the orofacial complications of radiotherapy. J Am Dent Assoc. 1987;114:316.
136 Rowles S. The inorganic composition of dental calculus. In: Blackwood HJ, editor. Bone and tooth. Oxford: Pergamon Press, 1964.
137 Sanchez-Sotres L, Van Huysen G, Gilmore H. A histologic study of gingival tissue response to amalgam, silicate and resin restorations. J Periodontol. 1969;42:8.
138 Schoff F. Periodontia: an observation on the attachment of calculus. Oral Surg. 1955;8:154.
139 Schroeder H. Inorganic content and histology of early dental calculus in man. Helv Odontol Acta. 1963;7:17.
140 Schroeder H. Crystal morphology and gross structures of mineralizing plaque and of calculus. Helv Odontol Acta. 1965;9:73.
141 Schroeder H. Formation and inhibition of dental calculus. Berne: Hans Huber; 1969.
142 Schroeder H, Bambauer H. Stages of calcium phosphate crystallization during calculus formation. Arch Oral Biol. 1966;11:1.
143 Schwartz A. Tissue changes incidental to orthodontic tooth movement. Ortho Oral Surg Rad Int J. 1932;18:331.
144 Scoman S. Study of the relationship between periodontal disease and the wearing of partial dentures. Aust Dent J. 1963;8:206.
145 Selvig J. Attachment of plaque and calculus to tooth surfaces. J Periodont Res. 1970;5:8.
146 Serio F, Siegel M, Slade B. Plasma cell gingivitis of unusual origin. J Periodontol. 1991;62:390.
147 Serio F, Hawley C. Periodontal trauma and mobility. Diagnosis and treatment planning. Dent Clin North Am. 1999;43:37.
148 Setz J, Diehl J. Gingival reaction on crowns with cast and sintered metal margins: a progressive study. J Prosthet Dent. 1994;71:442.
149 Sharawy A, Sabharwal K, Socransky S, et al. A quantitative study of plaque and calculus formation in normal and periodontally involved mouths. J Periodontol. 1966;37:495.
150 Silness J. Fixed prosthodontics and periodontal health. Dent Clin North Am. 1980;24:317.
151 Sorensen J, Doherty F, Newman M, et al. Gingival enhancement in fixed prosthodontics. Part I. Clinical findings. J Prosthet Dent. 1991;65:100.
152 Sorensen J, Larsen I, Jorgensen K. Gingival and alveolar bone response to marginal fit of subgingival crown margins. Scand J Dent Res. 1986;94:109.
153 Spieler E. Preventing toothbrush abrasion and the efficacy of the Alert toothbrush. Comp Continu Edu Dent. 1996;17:478.
154 Standford J. Analysis of the organic portion of dental calculus. J Dent Res. 1966;45:128.
155 Stanton G. The relation of diet to salivary calculus formation. J Periodontol. 1969;40:167.
156 Stewart R, Ratcliff P. The source of components of subgingival plaque and calculus. Periodont Abstr. 1966;14:102.
157 Sutcliffe P. Chronic anterior gingivitis: an epidemiological study in school children. Br Dent J. 1968;125:47.
158 Theilade J, Schroeder H. Recent results in dental calculus research. Int Dent J. 1966;16:205.
159 Tibbetts L, Kashiwa H. A histochemical study of early plaque mineralization. Abstract No. 616. J Dent Res. 1970;19:202.
160 Tomar SL, Winn DM. Chewing tobacco use and dental caries among U.S. men. J Am Dent Assoc. 1999;130:1601-1610.
161 Turesky S, Renstrup G, Glickman I. Effects of changing the salivary environment on progress of calculus formation. J Periodontol. 1962;33:45.
162 Volker J, Pinkerton D. Acid production in saliva carbohydrates. J Dent Res. 1947;26:229.
163 Volpe A, Kupczak L, King W, et al. In vivo calculus assessment. Part IV. Parameters of human clinical studies. J Periodontol. 1969;40:76.
164 Von der Fehr F, Brudevold F. In vitro calculus formation. J Dent Res. 1960;39:1041.
165 Waerhaug J. The source of mineral salts in subgingival calculus. J Dent Res. 1955;34:563.
166 Waerhaug J, Zander H. Reaction of gingival tissue to self-curing acrylic restorations. J Am Dent Assoc. 1957;54:760.
167 Walsh PM, Epstein JB. The oral effects of smokeless tobacco. J Can Dent Assoc. 2000;66:22-25.
168 Wasserman B, Mandel J, Levy BM. In vitro calcification of calculus. J Periodontol. 1958;29:145.
169 White D. Dental calculus: recent insights into occurrence, formation, prevention, removal and oral health effects of supragingival and subgingival deposits. Eur J Oral Sci. 1997;105:508.
170 Whittle JG, Lamden KH. Lip and tongue piercing: experiences and views of general dental practitioners in South Lancashire. Prim Dent Care. 2004;11:92-96.
171 Wise M, Dykema R. The plaque retaining capacity of four dental materials. J Prosthet Dent. 1975;33:178.
172 Yuodelis R, Weaver J, Sapkos S. Facial and lingual contours of artificial complete crowns and their effect on the periodontium. J Prosthet Dent. 1973;29:61.
173 Zadik Y, Sandler V, Bechor R, Salehrabi R. Analysis of factors related to extraction of endodontically treated teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:31-35.
174 Zander H. The attachment of calculus to root surfaces. J Periodontol. 1953;24:16.
175 Zander H, Hazen S, Scott D. Mineralization of dental calculus. Proc Soc Exp Biol Med. 1960;103:257.